Chapter 6 Contextual influences
nutrition, respiration and other factors
This text has as its primary focus the manual, biomechanical, evaluation and treatment approaches appropriate to care of dysfunction and pain problems. It is unwise, however, to restrict attention to a simplistic formula that suggests that there are only ‘mechanical solutions for mechanical problems’. A subtext, elaborated on in Volume 1, Chapters 4 and 7 and in this text’s ‘Essential information’ chapter, enunciates the view of complex, rather than simplistic, etiologies for most forms of dysfunction and pain. It is important that contextual influences always be considered, including chronobiological factors, nutrition, endocrine responses, anxiety and breathing patterns.
Even apparently straightforward conditions, such as sprains and strains, have a biochemical (and all too often an emotional) overlay and anyone dealing with such problems should be aware of the potential for assisting recovery through biochemical means. In this chapter, a number of background issues will be discussed that aim to broaden the understanding of features of pain and dysfunction that may be modified through manipulation of diet or through appropriate medication. The authors have focused their attention in this chapter on those influences that pertain to chronic pain management. This does not undervalue the tremendous potential influence that these perpetuating and influencing factors can have on other health concerns, such as cancer, arteriosclerosis, attention deficit disorders and a host of other conditions. Although all these are important, they are not within the scope of this text.
Implicit in this focus on biochemistry is a need for awareness of the influences of such factors as sleep and breathing patterns on the chemical processes involved in most conditions involving inflammation, pain and the healing of tissues (Adams 1977, Affleck 1996). Also of great importance is the need for the practitioner to operate within the scope of her license and training. Even if the license allows for the practice of counseling these factors, the need for appropriate training and maintenance of current continuing education requirements is important for the provision of optimal clinical care.
Chronobiology
Where inflammation is part of the cause of a painful condition, anything that reduces or modifies the inflammatory process is likely to reduce the level of perceived pain. However, while inflammation may not be pleasant, it is a vitally important process in repairing (or defending against) damage, irritation or infection. Therefore, strategies that try to modify inflammation need to aim at a limited degree of modulation, rather than total elimination of this healing process. See Volume 1, Chapter 7 for further discussion of a variety of influences on local and systemic inflammation.
Before assessing nutritional influences on inflammation and pain, note should be taken of research that demonstrates the existence of diurnal patterns that profoundly influence inflammatory processes and that explains why inflammation, of all sorts, is normally more intense at night. The normal pattern results in inflammatory processes alternating with those aspects of immune functions concerned with defense against infection; however, these diurnal patterns can be disrupted by a number of factors (Petrovsky & Harrison 1998, Petrovsky et al 1998).
Those systems of the body that defend against attack by bacteria or viruses are far more active between roughly 10am and 10pm. This involves key elements of the immune system’s surveillance and defense capabilities. For example, T helper cells 1 (Th1) assist B cells and other T cells, and are involved in the secretion of interleukin-2, interleukin-12 and gamma-interferon, promoting the transformation of CD8 suppressor cells into NK (natural killer) cytotoxic cells, which play a vital role in the inactivation of virally infected and mutagenic cells.
Defensive and repair processes, of which inflammation is a part, are more active between roughly 10pm and the following 10am. For example, Sutherland (2005) reports that as many as 75% of asthmatic subjects are awakened by asthma symptoms, at least once per week, with approximately 40% experiencing nocturnal symptoms on a nightly basis. A great deal of research demonstrates that nocturnal symptoms of cough and dyspnea accompany circadian (i.e. chrononbiological) variations in airway inflammation and physiologic variables.
Monro (2001) reports that: ‘A natural cycling between the defensive and repair modes of aspects of the immune system is disturbed in ill-health and a chronic cytokine shift may lock the body into a pro-inflammatory state’.
These patterns are, therefore, capable of being disrupted. Various events and circumstances, which can largely be described as ‘stressful events’, seem capable of altering the diurnal rhythms, so that the inflammatory phase can stay ‘switched on’ for most of the time, not just at night. When this happens the defensive phase of the cycle is relatively weakened, creating a greater likelihood of infection. This can occur because of:
• exposure to carbamate and organophosphate insecticides, which inhibit interleukin-2, essential for Th1 function
• intake of steroids, such as cortisone
• ‘Stress, both psychological and physical. Stress activates the hypothalamo–pituitary–adrenal axis and leads to increased production of cortisol. Excessive exercise and deprivation of food or sleep also result in a falling ratio of DHEA to cortisol and an increase in a Th1 to Th2 shift. It is known that Epstein-Barr virus antibody titers rise amongst students facing examinations and that this virus is usually controlled by a Th1 response. Stress causes increased viral replication and hence antibody production’ (Monro 2001).
• Cancer. ‘Many of the risk factors for cancer, such as carcinogenic chemicals or tobacco smoke also cause long-term inflammation and lower Th1 levels’ (Monro 2001).
Sleep and pain
Additional to these influences, disturbed sleep patterns can produce negative effects on pain and recovery from injury. Any disruption of stage 4 sleep results in reduction in growth hormone production by the pituitary gland, leading to poor repair of irritated, inflamed and damaged tissues and longer recovery times (Griep 1994, Moldofsky & Dickstein 1999).
‘The interaction of the circadian sleeping-waking brain and the cytokine-immune-endocrine system is integral to preserving homeostasis. … there may be host defense implications for altered immune and endocrine functions in sleep-deprived humans. Activation of cytokines and sleepiness occur during the acute phase response to bacterial or viral disease. There are disturbances in sleep and cytokine-immune functions in chronic protozoal and viral disease… Sleep-related physiological disturbances may play a role in autoimmune diseases, primary sleep disorders and major mental illnesses.’ (Monro 2001)
The stress factors listed by Monro, as well as awareness of the cyclical nature of inflammation, are both important informational features of which patients should be made aware. In addition, nutritional tools that may allow a degree of influence over inflammatory processes (without switching them off!) can offer the patient a sense of control over pain, a powerful empowerment, especially in chronic conditions.
Pain and inflammation: allergic, dietary and nutritional factors
Two major antiinflammatory nutritional methods are useful in most pain situations - the dietary approach and the enzyme approach - and both or either can be used, if appropriate.
Inflammation is a natural and mostly useful physical response to irritation, injury and infection. To drastically alter or reduce it may be counterproductive and, therefore, a mistake, as has been shown in the treatment of arthritis using non-steroidal antiinflammatory drugs (NSAIDs) over the past 30 years or so. Apart from the toxic nature of NSAIDs, untreated joints have commonly been shown to remain in better condition than those treated with NSAIDs (Pizzorno 1996, Werbach 1996).
Nutritional approaches for modulating inflammation (Bakker et al 2010, Sanders 2007)
The reasoning behind the importance of antiinflammatory dietary protocols for patients is given below. The advice for the patient (guidelines that can be copied for the patient’s use) is found in Chapter 7 and in the Appendix.
1. Reduce consumption of animal fats. Pain/inflammation processes involve particular prostaglandins and leukotrienes, which are (to a great extent) dependent upon the presence of arachidonic acid, which humans manufacture mainly from animal fats. Reducing animal fat intake cuts down access to the enzymes that help to produce arachidonic acid and, therefore, lowers the levels of the inflammatory substances released in tissues that contribute so greatly to pain (Donowitz 1985, Ford-Hutchinson 1985).
2. Eating fish or taking fish oil helps ease inflammation (Moncada 1986). Fish deriving from cold water areas such as the North Sea or Alaskan waters contain the highest levels of eicosapentanoic acid (EPA), which reduces levels of arachidonic acid in tissues and therefore helps to produce fewer inflammatory precursors. Fish oil provides these antiinflammatory effects without interfering with those prostaglandins, which protect the stomach lining and maintain the correct level of blood clotting. Over-the-counter drugs, such as NSAIDs, which reduce inflammation, commonly cause new problems by interfering with prostaglandin function as well as encouraging gut dysfunction, which may lead to intolerance or allergic reactions (see below).
Research has shown that the use of EPA in rheumatic and arthritic conditions offers relief from swelling, stiffness and pain, although benefits do not usually become evident until supplementation has been taken for 3 months, reaching their most effective level after about 6 months (Werbach 1991a).
Patients (unless intolerant to fish) should be advised to:
3. Antiinflammatory (proteolytic) enzymes, derived from plants, have a gentle but substantial antiinflammatory influence. These include bromelaine, which comes from the pineapple stem (not the fruit), and papain from the papaya plant. Around 2–3 g of one or other should be taken (bromelaine seems to be more effective) spread through the day, away from meal times as part of an antiinflammatory, pain-relieving strategy (Cichoke 1981, Taussig 1988).
Intolerances, allergies and musculoskeletal dysfunction
Specific individualized pathophysiological responses to particular foods and liquids account for a significant amount of symptom production, including pain and discomfort (Brostoff 1992). In order to make sense of a patient’s presenting symptoms, remain alert to the possibility that at least some of the pain, stiffness, fatigue, etc. may be deriving from, or being aggravated by, what is being consumed.
Two different responses seem to be involved: true food allergy, which is an immunological event (involving immunoglobulin E or IgE), and the less well-understood phenomenon of food intolerance, which involves adverse physiological reactions of unknown origin, without immune system intervention. It is possible that food intolerance may include an element of actual food toxicity or a very individual reaction to foods, probably related to enzyme deficiency (Anderson 1997).
Unfortunately, the terms food allergy (hypersensitivity) and food intolerance seem to have become the source of much confusion and little certainty.
Mitchell (1988) states:
The Royal College of Physicians … has directly addressed the problem of terminology. They recommend that the general term of food intolerance be used and that other terms such as food allergy and hypersensitivity be reserved for those situations where a pathogenetic mechanism is known or presumed.
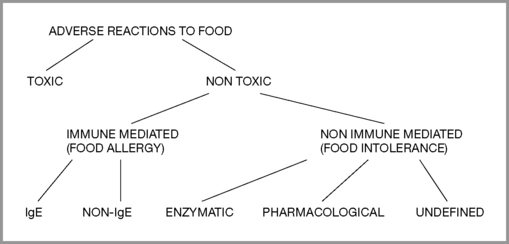
By definition (Royal College of Physicians 1984), food intolerance is a reproducible, unpleasant (i.e. adverse) reaction to a specific food or food ingredient that is not psychologically based (food aversion). Classification of adverse reactions (Ortolani & Vighi 1995) is divided into toxic and non-toxic, with toxic implying general human toxicity, not individual susceptibility. Non-toxic divisions include immune mediated (food allergy) and non-immune mediated (food intolerance). Provocation and other tests help to determine the level of reaction and whether desensitization might be attempted or if total avoidance should be recommended. (Figure 6.1)
Mechanisms
Food reaching the digestive system is usually processed enzymatically to molecular size (short-chain fatty acids, peptides and disaccharides) so that absorption or elimination can take place after the nutrients have been transferred across the mucous membrane into the bloodstream.
Unfortunately, in many instances food antigens and immune complexes also find their way across this mucosal barrier. How fast and in what quantity such undesirable substances enter the bloodstream from the gut seem to be directly linked to the quantity of antigenic material in the gut lumen (Mitchell 1988, Walker 1981).
The presence of specialised membranous epithelial cells… appears to allow active transport of antigen across the mucosa even when concentrations of antigen are low. Permeability is retarded by defensive mechanisms, including enzyme and acid degradation, mucus secretion and gut movement and barriers, which reduce absorption and adherence.
If permeability across the barrier to the bloodstream is compromised this signifies a failure of the defensive mechanisms, so the question arises as to what leads to this failure.
• Drugs (antibiotics, steroids, alcohol, NSAIDs – see discussion earlier in this chapter) (Bjarnason 1984, Jenkins 1991)
• Advancing age (Hollander 1985)
• Specific genetically acquired intolerances (allergies)
• Infections and overgrowths in the intestine, e.g. bacterial, yeast (Isolauri 1989)
• Chemicals contaminating ingested food (pesticides, additives, etc.) (O’Dwyer 1988)
• Maldigestion, constipation (leading to gut fermentation, dysbiosis, etc.) (Iacano 1995)
• Emotional stress that alters the gut pH, negatively influencing normal flora
• Major trauma, such as burns (possibly due to loss of blood supply to traumatized area) (Deitch 1990)
• Toxins that are not excreted or deactivated may end up in the body’s fat stores (O’Dwyer 1988)
All or any of these or other factors can irritate the gut wall and allow an increase in the rate of transportation of undesirable molecules into the bloodstream – the so-called leaky gut syndrome.
Research suggests that the relative health and efficiency of the individual’s liver, along with the age of first exposure, the degree of antigenic load and the form in which the antigen is presented, all play roles in deciding how the body responds, with some degree of adaptive tolerance being a common outcome (Mitchell 1988, Roland 1993). Early feeding patterns are one key factor in determining the way the body later responds to antibodies delivered via food and which foods are most involved, with eggs, milk, fish and nuts being among those most likely to produce problems (Brostoff 1992, Mitchell 1988).
Most people exhibit some degree of serum antibody responses to food antigens. Antibodies assist in elimination of food antigens by forming immune complexes, which are subsequently eliminated by the immune system. However, failure to remove such complexes may result in them being deposited in tissues, leading to subsequent inflammation (Brostoff 1992). Sometimes the immune response to food antigens involves IgE and sometimes it does not, in which case the response would attract a label of ‘food intolerance’.
Mast cells, immune responses and inflammation
Mast cells in the lungs, intestines, connective tissues and elsewhere in the body are critical to the allergic response. Mast cells in connective tissue play a role in the regulation of the composition of ground substance. They contain heparin, histamine and eosinophilic chemotactic factor and are involved in immediate hypersensitivity reactions. Mast cells have surface receptors with a high affinity for IgE, but they can also interact with non-immunological stimuli, including food antigens.
The violence of any reaction between mast cells and IgE (or other stimuli) depends on the presence in the tissues of a variety of biological substances, such as histamine and arachidonic acid (and its derivatives such as leukotrienes), all of which augment inflammatory processes (Holgate 1983, Wardlaw 1986). Histamine is secreted by mast cells during exposure to allergens and the result is local inflammation and edema as well as bronchiole constriction. This last effect is especially relevant to asthmatics but can affect anyone to some degree, creating breathing difficulties.
At times the response to ingested and absorbed antigens is very fast – a matter of seconds, however, it is also possible for hours or days to elapse before a reaction occurs (Mitchell 1988).
Muscle pain and allergy/intolerance
Copious evidence exists linking allergy and food intolerance to muscle pain, chronic fatigue, fibromyalgia syndrome and a host of other mysterious and complex symptoms. Lactose intolerance produced temporal pain, blurred vision, dizziness and tachycardia in one patient that lasted 26 years before diagnosis. (Matthews & Campbell 2000). Many people suffering perplexing symptoms are often labeled as psychosomatic patients, with the symptoms described as ‘somatoform’ – i.e. symptoms that cannot be traced to a specific physical cause. Rief et al (2001) report that the most frequent somatoform symptoms were back pain, joint pain, pain in the extremities, and headache, as well as abdominal symptoms (bloating or intolerance of several foods) and cardiovascular symptoms (palpitation). The question as to the validity of ‘intolerance’ as a non-psychosomatic condition continues to rage in medical circles. (Nettleton et al 2009) Answers other than ‘somatoform’ or ‘psychosomatic’ are emerging. For example, Fruhauf (2009) notes that:
More than 20% of the population in industrialized countries suffers from food intolerance or food allergy. The majority of cases of food intolerance are due to non-immunological causes. These causes range from pseudo-allergic reactions to enzymopathies, chronic infections and psychosomatic reactions that are associated with food intolerance. The prevalence of true food allergy, i.e., immunologically mediated intolerance reactions, is only 2% to 5% in adults and 5% to 10% in children.
The manifestation and severity of allergic rhinitis symptoms exhibit marked 24 hour variations; in most people symptoms are worse overnight, or early in the morning, and often compromise nighttime sleep, resulting in poor daytime quality of life, disturbed school and work performance, irritability and moodiness (Smolensky et al 2007).
A study evaluated the frequency of major symptoms as well as allergic symptoms, such as rhinitis, in a group of more than 30 patients with a diagnosis of ‘primary fibromyalgia’ compared with matched (age and sex) controls (Tuncer 1997). Symptom prevalence in the FMS group (apart from pain, which was 100%) was migraine 41%, irritable bowel syndrome (IBS) 13%, sleep disturbance 72% and morning stiffness 69%. There was a frequent finding of allergy history in the FMS group, with elevated (though not significantly) IgE levels. Sixty-six percent of the FMS patients tested were positive for allergic skin tests.
A study at the school of medicine of East Carolina University in 1992, involving approximately 50 people with hay fever or perennial allergic rhinitis, found that approximately half those tested fitted the American College of Rheumatology criteria for fibromyalgia (Cleveland et al 1992).
Four patients diagnosed with fibromyalgia syndrome for between 2 and 17 years, who had all undergone a variety of treatments with little benefit, all had complete, or nearly complete, resolution of their symptoms within months after eliminating monosodium glutamate (MSG), or MSG plus aspartame, from their diet. All patients were women with multiple co-morbidities prior to elimination of MSG. All have had recurrence of symptoms whenever MSG is ingested. The researchers note that excitotoxins are molecules, such as MSG and aspartame, that act as excitatory neurotransmitters and can lead to neurotoxicity when used in excess. They proposed that these four patients may represent a subset of fibromyalgia syndrome that is induced or exacerbated by excitotoxins or, alternatively, may comprise an excitotoxin syndrome that is similar to fibromyalgia (Werbach 1993).
Simons et al (1999) note that patients with active symptoms of allergic rhinitis as well as myofascial trigger points receive only temporary relief when specific therapy is given for the trigger points. ‘When the allergic symptoms are controlled, the muscle response to local TrP therapy usually improves significantly. Hyper-sensitivity to allergens, with histamine release, seems to act as a perpetuating factor for myofascial trigger points.’ They note that food allergies should be considered as a perpetuating factor for myofascial TrPs and that although the ‘shock organs for allergic reactions’ in most people are the upper respiratory tract, eyes, bronchi, skin or joints, ‘in other patients, the skeletal muscles appear to serve as the shock organ for allergies’.
Dr Anne Macintyre, medical adviser to ME Action, an active UK support group for patients with myalgic encephalomyelitis, fibromyalgia and chronic fatigue conditions, supports an ‘immune dysfunction’ model as the underlying mechanism for FMS. She states: ‘The immune dysfunction in ME may be associated with increased sensitivities to chemicals and/or foods, which can cause further symptoms such as joint pain, asthma, headache and IBS’ (Macintyre 1993).
For many years, Dr Theron Randolph recorded clinical changes as an individual passes through stages of ‘reaction’ to chemicals (in food or in the environment) (Randolph 1976). He divides these reactions into those that relate to the active stimulation of an immune reaction by the allergen and those that relate to withdrawal from it. During some of the stages, most notably ‘systemic allergic manifestations’, most of the major symptoms associated with FMS may become apparent, including widespread pain, fatigue, mental confusion, insomnia and irritable bowel. Where particular food allergens are consumed daily, reactions are usually not acute but may be seen to be chronically present. The clinical ecology model suggests that the individual may by then have become ‘addicted’ to the substance and that the allergy is then ‘masked’ by virtue of regular and frequent exposure to it, preventing the withdrawal symptoms that would appear if exposure was stopped. Feingold (1973) states:
If a reacting individual associates the stimulatory effect [of an allergen] with a given exposure, he tends to resort to this agent as often as necessary ‘to remain well’. The coffee addict for example who requires coffee to get started in the morning, tends to use it through the day as often as necessary and in the amount sufficient to keep going. Over a period of time, a person so adapting tends to increase the frequency of intake and the amount per dose to maintain the relatively desirable effect. The same holds true for other common foods.
Allergy-hyperventilation ‘masqueraders’
Blood chemistry can be dramatically modified (increased alkalosis) by a tendency to hyperventilation and this has profound effects on pain perception and numerous other symptoms including anxiety, sympathetic arousal, paresthesia and sustained muscular tonus (Lum 1981, Macefield & Burke 1991, Timmons & Ley 1994).
Brostoff (1992) states that some experts are actually dismissive of the concept of food intolerance and believe that many individuals so diagnosed are actually hyperventilators. He considers that: ‘Hyperventilation is relatively uncommon and can masquerade as food sensitivity’. Barelli (1994) has shown that a tendency to hyperventilation increases circulating histamines, making allergic reactions more violent and more likely.
So we have two phenomena – allergy and hyper-ventilation – both of which can produce symptoms reminiscent of the other (including many associated with chronic muscle pain), each of which can aggravate the effects of the other (hyperventilation by maintaining high levels of histamine and allergy by provoking breathing dysfunction, such as asthma), and both of which commonly co-exist in individuals with fibromyalgia and other forms of chronic pain.
Defining food intolerances
In the 1920s and 1930s, Dr A.H. Rowe demonstrated that widespread chronic muscular pains, often associated with fatigue, nausea, gastrointestinal symptoms, weakness, headaches, drowsiness, mental confusion and slowness of thought, as well as irritability, despondency and widespread bodily aching, commonly had an allergic etiology. He called the condition ‘allergic toxemia’ (Rowe 1930, 1972).
Randolph (1976) has described what he terms ‘systemic allergic reaction’, which is characterized by a great deal of pain, either muscular and/or joint related, as well as numerous symptoms common in FMS. Randolph says:
The most important point in making a tentative working diagnosis of allergic myalgia is to think of it. The fact remains that this possibility is rarely ever considered and is even more rarely approached by means of diagnostico-therapeutic measures capable of identifying and avoiding the most common environmental incitants and perpetuents of this condition – namely, specific foods, addictants, environmental chemical exposures and house dust.
Randolph points out that when a food allergen is withdrawn from the diet it may take days for the ‘withdrawal’ symptoms to manifest: ‘During the course of comprehensive environmental control [fasting or multiple avoidance] as applied in clinical ecology, myalgia and arthralgia are especially common withdrawal effects, their incidence being exceeded only by fatigue, weakness, hunger and headache’. The myalgic symptoms may not appear until the second or third day of avoidance of a food to which the individual is intolerant, with symptoms starting to recede after the fourth day. He warns that in testing for (stimulatory) reactions to food allergens (as opposed to the effects of withdrawal), the onset of myalgia and related symptoms may not take place for between 6 and 12 hours after ingestion (of an allergen-containing food), which can confuse matters as other foods eaten closer to the time of the symptom exacerbation may then appear to be at fault. Other signs that can suggest that muscle pain is allied to food intolerance include the presence of restless legs, a condition that also commonly co-exists with FMS and contributes to insomnia (Ekbom 1960).
When someone has an obvious allergic reaction to a food this may well be seen as a causal event in the emergence of other symptoms. If, however, the reactions occur many times every day and responses become chronic, the cause and effect link may be more difficult to make.
If symptoms such as muscular pain may at times be seen to be triggered by food intolerance or allergy, the major question remains – what is the cause of the allergy? (Box 6.1) As discussed earlier in this chapter, one possibility is that the gut mucosa may have become excessively permeable, so allowing molecules to enter the bloodstream where a defensive immune response is both predictable and appropriate. ‘Leaky gut’ can be seen to be a cause of some people’s allergy (Paganelli 1991, Troncone 1994). The trail does not stop there, however, because it is necessary to ask: what caused the leaky gut?
Box 6.1 Biological synchronicity
There are both linear and spatial ways of interpreting what happens in life in general and to the body in particular. Cause and effect represent the way many people in the West understand the relationships between events (causality), i.e. one thing causes or is caused, or at least strongly influenced, by another.
A different way of viewing two events is to see them as being part of a complex continuum, each being part of the same (larger) process but with neither event dependent on the other, linked by a synchronistic connective principle. The words ‘synchronicity’ or ‘simultaneity’ are used to describe this way of viewing patterns and events (Jung 1973).
• hyperventilation commonly leads to anxiety; therefore, we might assume that hyperventilation ‘causes’ anxiety; however
• anxiety commonly leads to hyperventilation; therefore, we might assume that anxiety causes hyperventilation; or it might be said that
• anxiety and hyperventilation not only ‘feed’ each other but can be triggered and/or aggravated by low blood sugar levels, increased progesterone levels, sympathetic arousal, toxic factors, adrenal stimulation, metabolic acidosis, climatic conditions, altitude, emotional stimuli, allergic reactions and so forth. Therefore, we might more comprehensively and appropriately assume that anxiety and hyperventilation are part of a continuum, involving all or any of these (and numerous other) factors, interacting with the unique genetic and acquired biochemical, biomechanical and psychological individuality of the person affected.
Similar complex continuities exist in most chronic conditions and, as indicated in this chapter, even in some apparently simple conditions.
This way of viewing the patient’s problem involves placing it in context: the problem within the patient (in all his/her acquired and inherited uniqueness and complexity), within the patient’s environment, and that environment within the broader environment, etc. This approach can be termed ‘biological synchronicity’ (Chaitow 2001) for if we are looking for ‘causes’ of symptoms we need to think as broadly as possible so that with a wide enough lens, we may discern a pattern, a web of influences, which we may be able to help the patient untangle.
Solutions may possibly be found in nutritional strategies, stress-reducing methods, psychological support, biomechanical balancing and any of numerous other approaches, none of which can ‘cure’ the individual but all of which can ‘allow’, or encourage, self-healing to take place. When treatment is seen in this way, it becomes another feature in the contextual pool of influences interacting within the individual. The therapeutic outcome should, therefore, not be seen as an effect resulting from a cause (treatment) but rather the emergence of (hopefully) positive change out of that particular complex context.
A way of discerning where the therapeutic encounter enters the picture requires a spatial vision of combinations of synchronous events, whether biochemical, biomechanical, psychosocial, energetic or spiritual, with ‘treatment’ designed to be a coherent, beneficial influence encouraging self-healing.
Allergy, the hyperreactive immune function and muscle pain
As part of the allergy link with myalgic pain, the immune system may at times be involved with multiple or chronic infections as well as with antigens, which keeps cytokine production at an excessively high level. For example, a viral connection has been suggested in the etiological progression to conditions predominated by chronic muscle pain. Macintyre (1993) offers research evidence for this:
The onset of ME [FMS] usually seems to be triggered by a virus, though the infection may pass unnoticed. Most common in the UK are enteroviruses, including coxsackie B and Epstein-Barr virus (Gow 1991)… Many people say they were fit and well before a viral infection which started their [condition]. But it is possible that in many such patients there have been other factors such as emotional stress, pesticide exposure, surgical or accidental trauma some months before the triggering infection.
Immune hyperactivity may, therefore, continue due to a persistent viral presence, the existence of some other toxic immune stimulant (pesticides, for example) or repetitive allergic responses, as suggested by Randolph. If so, high levels of cytokines resulting from excessive immune activation will produce a variety of flu-like symptoms, with characteristic persistent aching in the musculature (Oldstone 1989).
Treatment for ‘allergic myalgia’?
Randolph suggests: ‘Avoidance of incriminated foods, chemical exposures and sometimes lesser environmental excitants’. To achieve this in a setting other than a clinic or hospital poses a series of major hurdles for the practitioner and the patient. It makes perfect sense, if foods or other irritants can be identified, for these to be avoided, whether or not underlying causes (e.g. gut permeability) can be dealt with.
According to the Fibromyalgia Network, the official publication of FMS support groups in the USA, the most commonly identified foods that cause problems for many people with FMS are: wheat and dairy products, sugar, caffeine, Nutra-Sweet®, alcohol and chocolate (Fibromyalgia Network 1993, Uhde 1984). Note: The Fibromyalgia Network has specifically reported that Nutra-Sweet® (a form of aspartame) can exacerbate FMS symptoms in some people. All aspartame-containing foods should be used with caution in case they are aggravating symptoms, using strategies as outlined in Chapter 7 (exclusion diet).
Maintaining a wheat-free, dairy-free diet for any length of time is not an easy task, although many manage it. Issues involving patient compliance deserve special attention as the way information is presented and explained can make a major difference in the determination displayed by already distressed patients as they embark on potentially stressful modifications to their lifestyles.
Exclusion strategies, largely based on the original work of clinical ecologists such as Randolph, as well as the so-called ‘oligoantigenic’ dietary pattern based on the methods used at the Great Ormond Street Hospital for Children in London, are presented in Chapter 7.
CAUTION: When a food to which someone is strongly sensitive and has been consuming regularly is stopped, she may experience ‘withdrawal’ symptoms for a week or so, including flu-like symptoms, muscle and joint ache as well as anxiety, restlessness, etc. This will usually pass after a few days and can be a strong indication that whatever has been eliminated from the diet is responsible for a ‘masked’ allergy, which may be responsible for or aggravating symptoms. It is important for patients to be forewarned of this possibility.
Other therapeutic choices
Pizzorno (1996) has reviewed a range of detoxification and bowel enhancement methods that have been tested both clinically and in controlled studies. These studies demonstrate that if the bowel mucosa can be assisted to heal, gut flora replenished, liver function improved, allergens restricted, nutritional status evaluated and if necessary supplemented, marked improvements can be achieved in patients with chronic symptoms, such as those evident in the discussion of allergy, including chronic myalgic pain conditions (Bland 1995, Pizzorno 1996).
Testing for allergy/intolerance
Testing for intolerances and even frank allergies is not straightforward. Various factors may cause confusion, including the following (Roberson 1997).
• Demonstration of IgE antibodies in serum may not be possible because of the presence of other antibody classes.
• Cytotoxic blood tests commonly produce false-positive results.
• Skin testing is an effective means of demonstrating the presence of inhaled allergens but is not effective in confirming food allergens (Rowntree et al 1985, Simons et al 1999).
• Skin test responses to food may be lost when fairly young, even though IgE antibodies are present in serum.
• Skin testing is inefficient in assessing delayed sensitivities and fails to accurately evaluate metabolic intolerances to foods.
• James (1997) suggests that if there is a positive skin test and/or radioallergosorbent test (RAST), an elimination diet should be introduced to assess for food intolerances.
• An elimination diet involves a food or food family being excluded for 3–4 weeks, during which time symptoms are assessed. If there is an improvement, a challenge is performed by reintroducing the previously eliminated food.
• If symptoms are better when the food is excluded and symptoms reemerge when the food is reintroduced to the diet, the food is then excluded for not less than 6 months. This process offers the simplest, safest and most accurate method of assessing a food intolerance, but only when it is applied strictly.
Some evidence for exclusion diet benefits with allergy
• Seventy-four percent of 50 patients with asthma experienced significant improvement without medication following an elimination diet. Sixty-two percent were shown to have attacks provoked by food alone and 32% by a combination of food and skin contact (Borok 1990).
• When 113 individuals with IBS were treated by means of an elimination diet, marked symptomatic improvement was noted. Seventy-nine percent of the patients who also displayed atopic symptoms, including hay fever, sinusitis, asthma, eczema and urticaria, showed significant improvements in these symptoms as well (Borok 1994).
• A moderate to high intake of oily fish has been shown to be associated with reduced risk of allergic reactions, presumably due to high levels of EPA, which inhibits inflammatory processes (Hodge 1996, Thien 1996).
• A vegan diet that eliminated all dairy products, eggs, meat and fish as well as coffee, tea, sugar and grains (apart from buckwheat, millet and lentils) was applied to 35 asthmatics, of whom 24 completed the 1-year study. There was a 71% improvement in symptoms within 4 months and 92% after 1 year (Lindahl 1985).
Strategies
Oligoallergenic diets, elimination diets and rotation diets are variations in strategies that attempt to identify, and then minimize, the exposure to foods that provoke symptoms. Some of these dietary methods are discussed in Chapter 7.
The breathing connection
Anxiety is an aggravating factor in all chronic pain conditions (Wall & Melzack 1989), including muscular pain (Barlow 1959), and, as an emotional state, usually results in psychosocial therapeutic interventions.
The major influence on the biochemistry of the blood that triggers anxiety feelings relates to breathing pattern disorders, with hyperventilation being the most obvious and extreme (Timmons & Ley 1994). A variety of self-help measures are presented in Chapter 7 that might be useful while the patient is also being treated for the biomechanical concomitants of an upper chest respiratory pattern (short painful accessory breathing muscles, thoracic spine and rib cage restrictions, trigger point activity, etc.).
The biochemistry of hyperventilation (Chaitow 2004)
The pH scale runs from 1 to 14, with 1 being acidic and 14 alkaline, with the neutral midpoint being 7. ‘pH’ stands for partial pressure of hydrogen and the pH scale is an ‘alkalinity’ scale, where higher numbers indicate greater alkaline content. The physiological normal pH in the arterial blood is around 7.4, with an acceptable range from 7.35 to 7.45. Outside these limits lie ill effects of many kinds. The body will sacrifice many other things in order to maintain proper pH. A rise to 7.5 means more alkalinity, a drop to 7.3 more acidity. The term ‘acidosis’ means an excess of acid in the blood and tissues.
The acidity of the blood is determined largely by carbon dioxide (CO2), which is the end-product of aerobic metabolism. CO2 comes primarily from the site of energy production within the cells, the mitochondria. It is the biological equivalent of smoke and ash and is odorless, heavier than air and puts out fires, including ours. In its pure form, it quickly causes suffocation.
CO2 is extremely toxic and potentially lethal. For transportation to the lungs for exhalation, CO2 is turned into carbonic acid (H2CO3). The more H2CO3 in the blood, the more acidic it is and changes in breathing volume relative to CO2 production regulate the pH of the bloodstream (a job shared with the kidneys). The concentration of CO2, not oxygen, in the blood is the major regulator of breathing drive. Higher CO2 level immediately stimulates more breathing, apparently because excess of CO2 means that one is breathing oxygen-poor air, breathing has stopped or something else is happening that is likely to lead to suffocation.
During exercise, more CO2 is produced but more oxygen is needed also, so the need to keep pH constant is nicely linked with a greater drive to breathe. Gilbert (2002) explains with a formula:
High CO2 = high acidity = low pH = higher breathing drive. Conversely, reduced exertion reduces oxygen need, and also lowers CO2 production, which lessens the drive to breathe. Low CO2 = low acidity = high pH = lower breathing drive.
The biochemistry of anxiety and activity
Gilbert (2002) explains the links between anxiety, breathing and blood chemistry.
Anxiety is not merely a mental phenomenon. Perception of threat is supported by bodily changes designed to enhance readiness for action. Increased breathing is often one of those changes, and it is reasonable in the short run because it creates a mild state of alkalosis. This would help offset a possible surge of acid in the blood (not only carbonic acid, but lactic acid if muscle exertion is drastic enough, since lactic acid is given off by anaerobic metabolism). Long-distance runners, sprinters, and horse trainers have experimented successfully with doses of sodium bicarbonate, which supplements the natural bicarbonate buffer, and opposes the lactic acid load created by exercising muscles (Schott & Hinchcliff 1998, McNaughton et al 1999).
Once there is an increase in alkalinity, if action does not occur within a minute or two, homeostasis is disrupted. If perceived threat continues, physiological alarm also continues. The chemical cascade and eventual imbalance then become an additional disturbance. Gilbert (2002) continues:
Here is a likely sequence in the person prone to panic with hyperventilation, showing changes in the chemical, behavioral, and cognitive realms:
• initial threat perception (anxiety)
• increased breathing, mirroring the mind
• respiratory alkalosis and cerebral hypoxia
• appearance of symptoms in several body systems
• impairment of thought processes, disrupted mental stability
• hyper-emotionality, sustained anxiety, restricted reality-orientation, and limited awareness of available options for coping with the anxiety trigger.
Gilbert points out that some people are more susceptible than others to this sequence.
Using Doppler ultrasound to monitor changes in size of the basilar artery in panic patients, Gibbs (1992) found a wide variance in arterial diameter in response to the same degree of hyperventilation. Those with the strongest artery constriction, as much as 50%, were those with the greatest panic symptoms (Ball & Shekhar 1997).
Summary
• The body tries hard to maintain pH around 7.4 and ensure adequate oxygen supply and delivery.
• Overbreathing means more CO2 being eliminated than is being produced, so pH moves toward the alkalinity end.
• At the other extreme inadequate breathing retains more CO2 than is being produced, so pH drops toward the acidic end.
• The pH in the short term is adjusted by increases or decreases in breathing volume.
• Muscle contraction, or any increase in metabolism, produces more CO2 and normally the breathing increases to exhale more CO2.
• When respiration is matched to metabolic need, the level of CO2 and pH stays stable.
• But anticipated apprehension, anxiety, preparation for exertion, discomfort or chronic pain will increase breathing volume and if the exertion does not occur, CO2 will drop and alkalinity results.
• A deficit of CO2 promotes oxygen retention by the hemoglobin molecule and if this happens while vasoconstriction is being promoted by alkalosis, release of oxygen is further inhibited, leading to a range of symptoms including increased fatiguability of muscles, ‘brain fog’, increased neural sensitivity and pain perception.
Exercise, hyperventilation and asthma
Contextual factors can be seen in the example of asthma that is triggered by exercise.
Weiler et al (2007) have reported a wide variety of associated conditions and circumstances may be required before an asthmatic attack is triggered by exercise. These possibly include how much exercise, gastro-esophageal reflux, mouth rather than nasal breathing, vocal cord dysfunction, exercise induced hyperventilation, cold air, relative humidity, medications such as aspirin, consumption of various foods within a few hours of exercise (including shell-fish, wheat and celery) – and more.
The value of understanding context in any symptom is that simple linear conclusions are avoided. One of these factors, when alone, may not produce symptoms, but the cascade that occurs with combinations may produce the attack (see Box 6.1).
Breathing rehabilitation exercises are described in Chapter 7, and in Boxes 6.2 and 6.3. Autogenic training is described in Box 6.4.
Box 6.2 Alternate nostril breathing
In a healthy individual, at any given time, one nostril is more dominant than the other in terms of the volume of airflow. There is an alternation, with one nostril being more open than the other, every few hours throughout the day (Gilbert 1999).
Evidence suggests that whichever nostril is more open, the opposite hemisphere of the brain is slightly more active and in yoga this is utilized to enhance different activities related to particular hemispheric functions. These traditional yogic intuitions and observations have been confirmed by modern research in which EEG readings from the brain have been found to correlate increased hemispheric activity with the currently dominant nostril (Black et al 1989, Rossi 1991, Shannahoff-Khalsa 1991). The alternate nostril exercise has a calming and invigorating effect (Fig. 6.2). See p. 170 Box 7.17.
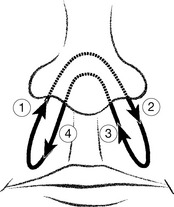
Figure 6.2 Alternate-nostril breathing. The air stream is directed alternately through each nostril by gently occluding the opposite nostril. This is thought to harmonize the two hemispheres of the brain, creating a balance between sympathetic and parasympathetic dominance
(reproduced with permission from Gilbert C 1999 Yoga and breathing Journal of Bodywork and Movement Therapies 3(1):44–54. Journal of Bodywork and Movement Therapies 1999; 3(1):50).
Box 6.3 Panic attack first aid
Rescue breathing techniques for risk situations that are likely to trigger symptoms (such as laughing, crying, high-intensity exercise, prolonged speech, humid or hot conditions, flying) include the following.
• Short breath-holds (to allow CO2 levels to rise) followed by low chest/low volume breathing. Great care must be taken to teach patients to breath-hold only to the point of slight discomfort and to avoid deep respirations on letting go (Innocenti 1987).
• Rest positions, e.g. arms forward, resting on a table or chairback to reduce upper chest effort and concentrate on nose/abdominal breathing, with focus on as slow an exhalation as possible.
• Hands on head or thumbs forward, hands on hips helps with breathlessness during exercise.
• Breathing into hands cupped over the nose and mouth for a minute or two helps patients identify and effectively separate symptoms from triggers.
• Use of a fan, with the sensation of moving air over the trigeminal nerve outlet on each side of the face, helps deepen and calm respiration (Bradley 2002).
Box 6.4 Autogenic training and progressive muscular relaxation
Relaxation exercises focus on the body and its responses to stress, trying to reverse these, while meditation tries to bring about a calming of the mind and, through this, a relaxation response.
Italian researchers compared the benefits of autogenic training (AT) and progressive muscular relaxation (PMR – also called Erickson’s technique) for patients with fibromyalgia (Rucco et al 1995). They found that both groups benefited in terms of pain relief if they carried out the exercise regularly and that, because PMR is easier and quicker to learn, patients are more likely to perform this regularly (compared with AT). Those learning AT complained of ‘too many intrusive thoughts’, which is precisely what AT is designed to eventually quieten – that is, the ‘training’ part of the exercise.
The modified form of AT described in Chapter 7 is an excellent way of achieving some degree of control over muscle tone and/or circulation and therefore over pain (Jevning 1992, Schultz 1959). See p. 170 Box 7.18.
Diet, anxiety and pain
If it could be shown that there exist common dietary factors that encourage anxiety, these triggers could be seen to be precursors to the pain and worthy of attention. This might offer the opportunity for relatively simple dietary interventions (exclusions) that could potentially reduce, or eliminate, the anxiety state that may represent the main precursor to their symptoms. A variety of such dietary triggers have been identified (Werbach 1991b) and some of the key features of this phenomenon are summarized below.
Buist (1985) has demonstrated a direct connection between clinical anxiety and elevated blood lactate levels, as well as an increased lactate:pyruvate ratio. This ratio is increased by alcohol, caffeine and sugar.
Glucose
Glucose loading has been shown to elevate blood lactate:pyruvate ratio in anxiety-prone individuals (Wendel & Beebe 1973). In a study involving 15 psycho-neurotics (seven with anxiety), 28 schizophrenics (eight with anxiety) and six healthy controls, the subjects consumed a cola drink containing 100 g of glucose. Blood lactate levels were markedly elevated during the third, fourth and fifth hours post glucose only in the anxiety-prone psychoneurotic and schizophrenic patients. The implication is that in anxiety-prone people, sugar intake should be moderated, if at all possible. See Box 6.5 for strategies to balance blood sugar.
Box 6.5 Strategies for balancing blood sugar levels
• Fluctuating blood glucose levels may trigger symptoms in patients with high carbohydrate diets that produce rapid rises followed by sharp falls to fasting levels – or below (Timmons & Ley 1994).
• Patients are recommended to eat breakfast (including protein) and to avoid going without food for more than 3 hours (Hough 1996).
• This fits in with a mid-morning and afternoon protein snack, as well as the usual (and possibly smaller) three meals a day.
• This is particularly relevant to patients who experience panic attacks or seizures, which have been shown to be more likely to strike when blood glucose levels are low. Paradoxically, this is more likely to happen when sugar intake is high (Timmons & Ley 1994)!
• The micronutrient chromium has been shown to improve glucose tolerance and stabilize blood sugar imbalances, in doses of 200 μg daily (Werbach 1991a).
Alcohol
In an experimental placebo-controlled study involving 90 healthy male volunteers, an increase was shown in state anxiety following administration of ethanol as compared with placebo (Monteiro 1990). The implication is that in anxiety-prone people, alcohol intake should be moderated or eliminated, if at all possible (Alberti & Natrass 1977).
When addressing chronic pain, Simons et al (1999) note that perpetuation of myofascial trigger points may be increased by regular consumption of excessive alcohol, which leads to poor eating habits (decreased intake of needed nutrients) and interferes with absorption of folic acid, pyridoxine, thiamine and other vitamins (while the body’s need is increased). ‘Some patients exhibit an idiosyncratic muscle reaction to alcoholic beverages, experiencing an attack of myofascial pain soon after or the day following indulgence.’
Caffeine
Caffeine was shown to have anxiogenic effects, particularly on those patients suffering panic disorders. In an experimental controlled study (Charney 1985), caffeine was found to produce significantly greater increases in subject-rated anxiety, nervousness, fear, nausea, palpitations, restlessness and tremors. The implication is that in anxiety-prone people, caffeine intake should be moderated or eliminated, if at all possible (Uhde 1984).
Regarding chronic pain and perpetuation of myofascial trigger points, Simons et al (1999) note:
Small to moderate amounts of caffeine may help to minimize TrPs by increasing vasodilation in the skeletal musculature. However, excessive intake of coffee and/or cola drinks that contain caffeine (more than two or three cups, bottles or cans daily) is likely to aggravate TrP activity. … Many combination analgesic drugs contain caffeine that may add significantly to the total caffeine load without the patient’s realizing it unless someone analyzes in detail the patient’s caffeine intake.
The authors of this text suggest that in some cases the degree of what should be considered ‘excessive’ might be much less than that indicated here, especially if the patient also has an intolerance (allergy) to the caffeine source (coffee, tea, chocolate, etc.). Where caffeine is part of the diet, it should be addressed as a suspect, eliminated to assess for improvement and, if reintroduced, attention paid to the reoccurrence of the painful state.
Anxiety and deficiency
Deficiency in various minerals, vitamins and amino acids has been associated with anxiety disorders.
5-HTP: a safe form of tryptophan
A plant source of 5-hydroxy-l-tryptophan (5-HTP), the immediate precursor to serotonin (5-hydroxytryptamine), is found abundantly in an African bean (Griffonia simplicifolia). Research has confirmed that this form of tryptophan safely converts into serotonin when it reaches the brain and is at least as effective as L-tryptophan in encouraging sleep and reducing anxiety levels (Caruso et al 1990). This has been found to be particularly helpful in assisting patients with fibromyalgia-type symptoms (Puttini & Caruso 1992). 5-HTP is available from health-food stores and pharmacists.
In an experimental double-blind study, 50 patients with primary fibromyalgia syndrome, with anxiety as one of their major presenting symptoms, randomly received either 5-HTP 100 mg three times daily or placebo. After 30 days there were significant declines in the number of tender points and in the intensity of subjective pain, and significant improvements in morning stiffness, sleep patterns, anxiety and fatigue in the patients receiving 5-HTP compared with the placebo group. Only mild and transient gastrointestinal side effects were reported by some individuals (Caruso et al 1990).
CAUTION: Tryptophan is an amino acid that has been widely used to treat stress symptoms and insomnia (Yunus et al 1992). The FDA removed tryptophan from over-the-counter sale in the early 1990s when Japanese manufacturers used a genetically engineered bacterial process to produce tryptophan, leading to eosinophilia-myalgia syndrome (EMS) (Belongia 1990). Tryptophan returned to the US market in 2002 with FDA warnings regarding potential risks for EMS.
Magnesium and vitamin B6
A dual deficiency of magnesium and B6 has been shown to increase the lactate:pyruvate ratio and is commonly associated with anxiety (Buist 1985). Supplementation (250–750 mg daily of magnesium and between 50 and 150 mg daily of B6) is claimed to be useful for anxiety, especially if taken with appropriate levels of calcium (Werbach 1991a).
CAUTION: Vitamin B6 (pyridoxine), in doses in excess of 200 mg daily taken for extended periods of time, is capable of producing sensory neuropathy (Waterston & Gilligan 1987). Such risks can be avoided by using the active coenzyme form of pyridoxal phosphate or ensuring a short duration of supplementation (a month or less) at moderate dosages (under 200 mg).
At the troponin binding site along the actin filaments within muscle cells, magnesium competes with calcium at the point where actin and myosin filaments bind together prior to contraction. Whereas calcium instigates contraction, magnesium controls the influx of calcium into the cell and can inhibit the contraction mechanism. Addition of sufficient magnesium not only may provide natural muscle relaxation and relief for muscle tension, cramps and tension headaches (Altura & Altura 2001), it may also prevent tetony-like reactions, which is especially pertinent to the health of the heart (Liao et all 1998).
Detoxification and muscle pain
Nutritional expert Jeffrey Bland has formulated a meal replacement product (Ultra-Clear), which is based on rice protein and rich in detoxifying nutrients. Avoiding allergenic foods while using products such as this provides a modified detoxification program that can be carried out while continuing with normal activities. Research has shown this to be helpful for many people with chronic muscular pain. A study of Bland’s detoxification methods involved 106 patients at different clinics, with either chronic fatigue syndrome or FMS (plus irritable bowel syndrome). The program called for avoidance of known food allergens, encouragement of intestinal repair, stimulation of liver detoxification and detoxification using the rice protein powder. Over a 10-week period there was a greater than 50% reduction in symptoms as well as laboratory evidence of improved liver and digestive function (Bland 1995).
Water
Approximately 60% of total body weight is water, although this percentage varies depending upon age, gender and body fat content. Water is essential to almost every reaction in the body and is abundant in blood and lymph, interstitial fluids and intracellular fluids. Deficiency of sufficient water to carry on normal functions at an optimal level (dehydration) is caused by inadequate intake of fluids, excessive loss of fluids or a combination of both. Dehydration carries with it consequences ranging from subtle changes in personality and mental status, such as feeling anxious, inability to concentrate, fatigue and sleepiness, to more serious repercussions of irritability, hyperreflexia, seizures, coma and death (Berkow & Fletcher 1992) (Box 6.6).
The water content of the body is managed by a combination of the thirst mechanism, antidiuretic hormone (ADH) manufactured by the posterior pituitary gland, and the kidneys. When water volume is sufficiently reduced, ADH is released to conserve the fluid content, even at the expense of toxicity. Electrolytes, which exist in the blood as acids, bases and salts (such as sodium, potassium, calcium, magnesium, chlorine), can be affected by dehydration, resulting in interference with normal transmission of electric charges.
Thirst is not apparently a good indicator of a need for rehydration (Mihill 2000), with some research suggesting that by the time thirst is recognized a person is already dehydrated to a level of 0.8–2% loss of body weight (Kleiner 1999). Sports nutritionists and physiologists suggest that dehydration of as little as 1% decrease in body weight results in impaired phyiological and performance responses (Kleiner 1999). Stamford (1993) postulates that muscle cramps may be related to hydration status. High sweat rates and dehydration probably disrupt the balance between the electrolytes potassium and sodium, leading to cramps.
Apart from hydration factors, the mineral content of water (unless distilled) will influence the value of appropriate intake, with a variety of research studies indicating benefits of mineral-rich water supplies. For example, bioavailability of magnesium in drinking water is said to affect conditions as diverse as migraines, atherogenesis (in mice), prostate cancer, breast cancer and preeclampsia in pregnancy (characterized by high blood pressure) (Melles & Kiss 1992, Sherer et al 1999, Yang et al 2000a, b). Magnesium is an important co-factor in many of the body’s enzyme systems and in all enzymatic processes involving ATP (Berkow & Fletcher 1992); therefore, its availability in the body is vital to normal metabolic processes.
One indicator as to the state of hydration and sufficiency of water intake is frequency of urination, which should be five to six times daily. Elevated body temperature, tasks that produce sweating, dry and/or hot climate, vomiting, diarrhea, or other dehydrating factors increase the need for more fluid intake in order to avoid dehydration.
Liver detoxification
Joseph Pizzorno ND, founder president of Bastyr University, Seattle, encourages liver detoxification by means of:
• increased intake of brassica family foods (cabbage, etc.)
• use of specific nutrients such as N-acetyl-cysteine and glutathione
• taking the herb Silybum marianum (milk thistle) 120 mg three times daily.
He states: ‘The strong correlation between chronic fatigue syndrome, fibromyalgia and multiple chemical sensitivities suggests that all may respond to hepatic (liver) detoxification, food allergy control and a gut restoration diet’ (Pizzorno 1996).
CAUTION: For recovering drug users, alcoholics, diabetics and those with an eating disorder, detoxification methods should not be applied without professional advice. If there is a co-existing bowel problem (constipation, ‘irritable bowel’) professional guidance to help normalize this should be sought.
Thyroid hormone imbalance and chronic musculoskeletal pain
Research has confirmed many of the connections between thyroid deficiency/thyroid hormone dysfunction and the symptoms of fibromyalgia, chronic muscle pain and chronic fatigue (Lowe 1997, 2000).
• Lowe (1997) suggests that when thyroid function is apparently normal (euthyroid), for example in patients with fibromyalgia, this may be the result of a failure of normal thyroid hormone to function correctly, due to ‘cellular resistance’ to the hormone.
• Lowe & Honeyman-Lowe (1998) have described the reasoning as to why thyroid hormone may not be functioning adequately, even when in ample supply: ‘To what do we attribute the inadequate thyroid hormone regulation in fibromyalgia?’. Hypothyroidism in adults results most frequently from autoimmune thyroiditis, but it often occurs following radiation exposure, surgical removal of part of the thyroid gland or pituitary failure (Oertel & LiVolsi 1991). For some FMS patients, contamination with dioxin or PCBs may be the source of interference with normal thyroid hormone regulation. These environmental contaminants are ubiquitous in our environment and are abundantly present in human breast milk, fat and blood (McKinney & Pedersen 1987). The contaminants cause the liver to eliminate thyroid hormone at an abnormally rapid rate (Van Den Berg et al 1988). They also displace thyroid hormone from the protein (transthyretin) that transports it into the brain, possibly reducing the concentration of the hormone in the brain (Lans et al 1993). PCBs and dioxin also appear to interfere with the binding of thyroid hormone to its receptors on genes. This interference alters transcription patterns and produces hypothyroid-like effects (McKinney & Pedersen 1987).
• Norwegian research has shown that there is a frequent incidence of thyroid dysfunction in people (especially women) who have chronic widespread musculoskeletal pain. This is not picked up when normal thyroid function tests are done, but shows up when antibodies to thyroid hormone are tested for. What this means is that these individuals may be producing adequate thyroid hormone but, for reasons that are not clear, their immune systems are deactivating this, giving an appearance of normal thyroid function yet with the symptoms (including widespread muscle pain) of under-active thyroid (Aarflot 1996).
• Chronic muscle pain resulting from the activity of myofascial trigger points is more severe when thyroid hormone and B vitamins are deficient (Gerwin 2005, Simons et al 1999).
The clinical signs of thyroid deficiency may include:
• increase in weight or difficulty losing weight
• dry skin, thinning hair (often including loss of outer third of eyebrows)
• persistently low core temperature (morning underarm temperature below 97.8°F (36.5°C)
Treatment requires expert assessment and monitoring and may involve the use of thyroid hormone replacement therapy (what Lowe calls metabolic rehabilitation) as well as nutritional and bodywork strategies.
Osteoporosis
Osteoporosis is an age-related disorder characterized by a decrease of bone mass that, because it affects the quantity of bone or causes atrophy of skeletal tissue, leads to increased susceptibility to fractures. Approximately 80% of those affected by osteoporosis are women, with this condition being responsible for 50% of fractures occurring in women over age 50. Compression fractures of the vertebrae, wrist fractures and traumatic fractures of the femoral neck are most common. Most elderly patients fail to recover normal activity after hip fracture, with the mortality rate within 1 year approaching 20%.
Under normal conditions, bone constantly undergoes remodeling, generally associated with the body’s attempts to maintain the concentration of calcium and phosphate in the extracellular fluid. When serum calcium levels decrease, parathyroid hormone secretion increases, which in turn stimulates osteoclastic activity (removal of bone) to raise the blood levels to normal (all of which may be associated with deficiency of vitamin D (Holick 2007)). When bone resorption occurs faster than bone formation, bone density changes result in a decline in bone mass. Osteomalacia (softening of the bone) may result from lack of calcium intake. Osteoporosis is a more complex condition.
Pizzorno & Murray (1999) explain.
The two conditions, osteomalacia and osteoporosis, are different in that in osteomalacia there is only a deficiency of calcium in the bone. In contrast, in osteoporosis there is a lack of both calcium and other minerals as well as a decrease of the non-mineral framework (organic matrix) of the bone. Little attention has been given to the important role that this organic matrix plays in maintaining bone structure.
Bone is a dynamic living tissue that is constantly being broken down and rebuilt, even in adults. Normal bone metabolism is dependent on an intricate interplay of many nutritional and hormonal factors, with the liver and kidney having a regulatory effect as well. Although over two dozen nutrients are necessary for optimal bone health, it is generally thought that calcium and vitamin D are the most important nutritional factors. However, hormones are also critical, as the incorporation of calcium into bone is dependent upon the estrogen.
The risk of osteoporosis is highest in postmenopausal women when estrogen levels naturally decrease. However, other risk factors include race, weight, dietary calcium intake, vitamin D levels, sedentary lifestyle, alcohol use and cigarette smoking. Weight-bearing exercise has been shown to be the most important determinant of bone density (Pizzorno & Murray 1999).
Stedman’s Dictionary (1998) points out:
Administration of estrogen at and after menopause does not simply halt the loss of bone, but actually increases bone mass. Hormone replacement with estrogen remains the most effective prevention and treatment for postmenopausal osteoporosis. … The benefits of estrogen therapy must be weighed against the increased risk of endometrial hyperplasia and endometrial carcinoma (which can be offset by concomitant administration of progestogen) and possibly of carcinoma of the breast.
Lee & Hopkins (1996) discuss at length the viewpoint that a wide range of conditions, including pre-menopausal symptoms and osteoporosis, may be more related to progesterone deficiencies rather than estrogen. While much of their premise has considerable validity, more research and investigation are needed into the role of progesterone, its safe application and the long-term effects of use.
The goals of osteoporosis treatment should include the need to preserve adequate mineral mass, prevent loss of the matrix and structural components of bone, and to assure optimal mechanisms which function to remodel damaged bone (Pizzorno & Murray 1999). A combination of weight-bearing exercise, intake of optimal nutrition (particularly calcium, magnesium, zinc, vitamins D and B6), exclusion of factors that leach calcium or block absorption (alcohol, caffeine, excessive protein, stress and smoking) while encouraging healthy hormonal balance, appear to be the most important steps the individual can take to avoid the development of osteoporosis. See also Box 6.7.
Box 6.7 Macro- and micronutrients
Adequate and balanced nutritional intake is necessary for optimal function of tissues throughout the body. Macronutrients are required in the greatest amount (e.g. carbohydrates, protein, fats), while micronutrients are essential factors required in only small amounts (e.g. vitamins, trace minerals). While a thorough discussion of this topic is outside the scope of this text, this brief overview is intended to remind the reader that nutrition is an important factor in wellness. These details regard the average adult body, with children and elderly needs being different.
Proteins are involved in structures, hormones, enzymes, muscle contraction, immunologic response and essential life functions. Proteins are composed of eight essential amino acids (AAs) including isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine. Arginine and histidine are ‘essential’ during growth periods and in some adults due to acquired or genetic factors but can usually be synthesized from the eight essential AAs listed above, during adult life. A normal active adult usually needs at least a minimum of 50 g to be healthy, with 60–80 g usually being ideal. Highly active or larger individuals may need more and there is evidence of genetic variations, with people of Oriental origin being capable of surviving in good health on lower protein levels than Caucasians (Stanbury 1983).
There is a need to distinguish between first- and second-class proteins. Vegetable protein sources do not contain all the essential AAs and dietary intake therefore requires a combination of different forms of vegetable protein, such as pulses (lentils, beans, etc.) + seeds or grains + pulses, so that the body can create first-class protein (protein synthesis), such as is found in fish, meat, eggs and dairy products. This awareness is particularly important for vegetarians and vegans, especially in childhood, pregnancy or when tissue repair is a factor.
From the essential AAs the body makes approximately 20 additional non-essential AAs, and from this available ‘pool’ the body then constructs tissues. Amino acids can also be used as energy sources but since the body cannot oxidize the nitrogen portion of AAs, a residue, urea or uric acid, remains (Brekhman 1980, Chaitow 1991).
Carbohydrates supply a source of quick, clean energy. Small amounts of carbohydrates are usually not a problem but intake of refined carbohydrates may raise insulin levels, upset blood sugar balances and produce excesses, which are then stored as body fat.
Essential fatty acids (EFAs) are needed by the body to transport fat-soluble vitamins (such as vitamins E and A), linoleic acid (LA) (omega 3) and alpha-linolenic acid (LNA) (omega 6).
Regarding micronutrients, 13 vitamins (A, B1, B2, B3, B5, B6, B7-biotin, B9-folic acid, B12, C, D, E, K) and 21 minerals (calcium, phosphorus, potassium, sulphur, sodium, chlorine, magnesium, silicon, iron, fluorine, zinc, strontium, copper, vanadium, selenium, manganese, iodine, nickel, molybdenum, cobalt, chromium) are needed in varying amounts, unique to the individual’s genetically acquired biochemical individuality (Williams 1979) and lifestyle. Of particular importance to the muscular system are the minerals iron, calcium, potassium and magnesium, and vitamins B1, B6, B12, folic acid and C (Simons et al 1999).
While calcium, iron, sodium and potassium are ‘popular’ minerals that most patients are aware of, magnesium is an extremely important but less well-known mineral. Magnesium plays an important structural role (along with calcium and phosphate) in bone formation, where about half of the body’s magnesium is stored. It is also one of the most abundant intracellular positive ions, being necessary for essentially all biochemical processes that involve the transfer of phosphate groups, for example, in the synthesis and use of ATP.
Supplementation of amino acids, vitamins and minerals may be necessary when intake is inadequate or is compromised due to use of alcohol, caffeine or medications that interfere with absorption or during periods of pregnancy, illness, tissue repair or major stress.
Vitamin D
Despite decades of research showing the importance of sufficient intake of vitamin D, deficiency of this nutrient remains common in both children and adults. Most cells in the body have receptors for vitamin D, possibly providing it with many roles in the prevention of chronic illnesses, including cancer, autoimmune diseases, infectious diseases, and cardiovascular disease (Holick 2007).
When the skin is exposed to sunlight (without sunscreen), solar ultraviolet B radiation is absorbed and converts 7-dehydrocholesterol to previtamin D3, which is rapidly changed to D3. The use of sunscreens and other skin products blocks the sun’s radiation, thereby interrupting this efficient process. Since the ‘sunshine vitamin’ is available in only a few foods, primarily oily fish, supplementation may be necessary to insure adequate levels, particularly for those in northern climates and during colder seasons, when sunlight is not readily available.
It is without question that this nutrient is vitally important. Its role with parathyroid hormone and calcium is crucial for bone health, and it is a potent immunomodulator. Holick (2007) provides a significant discussion, citing studies indicating that levels below 20 ng per milliliter are associated with a ‘30 to 50% increased risk of incident colon, prostate, and breast cancer, along with higher mortality from these cancers.’ Lapp (2009) notes ‘Epidemiologic associations have linked vitamin D with the risk of developing a host of health conditions, including multiple sclerosis, type 1 diabetes, rheumatoid arthritis, hypertension, cardiovascular disease, some cancers, and forms of depression.’ Gerwin (2005) links vitamin D deficiency as a perpetuator of trigger points and chronic myofascial pain.
It is important that manual practitioners consider these and other nutritional components of chronic pain, either within their own practices or through professional referral. The next chapter builds upon the concepts of this chapter, as the patient is encouraged to be an active member of the recovery team.
Aarflot T. Association between chronic widespread musculoskeletal complaints and thyroid autoimmunity. Scand J Prim Health Care. 1996;14(2):111-115.
Adams K. Sleep is for tissue restoration. J R Coll Physicians Edinb. 1977;11:376-388.
Affleck G. Sequential daily relations of sleep, pain intensity and attention to pain among women with FMS. Pain. 1996;68(2–3):363-368.
Alberti K., Natrass M. Lactic acidosis. Lancet. 1977;2:25-29.
Altura B.M., Altura B.T. Tension headaches and muscle tension: is there a role for magnesium? Med. Hypotheses. 2001;57(6):705-713.
Anderson J. Allergic diseases: diagnosis and treatment. Detroit: Henry Ford Health System, Allergy Division, 1997.
Bakker G.C.M., Van Erk M.J., Pellis L., et al. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: a nutrigenomics approach. The American Journal of Clinical Nutrition. 2010;91:1044-1059.
Ball S., Shekhar A. Basilar artery response to hyperventilation in panic disorder. Am J Psychiatry. 1997;154(11):1603-1604.
Barelli P. Nasopulmonary physiology. In: Timmons B., editor. Behavioral and psychological approaches to breathing disorders. New York: Plenum Press, 1994.
Barlow W. Anxiety and muscle tension pain. Br J Clin Pract. 13(5), 1959.
Belongia E. An investigation of the cause of the eosinophilia-myalgia syndrome associated with tryptophan use. N Engl J Med. 1990;323(6):357-365.
Berkow R., Fletcher A., editors. The Merck manual. New Jersey: Merck Research Laboratories, Rahway, 1992.
Bjarnason I. The leaky gut of alcoholism – possible route for entry of toxic compounds? Lancet. 1984;i:179-182.
Bland J. Medical food-supplemented detoxification program in management of chronic health problems. Alternative Therapies. 1995;1:62-71.
Block E., Arnott D., Quigley B., Lynch W. Unilateral nostril breathing influences lateralized cognitive performance. Brain Cogn. 1989;9(2):181-190.
Borok G. Childhood asthma – foods that trigger? S Afr Med J. 1990;77:269.
Borok G. IBS and diet. Gastroenterology Forum. April 29, 1994.
Bradley D. Breathing rehabilitation strategies. In: Chaitow L., Bradley D., Gilbert C., editors. Multidisciplinary approaches to breathing pattern disorders. Edinburgh: Churchill Livingstone, 2002.
Brekhman I. Man and biologically active substances. London: Pergamon Press, 1980.
Brostoff J. Complete guide to food allergy. London: Bloomsbury, 1992.
Buist R. Anxiety neurosis: the lactate connection. International Clinical Nutrition Review. 1985;5(1):1-4.
Caruso I., Puttini P., Cazzola M., Azzolini V. Double-blind study of 5-hydroxytryptophan versus placebo in the treatment of primary fibromyalgia syndrome. J Int Med Res. 1990;18(3):201-209.
Chaitow L. Breathing pattern disorders, motor control, and low back pain. Journal of Osteopathic Medicine. 2004;7(1):34-41.
Chaitow L. Thorsons’ guide to amino acids. London: Thorsons/HarperCollins, 1991.
Chaitow L. Unifying themes (keynote address). American Massage Therapy Association Conference, Quebec, Canada. October, 2001.
Charney D. Increased anxiogenic effects of caffeine in panic disorders. Arch Gen Psychiatry. 1985;42:233-243.
Cichoke A. The use of proteolytic enzymes with soft tissue athletic injuries. American Chiropractor. 1981:32. October
Cleveland C., Fisher R., Brestel E., Esinhart J., Metzger W. Chronic rhinitis and underrecognized association with fibromyalgia. Allergy Proc. 1992;13(5):263-267.
Deitch E. Intestinal permeability increased in burn patients shortly after injury. Surgery. 1990;107:411-416.
Donowitz M. Arachidonic acid metabolites and their role in inflammatory bowel disease. Gastroenterology. 1985;88:580-587.
Ekbom K. Restless legs syndrome. Neurology. 1960;10:868.
Feingold B. Hyperactivity in children. Presentation at the Kaiser Foundation Hospital, Sacramento, California, 3 December. 1973.
Fibromyalgia Network. Newsletter. 1993:12. October
Ford-Hutchinson A. Leukotrienes: their formation and role as inflammatory mediators. Federal Proceedings. 1985;44:25-29.
Fruhauf P. Food intolerance - Paediatric gastroenterologic view | [Intolerance potravin z pohledu dětského gastroenterologa]. Alergie. 2009;11(3):195-198.
Gerwin R. A review of myofascial pain and fibromyalgia – factors that promote their persistence. Acupunct Med. 2005;23(3):121-134.
Gibbs D.M. Hyperventilation-induced cerebral ischemia in panic disorder and effects of nimodipine. Am J Psychiatry. 1992;149:1589-1591.
Gilbert C. Yoga and breathing. Journal of Bodywork and Movement Therapies. 1999;3(1):44-54.
Gilbert C. The biochemistry of hyperventilation. In: Chaitow L., Bradley D., Gilbert C., editors. Multidisciplinary approaches to breathing pattern disorders. Edinburgh: Churchill Livingstone, 2002.
Gow J. Enteroviral sequences detected in muscles of patients with postviral fatigue syndrome. Br Med J. 1991;302:692-696.
Griep E. Pituitary release of growth hormone and prolactin in primary FMS. J Rheumatol. 1994;21(11):2125-2130.
Hodge L. Consumption of oily fish and childhood allergy risk. Med J Aust. 1996;164:136-140.
Holgate S. Mast cells and their mediators. In Holborrow E., Reeves W., editors: Immunology in medicine, ed 2, London: Academic Press, 1983.
Holick M. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
Hollander D. Aging-associated increase in intestinal absorption of macro-molecules. Gerontology. 1985;31:133-137.
Hough A. Physiotherapy in respiratory care. Cheltenham: Stanley Thornes, 1996.
Iacano G. Chronic constipation as a symptom of cow’s milk allergy. J Pediatr. 1995;126:34-39.
Innocenti D.M. Chronic hyperventilation syndrome. Cash’s textbook of chest, heart and vascular disorders for physiotherapists. ed 4. London: Faber and Faber; 1987.
Isolauri E. Intestinal permeability changes in acute gastroenteritis. J Pediatr Gastroenterol Nutr. 1989;8:466-473.
James J. Food allergy – what link to respiratory symptoms? Journal of Respiratory Diseases. 1997;18(4):379-390.
Jenkins A. Do NSAIDs increase colonic permeability? Gut. 1991;32:66-69.
Jevning R. The physiology of meditation – a wakeful hypometabolic integrated response. Neurosci Biobehav Rev. 1992;16:415-424.
Jung C.-G. Synchronicity: an acausal connecting principle. Princeton, New Jersey: Princeton University Press, 1973.
Kleiner S. Water: an essential but overlooked nutrient. J Am Diet Assoc. 1999;81(2):200-206.
Lapp J. Vitamin D: bone health and beyond. American Journal of Lifestyle Medicine. 2009;3(5):386-393.
Liao F., Folsom A., Brancati F. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1998;136(3):480-490.
Lans M.C., Klasson-Wehler E., Willemsen M., Meussen E., Safe S., Bouwer A. Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-p-dioxins and – dibenzofurans with human transthyretin. Chem Biol Interact. 1993;88(1):7-21.
Lee J., Hopkins V. What your doctor may not tell you about menopause. New York: Warner Books, 1996.
Lindahl O. Vegan diet regimen with reduced medication in the treatment of bronchial asthma. J Asthma. 1985;22:45-55.
Lowe J. Effectiveness and safety of T3 therapy in FMS. Clinical Bulletin of Myofascial Therapy. 1997;2(2/3):31-57.
Lowe J. The metabolic treatment of fibromyalgia. Tulsa, Oklahoma: McDowell Publishing Company, 2000.
Lowe J., Honeyman-Lowe G. Facilitating the decrease in fibromyalgic pain during metabolic rehabilitation. Journal of Bodywork and Movement Therapies. 1998;2(4):208-217.
Lum L. Hyperventilation – an anxiety state. J R Soc Med. 1981;74:1-4.
Macefield G., Burke D. Paraesthesia and tetany induced by voluntary hyperventilation: increased excitability of human cutaneous and motor axons. Brain. 1991;114:527-540.
Macintyre A. What causes ME? The immune dysfunction hypothesis. Journal of Action for ME. 1993;14:24-25. Autumn
Matthews S., Campbell A. When sugar is not so sweet. Lancet. 2000;355:1330.
McKinney J.D., Pedersen L.G. Do residue levels of polychlorinated biphenyls (PCBs) in human blood produce mild hypothyroidism? J Theor Biol. 1987;129:231-241.
McNaughton L., Dalton B., Palmer G. Sodium bicarbonate can be used as an ergogenic aid in high-intensity, competitive cycle ergometry of 1 h duration. Eur J Appl Physiol. 1999;80(1):64-69.
Melles Z., Kiss S.A. Influence of the magnesium content of drinking water and of magnesium therapy on the occurrence of preeclampsia. Magnes Res. 1992;12(5):4. 277–279
Mihill C. Water: the hidden fuel for performance. 2000. Water UK http://www.water.org.uk/magazine/bulletins/waterinfo/57.html
Mitchell E.B. Food intolerance. In: Dickerson W., Lee H., editors. Nutrition in the clinical management of disease. London: Edward Arnold, 1988.
Moldofsky H., Dickstein J.B. Sleep and cytokine-immune functions in medical, psychiatric and primary sleep disorders. Sleep Med Rev. 1999;3:325-337.
Moncada S. Leucocytes and tissue injury: the use of eicosapentanoic acid in the control of white cell activation. Wien Klin Wochenschr. 1986;98(4):104-106.
Monro J. Presentation at the Third International Symposium on Mushroom Nutrition in Milan, Italy on 10 March, 2001. 2001. (Copies available from Mycology Research Laboratories Ltd, email: info@aneid.pt)
Monteiro M. Subjective feelings of anxiety in young men after ethanol and diazepam infusions. J Clin Psychiatry. 1990;51(1):12-16.
Nettleton S., Woods B., Burrows R., et al. Food allergy and food intolerance: Towards a sociological agenda. Health. 2009;13(6):647-664.
O’Dwyer S. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459-1464.
Oertel J.E., LiVolsi V.A. Pathology of thyroid diseases. In Braverman L.E., Utiger R.D., editors: Werner and Ingbar’s the thyroid: a fundamental and clinical text, ed 6, New York: J B Lippincott, 1991.
Oldstone M. Viral alteration of cell function. Sci Am. 1989;261:34-40.
Ortolani C., Vighi G. Definition of adverse reactions to food. Allergy. 1995;50(Suppl 20):8-13.
Paganelli R. Intestinal permeability in patients with chronic urticaria-angiodema with and without arthralgia. Ann Allergy. 1991;66:181-184.
Petrovsky N., Harrison L. The chronobiology of human cytokine production. International Review of Immunology. 1998;16(5–6):635-649.
Petrovsky N., McNair P., Harrison L. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10(4):307-312.
Pizzorno J. Total wellness. Prima Publishing. California: Rocklin, 1996.
ed 2. Pizzorno J., Murray M., editors. Textbook of natural medicine. Vol 2. Edinburgh: Churchill Livingstone; 1999.
Puttini P., Caruso I. Primary fibromyalgia syndrome and 5-hydroxy-L-tryptophan: a 90-day open study. J Int Med Res. 1992;20(2):182-189.
Randolph T. Stimulatory withdrawal and the alternations of allergic manifestations. In: Dickey L., editor. Clinical ecology. Springfield, Illinois: Charles C Thomas, 1976.
Rief W., Hessel A., Braehler E. Somatization symptoms and hypochondriacal features in the general population. Psychosom Med. 2001;63(4):595-602.
Roberson K., editor. Asthma: clinical pearls in nutrition and complementary medicine. Sacramento, California: IT Services, 1997.
Roland N. Interactions between the intestinal flora and xenobiotic metabolizing enzymes and their health consequences. World Rev. Nutr. Diet.. 1993;74:123-148.
Rossi E. The twenty-minute break: using the new science of ultiadian rhythms. New York: Jeremy Tarcher, 1991.
Rowe A. Food allergy – its manifestation and control. Springfield, Illinois: Charles C Thomas, 1972.
Rowe A.H. Allergic toxemia and migraine due to food allergy. California West Medical Journal. 1930;33:785.
Rowntree S., Cogswell J., Platts-Mills T., Mitchell E. Development of IgE and IgG antibodies to food and inhalant allergens in children. Archives of Diseases in Children. 1985;60:727-735.
Royal College of Physicians. Food intolerance and food aversion. J R Coll Physicians Lond. 18(2), 1984.
Rucco V., et al. Autogenic training versus Erickson’s analogical technique in treatment of fibromyalgia syndrome. Rev European Sci Med Farmacol. 1995;17(1):41-50.
Sanders K., Sanders-Gendreau K. The College Student and the Anti-Inflammatory Diet. Explore. 2004;3:410-412.
Schott H.C.II, Hinchcliff. Treatments affecting fluid and electrolyte status during exercise. Vet Clin North Am Equine Pract. 1998;14(1):175-204.
Schultz J. Autogenic training – psychophysiological approach to psychotherapy. New York: Grune and Stratton, 1959.
Shannahoff-Khalsa D. Literalised rhythms of the central and autonomic nervous system. Int J Psychophysiol. 1991;11(3):222-251.
Sherer Y., Shaish A., Levkovitz H., et al. Magnesium fortification of drinking water suppresses atherogenesis in male LDL-receptor-deficient mice. Pathobiology. 1999;67(4):207-213.
Simons D., Travell J., Simons L. ed 2. Myofascial pain and dysfunction: the trigger point manual. Baltimore: Williams and Wilkins; 1999;1.
Smolensky M., Lemmer B., Reinberg A. Chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Adv Drug Deliv Rev. 2007;599(9–10):852-882.
Stamford B. Muscle cramps: untying the knots. Physical Sports Medicine. 1993;21:115-116.
Stanbury J. The metabolic basis of inherited disease. New York: McGraw-Hill, 1983.
Sutherland E.R. Nocturnal asthma. J Allergy Clin Immunol. 2005;116(6):1179-1186.
Taussig S. Bromelaine and its clinical application – update. J Ethnopharmacol. 1988;22:191-203.
Thien F. Oily fish and asthma. Med J Aust. 1996;164:135-136.
Timmons B.H., Ley R. Behavioral and psychological approaches to breathing disorders. New York: Plenum Press, 1994.
Troncone R. Increased intestinal sugar permeability after challenge in children with cow’s milk allergy or intolerance. Allergy. 1994;49:142-146.
Tuncer T. Primary fibromyalgia and allergy. Clin Rheumatol. 1997;16(1):9-12.
Uhde T. Caffeine and behaviour relationship to human anxiety, plasma MPHG and cortisol. Psychopharmacol Bull. 1984;20(3):426-430.
Van Den Berg K.J., Zurcher C., Brouwer A. Effects of 3,4,3′,4′- tetrachlorobiphenyl on thyroid function and histology in marmoset monkeys. Toxicol Appl Pharmacol. 1988;41:77-86.
Walker W. Antigen uptake in the gut. Immunol Today. 1981;2:30-34.
Wall P.D., Melzack R., editors. Textbook of pain, ed 2, Edinburgh: Churchill Livingstone, 1989.
Wardlaw A. Morphological and secretory properties of bronchoalveolar mast cells in respiratory diseases. Clin Allergy. 1986;16:163-173.
Waterston J., Gilligan B. Pyridoxine neuropathy. Med J Aust. 1987;146:640-642.
Weiler J., Bonini S., Coifman R. American Academy of Allergy, Asthma & Immunology work group report: Exercise-induced asthma. J Allergy Clin Immunol. 2007;119(6):1349-1358.
Wendel O., Beebe W. Glycolytic activity in schizophrenia. In: Hawkins D., Pauling L., editors. Orthomolecular Psychiatry. San Francisco: WH Freeman, 1973.
Werbach M. Nutritional influences on illness. Tarzana, California: Third Line Press, 1991.
Werbach M. Nutritional influences on mental illness. Tarzana, California: Third Line Press, 1991.
Werbach M. Nutritional influences on illness, ed 2. Tarzana, California: Third Line Press, 1993.
Werbach M. Natural medicine for muscle strain. Journal of Bodywork and Movement Therapies. 1996;1(1):18-19.
Williams R. Biochemical individuality. Texas: University Press, 1979.
Yang C., Chiu H., Tsai S Cheng M., Lin M., Sung F. Calcium and magnesium in drinking water and risk of death from prostate cancer. J Toxicol Environ Health. 2000;60(1):17-26.
Yang C.Y., Chiu H.F., Cheng M.F., Wu T.N., Hsu T.Y. Calcium and magnesium in drinking water and risk of death from breast cancer. J Toxicol Environ Health A. 2000;60(4):231-241.
Yunus W., Dailey J., Aldag J. Plasma tryptophan and other amino acids in primary FMS. J Rheumatol. 1992;19:90-94.