Radiation Physics
After completion of this chapter, the student will be able to do the following:
• Define the key words associated with radiation physics
• Identify the structure of the atom
• Describe the process of ionization
• Discuss the difference between radiation and radioactivity
• List the two types of ionizing radiation and give examples of each
• List the characteristics of electromagnetic radiation
• List the properties of x-radiation
• Identify the component parts of the x-ray machine
• Label the parts of the dental x-ray tubehead and the dental x-ray tube
• Describe in detail how dental x-rays are produced
• List and describe the possible interactions of x-rays with matter
To understand how x-rays are produced, the dental radiographer must understand the nature and interactions of atoms. A complete understanding of x-radiation includes an understanding of the fundamental concepts of atomic and molecular structure as well as a working knowledge of ionization, ionizing radiation, and the properties of x-rays. An understanding of the dental x-ray machine, x-ray tube, and circuitry is also necessary. The purpose of this chapter is to present the fundamental concepts of atomic and molecular structure, to define and characterize x-radiation, to provide an introduction to the x-ray machine, and to describe in detail how x-rays are produced. This chapter also includes a discussion of the interactions of x-radiation with matter.
Fundamental Concepts
Atomic and Molecular Structure
The world is composed of matter and energy. Matter is anything that occupies space and has mass; when matter is altered, energy results. The fundamental unit of matter is the atom. All matter is composed of atoms, or tiny invisible particles. An understanding of the structure of the atom is necessary before the dental radiographer can understand the production of x-rays.
Atomic Structure
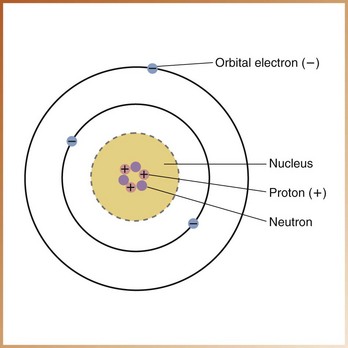
The atom consists of two parts: (1) a central nucleus and (2) orbiting electrons (Figure 2-1). The identity of an atom is determined by the composition of its nucleus and the arrangement of its orbiting electrons. At present, 105 different atoms have been identified.
Nucleus: The nucleus, or dense core of the atom, is composed of particles known as protons and neutrons (also known as nucleons). Protons carry positive electrical charges, whereas neutrons carry no electrical charge. The nucleus of an atom occupies very little space; in fact, most of the atom is empty space. For example, if an atom were imagined to be the size of a football stadium, the nucleus would be the size of a football.
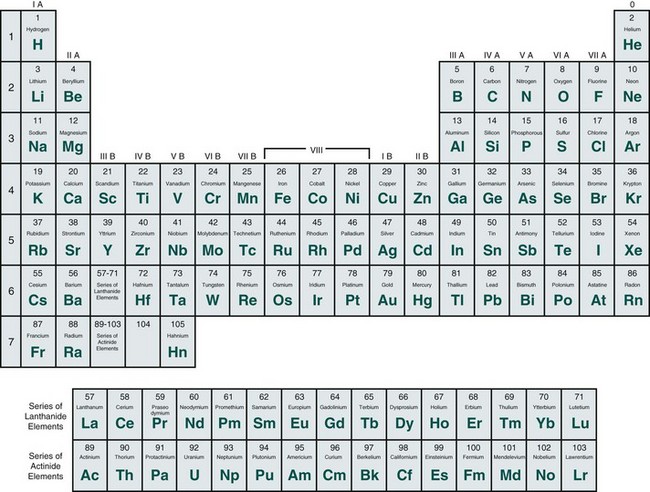
Atoms differ from one another on the basis of their nuclear composition. The number of protons and neutrons in the nucleus of an atom determines its mass number or atomic weight. The number of protons inside the nucleus equals the number of electrons outside the nucleus and determines the atomic number of the atom. Each atom has an atomic number, ranging from that of hydrogen, the simplest atom, which has an atomic number of 1, to that of hahnium, the most complex atom, which has an atomic number of 105. Atoms are arranged in the ascending order of atomic number on a chart known as the periodic table of the elements (Figure 2-2). Elements are substances made up of only one type of atom.
Electrons: Electrons are tiny, negatively charged particles that have very little mass; an electron weighs approximately 1/1800 as much as a proton or neutron. The arrangement of the electrons and neutrons in an atom resembles that of a miniature solar system. Just as the planets revolve around the sun, electrons travel around the nucleus in well-defined paths known as orbits or shells.
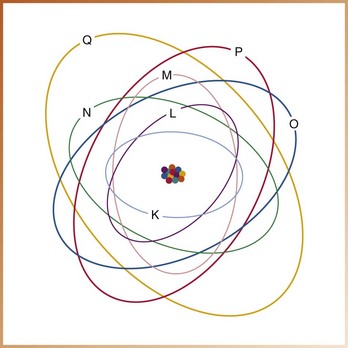
An atom contains a maximum of seven shells, each located at a specific distance from the nucleus and representing different energy levels. The shells are designated with the letters K, L, M, N, O, P, and Q; the K shell is located closest to the nucleus and has the highest energy level (Figure 2-3). Each shell has a maximum number of electrons it can hold (Figure 2-4).
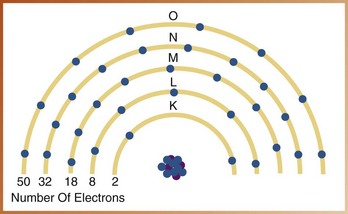
FIGURE 2-4 Maximum number of electrons that can exist in each shell of a tungsten atom. (Redrawn from Langlais RP: Exercises in oral radiology and interpretation, ed 4, St Louis, 2004, Saunders.)
Electrons are maintained in their orbits by the electrostatic force, or attraction, between the positive nucleus and the negative electrons. This is known as the binding energy, or binding force, of an electron. The binding energy is determined by the distance between the nucleus and the orbiting electron and is different for each shell. The strongest binding energy is found closest to the nucleus in the K shell, whereas electrons located in the outer shells have a weak binding energy. The binding energies of orbital electrons are measured in electron volts (eV) or kilo electron volts (keV). (One kilo electron volt equals 1000 electron volts.)
The energy required to remove an electron from its orbital shell must exceed the binding energy of the electron in that shell. A great amount of energy is required to remove an inner-shell electron, but electrons loosely held in the outer shells can be affected by lesser energies. For example, in the tungsten atom, the binding energies are as follows:
| 70 keV | K-shell electrons |
| 12 keV | L-shell electrons |
| 3 keV | M-shell electrons |
Note that the binding energy is greatest in the shell closest to the nucleus. To remove a K-shell electron from a tungsten atom, 70 keV (70,000 eV) of energy would be required, whereas only 3 keV (3000 eV) of energy would be necessary to remove an electron from the M shell.
Molecular Structure
Atoms are capable of combining with each other to form molecules. A molecule can be defined as two or more atoms joined by chemical bonds, or the smallest amount of a substance that possesses its characteristic properties. As with the atom, the molecule is also a tiny invisible particle. Molecules are formed in one of two ways: (1) by the transfer of electrons or (2) by the sharing of electrons between the outermost shells of atoms. An example of a simple molecule is water (H2O); the symbol H2 represents two atoms of hydrogen, and the symbol O represents one atom of oxygen (Figure 2-5).
Ionization, Radiation, and Radioactivity
The fundamental concepts of atomic and molecular structure just reviewed allow an understanding of ionization, radiation, and radioactivity. Before the dental radiographer can understand how x-rays are produced, a working knowledge of ionization and the difference between radiation and radioactivity is necessary.
Ionization
Atoms can exist in a neutral state or in an electrically unbalanced state. Normally, most atoms are neutral. A neutral atom contains an equal number of protons (positive charges) and electrons (negative charges). An atom with an incompletely filled outer shell is electrically unbalanced and attempts to capture an electron from an adjacent atom. If the atom gains an electron, it has more electrons than protons and neutrons and, therefore, a negative charge. Similarly, the atom that loses an electron has more protons and neutrons and thus has a positive charge. An atom that gains or loses an electron and becomes electrically unbalanced is known as an ion.
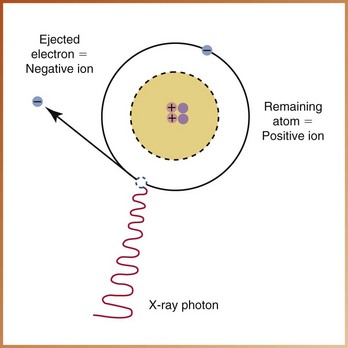
Ionization is the production of ions, or the process of converting an atom into ions. Ionization deals only with electrons and requires sufficient energy to overcome the electrostatic force that binds the electron to the nucleus. When an electron is removed from an atom in the ionization process, an ion pair results. The atom becomes the positive ion, and the ejected electron becomes the negative ion (Figure 2-6). This ion pair reacts with other ions until electrically stable, neutral atoms are formed.
Radiation and Radioactivity
Radiation, as defined in Chapter 1, is the emission and propagation of energy through space or a substance in the form of waves or particles. The terms radioactivity and radiation are sometimes confused; it is important to note that they do not have the same meaning.
Radioactivity can be defined as the process by which certain unstable atoms or elements undergo spontaneous disintegration, or decay, in an effort to attain a more balanced nuclear state. A substance is considered radioactive if it gives off energy in the form of particles or rays as a result of the disintegration of atomic nuclei.
In dentistry, radiation (specifically x-radiation) is used, not radioactivity.
Ionizing Radiation
Ionizing radiation can be defined as radiation that is capable of producing ions by removing or adding an electron to an atom. Ionizing radiation can be classified into two groups: (1) particulate radiation and (2) electromagnetic radiation.
Particulate Radiation
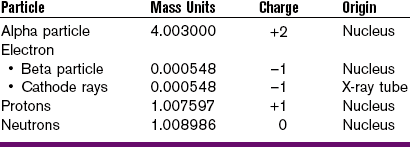
Particulate radiations are tiny particles of matter that possess mass and travel in straight lines and at high speeds. Particulate radiations transmit kinetic energy by means of their extremely fast-moving, small masses. Four types of particulate radiation are recognized (Table 2-1), as follows:
1. Electrons can be classified as beta particles or cathode rays. They differ in origin only.
a. Beta particles are fast-moving electrons emitted from the nucleus of radioactive atoms.
b. Cathode rays are streams of high-speed electrons that originate in an x-ray tube.
2. Alpha particles are emitted from the nuclei of heavy metals and exist as two protons and neutrons, without electrons.
3. Protons are accelerated particles, specifically hydrogen nuclei, with a mass of 1 and a charge of +1.
4. Neutrons are accelerated particles with a mass of 1 and no electrical charge.
Electromagnetic Radiation
Electromagnetic radiation can be defined as the propagation of wavelike energy (without mass) through space or matter. The energy propagated is accompanied by oscillating electric and magnetic fields positioned at right angles to one another, thus the term electromagnetic (Figure 2-7).
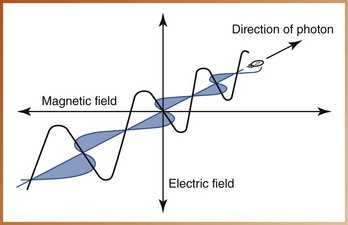
FIGURE 2-7 Oscillating electric and magnetic fields are characteristic of electromagnetic radiations.
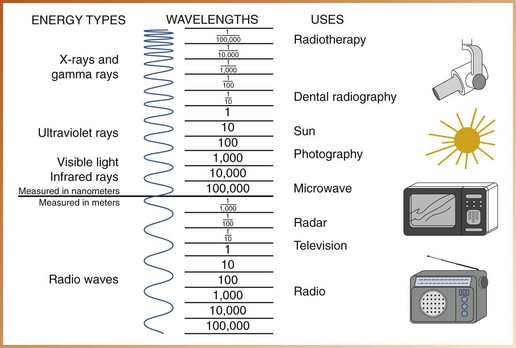
Electromagnetic radiations are man made or occur naturally; examples include cosmic rays, gamma rays, x-rays, ultraviolet rays, visible light, infrared light, radar waves, microwaves, and radio waves. Electromagnetic radiations are arranged according to their energies in what is termed the electromagnetic spectrum (Figure 2-8). All energies of the electromagnetic spectrum share common characteristics (Box 2-1). Depending on their energy levels, electromagnetic radiations can be classified as ionizing or non-ionizing. In the electromagnetic spectrum, only high-energy radiations (cosmic rays, gamma rays, and x-rays) are capable of ionization.
Electromagnetic radiations are believed to move through space as both a particle and a wave; therefore two concepts, the particle concept and the wave concept, must be considered.
Particle Concept: The particle concept characterizes electromagnetic radiations as discrete bundles of energy called photons, or quanta. Photons are bundles of energy with no mass or weight that travel as waves at the speed of light and move through space in a straight line, “carrying the energy” of electromagnetic radiation.
Wave Concept: The wave concept characterizes electromagnetic radiations as waves and focuses on the properties of velocity, wavelength, and frequency, as follows:
• Velocity refers to the speed of the wave. All electromagnetic radiations travel as waves or a continuous sequence of crests at the speed of light (3 × 108 meters per second [186,000 miles per second]) in a vacuum.
• Wavelength can be defined as the distance between the crest of one wave and the crest of the next (Figure 2-9). Wavelength determines the energy and penetrating power of the radiation; the shorter the distance between the crests, the shorter is the wavelength and the higher is the energy and ability to penetrate matter. Wavelength is measured in nanometers (nm; 1 × 10–9 meters, or one billionth of a meter) for short waves and in meters (m) for longer waves.
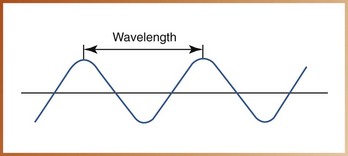
FIGURE 2-9 Wavelength is the distance between the crest (peak) of one wave and the crest of the next.
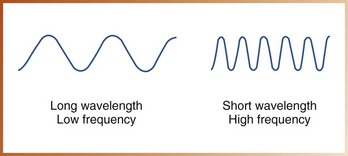
• Frequency refers to the number of wavelengths that pass a given point in a certain amount of time (Figure 2-10). Frequency and wavelength are inversely related; if the frequency of the wave is high, the wavelength will be short, and if the frequency is low, the wavelength will be long.
The amount of energy an electromagnetic radiation possesses depends on the wavelength and frequency.
Low-frequency electromagnetic radiations have a long wavelength and less energy. Conversely, high-frequency electromagnetic radiations have a short wavelength and more energy.
For example, communications media use the low-frequency, longer waves of the electromagnetic spectrum; the wavelength of a radio wave can be as long as 100 m, whereas the wavelength of a television wave is approximately 1 m. In contrast, diagnostic radiography uses the high-frequency, shorter waves in the electromagnetic spectrum; x-rays used in dentistry have a wavelength of 0.1 nm, or 0.00000000001 m.
X-Radiation
X-radiation is a high-energy, ionizing electromagnetic radiation. As with all electromagnetic radiations, x-rays have the properties of both waves and particles. X-rays can be defined as weightless bundles of energy (photons) without an electrical charge that travel in waves with a specific frequency at the speed of light. X-ray photons interact with the materials they penetrate and cause ionization.
X-rays have certain unique properties or characteristics. It is important that the dental radiographer be familiar with the properties of x-rays (Box 2-2).
X-Ray Machine
X-rays are produced in the dental x-ray machine. For learning purposes, the dental x-ray machine can be divided into three study areas: (1) the component parts, (2) the x-ray tube, and (3) the x-ray generating apparatus.
Component Parts
The dental x-ray machine consists of three visible component parts: (1) control panel, (2) extension arm, and (3) tubehead (Figure 2-11).

FIGURE 2-11 Three component parts of dental x-ray machine: A, control panel; B, extension arm; C, tubehead. (Courtesy Instrumentarium Dental, Inc. Milwaukee, WI.)
Control Panel
The control panel of the dental x-ray machine contains an on-off switch and an indicator light, an exposure button and indicator light, and control devices (time, kilovoltage, and milliamperage selectors) to regulate the x-ray beam. The control panel is plugged into an electrical outlet and appears as a panel or a cabinet mounted on the wall outside the dental operatory.
Extension Arm
The wall-mounted extension arm suspends the x-ray tubehead and houses the electrical wires that extend from the control panel to the tubehead. The extension arm allows for movement and positioning of the tubehead.
Tubehead
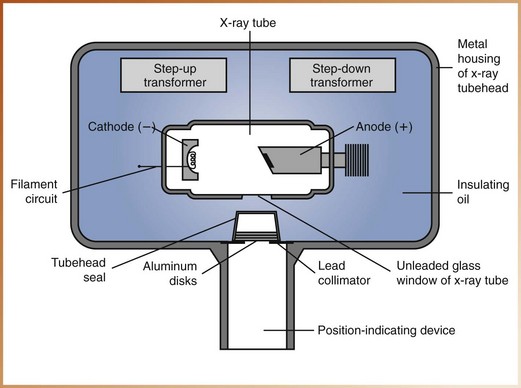
The x-ray tubehead is a tightly sealed, heavy metal housing that contains the x-ray tube that produces dental x-rays. The component parts of the tubehead include the following (Figure 2-12):
• Metal housing, or the metal body of the tubehead that surrounds the x-ray tube and transformers and is filled with oil—protects the x-ray tube and grounds the high-voltage components.
• Insulating oil, or the oil that surrounds the x-ray tube and transformers inside the tubehead—prevents overheating by absorbing the heat created by the production of x-rays.
• Tubehead seal, or the aluminum or leaded-glass covering of the tubehead that permits the exit of x-rays from the tubehead—seals the oil in the tubehead and acts as a filter to the x-ray beam.
• X-ray tube, or the heart of the x-ray generating system (discussed later) (Figure 2-13).

FIGURE 2-13 Actual dental x-ray tube. (From Bird DL, Robinson DS: Modern dental assisting, ed 10, St Louis, 2012, Saunders.)
• Transformer, or a device that alters the voltage of incoming electricity (also discussed later).
• Aluminum disks, or sheets of 0.5-mm–thick aluminum placed in the path of the x-ray beam—filter out the nonpenetrating, longer wavelength x-rays (Figure 2-14). Aluminum filtration is discussed in Chapter 5.

FIGURE 2-14 Aluminum filtration disk in x-ray tubehead. (From Bird DL, Robinson DS: Modern dental assisting, ed 10, St Louis, 2012, Saunders.)
• Lead collimator, or a lead plate with a central hole that fits directly over the opening of the metal housing, where the x-rays exit—restricts the size of the x-ray beam (Figure 2-15). Collimation is also discussed in Chapter 5.
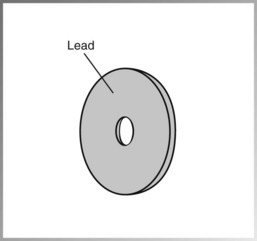
FIGURE 2-15 The lead collimator, or lead plate with a central opening, restricts the size of the x-ray beam.
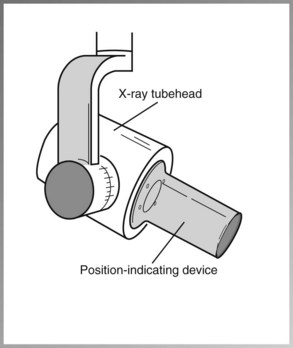
• Position-indicating device (PID), or open-ended, lead-lined cylinder that extends from the opening of the metal housing of the tubehead—aims and shapes the x-ray beam (Figure 2-16). The PID is sometimes referred to as the cone.
X-Ray Tube
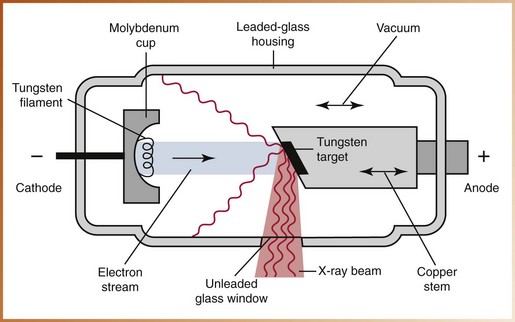
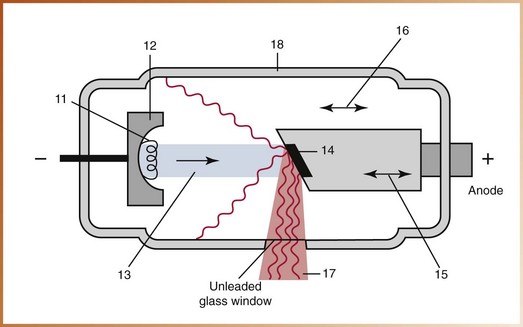
The x-ray tube is the heart of the x-ray generating system; it is critical to the production of x-rays and warrants a separate discussion from the rest of the x-ray machine. The x-ray tube is a glass vacuum tube from which all the air has been removed. The x-ray tube used in dentistry measures approximately several inches long by one inch in diameter. The component parts of the x-ray tube include a leaded-glass housing, negative cathode, and positive anode (Figure 2-17).
Leaded-Glass Housing
The leaded-glass housing is a leaded-glass vacuum tube that prevents x-rays from escaping in all directions. One central area of the leaded-glass tube has a “window” that permits the x-ray beam to exit the tube and directs the x-ray beam toward the aluminum disks, lead collimator, and PID.
Cathode
The cathode, or negative electrode, consists of a tungsten wire filament in a cup-shaped holder made of molybdenum. The purpose of the cathode is to supply the electrons necessary to generate x-rays. In the x-ray tube, the electrons produced in the negative cathode are accelerated toward the positive anode. The cathode includes the following:
X-Ray Generating Apparatus
To understand how the x-ray tube functions and how x-rays are produced, the dental radiographer must understand electricity and electrical currents, electrical circuits, and transformers.
Electricity and Electrical Currents
Electricity is the energy that is used to make x-rays. Electrical energy consists of a flow of electrons through a conductor; this flow is known as the electrical current. The electrical current is termed direct current (DC) when the electrons flow in one direction through the conductor. The term alternating current (AC) describes an electrical current in which the electrons flow in two, opposite directions. Rectification is the conversion of AC to DC. The dental x-ray tube acts as a self-rectifier in that it changes AC into DC while producing x-rays. This ensures that the current is always flowing in the same direction, more specifically, from cathode to anode.
Generators on older machines produced an x-ray beam with a wavelike pattern, whereas newer constant-potential x-ray machines produce a homogeneous beam of consistent wavelengths during radiation exposure. Constant-potential machines also reduce patient exposure to radiation by 20%, an important consideration for patient protection.
Amperage is the measurement of the number of electrons moving through a conductor. Current is measured in amperes (A) or milliamperes (mA). Voltage is the measurement of electrical force that causes electrons to move from a negative pole to a positive one. Voltage is measured in volts (V) or kilovolts (kV).
In the production of x-rays, both the amperage and the voltage can be adjusted. In the x-ray tube, the amperage, or number of electrons passing through the cathode filament, can be increased or decreased by the milliamperage (mA) adjustment on the control panel of the x-ray machine. The voltage of the x-ray tube current, or the current passing from the cathode to the anode, is controlled by the kilovoltage peak (kVp) adjustment on the control panel.
Circuits
A circuit is a path of electrical current. Two electrical circuits are used in the production of x-rays: (1) a low-voltage, or filament, circuit and (2) a high-voltage circuit.
The filament circuit uses 3 to 5 volts, regulates the flow of electrical current to the filament of the x-ray tube, and is controlled by the milliampere settings. The high-voltage circuit uses 65,000 to 100,000 volts, provides the high voltage required to accelerate electrons and to generate x-rays in the x-ray tube, and is controlled by the kilovoltage settings.
Transformers
A transformer is a device that is used to either increase or decrease the voltage in an electrical circuit (Figure 2-18). Transformers alter the voltage of the incoming electrical current and then route the electrical energy to the x-ray tube. In the production of dental x-rays, three transformers are used to adjust the electrical circuits: (1) the step-down transformer, (2) the step-up transformer, and (3) the autotransformer.
A step-down transformer is used to decrease the voltage from the incoming 110- or 220-line voltage to the 3 to 5 volts used by the filament circuit. A step-down transformer has more wire coils in the primary coil than in the secondary coil (see Figure 2-18). The coil that receives the alternating electrical current is the primary, or input, coil; the secondary coil is the output coil. The electrical current that energizes the primary coil induces a current in the secondary coil. The high-voltage circuit uses both a step-up transformer and an autotransformer. A step-up transformer is used to increase the voltage from the incoming 110- or 220-line voltage to the 65,000 to 100,000 volts used by the high-voltage circuit. A step-up transformer has more wire coils in the secondary coil than in the primary coil (see Figure 2-18). An autotransformer serves as a voltage compensator that corrects for minor fluctuations in the current.
Production of X-Radiation
With the component parts of the x-ray machine, the x-ray tube, and the x-ray generating apparatus reviewed, a discussion of the production of dental x-rays is now possible. Following is a step-by-step explanation of x-ray production (Figure 2-19):
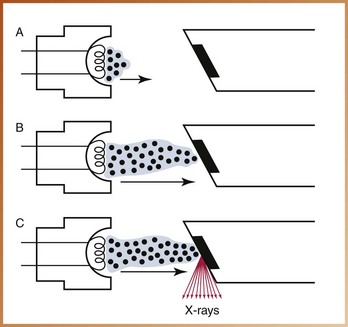
FIGURE 2-19 The production of dental x-rays occurs in the x-ray tube. A, When the filament circuit is activated, the filament heats up, and thermionic emission occurs. B, When the exposure button is activated, the electrons are accelerated from the cathode to the anode. C, The electrons strike the tungsten target, and their kinetic energy is converted to x-rays and heat.
1. Electricity from the wall outlet supplies the power to generate x-rays. When the x-ray machine is turned on, the electrical current enters the control panel through the cord plugged into the wall outlet. The current travels from the control panel to the tubehead through the electrical wires in the extension arm.
2. The current is directed to the filament circuit and step-down transformer in the tubehead. The transformer reduces the 110 or 220 entering-line voltage to 3 to 5 volts.
3. The filament circuit uses the 3 to 5 volts to heat the tungsten filament in the cathode portion of the x-ray tube. Thermionic emission occurs, defined as the release of electrons from the tungsten filament when the electrical current passes through it and heats the filament. The outer-shell electrons of the tungsten atom acquire enough energy to move away from the filament surface, and an electron cloud forms around the filament. The electrons stay in an electron cloud until the high-voltage circuit is activated.
4. When the exposure button is pushed, the high-voltage circuit is activated. The electrons produced at the cathode are accelerated across the x-ray tube to the anode. The molybdenum cup in the cathode directs the electrons to the tungsten target in the anode.
5. The electrons travel from the cathode to the anode. When the electrons strike the tungsten target, their energy of motion (kinetic energy) is converted to x-ray energy and heat. Less than 1% of the energy is converted to x-rays; the remaining 99% is lost as heat.
6. The heat produced during the production of x-rays is carried away from the copper stem and absorbed by the insulating oil in the tubehead. The x-rays produced are emitted from the target in all directions; however, the leaded-glass housing prevents the x-rays from escaping from the x-ray tube. A small number of x-rays are able to exit from the x-ray tube through the unleaded glass window portion of the tube.
7. The x-rays travel through the unleaded glass window, the tubehead seal, and the aluminum disks. The aluminum disks remove or filter the longer wavelength x-rays from the beam.
8. Next, the size of the x-ray beam is restricted by the lead collimator. The x-ray beam then travels down the lead-lined PID and exits the tubehead at the opening of the PID.
Types of X-Rays Produced
Not all x-rays produced in the x-ray tube are the same; x-rays differ in energy and wavelength. The energy and wavelength of x-rays vary based on how the electrons interact with the tungsten atoms in the anode. The kinetic energy of the electrons is converted to x-ray photons through one of two mechanisms: (1) general (braking) radiation and (2) characteristic radiation.
General Radiation
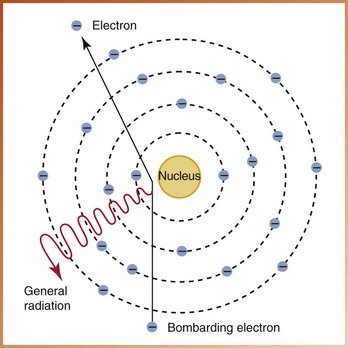
Speeding electrons slow down because of their interactions with the tungsten target in the anode. Many electrons that interact with the tungsten atoms undergo not one but many interactions within the target. The radiation produced in this manner is known as general radiation, or braking radiation (bremsstrahlung). The term braking refers to the sudden stopping of high-speed electrons when they hit the tungsten target in the anode. Most x-rays are produced in this manner; approximately 70% of the x-ray energy produced at the anode can be classified as general radiation.
General (braking) radiation is produced when an electron hits the nucleus of a tungsten atom or when an electron passes very close to the nucleus of a tungsten atom (Figure 2-20). An electron rarely hits the nucleus of the tungsten atom. When it does, however, all its kinetic energy is converted into a high-energy x-ray photon. Instead of hitting the nucleus, most electrons just miss the nucleus of the tungsten atom. When the electron comes close to the nucleus, it is attracted to the nucleus and slows down. Consequently, an x-ray photon of lower energy results. The electron that misses the nucleus continues to penetrate many atoms, producing lower energy x-rays before it imparts all of its kinetic energy. As a result, general radiation consists of x-rays of many different energies and wavelengths.
Characteristic Radiation
Characteristic radiation is produced when a high-speed electron dislodges an inner-shell electron from the tungsten atom and causes ionization of that atom (Figure 2-21). Once the electron is dislodged, the remaining orbiting electrons are rearranged to fill the vacancy. This rearrangement produces a loss of energy that results in the production of an x-ray photon. The x-rays produced by this interaction are known as characteristic x-rays.
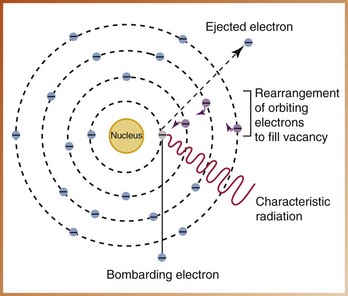
FIGURE 2-21 An electron that dislodges an inner-shell electron from the tungsten atom results in the rearrangement of the remaining orbiting electrons and the production of an x-ray photon known as characteristic radiation.
Characteristic radiation accounts for a very small part of x-rays produced in the dental x-ray machine. It occurs only at 70 kVp and above because the binding energy of the K-shell electron is approximately 70 keV.
Definitions of X-Radiation
Terms such as primary, secondary, and scatter are often used to describe x-radiation. Understanding the interactions of x-radiation with matter requires a working knowledge of these terms, as follows:
• Primary radiation refers to the penetrating x-ray beam that is produced at the target of the anode and that exits the tubehead. This x-ray beam is often referred to as the primary beam, or useful beam.
• Secondary radiation refers to x-radiation that is created when the primary beam interacts with matter. (In dental radiography, “matter” includes the soft tissues of the head, the bones of the skull, and the teeth.) Secondary radiation is less penetrating than primary radiation.
• Scatter radiation is a form of secondary radiation and is the result of an x-ray that has been deflected from its path by the interaction with matter. Scatter radiation is deflected in all directions by the patient’s tissues and travels to all parts of the patient’s body and to all areas of the dental operatory. Scatter radiation is detrimental to both the patient and the radiographer.
Interactions of X-Radiation
What happens after an x-ray exits the tubehead? When x-ray photons arrive at the patient with energies produced by the dental x-ray machine, one of the following events may occur:
• X-rays can pass through the patient without any interaction.
• X-ray photons can be completely absorbed by the patient.
• X-ray photons can be scattered (Figure 2-22).
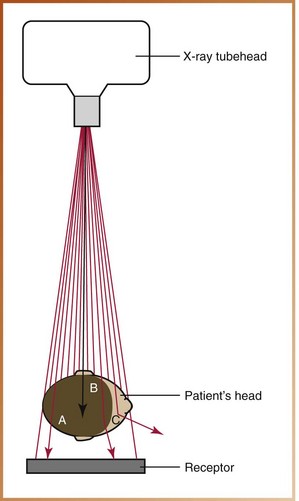
FIGURE 2-22 Three types of radiation interactions with the patient may occur. A, The x-ray photon may pass through the patient without interaction and reach the receptor. B, The x-ray photon may be absorbed by the patient. C, The x-ray photon may be scattered onto the receptor or away from the receptor.
A knowledge of atomic and molecular structure is required to understand such interactions and effects. At the atomic level, four possibilities can occur when an x-ray photon interacts with matter: (1) no interaction, (2) absorption or photoelectric effect, (3) Compton scatter, and (4) coherent scatter.
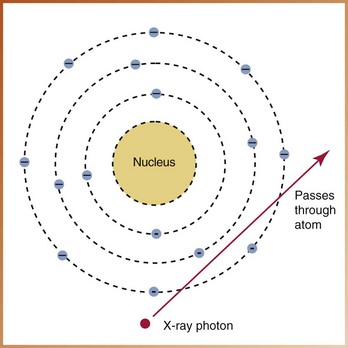
No Interaction
It is possible for an x-ray photon to pass through matter or the tissues of a patient without any interaction (Figure 2-23). The x-ray photon passes through the atom unchanged and leaves the atom unchanged. The x-ray photons that pass through a patient without interaction are responsible for producing densities and make dental radiography possible.
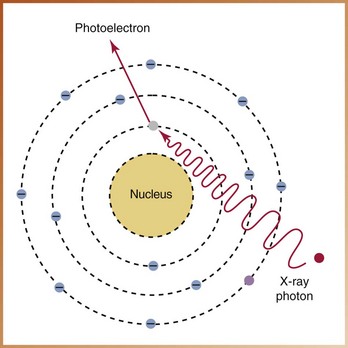
Absorption of Energy and Photoelectric Effect
It is possible for an x-ray photon to be completely absorbed within matter, or the tissues of a patient. Absorption refers to the total transfer of energy from the x-ray photon to the atoms of matter through which the x-ray beam passes. Absorption depends on the energy of the x-ray beam and the composition of the absorbing matter or tissues.
At the atomic level, absorption occurs as a result of the photoelectric effect. In the photoelectric effect, ionization takes place. An x-ray photon collides with a tightly bound, inner-shell electron and gives up all its energy to eject the electron from its orbit (Figure 2-24). The x-ray photon imparts all of its kinetic energy to the orbital electron, is absorbed, and ceases to exist. The ejected electron is termed a photoelectron and has a negative charge; it is readily absorbed by other atoms because it has very little penetrating power. The atom that remains has a positive charge. The photoelectric effect accounts for 30% of the interactions of matter with the dental x-ray beam.
Compton Scatter
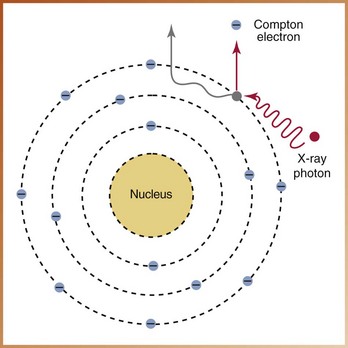
It is possible for an x-ray photon to be deflected from its path during its passage through matter. The term scatter refers to this type of radiation. At the atomic level, the Compton effect accounts for most of the scatter radiation.
In Compton scatter, ionization takes place. An x-ray photon collides with a loosely bound, outer-shell electron and gives up part of its energy to eject the electron from its orbit (Figure 2-25). The x-ray photon loses energy and continues in a different direction (scatters) at a lower energy level. The new, weaker x-ray photon interacts with other atoms until all its energy is gone. The ejected electron is termed a Compton electron, or recoil electron, and has a negative charge. The remaining atom is positively charged. Compton scatter accounts for 62% of the scatter that occurs in diagnostic radiography.
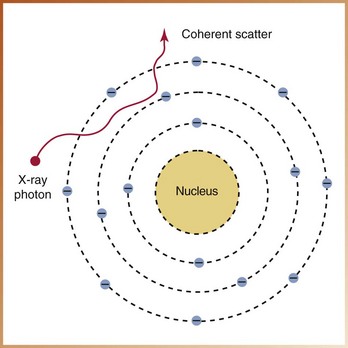
Coherent Scatter
Another type of scatter radiation that may take place when x-rays interact with matter is known as coherent scatter, or unmodified scatter. Coherent scatter involves an x-ray photon that has its path altered by matter (Figure 2-26). Coherent scatter occurs when a low-energy x-ray photon interacts with an outer-shell electron. No change in the atom occurs, and an x-ray photon of scattered radiation is produced. The x-ray photon is scattered in a different direction from that of the incident photon; no loss of energy and no ionization occur. Essentially, the x-ray photon is “unmodified” and simply undergoes a change in direction without a change in energy. Coherent scatter accounts for 8% of the interactions of matter with the dental x-ray beam.
Summary
• An atom consists of a central nucleus composed of protons, neutrons, and orbiting electrons.
• Most atoms exist in a neutral state and contain equal numbers of protons and neutrons.
• When unequal numbers of protons and electrons exist, the atom is electrically unbalanced and is termed an ion.
• The production of ions is termed ionization; an ion pair (a positive ion and a negative ion) is produced. The atom is the positive ion, and the ejected electron is the negative ion.
• Ionizing radiation is capable of producing ions and can be classified as particulate or electromagnetic.
• Electromagnetic radiations (e.g., x-rays) exhibit characteristics of both particles and waves and are arranged according to their energies.
• The energy of an electromagnetic radiation depends on wavelength and frequency.
• A low-energy radiation has a low frequency and a long wavelength; a high-energy radiation has a high frequency and a short wavelength.
• X-rays are weightless, neutral bundles of energy (photons) that travel in waves with a specific frequency at the speed of light.
• X-rays are generated in an x-ray tube located in the x-ray tubehead.
• The x-ray tube consists of a leaded-glass housing, a negative cathode, and a positive anode. Electrons are produced in the cathode and accelerated toward the anode; the anode converts the electrons into x-rays.
• After x-rays exit the tubehead, several interactions are possible: The x-rays may pass through the patient (no interaction), may be completely absorbed by the patient (photoelectric effect), or may be scattered (Compton scatter and coherent scatter).
Frommer, HH, Savage-Stabulas, JJ, Ionizing radiation and basic principles of x-ray generation. Radiology for the dental professional, ed 9, St. Louis, Mosby, 2011.
Johnson, ON, Thomson, EM, Characteristics and measurement of radiation. Essentials of dental radiography for dental assistants and hygienists, ed 8, Upper Saddle River, NJ, Pearson Prentice Hall, 2007.
Johnson, ON, Thomson, EM, The dental x-ray machine: Components and functions. Essentials of dental radiography for dental assistants and hygienists, ed 8, Upper Saddle River, NJ, Pearson Prentice Hall, 2007.
White, SC, Pharoah, MJ, Radiation physics. Oral radiology: principles and interpretation, ed 6, St Louis, Mosby, 2009.
Multiple Choice
____1. Which of the following electrons has the greatest binding energy?
____2. What type of electrical charge does the electron carry?
____3. Which term describes two or more atoms that are joined by chemical bonds?
____4. Which of the following describes ionization?
____5. Which term describes the process by which unstable atoms undergo spontaneous disintegration in an effort to attain a more balanced nuclear state?
____6. Which of the following is not a type of particulate radiation?
____7. Which of the following is not a type of electromagnetic radiation?
____8. Which of the following statements is incorrect?
a. Velocity is the speed of a wave.
b. Wavelength is the distance between waves.
c. Frequency is the number of wavelengths that pass a given point in a certain amount of time.
Identification
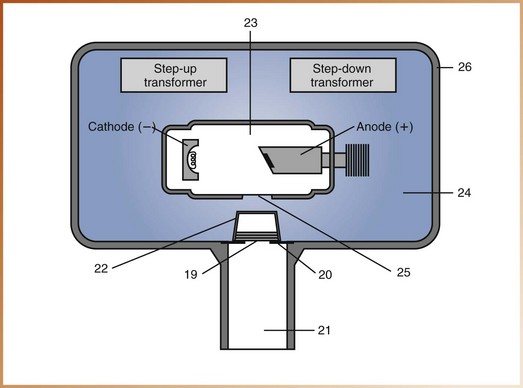
For questions 11 to 18, identify each of the labeled structures in Figure 2-27.
For questions 19 to 26, identify each of the labeled structures in Figure 2-28.
Multiple Choice
____27. Which of the following regulates the flow of electrical current to the filament of the x-ray tube?
____28. Which of the following is used to increase the voltage in the high-voltage circuit?
____29. Which of the following does not occur when the high-voltage circuit is activated?
a. The unit produces an audible and visible signal.
b. Electrons produced at the cathode are accelerated across the tube to the anode.
____30. Which of the following is the location where x-rays are produced?
____31. Which of the following is the location where thermionic emission occurs?
____32. Which of the following accounts for 70% of all the x-ray energy produced at the anode?
____33. Which of the following occurs only at 70 kVp or higher and accounts for a very small part of the x-rays produced in the dental x-ray machine?
____34. Which of the following describes primary radiation?
a. radiation that exits the tubehead
b. radiation that is created when x-rays come in contact with matter
c. radiation that has been deflected from its path by the interaction with matter
____35. Which of the following describes scatter radiation?
a. radiation that exits the tubehead
b. radiation that is more penetrating than primary radiation
c. radiation that has been deflected from its path by interaction with matter
____36. Which of the following type of scatter occurs most often with dental x-rays?
Identification
For questions 37 to 40, identify the x-radiation interaction with matter in Figures 2-29, 2-30, 2-31, and 2-32.
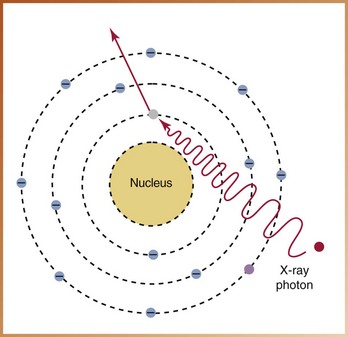
FIGURE 2-29
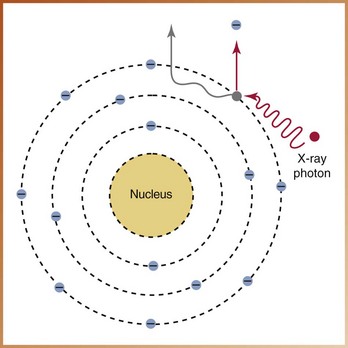
FIGURE 2-30
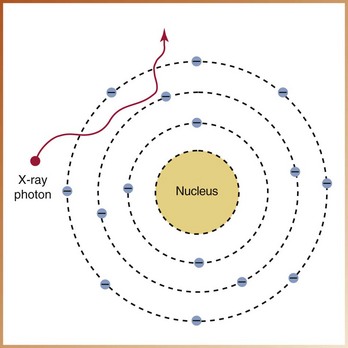
FIGURE 2-31
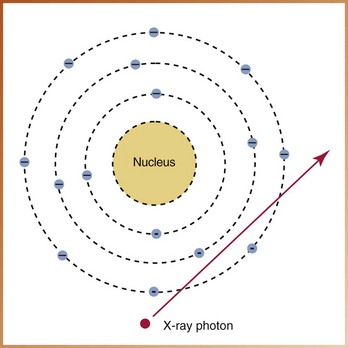
FIGURE 2-32
Multiple Choice
For questions 41 to 44, refer to Figures 2-29, 2-30, 2-31, and 2-32.
____41. The interaction of x-radiation with matter illustrated in Figure 2-29 demonstrates:
____42. The interaction of x-radiation with matter illustrated in Figure 2-30 demonstrates:
____43. The interaction of x-radiation with matter illustrated in Figure 2-31 demonstrates:
____44. The interaction of x-radiation with matter illustrated in Figure 2-32 demonstrates: