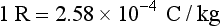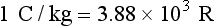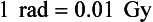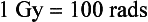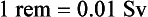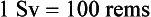Radiation Biology
After completion of this chapter, the student will be able to do the following:
• Define the key words associated with radiation injury
• Describe the mechanisms, theories, and sequence of radiation injury
• Define and discuss the dose–response curve and radiation injury
• List the determining factors for radiation injury
• Discuss the short-term and long-term effects as well as the somatic and genetic effects of radiation exposure
• Describe the effects of radiation exposure on cells, tissues, and organs
• Identify the relative sensitivity of a given tissue to x-radiation
• Define the units of measurement used in radiation exposure
• List common sources of radiation exposure
• Discuss risk and risk estimates for radiation exposure
All ionizing radiations are harmful and produce biologic changes in living tissues. The damaging biologic effects of x-radiation were first documented shortly after the discovery of x-rays. Since that time, information about the harmful effects of high-level exposure to x-radiation has increased on the basis of studies of atomic bomb survivors, workers exposed to radioactive materials, and patients undergoing radiation therapy. Although the amount of x-radiation used in dental radiography is small, biologic damage does occur.
The dental radiographer must have a working knowledge of radiation biology, the study of the effects of ionizing radiation on living tissue, to understand the harmful effects of x-radiation. The purpose of this chapter is to describe the mechanisms and theories of radiation injury, to define the basic concepts and effects of radiation exposure, to detail radiation measurements, and to discuss the risks of radiation exposure.
Radiation Injury
In diagnostic radiography, not all x-rays pass through the patient and reach the dental x-ray film; some are absorbed by the patient’s tissues. Absorption, as defined in Chapter 2, refers to the total transfer of energy from the x-ray photon to patient tissues. What happens when x-ray energy is absorbed by patient tissues? Chemical changes occur that result in biologic damage. Two specific mechanisms of radiation injury are possible: (1) ionization and (2) free radical formation.
Ionization
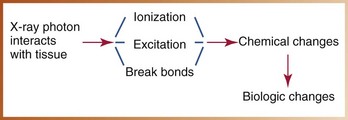
X-rays are a form of ionizing radiation; when x-rays strike patient tissues, ionization results. As described in Chapter 2, ionization is produced through the photoelectric effect or Compton scatter and results in the formation of a positive atom and a dislodged negative electron. The ejected high-speed electron is set into motion and interacts with other atoms within the absorbing tissues. The kinetic energy of such electrons results in further ionization, excitation, or breaking of molecular bonds, all of which cause chemical changes within the cell that result in biologic damage (Figure 4-1). Ionization may have little effect on cells if the chemical changes do not alter sensitive molecules, or such changes may have a profound effect on structures of great importance to cell function (e.g., DNA).
Free Radical Formation
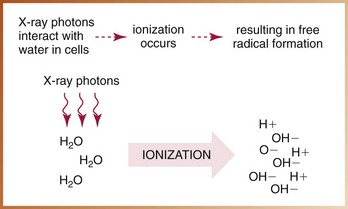
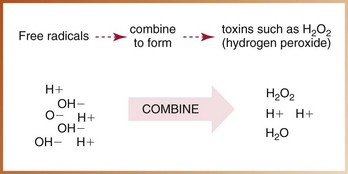
X-radiation causes cell damage primarily through the formation of free radicals.* Free radical formation occurs when an x-ray photon ionizes water, the primary component of living cells. Ionization of water results in the production of hydrogen and hydroxyl free radicals (Figure 4-2). A free radical is an uncharged (neutral) atom or molecule that exists with a single, unpaired electron in its outermost shell. It is highly reactive and unstable; the lifetime of a free radical is approximately 10–10 seconds. To achieve stability, free radicals may (1) recombine without causing changes in the molecule, (2) combine with other free radicals and cause changes, or (3) combine with ordinary molecules to form a toxin (e.g., hydrogen peroxide [H2O2]) capable of producing widespread cellular changes (Figure 4-3).
Theories of Radiation Injury
Damage to living tissues caused by exposure to ionizing radiation may result from a direct hit and absorption of an x-ray photon within a cell or from the absorption of an x-ray photon by the water within a cell accompanied by free radical formation. Two theories are used to describe how radiation damages biologic tissues: (1) the direct theory and (2) the indirect theory.
Direct Theory
The direct theory of radiation injury suggests that cell damage results when ionizing radiation directly hits critical areas, or targets, within the cell. For example, if x-ray photons directly strike the deoxyribonucleic acid (DNA) of a cell, critical damage occurs, causing injury to the irradiated organism. Direct injuries from exposure to ionizing radiation occur infrequently; most x-ray photons pass through the cell and cause little or no damage.
Indirect Theory
The indirect theory of radiation injury suggests that x-ray photons are absorbed within the cell and cause the formation of toxins, which, in turn, damage the cell. For example, when x-ray photons are absorbed by the water within a cell, free radical formation results. The free radicals combine to form toxins (e.g., H2O2), which cause cellular dysfunction and biologic damage. An indirect injury results because the free radicals combine and form toxins, not because of a direct hit by x-ray photons. Indirect injuries from exposure to ionizing radiation occur frequently because of the high water content of cells. The chances of free radical formation and indirect injury are great because cells are 70% to 80% water.
Dose–Response Curve
If all ionizing radiations are harmful and produce biologic damage, what level of exposure is considered acceptable? To establish acceptable levels of radiation exposure, it is useful to plot the dose administered and the damage produced. With radiation exposure, a dose–response curve can be used to correlate the “response,” or damage, of tissues with the “dose,” or amount, of radiation received.
When dose and damage are plotted on a graph, a linear, nonthreshold relationship is seen. A linear relationship indicates that the response of the tissues is directly proportional to the dose. A nonthreshold relationship indicates that a threshold dose level for damage does not exist. A nonthreshold dose–response curve suggests that no matter how small the amount of radiation received, some biologic damage does occur (Figure 4-4). Consequently, there is no safe amount of radiation exposure. In dental radiography, as mentioned earlier, although the doses received by patients are low, damage does occur.
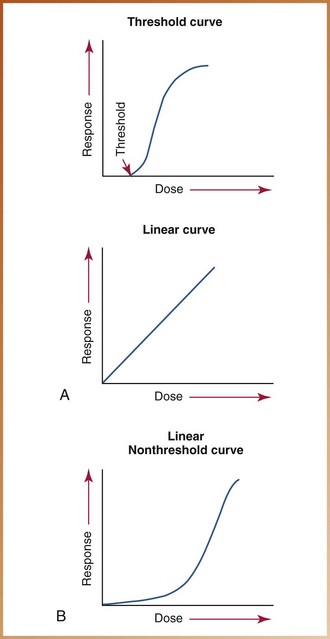
FIGURE 4-4 A, Threshold curve: This curve indicates that below a certain level (threshold), no response is seen. Linear curve: This curve indicates that response is proportional to dose. B, Linear nonthreshold curve: This dose–response curve indicates that a response is seen at any dose.
Most of the information used to produce dose–response curves for radiation exposure comes from studying the effects of large doses of radiation on populations, for example, atomic bomb survivors. In the low-dose range, however, minimal information has been documented; instead, the curve has been extrapolated from animal and cellular experiments.
Stochastic and Nonstochastic Radiation Effects
Biologic effects from radiation can be classified as stochastic or nonstochastic. Stochastic effects occur as a direct function of dose. The probability of occurrence increases with increasing absorbed dose; however, the severity of effects does not depend on the magnitude of the absorbed dose. As in the case of nonthreshold radiation effects, stochastic effects do not have a dose threshold. Examples of stochastic effects include cancer (i.e., tumor) induction and genetic mutations.
Nonstochastic effects (deterministic effects) are somatic effects that have a threshold and that increase in severity with increasing absorbed dose. Examples of nonstochastic effects include erythema, loss of hair, cataract formation, and decreased fertility. Compared with stochastic effects, nonstochastic effects require larger radiation doses to cause serious impairment of health.
Sequence of Radiation Injury
Chemical reactions (e.g., ionization, free radical formation) that follow the absorption of radiation occur rapidly at the molecular level. However, varying amounts of time are required for these changes to alter cells and cellular functions. As a result, the observable effects of radiation are not visible immediately after exposure. Instead, following exposure, a latent period occurs. A latent period can be defined as the time that elapses between exposure to ionizing radiation and the appearance of observable clinical signs. The latent period may be short or long, depending on the total dose of radiation received and the amount of time, or rate, it took to receive the dose. The more radiation received and the faster the dose rate, the shorter is the latent period.
After the latent period, a period of injury occurs. A variety of cellular injuries may result, including cell death, changes in cell function, breaking or clumping of chromosomes, formation of giant cells, cessation of mitotic activity, and abnormal mitotic activity.
The last event in the sequence of radiation injury is the recovery period. Not all cellular radiation injuries are permanent. With each radiation exposure, cellular damage is followed by repair. Depending on a number of factors, cells can repair the damage caused by radiation. Most of the damage caused by low-level radiation is repaired within the cells of the body.
The effects of radiation exposure are additive, and unrepaired damage accumulates in the tissues. The cumulative effects of repeated radiation exposure can lead to health problems (e.g., cancer, cataract formation, birth defects). Table 4-1 lists disorders that may result from the cumulative effects of repeated radiation exposure on tissues and organs.
Determining Factors for Radiation Injury
In addition to understanding the mechanisms, theories, and sequence of radiation injury, it is important to recognize the factors that influence radiation injury. The factors used to determine the degree of radiation injury include the following:
• Total dose: Quantity of radiation received, or the total amount of radiation energy absorbed. More damage occurs when tissues absorb large quantities of radiation.
• Dose rate: Rate at which exposure to radiation occurs and absorption takes place (dose rate = dose/time). More radiation damage takes place with high dose rates because a rapid delivery of radiation does not allow time for the cellular damage to be repaired.
• Amount of tissue irradiated: Areas of the body exposed to radiation. Total-body irradiation produces more adverse systemic effects than if small, localized areas of the body are exposed. An example of total-body irradiation is the exposure of a person to a nuclear energy disaster. Extensive radiation injury occurs when large areas of the body are exposed because of the damage to the blood-forming tissues.
• Cell sensitivity: More damage occurs in cells that are most sensitive to radiation, such as rapidly dividing cells and young cells (see later discussion).
• Age: Children are more susceptible to radiation damage than are adults.
Radiation Effects
Short-Term and Long-Term Effects
Radiation effects can be classified as either short-term or long-term effects. Following the latent period, effects that are seen within minutes, days, or weeks are termed short-term effects. Short-term effects are associated with large amounts of radiation absorbed in a short time (e.g., exposure to a nuclear accident or the atomic bomb). Acute radiation syndrome (ARS) is a short-term effect and includes nausea, vomiting, diarrhea, hair loss, and hemorrhage. Short-term effects are not applicable to dentistry.
Effects that appear after years, decades, or generations are termed long-term effects. Long-term effects are associated with small amounts of radiation absorbed repeatedly over a long period. Repeated low levels of radiation exposure are linked to the induction of cancer, birth abnormalities, and genetic defects.
Somatic and Genetic Effects
All the cells in the body can be classified as either somatic or genetic. Somatic cells are all the cells in the body except the reproductive cells. The reproductive cells (e.g., ova, sperm) are termed genetic cells. Depending on the type of cell injured by radiation, the biologic effects of radiation can be classified as somatic or genetic.
Somatic effects are seen in the person who has been irradiated. Radiation injuries that produce changes in somatic cells produce poor health in the irradiated individual. Major somatic effects of radiation exposure include the induction of cancer, leukemia, and cataracts. These changes, however, are not transmitted to future generations (Figure 4-5).
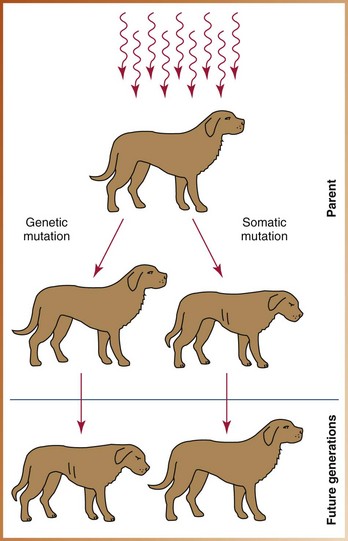
FIGURE 4-5 A somatic mutation produces poor health in the exposed animal but does not produce mutations in subsequent generations. In contrast, a genetic mutation does not affect the exposed animal but produces mutations in future generations.
Genetic effects are not seen in the irradiated person but are passed on to future generations. Radiation injuries that produce changes in genetic cells do not affect the health of the exposed individual. Instead, the radiation-induced mutations affect the health of the offspring (see Figure 4-5). Genetic damage cannot be repaired.
Radiation Effects on Cells
The cell, or basic structural unit of all living organisms, is composed of a central nucleus and surrounding cytoplasm. Ionizing radiation may affect the nucleus, the cytoplasm, or the entire cell. The cell nucleus is more sensitive to radiation than is the cytoplasm. Damage to the nucleus affects the chromosomes containing DNA and results in disruption of cell division, which, in turn, may lead to disruption of cell function or cell death.
Not all cells respond to radiation in the same manner. A cell that is sensitive to radiation is termed radiosensitive; one that is resistant is termed radioresistant. The response of a cell to radiation exposure is determined by the following:
• Mitotic activity: Cells that divide frequently or undergo many divisions over time are more sensitive to radiation.
• Cell differentiation: Cells that are immature or are not highly specialized are more sensitive to radiation.
• Cell metabolism: Cells that have a higher metabolism are more sensitive to radiation.
Cells that are radiosensitive include blood cells, immature reproductive cells, and young bone cells. The cell that is most sensitive to radiation is the small lymphocyte. Radioresistant cells include cells of bone, muscle, and nerve (Table 4-2).
TABLE 4-2
Tissue and Organ Sensitivity to Radiation
| Radiosensitive Cells | Radioresistant Cells |
| Small lymphocyte (high sensitivity) | Muscle tissue (low sensitivity) |
| Bone marrow (high sensitivity) | Nerve tissue (low sensitivity) |
| Reproductive cells (high sensitivity) | Mature bone and cartilage (fairly low sensitivity) |
| Intestinal mucosa (high sensitivity) | Salivary gland (fairly low sensitivity) |
| Skin (fairly high sensitivity) | Thyroid gland (fairly low sensitivity) |
| Lens of eye (fairly high sensitivity) | Kidney (fairly low sensitivity) |
| Oral mucosa (fairly high sensitivity) | Liver (fairly low sensitivity) |
Radiation Effects on Tissues and Organs
Cells are organized into the larger functioning units of tissues and organs. As with cells, tissues and organs vary in their sensitivity to radiation. Radiosensitive organs are composed of radiosensitive cells and include the lymphoid tissues, bone marrow, testes, and intestines. Examples of radioresistant tissues include the salivary glands, kidney, and liver.
In dentistry, some tissues and organs are designated as “critical” because they are exposed to more radiation than are others during radiographic procedures. A critical organ is an organ that, if damaged, diminishes the quality of a person’s life. Critical organs exposed during dental radiographic procedures in the head and neck region include the following:
Radiation Measurements
Radiation can be measured in the same manner as other physical concepts such as time, distance, and weight. Just as the unit of measurement for time is minutes, for distance miles or kilometers, and for weight pounds or kilograms, the International Commission on Radiation Units and Measurement (ICRU) has established special units for the measurement of radiation. Such units are used to define three quantities of radiation: (1) exposure, (2) dose, and (3) dose equivalent.
The dental radiographer must know radiation measurements to discuss exposure and dose concepts with the dental patient.
At present, two systems are used to define radiation measurements: (1) The older system is referred to as the traditional system, or standard system; and (2) the newer system is the metric equivalent known as the SI system, or Système International de’Unités (International System of Units).
The traditional units of radiation measurement include the following:
The SI units of radiation measurement include the following:
This text uses both the traditional and SI units of measurement; the dental radiographer should be familiar with both systems and know how to convert measurements from one system to the other (Table 4-3). In addition, the dental radiographer must be familiar with a number of physics terms used in the definitions of both traditional and SI units of radiation measurement (Table 4-4).
TABLE 4-3
Units of Radiation Measurement

QF, quality factor; J, joule; SI, International System of Units.
TABLE 4-4
| Term | Definition |
| Coulomb (C) | Unit of electrical charge; the quantity of electrical charge transferred by 1 ampere in 1 second. |
| Ampere (A) | Unit of electrical current strength; current yielded by 1 volt against 1 ohm of resistance. |
| Erg (erg) | Unit of energy equivalent to 1.0 × 10–7 joules or to 2.4 × 10–8 calories. |
| Joule (J) | SI unit of energy equivalent to the work done by the force of 1 newton acting over the distance of 1 meter. |
| Newton (N) | SI unit of force; the force that, when acting continuously on a mass of 1 kilogram, will impart to it an acceleration of 1 meter per second squared (m/sec2). |
| Kilogram (kg) | Unit of mass equivalent to 1000 grams or 2.205 pounds. |
Exposure Measurement
The term exposure refers to the measurement of ionization in air produced by x-rays. The traditional unit of exposure for x-rays is the roentgen (R). The roentgen is a way of measuring radiation exposure by determining the amount of ionization that occurs in air. A definition follows:
In measuring the roentgen, a known volume of air is irradiated. The interaction of x-ray photons with air molecules results in ionization, or the formation of ions. The ions (electrical charges) that are produced are collected and measured. One roentgen is equal to the amount of radiation that produces approximately two billion, or 2.08 × 109, ion pairs in one cubic centimeter (cc) of air.
The roentgen has limitations as a unit of measure. It measures the amount of energy that reaches the surface of an organism but does not describe the amount of radiation absorbed. The roentgen is essentially limited to measurements in air. By definition, it is only used for x-rays and gamma rays and does not include other types of radiation.
No SI unit for exposure that is equivalent to the roentgen exists. Instead, exposure is simply stated in coulombs per kilograms (C/kg). The coulomb (C) is a unit of electrical charge. The unit C/kg measures the number of electrical charges, or the number of ion pairs, in 1 kg of air. The conversions for roentgen and coulombs per kilogram can be expressed as follows:
Dose Measurement
Dose can be defined as the amount of energy absorbed by a tissue. The radiation absorbed dose, or rad, is the traditional unit of dose. Unlike the roentgen, the rad is not restricted to air and can be applied to all forms of radiation. A definition follows:
Using SI units, 1 rad is equivalent to 0.01 joule per kilogram (0.01 J/kg). The SI unit equivalent to the rad is the gray (Gy), or 1 J/kg. The conversions for rad and Gy can be expressed as follows:
Dose Equivalent Measurement
Different types of radiation have different effects on tissues. The dose equivalent measurement is used to compare the biologic effects of different types of radiation. The traditional unit of the dose equivalent is the roentgen equivalent (in) man, or rem. A definition follows:
To place the exposure effects of different types of radiation on a common scale, a quality factor (QF), or dimensionless multiplier, is used. Each type of radiation has a specific QF based on different types of radiation producing different types of biologic damage. For example, the QF for x-rays is equal to 1.
The SI unit equivalent of the rem is the sievert (Sv). Conversions for the rem and sievert can be expressed as follows:
Measurements Used in Dental Radiography
In dental radiography, the gray and sievert are equal, and the roentgen, rad, and rem are considered approximately equal. Smaller multiples of these radiation units are typically used in dentistry because of the small quantities of radiation used during radiographic procedures. The prefix “milli,” meaning 1/1000 (e.g., millirad, or mrad), allows the dental radiographer to express small quantities of exposure, dose, and dose equivalent.
Radiation Risks
To understand radiation risks, the dental radiographer must be familiar with the potential sources of radiation exposure. This knowledge can then be used to better understand the radiation risks associated with dentistry.
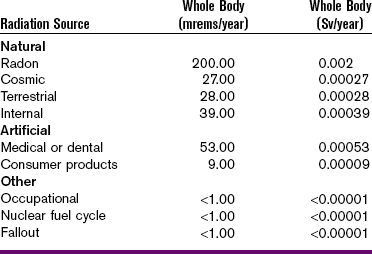
Humans are exposed daily to background radiation, a form of ionizing radiation that is ubiquitous in the environment. Naturally occurring background radiation includes cosmic radiation and terrestrial radiation. Cosmic radiation originates from the stars and the sun. A person’s exposure to cosmic radiation depends on altitude: the higher the altitude, the greater is the exposure to cosmic radiation. Terrestrial radiation also occurs naturally and is emitted from radioactive materials present in the earth and air. Examples of terrestrial radiation include potassium-40 and uranium.
In the United States, the average dose of background radiation received by an individual ranges from 150 to 300 mrads (0.0015–0.003 Gy) per year. This exposure may vary according to geographic location. For example, geographic areas that contain more radioactive materials are associated with increased amounts of terrestrial radiation, whereas areas at higher elevations (e.g., Denver) are associated with increased amounts of cosmic radiation.
In addition to naturally occurring background radiation, modern technology has created artificial, or man-made, sources of radiation. Consumer products (e.g., luminous wristwatches, televisions), fallout from atomic weapons, weapons production, and the nuclear fuel cycle are all sources of radiation exposure.
Medical radiation, another source of radiation exposure, is the greatest contributor to artificial radiation exposure. Medical radiation includes medical radiographic procedures, dental radiography, fluoroscopy, nuclear medicine, and radiation therapy. Medical radiation exposure equals the average yearly dose from all other exposures combined and typically accounts for half of the total exposure received.
Table 4-5 summarizes the sources of low-level radiation.
Risk and Risk Estimates
A risk can be defined as the likelihood of adverse effects or death resulting from exposure to a hazard. In dental radiography, risk is the likelihood of an adverse effect, specifically cancer induction, occurring from exposure to ionizing radiation.
The potential risk of dental radiography inducing a fatal cancer in an individual has been estimated to be approximately 3 in 1 million. The risk of a person developing cancer spontaneously is much higher, or 3300 in 1 million. To keep the concept of risk in perspective, the risk of incurring a fatal cancer from dental radiographic procedures should be compared with commonplace risks. For example, a 1-in-1-million risk of a fatal outcome is associated with each of the following activities: riding 10 miles on a bike, 300 miles in an auto, or 1000 miles in an airplane; or smoking 1.4 cigarettes per day. These risk estimates suggest that death is more likely to occur from common activities than from dental radiographic procedures and that cancer is much more likely to be unrelated to radiation exposure. In other words, the risks from dental radiography are not significantly greater than the risks of other everyday activities in modern life.
Dental Radiation and Exposure Risks
To calculate the risk from dental radiographic procedures, doses to critical organs must be measured. As previously defined, a critical organ, if damaged, diminishes the quality of an individual’s life. With dental radiographic procedures, the critical organs at risk include the thyroid gland and active bone marrow. The skin and eyes may also be considered critical organs.
Risk Elements
Thyroid Gland: Although the thyroid gland is not irradiated by the primary beam in dental radiographic procedures, thyroid radiation exposure does occur. An estimated dose of 6000 mrads (0.06 Gy) is necessary to produce cancer in the thyroid gland; such a large dose is not incurred in dental radiography. Instead, the average dose to the thyroid gland (rectangular collimation, D-speed film, long position-indicating device [PID], 20-film series) is 6 mrads (0.00006 Gy), or 1/1000 of the dose necessary to induce thyroid cancer.
Bone Marrow: The areas of the maxilla and mandible exposed during dental radiography account for a very small percentage of active bone marrow. The risk of cancer induction (leukemia) is directly associated with the amount of blood-producing tissues irradiated and the dose. Leukemia is induced most likely at doses of 5000 mrads (0.05 Gy) or more; a dose of such magnitude does not occur in dental radiography. The average bone marrow dose from periapical radiography is approximately 1 to 3 mrads (0.00001–0.00003 Gy) per film. Consequently, about 2000 to 5000 films would have to be exposed to induce leukemia.
Skin: A total of 250 rads (2.5 Gy) in a 14-day period causes erythema, or reddening, of the skin. To produce such changes, more than 500 dental films (F-speed film, exposure rate 0.7 R/second) in a 14-day period would have to be exposed. This is not a likely scenario in dental radiography.
Eyes: More than 200,000 mrads (2 Gy) are necessary to induce cataract formation (cloudiness of lens) in the eyes. Again, such high doses are not a consideration in dental radiography. Instead, the average surface dose to the cornea of the eye (D-speed film, long PID, 20-film series) is approximately 60 mrads (0.0006 Gy). In dental radiography, the chance of cataracts occurring is so unlikely that some scientists no longer consider the eyes a critical organ.
Patient Exposure and Dose
Dental patients must be protected from excess exposure to radiation. (Chapter 5 discusses patient protection in detail.) How much radiation exposure results from dental radiography? The amount of exposure varies, depending on the following:
• Film speed: Radiation exposure can be limited by using the fastest film available. The use of F-speed film instead of D-speed reduces the absorbed dose by 60%. Using F-speed film instead of E-speed reduces the absorbed dose by an additional 20%.
• Collimation: Radiation exposure can be limited by using rectangular collimation. The use of rectangular collimation instead of round collimation reduces the absorbed dose by 60% to 70%.
• Technique: Radiation exposure can be limited by increasing the source-to-film distance. The use of the long-cone paralleling technique and increased source-to-film distance reduces the skin dose.
• Exposure factors: Radiation exposure can be limited by using a higher kilovoltage peak. The use of higher kilovoltage peak reduces the skin dose.
Surface exposure, or the measure of the intensity of radiation at the patient’s skin surface in coulombs per kilogram or roentgens, is typically used when referring to patient exposure. A single intraoral radiograph (D-speed film, 70 kVp, long PID) results in a mean surface exposure of 250 milliroentgens (mR). With F-speed film, a single intraoral radiograph results in a mean surface exposure of 100 mR.
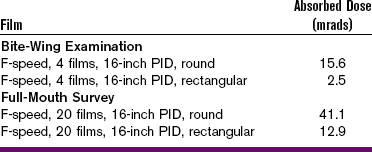
The concept of absorbed dose may also be used when referring to patient exposure and dose. The absorbed dose from a 20-film series of dental radiographs (round collimation, F-speed film, long PID) is estimated to be 41 mrads (0.00041 Gy). If rectangular collimation is used, the absorbed dose decreases to approximately 13 mrads (0.00013 Gy) (Table 4-6).
Risk Versus Benefit of Dental Radiographs
X-radiation is harmful to living tissues. Because biologic damage results from x-ray exposure, dental radiographs should be prescribed for a patient only when the benefit of disease detection outweighs the risk of biologic damage. When dental radiographs are properly prescribed and exposed, the benefit of disease detection far outweighs the risk of damage from x-radiation (see Chapter 5).
Summary
• All ionizing radiation is harmful and produces biologic changes in living tissue.
• Radiation injury results from ionization or free radical formation.
• A dose–response curve is used to demonstrate the response (damage) of tissues to the dose (amount) of radiation received.
• A threshold dose for damage does not exist, and the response of tissues is directly proportional to the dose received.
• Radiation injury follows a sequence of events: latent period, period of injury, and period of recovery.
• Radiation injury is affected by total dose, dose rate, amount of tissue irradiated, cell sensitivity, and patient’s age.
• Short-term radiation effects occur when large amounts of radiation are absorbed in a short period; long-term radiation effects occur when small amounts of radiation are absorbed over a long time.
• Radiation effects are classified as somatic (seen in the irradiated person) or genetic (passed on to future generations).
• Cellular response to radiation depends on mitotic activity, cell differentiation, and cell metabolism.
• Radiosensitive cells include blood cells, immature reproductive cells, young bone cells, and epithelial cells. Radioresistant cells include the cells of bones, muscle, and nerve.
• Exposure is the measurement of ionization in air produced by x-rays; the units for exposure are the roentgen (R) and coulombs per kilogram (C/kg).
• Dose is the amount of energy absorbed by a tissue; the units for dose are the radiation absorbed dose (rad) and the gray (Gy).
• Dose equivalent measurement is used to compare the biologic effects of different types of radiation; the units for dose equivalent are the roentgen equivalent (in) man (rem) and the sievert (Sv).
• The risks of radiation exposure involved in dental radiography are not significantly greater than other everyday risks in life.
• The amount of exposure a patient receives from dental radiographs depends on the film speed, collimation, technique, and exposure factors used.
• Dental radiographs should be prescribed only for a patient when the benefit of disease detection outweighs the risk of damage from x-radiation.
Bernstein, DI, Clark, SJ, Scheetz, JP, Farman, AG, Rosenson, B. Perceived quality of radiographic images after rapid processing of D- and F-speed direct-exposure intraoral x-ray films. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(4):486–491.
Frommer, HH, Savage-Stabulas, JJ, Biologic effects of radiation. Radiology for the dental professional, ed 9, St Louis, Mosby, 2011.
Johnson, ON, Thomson, EM, Effects of radiation exposure. Essentials of dental radiography for dental assistants and hygienists, ed 8, Upper Saddle River, NJ, Pearson Prentice Hall, 2007.
Miles, DA, Van Dis, ML, Williamson, GF, Jensen, CW, Radiation biology and protection. Radiographic imaging for the dental team, ed 4, St Louis, Saunders, 2009.
White, SC, Pharoah, MJ, Radiation safety and protection. Oral radiology: principles and interpretation, ed 6, St Louis, Mosby, 2009.
White, SC, Pharoah, MJ, Radiobiology. Oral radiology: principles and interpretation, ed 6, St Louis, Mosby, 2009.
Multiple Choice
____1. The latent period in radiation biology is the time between:
a. exposure of film and development
b. subsequent doses of radiation
b. has an unpaired electron in the outer shell
c. is highly reactive and unstable
____3. Direct radiation injury occurs when:
a. x-ray photons hit critical targets within a cell
b. x-ray photons pass through the cell
c. x-ray photons are absorbed and form toxins
____4. Indirect radiation injury occurs when:
a. x-ray photons hit critical targets within a cell
b. x-ray photons pass through the cell
c. x-ray photons are absorbed and form toxins
____5. Which of the following relationships describes the response of tissues to radiation?
____6. Which of the following factors contributes to radiation injury?
____7. Which of the following statements is correct?
a. Short-term effects are seen with small amounts of radiation absorbed in a short period.
b. Short-term effects are seen with small amounts of radiation absorbed in a long period.
c. Long-term effects are seen with small amounts of radiation absorbed in a short period.
d. Long-term effects are seen with small amounts of radiation absorbed in a long period.
____8. Radiation injuries that are not seen in the person irradiated but that occur in future generations are termed:
____9. Which of the following is most susceptible to ionizing radiation?
____10. The sensitivity of tissues to radiation is determined by:
____11. Which of the following is considered radioresistant?
____12. An organ that, if damaged, diminishes the quality of an individual’s life is termed a:
____13. The traditional unit for measuring x-ray exposure in air is termed:
____14. Which of the following radiation units is determined by the quality factor (QF)?
____15. The unit for measuring the absorption of x-rays is termed:
____16. Which of the following conversions is correct?
____17. Which of the following traditional units does not have an SI equivalent?
____18. Which of the following is used only for x-rays?
____19. Which of the following conversions is correct?
____20. What is the average dose of background radiation received by an individual in the United States?
a. 0 to 100 mrads (0–0.001 Gy)
b. 50 to 100 mrads (0.0005–0.001 Gy)
c. 150 to 300 mrads (0.0015–0.003 Gy)
____21. What is the greatest contributor to artificial radiation exposure?
____22. The amount of radiation exposure an individual receives varies depending on:
____23. A single intraoral radiograph (D-speed film, 70 kVp, long PID) results in a mean surface exposure of:
____24. What is the dose at which leukemia induction is most likely to occur?
*A free radical with no charge is denoted by a dot following the chemical symbol (e.g., H·). A free radical with a charge is an ion.