Chapter 29 Functional Anatomy of the Palmar Aspect of the Foot
The palmar portion of the foot consists of several important structures functioning to support the foot and the limb of the horse, as well as being an integral part of the energy dissipation mechanisms present within each limb. These palmar foot structures include the cartilages of the foot (also called the lateral collateral or ungual cartilages), the digital cushion, the frog, and an extensive vascular network. Although each of these structures is present in every foot, morphological features and tissue composition vary widely among horses, which may be responsible for differing efficiencies in ability to dissipate energy. Furthermore, such differences may in part account for differences between the feet of a sound horse and the feet of a horse with chronic lameness associated with the foot. Awareness of how these tissues interact and relate to one another during foot impact is important for understanding how the foot dissipates energy and how potential problems may arise to produce lameness when energy dissipation is not efficient and the concussive forces are transmitted to the bones and other connective tissues. The domestic horse spends considerable time standing, so the structure of the palmar aspect of the foot is important for support to the weight of the horse shifted dorsally toward the connective tissues at the toe of the foot.
The medial and lateral cartilages of the foot extend from the palmar surface of the distal phalanx to the bulbs of the heel as large vertical sheets, whereas the digital cushion lies between the medial and lateral cartilages of the foot and extends dorsally toward the solar surface of the distal phalanx distal to the deep digital flexor tendon (DDFT). Associated with each cartilage of the foot is a venous network that connects with the venous vessels under the distal phalanx and the vessels associated with the dermis of the hoof wall. The venous microvasculature forms a hydraulic system that is hypothesized to provide the mechanism for how the ground impact energies are dissipated, before these forces are transmitted to and damage the bone and other connective tissues within the foot. Horses with good or excellent hydraulic systems should be more efficient in dissipating the impact energy compared with horses with feet with less-well-developed hydraulic networks.
The cartilages of the foot lie beneath the skin and dermis and the coronary venous plexus and have previously been described as rhomboid-shaped, with a convex surface abaxially and a concave surface axially. Several ligaments secure the cartilages of the foot to the digital bones.1-4 However, these descriptions are overly simplistic and appear to have been obtained from examination of horses with underdeveloped structures of the palmar aspect of the foot. The morphological features of the cartilages of the foot vary greatly, with a range of shapes and thickness, the presence of an axial projection from its distal edge, and the extent of vascularity.5 The structure of the cartilages of the foot is best determined by viewing the foot in transverse sections (Figures 29-1 and 29-2). In frontal (dorsal) sections cut perpendicular to the ground, beginning at the bulbs of the heel, the cartilages of the foot have a C- to L-shaped configuration. Both the upright and the base parts of the L-shaped cartilage vary in thickness among horses. The mean thickness of the upright part at the level of the navicular bone ranges from 0.5 to 2.0 cm in an adult horse (450 to 550 kg body weight). The base part or axial projection of the L-shaped cartilage varies in its thickness and the distance that it extends toward the midline of the foot overlying the bars and the frog. The cartilages of the foot are thinnest in the heel region (0.45 to 1.3 cm) but become thicker closer to the distal phalanx (0.6 to 1.5 cm) and thin slightly as they attach onto the distal phalanx (0.5 to 1.0 cm). The cartilages of the foot are thicker in forelimbs than hindlimbs.
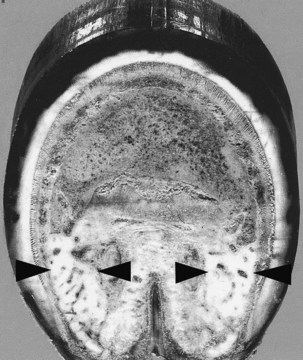
Fig. 29-1 Transverse section through the distal aspect of a foot of a Quarter Horse 25 years of age, with no history of foot problems. Arrows show the thick cartilages of the foot. Axial projection composed of fibrocartilage extends between each cartilage of the foot.
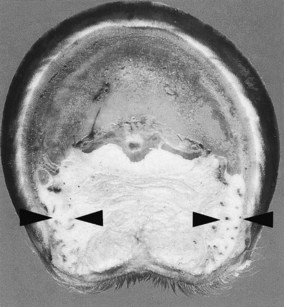
Fig. 29-2 Transverse section of the foot of a horse with chronic foot pain with thin cartilages of the foot (arrows). The digital cushion is primarily composed of fat and elastic tissue, with little fibrocartilage.
Overlying the abaxial surface of the cartilages of the foot are a plexus, the laminar dermis, and the hoof. Loose connective tissue extends from each cartilage to the DDFT. On the axial surface the axial projection of the cartilage extends toward the midline of the foot. In most feet, this axial projection extends from the dorsal half of the cartilage; the palmar half of the cartilage has virtually no, or very small, extension into the substance of the digital cushion. However, in some feet it extends the entire dorsal-palmar extent of the cartilages of the foot. This axial projection extends to overlie the epidermal ridges of the bars, with many fingerlike projections extending into the substance of the digital cushion, and may extend across the midline of the palmar aspect of the foot under the digital cushion to fuse with that of the opposite side of the foot. These white bundles of fibrous and fibrocartilaginous tissues are easily discerned from the surrounding yellow elastic, adipose, and collagen fibers of the digital cushion. The relative thickness of the axial projection in a distal-proximal orientation varies. In horses younger than 4 to 5 years of age the axial projection usually is not fully developed along the entire length of the cartilage, and in young foals there is only a thin sheet of fibrous tissue.
The cartilages of the foot contain primarily hyaline cartilage, but in many horses from 4 to 5 years of age the medial border of the cartilage develops fibrocartilage. A fibrocartilaginous ligament of variable thickness develops between the cartilage and the DDFT; this is consistently larger in forelimbs than hindlimbs. In some horses the cartilages of the foot can ossify. This may be genetically controlled, but any stress on the foot, that accentuates vibration and results in higher energy forces being transmitted through the foot, may promote ossification.
Several ligamentous attachments connect the cartilages of the foot to the distal and middle phalanges, as well as to the navicular bone.3,4 The chondroungular ligaments attach the cartilage to the distal phalanx along the palmar process, whereas the medial and lateral chondrocoronal ligaments attach the cartilage to the proximal half of the middle phalanx. The medial and lateral ligaments of the cartilages of the foot (collateral chondroungular ligaments) attach the cartilage to the angle of the distal phalanx. The paired chondrosesamoidean ligaments attach the axial surface of the cartilage to the navicular bone. A pair of elastic ligaments extends between the proximal phalanx and the proximal surface of the cartilages of the foot; these ligaments are most prominent in larger horses, such as draft breeds. Cruciate ligaments of the cartilages of the foot (cruciate chondroungular ligaments) connect the axial surface of the cartilage to the palmar process on the opposite side of the foot. Within the digital cushion are fiber tracts radiating from the connective tissue ventral to the attachment of the DDFT (digital torus), through the digital cushion, to the axial surface of the cartilages of the foot.
Each hoof cartilage is perforated by numerous vascular foramina, the number of which varies depending on the thickness of the cartilages of the foot. Within the vascular channels is a large central vein, with a rich network of microvessels termed veno-venoanastomoses.5-7 These microvessels exit the large central vein and, after a variable course within the vascular channels, reenter the same vein. More of the vascular channels are present at the distal level of the cartilages of the foot, but in feet with relatively thicker cartilages of the foot, there are more vascular channels proximally compared with feet with much thinner cartilages. The veins coalesce proximal to the cartilage into a venous plexus before uniting to form the medial and lateral palmar digital veins.7,8
Digital Cushion
The digital cushion consists of a meshwork of collagen and elastic fiber bundles, with small areas of adipose tissue, and lies between the cartilages of the foot, extending dorsally as a wedge-shaped tissue attached to the DDFT and the solar surface of the distal phalanx, near the distal attachment of the DDFT.1-4 It overlies the frog and its dermis and the axial projection from the cartilages of the foot. Proximally and dorsally the digital cushion fuses with the distal digital annular ligament and bulges into the bulbs of the heel, which are separated superficially by a central shallow groove. Some horses have areas of fibrocartilage in the digital cushion, and, if present, these extend between the cartilages of the foot and the DDFT. Two arteries pass through the digital cushion to the area distal to the digital cushion but proximal to the axial projection of the cartilages of the foot, and then branch extensively to supply the frog.2-6 Only a few vessels ramify through the digital cushion from these two arteries.
Energy Dissipation
The function of the digital cushion is controversial. The structural organization of the cartilages of the foot, the digital cushion, and the vasculature suggests a role in energy dissipation.5 The pressure theory suggests that at ground contact the frog stay is pushed upward into the digital cushion, forcing the cartilages of the foot outward. The depression theory emphasizes a downward movement of the pastern into the digital cushion during ground impact, forcing the cartilages of the foot to move outward. However, neither theory is consistent with measurement of negative pressure within the digital cushion during stance and locomotion.9 The hemodynamic hypothesis provides a hydraulic mechanism during ground contact, so that impact energy is transmitted to the fluid within the blood vessels.5 During ground impact the outward expansion of the cartilages of the foot probably occurs through the bars contacting the axial projections and the downward movement of the bony column within the hoof capsule. This creates a negative pressure within the digital cushion. At this brief moment of impact the venous blood within the vessels of the palmar aspect of the foot is forced into the microvenous vasculature within the vascular channels of the cartilages of the foot. Hydraulic resistance to flow through the microvasculature dissipates the high-frequency energy waves, which are potentially deleterious to bone and other tissues. Negative pressure in the foot enables refilling of the vasculature before the next footfall. In feet with thick cartilages of the foot, enclosing more microvessels within the vascular channels, more energy is dissipated on ground contact compared with feet with thin cartilages of the foot. The fibrocartilage content of the digital cushion also is crucial to energy dissipation, because the fibrocartilage has its own energy-absorbing mechanisms. The elastic tissue acts only like a spring and absorbs little energy on ground contact, serving to return the foot to its original position as the foot leaves the ground.
The shape of the foot appears to influence the development of the cartilages of the foot and the structure of the digital cushion. In well-balanced feet, with the frog on the ground along with the bars, cartilages of the foot tend to be thick with fibrocartilage in the digital cushion.5 In feet with a low heel and long toe, the site of ground contact of the hoof wall at the angle of the wall and the bars usually is beneath the bony part of the distal phalanx rather than underneath the cartilages of the foot. Therefore more of the energy of impact is transferred to the bone and hoof wall laminae, as the cartilages of the foot and the digital cushion are in essence bypassed during ground impact.
Navicular Suspensory Apparatus
The navicular suspensory apparatus consists of several ligaments functioning to suspend the distal sesamoid bone (navicular bone) on the palmar surface of the distal interphalangeal (DIP) joint.1-4 Proximally, paired collateral suspensory ligaments of the navicular bone (or the collateral sesamoidean ligament) arise from the distal surface of the proximal phalanx and pass in a distopalmar direction, attaching along the abaxial surface of the middle phalanx,10 to insert on the extremities of the navicular bone. In addition, small branches attach to the axial surface of the cartilages of the foot and the distal phalanx. The attachment along the middle phalanx is important biomechanically during forward movement of the limb, because high loads are created on the joint surfaces between the navicular bone and the middle phalanx and between the navicular bone and the distal phalanx.11 These ligaments are composed of collagen fibers with an abundance of elastic tissue fibers. Distally the distal sesamoidean impar ligament extends from the distal border of the navicular bone to the entire flexor surface of the distal phalanx adjacent to the insertion of the DDFT.1-4 At its insertion the distal sesamoidean impar ligament contains an extensive network of microvessels containing arteriovenous complexes and nerve fibers within loose connective tissue septae.10 The arteriovenous complexes are innervated by many peptidergic nerve fibers, including substance P, neurokinin A, and calcitonin gene-related peptide, which are present in the many sensory fibers innervating the foot.12 The neuropeptides substance P and neurokinin A also have pharmacological receptors, located on the small isolated microvessels and the arteriovenous complexes within the distal sesamoidean impar ligament, to control blood flow through this intricate vascular network. When these peptides are released from the sensory nerve fibers in the foot, they promote an active vasodilatation of these small vessels, presumably through a nitric oxide pathway. The locations of these arteriovenous complexes suggest that they may have two possible functions, including providing a protective mechanism for detection of high-pressure differences within the region during movement, and maintaining the hydration status of the distal sesamoidean impar ligament and other nearby connective tissues for optimal function.
The distal border of the navicular bone has a narrow, elongated facet for articulation with the distal phalanx. Between this and the attachment of the distal sesamoidean impar ligament is a fossa containing foramina for blood vessels. The proximal border also has several small foramina. The dorsal articular surface of the navicular bone and the articulation between the navicular bone and the distal phalanx create a substantial palmar extension of the articular surface of the DIP joint. During extension of the DIP joint, with fixation of the foot on the ground and the movement of the body over the distal limb, the middle phalanx contacts the dorsal articular surface of the navicular bone, and the navicular bone becomes a weight-bearing structure. Loads transmitted through the navicular bone are supported by the proximal and distal suspensory ligaments. The role of the DDFT is discussed further in Chapter 32.
Distal Interphalangeal Joint
The distal articular surface of the middle phalanx, the articular surface of the distal phalanx, and the articular surfaces of the navicular bone form the DIP joint. The relatively short medial and lateral collateral ligaments of the distal DIP joint arise from the distal ends of the middle phalanx to insert on the distal phalanx and the dorsal part of the cartilages of the foot. The joint capsule has a small dorsal pouch and an extensive palmar pouch, and blends with the collateral ligaments and common digital extensor tendon. The palmar pouch of the DIP joint is greatly expandable (25 to 30 mL in volume) and is subdivided into a proximal palmar pouch and a small distal palmar pouch extending between the navicular bone and the distal phalanx. In the midline the palmar pouch extends proximally beyond the two small secondary tendons of the DDFT, which attach to the distal end of the middle phalanx. The proximal palmar pouch almost surrounds the collateral sesamoidean ligaments.13,14 The DIP joint cavity also has several small abaxial, dorsal projecting outpouchings that are in close proximity to the sensory nerves of the medial and lateral palmar digital nerves. The DIP joint capsule therefore has a large surface area through which local anesthetic solution may diffuse. The comma-shaped navicular bursa is much smaller (approximately 3 mL in volume). Proximally it can extend over the proximal border of the navicular bone to protrude dorsally.
Innervation
The innervation of the equine distal forelimb is crucial to the horse because this is how the horse interacts with the environment. Thus the foot can be considered to be a neurosensory organ; touch, pressure, and proprioception, as well as nociception, are sensations conveyed in the nerves of the foot. The main innervation to the foot is via the medial and lateral palmar nerves and the medial and lateral palmar metacarpal nerves.l-4 The distal continuations of the palmar nerves course parallel to the accompanying artery and then obliquely across the abaxial surface of the ligament of the ergot to supply most tissues of the palmar half to third of the foot (see Chapter 10). The Editors point out that, according to their clinical experience and current research results, diagnostic analgesia of the digital nerves results in analgesia of most of the foot, including the solar surface. Several small nerves branch from the medial palmar digital nerve to course with the artery of the digital cushion and to supply the palmar aspect of the foot, including the dermis of the overlying skin and frog, parts of the digital cushion, laminae of the bulbs of the heel, cartilages of the foot, and portions of the quarters. A small branch of the lateral palmar nerve may supply the ligament of the ergot. The dorsal branches of the palmar nerve continue with the palmar digital vein to innervate the dorsal aspects of the foot, including the DIP joint, the laminar and solar dermis, and the dorsal part of the cartilages of the foot. An intermediate branch from this nerve occurs in approximately a third of horses. In some horses a branch of the medial palmar metacarpal nerve supplies the coronary band. No communication occurs between the palmar metacarpal nerves and the dorsal branches of the palmar digital nerves. Variable branches occur, including one from the lateral palmar nerve in the metacarpal region extending obliquely to the coronary band, and one from the medial palmar digital nerve to the navicular bursa. In the hindlimbs an additional nerve supplies the coronary and laminar dermis of the dorsal foot, provided by the medial and lateral dorsal metatarsal nerves (terminal branches of the deep fibular nerve). Rarely branches from the plantar metatarsal nerves course under the distal ends of the second and fourth metatarsal bones to supply the dermis of the periople and coronet. The widespread distribution of these nerves provides a broad sensory and sympathetic autonomic innervation pattern to the tissues and vasculature of the foot.
The sensory nerves have many diverse functions in addition to conveying consciously perceived sensations, including touch, proprioception, and pain. The palmar digital nerves are composed of both small, unmyelinated nerves and larger, myelinated nerves in a ratio of nearly 4 : 1. Approximately 25% of the unmyelinated fibers are sympathetic nerves, and 75% are afferent fibers. Much of the sensory information from the foot and the activity of the sympathetic autonomic nerves is conveyed to the spinal cord through the unmyelinated fibers, which are the more slowly conducting nerve fibers. Many neurochemicals are present within the nerves including noradrenaline and adrenaline, substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y, peptide histidine isoleucine, vasoactive intestinal peptide, and the enkephalins. These are released locally from the peripheral processes of the sensory nerves, and the effect on surrounding tissues depends on which neurochemical(s) is emitted.
Most of these peptides have been identified in the foot, usually in close association with blood vessels and other microvasculature. Within the dorsal hoof wall the sympathetic fibers containing noradrenaline and neuropeptide Y are present along the small arterioles within the dermal laminae and form dense plexuses around the arteriovenous anastomoses.15 The same neurotransmitters are present in the navicular region, including the arteriovenous complexes.13 Noradrenaline and neuropeptide Y promote vasoconstriction, whereas vasoactive intestinal peptide is a prominent dilator of the smooth muscle of the microvessels.16 The other peptides, such as substance P, calcitonin gene-related peptide, and peptide histidine isoleucine, are present in the sensory nerve fibers of the dorsal hoof wall and the distal aspect of the distal sesamoidean impar ligament10,12 and also promote vasodilation by means of an endothelial-dependent mechanism and the activation of a nitric pathway.17,18 Activation of these sensory nerves, either directly or indirectly through pain mechanisms, produces a measurable increase in the concentration of these peptides in joints and tissues as they interact with tissue elements, such as inflammatory cells and macrophages, and controls edema formation.l9
Other sensations, such as touch and proprioception, are mediated to the spinal cord through the larger myelinated fibers. The receptors of these nerve fibers are present in the bulbs of the heel and in association with the collateral sesamoidean ligaments.20-22 The locations of these lamellated receptors appear to be critical for the perception of proprioceptive stimuli by the horse during movement when heel-first landing occurs. Activation of these receptors at ground impact and the rapid conduction to the spinal cord through these thickly myelinated fibers enable this sensory information to become incorporated into the spinal cord reflex mechanisms controlling locomotion. During toe-first or flatfooted landings, activation of these sensory receptors is presumably less. If a horse is shod with a pad, it may shorten its stride because the pacinian receptors are not adequately stimulated. If a finger-sized piece of rubber is attached temporarily to the pad, within one or two steps the stride extends forward maximally, as the horse “realizes” the importance of this area of the foot, by activation of the sensory receptors. If the rubber is removed after several days, the gait does not revert. Together the unmyelinated and the myelinated nerves enable the horse to smoothly negotiate the varying surfaces of the terrain during locomotion and provide a means for monitoring changes in the peripheral tissues and controlling the physiological and pathological environment within the foot. In addition to these pain-related sensory nerves, the foot also contains numerous myelinated nerves involved in touch and pressure sensation, permitting the horse to realize where its feet are during stance. Their importance is realized when riders of barefooted horses often remark that their horses are able to “feel” the ground surface and more carefully place the foot than when peripherally loaded devices (shoes) are present. Further research suggests that the active engagement of the neural elements of the foot may be critically important in determining the overall health of the foot.