Chapter 60Cervical Stenotic Myelopathy
Cervical stenotic myelopathy (CSM; wobbler syndrome), a common spinal cord disease of horses, is characterized by malformation of the cervical vertebrae, stenosis of the vertebral canal, and spinal cord compression.1 The age of onset is typically 6 months to 3 years, although mature horses are also sometimes affected. Young horses with the disorder have commonly grown rapidly and are more likely to have developmental orthopedic disease of the appendicular skeleton than peers.2 Male horses are more frequently affected than females.3 CSM has been reported in most light and draft horse breeds, although Thoroughbred and Warmblood horses appear to be particularly predisposed.
Clinical Signs
The clinical signs of spinal cord compression are usually insidious in onset, although owners sometimes report a traumatic incident before recognizing any ataxia. Such traumatic incidents may occur because of mild or previously unrecognized neurological deficits (e.g., occasional tripping) that result in a fall.
Horses with CSM generally have neurological deficits that are recognizable in all limbs, characterized by symmetrical weakness, ataxia, and spasticity.4 In most instances the hindlimbs are more severely affected than the forelimbs, probably because of the more superficial location of hindlimb tracts in the white matter of the spinal cord. At rest, severely affected horses may have a base-wide stance and delayed responses to proprioceptive positioning, whereas at the walk, weakness may be manifest by stumbling and toe dragging. Horses with prolonged clinical signs of CSM may therefore have hooves or shoes that are chipped, worn, or squared at the toe. Ataxia (a sign associated with defective proprioception) is evident as truncal sway at a walk, inconsistent and erratic foot placement, and circumduction and posting (pivoting on the inside limb) of the hindlimbs during circling. Moderately to severely affected horses sometimes have lacerations on the heel bulbs and medial aspects of the forelimbs from overreaching and interference. Spasticity, characterized by a stiff-legged gait and exaggerated movements, may be observed in moderately affected horses, especially in the forelimbs or in the hindlimbs when stepping over curbs or poles. When prompted to back, horses may stand base-wide, lean backward, and drag the forelimbs. Occasionally, signs associated with the forelimbs may be more severe than those in the hindlimbs, particularly in horses with caudal cervical lesions, probably because of involvement of local spinal cord grey matter.
A grading scale (0 to 5) is often used to score horses with signs of spinal ataxia and weakness: 0, normal; 1, very mild deficits detectable only with complex movements (e.g., walking with head elevated, on an incline or when circling); 2, mild-moderate deficits that are detectable at the walk; 3, marked deficits obvious at the walk; 4, severe deficits that result in difficulty remaining standing; 5, recumbent. Some clinicians favor an approach in which individual limbs are scored separately for signs of ataxia and weakness, with a global score being used to summarize the total neurological deficit. Such an approach is helpful when evaluating disease progression and response to treatments.5
Asymmetrical ataxia and paresis are observed occasionally in horses with dorsolateral compression of the spinal cord caused by proliferative, degenerative articular processes and periarticular soft tissues.6 Infrequently, signs of compressive radiculopathy, such as cervical pain, atrophy of the cervical musculature, cutaneous hypalgesia, and hyporeflexia of cervical reflexes adjacent to the site of spinal cord compression may be evident. These signs are more commonly observed in horses older than 4 years of age with moderate-to-severe arthropathy of the fifth to seventh cervical vertebrae (C5 to C7) and usually result from peripheral nerve compression by proliferative articular processes as the nerve root exits the vertebral canal through the intervertebral foramen.7
In some instances arthropathy of the caudal cervical vertebral articular processes may produce forelimb lameness, caused by spinal nerve root compression, without producing clinical signs of spinal cord compression.8 Affected horses typically have a short cranial phase of the stride and a low forelimb foot arc and may stand or walk with the head and neck extended (see Chapter 53). Rarely, diskospondylosis of the cervical vertebrae produces a short-strided gait and cervical pain, with or without spinal ataxia (Figure 60-1). Horses with diskospondylosis or arthropathy of the caudal vertebrae may exhibit signs of lameness, pain, or stiffness with the neck in only certain positions or when the head and neck are manipulated or the horse is turned. For example, some affected horses may be unwilling to turn the neck laterally when offered food.
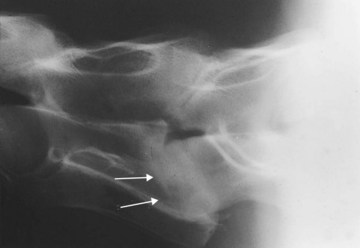
Figure 60-1 Lateral-lateral radiographic image of the caudal aspect of the neck of a 4-year-old Paint gelding with cervical pain and spinal ataxia. Cranial is to the left. Note the abnormal intercentral articulation between the sixth and seventh cervical vertebrae (arrows). This represents end-stage diskospondylosis.
Dynamic spinal cord compression usually occurs in younger horses (<2 years of age) and is associated with instability of the cervical vertebrae, particularly between C3 and C6. Dorsal laminar extension, caudal epiphyseal flare, or abnormal ossification patterns may contribute to the problem. Static vertebral canal stenosis (type II) is characterized by constant spinal cord compression, regardless of neck position (Figure 60-2), and is seen usually in older horses.9 It generally results from osteoarthritis (OA) of the articular processes and proliferation of periarticular soft tissue structures. Synovial cysts, which are often associated with OA of the articular processes, may produce waxing and waning, or acute-onset asymmetrical neurological signs. In some horses with static compression, flexion of the neck stretches the ligamentum flavum and relieves spinal cord compression, whereas extension exacerbates the problem.
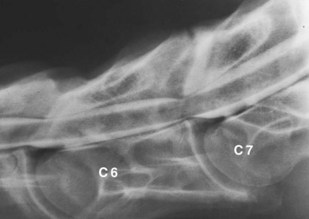
Figure 60-2 Lateral-lateral radiographic image during myelographic examination of the fifth to seventh (C7) cervical vertebrae of a 3-year-old Thoroughbred colt with static spinal cord compression. The dorsal and ventral contrast columns are attenuated by more than 50% at the fifth and sixth (C6) and sixth and seventh cervical vertebral articulations.
Diagnosis
The following neurological disorders should be considered potential differential diagnoses and may produce signs similar to or indistinguishable from CSM: equine protozoal myeloencephalitis (EPM), equine degenerative myeloencephalopathy (EDM), equine herpesvirus–1 (EHV-1) myelitis, occipitoatlantoaxial malformation, spinal cord trauma, vertebral fracture, vertebral abscess or neoplasia, and verminous myelitis (see Chapter 11).
Horses with traumatic cervical vertebral disorders usually exhibit pain during manipulation or palpation of the neck, and the disorder may sometimes be differentiated from CSM by standing radiographic examination. Occipitoatlantoaxial malformation (see Chapter 53) occurs primarily in Arabian horses and is diagnosed definitively by radiological evaluation (see Figure 53-4, B). EDM is diagnosed by exclusion (unremarkable cerebrospinal fluid [CSF] cytological examination findings, negative immunoblot analysis for Sarcocystis neurona, and negative radiological findings and myelographic examination findings). A veterinarian may suspect EDM based on the age (usually less than 18 months) and during neurological examination (hyporeflexia, and similar degrees of ataxia in the forelimbs and hindlimbs), but definitive diagnosis is achieved only by postmortem examination. Although several breeds have been reported with the disease, EDM appears to have a familial predisposition in Standardbred horses.10 Horses with EHV-1 myelitis may have urinary incontinence, poor tail tone, and hindlimb lower motor neuron weakness. Signs associated with cranial nerve involvement may occasionally be observed. In EHV-1 myelitis, CSF evaluation typically reveals xanthochromia and albuminocytological dissociation (high protein concentration, normal cell count); a rising EHV-1 serum antibody titer, virus isolation, and polymerase chain reaction (PCR) diagnosis all may be used to provide supportive evidence of EHV-1 myelitis. In areas where EPM is endemic (such as North and South America) or in horses exported from these regions, distinguishing between EPM and CSM can be difficult. Asymmetrical ataxia, focal sweating, and focal muscle atrophy should direct diagnostic efforts toward EPM; however, symmetrical spinal ataxia does not preclude a diagnosis of EPM. EPM-affected horses with symmetrical ataxia are differentiated from those with CSM on the basis of standing radiographic examination, CSF immunoblot analysis for S. neurona, and, in some circumstances, myelographic evaluation (see comments on equine myelography, later). Immunoblot analysis of CSF is frequently positive, however, if horses affected by CSM are in a geographical area with a high seroprevalence of EPM. Therefore differentiation of these two conditions should not be determined on the basis of CSF analysis alone. Findings of cytological analysis of CSF are usually unremarkable in horses with CSM, although mild xanthochromia or slightly increased protein concentration may be observed in affected horses, especially if signs have developed acutely, perhaps precipitated by trauma.
Plain radiography of the cervical vertebrae can be used to assess the likelihood of CSM in horses with spinal ataxia.11 Accurate assessment of cervical radiographs requires a precise lateral-lateral radiographic image of the cervical vertebrae,12 ensuring that the ventral prominences of the transverse processes are perfectly overlying each other. Radiographic obliquity results in indistinct margins of the ventral aspect of the vertebral canal, and in erroneous values for objective measurements. Obtaining precise lateral-lateral radiographs of the cervical vertebrae in recumbent horses is difficult, so whenever possible, plain or digital radiographs should be obtained in the standing, sedated horse.
Cervical radiographs should be evaluated subjectively and objectively. Subjective interpretation is based on examining for the presence of five characteristic malformations of the cervical vertebrae, which include (1) flare of the caudal epiphysis of the vertebral body (the so-called “ski-jump appearance”); (2) abnormal ossification of the articular processes; (3) subluxation or malalignment between adjacent vertebrae; (4) extension of the vertebral caudal dorsal lamina; and (5) OA of the articular processes (Figure 60-3). Estimating the importance of lesions identified through subjective interpretation can be difficult and is based on the clinician’s experience and interpreting the balance of probability. For example, OA of (especially the caudal) vertebral articular processes is recognized commonly in normal horses.13 Hence recognition of characteristic vertebral malformations is considered supportive in diagnosis at best.14
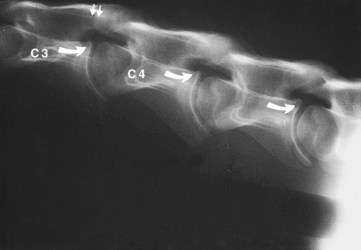
Figure 60-3 Lateral-lateral radiographic image of the midneck region of an 8-month-old Quarter Horse colt with spinal ataxia caused by cervical stenotic myelopathy. Bony malformations consistent with cervical stenotic myelopathy include flare of the caudal epiphyses (curved arrows), caudal extension of the third cervical vertebra dorsal lamina (arrows), and malalignment of the articulation of the third (C3) and fourth (C4) cervical vertebrae.
Objective assessment of vertebral canal diameter is more accurate than subjective evaluation of vertebral malformation for identifying young horses affected by CSM, but may lead to false negative diagnoses in older horses.9 Both intervertebral and intravertebral measurements are used. The sensitivity and specificity of the intravertebral sagittal ratio for identifying horses affected by CSM are approximately 90% for vertebral sites between the fourth and seventh cervical vertebrae, suggesting that generalized stenosis of the vertebral canal may be the most important factor in the development of CSM (especially in young horses).11 The intravertebral sagittal ratio is calculated by dividing the minimum sagittal diameter of the vertebral canal (the narrowest perpendicular distance from the dorsal aspect of the vertebral body to the ventral border of the dorsal lamina) by the maximum width of the cranial vertebral physis (from the vertebral canal) (Figure 60-4). Because the vertebral body is located within the same anatomical plane as the vertebral canal, this ratio negates effects of magnification resulting from variability in object-plate distance. In most normal horses, the sagittal ratio exceeds 52% from the fourth to sixth cervical vertebrae and 56% at the seventh cervical vertebrae in horses greater than 320 kg. The positive predictive value of such measurements is probably higher, and the negative predictive value lower, in ataxic horses from countries where conflicting diagnoses (such as EPM) are not routinely encountered (i.e., false-positive results are less likely, but false-negative results are more likely because the underlying prevalence of CSM in ataxic horses is higher). Similarly, the positive and negative predictive values of objective cervical radiography measurements in the absence of ataxia (e.g., during prepurchase radiography) have not been evaluated, but false-positive results are likely to be more common and false-negative results less common, because the prevalence of CSM in this population is much lower. Judicious use of additional diagnostic tests (e.g., CSF evaluation) to help rule out other differential diagnoses should be considered to increase pretest probability, especially in countries where other causes of spinal ataxia are common.
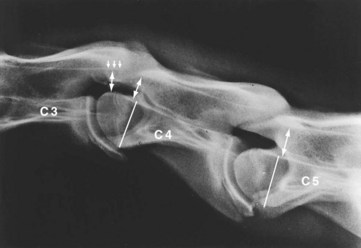
Figure 60-4 Lateral-lateral radiographic image of the third (C3), fourth (C4), and fifth (C5) cervical vertebrae of a 2-year-old Thoroughbred colt with cervical stenotic myelopathy. The sagittal ratio is determined by dividing the intravertebral minimum sagittal diameter (double arrow) by the width of the vertebral body (line). The intervertebral minimum sagittal diameter is measured from the caudoventral aspect of the dorsal lamina of C3 to the craniodorsal aspect of the vertebral body of C4 (double arrow below the smaller arrows). There is caudal extension of the dorsal lamina of C3 (smaller arrows), and there is malalignment at the articulation of C3 and C4.
Some clinicians advocate use of ratiometric measurements that take into account the distance between adjacent vertebrae (intervertebral ratios) (see Figure 60-4) based on the rationale that most compressive lesions occur between, rather than within, the vertebrae.15 Particularly high-quality radiographs are usually required for such measurements, but analysis suggests that this approach may be helpful in differentiating CSM from other conditions.16 Further comparison of both methods in a large group of horses is needed based on a gold standard diagnosis established at postmortem examination, because myelography is problematic (see discussion later). However, available postmortem material may be skewed toward severely affected horses because these horses may more often be euthanized.
A semiquantitative scoring system developed by Mayhew and colleagues17 is advocated in foals younger than 1 year of age for assessment of cervical radiographs for diagnosis of CSM. The scoring system combines objective measurement of vertebral canal diameter and subjective evaluation of vertebral malformation. Stenosis of the vertebral canal is assessed by determination of the intervertebral and intravertebral minimum sagittal diameter (see Figure 60-4). The intervertebral and intravertebral minimum sagittal diameters are corrected for radiographic magnification by dividing these values by the length of the vertebral body. The maximum score for cervical vertebral stenosis is 10 points. Cervical vertebral malformation is determined by subjective assessment of five categories: (1) encroachment of the caudal epiphysis of the vertebral body dorsally into the vertebral canal, (2) caudal extension of the dorsal lamina to the cranial physis of the adjacent vertebra, (3) angulation between adjacent vertebral bodies, (4) abnormal ossification of the physis, and (5) OA of the articular processes. The maximum score allotted for each category of bony malformation is 5 points. A total score of 12 or higher (maximum total score 35) confirms the radiological diagnosis of CSM. Stenosis of the vertebral canal and malalignment between adjacent vertebrae are the most discriminating parameters in this semiquantitative scoring system to differentiate normal from affected foals.
Radiology may be used to predict the likelihood of CSM, and in countries where conflicting differential diagnoses are rare (and in the absence of other signs to the contrary), radiology is often considered sufficient to make a presumptive diagnosis of cervical compression without the need for further tests. In countries where EPM or other conflicting differential diagnoses are possibilities, many clinicians favor myelography for diagnosis. Unfortunately, for most intervertebral sites, myelography results in a high number of false-positive and false-negative results.18 Myelography remains, however, a prerequisite if surgical intervention is considered a viable option on the basis of severity of signs and the owner’s wishes and expectations (see later). This is because standing radiography does not definitively pinpoint the actual site of the compressive lesion(s).11 Typically during myelography, however, clinicians obtain additional radiographic images, such as the lateral-lateral projections in neutral, extended, and flexed positions, although the last technique in particular results in more false-positive diagnoses.18 Radiography of the neck in different positions does, however, enable the clinician to attempt to differentiate between static and dynamic compressive lesions. Note that neck flexion and extension while horses are under anesthesia are contraindicated if there is evidence of compression on the initial neutral views. Ventrodorsal images may be attempted in small or young horses, especially in the cranial aspect of the neck, and may demonstrate an asymmetrical compressive lesion that might otherwise account for some false-negative diagnoses in larger horses.
Various techniques have been used for interpreting equine myelograms. In normal horses there should be no or minimal change to the width of the dorsal dye column, but it is common to have loss of the ventral dye column. Consequently, most commonly at intervertebral sites between C2 and C6, diagnosis of spinal cord compression is made on the basis of a 50% or greater decrease in the sagittal width of the dorsal and ventral contrast columns in comparison with the column width at the immediate cranial or caudal midvertebral site.12 At C6-C7, a reduction of more than 20% of the dural diameter measured in the midbody region is best used to diagnose compression, because this measurement has a relatively high sensitivity and specificity.18 Clinicians may favor use of different “cutoff” values for exclusion or inclusion of diagnosis based on the consequence of the derived decision (i.e., possible euthanasia or surgery).16 As in interpretation of plain radiographs, the positive predictive value for myelography is likely to be higher in ataxic horses from countries without many conflicting differential diagnoses because the prevalence of true vertebral compression in ataxic horses is relatively higher.
Horses should be monitored for 24 hours after the myelographic procedure for depression, fever, seizure, or more severe ataxia. Worsening of neurological status after myelography may result from spinal cord trauma during hyperflexion or as a result of the general anesthesia or recovery, iatrogenic puncture of the spinal cord, or chemical meningitis. Administration of phenylbutazone (4.4 mg/kg orally [PO] q24h) 1 day before until 1 day after myelographic examination is recommended.
Additional techniques that have been used or proposed for evaluating horses with compressive myelopathy include electromyography of the cervical musculature (examining for presence of signs of local muscle denervation caused by grey matter or peripheral nerve disease),19 transtentorial magnetic stimulation,20 and kinematic gait analysis,21 but such techniques require further validation before their widespread use is recommended. Antemortem diagnosis of CSM therefore has inherent problems and limitations, of which the clinician should be aware, but a combination of tests and methodologies taken in the context of the signalment, history, and comprehensive physical and neurological examinations likely optimizes accurate diagnosis.
Conservative Management
Successful conservative management of foals younger than 1 year of age with CSM has been achieved using the paced diet program.17,22 The goal of this dietary program is to retard bone growth, enhance bone metabolism, and allow the vertebral canal diameter to enlarge to relieve spinal cord compression. This dietary program is restricted in energy and protein (65% to 75% of National Research Council [NRC] recommendations) but maintains balanced vitamin and mineral intake (minimum 100% NRC recommendations). Vitamins A and E are provided at 3 times NRC recommendations, and selenium is supplemented to 0.3 ppm. Roughage is provided by pasture or low-quality (6% to 9% crude protein) timothy hay. Dietary regimens are individually formulated according to the age and weight of the foal. Solitary stall confinement is recommended to minimize repetitive spinal cord compression caused by dynamic instability.
Most clinicians advocate antiinflammatory therapy in all horses with CSM.9 Administration of glucocorticoids and/or nonsteroidal antiinflammatory drugs may reduce edema and provide transient improvement in neurological signs or may reduce compression from inflamed associated soft tissues. Some clinicians advocate use of dimethyl sulfoxide, particularly in horses in which clinical signs have developed acutely. Spontaneous recovery from CSM without dietary management or surgical intervention is not reported.
Horses with cervical pain and forelimb lameness caused by cervical vertebral arthropathy may benefit from intraarticular administration of corticosteroids or chondroprotective agents. Intraarticular administration of medication is performed with ultrasound guidance using a 15-cm (6-inch), 18-gauge spinal needle in a standing, sedated, or recumbent horse.23 The cranial articular process of the more caudal vertebra is superficial to the caudal process of the cranial vertebra (Figure 60-5, A). The articular space is accessed at the cranioventral opening of the articular facet, which is angled approximately 60 degrees from the ultrasound beam. The needle should be introduced 5 cm cranial to the joint and inserted at a 30-degree angle to the skin surface (Figure 60-5, B). Joint penetration should be confirmed by aspiration of synovial fluid. If the neck is extended, the transverse process of the cranial vertebra may obscure the path to the articulation. Intraarticular administration of triamcinolone acetonide (6 mg per joint) or methylprednisolone acetate (100 mg per joint) has produced a reduction in cervical pain in more than 50% of horses with arthrosis of the articular processes.24 The goal of intraarticular antiinflammatory therapy should be to improve cervical mobility, reduce cervical pain, and eliminate forelimb lameness. In my experience, intraarticular therapy rarely improves spinal ataxia presumed to be associated with articular process OA.
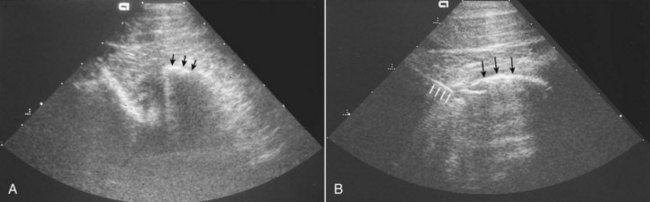
Figure 60-5 A, Ultrasonographic examination of the articulation of the fifth and sixth cervical vertebrae (coronal image). The cranial articular process of the sixth cervical vertebra (arrows) is superficial to the caudal articular process of the fifth cervical vertebra. B, Ultrasonographic examination during arthrocentesis of the articulation of the fifth and sixth cervical vertebrae (transverse image). A 15-cm spinal needle is entering the ventrolateral aspect of the joint (white arrows) deep to the cranial articular process of the sixth cervical vertebra (black arrows).
Surgical Treatment
Surgical intervention is the most widely reported therapeutic approach for (particularly adult) horses with CSM. Cervical vertebral interbody fusion (ventral stabilization) was first described in 1979 for horses with dynamic spinal cord compression, and it is widely performed, particularly in certain centers.25 The procedure fuses adjacent vertebrae in the extended position, which provides immediate relief of most dynamic spinal cord compressive lesions while preventing repetitive spinal cord trauma. Dorsal laminectomy (subtotal Funkquist type B) is described for horses with static CSM and provides immediate decompression of the spinal cord.26 Portions of the dorsal lamina, ligamentum flavum, and joint capsule overlying the site of spinal cord compression are removed during dorsal laminectomy. This procedure effectively decompresses the spinal cord. However, it has been associated with substantial postoperative complications27 and is not widely performed. Interbody fusion is an alternative to dorsal laminectomy for horses with static compressive lesions because after cervical vertebral fusion the articular processes remodel and soft tissue structures atrophy, resulting in delayed decompression of the spinal cord over several months.28
Cervical vertebral fusion improves the neurological status of 44% to 90% of horses with CSM, and 12% to 62% of horses return to athletic function.27,29 Of the horses that return to athletic function, approximately 60% are able to perform at the level of intended use, including racing, jumping, and pleasure performance activities. The anticipated magnitude of improvement is one to two neurological grades after cervical fusion. Occasionally, three grades of improvement in neurological status have been achieved. However, it is unusual for a horse with grade IV neurological deficits to become neurologically normal after cervical vertebral fusion. Rarely, a domino effect can occur in horses after vertebral fusion, wherein spinal cord compression develops at the intervertebral site adjacent to the site of fusion. This may result from added forces at the adjacent site or natural progression of the disease.30 Subtotal laminectomy is reported to improve the neurological status of 40% to 75% of horses with static compression.26
Fatal postoperative complications have been reported with surgical management of horses with static compressive lesions of the caudal cervical vertebrae (subtotal laminectomy and interbody fusion). Complications directly related to the surgical procedure include vertebral body fracture, spinal cord edema, and implant failure. Seroma formation is common after cervical vertebral interbody fusion and can be minimized by use of a pressure bandage maintained over the surgical site for 2 to 3 weeks.27
The most important horse-related factor for determining postoperative prognosis is duration of clinical signs before surgical intervention. Horses with neurological gait deficits present for less than 1 month before surgery are more likely to return to athletic function than are horses with signs of greater than 3 months’ duration.27 The number of spinal cord compressive sites and horse age do not appear to affect the long-term outcome of horses with cervical vertebral interbody fusion. Horses with dynamic compressive lesions appear to have a better postoperative result than those with static compressive lesions. Horses with lesions involving the sixth and seventh cervical vertebrae have a less favorable prognosis than those with lesions affecting the third to fifth cervical vertebrae.
The duration of convalescence and rehabilitation after cervical vertebral interbody fusion is approximately 12 to 18 months, and a horse’s neurological status may continue to improve throughout that time. An individualized exercise program dependent on capability, projected use, and neurological status should be designed to promote muscular strength and coordination. Extended exercise at slow speed, including ponying (being led from another horse), working over poles, and lunging on inclines, is recommended during rehabilitation to build muscular strength. The point at which the horse is competent to return to athletic function after cervical vertebral interbody fusion should be determined by neurological examination.