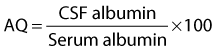Chapter 11 Neurological Examination and Neurological Conditions Causing Gait Deficits
Differentiating neurological gait deficits from lameness can sometimes be a dilemma for the clinician. Many repeated examinations and ancillary testing may be necessary, and even then experienced clinicians may give varied opinions about the same horse. Lack of definitive diagnostic tests to identify the origin of subtle gait changes, which in some horses may be perceived only by a rider or driver and not visible, promotes diagnoses that are based purely on opinions and individual prejudices. This chapter discusses the examination of a horse with gait deficits caused by disease of either the spinal cord, the most frequently documented cause of neurological gait deficits, or peripheral nerves. The chapter does not consider neurological syndromes characterized by signs of brain dysfunction, such as vestibular, cerebral, and cerebellar disorders. We also refer readers to a review on the equine spinal cord.1
Diagnosis
History is important but depends not only on asking the appropriate questions but also on many uncontrollable factors, such as how closely, impartially, and astutely the horse has been observed. The time of onset of signs and rate of progression, whether the gait deficit waxes and wanes or is affected by exercise or rest, whether one or more limbs are affected, whether the affected limb varies, what the horse was doing before onset of signs occurred, whether exercise or management has changed, whether the horse has been moved geographically, whether signs occurred after transport, what medications may have been given and any observed effects, and whether other horses on the farm or in the stable have had any recent illnesses or fever should be determined. It is also important to know if other horses on the same farm have similar clinical signs. For instance, a history of fever, respiratory disease, or abortions in horses in contact with the affected horse would make one suspect equine herpesvirus–1 (EHV-1) infection.
Clinical Examination
The clinician should observe whether the horse displays cranial nerve dysfunction; muscle hypertrophy, atrophy, or asymmetry; muscle trembling; abnormal hoof wear; or abnormal posture. Muscle atrophy may be caused by disease of the ventral horn cells of the gray matter of the spinal cord, peripheral nerve, or the muscle itself; it also can occur with disuse. Palpation can reveal abnormalities such as altered skin temperature, sweating, muscle fasciculations, abnormal sensitivity, or soreness. The horse should be observed on a flat surface, at a walk and trot, and in straight and curving lines. The horse should be evaluated on a surface that allows detection of abnormal hoof flight and placement, toe dragging, or excessive force when landing. The sound of the feet landing should be noted for consistency and loudness. ![]() Hard surfaces also may enhance abnormal hyperflexion in horses with stringhalt. Evaluation on a soft surface may be necessary if the horse is unstable or if the clinician is trying to determine whether the horse could have sore feet. Any abnormal head or neck movement associated with limb movement should be noted. It is important to permit normal neck and head movement when the horse is being led. The person leading the horse should hold the horse as loosely as is safely possible. Collapsing or sinking on a limb, knuckling, hyperflexion, spasticity, hesitance in any part of the stride, dragging of a toe, landing excessively hard, leaning to one side, or failing to track straight can indicate a neurological deficit.
Hard surfaces also may enhance abnormal hyperflexion in horses with stringhalt. Evaluation on a soft surface may be necessary if the horse is unstable or if the clinician is trying to determine whether the horse could have sore feet. Any abnormal head or neck movement associated with limb movement should be noted. It is important to permit normal neck and head movement when the horse is being led. The person leading the horse should hold the horse as loosely as is safely possible. Collapsing or sinking on a limb, knuckling, hyperflexion, spasticity, hesitance in any part of the stride, dragging of a toe, landing excessively hard, leaning to one side, or failing to track straight can indicate a neurological deficit. ![]() Various manipulations are used to diagnose whether proprioceptive or motor deficits exist and to localize the lesion. While being led, the horse should be evaluated while stopping and starting from a walk and trot, backing up, circling tightly in both directions, walking while sideways traction is applied and released on the tail, being pushed sideways from a standstill, and walking with its head elevated.
Various manipulations are used to diagnose whether proprioceptive or motor deficits exist and to localize the lesion. While being led, the horse should be evaluated while stopping and starting from a walk and trot, backing up, circling tightly in both directions, walking while sideways traction is applied and released on the tail, being pushed sideways from a standstill, and walking with its head elevated. ![]() Some clinicians also evaluate repositioning of the foot after placing the horse’s hoof in an abnormal position. We do not find this particularly helpful because a horse’s disposition, age, training, and distractions can affect its response. A horse with a normal gait may stand with its feet placed in an abnormal position for what seems an abnormally long time. Some clinicians also use wheelbarrowing and hopping reactions.1 We do not use these tests in mature horses because we believe responses may be inconsistent and difficult to evaluate accurately and safely. Observation of the horse walking or trotting up and down inclines can be helpful in revealing whether the horse “knows where its limbs are” (proprioception) and can adjust limb movement appropriately. It may be helpful to observe the horse while it is being ridden or lunged, and in some horses while it is loose in an enclosure. Watching the horse stop and start, turn, back up, and maintain its balance during many postural maneuvers allows detection of neurological deficits that may not be obvious when the horse is being led. Consider the following:
Some clinicians also evaluate repositioning of the foot after placing the horse’s hoof in an abnormal position. We do not find this particularly helpful because a horse’s disposition, age, training, and distractions can affect its response. A horse with a normal gait may stand with its feet placed in an abnormal position for what seems an abnormally long time. Some clinicians also use wheelbarrowing and hopping reactions.1 We do not use these tests in mature horses because we believe responses may be inconsistent and difficult to evaluate accurately and safely. Observation of the horse walking or trotting up and down inclines can be helpful in revealing whether the horse “knows where its limbs are” (proprioception) and can adjust limb movement appropriately. It may be helpful to observe the horse while it is being ridden or lunged, and in some horses while it is loose in an enclosure. Watching the horse stop and start, turn, back up, and maintain its balance during many postural maneuvers allows detection of neurological deficits that may not be obvious when the horse is being led. Consider the following:
In some horses it is necessary to observe the horse performing its usual activity, providing it is capable. However, gait deficits may be much less apparent at speed than when the horse is walking or trotting slowly. Basically, the clinician is trying to determine whether the horse moves symmetrically and smoothly with normal stride length and height of foot flight appropriate to the breed and use, whether it appears strong and consistently places its feet in the appropriate positions, and whether it moves in balanced harmonious fashion. It is sometimes difficult to determine whether certain postural or gait changes are a result of pain or weakness or are associated with motor or proprioceptive deficits. Is the horse flexing its hindlimbs excessively and holding its croup more ventrally and flexed because of pain or weakness? If the horse is shifting weight between the hind feet, is it because of weakness, as seen, for example, in lower motor neuron disease, or because of pain? When both limbs are affected, manipulation of a limb to try to localize pain may not be possible. Gaited horses can be extremely difficult to evaluate, especially if one is unfamiliar with the specific gaits. Conformation also can confound interpretation of clinical signs. It may be necessary to observe the horse on many occasions and compare its gait before and after exercise. Is the deficit consistent, or does it vary? If it worsens with exercise, is it because of pain or inability to compensate for a neurological deficit as the horse tires? Perineural analgesia may be helpful. Is there palpable evidence of muscle cramping with exercise or an increase in creatine kinase (CK) level, indicating rhabdomyolysis? A variable gait deficit and inconsistent alterations in foot flight or placement are more likely to represent a neurological deficit than lameness; single limb lameness may vary in intensity but usually remains similar in character. Painful and neurological conditions could coexist but may be difficult to differentiate even with use of commonly used analgesics such as phenylbutazone.
If the horse buckles in a limb, especially on turns, is easily pulled sideways by the tail when standing or walking, or trembles its limb, weakness of the extensor muscle groups should be suspected. ![]() When the flexor muscles are weak, the horse is unable to lift its limb normally, and the toe may be worn from dragging. Pushing the horse sideways or trying to pull on the halter and tail simultaneously can reveal weakness. If the horse is weak or has pain in one limb, it is not able to bear weight normally when the contralateral hoof is lifted from the ground. Neck flexion sideways and vertically should be evaluated for ease and range of movement. Skin sensation and the cutaneous trunci reflex and cervical reflexes should be evaluated. Tapping the trunk should elicit contraction of the cutaneous trunci muscle. Abnormalities can delineate a thoracic spinal cord lesion, because afferent input is through the dorsal thoracic nerves and cranially through the spinal cord white matter, and the efferent pathway involves the cranial thoracic motor neurons in the first thoracic and eighth cervical segments and the lateral thoracic nerve. Hypalgesia of the cutaneous trunci as assessed by response to a two-pinch test with a hemostat is rare and occurs only with severe thoracic spinal cord disease.1 Lack of a cervicofacial reflex (failure of the facial muscles to twitch when the ipsilateral side of the cranial aspect of the neck is tapped) can suggest a lesion in the cervical cord or a branch of cranial nerve VII. If tapping the side of the neck fails to elicit contraction of the cutaneous coli muscle, a cervical cord lesion could exist. If any abnormal response to skin stimulation is detected, the test should be repeated because the horse’s disposition can influence its responses. Limb reflexes usually are not used, although patellar reflexes can be elicited in horses. We do not consider the thoracolaryngeal reflex (slap test) to be helpful. Response is inconsistent in horses with cervical spinal cord lesions and may be absent in normal horses.
When the flexor muscles are weak, the horse is unable to lift its limb normally, and the toe may be worn from dragging. Pushing the horse sideways or trying to pull on the halter and tail simultaneously can reveal weakness. If the horse is weak or has pain in one limb, it is not able to bear weight normally when the contralateral hoof is lifted from the ground. Neck flexion sideways and vertically should be evaluated for ease and range of movement. Skin sensation and the cutaneous trunci reflex and cervical reflexes should be evaluated. Tapping the trunk should elicit contraction of the cutaneous trunci muscle. Abnormalities can delineate a thoracic spinal cord lesion, because afferent input is through the dorsal thoracic nerves and cranially through the spinal cord white matter, and the efferent pathway involves the cranial thoracic motor neurons in the first thoracic and eighth cervical segments and the lateral thoracic nerve. Hypalgesia of the cutaneous trunci as assessed by response to a two-pinch test with a hemostat is rare and occurs only with severe thoracic spinal cord disease.1 Lack of a cervicofacial reflex (failure of the facial muscles to twitch when the ipsilateral side of the cranial aspect of the neck is tapped) can suggest a lesion in the cervical cord or a branch of cranial nerve VII. If tapping the side of the neck fails to elicit contraction of the cutaneous coli muscle, a cervical cord lesion could exist. If any abnormal response to skin stimulation is detected, the test should be repeated because the horse’s disposition can influence its responses. Limb reflexes usually are not used, although patellar reflexes can be elicited in horses. We do not consider the thoracolaryngeal reflex (slap test) to be helpful. Response is inconsistent in horses with cervical spinal cord lesions and may be absent in normal horses. ![]() Blindfolding the horse usually is not part of our routine neurological examination unless vestibular disease is suspected. A complete physical examination should always be conducted. In some horses with hindlimb gait deficits, palpation per rectum of the pelvic bones, lumbar region, caudal aspect of the aorta, and iliac vessels may be necessary. Simple observation may not differentiate hindlimb weakness caused by spinal cord disease from that caused by partial aortoiliac thrombosis. Horses that do not “feel right” to the rider yet show no obvious deficits to the observer whether observed saddled or in hand are problematic. It may be necessary to observe a horse from a jog cart or carriage if the gait deficit about which a driver complains is not visible to the bystander. In attempting to differentiate between a musculoskeletal and neurological condition causing a gait deficit in a limb, diagnostic analgesia may be necessary. Obviously, this does not help differentiate pain from lameness emanating from a lesion proximal to the coxofemoral or scapulohumeral joints. A course of nonsteroidal antiinflammatory drugs (such as moderate doses of phenylbutazone for days or even several weeks) may be helpful in determining whether a gait deficit is caused by pain.
Blindfolding the horse usually is not part of our routine neurological examination unless vestibular disease is suspected. A complete physical examination should always be conducted. In some horses with hindlimb gait deficits, palpation per rectum of the pelvic bones, lumbar region, caudal aspect of the aorta, and iliac vessels may be necessary. Simple observation may not differentiate hindlimb weakness caused by spinal cord disease from that caused by partial aortoiliac thrombosis. Horses that do not “feel right” to the rider yet show no obvious deficits to the observer whether observed saddled or in hand are problematic. It may be necessary to observe a horse from a jog cart or carriage if the gait deficit about which a driver complains is not visible to the bystander. In attempting to differentiate between a musculoskeletal and neurological condition causing a gait deficit in a limb, diagnostic analgesia may be necessary. Obviously, this does not help differentiate pain from lameness emanating from a lesion proximal to the coxofemoral or scapulohumeral joints. A course of nonsteroidal antiinflammatory drugs (such as moderate doses of phenylbutazone for days or even several weeks) may be helpful in determining whether a gait deficit is caused by pain.
Hematology and Serology
In most horses serum chemistry screens and hematological tests are not particularly helpful; however, in horses with a gait deficit caused by an underlying muscle disease, evaluation of aspartate transaminase (AST) and CK levels may be helpful. Stage of training, exercise pattern, and whether the blood specimen was obtained after exercise preceded by a day of rest must be considered in evaluation of enzyme levels. If a horse consistently has abnormally elevated enzyme levels, then the horse has rhabdomyolysis, and the clinician must decide whether the condition is causing or contributing to the horse’s abnormal gait. Plasma CK and AST levels do not increase simply because of muscle atrophy; rhabdomyolysis must occur to increase the enzyme levels in the blood (see Chapter 83). Elevated plasma concentrations of CK and AST in horses that are not being exercised suggest a primary muscle disorder, such as (but not limited to) polysaccharide storage myopathy (see Chapter 83). An elevation in white blood cell count and fibrinogen level indicates inflammation. In our experience, elevation in fibrinogen level is a more consistent indicator of inflammation in the adult horse than is elevation in white blood cell count.
If clinical signs suggest equine lower motor neuron disease, serum levels of vitamin E (α-tocopherol) should be measured; levels of vitamin E have consistently been low in horses with confirmed equine motor neuron disease, unless the horse has been given supplements.2 Thus low vitamin E levels may be suggestive of, but are not specific for, equine lower motor neuron disease. Tocopherol concentrations can decrease during winter when horses lack access to green pasture.3 Daily variations in plasma levels may occur.4 Low levels also have been reported in clinically normal horses5-7 and in one horse with chronic gastrointestinal disease.8 The laboratory that performs the test should be contacted for any specific requirements for submission of samples and to ensure they have an established normal range for vitamin E levels.
Serological testing for antibodies to various infectious agents may be indicated. In EHV-1 infection, detection of an increase in antibody titer is considered diagnostic of the disease. A horse that shows signs of neurological disease secondary to EHV-1 should have an elevated serum antibody titer, and single high titers have been the basis for initial diagnosis in individual horses. Recent vaccination confounds interpretation. Rarely, high titers may be measured in horses with no history of recent vaccination and no obvious clinical signs of EHV-1 infection.
Antibody titers for Borrelia burgdorferi, the cause of Lyme disease, sometimes are measured in serum from horses with ill-defined gait deficits. High titers, or rising titers, have been used as a basis for treatment of the disease. A positive titer, however, does not mean the horse has active disease. Because of the geographical variation in exposure to B. burgdorferi, titers may vary greatly. Serological surveys in the United States have demonstrated positive test results in 1% of samples from nonendemic areas and up to 68% in endemic areas.9-11 Reports of horses “responding” to treatment exist,12,13 but to date we are unaware of any horses with Lyme disease in which neurological deficits mimic primary lameness. Currently the importance of Lyme disease as a cause of equine gait deficits is unclear.
A Western blot test for the evaluation of equine protozoal myelitis (EPM) was first made commercially available at the University of Kentucky by Dr. David Granstrom.14 Serological testing for the presence of antibodies to Sarcocystis neurona can be used only to indicate exposure to the organism. Through exposure to S. neurona, many horses develop antibodies in the absence of clinical disease Serological surveys in certain areas of the United States have shown that a high percentage of horses have positive antibody titers. A positive test result does not mean the horse has EPM. A negative test result could theoretically occur in horses with peracute disease or perhaps in severely immunocompromised animals. However, a negative test result usually indicates that disease caused by S. neurona is highly unlikely. The test result also could be negative in a horse with signs of EPM if another protozoan, such as Neospora, causes the spinal cord lesions. In a U.S. study of several hundred horses with neurological disease, test sensitivity was 89%, but specificity was only 71%, because 30% of horses with other neurological diseases also had antibodies to S. neurona. Although the positive predictive value was only 72% in horses with neurological diseases, the negative predictive value was almost 90%, indicating that a negative test result is useful in this population.14 In one study of 44 horses on a farm sampled for more than 1 year, all horses were seropositive for at least 50 weeks yet showed no neurological signs (see following discussion).15
Cerebrospinal Fluid Aspiration and Analysis
Cerebrospinal fluid (CSF) can be obtained from either the atlantooccipital or the lumbosacral space. The advantage of lumbosacral centesis is that it can be performed in the standing sedated horse, whereas atlantooccipital centesis requires general anesthesia. Fluid from the atlantooccipital site is considered easier to obtain and not as likely to be contaminated with blood. The atlantooccipital site is identified by palpating the cranial edge of the wings of the atlas. The hair is clipped and the site prepared aseptically. Atlantooccipital centesis is performed at the intersection of the median plane and a line drawn across the cranial edge of the wings of the atlas. In an adult horse, a 9-cm (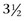 -inch), 18- or 20-gauge spinal needle is directed toward the horse’s lower lip with the head held in a flexed position. It is important that the needle remain on the midline as it is advanced, because otherwise it will be too far lateral to enter the subarachnoid space. The needle is initially inserted to a depth of approximately 2.5 cm (1 inch) and then gradually advanced. While the needle is gradually advanced to the subarachnoid space, it should be held carefully to prevent penetrating the spinal cord when advancing through the atlantooccipital membrane and the dura mater. Usually a “pop” is felt as the needle advances through the dura; however, this finding is not consistent and the stylette should be frequently removed to observe for flow of CSF. CSF usually flows from the needle once the subarachnoid space is entered; however, once a substantial depth has been reached (about 5 to 8 cm [2 to 3 inches] in an average-size horse), some clinicians advise gentle and frequent aspiration with a small syringe.
-inch), 18- or 20-gauge spinal needle is directed toward the horse’s lower lip with the head held in a flexed position. It is important that the needle remain on the midline as it is advanced, because otherwise it will be too far lateral to enter the subarachnoid space. The needle is initially inserted to a depth of approximately 2.5 cm (1 inch) and then gradually advanced. While the needle is gradually advanced to the subarachnoid space, it should be held carefully to prevent penetrating the spinal cord when advancing through the atlantooccipital membrane and the dura mater. Usually a “pop” is felt as the needle advances through the dura; however, this finding is not consistent and the stylette should be frequently removed to observe for flow of CSF. CSF usually flows from the needle once the subarachnoid space is entered; however, once a substantial depth has been reached (about 5 to 8 cm [2 to 3 inches] in an average-size horse), some clinicians advise gentle and frequent aspiration with a small syringe.
In preparation for aspiration from the lumbosacral space the type and degree of restraint is guided by the horse’s behavior, the horse’s stability, and the clinician’s personal preference. A nose twitch, stocks, sedation, or a combination of physical and chemical restraint are options. We prefer to use light sedation with xylazine, sometimes combined with butorphanol. However, lumbosacral CSF pressure can be transiently decreased up to 15 minutes after administration of a high dose of xylazine (1.1 mg/kg intravenously).16 The puncture site for lumbosacral centesis is identified by combining several landmarks, realizing that individual variation exists. A line drawn between the caudal edge of the tubera coxae and the intersection with the midline can be used to locate the lumbosacral space. The lumbosacral space is bordered cranially by the caudal edge of the sixth lumbar vertebra, caudally by the cranial edge of the sacrum, and laterally by the medial rim of the tubera sacrale. The dorsal spinous process of the last lumber vertebra is lower than the dorsal spinous process of the fifth lumbar vertebra. The V formed by the medial rim of the tubera sacrale is one of the more useful landmarks, and the appropriate site for puncture is within this V. The site should be prepared aseptically, and local anesthetic solution is placed subcutaneously. A small skin stab incision is usually made. The needle is inserted on the midline, at the depression palpated just caudal to the last lumbar vertebra, in the middle of the V formed by the tubera sacrale. A 15-cm, 18-gauge spinal needle is generally adequate for a horse that is 16 hands or less. A 20-cm needle may be necessary in a horse greater than 16 to 17 hands. While the clinician advances the needle, it is critical to remain on the midline. The needle can be advanced until a pop indicates it is advancing through the dura or until the horse responds as the needle stimulates nervous tissue. These responses can be unreliable and occasionally dangerous for the horse, the handler, and the individual performing the centesis. Because horses can react unpredictably (including rearing, bolting, collapsing, or kicking), it is safer to advance the needle gradually until it is near the spinal canal, approximately 12.5 cm (5 inches) in a 15- to 16-hand horse. Once the needle is near the canal, it should be advanced slowly with repeated frequent removal of the stylette and aspiration with a small syringe. The horse may move its tail when the dura is penetrated, but usually minimal reaction occurs. If fluid is obtained but the amount is small, the needle can be rotated 180 degrees. Jugular vein compression for at least 10 seconds (Queckenstedt’s test) is thought to elevate intracranial CSF pressure and aid fluid collection, provided flow is not obstructed. If a hemorrhagic sample is thought to be from iatrogenic causes, the syringe can be changed frequently until subsequent aliquots are clear. If fluid is not obtained on the first attempt, the needle is withdrawn and the procedure is repeated slightly cranial or caudal to the original location. CSF samples should be placed in sterile tubes and rapidly processed after collection.
Normal CSF is clear and colorless, and red discoloration indicates hemorrhage. However, normal fluid can sometimes appear mildly hazy when grossly examined, especially in a tube with ethylenediamine tetraacetic acid. Hemorrhage can be iatrogenic or caused by underlying disease. Fluid may appear clear even with red blood cell contamination, and studies indicate that subjective evaluation of spinal fluid is sensitive in detecting blood only when the red blood cells number more than 1200/mcL.17,18 Centrifugation of a bloody sample should produce a clear fluid with a pellet of red blood cells on the bottom of the sample tube. If hemorrhage occurred before collection and lysis of cells occurred, the supernatant may be slightly pink or xanthochromic (orange/yellow or yellow). Lysis of red blood cells reportedly can occur within 1 to 4 hours.19 Xanthochromic CSF results from red blood cell breakdown products (bilirubin) and suggests hemorrhage or vasculitis. A centrifuged xanthochromic sample does not become clear. Turbid CSF may appear with hypercellularity or epidural fat contamination. The latter is not uncommon with lumbosacral aspirates. Formulas used to differentiate between white cell or protein elevations caused by iatrogenic blood contamination of CSF versus pathological increases have been shown to be unreliable. Contamination with a few thousand red blood cells results in minimal increase in white blood cell count or protein content.18
The normal reported range for leukocyte counts has been variable; usually a range of 0 to 6/mcL is cited,20 but higher values have been reported.21,22 Diversity in techniques can account for different values in normal CSF. Undiluted fluid can be assayed in a hemocytometer, or acidified crystal violet can be added to accentuate the cells.20 It is important that equine reference values be determined in the laboratory the practitioner uses. As previously stated, the cell quality rapidly deteriorates in CSF, and samples for cytological testing should be processed rapidly or a portion fixed in 40% ethanol if processing must be delayed. For morphological and differential evaluation, cytocentrifugation or filtration through a glass fiber membrane filter is the preferred method of processing spinal fluid. In our experience, cell and differential counts are often normal in horses with spinal cord disease. Small lymphocytes and monocytes are normally seen. Neutrophils may be seen with blood contamination or inflammation. Eosinophilia is rarely seen in equine CSF but could occur secondary to parasite migration. Rarely, eosinophils have been seen in samples from horses with protozoal encephalomyelitis,21 but frequently spinal fluid from horses with EPM is normal. A relative neutrophilia, with or without an increase in cell count, indicates inflammation, and intracellular bacteria may be seen in horses with bacterial meningitis.
Reported values for protein content of CSF vary considerably among laboratories, probably because of diversity in measurement techniques. A range of 10 to 120 mg/dL is generally acceptable, although some authors consider 100 to 105 mg/dL the high end of normal range.20,23 Protein may increase because of vascular leakage (vasculitis), inflammatory lesions, trauma, iatrogenic blood contamination, or intrathecal globulin production. High-resolution protein electrophoresis of CSF has been reported in a small number of horses, but its value as a diagnostic test remains to be determined. Compared with normal horses (n = 18), horses with cervical cord compression (n = 14) often had a decreased β fraction and post-β peaks.24 However, divergent findings have been reported. Because CK is abundant in neural tissue (and in skeletal tissue and cardiac muscle) and is a large macromolecule that does not cross the blood-brain barrier, measurement was suggested to be a sensitive index of central nervous system lesions. Horses with EPM were reported to frequently have increased CSF CK concentrations, unlike horses with cervical vertebral malformation.25 However, another study showed that the sensitivity and specificity of CSF CK activity are inadequate for diagnostic use. Also, CSF simultaneously collected from the atlantooccipital and lumbosacral sites had disparate values for CK activity, which was not associated with site or other CSF parameters. Contamination of CSF with either epidural fat or dura, which is possible during collection, increases CK activity.26
Albumin is the predominant protein in normal CSF. Elevated albumin concentration can indicate hemorrhage or altered blood-brain barrier integrity. To eliminate serum albumin as a source of increased CSF protein and albumin, the following albumin quotient (AQ) has been suggested27:
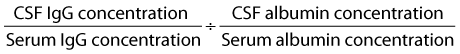
The AQ cited for normal equine CSF is 1.4 ± 0.04,23,27 and it was suggested that an increase above reference range indicated blood contamination during sample collection or compromise of the blood-brain barrier. The immunoglobulin G (IgG) index
was suggested to be useful for differentiating intrathecal IgG production from an increase secondary to blood contamination or increased blood-brain barrier permeability. Normal reference range has been reported to be 0.14 to 0.24.27 However, we do not consider these to be specific, and blood contamination can increase the IgG index without a concomitant change in AQ.18
Although CSF cell count, cytological examination findings, and total protein concentration often do not represent the extent or type of spinal cord or brain tissue disease, when abnormal, the values can be useful. For example, in central nervous system (CNS) disease caused by EHV-1 infection the fluid may be xanthochromic with a high protein level but normal cell count. This disassociation between elevation in protein level and normal cell count may help differentiate EHV-1 infection from EPM. Also, xanthochromic CSF indicates an alteration in the blood-brain barrier and could explain false-positive CSF immunoblot findings for S. neurona in a horse with positive serological test results. Unfortunately, except for EHV-1 infections and meningitis, CSF analysis with currently available tests frequently is not helpful in diagnosing spinal cord disease in horses.
In the United States, the frequency of performing CSF aspiration increased with the introduction of a Western immunoblot test for detecting S. neurona antibodies. Although limited data are available, the specificity and sensitivity of the immunoblot test on CSF from horses with clinical signs consistent with EPM were reported to be approximately 90%.14 However, positive test results have been found in clinically normal horses and in horses with neuropathological lesions other than EPM. Even minute amounts of contamination of CSF with blood can cause the test result to be positive in a horse with high serum antibody levels.19 When CSF was contaminated by even minute amounts of strongly immunoreactive blood (10−3 mcL of blood per milliliter of CSF), the fluid was falsely positive even though the AQ was normal.18 This small amount of blood contamination is grossly undetectable and can correlate with as little as eight red blood cells/mcL of CSF. Also, blood contamination, without increasing the AQ, can increase the IgG index. The IgG index is not specific for intrathecal IgG production. Although the red blood cell count may be a more sensitive indicator of blood contamination than the AQ, it does not correlate with the amount of antibody contamination. Minute amounts of highly immunoreactive blood may have a greater impact on CSF Western blot analysis than a greater amount of contamination with blood with low immunoreactivity.18 Any compromise to the blood-brain barrier regardless of cause allows antibodies to leak into the CSF from the serum, causing a false-positive test result. Although the test for immunoblot S. neurona antibodies was reported to have 85% positive predictive value in a study of horses with neurological disease,14 in the general equine population the test has poor positive predictive value. Many normal horses have positive antibody test results.28 In contrast the negative predictive value for the test is high. With what is currently known, interpretation of positive Western blot results must be made with caution. Negative Western blot test results are generally useful to rule out EPM.
Since the introduction of the original CSF Western blot analysis detecting antibodies to S. neurona, other tests have been developed. These tests include a modified Western blot, an enzyme-linked immunosorbent assay (ELISA), and an immunofluorescent antibody test. Large scale critical evaluation of these tests has not been performed. However early indications suggest that all of these tests may have some limitations. The immunofluorescent antibody test cross-reacts with Sarcocystis fayeri, therefore resulting in false-positive diagnosis of S. neurona infection.29 The ELISA is based on a surface antigen that is missing in some strains of S. neurona; therefore, false-negative results may occur.30 For the reasons discussed previously, both the original and modified Western blot tests may produce false-positive results. In conclusion, antibody testing for S. neurona infection must be used cautiously and in conjunction with other diagnostic tests in attempts to rule out other causes of neurological disease.
Polymerase chain reaction (PCR) testing detects DNA of infectious organisms and has been applied to CSF. Its value in the diagnosis of EPM is controversial, especially when positive results have been reported on CSF samples that were negative for S. neurona antibodies and from horses that did not exhibit overt neurological deficits. We do not find PCR testing for the diagnosis of EPM useful. Use of the PCR technique on CSF has been helpful in diagnosing neuroborreliosis in a horse. Similar to some human cases, the PCR test result was positive yet the CSF had a negative antibody titer.12
Radiography
The use of radiographs in evaluating traumatic or infectious injuries, congenital lesions, and developmental malformations of the spinal column is limited by the size of the horse. Radiographs are useful in diagnosing congenital abnormalities of vertebrae, narrowing of intervertebral disk spaces, stenosis of the cervical spinal canal, osteoarthritic changes, osteomyelitis or osseous cysts, vertebral neoplasia, malalignment, and fractures. However, in most mature horses, except for the cervical spine, general anesthesia may be required for adequate radiographs of the spine. Computed tomography (CT) and magnetic resonance imaging have tremendous potential for evaluating the equine central nervous system but also are limited by the size of the horse. At present, except in foals, CT is available only for evaluating the head and cranial midcervical regions. The primary use of radiology in evaluating horses with neurological disease is localization of cervical vertebral lesions or cervical vertebral malformation and diagnosis of cervical compressive myelopathy or stenotic myelopathy (see Chapter 60).
Survey radiology is useful in the diagnosis of cervical vertebral malformation and cord compression but can be misleading. Standing lateral radiographic images of the cervical vertebrae are routinely evaluated to detect vertebral malformation and to measure spinal canal diameter and can suggest the likelihood of cervical compressive myelopathy.1,31,32 In horses with cervical compressive myelopathy, malformations that characteristically may be identified include flare of the caudal epiphysis of the vertebral body (vertebral endplate modeling), caudal extension of the dorsal laminae, vertebral nonalignment, and osteoarthritis of articular facets. Modeling of the articular processes of the caudal cervical vertebrae is a common malformation identified in horses with cervical compressive myelopathy and in horses that do not have cervical compressive myelopathy. Radiological interpretation of changes is more difficult in older horses, because obvious changes may be seen, without impingement on the spinal canal. Subjective evaluation of articular facet abnormalities can result in a false-positive diagnosis of cervical compressive myelopathy. Identification of characteristic vertebral malformations supports but does not confirm the diagnosis of cervical compressive myelopathy, and subjective radiological evaluation of a malformation does not reliably differentiate between horses with or without cervical compressive myelopathy. Objective assessment of vertebral canal diameter is a more reliable indicator of cervical compressive myelopathy than the subjective evaluation of vertebral malformation. The minimum sagittal diameter (MSD) is the first described method of assessment of canal diameter based on lateral cervical radiographs.31 Determination of canal diameter using the sagittal ratio improves on the original measurements by adjusting for magnification and providing a more accurate adjustment for body size.32 The sagittal ratio measurements were developed using a population of affected (confirmed by myelogram or histopathological studies) versus nonaffected horses.32 The sagittal ratio is determined by dividing the MSD by the width of the corresponding vertebral body. Although a sagittal ratio percent at any cervical vertebra from the third to seventh cervical vertebrae less than 50% is a strong predictor of spinal cord compression, a few horses with no pathological evidence of spinal cord compression have had sagittal ratios of less than 50%. Recently intervertebral measurements of canal diameters were shown to improve diagnosis of cord compression, and addition of intervertebral sagittal ratio measurements was recommended to increase accuracy of plain radiographs.1
A semiquantitative scoring system for evaluating cervical radiographs in horses younger than 1 year of age has been published. This scoring system used neurological examination alone to determine affected versus nonaffected foals and combined subjective determination of radiographic vertebral malformation and objective determination of canal diameter.31 Vertebral canal stenosis is determined by measurement of intervertebral and intravertebral MSD. Dividing the MSD by the length of the vertebral body corrects for magnification. Malformation is determined by the subjective assessment of five categories. The most discriminating factors in the semiquantitative scoring system in differentiating affected from nonaffected foals are canal stenosis and the angle between adjacent vertebrae. The disadvantage of the semiquantitative scoring system is the inclusion of subjective determinations.
Myelographic examination is advised to obtain the best evidence of compression.1,33-35 Myelograms also can demonstrate compression from soft tissue masses, which are not evident radiologically, and suggest transverse compression. However, myelography may not be definitive and occasionally is misleading. A study to evaluate myelography critically and compare the results with necropsy findings in a large number of horses has not been done. A diagnosis of cord compression is assumed if a 50% reduction in the width of the dorsal dye column exists. However, the diagnostic criterion of 50% decrease in width of the dorsal dye column is not well documented34 and has been found in horses with no histological evidence of cord compression at the site of dye column decrease. Iohexol is currently the preferred contrast medium for myelography. It is important that the owner understand the advantages and disadvantages (including risks) of a myelogram before the procedure is undertaken.
Electromyography and Nerve Conduction Studies
Recording electrical activity of muscles can indicate whether evidence of denervation or a myopathy exists, although the distinction is not always clear-cut. Electromyographic examination findings in the early stages of disease or injury may be normal. Certain abnormal patterns can indicate denervation. However, depending on the specific areas to be examined, electromyography may require anesthesia or heavy sedation. It may be helpful in identifying abnormal muscles and indirectly the affected nerves. In a standing, awake horse, spontaneous muscle movement can hinder interpretation.
Values for sensory and motor nerve conduction velocities in horses and ponies were reported.36-39 Differences in speed of conduction occur in different nerves and horses’ sensory nerve conduction velocities are slower than those of ponies.39 However, similar motor nerve conduction velocities were reported for the median and radial nerves of ponies and horses.37 Location of the segment being measured may be important, because distal tapering of nerves may be associated with slower velocity. Skin temperature significantly affects nerve conduction velocity,39 and variability in technique can alter findings. Slower motor nerve conduction velocities were reported in horses older than 18 years of age.36 The procedure usually requires that the horse be anesthetized and, similar to electromyography, should be performed by a skilled person. The technique mainly has been used in research.
Nuclear Scintigraphy
Nuclear scintigraphy has been helpful in identifying lesions in the cervical, thoracic, and lumbar spinal column and pelvic areas not readily evaluated by radiography. It also has been used to evaluate vertebral changes identified radiologically, to determine whether active bone change has occurred. It has revealed hairline fractures and other unsuspected bone lesions in the appendicular skeleton as the cause of gait deficits, which sometimes had been suspected to be caused by spinal cord disease. Scintigraphic imaging from both sides of the horse can differentiate which side may have a lesion. The role of nuclear scintigraphy in diagnosing equine spinal cord disease is limited.
Ultrasonography
Ultrasonography has been used to diagnose aortoiliac thrombosis and to identify soft tissue masses near the spine or deep within muscles. It has also revealed bony proliferation or fractures of the pelvis in horses with obscure gait deficits that were originally suspected to be a result of spinal cord disease.
Specific Diseases and Syndromes
Equine Protozoal Myelitis (EPM)
EPM was first reported in 197440-43 and appeared to be the same condition originally reported as segmental myelitis of unknown cause.44 It is caused by infection with S. neurona. EPM currently appears to be limited to the Western hemisphere. It is particularly of concern in the United States, where in some regions high percentages of horses are infected. The actual number of horses confirmed as having neurological disease from EPM is much lower than the actual number of horses infected, but the disease does have a substantial and serious impact. EPM has not been confirmed in horses younger than 6 months of age, although antibodies were detected in serum from a 2-month-old foal.45 A recent comprehensive review of this disease should be consulted for details.46 Neospora species have been identified as a cause of EPM in horses from the western United States.47-50 CSF testing was positive for S. neurona antibodies by Western blot test, and no antemortem features distinguished Neospora infection from Sarcocystis infection.
The disease caused by S. neurona tends to occur in warm, temperate, nonarid areas with resident opossums. The horse is a “dead-end” host, and the disease is not contagious. The life cycle is not completely understood, although opossums have been identified as the definitive host. The proportion of infected horses that show clinical signs is low. This disease can cause gait deficits affecting one or all limbs and may be difficult or impossible to differentiate from musculoskeletal or other neurological diseases. Signs ascribed to EPM by veterinarians in the United States have been seen in horses in the United Kingdom, where horses have no known exposure to the organism.1 Infected horses and horses with confirmed EPM seen in Europe, Asia, or South Africa have been imported from the Western hemisphere.46 Horses frequently show asymmetrical deficits and may have focal or multifocal muscle atrophy or cranial nerve deficits. Horses may have profound or mild motor or proprioceptive gait deficits, and onset of signs can be acute or chronic, with slow or rapid progression. It may be difficult or impossible to differentiate subtle neurological deficits from those caused by subtle lameness or musculoskeletal pain. Behavior may change. Focal sweating may occur. Diagnosis is based on clinical signs and history, by eliminating other potential causes by radiography and other diagnostic tests, and by testing of serum or CSF for antibodies to S. neurona. No definitive antemortem test exists, although absence of serum antibodies to S. neurona makes it highly unlikely that a horse has EPM. If a horse demonstrates classic signs (e.g., asymmetrical motor deficits and muscle atrophy in the hindlimbs, asymmetrical motor deficits in one or more limbs, a limb deficit combined with cranial nerve deficits not deemed caused by peripheral nerve trauma) and has no other organ dysfunction, we would treat the horse for EPM if it has been in the United States and serological findings are positive. We would forgo CSF testing for reasons outlined earlier.
To date, drugs used to treat EPM have been a combination of trimethoprim-sulfa (sulfadiazine or sulfamethoxazole) and pyrimethamine, or sulfas and pyrimethamine, diclazuril, toltrazuril, and nitazoxanide. Because no definitive antemortem test exists to confirm the disease, evaluation of response to therapy is problematic, especially because the clinical syndrome as treated is so variable and often poorly defined. To date, no treatment trials of experimental infections have been reported. Confounding assessment of drug response is the fact that experimentally infected horses develop clinical signs that decrease over time, despite receiving no treatment.51 Numbers of organisms ingested, virulence factors, and the horse’s own immune status (which depends on heredity, previous exposure to S. neurona, stresses such as transport and parturition, lack of adequate nutrition, and other factors) all presumably can affect development of and recovery from the disease. In the United States the most widely used drug combination is one of the sulfa drugs and pyrimethamine. Because pyrimethamine reaches higher concentrations in the CSF and neural tissue, it is considered superior to trimethoprim. The usual dosage regimen is 20 mg of sulfadiazine per kilogram once or twice daily and 1 mg of pyrimethamine per kilogram once daily, both by mouth for at least 2 to 3 months. Diarrhea occasionally occurs in horses treated with trimethoprim-sulfamethoxazole, and anemia and leukopenia have been observed in some horses receiving 1 mg of pyrimethamine with sulfas per kilogram twice daily. Whether horses require such a prolonged course of treatment or continued high levels of pyrimethamine is unknown. Earlier treatment regimens used a lower dose, but to our knowledge no observations comparing dosages have been reported. A syndrome of bone marrow aplasia and hypoplasia, renal nephrosis or hypoplasia, and epithelial dysplasia was reported in three foals born from mares given sulfonamides, trimethoprim, pyrimethamine, vitamin E, and folic acid during gestation. The authors of that report suggested that administration of the folic acid reduced absorption of active folic acid and, combined with the folic acid inhibitors (trimethoprim and pyrimethamine), induced folic acid deficiency and lesions in the foals.52 We do not routinely add supplements for horses being treated with trimethoprim or pyrimethamine, but if sequential blood tests indicate anemia or leukopenia, the horse should be given folinic acid, a form of bioactive tetrahydrofolate. Folic acid should not be used because it is poorly absorbed in the horse, conversion to its active form is prevented by the dihydrofolate reductase inhibitors pyrimethamine and trimethoprim, and it can competitively decrease absorption of the active form of folic acid.46,52
Diclazuril, a coccidiostat, has anti–S. neurona activity in cell cultures infected with S. neurona53 and has been used to treat horses with suspected EPM.54 It is absorbed quickly after feeding. Dosage and therapeutic efficacy are being evaluated. Toltrazuril, like diclazuril, is a triazine-based anticoccidial drug. Because the drug has good lipid solubility and oral absorption and is absorbed into the CSF, it has potential for treating EPM.55 Ponazuril, a metabolite of toltrazuril, has in vitro activity against S. neurona.56 Ponazuril appeared to have favorable clinical results in a multicenter treatment study.46 The drug has undergone U.S. Food and Drug Administration (FDA) testing, has been approved, and is marketed under the trade name Marquis. Label recommended dosage is 5 mg/kg administered once per day orally. Studies have shown that Marquis has a wide range of safety. The lack of complicating side effects has led to numerous nonlabel dosage regimens. Some of these dosage regimens include double doses for the first 3 to 5 days of therapy, loading doses of 7 times the recommended dose followed by twice the recommended dose for the duration of therapy, and high doses given once weekly or monthly. These nonlabel uses have not been critically evaluated and should be used with caution.
Nitazoxanide kills S. neurona in cell cultures and has been tested in a field trial. Safety studies showed lethargy at twice the recommended dose and illness and death at four times the recommended dose. Gastrointestinal upset can be a complication of nitazoxanide therapy. Concurrent administration of a vegetable oil (corn oil) with nitazoxanide appears to increase small intestinal absorption and reduce gastrointestinal upset. Manufacturers have also recommended starting therapy with a reduced dose for several days. In seven horses with clinical signs compatible with EPM and positive immunoblot results for S. neurona antibodies in the CSF, clinical signs improved in six horses by the end of the trial (85 to 140 days).57 Clinical signs recurred in two horses when treatment was stopped, but signs improved when treatment was reinitiated. Another report described two horses with a diagnosis of EPM that improved after 28 to 42 days of treatment with 50 mg of nitazoxanide per kilogram once daily.58 Anorexia and depression were reported as side effects.58 The CSF remained positive for S. neurona antibodies. Until more information is available about this drug, we do not recommend its use. Although nitazoxanide received FDA approval and was marketed as Navigator, recently the drug was taken off the market presumably as a result of gastrointestinal complications.
To our knowledge, no evidence shows that concurrent use of immune stimulants, oral antioxidants, and antiinflammatory drugs has any beneficial effect. The use of corticosteroids is controversial, because some clinicians claim corticosteroid administration can exacerbate infection. Severity of neurological signs in horses infected with S. neurona reportedly was increased by corticosteroids,59 but in another study of induced disease, signs were less severe in horses given corticosteroids.60
Providing an accurate prognosis is difficult, given the inherent diagnostic problems. Some horses that recover or respond to treatment may not have EPM, and others may recover spontaneously. Economic factors influence duration of treatment and time allowed for convalescence. Even when a severely affected horse improves dramatically, if recovery of function is not complete, a return to previous performance levels is not possible. Signs also may recur in the same horse; whether this is caused by recrudescence of infection or reinfection is unknown. We usually give a guarded prognosis for full recovery of horses showing moderate gait deficits compatible with EPM.
Because the exact life cycle and natural intermediate hosts are unknown, definitive recommendations for control of the disease are difficult. Because the opossum is the definitive host and sheds sporocysts, which the horse ingests, fecal contamination of feedstuffs or water sources by this animal should be prevented. The role of other intermediate mammalian hosts is unclear. The efficacy of a recently introduced vaccine remains to be determined.
Cervical Spinal Cord Compression
Cervical vertebral malformations of various types have been described as the cause of cord compression and neurological signs.1,61,62 Occasionally it may be difficult to decide if a horse is mildly affected by cervical cord compression or is bilaterally lame in the hindlimbs. Mildly affected horses may show only a slightly stiff, stabbing gait at a walk and trot, only mild circumduction of the outside hindlimb when turning, and equivocal hindlimb dysfunction at a canter. Horses with bilateral osteochondrosis dissecans of the hocks or stifles may show similar signs but usually also have joint capsule distention. Thorough lameness and neurological examinations and radiographs are needed. With more severe compression, the gait deficits increase. Circumduction may be severe, and the horse may strike the distal aspect of the limb with the opposite hoof, causing hair loss or wounds from interference. A horse may lose balance or fall, especially when backing up or turning. If the caudal cervical spinal cord is compressed, thoracic limb motor deficits and hypometria, frequently asymmetrical, may occur. The horse may severely scuff or drag its toes and have abnormal hoof wear. Occasionally, substantial bony proliferation at the synovial articular facets can result in neck stiffness and decreased ability to turn in one direction. Cervical muscle atrophy is rare but can occur if the nerves or lower motor neurons are affected. An affected horse usually lacks hindlimb impulsion and may have a somewhat stiff, bouncy canter. The horse frequently is imprecise when stopping, and the hindquarters may sway or bounce. When compression of the cranial cervical spinal cord occurs, the horse may hold its neck and head higher than normal, in an extended position, and in horses with severe clinical signs all limbs may be affected. Signs may occur suddenly or have a more gradual onset, and progression is variable.
Various vertebral abnormalities have been reported in young horses, but clinical signs can be delayed, even when radiographs reveal chronic lesions. We suspect that trauma may cause a preexisting lesion to become clinically relevant. If a horse with vertebral malformation falls, acute spinal cord compression can occur. Acute cervical spinal cord compression caused by trauma can cause tetraparesis or recumbency, but signs may be delayed in the initial stages after injury and may become apparent only when muscle spasms subside, the unstable fracture displaces, or progressive hemorrhaging is present. In the neck the occipitoatlantoaxial and caudal cervical regions are predilection sites for spinal cord injury.1 Synovial cysts may also cause severe sudden signs of spinal cord compression, often asymmetrical and sometimes intermittent.1 The diagnosis of synovial cysts is usually made at necropsy.
Diagnosis of cervical cord compression is based on radiography and myelography. Numerous types of vertebral abnormalities have been described. Management depends on the nature of the lesion, severity of clinical signs, intended use of the horse, and financial considerations. Horses affected by cervical vertebral malformation and cord compression at less than a year of age may improve when exercise and energy intake are restricted.63 Although no controlled studies of a paced diet and restricted exercise program have been conducted, clinical experience supports its use in young horses with radiological evidence of cervical vertebral malformation.1,63 This treatment is not helpful for young horses with very severe stenosis, for defects such as occipitoatlantoaxial or other cranial cervical malformations, or for older horses. Prognosis with conservative management is poor. Surgical fusion of vertebrae is indicated in some horses and has been used successfully.62,64,65 This subject is discussed in Chapter 60.
Equine Degenerative Myeloencephalopathy and Neuroaxonal Dystrophy
Horses mildly affected by equine degenerative myeloencephalopathy and neuroaxonal dystrophy may be misdiagnosed as being lame. Clinical signs may be somewhat similar to those of cervical spinal cord compression. Because no definitive antemortem test exists, clinical diagnosis is based on clinical signs, sometimes supported by the presence of other affected horses on the same farm or in the same family.
Equine degenerative myelopathy is thought to be a vitamin E deficiency, with a likely genetic predisposition.66,67 Neuroaxonal dystrophy appears to have a genetic basis in Morgan horses.68 Various breeds and also Przewalski’s horses and zebras can be affected, and no geographical restriction is apparent. When horses are affected at a young age (i.e., <6 to 12 months old), signs are more severe and progressive than when signs are first noted in horses 2 years old or older. However, because signs can be mild and only slowly progressive, owners may not be aware of the abnormality. When a severely affected horse is identified on an individual farm, other, more mildly affected horses are often found on the same premises or among relatives. Signs tend to be most noticeable in the hindlimbs. Affected horses usually lift the hind feet too high and slap them down on the ground and frequently lift the hoof toward the midline and then place it more laterally. The gait is jerky and asynchronous and sometimes ataxic, with excessive sideways sway of the hindquarters. Interference may occur, with a hind hoof hitting the opposite hind fetlock or pastern region. The horse may have a jerky foot placement when stopping and may pivot on the hindlimbs when turning. Severely affected horses may show forelimb ataxia and weakness of all limbs. The gait lacks impulsion. Occasionally, middle-aged horses are examined because of inability to perform at collected gaits with impulsion and precision. No musculoskeletal cause is found, but the hindlimb gait is characteristic of mild equine degenerative myeloencephalopathy or neuroaxonal dystrophy. Mildly affected mature horses appear to function without substantial progression of signs. No ancillary diagnostic test confirms the disease. Vitamin E supplementation (5000 to 6000 units by mouth daily) has been used to treat affected horses, with some, but not total, improvement reported.69 Horses at risk for the disease should be given vitamin E supplements. Supplementation on farms with a number of affected horses was associated with a subsequent decrease in the incidence of disease.66 Mares and foals should have access to grass pasture, because lack of access to green pasture has been identified as a risk factor.
Equine Lower Motor Neuron Disease
Equine lower motor neuron disease has been diagnosed in many countries.70 Older horses and those lacking access to green pasture appear to be at risk to develop equine lower motor neuron disease. The disease is thought to be caused by deficiency of antioxidant activity in the central nervous system, leading to degeneration and loss of lower motor neurons in the brainstem and spinal cord.71 Affected horses lose muscle mass and have generalized muscle trembling, which may be more severe in the triceps and quadriceps and is exacerbated by transport. Other clinical signs include stiffness, shifting of weight between the hindlimbs, standing with all feet excessively under the body, excessive sweating, holding the tail elevated and trembling, long periods of recumbency, and sometimes excessively low head carriage. Trembling disappears when the horse lies down. Although the gait may be choppy, the horse has no lameness or ataxia. Horses move better than they stand, and therefore the condition is unlikely to be confused with lameness. Muscle atrophy may be profound. Ophthalmoscopic examination of horses with chronic equine lower motor neuron disease may reveal abnormal pigment deposition in the tapetum with a horizontal band of pigment at the tapetal-nontapetal junction.72 Diagnosis is based on clinical signs and low serum vitamin E concentrations in unsupplemented horses or biopsy of the sacrocaudalis dorsalis medialis (dorsolateral coccygeal) muscle. Affected horses that have not been given vitamin E usually have serum vitamin E concentrations less than or equal to 1 mcg/mL.73 Biopsy of a branch of the spinal accessory nerve, which had a high specificity and sensitivity in diagnosis of equine lower motor neuron disease, has been replaced by the muscle biopsy, which is technically much easier, can be performed in the standing horse, and has a similar diagnostic specificity and sensitivity.74 However, false-positive test results can occur in horses that have had “tail blocks.”74 The dorsolateral coccygeal muscle is ideal for biopsy because it contains a high percent of type I oxidative fibers, which are the main muscle fibers affected by the disease. Other muscles with a high proportion of type I fibers are not accessible for biopsy. Most limb muscles have high percentages of type II fibers and are not suitable for diagnosis of the disease.
Oral vitamin E supplementation (6000 to 10,000 units by mouth daily) improves horses, and green pasture is also helpful. However, athletic ability may remain impaired.71
Equine Herpesvirus 1 Infection
Neurological disease caused by EHV-1 can occur in individual horses or as an outbreak. Ataxia is variable but usually symmetrical. The hindlimbs are more severely affected, and recumbency can occur. Signs occur acutely and usually stabilize within 24 to 48 hours. Because this condition is unlikely to be confused with lameness, it is not discussed further and readers are referred to a review.1
Miscellaneous Diseases of the Spinal Cord
Spinal cord disease from migrating parasites could manifest as an asymmetrical gait deficit. Incidence appears to vary geographically, and clinical signs reflect the path of migration. Antemortem diagnosis is usually not possible, although eosinophilia in the CSF supports the diagnosis. Various parasites including Setaria species, Halicephalobus (Micronema) deletrix, Hypoderma, and Strongylus species have been identified. Treatment includes antiparasitic and antiinflammatory drugs.
Vertebral osteomyelitis, neoplasia, and diskospondylitis are rare causes of spinal cord disease. Signs reflect location of the lesion, which may be confirmed by radiography or scintigraphy. CSF may reflect the disease condition if it extends through the dura. Traumatically induced diskospondylitis has been described and may be difficult to differentiate from bacterial diskospondylitis.1,75 Spinal cord traumas may occur directly or from instability of intervertebral joints. External trauma can affect any horse, and clinical signs reflect the site of the lesions. Three predilection sites for injury are the occipitoatlantoaxial region, the caudal cervical region (the fifth cervical to the first thoracic vertebrae) and midthoracolumbar region.1 Clinical signs may initially be mild or peracute, and some horses develop severe progressive signs. It is often not possible to perform an adequate or accurate neurological examination on, or form a prognosis for, an acutely injured horse. Initial treatment includes first aid care, sedation if needed, and the administration of analgesics, antiinflammatory drugs, and mannitol. Radiographs can be useful, depending on site of injury and size of the horse. Repeated neurological evaluations are used for prognosis.
Peripheral Nerve Injuries
Except for stringhalt and radial nerve injury, peripheral nerve diseases affecting the gait are rarely diagnosed. Suprascapular nerve injury by itself does not alter the gait but results in atrophy of the supraspinatus and infraspinatus muscles (Sweeny; see Figure 6-20).76-78 However, injury to the nerve usually occurs with more general trauma to the region such as a collision or fall. This type of injury frequently can lead to damage to other nerves of the limb and soft tissue structures. If other nerve roots of the brachial plexus are simultaneously damaged, the shoulder joint may be unstable and may subluxate laterally. ![]() The horse may circumduct the limb during protraction. Rest and antiinflammatory drugs are usually used. Several surgical procedures have been advocated for suprascapular nerve injury.79
The horse may circumduct the limb during protraction. Rest and antiinflammatory drugs are usually used. Several surgical procedures have been advocated for suprascapular nerve injury.79
Radial nerve paresis or paralysis is recognized, usually secondary to trauma. Horses with radial nerve paralysis cannot flex the shoulder joint or extend the elbow, knee, fetlock, or interphalangeal joints (see Figure 5-14). The dorsum of the toe rests on the ground, and the elbow is dropped. Severely affected horses have difficulty rising and often collapse on the limb if it bears weight. More mildly affected horses may advance the leg by flinging or jerking it forward from the shoulder. Evaluation of skin sensation may not be helpful. Atrophy of the triceps and other limb extensor muscles occurs after 2 weeks, and denervation potentials can be found on electromyographic examination 3 to 4 weeks, or sooner, after radial nerve injury.79,80 Because of the difficulty of knowing whether the gait deficits result solely from radial nerve injury or muscle damage, an accurate prognosis can be difficult in horses with acute clinical signs. Signs of radial paralysis occurring after recumbency or general anesthesia are probably caused by ischemic myopathy, with possible ischemic neuropraxia, and these horses generally recover. Prognosis depends on the cause and extent of radial nerve injury, neither of which may be identified. Prognosis is obviously better in horses that are less severely affected and those that show early signs of improvement. However, some severely affected horses completely recover. Prognosis is worse if rapid severe atrophy of extensor muscle occurs. Signs of radial nerve dysfunction may be associated with trauma to caudal cervical or cranial thoracic nerve roots secondary to an injury to the head of a rib; other nerves and muscles may also be affected. Signs of radial nerve dysfunction can also occur from lesions in the caudal cervical and cranial thoracic ventral gray matter, but other signs of spinal cord disease usually coexist, especially in EPM. Physical therapy, including splinting to avoid flexural deformity, is very important, and electrical stimulation of muscles may also help prevent atrophy. Irreversible fibrosis and contracture are likely without intervention.
Lesions in the nerves supplying the flexor muscles of the thoracic limb are extremely rare, although signs of dysfunction can accompany brachial plexus or spinal cord lesions.77 If the ulnar nerve is sectioned, the horse may move its foot in a jerking fashion with decreased flexion of the fetlock and carpal joints. When the median nerve is cut, the horse drags the toe because of decreased flexion of the fetlock and carpus. Hypalgesia of the medial aspect of the pastern occurs, whereas with ulnar neurectomy, hypalgesia of the lateral metacarpal region occurs.77,79 After neurectomy of the proximal musculocutaneous nerve, the horse drags its toe because of decreased elbow flexion. Because natural disease syndromes affecting these nerves are not described, prognosis is difficult because gait deficits improve with time after neurectomy.79
If the femoral nerve is damaged, the horse cannot extend its stifle and rests the leg in a flexed position. The hip is lower than the opposite limb, and the horse cannot support weight normally or at all when walking. When both limbs are affected, the horse will appear crouched and have great difficulty rising. The patellar reflex is absent or depressed, and with time the quadriceps muscles atrophy. Damage to the nerve has occurred during general anesthesia with horses positioned in dorsal or lateral recumbency (usually with the affected limb having been positioned uppermost) or after overextension of the limb, after pelvic or femoral fractures, or in association with space-occupying masses impinging on the nerve.79,81,82 Lesions in the spinal cord ventral gray matter or nerve roots at the L5 or L6 lumbar vertebra can also cause signs of femoral nerve paralysis. Horses with patellar fractures and subsequent inability or reluctance to bear weight on the affected hindlimb may mimic those with femoral nerve injury.82 Complete neurological evaluation to detect other deficits may be difficult if signs of femoral nerve paralysis are severe. Because the condition is rare and rhabdomyolysis and postoperative myopathy can mimic the signs of femoral nerve damage, giving a prognosis is difficult. Antiinflammatory drugs are usually used. Most horses with postanesthetic femoral nerve paresis make a complete recovery.82
Signs of paresis or paralysis of the sciatic nerve can occur in horses with pelvic fractures, with deep muscle injections in foals, or with spinal cord lesions affecting the ventral gray matter or nerve roots of the fifth lumbar to third sacral nerves. Signs reflect flexor muscle weakness. The horse can support weight on the limb if the hoof is placed flat on the ground under the pelvis. Otherwise, the horse stands with the hock and stifle extended and the dorsum of the hoof on the ground behind it. When the horse walks, it drags or jerks the limb forward. With time all muscles distal to the stifle and those of the caudal aspect of the thigh atrophy. The prognosis is very poor if the nerve is severed. If the fibular (peroneal) nerve is damaged (usually because of blunt trauma), the horse cannot extend the fetlock and interphalangeal joints or flex the tarsus normally. At rest it stands with the hoof behind it, resting on its dorsal surface. If the hoof is placed flat on the ground under the horse, the horse can support weight. When the horse moves, it drags the foot cranially, then jerks it caudally, sliding it on the ground. Skin sensation is decreased over the dorsal and lateral aspects of the tarsus and metatarsal region. With time, muscle atrophy in the craniolateral aspect of the crus can occur. Treatment involves support and protection of the distal limb. Electrical stimulation of muscles might help prevent muscle atrophy. Many horses recover with time. Gait had returned to virtually normal within 3 months of experimental transection of the fibular nerve.79 Tibial nerve injury is uncommonly diagnosed, but a stringhalt-like gait has been described. When walking, the horse overflexes the limb and drops the foot straight to the ground when it reaches the end of the cranial phase of the stride. The gastrocnemius muscle reportedly atrophies. The horse stands with the fetlock flexed or partly knuckled, the tarsus flexed, and the hip lower than that of the unaffected leg.81 Obturator nerve damage, which can occur after foaling, results in signs varying from abduction or circumduction and stiffness of the affected limb when walking to paraplegia. Prognosis depends on severity of signs, whether both limbs are affected, and whether adequate supportive care can be provided.
Stringhalt is easily recognized from its exaggerated flexion of the hock, which can result in a bizarre hopping, jerking, and propulsive gait when both hindlimbs are affected (see Chapter 48). ![]() Horses may be so severely affected that they “freeze” in the abnormal position or are very reluctant or unable to move, particularly in those with bilateral stringhalt. They may strike the ventral abdomen with the hoof. The gait usually is worse when the horse is walking on a hard surface and when it is anxious or frightened. Horses with mild signs may show the exaggerated hock flexion only when backing up or turning or during the first few strides after walking from a standstill. Atrophy of the distal limb muscles may occur in horses with chronic stringhalt. Spasticity, toe scuffing, and stumbling of the thoracic limbs and left laryngeal hemiplegia have been described in some affected horses.83,84 A distal axonopathy of peripheral nerves has been described.85,86 The condition can be sporadic or occur in outbreaks. The cause frequently is unknown, especially when only one horse is affected. Outbreaks have been associated with particular pastures, and mycotoxins are a suspected cause.83,84 Lathyrism can also be a cause. Phenytoin and baclofen have been used with some success to decrease clinical signs.87,88 Tenectomy of the lateral digital tendon has also been used. The course is variable, and some horses recover spontaneously. However, because it is difficult to predict which horses will recover, the prognosis is guarded, especially in horses with severe clinical signs or in single horses unassociated with a pasture outbreak.
Horses may be so severely affected that they “freeze” in the abnormal position or are very reluctant or unable to move, particularly in those with bilateral stringhalt. They may strike the ventral abdomen with the hoof. The gait usually is worse when the horse is walking on a hard surface and when it is anxious or frightened. Horses with mild signs may show the exaggerated hock flexion only when backing up or turning or during the first few strides after walking from a standstill. Atrophy of the distal limb muscles may occur in horses with chronic stringhalt. Spasticity, toe scuffing, and stumbling of the thoracic limbs and left laryngeal hemiplegia have been described in some affected horses.83,84 A distal axonopathy of peripheral nerves has been described.85,86 The condition can be sporadic or occur in outbreaks. The cause frequently is unknown, especially when only one horse is affected. Outbreaks have been associated with particular pastures, and mycotoxins are a suspected cause.83,84 Lathyrism can also be a cause. Phenytoin and baclofen have been used with some success to decrease clinical signs.87,88 Tenectomy of the lateral digital tendon has also been used. The course is variable, and some horses recover spontaneously. However, because it is difficult to predict which horses will recover, the prognosis is guarded, especially in horses with severe clinical signs or in single horses unassociated with a pasture outbreak.
Shivers is somewhat similar to stringhalt, and the origin and pathogenesis are unknown (see Chapter 48). Affected horses tremble one or both pelvic limbs, primarily when backing up or lifting a hoof, and they elevate the tail.![]() Some affected horses cannot stand to have the hooves trimmed, even though the hindlimb gaits are relatively normal and otherwise functional. The clinical course seems variable, and the disease is thought to be progressive, at least in draft breeds.79 However, we have seen affected horses remain relatively static and functional, although hoof care can be difficult because of the inability to stand for the farrier. A group of horses exists with mild hindlimb deficits resembling a combination of stringhalt and shivers. Although these horses may continue to be functional for riding, the gait disability impairs dressage performance. The cause usually is undiagnosed.
Some affected horses cannot stand to have the hooves trimmed, even though the hindlimb gaits are relatively normal and otherwise functional. The clinical course seems variable, and the disease is thought to be progressive, at least in draft breeds.79 However, we have seen affected horses remain relatively static and functional, although hoof care can be difficult because of the inability to stand for the farrier. A group of horses exists with mild hindlimb deficits resembling a combination of stringhalt and shivers. Although these horses may continue to be functional for riding, the gait disability impairs dressage performance. The cause usually is undiagnosed.