Chapter 84Principles and Practices of Joint Disease Treatment
The joint is an organ, and there are a number of ways in which traumatic damage occurs to it, ultimately resulting in degradation of articular cartilage. In 1966, the concurrent damage to joint capsule and ligament attachments that accompanied osteochondral fragmentation and cartilage damage was described.1 However, the fact that synovitis and capsulitis could cause degradation of articular cartilage was not known until later. Experimentally, cartilage morphological damage and loss of glycosaminoglycan (GAG) staining occurred in the absence of instability or traumatic disruption of joint soft tissues.2 Surveys estimate that approximately 60% of lameness is related to osteoarthritis (OA).3,4 Rapid resolution of synovitis and capsulitis is critical in management of OA because synovitis induces cartilage matrix degradation. The goals of treatment of traumatic joint injuries are twofold: to return the joint to normal as quickly as possible and to prevent occurrence or to reduce the severity of OA, thus to minimize lameness and joint deterioration. Medical management is largely used to minimize OA, but timely surgery to remove osteochondral fragments, to reduce and repair large intraarticular fractures in an appropriate fashion, to diagnose accurately ligamentous and meniscal injuries using arthroscopy, and to manage manifestations of osteochondrosis can be critical to prevent OA. This chapter addresses both medical and surgical management.
Medical Treatment
The goal to manage acute synovitis, with or without capsulitis, is to return the joint to normal as quickly as possible. In addition to reducing lameness and returning the horse to work, suppression of synovitis and capsulitis is important to prevent the byproducts of inflammation from compromising articular cartilage and causing OA. Pain relief and minimizing the potential microinstability associated with excessive synovial effusion are both also critical. As information increases regarding potential targets for therapeutic intervention, the range of treatment options has increased. Medications that provide pain relief, but for which any therapeutic action at the level of cartilage matrix has not been defined, are termed symptom-modifying osteoarthritic drugs (SMOADs). Disease-modifying osteoarthritic drugs (DMOADs) are those agents that can positively influence either the articular cartilage or the synovial environment. DMOADs were previously called chondroprotective drugs.
Nonsteroidal Antiinflammatory Drugs
Nonsteroidal antiinflammatory drugs (NSAIDs) are antiinflammatory agents that inhibit some components of the enzyme system that converts arachidonic acid into prostaglandins and thromboxane. Prostaglandin (PG) E2 (PGE2) is the product associated with synovial inflammation and cartilage matrix depletion and was demonstrated in the synovial fluid of horses with OA.5,6 Phenylbutazone is the most commonly used NSAID in the horse, given at a dose of 2.2 mg/kg, once or twice daily. However, results are variable both in horses with naturally occurring OA7 and in experimental trials using an equine OA model developed at Colorado State University (CSU), which has been used to assess a number of commonly used medications.8 Lameness scores were lowest after administration of the combination of phenylbutazone with flunixin meglumine compared with phenylbutazone alone, but concerns with secondary side effects (including acute necrotizing colitis) were raised.8
All NSAIDs inhibit cyclooxygenase (COX) activity to some degree,5,6 but more recently two different isoenzymes called COX-l and COX-2 were reported with potential importance in the horse. COX-l maintains the “good” or “housekeeping” portion (constitutive part) of the COX pathway.9 COX-1 is important in the balance of normal physiology of the gastrointestinal and renal systems but plays a lesser role in the inflammatory COX cascade. COX-2 is associated with inflammatory events, especially those driven by macrophages and synovial cells, plays a minor role in normal physiology, and is considered the “bad” or “inducible” portion of the COX pathway. Drugs that preferentially inhibit COX-2 have been developed. Although it is logical that inhibition should minimize side effects, there is some suggestion that complete inhibition of COX-2 may not be optimal for either the joint or the horse.9-11 Whereas COX-l is mainly responsible for the protective functioning of prostaglandins, COX-2 may play an accessory constitutive role in the COX pathway.
The mainstream view is still that the beneficial effects of selective COX-2 inhibition in OA are ideal. Anecdotally, carprofen (Rimadyl, Pfizer, New York, New York, United States) was used at CSU in horses that developed high serum creatinine levels and diarrhea in association with phenylbutazone use. These side effects disappeared when horses were given carprofen; a protective effect was seen, implying there may be more preferential COX-2 inhibition than with phenylbutazone therapy. Firocoxib, a member of the class of drugs that selectively inhibits the COX-2 isoenzyme, is now approved for use in horses to control pain and inflammation associated with OA in general, and its pharmacokinetics during prolonged use have been determined.12
Although prostaglandin inhibition provides effective symptomatic relief, there may be long-term deleterious effects of some NSAIDs on cartilage metabolism.11 In vitro work in the horse had initially shown no evidence of deleterious effects on cartilage metabolism.13 However, in a more recent study, phenylbutazone was given to horses for 14 days, and serum was tested on articular cartilage explants in vitro. There was decreased proteoglycan synthesis to a degree similar to that with recombinant human interleukin–1β.14 However, in the absence of any clinical associations between phenylbutazone use and articular cartilage degeneration, continued appropriate use of NSAIDs is justified.
A new, licensed topical NSAID preparation, 1% diclofenac sodium cream (Surpass, IDEXX Laboratories, Greensboro, North Carolina, United States), is now available in the United States but not in Europe. Previous research in people indicated topical NSAID could be clinically beneficial while reducing systemic side effects. Antiinflammatory effects were demonstrated experimentally using an induced subcutaneous inflammation model.15 A clinical field trial of the topically applied diclofenac liposomal cream for the relief of joint inflammation showed promising results,16 and more recently its value was demonstrated in equine OA using an osteochondral fragment–exercise model (see the section on Intraarticular Corticosteroids).8
Intraarticular Corticosteroids
The use of intraarticular corticosteroids for treatment of equine OA was extensively reviewed in 1996, and the benefits and deleterious side effects were more recently clarified.17 Based on my observation of an apparent lack of correlation between the previous use of betamethasone esters (Betavet Soluspan, Schering-Plough Animal Health Corp., Union, New Jersey, United States) and articular cartilage degradation during arthroscopic surgery for osteochondral chip removal, experimental studies were initiated for the three most commonly used intraarticular corticosteroids. Methylprednisolone acetate (MPA) (Depo-Medrol, Pharmacia and Upjohn Co., Kalamazoo, Michigan, United States), triamcinolone acetonide (TA) (Vetalog, Bristol Myers Squibb for Fort Dodge, Fort Dodge, Iowa, United States), and betamethasone esters (Betavet Soluspan) were evaluated using an osteochondral fragment–exercise model.18-20 Betamethasone (Betavet Soluspan, now available as Celestone Soluspan) was tested first. Osteochondral fragments were created arthroscopically on the distal dorsal aspect of the radial carpal bone in both middle carpal joints in 12 horses, and one joint was treated with 15 mg of betamethasone at 14 and 35 days after surgery.18 The contralateral control middle carpal joint was injected with saline. No deleterious side effects on articular cartilage were demonstrated. Exercise produced no harmful effects in the presence of betamethasone.18
In subsequent studies with intraarticular corticosteroids (as well as other treatments), the research model was modified so that the contralateral joint was not used as a control. The chip fragment model was also modified to more effectively produce early osteoarthritic change. Eighteen horses were randomly assigned to three groups: MPA or TA was injected 14 and 28 days after surgery, and horses were exercised on a high-speed treadmill for 6 weeks, beginning on day 15 after surgery. Results were compared with control joints treated with corticosteroids but which had no osteochondral fragment, as well as with a second control osteochondral fragment group treated with polyionic fluid (Figure 84-1).19,20 In joints containing an osteochondral fragment and treated with MPA, there was a trend (not statistically significant) for lower lameness scores. However, there were significantly lower synovial fluid PGE2 concentrations and lower scores for intimal hyperplasia and vascularity (no effect on cellular infiltration in the synovium compared with placebo-treated joints) in MPA-treated joints compared with control joints. Of more importance, modified Mankin scores (a score of histopathological change in articular cartilage) were significantly increased in MPA-treated joints compared with control joints, suggesting deleterious effects of intraarticular administration of MPA.19 This is in contrast to the results with TA (Vetalog).20 Horses that were given 12 mg of TA in a joint containing a fragment (TA TX) were less lame than horses in the two control groups (see Figure 84-1). Horses treated with TA had lower protein and higher hyaluronan (HA) and GAG concentrations in synovial fluid. Synovium from treated groups had less inflammatory cell infiltration, intimal hyperplasia, and subintimal fibrosis. Analysis of articular cartilage morphological parameters evaluated using a standardized scoring system was significantly better from TA control (no fragment) and TA treatment groups compared with the control placebo-treated fragment group. The results supported favorable effects of TA on lameness scores, synovial fluid, synovium, and articular cartilage morphological parameters, both with direct intraarticular administration and remote site administration compared with placebo injections.20 Evaluation of intraarticular TA on subchondral bone showed no deleterious effects.21 In other work, repetitive intraarticular administration of MPA to exercising horses altered mechanical integrity of articular cartilage but had no effect on subchondral or cancellous bone.23
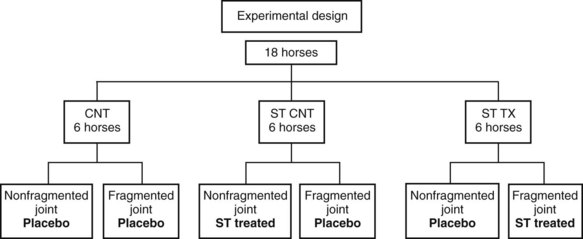
Fig. 84-1 Design of experiments to assess the value of direct intraarticular injection of a corticosteroid into an osteoarthritic joint (ST TX), as well as injection of intraarticular corticosteroid in a remote joint (ST CNT) compared with a saline-injected control (CNT).
(Reproduced with permission. McIlwraith CW: From arthroscopy to gene therapy—30 years of looking in joints, Proc Am Assoc Equine Pract 51:65, 2005.)
Based on these and recent in vitro results demonstrating a protective effect of TA,24 I recommend TA be used especially in high motion joints. Some have recommended low doses of MPA to alleviate potential negative effects. However, based on in vitro titration studies, commonly used “low doses” are unlikely to have the same clinical effects because a greater concentration of corticosteroid is needed to inhibit the catabolic compared with the anabolic effects on articular cartilage.25 On the other hand, clinical improvement is seen in horses administered low doses, an observation that is more important to a clinician than are experimental data.
Despite scientific studies demonstrating the efficacy and chondroprotective properties of TA, some practitioners fear the potential of TA to cause laminitis. Laminitis did not occur in 1200 horses treated with TA when the total dose did not exceed 18 mg.26 From this study, 18 mg was established as a maximum dose. A more recent study reported no association between the occurrence of laminitis and the intraarticular use of TA.27 There was a recent legal case in the United Kingdom in which a horse developed catastrophic laminitis after receiving 8 mg of TA in each tarsus and 20 mg of dexamethasone into its back.28 This led to a review of the literature, which revealed that there was a lack of good evidence linking laminitis to corticosteroid injection; it was suggested that a large-scale multicenter trial was needed.29 A related retrospective study from one clinician29,30 revealed that laminitis associated with intraarticular injection of corticosteroids had occurred in 3 of 2000 (0.15%) horses. TA was used the majority of the time, and the upper total dose ranged from 20 to 45 mg.30 The relationship between corticosteroid use and laminitis is discussed further in Chapter 34.
Another traditional cliché is that although it is better not to use MPA in high-motion joints, using it in low-motion joints (such as the distal tarsal joints) is appropriate. This implies that we do not care about the state of the articular cartilage in these joints, and perhaps corticosteroids may promote ankylosis. There is currently no evidence that ankylosis can be promoted in this fashion. The other side of this argument is that we should preserve articular cartilage whenever we can. Intraarticular injection of MPA or TA (with or without hyaluronan [HA]) in horses with OA of the distal hock joints led to a positive outcome in only 38% of horses (suggesting to the authors that surgical treatment may lead to better long-term prognosis).31 There was no significant difference between treatment with either MPA or TA, thus questioning any clinical advantages of the use of MPA.31 However, this was a relatively small study performed on a referral population of horses, and these results may not be representative of the overall response of horses with distal hock OA to intraarticular medication.
Intraarticular corticosteroids are commonly combined with HA, and there is a perception that the HA might be protective against any deleterious effects of corticosteroids (MPA). This perception is based on tradition rather than scientific proof but has become common thinking among equine practitioners.3 Some support can be gained from a 1-year, single-blind, randomized study in which 24 human patients were treated with intraarticular HA once weekly for 3 weeks and then again at 6 months (total of six injections).32 Sixteen of these patients also had TA before the first and fourth HA injections, and using the Western Ontario and McMaster Universities Index of OA (WOMAC) scores, the results were better with the combination of these two products. There was no progression of OA as evaluated using magnetic resonance imaging in either group.32 Two in vitro equine studies evaluated whether HA might have a mitigating effect against the deleterious effects of MPA. In the first study, HA addition had little effect on MPA-induced cartilage matrix proteoglycan catabolism in cartilage explants.33 In the second study, MPA combined with HA had beneficial effects on proteoglycan metabolism in interleukin-1 (IL-1)–treated equine chondrocytes (but there were no comparisons between HA alone and MPA plus HA).34 The combination of MPA and HA increased PG synthesis compared with IL-1–treated controls.34
Hyaluronan (Sodium Hyaluronate)
HA is a nonsulfated GAG, and the biological characteristics and therapeutic use of HA in equine OA were reviewed previously.35,36 HA has modest analgesic effects,37 but more emphasis is placed on its antiinflammatory effects that may be physical (steric hindrance) or pharmacological (inhibition of inflammatory cells and mediators).36 Various in vitro studies have shown HA protects against IL-1–driven prostaglandin synthesis and inhibits free radicals, but the ability of HA to inhibit matrix metalloproteinase (MMP) activity is questionable.38,39 Several inflammatory mediators can augment HA production by synovial fibroblasts in vitro; therefore elevated synthesis of HA in early OA may constitute a protective response by the synovium to joint inflammation.36 While providing a rationale for exogenous administration, it may explain the elevated levels of HA in response to intraarticular injection of a number of medications.19,20
In my opinion, HA alone is useful in horses with mild to moderate synovitis, but the adjunctive use of corticosteroids is necessary in most horses with OA. However, based on clinical evidence in people, although the immediate effects may not be dramatic, the evidence for long-term disease-modifying activity of HA is accumulating.40 Claims that HA preparations of molecular weight exceeding 1 × 106 Da may provide superior clinical results and chondroprotection than lesser-molecular-weight products remain controversial.36,41,42
In a randomized, double-blind, and placebo-controlled clinical study, 77 Standardbred trotters with moderate to severe lameness were treated with HA, polysulfated glycosaminoglycan (PSGAG), or placebo for 3 weeks. Mean initial lameness score was significantly reduced during treatment and at the last examination in all three groups (P < 0.01).43 Additionally, the prevalence of sound horses increased significantly from 1 to 3 weeks of treatment into the last examination in all three groups. Both drugs (250 mg of PSGAG intraarticularly four times or 20 mg of HA intraarticularly twice) were superior to placebo for reduction of lameness score during treatment and the total study period, time until soundness occurred, and the number of sound horses at the last examination. Thus placebo and drug therapy were effective in the treatment of naturally occurring traumatic arthritis in horses, but HA and PSGAG gave better results than placebo. In a second study, the researchers compared intraarticular saline with rest alone in 38 Standardbreds with traumatic arthritis.44 The mean lameness was significantly lower when 2.0 mL of 0.9% NaCl solution was injected compared with control horses.44 This raises the question: is this effect the result of withdrawing fluid and/or placing a needle in the joint?
Most recently, intraarticular HA was tested in the CSU equine OA model.45 OA was induced by the osteochondral fragment–exercise model in one middle carpal joint of 24 horses. Eight horses received HA (20 mg) (Hyvisc, Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany) and amikacin (125 mg) intraarticularly on study days 14, 21, and 28. A second group of eight horses received PSGAG (250 mg) and amikacin (125 mg) intraarticularly on study days 14, 21, and 28. The remaining eight horses were control horses. There were no adverse treatment-related problems. Synovial effusion was reduced with PSGAG compared with controls. No changes in other clinical signs (lameness, response to flexion, joint effusion, and radiological findings) were seen with PSGAG or HA compared with controls. Histologically, however, there was significantly less articular cartilage fibrillation seen with HA treatment compared with controls (despite no significant reduction in vascularity and subintimal fibrosis of the synovium). The conclusion was that HA had beneficial disease-modifying effects and was a viable therapeutic option in equine OA.45 The result of a questionnaire survey of 20 members of the American Association of Equine Practitioners (14 responses) showed that it was uncommon for the respondents to administer intraarticular HA initially or alone, particularly in horses with established OA.3 Twelve of 14 supplemented HA injections with other forms of treatment (usually intraarticular corticosteroids).3
The use of intravenous HA (Legend [or Hyonate], Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, Kansas, United States) in the treatment of OA is now common. Using intravenous HA in the experimental osteochondral fragment–exercise model, there was significant improvement in clinical lameness, decreased PGE2, and total protein levels in the synovial fluid, as well as decreased hyperemia and cellular infiltration of the synovium.46 However, in a survey, most clinicians were not impressed by the efficacy of intravenous HA and its short duration of action in the treatment of OA, particularly when used alone.3
Based on the reports from the manufacturing company, the majority of intravenous HA is used “prophylactically” in athletic horses with the attributed benefits being subjective. The prophylactic value of intravenous HA was studied in both Quarter Horse and Thoroughbred racehorses. One hundred and forty horses participated in the Quarter Horse study and received either intravenous saline or HA every 2 weeks for the duration of the 9-month study.47 Trends for HA-treated horses to race longer, require an intraarticular injection of corticosteroid earlier, have a better speed index, have a higher average number of starts, and earn more money were observed when compared with placebo-treated horses. The Editors note that the better performance results could be due to earlier corticosteroid treatment in the HA-treated compared with placebo-treated horses. A second unpublished study was conducted in Thoroughbred racehorses using synovial fluid markers and starting with horses without musculoskeletal problems. No significant differences were found between HA- and placebo-treated horses. However, there are anecdotal positive reports regarding the prophylactic use of intravenous HA from trainers in various equine disciplines.
Polysulfated Glycosaminoglycan
PSGAG belongs to a group of polysulfated polysaccharides and includes, in addition to PSGAG (Adequan, Luitpold Pharmaceuticals Inc., Animal Health Division, Shirley, New York, United States), pentosan polysulfate. These drugs are DMOADs, and therefore PSGAG has been traditionally used when cartilage damage is presumed present rather than in the treatment of acute synovitis.48 However, recent work questions this traditional approach (see below). Use of DMOADs is meant to prevent, retard, or reverse the morphological cartilaginous lesions of OA, with the major criterion for inclusion being prevention of cartilage degeneration. The principal GAG in PSGAG is chondroitin sulfate (CS), and the product is made from an extract of bovine lung and trachea modified by sulfate esterification.
Adequan was reviewed extensively in 1996.48 One in vitro study demonstrated that PSGAG was the only drug tested (others included phenylbutazone, flunixin meglumine, betamethasone, and HA) that inhibited stromelysin.49 There have been three other in vitro studies on the effect of PSGAG on equine cartilage that had contradictory results. PSGAG caused increased collagen and GAG synthesis in both articular cartilage explants and cell cultures from normal and osteoarthritic equine articular cartilage.50 However, other work found a dose-dependent inhibition of proteoglycan synthesis, little effect on proteoglycan degradation, and no effect on proteoglycan monomer size.51 Various studies have supported the value of intraarticular PSGAG (250 mg) in equine OA, including a clinical study,52 a study using a Freund’s adjuvant-induced OA model,53 and a carpal synovitis model using sodium monoiodoacetate.54 In the latter study, there was significant reduction of articular cartilage fibrillation erosion, less chondrocyte death, and markedly improved GAG staining.54 However, PSGAG had no benefit in healing preexisting articular cartilage lesions in the latter study54 or in a different study in ponies.55
I have traditionally recommended the use of intraarticular PSGAG after arthroscopic surgery when there is substantial loss of articular cartilage (most commonly in the carpus). I observed rapid resolution of synovitis and hemarthrosis after PSGAG administration that otherwise tended to be persistent after arthroscopy when there was secondary loss of articular cartilage. A recent study using the CSU equine osteochondral fragment–exercise model compared intraarticular PSGAG with either intraarticular HA or saline and revealed that synovial fluid effusion was significantly reduced with PSGAG compared with both saline and HA. The degree of vascularity and subintimal fibrosis of the synovium was significantly reduced with PSGAG treatment compared with controls.45 The main value of intraarticular PSGAG appears to be for severe (and acute) synovitis (most commonly seen after arthroscopic surgery when there is considerable debridement of bone). However, the U.K. data sheet expressly states, “Do not inject into actively inflamed joints. In the presence of active joint inflammation, therapy with a suitable antiinflammatory drug should be given prior to intraarticular treatment with Adequan.”
Administration of PSGAG intramuscularly has become popular. However, intramuscular PSGAG (500 mg every 4 days for seven treatments) produced relatively insignificant effects in horses with sodium monoiodoacetate–induced synovitis (limited to slightly improved GAG staining).56 In a more recent study using the osteochondral fragment–exercise model in which intramuscular PSGAG was used as a positive control (administered every 4th day for 28 days starting 14 days post-OA induction), decreased GAG levels in the serum 14 days posttreatment was the only significant beneficial effect.57 In this study, better improvement was seen in horses given extracorporeal shock wave therapy.
In a 1996 survey, PSGAG was considered more effective than HA for the treatment of subacute OA and less effective for idiopathic joint effusion and acute synovitis58; however, there is currently only weak evidence to justify intramuscular administration. It was reported that articular cartilage concentrations of PSGAG after intramuscular administration are capable of inhibiting some cartilage degrading enzymes,59 but the duration of effective concentration is unclear. A number of articular degradative enzymes were reduced in an in vitro study in other animals, but direct evidence of effectiveness in the horse is lacking.59,60
In the questionnaire survey of veterinarians cited previously, indications for use of intramuscular PSGAG varied widely, including acute and/or chronic OA.3 PSGAG was also used as a preventative measure, and information from the manufacturer reports that 90% of sales are for such “prophylactic” use. There have been no scientific studies on prophylactic use, and efficacy is difficult to prove or disprove.
A principal driving force for the persistent use of intramuscular PSGAG in preference to intraarticular administration is the work demonstrating a slightly increased risk of infection following intraarticular injection compared with corticosteroids and HA.61 However, a companion study found that all risks could be obviated with concurrent intraarticular injection of amikacin sulfate, 125 mg (0.5 mL).62 In a survey of 20 practitioners, it was reported that six of seven racehorse veterinarians used intraarticularly administered PSGAG, at least occasionally, whereas a similar number of nonracehorse veterinarians avoided the practice.3 Intraarticular use of PSGAG is still common in Europe. A recent multivariable analysis of factors influencing the outcome of two treatment protocols in 128 horses responding positively to intraarticular analgesia of the distal interphalangeal joint showed significant positive effects with an intraarticular PSGAG therapy protocol of three intraarticular injections approximately 8 days apart.63 Antimicrobial drugs were not administered, and no adverse results were reported.
Pentosan Polysulfate
The use of pentosan polysulfate (PPS) was reviewed in 1996, but at that time reports of its efficacy were only anecdotal.64 PPS is a heparinoid compound but is unique because it is derived from beechwood hemicellulose instead of animal sources. Commercial products available include Cartrophen Vet (Biopharm, Australia Pty Ltd, Bondi Junction, NSW, Australia) (licensed for use in small animals in Australasia and Europe, but not in horses) and more recently Pentosan Equine Injection (Nature Vet Pty Ltd., Glenorie, NSW, Australia; 250 mg/mL PPS sodium), which is licensed in Australasia. In experimental studies in sheep, weekly intraarticular injections of PPS for 4 weeks improved joint function and reduced mean radiological scores and Mankin histological scores of articular cartilage damage in femoral condyles.65
Recently, we found favorable results using PPS given at a dose of 3 mg/kg body weight once weekly for 4 weeks (Pentosan Equine Injection). Using the carpal osteochondral fragment–exercise model, there was a significant decrease in articular cartilage fibrillation (P < 0.05) and a strong trend (P = 0.06) for improvement in overall cartilage histological appearance (modified Mankin score). Furthermore, although there was improvement in most other parameters (lameness, joint flexion, synovial fluid total protein concentration, synovial fluid collagen degradation products, and aggrecan synthesis), results were not statistically significant.
Combination Intraarticular Therapy
HA and corticosteroids are commonly combined. Without corticosteroids, the efficacy of intraarticular HA (based on short-term clinical response) is limited to horses with mild to moderate synovitis but is markedly enhanced with corticosteroids. Some veterinarians have used HA in conjunction with MPA, thinking that the former would mitigate the effects of the latter, but this is questionable (see page 842). In human medicine, localized, severe, acute inflammatory reactions (SAIRs) were associated with the use of highly cross-linked HA products.66 Although no adverse reactions were reported using Hylan G-F 20 (Synvisc, Genzyme, Cambridge, Massachusetts, United States) in people with knee OA,67 others have reported SAIRs and reduction of the risk with concurrent intraarticular corticosteroid administration.68 The risk of adverse reactions after Hylan G-F 20 administration may increase with the second or third injection.69 This may be because of an immunogenic mechanism associated with fermented HA products; the problem was not seen with naturally extracted HA.69 Anecdotally, there have been more adverse reactions in horses following synthetic HA injections compared with naturally extracted HA. The combination of TA and insulin-like growth factor-I (IGF-I) has been evaluated in vitro with positive results.70
The Editors add that various other combinations of intraarticular products have been used by practitioners to manage horses with OA. A combination of hyaluronan and PSGAG (and amikacin sulfate) has been used, apparently quite safely, to manage chronic OA in Standardbred racehorses and anecdotal reports suggest this combination gives better results than injection of either product alone. Studies evaluating efficacy and safety of combination therapy have not been done. A new product, Polyglycan (Arthrodynamic Technologies, Versailles, Kentucky, United States), is a combination of HA, glucosamine, and CS and is marketed as a postsurgical lavage solution. This product is currently being used to manage OA in horses as an intraarticular or intramuscular injection and anecdotal reports suggest the efficacy of this combination approach is similar to that seen with PSGAG alone. Preliminary studies are underway.
Oral Joint Supplements
Oral joint supplements are loosely classified as nutraceuticals. The term “nutraceutical” combines the word “nutrient” (nourishing food or food component) with “pharmaceutical” (a medical drug)71 and describes a broad list of products sold under the premise of being a dietary supplement (i.e., a food) but for the expressed intent of treatment or prevention of disease. The claims (usually made by manufacturers) to aid in equine joint health are often very weak. Unlike a feed, a nutraceutical is unlikely to have an established nutritive value. Feeds are required to have nutritive value, and labels must be accountable. Joint supplements fall between food and drugs, and there is no requirement for labels to list ingredients or nutrient profiles as required for feeds. They are sold with the intent to treat or prevent disease without first undergoing proper drug approval.71 Joint supplements are fed to horses to heal the lame or make chronically unsound horses sound or to prevent joint problems from occurring. However, when an owner or trainer first uses oral supplements, the source of pain is rarely diagnosed. A role in prevention of OA is hard to disprove but serves as a rationale to use nutraceuticals and many licensed drugs. In 2005, nutraceutical sales in the United States reached more than 1 billion U.S. dollars for companion animals, and that figure was expected to double in the next 3 years. To equine practitioners, this is disturbing because this industry is, for the most part, unregulated by the Food and Drug Administration, and there is only weak in vivo scientific evidence to support use of the their products.72
None of the oral supplements or nutraceuticals are licensed, and proof of efficacy is generally lacking. Most products include variable amounts of glucosamine and/or CS along with other ingredients. The first oral GAG products available for equine use included a CS product extracted from bovine trachea (Flex-Free) and a complex of GAGs and other nutrients from the sea mussel Perna canaliculus (Syno-Flex)(Vita Flex Nutrition, Council Bluffs, Iowa, United States). More recently, glucosamine hydrochloride, CS, manganese, and vitamin C have been marketed as a nutraceutical (Cosequin) (Nutramax Laboratories, Inc., Edgewood, Maryland, United States), and a number of other products have simulated Cosequin. There are now many other commercially available products containing various concentrations of glucosamine, chondroitin sulfate, manganese, vitamin C, methylsulfonylmethane (MSM), fish oils, and other constituents. The different labeling methods make it extraordinarily difficult to compare objectively the relative amounts of the different constituents between products. Glucosamine sulfate is a precursor of the disaccharide subunits of cartilage proteoglycans. Glucosamine salts are well absorbed orally in people73; however, in the horse oral bioavailability of glucosamine hydrochloride was only 2.5%, interpreted as poor absorption, but with extensive tissue uptake thereafter.74 In dogs, glucosamine is poorly absorbed when given orally (12%), probably because of extensive first-pass metabolism in the gastrointestinal tract and/or liver.75
In a more recent study, eight adult female horses with no evidence of joint disease were randomly assigned to two groups (n = 4) in a crossover study.76 Glucosamine hydrochloride (20 mg/kg) was administered by nasogastric intubation or intravenous injection. The maximum serum and synovial fluid glucosamine concentrations were substantially higher after intravenous administration (288 ± 53 µM and 250 µM, respectively) than by the oral route (5.8 ± 1.7 µM and 0.3 to 0.7 µM). The levels of glucosamine found in synovial fluid after oral administration were lower than those that have been used in vitro to study glucosamine action in tissue culture.76
CS consists of alternating disaccharide subunits of glucuronic acid and sulfated N-acetylgalactosamine molecules and is a principal GAG of aggregating proteoglycan (aggrecan). CS is less sulfated but resembles PSGAG in structure and mechanism of action. Oral absorption of CS was tested in horses. A low-molecular-weight CS (0.80 kDa) was evaluated by quantifying the disaccharide content using a validated method that combined enzymatic digestion of plasma followed by fluorescence high-performance liquid chromatography.77 Low-molecular-weight CS was absorbed better than glucosamine; its absorption may be influenced by the molecular weight of the polymer.77
In vitro studies can potentially help to determine at what concentrations glucosamine or CS may inhibit a catabolic response in equine cartilage explants. A study of cartilage disks incubated with lipopolysaccharide (LPS) in varying concentrations of glucosamine, CS, or both revealed that glucosamine concentrations as low as 1 mg/mL decreased nitrogen oxide production, but that CS at either 0.25 or 0.5 mg/mL had no effect. Glucosamine concentrations as low as 0.5 mg/mL decreased PGE2 production, whereas CS did not affect PGE2 production. A combination of CS and glucosamine decreased MMP-9 activity but had no effect on MMP-2; there was a trend for decreasing MMP-13 protein concentrations.78
In vitro dose titration studies of glucosamine hydrochloride and CS alone and in combination were performed in our laboratory. There were no detrimental effects of either glucosamine hydrochloride, CS, or a combination of both on normal cartilage metabolism. High doses of either glucosamine or CS or the combination limited total GAG release, whereas intermediate doses enhanced GAG synthesis and total cartilage content.79 The same dosages tested on IL-l–conditioned articular cartilage explants revealed no treatment effects for either glucosamine or CS alone, but a protective effect of high doses of the two combined for total GAG release. This study suggested that a combination of CS and glucosamine might be beneficial to cartilage metabolism by preventing GAG degradation. However, the question of effective concentration of glucosamine after oral administration remains an issue.76
An oral HA product showed no efficacy in our osteochondral fragment–exercise model. However, a clinical study evaluating the use of an oral HA product after arthroscopic surgery for tarsocrural joint osteochondritis dissecans (OCD) revealed a significant reduction in postoperative synovial fluid effusion in treated horses compared with controls.80
The osteochondral fragment–exercise model was used to test avocado and soybean unsaponifiable (ASU) extracts.81 The placebo control group (n = 8) received molasses orally once daily, whereas the ASU-treated horses (n = 8) received 6 g of ASU plus a similar volume of molasses orally. At the termination of the study, horses treated with ASU had significantly improved total gross examination score (articular cartilage erosion plus synovial hemorrhage score) in the OA joint compared with placebo control horses.81 There were significant decreases in synovial intimal hyperplasia and the cartilage disease score and a trend for decrease in lameness scores. Although improvement was modest, it was greater than that seen with other parenteral (intramuscular PSGAG and intravenous HA) and oral (HA) products tested using the same model.
Platinum Performance (Platinum Performance Inc., Buellton, California, United States) is a combination of rare earth minerals and omega-3 fatty acids that is used postoperatively, but all information is anecdotal. There is potential value of using omega-3 polyunsaturated fatty acids (PUFAs) in the management of OA. PUFA administration reduces inflammatory mediators in equine monocytes, corresponding to an increase in the ratio of omega-3 to omega-6 fatty acids in cell membrane phospholipids.77,78 In an in vitro study, the role of α-linolenic acid and omega-3 PUFA, as well as its antiinflammatory potential for the reduction of equine synovial inflammation, was evaluated in an established LPS model.79 Whereas LPS significantly increased production of PGE2 and decreased production of HA, treatment with α-linolenic acid at the highest dose inhibited prostaglandin production.79
Summary of Use of Conventional Medications
Conventional medications still form a large part of the equine veterinarians’ armamentarium. Increased attention is being paid to physical therapy regimens and positive results demonstrated with shock wave therapy (see Polysulfated Glycosaminoglycan, page 843); these could possibly decrease the need for medication for equine joint disease. COX-2 inhibitors may be useful when a horse does not tolerate phenylbutazone well. Intraarticular corticosteroids continue to be the principal intraarticular therapy. The use of MPA has decreased appropriately, and the value of betamethasone esters and TA is recognized, but there is still an overuse of MPA, and I consider its use in high-motion joints to be below the standard of care. (One of the Editors [MWR] respectfully disagrees. Given the short-lived effect of TA, limited dosages [20 to 40 mg/joint] of MPA are still used in high-motion joints if a sustained effect of antiinflammatory therapy is needed.) Continued availability of licensed medication is a challenge. Intraarticular HA continues to be used in conjunction with corticosteroids. Recent research challenges the value gained from intramuscular PSGAG, but results have been positive with intraarticular use of the drug. It is predicted that PPS will become a licensed medication, and its value has been documented scientifically. Oral nutraceuticals continue to be somewhat of a “black box” as far as efficacy is concerned, but positive results in a controlled study with avocado and soybean extracts are exciting.
The Editors respectfully point out that the osteochondral fragment–exercise model of OA is not necessarily representative of naturally occurring OA in sports horses. Although it is a reliably reproducible model of OA, drugs may have a different effect in naturally occurring OA compared with this experimental model. Comparisons between the efficacy of various drugs and other treatment modalities using the experimental model are also not easily made because in most of the studies significant results are achieved with the tested drug compared with control horses for one or more of the many measured parameters. The studies are relatively short term compared with the career of many equine athletes and only assess short-term outcomes. The studies using this model have unquestionably enhanced knowledge about the potential efficacy of a variety of drugs, but these results are not necessarily transferable to all high- and low-motion joints with naturally occurring OA.
Newer Biologically Based Therapies
Biological therapy specifically modulates key mediators, and our improved understanding of critical mediators in equine traumatic arthritis and OA has led to the identification of new targets for therapy. Two obvious targets identified include MMPs and IL-1.
Inhibition of Metalloproteinases as a Therapeutic Approach
MMP inhibitors include peptide-based (including hydroxamic acids), non–peptide-based (including chemically modified tetracyclines such as doxycycline), and naturally occurring inhibitors (such as omega-3 fatty acids, i.e., fish oils; see the section on Oral Joint Supplements).
In vitro studies assessed the MMP inhibitor BAY 12-9566 using an IL-1 degradation model (tenfold concentration [1 nM : 10 µM] increases) in equine and canine articular cartilage explants.85 There was a significant dose-dependent reduction in the catabolic effect of IL-1α on the release of proteoglycans and type II collagen. In vivo assessment of MMP inhibition has not been performed in the horse. However, a canine study of experimentally induced OA (cranial cruciate ligament transection) failed to demonstrate efficacy with an MMP inhibitor,86 and the prospect for these being valuable therapeutically in the horse seems low.
Inhibiting Interleukin–1
The most commonly accepted mediator at the top of the cascade for cartilage degradation in OA is IL-1. However, tumor necrosis factor (TNF) has received most attention in rheumatoid arthritis in people, leading to the novel therapy of using an anti-TNF monoclonal antibody adalimumab (Humira, Abbott Laboratories, Abbott Park, Illinois, United States) and recombinant human TNF receptor etanercept (Enbrel, Wyeth Pharmaceuticals, Madison, New Jersey, United States).87
IL-1 activates MMP, aggrecanase, and PGE2 release by acting through IL-1 receptors on the cell membrane (Figure 84-2). IL-1 can be inhibited through the natural antagonist, IL-1 receptor antagonist (IL-1ra), which binds to the cell membrane IL-1 receptor to block IL-1. IL-1 can also be inhibited by using soluble receptors, where IL-1 receptors are released from the cell membrane and bind IL-1.87 Soluble receptors are used to inhibit TNF, but there are no current techniques for using IL-1–soluble receptors therapeutically. Cell membrane IL-1 receptors can be inhibited by using recombinant proteins or gene therapy (see Chapter 63). The first therapeutic application of IL-1ra for equine use involved the direct transfer of the equine IL-1ra gene sequence to synoviocytes using an adenoviral vector.88,89 Complete inhibition of carpal OA confirmed the critical role of IL-1 in the osteoarthritic process.89 Although both symptom- and disease-modifying effects were demonstrated and the magnitude of therapeutic value was greater than any other medication tested to date, repeated delivery of IL-1ra using gene transfer necessitates a better vector, which is currently being researched.
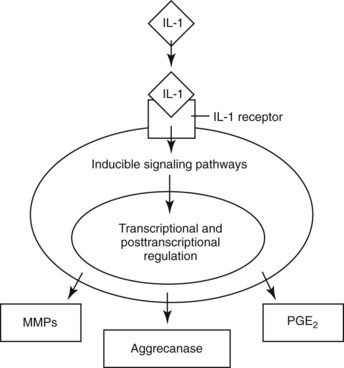
Fig. 84-2 Diagram of interleukin-1 (IL-1) activation of metalloproteinase, aggrecanase, and prostaglandin E2 release acting through IL-1 receptors on the cell membrane.
(Reproduced with permission. McIlwraith CW: From arthroscopy to gene therapy—30 years of looking in joints, Proc Am Assoc Equine Pract 51:65, 2005.)
The limitations with gene transfer therapy have led to the development of alternative methods of delivering IL-1ra to joints. The concept of IL-1ra, IRAP (Arthrex Vet Systems, Naples, Florida, United States) was developed in Europe as a product named Orthokine (Orthogen AG, Dusseldorf, Germany). To make IRAP, peripheral blood is collected in a syringe containing glass beads soaked in chromium sulfate and is incubated for 24 hours and is then centrifuged. The autologous modified serum (autologous conditioned serum [ACS]) is then used for one or more intraarticular injections. Marked elevation of IL-1ra without elevated expression of IL-1 or TNF, that is, preferential up-regulation of “good” cytokines without concurrent up-regulation of “bad” cytokines, was documented.90 In 2004, Arthrex Biosystems began marketing IRAP in the United States, and their unpublished data confirmed up-regulation of IL-1ra but not to the same extent as previously reported.90 Up-regulation of proteins is probably through stimulation of monocytes by the beads and syringe, and it is likely that more than IL-1ra is up-regulated, a concept currently being studied at CSU using mass spectroscopy.
IRAP was tested in a double-blinded, placebo-controlled, experimental study using the osteochondral fragment–exercise model and was shown to have both symptom and disease-modifying effects in horses.91 In eight placebo and eight ACS-treated horses, 6 mL of phosphate-buffered saline (PBS) solution or ACS, respectively, was injected into the OA-affected joint on days 14, 21, 28, and 35. No adverse treatment-related events were detected. Horses that were treated with ACS had significant improvement in lameness scores, significantly decreased synovial hyperplasia, and less gross cartilage fibrillation and synovial hemorrhage than PBS-injected horses. Synovial fluid concentration of IL-1ra (assessed using a mouse anti-IL-1ra antibody) was increased after treatment with ACS.91 All reports concerning clinical efficacy of ACS are anecdotal; there have been no controlled clinical trials.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) have been used intraarticularly in the horse for a number of “indications.” Both clinical experience and research studies are limited to adult-derived MSCs, and bone marrow remains the gold standard as a source of MSCs. However, other sources such as muscle, cartilage, and adipose tissue contain multipotent MSCs. MSCs from bone marrow or digestive tissue are isolated by simple adhesion and proliferation of MSCs to tissue culture surfaces, a technique that yields a near-homogenous MSC population and is called culture expansion.92 Work is proceeding on establishing cell surface antigens that characterize MSCs. There are distinct differences between cultured-expanded MSCs and those available through non–culture-expanded sources, such as the Vet-Stem technique (Vet-Stem Regenerative Veterinary Medicine, Poway, California, United States), in which harvested fat undergoes tissue digestion, producing a nucleated cell population called stromal vascular fraction. Stromal vascular fraction is believed to contain approximately 2% to 4% MSCs. Much of the other literature regarding culture-expanded adipose-derived MSCs cannot necessarily be compared with the Vet-Stem technique.
Intraarticular use of MSCs was investigated in a study comparing adipose-derived stromal cells, bone marrow–derived cultured MSCs, and placebo in the equine osteochondral fragment–exercise model. There were no significant treatment effects in any group, with the exception of improvement in synovial fluid PGE2 levels in horses treated with bone marrow–derived MSCs compared with the placebo group.93 Based on this study, we cannot recommend the use of MSCs for the treatment of OA. However, the results of an ovine OA model using medial meniscectomy and cranial cruciate ligament transection, evaluating intraarticular autogenous bone marrow–derived MSCs, were encouraging.94 There was regeneration of the medial meniscus and decreased progression of OA. Meniscal regeneration could be critical in decreasing OA in this model, whereas OA in the equine osteochondral fragment–exercise model has a different pathogenic pathway. Based on the ovine study, considerable interest was generated at CSU in using intraarticular MSCs to treat soft tissue injuries in the femorotibial joints after arthroscopy, apparently with good clinical results.
Calcitonin
There is some evidence to suggest a direct chondroprotective effect of calcitonin, which, together with its well-established effects on bone resorption, makes it a potential biological therapy for OA.95,96 Calcitonin is used to treat osteoporosis.97 In some in vitro work, calcitonin was shown to attenuate collagenase activity, targeting type II collagen.98 Dogs with OA induced using the cranial cruciate ligament transection model given 400 U/day of calcitonin had significantly reduced histological changes in the articular cartilage of unstable knees. Calcitonin enhanced HA content, as well as the size distribution and relative abundance of aggrecan aggregates in cartilage in both operated and nonoperated knees. In the calcitonin group, the cartilage content of keratin sulfate increased in the operated joints.96
Radiation Synovectomy
Synovectomy is hardly a specific biological therapy but is included so its potential use can be recognized. Synovectomy is a well-developed technique for removal of synovial-based inflammation in people with rheumatoid arthritis, but surgical synovectomy is technically difficult, time consuming, and requires general anesthesia.99 Samarium-153 (153Sm) bound to hydroxyapatite microspheres (153SmM) effectively ablated normal equine synovium, with minimum radiation hazards to the horse or medical personnel.99 Radiation emissions from 153SmM are primarily absorbed by synovium; thus the clinician can target this intraarticular tissue specifically. 153Sm-hydroxyapatite complex minimizes exposure to other organs or support personnel. In another study, the effects of 153SmM on reactive synovium in a surgically induced model of synovitis were investigated.100 Although there was a transient flare causing lameness, effusion, and edema for 48 to 72 hours, 153SmM destroyed inflamed synovium. There may be potential (with further testing) for radiation synovectomy in horses with persistent synovitis unresponsive to conventional therapy.100
Surgical Treatment
Arthroscopy has revolutionized management of joint disease (see Chapter 23).101-103 Readers are referred to Diagnostic and surgical arthroscopy in the horse104 for details including advantages and disadvantages of the technique. Arthroscopic surgery remains the gold standard for diagnosis and assessment of pathological joints. Diagnostic arthroscopy is especially valuable when response to medical treatment of a joint is suboptimal. In many instances, cartilage lesions are more extensive than suggested from radiographs but are consistent with the degree of clinical signs.
By 1990, equine arthroscopy had gone from being a diagnostic technique used by a few veterinarians to the accepted way of performing joint surgery.101 Prospective and retrospective data substantiated the value of arthroscopy in management of carpal chip fractures,105 fragmentation of the dorsal margin of the proximal phalanx,106 carpal slab fractures,107 OCD of the femoropatellar108,109 and scapulohumeral110 joints, subchondral cystic lesions of the femur,111 and various manifestations of osteochondrosis of the tarsocrural joint.112 Diagnostic arthroscopy led to the recognition of previously undescribed articular lesions, many of which are treated using arthroscopic techniques.
Since 1990, there have been further advances in diagnosis and management of joint disease based on new pathobiological knowledge gained from arthroscopy.102,113-118 Previously thought of as inaccessible, the palmar aspects of the carpal and distal interphalangeal joints are now routinely evaluated arthroscopically.119-121 Arthroscopy led to the discovery of soft tissue injuries, previously undiagnosed, such as tearing of the medial palmar intercarpal ligament.122-126 In the fetlock joint, we now know success rates after arthroscopy for horses with osteochondral fragments of the palmar/plantar aspect of the proximal phalanx,127,128 treatment of OCD of the distal dorsal aspect of the third metacarpal/metatarsal bones,129 and apical, abaxial, and basilar fragments of the proximal sesamoid bones.130-134 The arthroscopic approach to the plantar pouch of the tarsocrural joint first documented in 1990101 was more recently reviewed.135
Considerable advances have been made in arthroscopic surgery of the stifle joint. Results for the management of OCD of the femoropatellar joint and a syndrome of fragmentation of the distal aspect of the patella occurring after medial patellar desmotomy and success when treating certain patellar fractures were documented.101,136-138 The use of arthroscopic surgery to treat subchondral cystic lesions of the medial condyle of the femur139 and proximal tibia140 has been reported. In fact, pathogenesis of cystic lesions in the medial femoral condyle, once thought to only be a manifestation of osteochondrosis, was recently redefined. Subchondral cystic lesions were traumatically induced by surgically creating a defect, 3 mm deep and 5 mm wide, in the subchondral bone plate.141 Arthroscopic curettage and drilling into the cyst wall of naturally occurring subchondral cysts sometimes caused cyst enlargement and recurrent lameness; alternative methods for management have developed. Examination of the fibrous tissue of medial femoral subchondral cystic lesions demonstrated the production of local mediators and neutral MMPs, which caused bone resorption in vitro.142 Production of nitric oxide, PGE2, and MMPs in media of explant cultures of equine synovium and articular cartilage has also been demonstrated in normal and osteoarthritic joints.143 Therefore injection of corticosteroids into the lining membrane of subchondral cysts has been performed clinically with favorable results (Figure 84-3).144
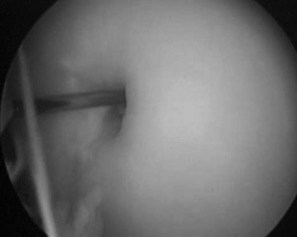
Fig. 84-3 Arthroscopic view of an 18-gauge spinal needle being used to inject triamcinolone acetonide into the lining of a subchondral cystic lesion.
(Reproduced with permission. McIlwraith CW: From arthroscopy to gene therapy—30 years of looking in joints, Proc Am Assoc Equine Pract 51:65, 2005.)
Cartilage lesions of the medial femoral condyle occur, but surgical manipulation is limited.145 Adjunctive therapy using IRAP or MSCs has become popular, but results are anecdotal. Arthroscopy has allowed great advances in the recognition and treatment of meniscal tears and cruciate ligament injuries.146-148 Horses with grade I and grade II meniscal tears can be managed successfully, but those with lesions that are not completely accessible do not respond well (Figure 84-4). Arthroscopy has been used to facilitate removal and repair of fragments from the intercondylar eminence of the tibia.149,150 Techniques to manage disease of the caudal pouches of the femorotibial joints are now published.147,151-154 Limited diagnostic and surgical arthroscopy of the coxofemoral joint can be performed.155,156 Arthroscopy is no longer confined to the limbs, and the arthroscopic anatomy of the temporomandibular joint was described recently.157
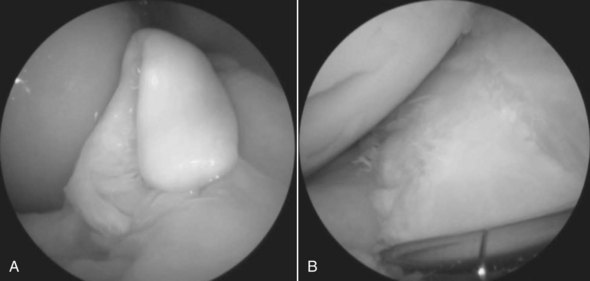
Fig. 84-4 A grade II tear of the medial meniscus before (A) and after removal and debridement of the torn portion (B).
(Reproduced with permission. McIlwraith CW: From arthroscopy to gene therapy—30 years of looking in joints, Proc Am Assoc Equine Pract 51:65, 2005.)
Arthroscopic-assisted repair with internal fixation of both nondisplaced and displaced articular fractures has become routine and includes fractures of the metacarpal/metatarsal condyles and carpal slab fractures (Figure 84-5).158-160 Techniques for evaluation and treatment of diseases of smaller joints161-164 and in large joints in which lameness is seldom encountered are well developed.165 Results of arthroscopic surgery are often limited by extensive cartilage damage, an observation that has led us back to scientific research as discussed below.
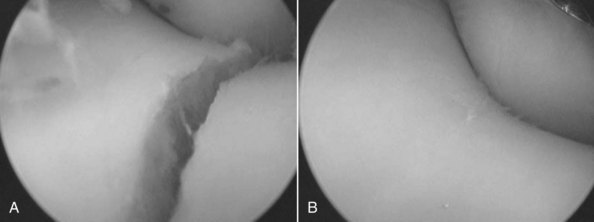
Fig. 84-5 Arthroscopic view of a displaced fracture of the lateral condyle of the distal aspect of the third metacarpal bone before (A) and after (B) reduction: under arthroscopic guidance and internal fixation.
(Reproduced with permission. McIlwraith CW: From arthroscopy to gene therapy—30 years of looking in joints, Proc Am Assoc Equine Pract 51:65, 2005.)
Recent Progress at Healing Articular Cartilage Lesions and Resurfacing Joints
Progressive loss of articular cartilage is the real challenge in horses with OA, and failure of osteochondral defects to heal is a major limiting factor in the prognosis after treatment of articular fractures. Arthroscopic techniques that enhance both the quantity and quality of cartilage repair tissue, while using the well-documented advantages of arthroscopic surgery, have been attempted.166-169 Repair, as occurs in full-thickness cartilage defects, is defined as replacement of damaged or lost cells and matrix with new cells and matrix, but the original structure and function may not be restored. Regeneration is a special form of repair, in which cells replace lost or damaged tissue with a tissue identical to the original, the aim of continuing investigations in resurfacing. Overall, attempts at improving the repair of articular defects can be divided into stimulation of endogenous repair and articular grafting.
Stimulation of Endogenous Repair
Stimulating endogenous repair involves techniques to provide access for marrow elements to populate the cartilage defect. The simplest method, debridement of the defect, provides fibrocartilaginous repair with high concentrations of type II collagen but low levels of proteoglycan (GAG).170,171 In addition to simple debridement, other methods of endogenous repair include partial-thickness chondrectomy, spongialization, subchondral bone drilling, abrasion arthroplasty, and most recently, micropicking or microfracture.
Debridement and the Need for Removal of Calcified Cartilage
The time-honored surgical principle of debridement to the level of subchondral bone is well known, but recent work at CSU revealed that if the calcified cartilage layer is left, healing is markedly restricted.169,172 If the calcified cartilage was removed, there was a significant increase in the amount of repair tissue in defects compared with controls at 4 and 12 months.173 There was also significant enhancement of repair tissue attachment and calcified cartilage reformation in defects from which the calcified cartilage had been removed (Figure 84-6).
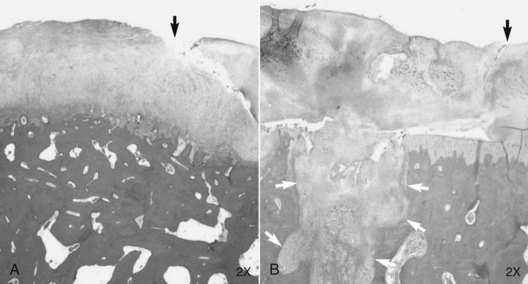
Fig. 84-6 Histology 12 months after articular cartilage defects in the medial femoral condyle were treated with microfracture and with the calcified cartilage removed (A) and retained (B). In addition to improved repair tissue, there is partial restoration of the calcified cartilage layer and better attachment of the repair tissue to the underlying bone in the section shown in A. Black arrows show the junction of repair tissue (left) and retained cartilage. White arrows in B show a micropick hole in subchondral bone.
(Reproduced with permission. McIlwraith CW: From arthroscopy to gene therapy—30 years of looking in joints, Proc Am Assoc Equine Pract 51:65, 2005.)
Partial-Thickness Chondrectomy
Partial-thickness chondrectomy down to relatively healthy chondral tissue (cartilage shaving) for partial-thickness defects and fibrillation smoothes the cartilaginous area and may decrease further tissue exfoliation, producing (in conjunction with joint lavage) an early remission of synovitis. However, controlled work in the horse is still necessary. In rabbits in which articular cartilage was shaved on the underside of the patella, there was no evidence of repair.174 Ultrastructural studies after arthroscopic cartilage debridement questioned any regeneration and suggested deleterious effects.175
Spongialization, Abrasion Arthroplasty, and Subchondral Bone Drilling
Spongialization is removal of sclerotic subchondral bone from the base of a full-thickness defect, a procedure sometimes done in horses. However, subchondral bone cysts can form, and I believe that an intact subchondral bone plate is essential for maintaining a foundation for repair.
Abrasion arthroplasty, also called superficial intracortical debridement, as opposed to deep cancellous debridement, is used on sclerotic degenerative lesions in people.176 The concept is controversial because it is necessary to expose cancellous bone to reach both blood supply and primitive MSCs. Critics have pointed to variables such as patient selection, arthroscopic debridement, and joint irrigation, as well as variation in the degree of pathological change in the joint.177 In an in vitro study, low oxygen tension was demonstrated to be chondrotrophic and led to the corollary that excessive oxygen tension is not conducive to cartilage formation. Deep debridement to cancellous bone exposes the defect to blood and MSCs, but also to higher oxygen tensions; some people have questioned whether increasing oxygen tension to the defect is beneficial or not.178 Follow-up biopsies after abrasion arthroplasty showed type I and type III collagen and only limited amounts of the much wanted type II collagen.176
Subchondral drilling had similar rationale in providing access through the cancellous bone plate, while still preserving most of the subchondral bone plate. In a study on full-thickness defects of the third carpal bone of horses, satisfactory functional healing was not achieved.179
Subchondral Micropicking (Microfracture)
Subchondral microfracture, or micropicking, was a technique developed by Dr. Richard Steadman and is extensively used in people.180,181 The technique was shown to provide equivalent repair to the commercially available autologous chondrocyte implantation process of Carticel (Genzyme, Cambridge, Massachusetts, United States).182 Micropicking is a simple and atraumatic way to provide pluripotential cells and growth factors while minimizing heat (associated with drilling with other techniques) and providing a rim of bone around the pick holes that may provide a foundation to enhance repair tissue attachment. Studies in the horse showed that the amount of repair tissue significantly increased when cartilage defects debrided down to subchondral bone were micropicked compared with debridement alone. A short-term study revealed that there was a significant increase in type II collagen mRNA production 8 weeks after microfracture.172 Although aggrecan production gradually increased between 2 and 8 weeks, this expression was not influenced by microfracture.172
Further Manipulation of Endogenous Healing Using Growth Factors (Protein Administration or Gene Therapy)
We may be able to manipulate cells to produce improved repair tissue matrix. Several naturally occurring polypeptide growth factors play an important role in cartilage homeostasis.183,184 However, endogenous manipulation not only involves the use of growth factors to promote synthesis of matrix components (anabolism) but also should inhibit proteinases and inflammatory factors that can cause ongoing degradation after surgery (catabolism) (Figure 84-7). IGF-I and transforming growth factor–β (TGF-β) have matrix anabolic activity and are also considered important in counteracting the degradatory and catabolic activities of cytokines and MMPs. Three-dimensional cultures of equine chondrocytes in fibrin gels were evaluated with either IGF-I or TGF-β and cultured without serum supplements; these two growth factors stimulated matrix component elaboration in a dose-dependent manner, with the most profound effects occurring at the highest concentration of IGF-I and TGF-β.185,186 In other cartilage explant studies using normal and IL-1–depleted cartilage, IGF-I had a positive effect on equine cartilage homeostasis.187 Because of these results, IGF-I was selected as a candidate growth factor for evaluation in the horse. Elution studies showed that IGF-I–laden equine fibrin had maximal stimulatory levels of IGF-I (>50 ng/mL) remaining for a minimum of 3 weeks.188 In vivo evaluation after placement of 25 µg of IGF-I in fibrin into cartilage defects in the femoropatellar joint showed improved cell populations, with more cartilage-like architecture after 6 months.189 However, type II collagen levels only increased to 47%, half of that found in normal articular cartilage, compared with a type II collagen content of 39% in the control defects, comparable with the levels seen in empty full-thickness defects.190
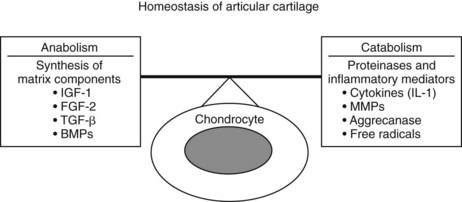
Fig. 84-7 Diagram of homeostatic pathways that can represent potential ways of endogenously manipulating articular cartilage. IGF, Insulin-like growth factor; FGF-2, fibroblast growth factor-2; TGF-β, transformin growth factor-β; BMPs, bone morphogenetic proteins; MMPs, matrix metalloproteinases; IL-1, interleukin-1.
(Reproduced with permission. McIlwraith CW: From arthroscopy to gene therapy—30 years of looking in joints, Proc Am Assoc Equine Pract 51:65, 2005.)
Other work in the same laboratory suggested IGF-I worked better in combination with a chondrocyte or a MSC graft, resulting in more complete cartilage repair.191 At 8 months after implantation of a mixture of chondrocytes in 25 mcg of IGF-I in femoropatellar defects in horses, there was improved joint surface, 58% type II collagen, and better neocartilage integration at the defect edges. Resurfacing of articular defects using autogenous fibrin laden with 50 mcg of IGF-I in 30 million chondrocytes/mL of fibrin was used in horses with OCD and subchondral cystic lesion of the fetlock and stifle joints. The chondrocytes were mixed with fibrinogen and IGF-I with activated thrombin to provide a two-component system for immediate injection. This polymerization process developed immediately on injection into the articular defect.167
Using gene therapy techniques (previously discussed with IL-1ra), transplanted chondrocytes were transfected with IGF-I.192,193 Transfecting transplanted chondrocytes with bone morphogenetic protein–7 accelerated cartilage repair.194
In a collaborative effort between CSU, Cornell University, University of Pittsburgh, and Harvard University, the usefulness of a combined gene therapy protocol using IL-1ra to decrease the effects of IL-1 on cartilage repair in combination with IGF-I (previously shown to enhance cartilage healing in an equine model, as well as to also reduce the deleterious effects of IL-1) was investigated.195-197 Using an osteoarthritic IL-1 coculture (synovium and articular cartilage) system, gene transduction of IGF-I and IL-1ra proteins was demonstrated using an adenoviral vector with protection of proteoglycan loss in the cartilage.195 There was also restoration of cartilage matrix without IL-1 present using the same in vitro system.196 The combination gene therapy protocol was then evaluated using full-thickness articular chondral defects treated with microfracture in the horse.197 This protocol enhanced the quality of the repair tissue in full-thickness equine chondral defects compared with microfracture alone, and there was increased type II collagen and aggrecan content in the defects.197
Articular Grafting
Reattachment of Cartilage Flaps and Periosteal and Sternal Grafts
Direct repair of large OCD defects by replacing the flap and providing fixation with polydioxanone (PDS) pins was done successfully in horses.198 However, attempts with direct grafting of periosteum or sternal cartilage met with disappointing results.170,171,199
Implantation of Autologous Chondrocytes
Early attempts at grafts of cultured chondrocytes or cartilage regenerative cells in a matrix were also relatively unsuccessful.200-202 Currently there is one commercially available technique of autologous chondrocyte implantation into human knees, and it is used principally for focal erosive defects203,204 and OCD.205 This is a two-stage procedure. After collection of cartilage, it is cultured; 3 weeks later, a piece of autogenous periosteum is sutured into the defect, and the cultured chondrocytes are injected beneath it. Despite reports implying excellent results, failures, particularly with detachment of the graft, can occur, and recently a clinical study with a 2-year follow-up period showed no superiority over microfracture alone.182 Grafting in this manner was used experimentally in 10-mm–diameter defects on the lateral trochlear ridge of talus and resulted in significantly improved defect filling with a well-integrated neocartilage and comparable expression of cartilage-specific markers.206
I have conducted experiments at CSU using a solid form of autologous chondrocyte culturing. Cartilage (300 mg) was harvested from the lateral trochlear ridge of the femur, and chondrocytes were cultured on a collagen membrane and reimplanted at 4 weeks. The results were significantly superior to empty defects and defects implanted with matrix alone. However, it is still a two-stage technique, and this remains a caveat.207
A one-stage technique for treatment of 15-mm defects in the trochlear ridge of the femur in the horse was evaluated.208 An articular cartilage biopsy was taken from the lateral trochlear ridge of the femur (follow-up evaluation at 12 months revealed no apparent morbidity associated with the cartilage biopsy). The cartilage was morselized into approximately 1 mm3 and suspended in fibrin on a membrane (a PDS-reinforced foam). This morselized cartilage-fibrin-PDS membrane combination was then placed into the defect with the membrane uppermost and fixed with three specially developed PDS-polyglycolic acid (PGA) staples. The follow-up results at 12 months were excellent. The technique is now being tested in people.
What about Stem Cells?
The use of MSCs in medical management of OA was previously discussed (see Mesenchymal Stem Cells, page 847). The relative chondrogenic potential of bone marrow–derived or adipose-derived MSCs was evaluated after expansion in monolayer culture and implantation into agarose or peptide gels.209 GAG accumulation in hydrogels seeded with culture-expanded MSCs and containing TGF-β after 21 days of culture was significantly enhanced with bone marrow–derived MSCs compared with adipose-derived MSCs, and there was also superior type II collagen expression. Implantation of MSCs (using fibrin as a vehicle) into femoral trochlear defects in the horse showed early benefit at 30 days, but there were no significant differences between treated and control joints at 8 months.210 Direct intraarticular injection of MSCs is currently being tested at CSU. We contend that MSCs act as trophic mediators; in addition to dividing and differentiating, MSCs secrete a variety of cytokines and growth factors, and these bioactive factors suppress local immune reactions, inhibit fibrosis and apoptosis, enhance angiogenesis, and stimulate mitosis and differentiation of tissue-intrinsic reparative or stem cells.211 These effects, which are referred to as trophic effects, are distinct from the direct differentiation of MSCs into repair tissue.
Osteochondral Grafts
The use of osteochondral plugs to repair experimentally created defects in the medial condyle of the femur was initially investigated using sternal osteochondral grafts.212,213 Techniques of autogenous and allogeneic osteochondral plug grafting using mosiacplasty were reported with some success, but the technique is challenging.214,215