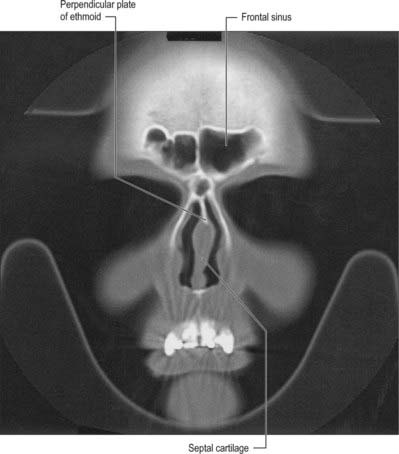
CHAPTER 32 Nose, nasal cavity and paranasal sinuses
The nose is the first part of the upper respiratory tract, and is responsible for warming, humidifying, and, to some extent, filtering inspired air. It also houses the olfactory epithelium which contains olfactory receptor neurones responsible for detecting airborne odorant molecules.
The nose may be subdivided into an external nose, which opens anteriorly onto the face through the nostrils or nares, and an internal chamber, divided sagittally by a septum into right and left cavities which open posteriorly into the nasopharynx through the posterior nasal apertures or choanae. The nasal cavities are housed in a supporting framework composed of bone and fibro-elastic cartilages. The larger bones in this framework contain air-filled spaces lined with respiratory epithelium, described collectively as the paranasal sinuses. The sinuses and the nasolacrimal ducts drain into the nasal cavity via openings in its lateral walls (Fig. 32.1).
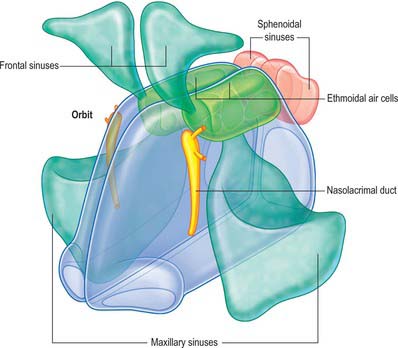
Fig. 32.1 Overview of spatial relationships between the nasal cavity, paranasal sinuses and nasolacrimal ducts.
(From Drake, Vogl and Mitchell 2005.)
EXTERNAL NOSE
The proportions of the nose and face, both from in front and the side, are of enormous significance to the rhinoplastic surgeon.
The shape of the external nose varies considerably between individuals. It is a pyramidal structure located in the midline of the midface and attached to the facial skeleton. Its upper angle or root is continuous with the forehead, and its free tip forms the apex which projects anteriorly. Its base contains two ellipsoidal apertures, the external nares or nostrils, which open onto its inferior surface, separated by the nasal septum and columella. The overall shape of the external nares is very variable. They usually measure 1.5–2 cm anteroposteriorly and 0.5–1 cm transversely and are narrower in front. The lateral surfaces of the nose unite in the median plane to form the dorsum. The alar sulcus is a groove in the skin bounding the nasal alae above and joining the nasiolabial sulcus. Below it curves towards the tip of the nose but does not reach it.
SKIN AND SOFT TISSUE
The skin and soft tissue covering the dorsum of the nose is usually thin, especially at the osseo-cartilaginous junction, and loosely connected to the nasal aponeurosis and the muscle fibres that fan out within it. Over the tip the skin is often thicker in men and has numerous large sebaceous glands but this varies considerably. These variations affect the final nasal contour and profile after nasal augmentation.
BONE AND CARTILAGE
Bony skeleton of the external nose
The piriform aperture has sharp edges. It is bounded below and laterally by the maxilla and above by the nasal bones (Fig. 32.2). The lateral part of the inferior edge of the piriform aperture merges into its lateral wall, which is formed by the frontal process of the maxilla. It is bounded above by the nasal part of the frontal bone and superomedially by the lateral edge of the nasal bone. The bony nasal septum articulates with the undersurface of the nasal bones and provides support to the dorsum of the nose. The nasal bones vary in thickness and width, which is of significance in planning osteotomies. They are thick and widest at the nasofrontal suture, narrow at the nasofrontal angle before they widen, and become thinner 9–12 mm below the nasofrontal angle.
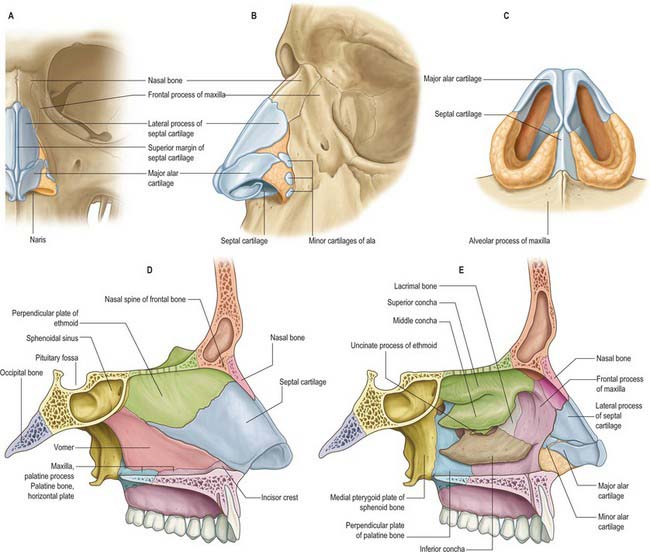
Fig. 32.2 The bony and cartilaginous skeletons of the nose. A, External nose, frontal view; B, External nose, lateral view; C, Inferior view of cartilages; D, Nasal cavity, medial wall; E, Nasal cavity, lateral wall (left side).
The commonest injury of the facial skeleton is a fracture of the nasal bones. In simple fractures the break usually occurs in the frontal process of the maxilla rather than at the nasofrontal suture. The terminal branch of the anterior ethmoidal nerve and its accompanying vessels are at risk when injuries involve the dorsum of the nose
Cartilaginous skeleton of the external nose
The cartilaginous framework consists of the paired lateral and major cartilages and several minor alar nasal cartilages (Fig. 32.2).
Lateral (superior) nasal cartilage
The lateral nasal cartilage is triangular, and its anterior margin is thicker than the posterior margin. The upper part is continuous with the septal cartilage, but anteroinferiorly it may be separated from it by a narrow fissure. The superior margin of the lateral nasal cartilage is attached to the nasal bone and frontal process of the maxilla, and the inferior margin is connected by fibrous tissue to the lateral crus of the major alar cartilage.
Major alar cartilage
The major alar cartilage is a thin flexible plate. It lies below the upper lateral cartilage and curves acutely around the anterior part of its naris. The medial part, the narrow medial crus (septal process), is loosely connected by fibrous tissue to its contralateral counterpart and to the anteroinferior part of the septal cartilage. The intermediate crus forms the margin of the apex of the nostril. The lateral crus lies lateral to the naris and runs superolaterally away from the margin of the nasal ala. The upper border of the lateral crus of the major alar cartilage is attached by fibrous tissue to the lower border of the lateral nasal cartilage. Its lateral border is connected to the frontal process of the maxilla by a tough fibrous membrane containing three or four minor alar cartilages. The junction between the lateral crura of the major alar and lateral cartilages is variable: the two edges may abut or overlap, in which case the lateral crus is then the more lateral at the junction. The lateral crus of the major alar cartilage is shorter than the lateral margin of the naris and runs away from the margin of the ala nasi. The lateral part of the margin of the ala nasi is fibroadipose tissue covered by skin. In front, the angulations or ‘domes’ between the medial and lateral crurae of the major alar cartilages are separated by a notch palpable at the tip of the nose.
MUSCLES
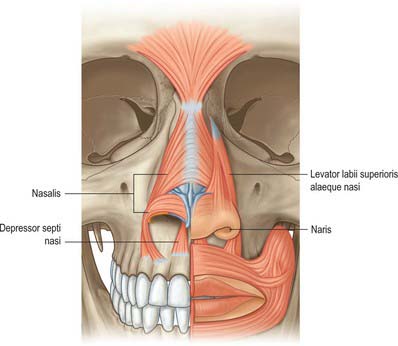
The nasal muscle group includes procerus, nasalis, dilator naris anterior, depressor septi and levator labii superioris alaequae nasi (Fig. 32.3). These muscles are involved in respiration, facial expression and in the production of some sounds during speech, when their activity is dependent upon the activity of orbicularis oris and the type of sound (see Clark et al 1998). Any or all of these muscles may be absent in cleft lip deformities with corresponding functional and aesthetic consequences.
Procerus
Procerus is a small pyramidal muscle that lies close to, and is often partially blended with, the medial side of the frontal part of occipitofrontalis. It arises from a fascial aponeurosis attached to the periosteum covering the lower part of the nasal bone, the perichondrium covering the upper part of the lateral nasal cartilage and the aponeurosis of the transverse part of nasalis. It is inserted into the glabellar skin over the lower part of the forehead between the eyebrows.
Nasalis
Nasalis consists of transverse and alar components. The transverse part (compressor naris) is attached to the maxilla above and lateral to the incisive fossa, and lateral to the alar part. Its fibres pass upwards and medially and expand into a thin aponeurosis which merges with its counterpart across the bridge of the nose. The merged aponeuroses blend with the aponeuroses of procerus and with fibres from levator labii superioris alaequae nasi. Fibres from the transverse part may also blend with the skin of the nasolabial and alar folds. The alar part (pars alaris or dilator naris posterior) is attached to the maxilla above the lateral incisor and canine, lateral to the bony attachment of depressor septi, and medial to the transverse part, with which it partly merges. Its fibres pass upwards and anteriorly and are attached to the skin of the ala above the lateral crus of the lower lateral cartilage, and to the posterior part of the mobile septum. The pars alaris helps to produce the upper ridge of the philtrum.
Nasalis is supplied by branches from the facial artery and from the infraorbital branch of the maxillary artery.
Nasalis is supplied by the buccal branch of the facial nerve. It may also be supplied by the zygomatic branch of the facial nerve.
The transverse part compresses the nasal aperture at the junction of the vestibule and the nasal cavity. The alar parts draw the alae and posterior part of the columella downwards and laterally and so assist in widening the nares and in elongating the nose. They are active immediately before inspiration.
Dilator naris anterior
Dilator naris anterior (also known as apicis nasi or the small dilator muscle of the nose) is a very small muscle attached to the upper lateral cartilage and the alar part of nasalis, and to the caudal margin of the lateral crus and the lateral alar crus. It encircles the naris and acts as a primary dilator of the nostril.
Dilator naris anterior and the alar part of nasalis (dilator naris posterior) probably function to prevent collapse of the nasal valve during inspiration. Their EMG activity is directly proportional to ventilatory resistance and is modified by signals that travel from pulmonary mechano- and pressure receptors via afferent vagal pathways to the brainstem respiratory centre: the efferent limb of the reflex arc runs in the facial nerve.
Depressor septi
Depressor septi lies immediately deep to the mucous membrane of the upper lip. It is usually attached to the periosteum covering the maxilla above the central and lateral incisors and the anterior nasal spine, and to the fibres of orbicularis oris above the central incisor. Its fibres pass to the columella, the mobile part of the nasal septum and the base of the medial crus of the nasal cartilage. A few muscle slips may pass between the medial crura into the nasal tip. Depressor septi may be absent or rudimentary.
Levator labii superioris alaequae nasi
Levator labii superioris alaequae nasi arises from the upper part of the frontal process of the maxilla and, passing obliquely downwards and laterally, divides into medial and lateral slips. The medial slip blends into the perichondrium of the lateral crus of the major alar cartilage of the nose and the skin over it. The lateral slip is prolonged into the lateral part of the upper lip, where it blends with levator labii superioris and orbicularis oris. Superficial fibres of the lateral slip curve laterally across the front of levator labii superioris and attach along the floor of the dermis at the upper part of the nasolabial furrow and ridge.
Levator labii superioris alaequae nasi is supplied by the facial artery and the infraorbital branch of the maxillary artery.
Levator labii superioris alaequae nasi is innervated by zygomatic and superior buccal branches of the facial nerve.
The lateral slip raises and everts the upper lip and raises, deepens and increases the curvature of the top of the nasolabial furrow. The medial slip pulls the lateral crus superiorly, displaces the circumalar furrow laterally, and modifies its curvature: it is a dilator of the naris. Depressor septi and the medial slip of levator labii superioris alaequae nasi are sometimes described as secondary nasal dilators: there is little evidence that either of these muscles has any direct influence on the nasal valve.
Anomalous nasal muscles
Anomalous, inconstant, nasal muscles have been described. They include anomalous nasi, attached to the frontal process of the maxilla, procerus, transverse part of nasalis and the upper lateral cartilage (i.e. in a region normally devoid of muscle) and compressor narium minor, which passes between the anterior part of the lower lateral cartilage and the skin near the margins of the nares. The existence of a small levator septi nasi has been questioned.
CUTANEOUS VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Nasal skin receives its blood supply from branches of the facial, ophthalmic and infraorbital arteries. The alae and lower part of the nasal septum are supplied by lateral nasal and septal branches of the facial artery and the lateral aspects and dorsum of the nose are supplied by the dorsal nasal branch of the ophthalmic artery and the infraorbital branch of the maxillary artery. The venous networks draining the external nose do not run parallel to the arteries but correspond to arteriovenous territories of the face. The frontomedian region of the face, including the nose, drains to the facial vein, and the orbitopalpebral area of the face, including the root of the nose, drains to the ophthalmic veins. The connections of the veins of the nose, upper lip and cheek with the drainage area of the ophthalmic veins are clinically significant because they can be the route which spreads infection and initiates thrombosis of the major intracranial sinuses. Lymph drainage is primarily to the submandibular group of nodes although lymph draining from the root of the nose drains to superficial parotid nodes. There is experimental evidence that nasal lymphatics might play a major role in CSF transport in animals such as the sheep: there is currently very little evidence that a similar pathway exists in humans (but see Johnston et al 2004).
NASAL CAVITY
The nasal cavity is an irregular space between the roof of the mouth and the cranial base. It is wider below than above, and widest and vertically deepest in its central region, where it is divided by a vertical osseocartilaginous septum that is approximately median in position. The bony part of the septum reaches the posterior limit of the cavity.
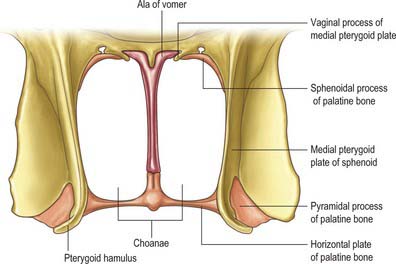
The nasal cavity communicates with the frontal, ethmoidal, maxillary and sphenoidal paranasal sinuses and opens into the nasopharynx through a pair of oval openings, the posterior nasal apertures or choanae. The latter are separated by the posterior border of the vomer, and each is limited above by the vaginal process of the medial pterygoid plates, laterally by the perpendicular plate of the palatine bone and the medial pterygoid plate, and below by the horizontal plate of the palatine bone (Fig. 32.4). The adult choana typically measures 2.5 cm in vertical height and 1.3 cm transversely: size is not usually affected by deviations of the nasal septum. The vomerovaginal and palatovaginal canals are found in the roof of this region.
Each half of the nasal cavity has a roof, floor, medial (septal) and lateral walls and a vestibule.
ROOF
The roof is horizontal in its central part and slopes downwards in front and behind (Fig. 32.3). The anterior slope is formed by the nasal spine of the frontal bones and by the nasal bones. The central region is formed by the cribriform plate of the ethmoid bone which separates the nasal cavity from the floor of the anterior cranial fossa. It contains numerous small perforations which transmit the olfactory nerves and their ensheathing meningeal layers, and a separate anterior foramen which transmits the anterior ethmoidal nerve and vessels. The posterior slope is formed by the anterior aspect of the body of the sphenoid, interrupted on each side by an opening of a sphenoidal sinus, and the sphenoidal conchae or superior conchae.
FLOOR
The floor of the nasal cavity is smooth and concave transversely, and slopes up from anterior to posterior apertures. The greater part is formed by the palatine processes of the maxillae which articulate posteriorly with the horizontal plates of the palatine bones at the palatomaxillary suture. Anteriorly, near the septum, a small infundibular opening in the bone of the nasal floor leads into the incisive canals that descend to the incisive fossa: this opening is marked by a slight depression in the overlying mucosa.
The floor of the nose may be deficient as a result of congenital clefting of the hard and/or soft palate.
MEDIAL WALL
The medial wall of each nasal cavity is the nasal septum, a thin sheet of bone (posteriorly) and cartilage (anteriorly), that lies between the roof and floor of the cavity (Fig. 32.2D).
Bony septum
The septum is usually relatively featureless but sometimes exhibits ridges or bony spurs. The posterosuperior part of the septum and its posterior border are formed by the vomer, which extends from the body of the sphenoid to the hard palate (for more details, see Chapter 29). Its surface is grooved by the nasopalatine nerves and vessels. The anterosuperior part of the septum is formed by the perpendicular plate of the ethmoid which is continuous above with the cribriform plate. Other bones which make minor contributions to the septum at the upper and lower limits of the medial wall are the nasal bones and the nasal spine of the frontal bones (anterosuperior), the rostrum and crest of the sphenoid (posterosuperior), and the nasal crests of the maxilla and palatine bones (inferior).
Cartilaginous septum
The septal cartilage is almost quadrilateral and may extend back (especially in children) for some distance between the vomer and the perpendicular plate of the ethmoid. Its anterosuperior margin is connected above to the posterior border of the internasal suture, and the distal end of its superior portion is continuous with the upper lateral cartilages. The anteroinferior border is connected by fibrous tissue on each side to the medial crurae of the major alar cartilage. The posterosuperior border joins the perpendicular plate of the ethmoid, while the posteroinferior border is attached to the vomer and anterior to that to the nasal crest and anterior nasal spine of the maxilla. The anteroinferior part of the nasal septum between the nares is devoid of cartilage and is therefore called the membranous septum: it is continuous with the columella anteriorly.
Above the incisive canals, at the lower edge of the septal cartilage, a depression pointing downwards and forwards is all that remains of the nasopalatine canal which connected the nasal and buccal cavities in early fetal life. Near this recess, a minute orifice leads back into a blind tubule, 2–6 mm long, which lies on each side of the septum and houses remnants of the vomeronasal organ.
Vomeronasal organ
In most amphibia, reptiles and mammals, the vomeronasal organ is the peripheral sensory organ of the accessory olfactory system. In these animals, paired vomeronasal organs are located either at the base of the nasal septum or in the roof of the mouth, and are involved in chemical communication that often, but not exclusively, is mediated via pheromones. In many macrosomatic animals the vomeronasal organ consists of a vomeronasal duct which contains chemosensory cells, and a vomeronasal nerve which terminates in the accessory olfactory bulb in the CNS. Whether adult humans possess a vomeronasal organ remains controversial; it is claimed that the duct sometimes persists beyond the embryonic stage (for detailed reviews, see Meredith 2001, Witt & Hummel 2006) but no neural connection appears to be present in humans after birth.
LATERAL WALL
The lateral wall of the nasal cavity is formed anteroinferiorly by the maxilla and its anterior and posterior fontanelles (bony deficiencies in the medial wall of the maxilla which are obliterated to varying degrees by fibrous tissue); posteriorly by the perpendicular plate of the palatine bone; and superiorly by the labyrinth of the ethmoid bone. It contains three projections of variable size, the inferior, middle and superior nasal conchae (turbinates) (Figs 32.2E, 32.5). The conchae curve generally inferomedially, each roofing a groove, or meatus, which is open to the nasal cavity. The middle conchae may also curve inferolaterally, or may be expanded by an enclosed air cell to form a so called ‘concha bullosa’ (30% of individuals) or have a concave medial surface, known as a paradoxical turbinate (10% of individuals).
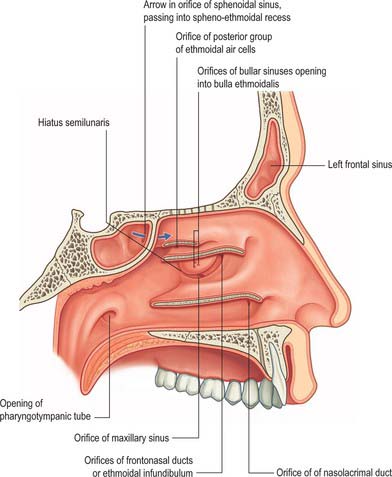
Fig. 32.5 Lateral wall of the nasal cavity. The conchae have been removed to show the positions of the ostia of the paranasal sinuses and the nasolacrimal duct.
(From Drake, Vogl and Mitchell 2005.)
Inferior concha and inferior meatus
The inferior concha is a thin, curved, independent bone (for more details, see Chapter 29). It articulates with the nasal surface of the maxilla and the perpendicular plate of the palatine bone. Its free lower border is gently curved and the subjacent inferior meatus reaches the nasal floor. The inferior meatus is the largest meatus, and it extends along almost all of the lateral nasal wall. It is deepest at the junction of its anterior and middle thirds, where it admits the inferior opening of the nasolacrimal canal (see Ch. 39). The canal is formed by the articulations between the lacrimal groove of the maxilla, the descending process of the lacrimal bone and the lacrimal process of the inferior concha. During postnatal development, the ostium of the nasolacrimal duct moves upwards and is increasingly hidden under the over-arching inferior concha.
Middle concha and middle meatus
The middle concha is a medial process of the ethmoidal labyrinth and may be pneumatized (conchal sinus). It extends back to articulate with the perpendicular plate of the palatine bone. The region beneath it is the middle meatus, which is deeper in front than behind, lies below and lateral to the middle concha, and continues anteriorly into a shallow fossa above the vestibule, termed the atrium of the middle meatus.
The main features of the lateral nasal wall are a rounded elevation, the bulla ethmoidalis, and a curved cleft, the hiatus semilunaris, formed by the posterior edge of the uncinate process, which constitutes the medial limit of the ethmoid infundibulum, a slit-like space that leads towards the maxillary ostium. The maxillary ostium is normally found lateral to the antero-inferior aspect of the uncinate process. The latter can be attached to the lateral nasal wall (50%), the anterior cranial fossa (25%) or the middle concha (25%). Where the uncinate process is attached determines whether the frontal sinus drains lateral to the ethmoid infundibulum or into it; if the uncinate process is attached to the lateral wall, the frontal sinus will drain into the middle meatus and not into the ethmoid infundibulum, but with the other configurations the sinus will drain into the infundibulum, and thus near or into the maxillary ostium. Agger nasi air cells are the name given to anterior ethmoid air cells that lie anterior to the ethmoid bulla (Fig. 32.5; see Fig. 32.12). The posterior fontanelle lies posterior to the uncinate process where there is no bone in the medial wall of the maxillary sinus, inferoposterior to the hiatus; it frequently has an accessory opening.
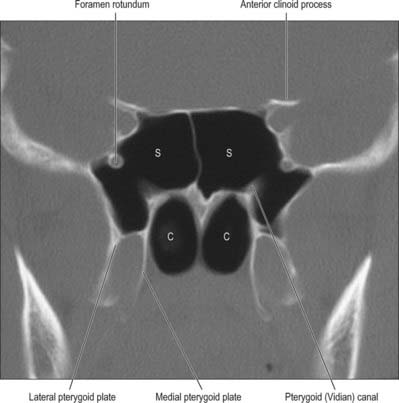
Fig. 32.12 Coronal CT scan showing the sphenoidal air sinus. S, Sphenoid sinus; C, Posterior choanae.
Sphenopalatine foramen
The sphenopalatine foramen, which is really a fissure, can be approached surgically through the middle meatus. It is posterior to the middle meatus and transmits the sphenopalatine artery and nasopalatine and superior nasal nerves from the pterygopalatine fossa (see Ch. 31). It is bounded above by the body and concha of the sphenoid, below by the superior border of the perpendicular plate of the palatine bone, and in front and behind by the orbital and sphenoidal processes of the palatine bone respectively.
Superior concha and superior meatus
The superior concha is a medial process of the ethmoidal labyrinth and presents as a small curved lamina, posterosuperior to the middle concha. It roofs the superior meatus and is the shortest and shallowest of the three conchae. Above the superior concha, the sphenoidal sinus opens into the triangular sphenoethmoidal recess which separates the superior concha and anterior aspect of the body of the sphenoid. The superior meatus is a short oblique passage extending about halfway along the upper border of the middle concha. The posterior ethmoidal sinuses open, via a variable number of apertures, into its anterior part.
Highest (supreme) nasal concha
Occasionally a fourth concha, the highest or supreme nasal concha, appears on the lateral wall of the sphenoethmoidal recess. The passage immediately below it is called the supreme nasal meatus; it may contain an opening for the posterior ethmoidal sinus.
Nasal vestibule
The nasal vestibule lies just inside the naris. It is limited above and behind by a curved ridge, the limen nasi, raised where the greater ala of the lateral cartilaginous crus overlaps the lower edge of the lateral nasal cartilage on each side. On the septal side of the nasal cavity the superior edge of the medial crus of the major alar cartilage (the medial intumescence) marks the boundary between the nasal vestibule and the nasal cavity. The medial wall of the vestibule is formed by a mobile septum consisting of the columella (which does not contain cartilage) and the underlying medial crura of the alar cartilages.
Nasal obstruction
The septum may be displaced by injury or by disproportionate growth of the cartilage that may cause it to bend; sometimes the deviation may cause unilateral nasal obstruction. Variations in the anatomy of the lateral nasal wall, usually associated with variations in the size and position of the anterior ethmoidal cells, may obstruct frontal or maxillary sinus drainage, e.g. frontal cells, supraorbital ethmoid cells and infraorbital or Haller’s cells, which represent an extension of anterior ethmoidal pneumatization along the infraorbital margin, sometimes within the roof of the maxillary sinus (see below).
NASAL AND OLFACTORY MUCOSAE
Nasal mucosa
The lining of the anterior part of the nasal cavity and vestibule is continuous with the skin, and consists of keratinized stratified squamous epithelium overlying a connective tissue lamina propria. Inferiorly the skin bears coarse hairs (vibrissae) which curve towards the naris and help to arrest the passage of particles in inspired air. In males, after middle age, these hairs increase considerably in size. Further posteriorly, at the limen nasi, this grades into a mucosa which is lined initially by non-keratinizing stratified squamous epithelium, and then by pseudostratified ciliated (respiratory) epithelium rich in goblet cells. Respiratory epithelium forms most of the surface of the nasal cavity, i.e. it covers the conchae, meati, septum, floor and roof, except superiorly in the olfactory cleft, where the olfactory epithelium is present. It is adherent to the periosteum or perichondrium of the neighbouring skeletal structures. In some areas, cells of the respiratory epithelium may be low columnar or cuboidal, and the proportion of ciliated to non-ciliated cells is variable.
There are numerous seromucous glands within the lamina propria of the nasal mucosa. Their secretions make the surface sticky so that it traps particles in the inspired air. The mucous film is continually moved by ciliary action (the mucociliary escalator or rejection current) posteriorly into the nasopharynx at a rate of 6 mm per minute. Palatal movements transfer the mucus and its entrapped particles to the oropharynx for swallowing, but some also enters the nasal vestibule anteriorly. The secretions of the nasal mucosa contain the bacteriocides lysozyme, β-defensin and lactoferrin, and also secretory immunoglobulins (IgA). The mucosa is continuous with the nasopharyngeal mucosa through the posterior nasal apertures, the conjunctiva through the nasolacrimal duct and lacrimal canaliculi, and the mucosa of the sphenoidal, ethmoidal, frontal and maxillary sinuses through their openings into the meati.
The mucosa is thickest and most vascular over the conchae, especially at their extremities, and also on the anterior and posterior parts of the nasal septum and between the conchae. The mucosa is very thin in the meati, on the nasal floor and in the paranasal sinuses. Its thickness reduces the volume of the nasal cavity and its apertures significantly. The lamina propria contains cavernous vascular tissue with large venous sinusoids.
Olfactory mucosa
Olfactory mucosa (Fig. 32.6) covers approximately 5 cm2 of the posterior upper parts of the lateral nasal wall, including the upper part of the vertical portion of the middle concha (where it is interspersed with respiratory epithelium in a checkerboard fashion) and the opposite part of the nasal septum, the superior concha, the sphenoethmoidal recess, the upper part of the perpendicular plate of the ethmoid and the portion of the roof of the nose that arches between the septum and lateral wall, including the underside of the cribriform plate (constituting the olfactory cleft or groove). It consists of a yellowish brown pigmented pseudostratified epithelium, containing olfactory receptor neurones, sustentacular cells and two classes of basal cell, lying on a subepithelial lamina propria containing subepithelial olfactory glands (of Bowman) and bundles of axons derived from the olfactory receptor neurones which course through the mucosa on their way to the cribriform plate. The glands secrete a predominantly serous fluid through ducts which open onto the epithelial surface. These secretions form a thin fluid layer in which sensory cilia and the microvilli of the sustentacular cells are embedded.
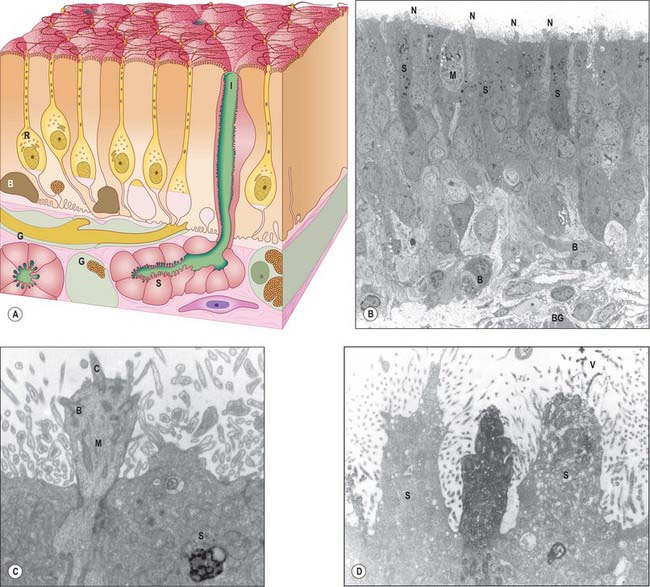
Fig. 32.6 A, The chief cytological features of the olfactory epithelium. Receptor cells (neurones) (R) are situated among columnar sustentacular cells. The axons of the receptor cells emerge from the epithelium in bundles enclosed by ensheathing glial cells (G). Rounded globose basal cells (B) and flattened horizontal basal cells (not shown) lie on the basal lamina and the subepithelial glands (of Bowman) (S) open on to the surface via their intraepithelial ducts (I). At the surface are cilia of the receptor cells and microvilli of the supporting cells. B, C and D are longitudinal sections through human olfactory epithelium: B, Ciliated olfactory receptor neurones with characteristic expanded ends (N) project into the nasal lumen. M, microvillar cell; S, supporting or sustentacular cells containing electron dense material; B, basal cells resting upon the basal lamina. The edge of a Bowman’s gland (BG) lies deeper in the lamina propria. C, Higher power view of the expanded end of an olfactory receptor neurone. The electron dense, osmiophilic material within the adjacent supporting cell (S) is thought to contribute to the pigmentation of the olfactory epithelium. C, olfactory cilium; B, basal body with projecting ‘feet’. D, Section of human olfactory epithelium immunostained with anti-OMP (olfactory marker protein): an immunopositive olfactory receptor neurone lies between two unstained supporting cells (S). V, microvilli.
(Parts B, C and D by courtesy of Professor Bruce Jafek, Department of Otolaryngology, University of Colorado, Denver, USA.)
Olfactory receptor neurones are bipolar. Their cell bodies and nuclei are located in the middle zone of the olfactory epithelium. Each neurone has a single unbranched apical dendrite, 2 μm diameter, which extends to the epithelial surface, and a basally directed unmyelinated axon, 0.2 μm diameter, which passes in the opposite direction, penetrates the basal lamina and enters the lamina propria. The tips of the dendrites project into the overlying secretory fluid and are expanded into characteristic endings (knobs) (Fig. 32.6B). Groups of up to 20 cilia radiate from the circumference of each ending and extend for long distances parallel to the epithelial surface. Internally, the short proximal part of each cilium has the ‘9 + 2′ pattern of microtubules typical of motile cilia, while the longer distal trailing end contains only the central pair of microtubules. The olfactory cilia lack dynein arms and are thought to be non-motile; their primary purpose is to increase the surface area of sensory receptor membrane available for the efficient detection of odorant molecules transferred across the mucous layer by odorant binding proteins. Mature olfactory neurones express olfactory marker protein (OMP), an abundant cytoplasmic protein involved in olfactory signal transduction (Fig. 32.6D). Each olfactory receptor neurone expresses receptors for a single (or very few) odorant molecules. In humans, over 1000 genes code for functional odorant receptors; the number of functional genes is much higher in macrosmotic animals (Buck & Axel 1991). Although neurones with the same receptor specificity are randomly distributed within anatomical zones of the epithelium, their axons all converge on the same glomerulus in the olfactory bulb. Specific odours activate a unique spectrum of receptor neurones which in turn activate restricted groups of glomeruli and their second order neurones.
The axons form small intraepithelial fascicles among the processes of sustentacular and basal cells. The fascicles penetrate the basal lamina, and are immediately surrounded by olfactory ensheathing cells. Groups of up to 50 such fascicles join to form larger olfactory nerve rootlets which pass through the cribriform plate of the ethmoid bone, wrapped in meningeal sheaths. They immediately enter the overlying olfactory bulbs, where they synapse in glomeruli with mitral cells and, to a lesser extent, with smaller tufted cells.
Microvillar cells occupy a superficial position in the olfactory epithelium. They are flask-shaped and electron-lucent, and the apical end of each cell gives rise to a tuft of microvilli that project into the mucus layer lining the nasal cavity (Fig. 32.6B). Cell counts in longitudinal sections reveal that microvillar cells occur with a density that is approximately one tenth of the density of ciliated olfactory neurones: their function and origin has yet to be determined.
Sustentacular, or supporting, cells are columnar cells that separate and partially ensheathe the olfactory receptor neurones. Their large nuclei form a layer superficial to the neuronal nuclei within the epithelium. The cells are capped by numerous long, irregular, microvilli which lie in the secretory fluid layer that covers the surface of the epithelium, intermingled with the trailing ends of the cilia on the olfactory receptor endings. Their expanded bases contain numerous lamellated dense bodies, which are the remnants of secondary lysosomes, and which contribute significantly to the pigmentation of the olfactory area (Fig. 32.6B,C). The granules gradually accumulate with age, and because these cells are long-lived, the intensity of pigmentation also increases with age. Neighbouring sustentacular cells are linked by desmosomes close to the epithelial surface, an arrangement that helps to stabilize the epithelium mechanically. Sustentacular cells and olfactory receptor neurones are linked by tight junctions at the level of the epithelial surface.
There are horizontal and globose basal cells. Horizontal basal cells are flattened against the basal lamina. Their nuclei are condensed and their darkly staining cytoplasm contains numerous intermediate filaments of the cytokeratin family, inserted into desmosomes between the basal cells and surrounding sustentacular cells. Globose cells are rounded or elliptical in shape, and have pale, euchromatic nuclei, and pale cytoplasm. They form a distinct zone that is slightly internal to the basal surface of the epithelium and characterized by mitotic figures: globose basal cells are the immediate source of new olfactory receptor neurones.
Olfactory ensheathing cells share properties with astrocytes and non-myelinating Schwann cells, but also possess distinctive features that indicate they are a separate class of glia. Developmentally they are derived from the olfactory placode rather than the neural crest. They ensheath olfactory axons in a unique manner throughout their entire course, and accompany them into the olfactory bulb, where they contribute to the glia limitans. In recent years, olfactory ensheathing cells have been the focus of intense experimental scrutiny in the search for a source of transplantable glia capable of supporting neuronal regeneration within the CNS, possibly in the treatment of paraplegia.
Olfactory (Bowman’s) glands are branched tubuloalveolar structures that lie beneath the olfactory epithelium and secrete their products onto the epithelial surface through narrow, vertical ducts. Their secretions, which include defensive substances, lysozyme, lactoferrin, IgA and sulphated proteoglycans, together with odorant-binding proteins which increase the efficiency of odour detection, bathe the dendritic endings and cilia of the olfactory receptors. The fluid acts as a solvent for odorant molecules, allowing their diffusion to the sensory receptors.
Turnover of olfactory receptor neurones
Olfactory receptor neurones are lost and replaced throughout life. Individual receptor cells have a variable lifespan, thought to average 1–3 months. Stem cells situated near the base of the epithelium undergo periodic mitotic division throughout life, giving rise to new olfactory receptor neurones which then grow a dendrite to the olfactory surface and an axon to the olfactory bulb. The cell bodies of these new receptor neurones gradually move apically until they reach the region just below the supporting cell nuclei. When they degenerate, dead neurones are either shed from the epithelium or are phagocytosed by sustentacular cells. The rate of receptor cell loss and replacement increases after exposure to damaging stimuli, but declines slowly with age, a phenomenon that presumably contributes to diminishing olfactory sensory function in old age. Biopsy specimens from normosmic adults have revealed that patchy replacement of olfactory with respiratory epithelium occurs even in young healthy adults (Paik et al 1992, Holbrook et al 2005).
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE OF THE NASAL CAVITY
Many of the vessels and nerves supplying the nasal cavities arise within the pterygopalatine fossa and these origins are described in Chapter 31.
Arteries
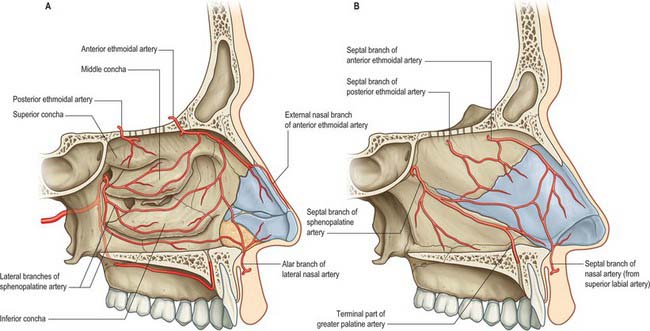
Branches of the ophthalmic, maxillary and facial arteries supply different territories within the walls, floor and roof of the nose (Fig. 32.7A,B). They ramify to form anastomotic plexuses within and deep to the nasal mucosa. Anastomoses also occur between some larger arterial branches. The anterior and posterior ethmoidal branches of the ophthalmic artery supply the ethmoidal and frontal sinuses and the roof of the nose (including the septum). The anterior ethmoidal artery usually runs within the bone of the anterior skull base unless this is well pneumatized with a supraorbital cell, in which case the artery is more likely to be positioned away from the skull base and to be more prone to surgical damage. The sphenopalatine branch of the maxillary artery supplies the mucosa of the conchae, meati and posteroinferior part of the nasal septum, i.e. it is the principal vessel supplying the nasal mucosa. The artery comes out of a fissure (erroneously termed a foramen), and normally divides before it enters the nasal cavity behind the crista ethmoidalis. The number and distribution of its branches show great variation. The greater palatine branch of the maxillary artery supplies the region of the inferior meatus. Its terminal part ascends through the incisive canal to anastomose on the septum with branches of the sphenopalatine and anterior ethmoidal arteries and with the septal branch of the superior labial artery. This septal region (Little’s area) is a common site of bleeding from the nose. The infraorbital artery and the superior, anterior, and posterior alveolar branches of the maxillary artery supply the mucosa of the maxillary sinus. The pharyngeal branch of the maxillary artery supplies the sphenoidal sinus.
Veins
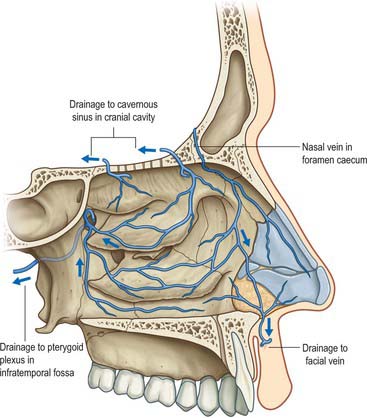
A rich submucosal cavernous plexus is especially dense in the posterior part of the septum and in the middle and inferior conchae (Fig. 32.8). Numerous arteriovenous anastomoses are present in the deep layer of the mucosa and around the mucosal glands. The cavernous conchal plexuses resemble those in erectile tissue: the nasal cavity is susceptible to blockage should they become engorged. Veins from the posterior part of the nose generally pass to the sphenopalatine vein that runs back through the sphenopalatine foramen to drain into the pterygoid venous plexus. The anterior part of the nose is drained mainly through veins accompanying the anterior ethmoidal arteries, and these veins subsequently pass into the ophthalmic or facial veins. A few veins pass through the cribriform plate to connect with those on the orbital surface of the frontal lobes of the brain. When the foramen caecum is patent, it transmits a vein from the nasal cavity to the superior sagittal sinus.
Nose bleeds
The vast majority of nosebleeds, particularly in children, occur as a result of digital trauma to the anastomosis of arterioles and veins in Little’s area (Kiesselbach’s plexus), on the nasal septum just inside the nasal vestibule. In older patients brisker bleeding may occur as a result of the spontaneous rupture of arteries further back in the nose. These may be controlled by applying pressure with a nasal pack but where this fails, knowledge of the pattern of arterial blood supply to the nasal cavity permits interruption of the appropriate blood supply by ligation or embolization of the feeding vessel. The sphenopalatine artery may be ligated under endoscopic visualization as it enters the nose. The ethmoidal arteries may be exposed within the orbit and ligated to arrest bleeding high up in the nasal cavity. The maxillary artery may be exposed surgically behind the posterior wall of the maxillary sinus and ligated, or alternatively it may be identified radiologically, using a radio-opaque dye, so that it may be blocked by embolization.
Lymphatic drainage
Lymph vessels from the anterior region of the nasal cavity pass superficially to join those draining the external nasal skin, and end in the submandibular nodes. The rest of the nasal cavity, paranasal sinuses, nasopharynx and pharyngeal end of the pharyngotympanic tube, all drain to the upper deep cervical nodes either directly or through the retropharyngeal nodes. The posterior nasal floor probably drains to the parotid nodes.
INNERVATION OF THE NASAL CAVITY
Olfaction is mediated via the olfactory nerves. General sensation (touch, pain and temperature) from the nasal mucosa is carried by branches of the ophthalmic and maxillary divisions of the trigeminal nerves (Fig. 32.9). Trigeminal fibres close to, and within, the epithelial layer are sensitive to noxious chemicals, e.g. ammonia and sulphur dioxide. Autonomic fibres innervate mucous glands and control cyclical and reactive vasomotor activity.
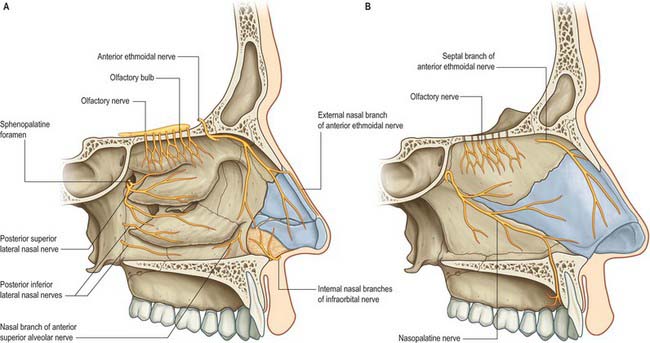
Fig. 32.9 The innervation of the nasal cavity. A, Lateral wall of the left nasal cavity. B, Medial wall of the left nasal cavity.
(From Drake, Vogl and Mitchell 2005.)
Trigeminal innervation
The anterior ethmoidal branch of the nasociliary nerve leaves the cranial cavity through a small slit near the crista galli and enters the roof of the nasal cavity where it runs in a groove on the inner surface of the nasal bone, supplying the roof of the nasal cavity. It gives off a lateral internal branch to supply the anterior part of the lateral wall and a medial internal branch to the anterior and upper parts of the septum, before emerging at the inferior margin of the nasal bone as the external nasal nerve to supply the skin of the external nose to the nasal tip. The infraorbital nerve supplies the nasal vestibule. The anterior superior alveolar nerve supplies part of the septum, the floor near the anterior nasal spine and the anterior part of the lateral wall as high as the opening of the maxillary sinus; the lateral posterior superior nasal and the posterior inferior nasal branches of the greater palatine nerve together supply the posterior three-quarters of the lateral wall, roof and floor; the medial posterior superior nasal nerves and the nasopalatine nerve supply the inferior part of the nasal septum; and branches from the nerve of the pterygoid canal supply the upper and posterior part of the roof and septum.
Autonomic innervation
Sympathetic postganglionic vasomotor fibres are distributed to the nasal blood vessels. Postganglionic parasympathetic fibres derived from the pterygopalatine ganglion provide the secretomotor supply to the nasal mucous glands, and are distributed via branches of the maxillary nerves.
Olfactory nerves
Olfactory nerves are bundles of very small axons derived from olfactory receptor neurons in the olfactory mucosa. The axons are unmyelinated, and in varying stages of maturity, reflecting the constant turnover of olfactory neurones that takes place in the olfactory epithelium. Bundles of axons surrounded by olfactory ensheathing cells form a plexiform network in the subepithelial lamina propria of the mucosa. The bundles unite into as many as 20 branches that cross the cribriform plate in lateral and medial groups, and enter the overlying olfactory bulb where they end in glomeruli. Each branch is ensheathed by dura mater and pia-arachnoid as it passes through the cribriform plate. The dura subsequently becomes continuous with the nasal periosteum, and the pia-arachnoid merges with the connective tissue sheaths surrounding the nerve bundles, an arrangement that may favour the spread of infection into the cranial cavity from the nasal cavity.
In severe injuries involving the anterior cranial fossa, the olfactory bulb may be separated from the olfactory nerves or the nerves may be torn, producing anosmia, i.e. loss of olfaction. Fractures may involve the meninges, so that cerebrospinal fluid may leak into the nose resulting in cerebrospinal rhinorrhoea. Such injuries open up avenues for intracranial infection from the nasal cavity.
NASAL AIRFLOW AND THE NASAL CYCLE
The paired nasal valves are critical regulators of nasal airflow and resistance. Each is divided into external and internal portions, although often only the internal portion is regarded as the ‘nasal valve’ or flow limiting segment. The external valve is made of the ala, the skin of the vestibule, the nasal sill and the contour of the medial crus of the lower lateral cartilage. The external valve has a tendency to collapse at high flow rates even in normal individuals.
The upper lateral cartilages are in continuity with the superior border of the nasal septum. A valve-like mechanism exists at its distal end that regulates nasal airflow. The nasal valve area and internal nasal valve are two entities that should not be confused. The nasal valve area is the narrowest portion of the nasal passage. It is bounded medially by the septum and the tuberculum of Zukerkandl, superiorly and laterally by the caudal margin of the upper lateral cartilages, its fibro-adipose attachment to the pyriform aperture, the anterior end of the inferior turbinate and inferiorly it is made of the floor of the pyriform aperture.
The entire nasal valve area averages 55 mm2 and 64 mm. The nasal valve, on the other hand, is the specific slit-like segment between the caudal margin of the upper lateral cartilage and the septum. It is measured in degrees and normally ranges between 10 and 15°. The dialator naris anterior muscle and the alar part of nasalis muscle support the nasal valves.
Airflow dynamics are recognized as playing a key role in conditioning (i.e. warming, humidifying and filtering) inspired air, however these dynamics are still poorly understood (Churchill et al 2004, Boyce & Eccles 2006). It is generally agreed that airflow within the nose is both laminar and turbulent. Inspired air is forced to pass through the nasal valve, and then expands as it passes further into the nasal cavity which offers little airflow resistance. This sudden change in speed and pressure produces turbulence and eddies. These currents allow adequate contact of inspired air with respiratory epithelium and facilitate odorant transport to the olfactory area. Chewing also affects nasal airflow: pulses of aroma-laden air are pumped out of the mouth into the retronasal region with each chew.
The cross sectional area of the nasal airway depends on the dimensions of the septal partition and inferior conchae, which are modulated by changes in the dimensions of the erectile tissue that covers them, and on the stability of the lateral nasal walls during breathing. The nasal cycle is an alternating fluctuation of nasal engorgement and airflow through the nasal passages, with period lengths ranging from approximately 1 to 5 hours. The mechanism involves changes in sympathetic tone to the venous erectile tissue of the nasal mucosa; increased sympathetic vasoconstriction causes resistance to fall. These alterations in the thickness and contours of the mucosal surfaces are visible as a swelling or shrinkage of the nasal lining, and may serve to protect the mucosae from desiccation. The pacemaker for the cycle is believed to lie within the suprachiasmatic nucleus. The rhythmicity of the cycle decreases with age.
Disturbances of the normal airflow pattern, whether produced by mucocutaneous or skeletal changes within the nose, affect normal nasal breathing and are usually perceived as some form of nasal obstruction.
PARANASAL SINUSES
The paranasal sinuses are the frontal, ethmoidal, sphenoidal and maxillary sinuses, housed within the bones of the same name (Figs 32.1, 32.10-32.13). They all open into the lateral wall of the nasal cavity by small apertures that permit both the equilibration of air between the various air spaces and the clearance of mucus from the sinuses into the nose via a mucociliary escalator. The detailed position of these apertures, and the precise form and size of each of the sinuses, varies enormously between individuals. Respiratory epithelium extends through the apertures of the paranasal sinuses to line their cavities, a feature that unfortunately favours the spread of infections. Sinus mucosa is thinner, less vascular and has fewer goblet cells than nasal mucosa. Cilia are always present in the mucosa near the apertures but less evenly distributed elsewhere within the sinuses.
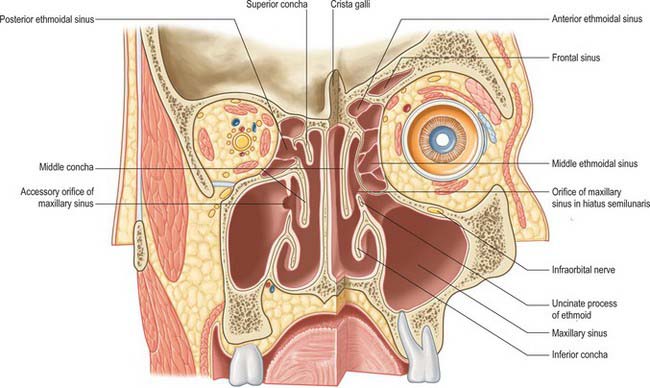
Fig. 32.10 A coronal section through the nasal cavity, viewed from the posterior aspect. The plane of the section on the right side is more anterior. The normal orifice of the maxillary sinus is shown on the right side and an accessory orifice on the left side.
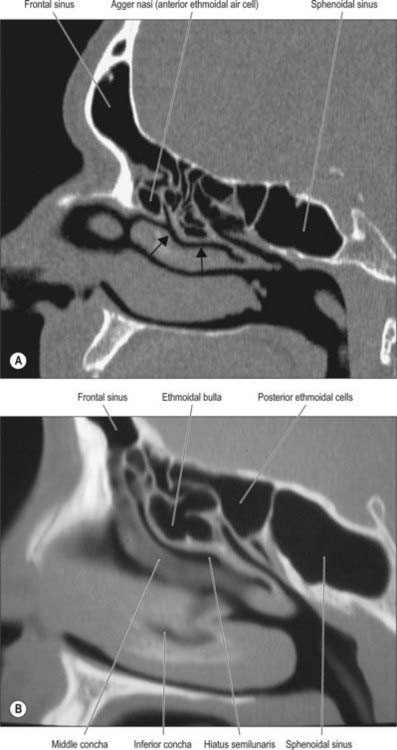
Fig. 32.13 A and B, Sagittal CT scans showing the frontal, ethmoidal and sphenoidal sinuses. Arrows indicate the hiatus semilunaris in A.
(A by courtesy of Professor D Bell.)
Most sinuses are rudimentary or absent at birth, but enlarge appreciably during the eruption of the permanent teeth and after puberty, events that significantly alter the size and shape of the face.
The functions of the paranasal sinuses remain speculative. They clearly add some resonance to the voice, and also allow the enlargement of local areas of the skull while minimizing a corresponding increase in bony mass. It is likely that such growth-related changes serve to strengthen particular regions, e.g. the alveolar process of the maxilla when the secondary dentition erupts, but they may also function in contouring the head to provide visual signals indicating the individual’s status in a social context (e.g. gender, sexual maturity and group identity).
FRONTAL SINUS
The paired frontal sinuses are posterior to the superciliary arches, between the outer and inner tables of the frontal bone (Figs 32.10, 32.11, 32.13A). Each usually underlies a triangular area on the surface of the face, its angles formed by the nasion, a point 3 cm above the nasion and the junction of the medial one-third and lateral two-thirds of the supraorbital margin. The two sinuses are rarely symmetrical, since the septum between them usually deviates from the median plane. Each sinus may be further divided into a number of communicating recesses by incomplete bony septa.
The average dimensions of an adult frontal sinus are: height 3.2 cm; breadth 2.6 cm; depth 1.8 cm. Each usually has a frontal portion that extends upwards above the medial part of the eyebrow, and an orbital portion that extends back into the medial part of the roof of the orbit. In 4% one or both sinuses may be hypoplastic or even absent; racial differences have been reported. The prominence of the superciliary arches is no indication of the presence or size of the frontal sinuses. A frontal sinus may extend posteriorly as far as the lesser wing of the sphenoid bone but does not invade it. The morphology of the frontal sinus may be specific enough to allow identification of individuals from radiological evidence for forensic purposes.
The aperture of each frontal sinus either opens into the anterior part of the corresponding middle meatus by the ethmoidal infundibulum either as a frontonasal recess (rather than a duct) or medial to the hiatus semilunaris if the uncinate process is attached to the lateral nasal wall or an agger nasi cell.
The frontal sinuses are rudimentary or absent at birth, generally well developed between the seventh and eighth years, but reach full size only after puberty. They are more prominent in males, and lend the forehead an obliquity that contrasts with the vertical or convex profile typical of children and females. In the presence of a persistent metopic suture, the frontal sinuses develop separately on either side of the suture, which can be helpful in excluding frontal fractures. (The frontal bone is described in Chapter 29.)
Vascular supply, lymphatic drainage and innervation
The frontal sinuses receive their arterial supply from the supraorbital and anterior ethmoidal arteries. The veins drain into an anastomotic vein in the supraorbital notch that connects the supraorbital and superior ophthalmic veins. Lymphatic drainage is to the submandibular nodes. The sinuses are innervated by branches of the supraorbital nerves (general sensation), and the orbital branches of the pterygopalatine ganglia (parasympathetic secretomotor fibres).
SPHENOIDAL SINUS
The sphenoidal sinuses are two large irregular cavities within the body of the sphenoid and therefore lie posterior to the upper part of the nasal cavity (Figs 32.12-32.15). Each opens into the corresponding sphenoethmoidal recess via an aperture high on the anterior wall of the sinus. At birth the sinuses are minute cavities, and their main development occurs after puberty. The average adult dimensions are: vertical height 2 cm; transverse breadth 1.8 cm; anteroposterior depth 2.1 cm. The sinuses are usually separated by a septum that deviates from the midline in 75%, so that they are unequal in size and form. Their lumina may be further partially divided by bony laminae, accessory septa, especially in the region of former synchondroses. Occasionally one sinus overlaps the other above and, rarely, they intercommunicate. Bony ridges, produced by the internal carotid artery, pterygoid canal, maxillary branch of trigeminal and, in 15% of individuals, the optic nerve, may project into the sinuses from their lateral walls. Dehiscences in the osseous walls of either sinus may occasionally leave their mucosa in contact with the overlying dura mater.
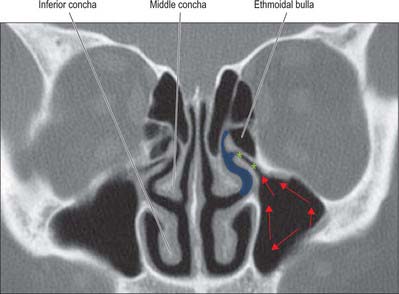
Fig. 32.14 Coronal CT scan through the ostiomeatal complex. Red arrows, direction of mucociliary flow; blue area, middle meatus; green stars, infundibulum.
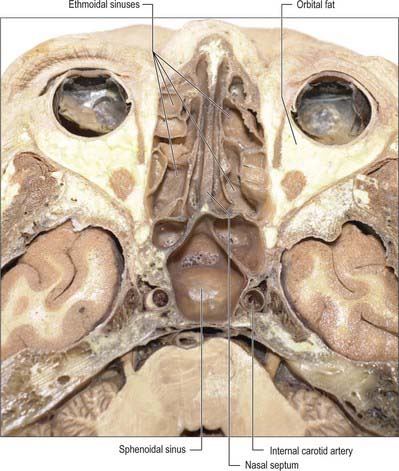
Fig. 32.15 Horizontal section of the head showing the ethmoidal and sphenoidal sinuses.
(By permission from Berkovitz BKB, Moxham BJ 2002 Head and Neck Anatomy. London: Martin Dunitz.)
The extent of pneumatization of surrounding bone is highly variable. In nearly 50% of skulls, a lateral recess may extend into the greater and lesser wings of the sphenoid or the pterygoid processes, and may even invade the basilar part of the occipital bone almost to the foramen magnum. A posterior ethmoidal sinus may extend posterosuperior to the relatively smaller sphenoidal sinuses. The sphenoidal sinuses are related above to the optic chiasma and hypophysis cerebri and on each side to the internal carotid artery and cavernous sinus (see Ch. 27). One or both sinuses may partially encircle the optic canal.
The shape and position of the sphenoidal sinus is of clinical importance in the trans-sphenoidal surgical approach to the hypophysis cerebri. The sinuses may be classified into three main types: sellar, the commonest type, where the sinus extends for a variable distance beyond the tuberculum sellae; presellar, where the sinus occasionally extends posteriorly towards, but not beyond, the tuberculum sellae; conchal, the rarest type, where a small sinus is separated from the sella turcica by 10 mm of trabecular bone. The anterior midline septum often becomes deviated to one side posteriorly: identification of this septation is important prior to surgery.
Vascular supply, lymphatic drainage and innervation
The sphenoidal sinuses receive their arterial supply from the posterior ethmoidal branches of the ophthalmic arteries and nasal branches of the sphenopalatine arteries. Venous drainage is through the posterior ethmoidal veins draining into the superior ophthalmic veins. Lymph drainage is to the retropharyngeal nodes. The sinuses are innervated by the posterior ethmoidal branches of the ophthalmic nerves (general sensation), and the orbital branches of the pterygopalatine ganglia (parasympathetic secretomotor fibres).
ETHMOIDAL SINUSES
The ethmoidal sinuses differ from the other paranasal sinuses in that they are formed of multiple thin-walled cavities in the ethmoidal labyrinth (Figs 32.10, 32.13-32.15) (for more details, see Chapter 29). The number and size of the cavities varies, from three large to 18 small sinuses on each side. They lie between the upper part of the nasal cavity and the orbit, and are separated from the latter by the paper-thin lamina papyracea or orbital plate of the ethmoid (this presents a poor barrier to infection which may therefore spread into the orbit). Pneumatization may extend into the middle concha, or into the body and wings of the sphenoid bone lateral to the sphenoid sinus.
The ethmoidal sinuses are divided clinically into anterior and posterior groups on each side, distinguished by their sites of communication with the nasal cavity. (Cells previously designated as belonging to a middle group are now included with the anterior group.) The anterior and posterior groups are separated from each other by the basal lamella of the middle concha; this may be indented by cells from either group and therefore it forms a rather tortuous barrier between them. In each group the sinuses are only partially separated by incomplete bony septa. The sinuses are of clinical significance at birth because they are susceptible to inflammation. They grow rapidly between the ages of 6 and 8 years and after puberty.
Anterior group
Peri-infundibular sinuses (anterior ethmoidal air cells)
Up to 11 anterior ethmoidal air cells drain into either the ethmoidal infundibulum or the frontonasal duct by one or more orifices. The most anterior group, agger nasi cells, are medial relations of the lacrimal sac and duct and invaginate beneath the agger nasi on the lateral wall of the nasal cavity anteriorly. Larger anterior and middle cells, Haller’s cells, may develop medially beneath the orbital floor. The most anterior supraorbital ethmoidal sinus cells may encroach on the frontal sinus.
Posterior group
Up to seven posterior ethmoidal air cells usually drain by a single orifice into the superior meatus; one may drain into the supreme meatus when present, and one or more into the sphenoidal sinus. The posterior group lies very close to the optic canal and optic nerve: optic nerve injury is a devastating potential complication of endoscopic sinus surgery, particularly if a spheno-ethmoid or Onodi cell is present. The reported incidence of an Onodi cell varies widely (3.5 to 51%), according to racial group: it is more common in non-Caucasians. The Onodi cell is usually regarded as the most posterior ethmoid cell that pneumatizes lateral and superior to the sphenoid sinus and is intimately associated with the optic nerve: it may contain a tuberculum nervi optici, where the optic canal bulges into the wall of the cell.
Vascular supply and innervation
The ethmoidal sinuses receive their arterial supply from nasal branches of the sphenopalatine artery and the anterior and posterior ethmoidal branches of the ophthalmic artery. Venous drainage is by the corresponding veins. The lymphatics of the anterior group drain to the submandibular nodes, and those of the posterior group to the retropharyngeal nodes. The sinuses are innervated by the anterior and posterior ethmoidal branches of the ophthalmic nerves (general sensation), and the orbital branches of the pterygopalatine ganglia (parasympathetic secretomotor fibres).
MAXILLARY SINUS
The maxillary sinus (maxillary antrum), is the largest of the paranasal sinuses. It fills the body of the maxilla (Chapter 29) and is pyramidal in shape (Fig. 32.10, Fig. 32.14). The base is medial and forms much of the lateral wall of the nasal cavity. The floor, which often lies below the nasal floor, is formed by the alveolar process and part of the palatine process of the maxilla. It is related to the roots of the teeth, especially the second premolar and first molar, but may extend posteriorly to the third molar tooth and/or anteriorly to incorporate the first premolar, and sometimes the canine. Defects in the bone overlying the roots are not uncommon. The roof of the sinus forms the major part of the floor of the orbit. It contains the infraorbital canal which may exhibit dehiscences. The lateral truncated apex of the pyramid extends into the zygomatic process of the maxilla, and may reach the zygomatic bone, in which case it forms the zygomatic recess which throws a V-shaped shadow over the antrum on a lateral radiograph.
The facial surface of the maxilla forms its anterior wall, and is grooved internally by a delicate canal (canalis sinuosus) which houses the anterior superior alveolar nerve and vessels as they pass forwards from the infraorbital canal. The posterior wall is formed by the infratemporal surface of the maxilla: it contains alveolar canals that may produce ridges in the sinus and that also conduct the posterior superior alveolar vessels and nerves to the molar teeth. The medial wall is deficient posterosuperiorly at the maxillary hiatus, a large opening which is partially closed in an articulated skull by portions of the perpendicular plate of the palatine bone, the uncinate process of the ethmoid bone, the inferior nasal concha, the lacrimal bone, and the overlying nasal mucosa, to form an ostium and anterior and posterior fontanelles. The ostium usually opens into the inferior part of the ethmoidal infundibulum, and thence into the middle meatus, via the hiatus semilunaris (the hiatus forms the area above the superior edge of the uncinate process). The fontanelles are covered only by periosteum and mucosa and may contain accessory ostia which may be visible on CT. All of the openings are nearer the roof than the floor of the sinus which means that the natural drainage of the maxillary sinus is reliant on an intact mucociliary escalator: the cilia of the sinus mucoperiosteum normally beat towards the ostium.
The maxillary sinus may be incompletely divided by septa; complete septa are very rare. The thinness of its walls is clinically significant in determining the spread of tumours from the maxillary sinus. A tumour may push up the orbital floor and displace the eyeball; project into the nasal cavity, causing nasal obstruction and bleeding; protrude onto the cheek, causing swelling and numbness if the infraorbital nerve is damaged; spread back into the infratemporal fossa, causing restriction of mouth opening due to pterygoid muscle damage and pain; or spread down into the mouth, loosening teeth and causing malocclusion. Extraction of molar teeth may damage the floor, and impact may fracture its walls. Hypoplasia of one maxillary antrum is present in up to 0.3% of the population.
Ostiomeatal complex
The term ostiomeatal complex, or ostiomeatal unit, refers to the area that includes the maxillary sinus ostium, ethmoid infundibulum and the hiatus semilunaris (Fig. 32.14). It is the common pathway for drainage of secretions from the maxillary and anterior group of ethmoidal sinuses; where the uncinate process attaches to the lateral nasal wall the complex also drains the frontal sinus.
Vascular supply, lymphatic drainage and innervation
The arterial supply of the maxilla is derived mainly from the maxillary arteries via the superior anterior, middle and posterior alveolar branches and from the infraorbital and greater palatine arteries. Branches of the posterior superior alveolar artery and the infraorbital artery form an anastomosis in the bony wall of the sinus, which also supplies the mucous membrane that lines the nasal chambers. An extraosseous anastomosis frequently exists between the posterior superior alveolar artery and the infraorbital artery. The intra- and extra-osseous anastomoses form a double arterial arcade which supplies the lateral antral wall and, partly, the alveolar process.
Veins corresponding to the arteries drain into the facial vein or pterygoid venous plexus on either side. Lymph drainage is to the submandibular nodes. The sinuses are innervated by the infraorbital and anterior, middle and posterior superior alveolar branches of the maxillary nerves (general sensation), and nasal branches of the pterygopalatine ganglia (parasympathetic secretomotor fibres).
For further details concerning the dimensions of the various air sinuses and the size and positions of their openings the reader is referred to Lang (1989).
IMAGING OF THE PARANASAL SINUSES
Standard radiological images are no longer recommended in the diagnosis of rhinosinusitis because of their poor specificity and sensitivity, although they may be used to confirm the presence of acute frontal or maxillary sinusitis that has failed to respond to medical treatment and requires urgent drainage. CT defines anatomical variations but should not be used in isolation for diagnosis because 1 in 3 asymptomatic individuals show incidental mucosal changes. Spiral CT provides good quality axial, coronal and sagittal images that facilitate an appreciation of the size and relationship of the paranasal sinuses.
SPREAD OF INFECTION TO CRANIAL CAVITY
The state of health of the paranasal sinuses depends on immunity, mucociliary clearance and aeration of the sinuses. The middle meatus forms the common drainage pathway for the anterior ethmoidal, frontal and maxillary sinuses, and can be readily examined with a fibreoptic endoscope (Simmen & Jones 2005). The posterior ethmoidal and sphenoidal sinuses drain into the superior meatus and sphenoethmoidal recess. Endoscopic examination will usually show infected mucus draining from these areas. Maxillary sinus infection may also spread from infected teeth.
Boyce J, Eccles R. Do chronic changes in nasal airflow have any physiological or pathological effect on the nose and paranasal sinuses? A systematic review. Clin Otolaryngol. 2006;31:15-19.
Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175-187.
The 2004 Nobel Prize in Physiology or Medicine was awarded to Axel and Buck for their discoveries of the genetic determinants of the olfactory receptors and the organization of the olfactory system in humans..
Churchill SE, Shackelford LL, Georgi JN, Black MT. Morphological variation and airflow dynamics in the human nose. Am J Hum Biol. 2004;16:625-638.
Doig TN, McDonald SW, McGregor OA. Possible routes of spread of carcinoma of the maxillary sinus to the oral cavity. Clin Anat. 1998;11:149-156.
Emanuel JM. Epistaxis. Cummings CW, et al, editors. Otolaryngology Head and Neck Surgery, 3rd edn., vol. 2. St Louis: Mosby, 1998;852-865.
Describes the blood supply to the nose and the surgical approaches to control epistaxis..
Holbrook EH, Leopold DA, Schwob JE. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope. 2005;115:2144-2154.
Jafek BW. Ultrastructure of human nasal mucosa. Laryngoscope. 1983;93:1576-1599.
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1:2.
Lang J. Clinical Anatomy of the Nose, Nasal Cavity and Paranasal Sinuses. Stuttgart: Thième, 1989.
McGowan DA, Baxter PW, James J. The Maxillary Sinus. Oxford: Wright, 1993.
Meredith M. Human vomeronasal organ function: a critical review of best and worst cases. Chem Senses. 2001;26:433-445.
Navarro JAC. The Nasal Cavity and Paranasal Sinuses: Surgical Anatomy. Berlin: Springer, 1997.
Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV. Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118:731-738.
Simmen D, Jones N. Manual of endoscopic sinus surgery and its extended applications. Stuttgart: Thieme, 2005.
Stammberger H, Kennedy DW. Paranasal sinuses: anatomic terminology and nomenclature. Ann Otol Rhinol Laryngol. 1995;104(Suppl 167):7-16. (10) Part 2
Traxler H, Windisch A, Geyerhofer U, Surd R, Solar P, Firbas W. Arterial blood supply of the maxillary sinus. Clin Anat. 1999;12:417-421.
Witt M, Hummel T. Vomeronasal versus olfactory epithelium: is there a cellular basis for human vomeronasal perception? Internat Rev Cytol. 2006;248:209-259.