CHAPTER 68 Liver
The liver is the largest of the abdominal viscera, occupying a substantial portion of the upper abdominal cavity. It occupies most of the right hypochondrium and epigastrium, and frequently extends into the left hypochondrium as far as the left lateral line (Fig. 68.1). As the body grows from infancy to adulthood the liver rapidly increases in size. This period of growth reaches a plateau around 18 years and is followed by a gradual decrease in the liver weight from middle age. The ratio of liver to body weight decreases with growth from infancy to adulthood. The liver weighs approximately 5% of the body weight in infancy and it decreases to approximately 2% in adulthood. The size of the liver also varies according to sex, age and body size. It has an overall wedge shape, which is in part determined by the form of the upper abdominal cavity into which it grows. The narrow end of the wedge lies towards the left hypochondrium, and the anterior edge points anteriorly and inferiorly. The superior and right lateral aspects are shaped by the anterolateral abdominal and chest wall as well as the diaphragm. The inferior aspect is shaped by the adjacent viscera. The capsule is no longer thought to play an important part in maintaining the integrity of the shape of the liver.
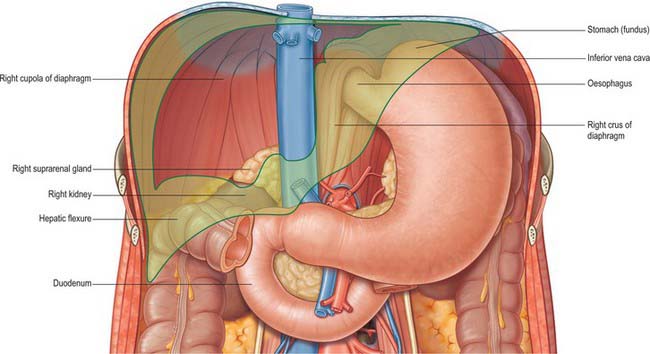
Fig. 68.1 The ‘bed’ of the liver. The outline of the liver is shaded green. The central bare area is unshaded.
Throughout life the liver is reddish brown in colour, although this can vary depending upon the fat content. Obesity is the most common cause of excess fat in the liver (also known as steatosis): the liver assumes a more yellowish tinge as its fat content increases. The texture is usually soft to firm, although it depends partly on the volume of blood the liver contains and the fat content.
The liver performs a wide range of metabolic activities required for homeostasis, nutrition and immune defence. For example, it is important in the removal and breakdown of toxic, or potentially toxic, materials from the blood and the regulation of blood glucose and lipids, the storage of certain vitamins, iron, and other micronutrients, and in breaking down or modifying amino acids. It is involved in a plethora of other biochemical reactions. Since the majority of these processes are exothermic, a substantial part of the thermal energy production of the body, especially at rest, is provided by the liver. The liver is populated by phagocytic macrophages, components of the mononuclear phagocyte system capable of removing particulates from the blood stream. It is an important site of haemopoiesis in the fetus.
SURFACES OF THE LIVER
The liver is usually described as having superior, anterior, right, posterior and inferior surfaces, and has a distinct inferior border (Figs 68.2, 68.3). However, the superior, anterior and right surfaces are continuous and no definable borders separate them. It would be more appropriate to group them as the diaphragmatic surface, which is mostly separated from the inferior, or visceral surface, by a narrow inferior border. At the infrasternal angle the inferior border is related to the anterior abdominal wall and is accessible to examination by percussion, but is not usually palpable. In the midline, the inferior border of the liver is near the transpyloric plane, about a hand’s breadth below the xiphisternal joint. In women and children the border often projects a little below the right costal margin.
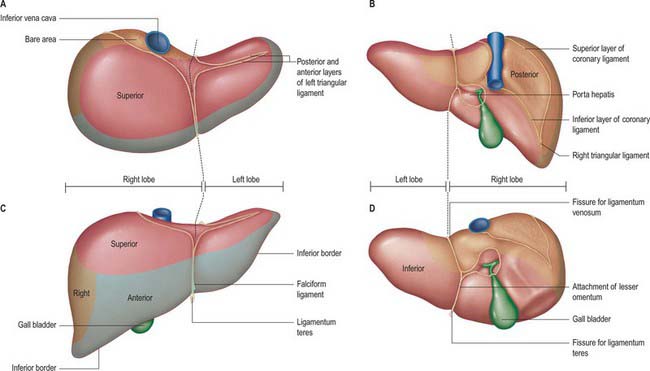
Fig. 68.2 The surfaces and external features of the liver. A, superior view; B, posterior view; C, anterior view; D, inferior view.
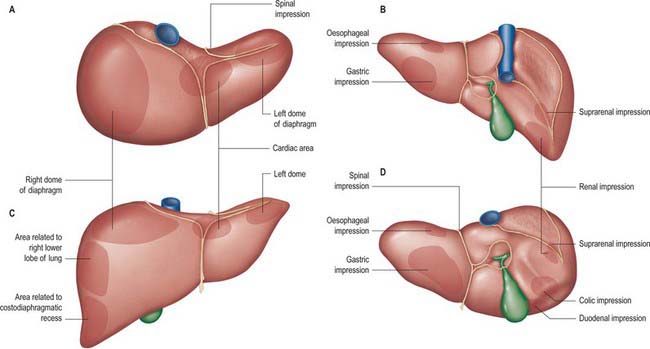
Fig. 68.3 Relations of the liver. A, superior view; B, posterior view; C, anterior view; D, inferior view.
The superior surface is the largest surface and lies immediately below the diaphragm, separated from it by peritoneum except for a small triangular area where the two layers of the falciform ligament diverge. The majority of the superior surface lies beneath the right dome, but there is a shallow cardiac impression centrally that corresponds to the position of the heart above the central tendon of the diaphragm. The left side of the superior surface lies beneath part of the left dome of the diaphragm.
The anterior surface is approximately triangular and convex and is covered by peritoneum except at the attachment of the falciform ligament. Much of it is in contact with the anterior attachment of the diaphragm. On the right the diaphragm separates it from the pleura and sixth to tenth ribs and cartilages, and on the left from the seventh and eighth costal cartilages.
The right surface is covered by peritoneum and lies adjacent to the right dome of the diaphragm which separates it from the right lung and pleura and the seventh to 11th ribs. The right lung and basal pleura both lie above and lateral to its upper third, between the diaphragm and the seventh and eighth ribs. The diaphragm, the costodiaphragmatic recess lined by pleura, and the ninth and tenth ribs all lie lateral to the middle third of the right surface. Lateral to the lower third, the diaphragm and thoracic wall are in direct contact. Rarely, the hepatic flexure and proximal transverse colon may lie on a long mesentery over the right and superior surfaces of the liver, referred to as Chilaiditi syndrome.
The posterior surface is convex, wide on the right, but narrow on the left. A deep median concavity corresponds to the forward convexity of the vertebral column close to the attachment of the ligamentum venosum. Much of the posterior surface is attached to the diaphragm by loose connective tissue, forming the triangular ‘bare area’. The inferior vena cava lies in a groove or tunnel in the medial end of the ‘bare area’. To the left of the caval groove the posterior surface of the liver is formed by the caudate lobe, and covered by a layer of peritoneum continuous with that of the inferior layer of the coronary ligament and the layers of the lesser omentum. The caudate lobe is related to the diaphragmatic crura above the aortic opening and the right inferior phrenic artery, and separated by these structures from the descending thoracic aorta.
The fissure for the ligamentum venosum separates the posterior aspect of the caudate lobe from the main part of the left lobe. The fissure cuts deeply in front of the caudate lobe and contains the two layers of the lesser omentum. The posterior surface over the left lobe bears a shallow impression near the upper end of the fissure for the ligamentum venosum that is caused by the abdominal part of the oesophagus. The posterior surface of the left lobe to the left of this impression is related to part of the fundus of the stomach. Together these posterior relations make up what is sometimes referred to as the ‘bed’ of the liver (Fig. 68.1).
The inferior surface is bounded by the inferior edge of the liver. It blends with the posterior surface in the region of the origin of the lesser omentum, the porta hepatis and the lower layer of the coronary ligament, and is marked near the midline by a sharp fissure which contains the ligamentum teres (the obliterated fetal left umbilical vein). The gallbladder usually lies in a shallow fossa, but this is variable: it may have a short mesentery or be completely intrahepatic and lie within a cleft in the liver parenchyma. The quadrate lobe lies between the fissure for the ligamentum teres and the gallbladder.
The inferior surface of the left lobe is related inferiorly to the fundus of the stomach and the upper lesser omentum. The quadrate lobe lies adjacent to the pylorus, first part of the duodenum and the lower part of the lesser omentum. Occasionally the transverse colon lies between the duodenum and the quadrate lobe. To the right of the gallbladder, the inferior surface is related to the hepatic flexure of the colon, the right suprarenal gland and right kidney, and the first part of the duodenum.
GROSS ANATOMICAL LOBES
Historically, the liver has been considered to be divided into right, left, caudate and quadrate lobes by the surface peritoneal and ligamentous attachments.
The right lobe is the largest in volume and contributes to all surfaces of the liver. It is divided from the left lobe by the falciform ligament superiorly and the ligamentum venosum inferiorly. On the inferior face to the right of the groove formed by the ligamentum venosum there are two prominences separated by the porta hepatis: the caudate lobe lies posterior, and the quadrate lobe anterior, to the porta hepatis. The gallbladder lies in a shallow fossa to the right of the quadrate lobe.
The left lobe is the smaller of the two main lobes, although it is nearly as large as the right lobe in young children.
It lies to the left of the falciform ligament with no subdivisions, and is substantially thinner than the right lobe, having a thin apex that points into the left upper quadrant.
The quadrate lobe is visible as a prominence on the inferior surface of the liver, to the right of the groove formed by the ligamentum venosum (and thus is incorrectly said to arise from the right lobe although it is functionally related to the left lobe). It lies anterior to the porta hepatis and is bounded by the gallbladder fossa to the right, a short portion of the inferior border anteriorly, the fissure for the ligamentum teres to the left, and the porta hepatis posteriorly.
The caudate lobe is visible as a prominence on the inferior and posterior surfaces to the right of the groove formed by the ligamentum venosum: it lies posterior to the porta hepatis. To its right is the groove for the inferior vena cava. Above, it continues into the superior surface on the right of the upper end of the fissure for the ligamentum venosum. In gross anatomical descriptions this lobe is said to arise from the right lobe, but it is functionally separate.
FUNCTIONAL ANATOMICAL DIVISIONS
Current understanding of the functional anatomy of the liver is based on Couinaud’s division of the liver into eight (subsequently nine) functional segments, based upon the distribution of portal venous branches and the location of the hepatic veins in the parenchyma (Couinaud 1957). Further understanding of the intrahepatic biliary anatomy, especially of the right ductal system, was enhanced by contributions from Hjortsjo (1948) and Healey & Schroy (1953) using the biliary system as the main guide for division of the liver (Fig. 68.4).
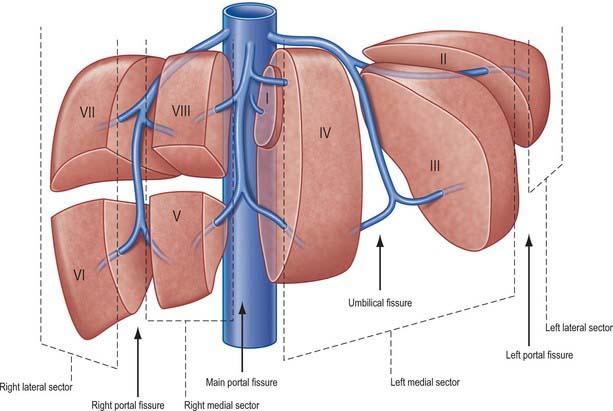
Fig. 68.4 The fissures and sectors of the liver. (Right lateral = right posterior; right medial = right anterior.)
The liver is divided into four portal sectors by the four main branches of the portal vein. These are right lateral, right medial, left medial and left lateral (sometimes the term posterior is used in place of lateral and anterior in place of medial). The three main hepatic veins lie between these sectors as intersectorial veins. These intersectoral planes are also called portal fissures (scissures). The fissures containing portal pedicles are called hepatic fissures. Each sector is sub-divided into segments (usually two) based on their supply by tertiary divisions of the vascular biliary sheaths.
Fissures of the liver
Knowledge of the fissures of the liver is essential for understanding liver surgery. Three major fissures, not visible on the surface, run through the liver parenchyma and harbour the three main hepatic veins (main, left and right portal fissures). Three minor fissures are visible as physical clefts of the liver surface (umbilical, venous and fissure of Gans).
The main fissure extends from the tip of the gallbladder back to the midpoint of the inferior vena cava and contains the middle (main) hepatic vein. It separates the liver into right and left hemi-livers. Segments V and VIII lie to the right and segment IV to the left of the fissure.
The left fissure divides the left hemi-liver into medial (anterior) and lateral (posterior) sectors. It extends from the mid point of the anterior edge of the liver between the falciform ligament and the left triangular ligament to the point which marks the confluence of the left and middle hepatic veins. It contains the left hepatic vein and separates the left anterior and left posterior sectors: segment III lies anteriorly and segment II posteriorly.
The right portal fissure divides the right hemi-liver into lateral (posterior) and medial (anterior) sectors. The plane of the right fissure is the most variable amongst the main fissures and runs approximately diagonally through the gross right lobe from the lateral end of the anterior border to the confluence of the left and middle hepatic veins. The fissure divides the right anterior sector to its left (segments V and VIII) from the right posterior sector to its right (segments VI and VII), and contains the right hepatic vein. The right fissure marks the thickest point of liver parenchyma which is commonly transected during liver resection.
The umbilical fissure separates segment III from segment VI within the left anterior sector and contains a main branch of the left hepatic vein (the umbilical fissure vein). It is marked by the attachment of the falciform ligament and sometimes covered by a ridge of liver tissue extending between the segments: it is often avascular and can be divided safely with diathermy during a surgical approach. It contains the umbilical portion of the left portal vein and the final divisions of the left hepatic duct and the left hepatic artery branches. The umbilical portion of the left portal vein is an important landmark: access to this vein and mobilization of the left portal vein are essential steps in surgery for hilar cholangiocarcinoma. A knowledge of the arrangement of the portal vein, hepatic artery and bile duct within the umbilical fissure is also essential when splitting the liver for an adult and paediatric recipient and for live donor liver transplantation for a child recipient.
Sectors and segments of the liver
Sectors
The sectors of the liver are made up of between one and three segments: right lateral sector = segments VI and VII; right medial sector = segments V and VIII; left medial sector = segments III and IV (and part of I); left lateral sector = segment II (Fig. 68.5). Segments are numbered in an ante-clockwise spiral centered on the portal vein with the liver viewed from beneath, starting with segment I up to segment VI, and then back clockwise for the most cranial two segments VII and VIII (Fig. 68.6).
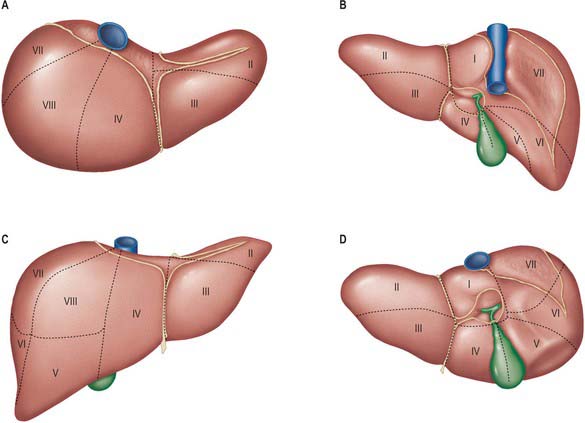
Fig. 68.5 Segments of the liver (after Couinaud). A, superior view; B, posterior view; C, anterior view; D, inferior view. The segments are sometimes referred to by number (name) – I (caudate) (sometimes subdivided into left and right parts called segment IX); II (left lateral superior); III (left medial inferior); IV (left medial superior) (sometimes subdivided into superior and inferior parts); V (right medial inferior); VI (right lateral inferior); VII (right lateral superior); VIII (right medial superior).
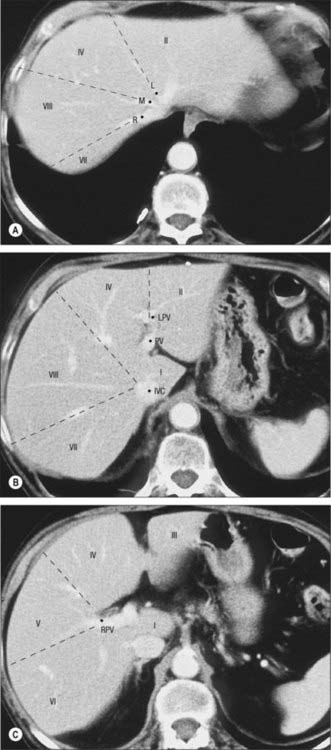
Fig. 68.6 Segments of the liver seen on axial CT scan. A, Contrast enhanced CT shows the left (L), middle (M), and right (R) hepatic veins at the superior aspect of the liver marking the left main and right portal fissures. B, Inferior to this the caudate lobe (segment I) lies between the inferior vena cava (IVC) and the main portal vein (PV). The left portal vein (LPV) separates segment II superiorly from segment III inferiorly. C, The right portal vein (RPV) divides segments V and VI inferiorly (C) from segments VII and VIII superiorly (B).
Segment I corresponds to the anatomical caudate lobe and lies posterior (dorsal) to segment IV with its left half directly posterior to segments II and II and its medial half surrounded by major vascular branches. The Glissonian sheaths to segment I arise from both right and left main sheaths: the segment therefore receives vessels independently from the left and right portal veins and hepatic arteries, and it drains independently into the inferior vena cava by multiple small branches (referred to as the lower group). They nearly always arise in the lower and occasionally from the middle third, but never from the upper third of the segment. The bile ducts draining the segment are closely related to the confluence of the right and left hepatic ducts such that excision of central bile duct tumours usually requires removal of segment IV.
Segment II is the only segment in the left lateral sector of the liver and lies postero-lateral to the left fissure. It often has only one Glissonian sheath and drains into the left hepatic vein. Rarely, a separate vein drains directly into the inferior vena cava.
Segment III lies between the umbilical fissure and the left fissure and is often supplied by one to three Glissonian sheaths: it drains into the left hepatic vein. The vein of the falciform ligament can provide an alternative drainage route for segment III.
Segment IV lies between the umbilical fissure and the main fissure, anterior to the dorsal fissure and segment I. Segment IV is supplied by three to five Glissonian sheaths, of which the majority arise in the umbilical fissure; their origins are often close to those that supply to segments II and III. Occasionally segment IV is supplied by branches from the main left pedicle. The main venous drainage segment is into the middle hepatic vein; the segment can also drain into the left hepatic vein through the vein of the falciform ligament.
Segment V is the inferior segment of the right medial sector and lies between the middle and the right hepatic veins. Its size is variable, as are the number of Glissonian sheaths that supply it. Venous drainage is into the right and middle hepatic veins.
Segment VI forms the inferior part of the right lateral sector posterior to the right portal fissure. It is often supplied by two to three branches from the right posterior Glisson’s sheath, but occasionally the Glisson’s sheath to segment VI can arise directly from the right pedicle. Venous drainage is normally into the right hepatic vein, but may be via the right inferior hepatic vein directly into the inferior vena cava.
Segment VII forms the superior part of the posterior sector and lies behind the right hepatic vein. The sheaths to segment VII are often single. The venous drainage is into the right hepatic vein; occasionally the segment can drain through the right middle hepatic vein directly into the inferior vena cava.
Supports of the liver
The liver is stabilized and maintained in its position in the right upper quadrant of the abdomen by both static and dynamic factors. Richelme & Bourgeon (1973) suggested a three-tier classification of the anatomical factors: the suspensory attachments at the posterior abdominal wall to the inferior vena cava, hepatic veins, coronary and triangular ligaments (primary factors); the support provided by the right kidney, right colonic angle and duodenopancreatic complex (secondary factors); the attachment to the anterior abdominal wall and diaphragm by the falciform ligament (tertiary factors).
The surgical implications of these different factors are important for traumatologists in terms of achieving a better understanding of the pathophysiology of liver trauma. The inferior vena cava and the supra-hepatic veins, especially the right hepatic vein, appear to be the most important anatomical structures that support the bulk of the liver. Other factors which are responsible for the maintenance of the position of the liver within the abdominal cavity include positive intra-abdominal pressure and the movement of the diaphragm during respiration.
Peritoneal attachments and ligaments of the liver
The liver is attached to the anterior abdominal wall, diaphragm and other viscera by several ligaments which are formed from condensations of the peritoneum.
Falciform ligament
The liver is attached in front to the anterior abdominal wall by the falciform ligament. The two layers of this ligament descend from the posterior surface of the anterior abdominal wall and diaphragm and turn onto the anterior and superior surfaces of the liver. On the dome of the superior surface, the right leaf runs laterally and is continuous with the upper layer of the coronary ligament. The left layer of the falciform ligament turns medially and is continuous with the anterior layer of the left triangular ligament. The ligamentum teres, which represents the obliterated left umbilical vein, runs in the lower free border of the falciform ligament and continues into a fissure on the inferior surface of the liver. In fetal life the left umbilical vein opens into the left portal vein: it is supposed to be obliterated in adult life, but frequently remains partially patent. This lumen may open up in conditions such as portal hypertension to form a collateral channel. The ligamentum teres has importance in abdominal surgery for several reasons. It is quite often divided in upper abdominal surgery to optimize access to the upper abdominal viscera or as the first step in the mobilization of the liver. The ligament is vascularized by numerous arterial branches, mainly from the segment IV artery, and these form an anastomotic connection with the branches of the internal thoracic artery: it is therefore important to ligate or coagulate the ligament during its division. The ligamentum teres is a landmark and guide to the segment III hepatic duct used in hepatocojejunostomy formation, and to the left portal vein lying in the umbilical fissure during creation of a mesentericoportal shunt.
Coronary ligament
The coronary ligament is formed by the reflection of the peritoneum from the diaphragm onto the posterior surfaces of the right lobe of the liver. A large triangular area of liver devoid of peritoneal covering, the ‘bare area’ of the liver, is defined between the two layers of the coronary ligament. Here the liver is attached to the diaphragm by areolar tissue and is in continuity inferiorly with the anterior pararenal space. The coronary ligament is continuous on the right with the right triangular ligament. On the left, the two layers become closely applied, and form the left triangular ligament. The upper layer of the coronary ligament is reflected superiorly onto the inferior surface of the diaphragm and inferiorly onto the right and superior surface of the liver. The lower layer of the coronary ligament is reflected inferiorly over the right suprarenal gland and right kidney, and superiorly onto the inferior surface of the liver. Surgical division of the right triangular and coronary ligaments allows the right lobe of the liver to be brought forward, and exposes the lateral aspect of the inferior vena cava behind the liver.
Triangular ligaments
The left triangular ligament is a double layer of peritoneum which extends to a variable length over the superior border of the left lobe of the liver. Medially the anterior leaf is continuous with the left layer of the falciform ligament and the posterior layer is continuous with the left layer of the lesser omentum. The left triangular ligament lies in front of the abdominal part of the oesophagus, the upper end of the lesser omentum and part of the fundus of the stomach. Division of the left triangular ligament allows the left lobe of the liver to be mobilized for exposure of the abdominal oesophagus and crura of the diaphragm. The left triangular ligament is an important stabilizing factor for the left lobe in operations involving removal of the right lobe of the liver. Its division will result in the left lobe being unstable to the extent that it can rotate and displace into the space vacated under the right hemidiaphragm: this extreme degree of rotation can compromise the venous outflow of the liver with consequent liver dysfunction. If it is divided, the left triangular ligament must be fixed at the end of the surgery, in addition to fixing back the falciform ligament.
The right triangular ligament is a short structure which lies at the apex of the ‘bare area’ of the liver and is continuous with the layers of the coronary ligament.
Lesser omentum
The lesser omentum is a fold of peritoneum which extends from the lesser curve of the stomach and proximal duodenum to the inferior surface of the liver. Its attachment to the inferior surface of the liver is L-shaped. The vertical component follows the line of the fissure for the ligamentum venosum, the fibrous remnant of the ductus venosus. More inferiorly the attachment runs horizontally to complete the L in the porta hepatis. At its upper end, the superior layer of the lesser omentum is continuous on the left with the posterior layer of the left triangular ligament, and the inferior layer is continuous on the right with the coronary ligament as it encloses the inferior vena cava. At its lower end, the two layers diverge to surround the structures of the porta hepatis. A thin fibrous condensation of fascia usually runs from the medial end of the porta hepatis into the fissure in the inferior surface which contains the ligamentum teres. This fascia is continuous with the lower border of the falciform ligament when the ligamentum teres re-emerges at the inferior border of the liver. Care should be taken when dividing the lesser omentum, because an aberrant left hepatic artery may run in the medial end: when present, it invariably extends to the liver at the base of the umbilical fissure, and may be identified by a pulsation in the lesser omentum close to the umbilical fissure.
Ligamentum venosum
The ligamentum venosum represents the obliterated venous connection that existed between the left portal vein and the left hepatic vein in fetal life. It is used as a guide and aid to control the left hepatic vein extrahepatically during surgery. By dividing the ligament close to its insertion into the left hepatic vein and retracting it laterally, the angle between the left and the middle hepatic veins, required for dissection and control of the left hepatic vein, may be accessed.
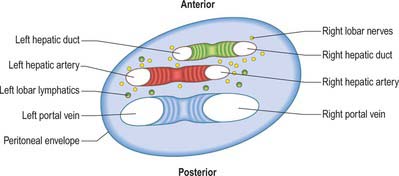
Porta hepatis, hepatoduodenal ligament and hilar plate
The porta hepatis is a deep fissure on the inferior surface of the liver. It is situated between the quadrate lobe in front and the caudate process behind, and contains the portal vein, hepatic artery and hepatic nervous plexuses as they ascend into the parenchyma of the liver, and the right and left bile ducts and some lymph vessels as they emerge from the liver. The hepatic ducts lie anterior to the portal vein and its branches, and the hepatic artery with its branches lies between the two (Fig. 68.7). All these structures are enveloped in the perivascular fibrous capsule, the hepatobiliary capsule of Glisson, a sheath of loose connective tissue which surrounds the vessels as they course through the portal canals in the liver and is also continuous with the fibrous hepatic capsule. The dense aggregation of vessels, supporting connective tissue, and liver parenchyma just above the porta hepatis is often referred to as the ‘hilar plate’ of the liver (Fig. 68.8). Understanding of the concept of hilar plate is important in surgical approaches to the hilar structures. Division or lowering of the hilar plate are essential for surgical access to the left hepatic duct. The hepatic artery, bile duct and portal vein extend from the porta hepatis towards the duodenum in the free edge of the hepatoduodenal ligament, which forms the anterior boundary of the epiploic foramen. Rapid control of the vessels entering the porta hepatis can be obtained by dividing the lesser omentum to the left of these structures and passing a tape around them from left to right through the epiploic foramen (a ‘Pringle’ manoeuvre).
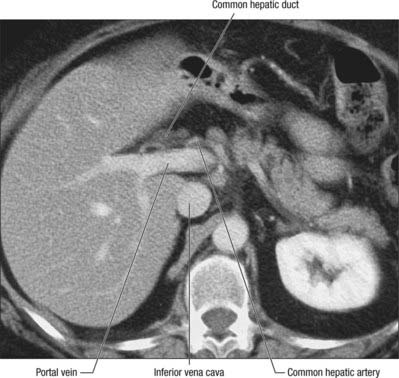
Fig. 68.8 Axial CT of the porta hepatis. The hepatic ducts lie anteriorly, the portal vein posteriorly, and hepatic artery between the two.
The left hepatic duct remains extrahepatic as it runs down to the bifurcation along the base of segment IV (the quadrate lobe). This extrahepatic length of duct is particularly useful when performing high biliary duct reconstructions where a length of jejunum is anastomosed to form a biliary-enteric bypass, to treat strictures of the common hepatic duct.
Glisson’s sheath
Glisson’s capsule condenses around the branches of the portal triad structures as Glissonian sheaths as they enter the liver parenchyma and extends as far the individual segments of the liver. This arrangement facilitates surgical control of both the main right and left pedicles of the liver as well as all the structures (biliary, autonomic and lymphatic elements) that supply individual sectors and segments in liver complex resectional surgery.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
The vessels connected with the liver are the portal vein, hepatic artery and hepatic veins. The portal vein and hepatic artery ascend in the lesser omentum to the porta hepatis, where each bifurcates. The hepatic bile duct and lymphatic vessels descend from the porta hepatis in the same omentum (Figs 68.7 and 68.8). The hepatic veins leave the liver via its posterior surface and run directly into the inferior vena cava.
Hepatic artery
In adults the hepatic artery is intermediate in size between the left gastric and splenic arteries. In fetal and early postnatal life it is the largest branch of the coeliac axis. The hepatic artery gives off right gastric, gastroduodenal and cystic branches as well as direct branches to the bile duct from the right hepatic and sometimes the supraduodenal artery (Fig. 68.9A). After its origin from the coeliac axis, the hepatic artery passes anteriorly and laterally below the epiploic foramen to the upper aspect of the first part of the duodenum. It may be subdivided into the common hepatic artery, from the coeliac trunk to the origin of the gastroduodenal artery, and the hepatic artery ‘proper’, from that point to its bifurcation. It passes anterior to the portal vein and ascends anterior to the epiploic foramen between the layers of the lesser omentum. Within the free border of the lesser omentum the hepatic artery is medial to the common bile duct and anterior to the portal vein. At the porta hepatis it divides into right and left branches before these run into the parenchyma of the liver. The right hepatic artery usually crosses posterior (occasionally anterior) to the common hepatic duct (Fig. 68.9B). This close proximity often means that the right hepatic artery is involved in bile duct cancer earlier than the left hepatic artery. Occasionally the right hepatic artery crosses in front of the common bile duct and may be injured in surgery of the common bile duct. It almost always divides into an anterior branch supplying segments V and VIII, and a posterior branch supplying segments VI and VII. The anterior division often supplies a branch to segment I and the gallbladder. The segmental arteries are macroscopically end-arteries although some collateral circulation occurs between segments via fine terminal branches.
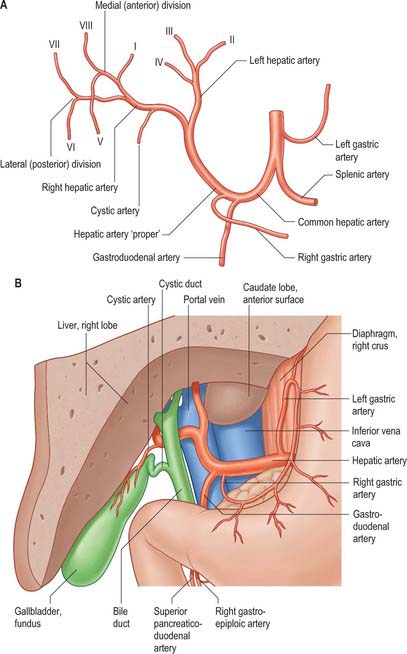
Fig. 68.9 The hepatic artery. A, Branches. B, Relations of the hepatic artery, bile duct and portal vein to each other in the lesser omentum: anterior aspect, portion of the liver removed.
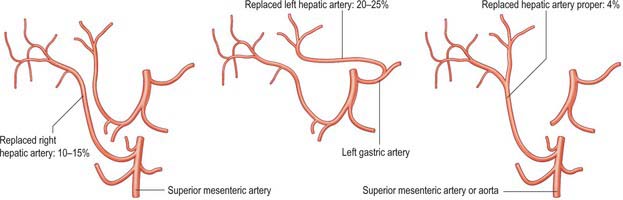
A small number of normal variants are important to demonstrate angiographically because they may influence surgical and interventional radiological procedures. A vessel that supplies a lobe in addition to its normal vessel is defined as an accessory artery. A replaced hepatic artery is a vessel that does not originate from an orthodox position and provides the sole supply to that lobe. Rarely a replaced common hepatic artery arises from the superior mesenteric artery (Fig. 68.10) and is identified at surgery by a relatively superficial portal vein (reflecting the absence of a common hepatic artery that would normally cross in front of the vein). More commonly a replaced right hepatic artery or an accessory right hepatic artery arises from the superior mesenteric artery (Fig. 68.11). In this case they run behind the portal vein and bile duct in the lesser omentum and can be identified at surgery by pulsation behind the portal vein. The accessory right hepatic artery may be injured during resections of the pancreatic head because the artery lies in close proximity to the portal vein. Occasionally, a replaced left hepatic artery or an accessory branch arises from the left gastric artery: these vessels provides a source of collateral arterial circulation in cases of occlusion of the vessels in the porta hepatis but may also be injured during mobilization of the stomach as it lies in the upper portion of the lesser omentum. Rarely, accessory left or right hepatic arteries may arise from the gastroduodenal artery or aorta. The presence of replaced arteries can be lifesaving in patients with bile duct cancer: because they are further away from the bile duct they tend to be spared from the cancer, making excision of the tumour feasible. Knowledge of these variations is also important in planning whole and split liver transplantation.
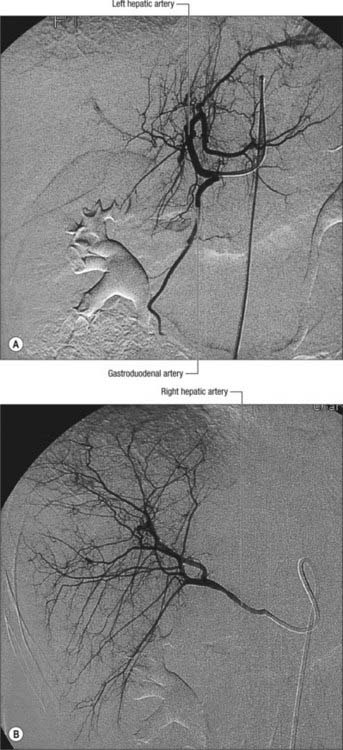
Fig. 68.11 Hepatic arteriogram. A, A selective hepatic arteriogram shows normal left hepatic artery branches and small right hepatic artery branches. B, The right hepatic artery is arising from the origin of the superior mesenteric artery.
Variations in the intrahepatic arteries are common and may be surgically important. For example, the segment VI artery most commonly arises from the left hepatic artery, but in about 10–20% of cases it arises from the right hepatic artery or the main hepatic artery. Failure to recognize this variation may compromise the blood supply to segment IV following right hepatectomy, and is especially important following right lobe donation for live donor liver transplantation.
Veins
The liver has two venous systems. The portal system conveys venous blood from the majority of the gastrointestinal tract and its associated organs to the liver (see Ch. 60). The hepatic venous system drains blood from the liver parenchyma into the inferior vena cava.
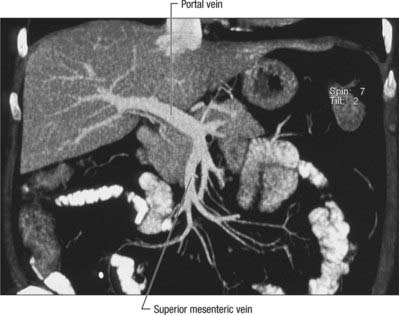
Portal vein
The portal vein begins at the level of the second lumbar vertebra and is formed from the convergence of the superior mesenteric and splenic veins (Fig. 68.12; see also Fig. 71.4 and Fig. 60.8. It is approximately 8 cm long and lies anterior to the inferior vena cava and posterior to the neck of the pancreas. It lies obliquely to the right and ascends behind the first part of the duodenum, the common bile duct and gastroduodenal artery. At this point it is directly anterior to the inferior vena cava. It enters the right border of the lesser omentum, ascends anterior to the epiploic foramen to reach the right end of the porta hepatis and then divides into right and left main branches which accompany the corresponding branches of the hepatic artery into the liver. In the lesser omentum the portal vein lies posterior to both the common bile duct and hepatic artery. It is surrounded by the hepatic nerve plexus and accompanied by many lymph vessels and some lymph nodes.
The main extrahepatic tributaries of the portal vein are the coronary or the left gastric vein, which ends in the left margin of the portal vein, and the posterior superior pancreatoduodenal vein nearer to the head of the pancreas. The portal vein divides into right and left branches at the hilum (Fig. 68.13). The left portal vein has a longer extraparenchymal course (4–5 cm) and tends to lie slightly more horizontally than the right portal vein, but is often of smaller calibre. It has horizontal and vertical portions. The horizontal portion runs along the base of segment VI and often gives branches to segment I and sometimes to segment VI in this part of its course. The branch to segment II continues laterally but the main left portal vein takes a more anterior and vertical course in the umbilical fissure (the vein of the umbilical fissure) where it gives branches to segments III and IV and receives the obliterated left umbilical vein (ligamentum teres). The majority of the supply to segment IV comes from the left portal vein, and only occasionally from the right via proximal branches of the main vein or branches from veins to segments V or VIII. The right portal vein is only 2–3 cm in length and usually divides into a right medial (anterior) sectoral division supplying segments V and VIII, and a right lateral (posterior) sectoral division supplying segments VI and VII. The medial division may give a branch to segment I.
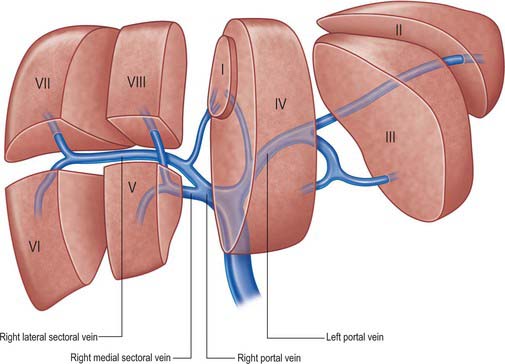
Fig. 68.13 The main portal vein and its intraheptic branches. (Right lateral = right posterior; right medial = right anterior.)
Variations usually involve the right portal vein: absence of a right portal vein with the resulting portal trifurcation in the form of left portal vein, right medial and right lateral portal veins, is present in 10–15% of livers. Occasionally the right medial vein arises from the left portal vein, a variant which is important to remember during left sided liver resection. The portal trifurcation has implications for split liver and live donor liver transplantation, where its presence might be considered as a relative contra-indication. On rare occasions, the portal bifurcation is absent, in which case the main portal vein enters the liver giving off the right segmental branches and then turns left to supply the left lobe of the liver (a contraindication to major liver surgery). Occasionally one or more of the segmental branches of the right lobe (especially segment IV) arises proximally.
Porto-systemic shunts
Increased pressure within the portal venous system may result in dilatation of the portal venous tributaries: a reversal of flow may occur where these veins form anastomoses with veins which drain into the systemic venous circulation. The common sites where porto-systemic shunts may occur, and the associated clinical implications, are listed in Table 68.1.
Table 68.1 Common sites of occurrence of porto-systemic shunts, and associated clinical implications.
| Portal veins | Systemic veins | Clinical presentations |
|---|---|---|
| Left gastric and lower oesophageal veins | Lower branches of oesophageal veins that drain into azygos and accessory hemiazygos veins | Oesophageal or gastric varices |
| Superior rectal veins | Middle and inferior rectal veins that drain into internal iliac and pudendal veins | Rectal varices |
| Persistent tributaries of left branch of portal vein, running in ligamentum teres | Peri-umbilical branches of superior and inferior epigastric veins | ‘Caput medusae’ |
| Intraparenchymal branches of right branch of portal vein, lying in liver tissue exposed in ‘bare area’ | Retroperitoneal veins that drain into lumbar, azygos and hemiazygos veins | Retroperitoneal dilated veins are at risk during surgery or interventional procedures |
| Omental and colonic veins in the region of the hepatic and splenic flexure | Retroperitoneal veins in the region of the hepatic and splenic flexure | |
| Patent ductus venosus connected to the left branch of the portal vein | Inferior vena cava | Is rare |
Hepatic veins
The liver is drained by three major hepatic veins into the suprahepatic part of the inferior vena cava and a multitude of minor hepatic veins that drain into the intrahepatic inferior vena cava. The three major veins are located between the four major sectors of the liver (Figs 68.6A, 68.14 and 68.15).
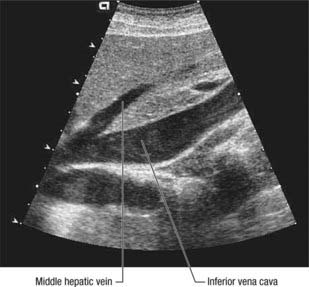
Fig. 68.14 Sagittal ultrasound of the middle hepatic vein. Middle hepatic vein draining into the inferior vena cava.
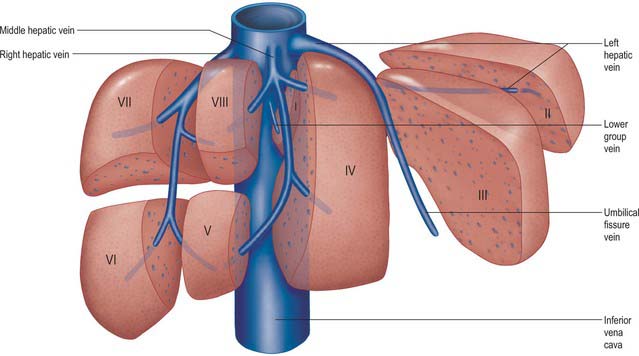
Fig. 68.15 Arrangement of the hepatic venous territories. Multiple lower group veins may be present. Individual segments may drain into more than one hepatic venous territory.
Right hepatic vein
This is the longest and the largest vein and also the most variable. It is usually single, but occasionally it remains as two trunks until it terminates by draining into the inferior vena cava. The right hepatic vein runs into the intersectoral plane between the right medial and right lateral sectors. It drains the whole of segments VI and VII and parts of segments V and VIII. The extent of its contribution to the drainage of segments V and VIII is variable, and depends upon the size of the veins that drain these segments into the middle hepatic vein. The right hepatic vein is formed near the anterior and inferior edge of the liver and lies in a coronal plane through most of its course. It drains into the inferior vena cava near the upper border of the caudate lobe. Of all the three major veins the right hepatic vein is the one which is the most variable in its size not only due to the differential contribution to segments V and VIII drainage along with the middle hepatic vein but also due to the occasional presence of an accessory right middle and right inferior veins.
Middle hepatic vein
The middle hepatic vein lies along the plane between the right medial and the left medial sectors of the liver. It usually joins with the left hepatic vein and terminates into the inferior vena cava as a common trunk. It drains the central part of the liver receiving constant branches from segments IV, V and VIII. The sizes of the branches draining segments V and VIII are variable and are of surgical importance in terms of right lobe living donation.
Left hepatic vein
The left hepatic vein lies between the left medial and left lateral sectors of the liver. It drains segments II, III and occasionally IV. Small veins draining segment II and occasionally the superior part of segment IV may drain directly into the inferior vena cava in the minority of livers. Usually a major tributary of the left hepatic vein, the umbilical fissure vein runs in the intersegmental plane between segments III and IV and contributes to their drainage. Occasionally the vein draining segment III ends separately in the confluence of the left and middle hepatic veins. These variations in the venous drainage are of significance in terms of split liver transplantation and live donor liver transplantation.
Minor veins
Segment I veins drain directly into the inferior vena cava and vary in number from one to five. Since it has an independent drainage from the rest of the liver in patients with Budd–Chiari syndrome, where all the major hepatic veins are blocked, segment I often continues to drain effectively and undergoes compensatory hypertrophy. Rarely there is an accessory right middle or inferior hepatic vein. When they are present they are of surgical importance especially if they are of more than 5 mm in diameter; they drain segments V and VI independently of the three major veins and therefore any tumour involving the three major veins can be safely resected as long as venous drainage from the accessory veins is ensured. In live donor and split liver transplantation these veins must be individually anastomosed to the recipient inferior vena cava to ensure adequate venous drainage.
Transjugular intraparenchymal porto-systemic shunt (TIPS) procedure for portal hypertension
In extreme cases of chronic portal hypertension, large calibre anastomoses between portal and systemic circulations may be formed within the liver parenchyma using a balloon catheter, introduced via the internal jugular vein and under radiological control, to puncture across and rupture through a thinned strip of liver parenchyma between hepatic veins and dilated portal branches.
Lymphatics
Lymph from the liver has abundant protein content. Lymphatic drainage from the liver is wide and may pass to nodes above and below the diaphragm. Obstruction of the hepatic venous drainage increases the flow of lymph in the thoracic duct. Hepatic collecting vessels are divided into superficial and deep systems.
Superficial hepatic vessels
The superficial vessels run in subserosal areolar tissue over the whole surface of the liver and drain in four directions. Lymph vessels from the majority of the posterior surface, the surface of the caudate lobe, and the posterior part of the inferior surface of the right lobe, accompany the inferior vena cava and drain into pericaval nodes. Vessels in the coronary and right triangular ligaments may directly enter the thoracic duct without any intervening node. Vessels from the majority of the inferior surface, anterior surface and most of the superior surface all converge on the porta hepatis to drain into the hepatic nodes. A few lymph vessels from the posterior surface of the lateral end of the left lobe pass towards the oesophageal opening to drain into the para-cardiac nodes. One or two lymph trunks from the right surface and right end of the superior surface accompany the inferior phrenic artery across the right crus to drain into the coeliac nodes.
Deep hepatic vessels
The great majority of the liver parenchyma is drained by lymphatic vessels within the substance of the liver. Fine lymphatic vessels merge to form larger vessels. Some run superiorly through the parenchyma to form the ascending trunks which accompany the hepatic veins and pass through the caval opening in the diaphragm to drain into nodes around the end of the inferior vena cava. Vessels from the lower portion of the liver form descending trunks which emerge from the porta hepatis and drain into the hepatic nodes.
INNERVATION
The liver has a dual innervation. The parenchyma is supplied by hepatic nerves which arise from the hepatic plexus and contain sympathetic and parasympathetic (vagal) fibres. They enter the liver at the porta hepatis and most accompany the hepatic arteries and bile ducts. A very few may run directly within the liver parenchyma. The capsule is supplied by some fine branches of the lower intercostal nerves, which also supply the parietal peritoneum, particularly in the area of the ‘bare area’ and superior surface: distension or disruption of the liver capsule causes quite well localized sharp pain.
Hepatic plexus
The hepatic plexus is the largest derivative of the coeliac plexus and receives branches from the anterior and posterior vagi. It accompanies the hepatic artery and portal vein and their branches into the liver, where its fibres run close to the branches of the vessels, supplying vasomotor fibres to the hepatic vessels and biliary tree, and innervating the hepatocytes directly. Multiple fine branches from the plexus supply the common and hepatic bile ducts directly; branches to the gallbladder form a delicate cystic plexus. The vagal fibres are motor to the musculature of the gallbladder and bile ducts and inhibitory to the sphincter of the bile duct.
Nerves from the hepatic plexus run with the branches of the common hepatic artery to supply, or contribute to the supply of, foregut derivatives. Branches run inferiorly from the plexus with the right gastric artery to contribute to the supply of the pylorus, with the gastroduodenal artery and its branches to reach the pylorus and the first part of the duodenum, and with the right gastroepiploic artery to provide a small contribution to the supply the right side of the stomach and the greater curvature. The superior pancreaticoduodenal extension supplies the descending part of the duodenum, the pancreatic head and the intrapancreatic part of the common bile duct.
Referred pain
Pain arising from the parenchyma of the liver is poorly localized. In common with other structures of foregut origin, pain is referred to the central epigastrium. Stretch of or involvement of the liver capsule by inflammatory or neoplastic processes rapidly produces well-localized pain of a ‘somatic’ nature.
MICROSTRUCTURE
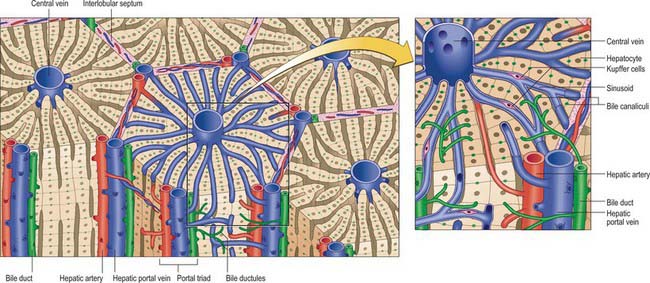
The liver is essentially an epithelial-mesenchymal outgrowth of the caudal part of the foregut, with which it retains its connection via the biliary tree. The surface of the liver facing the peritoneal cavity is covered by a typical serosa, the visceral peritoneum. Beneath this, and enclosing the whole structure, is a thin (50–100 μm) layer of connective tissue from which extensions pass into the liver as connective tissue septa and trabeculae. Branches of the hepatic artery and hepatic portal vein, together with bile ductules and ducts, run within these connective tissue trabeculae which are termed portal tracts (portal canals). The combination of the two types of vessel and a bile duct is termed a portal triad (Fig. 68.16); these structures are usually accompanied by one or more lymphatic vessels.
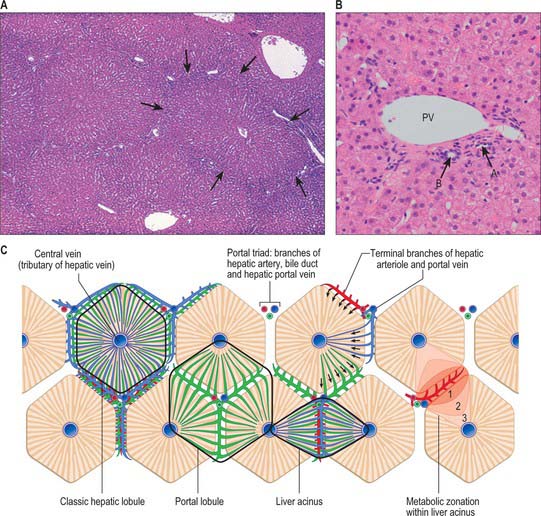
Fig. 68.16 The structural organization of human liver tissue. A, Lobules bordered by delicate connective tissue septa (arrows) in which run branches of the hepatic portal vein, hepatic artery and bile duct, grouped as portal triads. A central vein drains each lobule. B, A portal triad containing branches of the hepatic portal vein (PV; generally the largest profile), the hepatic artery (A) and a bile duct (B), with typical round epithelial nuclei. Other small bile ductule and arteriolar branches are also visible. C, A functional view in which the territories of the hepatic lobules are shown as regular hexagons (unlike their real appearance, which is highly variable). The different functions, such as blood flow from the territory of the acinus to sectors of adjacent hepatic lobules, are shown in different lobules, though in reality all occur in each lobule.
(By permission from Dr JB Kerr, Monash University, from Kerr JB 1999 Atlas of Functional Histology. London: Mosby.) (By courtesy of Mr Peter Helliwell and Dr Joseph Mathew, Department of Histopathology, Royal Cornwall Hospitals Trust, UK.)
The liver parenchyma consists of a complex network of epithelial cells, supported by connective tissue, and perfused by a rich blood supply from the hepatic portal vein and hepatic artery. The epithelial cells, hepatocytes, carry out the major metabolic activities of this organ, but additional cell types possess storage, phagocytic and mechanically supportive functions. In the mature liver, hepatocytes are arranged mainly in plates (or cords, as seen in two-dimensional sections) that are usually only one cell thick and separated by venous sinusoids which anastomose with each other via gaps in the plates. Until about seven years of age, plates are normally two cells thick.
Bile secreted by the hepatocytes is collected in a network of minute tubes (canaliculi). The hepatocytes can therefore be regarded as exocrine cells, secreting bile into the alimentary tract via the hepatic ducts and bile duct. Their other metabolic functions involve complex biochemical exchanges with the blood.
The fetal liver is a major haemopoietic organ: erythrocytes, leukocytes and platelets develop from the mesenchyme covering the sinusoidal endothelium.
Lobulation of the liver
The structural unit of the liver is the lobule: a roughly hexagonal arrangement of plates of hepatocytes, separated by intervening sinusoids which radiate outward from a central vein, with portal triads at the vertices of each hexagon (Fig. 68.16). The central vein is a tributary of the hepatic vein that drains the tissue. In some species, the classic lobular units are delimited microscopically by distinct connective tissue septa. However, the lobular organization of the human liver is not immediately evident in histological sections: the lobules do not have distinct boundaries, and connective tissue is sparse. The plates do not pass straight to the periphery of a lobule like the spokes of a wheel but run irregularly as they anastomose and branch.
Detailed studies of human liver, using three-dimensional reconstruction and morphometric analysis combined with histopathological observations, have revealed a highly ordered arrangement of functional units, the hepatic (portal) acini. Each acinus is an approximately oval mass of tissue, oriented around the afferent vascular system, i.e. a terminal branch of a hepatic arteriole and portal venule, and with its long axis defined by the territory between two adjacent central veins. It includes the hepatic tissue served by these afferent vessels, and is bounded by the territories of other acini.
The acinar definition of hepatic micro-organization has clarified important problems of liver histopathology, especially the development of zones of anoxic damage, glycogen deposition and removal, and of toxic trauma, which are all related to the direction of blood flow and thus tend to follow the acinar pattern. There are also real metabolic differences between hepatocytes within the acini, and they have been divided into three zones: zone 1 (periportal) is nearest to the terminal branches of afferent vessels; zone 2 is the intermediate zone; and zone 3 is the area closest to the central venous drainage.
Blood supply
Preterminal hepatic arterioles in the portal canals branch to convey arterial blood to the sinusoids by several routes. The main pattern is via a fine capillary plexus which drains to branches of the portal veins. Some arterial blood passes directly to the hepatic sinusoids, bypassing these capillary plexuses, but this represents only a small part of the total flow. Sinusoids thus contain mixed venous and arterial blood. Central veins from adjacent lobules form interlobular veins which unite as hepatic veins and drain to the inferior vena cava. Hepatic veins draining the tissue run quite separately with respect to the portal triad system, freely crossing the boundaries of triad territories.
Hepatic plates (cords)
The endothelial linings of the sinusoids are separated from hepatocytes forming the hepatic plates by a narrow gap, the perisinusoidal space of Disse, which is normally about 0.2–0.5 μm wide, but which distends in anoxic conditions. The space contains fine collagen fibres (mostly type III, with some types I and IV), the microvilli of adjacent hepatocytes, and occasional non-myelinated nerve terminals. There is no basal lamina within the space of Disse.
Minute bile canaliculi form nets with polygonal meshes in the hepatic plates. Each polygonal hepatocyte is surrounded by canaliculi (see below) except on the surfaces, at least two per cell, that face sinusoids. Hepatic plates thus enclose a network of canaliculi that pass to the lobular periphery, where they join to form narrow intralobular ductules (terminal ductules or the canals of Hering) lined by squamous or cuboidal epithelium. The intralobular ductules enter bile ductules, lined by cuboidal or columnar cells, in the portal canals. The flow of bile is thus towards the periphery of lobules, in the opposite direction to the blood flow, which is centripetal.
Cells of the liver
Cells of the liver include hepatocytes, hepatic stellate cells (also known as perisinusoidal lipocytes, or Ito cells), sinusoidal endothelial cells, macrophages (Kupffer cells), the cells of the biliary tree (cuboidal to columnar epithelium) and connective tissue cells of the capsule and portal tracts (Figs 68.16B and 68.17).
About 80% of the liver volume and 60% of its cell number are formed by hepatocytes (parenchymal cells) (Fig. 68.17). They are polyhedral, with 5–12 sides and are from 20 to 30 μm across. Their nuclei are round, euchromatic and often tetraploid, polyploid or multiple, with two or more in each cell. Their cytoplasm typically contains a considerable amount of rough and smooth endoplasmic reticulum, many mitochondria, lysosomes and well-developed Golgi apparatus, which are all features that indicate a high metabolic activity. Glycogen granules and lipid vacuoles are usually prominent. Numerous large peroxisomes and vacuoles containing enzymes, e.g. urease in distinctive crystalline forms, indicate the complex metabolism of these cells. Their role in iron metabolism is reflected by the presence of storage vacuoles containing crystals of ferritin and haemosiderin.
The surfaces of hepatocytes facing the sinusoids exhibit numerous microvilli, approximately 0.5 μm long, creating a large area of membrane, 70% of the hepatocyte surface, exposed to blood plasma. Elsewhere, hepatocytes are linked by numerous gap junctions and desmosomes. Lateral plasma membranes of adjacent hepatocytes form microscopic channels, the bile canaliculi, which are specialized regions of intercellular space formed by apposing grooves in hepatocyte plasma membranes, sealed from extraneous interstitial space by tight junctions. Numerous membrane-bound exocytotic vesicles cluster near the lumen of the canaliculi because the secretion of bile components is targeted to the canalicular plasma membrane. These canaliculi form the origins of the biliary tree and their tight junctions prevent bile from entering interstitial fluid or blood plasma: this is the blood–bile barrier.
Hepatic stellate cells are much less numerous than hepatocytes. They are irregular in outline and lie within the hepatic plates, between the bases of hepatocytes. They are thought to be mesenchymal in origin and are characterized by numerous cytoplasmic lipid droplets. These cells secrete most of the intralobular matrix components, including collagen type III (reticular) fibres. They store the fat-soluble vitamin A in their lipid droplets and are a significant source of growth factors active in liver homeostasis and regeneration. Hepatic stellate cells also play a major role in pathological processes. In response to liver damage, they become activated and predominantly myofibroblast-like. They are responsible for the replacement of toxically damaged hepatocytes with collagenous scar tissue, a process called hepatic fibrosis, that is seen initially in zone 3, around central veins. Fibrosis can progress to cirrhosis, where the parenchymal architecture and pattern of blood flow are destroyed, with major systemic consequences.
Hepatic venous sinusoids are generally wider than blood capillaries and are lined by a thin but highly fenestrated endothelium that lacks a basal lamina. The endothelial cells are typically flattened, each with a central nucleus and joined to each other by junctional complexes. The fenestrae are grouped in clusters with a mean diameter of 100 nm, allowing plasma direct access to the basal plasma membranes of hepatocytes. Their cytoplasm contains numerous typical transcytotic vesicles.
Kupffer cells are hepatic macrophages derived from circulating blood monocytes and originate in the bone marrow. They are long-term hepatic residents and lie within the sinusoidal lumen attached to the endothelial surface. Kupffer cells are irregular in shape, and have long processes that extend into the sinusoidal lumen. They form a major part of the mononuclear phagocyte system which is responsible for removing cellular and microbial debris from the circulation, and for secreting cytokines involved in defence. They remove aged and damaged red cells from the hepatic circulation, a function normally shared with the spleen, but fulfilled entirely by the liver after splenectomy.
Couinaud C. Le foié: etudes anatomique et chirurgicules. Paris: Masson, 1957.
The original description of hepatic segmentation by Couinaud..
Healey JE, Schroy PC. Anatomy of the biliary ducts within the human liver; analysis of the prevailing pattern of branchings and the major variations of the biliary ducts. Arch Surg. 1953;66:599-616.
Mitchell AWM, Dick R. Liver, gall-bladder, pancreas and spleen. In: Butler P, Mitchell AWM, Ellis H. Applied Radiological Anatomy. Cambridge: Cambridge University Press; 1999:239-258.