2 Prenatal development of the craniofacial region
During evolution, modern humans split from their last common ancestor with the apes around seven million years ago. Since that time, Homo sapiens have acquired features that make them unique as a species and separate them from other primates:
Many of these evolutionary changes are reflected in the form and function of the craniofacial region (Fig. 2.1). The essential morphology of the head and face is fundamental to being human and the development of this region is complicated, beginning early in life and requiring the coordinated growth and interaction of all primary cell populations within the embryo.
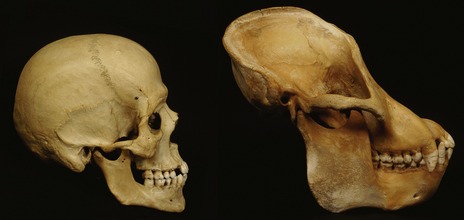
Figure 2.1 Comparison of a human (left) and orangutan (right) skull.
In the human skull, the cranium is enlarged and the forehead is upright above the eyes, reflecting the expansion of the frontal lobes. The human face is therefore positioned downwards and backwards relative to the forebrain.
Embryonic origins of the head and neck
The early embryo is derived from three primary germ layers of tissue (Table 2.1):
Table 2.1 Embryonic origins of the head and neck
| Ectoderm |
| Neural tube |
| Cranial neural crest |
| Endoderm |
| Mesoderm |
| Head mesoderm |
| Paraxial mesoderm |
There is also a further contribution from the neural crest (or fourth germ layer). Ectoderm and endoderm are derived from the epiblast and hypoblast, two fundamental cell populations within the bilaminar disc of the early embryo (Fig. 2.2). Mesoderm is produced during the process of gastrulation and the conversion of a bilaminar embryo into one with three primary tissue layers (Fig. 2.3).
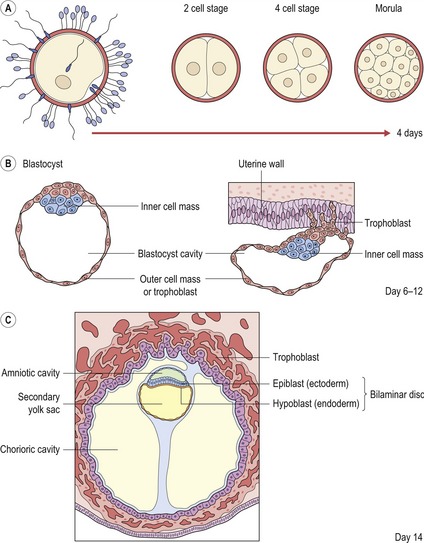
Figure 2.2 Early development of the embryo.
(A) Following fertilization, the zygote undergoes a series of mitotic cell divisions to produce a sixteen cell morula. (B) The cells within the morula are quickly organised into outer and inner cell masses and the early embryo is known as a blastocyst. Cells of the outer cell mass form the trophoblast, which mediates implantation of the blastocyst into the uterine wall and contributes to the placenta. The inner cell mass forms the embryo itself. (C) During implantation, the inner cell mass differentiates into two layers; the epiblast (future ectoderm) and hypoblast (future endoderm), which together form the bilaminar disc of the early embryo.
Redrawn from Sandler TW (ed.) (2003), Langman’s Medical Embryology, 9th edn (Baltimore: Lippincott Williams and Wilkins).
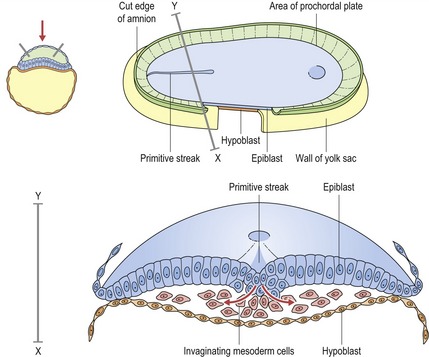
During the third week of embryonic development, the third germ layer or mesoderm is formed by the process of gastrulation. Cells of the epiblast migrate through the primitive streak, a raised structure on the surface of the epiblast, and detach to position themselves between epiblast and hypoblast and form the mesodermal cell layer.
Redrawn from Sandler TW (ed.) (2003), Langman’s Medical Embryology, 9th edn (Baltimore: Lippincott Williams and Wilkins).
In mammals, neural crest cells arise during formation of the neural tube and migrate extensively throughout the embryo in four overlapping domains:
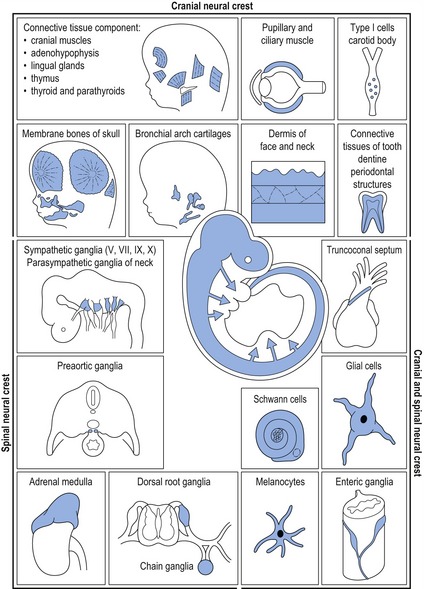
Cranial neural crest cells provide the building blocks for generating much of the skeleton and connective tissue of the head, in addition to the cranial ganglia and peripheral nerves innervating these skeletal structures. In the developing head, neural crest cells arising from the forebrain and midbrain regions populate the upper face, whilst those from the posterior midbrain and hindbrain migrate into the pharyngeal arch system (see Fig. 2.14). Cranial neural crest cells interact with ectodermal and mesodermal cell populations present within these regions, leading to the formation of craniofacial bones, cartilages and connective tissue (Fig. 2.4).
The prechordal plate—a molecular organizer for the brain and face
A region of fundamental importance for early development of the brain and face is the prechordal plate, an area of thickened endoderm that lies beneath the future forebrain of the early embryo (Fig. 2.5).
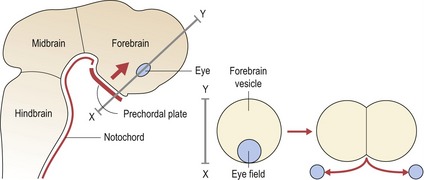
Figure 2.5 Signalling from the prechordal plate is important for patterning ventral regions of the early forebrain and producing bilateral subdivision of the eyefield.
Sonic hedgehog (SHH) signalling from the prechordal plate plays an important role in this process.
The prechordal plate acts as a true head organizer, producing molecular signals that pattern the forebrain and subdivide the eyefield into two. In the absence of normal signalling from this region, holoprosencephaly (HPE) or cyclopia can occur. In the most severe cases, the lower forebrain does not form and the upper part remains as a single undivided vesicle, rather than developing into paired cerebral hemispheres (Muenke & Beachy, 2000). This lack of midline development within the central nervous system has important consequences for the face, with a spectrum of facial deformity reflecting the severity of the brain malformation. Failure of forebrain division leads to a lack of separation of the optic primordia and the formation of a single cyclopic eye, which becomes situated below a rudimentary midline facial proboscis or nose-like structure (see Fig. 13.21).
Early organization of the craniofacial region
Primary architecture of the craniofacial region is established during early development and is based upon segmentation (Fig. 2.6). At the future head end of the human embryo, the neural tube is segmented into three vesicles, which will form:
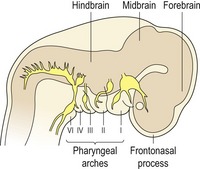
Figure 2.6 Segmentation of the craniofacial region in the early embryo.
The neural tube is segmented into forebrain, midbrain and hindbrain vesicles; the frontonasal process is situated over the developing forebrain and the segmented pharyngeal arches are situated ventrally.
On the lateral side of the head are the pharyngeal arches, which form:
In the upper region of the head is the frontonasal process, which surrounds the early forebrain and will form:
In the lower region of the head is the first pharyngeal arch, which will form:
The pharyngeal arches
In humans there are six pharyngeal arches, which appear progressively during the fourth week of embryonic development. Each arch is covered externally by ectoderm and internally by endoderm, whilst a core of mesodermal tissue exists within. As development proceeds, this central core becomes infiltrated by cranial neural crest cells that migrate into the arches from their site of origin adjacent to the roof of the neural tube. The junction of each arch is in close proximity with its neighbour, producing a pharyngeal cleft of ectoderm externally and a pouch of endoderm internally (Fig. 2.7).
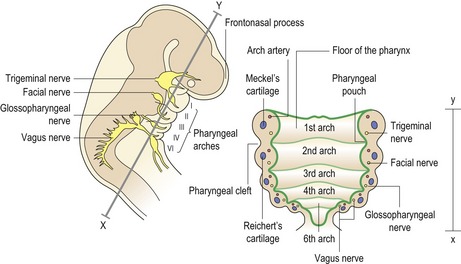
Figure 2.7 The pharyngeal arches.
Each arch is covered externally by ectoderm and internally by endoderm. Within each arch is a core of mesoderm, which becomes progressively infiltrated with migrating neural crest cells. In addition, a characteristic nerve, cartilage and artery are situated within each arch.
The pharyngeal arches give rise to a number of structures within the head and neck (Fig. 2.8):
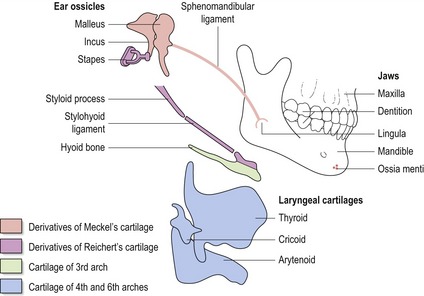
Figure 2.8 Skeletal derivatives of the pharyngeal arches.
The first arch gives rise to the upper and lower jaws, the dentition, the malleus and incus (middle ear ossicles) and sphenomandibular ligament.
The second arch gives rise to the styloid process, stylohyoid ligament, stapes (middle ear ossicle) and the lesser cornu and upper part of the body of the hyoid bone. The third arch gives rise to the greater cornu and lower part of the body of the hyoid bone. The fourth arch gives rise to the laryngeal cartilages. Those structures formed by the pharyngeal arch cartilages are indicated in colour.
Redrawn from Wendell-Smith CP, Williams RL, Treadgold S (1984), Basic Human Embryology, 3rd edn (London: Pitman Publishing).
The embryonic tissue components within each arch give rise to specific structures:
Within the pharyngeal arch system are also characteristic sets of bilaterally symmetrical arteries, nerves and cartilages. Those associated with the first two arches make contributions to the maxillary and stapedial arteries in the adult, whilst those within the lower pharyngeal arches are responsible for generating major arteries within the neck and cardiothoracic region. Neural crest cells that migrate into the third and fourth pharyngeal arches are known collectively as the cardiac neural crest, these cells making an important contribution to remodelling of the pharyngeal arch arteries and to the formation of a functional cardiac outflow tract and cardiothoracic vascular system. Any disruption within the embryonic pharyngeal region can have serious implications for normal development, which is exemplified by a group of related disorders known as the 22q11 deletion syndromes (Box 2.1).
Box 2.1 TBX1: a mediator of normal pharyngeal arch development
T-box genes encode a large group of transcription factors and TBX1 is thought to be a key player in the aetiology of the DiGeorge (DGS; OMIM 601362) and Velocardiofacial (VCFS; OMIM 192430) or 22q11 Deletion syndromes (22q11DS). These syndromes form part of a group of related human dysmorphic disorders that result from deletion or rearrangement of a large region of chromosome 22q11 (Baldini, 2005; Scambler, 2000). 22q11DS subjects are characterized by defects in the cardiac outflow tract and aortic arch, thymic and parathyroid aplasia or hypoplasia, and anomalies of the craniofacial region, which include micrognathia, cleft palate and a characteristic facial appearance. The principle clinical phenotypes present within these syndromes are thought to result from an absence of normal pharyngeal pouch signalling and localized disruption of neural crest migration within the pharyngeal arch system.
Three-dimensional facial scans of individuals with 22q11DS (left) and control subjects (right) aged between 2 weeks and 24 years. The 22q11DS face is only subtly different from the average, but careful examination reveals an exaggerated length of the nose, with a narrow nares and nasal base, but fullness above the tip. The eyes are upslanted and the ears cupped or unusual in shape (Hammond et al, 2005).
Reproduced with kind permission of Professor Peter Hammond, Institute of Child Health, University College London.
The candidacy of TBX1 for 22q11DS has been based upon its location within the deleted region of chromosome 22, its expression domain in endoderm and mesoderm of the developing pharyngeal arches and the finding that mice generated with a targeted deletion in Tbx1 exhibit a spectrum of phenotypic effects encompassing most of the common 22q11DS malformations (Lindsay et al, 2001). More recent genetic experiments in mice, designed to ablate the function of Tbx1 in different regions of the early pharynx at different developmental time points, have provided clues as to the role of this transcription factor during patterning and differentiation of the pharyngeal arch derivatives. In particular, Tbx1 activity influences the proliferation and expansion of endoderm lining the embryonic pharynx; a role that facilitates normal segmentation of the pharyngeal arches and the formation of structures derived from these regions.
Development of the face
Development of the face is a dynamic process, relying upon complex tissue interactions that are closely coordinated, both temporally and spatially (Fig. 2.9). Growth and development of this region is driven by neural crest migration and proliferation, which directs the formation and approximation of a series of swellings or processes. Ultimately, these processes fuse with each other to produce seamless regions of ectoderm and the characteristic features of a face. At the molecular level, a host of signalling molecules, transcription factors and extracellular matrix proteins control the cellular activities underlying these processes (Francis-West et al, 2003).
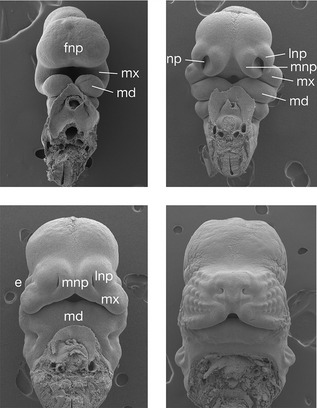
Figure 2.9 Early facial development.
Scanning electron micrographs of an early mouse embryo show development of the face from a series of rudimentary processes. In particular, the lateral part of the upper lip is formed from the maxillary processes, whilst the philtrum is derived from the paired medial nasal processes, which fuse in the midline. e, eye; fnp, frontonasal process; md, mandibular process; mx, maxillary process; lnp, lateral nasal process; mnp, medial nasal process; np, nasal placode.
Human facial development begins at approximately four weeks post conception, with the appearance of five processes, which surround the early oral cavity or stomodeum (Table 2.2):
Table 2.2 Building the head and neck
| Frontonasal process |
| Forehead including upper eyelids and conjunctiva |
| Medial nasal processes |
| Lateral nasal processes |
| First pharyngeal arch |
| Maxillary process |
| Mandibular process |
| Second pharyngeal arch |
| Third pharyngeal arch |
| Fourth pharyngeal arch |
| Sixth pharyngeal arch |
During the fourth week of development the frontonasal process rapidly enlarges as the underlying forebrain expands into bilateral cerebral hemispheres and the paired mandibular processes unite to provide continuity to the forbearer of the lower jaw and lip.
By five weeks of development, medial and lateral nasal processes form within the enlarged frontonasal process to surround an early ectodermal thickening, the nasal placode. The nasal placode gives rise to highly specialized olfactory receptor cells and nerve fibre bundles innervating the future nasal cavity. As the medial and lateral nasal processes enlarge, the nasal placodes sink into the nasal pits, which demarcates the nostrils.
Medial growth of the maxillary processes dominates subsequent development of the face, resulting first in contact and then fusion with the lateral nasal processes to form:
Further growth towards the midline pushes the lateral nasal processes superiorly and allows fusion of the maxillary processes with the medial nasal processes inferiorly, merging them together in the midline to form:
Thus, the upper lip is formed from the maxillary processes laterally and the medial nasal processes in the midline (Jiang et al, 2006).
Posteriorly, from the medial sides of the maxillary process, the secondary palate is formed via growth, elevation and subsequent fusion between the paired palatine processes. These processes also fuse with the nasal septum superiorly and the primary palate anteriorly, ultimately separating the oral and nasal cavities. The essential features of the human face have formed by eight weeks of development.
Development of the pharyngeal region
Endoderm lining the pharyngeal arches, in the form of the pharyngeal pouches, gives rise to a number of important structures associated with the mature pharynx (Fig. 2.10).
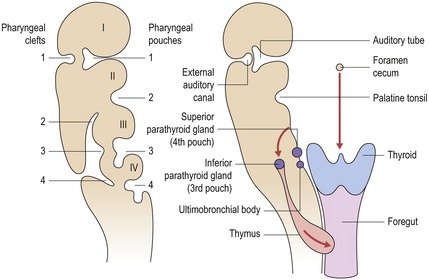
Figure 2.10 Derivatives of the pharyngeal clefts and pouches in the head and neck.
Redrawn from Sandler TW (ed.) (2003), Langman’s Medical Embryology, 9th edn (Baltimore: Lippincott Williams and Wilkins).
Externally, there are four pharyngeal clefts, but only one develops into a recognizable structure in the neonate. The first pharyngeal cleft forms the external auditory canal and contributes to the eardrum of the external ear. The remaining pharyngeal clefts are obliterated by downward growth of the second pharyngeal arch, disappearing as the cervical sinus.
The tongue arises from a series of swellings, which appear around the sixth week of development in the floor of the primitive pharynx (Fig. 2.11).
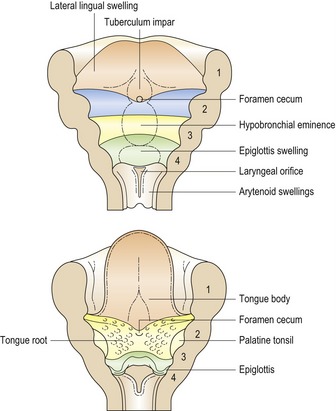
Figure 2.11 Embryonic origins of tongue.
The floor of the pharynx gives rise to a series of elevations at around the sixth week of embryonic development, which contribute to the anterior two-thirds and posterior third of the mature tongue.
Redrawn from Sandler TW (ed.) (2003), Langman’s Medical Embryology, 9th edn (Baltimore: Lippincott Williams and Wilkins).
Simultaneously with formation of the tongue, the thyroid gland is formed from a proliferation of endoderm at the foramen cecum, a site between the tuberculum impar and hypobranchial eminence in the pharyngeal floor. Descent of the primitive thyroid into the neck occurs over a period of a few weeks, resulting in the gland assuming its final position below the thyroid cartilage.
Development of the skull
The individual bones that make up the human skull are formed by two basic mechanisms:
With the exception of the clavicle, bones with an intramembranous origin are only found in the craniofacial region. Collectively, both endochondral and intramembranous bones of the skull originate from two embryonic cell populations:
The boundary of these two tissue contributions within the skull lies at the level of the coronal suture.
The skull can also be subdivided on an anatomical basis into two distinct regions:
The neurocranium is composed of the cranial vault (or desmocranium), which develops in membrane and surrounds the brain, and the cranial base (or chondrocranium), which develops from a cartilaginous template and forms the base of the skull. The viscerocranium or facial skeleton is also formed in membrane and develops from the facial processes and pharyngeal arches.
The cranial vault (desmocranium)
The cranial vault is formed entirely in membrane, being composed of the following bones:
These bones begin to appear during the fifth week of development and by around seven months ossification has progressed to the extent that they meet each other at specialized joints called sutures.
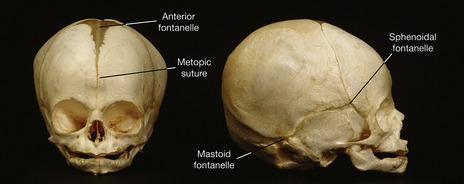
Sutures are specialized growth sites, which allow coordinated bone growth as the flat bones of the skull are displaced by growth of the brain and sensory capsules. In the neonatal skull, enlarged sutures or fontanelles are found in regions where three or more calvarial bones meet and a metopic suture exists between the initially paired frontal bones (Fig. 2.12). This provides a degree of flexibility to the infant skull, allowing it to pass down the birth canal. Sutures play an important role during postnatal growth of the skull and any form of premature fusion in these joints can lead to craniosynostosis (see Fig. 13.18).
The cranial base (chondrocranium)
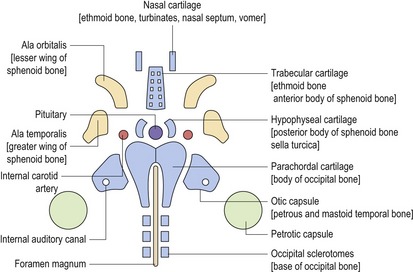
The cranial base is formed from a series of individual cartilages that lie between the early brain capsule and foregut, and begin to appear in the sixth week of development (Fig. 2.13). These cartilages form part of the primary cartilaginous skeleton within the embryo and extend from the cranial end of the notochord to the nasal capsule; both in the midline and more laterally.
In the midline, the occipital sclerotomes (which arise from the occipital somites) merge with the parachordal cartilage to form the basilar and condylar regions of the occipital bone. The hypophyseal (postsphenoid) and trabecular (presphenoid) cartilages form the body of the sphenoid bone whilst the trabecular and nasal cartilages form the perpendicular plate and crista galli of the ethmoid bone. Primary cartilages are also situated in the lateral regions of the skull base, the ala temporalis (alisphenoid) and ala orbitalis (orbitosphenoid), forming the greater and lesser wings of the sphenoid, respectively.
The early cranial base is also characterized by the presence of cartilaginous sensory capsules, which surround the developing sense organs and ultimately contribute to the skull base. The otic capsule contains the vestibular apparatus and cochlear, merging with the lateral part of the parachordal cartilage to form the petrous and mastoid temporal bone. The paired nasal capsules surround the olfactory cells situated at the base of the nasal pits. These cartilages merge with each other and the prechordal cartilage anteriorly to form the nasal cavity.
Facial skeleton (viscerocranium)
The bones of the facial skeleton or viscerocranium develop in membrane from neural crest cells that have migrated into the first and second pharyngeal arches and the facial processes. Ossification centres usually begin to appear within intramembranous condensations from around the seventh week of intrauterine development.
In the maxilla, ossification is first seen in the region of the deciduous canine; whilst in the mandible it occurs lateral to Meckel’s cartilage, between the mental and incisive branches of the inferior alveolar nerve. In both jaws, ossification spreads rapidly into the various processes of these bones. The bulk of Meckel’s cartilage is resorbed during this process of ossification, but some small regions do persist. Anteriorly, nodular remnants known as the ossia menti become incorporated into the mandibular symphysis; whilst posteriorly, the cartilage extends from the point where the inferior alveolar nerve enters the mandible, back towards the ear and forms a number of structures (see Fig. 2.8):
Embryonic development of the mandible is further complicated by the formation of three secondary cartilages at around 10 weeks of development:
These are known as secondary cartilages because they appear after the primary cartilaginous skeleton has been formed within the embryo. Secondary cartilages are found in intramembranous bones within the skulls of many birds and mammals, usually at the site of articulations or muscle attachments. The chondrocytes in secondary cartilage differentiate from progenitor cells within the periosteum of membrane bones. Mechanical stimulation in these regions causes these progenitor cells to differentiate into chondrocytes rather than osteoblasts (Hall, 1984).
Secondary cartilage is distinct from primary cartilage, both structurally and functionally, and can be regarded as an adaptation of intramembranous bone to allow growth in a field of compression. In humans, the coronoid and symphyseal cartilages are essentially transitory, disappearing during the first year of life and playing no significant role in development. However, the condylar cartilage persists until around 20 years of age and is an important centre of postnatal mandibular growth (see Box 3.3). It is the ability of this cartilage to adapt to external functional stimulation that has led many orthodontists to think that clinically significant growth of the mandibular condyle can be stimulated in an adolescent child with the use of a functional appliance.
Molecular regulation of early craniofacial development
In recent years, the advent of molecular biology and the ability to manipulate gene function in vivo has allowed biologists to make significant progress in understanding how embryonic development is controlled at the molecular level. This is particularly true of craniofacial development, where much information has been gathered on how this complex region is constructed within the embryo.
Early development of the head is based upon segmentation
Neural crest cells derived from the hindbrain are essential for normal formation of the face and neck. The early theme of segmentation within the embryonic craniofacial region (seen within the primary vesicles of the neural tube and the pharyngeal arches) is further reiterated in the hindbrain, which is also a segmented structure composed of seven subunits called rhombomeres.
Thus, the origin of neural crest cells can be traced for different regions of the head:
The even numbered rhombomeres (2, 4 and 6) contain the exit points for cranial nerves V, VII and IX; which will innervate structures within pharyngeal arches 1, 2 and 3, respectively. Thus, an axial level-specific code exists, established early in development prior to any neural crest migration; cells recognize each other and have a positional identity. Following their migration into the arches they produce the individual skeletal structures that make up the head in an orderly and integrated manner (Fig. 2.14).
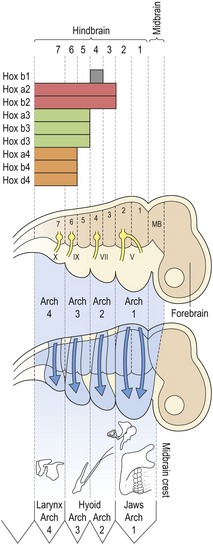
Figure 2.14 Neural crest migration and the formation of head structures.
The early neural tube is segmented into forebrain, midbrain and hindbrain, with the hindbrain being further segmented into rhombomeres. Neural crest cells migrate from their point of origin adjacent to the neural tube into the pharyngeal arches and express different combinations of Hox genes according to their axial origin. This migration is specific, neural crest destined for the first pharyngeal arch migrates from rhombomeres 1 and 2 (with a small contribution of crest from the midbrain region), whilst neural crest for the second and third pharyngeal arches migrates from rhombomeres 3 and 4 and 5 and 6, respectively. Rhombomeres 2, 4, 6 and 7 contain the exit points for cranial nerves V, VII, IX and X; these nerves will innervate structures derived from pharyngeal arches 1, 2, 3 and 4, respectively. Thus, early formation of the head is axial specific; neural crest migration is organized according to the region where these cells originate from.
Clearly, these mechanisms of craniofacial development are under genetic control. A number of genes and gene families that play a critical role in this process, establishing regional identity within the vertebrate head, have been identified.
Lessons from the fly
The fruitfly Drosophila melanogaster has proved to be a useful organism for studying early embryonic development. The Drosophila embryo, larva and ultimately, the adult fly is also based upon segmentation (Fig. 2.15). The basic fly body plan consists of:
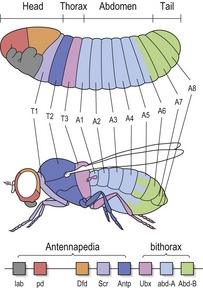
Figure 2.15 The body plan of the fly larva is segmented into a head, thorax, abdomen and tail.
Homeotic genes are found within two complexes on fly chromosome 3 (Antennapedia and bithorax), and are expressed along the body axis of the fly in the order that they appear along this chromosome. Each homeotic gene functions to specify the characteristic anatomical structures that develop within each individual segment.
Once these basic segments have been established, a group of genes known as homeotic genes specify their characteristic structure.
In the fly, homeotic genes are found in a single cluster within two complexes on chromosome 3:
In addition to their highly conserved structure, homeotic genes also demonstrate another remarkable feature. The axial level within the fly in which each gene functions displays a direct relationship with its position on the chromosome; a term known as colinearity. Those expressed in the most anterior, head end of the fly are found at the furthest end of the chromosome, whilst those in the thorax and abdomen are found progressively further along toward the opposite end. To put this more simply, these genes serve as a molecular representation of the anteroposterior axis of the fly embryo (Fig. 2.15).
Homeotic genes provide a combinatorial code for the specification of each regional embryonic segment in the fly embryo. Mutations in these genes can lead to bizarre homeotic transformations where one segment of the fly can assume the phenotype of another. As an example of the power of these genes, one of them, Antennapedia, specifies identity of the second thoracic segment; in the dominant mutation of Antennapedia this gene becomes expressed inappropriately in the head of the fly. As a result of this, there is a growth of thoracic legs from the head sockets, instead of antenna.
Hox genes and craniofacial development
The genetic control of development is more universal than one might imagine. A family of homeobox-containing genes very closely related to the homeotic genes of Drosophila are also found in vertebrates. These genes are called Hox genes and in the mouse and human genomes there are a total of thirty-nine, arranged in four clusters (instead of one in the fly) on four different chromosomes; Hoxa-d in mice and HOXA-D in man (Fig. 2.16). There are more of them because mice and humans have a structurally more complex body plan than flies. The presence of Hox genes means that humans and flies have a common ancestor. It might have taken several hundred million years of evolution to separate us, but the essential function of the genes that pattern the embryo has been retained.
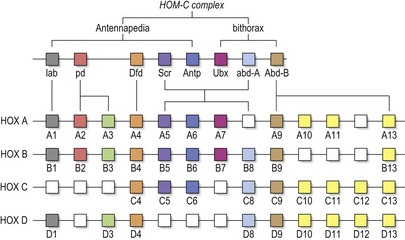
The 39 human HOX genes are organized within four clusters on four different chromosomes (HOX A-D). They are derived from a single ancestral cluster, from which the single HOM-C complex in Drosophila is also derived. HOM-C is itself composed of two clusters, Antennapedia (which contains five genes: labial, proboscipedia, Deformed, Sex combs reduced, Antennapedia) and bithorax (containing three genes: Ultrabithorax, abdominal A, Abdominal B). Cluster duplication during evolution has led to the concept of paralogous groups of Hox genes; thus groups of up to four genes derived from a common ancestral gene can be found. These paralogues can exhibit similar expression domains along the anteroposterior axis of the embryo, leading to redundancy between genes.
Diagram adapted from Scott MP (1992), Vertebrate homeobox gene nomenclature. Cell 71:551–553.
The expression of Hox genes in the vertebrate embryo can be seen within the neural tube, from the anterior region of the hindbrain through the length of the spinal cord. However, the expression patterns show a very precise spatial restriction. Each Hox gene is expressed in an overlapping domain along the anteroposterior axis of the embryo, but each gene has a characteristic segmental limit of expression at its anterior boundary. In the developing head, this spatially restricted expression pattern is seen in the hindbrain with the anterior limits of Hox gene expression corresponding to rhombomere boundaries. As neural crest cells migrate from rhombomeres of the neural tube into specific pharyngeal arches, the particular combination or code of Hox gene expression characteristic of the rhombomere is retained. Thus, cranial neural crest from each axial level conveys a unique and combinatorial Hox code. This code can be considered to specify form and pattern for the different pharyngeal arch-derived regions of the head and neck (Fig. 2.17).
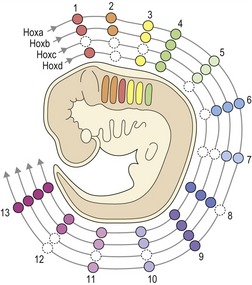
Figure 2.17 Hox gene expression in the mammalian embryo.
There are four Hox clusters in mammals (Hoxa-d) situated on four different chromosomes. Those Hox genes that occupy the same position in different clusters are paralogous and in mammals there are thirteen of these paralogous groups. The position of these paralogues on the chromosome reflects their expression along the anteroposterior axis of the embryo (i.e. Hoxa1 is expressed from the head end, whilst Hoxa13 begins at the tail end). This complex combinatorial expression of Hox genes represents a genetic blueprint for structure along the embryo.
Diagram adapted from Santagati F and Rijli FM (2003), Cranial neural crest and the building of the vertebrate head, Nat Rev Neurosci 4:806–818.
Patterning the first pharyngeal arch
Neural crest cells destined for the first pharyngeal arch, from which the maxilla and mandible develop, do not express Hox genes related to the homeotic homeobox. Indeed, it has been suggested that this loss of Hox gene expression from the first pharyngeal arch was the event that facilitated the evolution of a jaw. However, skeletal elements derived from the first and second arches are profoundly sensitive to the expression domains of the most anterior Hox gene, Hoxa2 (whose normal anterior boundary of expression lies at the border of pharyngeal arches 1 and 2) (see Fig. 2.14):
There is now experimental evidence to suggest that Hox genes control the mechanisms that result in morphogenesis of regions in the head and neck. One method of testing the Hox code is via the use of transgenic technology, by either disrupting or overexpressing a particular Hox gene in transgenic mice. Targeted disruption of the Hoxa2 gene, which is normally expressed in the second pharyngeal arch, leads to a loss of some specific second arch structures such as the stapes. In addition to this, there is also a duplication of proximal first arch structures, which are fused to those that have developed normally (Gendron-Maguire et al, 1993; Rijli et al, 1993). In other words, this gene deletion has produced a type of homeotic transformation. An absence of Hoxa2 leads to cells of the second arch adopting the identity of a first arch. Hoxa2 is clearly involved in patterning the second pharyngeal arch and its derivatives. Hoxa2 overexpression into the first arch leads to transformation of first arch skeletal elements into second (Grammatopoulos et al, 2000). Another Hox gene, Hoxd4, is normally expressed in the spinal cord, with an anterior limit of expression at the level of C1. If the expression domain of Hoxd4 is experimentally extended beyond C1 into the occipital region of the head, the resulting phenotype exhibits transformation of the skull occipital bones into additional cervical vertebrae (Lufkin et al, 1992).
The first pharyngeal arch also gives rise to the dentition and patterning of the tooth primordia that form along the primitive dental axis of the jaws is also under genetic control. Teeth represent a unique series of structures whose differences can be described in terms of changes in both shape and size, these changes being reflected in the position of the tooth within the future dental arch. Thus, in the human dentition central and lateral incisors are found in the anterior region of the dental arch, with canine, premolar and molar teeth situated progressively more posteriorly. Overexpression of Hoxa2 in the mandibular primordium does not affect tooth development, suggesting that patterning of the dentition and skeletal elements within the pharyngeal arches are independent (James et al, 2002).
If Hox genes do not pattern any of the structures derived from the first pharyngeal arch, either skeletal or dental; which genes are responsible? Subfamilies of homeobox genes, more diverged from the ancestral Hox genes, are expressed in spatially restricted patterns within the first pharyngeal arch and activity of these genes is important for patterning both the jaw skeleton and dentition (see Fig. 4.3).
Giving the jaws identity: Dlx genes
Distal-less (Dlx) genes incorporate a six-gene family of mammalian homeobox genes (Dlx1–3; 5–7) that exhibit nested domains of expression in neural crest of the pharyngeal arches during their development (Fig. 2.18). Within the mammalian genome, these genes are arranged in opposing pairs, with each pair having similar domains of expression:
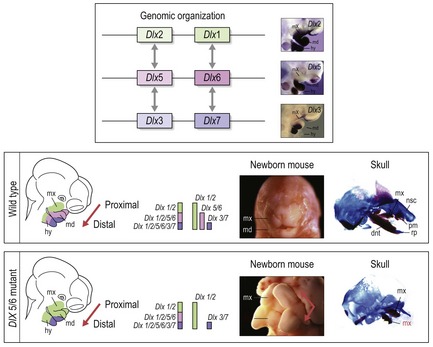
Figure 2.18 Patterning of the pharyngeal arches by Dlx genes.
The pharyngeal arches have individual identity, which can be visualized along the proximal to distal axis in relation to the skeletal structures they give rise to. In addition, the first pharyngeal arch is itself subdivided into a proximal (maxillary) and distal (mandibular) process. It is thought that different combinations of Dlx gene expression in these arches generate specific skeletal structures accordingly. The six Dlx genes within the mammalian genome are arranged in pairs on three separate chromosomes. Each pair demonstrates an expression pattern that is progressively more restricted in a distal direction. Thus, in the maxillary and mandibular processes, Dlx2/1 are expressed throughout, whilst Dlx5/6 are expressed from the mandibular process back (distally) and Dlx3/7 are expressed from the distal-most region of the mandibular process back (upper panel). By removing the function of both Dlx5 and Dlx6 in the mouse embryo, the mandibular process assumes the same code as the maxillary (the expression of Dlx2/1 only) and there is a homeotic transformation. The maxilla is duplicated because in essence there are two maxillary processes. This maxillary duplication can be clearly seen in the newborn mice and the skeleton of their skulls (lower panels).
Courtesy of Dr Michael Depew.
Dlx2/1 are expressed throughout the proximal-distal axis of the pharyngeal arches, whilst the expression domains of Dlx5/6 and Dlx3/7 become progressively more restricted in a distal direction:
Mice with a combined loss of Dlx1/2 function exhibit subtle anomalies in structures derived from more proximal regions of the pharyngeal arches, in particular the maxillary process of the first pharyngeal arch. Even though they are expressed in the distal pharyngeal arches, the loss of Dlx1/2 does not seem to affect the patterning of these regions, because of compensatory action by other Dlx genes. However, in mice lacking the function of both Dlx5 and Dlx6, genes that are only expressed in more distal regions of the pharyngeal arches, a homeotic transformation is found to occur; these mice have some conversion of mandibular arch structures to maxillary (Fig. 2.18) (Depew et al, 2002). This is because in essence, a loss of Dlx5/6 converts the code for a mandible into that for a maxilla. Thus, nested Dlx gene expression appears to play a fundamental role in establishing both the identity of different pharyngeal arches and the identity of the maxillary and mandibular processes of the first pharyngeal arch (Graham, 2002; Schilling, 2003).
Molecular biology of bone formation
Bone formation within the embryo begins with inductive signalling from epithelial and neural tissues, inducing the condensation of mesenchymal precursor cells. In the flat bones of the skull these cells then differentiate directly into osteoblasts; however, in the remainder of the skeleton, these condensations differentiate into chondrocytes and form a cartilaginous intermediate of the developing bone, which becomes progressively ossified with the ingress of osteoblasts.
Endochondral ossification
During the process of endochondral ossification (Fig. 2.19):
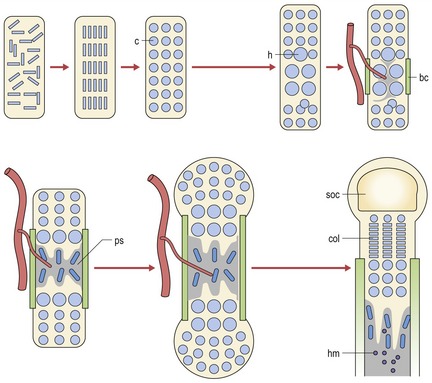
Figure 2.19 Endochondral ossification involves the differentiation of chrondrocytes and the formation of a cartilaginous template, which becomes vascularized and ossified via the ingress of osteoblasts. c, chondrocytes; h, hypertrophy; bc, bone collar; ps, primary spongiosum; col, proliferating chondrocytes; soc, secondary ossification; hm, haematopoetic marrow.
Adapted from Kronenberg HM (2003), Developmental regulation of the growth plate. Nature 423:332–336.
A number of transcription factors and signalling molecules have been identified as key regulators of endochondral bone formation. The SOX transcription factors are essential for chondrocyte differentiation. SOX9 is required for two crucial steps during this differentiation process:
whilst two other SOX transcription factors, L-SOX5 and SOX6, are required for the final step of chondrocyte differentiation.
Indian hedgehog (IHH) is a member of the Hedgehog family of signalling molecules and is a major regulator of:
Ihh mutant mice have shortened long bones and a dwarf-like phenotype (St-Jacques et al, 1999). Ihh is also a powerful inducer of parathyroid hormone-related protein (PTHrP), which acts to maintain chondrocytes in the proliferative state and therefore encourage cartilaginous bone growth.
Fibroblast growth factor (FGF) signalling is also an important regulator of:
Importantly, proliferation is inhibited through the FGF receptor FGFR3; Fgfr3 mutant mice have excessive chondrocyte proliferation (Colvin et al, 1996; Deng et al, 1996) and constitutively active mutations in human FGFR3 are the cause of achondroplasia or dwarfism (OMIM 100800) (Shiang et al, 1994).
Intramembranous ossification
Intramembranous ossification is dependent upon the direct proliferation of mesenchymal osteoprogenitor cells, which ultimately differentiate into osteoblasts. A key factor in the development of membrane bones within the skull is maintaining a correct balance between cellular proliferation and differentiation.
This process is directly influenced by activity of the MSX1 and MSX2 transcription factors (Ferguson, 2000). In particular, a lack of MSX2 activity in humans can lead to a reduction in calvarial ossification and a condition characterized by enlarged parietal foramina or bony defects in the skull vault (Wilkie et al, 2000). This condition is also seen in mice lacking Msx2 activity and interestingly, a lack of both Msx1 and Msx2 in the mouse leads to a lack of almost all membrane bone ossification in the skull, demonstrating the powerful combined effect of these proteins during development of the skull (Satokata et al, 2000). However, it is clear that the levels of MSX protein activity must be carefully controlled to coordinate normal skull development; mutations in the human MSX2 gene that increase transcriptional activity of the protein can produce too much bone formation and premature fusion of the cranial sutures or craniosynostosis (Ma et al, 1996).
Osteoblast differentiation
A degree of commonality does exist between intramembranous and endochondral bones in that osteoblast differentiation, a pre-requisite of osteogenesis regardless of the embryonic origin of the bone, requires activity of the Runx2 transcription factor. Mice lacking the function of Runx2 have no osteoblasts and consequently no bone, either endochondral or intramembranous. In the growth plate, Runx2 also induces the differentiation of hypertrophic chondrocytes and therefore plays an additional role in cartilaginous ossification.
Cobourne MT. Construction for the modern head: current concepts in craniofacial development. J Orthod. 2000;27:307-314.
Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4:806-818.
Meikle MC. Craniofacial Development, Growth and Evolution. Norfolk: Bateson; 2002.
Larsen WJ. Essentials of Human Embryology. Edinburgh: Churchill Livingstone; 1998.
Baldini A. Dissecting contiguous gene defects: TBX1. Curr Opin Genet Dev. 2005;15:279-284.
Colvin JS, Bohne BA, Harding GW, et al. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390-397.
Deng C, Wynshaw-Boris A, Zhou F, et al. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911-921.
Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381-385.
DUNCAN K, MCNAMARA C, IRELAND AJ, et al. Sucking habits in childhood and the effects on the primary dentition: findings of the Avon Longitudinal Study of Pregnancy and Childhood. Int J Paed Dent. 2008;18:178-188.
Ferguson MW. A hole in the head. Nat Genet. 2000;24:30-31.
Francis-West PH, Robson L, Evans DJ. Craniofacial development: the tissue and molecular interactions that control development of the head. Adv Anat Embryol Cell Biol. 2003;169(3–6):1-138.
Gendron-Maguire M, Mallo M, Zhang M, et al. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993;75:1317-1331.
Graham A. Jaw development: chinless wonders. Curr Biol. 2002;12:R810-R812.
Grammatopoulos GA, Bell E, Toole L, et al. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development. 2000;127:5355-5365.
Hall BK. Developmental processes underlying the evolution of cartilage and bone. Symposium, Zoological Society of London. 1984;52:155-176.
Hammond P, Hutton TJ, Allanson JE, et al. Discriminating power of localized three-dimensional facial morphology. Am J Hum Genet. 2005;77:999-1010.
James CT, Ohazama A, Tucker AS, et al. Tooth development is independent of a Hox patterning programme. Dev Dyn. 2002;225:332-335.
Jiang R, Bush JO, Lidral AC. Development of the upper lip: Morphogenetic and molecular mechanisms. Dev Dyn. 2006;235:1152-1166.
Lindsay EA, Vitelli F, Su H, et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97-101.
Lufkin T, Mark M, Hart CP, et al. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992;359:835-841.
Ma L, Golden S, Wu L, et al. The molecular basis of Boston-type craniosynostosis: the Pro148–>His mutation in the N-terminal arm of the MSX2 homeodomain stabilizes DNA binding without altering nucleotide sequence preferences. Hum Mol Genet. 1996;5:1915-1920.
Muenke M, Beachy PA. Genetics of ventral forebrain development and holoprosencephaly. Curr Opin Genet Dev. 2000;10:262-269.
Rijli FM., Mark M, Lakkaraju S, et al. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75:1333-1349.
Satokata I, Ma L, Ohshima H, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391-395.
Scambler PJ. The 22q11 deletion syndromes. Hum Mol Genet. 2000;9:2421-2426.
Schilling T. Evolution and development. Making jaws. Heredity. 2003;90:3-5.
Scott MP. Vertebrate homeobox gene nomenclature. Cell. 1992;71:551-553.
Shiang R, Thompson LM, Zhu YZ, et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335-342.
St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072-2086.
Wilkie AO, Tang Z, Elanko N, et al. Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification. Nat Genet. 2000;24:387-390.