3 Postnatal growth of the craniofacial region
The adult skull is composed of twenty-eight individual bones and represents one of the most complex regions of the body. The skull bones either develop from a cartilaginous template, ossify directly from membrane, or are composite, being formed following contributions from both mechanisms (Fig. 3.1). Growth of this region therefore represents a combination of endochondral and periosteal modes of osteogenesis.
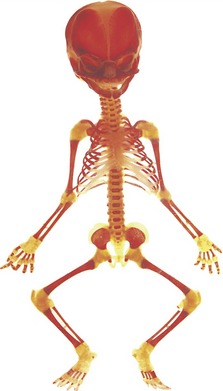
Figure 3.1 Human fetus at around 20 weeks of embryonic development, stained with Alizarin red and cleared.
With the exception of the clavicles, the axial and appendicular skeleton is characterized by endochondral ossification. The skull develops from a combination of endochondral and intramembranous ossification.
Courtesy of the Gordon Museum, King’s College London.
An understanding of the mechanisms underlying craniofacial growth is important for the orthodontist:
General growth of the body
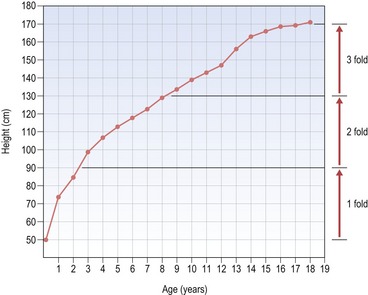
A simple plot of height versus age (or height-distance curve) for either males or females reveals a relatively smooth and constant increase that occurs from birth to the late teenage years and results in an approximate threefold increase in height (Fig. 3.2). However, the height versus age curve does not demonstrate the dynamic changes in growth rate or velocity that occur from birth to adulthood. To do this, an incremental plot of height change, or a height–velocity curve is required, which shows three general phases in the growth curve (Fig. 3.3):
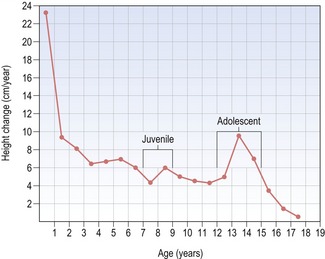
Figure 3.3 Height–velocity curve for a male from birth to 18 years of age.
Note rapid deceleration of growth during the first three years and then a gradual deceleration, briefly interrupted by a juvenile growth spurt at 8 years and the more significant adolescent growth spurt at around 13 years of age.
Whilst the general trends associated with height change are similar in males and females, some fundamental differences do occur between the sexes. In particular, the adolescent growth spurt is greater and occurs later in males, giving them a longer overall period of growth, greater acceleration during adolescence and generally an increased overall height.
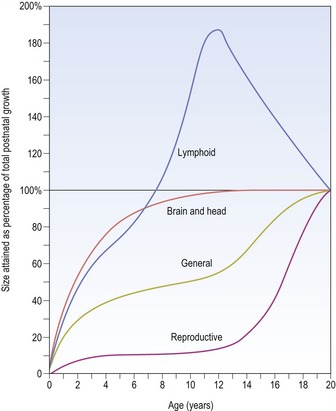
In contrast, other body tissues demonstrate quite different patterns of growth in comparison to height. For example, the central nervous system is well developed at birth and grows rapidly during the early years of life, being essentially complete by approximately 10 years of age; whilst the reproductive organs do not begin to increase in size until puberty (Fig. 3.4).
The skull at birth
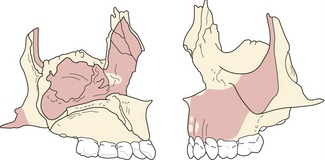
One of the most striking features of a newborn child is the large size of the head in relation to the rest of the body (Fig. 3.5). This is because at birth, the cranial vault is approximately two-thirds of its final dimension, due to extensive prenatal growth and development of the brain. However, despite this large size the skull of the neonate differs significantly from that of an adult (Fig. 3.6):
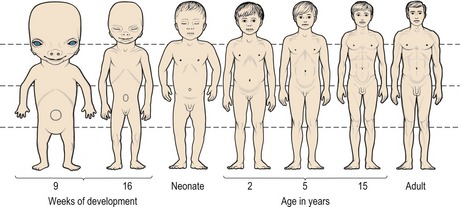
Figure 3.5 Changes in body proportions from the fifth month postconception to maturity.
Note the large size of the head in relation to the rest of the body at birth.
Redrawn from Medawar PB (1945), The shape of the human being as a function of time. Proc R Soc Lond 132:133–141.
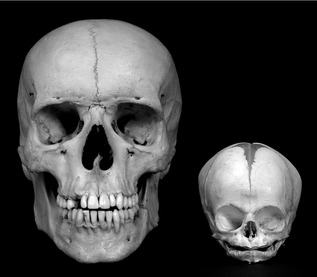
Figure 3.6 Comparison between adult and newborn skulls.
The infant face is wide because of the precociously large cranium and orbits, but also short because of a lack of development within the nasal complex and jaws. The floor of the nasal cavity is sandwiched between the orbits and there is little vertical development of the maxilla and mandible in the neonate. The adult skull is characterized by a nasal cavity situated below the orbits and significant vertical elongation of the jaws.
Rapid growth of the cranial vault continues for the first year after birth, but this progressively decreases during the next two years and remains at a low level until adulthood (Fig. 3.7). However, by 5 years of age around 90% of the adult cranial dimension has been attained. Significantly, the dimensions of the cranium are not affected by the pubertal growth spurt.
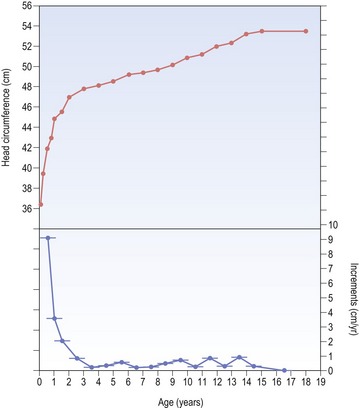
Figure 3.7 Growth in head circumference of a girl from birth until 18 years of age.
The upper plot is the head circumference–distance curve, whilst the lower plot is the head circumference–velocity curve. It can be seen that growth in head circumference is most rapid during the first three years of life.
In contrast to the cranial vault, the face is subject to a more significant change in postnatal dimension, which takes place over a longer period of time and does come under influence of the pubertal growth spurt. Facial growth results in anterior and vertical development of the nasal cavity and jaws relative to the cranial base and a significant change in overall proportions of the skull. By the mid-teenage years the cranial vault has attained adult dimensions, whilst the face is around 95% of its final size.
Mechanisms of craniofacial bone growth
It is clear from the direct comparison of different skull bones that postnatal growth of the craniofacial region does not result from a simple proportional enlargement of each individual bony element (Fig. 3.8). Endochondral bone growth occurs through cartilaginous replacement, whilst intramembranous bones grow as a result of periosteal remodelling. The complexity and diversity of the skull arises because the constituent bones enlarge differentially, in both a temporal and spatial manner (Fig. 3.9). The basic mechanisms underlying growth of the craniofacial region reflect this and produce:
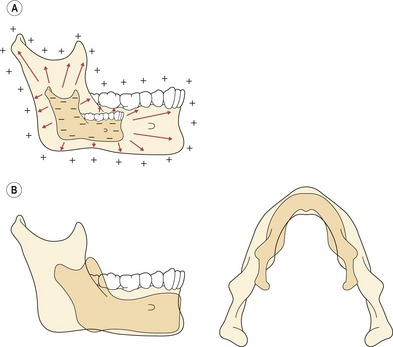
Figure 3.8 The mandible does not grow by a simple symmetrical enlargement (A); rather the condyle and ramus elongate in a posterior and superior direction, whilst the body of the mandible lengthens (B).
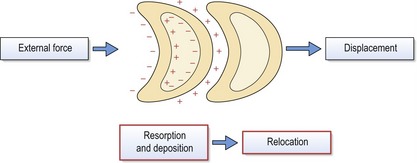
Figure 3.9 Periosteal resorption (−) and deposition (+) on the external and internal surfaces of a skull bone can produce differential changes in both size and shape, or relocation.
This relocation will follow the overall direction of external bony deposition. Displacement of individual bones under the influence of external force also takes place, occurring as an independent process but often simultaneous with relocation. However, it should be remembered that relocation and displacement can occur in opposite directions.
The relocation of a bone takes place via differential changes in both size and shape, which are mediated by surface deposition and resorption. This remodelling occurs on both the outer (periosteal) and inner (endosteal) surfaces of each bone and the relocation, or cortical drift, will follow the direction of external bony deposition.
The displacement of individual bones as single units also takes place, occurring as an independent process and often simultaneously with relocation. Displacement is mediated by the soft tissues, which apply external forces upon the bones, resulting in their displacement away from each other. Compensatory growth at the sutures maintains articulation of the bones as they move. The soft tissues include craniofacial muscles and connective tissues, primary and secondary cartilages and organs such as the brain and eyes. The relative importance and influence of these different forces upon craniofacial growth is controversial and they form the basis of several fundamental theories of growth control in this region.
Theories of craniofacial growth
Several theories that attempt to explain the mechanisms controlling postnatal growth of the craniofacial skeleton have been proposed. These theories have placed varying degrees of emphasis upon the role of genetic and environmental factors, or the significance of different tissues within this region; some being based upon experimental and biological observation and others having a more theoretical basis (Carlson, 2005).
The remodelling theory
The remodelling theory was presented by the anatomist James Couper Brash and represented the first attempt at a general theory explaining the fundamental mechanisms underlying craniofacial growth. This theory placed great emphasis upon remodelling as the primary mechanism by which all bones within the craniofacial complex grew. Thus, the cranial vault expanded via external deposition and internal resorption, whilst the facial bones grew downwards and forwards relative to the cranial vault by posterior resorption and anterior deposition.
The sutural theory
The sutural theory was largely the work of two anatomists, Joseph Weinmann and Harry Sicher, who suggested that primary growth of the craniofacial skeleton was genetically regulated, being controlled within the sutures and cartilages. Importantly, within this model, the sutures had an equivalent role to cartilage in being able to generate a tissue-separating force. For the cranial vault and maxillary complex, sutural growth was regarded as being the prime mediator of bony expansion and, in the case of the maxilla, downward and forward displacement relative to the anterior cranial base (Fig. 3.10).
The cartilaginous theory
In direct contrast to the sutural theory another anatomist, James Scott, suggested that sutures simply represented a continuation of the periosteum and endosteum of the craniofacial bones, in modified regions at their points of intersection. Growth in these regions should therefore be considered as periosteal in nature, being permissive rather than producing a tissue-separating force. Scott suggested that the bones abutting a suture could only be separated by the growth of an associated organ, such as the brain in the case of the cranial vault. Within this theory, great emphasis was placed upon the role of cartilage in producing the driving force of craniofacial growth: in particular, the nasal septal cartilage generating a downward and forward displacement of the maxillary complex, synchondroses elongating the cranial base and the condylar cartilage directing downward and forward growth of the mandible (Scott, 1953; 1954; ">1956).
The functional matrix theory
The functional matrix theory of Melvin Moss describes bone growth within the craniofacial skeleton as being influenced primarily by function (Moss and Salentijn, 1969). In contrast to both the sutural and cartilaginous theories, this theory does not assume that growth of the skull is genetically determined. Indeed, this theory suggests that genes play no major role in determining postnatal growth of the craniofacial region.
Moss suggests that the head simply represents a region where a number of specific functions occur, each being carried out by a ‘functional cranial component’. Functional cranial components consist of two elements:
The functional matrix represents all the tissues, organs and spaces that perform a given function, whilst skeletal units are the bones, cartilages and tendons that support this function. Two types of functional matrix exist:
The periosteal matrix consists of the soft tissues intimately related to a skeletal unit, such as muscles and tendons; whilst capsular matrices are the organs and tissue spaces associated with specific regions within the skull, such as the neurocranium, orbits and oropharynx.
Skeletal units are also further subdivided into:
Each skeletal unit does not necessarily represent an individual bone within the skull, some bones being composed of several microskeletal units or several bones uniting together to function as a single cranial component or macroskeletal unit. In general, the periosteal matrices primarily determine growth of microskeletal units, influencing the size and shape of bones; whilst macroskeletal growth is influenced more by the capsular matrices, producing displacement of cranial regions, such as the nasomaxillary complex or cranial vault (Fig. 3.11).
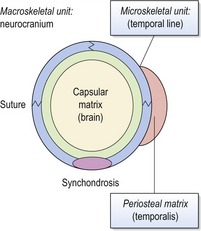
Figure 3.11 The functional matrix hypothesis applied to the cranial vault.
Primary growth of the brain or capsular matrix produces expansion of the flat bones and secondary growth at the sutures and synchondroses. This results in enlargement of the neurocranium or macroskeletal unit. During function, the temporalis muscle exerts pull on the periosteal matrix and bone growth of the temporal line (microskeletal unit).
Redrawn from Carlson DS (2005), Theories of craniofacial growth in the postgenomic era. Semin Orthod 11:172–183.
The servosystem theory
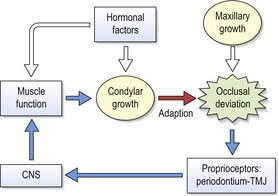
The primary cartilaginous skeleton of the craniofacial region is not influenced by the local and systemic environment to the extent that secondary cartilage of the mandibular condyle is. Based upon these observations, Alexandre Petrovic proposed that two principle factors determine growth of the craniofacial region:
This theory provides a ‘cybernetic’ model of craniofacial growth (Fig. 3.12), which is based upon established biological principles concerning growth and function of primary and secondary cartilages, and the sutures. A strength of this theory is that it incorporates both genetic and environmental influences and assumes a role for both cartilaginous and periosteal tissues during growth of the head.
Growth of the cranial vault
The cranial vault is composed of the squamous parts of the frontal, temporal and occipital bones, and the paired parietal bones. Growth of the cranial vault is intimately linked with growth and expansion of the brain, which passively displaces the individual bones of the skull vault in a concentric manner. As this displacement takes place, the intramembranous bones of the cranium grow in two ways (Fig. 3.13):
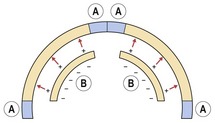
Figure 3.13 Bone growth in the cranial vault.
As growth of the brain passively expands the flat bones, compensatory bone growth at the sutures maintains patency (A). Whilst external and internal surface remodelling reduces the curvature and adjusts their relationship as they are displaced radially (B).
Sutures are specialized fibrous joints situated between adjacent intramembranous bones and they mediate growth along the osteogenic fronts of these bones as they are displaced away from each other. Sutures are tension-adapted; they do not generate the forces underlying bone displacement, but respond to them, adding new bone in equilibrium with bony separation and therefore maintaining patency. This process must be closely regulated; too much bone formation can lead to the premature fusion of one or a number of sutures within the skull and prevent growth of these regions. This results in excess, compensatory growth within other regions of the skull and distortion as the soft tissues expand, a condition called craniosynostosis.
In addition to growth at the sutures, bones of the cranial vault also undergo remodelling along their external and internal surfaces to reduce their curvature and adjust their relationship as they are displaced radially.
Growth of the cranial base
The cranial base develops from a primary cartilagenous chondrocranium, which undergoes a programme of endochondral ossification that is well advanced at birth. A number of bones contribute to the cranial base, including the frontal, ethmoid, sphenoid and occipital. Postnatal growth of this region is achieved by the following mechanisms:
Isolated regions of cartilage, or synchondroses, persist within the cranial base for variable periods of time and make a significant contribution to postnatal growth of this region. They mediate pressure-adapted primary endochondral growth and act directly to increase the anteroposterior dimension of the skull base (Box 3.1). Once growth in the synchondroses has ceased, the cartilage is replaced by bone to form a synostosis.
Box 3.1 How much does the anterior cranial base grow?
The anterior cranial base is frequently used as a plane of reference for the superimposition and comparison of serial cephalometric radiographs. It is therefore important to know the amount and duration of growth that occurs within this region and in particular, when this growth is complete.
From the age of 5 through to 20 years, the distance from sella to nasion (see Chapter 6) will increase approximately 8-mm in females and 10-mm in males, with this growth being essentially complete by the age of 14 and 17 years, respectively. Interestingly, in comparison to growth of the anterior cranial base as a whole, the distance from sella to the foramen caecum (situated between the frontal and ethmoid bones) demonstrates proportionately very little growth (around 3-mm). In contrast, the distance from foramen caecum to nasion increases between 5 and 7-mm. Given that the total length of this dimension in the adult is only on the order of 10 to 12-mm, this is proportionately a huge amount of growth (Bhatia & Leighton, 1993). These differences reflect the fact that anatomically, the anterior cranial base is a relatively stable region for use in regional superimposition (Björk, 1968; Melsen, 1974), but care should be taken when using nasion, because growth of the frontal sinus and remodelling of the frontal bone can significantly influence the position of this landmark.
Growth of the cranial base is not entirely endochondral in nature and considerable remodelling also occurs along its length. This is largely resorptive on the internal surface and depository externally, which contributes towards expansion and lateral relocation of the skull base. These patterns of remodelling activity within the cranial base have been mapped in some detail (Fig. 3.14) (Melsen, 1974). As the cranial base elongates and expands, via cartilagenous growth and surface remodelling, compensatory growth at the sutures maintains patency of the bony articulations within this region.
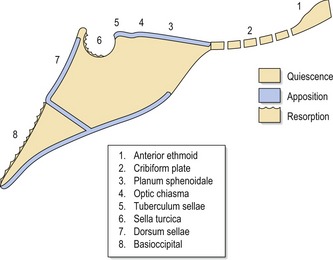
Figure 3.14 Surface remodelling within the cranial base.
The ethmoidal region is essentially stable after 4 years of age but bony apposition occurs along the planum sphenoidale (superior surface of the body of the sphenoid), optic chiasma and tuberculum sellae (anterior limit of the sella turcica) until the mid-teens. Within sella turcica, the anterior wall is stable from 5 years of age; however, the floor and posterior wall is resorptive until the late teens. Further posteriorly, the dorsum sellae is appositional, but the cerebral surface of the basioccipital bone is resorptive until around 17 years in females and 19 in males.
Adapted from Melsen B (1974), The cranial base. Acta Odontologica Scandinavica 32:1–126.
It is important to remember that coordinated growth of the cranial base does not occur in isolation within the skull and the influence of this region upon the face cannot be underestimated. The maxilla articulates with the anterior cranial base and the mandible is suspended beneath the middle cranial fossa, which is closely related to the posterior cranial base (Solow, 1980). Any degree of cranial base flexion between these two regions will directly affect the skeletal pattern of the jaws (Fig. 3.15). From the age of 12 there is very little change in this angle but individual variation in the size is high (Björk, 1955a).
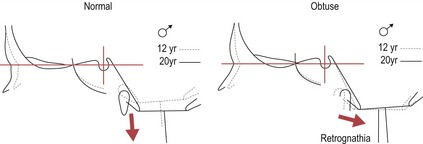
Figure 3.15 As the cranial base angle becomes more obtuse, the mandible becomes more retrognathic relative to the maxilla.
Conversely, a more acute angle makes the mandible more prognathic.
Redrawn from Björk A and Skieller V (1977), Growth of the maxilla in three dimensions as revealed radiographically by the implant method. Br J Orthod 4:53–64.
Growth of the nasomaxillary complex
The nasomaxillary complex forms the middle part of the facial skeleton and is dominated by the orbits, nasal cavity, upper jaw and zygomatic processes. A number of bones make contributions to this region, including the frontal, sphenoid, zygomatic, lacrimal, nasal, maxillary, palatine, ethmoid and vomer.
The maxilla grows downwards and forwards in relation to the anterior cranial base, accompanied by the orbits and nasal cavity, with all three regions increasing in volume as they grow. The cheekbones and zygomatic arches also grow laterally and are relocated in a posterior direction within the face. A complex pattern of surface remodelling and sutural growth achieves these bony changes.
The maxillary arch is lengthened and widened by posterior and lateral deposition, with this depository activity giving way to anterior resorption below the zygomatic buttress. Growth of the maxilla has been extensively described in three dimensions using the implant method (Box 3.2) (Fig. 3.16) (Björk and Skieller, 1977):
Box 3.2 The implant studies of Arne Björk
A great deal of information regarding postnatal craniofacial growth has been provided by a growth study carried out on children at the Royal Dental College in Copenhagen by Arne Björk. This landmark investigation began in the 1950s and combined the use of longitudinal cephalometric radiography with the placement of metallic implants into the jaws of around 100 children of each sex, covering an age period from 4 to 24 years (Björk, 1955b). The implants remained in position throughout the study and served as fixed reference points for radiographic superimposition. The stability of these implants meant that sites of growth and resorption could be identified within the individual jaws. This study highlighted many features of craniofacial growth that had not previously been recognized using cephalometric radiography alone. In particular were the findings that a significant amount of individual variation occurs in the pattern of facial growth when comparing subjects, that growth of the maxilla and mandible often contains a significant rotational component and that a number of naturally occurring and stable reference structures do exist within the craniofacial skeleton that can be used to compare serial cephalometric radiographs.
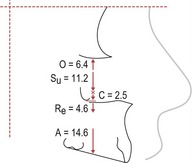
Figure 3.16 Average remodelling of the maxillary complex between 4 and 20 years of age in boys, as determined by the implant method.
Su = sutural lowering; O = apposition along the orbital floor; Re = resorption of the nasal floor; A = apposition on the alveolar process; C = apposition at the infrazygomatic crest.
Redrawn from Björk A and Skieller V (1977), Growth of the maxilla in three dimensions as revealed radiographically by the implant method. Br J Orthod 4:53–64.
These patterns of resorption and deposition occur over the surface of the maxilla (Fig. 3.17), as it is displaced downward and forward within the face. The origin of the displacing force has been a subject of some debate since the first studies on craniofacial growth were carried out. Indeed, the origin of the maxillary displacing force has formed a central theme for all of the major growth theories; be it periosteal growth at the sutures, cartilaginous growth at the nasal septum or the functional matrices associated with this bone.
Growth of the mandible
The mandible also grows downwards and forwards in relation to the cranial base and this is achieved by:
The ramus is remodelled in posterior, superior and lateral directions by bony resorption and deposition. This elongation and posterior relocation of the ramus translates the body of the mandible downwards and forwards and increases the posterior arch length. The regions of bone remodelling are complex, but essentially involve bony deposition and resorption along the posterior and anterior margins of the ramus, supplemented by distinct patterns of resorption and deposition along lateral and lingual regions of the condyle, coronoid, ramus and angle (Fig. 3.18).
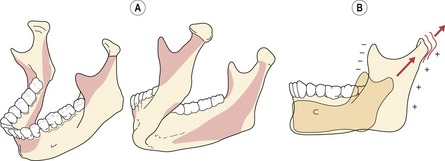
Figure 3.18 Mandibular growth.
Surface remodelling (A) and elongation of the condyle (B). Resorptive surfaces are represented by dark shading and depository surfaces are unshaded.
The condyle is also a major site of growth within the mandible, but controversy exists as to whether this contribution provides the primary force of mandibular displacement or whether this growth is more adaptive in nature.
Condylar cartilage
The condylar cartilage is a secondary cartilage that forms within the mandibular condyle at around 10 weeks of embryonic development. Initially, it forms a large carrot-shaped wedge within the whole of the condyle, but progressive ossification during early postnatal life results in a small cap of proliferating cartilage remaining beneath the fibrous articular surface of the condyle until around the end of the second decade.
In reality, the condylar cartilage represents an essential adaptation of the mandible, allowing bone growth to occur at the condyle, which during function is in a field of compression. This adaptation is necessary because the mandible is an intramembranous bone, which in the skull grow via a periosteal mode of osteogenesis within fields of tension on the surface periosteum, endosteum and at sutures. Periosteal osteogenesis is not pressure-adapted and intramembranous bones are unable to grow within fields of compression. During function, the mandibular condyle undergoes compressive loading within the temporomandibular joint; therefore an adaptation is required within this region to allow bone growth to occur. Endochondral bones, such as the long bones of the axial skeleton, are able to grow under compression because they retain regions of cartilage at the epiphyses or growth plates. The cartilagenous growth plates have inherent growth potential and are able to produce skeletal growth under compressive force. The condylar cartilage is more adaptive, maintaining articulation of the condyle within the glenoid fossa as the mandible is translated downward and forward through regional growth (Box 3.3).
Box 3.3 How does the condylar cartilage differ from an epiphyseal growth plate?
Superficially, the condylar cartilage resembles a primary growth plate such as an epiphysis or synchondrosis; however, considerable functional and anatomical differences exist between all of these structures.
The condylar cartilage is concerned with maintaining growth of an intramembranous bone (the mandible) within a field of multidirectional compression (the temporomandibular joint).
Epiphyseal growth plates are found within long bones and facilitate their elongation by endochondral ossification. Bone formation takes place within the peripheral calicified zone of the cartilage and growth is mediated by chondrocyte proliferation and cartilaginous replacement. An epiphyseal growth plate differs from the condyle in a number of respects:
Mandibular growth rotations
Growth in length of the mandibular ramus occurs essentially at the condyles, but this growth is variable in direction and often involves a component of rotation (Björk, 1955b; 1963). Large individual variation exists in the direction that is seen; with vertical, forward or backward growth taking place (Fig. 3.19). Three different types of mandibular growth rotation were originally described by Björk and Skieller, with the terminology associated with these different rotations being later simplified by Solow and Houston (Box 3.4) (Björk and Skieller, 1983; Solow and Houston, 1988):
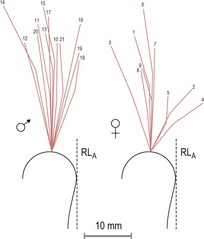
Figure 3.19 Variation in the direction of condylar growth direction.
Adapted from Björk A and Skieller V (1972), Facial development and tooth eruption. An implant study at the age of puberty. Am J Orthod 62:339–383.
Box 3.4 The confusing nomenclature of mandibular growth rotations
Solow and Houston (1988) updated the original nomenclature proposed by Björk and Skieller (1983) in an attempt to simplify the subject of mandibular growth rotation.
The sign convention for mandibular growth rotations was also clarified. With the head facing right, a forward or negative rotation is counterclockwise, whilst a backward or positive rotation is clockwise (Solow and Houston, 1988).
Mandibular growth rotations can take place in either a forward or backward direction, the total rotation representing the sum of the matrix and intramatrix rotations (Fig. 3.20). Forward rotations are the most common, associated with centres of rotation through the condyles, incisors or premolars; whilst backward rotations take place through centres in the condyles or the most distal-occluding molars (Björk, 1969). These different rotations all represent an imbalance in growth between anterior and posterior face height (Fig. 3.21). An excess of growth in the anterior face height will result in a total backward rotation of the mandible, whilst increased growth in posterior face height leads to a total forward rotation (Houston, 1988). In many cases of rotation, a normal occlusion is maintained because of dentoalveolar compensation; however, if the imbalance is severe, then a malocclusion such as anterior open bite or deep overbite may occur.
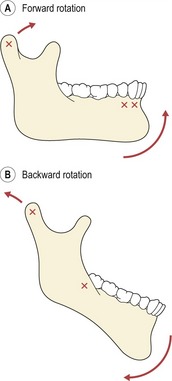
Figure 3.20 Mandibular growth rotations.
(A) Forward rotator. (B) Backward rotator. Centres of rotation are marked (X).
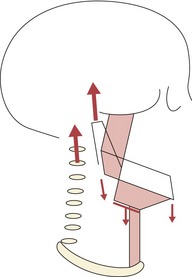
Figure 3.21 As the child grows, the cervical column increases in length and takes the head away from the shoulder girdle.
This is associated with growth and stretch of a chain of muscle groups extending from the mandible to the base of the skull superiorly and from the mandible to the hyoid bone, and hyoid bone to shoulder girdle inferiorly. This produces a descent of the mandibular symphysis and hyoid bone relative to the cranial base and an increase in anterior face height. Posterior face height increases via growth of the middle cranial fossa and the condyle. Extremes of growth in these dimensions can lead to excessive anterior or posterior facial growth and rotations of the mandible.
Redrawn from Houston WJ (1988), Mandibular growth rotations—their mechanisms and importance. Eur J Orthod 10:369–73.
The presence, or likelihood of a mandibular growth rotation can have important consequences for orthodontic treatment. Extremes of rotation can influence the eruptive paths of the teeth and skeletal relationships of the jaws. It is therefore important to detect these types of mandibular growth rotation if at all possible. Unfortunately, orthodontists rarely have the benefit of fixed metallic implants to superimpose their radiographs on, and a total growth rotation cannot be evaluated by simply measuring the outer bony contours of the mandible because remodelling will mask it. A structural method was therefore described, which was based upon identifying certain morphological features on a cephalometric radiograph that could be used to predict the presence and direction of a mandibular growth rotation (Björk, 1969). This method involves identifying and describing the following features (Fig. 3.22):
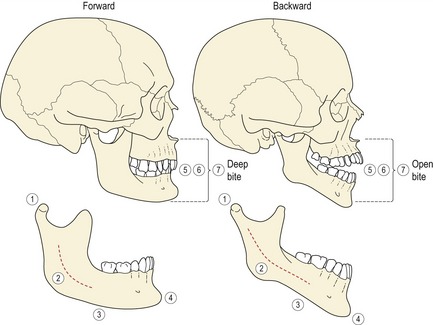
Figure 3.22 Structural signs of mandibular growth rotation.
Björk identified seven structural signs within the mandible that could be associated with different growth rotations. Not all of these signs are found in each individual but the greater the number present, the more reliable the prediction of a forward or backward rotation. In the forward rotating mandible: (1) the condyle is inclined forward; (2) the mandibular canal has a curvature greater than the mandibular contour; (3) the lower border of the mandible is rounded anteriorly and concave at the angle, due to bony deposition along the anterior region and symphysis, and resorption below the angle; (4) the symphysis is inclined forward within the face and the chin is prominent; (5) the interincisor angle, (6) interpremolar and intermolar angles are all increased; (7) the anterior lower face height is reduced with a tendency towards an increased overbite. In contrast, the backward rotating mandible is associated with: (1) a backward inclination of the condyles; (2) a flat mandibular canal; (3) a lower border that is thinner anteriorly and convex, due to minimal remodelling along the lower border of the mandible and bony deposition at the posterior border of the ramus; (4) the symphysis is inclined backward within the face and the chin is receding; (5) the interincisor angle, (6) interpremolar and intermolar angles are all decreased; (7) the lower anterior face height is increased and there is an anterior open bite.
There are conflicting results regarding the predictive ability of the structural method. An investigation using some of the more extreme cases from Björk’s original sample demonstrated a high prognostic estimate of mandibular growth rotation using combined measures of mandibular inclination (Skieller et al, 1984). However, an alternative study tested the ability of five experienced clinicians to differentiate extreme backward rotators from forward using cephalometric radiographs and found this to be no better than chance (Baumrind et al, 1984).
Dentoalveolar compensation
A considerable amount of individual variation exists in the amount and direction of maxillary and mandibular growth that occurs during postnatal development. The dentoalveolar compensatory mechanism attempts to maintain a normal interarch occlusal relationship in the presence of variation in the skeletal pattern (Solow, 1980). A number of different factors are responsible for dentoalveolar adaptation:
In the absence of adequate dentoalveolar compensation a malocclusion can therefore result. However, extremes of tooth position necessary to compensate for a jaw discrepancy might contribute towards crowding of the dental arches, exchanging one form of malocclusion for another. Alternatively, the size of the skeletal discrepancy might be such that successful compensation is not possible (Fig. 3.23). The amount of dentoalveolar compensation that has taken place in the presence of a skeletal discrepancy is an important factor when considering orthodontic treatment.
Adult craniofacial growth
Although most craniofacial growth is complete by the end of adolescence, longitudinal studies have demonstrated that a small amount continues during adult life. This tends to initially reflect the original growth pattern, especially when there is an underlying skeletal discrepancy; however, later in adult life, changes in the vertical dimension predominate. Rotational changes are also seen in the jaws, with males showing a greater tendency toward forward rotation of the mandible and females a backward rotation. It also appears that growth in females can re-accelerate in adulthood, especially during pregnancy. As well as these skeletal changes, considerable changes in the facial soft tissues take place with increasing age. In particular, the nose and chin tend to lengthen and the lips become more retrusive and less full with the passing of time.
Baumrind S, Korn EL, West EE. Prediction of mandibular rotation: an empirical test of clinician performance. Am J Orthod. 1984;86:371-385.
Bhatia SN, Leighton BC. A manual of facial growth. Oxford: Oxford University Press; 1993.
Björk A. Cranial base development. Am J Orthod. 1955;41:198-225.
Björk A. Facial growth in man, studied with the aid of metallic implants. Acta Odontol Scand. 1955;13:9-34.
Björk A. Variations in the growth pattern of the human mandible: longitudinal radiographic study by the implant method. J Dent Res. 1963;42(1):400-411.
Björk A. The use of metallic implants in the study of facial growth in children: method and application. Am J Phys Anthropol. 1968;29:243-254.
Björk A. Prediction of mandibular growth rotation. Am J Orthod. 1969;55:585-599.
Björk A, Skieller V. Growth of the maxilla in three dimensions as revealed radiographically by the implant method. Br J Orthod. 1977;4:53-64.
Björk A, Skieller V. Normal and abnormal growth of the mandible. A synthesis of longitudinal cephalometric implant studies over a period of 25 years. Eur J Orthod. 1983;5:1-46.
Carlson DS. Theories of craniofacial growth in the postgenomic era. Semin Orthod. 2005;11:172-183.
Houston WJ. Mandibular growth rotations—their mechanisms and importance. Eur J Orthod. 1988;10:369-373.
Melsen B. Time and mode of closure of the spheno-occipital synchrondrosis determined on human autopsy material. Acta Anat (Basel). 1972;83:112-118.
Melsen B. The cranial base. Acta Odontologica Scandinavica. 1974;32:1-126.
Moss ML, Salentijn L. The primary role of functional matrices in facial growth. Am J Orthod. 1969;55:566-577.
Scott JH. The cartilage of the nasal septum. Br Dent J. 1953;95:37-43.
Scott JH. The growth of the human face. Proc R Soc Med. 1954;47:91-100.
Scott JH. Growth at the facial sutures. Am J Orthod. 1956;42:381-387.
Skieller V, Björk A, Linde-Hansen T. Prediction of mandibular growth rotation evaluated from a longitudinal implant sample. Am J Orthod. 1984;86:359-370.
Solow B. The dentoalveolar compensatory mechanism: background and clinical implications. Br J Orthod. 1980;7:145-161.
Solow B, Houston WJ. Mandibular rotations: concepts and terminology. Eur J Orthod. 1988;10:177-179.