Chapter 33 Antenatal investigations
After reading this chapter, you will be able to:
Introduction
The field of antenatal investigations has grown greatly in the past few years. Tests are being offered today – and decisions from women are becoming necessary – that would have been unthinkable previously. Although NICE (2008) guidelines for antenatal care recommend a schedule of antenatal tests, there is still a wide variation in what tests are considered ‘routine’ in various parts of the UK. The increase in use of information technology has meant that women and their partners often have accessed much specialized information themselves, and this may shape their questions.
Therefore, midwives need to have a better-than-ever knowledge of what tests are being offered, in order that they can ensure women are making their choices based on up-to-date and comprehensive information. They also need to be effective counsellors as it is acknowledged that the skills and attitudes of midwives influence the uptake of screening tests (Heyman & Henriksen 2001, van den Berg et al 2007). The midwife should also appreciate that the complete clinical antenatal examination is one of the most effective and efficient screening and diagnostic tools, if undertaken systematically and skilfully.
Screening and diagnosis
Although the meanings of screening and diagnosis are very different, they are often confused, and the midwife must ensure that the woman fully understands the difference.
Screening can be defined as determining the risk or likelihood of a condition, whereas a diagnostic test will give a definite answer. Sometimes, action will be taken following the results of a screening test. For example, a low haemoglobin (Hb) result in pregnancy may be assumed to be caused by pregnancy-induced anaemia and iron tablets will probably be given without further investigation, although in a few rare cases the anaemia may be caused by uncommon conditions, such as chronic renal infection, which would need further investigations to obtain a diagnosis. However, it would not be cost-effective to do an infinite range of investigations for every woman who presented with a positive screening test where the usual cause can be easily treated.
Some screening tests will produce results which mean an invasive test will be necessary to obtain a diagnosis. This needs to be made clear to the woman by the midwife providing counselling – should a woman be undertaking a serum screening for Down syndrome test if she would not undergo amniocentesis in the case of a ‘high risk’ result? Some tests, such as ultrasound, can be both screening and diagnostic (see Table 33.1) – for instance, a scan can diagnose a missing limb or neural tube defect, but can also discover anomalies (for example, ‘soft markers’) which would need further investigations to determine a diagnosis. (See the NHS screening website for the timeline for antenatal tests.)
Table 33.1 Common procedures used for fetal assessment
| Test | Time | |
|---|---|---|
| Nuchal translucency (screening) | Chromosomal abnormality | 10–14 weeks |
| Chorionic villus sampling (diagnostic) | Chromosomal abnormality Genetic disease Metabolic disorders Haemoglobinopathies Infection |
>10 weeks |
| Amniocentesis (diagnostic) | Chromosomal abnormality Genetic disease Metabolic disorders Haemoglobinopathies Infection |
10–14 weeks (early) 15–18 weeks |
| Ultrasound (screening and diagnostic) | Assess fetus (dates/growth/viability/number) Diagnosis of some abnormalities (e.g. structural) Screening for abnormalities (e.g. soft markers) Assessment of placental site Liquor volume |
All gestations |
| Cordocentesis (diagnostic) | Obtain fetal blood sample | 2nd/3rd trimester |
| Doppler (screening) | Assess fetal/placental/uterine blood flow | 2nd/3rd trimester |
It is obviously not enough just to have the tests explained by the midwife – the implications of both positive and negative results also need to be explored before a woman can be said to be making an informed choice. As tests become more varied and complex and midwives’ time more limited, ensuring properly informed choice is becoming a greater challenge for midwives.
Blood tests
Blood is taken from a woman during pregnancy to detect conditions which may influence her wellbeing and that of the developing fetus.
Blood tests for assessment of maternal wellbeing
See Table 33.2 for normal blood laboratory values.
Table 33.2 Normal blood levels and specific diagnostic tests
| Non-pregnant | Pregnant | |
|---|---|---|
| General screening assays | ||
| Haemoglobin | 12–16 g/dL | 11–13 g/dL |
| Packed cell volume (PCV) | 37–45% | 33–39% |
| Red blood cell count (RBC) | 4.2–5.4 million/mm3 | 3.8–4.4 million/mm3 |
| Mean corpuscular volume (MCV) | 80–100 fL | 70–90 fL |
| Mean corpuscular haemoglobin (MCH) | 27–34 fL | 23–31 fL |
| Mean corpuscular haemoglobin concentration (MCHC) | 32–35 fL | 32–35 fL |
| Reticulocyte count | 0.5–1% | 1–2% |
| White blood cells (WBC) | 4–11 × 109/L | 6–16 × 109/L |
| Platelets | 150–400 × 109/L | 150–400 × 109/L |
| C-reactive protein (CRP) | 0–7 g/L | 0–7 g/L |
| Specific diagnostic tests | ||
| Serum iron | 50–100 mg/dL | 30–100 mg/dL |
| Unsaturated iron binding capacity | 250–300 mg/dL | 280–400 mg/dL |
| Transferrin saturation | 25–35% | 15–30% |
| Iron stores (bone marrow) | Adequate ferritin | Unchanged |
| Serum folate | 6–16 mg/mL | 4–10 mg/mL |
| Serum vitamin B12 | 70–85 ng/dL | 70–500 ng/dL |
| Serum ferritin | 15–300 pg/L | Unchanged |
ABO and rhesus blood grouping
Blood is typed as A, B, AB or O depending on specific agglutinogens on the erythrocytes. The rhesus factor identifies the blood group as negative or positive depending on whether the rhesus factor antigen is present. Because of the risk of anaemia, haemorrhage and shock in pregnancy and during birth, and the possible need to provide transfusion, it is important that the blood group is identified early in the pregnancy.
Antibodies
Maternal blood is examined for the presence of antibodies, particularly rhesus antibodies if the woman is rhesus negative. If the fetus is rhesus positive, antibodies can be stimulated by the occurrence of a fetomaternal haemorrhage, when ‘leaks’ occur and some fetal rhesus-positive cells pass into the maternal circulation. This can happen as pregnancy progresses, during procedures such as amniocentesis, chorionic villus sampling (CVS) or external cephalic version, in situations such as an antepartum haemorrhage, or at delivery. The rhesus-negative woman may respond by producing antibodies, in this or subsequent pregnancies, which may then cross the placenta to the fetal circulation and cause haemolysis in a rhesus-positive fetus. The administration of anti-D immunoglobulin is effective in preventing the production of these antibodies (MacKenzie et al 1991). Recent guidance from NICE (2008) suggests that routine antenatal anti-D prophylaxis should be offered to all non-sensitized, rhesus-negative women. It is crucial that careful discussion takes place regarding this prophylaxis as the woman must appreciate that she is being given a blood product. If the woman knows that the father of the child is rhesus negative also, prophylaxis will be unnecessary.
ABO incompatibility and less common antibodies such as Kell, Duffy and Kidd (Hoffbrand et al 2006) can also affect the fetus or newborn.
Full blood count
Full blood counts are taken routinely at booking and intervals during pregnancy, to detect a pathological fall in haemoglobin (Hb) which may indicate an iron deficiency anaemia. No woman should reach term with a potentially dangerous anaemia because this exposes her to the risk of excessive blood loss at delivery. It must be remembered, however, that other rare conditions may be discovered ‘accidentally’; for example, a low white cell count may lead to a diagnosis of leukaemia. It is important therefore that no abnormal result ever be disregarded.
Haemoglobin (Hb)
Because of physiological changes in pregnancy, haemoglobin levels will normally reduce, with the lowest reading expected at about 34 weeks. The World Health Organization (see website) cites 11 g/dL as the lowest acceptable reading, although other authorities quote figures down to 10 g/dL. A low Hb reading needs further investigation to establish the cause, so that appropriate treatment can be commenced.
Serum ferritin levels and total iron-binding capacity (TIBC) may be assessed (McGhee 2000) and causes of insidious blood loss, such as from chronic renal infection or parasitic infestation, may be investigated.
In the past, iron supplements were routinely given to pregnant women, but this is no longer considered appropriate (NICE 2008). Measurement of serum ferritin at booking may predict those who will develop anaemia during pregnancy and therefore treatment could be commenced before the Hb becomes low (Letsky 2002).
Mean corpuscular volume (MCV)
The earliest effect of iron deficiency is a reduced MCV. MCV is also reduced with alpha and beta thalassaemia minor. A raised MCV is associated with folate deficiency (high alcohol intake can reduce absorption of folic acid) or B12 deficiency.
Haemoglobinopathies
Haemoglobinopathies are a diverse group of inherited single-gene disorders involving abnormal haemoglobin patterns which constitute two major conditions: thalassaemia (minor or major) and sickle cell disorders: sickle cell trait (SCT or HbAS); sickle cell haemoglobin C disease (HbSC); and sickle cell disease/anaemia (HbSS).
Both sickle cell disease and thalassaemia are recessive conditions (see Ch. 26), therefore only those inheriting an affected gene from each parent will have the disease. If a woman is found to be carrying either the HbS gene or the thalassaemia trait (thalassaemia minor), it is necessary to test her partner before a prediction about the baby’s condition can be made. If both parents carry the gene, prenatal diagnosis can be made by chorionic villus sampling (CVS), amniocentesis or, more rarely, cordocentesis.
Currently, in high-prevalence areas, booking bloods for all women are automatically screened by hospital laboratories. In areas considered low prevalence, the Family Origin Questionnaire (DH 2007) should be used by midwives to identify who to test (see website).
Maternal infection screening
Rubella
This common viral infection is a significant condition in pregnancy because of the teratogenic effect on the developing fetus caused by transplacental transmission of the virus. Detection of rubella antibodies is carried out by serological testing to identify immunity (IgM and IgG antibodies).
The majority of women in the UK are immune as a result of routine vaccination against rubella at 11–14 years of age. However, since 1988, vaccination is now by measles, mumps and rubella (MMR) vaccine, usually administered before 15 months to male and female infants. It was hoped that with a universal take-up, rubella would be eradicated altogether. However, controversies over routine vaccination for infants reported in the media may compromise this.
All pregnant women are tested for rubella immunity at antenatal booking. Some women who have previously tested as immune have been known to become infected or test as susceptible, therefore testing in the preconception period is to be advised.
If a woman is not immune and comes into contact with rubella, she may develop the disease. Rubella can cause the loss of the pregnancy or the birth of a rubella-infected baby with various physical and mental anomalies. The fetus is most vulnerable up until 16 weeks, but the infection can cross the placenta at any gestation. To avoid the danger of rubella in future pregnancies, the non-immune woman is offered vaccination in the puerperium, together with contraceptive advice for a period of 3 months.
Hepatitis
Hepatitis means inflammation of the liver. There are several different viruses which affect the liver (A, B, C, D, E and F) but B and C are the types with the most direct relevance to midwives at present.
Hepatitis B (HBV)
Hepatitis B is an infectious blood-borne viral disease. It can cause a range of symptoms from very mild to life threatening. About 10% of adults infected become chronic carriers and this may then progress to serious liver disease. Transmission is by contact with body fluids or vertically to the fetus. However, although there is a high chance of perinatal transmission, interventions after birth can greatly reduce the risk of the baby becoming a chronic carrier, and therefore identification of the mother’s HBV status during pregnancy is important. All pregnant women should be screened for HBV infection (NICE 2008).
Because of its high level of infectivity, all healthcare workers (especially midwives) who have contact with body fluids should be vaccinated against HBV.
Hepatitis C (HCV)
Although HCV is very similar to hepatitis B, many more people infected with it will become chronic carriers and develop liver damage. There is no vaccine against HCV. At present, universal antenatal screening for HCV is not undertaken, but recent research has demonstrated a 0.8% prevalence rate in inner London, and in this study the majority of the infected women had no identified risk factors (Ward et al 2000).
Human immunodeficiency virus (HIV) infection
Department of Health (1999) guidelines state that HIV testing should be recommended to all women as part of routine antenatal testing, as there are now clearly identified strategies which can reduce transmission to the fetus. As with all tests, informed consent is necessary and the midwife must ensure her knowledge base, in this very fast-changing area, is up to date in order that she can offer explanations and answer questions. This is a condition where new research is almost continuously being published and therefore all maternity units should have an identified resource person to whom the more complex enquiries can be referred.
Toxoplasmosis
Toxoplasmosis is a parasitic infection caused by the protozoon Toxoplasma gondii which may cause congenital infection in the fetus. It can be transmitted from domestic cat faeces, soil, raw meat and unpasteurized milk. Pregnant women are also advised to avoid contact with sheep during lambing (DH 2000).
The test, which examines the immunity status of the woman by looking at IgG and IgM antibodies, should be performed in a toxoplasmosis reference laboratory, as diagnosis is not straightforward. NICE (2008) does not recommend routine testing.
Listeriosis
Listeriosis can cause upper respiratory disease, septicaemia and encephalic disease. In pregnancy it can result in preterm labour, stillbirth or meningitis (of mother or baby). It is caused by a common bacterium usually transmitted via contaminated food, and advice is given to pregnant women to specifically avoid soft cheeses and pâtés, and to ensure ‘cook-chill’ meals are well heated through. Pregnant women are advised to avoid contact with sheep during lambing (DH 2000). Diagnosis is made by culture of blood or cerebrospinal fluid.
Cytomegalovirus (CMV)
Cytomegalovirus is a herpesvirus that can be passed on by many routes, including sexual activities. CMV can lie latent in maternal tissues and become reactivated during pregnancy. The presence of CMV antibodies in the blood is indicative of infection and virus-specific IgM antibody is present in acute infections. It is the most common cause of intrauterine infection, and the fetus can be assessed by amniotic fluid studies (Antsaklis et al 2000).
Serology
Serological tests, both non-treponemal and treponemal, can be done in the antenatal period, and most women in the UK are routinely screened for syphilis at booking, as there is evidence that this is still an appropriate test (Cross et al 2005). It is possible to get false positive results with conditions such as malaria, tuberculosis and glandular fever, and those infected with pinta and yaws may test positive. Those who abuse narcotics can also test as a false positive.
Antenatal maternal blood tests to assess the fetus
Maternal serum screening for Down syndrome (MSSDS)
In the late 1980s, workers at St Bartholomew’s Hospital, London, developed a method of screening all women for the risk factor of Down syndrome (chormosone anomaly trisomy 21) in a current pregnancy by means of a maternal blood test (Loncar et al 1995). Since then, the test has been refined, expanded and nuchal translucency (NT) ultrasound added (see Ch. 26). Combined with the mother’s age (it has long been recognized that the incidence of Down syndrome increases with the age of the woman), these calculations result in an individual risk estimation.
Currently there are several variations of the test and local NHS Trust policies will determine the specific tests offered. NICE (2008) recommends the combined test between 11 weeks and 13 weeks 6 days, with the triple or quadruple test if booking later, at 15–20 weeks.
Because accurate dates are important in the assessment of serum levels, a ‘dating scan’ is often offered at booking, with or without the NT, if the woman plans to have serum screening. It is important the woman realizes that the outcome is only a risk assessment and if her result is considered a ‘screen positive’ she will probably need an amniocentesis for a diagnosis. It is obviously also possible that a ‘screen negative’ may well occur despite an affected fetus, and this also needs to be made clear.
An increased level of alpha-fetoprotein (AFP) has been previously used on its own as a screening test for neural tube defects (spina bifida and anencephaly), to be followed by amniocentesis to detect diagnostic levels in the amniotic fluid. Most neural tube defects are now diagnosed by ultrasound examination.
Not all pregnancies are suitable for routine MSSDS screening. Levels can be influenced by a multiple pregnancy, intrauterine bleeding, obesity or the woman being an insulin-dependent diabetic. Different values may also be necessary for IVF pregnancies (Wald et al 1999).
Assessment of fetal wellbeing
Fetal heart rate
In looking at the fetal heart rate as an indication of fetal wellbeing, it is usual practice to assess baseline rate, variability and alteration in heart rate in reaction to stress or movement.
The fetal heart rate varies through the antenatal period, ranging between 110 and 160 beats per minute (bpm), with an average baseline of:
During this time, variations around 20 bpm above and below these baselines are considered within normal limits and signify changes in fetal oxygenation. Tachycardia is more common in the preterm fetus, but may also indicate fetal infection, reaction to maternal medication, maternal pyrexia or tachycardia, acute blood loss, fetal anaemia, or conditions, such as Wolff–Parkinson–White disease. Tachycardia may be seen in fetal hypoxia but not usually without other indications.
Bradycardia (heart rate under 110 bpm) is most likely to be caused by hypoxia, fetal heart block, hypothermia or vagal nerve stimulation.
Fetal movements
Monitoring fetal movements as a test of fetal wellbeing was introduced by Sadovsky in the 1970s (Sadovsky & Polishuk 1977, Sadovsky et al 1983), and this led to extensive use of the Cardiff ‘Count to ten kick chart’. This required the woman to count 10 movements over a 12-hour period, with instructions to contact her GP or midwife should the 10 movements not be achieved. Despite many perceived problems (for example, non-compliance, increased anxiety), fetal movements are deemed to be an effective means of assessing wellbeing and reduced fetal activity is one of the most accurate means of identifying the fetus at risk of intrauterine death (Heazell & Froen 2008, James 2002); however, NICE (2008) suggests routine fetal movement counting should not be offered.
Maternity services have a variety of approaches and some still use ‘kick charts’. Whatever system is in place, it is important to encourage the woman to become familiar with her own baby’s pattern of movement, and for her to be aware of what actions she should take should there be a significant change in the movements.
Ultrasound
Today, ultrasound scanning (USS) (Fig. 33.1) is routine in antenatal care for women, as well as being an integral part of many of the specialized investigations. Ultrasound imaging is a non-invasive (when done with an abdominal transducer) screening and diagnostic technique using sound waves with a frequency well above the range of human hearing. (See website for background and method.) Although at present the usual method of ultrasound scanning is abdominal, vaginal ultrasound, using a special probe, is becoming more common in early pregnancy.
Ultrasound scans can be performed for a variety of reasons, from the earliest gestation up to and including when in labour, as well as postnatally to detect complications in the mother (for example, retained products) or to assess the baby. However, antenatal USS is most common, and it is important to remember that a scan for any reason during this period may result in findings apart from the purpose for which it was being done – for instance, a scan to assess the placental site may result in the discovery of a fetal abnormality. A woman undergoing ultrasound scanning should be aware of a scan’s capabilities, that a raised BMI can make ultrasound difficult and therefore less accurate, and that the finding of no abnormalities on USS is not a guarantee of no problems.
Reflective activity 33.1
With the woman’s and ultrasonographer’s permission, sit in on some ultrasound scans at various gestations in pregnancy, so you can become familiar with the findings of the ultrasonographer and with the questions women may ask.
Indications for first trimester ultrasound
Booking/early/dating scans
Historically, first trimester scans were only routinely offered to women unsure of their last menstrual period, and therefore an estimated delivery date (EDD) was able to be calculated from fetal measurements taken during scanning. However, with the increase in MSSDS and NT, a ‘dating’ scan is often routinely offered to ensure accurate timing of the tests.
Parameters that may be used to determine gestational age are crown–rump length, biparietal diameter, femur length, and head circumference. The gestational sac is sometimes assessed early in the first trimester to confirm an intrauterine pregnancy, to calculate the gestational age before the fetus is visible, or to diagnose an anembryonic pregnancy (no embryonic tissue).
For accuracy, the measurements to assess gestational age should be done in the first or early second trimester, as prediction of gestational age by ultrasound scan cannot be accurately made after 24 weeks, because of the wide spread of normal measurements. Measurements will be recorded, to act as a baseline in case fetal growth needs to be monitored later in pregnancy.
Diagnosis of pregnancy
The embryonic sac may be identified as early as 5 weeks’ gestation using a transabdominal probe, and at 4 weeks with a transvaginal probe. Fetal heart movements can be visualized at 6–7 weeks’ gestation and lack of fetal heart movement is a reliable method of diagnosing fetal death after this time. Actual movements of the fetus can be observed from 8–9 weeks. Doppler ultrasound equipment which produces amplified sound waves (that is, Sonicaid/Doptone) may be used to hear the fetal heart after about 12 weeks’ gestation, but failure to hear the fetal heart should not be assumed to indicate fetal death. Fetal viability should be checked by an ultrasound scan.
Ectopic pregnancy
This may be detected by ultrasound scan, the transvaginal route being more accurate than the abdominal route (Chudleigh & Thilaganathan 2004). Diagnosis is not always easy, but identification of high-risk groups, clinical examination and biochemical tests usually assist the diagnosis.
Miscarriage/missed abortion/vaginal bleeding
If a woman reports no longer ‘feeling pregnant’, or there are no signs of expected growth, an ultrasound scan may show the fetal sac failing to grow and a visible fetal pole but no fetal heartbeat. During research, some hospitals have shown that if a scan is done routinely at 11–14 weeks, a rate of 2–3% missed miscarriages may be identified. An advantage of a routine early scan may be the avoidance of traumatic bleeding and possible emergency admission for these women (Economides et al 1999).
Vaginal bleeding is not uncommon in early pregnancy and often the cause is never determined. Ultrasound is extremely valuable in assessing fetal viability when bleeding occurs, to determine what action, if any, should be taken.
Hydatidiform mole
Ultrasound scan confirms the diagnosis following clinical signs such as painless vaginal bleeding, a large-for-dates uterus, hyperemesis gravidarum, and absence of fetal heart sounds by 14 weeks’ gestation using Doppler ultrasound.
Cervical incompetence
In some cases, serial ultrasound from about 14 weeks can assess the condition of the cervical canal and detect shortening (Zlatnik et al 2000).
Multiple pregnancy
Multiple pregnancy can be identified by ultrasound from 4 weeks transvaginally, and 5 weeks abdominally. Initially it is suspected when more than one fetal sac is seen; the presence of two (or more) viable fetuses confirms the diagnosis.
Many twin pregnancies result in a singleton birth. Since the increased number of first trimester ultrasound scans, the ‘vanishing twin’ syndrome has been described, where twins are seen on an early scan but one is then lost – this is sometimes associated with vaginal bleeding, but often not (see Ch. 59). The figures for this are uncertain and range between 20% and 50% of twin pregnancies.
Nuchal translucency (see Ch. 26)
It is suggested that by examining fetal anatomy as well as measuring nuchal translucency at 12–13 weeks, the majority of structural and chromosomal abnormalities can be detected. However, significant defects can be missed (such as some heart and spine abnormalities); therefore, a later scan would also be recommended (Economides et al 1999).
Indications for second trimester ultrasound
Since the 1980s, women have been offered routine ultrasound assessment between 18 and 20 weeks’ gestation, often called the ‘anomaly’, ‘mid-pregnancy’ or ‘mid-trimester’ scan. By this time, most fetal organs are formed and many abnormalities can be seen. However, it must be stressed that not all structures and their functions can be assessed – some may need later scans and many abnormalities may not be able to be assessed by ultrasound at all. In spite of this, women frequently see this routine scan as a signal that ‘everything is alright’ and a guarantee of a problem-free pregnancy and baby, which can be a very misleading assumption.
Estimation of fetal age
If an earlier scan has not been done, the accuracy of the gestational age by dates can be confirmed by fetal measurements. These measurements are recorded at this time as a baseline to use if there is a suspected IUGR (intrauterine growth restriction) later in pregnancy.
Placental location
During all ultrasound examinations, identification of the placenta is made, but it is routinely done during this scan. If the placental site is low in the mid-trimester scan, depending on its position (NICE 2008), a repeat scan is usually offered in the third trimester, and the woman is advised as to what to do in the case of bleeding prior to this. Only a minority of placentae will fail to become fundal by 32 weeks (Chudleigh & Thilaganathan 2004), but if the placenta remains partially or wholly in the lower uterine segment it is a placenta praevia and appropriate care must be instigated.
Identification of fetal anomalies
Although fetal anomalies can be detected during any ultrasound scan, the mid-trimester scan is used routinely for this examination.
Fetal anatomy is assessed and many conditions, mainly structural, can be diagnosed (although some may need referral to a specialist centre for a definitive diagnosis). In addition, the ultrasonographer can also note any ‘soft markers’ – for example, extra digits, choroid plexus cysts or talipes. These can be benign anomalies which either disappear (for example, most choroid plexus cysts) or can be easily treated after birth. However, they can also be a manifestation of more serious underlying conditions, such as a chromosomal abnormality. An amniocentesis may be offered to exclude this. The use of soft markers is a controversial subject and can be a cause of great anxiety for many (Loughna 2006, Weisz et al 2007).
Indications for third trimester ultrasound
Assessment of fetal growth
Fetal growth is assessed clinically at every antenatal visit. If the midwife or doctor feels growth is suboptimal, a referral for ultrasound assessment is usually made, to confirm the clinical findings.
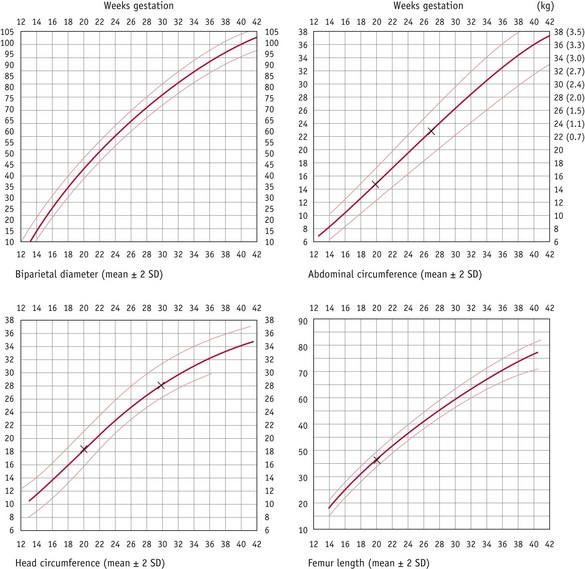
To assess fetal growth by ultrasound, the age of the fetus must be accurately established before 24 weeks’ gestation. Fetal growth may be monitored by serial ultrasound measurements of various parameters, every 2–4 weeks. Measurements of head and abdominal circumference are commonly used to estimate the growth in small-for-gestational-age fetuses, both asymmetrical and symmetrical, and large-for-gestational-age fetuses. In a fetus with symmetrical IUGR, the normal growth shows deviation below the 5th (or 10th) centile. In the asymmetrical condition, the abdominal circumference growth is slow and may stop, and eventually the head circumference growth also slows. IUGR can be diagnosed by plotting serial scans along centile lines previously defined as the normal growth pattern for that population. If IUGR occurs, a fall-off of growth can be seen (Fig. 33.2). Growth acceleration (large abdominal circumference) above the 90th centile may be due to maternal diabetes mellitus, especially if associated with polyhydramnios and a large placenta.
Estimation of fetal weight
Estimation of fetal weight can be made by using measurements obtained during ultrasound assessment. For the preterm fetus, and especially very preterm and multiples, ultrasound estimation of weight is the method of choice. This may provide vital information when consideration is being given to expediting a premature delivery.
However, at term it has been shown that parous women can often estimate the weight of the fetus as accurately as professionals using palpation (Diase & Monga 2002, Herrero & Fitzsimmons 1999). Clinical assessment can also be as accurate as ultrasound (Baum et al 2002) in estimating fetal weight around term – however, research studying this has specified using experienced professionals to do the assessments. It may be that a generation of practitioners, who are becoming increasingly dependent upon ultrasound in their practice, may not be able to replicate this research in the future. Since there will always be situations where ultrasound cannot be accessed, it is a reminder for all midwives to maintain their clinical skills of palpation and weight estimation.
Malpresentations/malpositions
Late in the third trimester an ultrasound scan can be used to confirm clinical findings regarding the presentation and position of the fetus (or each fetus in the case of a multiple pregnancy). This information can be used to help decision making if there is a question over the mode of delivery.
Ultrasound will also be used to guide the clinician if external cephalic version (ECV) is undertaken to turn a breech presentation.
Additional fetal assessment
Doppler ultrasound
As well as being used to monitor the fetal heart (for example, Sonicaid), this technique is also used to measure blood flow in the fetal and uterine/placental vessels from a waveform recording on a monitor screen. The blood flow pattern will change as an adaptation to poor placental function, so it is thought that alterations in the fetal umbilical blood flow may occur in early fetal compromise. Women will be referred for Doppler assessment in the second and third trimester because of oligohydramnios, differing growth in multiple pregnancies, IUGR (or a history of IUGR in a previous pregnancy) or maternal conditions (such as hypertensive disorders of pregnancy). It may also form part of post-dates assessment (NICE 2008).
Amniotic fluid measurement
As a routine clinical assessment during palpation in the second and third trimester, amniotic fluid quantity may be estimated to be reduced (oligohydramnios) or increased (polyhydramnios). Both these conditions, if suspected, need to be referred for ultrasound evaluation. Oligohydramnios may be associated with various fetal abnormalities, or with fetal compromise. Polyhydramnios may also be associated with fetal abnormality (for example, oesophageal atresia), or associated with maternal disease (such as diabetes mellitus) and a large fetus. All these conditions will need expert assessment, especially in determining timing and mode of delivery and planning aftercare. Amniotic fluid volume will also be observed as part of the assessment of fetal wellbeing for a woman with a medical condition (for example, pre-eclampsia) or as part of a post-term assessment.
See website for additional uses for ultrasound and for information on radiological and magnetic visualization techniques.
Invasive tests
Chorionic villus sampling (CVS)
Chorionic villus sampling can be undertaken at any gestation, but is used primarily as a first trimester test. Under continuous ultrasound visualization of the placenta, chorionic villi are obtained, usually by syringe, and these can be analysed for fetal chromosomal abnormalities. A provisional result is usually available within a few days. Depending on the position of the placenta, the procedure can be done either transabdominally or via the cervix.
The advantage of an early diagnostic test for chromosomal abnormality is that the woman would probably have the option of a first trimester termination, if that was her decision. Disadvantages include a rate of pregnancy loss usually quoted at about 2% although it has been suggested that rates of loss are reducing substantially (Evans & Andriole 2008). Difficulty in analysing the miscarriage rate is, however, complicated by the higher rate of spontaneous miscarriage in the first trimester. There is also a risk of results failure and studies have indicated a link between CVS and limb abnormality, probably restricted to procedures undertaken before 10 weeks’ gestation (RCOG 2005).
Following the procedure, rhesus-negative women will be given anti-D immunoglobulin to prevent possible isoimmunization.
Amniocentesis
Amniotic fluid can be used to test for fetal conditions such as chromosomal abnormalities, genetic diseases, or some fetal infections.
In the UK, amniocentesis is usually performed at about 15–18 weeks’ gestation (see website for information on ‘early’ amniocentesis) using ultrasound to visualize the uterus and its contents. A fine needle is passed through the abdominal wall into the uterus and about 20 mL of amniotic fluid is extracted. The fetal cells in the amniotic fluid must be cultured and the time taken for their growth (about 2–3 weeks) accounts for the wait for a diagnosis, which women find so difficult. There may be a possibility in the future of routinely using DNA analysis for all tests done on amniotic fluid, which would give a much quicker result.
Some amniocentesis will fail to give a result when the fetal cells do not grow, and the woman must be aware of this small risk (about 1 : 500), as well as the other disadvantages, before she can make an informed choice to have an amniocentesis. After the procedure, the fetal heart is auscultated or visualized on ultrasound and the woman should be allowed to hear/see this. She will usually be advised to rest for that day and avoid strenuous activity for a few days. Rhesus-negative women will receive anti-D immunoglobulin to prevent possible rhesus isoimmunization.
The risk of pregnancy loss following amniocentesis is about 0.5–1% but this can vary according to operator and centre. There is also a risk of infection following any invasive procedure.
Some tests on amniotic fluid, such as diagnosis of neural tube defects (which is now done by ultrasound) or assessing the lecithin:sphingomyelin ratio for fetal pulmonary maturity, are now no longer considered a reason for an invasive test.
Cordocentesis
This is an invasive investigation performed under ultrasound imaging, whereby a sample of fetal blood is obtained from the umbilical cord or intrahepatic vein, usually in the second or third trimester of pregnancy. The site of sampling is selected on considerations of accessibility, quality of visualization, gestational age and safety. The investigation was developed from a number of earlier interventions, including fetoscopy, for the purpose of antenatal diagnosis.
Cordocentesis carries a risk of miscarriage and also a risk of maternal infection and haemorrhage.
Conclusion
The suggestion of even a minor defect in the fetus can cause extreme anxiety for parents, especially as, even if all further tests show no abnormality, no professional can guarantee a ‘perfect’ baby.
There is some evidence that the anxiety engendered on identification of a potential problem does not go away even after a reassuring diagnosis (Lawrence 1999), and also that maternal anxiety during pregnancy may affect the physiological development of the fetus (Teixeira et al 1999).
However, the concept of prenatal screening is popular with most women and the ability to identify many abnormal fetuses leads to women having a choice of terminating the pregnancy. There are also many healthy children – and their mothers – alive today because of the provision of the tests described in this chapter.
The midwife’s role is to ensure that the woman receives accurate, evidence-based and up-to-date information in language she can understand, in order to make an informed decision. Where possible, the midwife should provide written information to support any discussions, and should also be aware of other sources of information which may be helpful, such as through the Internet and through voluntary groups. Whatever range of tests the woman and her family choose to access, for whatever reasons, the midwife should continue to provide support and respect throughout her care.