CHAPTER 31 Vitamin and Mineral Deficiencies
CHAPTER 31 Vitamin and Mineral Deficiencies
Micronutrients comprise 13 vitamins and 17 essential minerals. In industrialized societies, frank clinical deficiencies are unusual in healthy children, but they can and do occur in certain high-risk circumstances. Risk factors include diets that are consistently limited in variety, especially with the exclusion of entire food groups, malabsorption syndromes, and conditions causing high physiologic requirements. Various common etiologies of vitamin and nutrient deficiency states are highlighted in Table 31-1, and characteristics of vitamin deficiencies are outlined in Table 31-2.
TABLE 31-1 Etiology of Vitamin and Nutrient Deficiency States
| Etiology | Deficiency |
|---|---|
| DIET | |
| Vegans (strict) | Protein, vitamins B12, D, riboflavin |
| Breastfed infant | Vitamins K, D |
| Cow’s milk–fed infant | Iron |
| Bulimia, anorexia nervosa | Electrolytes, other deficiencies |
| Parenteral alimentation | Essential fatty acids, trace elements |
| Alcoholism | Calories, vitamin B1, B6, folate |
| MEDICAL PROBLEMS | |
| Malabsorption syndromes | Vitamins A, D, E, K, zinc, essential fatty acids |
| Cholestasis | Vitamins E, D, K, A, zinc, essential fatty acids |
| MEDICATIONS | |
| Sulfonamides | Folate |
| Phenytoin, phenobarbital | Vitamins D, K, folate |
| Mineral oil | Vitamins A, D, E, K |
| Antibiotics | Vitamin K |
| Isoniazid | Vitamin B6 |
| Antacids | Iron, phosphate, calcium |
| Digitalis | Magnesium, calcium |
| Penicillamine | Vitamin B6 |
| SPECIFIC MECHANISMS | |
| Transcobalamin II or intrinsic factor deficiency | Vitamin B12 |
| Other digestive enzyme | Carbohydrate, fat, protein deficiencies |
| Menkes kinky hair syndrome | Copper |
| Acrodermatitis enteropathica | Zinc |
| Reduced exposure to direct sunlight | Vitamin D |
WATER-SOLUBLE VITAMINS
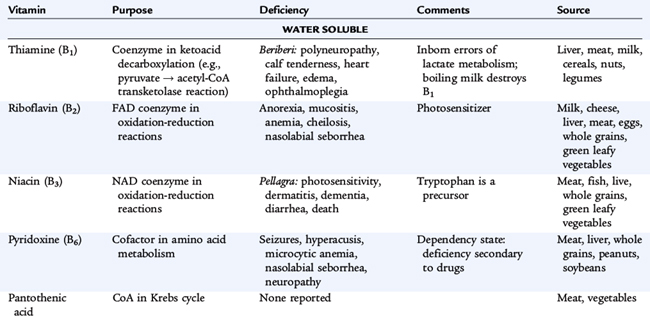
Water-soluble vitamins are not stored in the body except for vitamin B12; intake alters tissue levels. Absorption from the diet is usually high, and the compounds exchange readily between intracellular and extracellular fluids; excretion is via the urine. Water-soluble vitamins typically function as coenzymes in energy, protein, amino acid, and nucleic acid metabolism; as cosubstrates in enzymatic reactions; and as structural components.
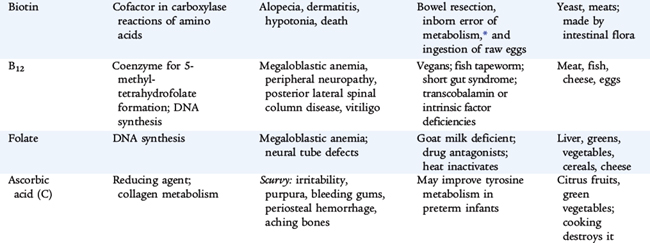
Ascorbic Acid
The principal forms of vitamin C are ascorbic acid and the oxidized form, dehydroascorbic acid. Ascorbic acid accelerates hydroxylation reactions in many biosynthetic reactions, including hydroxylation of proline in the formation of collagen. The needs of full-term infants for ascorbic acid and dehydroascorbic acid are calculated by estimating the availability in human milk.
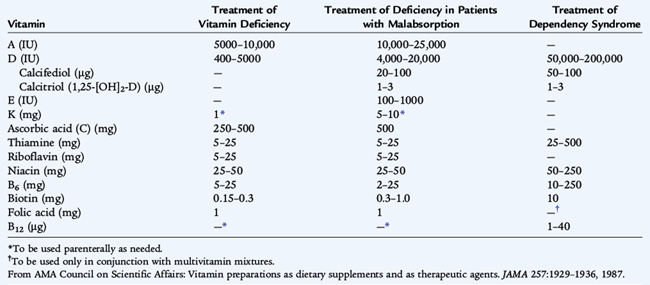
A deficiency of ascorbic acid results in the clinical manifestations of scurvy. Infantile scurvy is manifested by irritability, bone tenderness with swelling, and pseudoparalysis of the legs. The disease may occur if infants are fed unsupplemented cow’s milk in the first year of life or if the diet is devoid of fruits and vegetables. Subperiosteal hemorrhage, bleeding gums and petechiae, hyperkeratosis of hair follicles, and a succession of mental changes characterize the progression of the illness. Anemia secondary to bleeding, decreased iron absorption, or abnormal folate metabolism is also seen in chronic scurvy. Treatment of several vitamin related diseases is presented in Table 31-3.
B Vitamins
The B vitamins thiamine, riboflavin, and niacin are routinely added to enriched grain products; deficiencies in normal hosts are rare in the United States. Levels from human milk reflect maternal intake, and deficiency can develop in breastfed infants of deficient mothers.
Thiamine
Vitamin B1 functions as a coenzyme in biochemical reactions related to carbohydrate metabolism, decarboxylation of α-ketoacids and pyruvate, and transketolase reactions of the pentose pathway. Thiamine also is involved in the decarboxylation of branched-chain amino acids. Thiamine is lost during milk pasteurization and sterilization. Thiamine deficiency occurs in alcoholics and has been reported in adolescents who have undergone bariatric surgery for severe obesity. Infantile beriberi occurs between 1 and 4 months of age in breastfed infants whose mothers have a thiamine deficiency (alcoholism), in infants with protein-calorie malnutrition, in infants receiving unsupplemented hyperalimentation fluid, and in infants receiving boiled milk. Acute wet beriberi with cardiac symptoms and signs predominates in infantile beriberi. Anorexia, apathy, vomiting, restlessness, and pallor progress to dyspnea, cyanosis, and death from heart failure. Infants with beriberi have a characteristic aphonic cry; they appear to be crying, but no sound is uttered. Other signs include peripheral neuropathy and paresthesias.
Riboflavin
Vitamin B2 is a constituent of two coenzymes, riboflavin 5′-phosphate and flavin-adenine dinucleotide, essential components of glutathione reductase and xanthine oxidase, which are involved in electron transport. A deficiency of riboflavin affects glucose, fatty acid, and amino acid metabolism. Riboflavin and its phosphate are decomposed by exposure to light and by strong alkaline solutions.
Ariboflavinosis is characterized by an angular stomatitis; glossitis; cheilosis; seborrheic dermatitis around the nose and mouth; and eye changes that include reduced tearing, photophobia, corneal vascularization, and the formation of cataracts. Subclinical riboflavin deficiencies have been found in diabetic subjects, children in families with low socioeconomic status, children with chronic cardiac disease, and infants undergoing prolonged phototherapy for hyperbilirubinemia.
Niacin
Niacin consists of the compounds nicotinic acid and nicotinamide (niacinamide). Nicotinamide, the predominant form of the vitamin, functions as a component of the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). Niacin is involved in multiple metabolic processes, including fat synthesis, intracellular respiratory metabolism, and glycolysis.
In determining the needs for niacin, the content of tryptophan in the diet must be considered because tryptophan is converted to niacin. Niacin is stable in foods and withstands heating and prolonged storage. Approximately 70% of the total niacin equivalents in human milk are derived from tryptophan. Pellagra, or niacin deficiency disease, is characterized by weakness, lassitude, dermatitis, photosensitivity, inflammation of mucous membranes, diarrhea, vomiting, dysphagia, and, in severe cases, dementia.
Vitamin B6
Vitamin B6 refers to three naturally occurring pyridines: pyridoxine (pyridoxol), pyridoxal, and pyridoxamine. The phosphates of the latter two pyridines are metabolically and functionally related and are converted in the liver to the coenzyme form, pyridoxal phosphate. The metabolic functions of vitamin B6 include interconversion reactions of amino acids, conversion of tryptophan to niacin and serotonin, metabolic reactions in the brain, carbohydrate metabolism, immune development, and the biosynthesis of heme and prostaglandins. The pyridoxal and pyridoxamine forms of the vitamin are destroyed by heat; heat treatment was responsible for vitamin B6 deficiency and seizures in infants fed improperly processed formulas. Goat’s milk is deficient in vitamin B6.
Dietary deprivation or malabsorption of vitamin B6 in children results in hypochromic microcytic anemia, vomiting, diarrhea, failure to thrive, listlessness, hyperirritability, and seizures. Children receiving isoniazid or penicillamine may require additional vitamin B6 because the drug binds to the vitamin. Vitamin B6 is unusual as a water-soluble vitamin in that very large doses (≥500 mg/day) have been associated with a sensory neuropathy. Megadoses have been claimed to promote improved neurocognitive development in children with Down syndrome, but data from controlled trials are lacking, and the practice is not currently justified.
Folate
A variety of chemical forms of folate are nutritionally active. Folate functions in transport of single-carbon fragments in synthesis of nucleic acids and for normal metabolism of certain amino acids and in conversion of homocysteine to methionine. Food sources include green leafy vegetables, oranges, and whole grains; folate fortification of grains is now routine in the United States.
Folate deficiency, characterized by hypersegmented neutrophils, macrocytic anemia, and glossitis, may result from a low dietary intake, malabsorption, or vitamin-drug interactions. Deficiency can develop within a few weeks of birth because infants require 10 times as much folate as adults relative to body weight but have scant stores of folate in the newborn period. Folate is particularly heat labile. Heat-sterilizing home-prepared formula can decrease the folate content by half. Evaporated milk and goat’s milk are low in folate. Patients with chronic hemolysis (sickle cell anemia, thalassemia) may require extra folate to avoid deficiency because of the relatively high requirement of the vitamin to support erythropoiesis. Other conditions with risk of deficiency include pregnancy, alcoholism, and treatment with anticonvulsants (phenytoin) or antimetabolites (methotrexate). First occurrence and recurrence of neural tube defects are reduced significantly by maternal supplementation during embryogenesis. Because closure of the neural tube occurs before usual recognition of pregnancy, all women of reproductive age are recommended to have a folate intake of at least 400 μg/day as prophylaxis.
Vitamin B12
Vitamin B12 is one of the most complex of the vitamin molecules, containing an atom of cobalt held in a corrin ring (similar to that of iron in hemoglobin). The cobalt ion is at the active center of the ring and serves as the site for attachment of alkyl groups during their transfer. The vitamin functions in single-carbon transfers and is intimately related to folate function and interconversions. Vitamin B12 is essential for normal lipid and carbohydrate metabolism in energy production and in protein biosynthesis and nucleic acid synthesis.
In contrast to other water-soluble vitamins, absorption of vitamin B12 is complex, involving cleavage of the vitamin from dietary protein and binding to a glycoprotein called intrinsic factor, which is secreted by the gastric mucosa (parietal cells). The cobalamin–intrinsic factor complex is efficiently absorbed from the distal ileum. As vitamin B12 is absorbed into the portal circulation, it is transported bound to a specific protein, transcobalamin II. Its large stores in the liver also are unusual as a water-soluble vitamin. Efficient enterohepatic circulation normally protects from deficiency for months to years. Dietary sources of the vitamin are animal products only. Strict vegetarians should take a vitamin B12 supplement.
Vitamin B12 deficiency is rare. Early diagnosis and treatment of this disorder in childhood are important because of the danger of irreversible neurologic damage. Most cases in childhood result from a specific defect in absorption (see Table 31-2). Such defects include congenital pernicious anemia (absent intrinsic factor), juvenile pernicious anemia (autoimmune), and deficiency of transcobalamin II transport. Gastric or intestinal resection and small bowel bacterial overgrowth also cause vitamin B12 deficiency. Exclusively breastfed infants ingest adequate vitamin B12 unless the mother is a strict vegetarian without supplementation.
Depression of serum vitamin B12 and the appearance of hypersegmented neutrophils and macrocytosis (indistinguishable from folate deficiency) are early clinical manifestations of deficiency. Vitamin B12 deficiency also causes neurologic manifestations, including depression, peripheral neuropathy, posterior spinal column signs, dementia, and eventual coma. The neurologic signs do not occur in folate deficiency, but administration of folate may mask the hematologic signs of vitamin B12 deficiency, while the neurologic manifestations progress. Patients with vitamin B12 deficiency also have increased urine levels of methylmalonic acid. Most cases of vitamin B12 deficiency in infants and children are not of dietary origin and require treatment throughout life. Maintenance therapy consists of repeated monthly intramuscular injections, although a form of vitamin B12 is administered intranasally.
FAT-SOLUBLE VITAMINS
Fat-soluble vitamins generally have stores in the body, and dietary deficiencies generally develop more slowly than for water-soluble vitamins. Absorption of fat-soluble vitamins depends on normal fat intake, digestion, and absorption. The complexity of normal fat absorption and the potential for perturbation in many disease states explains the more common occurrence of deficiencies of these vitamins.
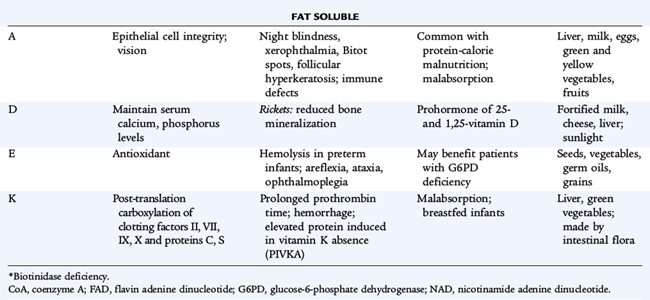
Vitamin A
The basic constituent of the vitamin A group is retinol. Ingested plant carotene or animal tissue retinol esters release retinol after hydrolysis by pancreatic and intestinal enzymes. Chylomicron-transported retinol esters are stored in the liver as retinol palmitate. Retinol is transported from the liver to target tissues by retinol-binding protein, releasing free retinol to the target tissues. The kidney then excretes the retinol-binding protein. Diseases of the kidney diminish excretion of retinol-binding protein, whereas liver parenchymal disease or malnutrition lowers the synthesis of retinol-binding protein. Specific cellular binding proteins facilitate the uptake of retinol by target tissues. In the eye, retinol is metabolized to form rhodopsin; the action of light on rhodopsin is the first step of the visual process. Retinol also influences the growth and differentiation of epithelia. The clinical manifestations of vitamin A deficiency in humans appear as a group of ocular signs termed xerophthalmia. The earliest symptom is night blindness, which is followed by xerosis of the conjunctiva and cornea. Untreated, xerophthalmia can result in ulceration, necrosis, keratomalacia, and a permanent corneal scar. Clinical and subclinical vitamin A deficiencies are associated with immunodeficiency; increased risk of infection, especially measles; and increased risk of mortality, especially in developing nations. Xerophthalmia and vitamin A deficiency should be urgently treated. Treatment is summarized in Table 31-3. Hypervitaminosis A also has serious sequelae, including headaches, pseudotumor cerebri, hepatotoxicity, and teratogenicity.
Vitamin E
Eight naturally occurring compounds have vitamin E activity. The most active of these, α-tocopherol, accounts for 90% of the vitamin E present in human tissues and is commercially available as an acetate or succinate. Vitamin E acts as a biologic antioxidant by inhibiting the peroxidation of polyunsaturated fatty acids present in cell membranes. It scavenges free radicals generated by the reduction of molecular oxygen and by the action of oxidative enzymes.
Vitamin E deficiency occurs in children with fat malabsorption secondary to liver disease, untreated celiac disease, cystic fibrosis, and abetalipoproteinemia. In these children, without vitamin E supplementation, a syndrome of progressive sensory and motor neuropathy develops; the first sign of deficiency is loss of deep tendon reflexes. Deficient preterm infants at 1 to 2 months of age have hemolytic anemia characterized by an elevated reticulocyte count, an increased sensitivity of the erythrocytes to hemolysis in hydrogen peroxide, peripheral edema, and thrombocytosis. All the abnormalities are corrected after oral, lipid, or water-soluble vitamin E therapy.
Vitamin D
Cholecalciferol (vitamin D3) is the mammalian form of vitamin D and is produced by ultraviolet irradiation of inactive precursors in the skin. Ergocalciferol (vitamin D2) is derived from plants. Vitamin D2 and vitamin D3 require further metabolism to become active. They are of equivalent potency. Clothing, lack of sunlight exposure, and skin pigmentation decrease generation of vitamin D in the epidermis and dermis.
Vitamin D (D2 and D3) is metabolized in the liver to calcidiol, or 25-hydroxyvitamin D (25-[OH]-D); this metabolite, which has little intrinsic activity, is transported by a plasma-binding globulin to the kidney, where it is converted to the most active metabolite calcitriol, or 1,25-dihydroxyvitamin D (1,25-[OH]2-D). The action of 1,25-(OH)2-D results in a decrease in the concentration of messenger RNA (mRNA) for collagen in bone and an increase in the concentration of mRNA for vitamin D–dependent calcium-binding protein in the intestine (directly mediating increased intestinal calcium transport). The antirachitic action of vitamin D probably is mediated by provision of appropriate concentrations of calcium and phosphate in the extracellular space of bone and by enhanced intestinal absorption of these minerals. Vitamin D also may have a direct anabolic effect on bone. 1,25-(OH)2-D has direct feedback to the parathyroid gland and inhibits secretion of parathyroid hormone.
Vitamin D deficiency appears as rickets in children and as osteomalacia in postpubertal adolescents. Inadequate direct sun exposure and vitamin D intake are sufficient causes, but other factors, such as various drugs (phenobarbital, phenytoin) and malabsorption, may increase the risk of development of vitamin-deficiency rickets. Breastfed infants, especially those with dark-pigmented skin, are at risk for vitamin D deficiency.
The pathophysiology of rickets results from defective bone growth, especially at the epiphyseal cartilage matrix, which fails to mineralize. The uncalcified osteoid results in a wide, irregular zone of poorly supported tissue, the rachitic metaphysis. This soft, rather than hardened, zone produces many of the skeletal deformities through compression and lateral bulging or flaring of the ends of bones.
The clinical manifestations of rickets are most common during the first 2 years of life and may become evident only after several months of a vitamin D–deficient diet. Craniotabes is caused by thinning of the outer table of the skull, which when compressed feels like a ping pong ball to the touch. Enlargement of the costochondral junction (rachitic rosary) and thickening of the wrists and ankles may be palpated. The anterior fontanelle is enlarged and its closure may be delayed. In advanced rickets, scoliosis and exaggerated lordosis may be present. Bowlegs or knock-knees may be evident in older infants, and greenstick fractures may be observed in long bones.
The diagnosis of rickets is based on a history of poor vitamin D intake and little exposure to direct ultraviolet sunlight. The serum calcium usually is normal but may be low; the serum phosphorus level usually is reduced, and serum alkaline phosphatase activity is elevated. When serum calcium levels decline to less than 7.5 mg/dL, tetany may occur. Levels of 24,25-(OH)2-D are undetectable, and serum 1,25-(OH)2-D levels are commonly less than 7 ng/mL, although 1,25-(OH)2-D levels also may be normal. The best measure of vitamin D status is the level of 25-(OH)-D. Characteristic radiographic changes of the distal ulna and radius include widening; concave cupping; and frayed, poorly demarcated ends. The increased space seen between the distal ends of the radius and ulna and the metacarpal bones is the enlarged, nonossified metaphysis.
The treatment of vitamin D–deficiency rickets and osteomalacia is presented in Table 31-3. Breastfed infants born of mothers with adequate vitamin D stores usually maintain adequate serum vitamin D levels for at least 2 months, but rickets may develop subsequently if these infants are not exposed to the sun or do not receive supplementary vitamin D. The American Academy of Pediatrics recommends vitamin D supplementation of all breastfed infants in the amount of 400 IU/day, started soon after birth and given until the infant is taking more than 500 mL/day of formula or vitamin D–fortified milk (for age >1 year). Toxic effects of excessive vitamin D include hypercalcemia, muscle weakness, polyuria, and nephrocalcinosis.
Vitamin K
The plant form of vitamin K is phylloquinone, or vitamin K1. Another form is menaquinone, or vitamin K2, one of a series of compounds with unsaturated side chains synthesized by intestinal bacteria. Plasma factors II (prothrombin), VII, IX, and X in the cascade of blood coagulation factors depend on vitamin K for synthesis and for post-translational conversion of their precursor proteins. The post-translational conversion of glutamyl residues to carboxyglutamic acid residues of a prothrombin molecule creates effective calcium-binding sites, making the protein active.
Other vitamin K–dependent proteins include proteins C, S, and Z in plasma and γ-carboxyglutamic acid–containing proteins in several tissues. Bone contains a major vitamin K–dependent protein, osteocalcin, and lesser amounts of other glutamic acid–containing proteins.
Phylloquinone is absorbed from the intestine and transported by chylomicrons. The rarity of dietary vitamin K deficiency in humans with normal intestinal function suggests that the absorption of menaquinones is possible. Vitamin K deficiency has been observed in subjects with impaired fat absorption caused by obstructive jaundice, pancreatic insufficiency, and celiac disease; often these problems are combined with the use of antibiotics that change intestinal flora.
Hemorrhagic disease of the newborn, a disease more common among breastfed infants, occurs in the first few weeks of life. It is rare in infants who receive prophylactic intramuscular vitamin K on the first day of life. Hemorrhagic disease of the newborn usually is marked by generalized ecchymoses, gastrointestinal hemorrhage, or bleeding from a circumcision or umbilical stump; intracranial hemorrhage can occur, but is uncommon. The American Academy of Pediatrics recommends that parenteral vitamin K (0.5 to 1 mg) be given to all newborns shortly after birth.
MINERALS
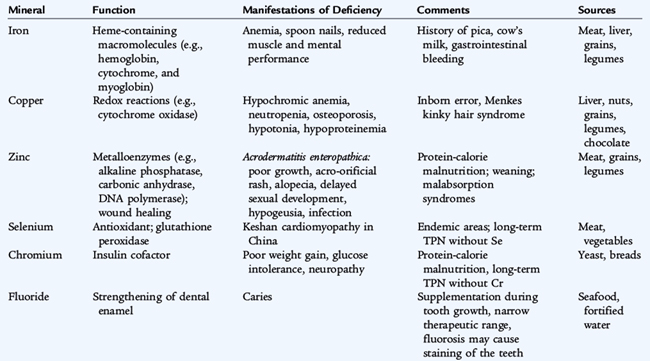
The major minerals are those that require intakes of more than 100 mg/day and contribute at least 0.1% of total body weight. There are seven essential major minerals: calcium, phosphorus, magnesium, sodium, potassium, chloride, and sulfur. Ten trace minerals, which constitute less than 0.1% of body weight, have essential physiologic roles. Characteristics of trace mineral deficiencies are listed in Table 31-4.
Calcium
Calcium is the most abundant major mineral. Ninety-nine percent of calcium is in the skeleton; the remaining 1% is in extracellular fluids, intracellular compartments, and cell membranes. The nonskeletal calcium has a role in nerve conduction, muscle contraction, blood clotting, and membrane permeability. There are two distinct bone calcium phosphate pools—a large, crystalline form and a smaller, amorphous phase. Bone calcium constantly turns over, with concurrent bone resorption and formation. Approximately half of bone mineral accretion occurs during adolescence. Bone mineral density peaks in early adulthood and is influenced by prior and concurrent dietary calcium intake, exercise, and hormone status (testosterone, estrogen).
Calcium intake can come from a variety of sources, with dairy products providing the most common and concentrated source. The calcium equivalent of 1 cup of milk (about 300 mg of calcium) is ¾ cup of plain yogurt, 1.5 oz of cheddar cheese, 2 cups of ice cream, ⅘ cup of almonds, or 2.5 oz of sardines. Other sources of calcium include some leafy green vegetables (broccoli, kale, collards); lime-processed tortillas; calcium-precipitated tofu; and calcium-fortified juices, cereals, and breads.
There is no classic calcium deficiency syndrome because blood and cell levels are closely regulated. The body can mobilize skeletal calcium and increase the absorptive efficiency of dietary calcium. Osteoporosis that occurs in childhood is related to protein-calorie malnutrition, vitamin C deficiency, steroid therapy, endocrine disorders, immobilization and disuse, osteogenesis imperfecta, or calcium deficiency (in premature infants). It is believed that the primary method of prevention of postmenopausal osteoporosis is to ensure maximum peak bone mass by providing optimal calcium intake during childhood and adolescence. Bone mineral status can be monitored by dual-energy x-ray absorptiometry.
No adverse effects are observed in adults with dietary calcium intakes of 2.5 g/day. There is concern that higher intakes may increase the risk of urinary stone formation, constipation, and decreased renal function and may inhibit intestinal absorption of other minerals (iron, zinc).
Iron
Iron, the most abundant trace mineral, is used in the synthesis of hemoglobin, myoglobin, and enzyme iron. Body iron content is regulated primarily through modulation of iron absorption, which depends on the state of body iron stores, the form and amount of iron in foods, and the mixture of foods in the diet. There are two categories of iron in food. The first is heme iron, present in hemoglobin and myoglobin, which is supplied by meat and rarely accounts for more than one fourth of the iron ingested by infants. The absorption of heme iron is relatively efficient and is not influenced by other constituents of the diet. The second category is nonheme iron, which represents the preponderance of iron intake consumed by infants and exists in the form of iron salts. The absorption of nonheme iron is influenced by the composition of consumed foods. Enhancers of nonheme iron absorption are ascorbic acid, meat, fish, and poultry. Inhibitors are bran, polyphenols (including the tannates in tea), and phytic acid, a compound found in legumes and whole grains. The percent intestinal absorption of the small amount of iron in human milk is 10%; 4% is absorbed from iron-fortified cow’s milk formula and from iron-fortified infant dry cereals.
In a normal term infant, there is little change in total body iron and little need for exogenous iron before 4 months of age. Iron deficiency is rare in term infants during the first 4 months, unless there has been substantial blood loss (see Chapter 62). After about 4 months of age, iron reserves become marginal, and, unless exogenous sources of iron are provided, the infant becomes progressively at risk for anemia as the iron requirement to support erythropoiesis and growth increases (see Chapter 150). Premature or low birth weight infants have a lower amount of stored iron because significant amounts of iron are transferred from the mother in the third trimester. In addition, their postnatal iron needs are greater because of rapid rates of growth and when frequent phlebotomy occurs. Iron needs can be met by supplementation (ferrous sulfate) or by iron-containing complementary foods. Under normal circumstances, iron-fortified formula should be the only alternative to breast milk in infants younger than 1 year of age. Premature infants fed human milk may develop iron deficiency anemia earlier unless they receive iron supplements. Formula-fed preterm infants should receive iron-fortified formula.
In older children, iron deficiency may result from inadequate iron intake with excessive cow’s milk intake or from intake of foods with poor iron bioavailability. Iron deficiency also can result from blood loss from such sources as menses or gastric ulceration. Iron deficiency affects many tissues (muscle and central nervous system) in addition to producing anemia. Iron deficiency and anemia have been associated with lethargy and decreased work capacity and impaired neurocognitive development, the deficits of which may be irreversible when onset is in the first 2 years of life.
The diagnosis of iron deficiency anemia is established by the presence of a microcytic hypochromic anemia, low serum ferritin levels, low serum iron levels, reduced transferrin saturation, normal to elevated red blood cell width distribution, and enhanced iron-binding capacity. The mean corpuscular volume and red blood cell indices are reduced, and the reticulocyte count is low. Iron deficiency may be present without anemia. Clinical manifestations are noted in Table 31-4.
Treatment of iron deficiency anemia includes changes in the diet to provide adequate iron and the administration of 2 to 6 mg iron/kg/24 hr (as ferrous sulfate) divided BID or TID. Reticulocytosis is noted within 3 to 7 days of starting treatment. Oral treatment should be continued for 5 months. Rarely, intramuscular or intravenous iron therapy is needed if oral iron cannot be given. Parenteral therapy carries the risk of anaphylaxis and should be administered according to a strict protocol, including a test dose.
Zinc
Zinc is the second most abundant trace mineral and is important in protein metabolism and synthesis, in nucleic acid metabolism, and in stabilization of cell membranes. Zinc functions as a cofactor for more than 200 enzymes and is essential to numerous cellular metabolic functions. Adequate zinc status is especially crucial during periods of growth and for tissue proliferation (immune system, wound healing, skin and gastrointestinal tract integrity); physiologic functions for which zinc is essential include normal growth, sexual maturation, and immune function.
Dietary zinc is absorbed (20% to 40%) in the duodenum and proximal small intestine. The best dietary sources of zinc are animal products, including human milk, from which it is readily absorbed. Whole grains and legumes also contain moderate amounts of zinc, but phytic acid inhibits absorption from these sources. On a global basis, poor bioavailability secondary to phytic acid is thought to be a more important factor than low intake in the widespread occurrence of zinc deficiency. Excretion of zinc occurs from the gastrointestinal tract. In the presence of ongoing losses, such as in chronic diarrhea, requirements can drastically increase.
Zinc deficiency dwarfism syndrome was first described in a group of children in the Middle East with low levels of zinc in their hair, poor appetite, diminished taste acuity, hypogonadism, and short stature. Zinc supplementation reduces morbidity and mortality from diarrhea and pneumonia and enhances growth in developing countries. Mild to moderate zinc deficiency is considered to be highly prevalent in developing countries, particularly in populations with high rates of stunting. Mild zinc deficiency occurs in older breastfed infants without adequate zinc intake from complementary foods or in young children with poor total or bioavailable zinc intake in the general diet. A high infectious burden also may increase the risk of zinc deficiency in developing countries. Acute, acquired severe zinc deficiency occurs in patients receiving total parenteral nutrition without zinc supplementation and in premature infants fed human milk without fortification. Clinical manifestations of mild zinc deficiency include anorexia, growth faltering, and immune impairment. Moderately severe manifestations include delayed sexual maturation, rough skin, and hepatosplenomegaly. The signs of severe deficiency include acral and periorificial erythematous, scaling dermatitis; growth and immune impairment; diarrhea; mood changes; alopecia; night blindness; and photophobia.
Diagnosis of zinc deficiency is challenging. Plasma zinc concentration is most commonly used, but levels are frequently normal in conditions of mild deficiency; levels in moderate to severe deficiency are typically less than 60 μg/dL. Acute infection also can result in depression of circulating zinc levels. The standard for the diagnosis of deficiency is response to a trial of supplementation, with outcomes such as improved linear growth or weight gain, improved appetite, and improved immune function. Because there is no pharmacologic effect of zinc on these functions, a positive response to supplementation is considered evidence of a preexisting deficiency. Clinically an empirical trial of zinc supplementation (1 mg/kg/day) is a safe and reasonable approach in situations in which deficiency is considered possible.
Acrodermatitis enteropathica is an autosomal recessive disorder that begins within 2 to 4 weeks after infants have been weaned from breast milk. It is characterized by an acute perioral and perianal dermatitis, alopecia, and failure to thrive. The disease is caused by severe zinc deficiency from a specific defect of intestinal zinc absorption. Plasma zinc levels are markedly reduced, and serum alkaline phosphatase activity is low. Treatment is with high-dose oral zinc supplements. A relatively uncommon condition associated with presentation of severe zinc deficiency is due to a defect in the secretion of zinc from the mammary gland, resulting in abnormally low milk zinc concentrations. Breastfed infants, especially those born prematurely, present with classic signs of zinc deficiency: growth failure, diarrhea, and dermatitis. Because there is no defect in the infant’s ability to absorb zinc, treatment consists of supplementing the infant with zinc for the duration of breastfeeding, which can be successfully continued. Subsequent infants born to the mother will also need zinc supplementation if breastfed. Zinc is relatively nontoxic. Excess intake produces nausea, emesis, abdominal pain, headache, vertigo, and seizures.
Fluoride
Dental enamel is strengthened when fluoride is substituted for hydroxyl ions in the hydroxyapatite crystalline mineral matrix of the enamel. The resulting fluoroapatite is more resistant to chemical and physical damage. Fluoride is incorporated into the enamel during the mineralization stages of tooth formation and by surface interaction after the tooth has erupted. Fluoride is similarly incorporated into bone mineral and may protect against osteoporosis later in life.
Because of concern about the risk of fluorosis, infants should not receive fluoride supplements before 6 months of age. Commercial formulas are made with defluoridated water and contain small amounts of fluoride. An infant older than 6 months of age who receives only ready-feed formula or is exclusively breastfed may benefit from supplemental fluoride. The fluoride content of human milk is low and is not influenced significantly by maternal intake. Fluoride levels of the water supply to which the child is exposed should be determined before fluoride supplements are prescribed. If the concentration of fluoride in the drinking water is less than 0.3 ppm, a supplement of 0.25 mg/day is recommended for infants and children 6 months to 3 years of age.
Barlow S.E. Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S165.
Centers for Disease Control: Provisional rates of any and exclusive breastfeeding by age among children born in 2005. http://www.cdc.gov/breastfeeding/data/NIS_data/2005/age.htm.
Daniels S.R., Greer F.R., the AAP Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198-208.
Gidding S.S., Dennison B.A., Birch L.L., et al. Dietary recommendations for children and adolescents: a guide for practitioners: consensus statement form the American Heart Association. Circulation. 2005;112:2061-2075.
ed 6. Kleinman R.E., editor. Pediatric Nutrition Handbook. Washington, DC: American Academy of Pediatrics; 2008.
Kliegman R.M., Behrman R.E., Jenson H.B., et al. Nelson Textbook of Pediatrics, 18th ed. Philadelphia: Saunders, 2007. Chap. 40–44
Kramer M.S., Guo T., Platt R.W., et al. Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. Am J Clin Nutr. 2003;78:291-295.
Krebs N.F. Food choices to meet nutritional needs of breast fed infants and toddlers on mixed diets. J Nutr. 2007;137(2):511S-517S.
Krebs N.F., Hambidge K.M. Trace elements. In: Duggan C., Watkins J.B., Walker W.A., editors. Nutrition in Pediatrics: Basic Science and Clinical Applications. 4th ed. Hamilton, Ontario: BC Decker; 2008:67-82.
Penny M.E. Protein-energy malnutrition: pathophysiology, clinical consequences, and treatment. In: Duggan C., Watkins J.B., Walker W.A., editors. Nutrition in Pediatrics: Basic Science and Clinical Applications. 4th ed. Hamilton, Ontario: BC Decker; 2008:127-142.
Wagner C.L., Greer F.R. Section on Breastfeeding and Committee on Nutrition, American Academy of Pediatrics: Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2008;122:1142-1152.