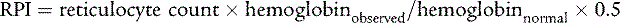CHAPTER 150 Anemia
CHAPTER 150 Anemia
ETIOLOGY
Anemia may be defined either quantitatively or physiologically. The diagnosis of anemia is determined by comparison of the patient’s hemoglobin level with age-specific and sex-specific normal values (see Table 149-2). The production of androgens at the onset of puberty in boys causes males to maintain a normal hemoglobin value about 1.5 to 2 g/dL higher than girls. The easiest quantitative definition of anemia is any hemoglobin or hematocrit value that is 2 standard deviations (SDs) (95% confidence limits) below the mean for age and gender. Nevertheless, in certain pathologic states, anemia may be present with a normal hemoglobin level, such as in cyanotic cardiac or pulmonary disease or when a hemoglobin with an abnormally high affinity for oxygen is present. In these circumstances, the physiologic definition is more appropriate. Anemia is often not a disease per se, but rather a manifestation of some other primary process and may accentuate other organ dysfunction.
Anemias are classified based on the size and hemoglobin content of the cells (Fig. 150-1). Hypochromic, microcytic anemia is caused by an inadequate production of hemoglobin. The most common causes of this type of anemia are iron deficiency and thalassemia. Most normocytic anemias are associated with a systemic illness that impairs adequate marrow synthesis of red blood cells (RBCs). Vitamin B12 and folic acid deficiencies lead to macrocytic anemia. Anemias may also occur from increased destruction. Hemolytic diseases are mediated either by intrinsic disorders of the RBC or by disorders extrinsic to the RBC itself. The most common RBC membrane disorders are hereditary spherocytosis and hereditary elliptocytosis. In both of these disorders, abnormalities of proteins within the cytoskeleton lead to abnormal RBC shape and function. Numerous RBC enzyme deficiencies may lead to hemolysis, but only two are common: glucose-6-phosphate dehydrogenase (G6PD) deficiency and pyruvate kinase deficiency. Immune-mediated hemolysis may be extravascular when RBCs coated with antibodies or complement are phagocytosed by the reticuloendothelial system. The hemolysis may be intravascular when antibody binding leads to complement fixation and lysis of RBCs.
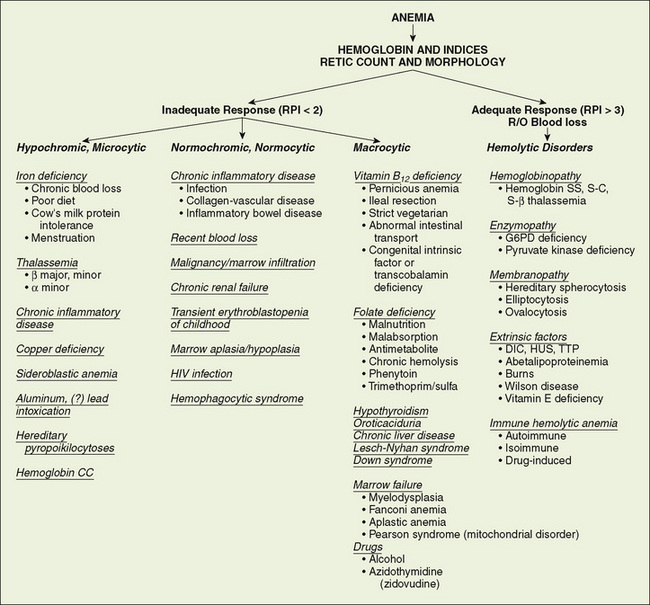
FIGURE 150-1 Use of the complete blood count, reticulocyte count, and blood smear in the diagnosis of anemia. DIC, disseminated intravascular coagulation; G6PD, glucose-6-phosphate dehydrogenase; HUS, hemolytic uremic syndrome; R/O, rule out; RPI, reticulocyte production index; TTP, thrombotic thrombocytopenic purpura.
CLINICAL MANIFESTATIONS
Acute onset of anemia can result in a poorly compensated state and may be manifested as an elevated heart rate, a flow murmur, poor exercise tolerance, headache, excessive sleeping (especially in infants) or fatigue, irritability, poor feeding, and syncope. In contrast, chronic anemia often is exceptionally well tolerated in children because of their cardiovascular reserve. Usually, children with chronic anemia will have tachycardia and a flow murmur on examination. The urgency of diagnostic and therapeutic intervention, especially the use of packed RBC transfusion, should be dictated by the extent of cardiovascular or functional impairment more than the absolute level of hemoglobin.
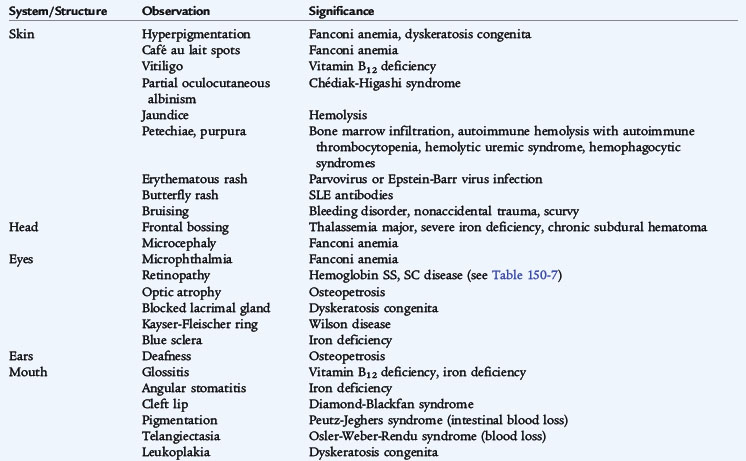
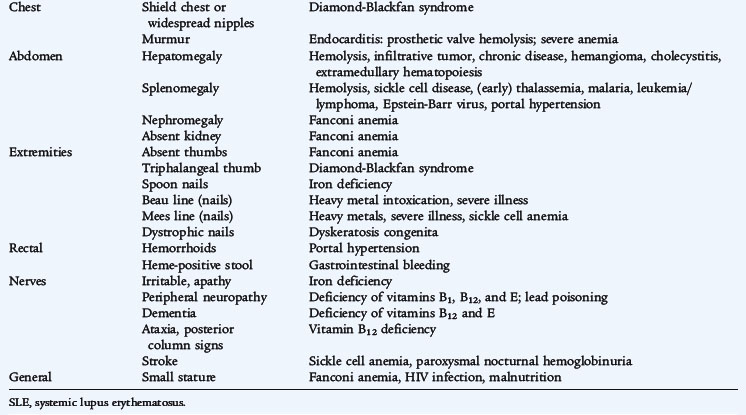
The causes of anemia often can be suspected from a careful history adjusted for the patient’s age (Tables 150-1 and 150-2). Anemia at any age demands a search for blood loss. A history of jaundice, pallor, previously affected siblings, drug ingestion by the mother, or excessive blood loss at the time of birth provides important clues to the diagnosis in newborns. A careful dietary history is crucial. The key findings in patients with hemolytic anemias are jaundice, pallor, and splenomegaly. Because of increased bilirubin production, gallstones (bilirubinate), a result of chronic hemolysis, are a common complication. Systemic complaints suggest acute or chronic illnesses as probable causes of anemia. In later childhood and adolescence, the presence of constitutional symptoms, unusual diets, drug ingestion, or blood loss, especially from menstrual bleeding, often points to a diagnosis. Congenital hemolytic disorders (enzyme deficiencies and membrane problems) often present in the first 6 months of life and frequently are associated with neonatal jaundice, although these disorders often go undiagnosed. A careful drug history is essential for detecting problems that may be drug-induced. Pure dietary iron deficiency is rare except in infancy, when cow’s milk protein intolerance causes gastrointestinal blood loss and further complicates an already inadequate iron intake.
TABLE 150-1 Historical Clues in Evaluation of Anemia
G6PD, glucose-6-phosphate dehydrogenase.
The physical examination also suggests the presence of anemia and may point to the potential causes (see Table 150-2). The first step is to assess the physiologic stability of the patient. Acute blood loss and acute hemolysis may manifest with tachycardia, blood pressure changes, and, most ominously, an altered state of consciousness. The presence of jaundice suggests hemolysis. Petechiae and purpura indicate a bleeding tendency. Hepatosplenomegaly and adenopathy suggest infiltrative disorders. Growth failure or poor weight gain suggests an anemia of chronic disease. An essential element of the physical examination in a patient with anemia is the investigation of the stool for the presence of occult blood.
LABORATORY STUDIES
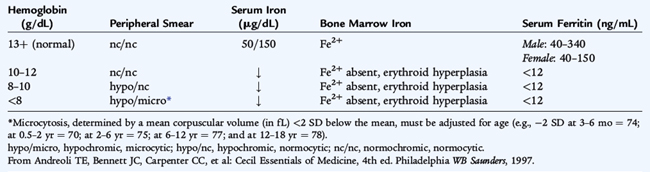
The initial laboratory evaluation of anemia involves a hemoglobin or hematocrit test to indicate the severity of the anemia. When the diagnosis of anemia has been substantiated, the workup should include a complete blood count with differential, platelet count, indices, and reticulocyte count. Examination of the peripheral blood smear assesses the morphology of RBCs (Fig. 150-2), white blood cells (WBCs), and platelets. All cell lines should be scrutinized to determine whether anemia is the result of a process limited to the erythroid line or a process that affects other marrow elements. Using data obtained from the indices and reticulocyte count, the workup can be organized on the basis of whether RBC production is adequate or inadequate and whether the cells are microcytic, normocytic, or macrocytic (see Fig. 150-1).
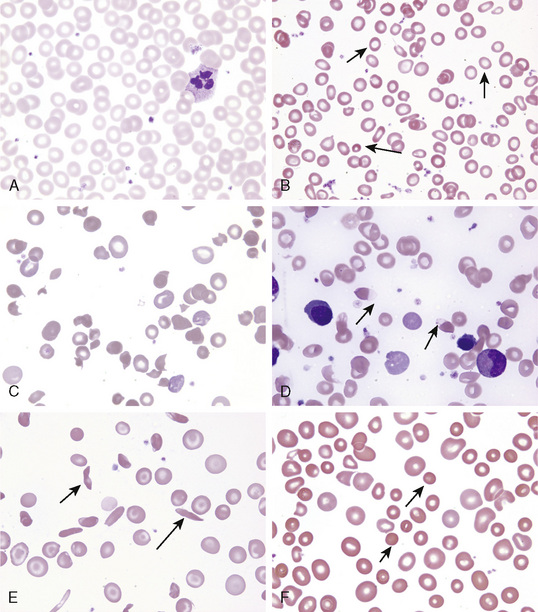
FIGURE 150-2 Morphologic abnormalities of the red blood cell. A, Normal. B, Hypochromic microcytes (iron deficiency). C, Schistocytes (hemolytic uremic syndrome D, Blister cells (glucose-6-phosphate dehydrogenase deficiency). E, Sickle cells (hemoglobin SS disease). F, Spherocytes (autoimmune hemolytic anemia.
(Courtesy of B. Trost and J.P. Scott.)
The reticulocyte production index (RPI), which corrects the reticulocyte count for the degree of anemia, indicates whether the bone marrow is responding appropriately. Reticulocytes routinely are counted per 1000 RBCs. A reduction in the denominator, which occurs in anemia, falsely increases the reticulocyte count. The formula for calculating the RPI is as follows:
An RPI greater than 3 suggests increased production and implies either hemolysis or blood loss, whereas an index below 2 suggests decreased or ineffective production for the degree of anemia. Reticulocytopenia signifies that the anemia is so acute in onset that the marrow has not had adequate time to respond, that reticulocytes are being destroyed in the marrow (antibody-mediated), or that intrinsic bone marrow disease is present. Some machines that perform complete blood counts report an absolute reticulocyte number that corrects the artifact caused by the degree of anemia.
When conducting a workup for hemolysis, the best indicators of the severity of hemolysis are the hemoglobin level and the elevation of the reticulocyte count. Biochemical evidence of hemolysis includes an increase in levels of bilirubin and lactate dehydrogenase and a decrease in haptoglobin.
DIFFERENTIAL DIAGNOSIS
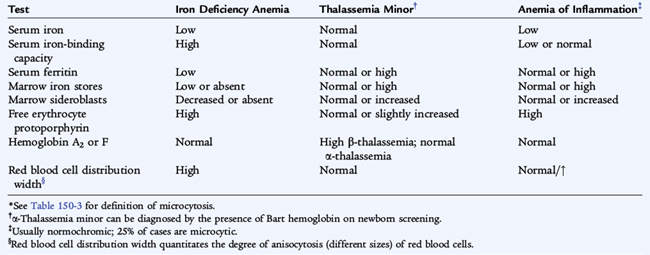
Hypochromic, Microcytic Anemia
Iron Deficiency Anemia
Etiology
Infants fed cow’s milk when younger than 1 year of age, toddlers fed large volumes of cow’s milk, and menstruating teenage girls who are not receiving supplemental iron are at high risk for iron deficiency. Dietary iron deficiency anemia is most common in bottle-fed toddlers who are receiving large volumes of cow’s milk and ingest few dietary substances high in iron (see Chapters 28 and 31). Iron deficiency anemia also may be found in children with chronic inflammatory diseases, even without chronic blood loss.
Epidemiology
The prevalence of iron deficiency, the most common cause of anemia in the world, is about 9% in toddlers, 9% to 11% in adolescent girls, and less than 1% in teenage boys. Iron deficiency anemia occurs in about one third of children who are iron deficient (Table 150-3). Some underprivileged minority populations in the United States may be at increased risk for iron deficiency because of poor dietary intake (see Chapter 31). Breastfed infants are less likely to have iron deficiency than bottle-fed infants because, although there is less iron in breast milk, this iron is more efficiently absorbed.
Clinical Manifestations
In addition to manifestations of anemia, central nervous system (CNS) abnormalities (apathy, irritability, poor concentration) have been linked to iron deficiency, presumably resulting from alterations of iron-containing enzymes (monoamine oxidase) and cytochromes. Poor muscle endurance, gastrointestinal dysfunction, and impaired WBC and T cell function have been associated with iron deficiency. Iron deficiency in infancy may be associated with later cognitive deficits and poor school performance.
Treatment
In an otherwise healthy child, a therapeutic trial of iron is the best diagnostic study for iron deficiency as long as the child is re-examined and a response is documented. The response to oral iron includes rapid subjective improvement, especially in neurologic function (within 24–48 hours) and reticulocytosis (48–72 hours); increase in hemoglobin levels (4–30 days); and repletion of iron stores (in 1–3 months). A usual therapeutic dose of 4 to 6 mg/day of elemental iron induces an increase in hemoglobin of 0.25 to 0.4 g/dL/day (a 1%/day increase in hematocrit). If the hemoglobin level fails to increase within 2 weeks after institution of iron treatment, careful re-evaluation for ongoing blood loss, development of infection, poor compliance, or other causes of microcytic anemia is required (Table 150-4; see Fig. 150-1).
Prevention
Bottle-fed infants should receive an iron-containing formula until 12 months of age, and breastfed infants older than 6 months of age should receive an iron supplement. The introduction of iron-enriched solid foods at 6 months of age, followed by a transition to a limited amount of cow’s milk and increased solid foods at 1 year, can help prevent iron deficiency anemia. Teenage girls who are menstruating should have a diet enriched with iron-containing foods. A vitamin with iron may also be used.
Thalassemia Minor
Etiology and Epidemiology
α-Thalassemia and β-thalassemia minor are common causes of microcytosis, either with or without a mild hypochromic, microcytic anemia. They are prevalent in certain ethnic groups (Mediterranean, Southeast Asian, African Americans). Individuals of Asian descent are at risk of having three or four α genes deleted, resulting in hemoglobin H disease (γ4) or hydrops fetalis with only Bart (α4) hemoglobin. (Table 150-5 and Fig. 150-3)
TABLE 150-5 Thalassemic Syndromes
| Disorder | Genotypic Abnormality | Clinical Phenotype |
|---|---|---|
| β-THALASSEMIA | ||
| Thalassemia major (Cooley’s anemia) | Homozygous β0-thalassemia | Severe hemolysis, ineffective erythropoiesis, transfusion dependency, iron overload |
| Thalassemia intermedia | Compound heterozygous β0- and β+-thalassemia | Moderate hemolysis, severe anemia, but not transfusion-dependent; main life-threatening complication is iron overload |
| Thalassemia minor | Heterozygous β0- and β+-thalassemia | Microcytosis, mild anemia |
| α-THALASSEMIA | ||
| Silent carrier | α–/αα | Normal complete blood count |
| α-Thalassemia trait | Mild microcytic anemia | |
| Hemoglobin H | α –/– – | Microcytic anemia and mild hemolysis; not transfusion-dependent |
| Hydrops fetalis | – –/– – | Severe anemia, intrauterine anasarca from congestive heart failure; death in utero or at birth |
From Andreoli T, Carpenter C, Griggs R, et al: Andreoli and Carpenter’s Cecil Essentials of Medicine, 7th ed. Philadelphia, Saunders, 2007.
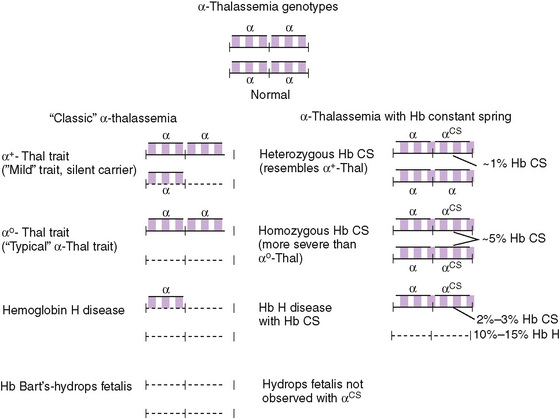
FIGURE 150-3 Genetic origins of the classic α-thalassemia syndromes due to gene deletions in the α-globin gene cluster. Hb Constant Spring (Hb CS) is an α-globin chain variant synthesized in such small amounts (1%–2% of normal) that it has the phenotypic impact of a severe nondeletion α-thalessemia allele; however, the αCS allele is always linked to a functioning α-globin gene, so it has never been associated with hydrops fetalis.
(From Hoffman: Hematology: Basic Principles and Practice, 5th ed. Philadelphia, Churchill Livingstone, 2008.)
Laboratory Testing
The thalassemia minor syndromes are characterized by a mild hypochromic, microcytic anemia with a low RPI (see Table 150-5). The RBC count is usually elevated. As a result, if the mean corpuscular volume (MCV) divided by the RBC count is less than 12.5 (Mentzer index), the diagnosis is suggestive of thalassemia trait. The blood smear reveals only microcytosis with α-thalassemia trait. Outside the neonatal period, when Bart hemoglobin is detectable, hemoglobin electrophoresis usually is normal in α-thalassemia minor (see Fig. 150-3). Blood smears of patients with β-thalassemia minor show microcytic RBCs. Target cells and basophilic stippled RBCs, caused by precipitation of alpha chain tetramers, also may be present. The diagnosis is based on an elevation of hemoglobin A2 and F levels in β-thalassemia.
Lead Poisoning
Lead poisoning may be associated with a hypochromic, microcytic anemia. Most patients have concomitant iron deficiency. The history of living in an older home (built before 1980) with chipped paint or lead dust should raise suspicion of lead poisoning, especially in a child with pica. Basophilic stippling on the blood smear is common. Lead intoxication rarely causes hemolytic anemia. Detection by routine screening, removal from exposure, chelation therapy, and correction of iron deficiency are crucial to the potential development of affected children.
Normocytic Anemia
Etiology and Treatment
Anemia is a common component of chronic inflammatory disease. Hepcidin, a protein made in the liver, plays a key role in iron homeostasis. Inflammation causes an increase in the production of hepcidin which interrupts the process of iron release by macrophages and also interrupts the absorption of iron from the intestines leading to anemia. The anemia of inflammation may be normocytic or, less often, microcytic. At times, this situation poses a clinical challenge, when children with inflammatory disorders that may be associated with blood loss (inflammatory bowel disease) exhibit a microcytic anemia. In these circumstances, the only specific diagnostic test that can differentiate the two entities clearly is a bone marrow aspiration with staining of the sample for iron (see Table 150-4). Low ferritin levels indicate concurrent iron deficiency. A trial of iron therapy is not indicated without a specific diagnosis in children who appear to be systemically ill.
Bone marrow infiltration by malignant cells commonly leads to a normochromic, normocytic anemia. The mechanism by which neoplastic cells interfere with RBC and other marrow cell synthesis is multifactorial. The reticulocyte count is often low. Immature myeloid elements may be released into the peripheral blood due to the presence of the offending tumor cells. An examination of the peripheral blood may reveal lymphoblasts; when solid tumors metastasize to the marrow, these cells are seldom seen in the peripheral blood. Teardrop cells may be seen in the peripheral blood. A bone marrow examination is frequently necessary in the face of normochromic, normocytic anemia.
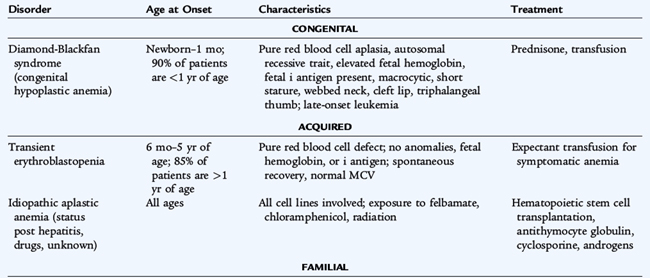
Congenital pure RBC aplasia (Diamond-Blackfan syndrome), a lifelong disorder, usually presents in the first few months of life or at birth with severe anemia and mild macrocytosis or a normocytic anemia. It is due to a deficiency of bone marrow red blood cell precursors (see Table 150-6). More than a third of patients have short stature. Many patients (50–66%) respond to corticosteroid treatment, but must receive therapy indefinitely. Patients who do not respond to corticosteroid treatment are transfusion-dependent and are at risk of the multiple complications of long-term transfusion therapy, especially iron overload. These patients have a higher rate of developing leukemia or other hematologic malignancies than the general population.
In contrast to the congenital hypoplastic anemias, transient erythroblastopenia of childhood, a normocytic anemia caused by suppression of RBC synthesis, usually appears after 6 months of age in an otherwise normal infant. Viral infections are thought to be the trigger, although the mechanism leading to RBC aplasia is poorly understood. The onset is gradual, but anemia may become severe. Recovery usually is spontaneous. Differentiation from Diamond-Blackfan syndrome, in which erythroid precursors also are absent or diminished in the bone marrow, may be challenging. Transfusion of packed RBCs may be necessary if the anemia becomes symptomatic before recovery.
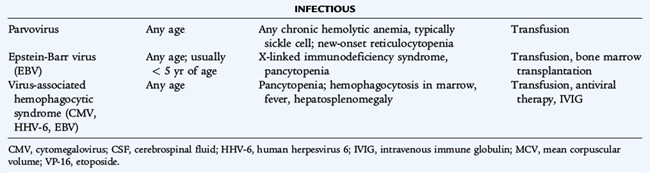
Aplastic crises may complicate any chronic hemolytic anemia. These periods of severe reticulocytopenia, during which the usual high rate of RBC destruction leads to an acute exacerbation of the anemia, may precipitate a cardiovascular decompensation. Human parvovirus B19 (the cause of fifth disease) infects erythroid precursors and shuts down erythropoiesis. Transient erythroid aplasia is without consequence in individuals with normal RBC survival. Recovery from parvovirus infection in hemolytic disease is spontaneous, but patients may need transfusion if the anemia is severe.
Macrocytic Anemia
See Figure 150-1.
Marrow Failure/Pancytopenia
Etiology
Pancytopenia is a quantitative decrease in formed elements of the blood—erythrocytes, leukocytes, and platelets. Patients more often exhibit symptoms of infection or bleeding than anemia because of the relatively short life span of WBCs and platelets compared with the life span of RBCs. Causes of pancytopenia include failure of production (implying intrinsic bone marrow disease), sequestration (hypersplenism), and increased peripheral destruction.
Differential Diagnosis
Features that suggest bone marrow failure and mandate an examination of bone marrow include a low reticulocyte count, teardrop forms of RBCs (implying marrow replacement, not just failure), presence of abnormal forms of leukocytes or myeloid elements less mature than band forms, small platelets, and an elevated mean corpuscular volume in the face of a low reticulocyte count. Pancytopenia resulting from bone marrow failure is usually a gradual process, starting with one or two cell lines, but later involving all three cell lines. Features suggesting increased destruction include reticulocytosis, jaundice, immature erythroid or myeloid elements on the blood smear, large platelets, and increased serum bilirubin and lactic dehydrogenase.
Aplastic Anemia
Etiology and Epidemiology
In a child with aplastic anemia, pancytopenia evolves as the hematopoietic elements of the bone marrow disappear and the marrow is replaced by fat. In developed countries, aplastic anemia is most often idiopathic. The disorder may be induced by drugs such as chloramphenicol and felbamate or by toxins such as benzene. Aplastic anemia also may follow infections, particularly hepatitis and infectious mononucleosis (Table 150-6). Immunosuppression of hematopoiesis is postulated to be an important mechanism in patients with postinfectious and idiopathic aplastic anemia.
Laboratory Studies
A bone marrow biopsy is crucial to determine cellularity or the extent of depletion of the hematopoietic elements.
Treatment
Survival rate is about 20% in severe aplastic anemia with supportive care alone, although the duration of survival may be years when vigorous blood product and antibiotic support is provided. For children with severe aplastic anemia—defined by an RPI less than 1%, absolute neutrophil count less than 500/mm3, platelet count less than 20,000/mm3, and bone marrow cellularity on biopsy specimen less than 25% of normal—the treatment of choice is hematopoietic stem cell transplantation (HSCT) from a sibling with identical HLA and compatible mixed lymphocytes. When HSCT occurs before the recipient is sensitized to blood products, survival rate is greater than 80%. The treatment of aplastic anemia without an HLA-matched donor for HSCT is evolving, with two major options: potent immunosuppressive therapy or unrelated or partially matched HSCT. Results of trials using immunosuppressive therapy with antithymocyte globulin, cyclosporine, and corticosteroids in combination with hematopoietic growth factors have been encouraging. Such therapy is often toxic, and relapses often occur when therapy is stopped.
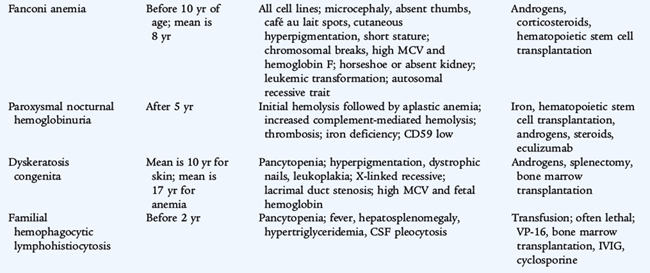
Fanconi Anemia
Etiology and Epidemiology
Fanconi anemia is a constitutional form of aplastic anemia that usually presents in the latter half of the first decade of life and may evolve over years. A group of genetic defects in proteins involved in DNA repair have been identified in Fanconi anemia which is inherited in an autosomal recessive manner. The diagnosis is based on demonstration of increased chromosomal breakage after exposure to agents that damage DNA. The repair mechanism for DNA damage is abnormal in all cells in Fanconi anemia, which may contribute to the increased risk of malignancies. Terminal acute leukemia develops in 10% of cases. Other malignancies include solid tumors of the head and neck, gastrointestinal tumors, and gynecologic tumors.
Clinical Manifestations
Patients with Fanconi anemia have numerous characteristic clinical findings (see Table 150-6).
Treatment
Hematopoietic stem cell transplantation can cure the pancytopenia caused by bone marrow aplasia. Many patients with Fanconi anemia and about 20% of children with aplastic anemia seem to respond for a time to androgenic therapy, which induces masculinization and may cause liver injury and liver tumors. Androgenic therapy increases RBC synthesis and may diminish transfusion requirements. The effect on granulocytes, and especially the platelet count, is less impressive.
Marrow Replacement
Marrow replacement may occur as a result of leukemia, solid tumors (especially neuroblastoma), storage diseases, osteopetrosis in infants, and myelofibrosis, which is rare in childhood. The mechanisms by which malignant cells impair marrow synthesis of normal hematopoietic elements are complex and incompletely understood. Bone marrow aspirate and biopsy are needed for precise diagnosis of the etiology of marrow synthetic failure.
Pancytopenia Resulting from Destruction of Cells
Pancytopenia resulting from destruction of cells may be caused by intramedullary destruction of hematopoietic elements (myeloproliferative disorders, deficiencies of folic acid and vitamin B12) or by the peripheral destruction of mature cells. The usual site of peripheral destruction of blood cells is the spleen, although the liver and other parts of the reticuloendothelial system may participate. Hypersplenism may be the result of anatomic causes (portal hypertension or splenic hypertrophy from thalassemia); infections (including malaria); or storage diseases (Gaucher disease; lymphomas; or histiocytosis). Splenectomy is indicated only when the pancytopenia is of clinical significance (e.g., increased susceptibility to bleeding or infection or produces high transfusion requirements).
Hemolytic Anemias
Major Hemoglobinopathies
Etiology
Because alpha chains are needed for fetal erythropoiesis and production of hemoglobin F (α2γ2), alpha chain hemoglobinopathies are present in utero. Four alpha genes are present on the two number 16 chromosomes (see Fig. 150-3 and Table 150-5). Single gene deletions produce no disorder (silent carrier state), but can be detected by measuring the rates of α and β synthesis or by using molecular biologic techniques. Deletion of two genes produces α-thalassemia minor with mild or no anemia and microcytosis. In individuals of African origin, the gene deletions occur on different chromosomes (trans), and the disorder is benign. In the Asian population, deletions may occur on the same chromosome (cis), and infants may inherit two number 16 chromosomes lacking three or even four genes. Deletion of all four genes leads to hydrops fetalis, severe intrauterine anemia, and death, unless intrauterine transfusions are administered. Deletion of three genes produces moderate hemolytic anemia with γ4 tetramers (Bart hemoglobin) in the fetus and ß4 tetramers (hemoglobin H) in older children and adults (see Table 150-5).
Beta chain hemoglobinopathies are more prevalent than alpha chain disorders possibly because these abnormalities are not symptomatic in utero. The major beta hemoglobinopathies include those that alter hemoglobin function, including hemoglobins S, C, E, and D, and those that alter beta chain production, the β-thalassemias. Because each RBC has two copies of chromosome 11 and they express both β-globin genes, most disorders of beta chains are not clinically severe, unless both beta chains are abnormal. By convention, when describing β-thalassemia genes, β0 indicates a thalassemic gene resulting in absent beta chain synthesis, whereas β+ indicates a thalassemic gene that permits reduced but not absent synthesis of normal β chains. Disorders of the beta chain usually manifest themselves clinically between 4 and 12 months of age, unless they have been detected prenatally or by cord blood screening.
β-Thalassemia Major (Cooley Anemia)
Etiology and Epidemiology
β-Thalassemia major is caused by mutations that impair beta chain synthesis. Because of unbalanced synthesis of alpha and beta chains, alpha chains precipitate within the cells, resulting in RBC destruction either in the bone marrow or in the spleen. β-Thalassemia major is seen most commonly in individuals of Mediterranean or Asian descent. The clinical severity of the illness varies on the basis of the molecular defect.
Clinical Manifestations
Signs and symptoms of β-thalassemia major result from the combination of chronic hemolytic disease, decreased or absent production of normal hemoglobin A, and ineffective erythropoiesis. The anemia is severe and leads to growth failure and high-output heart failure. Ineffective erythropoiesis causes increased expenditure of energy and expansion of the bone marrow cavities of all bones, leading to osteopenia, pathologic fractures, extramedullary erythropoiesis, and an increase in the rate of iron absorption.
Treatment
Treatment of β-thalassemia major is based on a hypertransfusion program that corrects the anemia and suppresses the patient’s own ineffective erythropoiesis, limiting the stimulus for increased iron absorption. This suppression permits the bones to heal, decreases metabolic expenditures, increases growth, and limits dietary iron absorption. Splenectomy may reduce the transfusion volume, but adds to the risk of serious infection. Chelation therapy with deferoxamine or deferasirox should start when laboratory evidence of iron overload (hemochromatosis) is present and before there are clinical signs of iron overload (nonimmune diabetes mellitus, cirrhosis, heart failure, bronzing of the skin, and multiple endocrine abnormalities). Hematopoietic stem cell transplantation in childhood, before organ dysfunction induced by iron overload, has had a high success rate in β-thalassemia major and is the treatment of choice.
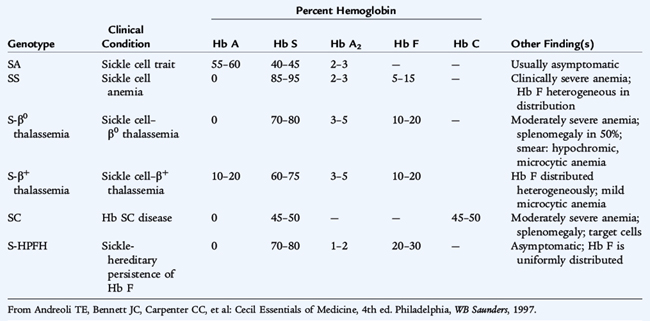
Sickle Cell Disease
Etiology and Epidemiology
The common sickle cell syndromes are hemoglobin SS disease, hemoglobin S-C disease, hemoglobin S-β thalassemia, and rare variants (Table 150-7). The specific hemoglobin phenotype must be identified because the clinical complications differ in frequency, type, and severity. As a result of a single amino acid substitution (valine for glutamic acid at the β6 position), sickle hemoglobin crystallizes and forms a gel in the deoxygenated state. When reoxygenated, the sickle hemoglobin is normally soluble. The so-called reversible sickle cell is capable of entering the microcirculation. As the oxygen is extracted and saturation declines, sickling may occur, occluding the microvasculature. The surrounding tissue undergoes infarction, inducing pain and dysfunction. This sickling phenomenon is exacerbated by hypoxia, acidosis, fever, hypothermia, and dehydration.
Clinical Manifestations and Treatment
A child with sickle cell anemia is vulnerable to life-threatening infection by 4 months of age. By that time, splenic dysfunction is caused by sickling of the RBCs within the spleen, resulting in an inability to filter microorganisms from the bloodstream in most patients. Splenic dysfunction is followed, eventually, by splenic infarction, usually by 2 to 4 years of age. The loss of normal splenic function makes the patient susceptible to overwhelming infection by encapsulated organisms, especially Streptococcus pneumoniae and other pathogens (see Table 150-8). The hallmark of infection is fever. A febrile patient with a sickle cell syndrome (temperature > 38.5°C) must be evaluated immediately (see Chapter 96). Current precautions to prevent infections include prophylactic daily oral penicillin begun at diagnosis and vaccinations against pneumococcus, Haemophilus influenzae type b, hepatitis B virus, and influenza virus.
TABLE 150-8 Clinical Manifestations of Sickle Cell Anemia*
| Manifestation | Comments |
|---|---|
| Anemia | Chronic, onset 3–4 mo of age; may require folate therapy for chronic hemolysis; hematocrit usually 18–26% |
| Aplastic crisis | Parvovirus infection, reticulocytopenia; acute and reversible; may need transfusion |
| Sequestration crisis | Massive splenomegaly (may involve liver), shock; treat with transfusion |
| Hemolytic crisis | May be associated with G6PD deficiency |
| Dactylitis | Hand-foot swelling in early infancy |
| Painful crisis | Microvascular painful vaso-occlusive infarcts of muscle, bone, bone marrow, lung, intestines |
| Cerebrovascular accidents | Large and small vessel occlusion → thrombosis/bleeding (stroke); requires chronic transfusion |
| Acute chest syndrome | Infection, atelectasis, infarction, fat emboli, severe hypoxemia, infiltrate, dyspnea, absent breath sounds |
| Chronic lung disease | Pulmonary fibrosis, restrictive lung disease, cor pulmonale, pulmonary hypertension |
| Priapism | Causes eventual impotence; treated with tranfusion, oxygen, or corpora cavernosa–to–spongiosa shunt |
| Ocular | Retinopathy |
| Gallbladder disease | Bilirubin stones; cholecystitis |
| Renal | Hematuria, papillary necrosis, renal concentrating defect; nephropathy |
| Cardiomyopathy | Heart failure |
| Skeletal | Osteonecrosis (avascular) of femoral or humeral head |
| Leg ulceration | Seen in older patients |
| Infections | Functional asplenia, defects in properdin system; pneumococcal bacteremia, meningitis, and arthritis; deafness from meningitis; Salmonella and Staphylococcus aureus osteomyelitis; severe Mycoplasma pneumonia |
| Growth failure, delayed puberty | May respond to nutritional supplements |
| Psychological problems | Narcotic addiction (rare), dependence unusual; chronic illness, chronic pain |
G6PD, glucose-6-phosphate dehydrogenase.
* Clinical manifestations with sickle cell trait are unusual but include renal papillary necrosis (hematuria), sudden death on exertion, intraocular hyphema extension, and sickling in unpressurized airplanes.
The anemia of SS disease is usually a chronic, moderately severe, hemolytic anemia that is not routinely transfusion dependent. The severity depends in part on the patient’s phenotype. Manifestations of chronic anemia include jaundice, pallor, variable splenomegaly in infancy, a cardiac flow murmur, and delayed growth and sexual maturation. Decisions about transfusion should be made on the basis of the patient’s clinical condition, the hemoglobin level, and the reticulocyte count.
Sickle cell disease is complicated by sudden, occasionally severe and life-threatening events caused by the acute intravascular sickling of the RBCs, with resultant pain or organ dysfunction (so-called crisis). In two different clinical situations, an acute, potentially life-threatening decline in the hemoglobin level may be superimposed on the chronic compensated anemia. Splenic sequestration crisis is a life-threatening, hyperacute decline in the hemoglobin level (blood volume) secondary to splenic pooling of the patient’s RBCs and sickling within the spleen. The spleen is moderately to markedly enlarged and the reticulocyte count is elevated. In an aplastic crisis, parvovirus B19 infects RBC precursors in the bone marrow and induces transient RBC aplasia with reticulocytopenia and a rapid worsening of anemia. Simple transfusion therapy is indicated for sequestration and aplastic crises when the anemia is symptomatic.
Vaso-occlusive painful events may occur in any organ of the body and are manifested by pain or significant dysfunction (Table 150-8). The acute chest syndrome is a vaso-occlusive crisis within the lungs with evidence of a new infiltrate on chest radiograph. It is often associated with infection and infarction. The patient may first complain of chest pain but within a few hours develops cough, increasing respiratory and heart rates, hypoxia, and progressive respiratory distress. Physical examination reveals areas of decreased breath sounds and dullness on chest percussion. Treatment involves early recognition and prevention of arterial hypoxemia. Oxygen, fluids, judicious use of analgesic medications, antibiotics, bronchodilators, and RBC transfusion (rarely exchange transfusion) are indicated in therapy for acute chest syndrome. Incentive spirometry may help reduce the incidence of acute chest crisis in patients presenting with pain in the chest or abdomen.
Pain crisis is the most common type of vaso-occlusive event. The pain usually localizes to the long bones of the arms or legs, but may occur in smaller bones of the hands or feet in infancy (dactylitis) or in the abdomen. Painful crises usually last 2 to 7 days. Vaso-occlusive crises within the femur may lead to avascular necrosis of the femoral head and chronic hip disease. Treatment of pain crises includes administration of fluids, analgesia (usually narcotics and nonsteroidal anti-inflammatory drugs) and oxygen if the patient is hypoxic. Although pain is often impossible to quantitate, the risk for drug dependency is highly overrated and appropriate use of analgesics is necessary.
Priapism occurs most typically in boys between 6 and 20 years old. The child experiences a sudden, painful onset of a tumescent penis that will not relax. Therapeutic steps for priapism include the administration of oxygen, fluids, transfusion to achieve a hemoglobin S less than 30% (often by partial exchange transfusion), and analgesia when appropriate. Fluid management requires recognition that renal medullary infarction results in loss of the ability to concentrate urine.
Overt stroke occurs in approximately 8% to 10% of patients with SS disease. These events may present as the sudden onset of an altered state of consciousness, seizures, or focal paralysis. Silent stroke, which is defined as evidence of cerebral infarction on imaging studies but a normal neurologic examination, is more common and occurs in approximately 20% of patients with SS disease. A significant change in school performance or behavior has been associated with silent stroke. Children with Hg SS disease over 3 years of age should be screened for increased risk of stroke using transcranial doppler (TCD).
Laboratory Diagnosis
The diagnosis of hemoglobinopathies is made by identifying the precise amount and type of hemoglobin using hemoglobin electrophoresis, isoelectric focusing, or high-performance liquid chromatography. Every member of an at-risk population should have a precise hemoglobin phenotype performed at birth (preferably) or during early infancy. Most states perform newborn screening for sickle cell disease.
Treatment
Direct therapy of sickle cell anemia is evolving. The mainstay of care are supportive measures. The use of chronic RBC transfusions to treat patients who have had a stroke has been very successful. Chronic RBC transfusions have also been used successfully for short time periods to prevent recurrent vaso-occlusive painful events, including acute chest syndrome and priapism. Hydroxyurea, which increases hemoglobin F, decreases the number and severity of vaso-occlusive events. Hematopoietic stem cell transplantation, utilizing a haploidentical sibling match, has cured many children with sickle cell disease. HSCT using alternative donors for children, without a suitable sibling match, is being studied.
Enzymopathies
Etiology
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an abnormality in the hexose monophosphate shunt pathway of glycolysis that results in the depletion of reduced nicotinamide adenine dinucleotide phosphate (NADPH) and the inability to regenerate reduced glutathione. Reduced glutathione protects sulfhydryl groups in the RBC membrane from oxidation. When a patient with G6PD is exposed to significant oxidant stress, hemoglobin is oxidized, forming precipitates of sulfhemoglobin (Heinz bodies), which are visible on specially stained preparations. The gene for G6PD deficiency is on the X chromosome.
The severity of hemolysis depends on the enzyme variant. In many G6PD variants, the enzymes become unstable with aging of the RBC and cannot be replaced because the cell is anucleated. Older cells are most susceptible to oxidant-induced hemolysis. In other variants, the enzyme is kinetically abnormal.
Epidemiology
The most common variants of G6PD deficiency have been found in areas where malaria is endemic. G6PD deficiency protects against parasitism of the erythrocyte. The most common variant with normal activity is termed type B and is defined by its electrophoretic mobility. The approximate gene frequencies in African Americans are 70% type B, 20% type A, and 10% type A−. Only the A− variant, termed the African variant, is unstable. Ten percent of black males are affected. A group of variants found in Sardinians, Sicilians, Greeks, Sephardic and Oriental Jews, and Arabs is termed the Mediterranean variant and is associated with chronic hemolysis and potentially life-threatening hemolytic disease. Because the gene for G6PD deficiency is carried on the X chromosome, clinical hemolysis is most common in males. Heterozygous females who have randomly inactivated a higher percentage of the normal gene may become symptomatic, as may homozygous females with the A− variant (0.5% to 1% of females of African descent).
Clinical Manifestations
G6PD deficiency has two common presentations. Individuals with the A− variant have normal hemoglobin values when well, but develop an acute episode of hemolysis triggered by serious (bacterial) infection or ingestion of an oxidant drug. The RBC morphology during episodes of acute hemolysis is striking. RBCs appear to have “bites” (cookie cells) taken out of them. These are areas of absent hemoglobin that are produced by phagocytosis of Heinz bodies by splenic macrophages; as a result, the RBCs appear blistered. Clinically evident jaundice, dark urine resulting from bilirubin pigments, hemoglobinuria when hemolysis is intravascular, and decreased haptoglobin levels are common during hemolytic episodes. Early on, the hemolysis usually exceeds the ability of the bone marrow to compensate, so the reticulocyte count may be low for 3 to 4 days.
Laboratory Studies
The diagnosis of G6PD deficiency is based on decreased NADPH formation. G6PD levels during an acute, severe hemolytic episode may be normal, however, because the most deficient cells have been destroyed and reticulocytes are enriched with G6PD. Repeating the test at a later time when the patient is in a steady-state condition, testing the mothers of boys with suspected G6PD deficiency, or performing electrophoresis to identify the precise variant present aids in diagnosis.
Treatment and Prevention
The treatment of G6PD deficiency is supportive. Transfusions are indicated when significant cardiovascular compromise is present. Maintaining hydration and urine alkalization protects the kidneys against damage from precipitated free hemoglobin. Hemolysis is prevented by avoidance of known oxidants, particularly long-acting sulfonamides, nitrofurantoin, primaquine, dimercaprol and moth balls (naphthalene). Fava beans (favism) have triggered hemolysis, particularly in patients with the Mediterranean variant. Infection also is a major precipitant of hemolysis in G6PD-deficient young children.
Pyruvate kinase deficiency is much less common than G6PD deficiency and represents a clinical spectrum of disorders caused by the functional deficiency of pyruvate kinase. Some individuals have a true deficiency state, and others have abnormal enzyme kinetics. The metabolic consequence of pyruvate kinase deficiency is adenosine triphosphate (ATP) depletion, impairing RBC survival. Pyruvate kinase deficiency is usually an autosomal disorder, and most children who are affected (and are not products of consanguinity) are double heterozygotes for two abnormal enzymes. Hemolysis is not aggravated by oxidant stress because patients with this condition tend to have a profound reticulocytosis. Aplastic crises are potentially life-threatening. The spleen is the site for RBC removal in pyruvate kinase deficiency. Most patients have amelioration of the anemia and a reduction of transfusion requirements after splenectomy.
Membrane Disorders
Etiology
The biochemical basis of hereditary spherocytosis and hereditary elliptocytosis are similar. Both conditions appear to have a defect in the protein lattice (spectrin, ankyrin, protein 4.2, band 3) that underlies the RBC lipid bilayer and provides stability of the membrane shape. In hereditary spherocytosis, pieces of membrane bud off as microvesicles because of abnormal vertical interaction of the cytoskeletal proteins and uncoupling of the lipid bilayer from the cytoskeleton. When the RBC loses membrane, cell shape changes from a biconcave disc to a spherocyte, which has the lowest ratio of surface area to volume. The RBC is less deformable when passing through narrow passages in the spleen. Hereditary elliptocytosis is a disorder of spectrin dimer interactions that occurs primarily in individuals of African descent. The transmission of the two variants is usually autosomal dominant, but spontaneous mutations causing hereditary spherocytosis are common. Hereditary pyropoikilocytosis (unusual instability of the erythrocytes when they are exposed to heat at 45°C) is the result of a structural abnormality of spectrin.
Clinical Manifestations
Hereditary spherocytosis varies greatly in clinical severity, ranging from an asymptomatic, well-compensated, mild hemolytic anemia that may be discovered incidentally to a severe hemolytic anemia with growth failure, splenomegaly, and chronic transfusion requirements in infancy necessitating early splenectomy. The most common variant of hereditary elliptocytosis is a clinically insignificant, morphologic abnormality without shortened RBC survival. The less common variant is associated with spherocytes, ovalocytes, and elliptocytes with a moderate, usually compensated, hemolysis. Far more significant hemolysis occurs in hereditary pyropoikilocytosis. The peripheral blood smear in hereditary pyropoikilocytosis often includes elliptocytes, spherocytes, fragmented RBCs, and striking microcytosis. Such patients may have bizarre blood smears in the newborn period with small, fragmented RBCs.
Laboratory Diagnosis
The clinical diagnosis of hereditary spherocytosis should be suspected in patients with even a few spherocytes found on the blood smear because the spleen preferentially removes spherocytes. An incubated osmotic fragility test confirms the presence of spherocytes and increases the likelihood of the diagnosis. The osmotic fragility test result is abnormal in any hemolytic disease in which spherocytes are present, however, especially antibody-mediated hemolysis.
Treatment
Splenectomy corrects the anemia and normalizes the RBC survival in patients with hereditary spherocytosis, but the morphologic abnormalities persist. Splenectomy should be considered for any child with symptoms referable to anemia or growth failure, but should be deferred until age 5 years, if possible, to minimize the risk of overwhelming postsplenectomy sepsis and to maximize the antibody response to the polyvalent pneumococcal vaccine. In several reports, partial splenectomy seems to improve the hemolytic anemia and maintain splenic function in host defense.
Hemolytic Anemia Caused by Disorders Extrinsic to the Red Blood Cell
Etiology and Clinical Manifestations
Isoimmune hemolysis is caused by active maternal immunization against fetal antigens that the mother’s erythrocytes do not express (see Chapter 62). Examples are antibodies to the A, B, and Rh D antigens; other Rh antigens; and the Kell, Duffy, and other blood groups. Anti-A and anti-B hemolysis is caused by the placental transfer of naturally occurring maternal antibodies from mothers who lack A or B antigen (usually blood type O). Positive results of the direct antiglobulin (Coombs) test on the infant’s RBCs (Fig. 150-4), the indirect antiglobulin test on the mother’s serum and the presence of spherocytes and immature erythroid precursors (erythroblastosis) on the infant’s blood smear confirm this diagnosis. Isoimmune hemolytic disease varies in clinical severity. There may be no clinical manifestations or the infant may exhibit jaundice, severe anemia, and hydrops fetalis.
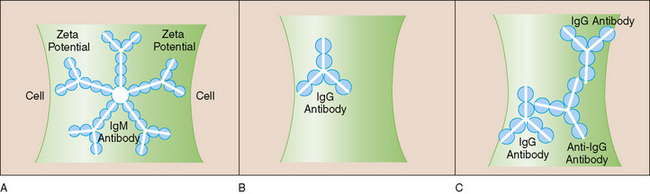
FIGURE 150-4 Coombs or direct antiglobulin test (DAT). In the DAT, so-called Coombs serum that recognizes human immunoglobulin (Ig) or complement (C) is used to detect the presence of antibody or C on the surface of the red blood cells (RBCs) by agglutination. A, An IgM antibody can bind two RBCs simultaneously because of its multiple antigen-binding sites. The great size of the IgM allows it to bridge the surface repulsive forces (zeta potential) between RBCs and cause agglutination. B, An IgG antibody is too small to bridge the zeta potential and cause agglutination. C, On the addition of Coombs serum, the zeta potential is bridged successfully, and RBCs agglutinate.
(Modified from Ware RE, Rosse WF: Autoimmune hemolytic anemia. In Orkin SH, Nathan DG, Look AT, Ginsburg D [eds]: Hematology of Infancy and Childhood, 6th ed. Philadelphia, WB Saunders, 2003, p 530.)
Autoimmune hemolytic anemia is usually an acute, self-limited process that develops after an infection (Mycoplasma, Epstein-Barr, or other viral infections). Autoimmune hemolytic anemia may also be the presenting symptom of a chronic autoimmune disease (systemic lupus erythematosus, lymphoproliferative disorders, or immunodeficiency). Drugs may induce a Coombs-positive hemolytic anemia by forming a hapten on the RBC membrane (penicillin) or by forming immune complexes (quinidine) that attach to the RBC membrane. Antibodies then activate complement-induced intravascular hemolysis. The third type of drug-induced immune hemolysis occurs during treatment with α-methyldopa and a few other drugs. In this type, prolonged drug exposure alters the RBC membrane, inducing neoantigen formation. Antibodies are produced that bind to the neoantigen; this produces a positive antiglobulin test result far more commonly than it actually induces hemolysis. In each of these conditions, the erythrocyte is an innocent bystander.
A second form of acquired hemolytic disease that is not antibody mediated is caused by mechanical damage to the RBC membrane during circulation. In thrombotic microangiopathy, the RBCs are trapped by fibrin strands in the circulation and physically broken by shear stress as they pass through these strands. Hemolytic uremic syndrome, disseminated intravascular coagulation (DIC), thrombotic thrombocytopenic purpura, malignant hypertension, toxemia, and hyperacute renal graft rejection all produce thrombotic microangiopathy. The platelets are usually large, indicating that they are young, but have a decreased survival even if the numbers are normal. Consumption of clotting factors is more prominent in DIC than in the other forms of thrombotic microangiopathy. The smear shows RBC fragments (schistocytes), microspherocytes, teardrop forms, and polychromasia. Other examples of mechanical injury to RBCs include damage by exposure to nonendothelialized surfaces (as in artificial heart valves) or as a result of high flow and shear rates in giant hemangiomas (Kasabach-Merritt syndrome).
Alterations in the plasma lipids, especially cholesterol, may lead to damage to the RBC membrane and shorten RBC survival. Lipids in the plasma are in equilibrium with lipids in the RBC membrane. High cholesterol levels increase the membrane cholesterol and the total membrane surface without affecting the volume of the cell. This condition produces spur cells that may be seen in abetalipoproteinemia and liver diseases. Hemolysis occurs in the spleen, where poor RBC deformability results in erythrocyte destruction. Circulating toxins (e.g., snake venoms and heavy metals) that bind sulfhydryl groups may damage the RBC membrane and induce hemolysis. Irregularly spiculated RBCs (burr cells) are seen in renal failure. Vitamin E deficiency can also cause an acquired hemolytic anemia as a result of abnormal sensitivity of membrane lipids to oxidant stress. Vitamin E deficiency may occur in premature infants who are not being supplemented with vitamin E or who have insufficient nutrition, in infants with severe malabsorption syndromes (including cystic fibrosis), and in infants with transfusional iron overload, which can lead to severe oxidant exposure.
Laboratory Diagnosis
The peripheral blood smear in autoimmune hemolytic anemia usually reveals spherocytes and occasionally nucleated RBCs. The reticulocyte count varies because some patients have relatively low reticulocyte counts as a result of autoantibodies which cross-reacts with RBC precursors.
Treatment and Prognosis
Transfusion for the treatment of autoimmune hemolysis is challenging because crossmatching is difficult, as the autoantibodies react with virtually all RBCs. In addition to transfusion, which may be lifesaving, management of autoimmune hemolytic anemia depends on antibody type. Management may involve administration of corticosteroids and, at times, intravenous immunoglobulin. Corticosteroids reduce the clearance of sensitized RBCs in the spleen. In drug-induced hemolysis, withdrawal of the drug usually leads to resolution of the hemolytic process. More than 80% of children with autoimmune hemolytic anemia recover spontaneously.