CHAPTER 58 Assessment of the Mother, Fetus, and Newborn
CHAPTER 58 Assessment of the Mother, Fetus, and Newborn
ASSESSMENT OF THE MOTHER
The optimal care of the newborn requires knowledge of the family history, prior and current pregnancies, and events of labor and delivery. Neonatal medicine requires a comprehensive understanding of the physiology of normal pregnancy; placental and fetal growth, function, and maturity; and any extrauterine or intrauterine pathologic events that affect the mother, placenta, or fetus. These latter adverse effects may result in an unfavorable neonatal outcome and include such significant maternal influences as poor nutrition, cigarette smoking, poverty, physical or psychological stresses, extremes of age (<16 years, >35 years), race, medical illness present before pregnancy, and medications as well as obstetric complications during the antepartum and intrapartum periods, perinatal infections, exposure to toxins and illicit drugs, and the inherent genetic predisposition of the fetus.
Pregnancies associated with perinatal morbidity or mortality are considered high risk. Identification of high-risk pregnancies is essential to the care of the infant because they may result in intrauterine fetal death, intrauterine growth restriction (IUGR), congenital anomalies, excessive fetal growth, birth asphyxia and trauma, prematurity (birth at <38 weeks) or postmaturity (birth at ≥42 weeks), neonatal disease, or long-term risks of cerebral palsy, mental retardation, and chronic sequelae of neonatal intensive care. Ten percent to 20% of women may be high risk at some time during their pregnancy. Although some obstetric complications are first seen during labor and delivery and cannot be predicted, more than 50% of perinatal mortality and morbidity results from problems identified before delivery as high risk. After a high-risk pregnancy is identified, measures can be instituted to prevent complications, provide intensive fetal surveillance, and initiate appropriate treatments of the mother and fetus.
A history of previous premature birth, intrauterine fetal death, multiple gestation, IUGR, congenital malformation, explained or unexplained neonatal death (e.g., group B streptococcal sepsis), birth trauma, preeclampsia, gestational diabetes, grand multipara status (five or more pregnancies), or cesarean section is associated with additional risk in subsequent pregnancies.
Pregnancy complications that increase the risk of a poor outcome can be secondary to maternal or fetal causes or both. Complications include placenta previa; abruptio placentae; preeclampsia; diabetes; oligohydramnios or polyhydramnios; multiple gestation; blood group sensitization; abnormal levels of unconjugated estriols, chorionic gonadotropin, or alpha-fetoprotein; abnormal fetal ultrasound; hydrops fetalis; maternal trauma or surgery; abnormal fetal presentation (breech); exposure to prescribed or illicit drugs; prolonged labor; cephalopelvic disproportion; prolapsed cord; fetal distress; prolonged or premature rupture of membranes; short cervical length (<25 mm) and the presence of fetal fibronectin in cervical secretions at less than 35 weeks’ gestation (a predictor of preterm labor); cervical infections and vaginosis; and congenital infections, including rubella, cytomegalovirus, herpes simplex, human immunodeficiency virus (HIV), toxoplasmosis, syphilis, and gonorrhea.
Maternal medical complications associated with increased risk of maternal and fetal morbidity and mortality include diabetes, chronic hypertension, congenital heart disease (especially with right-to-left shunting and Eisenmenger complex), glomerulonephritis, collagen vascular disease (especially systemic lupus erythematosus with or without antiphospholipid antibodies), lung disease (cystic fibrosis), severe anemia (sickle cell anemia), hyperthyroidism, myasthenia gravis, idiopathic thrombocytopenic purpura, inborn errors of metabolism (maternal phenylketonuria), and malignancy.
Obstetric complications often are associated with increased fetal or neonatal risk. Vaginal bleeding in the first trimester or early second trimester may be caused by a threatened or actual spontaneous abortion and is associated with increased risk of congenital malformations or chromosomal disorders. Painless vaginal bleeding that is not associated with labor and occurs in the late second or (more likely) third trimester often is the result of placenta previa. Bleeding develops when the placental mass overlies the internal cervical os; this may produce maternal hemorrhagic shock, necessitating transfusions. Bleeding also may result in premature delivery. Painful vaginal bleeding is often the result of retroplacental hemorrhage or placental abruption. Associated findings may be advanced maternal age and parity, maternal chronic hypertension, maternal cocaine use, preterm rupture of membranes, polyhydramnios, twin gestation, and preeclampsia. Fetal asphyxia ensues as the retroplacental hematoma causes placental separation that interferes with fetal oxygenation. Both types of bleeding are associated with fetal blood loss. Neonatal anemia may be more common with placenta previa.
Abnormalities in the volume of amniotic fluid, resulting in oligohydramnios or polyhydramnios, are associated with increased fetal and neonatal risk. Oligohydramnios (amniotic ultrasound fluid index ≤2 cm) is associated with IUGR and major congenital anomalies, particularly of the fetal kidneys, and with chromosomal syndromes. Bilateral renal agenesis results in diminished production of amniotic fluid and a specific deformation syndrome (Potter syndrome), which includes clubfeet, characteristic compressed facies, low-set ears, scaphoid abdomen, and diminished chest wall size accompanied by pulmonary hypoplasia and, often, pneumothorax. Uterine compression in the absence of amniotic fluid retards lung growth, and patients with this condition die of respiratory failure rather than renal insufficiency. Twin-to-twin transfusion syndrome (donor) and complications from amniotic fluid leakage also are associated with oligohydramnios. Oligohydramnios increases the risk of fetal distress during labor (meconium-stained fluid and variable decelerations); the risk may be reduced by saline amnioinfusion during labor.
Polyhydramnios may be acute and associated with premature labor, maternal discomfort, and respiratory compromise. More often, polyhydramnios is chronic and is associated with diabetes, immune or nonimmune hydrops fetalis, multiple gestation, trisomy 18 or 21, and major congenital anomalies. Anencephaly, hydrocephaly, and meningomyelocele are associated with reduced fetal swallowing. Esophageal and duodenal atresia, as well as cleft palate, interfere with swallowing and gastrointestinal fluid dynamics. Additional causes of polyhydramnios include Werdnig-Hoffmann and Beckwith-Wiedemann syndromes, conjoined twins, chylothorax, cystic adenomatoid lung malformation, diaphragmatic hernia, gastroschisis, sacral teratoma, placental chorioangioma, and myotonic dystrophy. Hydrops fetalis may be a result of Rh or other blood group incompatibilities and anemia caused by intrauterine hemolysis of fetal erythrocytes by maternal IgG-sensitized antibodies crossing the placenta. Hydrops is characterized by fetal edema, ascites, hypoalbuminemia, and congestive heart failure. Causes of nonimmune hydrops include fetal arrhythmias (supraventricular tachycardia, congenital heart block), fetal anemia (bone marrow suppression, nonimmune hemolysis, or twin-to-twin transfusion), severe congenital malformation, intrauterine infections, congenital neuroblastoma, inborn errors of metabolism (storage diseases), fetal hepatitis, nephrotic syndrome, and pulmonary lymphangiectasia. Twin-to-twin transfusion syndrome (recipient) also may be associated with polyhydramnios. Polyhydramnios is often the result of unknown causes. If severe, polyhydramnios may be managed with bed rest, indomethacin, or serial amniocenteses.
Premature rupture of the membranes, which occurs in the absence of labor, and prolonged rupture of the membranes (>24 hours) are associated with an increased risk of maternal or fetal infection (chorioamnionitis) and preterm birth. In the immediate newborn period, group B streptococcus and Escherichia coli are the two most common pathogens associated with sepsis. Listeria monocytogenes is a less common cause. Mycoplasma hominis, Ureaplasma urealyticum, Chlamydia trachomatis, and anaerobic bacteria of the vaginal flora also have been implicated in infection of the amniotic fluid. Infection with community-acquired methicillin-resistant Staphylococcus aureus must be considered for infants with skin infections or with known exposures. The risk of serious fetal infection increases, as the duration between rupture and labor (latent period) increases, especially if the period is greater than 24 hours. Intrapartum antibiotic therapy decreases the risk of neonatal sepsis.
Multiple gestations are associated with increased risk resulting from polyhydramnios, premature birth, IUGR, abnormal presentation (breech), congenital anomalies (intestinal atresia, porencephaly, and single umbilical artery), intrauterine fetal demise, birth asphyxia, and twin-to-twin transfusion syndrome. Twin-to-twin transfusion syndrome is associated with a high mortality and is seen only in monozygotic twins who share a common placenta and have an arteriovenous connection between their circulations. The fetus on the arterial side of the shunt serves as the blood donor, resulting in fetal anemia, growth retardation, and oligohydramnios for this fetus. The recipient, or venous-side twin, is larger or discordant in size, is plethoric and polycythemic, and may show polyhydramnios. Weight differences of 20% and hemoglobin differences of 5 g/dL suggest the diagnosis. Ultrasonography in the second trimester reveals discordant amniotic fluid volume with oliguria/oligohydramnios and hypervolemia/polyuria/polyhydramnios with a distended bladder, with or without hydrops and heart failure. Mortality is high if presentation occurs in the second trimester; however, most monochorionic twins have bidirectional balanced shunts and are not affected. Treatment includes amniocentesis and attempts to ablate the arteriovenous connection (using a laser). The birth order of twins also affects morbidity by increasing the risk of the second-born twin for breech position, birth asphyxia, birth trauma, and respiratory distress syndrome.
Overall, twinning is observed in 1:80 pregnancies; 80% of all twin gestations are dizygotic twins. The diagnosis of the type of twins can be determined by placentation, sex, fetal membrane structure, and, if necessary, tissue and blood group typing or DNA analysis.
Toxemia of pregnancy, or preeclampsia/eclampsia, is a disorder of unknown but probably vascular etiology that may lead to maternal hypertension, uteroplacental insufficiency, IUGR, intrauterine asphyxia, maternal seizures, and maternal death. Toxemia is more common in nulliparous women and in women with twin gestation, chronic hypertension, obesity, renal disease, positive family history of toxemia, or diabetes mellitus. A subcategory of preeclampsia, the HELLP syndrome (hemolysis, elevated liver enzyme levels, low platelets), is more severe and is often associated with a fetal inborn error of fatty acid oxidation (long-chain hydroxyacyl–coenzyme A dehydrogenase of the trifunctional protein complex).
FETUS AND NEWBORN
The late fetal–early neonatal period has the highest mortality rate of any age interval. Perinatal mortality refers to fetal deaths occurring from the 20th week of gestation until the 28th day after birth and is expressed as number of deaths per 1000 live births. Intrauterine fetal death accounts for 40% to 50% of the perinatal mortality rate. Such infants, defined as stillborn, are born without a heart rate and are apneic, limp, pale, and cyanotic. Many stillborn infants exhibit evidence of maceration; pale, peeling skin; corneal opacification; and soft cranial contents.
Mortality rates around the time of birth are expressed as number of deaths per 1000 live births. The neonatal mortality rate includes all infants dying during the period from after birth to the first 28 days of life. Modern neonatal intensive care allows many newborns with life-threatening diseases to survive the neonatal period, only to die of their original diseases or of complications of therapy after 28 days of life. This delayed mortality and mortality caused by acquired illnesses occur during the postneonatal period, which begins after 28 days of life and extends to the end of the first year of life.
The infant mortality rate encompasses the neonatal and the postneonatal periods. In the United States, it declined to 6.7:1000 in 2006. The rate for African-American infants was approximately 13:6000. The most common causes of perinatal and neonatal death are listed in Table 58-1. Overall, congenital anomalies and diseases of the premature infant are the most significant causes of neonatal mortality.
TABLE 58-1 Major Causes of Perinatal and Neonatal Mortality
FETUS
Abruptio placentae
Chromosomal anomalies
Congenital malformations (heart, CNS, renal)
Hydrops fetalis
Intrauterine asphyxia*
Intrauterine infection*
Maternal underlying disease (chronic hypertension, autoimmune disease, diabetes mellitus)
Multiple gestation*
Placental insufficiency*
Umbilical cord accident
PRETERM INFANT
Respiratory distress syndrome/bronchopulmonary dysplasia (chronic lung disease)*
Severe immaturity*
Congenital anomalies
Infection
Intraventricular hemorrhage*
Necrotizing enterocolitis
FULL-TERM INFANT
Birth asphyxia*
Birth trauma
Congenital anomalies*
Infection*
Macrosomia
Meconium aspiration pneumonia
Persistent pulmonary hypertension
CNS, central nervous system.
Low birth weight (LBW) infants, defined as infants having birth weights of less than 2500 g, represent a disproportionately large component of the neonatal and infant mortality rates. Although LBW infants make up only about 6% to 7% of all births, they account for more than 70% of neonatal deaths. IUGR is the most common cause of LBW in developing countries, whereas prematurity is the cause in developed countries.
Very low birth weight (VLBW) infants, weighing less than 1500 g at birth, represent about 1% of all births but account for 50% of neonatal deaths. Compared with infants weighing 2500 g or more, LBW infants are 40 times more likely to die in the neonatal period; VLBW infants have a 200-fold higher risk of neonatal death. The LBW rate has not improved in recent years and is one of the major reasons that the U.S. infant mortality rate is high compared with other large, modern, industrialized countries.
Maternal factors associated with a LBW caused by premature birth or IUGR include a previous LBW birth, low socioeconomic status, low level of maternal education, no antenatal care, maternal age younger than 16 years or older than 35 years, short interval between pregnancies, cigarette smoking, alcohol and illicit drug use, physical (excessive standing or walking) or psychological (poor social support) stresses, unmarried status, low prepregnancy weight (<45 kg), poor weight gain during pregnancy (<10 lb), and African-American race. LBW and VLBW rates for African-American women are twice the rates for white women. The neonatal and infant mortality rates are twofold higher among African-American infants. These racial differences are only partly explained by poverty.
In addition to the sociodemographic variables associated with LBW, specific, identifiable medical causes of preterm birth exist. Factors such as uterine anomalies, hydrops fetalis, and most medical illnesses are not seen more frequently in African Americans or in patients of lower socioeconomic status. Prematurity may be caused by spontaneous labor (50% of cases), spontaneous rupture of membranes (25%), or premature delivery for maternal or fetal indications (25%).
ASSESSMENT OF THE FETUS
Fetal size can be determined accurately by ultrasound techniques. Fetal growth can be assessed by determining the fundal height of the uterus through bimanual examination of the gravid abdomen. Ultrasound measurements of the fetal biparietal diameter, femur length, and abdominal circumference are used to estimate fetal growth. A combination of these measurements predicts fetal weight. Deviations from the normal fetal growth curve are associated with high-risk conditions.
IUGR is present when fetal growth stops and, over time, declines to less than the fifth percentile of growth for gestational age or when growth proceeds slowly, but absolute size remains less than the fifth percentile. Growth restriction may result from fetal conditions that reduce the innate growth potential, such as fetal rubella infection, primordial dwarfing syndromes, chromosomal abnormalities, and congenital malformation syndromes. Reduced fetal production of insulin and insulin-like growth factor I is associated with fetal growth restriction. Placental causes of IUGR include villitis (congenital infections), placental tumors, chronic abruptio placentae, twin-to-twin transfusion syndrome, and placental insufficiency. Maternal causes include severe peripheral vascular diseases that reduce uterine blood flow (chronic hypertension, diabetic vasculopathy, and preeclampsia/eclampsia), reduced nutritional intake, alcohol or drug abuse, cigarette smoking, and uterine constraint (noted predominantly in mothers of small stature with a low prepregnancy weight) and reduced weight gain during pregnancy. The outcome of IUGR depends on the cause of the reduced fetal growth and the associated complications after birth (Table 58-2). Fetuses subjected to chronic intrauterine hypoxia as a result of uteroplacental insufficiency are at an increased risk for the comorbidities of birth asphyxia, polycythemia, and hypoglycemia. Fetuses with reduced tissue mass due to chromosomal, metabolic, or multiple congenital anomaly syndromes have poor outcomes based on the prognosis for the particular syndrome. Fetuses born to small mothers and fetuses with poor nutritional intake usually show catch-up growth after birth.
TABLE 58-2 Problems of Intrauterine Growth Restriction and Small for Gestational Age
| Problem* | Pathogenesis |
|---|---|
| Intrauterine fetal demise | Placental insufficiency, hypoxia, acidosis, infection, lethal anomaly |
| Temperature instability | Cold stress, ↓ fat stores, hypoxia, hypoglycemia |
| Perinatal asphyxia | ↓ Uteroplacental perfusion during labor with or without chronic fetal hypoxia-acidosis, meconium aspiration syndrome |
| Hypoglycemia | ↓ Tissue glycogen stores; ↓ gluconeogenesis, hyperinsulinism, ↑ glucose needs of hypoxia, hypothermia, relatively large brain |
| Polycythemia-hyperviscosity | Fetal hypoxia with ↑ erythropoietin production |
| Reduced oxygen consumption/hypothermia | Hypoxia, hypoglycemia, starvation effect, poor subcutaneous fat stores |
| Dysmorphology | Syndrome anomalads, chromosomal-genetic disorders, oligohydramnios-induced deformations |
| Pulmonary hemorrhage | Hypothermia, polycythemia, hypoxia |
* Other problems are common to the gestational age–related risks of prematurity if born before 37 weeks.
Modified from Stoll BJ, Kliegman RM: The high-risk infant. In Behrman RE, Kliegman RM, Jenson HB, editors: Nelson Textbook of Pediatrics, 16th ed, Philadelphia, 2000, Saunders.
Fetal size does not always correlate with functional or structural maturity. Determining fetal maturity is crucial when making a decision to deliver a fetus because of fetal or maternal disease. Fetal gestational age may be determined accurately on the basis of a correct estimate of the last menstrual period. Clinically relevant landmark dates can be used to determine gestational age; the first audible heart tones by fetoscope are detected at 18 to 20 weeks (12 to 14 weeks by Doppler methods), and quickening of fetal movements usually is perceived at 18 to 20 weeks. However, it is not always possible to determine fetal maturity by such dating, especially in a high-risk situation, such as preterm labor or a diabetic pregnancy.
Surfactant, a combination of surface-active phospholipids and proteins, is produced by the maturing fetal lung and eventually is secreted into the amniotic fluid. The amount of surfactant in amniotic fluid is a direct reflection of surface-active material in the fetal lung and can be used to predict the presence or absence of pulmonary maturity. Because phosphatidylcholine, or lecithin, is a principal component of surfactant, the determination of lecithin in amniotic fluid is used to predict a mature fetus. Lecithin concentration increases with increasing gestational age, beginning at 32 to 34 weeks.
Methods used to assess fetal well-being before the onset of labor are focused on identifying a fetus at risk for asphyxia or a fetus who already is compromised by uteroplacental insufficiency. The oxytocin challenge test simulates uterine contractions through an infusion of oxytocin sufficient to produce three contractions in a 10-minute period. The development of periodic fetal bradycardia out of phase with uterine contractions (late deceleration) is a positive test result and predicts an at-risk fetus.
The nonstress test examines the heart rate response to fetal body movements. Heart rate increases of more than 15 beats/min, lasting 15 seconds, are reassuring. If two such episodes occur in 30 minutes, the test result is considered reactive (versus nonreactive), and the fetus is not at risk. Additional signs of fetal well-being are fetal breathing movements, gross body movements, fetal tone, and the presence of amniotic fluid pockets of greater than 2 cm, detected by ultrasound. The biophysical profile combines the nonstress test with these four parameters and offers the most accurate fetal assessment.
Doppler examination of the fetal aorta or umbilical arteries permits identification of decreased or reversed diastolic blood flow associated with increased peripheral vascular resistance, fetal hypoxia with acidosis, and placental insufficiency. Cordocentesis (percutaneous umbilical blood sampling) can provide fetal blood for PO2, pH, lactate, and hemoglobin measurements to identify a hypoxic, acidotic, or anemic fetus who is at risk for intrauterine fetal demise or birth asphyxia. Cordocentesis also can be used to determine fetal blood type, platelet count, microbial culture, antibody titer, and rapid karyotype.
In a high-risk pregnancy, the fetal heart rate should be monitored continuously during labor, as should uterine contractions. Fetal heart rate abnormalities may indicate baseline tachycardia (>160 beats/min as a result of anemia, β-sympathomimetic drugs, maternal fever, hyperthyroidism, arrhythmia, or fetal distress), baseline bradycardia (<120 beats/min as a result of fetal distress, complete heart block, or local anesthetics), or reduced beat-to-beat variability (flattened tracing resulting from fetal sleep, tachycardia, atropine, sedatives, prematurity, or fetal distress). Periodic changes of the heart rate relative to uterine pressure help determine the presence of hypoxia and acidosis caused by uteroplacental insufficiency or maternal hypotension (late or type II decelerations) or by umbilical cord compression (variable decelerations). In the presence of severe decelerations (late or repeated prolonged variable), a fetal scalp blood gas level should be obtained to assess fetal acidosis. A scalp pH of less than 7.20 indicates fetal hypoxic compromise. A pH between 7.20 and 7.25 is in a borderline zone and warrants repeat testing.
Fetal anomalies may be detected by ultrasonography. Emphasis should be placed on visualization of the genitourinary tract; the head (for anencephaly or hydrocephaly), neck (for thickened nuchal translucency), and back (for spina bifida); skeleton; gastrointestinal tract; and heart. Four-chamber and great artery views are required for detection of heart anomalies. Chromosomal anomaly syndromes may reveal choroid cysts, hypoplasia of the middle phalanx of the fifth digit, nuchal fluid, retrognathism, and low-set ears. In addition, chromosomal syndromes often are associated with an abnormal “triple test” (low estriols, low maternal serum alpha-fetoprotein levels, and elevated placental chorionic gonadotropin levels). If a fetal abnormality is detected, fetal therapy or delivery with therapy in the neonatal intensive care unit may be lifesaving.
ASSESSMENT OF THE NEWBORN
The approach to the birth of an infant requires a detailed history (Table 58-3). Knowing the mother’s risk factors enables the delivery room team to anticipate problems that may occur after birth. The history of a woman’s labor and delivery can reveal events that might lead to complications affecting either the mother or the neonate, even when the pregnancy was previously considered low risk. Anticipating the need to resuscitate a newborn as a result of fetal distress increases the likelihood of successful resuscitation.
TABLE 58-3 Components of the Perinatal History
DEMOGRAPHIC SOCIAL INFORMATION
Age
Race
Sexually transmitted infections, hepatitis, AIDS
Illicit drugs, cigarettes, ethanol, cocaine
Immune status (syphilis, rubella, hepatitis B, blood group)
Occupational exposure
PAST MEDICAL DISEASES
Chronic hypertension
Heart disease
Diabetes mellitus
Thyroid disorders
Hematologic/malignancy
Collagen-vascular disease (SLE)
Genetic history—inborn errors of metabolism, bleeding, jaundice
Drug therapy
PRIOR PREGNANCY
Abortion
Intrauterine fetal demise
Congenital malformation
Incompetent cervix
Birth weight
Prematurity
Twins
Blood group sensitization/neonatal jaundice
Hydrops
Infertility
PRESENT PREGNANCY
Current gestational age
Method of assessing gestational age
Fetal surveillance (OCT, NST, biophysical profile)
Ultrasonography (anomalies, hydrops)
Amniotic fluid analysis (L/S ratio)
Oligohydramnios-polyhydramnios
Vaginal bleeding
Preterm labor
Premature (prolonged) rupture of membranes (duration)
Preeclampsia
Urinary tract infection
Colonization status (herpes simplex, group B streptococcus)
Medications/drugs
Acute medical illness/exposure to infectious agents
Fetal therapy
LABOR AND DELIVERY
Duration of labor
Presentation—vertex, breech
Vaginal versus cesarean section
Spontaneous labor versus augmented or induced with oxytocin (Pitocin)
Forceps delivery
Presence of meconium-stained fluid
Maternal fever/amnionitis
Fetal heart rate patterns (distress)
Scalp pH
Maternal analgesia, anesthesia
Nuchal cord
Apgar score/methods of resuscitation
Gestational age assessment
Growth status (AGA, LGA, SGA)
AGA, average for gestational age; AIDS, acquired immunodeficiency syndrome; LGA, large for gestational age; L/S, lecithin-to-sphingomyelin ratio; NST, nonstress test; OCT, oxytocin challenge test; SGA, small for gestational age; SLE, systemic lupus erythematosus.
The transition from fetal to neonatal physiology occurs at birth. Oxygen transport across the placenta results in a gradient between the maternal and fetal PaO2. Although fetal oxygenated blood has a low PaO2 level compared with that of adults and infants, the fetus is not anaerobic. Fetal oxygen uptake and consumption are similar to neonatal rates, even though the thermal environments and activity levels of fetuses and neonates differ. The oxygen content of fetal blood is almost equal to the oxygen content in older infants and children because fetal blood has a much higher concentration of hemoglobin.
Fetal hemoglobin (two alpha and two gamma chains) has a higher affinity for oxygen than adult hemoglobin, facilitating oxygen transfer across the placenta. The fetal hemoglobin-oxygen dissociation curve is shifted to the left of the adult curve (Fig. 58-1); at the same PaO2 level, fetal hemoglobin is more saturated than adult hemoglobin. Because fetal hemoglobin functions on the steep, lower end of the oxygen saturation curve (PaO2, 20 to 30 mm Hg), however, oxygen unloading to the tissue is not deficient. In contrast, at the higher oxygen concentrations present in the placenta, oxygen loading is enhanced. In the last trimester, fetal hemoglobin production begins to decrease as adult hemoglobin production begins to increase, becoming the only hemoglobin available to the infant by 3 to 6 months of life. At this time, the fetal hemoglobin dissociation curve has shifted to the adult position.
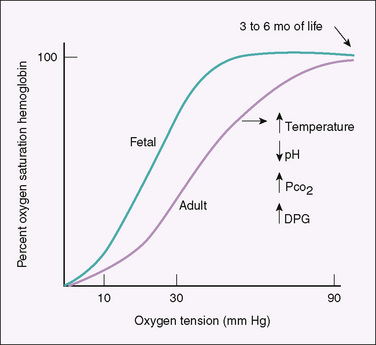
FIGURE 58-1 Hemoglobin-oxygen dissociation curves. The position of the adult curve depends on the binding of adult hemoglobin to 2,3-diphosphoglycerate (DPG), temperature, PCO2, and hydrogen ion concentration (pH).
A portion of well-oxygenated umbilical venous blood returning to the heart from the placenta perfuses the liver. The remainder bypasses the liver through a shunt (the ductus venosus) and enters the inferior vena cava. This oxygenated blood in the vena cava constitutes 65% to 70% of venous return to the right atrium. The crista dividens in the right atrium directs one third of this blood across the patent foramen ovale to the left atrium, where it subsequently is pumped to the coronary, cerebral, and upper extremity circulations by the left ventricle. Venous return from the upper body combines with the remaining two thirds of the vena caval blood in the right atrium and is directed to the right ventricle. This mixture of venous low-oxygenated blood from the upper and lower body enters the pulmonary artery. Only 8% to 10% of it is pumped to the pulmonary circuit; the remaining 80% to 92% of the right ventricular output bypasses the lungs through a patent ductus arteriosus and enters the descending aorta. The amount of blood flowing to the pulmonary system is low because vasoconstriction produced by medial muscle hypertrophy of the pulmonary arterioles and fluid in the fetal lung increases resistance to blood flow. Pulmonary artery tone also responds to hypoxia, hypercapnia, and acidosis with vasoconstriction, a response that may increase pulmonary vascular resistance further. The ductus arteriosus remains patent in the fetus because of low PaO2 levels and dilating prostaglandins. In utero, the right ventricle is the dominant ventricle, pumping 65% of the combined ventricular output, which is a high volume (450 mL/kg/min) compared with that pumped by an older infant’s right ventricle (200 mL/kg/min).
The transition of the circulation occurring between the fetal and neonatal periods involves the removal of the low-resistance circulation of the placenta, the onset of breathing, reduction of pulmonary arterial resistance, and closure of in utero shunts. Clamping the umbilical cord eliminates the low-pressure system of the placenta and increases systemic blood pressure. Decreased venous return from the placenta decreases right atrial pressure. As breathing begins, air replaces lung fluid, maintaining the functional residual capacity. Fluid leaves the lung, in part, through the trachea; it is either swallowed or squeezed out during vaginal delivery. The pulmonary lymphatic and venous systems reabsorb the remaining fluid.
Most normal infants require little pressure to spontaneously open the lungs after birth (5 to 10 cm H2O). A few infants require assistance with greater opening pressures (20 to 30 cm H2O). With the onset of breathing, pulmonary vascular resistance decreases, partly a result of the mechanics of breathing and partly a result of the elevated arterial oxygen tensions. The increased blood flow to the lungs increases the volume of pulmonary venous blood returning to the left atrium; left atrial pressure now exceeds right atrial pressure, and the foramen ovale closes. As the flow through the pulmonary circulation increases and arterial oxygen tensions rise, the ductus arteriosus begins to constrict. In a term infant, this constriction functionally closes the ductus arteriosus within 1 day after birth. A permanent closure requires thrombosis and fibrosis, a process that may take several weeks. In a premature infant, the ductus arteriosus is less sensitive to the effects of oxygen; if circulating levels of vasodilating prostaglandins are elevated, the ductus arteriosus may remain patent. This patency is a common problem in a premature infant with respiratory distress syndrome.
Ventilation, oxygenation, and normal pH and PCO2 levels immediately reduce pulmonary artery vasoconstriction by causing smooth muscle relaxation. Remodeling of the medial muscle hypertrophy begins at birth and continues for the next 3 months, resulting in a further reduction of pulmonary vascular resistance and a further increase of pulmonary blood flow. Persistence or aggravation of pulmonary vasoconstriction caused by acidosis, hypoxia, hypercapnia, hypothermia, polycythemia, asphyxia, shunting of blood from the lungs, or pulmonary parenchymal hypoplasia results in persistent pulmonary hypertension of the newborn (PPHN). Failure to replace pulmonary alveolar fluid completely with air can lead to respiratory distress (transient tachypnea of the newborn).
Routine Delivery Room Care and Resuscitation
Silver nitrate (1%) instilled into both eyes without being washed out is an indicated effective therapy for the prevention of neonatal gonococcal ophthalmia, which can result in severe panophthalmitis and subsequent blindness. Silver nitrate may produce a chemical conjunctivitis with a mucopurulent discharge and is not effective against C. trachomatis. Many hospitals use erythromycin drops to prevent neonatal gonococcal and chlamydial eye disease.
Bacterial colonization of a newborn may begin in utero if the fetal membranes have been ruptured. Most infants undergo colonization after birth and acquire the bacteria present in the mother’s genitourinary system, such as group B streptococci, staphylococci, E. coli, and clostridia. Colonization is common at the umbilicus, skin, nasopharynx, and intestine. Antiseptic skin or cord care is routine in most nurseries to prevent the spread of pathologic bacteria from one infant to another and to prevent disease in the individual infant. Staphylococcal bullous impetigo, omphalitis, diarrhea, and systemic disease may result from colonization with virulent S. aureus. For term infants, washing of the skin with 3% hexachlorophene is not routinely recommended but may prevent serious staphylococcal disease; preterm infants may absorb hexachlorophene, and neurotoxicity may develop. Triple antibiotic ointment (polymyxin B, neomycin, and bacitracin) or bacitracin may be applied to the umbilical cord to reduce its colonization with gram-positive bacteria. Epidemics of S. aureus nursery infections are managed with strict infectious disease control measures (cohorting, hand washing, and monitoring for colonization).
Vitamin K prophylaxis (intramuscular) should be given to all infants to prevent hemorrhagic disease of the newborn. Before discharge, infants should receive the hepatitis B vaccine and be screened for various diseases (Tables 58-4 and 58-5).
TABLE 58-4 Approximate Frequencies of Disorders Included in or Considered for Newborn Screening in the United States
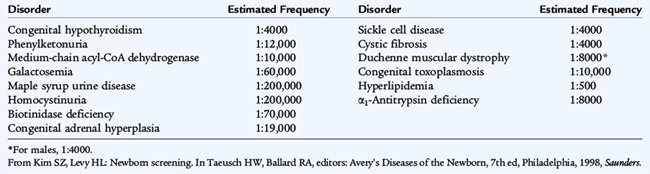
TABLE 58-5 Abnormal Newborn Screening Results: Possible Implications and Initial Action to Be Taken
| Newborn Screening Finding | Differential Diagnosis | Initial Action |
|---|---|---|
| ↑ Phenylalanine | PKU, non-PKU hyperphenylalaninemia, pterin defect, galactosemia, transient hyperphenylalaninemia | Repeat blood specimen |
| ↓ T4, ↑ TSH | Congenital hypothyroidism, iodine exposure | Repeat blood specimen or thyroid function testing, begin thyroxine treatment |
| ↓ T4, normal TSH | Maternal hyperthyroidism, thyroxine-binding globulin deficiency, secondary hypothyroidism, congenital hypothyroidism with delayed TSH elevation | Repeat blood specimen |
| ↑ Galactose (1-P) transferase | Galactosemia, liver disease, reducing substance, repeat deficiency variant (Duarte), transient | Clinical evaluation, urine for blood specimen. If reducing substance positive, begin lactose-free formula |
| ↓ Galactose-1-phosphate uridyltransferase | Galactosemia, transferase deficiency variant (Duarte), transient | Clinical evaluation, urine for reducing substance, repeat blood specimen. If reducing substance positive, begin lactose-free formula |
| ↑ Methionine | Homocystinuria, isolated liver dysfunction, tyrosinemia type I, transient hypermethioninemia | Repeat blood and urine specimen |
| ↑ Leucine | Maple syrup urine disease, transient elevation | Clinical evaluation including urine for ketones, acid-base status, amino acid studies, immediate neonatal ICU care if urine ketones positive |
| ↑ Tyrosine | Tyrosinemia type I or type II, transient tyrosinemia, liver disease | Repeat blood specimen |
| ↑ 17α-Hydroxyprogesterone | Congenital adrenal hyperplasia, prematurity, transient (residual fetal adrenal cortex), stress in neonatal period, early specimen collection | Clinical evaluation including genital examination, serum electrolytes, repeat blood specimen |
| S-hemoglobin | Sickle cell disease, sickle cell trait | Hemoglobin electrophoresis |
| ↑ Trypsinogen | Cystic fibrosis, transient, intestinal anomalies, perinatal stress, trisomies 13 and 18, renal failure | Repeat blood specimen, possible sweat test and DNA testing |
| ↑ Creatinine phosphokinase | Duchenne muscular dystrophy, other type of muscular dystrophy, birth trauma, invasive procedure | Repeat blood test |
| ↓ Biotinidase | Biotinidase deficiency | Serum biotinidase assay, biotin therapy |
| ↓ G6PD | G6PD deficiency | Complete blood count, bilirubin determination |
| ↓ α1-Antitrypsin | α1-Antitrypsin deficiency | Confirmatory test |
| Toxoplasma antibody (IgM) | Congenital toxoplasmosis | Infectious disease consultation |
| HIV antibody (IgG) | Maternally transmitted HIV, possible AIDS | Infectious disease consultation |
| ↑ Organic acids | Fatty acid oxidation defects (medium-chain acyl-CoA dehydrogenase deficiency) | Perform specific assay (tandem mass spectroscopy); frequent feeds |
G6PD, glucose-6-phosphate dehydrogenase; ICU, intensive care unit; PKU, phenylketonuria; T4, thyroxine; TSH, thyroid-stimulating hormone.
From Kim SZ, Levy HL: Newborn screening. In Taeusch HW, Ballard RA, editors: Avery’s Diseases of the Newborn, 7th ed, Philadelphia, 1998, Saunders.
Fetal or neonatal hypoxia, hypercapnia, poor cardiac output, and a metabolic acidosis can result from numerous conditions affecting the fetus, the placenta, or the mother. Whether in utero or after birth, asphyxia-caused hypoxic-ischemic brain injury is the result of reduced gaseous exchange through the placenta or through the lungs. Asphyxia associated with severe bradycardia or cardiac insufficiency reduces or eliminates tissue blood flow, resulting in ischemia. The fetal and neonatal circulatory systems respond to reduced oxygen availability by shunting the blood preferentially to the brain, heart, and adrenal glands and away from the intestine, kidneys, lungs, and skin.
Metabolic acidosis during asphyxia is caused by the combined effects of poor cardiac output secondary to hypoxic depression of myocardial function, systemic hypoxia, and tissue anaerobic metabolism. With severe or prolonged intrauterine or neonatal asphyxia, multiple vital organs are affected (Table 58-6).
TABLE 58-6 Effects of Asphyxia
| System | Effect |
|---|---|
| Central nervous system | Hypoxic-ischemic encephalopathy, IVH, PVL, cerebral edema, seizures, hypotonia, hypertonia |
| Cardiovascular | Myocardial ischemia, poor contractility, tricuspid insufficiency, hypotension |
| Pulmonary | Persistent pulmonary hypertension, respiratory distress syndrome |
| Renal | Acute tubular or cortical necrosis |
| Adrenal | Adrenal hemorrhage |
| Gastrointestinal | Perforation, ulceration, necrosis |
| Metabolic | Inappropriate ADH, hyponatremia, hypoglycemia, hypocalcemia, myoglobinuria |
| Integument | Subcutaneous fat necrosis |
| Hematology | Disseminated intravascular coagulation |
ADH, antidiuretic hormone; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia.
Many conditions that contribute to fetal or neonatal asphyxia are the same medical or obstetric problems associated with high-risk pregnancy (Table 58-7). Maternal diseases that interfere with uteroplacental perfusion (chronic hypertension, preeclampsia, and diabetes mellitus) place the fetus at risk for intrauterine asphyxia. Maternal epidural anesthesia and the development of the vena caval compression syndrome may produce maternal hypotension, which decreases uterine perfusion. Maternal medications given to relieve pain during labor may cross the placenta and depress the infant’s respiratory center, resulting in apnea at the time of birth.
TABLE 58-7 Etiology of Birth Asphyxia
| Type | Example |
|---|---|
| INTRAUTERINE | |
| Hypoxia-ischemia | Uteroplacental insufficiency, abruptio placentae, prolapsed cord, maternal hypotension, unknown |
| Anemia-shock | Vasa previa, placenta previa, fetomaternal hemorrhage, erythroblastosis |
| INTRAPARTUM | |
| Birth trauma | Cephalopelvic disproportion, shoulder dystocia, breech presentation, spinal cord transection |
| Hypoxia-ischemia | Umbilical cord compression, tetanic contraction, abruptio placentae |
| POSTPARTUM | |
| Central nervous system | Maternal medication, trauma, previous episodes of fetal hypoxia–acidosis |
| Congenital neuromuscular disease | Congenital myasthenia gravis, myopathy, myotonic dystrophy |
| Infection | Consolidated pneumonia, shock |
| Airway disorder | Choanal atresia, severe obstructing goiter, laryngeal webs |
| Pulmonary disorder | Severe immaturity, pneumothorax, pleural effusion, diaphragmatic hernia, pulmonary hypoplasia |
| Renal disorder | Pulmonary hypoplasia, pneumothorax |
Fetal conditions associated with asphyxia usually do not become manifested until delivery, when the infant must initiate and sustain ventilation. In addition, the upper and lower airways must be patent and unobstructed. Alveoli must be free from foreign material, such as meconium, amniotic fluid debris, and infectious exudates, which increases airway resistance, reduces lung compliance, and leads to respiratory distress and hypoxia. Some extremely immature infants weighing less than 1000 g at birth may be unable to expand their lungs, even in the absence of other pathology. Their compliant chest walls and surfactant deficiency may result in poor air exchange, retractions, hypoxia, and apnea.
The newborn (particularly a preterm infant) responds paradoxically to hypoxia with apnea rather than tachypnea as occurs in adults. Episodes of intrauterine asphyxia also may depress the neonatal central nervous system. If recovery of the fetal heart rate occurs as a result of improved uteroplacental perfusion, fetal hypoxia and acidosis may resolve. Nonetheless, if the effect on the respiratory center is more severe, a newborn may not initiate an adequate ventilatory response at birth and may undergo another episode of asphyxia.
The Apgar examination, a rapid scoring system based on physiologic responses to the birth process, is a good method for assessing the need to resuscitate a newborn (Table 58-8). At intervals of 1 minute and 5 minutes after birth, each of the five physiologic parameters is observed or elicited by a qualified examiner. Full-term infants with a normal cardiopulmonary adaptation should score 8 to 9 at 1 and 5 minutes. Apgar scores of 4 to 7 warrant close attention to determine whether the infant’s status will improve and to ascertain whether any pathologic condition is contributing to the low Apgar score.
By definition, an Apgar score of 0 to 3 represents either a cardiopulmonary arrest or a condition caused by severe bradycardia, hypoventilation, or central nervous system depression. Most low Apgar scores are caused by difficulty in establishing adequate ventilation and not by primary cardiac pathology. In addition to an Apgar score of 0 to 3, most infants with asphyxia severe enough to cause neurologic injury also manifest fetal acidosis (pH <7); seizures, coma, or hypotonia; and multiorgan dysfunction. Low Apgar scores may be caused by fetal hypoxia or other factors listed in Table 58-7. Most infants with low Apgar scores respond to assisted ventilation by face mask or by endotracheal intubation and usually do not need emergency medication.
Resuscitation of a newborn with a low Apgar score follows the same systematic sequence as that for resuscitation of older patients, but in the newborn period this simplified ABCD approach requires some qualification (Fig. 58-2). In the ABCD approach, A stands for securing a patent airway by clearing amniotic fluid or meconium by suctioning; A is also a reminder of anticipation and the need for knowing the events of pregnancy, labor, and delivery. Evidence of a diaphragmatic hernia and a low Apgar score indicate that immediate endotracheal intubation is required. If a mask and bag are used, gas enters the lung and the stomach, and the latter may act as an expanding mass in the chest that compromises respiration. If fetal hydrops has occurred with pleural effusions, a bilateral thoracentesis to evacuate the pleural effusions may be needed to establish adequate ventilation.
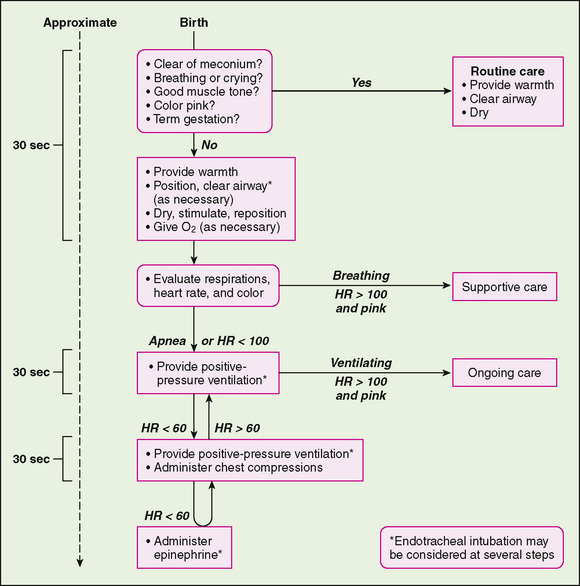
FIGURE 58-2 Algorithm for resuscitation of the newborn infant. HR, heart rate.
(From National guidelines for neonatal resuscitation. Pediatrics 106:E29, 2000.)
B represents breathing. If the neonate is apneic or hypoventilates and remains cyanotic, artificial ventilation should be initiated. Ventilation should be performed with a well-fitted mask attached to an anesthesia bag and a manometer to prevent extremely high pressures from being given to the newborn; 100% oxygen should be administered through the mask. If the infant does not revive, an endotracheal tube should be placed, attached to the anesthesia bag and manometer, and 100% oxygen should be administered. The pressure generated should begin at 20 to 25 cm H2O, with a rate of 40 to 60 breaths/min. An adequate response to ventilation includes good chest rise, return of breath sounds, well-oxygenated color, heart rate returning to the normal range (120 to 160 beats/min), normal end-tidal carbon dioxide, and, later, increased muscle activity and wakefulness. The usual recovery after a cardiac arrest first involves a return to a normal heart rate followed by disappearance of cyanosis, and noticeably improved perfusion. An infant may remain limp and be apneic for a prolonged time after return of cardiac output and correction of acidosis.
Breathing initially should be briefly delayed if meconium-stained amniotic fluid is present to avoid dissemination of meconium into the lungs, producing severe aspiration pneumonia. If meconium is noted in the amniotic fluid, the oropharynx should be suctioned when the head is delivered. After the birth of a depressed infant, the oral cavity should be suctioned again; the vocal cords should be visualized and the infant intubated, with suction applied while the tube is below the vocal cords. If meconium is noted below the cords, intubation should be repeated quickly to clear the remaining meconium. During this time, the infant should not be stimulated to breathe, and positive-pressure ventilation should not be applied.
C represents circulation and external cardiac massage. If artificial ventilation does not improve bradycardia, if asystole is present, or if peripheral pulses cannot be palpated, external cardiac massage should be performed at a rate of 120 compressions/min with compressions and breaths given at a ratio of 3:1. External cardiac massage usually is not needed because most infants in the delivery room respond to ventilation.
D represents the administration of drugs. If bradycardia is unresponsive to ventilation or if asystole is present, resuscitation drugs should be administered. Intravenous (IV) epinephrine (1:10,000), 0.1 to 0.3 mL/kg, should be given through an umbilical venous line or injected into the endotracheal tube. Additional medications for resuscitation include 2 mEq/kg of IV sodium bicarbonate if acidosis is prolonged and a rapid infusion of fluids (normal saline, or O-negative red blood cells if anemia is present) if poor perfusion suggests hypovolemia. Sodium bicarbonate should be given only if severe metabolic acidosis is suspected or is proven by blood gas analysis. Sodium bicarbonate should not be given unless the lungs are adequately ventilated. Before medications are administered in the presence of electrical cardiac activity with poor pulses, it is important to determine whether there is a pneumothorax. Transillumination of the thorax, involving the use of a bright light through each side of the thorax and over the sternum, may suggest pneumothorax if one side transmits more light than the other. Breath sounds may be decreased over a pneumothorax and there may be a shift of the heart tones away from the side of a tension pneumothorax.
If central nervous system depression in the infant may be due to a narcotic medication given to the mother, 0.1 mg/kg of naloxone (Narcan) can be given to the infant intravenously or endotracheally. Before this drug is administered, the ABCs should be followed carefully. Naloxone should not be given to a newborn of a mother who is suspected of being addicted to narcotics or is on methadone maintenance because the newborn may experience severe withdrawal seizures.
Physical Examination and Gestational Age Assessment
The first physical examination of a newborn may be a general physical examination of a well infant, an examination to confirm fetal diagnoses or to determine the cause of various manifestations of neonatal diseases. Problems in the transition from fetal to neonatal life may be detectable immediately in the delivery room or during the first day of life. Physical examination also may reveal effects of the labor and delivery resulting from asphyxia, drugs, or birth trauma. The first newborn examination is an important way to detect congenital malformations or deformations (Table 58-9). Significant congenital malformations may be present in 1% to 3% of all births.
TABLE 58-9 Life-Threatening Congenital Anomalies
| Name | Manifestations |
|---|---|
| Choanal atresia (stenosis) | Respiratory distress in delivery room, apnea, unable to pass nasogastric tube through nares |
| Pierre Robin syndrome | Micrognathia, cleft palate, airway obstruction |
| Diaphragmatic hernia | Scaphoid abdomen, bowel sounds present in left chest, heart shifted to right, respiratory distress, polyhydramnios |
| Tracheoesophageal fistula | Polyhydramnios, aspiration pneumonia, excessive salivation, unable to place nasogastric tube in stomach |
| Intestinal obstruction: volvulus, duodenal atresia, ileal atresia | Polyhydramnios, bile-stained emesis, abdominal distention |
| Gastroschisis/omphalocele | Polyhydramnios; intestinal obstruction |
| Renal agenesis/Potter syndrome | Oligohydramnios, anuria, pulmonary hypoplasia, pneumothorax |
| Hydronephrosis | Bilateral abdominal masses |
| Neural tube defects: anencephalus, meningomyelocele | Polyhydramnios, elevated α-fetoprotein; decreased fetal activity |
| Down syndrome (trisomy 21) | Hypotonia, congenital heart disease, duodenal atresia |
| Ductal-dependent congenital heart disease | Cyanosis, murmur, shock |
Appearance
The general appearance of the infant should be evaluated first. Signs such as cyanosis, nasal flaring, intercostal retractions, and grunting suggest pulmonary disease. Meconium staining of the umbilical cord, nails, and skin suggest fetal distress and the possibility of aspiration pneumonia. The level of spontaneous activity, passive muscle tone, quality of the cry, and apnea are useful screening signs to evaluate the state of the nervous system.
Vital Signs
The examination should proceed with an assessment of vital signs, particularly heart rate (normal rate, 120 to 160 beats/min); respiratory rate (normal rate, 30 to 60 breaths/min); temperature (usually done per rectum and later as an axillary measurement); and blood pressure (often reserved for sick infants). Length, weight, and head circumference should be measured and plotted on growth curves to determine whether growth is normal, accelerated, or retarded for the specific gestational age.
Gestational Age
Gestational age is determined by an assessment of various physical signs (Fig. 58-3) and neuromuscular characteristics (Fig. 58-4) that vary according to fetal age and maturity. Physical criteria mature with advancing fetal age, including increasing firmness of the pinna of the ear; increasing size of the breast tissue; decreasing fine, immature lanugo hair over the back; and decreasing opacity of the skin. Neurologic criteria mature with gestational age, including increasing flexion of the legs, hips, and arms; increasing tone of the flexor muscles of the neck; and decreasing laxity of the joints. These signs are determined during the first day of life and are assigned scores. The cumulative score is correlated with a gestational age, which is usually accurate to within 2 weeks (Fig. 58-5).
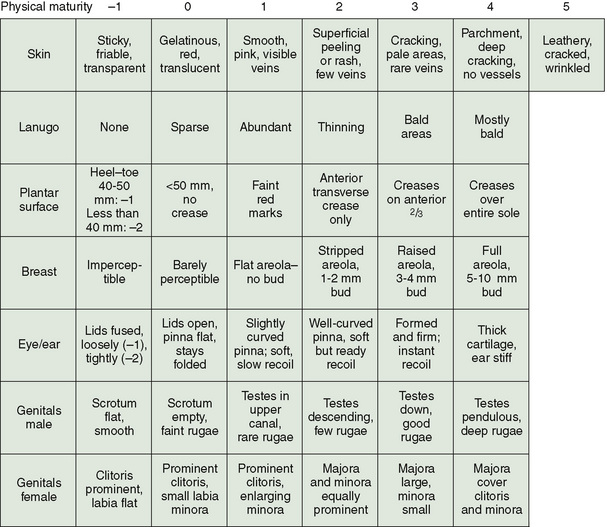
FIGURE 58-3 Physical criteria for assessment of maturity and gestational age. Expanded New Ballard Score (NBS) includes extremely premature infants and has been refined to improve accuracy in more mature infants.
(From Ballard JL, et al: New Ballard Score, expanded to include extremely premature infants. J Pediatr 119:417, 1991.)
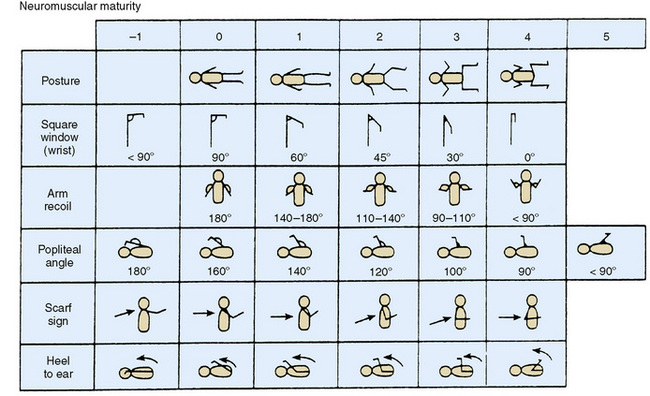
FIGURE 58-4 Neuromuscular criteria for assessment of maturity and gestational age. Expanded New Ballard Score (NBS) includes extremely premature infants and has been refined to improve accuracy in more mature infants.
(From Ballard JL, et al: New Ballard Score, expanded to include extremely premature infants. J Pediatr 119:417, 1991).
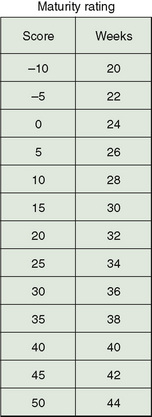
FIGURE 58-5 Maturity rating as calculated by adding the physical and neurologic scores, calculating the gestational age.
(From Ballard JL, et al: New Ballard Score, expanded to include extremely premature infants. J Pediatr 119:417, 1991).
Gestational age assessment permits the detection of abnormal fetal growth patterns, aiding in predicting the neonatal complications of largeness or smallness for gestational age (Fig. 58-6). Infants born at a weight greater than the 90th percentile for age are considered large for gestational age. Among the risks associated with being large for gestational age are all the risks of the infant of a diabetic mother and risks associated with postmaturity. Infants born at a weight less than 10th percentile for age (some growth curves use <2 standard deviations or the fifth percentile) are small for gestational age and have IUGR. Problems associated with small for gestational age infants include congenital malformations, in addition to the problems listed in Table 58-2.
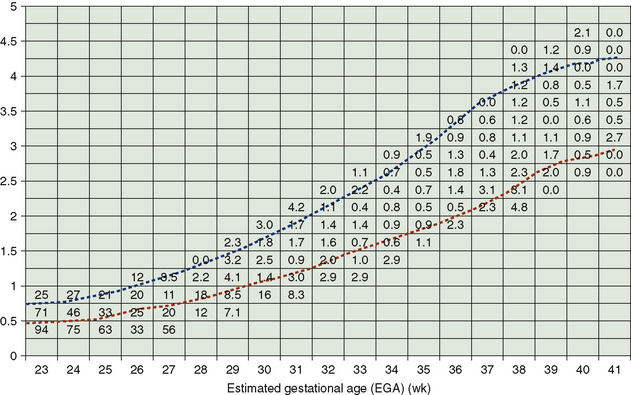
FIGURE 58-6 Birth weight–specific and estimated gestational age–specific mortality rates. The dashed lines of the figure represent the 10th and 90th percentile weights. The grid lines are plotted by each gestational age and in 250-g weight increments. Each number in the box is the percent mortality rate for the grid defined by gestational age and birth weight range.
(From Thomas P, Peabody J, Turnier V, et al: A new look at intrauterine growth and impact of race, attitude, and gender. Pediatrics 106:E21, 2000.)
Skin
The skin should be evaluated for pallor, plethora, jaundice, cyanosis, meconium staining, petechiae, ecchymoses, congenital nevi, and neonatal rashes. Vasomotor instability with cutis marmorata, telangiectasia, phlebectasia (intermittent mottling with venous prominence), and acrocyanosis (feet and hands) is normal in a premature infant. Acrocyanosis also may be noted in a healthy term infant in the first days after birth. The harlequin color change is a striking, transient, but normal sign of vasomotor instability and divides the body from head to pubis, through the midline, into equal halves of pink and pale color.
The skin is covered with lanugo hair, which disappears by term gestation. Hair tufts over the lumbosacral spine suggest a spinal cord defect. Vernix caseosa, a soft, white, creamy layer covering the skin in preterm infants, disappears by term. Post-term infants often have peeling, parchment-like skin. Mongolian spots are transient, dark blue to black pigmented macules seen over the lower back and buttocks in 90% of African-American, Indian, and Asian infants. Nevus simplex (salmon patch), or pink macular hemangiomas, is common, usually transient, and noted on the back of the neck, eyelids, and forehead. Nevus flammeus, or port-wine stain, is seen on the face and should cause the examiner to consider Sturge-Weber syndrome (trigeminal angiomatosis, convulsions, and ipsilateral intracranial tram-line calcifications).
Congenital melanocytic nevi are pigmented lesions of varying size noted in 1% of neonates. Giant pigmented nevi are uncommon but have malignant potential. Capillary hemangiomas are raised, red lesions, whereas cavernous hemangiomas are deeper, blue masses. Both lesions increase in size after birth, then resolve when the child is 1 to 4 years of age. When enlarged, these hemangiomas may produce high-output heart failure or platelet trapping and hemorrhage. Erythema toxicum is an erythematous, papular-vesicular rash common in neonates that develops after birth and involves eosinophils in the vesicular fluid. Pustular melanosis, more common in African-American infants, may be seen at birth and consists of a small, dry vesicle on a pigmented brown macular base. Erythema toxicum and pustular melanosis are benign lesions, but may mimic more serious conditions, such as the vesicular rash of disseminated herpes simplex or the bullous eruption of S. aureus impetigo. Tzanck smear, Gram stain, Wright stain, direct fluorescent antibody stain, polymerase chain reaction for herpes DNA, and appropriate cultures may be needed to distinguish these rashes. Other common characteristic rashes are milia (yellow-white epidermal cysts of the pilosebaceous follicles that are noted on the nose) and miliaria (prickly heat), which is caused by obstructed sweat glands. Edema may be present in preterm infants, but also suggests hydrops fetalis, sepsis, hypoalbuminemia, or lymphatic disorders.
Skull
The skull may be elongated and molded after a prolonged labor, which resolves 2 to 3 days after birth. The sutures should be palpated to determine the width and the presence of premature fusion or cranial synostosis. The anterior and posterior fontanelles should be soft and nonbulging, with the anterior larger than the posterior. A large fontanelle is associated with hydrocephalus, hypothyroidism, rickets, and other disorders. Soft areas away from the fontanelle are craniotabes; these lesions feel like a ping-pong ball when they are palpated. They may be a result of in utero compression. The skull should be examined carefully for signs of trauma or lacerations from internal fetal electrode sites or fetal scalp pH sampling; abscess formation may develop in these areas.
Face, Eyes, and Mouth
The face should be inspected for dysmorphic features, such as epicanthal folds, hypertelorism, preauricular tags or sinuses, low-set ears, long philtrum, and cleft lip or palate. Facial asymmetry may be a result of seventh nerve palsy; head tilt may be caused by torticollis.
The eyes should open spontaneously, especially in an upright position. Before 28 weeks’ gestational age, the eyelids may be fused. Coloboma, megalocornea, and microphthalmia suggest other malformations or intrauterine infections. A cloudy cornea greater than 1 cm in diameter also may be seen in congenital glaucoma, uveal tract dysgenesis, and storage diseases. Conjunctival and retinal hemorrhages are common and usually of no significance. The pupillary response to light is present at 28 weeks of gestation. The red reflex of the retina is shown easily. A white reflex, or leukokoria, is abnormal and may be the result of cataracts, ocular tumor, severe chorioretinitis, persistent hyperplastic primary vitreous, or retinopathy of prematurity.
The mouth should be inspected for the presence of natal teeth, clefts of the soft and hard palate and uvula, and micrognathia. A bifid uvula suggests a submucosal cleft. White, shiny, multiple transient epidermal inclusion cysts (Epstein pearls) on the hard palate are normal. Hard, marble-sized masses in the buccal mucosa are usually transient idiopathic fat necrosis. The tympanic membranes are dull, gray, opaque, and immobile in the first 1 to 4 weeks. These findings should not be confused with otitis media.
Neck and Chest
The neck appears short and symmetrical. Abnormalities include midline clefts or masses caused by thyroglossal duct cysts or by goiter and lateral neck masses (or sinuses), which are the result of branchial clefts. Cystic hygromas and hemangiomas may be present. Shortening of the sternocleidomastoid muscle with a fibrous tumor over the muscle produces head tilt and asymmetrical facies (neonatal torticollis). Arnold-Chiari malformation and cervical spine lesions also produce torticollis. Edema and webbing of the neck suggest Turner syndrome. Both clavicles should be palpated for fractures.
Examination of the chest includes inspection of the chest wall to identify asymmetry resulting from absence of the pectoralis muscle and inspection of the breast tissue to determine gestational age and detect a breast abscess. Boys and girls may have breast engorgement and produce milk; milk expression should not be attempted. Supernumerary nipples may be bilateral and may be associated with renal anomalies.
Lungs
Examination of the lungs includes observations of the rate, depth, and nature of intercostal or sternal retractions. Breath sounds should be equal on both sides of the chest, and rales should not be heard after the first 1 to 2 hours of life. Diminished or absent breath sounds on one side suggest pneumothorax, collapsed lung, pleural effusion, or diaphragmatic hernia. Shift of the cardiac impulse away from a tension pneumothorax and diaphragmatic hernia and toward the collapsed lung is a helpful physical finding for differentiating these disorders. Subcutaneous emphysema of the neck or chest also suggests a pneumothorax or pneumomediastinum, whereas bowel sounds auscultated in the chest in the presence of a scaphoid abdomen suggest a diaphragmatic hernia.
Heart
The position of the heart in infants is more midline than in older children. The first heart sound is normal, whereas the second heart sound may not be split in the first day of life. Decreased splitting of the second heart sound is noted in PPHN, transposition of the great vessels, and pulmonary atresia. Heart murmurs in newborns are common in the delivery room and during the first day of life. Most of these murmurs are transient and are due to closure of the ductus arteriosus, peripheral pulmonary artery stenosis, or a small ventral septal defect. Pulses should be palpated in the upper and lower extremities (over the brachial and femoral arteries). Blood pressure in the upper and lower extremities should be measured in all patients with a murmur or heart failure. An upper-to-lower extremity gradient of more than 10 to 20 mm Hg suggests coarctation of the aorta.
Abdomen
The liver may be palpable 2 cm below the right costal margin. The spleen tip is less likely to be palpable. A left-sided liver suggests situs inversus and asplenia syndrome. Both kidneys should be palpable in the first day of life with gentle, deep palpation. The first urination occurs during the first day of life in more than 95% of normal term infants.
Abdominal masses usually represent hydronephrosis or dysplastic-multicystic kidney disease. Less often, masses indicate ovarian cysts, intestinal duplication, neuroblastoma, or mesoblastic nephroma. Masses should be evaluated immediately with ultrasound. Abdominal distention may be caused by intestinal obstructions, such as ileal atresia, meconium ileus, midgut volvulus, imperforate anus, or Hirschsprung disease. Meconium stool is passed normally within 48 hours of birth in 99% of term infants. The anus should be patent. An imperforate anus is not always visible; the first temperature taken with a rectal thermometer should be taken carefully. The abdominal wall musculature may be absent, as in prune-belly syndrome, or weak, resulting in diastasis recti. Umbilical hernias are common in African-American infants. The umbilical cord should be inspected to determine the presence of two arteries and one vein and the absence of an urachus or a herniation of abdominal contents, as occurs with an omphalocele. The latter is associated with extraintestinal problems, such as genetic trisomies and hypoglycemia (Beckwith-Wiedemann syndrome). Bleeding from the cord suggests a coagulation disorder, and a chronic discharge may be a granuloma of the umbilical stump or, less frequently, a draining omphalomesenteric cyst. Erythema around the umbilicus is omphalitis and may cause portal vein thrombophlebitis and subsequent extrahepatic portal hypertension. The herniation of bowel through the abdominal wall 2 to 3 cm lateral to the umbilicus is a gastroschisis.
Genitalia
The appearance of the genitalia varies with gestational age. At term, the testes should be descended into a well-formed pigmented and rugated scrotum. The testes occasionally are in the inguinal canal; this is more common among preterm infants, as is cryptorchidism. Scrotal swelling may represent a hernia, transient hydrocele, in utero torsion of the testes, or, rarely, dissected meconium from meconium ileus and peritonitis. Hydroceles are clear and readily seen by transillumination, whereas testicular torsion in the newborn may present as a painless, dark swelling. The urethral opening should be at the end of the penis. Epispadias or hypospadias alone should not raise concern about pseudohermaphroditism. However, if no testes are present in the scrotum and hypospadias is present, problems of sexual development should be suspected (see Chapter 177). Circumcision should be deferred with hypospadias because the foreskin is often needed for the repair. The normal prepuce is often too tight to retract in the neonatal period.
The female genitalia normally may reveal a milky white or blood-streaked vaginal discharge as a result of maternal hormone withdrawal. Mucosal tags of the labia majora are common. Distention of an imperforate hymen may produce hydrometrocolpos and a lower midline abdominal mass as a result of an enlarged uterus. Clitoral enlargement with fusion of the labial-scrotal folds (labia majora) suggests adrenogenital syndrome or exposure to masculinizing maternal hormones.
Extremities
Examination of the extremities should involve assessment of length, symmetry, and presence of hemihypertrophy, atrophy, polydactyly, syndactyly, simian creases, absent fingers, overlapping fingers, rocker-bottom feet, clubfoot, congenital bands, fractures, and amputations.
Spine
The spine should be examined for evidence of sacral hair tufts, a dermal sinus tract above the gluteal folds, congenital scoliosis (a result of hemivertebra), and soft tissue masses such as lipomas or meningomyeloceles.
Hips
The hips should be examined for congenital dysplasia (dislocation). Gluteal fold asymmetry or leg length discrepancy is suggestive of dysplasia, but the examiner should perform the Barlow test and the Ortolani maneuver to evaluate the stability of the hip joint. These tests determine whether the femoral head can be displaced from the acetabulum (Barlow test) and then replaced (Ortolani maneuver) (see Chapter 198). The examiner’s long finger is placed over the greater trochanter, and the thumb is placed medially, just distal to the long finger. With the thighs held in midabduction, the examiner attempts to pull the femoral head gently out of the acetabulum with lateral pressure of the thumb and by rocking the knee medially. The reverse maneuver is performed by pressing the long finger on the greater trochanter and rocking the knee laterally. A clunking sensation is palpated when the femoral head leaves and returns to the acetabulum.
NEUROLOGIC ASSESSMENT
The neurologic examination should include assessment of active and passive tone, level of alertness, primary neonatal (primitive) reflexes, deep tendon reflexes, spontaneous motor activity, and cranial nerves (involving retinal examination, extraocular muscle movement, masseter power as in sucking, facial motility, hearing, and tongue function). The Moro reflex, present at birth and gone in 3 to 6 months, is one of the primary newborn reflexes. It is elicited by sudden, slight dropping of the supported head from a slightly raised supine position, which should elicit opening of the hands and extension and abduction of the arms, followed by upper extremity flexion and a cry. The palmar grasp is present by 28 weeks of age and gone by 4 months. Deep tendon reflexes may be brisk in a normal newborn; 5 to 10 beats of ankle clonus are normal. The Babinski sign is extensor or upgoing. The sensory examination can be evaluated by withdrawal of an extremity, grimace, and cry in response to painful stimuli. The rooting reflex (turning of the head toward light tactile stimulation of the perioral area) is present by 32 weeks of age.
Special Conditions Requiring Resuscitation in the Delivery Room
Cyanosis
Acrocyanosis (blue color of the hands and feet with pink color of the rest of the body) is common in the delivery room and is usually normal. Central cyanosis of the trunk, mucosal membranes, and tongue can occur at any time after birth and is always a manifestation of a serious underlying condition. Cyanosis is noted with 4 to 5 g/dL of deoxygenated hemoglobin. Central cyanosis can be caused by problems in many different organ systems, although cardiopulmonary diseases are the most common (Table 58-10). Respiratory distress syndrome, sepsis, and cyanotic heart disease are the three most common causes of cyanosis in infants admitted to a neonatal intensive care unit. A systematic evaluation of these and other causes of cyanosis is required for every cyanotic infant after prompt administration of oxygen, with or without assisted ventilation.
TABLE 58-10 Differential Diagnosis of Neonatal Cyanosis
| System/Disease | Mechanism |
|---|---|
| PULMONARY | |
| Respiratory distress syndrome | Surfactant deficiency |
| Sepsis, pneumonia | Inflammation, pulmonary hypertension, ARDS |
| Meconium aspiration pneumonia | Mechanical obstruction, inflammation, pulmonary hypertension |
| Persistent pulmonary hypertension of the newborn | Pulmonary hypertension |
| Diaphragmatic hernia | Pulmonary hypoplasia, pulmonary hypertension |
| Transient tachypnea | Retained lung fluid |
| CARDIOVASCULAR | |
| Cyanotic heart disease with decreased pulmonary blood flow | Right-to-left shunt as in pulmonary atresia, tetralogy of Fallot |
| Cyanotic heart disease with increased pulmonary blood flow | Mixing lesion as in single ventricle or truncus arteriosus |
| Cyanotic heart disease with congestive heart failure | Right-to-left shunt with pulmonary edema and poor cardiac output as in hypoplastic left heart and coarctation of aorta |
| Heart failure alone | Pulmonary edema and poor cardiac contractility as in sepsis, myocarditis, supraventricular tachycardia, or complete heart block; high-output failure as in PDA or vein of Galen or other arteriovenous malformations |
| CENTRAL NERVOUS SYSTEM (CNS) | |
| Maternal sedative drugs | Hypoventilation, apnea |
| Asphyxia | CNS depression |
| Intracranial hemorrhage | CNS depression, seizure |
| Neuromuscular disease | Hypotonia, hypoventilation, pulmonary hypoplasia |
| HEMATOLOGIC | |
| Acute blood loss | Shock |
| Chronic blood loss | Congestive heart failure |
| Polycythemia | Pulmonary hypertension |
| Methemoglobinemia | Low-affinity hemoglobin or red blood cell enzyme defect |
| METABOLIC | |
| Hypoglycemia | CNS depression, congestive heart failure |
| Adrenogenital syndrome | Shock (salt-losing) |
ARDS, acute respiratory distress syndrome; PDA, patent ductus arteriosus.
Life-Threatening Congenital Malformations
Various congenital anomalies can interfere with vital organ function after birth (see Table 58-9). Some malformations, such as choanal atresia and other lesions obstructing the airway, may complicate ventilation. Intrathoracic lesions, such as cysts or diaphragmatic hernia, interfere with respiration. Other malformations that obstruct the gastrointestinal system at the level of the esophagus, duodenum, ileum, or colon may lead to aspiration pneumonia, intestinal perforation, or gangrene. Gastroschisis and omphalocele are associated with exposed bowel on the abdominal wall. Omphalocele also is often associated with other malformations, whereas intestinal necrosis is more common in gastroschisis.
Many congenital malformations are obvious in the delivery room. Fetal ultrasound can detect many serious congenital anomalies in utero. Immediate palliative medical or surgical treatment or corrective surgery must be planned for most infants with major congenital malformations.
Shock
Shock in the delivery room is manifested by pallor, poor capillary refill time, lack of palpable pulses, hypotonia, cyanosis, and eventually cardiopulmonary arrest. Blood loss before or during labor and delivery is a common cause of shock in the delivery room. Blood loss may be caused by fetal-maternal hemorrhage, placenta previa, vasa previa, twin-to-twin transfusion syndrome, or displacement of blood from the fetus to the placenta as during asphyxia (asphyxia pallida). Hemorrhage into a viscus, such as the liver or spleen, may be noted in macrosomic infants, and hemorrhage into the cerebral ventricles may produce shock and apnea in preterm infants. Anemia, hypoalbuminemia, hypovolemia, and shock at birth are common manifestations of Rh immune hydrops.
Severe intrauterine bacterial sepsis may present with shock in the delivery room or immediately after the infant is transferred to the nursery. Typically these infants are mottled, hypotonic, and cyanotic and have diminished peripheral pulses. They have a normal hemoglobin concentration but may manifest neutropenia, thrombocytopenia, and disseminated intravascular coagulation. Peripheral symmetrical gangrene (purpuric rash) often is a sign of hypotensive shock in infants with severe congenital bacterial infections. Congenital left ventricular cardiac obstruction (critical aortic stenosis or hypoplastic left heart syndrome) also produces shock, although not usually in the delivery room.
Treatment of newborn infants with shock should involve the management approaches used for the sick infant. Problems may be anticipated through knowledge of the infant’s immune status, evidence of hydrops, or suspicion of intrauterine infection or anomalies. Stabilization of the airway and institution of respiratory support are essential. Hypovolemic shock should be managed with repeated boluses of 10 to 15 mL/kg of normal saline or lactated Ringer solution. If severe immune hemolysis is predicted, blood typed against the mother’s blood should be available in the delivery room and should be given to the newborn if signs of anemia and shock are present. Thereafter, all blood should be crossmatched with the infant’s and mother’s blood before transfusion. Drugs such as dopamine, dobutamine, epinephrine, or cortisol may improve cardiac output and tissue perfusion.
Birth Injury
Birth injury refers to avoidable and unavoidable injury to the fetus during the birth process. Caput succedaneum is a diffuse, edematous, often dark swelling of the soft tissue of the scalp that extends across the midline and suture lines. In infants delivered from a face presentation, soft tissue edema of the eyelids and face is an equivalent phenomenon. Caput succedaneum may be seen after prolonged labor in full-term and preterm infants. Molding of the head often is associated with caput succedaneum and is the result of pressure that is induced from overriding the parietal and frontal bones against their respective sutures.
A cephalhematoma is a subperiosteal hemorrhage that does not cross the suture lines surrounding the respective bones. A linear skull fracture rarely may be seen underlying a cephalhematoma. With time, the cephalhematoma may organize, calcify, and form a central depression.
Infants with cephalhematoma and caput succedaneum require no specific treatment. Occasionally a premature infant may develop a massive scalp hemorrhage. This subgaleal bleeding and the bleeding noted from a cephalhematoma may cause indirect hyperbilirubinemia requiring phototherapy. Retinal and subconjunctival hemorrhages are common but usually are small and insignificant. No treatment is necessary.
Spinal cord or spine injuries may occur in the fetus as a result of the hyperextended star gazing posture. Injuries also may occur in infants after excessive rotational (at C3–4) or longitudinal (at C7–T1) force is transmitted to the neck during vertex or breech delivery. Fractures of vertebrae are rare and may cause direct damage to the spinal cord, leading to transection and permanent sequelae, hemorrhage, edema, and neurologic signs. Rarely a snapping sound indicating cord transection rather than vertebral displacement is heard at the time of delivery. Neurologic dysfunction usually involves complete flaccid paralysis, absence of deep tendon reflexes, and absence of responses to painful stimuli below the lesion. Painful stimuli may elicit reflex flexion of the legs. Infants with spinal cord injury often are flaccid, apneic, and asphyxiated, all of which may mask the underlying spinal cord transection.
Injury to the nerves of the brachial plexus may result from excessive traction on the neck, producing paresis or complete paralysis. The mildest injury (neurapraxia) is edema; axonotmesis is more severe and consists of disrupted nerve fibers with an intact myelin sheath; neurotmesis, or complete nerve disruption or root avulsion, is most severe. Erb-Duchenne paralysis involves the fifth and sixth cervical nerves and is the most common and usually mildest injury. The infant cannot abduct the arm at the shoulder, externally rotate the arm, or supinate the forearm. The usual picture is one of painless adduction, internal rotation of the arm, and pronation of the forearm. The Moro reflex is absent on the involved side, and the hand grasp is intact. Phrenic nerve palsy (C3, C4, and C5) may lead to diaphragmatic paralysis and respiratory distress. Elevation of the diaphragm caused by nerve injury must be differentiated from elevation caused by eventration resulting from congenital weakness or absence of diaphragm muscle. Klumpke paralysis is caused by injury to the seventh and eighth cervical nerves and the first thoracic nerve, resulting in a paralyzed hand and, if the sympathetic nerves are injured, an ipsilateral Horner syndrome (ptosis, miosis). Complete arm and hand paralysis is noted with the most severe form of damage to C5, C6, C7, C8, and T1. Treatment of brachial plexus injury is supportive and includes positioning to avoid contractures. Active and passive range of motion exercises also may be beneficial. If the deficit persists, nerve grafting may be beneficial.
Facial nerve injury may be the result of compression of the seventh nerve between the facial bone and the mother’s pelvic bones or the physician’s forceps. This peripheral nerve injury is characterized by an asymmetric, crying face whose normal side, including the forehead, moves in a regular manner. The affected side is flaccid, the eye does not close, the nasolabial fold is absent, and the side of the mouth droops at rest. If there is a central injury to the facial nerve, only the lower two thirds of the face (not the forehead) are involved. Complete agenesis of the facial nucleus results in a central facial paralysis; when this is bilateral, as in Möbius syndrome, the face appears expressionless.
Skull fractures are rare, are usually linear, and require no treatment other than observation for very rare, delayed (1 to 3 months) complications (e.g., leptomeningeal cyst). Depressed skull fractures are unusual, but may be seen with complicated forceps delivery and may need surgical elevation. Fractures of the clavicle usually are unilateral and are noted in macrosomic infants after shoulder dystocia. Often a snap is heard after a difficult delivery, and the infant exhibits an asymmetrical Moro response and decreased movement of the affected side. The prognosis is excellent; many infants require no treatment or a simple figure of eight bandage to immobilize the bone.
Extremity fractures are less common than fractures of the clavicle and involve the humerus more often than the femur. Treatment involves immobilization and a triangular splint bandage for the humerus and traction suspension of the legs for femoral fractures. The prognosis is excellent.
Fractures of the facial bones are rare, but dislocation of the cartilaginous part of the nasal septum out of the vomeral groove and columella is common. Clinical manifestations include feeding difficulty, respiratory distress, asymmetrical nares, and a flattened, laterally displaced nose. Treatment reduces the dislocation by elevating the cartilage back into the vomeral groove.
Visceral trauma to the liver, spleen, or adrenal gland occurs in macrosomic infants and in extremely premature infants, with or without breech or vaginal delivery. Rupture of the liver with subcapsular hematoma formation may lead to anemia, hypovolemia, shock, hemoperitoneum, and disseminated intravascular coagulation. Infants with anemia and shock who are suspected to have an intraventricular hemorrhage but with a normal head ultrasound examination should be evaluated for hepatic or splenic rupture. Adrenal hemorrhage may be asymptomatic, detected only by finding calcified adrenal glands in normal infants. Infants with severe adrenal hemorrhage may exhibit a flank mass, jaundice, and hematuria, with or without shock.
Temperature Regulation
In utero, thermoregulation of the fetus is performed by the placenta, which acts as an efficient heat exchanger. Fetal temperature is nonetheless higher than the mother’s temperature. If maternal temperature becomes elevated during a febrile illness or on exposure to environmental heat, fetal temperature increases further. The immediate temperature at birth of an infant born to a febrile mother with chorioamnionitis is elevated regardless of the presence of infection in the infant, unless the infant has cooled in the delivery room.
After birth, a newborn remains covered by amniotic fluid and situated in a cold environment (20° C to 25° C). An infant’s skin temperature may decrease 0.3° C/min, and the core temperature may decrease 0.1° C/min in the delivery room. In the absence of an external heat source, the infant must increase metabolism substantially to maintain body temperature.
Heat loss occurs through four basic mechanisms. In the cold delivery room, the wet infant loses heat predominantly by evaporation (cutaneous and respiratory loss when wet or in low humidity), radiation (loss to nearby cold, solid surfaces), and convection (loss to air current). When the infant is dry, radiation, convection, and conduction (loss to object in direct contact with infant) are important causes of heat loss. After birth, all high-risk infants should be dried immediately to eliminate evaporative heat losses. A radiant or convective heat source should be provided for these high-risk infants. Normal term infants should be dried and wrapped in a blanket.
The ideal environmental temperature is the neutral thermal environment, the ambient temperature that results in the lowest rate of heat being produced by the infant and maintains normal body temperature. The neutral thermal environmental temperature decreases with increasing gestational and postnatal age. Ambient temperatures less than the neutral thermal environment first result in increasing rates of oxygen consumption for heat production, which is designed to maintain normal body temperature. If the ambient temperature decreases further or if oxygen consumption cannot increase sufficiently (due to hypoxia, hypoglycemia, or drugs), the core body temperature decreases.
Heat production by a newborn is created predominantly by nonshivering thermogenesis in specialized areas of tissue containing brown adipose tissue. Brown fat is highly vascular, contains many mitochondria per cell, and is situated around large blood vessels, resulting in rapid heat transfer to the circulation. The vessels of the neck, thorax, and interscapular region are common locations of brown fat. These tissues also are innervated by the sympathetic nervous system, which serves as a primary stimulus for heat production by brown adipose cells. Shivering does not occur in newborns.
Severe cold injury in an infant is manifested by acidosis, hypoxia, hypoglycemia, apnea, bradycardia, pulmonary hemorrhage, and a pink skin color. The color is caused by trapping of oxygenated hemoglobin in the cutaneous capillaries. Many of these infants appear dead, but most respond to treatment and recover. Milder degrees of cold injury in the delivery room may contribute to metabolic acidosis and hypoxia after birth. Conversely, hypoxia delays heat generation in cold-stressed infants.
Treatment of severe hypothermia should involve resuscitation and rapid warming of core (e.g., lung and stomach) and external surfaces. Fluid resuscitation also is needed to treat the hypovolemia seen in many of these infants. Reduced core temperature (32° C to 35° C) in the immediate newborn period often requires only external warming with a radiant warmer, incubator, or both.
Elevated Temperature
Exposure to ambient temperatures above the neutral thermal environment results in heat stress and an elevated core temperature. Sweating is uncommon in newborns and may be noted only on the forehead. In response to moderate heat stress, infants may increase their respiratory rate to dissipate heat. Excessive environmental temperatures may result in heatstroke or in hemorrhagic shock encephalopathy syndrome.
MISCELLANEOUS DISORDERS
Hypocalcemia
Hypocalcemia is common in sick and premature newborns. Calcium levels are higher in cord blood than in maternal blood because of active placental transfer of calcium to the fetus. Fetal calcium accretion in the third trimester approaches 150 mg/kg/24 hr, and fetal bone mineral content doubles between 30 and 40 weeks of gestation. All infants show a slight decline of serum calcium levels after birth, reaching a trough level at 24 to 48 hours, the point at which hypocalcemia usually occurs. Total serum calcium levels of less than 7 mg/dL and ionized calcium levels of less than 3 to 3.5 mg/dL are considered hypocalcemic.
The etiology of hypocalcemia varies with the time of onset and the associated illnesses of the child. Early neonatal hypocalcemia occurs in the first 3 days of life and is often asymptomatic. Transient hypoparathyroidism and a reduced parathyroid response to the usual postnatal decline of serum calcium levels may be responsible for hypocalcemia in premature infants and infants of diabetic mothers. Congenital absences of the parathyroid gland and DiGeorge syndrome also have been associated with hypocalcemia. Hypomagnesemia (<1.5 mg/dL) may be seen simultaneously with hypocalcemia, especially in infants of diabetic mothers. Treatment with calcium alone does not relieve symptoms or increase serum calcium levels until hypomagnesemia is also treated. Sodium bicarbonate therapy, phosphate release from cell necrosis, transient hypoparathyroidism, and hypercalcitoninemia may be responsible for early neonatal hypocalcemia associated with asphyxia. Early-onset hypocalcemia associated with asphyxia often occurs with seizures as a result of hypoxic-ischemic encephalopathy or hypocalcemia. Late neonatal hypocalcemia, or neonatal tetany, often is the result of ingestion of high phosphate–containing milk or the inability to excrete the usual phosphorus in commercial infant formula. Hyperphosphatemia (>8 mg/dL) usually occurs in infants with hypocalcemia after the first week of life. Vitamin D deficiency states and malabsorption also have been associated with late-onset hypocalcemia.
The clinical manifestations of hypocalcemia and hypomagnesemia include apnea, muscle twitching, seizures, laryngospasm, Chvostek sign (facial muscle spasm when the side of the face over the seventh nerve is tapped), and Trousseau sign (carpopedal spasm induced by partial inflation of a blood pressure cuff). The latter two signs are rare in the immediate newborn period.
Neonatal hypocalcemia may be prevented by administration of IV or oral calcium supplementation at a rate of 25 to 75 mg/kg/24 hr. Early asymptomatic hypocalcemia of preterm infants and infants of diabetic mothers often resolves spontaneously. Symptomatic hypocalcemia should be treated with 2 to 4 mL/kg of 10% calcium gluconate given intravenously and slowly over 10 to 15 minutes, followed by a continuous infusion of 75 mg/kg/24 hr of elemental calcium. If hypomagnesemia is associated with hypocalcemia, 50% magnesium sulfate, 0.1 mL/kg, should be given by intramuscular injection and repeated every 8 to 12 hours.
The treatment of late hypocalcemia includes immediate management, as in early hypocalcemia, plus the initiation of feedings with low phosphate formula. Subcutaneous infiltration of IV calcium salts can cause tissue necrosis; oral supplements are hypertonic and may irritate the intestinal mucosa.
Neonatal Drug Addiction and Withdrawal
Infants may become passively and physiologically addicted to medications or to drugs of abuse (heroin, methadone, barbiturates, tranquilizers, amphetamines) taken chronically by the mother during pregnancy; these infants subsequently may have signs and symptoms of drug withdrawal. Many of these pregnancies are at high risk for other complications related to IV drug abuse, such as hepatitis, acquired immunodeficiency syndrome (AIDS), and syphilis. In addition, the LBW rate and the long-term risk for sudden infant death syndrome are higher in the infants of these high-risk women.
Opiates
Neonatal withdrawal signs and symptoms usually begin at 1 to 5 days of life with maternal heroin use and at 1 to 4 weeks with maternal methadone addiction. Clinical manifestations of withdrawal include sneezing, yawning, ravenous appetite, emesis, diarrhea, fever, diaphoresis, tachypnea, high-pitched cry, tremors, jitteriness, poor sleep, poor feeding, and seizures. The illness tends to be more severe during methadone withdrawal. The initial treatment includes swaddling in blankets in a quiet, dark room. When hyperactivity is constant, and irritability interferes with sleeping and feeding, or when diarrhea or seizures are present, pharmacologic treatment is indicated. Seizures usually are treated with phenobarbital. The other symptoms may be managed with replacement doses of a narcotic (usually tincture of opium) to calm the infant; weaning from narcotics may be prolonged over 1 to 2 months.