CHAPTER 128 Esophagus and Stomach
CHAPTER 128 Esophagus and Stomach
GASTROESOPHAGEAL REFLUX
Etiology and Epidemiology
Gastroesophageal reflux (GER) is defined as the effortless retrograde movement of gastric contents into the esophagus. In infancy, GER is not always an abnormality. Physiologic GER (spitting up) is normal in infants younger than 8 to 12 months of age. Nearly half of all infants are reported to spit up at 2 months of age. Infants who regurgitate stomach contents meet the criteria for physiologic GER, so long as they maintain adequate nutrition and have no signs of respiratory complications or esophagitis. Factors involved in causing infantile GER include liquid diet; horizontal body position; short, narrow esophagus; small, noncompliant stomach; frequent, relatively large volume feedings; and an immature lower esophageal sphincter (LES). As infants grow, they spend more time upright, eat more solid foods, develop a longer and larger diameter esophagus, have a larger and more compliant stomach, and experience lower caloric needs per unit of body weight. Most infants stop spitting up by 9 to 12 months of age.
Clinical Manifestations
The presence of GER is easy to observe in an infant who spits up. In older children, the refluxate is usually kept down by reswallowing, but GER may be suspected by associated symptoms, such as heartburn, cough, dysphagia (trouble swallowing), wheezing, aspiration pneumonia, hoarse voice, failure to thrive, and recurrent otitis media or sinusitis. In severe cases of esophagitis, there may be laboratory evidence of anemia and hypoalbuminemia secondary to esophageal bleeding and inflammation.
Pathologic GER is diagnosed after 18 months of age or if there are complications, such as esophagitis, respiratory symptoms, or failure to thrive in younger infants. In older children, normal protective mechanisms against GER include antegrade esophageal motility, tonic contraction of the LES, and the geometry of the gastroesophageal junction. Abnormalities that cause GER in older children and adults include reduced tone of the LES, transient relaxations of the LES, esophagitis (which impairs esophageal motility), increased intra-abdominal pressure, cough, respiratory difficulty (asthma or cystic fibrosis), and hiatal hernia. When esophagitis develops as a result of acid reflux, esophageal motility and LES function are impaired further, creating a cycle of reflux and esophageal injury.
Laboratory and Imaging Studies
A clinical diagnosis is often sufficient in children with classic effortless regurgitation and no complications. Diagnostic studies are indicated if there are persistent symptoms or complications or if other symptoms suggest the possibility of GER. A child with recurrent pneumonia, chronic cough, or apneic spells without overt emesis may have occult GER. A barium upper gastrointestinal series helps to rule out gastric outlet obstruction, malrotation, or other anatomic contributors to GER. Because of the brief nature of the examination, a negative barium study does not rule out GER. Another study, a 24-hour esophageal pH probe monitoring, uses a pH electrode placed transnasally into the distal esophagus, with continuous recording of esophageal pH. Data typically are gathered for 24 hours, following which the number and temporal pattern of acid reflux events are analyzed. A similar test, which does not require the presence of acid in the stomach, is esophageal impedance monitoring, which records the migration of electrolyte-rich gastric fluid in the esophagus. Endoscopy is useful to rule out esophagitis, esophageal stricture, and anatomic abnormalities.
Treatment
In otherwise healthy young infants, no treatment is necessary. For infants with complications of GER, pharmacologic therapy with a proton-pump inhibitor should be offered (Table 128-1). Lesser benefits are obtained with H2 receptor antagonists. Prokinetic drugs, such as metoclopramide, are occasionally helpful by enhancing gastric emptying and increasing LES tone, but are seldom very effective. When severe symptoms persist despite medication, or if life-threatening aspiration is present, surgical intervention may be required. Fundoplication procedures, such as the Nissen operation, enhance the antireflux anatomy of the LES. In children with a severe neurologic defect who cannot tolerate oral or gastric tube feedings, placement of a feeding jejunostomy can be considered as an alternative to fundoplication.
TABLE 128-1 Treatment of Gastroesophageal Reflux
| Therapy | Comments |
|---|---|
| CONSERVATIVE MANAGEMENT | |
| Towel on caregiver’s shoulder | Cheap, effective; useful only for physiologic reflux |
| Thickened feedings | Reduces number of episodes, enhances nutrition |
| Smaller, more frequent feedings | Can be of some help; be careful not to underfeed child |
| Avoidance of tobacco smoke and alcohol | Always a good idea; may help control reflux symptoms |
| Abstaining from caffeine | Inexpensive, offers some benefit |
| Positional therapy—upright in seat, elevate head of crib or bed | Prone positioning with head up is helpful, but not for young infants due to risk of SIDS |
| MEDICAL THERAPY | |
| Proton pump inhibitor | Effective medical therapy for heartburn and esophagitis |
| Histamine H2 receptor antagonist | Reduces heartburn, less effective for healing esophagitis |
| Metoclopramide | Enhances stomach emptying and LES tone |
| Benefit often minimal | |
| SURGICAL THERAPY | |
| Nissen or other fundoplication procedure | For life-threatening or medically unresponsive cases |
| Feeding jejunostomy | Useful in child requiring tube feeds—delivering feeds downstream eliminates GERD |
GERD, gastroesophageal reflux disease; LES, lower esophageal sphincter; SIDS, sudden infant death syndrome.
ESOPHAGEAL ATRESIA AND TRACHEOESOPHAGEAL FISTULA
Etiology and Epidemiology
The esophagus and trachea develop in close proximity to each other during weeks 4 to 6 of fetal life. Defects in the mesenchyme separating the two structures result in a tracheoesophageal fistula (TEF), often in association with other anomalies (involving the kidneys, heart, spine, or limbs). This defect occurs in about 1:3000 live births. TEF is not thought to be a genetic defect because concordance between monozygotic twins is poor.
Clinical Manifestations
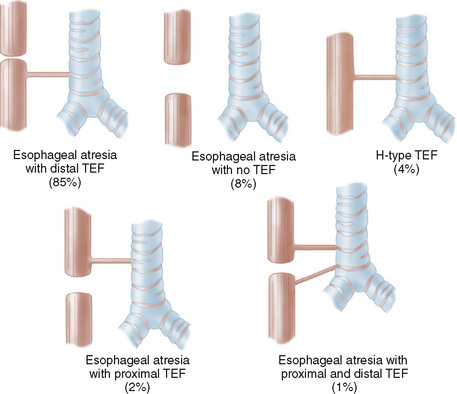
The most common forms of TEF occur with esophageal atresia; the H-type TEF without atresia is uncommon, as is esophageal atresia without TEF (Fig. 128-1). Associated defects include the VACTERL association—vertebral anomalies (70%), anal atresia (imperforate anus) (50%), cardiac anomalies (30%), TEF (70%), renal anomalies (50%), and limb anomalies (polydactyly, forearm defects, absent thumbs, syndactyly) (70%). A single artery umbilical cord is often noted. Infants with esophageal atresia have a history of polyhydramnios, exhibit drooling, and have mucus and saliva bubbling from the nose and mouth. Patients with TEF are vulnerable to aspiration pneumonia. When TEF is suspected, the first feeding should be delayed until a diagnostic study is performed.
Laboratory and Imaging Studies
The simplest test for TEF is to attempt gently to place a nasogastric tube via the mouth into the stomach. The passage of the tube is blocked at the level of the atresia. Confirmation is by chest x-ray with the tube coiled in the esophageal pouch. Air can be injected through the tube to outline the atretic pouch. Barium should not be used because of extreme risk of aspiration, but a tiny amount of dilute water-soluble contrast agent can be given carefully.
Treatment and Prognosis
The treatment of TEF is surgical. A thoracotomy provides access to the mediastinum via extrapleural dissection. The fistula is divided and ligated. The esophageal ends are approximated and anastomosed. In some cases, primary anastomosis cannot be performed because of a long gap between the proximal and distal portions of the esophagus. Various techniques have been described to deal with this problem, including pulling up the stomach, elongating the esophagus by myotomy, using intestinal segments to span the gap, and delaying the anastomosis and providing continuous suction to the upper pouch, allowing for growth.
ESOPHAGEAL FOREIGN BODIES
Etiology and Epidemiology
Young children often place nonfood items in their mouths. When these items are swallowed, they may become lodged in the upper esophagus at the thoracic inlet. The most common objects are coins. Smaller objects may pass harmlessly into the stomach, where they rarely cause symptoms. Other common esophageal foreign bodies include food items, small toys or toy parts, disc batteries, and other small household items. Children with a prior history of esophageal atresia or with poor motility secondary to GER are more prone to food impactions, which seldom occur in the normal esophagus.
Clinical Manifestations
Some children are asymptomatic, but most exhibit some degree of drooling, food refusal, or chest discomfort. Older children usually can point to the region of the chest where they feel the object to be lodged. Respiratory symptoms tend to be minimal, but cough may be present, especially when the esophagus is completely blocked by a large object, such as a piece of meat, which presses on the trachea.
Diagnosis
Plain chest and abdominal radiographs should be taken when foreign body ingestion is suspected. Metallic objects are easily visualized. A plastic object often can be seen on x-ray if the child is given a small amount of dilute contrast material to drink, although endoscopy is probably safer and more definitive.
Treatment
Endoscopy is ultimately necessary in most cases to remove an esophageal foreign body. Various devices can be used to remove the object, depending on its size, shape, and location. Coins usually are grasped and removed with a special-purpose forceps. Nets, baskets, and snares also are available. Whenever objects that may threaten the airway are being recovered, endoscopy should be performed with endotracheal intubation and under general anesthesia.
CAUSTIC INJURIES AND PILL ULCERS
Etiology and Epidemiology
In adolescents, caustic ingestion injuries are usually the result of suicide attempts. In toddlers, the accidental ingestion of household cleaning products is common. Typical injurious agents include drain cleaners, toilet bowl cleaners, dishwasher detergents, and powerful bleaching agents. Childproof lids for commercial products offer some protection, but have not eliminated the problem. Lye-based drain cleaners, especially liquid products, cause the worst injuries because they are swallowed easily and liquefy tissue rapidly. Solid-phase products are less likely to cause esophageal injury during accidental exposures because they burn the tongue and often are expelled before swallowing. Less caustic agents, such as bleach and detergents, cause less serious injury. Pill ulcers occur when certain medications (tetracyclines and nonsteroidal anti-inflammatory drugs) are swallowed without sufficient liquids, allowing prolonged direct contact of the pill with the esophageal mucosa.
Clinical Manifestations
Caustic burns cause immediate and severe mouth pain. The child cries out, drools, spits, and usually drops the container immediately. Burns of the lips and tongue are visible almost immediately. These burns indicate the possibility of esophageal involvement, although esophageal injury can occur in the absence of oral burns. Symptoms may not be present; further evaluation by endoscopy usually is indicated with any significant history of caustic ingestion. Pill injury causes severe chest pain and often prominent odynophagia (painful swallowing) and dysphagia.
Laboratory and Imaging Studies
A chest x-ray should be obtained to rule out aspiration and to inspect for mediastinal air. The child should be admitted to the hospital and given intravenous fluids until endoscopy. The true extent of burns may not be endoscopically apparent immediately, but delayed endoscopy can increase the risk of perforation. Most endoscopists perform the initial endoscopy soon after injury, when the patient has been stabilized. The extent of injury and severity of the burn should be carefully determined. Risk of subsequent esophageal strictures is related to the degree of burn and whether the injury is circumferential.
Treatment
A nasogastric tube can be placed at the time of the initial endoscopy to provide a route for feeding and to stent the esophagus. Systemic steroid use does not reduce the risk of stricture. Broad-spectrum antibiotics should be prescribed if infection is suspected.
Complications
Esophageal strictures, if they occur, usually develop within 1 to 2 months and are treated with balloon dilation. The risk of perforation during dilation is significant. When esophageal destruction is severe, surgical reconstruction of the esophagus using stomach or intestine may be necessary.
PYLORIC STENOSIS
Etiology and Epidemiology
Pyloric stenosis is an acquired condition caused by hypertrophy of the pyloric muscle, resulting in gastric outlet constriction. It occurs in 6 to 8 per 1000 live births and has a 5:1 male predominance. It is more common in first-born children.
Clinical Manifestations
Infants with pyloric stenosis typically begin vomiting during the first month of life, but onset of symptoms may be delayed. The emesis becomes increasingly more frequent and forceful as time passes. Vomiting in pyloric stenosis differs from spitting up because of its extremely forceful and often projectile nature. The vomited material does not contain bile because the gastric outlet obstruction is proximal to the duodenum. This feature differentiates pyloric stenosis from most other obstructive lesions of early childhood. Affected infants are ravenously hungry early in the course of the illness, but become more lethargic with increasing malnutrition and dehydration. The stomach becomes massively enlarged with retained food and secretions, and gastric peristaltic waves are often visible in the left upper quadrant. A hypertrophied pylorus (the olive) may be palpable. As the illness progresses, very little of each feeding is able to pass through the pylorus, and the child becomes progressively thinner and more dehydrated.
Laboratory and Imaging Studies
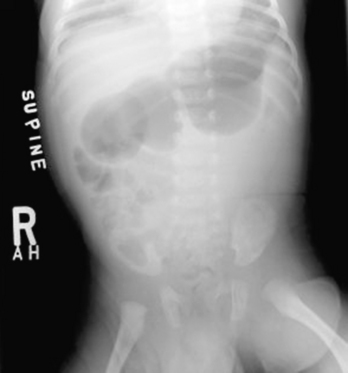
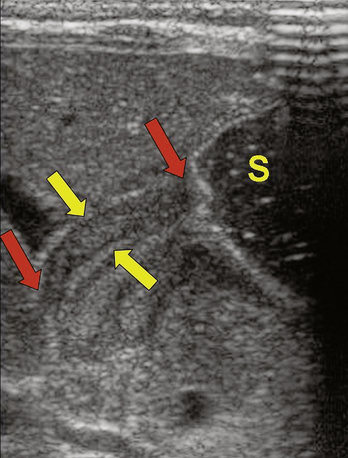
Repetitive vomiting of purely gastric contents results in loss of hydrochloric acid; the classic laboratory finding is a hypochloremic metabolic alkalosis with elevated blood urea nitrogen (BUN) secondary to dehydration. Jaundice with unconjugated hyperbilirubinemia also is common. Plain abdominal x-rays typically show a huge stomach and diminished or absent gas in the intestine (Fig. 128-2). Ultrasonography of the pylorus is the preferred study, showing elongation and thickening of the pylorus (Fig. 128-3). A barium upper gastrointestinal series, which is seldom necessary, shows a string sign caused by barium filling the elongated, constricted pyloric channel.
Treatment
Treatment of pyloric stenosis includes intravenous fluid and electrolyte resuscitation followed by surgical pyloromyotomy. Before surgery, dehydration and hypochloremic alkalosis must be corrected, generally with an initial normal saline fluid bolus followed by infusions of half-normal saline containing 5% dextrose. Potassium chloride is added to the solution after urine output is observed. For pyloromyotomy, a small incision is made, and the pyloric muscle is incised longitudinally to release the constriction. Care is taken not to cut into the mucosa itself.
PEPTIC DISEASE
Etiology and Epidemiology
Acid-related injury can occur in the esophagus, stomach, or duodenum. Table 128-2 lists risk factors for peptic ulcer disease in children. Helicobacter pylori is responsible for more than half of ulcers in the stomach and the duodenum in adults. H. pylori plays a significant but lesser role in childhood ulcer disease. Risk factors for acquisition of H. pylori are low socioeconomic status and poor sanitation, with the highest incidence in developing countries. GER (see Chapter 128) allows acidic gastric contents to injure the esophagus, resulting in esophagitis. Esophagitis is characterized by retrosternal and epigastric burning pain and is best diagnosed by endoscopy. It can range from minimal, with only erythema and microscopic inflammation on biopsy, to superficial erosions and finally to frank ulceration.
Clinical Manifestations
Typical symptoms are listed in Table 128-3. The presence of burning epigastric and retrosternal pain strongly suggests esophagitis. With duodenal ulcers, pain typically occurs several hours after meals and often awakens patients at night. Eating tends to relieve the pain. Gastric ulcers differ in that pain may be aggravated by eating, resulting in weight loss. Gastrointestinal bleeding from either can occur. Most patients report significant symptom relief with antacids or acid blockers.
TABLE 128-3 Peptic Disorders, Symptoms, and Clinical Investigation
| Syndrome and Associated Symptoms | Clinical Investigation |
|---|---|
| ESOPHAGITIS | |
| NONULCER DYSPEPSIA | |
| PEPTIC ULCER DISEASE | |
| Other symptoms | |
CBC, complete blood count; ESR, erythrocyte sedimentation rate; GI, gastrointestinal.
Laboratory and Imaging Studies
Whenever pain is localized to the upper abdomen or the retrosternal region, upper gastrointestinal endoscopy is indicated, although a short course of empiric therapy using a proton-pump inhibitor may be considered, with endoscopy reserved for nonresponders. The possibilities of inflammatory bowel disease, an anatomic abnormality such as malrotation, pancreatitis, and biliary disease should each be ruled out by appropriate testing when suspected (see Chapter 126 and Table 128-3 for recommended studies). Testing for H. pylori should be performed by biopsy during every endoscopy.
Treatment
If H. pylori is present in association with ulcers, it should be treated with a multidrug regimen, such as omeprazole-clarithromycin-metronidazole, omeprazole-amoxicillin-clarithromycin, or omeprazole-amoxicillin-metronidazole, given twice daily for 1 to 2 weeks. Other proton-pump inhibitors may be substituted for omeprazole. Tetracycline is useful in adults, but should be avoided in children. In the absence of H. pylori, esophagitis and peptic ulcer disease are treated with a proton-pump inhibitor, which yields higher rates of healing than H2 receptor antagonists. Gastric and duodenal ulcers heal in 4 to 8 weeks. Esophagitis requires 4 to 5 months of proton-pump inhibitor treatment for optimal healing.