CHAPTER 129 Intestinal Tract
CHAPTER 129 Intestinal Tract
MIDGUT MALROTATION
Etiology and Epidemiology
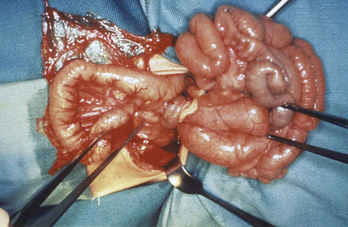
During early fetal life, the midgut is attached to the yolk sac and loops outward into the umbilical cord. Beginning at around 10 weeks’ gestation, the bowel reenters the abdomen and rotates counterclockwise around the superior mesenteric artery until the cecum arrives in the right lower quadrant. The duodenum rotates behind the artery and terminates at the ligament of Treitz in the left upper quadrant. The base of the mesentery becomes fixed along a broad attachment posteriorly, running from the cecum to the ligament of Treitz (Fig. 129-1A). When rotation is incomplete or otherwise abnormal, malrotation is present. Incomplete rotation occurs when the cecum stops near the right upper quadrant, and the duodenum fails to move behind the mesenteric artery; this results in an extremely narrow mesenteric root (see Fig. 129-1B) that makes the child susceptible to midgut volvulus (Fig. 129-2). It is common for abnormal mesenteric attachments (Ladd bands) to extend from the cecum across the duodenum, causing partial obstruction.
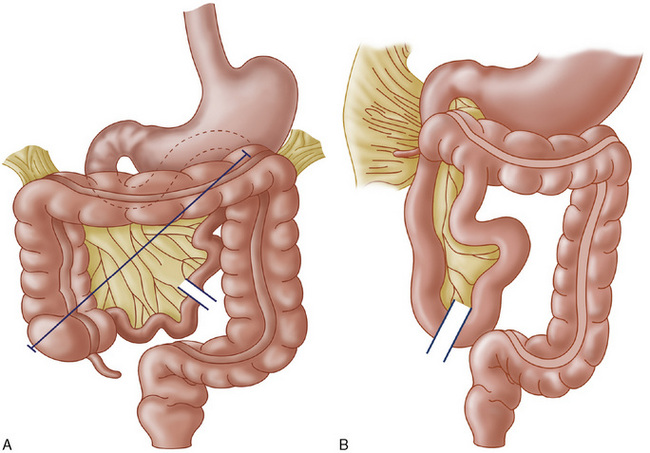
FIGURE 129-1 A, Normal rotation of the midgut. Note the long axis of mesenteric attachment (line). B, Midgut malrotation. Note the narrow mesentery, which predisposes to volvulus, and the presence of Ladd bands extending across the duodenum from the abnormally elevated cecum.
(From Donellan WJ [ed]: Abdominal Surgery of Infancy and Childhood. Luxembourg, Harwood Academic Publishers, 1996, pp 43/6, 43/8.)
Clinical Manifestations
About 60% of children with malrotation present with bilious vomiting during the first month of life; the remaining 40% present later in infancy or childhood. The emesis initially may be due to obstruction by Ladd bands without volvulus. When midgut volvulus occurs, the venous drainage of the gut is impaired, and congestion results in ischemia, pain, tenderness, and often bloody emesis and stools. The bowel eventually becomes necrotic, causing peritonitis and sepsis. Physicians caring for children must be alert to the possibility of malrotation in patients with vomiting and fussiness or abdominal pain.
Laboratory and Imaging Studies
Plain abdominal x-rays show evidence of obstruction. Abdominal ultrasound may demonstrate malrotation. An upper gastrointestinal (GI) series must be done and reveals absence of the normal C-loop of the duodenum, with the jejunum on the right side of the abdomen. When doubt exists about the normalcy of the duodenal course, the contrast material can be followed until it reaches the cecum. High placement of the cecum on follow-through (or by contrast enema) confirms the diagnosis. Laboratory studies are nonspecific, showing evidence of dehydration, electrolyte loss, or evidence of sepsis. A decreasing platelet count is a common indicator of bowel ischemia.
Treatment
Treatment is surgical. The bowel is untwisted. Ladd bands and other abnormal membranous attachments are divided. The mesentery is spread out and flattened against the posterior abdominal wall by moving the cecum to the left side of the abdomen. Sutures may be used to hold the bowel in position, but postoperative adhesions tend to hold the mesentery in place, resulting in a broad attachment and eliminating the risk of recurrent volvulus. Necrotic bowel is resected which may result in short gut syndrome.
INTESTINAL ATRESIA
Etiology and Epidemiology
Congenital partial or complete blockage of the intestine is a developmental defect that occurs in about 1:1500 live births. Atresia occurs in several forms (Fig. 129-3). One or more segments of bowel may be missing completely, there may be varying degrees of obstruction caused by webs or stenosis, or there may be obliteration of the lumen in cordlike bowel remnants. The end result is obstruction with upstream dilation of the bowel and small, disused intestine distally. When obstruction is complete or high grade, bilious vomiting and abdominal distention are present in the newborn period. In lesser cases, as in windsock types of intestinal webs, the obstruction is partial, and symptoms are more subtle.
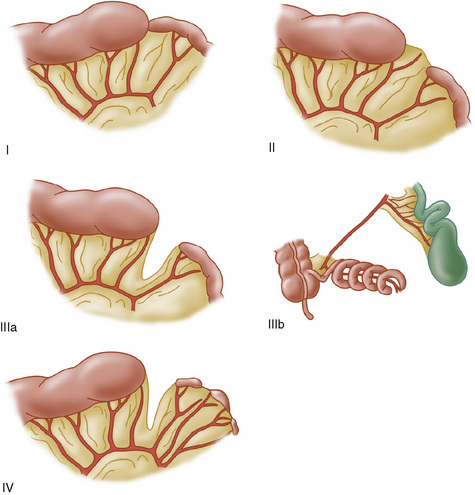
FIGURE 129-3 Types of intestinal atresia. I, internal web; II, cordlike remnant connecting proximal and distal bowel; IIIa, interrupted bowel with V-shaped mesenteric defect; IIIb, “apple peel” atresia with surviving bowel spiraling around a marginal artery; IV, multiple atresias.
(From Grosfeld JL, Ballantine TVN, Shoemaker R: Operative management of intestinal atresia based on pathologic findings. J Pediatr Surg 14:368, 1979.)
Clinical Manifestations
Intestinal atresia presents with a history of polyhydramnios and with abdominal distention and bilious vomiting in the neonatal period. If intestinal perforation is present, peritonitis and sepsis are inevitable.
Laboratory and Imaging Studies
Plain abdominal x-rays may localize the area of atresia and identify evidence of perforation, such as free air or calcifications typical of meconium peritonitis. Duodenal atresia appears as a double-bubble sign (gas in the stomach and enlarged proximal duodenum), with no gas distally. Atresias of the distal intestine are characterized by longer segments of dilated, air-filled bowel. Contrast studies are helpful if plain films are not sufficient. Atresia may also be a complication of meconium ileus associated with cystic fibrosis. Laboratory evaluation for cystic fibrosis (see Chapter 137) is indicated in cases of small bowel atresia. Other laboratory studies are not specific for atresia, but a complete blood count, serum electrolytes, liver functions, and amylase should be measured to identify dehydration, pancreatitis, and other complications.
OTHER CONGENITAL DISORDERS
Gastroschisis is an abdominal wall defect not involving the umbilicus, through which intestinal contents have herniated. In contrast to omphalocele, the bowel is not covered by peritoneum or amniotic membrane. As a result, prolonged contact with the amniotic fluid typically causes a thick, exudative peel on the exposed bowel. Gastroschisis is not associated with extraintestinal anomalies, but segments of intestinal atresia are common. After surgical reduction of the defect, return of normal bowel function may be slow and requires prolonged parenteral nutrition for infants with long atretic segments (short bowel syndrome) and infants with a thick peel.
Omphalocele is an abdominal wall defect at the umbilicus caused by failure of the intestine to return to the abdomen during fetal life. The bowel remains within the umbilical cord and is covered by peritoneum and amniotic membranes. This defect is associated with other congenital anomalies, especially cardiac defects, Beckwith-Wiedemann syndrome (somatic overgrowth, hyperinsulinemic hypoglycemia, risk for Wilms tumor), and intestinal complications. Treatment is surgical closure, which sometimes must be performed in stages to fit the bowel into a congenitally small abdominal cavity.
Anorectal malformations, including imperforate anus and its variants, are embryologic defects recognized at birth by the absence of a normal anal opening. Evaluation of these infants should include observation for emergence of meconium from the urethra or from fistulas on the perineum. A urinary catheter should be placed if urinary distention is present. In low lesions, a fistulous opening that drains meconium is present on the perineum. Low lesions commonly are also associated with fistulization between the bowel and bladder, vagina, or urethra. Lateral plain x-rays show the level of the defect and show gas in the bladder caused by a fistula. These children are treated initially by colostomy to divert the fecal flow, with subsequent anogenital reconstruction. The internal sphincter muscle is functionally absent in high lesions, and continence after repair is difficult to achieve. All children with imperforate anus require magnetic resonance imaging (MRI) of the lumbosacral spinal cord because of high incidence of tethered spinal cord. Urologic dysfunction is common and should be evaluated appropriately.
Hirschsprung disease is a motility defect caused by failure of ganglion cell precursors to migrate into the distal bowel during fetal life. The aganglionic distal segment does not exhibit normal motility and is obstructed secondary to spasm. In 75% of cases, the involved segment is limited to the rectosigmoid; total colonic involvement is seen in 8%. Rarely, long segments of small bowel also are aganglionic. Ultrashort segments involve only a few centimeters of distal rectum. Hirschsprung disease typically presents in the newborn period with failure to pass meconium by 24 hours of age. About 95% of normal infants pass stool spontaneously by this age; 95% of infants with Hirschsprung disease do not. Symptoms of distal bowel obstruction occur with distention and bilious vomiting. If the diagnosis is not made quickly, enterocolitis can result, associated with a high rate of mortality. Diagnosis is based on examination and one or more diagnostic studies. Abdominal distention is present in most cases. Digital rectal examination reveals an empty rectum that clenches around the examiner’s finger, giving the impression of an elongated sphincter. When the finger is withdrawn, a gush of retained stool is often expelled. A deep rectal biopsy specimen obtained surgically or by using a suction biopsy instrument is required for diagnosis. When no ganglion cells are shown in the submucosal plexus, accompanied by nerve trunk hyperplasia, the diagnosis is certain. Barium enema and anorectal manometry can demonstrate abnormalities, but false negative and false positive results occur. Therapy is surgical. When the bowel is markedly distended or inflamed, an initial colostomy usually is performed above the aganglionic segment, followed weeks later by one of several definitive repair procedures. With rectosigmoid involvement, transanal pull-through excises the aganglionic bowel and creates a primary colorectal anastomosis without laparotomy.
Meckel diverticulum is a remnant of the fetal omphalomesenteric duct and is an outpouching of the distal ileum present in 1% to 2% of the population. Although most are asymptomatic throughout life, some cause massive, painless GI bleeding due to acid secretion by ectopic gastric tissue. A Meckel diverticulum can also be a lead point for intussusception or may enable twisting (volvulus) of neighboring bowel around its vascular supply. Diverticulitis mimics appendicitis. Diagnosis may be made in most cases by technetium scan (Meckel scan), which labels the acid-producing mucosa. Because not all diverticula are seen, ultrasound and barium enteroclysis may be useful. When the level of suspicion is high, surgical or laparoscopic investigation is warranted. The treatment is surgical excision.
INFLAMMATORY BOWEL DISEASE
Epidemiology and Etiology
The peak incidence of inflammatory bowel disease (IBD) in children is in the second decade of life. IBD includes Crohn’s disease (CD), which can involve the entire gut, and ulcerative colitis (UC), which affects only the colon. The incidence of IBD is increasing, especially in industrialized countries, for reasons that are unclear. IBD is uncommon in tropical and developing countries. It is more common in Jewish than in other ethnic populations. Genetic factors play a role in susceptibility, with significantly higher risk if there is a family history of IBD. Having a first-degree relative with IBD increases the risk about 30-fold. Susceptibility has been linked to some HLA subtypes, and linkage analysis has identified multiple other susceptibility loci on several chromosomes. Environmental factors also play a role, because there is often nonconcordance among monozygotic twins. The environmental agents responsible have not been identified. Dietary triggers are unproved. Smoking doubles the risk of CD and halves the risk for UC.
Clinical manifestations depend on the region of involvement. UC involves only the colon, whereas CD can involve the entire gut, from mouth to anus. Colitis from either condition results in diarrhea, blood, and mucus in the stool; urgency; and tenesmus, a sensation of incomplete emptying after defecation. When colitis is severe, the child often awakens from sleep to pass stool. Toxic megacolon is a life-threatening complication characterized by fever, abdominal distention and pain, massively dilated colon, anemia, and low serum albumin owing to fecal protein losses. Symptoms of colitis always are present in UC and usually suggest the diagnosis early in its course. Extraintestinal manifestations of UC occur in a few patients and may include primary sclerosing cholangitis, arthritis, uveitis, and pyoderma gangrenosum (Table 129-1).
TABLE 129-1 Comparison of Crohn’s Disease and Ulcerative Colitis
| Feature | Crohn’s Disease | Ulcerative Colitis |
|---|---|---|
| Malaise, fever, weight loss | Common | Common |
| Rectal bleeding | Sometimes | Usual |
| Abdominal mass | Common | Rare |
| Abdominal pain | Common | Common |
| Perianal disease | Common | Rare |
| Ileal involvement | Common | None (backwash ileitis) |
| Strictures | Common | Unusual |
| Fistula | Common | Very rare |
| Skip lesions | Common | Not present |
| Transmural involvement | Usual | Not present |
| Crypt abscesses | Variable | Usual |
| Intestinal granulomas | Common | Rarely present |
| Risk of cancer* | Increased | Greatly increased |
| Erythema nodosum | Common | Less common |
| Mouth ulceration | Common | Rare |
| Osteopenia at onset | Yes | No |
| Autoimmune hepatitis | Rare | Yes |
| Sclerosing cholangitis | Rare | Yes |
* Colonic cancer, cholangiocarcinoma.
Symptoms can be subtle in CD. Small bowel involvement is associated with loss of appetite, crampy postprandial pain, poor growth, delayed puberty, anemia, and lethargy. Some symptoms may be present for some time before the diagnosis is made. Severe CD may cause partial or complete small bowel obstruction. Perineal abnormalities, including skin tags and fistulas, distinguish CD from UC. Other extraintestinal manifestations of CD include arthritis, erythema nodosum, and uveitis or iritis.
Laboratory and Imaging Studies
Blood tests should include complete blood count, erythrocyte sedimentation rate, and C-reactive protein and possibly serologic tests for IBD (Table 129-2). Anemia and elevated platelet counts are typical. Testing for abnormal serum antibodies can be helpful in diagnosing IBD and in discriminating between the colitis of CD and UC. Atypical perinuclear staining by antineutrophil cytoplasmic antibody is found in about 66% of UC patients and in only a few CD cases. Anti-Saccharomyces cerevisiae antibody is present in most CD patients and is uncommon in UC. Another IBD-specific antibody is anti-OmpC, directed against an Escherichia coli membrane protein. Because there is overlap between CD and UC, none of these tests can discriminate absolutely between the two conditions.
TABLE 129-2 Diagnostic Studies for Inflammatory Bowel Disease
| Study | Interpretation |
|---|---|
| BLOOD TESTS | |
| CBC with WBC differential | Anemia, elevated platelets suggest IBD |
| ESR | Elevated in many, but not all, IBD patients |
| C-reactive protein | Elevated in many, but not all, IBD patients |
| ASCA | Found in most CD patients and few UC patients |
| Atypical p-ANCA | Found in most UC patients and few CD patients |
| Anti-OmpC | Found in some UC and CD patients, rare in non-IBD |
| IMAGING STUDIES | |
| Upper GI series with SBFT | Essential to rule out ileal and jejunal CD |
| CT scan | Used to detect abscess, small bowel involvement |
| Tagged WBC scan | Sometimes helpful in determining extent of disease |
| ENDOSCOPY | |
| Upper endoscopy | Evaluate for CD of esophagus, stomach, and duodenum; obtain tissue for histologic diagnosis |
| Colonoscopy | Show presence or absence of colitis and terminal ileal CD; obtain tissue for histology |
| Capsule endoscopy | Emerging role in diagnosis of small bowel CD, more sensitive than upper GI series with SBFT |
Anti-OmpC, antibody to outer membrane protein C; ASCA, anti–Saccharomyces cerevisiae antibody; atypical p-ANCA, atypical perinuclear staining by antineutrophil cytoplasmic antibody; CBC, complete blood count; CD, Crohn’s disease; ESR, erythrocyte sedimentation rate; GI, gastrointestinal; IBD, inflammatory bowel disease; SBFT, small bowel follow-through; WBC, white blood cell.
In patients with suspected IBD, an upper GI series with small bowel follow-through or computed tomography (CT) scan is needed to detect small bowel involvement. Colonoscopy is preferred over contrast enema because biopsy specimens can be obtained and visual features can be diagnostic. Findings in UC include diffuse carpeting of the distal or entire colon with tiny ulcers and loss of haustral folds. Within the involved segment, no skip areas are present. In CD, ulcerations tend to be much larger with a linear, branching, or aphthous appearance; skip areas are usually present. Upper endoscopy cannot evaluate the jejunum and ileum, but is more sensitive than contrast studies in identifying proximal CD involvement. The capsule endoscope, a swallowed device that can visualize the entire small bowel, is potentially useful to visualize subtle small bowel disease (Fig. 126-1) when radiologic findings are absent.
Treatment
Ulcerative Colitis
UC is treated with the aminosalicylate drugs, which deliver 5-aminosalicylic acid (5-ASA, mesalamine) to the distal gut. Because it is rapidly absorbed, pure mesalamine must be specially packaged in coated capsules or pills or taken as a suppository to be effective in the colon. Other aminosalicylates (sulfasalazine, olsalazine, and balsalazide) use 5-ASA covalently linked to a carrier molecule. Sulfasalazine is the least expensive, but side effects resulting from its sulfapyridine component are common. When aminosalicylates alone cannot control the disease, steroid therapy may be required to induce remission. Whenever possible, steroids should not be used for long-term therapy. An immunosuppressive drug, such as 6-mercaptopurine or azathioprine, is useful to spare excessive steroid use in difficult cases. More potent immunosuppressives, such as cyclosporine or tacrolimus, are under investigation as rescue therapy when other treatments fail. Surgical colectomy with ileoanal anastomosis is curative and is an option for unresponsive severe disease or electively to end chronic symptoms and to reduce the risk of colon cancer, which is increased in patients with UC.
Crohn’s Disease
Inflammation in CD typically responds less well to aminosalicylates; oral or IV steroids are more important in inducing remission. To avoid the need for repetitive steroid therapy, immunosuppressive drugs, usually either azathioprine or 6-mercaptopurine, are started soon after diagnosis. CD that is difficult to control also may be treated with methotrexate or with agents that block the action of tumor necrosis factor-α. Infliximab is the most effective drug. Other drugs that inhibit white blood cell (WBC) migration or action, such as natalizumab, show promise. As with UC, surgery is sometimes necessary, usually because of obstructive symptoms, abscess, or severe, unremitting symptoms. Because surgery is not curative in CD, its use must be limited, and the length of bowel resection must be minimized.
CELIAC DISEASE
Etiology and Epidemiology
Celiac disease, or celiac sprue, is an immune-mediated injury to the mucosa of the small intestine triggered by the ingestion of gluten (a protein component) from wheat, rye, barley, and related grains. Rice does not contain toxic gluten and can be eaten freely, as can special pure preparations of oats not contaminated by other grains. In its severe form, celiac disease causes malabsorption and malnutrition. Nonetheless, many patients have few or no obvious or classic symptoms. Approximately 1 in 250 persons in the United States have celiac disease, only a few of which have been diagnosed due to severe manifestations. The disease is seen in association with diabetes and trisomy 21.
Clinical Manifestations
Symptoms can begin at the age when gluten-containing foods are given. Diarrhea, abdominal bloating, failure to thrive, irritability, decreased appetite, and ascites caused by hypoproteinemia are classic. Children may be minimally symptomatic or may be severely malnourished. Constipation is found in a few patients, probably because of reduced intake. A careful inspection of the child’s growth curve and determination of reduced subcutaneous fat and abdominal distention are crucial. Celiac disease should be considered in any child with chronic abdominal complaints.
Laboratory and Imaging Studies
Serologic markers include IgA antiendomysial antibody and IgA tissue transglutaminase antibody. Because IgA deficiency is common in celiac disease, total serum IgA also must be measured to document the accuracy of these tests. In the absence of IgA deficiency, either test yields a sensitivity and specificity of 95%. An endoscopic small bowel biopsy is essential to confirm the diagnosis and should be performed while the patient is still taking gluten. The biopsy specimen shows varying degrees of villous atrophy (short or absent villi), mucosal inflammation, crypt hyperplasia, and increased numbers of intraepithelial lymphocytes. When there is any question about response to treatment, a repeat biopsy specimen may be obtained several months later. Other laboratory studies should be performed to rule out complications, including complete blood count, calcium, phosphate, total protein and albumin, and liver function tests. Mild elevations of the transaminases are common and should normalize with dietary therapy.
Treatment
Treatment is complete elimination of gluten from the diet. The role of gluten in causing intestinal injury must be explained carefully. Consultation with a dietitian experienced in celiac disease is helpful, as is membership in a celiac disease support group. Lists of prepared foods that contain hidden gluten are particularly important for patients to use. Starchy foods that are safe include rice, soy, tapioca, buckwheat, potatoes, and (pure) oats. Many resources also are available via the Internet to help families cope with the large changes in diet and cooking that are required. Most patients respond clinically within a few weeks with weight gain, improved appetite, and improved sense of well-being. Histologic improvement lags behind clinical response, requiring several months to normalize.
MILK AND SOY PROTEIN INTOLERANCE (ALLERGIC COLITIS)
Etiology and Epidemiology
Dietary proteins are a common cause of intestinal inflammation with rectal bleeding in infants. The most commonly implicated agents are cow’s milk and, less often, soy proteins. Other dietary antigens are possible in breastfed infants; breast milk also contains a sampling of protein fragments from the entire range of foods consumed by the mother. Symptoms can appear from 1 to 2 weeks to 12 months of age.
Clinical Manifestations
Most infants with dietary protein intolerance appear healthy despite the presence of streaks of bloody mucus in their stools. This presentation often leads to an erroneous diagnosis of anal fissure. Careful examination of the anus is essential. There is no abdominal tenderness or distention and no vomiting. If these are present, other diagnoses, such as intussusception or volvulus, should be considered. Some children develop severe iron deficiency anemia; intestinal protein loss produces edema and a protein-losing enteropathy.
Laboratory and Imaging Studies
No blood test is particularly helpful. Peripheral eosinophilia generally is not present on complete blood count, which nevertheless should be performed to rule out an associated iron deficiency anemia. Most children are diagnosed clinically and treated empirically. For children with persistent symptoms or other concerns, the diagnosis can be confirmed safely and easily by rectal mucosal biopsy; this shows eosinophilic inflammation of the mucosa. Visual findings at proctoscopy usually include mucosal friability and lymphoid hyperplasia, giving a lumpy, mosquito-bitten appearance to the rectal mucosa.
Treatment
Infants who are bottle-fed should be switched to a hydrolyzed protein formula (e.g., Nutramigen, Pregestamil, or Alimentum). Breastfed infants may continue breastfeeding, but the mother should restrict dairy and soy products from her diet. Visible blood in stools typically resolves within a few days, although occult blood persists for several weeks. For infants with persistent bleeding, an amino acid–based formula is occasionally necessary. Nearly all of these infants lose their sensitivity to the offending protein by 1 year of age. The first intentional exposure to cow’s milk should be performed in the physician’s office because of a small risk of anaphylaxis. Treatment of iron deficiency also is indicated (see Chapter 150).
INTUSSUSCEPTION
Etiology and Epidemiology
Intussusception is the telescoping of a segment of proximal bowel into downstream bowel. Most cases occur in infants 1 to 2 years old. In infants younger than 2 years old, nearly all cases are idiopathic. In older children, the proportion of cases caused by a pathologic lead point increases. In young children, ileocolonic intussusception is common; the ileum invaginates into the colon, beginning at or near the ileocecal valve. When pathologic lead points are present, the intussusception may be ileoileal, jejunoileal, or jejunojejunal.
Clinical Manifestations
An infant with intussusception has sudden onset of crampy abdominal pain; the infant’s knees draw up, and the infant cries out and exhibits pallor with a colicky pattern occurring every 15 to 20 minutes. Feedings are refused. As the intussusception progresses, and obstruction becomes prolonged, bilious vomiting becomes prominent, and the dilated, fatigued intestine generates less pressure and less pain. As the intussuscepted bowel is drawn further into the downstream intestine by normal motility, the mesentery is pulled with it and becomes stretched and compressed. The venous outflow from the intussusceptum is obstructed, leading to edema, weeping of fluid, and congestion with bleeding. Third space fluid losses and “currant jelly” stools result. An unexpected feature of intussusception is lethargy. Between episodes of pain, the infant typically is glassy-eyed and groggy and appears to have been sedated. A sausage-shaped mass caused by the swollen, intussuscepted bowel may be palpable in the right upper quadrant or epigastrium.
Laboratory and Imaging Studies
The diagnosis depends on the direct demonstration of bowel-within-bowel. A simple and direct way of showing this is by abdominal ultrasound. If the ultrasound is positive, or if good visualization has not been achieved, a pneumatic or contrast enema under fluoroscopy is indicated. This is the most direct and potentially useful way to show and treat intussusception. Air and barium can show the intussusception quickly and, when administered with controlled pressure, usually can reduce it completely. The success rate for pneumatic reduction may be higher than hydrostatic reduction with barium and approaches 90% if done when symptoms have been present for less than 24 hours. The pneumatic enema has the additional advantage over barium of not preventing subsequent radiologic studies, such as upper GI series or CT scan. Nonoperative reduction should not be attempted if the patient is unstable or has evidence of perforation or peritonitis.
Treatment
Therapy must begin with placement of an IV catheter and a nasogastric tube. Before radiologic intervention is attempted, the child must have adequate fluid resuscitation to correct the often severe dehydration caused by vomiting and third space fluid losses. Ultrasound may be performed before the fluid resuscitation is complete. Surgical consultation should be obtained early as surgeons often prefer to be present during nonoperative reduction. If pneumatic or hydrostatic reduction is successful, the child should be admitted to the hospital for overnight observation of possible recurrence (risk is 5–10%). If reduction is not complete, emergency surgery is required. During surgery, gentle manual reduction is attempted, but resection of involved bowel because of severe edema, perforation, a pathologic lead point (polyp, Meckel diverticulum), or necrosis may be necessary.
APPENDICITIS
Etiology and Epidemiology
Appendicitis is the most common surgical emergency in childhood. The incidence peaks in the late teenage years, with only 5% of cases occurring in children younger than 5 years of age. Appendicitis begins with obstruction of the lumen, most commonly by fecal matter (fecalith), but appendiceal obstruction can occur secondary to hyperplasia of lymphoid tissue associated with viral infections or, rarely, the presence of neoplastic tissue (classically an appendiceal carcinoid tumor). Trapped bacteria proliferate and begin to invade the appendiceal wall, inducing inflammation and secretion. The obstructed appendix becomes engorged, its blood supply is compromised, and it finally ruptures. The entire process is rapid, with appendiceal rupture usually occurring within 48 hours of the onset of symptoms.
Clinical Manifestations
Classic appendicitis begins with visceral pain, localized to the periumbilical region. Nausea and vomiting occur soon after, triggered by appendiceal distention. As the inflammation begins to irritate the parietal peritoneum adjacent to the appendix, somatic pain fibers are activated, and the pain localizes to the right lower quadrant which is tender to palpation. Voluntary guarding is present initially, progressing to rigidity with rebound tenderness with onset of peritonitis. These classic findings may not be present in young children. The nature and location of pain can vary if the appendix is retrocecal, covered by omentum, or is located elsewhere. If typical history and physical examination findings are present, the patient is taken to the operating room. When doubt exists, imaging is helpful to rule out complications (right lower quadrant abscess, liver disease) and other disorders, such as mesenteric adenitis and ovarian or fallopian tube disorders. If some doubt remains following a negative workup, the child should be admitted to the hospital for close observation and serial examinations.
Laboratory and Imaging Studies
The history and examination are often enough to make the diagnosis, but laboratory and imaging studies are helpful when the diagnosis is uncertain. A WBC count greater than 10,000/mm3 is found in 89% of patients with appendicitis and 93% with perforated appendicitis. Unfortunately, this criterion is met by 62% of abdominal pain patients without appendicitis. Urinalysis is done to look for urinary tract infection, and a chest x-ray rules out pneumonia presenting as abdominal pain. Amylase, lipase, and liver enzymes are done to look for pancreatic or liver and gallbladder disease. The plain abdominal x-ray may reveal a calcified fecalith, which strongly suggests the diagnosis. When these studies are inconclusive, imaging is indicated with an abdominal ultrasound or CT scan, which may reveal the presence of an enlarged, thick-walled appendix with surrounding fluid. An appendiceal diameter of more than 6 mm is considered diagnostic.