CHAPTER 133 Respiratory System Assessment
CHAPTER 133 Respiratory System Assessment
ANATOMY OF THE RESPIRATORY SYSTEM
Air enters the nose and passes over the large surface area of the nasal turbinates. The large surface area and convoluted patterns of airflow warm, humidify, and filter the inspired air. Secretions draining from the paranasal sinuses are carried to the pharynx by the mucociliary action of the ciliated respiratory epithelium. Lymphoid tissue (adenoids) can obstruct the orifices of the eustachian tubes, which extend from the middle ear to the posterior aspect of the nasopharynx.
The epiglottis helps to protect the larynx during swallowing by deflecting material toward the esophagus. The arytenoid cartilages, which assist in opening and closing the glottis, are less prominent in children than in adults. The opening formed by the vocal cords (the glottis) is V-shaped with the apex of the V being anterior. Below the vocal cords, the walls of the subglottic space converge toward the cricoid portion of the trachea. In children under 3 years of age, the cricoid ring (first tracheal ring and a complete ring) is the narrowest portion of the airway, and in older children and adults the glottis is the most narrow. Rings of cartilage extending approximately 320 degrees around the airway circumference support the trachea and mainstem bronchi. The posterior wall of the trachea is membranous. Beyond the lobar bronchi, the cartilaginous support for the airways becomes discontinuous.
The right lung has three lobes (upper, middle, lower) and comprises approximately 55% of the total lung volume. The left lung has two lobes (upper, lower). The inferior division of the left upper lobe, the lingula, is analogous to the right middle lobe.
The lung has a tremendous capacity for growth. A full-term infant has approximately 25 million alveoli; an adult nearly 300 million alveoli. Most growth of new alveoli occurs during the first 2 years of life and is complete by 8 years of age after which lung volume increases with linear growth, but new alveoli are not usually formed.
PULMONARY PHYSIOLOGY
Pulmonary Mechanics
The major function of the lungs is to exchange oxygen (O2) and carbon dioxide (CO2) between the atmosphere and the blood. The anatomy of the airways, mechanics of the respiratory muscles and rib cage, nature of the alveolar-capillary interface, pulmonary circulation, tissue metabolism, and neuromuscular control of ventilation all influence gas exchange.
Air enters the lungs when the pressure in the thorax is less than that of the surrounding atmosphere. During inspiration, negative intrathoracic pressure is generated by contraction and lowering of the diaphragm. The accessory muscles of inspiration (external intercostal, scalene, and sternocleidomastoid muscles) are not used during quiet breathing but are recruited during exercise or in disease states to raise and enlarge the rib cage. Exhalation is normally passive but, with active exhalation, the abdominal and internal intercostal muscles are recruited.
Airway resistance is influenced by the diameter and length of the conducting airways, the viscosity of gas, and the nature of the airflow. During quiet breathing, airflow in the smaller airways may be laminar (streamlined), and resistance is then inversely proportional to the fourth power of the radius of the airway. At higher flow rates, turbulent flow, especially in the larger airways, increases resistance. Relatively small changes in airway diameter can result in large changes in airway resistance. When mechanical forces acting on the lung are at equilibrium (at the end of a normal relaxed breath), the volume of gas in the lung is termed the functional residual capacity (FRC) (Fig. 133-1). This gas volume maintains exchange of O2 during exhalation. Lung compliance (change in volume for a given change in pressure) is a measure of the ease with which the lung can be inflated. Processes that decrease lung compliance (surfactant deficiency, pulmonary fibrosis, pulmonary edema) may lead to decreases in FRC. Conversely, FRC may be increased in obstructive lung diseases (asthma and cystic fibrosis) secondary to gas trapping in the lungs.
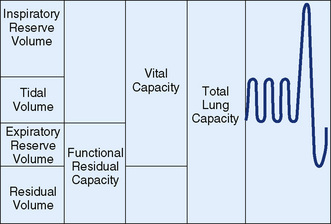
FIGURE 133-1 Lung volumes and capacities. Vital capacity and its subdivisions can be measured by spirometry, but calculation of residual volume requires measurement of functional residual capacity by body plethysmography, helium dilution, or nitrogen washout.
(From Andreoli TE, Bennett JC, Carpenter CJ, et al [eds]: Cecil Essentials of Medicine, 4th ed. Philadelphia, WB Saunders, 1997, p 127.)
During normal tidal breathing, lung volumes are usually in the mid-range of inflation (see Fig. 133-1). Residual volume (RV) is the volume of gas left in the lungs at the end of a maximal exhalation, and total lung capacity (TLC) is the volume of gas in the lungs at the end of maximal inhalation. Vital capacity (VC) is the maximal amount of air that can be expelled from the lungs and is the difference between TLC and RV.
Alveolar ventilation is defined as the exchange of carbon dioxide between the alveoli and external environment. Normally, about 30% of each tidal breath fills the conducting (non-gas-exchanging) airways (anatomic dead space). Because the anatomic dead space is relatively constant, increasing the tidal volume may increase the efficiency of ventilation. Conversely, if tidal volume decreases, then the dead space/tidal volume ratio increases, and alveolar ventilation decreases.
Excessive airway secretions, bronchospasm, mucosal edema and inflammation, airway stenosis, foreign bodies, loss of airway wall integrity (as with bronchiectasis), and airway compression may all produce symptomatic airway obstruction. Asthma and bronchiolitis are common causes of airway obstruction. Restrictive lung disease is less common and is characterized by normal to low FRC and RV, low TLC and VC, decreased lung compliance, and relatively normal flow rates. Restrictive lung disease can result from neuromuscular weakness, an alveolar filling process (lobar pneumonia, pulmonary edema), pleural disease (pleural effusion, inflammation, or mass), thoracic narrowing/stiffness (scoliosis, severe pectus excavatum), and abdominal distention.
Respiratory Gas Exchange
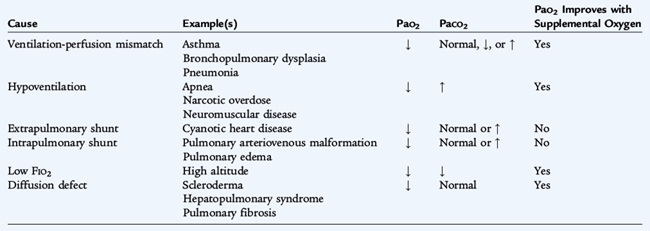
Gas exchange depends upon alveolar ventilation, pulmonary capillary blood flow, and the diffusion of gases across the alveolar-capillary membrane. Exchange of CO2 is determined by alveolar ventilation, while the exchange of O2 is influenced primarily by the regional matching of ventilation (V) with pulmonary blood flow (Q) (V/Q matching). V/Q matching is maintained, in part, by hypoxic pulmonary vasoconstriction (local constriction of the pulmonary vessels in areas that are hypoventilated). There are five causes of hypoxemia (Table 133-1). Disorders resulting in V/Q mismatching are the most common causes of hypoxemia.
Lung Defense Mechanisms
The lungs are constantly exposed to particles and infectious agents. The nose is the primary filter for large particles. The ciliated epithelium of the paranasal sinuses and nasal turbinates propagate filtered particles toward the pharynx. Particles less than 10 μm in diameter may reach the trachea and bronchi and deposit on the mucosa. Particles less than 1 μm may reach the alveoli. Ciliated cells lining the airways from the larynx to the bronchioles continuously propel a thin layer of mucus toward the mouth. Alveolar macrophages and polymorphonuclear cells engulf particles and pathogens that have been opsonized by locally secreted IgA antibodies or transudated serum antibodies.
Cough, important in protecting the lungs, is a forceful expiration that can clear the airways of debris and secretions. Cough may be voluntary or generated by reflex irritation of the nose, sinus, pharynx, larynx, trachea, bronchi, and bronchioles. The loss of the ability to cough results in poor secretion clearance and predisposes to atelectasis and pneumonia.
HISTORY
The complete respiratory history includes onset, duration, and frequency of respiratory symptoms (cough, noisy breathing, work of breathing/exercise tolerance, nasal congestion, sputum production), swallowing function (especially in infants), and exposure to others with respiratory illness. It is important to obtain information concerning the severity (hospitalizations, emergency department visits, missed school days) and pattern (acute, chronic, or intermittent) of symptoms. For infants, a feeding history should be obtained. Family history should include questions about asthma and atopy, immune deficiencies, and cystic fibrosis (CF). The environmental history includes exposure to smoke, pets, and pollutants. Travel history may also be relevant.
PHYSICAL EXAMINATION
Clothing should be removed from the upper half of the child’s body so that the thorax may be inspected. It is best to observe the respiratory pattern, rate, and work of breathing while the child is quiet, noting the shape and symmetry of the chest wall and the anteroposterior (AP) diameter.
Any factor that impairs respiratory mechanics is likely to increase the respiratory rate. However, nonrespiratory causes of tachypnea include fever, pain, and anxiety. Respiratory rates vary with age and activity (Table 133-2).
TABLE 133-2 Breathing Patterns
| Pattern | Features |
|---|---|
| Normal rate (breaths/min) | Preterm: 40–60; term: 30–40; 5 yr: 25; 10 yr: 20; 15 yr: 16; adult: 12 |
| Obstructive | |
| Mild | Reduced rate, increased tidal volume, slightly prolonged expiratory phase |
| Severe | Increased rate, increased use of accessory muscles, prolonged expiratory phase |
| Restrictive | Rapid rate, decreased tidal volume |
| Kussmaul respiration | Increased rate, increased tidal volume, regular deep respiration; consider metabolic acidosis or diabetic ketoacidosis |
| Cheyne-Stokes respiration | Cyclic pattern of waxing and waning of breathing interposed by central apneas/hypopneas; consider CNS injury, depressant drugs, heart failure, uremia (rare in children) |
| Biot respiration | Ataxic or periodic breathing with a respiratory effort followed by apnea; consider brainstem injury or posterior fossa mass |
| Gasping | Slow rate, variable tidal volume; consider hypoxia, shock, sepsis, or asphyxia |
It is important to observe the respiratory pattern and degree of effort (work of breathing). Hyperpnea (increased depth of respiration) may be observed with fever, metabolic acidosis, pulmonary and cardiac disease, or extreme anxiety. Hyperpnea without signs of respiratory distress suggests an extrapulmonary etiology (metabolic acidosis, fever, pain). When the work of breathing is increased, intercostal, supraclavicular, or substernal retractions are often observed. In children, increased inspiratory effort is also manifested by nasal flaring. Grunting (forced expiration against a partially closed glottis) suggests respiratory distress, but it may also be a manifestation of pain.
Causes of increased work of breathing during inspiration include upper airway obstruction (laryngomalacia), subglottic narrowing (croup, stenosis), and decreased pulmonary compliance (pneumonia, pulmonary edema). Increased expiratory work of breathing usually indicates intrathoracic airway obstruction (see Table 133-2).
Stridor, usually heard on inspiration, is a harsh sound emanating from the upper airway and caused by a partially obstructed extrathoracic airway. Wheezing is produced by partial obstruction of the lower airways and is usually heard more prominently during exhalation but may be present on inspiration. Wheezes can be harsh, monophonic, and low-pitched (usually from large, central airways) or high-pitched and musical (from small peripheral airways). Secretions in the intrathoracic airways may produce wheezing but more commonly result in irregular sounds called rhonchi. Fluid or secretions in small airways may produce sounds characteristic of crumpling cellophane (fine crackles or rales). Having the child take a deep breath and exhale forcefully will accentuate many abnormal lung sounds. Crackles may disappear after a few deep inspirations or a cough.
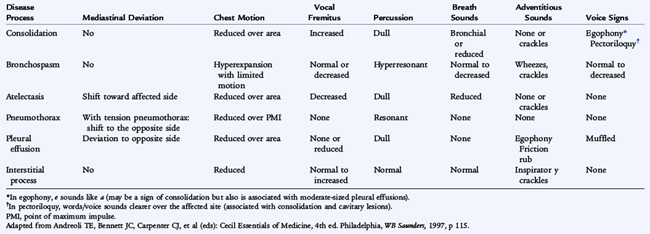
Normal breath sounds (vesicular) are characterized by long inspiratory and short expiratory phases. Bronchial breath sounds have short inspiratory and long expiratory phases and are normally heard over the trachea but suggest pulmonary consolidation or compression of the lung when heard elsewhere. Decreased breath sounds may be due to atelectasis, lobar consolidation (pneumonia), thoracic mass, or a pleural effusion. Observation of respiratory rate, work of breathing, tracheal and cardiac deviation, and chest wall motion, combined with percussion and auscultation help to identify intrathoracic disease (Table 133-3).
Digital clubbing is seen in CF and in some patients with other chronic pulmonary diseases. However, it may also be present in nonpulmonary chronic diseases (cyanotic heart disease, endocarditis, celiac disease, inflammatory bowel disease, chronic active hepatitis) or, rarely, as a familial trait.
Cough results from stimulation of irritant receptors in the airway mucosa. Acute cough generally is associated with respiratory infections or irritant exposure (smoke) and subsides as the infection resolves or the exposure is eliminated. The characteristics of the cough and the circumstances under which the cough occurs help in determining the cause. Sudden onset after a choking episode suggests foreign body aspiration. Morning cough may be due to the accumulation of excessive secretions during the night from sinusitis, allergic rhinitis, or bronchial infections. Nighttime coughing is a hallmark of asthma and can also be caused by gastroesophageal reflux disease. Cough exacerbated by lying flat may be due to postnasal drip, sinusitis, or allergic rhinitis. Recurrent coughing with exercise is suggestive of exercise-induced asthma/bronchospasm. Paroxysmal cough suggests pertussis or foreign body aspiration. A repetitive, staccato cough occurs in chlamydial infections in infants. A harsh, brassy, seal-like cough suggests croup, tracheomalacia, or psychogenic cough. The latter, which is most common in teenagers, disappears during sleep. Younger children can develop a throat-clearing, habit cough, which also disappears during sleep.
Chronic cough is defined as a daily cough lasting longer than 3 weeks. Common causes of chronic cough are asthma, postnasal drip syndromes (allergic rhinitis, sinusitis), and postinfectious tussive syndromes. It can also be caused by gastroesophageal reflux disease, swallowing dysfunction (infants), anatomic abnormalities (tracheoesophageal fistula, tracheomalacia), and chronic infection. Persistent cough may also be caused by exposure to irritants (tobacco and wood stove smoke), foreign body aspiration, or may be psychogenic in origin.
During the first several years of life, children experience frequent viral respiratory infections, especially if they attend day care or preschool. Cough that resolves promptly and is clearly associated with a viral infection does not require further diagnostic workup. However, cough persisting longer than 3 weeks warrants further evaluation.
DIAGNOSTIC MEASURES
Imaging Techniques
Chest radiographs are useful in assessing respiratory disease in children. In addition to determining lung abnormalities, they provide information about the bony thorax (rib or vertebral abnormalities), the heart (cardiomegaly, pericardial effusion), and the great vessels (right aortic arch/vascular rings, rib notching). Chest radiographs should be obtained in both the posteroanterior (PA) and lateral projections. Estimation of lung hyperinflation based on a single PA view is unreliable, whereas flattened diaphragms and an increased AP diameter on a lateral projection indicates hyperinflation. Expiratory views and fluoroscopy may detect partial bronchial obstruction due to an aspirated foreign body resulting in regional hyperinflation because the lung or lobe does not deflate on exhalation. Routine chest radiographs should be obtained after a full inspiration. Crowding of the blood vessels with poor inspiration can be misinterpreted as increased markings or infiltrates. External skin folds, rotation, and motion may produce distorted or unclear images.
A barium esophagogram is valuable in diagnosing disorders of swallowing (dysphagia) and esophageal motility, vascular rings (esophageal compression), tracheoesophageal fistulas, and, to a lesser extent, gastroesophageal reflux. When evaluating for a tracheoesophageal fistula, contrast material must be instilled under pressure via a catheter with the distal tip situated in the esophagus (see Chapter 128).
A computed tomography (CT) scan of the chest is the imaging test of choice for evaluating pleural masses, bronchiectasis, and mediastinal lesions as well as delineating pleural from parenchymal lesions. CT scans with intravenous contrast material provide excellent information about the pulmonary and great vessel vasculature and pulmonary embolism. High-resolution CT scans are used to assess lung parenchyma (pulmonary fibrosis, interstitial fluid) and the airways (bronchiectasis). The speed of current CT scanners makes it possible to scan most children without sedating them. However, sedation may be required to decrease motion artifact. Magnetic resonance imaging (MRI), useful in visualizing cardiac anatomy, is less useful for evaluation of pulmonary lesions.
Ultrasonography can be used to delineate some intrathoracic masses and is the imaging procedure of choice for assessing parapneumonic effusion/empyema. It is useful when assessing diaphragmatic motion.
Measures of Respiratory Gas Exchange
Measurements of oxygenation (PO2 and O2 saturation) and ventilation (PCO2) are important in the management of pulmonary diseases. A properly performed arterial blood gas analysis provides information about the effectiveness of both oxygenation and ventilation. However, arterial samples are more difficult to obtain, so capillary and venous blood samples are more commonly used. The PCO2 from a capillary sample is similar to that from arterial blood. The PCO2 in venous samples is approximately 6 mm Hg higher than arterial PCO2. The ratio of the serum bicarbonate concentration to PCO2 determines the pH. Capillary or venous samples should not be used to assess oxygenation.
There are both respiratory and metabolic causes of acidosis (see Chapter 37). In the presence of an alkalosis or acidosis, respiratory compensation (decreasing PCO2 to maintain a normal pH) can occur within minutes, but renal compensation (increasing the serum bicarbonate level) may not be complete for several days.
Pulse oximetry measures the O2 saturation of hemoglobin by measuring the blood absorption of two or more wavelengths of light. It is noninvasive, easy to use, and reliable. Because of the shape of the oxyhemoglobin dissociation curve, O2 saturation does not decrease much until the PO2 reaches approximately 60 mm Hg. Pulse oximetry may not accurately reflect true O2 saturation when abnormal hemoglobin is present (carboxyhemoglobin, methemoglobin), when perfusion is poor, or if no light passes through to the photodetector (nail polish).
The measurement of PCO2 is accomplished most reliably by blood gas analysis. However, there are noninvasive monitors that record end-expiratory PCO2 (end-tidal CO2), which is representative of alveolar PCO2. End-tidal PCO2 measurements are most commonly used in intubated and mechanically ventilated patients, but some devices can measure PCO2 at the nares. Transcutaneous electrodes can be used to monitor PCO2 and PO2 at the skin surface, but are not particularly accurate. Noninvasive techniques of CO2 measurement are best suited for detecting trends rather than for providing absolute values.
Pulmonary Function Testing
Measurement of lung volumes and airflow rates using spirometry are important in assessing pulmonary disease. The patient inhales to TLC and then forcibly exhales until no more air can be expelled. This test is often referred to as spirometry. During the forced expiratory maneuver the VC, the forced expired volume in the first second (FEV1), and forced expiratory flow (FEF) rates are measured. The predicted values for lung functions are based on patient age, gender, and race, but rely mostly on height. Most children above 6 years of age can perform spirometry. It is possible to perform these tests in infants using sedation and sophisticated equipment.
Airway resistance, FRC, and RV cannot be measured with spirometry and require other techniques. Body plethysmography can be used to measure airway resistance and lung volumes. Helium dilution can measure TLC and RV by determining the magnitude of dilution of inhaled helium in the air within the lung.
Abnormal results on pulmonary function testing can be categorized as indicating obstructive airway disease (low flow rates and increased RV or FRC) or a restrictive defect (low VC and TLC, with relative preservation of flow rates and FRC). When the FEV1 and flow rates are decreased to a greater extent than the VC, then airway obstruction is likely, but a proportional decrease in VC, FEV1, and flow rates suggests a restrictive lung defect. The mean midexpiratory flow rate (FEF25–75%) is a more sensitive measure of small airways disease than the FEV1, but is also more variable. The VC, FEV1, and FEF25–75% can be obtained with a simple spirometer. Pulmonary function testing can detect reversible airway obstruction characteristic of asthma with a significant improvement in FEV1 (>12%) or in FEF25–75% (>25%) following inhalation of a bronchodilator. Spirometry is also useful for longitudinal patient management. The peak expiratory flow rate (PEFR) can be obtained with a simple handheld device and may be useful for home monitoring of older children with asthma. However, it is highly dependent on patient effort and values must be interpreted with caution. Inhalation challenge tests using methacholine, histamine, or cold, dry air are used to assess airway hyperreactivity, but require sophisticated equipment and special expertise and should be performed only in a pulmonary function laboratory with experienced technicians.
Endoscopic Evaluation of the Airways
Endoscopic evaluation of the upper airways (nasopharyngoscopy) is performed with a flexible fiberoptic nasopharyngocope to assess adenoid size, patency of the nasal passages, and abnormalities of the larynx. It is especially useful in evaluating stridor and assessing vocal motion/function. Endoscopic evaluation of the intrathoracic airways can be done with either a flexible or rigid bronchoscope. Bronchoscopy is useful in identifying airway abnormalities (stenosis, malacia, endobronchial lesions, excessive secretions) and in obtaining airway samples for culture (bronchoalveolar lavage), especially in immunocompromised patients. Rigid bronchoscopy is the method of choice for removing foreign bodies from the airways and performing other interventions, and flexible bronchoscopy is most useful as a diagnostic tool and for obtaining lower airway cultures. Bronchoscopy requires deep sedation, but flexible nasopharyngoscopy can be performed without anesthesia. There are few absolute contraindications to bronchoscopy. Relative contraindications include bleeding diatheses, thrombocytopenia (<50,000/cm3), and clinical conditions too unstable to tolerate the procedure.
Examination of Sputum
Sputum specimens may be useful in evaluating lower respiratory tract infections, but they are difficult to obtain in young children. In addition, an expectorated specimen may not provide a representative sample of lower airway secretions. Specimens containing large numbers of squamous epithelial cells are either not from the lower airways or are heavily contaminated with upper airway secretions and may yield misleading results. Sputum in patients with lower respiratory tract bacterial infections often contains polymorphonucleated leukocytes and one predominant organism on culture. If sputum cannot be obtained, then bronchoalveolar lavage specimens (obtained by bronchoscopy) may be used for microbiologic diagnosis in selected situations. In patients with CF who cannot produce sputum, specially processed throat cultures are often used as surrogates for lower airway cultures.
Lung Biopsy
When less invasive methods fail to provide diagnoses in patients with pulmonary disease, a lung biopsy may be required. Although transbronchial lung biopsy through a bronchoscope is useful in adults, it is seldom done in children. Either a thoracoscopic procedure or a thoracotomy is preferred in children. Thoracotomy allows the surgeon to inspect and palpate the lung, which aids in choosing the best site for biopsy, but it is more invasive than thoracoscopy. In most cases, infants and children tolerate lung biopsy well.
THERAPEUTIC MEASURES
Oxygen Administration
Any child in respiratory distress should be treated with supplemental O2 to maintain normal O2 saturation levels. A nasal cannula is the easiest way to provide supplemental O2, but the oxygen concentration provided is variable and affected by the child’s own respiratory pattern. Supplemental O2 may also be delivered by a variety of facemask systems ranging from a simple facemask, which can provide 30 to 40% O2, to a nonrebreather mask with reservoir that can provide nearly 100% O2. For long-term administration of O2, a nasal cannula is the most widely used device, as it enables patients to eat and speak unhindered by the O2 delivery system.
The concentration of administered O2 should be high enough to relieve hypoxemia. Inspired O2 concentrations less than 40% are usually safe for long-term use. Patients requiring supplemental O2 should be monitored with pulse oximetry, either intermittently or continuously, or with arterial blood gas measurements of PO2 to allow titration to the lowest possible O2 concentration.
The acceptable O2 saturation depends on the patient and clinical situation. Generally, supplemental O2 should be administered to achieve a goal saturation level above 90%. Normal oxygen saturation is greater than 95%. It is unnecessary to achieve 100% saturation, especially if this requires potentially toxic levels of inspired O2 for extended periods of time.
Aerosol Therapy
Delivering therapeutic agents to the lower respiratory tract can be accomplished by inhalation of aerosol forms of the agents via dry powder inhalers (DPIs), metered dose inhalers (MDIs), or nebulizers. All of these devices are designed to generate relatively small particles that can bypass the filtering action of the upper airway and deposit in the lower airways. Many factors influence drug deposition including patient technique, the device used, age of the child (cooperation, inspiratory flow, and tidal volume), and breathing pattern. Plastic holding chambers (spacers) should be used with MDIs. Dry powder inhalers require a single rapid deep inhalation for optimal drug delivery, which is difficult for children under 6 years of age. MDIs and nebulizers can be used in children of all ages and are equally effective in delivering medications. The drugs most often delivered as aerosols are bronchodilators (albuterol, levalbuterol, ipratropium) and inhaled corticosteroids. Occasionally antibiotics (tobramycin) are delivered by aerosol.
Chest Physiotherapy and Clearance Techniques
When disease processes impair clearance of pulmonary secretions, airway clearance techniques may help maintain airway patency. One method is chest percussion, which moves secretions toward the central airways, from which they can be expectorated. Chest physiotherapy can also be performed effectively with the flutter valve, acapella device, and pneumatic vests. Chest physiotherapy is most beneficial in children with chronic airway secretions, especially those with CF. Children who are too weak to generate an effective cough benefit from the use of a mechanical cough assist device, used in conjunction with chest physiotherapy. Chest physiotherapy is not generally beneficial for patients with asthma or pneumonia, and its effectiveness in patients with atelectasis has not been clearly established.
Intubation
If the upper airway is obstructed or mechanical ventilation is needed, it may be necessary to provide the patient with an artificial airway. This is best done by placing an endotracheal tube via the mouth or nose into the trachea (intubation). Intubation alters the physiology of the respiratory tract in many ways, not all of which are beneficial. It interferes with the humidification, warming, and filtration of inspired air and prevents phonation. Intubation also stimulates secretion production. However, intubation with an endotracheal tube can be lifesaving.
Endotracheal tubes can damage the larynx and the airways if the tubes are of improper size and are not carefully maintained. The cricoid ring is the narrowest segment of a child’s airway and is completely surrounded by cartilage, which makes it vulnerable to damage, leading to subglottic stenosis. If the pressure created by the tube against the airway mucosa exceeds capillary filling pressure (roughly 35 cm H2O), mucosal ischemia develops, leading to necrosis. Therefore, a small air leak should be maintained around the endotracheal tube to minimize the risk of mucosal damage.
Artificial airways must be kept clear of secretions as mucous plugs in artificial airways can be fatal. Providing adequate humidification of inspired air and appropriate suctioning of the tube reduce the probability of occlusion by secretions. In addition to endotracheal tubes, the laryngeal mask can provide an artificial airway. This device consists of a tube with a soft mask at the distal end. The mask is placed over the larynx, creating a seal and allowing mechanical ventilation without the trachea being instrumented.
Tracheostomy
Tracheostomy is the surgical placement of an artificial airway into the trachea below the larynx. If prolonged intubation is anticipated, elective tracheostomy may be used to prevent laryngeal trauma, obviate the danger of accidental extubation, increase patient comfort, and facilitate nursing care. No clear guidelines are available as to how long patients can be intubated without sustaining airway damage or when a tracheostomy is indicated.
Children with severe chronic upper airway obstruction or those requiring long-term mechanical ventilation may benefit from tracheostomies. Because the tracheostomy tube hampers the ability to phonate and communicate, the child must be monitored carefully at all times. As with endotracheal tubes, tracheostomy tubes must be kept clear. Occlusion of the tube with secretions or accidental dislodgment of the tube can be fatal. Many children with tracheostomy tubes can be cared for at home provided the caregivers are well trained and adequately equipped.
Mechanical Ventilation
Patients who are unable to maintain adequate gas exchange may require mechanical ventilation. Most modes of mechanical ventilation involve inflation of the lungs with gas using positive-pressure ventilators. The inspiratory phase is active (air is pushed in) and exhalation is passive.
Positive-pressure ventilation often requires endotracheal intubation or tracheostomy, though it can be provided noninvasively via nasal or full facemasks. Noninvasive ventilation is particularly useful in patients with neuromuscular disease, but it can also be used to assist ventilation in patients in acute respiratory failure from a variety of causes.
No method of mechanical ventilation truly simulates natural breathing. All methods have their drawbacks and complications. Positive pressure is transmitted to the entire thorax and may impede venous return to the heart during inspiration. The airways and lung parenchyma may be damaged by inflation pressures and high concentrations of inspired O2. In general, inflation pressures should be limited to those necessary to provide sufficient lung expansion for adequate ventilation and the prevention of atelectasis. Pressure-cycled and volume-cycled ventilators (conventional ventilation) are the most widely used modalities in pediatrics, but high-frequency jet ventilation and high-frequency oscillation ventilators may be used in patients with severe lung disease who are failing conventional mechanical ventilation.