Skeletal Muscle
Structure
Understanding the normal structure and function of muscle, including gross, histologic, biochemical, physiologic, electrophysiologic, and ultrastructural features, is critical to understanding of muscle disease.
Structure of Myofibers
Structural and physiologic features of skeletal muscle determine much of its response to injury. Although muscle cells are frequently called muscle fibers or myofibers, they are in fact multinucleated cells of considerable length, which in some animals may approach 1 m. Myonuclei are located peripherally in the cylindrical myofiber (Fig. 15-1) and direct the physiologic processes of the cellular constituents in their area through a process known as nuclear domains. This anatomic arrangement allows segments of the cell to react independently of other portions of the cell. Myonuclei are considered terminally differentiated, with little or no capacity for mitosis and thus for regeneration.
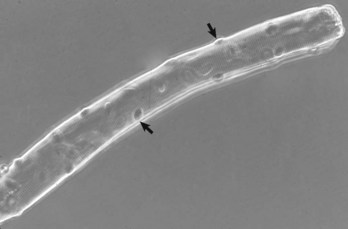
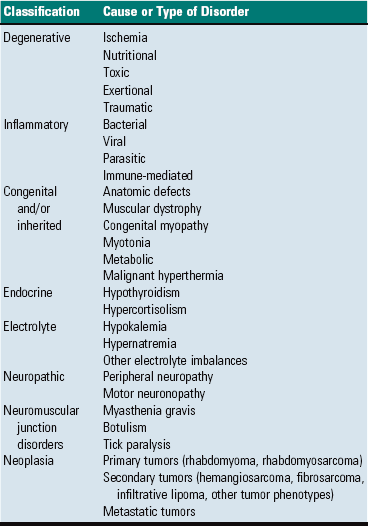
Fig. 15-1 Skeletal muscle, isolated intact myofiber.
Note the multiple peripherally located nuclei (arrows). Phase contrast microscopy. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Associated with myofibers are the satellite cells, also known as resting myoblasts (Web Fig. 15-1). These cells are distributed along the length of the myofiber, between the plasma membrane (sarcolemma) and the basal lamina. Satellite cells in skeletal muscle are very different from cells of the same name found within the peripheral nervous system. Muscle satellite cells are fully capable of dividing, fusing, and reforming mature myofibers. Thus, under favorable conditions, muscle cells (myofibers) are able to fully restore themselves after damage. Recent studies have found that pluripotent cells derived from bone marrow can also contribute to skeletal muscle repair, albeit only to a very small degree.
Web Fig. 15-1 Skeletal muscle myofibers, transverse section.
Note the satellite cell (resting myoblast) located between the sarcolemma (arrow) and the basal lamina (arrowhead). TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Each myofiber is surrounded by a basal lamina and outside of this by the endomysium, a thin layer of connective tissue containing capillaries. Myofibers are organized into fascicles surrounded by the perimysium, a slightly more robust layer of connective tissue (Web Fig. 15-2). Entire muscles are encased in the epimysium, a protective fascia that merges with the muscle tendon. This connective tissue framework is not inert, but in fact forms an integral part of the contractile function of muscle by storing and relaying force generated by myofiber contraction.
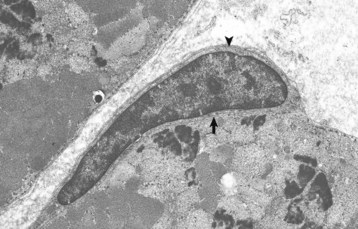
Web Fig. 15-2 Skeletal muscle, transverse section, normal mammalian muscle.
Each myofiber is surrounded by an endomysium of fine collagenous connective tissue. Myofibers are organized into fascicles, which are surrounded by a slightly thicker perimysium. Frozen section, reticulin stain. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Ultrastructural examination reveals that skeletal muscle is a highly and rigidly organized tissue, with what are perhaps the most highly structured cells in the body. Each myofiber is composed of many closely packed myofibrils containing actin and myosin filaments. The striations visible with light microscopy (Fig. 15-2) represent the sarcomeric arrangement of muscle cells, in which actin and myosin filaments attached to transverse Z bands form the framework, and other organelles and intracytoplasmic materials are interspersed within this framework (Fig. 15-3). The endoplasmic reticulum of myofibers is called the sarcoplasmic reticulum and is modified to contain terminal cisternae that sequester the calcium ions necessary to initiate actin and myosin interaction and thus contraction. Sarcolemmal invaginations that traverse the cell, the T (for transverse) tubules, allow rapid dispersion of a sarcolemmal action potential to all portions of the myofiber. The terminal cisternae of two adjacent sarcomeres and the T tubule form what is called the triad (Fig. 15-3, A).
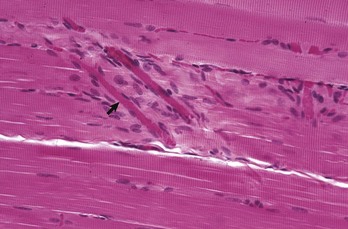
Fig. 15-2 Skeletal muscle, longitudinal section, normal mammalian muscle, cytoarchitectural characteristics.
Note the peripherally located myofiber nuclei and cross striations on the muscle fibers. The cross striations correspond to the A bands (dark lines) and I bands (light lines) in the transmission electron micrograph of Fig. 15-3, B. Myofibers are surrounded by an extensive capillary network (arrow). Formalin fixation, H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
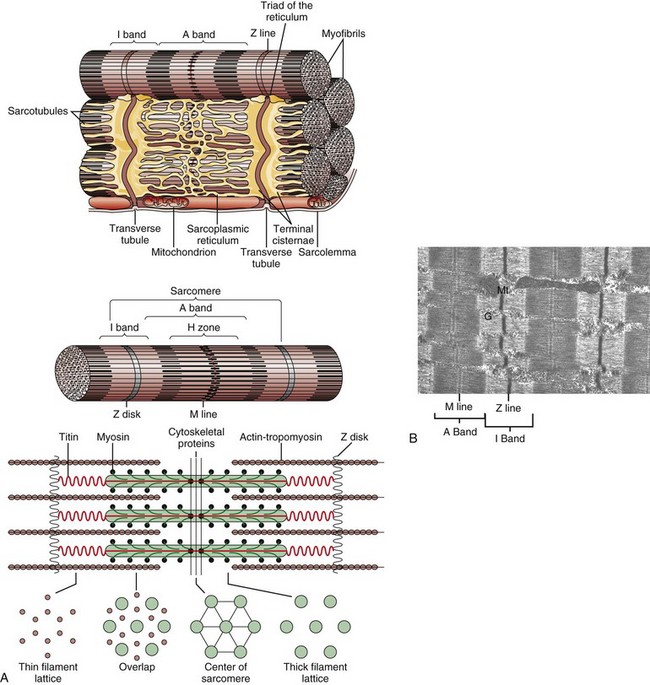
Fig. 15-3 Myofiber structure.
A, Schematic representation of myofiber orientation, secondary organelles, and ultrastructural arrangement of cytoskeletal proteins within sarcomeres. B, Skeletal muscle, longitudinal section, normal mammalian skeletal muscle. Sarcomeres are defined by Z lines, A bands composed of thick myosin filaments, and I bands composed of thin actin filaments. Dense M lines with adjacent clear H zones occur in the center of the A band. Mitochondria (Mt) and glycogen (G) are interspersed between the myofibrils. TEM. uranyl acetate and lead citrate stain. (A from Copstead-Kirkhorn LE, Banasik JL: Pathophysiology: biological and behavioral perspective, ed 3, St Louis, 2005, Saunders. B courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Neuromuscular junctions can only be visualized using electron microscopy or other specialized procedures (Fig. 15-4). Neuromuscular junctions occur only in specific zones within the muscle, usually forming an irregular circumferential “band” midway between myofiber origin and insertion.
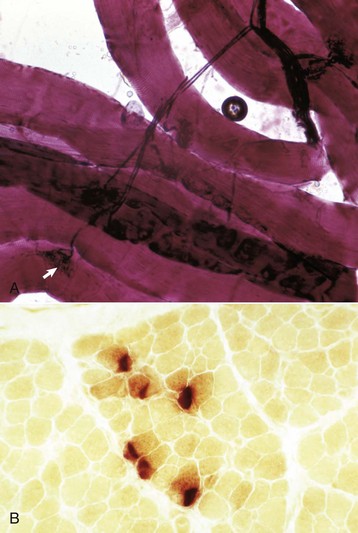
Fig. 15-4 Neuromuscular junctions.
A, An intramuscular nerve (top right) has given off axons, which terminate on a myofiber at a neuromuscular junction (arrow). Teased preparation, silver impregnation method. B, Neuromuscular junctions, transverse section through the center region of normal mammalian muscle. The neuromuscular junctions (red-brown stain) form a cluster. Nonspecific esterase stain, frozen section. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Types of Myofibers
Mammalian muscles are composed of muscle fibers of different contractile properties. A common classification of these fibers is based on three major physiologic features: (1) rates of contraction (fast or slow), (2) rates of fatigue (fast or slow), and (3) types of metabolism (oxidative, glycolytic, or mixed). These physiologic differences form the basis of histochemical methods that demonstrate fiber types. There are several fiber-type classifications. Classification of fibers into type 1, type 2A, and type 2B (Table 15-1) has proved to have practical application in muscle pathology. It is the classification used in this text. Type 1 fibers are rich in mitochondria, rely heavily on oxidative metabolism, and are slow-contracting and slow-fatiguing. Type 2 fibers have fewer mitochondria and are glycolytic, fast-contracting, and more easily fatigable. In most species, type 2 fibers can be subdivided into type 2A and type 2B. Type 2B fibers are the fast-contracting, fast-fatiguing, glycolytic fibers that depend on glycogen for their energy supply. Type 2A fibers are mixed oxidative-glycolytic and therefore, although fast-contracting, are also slow-fatiguing. Thus type 2A fibers are “intermediate” in the concentration of mitochondria, fat, and glycogen between type 1 and type 2B.
TABLE 15-1
| Fiber Type | Physiologic Characteristics | Morphologic Characteristics |
| 1 | Slow twitch, oxidative, fatigue resistant, “red muscle,” aerobic | High mitochondrial content, high fat content, low glycogen content |
| 2A | Fast twitch, oxidative and glycolytic, fatigue resistant | Intermediate mitochondria, fat, and glycogen content |
| 2B | Fast twitch, fatigue sensitive, glycolytic, “white muscle,” anaerobic | Low mitochondrial and fat content, high glycogen content |
Most muscles contain both type 1 and type 2 fibers, and these can be demonstrated by the myosin adenosine triphosphatase (ATPase) reaction (Fig. 15-5, A). Notice that the different fiber types are normally intermingled, forming what is called a mosaic pattern of fiber types. In most mature muscles, the staining pattern of the ATPase reaction reverses when sections are preincubated in an acid rather than an alkaline solution. There are examples of both patterns in the illustrations in this section. Acid preincubation can also be used to distinguish type 2A and type 2B fibers (Fig. 15-5, B). Regenerating fibers, classified as type 2C fibers, stain darkly in both acid and alkaline preparations, which is a distinguishing feature. In most species, oxidative enzyme reactions to demonstrate mitochondria also demonstrate fiber types to some degree (Fig. 15-6, A). Fiber typing can also be done by utilizing immunohistochemical procedures to identify specific myosin isoforms.
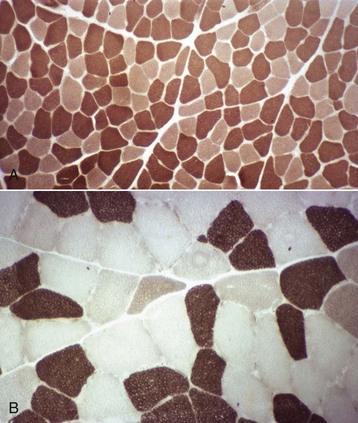
Fig. 15-5 Muscle fiber typing, myofibrillar adenosine triphosphatase (ATPase) reaction, normal skeletal muscle, transverse section.
A, Dog. Type 1 (light) and type 2 (dark) fibers are arranged in a mosaic pattern. Frozen section, ATPase pH 10.0. B, Horse. Acid preincubation allows differentiation of three fiber types, type 1 (dark), type 2A (light), and type 2B (intermediate = gray). Frozen section, ATPase 4.35. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
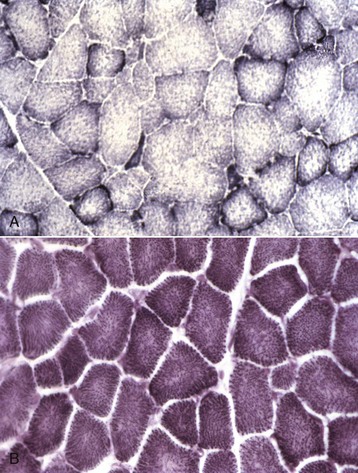
Fig. 15-6 Mitochondria, NADH reaction (blue stained), skeletal myocytes, normal skeletal muscle, transverse section.
A, Horse. Type 1 fibers contain the most mitochondria, type 2B the least, and mitochondrial content of type 2A fibers is intermediate between type 1 and type 2B. Frozen section, NADH reaction. B, Dog. All fiber types have a similar mitochondrial content, therefore this reaction cannot be used to identify different types of myofibers in canine muscle. Frozen section, NADH reaction. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
The percentage of each fiber type varies from muscle to muscle (Fig. 15-7). Type 1 fibers (slow-contracting, slow-fatiguing, and oxidative) are plentiful in those muscles in which the main function is slow, prolonged activity, such as those that maintain posture. Type 1 predominant postural muscles are most often located deep in the limb. Within the same muscle, the percentage of type 1 fibers often increases in the deeper portions. Muscles that contract quickly and for short periods of time, such as those designed for sprinting, contain more type 2B fibers. Only rarely are muscles composed of only one fiber type (e.g., the ovine vastus intermedius is type 1). Athletic training causes some type 2B fibers to be converted to 2A. There are also variations within breeds and differences in the same muscle in different species. For example, the dog has no type 2B purely glycolytic fibers; all canine fibers have strong oxidative capacity (see Fig. 15-6, B).
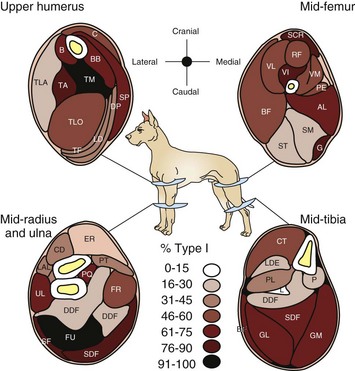
Fig. 15-7 Schematic diagram of the percentage of type 1 and type 2 myofibers in limb muscles in the dog.
There is a wide variation from muscle to muscle. Deeply located muscles have the most type 1 myofibers, indicative of their function in maintaining posture. (Redrawn from Armstrong RB, Sauber CW, Seeherman HJ, Taylor CR: Am J Anat 163:87-98, 1987.)
Innervation and Motor Units
The axons of the peripheral nerve trunks contain terminal branches that innervate multiple myofibers. The terminal branches form synapses with the myofibers at the neuromuscular junction. The myofibers innervated by a single axon form a motor unit, all fibers of which will contract simultaneously after stimulation. Different muscles have different sized motor units that relate to their function. For example, extraocular muscle function does not call for forceful contraction, but rather for many fine movements to smoothly move the globe. Therefore these muscles have very small motor units, with only a small number of myofibers (1 to 4) innervated by each axon. In contrast, the quadriceps muscle is not designed for fine movement, but instead is designed for generation of force, therefore motor units are quite large, with many myofibers (100 to 150 or more) innervated by a single axon.
Function
Skeletal muscle has many functions in the body. Some obvious and major functions are maintaining posture and enabling movement, including locomotion. The rhythmic contraction of the respiratory muscles (the intercostal muscles and the diaphragm) is essential for life. In addition, muscles play a major role in whole body homeostasis and are involved in glucose metabolism and maintenance of body temperature. On a purely esthetic level, muscle contributes to pleasing body contours.
The function of skeletal muscle is intimately related to the function of the peripheral nervous system. The physiologic attributes of a muscle fiber—its rate of contraction and type of metabolism (oxidative, anaerobic, or mixed)—are determined not by the muscle cell itself but by the motor neuron responsible for its innervation (Fig. 15-8). This fact is significant in evaluating histologic changes in muscle fibers. It is possible to divide changes in muscle fibers into two major classes: neuropathic and myopathic. Neuropathic changes are those that are determined by the effect or the absence of the nerve supply (e.g., atrophy after denervation). The term myopathy should be reserved for those muscle diseases in which the primary change takes place in the muscle cell, not in the interstitial tissue and not secondary to effects from the nerve supply. The term neuromuscular disease encompasses disorders involving lower motor neurons, peripheral nerves, neuromuscular junctions, and muscles.
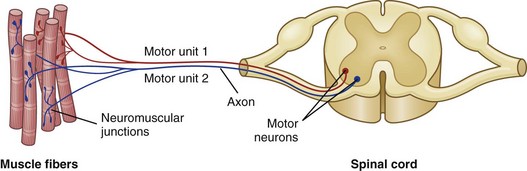
Fig. 15-8 Schematic diagram of motor units of a muscle.
Each motor unit consists of a motor neuron within the central nervous system and all the myofibers (muscle cells) supplied by the neuron and its axon branches. (From Huether SE, McCance KL: Understanding pathophysiology, ed 4, St Louis, 2008, Mosby.)
Metabolism and Ionic Homeostasis
Myofibers require a great deal of energy in the form of adenosine triphosphate (ATP) to generate force and movement. Type 1 oxidative and type 2A oxidative-glycolytic fibers use aerobic metabolism of glucose, stored in the muscle as glycogen, and fat. Type 2B glycolytic fibers rely primarily on anaerobic metabolism of glycogen for energy. Inherent or acquired metabolic defects that reduce skeletal muscle energy production can result in severe muscle dysfunction. A commonly encountered postmortem change, rigor mortis, illustrates the importance of ATP generation within skeletal muscle. The muscle contractile apparatus is still active immediately after death. ATP is necessary for the release of actin from myosin, the interaction that results in the sliding of myofilaments and contraction of muscle. After death, the absence of adequate ATP production causes the muscle fibers to undergo sustained contraction, which is known as rigor mortis. Rigor mortis eventually disappears because of muscle structural breakdown caused by autolysis or putrefaction (bacterial decomposition). The period of time for onset and release of rigor mortis varies, depending on physiologic (glycogen stores at the time of death) and environmental factors such as the environmental temperature (see Chapter 1).
Skeletal muscle is also excitable tissue, similar to that of the nervous system. Maintenance of proper ionic gradients across the sarcolemma is essential for initiation of the action potential. Internal ionic gradients, especially of calcium ions, are critical for initiation and termination of contraction. Alterations of ionic fluxes across the sarcolemma, or within the sarcoplasmic reticulum, can have a serious negative impact on myofiber function.
Examination of Muscle: Clinical, Gross, and Microscopic
The decision to closely examine muscle, either by a biopsy or at necropsy, relies on recognition of indicators of neuromuscular dysfunction.
Clinical Findings
Clinical signs of muscular disease are variable (Box 15-1). The most common manifestations are alteration in muscle size, muscle weakness, and abnormal gait. Depending on the nature of the disorder, clinical signs can be localized, multifocal, or generalized.
Alteration in muscle size is readily detected with careful physical examination. Unilateral atrophy is best appreciated by comparing muscles on both sides of the body. In cases of generalized atrophy, it is important to bear in mind the normal muscling of the breed. For example, the muscling of dairy cattle is less prominent than that of beef cattle, and mild generalized muscle atrophy in a draft horse breed is more difficult to detect than in a light horse breed.
Weakness can be obvious, as in an animal that is unable to rise or prefers to remain recumbent, or can be manifested primarily as exercise intolerance. Special attention should be paid to gait analysis. The gait of an animal with generalized weakness caused by muscle or peripheral nerve dysfunction will have a short stride and often be stiff, and all four legs are often positioned well under the body for support while standing. The abnormal gait of an animal with neuromuscular disease must be distinguished from a similar gait that can occur because of musculoskeletal disease (which is a misnomer, as these disorders affect bone and joint not muscle). Muscle or peripheral nerve dysfunction in the horse, with this species’ unique biomechanics of the pelvic limb, can result in mechanical lameness that can be mistaken for neurologic disease. Odd equine hindlimb gaits designated with such terms as shivers, stringhalt, and fibrotic myopathy are caused by muscle or peripheral nerve disorders. A fibrotic myopathy-like condition also occurs less commonly in the dog and can involve the forelimb. Severe denervating or progressive myopathic conditions that begin in utero or at an early age can cause joint contractures and limb deviation (see Fig. 15-44).
Animals with myotonia often exhibit a stiff gait and develop episodic muscle spasms that can lead to collapse. Percussion of muscle groups can cause a persistent muscle contraction known as dimpling.
In dogs, horses, and ruminants, the esophagus contains a large percentage of skeletal muscle. In dogs and camelids, myopathic, neuropathic, and neuromuscular junction disorders can involve these muscles, causing esophageal dysfunction and megaesophagus. Denervation can also contribute to esophageal dysfunction in cattle with vagal indigestion.
As far as can be determined by clinical evaluation and extrapolation from similar conditions in other species, most neuromuscular disorders in animals are not associated with pain. Muscle cramps, caused by either primary myopathy or partial denervation, and muscle swelling are exceptions to this rule.
Clinicopathologic Findings
If the plasma membrane of the myofiber is damaged or a segment of the myofiber becomes necrotic, some of the contents of the muscle cell will “leak out” and be taken up into the blood. The concentrations of some of these components in serum are used as an index of the extent of myofiber damage. The most commonly used is creatine kinase (CK), formerly called creatine phosphokinase (CPK). Aspartate aminotransferase (AST), formerly known as serum glutamic-oxaloacetic transaminase (SGOT), and lactic dehydrogenase (LDH) are also released but are not as specific an indicator of muscle damage because they are also present in other tissues. Because CK has a low renal threshold, it is quickly excreted in the urine. The half-life of circulating CK varies somewhat between species but is generally about 6 to 12 hours. The half-life of AST and LDH in the serum is much longer, and serum AST and LDH concentrations remain elevated for several days after muscle injury. Serum concentration of alanine aminotransferase (ALT) will also increase in all species from severe muscle cell necrosis. Other serum indicators of skeletal muscle injury include carbonic anhydrase III and fatty acid binding protein, but these latter proteins are not part of a routine serum chemistry panel. It has been speculated that the sarcolemma can become “leaky,” leading to release of CK and other enzymes, without the affected segment becoming overtly necrotic. This possibility is very hard to prove or disprove.
Although the laboratory testing for CK and AST is relatively standardized, laboratory normal ranges may vary considerably within and among laboratories. Determining the normal range of blood values for animals is a difficult task. Normal serum CK concentration in animals is generally less than 500 U/L. Normal serum concentrations of AST, ALT, and LDH vary greatly between species. Tests included in chemistry panels also vary in different laboratories. Some laboratories do not include CK in small animal chemistry panels, which can result in a misdiagnosis of hepatic disease in a dog or cat with a persistent increase in serum AST and ALT concentrations because of degenerative muscle disease. For the purposes of discussion in this chapter, a mild increase in CK or AST is considered to be up to 2 to 3 times normal, a moderate increase is 4 to 10 times normal, and a severe increase is 10 times normal or more.
It should be emphasized that myofibers can be dysfunctional without undergoing necrosis. Myopathic and neuropathic conditions resulting in atrophy, weakness, spasm, stiffness, or myotonia rarely result in significant increase in serum muscle enzyme concentrations. At this time there is no biochemical parameter that will assess muscle fiber function; only morphologic or structural muscle fiber integrity can be assessed.
Electromyography
Electromyography (EMG) can be a valuable tool when evaluating patients with suspected neuromuscular disease. Concentric needle EMG studies look for abnormal spontaneous activity generated by myofibers. In contrast to other electrodiagnostic studies, a flat line generated by a noncontracting muscle indicates a healthy muscle. Abnormal spontaneous activity includes wave forms designated as positive sharp waves, fibrillations, and myotonic bursts. These abnormal spontaneous electrical events are associated with characteristic sounds emitted by the EMG machine. Abnormal spontaneous activity, typically dense and sustained fibrillations and sharp waves, is generated in denervated muscle because of alteration in sodium channel activity in the membrane of denervated fibers. Spontaneous activity in degenerative myopathies, usually scattered fibrillations, positive sharp waves, and myotonic bursts, is likely related to ionic disturbances associated with fiber degeneration and regeneration; functional denervation after segmental necrosis of the segment containing the neuromuscular junction is also possible. Myotonic conditions result in notably abnormal ionic fluxes leading to waxing and waning of spontaneous potentials with a characteristic “dive bomber” sound. Severe denervating and degenerative disorders and canine cushingoid myotonia can be accompanied by myotonic bursts that start and stop abruptly, characteristic of pseudomyotonia.
Nerve conduction velocity studies evaluate the integrity and function of the peripheral nervous system. Primary demyelinating disorders result in severe reduction of nerve conduction velocity, but axons are intact and muscles are still technically innervated; therefore spontaneous activity does not occur. Repetitive nerve stimulation tests the function of the neuromuscular junctions.
Methods of Gross and Microscopic Examination of Muscle
A variety of examination techniques are often necessary to best appreciate changes occurring in muscle.
Gross Pathology and Muscle Sampling
Gross examination includes evaluation of changes in size (atrophied, hypertrophied, or normal), color, and texture. The gross pathologic appearance of skeletal muscle can be quite deceiving. What appear to be mild changes in muscle on gross examination often can be severe on microscopic examination, and what appear to be severe changes on gross examination can turn out to be artefact. Subjective evaluation of size can be highly unreliable unless control muscles (e.g., from normal animals or from the opposite sides) are available for weighing and measuring.
Color changes are common. The intensity of the red color of muscle varies, depending on the type of muscle, the age and species of animal, and the extent of blood perfusion. Pale muscle can indicate necrosis (Fig. 15-9, A and B; see Figs. 15-26; 15-34, A; 15-36, A; and 15-40) or denervation (Fig. 15-9, C; see Fig. 15-37) but is also common in young animals and anemic animals. Pale streaking of muscle most often reflects myofiber necrosis and mineralization (see Fig. 15-9, A and B) or infiltration by collagen or fat (see Fig. 15-9, C and D), and is one of the more reliable indicators of gross pathologic changes. Muscle parasites can be grossly visible as discrete, round to oval, pale and slightly firm zones (see Figs. 15-41 and 15-42, A). Dark red mottling of skeletal muscle can indicate congestion, hemorrhage, hemorrhagic necrosis (see Figs. 15-32, A, and 15-38), inflammation, or myoglobin staining after massive muscle damage (see Fig. 15-36, A) or can simply reflect vascular stasis (hypostatic congestion) after death. Hemorrhagic streaks within the diaphragm often accompany death caused by acute exsanguination. A green discoloration can indicate either eosinophilic inflammation (Fig. 15-10) or severe putrefaction. Lipofuscin accumulation in old animals, especially cattle, can cause a tan-brown discoloration of muscle. Black discoloration of the fascia occurs in calves with melanosis as an incidental finding and in older gray horses with metastasis of dermal melanoma to muscle fascia.
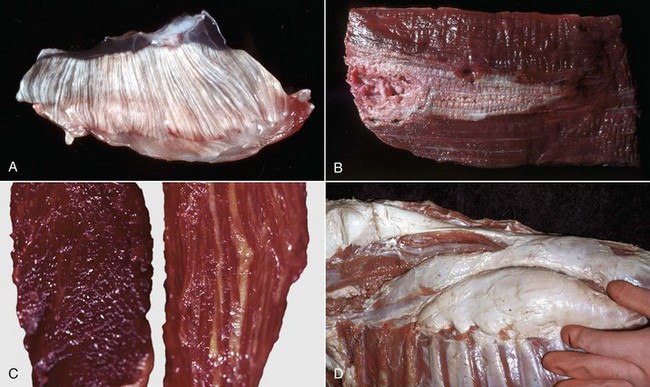
Fig. 15-9 Pathologic changes resulting in pale skeletal muscle.
A, Pale streaks, necrosis and mineralization, degenerative myopathy, canine X-linked muscular dystrophy, diaphragm (left side), dog. B, Localized pallor, necrosis, injection site of an irritant substance, semitendinosus muscle, cow. The irritant was injected just under the perimysium and caused necrosis and disruption of the myofibers. Some irritant seeped down between the fascicles to cause necrosis, but the fascicles of myofibers are still in place. C, Overall pale muscle with pale streaks from collagen and fat infiltration, denervation atrophy, equine motor neuron disease, horse. Equine motor neuron disease muscle (right) compared with normal muscle (left). D, Enlargement and pallor, steatosis, longissimus muscles, neonatal calf. The majority of the muscles have been replaced by fat. (A courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University. For histopathologic findings, see Fig. 15-45. B and D courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. For histopathologic findings, see Fig. 15-25. C courtesy Dr. A. de Lahunta, College of Veterinary Medicine, Cornell University. For histopathologic findings, see Figs. 15-19 and 15-37.)
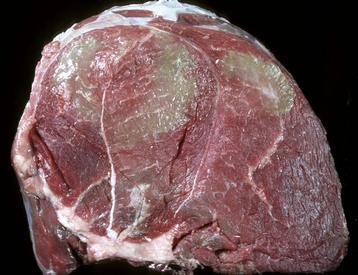
Fig. 15-10 Bovine eosinophilic myositis, gluteal muscles, cow.
Green discoloration of the muscle is due to inflammation that has abundant eosinophils. The inflammation is attributed to degenerating Sarcocystis spp. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. For histopathologic findings, see Fig. 15-39.)
Evaluation of texture is also important. Severely thickened and often calcified fascia occurs in cats with fibrodysplasia ossificans progressiva. Fat infiltration or necrosis can result in abnormally soft muscle. Decreased or increased muscle tone can be caused by denervation. Decreased tone can also occur as a result of a lack of muscle conditioning or postmortem autolysis.
Careful microscopic examination of multiple muscles is often required to detect lesions. In cases of suspected neuromuscular disease, multiple muscle samples should include active muscle (tongue, diaphragm, intercostals, and masticatory muscles), proximal muscle (lateral triceps, biceps femoris, semimembranosus, semitendinosus, and gluteal), and distal muscle (extensor carpi radialis, cranial tibial). For purposes of a biopsy, certain muscles (e.g., lateral triceps, biceps femoris, cranial tibial, semimembranosus, and semitendinosus) are easier to sample because of their parallel myofiber orientation. The ideal samples will also vary, depending on the suspected disorder, such as a type 1 predominant postural muscle for diagnosis of equine motor neuron disease; a type 2 predominant locomotory muscle for diagnosis of equine polysaccharide storage myopathy (EPSSM); and temporal or masseter muscle for diagnosis of masticatory myositis in dogs and masseter myopathy in horses. Short fibers, such as those in the intercostal muscle, are preferred for physiologic studies in which intact muscle fibers are necessary and for studies of neuromuscular junction zones.
To ensure proper fixation and orientation of sections prepared from fixed specimens, the sample should be a strip of muscle no more than 1 cm in diameter, with myofibers running lengthwise. Muscle maintains the ability to contract for some time after death, with the time varying, depending on the physiologic state.
Contraction of muscle after contact with fixative is the most common cause of an artefact called contraction band artefact. Contraction can be prevented or at least minimized by use of a specially designed muscle clamp (Web Fig. 15-3, A) or by placing the sample on a rigid surface, such as a portion of a tongue depressor, and fixing the ends with sutures, staples, or clamps before submersion in the fixative (Web Fig. 15-3, B).
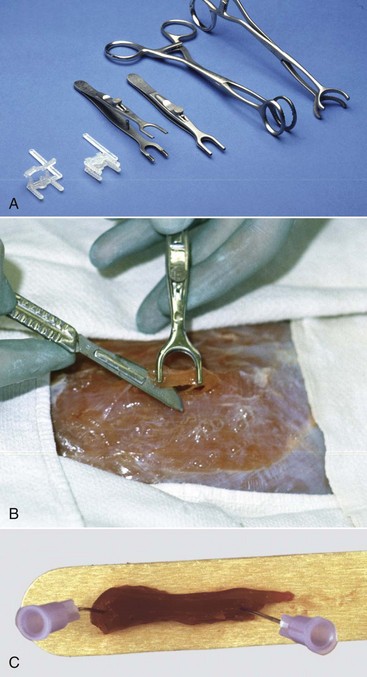
Web Fig. 15-3 Techniques for collection of muscle samples for histologic examination.
Clamps are used to prevent contraction of a fresh muscle specimen when it is immersed in 10% neutral-buffered formalin or EM fixative. A, Types of muscle clamps (from left to right): disposable plastic clamps (open and closed), stainless steel clamps (open and closed), a gallbladder clamp (unmodified), and a modified gallbladder clamp. The stainless steel clamps are autoclavable, the best but expensive. A suitable and economical clamp (not shown), can be made by welding a bar approximately 1 cm long, 3 to 5 mm wide, and 3 mm thick between the lower jaws of two small hemostats. B, Final excision stage. Initially two longitudinal incisions, approximately 5 mm apart and 15 mm long are made into the muscle in the direction of the myofibers. A horizontal cut is made 3 to 4 mm below the surface to undermine a piece of muscle. One jaw of the clamp is inserted under the muscle until its tip just exits on the other side. The clamp is lifted several mm above the surface of the muscle to ensure that the muscle fibers are tense and then the jaws are clamped. The clamped piece of muscle is excised by cutting at each end adjacent to the clamp as shown above. The muscle sample, still in the clamps, is placed in the fixative, usually 10% BNF for histopathologic examination and fixed overnight. For fixation for electron microscopic examination, the muscle in the clamp is placed into EM fixative for 1 to 2 hours. For histopathologic examination the muscle is trimmed by freeing the strip of muscle between the clamps by cutting immediately adjacent to the clamp jaws. Then a transverse section is cut from one end of this sample, avoiding any crushed area, and the remainder of the sample is cut longitudinally in the direction of the myofibers. Both samples are desirable for histopathologic examination. For electron microscopy, after fixation for 1 to 2 hours, slivers 0.5 to 1 mm thick are shaved from the outside of the sample. These are cut into pieces 0.2 mm in diameter and 0.5 mm long, with the longer dimension being in the direction of the myofibers. This long sample facilitates embedment so that the fibers are oriented either in cross section or longitudinally. Both sections are required for electron microscopy. C, Pinning strips of muscle onto a rigid surface, such as a piece of tongue depressor before immersion in 10% neutral-buffered formalin, will also minimize fixation artifacts but is not as effective as the clamps shown above. (A and B courtesy Dr M.D. McGavin, College of Veterinary Medicine, University of Tennessee. C courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Microscopic Examination
Frequently, lesions in muscles can be detected and evaluated only by microscopic examination. Proper microscopic examination requires evaluation of both transverse and longitudinal sections. Myofiber diameters, cytoarchitectural changes, and the percentage of abnormal myofibers are most reliably evaluated in transverse sections. Longitudinal sections reveal the length of changes such as segmental necrosis or regeneration or deposition of storage material. Improperly oriented samples, which result in sections that have obliquely oriented myofibers and thus neither longitudinal nor transverse myofibers, are difficult to evaluate. Use of a magnifying glass or dissecting microscope can aid in determining the orientation of myofibers during trimming of muscle before sectioning. Routine stains, such as hematoxylin and eosin (H&E), run the risk of offering the pathologist a “vast pink wasteland” for evaluation (Fig. 15-11, A) and are often inadequate for detecting subtle myopathic changes, lesions within intramuscular nerves, or presence of abnormal stored material. Various special stains, including reticulin, Masson trichrome, von Kossa, lipid (performed on frozen sections of fixed samples), and periodic acid–Schiff (PAS) for glycogen, are often invaluable in evaluation of routinely processed skeletal muscle (Web Table 15-1). Examples of many of these disorders can be found here in this chapter. Other valuable stains and reactions can only be performed on frozen sections of unfixed muscle samples (see Web Table 15-1).
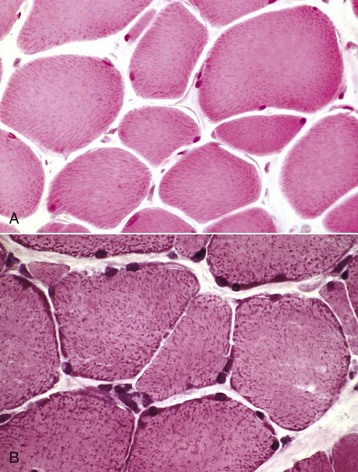
Fig. 15-11 Visibility of mitochondria, formalin-fixed vs. frozen sections, transverse sections of skeletal muscle.
A, Formalin-fixed, paraffin-embedded sample. Mitochondria stain faintly, and the myofibers lack detail when compared with B. H&E stain. B, Frozen section. Note the increased detail visible. Blue-stained mitochondria are seen throughout the cytoplasm and concentrated at the periphery of the myofibers. H&E stain. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
WEB TABLE 15-1
Useful Special Stains and Enzyme Reactions
ATPase, Adenosine triphosphatase; NADH, nicotinamide adenine dinucleotide dehydrogenase; PAS, periodic acid–Schiff; PTAH, phosphotungstic acid hematoxylin; SDH, succinate dehydrogenase.
For many decades, myofiber typing could be done only on frozen sections using the myosin ATPase reaction. Recently, immunohistochemical staining of myosin has been developed for demonstration of myofiber types in formalin-fixed muscle. This is a major advantage because fiber-type staining is often essential for the complete evaluation of muscle. It is most useful in demonstrating preferential involvement of a fiber type and alteration of the fiber-type pattern, the result of denervation and reinnervation.
Enzyme Histochemistry and Immunohistochemistry: There is no question that frozen section histochemistry of unfixed muscle samples is the “gold standard” of muscle pathology. Skeletal muscle may be the one tissue in which the morphology of cells and cellular components is best appreciated in frozen sections (Fig. 15-11, B). Routine frozen section histochemistry on muscle includes a battery of stains applied to serial sections. Examples of many of these stains are illustrated in this chapter. Stains used include H&E, modified Gomori’s trichrome, ATPase for fiber typing, nicotinamide adenine dinucleotide dehydrogenase (NADH), succinate dehydrogenase (SDH), cytochrome oxidase, and other mitochondrial enzyme stains, PAS for glycogen, alizarin red S for calcium, alkaline phosphatase and nonspecific esterase for macrophages and denervated fibers, and lipid stains. When indicated, frozen sections also allow for immunostaining for cytoskeletal proteins, such as dystrophin (see Fig. 15-46) and the dystrophin-associated proteins. Certain abnormal structures, such as nemaline rods formed by expansion of Z bands, as seen in nemaline rod myopathy, are not visible in routine sections but are readily identified in frozen sections stained with modified Gomori’s trichrome.
The major disadvantage of frozen section histochemistry is that unless a neuromuscular disease laboratory is readily available to immediately process unfixed muscle samples, careful preparation for overnight shipping, on ice, in a moist but not overly wet environment, is necessary. Any delay in shipment or overwetting or overheating of the sample results in nondiagnostic samples. In addition, preparation of frozen sections is time and labor intensive, and in most cases only a single transverse section approximately 1 cm in diameter is examined. This can create a significant sampling error when evaluating a small sample of a large muscle in which lesions may not be evenly distributed.
Complete evaluation, which includes morphometric examination and calculation of the percentage and mean diameter of each fiber type, detects changes in the percentage of each fiber type and fiber atrophy or hypertrophy. But at this time, morphometric analysis is not routinely performed on samples submitted for diagnostic purposes.
Frozen section histochemistry is always a powerful tool for evaluation of muscle disease. But in many disorders, it is possible to obtain diagnostic sections from routinely processed muscle samples when appropriate sample selection, handling, and processing are performed, and sections are examined by a pathologist familiar with muscle pathology.
Electron Microscopy: Although much of what used to be determined by electron microscopy has been supplanted by newer immunohistochemical procedures, electron microscopic evaluation of muscle is still important. Various structural alterations, such as abnormalities of neuromuscular junctions, mitochondria, sarcomeric disarray, sarcotubular dilation, Z-line streaming, and cytoplasmic inclusions, may be best visualized, and in some cases only visualized, by this method. Sampling and handling methods to minimize contraction and other artefacts and to allow for precise transverse and longitudinal sections are imperative.
Other Methods of Evaluation
Physiologic testing of isolated intact myofibers in vitro forms the basis for diagnosis of malignant hyperthermia (MH). Short fibers, such as from samples of intercostal muscle, are preferred. While maintained in a physiologic solution, myofiber bundles are exposed to various agents, such as caffeine and halothane, to detect abnormal contractural sensitivity. Biochemical and molecular biologic analysis of muscle samples can evaluate levels of muscle enzymes and other proteins, and genetic analysis can be performed to detect specific gene defects. These latter tests require fresh muscle samples snap-frozen in liquid nitrogen and maintained at −70° C until analysis.
Portals of Entry
Portals of entry are summarized in Box 15-2. Injury to muscle can occur secondary to trauma or infection. Muscle lying superficially can be damaged by penetrating wounds, including those created by intramuscular injections (Fig. 15-12; see also Fig. 15-9, B), which can also allow entry of infectious agents. Muscles located deeply are often injured after bone fracture. Crush injuries from external forces cause extensive muscle damage, and excessive tension can cause muscle tearing. Muscles are endowed with an extensive vascular network that can allow entry of blood-borne pathogens, immune complexes, antibodies and toxins, and inflammatory cells.
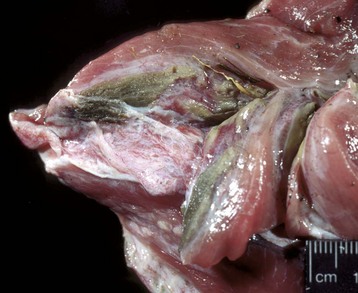
Fig. 15-12 Inflammation and myofiber necrosis, injection site, muscles, lateral thigh, cow.
Necrotic muscle has been stained green by the injected material, which has spread distally down the fascial plane between the two muscles from the original injection site (top right). (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Other routes by which muscle can become dysfunctional are summarized in Box 15-3. Some muscular disorders are genetically determined. Inherited or acquired dysfunction of motor neurons or nerves causes muscle injury in the form of atrophy. Toxins or an altered endocrine or electrolyte status can affect muscle, and physiologic damage can be caused by exhaustive or overexuberant exercise.
Defense Mechanisms
Defense mechanisms are summarized in Box 15-4. The thick encircling fascia (epimysium) of many muscles provides some protection from penetrating injuries and from extension of adjacent infection. This fascia can, however, also contribute to injury under circumstances that lead to increased intramuscular pressure causing hypoxia (compartment syndrome). Tissue macrophages are not typically found in normal muscle but are recruited rapidly from circulating monocytes in the vasculature. Macrophages can cross even an intact basal lamina and effectively clear debris from damaged portions of myofibers, allowing for rapid restoration of the myocyte through satellite cell activation. Neutrophils and other inflammatory cells are also recruited from the bloodstream in response to injury or infection. The extensive vascular network of muscle includes extensive collateral circulatory pathways that render muscle relatively resistant to ischemic damage caused by thrombosis or thromboembolism. Despite the high vascular density of muscle, metastasis of neoplasms to muscle is quite rare. There is some evidence to suggest that the capillary endothelium of skeletal muscle is inherently resistant to neoplastic cell adhesion and invasion.
Responses to Injury
It is often said that the range of response of muscle to injury is limited, consisting primarily of necrosis and regeneration. Actually, muscle is a remarkably adaptive tissue, with a wide range of response to physiologic and pathologic conditions. Myofibers can add or delete sarcomeres to cause elongation or shortening of the entire muscle. In addition to necrosis and regeneration, myofibers can atrophy and hypertrophy, they can split, they can undergo a variety of cytoarchitectural alterations, and they can completely alter their physiologic functions when undergoing fiber-type conversion. To describe muscle response to injury as stereotypical does not do justice to this inherent plasticity. What is true, though, is that it is frequently not possible to determine the cause of muscle injury based on gross or histologic lesions alone. Supplementary tests and clinical histories are often essential.
Necrosis and Regeneration
Myofiber necrosis can accompany a variety of disorders. Because of their multinucleate nature, myofibers often undergo segmental necrosis, with involvement of only one or several contiguous segments within the cell. Global necrosis of the entire length of the myofiber occurs only under severe duress, such as extreme pressure to the entire muscle causing crush injury, or widespread ischemia because of pressure on, or thromboembolism of, a large artery.
Necrotic portions of myofibers have several different histologic appearances. The earliest change is often segmental hypercontraction, resulting in segments of slightly larger diameter that are slightly darker staining (“large dark fibers”) that are best seen on transverse sections (Fig. 15-13, A). On longitudinal sections, “twisting” or “curling” of affected fibers is often seen. But similar changes occur as an artifactual change in improperly handled samples. The cytoplasm of fully necrotic portions of the fiber is often homogeneously eosinophilic and pale (hyaline degeneration), with loss of the normal cytoplasmic striations and the adjacent muscle nucleus. The affected cytoplasm then becomes floccular or granular as that portion of the myofiber starts to fragment (Fig. 15-13, B; see Fig. 15-15, B).
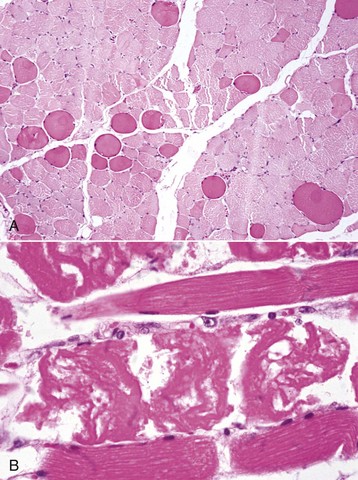
Fig. 15-13 Myofiber necrosis, skeletal muscle.
A, Hypercontraction, transverse section. Large, deeply stained fibers (“large dark red fibers”) are hypercontracted segments of a myofiber, the initial stage of necrosis. Note the rounded outline of these myofibers compared with the polygonal outlines of normal myofibers. Formalin fixation, H&E stain. B, Segmental necrosis, monensin toxicosis, longitudinal section, horse. Segments of the myofibers have undergone hypercontraction (center of figure), and the remaining cytoplasm is fragmented. Formalin fixation, H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Increased intracellular calcium is a common trigger of necrosis in all cells, and myofibers contain a high level of calcium ions stored in the sarcoplasmic reticulum. Therefore myofibers may be particularly sensitive to calcium-induced necrosis, either as a result of damage to the sarcolemma, causing influx of extracellular calcium, or from damage to the sarcoplasmic reticulum, releasing intracellular stores of calcium. Small wonder then that necrotic myofibers are often prone to overt mineralization. Overtly mineralized myofibers appear as chalky white streaks on gross examination (see Fig. 15-9, A) and as basophilic granular to crystalline material within myofibers on histologic examination. Large deposits of mineral can induce a foreign body granulomatous response. Although the presence or absence of myofiber mineralization has sometimes been used as a diagnostic aid, the circumstances under which a necrotic myofiber segment can become mineralized are so diverse that myofiber mineralization must be considered a nonspecific response, indicative only of myofiber necrosis. Myofiber mineralization can be confirmed with histochemical stains, such as alizarin red S and von Kossa. Histochemical staining for calcium in frozen sections also detects increased intracytoplasmic calcium in damaged myofibers that are not overtly necrotic or mineralized (Fig. 15-14, A).
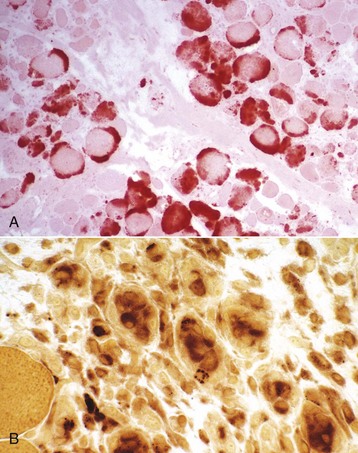
Fig. 15-14 Myofiber necrosis, skeletal muscle, transverse section.
A, There has been a massive influx of calcium (stained orange) into acutely necrotic fibers. Frozen section, alizarin red S stain. B, Macrophages with red-brown staining cytoplasm invading necrotic myofibers. Portions of intact fibers are in the lower left. Frozen section, nonspecific esterase stain. (A courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University. B courtesy Dr. B.J. Cooper, College of Veterinary Medicine, Oregon State University.)
Provided there is still an adequate blood supply, macrophages derived from transformation of blood monocytes rapidly infiltrate areas of myofiber necrosis (Fig. 15-14, B). Macrophages are able to traverse the basal lamina and rapidly clear cytoplasmic debris (Fig. 15-15, A). Other leukocytes, including neutrophils, eosinophils, and lymphocytes, can also be recruited to sites of extensive myonecrosis, presumably because of various cytokines released from damaged muscle. The infiltration of macrophages and other cells into areas of damaged muscle to clear away necrotic myofibers does not in any way constitute a form of myositis.
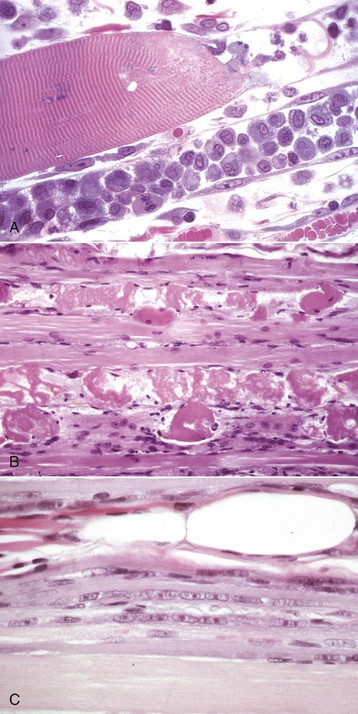
Fig. 15-15 Segmental necrosis and regeneration.
A, Monophasic segmental coagulation necrosis, skeletal muscle, longitudinal section of two myofibers. A segment of the upper fiber (right) and all the visible portion of the lower fiber have undergone necrosis, and macrophages have invaded through the intact basal lamina and cleared the cytoplasmic debris. Satellite cells on the inner surface of the basal lamina of the lower fiber are activated, and one (lower left side) is in mitosis. One-mm plastic-embedded section, H&E stain. B, Polyphasic injury, segmental coagulation necrosis and regeneration of myofibers, muscle, longitudinal section. Between each of the foci of coagulation necrosis in the lowest myofiber is a segment of small-diameter faintly basophilic cytoplasm lacking cross striations, in which there is an internal chain of euchromatic nuclei. This is a late stage of regeneration. Formalin fixation, H&E stain. C, Monophasic injury, late stage regeneration, skeletal muscle, longitudinal section. The regenerating segment of the myofiber consists of myotubes, which have small diameters, with slightly basophilic cytoplasm and internal rows of large euchromatic nuclei. Formalin fixation, H&E stain. (A courtesy Dr. A. Kelly, University of Pennsylvania. B courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University. C courtesy Dr. B.J. Cooper, College of Veterinary Medicine, Oregon State University.)
Because myonuclei are unable to divide, regeneration of muscle relies on satellite cell activation. Muscle satellite cells are resistant to many of the insults that result in myofiber necrosis, and activation of satellite cells is triggered by necrosis of adjacent segments of that myofiber. Therefore, as macrophages are clearing cytoplasmic debris, satellite cells are becoming activated and begin to divide in preparation for regeneration of the affected myofiber segment. If the myofiber basal lamina is still intact, it will leave an empty cylindrical space known as a sarcolemmal tube. This name is clearly a misnomer, dating from the days when the term sarcolemma was applied to the tube formed by the basal lamina that remains after segmental myofiber necrosis. Clearly what is now termed the sarcolemma (plasmalemma) of necrotic fiber segments is lost, but this is a misnomer that is firmly entrenched. The important concept to remember is that, if intact, the basal lamina forms a cylindrical scaffold to guide proliferating myoblasts and to keep fibroblasts out. Satellite cells may be seen undergoing mitosis, at which stage they are known as activated myoblasts, on the inner surface of this tube (see Fig. 15-15, A). Within hours, proliferating myoblasts will fuse end-to-end to form myotubes (Fig. 15-15, B and C), and within days the myotube produces thick and thin filaments and undergoes maturation to a myofiber, reestablishing myofiber integrity. If the basal lamina is ruptured, myotubes are said to be able to bridge gaps of 2 to 4 mm and larger ones heal by fibrosis (see later discussion). The process of myofiber regeneration recapitulates embryologic development of skeletal muscle and is depicted schematically in Fig. 15-16. A percentage of dividing satellite cells do not fuse with the forming myotube but instead become new satellite cells capable of future regeneration.
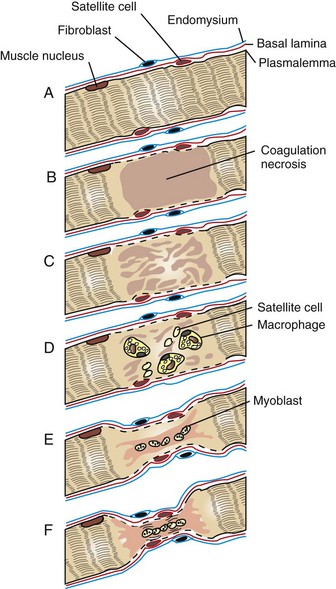
Fig. 15-16 Schematic diagram of segmental myofiber necrosis and regeneration.
A, Myofiber, longitudinal section. B, Segmental coagulation necrosis. C, The necrotic segment of the myofiber has become floccular and detached from the adjacent viable portion of the myofiber. The satellite cells are enlarging. D, The necrotic segment of the myofiber has been invaded by macrophages, and satellite cells are migrating to the center. The latter will develop into myoblasts. The plasmalemma of the necrotic segment has disappeared. E, Myoblasts have formed a myotube, which has produced sarcoplasm. This extends out to meet the viable ends of the myofiber. The integrity of the myofiber is maintained by the sarcolemmal tube formed by the basal lamina and endomysium. F, Regenerating myofiber. There is a reduction in myofiber diameter with central rowing of nuclei. There is early formation of sarcomeres (cross striations), and the plasmalemma has re-formed. Such fibers stain basophilically with H&E. (Redrawn with permission from Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
In summary, the success of muscle regeneration depends on (1) the presence of an intact basal lamina and (2) the availability of viable satellite cells. The stages of successful muscle regeneration are summarized in Box 15-5.
Thus myofibers undergoing segmental necrosis in which the basal lamina is preserved, as in metabolic, nutritional, and toxic myopathies, regenerate very successfully. However, when large areas of satellite cells are killed (e.g., by heat, intense inflammation, or infarction), the situation is very different. In this case, a return to normal is not possible, and healing is chiefly by fibrosis.
If the insult to the muscle is sufficient to disrupt the myofiber basal lamina but not enough to damage the satellite cells, regeneration attempts are ineffective. Because the basal lamina is not intact, there is no tube to guide the myoblasts proliferating from each end. Myoblast proliferation under these conditions results in formation of so-called muscle giant cells (Fig. 15-17). Thus the presence of muscle giant cells indicates that conditions for regeneration have not been optimal and occurs after destructive lesions, such as those caused by trauma that transects myofibers, infarction, and intramuscular bacterial infection or injection of irritants. Muscle giant cells are often accompanied by fibrosis, which will unite the ends of the damaged myofibers. This also occurs in muscle damaged by invasive or metastatic sclerosing carcinomas. Cytokines released from damaged muscle fibers contribute to the signaling pathways that initiate macrophage infiltration and regeneration, but they also contribute to interstitial fibroblast activation. Collagen is inelastic, and thus large areas of fibrosis inevitably reduce the ability of the muscle to contract and to stretch. Fibrosis within locomotory muscles often results in obvious alteration of the gait.
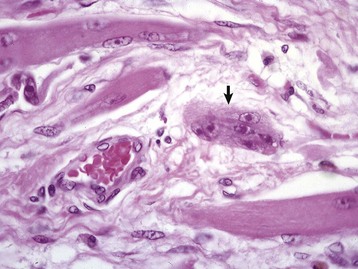
Fig. 15-17 Ineffectual regeneration.
Large, bizarre multinucleate muscle giant cells (arrow) are indicative of regeneration in an area in which the myofiber’s basal lamina has been damaged. Because the wall of the “myotube” of basal lamina is not intact, regenerating sarcoplasm exudes through the defect and in cross section this appears as a “muscle giant cell.” Formalin fixation, H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Because segmental necrosis and regeneration are such a common result of a wide variety of insults (e.g., overexertion, selenium deficiency, and toxic injury), a histologic diagnosis of segmental necrosis is often not helpful in determining the cause of the disease. Pathologic classification of lesions according to distribution (i.e., focal, multifocal, locally extensive, and diffuse) and duration (i.e., acute, subacute, chronic) has proven to be extremely useful in determining the possible causes of segmental muscle necrosis. Pathologic classification of degenerative myopathies has been further enhanced by Dr. Byron Kakulas, who introduced the terms monophasic necrosis and polyphasic necrosis. Monophasic lesions are of the same duration, indicative of a single insult. Polyphasic lesions indicate an ongoing degenerative process. Thus a focal monophasic lesion could be the result of a single traumatic incident such as an intramuscular injection (see Fig. 15-9, B). A multifocal monophasic lesion could represent a single episode of overly strenuous exercise (exertional myopathy) or a toxin being ingested on one occasion (e.g., a horse eating one dose of monensin; see Figs. 15-13, B, and 15-34, B). However, if the insult is repeated or ongoing, such as occurs in muscular dystrophy (see Fig. 15-45), selenium deficiency, or continuous feeding of a toxin, then new lesions (segmental necrosis) will form at the same time that regeneration is taking place; in other words, it will be a multifocal and polyphasic disease (see Fig. 15-15, B). Using this approach, it is sometimes possible to rule out a diagnosis (e.g., muscular dystrophy and selenium deficiency myopathy are typically polyphasic), but this is not an invariable rule. For example, in livestock with borderline concentrations of selenium, a sudden stress can cause a monophasic necrosis.
The term rhabdomyolysis is often encountered, particularly in the clinical arena, and especially in association with exercise-induced muscle injury (exertional rhabdomyolysis) in humans, horses, and dogs. Technically, rhabdomyolysis simply means necrosis (lysis) of striated muscle. This term was coined years ago to replace the term myoglobinuria as a description for the clinical entity of severe muscle injury associated with myoglobin release causing dark red discoloration of the urine. Thus rhabdomyolysis generally indicates the presence of a severe degenerative myopathy with a large degree of myofiber necrosis (see Fig. 15-36). In horses, the term exertional rhabdomyolysis has become firmly entrenched as a clinical entity in which exercise-induced muscle injury is the presenting sign. The term recurrent exertional rhabdomyolysis is often employed in cases in which repeated bouts of exercise-induced muscle damage have been documented.
Alteration in Myofiber Size
The normal myofiber diameter will vary, depending on fiber type, the muscle examined, the species, and the age of the animal. In some species (e.g., horse, cat, and humans), there are three distinct populations based on diameter: Type 1 fibers are the smallest, type 2B fibers the largest, and type 2A fibers are intermediate in size. Different sizes in diameters are in part a reflection of the oxidative needs of the fibers; oxygen diffuses more readily into the interior of small-diameter fibers. In the dog, all fiber types are oxidative, and fiber-type diameter is much more uniform. A histogram generated from morphometric analysis of fiber diameters will reveal the characteristics of individual muscles in various species. Not surprisingly, this type of detailed information is more readily available for human patients than for animals. Even without morphometric analysis, however, a pathologist experienced in examination of muscle can often determine whether there is a normal fiber-size distribution (based on fiber diameter in transverse section), or whether there is an increase in fiber-size variation. The finding of increased fiber-size variation suggests that something is wrong but in itself does not give any indication of cause. Increased fiber-size variation can be a result of fiber atrophy, fiber hypertrophy, or both and is considered part of the spectrum of changes included in the term chronic myopathic change (Box 15-6).
Atrophy
The term atrophy is used to imply either a reduction in the volume of the muscle as a whole or in the diameter of a myofiber. In the early stages of atrophy, it may be difficult or impossible to detect loss of muscle mass by gross observation, and morphometric evaluation of myofiber diameters may be required. Several cellular physiologic processes can be activated to result in muscle atrophy. These include induction of lysosomal action to result in autophagy of cytoplasmic components, apoptosis (programmed cell death), and activation of the cytoplasmic ubiquitin-proteosomal machinery. Lysosomal activation is prominent in denervation atrophy and is the basis for the positive reaction of denervated fibers in alkaline phosphatase and nonspecific esterase preparations. The causes of muscle fiber atrophy include physiologic and metabolic processes and denervation. In most instances, muscle atrophy is reversible provided the cause is corrected. The type of fiber undergoing atrophy varies, depending on the cause, therefore fiber typing is often required for a definitive diagnosis. Interestingly, type 2 fibers are the most likely to atrophy under a variety of circumstances (Box 15-7). Signalling molecules involved in muscle atrophy include tumor necrosis factor α (TNF-α) and interleukin (IL)-1 and IL-6.
Physiologic Muscle Atrophy: Decrease in myofiber diameter and therefore in the overall muscle mass is a physiologic response to lack of use (disuse atrophy), cachexia, and aging. Type 2 fibers are preferentially affected (Web Fig. 15-4). Disuse atrophy occurs relatively slowly, and only in muscles not undergoing normal contraction, as is caused by severe lameness or in muscles of a limb that is splinted or enclosed in a cast. The degree of disuse atrophy will be variable, but typically is not as severe as the atrophy of cachexia or denervation (see later discussion). Disuse atrophy is often asymmetric. Muscle atrophy caused by cachexia can be profound, especially in cases of cancer cachexia in which increased circulating levels of TNF alter the muscle metabolism, favoring catabolic processes rather than anabolic processes. Cachexia also develops relatively slowly and causes symmetric muscle atrophy. Muscle atrophy caused by aging can be considered a milder form of cachexia. Starvation, malnutrition, and chronic renal and cardiac diseases can also result in cachexia.
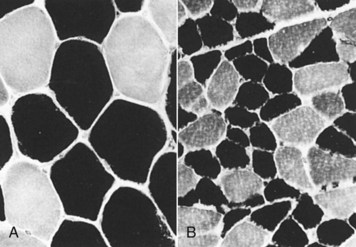
Web Fig. 15-4 Disuse atrophy, dog.
A, Transverse section of normal biceps femoris muscle. B, Same muscle, same magnification, 60 days after disuse. Both type 1 (light) and type 2 (dark) fibers are atrophic, but type 2 fibers are more severely affected. Frozen section, ATPase pH 9.8. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Atrophy Caused by Endocrine Disease: Preferential atrophy of type 2 fibers causing symmetric muscle atrophy also occurs because of various endocrine disorders. The most common are hypothyroidism and hypercortisolism in dogs. Aging horses with pituitary dysfunction or tumors (leading to equine Cushing’s syndrome) often develop type 2 muscle fiber atrophy. Myofibers contain a high concentration of surface receptors for several hormones, and atrophy caused by endocrine disease reflects the intimate interrelationship between the endocrine and the muscular systems.
Denervation Atrophy: Denervation atrophy, also known by the misnomer neurogenic atrophy, is not uncommon in veterinary medicine. Maintenance of normal myofiber diameter depends on trophic factors generated by an intact associated nerve. Loss of neural input results in rapid muscle atrophy, and more than half the muscle mass of a completely denervated muscle can be lost in a few weeks. This trophic effect is not dependent on contractile activity because denervation atrophy is not a feature of neuromuscular junction disorders such as botulism and myasthenia gravis. In these disorders, there is a failure of neuromuscular transmission, but the nerve to the muscle is intact; therefore the muscle is technically still innervated. Generalized neuropathies or neuronopathies, such as equine motor neuron disease, result in widespread and symmetric muscle atrophy. More commonly, however, only select nerve damage is present, resulting in asymmetric muscle atrophy. One example is equine laryngeal hemiplegia (roaring) secondary to damage to the left recurrent laryngeal nerve (Fig. 15-18). It should be pointed out that purely demyelinating disorders of peripheral nerves can cause profound neuromuscular dysfunction, but axons are still intact. Associated myofibers are not technically denervated and therefore do not undergo denervation atrophy.
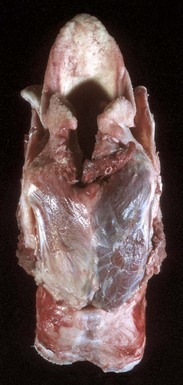
Fig. 15-18 Denervation muscle atrophy, left cricoarytenoideus dorsalis muscle, larynx, dorsal surface, horse.
Note the unilateral (left side) atrophy and pale gray-to-white discoloration of the muscle. This horse had a peripheral neuropathy, which led to laryngeal hemiplegia. (Courtesy College of Veterinary Medicine, University of Illinois.)
After denervation, fibers become progressively smaller in diameter as peripheral myofibrils disintegrate. If an atrophic fiber is surrounded by normal fibers, it will be pressed into an angular shape, called an angular atrophied fiber. The angular atrophied fibers of denervation atrophy most often occur either singly or in small contiguous groups (small group atrophy) (Fig. 15-19, A). In more severe denervating conditions, in which many fibers within muscle fascicles are undergoing denervation atrophy, there are no normal fibers to cause compression and angularity, and affected fibers occur as larger groups of small-diameter, rounded fibers (large group atrophy; Fig. 15-19, B). Although myofibrils disappear rapidly, muscle nuclei do not do so at the same rate, and therefore denervation atrophy is often associated with a notably increased concentration of myonuclei. The breakdown of glycogen in the myofiber is an early change in denervation atrophy, and therefore denervated fibers stain faintly or not at all with the PAS reaction.
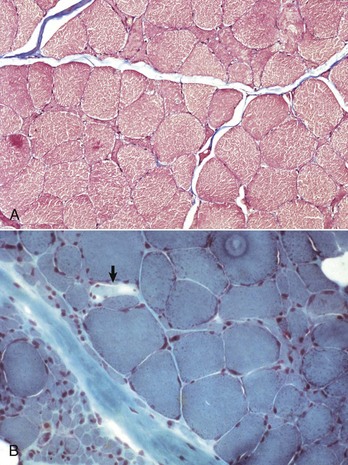
Fig. 15-19 Denervation atrophy, transverse sections.
Both sections are from horses with equine motor neuron disease. A, In relatively mild denervation, severely atrophied and angular fibers form small contiguous clusters indicative of small group atrophy. Formalin fixation, Masson trichrome stain. B, In severe denervation, entire fascicles of fibers undergo rounded atrophy characteristic of large group atrophy (lower left). Small group atrophy and admixed fiber hypertrophy are also present. A single pale stained fiber (arrow) is undergoing acute necrosis. There is also mild endomysial and perimysial fibrosis and mild fat infiltration (empty vacuoles in the upper right and lower left). Frozen section, modified Gomori’s trichrome stain. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
A histologic diagnosis of denervation atrophy may be suspected, based on the characteristic features of routinely processed muscles, but is most reliably documented with histochemistry or immunohistochemistry to detect fiber types. The loss of a nerve fiber to a muscle results in atrophy of all myofibers innervated by that nerve. Because of the intermingling of motor units forming a mosaic pattern of fiber types, myofibers undergoing denervation atrophy are scattered in a section of muscle. Because the motor neuron determines the histochemical myofiber type and because denervating diseases typically involve both type 1 and type 2 neurons or nerves, atrophy of both type 1 and type 2 myofibers in muscle fasciculi is the hallmark of denervation atrophy (Fig. 15-20, A).
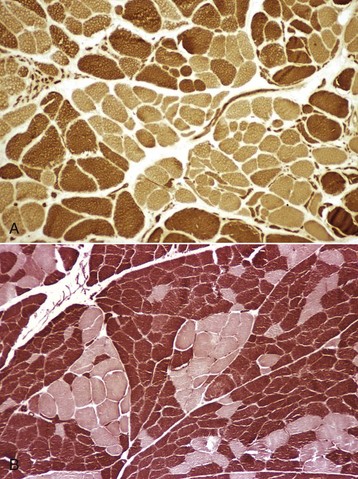
Fig. 15-20 Denervation atrophy and reinnervation, skeletal muscle, transverse sections.
A, Fiber typing reveals angular atrophy of both type 1 (light) and type 2 (dark) fibers, characteristic of denervation atrophy. In this case, there is also a loss of the normal mosaic pattern of fiber types, with groups of type 1 and of type 2 fibers indicative of reinnervation. This section is from a horse with laryngeal hemiplegia. Frozen section, ATPase pH 10.0. B, Fiber-type grouping in a dog indicative of denervation and reinnervation secondary to corticosteroid therapy. There is a loss of the normal mosaic pattern of fiber types, with grouping of type 1 (light) and type 2 (dark) fibers. The lack of angular atrophied fibers indicates that active denervation is not occurring at this time. Frozen section, ATPase pH 9.8. (A courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
In denervation atrophy, histologic examination of the intramuscular nerves is warranted because it may reveal axonal degeneration or loss of myelinated fibers. Masson trichrome stain can be useful here because it will differentiate myelin (red) from collagen (blue). If the nerve damage does not incapacitate the animal and the muscle can still be used (e.g., in locomotion), the remaining innervated myofibers often undergo notable hypertrophy because of increased workload. Often, the hypertrophied fibers in chronic denervation are type 1. Even without fiber typing, a pattern of severe small or large group atrophy (see Fig. 15-19, A), especially if associated with notable fiber hypertrophy (see Fig. 15-19, B), is strongly suggestive of denervation atrophy. A finding of damage in an associated peripheral nerve is definitive.
Under many circumstances, denervated muscle fibers can be reinnervated by subterminal sprouting of axons from adjacent normal nerves. Reinnervation results in return to normal myofiber diameter, but reinnervation is often from sprouts of a different type of nerve. Because muscle fiber type is a function of the motor neuron, the newly innervated myofiber takes on the fiber type determined by that neuron. This process results in a loss of the normal arrangement of type 1 and 2 myofibers and the formation of groups of the same fiber type adjacent to each other, called fiber-type grouping (see Fig. 15-20). Thus fiber-type grouping is the hallmark of denervation followed by reinnervation. What appears to be fiber-type grouping can also occur because of fiber-type conversion (most often to type 1 fibers) in chronic myopathic conditions. Careful evaluation of the structure and function of peripheral nerves helps distinguish neuropathic from myopathic changes. If previously reinnervated fibers are denervated again, the pattern includes large groups of atrophied fibers of a single fiber type, a process known as type-specific group atrophy. Type-specific group atrophy is far less common in animals than in humans. Fiber-type grouping and type-specific group atrophy can only be detected by methods that distinguish fiber types. Changes occurring as a result of denervation and reinnervation are illustrated in Fig. 15-21.
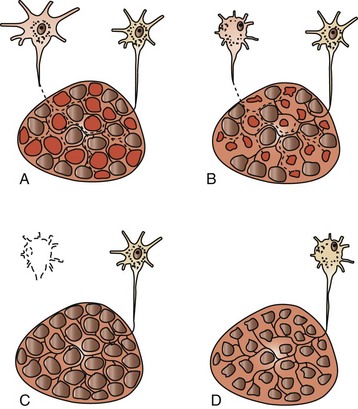
Fig. 15-21 Schematic diagram of motor units undergoing denervation and reinnervation.
A, Terminal axon branches innervate multiple myofibers, and myofiber type is determined by the electrical activity of the type of neuron innervating the myofiber. Normally the terminal axons of the motor units are intermingled, with the result that the differently stained myofiber types form a mosaic pattern. B, If a neuron (or axon) is damaged, the axon will undergo Wallerian degeneration, and the myofibers in that motor unit will undergo denervation atrophy. Small group atrophy is illustrated here. C, Axonal sprouts from a healthy neuron can reinnervate affected fibers and cause restoration of their normal diameter. The myofibers will assume the fiber type of the new motor unit, which often causes fiber-type conversion, leading to fiber-type grouping. D, If neuronal (or axonal) damage is progressive, denervation atrophy of large groups of fibers of a single type can occur, known as type-specific group atrophy. This type of atrophy is less common in animals than in humans. (Redrawn with permission from Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Atrophy Caused by Congenital Myopathy: Congenital myopathy in children is often associated with selective type 1 fiber atrophy. This finding is less common in the congenital myopathies identified thus far in animals. Selective type 1 atrophy is, however, a feature of feline nemaline myopathy, an animal model of congenital nemaline myopathy in children.
Hypertrophy
Myofibers increase in diameter by the addition of myofilaments. Physiologic hypertrophy is the normal process of myofiber enlargement that occurs with exercise conditioning. Compensatory hypertrophy occurs because of pathologic conditions that (1) decrease the number of functional myofibers and therefore increase the load on remaining fibers or (2) interfere with normal cellular metabolic or other physiologic processes. Compensatory myofiber hypertrophy is therefore considered a relatively nonspecific response to a variety of insults. Fibers undergoing compensatory hypertrophy can enlarge to more than 100 µm in diameter (normal is less than approximately 60 to 70 µm). Fiber hypertrophy often accompanies fiber atrophy, which contributes to increased fiber-size variation in various myopathic and neuropathic conditions.
Compensatory hypertrophy can occur because of a decrease in the number of functional myofibers. Thus, in a partially denervated muscle, the remaining innervated fibers hypertrophy (see Fig. 15-19, B), presumably as a result of increased workload. Pathologically hypertrophied fibers have less oxygen diffusion from interstitial capillaries to internal portions of the myofiber because of the increase in the distance from the capillary to the internal portions of the myofibers, which can lead to myofiber damage. Mechanical overloading of hypertrophied muscle fibers is also possible. For example, overloading of hypertrophied fibers can result in segmental necrosis of the hypertrophied fibers (see Fig. 15-19, B), or fibers can undergo longitudinal fiber splitting to generate one or more smaller-diameter “fibers,” all contained within the same basal lamina (Fig. 15-22). Serial sections of areas of fiber splitting generally reveals that splits do not extend the entire length of the myofiber. Fiber splitting is considered a form of cytoarchitectural alteration (see later discussion). Insulin-like growth factor 1 (IGF-1) is an important molecular signal involved in skeletal muscle hypertrophy. Genetic inactivation of the regulatory gene myostatin results in muscle hypertrophy caused by an increase in the number of myofibers.
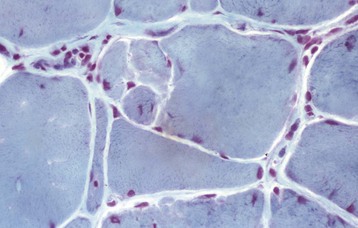
Fig. 15-22 Fiber type splitting of hypertrophied myofibers, nemaline myopathy, skeletal muscle, transverse section, cat.
Sarcolemmal ingrowth into the myofiber has resulted in multiple partitions with the formation of four myofibers; however, all myofibers are enclosed by one basal lamina. Frozen section, modified Gomori’s trichrome stain. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Cytoarchitectural Changes
In addition to fiber splitting, a variety of other cytoarchitectural changes can occur within myofibers. Some are degenerative, the result of an insult that damages the myofiber but does not culminate in myofiber necrosis. Others reflect underlying ultrastructural alterations that may be either pathologic or compensatory in nature. The functional significance of many of the myofiber cytoarchitectural changes is not known.
Vacuolar Change
Vacuolar change is a common cytoplasmic alteration. In formalin-fixed paraffin-embedded sections or in any sample subjected to less than ideal handling, true vacuolar change can be very difficult to distinguish from artifacts. Vacuoles can be an early manifestation of processes leading to necrosis, they can reflect underlying sarcotubular dilation as occurs in many myotonic conditions (see later discussion), and they can be caused by abnormal storage of carbohydrate or lipid, or they can reflect underlying myofibrillar abnormalities. Additional studies are often necessary to determine the nature of the vacuoles. When severe, such as in glycogen storage diseases, the term vacuolar myopathy is often employed.
Internal Nuclei
Myonuclei of mature myofibers in domestic animals are normally found peripherally, just beneath the sarcolemma. Nuclei located one nuclear diameter or more from the sarcolemma are known as internal nuclei. (note: The previously used term central nuclei is considered incorrect because few abnormally placed nuclei are exactly centrally located.) Internal nuclei are rare in normal mammalian muscle, but a small percentage can be found normally in avian and reptilian species. Rows of internal nuclei in small-diameter, slightly basophilic myofibers are characteristic of the myotubular stage of regeneration (see Fig. 15-15, B and C). In most species, myonuclei return to the normal peripheral location early in regeneration, within days of myotube formation. Rodents are the exception. In rodents, internal nuclei are retained after regeneration, which, in these species, provides a handy marker for identification of fibers that have undergone necrosis and regeneration. In other mammalian species, the presence of internal nuclei in normal or hypertrophied fibers is a nonspecific finding indicative of chronic myopathic change (Fig. 15-23; see Box 15-6). In hypertrophied fibers, the migration of myonuclei to the internal portion of the myofiber can precede the sarcolemmal infolding that creates longitudinal fiber splitting.
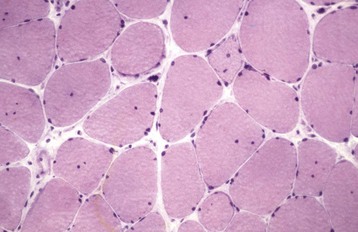
Fig. 15-23 Chronic myopathic change, medial triceps muscle, horse.
The variation in myofiber diameter and the presence of one or more internal nuclei in most myofibers are indicative of a chronic myopathic change. Frozen section, H&E stain. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Whorled and Ring Fibers
The cytoarchitectural rearrangements resulting in whorled and ring fibers are best appreciated in transverse sections. Whorled fibers contain spirals of cytoplasm with internally located nuclei. Whorled fibers can be seen in areas of chronic denervation, and also in areas in which myofiber necrosis with incomplete regeneration has occurred. Ring fibers (also known as ringbinden) contain a peripheral rim of sarcomeres oriented perpendicular to their normal orientation, resulting in peripheral radiating striations. Ring fibers are visible with many stains, both in frozen sections and in routinely processed sections. In either frozen or routine sections, they are best visualized in sections stained with PAS (Fig. 15-24, A) or iron hematoxylin. In humans, ring fibers are common in a specific form of inherited muscular dystrophy known as myotonic dystrophy, but they are also seen in other myopathic and in neuropathic conditions and therefore are not specific for myotonic dystrophy. Similarly, there is no animal disorder in which ring fibers are specific, and these fibers can be seen in a variety of myopathic and neuropathic conditions such as ovine congenital muscular dystrophy. The presence of ring fibers can only be considered a chronic myopathic change. For example, numerous ring fibers were found in muscle from the contralateral weight-bearing limb from a horse with long-standing, non–weight-bearing foreleg lameness.
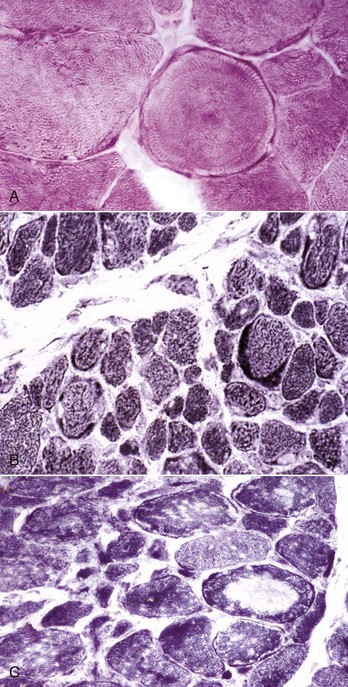
Fig. 15-24 Cytoarchitectural changes, skeletal muscle, transverse sections.
A, Ring fiber, extensor carpi radialis muscle, horse. A ring fiber is characterized by a peripheral rim of sarcomeres, arranged circumferentially around a myofiber and with their length at right angles to the long axis of the myofiber. Frozen section, PAS reaction. B, Irregular mitochondrial distribution with peripheral aggregates of mitochondria, Labrador centronuclear myopathy, temporalis muscle, dog. Frozen section, NADH reaction. C, Irregularity of mitochondrial distribution and “moth-eaten” fibers, polyneuropathy, dog. Fibers containing pale zones are characteristic of moth-eaten fibers. Frozen section, NADH reaction. (Courtesy Dr. B.A. Valentine, College of Veterinary Medicine, Oregon State University.)
Other Cytoarchitectural Changes
Many other cytoarchitectural changes reflect alterations in mitochondrial density or integrity and are best appreciated on examination of frozen sections, in which mitochondria can be visualized, or on ultrastructural examination. The presence of peripheral aggregates of mitochondria, which stain red with modified Gomori’s trichrome stain, form the basis of “ragged red” fibers. Ragged red fibers are a hallmark of mitochondrial myopathy in humans. In animals, however, ragged red fibers are common in various myopathic conditions and also occur in normal dog and horse muscle. Mitochondrial abnormalities are also detected by oxidative enzyme reactions such as NADH (Fig. 15-24, B and C) and SDH in frozen sections. Nemaline rods, formed by expansions of the Z-line material, stain purple to red with modified Gomori’s trichrome stain in frozen sections. These rods can also be seen in animals with other myopathic conditions. Moth-eaten fibers contain multiple pale zones because of loss of mitochondrial oxidative enzyme activity on frozen sections and occur in denervating disorders and in myopathic conditions (Fig. 15-24, C). Sarcoplasmic masses are pale-staining zones usually at the periphery of myofibers, but occasionally central. These can be seen in H&E-stained muscle sections and appear as light blue areas with few or no myofibrils. Ultrastructurally they often contain disarrayed myofilaments with or without degenerate mitochondria. Other less commonly encountered alterations in animal muscle are pale central cores visible with mitochondrial stains, tubular aggregates composed of sarcotubular membranes, and target fibers in which mitochondrial oxidative enzyme reactions reveal central clear zones surrounded by a thin rim of highly reactive cytoplasm. Other less commonly encountered alterations in H&E-stained sections of animal muscle are pale central cores. As demonstrated in sections stained by mitochondrial oxidative enzyme reaction (e.g., SDH) these are of three types: (1) cores rich in mitochondria and similar to the peripheral sarcoplasmic masses described above; (2) target fibers so designated because of a pale center surrounded by a rim of densely staining mitochondria; and (3) aggregates of sarcotubular membranes that do not stain with mitochondrial stains.
Chronic Myopathic Change
Evaluation of abnormal skeletal muscle often reveals chronic myopathic change, which includes alterations in myofiber diameter, cytoarchitectural alterations, and interstitial fibrosis and fat infiltration (see Box 15-6). Chronic myopathic change accompanies a variety of myopathic and neuropathic conditions. In particularly severe cases, a definitive cause may not be identified. Chronic inflammation or denervation and chronic degenerative myopathy resulting in repeated bouts of myonecrosis and regeneration often cause diffuse endomysial and perimysial fibrosis (see Figs. 15-19, B, and 15-48, B). Interstitial infiltration of muscle by mature adipocytes is less common than fibrosis and occurs most commonly in chronically denervated muscle (see Fig. 15-19, B), particularly neonatal muscle that lacks appropriate innervation (Fig. 15-25). Fat infiltration can also occur because of severe chronic degenerative myopathy. A chronically damaged or denervated muscle that develops profound fibrosis and/or fat infiltration can be grossly enlarged, despite atrophy or loss of myofibers—a condition known as pseudohypertrophy (see Fig. 15-9, D).
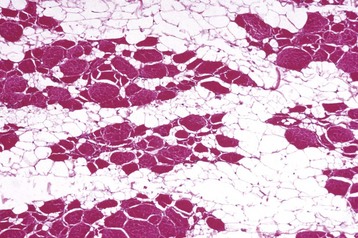
Fig. 15-25 Lipomatosis (steatosis), calf.
Lost myocytes have been replaced by mature adipocytes (clear [nonstaining] areas). Islands of remaining myofibers have groups of angular atrophied fibers admixed with hypertrophied fibers, suggestive of denervation atrophy. Formalin fixation, H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Disorders of Domestic Animals
Classification of muscle diseases based on lesions alone is not very satisfactory, and many classifications are based on cause (e.g., toxic myopathy or nutritional myopathy). An example of such a classification is given in Table 15-2. Myopathic conditions can be inherited or acquired. Inherited disorders can affect muscle metabolism or myofiber structure. Acquired muscle disease in livestock is often associated with nutritional deficiency or with ingestion of myotoxins, whereas acquired muscle disease in the dog is most often caused by immune-mediated inflammatory conditions. Other causes of acquired myopathies include ischemia, infectious agents, hormonal or electrolyte abnormalities, and trauma. There are also many neuropathic conditions that result in denervation atrophy (see peripheral nerve discussion). More information on most of the disorders described in this section can also be found under the appropriate species heading or in Web Appendix 15-1.
Degenerative
Degenerative myopathies are those resulting in segmental or global myofiber necrosis in which inflammatory cells are not the cause of the myofiber damage.
Disturbance of Circulation: Given the numerous capillary anastomosis and rich collateral circulation of skeletal muscle, only disorders that result in occlusion of a major artery or that cause widespread intramuscular vascular damage will result in myofiber necrosis (Box 15-8). Vascular occlusion of a major artery, most often aortoiliac thrombosis, occurs most commonly in cats (thromboembolism) and horses (mural thrombosis). Intramuscular vascular damage occurs in many species, and there are a variety of causes.
The basic factor in determining the effect of ischemia on muscle is the differential susceptibility of the various cells forming the muscle as a whole. Myofibers are the most sensitive, satellite cells less sensitive, and fibroblasts the least sensitive to anoxia. Thus obstruction of the blood supply to an area of muscle leads first to myofiber necrosis, then to death of satellite cells, and finally to the death of all cells, including the stromal cells. The size of skeletal muscle infarcts depends on the size of the vessel obstructed and the duration of blockage. Because of the numerous anastomoses, blockage of capillaries causes less severe ischemia but can result in segmental myofiber necrosis, which is usually multifocal and if the cause is ongoing, polyphasic, with regenerating and necrotic myofibers. However, when larger arteries are blocked, whole areas of muscle, including the satellite cells, are killed, resulting in a monophasic necrosis and healing by fibrosis. Ischemia can also cause peripheral nerve damage and neuropathy, leading to denervation atrophy of intact myofibers.
Increased intramuscular pressure can occur in a recumbent animal of sufficient weight after a prolonged period of recumbency, either because of disease or general anesthesia. Myofiber necrosis caused by recumbency can occur because of (1) decreased blood flow as a result of compression of major arteries, (2) reperfusion injury causing massive calcium influx into muscle cells when the animal moves or is moved and the compression relieved, (3) increased intramuscular pressure causing compartment syndrome (see later definition), or (4) any combination of these factors. Localized myonecrosis caused by recumbency is common in horses, cattle, and pigs; occurs only in large breeds of dogs; and is virtually unheard of in cats. In downer cows, the weight of the body of the animal in sternal recumbency can cause ischemia of the pectoral muscles and of any muscles of the forelimbs or hindlimbs that are tucked under the body. Ewes in advanced pregnancy with twins or triplets can develop an ischemic necrosis of the internal abdominal oblique muscle, which can lead to muscle rupture. Plaster casts or bandages that are too tight can put external pressure on muscles, leading to ischemia. The duration of ischemia determines the severity of necrosis and the success of regeneration (see the section on Necrosis and Regeneration). Postanesthetic myopathy is a monophasic, multifocal necrosis. In the downer cow, the lesions are multifocal to locally extensive (Fig. 15-26) and, depending on the duration since the onset of recumbency, can be either monophasic or polyphasic.
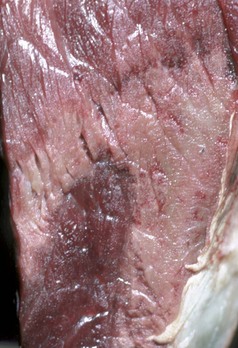
Fig. 15-26 Ischemic necrosis, downer cow syndrome, pectoral muscle, cow.
Increased intramuscular pressure during prolonged periods of recumbency has resulted in localized muscle pallor (lighter-colored areas of muscle) from myofiber necrosis secondary to decreased blood flow caused by compression of arteries. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Any severe insult, whether it be ischemia caused by recumbency or another myodegenerative disorder that causes myonecrosis within a muscle covered by a tight and nonexpansible fascia, can result in ischemic injury because early in the necrosis, there is increased intramuscular pressure. The resulting compromise of blood circulation leads to ischemic myodegeneration, which is known as compartment syndrome. The phenomenon of compartment syndrome is best illustrated in the anterior tibial muscle of humans after strenuous exercise. This condition is believed to be a consequence of swelling of the anterior tibial muscle, which is surrounded anteriorly by the inelastic anterior fascial sheath and posteriorly by the tibia. Swelling impedes blood supply, resulting in ischemia. A similar phenomenon occurs in muscles surrounded by tight fascia in animals, particularly horses. Horses that are recumbent because of general anesthesia can develop compartment syndrome affecting gluteal or lateral triceps muscles. Horses can also develop compartment syndrome in gluteal muscles because of exertional rhabdomyolysis and in temporal and masseter muscles because of selenium deficiency. Compartment syndrome is also possible in the temporal and masseter muscles of dogs with masticatory myositis.
Damage to intramuscular blood vessels will also cause myofiber necrosis. Vasculitis can cause areas of muscle damage (e.g., in horses with immune-mediated purpura hemorrhagica because of Streptococcus equi infection [see Fig. 15-33] and in pigs with erysipelas). Viral diseases that target blood vessels of many organs, such as bluetongue in sheep, can also affect muscle. Exotoxins produced by clostridial organisms cause myositis and severe localized vascular damage, leading to hemorrhage and myofiber necrosis (Table 15-3). The familial myopathy of Gelbvieh cattle is characterized by fibrinoid necrosis of intramuscular blood vessels and associated myonecrosis.
TABLE 15-3
Clostridial Toxins Causing Muscle Damage
| Toxin | Type | Action |
| α-Toxin | Calcium-dependent phospholipase | Hydrolyzes membrane phospholipids |
| θ-Toxin | Oxygen-labile cytotoxin (perfringolysin-O) | RBC and WBC lysis Induces platelet- activating factor leading to leukostasis and decreased tissue perfusion |
| κ-Toxin | Collagenase | Contributes to tissue lysis |
| µ-Toxin, γ- toxin | Hyaluronidases | Disruption of muscle integrity |
| ε-Toxin | Lipase | Lipid membrane lysis |
Nutritional Deficiency: Myofibers are particularly sensitive to nutritional deficiencies that result in the loss of antioxidant defense mechanisms. Nutritional myopathies are most common in livestock, including cattle, horses, sheep, and goats (Table 15-4). Although nutritional myopathy of livestock is often referred to as selenium/vitamin E deficiency, in the vast majority of cases, it is deficiency of selenium that is the cause of myofiber degeneration. The trace mineral selenium is a vital component of the glutathione peroxidase system, which helps to protect cells from oxidative injury. The high oxygen requirement combined with contractile activity makes striated muscle, both skeletal and cardiac, particularly sensitive to oxidative injury. Neonatal animals, which rely on stores of selenium accumulated during gestation, are most frequently affected. Affected muscle is pale as a result of necrosis (see Fig. 15-40); thus the common name white muscle disease. As should be evident from the previous discussion, a gross observation of pale muscle is not specific for necrosis caused by nutritional deficiency; therefore the term nutritional myopathy is much preferred.
TABLE 15-4
Nutritional and Toxic Myopathies
| Disorder | Species Affected | Cause |
| Nutritional myopathy | Horses, cattle, sheep, goats, camelids, pigs | Selenium or (less commonly) vitamin E deficiency |
| Ionophore toxicity | Horses, cattle, sheep, goats, pigs | Monensin, other ionophores used as feed additives |
| Plant toxicity | Horses, cattle, sheep, goats, pigs | Cassia occidentalis, other toxic plants; gossypol in cottonseed products |
| Pasture-associated myopathy (United Kingdom, midwestern United States) | Horses | Unknown—possible clostridial or fungal toxin |
Toxic Myopathies: Livestock are the animals most prone to develop a degenerative myopathy from the ingestion of a toxin (see Table 15-4). Myotoxins can be present in plants in pastures or hay and in plants or plant products in processed feed. Examples of toxic plants and plant products include Cassia (coffee senna), Karwinskia (coyotillo), Eupatorium (white snakeroot), Thermopsis spp., and gossypol present in cottonseed. Clinical signs are weakness, often leading to recumbency, and are accompanied by a moderate-to-severe increase in serum muscle enzyme concentrations. Gross and histologic findings of multifocal necrosis that can be either monophasic or polyphasic are typical. Diagnosis is based on identification of causative plants within feed, pasture, or stomach contents or when available, detection of toxic compounds in stomach content or liver.
Ionophore antibiotics, such as monensin, lasalocid, maduramicin, and narasin, are often added to ruminant feeds to enhance growth. Ionophores form lipid soluble dipolar reversible complexes with cations and allow movement of cations across cell membranes, often against the concentration gradient. This causes a disruption of ionic equilibrium that can be detrimental, especially to excitable tissue such as the nervous system, heart, and skeletal muscle. Ionophore toxicity results in calcium overload and death of skeletal (see Figs. 15-13, B, and 15-34) and cardiac muscle. Most domestic ruminants are quite tolerant of moderate ionophore levels, but toxicity occurs at very high levels. Most cases of ionophore toxicity involve the ingestion of monensin. The LD50 (the dose at which 50% of animals die) of monensin in cattle is 50 to 80 mg/kg, and the LD50 for sheep and goats is 12 to 24 mg/kg. Horses are exquisitely sensitive to ionophores and even very low levels are toxic, with an LD50 for monensin of only 2 to 3 mg/kg of body weight.
Exertional Myopathies: The ionic and physical events associated with myofiber contraction can under certain circumstances predispose a myofiber to necrosis. Exercise-induced myonecrosis, which can be massive, can occur because of simple overexertion. This outcome is well known in the capture and restraint of nondomesticated species, a syndrome known as capture myopathy. More often, however, exercise-induced myofiber damage occurs in animals with preexisting conditions such as selenium deficiency, muscular dystrophy, severe electrolyte depletion, or glycogen storage disease. The term exertional rhabdomyolysis (also known as exertional myopathy, azoturia, setfast, blackwater, Monday morning disease, and tying up) has long been applied to a syndrome recognized in horses (see Fig. 15-36). Only recently have underlying myopathic conditions been identified as the most common predisposing cause of equine exertional rhabdomyolysis (see the section on Muscular Disorders of the Horse). A similar disorder affects working dogs such as racing sled dogs and greyhounds, the cause of which is still unclear.
Trauma: External trauma to muscle includes crush injury, lacerations and surgical incisions, tearing caused by excessive stretching or exercise, burns, gunshot and arrow wounds, and certain injections. Some of these result in complete or partial rupture of a large muscle. The diaphragm is the most common muscle to rupture and in dogs and cats is most often the result of a sudden increase in intraabdominal pressure such as from being hit by a car. In horses, diaphragmatic rupture is thought to occur most often during falls in which the pressure of the abdominal viscera causes diaphragmatic damage. A partial rupture of a muscle results in a tear in the fascial sheath, through which the muscle can herniate during contraction. In racing greyhounds, spontaneous rupture of muscles, such as the longissimus, quadriceps, biceps femoris, gracilis, triceps brachii, and gastrocnemius, can occur during strenuous exercise. In horses, damage to the origin of the gastrocnemius muscle has been linked to overexertion during exercise or while struggling to rise. Tearing of muscle fibers occurs in the adductor muscles of the hind limbs of cattle doing the “splits” (sudden bilateral abduction) on a slippery floor. Because there is often extensive disruption of the myofibers’ basal laminae, most of the healing is accomplished by fibrosis. If muscle trauma is accompanied by fractures of bones and the animal moves the limb, further trauma by laceration by sharp bone fragments can result.
An abnormal response to localized muscle trauma is thought to be a possible underlying cause of two uncommon reactions of muscle: myositis ossificans and musculoaponeurotic fibromatosis. The term myositis ossificans is a misnomer, as the lesion does not involve inflammation, but it has attained the status of acceptance by common usage. Myositis ossificans is a focal lesion usually confined to a single muscle and has been seen in horses, dogs, and humans. The lesion is essentially a focal zone of fibrosis with osseous metaplasia, often with a zonal pattern. The central zone contains proliferating undifferentiated cells and fibroblasts; the middle one, osteoblasts depositing osteoid and immature bone; and the outer one, trabecular bone, which may be being remodeled by osteoclasts. These lesions can cause pain and lameness, which are often cured by surgical excision. A connective tissue disorder in cats, fibrodysplasia ossificans progressiva, has been inappropriately called myositis ossificans. Musculoaponeurotic fibromatosis has so far been described only in horses and humans. It is a progressive intramuscular fibromatosis that has also been called a desmoid tumor. Musculoaponeurotic fibromatosis is not, however, considered to be a true neoplastic process. Progressive dissecting intramuscular fibrosis accompanied by myofiber atrophy are the characteristic features. In most cases, the extent of intramuscular involvement makes surgical excision impossible, although wide excision of early lesions has proved to be curative.
Inflammatory Myopathies (Myositis, Myositides [Plural])
In addition to the misnomer “myositis ossificans,” the term myositis has been inappropriately applied to various other veterinary disorders, such as exertional and nutritional myopathy, in the horse. These two disorders are degenerative myopathies not inflammatory myopathies. It is vitally important to distinguish between a true myositis and a degenerative myopathy in which there is a secondary inflammatory response. In the normal response to the myofiber necrosis, the necrotic segment is infiltrated by macrophages recruited from the circulating monocyte population (see Figs. 15-14, B, and 15-15, A), which phagocytose the cellular debris. Severe acute necrotizing myopathy can also be accompanied by a certain degree of infiltrating lymphocytes, plasma cells, neutrophils, and eosinophils. Cytokines released from damaged muscle fibers are likely to recruit a variety of inflammatory cells under various circumstances, but these cells are not involved in causing the muscle cell damage. True myositis occurs only when inflammatory cells are directly responsible for initiating and maintaining myofiber injury and when inflammation is directed at the myofibers and not at the stroma. In some cases, it may take careful evaluation of the overall tissue changes, an understanding of the probable underlying cause, and years of experience with muscle pathology to differentiate a florid cellular response with macrophages on a “clean-up” mission from true inflammation. Lymphocytic myositis must also be distinguished from lymphoma involving skeletal muscle (see the section on Neoplasia).
Bacterial: Bacterial infections of muscle are not uncommon, particularly in livestock. Bacteria can cause suppurative and necrotizing, suppurative and fibrosing, hemorrhagic, or granulomatous lesions (Table 15-5). Bacterial infection can be introduced by direct penetration (wounds or injections), hematogenously, or by spread from an adjacent cellulitis, fasciitis, tendonitis, arthritis, or osteomyelitis (see the section on Portals of Entry).
TABLE 15-5
| Infectious Agent | Species Affected |
| Clostridium spp. causing myositis (e.g. Cl. septicum, Cl. chauvoei, | Horses, cattle, sheep, goats, pigs |
| Clostridium botulinum | Horses, cattle, sheep, goats, dogs |
| Pyogenic bacteria (e.g., Arcanobacterium pyogenes, Corynebacterium pseudotuberculosis) | Horses, cattle, sheep, goats, pigs, cats |
| Bacteria causing fibrosing and granulomatous lesions (e.g., Actinomyces bovis, Actinobacillus lignieresii) | Cattle, sheep, goats, pigs |
Various clostridial species, particularly Clostridium perfringens, Clostridium chauvoei, Clostridium septicum, and Clostridium novyi, can elaborate toxins (see Table 15-3) that damage myofibers and intramuscular vasculature, resulting in hemorrhagic myonecrosis (see Figs. 15-32 and 15-38). Toxemia is typical and often lethal. Clostridial myositis is most common in cattle and horses. Clostridial myositis has also been called gas gangrene and malignant edema in horses and blackleg in cattle.
Pyogenic bacteria introduced into a muscle usually cause localized suppuration and myofiber necrosis. This may resolve completely or become localized to form an abscess. In some cases, the infection can spread down the fascial planes (see Fig. 15-12). For example, a nonsterile intramuscular injection into the gluteal muscles of cattle can cause an infection that extends down the fascial planes of the muscles of the femur and tibia and erupts to the surface through a sinus proximal to the tarsus. Although the majority of inflammation involves fascial planes, some bacteria extend into and cause necrosis of adjacent muscle fasciculi. Streptococcus zooepidemicus (horses), Arcanobacterium pyogenes (cattle and sheep), and Corynebacterium pseudotuberculosis (horses, sheep, and goats) are common causes of muscle abscesses. After bite wounds from other cats, cats can develop cellulitis caused by Pasteurella multocida that extends into the adjacent muscle.
Bacteria causing single or multiple granulomas (focal or multifocal granulomatous myositis) are relatively uncommon. Most such lesions are caused by Mycobacterium bovis (tuberculosis), usually in cattle and pigs, but this disease is rare in North America.
Chronic fibrosing nodular myositis of the tongue musculature in cattle is the result of infection with Actinobacillus lignieresii (wooden tongue) or Actinomyces bovis (the agent causing lumpy jaw). A similar lesion caused by Staphylococcus aureus is known as botryomycosis and is most commonly seen in horses and pigs. It is most often wound related and can occur at a variety of sites. Histologically, actinobacillosis, actinomycosis, and botryomycosis are similar in that the lesions are encapsulated inflammatory lesions containing a central focus of “radiating clubs” of amorphous eosinophilic material associated with bacteria and neutrophils (Splendore-Hoeppli reaction). Neutrophils admixed with macrophages (pyogranulomatous inflammation) can also be seen. Gram-stained tissue can be used to differentiate between the clusters of Gram-positive cocci in Staphylococcus infection, the Gram-positive bacilli causing actinomycosis (Actinomyces bovis), and the Gram-negative bacilli causing actinobacillosis (Actinobacillus lignieresii).
Viral: Relatively few of these are recognized in veterinary medicine. Spontaneous ones are listed in Table 15-6. Gross lesions may or may not be visible and if present, are small, poorly defined foci or streaks. Muscle lesions induced by viruses are either infarcts secondary to a vasculitis, as seen in bluetongue in sheep, or multifocal necrosis, presumably because of a direct effect of the virus on the myofibers.
Parasitic: Parasitic infections of the skeletal muscles of domestic animals are not uncommon and include protozoal organisms and nematodes. The most important ones are listed in Table 15-7 and are discussed under the appropriate species heading. Most parasitic diseases have little pathologic or economic importance, with the exceptions of Neospora caninum, Hepatozoon americanum, and Trypanosoma cruzi in dogs and Trichinella spiralis in pigs.
TABLE 15-7
| Infectious Agent | Type of agent | Species Affected |
| Sarcocystis spp. | Protozoan | Horses, cattle, sheep, goats, camelids, pigs |
| Trichinella spiralis | Nematode | Pigs |
| Neospora caninum | Protozoan | Dogs, fetal cattle |
| Trypanosoma cruzi | Protozoan | Dogs |
| Cysticercus spp. | Cestode (larval form) | Cattle, sheep, goats, pigs |
| Nematode larval migrans | Nematode | Dogs |
| Hepatozoon americanum | Protozoan | Dogs |
As the name Sarcocystis suggests, intramyofiber protozoal cysts caused by Sarcocystis spp. are a common finding. This protozoal organism is a stage in the life cycle of an intestinal coccidium of carnivores that uses birds, reptiles, rodents, pigs, and herbivores as an intermediate host. Ingestion of oocysts by an intermediate host releases sporozoites that penetrate through the intestinal wall, enter blood vessels, and are hematogenously disseminated and invade tissue, including muscle. This parasite rarely causes clinical disease and is therefore most often considered an incidental finding. Sarcocystis infection of muscle is seen most often in horses, cattle, and small ruminants and occasionally in cats. Because they are intracellular, cysts are protected from the host’s defense mechanisms; thus there is no inflammatory response (Fig. 15-27).
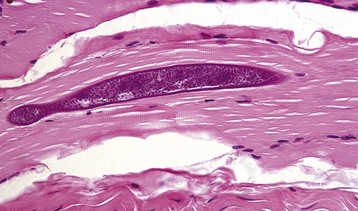
Fig. 15-27 Sarcocystosis, skeletal muscle, longitudinal section, cow.
The elongate encysted intramyofiber protozoan is characteristic of Sarcocystis spp. There is no associated inflammation. These parasites are common in the muscles of many species of domestic animals and are usually an incidental finding. Formalin fixation, H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Immune-Mediated: Immunologically induced myositis, not associated with vascular injury, has been recognized primarily in the dog. Rarely, immune-mediated myositis occurs in cats and horses. Infiltrating lymphocytes, most often cytotoxic T lymphocytes, are the cause of myofiber injury. Although cytotoxic T lymphocytes are the effector cells causing myofiber damage, the inflammatory infiltrate is a mixture of lymphocyte types. The characteristic histologic pattern of immune-mediated myositis is an interstitial and perivascular lymphocytic infiltration (Fig. 15-28, A, see also Fig. 15-48), often with invasion of intact myofibers by lymphocytes (Fig. 15-28, B). A variety of forms of immune-mediated myositis occur in the dog and can be localized to specific muscles, presumably because of unique myosin isoforms within those muscles. These are listed in Table 15-8. Acquired myasthenia gravis is also an immune-mediated disease and is included in this table for completeness, but this is a disorder causing damage to the neuromuscular junction rather than to myofibers. In cats, feline immunodeficiency virus infection is a cause of immune-mediated myositis. In horses, lesions consistent with immune-mediated myositis are occasionally found after exposure to Streptococcus equi ssp. equi or infection with equine influenza virus. It should be pointed out that small perivascular and interstitial infiltrates of lymphocytes, with no apparent myofiber damage, are a frequent incidental finding in equine muscle.
TABLE 15-8
Immune-Mediated Muscle Disorders
| Disorder | Species Affected |
| Purpura hemorrhagica | Horses |
| Viral-associated | Horses, cats |
| Polymyositis | Dogs, horses (rare) |
| Masticatory myositis | Dogs |
| Extraocular muscle myositis | Dogs |
| Acquired myasthenia gravis | Dogs, cats |
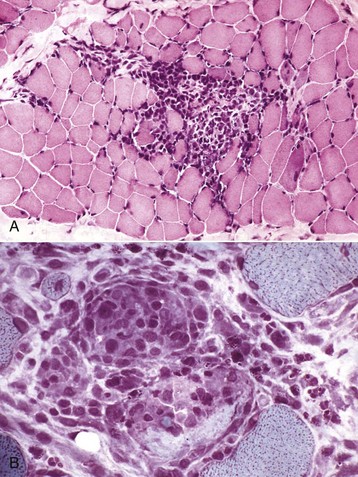
Fig. 15-28 Immune-mediated myositis, canine polymyositis, skeletal muscle, transverse sections, dog.
A, There is a dense interstitial infiltrate of primarily mononuclear inflammatory cells. Formalin fixation, H&E stain. B, Note the interstitial infiltrate of mononuclear inflammatory cells and mononuclear cells that have invaded intact myofibers causing myofiber necrosis. Frozen section, modified Gomori’s trichrome stain. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. B.J. Cooper, College of Veterinary Medicine, Oregon State University.)
Immune-mediated vasculitis resulting in muscle injury occurs in horses and is known as purpura hemorrhagica. Purpura hemorrhagica has been classically associated with Streptococcus equi ssp. equi, but other bacteria, such as Corynebacterium pseudotuberculosis, can also cause purpura hemorrhagica.
Congenital and Inherited Disorders
Muscle is subject to numerous hereditary, congenital, and neonatal defects (Web Table 15-2). Muscular disorders that are apparent at birth are congenital, but they may or may not be inherited. Inherited disorders can manifest at birth or soon thereafter, or they may not be apparent for many years. Molecular biologic studies and development of molecular genetic tests have greatly enhanced our understanding of several muscular disorders of animals and the ability to detect affected and carrier animals.
WEB TABLE 15-2
Confirmed or Suspected Inherited Myopathies in Animals
HYPP, Hyperkalemic periodic paralysis.
Question mark denotes that inheritance pattern is suspected but not proved.
Anatomic Defects: Anatomic defects in skeletal muscle are apparent at birth or soon thereafter. These defects can be either genetic or acquired and result from either abnormal in utero muscle development or abnormal innervation.
Innervation Defects: Congenital defects in the lower motor neuron system, involving motor neurons or peripheral nerves, result in severe alteration of myofiber development. Denervation occurring in fetal and neonatal animals can result in very complex muscle lesions because of the importance of innervation in myofiber development and maturation. Depending on the nature of the nervous system defect, muscular lesions can reflect failure of innervation, denervation of previously innervated fibers, or a combination of both. The most common example of this is arthrogryposis in cattle and sheep in which in utero infection or toxin ingestion causes nervous system lesions that lead to failure of innervation or to denervation of skeletal muscle. Additionally, a disorder thought to have a genetic basis has been reported in black Angus cattle and results in failure of innervation of skeletal muscle. Failure of innervation or severe denervation injury in utero most often result in failure of the myofibers to develop and their subsequent replacement by adipose tissue (fatty infiltration). This outcome can be severe in affected muscle and may be the basis for some cases of congenital muscular steatosis in livestock (see Figs. 15-9, D, and 15-25).
Genetic Defects: Congenital muscular hyperplasia (double muscling) is a genetic disease causing a congenital anatomic skeletal muscle defect (increased number of myofibers) in cattle, dogs, and children. This disorder is caused by defects in the myostatin gene, which controls in utero muscle development. There is more information on this disease in Web Appendix 15-1. With continued selective breeding and advancement in molecular biologic techniques, it is likely that other genetic defects affecting muscle structure may occur or be recognized.
Failure of Normal Development: In addition to failure of myofiber maturation caused by innervation defects, inherent myofibrillar developmental defects can occur. This is exemplified by myofibrillar hypoplasia causing splay leg in neonatal pigs. A similar condition has been reported in a calf.
Congenital defects in the diaphragmatic muscle (diaphragmatic hernia) can occur in all species but are most well documented in dogs and rabbits. A genetic basis with a multifactorial inheritance is suspected. Clinical signs of respiratory distress caused by herniation of abdominal viscera into the thoracic cavity generally occur at or shortly after birth. Defects in the left dorsolateral and central portions of the diaphragm because of failure of closure of the left pleuroperitoneal canal are most common.
Muscular Dystrophy: The term muscular dystrophy has been grossly misused in the veterinary literature. In the 1930s, the term nutritional muscular dystrophy was applied to a disease more appropriately classified as nutritional myopathy, and the misuse of this term has been a source of confusion for years as to exactly what is meant by muscular dystrophy. Using the definition applied to humans, muscular dystrophy is an inherited, progressive, degenerative primary disease of the myofiber characterized histologically by ongoing myofiber necrosis and regeneration (polyphasic necrosis). Several types of muscular dystrophy occur in humans and animals. The enormous recent advances in genetic and molecular characterization of muscle diseases have resulted in defining their exact genetic defects, such as those in the dystrophin gene responsible for Duchenne’s muscular dystrophy and trinucleotide repeat sequences in myotonic dystrophy, and in the reclassification of others. Similarly, reevaluation of some inherited disorders previously classified as muscular dystrophy, such as muscular dystrophy in sheep and cattle, suggests that they would be better classified as progressive congenital myopathies.
Congenital Myopathies: Those inherited disorders of muscle that do not qualify as anatomic defects, muscular dystrophy, myotonia, or a metabolic myopathy (see later discussion) are classified as congenital myopathies. These include structural defects leading to abnormal myofiber cytoarchitecture. In some cases, the defective gene is known, whereas the cause of others remains undetermined.
Myotonia (Channelopathies): Myotonia is defined as the inability of skeletal muscle fibers to relax, resulting in spasmodic contraction. Various inherited myotonic conditions have been recognized in humans and animals for many years. Only recently has the basis for many of these myopathies been determined. Most have been found to be related to inherited defects resulting in abnormal ion channel function. Maintenance of ionic equilibrium and control of the ionic fluxes of excitable tissue, such as muscle, are critical to normal muscle functioning. A variety of sarcolemmal ion channels exist that control fluxes of ions such as sodium, potassium, chloride, and calcium. Defective sodium or chloride channels most often result in myotonia.
Metabolic Myopathies: Inherited disorders of muscle metabolism (see Web Table 15-2) are characterized by reduced muscle cell energy production. Clinical signs include exercise intolerance, exercise-induced muscle cramps, and rhabdomyolysis (acute segmental myofiber necrosis). Metabolic defects can involve glycogen metabolism, fatty acid metabolism, or mitochondrial function. Metabolic disorders often cause increased blood lactate after exercise. Inheritance patterns vary. Glycolytic, glycogenolytic, and nonmitochondrial DNA–encoded enzyme defects are generally inherited in an autosomal recessive manner. Defects involving mitochondrial DNA–encoded enzymes are inherited through the dam because all mitochondria are contributed by the oocyte.
The pathways of glycolysis and glycogenolysis are complex, involving a cascade of enzymatic reactions. Deficiency of a glycolytic or glycogenolytic enzyme leads to accumulation of glycogen and in some cases glycogen-related proteoglycans. There are many different types of glycogen storage diseases and their categorization is dependent on which enzyme is deficient. Of the types of glycogenoses recognized in humans, five types (II, III, IV, V, and VII) cause glycogen accumulation in muscle. Of the glycogenoses affecting muscle, only types II (acid maltase deficiency), IV (glycogen branching enzyme deficiency), V (myophosphorylase deficiency), and VII (phosphofructokinase deficiency) have so far been recognized in animals. Storage diseases in which glycogen accumulates in muscle have been described in horses, cattle, sheep, dogs, and cats.
Inherited lipid storage myopathies have not yet been described in animals, although dogs appear to have a predilection for development of neuromuscular weakness because of acquired lipid storage myopathy with concurrent reduction in skeletal muscle carnitine activity. Mitochondrial myopathies are rarely recognized in animals, perhaps because of the difficulty in confirming mitochondrial defects. A few such disorders have been described in dogs, and a mitochondrial myopathy has been reported in one Arabian horse. Mitochondrial disorders may affect only muscle, or muscle involvement may be part of an encephalomyopathic condition.
Malignant Hyperthermia (MH): MH is a condition characterized by unregulated release of calcium from the sarcoplasmic reticulum, leading to excessive myofiber contraction that generates heat, resulting in a severe increase in body temperature. MH is often fatal. In humans, pigs, horses, and dogs, a congenital defect in the sarcoplasmic reticulum calcium-release channel, the ryanodine receptor, causes dysregulation of excitation-contraction coupling leading to MH. Episodes in affected individuals can be triggered by general anesthetic agents, especially halothane, or by stress, thus the name porcine stress syndrome for the disorder in pigs (see Fig. 15-43).
A MH-like condition can also occur because of other myopathic conditions, especially those that result in uncoupling of mitochondrial oxidative phosphorylation from the electron transport chain. Inherently uncoupled mitochondria within brown fat are the physiologic basis for production of heat during breakdown of this fat in neonates, and pathologically uncoupled or loosely coupled mitochondria in muscle as a result of an underlying myopathy release energy as heat.
Gross and microscopic lesions are described in the discussion on the disorder in the section on Disorders of Pigs.