Uvea
The uveal tract, like any other lamina propria, is in free communication with peripheral blood and is therefore not protected against any noxious agents in circulation. It is protected from physical injury (as are most other parts of the globe) by the thick fibrous tunic of the sclera and by the bony orbit.
Direct uveal injury, however, seems to be less important to the globe than the spread of the uveal disease to other parts of the eye that are less able to resist an insult or regenerate (particularly the lens and retina). The protection of other portions of the globe from inflammation in the uvea rests on two main mechanisms: the blood-ocular barrier and the unique immunologic phenomenon known as anterior chamber immune deviation.
The blood-ocular barrier is created by tight junctions between endothelial cells of the iris and retinal blood vessels and tight junctions between adjacent epithelial cells of the inner nonpigmented ciliary epithelium and the retinal pigment epithelium. Unless the damage to the uvea itself is so severe as to disrupt these tight junctions, they prevent most chemical and infectious agents from gaining access to vulnerable intraocular tissues such as the lens and retina.
The location of this barrier is important in understanding the different reactions to injury by different parts of the uveal tract. For example, the presence of tight junctions between the RPE cells is extremely important in preventing exudate from within the choroid gaining access to the subretinal space. Subretinal fluid accumulation can cause vision-threatening retinal detachment, so the blood-eye barrier at that site provides important protection.
In the anterior chamber, however, the situation is quite different. Here the barrier is at the level of the endothelial cells of the iris. During any substantial inflammation, this barrier is broken as part of the increased vascular permeability inherent in any acute inflammation. This allows infectious agents, inflammatory mediators, and leukocytes to flood into the stroma of the iris. Because the iris has no “barrier” along its anterior surface, these agents, cells, and chemicals then quickly diffuse into aqueous humor of the anterior chamber. From here, they may disperse throughout the globe and damage the lens or even the retina.
Anterior chamber-associated immune deviation (ACAID) is a specialized immune response by which infectious agents and many other antigens introduced into the anterior chamber cause only a highly controlled immune response that effectively eliminates the provoking antigen, while producing minimal bystander tissue injury that would threaten nearby ocular tissue. ACAID protects the eye from antigen-specific, immune-mediated injury from delayed-type hypersensitivity (DTH). The eye contains no lymphoid tissue. To initiate an immune response, antigens within the anterior chamber must first be captured by intraocular antigen presenting cells, exit the globe through the normal aqueous outflow pathways, and reach the marginal zones of the spleen. Here antigen-specific T lymphocytes are activated to differentiate into regulatory cells that interfere with the induction of DTH, thus muting DTH and sparing the eye from inflammation induced by DTH.
Lens
The defenses of the lens against injury are almost purely passive. They are the fibrous tunic of the cornea and sclera, the bony orbit, the eyelids, and a thick collagenous resilient lens capsule.
Retina And Vitreous
The retina and vitreous, like the lens, rely mostly on their protected intraocular niche, and like the lens, their active defense mechanisms are negligible. They are protected from most physical and chemical injury by the bony orbit, the fibrous shell of cornea and sclera, and by the uvea and from infectious (and to some extent, from toxic and metabolic) diseases by the blood-eye barrier created by tight junctions between RPE cells and by the tight junctions of the retinal vascular endothelium. The retina has essentially no defense against infectious agents, radiation, or noxious chemicals arriving through the vitreous. Ischemic injury is a major threat to retinal viability. It is protected by having an autoregulated vascular system that allows retinal perfusion to remain relatively normal despite wide fluctuations in systemic blood pressure. The injured retina also produces angiogenic growth factors and has a powerful system of scavengers to counteract the damaging effects of excitatory neurotoxins, nitric oxide, and other potentially damaging by-products of ischemia. The retina and vitreous have no resident phagocytes or other cellular components of the immune system.
Eyelids, Conjunctiva, And Orbit
Eyelids: Like skin anywhere else, the defenses of eyelid against injury include both structural and cellular defenses, including hair, keratin, epithelial tight junctions, and a potent intraepithelial and superficial dermal immune system (see discussion on skin). Like skin, it becomes susceptible to infectious disease when those defenses are compromised by excessive moisture, metabolic disease, or mechanical injury. The eyelid is susceptible to all of the same infectious, nutritional, and immune diseases as skin in any other location.
Conjunctiva: The conjunctiva is protected from most physical and chemical injuries by the eyelids and by the tear film. It has tight junctions between the epithelial cells to prevent easy access by infectious or chemical agents into the underlying lamina propria. It is capable of rapid replication in the event of injury and readily undergoes squamous metaplasia as an adaptive survival mechanism in response to chronic low-grade irritation of any type. The resident mucosal immune system (MALT) functions similarly to immune systems in other mucosal sites, such as the upper respiratory tract, lungs (bronchus-associated lymphoid tissue [BALT]), and gastrointestinal tract (gut-associated lymphoid tissue [GALT]) (see Chapter 13).
Responses To Injury
Injury to the corneal epithelium occurs from chemical or physical injury, from deficiency in the quantity or quality of the tear film, or by colonization of the corneal epithelium by infectious agents specifically adapted to that environmental niche. Common examples of such damage include abrasion from misdirected eyelashes or improperly structured eyelids, irritating plant or other foreign bodies within the conjunctival sac, or lacerations inflicted in the course of fighting or running through heavy brush. The cornea is also very susceptible to changes in the quality or quantity of the tear film that may result from primary disease of the lacrimal gland or from desiccation caused by enlargement of the globe or impaired closure of the eyelids.
The corneal endothelium may be injured by increased intraocular pressure (glaucoma), mechanical damage from an anteriorly displaced lens, or from injury mediated by leukocytes (so-called corneal endothelialitis).
The corneal stroma is most frequently injured as a sequela to epithelial damage. It may also be directly injured by lacerations and penetrating injury, implantation of infectious agents subsequent to penetrating injury, or rarely by percolation of chemical or infectious agents into the corneal stroma from the blood vessels at the limbus. Injury to the corneal endothelium will also affect the stroma, allowing the dehydrated but strongly hydrophilic stroma to absorb fluid from the aqueous humor. This results in corneal edema and substantial visual impairment (Box 20-10).
The great majority of corneal lesions encountered in clinical practice result from epithelial injury as a result of trauma or desiccation. Almost all corneal diseases listed in clinical textbooks reflect one or more of only three pathologic processes: adaptive cutaneous metaplasia in response to mild irritation, epithelial and/or stromal necrosis, and repair (wound healing) (Box 20-11). The varied clinical manifestations of these three fundamental processes are often given specific clinical names that serve to confuse rather than clarify.
Adaptive Cutaneous Metaplasia to Mild Persistent Irritation: The usual response of the cornea to persistent mild irritation is cutaneous metaplasia, which is a combination of keratinization, epithelial hyperplasia, epithelial pigmentation, subepithelial fibrosis, and vascularization (Figs. 20-59 and 20-60). In short, a cornea that is challenged to adapt to mild persistent irritation does so by recalling its genetic heritage as skin and comes to resemble skin in every way except for the acquisition of hair follicles. Not all examples of cutaneous metaplasia involve the entire range of adaptive changes; it is possible, for example, to have keratinization without pigmentation or epidermal metaplasia without accompanying stromal fibrosis and vascularization. It depends on the nature of the irritation. The most common causes for such adaptation include chronic desiccation and mechanical irritation from eyelid diseases such as entropion or anomalous placement or direction of eyelashes. Desiccation may result from defective production of tears as in keratoconjunctivitis sicca, from improper distribution of tears because of improper eyelid structure or function, or because of ocular enlargement that prevents the lids from closing. Because the cornea is permitted to exist in its privileged state only because it is constantly bathed in a protective and nourishing tear film, the partial or complete removal of that tear film represents a significant challenge to corneal homeostasis. If the change occurs slowly, the cornea successfully adapts by undergoing cutaneous metaplasia. If the desiccation occurs very rapidly, then the cornea will likely ulcerate.
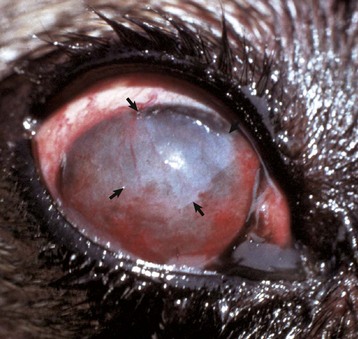
Fig. 20-59 Corneal cutaneous metaplasia, keratoconjunctivitis sicca, cornea, dog.
The diffuse corneal opacity (outlined by arrows) is caused by a combination of epithelial hyperplasia and keratinization, as well as stromal scarring and vascularization. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
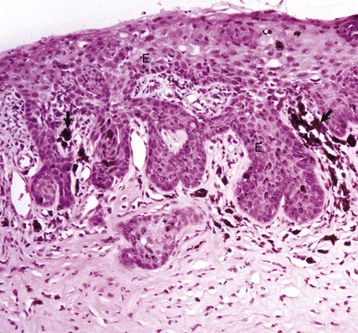
Fig. 20-60 Corneal cutaneous metaplasia, keratoconjunctivitis sicca, cornea, dog.
Chronic desiccation has caused adaptive epithelial hyperplasia (E), epithelial and stromal pigmentation (arrows), and stromal scarring. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Cutaneous metaplasia is a complex event that probably reflects the failure of conjunctival epithelial cells and stroma to acquire corneal phenotypes as they grow toward the center of the cornea to replace injured corneal tissue. The corneal epithelium has a finite number of replicative cycles. Persistent injury that exceeds the replicative ability of the corneal epithelium itself will require ingrowth of germinal cells that reside in the bulbar conjunctiva at the limbus. Similarly, injured corneal stroma often needs to recruit fibroblasts from the adjacent conjunctival lamina propria or sclera. Those chemical signals required to induce conjunctival or scleral tissue to undergo maturation into more appropriate “corneal” structure remain unknown.
Some of the lesions of cutaneous metaplasia are reversible, albeit only very slowly. Stromal fibrosis, however, is a permanent change, and the corneal stroma never again recovers complete transparency (see discussion on corneal wound healing). It is easy to think of corneal cutaneous metaplasia as an undesirable pathologic event, but in fact it is an eye-saving response to a pathologic change in the corneal environment. Failure to undergo cutaneous metaplasia would lead to corneal disintegration and subsequent destruction of all of the intraocular tissues.
Clinical entities characterized by corneal cutaneous metaplasia include pigmentary keratitis (corneal desiccation in brachycephalic dogs), keratoconjunctivitis sicca, band keratopathy secondary to eyelid closure defects, chronic keratitis secondary to irritation from eyelids or eyelashes, and chronic corneal desiccation as a consequence of chronic glaucoma.
Epithelial and/or Stromal Necrosis: Injuries to the cornea that occur too quickly or with too much severity to permit cutaneous metaplasia result in corneal necrosis. Because most of the injuries are from external insults, it is the corneal epithelium that is most commonly affected. The usual result is corneal ulceration. The causes are numerous, including rapidly progressing desiccation, severe mechanical injury, injury from exogenous chemicals, and a few niche-adapted infectious diseases such as herpesvirus infection in cats and Moraxella bovis infection in cattle.
After full-thickness epithelial loss, there is immediate osmotic absorption of water from the tear film into the anterior stroma, resulting in focal superficial stromal edema that is the clinical hallmark of corneal ulceration. The absorption of water increases the spacing between the stromal collagen fibers and results in increased scattering of light, which clinically appears as corneal opacity. Coloring the tear film with water-soluble dyes like fluorescein is a very common clinical diagnostic technique to accentuate the edema within the stroma. Within hours after injury, the early healing process begins and neutrophils from the tear film are also absorbed into the cornea (Fig. 20-61). In small “physiologic” numbers, they aid in defending the denuded cornea against opportunistic infection, provide growth factors for subsequent wound healing, and help with a small amount of corneal stromal débridement necessary for wound healing. If the wound becomes infected and the neutrophils migrate from the tear film and limbus in large numbers, their lytic enzymes create enough bystander injury to result in stromal destruction, known as suppurative keratomalacia (Fig. 20-62).
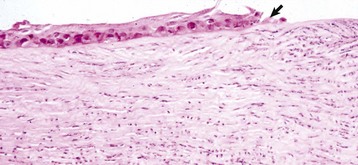
Fig. 20-61 Early healing, shallow ulcer, cornea, dog.
The corneal epithelium (arrow) is sliding along the surface of the denuded intact stroma that now has substantial staining pallor and separation of collagen fibers typical of edema. Numerous neutrophils have migrated into the stroma from the tear film. The neutrophils, although necessary in moderation, have the potential to cause enzymatic digestion of the stroma (keratomalacia) and are sources of fibroblastic and angioblastic growth factors. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
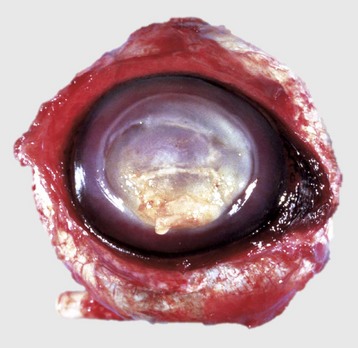
Fig. 20-62 Suppurative keratomalacia, enucleated globe, horse.
An initial corneal laceration became infected with Pseudomonas spp. The infection has resulted in proteolytic destruction of the stroma caused mostly by the release of digestive enzymes from neutrophils in the inflammatory exudate (center to lower half of pupil). Healing will result in a large amount of stromal scarring. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Corneal injuries of greater magnitude result in deep ulcers with substantial stromal loss, even to the point of disappearance of the entire stroma exterior to Descemet’s membrane. This is most commonly seen in rapidly progressing ulcers contaminated with bacteria, resulting in neutrophil-induced keratomalacia. These ulcers, known as melting ulcers, may progress over just a day or two to full-thickness stromal dissolution. Descemet’s membrane is the last barrier. It will bulge anteriorly into the defect created by the loss of the overlying stroma and epithelium to create a descemetocele (Fig. 20-63). In most instances, this fragile membrane (Descemet’s membrane) ruptures, resulting in a perforating ulcer, which leads to the loss of anterior chamber fluid and the strong probability of iris prolapse. Iris prolapse may also result from corneal perforation from trauma.
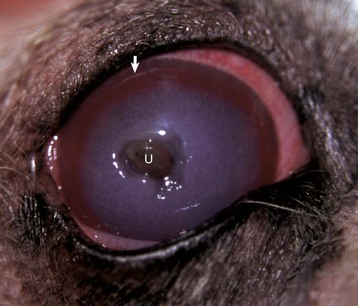
Fig. 20-63 Deep central corneal ulcer (U) (with descemetocele and early iris prolapse), dog.
Note the diffuse corneal edema (diffuse gray appearance) caused mostly by imbibition of tear fluid, with some contribution from the blood vessels which are growing (angiogenesis) into the injured cornea as part of wound healing (the circumferential red “brush border” from the limbus [arrow]). (Courtesy Dr. J. Wolfer, Islington Animal Clinic.)
Keratitis is a common clinical term indicating inflammation of the cornea. Because the normal cornea is avascular, true inflammation cannot occur until later in the disease process, after the injured cornea has been vascularized as part of wound healing (see later discussion). Nonetheless, corneal ulcers are very susceptible to opportunistic infection from normal conjunctival flora or organisms from the environment. This is probably particularly true of mycotic keratitis in horses, particularly when the fungal infection is given an unfair advantage because of inappropriate use of antibiotics and/or corticosteroids in the management of minor corneal lacerations. The presence of such organisms within the cornea results in a marked acceleration of leukocyte immigration from the tear film and blood vessels of the limbus. Although these leukocytes may be effective in neutralizing the infectious agent, they usually create substantial bystander injury to the stroma or the epithelium, resulting in a delay in wound healing and an increase in scarring.
Repair (Wound Healing): Those injuries limited to just a portion of the epithelial thickness are rapidly repaired by epithelial sliding and eventual mitotic regeneration. Such injuries are unlikely to ever receive veterinary attention. Deeper defects that involve the full thickness of the epithelium and varying depths of stroma initiate a stereotypic series of events that are clinically significant, easily observed in clinical practice, and amenable to both medical and surgical intervention. Because the cornea is optically clear and readily accessible for continuous observation after injury, it is a favorite model for the in vivo study of the basic events of wound healing in mammalian tissue. From a pragmatic clinical perspective, the vast majority of corneal lesions encountered in clinical practice are already past the stage of initial injury and are at the stage of corneal wound healing. If healing is less than optimal, an array of antimicrobial agents, surgical procedures, and chemical agents to modify the wound healing response is commonly used.
Most models of corneal wound healing use a mild, controlled nonseptic epithelial injury. The sequence of events and the biochemical signaling involved in such injury are not necessarily the same as those in naturally occurring massive corneal injuries and subsequent infections seen in veterinary practice, but nonetheless these carefully controlled models provide the only information available.
After full-thickness destruction of a portion of the corneal epithelium, the sequence of events is depicted in Fig. 20-64. Briefly, the injured epithelial cells release cytokines, such as interleukin-1 and platelet-derived growth factor (PDGF). Within minutes of lethal epithelial injury, these chemical mediators cause necrosis and/or apoptosis of stromal cells within the very superficial corneal stroma. At the same time, injured epithelial cells at the margin of the ulcer release epidermal growth factor (EGF), keratocyte growth factor (KGF), and hepatocyte growth factor (HGF) to stimulate epithelial migration and proliferation. Additionally, the production of these growth factors is upregulated in the tear film. The increase in EGF and other growth factors results in flattening and sliding of viable suprabasilar epithelial cells from the adjacent intact epithelium. These cells dissolve their intercellular junctions and migrate across the exposed stromal surface, adhering to a temporary scaffold of fibronectin and other adhesion molecules (Fig. 20-65). As long as the underlying stroma is healthy enough to support epithelial migration, excessive numbers of neutrophils are not present, and if the event that caused the original corneal injury is no longer active, those epithelial cells slide with remarkable speed (as much as 1 mm per day) across the denuded surface in an attempt to close the defect. There are many other cytokines and adhesion molecules involved in corneal epithelial sliding and regeneration, suggesting that rapid sealing of the defect must be exceedingly important. Closure of the defect by even a thin layer of epithelium halts the continued recruitment of neutrophils, which are responsible for the keratomalacia, vision-impairing stromal fibroplasia, and vascularization from the limbus. Closure of the corneal defect also prevents further edema and limits access by potentially injurious infectious agents.
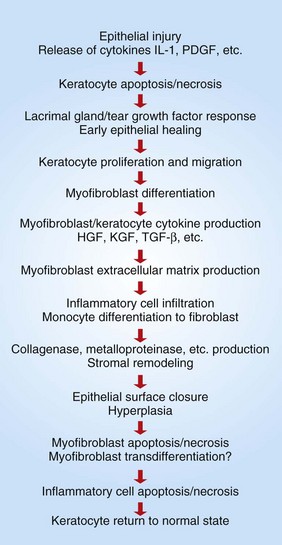
Fig. 20-64 Schematic diagram of the corneal wound healing cascade.
HGF, Hepatocyte growth factor; IL-1, interleukin-1; KGF, keratocyte growth factor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β. (Redrawn from Klenkler B, Sheardown H: Exp Eye Res 79:677-688, 2004.)
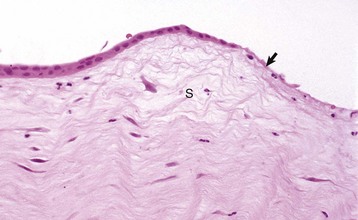
Fig. 20-65 Corneal epithelial cell migration (epithelial sliding), early wound healing, cornea, dog.
Corneal epithelial cells are migrating (sliding) to cover the corneal ulcer (arrow). The stroma is edematous (S) and contains a few neutrophils derived from the tear film. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Lagging behind the epithelial activation and sliding, which occur within minutes to hours of injury, is rebuilding of the injured stroma, which starts within a few days. It is not clear how the cornea recognizes the need for stromal rebuilding. There are some cases in which even a deep stromal defect does not initiate ingrowth of fibroblasts and blood vessels to rebuild the stroma. Instead, the epithelium simply slides across whatever small amount of normal stroma remains. This results in an optically clear but precariously thin healed cornea (Fig. 20-66). Migration of neutrophils from the tear film and from the limbus into the corneal wound is a powerful stimulus for the recruitment of both blood vessels and fibroblasts from the limbus. Neutrophil immigration begins about 12 hours after mild injury in response to chemotactic factors released by injured epithelium and stroma. This delay in neutrophil recruitment presumably allows the epithelium to slide and seal small defects and thus prevents excessive immigration of neutrophils (and its negative consequences as outlined later). Additional stimulation of stromal fibroplasia and angiogenesis comes from release of growth factors, such as PDGF, vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGF-β) from the injured epithelium, migrating neutrophils, and the injured stromal keratocytes. Not only does injury stimulate activation and migration of fibroblasts and angioblasts from the limbus, but it also changes the morphology and activity of the resident stromal fibroblasts (keratocytes). These cells undergo myofibroblastic metaplasia, become mobile and contractile, and produce increased amounts of various matrix proteins, growth factors, and metalloproteinases essential for stromal remodeling.
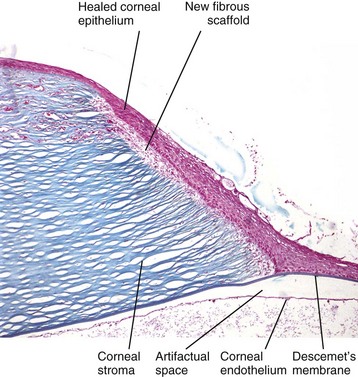
Fig. 20-66 Chronic descemetocele, cornea, dog.
On the right, at the site of a former deep corneal ulcer, the epithelium has healed by corneal epithelial cell migration (sliding) into the ulcer, with subsequent epithelial hyperplasia and return of the corneal epithelium to normal or even increased thickness. However, because there has been no replacement or remodeling of the stroma (right), the epithelium is positioned directly on the surface of Descemet’s membrane instead of on a reconstituted fibrous stroma. This process is “unsuccessful” wound healing, because the “healed” cornea, lacking stroma, is too fragile to survive. Masson trichrome stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The histologic events of stromal rebuilding after significant injury are less complicated than the innumerable biochemical signaling interactions involved. Mild stromal injuries are repaired by in situ enlargement and sluggish proliferation of resident keratocytes, which undergo fibroblastic metaplasia. After significant stromal injury, however, repair using just local keratocytes/monocytes is not adequate. Such repair must await the arrival of reinforcements in the form of fibroblasts and blood vessels from the limbus. Histologically, enlargement and hyperchromasia of limbal fibroblasts and angioblasts is visible within a day or two of injury, but detectable migration is not evident until about 4 days. Fibroblasts and blood vessels, preceded by a halo of edema and stromal proteolysis, migrate with a maximum speed of 1 mm per day until they reach the site of injury (Fig. 20-67). Here, they rebuild the injured stroma to provide a scaffold suitable for the migration, adhesion, and eventual normalization of the epithelium. Over time, this fibroblastic-angioblastic matrix of granulation tissue matures to histologically resemble normal stroma. However, it is never able to replicate the very specific lamellar structure of normal stroma and even with optimal stromal wound healing, corneal clarity is permanently impaired.
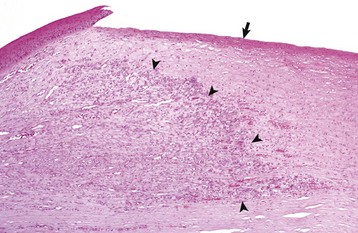
Fig. 20-67 Normal wound healing, cornea, dog.
Following shallow ulceration (arrow) that had damaged both the epithelium and the superficial stroma, the stroma is repaired and returned to near normal function by ingrowth of blood vessels and fibroblasts (wound healing) from the limbus (leading edge of healing is shown by arrowheads). Such repair of the stroma is usually a prerequisite for epithelial regeneration following any large corneal ulceration. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
It is worth mentioning here that a lamellar ingrowth of blood vessels from the limbus into the middle third of a perfectly normal corneal stroma occurs as a bystander effect of chronic uveitis (see the discussion on uveitis in the section on Delayed Responses to Injury).
To summarize, the epithelial and stromal events of wound healing are stimulated by a complex interaction of dozens of growth factors, adhesion molecules, and other chemical signals. It is far from understood exactly how these interact. It is clear that the cellular responses are greatly influenced not only by what chemicals are present but also by the context into which they are introduced. The action triggered by any of these mediators is influenced by other mediators in the environment, when the mediators are generated in relation to the wound healing process, and by what cells are targeted (Fig. 20-68).
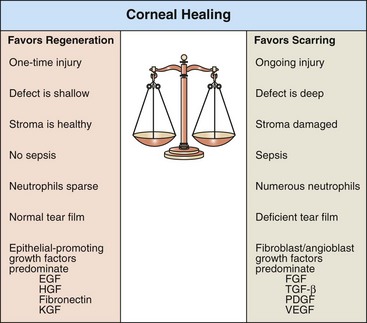
Fig. 20-68 Schematic diagram of corneal wound healing.
The outcome of corneal wound healing depends in part on the balance among growth factors coming from the tear film, injured epithelium and stroma, and immigrating leukocytes. EGF, Epidermal growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; KGF, keratocyte growth factor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
Uvea
Uveal Inflammation: The nomenclature of uveal inflammation is the same as that used in clinical ophthalmology (Box 20-12). Hypopyon is the accumulation of neutrophils and fibrin that settles ventrally within the anterior chamber (Fig. 20-69). Inflammation within the iris and ciliary body is usually referred to as anterior uveitis, although iridocyclitis is equally accurate. Inflammation limited to the choroid is choroiditis; inflammation limited to the vitreous is hyalitis; inflammation throughout the uveal tract is panuveitis; and inflammation involving the uveal tract and the adjacent ocular cavities (anterior chamber, posterior chamber, and vitreous) is endophthalmitis. Inflammation that spreads to involve sclera is known as panophthalmitis. Although these terms are widely used in clinical ophthalmology, in reality, the vascular tunic within the globe is a unified structure and virtually all examples of clinically significant uveal inflammation involve all portions of the uvea at least to some degree and have at least a little bit of effusion into the ocular chambers. Furthermore, the globe is a sealed sphere filled with fluid, so that inflammatory mediators or toxic products of infectious agents are likely to be widely disseminated within the globe. Thus from a purely histologic perspective, virtually all cases of uveitis can be correctly classified as endophthalmitis.

Fig. 20-69 Hypopyon (bilateral), feline infectious peritonitis, cat.
A mixture of fibrin and neutrophils (white-gray opacity) is present within the anterior chamber of the eyes. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
A further source of confusion between histopathologic and clinical terminology is a consequence of identification of the predominant leukocyte in the exudate. As is true of lesions in many other tissues, histopathologic examination underestimates the number of rapidly migrating granulocytes and is misled by the gradual accumulation of nonemigrating mononuclear leukocytes. Neutrophils rapidly accumulate within the aqueous or vitreous humors where they can be detected by clinical or cytologic examination. Unless bound up in fibrin, those leukocytes are washed away during histologic processing, leaving only the predominantly mononuclear population within the uveal stroma. This is not unique to the globe: An identical opportunity for confusion exists in correlating cytologic and histologic lesions in immune-mediated joint disease, chronic rhinitis, and pyometra (among others).
Causes of Uveitis: Uveitis can be initiated by a wide array of infections, immune responses, and trauma. Tissues of the globe usually act as an integrated unit, so injury to one part almost always has at least some influence on the health of other parts of the globe. The globe is filled with fluid, thereby allowing chemical messengers generated in one part of the globe to be absorbed by distant intraocular tissue. The iris stroma, in particular, is highly reactive because there is free communication between it and the aqueous humor. Any toxins, chemical mediators of inflammation, or growth factors secreted into the aqueous are absorbed by the iris, causing that portion of the uveal tract to react. (See later discussion on preiridal fibrovascular membrane in the section on Delayed Responses to Injury).
Most of the infectious causes of uveitis are ocular responses to systemic viral, bacterial, or parasitic diseases in which the uveal tract is only one of many tissues affected. Endophthalmitis as the sole manifestation of infectious disease is usually seen only as a sequela to penetrating injuries or perforating ulcers that allow the entry of environmental organisms into the globe. There are no viral causes of endophthalmitis, although there are a few systemic viral infections that cause vasculitis or retinitis that result in a uveal inflammatory response. Aberrant migration of nematode or trematode larvae occasionally causes endophthalmitis, as does ocular colonization by a variety of protozoal parasites that cause systemic disease (toxoplasmosis and encephalitozoon in particular). Uveal involvement in systemic mycoses and protothecosis is particularly common in animals in certain geographic regions.
Immune-mediated uveitis is a particularly frequent finding. In only a few types is there a reasonable understanding of the pathogenesis, as in uveodermatologic syndrome and phacoclastic uveitis (see later discussion). In the great majority, there is a chronic lymphocytic-plasmacytic endophthalmitis with no demonstrable infectious agent and a moderately successful clinical response to immunosuppressive therapy (see the later discussion on idiopathic lymphonodular uveitis in the section on Diseases of the Uvea). The lesion is clearly the result of an immunologic response, but it is not known whether such a lesion reflects primary immune-mediated disease or simply a response to an elusive infectious agent that has long since disappeared.
Ocular trauma is a frequent cause of transient endophthalmitis. The uvea also responds to afferent neural signals and chemical factors released from an injured cornea, so that with any significant corneal ulceration or keratitis, one can expect to have at least a mild anterior uveitis.
Immediate Response to Injury: Uveitis: Because the uveal tract has a fibrovascular stroma similar to the lamina propria of intestine or the dermis of skin, it undergoes all of the usual inflammatory reactions. There is nothing at all unique about these reactions. What is unique, however, is the importance of the consequences of that inflammation for other parts of the globe.
Delayed Responses to Injury: What is most distinctive about uveitis is not the macroscopic or microscopic character of the inflammatory reaction itself, but the consequences of that inflammation for adjacent portions of the globe. These bystander effects, which include anterior and posterior synechiae, retinal detachment, cataract, corneal vascularization, preiridal fibrovascular membrane, and glaucoma, are clinically significant and are frequently the targets of therapeutic intervention.
Synechiae are adhesions between the inflamed sticky iris and either the lens or cornea. Adhesion to the anterior capsular surface of the lens (in the normal globe, the iris lies against the lens capsule) is known as posterior synechia. The adhesion is initially fibrinous but will become a firm fibrous adhesion if allowed to persist. If that adhesion is sufficiently extensive around the pupillary margin (i.e., approaching the full circumference of the pupil), there is significant impairment of aqueous outflow from the posterior chamber to the anterior chamber (pupillary block) and inevitable secondary glaucoma. Increased pressure within the posterior chamber in the presence of a circumferential posterior synechia results in anterior bowing of the iris known as iris bombé (Fig. 20-70, A).
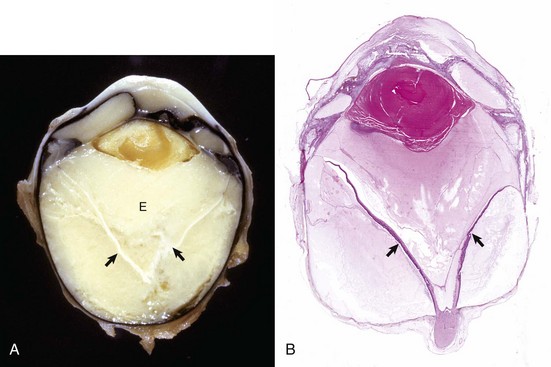
Fig. 20-70 Posterior synechia, pupillary block and iris bombé, eye, sagittal section, dog.
A, The iris has adhered to the lens, creating a pupillary block and subsequent iris bombé. Cloudy exudate (E) fills the vitreous, and its accumulation in the subretinal space has caused complete retinal detachment (arrows). These changes occurred as a consequence of corneal perforation and secondary septic endophthalmitis. B, Same globe as A. The serous effusion from the choroid has caused retinal detachment (arrows), which has serious implications for retinal survival. Additional lesions include posterior synechia, iris bombé, ciliary-lenticular adhesions, and circumferential cortical cataract. The combination of posterior synechia, pupillary block, iris bombé, and secondary peripheral anterior synechia is an exceedingly common sequence in the pathogenesis of glaucoma secondary to uveitis. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Anterior synechia is a focal-to-diffuse iridocorneal adhesion. Because the iris is not normally in proximity to the cornea, anterior synechia is much less prevalent than posterior synechia. It is most commonly seen as a sequela to iris prolapse, in which the flexible iris is literally “sucked up” into the corneal defect, where it then becomes anchored by fibrin and later by fibrosis. Peripheral anterior synechia commonly accompanies iris bombé and preiridal fibrovascular membranes (see later discussion).
Retinal detachment is a common sequela to uveitis and/or endophthalmitis by one of two mechanisms. Increased vascular permeability within the choroid results in effusion of fluid and cells into the subretinal space, resulting in so-called exudative detachment (Fig. 20-70, B). The normal retina is not actually adhered to the RPE. Fluid leaving the choroid during inflammation preferentially accumulates in the potential space between the retina and RPE because its only alternative would be to try to escape through the dense fibrous shell of the sclera. Alternatively, replacement of fibrinous exudates by fibrous tissue within the vitreous may result in tractional detachment. The immediate and delayed visual consequences of retinal detachment are discussed later (see section on Diseases of the Retina). If there is a lot of fibrovascular ingrowth as a consequence of organization of vitreal exudate, the maturing fibrovascular tissue routinely creates a dense membrane across the anterior surface of the vitreous (just posterior to the lens). This cyclitic membrane is anchored into the ciliary epithelium and often incorporates remnants of the detached retina.
Cataracts are frequent sequelae to uveitis, but the exact mechanisms are poorly understood. We do know that the avascular lens entirely depends on the aqueous humor for the delivery of nutrients and the removal of metabolic waste products. In eyes with uveitis, there is a notable drop in the production of aqueous humor (ocular hypotension is one of the clinical features of uveitis), resulting in lens “malnutrition.” It is assumed that the lens is also susceptible to injury by mediators of inflammation and other toxic products in the aqueous humor of inflamed globes.
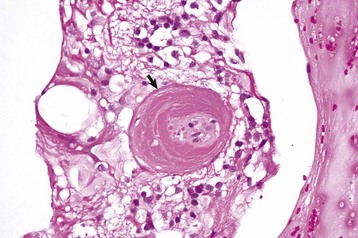
Corneal endothelialitis is characterized by an infiltration of neutrophils and/or lymphocytes into the corneal endothelium (Fig. 20-71). It usually is seen in globes with other lesions of chronic uveitis, but sometimes it is the most obvious histologic lesion of a uveitis that has almost disappeared (at least histologically) from other parts of the globe. It is much more prevalent in feline globes with feline infectious peritonitis (FIP) and idiopathic lymphonodular uveitis than in any others. Its pathogenesis is unknown. It was once a common lesion as a delayed sequela to vaccination with modified live vaccines containing canine adenovirus 1 and occasionally to canine adenoviral hepatitis. Complement-fixing antibodies against the virus were present within the endothelium as a result of a previous viral infection, resulting in attraction of neutrophils and subsequent bystander injury to the endothelium. If the endothelial damage was of sufficient magnitude, the result was the development of severe and sometimes permanent corneal edema, commonly referred to as blue eye.
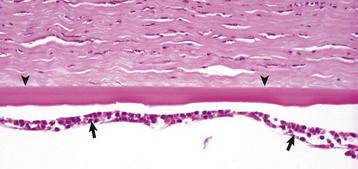
Fig. 20-71 Corneal endothelialitis, cornea, cat.
Neutrophils (arrows) adhere to and have accumulated on and in the corneal endothelium. When numerous, they separate the corneal endothelial cells from the adjacent Descemet’s membrane (arrowheads). This is a relatively frequent complication of anterior uveitis in cats and especially cats with feline infectious peritonitis. The leukocytes may be predominantly neutrophils or lymphocytes, depending on the pathogenesis and the duration of the uveitis. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Preiridal fibrovascular membrane refers to a layer of granulation tissue on the anterior surface of the iris. It is created by budding and migration of capillaries from the iris stroma and recruitment of fibroblasts as a routine response to cytokine mediators of wound healing (Fig. 20-72). It is no different from granulation tissue anywhere else in the body, but in typical ocular fashion, it achieves special significance within the globe. If the granulation tissue migrates across the anterior face of the lens, it creates a pupillary block resulting in secondary glaucoma. Alternatively, this granulation tissue can also migrate across the anterior face of the filtration angle to create a peripheral anterior synechia and once again cause secondary glaucoma (Fig. 20-73). Like any immature granulation tissue, it is also susceptible to hemorrhage and is a frequent cause of anterior chamber hemorrhage, known as hyphema. Wound healing after uveitis is only one of several mechanisms for the development of preiridal fibrovascular membranes, which are described in greater detail in the section on Glaucoma.
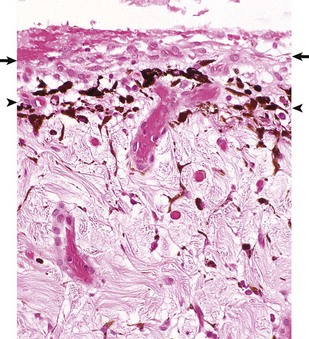
Fig. 20-72 Preiridal fibrovascular membrane, iris, dog.
A preiridal fibrovascular membrane (between arrows) formed by proliferating fibroblasts and capillaries has grown out from the iris stroma and has adhered to the anterior surface of the iris (represented here by the thin layer of pigmented melanocytes [between arrowheads]). These membranes form in response to growth factors within the aqueous humor, originating from such varied sources as intraocular neoplasms, or follow retinal detachment or chronic uveitis. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
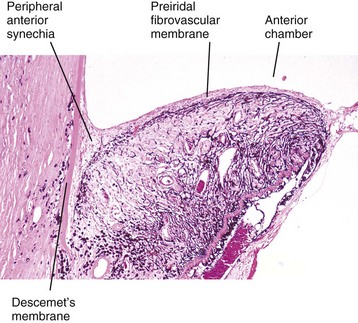
Fig. 20-73 Mature preiridal fibrovascular membrane, iris, dog.
A layer of mature granulation tissue (preiridal fibrovascular membrane) has adhered to the anterior surface of the iris. Contraction of the maturing membrane has distorted the shape of the iris and has caused it to adhere to the posterior surface of the cornea (peripheral anterior synechia). If extensive, the anterior synechia will result in secondary glaucoma. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Midstromal corneal vascularization is an extremely common and clinically useful clue pointing to the presence of chronic uveitis. The vessels grow inwardly from the limbus. It has no known functional significance and appears to be a purely accidental lesion because the blood vessels of the limbus respond to angiogenic growth factors being produced within the globe as part of the ongoing inflammation and repair.
Phthisis bulbi refers to a shrunken, disorganized endstage globe. It is a sequela not only to uveitis, but also severe uveitis is the most common cause.
Lens
The stereotypic histologic response of the lens to injury is hydropic swelling of the injured lens fibers, fiber disintegration resulting in cortical liquefaction, and abortive efforts at regeneration. This combination of changes is essentially identical, regardless of pathogenesis. They all result in opacification of the lens, referred to by the generic term cataract.
In clinical ophthalmology, cataracts are extensively subclassified by location within the lens, by age of onset, by the macroscopic appearance, or by the state of progression. This gives rise to an exceedingly complicated list of purely descriptive adjectives that are not at all reflective of the pathogenesis. Such classification is used mostly when dealing with inherited cataracts in dogs to ensure that the data being collected about the frequency or behavior of a cataract in a specific breed are indeed referring to the same disease.
The stereotypic microscopic changes of cataracts (Figs. 20-74 and 20-75) are various combinations of the following, listed in order of overall frequency:
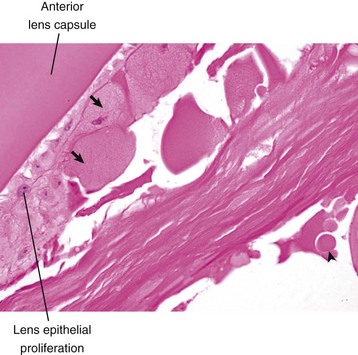
Fig. 20-74 Cataract, lens, dog.
There is epithelial hyperplasia of the lens with fibroblastic metaplasia, formation of bladder cells (arrows), and a Morgagnian globule (arrowhead). H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
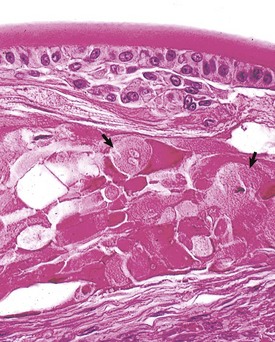
Fig. 20-75 Anterior cortical cataract, lens, fish.
Note the lens epithelial proliferation, fibroblastic metaplasia, and prominent bladder cells (arrows). (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
1. Fragmentation and liquefaction of cortical fibers, creating spherical globules of denatured lens protein known as Morgagnian globules.
2. Hydropic swelling of cells, known as bladder cells, attempting but failing to regenerate.
3. Hyperplasia and fibrous metaplasia of lens epithelium; the epithelial hyperplasia may create plaque-like thickening with or without fibroblastic metaplasia.
4. Posterior lens epithelial migration; the lens epithelium migrates from the equator to lie under the posterior lens capsule; the normal adult lens has no epithelium posterior to the equator.
5. More variable changes include lens swelling in acute cataracts, lens shrinking with wrinkling of the lens capsule in advanced (“hypermature”) cataracts, and intralenticular mineralization.
The lens is capable of additional responses to injury other than those associated with cataracts, but these are much less frequent. As discussed in the section on phacoclastic uveitis, rupture of the lens capsule allows regenerative lens epithelium to escape from the lens, undergo fibroblastic metaplasia, and migrate within the anterior and posterior chambers, sometimes with devastating consequences. The same type of proliferation complicates cataract surgery, producing thick plaques of fibroblast-like epithelial cells that are aesthetically displeasing and reduce visual acuity.
Retina And Vitreous
The general pathology of the retina resembles that of the brain. The neuronal elements of the adult retina of higher mammals do not regenerate; the outer segments of the photoreceptors, however, have a rapid turnover and have among the highest metabolic activity in the body. As long as the cell body within the outer nuclear layer remains viable, photoreceptors can be quickly regenerated.
The RPE remains mitotically active throughout life. Like other epithelia, it repairs itself, first by sliding viable cells into the area where cells have been lost and then by mitosis. Fibroblastic metaplasia is common (see later discussion).
The glial elements of the retina, particularly the astrocytes that reside within the inner nuclear layer (Müller cells), are exceedingly hardy and capable of proliferation. Repair of most cases of retinal necrosis occurs primarily by proliferation of Müller cells, which eventually form a dense glial scar, sometimes with a contribution from migrating RPE cells that can also undergo fibroblastic metaplasia (Fig. 20-76). Occasionally, the astrocytes proliferate along the vitreal face of the retina, forming a preretinal fibroglial membrane. Subretinal membranes (between the photoreceptors and the RPE) of a similar microscopic appearance are seen with chronic detachments and originate from migrating Müller cells or from retinal pigment epithelium that has undergone fibroblastic metaplasia.
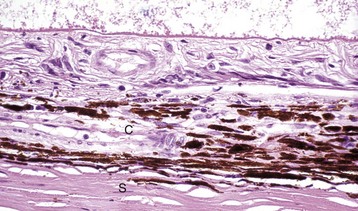
Fig. 20-76 Retinal atrophy (advanced), retina, dog.
There is virtually complete loss of all retinal layers, including photoreceptors, leaving only a few surviving blood vessels and glial cells. The impression of retinal fibrosis is mostly the result of fibrous astrocytosis originating within the inner nuclear layer. This lesion could be the end stage of almost any severe retinitis or retinal necrosis (including retinal detachment, retinal hypertension, glaucoma, or trauma). C, Choroid; S, sclera. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The vast majority of retinal lesions fall into the following three categories:
1. Inflammation as a result of extension from endophthalmitis or encephalitis. Inflammation targeting the retina specifically is exceedingly uncommon. The character of the inflammation within the retina is exactly the same as it is within the brain: neuronal necrosis, perivascular cuffing, and gliosis. What is different about the retina, however, is its susceptibility to exudative retinal detachment. Fluid and cells escaping from inflamed retinal vessels are most likely to accumulate in the subretinal space and result in the separation of the photoreceptors from the RPE and the choroidal vasculature (Fig. 20-77). The photoreceptors are therefore likely to become necrotic, not only from the by-products of inflammation but also from ischemia and other forms of malnutrition resulting from their anatomic dislocation from the RPE (remember that the photoreceptors are entirely dependent on their intimate contact with the RPE and their proximity to the choroid for their nutrition). Serious photoreceptor damage occurs within days.
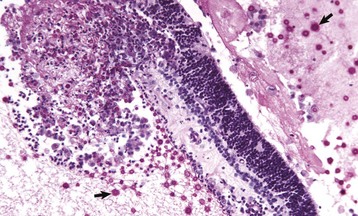
Fig. 20-77 Retinitis, retinal necrosis (with exudative retinal detachment), cryptococcosis, retina, dog.
Cryptococcus neoformans organisms (arrows) are numerous within the subretinal exudate. A mixture of fibrin, leukocytes, and organisms has replaced a portion of retina and created a focal preretinal exudate. Periodic acid–Schiff reaction. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
2. Noninflammatory photoreceptor degeneration from inherited metabolic disease, retinal detachment, or toxicity. Histologically, almost all of these photoreceptor diseases are indistinguishable from one another. The changes are the same regardless of whether the damage is from inborn metabolic errors (especially the innumerable inherited photoreceptor dysplasias of purebred dogs), excessive exposure to light or other radiation, or toxic chemicals.
3. Destruction of the neural elements of the inner retina (nerve fiber layer, ganglion cells, and inner nuclear layer) as a result of glaucoma. The pathogenesis of the inner retinal destruction and destruction of the optic nerve remain a source of great controversy and probably varies among species and with the type of glaucoma. Some of the damage is probably from pressure-induced collapse of retinal and even choroidal blood vessels, and some is probably the result of pressure-induced interference with the axoplasmic flow of nutrients within the optic nerve and nerve fiber layer (see the section on Glaucoma).
Retinal Detachment: The neurosensory retina (not including the RPE) is physically anchored only at the ora ciliaris and at the optic disc. It is held in apposition to the RPE partly by the physical presence of the gel-like vitreous and partially by the membrane forces related to the intricate interdigitations between photoreceptors and surface crevices in the RPE. The potential space between the photoreceptors and the RPE is the remnant of the lumen of the primary optic vesicle, and it persists throughout life. Retinal detachment (sometimes referred to as retinal separation by those who correctly point out that the retina is never really attached at all) is a frequent and serious complication of many different ocular diseases. It may be focal or more diffuse. The distance of separation between the photoreceptors and the RPE may only be slight (so-called flat detachments) or the entire retina may be separated and suspended in the vitreous (Fig. 20-78), supported only by its durable attachments to the ciliary body and optic nerve (so-called morning glory detachment because of its resemblance to a tubular flower when viewed by clinical examination through the pupil).
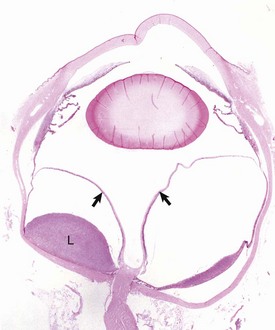
Fig. 20-78 Retinal detachment (complete), globe, sagittal section, dog.
Serous effusion from the growth of metastatic lymphoma (L) within the choroid and subretinal space has caused a complete retinal detachment (arrows). H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The most frequent types of retinal detachment are as follows:
1. Exudative retinal detachment: accumulation of serous, fibrinous, or cellular exudates (or hemorrhage) within the subretinal space as a consequence of choroiditis, retinitis, or retinal vascular hypertension (see Fig. 20-78).
2. Hematogenous retinal detachment: leakage of liquefied vitreous into the subretinal space through traumatic or degenerative breaks in the peripheral retina. This may occur secondary to trauma or to a very frequent aging lesion known as microcystoid peripheral retinal degeneration (Fig. 20-79).
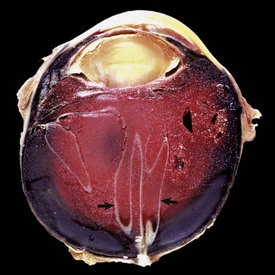
Fig. 20-79 Retinal detachment (traumatic), globe, sagittal section, dog.
The detachment has been caused by accumulation in the posterior chamber of subretinal hemorrhage. The retina (arrows) remains attached only at the sites of true anatomic attachment: the optic disc and its junction with the pars plana of the ciliary body. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
3. Tractional retinal detachment: maturation of hemorrhage or fibrin within the vitreous, which pulls the retina away from the choroid. As it matures, the combination of fibrous tissue, fibrin, and detached retina lies as a membrane stretching from ciliary body to ciliary body across the posterior surface of the lens, a lesion known as a cyclitic membrane.
Retinal detachment is immediately significant because the image on the retina is out of focus, but more serious is the rapidity with which the photoreceptors are damaged. The outer segments of the photoreceptors, responsible for light absorption and electrical signal generation, are lost within 2 weeks after experimental saline-induced flat detachment (Fig. 20-80). Histologic and clinical evidence in natural disease suggests that this degeneration occurs much more rapidly (a few days at most) in the presence of toxic by-products of inflammation, or when the distance between the retina and the RPE is great. The inner segments of the photoreceptors and the cell bodies within the outer nuclear layer are much more resistant to injury and may remain viable for months (Fig. 20-81).
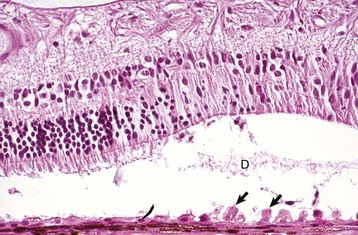
Fig. 20-80 Retinal detachment (serous), retina, dog.
Floccular eosinophilic debris (D), the histologic counterpart of a serous exudate, has pushed the retina away from the retinal pigment epithelium (RPE). There is hypertrophy (arrows) of the RPE, degeneration of the photoreceptors, and focal loss of neurons from the outer nuclear layer. RPE hypertrophy occurs after just a few hours of detachment. The rapidity of progression of the photoreceptor degeneration depends on the magnitude of the detachment and the toxicity of the fluid within the subretinal space. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
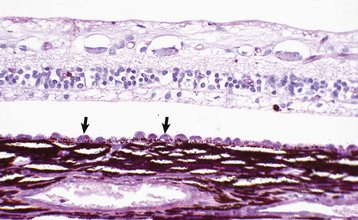
Fig. 20-81 Retinal detachment (chronic), retina, dog.
There is hypertrophy of the retinal pigment epithelium (arrows), with loss of nuclei from the outer nuclear layer as well as disintegration of the photoreceptors. At this stage, the loss of vision from this portion of retina is irreversible. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The retinal consequences of detachment probably vary by species, depending on how much intrinsic retinal vasculature is present. There is virtually no published information, but the almost avascular retina of horses should be much more susceptible to full-thickness ischemic injury than the well-vascularized retinas of dogs or cats (in which the inner half of the retina remains unaffected by detachment).
The detached retina in every species produces a variety of angiogenic growth factors presumably intended to increase its own blood supply. In species other than primates, however, there is little evidence of stimulation of retinal angiogenesis. Instead, these growth factors percolate through the vitreous and are absorbed into the iris stroma where they stimulate the production of a preiridal fibrovascular membrane. The result is a substantial risk of secondary glaucoma from either pupillary block or occlusion of the filtration angle (see the section on Glaucoma).
Eyelids, Conjunctiva, And Orbit
Eyelids: The responses of the eyelids to injury, the spectrum of potentially injurious agents, and the susceptibility to neoplasia are exactly the same as for skin and are not discussed further.
Although the responses of the eyelid itself to injury may be identical to those of skin elsewhere, there may be unique consequences for other parts of the globe, particularly the nearby conjunctiva and cornea. There may be direct spread of inflammation or neoplasia from the eyelid to the nearby conjunctiva or even to the cornea. Physical distortion of the eyelid from inflammation, scarring, or neoplasia may interfere with the ability of the eyelid to distribute the tear film, or to properly protect the cornea from injury. The abnormal eyelid may even cause mechanical injury by scraping the cornea that it was designed to protect.
There are only a few inflammatory diseases and neoplasms with a predilection for the eyelids, and these are discussed later under specific diseases. It is appropriate to be skeptical when attempting to prove an infectious cause for blepharitis in any species. There is a tendency to assume that whatever infectious agents are isolated from swabs of the eyelid margin or from expressed Meibomian secretion must be the cause of the blepharitis. This is flawed logic because ordinarily those same organisms are readily isolated from the conjunctival sac, or the eyelid margin, of perfectly healthy animals.
Conjunctiva: The reaction of conjunctiva to injury is the same as that of any other mucous membrane. It has a narrow spectrum of responses to injury and only seldom can an etiologic diagnosis be made by histologic examination of a conjunctival biopsy. The epithelium responds to acute injury with ulceration. With chronic mild injury, there is squamous metaplasia. In animals with pigmented conjunctivae, chronic irritation also stimulates hyperpigmentation, just as it often does in skin. If there is desiccation, the areas of squamous metaplasia are often keratinized.
The underlying lamina propria usually responds with a stereotypic lymphocytic-plasmacytic accumulation, with the development of hyperplastic lymphoid nodules if the stimulation is persistent. Neutrophils are seldom numerous within the tissue; if present at all, they are usually found migrating through the epithelium en route to the conjunctival sac where they will do battle with the infectious agents that might be present as opportunistic pathogens. Eosinophils are occasionally present in poorly defined allergic conjunctivitis in dogs, cats, and horses. They are present in greater numbers in the more discrete collagenolytic granulomas in horses with habronemiasis.
Looking for specific infectious agents in sections of conjunctiva is usually a waste of time. It is true that some of the viral diseases and chlamydial infections can leave specific footprints in the form of inclusion bodies, but these are usually present only very early in the course of these diseases, at a time before the lesion is assessed by conjunctival scraping or biopsy examination.
Orbit: There is nothing specific about the reaction of orbital tissues to injury. Each constituent (bone, fat, and muscle) reacts to injury in an identical fashion to that in the same type of tissue elsewhere. As has been a recurring theme in discussions of diseases in other parts of the globe and ocular adnexa, an event at one site almost always causes significant consequences for other parts of the globe, such as the following:
1. Accumulation of fluid, cellular exudates, or tumor cells in the orbit, resulting in protrusion of the globe (exophthalmus) with the resulting risk of corneal ulceration secondary to desiccation.
2. Destruction of orbital tissue and loss of fat from emaciation having the opposite effect; the globe sinks into the orbit, creating the risk of secondary entropion and resultant corneal irritation.
3. Injury to extraocular muscles, resulting in abnormal positioning of the globe, thus predisposing the cornea to ulceration from eyelid trauma or desiccation.
4. Significant damage to the function of the lacrimal gland, resulting in injury to the cornea via desiccation.
5. Space-occupying orbital lesions, particularly neoplasms, which may cause injury to the optic nerve and result in blindness.
Disorders Of Domestic Animals
Anomalies Of The Globe As A Whole
The globe has a complex embryogenesis involving carefully orchestrated interactions of neuroectoderm, surface ectoderm, and periocular mesenchyme throughout embryogenesis and in carnivores into the fifth or sixth week after birth. The opportunity for developmental error is thus substantial. This also means that all developmental errors, especially in carnivores, are not necessarily congenital. Because the globe is not essential for postnatal survival, even severe anomalies are encountered with considerable frequency. Selective breeding practices have increased the frequency of ocular anomalies. Those that are important or prevalent are discussed in sections dealing with diseases of the specific ocular segment affected.
As a general framework for understanding ocular anomalies, they are most conveniently divided into failures of initial induction, failures in remodeling, and late failures in atrophy. Included in this section are only those anomalies in very early induction that affect the eye as a whole. Those lesser anomalies that result from later defects in remodeling or atrophy are discussed in the sections devoted to diseases of the most severely affected components of the eye.
The eye begins very early in gestation as an outgrowth from the primitive forebrain. This primary optic vesicle grows outwardly from the brain toward the overlying surface ectoderm, remaining connected to the brain by the optic stalk. As the primary optic vesicle approaches the overlying ectoderm, it induces a focal thickening in that ectoderm, known as the lens placode. The placode thickens, separates from the surface ectoderm, and migrates inwardly to indent the spherical optic vesicle. As the lens pushes into that optic vesicle, the vesicle collapses on itself to form a bilayer optic cup (Fig. 20-82). When the lens vesicle separates from the surface ectoderm, that ectoderm re-forms over the lens and forms the presumptive corneal epithelium. The presence of the epithelium seems to act as a magnet to attract one or more waves of periocular mesenchyme that forms the primitive corneal stroma and endothelium. This periocular mesenchyme is derived from the neural crest and eventually also forms the sclera, the uveal stroma, and a well-developed but transient intraocular network of blood vessels (hyaloid artery and tunica vasculosa lentis) that nourish the developing retina and lens (Fig. 20-83). The eyelids, extraocular muscles, lacrimal gland, and orbit are formed more or less independent of the globe and are generally not affected by those diseases that impair development of the eye itself.
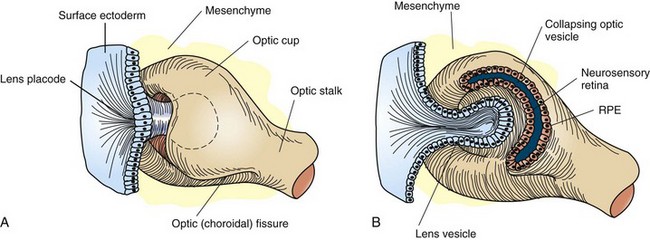
Fig. 20-82 Schematic diagram of the formation of the primary optic vesicle and optic cup.
Note that the optic fissure is present because the optic cup is not yet fused inferiorly. A, Formation of lens vesicle and optic cup with inferior choroidal or optic fissure. Mesenchyme surrounds the invaginating lens vesicle. B, Surface ectoderm forms the lens vesicle with a hollow interior. Note that the optic cup and optic stalk are of surface ectoderm origin. RPE, Retinal pigment epithelium. (From Cook CS, Sulik K, Wright K: Embryology. In Wright KW, ed: Pediatric ophthalmology and strabismus, St Louis, 1995, Mosby.)
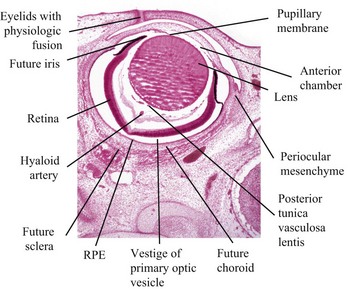
Fig. 20-83 Fetal globe, gestational age day 34, dog.
The periocular mesenchyme is organizing to form the choroid and sclera. The anterior chamber has been formed, but the anterior lip of the optic cup has not yet folded inwardly to induce the formation of the iris and ciliary body. The relatively large lens is surrounded by a rich vascular tunic derived from the hyaloid artery and pupillary membrane. RPE, Retinal pigment epithelium. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Anophthalmia is a very rare condition in which there is no detectable development even of the primary optic vesicle. It is usually bilateral. The vast majority of the cases clinically diagnosed as “anophthalmia” are in fact severe microphthalmia, and some vestige of a globe is found somewhere within the orbit.
Microphthalmia is the presence of a miniature, disorganized globe in an orbit of relatively normal size. In the great majority of cases, the anomaly does not reflect a primary maldevelopment but rather involution after some type of exogenous injury to a globe that up to that stage was normal in its development. This includes in utero trauma, ischemic injury, and infection. Such globes can be remarkably small; usually it is a tiny pigmented nodule somewhere within the abundant fat and muscle still present within the orbit (Fig. 20-84). Microscopically the most durable and therefore the most recognizable remnants of the previously normal globe are the pigmented ciliary processes. If they are present, then one can be sure that the globe had reached a reasonably advanced state of embryologic development before undergoing regression.
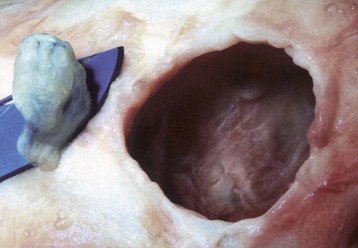
Fig. 20-84 Microphthalmia, globe and orbit, calf.
Note that the orbit (right) remains relatively normal, probably indicating that the initial development of the globe was normal and that its current small size (on the scalpel blade, left) is a result of in utero injury and subsequent atrophy (indicating so-called secondary microphthalmia rather than a primary failure of ocular development). (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Congenital cystic eye results from the failure of the primary optic vesicle to invaginate under the influence of the developing lens.
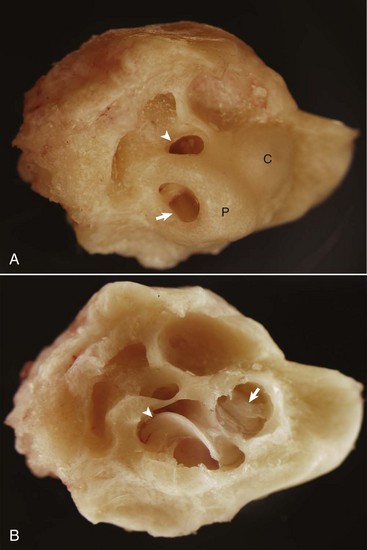
Cyclopia and synophthalmia are perceived clinically as a single midline globe and virtually always occur in animals with other major developmental anomalies. They reflect failure of division of the very primitive optic primordium into paired symmetric optic stalks and vesicles (or perhaps subsequent fusion), which therefore results in a single midline globe. Most examples have some degree of duplication of intraocular structures, such as the retina or lens, and are properly termed synophthalmia (Fig. 20-85). As naturally occurring developmental anomalies, cyclopia and/or synophthalmia are exceedingly rare. However, ewes grazing pastures of the alpine legume Veratrum californicum on day 15 of gestation give birth to lambs with this malformation. Ingestion of the plant before day 15 results in fetal death but no anomalies. Ingestion after day 15 results in various skeletal anomalies and cleft palate, but the globes are apparently normal. Similar ocular lesions have been experimentally induced in goats and cattle.
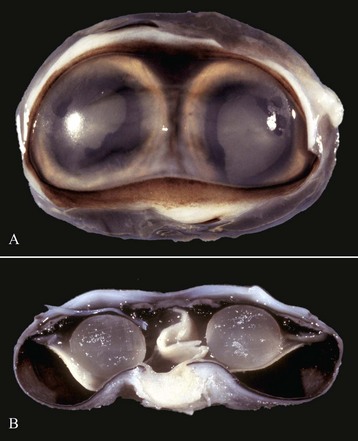
Fig. 20-85 Synophthalmia, globe, calf.
A, This fused globe has two lenses, two corneas, and partial duplication of the retina. B, Horizontal section of the fused globe revealing two lenses but a shared fused midline retina. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Coloboma is the least severe of the developmental abnormalities affecting the globe as a whole. When used in this context, the term refers to a defect in the closure of the optic fissure, which is a normal channel in the floor of the optic cup that remains open long enough to allow the entry of the blood vessels that form the hyaloid artery and the perilenticular vascular tunic. This fissure normally closes in the last third of gestation, persisting longest near the posterior pole of the globe just ventral to the optic disc. If it persists for too long, there is the possibility that the developing retina will accidentally grow outwardly through this defect (i.e., coloboma) to cause a retrobulbar cyst lined by retinal neuroectoderm (Fig. 20-86). This is a lesion made famous by its occurrence as one of the hallmarks of collie eye anomaly. The same defect occurs sporadically in other breeds of dogs and in horses, cattle, and cats. In Charolais cattle, colobomas at or near the optic disc are inherited as an autosomal dominant trait with incomplete penetrance.
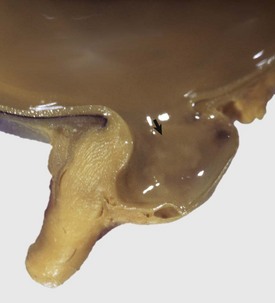
Fig. 20-86 Posterior polar coloboma, collie eye anomaly, globe, sagittal section, dog.
Failure of closure of the most posterior portion of the optic fissure has allowed outpouching (arrow) of the developing retina, adjacent to the optic nerve. The protruding retina is covered by sclera. This result has prevented proper local formation of choroid and sclera, resulting in so-called scleral ectasia. Such globes always have choroidal hypoplasia. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The stepwise closure of the optic fissure appears to be orchestrated by soluble factors released from the retinal pigmented epithelium, which is developing from the neuroectoderm of the posterior layer of the primitive optic cup. Because such colobomas are most commonly found in color-dilute animals, we assume that the proper chemical signaling is somehow related to the proper acquisition of pigment (or pigment-producing capability) by this neuroectoderm (see later section on Choroidal Hypoplasia).
Diseases Of The Globe As A Whole
Glaucoma: Glaucoma is not a single disease but a group of diseases sharing specific physiologic and structural characteristics. It is a clinical syndrome characterized by a sustained increase in intraocular fluid pressure that is detrimental to the health of the optic nerve and the retina, resulting in loss of vision and eventual blindness. Glaucoma causes changes in virtually every tissue within the globe, but changes in the retina and optic nerve are the most important. The syndrome is most prevalent in dogs, followed by cats and horses. It is rarely documented in other species but undoubtedly exists in all. Glaucoma is extremely prevalent as a cause for ocular pain and blindness in dogs, and it is by far the leading reason for surgical removal of the globe (enucleation). It is relatively less common in cats, yet it is still the leading cause for enucleation in that species. Its frequency in horses may be greatly underestimated because of its variable clinical presentation in that species and because ocular pressure is not routinely measured in horses.
Theoretically, glaucoma may result from an increase in the production of aqueous humor or a decrease in its removal. In practical terms, however, all examples of glaucoma in domestic animals result from impairment of aqueous outflow. Glaucoma is usually divided into primary and secondary glaucoma. Primary glaucoma refers to those examples occurring without any known acquired intraocular disease to explain the increase in pressure. The great majority of these result from developmental errors in the structure and function of the aqueous drainage pathways. Secondary glaucoma refers to those examples in which there are changes, such as lens luxation, pupillary block, or intraocular neoplasia, to explain the impairment of aqueous humor outflow.
The aqueous humor that fills the anterior and posterior chambers is a low-protein transparent fluid just slightly denser than water. It is formed continuously by a combination of plasma filtration and active secretion by ciliary epithelium. Its chemical composition varies somewhat among species, but it always has a very low total protein concentration (about 5% of total plasma protein) but an amino acid concentration 50% higher than that of plasma. It contains glucose and electrolytes in concentrations roughly equivalent to those in plasma. The aqueous humor produced within the ciliary body circulates past the lens to provide nutrients to it and remove waste products, enters the anterior chamber through the pupil, circulates within the anterior chamber to nourish corneal endothelium and stroma, and then exits through slitlike spaces at the junction between peripheral cornea and iris. This iridocorneal angle extends circumferentially around the globe and normally has tremendous reserve capacity to accommodate fluctuations in aqueous production and to provide a substantial margin of safety against the development of glaucoma secondary to partial blockage of the aqueous outflow by accumulations of blood or inflammatory debris.
The maintenance of intraocular fluid pressure is a balance between aqueous production and outflow and in domestic animals is influenced primarily by resistance to outflow. The rate of production varies from 2.5 µl per minute in dogs to 15 µl per minute in cats. The outflow pathway is through the iridocorneal angle and more specifically through a series of perforations in the connective tissue of the peripheral cornea, sclera, and iris stroma that makes up the trabecular meshwork. The microscopic anatomy of this outflow pathway varies by species, but all examples are quite similar in general design.
The trabecular meshwork is a series of mesenchymal sieves that occupies the iridocorneal angle and extends circumferentially around the globe. Embryologically, the trabecular meshwork is formed by rarefaction of the same mesenchyme that forms iris stroma. In carnivores this remodeling continues for several weeks after birth (Fig. 20-87). This area of perforated mesenchyme is referred to as the ciliary cleft. It is bordered externally by sclera, posteriorly by the muscles of the ciliary body, and internally by the iris stroma. Its anterior border, separating it from the anterior chamber, is formed by the pectinate ligament. With proper instrumentation (gonioscopy), the pectinate ligament is visible through the cornea as a series of cobweb-like branching cords (carnivores) or a fenestrated sheet (ungulates), stretching from the termination of Descemet’s membrane to the anterior portion of the iris root (Fig. 20-88).
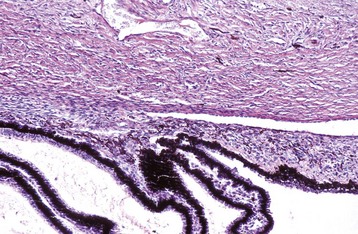
Fig. 20-87 Normal neonatal globe, immature ciliary cleft, trabecular meshwork and iris stroma, dog.
The trabecular meshwork will develop by rarefaction and remodeling of this ciliary cleft over a period of a few weeks during normal maturation. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
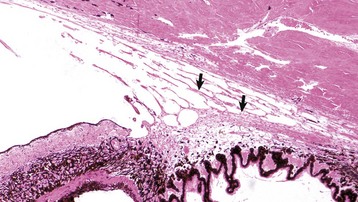
Fig. 20-88 Normal adult ciliary cleft, trabecular meshwork and other portions of the drainage pathway, cat.
The arrows indicate the predominant pathway for aqueous outflow in carnivores. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Ultrastructurally, the trabecular meshwork is a series of connective tissue beams covered (completely or incompletely, depending on the species) by a single layer of trabecular endothelial cells connected to each other by delicate cytoplasmic tendrils. The cells are phagocytic and are probably important in regulating the outflow of aqueous humor. Most of that flow is probably by transcellular movement of pinocytotic vesicles across the cytoplasm of the trabecular endothelium.
In most species, the most functionally significant portion of the trabecular meshwork exists in the deep peripheral corneal stroma and inner sclera, and is known as the corneoscleral meshwork. Aqueous humor passing through this portion of the meshwork then enters a network of large veins, known as the scleral venous plexus, which is embedded in the peripheral sclera. The aqueous humor entering these veins is then returned to the systemic circulation. A small percentage of the aqueous humor entering the filtration angle will exit via a more posterior route known as the uveoscleral meshwork, percolating into the veins of the ciliary body and choroid rather than into the scleral venous plexus (see Fig. 20-88). The proportion of aqueous humor leaving the globe by this more posterior route varies by species: 3% in cats, 15% in dogs, and a larger (but undetermined) percentage in horses. Differences in the proportion of fluid leaving the globe by different routes might explain differing susceptibilities to different types of glaucoma among the domestic animals, and it also creates at least the potential for different types of therapeutic interventions.
These outflow pathways are not just passive conduits through which the aqueous humor can flow. There is an important physiologic resistance to outflow responsible for the maintenance of normal intraocular pressure. The exact anatomic and physiologic constituents of this outflow resistance remain incompletely defined but include important contributions from the endothelial cells lining the collagen beams within the trabecular meshwork, the glycosaminoglycans embedded in the matrix supporting those endothelial cells, and blood pressure within the scleral venous plexus.
The clinical (macroscopic) lesions of glaucoma are related to the secondary effects of increased pressure on various components of the globe. Although the increase in pressure is the result of obstruction of outflow in the anterior chamber, the pressure elevation is distributed throughout the fluid medium of the globe, and the effects are thus felt by all parts of the globe. These effects are the same, regardless of the pathogenesis of the glaucoma, and they vary with the rapidity of onset, the magnitude of the pressure elevation, and the duration of the elevation. They are also influenced by the age of the patient and by the species. The most obvious of the macroscopic changes include ocular enlargement, corneal cloudiness because of edema, pupillary dilation, and excavation of the optic disc. The most frequent microscopic changes in glaucoma are listed in Box 20-13.
Morphologic Changes Secondary to Glaucoma: Buphthalmos (megaloglobus) is stretching of the globe secondary to increased intraocular pressure. It is most obvious in dogs and least obvious in horses (Fig. 20-89). Histologically the sclera becomes thin, and the anterior chamber increases in its anterior-posterior dimension. The enlargement is significant because it probably causes pain, and it prevents the eyelids from closing over the cornea, thus resulting in corneal desiccation, which can lead to either corneal cutaneous metaplasia or ulceration (depending on the speed of onset and the severity of the desiccation).

Fig. 20-89 Bilateral primary glaucoma (inherited), eye, rabbit.
The eyes are protruding and have diffuse gray corneal opacity typical of edema. Distinguishing ocular enlargement caused by glaucoma from exophthalmos caused by a retrobulbar mass is based on clinical assessment of intraocular pressure, the ability to push the globe more deeply into the orbit (retropulsion), and the presence or absence of an orbital mass as detected by radiography or other imaging techniques. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Corneal edema develops when the aqueous pressure exceeds the ability of the sodium pump within the corneal endothelium (in dogs, at about 40 mm Hg). More severe corneal edema then develops as a result of pressure-induced injury to that endothelium and that injury may become permanent if the endothelial injury is so extensive that it exceeds the capacity of the corneal endothelium to repair itself by sliding, usually when there is a loss of more than 50% of corneal endothelial cells. Corneal edema secondary to glaucoma is much more frequent in dogs than in cats for reasons that remain unclear.
Corneal striae are breaks in Descemet’s membrane occurring secondary to corneal stretching. They are visible on clinical examination as serpentine tracts of deep corneal stromal opacity.
Atrophy of the iris and ciliary processes occurs late in the course of glaucoma, probably as a consequence of chronic pressure-induced ischemia. Atrophy of the iris causes the initial physiologic pupillary dilation typical of glaucoma to become permanent. Atrophy of the ciliary processes eventually leads to normalization of intraocular pressure and even eventual hypotony, seen as part of very endstage glaucoma.
Cataracts are common in glaucoma, presumably as a result of the stagnation of aqueous flow that results in inadequate delivery of nutrients to and impaired removal of metabolic waste products from the lens.
Lens luxation probably results from stretching of the zonules secondary to ocular enlargement. Pressure-induced degenerative change in the zonules is another possibility. The luxation may be into the anterior chamber or into the posterior chamber. Luxation into the posterior chamber requires that the vitreous has already liquefied. Such vitreal liquefaction is a common sequela to inflammation that may have preceded the glaucoma, and it also occurs as a sequela to the glaucoma itself. In globes with vitreal liquefaction, whether the dislocated lens moves anteriorly or posteriorly seems to be just a matter of chance.
Retinal degeneration is the most important secondary change in glaucoma and is the one against which most therapeutic efforts are directed. It is very important because it causes blindness as a result of damage to the ganglion cells, which cannot regenerate, even if the glaucoma is eventually brought under control. The exact pathogenesis for the characteristic retinal and optic nerve changes probably varies among species and among different types of glaucoma. At least some of the contributing factors include the following:
1. Pressure-induced ischemic damage after collapse of blood vessels in the retina, optic nerve, or choroid in response to increased pressure in the vitreous.
2. Interference with ganglion cell nutrition by impairment of axoplasmic flow caused by pressure-induced compression of the axons passing through the lamina cribrosa.
3. Direct damage to ganglion cells by elevated local production of excitatory amino acids, notably glutamate, from injured optic nerve and retina.
The degeneration characteristically causes atrophy of the nerve fiber layer and ganglion cells, and later loss of neurons from the inner nuclear layer. The hardy astrocytes within the inner nuclear layer (Müller cells) remain intact. In classic glaucomatous retinal atrophy, the outer nuclear layer and photoreceptors remain unaffected for years (Fig. 20-90). This pattern of “inner retinal atrophy” with sparing of the outer nuclear layer and photoreceptors is virtually unique to glaucoma (remembering that inherited, nutritional, and toxic retinopathies all target photoreceptors). In dogs with high-pressure glaucoma, however, it is common to also see destruction even of outer portions of the retina. Such destruction sometimes occurs quite quickly, presumably in response to pressure-induced collapse of the superficial choroidal blood vessels (choriocapillaris) responsible for the nutrition of the photoreceptors and outer nuclear layer. In such cases, there is also necrosis of RPE.
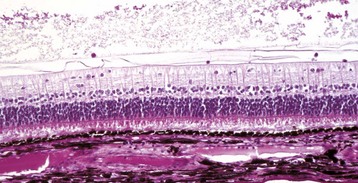
Fig. 20-90 Retinal atrophy, glaucoma, retina, horse.
There is loss of density in the nerve fiber layer and loss of virtually all the ganglion cells (top), but excellent preservation of the outer nuclear layer and photoreceptors. Retinal microscopic changes in glaucoma vary with its severity and duration and with the species affected. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Optic nerve changes are among the most controversial lesions seen in glaucoma. Much of the controversy centers on whether the optic nerve changes are just a consequence of glaucoma or whether they can occur independently of the pressure changes. The answer probably varies with the species and with the type of glaucoma under consideration. The situation is greatly confused by incautious extrapolation of the results from human glaucoma research. Glaucoma in humans is substantially different from that seen in most of our domestic animals. Microscopic changes in the optic nerve include degeneration of axons (Wallerian degeneration) and astrogliosis secondary to the loss of the ganglion cells and pressure-induced posterior displacement of the lamina cribrosa. Optic disc cupping is almost pathognomonic for glaucoma. It may occur very quickly as a result of pressure-induced posterior displacement of the lamina cribrosa or more slowly as a result of atrophy of the optic nerve itself from the loss of axons (Fig. 20-91).
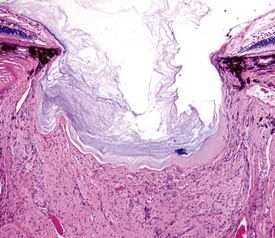
Fig. 20-91 Cupping of the optic disc, glaucoma, eye, dog.
The exact pathogenesis for the cupping in response to increased intraocular pressure probably involves many mechanisms and varies from species to species and among the different types of glaucoma. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The Classification of Glaucoma:
Primary Glaucoma: Primary glaucomas are those glaucomas occurring without any significant contribution from disease elsewhere within the globe. Primary glaucomas are subdivided into those cases in which there is detectable maldevelopment of the trabecular meshwork (goniodysgenesis) and those cases in which there are no primary histologic lesions (open-angle glaucoma). A very small proportion of these are truly congenital glaucomas in which clinical signs of glaucoma are evident in the first few weeks of life. The vast majority, however, have no clinically detectable increase in pressure or clinical signs related to glaucoma until middle-age or even older. The reasons for this delay in clinical onset are unknown.
Goniodysgenesis: Goniodysgenesis is a general term denoting imperfect development of the trabecular meshwork. It reflects incomplete remodeling of the solid mass of anterior chamber mesenchyme that gives rise to the stroma of the cornea and anterior uvea. In carnivores, most of this remodeling occurs in the first few weeks of life and involves rarefaction of what was previously a solid mesenchymal mass. In truly congenital glaucoma, there may be virtually no rarefaction (so-called trabecular hypoplasia), but in the great majority of cases there is reasonably complete development of the trabecular meshwork and other portions of the drainage system. The most common histologic anomaly is failure of the most anterior portion of the trabecular meshwork to be adequately remodeled, resulting in what looks like an abnormal solid or imperforate pectinate ligament (Figs. 20-92 and 20-93). The majority occurs as inherited disease in purebred dogs, with at least 20 different breeds known to be affected. The correlation between the severity of the goniodysgenesis and the frequency of glaucoma varies among breeds. It is sometimes quite poor, suggesting that there are other factors, perhaps functional rather than morphologic, involved in the pathogenesis of glaucoma.
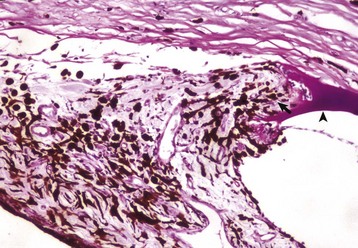
Fig. 20-92 Goniodysgenesis, primary glaucoma, dog.
Note the absence of a normal pectinate ligament. Descemet’s membrane (arrowhead) inserts directly into a broad band of stroma (arrow) continuous with the iris stroma. The rest of the trabecular meshwork is microscopically normal. Periodic acid–Schiff reaction. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
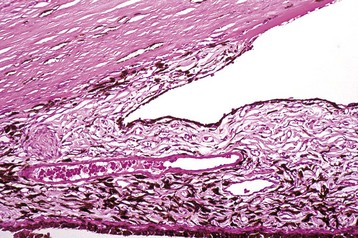
Fig. 20-93 Goniodysgenesis, primary glaucoma, dog.
Note the almost complete hypoplasia of the trabecular meshwork, the result of a profound arrest in remodeling of the mesenchyme within the ciliary cleft. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The reasons for the delay in the onset of clinical signs of glaucoma, often for 5 years or more, are poorly understood. There may be age-related changes in the composition of trabecular meshwork matrix components or in lens position that result in a small decrease in aqueous outflow. Such minor decreases would normally be insignificant, but they may be critical to a dog that already has impaired aqueous drainage. Alternatively, acquired changes, such as preiridal fibrovascular membrane or minor cellular infiltrates associated with a normally insignificant uveitis, might provide the “final straw” that triggers glaucoma in these predisposed individuals. There is no reason to presume that the triggering events are the same in each individual.
Primary open-angle glaucoma: Some examples of primary glaucoma in dogs, cats, and horses occur in globes in which there is no visible abnormality in the structure of the trabecular meshwork or other portions of the aqueous outflow pathways. The best known is open-angle glaucoma in beagles. It has been widely used as a laboratory model, but its frequency outside of the laboratory appears to be very low. These dogs develop increased intraocular pressure by about a year of age but usually do not have clinical signs of glaucoma (such as conjunctival congestion, corneal edema, and pupillary dilation) until they are between 2 and 4 years of age. In the early stages of glaucoma, all portions of the aqueous outflow pathways are histologically and ultrastructurally normal.
Secondary Glaucoma: Anything capable of obstructing the flow of aqueous through the pupil or its exit through the trabecular meshwork can cause secondary glaucoma. The most common are listed next more or less in the order of frequency in dogs, the species in which glaucoma is by far the most prevalent. These mechanisms are not mutually exclusive, and frequently several different mechanisms act together to produce glaucoma. It is also undoubtedly true that dogs with goniodysgenesis are particularly prone to the development of secondary glaucoma, even when the degree of pupillary or trabecular occlusion is less than would be required to produce glaucoma in normal dogs.
Pupillary block: The passage of aqueous through the pupil may be blocked by the anterior movement of the vitreous after primary anterior lens luxation by posterior synechiae as a sequela to fibrinous iridocorneal adhesions or by extension of a preiridal fibrovascular membrane (Fig. 20-94). Blockage may also occur from migration and fibroblastic metaplasia of lens epithelium that has been freed by rupture of the lens capsule or by massive swelling of the lens in what is known as intumescent cataract (Fig. 20-95). Displacement of the lens into the posterior chamber (posterior lens luxation) does not result in prolapse of the vitreous or in glaucoma.
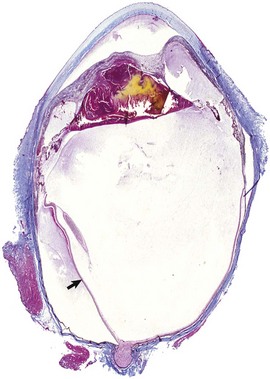
Fig. 20-94 Secondary glaucoma (from posterior synechia), iris bombé and peripheral anterior synechia, globe, sagittal section, dog.
The original injury was a penetrating wound of the cornea that caused phacoclastic uveitis. The inflamed iris adhered to the ruptured lens, causing a pupillary block and then iris bombé. The anterior displacement of the iris resulted in narrowing of the iridocorneal angle, in this case with adhesion of the base of the iris to the peripheral cornea (peripheral anterior synechia). Note the retinal detachment (arrow) and the abnormal shape of the lens (the latter resulting from loss of lens material at the time of perforation). Masson trichrome stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
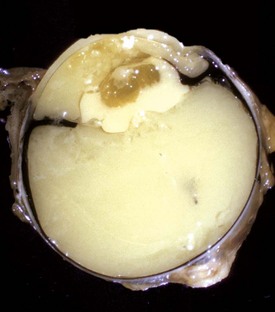
Fig. 20-95 Secondary glaucoma (from intumescent cataract), globe, sagittal section, dog.
Hydropic swelling of the lens (intumescent cataract) secondary to diabetes mellitus has caused the iris to be compressed against the peripheral cornea, resulting in a functional peripheral anterior synechia and a secondary glaucoma. The lens has subsequently ruptured, triggering a phacoclastic uveitis that explains the coagulated protein visible within the lens and ocular chambers. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Trabecular occlusion: The trabecular meshwork extends the full circumference around the iridocorneal angle, and no one is sure how much occlusion is required to produce glaucoma. The general consensus among ophthalmologists is that it must be at least 80%. The most common causes for this type of diffuse occlusion are infiltration by neoplastic cells (Fig. 20-96 and Box 20-14), peripheral iridocorneal adhesions by preiridal fibrovascular membranes (Fig. 20-97), or mechanical compression of the base of the iris by intumescent cataract or by large posterior chamber neoplasms. The tumors that most frequently cause glaucoma by infiltration of the trabecular meshwork are anterior uveal melanocytoma and metastatic lymphoma in dogs and diffuse iris melanoma and metastatic lymphoma in cats. Glaucoma caused by compression of the base of the iris is less frequent because it requires a very large tumor to cause the nearly circumferential occlusion. Only anterior uveal melanocytomas in dogs are likely to fulfill this requirement.
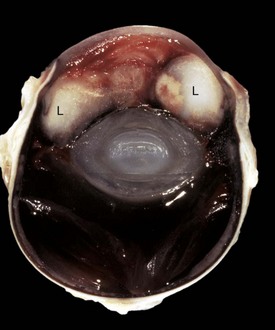
Fig. 20-96 Secondary glaucoma (from metastatic lymphoma), globe, sagittal section, dog.
The trabecular meshwork has been occluded by metastatic lymphoma (L) within the anterior uvea and anterior chamber. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
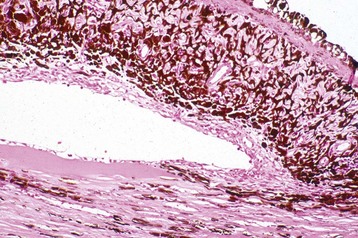
Fig. 20-97 Secondary glaucoma (from preiridal fibrovascular membrane), globe, sagittal section, dog.
The mature membrane has grown across the anterior surface of the pectinate ligament, creating peripheral anterior synechia. The trigger for the formation of the preiridal fibrovascular membrane was a small, otherwise insignificant iridociliary adenoma (not visible here). H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Very occasionally, glaucoma is caused by the occlusion of the trabecular meshwork by particulate material such as erythrocytes or leukocytes. It might seem that such occlusion would be prevalent, but such material usually settles by gravity and temporarily occludes only the ventral portion of the filtration angle.
Diseases Of The Cornea
Dermoid: Dermoid is the only corneal anomaly that is reasonably prevalent. It reflects the failure of the fetal ectoderm to undergo complete corneal “metaplasia,” with the result that a portion of the cornea remains as skin. Clinically, it is visible as a focus of corneal opacity in which there is hair and histologically as a segment of cornea that is more or less identical to normal skin (Fig. 20-98). The degree of skinlike change varies from complete development of skin and hair follicles to a less extreme version in which only vestigial hair follicles are present.
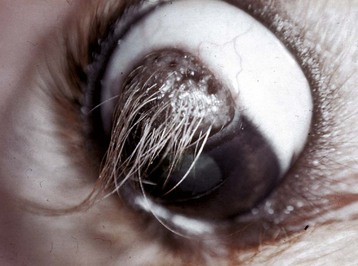
Fig. 20-98 Dermoid, cornea, dog.
A plaque of haired skin exists in place of the normal peripheral cornea. What triggers the failure of normal corneal maturation from pluripotential fetal ectoderm is unknown. Its clinical significance depends on how much corneal irritation is caused by the hair. Such dermoids may arise in cornea or, more commonly, bulbar conjunctiva. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Superficial Stromal Sequestration/Canine Persistent Ulcer: In dogs, cats, and horses (and probably in other species), there is a syndrome in which injury to the corneal epithelium is followed by an increased amount of superficial stromal apoptosis above the small amount that normally follows any corneal epithelial injury. In dogs and horses, this results in an inability of the sliding corneal epithelium to properly adhere to the underlying stroma, which is a prerequisite for full epithelial regeneration. Initially, adhesion of the sliding epithelium is to fibronectin and other transient adhesion molecules, but permanent adhesion requires the reformation of basement membrane, hemidesmosomes, and hemidesmosomal anchoring filaments, which extend through the basement membrane to anchor into the superficial stroma. If that stroma is abnormal, these filaments cannot grip and the regenerating epithelium is easily swept away with even the mildest trauma (Fig. 20-99). The cause for the excessive amount and/or persistence of the superficial stromal degeneration is not known. The most effective therapy is to surgically remove the superficial stroma (i.e., superficial keratectomy) to permit the epithelium to anchor into the normal deeper stroma.
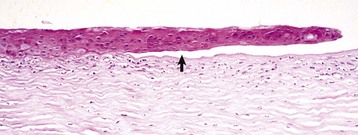
Fig. 20-99 Persistent ulcer, cornea, dog.
The epithelium is thickened and disorganized and has failed to adhere (arrow) to the adjacent corneal stroma. This failure of adhesion results in ineffective wound healing, seen clinically as either persistent or repeated ulceration. The failure of epithelial adhesion probably occurs because the hemidesmosomal anchoring filaments are unable to “anchor” in the injured stroma. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
In cats, the magnitude of the stromal devitalization is much greater, and there is imbibition of brown-colored pigment (thought to be derived from porphyrins in the tear film) into the dead stroma. This results in a very characteristic central corneal dark brown pigmentation that is the predominant and essentially pathognomonic feature of this disease (Figs. 20-100 and 20-101). The overlying epithelium may be ulcerated as it is in dogs or horses, but in many cases, it re-forms over the surface of the dead stroma. The frequency of this disease is much higher in those breeds with flat faces and prominent eyes, such as Persians and Himalayans, presumably because these eyes are predisposed to desiccation and other types of mechanical corneal injury. In non-Persian breeds with normal facial configuration, there is some evidence that ulceration caused by the herpesvirus is the most common predisposing cause.
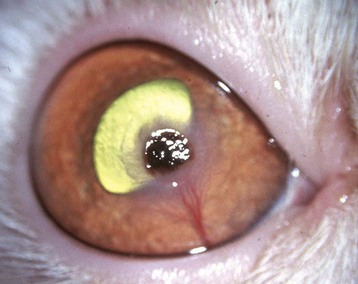
Fig. 20-100 Corneal sequestrum, cornea, cat.
The axial corneal ulcer has been accentuated by the absorption of a brown pigment, probably derived from porphyrins in the tear film. The gray halo of corneal edema and the prominent vascular ingrowth from the limbus to the edge of the ulcer are frequent but inconsistent features. (Courtesy Dr. M. Zigler, Mississauga-Oakville Veterinary Emergency Clinic.)

Fig. 20-101 Corneal sequestrum, cornea, cat.
Note the complete loss of epithelium on the right two thirds of the cornea (ulcer) and the necrosis of the remaining epithelium (left third). The stroma under the ulcer is necrotic (deeper eosinophilic) to a depth of approximately half the thickness of the cornea. The necrotic epithelium, the thickened basement membrane, and some of the necrotic stroma are accentuated by brown porphyrin pigment from the tears. Corneal sequestra also occur in the dog and horse, but they do not become pigmented. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Suppurative Keratomalacia (“Melting Ulcer”): Ulcers that become contaminated with bacteria or fungi are prone to suppurative destructive keratomalacia. Most of the stromal dissolution is probably the result of bystander injury from neutrophils recruited because of infection, but part of the damage may be associated with proteases of bacterial or fungal origin. Pseudomonas and streptococcus are the bacteria most likely to cause suppurative keratomalacia, which sometimes rapidly progresses to descemetocele and corneal perforation (see Fig. 20-62). Contamination by opportunistic hyphal fungi (especially Aspergillus spp.) is a particularly frequent cause of keratomalacia in horses (see Equine Keratomycosis).
Infectious Bovine Keratoconjunctivitis: See the discussion on infectious bovine keratoconjunctivitis in the section on Disorders of Ruminants.
Feline Herpetic Keratitis: See the discussion on feline herpetic keratitis in the section on Disorders of Cats.
Eosinophilic Keratitis: See the discussion on eosinophilic keratitis in the section on Disorders of Cats.
Canine Pannus Keratitis (Chronic Superficial Keratitis, Uberreiter’s Syndrome): See the discussion on canine pannus keratitis in the section on Disorders of Dogs.
Equine Keratomycosis: See the discussion on equine keratomycosis in the section on Disorders of Horses.
Corneal Dystrophies and Depositions: Macroscopic examination of the cornea often reveals various types of colored or refractile stromal deposits. Some of these have already been discussed (superficial stromal pigmentation as part of cutaneous metaplasia or accumulation of leukocytes as part of stromal inflammation). Most other corneal deposits are composed of lipid and/or mineral. Among the domestic animals, they are most often seen in dogs. These deposits generally have characteristic clinical features (breed, age, exact anatomic location, and macroscopic appearance) that allow a diagnosis to be made without the need for histopathologic examination. They fall into two broad categories: inborn errors of metabolism known as corneal dystrophies and acquired corneal deposits, resulting from previous corneal disease or as incidental manifestations of systemic metabolic abnormalities that have “overflowed” into the cornea.
Genetically conditioned canine corneal stromal dystrophies include a seemingly endless array of lipid or mineral deposits somewhere within the corneal stroma. By definition, the defect is bilateral and congenital, even though the clinical or histologic manifestation of the abnormality may not be visible until later in life. The list of affected breeds grows daily. In each breed, the location, clinical character, and progression of the lesion are characteristic. In many, the exact nature of the deposit has not been characterized, but cholesterol, phospholipid, or lipid-calcium complexes, either alone or in combination, have been identified in some.
Corneal endothelial dystrophy is seen in several breeds of dogs as bilateral, diffuse corneal edema secondary to the progressive destruction of corneal endothelial cells. The edema is not accompanied by any evidence of inflammation or stromal fibrosis (Fig. 20-102) and usually begins at the lateral peripheral cornea and progresses over months-to-years to create diffuse edema. The microscopic lesion is extremely difficult to see because the corneal endothelial cells are generally not well preserved in histologic sections. Scanning electron microscopy and other specialized techniques demonstrate a progressive decrease in the number of corneal endothelial cells over time. The exact pathogenesis remains unknown.
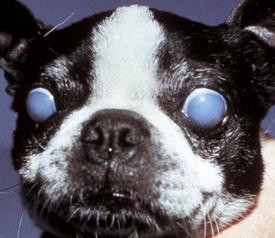
Fig. 20-102 Corneal endothelial dystrophy (inherited), cornea, Boston terrier, dog.
The blue-gray appearance of the corneas is due to severe diffuse corneal edema. The edema is secondary to progressive loss of corneal endothelial cells. The pathogenesis of this loss is unknown, but because these cells are critical in regulating the movement of water and electrolytes from the aqueous humor into the corneal stroma, their loss results in passive absorption of water into the cornea from the aqueous humor. (Courtesy Dr. M. Nasisse, Carolina Veterinary Specialists.)
Acquired corneal lipidosis (lipid keratopathy) results in milky or crystallin stromal deposits of serum lipids within the corneal stroma. The location depends on the pathogenesis. Any animal with an excessively high serum lipid concentration is predisposed to the deposition of neutral fat and cholesterol within the peripheral corneal stroma, reflecting an overflow from the limbal blood vessels. The lipid deposits become greatly accentuated if there is corneal inflammation because vascularization is increased and immature blood vessels are notoriously leaky.
The microscopic changes associated with corneal lipid deposition include the presence of the lipid material and variable degrees of reaction to those deposits. Stromal keratocytes may accumulate small lipid vacuoles, and clefts of cholesterol are usually found among the stromal fibers. In some cases there is virtually no inflammatory reaction, whereas in other dogs there is a well-developed granulomatous inflammatory reaction.
Diseases Of The Uvea
Uveal Anomalies: The epithelium on the posterior surface of the iris, the inner surface of the ciliary body, and the retinal pigment epithelium on the inner surface of the choroid are all derived from portions of the original optic vesicle. The bulk of the uvea is fibrovascular stroma derived from the periocular mesenchyme that originates from the neural crest (see Fig. 20-83). After outgrowth and later invagination of the primary optic vesicle to form the optic cup, the intraocular migration of this mesenchyme and its subsequent remodeling seem to be guided by soluble factors released from the neuroectoderm; failures of proper induction, remodeling, and eventual atrophy of portions of this mesenchyme are among the most prevalent of ocular anomalies.
The anomalies of the uveal tract can be divided into those resulting from a failure of initial induction or migration, a failure of later remodeling, or a failure of eventual atrophy (Table 20-3). During early embryogenesis, there is a persistent gap between the anterior lip of the optic cup and the overlying corneal epithelium. Several waves of periocular mesenchyme migrate through this gap to form the corneal stroma and endothelium, the stroma of the anterior uvea, and the anterior portion of the perilenticular vascular tunic. The ingrowth of mesenchyme to form the iris stroma is guided by the infolding of the most anterior margin of the optic cup, which will form the two layers of the future iris epithelium. Later, papillary proliferation of that iris epithelium gives rise to the epithelium of the ciliary processes. Proper inward migration of the neuroectoderm at the anterior lip of the optic cup seems to be a prerequisite for the subsequent migration of the mesenchyme to form the stroma of the iris and ciliary body (Fig. 20-103). Similarly, proper maturation of the future retinal pigment epithelium from the posterior neuroectoderm of the optic cup is required for the proper maturation of the retina, choroid, and overlying sclera.
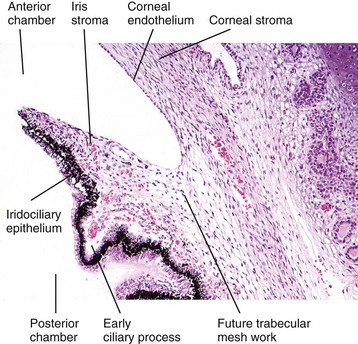
Fig. 20-103 Normal anterior uvea, 1-day-old puppy.
The iris is just beginning to be formed by ingrowth of neuroectoderm and associated mesenchyme from the anterior lip of the optic cup. Formation of the ciliary processes and trabecular meshwork will follow. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Iris Hypoplasia: Failure of ingrowth of the future iris epithelium results in the relatively rare anomaly of extreme iris hypoplasia, clinically referred to as aniridia (Fig. 20-104). This is relatively more frequent in horses than in other species. At least in some cases (and probably in most), it is inherited.
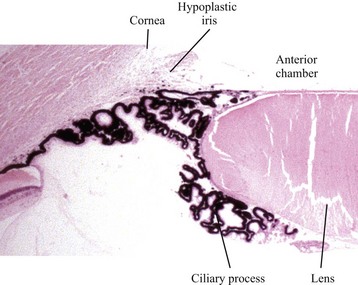
Fig. 20-104 Iris hypoplasia, piglet.
There is no ingrowth of iris epithelium, and the periocular mesenchyme has therefore not migrated inward to form the iris stroma. Unexpectedly, the ciliary processes have formed normally but have arrested maturation in that they have not retracted from the lens capsule. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Choroidal Hypoplasia: Choroidal hypoplasia is a common anomaly in the eyes of dogs and cats and is often associated with inadequate pigmentation of the retinal pigment epithelium and choroid. There are many examples in which lack of pigmentation and thinning of the choroid seems to have no effect on vision or any other aspect of ocular health (the so-called subalbinotic fundus). There is often an association between choroidal hypopigmentation, choroidal hyperplasia, and color dilution within the hair coat. Some are accepted as “normal for the breed” by genetic screening registries, although perhaps unwisely.
There are other instances in which choroidal hypoplasia is part of a significant developmental anomaly involving the choroids, retina, optic nerve, and sclera. By far, the most important example is collie eye anomaly. There are very similar anomalies in phenotypically related breeds, such as the Shetland sheepdog and Australian shepherd dog.
Collie eye anomaly was first described in 1953 as a widespread disease in smooth and rough collies in North America, Europe, and England. During the 1970s, it was found in more than 70% of collies within the United States, often with devastating consequences for vision. The multitude of anomalies can be explained by a single fundamental defect in the proper induction of the retinal pigment epithelium. The most basic and most prevalent of those inductive failures is hypoplasia and hypopigmentation of the choroid, which is why the disease is included here.
The fundamental defect in this anomaly appears to be improper inductive chemical signaling from the developing RPE. Choroidal development (in dogs and many other species) is in some way linked to the acquisition of melanin pigmentation. As a result of this defective signaling, the periocular mesenchyme destined to form the choroid and the sclera receives insufficient stimulation. In the choroid, this outcome is manifest as hypopigmentation and hypoplasia, including hypoplasia of the tapetum (Fig. 20-105). Within the fibrous tunic of the sclera, the most common manifestation is arrested growth, which results in delayed closure of the optic fissure along the ventral floor of the optic cup. This delay in closure allows the growing retina to bulge outwardly through the most posterior portion of this optic fissure. The outward protrusion of retina, usually just ventral to the optic disc, permanently prevents the proper closure of the embryonic fissure and sclera. The combined defect is referred to as posterior polar (or optic disc) coloboma, which produces a bulging of the sclera known as scleral ectasia (Fig. 20-106).
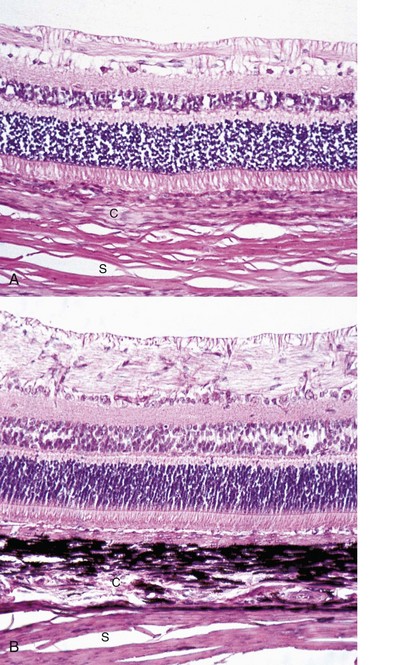
Fig. 20-105 Choroidal hypoplasia, collie eye anomaly, choroid, dog.
A, The choroid is only about half normal thickness and has no pigment. These lesions are the most consistent of those described for collie eye anomaly, but the molecular basis remains unknown. H&E stain. B, Normal canine retina and choroid, age-matched control. C, Choroid; S, sclera. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
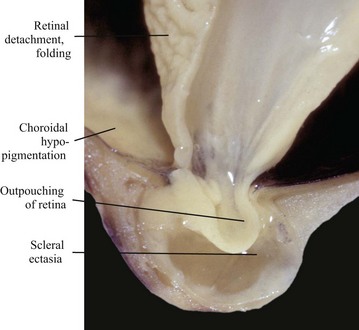
Fig. 20-106 Posterior polar scleral ectasia (at a site of coloboma), collie eye anomaly, eye, posterior pole, sagittal section, dog.
The retina protrudes through a defect in the posterior pole of the globe, a sequel to the delayed closure of the posterior margin of the embryonic fissure. The protruding retina is covered by sclera that also bulges posteriorly to accommodate the retinal protrusion. The lack of synchronization between the retinal and scleral growth rate that is part of collie eye anomaly causes retinal folding and, later, retinal detachment. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Other common defects in collie eye anomaly include mild microphthalmia, congenital retinal folding, tapetal hypoplasia, and retinal detachment. Detachment is usually delayed until the puppy is a few months of age and is not present at birth. Both the microphthalmia (which is usually just mild) and the retinal folding (see later discussion in the section on Retinal Dysplasia) probably represent improper coordination of the rates of retinal and scleral growth and may be another manifestation of improper signaling from the RPE. A retinal growth rate that exceeds that of the scleral shell inevitably results in folding of the redundant retina (interestingly, these retinal folds usually disappear as retinal and scleral growth eventually normalize). Conversely, normal growth of the scleral shell, accompanied by deficient retinal growth, stretches the retina and eventually results in complete retinal detachment (Fig. 20-107).
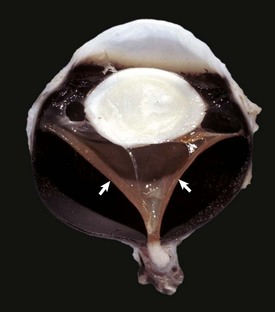
Fig. 20-107 Retinal detachment (complete), collie eye anomaly, globe, sagittal section, dog.
The retina (arrows) remains attached only at the sites of its true anatomic attachment: the optic disc and its junction with the pars plana of the ciliary body. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Other Uveal Abnormalities: Goniodysgenesis is maldevelopment of the filtration angle and is exceedingly common as a cause of primary glaucoma in dogs, but it is much less frequent in other species. It results from incomplete atrophy of the mesenchyme at the base of the iris. Most of this remodeling occurs in the first few weeks of life. For reasons that are poorly understood, clinical manifestations of glaucoma attributed to this developmental anomaly are not usually detected until middle-age or even later. Goniodysgenesis is described in more detail in the section on Glaucoma.
Persistent pupillary membranes and persistent primary vitreous refer to abnormal persistence of portions of the perilenticular vascular tunic or the vascular network within the developing vitreous. Such anomalies are common in dogs. The embryonic lens is encased in a network of blood vessels known as the tunica vasculosa lentis (Fig. 20-108). This network is created by contributions from the same mesenchyme that forms the iris stroma and from vasogenic mesenchyme growing into the developing vitreous through the posterior portion of the slowly closing optic fissure. The latter vessels, growing in from near the optic disc, are the hyaloid artery system (see Fig. 20-83). Together with other nonangiogenic mesenchymal elements, these vessels form the primary vitreous. This embryonic hyaloid artery system creates a temporary vascular network along the surface of the developing retina and also joins with the anterior chamber vessels to complete the tunica vasculosa lentis. All portions of this elaborate vascular system undergo atrophy before maturation of the globe. Persistence of one or more portions is common. The most common is persistence of the anterior portion of the tunica vasculosa lentis. This is usually referred to as persistent pupillary membrane. Macroscopically, these are seen as fine threads originating from the minor arterial circle of the iris. They are usually bloodless but are often pigmented. They may be inserted into the anterior stroma of the iris, or they may contact the surface of the lens (Fig. 20-109). Occasionally, in what is probably a more significant anomaly, they insert into the cornea. These membranes become clinically significant if they contact the lens or cornea, where they interfere with proper development of corneal or lens epithelium, or their associated basement membranes (Descemet’s membrane and lens capsule, respectively). Histologically, persistent pupillary membranes are thin endothelial tubes accompanied by varying amounts of mesenchymal stroma. At sites of corneal contact, they may cause fibrous metaplasia of the corneal endothelium. Where they contact the lens, there is usually epithelial proliferation and dysplasia of the lens capsule, resulting in a permanent focal cataract (Fig. 20-110). Those cases in which the pupillary membranes contact the cornea are probably more correctly classified as a minor expression of anterior segment dysgenesis (see later discussion).
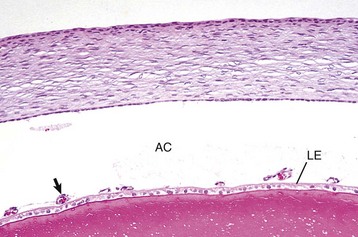
Fig. 20-108 Tunica vasculosa lentis (anterior portion [arrow]), 1-day-old kitten.
The vessels on the anterior surface of the lens will gradually disappear during the first few weeks of postnatal life. AC, Anterior chamber; LE, lens epithelium. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
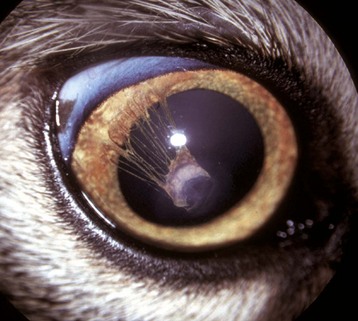
Fig. 20-109 Persistent pupillary membrane, 6-month-old dog.
These persisting portions of the tunica vasculosa lentis have adhered to the anterior surface of the lens, causing anterior polar lens opacity. (Courtesy pathology archives, Ontario Veterinary College.)
Fig. 20-110 Persistent pupillary membrane, dog.
These membranes (arrows) have adhered to the anterior lens capsule, resulting in disorganized proliferation of lens epithelium and lens capsule. The result is an anterior polar cataract. Periodic acid–Schiff reaction. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Persistence of various portions of the hyaloid artery system, with or without other portions of the primary vitreous, include much less common anomalies known as persistent hyaloid artery, and persistent (hyperplastic) primary vitreous (Fig. 20-111). When persistent blood vessels are accompanied by hyperplastic nonangiogenic mesenchymal spindle cells, the resulting anomaly is known as persistent hyperplastic primary vitreous. It has been described as a prevalent familial lesion in several breeds of dogs, particularly Doberman pinschers. The nonangiogenic mesenchyme undergoes notable fibroblastic proliferation, sometimes with cartilaginous metaplasia. Most affected dogs have concurrent anomalies, such as persistent pupillary membrane, microphthalmia, congenital cataract, and abnormal lenticular shape. Many of the described cases do not have the full range of lesions required for the diagnosis of persistent hyperplastic primary vitreous. Most would be better classified as persistent posterior tunica vasculosa lentis because the “hyperplastic” lesions of vitreal mesenchymal proliferation are absent.
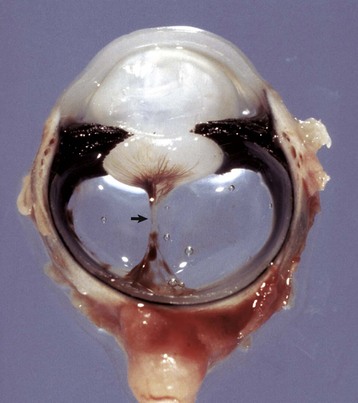
Fig. 20-111 Persistent hyaloid artery and posterior portions of the tunica vasculosa lentis, globe, sagittal section, dog.
A stalk (arrow) of fibrovascular tissue (the fetal hyaloid artery and associated mesenchyme) extends from the optic disc to ramify over the posterior surface of the lens. Opacity and deformity of the posterior pole of the lens are frequent complications. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Anterior segment dysgenesis is a blanket term for a variety of rare anomalies in which there is a failure of remodeling of the periocular mesenchyme destined to create the corneal stroma, corneal endothelium, iris stroma, and the anterior portion of the tunica vasculosa lentis. The usual clinical observation is absence of anterior chamber and the apparent fusion of the iris stroma to the corneal stroma and no corneal endothelium or Descemet’s membrane. The vast majority of these cases are not primary developmental anomalies but the result of perinatal corneal perforation. The loss of the anterior chamber is thus the result of a diffuse anterior synechia, usually secondary to traumatic corneal perforation or perforating ulcers that result in subsequent iris prolapse.
Acquired Uveal Diseases: There are in excess of 50 different etiologic types of uveitis in dogs alone, but in most of these the uveitis is only an incidental part of a systemic disease. The macroscopic or microscopic lesions within the globe are usually not distinctive, and diagnosis of the disease is rarely made on the basis of ocular lesions. Even when uveitis is the only clinical sign of disease, rarely is an etiologic diagnosis made. Many of the specific examples of uveitis listed in clinical textbooks are syndromes made distinctive by clinical features or by identification of a causal agent, rather than by distinctive histologic lesions. Listed next and in Table 20-4 are the most common and most important examples of uveitis that do have distinctive histologic features.
Idiopathic Lymphonodular Uveitis: Although not really a specific disease, idiopathic lymphonodular uveitis is by far the most common histologic pattern seen in uveitis. This may reflect in part a genuinely high frequency of immune-mediated uveitis. More likely, however, it is simply an indication of the chronicity of the uveitis because microscopic evaluation of globes with uveitis is usually done only in the chronic stages of disease, after all attempts at therapy have failed. Lymphonodular uveitis may therefore imply nothing more than a stereotypic endstage of uveitis that initially had a more distinctive and more variable histologic appearance. Lymphonodular uveitis was made famous by recurrent uveitis in horses. It is the only example of lymphonodular uveitis for which we have some insight into the actual cause, and for that reason it serves as the archetype for this group.
Equine Recurrent Uveitis: See the section on Disorders of Horses.
Feline Idiopathic Lymphonodular Uveitis: See the section on Disorders of Cats.
Canine Lymphocytic Uveitis: See the section on Disorders of Dogs.
Uveodermatologic Syndrome in Dogs (Vogt-Koyanagi-Harada Syndrome): See the section on Disorders of Dogs.
Systemic Mycoses: Systemic mycoses, such as blastomycosis, cryptococcosis, histoplasmosis, and coccidioidomycosis, are frequent causes of severe uveitis in those geographic areas where the organisms are common environmental contaminants. Immunodeficient animals may develop endophthalmitis as part of generalized disease caused by saprophytic fungi such as Aspergillus spp. or Candida spp., but these cases are rare; these same agents occasionally cause endophthalmitis when introduced by penetrating plant foreign bodies.
The frequency with which endophthalmitis accompanies systemic mycosis is unknown. The majority of cases are found in dogs, except for the inexplicable predilection of cryptococcosis for cats. Ocular involvement is part of systemic disease, but fairly frequently, ocular disease is the only obvious clinical sign. Blastomycosis is by far the most prevalent example of an endophthalmitis caused by systemic mycosis, and it serves here as the prototype for this group.
Blastomycosis is the most frequently reported intraocular mycosis in dogs, but it is rare in cats. It is estimated that about 25% of dogs with the systemic disease have clinically obvious ocular disease: unilateral or bilateral endophthalmitis with a very high frequency of exudative retinal detachment. The microscopic lesion is severe diffuse pyogranulomatous endophthalmitis, which tends to be more severe in the choroid and subretinal space than in the anterior uvea. The greatest accumulation of both leukocytes and organisms is usually in the subretinal space. The disease is pyogranulomatous and very destructive. The organisms may be numerous or extremely sparse, probably depending on the duration of the disease and on therapy. They are either free or within the cytoplasm of macrophages and have the typical features of Blastomyces spp.: thick-walled yeast 4 to 20 µm in diameter, with occasional broad-based budding. The diagnosis can often be made by cytologic evaluation of subretinal exudates in eyes that are already blind because of retinal detachment (one would probably not dare to attempt aspiration of the subretinal space in a globe that still had vision). Other lesions in affected globes are those seen in any severe uveitis: intraocular hemorrhage, posterior synechia, preiridal fibrovascular membrane, or cataract. Spread into the optic nerve and even orbit is an infrequent complication.
Cryptococcosis is similar to blastomycosis in that the lesions are predominantly within the retina, choroid, and optic nerve. As mentioned earlier, ocular cryptococcosis is immeasurably more prevalent in cats than in dogs or any other domestic animal. As is typical of cryptococcosis in other feline tissue, the granulomatous inflammatory response is often minimal. Large collections of poorly stained pleomorphic yeasts, surrounded by wide capsular halos, impart a typical “soap-bubble” appearance in hematoxylin and eosin (H&E)-stained sections. The organisms are usually numerous and vary greatly in size and shape. In a few cases, the granulomatous reaction is much more severe and mimics that seen in blastomycosis. In such lesions, organisms are typically scarce.
The ocular disease caused by Coccidioides immitis resembles blastomycosis but is more suppurative, more destructive, and more prone to progress to outright panophthalmitis. Involvement of the anterior uvea is perhaps more common than in the other systemic mycoses. The disease is seen only in animals that live in (or have visited) the very restricted geographic regions in which the organism is common. The great majority of cases are seen in dogs from the desert regions of the American Southwest (Fig. 20-112).
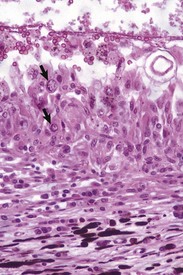
Fig. 20-112 Granulomatous choroiditis (destructive), coccidioidomycosis, choroid, dog.
The retina is absent because inflammatory exudate has caused retinal detachment. Organisms are usually difficult to find, but two spherules (arrows) are amid the macrophages. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The lesions caused by ocular infection with Histoplasma capsulatum are distinctive and quite different from those of the other systemic mycoses. There is usually a diffuse granulomatous and lymphocytic choroiditis with little suppuration and without much of the destruction that characterizes blastomycosis and coccidioidomycosis. Organisms are usually very numerous and visible as small spherical bodies within the cytoplasm of macrophages.
Protothecosis is included here because the organisms can easily be mistaken for yeast and because the clinical and histologic features closely resemble those of the systemic mycoses described earlier. Prototheca are colorless saprophytic algae capable of causing enteric, cutaneous, or generalized granulomatous disease in a variety of mammalian species. Ocular lesions have been described only in dogs with the disseminated form of the disease. The lesions are essentially identical to those seen with blastomycosis and are distinguishable only by the detection of the pleomorphic algae (which usually are very numerous). In histologic section, the algae are free or within macrophages. The organisms are spherical to oval, from 2 to 20 µm in diameter, and have a refractile cell wall that stains with periodic acid–Schiff (PAS) reaction or Gomori’s methenamine silver stain. Prototheca reproduces by asexual multiple fission, so that multiple daughter cells form enclosed within a single cell wall. There is no budding as with blastomyces and cryptococcus.
Feline Infectious Peritonitis–Associated Uveitis: See the section on Disorders of Cats.
Bovine Malignant Catarrhal Fever–Associated Uveitis: See the section on Disorders of Ruminants.
Lens-Induced Uveitis: Phacolytic uveitis is an extremely common, mild lymphocytic-plasmacytic anterior uveitis that occurs in a large proportion of animals with cataracts in which the lens protein is beginning to disintegrate and leak through the intact lens capsule. Although seldom significant as the cause of clinical signs, it significantly reduces the success of cataract surgery, unless the surgery is preceded by a course of antiinflammatory drugs.
Phacoclastic uveitis is predominantly an immune-mediated disease in response to the release of large amounts of intact lens protein through a traumatically ruptured lens capsule. The severity and character of the reaction seem to be influenced by the amount of lens material lost, the speed of the loss, the age of the animal, and the species. The disease is most commonly seen in dogs because of their habit of running into sharp objects or having unpleasant encounters with the neighborhood cat. Lens rupture may also occur after blunt trauma, in which case the rupture is usually of the thin posterior capsule. Phacoclastic uveitis also occurs after spontaneous lens rupture not associated with trauma. This occurs in rabbits as a result of penetration of the lens by Encephalitozoon cuniculi and in dogs with rapidly progressing diabetic cataracts.
The clinical syndrome is quite distinctive: the initial clinical signs are related to corneal perforation and uveitis as direct consequences of the penetrating injury and/or subsequent sepsis. Those are often managed successfully with antiinflammatory and antibiotic therapy, and everything seems to be healing very well until the development of a severe and therapeutically refractory anterior uveitis that most commonly occurs about 2 weeks after the initial injury. Once the phacoclastic uveitis is initiated, the prognosis for saving the globe becomes poor.
There are two different types of lesions of phacoclastic uveitis, and these usually occur concurrently. The typical lesions in acute disease include rupture of the lens capsule, accumulation of intralenticular neutrophils, and a perilenticular inflammatory reaction that is initially neutrophilic. With time, it becomes progressively more granulomatous but remains distinctly perilenticular (Fig. 20-113). After a few days, the inflammatory reaction is accompanied by proliferation, fibroblastic metaplasia, and perilenticular migration of lens epithelial cells that have escaped through the site of perforation (Fig. 20-114). Because the perforating injuries causing phacoclastic uveitis may also implant bacteria or foreign material into the globe, it is not always possible to determine what part of the posttraumatic lesion is phacoclastic uveitis and what part is in response to a foreign body or infection. True phacoclastic uveitis is distinctly perilenticular, with minimal reaction in more distant portions of the uvea such as the choroid.
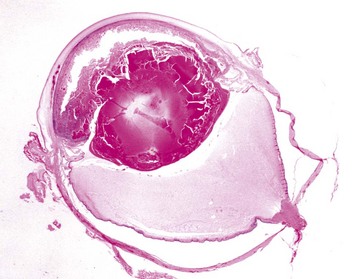
Fig. 20-113 Phacoclastic uveitis, encephalitozoonosis, eye, sagittal section, rabbit.
The anterior lens capsule has disintegrated, with substantial loss of lens material from the anterior cortex. The scalloped remnant of lens is now covered by a mixture of fibrin and leukocytes that fills the anterior chamber. In all species, the sudden release of intact lens protein triggers massive suppurative and granulomatous perilenticular inflammation that is typically delayed until 10 to 14 days after lens rupture. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
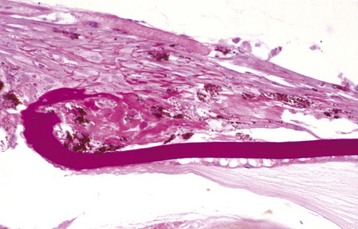
Fig. 20-114 Chronic phacoclastic uveitis, eye, horse.
The predominant lesion is now a plaque of fibroblast-like cells on the anterior surface of the lens adjacent to the site of capsular rupture (lower left). This plaque is created by metaplasia of lens epithelium that has escaped through the capsular defect. These proliferating cells frequently cause pupillary blockage and secondary glaucoma. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The pathogenesis of the inflammatory component of phacoclastic uveitis is probably an immunologic response to the release of large amounts of strongly antigenic lens protein into the aqueous humor. Although lens protein is not truly “foreign,” the body has tolerance for only small amounts of lens protein. The sudden release of large amounts following capsular rupture overwhelms this immune tolerance, resulting in a suppurative-to-pyogranulomatous reaction centered on the lens. This immune pathogenesis explains the lag between lens rupture and development of the phacoclastic uveitis. It also explains why small perforations might not cause the disease and why removal of the injured lens shortly after perforating injury prevents the development of the disease.
The fibrous metaplasia and migration of lens epithelium are critical prognostic events. This epithelium, once out of the lens, recognizes no barriers to its proliferation and causes pupillary block, occlusion of the filtration angle, and secondary glaucoma. In cats, probably it is this same epithelium that undergoes malignant transformation to the clinically significant and uniquely feline entity of posttraumatic primary ocular sarcoma (see discussion on neoplasia).
Phacoclastic uveitis can be a serious complication of cataract surgery in which too much lens material is left behind within the lens capsular bag. In most instances, complications arise primarily from the migration and fibroblastic metaplasia of this residual lens epithelium. This can result in opacification of the pupil (and of any artificial lens implant) and possibly pupillary block, causing secondary glaucoma.
Neoplasms of the Uvea: Primary uveal neoplasms are common only in dogs and cats, but they are exceedingly important in both species and vie with glaucoma as the most common reason for enucleation (Table 20-5). Primary tumors (melanocytic tumors and iridociliary epithelial tumors) are considerably more prevalent than metastatic tumors in the globe, with the possible exception of metastatic uveal lymphoma in cats.
In all species, melanocytic tumors are by far the most common of all ocular tumors. Those tumors predicted to be behaviorally benign are referred to as melanocytomas; those predicted to be malignant are classified as melanomas. As with melanocytomas and melanomas elsewhere, there is substantial variation in biologic behavior, depending on species and location. These site and species variables are more important in establishing the prognosis than are many of the histologic and cytologic features.
Canine anterior uveal melanocytoma is by far the most common primary intraocular tumor of dogs, arising from the melanocytes within the stroma of the iris or ciliary body. They ordinarily form a solid, deeply pigmented mass that protrudes into the anterior or posterior chamber. Growth is expansive rather than invasive (Fig. 20-115). The typical tumor has an inconspicuous germinal population of cytologically bland and poorly pigmented spindle cells, and a much larger proportion of huge ballooned “plump cells” distended with pigment (Fig. 20-116), which are assumed to be postmitotic endstage neoplastic melanocytes. These tumors have very little metastatic potential, and the few that are likely to metastasize can be identified by a notable increase in the numbers of mitotic figures. Sooner or later, all of these tumors grow large enough to cause obstruction of aqueous outflow and secondary glaucoma.
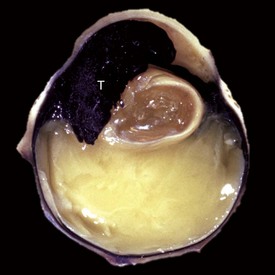
Fig. 20-115 Anterior uveal melanocytoma, eye, sagittal section, dog.
A large black tumor (T) has replaced the anterior half of the uvea and filled much of the anterior chamber. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
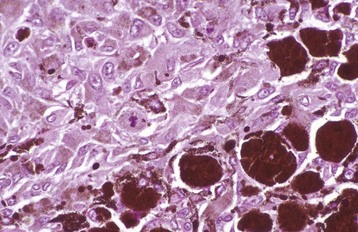
Fig. 20-116 Anterior uveal melanocytoma, iris, dog.
Large, heavily pigmented polygonal “plump cells” predominate (lower right). The smaller number of lightly pigmented spindle cells are germinal cells. The paucity of mitotic figures is a predictor that the tumor is benign. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Histologically identical melanocytoma occasionally arises within the choroid as a slowly expanding subretinal mass. They are significant because they cause retinal detachment.
Feline diffuse iris melanoma has a distinctive clinical presentation: unilateral coalescing hyperpigmentation of the iris that slowly (often over many years) progresses to diffuse iris thickening and secondary glaucoma (Fig. 20-117). This is a uniquely feline disease. The tumor originates from the layer of melanocytes that forms the anterior border layer of the normal iris. The transformed cells have enlarged nuclei and hyperchromasia and proliferate slowly to replace the normal iris architecture. The fully developed tumor, as the name implies, results in a diffuse iridal infiltration by pleomorphic round-to-epithelioid melanocytes. Their appearance in cytologic preparations is extremely variable. Tumors vary from amelanotic to heavily pigmented, and from round cell to epithelioid to spindle cell (and mixtures of all of the above). Mononuclear gigantism is frequently seen (Fig. 20-118). None of these variables has any prognostic significance.
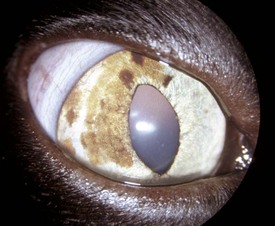
Fig. 20-117 Diffuse iris melanoma, eye, cat.
Coalescing areas of brown pigmentation have caused thickening of the iris. In many cats, the extent of the pigmentation and thickening progresses over many years, and eventually the tumor may cause glaucoma secondary to trabecular occlusion. Enucleation may then be necessary. (Courtesy pathology archives, Ontario Veterinary College.)
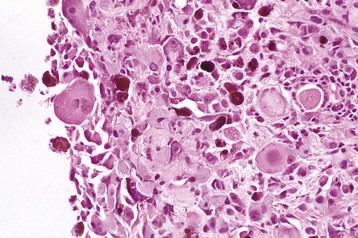
Fig. 20-118 Diffuse iris melanoma, iris, cat.
Note the extreme variation in cellular morphology: threefold variation in cellular size, nuclear gigantism, and variation in cell shape from round to spindle. The marked variation in the histologic and cytologic appearance of this tumor from case to case has no proven prognostic significance. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The prognosis has been the subject of great debate over the past 10 years. Retrospective studies that have attempted to establish histologic predictors of behavior have been thwarted by poor overall follow-up data (cats lost to follow-up, very few necropsies). Nonetheless, emerging from these studies is the general consensus that although these tumors are substantially more likely to eventually metastasize than are canine uveal melanomas, the overall risk of an affected cat developing clinical signs related to metastatic disease seems to be low. The probability that the affected eye will eventually develop glaucoma secondary to tumor infiltration of the trabecular meshwork is exceedingly high, although it may take years.
Rarely, cats develop canine-like nodular uveal melanocytomas (and dogs occasionally develop feline-like diffuse melanomas).
Iridociliary tumors vary from well-differentiated papillary adenomas to solid carcinomas. They all arise from the neuroectoderm of the posterior iris or ciliary body. The histologic distinctions have no apparent relevance to biologic behavior, because all of these tumors are benign. These are relatively common intraocular tumors in dogs (second in frequency only to melanocytoma), occasionally seen in cats, and infrequently seen in other species. They usually grow as discrete, expansile nodules that protrude into the posterior chamber and are thus visible through the pupil. They are populated by cuboidal and columnar epithelial cells resembling normal iridociliary epithelium and usually form cords and papillary structures reminiscent of disorganized ciliary processes (Fig. 20-119). Some have a more primitive cytologic and histologic appearance, but extraocular metastasis is so rare as to be nonexistent. Even small tumors may become clinically significant because they habitually produce fibroblastic and angiogenic growth factors that stimulate the development of preiridal fibrovascular membranes, causing secondary glaucoma or intractable hyphema.
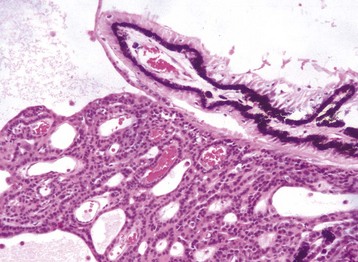
Fig. 20-119 Iridociliary adenoma (lower half of image), well-differentiated, posterior chamber, dog.
Although almost always benign, even small tumors of this type may induce the formation of a preiridal fibrovascular membrane that leads to secondary glaucoma. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Medulloepithelioma is a relatively rare congenital counterpart of iridociliary adenoma. It has the distinction of being the most common primary intraocular tumor of horses. The histologic appearance reflects its embryonic origin from primitive neuroectoderm still capable of both iridociliary and retinal differentiation. The tumor is populated by hyperchromatic cuboidal cells that make structures reminiscent of ciliary processes and sometimes tubular structures resembling the rosettes seen in retinal dysplasia. In horses, they often incorporate foci of cartilage, bone, and even brain tissue; these are known as teratoid medulloepitheliomas. Although they are by definition congenital tumors, their growth is slow and they may not be diagnosed until many years later.
Feline primary ocular sarcoma (posttraumatic sarcoma) is a high-grade spindle cell neoplasm that probably arises from lens epithelial cells that have escaped through ruptures in the lens capsule. These cells routinely undergo fibroblastic metaplasia as part of ineffective wound healing (see discussion on phacoclastic uveitis), but the metaplasia progresses to outright sarcoma only in cats. The tumor begins as a distinctly perilenticular mass and then grows to fill the globe (Fig. 20-120). As with histologically similar postvaccinal sarcomas, these tumors may have fibroblastic, osteoblastic, or cartilaginous areas in the same tumor (Fig. 20-121). The interval between trauma and detection of tumor can be many years, but the key word here is “detection.” It is very common for cats with this tumor to be presented for veterinary attention only after the tumor has filled the globe. We do not know the rapidity of microscopic tumor development following injury. These tumors frequently invade the optic nerve and then extend into the brain. They are also capable of distant metastasis.
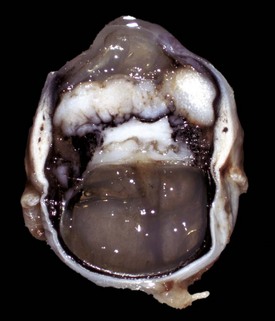
Fig. 20-120 Primary ocular sarcoma, eye, sagittal section, cat.
A solid white stromal sarcoma surrounds remnants of a ruptured lens. Wrinkled remnants of the lens capsule are still visible near the posterior margin of the tumor. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
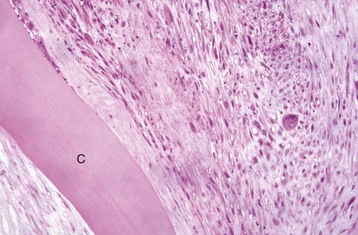
Fig. 20-121 Primary ocular sarcoma (right half of image), eye, cat.
Pleomorphic fibroblast-like cells, thought to be derived from lens epithelium, lie adjacent to remnants of lens capsule (C). Virtually all cases are preceded by rupture of the lens capsule. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Neoplasms metastatic into the globe are much less frequent than tumors originating within the globe. Virtually any metastatic neoplasm can localize within the uveal tract and spread from there into other portions of the globe. Their histologic appearance is the same as that of the primary tumor. By far the most prevalent is lymphoma (lymphosarcoma), seen in many species but particularly prevalent in cats. It causes diffuse thickening and pallor of the iris and less frequently the choroid. It is clinically almost indistinguishable from severe idiopathic uveitis, and the distinction is usually made on the basis of additional clinical evidence.
Diseases Of The Lens
Anomalies of Lens: The lens is derived from the thickening of the ectoderm induced by contact with the primary optic vesicle. This lens placode then migrates inwardly to cause the optic vesicle to invaginate on itself to form the primary optic cup. As it does, the lens placode grows to become a lens vesicle and separates from the overlying ectoderm, which will become corneal epithelium (see Fig. 20-83). This vesicle initially is just a single layer of cuboidal epithelial cells surrounded by a very thin capsule. The epithelial cells along its posterior surface elongate to obliterate the lumen of this primitive vesicle, creating the primary lens fibers that persist throughout life as the lens nucleus. The subsequent development of the cortical fibers of the postnatal lens depends entirely on mitotic activity from the anterior lens epithelium. No epithelium remains along the posterior half of the lens any time after the stage of the primary lens vesicle.
Anomalies of the lens are of minor importance when contrasted with anomalies of other portions of the globe. The lens has a central inductive role in ocular development, so significant anomalies of the lens are almost always accompanied by multiple ocular anomalies such as microphthalmia. It is likely that many of the lens anomalies reflect acquired degenerative changes (even if occurring in utero), resulting in regression of what was a normally developing lens. Such changes include an abnormally small lens (microphakia), an abnormally shaped lens (lenticonus and lentiglobus), and a congenital lens luxation, which is presumably secondary to some congenital abnormality in the zonules.
Lens Luxation: Dislocation of the lens may be partial (subluxation) or complete (luxation). It may fall forward into the anterior chamber, or it may remain trapped in the posterior chamber. A completely dislocated lens is likely to develop a diffuse cataract, presumably because of its inadequate access to aqueous humor. Anterior lens luxation (Fig. 20-122) is much more significant because it predisposes to glaucoma (see the section on Glaucoma). Lens luxation may be primary or secondary.
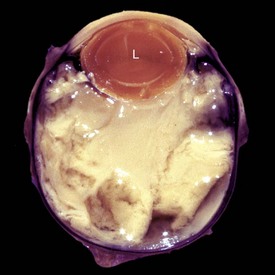
Fig. 20-122 Anterior lens luxation, eye, sagittal section, dog.
The dislocated, swollen lens (L) has moved anteriorly to lie against the posterior surface of the cornea and has compressed the iris, creating pupillary blockage and secondary glaucoma. There is coagulation and opacification of the aqueous humor and the vitreous because of increased protein from inflammation. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Primary lens luxation refers to that occurring without any known trauma or other ocular disease. It may be congenital or may be seen later in life. Congenital luxation is usually the result of a developmental error that causes abnormal or insufficient zonules. Much more prevalent are spontaneous luxations that occur in young adult dogs of specific breeds (notably terriers). The luxation is almost always bilateral, even if not simultaneous in onset. The ultrastructural and biochemical defect within the lens zonules have not been defined.
Secondary lens luxation is almost always a manifestation of blunt trauma that causes avulsion of the zonules, or excessive stretching of zonules within a globe that has become greatly enlarged secondary to glaucoma. It may also occur as a result of lysis of zonules by neutrophil enzymes or perhaps bacterial proteases in septic endophthalmitis.
The potential consequences of lens luxation are numerous, but the most significant is glaucoma. This is particularly frequent with anterior lens luxation. The pathogenesis may involve several factors including anterior dislocation of the vitreous causing pupillary block and accumulation of degenerate zonular material within the trabecular meshwork. It is sometimes impossible to decide whether the luxation caused the glaucoma, or the glaucoma caused the luxation.
Diabetic Cataract: Rapidly progressing bilateral cataracts develop in at least 70% of spontaneously diabetic dogs (but not in cats). Progression to complete cortical opacity usually occurs within a few weeks. The swelling may be so rapid that the lens actually ruptures. The pathogenesis of the cataract has traditionally been ascribed to the excessively high level of glucose within the aqueous. Glucose is normally the major energy source for lens fibers via anaerobic glycolysis. When the rate-limiting enzyme of this pathway, hexokinase, is overloaded with glucose, most of the excess glucose absorbed by the lens is shunted to the sorbitol pathway where it is transformed into the polyalcohol sorbitol, which is then slowly reduced to a ketose. Sorbitol may accumulate to very high concentrations within the lens where it osmotically attracts water, resulting in rapid swelling of the lens and disruption of its critical architecture. Sorbitol-induced osmotic disruption alone is not sufficient to explain all of the structural and metabolic changes in sugar-induced cataracts. The efficacy of antioxidants in slowing the progression of such cataracts, the nature of intralenticular biochemical alterations, and detection of increased intralenticular oxidants all point to some kind of oxidative damage as an additional promoter of cataracts.
Diseases Of The Retina
Retinal Dysplasia: Retinal dysplasia is a general term denoting an abnormal retinal differentiation characterized by jumbling of retinal layers. In the past, the term has been used rather loosely to include not only the rare genuine primary developmental anomalies but also postnecrotic and/or postinflammatory retinal scarring within the developing retina and retinal folding without any true disorganization. They should be considered as separate entities.
Primary retinal dysplasia is a rare anomaly that probably results from improper induction of retinal maturation by the RPE. Whether the failure relates to delayed and/or inadequate apposition between the two layers or a failure in the production of appropriate signaling molecules by the RPE is not known. The result is a retina with patchy-to-diffuse jumbling of the retinal layers (Fig. 20-123). Primary retinal dysplasia as the sole anomaly is seen as an inherited disease in a few breeds of dogs, but most examples are a part of multiple ocular anomalies. The significance of the lesion varies with the extent of dysplasia. Such retinas are prone to detachment (and in severe cases, the retina may never have been attached at all).
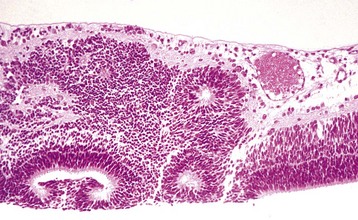
Fig. 20-123 Primary retinal dysplasia, retina, puppy.
The cells of the retinal layers are poorly organized and arranged haphazardly, sometimes creating acinar-like structures known as retinal rosettes. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Postnecrotic retinal dysplasia is a much more frequent phenomenon, seen as a sequel to acquired retinal necrosis of the developing retina. In dogs and cats, the period of susceptibility extends for at least 6 weeks after birth, during which time the retina continues to develop. Most of the documented examples are sequelae to viral infection, but in theory virtually any kind of retinal injury during its development could result in postnecrotic retinal “dysplasia.” In contrast to the adult retina, which retains no mitotic capability within the neurons of its various layers, the developing retina can still react with at least some neuronal regeneration. Such regeneration is usually mixed with glial scarring and does not restore perfect retinal architecture (Fig. 20-124).
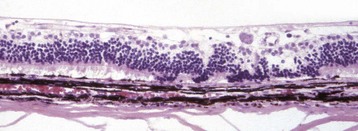
Fig. 20-124 Postnecrotic retinal dysplasia (so-called), in utero bovine viral diarrhea (BVD) virus infection, retina, calf.
There is loss of nuclei from all retinal layers as a sequela to the in utero destruction of retinal neurons by BVD virus. In some instances, this loss is followed by efforts at retinal regeneration, resulting in disorganization that may be considered as a retinal dysplasia. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The viruses most often implicated in causing retinal dysplasia in domestic animals are bovine virus diarrhea (BVD) virus in cattle, bluetongue virus in sheep, herpesvirus in dogs, and both parvovirus and choroid virus in cats. The histologic lesions can be quite variable, but usually there is a combination of very subtle residual inflammation along with postnecrotic scarring in the retina, optic nerve, and perhaps the choroids. Only if these viral infections damage the retina while its neurons still have proliferative capacity, will there be additional findings of disorganized neuronal proliferations with blending of nuclear layers. Proliferation also occurs in the RPE, which may be stimulated to migrate into the overlying scarred retina or to proliferate in situ as plaques of fibroblast-like cells. The pathogenesis of the “dysplasia” is the same as for lesions caused by these agents in the brain: infection and subsequent destruction of neurons (and possibly other cell types). The dysplasia is not specific to the mechanism of the cellular injury; it reflects only the unsuccessful attempts at postnecrotic healing in a retina still capable of at least some neuronal replication.
The window of susceptibility for the development of retinal dysplasia depends on the species (simply because the timetable for retinal development varies with the species). Infection of calves with BVD virus between 79 and 150 days gestation regularly results in postnecrotic retinal dysplasia, which is the most frequent and certainly the most studied of the virally induced retinal dysplasias. The initial ocular lesion is necrotizing lymphocytic endophthalmitis with random retinal necrosis. The inflammation gradually subsides, so there is scant evidence of inflammation in fetuses aborted later or in dead neonatal calves. Those ocular structures (cornea, uvea, optic nerve) that are already well differentiated at the time of the endophthalmitis may remain normal or exhibit some postnecrotic scarring; only the retina will exhibit abortive efforts at regeneration. Because the peripheral retina remains mitotically active for several weeks after the central retina has matured, dysplastic lesions may be found only in the peripheral retina. It is said that all calves with BVD-associated retinal dysplasia also have cerebellar hypoplasia. The same is probably true of cats with retinal dysplasia caused by in utero or perinatal infection with panleukopenia virus.
Retinal folding is by far the most common type of so-called retinal dysplasia. The great majority of such lesions are seen in juvenile purebred dogs. Most examples are inherited as autosomal recessive traits (sadly, so common in some breeds that they are now accepted as “normal variants”). The ophthalmoscopic findings and effect on vision vary from breed to breed. The histologic lesion is different from true retinal dysplasia and postnecrotic retinal dysplasia in that there is no jumbling of retinal layers, and there is no scarring. The exact pathogenesis for these retinal folds has not been determined, and it may not be the same among all breeds. What seems logical, however, is that the retinal folds represent nothing more than buckling of excessive retina within a globe because growth of the retina has been greater than that of the surrounding choroidal/scleral shell (Fig. 20-125). Supporting this speculation is the observation that such retinal folds are often transient and disappear as the puppy ages (implying that the growth rate of the scleral shell finally “catches up” to that of the retina). The most severe examples occur in dogs with developmental retinal nonattachment. These have extensively folded retinas because the distance from optic disc to ora ciliaris in a straight line is shorter than the convex route taken by a normally attached retina.
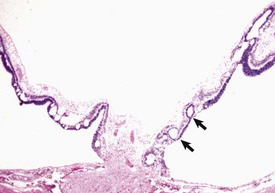
Fig. 20-125 Inherited retinal folds and retinal detachment, eye, posterior chamber, dog.
The acinar-like structures (arrows) within the detached retina are transverse sections through retinal folds. These folds probably arise because the growth rate of the retina temporarily exceeds that of the sclera, forcing the retina to fold on itself. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Optic Nerve Hypoplasia: The true frequency of optic nerve hypoplasia is unknown. Most examples are seen in toy breeds of dogs, with the diagnosis being made by clinical examination. Because there is no apparent visual defect, these eyes are almost never available for histologic assessment and the exact nature of the defect (true hypoplasia versus atrophy) remains unknown. Genuine hypoplasia of the optic nerve is the expected result of prenatal or neonatal destruction of ganglion cells from any intraocular disease (including congenital glaucoma, viral infection, and neonatal septicemia with embolic endophthalmitis). The lesion should therefore be correctly classified as atrophy rather than hypoplasia, although the distinction becomes muddy when talking about injuries in the prenatal and perinatal periods.
A distinctly different pathogenesis explains optic nerve hypoplasia in calves born from cows with vitamin A deficiency or in young calves with persistent perinatal dietary deficiency. Atrophy of the nerve results from compression as it passes through an abnormally flattened foramen. The reason for the flattening is improper regulation of osteoblastic activity around the periphery of the developing foramen. Optic nerve damage is not seen if the deficiency in vitamin A occurs after 2 years of age.
Acquired Retinal Disease (Tables 20-6 and 20-7):
Canine Progressive Retinal Atrophy: See the section on Disorders of Dogs.
TABLE 20-7
Acquired Retinal Diseases (Classified by Pathogenesis)

*The role of ischemia in glaucomatous retinal atrophy is controversial and probably varies by type of glaucoma and by species.
Photoreceptor Dysplasias: See the section on Disorders of Dogs.
Photoreceptor Degenerations: See the section on Disorders of Dogs.
Other Inherited Retinal Degenerations and Dysplasias: Inherited retinal dysplasias and degenerations have been reported as sporadic occurrences in a variety of cat breeds, but the syndrome has been adequately studied only in the Abyssinian breed. In this breed, there are two different diseases: early onset rod-cone dysplasia and late onset retinal degeneration affecting rods much sooner than cones. The early onset dysplasia is inherited as an autosomal dominant trait and is almost identical to the rod-cone dysplasia of Irish setter dogs. Retinal function deteriorates rapidly, with virtually all affected cats being blind before 1 year of age. In contrast, the late-onset retinal degeneration is inherited as an autosomal recessive, and affected cats usually show no clinical signs until about 2 years of age; they may retain at least some useful vision throughout life. The earliest structural changes are loss of neurons from the outer nuclear layer and disintegration of rod outer segments.
Congenital stationary night blindness in horses (nyctalopia) is a poorly documented disease affecting the Appaloosa breed (occasionally others). At least in Appaloosas, it seems to be inherited. The visual defects and associated behavioral abnormalities are seen in foals in dim light. In most cases, it does not progress sufficiently to cause obviously defective vision in daytime. Retinal histology is normal.
Other (Noninherited) Photoreceptor Diseases: Sudden acquired retinal degeneration (SARD) is an enigmatic, rapidly progressing, photoreceptor degeneration that is histologically identical to those of the inherited progressive retinal atrophies. Blindness occurs very rapidly (over a period of a few days to a few weeks). Affected dogs are adult or even elderly, and the disease can affect any breed or crossbreed. The funduscopic lesion is bilaterally symmetric and diffuse across the retina. Histologic studies of the early lesions are very few because there is no ethical justification for the surgical removal of these globes as the dogs are otherwise healthy. The disease has not been experimentally reproduced. The cause is unknown, but its occurrence is sometimes associated with polyuria, polydipsia, elevated serum cholesterol concentration, and increased serum alkaline phosphatase activity. Some but not even the majority of the affected dogs have adrenal cortical hyperfunction. How this malfunction causes the irreversible retinopathy, if indeed it does, is unknown. One small study demonstrated circulating, complement-fixing antibody to retinal S-antigen and interphotoreceptor retinoid-binding protein, raising the possibility that the disease is a cytotoxic autoimmune phenomenon.
Light-induced retinopathy is an important cause of blindness in animals maintained under inappropriate lighting conditions. The intensity and especially the duration of the light are important. Those species that are preferentially nocturnal in their natural habitat are particularly susceptible. The most susceptible group of all are deep-water fish maintained in aquariums with continuous artificial lighting or in shallow outdoor tanks without protection from sunlight. Outbreaks of blindness in different species of laboratory animals (especially albino rats) housed under continuous fluorescent lighting were the first to focus attention on this phenomenon.
The initial lesion is disruption of rod outer segment disks, followed by destruction of all photoreceptors and then their nuclei. At higher light intensities, but still within the range for “normal” room lighting, there may also be damage to neurons of the inner nuclear layer and to astrocytes and Müller cells. The histologic changes are the same as those for most other photoreceptor diseases, so this specific etiologic diagnosis is made based on the circumstantial evidence of abnormally bright light or an inappropriate balance between high-light and low-light intervals. The mechanism by which “ordinary” light damages the retina is still incompletely understood. Even normal vision is a “destructive” event, with light activation of photopigments generating a lot of free radicals and requiring physiologic replacement of photopigments and photoreceptor membrane discs during periods of sleep. The most popular theory is that of light-induced excessive oxidation of the very abundant polyunsaturated long-chain fatty acids of the rod discs, with the generation of free radicals. These cause cell membrane damage, and without adequate dark intervals there is reduced opportunity for regeneration.
Taurine deficiency as a cause for photoreceptor degeneration is seen only in cats. It is now largely a historical disease because all commercial feline diets are supplemented with taurine. Cats are unable to synthesize taurine from cysteine in amounts adequate for retinal function. Cats eating inappropriate diets (most often, stealing dog food) develop focal retinal atrophy in a horizontal streak dorsal to the optic disc. The disease, at least in some cases, progresses slowly to generalized retinal atrophy and blindness. The clinical features are identical to those of a historic disease known as feline central retinal degeneration. It is now assumed that the two diseases are the same but that may not be true in every single case. The histologic lesion is photoreceptor degeneration, initially involving cone outer segments, but eventually affecting rods as well. The rods of the peripheral retina are the last to degenerate.
Hypovitaminosis A as a cause for retinopathy is occasionally seen in groups of cattle or pigs receiving a ration deficient in vitamin A over a prolonged interval. Because most pastures have more than adequate vitamin A, deficiencies in cattle are likely to be seen only in animals raised in confinement and fed a poor quality ration (especially hay or grain that has been stored for a very long time). In adult animals, the effects of hypovitaminosis A first involve photoreceptor outer segments, slowly progressing to diffuse photoreceptor atrophy, loss of the outer nuclear layer, and eventually to complete retinal atrophy. These lesions have been reproduced in all domestic animals by feeding them specially formulated diets, but naturally occurring retinal lesions are seen almost exclusively in cattle. The deficiency of vitamin A results in a deficiency of rhodopsin within the photoreceptors. The initial ultrastructural lesion is swelling, followed by disintegration and fragmentation of the outer segments. Initially this can be reversed with vitamin A therapy until inner segments have also been affected.
Ischemic retinopathy may occur as a sequela to vascular occlusion within the retina itself or within the choroidal vasculature by tumor metastases or thromboemboli, as a sequela to retinal detachment, or as one of the mechanisms for retinal atrophy in glaucoma. Retinal ischemic injury may also occur subsequent to retinal or choroidal vasculitis associated with immune disease or a few infectious diseases such as thrombotic meningoencephalitis of cattle or Rocky Mountain spotted fever or ehrlichiosis in dogs (Fig. 20-126). The microvascular disease associated with diabetes mellitus is an exceedingly important cause of ischemic retinopathy in humans, but examples in domestic animals with spontaneous diabetes are rare.
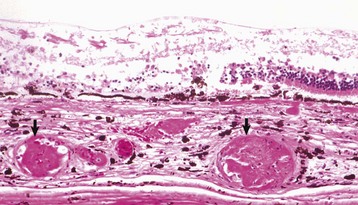
Fig. 20-126 Choroidal vasculitis with thrombosis, choroid and retina, horse.
Dilated choroidal blood vessels (arrows) contain fibrin thrombi and are surrounded by edema and some hemorrhage. There is ischemic necrosis of the overlying retina. The horse retina, with its very limited intrinsic vasculature, should theoretically be more susceptible to infarction secondary to choroidal vascular disease than the retina of any other domestic mammal. Such lesions are occasionally seen with disseminated intravascular coagulation regardless of the pathogenesis, as well as with idiopathic coagulopathies. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Vascular hypertension is an additional and relatively common cause for devastating retinal ischemic damage in dogs and cats. It is usually secondary to chronic renal failure. At least 60% of dogs with chronic renal failure are hypertensive.
The clinical disease is characterized by hemorrhage within the retina or from the retina into the posterior vitreous and retinal detachment secondary to serous effusion from injured retinal and choroidal blood vessels. The histologic lesions are primarily in retinal and choroidal vessels, but they may be found throughout the uvea. Characteristic changes are most easily found in the small retinal muscular arteries and larger arterioles. The vessel lesions include mural edema that may progress to fibrinoid necrosis of the tunica media and perivascular fibrosis. Muscular hypertrophy may occur in larger vessels, particularly those of the choroid. Changes occurring as a consequence of the vascular damage include localized retinal necrosis, serous retinal separation with resultant atrophy of photoreceptors and hypertrophy of retinal pigmented epithelium, and intraretinal hemorrhage (Fig. 20-127). There may be necrosis and/or repair of cells of the RPE and sclerosis within the choroid. The severity of the retinal necrosis varies from focal infarcts in the inner retina to (more commonly) extensive full-thickness hemorrhagic infarcts in large segments of retina. The larger lesions probably reflect the effects of widespread choroidal and retinal vascular degeneration. At least some of the retinal lesions are not the direct result of vascular necrosis but are the result of exaggerated autoregulatory vasoconstriction of precapillary sphincters attempting to protect the retinal capillary bed from the systemic hypertension. Sustained vasoconstriction leads to ischemic necrosis of the deprived retina, RPE or choroid, and necrosis of vascular endothelium distal to the constricted precapillary sphincters. Any blood that does manage to pass through the constricted sphincters into the capillaries enters blood vessels already damaged by a drop in perfusion pressure, so there is inevitable transmural leakage of serum and even blood.
Retinitis: Retinitis as the sole ocular lesion is rare. When it does occur, however, it is almost always in the course of neurotropic virus infections, such as canine distemper, rabies, and pseudorabies. Additional diseases that frequently cause prominent retinal lesions (albeit virtually never retinal lesions alone) include toxoplasmosis, canine ehrlichiosis and Rocky Mountain spotted fever, and thrombotic meningoencephalitis of cattle (Fig. 20-128). The lesions in the retina are the same as those in any other tissue affected by these systemic diseases. Retinal lesions also occur in the course of visceral larval migrans caused by the migration of the larvae of Toxocara canis and Baylisascaris procyonis.
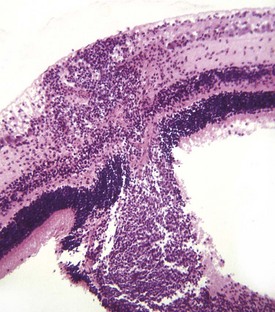
Fig. 20-128 Suppurative embolic retinitis, retina, steer.
There is focal obliteration of the retina by a combination of necrosis and inflammation. The leukocytes extend through the outer nuclear layer into subretinal space. This lesion is common in bovine thrombotic meningoencephalitis but is occasionally seen in other bacteremic or septicemic diseases. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Diseases Of The Eyelids
Developmental Anomalies: Anomalies in the formation of eyelids, including the shape of the palpebral fissure, are exceedingly common in dogs because “eye shape” has been so extensively manipulated during the evolution of the various breeds. In some instances, the “anomaly” is even a required feature for the breed (e.g., as with ectropion in bloodhounds and Saint Bernards). Most of these anomalies are not examined microscopically because they are macroscopically obvious. They are important in clinical ophthalmology and are considered in much greater detail in clinical textbooks than they are here.
Eyelid Agenesis and Coloboma: There may be partial or complete absence of an eyelid, particularly in cats. The most common by far is a partial defect (i.e., coloboma) involving only the upper eyelid. The notchlike defect results in localized corneal desiccation followed by cutaneous metaplasia.
Premature Eyelid Separation: The normal fusion of eyelids (known as physiologic ankyloblepharon) in carnivores is essential to protect the immature cornea from infection and desiccation. Premature eyelid separation (technically a “maldevelopment” because it occurs before the maturation of the globe) seriously predisposes the eye to infectious keratitis, desiccation, and even corneal rupture.
Entropion and Ectropion: Entropion is the inward rolling of the eyelid margin because of inadequate overall length. The usual result is irritation of the cornea by the eyelid skin, cilia, and/or hair. It is a very common anomaly in purebred dogs because they have been selected for breeding based partly on the shape of the palpebral fissure, which is a factor in determining facial “expression.” The extent and magnitude of the defect varies greatly among individual animals but tends to be relatively uniform within an affected breed. Clinical textbooks have extensive accounts of the manifestations of entropion and ectropion in different breeds and the innumerable techniques for their repair. The outcome for the cornea varies from corneal cutaneous metaplasia to outright ulceration, depending on the severity of the irritation. Entropion may also be acquired as the result of inflammation and subsequent contracture of a scar in the eyelid.
Ectropion is created by undue laxity of an excessively long eyelid, resulting in an outward gaping of the eyelid margin. As with entropion, its extent and severity vary greatly among individual animals and among breeds. The lower eyelid is more frequently affected to a clinically significant degree than the upper, presumably because the effect of gravity on the upper eyelid makes ectropion there less obvious. The anomaly has less significance than entropion because there is no direct corneal irritation. The protruding conjunctiva may entrap debris and become chronically inflamed and because of the failure of the lids to close properly, there may be some chronic exposure keratitis.
Anomalies of Eyelashes: Trichiasis, Distichiasis, and Ectopic Cilia: Anomalies of eyelashes are prevalent in dogs and may or may not cause clinical signs, depending on whether they irritate the cornea or not. They are mentioned here only for the sake of completeness because these are almost never evaluated histologically. Distichiasis is the presence of an ectopic row of cilia originating from the ducts of the Meibomian glands. The defect is usually bilateral. It may be clinically silent or cause corneal irritation. Trichiasis is misdirection of the normal cilia so that they contact the cornea. Ectopic cilia are abnormally placed cilia within the lamina propria of the conjunctiva. Their emergence through the palpebral conjunctiva may result in profound corneal irritation.
Acquired Diseases: Chalazion is sterile granulomatous inflammation in response to the leakage of Meibomian secretion into the surrounding dermis. It is much more common in dogs than in any other species. Although it can theoretically occur in response to any type of injury to the Meibomian gland, in almost all cases, the inflammation is found adjacent to Meibomian adenomas. Histologically, the inflammation is an accumulation of large foamy macrophages and multinucleated cells around the abnormal Meibomian gland. Some of the lipid may occur in the form of extracellular lakes of unstained free lipid, especially in cats. The macrophages contain distinctive slender, birefringent, intracellular crystals, which are readily visible under polarized light.
Idiopathic granulomatous marginal blepharitis is seen only in dogs as a series of coalescing nodules that eventually create a diffuse thickening of one or both eyelid margins. The histologic lesion is a coalescence of suppurating granulomas in the subconjunctival tissue of the eyelid margin, without any proven association with any particular adnexal structure, such as a Meibomian gland or hair follicle. The lesion is very similar to those of cutaneous sterile pyogranuloma syndrome and other idiopathic granulomatous panniculitides, for all of which the pathogeneses are unknown. No infectious agent has ever been identified.
Neoplasms of the Eyelids: Meibomian adenoma is by far the most common tumor of the canine eyelid, accounting for in excess of 80% of all eyelid tumors in that species. Eyelid tumors in any other species are infrequent to rare. The tumor is an exact counterpart of sebaceous adenomas seen elsewhere in skin: a smooth expansile growth populated by intermingled basal cells and mature sebaceous cells. The tumor usually retains well-developed lobular architecture and in some cases differs from a normal gland only by its much greater overall size (Fig. 20-129). Many are populated almost exclusively by basal cells with just a few foci of sebaceous differentiation. Many contain substantial amounts of melanin pigment. Behaviorally, they are benign and are easily cured by excision.
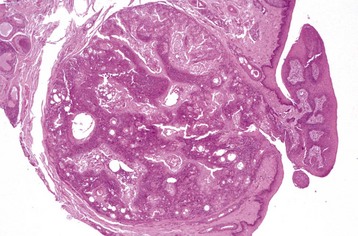
Fig. 20-129 Meibomian adenoma, eyelid, dog.
The tumor is a well-demarcated, encapsulated, expansile nodule consisting of intermingled basal and sebaceous cells. The accompanying papillary hyperplasia of the overlying epidermis is a common secondary reactive change. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Melanocytoma is the second most common tumor of the canine eyelid. It is identical in all respects to superficial dermal melanocytomas found elsewhere in the skin, and it is universally benign (Fig. 20-130).
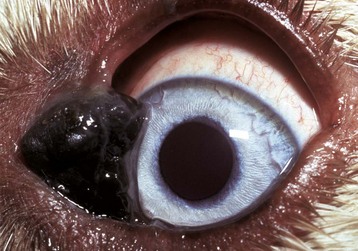
Fig. 20-130 Cutaneous melanocytoma, eyelid skin, dog.
A nodule of firm black tissue has replaced a wedge of the eyelid skin at its margin. Determining the exact location of the mass is clinically critical because a similar tumor arising even a few millimeters away on the palpebral conjunctiva would probably be behaviorally malignant. (Courtesy Dr. R. Peiffer, University of North Carolina.)
Other neoplasms affecting the eyelid with greater-than-usual frequency include nerve sheath tumors in dogs and cats, squamous cell carcinoma and mast cell tumors in cats, and sarcoids in horses. They are identical in histologic appearance and behavior to those tumors occurring anywhere else in skin.
Diseases Of The Conjunctiva
Conjunctival Dermoid: As with corneal dermoid, this is the presence of ectopic hair follicles and adnexal glands within the conjunctiva. Ordinarily, it is the bulbar conjunctiva that is affected. The ectopic tissue ranges from just a few scattered sebaceous glands to mature hair follicles with a full complement of adnexa.
Acquired Conjunctival Diseases:
Infectious Diseases: Infectious bovine rhinotracheitis (bovine herpesvirus 1) is usually accompanied by serous to purulent conjunctivitis, which can be confused clinically with infectious bovine keratoconjunctivitis caused by Moraxella bovis. However, corneal involvement with rhinotracheitis is uncommon, and it is never the major presenting complaint and never reaches the severity seen in infectious bovine keratoconjunctivitis. After the acute serous conjunctivitis, hyperplasia of the resident conjunctival lymphoid nodules (follicular lymphoid hyperplasia) frequently occurs. Macroscopically, this is evident as a series of glistening gray-white protruding nodules a few millimeters in diameter, visible through the epithelium of the bulbar and palpebral conjunctiva.
Feline infectious conjunctivitis is caused by mycoplasma, chlamydia, or herpesvirus. The microscopic lesions are not specific except for the presence of inclusion bodies characteristic of the specific infectious agent. Unfortunately, inclusion bodies are present only during the first few days of disease and are almost never present by the time cytologic or histologic samples are obtained. Thus the presumptive diagnosis is usually based on the clinical characteristics of the conjunctival disease and especially on the presence of other clinical signs. Because these agents are all quite widely distributed in the healthy feline population, one must use great caution in interpreting a positive result of a serologic or polymerase chain reaction (PCR) test as indicating a causal role for that organism in the disease in that patient.
Feline herpesvirus 1 (FHV-1) causes a combination of conjunctivitis, keratitis, and upper respiratory disease when it first affects young cats, but it may cause conjunctivitis alone as a recurring infection in older cats that have recovered from the initial, more widespread disease. Herpesvirus is a more significant cause of keratitis in cats.
Mycoplasma felis and Mycoplasma gatae have been reported to cause suppurative, erosive conjunctivitis. However, a review of the available evidence suggests that it is more likely that these mycoplasmas, which are members of the normal feline conjunctival flora, act only as significant opportunists in disease initiated by herpesvirus or chlamydia.
Chlamydia psittaci usually causes unilateral conjunctivitis in cats of any age, without any other associated disease. The conjunctivitis is initially neutrophilic but rapidly becomes a subepithelial mixture of neutrophils, macrophages, lymphocytes, and plasma cells. Early in the disease (between days 7 and 14), typical intracytoplasmic inclusion bodies can be seen, and their detection is improved by immunofluorescent staining. Because the clinical signs are characteristic and disease is easily treated, histologic assessment is rarely required. By the time histologic assessment is required in the few cases not responding to therapy, it is too late because lesions have become completely nonspecific lymphonodular conjunctivitis with no visible inclusion bodies.
Thelazia are thin, rapidly motile nematodes 7 to 20 mm in length that inhabit the conjunctival sac and lacrimal duct of a variety of wild and domestic mammals. They are perhaps not exactly “normal” in the conjunctival sac, but only a small proportion of animals infected with the parasite have clinical disease. They are transmitted from animal to animal by face flies, which ingest larvae in lacrimal secretions. In North America, they are of minor significance as parasites of horses and cause only a mild lymphonodular conjunctivitis.
Habronemiasis is a far more significant disease in horses. It causes oozing eosinophilic granulomas, up to 1 cm in diameter, in the palpebral and bulbar conjunctivae. The macroscopic lesion is a firm nodule with yellow, caseous gritty debris in the center. The lesion is in response to nematode larvae deposited by a fly intermediate host, usually Musca domestica or Stomoxys calcitrans. They are attracted to this location by the moisture collecting in the medial canthus. Larvae of Habronema muscae, Habronema microstoma, or Draschia (Habronema) megastoma are also capable of causing the same histologic lesion that is identical to cutaneous habronemiasis, a chronic eosinophilic and granulomatous inflammation surrounding live or dead larvae that are difficult to find in histologic sections (Fig. 20-131).
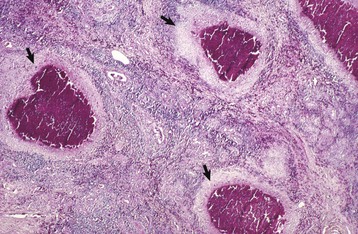
Fig. 20-131 Granulomatous conjunctivitis with eosinophils (focal), habronemiasis, conjunctiva, horse.
Note the multiple coalescing eosinophil-rich granulomas (arrows) within the conjunctival lamina propria. These granulomas are old larval migration tracts, but habronema larvae are rarely visible in them by the time such lesions are sampled for histological examination (usually to rule out neoplasia). H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Idiopathic and Immune Diseases: Idiopathic eosinophilic conjunctivitis is probably the conjunctival counterpart of the eosinophilic keratitis syndrome seen in cats and occasionally in horses. Rarely, the conjunctival lesion exists without any obvious corneal involvement. Lesions may be unilateral or bilateral. The microscopic lesions are greatly influenced by age and the previous antiinflammatory therapy, so it is hard to know what the “real” lesions are. In biopsy samples, the usual changes include ulceration, epithelial hyperplasia, squamous metaplasia, and a notable lymphocytic infiltration with a large number of eosinophils. The presence of eosinophils is a requirement for the diagnosis. They are not present as part of a granulomatous disease as in habronemiasis nor are they associated with collagenolysis as seen in insect bite reactions.
Protrusion and/or prolapse of the gland of the third eyelid is quite common in dogs and is thought to be the result of laxity in the connective tissue anchoring the gland to the cartilage of the third eyelid. Because the prolapsed mass is unsightly and resembles a neoplasm, it is sometimes excised and submitted for histologic examination, which reveals that the gland is usually completely normal or has mild nonspecific changes in response to desiccation or chronic edema.
Nodular granulomatous episcleritis (NGE) is a prevalent nodular lesion of the conjunctival lamina propria of dogs. It has been known by many names over the years, reflecting confusion about its exact status as an immune-mediated proliferative disease or a histiocytic neoplasm. Some of the synonyms include ocular nodular fasciitis, fibrous histiocytoma, or collie granuloma. It is not known whether this is one disease or several different diseases with the same histologic appearance. The disease is defined by its histopathologic changes: a discrete nodular accumulation of macrophages, fibroblasts, lymphocytes, and plasma cells anywhere in the conjunctival lamina propria. These cells are uniformly intermingled, without the formation of discrete granulomas. There is no collagenolysis, and usually there are not a significant number of granulocytes. The lateral limbus is the most frequent site, but the third eyelid is also frequently affected. In most instances, the lesion is unilateral and solitary, but bilateral involvement is not uncommon.
Necrotic scleritis is another canine idiopathic “immune-mediated” disease that may be mistaken for NGE. It often begins as a nodular thickening of the bulbar conjunctiva or underlying sclera just posterior to the limbus. Microscopically, however, it is a more destructive lesion with collagenolysis and (at least in the early disease) numerous eosinophils in addition to the macrophages, fibroblasts, and lymphocytes, which are also seen in NGE. It is a rapidly progressive and destructive lesion, capable of spreading rapidly to involve large areas of the sclera. Late in the disease it may spread into the globe, causing destructive coalescing granulomas throughout the uvea.
Neoplasms of the Conjunctiva: Squamous cell carcinoma is an exceedingly prevalent and economically significant neoplasm affecting the sunlight-exposed, nonpigmented epithelium of the eyelids, bulbar conjunctiva, usually at the lateral limbus and third eyelid of cattle living outdoors in sunny environments. The frequency is highest in areas in which livestock are likely to be exposed to ultraviolet radiation: areas with high altitude and lots of sunshine. Animals with poor pigmentation of eyelids and conjunctiva are particularly susceptible because this increases the risk of chronic cellular injury from solar radiation. Viruses, such as bovine papillomavirus and bovine herpesvirus 5, have been detected within bovine ocular squamous cell carcinomas, but their causal role has not been proven. An identical tumor occurs in horses living in the same environments.
As occurs with sunlight-induced squamous cell carcinoma in other cutaneous locations, the ocular tumor goes through a series of precancerous changes in response to actinic injury. The sequence of lesions is squamous plaque (acanthosis), keratoses (localized foci of hyperkeratosis), squamous papilloma, dysplasia, squamous carcinoma in situ, and eventually invasive squamous cell carcinoma (Fig. 20-132). Keratoses can sometimes develop into cutaneous horns. These are usually conical, can reach a length of a few centimeters, and consist of compacted laminated keratin (Fig. 20-133). Occasionally, they are on the surface of a papilloma. Like actinic keratoses they are considered to be premalignant. The stepwise transition from acanthosis to eventual carcinoma in situ is characterized by increasing nuclear pleomorphism, hyperchromasia, and loss of polarity of the cells and maturational jumbling of cells within the stratum spinosum. The only absolutely unequivocal proof of malignant transformation is invasion by cords of malignant cells through the basement membrane into the underlying lamina propria (Fig. 20-132, C). The mass of invading cells is usually surrounded by an intense lymphocytic-plasmacytic inflammatory reaction, a reaction that is interpreted as an immune response to the developing tumor. Not all precursor lesions develop into carcinomas. The efficacy of the immune response in destroying precursor lesions and early carcinomas is unknown.
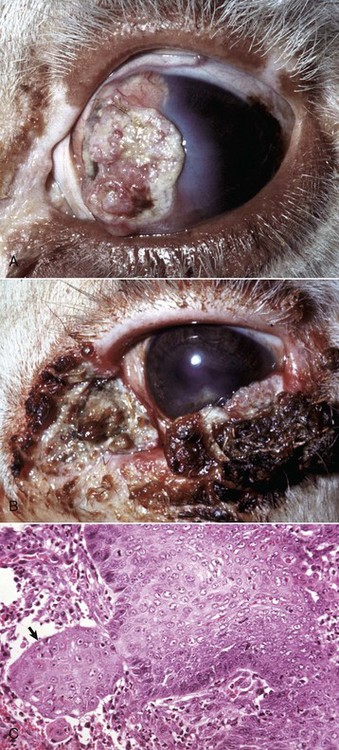
Fig. 20-132 Squamous cell carcinoma, eye and eyelids, Hereford cow.
A, Medial limbus. The carcinoma has spread over the cornea as an exophytic growth from its original site on the medial limbus. Note the corneal edema (gray area) adjacent to the margin of the carcinoma. Pigmentation of the eyelids, as shown here, does not protect the conjunctiva or the limbus from developing an actinic (exposure to sunlight) squamous cell carcinoma. B, Third and lower eyelids. Note the early exophytic squamous cell carcinoma on the third eyelid, nodules of acanthosis on the lateral half of the lower lid conjunctiva and on the skin of the medial canthus, and keratoses on the lower lid. C, Early infiltrative squamous cell carcinoma, lower lid. A small group (arrow) of epithelial cells has infiltrated through the basement membrane of the mucosal squamous epithelium. Note that these cells are atypical. The basal cells have lost their polarity and now lie horizontal to the edge of the infiltrating mass instead of vertically, as do normal basal cells (perpendicular to the basement membrane). H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Fig. 20-133 Cutaneous horn, lower eyelid, mucocutaneous junction.
The cutaneous horn on the lower lid is an exaggerated form of a keratosis. The unpigmented skin of the lower lid and medial canthus is erythematous, and the surface of the medial aspect of the lower lid is slightly roughed by white foci of acanthosis. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
In cats, squamous cell carcinoma usually affects the skin of the eyelid itself rather than conjunctiva. Squamous cell carcinoma is inexplicably rare in either the eyelids or the conjunctiva of dogs.
Benign squamous papillomas with no inclination for malignant progression are frequent tumors of the bulbar conjunctiva of dogs. They are formed by multiple slender fronds consisting of conjunctival lamina propria covered by hyperplastic but otherwise orderly and mature stratified squamous nonkeratinized epithelium. There is often substantial melanin pigment within the basal cells and lamina propria, so these tumors can be mistaken on clinical examination for conjunctival melanomas. There is no evidence that these papillomas are caused by a viral infection.
Primary conjunctival melanomas arise within the epithelium of the bulbar conjunctiva of dogs and cats. In dramatic contrast to the benign melanotic tumors of the haired skin of the eyelid or those arising deeper within the stroma of the limbus (see later discussion), these are aggressively invasive with a high risk of metastasis. They resemble oral melanomas in histologic and cytologic appearance and in the frequency of being amelanotic. Microscopically, they form solid endocrine-like packets of 20 to 30 cells supported by a delicate fibrous stroma. Intraepithelial clusters (junctional activity) are often present. The cells generally are pleomorphic epithelioid cells with frequent nuclear gigantism and hyperchromasia. They are markedly invasive, a feature often visible in histologic sections. Postoperative recurrence is extremely frequent. Pigment, when present, is most frequently found in tumor cells within or near the epithelium.
Limbal melanocytomas are relatively common tumors in dogs. They are infrequent but not rare in cats. They arise from the pigmented cells within the limbus, which is the junction of the corneal stroma and sclera. They form a slowly expanding, heavily pigmented discrete nodule populated by heavily pigmented large plump cells identical to those seen in the more common anterior uveal melanocytoma. They have no metastatic potential, and surgical excision is curative. They may bulge outwardly to distend the overlying conjunctiva, but histologic examination reveals that they do not infiltrate the epithelium and are therefore easily distinguished from the more dangerous primary conjunctival malignant melanoma.
Hemangioma and hemangiosarcoma are vascular endothelial neoplasms arising within the conjunctival lamina propria at the lateral edge of the third eyelid, and in the lateral bulbar conjunctiva of dogs, cats, and (occasionally) horses. About 30% of canine cases are bilateral. The sites of predilection and the increased risk of disease in outdoor dogs in sunny climates and high altitudes suggest that this disease is probably triggered by chronic actinic radiation injury (as is true for at least some examples of cutaneous hemangioma and hemangiosarcoma in dogs). Those tumors that are well circumscribed and consist of bland endothelium are classified as hemangiomas, and those formed by hyperchromatic endothelium with at least moderate anisokaryosis and peripheral invasion are classified as hemangiosarcomas. However, there is a continuum in the histologic appearance from hemangioma to hemangiosarcoma. Many are intermediate, or even show a continuum of lesions from benign to malignant within the same tumor. Distinguishing benign from malignant is based primarily on the degree of peripheral invasion. At least in dogs (the species most often affected), this distinction appears to have no behavioral significance, as almost all are benign and surgically curable.
In horses, some (but not all) of the vascular tumors involving the third eyelid are solid primitive hemangiosarcomas. Some of these tumors are very invasive and have a high risk of metastasis to the regional lymph node.
Adenocarcinoma of the gland of the third eyelid is a tumor of very old dogs (rarely cats), which is usually seen as a well-differentiated, slowly expanding tubular adenocarcinoma at the dorsal margin of the third eyelid. It replaces the normal gland of the third eyelid but only slowly invades adjacent tissue. The risk of metastasis is very low.
Lymphoma (lymphosarcoma) occasionally arises within the lamina propria of the third eyelid in animals (most often in cats) that do not have any evidence of disseminated lymphosarcoma. The cells are similar in histologic appearance to those of lymphomas elsewhere, but it takes courage to make this diagnosis when the tumor appears to be present in only a single, unlikely location, such as the third eyelid.
Diseases Of The Orbit
Orbital cellulitis is not a specific disease but inflammation of soft tissue in response to infectious agents introduced via a penetrating wound, a migrating foreign body, or an inflammatory focus from some adjacent tissue (often, a tooth root abscess). Only rarely does panophthalmitis extend into the orbit to cause orbital cellulitis because the sclera seems to be an effective barrier to the migration of leukocytes and infectious agents.
Lymphocytic interstitial dacryoadenitis is a progressive idiopathic lymphocytic inflammation of the lacrimal gland and/or gland of the third eyelid of dogs. It is present as a histologic lesion in most cases of spontaneous keratoconjunctivitis sicca. The initial lesion is an interstitial lymphocytic infiltration with epithelial necrosis, followed by glandular atrophy and extensive interstitial fibrosis with residual nests of lymphocytes. Because no infectious agent can be found and because the disease can be successfully managed with immunosuppressive therapy (particularly cyclosporin), it is assumed to represent a T lymphocyte–dependent autoimmune disease.
Orbital extraocular myositis affects all the extraocular muscles except the retractor bulbi. It is a rare disease of dogs. Histologically, there is a lymphocytic myositis that results in myonecrosis, followed by attempts at regeneration and eventually muscle atrophy and fibrosis. The acute disease causes enough swelling to result in pain and exophthalmos. In the chronic disease, there is a notable enophthalmos (retraction of the globe into the orbit). An immune-mediated attack directed specifically against the extraocular muscles is suspected to be the cause of this disorder. Because the extraocular muscles are a difficult site from which to obtain a biopsy, diagnosis is generally based on the typical clinical findings. Corticosteroid therapy is effective, but episodes can recur.
Orbital neoplasms are surprisingly frequent in dogs. They are rare in other domestic animals with the exception of cattle, in which malignant lymphoma occurs. Most orbital tumors are not discovered until they are large enough to cause exophthalmos or blindness, by which time the majority of cases are no longer amenable to surgical cure. Many different tumors in the orbit have been reported, but the only ones that are relatively frequent are optic nerve meningioma and multilobular osteochondroma in dogs, nasal carcinoma and gingival squamous cell carcinoma in dogs and cats, and malignant lymphoma in cattle. These tumors are all histologically identical to those in other locations.
Disorders Of Horses
Equine keratomycosis (mycotic keratitis) is probably always a manifestation of opportunistic contamination of corneal wounds by fungi that are common within the environment around horses. Aspergillus spp. is by far the most common agent isolated. Similar infection occurs much less frequently in dogs, cats, and other species. Alteration of the corneal defenses or the normal microbial environment by the prolonged use of antibiotics or corticosteroids in the management of corneal wounds is a strong predisposing factor in all species. In all species there are two different syndromes: (1) opportunistic infection of dead superficial stroma by huge numbers of fungal hyphae in the almost complete absence of inflammation or (2) deep stromal infection that evokes an intense suppurative keratomalacia that, if untreated, often progresses to corneal perforation and iris prolapse. The deep stromal keratitis in cases in which the stroma and epithelium have healed over the top of a deep stromal infection is referred to as a stromal abscess. The fungi exhibit strong tropism for Descemet’s membrane (as they do for basement membranes in other tissues) and may be absent from the stroma of the superficial (anterior) half of the cornea (Fig. 20-134). This explains why confirmation of infection by means of corneal scrapings is often unsuccessful. It also explains why topical application of antifungal medication alone is rarely successful. In many cases, successful management of the disease requires surgical débridement of the cornea to remove most of the infected tissue and the damaging leukocytes. One peculiarity of this disease is that the infection virtually never spreads into the globe itself, even though the fungi can be seen dangling within the anterior chamber. There must be something unique to the corneal environment that is essential for fungal virulence or survival.
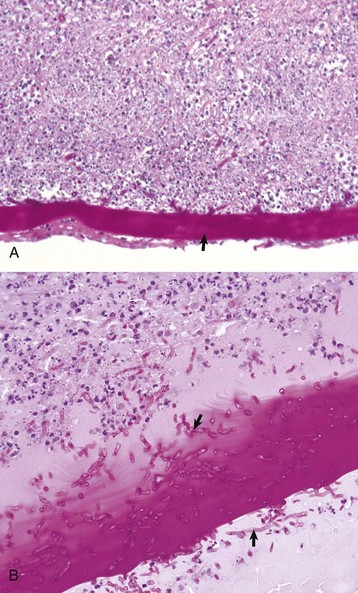
Fig. 20-134 Suppurative keratomalacia (deep stromal), posterior cornea, keratomycosis, horse.
A, The deep portion of the corneal stroma contains numerous neutrophils, resulting in stromal destruction (keratomalacia). Descemet’s membrane (arrow) is thickened and irregular and contains fungal hyphae. This is the most common histologic form of the disease. H&E stain. B, Higher magnification to show fungal hyphae (arrows) within and adjacent to Descemet’s membrane. Note the loss of the normal appearance of the stroma and the infiltration by neutrophils and edema fluid. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Eosinophilic Keratitis
Eosinophilic keratitis occurs predominately in cats and occasionally in horses (see the section on Disorders of Cats).
Equine Recurrent Uveitis
Equine recurrent uveitis (ERU), also referred to as periodic ophthalmia and moon blindness, is a frequent cause of blindness in horses. It is characterized by unpredictable episodes of severe uveitis with a tendency to become increasingly frequent and increasingly severe over time. It is likely that several different diseases are included under this diagnostic umbrella, but at least one of the causes is an immunologic reaction against intraocular leptospiral antigens. It is thought to be a delayed reaction to a systemic infection with Leptospira interrogans (most often serovar Pomona). The evidence supporting a role for leptospirosis in the pathogenesis of equine recurrent uveitis is substantial even if not absolutely conclusive:
1. Horses with high serum titers to Leptospira interrogans are 13 times more likely to have uveitis than horses with no such titers.
2. Antileptospiral antibodies rise during episodes of uveitis and decrease during quiescent phases.
3. The disease can be reproduced by infection of naive horses with Leptospira interrogans serovar Pomona. Not all infected horses developed uveitis, and the onset of ocular signs is usually delayed for a year or more after infection. In the original study, 22 of 36 of the eyes of Shetland ponies inoculated subcutaneously with small numbers of Leptospira interrogans serovar Pomona eventually developed classic recurrent uveitis. All ponies developed leptospiremia soon after inoculation, but none developed ocular disease until at least 50 weeks later.
4. There is cross-reaction between leptospiral antigens and various intraocular antigens, particularly those of the corneal endothelium and lens. This creates the possibility that the ongoing immune-mediated inflammation is not necessarily in response to persisting leptospiral antigens. Leptospirosis may cause the initial uveitis but may not be responsible for its perpetuation.
The most common alternative agent implicated in a syndrome clinically indistinguishable from leptospira-associated recurrent uveitis is Onchocerca cervicalis. Intraocular dead or dying microfilaria have been proposed as sources of antigen responsible for the disease.
The microscopic lesions depend on the stage of the disease and represent a continuum from anterior uveitis to endophthalmitis with retinal scarring or even phthisis bulbi. The earliest lesion is an anterior uveal inflammation that is transiently neutrophilic but rapidly becomes predominantly lymphocytic. Even during intervals in which the clinical disease is quiescent, the histologic lesions of perivascular lymphocytic anterior uveitis persist (Fig. 20-135). Peripheral corneal vascularization becomes increasingly prominent, eventually extending across the entire diameter of the corneal stroma. Choroidal inflammation is usually most obvious near the optic disc and may cause exudative retinal detachment. Even if the retina reattaches, there are residual lesions of photoreceptor loss or even full thickness retinal scarring as a result of ischemic injury during periods of detachment. This peripapillary retinal scarring is sometimes the only lesion seen in horses with a clinical history suggesting chronic, episodic ERU.
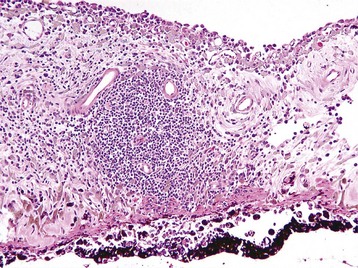
Fig. 20-135 Equine recurrent uveitis, iris, horse.
Perivascular lymphocytic-plasmacytic aggregates (center of image) within the iris stroma are typical of equine recurrent uveitis, but the same lesion is commonly seen in idiopathic uveitis in various species. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
A characteristic lesion of chronic ERU is an eosinophilic hyaline membrane that seems to cover the ciliary epithelium. This material in fact lies within the apical cytoplasm of the nonpigmented ciliary epithelium and resembles amyloid. Its pathogenesis is unknown, but it serves as a useful marker for the disease.
Disorders Of Ruminants (Cattle, Sheep, And Goats)
Infectious Bovine Keratoconjunctivitis
Infectious bovine keratoconjunctivitis (commonly known as pink eye) is a worldwide contagious disease of considerable economic importance that has been recognized for more than 100 years. It is caused by specific strains of the Gram-negative bacillus Moraxella bovis, which is part of the normal conjunctival and nasal flora of cattle. Virulent strains have capsular pili, which facilitate corneal colonization, and most of the virulent strains are also hemolytic. In contrast, most of the strains recovered from the conjunctiva of healthy animals are nonhemolytic and nonpiliated. It is not clear exactly what is responsible for emergence of the virulent strains. Moraxella bovis is transmitted from animal to animal by mechanical vectors such as flies, by direct contact, and by fomites. In natural outbreaks, face flies (Musca autumnalis) appear to be the most important vectors. The concurrent presence of other infectious agents, such as Mycoplasma or infectious rhinotracheitis virus, often increases the severity of the disease.
The initial lesions are shallow corneal ulcers, which are probably the direct result of epithelial cytotoxin produced by adherent Moraxella. The organisms then gain access to the stroma, which results in attraction of large numbers of neutrophils from the limbus and from conjunctival blood vessels via the tear film. Inevitably, there is bystander injury from the degranulating neutrophils, but Moraxella also produces a potent cytotoxin, which accelerates neutrophil destruction. Lesions are initially unilateral but eventually become bilateral. Progression of a lesion is rapid during the initial 48 to 72 hours after infection and usually results in a shallow painful corneal ulcer and foci of suppurative superficial stromal keratitis that gradually heal over the next few weeks. There is usually severe conjunctival hyperemia, followed by circumferential superficial vascular ingrowth from bulbar conjunctiva toward the central ulcers (Fig. 20-136). Healing involves the usual events of fibrovascular ingrowth from the limbus, resulting in permanent central corneal scarring, which is usually not functionally significant. In severe cases, the ulcer may progress to descemetocele, iris prolapse, and destruction of the globe.
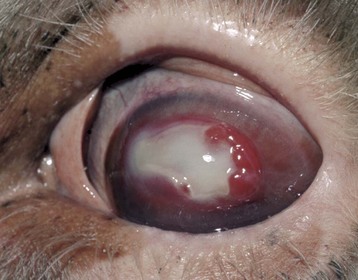
Fig. 20-136 Infectious bovine keratoconjunctivitis (“pink-eye”), cow.
The axial half of the ulcerated cornea is filled with neutrophils (suppurative keratomalacia) and surrounded by a border of red fleshy granulation tissue. The initial lesions are shallow corneal ulcers and foci of suppurative superficial stromal keratitis with conjunctival hyperemia, followed by circumferential superficial vascular ingrowth from bulbar conjunctiva toward the central ulcers. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Similar clinical and histologic entities exist in sheep, goats, and pigs associated with corneal colonization by Chlamydia spp. and Mycoplasma spp.
Bovine Malignant Catarrhal Fever–Associated Uveitis
The majority of cattle with malignant catarrhal fever have prominent ocular lesions in the form of peripheral midstromal corneal vascularization and obvious anterior uveitis. These lesions help to clinically distinguish this disease from mucosal disease and acute severe BVD. The histologic lesions within the eye resemble those elsewhere in the body: arterial necrosis with perivascular and intramural accumulation of lymphoblasts, among which are a few with mitotic figures. The vasculitis is most often identified within the iris, but it can be found anywhere within the uveal tract or retina. The accompanying uveitis is predominantly lymphocytic right from the start. Of great diagnostic significance from a clinical perspective is a ring of peripheral corneal stromal vascularization extending into the cornea from the limbus. The vascular ingrowth is accompanied by notable corneal edema. Although this is a routine lesion seen in any chronic severe uveitis, it is not seen in keratoconjunctivitis associated with several other bovine infectious diseases. There may be a concurrent lymphocytic corneal endothelialitis contributing to the corneal edema.
The pathogenesis of the disease remains controversial. The appearance of the vasculitis suggests that it should be an immune-mediated vasculitis, yet intramural deposition of immunoglobulin or complement is not a significant feature of the vascular lesion. A T lymphocyte–dependent, type IV immune pathogenesis has been suggested.
Squamous Cell Carcinoma
See the discussion on neoplasms of the conjunctiva in the section on Disorders of Domestic Animals.
Disorders Of Dogs
Canine Pannus Keratitis (Chronic Superficial Keratitis, Uberreiter’s Syndrome)
Pannus is a clinically and histologically distinctive superficial stromal keratitis seen primarily but not exclusively in German shepherd dogs. The disease usually begins at the lateral limbus as red conjunctival thickening. The lesion spreads toward the axial cornea as a superficial, fleshy, vascularized stromal infiltrate, involving both eyes (Fig. 20-137). Older lesions become intensely pigmented, and eventually the entire superficial stroma may be vascularized, scarred, and pigmented. The frequency and the severity of the disease increase proportionately as altitude increases, suggesting that sunlight exposure is important either in the initiation or the progression of the disease.
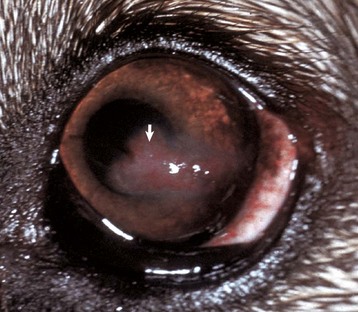
Fig. 20-137 Pannus keratitis, cornea, German shepherd dog.
Note the fleshy gray-pink superficial stromal ingrowth (arrow) from the lateral limbus (right) into the anterior stroma of the cornea. This appearance is classic for pannus. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The histologic changes are almost identical to those seen in cutaneous discoid lupus, another disease with a predilection for German shepherds and related breeds. Discoid lupus is also exacerbated by sunlight. The early lesion is characterized by infiltration of plasma cells and smaller numbers of lymphocytes into the basilar half of the epithelium and the adjacent superficial corneal stroma near the limbus. There is vacuolation and then patchy loss of basal cells, followed by the dispersal of pigment from the injured epithelial cells. This is phagocytosed by macrophages, which accumulate within the superficial stroma. As the disease progresses, cellular infiltration is accompanied by corneal vascularization and fibrosis.
The disease is successfully treated (but never cured) by immunosuppressive therapy identical to that used to treat cutaneous discoid lupus. The inability to identify an infectious agent and the response to immunosuppressive therapy suggests that the disease has an immunologic pathogenesis, but the exact mechanism remains elusive.
Collie Eye Anomaly
See the discussion on choroidal hypoplasia in the section on Disorders of Domestic Animals.
Uveodermatologic Syndrome In Dogs (Vogt-Koyanagi-Harada Syndrome)
Despite the exotic-sounding name, uveodermatologic syndrome is relatively frequent in the most susceptible breeds (Akitas, Siberian huskies, and Samoyeds). The clinical syndrome of facial dermal depigmentation and severe bilateral uveitis is distinctive, although many dogs with the uveitis do not have skin lesions. The canine syndrome closely parallels that of the human disease, except that the encephalitis, which is the least frequent part of the human syndrome, has not been confirmed in dogs. The histologic lesion is a destructive granulomatous endophthalmitis with abundant dispersal of melanin, a consequence of T lymphocyte–mediated destruction of melanin-producing cells of the RPE and urea. The disease is slowly progressive and is always eventually bilateral even though initially it may not affect both eyes.
Canine Progressive Retinal Atrophy
Progressive retinal atrophy is the traditional designation for a large group of inherited bilateral degenerative retinopathies of dogs. These have become widespread because of deliberate restrictions of genetic diversity imposed by current line-breeding practices in purebred dogs. Innumerable breeds are affected, and the list grows daily. They all have a similar clinical and microscopic appearance but that is where the uniformity ends. In reality, this group of diseases includes a genetically and biochemically diverse collection of photoreceptor dysplasias and degenerations that looked similar only when our methods of detection were limited to ophthalmoscopic evaluation and histopathologic examination. Its inclusion here as an acquired retinal disease rather than as a developmental disease is in deference to its clinical presentation and because at least some of this group are indeed true degenerations.
The stereotypic features seen by light microscopy are loss of photoreceptor outer segments followed by progressive loss of inner segments and eventually loss of neurons from the outer nuclear layer. The result is collapse and thinning of the retina. Other portions of the retina remain normal, and there is no inflammation.
Currently, these bilateral noninflammatory photoreceptor dysfunctions are divided into developmental photoreceptor dysplasias and later-onset, purely degenerative diseases. The classification of these various syndromes is under continuous review because in some cases what was once thought to be a degenerative disease affecting previously normal photoreceptors has been discovered to be an inborn biochemical error with a delayed phenotypic manifestation. The list of breeds in which photoreceptor dysplasia or degeneration has been described grows daily, so this brief discussion is not intended to be inclusive.
Photoreceptor Dysplasias
The majority of the photoreceptor dysplasias cause clinical signs of bilateral, progressive blindness in juvenile dogs. The fundamental defect varies by breed; the defect may specifically target rods or cones or affect both. Most examples have a simple, autosomal recessive inheritance pattern. The most thoroughly studied is the combined rod-cone dysplasia of Irish setter dogs. It is beyond the scope of this text to discuss all of the various photoreceptor dysplasias, so this one example serves to illustrate the general model.
Rod-cone dysplasia of Irish setters is inherited as a simple autosomal recessive trait. Dogs homozygous for the defect have arrested differentiation and then deterioration of the rod external segments, with cones being much less affected. The defect is detected ultrastructurally as early as 16 days after birth, at which time the outer rod segments adjacent to the retinal pigment epithelium should be developing. There is essentially diffuse loss of all rod photoreceptors by 12 weeks of age. This is followed by loss of cones and cells of the outer nuclear layer. The central retina is affected earlier and more severely than is the peripheral retina. Biochemical abnormalities are detected as early as 1 week of age. There is a severe deficiency of the activity of the phosphodiesterase responsible for the continuous hydrolysis of cyclic guanine monophosphate (cGMP) within outer segments. This results in a tenfold excess of cGMP, which has been shown to be toxic to photoreceptors in vitro, and also inhibits photoreceptor development. The basic defect is defective messenger RNA in the gene encoding the outer segment-specific phosphodiesterase.
Photoreceptor Degenerations
Photoreceptor degeneration refers to an equally diverse group of diseases in which early development is (as far as we can currently detect) normal, and degeneration occurs later in life. Prototype for this group is a rod-cone degeneration in toy and miniature poodles (with virtually identical diseases seen in English and American cocker spaniels, Labrador retrievers, and Portuguese water dogs). This disease is classified as a true degeneration in that photoreceptor differentiation seems to be normal until 6 to 9 weeks of age. After this time and progressing at an unpredictable rate, photoreceptor outer segments degenerate. Rods are affected earlier and with greater severity than are cones. The disease progresses at an unpredictable rate; affected dogs may not have clinical or ophthalmoscopic evidence of disease until 3 to 5 years of age (which in breeding dogs means that they will already have passed on the genetic defect). The macroscopic and histologic lesions begin centrally, around the optic disc, but eventually (by 6 to 7 years of age), the entire retina is affected and the dog is blind. The biochemical basis for the degeneration remains unknown.
Canine Lymphocytic Uveitis
Canine lymphocytic uveitis has the most frequent histologic pattern of uveitis seen in dogs (as it is in cats and horses), a lymphocytic-plasmacytic panuveitis that tends to be more severe in the anterior uvea than in the choroid. It should not really be considered a specific disease but rather a histologic pattern that is probably shared by many different diseases. As in the other species, the disease may be unilateral or bilateral and tends to occur in multiple episodes interrupted by periods of quiescence. The cause remains unknown, and it is likely that there are multiple different agents capable of inducing this identical histologic change. In contrast to the situation in cats, it does not cause glaucoma. The main differential diagnosis is phacolytic uveitis, and indeed in some cases the histologic distinction is impossible.
Canine Anterior Uveal Melanocytomas
See the discussion on neoplasms of the uvea in the section on Disorders of Domestic Animals.
Meibomian Adenomas
See the discussion on neoplasms of the eyelids in the section on Disorders of Domestic Animals.
Melanocytomas
See the discussion on neoplasms of the eyelids in the section on Disorders of Domestic Animals.
Limbal Melanocytomas
See the discussion on neoplasms of the conjunctiva in the section on Disorders of Domestic Animals.
Disorders Of Cats
Feline herpesvirus 1 (FHV-1) is an exceedingly common cause of acute-to-chronic keratitis in cats. Viral infection of the corneal epithelial cells results in shallow intraepithelial branching tracts of necrosis, known as dendritic ulcers. FHV-1 can also cause a more severe delayed, deeper stromal accumulation of lymphocytes and plasma cells that appears to be a syndrome separate from the dendritic ulcers. In cats with deeper stromal keratitis, there is concurrent nondendritic regional epithelial ulceration, necrosis of superficial stroma, and an intense accumulation of lymphocytes and plasma cells that presumably have migrated from the limbus. The histologic pattern is nonspecific, and a more definitive etiologic diagnosis requires the demonstration of viral antigen within the injured epithelium or in the deep stromal lesion. Most cases of herpetic stromal keratitis probably result from activation of latent infections precipitated by concurrent disease or the topical application of corticosteroids. The specific etiologic diagnosis can be problematic because viral antigen can be difficult to demonstrate in chronic lesions laden with a lot of neutralizing antibody. In addition, many cats are asymptomatic carriers and so the mere demonstration of the FHV-1 (by immunohistochemistry or PCR done on diseased corneas) is not sufficient to establish causation.
Eosinophilic Keratitis
Eosinophilic keratitis is a unique disease that occurs predominantly in cats but has been seen occasionally in horses. The macroscopic appearance is characteristic: a granular white proliferative lesion extending inwardly along what seems to be the corneal surface from the medial or lateral limbus. Many cases have similar lesions in the adjacent conjunctiva, and in a few cases the lesions are exclusively conjunctival. Most begin as a unilateral disease, but soon both eyes are involved. The histologic lesion is a nonulcerative superficial stromal infiltration of a mixture of eosinophils, plasma cells, mast cells, and macrophages (Fig. 20-138). The percentage of each cell type within the lesion varies with the duration of the disease and is significantly influenced by therapy, but eosinophils are always present and are a requirement for the diagnosis. The granular appearance of the macroscopic lesion is created by the degranulation of eosinophils, which create a thick refractile eosinophilic coagulum along the surface of the lesion (see Fig. 20-138). The diagnosis is usually made on the basis of clinical appearance and the presence of eosinophils in a superficial corneal scraping. Even a few eosinophils in a superficial stromal keratitis with compatible clinical signs should be diagnosed as chronic eosinophilic keratitis, even though the majority of the leukocytes are plasma cells or other mononuclear leukocytes.
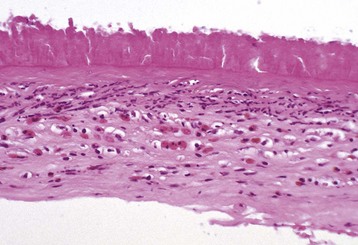
Fig. 20-138 Feline eosinophilic keratitis, cornea (keratectomy sample), cat.
The corneal epithelium and superficial stroma have been replaced by a coagulum of eosinophil granules. A mixture of leukocytes, predominantly eosinophils, has infiltrated the underlying stroma. The histologic changes are highly variable depending on the stage of the disease, but eosinophils are always present. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The cause and pathogenesis are unknown. Affected cats do not have concurrent skin lesions of eosinophilic granuloma complex or any other concurrent disease. Between 70% and 80% of lesions contain the DNA of FHV-1; the problem is that ocular herpesvirus infection is so common in cats that a definitive causal link is difficult to establish.
Feline Infectious Peritonitis–Associated Uveitis
The coronavirus of feline infectious peritonitis (FIP) causes a diffuse uveitis that is probably immune-mediated. The frequency of ocular lesions is unknown because the eyes are not regularly examined in cats with the disease. The histologic lesions in the globe are always bilateral and are similar to those seen in any other organ affected with the disease. As elsewhere, the lesions vary in the relative proportions of the different leukocyte types, the amount of fibrin, and the amount of necrosis. In most cases the disease is primarily an anterior uveitis with a mixture of lymphocytes, plasma cells, neutrophils, and macrophages. The often-repeated statement that FIP causes “pyogranulomatous” inflammation, especially within the globe, is greatly exaggerated. The exudate within the anterior chamber is usually predominantly neutrophilic, yet in the choroid the inflammation is usually lymphocytic-plasmacytic. Formation of keratic precipitates and a neutrophilic corneal endothelialitis are strong indicators of an FIP-associated uveitis (Fig. 20-139). In many cases, there are also typical histologic lesions in the orbital soft tissue adjacent to the sclera and in the meninges of the optic nerve. There is no other cause of uveitis in cats that is also routinely involved in orbital cellulitis. Classic necrotizing vasculitis is seen only rarely, and if present, it is likely to be in the retina. Sequelae such as pupillary block and secondary glaucoma are rarely seen, simply because cats with ocular lesions of FIP are usually in the advanced stages of the systemic disease and do not survive long enough for those potential sequelae to develop.
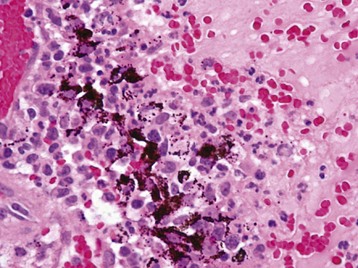
Fig. 20-139 Lymphocytic, granulomatous, and neutrophilic iridocyclitis (destructive), feline infectious peritonitis, ciliary body, cat.
Lymphocytes and plasma cells tend to predominate within the uveal stroma (left), whereas neutrophils, fibrin, and macrophages predominate within the adjacent aqueous humor of the posterior chamber (right). These lesions are typical of feline infectious peritonitis. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
Feline Idiopathic Lymphonodular Uveitis
Feline idiopathic lymphonodular uveitis is by far the most frequent histologic pattern of the uveitis in cats. It is important as a cause of the clinical signs of uveitis itself, but it is even more important as a very common cause of glaucoma in cats. The mechanism by which the uveitis causes the glaucoma is unknown; it is not by the usual mechanisms of posterior synechia or preiridal membrane formation. At the initial clinical presentation, the lesion may be unilateral, but in most cases the uveitis eventually becomes bilateral.
The microscopic lesions are essentially identical to those seen in equine recurrent uveitis: perivascular accumulations of lymphocytes and plasma cells throughout the anterior uvea, and with less regularity, within the choroid and around small vessels in the retina. The iris tends to have the greatest accumulation, and here the lymphocytic-plasmacytic perivascular aggregates may become so large as to be clinically visible (Fig. 20-140). The degree of iris thickening and pallor may be sufficient to make this lesion macroscopically indistinguishable from that of lymphoma (lymphosarcoma).
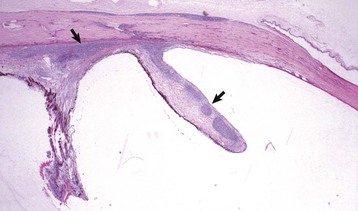
Fig. 20-140 Idiopathic lymphonodular uveitis, anterior uvea, cat.
The iris and ciliary body contain numerous coalescing perivascular lymphocytic-plasmacytic nodules (arrows). This lesion is the most common cause of glaucoma in cats (vying with diffuse iris melanoma for that distinction), but the mechanism by which it causes glaucoma is unknown. H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
The cause remains a complete mystery despite considerable investigation. The syndrome is presumed to be immune-mediated, but the identity of the antigen or antigens is unknown. Affected cats are no more likely to be seropositive or PCR-positive for the various feline systemic infectious agents (feline immunodeficiency virus [FIV], FIP, leukemia virus, etc.) than are healthy cats, but no credible cause has been demonstrated by immunohistochemical or PCR investigation of affected globes. Some believe strongly that a previous infection with Toxoplasma is somehow involved, but the evidence remains inconclusive.
Feline Diffuse Iris Melanoma
See the discussion on neoplasms of the uvea in the section on Disorders of Domestic Animals.
Feline Primary Ocular Sarcoma (Posttraumatic Sarcoma)
See the discussion on neoplasms of the uvea in the section on Disorders of Domestic Animals.