The Ear and Eye*
Ear
The ear is a specialized sense organ formed by a highly organized mixture of cutaneous, nasopharyngeal, osseous, and neurologic tissues. Within the ear are several air- and fluid-filled interfaces involved in the transduction of sound and pressure waves to action potentials that are conducted by the nervous system to the brain for interpretation and appropriate motor and cognitive responses.
In the context of interacting with animals, hearing is often considered of secondary or tertiary importance when compared to vision or olfaction. For many years, specific breeds predisposed to auditory dysfunction were maintained in colonies to facilitate investigation as animal models of human disease. More recently, throughout the world, deaf and hearing-impaired people are greatly benefiting from professionally trained hearing dogs. In addition, animal trainers, pet owners, and producers rely on a fully functioning auditory system to train, address, keep safe, or herd their animals. Therefore a much better understanding of conditions affecting this special sense is needed.
Many current textbooks focus on one species or one aspect of the ear. The focus of this chapter is to (1) clarify the anatomy of the ear by comparing and contrasting anatomic features of the domestic animal species, (2) address the responses to injury and the defense mechanisms protecting against injury, and (3) delineate various otic diseases that either affect many different species or are more unique to certain species.
Structure And Function
The external ear comprises the auricle (also known as pinna) and external acoustic meatus terminating medially at the tympanic membrane. Developmentally, the external ear arises from tissue elevations called auricular hillocks, three from the first branchial or pharyngeal arch and three from the second branchial or pharyngeal arch. The first pharyngeal groove or cleft between the two arches forms the external acoustic meatus. As they come into apposition early in development, the auricle and external acoustic meatus form. The auricle is a highly mobile and flexible, cartilaginous structure that is covered by haired skin with adnexa more dense on the convex than concave surface. The structural characteristics of auricles are closely tied to breed specifications. Among the species and breeds covered in this chapter, auricles can be erect, semi-erect, lop-eared, pendulous, microtic, or folded.
The auricle functions to collect, focus, and direct sound down the funnel-shaped external acoustic meatus to the tympanic membrane (Fig. 20-1). Because the rostroauricular and caudoauricular muscles are innervated by motor branches of the facial nerve, the flexible (cartilaginous) auricle is able to move rostrally, laterally, and caudally about a central auricular axis. The position of the auricle can signal an animal’s behavior or emotion. Fearful cats often flatten their ears in a defensive posture, whereas angry horses often completely flatten their ears caudally as an initial warning before they strike. Alternatively, a dog may flatten its ears when content or when it is being verbally scolded.
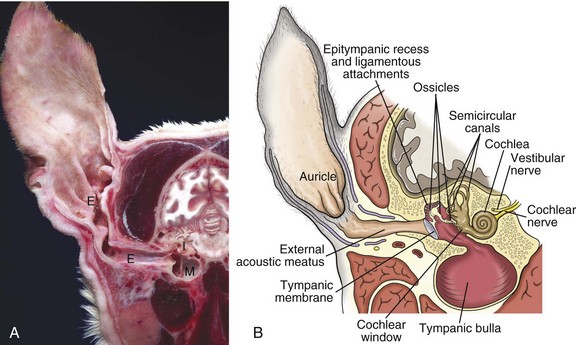
Fig. 20-1 Major regions of the ear.
A, Cross-section, head through the right ear, rostral surface, dog. External (E), middle (M), and inner (I) ear are illustrated. The tympanic membrane has been removed in this section. The external ear consists of the auricle and external acoustic meatus; the middle ear consists of the ossicles, tympanic cavity, and bulla; and the inner ear consists of the cochlea and semicircular canals. B, Schematic diagram depicting a cross-section through the external, middle, and inner ear of a dog. (A courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
The external acoustic meatus (ear canal) is a conical opening made up of elastic cartilage and bone (Fig. 20-2). The more lateral portions are composed of auricular cartilage that narrows and overlaps with annular cartilage. Dense fibrous connective tissue forms a bridge between the annular cartilage ring and the osseus portion of the external acoustic meatus. In cats and dogs, the osseous portion (1) is a very narrow rim of bone and (2) is a broad opening exposing the tympanic membrane that is readily visible during otic examination. In horses, ruminants, and pigs, the osseous portion of the external acoustic meatus is an elongate cylinder of bone with a narrow lumen (Fig. 20-3). In horses, the junction between the cartilaginous and osseous portions of the external acoustic meatus is grossly identified by an abrupt change from pigmented to nonpigmented epithelium. Visualization of the deeper portions of the external acoustic meatus in livestock species requires specialized equipment and heavy sedation.
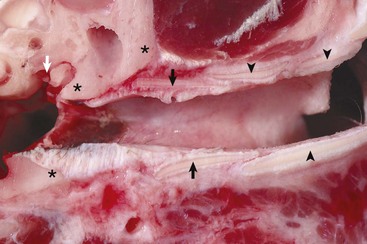
Fig. 20-2 External acoustic meatus, osseous and cartilaginous portions, cross-section through the left ear, dog.
Annular (black arrows) and auricular cartilage (black arrowheads) form the structure of the cartilaginous portion of the external acoustic meatus. Dense, white, fibrous connective tissue attaches the annular cartilage to the osseous rim of the external acoustic meatus (asterisks). The incudostapedius joint is visible in this image (white arrow). The tympanic membrane is removed in this section. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
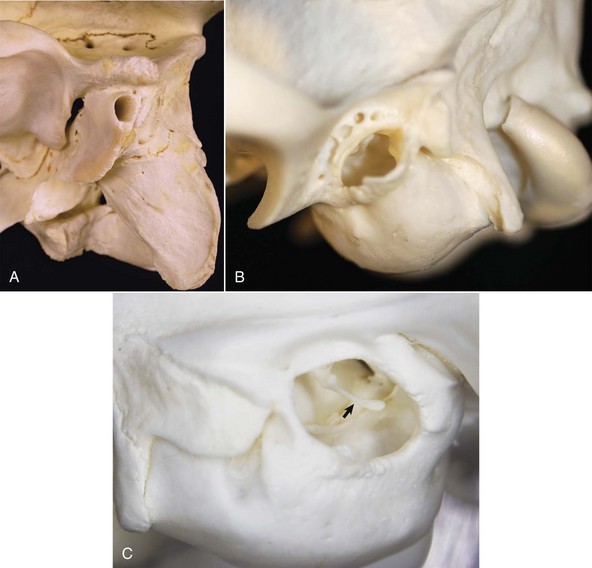
Fig. 20-3 Osseous external acoustic meatus, macerated specimens.
A, Ox. The bony external acoustic meatus in most livestock is much longer and narrower when compared to dogs and cats. Visualization of the middle ear is obscured by the elongate osseous external acoustic meatus. B, Dog. The osseous portion of the external acoustic meatus is a thin rim of bone allowing easy visualization of the middle ear. C, Cat. The osseous portion of the external acoustic meatus is very thin and the opening is very large, allowing easy visualization of the middle ear and malleus (arrow). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Although there are wide species variations, the cartilaginous and osseous portions of the external acoustic meatus are lined by a thin epidermis formed by stratified squamous epithelium and a thin dermis that contains a relatively uniform allotment of sebaceous glands, fewer hair follicles, and greater ceruminous glands when comparing medial to lateral portions (Fig. 20-4). Sebaceous glands are composed of 6 to 10 club-shaped acini surrounded by thin fibrous tissue with ducts opening into associated hair follicles. Relative to ceruminous glands, they tend to be located in the more superficial dermis. Ceruminous glands are simple, coiled, tubular glands that resemble apocrine sweat glands. Their ducts open either into hair follicles or directly to the epidermal surface.
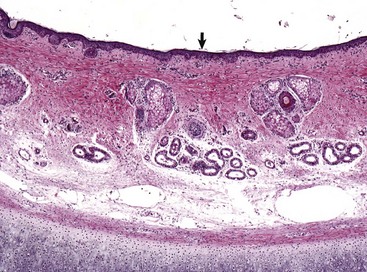
Fig. 20-4 External acoustic meatus epithelium and adnexa, pig.
Stratified squamous epithelium (arrow) lines the external acoustic meatus. Adnexa present are mixtures of sebaceous glands, most often flanking hair follicles, and deeper eccrine glands referred to as ceruminous glands. Auricular cartilage is present along the lower edge of the image. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Although motor innervation of muscles of the external acoustic meatus is provided by the facial cranial nerve, sensory innervation is more complex. Branches of the trigeminal, facial, and vagal cranial nerves and branches of the second cervical spinal nerve innervate the skin. Sensory innervation to the mucosa of the external acoustic meatus is provided by the mandibular branch of trigeminal and auriculotemporal cranial nerves. The primary blood supply to the ear is through the caudal auricular artery, which branches into the lateral, intermediate, deep, and medial auricular arteries. The caudal auricular artery is a main branch of the external carotid artery.
Middle Ear
Tympanic Membrane (Tympanum): The tympanic membrane, also known as the tympanum, is an extremely thin, three-layered, semitransparent membrane peripherally suspended from the tympanic ring by a fibrocartilaginous ring. The tympanic membrane is formed when the endoderm of the first pharyngeal pouch comes into close contact with the ectoderm of the first pharyngeal cleft or groove. Most of the tympanic membrane is held under tension so it can be deformed by and responsive to sound waves (Fig. 20-5). It covers the medial extremity of external acoustic meatus, demarcating the junction between the external ear and middle ear. Both surfaces of the tympanic membrane have an air interface.
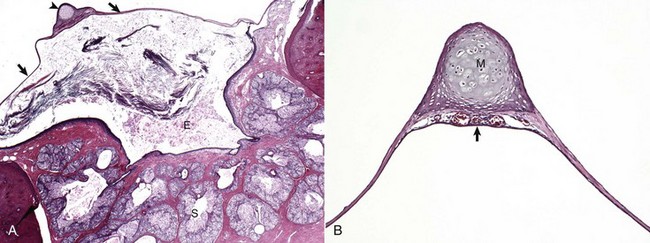
Fig. 20-5 Tympanic membrane.
A, Cat. Cross-section through the tympanic membrane (arrows), manubrium of the malleus (arrowhead), and external acoustic meatus (E). An intact tympanic membrane at the level of the pars tensa is very thin. It is lined by a single layer of cornified squamous epithelial cells externally, and low cuboidal to noncornified squamous epithelium line the inner surface. The abundant keratin within the external acoustic meatus is not uncommon. The prominent, multiple, branching sebaceous glands (S) are common in the deepest portions of the external acoustic meatus. H&E stain. B, Higher magnification of the tympanic membrane and malleus, pig. The manubrium (M) of the malleus is embedded in the tympanic membrane. Numerous blood vessels are present beneath the manubrium (arrow) and are located in the middle layer of the tympanic membrane beneath the external concave surface, which corresponds with the region of germinative epithelium. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
In most species, the tympanum is an oval-to-round structure, whereas in ruminants, it is shaped more like a broad triangle (Fig. 20-6). Embedded in the tympanum is the manubrium of the malleus. The placement of the manubrium in the tympanic membrane is highly variable between species. It is more centrally located in horses versus a more rostromedial location in ruminants and pigs.
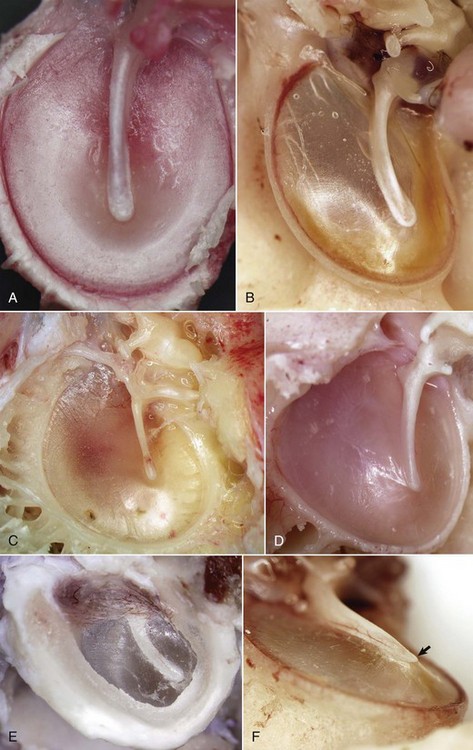
Fig. 20-6 Tympanic membrane.
A, Horse. In the horse, the tympanic membrane (tympanum) is more round than other species. The manubrium of the malleus forms a very shallow arc and is centrally located in the tympanum. B, Dog. The tympanum is oval to comma-shaped in dogs, and the manubrium of the malleus is C-shaped. C, Pig. The shape of the tympanic membrane is similar to that of the dog, but the manubrium of the malleus is shorter and straighter. D, Goat. Ruminants tend to have a more triangular-shaped tympanic membrane. In both pigs and ruminants, the manubrium of the malleus is more rostral and medial than in other species. E, Dog. Lateral view of tympanic membrane. The transparent portion of the tympanic membrane held under tension and associated with the manubrium of the malleus is the pars tensa. The highlights depict the radial striations of the normal pars tensa. Dorsally, the tympanic membrane is thicker, highly vascular and is under much less tension, and is designated as the pars flaccida. F, Dog. The tympanic membrane is convex on its medial surface in the tympanic cavity. The ventromedial extremity of the manubrium is called the umbo (arrow). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
The tympanum is divided into two sections, the pars tensa and the pars flaccida. The majority of the tympanic membrane is made up of the pars tensa, which is a very thin, translucent, and taut membrane that bulges convexly into the tympanic cavity (Fig. 20-6, E). The pars tensa is made up of three layers: (1) outer layer of keratinizing squamous epithelium derived from ectoderm of the first pharyngeal groove; (2) middle layer of thin, variably vascularized fibrous connective tissue originating from the pharyngeal wall; and (3) inner layer of very low cuboidal to nonkeratinizing squamous epithelium, which is of pharyngeal pouch origin.
The dorsal most portion of the tympanic membrane is the pars flaccida, which is roughly triangular, thicker, more vascular, and flaccid when compared to the pars tensa (see Fig. 20-6, E). Overlain by keratinized epithelium, the underlying stroma of the pars flaccida in dogs is made of loosely arranged collagen, rare mast cells, and few elastin fibers. This latter feature is in direct contrast to humans in which there are abundant elastin fibers. Visualized from the external acoustic meatus, this portion of the tympanic membrane may bulge into or away from the middle ear.
The tympanic membrane is placed roughly at a 45-degree angle relative to the central axis of horizontal portion of the external acoustic meatus (Fig. 20-7, A). However, commonly, the actual placement of the tympanic membrane is more variable with the external, concave surface of the tympanic membrane angled more rostrally (Fig. 20-7, B and C). Cats have a similar orientation to their tympanum (Fig. 20-8). Interestingly, the surface area of the tympanic membrane from an approximately 550-kg horse is larger than the tympanic membrane of a Maltese dog but is approximately 15% smaller than the surface area of the tympanic membrane of a German shepherd dog. Additionally, the tympanum of the fetal goat is approximately 20% larger than that of large dog breeds.
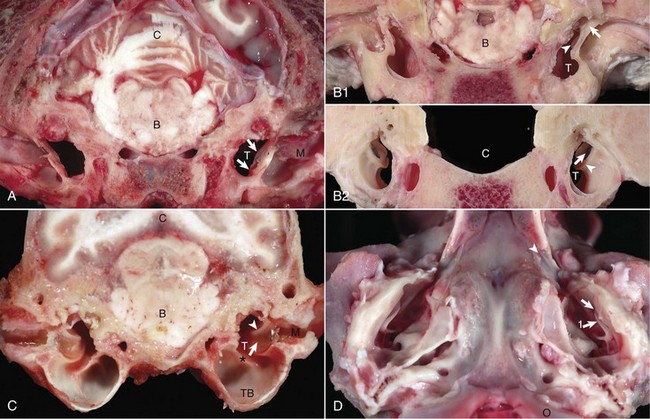
Fig. 20-7 External acoustic meatus, tympanic membrane, tympanic cavity. Compare unlabeled contralateral side with labeled side for more structural detail.
A, Transverse section, rostral surface, dog. The tympanic membrane (arrows) extends medially towards the tympanic cavity (T) at an approximate 45-degree angle from dorsal to ventral, in relation to the central axis of the horizontal part of the external acoustic meatus (M). Portions of the rostral edge of the tympanic ring have been inadvertently removed during sample preparation. Brainstem (B); cerebellum (C). B, Transverse section, rostral (1) and caudal (2) surfaces, goat. The tympanic cavity (T) has been opened bilaterally. In the cranial view (1), the tympanic membrane is not visualized because it is hidden by the tympanic ring (arrows), which surround the membrane. The manubrium of the malleus (arrowheads) is minimally visible. However, from the caudal view (2), the tympanic membrane (arrows) is clearly visible and is positioned so that the concave or external surface is tilted rostrally. Brainstem (B); calvarium where brainstem would be positioned (C); tympanic ring (arrowheads). C, Transverse section, caudal surface, dog. The external or concave surface of the tympanic membrane (arrow) is angled almost fully rostral rather than lateral. Note that the septum bullae (asterisk) are short and incomplete in the dog when compared with a cat (see Fig. 20-10). Brainstem (B); Cerebellum (C); Tympanic cavity (T); Manubrium of the malleus (arrowheads); External acoustic meatus (M), Tympanic bullae (TB). D, Ventral-dorsal view, opened bullae. Bilaterally, the concave surface of the tympanic membranes (arrow) are tilted rostrally. Occipital condyles (O) appear at the bottom of the image. Extending rostrally and medially from the tympanic cavity are the auditory tubes (arrowhead), which provide direct communication between the tympanic cavity and nasopharynx. Manubrium of the malleus (arrow 1). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
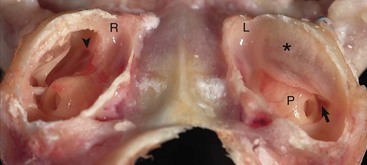
Fig. 20-8 Tympanic bullae, cat.
Caudoventral section, with both tympanic bullae opened ventrally. The septum bulla (asterisk) is intact in the left bulla (L) and opened ventrally in the right bulla (R). From rostral to caudal, the septum bulla dorsally abuts the petrous portion of the temporal bone. At its caudal extreme is an opening that allows communication between the two cavities (arrow). The auditory tube opening into the tympanic cavity is observed in the dorsal, rostral extremity of the right epitympanic cavity (arrowhead). The large bulge rostral to the round window corresponds to the start of the cochlea and is called the promontory (P). In both specimens the tympanic membrane is tilted rostrally. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Tympanic Cavity: The tympanic cavity is an air-filled compartment surrounded by bone that is separated from the external ear by a thin tympanic membrane (tympanum) and is in direct communication with the pharynx via the auditory tube (also known as the eustachian or pharyngotympanic tube). Both the tympanic cavity and the auditory tube are derived from the endoderm of the first pharyngeal pouch. The epitympanic recess is the dorsal extremity of the tympanic cavity within which lies the head of the malleus and short curs of the incus. Ligaments stabilize and anchor the incudomallearis joint and the short crus of the incus within this recess (Fig. 20-9).
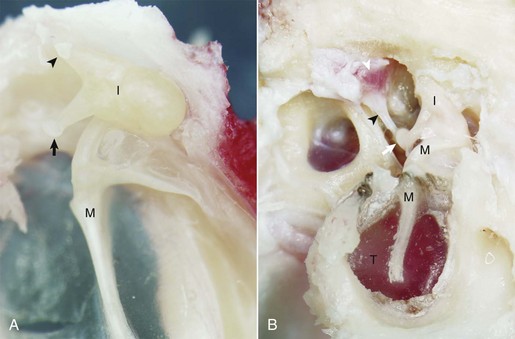
Fig. 20-9 Malleus, incus, incudomallearis joint, epitympanic recess.
A, Cat. Medial view. Seated in the epitympanic recess is the rounded head of the malleus (M) and the incus (I); together they are articulated to form the incudomallearis joint. The smaller crus of the incus (arrowhead) and the head of the malleus are anchored to the epitympanic recess by ligaments (see Fig. 20-1). At the end of the longer crus of the incus is the lenticular process (arrow). B, Giraffe. Lateral view, right ear. The tympanic membrane is intact (T). The small crus of the incus (I) along with the head of the malleus (M) are firmly anchored in the epitympanic recess by ligamentous attachments. The lenticular process of the long crus articulates with the head of the stapes seated in the oval window to form the incudostapedius joint (white arrow). The facial nerve has been removed in order to expose the stapedius muscle (white arrowhead), which is firmly attached to the stapes via its tendon (black arrowhead). (Courtesy Dr. B.L. Njaa, The Center for Veterinary Health Sciences, Oklahoma State University.)
In many species, there is a bulbous, ventral portion of the tympanic cavity called the tympanic bulla (see Fig. 20-3, B and C). Within the bulla of the dog and cat is a bony septum referred to as the septum bulla. In the cat, the septum bulla abuts the petrous portion of the temporal bone and separates the tympanic cavity into two compartments: the dorsolateral epitympanic cavity and the ventromedial tympanic cavity (see Fig. 20-8). This separation is incomplete, which allows communication between the two compartments through a narrow opening between the septum bulla and petrous portion of the temporal bone and a larger opening at its caudal edge. In the dog, this septum is a much smaller, incomplete bony ridge that only makes contact with the petrous portion of the temporal bone rostrally and often has tiny, elongate bony spicules with bulbous ends (see Fig. 20-7, B). The mucosal surfaces of the tympanic bullae of cats and dogs are lined by an epithelium that varies, depending on location. Dorsally, close to the auditory tube opening, the mucosa is composed mostly of ciliated columnar cells mixed with goblet cells and basal cells similar to the cells in the nasopharyngeal mucosae (Fig. 20-10 and see Fig. 20-20). Ventrally, the number of ciliated cells and goblet cells decreases and the number of cuboidal, less differentiated cells increases. The surfaces of the petrous portion of the temporal bone, auditory ossicles, and tympanic membrane are lined by cuboidal to noncornifying squamous epithelium. In ruminants, camelids, and pigs, the ventral portion of the tympanic cavity or bulla is made of more numerous bony compartments lined by noncornifying squamous epithelium (Fig. 20-11). In cattle and pigs, these compartments are air-filled with direct communication with the tympanic cavity. In camelids and small ruminants, the tympanic cavity does not appear to directly communicate with the bullae. Horses do not have readily identifiable tympanic bullae.
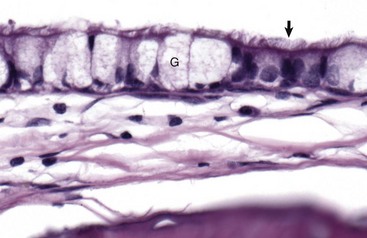
Fig. 20-10 Tympanic cavity mucosa, cat.
The mucosa of the tympanic cavity is not uniform. In cats and dogs, the mucosal epithelium in the more dorsal portions of the bullae morphologically mirrors mucosae of the nasopharynx. Included are ciliated columnar epithelial cells (arrow) and goblet cells (G) mixed with fewer nonciliated columnar epithelial cells. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
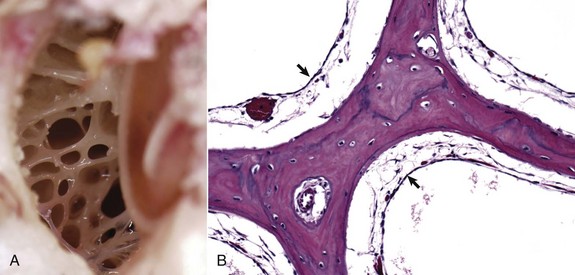
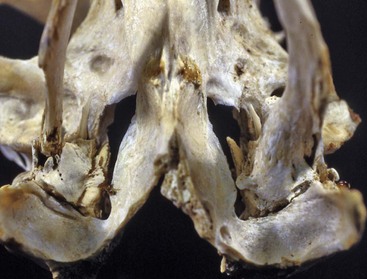
Fig. 20-11 Tympanic cavity and bulla.
A, Dorsal view into the middle ear, ox. Ruminants and pigs have small tympanic cavities but much larger tympanic bullae. The bullae are made of multiple, arborizing air-filled channels with numerous bony septa as shown here. B, Histologic section of tympanic bulla, pig. Bony septa are lined by low cuboidal to nonkeratinizing squamous epithelial cells (arrows). H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Auditory Ossicles: A chain of three bones or auditory ossicles forms the mechanical transduction system of hearing: the malleus, incus, and stapes (Figs. 20-12 and 20-13). The malleus and the incus, as well as the tensor tympani, are derived from the mesenchyme of the first branchial or pharyngeal arch. The stapes and the stapedius muscle originate from the mesenchyme of the second branchial or pharyngeal arch.
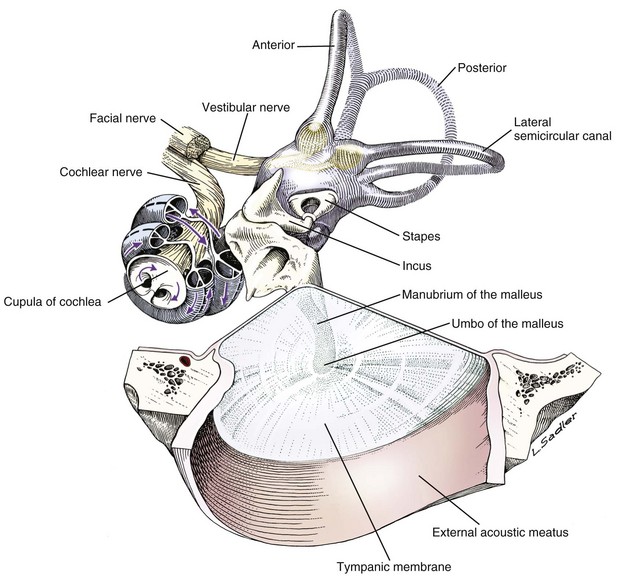
Fig. 20-12 Schematic diagram of the middle and inner ear, dog.
Tympanic membrane, auditory ossicles, and membranous labyrinth. The bony labyrinth has been removed to demonstrate the orientation of the cochlea and semicircular canals relative to the auditory ossicles and tympanic membrane. The facial nerve and vestibulocochlear nerve enter the ear together through the internal acoustic meatus.
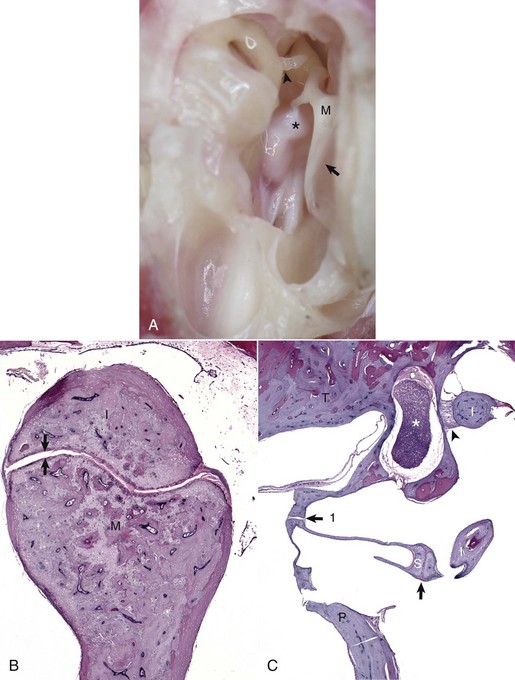
Fig. 20-13 Auditory ossicles and ossicular joints, cat.
A, Tympanic cavity, tympanic membrane, auditory ossicles, petrous portion of the temporal bone, auditory muscles, ventral view. The manubrium of the malleus is embedded in the tympanic membrane. The head of the malleus and incus are anchored in the epitympanic recess and form the incudomallearis joint. The long crus of the incus is shown articulating with the stapes to form the incudostapedius joint (arrowhead). Attached to the muscular process of the malleus (M) is the tensor tympani muscle (asterisk). Tympanic membrane (arrow). B, Histologic section of the incudomallearis joint (between the arrows), normal. The articulation of the malleus (M) and incus (I) is shown in the epitympanic recess. Similar to the petrous portion of the temporal bone, these ossicular bones lack a medulla. C, Histologic section of the incudostapedius joint. Positioned in the oval window is the stapes (S), held in place by a syndesmosis (arrow 1). The more ventral edge of the stapes has artifactually fractured. Articulating with the head of the stapes is the lenticular process of the long crus of the incus (I) to form the incudostapedius joint (arrow). The lenticular process of the incus is to the right of the incudostapedius joint. A portion of the short crus is positioned in the epitympanic recess and anchored by a ligamentous attachment (arrowhead). The facial nerve is present coursing through the facial groove (asterisk). Note the lack of complete bony encasement allowing communication with the tympanic cavity. Promontory (P), petrous portion of the temporal bone (T). H&E stain. (A and B courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Malleus: The largest of the ossicles is the malleus. The manubrium of the malleus is embedded in the tympanic membrane (see Fig. 20-5). The most ventromedial convexity of the malleus is the “umbo” (see Figs. 20-6, F, and 20-12). The muscular process of the manubrium near the neck of the malleus is the attachment site of a thin tendinous portion of the tensor tympani muscle. Various ligaments stabilize the malleus in the epitympanic cavity by anchoring the long, thin rostral process, the neck, and the head of the malleus. The head of the malleus articulates with the articular surface of the body of the incus forming the incudomallearis joint (see Figs. 20-9, 20-12, and 20-13). In the horse and cow and in aged dogs and cats, the incudomallearis joint capsule is a narrow but thick ligament that makes disarticulation difficult and gives the external appearance of a falsely fused joint. In younger dogs and cats, the incudomallearis ligament is not nearly as tenacious and disarticulation is much less difficult.
Incus: The incus is a bicuspid-shaped bone that lies caudal and dorsal to the malleus. It has two crura, one designated as the short crus, which is anchored in the epitympanic recess along with the body of the incus by a band of narrow connective tissue, and the other designated as the long crus, which transmits vibrations to the stapes. The lenticular process is at the end of the long crus (see Fig. 20-13), and in young animals, the lenticular process is a separate bone. In older animals, it fuses with the distal end of the long crus of the incus and articulates with the head of the stapes. Regardless of age, the incudostapedius joint capsular ligament is more translucent than the incudomallearis joint capsule and there is inherently more joint laxity.
Stapes: The stapes, named for its close resemblance to stirrups of a saddle, is often considered the smallest bone in the body.* However, its size and shape is somewhat variable, depending on species (Fig. 20-14). Its base or footplate is convex and firmly seated in the oval or vestibular window of the petrous portion of the temporal bone and anchored by the annular ligament of the stapes. This arrangement forms a syndesmosis between the stapes base and cartilage of the oval window (Fig. 20-15). The stapedius muscle, fittingly referred to as the smallest muscle in the body, is attached to the muscular process of the shorter caudal crus close to the head of the stapes. Vibrations of the tympanic membrane are linearly transduced to stapes vibrations that lead to fluid waves of the perilymph of the internal ear.
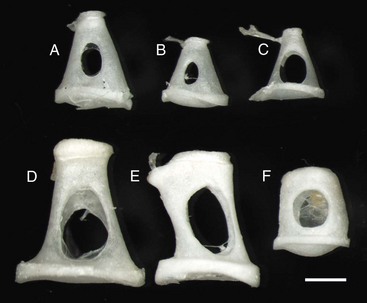
Fig. 20-14 Stapes, species variations.
Stapes are highly variable in size and shape, depending on the species. The first two stapes, beginning from the top row left are from different sized dogs. The larger stapes is from a 20 kg mixed breed dog (A). The smaller stapes is from a Maltese dog (B). The third stapes of the upper row is from a cat (C). The lower row depicts stapes from a horse (D), cow (E), and slaughter-age pig (F). In all cases, the lower plate of the stapes is convex. Also, in each case, the tendinous attachment of the stapedius muscle is affixed to the shorter crus or limb of the stapes. Scale bar = 1 mm. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
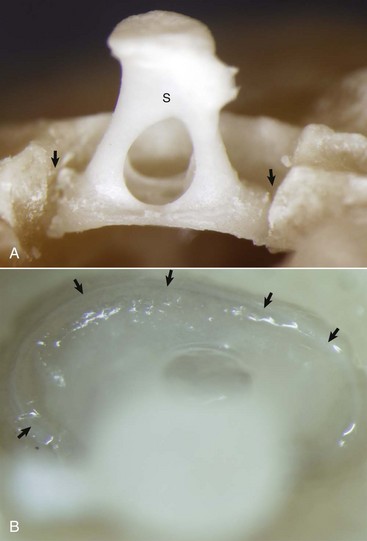
Fig. 20-15 Stapes in situ, horse.
A, Partially opened oval window. The stapes (S) is seated in the oval window connected to the petrous portion of the temporal bone by the annular ligament (arrows). B, Ventrolateral view of the stapes, in situ. A thin rim of annular cartilage is visible denoting the syndesmosis formed between the stapes and cartilage of the oval window of the petrous portion of the temporal bone (arrows). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Middle Ear Muscles And Nerves
The middle ear has two muscles associated with the auditory ossicles that help modulate auditory transduction and a third muscle that controls patency of the auditory tube. The tensor tympani muscle originates rostrally and medially from the bony recess in the petrous portion of the temporal bone and makes its tendinous insertion onto the muscular process of the neck of the malleus (Fig. 20-16; see Fig. 20-13, A). It receives its innervation via a motor branch of the trigeminal nerve. Contraction of the tensor tympani muscle pulls the tympanic membrane medially and rostrally, placing greater tension on the auditory ossicle chain, which results in an increased resonant frequency of the auditory sound conduction system.
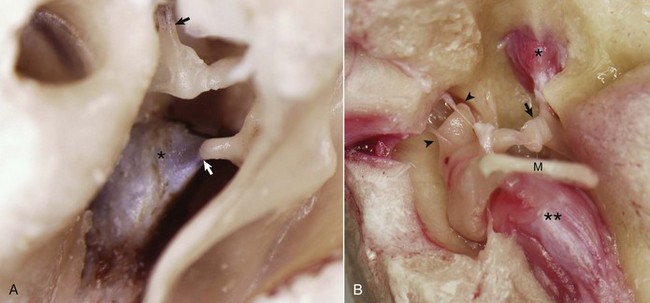
Fig. 20-16 Auditory ossicular muscles.
A, Middle ear, caudal view, ox. The external acoustic meatus is to the right on this image with the caudal edge of the tympanic membrane removed. The tensor tympani muscle (asterisk) is attached via its tendon to the muscular process of the malleus (arrow). The tendon of the stapedius muscle (arrowhead) is attached to the stapes bone near the head of the stapes. B, Middle ear, ventral view, horse. The bony external acoustic meatus, tympanic membrane, and associated connective tissue have been removed. The articulated incus and malleus form the incudomalleolar joint and are anchored in the epitympanic recess (arrowheads). The incudostapedius joint is opened (arrow). The facial nerve and some of the surrounding bone have been removed to expose the stapedius muscle (asterisk) attached to the stapes seated in the oval window. The muscular process of the malleus is obscured by the position of the manubrium (M) of the malleus, which is attached to the tensor tympani muscle (two asterisks). (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
The stapedius muscle originates in the stapedius muscular fossa located dorsomedial to and obscured by the facial nerve as it courses through the facial groove of the temporal bone (Fig. 20-17). The stapedial branch of the facial nerve innervates this muscle as it converges into a thin tendon that inserts onto the muscular process of the short crus of the incus close to the head of the stapes. In the cat, the displacement variance of the stapes in its vestibular window is approximately 0.2 µm, whereas the maximal contraction of the stapedius muscle leads to dorsal and caudal stapedial bone displacement of between 40 and 60 µm. This displacement, which is perpendicular to the normal movement of the stapes, maximally attenuates sound transmission up to 30 decibels. Contraction of the stapedius muscle is an integral part of what is termed the acoustic reflex, defined as consensual, reflexive contraction of the muscle in response to stimuli (frequently sound) that leads to attenuated acoustic transmission.
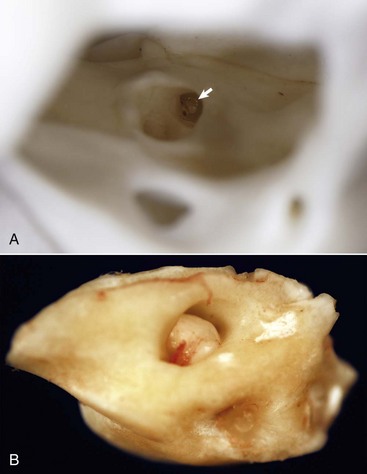
Fig. 20-17 Internal acoustic meatus, cat.
A, Right internal acoustic meatus. Viewed through the open left external acoustic meatus, the right internal acoustic meatus (arrow) represents the bony opening into the petrous portion of the temporal bone, through which the vestibulocochlear nerve and facial nerve exit the cranial cavity. B, Petrous portion of the temporal bone, cat. The yellow hue is very typical of this bone in all species. The large central opening is the internal acoustic meatus within which lie the vestibular and cochlear nerves. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
The tensor veli palatine muscle arises from a groove in the petrous portion of the temporal bone medial and ventral to the tensor tympani muscle. Along with its nerve, the tensor veli palatine nerve, a branch of the trigeminal nerve, this long, slender muscle extends rostrally from the tympanic cavity parallel to the auditory tube. In concert with the levator veli palatine muscle, which is innervated by the facial nerve, coordinated contraction of these muscles open the pharyngeal orifice of the auditory tube.
Two cranial nerves provide motor branches to the muscles of the middle ear. Branches of the trigeminal nerve named for their respective muscles innervate the tensor tympani and tensor veli palatine muscles within the tympanic cavity. The facial nerve initially leaves the cranial cavity through the internal acoustic meatus (see Fig. 20-17), along with the vestibulocochlear cranial nerve, and then courses through the facial groove of the petrous portion of the temporal bone in close proximity to the oval window (Fig. 20-18). Several millimeters medial and lateral to the tendon of the stapedius muscle, the bony casing of the facial groove is incomplete, which allows direct communication between the epineurial connective tissue of the facial nerve and the tympanic cavity (see Fig. 20-18). This proximity and exposure of the facial nerve to the tympanic cavity explains why middle ear disease can manifest as facial nerve dysfunction. The facial nerve emerges from the middle ear through the stylomastoid foramen immediately caudal to the external acoustic meatus.
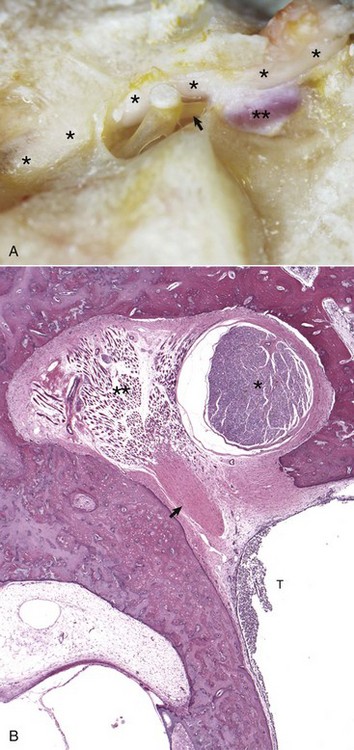
Fig. 20-18 Stapedius muscle and its proximity to the facial nerve.
A, Horse. The facial nerve (asterisks) courses through the facial groove in close proximity to the stapes and partially obscures the stapedius muscle (two asterisks). The tendon of the stapedius muscle (arrow) is shown attached to the short arm of the stapes, near the head of the stapes. B, Dog. Histology of facial nerve and stapedius muscle. The facial nerve (asterisk) is present within the facial groove partially obscuring the stapedius muscle (two asterisks) anchored in its stapedius muscular fossa. The tendon of the stapedius muscle (arrow) is observed in oblique, transverse section, but the stapes is not in this plane of section. Within the tympanic cavity (T) are moderate numbers of neutrophils, indicative of suppurative otitis media. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Auditory Tube (Eustachian Or Pharyngotympanic Tube)
In most mammalian species, the middle ear communicates with the pharynx through the auditory tube, which originates from the first pharyngeal pouch (Fig. 20-19). In the middle ear, the auditory tube opens into the most rostral and dorsal portion of the tympanic cavity called the epitympanic cavity. In the pharynx, the auditory tube originates from a narrow slit-like opening in the nasopharyngeal cavity and is lined by epithelium that is contiguous with the nasopharynx, namely ciliated, columnar pseudostratified epithelium mixed with goblet cells (Fig. 20-20). In many species, flanking the auditory tube are clusters of lymphocytes referred to as the tubal tonsil.
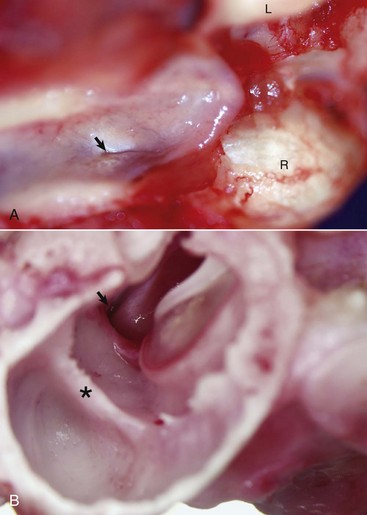
Fig. 20-19 Auditory tube.
A, Nasopharynx, cat. A thin slit-like opening, normally maintained in a closed position, represents the opening of the right auditory tube into the nasopharynx (arrow). L, Left occipital condyle; R, right occipital condyle. B, Middle ear, tympanic bulla, right ear, caudal, oblique view, dog. The auditory tube (arrow) is located dorsal, medial, and rostral to the tympanic ring of the tympanic membrane just to the left of the tympanic membrane and manubrium of the malleus. The septum bulla (asterisk) is short and incomplete when compared to the cat. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
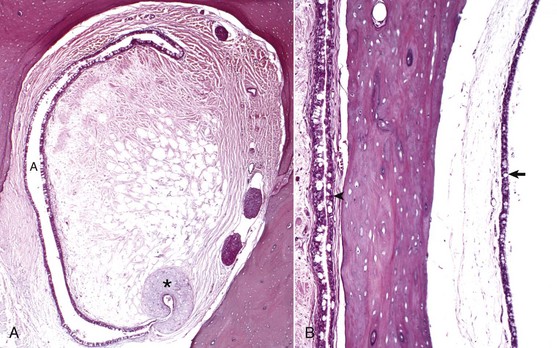
Fig. 20-20 Histology of the auditory tube mucosa, cat.
A, Rostral opening of the auditory tube. The auditory tube (A) in cross-section is typically C-shaped. Comma-shaped cartilage (asterisk) provides structural support to portions of the auditory tube. The mucosal lining is composed of ciliated, pseudostratified, columnar epithelium mixed with goblet cells and non-ciliated epithelial cells. H&E stain. B, Cross-section through tympanic bulla and auditory tube. The mucosae of the tympanic bulla (arrow) and the lining of the auditory tube (arrowhead) are composed of pseudostratified, ciliated columnar epithelial cells mixed with goblet cells and basal cells. This mucosa is virtually identical to the nasopharyngeal mucosa. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Infectious organisms can migrate via the auditory tube between the nasopharynx and the middle ear, thus serving as a portal of entry for each area. Additionally, the auditory tube is an important route for clearance of an infectious organism from the middle ear via the nasopharynx to the alimentary system.
Unique to the horse and other Equidae, guttural pouches (see Chapters 9 and 17) are enlarged diverticula of the auditory tubes that extend further rostrally, medially, and ventrally when compared to auditory tubes of other mammalian species. Although the precise function of guttural pouches remains controversial, their proximity to internal carotid arteries and their ability to inflate during vigorous exercise makes the idea of an extracalvarial brain cooling apparatus a provocative hypothesis.
Internal Ear
The internal ear is confined to a single bone, the petrous portion of the temporal bone. In most mammalian species, it is a triangular, wedge-shaped bone that forms the dorsomedial margin of the tympanic cavity. Often referred to as the hardest bone of the body, it is frequently slightly more yellow than surrounding bone and lacks the cancellous bony arrangement or medullary cavities present in other portions of the temporal bone. The internal ear is derived from a focal area of ectoderm referred to as the otic placode. This eventually forms an otic vesicle and through interaction with surrounding embryonic tissues, differentiates into this highly specialized tissue.
The internal ear is essentially made up of several membranous compartments, collectively known as the membranous labyrinth, derived from ectoderm, that contain endolymph. The membranous labyrinthine compartments include the cochlea, sacculus, utricle, and each semicircular canal with associated ampullae (Fig. 20-21). Surrounding the membranous labyrinth is a protective bony shell, derived from mesoderm, known as the osseous labyrinth (i.e., petrous portion of the temporal bone). The membranous labyrinth is classically a rostrally coiled tube or cochlea with an intermediate compartment or vestibule and a caudal semicircular canal region. However, the shape of the petrous portion of the temporal bone is very different. The beginning of the cochlea is denoted by the prominent bulge in the petrous portion of the temporal bone known as the promontory. Caudally, portions of this bone give the appearance of the tubular membranous labyrinth that likely represent the semicircular canals. Within the membranous labyrinth are biologic mechanosensory hair cells (see later) responsible for hearing (the auditory compartment) and for assessing head position, acceleration, and balance (the vestibular compartment).
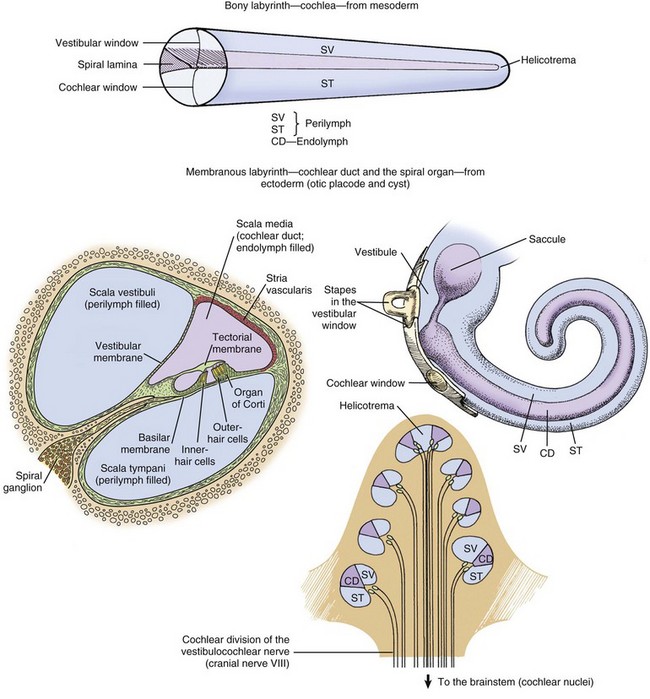
Fig. 20-21 Schematic diagram of the structure of the cochlea, cochlear duct, and spiral organ. (Modified from de Lahunta A, Glass E: Veterinary neuroanatomy and clinical neurology, ed 3, St Louis, 2009, W.B. Saunders.)
Cochlea: The cochlea is the most complex portion of the membranous labyrinth comprising two closed-end tubular structures that are highly coiled (see Fig. 20-21). The central core of bony labyrinth around which the cochlea spirals nearly three times is the modiolus. Sound waves vibrate the tympanic membrane and are converted by coordinated movements of the malleus, incus, and stapes to fluid waves within the perilymph by vibrations of the vestibular or oval window. Fluid waves travel through the scala vestibuli toward the cupula, reaching the helicotrema, and then returning within the scala tympani toward the round (also known as cochlear) window. Positioned between the scala vestibuli and the scala tympani is the second closed compartment known as the cochlear duct or scala media. The cochlear duct is separated from the scala vestibuli by the vestibular membrane (also known as Reissner’s membrane), which transmits fluid waves of perilymph in the scala vestibuli into fluid waves of endolymph in the cochlear duct. Within the cochlear duct is a ribbonlike strip of extracellular matrix called the tectorial membrane. It is composed of several genetically distinct types of collagen (collagen types II, IX, and XI) and three distinct noncollagenous glycoproteins (α-tectorin, β-tectorin, and otogelin). The tectorial membrane rests on and is affixed to tips of hair cells that make up the mechanosensory portion of the organ of Corti that lies on the basilar membrane (Fig. 20-22). There are a series of three rows of outer hair cells and a single row of inner hair cells (see Fig. 20-21). Fluid waves in the cochlear duct result in movement of the tectorial membrane, which distorts inner and outer hair cells (neural-like cells), resulting in their depolarization and afferent transmission of action potentials via the cochlear branch of the vestibulocochlear cranial nerve to the vestibular nucleus.
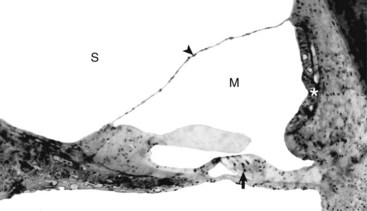
Fig. 20-22 Cochlear duct, cat.
The tectorial membrane is positioned over the inner and outer hair cells of the organ of Corti (arrow). The intact vestibular membrane (arrowhead) becomes distorted when auditory ossicular movements create fluid waves in the perilymph of the scala vestibuli (S). The stria vascularis (asterisk) is the source of endolymph production and maintenance. See Fig. 20-21 for a diagram of the structure. M, Scala media. Thick tissue section, toluidine blue stain. (Courtesy Dr. D.A. Ryugo; from Ryugo DA, Cahill HB, Rose LS, et al: Hear Res 181:73-84, 2003.)
Vestibular System: The vestibular system is made of several endolymph-filled compartments located in the caudal third to half of the petrous portion of the temporal bone. It represents a major sensory system that (1) maintains balance in concert with general proprioception and visual systems, (2) coordinates body posture, and (3) helps maintain ocular position in relation to the position or motion of the head. Included in the vestibular system are the semicircular canals, utricle, saccule, vestibular ganglia, vestibular portion of cranial nerve VIII (vestibulocochlear nerve), vestibular nuclei, and vestibular lobules of the cerebellum.
There are three semicircular canals oriented at right angles relative to each other occupying three planes. Each canal has a terminal dilation or ampulla that contains a specialized surface sensory organ called the crista. In aggregate, the sensory portion is referred to as crista ampullaris. Each crista is lined by specialized sensory hair cells that send continuous tonic neural signals to the vestibular nucleus. Deflection of these sensory hair cells during acceleration, deceleration, or rotation results in variation of the tonic signals sent to the vestibular nucleus. However, the hair cells are not activated during constant velocity.
Maculae are receptors located within the membranous utricle and saccule of the vestibule. The saccular macula is oriented in the vertical plane, whereas the macula of the utriculus is oriented in the horizontal plane. Surface lining neuroepithelial hair cells of the macula project into an otolithic membrane. Movement of the otolithic membrane causes deflection of the hair cells and triggers action potential. As in the case of the crista ampullaris, macular receptors provide a continuous tonic nervous input, with a net effect of maintaining static head positioning relative to gravity.
On stimulation of sensory nerve endings, action potentials are transmitted through bipolar cells whose cell bodies are located in the vestibular ganglia of the vestibular branch of the vestibulocochlear nerve. Signals travel to the vestibular nuclei in the medulla. From the vestibular nuclei, connections are made with the oculomotor, trochlear, and abducent nuclei of the rostral brainstem via the medial longitudinal fasciculus, the vestibulocerebellum by the caudal cerebellar peduncle, and the spinal cord via the vestibulospinal tract located in the ventral funiculus.
Histologic Evaluation of the Internal Ear: See Web Appendix 20-1.
Portals Of Entry
Portals of entry into the ear are listed in Box 20-1.
External Ear
Extension from the External Environment: Extension from the external environment is a common portal of entry into the external ear. It is a specialized invagination of the skin that terminates medially at the tympanic membrane of the middle ear. As the external acoustic meatus gradually narrows, its funnel shape is conducive in directing foreign materials, fomites, parasites, and/or infectious microorganisms into the external ear and toward the tympanic membrane of the middle ear. Additionally, its moist environment favors colonization of skin and/or mucosa by pathogenic microorganisms (Table 20-1). Although dermatitides can affect any part of the dermis, including the external ear, occasionally, involvement of the ear is an important feature used to arrive at a definitive diagnosis.
TABLE 20-1
Predisposing Factors and Primary Causes of Otitis Externa
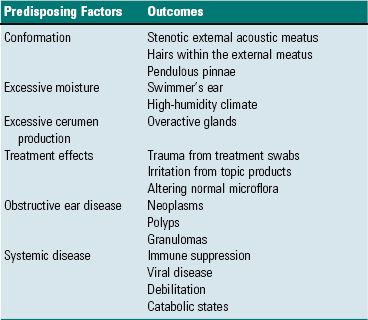
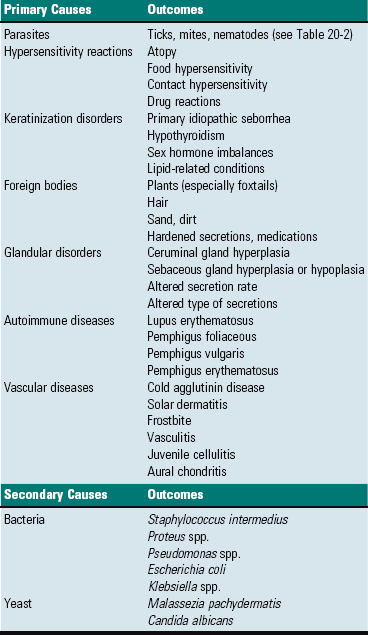
Modified from Griffin CE, Kwochka KW, MacDonald JM: Otitis externa and media. In Current veterinary dermatology: The art and science of therapy, St. Louis, 1993, Mosby; and Scott DW, Miller WH, Griffin CE: Muller & Kirk’s small animal dermatology, ed 6, Philadelphia, 2001, WB Saunders.
Hematogenous Spread: Hematogenous spread is a portal of entry into the external ear. Septicemias and/or specific types of viremias are thought to contribute to the development of external ear disease. Otitis externa and possibly deafness have been attributed to canine distemper virus, but it is unclear if the virus is a primary cause or one of several factors leading to otic disease. In an animal with septicemia, circulating bacteria have the potential to adhere to the endothelium of the capillary beds of the external ear, colonize the endothelium, and spread into adjacent tissues (see Chapter 4).
Extension from the Middle Ear: Extension from the middle ear is another portal of entry into the external ear, especially in cavalier King Charles spaniels with primary secretory otitis media. In primary secretory otitis media, the external ear is typically unaffected unless the tympanic membrane is ruptured and mucoid debris is spread into the external acoustic meatus. However, it has been suggested that this breed may have underlying failure of mucosae of the middle ear predisposing these spaniels to auditory tube dysfunction and thus otitis media leading to rupture of the tympanic membrane.
Middle Ear
Extension through Perforation of the Tympanic Membrane: Extension from the external ear through a perforated tympanic membrane is a portal of entry into the middle ear (Fig. 20-23). In dogs with chronic otitis externa, secondary otitis media may occur in as many as 80% of affected dogs. At the time of clinical diagnosis, the tympanic membrane is most often intact, although some studies report perforations in over 40% of cases with otitis externa and concurrent otitis media. Based on the results of bacteriologic studies, it has been shown that a majority of dogs with concurrent otitis externa and otitis media have different bacteria isolated from each compartment. Thus it is unclear if the portal of entry in otitis media involves perforation of the tympanic membrane and spread of otitis externa into the middle ear with subsequent healing of the tympanic membrane or if otitis media results from bacteria ascending a poorly functioning auditory tube. Multiple studies implicate the latter mechanism (see later).
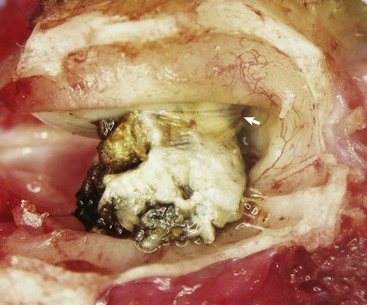
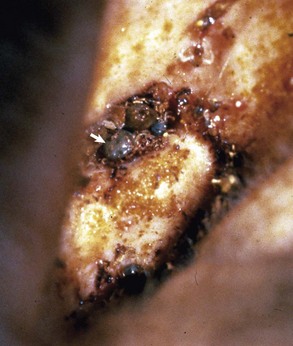
Fig. 20-23 Ruptured tympanic membrane, dog.
A large aggregate of cerumen bulges through a tear in the caudal portion of the pars tensa between the manubrium of the malleus and the caudal bony tympanic ring into the tympanic cavity. The wrinkled flap of the torn pars tensa can be seen (arrow). There was no evidence of otitis media and likely the tear was acute. A ceruminous gland adenoma was identified in the horizontal portion of the external acoustic meatus unassociated with the tympanic membrane causing complete obstruction. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Ascension of the Auditory Tube: Ascension up the auditory tube is a portal of entry into the middle ear. It appears that dysfunction of the auditory tube is a necessary precursor for development of otitis media and likely leads to impaired clearance of middle ear effusions and prolonged periods of negative pressure within the tympanic cavity. Dysfunction may be related to impairment of the opening of the auditory tube into the pharynx during swallowing when pressure equalization takes place or it could be related to alterations of mucociliary clearance facilitated by epithelial cells lining the tube. Via either mechanism, it appears that microorganisms can use this portal to reach the middle ear.
Extension Via Degeneration of the Temporohyoid Joint: Direct extension into the middle ear can occur from degeneration of the temporohyoid joint and release of microorganisms. See the discussion on temporohyoid osteoarthropathy in the section on Disorders of Horses.
Extension Via Erosion through the Tympanic Bulla: Erosion through the tympanic bullae is a rare portal of entry into the middle ear. Neoplastic processes, such as oral squamous cell carcinomas, regional lymphosarcoma, or local compression by an abscess, can lead to bone remodeling, as well as bone lysis, with subsequent spread into the middle ear.
Migration along Vascular or Neural Pathways: Branches of the caudal auricular artery and the facial nerve traverse within the middle ear and have the potential to serve as pathways for spread of microorganisms and neoplasms from the brain to the middle ear. This migratory process likely occurs via the extracellular matrix of arteries and nerves (see Chapters 3, 10, and 14) and via anterograde axonal transport in nerves (see Chapter 14). As an example, cranial nerve sheath tumors (see Chapter 14) in the brain have been reported to migrate along branches of the facial nerve and vestibulocochlear nerves and enter the ear via the internal acoustic meatus.
Internal Ear
Extension from the Middle Ear: Extension from the middle ear is a portal of entry into the internal ear, thus otitis interna or labyrinthitis is most commonly thought of as occurring by direct extension from an infection of the middle ear. The most likely portal is the round or cochlear window. Based on studies in cats, the permeability of the round window is increased for elements (sodium) and macromolecules (tritiated albumin) during mild cases of experimentally induced otitis media. Penetration through the oval or vestibular window is less likely because of the annular syndesmosis that is formed between the petrous portion of the temporal bone and the stapes (Fig. 20-24). Although otitis media may be diagnosed in isolation, otitis interna is rarely diagnosed without concurrent otitis media.
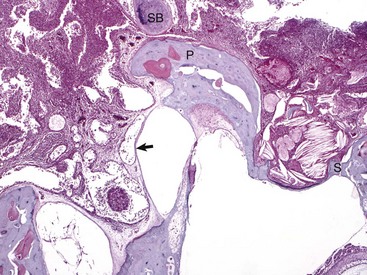
Fig. 20-24 Chronic otitis media, cat.
Histologic section of middle ear, petrous portion of the temporal bone through the round and oval window with the stapes in situ. The syndesmosis formed between the bone of the oval or vestibular window of the petrous portion of the temporal bone seems to prevent otitis media from spreading into the inner ear. Conversely, the membranous covering of the round or cochlear window is infiltrated by inflammatory cells. During episodes of otitis media, the permeability of this membrane is increased. Otitis interna was diagnosed in this cat (not depicted in this image). At the top of the image is the edge of the septum bulla abutting the petrous portion of the temporal bone, anatomic indication that this is from a cat. Edge of stapes (S); round window membrane (arrow); promontory (P); septum bulla (SB). H&E stain. (Courtesy Dr. B. L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Hematogenous Spread: Entry through hematogenous spread occurs in the internal ear (see the previous discussion on the middle ear in the section on Portals of Entry for details).
Migration along Vascular or Neural Pathways: Migration along vascular or neural pathways as a portal of entry occurs in the internal ear (see the previous discussion on the middle ear in the section on Portals of Entry for details).
Responses To Injury
The ear’s responses to injury are listed in Box 20-2.
External Ear
The external ear is an extension of the integument, and it responds to inflammatory stimuli similarly. All of the hallmarks of inflammation occur in otitis externa. Initially, there is reddening and warmth of the affected auricle associated with otitis externa caused by vascular dilatation and hyperemia. Transudation of fluid out of leaky vessels leads to edema affecting both the auricle (see Fig. 17-13) and external acoustic meatus. Edema within the tissues results in swelling of the tissues and discomfort when the tissues are touched. As the inflammatory response progresses, the transudate becomes an exudate, infiltrating the dermis of the external ear. Epithelial and adnexal changes, described later, result in further expansion of the external ear dermis. Eventually, the lumen of the external acoustic meatus may become so stenotic that hearing function becomes impaired.
As has already been described, the external acoustic meatus and auricle are lined by haired skin. Large, abundant, multiple, branching, and actively secreting sebaceous glands are most prominent in the deeper portions of the external acoustic meatus associated with hair follicles (see Fig. 20-5, A). Smaller, tubular, eccrine sweat glands, referred to as ceruminous glands, are located in the deeper layers of dermis. The epidermis, which is best studied in dogs, in response to inflammation, becomes hyperplastic and hyperkeratotic, although in some conditions it becomes ulcerated. Glandular changes include smaller, less abundant, less active sebaceous glands and more numerous, typically large, dilated ceruminous glands. Neutrophils, lymphocytes, and macrophages typically infiltrate the dermis, as well as the ectatic ceruminous glands (Fig. 20-25). Aggregates of lymphocytes form when the process is chronic. With increased chronicity, there is greater infiltration by fibroblasts and collagen, which can lead to more permanent stenotic changes. Finally, soft tissues, such as the dermis or auricular cartilage, may become ossified through metaplasia in the most chronically inflamed ears.
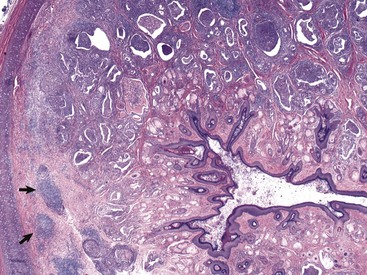
Fig. 20-25 Otitis externa, external acoustic meatus, dog.
In this histologic section, the dermis contains increased numbers of ceruminous glands that are dilated and filled with inflammatory cells, typically neutrophils, macrophages, lymphocytes, and plasma cells. These inflammatory cells are also present in the periadnexal dermis. Lymphoid aggregates form nodules (arrows) in the deep dermis near the cartilaginous rings of the external acoustic meatus. The overlying epidermis is mildly to moderately thickened (acanthosis). The luminal diameter has been markedly reduced, which can further exacerbate the problem of otitis externa. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Auricles of lightly pigmented cats chronically exposed to ultraviolet (UV) light are prone to developing squamous cell carcinoma (described later in the chapter; also see Chapter 17). UVB light leads to cellular transformation of the epithelial cells leading to a clonal population of neoplastic squamous epithelial cells.
Middle Ear
Myringitis: Inflammation of the tympanic membrane is called myringitis and is most commonly caused by bacterial infection of the external or middle ear (see Fig. 20-39, B). Macroscopically, the tympanic membrane may be congested, hemorrhagic, or thickened, resulting in opacity. Microscopically, the tympanic membrane has all of the characteristics of acute inflammation and if the inciting cause is unresolved results in chronic inflammation (see Chapter 3). Prolonged and severe myringitis may lead to perforation of the tympanic membrane and prevent its healing.
Healing of the Tympanic Membrane: The tympanic membrane is a regionally well-vascularized membrane that has an air-interface along each surface. It can be perforated by traumatic injury, sudden exposures to high pressures, degradative enzymes and pressures from acute and chronic inflammation, chronic mite infestations, and neoplasms. Unique to the tympanic membrane, and likely related to its function, is its inherent ability to heal rapidly while maintaining its thin structure during healing through a process called epithelial migration. Unlike most other tissues, in which granulation tissue forms and bridges the defect followed by reepithelialization, the tympanic membrane closes the defect, first with migrating epithelial cells followed by a granulation tissue response that closes the mesenchymal portion of the tympanic membrane.
Within minutes to hours of the initial perforation, injured tissue at the edge of the perforation initiates an acute inflammatory response. Within hours, epithelial cells proliferate initially along the annulus and along the margin of the manubrium, corresponding to areas in which the blood supply is most prominent and epithelial stem cells of the tympanum are thought to reside. Proliferating epithelium advances toward the perforation using keratin as a scaffolding to initially bridge and reepithelialize the defect. As this epithelium is migrating across the defect, granulation tissue forms on the inner edge of the perforated membrane. Anchored to the epithelium that initially closes the perforation, the middle, mesenchymal layer of the tympanic membrane is repaired by this advancing granulation tissue. As granulation tissue remodels to its normal thin layer, the inner epithelial layer finally bridges the defect to complete the healing process. In an experimental animal model, 2.5-mm perforations were completely healed by 9 days after injury.
Goblet Cell Metaplasia and Impaired Mucociliary Clearance: An important defense mechanism of the middle ear is the mucociliary apparatus of the auditory tube and contiguous tympanic cavity. Chronic otitis media causes a decrease in the number of ciliated cells (ciliary atrophy) in the mucosa of the auditory tube. Studies in cats determined that neutrophil lysate (likely consisting of degradative enzymes) or lipopolysaccharide did not significantly reduce the number of ciliated cells in the auditory tube, but auditory tube obstruction and likely increased pressure in the middle ear resulted in a dramatic decrease in the number of ciliated epithelial cells. In the same study, there was a marked increase in the total number of goblet cells lining the tympanic cavity and bulla. It was hypothesized that obstruction of the auditory tube elevated partial pressure of carbon dioxide (pCO2) in the mucus layer of the mucosa, triggering mucosal stem cells to differentiate toward goblet cells rather than ciliated cells. The viscoelasticity of the mucus produced by these goblet cells was greater than normal and resulted in a marked decrease of mucociliary clearance of the middle ear, probably further contributing to auditory tube obstruction, increased pressure in the middle era, and metaplasia of ciliated cells to goblet cells.
Osteosclerosis of the Tympanic Bulla: In infections of the middle ear caused by microorganisms, mediators of acute and chronic inflammation, such as cytokines and degradative enzymes, may lead to excessive periosteal proliferation of new bone and osteosclerosis (thickening) of the wall of the tympanic bulla (Fig. 20-26; see Chapter 16). The bulla may become grossly distorted, and the lumen volume typically decreases. Inflammation also can contribute to bone lysis.
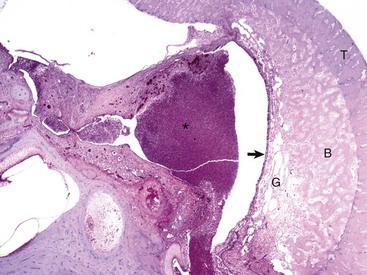
Fig. 20-26 Chronic otitis media with osteosclerosis, guinea pig.
The tympanic cavity is partially filled with a suppurative exudate (asterisk, center of the image). The inner surface of the tympanic bulla is lined by a thickened hyperplastic mucosa (arrow). Below the mucosa is an area of granulation tissue (G) that overlies the formation newly remodeled bone (B), a reparative or healing response to injury. Tinctorially, the tympanic bulla (T) is the darker blue area along the right margin of the image. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Formation of Inflammatory Polyps: Inflammatory polyps are discussed later but are thought to represent both inflammatory and hyperplastic responses to injury induced by otitis media. Inflammation of the middle ear results in the formation of polypoid masses by an undetermined mechanism. These masses then result in clinical disease referable to where they exert their greatest effect. Polyps that involve the auditory tube and the nasopharynx can result in dysphagia and dyspnea, whereas those involving the tympanic membrane and external acoustic meatus lead to signs of otitis externa.
Horner’s Syndrome/Pourfour Du Petit Syndrome: Postganglionic sympathetic fibers that innervate the eye course through the middle ear with branches of the internal carotid artery via the tympano-occipital fissure. Smooth muscle that lifts the upper eye, retractor muscles of the third eyelid, smooth muscle that tonically pulls the eyes rostrally, and dilator muscles of the pupil are all innervated by these fibers. Thus, in otitis media, cells and mediators of inflammation can act on postganglionic sympathetic fibers that innervate the eye as they traverse through the middle ear, leading to primary demyelination and axonal injury.
Most commonly, injury resulting in partial paralysis of postganglionic sympathetic fibers causes Horner’s syndrome. This syndrome has the following clinical signs: miosis, enophthalmos, ptosis, a narrowed palpebral aperture, protrusion of the third eyelid, and peripheral vasodilation of the skin of the face. Resolution of signs typically follows clearance of the cause.
Injury to the postganglionic sympathetic fibers can much less commonly result in hyperexcitability or hyperirritability. This is most commonly reported in cats that have had their ears examined, sampled, and flushed while under anesthesia. Clinical signs include mydriasis, exophthalmos, widening of the palpebral aperture and cool skin over the face. In all cases reported, clinical signs resolved spontaneously. This hyperirritability syndrome is the opposite of Horner’s syndrome and is referred to by some as the Pourfour du Petit syndrome.
Internal Ear
Sensory Cell Degeneration/Death: Within the organ of Corti are the outer and inner sensory hair cells, as well as spiral ganglion neurons. The sensory hair cells are the most vulnerable to injury. Damage to the sensory cells or ganglion cells results in impaired function that is often permanent. Whether the cause is labyrinthitis, noise-induced hearing loss (85 dB sound pressure level or higher), chemical ototoxins, or the poorly understood disorder called presbycusis (i.e., age-related hearing loss), sensory hair cells are targeted and undergo degeneration or death leading to hearing impairment or hearing loss. Bursts of intense noise are thought to result in disarrangement or breakage to the stereocilia, whereas continuous exposure to damaging noise leads to death of the hair cells.
Auditory Ossicular Chain Damage: Chronic otitis media can potentially lead to conductive hearing loss by damaging the auditory ossicles. Chronic exposure to infection, bacterial or fungal toxins, and degradative enzymes from inflammation can result in bony lysis. Excessively lytic bones may develop pathologic fractures. Additionally, chronic inflammation can result in fibrosis, which may restrict the movement of the ossicles. Finally, chronic inflammation can damage the articulations of the ossicles, thus impairing transmission of vibrations.
Defense Mechanisms
Defense mechanisms of the ear are listed in Box 20-3.
External Ear
Integumentary Defenses: Defense mechanisms of the skin are discussed in Chapters 3 and 17.
Epithelial Migration: Migration of cornified epithelial cells to the periphery of the tympanic membrane and onto the external acoustic meatus continuously replaces the effete epidermal layer of the tympanic membrane. Epithelial migration is thus an important means of maintaining tympanic membrane thickness, clearance of debris and likely microorganisms and parasites from the tympanic membrane and external acoustic meatus, and retaining vibratory sensitivity. Alterations in this epithelial migratory pattern can lead to or may be the cause of disease of the external or middle ear. As discussed previously, epithelial migration is also the method by which perforations of the tympanic membrane are repaired.
Adnexa and Cerumen: Cerumen is an oily emulsion that coats and protects the integument of the external acoustic meatus. Its naturally hydrophobic properties make it an important barrier to the entry of excessive moisture into the epidermal cells or underlying dermis. In normal ears of dogs, cerumen is made of sloughed superficial squamous cells mixed with ceruminous and sebaceous gland secretions. There is a high lipid content in cerumen made up of neutral lipids. In otitic ears, the cerumen changes because ceruminous glands are typically more numerous and active during periods of inflammation and thus contribute more to the content of cerumen (see Fig. 20-25). One consequence is a decrease in the lipid content, a decrease in hydrophobicity, and impairment of a natural barrier.
Cerumen in otitic ears becomes more acidic than normal, which is believed to impair bacterial growth. This outcome is due to greater contribution by ceruminous glands during periods of external ear inflammation. The pH of the external acoustic meatus is variable in dogs, ranging from 4.6 to 7.2. During periods of inflammation, the mean pH in acute otitis externa may decrease, whereas the mean pH in chronic otitis externa tends to increase. The mechanistic consequences of these changes are poorly understood.
Immunoglobulins IgA, IgG, and IgM have been identified in canine cerumen; however, the predominant immunoglobulin is IgG. This high level of IgG is believed to be related to transudation of serum in the inflamed external ear. Lysozyme and interleukins have also been identified in cerumen and provide an antimicrobial function.
Commensal Organisms: A mixture of bacteria and yeast normally populates the external acoustic meatus. Various species of “potentially pathogenic” microorganisms have been cultured from external ears of dogs with no evidence of disease. They include Bacillus spp., Corynebacterium spp., Escherichia coli, Micrococcus spp., Staphylococcus spp., Streptococcus spp., and yeast organisms, most commonly Malassezia spp. Rarely, Pseudomonas spp. and Proteus spp. have been isolated. Based on a single study of horses, Corynebacterium spp. and Staphylococcus intermedius were isolated from normal ears. Presumably, these organisms live symbiotically and defense mechanisms limit or prevent proliferation of a monoculture. However, the spectrum of microorganisms cultured from diseased external ears closely mirrors this list.
Osseous External Acoustic Meatus: The length and diameter of the osseous portion of the external acoustic meatus are highly variable among species and provide a unique structural defense mechanism. Animals with longer and narrower osseous portions have tympanic cavities and tympanic membranes that are better protected than those with shorter osseous portions.
Middle Ear
Mucociliary Apparatus: Various portions of the middle ear are lined by epithelium similar to that found in the nasopharynx. A combination of goblet cells and ciliated columnar epithelial cells extend through the auditory tube into the tympanic cavity. See Chapters 4 and 9 for discussion of the mucociliary apparatus.
Surfactant: Surfactant in the lung has long been recognized for its central role in reducing surface tension in alveoli, allowing them to expand and collapse normally during inspiration and expiration, respectively (see Chapter 9). However, recently, it has been shown that surfactant has other roles, especially in the function of the auditory tube. Surfactant is a complex mixture made up of 90% lipid and phospholipid and 10% surfactant proteins, designated surfactant protein-A (SP-A), SP-B, SP-C, and SP-D. Cuboidal epithelial cells lining the auditory tube are the source of surfactant and contain apical secretory granules analogous to what are seen in type II pneumocytes of the lung. One major difference between pulmonary surfactant and auditory tube surfactant is the ratio of phosphatidylcholine to sphingomyelin. Pulmonary surfactant has a ratio of 67 to 1, whereas auditory tube surfactant has a ratio of 2 to 1. This dramatic difference in auditory tube surfactant phospholipid content is reflected in a reduced ability to modify surface tension.
The most abundant surfactant protein in lung surfactant is SP-B, a strongly hydrophobic protein with high surface activity. However, auditory tube surfactant has a paucity of SP-B and relative abundance of SP-A and SP-D, two highly hydrophilic surfactant proteins. This difference may indicate a different function for auditory tube surfactant, namely, acting as a release agent and anti-adhesive rather than a surface tension modifying substance. Anti-adhesive properties would facilitate opening of the auditory tube.
Surfactant proteins are collectins that have two domains: (1) a lectin moiety that binds to the surface of foreign substances and organisms and (2) a collagenous domain that functions as a ligand for phagocytosis and complement activation (see Chapter 3). Finally, surfactant is believed to play a protective role against free radicals by early termination of free radical propagation, and protecting against oxidative injury. A lipid-binding pocket in surfactant proteins may prevent free radicals from propagating.
Auditory Tube–Associated Lymphoid Tissue (ATALT): Dorsal to the opening of the auditory tube is an aggregate of lymphoid tissue. In humans and rodents, this aggregate is referred to as the tubal tonsil. Based on a limited number of dissections of presumed normal auditory tubes, lymphoid aggregates have not commonly been identified in the species covered in this text; however, it is reasonable to assume that they exist in this location (see Chapters 4 and 9) and should be thought of as a component of MALT. Lymphoid tissue in the nasopharynx may proliferate and become locally prominent in response to inflammation or infection.
Commensal Microorganisms: Resident microflora in the middle ear are similar to microbes found in the nasopharynx and the external ear. They include aerobic and anaerobic bacteria and yeast in small numbers. Staphylococcus spp., Streptococcus spp., E. coli, Branhamella spp., Bordetella bronchiseptica, Enterococcus spp., and Bacillus spp. have been isolated from the middle ear of normal dogs. Clostridium perfringens has also been isolated from tympanic cavities of normal dogs.
Internal Ear
Petrous Portion of the Temporal Bone: Referred to as the hardest bone in the body, the petrous portion of the temporal bone is the bony labyrinth that encases and protects the membranous labyrinth held within. The thick and extremely dense bony labyrinth ensures maximal protection to the sensory portions of the internal ear. In addition, its dorsomedial location in the body deep to the external acoustic meatus provides one of the most protected sites in the body.
Acoustic Reflex: Reflexive contraction of the tensor tympani and stapedius muscles in response to loud and injurious noise functions to protect the internal ear by dampening sound conductance. Contraction of the tensor tympani pulls the malleus rostrally and applies tension to the tympanic membrane. The stapedius muscle applies tension to the stapes in a dorsocaudal direction, relative to the tensor tympani muscle; parallel to the long axis of the oval window and perpendicular to the direction the stapes oscillates in the oval window. Thus the delicate structures of the internal ear are protected.
Disorders of Domestic Animals
Developmental Anomalies Of The External Ear
Auricular Agenesis/Aplasia: Auricular agenesis/aplasia (also known as anotia) has been reported in sheep, cattle, and a dog. Auricular hillocks or ridges of the first and second branchial (pharyngeal) arches, which normally interact to form the auricle and the first groove or cleft that normally forms between the two arches, which becomes the external acoustic meatus, fail to interact properly during embryologic development. This failure to develop normally results in anotia and a lack of an external acoustic meatus. It can manifest as a unilateral or bilateral lesion. In sheep, auricular agenesis has been associated with deafness, as well as other congenital anomalies.
Auricular Hypoplasia: Auricular hypoplasia (also known as microtia) is a normal feature of certain animal breeds. Auricles are what distinguish the La Mancha breed from other goat breeds. Ear length in goats is inherited as an incompletely dominant trait. Two auricular phenotypes exist in the breed: “gopher ears” and “elf ears.” Goats with gopher ears have microtic ears that possess little or no auricular cartilage and either fold dorsally or ventrally. La Mancha goats with elf ears have microtic auricles that are larger than gopher ears, up to 5 cm in length with folded, distorted auricular cartilage (Fig. 20-27, A) that either folds upward or downward.
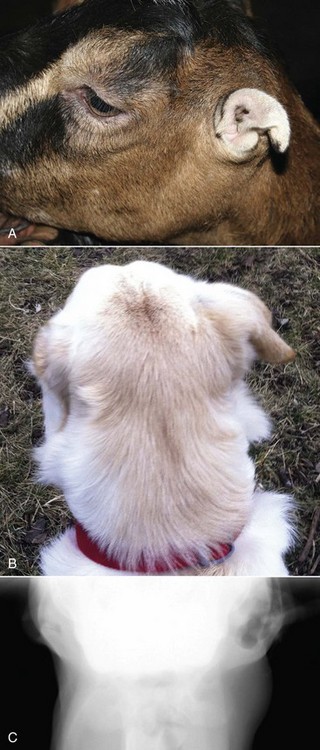
Fig. 20-27 Microtic and distorted ears, goat and cat.
A, La Mancha doe. Elf ears are one of two breed-standard auricle phenotypes accepted for La Mancha goat registry. B, Labrador retriever dog. The left auricle is smaller than the right and has always been. The dog is deaf as a result of impaired development of the ear. C, Radiograph of the same dog in B. The left external acoustic meatus, middle ear, and bulla are very small and underdeveloped when compared to the right side. (A courtesy Dr. M. Smith, College of Veterinary Medicine, Cornell University; B and C courtesy Dr. T. Lykins, Pet Pro, and Ms. T. Maxey, owner.)
Scottish fold cats have slightly microtic ears with a characteristic forward or rostrally folded auricular cartilage. Cats with this simple autosomal dominant inherited trait have unaffected auricles at birth, but the fold of the auricle begins to develop at 3 to 4 weeks of age. Defective cartilage is believed to be the primary problem in these cats, whereby the auricular cartilage either develops abnormally or lacks resiliency succumbing to gravitational forces. Presumably, present-day Scottish fold cats are descendants of a single Scottish female cat that had a spontaneous mutation of the folded ear allele (Fd). It is believed that all Scottish fold cats with the folded-ear phenotype, whether homozygous or heterozygous, suffer with mild or severe, respectively, forms of osteochondrodysplasia of their distal limbs and tails. The mode of inheritance for this skeletal abnormality is also believed to be incomplete dominant.
Unilateral microtia was first reported in a 1950s report involving six slaughter-age pigs in Denmark. Affected auricles were one-third their normal size, and there was atresia of both the bony and cartilaginous portions of the external meatus. In addition, the tympanic cavity was poorly developed with complete absence of tympanic membranes and auditory ossicles. Specimens were never examined histologically. Other species, such as dogs, may be affected sporadically (Fig. 20-27, B).
Auricular Agenesis/Aplasia: Preauricular malformations (tags, cysts, pits, fissures, and sinuses) are a group of developmentally related disorders of soft tissues that occur rostral to the auricle (Web Fig. 20-1). Preauricular tags are pedunculated outgrowths of skin that contain no bony, cartilaginous, or cystic components and do not communicate with the external or middle ear. The remaining malformations often have an opening rostral to the auricle that is contiguous with a sinus tract that travels under the skin following alongside the cartilage of the external acoustic meatus. These latter malformations are lined by squamous epithelial cells, which may lead to the formation of cysts filled by squamous cell debris or if infected with microorganisms can result in abscesses and with rupture cellulitis.
Preauricular malformations should be differentiated from branchial cysts that arise developmentally from the first branchial cleft. Cleft malformations are closely linked with other structures that may develop normally from the clefts and arches such as the external acoustic meatus, tympanic membrane, and facial nerve. Animals with preauricular or cleft malformations should be examined for congenital anomalies affecting other organ systems.
Cropped or Notched Ears: Cropped or notched ears was a deformity identified in a group of Highland cattle, affecting 45 of 46 progeny from one breeding sire. The inheritance was determined to be incomplete dominance of a single autosomal gene. Other auricular anomalies that have been reported, primarily in sheep, include polyotia (presence of more than one auricle), macrotia (enlarged auricles), synotia (fusion of auricles), and misplaced auricles (heterotopic otia). A single case report of a Friesian cross calf with epitheliogenesis imperfecta had one ear deformed from rolling of its lateral margins followed by fusion of the surfaces after being brought into close proximity.
Atresia of the External Acoustic Meatus: The external acoustic meatus is normally atretic or stenotic for the first days of life in altricial animals but is open at birth in precocial animals.* For example, beyond this initial neonatal period, atresia of the external acoustic meatus is reported as congenital or secondary to trauma (Fig. 20-28). When congenital, it occurs in animals as a lone defect or with other congenital abnormalities such as anotia, microtia, middle ear hypoplasia, auditory ossicular malformation or agenesis, agenesis of ossicular musculature, abnormal positioning of the facial nerve, hydrocephalus, and soft palate hypoplasia. The external acoustic meatus arises from the first branchial cleft or groove and disturbances of its embryonic development result in congenital atresia. Atresia has been described in all species typically covered in this text.
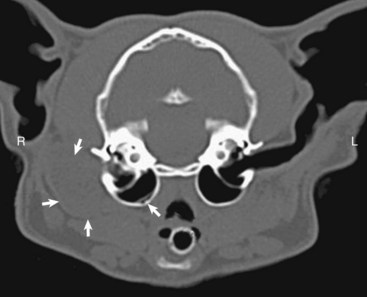
Fig. 20-28 Congenital, unilateral external acoustic meatus atresia, right ear, cat.
Axial computed tomography (CT) scan of the head of the cat demonstrating replacement of the right horizontal canal by a hypodense round, well-marginated fluid attenuating material located immediately lateral to the right tympanic bulla, outlined by the solid white arrows. The open white arrow illustrates the soft-tissue material accumulating in the dependent portion of the bulla. R, Right side; L, left side. (With permission of J Feline Med Surg 11:864-868, 2009.)
Stenosis of the External Acoustic Meatus (Congenital Hypothyroidism): Stenotic external acoustic meatuses can occur in toy fox terriers that develop goiter caused by a fully penetrant, autosomal recessive nonsense mutation of the thyroid peroxidase gene resulting in dyshormonogenesis and congenital hypothyroidism. Clinically, puppies are lethargic, do not walk, and are able to hear normally.
A congenital hypothyroidism and dysmaturity syndrome with multiple congenital musculoskeletal abnormalities has been described in foals in western Canada. In addition to tendon laxity and bony changes, some foals have floppy ears that likely are caused by congenital defects in the formation of auricular cartilage. External acoustic meatus, middle ear, or internal ear abnormalities have never been investigated in foals affected by this syndrome.
Otognathia: Otognathia, a rudimentary accessory mouth found at the base of the ear, is an unusual condition reported in sheep and cattle. This accessory orifice is either blind or continuous with the pharynx, lined by a mucous membrane, and may contain rudimentary teeth, mandible-like bones, and lateral extensions of the tongue. Both unilateral and bilateral otognathia have been reported. Embryologically, the first branchial pouch (endodermal origin) makes contact with the first branchial cleft (ectodermal origin), eventually thinning to form a membrane, which normally becomes the tympanic membrane. In otognathia, during development, this membrane between the developing first pharyngeal cleft and first pharyngeal pouch ruptures and forms persistent fistulae. Dentigerous cysts, most commonly reported in horses and described later, may have a similar embryologic origin.
Inflammation Of The External Ear
Otitis Externa: Otitis externa is rarely a primary condition but arises from the interaction of predisposing factors, primary causes, and secondary causes (see Table 20-1). Predisposing factors, such as conformation, auricular phenotype, breed, and external ear moisture, act as inherent risk factors for the development of otitis externa but are not directly causative. Primary causes, such as ectoparasites, keratinization defects, foreign bodies, hypersensitivity reactions, and systemic immune-mediated disorders that affect the integument, may initiate inflammation of the external acoustic meatus. Secondary factors, such as bacterial and fungal infections, tend to intensify the inflammatory reaction and subsequent severity of otitis externa.
Dogs are most severely affected and therefore examined the most. The most common primary causes appear to be atopic dermatitis, adverse food reactions, ectoparasites, and foreign bodies. It is estimated that the prevalence range of otitis externa in dogs is 15% to 20.4%, which is much higher than the estimated prevalence of 4% in cats. In addition, long-standing chronic external ear infections that have excessive healing responses with extensive fibrosis, cartilage remodeling, and osseous metaplasia can make an eventual return to normal structure and function difficult or nearly impossible.
Macroscopically, otitis externa may include discharge from the ear (also known as otorrhea), hemorrhage from the ear (also known as otorrhagia), and pain elicited when palpating the ear (also known as otodynia or otalgia). The auricles tend to be red, warm, and edematous (see Fig. 17-13) due to increased congestion of dermal blood vessels (Fig. 20-29). With chronicity, the epidermis may become thickened and bosselated, as well as hyperpigmented. The normally pliable auricular cartilage may be modified by fibrosis or osseous metaplasia and become stiffer. The most severely affected ears may develop acquired stenosis of the external acoustic meatus. This feature is an important perpetuating factor that promotes recurrent episodes of otitis externa and prevents satisfactory resolution.
Fig. 20-29 Otitis externa, dog.
The auricle is thickened, reddened, and painful (otalgia) with obvious otorrhea and presumed otiasma. (Courtesy Dr. W.H. Miller, College of Veterinary Medicine, Cornell University).
Microscopically, many of the changes have already been discussed. The dermis thickens initially because of edema followed by exudation and infiltration by inflammatory cells. Initially, neutrophils predominate but later, with more chronic inflammation, macrophages are prominent, mixed with lymphocytes and plasma cells. The formation of multiple lymphoid aggregates is not uncommon (see Fig. 20-25). The adnexa transforms from an initially dominant sebaceous gland population to an overabundance of ceruminous glands that tend to be large, ectatic, and infiltrated by inflammatory cells. The overlying epidermis is typically both hyperplastic and hyperkeratotic, although it may be multifocally eroded or ulcerated in acute episodes. All of this thickening in aggregate leads to a reduction in the luminal diameter of the external acoustic meatus.
In chronically affected ears, thick bands of dense fibrous connective tissue replace dermal adnexa. The lack of ceruminous and sebaceous glands results in a lack of cerumen production. Without this natural, typically hydrophobic barrier, the epidermal surface is perpetually hyperplastic and hyperkeratotic. The reduced hydrophobicity results in an increased amount of moisture, superficially resulting in more intercellular edema (see Fig. 17-13) of the epidermis. Cartilage may undergo metaplastic change, resulting in the formation of cartilaginous nodules or more commonly undergo osseous metaplasia. The net affect is a permanent, severe reduction in the luminal diameter of the external acoustic meatus.
Clinically, affected animals have an increased tendency to scratch their ears or shake their heads incessantly. Otitis externa is frequently associated with a malodorous aural discharge or otiasma.* In general, animals have a reluctance to allow examination of affected ears. For those animals that are chronically affected, signs of middle and internal ear disease may develop.
Vascular Injury Of The External Ear
Infarction: Vascular injury to the auricle occurs in all domestic animal species and results in responses ranging from ear tip necrosis to sloughing of the entire auricle. Various causes, such as bacterial septicemia, immune-mediated vasculitis, frostbite, and toxins, injure and activate endothelium, platelets, and clotting cascades often resulting in vascular thrombosis.
• In pigs, septicemic salmonellosis and its endotoxins can injure vascular endothelium leading to thrombosis of small vessel and tissue infarction of the ear tip and tail (see Chapter 3 for mechanistic details). Ears initially are dark red to purple-black and painful and then become dry (dry gangrene) and scabbed and eventually slough.
• In cats with feline infectious peritonitis (FIP) and also hypothesized in German shepherd dogs with familial vasculopathy (autosomal recessive inheritance pattern), immune complex–mediated vasculitis (type III hypersensitivity) has the potential to cause systemic or cutaneous vasculitides of extremities such as the ears, tails, and potentially feet.
• Severe drops in ambient temperature in newborn animals or animals that are poorly sheltered can result in frostbite. In these cases, blood is shunted away from the extremities to preserve the core body temperature and the poorly perfused extremities, such as ears, tails, feet, and nose, are compromised. Affected tissues demonstrate a clear line of demarcation between tissue that has died or has become infarcted as the result of inadequate circulation and the tissue that retained adequate circulation. The dead tissue darkens, is cool to the touch, and becomes dry from dry gangrene (Fig. 20-30).
Fig. 20-30 Auricular infarction, frostbite, goat.
Note that the distal (upper) half of the ear has dry gangrene from frost-bite. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
• Ingested preformed toxins, such as ergot alkaloids, cause vasoconstriction leading to vascular compromise, infarction, and sloughing of extremities.
Hematomas: Animals with chronic external or middle ear disease often respond clinically to discomfort by continuously and vigorously shaking their heads. This action applies severe centrifugal shearing forces (trauma) to blood vessels and auricular cartilage at the sides and tips of the auricles. Since auricular cartilage is elastic and the blood vessels pass through perforations, repeated trauma results in fractures of auricular cartilage, most likely at these areas of perforation. Presumably, the sharp edges of the fracture cartilage and the shearing forces lead to laceration of associated blood vessels. Hemorrhage from these ruptured auricular blood vessels is the genesis of aural hematomas with blood collecting either intrachondrially or subparachondrially.
Aural hematomas are most common in dogs, pigs, and cats but have been reported in sheep, goats, a cow, and a foal. Affected auricles are markedly swollen, most obvious on the concave surface, and are warm, hyperemic, painful, and heavy and tend to droop (Fig. 20-31, A). Large breed dogs, in particular golden retrievers and Labrador retrievers, and middle-aged to older dogs with ear disease are prone to develop aural hematomas. If untreated, hematomas eventually heal by fibrosis resulting in a very firm to hard, thickened, and permanently malformed auricle. When opened, the cavity that forms is filled with clotted blood and a meshwork of fibrin (Fig. 20-31, B). Microscopic changes typically include cartilage fractures and splitting, cartilage erosion, and granulation tissue formation.
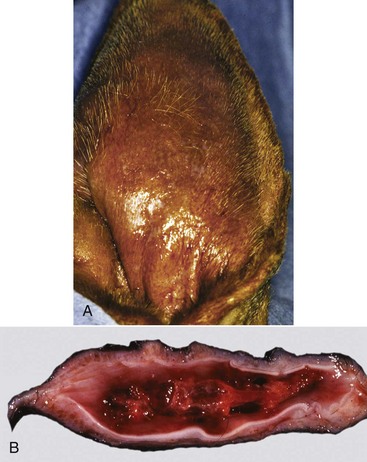
Fig. 20-31 Aural hematoma, dog.
A, Left auricle. The concave aural surface has become convex due to expansion of the underlying connective tissue by mixtures of blood and fibrin. B, Auricle, cross-section. Portions of the auricular cartilage are present on both sides of the cavity. Within the cavity are abundant strands of lighter, eosinophilic fibrin strands mixed with thicker, larger dark red blood clots. (A courtesy Dr. M.C. Rochat, Center for Veterinary Health Sciences, Oklahoma State University; B courtesy Dr. D.D. Harrington, School of Veterinary Medicine, Purdue University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Parasitic Diseases Of The External Ear
Numerous ectoparasites parasitize or infest domestic and wild animals, including mites, ticks, and nematodes (Table 20-2). Most affect their hosts by infesting various parts of the integumentary system (see Chapter 17). A small proportion of these organisms preferentially infest the ear. Reactions vary from minimal irritation to gross deformity. External ear infections may be isolated or extend to the middle and internal ears. The clinical signs are prototypic, including head shaking, repeated ear twitching, excessive ear itching, and traumatization of the auricle and base of the ear.
TABLE 20-2
Ticks, Mites, and Nematodes Affecting the External or Middle Ears of Animals
| Name | Type | Host |
| Notoedres cati | s, e, m | Cat. Rabbits, dogs |
| Sarcoptes scabiei | s, m | Dog, pig |
| Otodectes cynotis | e, m | Cat, dog, other carnivores |
| Psoroptes cuniculi | e, m | Rabbit, sheep, goat, horse, deer |
| Raillietina species | e, m | Cattle, buffalo, goat, others |
| Otobius megnini | e, t | Dog, cat, horse, ruminants, swine |
| Otobius lagophilus | e, t | Rabbits |
| Rhipicephalus species | s, e, t | Dog, horse, ruminants, others |
| Amblyomma species | s, t | Dog, horse, ruminants |
| Amblyomma maculatum | e, t | Ruminants, others |
| Amblyomma americanum | s, e, t | Ruminants, others |
| Boophilus species | s, t | Ruminants, horse |
| Dermacentor nitens | s. t | Horse, ruminants |
| Haemaphysalis leporispalustris | s, t | Horse, ruminants |
| Ixodes ricinus | s, t | Dog, horse, ruminant |
| Rhabditis spp. | e, n | Cattle |
| Mammomonogamus auris | e, n | Cat (otitis media) |
s, Skin; e, ear; n, nematode; m, mite; t, tick,
Modified from Thomson RG: Special veterinary pathology, Toronto, 1988, BC Decker.
Ear Mite Infestations (Otoacariasis): Mites are categorized as burrowing or nonburrowing. Many species, such as Sarcoptes spp. (see Fig. 17-57) and Demodex spp., can cause more generalized lesions with possible involvement of the ear. However, there are few mites that spend much or all of their life cycle within the concave portion of the auricle or in the external acoustic meatus.
Otodectes Cynotis: Otodectes cynotis infest the external acoustic meatus of domestic and wild cats, dogs, and occasionally ruminants. The main route of infestation is from dam to offspring. Other routes of spread include contaminated combs, brushes, bedding, or other grooming accessories. They are nonburrowing and feed on the cerumen, keratin, and lipids. These mites are a prominent primary cause of otitis externa with up to 50% of cats and 10% of dogs developing otitis externa. They cause intense irritation by mechanisms not well understood. Copious cerumen production ensues until a thick, waxy, dark brown exudate obstructs the external acoustic meatus. Auricles are often alopecic and have “scratching” wounds from trauma that form in response to intense pruritus. These areas may also become infected secondarily with bacteria. The mucosa becomes hyperkeratotic with acanthosis and parakeratotic hyperkeratosis with crusts that contain mites and mite detritus mixed with cerumen. Small-to-moderate numbers of lymphocytes and macrophages infiltrate the dermis and subcutis. Ceruminous glands are typically hypertrophied and hyperplastic.
While a majority of cats are infested, a minority manifest clinical disease possibly owing to early exposure as kittens and the development of Arthus- and immediate-type hypersensitivity reactions.
Notoedres Cati: Notoedres cati is primarily a cat pathogen but can infest dogs, foxes, rabbits, and rarely people. Usually, infestations are restricted to the auricles, head, face, neck, and shoulders. Infestation produces alopecia, pruritus, thick crusting, and excoriation of the rostral pinnae as the female mite burrows in the stratum corneum and occasionally penetrates hair follicles and sebaceous glands. Microscopic lesions include epidermal hyperplasia and spongiosis with perivascular eosinophilic dermatitis and crusting.
Raillietina Species: Raillietina spp. of mites occur most commonly in cattle, buffalo, and goats of nearly every continent. Cattle are most commonly parasitized by Raillietina auris, whereas the mite infesting goat ears is Raillietina caprae. Raillietina flechtmanni is found in buffalo and cattle ears. These mites often go undetected because of their small size and their tendency to reside deep in the external acoustic meatus adjacent to the tympanic membrane, and they do not commonly cause clinical disease. When clinically apparent, there is often a thick plug of cerumen and debris, with variable suppuration, behind which the mites reside. Additionally, the external acoustic meatus may be ulcerated. Affected animals rarely may develop central nervous system (CNS) signs related to heavy infestations that penetrate the middle and internal ear. Otitis externa is more severe with concurrent bacterial infections or if the host is additionally infected with Rhabditis spp. of nematodes. In goats, there are often concurrent infections with Raillietina caprae and pathogenic Mycoplasma spp.
Psoroptes cuniculi: Psoroptic otoacariasis is most commonly caused by Psoroptes cuniculi, infesting sheep, goats, deer, horses, donkeys, mules, and antelope. Although capable of feeding on any part of the animal, it prefers the ear in goats, sheep, and horses. They live on the surface feeding on lipids, keratin, crusts, and cerumen. Pruritus can be intense and is related to surface irritation by infesting mites and hypersensitivity reactions leading to self-trauma to the auricle and periauricular skin. As described (see Chapter 17), histologic lesions include eosinophilic perivascular dermatitis that can be spongiotic, hyperplastic, hyperkeratotic, or exudative. In affected goats, care must be taken to check that the infestation is not complicated by Raillietina spp.
Ticks: Ticks and tick-borne diseases rank as some of the most important health constraints of livestock in many parts of the world. Ticks serve as vectors for the spread of disease, affect production parameters such as weight gain or milk production, can lead to significant anemia, may impair individual or herd immunity, and can be a source of aggravation or irritation. Sites of attachment and feeding may occur in multiple locations, but many species of ticks have preferred sites. What follows is a discussion of a few species of mites that preferentially feed in the ears.
Rhipicephalus Species: The brown ear tick, Rhipicephalus appendiculatus, is found most commonly in southern and southeastern African countries. Adults primarily attach to the ears of domestic and wild ruminants whilst larvae and nymphs attach to ears, head, and neck of most ruminant species, as well as equids, carnivores, and hares. Bos indicus cattle become fairly resistant to tick infestations, but exotic Bos taurus cattle can have severe production losses and very significant ear damage. R. appendiculatus secrete proteins in their saliva, some of which react with the host animal enzymes to form a hard feeding cone and others have inherent enzymatic activity. The protein that forms the cone is called cement, and it provides an anchor for feeding ticks and may interfere with a robust inflammatory reaction by the host animal. Affected ears can range from minimally injured to very deformed and sometimes can appear shredded. Microscopically, at the feeding sites, neutrophils predominate in the dermis immediately surrounding the cement layer and are also mixed with macrophages and variable numbers of eosinophils. Brown ear tick toxicosis is a poorly understood condition that occurs in susceptible cattle, presumably the result of tick saliva toxins. Some saliva-derived toxins have an immunosuppressive effect, although the exact mechanism is not understood. These animals may have suppression of adaptive immune responses and lose protection against other environmental pathogens that they have encountered and resisted. In some instances, tick toxicosis can be fatal.
Gulf Coast Ear Tick: Another tick that preferentially infests the ear is variably named the Gulf coast tick or Gulf coast ear tick. Amblyomma maculatum is native to North, Central, and South America. In North American, this tick can be found all along the Gulf coast, extending as far inland as Oklahoma, Kansas, and Arkansas and along the southern Atlantic coast. Adult ticks preferentially feed on cattle, sheep, horses, and mules by attaching to the external ears but can also infest deer, cats, foxes, dogs, and pigs. Heavy infestations cause intense auricular inflammation and swelling and may even cause the destruction of the auricular cartilage resulting in a droopy ear sometimes referred to as “gotch ear”* (Fig. 20-32). A. maculatum is an experimental vector for transmitting Cowdria ruminantium, the causative agent for heartwater disease, but it has not been implicated in field outbreaks in North America. It has also been shown that the Gulf coast ear tick in Florida, Georgia, Kentucky, Mississippi, Oklahoma, and South Carolina may serve as a reservoir for Rickettsia parkeri.
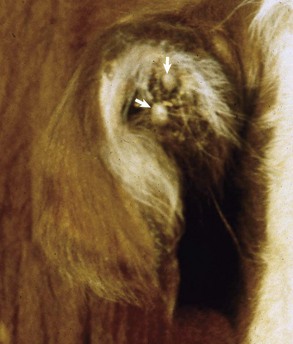
Fig. 20-32 Gotch ear, bovine.
The left ear of a Hereford cow is severely deformed because of infestation by numerous Gulf coast ear ticks, Amblyomma maculatum. The bodies of several ticks (arrows) are attached to the concave surface of the medially, ventrally, and rostrally deviated auricle. (Courtesy Dr. J.A. Hair, Oklahoma State University.)
Spinose Ear Tick: Otobius megnini, the “spinose ear tick,” has a broad host range, including ungulates, sheep, goats, cattle, horses, dogs, and humans. Adults are free living and nonparasitic, but nymph and larval stages are parasitic. Newly hatched larvae remain in the environment until they climb onto a suitable host. Owing to their small size and propensity for living very deep in the external acoustic meatus, larvae are rarely identified in host ears. As these ticks moult and mature, some can be found attached to and blood feeding in the more shallow portions of the external acoustic meatus or attached to the auricular skin (Fig. 20-33). After several months of feeding in or on host ears, nymphs drop off the host, seeking dry, sheltered places where they moult into adults. Lesions are attributable to blood feeding, which results in local irritation and secondary otitis externa. As has already been described, local reactions to the feeding sites include perivascular to interstitial dermatitis laden with neutrophils and eosinophils. Infested animals may develop clinical symptoms such as head shaking and pruritus related to nymph feeding on blood and lymph of the skin of the external acoustic meatus.
Bacterial Diseases Of The External Ear
Dermatophilosis (Streptothrichosis): Dermatophilosis (also known as streptothrichosis) is caused by Dermatophilus congolensis and is a zoonotic bacterium of the skin and mucosae of the nose, commissures of the lips, distal or proximal limbs, and ears but may proliferate virtually anywhere on the body. The bacterium prefers moist areas and requires the integrity (i.e., barrier) of the skin to be impaired. Thus damaged skin with scabs and crusts on the face and ears are sites of colonization by D. congolensis in younger animals. D. congolensis is transferred during nursing from damp and traumatized skin of the inguinal region of lactating dams to the ears of nursing kids (Fig. 20-34). Colonization begins by invasion of flagellated zoospores that penetrate the epidermis and reach the level of the basement membrane in which they transform to a filamentous morphology. They also penetrate into the dermis and follicular adnexa, inciting a prominent neutrophilic response. This acute inflammation halts further invasion. However, residual bacteria colonies are able to invade nascent, regenerated epidermis. This cycle of bacterial growth, inflammation, and epidermal regeneration leads to the multi-laminated pustular crusts so typical of dermatophilosis. Damage to the cutaneous barrier system can also be caused by other invasive bacteria, fungi, or ectoparasites such as mites and ticks. Macroscopically, lesions are characterized by a crusting and exudative dermatitis (see Chapter 17) with thick crusts covering an epidermis that is ulcerated and hemorrhagic. Microscopically, crusts are made of alternating layers of markedly parakeratotic hyperkeratosis, degenerate neutrophils, and coagulative necrosis all laden with Dermatophilus congolensis. It can be identified by its filamentous morphology with longitudinal and transverse septa (see Fig. 17-48).
Fig. 20-34 Cutaneous dermatophilosis, goat.
The auricular skin, as well as skin of the muzzle, face, and periocular region, is matted from a severely exudative dermatitis caused by Dermatophilus congolensis. (Courtesy Dr. K.G. Thompson, Institute of Veterinary, Animal, and Biomedical Science, Massey University.)
Neoplasms Of The External Ear
Most frequently, aural neoplasia is a unilateral disease; bilateral involvement is rare. Sebaceous gland tumors, histiocytomas, plasmacytomas, and mast cell tumors (see Chapter 6) are the most common types of aural neoplasms in dogs. Sebaceous gland tumors are most often benign, but diagnoses of aural masses resembling sebaceous glands include sebaceous gland hyperplasia, sebaceous adenoma, sebaceous epithelioma, and sebaceous adenocarcinoma. Differentiating between histiocytomas, plasmacytomas, and mast cell tumors requires a surgical biopsy (see Chapter 17) and histologic examination.
In cats, basal cell tumors, vascular tumors, and squamous cell carcinomas are the most common types of aural neoplasms. Vascular neoplasms and squamous cell carcinomas arise on lightly pigmented pinna of outdoor cats exposed to the sun for prolonger periods of time as a result of UVB light-induced neoplastic transformation (see Chapter 6). With squamous cell carcinomas, the margins at the base and tips of the ears are most often affected (Fig. 20-35; also see Table 17-3). However, they can also arise within the external acoustic meatus and the tympanic cavity and bulla. Macroscopically, they are raised, ulcerated, and hemorrhagic neoplasms. Growth is often a combination of local stromal invasion and concurrent vascular invasion with metastasis via regional lymphatic vessels. Initially, these tumors can be misdiagnosed as exudative dermatitic lesions.
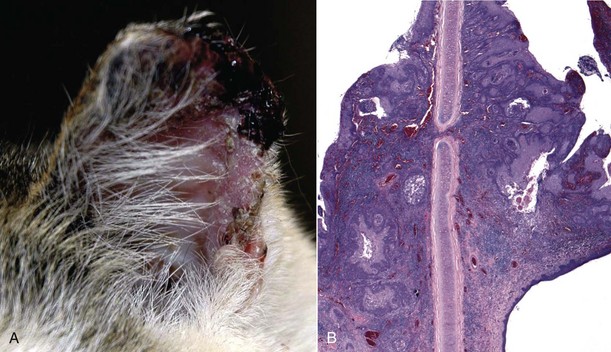
Fig. 20-35 Aural squamous cell carcinoma, cat.
A, Lateral view. The lateral margin and tip of the left auricle are ulcerated and covered by a serohemorrhagic crust caused by an underlying squamous cell carcinoma. B, Histologic section of aural squamous cell carcinoma. Note the cords and islands of anaplastic squamous epithelial cells that have infiltrated the dermis. The tumor may penetrate through the auricular cartilage as it grows. H&E stain. (A courtesy Dr. W.H. Miller, College of Veterinary Medicine, Cornell University; B courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Neoplasms of the external acoustic meatus in the dog or cat are infrequently diagnosed but the most common types include ceruminous gland tumors, sebaceous tumors, and epithelial neoplasms of undetermined origin. In fact, the majority (85%) of feline aural neoplasms are malignant compared to 60% of canine aural neoplasms being malignant. Of these, ceruminous gland adenocarcinomas are the most common neoplasm diagnosed in the external acoustic meatus of both dogs and cats. Typically, middle-aged to older animals are affected.
Canine ceruminous gland adenomas are more commonly diagnosed than adenocarcinomas. By comparison, adenomas are multiple, small, pedunculate, irregular, and firm masses. These masses may develop secondarily to recurrent bouts of chronic otitis externa. Some speculate that the chemical mediators of chronic inflammation and products of cerumen decomposition are carcinogenic and contribute to dysplasia and neoplastic transformation. Ceruminous gland adenocarcinomas tend to be locally invasive and expansile (Fig. 20-36, A). There is a tendency for metastasis to regional lymph nodes, lungs, and systemic viscera.
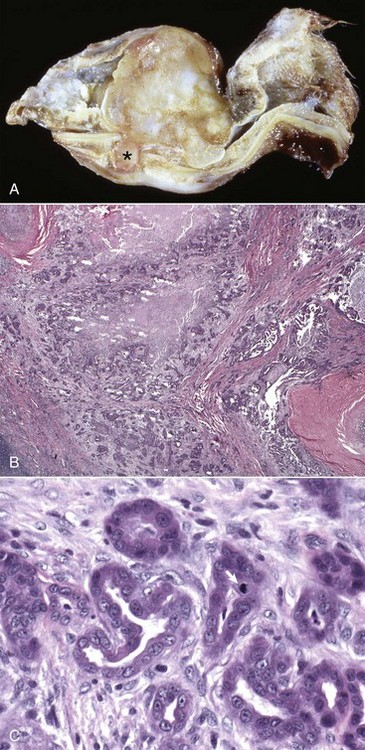
Fig. 20-36 Ceruminous gland adenocarcinoma.
A, Cross-section of external acoustic meatus. This ceruminous gland adenocarcinoma fills the external acoustic meatus and expands its luminal diameter. Ventrally, there is a small extension of the neoplasm (asterisk) either through or between overlapping layers of auricular and annular cartilage. B, Histology of ceruminous gland carcinoma. Neoplastic ceruminous glands induce a prominent desmoplastic response and are shown invading through the cartilage (bottom center of image). H&E stain. C, Higher magnification of B. The neoplasm has haphazardly arranged and formed tubules and acini with evidence of anaplastic and mitotically active cells (arrow). It is shown growing through the auricular cartilage. H&E stain. (A courtesy Dr. W.N. Evering, Pfizer Global Research; B and C courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
By contrast, ceruminous gland adenocarcinomas are much more frequently diagnosed in cats than adenomas and account for up to 2% of all feline neoplasms. They tend to be locally invasive and up to 50% are reported to metastasize to regional lymph nodes, lungs, or systemic viscera. There is a greater tendency for adenocarcinomas to be diagnosed in aged male cats.
These neoplasms are readily capable of growing between overlapping areas of auricular cartilage extending into the periauricular dermis and subcutis (Fig. 20-36, B). Histologically, the neoplastic cells tend to form acini, small ducts, irregular tubular structures, or small clusters (Fig. 20-36, C). Neoplastic cells are typically basaloid to low columnar with intensely basophilic cytoplasm. Variation in nuclear and cytoplasmic size is variable, but neoplastic cells may be quite uniform. The occurrence and number of mitotic figures are highly variable. There tends to be a very prominent desmoplastic response to the surrounding tissue. Large areas of necrosis are not uncommon.
Miscellaneous Disorders Of The External Ear
Pinnal Alopecia: Pinnal alopecia occurs as a congenital disease in cattle or an acquired disease in dogs and cats. In Polled Hereford calves, this congenital disease begins at birth as an alopecic disease of the muzzle, auricular margins, and base of the ears. Pedigree analysis suggests this disorder is an inherited disease but the mode of inheritance remains undermined. A detailed analysis of this condition consistently found evidence of epidermal maturation and keratinization defects. At birth, the hair coat was wiry and tightly curled; it epilated easily. Alopecia and hyperkeratosis became generalized by 3 months of age. Marked wrinkling of the skin developed over the face and neck as the cattle aged. Microscopically, the density of hair follicles was normal, but a higher proportion of these follicles were in telogen phase. Additionally, there was premature keratinization and degeneration of the internal root sheath of the follicle, sebaceous gland atrophy, and dilated sweat glands that were lined with a thinned epithelium. This process resulted in easy epilation of hair. In older affected calves, the hyperkeratotic skin was also inflamed with a mild, superficial, perivascular, lymphocytic perivascular dermatitis. Interestingly, hyperkeratotic lesions also affected the rumen mucosa. Clinically, the anemia was characterized as nonregenerative, normocytic to macrocytic, and normochromic.
Acquired pinnal alopecia occurs primarily in dogs and cats (Box 20-4). The condition in dogs primarily affects dachshunds, but has also been reported in Chihuahuas, Boston terriers, whippets, and Italian greyhounds. Siamese cats are more commonly diagnosed with acquired pinnal alopecia that is patchy to complete but unlike the dog spontaneously resolves. In dogs, it is often symmetrical, and auricular hair of the convex surface becomes miniaturized. Then gradually over time, auricles become alopecic (Fig. 20-37). Rarely, the auricle becomes completely alopecic; however, the remainder of the body hair coat is typically unaffected. Biopsies show affected anagen hair follicles that are smaller diameter or shorter.
Fig. 20-37 Pinnal alopecia.
A, Dorsal view, cat. Alopecia is present as bilaterally symmetric lesions most severely affecting the convex surface of the auricles of the distal half of each ear (light pink areas). In the dog, this lesion tends to be permanent, whereas in the cat, typically Siamese breeds, this often spontaneously resolves. B, Auricle, convex surface, calf. Note the absence of hair on the convex surface of the ear. This case is an example of congenital pinnal alopecia in Polled Hereford calves. (A courtesy Dr. W.H. Miller, College of Veterinary Medicine, Cornell University. B courtesy Drs. W. Crowell and D.E. Tyler, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Developmental Anomalies Of The Middle Ear
Primary Ciliary Dyskinesia (Immotile Cilia Syndrome): Described most completely in the dog, primary ciliary dyskinesia (also known as immotile cilia syndrome) has also been suspected or confirmed in horses, ruminants, pigs, and cats. Typically, affected animals are under a year of age, have a persistent cough and nasal discharge related to chronic rhinitis, and have chronic recurrent respiratory disease related to impaired or ineffective mucociliary clearance without evidence of immunologic impairment.
In dogs, the most common ciliary defects include dynein arm deficiency, abnormal microtubular patterns, random orientation of the microtubules, and electron-dense inclusions in the basal body that anchors the cilium to the cell. In horses, the total number of cilia may be decreased and the vast majority have central microtubule pair defects. The middle ear relies in part on fully functioning ciliated epithelial cells and mucus-producing goblet cells for clearance of fluid and debris through the auditory tube. Thus abnormal cilia equates to abnormal auditory tube clearance.
Macroscopic lesions include bilateral mucopurulent rhinitis, sinusitis, and chronic tracheitis. There is frequently unilateral or bilateral otitis media evident with mucopurulent exudate or with sterile, gelatinous material filling the tympanic cavity. Tympanic bullae may be thickened or sclerotic. Microscopically, the tympanic cavity contains proteinaceous material mixed with variable numbers of inflammatory cells, typically neutrophils and macrophages with variable aggregates of lymphocytes and plasma cells.
Clinically, animals present with a chronic cough and persistent nasal discharge, and the symptoms are unresponsive to antibiotics. Typically, middle or internal ear signs are not evident clinically. Frequently, animals are neutrophilic, likely caused by persistent secondary bacterial infections of the upper respiratory tract. Affected males are frequently infertile.
Inflammation Of The Middle Ear
Otitis Media: Infections of the middle ear affect all domestic animal species but vary in prevalence and which pathogens are isolated. Ruminants and pigs are most severely affected, whereas cats are less commonly affected.
Otitis media in pigs occurs naturally as a result of nasopharyngeal ascent of bacteria through the auditory tubes. Pasteurella multocida, Arcanobacterium pyogenes, and Mycoplasma hyorhinis are likely pathogens, separately or concurrently, that first colonize the nasopharynx and ascend the auditory tube to gain entry and cause otitis media. Studies of the pathogenesis of otitis media in young piglets suggest that dysfunction of the auditory tube is an initial contributing factor. Dysfunction is linked to acute inflammation, goblet cell hyperplasia of mucosae and occasional acute inflammation of the tympanic cavity. In older pigs, otitis media was more severe and suppurative and auditory tube lesions were more severe and characterized by interepithelial infiltration by neutrophils, intraluminal exudation of neutrophils and fibrin, marked goblet cell hyperplasia, mucosal erosion to ulceration, and infiltration of the propria submucosa by lymphocytes and macrophages. Bacterial colonies of Pasteurella multocida and Arcanobacterium pyogenes were present in the exudate. Colonization of the tympanic cavity resulted in lysis of bone of the wall and of the osseous septa of tympanic bullae. In severe cases, lesions spread and caused otitis interna and acute fibrinous inflammation of the cochlea and vestibule, as well as extension into the leptomeninges and neuropil of the brain. Pigs older than 4 months more commonly had severe, chronic otitis media with extensive expansion as described above.
In ruminants, Histophilus somni, Pasteurella multocida, Arcanobacterium pyogenes, Mycoplasma bovis, and Streptococcus spp. have been isolated from animals with otitis media. Various mites (see previous discussion) and nematodes may also contribute to the occurrence and severity of disease. As in pigs, otitis media occurs as a result of nasopharyngeal ascent of bacteria through the auditory tubes. In natural infections, potential routes of initial exposure to these bacteria include congenital infection, ingestion of colostrum, ingestion of contaminated milk, exposure to infected vaginal secretions, or ingestion or inhalation of respiratory secretions leading to nasopharyngeal colonization. Other factors include concurrent bacterial or viral infections, nutritional status of the herd, level of contamination in the environment, and host immune status.
Macroscopically, lesions may be unilateral or bilateral. Mucosae lining tympanic bullae are congested and edematous (see Fig. 17-13). Bullae can be filled with fibrinopurulent to caseous exudate and the underlying mucosa is often ulcerated (Fig. 20-38). There may be remodeling of bony septa of the bullae or osteolysis, as well as osteolysis of auditory ossicles. With chronicity, the mucosa of the tympanic cavity and bullae becomes markedly thickened by fibrosis and granulation tissue. In severely affected animals, tympanic membranes and auditory ossicles are missing. Microscopic lesions reflect what is seen grossly, namely bony lysis of bullae septa, infiltration by mixtures of neutrophils and macrophages, abundant cellular debris, and variable numbers of bacteria. When examined, auditory ossicles have superficial erosion of bone. The epithelium of the tympanic cavity is highly variable, with areas lined by squamous epithelium, whereas others are lined by ciliated, pseudostratified columnar epithelium mixed with goblet cells. The latter mucosae often form small cystic cavities surrounded by exudate. The propria submucosa is infiltrated by variable numbers of lymphocytes, plasma cells, macrophages, and neutrophils.
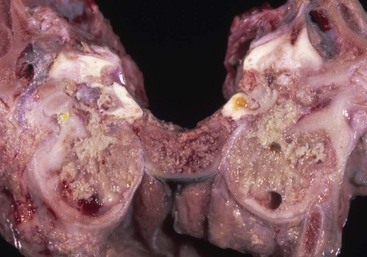
Fig. 20-38 Suppurative otitis media, rostral view, cow.
Bilaterally, tympanic cavities and bullae are filled with suppurative exudate. (Courtesy Dr. B.L. Njaa, College of Veterinary Medicine, Cornell University.)
Clinical signs frequently include facial nerve paralysis, head tilt, drooping ears, epiphora, and mucopurulent nasal discharge. Severe otitis media can result in tympanic membrane rupture leading to purulent otorrhea and otiasma. Rarely, infections spread to cause meningitis.
Otitis media is an uncommon disease in cats. Numerous bacteria have been isolated including E. coli, Enterobacter spp., Enterococcus spp., Streptococcus spp., β-hemolytic Streptococcus spp., Staphylococcus spp., Proteus spp., and Clostridium spp. Although more frequently a unilateral disease, bilateral disease is reported. Grossly, any tympanic bulla that contains fluid of any kind should be suspected as being otitis media. Tympanic cavity effusions may be translucent, gray mucoid, yellow-green, red-tinged, or hemorrhagic (Fig. 20-39, A). Flushing the effusion may allow visualization of ruptured tympanic membranes, bony proliferation of the tympanic bulla, or deformities to the auditory ossicles, when examined with the use of a dissecting microscope. Microscopically, there are abundant neutrophils, typically degenerate, mixed with macrophages, and variable numbers of lymphocytes and plasma cells (Fig. 20-39, B). Often, the pseudostratified, ciliated columnar epithelium of the tympanic bulla is undermined by the infiltrate and frequently forms smaller, gland-like structures. Areas of cholesterol cleft formation are common as are areas of hemorrhage. The amount of fibrosis within the tympanic cavity is variable and relates to the chronicity of the otitis media. Osseous proliferation of the tympanic bulla is seen more often than bony erosions. The earliest necropsy survey of cats submitted for routine postmortem examinations found that 9.1% had evidence of middle ear inflammation. A recent Journal of the American Veterinary Medical Association (JAVMA) study suggests a much lower prevalence; however, more importantly, the study confirmed that clinical disease caused by otitis media is rare when compared to the number of cats with gross evidence of disease. Therefore both bullae should be routinely opened when performing postmortem examinations of cats.
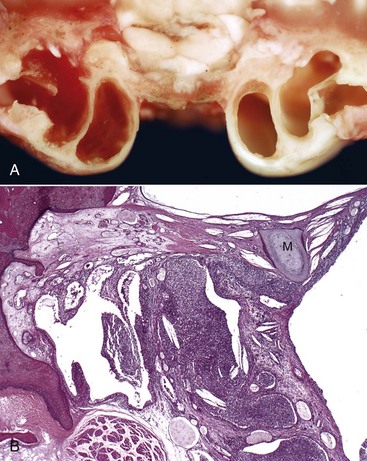
Fig. 20-39 Otitis media, cat.
A, Rostral view. Red-tinged, viscous fluid fills the tympanic compartments of the left ear. The right ear is normal with no luminal exudate. The septum bulla is complete and separates the tympanic cavity into the dorsolateral epitympanic cavity and the ventromedial tympanic cavity. B, Histologic section of middle ear. The tympanic cavity contains abundant suppurative exudate (center) mixed with numerous cholesterol clefts. The propria submucosa of the tympanic cavity is thickened by edematous fibrous connective tissue. Throughout are numerous cystic cavities lined by mucosa. The manubrium (M) of the malleus is embedded in a tympanic membrane that is thickened as a result of chronic inflammation (myringitis) with fibrosis and cholesterol cleft formation. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Nasopharyngeal Polyps (Inflammatory Polyps): Nasopharyngeal polyps are the most common, nonneoplastic, inflammatory masses affecting cats often under 2 years of age. They also occur much less frequently in dogs and have been reported once in a horse. A confirmed cause has not yet been determined, but proposed etiologies include chronic upper respiratory tract infections, otitis media, ascending middle ear infections via the auditory tube, or congenital defects such as aberrant growths from remnants of the branchial arches. As their name suggests, inflammatory polyps are pedunculated and polypoid, often with a smooth surface (Fig. 20-40). They may be confined to the middle ear, protrude through the auditory tube into the nasopharynx, or penetrate through a ruptured tympanic membrane into the external acoustic meatus. Histologically, inflammatory polyps have a fibrovascular core that is infiltrated with lymphocytes, plasma cells, and macrophages of varying numbers and are covered by epithelium. The overlying epithelium reflects the tissue of origin whether arising from the nasopharynx, auditory tube, tympanic bulla, or other portions of the tympanic cavity and varies from stratified squamous epithelium to pseudostratified ciliated columnar epithelium mixed with goblet cells (Fig. 20-41). Signs of disease vary, depending on the location of the polyps. Signs of otitis externa or otitis media may commonly indicate the presence of polyps. Other symptoms include nasal, otic, or ocular discharge; sneezing; dyspnea; stridor; voice change; dysphagia; head tilt; Horner’s syndrome; nystagmus; and ataxia. Much less commonly, obstructing polyps may lead to cyanosis and syncope.
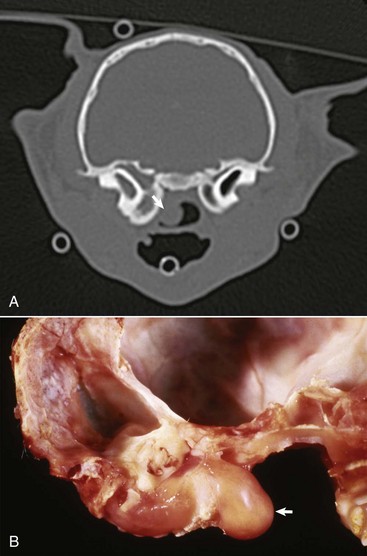
Fig. 20-40 Nasopharyngeal polyp, cat.
A, Computed tomography (CT) of tympanic bullae and pharynx. A small, central bulge of hypoechoic tissue (arrow) extends from the left tympanic cavity into the nasopharynx, presumably through the auditory tube. B, Cross-section through the left tympanic bulla and nasopharynx. A nasopharyngeal polyp (arrow) fills the tympanic bulla and extends through the auditory tube into the nasopharynx. The smooth, shiny surface indicates it is covered by epithelium. The inner ear is opened in this view, and the rostral, ventral spiral turns of the cochlea are present. (A courtesy Dr. N. Dykes, College of Veterinary Medicine, Cornell University; B courtesy Dr. J. Render, North American Science Associates.)
Fig. 20-41 Nasopharyngeal inflammatory polyps, cats.
A, Histologic section of the polyp depicted in Fig. 20-40, A. The mucosa covering the surface of this polyp is primarily hyperplastic stratified squamous epithelium but in some cases polyps may also by covered by ciliated columnar epithelium mixed with goblet cells. The lamina propria contains abundant chronic inflammatory cells. H&E stain. B, The lamina propria of this polyp is partially obscured by dense infiltrates of lymphocytes, plasma cells, and macrophages. The mucosa is composed of ciliated, columnar, pseudostratified epithelial cells mixed with goblet cells and is focally eroded or ulcerated. H&E stain. (A courtesy Dr. D. Schafer, College of Veterinary Medicine, Cornell University; B courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Inflammatory polyps reported in dogs and horses occur much less frequently. Based on published accounts, the age of onset is typically much older. In the few reports of inflammatory polyps in dogs, they primarily occur in male dogs. Macroscopic and histologic features as well as clinical signs are virtually identical to what has been described in cats.
Neoplasms Of The Middle Ear
Neoplasms occur rarely in the middle ear. Squamous cell carcinomas have been diagnosed most frequently in dogs and cats. Neoplasms may originate within the middle ear or arise in the external ear canal and penetrate through the tympanic membrane into the tympanic cavity. Neoplastic growth tends to be expansile and infiltrative with evidence of bony lysis of bullae, lysis of the petrous portion of the temporal bone, or intracranial invasion. Local damage to the facial nerve or vestibulocochlear nerve may lead to vestibular signs, facial nerve paralysis, or Horner’s syndrome. In both dogs and cats, pain on opening the mouth is very common.
Inflammation Of The Internal Ear
Otitis Interna (Labyrinthitis): Otitis interna results from extension of otitis media. It may occur with or without osteomyelitis of the petrous portion of the temporal bone. With time and severity, lesions progress retrograde through the internal acoustic meatus into the cranial cavity, resulting in meningitis, ventriculitis, and encephalitis. Macroscopically, there is typically a middle ear exudate that varies from serosanguineous to suppurative to granulomatous. Microscopically, the inflammatory infiltrate affecting the middle ear is typically composed of neutrophils, macrophages, lymphocytes, and plasma cells. Within the membranous labyrinth, smaller numbers of neutrophils mixed with fibrin can be found in the perilymph (Fig. 20-42). Fewer lymphocytes and plasma cells may infiltrate the lamina propria of the internal ear mucosa. The most plausible portal of entry is through the membranous covering of the round or cochlear window (see Fig. 20-21).
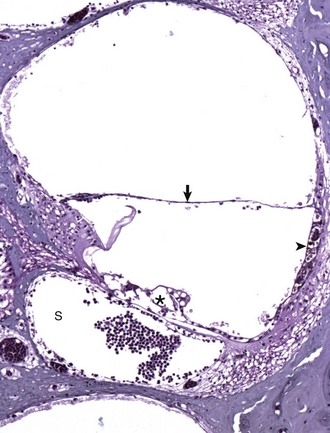
Fig. 20-42 Otitis interna, guinea pig.
The ventral compartment known as the scala tympani (S) contains a large luminal aggregate of inflammatory cells, mainly heterophils (neutrophils). Fewer inflammatory cells are present in the cochlear duct and scala vestibuli. The vestibular membrane (arrow) is intact. The tectorial membrane is artifactually separated from the organ of Corti that is also presumably artifactually distorted (asterisk). Stria vascularis (arrowhead). See Fig. 20-21 for a diagram of the structure. H&E stain. (Courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University).
Vestibular Disease Of The Internal Ear
Injury to any portion of the vestibular system (see the previous section on the Vestibular System) leads to a condition collectively referred to as vestibular disease. Although the lesion may be located peripherally (internal ear sensory receptors, vestibular ganglia, or peripheral axons of cranial nerve [CN] VIII) or centrally (vestibular nuclei of the medulla, vestibular projections to the rostral brainstem, cerebellum, or spinal cord), affected animals typically exhibit a head tilt, nystagmus, asymmetric ataxia, circling, and variable facial paralysis.
Congenital vestibular disease has been reported to occur in certain breeds of dogs (Doberman pinschers, German shepherd dogs, Cocker Spaniels, Beagles, or Akitas) and more commonly in oriental breeds of cats (Siamese, Tonkinese, or Burmese). The cause of this disease is not well understood largely because most affected animals recover spontaneously or compensate within the first few weeks of life. Gross lesions are typically absent. As a result of the clinical course, histologic lesions are usually not reported. However in one report, a small number of Doberman pinscher puppies had evidence of intense lymphocytic infiltration of the middle and internal ear propria submucosa. The most common clinical sign is a head tilt with varying degrees of ataxia. Nystagmus is reported but a less prominent feature. Deafness has been reported in some cases.
Concurrent otitis media and otitis interna is the most common cause of peripheral vestibular disease. Otitis media in isolation will not result in vestibular disease, but if deficits are detected that are compatible with peripheral vestibular disease, concurrent internal ear involvement is confirmed. Causes include (1) viral infections such as those caused by canine distemper virus and feline infectious peritonitis virus and (2) various bacterial, rickettsial (Rocky Mountain spotted fever, ehrlichiosis, bartonellosis), protozoal (toxoplasmosis, neosporosis), and mycotic (cryptococcosis, blastomycosis, histoplasmosis, coccidiomycosis) infections. A small group of affected horses had concurrent facial nerve paralysis as well. Refer to previous discussions of gross and histologic features of otitis media and otitis interna.
Idiopathic peripheral vestibular disease is considered the second most common form of this disease diagnosed in dogs and cats. Dogs are typically geriatric at the time of diagnosis. In cats, age is less important, but most cases are diagnosed in the summer and fall months in the northeastern and mid-Atlantic regions of the United States. A cause has not been determined and specific lesions have not been identified. One key clinical feature in making this diagnosis is the presence of peripheral vestibular disease signs in the absence of concurrent facial nerve paralysis or Horner’s syndrome.
Aural or intracranial neoplasia may arise within, compress on, or infiltrate into the labyrinthine or neural portions of the vestibular system. Vestibular neurofibromas or schwannomas may primarily arise within the vestibulocochlear nerve (see Figs. 14-114 and 14-115. Compression or invasion of soft tissues of the head and ear, the skull, brainstem, or cerebellum can result in significant vestibular deficits. Based on diagnostic imaging, lytic lesions of the tympanic bullae or petrous portions of the temporal bone are more often associated with aural neoplasia than with otitis media or otitis interna. Signs associated with intracranial neoplasia depend more on the location of the neoplasm versus the kind of neoplasm. Grossly, the neoplasm may arise within or infiltrate a portion of the central vestibular system. Alternatively, the mass may cause obstructive hydrocephalus or brain herniation, resulting in more widespread neurologic deficits.
Various therapeutic agents can cause vestibular disease. Ototoxic agents, such as aminoglycoside antimicrobials, furosemide, platinum-containing antineoplastic agents, and salicylates, are a few categories of chemicals that cause injury to or death of the neurosensory hair cells of the membranous labyrinth. In most instances, deafness that develops is permanent, whereas vestibular disease from other nontoxic causes may improve or completely resolve with time. Metronidazole is an antimicrobial agent capable of causing neurotoxicity with resultant vestibular disease. The exact mechanism is not fully known but theorized to be modulated by γ-aminobutyric acid (GABA) receptors in the vestibulocerebellum and may result in axonal degeneration. The signs typically resolve on cessation of therapy. There is very little published documenting lesions associated with metronidazole therapy.
Hearing Loss and Deafness: Hearing loss and deafness can be congenital or acquired, and each type further categorized as conduction or neurosensory deafness. Deafness in animals with a patent external acoustic meatus is likely congenital in origin. Audiometrics first detect sound perception in kittens 5 days old and puppies 14 days old. Animals that are known to hear normally that subsequently undergo hearing loss have acquired deafness. A patent external acoustic meatus, intact tympanic membrane, functioning auditory ossicles, and normal perilymph and endolymph of the cochlea define the conduction portion of hearing and thus any damage or loss of these structures leads to conduction deafness. The neurosensory contribution to hearing includes the membranes and neurosensory cells of the membranous labyrinth and associated neural connections. Any interference with the neural pathways or injury to the mechanosensory hair cells leads to neurosensory deafness.
Causes of conduction hearing loss include (1) external acoustic meatus stenosis that may be congenital or acquired secondary to otitis externa, (2) chronic otitis externa leading to narrowing of the external acoustic meatus or tympanic membrane rupture and impaired sound transmission, (3) auditory ossicle damage or tympanic membrane injury from otitis media, and (4) damage from neoplasms affecting any portion of the conduction system. Repair or removal of the cause of the conduction deafness can result in return to normal function and hearing.
Congenital inherited sensorineural deafness is a common disorder, especially in dogs and cats. Alterations in the function of microphthalmia-associated transcription factor (MITF) occurs in Dalmatian dogs with congenital sensorineural deafness and blue iris color. The merle color pattern is a pigment dilution related to variable expression of the merle allele of the pigment locus mouse Silver pigment locus homolog (SILV). Dogs homozygous for the dominant merle allele are significantly more likely to be deaf than heterozygotes. The piebald gene, which results in a coat color that alternates between white and darkly pigmented, also has been linked with deafness. Both the piebald and merle genes cause the blue eye phenotype by suppressing melanocytes in the iris and deafness by suppressing melanocytes in the stria vascularis of the cochlear duct.
Congenital sensorineural deafness has been classified into two major categories based on mechanism: albinotic and abiotrophic. The albinotic form or Scheibe deformity is also known as cochleosaccular degeneration. It is associated with hypopigmentation and stria vascularis dysfunction. Deafness due to stria vascularis dysfunction is related to lack of melanocytes. Endolymph is produced and maintained by the stria vascularis, but in the absence of melanocytes, this function is inhibited. Abnormal endolymph leads to degeneration of hair cells of the cochlea characterized by atrophy of stria vascularis, collapse of the cochlear duct, degeneration of the organ of Corti, abnormal tectorial membrane, delayed spiral ganglion degeneration, and collapse of the saccules (Fig. 20-43). Deafness may be unilateral or bilateral with up to 50% of white-coated, blue-eyed cats affected. Dalmatian dogs have a 30% incidence of the albinotic form, which is inherited as an autosomal dominant gene.
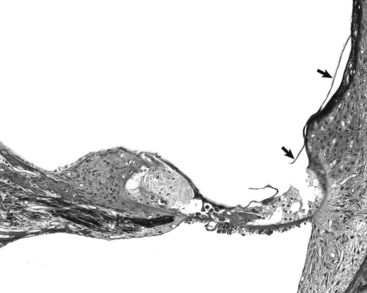
Fig. 20-43 Albinotic or Scheibe-type deafness, cat.
The vestibular membrane (arrows) is ruptured and collapsed onto the organ of Corti (asterisk) and the tectorial membrane is difficult to discern in the section. The scala media or cochlear duct is obliterated. See Fig. 20-21 for a diagram of the structure. Thick tissue section, toluidine blue stain. (Courtesy Dr. D.A. Ryugo; from Ryugo DA, Cahill HB, Rose LS, et al: Hear Res 181:73-84, 2003.)
The abiotrophic form is caused by abiotrophy of sensory hair cells (neuroepithelial) with subsequent atrophy of the organ of Corti, yet the cochlear duct and stria vascularis are normal. Typically a bilateral condition, hair cells of the vestibular system and cochlear system are affected. This form of deafness is a progressive condition, most often recognized at a later age. This mechanism may be the cause of deafness in cavalier King Charles spaniels, which typically is not recognized until 3 to 4 years of age. Although much is known clinically and functionally about this disease, because of the need for rapid fixation and decalcification of affected tissues, little is known about the pathogenesis or histopathology of this form.
Acquired sensorineural deafness most commonly occurs secondary to otitis media. Inflammation extends through the round or cochlear window into the membranous labyrinth. Inflammation causes damage to the sensorineural hair cells resulting in both vestibular signs and deafness. Less commonly, neoplasia involving the petrous portion of the temporal bone and associated nerves can cause deafness via compression.
Presbycusis refers to age-associated deafness that occurs as an animal ages. The pathogenesis of presbycusis may relate to arthrosis of the auditory ossicle articulations or more likely to a late form of abiotrophy of the sensory hair cells of the cochlea. Thus it represents an acquired form of deafness that may be the result of conduction or neurosensory abnormalities. Histologic studies have demonstrated profound hair cell loss and neuronal loss, most prominent in the basal coils of the cochlea. This is comparable with presbycusis in people.
Acoustic noise trauma is a leading cause of deafness in people. The injury is primarily focused on the organ of Corti. Tears form in the supporting structures of the organ of Corti, causing release of hair cells into the endolymph and vascular injury resulting in bleeding into the compartments of the cochlea. Several weeks after the initial injury, the organ of Corti is completely degenerated caused initially by outer hair cell loss followed by inner hair cell loss. There is also a retrograde degeneration of the associated nerve tracts.
Ototoxicity is another means of acquired deafness. Many compounds are known to be ototoxic, including aminoglycoside antimicrobials, platinum-containing chemotherapeutic agents, furosemide, salicylates, and otic cleansers. To exert toxicity, ototoxins much reach the internal ear. This process may occur via hematogenous spread, enhanced diffusion through a tympanic membrane under the influence of otitis media, or through a perforated tympanic membrane. Once in the tympanic cavity, ototoxic compounds gain access to the internal ear by diffusing through the round window. The target of ototoxicity is the hair cells. As has already been discussed, hair cells of the vestibular and cochlear systems succumb and become injured or die from apoptosis. Deafness tends to be permanent, whereas vestibular disease is more transient.
Disorders Of Horses
Dentigerous Cysts (Temporal Odontomatas, Periauricular Cysts): Dentigerous cysts (also known as temporal odontomatas, temporal teratomas, temporal cysts, ear or aural fistulas, ear teeth, and heterotopic polydontia) are rare, nonheritable, congenital cysts that occur rostral and ventral to the base of the ear pinna (Fig. 20-44). They also occur in the forehead, paranasal sinuses, and petrous portion of the temporal bone. Typically flask-shaped due to a long narrow fistula that drains to the surface, dentigerous cysts are most often unilateral arising in the temporal region as the result of malpositioned tooth stem cells from the first branchial arch that are displaced within the first branchial cleft. Microscopically, the fistula and cavity are lined by stratified squamous epithelium and teeth of varied stages of differentiation composed of dentin, cementum, and enamel. Teeth are loosely or firmly attached to underlying temporal or parietal bones of the skull. Salivary gland tissue may also be present. When dentigerous cysts do not contain teeth or dental structures, they are called dermoid cysts. In sheep, dentigerous cysts occur in the mandibular incisor region and are called ovine odontogenic cysts.
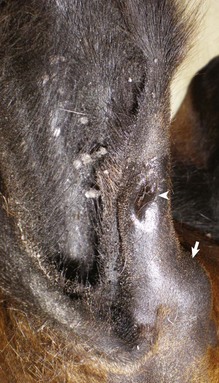
Fig. 20-44 Dentigerous cyst, right auricle, horse.
Within the craniomedial edge of the right auricle is a firm mass that represents a dentigerous cyst (arrow). Along the craniodorsal edge of this mass is an external opening to a fistulous tract (arrowhead). Scattered over the concave surface of the auricle are several pale, aural plaques (see Fig. 20-46). (Courtesy Dr. P. Fretz, The Western College of Veterinary Medicine, University of Saskatchewan.)
Miscellaneous Disorders
Auricular Chondrosis: Auricular chondrosis is a condition in horses with similarities to auricular chondritis (see the section on Disorders of Dogs and Disorders of Cats) but with one main difference and that is a lack of inflammation and chondritis. A cause for this condition has not been determined. The lack of inflammation and lack of response to corticosteroids in the lone case report indicate the pathogenesis is likely distinct from auricular chondritis. Macroscopically, lesions were characterized by nodular thickenings of both pinnae that ranged from 3 to 8 mm in diameter palpable below the surface with no apparent involvement of the overlying epidermis. With the exception of mild parakeratotic hyperkeratosis, microscopic lesions were primarily confined to the auricular cartilage. Cartilage plates were markedly thickened with central areas that lacked chondrocytes and had areas of degeneration and necrosis. Other regions contained adipocytes and were invaded by small blood vessels. Along the margins of the damaged cartilage were areas of nascent cartilage formation with macrocytic chondrocytes and deeply basophilic matrix present at the interface. However, there was no evidence of infiltration of the cartilage or peripheral soft tissue by inflammatory cells. In the lone reported case, nodules were nonpainful and persistent.
Temporohyoid Osteoarthropathy: The temporohyoid joint is a permanent synchondrosis connecting the proximal stylohyoid bone to the petrous portion of the temporal bone via tympanohyoid cartilage. This articulation is in very close proximity to the middle and inner ear. A complete understanding of normal joint movement is lacking but the temporohyoid joint is believed to function by dampening movement of the hyoid apparatus during tongue movement. Temporohyoid osteoarthropathy is a bony proliferative disease of the temporohyoid articulation that may result from (1) infectious agents inciting the disease by local extension of otitis media/interna, hematogenous spread, ascending infection from the respiratory tract, or extension from guttural pouch disease or (2) premature degenerative joint disease of the temporohyoid joint.
Infectious Microorganisms: Otitis media causes ventral osteitis of the tympanic cavity and because of proximity to the temporohyoid joint, osteoarthritis of the joint ensues by extension of inflammation. Osteomyelitis and periosteal proliferation of bone results in ankylosis of the temporohyoid joint and fusion of the stylohyoid bone to the petrous portion of the temporal bone (Fig. 20-45). Additionally, the bony portion of the external acoustic meatus is also in close apposition and may become narrowed as a result of exostosis and osteoarthritis. Infectious agents may also affect this region via other routes leading to similar lesions.
Premature Degenerative Joint Disease: Thickening of the proximal stylohyoid and ankylosis of the temporohyoid joint can result from degenerative joint disease. In a recent study, age-related, bony remodeling changes were documented in the temporohyoid joint, including (1) club-shaped development to the proximal stylohyoid bone; (2) rounding of the synostosis with the petrous portion of the temporal bone; and (3) extension of osteophytes from the petrous portion of the temporal bone, typically enveloping the stylohyoid head and in some cases bridging the joint. These proliferative changes were typically observed bilaterally but were never as severe as is seen in temporohyoid osteoarthropathy. Histologic changes were most pronounced in the oldest horses in the study. There was marked disorganization of the joint with marked irregularity in the depth of the joint fibrocartilage, a variation of lipping to fragmentation of the petrous portion of the temporal bone along the joint margin, markedly irregular chondro-osseous junctions, extensive fibrous replacement of periarticular bone, marked periosteal thickening, and marked clustering of chondrocytes with heterogeneity of the surrounding and intervening matrix. There were no areas of active inflammation in any of the examined sections.
Clinical disease is often categorized into two syndromes. In the first scenario, horses demonstrate abnormal behavior, such as shaking their heads, ear rubbing, and problems chewing, and resent having a bit in their mouths. The second syndrome is related to a fracture through the ankylosed joint leading to signs attributable to acute vestibular or facial nerve injury. Rarely, affected horses may develop meningitis.
Aural Plaques (Aural Papillomatosis): Aural plaques (also known as equine ear papillomas, papillary acanthoma, hyperplastic dermatitis of the ear, or “ear fungus”) occur in horses over 1 year of age and are thought to be caused by papilloma virus spread between horses by fly bites. Macroscopically, lesions are characterized as raised, well-demarcated, hypopigmented, hyperkeratotic plaques arising from the inner concave surface of the auricle (Fig. 20-46, A). Plaques are typically 1 to 3 mm in diameter but can overlap to involve larger areas. Microscopically, the epidermis is moderately hyperplastic with a stratum corneum that is variably hyperkeratotic. The granulosis layer tends to be quite prominent. Scattered through the affected epidermis are singlets or clusters of koilocytes. Basal epithelial cells tend to be more poorly pigmented when compared to peripheral, more normal epidermis (Fig. 20-46, B). Various molecular techniques have been used to demonstrate papillomavirus in the epidermis of the plaques. Clinically, aural plaques rarely resolve spontaneously, but they are usually of little clinical significance unless infected by bacteria secondary to trauma.
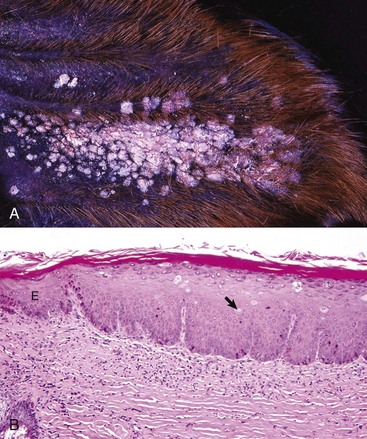
Fig. 20-46 Aural plaques, horse.
A, Concave surface of right auricle. Multiple, hypopigmented, exophytic gray-to-white masses cover the central portion of the auricle. Often, these plaques coalesce to form a large mass. B, Histologic section of an aural plaque. The stratified squamous epithelium is hyperplastic. When compared to the adjacent more normal and basally pigmented epithelium (E), the basal epithelial cells are hypopigmented. Scattered through the sessile mass are koilocytes (arrow). H&E stain. (A courtesy Dr. Rob Fairley, The Western College of Veterinary Medicine, University of Saskatchewan; B courtesy Dr. Rob Bildfell, College of Veterinary Medicine, Oregon State University.)
Guttural Pouch Disease: See Chapters 9 and 17.
Disorders Of Ruminants (Cattle, Sheep, And Goats)
β-Mannosidosis of Cattle and Goats: β-Mannosidosis is an autosomal recessive lysosomal storage disease caused by a deficiency of glucohydrolase β-d-mannosidase leading to the accumulation of oligosaccharide substrates of β-mannosidase in lysosomes of multiple cell types located in nervous, renal, thyroid, and lymphoid tissues. This disease was first described in Nubian kids and has subsequently been reported in Salers calves. Newborn animals were unable to rise and had dome-shaped heads, intention tremors, nystagmus, and bilateral Horner’s syndrome. Macroscopically, affected animals have bilaterally kinked and folded auricles that are most severe in Nubian kids but less severe in Salers calves. Additionally, Nubian kids have sensorineural deafness, Salers calves have normal auditory function. In both species, there is narrowing of the cartilaginous portions of the external acoustic meatus. Tympanic bullae are normal in the goats but smaller in the calves. Mucosa of the middle ear of the goats forms prominent polypoid projections, thus reducing the volume of the tympanic cavity.
Microscopically in both species, prominent intracytoplasmic vacuoles (lysosomes, see Chapter 14) are present in cells of the cochlear duct, including cochlear hair cells, supportive cells of the organ of Corti, cells of the stria vascularis, mesothelial cells covering the scala tympani, neurons of the spiral ganglion, cells of Reissner’s membrane, endothelial cells, and fibroblasts.
Parasitic Diseases
Stephanofilarial Otitis: Stephanofilarial otitis, commonly referred to as ear-sore, occurs in cattle and buffalo and is caused by Stephanofilaria zaheeri. Biting flies have been implicated in the transmission of the parasite to the auricle and infestation is painful. Macroscopic lesions are most evident on the concave surface of the auricle and vary from congestion to inflammation with hemorrhage, to severe crusting (parakeratosis) if chronic, and to alopecia with depigmentation. Microscopically, microfilaria are present in the auricular epidermis and dermis along with lymphocytes, macrophages, hyperplastic or degenerate sebaceous glands, and hemorrhage. Microfilaria in the dermis are usually dead and located in areas that are infiltrated by macrophages, eosinophils, and plasma cells. It has been hypothesized that this lesion may represent a form of immune-mediated response. Chronic cases of “ear-sore” can result in dysplastic or neoplastic transformation of cells of the epidermis likely linked to excessive cellular mitoses and mutations of genes that arise in rapidly dividing somatic cells (see Chapters 1 and 6).
Rhabditis Species Otitis: A free-living, saprophytic, rhabditiform-nematode of the genus Rhabditis is a cause of otitis externa in cattle. Concurrent infections with the ear mite Raillietina auris and the yeast Malassezia spp. are also common. Rhabditis spp. occur in tropical and subtropical climate zones that occur in Africa and some regions of Brazil. Rhabditis spp. infect and proliferate in the external acoustic meatus. Infested cattle may be asymptomatic or may have depression, otorrhea, otitis media and interna, cranial nerve paralysis, meningitis, circling, recumbency, and death. Elevated environmental temperature and high humidity are primary risk factors that contribute to the occurrence of this disease; additional risk factors include the breed, presence of horns, irritation or injury of the auricular skin caused by certain types of insecticide dips, and age. Examples of risk factors are (1) purebred and crossbred Bos indicus cattle, such as Gyr, have long, pendulous auricles that provide a more favorable environment for infection; (2) in Bos indicus cattle breeds, horns are thought to compress the external acoustic meatus and thus increase their susceptibility to infection; (3) cattle “dip-treated” with an acaricide had greater infestations with Rhabditis spp. compared to cattle treated by a spray method; and (4) because older cattle accumulate more organic matter in their external acoustic meatuses, it is thought that this outcome provides a better environment for infestation with Rhabditis spp.
Neoplasms
Aural Melanomas of Angora Goats: UV radiation (see Chapter 6) plays a significant role in the induction of malignant melanomas on the dorsal surface of the auricle of Angora goats (Fig. 20-47); a less common site is the base of the horns and coronary band of the hooves. Macroscopically, this neoplasm is characterized by single or multiple black nodules either superficially or subcutaneously. Aural melanomas are highly aggressive and spread initially by local invasion to areas, such as the frontal sinuses, followed by rapid and widely disseminated metastases to regional lymph nodes and other organ systems, such as the liver. Microscopically, these neoplastic cells are polygonal to spindle shaped, typically heavily pigmented, and moderately pleomorphic. Mitotic figures can be numerous. Typically, neoplastic cells stain strongly for melanin A. One report determined strong expression of p53 in neoplastic cells but its significance remains undetermined (see Chapter 6).

Fig. 20-47 Auricular melanomas, white Angora goat.
Two large exophytic, dark black, ulcerated melanomas are growing from the convex surface of the auricle. Closer inspection reveals several smaller melanomas. These neoplasms are highly malignant and are believed to be caused by ultraviolet (UV) irradiation. (Courtesy Dr. K.G. Thompson, Institute of Veterinary, Animal & Biomedical Sciences, Massey University.)
Disorders Of Pigs
Aural Chewing (Ear Chewing or Ear Cannibalism): Aural chewing (also known as ear chewing or ear cannibalism) appears to be linked to the practice of intensified confinement rearing systems and docking of tails. High population density, heightened competition for food, low protein or inadequate nutrition, boredom, and inadequate microclimatic conditions probably increase the stress on and irritability of piglets, resulting in restlessness and aberrant behaviors. It is likely that tail docking simply redirects the focus of such behavior to the ears, as they represent two protruding and readily assessable objects. Eventually, piglets begin to suck or chew the ears of penmates until they become reddened and ulcerated. The appearance of these lesions and the taste of the serum and blood likely attract additional piglets to suck or chew the ears, thus worsening the lesions. At this stage, the affected piglets experience intense pain. Macroscopically, lesions are typically bilateral most often affecting the ventral portions of the ears and are characterized by torn and traumatized auricular edges with crusting. The remainder of the auricle is typically red and thickened. Repeated trauma may lead to localized intradermal or intrachondral abscess formation. It may also represent a nidus of infection that disseminates systemically as a septicemia such as Streptococcus spp. Microscopically, lesions are consistent with those tissue changes described in acute inflammation (see Chapter 3).
Ear Necrosis (Necrotic Ear Syndrome and Ulcerative Spirochetosis of the Ear): Ear necrosis, which is also known as necrotic ear syndrome and ulcerative spirochetosis of the ear, occurs in 6- to 9-week-old piglets and is thought to be caused by a spirochetal bacterium of the genus Treponema that is transmitted between animals through broken skin caused by biting of ears. Macroscopically, lesions appear as ulcerated, hemorrhagic, and crusted areas along the lower margin of the auricle near the base of the ear. When severe, the entire ear margin may be affected. The dermis and subcutis beneath the crusts are typically thickened due to edema and active hyperemia and vary in color from gray-black to red. Microscopically, thick serocellular crusts often containing cocci or coccobacilli cover the ulcerated epidermis that also contains large numbers of neutrophils mixed with cellular debris (acute inflammation). Vasculitis is evident in both arterioles and venules of the deeper dermis with fibrinoid degeneration, medial hyperplasia, and thrombosis. Adjacent, nonulcerated epidermis is hyperplastic with prominent, deeply invaginating epidermal pegs (reparative response). The Warthin-Starry stain (silver impregnation methodology) has been used to identify spirochetes in the junction between necrotic and healing tissues, as well as in the deeper dermis that has vasculitis, edema, and hemorrhage.
Disorders Of Dogs
Auricular Parasitic Dermatitis: Stomoxys calcitrans: The adult stable fly, Stomoxys calcitrans, is responsible for a hemorrhagic, crusting dermatitis in dogs. Although infestations may also affect the face, these stable flies have a tendency to feed at the ear tips of dogs with erect ears or along ear margins of pendulous ears. Affected ears are typically red and alopecic with dark red, crusted hemorrhage over the surface. Chronically, ears may become very thickened and firm owing to a granulomatous inflammatory response. Histologically, there is an intense perivascular to interstitial dermatitis with areas of necrosis and infiltration by numerous eosinophils, plasma cells, and macrophages. The overlying epidermis is irregularly acanthotic with compact orthokeratotic or parakeratotic hyperkeratosis. Chronically affected ears tend to have more dense infiltrates of macrophages mixed with lymphocytes, mast cells, eosinophils, and plasma cells that obscure normal adnexal architecture. There may be prominent lymphoid aggregates. Discrete granulomas may be present. Dogs typically experience intense pruritus and pain.
Inflammation
Otitis Externa, Media, and Interna: See the section on Disorders of Domestic Animals.
Canine Leproid Granuloma: Canine leproid granuloma is an uncommon mycobacterial disease affecting the subcutis and dermis of dogs. A causative agent has never been cultured but through molecular techniques the cause of canine leproid granuloma is a novel, slow-growing mycobacterium of the Mycobacterium simiae–related group. Thought to be an environmental saprophytic organism, lesions form as a result of dermal inoculation through traumatic wounds or arthropod vectors. Single to multiple, firm, painless nodules most frequently arise on the dorsal surface of the auricle at its base but may affect the auricular tip, the head, or distal forelimbs. The largest lesions, upward of 5 cm in diameter, are alopecic and frequently ulcerated.
Histologically, a multinodular to diffuse, pyogranulomatous infiltrate extends from the dermis into the subcutis (Fig. 20-48). Macrophages may be large with abundant cytoplasm and may form multinucleated giant cells. Neutrophils are intermixed with the macrophages or may aggregate into focal clusters. The occurrence and number of lymphocytes and plasma cells are highly variable, often scattered throughout, and more prominent along the deep edge of the mass. The morphology of the mycobacteria is not uniform and includes filamentous to bacilliform morphologies with or without beading, to coccoid. In contrast to rapidly growing mycobacteria of the Runyon group IV, which typically have discrete pyogranulomas centered on clear spaces that contain acid-fast positive bacteria, this morphology is absent in leproid granulomas. In addition, caseous exudation and mineralization are not features typical of leproid granulomas. This condition is a self-limiting disease that typically resolves spontaneously within 6 months of the initial diagnosis. Neither regional lymph node involvement nor systemic disease has ever been reported. Shorthaired dog breeds are predisposed to developing canine leproid granulomas with no sex predilection. Based on Australian studies, more than half of the cases were described in Boxers and Boxer crossbred dogs. In a much smaller North American study, German Shepherd dogs were overrepresented.
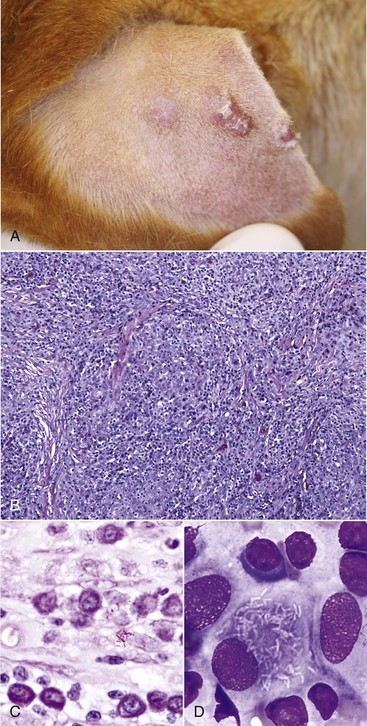
Fig. 20-48 Canine leproid (lepromatous) granulomas, auricle, dog.
A, Several pyogranulomas are present in the subcutis of the convex surface of the external ear. Overlying skin is eroded or ulcerated; the leftmost ulcer has healed. B, The granulomas contain numerous large macrophages, many lymphocytes, and fewer plasma cells and neutrophils. H&E stain. C, Higher magnification of B. Short to long acid-fast bacilli (red color), some with a beaded morphology, are present in the granulomatous exudate. Acid-fast stain. D, Fine needle aspirate of a mass. In this cytologic preparation, there is a large macrophage that contains numerous bacilli consistent with a diagnosis of a canine leproid granuloma. Aqueous Romanowsky stain. (A courtesy Dr. D. Crow, Animal Dermatology Clinic, Dallas, TX; B courtesy Dr. B.L. Njaa, The Center for Veterinary Health Sciences, Oklahoma State University; C and D courtesy Dr. R.W. Allison, The Center for Veterinary Health Sciences, Oklahoma State University.)
Cholesterol Granulomas: Cholesterol granulomas are an uncommon condition of dogs and cats that develop in the middle ear. Predisposing factors include hemorrhage into the middle ear, impairment of auditory tube drainage, and diminished or obstructed ventilation of the middle ear. These benign, expansile masses develop most often in association with concurrent otitis media or some other middle ear disease (Fig. 20-49). A prominent microscopic feature for which these masses are named are scattered and clustered acicular clefts (cholesterol clefts), derived from red cell membranes in areas of hemorrhage. Surrounding these clefts are mixtures of macrophages, plasma cells, lymphocytes, and variable numbers of neutrophils, depending on the duration and type of otitis media. In addition to fibroplasia, there may be areas of necrosis and other regions of mineralization.
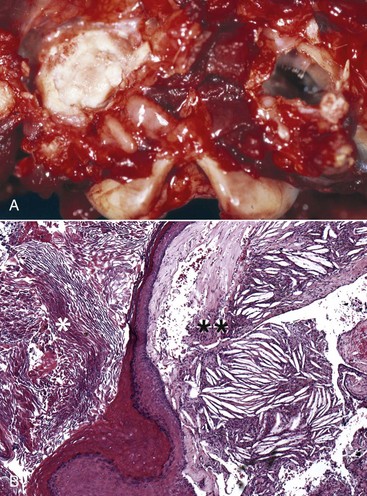
Fig. 20-49 Aural cholesteatoma with middle ear cholesterol granuloma, dog.
A, Ventral-dorsal view. The right tympanic cavity is filled with thick white cholesterol-laden material that obliterates its lumen. The left tympanic cavity is considered within normal limits. Note the fine spicules of bone with bulbous ends that represent a typical septum bulla in dogs. B, Histologic section of the tympanic cavity depicted in A. The left half of the figure shows an epithelial lined cyst filled with keratin flakes. This is a cholesteatoma (asterisk). The right half of the figure shows a mass composed of fibrous connective tissue mixed with numerous acicular spaces representing cholesterol clefts. This mass is a cholesterol granuloma (two asterisks). In this case, both a cholesteatoma and cholesterol granuloma are present in the same middle ear. H&E stain. (A courtesy Dr. M. Rozmanec, College of Veterinary Medicine, Cornell University; B courtesy Ms. J.M. Cramer and Dr. A. Alcaraz, College of Veterinary Medicine, Cornell University.)
Miscellaneous Disorders
Aural Cholesteatoma (Aural Epidermal Cyst): Aural cholesteatomas are masses most commonly found in the middle ear of middle-aged to aged dogs, possibly more commonly in cocker spaniels (see Fig. 20-49). Although aural cholesteatomas are benign cysts, by virtue of their location, concurrent otitis, and expansile growth, they can be locally destructive and rarely lead by extension to meningitis.
Two main theories exist that account for the origin of these epithelial cysts. The first and least popular is the congenital theory in which embryonic epithelial rests are the genesis for their formation. However, the precedent of otitis media and otitis externa in all canine cases strongly supports cyst formation as an acquired change. The more likely pathogenesis is a combination of otitis media and otitis externa resulting in prolonged negative pressure within the middle ear leading to deformity of and likely perforation or invagination of a portion of the tympanic membrane into the middle ear forming a cyst lined by cornifying, stratified squamous epithelium. Because of the inherent epidermal migration of the external surface of the tympanic membrane, these cysts continue to expand as a result of continuous keratin production. In addition, chronic otitis externa can lead to external acoustic meatal stenosis or obstruction preventing the normal flow of keratin from the external tympanic membrane out the external acoustic meatus. Membrane rupture may ensue (see Fig. 20-23) and a cholesteatoma may develop.
Macroscopically, although the name implies something very different, cholesteatomas are not true neoplasms, but expansile cysts lined by keratinizing stratified squamous epithelium and filled with abundant laminated keratin. Thus more appropriate nomenclature for this entity is aural epidermal cyst or epidermal cyst of the middle and external ear. Expansion of the cyst within the middle ear can result in enlargement of the tympanic bulla, as well as bony lysis of bullae. Lysis of the petrous portion of the temporal bone may occur and is always associated with neurologic signs. Sclerosis or periosteal reactions of the ipsilateral temporomandibular joint is a common sequela.
Microscopically, aural cholesteatomas resemble epidermal cysts commonly diagnosed elsewhere in the skin. The lumen contains abundant layers of keratin flakes. A band of well-vascularized fibrous connective tissue that may contain neutrophils, lymphocytes, and macrophages forms the wall of the cyst. Outside of the wall is mature granulation tissue, most likely a reparative response to chronic, recurrent otitis media. Cholesterol granulomas may develop in concert with cholesteatomas. Depending on the duration, severity of otitis media, and size of the cholesteatoma, the tympanic bulla may be osteosclerotic or osteolytic.
Clinical signs are referable to concurrent otitis externa and otitis media and may include facial nerve palsy, head tilt, ataxia, nystagmus, and circling. Lesions are most typically unilateral. Hearing may be impaired from damage to the tympanic membrane or ossicular chain. Temporomandibular joint involvement often results in pain when opening the mouth.
Craniomandibular Osteopathy: Craniomandibular osteopathy is a proliferative, non-neoplastic lesion affecting bones of the head, in particular of the tympanic bullae (see Chapter 16 for more detail). It is an autosomal recessive disorder of terrier breeds, especially West Highland white terriers. Macroscopically, tympanic bullae are markedly enlarged and filled with new bone. Typically a bilateral lesion, affected bullae fuse with the adjacent mandible, restricting movement of the mandible. Microscopically, normal lamellar bone undergoes osteoclastic resorption and is replaced by a primitive, coarse type of bone that expands beyond the normal confines of the periosteum. Lymphocytes, plasma cells, and neutrophils invade the periphery of the affected bone. Presumably, filling of tympanic bullae with new bone impairs hearing by interfering with auditory ossicular function; however, there is a paucity of confirmatory reports.
Disorders Of Cats
Feline Lysosomal Storage–Induced Microtia: Mucopolysaccharidosis (MPS) VI is a lysosomal storage disease reported most commonly in cats (see Chapter 14). Affected cats have mutations in their 4-sulfatase gene that leads to the accumulation of dermatan and chondroitin sulphate in lysosomes of cells. This accumulation is thought to lead to inhibition of other lysosomal enzymes and possibly the accumulation of other glycolipids. Aside from other features of facial dysmorphism, one characteristic of MPS VI is microtia or small auricles that are typically set lower on their heads (Fig. 20-50). The gross lesions can be extremely subtle unless a direct comparison is made to age-matched, unaffected cats. There are no histologic features specific to the auricles. Clinical disease is referable to neurologic symptoms (see Chapter 14).
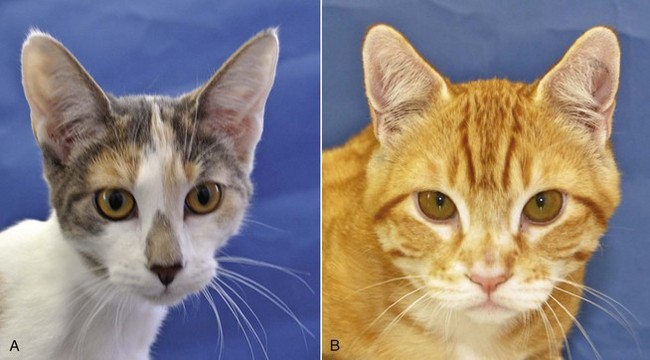
Fig. 20-50 Mucopolysaccharidosis (MPS) VI, cat.
A, Normal cat. B, Cat with MPS VI. The auricles in the cat with MPS VI are smaller than the normal cat. The bridge of the nose is also broader in the cat with MPS VI when compared with the normal cat. (Courtesy Dr. M.E. Haskins, School of Veterinary Medicine, University of Pennsylvania.)
Inflammation
Otitis Externa, Media, and Interna: See Disorders of Domestic Animals.
Auricular Chondritis (Relapsing Polychondritis): Auricular chondritis (also known as relapsing polychondritis) is a rare inflammatory condition in cats (and dogs) affecting cartilage. It is thought to be caused by an immune-mediated response to type II collagen in cartilage. Macroscopically, lesions are characterized by a bilateral auricular chondritis and rarely by concurrent nonerosive polyarthritis, tracheal and laryngeal chondritis, nasal chondritis, ocular inflammation, and audiovestibular damage. Auricles are swollen, erythematous, painful, pruritic, variably alopecic, and curled. Over time, they become permanently deformed, thickened, and firm. Microscopically, auricular cartilage is distorted and wrinkled rather than being a typical straight band caused by cartilage degeneration (Fig. 20-51). Inflammation characterized by infiltration with lymphocytes, plasma cells, macrophages, multinucleated giant cells, and neutrophils is observed. Distinct lymphoid follicles are typically seen peripheral to the damaged cartilage. In the severe chronic cases, cartilage is necrotic with areas of regenerative or dysplastic chondroid nodule formation, neovascularization, and extensive fibrosis throughout the auricle.
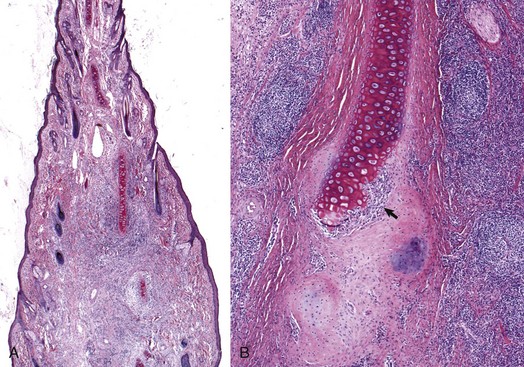
Fig. 20-51 Auricular chondritis, cat.
A, Histologic section of an auricle. The auricle is thickened and expanded by a chronic inflammatory exudate containing lymphocytes and fewer numbers of macrophages. The auricular cartilage is incomplete and made of individual cords surrounded by inflammatory cells and nascent cartilage. H&E stain. B, Higher magnification of A. Abundant lymphocytes have migrated into the dermis of the auricle. There is both calcified and uncalcified cartilage present adjacent to the preexisting auricular cartilage. A thick layer of neutrophils (arrow) are shown invading and destroying the preexisting auricular cartilage. H&E stain. (Courtesy Ms. J.M. Cramer, College of Veterinary Medicine, Cornell University.)
Proliferative, Necrotizing Otitis Externa: Otitis externa is an uncommon condition in cats. A rare, unique condition of unknown etiology is proliferative necrotizing otitis externa that affects cats. Large, well-demarcated erythematous plaques develop over the concave auricular surface and are covered by thick, tan-to-brown keratinous debris (Fig. 20-52, A). These lesions may extend into and partially occlude the external acoustic meatus. Microscopically, these plaques are sharply demarcated from the adjacent normal skin. The key diagnostic features include (1) superficial acanthosis with pronounced hair follicle outer root sheath hyperplasia, (2) marked neutrophilic luminal folliculitis, (3) mild to moderate follicular hyperkeratosis, and (4) scattered individually necrotic keratinocytes of the outer root sheath of hair follicles (Fig. 20-52, B). Cats may range from 2 months to 5 years of age. Lesions may cause no discomfort or mild pruritus and pain. Lesions typically resolve spontaneously.
Fig. 20-52 Proliferative, necrotizing otitis externa, cat.
A, A large, thick proliferative mass grows from the inner, concave surface of the auricle and extends into the external acoustic meatus. B, The epithelium of the hair follicle is markedly hyperplastic and hyperkeratotic with luminal folliculitis. Numerous necrotic keratinocytes are present within the follicular lumen mixed with necrotic inflammatory cells and cellular debris. H&E stain. (Courtesy Dr. E.A. Mauldin, School of Veterinary Medicine, University of Pennsylvania.)
Parasitic Diseases
Mammomonogamus auris: Mammomonogamus auris, a strongyloid nematode, is a rare and regionally distinct cause of otitis media in cats of the Asian pacific region. It is unknown how the adult nematode infects and spreads to and in the middle ears of affected cats. The tympanic membrane is not considered a portal of entry because it is always intact at the time of diagnosis. The need for an intermediate host has been speculated but as of yet has not been identified. However, after infection, some nematodes mature in the trachea, larynx, nasal sinuses, or middle ear and can readily pass in and out of these locations via the auditory tube, nasopharynx, and laryngopharynx. Frequently, a unilateral infection with a single worm pair is observed, although bilateral infections occur and up to eight pairs have been recovered from middle ears of affected cats (Fig. 20-53). Samples of the middle ear have never been collected for microscopic or microbiologic examination. Most cats infected with M. auris are asymptomatic, although head shaking can be a feature of this disease.
Miscellaneous Disorders
Acquired Folding of the Auricle: Acquired folding of the auricle is a disease of sudden onset in adult cats. In nearly all cases, affected cats have a prolonged history of treatment with otic preparations containing glucocorticoids. Affected cats typically have biochemical evidence of iatrogenic adrenocortical insufficiency. The lesion consists of bilateral rostral and lateral folding of the distal or apical third of the auricles. They are cool and thin and appear to lack palpably normal cartilage. Histologic features have not been reported.
Feline Ceruminous Cystomatosis: The cause of feline ceruminous cystomatosis is unknown. It is characterized by benign, cystic, non-neoplastic proliferation of ceruminous glands on the medial surface of the auricle, the base of the auricle, and extending to variable depths into the external acoustic meatus. These glands are markedly dilated, clustered, and filled with brown, inspissated ceruminous secretion and are dark blue to black. As a result, they may be misdiagnosed as melanocytic or vascular neoplasms (Fig. 20-54). Microscopically, cysts are massively dilated, cerumen-filled glands surrounded by minimal to moderate, mononuclear inflammation. Typically, affected cats exhibit no signs attributable to these nodules.
Fig. 20-54 Feline ceruminous cystomatosis, auricle, Abyssinian cat.
A, Multiple, dark grey to blue to black nodules extend from the concave surface of the auricle and are characteristic of feline ceruminous cystomatosis, a cystic dilatation and hyperplasia of ceruminous glands. B, Flattened, squamous epithelial cells line the surface of the cyst. The lumen is filled with gray cerumen. H&E stain. (A courtesy B. Moyes and Dr. B. Milleson, Briarglen Veterinary Clinic, Tulsa, OK; B courtesy Dr. B.L. Njaa, Center for Veterinary Health Sciences, Oklahoma State University.)
Eye
Because of its superficial anatomic location and the transparency of its anterior pole, the globe is the only organ that can be directly examined in great detail by the clinician. As a result, the evaluation of the gross lesions of the eye is really the realm of the clinical practitioner and especially the clinical ophthalmologist. The terminology of ophthalmic pathology is identical to the terminology of clinical ophthalmology, which unfortunately is complex and seemingly designed to intimidate anyone other than the most determined.
Examining a globe is really a miniature postmortem in itself. The embryologic development, general reactions to injury, and specific diseases are as different among the cornea, uvea, and retina as they are among the lung, liver, and kidney. Therefore it is traditional to consider the diseases of the eye as a series of relatively separate topics defined by the anatomic portion of the eye under consideration. There are certainly some diseases affecting the eye as a whole, but they are actually in the minority.
All adult mammalian globes have similar general anatomy, depicted in Fig. 20-55. The globe is a spherical biologic camera with an elaborate autofocus lens derived from surface ectoderm and a light-absorbing film plate (retina) created by an outgrowth of specialized neurons from the brain. All other structures within and surrounding the globe are simply there to ensure the optimal function of the cornea, lens, and retina.
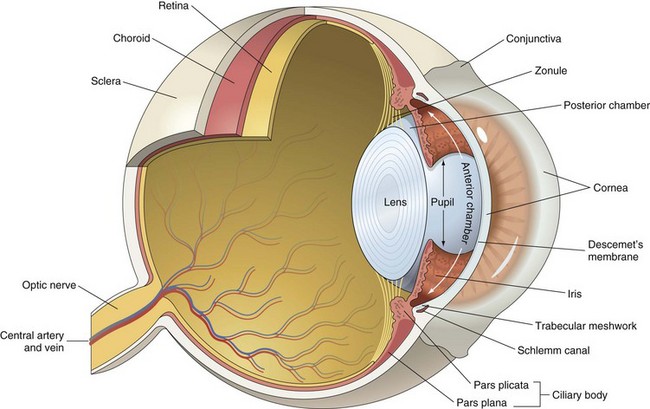
Fig. 20-55 Schematic diagram of the anatomy of the eye. (Modified from Kumar V, Abbas AK, Fausto N: Robbins & Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders; and Slatter D: Fundamentals of veterinary ophthalmology, ed 3, Philadelphia, 2001, Saunders.)
The eye develops as a tentacle of brain tissue that reaches out to just under the skin surface of the developing embryo. The eye’s purpose is to gather sensory information in the form of photons of light, which are absorbed by neurons specifically adapted to convert light into electrical energy. To facilitate access by those photons to the light-sensitive neurons of the retina, the surface ectoderm, which would normally form ordinary skin, undergoes specialized differentiation into the cornea and lens as the tentacle of the brain comes into proximity with it. Details of embryogenesis are discussed in later sections of this chapter.
The details of ocular anatomy can be found in any histology text, but there are some specific features that are particularly relevant to ocular pathology. A few of the most important features are emphasized in the following:
1. The globe is a sealed, fluid-filled sphere, which is protected from noxious influences by a bony orbit, mobile eyelids, a thick fibrous outer shell of cornea and sclera, and a series of vascular and epithelial tight junctions within the uvea and retina referred to as the blood-eye barrier.
2. Although the anatomy and physiology of the globe serve to protect it from many injuries affecting other parts of the body, the same unique features make it extremely susceptible to the propagation of injury once those defenses have been overcome. The fluid media within the globe allows diffusion of infectious agents and chemical mediators of inflammation throughout the globe. The same defenses that prevent entry of various types of chemical or biologic agents also prevent drainage of dangerous by-products of tissue injury and inflammation.
3. Proper visual function requires that very precise anatomic relationships be maintained among the constituent parts of the globe. Minor changes in those relationships that would be insignificant in most other tissues can have devastating results within the globe. Common examples include blindness resulting from minor accumulations of serous exudate behind the retina (serous retinal detachment), or accumulation of edema fluid within the cornea that significantly alters corneal clarity even to the point of blindness. Many of the critical tissues within the globe are unforgiving even of minor changes associated with inflammation or wound healing that in most other tissue would be regarded as insignificant.
4. Most of the visually critical tissues within the globe have negligible regenerative capacity. Some, like the adult retina, are essentially postmitotic and cannot regenerate at all. Others, such as the lens and cornea, are capable of limited regeneration, but the regeneration never recreates a perfect structural or functional replica of the original tissue. Many of the most significant intraocular lesions are related to events of wound healing (epithelial proliferation, angiogenesis, and fibrosis), which are desirable events in most tissue but cause serious functional impairments in the globe.
5. Unlike most other tissue, the eye has essentially no reserve capacity. In many tissues, we struggle to determine whether a lesion is or is not “functionally significant.” There is probably no such thing as a functionally insignificant lesion within the globe. Every single focus of necrosis, inflammation, or scarring probably has a visual consequence even if the degree of impairment is not easily measured.
6. Vision requires that the cornea, lens, and fluid media within the globe remain optically clear. This means that accumulation of exudates, or changes in refractive properties related to conditions such as edema and fibrosis, are extremely damaging even though these reactions to injury may be beneficial to the survival of the globe itself. There is not much point in having a globe that has survived but has forfeited vision.
7. Ocular bystander injury occurs when injury to one component of the globe “spills over” and affects other parts of the globe. Examples include the following:
• Inflammatory effusion from choroiditis, which can cause retinal detachment
• Alteration in aqueous composition and flow, which can lead to cataracts
• Organization of intraocular exudates, which can cause tractional retinal detachment or glaucoma secondary to pupillary block or peripheral anterior synechia
• Chemical mediators of wound healing in chronic uveitis that stimulate preiridal fibrovascular membranes and corneal stromal vascularization
Structure And Function
The cornea is the anterior third of the fibrous tunic that provides structural support for the retinal and uvea. It is a three-layered sandwich about 1 mm thick. It has a thick center of densely compacted collagen fibrils covered on its anterior surface by stratified squamous epithelium derived from fetal surface ectoderm. On its inner (posterior) surface, it is covered by a single layer of cuboidal epithelial cells, known as the corneal endothelium, which is derived from periocular mesenchyme (Fig. 20-56). The corneal epithelium is 6 to 10 cells thick and has a turnover time of 5 to 7 days. The adult corneal endothelium of most domestic mammals does not replicate.
The cornea is an adaptation of skin. These adaptations to the epidermis are designed specifically to render it transparent. The surface epithelium differs from that of skin in that there is no keratinization, pigmentation, hair follicles, or adnexal glands. The corneal stroma resembles dermis, except that it lacks blood vessels, hair follicles, and leukocytes. It has relatively few fibroblast-like cells (keratocytes) and a highly regimented organization of its collagen fibrils. These fibrils are arranged in compact lamellae with a space between them that matches the wavelength of visible light. Thus the cornea allows the passage of light without any scattering. To further facilitate the unimpaired passage of light, the corneal stroma is maintained in a dehydrated state compared with that of most other tissue. That dehydrated state is maintained passively by intercellular junctions within the corneal epithelium and endothelium, which exclude water from the tear film and anterior chamber, respectively. It is further maintained by the active removal of electrolytes (and thus water) by energy-dependent membrane pumps within the corneal endothelium and to a much lesser degree the overlying epithelium. The avascular cornea is permitted to exist in this highly privileged anatomic and physiologic state only as long as its “support system” is working properly. It must be protected from desiccation and irritation by properly functioning eyelids and lacrimal secretion, nourished by a tear film that is biochemically normal and delivered in adequate amounts, and replenished by germinal cells resident in the adjacent conjunctiva and sclera. The failure of any of these support systems results in corneal decompensation, seen clinically either as necrosis or as reversion of the cornea to a skinlike state, which although much more durable is unfortunately also opaque.
Uvea
The uvea is the nourishing vascular tunic of the globe. It is made up of the iris, ciliary body, and choroid. It includes the pigmented stroma and muscles of the iris and ciliary body and the light-reflecting mirror known as the tapetum, which is buried within the dorsal choroid of all domestic species except the pig. It borders the fluid-filled cavities of the globe: the anterior chamber, posterior chamber, and vitreous. The uvea is surrounded exteriorly by the sclera.
The uvea is formed in part by neuroectoderm from the primary optic vesicle, and in part by periocular mesenchyme. Its primary function is to provide nutrients to the avascular lens and to the outer half of the retina. In its general structure, it is similar to the lamina propria of the various tubular organs such as the intestine or reproductive tract. It is rich in fibrous tissue, nerves, blood vessels, and melanocytes. In contrast to the lamina propria of other tissue, however, it contains virtually no resident lymphocytes or plasma cells. Although the vasculature of the uvea is in free communication with systemic vasculature, access by infectious agents or tumor cells from the blood into the globe via the uvea is normally prevented by a series of strategically located tight junctions, which are collectively referred to as the blood-eye barrier (see later discussion in the section on Defense Mechanisms).
Lens
The lens is a biconvex, avascular, transparent accumulation of elongated epithelial cells, suspended within the pupillary aperture. Its shape and elasticity vary greatly by species and with age. It is surrounded by the lens capsule, which is a thick basement membrane (predominantly type IV collagen) produced throughout life by the lens epithelium. Germinal epithelium forms a single layer of cuboidal epithelial cells just below the capsule along the anterior surface of the lens. The epithelium is absent from the posterior surface of the lens. Near the equator of the lens, these epithelial cells migrate inwardly and elongate to become lens fibers. As they elongate, the cells also lose most of their cytoplasmic organelles and eventually lose their nuclei. They develop complex “ball and socket” interdigitations of their plasma membranes and many gap junctions that allow each lens fiber to adhere tightly to all of its adjacent fibers.
New lens fibers are recruited from the germinal cells of the lens epithelium throughout life, so the lens continues to grow. Its net growth is partially counterbalanced by a progressive increase in the density of the center of the lens (the lens nucleus), which is formed by the oldest lens fibers. Gradually the increasing number of aged fibers within the progressively expanding lens nucleus makes the lens less transparent (so-called nuclear sclerosis) and less flexible, so that at least in some species, there is a loss of focusing ability (accommodation) with age.
The purpose of the lens is to further refract light that has passed through the cornea and to focus that light onto the retina. To accomplish this, the lens obviously must remain transparent and in its proper location within the pupillary aperture. Its transparency depends on its highly regimented structure, the paucity of cytoplasmic organelles, the very precise molecular character of its intracellular crystalline proteins, and on its maintenance of a critical state of dehydration. This dehydration is maintained primarily by excretion of electrolytes through an active sodium-potassium–dependent adenosinetriphosphatase pump, located mostly in the anterior lens epithelium.
The location of the lens within the pupillary aperture (with the iris resting on its anterior surface and its posterior surface embedded in a depression in the anterior surface of the vitreous) is maintained by a ring of transparent elastin-like fibers (the lens zonules or zonular fibers) stretching from the lens capsule to the nonpigmented ciliary epithelium. The exact arrangement of these fibers varies greatly by species. Contraction and relaxation of the ciliary muscles alters tension on these fibers and allows the lens to change shape or position, and thus to focus.
The health of the lens almost totally depends on the aqueous humor delivering nutrients and removing its waste products. Lens metabolism is primarily via anaerobic glycolysis. Glucose is delivered via the aqueous humor and is absorbed across the lens capsule, which is impermeable to most other substances. This metabolic peculiarity of the lens is vital in understanding the development of diabetic cataracts (see later discussion).
Retina And Vitreous
The retina is the raison d’être for the entire globe. The cornea, lens, uveal tract, and sclera are simply a “supporting cast” to allow the retina to do its job of converting photons of visible light into electrical impulses, which are transmitted to the visual cortex of the brain. It is the most fascinating of the ocular tissues, yet at the same time the one that we can influence the least. It is one of the sad realities of ocular disease that we are often powerless spectators, able to view the evidence of active or previous retinal disease, yet with virtually no ability to influence it. Most frequently, at the time of clinical examination, the eye is already blind from retinal detachment, inherited photoreceptor disease, or the devastating effects of glaucoma.
The retina is literally an outpouching of brain tissue. It is formed from the neuroectoderm of the anterior half of the primitive optic vesicle. As the optic vesicle invaginates to form the optic cup, the presumptive retinal neuroectoderm is pushed into apposition with the neuroectoderm from the posterior half of the optic vesicle, which will form the retinal pigment epithelium (RPE). The proper development of the retina requires that proximity to the retinal pigment epithelium. This dependence of retina on the retinal pigment epithelium continues throughout life. It is impossible to review all significant features of the very complex retinal structure and function here, but those features that are most important in understanding the retinal diseases are as follows:
1. Macroscopically the retina resembles a slightly opaque vascularized membrane, resembling tissue paper, held against the RPE, choroid, and sclera by the gel-like vitreous. It is not actually attached to that RPE and/or choroid except at the optic disc and at its very periphery, where it becomes continuous with the epithelium of the pars plana of the ciliary body (the site of transition is known as the ora ciliaris retinae).
2. The retina consists of three layers of neurons (ganglion cells, inner nuclear layer, and outer nuclear layer) separated by cell-free layers created by the intermingling of the axons and dendrites of those neurons. The innermost layer, adjacent to the vitreous, is the nerve fiber layer. It is made up of the ganglion cell axons that, as they exit the globe, become the optic nerve. The RPE is usually listed as the outermost layer of the retina, but it is derived from the posterior half of the optic vesicle and is not directly involved in vision. Anatomically and functionally, the RPE is more appropriately considered a part of the choroid. The combination of retina, RPE, choroid, and tapetum that is responsible for the ophthalmoscopic appearance of the posterior pole of the globe is known as the ocular fundus (Fig. 20-57).
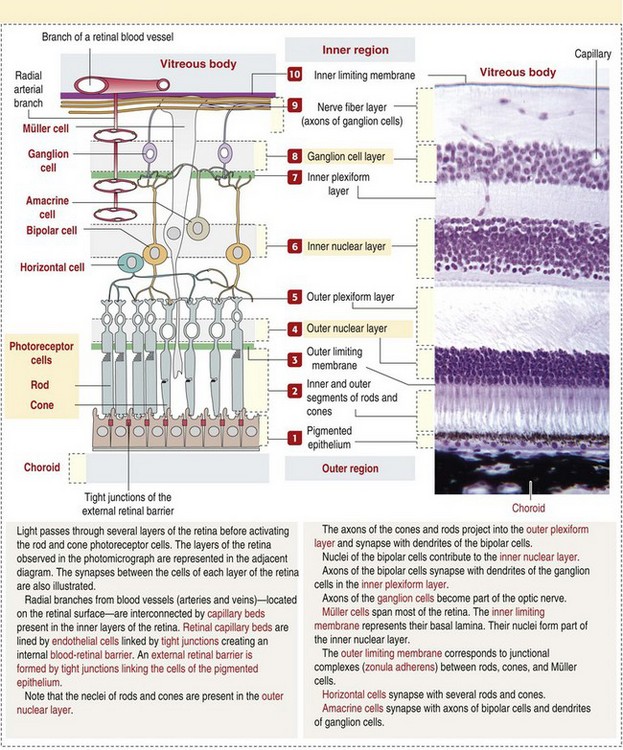
Fig. 20-57 Normal mammalian retina. (From Kierszenbaum AL: Histology and cell biology: an introduction to pathology, St Louis, 2002, Mosby.)
3. The outer nuclear layer is formed by the cell bodies of the photoreceptors, which are specialized cilia containing photoactivated pigments that are responsible for converting light into electrical impulses. These photoreceptors are embedded into crevices within the surface of the adjacent RPE, but there are no actual cellular junctions. This intimate contact is essential to allow the RPE to deliver nutrients to and remove waste products from the metabolically demanding photoreceptors. The potential space between the photoreceptors and the RPE is the remnant of the lumen of the primary optic vesicle. It is in this space that hemorrhage, edema fluid, and inflammatory exudate accumulate, resulting in exudative retinal detachment.
4. The inner (vitreal) half of the retina in most species is supplied by blood vessels entering the globe through the optic nerve. In contrast, the outer nuclear layer, photoreceptors, and RPE are totally dependent on diffusion of nutrients from the adjacent choroid. Separation of the retina from the RPE results in rapid ischemic degeneration of photoreceptors, and eventually of the outer nuclear layer. In the horse, in which retinal blood vessels are almost absent, the assumption is that the retina relies almost completely on choroidal diffusion to obtain nutrients, and the consequences of retinal detachment should therefore be even more devastating.
5. The optic nerve is the continuation of the nerve fiber layer of ganglion cell axons into the brain and uses the preexistent tube formed by the embryonic optic stalk. Ganglion cell axons exit the globe through a series of perforations, known as the lamina cribrosa, in the sclera at the posterior pole of the globe. In most species, axons become myelinated at about the level of the lamina cribrosa, as they leave the globe. The combination of axons, myelin, and supporting glia on the vitreal side of that lamina cribrosa forms the optic disc. The amount of myelin and how far the myelinated fibers extend into the retina are responsible for the ophthalmoscopic appearance of the optic disc, which varies among species (Fig. 20-58). In dogs, the myelin extends several millimeters internal (anterior) to the lamina cribrosa and this extension is responsible for the prominence of the optic disc in that species. Because the optic nerve is a tract of the brain rather than a true peripheral nerve, the myelin is laid down by oligodendrocytes instead of Schwann cells (see Chapter 14).
Fig. 20-58 Normal optic disc, dog.
The dense fibrous tunic of the sclera becomes fenestrated to permit the exit of the nerve, creating the lamina cribrosa (arrow). H&E stain. (Courtesy Dr. B. Wilcock, Ontario Veterinary College.)
6. Vision requires that the light gathered and refracted by the cornea is focused by the lens onto the photoreceptors. Light passes through the minimally absorptive inner two-thirds of the retina so that most of it can be absorbed by the pigments within the photoreceptors. The electrical signal generated by activation of the photopigment is transmitted in a stepwise fashion from the outer nuclear layer to the neurons of the inner nuclear layer, then to the ganglion cells, and finally via the nerve fiber layer to the optic nerve and brain. The number of photoreceptors “feeding” to a single ganglion cell varies greatly among species and is one of the variables determining visual acuity and the efficacy of dim-light vision. In all domestic animals except the pig, photons not absorbed on the initial pass through the photoreceptors are then reflected back by the cytoplasmic crystals embedded within the tapetum to stimulate the photoreceptors a second time. The tapetum is therefore assumed to be a choroidal adaptation to increase the efficiency of vision in dim light.
The vitreous is an optically clear, gelatinous mass that fills the interior of the globe from the back of the lens to the retina. It is about 99% water, with collagen and hyaluronic acid making up most of the remaining 1%. Little is known about the production and turnover of the vitreous humor. It is thought to be made mostly by secretion from nonneuronal cells of the retina and the nonpigmented epithelium of the ciliary body. The vitreous contains only a scattering of histiocyte-like cells, known as hyalocytes, and a few fibroblasts. Along the anterior surface of the vitreous is a shallow depression known as the hyaloid fossa, in which lies the posterior surface of the lens. The anterior surface of the vitreous undergoes condensation to form the anterior hyaloid membrane, which separates the vitreous from the aqueous humor. The vitreous seems to function mainly to maintain the shape of the globe and to help support the retina in its normal position against the RPE and choroid.
Eyelids, Conjunctiva, And Orbit
The central theme in ophthalmology is the degree to which proper ocular function rests on the ability of the critical visual tissues, such as the retina and lens, to function thanks to a carefully controlled, protected environment. Thus the eye depends entirely on a long list of factors in a “supporting cast” to protect the sensitive visual tissues against the external environment. The first line of defense is the eyelids, conjunctiva, and bones of the orbit that create a wall around the globe. This protective wall is designed to let in light and nutrients but to exclude the many elements of the external environment that might injure this very sensitive organ.
From the perspective of a pathologist, the diseases of the eyelids, conjunctiva, and orbit are much less intriguing than those of the globe itself. From a clinical perspective, however, the diseases of the eyelid and conjunctiva form a major part of what the primary care veterinarian is likely to diagnose and treat.
Eyelids: The epithelium of the eyelids develops from the surface ectoderm adjacent to the cornea. After separation of the lens vesicle, the surface ectoderm regains continuity to form the cornea. Ectoderm at the periphery of the cornea then migrates over the surface of the embryonic cornea, accompanied by some underlying periocular mesenchyme to form the eyelids. The ectoderm forms the surface epithelium and glands; the accompanying periocular mesenchyme forms the deeper dermis and the eyelid muscles. These ingrowing lid buds fuse with each other over the central cornea. How long this fusion persists varies with the species. In dogs and cats, it persists until about 2 weeks after birth and provides the immature cornea with a secure, sterile environment in which to complete its embryologic development.
The mature eyelids are movable folds of skin that slide across the surface of the cornea on a film of mucus and fluid known as the tear film. This blinking movement serves to help distribute the protective tear film across the corneal surface and to remove unwanted particulate debris from the corneal surface. Each eyelid has an anterior surface of haired skin, with all of the adnexal glands as seen in skin anywhere else. The dermis is modified by the addition of striated muscle (orbicularis oculi and levator muscles). The inner surface of the eyelid, which rests against the cornea, is covered by a mucous membrane known as the palpebral conjunctiva (see the next section on the Conjunctiva). The site of transformation from eyelid skin to palpebral conjunctiva at the eyelid margin is characterized by the presence of a row of very large modified sebaceous glands known as Meibomian glands and by the emergence of several rows of large modified hairs that are the eyelid cilia (eyelashes). The Meibomian glands contribute a lipid component to the tear film, which aids in the dispersal of the aqueous component of the tear film and helps prevent evaporation. The cilia are largest and most numerous along the margin of the upper eyelid; they may be infrequent or absent along the lower eyelid.
Conjunctiva: The conjunctiva is a mucous membrane extending from the palpebral margin of the eyelid to the periphery of the cornea. The portion of conjunctiva that covers the posterior surface of the eyelid is the palpebral conjunctiva, and the portion attached to the surface of the globe and continuous with the peripheral cornea at the limbus is the bulbar conjunctiva.
The palpebral conjunctiva consists of stratified squamous nonkeratinized epithelium near its origin at the eyelid margin but quickly becomes stratified columnar epithelium with numerous goblet cells. At the base of the eyelid, it is reflected back onto the surface of the sclera. Now named the bulbar conjunctiva, it extends over the eyeball as far as the periphery of the cornea. The epithelium of the bulbar conjunctiva lacks goblet cells. It becomes continuous with the corneal epithelium at the limbus. At the junction between bulbar conjunctiva and corneal epithelium, there is a population of germinal cells that are the permanent replicative cells of the corneal epithelium. They are the source of replacement corneal epithelial cells in cases of persistent corneal ulceration.
The space between the palpebral and bulbar conjunctiva is the conjunctival sac. The space between the upper and lower eyelid is known as the palpebral fissure. The medial (nasal) limit of the palpebral fissure (where the upper and lower eyelids join) is the medial canthus. The lateral (temporal) margin of the palpebral fissure is known as the lateral canthus. It is the palpebral fissure that determines what most people would regard as the “shape of the eye,” which has considerable importance in the breeding of purebred dogs. It is the careless genetic manipulation of the shape of the palpebral fissure that is responsible for the frequency of some of the most common eyelid diseases (see later discussion of entropion and ectropion in the section on Diseases of the Eyelid).
The lamina propria of both the palpebral and bulbar conjunctiva resembles the lamina propria of any other mucous membrane. It has an abundance of blood vessels, loose connective tissue, variable numbers of lymphocytes, plasma cells, and diffuse lymphoid tissue and lymphoid nodules (mucosa-associated lymphoid tissue [MALT]). The MALT responds immunologically to the microbial flora within the conjunctival sac. In patients with chronic conjunctivitis, the lymphoid nodules are often grossly visible, indicating the intensity and duration of the response of the MALT to antigenic stimuli.
The ventral conjunctiva, as it transforms from palpebral to bulbar conjunctiva, undergoes an additional specialization in the form of the third eyelid or nictitating membrane. This large fold of conjunctiva protrudes from the ventral-medial canthus over the anterior surface of the cornea and contains a central supporting plate of cartilage and a stroma of dense fibrous tissue, containing an accessory lacrimal gland. Both its anterior and posterior surfaces are covered by stratified squamous nonkeratinized epithelium. In most domestic species, its movement is passive, serving to cover the globe and provide an extra level of protection when the globe is retracted into the orbit by the retractor bulbi muscle.
Orbit: The orbit is the bony fossa that surrounds most of the globe, except the cornea, and separates the globe from the brain. Along its posterior border are numerous foramina through which blood vessels and nerves reach or leave the globe. The orbit is formed by the fusion of five to seven bones, depending on the species. It is a complete bony shell, except in dogs, cats, and pigs, in which the dorsal roof of the orbit is formed only by the supraorbital ligament that extends from the frontal bone to the zygomatic bone, leaving the dorsal orbit incomplete. The orbit contains the globe itself but also the extraocular muscles, abundant fat, lacrimal gland, zygomatic salivary gland, and all the muscles and nerves that support these structures.
The lacrimal gland is a specialized serous salivary gland located in the orbit, dorsolateral to the eyeball. Along with the histologically similar gland of the third eyelid, it is responsible for the production of the serous component of tears. It empties through 15 to 20 small excretory ducts at the lateral part of the fornix of the superior conjunctival sac.
It is unclear how much interdependence there is between the ocular and orbital embryologic development. On one hand, animals with severe microphthalmia often have a relatively normal orbit, but usually there is some reduction in size and distortion of shape. It may be that the critical issue is the stage of embryogenesis at which the developing globe is injured and subsequently atrophies (remember that most microphthalmia in domestic animals is “secondary” and is the result of in utero atrophy rather than true hypoplasia).
Portals Of Entry
Portals of entry into the cornea are listed in Box 20-5. Injury to the cornea may arise from altered homeostasis of the corneal surface, by penetrating injury through the surface epithelium, secondary to injury to corneal endothelium, or rarely by migration into the cornea from the blood vessels at the limbus. Regardless of the route of entry, virtually all significant corneal diseases reflect stromal injury, whether this stromal injury was the primary lesion or simply a consequence of injury to the surface epithelium or to the endothelium of the cornea.
Uvea
Portals of entry into the uvea are listed in Box 20-6. Microbes and other injurious agents enter the uvea via the bloodstream (hematogenous), penetrating injury, or aqueous or vitreous. Hematogenous entry is used by toxins, infectious agents, and metastatic neoplasia. Injury to the blood vessels themselves (thrombosis, occlusion by neoplastic emboli) may cause ischemic damage. Penetrating injury provides infectious agents entry into one or more of the ocular chambers, causing uveal injury either by production of toxins or (more often) by stimulating a uveal inflammatory response. Injury that causes lens rupture can initiate a phacoclastic uveitis (see later discussion). Chemical mediators of inflammation released from an injured cornea, lens, or retina diffuse into and throughout the aqueous or vitreous, thus providing the opportunity for additional injury in contiguous tissues.
Lens
Portals of entry into the lens are listed in Box 20-7. Although the lens is occasionally injured by a direct perforating injury (see later discussion on phacoclastic uveitis in the section on Lens-Induced Uveitis) or becomes displaced by blunt trauma (lens luxation), the vast majority of injuries to the lens are related to defective lens nutrition. This involves either inadequate delivery of nutrients because of defective flow of aqueous humor or chemically abnormal aqueous humor. The aqueous humor may contain by-products of intraocular necrosis or inflammation, or excessive/deficient levels of specific chemicals. Common examples include cataracts caused by excessive glucose levels in animals with diabetes mellitus and cataracts associated with systemic hypocalcemia. Degenerative changes in the lens are also seen in dogs with inherited photoreceptor disorders (so-called progressive retinal atrophy), probably caused by diffusion of toxic by-products of photoreceptor degeneration. Inherited cataracts in dogs are the single most frequent type of cataract seen in veterinary practice, but virtually nothing is known about the specific biochemical pathogenesis.
Among the more exotic causes of lens injury are injury from therapeutic radiation; electrical shock, including lightning strike; and penetrating intralenticular metallic foreign bodies. Dietary imbalances, excessive sunlight exposure, and parasitic migration are common causes of cataracts in some nonmammalian species (especially fish) but have rarely been reported in domestic mammals. Cataracts have been reported in puppies and wolf cubs fed a commercial milk replacer, and in kittens fed feline milk replacer. Cataract development was attributed to a dietary deficiency of arginine, although the precise nature of the dietary deficiency was not identified in every instance.
Because the lens is avascular and surrounded by a dense collagenous capsule, it is relatively impervious to the entry of infectious agents from elsewhere in the globe. The one rare exception in domestic mammals is the seemingly specific targeting of the lens capsule by systemic aspergillosis and presumably by other species of hyphal fungi occurring as a sequela to mycotic abomasitis in calves or opportunistic mycoses in dogs immunosuppressed by cancer chemotherapy. These fungi exhibit a tropism for basement membranes throughout the body, including the lens capsule. In rabbits, the lens capsule is frequently penetrated by the protozoon Encephalitozoon cuniculi, which causes spontaneous lens rupture. In fish, cataracts induced by the selective intralenticular penetration by fluke larvae are exceedingly prevalent. Several viral diseases, notably bovine viral diarrhea, are occasionally associated with congenital cataracts as a consequence of generalized systemic infection in utero before the establishment of the blood-eye barrier.
Retina And Vitreous
It has been said that anything in notable excess or deficiency is harmful. This statement is particularly applicable to the retina, which, despite its sheltered intraocular niche, probably is injured by a wider range of noxious stimuli arriving by a wider variety of routes than any other tissue in the body.
Portals of entry into the retina are listed in Box 20-8. The retina can be injured by light and other types of radiation arriving through the cornea and lens, by hematogenous dissemination of chemical or infectious agents, by objects penetrating through the cornea or through the sclera, and by microorganisms arriving via the optic nerve. Because the retina is part of the brain, it is susceptible to most of the infectious, degenerative, and metabolic diseases of the brain, including the storage diseases. Exactly how frequently the retina is involved in diseases affecting primarily the brain is unknown, but the risk appears to be quite low.
The retina is sensitive to injury from chemical mediators of inflammation and toxic products of infectious agents within the vitreous and is particularly sensitive to increased intraocular pressure. Especially in purebred dogs, there is also a huge list of inherited defects in photoreceptor metabolism, which result in progressive destruction of photoreceptors and their nuclei.
The most important and most frequent causes of injury are those related to retinal detachment (see later discussion), increased intraocular pressure, vascular hypertension, and genetically determined photoreceptor diseases. It is important to view this list in perspective. Despite the long list of potentially injurious stimuli and the innumerable routes by which such stimuli can impact the retina, retinal disease is actually quite infrequent.
Eyelids, Conjunctiva, And Orbit
Eyelids: The outer surface of the eyelid is skin and therefore is susceptible to the same diseases as the skin elsewhere on the body. For palpebral skin, the portals of entry are the same as for skin at other sites, as follows:
Conjunctiva: The conjunctiva is a mucous membrane similar in structure to other mucous membranes and is therefore susceptible to injury from the same range of physical and chemical injuries affecting any other mucous membrane. The routes of entry are predictable and as follows:
Orbit: Portals of entry into the orbit are as follows:
1. Orbital fractures from external trauma
2. Extension of inflammatory or neoplastic disease originating within the gingiva or the nasal cavity
3. Extension of intraocular inflammatory or neoplastic disease through the sclera
4. Direct extension of inflammatory or neoplastic foci from the conjunctiva or eyelid
5. Penetrating injuries through the skin or upwardly through the mouth, or migrating foreign bodies implanting infectious agents
6. Hematogenous localization of microorganisms, neoplasms, and influx of antibodies in immune-mediated diseases (muscle, lacrimal gland)
7. Extension from the cranial vault via the optic nerve or through one of the other foramina; this is rare
Of these portals of entry, infectious orbital cellulitis resulting from penetrating foreign body or migration from tooth root cellulitis is the most common. In dogs and cats, spread of osteolytic nasal carcinoma, oral squamous cell carcinoma, or malignant melanoma into the orbit is relatively frequent. In cattle, the orbit is a frequent location for metastatic malignant lymphoma in those animals that survive long enough with the disease.
Defense Mechanisms
The cornea is defended against injury by its protected anatomic location, by several reflexes, and by the tear film (Box 20-9). Passive (anatomic) protection is provided by the eyelids, the eyelashes, and the bony orbit. Various reflexes are activated by mechanical stimulation of eyelids, eyelashes, or the cornea itself. The blink reflex causes the eyelids to close when the eyelids or eyelashes are stimulated by contact with any solid material or even strong air currents. The menace reflex causes the eyelids to blink when there is visual perception of a threat to the globe. The corneal reflex causes the eyelids to close when the cornea itself is irritated by external stimuli. Reflex retraction of the globe into the orbit, with subsequent passive sliding of the third eyelid to cover the cornea, occurs in response to corneal trauma.
The tear film is a serous secretion produced by the lacrimal gland and by the gland of the third eyelid, with contributions from goblet cells in the conjunctiva and several accessory glands within the conjunctival lamina propria. It not only provides the majority of the nourishment for the avascular cornea but also provides a mucus coat to prevent evaporation of the protective fluid, soluble antibacterial chemicals, and a flushing action to protect the cornea against colonization by bacteria and fungi that live in the conjunctival sac.