Chapter 2 First Week of Human Development
Human development begins at fertilization when a sperm fuses with an oocyte to form a single cell, a zygote. This highly specialized, totipotent cell marks the beginning of each of us as a unique individual. The zygote, just visible to the unaided eye, contains chromosomes and genes (units of genetic information) that are derived from the mother and father. The zygote divides many times and becomes progressively transformed into a multicellular human being through cell division, migration, growth, and differentiation.
Gametogenesis
Gametogenesis (gamete formation) is the process of formation and development of specialized generative cells, gametes (oocytes or sperms). This process, involving the chromosomes and cytoplasm of the gametes, prepares these sex cells for fertilization. During gametogenesis, the chromosome number is reduced by half and the shape of the cells is altered. A chromosome is defined by the presence of a centromere, the constricted part of a chromosome. Before DNA replication in the S phase of the cell cycle, chromosomes exist as single-chromatid chromosomes. A chromatid consists of parallel DNA strands. After DNA replication, chromosomes are double-chromatid chromosomes.
The sperm and oocyte, the male and female gametes, are highly specialized sex cells. Each of these cells contains half the number of chromosomes (haploid number) that are present in somatic (body) cells. The number of chromosomes is reduced during meiosis, a special type of cell division that occurs during gametogenesis. Gamete maturation is called spermatogenesis in males and oogenesis in females (Fig. 2-1). The timing of events during meiosis differs in the two sexes.
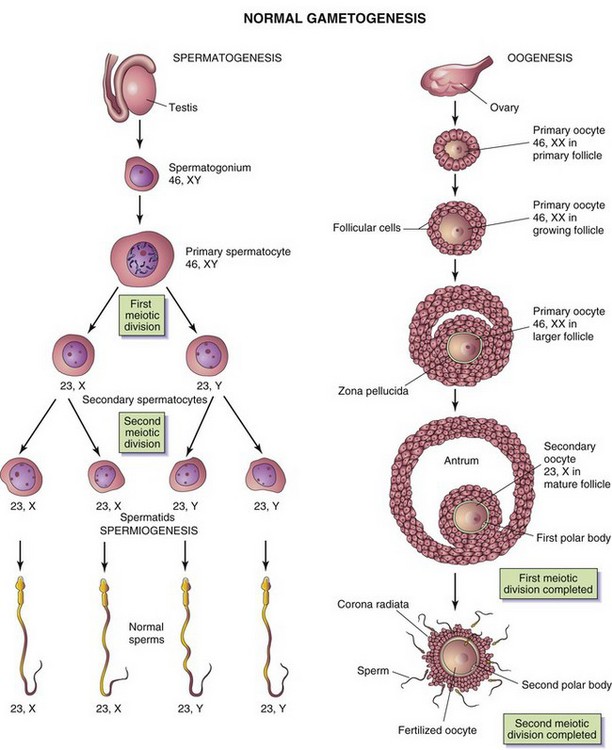
FIGURE 2–1 Normal gametogenesis: conversion of germ cells into gametes (sex cells). The drawings compare spermatogenesis and oogenesis. Oogonia are not shown in this figure because they differentiate into primary oocytes before birth. The chromosome complement of the germ cells is shown at each stage. The number designates the total number of chromosomes, including the sex chromosome(s) shown after the comma. Notes: (1) Following the two meiotic divisions, the diploid number of chromosomes, 46, is reduced to the haploid number, 23. (2) Four sperms form from one primary spermatocyte, whereas only one mature oocyte results from maturation of a primary oocyte. (3) The cytoplasm is conserved during oogenesis to form one large cell, the mature oocyte. The polar bodies are small nonfunctional cells that eventually degenerate.
Meiosis
Meiosis is a special type of cell division that involves two meiotic cell divisions; it takes place in germ cells only (Figs. 2-2 and 2-3). Diploid germ cells give rise to haploid gametes (sperms and oocytes).
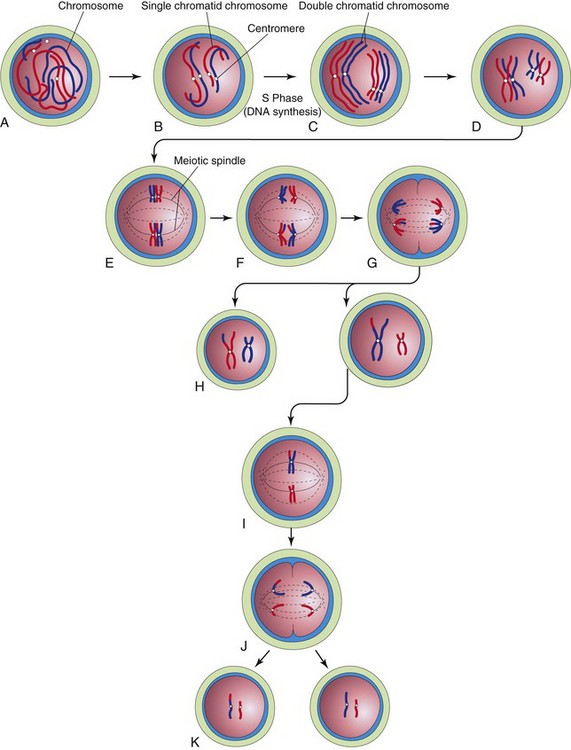
FIGURE 2–2 Diagrammatic representation of meiosis. Two chromosome pairs are shown. A to D, Stages of prophase of the first meiotic division. The homologous chromosomes approach each other and pair; each member of the pair consists of two chromatids. Observe the single crossover in one pair of chromosomes, resulting in the interchange of chromatid segments. E, Metaphase. The two members of each pair become oriented on the meiotic spindle. F, Anaphase. G, Telophase. The chromosomes migrate to opposite poles. H, Distribution of parental chromosome pairs at the end of the first meiotic division. I to K, Second meiotic division. It is similar to mitosis except that the cells are haploid.
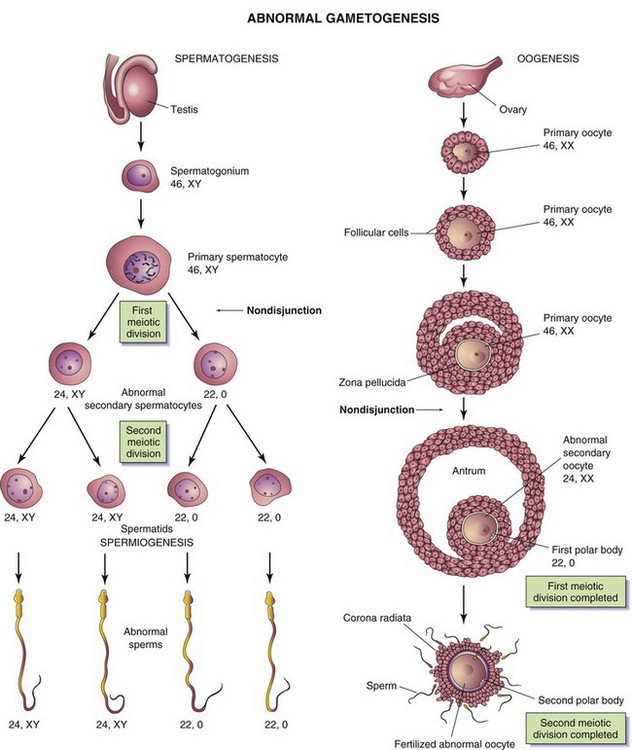
FIGURE 2–3 Abnormal gametogenesis. The drawings show how nondisjunction (failure of one or more pairs of chromosomes to separate at the meiotic stage) results in an abnormal chromosome distribution in gametes. Although nondisjunction of sex chromosomes is illustrated, a similar defect may occur in autosomes. When nondisjunction occurs during the first meiotic division of spermatogenesis, one secondary spermatocyte contains 22 autosomes plus an X and a Y chromosome, and the other one contains 22 autosomes and no sex chromosome. Similarly, nondisjunction during oogenesis may give rise to an oocyte with 22 autosomes and two X chromosomes (as shown) or may result in one with 22 autosomes and no sex chromosome.
The first meiotic division is a reduction division because the chromosome number is reduced from diploid to haploid by pairing of homologous chromosomes in prophase and their segregation at anaphase. Homologous chromosomes or homologs (one from each parent) pair during prophase and separate during anaphase, with one representative of each pair randomly going to each pole of the meiotic spindle. The spindle connects to the chromosome at the centromere. At this stage, they are double-chromatid chromosomes. The X and Y chromosomes are not homologs, but they have homologous segments at the tips of their short arms. They pair in these regions only. By the end of the first meiotic division, each new cell formed (secondary spermatocyte or secondary oocyte) has the haploid chromosome number, that is, half the number of chromosomes of the preceding cell. This separation or disjunction of paired homologous chromosomes is the physical basis of segregation, the separation of allelic genes during meiosis.
The second meiotic division follows the first division without a normal interphase (i.e., without an intervening step of DNA replication). Each double-chromatid chromosome divides, and each half, or chromatid, is drawn to a different pole; thus, the haploid number of chromosomes (23) is retained and each daughter cell formed by meiosis has the reduced haploid number of chromosomes, with one representative of each chromosome pair (now a single-chromatid chromosome). The second meiotic division is similar to an ordinary mitosis except that the chromosome number of the cell entering the second meiotic division is haploid.
Abnormal Gametogenesis
Disturbances of meiosis during gametogenesis, such as nondisjunction (Fig. 2-3), result in the formation of chromosomally abnormal gametes. If involved in fertilization, these gametes with numerical chromosome abnormalities cause abnormal development such as occurs in infants with Down syndrome (see Chapter 20).
Spermatogenesis
Spermatogenesis is the sequence of events by which spermatogonia are transformed into mature sperms. This maturation process begins at puberty. Spermatogonia are dormant in the seminiferous tubules of the testes during the fetal and postnatal periods. They increase in number during puberty. After several mitotic divisions, the spermatogonia grow and undergo changes.
Spermatogonia are transformed into primary spermatocytes, the largest germ cells in the seminiferous tubules. Each primary spermatocyte subsequently undergoes a reduction division—the first meiotic division—to form two haploid secondary spermatocytes, which are approximately half the size of primary spermatocytes. Subsequently, the secondary spermatocytes undergo a second meiotic division to form four haploid spermatids, which are approximately half the size of secondary spermatocytes. The spermatids are gradually transformed into four mature sperms by a process known as spermiogenesis (Fig. 2-4). The entire process of spermatogenesis, which includes spermiogenesis, takes approximately 2 months. When spermiogenesis is complete, the sperms enter the seminiferous tubules.
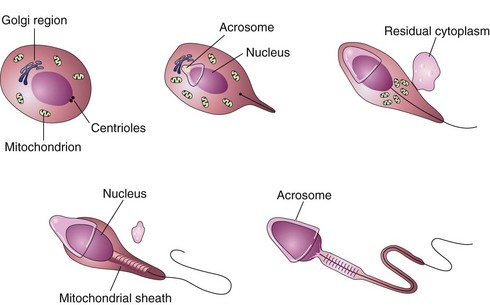
FIGURE 2–4 Illustrations of spermiogenesis, the last phase of spermatogenesis. During this process, the rounded spermatid is transformed into elongated sperm. Note the loss of cytoplasm, development of the tail, and formation of the acrosome. The acrosome, derived from the Golgi region of the spermatid, contains enzymes that are released at the beginning of fertilization to assist the sperm in penetrating the corona radiata and zona pellucida surrounding the secondary oocyte.
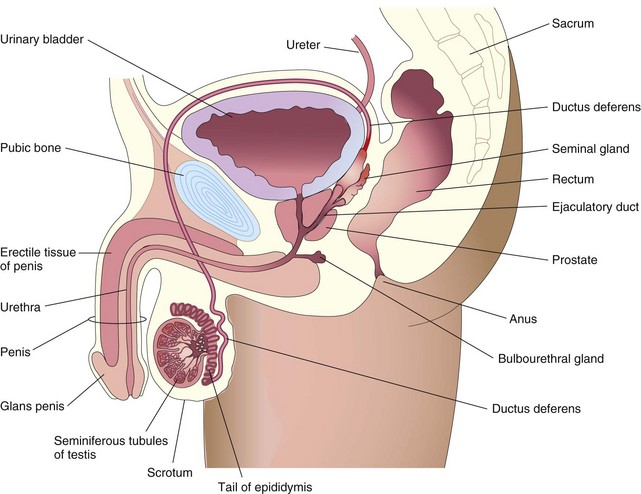
Sertoli cells lining the seminiferous tubules support and nurture the germ cells and may be involved in the regulation of spermatogenesis. Sperms are transported passively from the seminiferous tubules to the epididymis, where they are stored and become functionally mature during puberty. The epididymis is the elongated coiled duct along the posterior border of the testis (see Fig. 2-12). It is continuous with the ductus deferens (vas deferens), which transports the sperms to the urethra.
Mature sperms are free-swimming, actively motile cells consisting of a head and a tail (Fig. 2-5A). The neck of the sperm is the junction between the head and tail. The head of the sperm forms most of the bulk of the sperm and contains the haploid nucleus. The anterior two thirds of the head is covered by the acrosome, a cap-like saccular organelle containing several enzymes. When released, these enzymes facilitate dispersion of the follicular cells of the corona radiata and sperm penetration of the zona pellucida during fertilization. The tail of the sperm consists of three segments: middle piece, principal piece, and end piece (Fig. 2-5A). The tail provides the motility of the sperm that assists its transport to the site of fertilization. The middle piece of the tail contains mitochondria, which provide the adenosine triphosphate (ATP) necessary for activity.
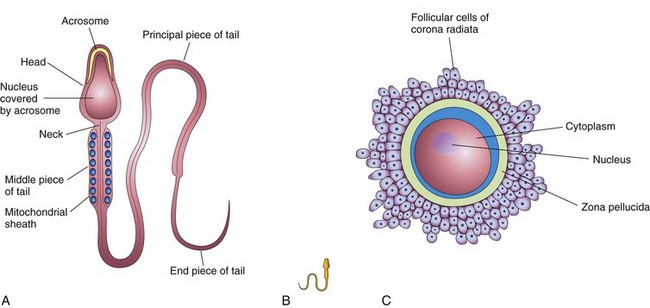
FIGURE 2–5 Male and female gametes (sex cells). A, The main parts of a human sperm (×1250). The head, composed mostly of the nucleus, is partly covered by the cap-like acrosome, an organelle containing enzymes. The tail of the sperm consists of three regions: the middle piece, principal piece, and end piece. B, A sperm drawn to approximately the same scale as the oocyte. C, A human secondary oocyte (×200), surrounded by the zone pellucida and corona radiata.
Many genes and molecular factors are implicated in spermatogenesis. For example, recent studies indicate that proteins of the Bcl-2 family are involved in the maturation of germ cells, as well as their survival at different stages. For normal spermatogenesis, the Y chromosome is essential; microdeletions result in defective spermatogenesis and infertility.
Oogenesis
Oogenesis is the sequence of events by which oogonia are transformed into mature oocytes. This maturation of the oocytes begins before birth and is completed after puberty. Oogenesis continues to menopause, which is the permanent cessation of the menstrual cycle.
Prenatal Maturation of Oocytes
During early fetal life, oogonia proliferate by mitosis, a special type of cell division (Fig. 2-2). Oogonia (primordial female sex cells) enlarge to form primary oocytes before birth; for this reason, no oogonia are shown in Figures 2-1 and 2-3. As the primary oocytes form, connective tissue cells surround them and form a single layer of flattened, follicular cells (see Fig. 2-8). The primary oocyte enclosed by this layer of cells constitutes a primordial follicle (see Fig. 2-9A). As the primary oocyte enlarges during puberty, the follicular epithelial cells become cuboidal in shape and then columnar, forming a primary follicle (see Fig. 2-1). The primary oocyte soon becomes surrounded by a covering of amorphous acellular glycoprotein material, the zona pellucida (see Figs. 2-8 and 2-9B). Scanning electron microscopy of the surface of the zona pellucida reveals a regular mesh-like appearance with intricate fenestrations.
Primary oocytes begin the first meiotic divisions before birth, but completion of prophase does not occur until adolescence. The follicular cells surrounding the primary oocytes secrete a substance, oocyte maturation inhibitor, which keeps the meiotic process of the oocyte arrested.
Postnatal Maturation of Oocytes
Beginning during puberty, usually one follicle matures each month and ovulation occurs, except when oral contraceptives are used. The long duration of the first meiotic division (up to 45 years) may account in part for the relatively high frequency of meiotic errors, such as nondisjunction (failure of paired chromatids of a chromosome to dissociate), that occur with increasing maternal age. The primary oocytes in suspended prophase (dictyotene) are vulnerable to environmental agents such as radiation.
No primary oocytes form after birth, in contrast to the continuous production of primary spermatocytes (Fig. 2-3). The primary oocytes remain dormant in the ovarian follicles until puberty. As a follicle matures, the primary oocyte increases in size and shortly before ovulation, completes the first meiotic division to give rise to a secondary oocyte and the first polar body. Unlike the corresponding stage of spermatogenesis, however, the division of cytoplasm is unequal. The secondary oocyte receives almost all the cytoplasm (Fig. 2-1), and the first polar body receives very little. This polar body is a small, nonfunctional cell. At ovulation, the nucleus of the secondary oocyte begins the second meiotic division, but progresses only to metaphase, when division is arrested. If a sperm penetrates the secondary oocyte, the second meiotic division is completed, and most cytoplasm is again retained by one cell, the fertilized oocyte (Fig. 2-1). The other cell, the second polar body, is also a small nonfunctional cell. As soon as the polar bodies are extruded, maturation of the oocyte is complete.
There are approximately 2 million primary oocytes in the ovaries of a newborn female, but most regress during childhood so that by adolescence no more than 40,000 remain. Of these, only approximately 400 become secondary oocytes and are expelled at ovulation during the reproductive period. Few of these oocytes, if any, are fertilized and become mature. The number of oocytes that ovulate is greatly reduced in women who take oral contraceptives because the hormones in them prevent ovulation from occurring.
Comparison of Gametes (sex Cells)
The gametes (oocytes or sperms) are haploid cells (have half the number of chromosomes) that can undergo karyogamy (fusion of the nuclei of two sex cells). The oocyte is a massive cell compared with the sperm and is immotile (Fig. 2-5), whereas the microscopic sperm is highly motile. The oocyte is surrounded by the zona pellucida and a layer of follicular cells, the corona radiata (Fig. 2-5C).
With respect to sex chromosome constitution, there are two kinds of normal sperms: 23, X and 23, Y, whereas there is only one kind of normal secondary oocyte: 23, X (Fig. 2-1). By convention, the number 23 is followed by a comma and an X or Y to indicate the sex chromosome constitution; for example, 23, X indicates that there are 23 chromosomes in the complement, consisting of 22 autosomes and one sex chromosome (an X in this case). The difference in the sex chromosome complement of sperms forms the basis of primary sex determination.
Abnormal Gametes
The ideal biological maternal age for reproduction is considered to be from 18 to 35 years. The likelihood of chromosomal abnormalities in the embryo gradually increases as the mother ages. In older mothers, there is an appreciable risk of Down syndrome or some other form of trisomy in the infant (see Chapter 20). The likelihood of a fresh gene mutation (change in DNA) also increases with age. The older the parents are at the time of conception, the more likely they are to have accumulated mutations that the embryo might inherit.
During gametogenesis, homologous chromosomes sometimes fail to separate, a pathogenic process called nondisjunction; as a result, some gametes have 24 chromosomes and others only 22 (Fig. 2-3). If a gamete with 24 chromosomes unites with a normal one with 23 chromosomes during fertilization, a zygote with 47 chromosomes forms (see Fig. 20-2). This condition is called trisomy because of the presence of three representatives of a particular chromosome instead of the usual two. If a gamete with only 22 chromosomes unites with a normal one, a zygote with 45 chromosomes forms. This condition is called monosomy because only one representative of the particular chromosome pair is present. For a description of the clinical conditions associated with numerical disorders of chromosomes, see Chapter 20.
As many as 10% of sperms ejaculated are grossly abnormal (e.g., with two heads), but it is believed that these abnormal sperms do not fertilize oocytes due to their lack of normal motility. Most morphologically abnormal sperms are unable to pass through the mucus in the cervical canal. Measurement of forward progression is a subjective assessment of the quality of sperm movement. Radiography, severe allergic reactions, and certain antispermatogenic agents have been reported to increase the percentage of abnormally shaped sperms. Such sperms are not believed to affect fertility unless their number exceeds 20%.
Although some oocytes have two or three nuclei, these cells die before they reach maturity. Similarly, some ovarian follicles contain two or more oocytes, but this phenomenon is rare.
Uterus, Uterine Tubes, and Ovaries
A brief description of the structure of the uterus, uterine tubes, and ovaries is presented as a basis for understanding reproductive ovarian cycles and implantation of the blastocyst (see Figs. 2-7 and 2-19).
Uterus
The uterus is a thick-walled, pear-shaped muscular organ, averaging 7 to 8 cm in length, 5 to 7 cm in width at its superior part, and 2 to 3 cm in wall thickness. The uterus consists of two major parts (Fig. 2-6A): the body, the superior two thirds, and the cervix, the cylindrical inferior one third.
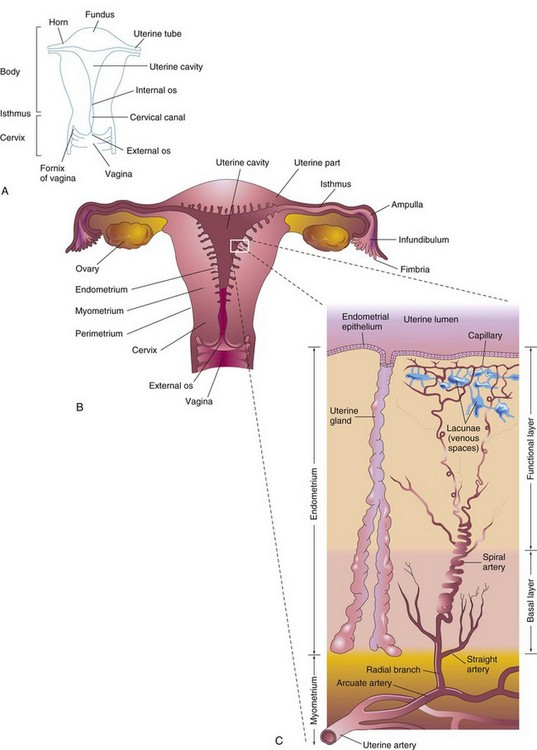
FIGURE 2–6 A, Parts of the uterus and vagina. B, Diagrammatic frontal section of the uterus, uterine tubes, and vagina. The ovaries are also shown. C, Enlargement of the area outlined in B. The functional layer of the endometrium is sloughed off during menstruation.
The body of the uterus narrows from the fundus, the rounded superior part of the body, to the isthmus, the 1-cm-long constricted region between the body and the cervix. The cervix of the uterus is its tapered vaginal end that is nearly cylindrical in shape. The lumen of the cervix, the cervical canal, has a constricted opening at each end. The internal os (opening) of the uterus communicates with the cavity of the uterine body and the external os communicates with the vagina. The walls of the body of the uterus consist of three layers (Fig. 2-6B):
The perimetrium is a peritoneal layer that is firmly attached to the myometrium. During the luteal (secretory) phase of the menstrual cycle, three layers of the endometrium can be distinguished microscopically (Fig. 2-6C):
At the peak of its development, the endometrium is 4 to 5 mm thick. The basal layer of the endometrium has its own blood supply and is not sloughed off during menstruation. The compact and spongy layers, known collectively as the functional layer, disintegrate and are shed during menstruation and after parturition (delivery of a baby).
Uterine Tubes
The uterine tubes, approximately 10 cm long and 1 cm in diameter, extend laterally from the horns of the uterus (Fig. 2-6A). Each tube opens at its proximal end into the horn of the uterus and into the peritoneal cavity at its distal end. For descriptive purposes, the uterine tube is divided into four parts: infundibulum, ampulla, isthmus, and uterine part. The tubes carry oocytes from the ovaries and sperms entering from the uterus to reach the fertilization site in the ampulla (Fig. 2-6B). The uterine tube also conveys the cleaving zygote to the uterine cavity.
Ovaries
The ovaries are almond-shaped reproductive glands located close to the lateral pelvic walls on each side of the uterus that produce oocytes (Fig. 2-6B). The ovaries also produce estrogen and progesterone, the hormones responsible for the development of secondary sex characteristics and regulation of pregnancy.
Female Reproductive Cycles
Commencing at puberty, females undergo reproductive cycles (sexual cycles), involving activities of the hypothalamus of the brain, pituitary gland, ovaries, uterus, uterine tubes, vagina, and mammary glands (Fig. 2-7). These monthly cycles prepare the reproductive system for pregnancy.
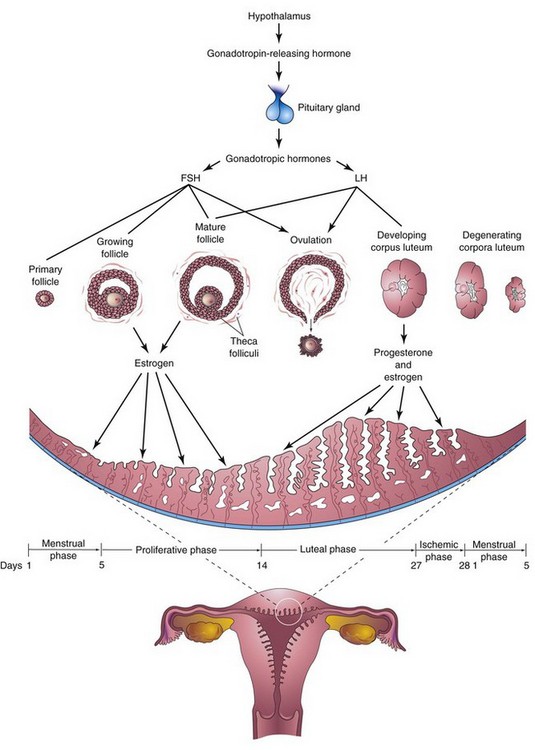
FIGURE 2–7 Schematic drawings illustrating the interrelations of the hypothalamus of the brain, pituitary gland, ovaries, and endometrium. One complete menstrual cycle and the beginning of another are shown. Changes in the ovaries, the ovarian cycle, are induced by the gonadotropic hormones (follicle-stimulating hormone and luteinizing hormone). Hormones from the ovaries (estrogens and progesterone) then promote cyclic changes in the structure and function of the endometrium, the menstrual cycle. Thus, the cyclical activity of the ovary is intimately linked with changes in the uterus. The ovarian cycles are under the rhythmic endocrine control of the pituitary gland, which in turn is controlled by the gonadotropin-releasing hormone produced by neurosecretory cells in the hypothalamus.
A gonadotropin-releasing hormone is synthesized by neurosecretory cells in the hypothalamus.
The gonadotropin-releasing hormone is carried by the hypophysial portal system to the anterior lobe of the pituitary gland. This hormone stimulates the release of two hormones produced by this gland that act on the ovaries:
Ovarian Cycle
FSH and LH produce cyclic changes in the ovaries—the ovarian cycle (Fig. 2-7)—development of follicles (Fig. 2-8), ovulation, and corpus luteum formation. During each cycle, FSH promotes growth of several primordial follicles into 5 to 12 primary follicles (Fig. 2-9A); however, only one primary follicle usually develops into a mature follicle and ruptures through the surface of the ovary, expelling its oocyte (Fig. 2-10).
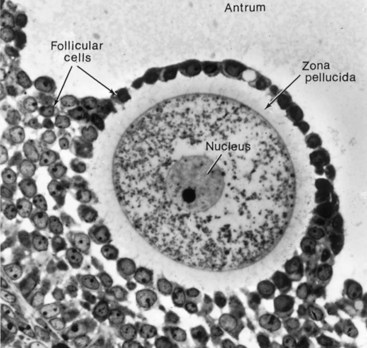
FIGURE 2–8 Photomicrograph of a human primary oocyte in a secondary follicle, surrounded by the zona pellucida and follicular cells. The mound of tissue, the cumulus oophorus, projects into the antrum.
(From Bloom W, Fawcett DW: A Textbook of Histology, 10th ed. Philadelphia, WB Saunders, 1975. Courtesy of L. Zamboni.)
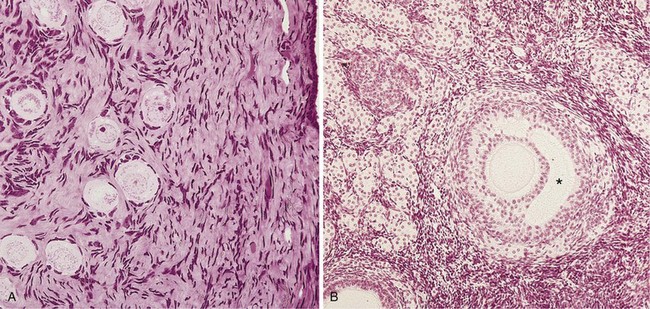
FIGURE 2–9 Micrographs of the ovarian cortex. A, Several primordial follicles are visible (×270). Observe that the primary oocytes are surrounded by follicular cells. B, Secondary ovarian follicle. The oocyte is surrounded by granulosa cells of the cumulus oophorus (×132). The antrum can be clearly seen (*).
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 2nd ed. Philadelphia, WB Saunders, 2001.)
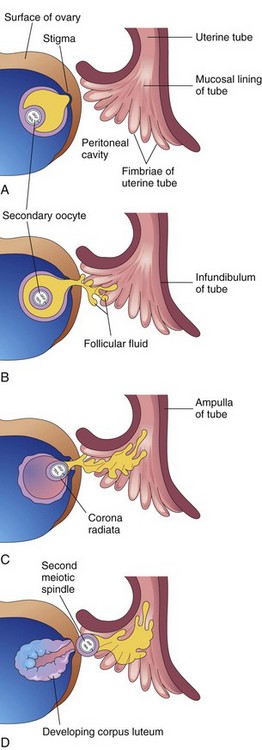
FIGURE 2–10 Illustrations of ovulation. Note that fimbriae of the infundiulum of the uterine tube are closely applied to the ovary. The finger-like fimbriae move back and forth over the ovary and “sweep” the oocyte into the infundibulum. When the stigma (swelling) ruptures, the secondary oocyte is expelled from the ovarian follicle with the follicular fluid. After ovulation, the wall of the follicle collapses and is thrown into folds. The follicle is transformed into a glandular structure, the corpus luteum.
Follicular Development
Development of an ovarian follicle (Figs. 2-8 and 2-9) is characterized by:
As the primary follicle increases in size, the adjacent connective tissue organizes into a capsule, the theca folliculi (Fig. 2-7). The theca soon differentiates into two layers, an internal vascular and glandular layer, the theca interna, and a capsule-like layer, the theca externa. Thecal cells are thought to produce an angiogenesis factor that promotes growth of blood vessels in the theca interna (Fig. 2-9B), which provide nutritive support for follicular development. The follicular cells divide actively, producing a stratified layer around the oocyte (Fig. 2-9B). The ovarian follicle soon becomes oval and the oocyte eccentric in position. Subsequently, fluid-filled spaces appear around the follicular cells, which coalesce to form a single large cavity, the antrum, which contains follicular fluid (Figs. 2-8 and 2-9B). After the antrum forms, the ovarian follicle is called a vesicular or secondary follicle.
The primary oocyte is pushed to one side of the follicle, where it is surrounded by a mound of follicular cells, the cumulus oophorus, that projects into the antrum (Figs. 2-9B). The follicle continues to enlarge until it reaches maturity and produces a swelling on the surface of the ovary (Fig. 2-10A).
The early development of ovarian follicles is induced by FSH, but final stages of maturation require LH as well. Growing follicles produce estrogen, a hormone that regulates development and function of the reproductive organs. The vascular theca interna produces follicular fluid and some estrogen. Its cells also secrete androgens that pass to the follicular cells (Fig. 2-8), which, in turn, convert them into estrogen. Some estrogen is also produced by widely scattered groups of stromal secretory cells, known collectively as the interstitial gland of the ovary.
Ovulation
Around midcycle, the ovarian follicle, under the influence of FSH and LH, undergoes a sudden growth spurt, producing a cystic swelling or bulge on the surface of the ovary. A small avascular spot, the stigma, soon appears on this swelling (see Fig. 2-10A). Before ovulation, the secondary oocyte and some cells of the cumulus oophorus detach from the interior of the distended follicle (Fig. 2-10B).
Ovulation is triggered by a surge of LH production (Fig. 2-11). Ovulation usually follows the LH peak by 12 to 24 hours. The LH surge, elicited by the high estrogen level in the blood, appears to cause the stigma to balloon out, forming a vesicle (Fig. 2-10A). The stigma soon ruptures, expelling the secondary oocyte with the follicular fluid (Fig. 2-10B to D). Expulsion of the oocyte is the result of intrafollicular pressure and possibly contraction of smooth muscle in the theca externa owing to stimulation by prostaglandins. Mitogen-activated protein kinases 3 and 1 (MAPK 3/1), also known as extracellular signal-regulated kinases 1 and 2 (ERK1/2) in ovarian glanulosa cells seem to regulate signaling pathways that control ovulation. Plasmins and matrix metalloproteins appear also to play a role in controlling rupture of the follicle. The expelled secondary oocyte is surrounded by the zona pellucida and one or more layers of follicular cells, which are radially arranged as the corona radiata (Fig. 2-10C), forming the oocyte-cumulus complex. The LH surge also seems to induce resumption of the first meiotic division of the primary oocyte. Hence, mature ovarian follicles contain secondary oocytes (Fig. 2-10A and B). The zona pellucida (Fig. 2-8) is composed of three glycoproteins (ZPA, ZPB, ZPC), which usually form a network of filaments with multiple pores. Binding of the sperm to the zona pellucida (sperm–oocyte interactions) is a complex and critical event during fertilization.
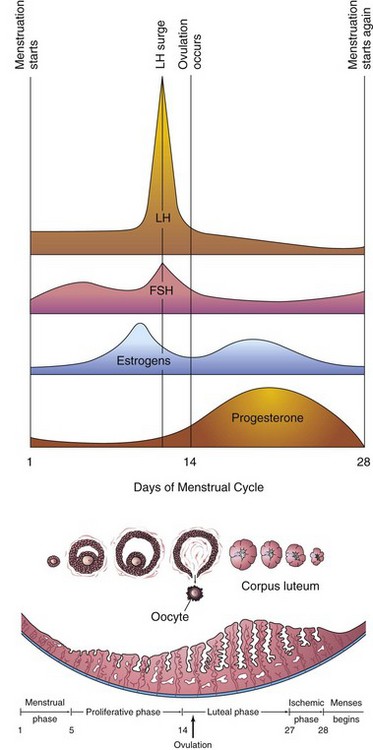
FIGURE 2–11 Illustration of the blood levels of various hormones during the menstrual cycle. Follicle-stimulating hormone (FSH) stimulates the ovarian follicles to develop and produce estrogens. The level of estrogens rises to a peak just before the luteinizing hormone (LH) surge. Ovulation normally occurs 24 to 36 hours after the LH surge. If fertilization does not occur, the blood levels of circulating estrogens and progesterone fall. This hormone withdrawal causes the endometrium to regress and menstruation to start again.
Mittelschmerz and Ovulation
A variable amount of abdominal pain, mittelschmerz (German mittel, mid + schmerz, pain), accompanies ovulation in some women. In these cases, ovulation results in slight bleeding into the peritoneal cavity, which results in sudden constant pain in the lower abdomen. Mittelschmerz may be used as a symptom of ovulation, but there are better symptoms, such as the slight drop in basal body temperature.
Anovulation
Some women do not ovulate (cessation of ovulation—anovulation) because of an inadequate release of gonadotropins. In some of these women, ovulation can be induced by the administration of gonadotropins or an ovulatory agent such as clomiphene citrate. This drug stimulates the release of pituitary gonadotropins (FSH and LH), resulting in maturation of several ovarian follicles and multiple ovulations. The incidence of multiple pregnancy increases as much as tenfold when ovulation is induced. Rarely do more than seven embryos survive.
Corpus Luteum
Shortly after ovulation, the walls of the ovarian follicle and theca folliculi collapse and are thrown into folds (see Fig. 2-10D). Under LH influence, they develop into a glandular structure, the corpus luteum, which secretes progesterone and some estrogen, causing the endometrial glands to secrete and prepare the endometrium for implantation of the blastocyst.
If the oocyte is fertilized, the corpus luteum enlarges to form a corpus luteum of pregnancy and increases its hormone production. Degeneration of the corpus luteum is prevented by human chorionic gonadotropin, a hormone secreted by the syncytiotrophoblast of the blastocyst (see Fig. 2-19B). The corpus luteum of pregnancy remains functionally active throughout the first 20 weeks of pregnancy. By this time, the placenta has assumed the production of the estrogen and progesterone necessary for the maintenance of pregnancy (see Chapter 7).
If the oocyte is not fertilized, the corpus luteum involutes and degenerates 10 to 12 days after ovulation. It is then called a corpus luteum of menstruation. The corpus luteum is subsequently transformed into white scar tissue in the ovary, called a corpus albicans. Ovarian cycles terminate at menopause, the permanent cessation of menstruation due to ovarian failure; menopause usually occurs between the ages of 48 and 55. The endocrine, somatic (body), and psychological changes occurring at the termination of the reproductive period are called the climacteric.
Menstrual Cycle
The menstrual cycle is the time during which the oocyte matures, is ovulated, and enters the uterine tube. The hormones produced by the ovarian follicles and corpus luteum (estrogen and progesterone) produce cyclic changes in the endometrium (Fig. 2-11). These monthly changes in the internal layer of the uterus constitute the endometrial cycle, commonly referred to as the menstrual cycle or period because menstruation (flow of blood from the uterus) is an obvious event.
The endometrium is a “mirror” of the ovarian cycle because it responds in a consistent manner to the fluctuating concentrations of gonadotropic and ovarian hormones (Figs. 2-7 and 2-11). The average menstrual cycle is 28 days, with day 1 of the cycle designated as the day on which menstrual flow begins. Menstrual cycles normally vary in length by several days. In 90% of women, the length of the cycles ranges between 23 and 35 days. Almost all these variations result from alterations in the duration of the proliferative phase of the menstrual cycle.
Anovulatory Menstrual Cycles
The typical menstrual cycle, illustrated in Figure 2-11, is not always realized because the ovary may not produce a mature follicle and ovulation does not occur. In anovulatory cycles, the endometrial changes are minimal; the proliferative endometrium develops as usual, but no ovulation occurs and no corpus luteum forms. Consequently, the endometrium does not progress to the luteal phase; it remains in the proliferative phase until menstruation begins. Anovulatory cycles may result from ovarian hypofunction. The estrogen, with or without progesterone, in oral contraceptives (birth control pills) acts on the hypothalamus and pituitary gland, resulting in inhibition of secretion of gonadotropin-releasing hormone and FSH and LH, the secretion of which is essential for ovulation to occur.
Phases of the Menstrual Cycle
Changes in the estrogen and progesterone levels cause cyclic changes in the structure of the female reproductive tract, notably the endometrium. The menstrual cycle is a continuous process; each phase gradually passes into the next one (see Fig. 2-11).
Menstrual phase. The functional layer of the uterine wall (Fig. 2-6C) is sloughed off and discarded with the menstrual flow, called menses (monthly bleeding), which usually lasts 4 to 5 days. The blood discharged through the vagina is combined with small pieces of endometrial tissue. After menstruation, the eroded endometrium is thin.
Proliferative phase. This phase, lasting approximately 9 days, coincides with growth of ovarian follicles and is controlled by estrogen secreted by these follicles. There is a two- to three-fold increase in the thickness of the endometrium and in its water content during this phase of repair and proliferation. Early during this phase, the surface epithelium reforms and covers the endometrium. The glands increase in number and length and the spiral arteries elongate.
Luteal phase. The luteal or secretory phase, lasting approximately 13 days, coincides with the formation, functioning, and growth of the corpus luteum. The progesterone produced by the corpus luteum stimulates the glandular epithelium to secrete a glycogen-rich material. The glands become wide, tortuous, and saccular, and the endometrium thickens because of the influence of progesterone and estrogen from the corpus luteum, and because of increased fluid in the connective tissue. As the spiral arteries grow into the superficial compact layer, they become increasingly coiled (Fig. 2-6C). The venous network becomes complex and large lacunae (venous spaces) develop. Direct arteriovenous anastomoses are prominent features of this stage.
If fertilization does not occur:
Ischemic phase. The phase occurs when the oocyte is not fertilized. Ischemia (reduced blood supply) occurs as the spiral arteries constrict, giving the endometrium a pale appearance. This constriction results from the decreasing secretion of hormones, primarily progesterone, by the degenerating corpus luteum. In addition to vascular changes, the hormone withdrawal results in the stoppage of glandular secretion, a loss of interstitial fluid, and a marked shrinking of the endometrium. Toward the end of the ischemic phase, the spiral arteries become constricted for longer periods. This results in venous stasis and patchy ischemic necrosis (death) in the superficial tissues. Eventually, rupture of damaged vessel walls follows and blood seeps into the surrounding connective tissue. Small pools of blood form and break through the endometrial surface, resulting in bleeding into the uterine cavity and from the vagina. As small pieces of the endometrium detach and pass into the uterine cavity, the torn ends of the arteries bleed into the cavity, resulting in a loss of 20 to 80 ml of blood. Eventually, over 3 to 5 days, the entire compact layer and most of the spongy layer of the endometrium are discarded in the menses. Remnants of the spongy and basal layers remain to undergo regeneration during the subsequent proliferative phase of the endometrium. It is obvious from the previous descriptions that the cyclic hormonal activity of the ovary is intimately linked with cyclic histologic changes in the endometrium.
Pregnancy phase. If pregnancy occurs, the menstrual cycles cease and the endometrium passes into a pregnancy phase. With the termination of pregnancy, the ovarian and menstrual cycles resume after a variable period (usually 6 to 10 weeks if the woman is not breast-feeding her baby). Except during pregnancy, the reproductive cycles normally continue until menopause.
Transportation of Gametes
Oocyte Transport
The secondary oocyte is expelled at ovulation from the ovarian follicle with the escaping follicular fluid (Fig. 2-10C and D). During ovulation, the fimbriated end of the uterine tube becomes closely applied to the ovary. The finger-like processes of the tube, fimbriae, move back and forth over the ovary. The sweeping action of the fimbriae and fluid currents produced by the cilia of the mucosal cells of the fimbriae “sweep” the secondary oocyte into the funnel-shaped infundibulum of the uterine tube. The oocyte then passes into the ampulla of the tube, mainly as the result of peristalsis—movements of the wall of the tube characterized by alternate contraction and relaxation—that pass toward the uterus.
Sperm Transport
The sperms are rapidly transported from the epididymis to the urethra by peristaltic contractions of the thick muscular coat of the ductus deferens (Fig. 2-12). The accessory sex glands—seminal glands (vesicles), prostate, and bulbourethral glands—produce secretions that are added to the sperm-containing fluid in the ductus deferens and urethra.
From 200 to 600 million sperms are deposited around the external os of the uterus and in the fornix of the vagina during intercourse (see Fig. 2-6A). The sperms pass through the cervical canal by movements of their tails. The enzyme vesiculase, produced by the seminal glands, coagulates some of the semen or ejaculate and forms a vaginal plug that may prevent the backflow of semen into the vagina. When ovulation occurs, the cervical mucus increases in amount and becomes less viscid (sticky), making it more favorable for sperm transport.
The reflex ejaculation of semen may be divided into two phases:
Passage of sperms through the uterus into the uterine tubes results mainly from muscular contractions of the walls of these organs. Prostaglandins in the semen are thought to stimulate uterine motility at the time of intercourse and assist in the movement of sperms to the site of fertilization in the ampulla of the uterine tube. Fructose, secreted by the seminal glands, is an energy source for the sperms in the semen.
The volume of ejaculate (sperms mix with secretions from the accessory sex glands) averages 3.5 ml, with a range of 2 to 6 ml. The sperms move 2 to 3 mm per minute, but the speed varies with the pH of the environment. They are nonmotile during storage in the epididymis, but become motile in the ejaculate. They move slowly in the acid environment of the vagina, but move more rapidly in the alkaline environment of the uterus. It is not known how long it takes sperms to reach the fertilization site in the ampulla, but the time of transport is probably short. Motile sperms have been recovered from the ampulla 5 minutes after their deposition near the external uterine os. Some sperms, however, take as long as 45 minutes to complete the journey. Only approximately 200 sperms reach the fertilization site; most sperms degenerate and are absorbed in the female genital tract.
Maturation of Sperms
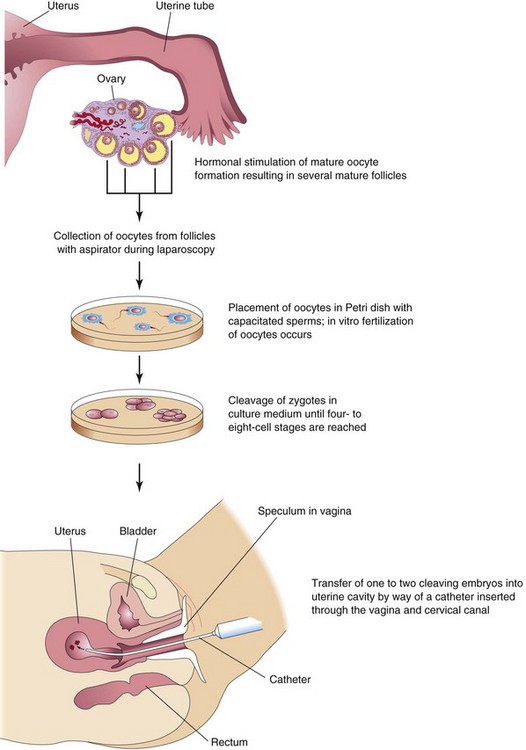
Freshly ejaculated sperms are unable to fertilize oocytes. Sperms must undergo a period of conditioning—capacitation—lasting approximately 7 hours. During this period, a glycoprotein coat and seminal proteins are removed from the surface of the sperm acrosome. The membrane components of the sperms are extensively altered. Capacitated sperms show no morphologic changes, but they are more active. Sperms are usually capacitated in the uterus or uterine tubes by substances secreted by these parts of the female genital tract. During in vitro fertilization, capacitation is induced by incubating the sperms in a defined medium for several hours (see Fig. 2-15). Completion of capacitation permits the acrosome reaction to occur.
The acrosome of the capacitated sperm binds to a glycoprotein (ZP3) on the zona pellucida. Studies have shown that the sperm plasma membrane, calcium ions, prostaglandins, and progesterone play a critical role in the acrosome reaction. This reaction of sperms must be completed before the sperms can fuse with the oocyte. When capacitated sperms come into contact with the corona radiata surrounding a secondary oocyte (Fig. 2-13), they undergo complex molecular changes that result in the development of perforations in the acrosome. Multiple point fusions of the plasma membrane of the sperm and the external acrosomal membrane occur. Breakdown of the membranes at these sites produces apertures. The changes induced by the acrosome reaction are associated with the release of enzymes, including hyaluronidase and acrosin, from the acrosome that facilitate fertilization. Capitation and the acrosome reaction appear to be regulated by a tyrosine kinase, src kinase.
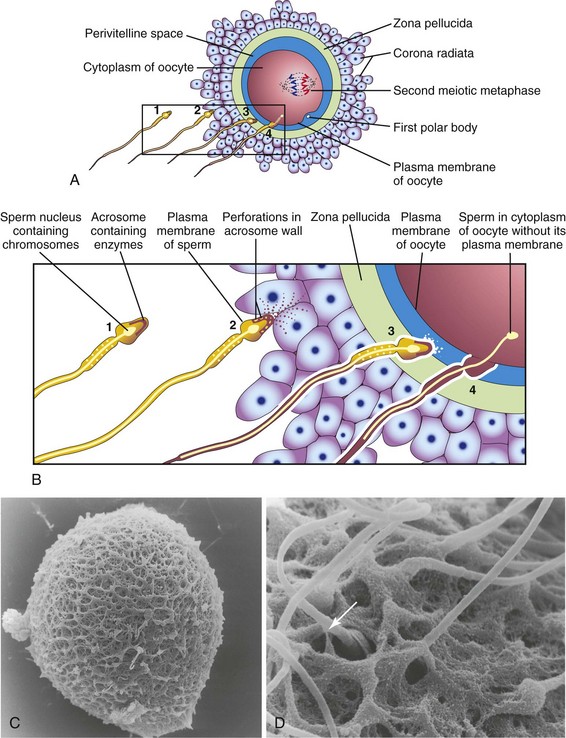
FIGURE 2–13 Acrosome reaction and sperm penetrating an oocyte. The detail of the area outlined in A is given in B. 1, Sperm during capacitation, a period of conditioning that occurs in the female reproductive tract. 2, Sperm undergoing the acrosome reaction, during which perforations form in the acrosome. 3, Sperm digesting a path through the zona pellucida by the action of enzymes released from the acrosome. 4, Sperm after entering the cytoplasm of the oocyte. Note that the plasma membranes of the sperm and oocyte have fused and that the head and tail of the sperm enter the oocyte, leaving the sperm’s plasma membrane attached to the oocyte’s plasma membrane. C, Scanning electron microscopy of an unfertilized human oocyte showing relatively few sperms attached to the zona pellucida. D, Scanning electron microscopy of a human oocyte showing penetration of the sperm (arrow) into the zona pellucida.
(Courtesy of P. Schwartz and H.M. Michelmann, University of Goettingen, Goettingen, Germany.)
Male Fertility
During evaluation of male fertility, an analysis of semen is made. Sperms account for less than 10% of the semen. The remainder of the ejaculate consists of the secretions of the seminal glands, prostate, and bulbourethral glands. There are usually more than 100 million sperms per milliliter of semen in the ejaculate of normal males. Although there is much variation in individual cases, men whose semen contains 20 million sperms per milliliter, or 50 million in the total specimen, are probably fertile. A man with fewer than 10 million sperms per milliliter of semen is likely to be sterile, especially when the specimen contains immotile and abnormal sperms. For potential fertility, 50% of sperms should be motile after 2 hours and some should be motile after 24 hours. Male infertility may result from a low sperm count, poor sperm motility, medications and drugs, endocrine disorders, exposure to environmental pollutants, cigarette smoking, abnormal sperms, or obstruction of a genital duct such as in the ductus deferens (see Fig. 2-12). Male infertility is detectable in 30% to 50% of involuntary childless couples.
Vasectomy
The most effective method of permanent contraception in men is vasectomy, or excision of a segment of each ductus deferens (vas deferens). Following vasectomy, there are no sperms in the semen or ejaculate, but the volume is essentially the same. Reversal of vasectomy is technically feasible by microsurgical techniques; however, the success rate is variable.
Dispermy and Triploidy
Although several sperms attach to the corona radiata and zona pellucida, usually only one sperm penetrates the oocyte and fertilizes it. Two sperms may participate in fertilization during an abnormal process known as dispermy, resulting in a zygote with an extra set of chromosomes. Triploid conceptions account for approximately 20% of chromosomally abnormal spontaneous abortions. Triploid embryos (69 chromosomes) may appear normal, but they nearly always abort or die shortly after birth.
Viability of Gametes
Studies on early stages of development indicate that human oocytes are usually fertilized within 12 hours after ovulation. In vitro observations have shown that the oocyte cannot be fertilized after 24 hours and that it degenerates shortly thereafter. Most human sperms probably do not survive for more than 48 hours in the female genital tract. After ejaculation, sperms are stored in folds of the mucosa of the cervix and are gradually released into the cervical canal and pass through the uterus into the uterine tubes. The short-term storage of sperms in the cervix provides a gradual release of sperms and thereby increases the chances of fertilization. Sperms and oocytes can be frozen and stored for many years and can be used for in vitro fertilization.
 Fertilization
Fertilization
The usual site of fertilization is in the ampulla of the uterine tube (Fig. 2-6B). If the oocyte is not fertilized there, it slowly passes along the tube to the body of the uterus, where it degenerates and is resorbed. Although fertilization may occur in other parts of the tube, it does not occur in the body of the uterus. Chemical signals (attractants), secreted by the oocyte and surrounding follicular cells, guide the capacitated sperms (sperm chemotaxis) to the oocyte.
Fertilization is a complex sequence of coordinated molecular events that begins with contact between a sperm and an oocyte (see Fig. 2-13), and ends with the intermingling of maternal and paternal chromosomes at metaphase of the first mitotic division of the zygote, a unicellular embryo (Fig. 2-14E).
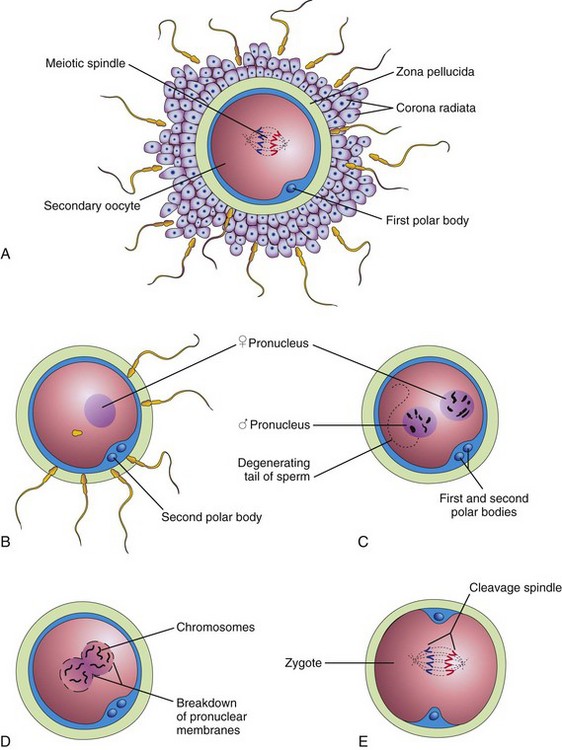
FIGURE 2–14 Illustrations of fertilization, the procession of events beginning when the sperm contacts the secondary oocyte’s plasma membrane, and ending with the intermingling of maternal and paternal chromosomes at metaphase of the first mitotic division of the zygote. A, Secondary oocyte surrounded by several sperms, two of which have penetrated the corona radiata. (Only 4 of the 23 chromosome pairs are shown.) B, The corona radiata is not shown, a sperm has entered the oocyte, and the second meiotic division has occurred, forming a mature oocyte. The nucleus of the oocyte is now the female pronucleus. C, The sperm head has enlarged to form the male pronucleus. This cell, now called an ootid, contains the male and female pronuclei. D, The pronuclei are fusing. E, The zygote has formed; it contains 46 chromosomes, the diploid number.
Defects at any stage in the sequence of these events might cause the zygote to die. The fertilization process takes approximately 24 hours. Transgenic and gene knockout studies in animals have shown that carbohydrate-binding molecules and gamete-specific proteins on the surface of the sperms are involved in sperm–egg recognition and their union.
Phases of Fertilization
Fertilization is a sequence of coordinated events (see Figs. 2-13 and 2-14):
The zygote is genetically unique because half of its chromosomes came from the mother and half from the father. The zygote contains a new combination of chromosomes that is different from that in the cells of either of the parents. This mechanism forms the basis of biparental inheritance and variation of the human species. Meiosis allows independent assortment of maternal and paternal chromosomes among the germ cells (see Fig. 2-2). Crossing over of chromosomes, by relocating segments of the maternal and paternal chromosomes, “shuffles” the genes, thereby producing a recombination of genetic material. The embryo’s chromosomal sex is determined at fertilization by the kind of sperm (X or Y) that fertilizes the oocyte. Fertilization by an X-bearing sperm produces a 46, XX zygote, which develops into a female, whereas fertilization by a Y-bearing sperm produces a 46, XY zygote, which develops into a male.
Fertilization
Preselection of Embryo’s Sex
Because X and Y sperms are formed in equal numbers, the expectation is that the sex ratio at fertilization (primary sex ratio) would be 1.00 (100 boys per 100 girls). It is well known, however, that there are more male babies than female babies born in all countries. In North America, for example, the sex ratio at birth (secondary sex ratio) is approximately 1.05 (105 boys per 100 girls). Various microscopic techniques have been developed in an attempt to separate X and Y sperms (gender selection) using:
The use of a selected sperm sample in artificial insemination may produce the desired sex.
Assisted Reproductive Technologies
In Vitro Fertilization and Embryo Transfer
In vitro fertilization (IVF) of oocytes and transfer of the cleaving zygotes into the uterus have provided an opportunity for many women who are sterile (e.g., owing to tubal occlusion) to have children. The first of these babies was born in 1978. Since then, several million children have been born after an in vitro fertilization procedure. The steps involved during in vitro fertilization and embryo transfer are as follows (Fig. 2-15):
Cryopreservation of Embryos
Early embryos resulting from in vitro fertilization can be preserved for long periods by freezing them in liquid nitrogen with a cryoprotectant (e.g., glycerol or dimethyl sulfoxide, DMSO). Successful transfer of four- to eight-cell embryos and blastocysts to the uterus after thawing is now a common practice. The longest period of sperm cryopreservation that resulted in a live birth was reported to be 21 years.
Intracytoplasmic Sperm Injection
A sperm can be injected directly into the cytoplasm of a mature oocyte. This technique has been successfully used for the treatment of couples for whom in vitro fertilization failed or in cases where there are too few sperms available.
Assisted In Vivo Fertilization
A technique enabling fertilization to occur in the uterine tube is called gamete intrafallopian (intra tubal) transfer. It involves superovulation (similar to that used for IVF), oocyte retrieval, sperm collection, and laparoscopic placement of several oocytes and sperms into the uterine tubes. Using this technique, fertilization occurs in the ampulla, its usual location.
 Cleavage of Zygote
Cleavage of Zygote
Cleavage consists of repeated mitotic divisions of the zygote, resulting in a rapid increase in the number of cells (blastomeres). These embryonic cells become smaller with each successive cleavage division (Figs. 2-16 and 2-17). Cleavage occurs as the zygote passes along the uterine tube toward the uterus (see Fig. 2-20). During cleavage, the zygote is within the zona pellucida. Division of the zygote into blastomeres begins approximately 30 hours after fertilization. Subsequent cleavage divisions follow one another, forming progressively smaller blastomeres. After the nine-cell stage, the blastomeres change their shape and tightly align themselves against each other to form a compact ball of cells. This phenomenon, compaction, is probably mediated by cell-surface-adhesion glycoproteins. Compaction permits greater cell-to-cell interaction and is a prerequisite for segregation of the internal cells that form the inner cell mass or embryoblast of the blastocyst (Fig. 2-16E and F). When there are 12 to 32 blastomeres, the developing human is called a morula. Internal cells of the morula are surrounded by trophoblastic cells. The morula forms approximately 3 days after fertilization as it enters the uterus (Fig. 2-16D).
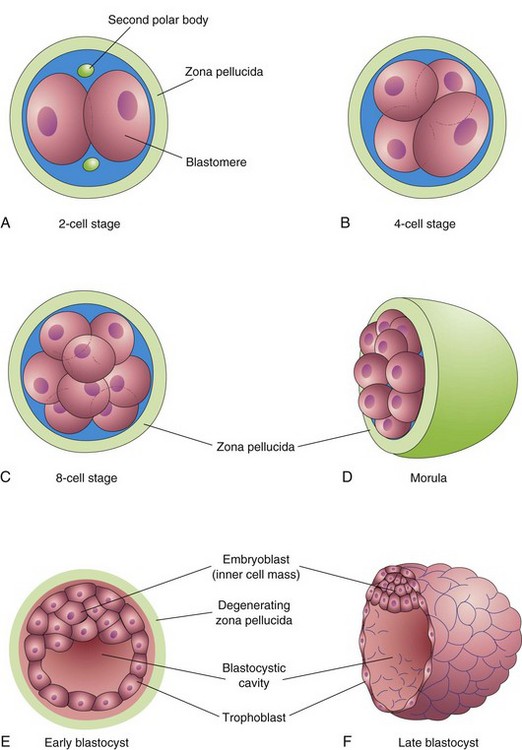
FIGURE 2–16 Illustrations of cleavage of the zygote and formation of the blastocyst. A to D, Various stages of cleavage of the zygote. The period of the morula begins at the 12- to 16-cell stage and ends when the blastocyst forms. E and F, Sections of blastocysts. The zona pellucida has disappeared by the late blastocyst stage (5 days). The second polar bodies shown in A are small, nonfunctional cells. Cleavage of the zygote and formation of the morula occur as the dividing zygote passes along the uterine tube. Blastocyst formation occurs in the uterus. Although cleavage increases the number of blastomeres, note that each of the daughter cells is smaller than the parent cells. As a result, there is no increase in the size of the developing embryo until the zona pellucida degenerates. The blastocyst then enlarges considerably (F).
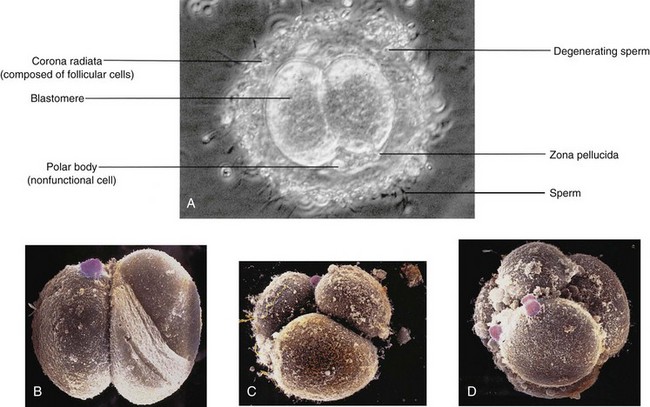
FIGURE 2–17 A, Two-cell stage of a cleaving zygote developing in vitro. Observe that it is surrounded by many sperms. B, In vitro fertilization, two-cell stage human embryo. The zona pellucida has been removed. A small rounded polar body (pink) is still present on the surface of a blastomere (artificially colored, scanning electron microscopy, ×1000). C, Three-cell stage human embryo, in vitro fertilization (scanning electron microscopy, ×1300). D, Eight-cell stage human embryo, in vitro fertilization (scanning electron microscopy, ×1100). Note the rounded large blastomeres with several spermatozoa attached.
(A, Courtesy of M.T. Zenzes, In Vitro Fertilization Program, Toronto Hospital, Toronto, Ontario, Canada; D, From Makabe S, Naguro T, Motta PM: Three-dimensional features of human cleaving embryo by ODO method and field emission scanning electron microscopy. In Motta PM: Microscopy of Reproduction and Development: A Dynamic Approach. Rome, Antonio Delfino Editore, 1997.)
Mosaicism
If nondisjunction (failure of a chromosome pair to separate) occurs during an early cleavage division of a zygote, an embryo with two or more cell lines with different chromosome complements is produced. Individuals in whom numerical mosaicism is present are mosaics; for example, a zygote with an additional chromosome 21 might lose the extra chromosome during an early division of the zygote. Consequently, some cells of the embryo would have a normal chromosome complement and others would have an additional chromosome 21. In general, individuals who are mosaic for a given trisomy, such as the mosaic Down syndrome, are less severely affected than those with the usual nonmosaic condition.
 Formation of Blastocyst
Formation of Blastocyst
Shortly after the morula enters the uterus (approximately 4 days after fertilization), a fluid-filled space, the blastocystic cavity, appears inside the morula (Fig. 2-16E). The fluid passes from the uterine cavity through the zona pellucida to form this space. As fluid increases in the blastocystic cavity, it separates the blastomeres into two parts:
Early pregnancy factor, an immunosuppressant protein, is secreted by the trophoblastic cells and appears in the maternal serum within 24 to 48 hours after fertilization. Early pregnancy factor forms the basis of a pregnancy test during the first 10 days of development.
During this stage of development—known as blastogenesis—the conceptus is called a blastocyst (Fig. 2-18). The embryoblast now projects into the blastocystic cavity and the trophoblast forms the wall of the blastocyst. After the blastocyst has floated in the uterine secretions for approximately 2 days, the zona pellucida gradually degenerates and disappears (Figs. 2-16F and 2-18A). Shedding of the zona pellucida and hatching of the blastocyst have been observed in vitro. Shedding of the zona pellucida permits the hatched blastocyst to increase rapidly in size. While floating in the uterus, the embryo derives nourishment from secretions of the uterine glands.
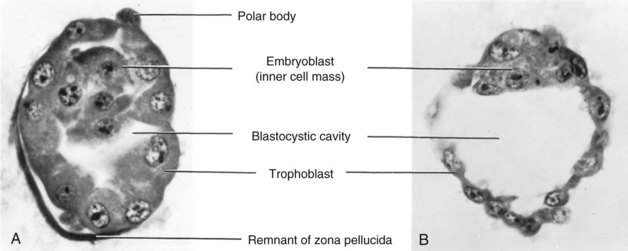
FIGURE 2–18 Photomicrographs of sections of human blastocysts recovered from the uterine cavity (×600). A, At 4 days: the blastocystic cavity is just beginning to form and the zona pellucida is deficient over part of the blastocyst. B, At 4.5 days; the blastocystic cavity has enlarged and the embryoblast and trophoblast are clearly defined. The zona pellucida has disappeared.
(From Hertig AT, Rock J, Adams EC: Am J Anat 98:435, 1956. Courtesy of the Carnegie Institution of Washington.)
Approximately 6 days after fertilization (day 20 of a 28-day menstrual cycle), the blastocyst attaches to the endometrial epithelium, usually adjacent to the embryonic pole (Fig. 2-19A). As soon as it attaches to the endometrial epithelium, the trophoblast proliferates rapidly and differentiates into two layers (Fig. 2-19B):
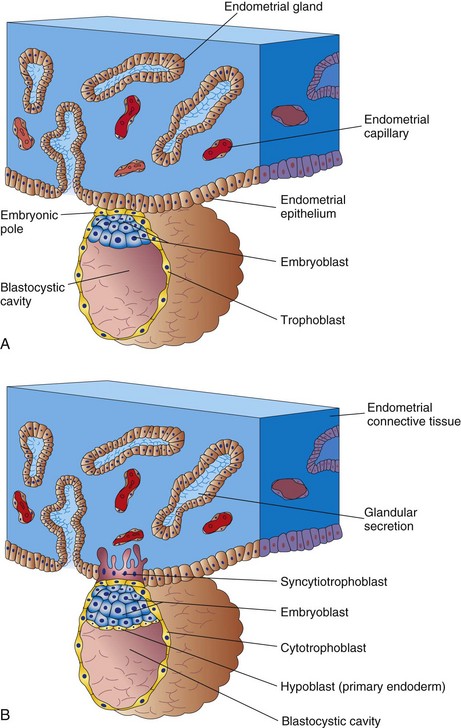
FIGURE 2–19 Attachment of the blastocyst to the endometrial epithelium during the early stages of implantation. A, At 6 days: the trophoblast is attached to the endometrial epithelium at the embryonic pole of the blastocyst. B, At 7 days: the syncytiotrophoblast has penetrated the epithelium and has started to invade the endometrial connective tissue. Note: Some students have difficulty interpreting illustrations such as these because in histologic studies, it is conventional to draw the endometrial epithelium upward, whereas in embryologic studies, the embryo is usually shown with its dorsal surface upward. Because the embryo implants on its future dorsal surface, it would appear upside down if the histologic convention were followed. In this book, the histologic convention is followed when the endometrium is the dominant consideration (e.g., Fig. 2-6C), and the embryologic convention is used when the embryo is the center of interest, as in the adjacent illustrations.
Both intrinsic and extracellular matrix factors modulate, in carefully timed sequences, the differentiation of the trophoblast. Transforming growth factor-β (TGF-β) regulates the proliferation and differentiation of the trophoblast by interaction of the ligand with Type I and Type II receptors, serine/threonine protein kinases. At approximately 6 days, the finger-like processes of syncytiotrophoblast extend through the endometrial epithelium and invade the connective tissue. By the end of the first week, the blastocyst is superficially implanted in the compact layer of the endometrium and is deriving its nourishment from the eroded maternal tissues (Fig. 2-19B). The highly invasive syncytiotrophoblast expands quickly adjacent to the embryoblast, the area known as the embryonic pole (Fig. 2-19A). The syncytiotrophoblast produces enzymes that erode the maternal tissues, enabling the blastocyst to “burrow” into the endometrium. At approximately 7 days, a layer of cells, the hypoblast (primary endoderm), appears on the surface of the embryoblast facing the blastocystic cavity (Fig. 2-19). Comparative embryologic data suggest that the hypoblast arises by delamination of blastomeres from the embryoblast.
Preimplantation Genetic Diagnosis
Preimplantation genetic diagnosis can be carried out 3 to 5 days after in vitro fertilization of the oocyte. One or two cells (blastomeres) are removed from the embryo known to be at risk of a single gene defect or chromosomal anomaly. These cells are then analyzed before transfer into the uterus. The sex of the embryo can also be determined from one blastomere taken from a six- to eight-cell dividing zygote and analyzed by polymerase chain reaction and fluorescence in situ hybridization (FISH) techniques. This procedure has been used to detect female embryos during in vitro fertilization in cases in which a male embryo would be at risk of a serious X-linked disorder. The polar body may also be tested for diseases where the mother is the carrier (Fig. 2-14A).
Abnormal Embryos and Spontaneous Abortions
Many zygotes, morulae, and blastocysts abort spontaneously. Early implantation of the blastocyst is a critical period of development that may fail to occur owing to inadequate production of progesterone and estrogen by the corpus luteum. Clinicians occasionally see a patient who states that her last menstrual period was delayed by several days and that her last menstrual flow was unusually profuse. Very likely such patients have had early spontaneous abortions. The overall early spontaneous abortion rate is thought to be approximately 45%. Early spontaneous abortions occur for a variety of reasons, one being the presence of chromosomal abnormalities. More than half of all known spontaneous abortions occur because of these abnormalities. The early loss of embryos appears to represent a removal of abnormal conceptuses that could not have developed normally, that is, natural screening of embryos, without which the incidence of infants born with birth defects would be far greater.
Summary of First Week (Fig. 2-20)
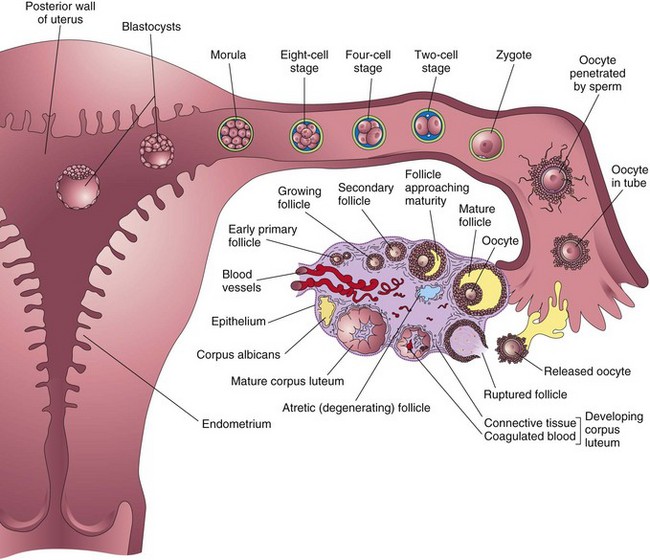
FIGURE 2–20 Summary of the ovarian cycle, fertilization, and human development during the first week. Stage 1 of development begins with fertilization in the uterine tube and ends when the zygote forms. Stage 2 (days 2 to 3) comprises the early stages of cleavage (from 2 to approximately 32 cells, the morula). Stage 3 (days 4 to 5) consists of the free (unattached) blastocyst. Stage 4 (days 5 to 6) is represented by the blastocyst attaching to the posterior wall of the uterus, the usual site of implantation. The blastocysts have been sectioned to show their internal structure.
Clinically Oriented Problems
Discussion of these problems appears at the back of the book.
References and Suggested Reading
Alfarawati S, Goodall N, Gordon T, et al. Cytogenetic analysis of human blastocysts with the use of FISH, CGH and aCGH: scientific data and technical evaluation. Hum Reprod. 2010;25(Suppl 1):i41.
American Society for Reproductive Medicine. Revised guidelines for human embryology and andrology laboratories. Fertil Steril. 2008;90(Supplement):s45.
Antonucc N, Stronati A, Manes S, et al. Setup of a novel in-vitro sperm head decondensation protocol for a rapid flow cytometric measurement of the DNA content. Hum Reprod. 2010;25(Suppl 1):i24.
Bouffard C, Viville S, Knoppers BM. Genetic diagnosis of embryos: clear explanation, not rhetoric, is needed. CMAJ. 2009;181:387.
Barratt CLR, Kay V, Oxenham SK. The human spermatozoa—a stripped down but refined machine. J Biol. 2009;8:63.
Clermont Y, Trott M. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198.
Duggavathi R, Murphy BD. Ovulation signals. Science. 2009;324:890.
Fragouli E, Lenzi M, Ross R, et al. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Hum Reprod. 2008;23:2596.
Frey KA. Male reproductive health and infertility. Prim Care Clin Office Prac. 2010;37:643.
Hampton T. Researchers discover a range of factors undermine sperm quality, male fertility. JAMA. 2005;294:2829.
. Preimplantation Genetic Diagnosis. Harper J, editor, 2nd ed. Cambridge University Press, Cambridge, 2009.
Hertig AT, Rock J, Adams EC, Menkin MC. Thirty-four fertilized human ova, good, bad, and indifferent, recovered from 210 women of known fertility. Pediatrics. 1959;23:202.
Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39.
Gunby J, Bissonnette F, Librach C, Cowan L. Assisted reproductive technologies (ART) in Canada: 2007 results from the Canadian ART Register. Fertil Steril. 2011;95:542.
Kader AA, Choi A, Orief Y, et al. Factors affecting the outcome of human blastocyst. Reprod Biol Endocrin. 2009;7:99.
Myers M, Pangas SA. Regulatory roles of transforming growth factor beta family members in folliculogenesis. WIREs Syst Biol Med. 2010;2:117.
Nusbaum RL, McInnes RR, Willard HF. Thompson & Thompson Genetics in Medicine, 6th ed. Philadelphia: WB Saunders; 2004.
Pauli S, Berga SL, Shang W, et al. Current status of the approach to assisted reproduction. Pediatr Clin North Am. 2009;56:467.
Robertson SA. Immune regulation of embryo implantation – all about quality control. J Reprod Immun. 2009;81:113.
Rock J, Hertig AT. The human conceptus during the first two weeks of gestation. Am J Obstet Gynecol. 1948;55:6.
Shi L. Epigenetic regulation in mammalian preimplantation embryo development. Rep Biol Endocrin. 2009;7:59.
Steptoe PC, Edwards RG. Birth after implantation of a human embryo. Lancet. 1978;2:36.
Wasserman PM. Mammalian fertilization: the strange case of sperm protein 56. Bioessays. 2009;31:153.
Weremowicz S, Sandstrom DJ, Morton CC, et al. Fluorescence in situ hybridization (FISH) for rapid detection of aneuploidy: experience in 911 prenatal cases. Prenat Diagn. 2001;21:262.
Wilmut I, Schnieke AE, McWhir J, et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810.