Chapter 7 Placenta and Fetal Membranes
The placenta and fetal membranes separate the fetus from the endometrium—the inner layer of the uterine wall. An interchange of substances, such as nutrients and oxygen, occurs between the maternal and fetal bloodstreams through the placenta. The vessels in the umbilical cord connect the placental circulation with the fetal circulation. The fetal membranes include the chorion, amnion, umbilical vesicle (yolk sac), and allantois.
Placenta
The placenta is the primary site of nutrient and gas exchange between the mother and fetus. The placenta is a fetomaternal organ that has two components:
The placenta and umbilical cord form a transport system for substances passing between the mother and fetus. Nutrients and oxygen pass from the maternal blood through the placenta to the fetal blood, and waste materials and carbon dioxide pass from the fetal blood through the placenta to the maternal blood. The placenta and fetal membranes perform the following functions and activities: protection, nutrition, respiration, excretion, and hormone production. Shortly after birth, the placenta and fetal membranes are expelled from the uterus as the afterbirth.
Decidua
The decidua is the endometrium of the uterus in a pregnant woman. It is the functional layer of the endometrium that separates from the remainder of the uterus after parturition (childbirth). The three regions of the decidua are named according to their relation to the implantation site (Fig. 7-1):
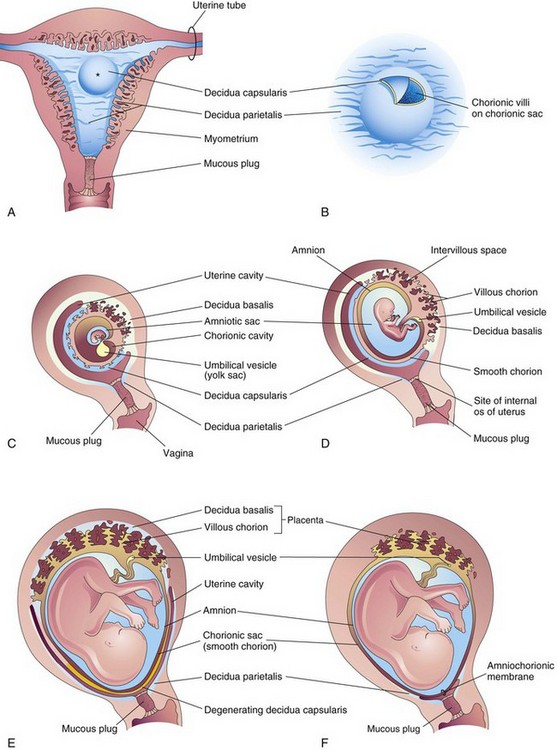
FIGURE 7–1 Development of the placenta and fetal membranes. A, Frontal section of the uterus showing elevation of the decidua capsularis by the expanding chorionic sac of a 4-week embryo implanted in the endometrium on the posterior wall (*). B, Enlarged drawing of the implantation site. The chorionic villi were exposed by cutting an opening in the decidua capsularis. C to F, Sagittal sections of the gravid or pregnant uterus from weeks 5 to 22 showing the changing relations of the fetal membranes to the decidua. In F, the amnion and chorion are fused with each other and the decidua parietalis, thereby obliterating the uterine cavity. Note in D to F that the chorionic villi persist only where the chorion is associated with the decidua basalis.
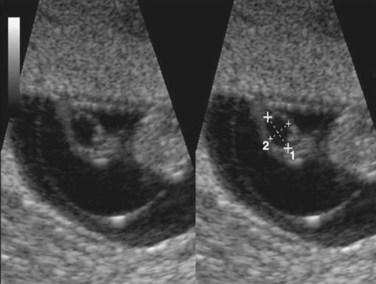
In response to increasing progesterone levels in the maternal blood, the connective tissue cells of the decidua enlarge to form pale-staining decidual cells. These cells enlarge as glycogen and lipid accumulate in their cytoplasm. The cellular and vascular changes occurring in the endometrium as the blastocyst implants constitute the decidual reaction. Many decidual cells degenerate near the chorionic sac in the region of the syncytiotrophoblast and, together with maternal blood and uterine secretions, provide a rich source of nutrition for the embryo. It has also been suggested that they protect the maternal tissue against uncontrolled invasion by the syncytiotrophoblast and they may be involved in hormone production. Decidual regions, clearly recognizable during ultrasonography, are important in diagnosing early pregnancy.
Development of Placenta
Early placental development is characterized by the rapid proliferation of the trophoblast and development of the chorionic sac and chorionic villi (see Chapters 3 and 4). By the end of the third week, the anatomic arrangements necessary for physiologic exchanges between the mother and embryo are established. A complex vascular network is established in the placenta by the end of the fourth week, which facilitates maternal-embryonic exchanges of gases, nutrients, and metabolic waste products.
Chorionic villi cover the entire chorionic sac until the beginning of the eighth week (Figs. 7-1C, 7-2, and 7-3). As this chorionic sac grows, the villi associated with the decidua capsularis become compressed, reducing the blood supply to them. These villi soon degenerate (see Figs. 7-1D and 7-3B), producing a relatively avascular bare area, the smooth chorion (chorion laeve). As the villi disappear, those associated with the decidua basalis rapidly increase in number, branch profusely, and enlarge. This bushy area of the chorionic sac is the villous chorion (chorion frondosum).
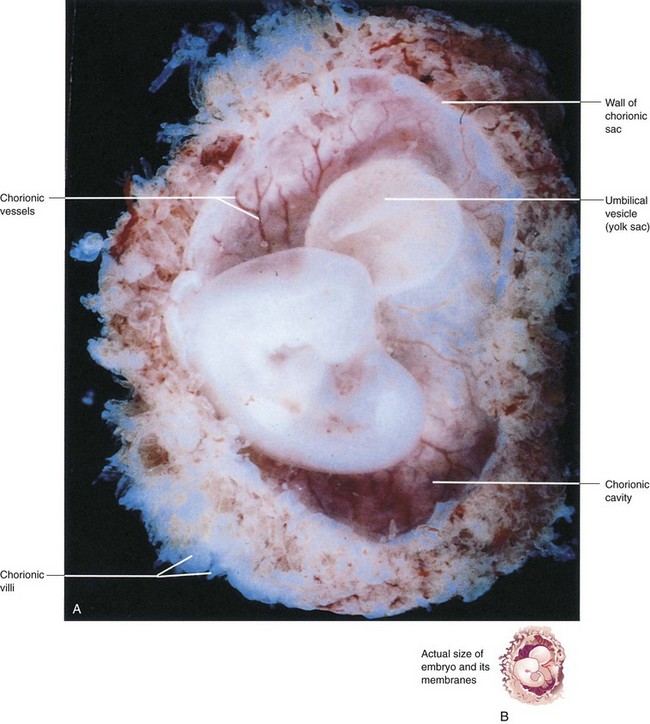
FIGURE 7–2 A, Lateral view of a spontaneously aborted embryo at Carnegie stage 14, approximately 32 days. The chorionic and amniotic sacs have been opened to show the embryo. Note the large size of the umbilical vesicle at this stage. B, The sketch shows the actual size of the embryo and its membranes.
(A, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
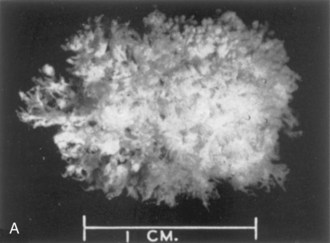
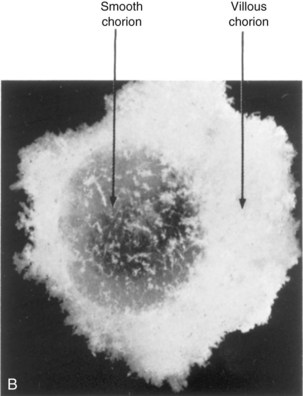
FIGURE 7–3 Spontaneously aborted human chorionic sacs. A, At 21 days. The entire sac is covered with chorionic villi (x4). B, At 8 weeks. Actual size. Some of the chorionic villi have degenerated forming the smooth chorion.
(From Potter EL, Craig JM: Pathology of the Fetus and the Infant, 3rd ed. Copyright 1975 by Year Book Medical Publishers, Chicago.)
Ultrasonography of Chorionic Sac
The size of the chorionic sac is useful in determining gestational age of embryos in patients with uncertain menstrual histories. Growth of the chorionic sac is extremely rapid between weeks 5 and 10. Modern ultrasound equipment, especially instruments equipped with intravaginal transducers, enables ultrasonographers to detect the chorionic sac, or gestational sac, when it has a median sac diameter of 2 to 3 mm (see Fig. 3-7). Chorionic sacs with this diameter indicate that the gestational age is 4 weeks and 3 to 4 days, that is, approximately 18 days after fertilization.
The uterus, chorionic sac, and placenta enlarge as the fetus grows. Growth in the size and thickness of the placenta continues rapidly until the fetus is approximately 18 weeks old. The fully developed placenta covers 15% to 30% of the decidua and weighs approximately one sixth that of the fetus.
The placenta has two parts (Figs. 7-1E and F and 7-4):
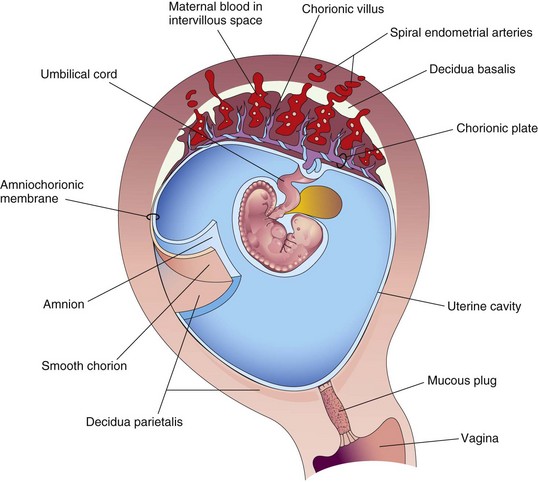
FIGURE 7–4 Drawing of a sagittal section of a gravid uterus at 4 weeks shows the relation of the fetal membranes to each other and to the decidua and embryo. The amnion and smooth chorion have been cut and reflected to show their relationship to each other and the decidua parietalis.
The fetal part of the placenta is attached to the maternal part of the placenta by the cytotrophoblastic shell, the external layer of trophoblastic cells on the maternal surface of the placenta (Fig. 7-5). The chorionic villi attach firmly to the decidua basalis through the cytotrophoblastic shell and anchor the chorionic sac to the decidua basalis. Endometrial arteries and veins pass freely through gaps in the cytotrophoblastic shell and open into the intervillous space.
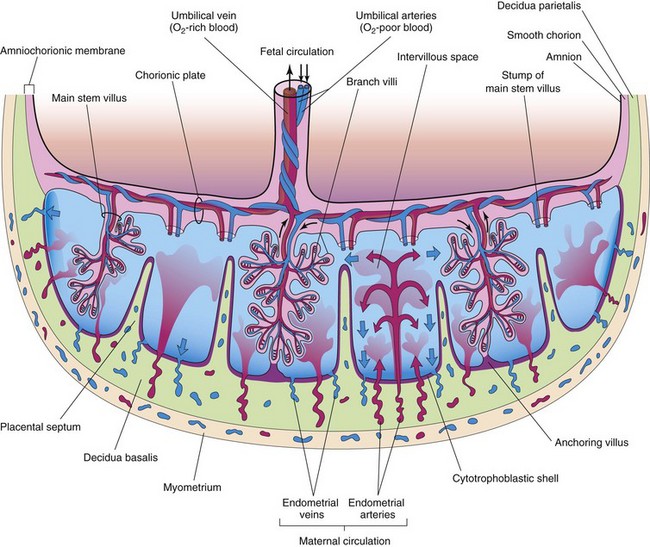
FIGURE 7–5 Schematic drawing of a transverse section through a full-term placenta, showing (1) the relation of the villous chorion (fetal part of placenta) to the decidua basalis (maternal part of placenta), (2) the fetal placental circulation, and (3) the maternal placental circulation. Note that the umbilical arteries carry poorly oxygenated fetal blood (shown in blue) to the placenta and that the umbilical vein carries oxygenated blood (shown in red ) to the fetus. Note that the cotyledons are separated from each other by placental septa, projections of the decidua basalis. Each cotyledon consists of two or more main stem villi and many branch villi. In this drawing, only one stem villus is shown in each cotyledon, but the stumps of those that have been removed are indicated.
The shape of the placenta is determined by the persistent area of chorionic villi (Fig. 7-1F). Usually this is a circular area, giving the placenta a discoid shape. As the chorionic villi invade the decidua basalis, decidual tissue is eroded to enlarge the intervillous space. This erosion produces several wedge-shaped areas of decidua, the placental septa, that project toward the chorionic plate, the part of the chorionic wall related to the placenta (see Fig. 7-5). The placental septa divide the fetal part of the placenta into irregular convex areas—cotyledons. Each cotyledon consists of two or more stem villi and their many branch villi (Fig. 7-6A). By the end of the fourth month, the decidua basalis is almost entirely replaced by the cotyledons. Expression of the transcription factor Gcm1 (glial cells missing-1) in trophoblast stem cells regulates the branching process of the stem villi to form the vascular network in the placenta.
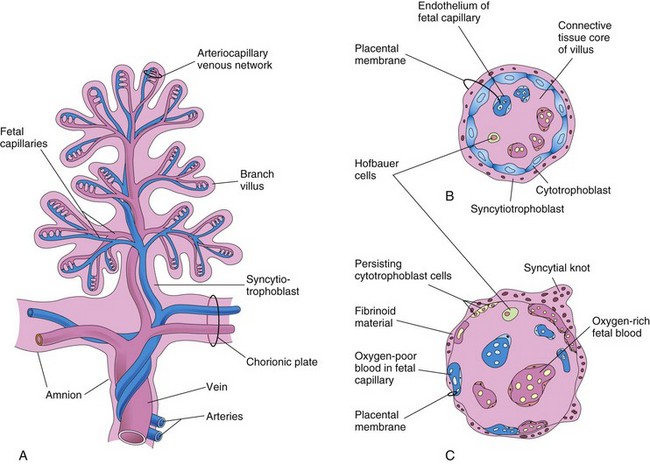
FIGURE 7–6 A, Drawing of a stem chorionic villus showing its arteriocapillary-venous system. The arteries carry poorly oxygenated fetal blood and waste products from the fetus, whereas the vein carries oxygenated blood and nutrients to the fetus. B and C, Drawings of sections through a branch villus at 10 weeks and full term, respectively. The placental membrane, composed of extrafetal tissues, separates the maternal blood in the intervillous space from the fetal blood in the capillaries in the villi. Note that the placental membrane becomes very thin at full term. Hofbauer cells are thought to be phagocytic cells.
The decidua capsularis, the layer of decidua overlying the implanted chorionic sac, forms a capsule over the external surface of the sac (Fig. 7-1A to D). As the conceptus enlarges, the decidua capsularis bulges into the uterine cavity and becomes greatly attenuated. Eventually the decidua capsularis contacts and fuses with the decidua parietalis on the opposite wall, thereby slowly obliterating the uterine cavity (Fig. 7-1E and F). By 22 to 24 weeks, the reduced blood supply to the decidua capsularis causes it to degenerate and disappear. After disappearance of the decidua capsularis, the smooth part of the chorionic sac fuses with the decidua parietalis. This fusion can be separated and usually occurs when blood escapes from the intervillous space (see Fig. 7-4). The collection of blood (hematoma) pushes the chorionic membrane away from the decidua parietalis, thereby reestablishing the potential space of the uterine cavity.
The intervillous space of the placenta, which contains maternal blood, is derived from the lacunae that developed in the syncytiotrophoblast during the second week of development (see Chapter 3). This large blood-filled space results from the coalescence and enlargement of the lacunar networks. The intervillous space of the placenta is divided into compartments by the placental septa; however, there is free communication between the compartments because the septa do not reach the chorionic plate (see Fig. 7-5).
Maternal blood enters the intervillous space from the spiral endometrial arteries in the decidua basalis (see Figs. 7-4 and 7-5). The spiral arteries pass through gaps in the cytotrophoblastic shell and discharge blood into the intervillous space. This large space is drained by endometrial veins that also penetrate the cytotrophoblastic shell. Endometrial veins are found over the entire surface of the decidua basalis. The numerous branch villi—arising from stem villi—are continuously showered with maternal blood that circulates through the intervillous space (Fig. 7-5). The blood in this space carries oxygen and nutritional materials that are necessary for fetal growth and development. The maternal blood also contains fetal waste products such as carbon dioxide, salts, and products of protein metabolism.
The amniotic sac enlarges faster than the chorionic sac. As a result, the amnion and smooth chorion soon fuse to form the amniochorionic membrane (Figs. 7-4 and 7-5). This composite membrane fuses with the decidua capsularis and, after disappearance of the latter, adheres to the decidua parietalis (Fig. 7-1F). It is the amniochorionic membrane that ruptures during labor. Preterm rupture of this membrane is the most common event leading to premature labor. When the membrane ruptures, amniotic fluid escapes through the cervix and vagina to the exterior.
Placental Circulation
The branch chorionic villi of the placenta provide a large surface area where materials may be exchanged across the very thin placental membrane (“barrier”) interposed between the fetal and maternal circulations (see Figs. 7-5 and 7-6). It is through the numerous branch villi that arise from the stem villi that the main exchange of material between the mother and fetus takes place. The circulations of the fetus and the mother are separated by the placental membrane consisting of extrafetal tissues (see Fig. 7-6B and C).
Fetal Placental Circulation
Poorly oxygenated blood leaves the fetus and passes through the umbilical arteries to the placenta. At the site of attachment of the umbilical cord to the placenta, these arteries divide into several radially disposed chorionic arteries that branch freely in the chorionic plate before entering the chorionic villi (Fig. 7-5). The blood vessels form an extensive arteriocapillary-venous system within the chorionic villi (Fig. 7-6A), which brings the fetal blood extremely close to the maternal blood. This system provides a very large surface area for the exchange of metabolic and gaseous products between the maternal and fetal bloodstreams. There is normally no intermingling of fetal and maternal blood; however, very small amounts of fetal blood may enter the maternal circulation when minute defects develop in the placental membrane (Fig. 7-6B and C). The well-oxygenated fetal blood in the fetal capillaries passes into thin-walled veins that follow the chorionic arteries to the site of attachment of the umbilical cord. They converge here to form the umbilical vein. This large vessel carries oxygen-rich blood to the fetus (Fig. 7-5).
Maternal Placental Circulation
The maternal blood in the intervillous space is temporarily outside the maternal circulatory system. It enters the intervillous space through 80 to 100 spiral endometrial arteries in the decidua basalis. These vessels discharge into the intervillous space through gaps in the cytotrophoblastic shell. The blood flow from the spiral arteries is pulsatile and is propelled in jet-like fountains by the maternal blood pressure (Fig. 7-5). The entering blood is at a considerably higher pressure than that in the intervillous space and spurts blood toward the chorionic plate that forms the “roof” of the intervillous space. As the pressure dissipates, the blood flows slowly over the branch villi, allowing an exchange of metabolic and gaseous products with the fetal blood. The blood eventually returns through the endometrial veins to the maternal circulation.
The welfare of the embryo and fetus depends more on the adequate bathing of the branch villi with maternal blood than on any other factor. Reductions of uteroplacental circulation result in fetal hypoxia (below normal levels of oxygen) and intrauterine growth restriction (IUGR). Severe reductions of uteroplacental circulation may result in fetal death. The intervillous space of the mature placenta contains approximately 150 ml of blood that is replenished three or four times per minute.
Placental Membrane
The placental membrane is a composite structure that consists of the extrafetal tissues separating the maternal and fetal blood. Until approximately 20 weeks, the placental membrane consists of four layers (Figs. 7-6 and 7-7): syncytiotrophoblast, cytotrophoblast, connective tissue of villus, and endothelium of fetal capillaries. After the 20th week, cellular changes occur in the branch villi that result in the cytotrophoblast in many of the villi becoming attenuated. Eventually cytotrophoblastic cells disappear over large areas of the villi, leaving only thin patches of syncytiotrophoblast. As a result, the placental membrane consists of three layers in most places (Fig. 7-6C). In some areas, the placental membrane becomes markedly thinned and attenuated. At these sites, the syncytiotrophoblast comes in direct contact with the endothelium of the fetal capillaries to form a vasculosyncytial placental membrane.
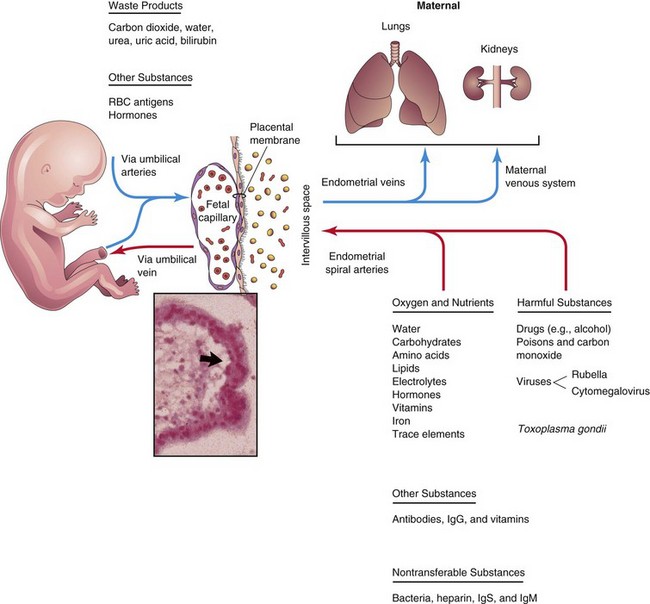
FIGURE 7–7 Diagrammatic illustration of transfer across the placental membrane (barrier). The extrafetal tissues, across which transport of substances between the mother and fetus occurs, collectively constitute the placental membrane. Inset, Light micrograph of chorionic villus showing a fetal capillary and the placental membrane (arrow).
Sometimes the placental membrane is called the placental barrier, an inappropriate term because there are only a few substances, endogenous or exogenous, that are unable to pass through the placental membrane in detectable amounts. The placental membrane acts as a barrier only when the molecule is of a certain size, configuration, and charge such as heparin. Some metabolites, toxins, and hormones, although present in the maternal circulation, do not pass through the placental membrane in sufficient concentrations to affect the embryo/fetus.
Most drugs and other substances in the maternal plasma pass through the placental membrane and enter the fetal plasma (Fig. 7-7). Electron micrographs of the syncytiotrophoblast show that its free surface has many microvilli that increase the surface area for exchange between the maternal and fetal circulations. As pregnancy advances, the placental membrane becomes progressively thinner so that blood in many fetal capillaries is extremely close to the maternal blood in the intervillous space (Figs. 7-6C and 7-7).
During the third trimester, numerous nuclei in the syncytiotrophoblast aggregate to form multinucleated protrusions called nuclear aggregations or syncytial knots (Fig. 7-6B and C). These aggregations continually break off and are carried from the intervillous space into the maternal circulation. Some knots lodge in capillaries of the maternal lungs where they are rapidly destroyed by local enzyme action. Toward the end of pregnancy, eosinophilic fibrinoid material forms on the surfaces of villi (Fig. 7-6C) and appears to reduce placental transfer.
Functions of Placenta
The placenta has three main functions:
These comprehensive activities are essential for maintaining pregnancy and promoting normal fetal development.
Placental Metabolism
The placenta, particularly during early pregnancy, synthesizes glycogen, cholesterol, and fatty acids, which serve as sources of nutrients and energy for the embryo/fetus. Many of its metabolic activities are undoubtedly critical for its other two major placental activities (transport and endocrine secretion).
Placental Transfer
The transport of substances in both directions between the fetal and maternal blood is facilitated by the great surface area of the placental membrane. Almost all materials are transported across the placental membrane by one of the following four main transport mechanisms: simple diffusion, facilitated diffusion, active transport, and pinocytosis.
Passive transport by simple diffusion is usually characteristic of substances moving from areas of higher to lower concentration until equilibrium is established. In facilitated diffusion, there is transport through electrical gradients. Facilitated diffusion requires a transporter but no energy. Such systems may involve carrier molecules that temporarily combine with the substances to be transported. Active transport is the passage of ions or molecules across a cell membrane. Pinocytosis is a form of endocytosis in which the material being engulfed is a small amount of extracellular fluid. This method of transport is usually reserved for large molecules. Some proteins are transferred very slowly through the placenta by pinocytosis.
Other Placental Transport Mechanisms
There are three other methods of transfer across the placental membrane. In the first, fetal red blood cells pass into the maternal circulation, particularly during parturition, through microscopic breaks in the placental membrane. Labeled maternal red blood cells have also been found in the fetal circulation. Consequently, red blood cells may pass in either direction through very small defects in the placental membrane. In the second method of transport, cells cross the placental membrane under their own power, such as maternal leukocytes and Treponema pallidum, the organism that causes syphilis. In the third method of transport, some bacteria and protozoa such as Toxoplasma gondii infect the placenta by creating lesions and then cross the placental membrane through the defects that are created.
Transfer of Gases
Oxygen, carbon dioxide, and carbon monoxide cross the placental membrane by simple diffusion. Interruption of oxygen transport for several minutes endangers survival of the embryo or fetus. The placental membrane approaches the efficiency of the lungs for gas exchange. The quantity of oxygen reaching the fetus is primarily flow limited rather than diffusion limited; hence, fetal hypoxia (decreased levels of oxygen) results primarily from factors that diminish either the uterine blood flow or fetal blood flow. Maternal respiratory failure (for example, from pneumonia) will also reduce oxygen transport to the fetus.
Nutritional Substances
Nutrients constitute the bulk of substances transferred from the mother to the embryo/fetus. Water is rapidly exchanged by simple diffusion and in increasing amounts as pregnancy advances. Glucose produced by the mother and placenta is quickly transferred to the embryo/fetus by facilitated diffusion. There is little or no transfer of maternal cholesterol, triglycerides, or phospholipids. Although there is transport of free fatty acids, the amount transferred appears to be relatively small. Amino acids are actively transported across the placental membrane and are essential for fetal growth. For most amino acids, the plasma concentrations in the fetus are higher than in the mother. Vitamins cross the placental membrane and are essential for normal development. Water-soluble vitamins cross the placental membrane more quickly than fat-soluble ones.
Hormones
Protein hormones do not reach the embryo or fetus in significant amounts, except for a slow transfer of thyroxine and triiodothyronine. Unconjugated steroid hormones cross the placental membrane rather freely. Testosterone and certain synthetic progestins cross the placental membrane and may cause masculinization of female fetuses (see Chapter 20).
Electrolytes
These compounds are freely exchanged across the placental membrane in significant quantities, each at its own rate. When a mother receives intravenous fluids with electrolytes, they also pass to the fetus and affect its water and electrolyte status.
Maternal Antibodies and Proteins
The fetus produces only small amounts of antibodies because of its immature immune system. Some passive immunity is conferred on the fetus by the placental transfer of maternal antibodies. IgG gamma globulins are readily transported to the fetus by transcytosis. Maternal antibodies confer fetal immunity to some diseases such as diphtheria, smallpox, and measles; however, no immunity is acquired to pertussis (whooping cough) or varicella (chickenpox). A maternal protein, transferrin, crosses the placental membrane and carries iron to the embryo or fetus. The placental surface contains special receptors for this protein.
Hemolytic Disease of the Newborn
Small amounts of fetal blood may pass to the maternal blood through microscopic breaks in the placental membrane. If the fetus is Rh-positive and the mother Rh-negative, the fetal blood cells may stimulate the formation of anti-Rh antibodies by the immune system of the mother. These antibodies pass to the fetal blood and cause hemolysis of the fetal Rh-positive blood cells, jaundice, and anemia in the fetus. Some fetuses with hemolytic disease of the newborn, or fetal erythroblastosis, fail to make a satisfactory intrauterine adjustment. They may die unless delivered early or given intrauterine, intraperitoneal, or intravenous transfusions of packed Rh-negative blood cells until after birth (see Chapter 6). Hemolytic disease of the newborn is relatively uncommon now because Rh (D) immunoglobulin given to the mother usually prevents development of this disease in the fetus.
Waste Products
Urea and uric acid pass through the placental membrane by simple diffusion. Conjugated bilirubin (which is fat soluble) is easily transported by the placenta for rapid clearance.
Drugs and Drug Metabolites
Drugs taken by the mother can affect the embryo/fetus directly or indirectly by interfering with maternal or placental metabolism. The amount of drug or metabolite reaching the placenta is controlled by the maternal blood level and blood flow through the placenta. Most drugs and drug metabolites cross the placenta by simple diffusion, the exception being those with a structural similarity to amino acids, such as methyldopa and some antimetabolites.
Some drugs cause major birth defects (see Chapter 20). Fetal drug addiction may occur after maternal use of drugs such as heroin and 50% to 75% of these newborns experience withdrawal symptoms. Because psychic dependence on these drugs is not developed during the fetal period, no liability to subsequent narcotic addiction exists in the infant after withdrawal is complete.
Most drugs used for the management of labor readily cross the placental membrane. Depending on the dose and its timing in relation to delivery, these drugs may cause respiratory depression of the newborn infant. All sedatives and analgesics affect the fetus to some degree. Neuromuscular blocking agents that may be used during operative obstetrics cross the placenta in only small amounts. Inhaled anesthetics can also cross the placental membrane and affect fetal breathing if used during parturition.
Infectious Agents
Cytomegalovirus, rubella, coxsackie viruses, and viruses associated with variola, varicella, measles, herpes virus, and poliomyelitis may pass through the placental membrane and cause fetal infection. In some cases, such as the rubella virus, birth defects, such as cataracts, may be produced (see Chapter 20). Microorganisms such as Treponema pallidum, which causes syphilis, and Toxoplasma gondii, which produces destructive changes in the brain and eyes, also cross the placental membrane, often causing severe birth defects and/or death of the embryo or fetus.
Placental Endocrine Synthesis and Secretion
Using precursors derived from the fetus and/or the mother, the syncytiotrophoblast of the placenta synthesizes protein and steroid hormones. The protein hormones synthesized by the placenta follow:
The glycoprotein hCG, similar to luteinizing hormone, is first secreted by the syncytiotrophoblast during the second week; hCG maintains the corpus luteum, preventing the onset of menstrual periods. The concentration of hCG in the maternal blood and urine increases to a maximum by the eighth week and then declines. The steroid hormones synthesized by the placenta are progesterone and estrogens. Progesterone can be found in the placenta at all stages of gestation, indicating that it is essential for the maintenance of pregnancy. The placenta forms progesterone from maternal cholesterol or pregnenolone. The ovaries of a pregnant woman can be removed after the first trimester without causing an abortion because the placenta takes over the production of progesterone from the corpus luteum. Estrogens are also produced in large quantities by the syncytiotrophoblast.
The Placenta as an Allograft*
The placenta can be regarded as an allograft with respect to the mother. The fetal part of the placenta is a derivative of the conceptus, which inherits both paternal and maternal genes. What protects the placenta from rejection by the mother’s immune system? This question remains a major biologic enigma in nature. The syncytiotrophoblast of the chorionic villi, although exposed to maternal immune cells within the blood sinusoids, lacks major histocompatibility (MHC) antigens and thus does not evoke rejection responses. However, extravillous trophoblast (EVT) cells, which invade the uterine decidua and its vasculature (spiral arteries), express class I MHC antigens. These antigens include HLA-G, which, being nonpolymorphic (class Ib), is poorly recognizable by T lymphocytes as an alloantigen, as well as HLA-C, which, being polymorphic (class Ia), is recognizable by T cells. In addition to averting T cells, EVT cells must also shield themselves from potential attack by natural killer (NK) lymphocytes and injury inflicted by activation of complement. Multiple mechanisms appear to be in place to guard the placenta:
Placenta as an Invasive Tumor-like Structure
The placenta in many species, including humans, is a highly invasive tumor-like structure that invades the uterus to tap in on its blood supply to establish an adequate exchange of key molecules between the mother and the fetus. What protects the uterus from placental overinvasion? Following the development of chorionic villi, the invasive function of the placenta is provided by the subset of cytotrophoblastic cells (EVT cells), which are produced by proliferation and differentiation of stem cells located in the cytotrophoblast layer of certain chorionic villi (anchoring villi). They break out of the villous confines and migrate as cell columns to invade the decidua where they reorganize as distinct subsets: a nearly continuous cell layer (cytotrophoblastic shell) separating the decidua from maternal blood sinusoids; cells dispersed within the decidua (interstitial trophoblast); multinucleate placental-bed giant cells produced by EVT cell fusion; and endovascular trophoblast, which invade and remodel the uteroplacental (spiral) arteries within the endometrium and a part of the myometrium. Optimal arterial remodeling (loss of tunica media and replacement of the endothelium by the EVT cells) allows steady placental perfusion with maternal blood unhindered by the presence of vasoactive molecules. Inadequate EVT cell invasion leading to poor placental perfusion underlies the pathogenesis of preeclampsia (a major pregnancy-associated hypertensive disorder in the mother) and certain forms of IUGR of the fetus, whereas excessive invasion is a hallmark of gestational trophoblastic neoplasias and choriocarcinomas.
Trophoblastic stem cells have been successfully propagated from the murine (mice) but not from the human placenta. However, normal human EVT cells have been successfully propagated from first-trimester human placentas. Using these cells for functional assays in vitro, it was shown that molecular mechanisms responsible for their invasiveness are identical to those of cancer cells, whereas their proliferation, migration, and invasiveness are stringently regulated in situ by a variety of locally produced molecules: growth factors, growth factor binding proteins, proteoglycans, and components of the extracellular matrix. Numerous growth factors, such as epidermal growth factor, TGF-a, amphiregulin, colony-stimulating factor 1, vascular endothelial growth factor, and placental growth factor were shown to stimulate EVT cell proliferation without affecting migration or invasiveness, whereas insulin-like growth factor II and an insulin-like, growth factor-binding protein, IGFBP-1, were shown to stimulate EVT cell migration and invasiveness without affecting proliferation. TGF-β, primarily produced by the decidua, was shown to provide the key control of EVT cell proliferation, migration, and invasiveness, whereas trophoblastic cancer (choriocarcinoma) cells were shown to be resistant to the inhibitory signals of TGF-β. Thus, it appears that the decidua plays a dual role in uteroplacental homeostasis by immunoprotection of the placenta and protection of the uterus from placental overinvasion.
Preeclampsia
Preeclampsia is a serious disorder that occurs during pregnancy, usually after the 20th week of gestation. Maternal hypertension, proteinuria, and edema are essential features of this condition. Preeclampsia can lead to eclampsia resulting in miscarriage and maternal death. The cause of preeclampsia is uncertain but recent studies have implicated the rennin-angiotensin system in the development of the high blood pressure and edema. In eclampsia, extensive infarcts are present that reduce uteroplacental circulation. This can lead to fetal malnutrition, fetal growth restriction, miscarriage, or fetal death.
Uterine Growth during Pregnancy
The uterus of a nonpregnant woman lies in the pelvis. To accommodate the growing conceptus, the uterus increases in size (Fig. 7-8A). It also increases in weight and its walls become thinner (Fig. 7-8B and C). During the first trimester, the uterus moves out of the pelvis and by 20 weeks reaches the level of the umbilicus. By 28 to 30 weeks, the uterus reaches the epigastric region, the area between the xiphoid process of the sternum and the umbilicus. The increase in size of the uterus largely results from hypertrophy of preexisting smooth muscular fibers and partly from the development of new fibers.
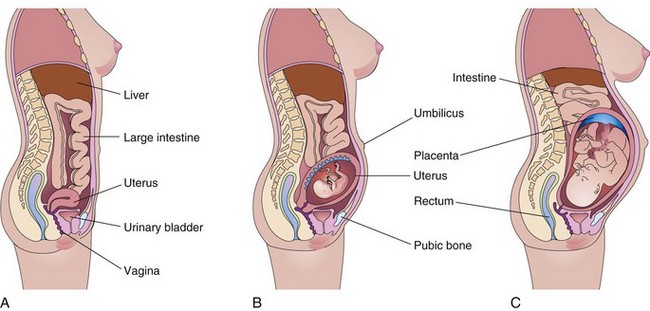
FIGURE 7–8 Drawings of median sections of a woman’s body. A, Not pregnant. B, Twenty weeks pregnant. C, Thirty weeks pregnant. Note that as the conceptus enlarges, the uterus increases in size to accommodate the rapidly growing fetus. By 20 weeks, the uterus and fetus reach the level of the umbilicus, and by 30 weeks, they reach the epigastric region. The mother’s abdominal viscera are displaced and compressed, and the skin and muscles of her anterior abdominal wall are stretched.
Parturition
Parturition (childbirth) is the process during which the fetus, placenta, and fetal membranes are expelled from the mother’s reproductive tract (Fig. 7-9). Labor is the sequence of involuntary uterine contractions that result in dilation of the uterine cervix and expulsion of the fetus and placenta from the uterus. The factors that trigger labor are not completely understood, but several hormones are related to the initiation of contractions. The fetal hypothalamus secretes corticotropin-releasing hormone, which stimulates the anterior hypophysis (pituitary) to produce adrenocorticotropin. This hormone causes the secretion of cortisol from the suprarenal (adrenal) cortex. Cortisol is involved in the synthesis of estrogens. Many steroids are involved in uterine contraction.
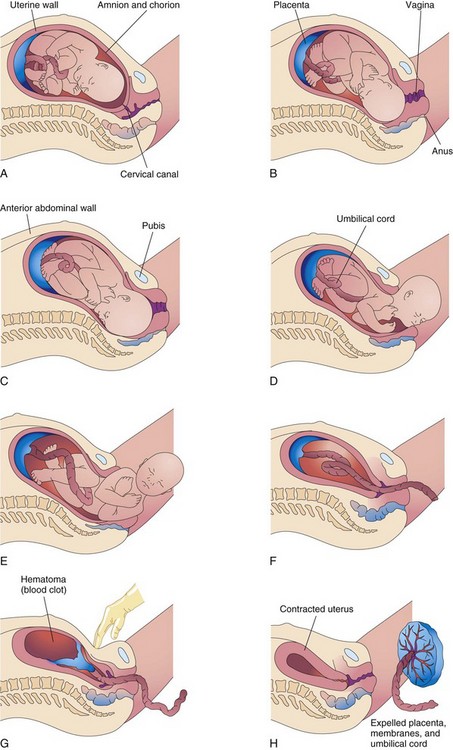
FIGURE 7–9 Drawings illustrating parturition (childbirth). A and B, The cervix is dilating during the first stage of labor. C to E, The fetus is passing through the cervix and vagina during the second stage of labor. F and G, As the uterus contracts during the third stage of labor, the placenta folds and pulls away from the uterine wall. Separation of the placenta results in bleeding and formation of a large hematoma (mass of blood). Pressure on the abdomen facilitates placental separation. H, The placenta is expelled and the uterus contracts.
Peristaltic contractions of uterine smooth muscle are elicited by oxytocin, which is released by the neurohypophysis of the pituitary gland. This hormone is administered clinically when it is necessary to induce labor. Oxytocin also stimulates release of prostaglandins from the decidua increasing myometrial contractility by sensitizing the myometrial cells to oxytocin.
Estrogens also increase myometrial contractile activity and stimulate the release of oxytocin and prostaglandins. From studies carried out in sheep and nonhuman primates, it seems that the duration of pregnancy and the process of birth are under the direct control of the fetus.
Labor is a continuous process; however, for clinical purposes, it is usually divided into three stages:
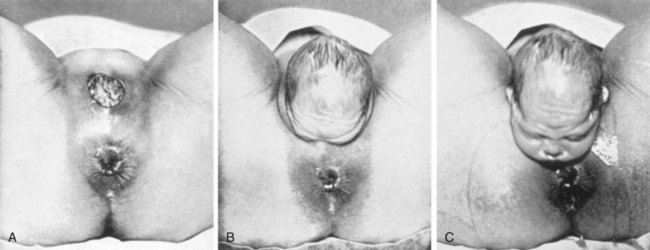
FIGURE 7–10 Delivery of the baby’s head during the second stage of labor is shown. A, The crown of the head distends the mother’s perineum. B, The perineum slips over the head and face. C, The head is delivered: subsequently, the body of the fetus is expelled.
(From Greenhill JB, Friedman EA: Biological Principles and Modern Practice of Obstetrics. Philadelphia, WB Saunders, 1974.)
Retraction of the uterus reduces the area of placental attachment (Fig. 7-9G). A hematoma, a localized mass of extravasated blood, soon forms deep to the placenta and separates it from the uterine wall. The placenta and fetal membranes are expelled through the vaginal canal. The placenta separates through the spongy layer of the decidua basalis (see Chapter 2). After delivery of the baby, the uterus continues to contract (Fig. 7-9H). The myometrial contractions constrict the spiral arteries that supplied blood to the intervillous space. These contractions prevent excessive uterine bleeding.
Placenta and Fetal Membranes after Birth
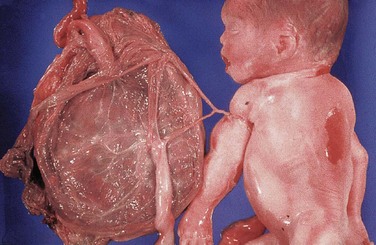
The placenta commonly has a discoid shape, with a diameter of 15 to 20 cm and a thickness of 2 to 3 cm (Fig. 7-11). It weighs 500 to 600 g, which is approximately one sixth the weight of the average fetus. The margins of the placenta are continuous with the ruptured amniotic and chorionic sacs (Fig 7-11).
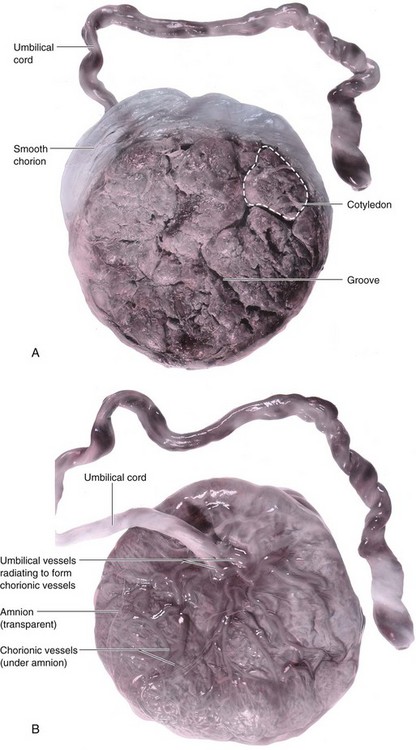
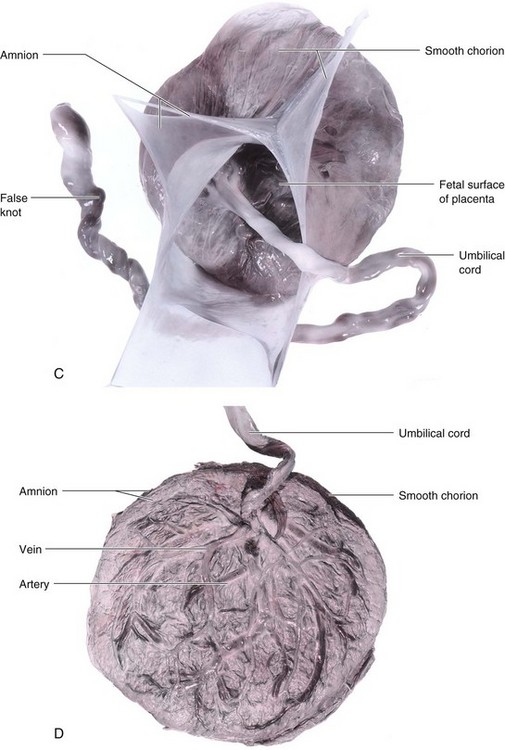
FIGURE 7–11 Placentas and fetal membranes after birth, approximately one third actual size. A, Maternal surface showing cotyledons and the grooves around them. Each cotyledon consists of a number of main stem villi with their many branch villi. The grooves were occupied by the placental septa when the maternal and fetal parts of the placenta were together (see Fig. 7–5). B, Fetal surface showing blood vessels running in the chorionic plate deep to the amnion and converging to form the umbilical vessels at the attachment of the umbilical cord. Placentas and fetal membranes after birth, approximately one third of actual size. C, The amnion and smooth chorion are arranged to show that they are fused and continuous with the margins of the placenta. D, Placenta with a marginal attachment of the cord, often called a battledore placenta because of its resemblance to the bat used in the medieval game of battledore and shuttlecock.
(From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
When chorionic villi persist on the entire surface of the chorionic sac (an uncommon occurrence), a thin layer of placenta attaches to a large area of the uterus. This type of placenta is a membranous placenta (placenta membranacea). When villi persist elsewhere, several variations in placental shape occur: accessory placenta (Fig. 7-12), bidiscoid placenta, and horseshoe placenta. Although there are variations in the size and shape of the placenta, most of them are of little physiologic or clinical significance.
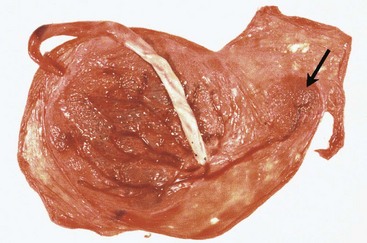
FIGURE 7–12 A full-term placenta and an accessory placenta (arrow). The accessory placenta developed from a patch of chorionic villi that persisted a short distance from the main placenta.
Gestational Choriocarcinoma
Abnormal proliferation of the trophoblast results in gestational trophoblastic disease, a spectrum of lesions including highly malignant tumors. The cells invade the decidua basalis, penetrate its blood vessels and lymphatics, and may metastasize to the maternal lungs, bone marrow, liver, and other organs. Gestational choriocarcinomas are highly sensitive to chemotherapy and cures are usually achieved.
Maternal Surface of Placenta
The characteristic cobblestone appearance of the maternal surface is produced by slightly bulging villous areas—cotyledons—which are separated by grooves that were formerly occupied by placental septa (Figs. 7-5 and 7-11A). The surface of the cotyledons is covered by thin grayish shreds of the decidua basalis that separated from the uterine wall when the placenta was extruded. Most of the decidua is temporarily retained in the uterus and is shed with the uterine bleeding after delivery of the fetus.
Examination of the placenta prenatally by ultrasonography or magnetic resonance imaging (Fig. 7-13) or postnatally by gross and microscopic study may provide clinical information about the causes of IUGR, placental dysfunction, fetal distress and death, and neonatal illness. Placental studies can also determine whether the expelled placenta is complete. Retention of a cotyledon or accessory placenta (Fig. 7-12) in the uterus may cause severe uterine hemorrhage.
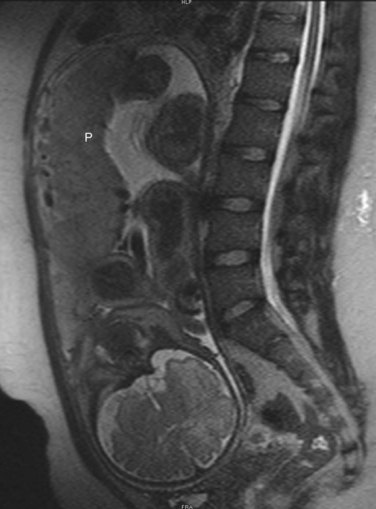
FIGURE 7–13 Sagittal magnetic resonance image of the pelvis of a pregnant woman. The vertebral column and pelvis of the mother are visible, as are the fetal brain, limbs and placenta (P).
(Courtesy of Stuart C. Morrison, Section of Pediatric Radiology, The Children’s Hospital, Cleveland Clinic, Cleveland, OH.)
Fetal Surface of Placenta
The umbilical cord usually attaches to the fetal surface of the placenta, and its epithelium is continuous with the amnion adhering to the fetal surface (Figs. 7-5 and 7-11B).The fetal surface of a freshly delivered placenta is smooth and shiny because it is covered by the amnion. The chorionic vessels radiating to and from the umbilical cord are clearly visible through the transparent amnion. The umbilical vessels branch on the fetal surface to form chorionic vessels, which enter the chorionic villi and form the arteriocapillary-venous system (Fig. 7-6A).
Placental Abnormalities
Abnormal adherence of chorionic villi to the myometrium is called placenta accreta (Fig. 7-14). When chorionic villi penetrate the full thickness of the myometrium to or through the perimetrium (peritoneal covering), the abnormality is called placenta percreta. Third-trimester bleeding is the common presenting sign of these placental abnormalities. Most patients with placenta accreta have normal pregnancies and labors. After birth, the placenta fails to separate from the uterine wall and attempts to remove it may cause hemorrhage that is difficult to control.
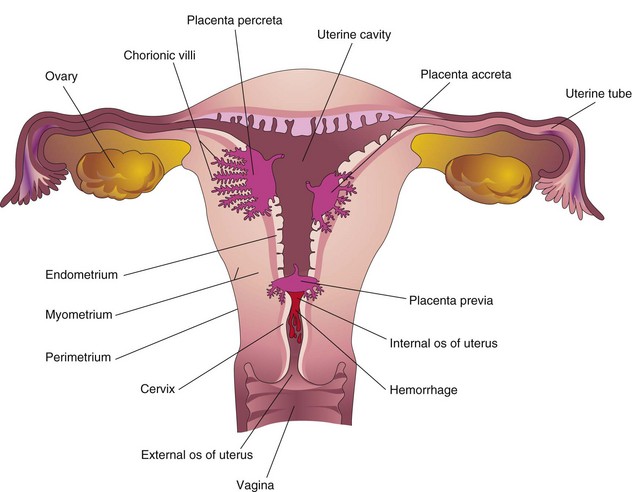
FIGURE 7–14 Placental abnormalities. In placenta accreta, there is abnormal adherence of the placenta to the myometrium. In placenta percreta, the placenta has penetrated the full thickness of the myometrium. In this example of placenta previa, the placenta overlies the internal os of the uterus and blocks the cervical canal.
When the blastocyst implants close to or overlying the internal os of the uterus, the abnormality is called placenta previa. Late pregnancy bleeding may result from this placental abnormality. The fetus has to be delivered by cesarean section when the placenta completely obstructs the internal uterine os. Ultrasound scanning of the placenta is invaluable for clinical diagnosis of placental abnormalities.
Umbilical Cord
The attachment of the umbilical cord to the placenta is usually near the center of the fetal surface of this organ (Fig. 7-11B), but it may attach at any point. For example, insertion of the cord near the placental margin produces a battledore placenta (see Fig. 7-11D). The attachment of the cord to the fetal membranes is termed a velamentous insertion of the cord (Fig. 7-15).
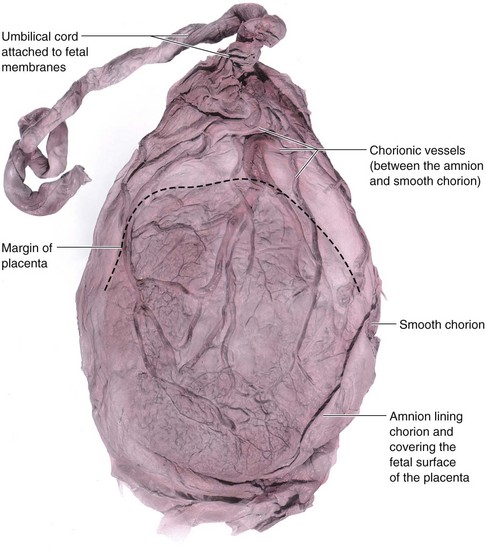
FIGURE 7–15 A placenta with a velamentous insertion of the umbilical cord. The cord is attached to the membranes (amnion and chorion), not to the placenta. The umbilical vessels leave the cord and run between the amnion and chorion before spreading over the placenta. The vessels are easily torn in this location, especially when they cross over the inferior uterine segment; the latter condition is known as vasa previa. If the vessels rupture before birth, the fetus loses blood and could be near exsanguination when born.
(From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000.)
Color flow Doppler ultrasonography may be used for the prenatal diagnosis of the position and structural abnormalities of the umbilical cord and its vessels. The umbilical cord is usually 1 to 2 cm in diameter and 30 to 90 cm in length (average, 55 cm). Excessively long or short cords are uncommon. Long cords have a tendency to prolapse and/or to coil around the fetus (see Fig. 7-19). Prompt recognition of prolapse of the umbilical cord is important because the cord may be compressed between the presenting body part of the fetus and the mother’s bony pelvis, causing fetal hypoxia or anoxia. If the deficiency of oxygen persists for more than 5 minutes, the baby’s brain may be damaged. A very short cord may cause premature separation of the placenta from the wall of the uterus during delivery.
The umbilical cord usually has two arteries and one vein that are surrounded by mucoid connective tissue (Wharton jelly). Because the umbilical vessels are longer than the cord, twisting and bending of the vessels are common. They frequently form loops, producing false knots that are of no significance; however, in approximately 1% of pregnancies, true knots form in the cord, which may tighten and cause fetal death resulting from fetal anoxia (Fig. 7-16). In most cases, the knots form during labor as a result of the fetus passing through a loop of the cord. Simple looping of the cord around the fetus occasionally occurs (see Fig. 7-19B). In approximately one fifth of deliveries, the cord is loosely looped around the neck without increased fetal risk.
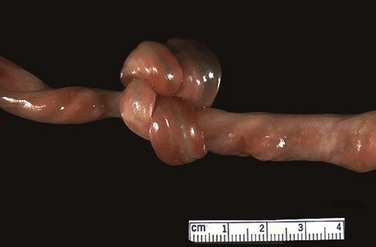
FIGURE 7–16 Photograph of an umbilical cord showing a true knot. Such a knot will cause severe anoxia (decreased oxygen in the fetal tissues and organs).
(Courtesy of Dr. E.C. Klatt, Department of Biomedical Sciences, Mercer University School of Medicine, Savannah, GA.)
Umbilical Artery Doppler Velocimetry
As gestation and trophoblastic invasion of the decidua basalis progress, there is a progressive increase in the diastolic flow velocity in the umbilical arteries. Doppler velocimetry of the uteroplacental and fetoplacental circulation is used to investigate complications of pregnancy such as IUGR and fetal distress resulting from fetal hypoxia and asphyxia (Fig. 7-17). For example, there is a statistically significant association between IUGR and abnormally increased resistance in an umbilical artery.
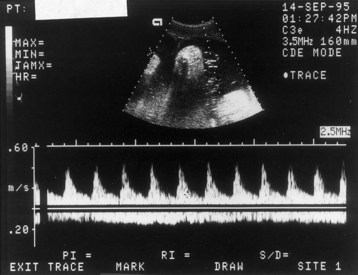
FIGURE 7–17 Doppler velocimetry of the umbilical cord. The arterial waveform (top) illustrates pulsatile forward flow, with high peaks and low velocities during diastole. This combination suggests high resistance in the placenta to placental blood flow. Because this index changes over gestation, it is important to know that the pregnancy was 18 weeks’ gestation. For this period, the flow pattern is normal. The nonpulsatile flow in the opposite, negative direction represents venous return from the placenta. Both waveforms are normal for this gestational age.
(Courtesy of Dr. C.R. Harman, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Maryland, Baltimore, MD.)
Absence of an Umbilical Artery
In approximately 1 in 100 neonates, only one umbilical artery is present (Fig. 7-18), a condition that may be associated with chromosomal and fetal abnormalities. Absence of an umbilical artery is accompanied by a 15% to 20% incidence of cardiovascular defects in the fetus. Absence of an artery results from either agenesis or degeneration of one of the two umbilical arteries. A single umbilical artery and the anatomic defects associated with it can be detected before birth by ultrasonography.
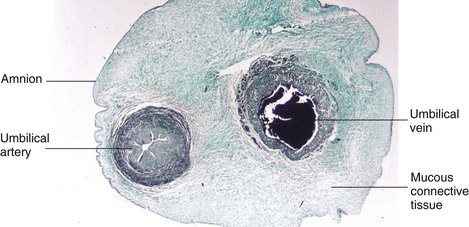
FIGURE 7–18 Transverse section of an umbilical cord. Observe that the cord is covered by epithelium derived from the enveloping amnion. It has a core of mucous connective tissue (Wharton jelly). Observe also that the cord has one umbilical artery and one vein; usually there are two umbilical arteries.
(Courtesy of Professor V. Becker, Pathologisches Institut der Universität, Erlangen, Germany.)
Amnion and Amniotic Fluid
The thin but tough amnion forms a fluid-filled, membranous sac that surrounds the embryo and later the fetus. The sac contains amniotic fluid (Figs. 7-19 and 7-20). As the amnion enlarges, it gradually obliterates the chorionic cavity and forms the epithelial covering of the umbilical cord (Figs. 7-18 and 7-20C and D).
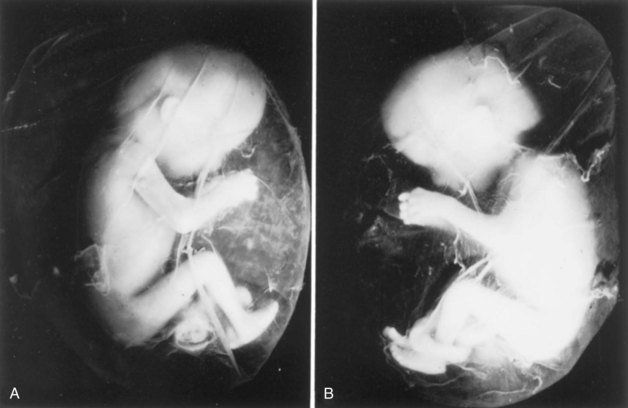
FIGURE 7–19 A, 12-week fetus within its amniotic sac. The fetus and its membranes aborted spontaneously. It was removed from its chorionic sac with its amniotic sac intact. Actual size. B, Note that the umbilical cord is looped around the left ankle of the fetus. Coiling of the cord around parts of the fetus affects development when the coils are so tight that the circulation to the parts is affected.
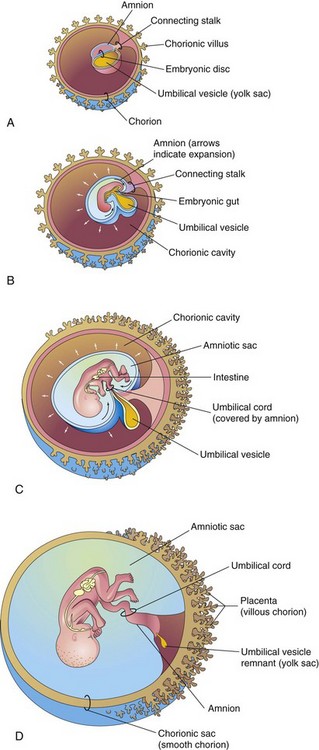
FIGURE 7–20 Illustrations showing how the amnion enlarges, obliterates the chorionic cavity, and envelops the umbilical cord. Observe that part of the umbilical vesicle is incorporated into the embryo as the primordial gut. Formation of the fetal part of the placenta and degeneration of chorionic villi are also shown. A, At 3 weeks. B, At 4 weeks. C, At 10 weeks. D, At 20 weeks.
Amniotic Fluid
Amniotic fluid plays a major role in fetal growth and development. Initially, some amniotic fluid is secreted by cells of the amnion.
Most fluid is derived from maternal tissue and interstitial fluid by diffusion across the amniochorionic membrane from the decidua parietalis (Fig. 7-5). Later there is diffusion of fluid through the chorionic plate from blood in the intervillous space of the placenta.
Before keratinization of the skin occurs, a major pathway for passage of water and solutes in tissue fluid from the fetus to the amniotic cavity is through the skin; thus, amniotic fluid is similar to fetal tissue fluid. Fluid is also secreted by the fetal respiratory and gastrointestinal tracts and enters the amniotic cavity. The daily rate of contribution of fluid to the amniotic cavity from the respiratory tract is 300 to 400 ml. Beginning in the 11th week, the fetus contributes to the amniotic fluid by excreting urine into the amniotic cavity. By late pregnancy, approximately 500 ml of urine is added daily. The volume of amniotic fluid normally increases slowly, reaching approximately 30 ml at 10 weeks, 350 ml at 20 weeks, and 700 to 1000 ml by 37 weeks.
Circulation of Amniotic Fluid
The water content of amniotic fluid changes every 3 hours. Large amounts of water pass through the amniochorionic membrane into the maternal tissue fluid and enter the uterine capillaries. An exchange of fluid with fetal blood also occurs through the umbilical cord and where the amnion adheres to the chorionic plate on the fetal surface of the placenta (Figs. 7-5 and 7-11B); thus, amniotic fluid is in balance with the fetal circulation.
Amniotic fluid is swallowed by the fetus and absorbed by the fetus’s respiratory and digestive tracts. It has been estimated that during the final stages of pregnancy, the fetus swallows up to 400 ml of amniotic fluid per day. The fluid passes into the fetal bloodstream and the waste products in it cross the placental membrane and enter the maternal blood in the intervillous space. Excess water in the fetal blood is excreted by the fetal kidneys and returned to the amniotic sac through the fetal urinary tract.
Disorders of Amniotic Fluid Volume
Low volumes of amniotic fluid—oligohydramnios—result in many cases from placental insufficiency with diminished placental blood flow. Preterm rupture of the amniochorionic membrane occurs in approximately 10% of pregnancies and is the most common cause of oligohydramnios. When there is renal agenesis (failure of kidney formation), the absence of fetal urine contribution to the amniotic fluid is the main cause of oligohydramnios. A similar decrease in fluid occurs when there is obstructive uropathy (urinary tract obstruction).
Complications of oligohydramnios include fetal birth defects (pulmonary hypoplasia, facial defects, and limb defects) that are caused by fetal compression by the uterine wall. Compression of the umbilical cord is also a potential complication of severe oligohydramnios.
High volumes of amniotic fluid—polyhydramnios—result when the fetus does not swallow the usual amount of amniotic fluid. Most cases of polyhydramnios (60%) are idiopathic (unknown cause), 20% are caused by maternal factors, and 20% are fetal in origin. Polyhydramnios may be associated with severe defects of the central nervous system, such as meroencephaly (anencephaly). When there are other anomalies, such as esophageal atresia (blockage), amniotic fluid accumulates because it is unable to pass to the fetal stomach and intestines for absorption. Ultrasonography has become the technique of choice for diagnosing oligo- and polyhydramnios.
Composition of Amniotic Fluid
Amniotic fluid is an aqueous solution in which undissolved material (desquamated fetal epithelial cells) is suspended. Amniotic fluid contains approximately equal portions of organic compounds and inorganic salts. Half of the organic constituents are protein; the other half consists of carbohydrates, fats, enzymes, hormones, and pigments. As pregnancy advances, the composition of the amniotic fluid changes as fetal excreta (meconium [fetal feces] and urine) are added. Because fetal urine enters the amniotic fluid, studies of fetal enzyme systems, amino acids, hormones, and other substances can be conducted on fluid removed by amniocentesis (see Fig. 6-13). Studies of cells in the amniotic fluid permit diagnosis of chromosomal abnormalities such as trisomy 21 (Down syndrome). High levels of alpha fetoprotein usually indicate the presence of a severe neural tube defect. Low levels of alpha fetoprotein may indicate chromosomal aberrations such as trisomy 21 (see Chapter 20).
Significance of Amniotic Fluid
The embryo, suspended in amniotic fluid by the umbilical cord, floats freely. Amniotic fluid has critical functions in the normal development of the fetus:
Premature Rupture of Fetal Membranes
Rupture of the amniochorionic membrane is the most common event leading to premature labor and delivery, and the most common complication resulting in oligohydramnios. Loss of amniotic fluid removes the major protection that the fetus has against infection. Rupture of the amnion may cause various fetal defects that constitute the amniotic band syndrome (ABS), or the amniotic band disruption complex (Fig. 7-21). The incidence of the ABS is approximately 1 in every 1200 live births. Prenatal ultrasound diagnosis of ABS is now possible. The defects caused by ABS vary from digital constriction to major scalp, craniofacial, and visceral defects. The cause of these anomalies is probably related to constriction by encircling amniotic bands (Figs. 7-19 and 7-21).
 Umbilical Vesicle
Umbilical Vesicle
The umbilical vesicle (yolk sac) can be observed sonographically early in the fifth week. Early development of the umbilical vesicle was described in Chapters 3 and 5. At 32 days, the umbilical vesicle is large (Figs. 7-1C and 7-2). By 10 weeks, the umbilical vesicle has shrunk to a pear-shaped remnant approximately 5 mm in diameter (Fig. 7-20) and is connected to the midgut by a narrow omphaloenteric duct (yolk stalk). By 20 weeks, the umbilical vesicle is very small (Fig. 7-20C); thereafter, it is usually not visible. The presence of the amniotic sac and umbilical vesicle enables early recognition and measurement of the embryo. The umbilical vesicle is recognizable in ultrasound examinations until the end of the first trimester.
Significance of Umbilical Vesicle
Although the umbilical vesicle is nonfunctional as far as yolk storage is concerned (hence the name change), its presence is essential for several reasons:
Fate of Umbilical Vesicle
At 10 weeks, the small umbilical vesicle lies in the chorionic cavity between the amniotic and chorionic sacs (Fig. 7-20C). It atrophies as pregnancy advances, eventually becoming very small (Fig. 7-20D). In very unusual cases, the umbilical vesicle persists throughout pregnancy and appears under the amnion as a small structure on the fetal surface of the placenta near the attachment of the umbilical cord. Persistence of the umbilical vesicle is of no significance. The omphaloenteric duct usually detaches from the midgut loop by the end of the sixth week. In approximately 2% of adults, the proximal intra-abdominal part of the omphaloenteric duct persists as an ileal diverticulum (Meckel diverticulum [see Chapter 11]).
 Allantois
Allantois
Early development of the allantois is described in Chapter 4. In the third week, it appears as a sausage-like diverticulum from the caudal wall of the umbilical vesicle that extends into the connecting stalk (Fig. 7-22A). During the second month, the extraembryonic part of the allantois degenerates (Fig. 7-22B). Although the allantois is not functional in human embryos, it is important for three reasons:
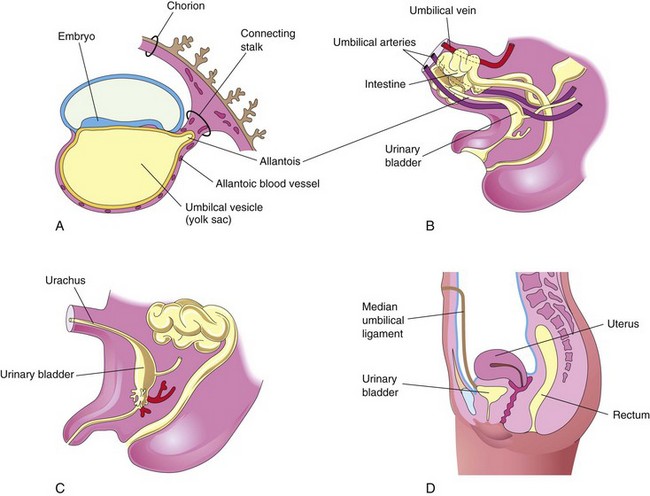
FIGURE 7–22 Illustrations of the development and usual fate of the allantois. A, A 3-week embryo. B, A 9-week fetus. C, A 3-month male fetus. D, Adult female. The nonfunctional allantois forms the urachus in the fetus and the median umbilical ligament in the adult.
Allantoic Cysts
A cystic mass in the umbilical cord may represent the remains of the extraembryonic part of the allantois (Fig. 7-23). These cysts usually resolve but may be associated with an omphalocele—congenital herniation of viscera into the proximal part of the umbilical cord (see Chapter 11).
Multiple Pregnancies
The risks of chromosomal anomalies and fetal morbidity and mortality are higher in multiple gestations than in single pregnancies. As the number of fetuses increases, the risks are progressively greater. In most countries, multiple births are more common now because of greater access to fertility therapies, including induction of ovulation that occurs when exogenous gonadotropins are administered to women with ovulatory failure, and to those being treated for infertility by assisted reproductive technologies. In North America, twins normally occur approximately once in every 85 pregnancies, triplets approximately once in 902 pregnancies, quadruplets once in 903 pregnancies, and quintuplets approximately once in every 904 pregnancies.
Twins and Fetal Membranes
Twins that originate from two zygotes are dizygotic (DZ) twins or fraternal twins (Fig. 7-24), whereas twins that originate from one zygote are monozygotic (MZ) twins or identical twins (Fig. 7-25). The fetal membranes and placentas vary according to the origin of the twins (Table 7-1). In the case of MZ twins, the type of placenta and membranes formed depends on when the twinning process occurs. Approximately two thirds of twins are DZ. The frequency of DZ twinning shows marked racial differences, but the incidence of MZ twinning is approximately the same in all populations. In addition, the rate of MZ twinning shows little variation with the mother’s age, whereas the rate of DZ twinning increases with maternal age.
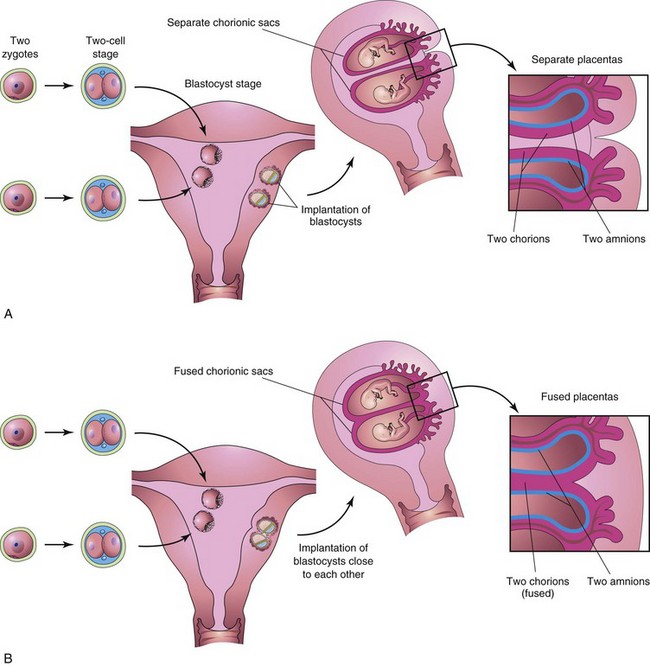
FIGURE 7–24 Diagrams illustrating how dizygotic twins develop from two zygotes. The relationships of the fetal membranes and placentas are shown for instances in which the blastocysts implant separately (A) and the blastocysts implant close together (B). In both cases, there are two amnions and two chorions. The placentas are usually fused when they implant close together.
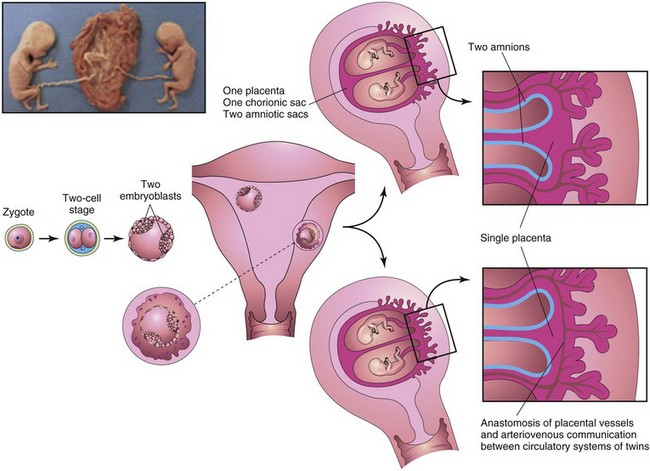
FIGURE 7–25 Diagrams illustrating how approximately 65% of monozygotic twins develop from one zygote by division of the embryoblast of the blastocyst. These twins always have separate amnions, a single chorionic sac, and a common placenta. If there is anastomosis of the placental vessels, one twin may receive most of the nutrition from the placenta. Inset, monozygotic twins, 17 weeks’ gestation.
(Courtesy of Dr. Robert Jordan, St. Georges University Medical School, Grenada.)
Table 7–1 Frequency of Types of Placentas and Fetal Membranes in Monozygotic (MZ) and Dizygotic (DZ) Twins

The study of twins is important in human genetics because it is useful for comparing the effects of genes and environment on development. If an abnormal condition does not show a simple genetic pattern, comparison of its incidence in MZ and DZ twins may reveal that heredity is involved. The tendency for DZ, but not MZ, twins to repeat in families is evidence of hereditary influence. Studies in a Mormon population showed that the genotype of the mother affects the frequency of DZ twins, but the genotype of the father has no effect. It has also been observed that if the firstborn are twins, a repetition of twinning or some other form of multiple birth is approximately five times more likely to occur with the next pregnancy than in the general population.
Dizygotic Twins
Because they result from fertilization of two oocytes, DZ twins develop from two zygotes and may be of the same sex or different sexes (Fig. 7-24). For the same reason, they are no more alike genetically than brothers or sisters born at different times. The only thing they have in common is that they were in their mother’s uterus at the same time. DZ twins always have two amnions and two chorions, but the chorions and placentas may be fused. DZ twinning shows a hereditary tendency. Recurrence in families is approximately three times that of the general population. The incidence of DZ twinning shows considerable racial variation, being approximately 1 in 500 in Asians, 1 in 125 in whites, and as high as 1 in 20 in some African populations.
Anastomosis of Placental Blood Vessels
Anastomoses between blood vessels of fused placentas of DZ twins may result in erythrocyte mosaicism. The members of these DZ twins have red blood cells of two different blood groups because red cells were exchanged between the circulations of the twins. In cases in which one fetus is a male and the other is female, masculinization of the female fetus does not occur.
Monozygotic Twins
Because they result from the fertilization of one oocyte and develop from one zygote (see Fig. 7-25), MZ twins are of the same sex, genetically identical, and very similar in physical appearance. Physical differences between MZ twins are environmentally induced, such as anastomosis of placental vessels (Fig. 7-26). MZ twinning usually begins in the blastocyst stage, approximately at the end of the first week, and results from division of the embryoblast into two embryonic primordia. Subsequently, two embryos, each in its own amniotic sac, develop within the same chorionic sac and share a common placenta—a monochorionic—diamniotic twin placenta. Uncommonly, early separation of embryonic blastomeres (e.g., during the two- to eight-cell stages) results in MZ twins with two amnions, two chorions, and two placentas that may or may not be fused (Fig. 7-27). In such cases, it is impossible to determine from the membranes alone whether the twins are MZ or DZ.
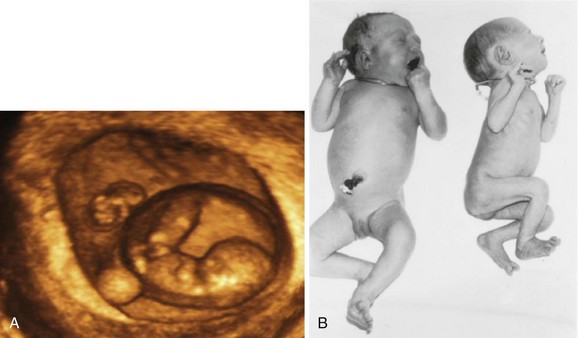
FIGURE 7–26 A, Three-dimensional ultrasound scan of a 6-week monochorionic diamniotic discordant twins. The normal twin (right) is seen surrounded by the amniotic membrane and adjacent to the umbilical vesicle. The arms and legs can also be seen. The smaller fetus is also visible (above left). B, Monozygotic, monochorionic, diamniotic twins showing a wide discrepancy in size resulting from an uncompensated arteriovenous anastomosis of placental vessels. Blood was shunted from the smaller twin to the larger one, producing the twin transfusion syndrome.
(A, Courtesy of Dr. E.A. Lyons, Professor of Radiology, Obstetrics and Gynecology, and Anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
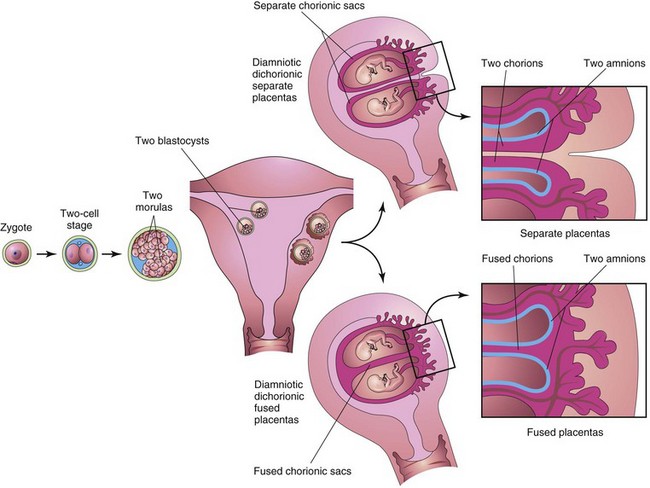
FIGURE 7–27 Diagrams illustrating how approximately 35% of monozygotic twins develop from one zygote. Separation of the blastomeres may occur anywhere from the two-cell stage to the morula stage, producing two identical blastocysts. Each embryo subsequently develops its own amniotic and chorionic sacs. The placentas may be separate or fused. In 25% of cases, there is a single placenta resulting from secondary fusion, and in 10% of cases, there are two placentas. In the latter cases, examination of the placenta would suggest that they were dizygotic twins. This explains why some monozygotic twins are wrongly stated to be dizygotic twins at birth.
Twin Transfusion Syndrome
This syndrome occurs in as many as 30% of monochorionic-diamniotic MZ twins. There is shunting of arterial blood from one twin through arteriovenous anastomoses into the venous circulation of the other twin. The donor twin is small, pale, and anemic (Fig. 7-26), whereas the recipient twin is large and has polycythemia, an increase above the normal in the number of red blood cells. The placenta shows similar abnormalities; the part of the placenta supplying the anemic twin is pale, whereas the part supplying the polycythemic twin is dark red. In lethal cases, death results from anemia in the donor twin and congestive heart failure in the recipient twin. Treatment by laser ablation of the shunt can be performed in some cases.
Establishing the Zygosity of Twins
Establishing the zygosity of twins is important in tissue and organ transplantation (e.g., bone marrow transplantations). The determination of twin zygosity is now done by molecular diagnosis because any two people who are not MZ twins are virtually certain to show differences in some of the large number of DNA markers that can be studied.
Late division of early embryonic cells, such as division of the embryonic disc during the second week, results in MZ twins that are in one amniotic sac and one chorionic sac (Fig. 7-28A). A monochorionic-monoamniotic twin placenta is associated with a fetal mortality rate approaching 50%. These MZ twins are rarely delivered alive because the umbilical cords are frequently so entangled that circulation of blood through their vessels ceases and one or both fetuses die. Sonography plays an important role in the diagnosis and management of twin pregnancies (Figs. 7-26A and 7-29). Ultrasound evaluation is necessary to identify various conditions that may complicate MZ twinning such as IUGR, fetal distress, and premature labor.
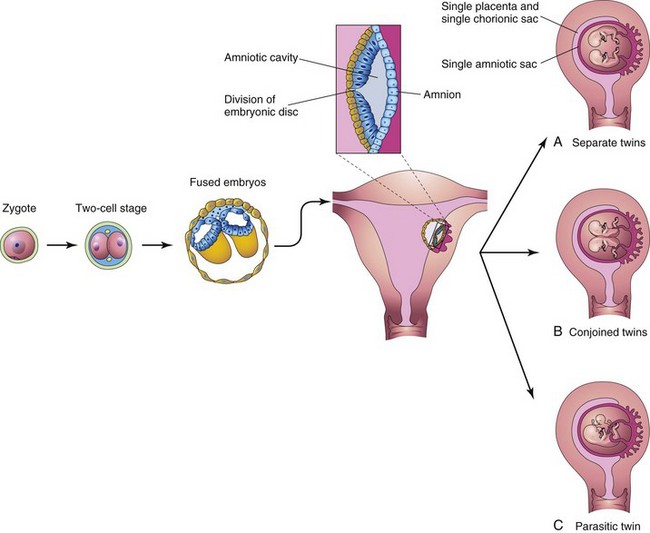
FIGURE 7–28 Diagrams illustrating how some monozygotic twins develop. This method of development is very uncommon. Division of the embryonic disc results in two embryos within one amniotic sac. A, Complete division of the embryonic disc gives rise to twins. Such twins rarely survive because their umbilical cords are often so entangled that interruption of the blood supply to the fetuses occurs. B and C, Incomplete division of the disc results in various types of conjoined twins.
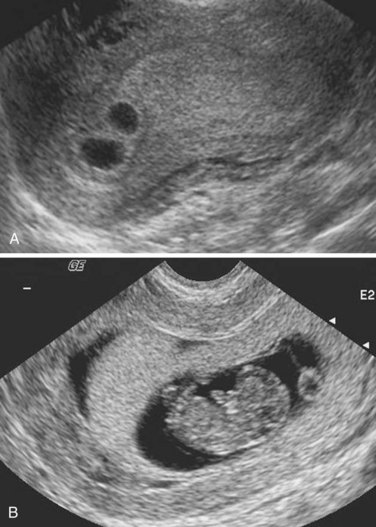
FIGURE 7–29 Serial ultrasound scans of a dichorionic pregnancy. A, At 3 weeks gestation. B, At 7 weeks gestation.
(Courtesy of Dr. E.A. Lyons, Professor of Radiology, Obstetrics and Gynecology, and Anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
MZ twins may be discordant for a variety of birth defects and genetic disorders, despite their origin from the same zygote. In addition to environmental differences and chance variation, the following have been implicated:
Early Death of a Twin
Because ultrasonographic studies are a common part of prenatal care, it is known that early death and resorption of one member of a twin pair is fairly common. Awareness of this possibility must be considered when discrepancies occur between prenatal cytogenetic findings and the karyotype of an infant. Errors in prenatal cytogenetic diagnosis may arise if extraembryonic tissues (e.g., part of a chorionic villus) from the resorbed twin are examined.
Conjoined Monozygotic Twins
If the embryonic disc does not divide completely, or adjacent embryonic discs fuse, various types of conjoined MZ twins may form (Figs. 7-28B and C, 7-30, 7-31, and 7-32). These attached twins are named according to the regions that are attached; for instance, thoracopagus indicates that there is anterior union of the thoracic regions. It has been estimated that the incidence of conjoined twins is 1 in 50,000 to 100,000 births. In some cases, the twins are connected to each other by skin only or by cutaneous and other tissues, such as fused livers (Fig 7-31). Some conjoined twins can be successfully separated by surgical procedures (see Fig. 7-30B); however, the anatomic relations in many conjoined twins do not permit surgical separation with sustained viability (see Fig. 7-32).
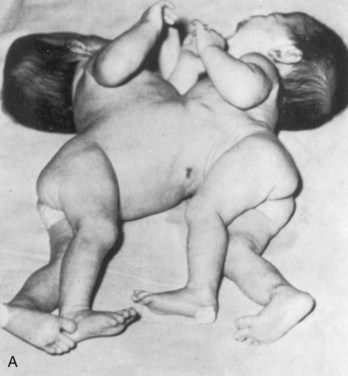
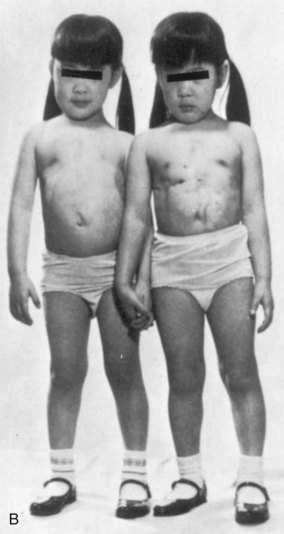
FIGURE 7–30 A, Newborn monozygotic conjoined twins showing union in the thoracic regions (thoracopagus). B, The twins approximately 4 years after separation.
(From deVries PA: Case history—The San Francisco twins. In Bergsma D [ed]: Conjoined Twins. New York, Alan R. Liss for the National Foundation-March of Dimes, DBOAS III, [1], 141–142, 1967, with permission of the copyright holder.)
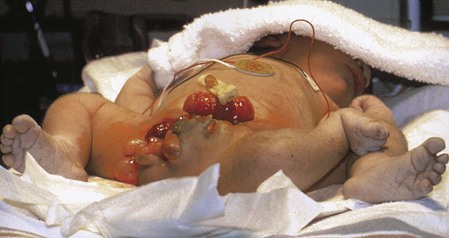
FIGURE 7–31 Parasitic twins, anterior view. Note normal tone and posture of fully developed host twin with meconium staining, exstrophy of the bladder in both host and parasitic twins, exposed small bowel in parasitic twin, and fully formed right lower limb with normal tone and flexion in the parasitic twin.
(Courtesy of Dr. Linda J. Juretschke, The Ronald McDonald Children’s Hospital of Loyola University Medical Center, Maywood, IL.)
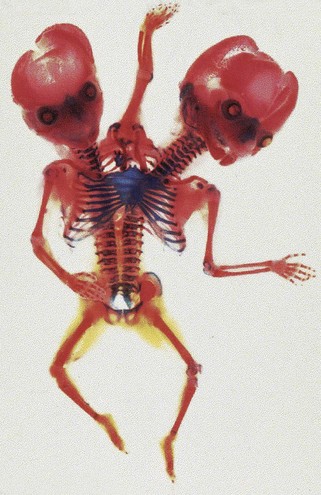
FIGURE 7–32 Dicephalic (two heads) conjoined twins, alizarin stained, showing bone (red) and cartilage (blue). Note the two clavicles supporting the midline upper limb, fused thoracic cage, and parallel vertebral columns.
(Courtesy of Dr. Joseph R. Siebert, Children’s Hospital and Regional Center, Seattle, WA.)
Summary of Placenta and Fetal Membranes
Clinically Oriented Problems
Case 7–4
A pathologist asked you to examine a section of an umbilical cord. You observed that there was only one umbilical artery.
Discussion of these problems appears at the back of the book.
References and Suggested Reading
Abuhamad AZ. Doppler ultrasound in obstetrics. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Alexander GR, Wingate MS, Salihu H, et al. Fetal and neonatal mortality risks of multiple births. Obstet Gynecol Clin North Am. 2005;32:1.
Baschatt AA. Fetal growth restriction: from observation to intervention. J Perinat Med. 2010;38:239.
Benirschke K, Kaufmann P. Pathology of the Human Placenta, ed 4. New York: Springer-Verlag; 2000.
Bronsan PG. The hypothalamic pituitary axis in the fetus and newborn. Semin Perinatol. 2001;25:371.
Callen PW. The role of amniotic fluid volume in fetal health and disease. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Chauhan SP, Scardo JA, Hayes E, et al. Twins: Prevalence, problems, and preterm births. Am J Obstet Gynecol. 2010;203:305.
Collins JH. Umbilical cord accidents: Human studies. Semin Perinatol. 2002;26:79.
Cross JC. Formation of the placenta and extraembryonic membranes. Ann N Y Acad Sci. 1998;857:23.
Cunningham FG, Leveno KJ, Bloom SL, et al. Williams’ Obstetrics, ed 23. New York: McGraw-Hill; 2009.
Egan JFX, Borgida AF. Ultrasound evaluation of multiple pregnancies. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Feldstein VA, Harris RD, Machin GA. Ultrasound evaluation of the placenta and umbilical cord. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
James JL, Whitley GS, Cartwright JE. Pre-eclampsia: fitting together the placental, immune and cardiovascular pieces. J Pathol. 2010;221:363.
Jirasel JE. An Atlas of Human Prenatal Developmental Mechanics: Anatomy and Staging. London and New York: Taylor & Francis; 2004.
Kazandi M. Conservative and surgical treatment of abnormal placentation: report of five cases and review of the literature. Clin Exp Obstet Gynecol. 2010;37:310.
Laing FC, Frates MC, Benson CB. Ultrasound evaluation during the first trimester. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Kodaman PH, Taylor HS. Hormonal regulation of implantation. Obstet Gynecol Clin North Am. 2004;31:745.
Liao JB, Buhimschi CS, Norwitz ER. Normal labor: Mechanism and duration. Obstet Gynecol Clin North Am. 2005;32:145.
Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203:531.
Moore KL, Dalley AD, Agur AMR. Clinically Oriented Anatomy, ed 6. Baltimore: Williams & Wilkins; 2010.
Mundy CA. Intravenous immunoglobulin in the management of hemolytic disease of the newborn. Neonat Netwk. 2005;24:17.
Redline RW. Placental pathology. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Robertson SA. Immune regulation of embryo implantation—All about quality control. J Reprod Immun. 2009;81:113.
Spencer R. Theoretical and analytical embryology of conjoined twins. Part I: Embryogenesis. Clin Anat. 2000;13:36.
Spencer R. Theoretical and analytical embryology of conjoined twins. Part II: Adjustments to union. Clin Anat. 2000;13:97.
Silasi M, Cohen B, Karumanchi SA, et al. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynec Clinic NA. 2010;37:239.
Williams PJ, Mistry HD, Innes BA, et al. Expression of AT1R, AT2R and AT4R and their roles in extravillous trophoblast invasion in the human. Placenta. 2010;31:448.
Yagel S. The developmental role of natural killer cells at the fetal-maternal interface. Am J Obstet Gynecol. 2009;201:344.