Chapter 12 The Cerebellum
1. The cerebellum constantly compares the intended movement with the actual movement and makes appropriate adjustments.
2. Cerebellar histology and phylogeny give clues to cerebellar function.
3. The vestibulocerebellum helps coordinate balance and eye movements.
4. The spinocerebellum helps coordinate muscle tone as well as limb movement.
5. The cerebrocerebellum helps with planning coordinated, properly timed movement sequences.
The preceding chapters, which describe the physiology of movement, discuss the function of lower motor neurons, the “final common pathway” through which the central nervous system (CNS) can initiate and control movement through the contraction of skeletal muscle. The corticospinal system and the descending brainstem motor system are described in those previous chapters as major subgroups of upper motor neurons that influence the lower motor neuron. More medial portions of those systems coursing through the spinal cord are primarily responsible for the control of axial and proximal antigravity extensor muscles. The more lateral portions primarily control more skilled, learned, voluntary movements caused by contraction of distal flexor muscles. This chapter describes the function of the cerebellum, part of another subgrouping of upper motor neurons critical for proper movement.
The cerebellum (Latin for “little brain”) is caudal to the cerebral cortex and dorsal to the brainstem (Figure 12-1). Although it constitutes only about 10% of the gross brain volume because of its highly convoluted structure, the cerebellum contains more than half of all the brain’s neurons. The outer layer of cerebellar gray matter, the cerebellar cortex, has a highly regular, three-layered, histological appearance, which suggests that all cerebellar regions may perform a common underlying task. As with the cerebral cortex, the particular inputs to a given region of cerebellar cortex, and the particular output targets that it influences, in large part account for the functional differences between cerebellar regions. Two large pairs of white matter stalks, called cerebellar peduncles, primarily carry axons into the cerebellum, and a third pair of cerebellar peduncles carries axons out from the cerebellum. In addition to the cerebellar cortex and the cerebellar white matter axons entering and leaving the cortex, a group of deep cerebellar nuclei is embedded within the cerebellar white matter. The cells of these nuclei are a principal origin of the axons leaving the cerebellum.
FIGURE 12-1 The cerebellum (Latin for “little brain”) is caudal to the cerebral hemispheres and dorsal to the brainstem.
(Redrawn from Miller ME, Christiansen GC, Evans HE: The anatomy of the dog, Philadelphia, 1964, Saunders.)
The cerebellum is not necessary for sensation or the initiation of movement. Muscle strength remains largely intact with complete destruction of the cerebellum. However, the cerebellum plays a crucial role in the timing and coordination of movement initiated by the parts of the motor system hierarchy discussed in Chapter 10. It does so by adjusting and modulating the output of the motor cortices, corticospinal tract, descending brainstem motor pathways, and spinal cord. Lesions of the cerebellum lead to major clinical deficits in the precision and grace with which movement is accomplished.
The Cerebellum Constantly Compares the Intended Movement with the Actual Movement and Makes Appropriate Adjustments
In performing the essential role of adjusting the timing and coordination of movement, the cerebellum first receives information from components of the motor system hierarchy about the movement it has commanded. It also receives information from muscle spindles, the vestibular and visual systems, and other sensory receptors about the movement the body is actually performing. When the intended movement and the actual movement are not the same, the cerebellum’s job is to perform the adjustments necessary to make them the same. For example, if the brain intends that a cat move its mouth to a piece of food in a dish, but sensory receptors inform the cerebellum that the trajectory of the head will cause the mouth to miss the dish, the cerebellum makes appropriate adjustments in the components of the motor system hierarchy to correct the head’s trajectory. The correction can be made to the movement in progress and to the plan for subsequent movement.
This chapter describes cerebellar histology and phylogenetic anatomy as clues to cerebellar function. Movement disorders resulting from lesions of the cerebellum are described as further clues to the function of this part of the brain.
Cerebellar Histology and Phylogeny Give Clues to Cerebellar Function
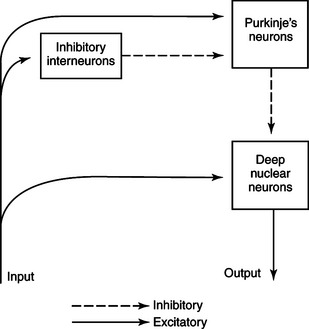
The cortex throughout the cerebellum consists of three layers and only five types of neurons: stellate, basket, Golgi, granule, and Purkinje cells (Figure 12-2). The outermost layer is the molecular layer and consists primarily of granule cell axons, known as parallel fibers; dendrites of neurons located in deeper layers; and scattered inhibitory interneurons: the stellate and basket cells (Figure 12-3). The middle Purkinje cell layer consists of the large cell bodies of Purkinje neurons, which have a flat but extremely expansive dendritic field oriented at right angles to the parallel fibers (see Figure 12-2). This arrangement, and because a parallel fiber contacts the dendrites of many Purkinje cells, is concordant with the cerebellum’s role in the timing of movement components. Axons of the Purkinje neurons go to the deep cerebellar nuclei, located outside of the cerebellar cortex. The Purkinje cells are the only output neurons of the cerebellar cortex and are all inhibitory, using gamma-aminobutyric acid (GABA) as their transmitter. They can inhibit the spontaneously active neurons of the deep cerebellar nuclei, whose axons leave the cerebellum. The innermost granule cell layer of the cerebellar cortex contains a vast number of small granule cells and occasional Golgi cells.
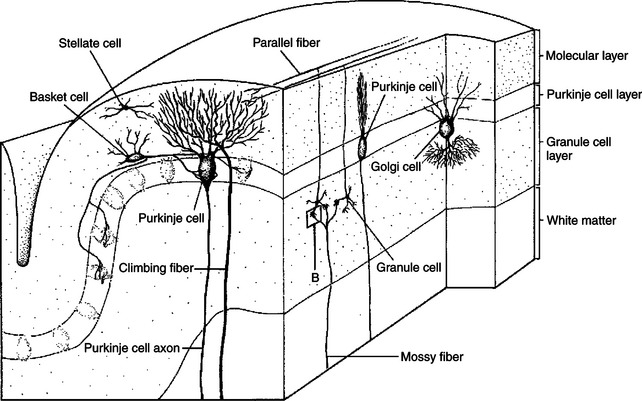
FIGURE 12-2 Five types of neurons are organized into three layers in the cerebellar cortex. A single cerebellar folium is sectioned vertically, in both longitudinal and transverse planes, to illustrate the general organization of the cerebellar cortex.
(From Kandel ER, Schwartz JH: Principles of neural science, ed 2, New York, 1985, Elsevier Science Publishing.)
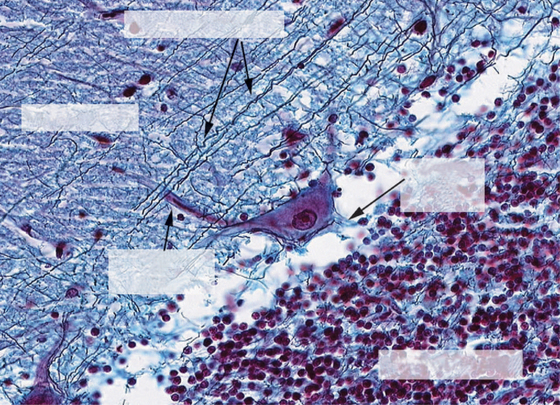
FIGURE 12-3 High-power photomicrograph of the three layers of the cerebellar cortex: granule cell layer, Purkinje cell layer (not labeled), and molecular layer. The image provides a good example of how the parallel fibers, labeled “granule cell processes,” cross the dendritic region of the Purkinje cell in the molecular layer. However, the elaborate branching pattern of the Purkinje cell dendrites is not apparent with this stain.
(Image courtesy Dr. Tom Caceci, Department of Biomedical Sciences and Pathobiology, College of Veterinary Medicine, Virginia Tech.)
The two primary groups of input axons to the cerebellum are the mossy fiber and climbing fiber axons (see Figure 12-2). Both are excitatory; they cause excitatory postsynaptic potentials (EPSPs) within the cerebellar cortex and, through collateral axons, within the deep cerebellar nuclei. The mossy and climbing fibers collectively carry information from components of the motor system hierarchy and from peripheral sensory receptors regarding the planning and execution of the movement. The shorter input/output circuit of the cerebellum consists of the climbing and mossy fiber stimulation to the deep cerebellar nuclei, whose output in turn leaves the cerebellum to modify components of the motor system hierarchy (Figure 12-4). However, the output of the deep cerebellar nuclei is itself modified by inhibition from Purkinje cell axons. The Purkinje cell inhibition of deep cerebellar nuclei is based on the cerebellar cortex’s own integration of mossy and climbing fiber inputs.
Although the cortical synaptology is understood, just how the cerebellum integrates movement feedback with the motor plan and then modifies the output of the deep nuclear neurons is not clear. As noted earlier, because the histological appearance of the cortex is similar throughout the cerebellum, it seems likely that a similar underlying modulation process occurs regardless of the cerebellar region. However, regional variation of inputs from and outputs to different parts of the nervous system would render different motor results from different cerebellar regions. Therefore, it is useful to examine the three major, phylogenetically different divisions of cerebellum.
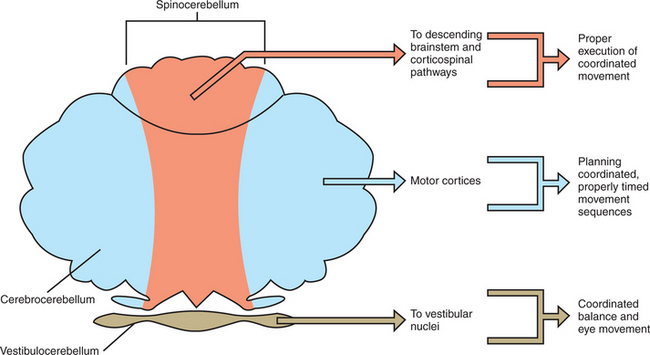
The cerebellum can be divided into three distinct regions from both a functional perspective and a phylogenetic perspective: the vestibulocerebellum, the spinocerebellum, and the cerebrocerebellum (Figures 12-5 and 12-6).
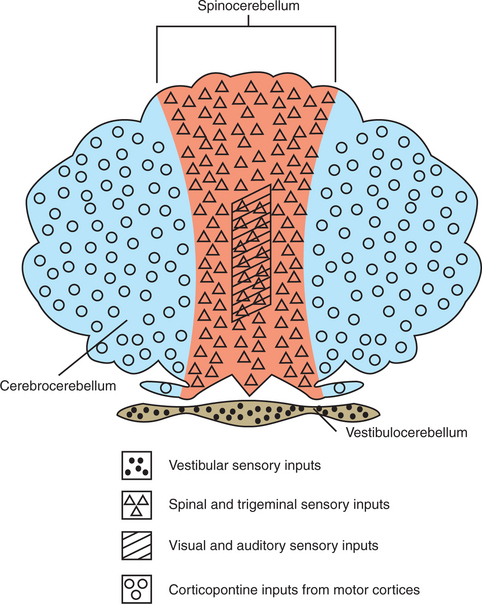
FIGURE 12-5 The cerebellum can be divided into three distinct regions, illustrated here with their respective major inputs, from both a functional perspective and a phylogenetic perspective.
(Modified from Kandel ER, Schwartz JH: Principles of neural science, ed 2, New York, 1985, Elsevier Science Publishing.)
The Vestibulocerebellum Helps Coordinate Balance and Eye Movements
The vestibulocerebellum occupies the flocculonodular lobe and receives most of its afferent input from the vestibular system (Figures 12-5 and 12-6). Its efferent output returns to the vestibular nuclei, either directly from cerebellar cortex or by way of the deep cerebellar nuclei (specifically the fastigial nucleus). The cerebellar output to the vestibular nuclei helps to coordinate the axial and proximal muscles controlling balance, through the vestibulospinal tract, and helps to coordinate head and eye movements through the medial longitudinal fasciculus (MLF; see Chapter 11). In short, the vestibulocerebellum adjusts the coordination of vestibular reflexes. Because this part of the cerebellum was the first to appear in vertebrate evolution, it is sometimes called the archicerebellum.
The Spinocerebellum Helps Coordinate Muscle Tone as Well as Limb Movement
The spinocerebellum extends rostrocaudally through the medial portion of the cerebellum (Figures 12-5 and 12-6). It receives sensory inputs from muscle and cutaneous receptors through the spinal cord and trigeminal nuclei. It also receives input from neurons in spinal reflex circuits, some of which receive information from corticospinal or descending brainstem pathways. Some input to the spinocerebellar region also comes directly from the primary motor and primary somatosensory cortices. The spinocerebellum therefore receives information about commands for movement and significant information about the execution of the movement itself. Its outputs travel, through its deep cerebellar nuclei (specifically the fastigial and interpositus), to brainstem nuclei controlling the antigravity musculature (e.g., reticular nuclei), as well as to a brainstem nucleus controlling distal limb musculature (e.g., red nucleus). Some of the spinocerebellar output travels to the primary motor cortex by way of the thalamus. Through these output projections, the spinocerebellum can adjust the timing and coordination of movement and muscle tone. Such adjustments are presumably based on a comparison of spinocerebellar input regarding the movement command (e.g., from primary motor cortex) with feedback about the ongoing movement itself (e.g., from muscle, joint, and skin inputs). Because this portion of the cerebellum appeared next in evolution, it is sometimes called the paleocerebellum.
The Cerebrocerebellum Helps with Planning Coordinated, Properly Timed Movement Sequences
The cerebrocerebellum occupies the lateral cerebellar hemispheres (Figures 12-5 and 12-6). This region also receives input from the primary motor cortex, but more important, receives a substantial input from premotor and supplementary motor cortices. These cortical inputs reach the cerebellum by way of the corticopontine-cerebellar system. This area of the cerebellum does not have direct access to information from peripheral receptors as does the spinocerebellum. Its outputs return to the motor cortices by way of the thalamus. Therefore the cerebrocerebellum is part of a communication loop with regions of motor cortex that are involved in the planning of, and preparation for, movement. Whereas the spinocerebellum helps coordinate the execution of movement, it appears that the cerebrocerebellum helps the motor cortices with planning ahead for the next appropriate movement so that there will be smooth and appropriately timed transitions between components of a movement sequence. The dramatic growth of the cerebrocerebellum and cerebral cortex was the major phylogenetic addition to the brain during primate evolution, and thus it is often called the neocerebellum. Presumably this is linked to the primate’s ability to perform graceful, intricate, appropriately timed voluntary movements, such as coordinated finger movements and the mouth and tongue movements necessary for speech.
The Cerebellum Plays a Role in Motor Learning
Several lines of evidence suggest that the cerebellum plays a significant role in motor learning. For example, functional magnetic resonance imaging (fMRI) studies have shown that the cerebellum is very active when learning a new sequence of movements, but it is not as active once the movement becomes relatively automatic. This suggests that the cerebellum is likely involved in the transition from having to concentrate on learning of a new motor skill, such as where to place the individual fingers on the piano keys to form a chord, to being able to perform that skill automatically, with limited thought. Some reflex behaviors, such as the vestibulo-ocular reflex (see Chapter 11), although automatic, need to be fine-tuned or adjusted (e.g. with respect to the amount of eye rotation necessary to counteract a given amount of head rotation to keep the gaze fixed on a target) as the proportions of the head change during growth. Damage to certain regions of the cerebellum can prevent this type of adaptive adjustment. In addition, some forms of associative learning, such as some classically conditioned responses, can be abolished after cerebellar lesions. The ability to make motor adaptations after alterations of the visual world, such as learning to throw darts accurately after wearing prism glasses, can also be severely impaired in individuals with cerebellar damage.
Structural and functional changes in cerebellar circuitry have also been observed during motor learning. For example, increases in the number of parallel fiber and climbing fiber synaptic contacts on Purkinje cells have been observed following the learning of complex motor behavior. Furthermore, simultaneous activation of these two types of fibers synapsing on a Purkinje cell can produce a long-term depression of Purkinje cell activity. Such depression can have a profound effect on the activity of neurons of the deep cerebellar nuclei that leave the cerebellum to control components of the motor hierarchy.
Cerebellar Disease Causes Abnormalities of Movement
As discussed, the cerebellum constantly compares the intended movement with the actual movement and makes appropriate adjustments. In cerebellar disease, these appropriate adjustments are not made, resulting in a variety of movement disorders. Affected animals often place their paws far apart (wide-based gait) and walk in an uncoordinated manner (ataxia), which reflects the inability of the vestibulocerebellum and spinocerebellum to coordinate balance and movement of the axial skeleton. Affected animals also have various degrees of dysmetria (inappropriate measure of muscular contraction), in which movements either continue too long or not long enough. In animals this is often manifested as difficulty in bringing the muzzle to a fixed point in space, such as a food dish, and as exaggerated “goose stepping” walking movements. Asynergia, a failure of the components of a complex movement to occur in a coordinated fashion, may also be seen. Intention tremor (action tremor), an oscillating movement disorder (tremor) that is worse when the animal is moving, especially near the end of the movement, is common in cerebellar disease as well. Intention tremors are much less severe when the animal is relaxed and not moving and worsen when a movement is being performed. In animals, intention tremors seem worse in the head and axial (proximal) antigravity muscles. If the vestibulocerebellum is damaged, nystagmus may also be seen (see Chapter 11). These commonly-associated clinical signs resulting from cerebellar disease exemplify how the mechanism of disease can be understood through knowledge of normal physiology.
CLINICAL CORRELATIONS
Cerebellar Hypoplasia
History.
An 11-week-old female barn kitten is brought to your clinic for examination. The owner states that this kitten and several others in the litter have been uncoordinated since they began to walk.
Clinical Examination.
Physical examination abnormalities are limited to the nervous system. The kitten is bright, alert, and responsive and seems to be of normal size for her age. All cranial nerve and spinal segmental reflexes and intersegmental responses are within normal limits. There is no atrophy. The kitten is uncoordinated (ataxic) when she moves and tends to raise her front paws higher than normal when walking (“goose stepping” hypermetria). She holds her paws far apart when walking. There are coarse, rhythmic movements of her head and proximal antigravity muscles that are absent at rest and severe when she is attempting a precise movement, such as getting her head to a food dish (intention tremor). Her complete blood count and serum chemistry results are within normal limits.
Comment.
This kitten demonstrates classic signs of cerebellar disease. The cerebellum constantly compares the intended movement with the actual movement and, when these are not the same, makes the appropriate adjustments. When the cerebellum cannot do this, movement disorders characterized by wide-based gaits, ataxia, dysmetria, asynergia, and intention tremor occur. These movement disorders are worse with precise movement and nearly absent at rest.
This kitten’s clinical signs are likely caused by cerebellar hypoplasia, in which the cerebellum never developed completely in utero. The in utero infection of feline panleukopenia virus results in destruction of the actively dividing granule cells (neurons), with an underdevelopment (hypoplasia) of the granular cell layer of the cerebellum. Purkinje cells may also be affected. Barn cats are often not vaccinated for this disease, and often several kittens in a litter are affected.
Newborn Calf Unable to Rise
History.
A producer calls to ask about an Angus heifer calf born early today that has not stood. The calf makes efforts but does not seem coordinated enough to stand. The producer has fed the calf with colostrum by tube and wants her examined. This is the second calf this season that has had this problem. They euthanized the other calf after she had not improved over 2 to 3 days. The calves are very valuable, and the producer would like to keep this calf in the herd. Further questioning of the owner reveals an increased percentage of abortions this year. She also bought several new replacement cows last fall that have been introduced to the herd.
Clinical Examination.
The calf has a normal temperature, pulse, and respiration. She appears responsive to noise, almost hyperexcitable. There is no evidence of trauma. When the calf is placed in a standing position, she sways back and forth; she tries to maintain a base-wide stance but sometimes falls over or backs up. She appears extremely uncoordinated and hypermetric. She scores a 4/5 on ataxia, with 5 being the most severe. Other abnormalities include a greatly delayed menace response; it is difficult to tell if her vision is within normal limits because she is bumping into things when she tries to walk. When she is laid back down and her reflexes are assessed, the calf is hyperreflexic in all her responses.
Comment.
Based on this history of the herd and the calf, this herd most likely has a problem with bovine viral diarrhea virus (BVDV). This was likely introduced by replacement cows. BVDV would explain the abortions as well as the two affected calves. With BVDV, the virus infects the germinal cells within the cerebellum and also kills the Purkinje cells. Infection of these cells results in local inflammation, cell death, hemorrhage, and necrosis. Because of the damage to the Purkinje cells, inhibitory function is disrupted, which affects the vestibulocerebellum, spinocerebellum, and cerebrocerebellum. The deficits in these areas are associated with clinical signs of abnormalities in balance (vestibulocerebellum), eye movement (vestibulocerebellum), ataxia and base-wide stances (vestibulo and spinocerebellum), and motor coordination and sequencing (spino and cerebrocerebellum).
Treatment.
Because BVDV causes irreversible cell damage, the prognosis for this calf is poor. Even if treatment were available, the calf most likely has BVDV and would shed virus if reintroduced into the herd. Euthanasia is the best option for this calf. The owner should screen the herd and identify infected and persistently infected (PI) animals. Additionally, vaccination with a live versus a killed BVDV vaccine may improve overall outcome.
Brodal P. The central nervous system: structure and function, ed 3. New York: Oxford University Press, 2004.
Brodal P. The central nervous system: structure and function, ed 3. New York: Oxford University Press, 2004.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saundes, 2006.
Haines DE. Fundamental neuroscience for basic and clinical applications. Philadelphia: Churchill Livingstone, 2006.
Jennings DP. Supraspinal control of posture and movement. Reece WO, ed. Duke’s physiology of domestic animals, ed 12, Ithaca, NY: Comstock Cornell University Press, 2004.
Kandel ER, Schwartz JH, Jessell TM. Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
PRACTICE QUESTIONS
1. Which of the following is principally involved in planning ahead for the next appropriate movement?
2. Loss of the cerebellum causes specific sensory deficits and prevents the initiation of movement.
3. Which of the following is true regarding cerebellar Purkinje cells?
4. Loss of the cerebellum causes loss of the muscle stretch reflex.
5. Cats with congenital malformations of the cerebellum often have ataxia, intention tremor, and wide gait.