Chapter 10 The Central Control of Movement
1. The central nervous system structures controlling movement have a hierarchical organization.
2. The spinal cord is the most caudal and simplest level of the movement control hierarchy.
3. Brainstem upper motor neuron pathways are the source of all descending motor system input to the spinal cord, except for one other major pathway.
4. Medial and lateral descending brainstem motor pathways respectively control proximal muscles of posture and more distal muscles of skilled movement.
5. The reticulospinal and vestibulospinal tracts are medial brainstem motor pathways important for keeping the body upright against the pull of gravity.
6. The rubrospinal tract is a lateral brainstem motor pathway that can control distal limb musculature associated with skilled movement.
7. The corticospinal (pyramidal) tract is a direct projection from cerebral cortex to spinal cord responsible for the most skilled voluntary movements of mammals.
8. The corticospinal tract has a massive lateral component controlling the distal musculature and a minor medial component controlling the axial and proximal musculature.
9. The motor cortices of the frontal lobe, the highest level of the motor control hierarchy, consist of three different functional regions.
10. Corticospinal tract co-activation of both alpha (α) and gamma (γ) lower motor neurons may help with small automatic corrections of voluntary movements.
11. The motor system shares some organizational principles with sensory systems.
12. The basal ganglia and cerebellum modulate the activity of motor system components for the respective selection and adjustment of movement
Unlike the sensory system, which transforms physical energy into neural information, the motor system transforms neural information into physical energy. All movement is the result of the contraction of varying numbers of extrafusal skeletal muscle fibers within varying numbers of motor units. These extrafusal muscle fibers do not contract until commanded to do so by the alpha (α) lower motor neuron. The α motor neuron, in turn, does not send such an action potential command until signaled to do so by descending upper motor neurons or from incoming sensory neurons (or interneurons) in a reflex arc. Thus the α motor neuron is the “final common neural pathway” by which the nervous system can initiate the extrafusal muscle contractions that result in movement.
Movement can be divided into two general forms. The first is a largely learned, voluntary, conscious, and skilled form, often dominated by flexor muscle activation. The second form is characterized by postural, antigravity muscle activity that is generally subconscious, involuntary, and dominated by extensor muscle contraction. The skilled movement results from fairly discrete contraction of a few muscle groups, many of which are distal to the spinal column. The maintenance of posture often includes longer-term contraction of larger groups of muscles, many of which are located closer (proximal) to the spinal column. Correspondingly, in the spinal cord gray matter, the α motor neurons that control the more distal muscles tend to be located laterally; those controlling the more proximal and axial muscles for posture are located more medially.
Initiating the learned, skilled, voluntary movement of the distal musculature is largely the responsibility of a subgroup of upper motor neuron tracts that project through more lateral regions of the spinal cord white matter and terminate in lateral regions of the spinal cord gray matter. Initiating antigravity and postural muscle activity is the responsibility of upper motor neuron tracts that are associated with more medial regions of the spinal cord white and gray matter, respectively. This lateral-medial distinction is a significant organizational principle in central nervous system (CNS) motor control. Skilled, voluntary movement of the distal musculature is primarily controlled by a lateral system of lower motor neurons and upper motor neuron spinal tracts. More medial systems of such neurons and tracts primarily control postural and antigravity activity of the proximal and axial musculature.
The Central Nervous System Structures Controlling Movement Have a Hierarchical Organization
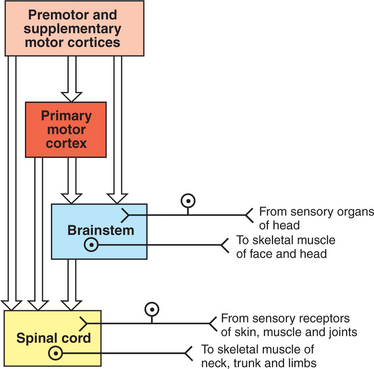
Another organizational principle of the neural control of movement is that it consists of a hierarchy. Generally, simpler movements or movement patterns are organized by more caudal parts of the CNS, and more complex and skilled patterns are organized by progressively more rostral regions (Figure 10-1).
The Spinal Cord Is the Most Caudal and Simplest Level of the Movement Control Hierarchy
The spinal cord contains the lower motor neurons that represent the final common path to the muscles of the trunk and limbs (Figure 10-1). As noted in Chapter 6, an α lower motor neuron innervates several extrafusal muscle fibers of a single muscle, forming a motor unit (see Figure 6-6). The neuronal cell bodies of the motor units of a given muscle are clustered into a motor neuron pool located in the ventral horn of spinal cord gray matter. The motor neuron pool of a muscle has a cigar-shaped, longitudinal organization in the cord, often extending rostrocaudally over a few spinal cord segments (e.g., L1-L3; see Figure 10-2). These motor neuron pools have a somatotopic organization in the ventral horn; that is, their relative position in the CNS corresponds to the relative body position of the muscles that their neurons innervate. In other words, motor neuron pools whose neurons innervate distal muscles of the limbs tend to be located in more lateral parts of the ventral horn, whereas motor neuron pools associated with axial and proximal musculature tend to be located more medially within the ventral horn.
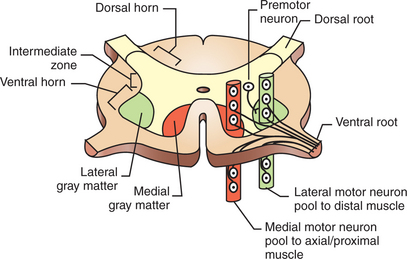
FIGURE 10-2 Somatotopic organization of lower motor neurons in the ventral horn of the spinal cord that respectively supply the distal and axial/proximal musculature. Cell bodies of motor units supplying a given muscle are arranged in longitudinal columns within the ventral horn called motor neuron pools. Motor neuron pools to more distal muscles lie laterally to those supplying the axial and proximal musculature. Spinal premotor neurons, which synapse on the motor neurons supplying muscles, are located in the intermediate zone of spinal cord gray matter and also have a somatotopic organization.
(Modified from Kandel ER, Schwartz JH, Jessell TM: Principles of neural science, ed 3, New York, 1991, Elsevier Science Publishing.)
Lower motor neurons projecting out to muscles are often synaptically activated by premotor neurons whose cell bodies are usually located in the intermediate zone of spinal cord gray matter (Figure 10-2). Activating a premotor neuron in the lateral part of the intermediate zone on one side of the body will generally activate a modest number of α motor neurons, in the lateral part of the ventral horn, on the same side of the body. This in turn will result in the activation of a modest number of distal limb muscles that would generally be used for skilled, voluntary movement. Premotor neuron activation in the medial part of the intermediate zone on one side of the body will generally activate a larger number of α motor neurons, in the medial part of the ventral horn, often on both sides of the body and often over more than one spinal cord segment. This in turn will result in the extensive activation of axial or proximal antigravity muscles on both sides of the body. Such a complement of muscles would be required for the involuntary stabilization or adjustment of posture. It can therefore be seen that more lateral parts of the spinal cord gray matter are involved in control of the distal limb musculature of skilled voluntary movement, whereas more medial parts are associated with the axial and proximal musculature of postural control.
The simplest type of motor behavior, the spinal segmental reflex (e.g., the knee jerk reflex; see Chapter 7), can be organized at the level of the spinal cord, without significant control from more rostral divisions of the CNS (e.g., the brain). Under different circumstances, however, the same spinal premotor and α motor neurons that participate in a simple spinal reflex could be activated by the brain to participate in an elegant and skilled sequence of movement.
Brainstem Upper Motor Neuron Pathways Are the Source of All Descending Motor System Input to the Spinal Cord, Except for One Other Major Pathway
Four major axon tracts originate in the brainstem and descend to the spinal cord to influence spinal lower motor neurons: the vestibulospinal tract, the reticulospinal tract, the tectospinal tract, and the rubrospinal tract (Figure 10-3). Collectively, the first three are involved in the involuntary maintenance and adjustment of posture and in reflex orientation of the head. Therefore they are principally involved in the control of axial and proximal musculature. The rubrospinal tract is mainly involved in control of distal limb musculature of the type that mediates voluntary skilled movements. These four tracts (often along with components of the basal ganglia and cerebellum) are sometimes referred to as the “extrapyramidal” motor system. This is in contrast to the “pyramidal” motor system that originates in the cerebral cortex, the other major descending motor pathway to the spinal cord, as discussed later. Because the term “extrapyramidal” can encompass such a diverse group of structures, and because it is often applied inconsistently, it is being used less frequently. The four tracts from brainstem to spinal cord are collectively referred to here as the descending brainstem motor pathways.
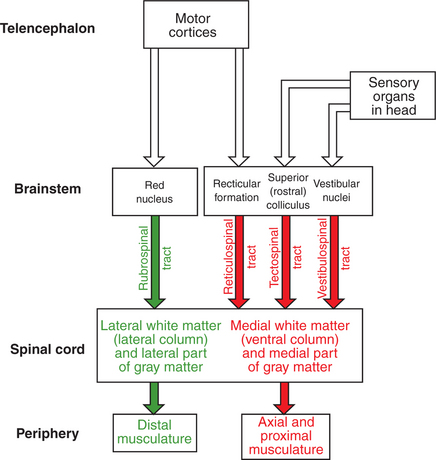
FIGURE 10-3 Organization of the descending brainstem motor pathways to the spinal cord. The medial brainstem motor pathways are the reticulospinal, vestibulospinal, and tectospinal tracts. They travel in more medial regions of the spinal cord white matter and synapse within more medial regions of the spinal cord gray matter controlling the axial and proximal musculature. The rubrospinal tract is a lateral brainstem motor pathway that travels in more lateral regions of the spinal white matter and synapses within more lateral regions of the spinal gray matter controlling the distal limb musculature. Crossing of some of the pathways is not represented.
The brainstem, like the spinal cord, contains lower motor neurons that can synaptically activate skeletal muscles, in this case the face and head muscles (see Figure 10-1). The cell bodies of these α motor neurons reside in various cranial nerve nuclei (e.g., facial motor, hypoglossal, oculomotor). The brainstem also receives direct input from sensory organs in the face and head (e.g., eye, vestibular apparatus). Therefore, as in the spinal cord, some fairly simple segmental reflexes can be organized at the brainstem level without significant control from other levels of the motor system. Because the brainstem also contains the descending motor pathways to the spinal cord previously noted, however, the brainstem also provides a means by which input from sensory organs in the face and head can reach and control lower motor neurons of the spinal cord that operate muscles of the trunk and limbs (see Figure 10-3). Some of the descending brainstem motor pathways also provide a means by which more rostral regions of the motor system (e.g., motor cortex) can indirectly influence spinal lower motor neurons.
Medial and Lateral Descending Brainstem Motor Pathways Respectively Control Proximal Muscles of Posture and More Distal Muscles of Skilled Movement
The descending brainstem motor pathways to the spinal cord can be divided into a medial group and a lateral pathway. The vestibulospinal, reticulospinal, and tectospinal tracts constitute the medial brainstem motor pathways, whereas the rubrospinal tract represents the lateral brainstem motor pathway (see Figure 10-3). The groupings are generally based on the relative position of these tracts within the spinal cord white matter. The axons of the tracts that represent the medial brainstem motor pathways (vestibulospinal, reticulospinal, tectospinal) will travel in more medial regions of the spinal cord white matter (e.g., ventral column) and will synapse within more medial regions of the spinal cord gray matter. Those medial regions of the spinal gray matter contain medial premotor neurons and medial α motor neurons that control the axial and proximal extensor musculature primarily involved in involuntary maintenance and adjustment of posture. Axons of the lateral brainstem motor pathway (rubrospinal) run in a more lateral region of the spinal white matter (lateral column) and synapse in the more lateral spinal gray matter. The premotor and α motor neurons of this region principally control the distal flexor musculature involved in voluntary skilled movement.
Thus, medial brainstem motor pathways project to medial regions of the spinal cord gray matter whose neurons control the more medially located (axial and proximal) extensor muscles of posture, whereas the lateral brainstem motor pathway projects to lateral regions of the spinal gray matter whose neurons control the more laterally located (distal) flexor muscles of skilled movement.
The Reticulospinal and Vestibulospinal Tracts Are Medial Brainstem Motor Pathways Important for Keeping the Body Upright Against the Pull of Gravity
A major responsibility of the medial descending brainstem motor pathways is to maintain the body subconsciously in an upright position against the pull of gravity. The reticulospinal and vestibulospinal tracts play a major role in this involuntary control of the axial and proximal extensor musculature that prevents the animal from falling to the ground. The reticulospinal tract is particularly important in controlling the magnitude of the steady-state contraction level, or muscle tone, of these antigravity muscles. The vestibulospinal tract plays an essential role in activating the antigravity muscles in response to destabilization of the body with respect to gravity. Keep in mind that subconscious control of the postural musculature is an integral part of the ability to execute skilled voluntary movement of the distal musculature successfully, because voluntary movement requires a stable “platform” on which it can proceed.
The reticulospinal tract originates from cell bodies in the reticular formation of the brainstem (see Figure 10-3). This is a netlike complex of many small clusters of cell bodies (nuclei) and loosely organized axonal projections, located near the midline. Once thought to be a diffuse and fairly nonspecific system, the reticular formation is now known to contain a number of functionally specific nuclei. In addition to being the origin of a medial descending brainstem motor pathway to the spinal cord, ascending projections of the reticular formation play an important role in modulating consciousness, arousal, and attention. The reticular formation receives a vast array of sensory information and also plays an important role in pain perception, respiration, and circulatory function.
Axons of the reticulospinal tract synapse within medial regions of the spinal cord gray matter that primarily control the axial and proximal extensor musculature (see Figure 10-3). Collectively, the tract projects to virtually all rostrocaudal levels of the cord. Portions of the reticulospinal tract that originate from cells in reticular nuclei of the pons tend to have an excitatory effect on lower motor neurons to the antigravity muscles. Portions of the tract coming from the reticular nuclei of the medulla tend to have an inhibitory effect on the lower motor neurons to antigravity muscles. These opposing portions of the reticulospinal tract interact to regulate antigravity muscle tone. Influences from other regions of the brainstem, the cerebellum, and the spinal cord endow the pontine reticular nuclei with a high level of spontaneous activity. The effects of such spontaneous excitatory activity on antigravity muscle tone can be tempered by activation of the inhibitory medullary reticular nuclei. Descending projections from the cerebral cortex to the brainstem represent a significant forebrain source of relative control over the two portions of the reticulospinal tract. This cortico-reticulospinal route emphasizes the point that some of the descending brainstem motor pathways provide an indirect way for more rostral levels of the motor system hierarchy to influence spinal lower motor neurons (see Figure 10-3).
The descending cortical projections to the origins of the reticulospinal tract endow that tract with two important motor functions, in addition to its critical role in the subconscious modulation of antigravity muscle tone. The first function is related to skilled voluntary movement requiring a stable postural background, as previously noted. Just before the execution of such a voluntary movement, the reticulospinal tract subconsciously activates the appropriate axial and proximal musculature that will compensate for the postural destabilization that will be produced by the intended voluntary movement (usually of the distal musculature). The reticulospinal tract also plays a role in the voluntary execution of crude (nonskilled), stereotypical movements of the proximal limb musculature, such as those involved in simple pointing or locomotion.
As noted in Chapter 8, γ motor neurons are usually activated along with α motor neurons so that muscle spindles maintain their sensitivity to stretch even when the muscle is shortened during contraction. This α−γ co-activation is a principle common to the excitation of lower motor neurons by upper motor neurons. Under certain circumstances, however, it appears that this process can be dissociated, such that the γ motor neuron–mediated sensitivity of the muscle spindle, and thus the sensitivity of the stretch reflex, can be adjusted apart from extrafusal muscle contraction. Although the reticulospinal tract participates in α−γ co-activation of lower motor neurons, it appears to be strongly associated with the ability to regulate γ motor neuron activity independently. It is likely that this ability of the reticulospinal tract to modulate independently the sensitivity of the stretch reflex underlies its significant role in adjusting antigravity muscle tone.
The vestibulospinal tract originates from cell bodies in the vestibular nuclear complex, which lies primarily in the medulla, just ventral to the fourth ventricle. This complex consists of several subnuclei that receive their principal synaptic input from eighth cranial nerve fibers carrying sensory input from the vestibular apparatus of the inner ear (see Chapter 11). The vestibular apparatus provides sensory information about the position of the head with respect to gravity and about acceleration of the head through space, thus indicating body position and disturbances of balance. The vestibular nuclear complex also receives significant input from the cerebellum, but not from forebrain levels of the motor system hierarchy.
As in the reticulospinal tract, axons of the vestibulospinal tract synapse within medial regions of the spinal cord gray matter that primarily control the axial and proximal extensor musculature (see Figure 10-3). Also like the reticulospinal tract, vestibulospinal tract axons collectively project to virtually all rostrocaudal levels of the spinal cord. A disturbance of balance detected by the vestibular apparatus results in excitation of the antigravity musculature in an attempt to counteract the disturbance. Although the vestibulospinal tract principally functions to produce compensatory adjustments to postural disturbances, it seems to make some contribution to antigravity muscle tone as well.
Some aspects of the functions of these two descending brainstem motor pathways can be better understood by considering the clinical state called decerebrate rigidity. This condition occasionally results from severe forebrain disease. It also results from surgical transection of the brain at the rostral midbrain level, as discovered by the British neurophysiologist Charles Sherrington. As noted earlier, the portion of the reticulospinal tract originating in the pons, which excites lower motor neurons to antigravity muscles, has a high degree of spontaneous activity. Excitation of the portion of the reticulospinal tract originating in the medulla inhibits the lower motor neurons to antigravity muscles. When the forebrain is disconnected from the brainstem, descending projections from cerebral cortex cannot excite these medullary reticular neurons projecting to the spinal cord, and thus a significant source of inhibition to the lower motor neurons of antigravity muscles is removed. The excitation of the lower motor neurons produced by the spontaneous activity of the pontine reticulospinal neurons has now lost a significant source of opposition, and therefore much greater muscle tone exists in the antigravity muscles. The animal now assumes a hobbyhorse-like posture, often so rigid that the animal stands in a fixed position. Subsequently cutting a portion of the vestibulospinal tract reduces some of this rigidity, so the tract apparently plays some normal role in regulating antigravity muscle tone, in addition to its principal role in responding to postural destabilization with respect to gravity.
As noted, the reticulospinal and vestibulospinal tracts make important contributions to the control of the axial and proximal musculature to keep the body upright. However, the to-and-fro rhythmicity of walking and running is organized by circuits of spinal interneurons that control the lower motor neurons in a repetitive, oscillating manner. Although these spinal neural networks are capable of producing this simple oscillatory behavior without control by more rostral parts of the motor system hierarchy, the reticulospinal tract plays an important role in initiating this locomotor behavior and in controlling its speed.
The tectospinal tract is a medial brainstem motor pathway that is principally involved in reflex orientation of the head toward environmental stimuli. The cells of origin of the tectospinal tract are located in the superior colliculus of the midbrain (often called “rostral colliculus” in quadrupeds; see Figure 10-3). As in the other two medial brainstem motor pathways to the spinal cord, the tectospinal tract axons synapse within medial regions of the spinal cord gray matter that primarily control the axial and proximal musculature. However, these axons only project as far as the upper cervical regions of the cord. This is consistent because the tectospinal tract principally controls the musculature that moves the head. The superior colliculus processes visual, auditory, and somatosensory information about the relative position of stimuli in the environment with respect to the organism. The superior colliculus can also control rapid reflex movements (saccades) of the eyes to the stimulus. The tectospinal tract is involved in producing a movement of the head toward the stimulus that corresponds with the rapid eye movement so that the animal’s gaze is fixated directly on the stimulus.
The Rubrospinal Tract Is a Lateral Brainstem Motor Pathway That Can Control Distal Limb Musculature Associated with Skilled Movement
As noted, the reticulospinal, vestibulospinal, and tectospinal tracts are medial descending brainstem motor pathways whose axons run rostrocaudally within more medial portions of the spinal white matter and synapse in more medial portions of the spinal gray matter. This region of the spinal gray matter exerts extensive, often bilateral control of the axial and proximal musculature involved in postural control and head orientation. In contrast, the rubrospinal tract is a lateral descending brainstem motor pathway whose axons course within more lateral regions of the spinal white matter and synapse in more lateral portions of the spinal gray matter (see Figure 10-3). This region of the spinal gray matter exerts unilateral control over a limited complement of muscles of the distal limbs, often flexors, associated with skilled movements of the extremities.
The rubrospinal tract axons originate in cells of the red nucleus (nucleus ruber) of the mesencephalon. The red nucleus receives a very significant descending input from higher levels of the motor system hierarchy in the cerebral cortex. This cortico-rubrospinal route provides a means for the motor cortices to influence indirectly the spinal lower motor neurons that operate the distal limb flexor musculature. Therefore the cortico-rubrospinal route is involved in the voluntary control of musculature that participates in skilled, often manipulative movements of the extremities (although not in the most dexterous movements of the digits). The rubrospinal tract is more important for these types of movements in quadrupeds compared with primates. In primates, direct projections from motor cortices to the spinal cord (the corticospinal tract, described next) are more important than the rubrospinal tract in the control of voluntary skilled movement of the extremities. As for most nuclei giving rise to tracts that play a direct role in movement, the red nucleus also receives a significant input from the cerebellum. The role of the cerebellum in motor control is briefly described later and in more detail in Chapter 12.
The Corticospinal (Pyramidal) Tract Is a Direct Projection from Cerebral Cortex to Spinal Cord Responsible for the Most Skilled Voluntary Movements of Mammals
The motor cortices of the forebrain constitute the portion of the motor system hierarchy above that of the brainstem and represent the most complex level. As mentioned earlier, these cortical regions are collectively capable of operating spinal lower motor neurons indirectly through some of the descending brainstem motor pathways to the spinal cord (e.g., cortico-reticulospinal route, cortico-rubrospinal route). In mammals a more efficient system exists for the cortical control of spinal lower motor neurons: a direct projection from cells in the motor cortices to the gray matter of the spinal cord. This direct corticospinal tract, also referred to as the “pyramidal tract,” is responsible for the most elaborate and dexterous voluntary movement sequences of which mammals are capable, especially movements involving the extremities. However, this tract also participates in less elaborate voluntary movements of the distal musculature and can exert some voluntary control over the postural muscles as well.
The Corticospinal Tract Has a Massive Lateral Component Controlling the Distal Musculature and a Minor Medial Component Controlling the Axial and Proximal Musculature
The corticospinal tract axons primarily originate from cells located in the motor cortices of the frontal lobe of the cerebral hemisphere (Figure 10-4). All cells contributing to the tract are located in the fifth of the six histological layers of cortical tissue. Along their route from the cerebral cortex, these corticospinal axons pass through the internal capsule of the forebrain, the cerebral peduncles on the ventral surface of the midbrain, and the pontine nuclei within the ventral pons, and they emerge on the ventral surface of the medulla, adjacent to the midline, as the pyramids. These appear pyramid-shaped in cross section, partly inspiring the name pyramidal tract for axons that pass through them.
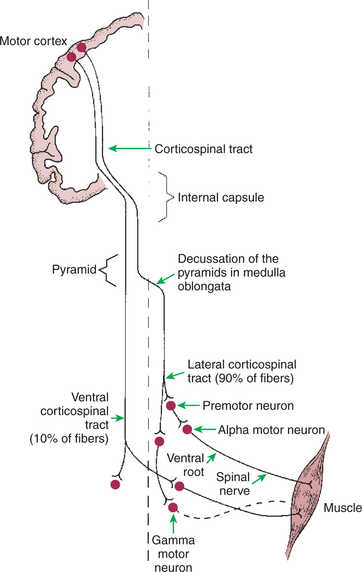
FIGURE 10-4 Corticospinal tract is a direct route primarily from the motor cortices to the contralateral spinal cord gray matter. Most axons of the tract synapse on premotor neurons of the intermediate zone, but some, depending on species phylogeny, synapse directly on α and γ lower motor neurons. About 90% of the axons of the tract cross the midline at the spinomedullary border to form the lateral corticospinal tract, and about 10% remain on the same side to form the ventral corticospinal tract.
As the corticospinal tract axons reach the spinomedullary border, the vast majority (85%–90% in primates) cross the midline at a structure called the pyramidal decussation (Figure 10-4). The crossing axons then form the lateral corticospinal tract, located in the lateral spinal cord white matter, and synapse within lateral regions of the spinal cord gray matter (Figure 10-5). As noted earlier, the lateral regions of the spinal gray matter contain premotor and α motor neurons that primarily control the distal flexor musculature of the extremities that participate in skilled, manipulative, usually voluntary movements. Given this organization, damage to the motor cortices on one side of the body has devastating effects on voluntary skilled movement of the distal flexor musculature on the opposite side of the body. A much smaller percentage of axons traveling in the medullary pyramid do not cross the midline at the pyramidal decussation and remain on the same side of the body to form the much smaller ventral corticospinal tract (see Figure 10-4). The axons of this tract are located in more medial regions of the spinal white matter and synapse in more medial regions of the spinal gray matter that control the axial and proximal postural musculature (see Figure 10-5). The ventral corticospinal tract provides a direct means of voluntary control over muscles that are normally involved in a subconscious antigravity function.
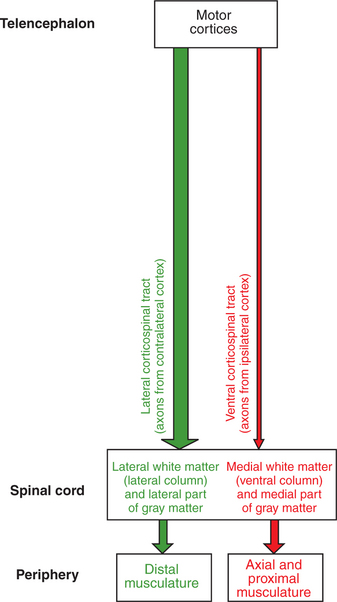
FIGURE 10-5 Somatotopic relationship of corticospinal tract components. As with the descending brainstem motor pathways, the corticospinal tract can be divided into components that respectively travel in more lateral or more medial regions of the spinal cord white matter. The massive and laterally located lateral corticospinal tract synapses in more lateral regions of the spinal cord gray matter that control the distal limb musculature. The axons of this tract originate from the contralateral motor cortices. The much smaller ventral corticospinal tract, whose axons originate from the ipsilateral motor cortices, travels in more medial regions of the spinal white matter and synapses in more medial regions of the spinal gray matter that control the axial and proximal musculature.
The ability of the corticospinal tract to control the most dexterous, skilled movements of the body derives from the synaptic termination pattern of several of its axons. The greater the number of synapses between a neuron in the motor cortices and an α motor neuron in the spinal cord ventral horn, the greater is the number of α motor neurons activated and the less precise the control of the musculature. This is true because each neuron that is excited in the pathway usually activates several postsynaptic neurons. Corticospinal axons bypass synapsing with neurons of the brainstem motor pathways to the cord, but more significantly, some corticospinal axons can bypass synapsing with premotor neurons of the spinal gray matter, contacting α motor neurons directly. Therefore a given corticospinal neuron can ultimately control smaller numbers of α motor neurons and a smaller complement of the musculature. This permits increased fractionation of movement, the increased independence of the actions of different muscles (e.g., the ability to move individual fingers instead of all the fingers together). The proportion of corticospinal neurons making monosynaptic connections with spinal α motor neurons is related to phylogeny. There are no such connections in cats, a small proportion in monkeys, a larger proportion in the anthropoid apes, and a still larger proportion in humans, where the most skilled, fractionated, manipulative movements take place.
As noted, cranial nerve nuclei in the brainstem contain lower motor neurons controlling muscles of the face and head. A significant complement of the axons that leave the motor cortices to form the corticospinal tract will not continue to the spinal cord, but will leave the tract to synapse at the cranial nerve motor nuclei of the brainstem. This complement of axons is referred to as the corticobulbar tract (“bulb” being an archaic term for portions of the brainstem). A given cranial nerve nucleus generally receives significant corticobulbar input from both cerebral hemispheres.
As discussed earlier, the motor cortices send axons to synapse in the brainstem reticular nuclei and in the red nucleus of the mesencephalon as part of an indirect route (e.g., cortico-reticulospinal, cortico-rubrospinal) for cortical control of spinal lower motor neurons. However, such cortical projections to brainstem nuclei, other than to cranial nerve motor nuclei, are not usually viewed as belonging to the corticobulbar tract. Another significant example of such a projection synapses on diffuse aggregates of cells in the ventral pons called the pontine nuclei. The cells of the pontine nuclei then project to the contralateral cerebellum. It is thought that this cortical route to the cerebellum is used to inform the cerebellum of movement intended by the cerebral cortex, so that if the actual movement is not the one intended, the cerebellum can initiate appropriate adjustments.
The Motor Cortices of the Frontal Lobe, the Highest Level of the Motor Control Hierarchy, Consist of Three Different Functional Regions
The motor cortices of the frontal lobe, the origin of most of the corticospinal tract axons, are composed of the primary motor cortex, the supplementary motor cortex, and the premotor cortex (Figure 10-6; see also Figure 10-1). Although these forebrain regions collectively represent the highest level of the motor control hierarchy, the areas appear to differ with respect to the complexity of motor functions controlled.
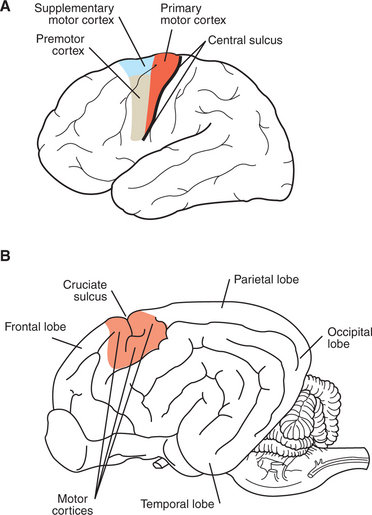
FIGURE 10-6 Motor cortices. A, Location of primary motor, supplementary motor, and premotor cortices in the human brain. B, Vicinity of the motor cortices in the canine brain.
In primates the primary motor cortex (MI) is located just rostral to the prominent central sulcus and therefore lies along the precentral gyrus (Figure 10-6, A). In many nonprimate mammalian species a central sulcus is not present, and MI appears to lie near the cruciate sulcus (Figure 10-6, B). Low-level electrical stimulation of a very small region of MI is capable of activating a small number of functionally related muscles. Furthermore, an orderly relationship exists between the region of the body where the muscles are activated and the region of MI stimulated. In this somatotopic map of the body musculature in MI, muscles in the caudal part of the body (or the feet in bipeds) can be most easily activated from more dorsomedial parts of MI, whereas muscles in the rostral part of the body (or head in bipeds) can be most easily activated from more ventrolateral parts of MI, with a fairly orderly representation of the other regions of the body between those parts of MI. As shown in Figure 10-7, the musculature of different parts of the body is not equally represented in the somatotopic map. Regions depicted as larger have a larger area of MI devoted to their voluntary muscular control, and thus the movements of that region will generally be that much more precise and fractionated. In the somatotopic map of MI in humans, the hand and the mouth musculatures have a very large proportional representation, reflecting the respective importance of these areas in manipulating objects with the fingers and in articulating speech. The proportional representation of the musculature of the different body parts in MI varies with phylogeny, but the somatotopic maps of the primates tend to be the most detailed, reflecting the most precise control over skilled, voluntary movements.
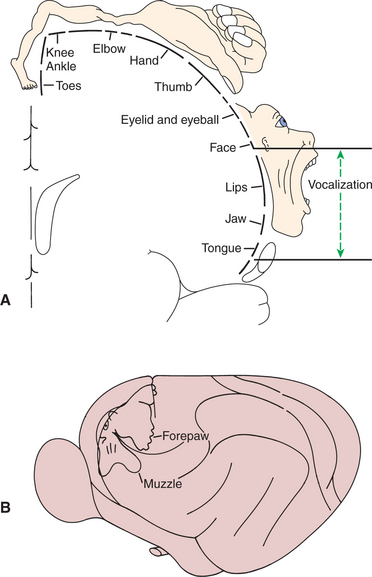
FIGURE 10-7 Somatotopic map of primary motor cortex (MI) showing the origins of axons going to the different skeletal muscles of the body. Body parts represented as proportionally larger have a larger area of MI devoted to their voluntary control, and the movement of that part will generally be that much more precise and fractionated. A, In the human, muscles controlling the hand/digits and the mouth are disproportionately represented because these muscles are needed for the critical and precise movements of grasping/manipulation and speech. B, primary motor cortex of a cat.
(A redrawn from Penfield W, Rasmussen T: The cerebral cortex of man, New York, 1950, Macmillan; from Berne RM, Levy MN: Physiology, ed 2, St Louis, 1988, Mosby; B from Prosser CL: Comparative animal physiology, ed 3, New York, 1988, Wiley.)
The supplementary motor cortex and the premotor cortex are also located in the frontal lobe, just rostral to MI, with supplementary motor cortex positioned dorsomedial to premotor cortex (see Figure 10-6, A). Both areas also have a somatotopic map of the body musculature, but it is less precise than in MI. In addition to corticospinal and corticobulbar tract axons, both areas also collectively give rise to axonal projections to nuclei of origin of some descending brainstem motor pathways (see Figure 10-1). Most significantly, however, the supplementary motor and premotor cortices send axons to synapse within MI and thus may represent “supramotor” areas, with an even higher status in the motor control hierarchy than MI; these areas may instruct MI to organize its fairly discrete muscle actions into more elaborate movement patterns. This concept is supported by the fact that, for voluntary movement, neurons in these supramotor areas become active before those of MI.
Evidence indicates that the supplementary motor cortex is particularly important in the planning and organizing of complex sequences of the discrete movements normally carried out by MI. For example, supplementary motor cortex appears to be particularly active when an individual mentally rehearses a specific sequence of finger movements. Supplementary motor cortex also appears to be important for instructing the limbs (particularly the forelimbs) on the two sides of the body to work together, simultaneously, to accomplish a task. Premotor cortex appears to play an important role in preparatory orientation of the body for the execution of a particular motor task. An example in primates would be rotation of the shoulders and movement of the arms toward a target that is to be manipulated by the hands. interestingly, both these areas receive integrated sensory input and visuospatial information from the posterior parietal cortex, which most likely plays a role in the sequence-planning and preparatory orientation functions.
Using the analogy of playing the piano, we could view MI as being responsible for the simplest muscle activation necessary to press a single piano key, supplementary motor cortex as responsible for planning and organizing the sequence of such finger movements necessary to play a melody, and premotor cortex as responsible for orienting the arms and hands to the correct region of the keyboard to play the various sequences. Of course, the interaction among these areas in determining the appropriate corticospinal (and corticobulbar) tract activity necessary to produce the voluntary movement is certainly more complex than this, and the functional role of these areas in motor control, and how they work together, is still under investigation.
The severity of the deficits resulting from lesions of the corticospinal (pyramidal) system varies with the phylogenetic status of the animal. In primates, such as humans, in whom the pyramidal system is developed extensively, corticospinal tract lesions rostral to the pyramidal decussation cause a dense weakness of the contralateral side of the body. Such one-sided weakness is called hemiparesis and is most extensive in the hand and facial muscles (symptoms common in “stroke” in humans). These symptoms are understandable because a huge percentage of the corticospinal tract axons in primates cross the midline at the spinomedullary border (the location of the pyramidal decussation), and the hand and face have the largest proportional representation in primary motor cortex (see Figures 10-4 and 10-7).
In most veterinary species the corticospinal system is not as well developed as in humans, and supraspinal lesions of this system cause much less severe contralateral weakness and almost no alteration of gait. However, clinical examination can reveal more subtle deficits in voluntary movements of the contralateral limbs. An example is the proprioceptive positioning reaction, the ability of an animal to return its paw to a normal, pads-down posture after the clinician flexes it to make the dorsal surface touch the floor or tabletop. This response requires the animal’s conscious awareness that the paw is in the flexed position (conscious proprioception) and then requires that the animal be able to respond consciously by returning the paw to its normal posture. This latter motor response in turn is affected by the integrity of the upper motor neurons of the corticospinal tract. When these corticospinal tract neurons are damaged, the animal is slow to return its paw to a normal posture. In addition, toes tend to be dragged on the ground as the leg is drawn forward in normal gait. It should be noted that such deficits could also be produced by damaged corticorubral axons originating in the motor cortices. Noting these conscious positioning deficits and other subtle gait changes is important in localizing lesions within the CNS.
Corticospinal Tract Co-Activation of Both Alpha (α) and Gamma (γ) Lower Motor Neurons May Help with Small Automatic Corrections of Voluntary Movements
As noted earlier, α−γ co-activation is a principle common to the excitation of lower motor neurons by upper motor neurons. It has been suggested that such co-activation may permit the muscle spindle to function as an “automatic error correction system” when voluntary movement against a load results in a small deviation from the intended result.
As discussed in Chapter 8, the activation of γ motor neurons along with α motor neurons ensures that the intrafusal muscle fibers remain taut enough to transduce stretch even as the muscle reaches a shorter length on contraction of the extrafusal fibers. The γ motor neuron activation tightens the intrafusal fibers by causing contraction of their polar ends, resulting in adjustment of the muscle spindle sensitivity to the new length of the muscle. It is thought that the α−γ co-activation resulting from a voluntary motor command is meant to produce a contraction of intrafusal fibers that is concordant with extrafusal fiber contraction, such that the muscle spindle is made just sensitive enough to transduce stretch at the new muscle length. Under these circumstances, if the load is more than expected, the α motor neuron activity will not have produced enough extrafusal fiber contraction to shorten the muscle to the new desired length. However, the γ motor neuron activity will have produced the appropriate intrafusal fiber contraction to adjust the muscle spindle sensitivity for the new desired length. This mismatch, where the spindle sensitivity has been adjusted for the new muscle length but the extrafusal fibers have not contracted enough to reach that length, results in a stretching of the muscle spindle and activation of segmental stretch reflex mechanisms. That is, the stretching of the muscle spindle results in more excitatory postsynaptic potentials (EPSPs) on the α motor neurons to the muscle, increasing their action potential firing and increasing the extrafusal fiber contraction to assist with reaching the new desired length.
This type of error correction, in which segmental stretch reflex mechanisms help to accomplish the intended muscle shortening when the corticospinal pathway has not produced the sufficient α motor neuron activity, is called a servo-assist function. Thought to result from α−γ co-activation, this servo-assist function is analogous to the power steering in a car, where a compressor in the motor adds power to the driver’s turning of the steering wheel when significant resistance is encountered by the tires.
The Motor System Shares Some Organizational Principles with Sensory Systems
With most of the major components and pathways of the motor system now described, it appears that the motor system shares principles of organization common to other brain systems (e.g., sensory systems). One such organizational principle is the existence of topographic maps of the body. As noted, there are organized somatotopic maps of the body’s musculature in the motor cortices. Topographic organization also exists in many sensory systems, except it is the peripheral receptor surface that is topographically mapped. For example, CNS components of the somatosensory (touch) system, such as primary somatosensory cortex, contain an organized somatotopic map of the different regions of the skin surface.
Two other principles of organization shared by the motor system and sensory systems are serial and parallel processing of nervous system information. In sensory systems, serial processing generally refers to the passage of information from the periphery to successively more rostral regions of the nervous system, in a serial fashion. For example, in the visual system, axons of cells in the retina synapse in the lateral geniculate nucleus of the thalamus, and these thalamic neurons in turn send their axons to synapse in primary visual cortex. Often, in serial processing within the sensory systems, the information collected at successively more rostral levels of the nervous system is organized into a more sophisticated form. Serial processing can also be observed in the motor system, although in a different direction; from more rostral regions to more caudal regions. The cortico-reticulospinal route is an example of this.
Parallel processing refers to the different pathways within a given sensory system operating in parallel, respectively, to carry qualitatively different forms of information. Again, using the somatosensory system as an example, there are separate pathways to cerebral cortex to carry information about gentle touch of the skin and about intense skin contact usually perceived as painful. In the motor system, an example of parallel processing is the respective control of the proximal antigravity musculature by one set of descending brainstem motor pathways (vestibulospinal, reticulospinal) and control of the distal flexor musculature by a different descending brainstem motor pathway (rubrospinal).
Undoubtedly, a combination of both serial and parallel processing is necessary for the integrated function of sensory, as well as motor systems.
The Basal Ganglia and Cerebellum Modulate the Activity of Motor System Components for the Respective Selection and Adjustment of Movement
Portions of the motor system are important for proper motor function but do not appear to be directly involved in initiating movement. These structures—the basal ganglia and the cerebellum—serve primarily to modulate the activity of other motor system structures without directly producing movement.
The basal ganglia are a group of nuclei, the majority of which are deep within the cerebral hemispheres. They include the caudate nucleus and putamen (known collectively as the striatum), the globus pallidus, the substantia nigra, and the subthalamic nucleus. The internal neural circuitry of this multinuclear functional unit is extremely complex and participates in several parallel pathways running through the basal ganglia. The basal ganglia receive input from the motor cortices and many other areas of cerebral cortex and, by way of the thalamus, project output back to the motor cortices, particularly the supplementary motor and premotor cortices. Again, these regions are important in the planning and preparation for movement. Some basal ganglia output projects directly to brainstem nuclei controlling movement.
Generally it is thought that the basal ganglia use the information received from the cortex, including information about the movement plan and the context of the situation, to help select the appropriate movement pattern while suppressing less appropriate, competing patterns. Two principal circuits within the basal ganglia play an important role in this process. One circuit acts to facilitate inhibitory output of the basal ganglia, presumably acting to suppress the inappropriate, competing movement pattern. The other circuit acts to reduce inhibitory output of the basal ganglia, presumably “removing the brakes” from the appropriate movement pattern. Dopamine-containing neurons that project from the substantia nigra of the basal ganglia to the striatum of the basal ganglia play an important role in regulating these two circuits. In humans, when these dopamine-containing neurons degenerate in Parkinson’s disease, severe motor deficits develop, such as difficulty beginning the appropriate movement, slowness of movement, rigidity, and resting tremor. Parkinson’s disease does not occur naturally in veterinary species, but some toxins can selectively destroy these dopamine-projecting neurons in nonhuman species, producing some motor deficits seen in the human disease. In horses, ingestion of the yellow star thistle can produce damage of the basal ganglia. Some of the abnormal movements resulting from this damage, involving the lips and tongue of the horse, are reminiscent of such abnormal movements seen in the fingers of human patients with Parkinson’s disease. interestingly, the respective structures in the two species are both used in grasping movements.
The structure and function of the cerebellum and its role in motor control are discussed in Chapter 12 and are mentioned only briefly here. The cerebellum’s importance in motor control is indicated by the earlier observation that virtually all the nuclei giving rise to the brainstem motor pathways receive output from the cerebellum. Also, the cerebellum indirectly receives input (through pontine nuclei) from the motor cortices (MI, supplementary motor cortex, premotor cortex). As with the basal ganglia, the cerebellum not only receives information from the motor cortices, but indirectly sends information back to them as well. Importantly, the cerebellum receives much sensory information from the skin, joints, muscles, vestibular apparatus, and even the visual system. Therefore the cerebellum receives information about the planning and initiation of movement, as well as continuous sensory feedback about the progress of the movement. The cerebellum in turn can influence activity in the motor cortices and in the brainstem motor pathways to the spinal cord.
Through this organization, it is thought that the cerebellum acts to compare information about the movement plan with information about how the movement is actually being carried out. It can then presumably make adjustments to the ongoing movement itself, or even adjust the movement plan. Within this framework, the cerebellum appears particularly concerned with gathering sensory feedback about, and with adjusting the control of, the timing of movement. Both experimental and clinical studies have shown that cerebellar damage produces significant deficits in the coordination and smoothness of complex movements. These deficits presumably arise because of problems in the timing of muscle contraction components of the movement. If the muscle contraction components are not properly timed, the movement can appear jerky and uncoordinated, may exhibit improper force, and may not stop at the appropriate time.
CLINICAL CORRELATIONS
Focal Lesion of the Motor Cortex
History.
You examine an 11-year-old female boxer dog. Her vaccination history is current. She had an adenocarcinoma of the mammary gland removed 6 months before your examination.
The owner states that over the past few days the dog has become progressively weaker in the left front and left rear legs and occasionally stands with the left front paw flexed such that the dorsal surface touches the ground. On the previous day the dog had a seizure.
Clinical Examination.
On physical examination of the patient, you find several routine old-age changes and the results of the mammary surgery. You find also that the dog seems drowsy and is weak on the left front and left rear legs. She has a proprioceptive positioning reaction deficit of both the left front and the left rear leg. Radiographic study of the chest reveals metastatic, neoplastic lesions in the lungs.
Comment.
The proprioceptive positioning reaction (or response) is tested by flexing the animal’s paw, dorsal side down, while gently supporting her weight. A normal dog senses (conscious proprioception) that the paw is upside down and returns it to the normal pads-down posture (motor response). This is called a “response” (or reaction), rather than a reflex, because it involves a degree of conscious control. This particular response requires normal function of skin and joint receptors and the peripheral nerve in the tested leg and of the sensory neuron tracts that ascend toward the brain along the ipsilateral (same) side of the spinal cord. Traveling along a multisynaptic pathway, the sensory information crosses to the contralateral (opposite) side of the brain in the brainstem and reaches the contralateral (with respect to the side of the original stimulus) cerebral cortex. As the animal becomes consciously aware that the paw is in an unusual position, action potentials are sent back, down the corticospinal tract, to the lower motor neurons of the muscles of the leg, causing the paw to return to the normal position.
With the wiring diagram of this response in mind, you can see that a deficit in the proprioceptive positioning reaction of the left front and left rear legs could be caused by a lesion of the left cervical spinal cord, the right motor cortex, or supraspinal portions of the right corticospinal tract. This dog’s seizure (a manifestation of brain disease) at about the same time suggests that the lesion is in the right cerebral cortex. The brain is a common site for metastasis, and the radiographic lung lesions suggest that the mammary tumor has spread to both the lung and the right side of the brain. The lung contains the first capillary bed that a metastatic cancer cell is likely to encounter when it enters the venous system of the mammary gland. Some cells stop here and grow.
Anderson ME, Binder MD. Spinal and supraspinal control of movement and posture. Patton HD, Fuchs AF, Hille B, et al. Textbook of physiology, ed 21, Philadelphia: Saunders, 1989.
Bear MF, Connors BW, Paradiso MA. Neuroscience: exploring the brain, ed 3. Philadelphia: Lippincott Williams & Wilkins, 2007.
Brodal P. The central nervous system: structure and function, ed 3. New York: Oxford University Press, 2004.
Fetz EE. Motor functions of cerebral cortex. Patton HD, Fuchs AF, Hille B, et al. Textbook of physiology, ed 21, Philadelphia: Saunders, 1989.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Haines DE. Fundamental neuroscience for basic and clinical applications. Philadelphia: Churchill Livingstone, 2006.
Jennings DP. Supraspinal control of posture and movement. Reece WO, ed. Duke’s physiology of domestic animals, ed 12, Ithaca, NY: Comstock Cornell University Press, 2004.
Kandel ER, Schwartz JH, Jessell TM. Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
Lorenz MD, Kornegay J. Oliver and Lorenz’s handbook of veterinary neurology, ed 4. Philadelphia: Saunders, 2004.
Nichols JG, Martin AR, Wallace BG, Fuchs PA. From neuron to brain, ed 4. Sunderland, Mass: Sinauer Associates, 2001.
Purves D, Augustine G, Fitzpatrick D, et al. Neuroscience, ed 3. Sunderland, Mass: Sinauer Associates, 2004.
PRACTICE QUESTIONS
1. A motor neuron pool located most laterally in the ventral horn of the spinal cord is most likely to operate a muscle controlling movement of the:
2. Which of the following is true regarding decerebrate rigidity?
3. Which of the following descending brainstem motor pathways controls distal limb musculature associated with skilled movement?
4. The corticospinal (pyramidal) tract, in general, initiates what form of movement?
5. You are presented with a dog with a dense weakness, and proprioceptive placing reaction deficit, of his left front and left back legs. A single pathological site could cause these signs if it were located in the:
6. The corticospinal tract simultaneously co-activates both the α and the γ lower motor neurons. If the initial co-activation fails to be sufficient to cause the intended shortening of the muscle, sensory neuron activity of the muscle spindle of that muscle will have what influence on the α motor neurons to the same muscle?