Chapter 33 The Endocrine System
1. Hormones are chemicals produced by specific tissues that are transported by the vascular system to affect other tissues at low concentrations.
2. The endocrine and nervous systems are integrated in their control of physiological processes.
1. Protein hormones are initially synthesized as preprohormones and then cleaved in the rough endoplasmic reticulum to form prohormones and in the Golgi apparatus to form the active hormones, which are stored in granules before being released by exocytosis.
2. Steroids are synthesized from cholesterol, which is synthesized by the liver; steroids are not stored but are released as they are synthesized.
Transport of Hormones in the Blood
1. Protein hormones are hydrophilic and carried in the plasma in dissolved form.
2. Steroids and thyroid hormones are lipophilic and carried in plasma in association with both specific and nonspecific binding proteins; the amount of unbound, active hormone is relatively small.
1. Protein hormones have specific receptors on target tissue plasma membranes, whereas steroids have specific receptors within the cytoplasm or nucleus.
1. Steroids interact directly with the cell nucleus through the formation of a complex with its cytosolic receptor, whereas protein hormones need a messenger because they cannot enter the cell.
1. Steroid hormones are metabolized by conjugation with sulfates and glucuronides, which makes steroids water soluble.
1. The most important feedback control for hormones is the negative-feedback system, in which increased hormone concentrations result in less production of the hormone, usually through an interaction with the hypothalamus or pituitary gland.
2. Endocrine secretory patterns can be influenced by factors such as sleep or light and can produce circadian rhythms.
1. The hypothalamus coordinates the activity of the pituitary gland through the secretion of peptides and amines.
1. The neurohypophysis has cell bodies that originate in the hypothalamus, with cell endings that secrete oxytocin and vasopressin.
2. Oxytocin and vasopressin are synthesized in cell bodies within the hypothalamus and are carried by axon flow to the posterior lobe, where they are released.
3. The main effects of oxytocin are on the contraction of smooth muscle (mammary gland and uterus); the effects of vasopressin are primarily on the conservation of water (antidiuresis) and secondarily on blood pressure.
4. Plasma osmolality controls the secretion of vasopressin.
5. The anterior pituitary produces growth hormone, prolactin, thyroid-stimulating hormone, follicle-stimulating hormone, luteinizing hormone, and corticotropin.
6. Adenohypophyseal activity is controlled by hypothalamic releasing hormones, which are released into the portal system, which in turn connects the median eminence of the hypothalamus and the anterior pituitary gland.
GENERAL CONCEPTS
Hormones Are Chemicals Produced by Specific Tissues That Are Transported by the Vascular System to Affect Other Tissues at Low Concentrations
The endocrine system has evolved to allow physiological processes to be coordinated and regulated. The system uses chemical messengers called hormones. Hormones have traditionally been defined as “chemicals that are produced by specific endocrine organs, are transported by the vascular system, and are able to affect distant target organs in low concentration.” Although this definition is useful from a veterinary medical point of view, it should be recognized that some substances, such as prostaglandins and somatomedins, are produced by many other tissues and are still considered hormones.
Other types of control systems use chemical substances that are not transported in the vascular system to influence distant cell activity. These systems serve as means of local integration among or between cells, as follows:
1. Paracrine effectors, in which the messenger diffuses through the interstitial fluids, usually to influence adjacent cells; if the messenger acts on the cell of its origin, the substance is called an autocrine effector (Figure 33-1).
2. Neurotransmitters, which affect communication between neurons, or between neurons and target cells; the substances are limited in the distance traveled and the area of the cell influenced (Figure 33-2).
3. Exocrine effectors, such as hormones produced by the pancreas, are released into the gastrointestinal tract.
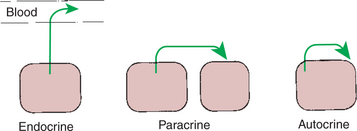
FIGURE 33-1 Types of cell communication via chemical mediators.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
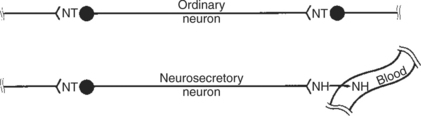
FIGURE 33-2 Comparison of functional arrangements of an ordinary neuron releasing its neurotransmitter (NT) into a synapse and a neurosecretory neuron releasing its neurohormone (NH) into a blood vessel.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
The Endocrine and Nervous Systems Are Integrated in Their Control of Physiological Processes
The endocrine system interacts with the other main regulatory system, the nervous system, which coordinates activities that require rapid control. An example of the close interaction of the two systems is the reflex in which suckling causes the release of milk. Suckling initiates the transmission of nerve impulses from the mammary gland to the hypothalamus (by way of the spinal tract). Neurosecretory neurons within the supraoptic and paraventricular nuclei are stimulated to synthesize oxytocin. Oxytocin is transported along axons of these nerves and is released from nerve endings in the posterior pituitary into the blood vascular system. Oxytocin is then carried to the mammary gland, where it causes contraction of myoepithelial cells. These cells surround the smallest unit of milk-secreting cells, called an alveolus. This results in the movement of milk into the large cisternae adjacent to the teat and subsequently into the teat.
The interaction between the nervous and endocrine systems can be even more direct. For example, endocrine cells of the adrenal medulla are directly controlled by preganglionic neurons of the adrenal medulla, and the medullary hormones are released immediately in response to stressful stimuli. The endocrine and nervous systems also share transmitters; substances such as epinephrine, dopamine, histamine, and somatostatin are found in both endocrine and neural tissues.
The endocrine system is involved in control of physiological functions, including metabolism, growth, and reproduction. Metabolism can be divided into two forms: energy and mineral. The hormones that control energy metabolism include insulin, glucagon, cortisol, epinephrine, thyroid hormone, and growth hormone. The hormones that control mineral metabolism include parathyroid hormone, calcitonin, angiotensin, and renin. The hormones that control growth include growth hormone, thyroid hormone, insulin, estrogen and androgen (both reproductive hormones), and a large number of growth factors. The hormones that control reproduction include estrogen, androgen, progesterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin (PRL), and oxytocin.
One of the important characteristics of the endocrine system is the amplification of the signal. The action of one steroid molecule to activate a gene can result in the formation of many messenger ribonucleic acid (mRNA) molecules, and each of these can induce the formation of many enzyme molecules. Also, one protein molecule can influence the formation of many cyclic adenosine 3′,5′-monophosphate (cAMP) molecules, and each of these can activate many enzymes. Amplification is the basis for the sensitivity of the endocrine system, which allows small amounts of hormones in plasma (10−11 to 10−12 mol) to produce significant biological effects. Hormone action also influences rates of existing enzyme reactions, but not the initiation of new reactions. This implies that there are certain basal levels of enzyme activities even in the absence of hormones. Hormone action is relatively slow and prolonged, with the effects of hormones lasting minutes to days. This contrasts with the nervous system, in which the response is rapid and short (milliseconds to seconds).
SYNTHESIS OF HORMONES
Protein Hormones Are Initially Synthesized as Preprohormones and Then Cleaved in the Rough Endoplasmic Reticulum to Form Prohormones and in the Golgi Apparatus to Form the Active Hormones, Which Are Stored in Granules Before Being Released by Exocytosis
The major classes of hormones include proteins (e.g., growth hormone, insulin, corticotropin [previously called adrenocorticotropic hormone, or ACTH]); peptides (e.g., oxytocin and vasopressin); amines (e.g., dopamine, melatonin, epinephrine); and steroids (e.g., cortisol, progesterone, vitamin D). The protein and peptide hormones are initially synthesized on ribosomes as larger precursor proteins, which are referred to as preprohormones (Figure 33-3). Synthesis of protein hormones begins in ribosomes, with the “pre” portion immediately attaching to the rough endoplasmic reticulum (RER), which pulls the ribosomes into close apposition with the RER. During synthesis, the preprohormone is secreted into the interior of the RER. The presence of a peptidase within the wall of the RER allows the “pre” portion of the molecule to be rapidly removed and the prohormone to leave the RER in vesicles that have been pinched off from the RER. These vesicles then move to the Golgi apparatus, where they coalesce with Golgi membranes to form secretory granules. The prohormone is cleaved during this process, so most of the hormone is in its final form within the Golgi apparatus, although some prohormone can also be found.
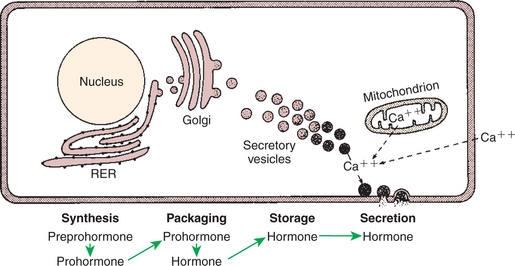
FIGURE 33-3 Subcellular components of peptide hormone synthesis and secretion. RER, Rough endoplasmic reticulum.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
Protein hormones are stored in granules within the gland until needed for release. Although some of the hormone is secreted on a continuous basis, most is secreted through the process of exocytosis of granules in response to a specific signal. The process of exocytosis requires adenosine triphosphate (ATP) and calcium (Ca2+). Increased cytoplasmic calcium results from intracellular release of Ca2+ from mitochondria, or endoplasmic reticulum, or from the influx of extracellular Ca2+.
Steroids Are Synthesized from Cholesterol, Which Is Synthesized by the Liver; Steroids Are Not Stored but Are Released as They Are Synthesized
Steroids represent a class of hormones that, unlike protein hormones, are lipophilic. In general, they belong to one of two categories: adrenocortical hormones (glucocorticoids, mineralocorticoids) and sex hormones (estrogens, progesterone, androgens). They have a common four-ring, 17-carbon skeleton that is derived from cholesterol (Figure 33-4). Although the steroids can be synthesized de novo within the cell from the two-carbon molecule acetate, the majority of steroids are formed from cholesterol, which is synthesized by the liver (Figure 33-5). Low-density lipoproteins (LDLs) enter steroid-producing cells through interaction with a membrane receptor. Cholesterol is released through the degradation of LDLs by lysosomal enzymes. Cholesterol is either used immediately for steroid synthesis or stored in granules in an ester form within the cell. The first step in the synthesis of all steroid hormones from cholesterol involves cleavage of the side chain of cholesterol to form pregnenolone; this step occurs within the mitochondrion. Subsequent modifications of the steroid molecule may occur within the mitochondrion or may involve movement to other compartments of the cell (Figure 33-6). The control of movement of steroids among cell compartments during the synthesis process is not well understood.
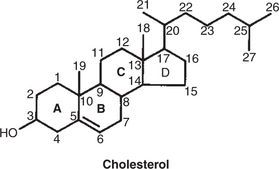
FIGURE 33-4 The ring structure and numbering system of the carbon atoms in steroid hormones, illustrated for the cholesterol molecule.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
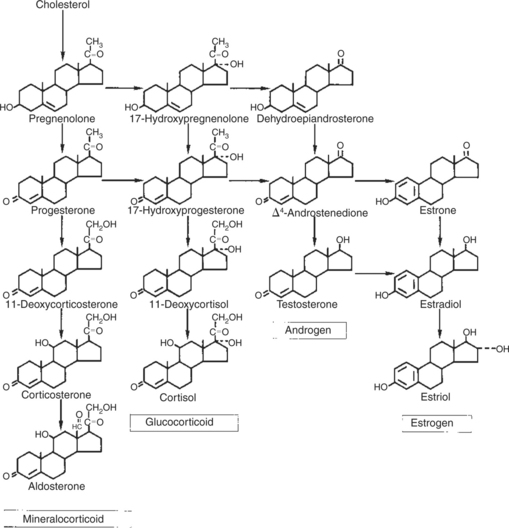
FIGURE 33-5 Pathways involved in the production of the major steroid hormones.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
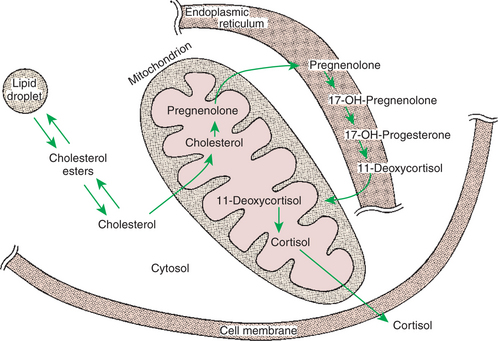
FIGURE 33-6 Subcellular compartmentalization of cortisol biosynthesis.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
The type of steroid hormone that is eventually synthesized depends on the presence of specific enzymes within the particular cell. For example, only cells of the adrenal cortex contain enzymes (hydroxylases) that result in hydroxylation of the 11th and 21st carbon molecules, a process that is essential for the production of glucocorticoids and mineralocorticoids. The pattern for sex steroid biosynthesis is for pregnenolone to be modified in a sequence that involves progesterone, androgens, and finally estrogens. Cells that synthesize androgens (e.g., Leydig cells of the testis) have the enzymes required for the formation of pregnenolone and progesterone, as well as for the modification of progesterone to androgen, but lack the enzymes necessary to modify androgens into estrogens. Although the sex steroid–forming cells do not have enzymes that allow the formation of adrenocortical hormones, the adrenal cortex contains the enzyme systems necessary for the formation of both adrenocortical hormones and sex hormones, although the former are emphasized. As a result, the adrenal cortex normally produces small amounts of sex steroids and produces larger amounts in certain pathophysiological conditions.
There is no provision for the storage of steroid hormones within the cell; they are secreted immediately after formation by simple diffusion across the cell membrane because of their lipophilic structure. Thus, synthesis and secretion of steroid hormones occur in a tightly coupled manner, whereby the rate of hormone secretion is controlled by the rate of synthesis. The only storage form of steroids within these cells involves that of the precursor molecule, cholesterol, as an ester.
TRANSPORT OF HORMONES IN THE BLOOD
Protein Hormones Are Hydrophilic and Carried in the Plasma in Dissolved Form
This chapter focuses mainly on hormones that are transported to target tissues in the vascular system. The means by which hormones are transported in the blood varies according to the solubility of the hormone. Protein and peptide hormones are hydrophilic and are carried in the plasma in dissolved form. The protein hormones may circulate in monomeric (single-unit) or polymeric (multiple-unit) form (e.g., insulin). Hormones that have subunits can appear in the circulation in subunit form, although this reduces the biological potency of the molecule.
Steroids and Thyroid Hormones Are Lipophilic and Carried in Plasma in Association with Both Specific and Nonspecific Binding Proteins; the Amount of Unbound, Active Hormone Is Relatively Small
The transport of steroid and thyroid hormones is more complicated than that of protein hormones, because the steroid and thyroid hormones are lipophilic and thus have limited solubility in aqueous solutions. These hormones are transported in the blood through association with various types of proteins. Some of the proteins that bind steroids have a high affinity for a particular steroid; for example, a globulin, transcortin, has a high affinity for cortisol and corticosterone but also serves as an important transport vehicle for progesterone, even though it has a lower affinity for this hormone. The carrier proteins that have high affinities have low capacity because of their low plasma concentration. In contrast, the general class of plasma proteins called albumins have low affinities for steroid hormones but have a high capacity for steroid transport because of their high concentration in plasma.
A hormone must be in the free, or unbound, form before it can penetrate a target cell and elicit biological activity. This is accomplished by the establishment of equilibrium between bound and free hormone levels in the plasma. The free form usually represents only about 1% of the total amount of hormone in the plasma (up to 10% of cortisol may be in the free form). The system is responsive to use of the free form, and the free form is replenished quickly by dissociation of bound hormone from the protein. The total amount of the hormone is usually measured, with the exception of thyroid hormone, for which attempts are usually made to estimate the amounts of bound and free. As indicated for steroid hormones, synthesis and release are tightly linked, and because metabolic clearance rates are usually constant, concentrations of steroids in plasma are usually a good reflection of the secretion rate. Under certain physiological conditions, such as pregnancy in humans, metabolism of estrogens can change because of the increased production of estrogen-binding proteins.
HORMONE-CELL INTERACTION
Protein Hormones Have Specific Receptors on Target Tissue Plasma Membranes, Whereas Steroids Have Specific Receptors Within the Cytoplasm or Nucleus
A central question in endocrinology is how hormones and target cells of a particular tissue interact in a specific manner. The problem seems almost overwhelming for steroids because they are lipid soluble and able to permeate all cells of the body. The solution is that target cells have receptors that are specific for a particular hormone. For steroids, the receptors are located in the cytoplasm or nucleus of the target cells, whereas receptors for protein and peptide hormones are located on the plasma membrane of the cell. In addition to specificity, receptors have a high affinity for their respective hormone. These characteristics of the receptor allow hormones to be in low concentration in the blood but effective in producing significant tissue response.
The greater the affinity of the receptor for the hormone, the longer is the biological response. Termination of the action of a hormone usually requires dissociation of the hormone from the receptor. This occurs most often as a result of a decrease in plasma concentrations of the hormone; the binding of receptor and hormone is noncovalent, and declining hormone concentrations favor a chemical equilibrium of dissociation over association. Termination of hormone action can also result from internalization of the receptor-hormone complex through the process of endocytosis. The hormone is degraded by lysosomal enzymes, whereas the receptor, protected because of its association with the vesicle membrane, can be recycled to the plasma membrane.
Receptors are present on cells in much greater numbers than required for the elicitation of a biological response. Occupancy by a hormone of considerably less than 50% of the receptors usually elicits a maximal biological response. Even so, changes in receptor numbers that affect the sensitivity of the cell, although not its maximal responsiveness, can occur. Changes in receptor number affect the probability that an interaction will occur between receptor and hormone. Receptor synthesis can be stimulated by a hormone that is different from the hormone that interacts with the receptor. For example, predominant gonadotropin receptors on ovarian granulosa cells change from FSH to LH receptors late in the ovarian follicle phase because of the influence of FSH. This allows the control of the ovarian follicle to pass from FSH to LH, which facilitates ovulation and the formation of a corpus luteum. Conversely, receptor numbers can decrease in conjunction with continued interaction of receptor and hormone. This often occurs when an agonist that has great affinity for the receptor is administered or when amounts of hormone are pathologically elevated. The receptor numbers are downregulated in this situation. The end result is that the animal becomes resistant to continued therapy with the hormone in question.
POSTRECEPTOR CELL RESPONSES
Steroids Interact Directly with the Cell Nucleus Through the Formation of a Complex with Its Cytosolic Receptor, Whereas Protein Hormones Need a Messenger Because They Cannot Enter the Cell
The events that follow binding of the hormone and receptor depend on whether a steroid, protein, or peptide hormone is involved. With steroids, the hormone is able to interact within the cell because of its ability to penetrate the lipoprotein plasma membrane (Figure 33-7). The interaction of receptor and steroid hormone results in activation of the subsequent complex translocation to the nucleus, where it interacts with specific sites on the chromatin. The result is the production of mRNA, which, when translocated to the ribosomes, directs synthesis of proteins that produce the desired biological result.
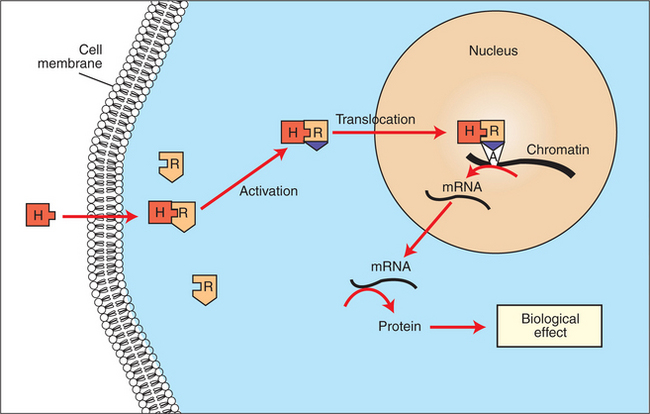
FIGURE 33-7 Subcellular mechanism of action of a lipophilic hormone (H) via an intracellular receptor (R). The H-R complex induces messenger ribonucleic acid (mRNA) synthesis by binding to an acceptor site (A) on the chromatin.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
Protein or peptide hormones require an intermediary to act in their behalf because they are not able to penetrate the plasma membrane of the cell; the intermediary substance is known as a second messenger (Figure 33-8). The best-documented second messenger is cAMP, which is produced by the activation of an enzyme, adenyl cyclase, through interaction of the hormone and receptor in the plasma membrane. The activation of adenyl cyclase and the production of cAMP result in the phosphorylation of protein kinases, which are responsible for the biological response. Other second messengers include cytosolic calcium and its associated phosphodiesterase, calmodulin, as well as inositol triphosphate (IP3) and diacylglycerol, both of which are products of phosphatidylinositol metabolism. An important action of IP3 is the stimulation of intracellular calcium release. One important response to diacylglycerol is the activation of phospholipase A and the formation of arachidonic acid, which leads to formation of members of the prostaglandin family of molecules. The biological response to a protein or peptide hormone– receptor interaction is more rapid than that to steroids; preexisting enzymes are activated, whereas the biological response to steroid requires the synthesis of enzyme protein.
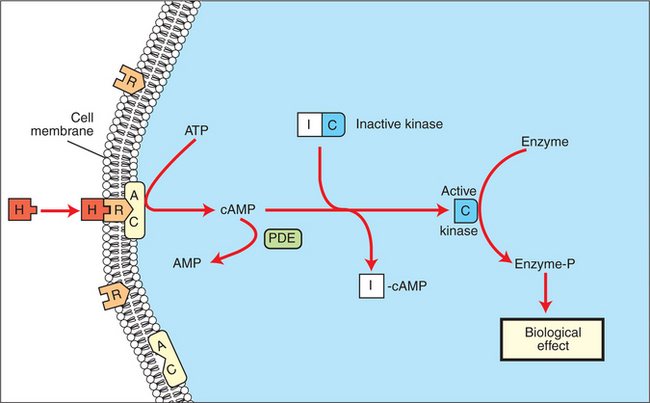
FIGURE 33-8 Subcellular mechanism of action of a hydrophilic hormone (H) via a membrane receptor (R), adenyl cyclase (AC), and cyclic adenosine monophosphate (cAMP). ATP, Adenosine triphosphate; I and C, inhibitory and catalytic subunits of the kinase, respectively; PDE, phosphodiesterase.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
METABOLISM OF HORMONES
Steroid Hormones Are Metabolized by Conjugation with Sulfates and Glucuronides, Which Makes Steroids Water Soluble
Hormone activity is limited by the metabolism of hormones. The metabolism of steroids usually involves reduction of the molecule, followed by conjugation with sulfates and glucuronides, which increases the water solubility of the steroids, allowing them to be excreted in urine. The liver is the main organ responsible for this process. Iodine molecules are removed from thyroid hormones during metabolism. Protein hormones are cleaved by peptidases; this is preceded by reduction of disulfide bonds if this is a characteristic of the molecule. Although a metabolite is usually less biologically potent than the original molecule, some evidence suggests that conjugates of steroids can have significant biological activity. This raises the question of whether the conversion of hormones intracellularly, such as testosterone to dihydrotestosterone, represents metabolism, because dihydrotestosterone is more potent biologically than testosterone. Another example, the conversion of estradiol-17β to estrone by peripheral tissues, including adipose cells, is described as a form of metabolism; however, estrone is a natural, and relatively potent, estrogen.
Although in some situations the rate of clearance of a hormone can change (e.g., decrease as a result of increased hormone-binding plasma proteins during pregnancy, or increase as the result of decreased hormone-binding plasma proteins in conjunction with liver disease), metabolism of hormones is relatively constant, and the concentration of a hormone usually reflects the other determinant of hormone activity: the rate of synthesis of the hormone.
FEEDBACK CONTROL MECHANISMS
The Most Important Feedback Control for Hormones Is the Negative-Feedback System, in Which Increased Hormone Concentrations Result in Less Production of the Hormone, Usually Through an Interaction with the Hypothalamus or Pituitary Gland
The effects of hormones are proportional to their concentrations in blood, and therefore control of these concentrations is an important aspect in ensuring that physiological function is normal. As indicated previously, the primary factor affecting hormone concentrations in blood is the secretion rate by a particular organ. Feedback-loop control systems have evolved in which concentrations of hormones are monitored at the controlling point either to increase or to decrease secretion of a hormone by an endocrine organ. The most common feedback system is negative feedback, in which continuous monitoring allows the system to counteract changes in hormone secretion or to maintain a relatively constant environment.
An example of systems in which negative-feedback control involves both the endocrine and the nervous system is shown in Figure 33-9. The hypothalamus, which controls secretion of tropic hormones in the anterior pituitary through the secretion of peptide-releasing hormones, has cells with a certain set point by which they compare concentrations of hormone in the blood with the output of releasing hormones. If blood concentrations fall below the physiological set point, releasing-hormone output increases; this in turn increases production of tropic hormones by the anterior pituitary and subsequently the secretion of the hormone by the target organ. Conversely, if the hormone concentration increases above acceptable physiological limits, a shutdown of releasing-hormone production occurs within the hypothalamus, tropic hormone secretion by the anterior pituitary decreases, and production of the hormone by the target organ decreases. This type of control system is not an “all-or-none” arrangement because changes and adjustments are being continuously made to maintain an optimal concentration of hormone.
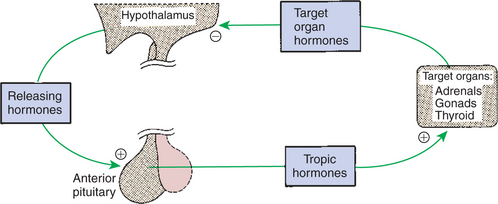
FIGURE 33-9 Negative feedback of tropic and releasing hormones by target organ hormones. Plus signs indicate stimulation, and the minus sign indicates inhibition. In some cases, such inhibition occurs at the pituitary gland.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
In the negative-feedback system an increase in secretion of hormone results in a decrease in tropic hormone secretion. It is also possible to have a negative-feedback system in which an increase in a physiological substance, such as glucose, causes an increase in a hormone, in this case insulin, which plays an important role in glucose metabolism. This is considered to be a negative-feedback system because blood glucose concentrations are being “dampened,” or returned toward normal levels, through the action of insulin.
Positive-feedback systems also exist, although they are much less common than negative-feedback systems. One example is the preovulatory release of LH, in which the pulsatile rate of LH secretion greatly increases during the late stages of ovarian follicular development because of increased estrogen production by the follicle. In this situation, there is a definitive end point: ovulation results in a decline in the stimulus, estrogen, although the duration of the LH surge is probably determined within the hypothalamus, and therefore the LH response to estrogen is modulated.
Endocrine Secretory Patterns Can Be Influenced by Factors Such as Sleep or Light and Can Produce Circadian Rhythms
Endocrine secretion patterns can occur outside the control of negative-feedback inhibition. Hormone patterns can change on an approximate 24-hour basis, a process referred to as a diurnal rhythm, or circadian rhythm. Circadian is the preferred term because diurnal refers to activity in the daytime; nocturnal should be used for the rhythms that are active at night. Most of the daily rhythms have some aspects of light, or lack of light, as a major influence in the rhythm. Rhythmic changes in hormone patterns that occur at shorter intervals, often in the range of an hour, are called ultradian rhythms.
THE HYPOTHALAMUS
The Hypothalamus Coordinates the Activity of the Pituitary Gland Through the Secretion of Peptides and Amines
As indicated previously, the two major controlling systems are the nervous and the endocrine systems. The interface between these systems occurs, for the most part, in the hypothalamus. The hypothalamus is an area of the diencephalon that forms the floor of the third ventricle and includes the optic chiasma, tuber cinereum, mammillary bodies, and the median eminence. Often not included in this classification are the infundibulum and the neurohypophysis (stalk of the posterior lobe and the posterior lobe, respectively), although both tissues represent extensions of the hypothalamus into the pituitary gland.
The hypothalamus produces peptides and amines that influence the pituitary gland to produce (1) tropic hormones (e.g., corticotropin), which in turn influence the production of hormones (e.g., cortisol) by peripheral target endocrine tissues, or (2) hormones that directly cause a biological effect in tissues (e.g., PRL). The hypothalamus is also the center for the control of a large number of autonomic nervous system control pathways.
THE PITUITARY GLAND
The pituitary gland, or hypophysis cerebri, is composed of the adenohypophysis (pars distalis, or anterior lobe), the neurohypophysis (pars nervosa, or posterior lobe), the pars intermedia (intermediate lobe), and the pars tuberalis (Figure 33-10). The adenohypophysis is formed from an area of the roof of the embryonic oral ectoderm called Rathke’s pouch, which extends upward to meet the neurohypophysis, which extends downward as an outpouching of neural ectoderm from the floor of the third ventricle.
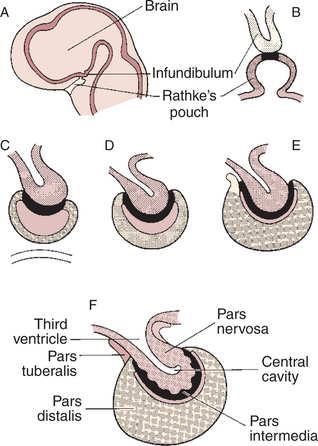
FIGURE 33-10 Diagrams showing progressive stages in the embryonic development of the pituitary gland. Rathke’s pouch becomes detached from the oral epithelium at stage C.
(From Villee CA, Walker WF Jr, Smith FE: General zoology, ed 2, Philadelphia, 1963, Saunders, and from Turner CD, Bagnara JT: General endocrinology, ed 6, Philadelphia, 1977, Saunders.)
The Neurohypophysis Has Cell Bodies That Originate in the Hypothalamus, with Cell Endings That Secrete Oxytocin and Vasopressin
The neurohypophysis is composed of axons whose neural origin is largely within the supraoptic and paraventricular nuclei of the hypothalamus. The neurohypophysis is an extension of the hypothalamus into the pituitary; that is, the cell bodies are in the hypothalamus. The axons form the stalk of the posterior lobe, and the nerve endings are in the lobe proper (Figure 33-11).
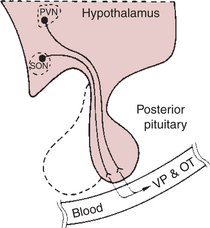
FIGURE 33-11 The hypothalamoneurohypophyseal system, which secretes vasopressin (VP) and oxytocin (OT). PVN, Paraventricular nucleus; SON, supraoptic nucleus.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
The endocrine-secretory neurons that constitute the neurohypophysis differ from neurons involved in the transmission of neural signals in several ways: (1) neurosecretory neurons do not innervate other neurons, even though they are innervated; (2) the secretory product of neurosecretory neurons is secreted into the blood; and (3) the secretory product can act at distances greatly removed from the neuron. Also, in contrast to anterior pituitary hormones, which influence other tissues to produce hormones, posterior lobe hormones can directly cause the desired tissue response.
The first indication of the physiological activity of the neurohypophyseal lobe was the finding of Oliver and Schafer in 1895 that the injection of whole pituitary extracts caused a rise in blood pressure. This effect was soon found to be associated with the pars nervosa. This action represents the effects of one of the main neurohypophyseal hormones, vasopressin. The existence of the other main neurohypophyseal hormone, oxytocin, was first indicated in 1915, when Gaines showed that the injection of posterior pituitary gland extracts caused milk ejection. In 1941, Ely and Peterson showed that a denervated mammary gland could eject milk if the gland was perfused with blood that had been enriched with posterior pituitary extract. Both neurohypophyseal hormones were isolated and sequenced by du Vigneaud in 1954. These were some of the first proteins whose amino acid sequences were elucidated.
Oxytocin and Vasopressin Are Synthesized in Cell Bodies Within the Hypothalamus and Are Carried by Axon Flow to the Posterior Lobe, Where They Are Released
As indicated, the two important hormones produced by the neurohypophysis are vasopressin and oxytocin. Although it was previously thought that the two hormones were produced in separate nuclei, evidence now indicates that both hormones are produced in both the supraoptic and the paraventricular nucleus. The cell bodies that synthesize the hormones are large and thus are called magnocellular nuclei. The synthesis of vasopressin and oxytocin, as described previously for protein and peptide hormones, involves first the production of a preprohormone, prepropressophysin for vasopressin and prepro-oxyphysin for oxytocin, at the level of the cell body within the hypothalamus (Figure 33-12). The “pre” portion of the molecule is cleaved before the molecules are packaged into granules. During passage of the granules down the axon, the prohormone is cleaved to produce either oxytocin or vasopressin; the remaining peptide fragments are called neurophysin I or neurophysin II, respectively. Neurophysin I, which is released into the vascular system along with oxytocin, has been quantified as an alternative means of following the release of oxytocin. At present, the physiological function of the neurophysins is not known.
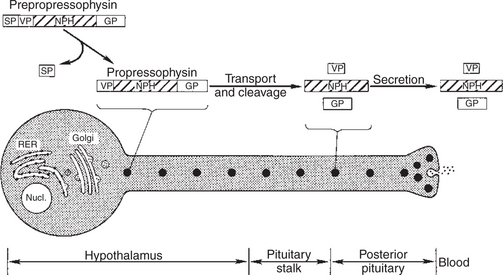
FIGURE 33-12 Diagram of a vasopressin-secreting neuron illustrating the subcellular components involved in synthesis and secretion. This process begins with the synthesis of prepropressophysin, which consists of (1) a signal peptide (SP), (2) vasopressin (VP), (3) neurophysin (NPH), and (4) a glycoprotein (GP). The production and release of oxytocin is identical except that no glycoprotein is involved. RER, Rough endoplasmic reticulum; Nucl., nucleus.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
The release of the posterior lobe peptide hormones is initiated in the hypothalamus as a result of depolarization of the cell body because of stimulation by neural afferents. The action potential generated extends down the axon to the nerve terminal, where the secretory granules containing the hormone are stored. The depolarization of the nerve cell membrane allows the influx of calcium ions, which initiates the release of hormone through the process of exocytosis.
The Main Effects of Oxytocin Are on the Contraction of Smooth Muscle (Mammary Gland and Uterus); the Effects of Vasopressin Are Primarily on the Conservation of Water (Antidiuresis) and Secondarily on Blood Pressure
The main effects of oxytocin involve the contraction of the myoepithelial cells, which surround the alveoli in the mammary gland and the myometrium of the uterus (see Chapters 38 and 39).
The main activity of vasopressin belies its name because its main effect is antidiuretic, the enhancement of water retention by the kidney. As a consequence, the hormone is often called antidiuretic hormone (ADH) (Figure 33-13). Vasopressin is the most important hormone for the control of water balance. Vasopressin also has a pressor effect, which involves the contraction of smooth muscle of the vascular system and therefore has an effect on blood pressure. The main form of vasopressin in most species is arginine vasopressin, whereas in pigs it is lysine vasopressin, and in birds arginine vaso-tocin.
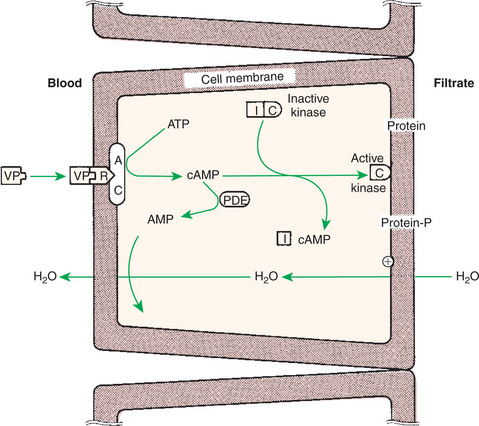
FIGURE 33-13 Antidiuretic mechanism of action of vasopressin (VP) on cells of the distal tubule and collecting ducts. AC, Adenyl cyclase; AMP, adenosine monophosphate; ATP, adenosine triphosphate; cAMP, cyclic AMP; I and C, inhibitory and catalytic subunits of the kinase, respectively; PDE, phosphodiesterase; R, receptor.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
Plasma Osmolality Controls the Secretion of Vasopressin
The control of vasopressin secretion as a result of changes in plasma osmolality is through osmoreceptors located in the hypothalamus as well as through receptors located in the esophagus and stomach that immediately sense water intake (Figure 33-14). An increase in osmolality of body fluids increases the rate of action potential firing in the osmoreceptors, which in turn activates hypothalamic cells that synthesize vasopressin. This negative-feedback system is sensitive to changes in osmolality, and the solute-to-water ratio is maintained within 1% to 2% of the normal values. The regulation of the pressor effect of vasopressin—that is, through blood volume— is achieved by increasing the number of action potentials in stretch receptors located in the atria. A decrease in blood volume activates the stretch receptors, which inhibit activity of neurons, vagal in origin, that inhibit the osmoreceptor cells. Blood volume changes that decrease blood pressure also affect vasopressin release through activation of baroreceptors in the carotid sinus and aortic arch.
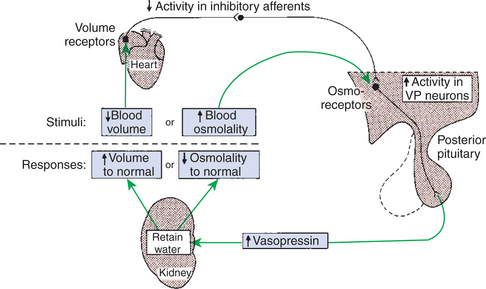
FIGURE 33-14 Major mechanisms regulating vasopressin (VP) secretion. A perturbation in either blood volume or osmolality modifies vasopressin secretion to restore these parameters to their normal values. However, this restoration requires appropriate water intake adjustments by thirst as well as by the modulation of water retention depicted. Also, the two responses indicated may be affected by simultaneous changes in sodium balance.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
Diabetes insipidus (DI) is a disorder of water metabolism characterized by polyuria, urine of low specific gravity or osmolality, and polydipsia. It is caused by defective secretion of ADH (central DI) or by the inability of the renal tubule to respond to ADH (nephrogenic DI). Deficiency of ADH can be partial or complete. Central DI is characterized by an absolute or relative lack of circulating ADH and is classified as primary (idiopathic and congenital) or secondary. Secondary central DI usually results from head trauma or neoplasia.
Central DI may appear at any age, in any breed of dog or cat, and in either gender; however, young adults (6 months of age) are most often affected. The major clinical signs of DI are profound polyuria and polydipsia (>100 mL/kg/day; normal range, 40-70 mL/kg/day), nocturia, and incontinence, usually of several months’ duration. The severity of the clinical signs varies because DI may result from a partial or complete defect in ADH secretion or action. Other, less consistent signs include weight loss (because these animals are constantly seeking water) and dehydration.
Routine complete blood cell count and serum biochemical and electrolyte profiles are usually normal in animals with DI. Plasma osmolality is often high (>310 mOsm/L) in central or nephrogenic DI as a result of dehydration. Animals with primary polydipsia often exhibit low plasma osmolality (<290 mOsm/L) as a result of overhydration. When present on initial evaluation, abnormalities (e.g., slightly increased hematocrit, hypernatremia) are usually secondary to dehydration from water restriction by the pet owner. In DI the urinalysis is unremarkable except for the finding of a persistently dilute urine (urine specific gravity, 1.004-1.012).
Diagnostic tests to confirm and differentiate central DI, nephrogenic DI, and psychogenic polydipsia include the modified water deprivation test or response to ADH supplementation. The modified water deprivation test is designed to determine whether endogenous ADH is released in response to dehydration and whether the kidneys can respond to ADH. The more common causes of polyuria and polydipsia should be ruled out before this procedure is performed. Failure to recognize renal failure before water deprivation may lead to an incorrect or inconclusive diagnosis or may cause significant morbidity in the patient.
Hypersecretion of vasopressin in the absence of osmotic or volumetric stimulation is called the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Neoplastic processes are often involved in SIADH; ectopic tumors, often located in the lung, are the neoplasms most frequently involved.
The Anterior Pituitary Produces Growth Hormone, Prolactin, Thyroid-Stimulating Hormone, Follicle-Stimulating Hormone, Luteinizing Hormone, and Corticotropin
The adenohypophysis comprises the pars distalis and the pars intermedia. The major hormones produced by the anterior pituitary are growth hormone (GH, also called somatotropin), PRL, thyroid-stimulating hormone (TSH), FSH, LH, and corticotropin (Table 33-1). GH is produced by acidophilic somatotropes, and PRL is produced by lactotropes; both are classified as somatomammotropins. GH and PRL are single-chain proteins that contain two and three disulfide bonds, respectively. There is overlap of activity between GH and PRL; this overlap is based on the approximately 50% homology of their amino acid sequences. Of these two major somatomammotropins, GH is uniquely species specific as to its activity.
Table 33-1 Six Major Hormones Secreted by the Anterior Pituitary Gland
| Hormone | Abbreviations |
|---|---|
| Glycoproteins | |
| Follicle-stimulating hormone | FSH |
| Luteinizing hormone (interstitial cell–stimulating hormone) | LH (ICSH) |
| Thyroid-stimulating hormone (thyrotropin) | TSH |
| Somatotropins | |
| Growth hormone (somatotropin) | GH |
| Prolactin | PRL |
| Pro-opiomelanocortins | |
| β-Lipotropin | |
| Corticotropin (adrenocorticotropic hormone) | ACTH |
TSH, produced by thyrotropes, and FSH and LH, produced by gonadotropes, are classified as glycoproteins because all three molecules have carbohydrate moieties. These hormones have α and β subunits that are linked by noncovalent binding. The α subunits are identical (and interchangeable) among the three glycoproteins. The β subunits, unique for each hormone, impart the specific action of each hormone. Other members of this family of hormones that are not of anterior pituitary origin include equine chorionic gonadotropin (also called “pregnant mare’s serum gonadotropin”) and primate chorionic gonadotropin, which are produced by cells of the placental chorion.
Corticotropin and β-lipotropin belong to the pro-opiomelanocortin family in that they originate from a common prohormone (Figure 33-15). Cells in both the pars distalis and the pars intermedia synthesize pro-opiomelanocortin molecules. The emphasis on the type of hormone produced is different in the end product; corticotropin is produced by pars distalis corticotropes. In the pars intermedia, corticotropin is cleaved by corticotropes to form α-melanocyte–stimulating hormone (α-MSH), the predominant hormone of this lobe. The remaining peptide fragment is known as corticotropin-like intermediate lobe peptide; the physiological activity of this peptide fragment is not known. In both the pars distalis and the pars intermedia, β-lipotropin is cleaved to form β-endorphins and γ-lipotropin. Endorphins have opioid activity and appear to modulate gonadotropin secretion.
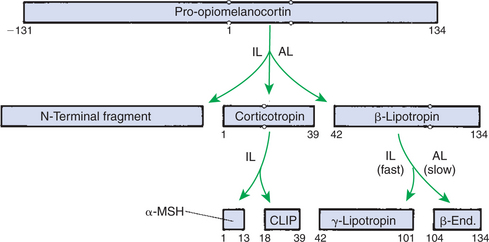
FIGURE 33-15 Cleavage of pro-opiomelanocortin to yield corticotropin and related peptides. By convention, the numbering of the amino acids begins with the first one of corticotropin and then increases positively toward the carboxy terminal and negatively toward the amino terminal. Cleavage occurs at pairs of basic amino acids indicated by the circles. AL, Anterior lobe; α-MSH, alpha-melanocyte–stimulating hormone; β-End., beta-endorphin; CLIP, corticotropin-like intermediate lobe peptide; IL, intermediate lobe.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
Control of adenohypophyseal activity was not understood for many years, primarily because the functional connection between the brain and the anterior pituitary gland was not understood. In the 1930s, Popa and Fielding, Budapest medical student and university professor, respectively, described the vascular system that connects the hypothalamus with the pituitary gland but were unable to determine the direction in which blood flowed. In about 1950, Geoffrey Harris drew the important conclusion that the linkage involved blood passage from the hypothalamus to the anterior pituitary gland through the portal blood system previously described by Popa and Fielding (Figure 33-16). The dorsal hypophyseal artery, which supplies nutrients and oxygen to the adenohypophysis (the ventral hypophyseal artery supplies the neurohypophysis), terminates in the median eminence as a capillary plexus. Blood from these plexus is drained by two veins that empty into sinusoidal capillaries of the pars distalis, completing the portal venous system (one vein supplies the ventral, central part of the pars distalis; the other supplies the dorsal, peripheral areas).
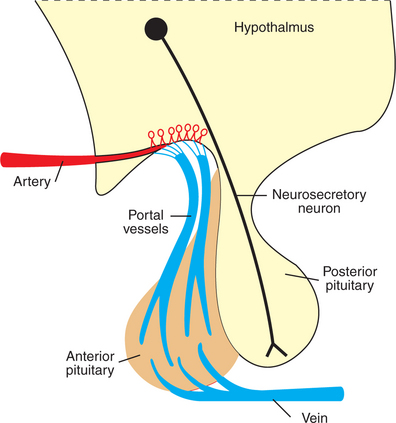
FIGURE 33-16 Diagram of the hypothalamopituitary unit, contrasting the vascular connection between the brain and the anterior pituitary gland with the neuronal connection between the brain and the posterior pituitary gland.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
Adenohypophyseal Activity Is Controlled by Hypothalamic Releasing Hormones, Which Are Released into the Portal System, Which in Turn Connects the Median Eminence of the Hypothalamus and the Anterior Pituitary Gland
Whereas neurons that compose the neurohypophysis are influenced directly by neural input within the hypothalamus, the imposition of a vascular system between the hypothalamus and the adenohypophysis requires a different type of control system. The hypothalamus produces regulatory or hypophysiotropic hormones, which are transported to and released within the median eminence (comparable to posterior lobe hormones) (Figure 33-17). These regulatory hormones then pass via the portal venous system to the adenohypophysis, where they stimulate the release of the various anterior pituitary hormones. The synthesis of adenohypophyseal regulatory hormones is controlled by both neural and hormonal inputs at the level of the hypothalamus. Some of the hypophyseal hormones have been found in other areas of the brain and extraneural sites, including the gastrointestinal tract and the pancreas.
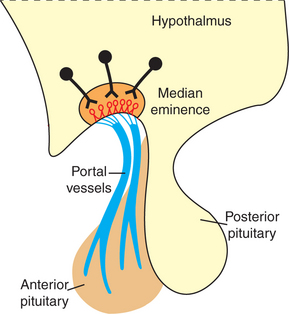
FIGURE 33-17 Hypothalamic neurosecretory neurons and hypothalamohypophyseal portal vessels.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.)
The initial isolation and identification of the hypothalamic hormones required large amounts of tissue as well as expertise in biochemistry. The first identified hypothalamic hormone, which controls corticotropin release, was originally called corticotropin-releasing factor (now changed from factor to hormone). The initial work, done by Guillemin’s group at the University of Houston in the early 1960s, required the collection, freezing, and transport of several hundred thousand sheep brains from abattoirs located in the western United States, as well as the subsequent dissection of the hypothalami. The hypothalamic hormones that have been characterized and the hormones they release include the following (Table 33-2):
1. Corticotropin-releasing hormone (CRH), a 41–amino acid polypeptide that stimulates corticotropes to release all components of the pro-opiomelanocortin family of molecules.
2. Gonadotropin-releasing hormone (GnRH), a decapeptide that stimulates gonadotrope secretion of both FSH and LH.
3. Thyrotropin-releasing hormone (TRH), a tripeptide that stimulates thyrotrope secretion of TSH.
4. Dopamine, a catecholamine precursor of norepinephrine that inhibits lactotrope secretion of PRL and thyrotrope secretion of TSH.
5. Somatostatin, a tetradecapeptide that inhibits somatotrope secretion of GH.
6. Growth hormone–releasing hormone (GHRH), a 44–amino acid polypeptide that stimulates somatotrope secretion of GH.
Table 33-2 Major Hypophysiotrophic Hormones
| Hormone | Abbreviation | Site of origin |
|---|---|---|
| Thyrotropin-releasing hormone | TRH | Paraventricular nucleus |
| Gonadotropin-releasing hormone | GnRH | Preoptic area of hypothalamus |
| Growth hormone–inhibiting hormone (somatostatin) | GHIH | Anterior hypothalamic area |
| Growth hormone–releasing hormone | GHRH | Arcuate nucleus |
| Corticotropin-releasing hormone | CRH | Paraventricular nucleus |
| Prolactin-releasing factor | PRF | ? |
| Prolactin-inhibiting hormone (or dopamine) | PIH | Arcuate nucleus |
Modified from Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.
Except for dopamine, all these hypophysiotropic hormones are peptides.
Previously, only four of the anterior pituitary hormones (FSH, LH, TSH, and corticotropin) were considered to be “tropic”; that is, their main effect was stimulation of hormone secretion by specific endocrine organs located peripheral to the pituitary gland. More recently, GH has been added to this list because GH stimulates the liver to produce somatomedins, which have a negative-feedback effect on GH secretion. PRL remains as the only pars distalis hormone for which negative-feedback inhibition has not been demonstrated through hormones produced by PRL target tissues.
The most important regulation of secretion of the protein hormones by the pars distalis is by feedback inhibition. One feedback system involves negative-feedback inhibition of the tropic pituitary hormone by interaction of the target organ hormone with the hypothalamus, as well as with the pituitary gland; this system is called a long-loop feedback system (Figure 33-18). For example, cortisol is produced by the adrenal cortex as a result of corticotropin stimulation, and cortisol in turn has a negative-feedback effect on corticotropin production at the level of the hypothalamus and the anterior pituitary gland. Short-loop feedback systems have also been described; an anterior pituitary hormone such as corticotropin has a direct negative-feedback inhibition of hormone secretion, in this case CRH, within the hypothalamus.
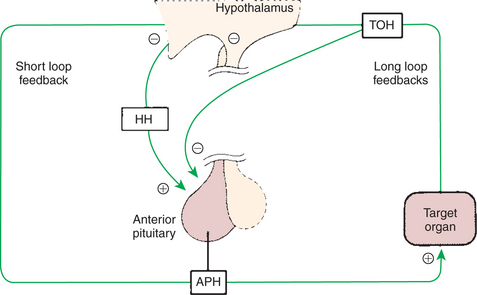
FIGURE 33-18 Regulation of anterior pituitary hormone (APH) secretion by hypophysiotropic hormones (HH), short-loop negative feedback, and long-loop negative feedback by target organ hormones (TOH). Plus signs indicate stimulation, and minus signs indicate inhibition.
(From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.) Modified from Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders.
Even under conditions of negative-feedback inhibition, the secretion of anterior pituitary hormones is not constant. For example, even though estrogens exert a continuous, potent negative-feedback inhibition on gonadotropin secretion, gonadotropin secretion alternates between secretion and no secretion, with pulses of gonadotropins released into the vascular system. In the case of gonadotropins, the ovarian endocrine status influences the pulse rate and the amplitude of each pulse. Progesterone domination is associated with a decreased pulse rate and an increased pulse amplitude, whereas estrogen causes the opposite effect. The work of Irvine and Alexander has provided the best documentation of the precise relationship between secretory activity of hypothalamic regulatory and anterior pituitary hormones. Their data were obtained through analysis of hormones obtained from the intercavernal sinus, which collects venous blood from the pituitary gland of the horse.
Clinical syndromes of somatotropin deficiency and excess include pituitary dwarfism in the dog and acromegaly in the cat, respectively. Pituitary dwarfism results from destruction of the pituitary gland through a neoplastic, degenerative, or anomalous process. It may be associated with decreased production of other pituitary hormones, including TSH, ACTH, LH, FSH, and GH. Pituitary dwarfism is most common in German shepherd dogs aged 2 to 6 months. Other affected breeds include Carnelian bear dogs, spitz, toy pinschers, and Weimaraners. In German shepherd dogs the disease is inherited as a simple autosomal recessive trait and occurs as a result of cystic Rathke’s pouch. The first observable clinical signs of pituitary dwarfism are slow growth, noticed in the first 2 to 3 months of life, and mental retardation, usually manifested as difficulty in house training. Physical examination findings may include proportionate dwarfism, retained puppy hair coat, hypotonic skin, truncal alopecia, cutaneous hyperpigmentation, infantile genitalia, and delayed dental eruption. Clinicopathological features include eosinophilia, lymphocytosis, mild normocytic-normochromic anemia, hypophosphatemia, and occasionally hypoglycemia resulting from secondary adrenal insufficiency. Differential diagnoses include other causes of stunted growth, such as hypothyroid dwarfism, portosystemic shunt, diabetes mellitus, hyperadrenocorticism, malnutrition, and parasitism. Diagnosis is made by measuring serum growth hormone concentrations (assay no longer commercially available) or serum somatomedin C (insulin-like growth factor 1 [IGF-1]). The advantage of IGF-1 is that it is not species specific. There is usually a subnormal response to exogenous TSH and ACTH stimulation tests; furthermore, endogenous TSH and ACTH are decreased in affected dogs as a result of panhypopituitarism.
Acromegaly, or hypersomatotropism, is the condition resulting from chronic excessive GH secretion in the adult animal. Canine acromegaly is an extremely rare disorder observed after the administration of progestational compounds for suppression of estrus in intact female dogs. The disease is caused by excessive secretion of GH from mammary cells under the influence of exogenous progesterone. Acromegaly in cats, as in humans, is caused by a GH-secreting tumor of the anterior pituitary gland. Such tumors in cats grow slowly and may be present for a long time before the onset of clinical signs. Feline acromegaly occurs in older (8- to 14-year-old) cats and occurs more frequently in males. Canine acromegaly occurs in intact female dogs given progestational compounds for estrus prevention.
Clinical signs of uncontrolled diabetes mellitus (DM) are often observed as the first manifestations of acromegaly; therefore polydipsia, polyuria, and polyphagia are the most common presenting signs. Net weight gain of lean body mass in animals with uncontrolled DM is a key sign of acromegaly. Organomegaly, including renomegaly (observed in both cats and humans with acromegaly), hepatomegaly, and enlargement of endocrine organs, is also observed. Some dogs and cats show the classic enlargement of extremities, body size, jaw, tongue, and forehead that is characteristic of acromegaly in humans. Some of the most striking manifestations of acromegaly occur in the musculoskeletal system, such as an increase in muscle mass and growth of the acral segments of the body, including the paws, chin, and skull. Cardiovascular abnormalities, such as cardiomegaly (as determined radiographically and echocardiographically), systolic murmurs, and congestive heart failure, develop late in the course of the disease. Azotemia develops late in the disease in approximately 50% of acromegalic cats. Neurological signs of acromegaly in humans, such as peripheral neuropathies (paresthesias, carpal tunnel syndrome, sensory and motor defects), and parasellar manifestations, such as headache and visual field defects, are not generally detected in acromegalic small animals.
Impairments in glucose tolerance and insulin resistance that result in DM are observed in all cats and most dogs with acromegaly. Measurement of endogenous insulin reveals dramatically increased serum insulin concentrations. Despite severe insulin resistance and hyperglycemia, ketosis is rare in acromegalic animals. Feline acromegaly should be suspected in any diabetic cat (especially males) that has severe insulin resistance (insulin requirement >20 U/cat/day). Hypercholesterolemia and mild increases in serum activities of liver enzymes are attributed to the diabetic state. Hyperphosphatemia without azotemia is also a common clinicopathological finding, perhaps as a result of GH-stimulated bone growth. Urinalysis findings are unremarkable except for persistent proteinuria, probably as a result of systemic hypertension and glomerulosclerosis.
A definitive diagnosis of acromegaly requires documentation of increased plasma G or somatomedin C concentrations. Unfortunately, feline and canine GH assays are no longer commercially available.
Cushing’s syndrome and hyperthyroidism should be ruled out before measurement of GH. At this time the most definitive test for the diagnosis of acromegaly in cats is computed tomography (CT) of the pituitary region. CT findings, coupled with the exclusion of other disorders that cause insulin resistance (hyperthyroidism, hyperadrenocorticism), in cats that exhibit clinical signs of acromegaly should lead the clinician to a diagnosis of acromegaly.
CLINICAL CORRELATIONS
Equine Cushing’s Disease
History.
You are called to examine a 15-year-old mare whose owner complains that the mare has had stiffness in her legs for the past 9 months. The mare has been used as a broodmare and delivered a foal the past spring (and for the 7 previous years). She failed to conceive last spring, and now, in the early summer of the next year, she has yet to exhibit normal estrous cycles.
Clinical Examination.
As you gain a general perspective on the mare, you notice that she appears to have been clipped recently. She is not a show mare, and because it is early summer, you inquire why she has been clipped. The owner indicates that the mare has been slow to shed this spring, and she is tired of seeing the mare with a rough hair coat. The finding of a long hair coat out of season prompts you to ask about the water consumption of the mare; the owner indicates that the mare drank more water (and urinated accordingly) than would be expected. You examine the feet and find that the soles appear slightly “dropped”; you find a small abscess in the sole of one of the feet.
Comment.
The main clue regarding the nature of the disease is the presence of a long hair coat out of season; this is the sine qua non of the disease. The usual complaints of owners of horses with Cushing’s disease are related to chronic processes, such as pneumonia, laminitis, or weight loss, the latter often associated with parasitism and an inability to masticate properly because of tooth problems. Broodmares progressing into Cushing’s disease often have a recent history of infertility after a successful broodmare career. Although the cause of infertility is not known, a disturbance of gonadotropin secretion is likely, along with a perturbation of the pro-opiomelanocortin system.
The disease represents a classic case of loss of control of the intermediate lobe of the pituitary gland by the hypothalamus, in this case the loss of dopaminergic control. Under normal conditions, melanotropes of the intermediate lobe process pro-opiomelanocortin to α-MSH and acetylated β-endorphin, residues 1 to 31, and nonopiate active carboxy-terminally shortened 1 to 26, or 1 to 27, endorphin. In the absence of dopamine, the melanotropes produce α-MSH, as well as β-endorphin, 1 to 31 (the active form), and small amounts of corticotropin; the latter stimulates glucocorticoid production by the adrenal cortex. The negative-feedback control system fails in this situation because the melanotropes do not have glucocorticoid receptors, even under normal conditions. The result is unchecked synthesis and secretion of melanotrope products, including corticotropin, and unchecked glucocorticoid secretion. Activity of the corticotropes in the pars distalis is decreased because of negative-feedback inhibition by the glucocorticoids. One of the long-term effects of excess glucocorticoid secretion is muscle wasting, a common finding in these animals. Also, typical manifestations of the disease include polydipsia and polyuria, which result from compression of the pars nervosa by the enlarging pars intermedia and reduction in ADH synthesis.
Although there is hyperplasia of the intermediate lobe in Cushing’s disease, it has not been established whether this disease occurs because of autonomous hyperplasia of the intermediate lobe or, conversely, whether the hyperplasia occurs because of gradual loss of dopaminergic control by the hypothalamus. One theory is that chronic stress, such as that occurring with laminitis, could affect dopamine secretion by the hypothalamus, leading to loss of control of the intermediate lobe and the development of hyperplasia.
Eiler H. Endocrine glands. Reece WO, ed. Dukes’ physiology of domestic animals, ed 12, Ithaca, NY: Cornell University Press, 2004.
Feldman EC, Nelson RW. Canine and feline endocrinology and reproduction, ed 3, Philadelphia: Saunders, 2004.
Foster DW, Kronenberg HM, Larsen PR, Wilson JD. Williams textbook of endocrinology, ed 9. Philadelphia: Saunders, 1998.
Hedge GA, Colby HD, Goodman RL. Clinical endocrine physiology. Philadelphia: Saunders, 1987.
Martin R. Endocrine physiology. New York: Oxford University Press, 1985.
Pineda MH, Dooley MP. McDonald’s veterinary endocrinology and reproduction, ed 5, Ames: Iowa State University Press, 2003.
Tepperman J, Tepperman M. Metabolic and endocrine physiology, ed 5. Chicago: Year Book Medical Publishers, 1987.
PRACTICE QUESTIONS
1. In general, hormones are classified as proteins, peptides, and steroids. Which one of the following hormones is a peptide?
2. In general, steroid hormones are classified as mineralocorticoid, glucocorticoid, and sex steroids. Which one of the following hormones is a glucocorticoid?
3. Direct feedback control of corticotropin-releasing hormone by corticotropin is termed:
4. Hormones from the pro-opiomelanocortin family are synthesized from precursor hormones produced in either the pars distalis or the pars intermedia. The two main hormones produced by these two lobes (in respective order) are:
5. Increased hormonal activity that occurs during daylight hours is termed _____________ rhythm.