Chapter 43 Water Balance
1. The kidney maintains water balance.
2. The proximal tubule reabsorbs more than 60% of filtered water.
3. The kidney can produce either concentrated or diluted urine.
4. A hypertonic medullary interstitium is needed to form concentrated urine.
5. The juxtamedullary nephrons extend deep into the inner medulla.
6. Solute reabsorption by the medullary thick ascending limb and collecting duct generates and maintains medullary hypertonicity.
7. The countercurrent mechanism increases medullary interstitial osmolality with minimal energy expenditure.
8. Countercurrent exchange in the vasa recta removes water from the medullary interstitium without reducing medullary interstitial hypertonicity.
9. Active sodium chloride reabsorption in the thick ascending limb and the distal convoluted tubule dilutes the tubule fluid.
10. Antidiuretic hormone regulates collecting duct water permeability to determine the final urine osmolality.
11. Cells in the inner medulla adapt to interstitial hyperosmolality by accumulation of organic osmolytes.
The Kidney Maintains Water Balance
One of the most important functions of the kidney is maintaining the water content of the body and the tonicity of the plasma. Terrestrial animals constantly guard against desiccation, and thus their kidneys evolved to reabsorb most of the water in the glomerular filtrate. Under normal conditions, a 10-kg beagle that produces 53.3 L of glomerular filtrate every day may reabsorb more than 99% of the water contained in the glomerular filtrate, excreting only 0.2 to 0.25 L of urine. When water deprived, a normal dog is able to produce urine that has seven to eight times the osmolality of plasma, significantly higher than 2000 milliosmoles per kilogram of water (mOsm/kg H2O). However, the kidney also can produce hypotonic urine in response to a water overload. After a water load, the same dog can excrete urine with an osmolality as low as 100 mOsm/kg H2O, approximately one-third that of plasma. This chapter discusses how the kidney accomplishes these feats.
The Proximal Tubule Reabsorbs More Than 60% of Filtered Water
The proximal tubule reabsorbs the majority of the glomerular filtrate. It takes up solutes from the tubule fluid by both active and passive means. The sodium-potassium– adenosinetriphosphatase (Na+,K+-ATPase) pump in the basolateral plasma membrane actively transports Na+ and drives carrier-mediated secondary active transport and passive uptake of solutes and water. As Na+ is actively transported from the cell into the interstitial fluid, Na+ and other solutes are removed from the tubule fluid by secondary active transport or passive diffusion. Removal of solute from the tubule fluid creates a slight gradient favoring the movement of water into the cells and the intercellular spaces. The proximal tubule apical brush border and complex basolateral plasma membrane create a large surface area for reabsorption that is highly permeable to water because of abundant water channels, aquaporin-1 and aquaporin-7, in the membranes. Thus the small chemical gradient results in rapid movement of water from the tubule fluid to the interstitial fluid. The high oncotic pressure and low hydrostatic pressure in the peritubular capillaries favor the movement of reabsorbed water and solute from the interstitial fluid to the blood.
During the course of a day, 32 to 37 L of water are reabsorbed by the proximal tubules in the kidneys of our 10-kg beagle. However, because the water is reabsorbed nearly isotonically with salt, the osmolality of the tubule fluid remains similar from Bowman’s space to the beginning of the thin descending limb of Henle’s loop.
The Kidney Can Produce Either Concentrated or Diluted Urine
An elegant system has evolved in the mammalian kidney that allows excretion of either concentrated or diluted urine as circumstances warrant. This system has three main components: (1) generation of a hypertonic medullary interstitium, which allows excretion of concentrated urine; (2) dilution of the tubule fluid by the thick ascending limb and the distal convoluted tubule, which allows excretion of dilute urine; and (3) variability in the water permeability of the collecting duct in response to antidiuretic hormone (ADH, vasopressin), which determines the final urine concentration. The beauty of this system is that all the factors necessary for urine concentration and dilution are operative at any given time, so the kidney can respond immediately to changes in ADH levels with corresponding changes in urine osmolality and water excretion.
A Hypertonic Medullary Interstitium Is Needed to Form Concentrated Urine
Terrestrial mammals usually produce urine that is concentrated well above the plasma osmolality. Excretion of concentrated wastes conserves water and thus reduces the volume of water that must be consumed each day to prevent dehydration. Two of the three factors just mentioned are responsible for the formation of concentrated urine: (1) generation of a hypertonic medullary interstitium and (2) enhanced water permeability in the collecting duct in the presence of ADH.
The hypertonicity of the medullary interstitium is produced and maintained primarily by (1) the reabsorption of osmotically active substances by tubules in the medulla and (2) the removal of water from the medullary interstitium by the vasa recta.
The Juxtamedullary Nephrons Extend Deep into the Inner Medulla
The anatomical arrangement of the renal tubules in the medulla is a crucial element of the urine-concentrating mechanism. The nephrons of the mammalian kidney are subdivided into superficial and juxtamedullary nephrons based on the location of their respective glomeruli (see Figure 41-1). Superficial nephrons have short loops of Henle that extend only into the inner stripe of the outer medulla. Juxtamedullary nephrons have long loops of Henle that extend deep into the inner medulla. The juxtamedullary nephrons are particularly responsible for the kidney’s ability to concentrate urine at a much higher level than the osmolality of plasma.
As discussed in Chapters 41 and 42, avian kidneys contain both mammalian-type and reptilian-type nephrons. The reptilian-type nephrons have glomeruli that are near the surface in the renal cortex and have no loops of Henle. The mammalian-type nephrons have glomeruli that are deeper in the cortex and have either short or long loops of Henle that extend into the medullary cone. The mammalian-type nephrons are believed to be at least partly responsible for the ability of birds to excrete urine that is hypertonic relative to plasma.
Solute Reabsorption by the Medullary Thick Ascending Limb and Collecting Duct Generates and Maintains Medullary Hypertonicity
The thick ascending limb of Henle’s loop actively takes up sodium chloride (NaCl) but is impermeable to water. Therefore, this segment raises the osmolality of the interstitial fluid, thus generating medullary interstitial hypertonicity and an osmotic gradient.
The inner medullary collecting ducts also actively reabsorb NaCl, but their more important contribution to the medullary hypertonicity is the reabsorption of urea (Figure 43-1). Although the cortical and outer medullary collecting ducts are impermeable to urea, the terminal inner medullary collecting duct (IMCD) is highly permeable to urea through carrier-mediated urea transport. Thus, urea is conserved in the tubule fluid until the terminal IMCD deep in the medulla. There, urea reabsorption into the interstitial fluid is enhanced by ADH, so when conditions demand water conservation, urea reabsorption is enhanced. Increased urea uptake increases the osmotic gradient for water uptake. Because the thin limbs of Henle’s loop are permeable to urea, whereas the tubule segments that intervene between the thin ascending limb and the terminal IMCD are impermeable to urea, the urea that is reabsorbed from the terminal IMCD is recycled back to the IMCD. In mammals this system of urea recycling enhances the efficiency of the urine-concentrating mechanism. In birds, however, urea is nearly absent in the medullary interstitium; urates do not contribute appreciably to osmotic pressure because they have low water solubility. Thus, medullary hypertonicity in birds depends on single-solute (NaCl) recycling.
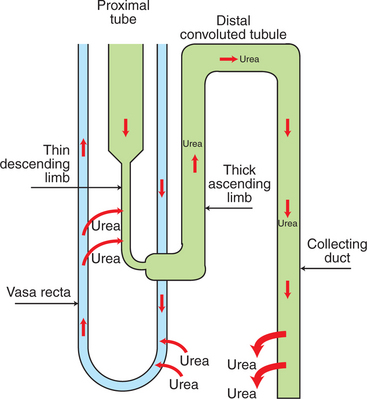
FIGURE 43-1 Urea recycling in the kidney. Filtered urea is reabsorbed in the inner medullary collecting duct by carrier-mediated, facilitated transport and diffuses down its concentration gradient into the vasa recta. It is believed that urea diffuses out of the fenestrated ascending vasa recta down its concentration gradient and returns to the tubule lumen by transport into the descending thin limbs of Henle’s loop. Urea uptake in the descending thin limbs and descending vasa recta is enhanced by the presence of urea transporters. The accumulation of urea in the medullary interstitium contributes to the osmotic pressure and is an important component of renal regulation of water balance.
The Countercurrent Mechanism Increases Medullary Interstitial Osmolality with Minimal Energy Expenditure
The countercurrent mechanism is responsible for the amplification of the medullary hypertonicity initiated by the active reabsorption of solutes by the thick ascending limb of Henle’s loop and the medullary collecting duct. This function is accomplished with minimal energy expenditure because of two characteristics: (1) the anatomical arrangement of the thin limbs of Henle’s loop and the vasa recta and (2) the differential water and salt permeabilities of the descending and ascending thin limbs.
The thin limbs of Henle’s loop in juxtamedullary nephrons extend deep into the inner medulla. The descending and ascending thin limbs are joined by a sharp, “hairpin” turn. Thus the descending and ascending thin limbs are parallel and juxtaposed, with the tubule fluid flow in opposite directions. The vasa recta are arranged similarly. This arrangement of parallel, adjacent conduits with opposite directions of flow enables energy-efficient conservation of solute in the region, similar to heat conservation mechanisms in the extremities of arctic animals (Figure 43-2) and in heat pumps used for home heating.
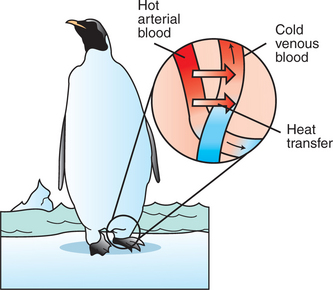
FIGURE 43-2 Countercurrent blood flow for conservation of heat. In many cases, aquatic birds and aquatic mammals have a countercurrent arrangement of blood vessels in their extremities in order to conserve body heat despite constant exposure to cold ambient temperatures without the insulation provided by feathers or subcutaneous fat. Arteries carrying hot blood to the distal extremities, such as the penguin’s foot, are closely intertwined with the veins returning cooled blood from the capillary bed. In this way the arterial blood entering the foot warms the returning venous blood. This arrangement minimizes the loss of heat (energy) to the cold environment and cooling of the body core by the return of chilled venous blood. The countercurrent flow provides efficient energy conservation by creating a temperature gradient between the arterial and venous systems at every level.
(Redrawn from Berl T, Schrier RW: Disorders of water metabolism. In Schrier RW, editor: Renal and electrolyte disorders, ed 4, Boston, 1992, Little, Brown.)
First, consider the contribution of the thin limbs of Henle’s loop to solute and water reabsorption (Figure 43-3). The descending thin limb originates from the straight portion of the proximal tubule and is aligned with the thick ascending limb of Henle’s loop. The tubule fluid entering the thin limb is essentially isosmotic to plasma. The surrounding interstitial fluid is hyperosmotic because of active Na+ reabsorption by the water-impermeable thick ascending limb. Therefore a gradient for water and solutes is established between the descending-thin-limb tubule fluid and the interstitial fluid. The descending thin limb contains aquaporin-1 water channels and is highly permeable to water. In addition, portions of the descending thin limb contain urea transporters that facilitate the movement of urea across the epithelium. However, the descending thin limb is not permeable to salt. Thus the tubule fluid equilibrates with the interstitial fluid by the movement of water into the interstitium and urea into the tubule lumen, and the tubule fluid osmolality rises.
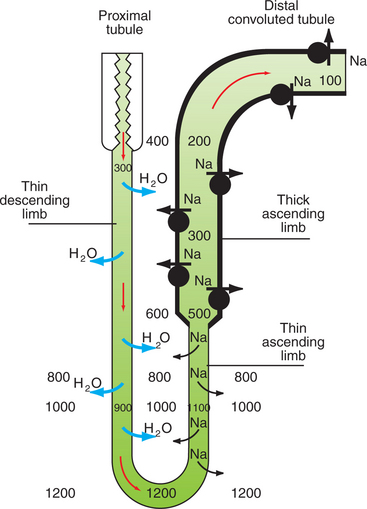
FIGURE 43-3 Roles of the thin limbs of Henle’s loop and the distal nephron to the generation and maintenance of a medullary interstitial concentration gradient and a dilute tubule fluid. The osmolality of the tubule fluid is approximately 300 mOsm/kg H2O when it leaves the proximal tubule and enters the environment of a progressively more concentrated medullary interstitium. Because the thin descending limb is impermeable to sodium (Na) but is permeable to water (H2O), the gradient between the tubule fluid and the interstitial fluid is reduced by diffusion of water into the interstitium, thus raising the osmolality of the tubule fluid. After the hairpin turn deep in the inner medulla, the concentrated tubule fluid enters regions of lower interstitial osmolality as it flows through the ascending thin limb of Henle’s loop. Because this segment is impermeable to water but is permeable to sodium, the gradient between the tubule fluid is minimized by movement of sodium into the interstitium from the tubule fluid. The differential permeabilities of the descending and ascending thin limbs and the countercurrent arrangement preserve the medullary interstitial concentration gradient. The thick ascending limb of Henle’s loop is impermeable to water and actively transports sodium into the interstitium, thus diluting the tubule fluid and generating the medullary concentration gradient. The process of dilution of the tubule fluid is continued in the distal convoluted tubule so that the osmolality of the tubule fluid delivered to the collecting duct system is approximately 100 mOsm/kg H2O, much less than that of plasma (295-300 mOsm/kg H2O).
The osmolality of the medullary interstitial fluid is progressively higher in the deeper regions of the medulla. As the water-permeable descending thin limb reaches regions of higher and higher interstitial osmolality, the tubule fluid continues to equilibrate by diffusion of water into the interstitium, and the tubule fluid osmolality progressively rises until it reaches its maximal concentration at the hairpin turn.
At this point, the thin limb ascends through regions of progressively lower interstitial osmolality, and once again the tubule fluid equilibrates with the interstitial fluid. However, the ascending thin limb is impermeable to water and permeable to NaCl, so the equilibration occurs not by movement of water into the tubule fluid, but by diffusion of NaCl from the tubule fluid into the interstitial fluid. Thus the tubule fluid osmolality decreases and the interstitial osmolality increases. This process continues until the ascending thin limb merges with the thick ascending limb in the outer medulla. At the transition to the thick ascending limb, the tubule fluid osmolality is only moderately hypertonic.
At this point, what has been accomplished? By passive means, the thin limbs have reabsorbed both water and salt. The water was reabsorbed from the descending thin limb, and the salt was reabsorbed from the ascending thin limb. At the same time, the countercurrent flow in these two segments has helped to amplify the medullary hypertonicity.
Countercurrent Exchange in the Vasa Recta Removes Water from the Medullary Interstitium Without Reducing Medullary Interstitial Hypertonicity
The diffusion of water from the descending thin limb into the interstitium would dilute the effect of salt and urea transport into the interstitium and cause swelling of the inner medulla if it were not for the ability of the vasa recta to remove the reabsorbed fluid from the medullary interstitium. The walls of the vasa recta are permeable to water, salts, and urea. The relatively high plasma oncotic pressure in the vasa recta entering the medulla favors the movement of water into the capillary lumen, and NaCl and urea equilibrate with the interstitial concentration. Thus, as the vessels descend in the inner medulla, the plasma osmolality and urea concentration rise as the vasa recta near the hairpin turn, then fall as it ascends out of the medulla (Figure 43-4).
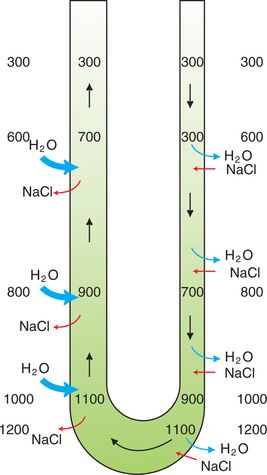
FIGURE 43-4 Countercurrent exchange in the vasa recta. The walls of the vasa recta are permeable to both water and salt (NaCl). Plasma entering the medulla in the descending vasa recta has an osmolality of approximately 300 mOsm/kg H2O, which increases as plasma traverses deeper regions of the medulla, where the interstitial osmolality is elevated. Similarly, after the hairpin turn, the plasma osmolality progressively decreases as the vessel ascends into regions of lower interstitial osmolality. In both arms of the vasa recta, the gradient between the plasma and interstitial osmolality is reduced by movement of water and solute in opposite directions. This system prevents the dissipation of the medullary concentration gradient. In addition, there is net removal of water from the interstitium because of the relatively low hydrostatic pressure and relatively high oncotic pressure in the vasa recta.
What is the net effect of passive equilibration of the interstitial fluid with the plasma in the vasa recta? Two observations indicate that by the time the ascending vasa recta leave the medulla, there has been net movement of fluid into the capillary: (1) the plasma oncotic pressure has fallen, and (2) the blood flow in the ascending vasa recta is approximately double that in the descending vasa recta. Thus the countercurrent arrangement of the vasa recta, the passive equilibration of the plasma with the changing interstitial osmolalities in different regions of the medulla, and the relatively high initial plasma oncotic pressure allow the removal of both water and solute from the medullary interstitium, without dissipating the medullary hypertonicity.
Active Sodium Chloride Reabsorption in the Thick Ascending Limb and the Distal Convoluted Tubule Dilutes the Tubule Fluid
The thick ascending limb and the distal convoluted tubule actively reabsorb Na+, which drives Cl− reabsorption. Because these segments are impermeable to water, active solute reabsorption causes a progressive decline in the tubule fluid osmolality. Thus the thick ascending limb and distal convoluted tubule are often called the diluting segments. The result is that the tubule fluid delivered to the collecting duct is hypotonic, regardless of the physiological state of the animal.
Antidiuretic Hormone Regulates Collecting Duct Water Permeability to Determine the Final Urine Osmolality
The generation of medullary hypertonicity and dilution of the tubule fluid in the distal nephron segments set the stage for the elimination of either concentrated or dilute urine, as warranted by the fluid volume status, plasma tonicity, and blood pressure of the animal. The water permeability of the collecting duct, which is regulated by ADH (arginine vasotocin in birds), determines the osmolality of the excreted urine.
During water overload, ADH is absent, and the collecting duct is relatively impermeable to water. The tubule fluid delivered by the distal convoluted tubule remains hypotonic because the water is confined within the collecting duct. Thus, in the absence of ADH, dilute urine is formed, and excess water is excreted (Figure 43-5).
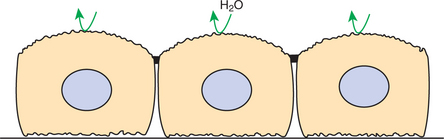
FIGURE 43-5 Collecting duct epithelium in the absence of antidiuretic hormone (ADH). When ADH is absent, the apical plasma membrane is impermeable to water, and dilute urine is excreted.
Under conditions perceived as dehydration or volume depletion, ADH is released from the pituitary. ADH release is triggered by a rise in plasma osmolality of as little as 3 to 5 mOsm/kg H2O resulting from dehydration or salt overload and by decreased blood pressure as a result of systemic vasodilation, heart failure, or isosmotic volume depletion from vomiting, diarrhea, or hemorrhage. In these circumstances, the animal needs to reduce the plasma osmolality to normal or to restore fluid volume or blood pressure.
ADH acutely regulates the water permeability of the collecting duct by regulating the location of the water-channel protein aquaporin-2 (AQP2) in collecting duct cells. When ADH is absent, AQP2 is contained in cytoplasmic vesicles in principal cells and IMCD cells. ADH secretion stimulates the insertion of AQP2 into the apical plasma membrane of these cells, and water freely passes through these channels. In addition, ADH may activate AQP2 already present in the plasma membrane. Chronic ADH stimulation leads to an overall increase in the amount of AQP2 in the collecting duct. Forms of the clinical condition known as nephrogenic diabetes insipidus, which is characterized by renal unresponsiveness to ADH, are caused by either abnormalities in or a deficiency of AQP2 proteins. Another water channel, aquaporin-3, is present in the basolateral plasma membrane of collecting duct cells, regardless of ADH status, and allows the movement of water from inside the cell to the interstitial space.
Thus, when ADH is present, water flows from the dilute tubule fluid into the interstitium down the concentration gradient, producing structural alterations that include cell swelling and dilation of the intercellular spaces (Figure 43-6). As the now water-permeable collecting duct traverses the inner medulla through regions of progressively higher interstitial fluid osmolality, the tubule fluid equilibrates by diffusion of water into the interstitium, and a highly concentrated urine is eliminated.
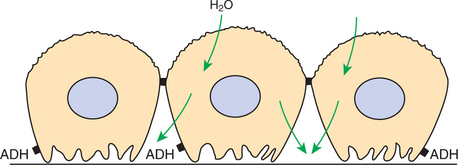
FIGURE 43-6 Water permeability of apical plasma membrane of collecting duct epithelium in the presence of antidiuretic hormone (ADH). ADH stimulates the insertion of water channels into the apical plasma membrane, which enhances its water permeability. Water rushes into the cells and across the water-permeable basolateral plasma membrane into the lateral intercellular spaces, where solute concentrations are high in relation to the tubule fluid. Morphological changes that have been observed include translocation of the membrane containing the aquaporin-2 water channels from intracytoplasmic vesicles to the apical plasma membrane, cell swelling into the tubule lumen, and dilation of the lateral intercellular spaces.
In birds, salt and water reabsorption occurs distal to the collecting ducts. Birds lack a urinary bladder; urine travels from the kidneys via the ureters to the cloaca, where both salt and water are reabsorbed. Furthermore, cloacal urine passes retrograde into the digestive tract, where additional salt and water are reabsorbed.
Cells in the Inner Medulla Adapt to Interstitial Hyperosmolality by Accumulation of Organic Osmolytes
Cells in the inner medulla not only exist in a hypertonic environment, but also regulate cell volume during changes in ambient osmolality. These cells accomplish this by accumulating organic osmolytes that maintain the intracellular osmotic pressure and prevent cell shrinkage without marked increases in the concentration of intracellular electrolytes. These substances include sorbitol, betaine, inositol, and glycerophosphorylcholine. The intracellular concentrations of these osmolytes vary with the diuretic state of the animal, increasing during periods of urine concentration, when the medullary interstitial osmolality is maximized, and decreasing during diuresis, when medullary interstitial osmolality decreases. Changes in the intracellular content of organic osmolytes in response to changes in ambient osmolality occur by parallel changes in either the production (sorbitol) or transmembrane transport (betaine) of the osmolytes.
CLINICAL CORRELATIONS
Diabetes Insipidus
History.
A client presents her 6-month-old female Boston terrier with the complaint of excessive water consumption and urination.
Clinical Examination.
The physical examination reveals no abnormalities. The dog is alert and active. A urinalysis is normal, and the urine specific gravity is 1.002 (osmolality, 152 mOsm/kg H2O). The serum chemistry profile and a complete blood cell count (CBC) are normal.
You admit the dog to your clinic for a modified water deprivation test. The urine fails to become concentrated despite a 5% loss of body weight. You administer vasopressin (ADH), and the urine specific gravity is 1.029 (osmolality, 852 mOsm/kg H2O) 1 hour later.
Comment.
The dog has central diabetes insipidus (DI), which is a deficiency of antidiuretic hormone (ADH). The urine is diluted by the thick ascending limb of Henle’s loop and the distal convoluted tubule. Solute-free water absorption in the collecting duct depends on the action of ADH. In the absence of ADH, excessive volumes of water are excreted, and the dog drinks voraciously to prevent dehydration.
Other causes of excretion of dilute urine (urine osmolality much lower than serum osmolality) are psychogenic polydipsia, hyperadrenocorticism, glucocorticoid therapy, hypercalcemia, hypokalemia, and nephrogenic DI. Most of these can be detected from a thorough history, physical examination, CBC, and serum chemistry profile. When only psychogenic polydipsia, central DI, and nephrogenic DI remain in the differential diagnosis, the diagnosis usually can be made using the modified water deprivation test. Animals with psychogenic polydipsia can secrete ADH and have normal kidneys; therefore they concentrate their urine after water deprivation. Dogs with DI can concentrate their urine minimally or not at all after water deprivation. If the problem is insufficient ADH release (central DI), the urine concentration increases in response to exogenous ADH. If the kidney is unresponsive to ADH (nephrogenic DI), the urine concentration does not increase further in response to additional ADH.
Chronic Renal Insufficiency
History.
You recommend routine dentistry for a 15-year-old male miniature schnauzer that appears to be in good health. Before administering general anesthetic to this aged animal, you obtain a CBC, serum chemistry profile, and urinalysis to detect any subclinical organ dysfunction.
Clinical Examination.
The CBC and serum chemistry profile are normal. The urinalysis is normal with a specific gravity of 1.010 (osmolality, 352 mOsm/kg H2O). You ask the owner to submit a sample of the dog’s urine from the first void of the day. The specific gravity of this sample is 1.012 (osmolality, 401 mOsm/kg H2O).
Comment.
Chronic renal insufficiency is common in geriatric patients and is probably responsible for the two urine specific gravity values in the “fixed range” of 1.008 to 1.012. These values correspond to osmolalities similar to or slightly higher than normal plasma osmolality. Additional evaluation would have to be performed to verify that this animal could neither dilute nor concentrate his urine significantly; however, in an animal of advanced age and in the absence of other clinical or laboratory abnormalities, further evaluation is probably not indicated.
In chronic renal insufficiency the loss of functional nephrons is first manifested by the inability to alter significantly the urine concentration in response to a water load or water deprivation. The residual nephrons are able initially to sustain adequate filtration rates to prevent azotemia (elevated serum creatinine and urea nitrogen levels), but the compensatory increase in flow rates in individual nephrons probably exceeds the capacity of the thick ascending limb and distal convoluted tubule to dilute the tubule fluid significantly. The residual nephrons are also unable to generate a steep medullary concentration gradient, and thus the tubule fluid cannot be concentrated greatly above the level of the plasma osmolality. If there is progressive nephron loss, the glomerular filtration rate will continue to decline, and renal failure will ensue.
Treatment.
It is important to be aware that your patient has chronic renal insufficiency and is unable to respond efficiently to changes in fluid and salt intake. Water should be withheld only briefly, and caution should be exercised in supporting the animal with intravenous fluids during anesthesia while avoiding fluid overload.
A highly bioavailable, low-protein diet that is also low in sodium and phosphorus may hinder the progression of chronic renal disease and delay the onset of renal failure, at least in some species.
Brenner BM, ed. Brenner & Rector’s the kidney, ed 7, E-dition, Philadelphia: Saunders, 2004.
DiBartola SP. Fluid therapy in small animal practice, ed 2. Philadelphia: Saunders, 2000.
Nielsen S, Frøkiær J, Marples D, et al. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205.
Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol. 2003;284:F253.
Seldin SW, Giebish G. The kidney: physiology and pathophysiology, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2000.
Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol. 2005;288:F881.
PRACTICE QUESTIONS
1. The bulk of filtered water is reabsorbed by which renal tubule segment?
2. The kidney responds rapidly to changing water requirements. The ability to alter quickly the rate of water excretion by greatly concentrating or diluting the urine is the result of several factors. Which of the following does not contribute to this ability?
3. The hypertonic medullary interstitium is generated in large part by:
4. In dehydration, ADH is released, which reduces water excretion by:
5. In clinical situations the excretion of dilute urine may be caused by all the following except: