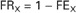Chapter 42 Solute Reabsorption
1. The renal tubule reabsorbs filtered substances.
2. Renal tubule function may be assessed by determining fractional excretion and fractional reabsorption rates.
3. The proximal tubule reabsorbs the bulk of filtered solutes.
4. The proximal tubule secretes organic ions.
5. The distal tubule segments reabsorb salts and dilute the tubule fluid.
6. The collecting duct reabsorbs sodium chloride and can secrete or reabsorb potassium.
7. The distal tubule and collecting duct respond to systemic signals to alter salt excretion as required.
8. Aldosterone enhances sodium reabsorption and potassium secretion.
9. Other hormones regulate sodium transport, including angiotensin II, antidiuretic hormone, endothelin, and atrial natriuretic peptide.
10. Calcium is reabsorbed in the distal nephron and connecting segment, regulated by parathyroid hormone, vitamin D3, and calcitonin.
The Renal Tubule Reabsorbs Filtered Substances
The bulk of the ultrafiltrate formed in the glomerulus must be reabsorbed by the renal tubules rather than excreted in the urine. To understand the importance of tubule reabsorption of filtered substances, consider the 10-kg beagle that forms 53.3 L of glomerular filtrate each day. The ultrafiltrate contains virtually the same concentration of salts and glucose as plasma; therefore, without tubular reabsorption, the urinary loss of sodium, chloride, potassium, bicarbonate, and glucose alone would total more than 500 g of solute. In the absence of tubular reabsorption, the beagle would need to replace these chemicals constantly throughout the day by eating more than a pound of salts and drinking more than 50 L of water at the same rate as the urinary loss to maintain fluid and salt balance.
Fortunately, the renal tubule efficiently retrieves these and other constituents of the ultrafiltrate. Figure 42-1 illustrates the percentages of various filtered substances that remain in the tubule fluid at different points along the tubule. One hundred percent of the filtered glucose is reabsorbed by the proximal tubule; by the time the final urine is formed in the terminal collecting duct, approximately 99% of the filtered water and sodium has been retrieved.
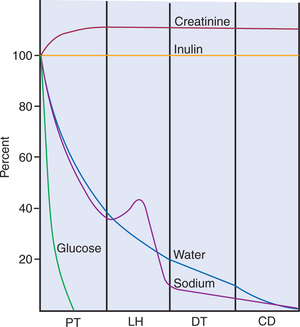
FIGURE 42-1 Illustration of the percentage of filtered substances [(UX/PX) × 100/(Uinulin/Pinulin)] remaining in the tubule fluid in various tubule segments. Creatinine is secreted by the proximal tubule and is excreted at a greater rate than the reference substance, inulin. CD, Collecting duct; DT, distal tubule; LH, loop of Henle; PT, proximal tubule.
(Modified from Sullivan LP, Grantham JJ, editors: Physiology of the kidney, ed 2, Philadelphia, 1982, Lea & Febiger.)
Renal Tubule Function May Be Assessed by Determining Fractional Excretion and Fractional Reabsorption Rates
The percentage of a filtered substance that is excreted in the urine is called the fractional excretion rate. It is the net result of the tubular reabsorption and secretion of the filtered substance. Mathematically, the fractional excretion rate of a substance X is the ratio of the urinary concentration of X (UX) to the plasma concentration of X (PX) divided by the urinary/ plasma (U/P) ratio of a reference substance that is neither secreted nor reabsorbed. Relating UX/PX to the U/P ratio of a reference substance eliminates the confounding effect of water reabsorption on the urinary concentration of X. In experimental settings the urinary and plasma concentrations of inulin during a constant inulin infusion may be used for reference. However, it is more practical in clinical situations to use creatinine as the reference substance. Therefore the fractional excretion rate of X (FEX) is determined by the following equation:
where Ucreatinine and Pcreatinine are the urinary and plasma concentrations of creatinine, respectively. By multiplying FEX by 100, the fractional excretion rate is expressed as the percentage of filtered X that is excreted.
The fractional reabsorption rate of X (FRX) represents the proportion of filtered X that is reabsorbed by the tubule. FRX is mathematically determined by the following equation:
This can be expressed as a percentage by multiplying FRX by 100.
The Proximal Tubule Reabsorbs the Bulk of Filtered Solutes
The rate of reabsorption and secretion of filtered substances varies among segments of the renal tubule. In general, the proximal tubule reabsorbs more of the ultrafiltrate than other tubule segments, at least 60% of most filtered substances.
The structure of the proximal tubule and its proximity to the peritubular capillary facilitate the movement of tubule fluid components into the blood through two pathways: the transcellular pathway and the paracellular pathway. The tubule fluid flows over the apical surface of the proximal tubule epithelial cell. Substances transported through the transcellular pathway cross the apical plasma membrane and discharge across the basolateral plasma membrane into the interstitial fluid on the blood side of the cell. Passage across the apical and basolateral plasma membranes occurs largely by carrier-mediated transport. A vast surface area is available for transport of tubule fluid components into the cell because of extensive microprojections of the apical plasma membrane, called microvilli, which collectively create a beautiful membranous structure known as the brush border (Figures 42-2 and 42-3). On the blood side of the cell, complex infoldings of the basolateral plasma membrane enhance the surface area available for transport from the cell into the interstitial fluid that bathes the peritubular capillary.
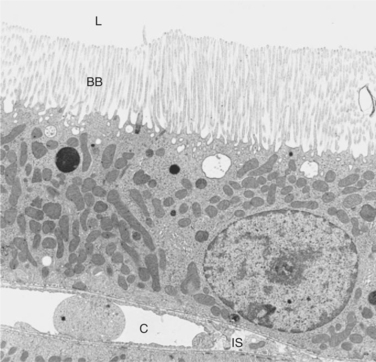
FIGURE 42-2 Transmission electron micrograph of cross section of rat proximal tubule. The brush border (BB) of the apical plasma membrane extends from the epithelial cells into the tubule lumen (L), where it is bathed by the tubule fluid. On the basal side of the cell is the interstitial space (IS) and the peritubular capillary (C). (Magnification ×3400.)
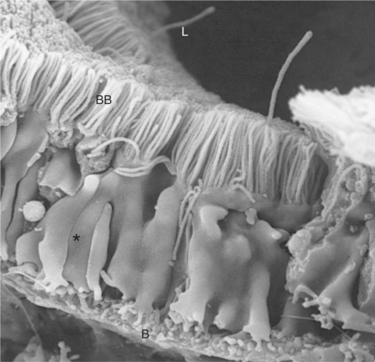
FIGURE 42-3 Scanning electron micrograph of rat proximal tubule, viewed from the lateral intercellular space. The lush brush border (BB) carpets the luminal aspect (L). Lateral cellular processes (asterisk) interdigitate with those of neighboring cells. The surface of the basal plasma membrane (B) is amplified by extensive membrane infoldings, creating numerous processes called micropedici (“tiny feet”). (Magnification ×4400.)
The second route of transport in the proximal tubule is the paracellular pathway. Substances pass through the paracellular pathway from the tubule fluid across the zonula occludens, a permeable structure that attaches the proximal tubule cells to each other at the junction of the apical and basolateral plasma membrane domains (Figure 42-4). Paracellular transport occurs by passive diffusion or by solvent drag, which is the entrainment of solute by the flow of water. Substances crossing the zonula occludens enter the lateral intercellular space, which is thought to communicate freely with the interstitial fluid; from there, reabsorbed substances can be taken up by the peritubular capillary.
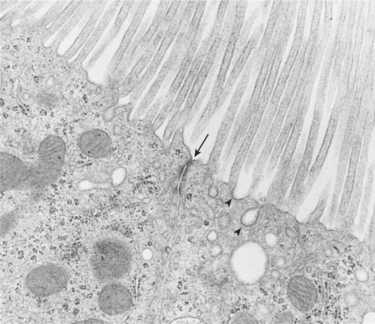
FIGURE 42-4 Transmission electron micrograph of apical region of rat proximal tubule viewed in cross section. The zonula occludens (arrow) joins adjacent proximal tubule cells. The zonula occludens divides the apical plasma membrane from the basolateral plasma membrane and separates the tubule fluid from the fluid of the lateral intercellular space. Also seen are coated pits (arrowheads) that contain the binding sites for substances reabsorbed by receptor-mediated endocytosis. (Magnification ×19,600.)
Movement of water and solute from the interstitial fluid into the bloodstream is driven by Starling’s forces and aided by the proximity of the peritubular capillary. In mammals the peritubular capillary originates at the glomerular efferent arteriole, subdivides, and wraps closely around the basal aspect of the proximal tubule (Figure 42-5). The plasma leaving the glomerulus has a high oncotic pressure because water and salts are filtered, but proteins are retained in the capillary. The peritubular capillary has low resistance, and thus the hydrostatic pressure in the capillary is low. Both these conditions—high peritubular plasma oncotic pressure and low peritubular capillary hydrostatic pressure—favor fluid and solute uptake from the interstitium into the bloodstream.
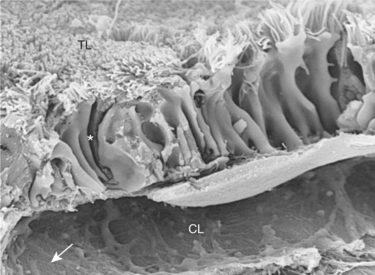
FIGURE 42-5 Scanning electron micrograph of rat proximal tubule and peritubular capillary. The peritubular capillary wraps around the basal aspect of the proximal tubule cells. Substances retrieved from the tubule lumen (TL) are delivered through either the transcellular pathway or the paracellular pathway into the fluid bathing the basolateral aspect of the epithelial cells. Water and solutes enter the interstitial space and diffuse into the peritubular capillary lumen (CL). Asterisk represents lateral intercellular space; arrow represents fenestrae of peritubular capillary endothelium. (Magnification ×7000.)
In birds the effect of the peritubular blood supply on tubular reabsorption and secretion is complicated by the presence of a renal portal circulation. Renal portal veins anastomose with the efferent glomerular arterioles and supply peritubular blood to the reptilian-type nephrons and the proximal and distal tubules of mammalian-type nephrons; thus these tubules, but not the loops of Henle of the mammalian-type nephrons, are supplied with a mixture of portal venous and arterial blood. The rate of flow to the renal portal supply varies and is controlled by a smooth muscle valve.
Reabsorption of solutes takes place by a number of transport mechanisms, including passive diffusion, solvent drag, primary active transport, and carrier-mediated secondary active transport. (Transport mechanisms are described in Chapter 1.) In the proximal tubule, much of the transport of substances from the tubule fluid to the blood is driven by the active transport of sodium ions (Na+) by the sodium-potassium– adenosinetriphosphatase (Na+,K+-ATPase) pump located in the basolateral plasma membrane, which extrudes 3 Na+ ions and takes up 2 K+ ions on each turnover of the pump (Figure 42-6).
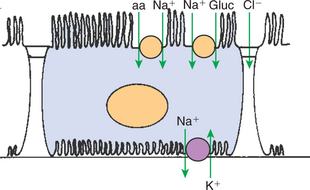
FIGURE 42-6 Schematic illustration of transport processes in the proximal tubule epithelial cell. Virtually all transport is believed to be driven by active reabsorption of Na+ by the Na+,K+-ATPase located in the basolateral plasma membrane. Glucose (gluc) and amino acids (aa), as well as many other solutes, enter the cell by secondary active transport with Na+, driven by the low intracellular Na+ concentration resulting from the active transport of Na+ out of the cell. Cl− diffuses across the zonula occludens into the lateral intercellular spaces down its electrochemical gradient.
The normal operation of the Na+,K+-ATPase pump reduces the intracellular Na+ concentration and increases the intracellular K+ concentration. Subsequent outward diffusion of K+ down its chemical gradient through K+ channels causes the interior of the cell to become electrically negative relative to the exterior. Thus an electrochemical gradient for Na+ across the apical plasma membrane is established, favoring Na+ uptake from the tubule fluid into the cell. Na+ uptake across the apical cell membrane is facilitated by specific transporters in the membrane that couple the movement of other solutes either in the same direction as Na+ (co-transport) or in the opposite direction (counter-transport). Glucose, amino acids, phosphate, sulfate, and organic anions are taken up from the proximal tubule fluid by this mechanism (secondary active transport). The active uptake of these substances increases their intracellular concentration, and they move across the basolateral plasma membrane and into the blood down their electrical or chemical gradient by passive or facilitated diffusion.
Bicarbonate (HCO3−) reabsorption in the proximal tubule is also driven by the Na+ gradient, although indirectly. The chemical gradient for Na+ drives Na+ and proton (hydrogen ion, H+) counter-transport across the apical plasma membrane through a Na+/H+ exchanger. Secreted H+ combines with filtered HCO3− in the tubule fluid to form water (H2O) and carbon dioxide (CO2), catalyzed by the enzyme carbonic anhydrase in the apical plasma membrane of proximal tubule cells. CO2 diffuses passively across the apical plasma membrane into the cell. There, cytoplasmic carbonic anhydrase catalyzes the hydroxylation of CO2 with OH− donated from H2O, forming H+ and HCO3− within the cell. The HCO3− moves across the basolateral plasma membrane primarily through a Na+,3-(HCO3−) co-transporter as well as a HCO3−/Cl− exchanger. The H+ is transported into the tubule fluid primarily through the Na+/H+ antiporter. By this complex mechanism, illustrated in Figure 42-7, the proximal tubule reabsorbs 60% to 85% of filtered HCO3−.
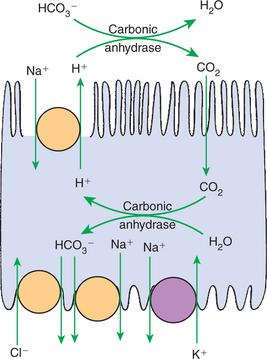
FIGURE 42-7 Schematic illustration of bicarbonate (HCO3−) reabsorption and acid secretion in the proximal tubule. The active reabsorption of Na+ by the basolateral Na+,K+-ATPase pump drives the secretion of H+ through the Na+/H+ exchanger in the apical plasma membrane. In the lumen the secreted H+ and filtered HCO3− form H2O and CO2 under the influence of apical membrane–associated carbonic anhydrase. The CO2 readily diffuses across the apical plasma membrane into the cell and combines with intracellular H2O to form H+ and HCO3−. This process is catalyzed by cytoplasmic carbonic anhydrase. The H+ is secreted into the tubule fluid, and the HCO3− is transported to the blood side of the cell through co-transport with Na+ or counter-transport with Cl−.
Chloride ion (Cl−) reabsorption in the proximal tubule is also indirectly powered by the Na+,K+-ATPase pump and occurs through both paracellular and transcellular routes. As Na+, HCO3−, glucose, amino acids, and other solutes are selectively reabsorbed and water is taken up along with these solutes, the concentration of Cl− in the tubule fluid rises, establishing a chemical gradient for Cl− movement toward the blood side of the epithelium. In addition, in the early proximal tubule, the selective uptake of Na+ exceeds that of anions, resulting in a net positive charge on the blood side. This creates a small electrical gradient favoring anion reabsorption. Thus, in the early proximal tubule, the chemical gradient and the electrical gradient favor Cl− reabsorption. The zonula occludens is highly permeable to Cl−, thus passive, paracellular transfer of Cl− from the tubule lumen to the interstitial fluid occurs. It is now known that transcellular Cl− absorption also occurs in the proximal tubule. Cl−-coupled transporters in both the apical and basolateral plasma membranes and Cl− channels in the basolateral plasma membrane facilitate transmembrane Cl− transport, which is also driven indirectly by Na+,K+-ATPase.
In distal portions of the proximal tubule, the tubule fluid becomes depleted of many of the substances necessary for Na+ reabsorption by co-transport. There, the Na+,K+-ATPase pump continues to move Na+ from the cell into the interstitial fluid; Na+ uptake from the lumen occurs predominantly by electrically neutral sodium chloride (NaCl) uptake facilitated by coordinated Na+- and Cl−-coupled transporters and by passive reabsorption of Na+ through the paracellular pathway. Paracellular transport of Na+ is made possible here by the chemical gradient for Cl− established by the selective reabsorption of other solutes in the early proximal tubule. As Cl− moves down its chemical gradient from the tubule lumen to the blood side, it carries Na+ along with it by electrostatic attraction. The passage of Cl− down its chemical gradient also abolishes the small, lumen-negative charge and in fact establishes a small, lumen-positive charge in the late proximal tubule, which further favors the passive transfer of Na+ to the blood side.
Other filtered solutes, such as potassium (K+) and calcium (Ca2+) ions, are present in the tubule fluid in low concentrations and are reabsorbed by the proximal tubule. Approximately 65% of filtered Ca2+ is reabsorbed in the proximal tubule. About 90% of the Ca2+ uptake in the proximal tubule is paracellular because of a favorable electrochemical gradient in the late proximal tubule and solvent drag. The majority of K+ reabsorption in the proximal tubule also occurs by passive mechanisms, primarily through the paracellular route but also through K+ channels in the apical and basolateral plasma membranes.
The proximal tubule also reabsorbs filtered peptides and low-molecular-weight proteins. A large proportion of filtered peptides are degraded to amino acids by peptidases in the proximal tubule brush border and are reabsorbed by co-transport with Na+ across the apical plasma membrane. Short-chain peptides are themselves transported through co-transport with H+ on specific transporters (PEPT1 and PEPT2) in the proximal tubule brush border, driven by the proton gradient between the tubule fluid and cytoplasm. Most of these dipeptides and tripeptides are degraded by intracellular peptidases, although some may exit intact to the blood side through another peptide transporter.
Low-molecular-weight proteins are avidly reabsorbed by the proximal tubule, but by a different mechanism. Filtered proteins such as insulin, glucagon, and parathyroid hormone are taken up at the apical plasma membrane by carrier-mediated endocytosis (see Figure 42-4). The proteins are delivered by the endocytic vesicles to intracellular organelles called lysosomes (Figure 42-8). Proteolytic enzymes within the lysosomes degrade the reabsorbed proteins, and the resultant amino acids are transported into the interstitial fluid and returned to the blood.
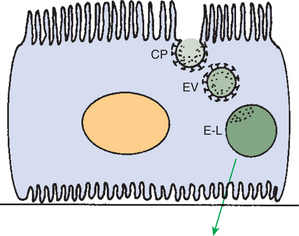
FIGURE 42-8 Schematic illustration of receptor-mediated endocytosis of filtered proteins in the proximal tubule. Filtered proteins bind with their receptors in the membrane of coated pits (CP) in the apical plasma membrane. The coated pits invaginate and form endocytic vesicles (EV) that transport the proteins to the endosomal-lysosomal system (E-L), from which the reabsorbed proteins or their degradation products are returned to the circulatory system.
The Proximal Tubule Secretes Organic Ions
The proximal tubule secretes a wide variety of organic ions into the tubule fluid. Many organic ions, including both endogenous waste products and exogenous drugs or toxins, are protein bound in the plasma and thus are poorly filtered by the glomerulus. However, the proximal tubule takes up these substances from the blood and extrudes them into the tubule fluid by a carrier-mediated process, thus clearing them from the plasma. Endogenous organic compounds secreted by the proximal tubule include bile salts, oxalate, urate, creatinine, prostaglandins, epinephrine, and hippurates. Drugs and toxins secreted by the proximal tubule include antibiotics (e.g., penicillin G, trimethoprim), diuretics (e.g., chlorothiazide, furosemide), the analgesic morphine and many of its derivatives, and the potent herbicide paraquat.
This aspect of proximal tubule function has broad practical applications. Tubule secretion of endogenous organic ions, drugs, and toxins provides the basis for urine testing for hormones and foreign substances as a reflection of blood levels that may be only transiently elevated. Tubule secretion of exogenous p-aminohippurate is used to estimate renal plasma flow. Tubule secretion of certain antibiotics is important in determining which antibiotics can reach high concentrations in the urine for more effective treatment of urinary tract infections. Similarly, secretion of diuretics such as furosemide enhances delivery of these drugs to their site of action in the thick ascending limb of Henle’s loop. Finally, tubule secretion of certain drugs determines in part their excretion rate and affects appropriate dosing, which can be particularly important in patients with compromised renal function.
Tubule secretion plays a larger role in birds than in mammals. The end product of protein metabolism in mammals is urea, which is excreted primarily through glomerular filtration. In birds the end product of protein metabolism is uric acid. Uric acid is produced by the liver and the kidney in birds and is excreted primarily by tubule secretion. In fact, in starlings the total amount of uric acid excreted by the kidney is more than five times the amount filtered. The principal site of uric acid secretion in the avian kidney is believed to be the proximal portion of reptilian-type nephrons.
The Distal Tubule Segments Reabsorb Salts and Dilute the Tubule Fluid
The structure of the tubule epithelium changes abruptly and dramatically at the end of the proximal tubule. The proximal tubule, with its abundant mitochondria, luxuriant brush border, and pronounced basolateral plasma membrane infoldings, is suited for high-volume transport of a large variety of substances by both active and passive mechanisms. The segments that follow the proximal tubule each have a unique structure, reflecting their specialized functions. Immediately downstream from the straight portion of the proximal tubule is the thin limb of Henle’s loop, which is a low epithelium with few mitochondria and few membranous infoldings (Figure 42-9). As might be expected, physiological studies suggest that active transport of solutes in this segment is virtually nonexistent. The function of the thin limb is determined by its passive permeability properties and its spatial orientation in the medulla. These characteristics are essential to its role in water reabsorption, as discussed in Chapter 43.
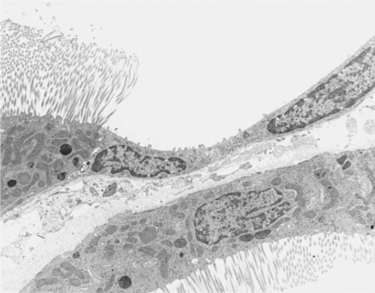
FIGURE 42-9 Transmission electron micrograph of rat kidney illustrating the transition from the proximal tubule to the thin descending limb of Henle’s loop. The tall epithelium of the proximal tubule with the extensive brush border and abundant mitochondria abruptly changes to the low epithelium of the thin limb of Henle’s loop. Epithelial cells of the thin limb have a smooth, simple plasma membrane surface and few mitochondria, which is consistent with the absence of active transport functions. (Magnification ×5900.)
In the ascending limb of Henle’s loop, the low epithelium of the thin ascending limb abruptly changes to the relatively tall epithelium of the thick ascending limb. The thick ascending limb has many mitochondria and basolateral plasma membrane infoldings, reflecting its high capacity for active solute transport (Figure 42-10). The distal convoluted tubule follows with an even taller epithelium and a dense array of mitochondria. Next is the connecting segment, a heterogeneous segment that connects the nephrons to the collecting duct system.
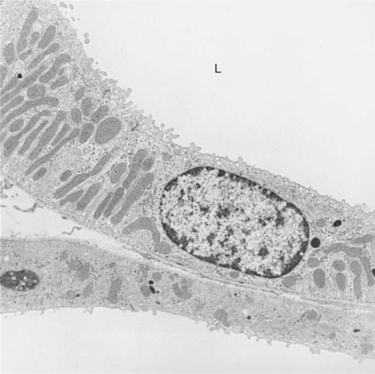
FIGURE 42-10 Transmission electron micrograph of thick ascending limb of Henle’s loop in the rat. In accordance with its important role in active Na+ reabsorption, the thick ascending limb is a tall epithelium, with extensive basolateral plasma membrane infoldings and numerous mitochondria. A collecting duct is adjacent to the basolateral aspect of the thick limb. L, Tubule lumen. (Magnification ×5600.)
The distal tubule segments, which include the thick ascending limb of Henle’s loop and the distal convoluted tubule, reabsorb Na+, K+, Cl−, and the divalent cations Ca2+ and Mg2+. These segments reabsorb solutes against a high gradient. By the time the tubule fluid leaves the distal convoluted tubule, more than 90% of the filtered salts have been reabsorbed, and the osmolality of the tubule fluid is typically reduced from approximately 300 to 100 mOsm/kg H2O.
As in the proximal tubule, salt reabsorption in the distal tubule segments is driven by the Na+,K+-ATPase pump in the basolateral plasma membrane. In the thick ascending limb of Henle’s loop, the basolateral Na+,K+-ATPase pump actively transports Na+ from the cell into the interstitial fluid, creating an electrochemical gradient for Na+ across the apical plasma membrane. This gradient drives ion transport through the Na+,K+,2Cl− co-transporter in the apical plasma membrane, and these ions enter the cell (Figure 42-11). The Cl− diffuses down its chemical gradient into the interstitial fluid through Cl− channels in the basolateral plasma membrane. The K+ moves extracellularly down its concentration gradient through K+ channels across both the basolateral plasma membrane and the apical plasma membrane. The Cl− absorption and K+ secretion cause a lumen-positive voltage (electrical potential) relative to the interstitium, which creates a lumen-to-blood electrical gradient for cations that then diffuse into the interstitial fluid through the paracellular pathway. The apical Na+,K+,2Cl− co-transporter in the thick ascending limb is inhibited by the loop diuretics, such as bumetanide and furosemide, which are often used in clinical veterinary medicine.
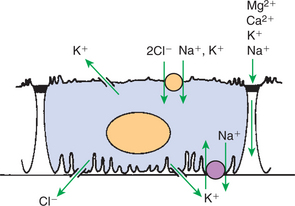
FIGURE 42-11 Schematic illustration of transport functions of the thick ascending limb. Na+ is actively reabsorbed through the basolateral Na+,K+-ATPase pump. Na+, K+, and Cl− enter the cell from the luminal fluid through secondary active co-transport. Cl− diffuses down its concentration gradient across the basolateral plasma membrane through a Cl− channel. K+ leaves the cell through both an apical and a basolateral K+ channel. A lumen-to-blood gradient for cations is present in this segment, which drives reabsorption of Na+, K+, Ca2+, and Mg2+ through the cation-selective paracellular pathway.
The distal convoluted tubule contains a NaCl co-transporter, and the connecting segment contains a Na+ channel in the apical plasma membrane that permits transport of Na+ from the tubule fluid down the chemical gradient for Na+ generated by the basolateral Na+,K+-ATPase pump. Cl− moves from the cell to the interstitial fluid through a basolateral Cl− channel, driven by the electrical gradient. The activity of the apical NaCl co-transporter is inhibited by thiazide diuretics.
Both the thick ascending limb and the distal convoluted tubule are impermeable to water. The avid reabsorption of salts without water results in a hypotonic tubule fluid, and thus these segments are sometimes called the diluting segments. Dilution of the tubule fluid takes place regardless of the volume status of the animal. Dilution of the tubule fluid in these segments is an important component of fluid volume regulation, allowing the kidney to excrete excess water without salt, thereby preventing water overload and plasma hypotonicity. The role of the thick ascending limb and the distal convoluted tubule in water balance is discussed further in Chapter 43.
The Collecting Duct Reabsorbs Sodium Chloride and Can Secrete or Reabsorb Potassium
The collecting duct system begins with the connecting segment, which is a transition region from the distal convoluted tubule to the initial collecting tubule. Depending on the species, the connecting segment contains several distinct epithelial cell types, including distal convoluted tubule cells, connecting segment cells, intercalated cells, and principal cells. Each of these structurally distinct cell types has specific physiological functions.
The initial collecting tubules converge and empty into the cortical collecting duct, which descends through the cortex and medulla to the papillary tip, where the tubule fluid (urine) discharges into the renal pelvis. Throughout most of the collecting duct system, two main cell types exist: the intercalated cell, which has many intracytoplasmic vesicles and mitochondria, and the principal cell, which has fewer intracytoplasmic vesicles and mitochondria but extensive basolateral plasma membrane infoldings (Figure 42-12). The principal cell is the major cell type in the initial collecting duct, the cortical collecting duct, and the outer medullary collecting duct, accounting for approximately two thirds of the cells in most regions. The intercalated cell accounts for the remainder of the cortical and outer medullary collecting duct cells, and in some species (rat, mouse, and human, at least) it persists even in the inner medullary collecting duct.
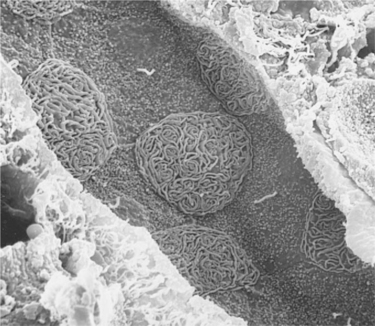
FIGURE 42-12 Scanning electron micrograph of outer medullary collecting duct in the rat, viewed from the luminal surface. Two cell types are evident: the principal cell, with short, small projections over the apical surface and a single central cilium, and the intercalated cell, with extensive, complex membrane folds (microplicae) over the apical surface. (Magnification ×2000.)
The principal cell reabsorbs NaCl in the collecting duct. Its extensive basolateral plasma membrane infoldings contain Na+,K+-ATPase. As in other tubule segments, Na+ is actively transported by this pump into the interstitial fluid, which establishes an electrochemical gradient for Na+ uptake from the tubule fluid through Na+ channels in the apical plasma membrane. The resulting lumen-negative electrical potential drives Cl− absorption through the paracellular pathway. At the same time, K+ is pumped actively from the interstitial fluid into the cell by Na+,K+-ATPase, raising the intracellular K+ concentration above that of the interstitial fluid and the tubule fluid. Intracellular K+ exits the cell down the chemical gradient through K+ channels present in the apical and basolateral plasma membranes. Under normal circumstances, however, net K+ secretion occurs for two reasons: (1) the apical K+ channel is more permeable than the basolateral K+ channel, and (2) the lumen-negative electrical potential favors K+ secretion (Figure 42-13).
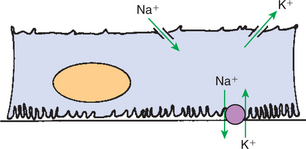
FIGURE 42-13 Schematic illustration of transport in the principal cell of the collecting duct. The extensive basal plasma membrane infoldings contain large amounts of Na+,K+-ATPase. Active transport of Na+ by this pump drives passive diffusion of Na+ from the tubule lumen into the cell through a Na+-selective channel in the apical plasma membrane. A K+-selective channel in the apical plasma membrane provides a route for passive diffusion of intracellular K+ into the tubule fluid. The hormone aldosterone enhances Na+,K+-ATPase activity and increases the Na+ and K+ permeability of the apical plasma membrane, thus enhancing Na+ reabsorption and K+ secretion in this segment.
The collecting duct can also reabsorb K+, and the intercalated cell appears to be responsible for this function. Potassium ions are actively transported from the cytoplasm across the apical plasma membrane of the intercalated cell in exchange for hydrogen ions by an H+,K+-ATPase pump similar to that in the parietal cell of the stomach. This pump also contributes to the acidification of the urine (see Chapter 44).
The Distal Tubule and Collecting Duct Respond to Systemic Signals to Alter Salt Excretion as Required
In the proximal tubule, filtered solutes and water are generally reabsorbed regardless of the animal’s physiological state. In contrast, the distal tubule and collecting duct control the ultimate rate of excretion of electrolytes and water to maintain homeostasis despite variations in dietary intake and extrarenal losses of salts and water. The specific homeostatic responses of these segments are controlled in large part by several hormones, including aldosterone, angiotensin II, antidiuretic hormone, endothelin, atrial natriuretic peptide, parathyroid hormone, 1α,25-(OH)2–vitamin D3, and calcitonin. In birds the relative importance of the reptilian-type and the mammalian-type nephrons in regulation of salt balance has not been established. Furthermore, in many species of birds, particularly marine and desert species, sodium balance is regulated largely by secretion of NaCl by the nasal (supraorbital) gland, rather than by regulation of renal excretion. Finally, ureteral urine delivered to the cloaca moves in a retrograde manner into the digestive tract, where additional salt reabsorption occurs.
Aldosterone Enhances Sodium Reabsorption and Potassium Secretion
Aldosterone is a mineralocorticoid hormone that is secreted by the adrenal cortex. Aldosterone release is stimulated by systemic hypotension through the renin-angiotensin system. Aldosterone acts on connecting segment cells and the principal cells of the collecting duct to enhance Na+ reabsorption, which in turn enhances water reabsorption to correct perceived volume depletion. At the cellular level, aldosterone increases the permeability of the apical plasma membrane Na+ channels and stimulates Na+,K+-ATPase activity, thereby enhancing Na+ reabsorption. Chronic aldosterone stimulation even causes a structural adaptation in these cells, the proliferation of the basolateral plasma membrane, where Na+,K+-ATPase resides. In addition, chronic aldosterone stimulation increases transcription of the apical NaCl co-transporter in the distal convoluted tubule and the epithelial Na+ channel (ENaC) in the collecting duct and trafficking of these transport proteins to the apical plasma membrane.
Aldosterone release is also stimulated by hyperkalemia (elevated plasma K+ level) and has an important role in regulating K+ homeostasis. The acute effect of aldosterone on K+ is to enhance its entry into aldosterone-responsive cells by stimulating Na+,K+-ATPase activity; this lowers serum K+ levels but has little effect on renal K+ excretion. More chronic aldosterone stimulation increases the number of K+ channels in the apical plasma membrane of connecting segment cells and principal cells, thereby increasing the apical K+ permeability and K+ secretion. Therefore the immediate effect of aldosterone is a redistribution of K+ from extracellular to intracellular compartments, but with continued aldosterone stimulation, renal elimination of K+ is augmented.
Other Hormones Regulate Sodium Transport, Including Angiotensin II, Antidiuretic Hormone, Endothelin, and Atrial Natriuretic Peptide
In addition to enhancing sodium transport by stimulating aldosterone release, angiotensin II directly enhances sodium reabsorption in the proximal tubule, thick ascending limb of Henle’s loop, and the collecting duct. These segments contain specific angiotensin II receptors (AT1 receptors) that, when activated, increase Na+ transport. In the proximal tubule, angiotensin II stimulates Na+ uptake through the apical Na+/H+ exchanger and the basolateral Na+(HCO3−)3 co-transporter and Na+,K+-ATPase. Angiotensin II also increases expression of the apical Na+/H+ exchanger and the Na+,K+,2Cl− transporter in the thick ascending limb. In the collecting duct, angiotensin II enhances Na+ transport via the ENaC.
In some species, antidiuretic hormone (ADH, vasopressin), which is released when an animal is volume depleted, dehydrated, or hypotensive, enhances salt reabsorption from the thick ascending limb and the collecting duct. The increased salt transport partly results from vasopressin-stimulated increases in the apical Na+,K+,2Cl− co-transporter in the thick ascending limb and the ENaC in the collecting duct. Although ADH stimulation of salt reabsorption in the thick ascending limb has the seemingly paradoxical effect of enhancing the dilution of the tubule fluid, this in fact allows maximal salt and water conservation because water reabsorption in the collecting ducts is enhanced (see Chapter 43).
Endothelin is a peptide hormone produced in the kidney in the collecting duct, endothelial cells, and the thick ascending limb of Henle’s loop. Endothelin acts on specific receptors in the collecting duct and thick ascending limb and increases renal NaCl and water excretion by effects on epithelial transport and renal microcirculation, mediated by nitric oxide and prostaglandins. The transport mechanisms that are inhibited include Na+,K+-ATPase and the apical ENaC.
Atrial natriuretic peptide is produced in the cardiac atria and enhances Na+ excretion in the distal tubules and collecting ducts by inhibiting aldosterone release and also by inhibiting vasopressin effects in the collecting ducts.
Calcium Is Reabsorbed in the Distal Nephron and Connecting Segment, Regulated by Parathyroid Hormone, Vitamin D3, and Calcitonin
The kidney reabsorbs the majority of filtered calcium (Ca2+) and contributes significantly to the regulation of systemic Ca2+ balance. Approximately 65% of filtered Ca2+ is absorbed in the proximal tubule; the majority of Ca2+ reabsorption in the proximal tubule is paracellular and passive, driven by electrical and chemical gradients. Approximately 20% of filtered Ca2+ is reabsorbed in the thick ascending limb of Henle’s loop. Ca2+ reabsorption in this segment occurs both by passive, paracellular means, driven by electrochemical gradients, and by active, transcellular transport. Ca2+ transport in the thick ascending limb is suppressed when serum Ca2+ is elevated, through activation of the basolateral calcium-sensing receptor (CaSR). The distal convoluted tubule and the connecting segment reabsorb an additional 10% of the filtered Ca2+, primarily by active transcellular transport. The basolateral plasma membrane of distal convoluted tubule and connecting segment cells contains a Ca2+-ATPase pump that actively extrudes intracellular Ca2+ into the interstitial fluid. Ca2+ is also transported across the basolateral plasma membrane by a Na+/Ca2+ antiporter that exchanges extracellular Na+ for intracellular Ca2+. Ca2+ in the tubule fluid enters the cell across the apical plasma membrane through a Ca2+ channel, and diffusion to the basolateral side of the cell is facilitated by binding to a Ca2+-binding protein, calbindin 28k. Finally, 1% to 2% of the filtered Ca2+ is reabsorbed in the collecting ducts; the mechanisms of Ca2+ transport in the collecting duct have not been determined.
Regulation of Ca2+ transport occurs in the distal convoluted tubule, the connecting segment, and the cortical thick ascending limb of Henle’s loop. Parathyroid hormone, 1α,25-(OH)2–vitamin D3, and calcitonin have important roles in controlling renal Ca2+ excretion.
Hypocalcemia (low plasma Ca2+ level) stimulates parathyroid hormone release, which affects bone, the intestines, and the kidneys to raise the plasma Ca2+ level. The response of the kidney occurs in the cortical thick ascending limb, the distal convoluted tubule, and the connecting segment. Parathyroid hormone (PTH) is believed to increase apical uptake of Ca2+ in these segments by increasing the activity of the apical Ca2+ channel. Furthermore, at least in the distal convoluted tubule, PTH increases the Cl− conductance at the basolateral plasma membrane, which hyperpolarizes the cells (the interior becomes more electrically negative) and thus increases the driving force for Ca2+ entry. PTH also inhibits inorganic phosphate (Pi) reabsorption in the proximal tubule by causing internalization of sodium-dependent Pi transporters (NaPi-2) in the brush border.
The hormone 1α,25-(OH)2–vitamin D3 is converted to its active form in the proximal convoluted tubules; this process is stimulated by PTH. Receptors for vitamin D3 are located predominantly in the distal convoluted tubule and connecting segment, where vitamin D3 increases the cellular content of the Ca2+-binding protein, calbindin 28k, and thus contributes to enhanced Ca2+ reabsorption. Vitamin D3 also increases expression of the NaPi-2 in the proximal tubule and thus enhances phosphate reabsorption.
Calcitonin reduces the serum Ca2+ concentration, largely by stimulating Ca2+ deposition in bone. Although pharmacological doses of calcitonin may enhance renal Ca2+ excretion, physiological doses reduce renal Ca2+ excretion. Calcitonin enhances Ca2+ reabsorption in the thick ascending limb, the distal convoluted tubule, and the connecting segment. It is believed that calcitonin hyperpolarizes cells in these segments and thereby enhances Ca2+ uptake from the tubule fluid.
CLINICAL CORRELATIONS
Glucosuria
History.
A client presents her 10-year-old female miniature schnauzer with the complaint of a dramatic increase in water consumption and urine volume over the previous 2 weeks.
Clinical Examination.
No major abnormalities are found on physical examination. The dog appears alert and is moderately overweight. The urinalysis reveals 4+ glucose (normally negative) and urine specific gravity of 1.030. The plasma glucose level is tested immediately and is 275 mg/dL (normal, 80-120 mg/dL).
Comment.
The dog has diabetes mellitus, which results from a relative or absolute deficiency of insulin secreted from the β cells of the pancreas. The insulin deficiency results in elevated plasma glucose levels. Glucose is freely filtered by the glomerulus and normally is entirely reabsorbed by the proximal tubule. As the plasma glucose level rises, the glucose concentration in the glomerular filtrate rises. When it exceeds the reabsorptive capacity of the proximal tubule (the renal threshold) of approximately 180 mg/dL, glucose appears in the urine (glucosuria). The glucose acts as an osmotic agent and increases the volume of urine excreted. The dog then drinks more water to replace the excessive fluid loss.
Treatment.
Treatment of diabetes mellitus in veterinary patients usually involves the administration of insulin injections two to three times each day, with adjustments of the dose in accordance with frequent evaluations of the plasma or urine glucose values. When the insulin dosage is appropriate, the plasma glucose level is normalized, the glucosuria disappears, and the urine volume and water consumption decrease.
Hypoadrenocorticism
History.
A concerned client presents her 1-year-old spayed female Samoyed with the complaint of severe weakness, inappetence, and vomiting since the previous day.
Clinical Examination.
The dog is lethargic, weak, and extremely dehydrated. The heart rate is normal, but pulses are weak. No other abnormalities are detected on physical examination. Samples of blood and urine are collected immediately for a complete blood count, serum chemistry profile, and urinalysis, and then an intravenous catheter is placed to start volume replacement therapy with a balanced electrolyte solution. The urinalysis is normal, with a specific gravity of 1.025. Abdominal radiographs are normal, but the thoracic radiographs demonstrate a small cardiac silhouette and small thoracic vessels. The serum creatinine level is 2.5 mg/dL (normal, 0.6-1.2 mg/dL), serum K+ level is 6.5 mEq/L (normal, 3.6-5.6 mEq/L), serum Na+ is 129 mEq/L (normal, 141-155 mEq/L), serum Cl− is 97 mEq/L (normal, 103-115 mEq/L), and serum HCO3− is 12 mEq/L (normal, 18-24 mEq/L).
Comment.
The dog has hypoadrenocorticism. The metabolic disturbances result from a deficiency of the mineralocorticoid hormone aldosterone. In a normal animal, aldosterone stimulates Na+,K+-ATPase activity and enhances the apical plasma membrane Na+ and K+ permeability in the connecting segment and the collecting duct, thereby enhancing Na+ reabsorption and K+ secretion. In the absence of aldosterone, Na+ conservation and K+ secretion in these segments are impaired. Cl− and water follow the path of Na+ and also are excreted excessively by the kidney. Hyperkalemia (high serum K+ level) has profound effects on excitable tissue, including nerve and muscle cells, and results in muscle weakness, decreased cardiac output, hypotension, and cardiac arrhythmias. The loss of Na+ and water results in volume depletion and decreased size of the heart and thoracic blood vessels and exacerbates the hypotension and poor tissue perfusion.
Poor perfusion of the kidneys is probably the major cause of the elevated serum creatinine level (azotemia) because inadequate renal blood flow and reduced glomerular capillary hydrostatic pressure prevent adequate glomerular filtration. This is called prerenal azotemia. In most cases of prerenal azotemia the urine is maximally concentrated in an attempt to retain fluid and restore blood volume, but in hypoadrenocorticism, this response is often blunted, possibly by the hyponatremia (reduced serum Na+ level) or by the absence of glucocorticoids, which have been shown to have a permissive effect on maximal urine concentration (see Chapter 43). The decreased serum bicarbonate level indicates metabolic acidosis, which is the result of both the diminished renal ability to secrete H+ and reabsorb HCO3− (see Chapter 44) and the increased production of acid from poorly perfused tissue.
Treatment.
Prompt treatment is important for the animal’s survival because the hyperkalemia and acidosis can cause fatal cardiac arrhythmias. Volume repletion with normal saline and correction of the base deficit (low serum HCO3− level) often stabilizes the animal. Replacement therapy with mineralocorticoid hormones (e.g., desoxycorticosterone acetate, desoxycorticosterone pivalate, fludrocortisone acetate) restores apical Na+ channel and basolateral Na+,K+-ATPase activity and should be started as soon as possible. Frequently, glucocorticoid hormones are given early to treat shock even before the electrolyte status is known; these hormones are beneficial for two reasons. First, hypoadrenocorticism usually results in glucocorticoid deficiency, sometimes manifested by hypoglycemia, and replacement therapy is indicated. Second, the mineralocorticoid activity in many glucocorticoid preparations may be beneficial in correcting the hyperkalemia and hyponatremia.
A definitive diagnosis may be obtained by an adrenocorticotropic hormone (ACTH, corticotropin) challenge test, which maximally stimulates the release of cortisol from the adrenal gland and shows little or no response in animals with hypoadrenocorticism.
Chronic maintenance usually involves oral therapy with fludrocortisone acetate; the proper dosage is determined by periodic evaluations of the serum K+ and Na+ levels. Chronic replacement of glucocorticoids is also recommended.
Brenner BM, ed. Brenner & Rector’s the kidney, ed 7, e-dition, Philadelphia: Saunders, 2004.
Grantham JJ, Wallace DP. Return of the secretory kidney. Am J Physiol Renal Physiol. 2002;282:F1.
Loffing J, Kaissling B. Sodium and calcium transport pathways along the mammalian distal nephron: from rabbit to human. Am J Physiol Renal Physiol. 2003;284:F628.
Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol. 2004;287:F593.
Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol. 2002;282:F777.
Planelles G. Chloride transport in the renal proximal tubule. Pflugers Arch Eur J Physiol. 2004;448:561.
Seldin SW, Giebish G. The kidney: physiology and pathophysiology, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2000.
PRACTICE QUESTIONS
1. Which segment of the renal tubule is responsible for the reabsorption of the bulk of filtered solutes?
2. The main driving force for the reabsorption of solutes from the tubule fluid is:
3. The net rate of reabsorption of a solute in the proximal tubule fluid is determined by the:
4. The ultimate rate of excretion of K+ in the urine is determined by the:
5. Which of the following are effects of aldosterone on Na+ transport in the connecting segment and collecting duct?