Chapter 18 Treatment of Urinary Disorders
Management of Acute Renal Failure
Pathophysiology
Acute renal failure results from a sudden, severe decline in renal function, which may be initiated by prerenal, renal, or postrenal causes. Although several specific renal diseases can cause acute renal decompensation, in veterinary medicine acute intrinsic renal failure is most commonly caused by a nephrotoxicant or an infectious or ischemic injury. The proportionately high blood flow and large capillary surface area in the kidney increase the organ’s sensitivity to blood-borne toxicants. Additionally, the sophisticated transport mechanisms, intensive metabolic activity, and refined concentrating mechanisms found in renal tubules increase the likelihood of toxic injury. Glomeruli, too, are susceptible to direct destruction and immunologic injury. The most common nephrotoxicants encountered in veterinary medicine are ethylene glycol and therapeutic agents such as aminoglycosides, amphotericin B, cisplatin, radiographic contrast agents, and analgesics. Additional nephrotoxins include lilies, which are toxic in cats only, and raisins and grapes, which are toxic in dogs.1
Ischemic injury may result from any insult that compromises perfusion of afferent arteriolar blood flow. Hypoperfusion from shock, dehydration, or hypotension is the most common mechanism of renal ischemia. Trauma, anesthesia, cardiac output failure, and persistent vomiting or diarrhea are potential ischemic events encountered in small animals. Thrombosis, hyperviscosity, and polycythemia are additional, less common disorders that interfere with renal blood flow. Angiotensin-converting enzyme (ACE) inhibitors, widely used in the management of congestive heart failure in dogs and proteinuric disorders, inhibit production of the vasopressor angiotensin II. In the glomerulus angiotensin II blockade preferentially dilates efferent arterioles, which may lead to loss of glomerular capillary pressure and reduction in glomerular filtration (see Chapter 14). The vasodilatory effect is most prominent in diseased or poorly perfused kidneys and can lead to progressive azotemia or overt acute renal failure in treated patients.2,3
The administration of nonsteroidal antiinflammatory drugs (NSAIDs) may inhibit vasodilatory prostaglandin production in the kidneys. The effect of NSAIDs is minimal in healthy kidneys but can be devastating when superimposed on marginally functioning kidneys, hypovolemia, or other vasoconstrictive states (anesthesia, surgery, sepsis, heart failure, liver failure, nephrotic syndrome).4 In these disorders renal blood flow and glomerular filtration rate become increasingly dependent on prostaglandin synthesis; administration of NSAIDs can precipitate renal ischemia and failure. Many systemic diseases increase the risk of acute renal failure by ischemic or vascular mechanisms. These disorders include pancreatitis, hepatic failure, immune-mediated hemolytic anemia, heat stroke, disseminated intravascular coagulopathy, rickettsial disease, babesiosis, and bacterial endocarditis.5,6
Both toxicant and ischemic insults to nephrons lead to impairment of cellular transport mechanisms, cellular swelling, and death. Cellular hypoxia and intracellular calcium overload lead to additional membrane damage and oxygen free radical formation. Vascular congestion and tubular obstruction result from cellular swelling and act as common mechanisms perpetuating renal ischemia and renal failure.7 Therapeutic measures employed in acute renal failure attempt to support renal excretory function, attenuate cellular damage, and favor renal recovery.
General Considerations in Management
Goals of management of established acute renal failure are to (1) treat or minimize underlying disease processes; (2) correct fluid, electrolyte, and acid–base disorders; (3) initiate a diuresis; (4) manage systemic complications; and (5) establish a prognosis. The first principle in managing any disease process is to “treat the treatable.” In acute renal failure “treatable” problems may include dehydration or hypovolemia, postrenal obstruction, cardiac or hepatic disease, leptospirosis, rickettsial disease, bacterial endocarditis, pyelonephritis, hypercalcemia, renal lymphoma, and hemoglobinuria. Early recognition of potential toxicant-induced renal failure allows for treatment with specific antidotes (e.g., 4-methylpyrazole or ethanol for ethylene glycol) or with nonspecific measures such as gastric lavage, fluid therapy, and cathartics. Administration of any potentially nephrotoxic agents should be stopped.
Fluid Therapy
After identification of underlying disorders, the management of acute renal failure relies largely on management of fluid, electrolyte, and acid–base imbalances. Fluid deficits are estimated (estimated percentage dehydration × body weight in kilograms = liters required) and replaced rapidly, within 4 to 6 hours. Initial fluid choices include 0.9% saline or other replacement solutions. Low-sodium fluids such as 0.45% saline/2.5% dextrose or half-strength lactated Ringer’s solution in 2.5% dextrose may be used for patients with cardiac insufficiency or hypernatremia. Fluids for maintenance requirements (40 to 60 mL/kg per day) and ongoing losses (polyuria, vomiting, diarrhea) should be added to the daily fluid total. In most cases rehydration fluid requirements will equal two to three times maintenance requirements; careful calculation of deficits and ongoing needs is recommended to prevent underestimation of fluid needs (Table 18-1).
Urine output should be measured during the rehydration phase to document appropriate diuresis and to calculate future fluid requirements. After adequate volume replacement, urine output should reach at least 1 to 2 mL/kg per hour. Oliguric patients, in which urine output is less than 1 mL/kg per hour, require additional treatment. If the animal is not overhydrated, mild volume expansion may be considered. Administration of an additional 3% to 5% of the animal’s body weight in fluid should eliminate any remaining, undetected volume deficits and enhance renal perfusion and glomerular filtration rate (GFR).8 If volume expansion is attempted, the patient must be carefully monitored for signs of overhydration, including inappropriate weight gain, systemic hypertension, increased bronchovesicular sounds, tachypnea, tachycardia, restlessness, chemosis, and serous nasal discharge. Note that dry mucous membranes can be a consequence of uremia and are not good indicators of hydration status in acute renal failure.9 Appropriate volume expansion is documented by a modest increase in body weight and modest reductions in the hematocrit and plasma protein concentrations. Volume overload is a common complication of fluid therapy in oligoanuric renal failure patients. Roughly two thirds of dogs and cats referred for hemodialysis management of uremic crises are hypervolemic. Without dialytic support this complication can be difficult to reverse unless urine production increases.9
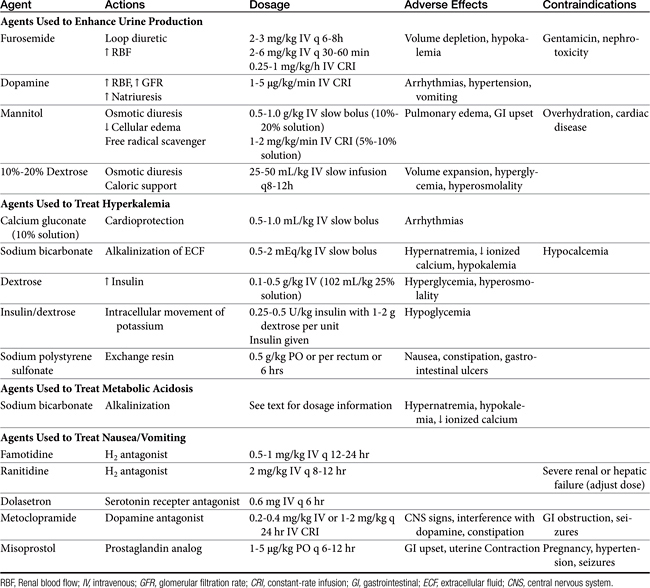
Methods to Enhance Urine Production
If urine production remains poor after rehydration and volume expansion, pharmacologic manipulation of oliguria is warranted. Furosemide, dopamine, and osmotic diuretics have been standard options for management of oliguric and anuric renal failure despite a lack of clinical studies confirming efficacy (see Chapter 17 and for dopamine, see Chapter 14). Despite the lack of proven clinical efficacy, furosemide (2 to 3 mg/kg intravenously every 6 to 8 hours) is often chosen as an initial treatment for oliguria because it is readily available and easy to administer. A constant-rate infusion (CRI) of furosemide (1 mg/kg per hour) has also been recommended.8,10
As a loop diuretic, furosemide helps increase tubular flow and improve renal blood flow but does not significantly affect GFR.7 It is also speculated that the activity of furosemide may protect cells of the thick ascending loop of Henle by reducing active transport at this site. Furosemide may be useful in managing overhydration and hyperkalemia and enhancing toxin elimination in acute renal failure.9 Furosemide has been shown to exacerbate gentamicin toxicity and should be avoided in patients recently treated with aminoglycosides.11 If urine output does not increase in 30 to 60 minutes, furosemide may be repeated at 4 to 6 mg/kg intravenously at 30- to 60-minute intervals; concurrent dopamine administration should also be considered. The efficacy of furosemide in reversing oliguria appears to be improved with the concurrent administration of dopamine.12 Dopamine in this instance may improve delivery of furosemide to sites of activity. If diuresis is established with furosemide administration, intravenous bolus doses may be repeated every 6-8 hours or a CRI can be maintained at 0.25 to 1 mg/kg/hr.9 In healthy Greyhounds a furosemide infusion resulted in more diuresis, natriuresis, and calciuresis and less kaliuresis than bolus doses.13 High-dose furosemide did not increase survival rates in people with acute renal failure in a prospective, double-blinded, randomized placebo-controlled trial.14 An influence on survival in cats and dogs has not been established. Extracellular fluid volume and potassium requirements should be carefully addressed during furosemide treatment.
Dopamine is a catecholamine (a norepinephrine precursor) that in low doses causes increases in renal blood flow. In dogs dopamine acts at specific splanchnic and renal receptors to cause efferent arteriolar vasodilation, enhancing renal blood flow and sodium excretion. Dilation of mesenteric, coronary, and intracerebral vascular beds also is expected. Effects on GFR are modest.15 In cats dopamine appears to stimulate α-adrenergic receptors, leading to increased blood pressure and natriuresis.16 Dopamine must be administered as a CRI, ideally with an automated fluid infusion pump. Dopamine is administered diluted in nonalkaline fluids, usually normal saline or dextrose solutions. Infusion rates of 1 to 5 μg/kg per minute are recommended. Infusion is usually started at 1 to 2 μg/kg per minute while the patient is monitored for changes in heart rate or rhythm. Tachycardia, ectopic or premature ventricular beats, nausea, vomiting, and hypertension are adverse effects, predominantly seen at higher doses. The pressor effects of dopamine are variable and can be detrimental to renal function; monitoring of urine output and degree of azotemia is imperative in individual patients. The half-life of dopamine is approximately 2 minutes; effects are withdrawn within 10 minutes after the infusion is discontinued. The drug is metabolized to inactive compounds by monoamine oxidase and catechol-O-methyltransferase in the kidney, liver, and plasma.17 Recent prospective, randomized, double-blinded, placebo-controlled trials in humans have failed to show efficacy of low-dose dopamine in management of acute renal failure. Dopamine may also cause detrimental gastrointestinal, respiratory, endocrine, and immunologic effects.18 Use of dopamine for management of acute renal failure has fallen out of favor, except as needed for pressor response.
Fenoldopam and other new selective dopamine subtype DA-1 receptor agonists may more effectively increase renal blood flow in dogs8 and possibly in cats. Results of these selective dopaminergic compounds have not been reported in clinically affected patients in acute renal failure.
KEY POINT 18-3
The primary benefit of diuretic administration is to increase urine production and simplify management. Minimal, if any, benefits on glomerular filtration rate or survival are likely.
Osmotic diuretics currently represent the optimal pharmacologic option for enhancing urine flow. Osmotic agents such as mannitol enhance urine production by increasing both intravascular volume and tubular fluid flow. Mannitol is freely filtered at the glomerulus and poorly reabsorbed in renal tubules, creating an osmotic effect such that water is not reabsorbed from the tubular lumen. Osmotic agents also prevent tubular and vascular obstruction by minimizing cellular swelling. Mannitol also possesses weak renal vasodilatory and cellular free radical scavenging actions.19 Adverse effects of mannitol infusion include volume overload and pulmonary edema, gastrointestinal upset, and central nervous system effects (usually at high doses). The drug is contraindicated for overhydrated or dehydrated patients and for patients with preexisting cardiac disease or suspected intracranial hemorrhage. Mannitol (20% to 25% solution) may be administered at a dosage of 0.5 to 1.0 g/kg intravenously as a slow bolus (over 15 to 20 minutes).20 Another protocol entails administration of partial dosages (0.5 mg/kg each) every 15 minutes for three treatments.21 Urine output should improve within 1 hour. A second bolus may be attempted if the agent is unsuccessful, but the potential for volume overexpansion and edema formation increases. When mannitol is beneficial, intermittent bolus injections (0.5 to 1 g/kg intravenously every 6 to 8 hours) or CRI of a 5% to 10% solution (2 to 5 mL/min) may be given up to 2 g/kg per day.13 Lower doses given more frequently (0.25 to 0.5 g/kg intravenously every 4 hours) or a CRI of 1 to 2 mg/kg/min also have been recommended.9 One author recommends maintaining diuresis with an infusion of mannitol diluted in lactated Ringer’s solution.8
Hypertonic dextrose solutions have been useful as an alternative osmotic agent. Once the renal threshold for glucose transport has been exceeded, dextrose solutions create effects similar to those of mannitol on tubular flow and urine output. Solutions of 10% or 20% dextrose are formulated and administered as intermittent slow boluses of 25 to 50 mL/kg (over 1 to 2 hours) two or three times per day. The initial infusion rate may be as high as 2 to 10 mL/min in order to rapidly create hyperglycemia. The infusion rate may subsequently be dropped to 1 to 5 mL/min.21,22 Advantages of dextrose solutions include low cost, availability, and relative safety. Dextrose solutions also provide nominal caloric supplementation. Urine glucose is easily monitored to ensure that sufficient hyperglycemia and filtration of glucose are continuing; urine volume still must be quantitated because glycosuria can occur without significant increases in urine production. Dextrose solutions may be inferior to mannitol in other respects, however, because the osmotic effects on cellular swelling and tubular obstruction will be minimized by intracellular equilibration of glucose across cell membranes, an effect that does not occur with mannitol.8 Hypertonic glucose also lacks the vasodilatory and free radical scavenging effects of mannitol. The rapid movement of glucose intracellularly does, however, minimize the potential development of vascular overload and pulmonary edema.
For patients who become fluid overloaded and have decreased renal output, fluid removal is an essential part of management. Standard treatment in human medicine that is also available for veterinary patients includes peritoneal dialysis and hemodialysis. A promising new treatment is cross-linked polyelectrolyte sorbents. This oral solution can absorb as much as 50 times its weight in gastrointestinal water (up to a liter of fluid in a 30 kg dog) as well as urea, creatinine, and potassium, allowing an alternative to renal excretion of excessive fluid and solutes.23
The choice of initial treatment protocol for oliguria varies with clinician preference, experience, available technical support, and patient variables. Furosemide or dopamine (or both) historically were employed initially, but this practice must be critically assessed because dopamine may actually cause detrimental effects. Mannitol is probably, however, the preferred agent for treatment of nephrotoxic and ischemic renal failure in patients that are not overhydrated. If one protocol is ineffective, another protocol may be attempted. Polyuric renal failure generally is easier to manage and has a better prognosis than oliguric renal failure. The effects of all measures to reverse oliguria appear to diminish as the duration of oliguria is prolonged.
Management of Hyperkalemia and Metabolic Acidosis
Patients with acute renal failure may be hypokalemic, normokalemic, or hyperkalemic. Hyperkalemia is most likely observed with oliguric or anuric renal failure. Management of hyperkalemia and other electrolyte disturbances is ideally based on serum electrolyte determinations; however, an estimate of potassium status can often be made on the basis of an electrocardiogram. Administration of potassium-free fluids and initiation of a diuresis is usually sufficient to correct mild to moderate hyperkalemia. Longer-term control of mild hyperkalemia may be gained with exchange resins. Sodium polystyrene sulfonate (Kayexalate) is given as a suspension in 20% sorbitol (2 g/kg/day by mouth or by rectum in 3 to 4 divided doses). Nausea, constipation, and gastrointestinal ulceration or erosion are possible complications of this product and have limited its tolerance in veterinary patients.9
Peaked T waves, bradycardia, prolonged PR intervals, flattened P waves, and widened QRS complexes may be seen with moderate elevations in serum potassium. Severe hyperkalemia may result in a loss of P waves, idioventricular rhythms, atrial standstill, or ventricular fibrillation and represents a life-threatening emergency. With severe electrocardiographic changes, administration of calcium gluconate (0.5 to 1 mL/kg of a 10% solution given intravenously over 10 to 15 minutes) offers cardioprotective actions. Calcium ions counteract potassium without lowering serum potassium; other measures must be initiated to prevent subsequent cardiac toxicity.24
Bicarbonate administration facilitates an intracellular shift of potassium ions and is another useful initial treatment for moderate hyperkalemia. Sodium bicarbonate is administered as a slow intravenous injection of 0.5 to 2 mEq/kg.24 Alternatively, bicarbonate deficits can be determined on the basis of serum bicarbonate, total CO2, or base deficit measurement. The deficit is calculated by the formula: 0.3 × body weight (kg) × base deficit or (20 − serum bicarbonate or total CO2 concentration). A portion of the deficit (usually one fourth or one half) is given as a slow bolus or in fluids, and the acid–base status is reassessed. An advantage of sodium bicarbonate administration is concurrent correction of coexisting metabolic acidosis. In the absence of hyperkalemia, bicarbonate administration is reserved for severe acidosis (blood pH <7.2 or total CO2 <12 to 15 mEq/L). Overzealous bicarbonate administration may have serious detrimental results, including hypernatremia, hyperosmolality, ionized calcium deficits, reduced plasma potassium concentrations, metabolic alkalosis, and paradoxical acidosis of the cerebrospinal fluid.
An alternative method of therapy for acute hyperkalemia includes the administration of glucose (dextrose 0.1 to 0.5 g/kg as a 20% solution or 1 to 2 mL/kg 50% dextrose diluted to 25%).20 Administration of glucose triggers endogenous insulin secretion; both glucose and insulin facilitate intracellular movement of potassium. Protocols utilizing insulin and glucose (0.25 to 0.5 U/kg insulin followed by 1 to 2 g glucose per unit of insulin administered) have also been recommended. Exogenous insulin administration can promote hypoglycemia; blood glucose monitoring is required.
Disorders of calcium are occasionally found in acute renal failure. Hypercalcemia may be a cause of acute renal damage. Calcium levels usually drop with fluid or diuretic administration; investigation into the etiology of hypercalcemia should proceed, however. Severe hypocalcemia is rare except in ethylene glycol intoxication. Alkalinizing therapy may further reduce ionized calcium levels, resulting in symptomatic hypocalcemia. Calcium administration may be necessary in some cases.
Maintenance Fluid Therapy
Once a diuresis has been established, and in cases of nonoliguric acute renal failure, fluid therapy should be tailored to match urine volume and other sensible and insensible losses. Insensible losses (e.g., water lost from respiration) are estimated at 13 to 22 mL/kg per day. Urine output (the most variable sensible loss in patients with renal failure) is quantitated during 6- or 8-hour intervals; the amount lost is replaced during an equivalent period. Ongoing gastrointestinal losses also are estimated and replaced. Some clinicians factor in a 3% to 5% estimate to provide for subclinical dehydration each day, regardless of physical examination findings (except for signs of overhydration). Intervals can be extended as the animal is stabilized.
Fluid composition during maintenance therapy should be tailored to the individual. Polyionic replacement solutions that provide buffering activity and electrolyte replacement (e.g., lactated Ringer’s solution, Normosol-R, Plasma-Lyte 56) may be administered during the first few days of treatment, especially if gastrointestinal or electrolyte losses are great. For longer-term therapy, lower sodium solutions designed to meet maintenance fluid needs (e.g., half-strength lactated Ringer’s solution or 0.45% saline in 2.5% dextrose, Normosol-M, or Plasma-Lyte 56) are preferred, insofar as most ongoing losses will consist of free water losses in polyuria.8 Alternating administration of 5% dextrose solutions with high-sodium replacement solutions may also be effective in preventing hypernatremia in patients requiring long-term fluid therapy.21 Potassium supplementation in excess of amounts supplied in commercial fluids is usually required during the maintenance phase of treatment; a total of 20 to 30 mEq KCl per liter of fluid administered is typically sufficient.
Other Considerations
Multiple complications may be encountered during the course of treatment of acute renal failure. Complications are usually a result of uremia and include oral ulceration, vomiting, diarrhea, malnutrition, infection, hemorrhage, anemia, hypertension, and neurologic deterioration. Most of these complications are best ameliorated by minimizing azotemia. Anorexia and vomiting are typically due to activation of the chemoreceptor trigger zone, uremic gastritis, and mucosal intestinal ulceration. Management of gastrointestinal complications of uremia is described in the later discussion of chronic renal failure. Note that metoclopramide and histamine-2 receptor blockers such as ranitidine and famotidine are renally excreted; doses should be modified for severely uremic animals. Aggressive nutritional support may be required in patients with acute renal failure undergoing long periods of treatment. A diet providing 2 to 3 g protein/kg per day and 70 to 110 kcal/kg per day is optimal for critically ill patients with renal failure. A reduced protein diet is designed to minimize uremia and acidosis associated with acute renal failure. In recovering, mildly azotemic patients, a high-protein diet may enhance renal recovery.25
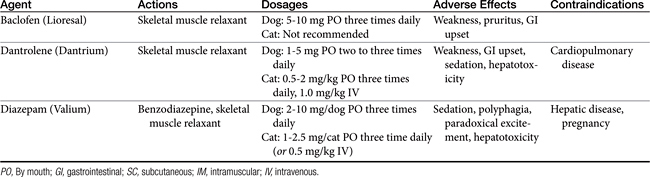
Although often considered a hallmark of chronic disease, anemia may become severe in the course of acute renal failure because of depressed erythropoiesis and hemorrhage. Transfusion support and attention to gastrointestinal or generalized hemorrhage may be needed. Careful attention to aseptic care of intravenous catheters, urinary catheters, and wounds is important to prevent infection in patients with acute renal failure. Neurologic disturbances, including ataxia, stupor, tremors, head bobbing, and seizures, may be observed in animals with severe anemia. Neurologic symptoms may be attributed to hypocalcemia, uremic encephalopathy, cerebral edema, dialysis, or an underlying toxicant (e.g., ethylene glycol). Resolution of uremia, control of hypocalcemia, or administration of low-dose diazepam may be required for management.
Cellular Protectants
Many agents have been investigated as potential cellular protectants or stimulants of cellular regeneration in acute renal failure. Agents such as magnesium, adenosine triphosphate (ATP), thyroxine, and glycine have been considered for their potential to restore intracellular energy stores. Atrial natriuetic peptide (ANP), brain natriuetic peptide (BNP), and other similar peptides increase GFR and have renoprotective effects in ischemic renal injury. ANP has caused severe hypotension in patients with renal failure, but BNP appears to increase GFR without causing systemic hypotension.26 Oxygen free radical scavengers and calcium channel blockers have been investigated as methods of alleviating reperfusion injury in renal epithelial cells. Growth factors may promote cellular repair and regeneration. Most of these agents remain in the experimental stages, however, and have found limited clinical application in human or veterinary medicine. Manipulation of the cell biology of acute renal failure is likely to provide therapeutic options in the future, however.27,28
Management of Chronic Kidney Disease
Many varied insults can lead to progressive renal dysfunction in small animals. Infectious diseases, obstructive disorders, hypercalcemia, glomerular disease, and some neoplastic disorders may be identified and specific treatment pursued. In many cases, however, a specific etiology is not determined, and management is directed toward alleviation of clinical signs; correction of metabolic consequences; and, ideally, slowed progression of the disease process. Principles of medical management are to (1) stage the disease and pursue appropriate diagnostic strategies, (2) consider renoprotective maneuvers that may retard progression of renal damage, (3) identify and manage sequelae of renal failure (hypertension, anemia, metabolic acidosis, and gastrointestinal ulceration), (4) intervene as necessary in crises, and (5) plan and initiate appropriate monitoring and follow-up evaluations. Excellent reviews of staging criteria and management considerations are available,29 and updated guidelines are available from the International Renal Interest Society (IRIS) at iris-kidney.org. In general, dietary and other therapeutic maneuvers should be instituted in a stepwise approach, with serial monitoring implemented to tailor management (Figure 18-1).
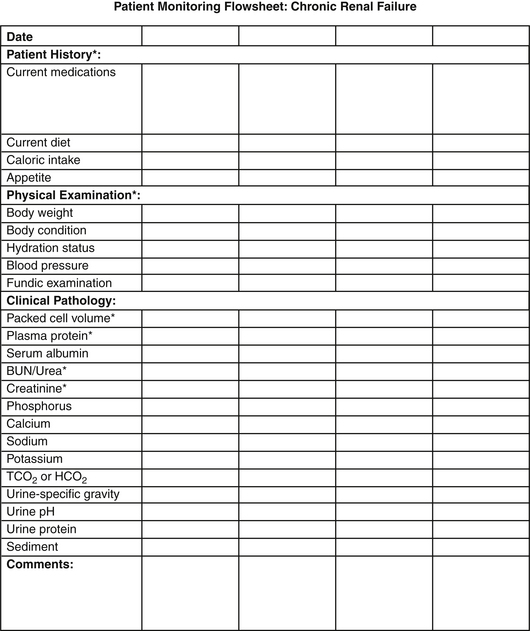
Figure 18-1 Example of a flowsheet for monitoring the clinical and clinicopathologic features of chronic renal failure.
Pathophysiology
The clinical and pathophysiologic consequences of renal disease result from complex events set into motion as excretory, homeostatic, and other renal functions are lost. When approximately 66% of total nephron mass is lost, fluid excretion per nephron is increased to facilitate waste excretion. Solute diuresis in remaining nephrons and developing tubular dysfunction lead to polyuria and compensatory polydipsia. As nephron loss progresses to 75% or greater, excretory function is compromised and azotemia develops. With progressive reduction in GFR, excretion of phosphorus and endogenous acids is impaired, leading to hyperphosphatemia, hypocalcemia, metabolic acidosis, and secondary hyperparathyroidism. Diseased kidneys also fail to produce or regulate other important metabolic and endocrine compounds, leading to systemic hypertension, anemia, and a catabolic state. Widespread polysystemic effects of uremia are possible as well, affecting gastrointestinal mucosa, neuromuscular function, cardiopulmonary function, and immunologic function. Management strategies are designed to blunt these effects of progressive renal dysfunction (Table 18-2).
Dietary Strategies
Protein
Reduction in protein intake (compared with protein content of maintenance commercial dog foods) has been advocated for dogs and cats with renal disease. Dietary protein restriction has been advocated on the basis of the hyperfiltration theory of progressive renal disease.30 In rats with induced renal disease, the compensatory response of remaining nephrons includes increases in single nephron blood flow, single nephron filtration rate, and elevated glomerular capillary pressure.31 These responses are ultimately detrimental in rodent models, leading to progressive renal injury,30 an effect that can be blunted by reduced protein diets that minimize glomerular hypertension.
Although glomerular hypertension and hypertrophy occur in dogs with experimental renal disease,32 a significant effect of protein restriction alone on the course of renal failure in dogs or cats has not yet been demonstrated.33-36 Reduction in protein intake is undeniably beneficial in moderately to severely affected patients by reducing the production of nitrogenous wastes and acid by-products that contribute to uremia and metabolic acidosis. In such patients (usually with blood urea nitrogen >60 to 75 mg/dL or mmol/L), moderate restriction of protein intake can be expected to reduce blood urea concentrations, alleviate metabolic acidosis, and indirectly minimize phosphorus intake.37 Recommended dietary protein intake for initial management is 2 to 3.5g/kg per day in dogs. This level is generally provided by diets containing high biologic value protein at approximately 13% of gross energy when fed at maintenance caloric requirements.37 The protein requirement for dogs in renal failure is higher than the minimum protein requirements for healthy dogs; however, most commercial maintenance diets are 20% to 30% protein. In cats protein requirements are 3.5 to 4.0 g/kcal per day and may be provided by diets containing approximately 21% of gross energy as protein.37 Products that provide high-quality protein in homemade diets include eggs, liver, cottage cheese, and lean meats.
Further reduction in protein intake should be reserved for refractory patients in which signs of uremia persist on the previously described diet. Excessive protein restriction may lead to protein malnutrition, hypoalbuminemia, and anemia. Protein or other nutrient deficiencies can inadvertently develop if adequate quantities of a moderately restricted diet are not consumed; intake of adequate energy should take precedence in dietary formulations. Protein depletion also may adversely affect renal function by contributing to alterations in renal hemodynamics and accentuating muscle catabolism, anemia, and acidosis. Animals with protein malnutrition exhibit weight loss, poor hair coats, and muscle wasting. Although reduced protein intake may improve clinical signs of renal disease, this dietary maneuver is unlikely to prevent renal disease in normal animals, dramatically slow progression of renal disease, or enhance renal function. Thus the role of reduced protein intake in animals with early renal disease is less clear. Again, moderate restriction of protein may be appropriate in these individuals, with regular monitoring for evidence of protein malnutrition and for progression of azotemia.
Phosphorus
Restriction of dietary phosphorus is advocated for patients with renal failure to minimize hyperphosphatemia, secondary hyperparathyroidism, and dystrophic mineralization. Feeding to maintain a calcium × phosphorus solubility product below 60 to 70 is recommended to minimize soft tissue and renal mineralization. In experimental studies in dogs with induced renal disease, phosphorus and calcium restriction improved survival times but did not prevent renal mineralization.38 In a prospective clinical study in cats, feeding a phosphorus- and protein-restricted diet, and administering phosphorus binders if needed, improved survival times from a mean of 383 days to 616 days.39 Cats fed the renal diet also had reduced plasma urea and phosphorus concentrations when compared with cats fed other diets.
Although phosphorus restriction can be advocated more reliably and earlier than protein restriction, most diets formulated for renal disease are restricted in both protein and phosphorus content because meat proteins are the primary source of phosphorus in the diet. Appropriate canine diets are 0.13% to 0.28% phosphorus on a dry weight basis, providing 0.3 to 0.5 mg phosphorus/kcal, whereas feline diets are approximately 0.5% phosphorus, providing 0.9 mg phosphorus/kcal.40 Supplemental phosphate-binding agents may be required if dietary restriction is inadequate to minimize hyperphosphatemia and normalize the calcium × phosphorus solubility product (see later discussions of dietary supplements and secondary hyperparathyroidism).
Sodium
Moderate sodium restriction is beneficial for dogs with renal disease, particularly those with systemic hypertension. Although single nephron adaptive responses are remarkably efficient for maintaining solute and water balance in renal disease, handling of large fluid and solute loads is limited, and conservation of water and solute is impaired. Sodium excretion increases with declining GFRs to maintain homeostasis; however, response to a sodium challenge may be impaired, and excess sodium intake could lead to volume expansion. Conversely, sodium cannot be maximally conserved in the presence of acute restriction in intake or volume depletion. Diets should provide 15 to 50 mg/kg per day, usually 0.1% to 0.3% on a dry matter basis.41 Changes in sodium intake should be made gradually if possible to prevent rapid changes in fluid homeostasis and extracellular fluid volume. A recent study of the effects of dietary sodium intake on renal function in normal cats and cats with experimentally induced renal disease indicated that low sodium intake may actually contribute to hypokalemic nephropathy and progressive renal injury in cats.42 More research must be done to determine the optimal level of sodium intake for cats with renal insufficiency or failure.
Lipids
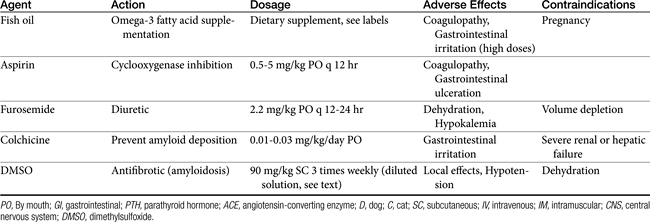
Abnormalities of lipid metabolism in renal disease may lead to hypercholesterolemia, hypertriglyceridemia, and elevated low-density lipoprotein concentrations. High saturated fatty acid intake has been shown to accelerate glomerulosclerosis and progressive renal injury in rat models. Dietary lipid composition may be manipulated to minimize hypercholesterolemia, minimize inflammation, and protect renal hemodynamic function. In one study of dogs with induced renal failure, dogs fed a diet supplemented with omega-3 polyunsaturated fatty acids (provided by menhaden fish oil) had reduced intraglomerular pressure, reduced proteinuria, and better indices of renal function than dogs supplemented with omega-6 polyunsaturated fatty acids.43 Supplementation of omega-3 polyunsaturated fatty acids may be expected to favor vasodilatory eicosanoid production, inhibit intrarenal platelet aggregation, and minimize systemic and glomerular hypertension. Whether any appreciable effect of dietary lipid manipulation will be seen over the long-term course of chronic renal failure remains unknown.43,44
Energy
Appropriate caloric intake is a frequently overlooked goal of dietary management of renal failure patients. A catabolic state may be perpetuated despite the best manipulations of dietary content if sufficient calories for body energy requirements are not ingested. Energy depletion and protein malnutrition in turn exacerbate azotemia and hamper renal compensatory or regenerative responses. Energy requirements for patients with chronic renal failure have been estimated at 60 to 110 kcal/kg per day; a reasonable starting point is 75 kcal/kg per day.31 Frequent monitoring of body weight and body condition is imperative to ensure that appropriate weight is maintained.
As renal failure progresses, intake of appropriate calories becomes more important than the composition of the diet. Caloric supplements composed of fat and carbohydrate sources may be offered to provide additional energy. Occasionally, obese patients with renal disease are encountered. Because obesity may contribute to systemic hypertension and impair other organ system functions, weight reduction is desirable in these patients. Adjustments in weight must be gradual, however, and excessive restriction of calories and protein intake should be avoided.
Dietary Supplements
Intestinal Phosphate-Binding Agents
If dietary phosphorus reduction is ineffective in maintaining a serum phosphorus level of less than 6 mg/dL and a calcium × phosphorus solubility product less than 70, phosphorous-binding agents may be administered.45 These agents are generally ineffective if dietary phosphorus is not restricted concurrently and are most effective when given just before or with a meal. Liquid or encapsulated preparations are preferable to tablet forms because they more readily mix with ingesta in the intestinal tract. These agents prevent absorption of ingested phophorus or phosphorus secreted in saliva, bile, or intestinal fluid. Tablet forms can be crushed and given with food. Aluminum-based products (aluminum hydroxide, aluminum carbonate) are widely available and are administered at daily dosages of 30 to 100 mg/kg divided into two or three feedings.46 Magnesium-based products should be avoided in patients with renal failure.45
Calcium-based products (calcium acetate, 60 to 90 mg/kg per day; calcium carbonate, 90 to 150 mg/kg per day) are alternative phosphorus-binding agents with additional alkalinizing effects. Calcium-based products can also be used to minimize or correct hypocalcemia. Calcium acetate is recommended in normocalcemic to mildly hypercalcemic patients, insofar as calcium carbonate is more likely to lead to hypercalcemia. Calcium-based products and aluminum-based agents also may be administered concurrently for added phosphorus-binding effects.37,45 Serum calcium and phosphorus concentrations should be monitored every 2 weeks initially, then monthly or as needed during chronic therapy. Adverse effects of phosphorus-binding agents include nausea, gastrointestinal upset, constipation, and hypophosphatemia. Toxic effects of aluminum are theoretically possible with long-term administration, including anemia, encephalopathy, and osteomalacia.47 Aluminum toxicity appears to be unlikely in dogs and cats.
Alkalinizers
Although dietary protein restriction helps reduce acid metabolites and metabolic acidosis, alkalinization therapy may be required in animals with moderate to severe metabolic acidosis. Chronic untreated metabolic acidosis may accelerate protein catabolism and azotemia, promote renal ammoniagenesis contributing to progressive renal tissue damage, and lead to increased calcium and potassium losses. Acid–base derangements also likely contribute to the clinical manifestations of renal failure, including anorexia, vomiting, and weight loss.48,49 Alkalinizing therapy is ideally planned on the basis of serial blood gas analyses. Serum total CO2 (Tco2) measurement is a reasonable guide to management in most patients. Oral alkalinization is recommended when bicarbonate or Tco2 measurements fall below 15 to 17 mmol/L, whereas parenteral supplementation may be needed if the Tco2 falls below 10 to 12 mmol/L.
Alkalinizing agents include potassium citrate (35 mg/kg orally every 8 hours or 0.3 to 0.5 mEq potassium/kg orally every 12 hours) and calcium carbonate or calcium acetate (100 mg/kg daily).31,50 These agents are particularly valuable when hypokalemia (potassium citrate), hyperphosphatemia, or hypocalcemia (calcium-based agents) is a concurrent problem (discussed elsewhere). Oral sodium bicarbonate may be administered at 8 to 12 mg/kg every 8 to 12 hours (1 mEq/cat every 8 to 12 hours for cats). Household baking soda supplies approximately 4000 mg bicarbonate per teaspoon (or 12 mEq bicarbonate/g); 5- and 10-grain tablet preparations are also available. Alternatively, a 1 mEq/mL solution of bicarbonate can be prepared by adding 5 or 6 tablespoons of baking soda to 1 L of water (or one third of an 8 ounce box is added to 1 quart of water).48 Because of the added sodium intake, sodium bicarbonate may be inadvisable in hypertensive patients, and some clinicians prefer to use alternative alkalinizing agents in all renal failure patients. It is questionable, however, whether the sodium salt in sodium bicarbonate contributes to hypertensive disease in dogs and cats.
Dosages of all alkalinizing agents may be titrated to effect. The goal of treatment is to modify bicarbonate concentrations to approximately 18 to 24 mmol/L. Overcorrection of acidosis can lead to metabolic alkalosis, hypokalemia, or ionized calcium deficits.
Potassium
Renal failure and metabolic acidosis have been identified as risk factors for hypokalemic myopathy in cats.51,52 Increased fractional excretion of potassium is observed, although 24-hour potassium loss is variable. Hypokalemia may be exacerbated by chronic metabolic acidosis, especially in cats fed acidifying diets. Potassium depletion in turn induces acidosis and depresses GFR, intensifying renal disease and potassium loss.52-54 From these observations it has been hypothesized that supplementation of potassium in cats with renal insufficiency may stabilize or improve renal function. A beneficial effect on renal function and overall outcome in chronic kidney disease has not yet been proven; however, potassium supplementation can increase total body potassium somewhat and appears to be well tolerated.
Most commercial renal diets have been adjusted to provide potassium beyond requirements for healthy animals, but cats with mild to moderate hypokalemia (K 3.5 to 4.5 mEq/L) will benefit from potassium supplementation at 2 to 5 mEq/day. Low-dose supplementation (2 mEq/cat per day) may be justified in normokalemic cats to prevent potassium depletion.52 Cats with severe hypokalemia may require intensive replacement with intravenous potassium chloride or increased supplementation (5 to 10 mEq/day). Potassium supplementation may be initiated at 1 to 6 mEq/kg per day if other dietary measures do not correct the hypokalemia.31 Potassium concentration should be carefully monitored in cats and in dogs undergoing fluid diuresis for renal disease. Chronically, dogs with polyuric renal failure also may become hypokalemic but seem more resistant to this sequela of renal disease. Some renal failure diets may in fact oversupply potassium for canine needs.
Potassium supplements are available in powder or liquid form for long-term oral administration. Potassium gluconate powder mixed in food appears to be the most palatable and best-tolerated product; flavored potassium gluconate elixirs also are available. A relatively new flavored gel preparation also may be acceptable to cats. Potassium citrate solution or diluted potassium chloride for injection (dilute 1:1 with water) also may be given orally. Gastrointestinal ulceration, nausea, vomiting, and food aversion may develop with liquid preparations.
Summary of Dietary Recommendations
On the basis of current information, the ideal diet for small animals in mild to moderate chronic renal failure should be moderately reduced in protein, phosphorous, and sodium content; contain high-quality protein sources; be highly digestible; and provide adequate potassium, nutrient, and caloric density. In a recent study, feeding a diet appropriately modified in protein, phosphorus, lipids, and sodium was associated with stable renal function and delayed onset of uremia in dogs with moderate chronic renal failure.55 These dogs had a better perceived quality of life, lower risk of death, and prolonged survival (>13 months) compared with dogs fed a maintenance diet. In these studies other treatments appropriate to the stage and consequences of renal failure were initiated as needed, lending support to the long-term benefits of a carefully crafted and monitored treatment regimen for chronic renal diseases. Similarly, in 45 cats with mild to moderate renal failure followed for up to 2 years, cats fed a renal diet had significantly lower all-cause mortality rates and no uremic crises or renal mortality compared to cats fed a maintenance diet. Cats fed the diet formulated for renal disease also had reduced azotemia and acidosis during the study period.56 The composition and nutrient profiles for commercial renal diets are available in manufacturers’ product information and summarized in a review by Bartges and Brown.57Dietary supplements or homemade diets may be required to meet the needs of individual patients.
Management of Anorexia and Vomiting
The best dietary strategy is ineffective if the patient becomes anorectic, cannot consume adequate calories for energy needs, or is vomiting and intolerant of enteral feeding. Many metabolic consequences of renal failure may affect appetite, including hydration status, severity of uremia, degree of anemia, acidosis, secondary hyperparathyroidism, gastrointestinal complications, and electrolyte imbalances. In the sick, uremic animal, correction of dehydration, acidosis, electrolyte abnormalities, and gastrointestinal complications should be accomplished before attempting to introduce a therapeutic renal diet58 Supplementation of water-soluble vitamins and correction of anemia also may improve appetite. ACE inhibitors, some antimicrobials, and many other therapeutic agents can contribute to anorexia, and their potential benefit should be reviewed critically in intolerant patients.
In anorectic patients with chronic renal failure, a review of previous diets, dietary habits, and drug therapy is advised. As with all dietary changes, new diets should be introduced gradually, and small, more frequent meals may be preferable for many patients. Owners and nursing staff can tailor the feeding schedule and feeding environment to enhance appetite by avoiding hurried, noisy feeding or feeding in close association with painful or stressful procedures. Some animals respond to hand feeding or feeding during petting and socialization, especially in a quiet ward or outside the hospital area. Warming of food, moistening food, and ensuring easy access to food are practical methods of improving acceptance.58 Flavoring agents can also be added, including animal fat, bouillon, clam juice, tuna broth, brewer’s yeast, garlic, butter, or cottage cheese.37 An added benefit of flavored liquids is enhanced fluid intake, although broths high in sodium and phosphorus should be avoided. Supplementation of vegetable oils, margarine, cream, or complex sugars may be used to increase caloric intake.31 If oral ulcers that limit food intake are observed, application of xylocaine gels or cool tea flushes may be used to alleviate pain. Enteral feeding using esophagostomy or gastrostomy tubes are another option for managing chronic renal failure in dogs and cats. They allow easy administration of medications and fluids in addition to providing adequate nutrition to an anorectic or hyporexic patient.
Gastrointestinal effects of uremia include mucosal irritation from nitrogenous waste products, impaired gastrointestinal mucosal barriers, and hypergastrinemia. Central receptors for appetite and nausea also are affected by retained substances and increased parathyroid hormone (PTH) concentrations. Anorexia, vomiting, and diarrhea are common complications of advanced renal failure. In patients with chronic renal failure, sporadic vomiting, nausea, and anorexia may be alleviated by the administration of histamine blockers such as cimetidine (5 mg/kg orally, intramuscularly, or intravenously every 6 to 8 hours), famotidine (0.5 to 1 mg/kg orally every 12 to 24 hours), or ranitidine (0.5 to 2 mg/kg orally every 12 hours). Cimetidine inhibits hepatic metabolism of many drugs, including β-blockers and calcium channel blockers, and should be avoided in patients receiving these drugs. Alternately, administration of a proton pump inhibitor (omeprazole 0.7 to 1.5 mg/kg orally every 12 to 24 hours in cats or 0.5-1 mg/kg orally every 24 hours) may be effective in cases refractory to histamine blockers. The addition of sucralfate, a gastrointestinal mucosal protectant, may be useful for patients with severe gastritis or suspected gastrointestinal hemorrhage. Because sucralfate is most effective in an acidic stomach environment, other antiemetics or antacid medications should be given at least 30 minutes after administration of sucralfate when used concurrently. For refractory vomiting metoclopramide (0.2 to 0.4 mg/kg subcutaneously, intramuscularly, or orally every 8 hours) may be administered to improve gastric emptying and reduce centrally mediated nausea. Dolasetron is a serotonin receptor antagonist that has been used extensively in people to decrease nausea and vomiting associated with chemotherapy and anesthesia. Little published information is available about the efficacy and pharmacokinetics of this drug in dogs or cats, but its use in veterinary medicine is growing. It has potential use in managing nausea and vomiting in dogs and cats caused by chemotherapy; anesthesia; enteritis; and metabolic diseases, including renal failure. A dose of 0.6 mg/kg intravenously or orally every 24 hours is recommended to prevent nausea and vomiting, whereas a higher dose of 1 mg/kg intravenously or orally every 24 hours is recommended for treatment of clinical emesis and nausea. It can be used in combination with other antiemetics, including metoclopramide.59 Ondasetron, another serotonin receptor antagonist, was shown to be about twice as effective as metoclopramide in alleviating nausea and vomiting in uremic human patients.60 Maropitant, a novel synthetic nonpeptide neurokinin type 1 (NK1) selective receptor antagonist, prevents and treats emesis and is licensed for use in dogs. The dose of 1 mg/kg orally or subcutaneously every 24 hours for up to 5 days is recommended for dogs to manage nausea and vomiting caused by a variety of factors, whereas a higher dose is recommend for prevention of motion sickness.61 Maropitant has not been approved for use in cats. One published study concerning its use in cats demonstrated that it is safe and effective in reducing the incidence of vomiting induced in laboratory cats by xylazine administration or motion sickness.62
Misoprostol, a synthetic prostaglandin analog, inhibits gastric acid and pepsin secretion and has a cytoprotective effect on gastric mucosa. The drug may be useful in renal failure–induced gastritis at a dosage of 1 to 5 μg/kg orally every 6 to 8 hours. Transient gastrointestinal upset is a possible adverse effect of misoprostol administration that may be managed by adjusting the drug dosage and giving the drug with food.17 Anorexia or gastrointestinal complications of drug administration must be addressed quickly in patients with renal failure, however, because dehydration and renal decompensation can occur.
Pharmacologic manipulation of appetite also has been attempted in anorectic patients. In the short term, intravenous administration of low-dose diazepam (0.05 to 0.15 mg/kg intravenously) may be successful in reviving appetite or stimulating food intake. Oral administration of benzodiazepines, such as oxazepam, may result in unacceptable sedation. Oral diazepam also has been associated with behavior changes and incidences of hepatic failure in cats. The metabolism of benzodiazepines may be reduced with concurrent administration of cimetidine. Cyproheptadine, dosed at 2 to 4 mg/cat orally every 12 to 24 hours, is an additional appetite stimulant. Other agents, such as anabolic steroids, glucocorticoids, and progestins, are of questionable benefit in stimulating appetite. Glucocorticoids are not recommended for most patients with renal failure because they may promote tissue catabolism, contribute to gastrointestinal ulceration, and result in fluid and sodium retention and glomerular hyperfiltration. Mirtazapine is a noradrenergic and specific serotonergic antidepressant that has been used in dogs and cats for management of nausea and vomiting, although no published data confirm its efficacy and safety.
Management of Systemic Hypertension
In animals with chronic kidney disease, the afferent arteriole dilates, which leads to increased intraglomerular pressure. The kidney is susceptible to hypertensive damage, on account of both elevated systemic arterial blood pressure and intraglomerular pressure. In dogs there is a close association between elevated intraglomerular pressure and progressive renal injury. Systemic hypertension is observed in more than 60% of dogs and cats with renal disease, particularly in animals with glomerular disorders, renal vascular disease, and renal neoplasia.41 Multiple mechanisms may contribute to the development of hypertension in renal failure, including decreased glomerular filtration, impaired sodium and water handling, local activation of the renin–angiotensin–aldosterone system, and impaired production of renal vasodilatory substances. High systolic blood pressure (>163 mm Hg) in dogs at the time of diagnosis of chronic renal failure was associated with increased risk of developing a uremic crisis and dying, compared with dogs that had lower blood pressure.63 Controlling hypertension may decrease the rate of progression of chronic renal failure. Clinical signs of hypertension in small animals are usually manifestations of ocular complications, including blindness, retinal hemorrhages, retinal detachment, and glaucoma, but they may include cardiac failure, neurologic signs, hemorrhage, and effusions. Overt signs are often inapparent, however, and blood pressure recordings should be routinely monitored in patients with renal disease.
Moderate restriction of sodium intake is one step in management of mild systemic hypertension. Dietary sodium content of 0.1% to 0.3% sodium by dry matter is recommended for initial management.41,64 Most commercial “renal” diets provide appropriate sodium content, limiting sodium intake to 10 to 40 mg/kg per day. Sodium restriction should be gradual so as not to precipitate volume depletion. If necessary, additional sodium restriction may be accomplished by feeding homemade diets or diets formulated for cardiac disease.
Pharmacologic manipulation of blood pressure may be indicated in animals with moderate to severe hypertension (systolic blood pressure >180-200 mm Hg), clinical signs attributable to hypertension, or persistent hypertension despite sodium restriction. There is evidence that dogs with renal failure have great variability in blood pressure, and it may be appropriate to initiate antihypertension drugs in dogs with intermittently elevated blood pressure (>160/100 mm Hg).46 The choice of agent must be based on the potential risks and benefits in the individual animal and the clinician’s experience and preference. A variety of agents have been proposed for use in hypertension, including ACE inhibitors and calcium channel blockers, diuretics and β-blockers.
In dogs ACE inhibitors are the first choice for management of hypertension. Inhibition of angiotensin II production leads to decreased aldosterone secretion, decreased blood pressure, efferent arteriolar dilation, and reduced intraglomerular capillary pressure. In glomerular disease ACE inhibitors are helpful in controlling hypertension and minimizing proteinuria. In dogs with experimentally induced chronic renal insufficiency, enalapril was shown to decrease proteinuria and glomerular capillary pressure after 3 and 6 months of therapy, respectively. Dogs receiving enalapril had fewer glomerular and tubulointerstitial lesions than the placebo-treated group.65
KEY POINT 18-7
Angiotensin-converting enzyme inhibitors are the first choice antihypertensive agent for dogs. They are administered at low doses initially and titrated to achieve ideal effect without worsening azotemia or electrolyte disturbances.
Potential risks of ACE inhibitor administration include hypotension; decreased renal perfusion; hyperkalemia; gastrointestinal upset; and, rarely, myelosuppression or seizures. Excessive reductions in renal perfusion and GFR are most worrisome because they may lead to acute decompensation of renal failure. To prevent this complication, administration of ACE inhibitors is initiated at a low dosage while blood pressure, blood urea nitrogen, and creatinine concentration are measured. The drug may be slowly increased to an effective dosage. Starting dosages of enalapril and benazepril are 0.25 to 0.5 mg/kg orally per day. Benazepril is less likely to cause renal damage than enalapril and has been shown to decrease systolic blood pressure in dogs and cats. A dose of 0.5 to 1 mg/kg orally every 24 hours successfully decreased blood pressure in cats with experimentally induced renal disease, without decreasing GFR.66 Irbesartan (5 mg/kg orally every 12 to 24 hours) is an angiotensin II–receptor blocker that will also lower blood pressure in dogs.67 Further research is needed to determine whether all cats and dogs with chronic renal failure or renal insufficiency would benefit from ACE inhibitor therapy. This class of drugs may be renoprotective without lowering systemic blood pressure.
Calcium channel blockers such as diltiazem or amlodipine also are attractive agents for the management of hypertension in patients with renal failure. Amlodipine (0.625 to 1.25 mg/cat daily by mouth) has become the preferred agent for cats.68 In dogs that are nonresponsive to ACE inhibitors or in which the drugs are contraindicated, amlodipine (0.05 to 0.25 mg/kg orally every 24 hours) can be administered. Calcium channel blockers reduce blood pressure by peripheral vasodilatory effects; potency varies with the preparation. Calcium channel blockers increase peripheral resistance, leading to a decrease in blood pressure, but they also dilate afferent renal arteriole, which can be detrimental. There are some concerns about possible detrimental effects of calcium channel blockers, which were associated with exacerbation of renal injury, proteinuria, or both in studies of people and diabetic dogs. Newer classes of calcium channel blockers may offer increased renoprotective effects by dilating both the efferent and afferent renal arterioles.67 Calcium channel blockers also possess cytoprotective qualities that may be helpful in acute or chronic renal damage. Calcium channel blockers are negative inotropes and may cause hypotension, cardiac arrhythmias, and gastrointestinal upset in some patients.
KEY POINT 18-8
The calcium channel blocker amlodipine is the first-line antihypertensive agent for cats.
Diuretics are the mainstay of treatment of early volume-dependent hypertension in human patients; however, they may contribute to dehydration and potassium loss and may be inadvisable for patients with chronic renal failure. The diuretic spironolactone acts by inhibiting aldosterone. Aldosterone has hemodynamic effects that preferentially dilate the afferent arteriole, raising intraglomerular pressure; thus spironolactone and eplerenone (another aldosterone antagonist) may be useful drugs in the management of patients with renal failure.67 Further studies are necessary.
Beta-blockers such as propranolol and atenolol are agents with negative inotropic and vasodilatory actions. Atenolol (2 mg/kg daily for cats, 0.25 to 2 mg/kg daily for dogs) may be preferred over propranolol because of its duration of action and β1-receptor specificity. Atenolol is less likely to cause bronchoconstrictive side effects than propranolol.64 Both diuretics and β-blocking agents appear to be minimally effective in dogs with hypertension of renal failure, although β-blockers may be effective in cats.69 Clinical application now is limited.
Serial monitoring of blood pressure, hydration status, and renal and cardiac function is imperative for the appropriate management of hypertensive disease (reduction to approximately 150 to 170 mm Hg). Weekly blood pressure recordings should be made initially as dietary and then pharmacologic management is initiated. Biweekly or monthly recordings can be continued during maintenance treatment. Refractory hypertension may respond to combination therapy or addition of a direct vasodilator such as hydralazine. Administration of a calcium channel blocker with a β-blocking agent is not recommended because of additive negative inotropic effects. Control of hypertension in patients with renal failure may slow the progression of disease and minimize the ocular, cardiovascular, and neurologic complications that can develop with uncontrolled hypertension.
Management of Secondary Hyperparathyroidism
Hyperphosphatemia, hypocalcemia, and impaired activation of vitamin D metabolites contribute to the development of secondary hyperparathyroidism in animals with renal failure. PTH plays an important role in regulating plasma calcium and phosphorous concentrations through effects on the gastrointestinal tract, kidney, and bone. The primary stimulus for PTH release is a drop in plasma calcium concentration; in renal failure phosphorous retention and hyperphosphatemia may lead to hypocalcemia. Additionally, and perhaps more important, impaired conversion of 25-hydroxycholecalciferol (25-hydroxyvitamin D) to the active 1,25-hydroxycholecalciferol (1,25-hydroxyvitamin D, or calcitriol) by 1-α-hydroxylase impairs gastrointestinal absorption of calcium. Calcitriol also plays an important role in the regulation of PTH by exerting an inhibitory (negative feedback) effect on PTH production and release.
The classic effect of secondary hyperparathyroidism in animals with renal failure is the development of renal osteodystrophy, usually seen as “rubber jaw,” which results from excessive calcium and phosphorous removal from bone. This complication appears to be rare in dogs and cats but may develop with long-standing disease or juvenile renal disease. Secondary hyperparathyroidism has, however, been implicated as a contributor to many other manifestations of uremia in human patients, including anemia, glucose intolerance, hyperlipidemia, encephalopathies, neuropathies, cardiac damage, muscle damage, and immunologic dysfunction. Furthermore, soft tissue mineralization associated with hyperparathyroidism may contribute to the progression of renal failure.
Dietary restriction of phosphorus, administration of phosphorus-binding agents, and calcium supplementation may be sufficient to minimize secondary hyperparathyroidism in early chronic renal failure (see earlier discussions of phosphorus binders and alkalinizers). Supplementation of the active vitamin D metabolite calcitriol may be valuable in further management of hyperparathyroidism and renal failure. Calcitriol acts to enhance calcium absorption in the intestines, enhance reabsorption of calcium in the kidneys, facilitate PTH-mediated removal of calcium from bone, and directly inhibit PTH secretion.70,71 Calcitriol supplementation should help normalize plasma calcium and phosphorous concentrations and minimize the clinical and clinicopathologic effects of secondary hyperparathyroidism.
Calcitriol supplementation has been recommended in low-dose (2.5 to 3.5 ng/kg per day)70 and high-dose (6.6 ng/kg per day)71 protocols. Formulations of 250- and 500-ng capsules are available; other doses may be prepared by special order from compounding pharmacies. Serum calcium concentrations should be normal, and serum phosphorus concentrations should be maintained at less than 6 mg/dL before initiation of calcitriol supplementation. Frequent monitoring of calcium and phosphorus concentrations is required during administration. The first assessment should be completed 7 to 14 days after initiation of treatment, followed by monthly rechecks. Ideally, efficacy of treatment should be assessed by PTH measurements on pooled blood samples obtained before and during the first 6 months of treatment. The major complication of calcitriol supplementation is the development of hypercalcemia. Hypercalcemia can be managed by adjusting calcium-based phosphorus-binding agents used concurrently, reducing the dosage of calcitriol, or discontinuing administration of calcitriol temporarily and reinstituting the drug at a lower dosage when calcium concentrations return to normal.70 If phosphorus-binding agents are required to help normalize serum phosphorus, calcium acetate or aluminum-based binding agents may be preferable to calcium carbonate to minimize the propensity for hypercalcemia.
KEY POINT 18-9
When administered and monitored appropriately, calcitriol appears to improve quality and length of life in dogs with moderate-stage chronic kidney disease.
Calcitriol administration reportedly results in rapid reduction of serum PTH levels, normalization of serum calcium concentrations, and subjective improvement in the general well-being of treated dogs.70 Some investigators advocate its use early in renal failure as a method of improving quality of life in patients with renal failure and as a method of potentially slowing progression of renal disease. However, other investigators reserve calcitriol supplementation for animals in which hyperparathyroidism has been documented.71 Preliminary results from a double-blind randomized controlled clinical trial of calcitriol administration in dogs with spontaneous mild to moderate chronic kidney disease indicate prolonged survival and reduced mortality in dogs receiving calcitriol. PTH measurements and ionized calcium measurements were conducted frequently so that the dosage could be tailored appropriately; effective doses ranged from 0.75 to 5.0 ng/kg/day.72 As with other medical manipulations in patients with renal failure, careful monitoring of treatment is essential, with the potential benefits and possible risks weighed for each individual patient.
Management of Anemia
Progressive, nonregenerative anemia is a common complication of chronic renal dysfunction. Moderate to severe anemia is responsible for many of the clinical signs of renal disease, including apathy, lethargy, weakness, poor appetite, and poor body condition. A number of pathophysiologic mechanisms probably contribute to the anemia observed in renal failure, including depressed erythrocyte production, shortened red blood cell life spans, concurrent chronic inflammatory disease, and blood loss caused by gastrointestinal bleeding.73 Gastrointestinal blood loss and erythropoietin lack appear to be the most important mechanisms of anemia. Dramatic responses to erythropoietin supplementation are seen in some cases.
Recombinant human erythropoietinrh (EPO), a genetically engineered replica of human erythropoietin, became available in the late 1980s and has been used in dogs and cats with anemia of renal failure. Erythropoietin is administered at an initial dosage of 100 U/kg subcutaneously three times weekly until the hematocrit is normalized. A rapid loading phase of 150 U/kg/day for the first week has been recommended for severely anemic (<14% packed cell volume) patients.46 Target hematocrits are 0.37 to 0.45 L/L in dogs and 0.30 to 0.40 L/L in cats. Initial monitoring includes reevaluation of packed cell volume or hematocrit measurements at 7- to 14-day intervals. In most cases rapid, progressive increases in red blood cell count, hemoglobin concentration, and hematocrit are observed, along with improvement in clinical parameters such as appetite, body condition, alertness, and activity.74,75 Lower initial dosages (50 to 75 U/kg) may be used if a slower response is desired, in hypertensive patients, or if the drug is cost prohibitive. If a response is not observed in 3 to 4 weeks, the dosage may be increased incrementally to 125 to 150 U/kg three times weekly. If a poor response is seen, the animal should be evaluated for untreated blood loss, iron deficiency, concurrent inflammatory disease. As target hematocrits are reached, the dosage and frequency of administration are tapered, with required maintenance dosages usually at 75 to 100 U/kg every 4 to 7 days. Follow-up monitoring can then be performed at monthly intervals if a stable clinical course has been observed.
Unfortunately, resistance to rhEPO is observed in some dogs and cats after several weeks of treatment. Anti-rhEPO antibodies are formed in 25% to 50%74 of treated patients, blocking the erythropoietin effect. A rapid decline in hematocrit, red blood cell count, and hemoglobin concentration, along with erythroid hypoplasia of the bone marrow, is observed 4 to 16 weeks after initiation of therapy. Anti-rhEPO antibodies may also interfere with remaining endogenous erythropoietin and result in life-threatening anemia or a transfusion-dependent anemia. Because of the potential development of cross-reacting antibodies, rhEPO treatment is generally reserved for dogs and cats with symptomatic severe anemia, usually when the hematocrit drops below 20% to 25% in dogs or 17% to 20% in cats. Anecdotal evidence suggests that darbepoetin, a second-generation erythropoietin analog with a longer duration of action, may be less antigenic in dogs and cats than rhEPO.
A suboptimal response to rhEPO treatment also may be attributed to depleted iron stores. Iron status, including serum iron concentration and total iron-binding capacity, should be evaluated before initiating treatment and reevaluated monthly or if apparent resistance to treatment is observed. Some authors recommend iron supplementation for all animals treated with rhEPO.74 Ferrous sulfate is given orally at a dosage of 100 to 300 mg/day (dogs) or 50 to 100 mg/day (cats). Gastrointestinal side effects may be minimized by dividing the dose into smaller doses. Iron dextran can be given intramuscularly to ensure administration; however, anaphylaxis and iron overload are possible. Systemic and intrarenal hypertension are additional consequences of rhEPO administration that may develop as a result of the increased red cell volume and adaptive increased peripheral vascular resistance. Initiation of rhEPO treatment is contraindicated for patients with uncontrolled hypertensive disease. Other adverse effects uncommonly observed with rhEPO administration include allergic reactions, fevers, seizures, vomiting, and polycythemia.76
Ideally, recombinant feline and canine EPO would be used in anemic patients with chronic renal failure, but these products are not available commercially. Recombinant canine EPO (rcEPO) was shown to be effective in stimulating erythropoiesis in 19 dogs with anemia caused by chronic renal failure but had less efficacy in dogs with red cell aplasia caused by previous therapy with rhEPO.77
The androgenic effects of anabolic steroids also may stimulate red blood cell production in renal failure patients. Androgens increase renal and extrarenal erythropoietin secretion, stimulate erythroid precursors in the bone marrow, and may stimulate heme synthesis. Testosterone esters, nandrolone decanoate, and stanozol are readily available and inexpensive agents, but they may be controlled substances in some areas. These agents have been recommended for their nonspecific effects on appetite, strength, body condition, and general well-being, effects that are largely anecdotal. Administration of anabolic steroids may promote sodium and fluid retention and cause hepatotoxicity, so their use in animals with renal or cardiac dysfunction is not entirely innocuous. For the most part, rhEPO has replaced anabolic steroids in the management of renal failure.46
Management of Glomerular Disease
Pathophysiology and General Considerations
Glomerular disease is a common cause of proteinuria and progressive renal disease in dogs and is encountered occasionally in cats. In glomerulonephritis (GN) the deposition of immune complexes in glomerular capillary walls initiates a local inflammatory response, including complement activation, activation of the membrane attack complex, chemotaxis of neutrophils and macrophages, and production of oxygen free radicals. Immune complexes may form in circulation in response to numerous antigens or when antibodies react with endogenous or planted glomerular antigens in situ. GN in dogs has been associated with numerous systemic infectious and inflammatory diseases, including canine adenovirus, bacterial endocarditis, brucellosis, dirofilariasis, ehrlichiosis, borreliosis, neoplasia, pancreatitis, systemic lupus erythematosus, and other immune-mediated and chronic inflammatory disorders (including neoplastic disease). In cats feline leukemia virus, feline infectious peritonitis, polyarthritis, pancreatitis, and other immune-mediated diseases are implicated. Many of these are treatable diseases; however, a source of antigen is not identified in many cases of GN; familial and idiopathic glomerulopathies are recognized.78
Proteinuria is a typical sequela of glomerular damage and may be the earliest detectable laboratory abnormality. Leakage of protein across the glomerulus can lead to tubulointerstitial injury and progression of renal damage. In humans, dogs, and cats, proteinuria is linked to progression of renal disease. Two studies have shown that higher urine protein:creatinine ratios in cats are inversely related to survival in cats with chronic renal failure and proteinuria. Benazepril has been shown to decrease proteinuria in cats.79
In renal amyloidosis deposition of amyloid A, derived from the acute phase reactant serum amyloid A, predominates. In dogs and cats, renal amyloidosis is usually a component of reactive systemic amyloidosis triggered by chronic inflammatory disease.80 Familial forms of systemic amyloidosis are observed in the Abyssinian cat and Chinese Shar-Pei dog. In both GN and renal amyloidosis, glomerular surface area, function, and permeability are affected, leading to proteinuria and glomerular hyperfiltration. Ultimately, the nephron becomes nonfunctional; azotemia and renal failure ensue.81
Other sequelae of progressive glomerular disease include systemic hypertension, hypercoagulability, hyperlipidemia, and nephrotic syndrome.41,82 Systemic hypertension develops commonly in glomerular disease as a result of sodium retention and complex intrarenal mechanisms leading to depressed vasodilatory and enhanced vasoconstrictive responses. A hypercoagulable state is favored not only by loss of antithrombin III but also by increased concentrations of fibrinogen; factors V, VIII, and X; and enhanced platelet aggregability in glomerular disease.82 Dogs with antithrombin III concentrations less than 70% of normal and with fibrinogen concentrations of more than 300 mg/dL are at high risk for thromboembolic events.
Goals of management of glomerular disease are (1) to identify and treat the underlying disease process if possible, (2) to minimize proteinuria, and (3) to manage the consequences of glomerular disease and renal failure as they occur. Additional information about the disease and improved management strategies can be expected if renal lesions are appropriately characterized in biopsy specimens.78
KEY POINT 18-10
Pharmacologic treatment should be initiated in dogs and cats with persistent proteinuria (urine protein creatinine > 2.0 or >0.5, respectively). Medical management is most effective when initiated early in the disease process (before azotemia) and when a treatable underlying disease can be identified.
Glomerulonephritis
If an underlying disease process is not found or is not reversible, adjunctive treatments are initiated. Intervention is recommended early in the disease process and should be instigated before the development of renal azotemia when possible. Once a dog or cat is diagnosed with persistent proteinuria (urine protein:creatinine ratio >2 or >0.5, respectively), medical intervention should be considered.83
ACE inhibitor therapy should be initiated at the time of diagnosis of GN. Enalapril (0.5 mg/kg orally every 12 to 24 hours) has been shown to decrease proteinuria, serum creatinine, and blood pressure, compared with a placebo, in dogs with idiopathic membranous and membranoproliferative GN.84 Enalapril is used in proteinuric, normotensive dogs as well as hypertensive dogs with GN. Benazepril has not been objectively assessed for treatment of GN in dogs but may be as efficacious. Dosages should be started low and titrated to effect while renal function and blood pressure are monitored carefully because administration of ACE inhibitors can cause acute decompensation of renal function, especially if volume status is poor.
Immunosuppressive therapy is often considered to counter the immunologic components of glomerular diseases. Corticosteroids, although often potent immunosuppressive and antiinflammatory agents, have potential disadvantages in the treatment of glomerular disease. Steroids may increase glomerular permeability and worsen proteinuria. Steroid treatment may accelerate muscle catabolism, worsen azotemia, contribute to hypercoagulability and thromboembolism, exacerbate hypertension, and immunosuppress already-debilitated patients.85 Because of these effects and the lack of convincing evidence of the efficacy of steroids in glomerular disease, steroid treatment is generally reserved for patients in which the underlying disease process is steroid responsive, such as systemic lupus erythematosus.81 One histologic variant of glomerular disease in humans, minimal change disease, is responsive to corticosteroids; if lesions are consistent with this diagnosis in a dog, corticosteroid treatment should be more strongly considered.78 Other cytotoxic agents such as azathioprine, cyclophosphamide, and chlorambucil may be chosen for immunosuppressive effects in cases of rapidly progressive GN. Human patients with membranous nephropathy respond best to a combination of corticosteroid and alkylating agent.78 However, efficacy of many of these agents as single agents or in combination has not been adequately determined in dogs. Cyclosporine was not beneficial for dogs with idiopathic GN in one study.86
Inflammation may be modified by other means. Thromboxane synthetase inhibitors are effective in decreasing proteinuria, platelet aggregation, and thromboxane generation in experimental models of GN and can prevent histologic development of GN if administered at the time of the glomerular insult.87 The drug may decrease proteinuria even when administered after the insult.88 The NSAID aspirin is advocated in glomerular disease for its antithrombotic effects, but it may also influence glomerular inflammation by inhibiting platelet activation and aggregation. Low-dose aspirin administration (0.5 to 5 mg/kg every 12 hours) is designed to allow inhibition of platelet cyclooxygenase without affecting prostacyclin formation, an important vasodilatory compound and an antagonist of platelet aggregation.89 Low-dose aspirin administration also may reduce the risk of thromboembolic complications in dogs with glomerular disease.78
Dietary lipid composition has been shown to affect glomerular hypertrophy, glomerular capillary pressure, and renal function in dogs with experimentally reduced renal mass (15/16 nephrectomy).90 In humans with nephrotic syndrome, omega-3 fatty acid supplementation has been effective in reducing triglyceride concentration and platelet aggregation.91 These effects may be important because hypercholesterolemia may contribute to progressive glomerular damage and increased proteinuria.
Dietary protein is also manipulated in glomerular disease. Although protein supplementation may appear logical in patients with urinary protein loss and hypoalbuminemia, moderate restriction of protein has been more effective in minimizing proteinuria and effective protein loss. Recommendations are similar to those for other types of chronic renal failure. Certainly, protein synthesis is affected by dietary protein intake, however, and appropriate dietary protein levels must be determined for individual patients. Sodium restriction also is implemented, as for chronic renal failure, and is particularly important in glomerular disease to prevent or minimize hypertension and edema.
Diuretics may be required for management of the edematous patient with nephrotic syndrome. Furosemide (2.2 mg/kg orally every 12 to 24 hours) is reasonably effective. Patients should be monitored for volume depletion or hypokalemia associated with furosemide administration.
A logical overall approach to the patient with GN is as follows. Baseline measurements of renal function, albumin, blood pressure, antithrombin III levels, and urinary protein loss are measured. Dietary manipulation, ACE inhibitor therapy, fatty acid supplementation and aspirin administration are initiated, and the patient is reevaluated in 1 to 2 weeks. Reductions in the urine protein:creatinine ratio, along with stable renal function, indicate good response. Ancillary treatment for renal failure or nephrotic syndrome may be initiated as needed in individual cases. Although some cases resolve with treatment of underlying disease or spontaneous remission, many cases are steadily progressive. Patients with nephrotic syndrome or established azotemia have a poor prognosis.
KEY POINT 18-11
Dietary management, angiotensin-converting enzyme inhibitor administration, fatty acid supplementation, and aspirin provide the cornerstones for management of glomerular disease. Goals of treatment include a reduction in urine protein creatinine, avoidance of complications, and stable renal function.
Renal Amyloidosis
Renal amyloidosis carries a poor prognosis, especially if renal failure and uremia are evident. Underlying inflammatory and neoplastic diseases should be identified and managed if possible; however, few interventions will affect established amyloid deposits. Dimethylsulfoxide (DMSO) has been suggested in the treatment of amyloidosis. The drug may enhance solubilization of amyloid fibrils, reduce serum amyloid A protein concentrations, and reduce associated interstitial inflammation and fibrosis. The latter effect is most likely to be beneficial in improving renal function and reducing proteinuria. Long-term management of a few dogs with DMSO injections has been described.81,92 DMSO (90% solution) may be diluted 1:4 with sterile water and administered subcutaneously at a dosage of 90 mg/kg three times weekly.81 Adverse effects of DMSO include nausea, a garliclike odor, and pain on injection. Oral daily dosages have been described, ranging from 250 to 300 mg/kg per day.93,94
Colchicine is another agent that impairs the release of serum amyloid A from hepatocytes and may prevent the production of amyloid-enhancing factor.78 The agent is used to prevent development and progression of amyloidosis in human patients with familial Mediterranean fever. Like DMSO, the agent is unlikely to be helpful after the development of renal failure. Low doses of colchicine (0.01 to 0.03 mg/kg daily) may be considered prophylactically for Chinese Shar-Pei dogs with recurrent fevers and joint disease, which may be precursors to systemic or renal amyloid deposition.78,81 Both colchicine and DMSO are most effective in the early phases of amyloid deposition; colchicine may reduce proteinuria as well. As with other types of glomerular disease, metabolic complications such as hypertension and hypercoagulability must be identified and addressed.
Dialytic Therapy
Dialytic therapy is available to remove excess water or solutes from plasma using osmotic gradients across a semipermeable membrane. In hemodialysis the membrane is an extracorporeal synthetic membrane, whereas in peritoneal dialysis the peritoneum serves as the membrane for exchange. By these methods urea, creatinine, and other retained molecules can be eliminated in the patient with renal failure. Although peritoneal and hemodialysis techniques have been described in many animal models, their clinical application in small animal veterinary medicine has been limited by the extensive technical, equipment, and financial requirements involved.95-102 Dialytic therapy is generally considered most appropriate as a temporary, short-term measure in reversible renal and postrenal disorders. It can, however, be an effective means of supplementing medical management in refractory end-stage chronic renal failure. It is also used to manage feline transplant recipients and cats with ureteral obstruction before surgical intervention. Intermittent hemodialysis has been successful in reducing the average urea concentration in dogs and cats with chronic renal disease and moderate azotemia.100,101,103 Indications for dialytic therapy in acute renal failure include failure of conservative therapy; refractory oliguria or anuric, life-threatening fluid overload; or life-threatening electrolyte or acid–base disturbances. Hemodialysis also is useful in the early management of toxicoses, especially ethylene glycol intoxication, as well as adjunctive treatment of refractory leptospirosis. In chronic renal failure, dialysis is considered when uremic signs are unresponsive to therapy, usually when azotemia is advanced. The procedure requires reliable vascular access, an appropriate hemodialyzer and dialysis delivery system, and dedicated technical team.101,103 Currently, hemodialysis is available at the University of California–Davis Companion Animal Dialysis Unit, Davis, California, and San Diego, California; and the Animal Medical Center, New York, New York. Continuous renal replacement may be available at additional specialty centers or veterinary teaching hospitals. The development of additional centers for intermittent dialysis in dogs may increase the application of the technique for improved management and prolonged survival in selected cases. Peritoneal dialysis may be initiated in the practice or referral center setting after placement of an intraabdominal dialysis catheter.98 Straight, acute peritoneal dialysis catheters are available for emergency dialysis, whereas column disk or T-fluted catheters are preferred for long-term dialysis. Dialysate solutions of 1.5% to 4.25% dextrose are infused to create an osmotic gradient within the abdomen. Substances such as urea, creatinine, phosphorus, electrolytes, and other uremia molecules can pass through intercellular channels of the peritoneum into the dialysate for removal. A dedicated technical support team is required to manage the frequent exchanges and potential complications of the procedure. Peritonitis, hypoalbuminemia, electrolyte abnormalities, and leakage around the catheter are common complications.99
Renal Transplantation
Renal transplantation is the definitive mode of management of chronic renal diseases in human patients and has become a viable option in veterinary medicine in selected circumstances. A successful clinical renal transplantation program has been developed for cats at the Veterinary Medical Teaching Hospital, University of California, Davis,104-106 and is now available at several veterinary referral hospitals in the United States and Canada. With transplantation of a healthy donor kidney and appropriate immunosuppressive therapy, uncomplicated cases can expect good-quality posttransplant survival times of 1 to 3 years. Of 61 cats receiving renal transplants between 1996 and 1999 at the University of Davis, approximately 60% survived 6 months after the operation, whereas the 3-year survival rate was about 40%.107 Cats 10 to 14 years of age had a higher risk of death, especially during the 6-month postoperative period.107 The best candidates for renal transplantation are cats in early renal failure, with less than 20% weight loss and no other disease conditions. Candidates are screened for cardiac disease, neoplasia, concurrent metabolic disease, urinary tract infection, feline leukemia virus, and feline immunodeficiency virus.
Immunosuppressive therapy with cyclosporine and prednisolone is initiated perioperatively and continued indefinitely. Treatment is monitored by frequent measurements of trough blood levels of cyclosporine. Potential complications of transplantation include anesthetic and surgical complications, obstruction of the transplanted ureter, infections caused by immunosuppression, pyelonephritis, postoperative hypertension, central nervous system disease, hypercalcemia, and acute or chronic graft rejection.107,108 De novo malignant neoplasia was diagnosed in 9.5% of 95 feline transplant recipients 2 to 28 months after transplantation and may be associated with immunosuppressive therapy.109Transplantation cannot be regarded as a cure for renal disease or an option for emergency treatment of renal failure, but it can be expected to provide an improved quality of life and enhanced survival in some cats with renal failure. Details of the program, surgical procedure, and criteria for case selection are available.105,106 Canine transplantation remains problematic, although newer immunosuppressive strategies have been developed.110
Management of Micturition Disorders
Physiology of Micturition
The storage phase of micturition is characterized by sympathetic dominance, with sympathetic innervation to the bladder and urethra supplied by the hypogastric nerve. Activation of β-adrenergic receptors in the urinary bladder facilitates relaxation of the detrusor muscle, whereas stimulation of α-adrenergic receptors in the bladder neck and urethra facilitates smooth muscle contraction and closure of the outlet. Additional urethral resistance is supplied by the striated muscle of the external urethral sphincter. As bladder volume and pressure increase with filling, afferent information is transmitted to the central nervous system by way of the pelvic nerve and spinal afferent pathways. Voiding is initiated by voluntary control centers in the cerebral cortex and midbrain. Efferent impulses are transmitted by spinal pathways and the pelvic nerve in the parasympathetic system, initiating contraction by stimulating cholinergic receptors in the detrusor muscle of the urinary bladder. The sympathetic input to the bladder and urethra is inhibited, allowing outlet resistance to drop appropriately. After complete voiding, the system is reset for storage.111
Disorders of urine storage usually result in urine leakage, whereas disorders of voiding result in urine retention, incomplete voiding, or incontinence. Most disorders of micturition can be classified and managed according to the status of urinary bladder (hypocontractile or hypercontractile) and urethral (hypotonic or hypertonic) function.112,113 Diagnosis is usually based on evaluation of historical, physical, and observational findings, although specialized urodynamic testing is required in some instances. Pharmacologic agents are valuable in the management of functional micturition disorders; manipulation of urinary bladder or urethral smooth muscle tone can aid in facilitating normal micturition. Because pharmacologic activity is directed at the end organ (postganglionic receptors in the urinary bladder or urethra), agents are applied similarly in both neurogenic and non-neurogenic disorders (Table 18-3). Practical reviews regarding the management of major micturition disorders are available.114,115
The Hypocontractile Urinary Bladder
Problems resulting in hypocontractile urinary bladders include sacral or suprasacral neurologic lesions, acute or chronic overdistention of the urinary bladder, disorders causing general muscle weakness, or dysautonomia. Urinary bladder contraction is primarily controlled by parasympathetic (cholinergic) input. Cholinergic agents have been used to promote bladder emptying in atonic bladders, although the success of orally administered agents is unreliable.116 Bethanechol chloride is administered at starting dosages of 1.25 to 2.5 mg (cats), 5 mg (small dogs), and 10 mg (larger dogs) every 8 to 12 hours. Full effects of the drug should be apparent within 1 to 2 days. When effective, voiding is usually observed within 2 hours. The dosage may be increased by 2.5- to 5-mg increments up to 25 mg every 8 hours in dogs and 7.5 mg every 8 hours in cats if ineffective.113 Parenteral administration of bethanechol (2.5 to 10 mg given subcutaneously every 8 hours) may be effective in refractory dogs with bladder atony; however, the likelihood of adverse effects is increased with this route.117,118
Adverse effects of cholinergic agents include muscarinic effects such as salivation, defecation, and abdominal cramping. Vomiting, diarrhea, and anorexia also are possible complications. Overdosage or parenteral administration can (rarely) result in a cholinergic crisis and death; atropine is useful as an antidote. Parasympathomimetic agents are contraindicated in the face of urinary or gastrointestinal obstruction and should be used with caution in animals with bronchial disease or ulcerative gastrointestinal disease. Bethanechol administration may increase smooth muscle tone at the bladder neck and outlet;119 urethral resistance must be minimized with α-antagonists, striated muscle relaxants, or both before bethanechol treatment is initiated. Intermittent urinary catheterization or indwelling urinary catheterization may be necessary during early therapy to ensure a patent outlet and to maintain a small urinary bladder, facilitating recovery of smooth muscle function.
Recovery of urinary contractile function is most likely in animals with acute overdistention of urinary bladder or with reversible neurologic lesions creating detrusor atony. Alternative pharmacologic agents enhancing bladder motility are lacking; cholinesterase inhibitors, β-antagonists, dopamine antagonists, and prostaglandin treatments have been investigated in human beings but have not received much attention in veterinary patients. Increased urinary frequency and enhanced contractile indices have been observed with cisapride administration in human patients.120,121 This prokinetic agent increases acetylcholine release at neuromuscular junctions and may ultimately be valuable in stimulating bladder smooth muscle in dogs and cats, but urinary effects remain unproved and availability is limited to veterinary compounding pharmacies.115,122
The Hypercontractile Urinary Bladder
Accommodation, or compliance, of the urinary bladder may be affected by congenital disorders, chronic inflammation, infiltrative masses, neurologic disorders, or idiopathic causes. In cats bladder hypercontractility (detrusor instability) has been described in feline leukemia–associated urinary incontinence.123 Filling of the bladder is impaired, and involuntary bladder contractions occur at low bladder pressures and volumes. Clinically, disorders of bladder accommodation are manifested by urinary incontinence and pollakiuria. Management of urinary bladder storage dysfunction may include treatment of urinary tract infections, correction of neurologic disorders, or pharmacologic intervention.
KEY POINT 18-13
Urinary bladder overactivity is rare in small animals but responds well to pharmacologic treatment. Treatment of urinary tract infection and inflammation and consideration of behavioral disorders should precede trial treatment.
Agents with anticholinergic properties may be used to alleviate the signs associated with bladder contractility or reduced bladder storage function. These agents appear to be quite effective in dogs and cats with idiopathic and feline leukemia–associated urinary incontinence but may be less effective in bladders with severe inflammatory disease, neoplastic diseases, or fibrotic changes. Oxybutynin, with anticholinergic, antispasmodic, and local anesthetic actions on the urinary bladder, has been used in veterinary patients and is available in tablet form (5-mg tablets) and in a liquid syrup. In dogs dosages of approximately 0.2 mg/kg have been effective.123 Small dogs usually respond to 0.75 to 1.25 mg oxybutynin every 8 to 12 hours, whereas larger dogs may require 2.5 to 5 mg every 8 to 12 hours. In cats a dosage of 0.5 to 1.25 every 8 to 12 hours is recommended.124 Long-acting formulations have recently become available. Tolterodine is a competitive, pure muscarinic receptor antagonist that is the drug of choice in humans for treating destrusor instability in human beings, which results in a hyperactive or hyperreflexive bladder. It has fewer side effects than oxybutynin, which was previously the drug of first choice.125 At this time there is not information on appropriate doses in veterinary patients.
Other agents with anticholinergic or antispasmodic activity include dicyclomine, tricyclic antidepressants, propantheline, and tolterodine. Dicyclomine is a similar, less expensive agent that has been as effective as oxybutynin in preliminary studies in dogs. Dosages of 5 to 10 mg every 8 hours are recommended in dogs.126 Use of this drug in cats has not been reported.
The tricyclic antidepressant imipramine is another agent with anticholinergic properties. Imipramine also has mild stimulatory effects on α- and β-receptors in the bladder and urethra, which serve to further facilitate urine storage. Recommended dosages of imipramine are 5 to 15 mg orally every 8 to 12 hours in dogs and 2.5 to 5 mg orally every 12 hours in cats.112 Propantheline is an alternative anticholinergic agent. Recommended dosages of propantheline range from 5 to 7.5 mg as needed in cats (frequency varies from every 8 hours to every 2 or 3 days)112,127,128 and 7.5 to 30 mg every 12 hours in dogs.113 Starting doses of 5 mg/day in cats and 7.5 to 15 mg every 12 hours in dogs are reasonable.
Ptyalism is a common complication of anticholinergic administration in cats that can be minimized by placing the product in gelatin capsules. Other adverse effects of anticholinergic agents include drowsiness, ileus and vomiting, constipation, and urine retention. Dry mouth, dry eyes, and mydriasis have been reported in humans. Anticholinergic agents are contraindicated in animals with glaucoma. Many alternative agents have been employed for detrusor instability in people, including tolterodine, β-agonists, calcium channel blockers, and other smooth muscle relaxants. Success rates have varied, and these agents have not been investigated in veterinary medicine.
The Hypotonic Urethra
Reproductive Hormones
Poor outlet resistance (urethral incompetence) is a common disorder of middle-aged, neutered, medium- to large-breed dogs, and predisposing factors include caudal bladder position, a short urethra, poor urethral tone, neutering, breed, and obesity. German Shepherd Dogs, Doberman Pinschers, Old English Sheepdogs, Springer Spaniels, Boxers, Rottweilers, Weimaraners, and Irish Setters are overrepresented.129 Urethral incompetence can also be attributed to congenital, inflammatory, and neurogenic disorders. Because of the prevalence of this problem in older, neutered animals, reproductive hormone supplementation has been used extensively with good results. Affected animals do not appear to be deficient in reproductive hormones; improved continence observed with reproductive hormone administration is likely due to a variety of effects on the urethra. The major action of reproductive hormones in the lower urinary tract may be sensitization and upregulation of α-adrenergic receptors in the bladder neck and urethra. Mucosal integrity, collagen content, and capillary vascularity in the urethra also are enhanced by estrogens, contributing to a more effective urethral mucosal “seal.”130
KEY POINT 18-14
Urethral sphincter mechanism incompetence is the most common cause of urinary incontinence in dogs. Most affected dogs leak urine intermittently at rest and respond well to alpha-adrenergic agents, reproductive hormones, or a combination of the two.
Diethylstilbestrol and stilbestrol are effective and reasonably safe choices for female dogs with urethral incompetence. Diethylstilbestrol is initially administered at a total dosage of 0.1 to 1 mg (approximate 0.02 mg/kg) orally each day for 5 to 7 days, followed by a similar dosage administered every 5 to 14 days.112 Daily estrogen treatment, using minimal dosages of stilbestrol, also has been recommended. The protocol includes starting dosages of 0.04 to 0.06 mg orally administered daily for 1 week and then reduced at weekly intervals to 0.01 mg daily. After 4 weeks the treatment is discontinued. A prolonged residual effect may be observed. If incontinence recurs, the protocol may be repeated or the drug may be administered indefinitely at 0.01 to 0.02 mg/dog daily.131 Commercially available alternative estrogens include conjugated estrogens (Premarin, 0.02 mg/kg orally every 2 to 4 days and estriol, 0.5 to 2.0 mg/dog every 2 to 3 days). As for diethylstilbesterol, daily loading doses are advised for the first 5 to 7 days. More frequent dosing (two to three times per week) are usually necessary with these preparations.
Response to estriol was studied in a group of 129 incontinent adult spayed female dogs in an open label trial. Dogs were given 2 mg of estriol daily for 1 week, then the dose was reduced at weekly intervals to the minimal effective dose (0.5 to 2 mg/dog every 24 to 48 hours). Veterinarians reported continence in 61% and improvement in an additional 22% of the dogs; owner-reported responses were slightly less favorable.132 Favorable experience with natural, conjugated estrogen (similar to Premarin) has been described in nine incontinent large-breed dogs followed in a prospective manner. All dogs responded well to estrogen administration; daily administration was continued until 2 weeks of continence had been achieved. In seven of nine dogs in which dose information was reported, maintenance dosages ranged from 0.625 mg to 1.25 mg per dog, administered orally every 12 to 72 hours. In the remaining two dogs, administration every 4 to 7 days was effective.133 No hematologic effects of estrogen were observed in estrogen-treated dogs in either study.132,133
Significant inter-animal variation in accumulation and recirculation of the drug was noted in pharmacokinetic studies.134 Potential adverse effects of estrogen administration include bone marrow suppression, alopecia, behavioral changes, and signs of estrus, although the risks are minimal when the drug is used properly. Periodic monitoring of complete blood counts is advised for dogs receiving long-term estrogen administration. In addition to toxic effects, estrogens frequently cause signs of estrus in cats and are not recommended in that species.112
Many spayed female dogs respond well to estrogen administration. A response rate of 60% to 70% can be expected.131 A residual effect may be observed in some dogs such that the drug can be discontinued intermittently; however, most dogs require constant treatment and ultimately become refractory to the drug. Dosage and frequency adjustments can be attempted when treatment failure occurs; however, switching to or combining with an alternative agent may be more effective.
Testosterone administration may be used similarly for the treatment of urethral incompetence in male dogs and cats. The potential for adverse effects is significant; effects include aggression, other behavioral changes, prostatic disease, and aggravation of disorders such as perianal adenomas and perineal hernias. Other disadvantages of the drug include the ineffectiveness of oral preparations and its classification as a controlled drug. Testosterone proprionate may be administered parenterally at dosages of 2.2 mg/kg subcutaneously or intramuscularly (dogs) or 5 to 10 mg/cat every 2 to 3 days. Testosterone cypionate or testosterone enanate may be administered every 30 to 60 days at a similar dosage. Some dogs require higher doses; a dosage of 200 mg/dog administered intramuscularly has been effective.135 Experiences with oral preparations (methytestosterone) for urinary incontinence have not been reported. The many disadvantages of this drug limit its application in the treatment of urinary incontinence, although it may be useful in some neutered male dogs with acquired urethral incompetence. If the product is minimally effective or frequent injections are required, alternative agents should be used.
α-Adrenergic Agonists
Sympathomomimetic agents (Alpha-agonists) are effective agents for the treatment of urethral incompetence. α-adrenergic agents probably enhance urethral closure through release of endogenous norepinephrine and direct stimulation of α-receptors in the bladder neck and urethra. The agents are usually well tolerated and may be used in animals of either sex. Phenylpropanolamine preparations are effective and available from veterinary suppliers. The agent is no longer found in over-the-counter decongestant preparations. Approximate dosages of 1.5 to 3 mg/kg orally every 8 to 12 hours are recommended. The best responses appear to be gained by starting at a dosage of at least 1.5 mg/kg every 8 hours and then adjusting the dosing frequency after a few weeks. Many dogs can be maintained with once- or twice-daily administration of phenylpropanolamine, especially in timed-release formulations. Ephedrine (ephedrine, pseudoephedrine) is an alternative α-agonist also available in over-the-counter preparations. However, many of these preparations are also under pharmacist control at this time and cannot be purchased in bulk amounts. Dosages are similar to phenylpropanolamine (1.2 mg/kg orally every 8 to 12 hours).112 Dogs usually receive 12.5 to 50 mg orally two to three times daily. Efficacy of ephedrine compounds is slightly less predictable than that of phenylpropanolamine.
KEY POINT 18-16
Phenylpropanolamine remains the most reliable pharmacologic treatment for urethral incompetence in dogs. Adjustments in dose, frequency, and type of product may improve response in the small percentage of affected dogs with refractory incontinence.
In cats the administration of α-agonists is somewhat problematic. One-half of a 25-mg phenylpropanolamine tablet may be given orally every 8 to 12 hours. Some experts recommend sprinkling 1⁄12 of a 75-mg capsule onto food twice daily.136 The effectiveness of α-agonists in cats is questionable, however. Only small portions of the urethra are composed predominantly of smooth muscle and expected to respond to α-agonists. Fortunately, pure urethral incompetence is rare in cats.
Adverse effects of α-agonists include anorexia, weight loss, hyperexcitability, and tachycardia. The author has observed occasional instances of gastrointestinal upset and skin eruption with phenylpropanolamine administration. Systemic hypertension is a serious theoretical complication, although it has not yet been reported with clinical use.137,138 The drug should be avoided or used cautiously in dogs with cardiac or hypertensive disorders, including renal disease and diabetes mellitus. In human patients the drug is contraindicated in the face of prostatic hypertrophy, hyperthyroidism, and glaucoma.
Response to appropriate dosages of phenylpropanolamine are usually good. Excellent clinical responses or “cures” can be expected in 75% to 90% of dogs,131,137,139,140 with significant improvement noted in almost all patients treated with the drug. In a large (n = 50) prospective, blinded, placebo-controlled study, Scott et al.141 found 55% of treated dogs (phenylpropanolamine 1 mg/kg orally every 8 hours) became continent after 7 days of treatment, as opposed to 26% of placebo-treated dogs. After 28 days the percentage of continent dogs rose to 85.7% in the phenylpropanolamine group, as opposed to 33% of placebo-treated dogs.141 In another randomized, double-blinded study, female dogs responded well to phenylpropanolamine (1.5 mg/kg every 12 hours); 21 of 24 dogs were continent and another 2 improved.142
Although most resources recommend administration of phenylpropanolamine two or three times daily for best effect, the dose is adjusted to the minimal amount needed to achieve continence. As with reproductive hormones, it is usually beneficial to start with the optimal dose until continence is achieved. However, a recent investigation in healthy intact Beagles challenges this assumption. Dogs were treated with phenylpropanolamine at once-, twice-, or thrice-daily intervals. Urodynamic changes (increased urethral pressure) were similar for all dosing frequencies, leading the authors to recommend once-daily treatment for incontinent dogs.143 Desensitization of receptors was suspected with thrice-daily administration. Responses in intact Beagles may not parallel those in incontinent spayed dogs; however, reduced frequency of administration may be effective in some dogs and may prevent development of tolerance during long-term treatment.
Although over-the-counter availability of ephedrine and pseudoephedrine makes their use convenient, outcomes are slightly less favorable with these agents. With ephedrine compounds, 82.4%144 and 74%139 continence rates are reported. In another group of nine female dogs studied in a crossover design, improvement in continence score, maximal urethral closure pressure, and functional area of the urethral pressure profile was observed after pseudoephedrine (1.5 mg/kg every 8 hours) administration.138
Although most dogs exhibit excellent response to alpha agonists, refractory incontinence does occur. Patients that do not respond tend to be younger dogs with congenital urethral incompetence or dogs in which incontinence develops before or soon after ovariohysterectomy. These patients may respond to an increased therapeutic dosage; combination treatment with reproductive hormones; or other treatment modalities, such as urethral injection of collagen.
Alternative agents currently under investigation include gonadotropin-releasing hormone (GnRH) analogs and duloxetine. Reichler145 reported their experience with luprolide, buserelin, and deslorelin, GnRH analogs that suppress the release of sex hormone. Their use in incontinence is based on the theory that chronically unsuppressed follicle-stimulating hormone (FSH) and luteinizing hormone (LH) release (due to lack of negative feedback) in ovariectomized dogs may contribute to urinary incontinence. Administration of analogs paradoxically results in reduced FSH and LH over time. In 12 of 13 dogs with refractory incontinence, the drug appeared useful, either alone or in combination with alpha agonists. Deslorelin (5 to 10 mg depot injection) became the preferred treatment in this study. In a subsequent trial, 9 of 23 incontinent dogs treated with long-acting leuprolide were continent for prolonged periods (70 to 575 days); another 10 of the dogs had partial response. These 23 dogs, however, also responded to phenylpropanolamine, with 92% overall reduction in urine leakage.146 Urethral closure pressures did not increase in these dogs, however. The only urodynamic parameter that changed after depot leuprolide injection in spayed Beagles was cystometric threshold volume (an indicator of capacity and accommodation).147 There were no apparent adverse effects of GnRH treatment reported in this study, but long-term use of these drugs in dogs has not been evaluated. GnRH analogs may prove to be a valuable long-acting treatment that would alleviate the need for daily or weekly medication; however, availability and cost of GnRH analogs limit their use in the United States.
Duloxetine, a serotonin and norepinephrine reuptake inhibitor, has proven useful in women with stress incontinence and may improve striated muscle resistance as well as bladder capacity.148 Adverse effects included nausea, fatigue, insomnia, constipation, diarrhea, and headache, but these symptoms were infrequent and mild. Experience is limited in small animals at this time.
For incontinent dogs that fail to respond to appropriate pharmacologic treatment, a search for underlying urinary tract infection, neurologic abnormalities, and other causes of incontinence is indicated. For refractory female dogs, a bulking agent such as collagen can be injected into the urethral submucosa to generate increased urethral resistance. This minimally invasive, endoscopic-assisted procedure provides significant improvement for up to several years and can be repeated when effectiveness wanes.149-151 Alternatively, surgical options, including episioplasty, colposuspension, or urethropexy, are reasonable for some dogs that fail to respond to medical management.152,153 Many dogs undergoing urethral injections or surgical procedures require phenylpropanolamine treatment after surgery to achieve the best outcome.
The Hypertonic Urethra (Functional Urethral Obstruction)
Inappropriate urethral resistance may lead to functional urethral obstruction and urine retention. Urethral inflammation or spasm can develop after urethral obstruction with uroliths or urethral plugs. Urethral resistance also may be uncoordinated with bladder contraction as a result of neurologic disorders or idiopathic causes. Urethral resistance also may increase when bethanechol is administered for hypocontractile urinary bladders; the drug also stimulates contractions of musculature at the bladder neck. In these situations pharmacologic manipulation may be instituted to decrease smooth or striated muscle contractility in the urethra and reduce outlet resistance.
Alpha-Adrenergic Antagonists
Alpha-adrenergic antagonists are the preferred agents for decreasing urethral smooth muscle tone and are experimentally effective in reducing overall urethral resistance in dogs.154Their activity is less predictable in cats, in which striated muscle predominates in the urethra. Phenoxybenzamine has been a commonly used α-antagonist in both dogs and cats.155,156 Phenoxybenzamine irreversibly inactivates α-receptors and may have a central effect on striated musculature.157 The drug is available in 10-mg capsules and is dosed at approximately 0.25 mg/kg orally every 12 to 24 hours.112 Total dosages usually range from 5 to 20 mg (dogs) and 2.5 to 5 mg (cats). Efficacy may be discerned by judging the quality of urine stream produced. Phenoxybenzamine is expensive and unavailable in some areas. The drug’s availability may be further limited because of the discovery of its carcinogenic potential in rats157 as well as its obsolescence relative to newer alpha antagonists used in human beings.
An alternative, and more selective, α1-antagonist is prazosin, a drug that has been recommended in the management of heart failure and systemic hypertension in dogs. Extrapolated dosages are 1 mg per 15 kg body weight orally every 8 to 12 hours in dogs; dosages of 0.25 to 0.5 mg every 12 to 24 hours seem reasonable for cats. Because total dosages of 1.5 to 3 mg orally every 8 hours are used for functional urethral obstruction in humans, however, lower dosages may be effective in small animals. Prazosin was shown to produce superior urethral pressure reduction compared with phenoxybenzamine, although prazosin therapy was associated with a significant drop in blood pressure.158 Additional α-blocking agents have included terazosin and doxazosin.157,159 No reports are available regarding their use in small animals, although a starting dose of 0.125 to 0.25 mg/dog orally every 24 hours can be extrapolated from human dose recommendations.
Tamsulosin is a newer antagonist that exhibits strong selectivity for urinary α1 receptors (α1A) over vascular α1 receptors (α1B), minimizing the risk of hypotension.160,161 Because glucuronidation is probably required for metabolism, tamsulosin cannot currently be recommended for use in cats. In experimental studies in dogs, doses of 1 to 100 μg/kg intravenously and orally have been used to evaluate the effect of tamsulosin on urethral pressure and arterial blood pressure.160,162,163 Oral tamsulosin doses of 1 to 10 μg/kg produced dose-dependent blockade of phenylephrine-induced urethral pressure.163 For clinical application in dogs, 10 μg/kg orally every 24 hours is recommended on the basis of anecdotal evidence.
Silodosin is another commercially available α1 antagonist that shows even greater α1A receptor selectivity in canine tissues than does tamsulosin and may prove to be a useful therapy, but veterinary clinical data and experience with silodosin are lacking at this point.164
Adverse effects of α-antagonists include hypotension, reflex tachycardia, and gastrointestinal irritation. Nausea can be minimized by administration of the drug with food. The drug may be dangerous to animals with cardiac disease receiving other vasodilators or diuretics and to animals with renal disease, in which drops in perfusion pressure could precipitate an acute crisis. Any evidence of weakness should prompt withdrawal of the drug or adjustment of the dosage.
Striated Muscle Relaxants
If manipulation of urethral smooth muscle does not sufficiently improve voiding, addition of a striated muscle relaxant may be considered. Striated muscle relaxants are particularly useful in dysuric patients with upper motor neuron lesions and in cats with functional urethral obstruction. In this species striated muscle predominates in the distal urethra and likely contributes most to functional obstruction. The most common muscle relaxant used for urethral resistance is the benzodiazepine diazepam. The drug serves as a short-acting muscle relaxant by centrally mediated actions, and its effect on urethral striated muscle is variable.
Diazepam (0.2 to 0.5 mg/kg orally) is recommended as a temporary agent to facilitate bladder expression or to augment weak voiding; the agent is given 15 to 30 minutes before expression.156 Dosages for cats range from 1.25 to 2.5 mg/cat every 8 to 12 hours; sedation is common with higher dosages.155,156 In dogs total dosages of 2 to 10 mg/dog are given.117,118 Adverse effects of diazepam administration include sedation, weakness, and paradoxical excitement. In cats behavior changes and idiosyncratic hepatotoxicity are additional concerns. Alprazolam is a reasonable alternative if chronic administration is needed.
Dantrolene is an alternative striated muscle relaxant that has been investigated in cats. Dantrolene acts as a direct muscle relaxant by inhibiting calcium movement from the sarcoplasmic reticulum in muscle cells. The agent (1 mg/kg, administered intravenously) was effective in reducing segmental urethral pressures in healthy male cats165 and in moderately reducing urethral pressures in a small group of recently obstructed cats.166 The effect on urethral musculature was enhanced by the concurrent administration of prazosin.166 Recommended oral dosages are 1 to 5 mg/kg orally every 8 hours in dogs and 0.5 to 2 mg/kg orally every 8 hours in cats.167 Potential adverse effects include sedation, dizziness, weakness, and gastrointestinal upset; the drug is contraindicated in patients with cardiopulmonary disease. Hepatotoxicity is a worrisome adverse effect of dantrolene administration in humans, usually after long-term treatment at high dosages.17 Although oral dantrolene has been recommended for several years, clinical reports regarding its use in small animals are lacking. Other striated muscle relaxants include baclofen, cyclobenzaprine, and botulinum toxin injections.159 On the basis of anecdotal reports relayed to the authors, baclofen cannot be recommended because of poor tolerance of the drug in dogs and cats.
Treatment of detrusor–urethral dyssynergia has been unrewarding in reported cases.155,168-170 In the authors’ experience, dogs with dysfunctional voiding in the absence of compressive neurologic disease are often responsive to manipulation of smooth muscle resistance.171 Pharmacologic manipulation of urethral tone in cats with dyssynergia or functional obstruction and dogs with suspected striated urethral dyssynergia is less rewarding. Recovery is also related to the degree of detrusor damage sustained by the time of diagnosis. In chronic cases circumvention of urethral resistance using intermittent urinary catheterization or urethral stents may be necessary for temporary or prolonged relief of obstruction.159
Pharmacologic Agents that Target Ureteral Motility
With an increase in obstructive ureteroliths observed in small animal practice, interest in pharmacologic strategies to promote passage of ureteroliths through the urethra has increased. Fluid therapy and diuretics may be useful in increasing urine flow and hydrostatic pressure proximal to a ureterolith, but this approach must be done with caution so that affected animals, especially those with complete obstruction, are not overhydrated. Alleviating ureteral spasm can be attempted. Alpha antagonists, calcium channel blocking agents, and antiinflammatory agents can facilitate ureteral relaxation.172 These drugs do appear to facilitate ureterolith passage in humans but have not been well studied in dogs or cats. Glucagon appears to reduce ureteral contractions in dogs and has been used in cats with ureteral obstruction. In cats, however, the drug did not appear overly efficacious and can lead to acute adverse reactions, characterized by tachypnea, dyspnea, and gastrointestinal signs.173 Amitriptyline, a tricyclic antidepressant, reduced smooth muscle activity in human and pig ureteral segments in ex vivo studies. Further study of the effects of amitriptyline on feline ureters is warranted. Tamsulosin (see the discussion of the hypertonic urethra in this chapter) has become the agent of choice for medical management of small ureteroliths in humans.
Drugs Used in the Management of Feline Lower Urinary Tract Disease
Idiopathic hematuria, dysuria, and urethral obstruction represent a common clinical problem encountered in cats. Feline urologic syndrome, idiopathic feline lower urinary tract disease (iFLUTD), idiopathic feline cystitis, and feline interstitial cystitis all are terms that have been used to describe this combination of clinical signs when urinary tract infection, urolithiasis, neoplasia, and other causes have been ruled out. Many pharmacologic agents and treatment strategies have been proposed to manage this disorder and prevent its recurrence. The apparent efficacy of any treatment for cats with idiopathic disease should be considered in light of the usual self-limiting nature of this disorder, and pharmacologic agents should be administered only after assessment of the likely benefits and possible risks of each treatment.127,174 Recent publications suggest a relationship among stress, alterations in the feline nervous system, and the development of iFLUTD, with an increased emphasis on environmental and behavioral influences.175-177 Moisture intake and environmental modifications appear to be the most effective treatments in cats with recurrent idiopathic disease; however, pharmacologic agents have a place in short-term symptomatic relief and in reducing discomfort and recurrence in refractory cases.
Historical Treatment Stragegies
Urinary Acidifiers
Manipulation of urine pH, usually by dietary means, has been a long-standing strategy for management of feline lower urinary tract disease (FLUTD). The strategy is most likely to be helpful in cats in which struvite crystalluria is a significant component of obstructive FLUTD and does not eliminate recurrence in all cats with idiopathic disease.127,178 Most urologic diets are designed to promote acid to neutral urine in fed cats; additional acidification is rarely necessary or wise.
Overacidification is another possible complication of urine acidification in cats; young cats and cats with renal insufficiency are most susceptible. Long-term administration of urinary acidifiers may contribute to metabolic acidosis, hypokalemia, bone and mineral imbalances, renal failure, and calcium oxalate urolithiasis.179-181 Heinz body anemia and methemoglobinemia have been observed in kittens treated with dl-methionine as well as in adult cats treated with high dosages.182 Arrhythmias and central nervous system depression are serious complications of high-dose ammonium chloride administration. Both agents are contraindicated in patients with hepatic disease because the administration of an ammonium load may potentiate hepatoencephalopathy. Serial monitoring of clinical status, complete blood counts, acid–base, and electrolytes is recommended during therapy.
Urinary Antiseptics
Urinary antiseptic agents have been considered as adjunctive treatment in FLUTD because of their antiviral activity.127 Viruses including herpesvirus, feline syncytia-forming virus, and feline calicivirus have been implicated in the etiopathogenesis of FLUTD, although a consistent cause-and-effect relationship has been difficult to establish. The agents are more commonly used for antibacterial and antifungal properties, but these are uncommon causes of urinary disease in cats. The most commonly suggested antiseptic for small animal usage is methenamine, a cyclic hydrocarbon administered in combination with either mandelic acid (methenamine mandelate) or hippuric acid (methenamine hippurate). Mandelic acid and hippuric acid serve to acidify the urine and may exhibit some additional antimicrobial activity. With sufficient contact time in acidic urine, methenamine is converted to formaldehyde, a potent antimicrobial agent. Methenamine is contraindicated for patients with renal or hepatic insufficiency, and its use may contribute to overacidification like other urinary acidifiers. Use of other antiseptics or analgesics containing methylene blue or azodyes is discouraged in cats because of the potential for development of Heinz body hemolytic anemia.
Glucocorticoids
Glucocorticoids have been recommended to alleviate urinary bladder or urethral inflammation in cats with iFLUTD. Antiinflammatory effects of glucocorticoids on leukocyte migration, vascular permeability, and arachidonic acid metabolism would be expected to suppress the inflammatory symptomatology and hematuria associated with this disorder. Treatment is based on the assumption that persistent inflammation leads to hematuria. Although it appears that glucocorticoids do little to alter the course of typical iFLUTD,183 some clinicians recommend glucocorticoid administration (prednisone or prednisolone 1 to 2 mg/kg every 24 hours) in cats exhibiting chronic, recurrent, or refractory idiopathic hematuria and dysuria.184 No convincing evidence has been presented to establish that glucocorticoids alter the natural course of iFLUTD or prevent recurrence, however.
Glucocorticoid administration also presents certain risks. Refractory urinary tract infection and pyelonephritis may develop, especially when glucocorticoids are administered to cats with indwelling urinary catheters.185 Prophylactic antimicrobial treatment does not appear to reduce the risk of catheter-induced infection. Glucocorticoids must be considered contraindicated for cats with urinary catheters in place or with evidence of bacteriuria. The catabolic effects of glucocorticoids also may be hazardous in debilitated, azotemic, or dehydrated cats.127
Contemporary Treatment Strategies
Antispasmodic Agents
Agents that relax smooth or striated muscle of the urinary tract have been advocated for symptomatic relief of pollakiuria, dysuria, and stranguria in cats with FLUTD.128,184,186 The anticholinergic agents propantheline and oxybutynin have been recommended for their antispasmodic effects on the urinary bladder. In one small controlled study, propantheline administration did not affect resolution of clinical signs at 5 days after treatment compared with placebo administration;187 however, this agent has little direct smooth muscle relaxant properties. If antispasmodic agents are administered, cats should be monitored for urine retention; the loss of a frequent mechanical washout of urine theoretically could delay resolution of inflammation or predispose cats to urinary tract infection.
Agents acting on urethral musculature also have been recommended to facilitate urination in dysuric cats and to alleviate functional urethral obstruction in postobstructed cats. Phenoxybenzamine and prazosin are α-adrenergic antagonists that serve to inhibit urethral smooth muscle contracture. These agents may be helpful in minimizing resistance in the preprostatic and prostatic portions of the urethra in cats;188-189 striated muscle components of the urethra are not affected. Diazepam or dantrolene may be more effective in relaxing skeletal muscle in the postprostatic urethra.165,166,189 Phenothiazine derivatives, such as acepromazine188 and aminopropazine, may also be effective as direct smooth and striated muscle relaxants and can be particularly useful in minimizing anxiety of acutely affected cats.
Analgesia
For acute flare-ups of lower urinary tract signs, short-term analgesic treatments may be useful to reduce the discomfort associated with bladder and urethral inflammation. Butorphanol (0.5 to 1.25 mg/cat orally every 4 to 6 hours) has been recommended;190 longer-acting buprenorphine can be considered as well. Both agents can be given as subcutaneous injections if this is less stressful to the cat.186 NSAIDs have also been recommended for analgesic and antiinflammatory effects. No controlled studies are available to demonstrate a response from any of these agents.
Anxiolytic Agents
A variety of other agents have been considered for treatment of nonseptic, idiopathic inflammatory cystitis (interstitial cystitis [IC]) in human patients, and many have been suggested for similar usage in cats. In some ways the disease in cats does appear to mimic IC in women, a disorder characterized by dysuria, pollakiuria, and painful urination without demonstrable cause. Pathophysiologic mechanisms identified in women with IC have been documented in affected cats,178,191-193 including increased mast cell numbers in the urinary bladder, decreased urinary glycosaminoglycan excretion,191 and altered urinary bladder permeability.193Additional investigations have focused on the influence of neurogenic mediators of inflammation in IC, in which sensory input from afferent neurons in the urinary bladder may trigger inflammatory and pain responses.192
A variety of anxiolytic and antidepressant agents have been investigated in IC, including antihistamines, doxepin, and amitriptyline (Elavil). Amitriptyline is a tricyclic antidepressant with multiple actions, including (1) potentiation of neurotransmitter activity in the central nervous system, (2) inhibition of histamine release, (3) potent antihistaminic properties, and (4) anticholinergic activity.194 As an antidepressant and antianxiety agent, amitriptyline has been used in small animals for behavioral modification, elimination disorders, chronic pruritus, and self-mutilation.195,196 The agent has shown promise for the treatment of IC in women and has been recommended for alleviation of anxiety and pain associated with iFLUTD.192 Eleven of fifteen cats treated with amitriptyline were free of chronic iFLUTD signs for 6 months in one study, and nine were asymptomatic for 12 months or longer.197
Amitriptyline may be less effective for treating acute than chronic episodes of iFLUTD. Amitriptyline administration (5 mg/day orally) had no beneficial effect in treating 31 cats with acute, nonobstructive iFLUTD, and they were significantly more likely to have recurrence of hematuria and pollakiuria within 7 days of discontinuation of amitriptyline, compared with cats administered a placebo. Clinical signs usually resolved within 7 days, regardless of whether a placebo or amitriptyline was administered.198 A second placebo-controlled study of 24 cats with iFLUTD did not show a benefit of daily amitriptyline (10 mg/cat) administered for 1 week.199
For cats in which amitriptyline is indicated, a starting dosage of 5 mg/cat every 24 hours is empirically recommended; the dose is adjusted to effect a mild calming behavior in the cat, which is usually achieved with dosages of 2.5 to 12.5 mg/cat per day.192 Long-term treatment is recommended, along with elimination of environmental stresses, in cats affected by severe, recurrent idiopathic disease. Adverse effects of amitriptyline administration in human patients include anticholinergic effects (dry mouth, blurred vision, constipation), hypotension, drowsiness, and cardiac arrhythmias.194 Sedation, vomiting, and disorientation have been reported in dogs treated with the drug,195 whereas transient sedation has been the most common adverse effect observed in cats. Amitriptyline may also predispose to bacterial cystitis by causing urinary retention and decreased frequency of urination.198 Clomipramine (0.5 mg/kg/day) is a good alternative anxiolytic agent that seems to be more tolerable for cats.
Behavioral-modification drugs have been used in treatment of iFLUTD, but results are only anecdotal. Fluoxetine and paroxetine, two selective serotonin reuptake inhibitors, have been used with some success to treat compulsive disorders in cats and dogs. They are dosed at 0.5 to 1 mg/kg orally every 24 hours. Although considered safe, sedation, anxiety, poor appetite, constipation, lowered seizure threshold, and difficulty in controlling diabetic patients are possible side effects. These drugs should not be used concurrently with monoamine oxidase inhibitors, selegiline, or L-tryptophan.200 In a recent prospective clinical trial involving 12 cats, feline facial pheromone was compared with a placebo as treatment for iFLUTD. Although there was no statistical differences between the two groups, more of the pheromone-treated cats had less severe and fewer episodes of iFLUTD,201 and further studies are warranted. Modification of environment and attention to behavioral issues must be done concurrently to minimize the neuroendocrine influences on the disease. Environmental enrichment, reduction in intercat conflict or aggression, change of feeding method, and litter box management are included in the potential modifications.176
Glycosaminoglycans
Pentosan polysulfate (PPS; Elmiron) is a synthetic polysaccharide that augments the protective glycosaminoglycan layer of the urinary bladder. Orally administered PPS has resulted in good long-term responses (>6 to 12 months) in some women with IC202 and may be effective in reducing clinical episodes in cats with recurrent or chronic idiopathic disease. The currently recommended dosage for cats is 8 mg/kg orally every 12 hours.190 Oral glucosamine has been suggested as an alternative glycosaminoglycan. In a controlled study of 40 cats treated for 6 months, there was no difference in owner assessments of each cat’s health and number of days with lower urinary tract signs between glucosamine-treated cats and placebo-treated cats.201 Cats in both groups were somewhat improved owing to the introduction of canned cat food; however, recurrences were still frequent.
Intravesicular Agents used in Lower Urinary Tract Disease
Infusion of antimicrobial and antiinflammatory agents into the urinary bladder has been attempted as a form of local therapy in dogs and cats with lower urinary tract disease. The idea of directly applying antiseptic, antiinflammatory, or analgesic agents to diseased mucosa is enticing. Even saline infusion may provide symptomatic relief of lower urinary tract symptoms. Simple urohydrodistention provides temporary relief of symptoms in some women with IC.203 Presumably, extreme distention of the urinary bladder may stimulate and exhaust mast cell degranulation, induce urinary glycosaminoglycan production, and cause ischemic degeneration of bladder sensory nerve endings. Released inflammatory mediators also can be flushed with the infused solution. Instillation of solutions into the urinary bladder requires placement of a urinary catheter, however, and urohydrodistention requires general or regional anesthesia. Furthermore, agents instilled into the urinary bladder often are rapidly voided and may be altered with inflammation; enhanced permeability to salicylate infusion has been documented in cats with idiopathic cystitis.193 Thus the risk of significant systemic absorption of intravesicular agents is difficult to predict.
Antifungal Agents
Primary fungal infections of the lower urinary tract are rare, and Candida spp. are the most common cause of funguria in dogs and cats. Candida spp. are normal florae of the gastrointestinal system, upper respiratory tract, and genital mucosa. Infections in dogs and cats are usually due to Candida albicans, although other species have been reported. Candiduria in humans occurs when predisposing factors are present, such as indwelling urinary catheters, diabetes mellitus, drug therapy (antibiotic, steroids, cancer chemotherapeutic), and lower urinary disease. In dogs and cats, these same factors are likely to be relevant. In a report of 31 cases of candiduria in dogs and cats, 35% of the patients had diabetes mellitus.204
Unlike management of funguria in humans, in which correction of the underlying condition often resolves the infection, treatment of all cases of funguria in dogs and cats is recommended. The drug of first choice in humans with a persistent infection is fluconazole, and this is likely true for dogs and cats. The condition should be managed as a complicated urinary tract infection, and treatment should be continued for at least 2 to 4 weeks, with resolution confirmed by negative urine culture. If the infection persists beyond 4 weeks of therapy, then antifungal susceptibility testing should be done because Candida organisms can develop resistance to azole drugs.204 Previously reported doses of fluconazole are 2.5 to 5 mg/kg orally every 24 hours in dogs and 2.5 to 10 mg/kg orally every 12 hours in cats.17 An alternative treatment to oral or injectable antifungals is intravesicular infusions of 1% clotrimazole into the urinary bladder, using a Foley catheter. A dose of 9 mL/kg infused by a urinary catheter weekly for 3 weeks was successful in treating a diabetic cat. A diabetic dog was successfully treated with 7 mL/kg injected into the bladder, with ultrasound assistance, for four treatments. This treatment may be a useful alternative to oral fluconazole, although further studies are needed to prove efficacy and safety.204
Dimethylsulfoxide
The free radical scavenging agent DMSO has been applied intravesicularly as a local antiinflammatory and analgesic agent. The drug and its metabolites neutralize free radical hydroxides and free radical oxygen, modulate platelet aggregation and prostaglandin metabolism, decrease fibroplasia, and provide local analgesia. In high concentrations DMSO may have antibacterial activity, but it also creates mucosal edema and hemorrhage.205 The agent has been used in the management of interstitial and radiation-induced cystitis in human patients, cyclophosphamide-induced cystitis in dogs, and idiopathic cystitis in cats.184,203,206 DMSO infusion may be considered for cats with chronic, refractory idiopathic disease, especially those with thickened urinary bladder walls.
The treatment regimen for cats involves instillation of 10 to 20 mL of 10% medical grade DMSO (Rimso-50) into the urinary bladder with the cat under general anesthesia. The solution is left in the urinary bladder for 10 minutes and then removed. The process can be repeated 2 weeks after the initial application if needed.184 Up to 25 mL of 25% to 50% intravesicular DMSO is empirically recommended for dogs. In cats instillation of 45% veterinary grade DMSO for 3 days after induction of salicylate/ethanol bladder wall injury did not alter the subsequent inflammatory response or infection rate.185 The drug also may have contributed to renal lesions observed in these cats and appeared to be locally irritating.185 Intravascular hemolysis and hemoglobinuria may result if significant quantities of DMSO are absorbed.
Bacillus Calmette–Guérin Therapeutic
Bacillus Calmette–Guérin (BCG) Therapeutic is a bacterial product of attenuated Mycobacterium bovis, with muramyl dipeptide as the predominant active compound. Intravesicular BCG cell wall infusion is used as an alternative to radical surgery in human patients with carcinoma of the urinary bladder. The agent promotes a local inflammatory reaction that appears to suppress superficial cancerous lesions by incompletely understood mechanisms.207 Effects of T cells and natural killer cells also lead to its use as an immunostimulant. The agent must be administered by way of nontraumatic urethral catheterization and handled as hazardous infectious material. Local irritation and hematuria are commonly observed after infusion; systemic adverse effects are rare but may include fever, nausea, diarrhea, anemia, leukopenia, ureteral obstruction, shock, and death. The agent is contraindicated for patients with urinary tract infections or fevers and those receiving immunosuppressive therapy. In dogs injection of intralesional BCG has been variably helpful during surgical resection of transitional cell carcinomas. Severe granulomatous reactions are possible.
Infusion of other chemotherapeutic agents (doxorubicin, thiotepa) is rarely attempted in veterinary medicine because of the advanced stage of disease usually present at diagnosis. Local infusions are not expected to penetrate beyond the submucosa, whereas most neoplasms have infiltrated the muscularis or serosa in small animals.208
Piroxicam
Piroxicam (Feldene), a potent NSAID, appears to have antitumor activity in some animals with urinary tract neoplasia.209 In dogs with transitional cell carcinoma, sustained remissions are possible in selected individuals, whereas maintenance of stable disease may be obtained in many. For neoplastic disease, piroxicam is administered daily at 0.3 mg/kg/day. Piroxicam (administered daily or every other day) also has been considered for the treatment of chronic inflammatory bladder disorders. Clinical experience supports this practice; the safety and efficacy of the drug have not been critically evaluated, however.174 Gastrointestinal ulceration is common; concurrent administration of gastrointestinal protectants is recommended.
1. Mazzaferro E.M., Eubig P.A., Hackett T.B., et al. Acute renal failure associated with raisin or grape ingestion in 4 dogs. J Veter Emerg Crit Care. 2004;14:203.
2. Toto R.D. Renal insufficiency due to angiotensin-converting-enzyme inhibitors. Miner Electrolyte Metab. 1994;20:193.
3. Longhofer S.L., Ericsson G.F., Cifelli S., Benitz A.M. Renal function in heart failure dogs receiving furosemide and enalapril maleate. J Vet Intern Med. 1993;7:123.
4. Murray M.D., Brater D.C. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol. 1993;32:435.
5. Forrester S.D., Lees G.E. Renal manifestations of polysystemic diseases. In: Osborne C.A., Finco D.R., editors. Canine and feline nephrology and urology. Baltimore: Williams & Wilkins; 1995:491.
6. Lobetti R.G., Jacobson L.S. Renal involvement in dogs with babesiosis. J S Afr Vet Assoc. 2001;72:23.
7. Lane I.F., Grauer G.F., Fettman M.J. Acute renal failure: Part I: Risk factors, prevention and strategies for protection. Compend Contin Educ Pract Vet. 1994;16:15.
8. Chew D.J. Fluid therapy during intrinsic renal failure. In: Dibartola S.P., editor. Fluid therapy in small animal practice. Philadelphia: Saunders; 1992:554.
9. Cowgill L.D., Francey T. Acute uremia. In Ettinger S.J., Feldman E., editors: Textbook of veterinary internal medicine, ed 6, St Louis: Saunders, 2005.
10. MacIntyre DK, Royer N: Management of acute renal failure in critical patients: technical concerns, Lake Buena Vista, Fla, 1995, Proc 13th Am Coll Vet Intern Med Forum, p. 10.
11. Adelman R.D., Spangler W.L., Beasom F., et al. Furosemide enhancement of experimental gentamicin nephrotoxicity: comparison of functional and morphological changes with activities of urinary enzymes. J Infect Dis. 1979;140:342.
12. Lindner A., Cutler R.E., Goodman W.G. Synergism of dopamine plus furosemide in preventing acute renal failure in the dog. Kidney Int. 1979;16:158.
13. Adin D.B., Taylor A.W., Hill R.C., et al. Intermittent bolus injection versus continuous infusion of furosemide in normal adult greyhound dogs. J Vet Intern Med. 2003;17:632.
14. Cantarovich F., Rangoonwala B., Lorenz H., et al. High-dose furosemide for established ARF: a prospective, randomized, double-blinded, placebo-controlled, multicenter trial. Am J Kidney Dis. 2004;44:402.
15. Denton M.D., Chertow G.M., Brady H.R. “Renal-dose” dopamine for the treatment of acute renal failure: scientific rationale, experimental studies and clinical trials. Kidney Int. 1996;49:4.
16. Clark K.L., Robertson J.M., Drew G.M. Do renal tubular dopamine receptors mediate dopamine-induced diuresis in the anesthetized cat? J Cardiovasc Pharmacol. 1991;17:267.
17. Plumb D.C. Veterinary drug handbook, ed 5. Philadelphia: Wiley-Blackwell; 2005.
18. Debaveye Y.A., van den Berghe G.H. Is there still a place for dopamine in the modern intensive care unit? Anesth Analg. 2004;98:461.
19. Burnier M., Schrier R.W. Protection from acute renal failure. Adv Exp Med Biol. 1986;212:275.
20. Kirby R. Acute renal failure as a complication in the critically ill animal. Vet Clin North Am Small Anim Pract. 1989;19:1189.
21. Ross L.A. Fluid therapy for acute and chronic renal failure. Vet Clin North Am Small Anim Pract. 1989;19:343.
22. Finco D.R., Low D.G. Intensive diuresis in polyuric renal failure. In: Kirk R.W., editor. Current veterinary therapy VII, small animal practice. Philadelphia: Saunders; 1980:1091.
23. Cowgill L.D., Francey T., Strickland A.D., et al. Reduction of dialysis dependency with an oral cross-linked polyelectrolyte sorbent (CLP) in ESRD (abstract). ASAIO J. 2002;48(2):177.
24. Kintzer P.P., Peterson M.E. Hypoadrenocortism. In: Bonagura J.D., Twedt D.C., editors. Kirk’s current veterinary therapy XIV. St Louis: Saunders, 2009.
25. White J.V., Finco D.R., Crowell W.A., et al. Effect of dietary protein on functional, morphologic and histologic changes of the kidney during compensatory hypertrophy. Am J Vet Res. 1991;52:1357.
26. Venkataraman R., Kellum J.A. Novel approaches to the treatment of acute renal failure. Expert Opin Invest Drugs. 2003;12:1353.
27. Brady H.R., Brenner B.M., Lieberthal W. Acute renal failure. In: Brenner B.M., editor. Brenner and Rector’s the kidney. ed 5. Philadelphia: Saunders; 1996:1200.
28. Schrier R.W., Wang W., Poole B., et al. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5.
29. Elliott J., Watson A.D.J. Chronic kidney disease: staging and management. In: Bonagura J.D., Twedt D.C., editors. Kirk’s current veterinary therapy XIV. St Louis: Saunders; 2009:883-892.
30. Hostetter T.H., Olson J.I., Rennke H.G., et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85.
31. Brown S.A. Canine renal disease. In: Wills J.M., Simpson K.W., editors. The Waltham book of clinical nutrition of the dog and cat. Oxford, England: Pergamon; 1994:313.
32. Brown S.A., Finco D.R., Crowell W.A., et al. Single-nephron adaptations to partial renal ablation in the dog. Am J Physiol. 1990;258:F495.
33. Finco D.R., Brown S.A., Crowell W.A., et al. Effects of dietary phosphorus and protein in dogs with chronic renal failure. Am J Vet Res. 1992;53:2264.
34. Finco D.R., Brown S.A., Crowell W.A., et al. Effects of dietary protein intake on geriatric dogs with reduced renal mass. Am J Vet Res. 1994;55:867.
35. Polzin D.J., Osborne C.A., O’Brien T.D., et al. Effects of protein intake on progression of canine chronic renal failure. J Vet Intern Med. 1993;7:125.
36. Adams L.G., Polzin D.J., Osborne C.A., et al. Effects of dietary protein and calorie restriction in clinically normal cats and in cats with surgically induced chronic renal failure. Am J Vet Res. 1993;54:1653.
37. Polzin D.J., Osborne C.A. Conservative medical management of chronic renal failure. In: Osborne C.A., Finco D.R., editors. Canine and feline nephrology and urology. Baltimore: Williams & Wilkins; 1995:508.
38. Brown S.A., Crowell W.A., Barsanti J.A., et al. Beneficial effects of dietary mineral restriction in dogs with marked reduction of functional renal mass. J Am Soc Nephrol. 1991;1:1169.
39. Elliott J., Rawlings J.M., Markwell P.J., et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract. 2000;41:242.
40. Polzin D., Osborne C., Ross S. Chronic kidney disease. In: Ettinger S., Feldman E., editors. Textbook of Veterinary Internal Medicine. St Louis: Elsevier, 2005.
41. Acierno M.J. Systemic hypertension in renal disease. In: Bonagura J.D., Twedt D.C., editors. Kirk’s current veterinary therapy XIV. St Louis: Saunders, 2009.
42. Buranakarl C., Mathur S., Brown S.A. Effects of dietary sodium chloride intake on renal function and blood pressure in cats with normal and reduced renal function. Am J Vet Res. 2004;65:620.
43. Brown SA: Dietary fatty acid supplementation and chronic renal disease, Lake Buena Vista, Fla, 1995, Proc 13th Am Coll Vet Intern Med Forum, p. 470.
44. Bauer JE: Management of spontaneous canine renal disease by dietary polyunsaturated fatty acids, Lake Buena Vista, Fla, 1995, Proc Am Coll Vet Intern Med Forum, p. 477.
45. Chew D.J., Dibartola S.P., Nagode L.A., et al. Phosphorus restriction in the treatment of chronic renal failure. In: Kirk R.W., Bonagura J.D., editors. Current veterinary therapy XI, small animal practice. Philadelphia: Saunders, 1992.
46. Polzin D.J., Osborne C.A., Ross S. Chronic kidney disease. In Ettinger S.J., Feldman E., editors: Textbook of veterinary internal medicine, ed 6, St Louis: Saunders, 2005.
47. Llach F., Bover J. Renal osteodystrophy. In: Brenner B.M., editor. Brenner and Rector’s the kidney. ed 5. Philadelphia: Saunders; 1996:2187.
48. Autran de Morais H. Dibartola SP: Acid-base disorders. In: Bonagura J.D., Twedt D.C., editors. Kirk’s current veterinary therapy XIV. St Louis: Saunders, 2009.
49. Giovanetti S., Cupisti A., Barsotti G. The metabolic acidosis of chronic renal failure: pathophysiology and treatment,. Contrib Nephrol. 1992;100:48.
50. Lulich J., Osborne C.A., O’Brien T., et al. Feline renal failure: questions, answers, questions. Compend Contin Educ Pract Vet. 1992;14:127.
51. Dow S.W., Lecouteur R.A., Fettman M.J., et al. Potassium depletion in cats: hypokalemic polymyopathy. J Am Vet Med Assoc. 1987;191:1563.
52. Dow S.W., Fettman M.J. Renal disease in cats: the potassium connection. In: Kirk R.W., Bonagura J.D., editors. Current veterinary therapy XI, small animal practice. Philadelphia: Saunders; 1992:820.
53. Dow S.W., Fettman M.J., Smith K.R., et al. Effect of dietary acidification and potassium depletion on acid-base balance, mineral metabolism and renal function in adult cats. J Nutr. 1990;120:569.
54. DiBartola S.P., Buffington C.A., Chew D.J., et al. Development of chronic renal disease in cats fed a commercial diet. J Am Vet Med Assoc. 1993;202:744.
55. Jacob F., Polzin D.J., Osborne C.A., et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in dogs. J Am Vet Med Assoc. 2002;220:1163.
56. Ross S., Osborne C., Polzin D., et al. Clinical evaluation of effects of dietary modification in cats with spontaneous chronic renal failure (abstr.). J Vet Intern Med. 2005;19(3):433.
57. Bartges J.W., Brown S.A. Summary of dietary recommendations in urinary diseases. In: Bonagura J., editor. Kirk’s current veterinary therapy XIII. Philadelphia: Saunders; 2000:841.
58. Osborne C.A., Lulich J.P., Sanderson S.L., et al. Treatment of uremic anorexia. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XII, small animal practice. Philadelphia: Saunders; 1995:966.
59. Ogilvie G.K. Dolasetron: a new option for nausea and vomiting. J Am Anim Hosp Assoc. 2000;36:481.
60. Ljutic D., Perkovic D., Rumboldt Z., et al. Kidney. Blood Press Rev. 2002;25:61.
61. de la Puente-Redondo V.A., Siedek E.M., Benchaoui H.A., et al. The anti-emetic efficacy of maropitant (Cerenia) in the treatment of ongoing emesis caused by a wide range of underlying clinical aetiologies in canine patients in Europe. J Small Anim Pract. 2007;48:93-98.
62. Hickman M.A., Cox S.R., Mahabir S., et al. Safety, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (Cerenia) for the prevention of emesis and motion sickness in cats. J Vet Pharmacol Ther. 2008;31:220-229.
63. Jacob F., Polzin D.J., Osborne C.A., et al. Association between initial systolic blood pressure and risk of developing a uremic crisis or of dying in dogs with chronic renal failure. J Am Vet Med Assoc. 2003;222:322.
64. Stepien R.L., Henik R.A. Systemic hypertension. In: Bonagura J.D., Twedt D.C., editors. Kirk’s current veterinary therapy XIV. St Louis: Saunders, 2009.
65. Brown S.A., Finco D.R., Brown C.A., et al. Evaluation of the effects of inhibition of angiotensin converting enzyme with enalapril in dogs with induced renal insufficiency. Am J Vet Res. 2003;64:321.
66. Brown S.A., Brown C.A., Jacobs G., et al. Effects of the angiotensin converting enzyme inhibitor benazepril in cats with induced renal insufficiency. Am J Vet Res. 2001;62:375.
67. Brown SA: Renoprotective mechanisms of angiotensin inhibition, Minneapolis, 2004, Proc 22nd American Coll of Vet Intern Med, p. 712.
68. Henik RA, Snyder PS, Volk LM: Amlodipine bisylate therapy in cats with systemic arterial hypertension secondary to chronic renal disease (abstr.), San Francisco, 1994, Proc 12th ACVIM Forum, p. 976.
69. Ross L.A. Hypertension and chronic renal failure. Semin Vet Med Surg Small Anim. 1992;7:221.
70. Chew DJ, Nagoda LA, Carothers MA et al: Calcitriol treatment of renal secondary hyperparathyroidism in dogs and cats, Washington, DC, 1993, Proc 11th Am Coll Vet Intern Med Forum, p. 164.
71. Polzin D.J., Ross S., Osborne C.A. Calcitriol. In: Bonagura J.D., Twedt D.C., editors. Kirk’s current veterinary therapy XIV. St Louis: Saunders, 2009.
72. Polzin D.J., Ross S., Osborne C., et al. Clinical benefit of calcitriol in canine chronic kidney disease (abstr.). J Vet Intern Med. 2005;19(3):433.
73. King L.G., Giger U., Diserens D., et al. Anemia of chronic renal failure in dogs. J Vet Intern Med. 1992;6:264.
74. Cowgill L.D. Medical management of the anemia of chronic renal failure. In: Osborne C.A., Finco D.R., editors. Canine and feline nephrology and urology. Baltimore: Williams & Wilkins; 1995:539.
75. Cowgill L.D., Feldman B., Levy J., et al. Efficacy of recombinant human erythropoietin (rHuEPO) for anemia in dogs and cats with renal failure. J Vet Intern Med. 1990;4:126.
76. Cowgill L.D. CVT Update: use of recombinant human erythropoietin. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XII, small animal practice. Philadelphia: Saunders; 1995:961.
77. Randolph J.F., Scarlett J., Stokol T., et al. Clinical efficacy and safety of recombinant canine erythropoietin in dogs with anemia of chronic renal failure and dogs with recombinant human erythropoietin-induced red cell aplasia. J Vet Intern Med. 2004;18:81.
78. Vaden S. Glomerular diseases. In: Ettinger S.J., Feldman E.C., editors. Textbook of veterinary internal medicine. ed 6. Philadelphia: Saunders; 2005:1786.
79. Elliot J: Importance of proteinuria in cats with chronic renal failure, Minneapolis, 2004, Proc 22nd American Coll Vet Intern Med, p. 708.
80. DiBartola S.P., Benson M.D. Pathogenesis of reactive systemic amyloidosis. J Vet Intern Med. 1989;3:31.
81. Grauer G.F., DiBartola S.P. Glomerular diseases. In: Ettinger S.J., Feldman R.N., editors. Textbook of veterinary internal medicine. ed 4. Philadelphia: Saunders; 1996:1760.
82. Relford R.L., Green R.A. Coagulation disorders in glomerular diseases. In: Kirk R.W., Bonagura J., editors. Current veterinary therapy XI, small animal practice. Philadelphia: Saunders; 1992:827.
83. Lees G.E., Brown S.A., Grauer G.F., et al. Assessment and management of proteinuria in dogs and cats. 2004 ACVIM Forum Consensus Statement (Small Animal). 2004.
84. Grauer G.F., Greco D.S., Getzy D.M., et al. Effects of enalapril versus placebo as a treatment for canine idiopathic glomerulonephritis. J Vet Intern Med. 2000;14:526.
85. Llach F. Hypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndrome. Kidney Int. 1985;28:429.
86. Vaden S. The effects of cyclosporin versus standard care in dogs with naturally occurring glomerulonephritis,. J Vet Intern Med. 1995;9:259.
87. Longhofer S.L., Culham C.A., Frisbie D.D., et al. Effects of thromboxane synthetase inhibition on immune complex glomerulonephritis. Am J Vet Res. 1991;52:480.
88. Grauer G.F., Frisbie D.D., Snyder P.S., et al. Treatment of membranoproliferative glomerulonephritis and nephrotic syndrome in a dog with a thromboxane synthetase inhibitor. J Vet Intern Med. 1992;6:77.
89. Vaden S.L., Brown C.A. Glomerular disease. In: Bonagura J., Twedt D.C., editors. Kirk’s current therapy XIV. St Louis: Saunders, 2009.
90. Brown S.A., Brown C.A., Crowell W.A., et al. Dietary lipid composition alters the chronic course of canine renal disease. J Vet Intern Med. 1996;10:168.
91. Hall A.V., Parbtani A., Clark W.F., et al. Omega-3 fatty acid supplementation in primary nephrotic syndrome: effects on plasma lipids and coagulopathy. J Am Soc Nephrol. 1992;3:1321.
92. Spyridakis L., Brown S.A., Barsanti J.A., et al. Amyloidosis in a dog: treatment with dimethylsulfoxide. J Am Vet Med Assoc. 1986;189:690.
93. Gruys E., Sijens R.J., Biewenga W.J. Dubious effect of dimethylsulfoxide therapy on amyloid deposits and amyloidosis. Vet Res Commun. 1981;5:21.
94. Cowgill L.D. Diseases of the kidney. In: Ettinger S.J., editor. Textbook of veterinary internal medicine. ed 2. Philadelphia: Saunders; 1983:1843.
95. Carter L.J., Wingfield W.E., Allen T.A. Clinical experience with peritoneal dialysis in small animals. Compend Contin Educ Pract Vet. 1989;11:1335.
96. Crisp M.S., Chew D.J., DiBartola S.P., et al. Peritoneal dialysis in dogs and cats: 27 cases (1976-1987). J Am Vet Med Assoc. 1989;195:1262.
97. Thornhill J.A., Hartman J., Boon G.D., et al. Support of an anephric dog for 54 days with ambulatory peritoneal dialysis and a newly designed peritoneal catheter. Am J Vet Res. 1984;45:1156.
98. Ross L.A., Labato M.A. Peritoneal dialysis. In DiBartola S.P., editor: Fluid, electrolyte, and acid-base disorders in small animal practice, ed 3, St Louis: Saunders, 2006.
99. Lane I.F., Carter L.J., Lappin M.R. Peritoneal dialysis: an update on methods and usefulness. In: Kirk R.W., editor. Current veterinary therapy XI, small animal practice. Philadelphia: Saunders; 1992:865.
100. Cowgill LD: Veterinary hemodialysis: state of the art/science, San Antonio, Texas, 1996, Proc 14th Am Coll Vet Intern Med Forum, p. 368.
101. Langston C.E. Hemodialysis. In: Bonagura J., Twedt D.C., editors. Kirk’s current veterinary therapy XIV. St Louis: Saunders, 2009.
102. Cowgill L.D., Francey T. Hemodialysis. In DiBartola S.P., editor: Fluid, electrolyte, and acid-base disorders in small animal practice, ed 3, St Louis: Saunders, 2006.
103. Langston C.E., Cowgill L.D., Spano J. The application of hemodialysis in uremic cats: a review of 24 cases. J Vet Intern Med. 1996;10:168.
104. Gregory C.R., Gourley I.M., Kochin E.J., et al. Renal transplantation for treatment of end-stage renal failure in cats. J Am Vet Med Assoc. 1992;201:285.
105. Gregory C.R. Renal transplantation in cats. Compend Contin Educ Pract Vet. 1993;15:1325.
106. Gregory C.R., Gourley I.M. Organ transplantation in clinical veterinary medicine. In: Slatter D.H., editor. Textbook of small animal surgery. ed 2. Philadelphia: Saunders; 1993:95.
107. Adin C.A., Gregory C.R., Kyles A.E., et al. Diagnostic predictors of complications and survival after renal transplantation in cats. Vet Surg. 2001;30:515.
108. Aronson L.R., Drobatz K.J. Hypercalcemia following renal transplantation in a cat. J Am Vet Med Assoc. 2000;21:1034.
109. Wooldridge J.D., Gregory C.R., Mathews K.G., et al. The prevalence of malignant neoplasia in feline renal-transplant recipients. Vet Surg. 2002;31:94.
110. Lothrop C: Simultaneous bone-marrow transplantation and renal transplantation for end-stage renal disease in dogs, 2005, Proc 23rd Annual Forum of the American College of Veterinary Internal Medicine, p. 515.
111. deGroat W.C., Booth A.M. Physiology of the bladder and urethra. Ann Intern Med. 1980;92:312-315.
112. Lane I.F., Westropp J.L. Urinary incontinence and micturition disorders: pharmacologic management. In: Bonagura J.D., Twedt D.C., editors. Current veterinary therapy XIV. St Louis: Saunders, 2009.
113. Barsanti J.A. Urinary incontinence. In: Lorenz M.D., Cornelius L.M., editors. Small animal medical diagnosis. ed 2. Philadelphia: Lippincott; 1993:345.
114. Lane I.F. Treating urinary incontinence. Vet Med. 2003;98:58.
115. Fischer J.R., Lane I.F. Treating functional urinary obstruction. Vet Med. 2003;98:67.
116. Finkbeiner A.E. Is bethanechol chloride effective in promoting bladder emptying? J Urol. 1985;134:443.
117. Labato M.A. Disorders of micturition. In Morgan R., editor: Handbook of small animal practice, ed 5, St Louis: Churchill Livingstone, 2008.
118. Rosin A.H., Ross L. Diagnosis and pharmacologic management of disorders of urinary continence in the dog. Compend Contin Educ Pract Vet. 1981;3:601.
119. EI-Salmy S., Downie J.W., Awad S.A. Urethral function after chronic cauda equina lesions in cats: I. The contribution of mechanical factors and sympathetic innervation to proximal sphincter dysfunction. J Urol. 1990;144:1022.
120. Boyd I.W., Rohan A.P. Urinary disorders associated with cisapride. Med J Aust. 1994;160:579.
121. Carone R., Vercelli D., Bertapelle P. Effects of cisapride on anorectal and vesicourethral function in spinal cord injured patients. Paraplegia. 1993;31:125.
122. Coates: 2004. Neurogenic micturition disorders, Proc 22nd Am College of Vet Intern Med Forum, Minneapolis, 2004, pp.324–326.
123. Lappin M.R., Barsanti J.A. Urinary incontinence secondary to idiopathic detrusor instability: cystometrographic diagnoses and pharmacologic management in 2 dogs and a cat. J Am Vet Med Assoc. 1987;191:1439.
124. Lane I.F. Disorders of micturition. In: Osborne C., Finco D.R., editors. Canine and feline nephrology and urology. Baltimore: Williams & Wilkins; 1995:693.
125. Wefer J., Truss M.C., Jonas U. Tolterodine: an overview. World J Urol. 2001;19:312-318.
126. Lane I.F. Use of anticholinergic agents in lower urinary tract disease. In: Bonagura J., editor. Kirk’s current veterinary therapy XIII. Philadelphia: Saunders; 2000:899.
127. Kruger J.M., Osborne C.A., Lulich J.P. Management of nonobstructive idiopathic feline lower urinary tract disease. Vet Clin North Am Small Anim Pract. 1996;26:571.
128. Ling G.V. Feline urologic syndrome. In: Ling G.V., editor. Lower urinary tract diseases of dogs and cats: diagnosis, medical management, and prevention. St Louis: Mosby; 1995:179.
129. Bacon N.J., Oni O., White A.S. Treatment of urethral sphincter mechanism incompetence in 11 bitches with a sustained-release formulation of phenylpropanolamine hydrochloride. Vet Rec. 2002;151:373.
130. Tapp A.J.S. The effect of sex hormones on the female lower urinary tract. In: Cardozo L., editor. Update on drugs and the lower urinary tract. London: Royal Society of Medicine Services; 1988:15.
131. Arnold S. Relationship of incontinence to neutering. In: Kirk R.W., editor. Current veterinary therapy XI, small animal practice. Philadelphia: Saunders; 1992:875.
132. Mandigers P.J., Nell T. Treatment of bitches with acquired urinary incontinence with oestriol. Vet Rec. 2001;149:765.
133. Angioletti A., DeFrancesco I., Vergottini M., et al. Urinary incontinence after spaying in the bitch: incidence and oestrogen-therapy. Vet Res Comm. 2004;28:153-155.
134. Hoeijmakkers M., Janszen B., Coert A., et al. Pharmacokinetics of oestriol after repeatd oral administration to dogs. Res Vet Sci. 2003;75:55.
135. Barsanti J.A., Edwards P.D., Losonsky J. Testosterone responsive urinary incontinence in a castrated male dog. J Am Anim Hosp Assoc. 1981;17:117.
136. Ling G.V. Disorders of urination. In: Ling G.V., editor. Lower urinary tract diseases of dogs and cats: diagnosis, medical management, and prevention. St Louis: Mosby; 1995:192.
137. Richter K.P., Ling G.V. Clinical response and urethral pressure profile changes after phenylpropanolamine administration in dogs with primary sphincter incompetence. J Am Vet Med Assoc. 1985;187:605.
138. Byron J.K., March P.A., Chew D.J., et al. Effect of phenylpropanolamine and pseudoephedrine on the urethral pressure profile and continence scores of incontinent female dogs. J Vet Intern Med. 2007;21:47.
139. Arnold S., Arnold P., Hubler M., et al. Incontinentin urinae bei der kastrierten huendin: haeufigkeit und rassedisposition. Schweiz Arch Tierheilkd. 1989;131:259.
140. White R.A.S., Pomeroy C.J. Phenylpropanolamine: an α-adrenergic agent for the management of urinary incontinence in the bitch associated with urethral sphincter mechanism incompetence. Vet Rec. 1989;125:478.
141. Scott L., Leddy F., Bernay F., et al. Evaluation of phenylpropanolamine in the treatment of urethral sphincter mechanism incompetence in the bitch. J Small Anim Pract. 2002;43:493.
142. Burgherr T., Reichler I., Hung L., et al. Efficacy, tolerance and acceptability of Incontex in spayed bitches with urinary incontinence. Schweiz Arch Tierheikd. 2007;149:307-313.
143. Carofiglio F., Hamaide A.J., Farnir F., et al. Evaluation of the urodynamic and hemodynamic effects of orally administered phenylpropanolamine and ephedrine in female dogs. Am J Vet Res. 2006;67:723-730.
144. Nendick P.A., Lark W.T. Medical therapy of urinary incontinence in ovariectomized bitches: a comparison of the effectiveness of diethylstilbesterol and pseudoephedrine. Aus Vet J. 1987;64:117.
145. Reichler I.M., Hubler M., Jochle W., et al. The effect of GnRH analogs on urinary incontinence after ablation of the ovaries in dogs,. Theriogenology. 2003;60:1207.
146. Reichler I.M., Jochle W., Piche C.A., et al. Effect of a long acting GnRH analogue or placebo on plasma LH/FSH, urethral pressure profiles and clinical signs of urinary incontinence due to sphincter mechanism incompetence in bitches. Theriogenology. 2006;66:1227.
147. Reichler I.M., Barth A., Piche C., et al. Urodynamic parameters and plasma LH/FSH in spayed Beagle bitches before and 8 weeks after GnRH depot analogue treatment. Theriogenology. 2006;66:2127.
148. Norton P.A., Zinner N.R., Yalcin I., et al. Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol. 2002;187(1):40-48.
149. Arnold S., Hubler M., Lot-Stolz G., Rusch P. Treatment of urinary incontinence in bitches by endoscopic injection of glutaraldehyde cross-linked collagen. J Small Anim Pract. 1996;37:163.
150. Barth A., Reichler I.M., Hubler M., et al. Evaluation of long-term effects of endoscopic injection of collagen into the urethral submucosa for treatment of urethral sphincter incompetence in female dogs: 40 cases (1993-2000). J Am Vet Med Assoc. 2005;226:73.
151. Byron J.B., Chew D.J., McLaughlin M.A., et al. Transurethral collagen implantation for treatment of canine urinary incontinence. ACVIM Forum. 2005. Abstract 120
152. Hoelzler M.G., Lidbetter D.A. Surgical management of urinary incontinence. Vet Clin North Am Small Anim Pract. 2004;34:1057.
153. Rawlings C., Barsanti J.A., Mahaffey M.B., et al. Evaluation of colposuspension for treatment of incontinence in spayed female dogs. J Am Vet Med Assoc. 2001;219:770.
154. Khanna O.P., Gonick P. Effects of phenoxybenzamine on canine lower urinary tract. Urology. 1975;6:323.
155. Barsanti J.A., Coates J.R., Bartges J.W. Detrusor-sphincter dyssynergia. Vet Clin North Am Small Anim Pract. 1996;26:327.
156. Lees G.E. Management of voiding disability following relief of urethral obstruction. In: August J.R., editor. Consultations in feline internal medicine 2. Philadelphia: Saunders; 1994:365.
157. Wein A.J. Drug therapy for neurogenic and non-neurogenic bladder dysfunction. In: Seidmon E.J., Hanna P.M., editors. Current urologic therapy. Philadelphia: Saunders; 1994:291.
158. Fischer J.R., Lane I.F., et al. Urethral pressure profile and hemodynamic effects of phenoxybenzamine and prazosin in non-sedated male beagle dogs. Can J Vet Res. 2003;67:30.
159. Lulich JP: Managing functional urethral obstruction, Minneapolis, 2004, Proc 22nd Forum of the American College of Veterinary Internal Medicine, p. 514.
160. Ohtake A., Sato S., Sasamata M., et al. Effects of tamsulosin on resting urethral pressure and arterial blood pressure in anaesthetized female dogs. J Pharm Pharmacol. 2006;58(3):345-350.
161. Sato S., Ohtake A., Hatanaka T., et al. Relationship between the functional effect of tamsulosin and its concentration in lower urinary tract tissues of dogs. Biol Pharm Bull. 2007;30:481-486.
162. Sudoh K., Tanaka H., Inagaki O., et al. Effect of tamsulosin, a novel alpha 1-adrenoceptor antagonist, on urethral pressure profile in anaesthetized dogs. J Auton Pharmacol. 1996;16(3):147-154.
163. Witte D.G., Brune M.E., Katwala S.P., et al. Modeling of relationships between pharmacokinetics and blockade of agonist-induced elevation of intraurethral pressure and mean arterial pressure in conscious dogs treated with alpha(1)-adrenoceptor antagonists. J Pharmacol Exp Ther. 2002;300(2):495-504.
164. Tatemichi S., Tomiyama Y., Maruyama I., et al. Uroselectivity in male dogs of silodosin (KMD-3213), a novel drug for the obstructive component of benign prostatic hyperplasia. Neurolurol Urodyn. 2006;25:792-799.
165. Straeter-Knowlen I.M., Knowlen G.G., Speth R.C., et al. Effect of succinyl choline, diazepam and dantrolene on the urethral pressure profile of healthy, sexually intact male cats. Am J Vet Res. 1994;55:1739.
166. Straeter-Knowlen I.M., Marks S.L., Rishniw M., et al. Urethral pressure response to smooth and skeletal muscle relaxants in anesthetized, adult male cats with naturally acquired urethral obstruction. Am J Vet Res. 1995;56:919.
167. Polzin D.J., Osborne C.A. Diseases of the urinary tract. In: Davis L.E., editor. Handbook of small animal therapeutics. New York: Churchill-Livingstone; 1985:333.
168. Blackwell N.J. Reflex dyssynergia in the dog. Vet Rec. 1993;132:516.
169. Collins B.K., Moore C.P., Hagee J.H. Sulfonamide-associated keratoconjunctivitis sicca and corneal ulceration in a dysuric dog. J Am Vet Med Assoc. 1986;189:924.
170. Gookin J.L., Bunch S.E. Detrusor-striated sphincter dyssynergia in a dog. J Vet Intern Med. 1996;10:339.
171. Lane I.F., Fischer J.R., Miller E.M., Grauer G. Functional urethral obstruction in three dogs: urethral pressure profile results and response to alpha adrenergic antagonists. J Vet Intern Med. 1998;14:43.
172. Porpiglia F., Ghignone G., Fiori C., et al. Nifedipine versus tamsulosin for the management of lower ureteral stones. J Urol. 2004;172:568.
173. Forman M.A., Francey T., Fischer J.R., et al. Use of glucagons in the management of acute ureteral obstruction in 25 cats (abstr.). J Vet Intern Med. 2004;18:417.
174. Kalkstein T.S., Kruger J.M., Osborne C.A. Feline idiopathic lower urinary tract disease. Part IV: Therapeutic options. Compend Contin Educ Pract Vet. 1999;21:497.
175. Buffington C.A.T. External and internal influences on disease risk in cats. J Am Vet Med Assoc. 2002;220:994.
176. Westropp J., Buffington C.A.T., Chew D. Feline lower urinary tract diseases. In Ettinger S.J., Feldman E., editors: Textbook of veterinary internal medicine, ed 6, St Louis: Saunders, 2006.
177. Cameron M.E., Casey R.A., Bradshaw J.W., et al. A study of environmental and behavioural factors that may be associated with feline idiopathic cystitis,. J Small Anim Pract. 2004;45:144.
178. Buffington C.A.T., Chew D.J. Lower urinary tract disease in cats: new directions. Vet Clin Nutr. 1994;1:53.
179. Ching S.V., Fettman M.J., Hamar D.W., et al. The effect of chronic dietary acidification using ammonium chloride on acid-base and mineral and bone metabolism in adult cats. Am J Vet Res. 1992;53:2125.
180. Dow S.W., Fettman M.J., LeCouteur R.S. Potassium depletion in cats: renal and dietary influences. J Am Vet Med Assoc. 1987;191:1569.
181. Kirk C.A., Ling G.V., Franti C.E., et al. Evaluation of factors associated with development of calcium oxalate urolithiasis in cats. J Am Vet Med Assoc. 1995;207:1429.
182. Maede Y., Hoshino T., Inaba M. Methionine toxicosis in cats. Am J Vet Res. 1987;48:289.
183. Osborne C.A., Kruger J.M., Lulich J.P., et al. Prednisolone therapy of idiopathic feline lower urinary tract disease: a double-blind clinical study. Vet Clin North Am Small Anim Pract. 1996;26:563.
184. Ross L.A. Treating FUS in unobstructed cats and preventing its recurrence. Vet Med. 1990;85:1218.
185. Barsanti J.A., Shotts E.B., Crowell W.A., et al. Effect of therapy on susceptibility of urinary tract infection in male cats with indwelling urethral catheters. J Vet Intern Med. 1992;6:64.
186. Bernard M.A. Feline urologic syndrome: a study of seasonal incidence, frequency of repeat visits and comparison of treatments. Can Vet J. 1978;19:284.
187. Barsanti J.A., Finco D.R., Shotts E.B., et al. Feline urologic syndrome: further investigation into therapy. J Am Anim Hosp Assoc. 1982;18:391.
188. Marks S.L., Straeter-Knowlen I.M., Knowlen G.G., et al. The effects of phenoxybenzamine and acepromazine maleate on urethral pressure profiles of anesthetized healthy male cats,. J Vet Intern Med. 1993;7:122.
189. Mawby D.I., Meric S.M., Crichlow E.C., et al. Pharmacologic relaxation of the urethra in male cats: a study of the effects of phenoxybenzamine, diazepam, nifedipine and xylazine. Can J Vet Res. 1990;55:28.
190. Lane I.F., Bartges J.W. Treating refractory idiopathic lower urinary tract diseases in cats. Vet Med. 1999;94:633.
191. Buffington C.A.T., Blaisdell J.L., Binns S.P., et al. Decreased urinary glycosaminoglycan in cats with idiopathic lower urinary tract disease. J Vet Intern Med. 1993;7:126.
192. Buffington C.A.T., Chew D.J., DiBartola S.P. Interstitial cystitis in cats. Vet Clin North Am Small Anim Pract. 1996;26:317.
193. Gao X., Buffington C.A.T., Au J.L.S. Effect of interstitial cystitis on drug absorption from urinary bladder. J Pharmacol Exp Ther. 1994;271:818.
194. Baldessarini R.J. Drugs and the treatment of psychiatric disorders. In: Gilman A.G., Rall T.W., Niew A.S., et al, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. ed 8. New York: Pergamon; 1990:383.
195. Miller W.H., Scott D.W., Wellington J.R. Nonsteroidal management of canine pruritus with amitriptyline. Cornell Vet. 1992;82:53.
196. Marder A. Psychotropic drugs and behavioral therapy. Vet Clin North Am Small Anim Pract. 1991;21:329.
197. Chew D.J., Buffington C.A.T., Kendall M.S., et al. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Assoc. 1998;213:1282.
198. Kruger J.M., Conway T.S., Kaneene J.B., et al. Randomized controlled trial of the efficacy of short-term amitriptyline administration for treatment of acute, nonobstructive, idiopathic lower urinary tract diseases in cats. J Am Vet Med Assoc. 2003;222:749.
199. Kraijer M., Fink-Gremmels J., Nickel R.F. The short-term clinical efficacy amitriptyline in the management of idiopathic feline lower urinary tract disease: a controlled clinical study,. J Feline Med Surg. 2003;5:191.
200. Virga V. Behavioral dermatology. Vet Clin North Am Small Anim Pract. 2003;33:231.
201. Gunn-Moore D.A., Cameron M.E. A pilot study using synthetic feline facial pheromone for the management of feline idiopathic cystitis,. J Feline Med Surg. 2004;6:133.
202. Parson C.L. The therapeutic role of sulfated polysaccharides in the urinary bladder,. Urol Clin North Am. 1994;21:90-100.
203. Messing E.M. Interstitial cystitis and related syndromes. In: Walsh P.C., Retik A.B., Stamey T.A., et al, editors. Campbell’s urology. ed 6. Philadelphia: Saunders; 1992:982.
204. Pressler B: Fungal urinary infections, Minneapolis, 2004, Proc 22nd Am Coll Vet Intern Med Forum, p. 555.
205. Barsanti J.A., Finco D.R., Brown S.A. The role of dimethyl sulfoxide and glucocorticoids in lower urinary tract diseases. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XII. Philadelphia: Saunders; 1995:1011.
206. Laing E.J., Miller C.W., Cochrane S.M. Treatment of cyclophosphamide-induced hemorrhagic cystitis in five dogs. J Am Vet Med Assoc. 1988;193:233.
207. Friberg S. BCG in the treatment of superficial cancer of the bladder: a review. Med Oncol Tumor Pharmacother. 1993;10:31.
208. Knapp D.W. Tumors of the urinary system. In Withrow S.J., editor: Withrow and MacEwen’s small animal clinical oncology, ed 4, St Louis: Saunders, 2007.
209. Knapp D.W., et al. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 1994;8:273.