Appendix 1 Regulatory Issues
The Food and Drug Administration
The Food and Drug Administration (FDA) is one of several agencies that regulate the use of drugs, biologics, and medical devices in humans and nonhuman animals (Table 1). The FDA focuses its activities on ensuring the efficacy and safety of drugs to animals (including humans), drug handlers, and the environment. As such, the FDA develops and enforces regulations and written policies for statutory responsibilities in protecting public health through public hearings, public notices, and consultants. The history of the FDA begins in the mid-1800s, although the United States Pharmacopeia (USP) was established earlier by 11 physicians in 1820. The USP was the first organization to address drugs through the formulation of the first compendium of drug standards in the United States, which is one of many activities it continues to do today. The activities of the FDA began when the Bureau of Chemistry was formed as part of the newly created US Department of Agriculture (USDA) during Abraham Lincoln’s presidency. Its primary function was analysis of agricultural products. The regulatory activities of the FDA were formalized to include prohibition of interstate commerce of misbranded or adulterated drugs with the passage of the Pure Food and Drug Act of 1906. In 1927, the agency was named the Food, Drug and Insecticide Administration, and its current name was acquired in 1930. In 1937, 107 persons, including many children, died after ingestion of the antibiotic sulfanilamide prepared in a diethylene glycol vehicle. In response to this incident, Congress empowered the FDA to assure safety of drug products with passage of the Federal Food, Drug, and Cosmetic Act in 1938. In 1962, in response to birth defects associated with the sleeping pill thalidomide, Congress passed the Kefauver-Harris Drug Amendment, which not only empowered the FDA to have greater input regarding drug safety but also provided regulation for assurance of drug efficacy. It was not until 1968, with passage of the Animal Drug Amendment, that animal drugs were defined and placed under the regulatory actions of the FDA. The current offices of the FDA are delineated in Figure 1. Other acts involving the FDA’s oversight of veterinary or human issues can be found at the FDA website (see Table 1).
Table 1 Federal Regulatory Agencies Dealing with Animal Drugs, Biologics, and Medical Devices
| Agency | Function |
|---|---|
| Department of Interior: Environmental Protection Agency (EPA) | Regulates human drugs, biological devices, radiation products and issues, and food safety |
| Food and Drug Administration (FDA) | Regulates human drugs, biological devices, radiation products and issues, food safety, cosmetics, and veterinary drugs (through the Center for Veterinary Medicine [CVM]) |
| US Department of Agriculture: Animal and Plant Health Inspection Service | Regulates animal biologics, helps the FDA monitor proper use of drugs in animals, prohibits repackaging and relabeling of veterinary biologics for over-the-counter (OTC) sale or distribution |
| Department of Justice: Drug Enforcement Agency (DEA) | Regulates and enforces the Controlled Substances Act of 1970 |
| Department of Interior | Controls licensing of topical animal pesticide use and distribution under the Federal Insecticide, Fungicide, and Rodenticide Act |
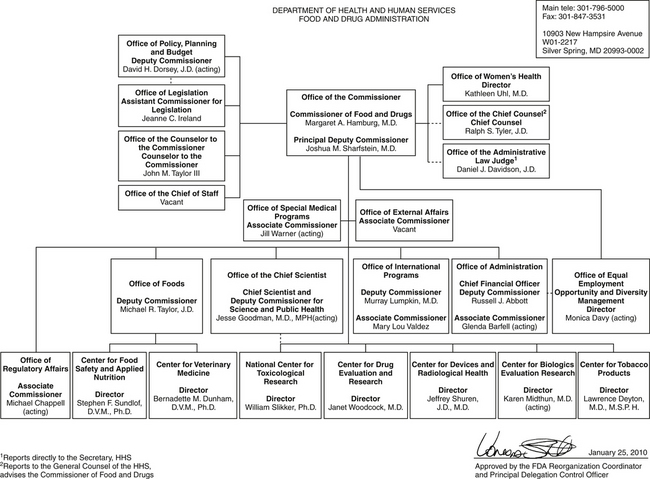
Figure 1 Extralabel drug use algorithm offered by the Food and Drug Administration (FDA) to veterinarians. ∗Record requirements include animal identification (individuals or as group; species; number treated; condition; drug name and active ingredient; dosing regimen; duration; and specified withdrawal, withholding, or discard time(s) (both label and that specified by veterinarian) when applicable (meat, milk, eggs, or animal-derived food). ∗∗Label requirements include name and address of prescribing veterinarian, name of drug, specified direction for use (class/species; identification of the animal or group; dosing regimen including route; and duration of therapy), and cautionary statements. ∗∗∗The compounding of preparations from bulk drugs is generally considered by the FDA to be illegal.
Laws regarding food and drugs are not passed by the FDA; rather, they are passed by Congress and enforced by the FDA through its regulations. Current activities of Congress, including those related to drugs, are noted in the Federal Register. FDA regulations (Table 2) also are printed in Title 21, Code of Federal Regulations (21CFR), which is updated on April 1 of each year. These books can be purchased from the US Government Printing Office. To facilitate implementation of its regulations by field officers, the FDA also publishes compliancy policy guidelines. Although not legally binding, they do provide insight into the Agency’s current thinking.
Table 2 Drug Laws or Guidelines Affecting the Use of Human and Veterinary Drugs
| Law or Guideline | Year | Action |
|---|---|---|
| Federal Pure Food and Drug Act | 1906 | Established standards for safety and purity. |
| Federal Food, Drug, and Cosmetic Act | 1938 | Prohibited marketing of new drugs until adequately tested under label conditions for safety. |
| Durham-Humphrey Amendment to the Food, Drug, and Cosmetic Act | 1952 | Defined over-the-counter (OTC) products by distinguishing them from prescription-only products. |
| Kefauver-Harris Amendment to the Food, Drug, and Cosmetic Act | 1962 | Required scientific proof of efficacy and safety before marketing of a drug and that the Food and Drug administration (FDA) be notified before testing of drugs in humans. Investigational new drug (IND) applications established. Safety and efficacy data needed retroactive to all drugs introduced between 1938 and 1962. Drugs introduced before 1938 are considered “grandfather” drugs as long as labeled use does not change (e.g., phenobarbital, levothyroxine, digoxin). |
| Animal Drug Amendments to the Food, Drug, and Cosmetic Act | 1968 | Animal drug regulations placed under one section of the Food, Drug, and Cosmetic Act; the use of animal drugs is restricted to the species and usage as specified on the label. |
| Poison Prevention Packaging Act | 1970 | Required that hazardous substances be dispensed in child-resistant containers. |
| Comprehensive Drug Abuse Prevention and Control Act (Controlled Substances Act) | 1970 | Controlled the manufacture and prescription of habit-forming drugs. |
| Orphan Drug Act | 1983 | Addressed the development of drugs indicated for rare diseases. |
| Compliance Policy Guidelines | 1984 | Addressed extralabel use of new animal drugs in food-producing animals and distribution and use of human-labeled drugs for animals. Note, however, that the policies and guidelines were in contradiction to the Food, Drug, and Cosmetic Act. Created the legal veterinary prescription. |
| Drug Price Competition and Patent Restoration Act | 1984 | Addressed new drug applications for generic drug products. |
| Generic Animal Drug and Patent Term Restoration Act (GAPTRA) | 1988 | Extended to veterinary products the right of companies to produce and sell generic versions of animal drugs approved after October 1962 without duplicating research done to prove them safe and effective. |
| Compliance Policy Guides | 1991 | Same as the 1984 Guidelines. |
| Animal Medicinal Drug Use Clarification Act (AMDUCA) | 1994 | Legalized extralabel drug use of certain approved animal drugs and approved human drugs for animals as long as specified criteria are met. Final act effective in 1996. |
| Dietary Supplement Health and Education Act | 1994 | Established specific labeling requirements, provided a regulatory framework, and authorized FDA to promulgate good manufacturing practice regulations for dietary supplements. This act defined “dietary supplements” and “dietary ingredients” and classified them as food. The act also established a commission to recommend how to regulate claims. The Center for Veterinary Medicine (CVM) subsequently determined that this act does not apply to products intended for use in animals. |
| Animal Drug Availability Act (ADAA) | 1996 | Provided additional legislation to address the lack of legally available animal drugs. Facilitated approval of new animal drugs through flexibility in the approval process. |
| Food and Drug Administration Modernization Act (FDAMA) | 1997 | Enhanced FDA’s mission in ways that recognized the Agency would be operating in a twenty-first century characterized by increasing technologic, trade, and public health complexities. Many issues were addressed in the ADAA. |
The Center for Veterinary Medicine (CVM) identifies itself as a consumer protection agency that fosters public and animal health by approving safe and effective products for animals and by enforcing other applicable provisions of the Federal Food, Drug, and Cosmetic Act and other authorities. The organization of the FDA reveals that only a fraction of its efforts are directed toward the CVM. The organization of the CVM is shown in Figure 1. The CVM website at the time of printing was http://www.fda.gov/AnimalVeterinary/default.htm. A publication, FDA and the Veterinarian, is available at the FDA website.
Several publications define the FDA’s laws and regulations: Requirements of Laws and Regulations Enforced by the U. S. Food and Drug Administration, which can be obtained from the US Department of Human Health Services, and Code of Federal Regulations (CFR). A more user-friendly account of the laws regulating veterinarians can be found in FDA and the Veterinarian. The most recent issue was published in 1989, but a newer version is being prepared. Finally, James E. Wilson, DVM, JD, has written a book, Law and Ethics of the Veterinary Profession, that provides a more focused perspective on the use of drugs in animals.
Keeping abreast of changes in the FDA’s response to veterinary use of drugs can be difficult. Generally, the Journal of the American Veterinary Medical Association and the American Veterinary Medical Association (AVMA) have done an excellent job in putting together and publishing symposia that delineate and discuss the implications of FDA actions. In addition, the American College of Veterinary Clinical Pharmacology and the American Academy of Veterinary Pharmacology and Therapeutics provide guidance through publications and consultation. Finally, the FDA appears to be willing to answer any questions or concerns one might have regarding the use of drugs in animals. In the CVM, the Office of New Animal Drug Evaluation consists of the Division of Therapeutic Drugs for Non-Food, which in turn contains a section for Companion and Wildlife Drugs. Note that there are other federal agencies that regulate the use of drugs in animals, including the Environmental Protection Agency (EPA), the Animal and Plant Health Inspection Service, and others (see Table 1).
Drugs Defined
A drug is well defined by the FDA (see Table 1). It must be recognized as such (e.g., by the USP); intended for diagnosis, cure, mitigation, or prevention of disease in humans or other animals (note that the FDA defines humans as animals); intended to affect body structure or function; or a component of any of these. In 1968, the FDA first made a distinction between human and veterinary-labeled drugs. As with most regulations since that time, the distinction reflected a concern for human food safety. At that time, an animal drug was adulterated if used in an extralabel fashion. Thus a veterinarian could not modify a dosing regimen, therapeutic intent, and so on of a drug without being liable in both criminal and civil courts. Currently, a new animal drug (NAD) is “any drug intended for use in animals other than man … not recognized … as safe and effective under conditions on the label.” A drug’s label includes the label on the product, as well as any accompanying material (see Box 1). A prescription drug is defined by whether or not adequate directions can be prepared for use of the drug by a layperson. Any drug for which this is possible must be sold as an over-the-counter (OTC) preparation; any other product is a prescription (Rx) product that must bear the phrase: “Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.” Trying to identify the approval status of a drug can be difficult. Obviously, if the label has this cautionary statement, a new animal drug application (NADA) exists for the drug and it can be used legally according to the label specifications. Determining whether an NADA exists for a drug can, however, be difficult. If the drug is listed in the veterinary versions of the Physician’s Desk Reference (i.e., the Veterinary Drugs and Biological Products, published by Medical Economics, or the Compendium of Veterinary Products, published by Bayer Animal Health [both basically are copies of package inserts]), then the drug has an NADA. Note that drugs listed in veterinary textbooks, including formularies, pharmacology texts, internal medicine texts, and so on, do not necessarily have an NADA. Other sources include members of the American College of Veterinary Clinical Pharmacology, the FDA Green Book (http://www.fda.gov/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/ucm042847.htm, last accessed May 25, 2010), or the Handbook of New Animal Drugs, published by Shotwell and Carr.
The Drug Approval Process
From discovery of a new compound (including isolation and synthesis) through its development (including establishing safety and efficacy) to its marketing involves researchers and clinicians and a consortium of regulatory, industrial, and often academic investigators.
Human Drugs
The approval process for human drugs in the United States has been described as the most vigorous in the world, costing on average $359 million to move a drug from the laboratory to the patient. After identification and isolation of a compound, its safety is established in laboratory animals. Preclinical testing studies include acute and chronic toxicity studies focusing on the reproductive status, mutagenicity, and carcinogenicity of the drug. A safe dosing range is established, requiring both pharmacokinetic and pharmacodynamic (dose-response) studies. At this point, if the compound is considered a potential candidate for approval, an investigational new drug (IND) is filed by the sponsor, with protocols for clinical testing. This phase requires approximately 5 to 8 years, and generally only 1 drug in 5000 evaluated succeeds in this phase. At this point, if the compound is considered a potential candidate for approval, an IND is filed by the sponsor, along with protocols for clinical testing. Approximately 2000 INDs are filed with the FDA each
Box 1 Package Drug Inserts/Labels
Defined
The drug package insert (DPI) is a legal document that accompanies a finished dosing form of an approved drug product. As such, it applies only to the approved product that it accompanies. The DPI for a product includes the label on the product itself as well as any and all accompanying materials (i.e., inside the package. All information included must be approved by the Food and Drug Administration). The label does not include technical monographs or advertisements that might be distributed by a manufacturer. Although the manufacturer may sponsor post-market surveillance studies that can be cited in and provide the basis for information in monographs used for promotion of the product, care must still be taken not to promote a use not stated on the label. Changes to labels that might accommodate new findings regarding the disease targeted by the drug or its use require approval by regulatory agencies, a cost the manufacturer may not want to pursue. Any use of the product beyond that specifically delineated in the label constitutes extra label drug use (EDLU). Data provided in current drug package inserts (DPIs) might be categorized as Product Description, Product Efficacy, or Product Safety with some overlap among the categories.
Product Description: Drug or Drug Product Name
Product Efficacy
Product Safety
Box 2 What’s in a (Drug) Name?
A drug by any other name may not be the same. The name of the active moiety (AM; drug substance) must be distinguished from the drug product name. The AM will have a chemical name (determined by the Chemical Analytical Society [CAS] based on its structure) and a generic name. In contrast, the drug product will have a non-proprietary (established) name and proprietary (brand) name. In the United States, the generic AM is initially named by the United States Adopted Name [USAN] Council. Ideally, the name will also be approved by the International Nonproprietary Name (INN) Expert Committee, such that the generic name is globally applicable. As such, only one generic name should exist for any AM, although exceptions exist (e.g., epinephrine versus adrenaline; acetaminophen versus paracetamol, and occasional differences in spelling [alfa versus alpha]).
The generic AM name consists of three parts that logically apply to the AM activity. The stem, generally located at the end of the name, is the most important. It indicates an action unique to and shared by all drugs with the same stem. The stem can be based on a chemical relationships (e.g., azepams, cefalosporins), therapeutic activity (terone for antiandrogens or poietins for erythropoiten), or mechanism of action (statin for cholesterol-lowering drugs). Currently more than 300 stems exist. The prefix of a generic AM is used to discriminate among members having the same stem (e.g., diazepam, clonazepam). The importance of racemates in the past decade has led to the use of logical prefixes indicating the enantiomer status of an AM. These include dextrorotatory (dextromethorphams) or R (arformeterol), and levorotatory (levofloxacin, levatiracetam) or S (esmolol). The infix of the drug name is irrelevant unless further subclassification is necessary. Because many approved drug products may have the same AM, it is the generic AM that is preferred for general use in order to facilitate the flow of scientific information within and among nations.
Some drug substances are accompanied by a salt or ester. In such cases, USAN will also provide an API that contains the AM (e.g., zonisamide, pancuronium, triamcinolone, or penicillin) is added to the associated salt (e.g., sodium zonisamide or pancuronium bromide) or ester (e.g., triamcinolone acetate or procaine penicillin). The AM (or API) becomes part of the established (non-proprietary) drug product name, which is established nationally (e.g., the United States Pharmacopeia) and approved by the regulatory agency (e.g., the FDA). Generally, the non-proprietary drug product name includes the USAN generic AM or API (with the choice by USP depending on how important the salt or ester is to drug use), the route of administration (e.g., oral [excluded if implied by the dosing form], topical, ophthalmic) and the dosage form (e.g., tablet, solution, lotion, suspension, aerosol).
Other descriptors (e.g., altered release rate [e.g., delayed, extended release] or delivery system [e.g., transdermal patch) may be included. It is the USP generic drug product name (e.g., carprofen tablet, enrofloxacin otic solution) that is generally on the package insert of the drug product approved by the Food and Drug Administration. The USAN names and the possible options for drug product names can be found in the United States Pharmacopeia Dictionary and National Formulary (USP-NF), respectively, each published annually by the USP. Note that the AM (API) is likely to be only one of several ingredients found in the finished drug product that is approved by the regulatory agency (e.g., the Food and Drug Administration [FDA] in the United States); other ingredients might be excipients intended as filler, stabilizers or antibacterial.
The proprietary or brand name of the approved drug product (e.g., Rimadyl, Baytril Otic) belongs to the manufacturer, although the FDA offers guidelines that are intended to avoid inappropriate public perceptions of drug action or safety based on the brand name. A generic drug product that has been demonstrated by the manufacturer to be therapeutically equivalent to the pioneer proprietary drug product may be subsequently approved by the FDA. The two products will have the same USP established drug product name. However, the generic drug product must either not have a brand name or have a brand name that differs from the pioneer drug product (e.g., Novox as a generic Rimadyl). Finally, it is important to note that a manufacturer may actually change the AM (or API) associated with a brand name product; this is particularly common to over-the-counter products (e.g., Kaopectate). For other information that may be found on a package drug inserts, see Box 1.
year. Long-term safety studies continue in animals as the drug enters Phase I clinical trials in humans.
The clinical phase of drug approval involves three distinct phases of clinical trials that provide the basis of the drug label. Phase I is conducted on a small number of normal volunteers (generally 20 to 80; most commonly, young adult Caucasian males are studied) to determine a safe dosing range and the disposition of the drug (pharmacokinetics). Phase II begins studies in clinical effectiveness and safety in several hundred persons with target illnesses. In Phase III, the number increases to several thousand to establish risk:benefit ratios. Phases I through III last approximately 3 to 10 years; if the drug demonstrates a favorable risk:benefit ratio, the sponsor can submit a new drug approval (NDA). A typical NDA is approximately 100,000 pages or more in length. The FDA, by law, must review the NDA within 6 months, although generally this time period is exceeded. During this phase, the sponsor and FDA determine the detailed information that will accompany the label, including contraindications, precautions, side effects, dosages, routes of administration, and frequency of administration. Only one of five drugs studied for human use receives FDA approval.
After approval, the manufacturer can promote the drug, but large-scale clinical trials will continue to further define the safety profile. Additional Phase IV studies may be required. Once the drug is in widespread use, adverse effects previously undetected may be recognized. Occasionally, if the adversities are serious (fatal), the drug may be withdrawn from the market. Postmarket studies also may identify efficacy for indications not previously identified during the approval process. New information from postmarket studies are used to update the NDA. During this time period, reports of adverse reactions, particularly those not previously recognized or unexpected, are important to evaluating the safety of the drug. For human drugs, reports can be made on the Drug Experience Form, through MedWatch (a voluntary reporting program), and through the sponsoring pharmaceutical company.
Animal Drugs
The approval process for new animal drugs is not as clear cut as for human drugs. This reflects in part the variabilities presented by species differences, economic considerations, and the importance of food (human) safety. Thus, although the legal standards for safety and efficacy data are the same for both human and animal drugs, design of safety and efficacy studies and the criteria for approval differ, and the approval process for an NAD generally is tailored to the particular drugs. The differences are most marked for food animal drugs for which efficacy and safety data in the target species must be weighed in the context of economic considerations. The path of human drug approval does not apply to animals, although many of the same data are collected. The regulations for the approval of an animal drug can be found in 21CFR 514.1; however, several recent drug laws (e.g., the FDA Modernization Act and the Animal Medicinal Drug Use Clarification Act) and the Generic Drug Law of 1988 have impacted several aspects of this law, which is currently being updated by the FDA.
The approval process for animal drugs is changing but currently occurs in two distinct phases. (http://www.fda.gov/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm123821.htm; last accessed May 25, 2010). During the investigational new animal drug (INAD) phase, five technical areas are reviewed by the FDA. These include composition, manufacturing, and chemistry (CMC, basic manufacturing data); target animal safety; evidence of clinical efficacy; environmental considerations; and human food safety, which includes tissue residue data, for animal tissues intended for human consumption. During this phase, the FDA, in concert with the sponsor, determines product development plans, reviews protocols for the study design, implements the studies, and generates the raw data intended to support approval; the FDA then decides whether the data are sufficient to address concerns. The protocols for toxicology and tissue residue chemistry studies are straightforward, but more creative and innovative approaches are taken for target animal safety and efficacy because of the diverse issues surrounding new animal drugs.
Once the technical sections are completed, the second phase, the NADA is filed. This phase is in transition and represents a new approach to drug approval by the FDA (an “administrative” NADA) in that the majority of data supporting the approval of a drug may have already been reviewed by the time this phase is begun. The drug may be known to be approvable before this phase is reached because of the phased INAD review. The review process becomes largely interactive through the phased review process (http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM052464.pdf).
Orphan Drugs
The Orphan Drug Act of 1983 provided incentive for development of drugs used to treat rare diseases, which did not present sufficient economic incentive for a sponsor to undergo the traditional approval process because they benefit only a small number of patients. The added incentives include tax advantages and marketing exclusivity to the sponsoring company. The National Institutes of Health often participates in the development of orphan drugs. Examples of the 300 human drugs given orphan status include erythropoietin, α1-antitrypsin, and human growth hormone. Criteria that a drug must meet to become an orphan drug include intent to treat a serious or life-threatening disease; lack of a comparable or satisfactory alternative; involvement in a clinical trial as an IND; and active pursuit of full approval by the sponsor. If these criteria are not met, the drug may still be obtained for compassionate use. The clinician in essence becomes the investigator by submitting a treatment IND.
Generic Drugs
Pharmacists can dispense an equivalent, less expensive, nonproprietary (generic) drug without prescriber approval. An exception occurs if a state has a mandatory substitution law or if the brand name product is dispensed along with a Dispensed as Written (DAW.) order. Generics may be pharmaceutically equivalent but may not be therapeutically equivalent. Those tested by the FDA and found to be therapeutically equivalent are listed in Approved Drug Products with Therapeutic Equivalence Evaluations, known as the Orange Book (http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm; last accessed May 25, 2010). Generic products not only contain the same active ingredient as the proprietary drug but also meet bioequivalence standards. Substitutions of generic drugs for proprietary drugs are recommended only for those drugs shown to be therapeutically equivalent. Examples of drugs that are not therapeutically equivalent to their brand name counterparts include digoxin, phenytoin, conjugated estrogens, and slow-release theophyllines. The Orange Book addresses therapeutic equivalence in human medicine. Veterinary clinicians should not mistake therapeutic equivalence established in humans to be the same in animals.
The Drug Availability Crisis
In the mid-1990s, the Animal Health Institutes focused on an issue they termed the drug availability crisis. It is based on the fact that veterinarians are faced with the need and desire for improved veterinary care for their patients; there is a great availability of human-labeled drugs but fewer NADA applications. Simplistically, the FDA requirements of NAD approval have not allowed for flexibility in keeping up with the scientific advancements in the diagnosis, treatment, and prevention of animal diseases. For example, in 1994, for dogs, there were only 369 Rx drug products and 67 OTC drug products. For cats, there were only 169 Rx and 40 OTC drug products. For cats, these 209 products reflect only 84 drugs, many of which are no longer used. Approximately 15% of drugs discussed by the author used to treat or prevent illnesses in dogs are approved for that use; the number is even smaller for cats (less than 10%).
Two potential reasons preclude pharmaceutical companies pursuing the approval of human drugs for animals. First, adverse reactions that may occur in animals receiving the drug may impact the human market even if the adverse reaction is not likely to occur in humans. The second detractor is economic recovery. The cost of approving drugs has progressively increased, in part because of the “moving target” presented by the FDA to pharmaceutical companies. Requirements are constantly changing, and it is often difficult for the company to predict or keep up with changes. All changes are costly. A common misconception is that veterinary drugs do not have to undergo the same intensive scrutiny for approval as do human drugs. In fact, the opposite might be considered true. Because of the concern with tissue residues, far more time and effort might be put into animal drug approval, particularly food animal approval, than human drug approval. Environmental impact studies may also be more intense. Thus the cost for approval of an animal drug is disproportionately higher than of a human drug, particularly when the cost is compared with the recovery of costs. The animal market is very small compared with the human pharmaceutical market (millions compared with billions). The time for development of a drug (from identification of a potential compound to its final FDA approval) is 5 to 10 years, and the cost is approximately 1 to 2 million dollars per year (the longer time for food animals). As drug approval costs increase, the number of NADAs may decline. Extralabel use of human generic versions of animal-approved drugs or compounding in lieu of prescribing approved drugs are also disincentives for manufacturers to pursue approval of a veterinary drug.
Extralabel Drug Use
If a new NADA exists for a drug, to use the drug in a legal manner the veterinarian must adhere to the specifications noted on the label (which includes both the label adhered to the medication and the accompanying package insert). Otherwise, an NAD is used in an extralabel manner. In 1994, Congress passed the Animal Medicinal Drug Use Clarification Act (AMDUCA), which legalized extralabel drug use (ELDU) by veterinarians as long as specific criteria or restrictions are met (see Table 2). ELDU, whether actual or intended, occurs when the drug is used in a manner that is not in accordance with the approved label directions. This includes but is not limited to a different dose, interval, route, indication, or species. Veterinary ELDU is legalized by AMDUCA (see Table 2) only for approved drugs (human or animal) and not for products intended for use as drugs but are not approved drugs. The latter substances are perceived by the FDA to be unapproved drugs and as such, fall under their regulatory jurisdiction. This includes novel ingredients, such as herbs and nutraceuticals, as well as products compounded outside the stipulations of AMDUCA. However, the FDA recognizes that there are diseases in animals for which there is no approved drug treatment and that strict enforcement of their law precludes the practice of veterinary medicine. To address these concerns, in 1984 and 1991, the FDA published Compliance Policy Guidelines for ELDU, including ELDU of animal drugs (Compliance Policy Guideline 7125.06) and ELDU of human drugs in animals (Compliance Policy Guideline 7125.35). The guidelines focused on ELDU in food animals and have been updated (http://www.fda.gov/AnimalVeterinary/ResourcesforYou/FDAandtheVeterinarian/ucm077390.htm). These include EDLU is permitted only by or on the order of a veterinarian; it is allowed only for FDA-approved animal and human drugs (which excludes compounded products; see Compounding); it must be implemented in the context of an existing valid veterinary-client-patient relationship (VCPR; (Box 3). For food animals,
Box 3 Requirements of a Valid Veterinary-Client-Patient Relationship (VCPR) as Defined by the Food and Drug Administration (FDA)
further restrictions apply. An interactive algorithm is available from the AVMA that directs the veterinarian using ELDU (http://www.avma.org/reference/amduca/amduca1.asp).
Compounding
The guidelines regarding the compounding of pharmaceuticals under the direction of a veterinarian are delineated in Compliance Policy Guideline 7125.40. Conditions under which compounding is legal are specified in the AMDUCA. Compounding includes any manipulation of the drug beyond that stipulated on the label (such as reconstitution of a powdered drug) (Table 4). Conditions under which compounding is not subject to regulatory actions include a legitimate practice (pharmacy or veterinary; includes licensure), operation within the conformity of state law, for pharmacists in response to a prescription, and for veterinarians in response to a valid VCPR. Compounding is likely to result in conversion of an approved animal drug into one that is unapproved. Compounding of human drugs and occasionally bulk drugs into appropriate dosage forms may be acceptable in certain circumstances (e.g., combinations of anesthetics to titrate administration, dilution of drugs for pediatric or small exotic animals). A legitimate medical need must be identified (e.g., health or life of the animal is threatened or suffering may occur). Additionally, there must be no marketed, approved animal or human drug, regardless of whether it is used in a labeled or extralabeled fashion that may be substituted for the compounded agent. Occasionally, other rare circumstances may be considered.
The compounded product must be dispensed by a veterinarian or prescribed and subsequently dispensed by a pharmacist. For companion animals, the safety and efficacy of the compounded drug must be consistent with current standards, appropriate steps should be taken to minimize the risk of human exposure to harmful ingredients, patient records must be kept, and the compounded drug must bear labeling information to ensure adequate and proper use of the product (including name and address of the veterinarian; the active ingredient; date dispensed and expiration date; directions for use; cautionary statement; and if dispensed by the pharmacist, appropriate pharmacist information). The compounded preparation cannot be sold to another veterinarian or pharmacist.
A number of pharmacists throughout the United States compound drugs for veterinarians. The Professional Compounding Center of America (PCCA) is a resource for education in compounding drugs for both human and veterinary medicine. Veterinarians seeking compounded products would be prudent to work with a pharmacist who is a member of the PCCA to be more certain of quality control concerns. The PCCA can be reached at 800-331-2498.
Compounded products are not regulated; assurance of quality, safety and efficacy is incumbent on the prescribing veterinarian. Because compounded products may be less safe that an approved product (other than increase accuracy in dosing), attention to patient response is important. Therapeutic failure may be a common adverse event that is easily forgotten with compounded products. Care must be taken to not misinterpret the availability of a compounded product as evidence of safety or efficacy. The discerning clinician should ensure, through contact with the pharmacist, that state pharmacy laws are followed. Sources of drugs should be confirmed with regard to quality. Manufactured products should be avoided for both ethical and safety reasons. The Pharmacy Compounding Accreditation Board (http://www.pcab.info/) offers accreditation to pharmacies willing to meet a robust set of criteria that ensure products meet both quality and ethical standards as intended by both the Food and Drug Administration and the pharmacy associations. Veterinarians are encouraged to identify those pharmacies that are PCAB accredited.
Alternative Mechanisms for Use of Human Drug in Animals
There are two other mechanisms by which a practitioner can legally use a human drug. Regulatory discretion (discretionary enforcement) has been applied by the FDA to selected drugs with no NAD. Recommendations for regulatory discretion of a drug are made by the Division of Drug for Non-Food Animals to the Division of Compliance. Digoxin is an example for which regulatory discretion has existed for a long time; labeling for animal use is even allowed for this product because it is so old. Newer regulations, however, prevent labeling for animals without an NADA. If a drug has been shown through illegal use to be safe and efficacious for the treatment of a disease in animals, the FDA will allow its use without an NADA (notification by letter). Examples include potassium bromide for treatment of refractory seizures, 4-methylpyrrazole for treatment of ethylene glycol toxicity, and calcium ethylene diaminetetra-acetic acid for treatment of lead poisoning. To obtain regulatory discretion, the practitioner needs to contact the Division of Compliance (see Box 3).
An alternative route to using a drug in an extralabel fashion legally for an animal is to procure an INAD application from the Division of Drug for Non-Food Animals (301-594-1722). The INAD provides statutory authority to exempt the drug from the NADA requirement. It limits the use of the drug to experts qualified by training and experience. A compassionate use INAD can be obtained in 1 day by calling the previous telephone number to treat an animal whose life is threatened. Note that the INAD is not necessary if the drug is approved for use in any species. The INAD is needed only if the drug is unapproved, meaning that there is no approved version for humans or animals (i.e., a drug approved in Canada, Mexico, or Europe but not the United States). Occasionally, the FDA may recommend that an INAD be obtained for the use of a drug recently approved for use in humans because data regarding the safety of the drug are probably still being collected for humans. An INAD may also be recommended if the drug is toxic (especially carcinogenic) or it is scheduled (regulated by the Drug Enforcement Agency). If an INAD is obtained, veterinarians must keep records regarding the use of the drug and must notify the FDA every time a shipment is requested or an adverse reaction occurs.
Extrapolation of human drugs to animals should be accompanied, whenever possible, by scientific studies that support proper dosing regimens. Recommendations regarding the extrapolation of dosing regimens among species is addressed in Chapter 2.
Schedule Drugs
Drug (substances) considered to be associated with a potential for abuse or physical dependence are restricted (controlled) by the FDA. The Controlled Substances Act delineates requirements that must be met when such drugs are administered, dispensed, or prescribed. The Drug Enforcement Agency (DEA) is charged with enforcement of the act, its amendments, and their regulations. The act includes federal requirements regarding the purchase, storage, prescription, and record keeping for controlled substance use. States may have additional requirements regarding purchase, dispensing, inventory, record keeping, and storage. Requirements are likely to vary from state to state (http://www.legislature.state.al.us/CodeofAlabama/1975/50719.htm).
Requirements for controlled substance use vary for practitioners, researchers, teachers, and pharmacists. Practitioners dispensing or prescribing Class I and II substances annually must obtain both federal and state registration; an exception is made if done in the confines of an employer (hospital)/employee (student) relationship and the employer is registered. Federal registration occurs through the DEA. For practitioners, use of Classes III to V must be preceded by a notice of intent to the DEA, although registration also may be required for Classes III to V, if certain conditions are not met (www.dea.gov). Registering practitioners must submit evidence of a professional degree. Licensure must be voluntarily surrendered on retirement. Mechanisms for state registration also vary; in the state of Alabama, registration of veterinarians occurs through the State Board of Veterinary Medical Examiners.
The DEA publishes a Practitioner’s Manual (http://www.deadiversion.usdoj.gov/pubs/manuals/pract/index.html; last accessed May 25, 2010), which delineates requirements for the use of these substances. Among the major requirements, selected states require a special prescription blank (and often multiple copies). Generally, both state controlled substance registration (SCSR) and federal (DEA) numbers must appear on prescriptions. Purchase of controlled substances also requires a special triplicate form obtained from the DEA. All actions with controlled substances must be within the confines of a veterinary client–patient relationship. Containers must be designed such that children under the age of 5 cannot open the product, and a label stating “This Package for Households Without Young Children” must be applied when a childproof container is not used. Specific requirements also vary with the class or schedule of the controlled substance.
The classification of controlled substances reflects the perceived level of potential abuse or physiologic or psychological dependence (Table 3). The schedule of a controlled substance is indicated on the label of the product. Schedule I drugs have the highest potential of abuse and physical dependence. These drugs have no acceptable or safe medical use in the United States and are not to be prescribed, but they can be obtained for research purposes. Heroin is the most notable example. Schedule II drugs, exemplified by cocaine and morphine, are considered to have a high potential for abuse and dependence but have some medicinal worth. These drugs must be stored in a securely locked, well-constructed cabinet or safe. Loss or theft requires immediate contact of the nearest DEA field office. Prescription refills are not allowed for these drugs, and they cannot be prescribed by telephone unless in an emergency (with a follow-up written prescription provided within 72 hours). Schedule III drugs, exemplified by selected opioids and barbiturates, have an accepted therapeutic use. Although abuse of the substance may lead to moderate or low physical dependence, the risk of abuse is less than that of Schedule I or II drugs. Schedule IV drugs, exemplified by diazepam and butorphanol, are considered to present a low potential for dependence or abuse. These drugs can be refilled but only to a maximum of five times; the prescription is effective only for 6 months. Schedule V drugs, such as diphenoxylate, have the lowest potential of abuse or dependence. Restrictions for drugs in this schedule are limited to age (humans), distribution by a pharmacist, and purchase in limited quantities. Schedule V drugs include narcotics in Schedules II to IV included as part of a combination with other drugs (e.g., antitussives, antidiarrheals) with restrictions, including the concentration of the substance in the final product.
| Schedule | Definition | Drugs (lists are NOT inclusive) |
|---|---|---|
| I | Highest risk of abuse and dependence and no medicinal use | Heroin, hallucinogens (LSD, mescaline, marijuana), amphetamines |
| II | High risk of abuse and dependence and limited medicinal use | Carfentanil, cocaine, codeine, diprenorphine, etorphine, fentanyl, hydrocodone, hydromorphone, methylphenidate, morphine, meperidine, opium extracts and so on, oxymorphone, pentobarbital, sufentanil, thebaine |
| III | Moderate potential for abuse and dependence, but accepted therapeutic use | Anabolic steroids, barbiturates, buprenorphine, codeine combinations, hydrocodone combinations, ketamine, morphine combinations, pentobarbital combinations, thiamylal, thiobarbiturate, tiletamine-zolazepam |
| IV | Low potential for abuse or dependence | Alprazolam, butorphanol, chloral hydrate, chlordiazepoxide, clonazepam, clorazepate, diazepam, flurazepam, lorazepam, midazolam, oxazepam, pentazocine, phenobarbital, propoxyphene |
| V | Lowest potential for abuse | Diphenoxylate; selected (low strength) codeine preparations; hydrocodone and so on, as part of drug combination product; low-strength opium preparations |
From http://www.justice.gov/dea/pubs/scheduling.html (last accessed May 25, 2010); note that the list of drugs may vary with some states.
Reporting Adverse Drug Reactions
Adverse drug reactions are described in Chapter 3. Report of adverse reactions is an important means of monitoring the safety of products, as well as communicating unexpected adversities. In addition to the vehicles listed here, letters to the editors or case reports in veterinary journals or continuing education programs can be effective tools for reporting adverse drug reactions. Additionally, the adversity should be reported to the pharmaceutical company. Note that adverse events to biologics and pesticides should be reported to the respective agency (see Table 1). The steps to reporting an adverse event include contacting the technical services of the appropriate pharmaceutical manufacturer, if it is an animal-approved drug. If it is not an FDA-approved product for animals or if direct reporting to the FDA is desired, a report may be submitted via telephone at 1-888-FDA-VETS, or more appropriately, to the FDA on Form 1932a: Veterinary Adverse Drug Reaction, Lack of Effectiveness, Product Defect Report (postage prepaid and preaddressed). The form can be downloaded at the CVM website (http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/AnimalDrugForms/UCM048810.pdf; last accessed May 25, 2010) or can be obtained by written request at ADE Reporting System, Center for Veterinary Medicine, US Food & Drug Administration,7500 Standish Place, Rockville, MD 20855-2773.
Prescription Writing
Prescriptions are intended to provide direction to the pharmacist regarding the dispensing of a specific medication to a specific patient and must be written for all legend drugs. Specific guidelines regarding who may legally issue a prescription vary among states; however, licensed professionals may prescribe only within the profession in which they are licensed. Prescriptions must be issued only in the context of a valid VCPR (see Box 3). Prescriptions should be written in ink (includes typewritten and computer-generated forms) and must be signed by the prescriber. Pharmacies are not likely to stock veterinary drugs but may be willing to order them or infrequently used human products. The basic elements of a prescription (Box 4) may vary among states. Common mistakes made in prescription writing that can lead to mismedication include the inappropriate use of decimal points (a decimal point with a zero [e.g., 1.0] should not be used after a whole number because the decimal may be missed; a zero is always used to designate a fraction [e.g., 0.5] because the decimal may be missed) and the use of abbreviations that are similar (e.g., use of U for units [easily mistaken for a 0]). Writing numbers as words in lieu of or in addition to numerals may facilitate safety, particularly with toxic drugs.
Labels for prescription drugs should include the name, address, and phone number of the prescriber; the patient and owner’s name (and for controlled substances, address and phone number); animal name (if appropriate) and species; dispensing date; drug name, quantity, and strength; instructions
for use; and appropriate precautionary statements. State regulations regarding the contents of the drug label may vary. Drugs should be dispensed in a childproof container unless requested otherwise by the owner; a signed request should be kept in the record if so requested.
Ethical Considerations in Clinical Trials∗
Assurance of Ethical Use of Animals
The use of animals in prospective research studies is becoming increasingly controversial. In this section, considerations for client-owned animals are contrasted with experimental animals that are owned by the research facility. Included in this latter group are animals that have been donated by clients. This section also discusses ethical consideration for humans, experimental animals, and client-owned animals in clinical trials. General ethical considerations for all clinical trials are also be presented.
Rights of Human Subjects
It is not unreasonable to expect the same concern and consideration for veterinary patients that is given human patients used as clinical research subjects. Human patients involved in clinical research are very well protected against inhumane use. The Nuremberg Code of Ethics in Medical Research (1948) emphasizes the rights of the experimental human subject. The Declaration of Helsinki (1964) as adopted by the World Medical Association went further and mandated the following:
The Declaration emphasized the right of informed consent and specifically addressed human medical research that is combined with professional patient care. Research facilities, such as academic institutions that direct clinical research in humans, are guided by Institutional Review Boards whose primary charge is to ensure compliance with the Nuremburg Code and Declaration of Helsinki. With minor modifications, most of the guidelines delineated in these two documents also are applicable to the veterinary patient (client-owned animal) used in clinical research. Like human medical counterparts, veterinary clinical research facilities should institute a mechanism by which adherence to these guidelines is assured.
Welfare of Experimental Animals
Experimental animals are protected by guidelines offered by the Animal Welfare Act of 1966 and its subsequent amendments. The Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals (i.e., the National Institutes of Health Policy) requires compliance with this act of all institutions receiving PHS funds. Most research facilities (academic institutions) have laboratory animal care committees that assure compliance by individual investigators with the PHS policy. However, these committees are not necessarily charged with the care of client-owned animals and often (as at Texas A&M University) agree that research involving client-owned animals does not fall under their purview.
Welfare of Client-Owned Animals
To protect the welfare of client-owned animals, some type of review board should be in place. In a university setting, a Hospital Review Committee evaluates clinical trials and other types of clinical research. The term Health Review Committee (HRC) is used in this manuscript to distinguish from Institutional Review Boards (IRB) that address human subjects in clinical research. The goals of an HRC should be to protect the patient, client, institution and attending veterinarian(s) from the intended or inadvertent application of investigations that are inhumane or unethical and to promote the advancement of science through clinical research. The HRC functions to safeguard the welfare of the patient through the approval of proposals involving clinical research. Although its mission is not to provide rigorous review of the scientific merits of a proposed study, decisions regarding the ethical nature of a proposed clinical trial may require the HRC to question the scientific basis and the scientific and statistical design of any proposals.
General Ethical Considerations
General considerations on the ethical use of animals in clinical trials arise from the human research guidelines. Some of the following considerations are most applicable for clinical trials using client-owned animals. An over-arching truth is that it is more ethical to perform a randomized clinical trial than to use treatments of unproven efficacy. The patient’s best interest cannot be forsaken for the sole purpose of “therapeutic progress”; rather, treatment of the patient is the priority. Completion of the trial must not be rushed if it creates risks to the patient. In the study design phase, protocols should be developed for use in the situation where the risk:benefit ratio of the therapeutic intervention becomes too high during the course of therapy (e.g., the trial will be terminated, the currently recommended therapy will be used, and so on). Trials should include methods to evaluate the incidence, frequency, type, and severity of side effects of test treatments. This is especially important if client-owned animals are used.
In determining the treatment protocol, if periodic therapeutic withdrawal or use of a placebo is planned, assurance must be provided that the subjects’ life or comfort is not threatened. The use of an inactive placebo is not appropriate (except in very unusual, experimental situations) if there is a standard treatment protocol for the disease to be investigated. The double-blind technique (defined here as blinding of the investigator and client or caretaker) should be abandoned if one treatment can be clearly recognized by the investigators to be preferred because of its beneficial effects, or if a treatment entails any risks that prove to be unreasonable. The code indicating a subject’s treatment must always be available to appropriate participants in case of an emergency. For trials using client-owned animals, if a client withdraws an animal from the study, assurance should be given that patient care will remain available. There should be a system of verification of results that avoids the possibility of manipulating results after the study.
Additional questions regarding the scientific merits of the study that should be answered by the investigator include the following:
Guidelines for Completing an Informed Consent
The informed consent should be perceived as a document that provides information to the owner, in layman’s terms. The information should be pertinent to the study and should include anything that is likely to be important to the animal owner. This recommendation might seem nebulous and needlessly all-inclusive. However, the intent of the consent form might best be appreciated by answering the following questions: If you (or your child) were the subject of this study, what would you want to know about it? Is there anything left out of the consent form that the client is likely to get mad about when he/she finds out? The informed consent should be succinct, clear, and above all else informative to the animal owner. Bolding might be used to emphasize points that the investigator feels are particularly important to the client.
The following consent form is organized for purposes of discussion. The organization should be tailored to the study in a manner that is not confusing to the animal owner. Use of lay terminology that the client can understand is paramount to an appropriate informed consent. Information should be clear and succinct.
Suggestions regarding generation of an informed consent follow. The first paragraph identifies the animal and animal owner. The reason for the animal’s inclusion should be stated. Additional information might be included in a brochure, which should be submitted along with the protocol. Note that information in the brochure is not part of the informed consent and cannot replace the information required in the consent document. The second paragraph should provide information about the experimental protocol. The following information should be included:
Those that are experimental should be noted as such. Additional information might include the funding agency and the number of animals to be studied.
The third paragraph might focus on the risks and benefits associated with the study. The following must be included:
If a placebo or negative control is included in the study, it must be clear to the animal owner that there is a possibility that the animal may receive no therapeutic benefit from the study.
Additional information that might be included in this section is as follows:
This last statement might include costs to be incurred by the owner for participating in the study, the number of follow-up visits to a veterinarian, record-keeping, and telephone calls.
The fourth paragraph may focus on client options should an adverse reaction occur or client withdrawal be desired. Relevant information that should be included in this section is as follows:
The fifth paragraph ensures the confidentiality of the study. Points to be included are as follows:
Each page of the informed consent should be numbered and accompanied by a place for the client’s initials next to the number.
Informed consent may be waived under extenuating circumstances if the investigator and an unbiased clinician (one not participating in the study) certify in writing all of the following:
Reporting and Reviewing Clinical Trials
Data Reporting
A clinical trial is not complete until the information is disseminated. Publications of results should be prepared and made available as soon as possible. Although most manuscripts are prepared after closeout, in certain instances, interim publications are prepared.
Interim publications provide access to study results as they occur. Additional benefits might include easier preparation of the final manuscript and greater exposure of the study to the public. However, there are several disadvantages to interim reporting (Meinert, 1986). Results that are inconclusive may be confusing. If the results are discouraging, investigator enthusiasm may wane. Most critical is the possibility of bias in subsequent treatment assignment and data collection. Finally, data analysis and presentation may differ from and thus diminish the impact of the final report. Results of the study can be disseminated through publications in peer-reviewed journals and presentations at national meetings. Some journals (those that focus predominantly on human medicine) do not publish papers that have been presented nationally. The time gap between presentation and publication should be minimal. The choice of journal should be limited to referred journals that are covered in Index Medicus and Index Veterinarius. Unreferred journals should be avoided if they lack a critical review process. Such journals may reach a smaller public and thus may be more difficult for other investigators to identify or retrieve. A specialty journal (i.e., Internal Medicine, Neurology, or Surgery) might be considered if the results are of primary interest to the specialty group.
The potential importance of many veterinary clinical trials is not realized because of failure to publish the appropriate information. The goal of the publication should be to provide a clear, concise description of the study. Studies that report statistically insignificant findings should also be published. The clinical trial may only result in a single manuscript that is published on completion of the trials. The organization of the manuscript varies with the targeted journal. Typical components include the following.
The title is one of the most important components of the publication. It should be concise and as short as possible while indicating the main thrust of the paper. The term clinical trial should be included in the title. The title section should also include the authors, source of financial support, acknowledgements, and address for reprints. Finally, a list of key words selected by the author should be included to allow for retrieval.
The abstract is often the only part of a paper that is read and as such should provide a summary of the paper. The abstract will be included in Medline, the computerized version of Index Medicus, or other computerized databases. The abstract should include the study purpose or objective, primary outcome measure, intervention; type of control, method of allocation, blinding procedures, number of animals enrolled and studied, and conclusions.
The introduction should be short and succinct. Its purpose is to provide a historic background for the study. Included is a literature review, what led to the initiation of the study, the study objectives, and the rationale for the study. This might include a rationale for the study design, intervention, or outcome measurements.
The methods section should be sufficiently detailed to allow readers to make informed judgments regarding the quality of the methods. Citation of a previously published paper describing the methods can reduce the content if the paper was devoted primarily to the design and methods of the trial.
The results section is generally the longest. The crux of the paper should be represented by tables, charts, and figures, which should be understandable without reference to the text.
The discussion should highlight the important findings of the study. Positive and negative findings should be reported. The clinical implications of these results and consistency with previous findings should be discussed.
The conclusion may either stand alone or complete the discussion. The conclusions must be drawn from the results of the trial. If appropriate, the statistical power of the study should be noted if the conclusion favors the null hypothesis. Finally, a statement regarding the extent of generalization should be included.
References should be limited to those that support the rationale of the objectives and document methods of data collection and analysis. The journal of publication will dictate the organization of the references. Original articles should be referenced whenever possible. Secondary sources are acceptable if the primary cannot be found; if the primary is published in a foreign language; or if the secondary expands on information provided by the primary paper. Checks of accuracy of title name, spelling of author’s name, and so on should be based on the article itself and not on citations listed in other bibliographies (e.g., Medline).
The appendix section is optional and may not be allowed by some journals. Contents should be limited to information regarding methods that is too technical or detailed to include in the body of the text. Examples of information to be contained in the appendices are details of sample size calculations; sample data forms; data collection schedules; special charts, figures, and equations; consent statements; and data listings.
The submission process can be facilitated if the manuscript is reviewed before submission. Authors should review the paper for inconsistencies in format and style (including tables and figures), for redundancy, and for reporting deficiencies. Figure and table numbers should match text citations. Citations should be reviewed to ensure that information cited is correct. Colleagues should provide the second review. Their primary function is to identify confusing aspects of the manuscript. Total rewrites may result from this internal review. The final review by the authors should focus on the format of the journal to which the manuscript is to be submitted. The number of copies and prints submitted, title page, style and format, and so on should match the specifications set forth in the journal’s “Instructions to Authors.” After publication, the primary authors should establish an archive consisting of all documents related to the paper, beginning with raw data and finishing with a copy of the printed manuscript. This information should be kept at least 3 years (Meinert, 1986).
Reviewing a Clinical Trial
When reviewing a manuscript that reports the results of a clinical trial, the reviewer should be unbiased regarding the results. The reviewer’s opinion should be based on the merits of the study rather than on the opinions and critiques of others. The purpose of the clinical report should be strongly considered, particularly if sponsor support was critical to the study and the sponsor stands to gain financially from it. The information provided in the report should allow adequate review of methodology so that critical aspects of the report can be evaluated. Reproducibility of results and generalization of results to a large population (rather than a small subset) should be assessed. Exclusion of patients should be well justified. The study design should be well safeguarded against biases during the assignment and administration of the intervention and during data collection and analysis. Methods used to edit the data for errors or inadequacies should be cited. Statistical methods should be appropriately sophisticated. Major differences in baseline group comparability, dropout rates, or compliance should be evaluated for a possible role in treatment differences.