29 The Pelvis and Reproductive Organs of the Ruminant
This chapter is concerned with the pelvic cavity, the intrapelvic and extrapelvic reproductive organs of both sexes, and the udder.
THE PELVIC CAVITY
The pelvic cavity of the cow becomes progressively narrower between the entrance and the exit. It also loses depth but in less regular fashion because a pronounced dip of the middle part of the floor results in a local increase in height before the caudal part slopes steeply upward to the shallow exit (Figure 29–1).
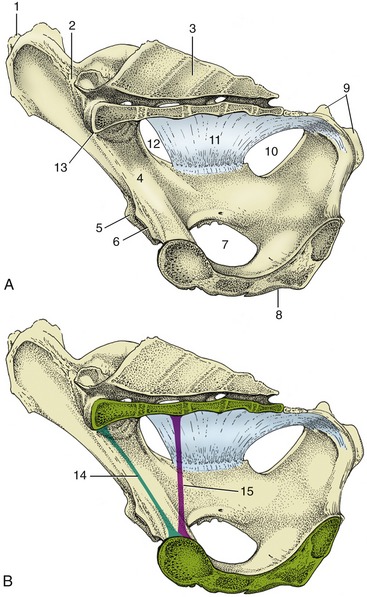
Figure 29–1 A-B, Median section of the bony pelvis of a cow. Certain obstetrical terms are illustrated in B. 1, Coxal tuber; 2, sacroiliac joint; 3, sacrum; 4, shaft of ilium; 5, cranial border of acetabulum; 6, pecten pubis; 7, obturator foramen; 8, symphysis; 9, ischial tuber; 10, lesser sciatic foramen; 11, sacrosciatic ligament; 12, greater sciatic foramen; 13, promontory; 14, conjugate—the line connecting the promontory with the pecten; 15, vertical diameter—the vertical line between the pectin and the pelvic roof.
The entrance faces ventrocranially at an angle that carries the pecten of the pubis below the second intersacral joint (Figure 29–1/15). Behind the iliac shaft, the width is reduced by inflection of the high ischial spine, and it becomes further reduced by the encroachment of the massive ischial tuber on the exit (Figure 29–2). The conspicuously cramped exit is roughly triangular; the third caudal vertebra and the tubercular ischial tubers are its corners. The lateral border is completed by the sacrotuberous ligament (the edge of the sacrosciatic ligament), while the caudal margin of the floor is cut away at the ischial arch. The strong development of the ischial crest and tuber combine to reduce the contribution to the lateral wall that is made by the sacrosciatic ligament (Figure 29–2/4).
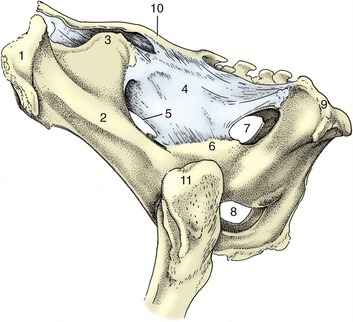
Figure 29–2 Lateral view of the bony pelvis of a cow. 1, Coxal tuber; 2, shaft of ilium; 3, sacral tuber; 4, sacrosciatic ligament; 5, greater sciatic foramen; 6, ischial spine; 7, lesser sciatic foramen; 8, right and left obturator foramina; 9, ischial tuber; 10, sacrum; 11, greater trochanter.
There are certain variations associated with age and gender. The entrance is almost uniformly wide in mature cows but considerably narrowed in its ventral part in heifers. In these younger animals the cranial part of the floor raises a ridge over the symphysis; in older cows, especially those that have carried several calves, the same region is level or sunken. The male girdle, despite being significantly more robust, encloses a cavity that is clearly less capacious; it is even more confined at the entrance, and beyond this the cranial part of the floor tends to be domed.
In sheep and goats the long, slender iliac shafts approach the vertebral column at an acute angle that, in combination with the shortness of the sacrum, places the pecten below the second joint of the tail (see Figure 26–2).
The sacroiliac joints (Figure 29–3) are complemented by strong ligaments binding the two bones together; virtually no movement is normally allowed. About the time of parturition there is some hormone-induced slackening of the collagenous structures of the pelvis, and a modest but potentially significant mobility may then become possible (p. 214).
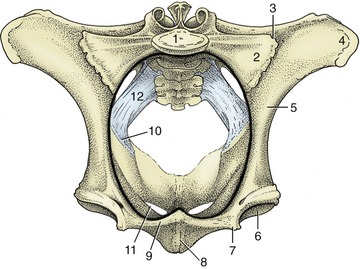
Figure 29–3 Cranial view of the bony pelvis of a cow. The terminal line (black) is indicated. 1, Body of first sacral vertebra; 2, wing of sacrum; 3, sacroiliac joint; 4, coxal tuber; 5, shaft of ilium; 6, acetabulum; 7, iliopubic eminence; 8, symphysis; 9, pecten pubis; 10, ischial spine; 11, obturator foramen; 12, sacrosciatic ligament.
Ankylosis of these joints, accompanied by lumbar spondylosis, is common in aging bulls and, when severe, may disable the animal for service.
The perineal region is extensive because those parts of the hamstring musculature that in the horse provide it with very prominent lateral boundaries are lacking in cattle.
By convention, the region is considered to extend ventrally to include the nearest part of the udder (or scrotum). The increase in breadth exposes the sacrotuberous ligaments, the ischial tubers, and the ischiorectal fossae as visible and palpable surface landmarks. The anus and vulva, the most obvious features of the dorsal and ventral perineal regions, respectively, are considered later (see Figure 29–10).
The blood supply to pelvic structures is delivered by the small median sacral artery and the much larger, paired internal iliacs (Figure 29–4). The first or, more accurately, its continuation as the median caudal artery has already been encountered (p. 668). The internal iliac artery serves both parietal and visceral structures, contrary to the usual arrangement. It enters the pelvic cavity close to the sacroiliac joint and continues down the ilium to reach the vicinity of the lesser sciatic foramen (Figure 29–1/10) before dividing into internal pudendal and caudal gluteal arteries. The latter, like other parietal branches, is of no present concern. The internal iliac’s first visceral branch, detached close to the origin of the parent trunk, is the umbilical artery. This term, though appropriate to its role in the fetus, is misleading because the vessel is now almost exclusively concerned with supplying blood to the uterus through a large uterine artery; the continuation of the umbilical, reduced to a fibrous cord with a vestigial lumen, is better known as the round ligament of the bladder. The male homologue of the uterine artery is the deferential. (The distribution of the arteries to the viscera is considered with the organs they supply.) The second visceral branch, the vaginal artery, is detached close to the termination of the internal iliac trunk and supplies the bulk of the pelvic viscera. The male homologue is the prostatic artery. The internal pudendal artery supplies both parietal structures, including the muscles of the pelvic diaphragm, and viscera, including the female tract from the caudal vagina to the vestibule. The depleted trunk leaves the pelvis, through an opening in the fascia directly above the symphysis, to supply branches to the clitoris and labia and other branches to the perineum, some of which reach the caudal part of the udder (or scrotum and prepuce).
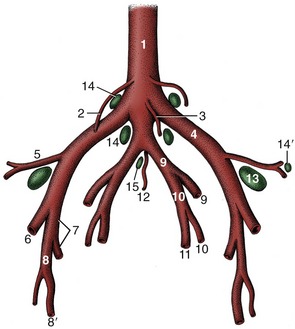
Figure 29–4 Branching pattern of the caudal part of the bovine abdominal aorta. 1, Aorta; 2, ovarian artery; 3, caudal mesenteric artery; 4, external iliac artery; 5, deep circumflex iliac artery; 6, femoral artery; 7, deep femoral artery; 8, pudendoepigastric trunk; 8′, external pudendal artery; 9, internal iliac artery; 10, umbilical artery; 11, uterine artery; 12, median sacral artery; 13, deep inguinal (iliofemoral) lymph node; 14, 14′, medial and lateral iliac lymph nodes; 15, sacral lymph nodes.
The nerves within the pelvis fall into two groups (Figure 29–5). The first comprises the obturator and sciatic nerves that, despite their vulnerability to injury at parturition, will be described with the hindlimb. The second group comprises the pudendal, caudal rectal, and pelvic nerves, of which all are purely sacral in origin and concerned with the supply of the pelvic viscera and the perineum. The significant divisions of the pudendal nerve are the deep perineal and distal cutaneous branches and the continuation of the main trunk. The deep perineal supplies both visceral and somatic structures of the caudal pelvic region. The distal cutaneous branch supplies structures of the ventral perineum (before it becomes superficial by emerging from the ischiorectal fossa), crosses the medial process of the ischial tuber (where it may be palpated), and supplies the vulva and perineal skin; some branches extend as far as the nearest part of the udder. The depleted trunk passes ventral to the vagina before leaving the pelvis in company with the internal pudendal artery; it supplies the dorsal nerve of the clitoris and supplies other branches to the skin of the udder. (In the male, the corresponding branches are the dorsal nerve of the penis and cutaneous branches to the scrotum and prepuce.)

Figure 29–5 Nerves and vessels on the medial surface of the bovine pelvic wall. Local anesthesia of the pudendal nerve can be obtained by injections at A and B; anesthesia of the caudal rectal nerves is possible by an injection at C. 1, Sacrum; 2, pelvic symphysis; 3, rectum (reflected); 4, vagina (reflected); 5, sciatic n.; 6, obturator n.; 7, pudendal n.; 7′, distal cutaneous branch of pudendal n.; 7″, proximal cutaneous branch of pudendal n.; 7′″, deep perineal n.; 7″″, continuation of pudendal n. to clitoris; 8, caudal rectal nn.; 9, pelvic n.; 10, internal iliac a.; 10′, caudal gluteal a.; 11, vaginal a.; 12, internal pudendal a.; 13, caudal border of sacrosciatic ligament; 14, retractor clitoridis.
THE RECTUM AND ANUS
Although the origin of the rectum is arbitrarily defined, its most caudal part is distinguished from the colon by a wider caliber and more muscular wall. The interior, marked by impermanent transverse folds, is generally distended with feces (Figure 29–6).
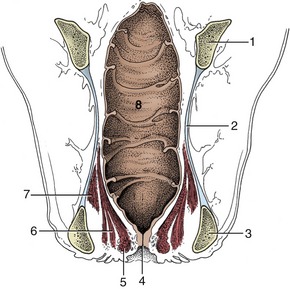
Figure 29–6 Dorsal section of the bovine rectum and adjacent structures. Note especially the topography of the pelvic diaphragm (6, 7).
1, Shaft of ilium; 2, sacrosciatic ligament; 3, ischial tuber; 4, anus; 5, external anal sphincter; 6, levator ani; 7, coccygeus; 8, rectum.
The colic mesentery continues as the mesorectum, which abruptly shortens to a mere 3 cm, before gradually decreasing further until it eventually disappears (Figures 29–7 and 29–8), which brings the rectum into broad contact with the pelvic roof. In this process more and more of the rectal circumference becomes denuded of serosa until the last part is completely embedded in fat, which provides the cushion that allows the gut to adjust to changing circumstances. The close connection with the pelvic roof and walls is a handicap to rectal explorations, and for many purposes the hand must be carried forward into the more mobile colon (Figure 29–9) (p. 720).
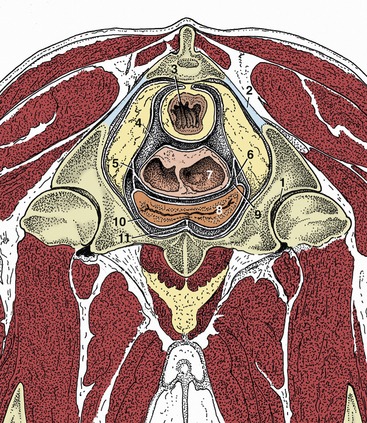
Figure 29–7 Transverse section of the pelvis of a cow at the level of the hip joint (cranial surface). Note the large amount of retroperitoneal fat in the pelvis. (See Figure 29–11 for the level of this section.)
1, Hip joint; 2, sacrosciatic ligament; 3, rectum; 4, rectogenital pouch; 5, broad ligament of uterus; 6, lateral ligament of bladder; 7, uterus sectioned where the two horns are conjoined; 8, bladder; 9, vesiocogenital pouch; 10, pubovesical pouch; 11, median ligament of bladder.
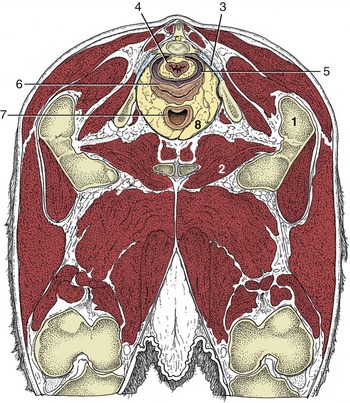
Figure 29–8 Transverse section of the bovine pelvis at the level of the first caudal vertebra (cranial surface). The section passes through the obturator foramina. Note that the peritoneum covers only the dorsal surface of the vagina; the lateral and ventral surfaces are retroperitoneal at this level. (See Figure 29–11 for the level of this section.)
1, Greater trochanter; 2, obturator foramen; 3, sacrosciatic ligament; 4, rectum; 5, rectogenital pouch; 6, vagina; 7, neck of bladder; 8, retroperitoneal fat.
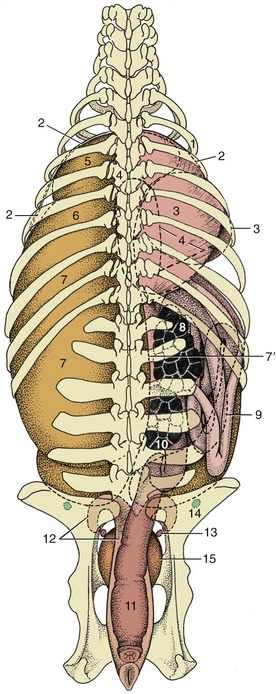
Figure 29–9 Relationship of the principal abdominal and pelvic organs to the bovine skeleton, dorsal view. 1, Sixth rib; 2, cranial extent of diaphragm; 3, omasum, most of it covered by the liver; 4, outline of abomasum; 5, reticulum; 6, atrium ruminis; 7, dorsal sac; 7′, right face of rumen; 8, right kidney; 9, descending duodenum; ventral to it is the intestinal mass; 10, left kidney; 11, rectum; 12, uterus; 13, ovary; 14, lateral iliac lymph node; 15, bladder.
The anal canal is embraced by the pelvic diaphragm; the postdiaphragmatic part forms a low eminence presenting a short transverse slit through which the skin continues to provide the last stretch of the canal with a cutaneous epithelial covering. The anus is guarded by the usual two sphincters, and the striated external one exchanges fascicules with other muscles of the perineum (Figure 29–10).
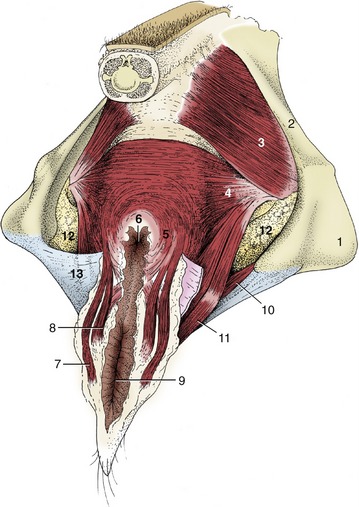
Figure 29–10 The perineal muscles of a cow. 1, Ischial tuber; 2, sacrosciatic ligament; 3, coccygeus; 4, levator ani; 5, external anal sphincter; 6, anus; 7, retractor clitoridis; 8, constrictor vulvae; 9, vulva; 10, urogenital diaphragm; 11, constrictor vestibuli; 12, fat in ischiorectal fossa; 13, perineal fascia (partly removed on the right side).
Most of the rectum is supplied from the cranial rectal artery, a branch of the caudal mesenteric, but the terminal section and the anal region are supplied by twigs from the caudal rectal, an indirect branch of the vaginal artery. The venous drainage is divided between the portal and systemic systems.
THE BLADDER AND URETHRA
The bladder is intraabdominal in the young calf. In the adult it is confined to the pelvic cavity when empty but extends forward over the abdominal floor when distended. The neck within the pelvis is without a peritoneal covering and is attached to the pelvic floor by fat and loose connective tissue (see Figures 29–7 and 29–8). Urine escaping from a ruptured bladder, which is a relatively common mishap, especially in steers, may infiltrate this tissue or enter the peritoneal cavity according to the site of the tear. There are the usual lateral and median ligaments.
The relations of the bladder naturally vary. In the cow, it is always in contact with the cranial part of the vagina and the cervix and often with the body and horns of the uterus. Within the abdomen it makes contact with the dorsocaudal blind sac of the rumen and with the intestines (Figure 29–11).
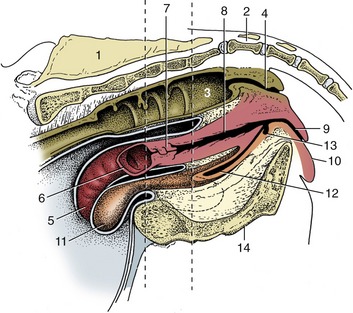

Figure 29–11 Median section of the bovine pelvis. The two vertical broken lines indicate the levels of the transverse sections in Figures 29–7 and 29–8. The position of the obturator foramen is indicated by a broken outline. 1, Sacrum; 2, first caudal vertebra; 3, rectum; 4, anal canal; 5, right uterine horn; 6, left uterine horn, mostly removed; 7, cervix; 8, vagina; 9, vestibule; 10, vulva; 11, bladder; 12, urethra; 13, suburethral diverticulum; 14, symphysis.
Figure 29–13 Various functional stages of the bovine ovary. A, Ovaries with small secondary follicles. B, Ovaries with mature follicle ready to rupture. C, Ovary with recently ruptured follicle; the scar is small and round. D, Ovary with mature corpus luteum.
The urethra is much narrower than that of the mare and runs below the vagina, to which it becomes increasingly attached as it proceeds caudally. It opens into the vestibule through a median slit that is shared with the suburethral diverticulum (Figure 29–11/13), a blind pouch extending cranially that is large enough to admit the end joint of a finger. The pouch can be a nuisance when catheterization is attempted. The urethralis muscle only covers the caudal part of the urethra, which more cranially is anchored to the floor by a short but strong ligament. The cranial fascicules of the urethralis muscle insert on a dorsal raphe that completes the encirclement of the urethra; the more caudal ones form a U that attaches to each side of the vagina and vestibule, enclosing both the diverticulum and the urethra.
The blood supply to these organs comes from the umbilical and vaginal arteries.
THE FEMALE REPRODUCTIVE ORGANS
The topographical peculiarities of the reproductive organs of the female ruminant are the consequence of the descent of the fetal ovaries to the most caudal part of the abdomen, which is a more considerable descent than in other domestic species; as a result, the horns of the uterus are drawn back toward their ovarian attachments and do not range far into the abdomen except in advanced pregnancy. The following account refers primarily to the organs of the mature, nonpregnant cow.
THE OVARY AND UTERINE TUBE
The ovary is a firm, rather irregular ovoid body, small (4 × 2.5 × 1.5 cm) in relation to body size. Joined to the body wall and to the reproductive tract by inclusion in the broad ligament, it is related to the ventral part of the shaft of the ilium, level with the bifurcation of the uterus. Follicles and corpora lutea may project from any part of the surface (Figure 29–13).
The largest follicles attain a diameter of 2 cm, but even those as small as 5 mm in diameter may be detected on palpation per rectum. Because the estrus cycle is short (generally 21 days), follicles and corpora lutea of some size may be present together.
The uterine tube is rather long, but its flexuous course brings its beginning and end close together (Figure 29–14, A-B). The thin-walled infundibulum lies over the lateral wall of the ovary in the free margin of the mesosalpinx. The succeeding, narrower part of the tube winds within the lateral wall of the ovarian bursa to reach the tip of the uterine horn. It is divided into ampulla and isthmus, approximately in the ratio of 2:1, but the distinction is only obvious at certain stages of the cycle. The transition of isthmus to horn is gradual and marked by muscular thickening.
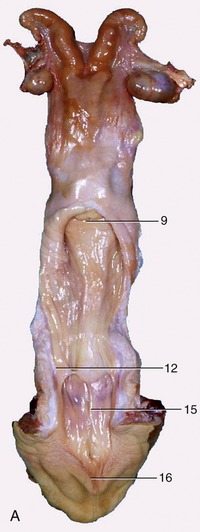
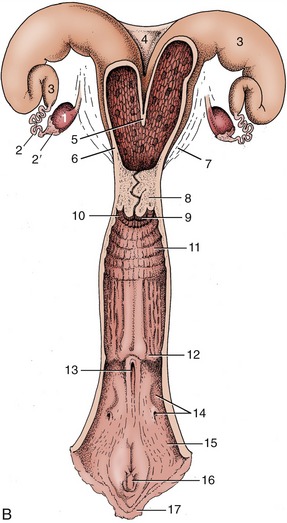
Figure 29–14 The bovine reproductive organs, dorsal view. A, The vagina and the vestibule have been opened in the specimen. B, The greater part of the tract is shown opened in the schema. 1, Ovary; 2, uterine tube; 2′, infundibulum; 3, uterine horn; 4, intercornual ligament; 5, wall of uterus dividing the two horns; 6, body of uterus with caruncles; 7, broad ligament; 8, cervix; 9, vaginal part of cervix; 10, fornix; 11, vagina; 12, position of former hymen; 13, external urethral orifice and suburethral diverticulum; 14, major vestibular gland and its excretory orifice; 15, vestibule; 16, glans of the clitoris; 17, right labium.
Apart from features associated with the frequency of twin and multiple pregnancies, the ovaries as well as the tubes of sheep and goats are very similar to those of cows.
THE UTERUS
At first sight, the uterus appears to consist of a relatively long body succeeded by two divergent, tapering horns coiled ventrally on themselves (Figure 29–15).
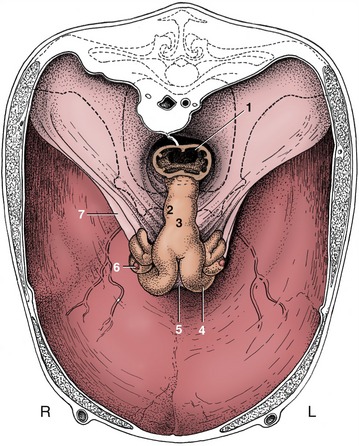
Figure 29–15 The reproductive organs of a cow in situ, cranial view. The bony pelvis is indicated by broken lines. The uterus sags within this largely eviscerated abdomen. 1, Rectum; 2, cervix; 3, body of uterus; 4, left uterine horn; 5, intercornual ligament; 6, right ovary; 7, broad ligament.
This impression is misleading. Most of the apparent body is furnished by the two horns lying side by side within shared serosal and muscular coats, which is an arrangement suggested by a dorsal groove that becomes more pronounced toward the bifurcation. Where the horns diverge, the superficial tissues initially bridge the gap, forming short dorsal and ventral intercornual ligaments (Figure 29–14/4) that bound a small pocket conveniently arranged to allow the organ to be fixed by a finger during rectal examinations. The tight winding of the horns is not constant but results from stimulation of the muscle of the organ and of the broad ligament. The stimulus is provided by handling, which explains why the form of the uterus appears to become more definite and its consistency firmer in the course of a rectal examination. The effect is most noticeable during estrus.
The firmness of the cervix permits recognition of the caudal limit of the body when the intact organ is handled, but there is nothing to indicate its cranial limit. It is surprising to discover, when the organ is laid open, that the body is a mere 3 cm in length. Each horn measures 35 cm or so, of which about one third is incorporated in the “pseudobody”; the cervix measures 8 to 10 cm.
The most characteristic feature of the interior is the presence of the caruncles, the attachment sites of the fetal membranes in pregnancy. About forty of these are arranged in four more or less regular rows in the wider parts of the horn, reducing to a double line toward the tip.
The cervix begins at the constriction of the internal uterine ostium, beyond which the passage is occluded by the interlocking of projections from the walls; these consist of three or four circular folds in virgin animals, but these become broken and irregular in multipara. The most caudal fold projects into the vagina, where it is surrounded by an annular fornix. The cervical mucosa also shows longitudinal folds that, on reaching the external ostium, radiate in a fashion recalling the segments of an orange (Figure 29–16, A-B). If parturition is disregarded, the cervix is most easily passed by an instrument at estrus, but the difficulties experienced at other times can be overcome and indeed must be overcome to allow embryo transfer.
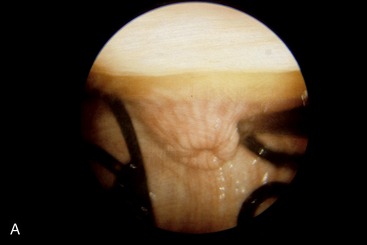
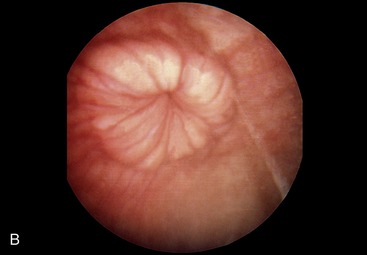
Figure 29–16 The appearance of the vaginal part of the bovine cervix during pregnancy (A) and during estrus (B).
Most features that distinguish the uterus of the small ruminants are of little practical importance. The free surfaces of the caruncles are concave, most obviously in the ewe (Figure 29–17). Certain features of the cervix are more significant. Many irregular, originally circular folds project into the lumen, fitting closely together; the last one is sunken into a recess of the vaginal wall. In combination, these features make catheterization of the uterus very difficult if not impossible at most stages of the cycle.
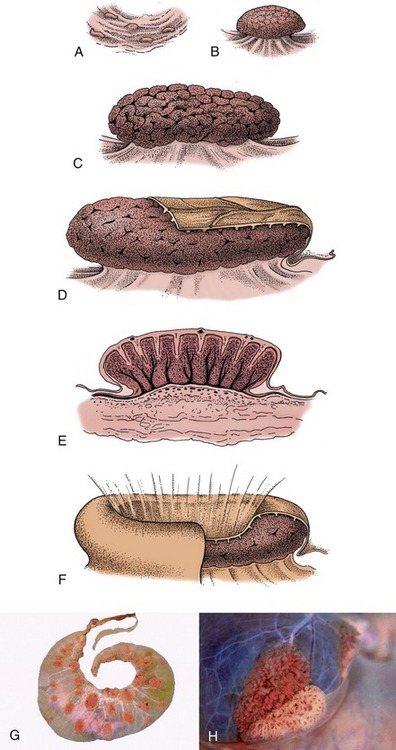
Figure 29–17 A–E, Development of caruncles in the wall of the bovine uterus. A, Caruncle in a nongravid uterus. B, Caruncle in a 2-week gravid uterus. C, Caruncle in a 6-month gravid uterus. D, Caruncle near term, covered in part by a cotyledon (fetal tissue). E, Section of a placentome. F, Placentome of a sheep. G, Cotyledonary placenta (ruminant). H, Partial separation of maternal and fetal parts of placentome (cow).
THE VAGINA
The remaining part of the genital tract is divided between the vagina and vestibule, approximately in the ratio of 3:1; the boundary is a few centimeters cranial to the ischial arch (see Figure 29–11). Because the vagina is capable of great expansion, in length and in diameter, its passive dimensions are not of great significance. The lining exhibits low folds, both circular and longitudinal, and the lumen is closed by the falling together of the roof and floor (see Figure 29–8).
It is usual to find the caudal part ventrally narrowed, especially in young animals; this feature, not to be confused with the hymen (which is rarely much in evidence), is ascribed to the urethralis muscle.
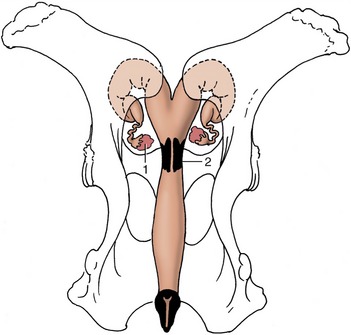
Figure 29–12 Dorsal view of the bony pelvis and related (nongravid) bovine reproductive organs. Note the position of the ovaries in relation to the pecten pubis. 1, Ovary; 2, cervix.
The cranial two thirds of the dorsal wall faces into the rectogenital pouch, but caudal to this the vagina and rectum are joined by a wedge of tissue (see Figure 29–11). The ventral surface has a less complete peritoneal covering and is related to the bladder and urethra and to the packing tissues about the urethra. The lateral walls are also largely without peritoneum, being cranially included in the broad ligament and more caudally sharing in the general retroperitoneal arrangement (see Figures 29–7 and 29–8). This limitation of the peritoneum is relevant to the prognosis of wounds to the vaginal wall. The peritoneal covering of the dorsal fornix region provides a convenient route for surgical access to the abdominal cavity, most often used for operations on the ovary; it has the additional advantage of avoiding the major vessels that pass below and to the sides of the vagina (see Figure 26–19).
Vestiges of the mesonephric ducts may be found below the mucosa of the floor near the junction with the vestibule; they are sometimes the origin of cysts.
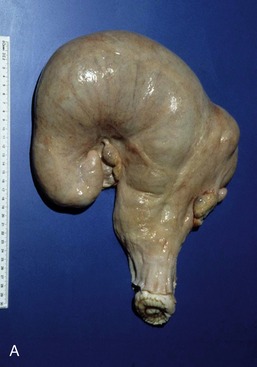
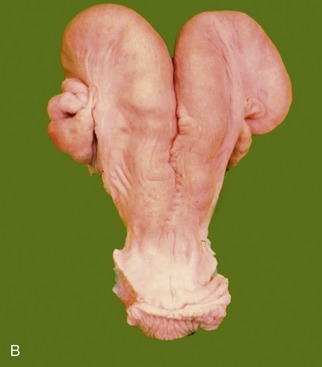
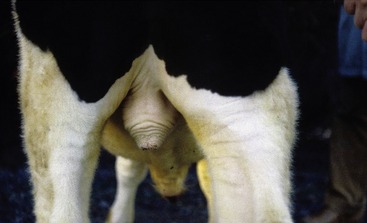
The vagina is almost absent in the freemartin (p. 712), whose abnormally short tract is evident on examination of the vestibule. Aplasia or constriction of the vagina also occurs in white heifer disease, another congenital anomaly.
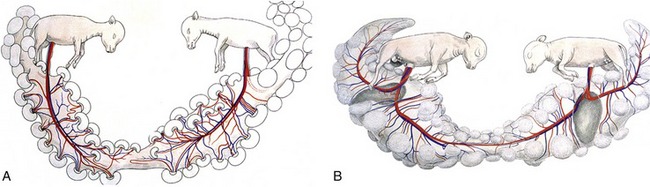
The freemartin is found after a twin pregnancy in which the female fetus is adversely affected by the male twin (Figure 29–18).
THE VESTIBULE AND VULVA
The vestibule slopes ventrally to open between the labia (see Figure 29–11). It is less distensible than the vagina and its side walls are normally in contact. When drawn apart, the opening of the urethra is exposed at the cranial end of the vestibule and, at the other, the fossa containing the glans of the clitoris (see Figure 29–14). A large depression caudolateral to the urethral opening marks the location of the major vestibular gland, about 3 cm long, which is enclosed within the urogenital diaphragm. The vestibular mucosa is generally darkened over the gland.
The rounded, rather low labia are often marked by trauma sustained at previous calvings. Simple inspection exposes relatively little of the slender clitoris because the glans is fused with the prepuce. The vulva of the freemartin, abnormally small, is surrounded by unusually long hair.
The vestibule penetrates the urogenital diaphragm (perineal membrane), which fills the gap between the rectovaginal septum and the pelvic floor. The fascia of the diaphragm arises from the pelvic floor, bends around and attaches to the wall of the vestibule, and merges with the rectovaginal septum, the lower edge of the pelvic diaphragm, and the parietal pelvic fascia. One importance of the arrangement lies in its anchorage of the genital tract, opposing the drag of the gravid uterus as it sinks into the abdomen and the backward drag during calving.
Constrictor vestibularis and constrictor vulvae muscles are associated with the vestibule and vulva. The former, the more important, incorporates some fascicules that continue from the levator ani and form the perineal body. It runs over the wall of the vestibule caudal to the diaphragm and passes below the vestibule to join its fellow; on contraction it narrows the genital passage and raises a ridge in its floor. The constrictor vulvae, through its insertion to the vulva and adjacent skin, may cause the opening to gape.
VASCULARIZATION
The relatively small ovarian artery, a direct branch of the aorta in cattle, supplies the ovary, the uterine tube, and the adjoining part of the horn of the uterus. The ovarian artery is distinguished by an extraordinarily convoluted course within the cranial part of the broad ligament and has extensive contact with the plexiform ovarian vein (Figure 29–19). These features facilitate the transfer of prostaglandins from venous to arterial blood. The uterine artery arises from the internal iliac and enters the pelvic cavity within the broad ligament. It is ostensibly a branch of the umbilical but appropriates virtually the entire flow of its parent (see Figure 29–4). It is the largest of the arteries to the female tract, and before reaching the uterus, it divides into cranial and caudal parts, each the source of about half a dozen stem vessels that reach the mesometrial border of the uterus. Branches from these run over the uterine walls following courses that appear to coincide with the locations of the caruncles internally. The arrangement leaves the antimesometrial border of the uterus less well supplied and thus less prone to bleeding when incised. The vaginal artery, branching from the internal iliac near the ischial spine, runs over the dorsolateral surface of the vagina before swinging forward over the lateral wall, where it risks involvement, with possibly fatal outcome, in vaginal rupture, a relatively common calving catastrophe in heifers. Various branches pass to the caudal genital tract and to the bladder and urethra.
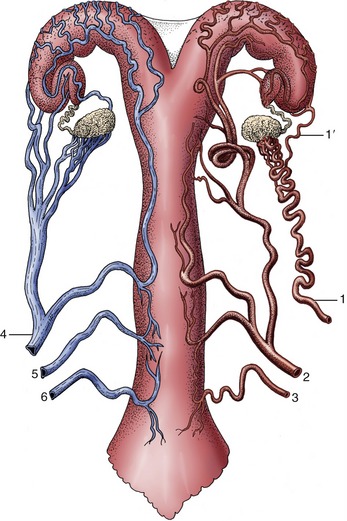
Figure 29–19 Semischematic, ventral view of the blood supply to the bovine reproductive tract (cow). The arteries are depicted on the right side, the veins on the left. 1, Ovarian artery; 1′, uterine branch; 2, uterine artery; 3, vaginal artery; 4, ovarian vein; 5, accessory vaginal vein; 6, vaginal vein.
A very large and conspicuous venous plexus lies in the parametrial tissues of the broad ligament and over the ventral surface of the uterus and vagina, partly covered by the outer layers of muscle. It constitutes a blood pool that can drain in several directions (see Figure 29–19). The ovarian vein, the largest emissary vessel, runs in the cranial part of the broad ligament; the vaginal veins, including a surprisingly small vein that corresponds to the large uterine artery, play a secondary role. Both sympathetic and parasympathetic nerves supply the genital tract.
GROWTH AND CYCLICAL CHANGES
The growth of the reproductive organs, isometric in the very young, accelerates in response to the production of ovarian hormones after the initiation of the estrus cycle, generally when a heifer is about 8 to 10 months old. The cumulative effects of a few cycles produce a striking increase in the dimensions and a clearer differentiation of the component tissues of the tract.
In each cycle a follicle becomes identifiable on rectal examination about the 16th day and attains its full size a couple of days later. Its rupture is preceded by a reduction in internal pressure, recognizable on rectal palpation; the clot that succeeds the moderate ensuing hemorrhage is soon replaced by a corpus luteum. This reaches its maximal size, approximately that of the follicle it replaces, after about a week; regression then begins, and by the 21st day, the time of the next estrus, it has already shrunk by about two thirds. It is eventually replaced by a scar. The waxing and waning of the corpus luteum are marked by color changes progressing from brown to ochre and then through orange, brick red, and dirty white in regression.
The ampulla becomes noticeably wider after ovulation, when the sphincter action of the isthmus delays the entry of the egg into the uterus. The uterine changes that commence in proestrus and continue into metestrus involve hyperemia and edema thickening the endometrium; the moderate hemorrhage that sometimes accompanies their subsidence appears to be the origin of the increasing pigmentation of the uterine wall of older animals.
An increase in the size, complexity, and activity of the endometrial glands culminates a week or so after ovulation. The activity of the myometrium, whether spontaneous or in response to external stimuli, is greatest immediately before and during estrus.
The greater activity of the cervical mucosa during estrus spreads to the mucosa that lines the cranial part of the vagina. The transparent mucus of low viscosity that is produced is eventually discharged and may be tinged with blood when bleeding at metestrus is pronounced. There is no distinct cycle of cornification of the vaginal epithelium.
The bovine estrus cycle is repeated at intervals of 21 days. The small ruminants are seasonally polyestrous, largely in the fall and early winter; the cycle lasts 16 or 17 days in sheep and 20 in goats.
GESTATION AND PARTURITION
Gestation lasts 280 days in cattle, 147 days in sheep, and 154 days in goats. During this time every part of the reproductive system shows some changes, but obviously the most striking are in the uterus, which increases its weight 15-fold (100-fold when its content is included).
The ovary is distinguished by the presence of the corpus luteum of pregnancy, which persists beyond the life span of the periodic body of the infertile cycle. Its survival is not always accompanied by total suppression of follicular activity; a few cows come into heat and ovulate in early pregnancy. The corpus luteum is not necessary for the support of pregnancy during the last 3 months and usually begins to regress about a month before term (Figures 29–20 and 29–21).
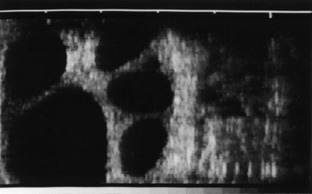
Figure 29–20 Ultrasonographic transrectal scan of the ovary of a cow that was stimulated with gonadotropin to induce superovulation. The black spots represent sections of large tertiary follicles.
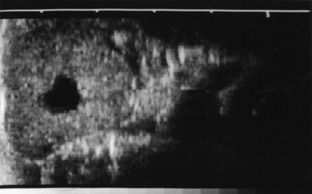
Figure 29–21 Ultrasonographic transrectal scan of a corpus luteum of a cycling cow; the corpus is marked by a cavity (black spot).
The progestational changes that are part of every cycle persist and intensify in the presence of an embryo. Although the blastocyst is initially confined to one horn, the membranes soon spread to the other; however, the embryo, later the fetus, is almost invariably restricted unilaterally, and a developing asymmetry is one of the first detectable signs of pregnancy. The amniotic sac becomes palpable about the 30th day, the fetus itself about the 70th. The caruncles of the gravid horn gradually increase from low, smooth-surfaced bumps to become large, pedunculated swellings with surfaces pitted for the reception of the chorionic villi; by term the largest may attain the size of a clenched fist (Figures 29–17, 29–22, and 29–23). Those in the nongravid horn later also enlarge but to a lesser degree.

Figure 29–22 A, Transrectal ultrasonic view of a placentome (1) and fetal head (2) at 3 months’ gestation. The two lower jaws of the fetus are at 3. B, Transrectal ultrasonic view of a placentome (+) at 5 months’ gestation. Ultrasonic views of placentomes are diagnostic of pregnancy if the fetus itself cannot be visualized.
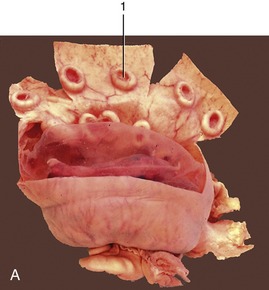
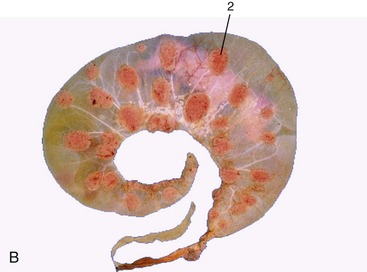
Figure 29–23 A, A gravid uterus, partly opened. B, A bovine fetus within its membranes. The villi are mainly restricted to the cotyledons. 1, Caruncle; 2, cotyledon.
The enlargement of the uterus does not affect all parts equally. The lesser curvature, being tethered by the broad ligament, most resists expansion, which causes the horn to alter shape: the greater curvature and adjacent parts grow away from the attachment. Hypertrophy of the tissues of the broad ligament restrains the uterus from sinking into the abdomen for a time, but by the third month this resistance is overcome and the uterus begins to slip forward over the abdominal floor. The supply of blood to the gravid uterus is necessarily greatly increased; all uterine vessels contribute to this, but the major role is played by the uterine artery of the gravid side, which increases in diameter from a few millimeters to a centimeter or more. It loses its flexuous character and now passes forward into the abdomen, where it is easily found on palpation against the ilium; identification is assisted by the characteristic vibration (fremitus) it now displays.
The topography is not the same in every pregnancy. The enlarging uterus usually enters the supraomental recess but sometimes may slip forward against the right or the left flank. As it expands, it sinks within the abdomen and for a time passes out of reach of a hand within the colon; this inability to reach the uterus at about the fifth month is as diagnostic of pregnancy as its palpable enlargement at earlier and later times. The descent into the abdomen stretches the vagina and carries the cervix over the pubic brim. Toward term, the uterus occupies most of the ventral and right sections (in the common arrangement) of the abdomen, which raises the rumen dorsally and crushes the intestines upward (Figure 29–24). It makes contact with the liver and diaphragm, on which it exerts increasing pressure. During the first months the calf enjoys freedom to move and adjust position within the amniotic fluid, but as pregnancy continues, it is forced to adapt to the form and dimensions of the uterine horn.
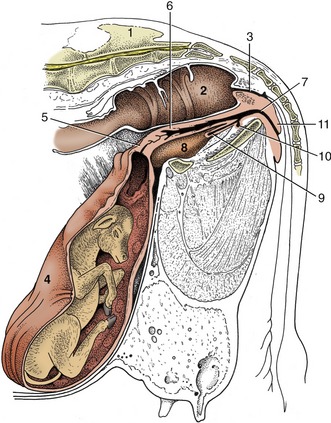
Figure 29–24 Paramedian section of the caudal abdomen and pelvis of a pregnant cow. The section is not quite vertical because it cuts through the vertebral canal and an obturator foramen. Note the large placentomes. 1, Sacrum; 2, rectum; 3, anal canal; 4, uterus; 5, cervix; 6, vagina; 7, vestibule; 8, bladder; 9, urethra; 10, suburethral diverticulum; 11, vulva.
The cervical canal is closed by a mucous plug, developing from the first month and later projecting through the external cervical ostium. The first changes in the vagina are due to traction, but the wall later becomes increasingly elastic and the lumen potentially roomier. Enlargement of the vulva is evident by the end of the first trimester in animals carrying their first calf, but in multipara, in which the vulva tends to be permanently enlarged, there may be no change obvious until shortly before birth.
Changes that signal the approach of parturition include softening of the sacrosciatic ligament, with insinking beside the tail head (Figure 29–25, A-B); a similar loosening of other pelvic ligaments allows some relaxation of the sacroiliac joints. The connective tissues of the cervix and caudal reproductive tract and vulvar and perineal skin share in these changes that, though spread over several weeks, are much intensified in the last few days. When parturition actually impends, edema of the soft parts may cause the vulva to gape.
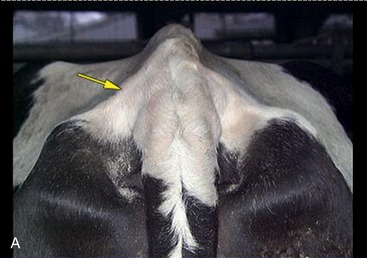
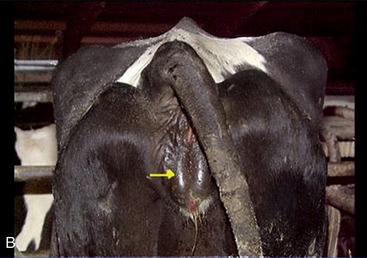
Figure 29–25 Indications of impending parturition. A, Relaxation of the sacrosciatic ligament. B, Swelling of the vulva.
The earlier description of the bony pelvis of cows will have suggested that it is not particularly favorable to easy parturition. Its dimensions are relatively small, and the axis of the birth canal is broken where it passes over the pubic brim and again where the floor changes direction to rise toward the exit. Some increase in the vertical diameter is possible if the pelvis can rotate about the relaxed sacroiliac joints, but this relief is clearly denied to the standing cow. The principal soft tissue impediments to easy birth are the cervix, the caudal end of the vagina, and the vulva. Normally these parts also loosen under hormonal influence.
The umbilical cord ruptures when the cow gives birth and, being relatively short, often before delivery is complete. Its constituents part at different levels.
The fetal membranes (the “cleansings” in lay speech) normally separate from the endometrium and are expelled shortly after delivery; it is a process hastened by suckling, which stimulates the release of oxytocin. Retention with corruption in utero may require human intervention to accomplish their removal.
After parturition, the tract tends to return to its former state, but first pregnancies leave a permanent legacy in the form of thickening and loss of symmetry (Figure 29–26). The uterus contracts as soon as it empties, undergoing a very rapid atrophy in which a third of its weight is lost within a couple of days; the second third is lost before the week is out. The decline is slower thereafter, but should a cow remain “empty,” a period of superinvolution (lactation atrophy) may follow, in which the size of the uterus drops below the resting norm. Involution of the vagina, vestibule, and vulva is slower.
SOME ASPECTS OF DEVELOPMENT
Only a few points need be raised to supplement the general account given in Chapter 5.
Most unusually, ovulation in cattle does not occur until some hours after the end of estrus. Cleavage commences in the uterine tube, where fertilized ova are detained for several days before being released by the isthmus into the horn of the uterus. The small, spherical blastocyst that is first formed undergoes very rapid elongation from about the 13th day, first extending as a threadlike structure through the entire length of that horn and then, by about the 18th day, passing through the body to invade the contralateral horn. In this way, a single embryo takes maximal advantage of the endometrium available for its support. When twins are present, each claims one horn, and because both usually derive from the same ovary, transuterine migration seems to be readily accomplished. Contact between the two chorionic sacs is inevitable and results in fusion and anastomoses of the twin sets of vessels (with potentially unfortunate results; see Figure 29–28).
The account of the development of the embryonic membranes and establishment of the cotyledonary placenta (see Figure 29–17) already given requires no amplification.
The placenta is a barrier to the intrauterine exchange of immune bodies in utero in ruminant species, and the newborn relies on colostrum for its early immunological protection.
Although the incidence of twin pregnancy in cattle is not high (1% to 4% according to breed), twinning has attracted much attention because of the virtual certainty that the female partner of a male calf will exhibit intersex characteristics. The masculinization of the female, the so-called freemartin, is due to exchange between the two circulations. It was long thought that exposure to androgens was the causal factor, but this is now believed to be of little importance. In the prevailing view, what is significant is the transfer of antimüllerian hormone (causing regression of the müllerian ducts) and descending (causing gubernacular outgrowth) and the exchange of cells between the two embryos, which are in fact chimeras (see Figure 29–18).
Support for the last point is obtained from the fact that most cattle twins, presumably those that shared a common placental circulation, accept grafts of their partner’s skin in adult life, which indicates that cellular exchange had taken place when they were immunologically tolerant.
Twins and triplets are of course common in sheep and goats. The incidence varies with the breed and reflects the clemency or severity of the environment in which that breed evolved.
It is often convenient to be able to estimate the age of an aborted fetus in the field. There are many tables relating various measurements to age, but all suffer from the disadvantage of recording average values for parameters that vary considerably with breed, nutritional status, and other factors. One guide, easily memorized, allows 1 cm crown–rump length for each of the first 12 weeks’ gestation and 2.5 cm for each week thereafter. Except with the youngest embryos, it is rarely more than 2 weeks off, and greater accuracy is hardly to be expected of any rule-of-thumb method.
Qualitative methods that consider the external and internal anatomy are more accurate, but information on these matters must be sought elsewhere.
A few of the most obvious features are given in Tables 29–1 and 29–2.
Table 29–1 Guide to the Aging of Cattle Fetuses
From Evans HE, Sack WO: Prenatal development of domestic and laboratory animals. Growth curves, external features and selected reference. Anat Histol Embryol 2:11–45, 1973.
| Age (mo) | Crown–Rump Length (cm) | External Features |
|---|---|---|
| 1 | 1 | Head and limb buds are distinguishable |
| 2 | 6 | Digits are distinguishable |
| 3 | 10 | Scrotal (male) or mammary (female) swelling is distinct |
| 4 | 20 | First hairs appear about the eyes; horn buds are present |
| 5 | 30–40 | Hairs appear about the mouth; testes are within the scrotum |
| 6 | 40–60 | Hair is present on the tail extremity |
| 7 | 50–70 | Hair is present on the proximal parts of the limbs |
| 8 | 60–80 | The haircoat is general but still short and sparse over the belly |
| 9 | 70–90 | The appearance is mature and the body is well haired; the incisors have erupted |
| Full term (278–290 days) |
Table 29–2 Guide to the Aging of Sheep Fetuses
| Age (mo) | Crown–Rump Length (cm) | External Features |
|---|---|---|
| 1 | 2 | Pinna triangular; eyelids forming; tactile hair follicles beginning to appear around eyes; principal forelimb digits prominent |
| 1.5 | 6 | Eyelids fused; external genitalia differentiated; teats present |
| 2 | 11 | Hair begins to cover the body |
| 3 | 24 | Tactile hairs appear on face; testes in upper part of scrotum |
| 4 | 38 | Woolly hair begins to grow; eyes open again |
| Full term (147–155 days) |
From Evans HE, Sack WO: Prenatal development of domestic and laboratory animals. Growth curves, external features and selected reference. Anat Histol Embryol 2:11–45, 1973.
Maturity in the sense of the capacity to make the integrated physiological responses necessary for survival outside the uterus is not achieved until late in gestation. In lambs the mortality is 100% in those delivered at 135 days and is still very high in those delivered at 140 days. Unfortunately, reliable information on these matters for cattle is not readily available.
THE MALE REPRODUCTIVE ORGANS
THE SCROTUM AND TESTES
The pendulous scrotum is contained between the cranial parts of the thighs and may reach the level of the hocks. A constricted neck joins it to the trunk, just caudal to the superficial inguinal ring, while its lower part is molded on the testes (Figure 29–27). A mass of fat (“cod fat”) is commonly found about the cord stump of the castrate; when present in excess, it may dilate the inguinal canal and produce a pseudohernia inguinalis. Although the rudimentary teats often found on the cranial face of the scrotum possess little intrinsic interest, their number and spacing receive attention in dairy bulls because the corresponding characters are likely to be transmitted to their female offspring. The scrotal nerve supply is diffuse; it comes from the first two lumbar, the genitofemoral, and the pudendal nerves.
Wool covering the scrotum of the ram may cause infertility by impairing the dissipation of heat.
Each testis is ellipsoidal, large in relation to body size (especially in the smaller ruminants), and hangs vertically in the scrotum, where it may be palpated (Figure 29–28). It carries a large epididymis along the medial or caudomedial border that is turned to face its fellow. The epididymis is firmly attached to this border of the testis; the head extends a considerable distance down the free border, while the large, conical, and very distinctly palpable tail projects ventrally. The capsule of the testis displays a distinctive winding pattern of vessels and contains the parenchyma under slight pressure; delicate partitions detached from the capsule merge to form a prominent mediastinum (see Figures 5–37 and 5–38).
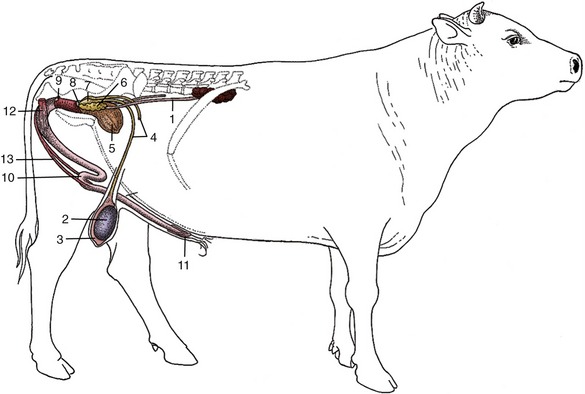
Figure 29–28 Disposition of the urogenital organs of a bull. 1, Ureter; 2, right testis; 3, epididymis; 4, deferent duct; 5, bladder; 6, vesicular gland; 7, ampulla of deferent duct; 8, body of prostate; 9, bulbourethral gland; 10, sigmoid flexure of penis; 11, glans penis; 12, ischiocavernosus; 13, retractor penis.
After emerging from the tail, the deferent duct ascends along the medial border of the epididymis but is separated from this by the mesorchium, which is a relationship that advises a cranial approach in vasectomy operations. The duct is easily recognized on palpation as a firm, narrow strand. The conical, dorsally tapering spermatic cord is largely composed of the exceptionally convoluted testicular artery embedded in the pampiniform plexus (see Figure 5–43). The significance of the arteriovenous anastomoses found here remains obscure: they may be related to the varicoceles occasionally found in the cords of castrated animals (see Figure 5–46). The part of the cord located within the scrotal neck is selected for crushing in the Burdizzo method of castration.
The lymphatic drainage of the testis is to the medial iliac nodes; that of the scrotum is to the superficial inguinal node by the scrotal neck.
THE PELVIC REPRODUCTIVE ORGANS
The constituents of the spermatic cord disperse at the vaginal ring, from whence the deferent duct may be traced over the dorsal surface of the bladder. It passes under the body of the prostate to reach the urethra, and in the last part of its course it is combined with the duct of the vesicular gland in a very short common passage. The subterminal stretch (ca. 10 to 12 cm) lies beside its fellow in the genital fold; the wall of this part is swollen to form the cylindrical ampulla or ampullary gland. A median vestige of the fused paramesonephric ducts is sometimes present between the two ampullae (Figure 29–29).
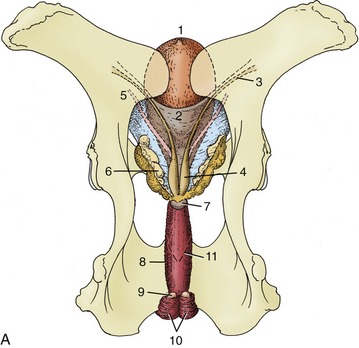
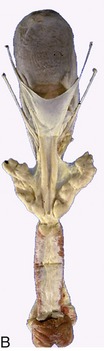
Figure 29–29 Dorsal view of the bull’s pelvis and related urogenital organs. A, Schema. 1, Bladder; 2, genital fold; 3, right deferent duct; 4, ampulla of deferent duct; 5, left ureter; 6, vesicular gland; 7, body of prostate; 8, urethralis (surrounding urethra); 9, bulbourethral gland; 10, bulbospongiosus; 11, caudal extent of the rectogenital pouch (broken line).
The urethra runs over the pelvic floor from the bladder (Figure 29–30) and leaves the pelvic cavity by bending around the ischial arch. Level with the arch, the lumen presents a dorsal diverticulum guarded at its entrance by a mucosal flap. The flap splits at its caudal extremity into two folds that constrict the urethral lumen by attaching to the walls. The tip of a catheter almost inevitably engages in this diverticulum, which makes catheterization of the bladder impossible if surgical access to the urethra is not gained first. (Even without the diverticulum, the sigmoid flexure of the penis presents a formidable complication.)

Figure 29–30 Transverse section of the bovine pelvic urethra immediately caudal to the body of the prostate. 1, Urethra; 2, spongy tissue (stratum spongiosum); 3, disseminate part of prostate; 4, urethralis; 5, dorsal aponeurosis of urethralis.
The pelvic urethra is encircled by the striated urethral muscle, completed dorsally by a stout aponeurotic plate. A thin sleeve of spongy tissue directly surrounds the lumen; when followed caudally, it expands to form the bulb of the penis. The penile urethra is narrower, especially at the sigmoid flexure, where calculi most often lodge, particularly in castrated animals.
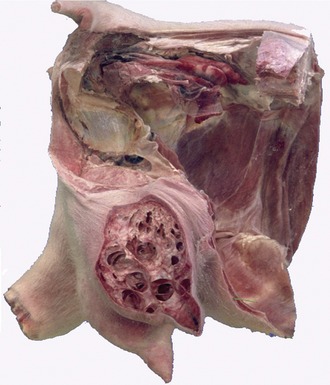
The vesicular glands are very large (10 × 3 to 15 × 5 cm) and contribute the bulk of the seminal fluid. They are flexed on themselves, grossly lobulated with narrow branching lumina, and lie within the genital folds, mainly lateral to the ampullary glands (Figure 29–29, A-B). The prostate of the bull consists of a disseminate part stretching along the length of the urethra, largely dorsal to the lumen and diminishing in thickness when followed caudally, and a compact part (body) consisting of paired lobes that have broken through the urethral aponeurosis and together form a bar lying across the first part of the urethra (4 × 1 cm).
The small bulbourethral glands, located by the ischial arch, are flattened and covered by the bulbospongiosus muscle (Figure 29–29, B). Their watery secretion is discharged into the diverticulum and flushes the urethra in advance of the main ejaculate.
Apart from the body of the prostate, which is specific to the bull, the pelvic reproductive glands are very similar in the three domestic ruminants.
THE PENIS AND PREPUCE
The penis of an adult bull is almost 1 m long, but about a quarter of its length is taken up by the sigmoid flexure located above and behind the scrotum (Figures 29–31, 29–32, and 29–33).
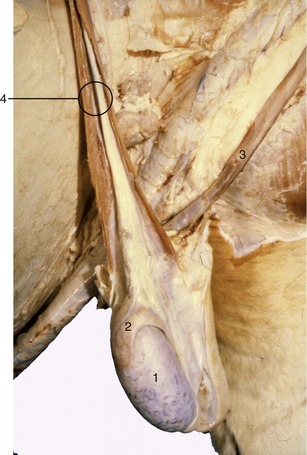
Figure 29–31 Scrotum opened, and testis and epididymis exposed. Note tortuous veins on surface of the testis. 1, Testis; 2, epididymis; 3, retractor penis muscle; 4, spermatic cord.

Figure 29–32 The fibroelastic bovine penis and its retractor muscle. 1, Sigmoid flexure; 2, retractor penis muscle; 3, preputial skin.
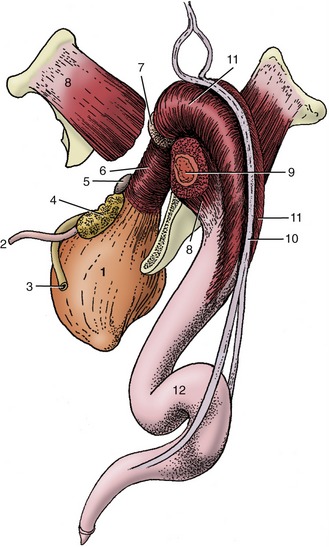
Figure 29–33 The bovine penis and its muscles; caudolateral view. 1, Bladder; 2, ureter; 3, deferent duct; 4, vesicular gland; 5, body of prostate; 6, urethralis; 7, bulbourethral gland; 8, ischiocavernosus; 9, crus of penis (in transverse section); 10, retractor penis; 11, bulbospongiosus; 12, sigmoid flexure.
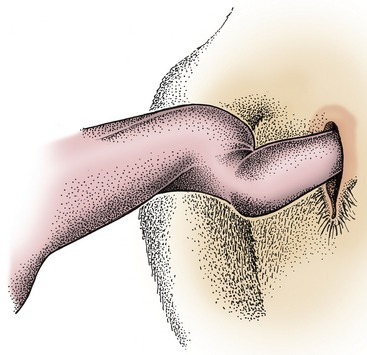
Being of the fibroelastic type, it is relatively rigid at all times. The rodlike, laterally compressed crura are almost surrounded by the powerful ischiocavernosus muscles and contain more generous cavernous spaces than are present in other parts of the organ. The construction of the body of the penis is not immediately evident because its constituents, the crura and the urethra, are enclosed within a common tunica albuginea (Figure 29–34). Paired ligaments suspend the caudal part of the body from the symphysial tendon; their occasional rupture causes the penis to sag. The extremity of the quiescent penis is capped by a cushion of softer tissue, forming an asymmetrical, ventrally bent, and slightly spiraled glans that is contained within the caudal part of the prepuce. The glans exhibits a raphe or seam over its right aspect; the urethra follows this to open on the summit of a low process (Figure 29–35).
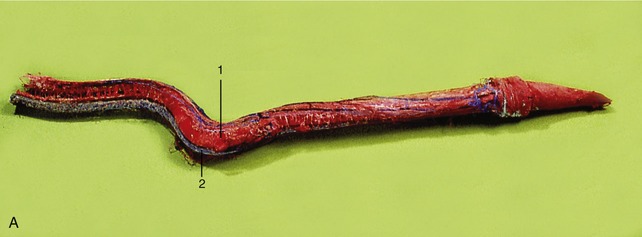

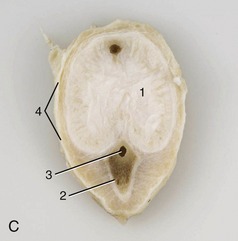
Figure 29–34 Cast of the cavernous spaces of the bovine penis (A) and transverse sections caudal (B) and cranial (C) to the sigmoid flexure. 1, Corpus cavernosum; 2, corpus spongiosum; 3, urethra; 4, tunica albuginea.
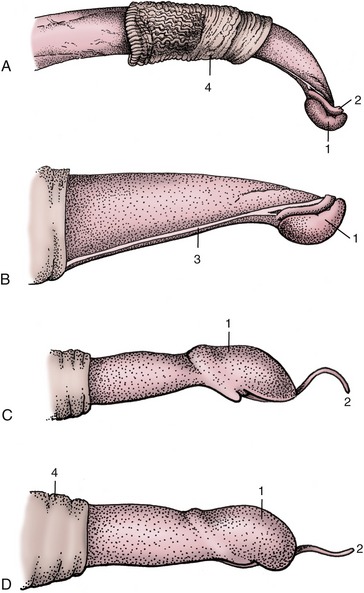
Figure 29–35 Right lateral view of the distal end of the bull’s penis, flaccid (A) and erect (B); the distal end of the ram’s (C) and buck’s (D) penis. 1, Glans; 2, urethral process; 3, raphe; 4, preputial skin.
The prepuce shows the usual disposition and encloses a cavity that is both long and narrow. The prepuce droops behind the umbilicus, most obviously in beef bulls, which makes it vulnerable to injury by sharp grasses.
The penis obtains its blood supply from branches of the internal pudendal artery that are detached within the pelvis. One, the artery of the bulb, supplies the bulb and corpus spongiosum; a second, the deep artery of the penis, supplies the crus; and a third, the dorsal artery, travels along the upper border to reach the glans, detaching twigs to the prepuce en route. All three are accompanied by satellite veins that drain both the tissues and the blood spaces within the spongy and cavernous bodies. The crura and corpus cavernosum constitute a single unit into which blood is transferred during erection. Venous blood leaving this unit reaches the systemic circulation via pelvic channels. The bulb, the corpus spongiosum, and the glans form a second unit that also drains via pelvic channels but possesses an additional more cranial outlet. Consequently, drainage of the spongiosus system is not completely arrested by contraction of the bulbospongiosus.
The paired dorsal nerves, which run with the dorsal arteries, overlap in their distribution. Since stimulation of the apex of the penis is necessary for the attainment of full erection, the integrity of these nerves is essential for reproductive competence. The preputial skin, including that over the penis, is supplied from the first two lumbar, the genitofemoral, and the pudendal nerves.
Cranial preputial muscles that arise in the xiphoid region and insert beside and behind the preputial orifice are able to draw the prepuce craniodorsally, which constricts its orifice. Anomalies of these muscles may prevent protrusion or impair the return of the penis to the prepuce. Caudal preputial muscles of inconstant occurrence appear to have little significance.
The usual suite of muscles is associated with the penis (see Figure 29–33). The well-developed retractor penis possesses particular interest as it must relax to allow exposure of the penis for examination or treatment. It arises from the caudal vertebrae, passes to the side of the rectum, and reaches the penis at the second bend of the flexure; some fibers attach here, but others continue to more distal and diffuse insertions. The local contractions of the retractor that help maintain the flexure are controlled by a sympathetic innervation that is conveyed within the pudendal and caudal rectal nerves; these must be blocked to allow withdrawal of the penis for examination. The administration of an antiadrenergic tranquilizer has the same effect. A low lumbar epidural block is additionally required when anesthesia is indicated.
The lymphatics from the prepuce pass to the superficial inguinal node.
The penis of the small ruminants is chiefly distinguished by the length of the slender, erectile urethral process, which projects 2 to 3 cm beyond the glans in bucks and 3 to 4 cm in rams (see Figure 29–35). In former times, as in primitive societies today, amputation of the process was performed with the intention of depriving rams of their fertilizing capacity. The sheath is also relatively short in these species.
GROWTH AND FUNCTIONAL CHANGES
The bovine testes have arrived in the scrotum by midgestation, a surprisingly early period. They are very small at birth but grow more rapidly than the body as a whole from the first week and at an accelerated rate when the young bull approaches puberty. Growth for a time then keeps pace with general development; in older bulls some shrinkage is demonstrable. Libido may develop before spermatogenesis is achieved, which is generally about the 10th month. Epididymal growth lags a little behind that of the testes.
Progress in the development of the secondary reproductive glands is testosterone dependent and follows after testicular maturation. They are all initially small, but to varying degrees, and take some time to acquire their adult sizes and conformations.
Less than half its final length, the neonatal penis is very slender. It is without a sigmoid flexure, contains little erectile tissue, and is fused with the prepuce at its apex. The preputial cavity, which does not extend proximally beside the penis, is occupied by low folds. The characteristic bends begin to develop about the 3rd month. Growth is slow, and, though it quickens from puberty, the final size is not attained until well into the 2nd year. Separation from the sheath is first confined to the left side of the apex but later spreads around the whole circumference and extends proximally. A narrow frenulum persists for some time, and tags may remain until ruptured at first service. The occasional persistence of the frenulum may produce a ventral deflection of the apex.
Castration frustrates normal development and, if performed late, may result in regressive changes. The accessory glands are especially sensitive to the endocrine status. The reactions of these organs to artificially administered estrogens has attracted much notice because of the danger that hormone residues within the carcass may represent to human health. The practice has been made unlawful in many countries. Its occurrence is most easily detected by histological examination of the prostate. Failure of the apex of the penis to separate from the prepuce causes the castrate to urinate deep within the preputial cavity.
Erection involves only a slight increase in length and diameter; protrusion results from effacement of the flexure. Relatively little extra blood is required to engorge the cavernous spaces; this is initially produced by relaxation of the supplying arteries, which increases the pressure within these spaces from the low resting level (5 to 16 mm Hg) to the arterial pressure (75 to 80 mm Hg). The apex protrudes at this stage. Contractions of the ischiocavernosi raise the pressure further and, by compressing the vessels against the ischial arch, occlude the venous drainage route. These contractions impel blood forward through certain thick-walled veins of the corpus cavernosum to discharge at the sigmoid flexure (see Figure 29–33). Effacement of this flexure now causes the apex to protrude considerably (25 to 40 cm); contact with the vaginal wall after intromission stimulates the completion of erection. For a short period, pressure within the corpus cavernosum rises to a remarkable level; it is asserted that it can be as much as 60 to 100 times the arterial pressure. Ejaculation follows, and the semen is rapidly impelled through the urethra by the coordinated activity of the urethralis and bulbourethralis muscles.
The free part of the penis spirals in the later stages of erection, following a left-hand thread around the raphe (Figure 29–36). This is due to the apical ligament, a local concentration of collagen within the tunica albuginea. Since precocious or exaggerated spiraling makes intromission impossible, there are occasional indications for the surgical division of this ligament. Another problem, fortunately only of occasional occurrence, is rupture of a tunica albuginea unable to withstand the extreme pressure briefly developed in the late stage of erection; the weakest region appears to be the distal bend of the sigmoid flexure.
THE ANATOMY OF RECTAL PALPATION IN CATTLE*
As in the horse, rectal exploration in cattle is not free from risk of injury to the mucosa or even, in extreme cases, perforation of the intestinal wall—a mishap most likely to occur when invasion of the rectum induces straining. The novice should not attempt the procedure without appropriate supervision. Although rectal examinations of cows are most frequently performed to obtain information on the functional or pathological status of the reproductive organs, it is necessary to be familiar with the larger anatomy that can be appreciated with the use of this procedure. The territory that can be explored when the hand is carried forward into the descending colon is more extensive than might be supposed.
The parts of the pelvic and abdominal walls that are accessible include the bones bounding the pelvic cavity and the regions of the deep inguinal rings. Dorsally, the caudal segment of the aorta and its bifurcation are within reach, and, scattered about the vessels, the larger lymph nodes of the medial iliac and deep inguinal groups (see Figure 29–4/13,14) can be palpated. The deep inguinal nodes are particularly important in connection with mastitis. The caudal part of the rumen is very obvious directly before the pelvic inlet, and it can be confirmed that ventrally the rumen extends into the right half of the abdomen. The caudodorsal blind sac may even intrude into the pelvic cavity when distended with gas. However, much of the rumen and the remaining compartments of the stomach are inaccessible, as are the liver and the spleen. The one necessary qualification of this statement refers to the abomasum, part of which is brought into reach in certain displacements. The right dorsal quadrant of the abdomen is occupied by small intestine, cecum, and colon, which together form a soft, fluctuating mass in which individual parts are mostly not identifiable when normal; the most common exception is the rounded tip of the gas-filled cecum.
Most of the left kidney, pushed to the right by the rumen and suspended from the abdominal roof, may be palpated; only the caudal pole of the right kidney is within reach and then only in smaller subjects. Healthy ureters are not detectable unless the initial portion of the left one can be appreciated where it passes over the surface of the kidney. The impression made by the bladder varies greatly because it forms a firm mass over the most cranial part of the pelvic floor when contracted but extends well forward into the abdomen as a fluctuating structure when distended. The intervention of the female reproductive tract makes this organ far less accessible in cows than in male animals.
Passing attention has already been given to the inspection per rectum of the reproductive organs, and we gather together here only the principal features. A systematic examination is best begun by locating the cervix, easily recognized by its firmness and dimensions, although its location varies greatly according to the present status and past history of the animal. The short body of the uterus lies forward of the cervix, and the uterus may be fixed by the insertion of a finger between the intercornual ligaments to allow examination and comparison of the horns that diverge to each side. Frequently, these manipulations stimulate contraction of the uterine muscle, which can sometimes be quite powerful. The reader is reminded that in certain circumstances the uterus passes far into the abdomen. If not too much enlarged, it may be retrieved by passing the hand forward and downward into the ventral part of the abdomen on the right side and then withdrawing the hand with the fingers flexed toward the palm to enclose the uterus. The broad ligaments proceeding to the horns of the uterus are distinct, but the uterine tubes, which run near the free cranial margins of the ligaments, are less certainly discoverable because, although fairly firm, they are only about 2 mm wide. The free margins of the broad ligaments also provide a guide to the location of the ovaries, which lie on the floor of the pelvic cavity in the young virgin animal but are displaced cranially and ventrally into the abdomen in older, more sexually experienced cows. An indication has already been given of the features of the follicles and corpora lutea that may be appreciated by examination of the ovarian surface. The reader is also reminded that the forward and downward movement of the reproductive organs in pregnancy may carry them out of reach for a time (Figure 29–37).
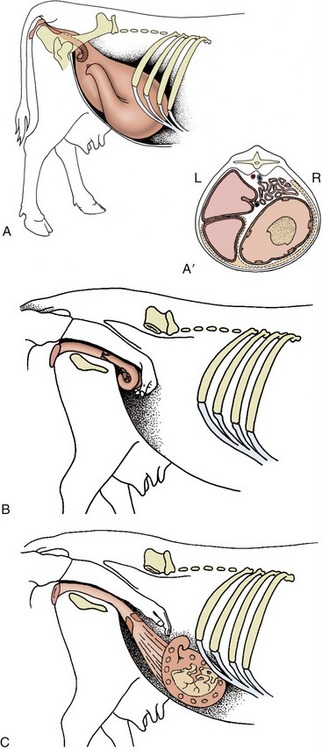
Figure 29–37 The position of the nongravid uterus and various stages of the gravid uterus in lateral view. A, Nongravid and 6-months gravid uterus. A′, The topography of the 6-months gravid uterus in transverse section. B, At 2 to 3 months the uterus has begun to slide down the caudal abdominal wall, but it can be scooped up by the hand in the colon. C, At 5 months the uterus is temporarily out of reach.
THE UDDER
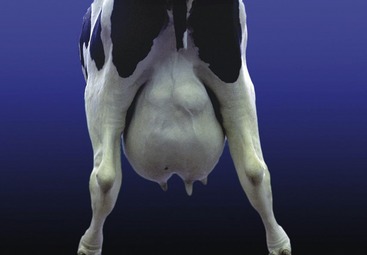
The four mammary glands of the cow are consolidated in a single mass, the udder, placed below the caudal part of the abdomen and extending between the thighs. The udder is divided into quarters corresponding to the four glands, and each bears a principal teat. A median groove divides the udder into right and left halves, but the boundary between a forequarter and a hindquarter is rarely distinct. Most of the dorsal base is shaped to fit against the belly wall, but the part below the pelvis is narrower because it is compressed between the thighs (Figure 29–38). The skin over the udder is thin, supple, and mobile, except over the teats, where it is tightly bound down and naked.
The udder is suspended by strong sheets of fascia that surround and enclose the gland substance and are continuous with the connective tissue framework that permeates the entire organ. The fascia forms a continuous investment over each half, but it is customary to describe medial and lateral laminae as though these were independent formations. The medial lamina arises mainly from the tunica flava, in small part from the symphysial tendon, and is largely composed of elastic tissue. The lateral lamina arises from the external crus of the inguinal ring and, behind this, from the medial femoral fascia and is composed of dense connective tissue (Figures 29–39 and 29–40). Both laminae thin when followed ventrally, which is the result of their detachment of numerous leaves that interdigitate with layers of glandular tissue. The different natures of the two laminae explain the sagging of the medial part of the heavily laden udder. Ever increasing demands for milk production place a heavy and sometimes unsustainable burden on the suspensory apparatus, which occasionally ruptures—a disastrous happening.
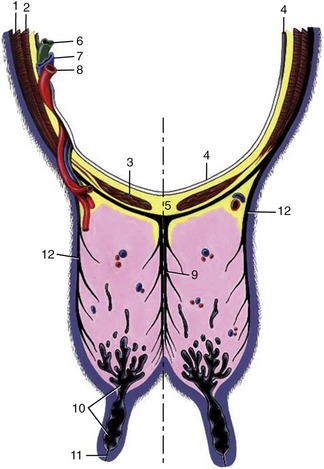
Figure 29–39 Transverse section of the abdominal floor and cranial quarters of the bovine udder. 1, External abdominal oblique; 2, internal abdominal oblique; 3, rectus abdominis; 4, peritoneum; 5, linea alba; 6, lymph vessel; 7, external pudendal vein; 8, external pudendal (mammary) artery; 9, medial laminae of suspensory apparatus; 10, lactiferous sinus; 11, papillary duct; 12, lateral laminae of suspensory apparatus.
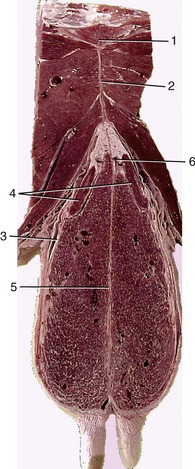
Figure 29–40 Transverse section of the pelvic floor and caudal quarters of the bovine udder. 1, Pelvic symphysis; 2, symphysial tendon; 3, lateral suspensory laminae; 4, mammary (superficial inguinal) lymph node; 5, medial suspensory laminae; 6, tributary of external pudendal vein.
Each gland is constructed about a branching duct system, separated from its neighbors by connective tissue. The alveolar secretory units lead to small excretory ducts that combine with others until, after several successive unions, about a dozen wide lactiferous ducts are produced; these converge on a large sinus situated in the lower part of the quarter and extend into the teat (Figure 29–41). The lactiferous ducts are unusual in demonstrating alternating wider and narrower sections. The more superficial dilations, which may be 3 cm or more in caliber, may be palpable when distended with milk and are then known as “milk knots.” Although the duct systems are independent, infection readily spreads between the quarters of the same side.

Figure 29–41 Sagittal section of udder, showing teat and gland sinuses and lactiferous ducts filled with latex (cranial quarter [green]; caudal quarter [blue]).
The lactiferous sinus has a capacity of several hundred milliliters and is divided by a mucosal fold into gland and teat parts. The fold, based on a submucosal ring of veins, varies in prominence; occasionally it may be sufficiently pronounced to impede milk flow.
The teats, though variable, are most often cylindrical and about 8 cm long. The teat wall, generally about 6 mm thick, increases to about 1 cm at the lower end, where it is traversed by the papillary duct (Figure 29–42). The wall consists of a dry, outer skin, an intermediate layer that includes smooth muscle and many veins and constitutes a form of erectile tissue, and an inner mucosal layer marked by folds. The lining, generally yellowish, is white in the papillary duct, where it shows a pattern of low ridges; these, when followed proximally, are found to radiate from the upper opening, although it must be admitted that the arrangement is rarely as distinct as traditionally described (Figure 29–43). Desquamation of the epithelium provides a bacteriostatic substance that helps occlude the passage. A more effective means of closure is provided by a sphincter muscle, reinforced by elastic tissue.

Figure 29–42 Variations in the form of the bovine teat extremity. A, Funnel-shaped. B, Dish-shaped. C, Rounded. D, Pointed.
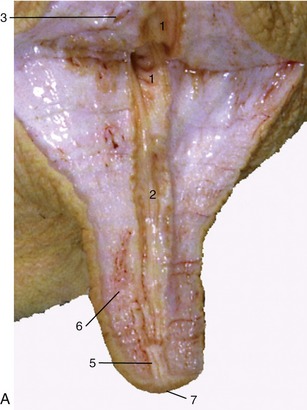
Figure 29–43 A, B, Section of a cow’s teat and lactiferous sinus. 1, 2, Lactiferous sinus; 1, gland sinus; 2, teat sinus; 3, openings of lactiferous ducts; 4, submucosal venous ring; 5, papillary duct; 6, venous plexus in teat wall; 7, teat orifice.
Accessory teats, sometimes associated with functional glandular tissue, are very common. They are undesirable as they may be a complication at milking.
The vascular arrangements are necessarily generous. The main supply, which continues the external pudendal artery, has a diameter that may exceed 15 mm where it passes through the inguinal canal accompanied by a satellite vein, lymphatics, and nerves (see Figure 29–39). On reaching the base of the udder, it divides into divergent branches, one passing cranially, the other caudally; both are partially or wholly embedded in the gland substance. The caudal mammary branch anastomoses with a division of the ventral perineal artery, which restricts its distribution to the mammary lymph nodes and a limited portion of the hindquarter.
The pattern of the veins is complicated. A venous ring above the udder is formed by paired veins connected across the midline by transverse vessels (Figure 29–44). Drainage is effected by the external pudendal veins, which pass through the inguinal canals, and by the subcutaneous abdominal (“milk”) veins, which pursue very flexuous subcutaneous courses over the abdomen before disappearing through palpable openings (“milk wells”) in the body wall to discharge into the internal thoracic veins (Figure 29–45).

Figure 29–44 The venous drainage of the udder. 1, Subcutaneous abdominal (milk) v.; 2, milk “well”; 3, internal thoracic v.; 4, cranial vena cava; 5, external pudendal v.; 6, internal pudendal v.; 6′, ventral labial v. (connecting ventral perineal v. with caudal mammary veins); 7, caudal vena cava; 8, diaphragm; 9, costal arch; 10, first rib.
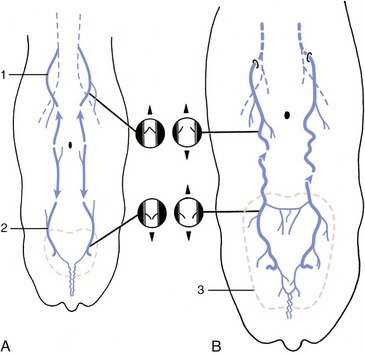
Figure 29–45 Development of the subcutaneous abdominal veins (schematic dorsal view). A, The region drained by the cranial superficial epigastric vein (1) is separated from that of the caudal superficial epigastric vein (2) in the calf and heifer. The valves in the cranial superficial epigastric vein direct blood cranially, while those in the caudal superficial epigastric vein direct blood caudally. B, The subcutaneous abdominal vein is formed during pregnancy. The increased blood flow through the enlarging udder (3) causes the veins to distend, the valves to become inefficient, and the two drainage regions to unite, which allows blood to flow in both directions.
Connections of the caudal part of the ring with ventral labial veins are of uncertain significance. The arrangement described is characteristic of the adult lactating cow and includes features that developed during the first pregnancy, a time when increased mammary blood flow led to venous congestion and dilation, followed by valvular incompetence and breakdown. This opened a continuous channel connecting the cranial and caudal superficial epigastric veins, which previously drained in opposite directions (see Figure 29–45).
The significance of the mature arrangement lies in its assurance of effective venous drainage should some channels be occluded in the recumbent cow. The milk vein is sometimes used for intravenous injection or blood sampling, but it is not a wise choice; its varicosed structure predisposes it to potentially troublesome leakage.
The teats and gland substance are permeated by a rich lymphatic plexus from which emerge larger vessels that run to the mammary lymph nodes situated above the caudal part of the udder. Many of these large lymphatic vessels reveal their positions through the skin and, running caudodorsally (Figure 29–46), are readily distinguished from the subcutaneous veins that run craniodorsally. The mammary lymph nodes, generally two on each side—one large and one much smaller—lie deep to the lateral lamina of the suspensory apparatus, where the larger one may be reached on deep palpation from behind (Figure 29–47). The efferent flow is to the deep inguinal node in the angle between the deep circumflex and external iliac arteries. This node may be palpated per rectum.
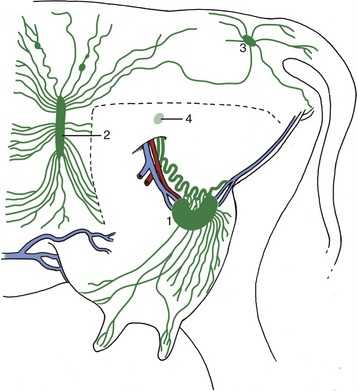
Figure 29–46 Lymph drainage of the udder. The broken line indicates where the left limb was removed to expose the udder. 1, Mammary (superficial inguinal) lymph node; 2, subiliac lymph node; 3, ischial lymph node; 4, position of deep inguinal (iliofemoral) node.
The cutaneous innervation of the udder is inconveniently diffuse; innervation is obtained from three sources: ventral branches of the first two lumbar nerves, the genitofemoral nerve, and mammary branches of the pudendal nerve. The gland substance and the deeper tissues of the teat wall are supplies by the genitofemoral nerve alone; this reaches the udder through the inguinal canal.
At full term, the mammary glands exhibit short but well-formed teats, small sinuses, and the first branches of the duct systems. The bulk of the udder consists of fat. During the next few months growth keeps pace with the general growth of the body and is entirely due to deposition of fat. Thereafter, and thus commencing well before puberty, growth quickens; the rapid development of both the duct system and the gland tissue is probably due to the cyclical production of estrogen because spurts of activity occur directly before ovulation. Although a well-developed duct system is present by the time a heifer first conceives, additional growth of the ducts predominates in the first months of pregnancy; growth of the secretory tissue occurs in the second half.
Growth in late pregnancy is dependent on prolactin and growth hormone of hypophysial origin, in addition to progesterone and estrogen. Secretion of milk is later maintained by corticotropin, thyroid-stimulating hormone, and somatotropin. Regular milking is also necessary to maintain production. Since the act of milking stimulates the release of prolactin, oxytocin, and corticotropin, more frequent milking, within limits, increases the yield.
The mammary gland is composed of tubuloalveolar secretory units grouped to form lobules defined by connective tissue septa (Figure 29–48, A). The secretory alveoli are lined by a simple epithelium that changes markedly in height during the cycle of activity. The cells demonstrate maximal activity in those alveoli prepared to release milk when stimulated by suckling (or milking). After this the alveolar lumina are collapsed and irregular (Figure 29–48, B), and the epithelium is much reduced in height. All lobules within one gland do not necessarily exhibit the same stage of the secretory cycle, and both active and nonactive lobules may be present concurrently. The milk is forced from the secretory units into the duct system by contraction of surrounding myoepithelial cells (Figure 29–49). The interlobular and intralobular connective tissue provides important structural support and conveys blood, lymph vessels, and nerves.
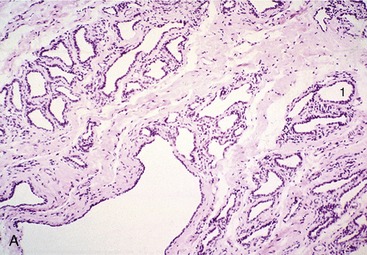

Figure 29–48 Section of (A) nonlactating and (B) lactating mammary glands; a compound tubuloalveolar gland (70×).
The udder of the small ruminants combines two glands that are more (goats) or less (sheep) distinctly demarcated externally. In milk goats the udder is large in relation to body size, deep, and conical (Figure 29–50); in ewes it is smaller and more hemispherical, although inclining toward the caprine form in breeds used for cheese production. The teats are cylindrical in the young, but in older animals, especially in goats of high productivity, they tend to become conical and blend more smoothly with the contours of the gland (Figure 29–51). Accessory teats are not uncommon in goats. The udder skin is finely haired in goats; in sheep the upper part may cover the fleece.
The structure, suspension, and vascular arrangements generally resemble those of the bovine udder. However the teats are not wholly naked. In sheep, closure of the papillary duct is achieved without the presence of a sphincter muscle.