5 The Urogenital Apparatus
The official nomenclature brings the urinary and reproductive organs together under one heading, apparatus urogenitalis. The chief justification for this convention lies in the common origin of certain elements of both organ complexes in the intermediate mesoderm and adjacent part of the celomic epithelium. In addition, the urinary and reproductive systems of the adult share the final portions of the tracts that deliver their products to the exterior; the part used in common is limited to the urethra in the male and the vestibule in the female.
Because of the close developmental associations of the urinary and reproductive systems, we have chosen in this chapter to precede the account of the adult anatomy by a review of the development. The uninitiated reader is therefore advised to consult Figures 5–1 and 5–2, which show the general layout of the urogenital apparatus in each sex, before reading further.
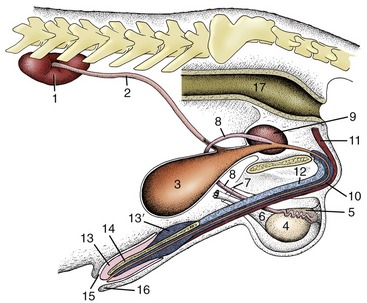
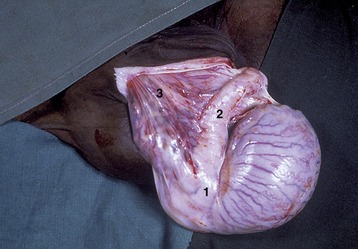
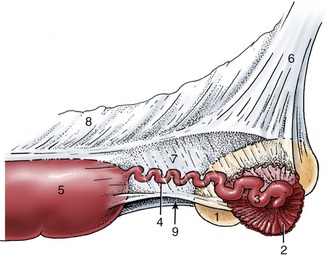
Figure 5–1 The urinary and male reproductive organs (dog). 1, Right kidney; 2, ureter; 3, bladder; 4, testis; 5, epididymis; 6, spermatic cord; 7, vaginal ring; 8, deferent duct; 9, prostate; 10, corpus spongiosum (spongy body); 11, retractor penis; 12, corpus cavernosum (cavernous body); 13, glans penis; 13′, bulb of glans; 14, os penis; 15, preputial cavity; 16, prepuce; 17, rectum.
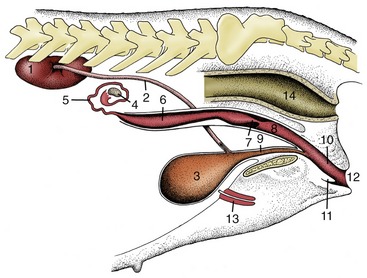
Figure 5–2 The urinary and female reproductive organs (bitch). 1, Right kidney; 2, ureter; 3, bladder; 4, ovary; 5, uterine tube; 6, uterine horn; 7, cervix; 8, vagina; 9, urethra; 10, vestibule; 11, clitoris; 12, vulva; 13, vaginal process; 14, rectum.
THE DEVELOPMENT OF THE UROGENITAL APPARATUS
DEVELOPMENT OF THE URINARY ORGANS
The intermediate mesoderm reflects in muted fashion the segmentation that is so evident in the adjoining somites. It soon forms in its caudal domain a continuous solid longitudinal (nephrogenic) thickening from which arise, in craniocaudal and temporal sequence, three attempts at the formation of an excretory organ. The first attempt constitutes the pronephros, which forms in the presumptive neck region; this has a transient existence and is not functional in mammals. The second attempt, the mesonephros, forms in the thoracic and lumbar regions and is more successful; it is functional through a large part of embryonic life. The third attempt, the metanephros, forms in the lumbar region; it becomes the adult kidney (Figure 5–3).
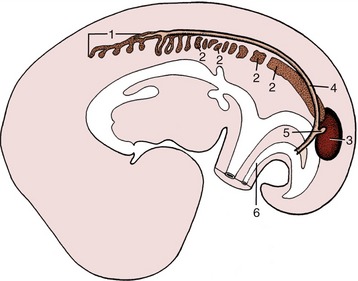
Figure 5–3 Differentiation of the intermediate mesoderm. 1, Pronephros; 2, mesonephros, segmented cranially but continuous caudally; 3, metanephros; 4, pronephric (later mesonephric) duct; 5, ureteric bud; 6, urachus.
All three structures have a series of excretory tubules as their essential histological feature. In the pronephros one end of each tubule turns caudally to meet its neighbor, and in this way a continuous pronephric duct is formed (Figure 5–3/4), which at its caudal end grows toward and opens into the cloaca. The duct survives the regression of the pronephric tubules and is adopted as the means of drainage of the mesonephric tubules that now appear. Because the pronephric tubules are nonfunctional, their peculiarities of construction need not be noted.
The mesonephric tubules are much more numerous. Each resembles a rather simple version of the nephron of the adult kidney in structure and function (see Figure 5–27). The blind end is invaginated by a capillary tuft to form a filtration mechanism while the connection of the other end with the pronephric duct, now more appropriately termed the mesonephric duct, provides an outlet for the urine that is formed. The mesonephros may be a very prominent organ at its apogee, when it projects from the roof of the abdomen (Figure 5–4). Its size varies among species and is in inverse proportion to the permeability (and thus the excretory efficiency) of the placenta. The mesonephros is supplanted by the metanephros when it begins to regress, which is a process that occurs in a craniocaudal direction. Parts, however, survive to be given fresh use by the male reproductive system (Figure 5–5).
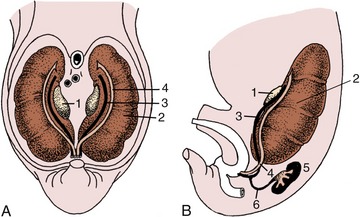
Figure 5–4 Ventral (A) and lateral (B) views of the abdominal roof in a pig embryo of 2.5 cm. The pronephric duct drains the mesonephros and is now more aptly termed the mesonephric duct. 1, Developing gonad; 2, mesonephros; 3, mesonephric duct; 4, paramesonephric duct; 5, metanephros; 6, ureter.
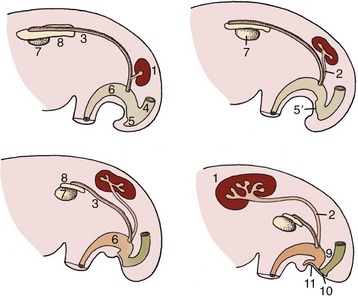
Figure 5–5 The development of the metanephros from two primordia (metanephric cord and ureteric bud). Note the gradual regression of the mesonephros. 1, Metanephros; 2, ureteric bud (future ureter); 3, mesonephric (deferent) duct; 4, rectum; 5, cloaca; 5′, cloacal membrane; 6, urogenital sinus; 7, gonad; 8, remnant of mesonephros (future epididymis); 9, urorectal septum; 10, anal membrane; 11, urogenital membrane.
The metanephros has two primordia. One is provided by an outgrowth, the ureteric bud, from the lower end of the mesonephric duct close to its opening into the cloaca. This bud grows cranially into the metanephric blastema constituted by the caudal part of the nephrogenic cord (Figure 5–3/5). The extremity of the bud undergoes a dozen or so dichotomous divisions. Branches of the later orders become the collecting tubules of the kidney, whereas those of the first few orders are later reabsorbed into the terminal expansion of the duct in a variable fashion that accounts for the specific forms of the renal pelvis and calices. The outer part of the metanephric mass forms the capsule and interstitium of the kidney, while cellular condensation in the inner part creates the cell cords that are transformed into nephrons. One end of each cell cord makes contact with a connecting duct, and once canalization has occurred, a continuous passage is established (Figure 5–6). The other extremity of the nephron becomes invaginated by a vascular tuft supplied from a local branch of the aorta; this forms the glomerulus (see also Figure 5–27).
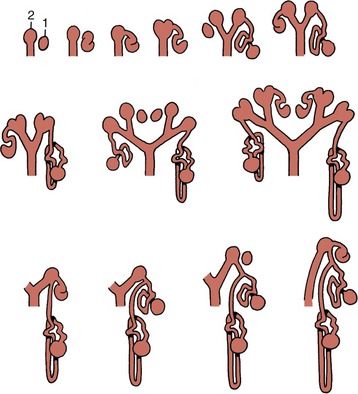
Figure 5–6 This series of schematic drawings depicts the connections between developing nephrons (1) and branches (2) of the ureteric bud. Note the dichotomous division of the drainage system (ureteric bud).
The lower urinary passages are formed by the horizontal division of the cloacal region of the hindgut. The division is effected by the caudal growth of a wedge of mesoderm present within the angle between the hindgut and the allantoic bud. This wedge, the urorectal septum, eventually reaches the cloacal membrane, which is thus divided into dorsal (anal) and ventral (urogenital) parts (Figure 5–5/9). The fusion site corresponds to the perineal body. When the anal membrane breaks down, the dorsal passage becomes a continuous rectoanal canal. A similar rupture of the urogenital membrane provides the ventral passage with a separate opening to the surface of the body. This urogenital passage differentiates into a cranial part, the future bladder and allantois, and a caudal part from which the urethra is formed.
The bladder then appears as a widening that is continued cranially by the allantoic duct and caudally by an undilated urethra. The allantoic duct or urachus (Figure 5–3/6) can be followed through the umbilical opening to an extraembryonic expansion (the allantois) in which urine accumulates and which is discarded at birth. The part of the duct within the fetus then shrivels and is finally represented only by the cicatrix or scar on the apex of the bladder. The caudal part of the primordium is transformed into the urethra—the entire urethra in the female but only the short pelvic urethra in the male (in which the penile urethra develops with the genital system). The definitive positions of the openings of the mesonephric and metanephric ducts result from the incorporation of their lower ends within the larger passage. The rearrangement brings the opening of the metanephric duct (ureter) into the bladder, while that of the mesonephric duct (deferent duct) becomes situated more caudally within the urogenital sinus (see Figure 5–5). In this process the mesoderm of the mesonephric duct provides the epithelium of the dorsal trigonal region (p. 183) of the bladder, while the epithelium of the remaining part is provided by hindgut endoderm. The outer layers of the bladder wall differentiate from local mesoderm.
DEVELOPMENT OF THE MALE REPRODUCTIVE ORGANS
Although the genetic sex of the embryo is decided when the male and female gametes combine, the early stages of morphological differentiation of the reproductive organs follow an indifferent pattern that is common to the two sexes. In both, the gonadal primordium appears as a thickening of the celomic epithelium on the medial aspect of the mesonephros. It projects as a swelling when the underlying mesenchyme proliferates (Figure 5–7, A/5). Cords of cells that develop from the covering epithelium penetrate the interior of the swelling (Figure 5–7, B/5). These cords shortly incorporate the primordial germ cells, which, rather surprisingly, have a distant origin in the endoderm of a restricted portion of the yolk sac, where they are identifiable by their large size. They reach the gonad by migration over the gut and its mesentery, but carriage in the bloodstream also seems possible.
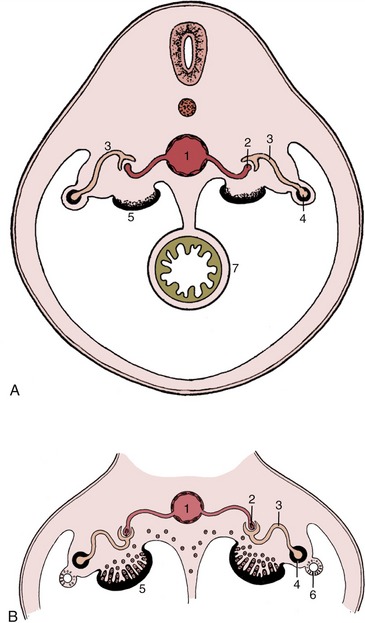
Figure 5–7 A, Early development of the indifferent gonad. B, Invasion of the gonad by epithelial cords, which then incorporate primordial germ cells. 1, Aorta; 2, capillary tuft (in nephron); 3, nephron (tubule); 4, mesonephric duct; 5, gonad; 6, paramesonephric duct; 7, gut.
An early indication that the gonad will become a testis is provided by a marked mesenchymal condensation (tunica albuginea) below the celomic epithelium. Now isolated from the surface epithelium, the cords increase in size and in complexity of arrangement (Figure 5–8/3). They connect to a plexus or network (rete) within the testis. On the other side the plexus makes contact with the blind ends of the few tubules that have survived the general regression of the mesonephros (Figure 5–8, B/3-5). Differentiation within the cell cords permits recognition of two cell lineages. One provides the sustentacular (Sertoli) cells of the seminiferous tubules; the second, contributed by the primordial germ cells, provides the germinal epithelium. During fetal development the primordial germ cells differentiate into gonocytes, which after birth give rise to spermatogonia. At puberty, the spermatogonia proliferate and differentiate to supply cells that undergo meiosis and spermiogenesis to form male gametes (see Figure 5–39). Sections through the adult testis show seminiferous tubules cut in various planes. The walls of the highly convoluted tubules are lined by a stratified germinal epithelium consisting of cells in various stages of differentiation. Supporting Sertoli cells nourish the germ cells. Cells of an additional type can be identified. These, the Leydig cells, produce the steroid testosterone that is essential if spermatogenesis is to continue. Their progenitors, like those of Sertoli and primordial germ cells, presumably migrate from the mesonephros during fetal development to become embedded in a mesenchymal interstitium, and around puberty, when the process of spermatogenesis is initiated, a second generation of Leydig cells develops. The initial formation of the seminiferous cords is followed in later fetal life by canalization of the cords to create a series of passages leading to the mesonephric duct, which thus becomes the outlet for the gamete products of the testis. The peripheral parts of the cords become seminiferous tubules, the central parts become the rete testis, and the mesonephric tubules become the efferent ductules (Figure 5–8, C). The first part of the mesonephric duct convolutes and forms the duct of the epididymis within the dense connective tissue of that organ; the remaining part retains a straighter course, and as the deferent duct (Figure 5–5/3), it opens into that part of the cloaca that becomes the urogenital sinus (Figure 5–5/6). Glandular proliferation of the lining of the duct toward its termination produces the ampullary thickening, while in most species, but not in carnivores, a subterminal budding enlarges as the vesicular gland (Figure 5–9/5). In some species a final short passage, the ejaculatory duct, persists, but in others later adjustments cause the deferent and vesicular ducts to open separately. Gonadal enlargement causes the testis to hang within a fold (mesorchium) arising from the regressing mesonephros. The duct is carried within this supporting fold, which in its caudal stretch inclines medially to form with its neighbor the genital fold of peritoneum that helps subdivide the peritoneal cavity of the pelvis. The testis later migrates outside the abdomen (p. 173) before the initiation of spermatogenesis.
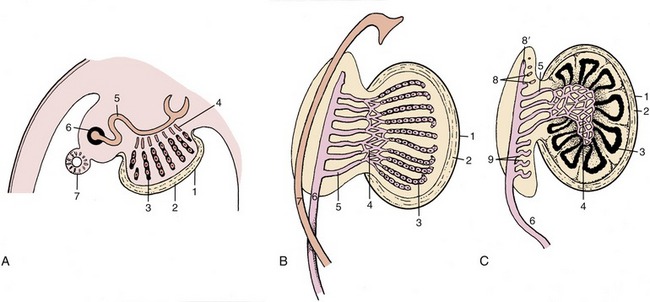
Figure 5–8 Three stages in the development of the testis. A, The epithelial cords are isolated from the surface epithelium by the formation of the tunica albuginea. B, The epithelial cords, rete, and mesonephric tubules have interconnected. C, The epithelial cords become seminiferous tubules, and the mesonephros is gradually transformed into part of the epididymis. 1, Celomic epithelium; 2, tunica albuginea; 3, epithelial cords, seminiferous tubules; 4, rete testis; 5, mesonephric tubules, efferent ductules; 6, mesonephric (later deferent) duct; 7, paramesonephric duct; 8, cranial remnant of mesonephric tubules (aberrant ductules); 8′, remnant of 6 (appendix of epididymis); 9, caudal remnant (paradidymis).
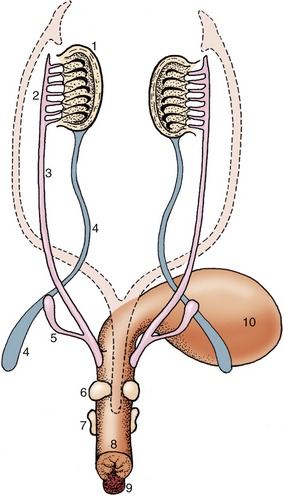
Figure 5–9 Differentiation of the urogenital sinus. Note the budding of the prostate and bulbourethral glands and the enlargement of the genital tubercle. The regressed paramesonephric ducts are indicated by the broken lines. 1, Testis; 2, epididymis; 3, deferent duct; 4, gubernaculum; 5, vesicular gland; 6, prostate; 7, bulbourethral gland; 8, urogenital sinus (urethra); 9, genital tubercle; 10, bladder.
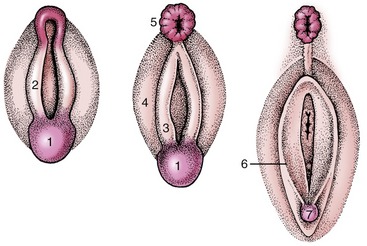
The division of the cloaca has been described (p. 147). The caudal part of the sinus constitutes the pelvic part of the urethra. Outgrowths from its lining differentiate into the prostate and bulbourethral glands in a species-characteristic fashion (see Figure 5–9). The greater part of the male urethra lies within the penis and has a different origin. Thickenings appear around the margin of the urogenital membrane in the indifferent stage (Figure 5–10). One, ventral and median, constitutes the genital (phallic) tubercle or swelling (Figure 5–10/1), which gives rise to the greater part of the penis; other thickenings that are more lateral in position contribute the scrotum. A further urogenital fold that appears medial to each scrotal swelling makes an additional contribution to the penis. A groove extends along the (initially) dorsal surface of the genital tubercle; it is gradually closed by the approach and mergence of these urogenital folds. This process is rather complex as the lining of the penile urethra is provided by an extension of the endoderm of the urogenital sinus, although the initial swellings have ectodermal coverings. The corpus spongiosum (spongy body) of the penile urethra directly continues the bulbar tissue of the pelvic urethra, while the corpus cavernosum penis forms within the genital swelling. The lateral swellings grow and join together to form the scrotum, which retains evidence of its bilateral origin in a median raphe and septum.
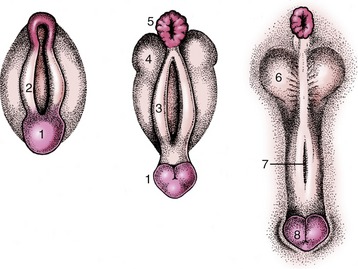
Figure 5–10 Development of the male external genitalia. 1, Genital tubercle; 2, cloacal fold; 3, urogenital fold; 4, lateral (scrotal) swelling; 5, anus; 6, scrotum; 7, groove closing to form the penile urethra; 8, glans penis.
Differentiation of the male efferent duct system, accessory glands, and external genitalia depends on the presence of testosterone, the male sex hormone produced by the developing testes. The testes also produce several other hormones, for example, the antimüllerian hormone (AMH) and insulin-like factor 3 (descendine), respectively responsible for the disappearance of the müllerian duct and the outgrowth of the gubernaculum. Without exposure to these three hormones the genital tract would develop in the female direction. Removal of the pituitary by decapitation in the fetal period does not disturb the production of these hormones by the testis (Figure 5–11, A-B).
DEVELOPMENT OF THE FEMALE REPRODUCTIVE ORGANS
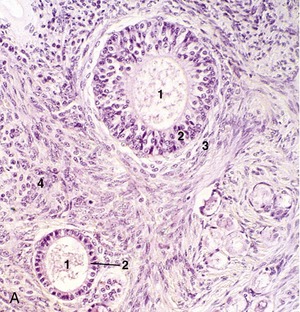
The initial stages of gonadal development resemble those described for the male. Later, the cell cords fragment into cell clusters, each enclosing an immigrant germ cell. The cords penetrate less deeply into the interior of the gonad than in the male. The primordial follicles are formed here. Rete formation is less pronounced in the ovary, and because no connection is established with mesonephric tubules, no uninterrupted tubular outlet for the escape of gametes is created (Figure 5–12).
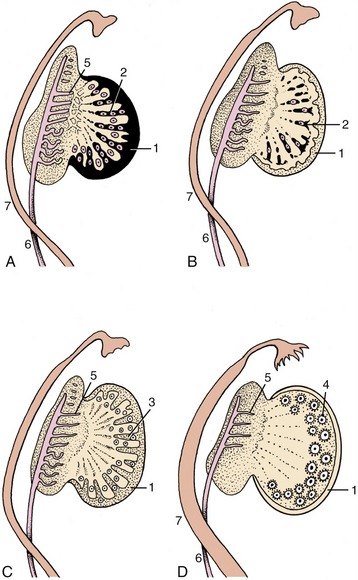
Figure 5–12 Successive stages in the development of the ovary. 1, Celomic epithelium; 2, epithelial cords, penetrating (A) and regressing (B); 3, second formation of sex cords (C); 4, primitive follicles; 5, remnants of mesonephric tubules; 6, mesonephric duct; 7, paramesonephric duct (D).
Consequently, follicular rupture releases the female gametes at the surface of the ovary by tissue breakdown, a process made easier by the absence of a thick tunica albuginea. The same feature allows for the formation of further sex cords and the establishment of additional follicles during a large part of prenatal life; indeed in certain species this process may continue for a time after birth. Even so, it ceases eventually, and the number of female gametes is then at its maximum; it is afterward depleted by loss through atresia and, to a much smaller extent, through ovulation. Ovarian descent is very limited in most species, being greatest in the ruminants in which the ovaries shift caudally to the abdominopelvic boundary. The duct system of the female is largely provided by the paramesonephric ducts (Figure 5–12/7), which have only vestigial importance in the male. These ducts first develop by invagination of the celomic epithelium lateral to the mesonephric ducts and secondly by active growth in the direction of the urogenital sinus within the genital folds. In contrast, the mesonephric ducts regress in craniocaudal sequence (Figure 5–13), and only remnants survive within the broad ligaments and in the vaginal wall (ducts of Gartner, ductus epoöphori longitudinales), where they are occasionally the seat of anomalous processes. The cranial part of each paramesonephric duct runs lateral to the mesonephric duct, but it crosses this more caudally where it inclines to meet and fuse with its fellow (Figure 5–14/6). The cranial end of each paramesonephric duct remains open to the peritoneal cavity (abdominal ostium of the uterine tube), but the caudal end of the united duct initially ends blindly against a solid outgrowth from the dorsal wall of the urogenital sinus (Figure 5–15). The uterine tubes and the horns, body, and cervix of the uterus form from the paramesonephric ducts; their caudal parts fuse to an extent that varies with the species and accounts for the very different form and proportions of the uterus of adult animals (p. 199) (Figure 5–16). The supporting genital fold becomes the broad ligament with its various parts. The vaginal lumen appears within the solid outgrowth from the sinus, although a tissue partition, the hymen, may persist near the junction with the fused paramesonephric ducts. A hymen is present only in virgin animals and is rarely well formed in domestic species. Some dispute exists over the contribution of the urogenital and paramesonephric epithelia to the lining of the vagina in the adult, and some suggest that the boundary may divide regions with different responses to hormonal influences that are observed in some species.
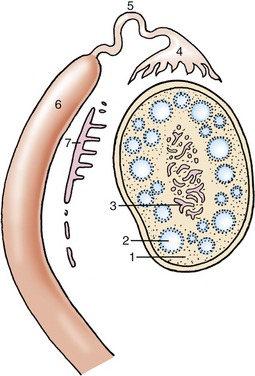
Figure 5–13 Differentiation of paramesonephric duct and regression of mesonephric duct. 1, Interstitial tissue of the ovary; 2, primitive follicles; 3, ovarian rete; 4, infundibulum; 5, uterine tube; 6, uterine horn (4, 5, and 6 differentiate from paramesonephric duct); 7, remnants of the mesonephric tubules and duct (epoöphoron and paroöphoron).
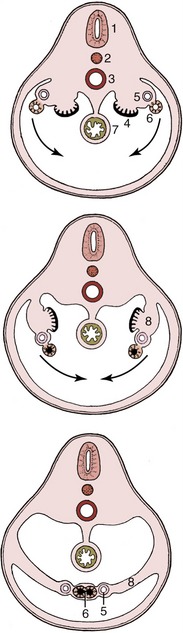
Figure 5–14 Transverse sections (from cranial to caudal) through the caudal part of the abdomen, illustrating the creation of the genital fold in the female embryo. 1, Neural tube; 2, notochord; 3, aorta; 4, gonad; 5, mesonephric duct (regressing); 6, paramesonephric duct (merged in the caudal section); 7, gut; 8, genital fold.

Figure 5–15 The fusion of the combined paramesonephric ducts with a bud from the urogenital sinus forms the vagina. 1, Rectum; 2, caudal part of urogenital sinus (vestibule); 3, cranial part of urogenital sinus (bladder, urethra); 4, bud from urogenital sinus; 5, fused paramesonephric ducts; 6, vagina; 7, cervix uteri; 8, uterine horn.
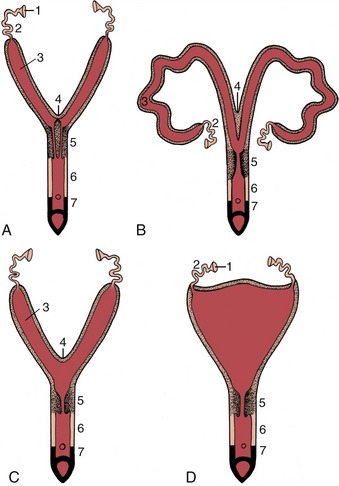
Figure 5–16 Different degrees of fusion of the paramesonephric ducts. A, Uterus duplex (rabbit). B, Uterus bicornis (small body: sow, cow). C, Uterus bicornis (large body: mare). D, Uterus simplex (woman). 1, Infundibulum; 2, uterine tube; 3, uterine horn; 4, fusion site of the two ducts; 5, cervix; 6, vagina; 7, vestibule.
The urogenital sinus becomes the vestibule with relatively little further change. Epithelial outgrowths form the vestibular glands in species-variable fashion. The external genital parts are formed from the same structures as in the male; the genital tubercle and lateral folds (swellings) appear first (Figure 5–17). The former produces the clitoris, while the lateral folds, which form the labia majora of human anatomy, regress—with a possible reservation for the bitch. The labia of the vulva of the domestic species are provided by the urogenital folds (Figure 5–17/3) that appear medial to the lateral swellings and correspond to the labia minora of women.
THE PROCESS OF TESTICULAR DESCENT
The descent of the testis into a scrotal position is necessary in most mammals to obtain normal fertility. The process depends on the existence of a mesenchymal condensation, the gubernaculum testis, within a detachment from the genital fold that leads from the testis toward and through the inguinal canal (Figure 5–18). At a certain critical period of development (which varies in timing among different species) the distal part of the gubernaculum, which extends through the inguinal canal to the groin, enlarges very rapidly and considerably (Figure 5–19, A-B). The gubernaculum is invaded by an extension of the peritoneal lining of the abdomen. In this way the vaginal process, which provides the space into which the testis will be drawn, is formed (Figure 5–18/3). The invasion by the vaginal process divides the gubernaculum into three parts: the proximal part (pars propria) is enclosed by the inner (future visceral) peritoneal lining of the process; the second part (pars vaginalis) surrounds the outer (future parietal) peritoneal lining of the process; and the third part (pars infravaginalis) lies distal to the invagination and is thus continuous with the other parts. The swelling of the gubernaculum commences distally, causing it to exert pressure on the body wall about the superficial ring of the inguinal canal. This displaces the testis distally, toward the abdominal entrance of the canal. The swelling then gradually extends proximally, and at its peak the part adjacent to the testis (and within the inguinal canal) is as thick as the testis itself (see Figure 5–19, A-B). At this stage any slight increase in intraabdominal pressure may be sufficient to expel the testis from the abdomen into the inguinal canal, although for a time its return to the abdomen is still possible. The descent is complete and irreversible once the core of the gubernaculum has regressed (Figure 5–20). A well-timed gubernacular regression is therefore as indispensable to normal descent as is the earlier swelling. Because the timing is critical and the process is subject to various disturbances, it is not surprising that abdominal retention and abnormal descent are both relatively frequent. Failure of the testis to appear in the groin is known as cryptorchidism (hidden testis). It takes various forms: it may be unilateral or bilateral and may present the testis held within the abdomen or trapped within the inguinal canal. As a result of the higher temperature to which an undescended testis is exposed, spermatogenesis is not initiated at puberty. The condition is clearly undesirable and, although unilaterally cryptorchid animals may be fertile, they should be excluded from breeding because the condition is often hereditary.
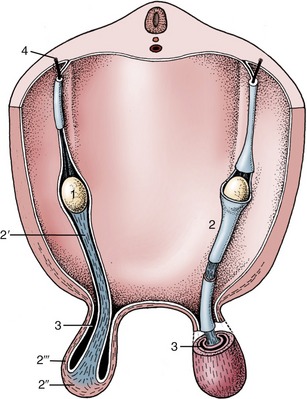
Figure 5–18 Schematic representation of the testis and gubernaculum within the peritoneal fold in which descent takes place. 1, Testis; 2, gubernaculum; 2′, pars propria; 2″, pars infravaginalis; 2′″, pars vaginalis; 3, vaginal process; 4, testicular artery.
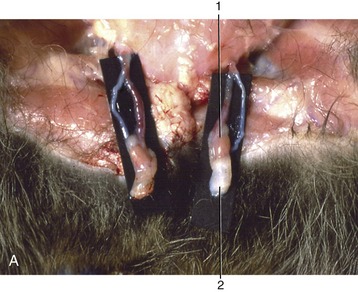
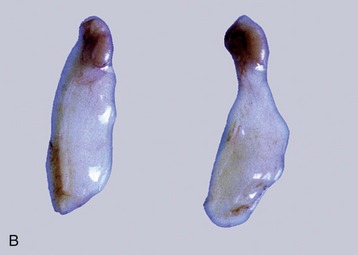
Figure 5–19 Stages in the process of gubernacular swellings. The testis and gubernaculum have already passed the inguinal canal. Inguinal area of newborn pup. A, 1, Testis; 2, exposed gubernaculum. B, Testis and gubernaculum of pig fetus (110 days).
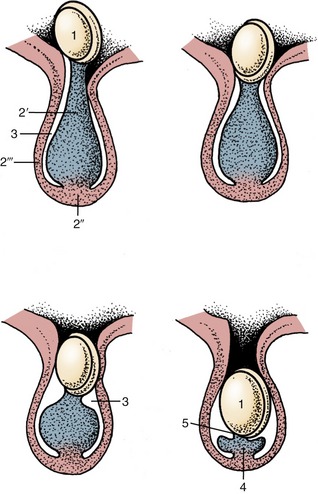
Figure 5–20 Successive stages in gubernacular regression in the pig fetus. Observe the migration of the testis caused by this regression. 1, Testis and epididymis; 2, gubernaculum; 2′, pars propria; 2″, pars infravaginalis; 2′″, pars vaginalis; 3, vaginal process; 4, ligament of the tail of the epididymis; 5, proper ligament of the testis.
Similar structures are formed in the female sex but do not develop significantly, except in the bitch among domestic mammals, in which the existence of the vaginal process is occasionally troublesome (p. 461).
In several species when a twin pregnancy occurs, the circulation of the two fetuses can become interconnected, which results in not only the exchange of cells but also hormones (Figure 29–18). The hormonal influence of the male fetus can interfere with the development of the female co-twin. In cattle this can result in a “freemartin,” in which the ovary and the female duct system is severely underdeveloped or absent. It can also result in the outgrowth of the gubernacula in the female twin (see Figure 35–8, A-B). Very seldom, this can also occur in a pig fetus that is interconnected with a male fetus in utero.
THE URINARY ORGANS
The urinary system comprises paired kidneys that form the urine from the blood; ureters that convey the urine from the kidneys; the bladder, where urine is stored until it can be discharged conveniently; and the urethra, through which it finally passes to the exterior. As almost the entire male urethra also conveys the reproductive products, it is usual to describe it with the reproductive organs.
THE KIDNEYS
The kidneys have the maintenance of the milieu intérieur as their prime task. They do this by filtering the plasma, initially extracting an enormous volume of fluid before subjecting this ultrafiltrate to further processing in which useful substances are selectively reabsorbed, waste substances are concentrated for elimination, and the volume is adjusted by the conservation of sufficient water to maintain the composition of the plasma within the appropriate range. Some figures may give an impression of the dimensions of this task. In large dogs (and animals of similar size), 1000 to 2000 L of blood perfuse the kidneys daily; the 200 to 300 L of fluid that are filtered from this volume are later reduced by reabsorption until only 1 or 2 L of urine remain to be discharged.
The endocrine function of the kidneys consists of the production and release of two hormones: renin, which plays a vital role in the regulation of systemic blood pressure, and erythropoietin, which influences erythropoiesis. Both are produced within the juxtoglomerular complexes, localized regions of intimate association between arterioles formed by the union of afferent glomerular capillaries with adjacent portions of the distal convoluted tubules (p. 222).
The kidneys are firm, reddish-brown glands whose appearance varies considerably among mammals (Figure 5–21). The most familiar form, that which has introduced the term kidney-shaped to the common vocabulary, is encountered in the dog (Figure 5–21, D), cat, and small ruminants. The kidneys of the pig (Figure 5–21, C) are a much flattened version, whereas those of the horse (Figure 5–21, E) are more heart-shaped. In contrast, the bovine kidneys (Figure 5–21, B) are very dissimilar and have a surface deeply fissured to outline many lobes. Even greater subdivision is shown by the kidneys of certain marine species (Figure 5–21, A), which resemble trusses of grapes that have the lobes only slightly fused and mainly held together by the branching “stalk.”
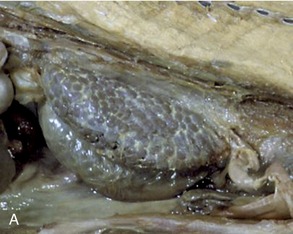

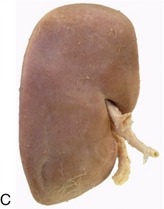

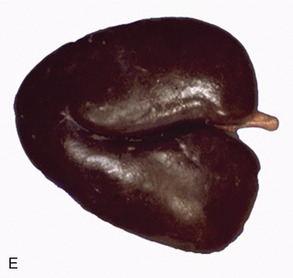
Figure 5–21 Kidney of a dolphin (A), kidney of a cow (B), kidney of a pig (C), kidney of a dog (D), and kidney of a horse (E).
The kidneys are usually found pressed against the abdominal roof, one to each side of the vertebral column, and predominantly in the lumbar region, although often extending forward under the last ribs. Their positions change with the excursions of the diaphragm, and they move, perhaps by half the length of a vertebra, with each breath. They are rarely symmetrical; in domestic animals, other than pigs, the right one is about half a kidney-length in advance of its fellow. The cranial extremity of the right kidney commonly fits into a fossa of the liver, which helps fix its position. The left one, lacking this lodgment, is more mobile and is more likely to sag within the abdomen. The pendulous left kidney of ruminants is thrust into the right half of the abdomen by the enormous development of the stomach. In general, kidneys pressed against the abdominal roof are largely retroperitoneal, whereas those suspended at a lower level have a more extensive peritoneal covering (Figure 5–22).
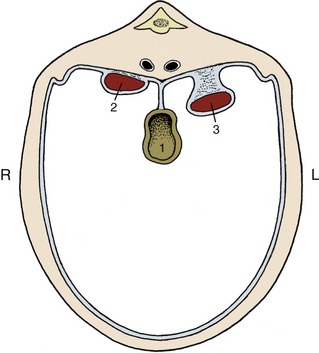
Figure 5–22 Schematic representation of the position of the kidneys in relation to the peritoneal cavity. 1, Gut; 2, right kidney (retroperitoneal); 3, left kidney (intraperitoneal: pendulous or “floating”).
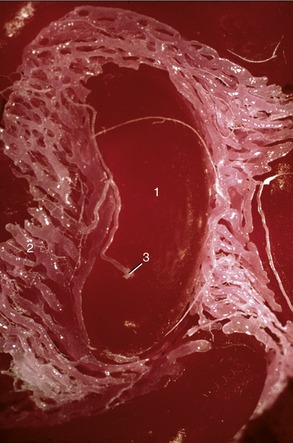
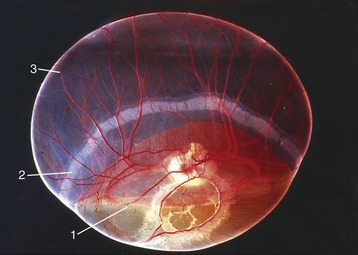
Each kidney lies within a splitting of the sublumbar fascia, which also holds considerable fat (sometimes enough to hide the kidney completely). The fat protects against distorting pressures from neighboring organs. The surface of a kidney is generally smoothly convex except for an indentation of the medial border. This indentation leads to a concealed space (renal sinus; Figure 5–23) occupied by the dilated origin (renal pelvis) of the ureter, the vessels and nerves passing to and from the renal hilus, and more fat.

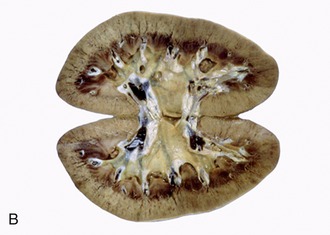
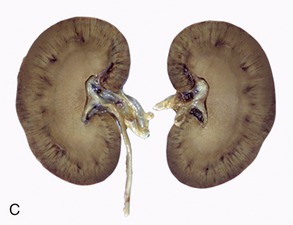

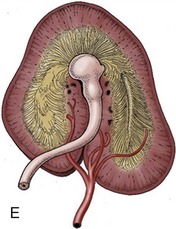
Figure 5–23 Sectioned kidney. Notice that the complexity of the renal pelvis decreases from cow to horse. Cow (plastinated specimen) (A), pig (B), dog (C), cat (D), horse (E).
The general organization of the kidney is most conveniently shown in a section that divides the organ into dorsal and ventral “halves.” Such a section shows that the parenchyma is enclosed within a tough fibrous capsule. The capsule restricts the kidney’s ability to expand; the swelling that occurs in certain disease conditions therefore tends to compress the tissue and narrow the internal passages. The capsule strips readily from the healthy kidney but adheres where the underlying substance has been scarred by former lesions.
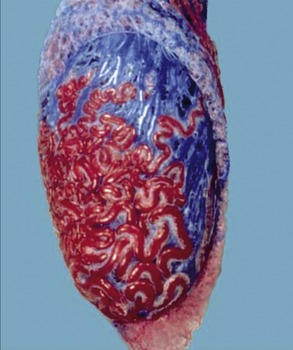
The parenchyma is visibly divided into an outer cortex and an inner medulla (see Figure 5–23). The cortex is distinguished by its reddish-brown color and finely granular appearance. The medulla consists of a dark, purplish outer zone, from which stripes (medullary rays) extend into the cortex, and a paler, grayish-red, and radially striated inner zone that extends toward the renal sinus. The gross arrangement of the medulla shows very marked species differences. In many species the medulla is arranged as several (or even many) discrete masses, each roughly pyramidal in form. In kidneys of this type a portion of the cortex is associated with each pyramid and caps its base, the aspect directed toward the outer surface. The apex of the pyramid points toward the renal sinus and forms a papilla that fits into a cuplike expansion (calix) of the renal pelvis. Each medullary pyramid with its associated cortex constitutes a renal lobe. Kidneys that retain this organization are said to be multipyramidal or multilobar. In some multipyramidal kidneys, such as those of cattle (Figure 5–23, A), the boundaries between the lobes are revealed by the fissures that penetrate from the surface; in others, including those of pigs, no external evidence of lobation is present (Figure 5–23, B).
All mammalian kidneys pass through a multipyramidal phase in their development, although in most species the number of lobes is later drastically reduced (Figure 5–24). In some species, including the dog, horse, and sheep, all the pyramids finally fuse to form a single medullary mass that confines the cortex to the periphery, where it forms a continuous shell. Even this unipyramidal or unilobar type of kidney retains some evidence of its complex ontogeny; a slight scalloping of the corticomedullary junction, punctuated by the arteries that mark the interlobar boundaries, shows where the pyramids fused. The fusion joins the papillae in a common crest (Figures 5–25 and 5–26) that may be modeled to reveal its composite origin; it is so modeled in the dog and goat but not in the horse.
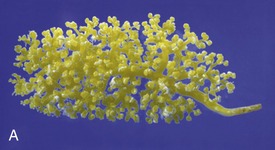

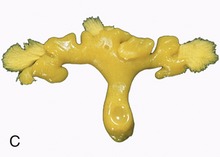
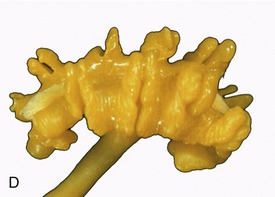

Figure 5–24 Corrosion casts of the renal pelvis of: A, Dolphin, note the branched renal pelvis with many calices. B, Cow, note the papillary ducts extending from the calices. C, Pig, the renal pelvis becomes confluent; again note the papillary ducts. D, Dog, the renal pelvis is one cavity but note the ridges between the renal papilla. E, Horse, one simple renal pelvis and many papillary ducts opening into the renal pelvis.
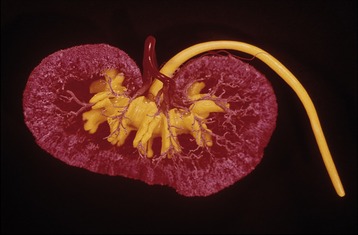
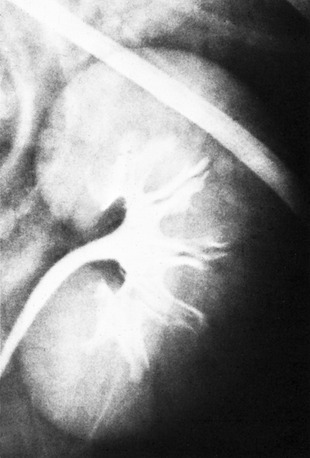
Figure 5–25 Corrosion cast of canine kidney. The renal pelvis and ureter are filled with yellow plastic. Notice the indentations in the pelvis corresponding with the crests of the renal papillae. The ramifications of the renal artery (red) are clearly visible.

Figure 5–26 Corrosion cast of renal pelvis, renal artery, and renal veins of a goat. The depressions of the ridges of the renal papillae are clearly visible.
The functional units within the kidney are known as renal tubules or nephrons. These epithelial tubules are supported by a connective tissue interstitium and are estimated to number several hundred thousand or even a million in canine kidneys. The structure and the functions of the nephron are more appropriately described in texts of microscopic anatomy and physiology; only a few points, mainly those discernible to the naked eye, are mentioned here.
Each nephron begins with a blind expansion that is invaginated by a cluster of capillaries known as a glomerulus (Figures 5–27/1 and 5–28). The glomerulus and its epithelial covering together constitute a renal corpuscle (Figure 5–27/1′), a structure just large enough to be visible to the unaided eye, especially if the capillaries are congested. The corpuscles are scattered throughout the cortex and give it a finely granular appearance.
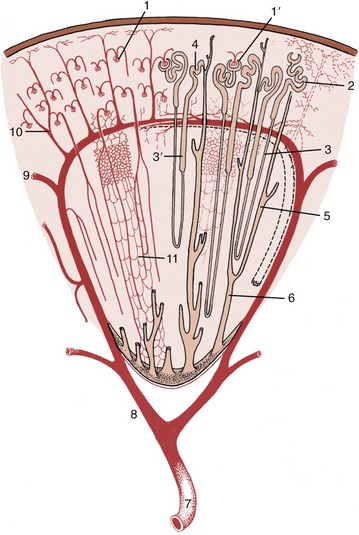
Figure 5–27 Schematic drawing of a kidney lobe. 1, Glomerulus; 1′, renal corpuscle; 2, proximal convoluted tubule; 3, descending limb of nephron; 3′, ascending limb; 4, distal convoluted tubule; 5, collecting tubule; 6, papillary duct; 7, renal artery; 8, interlobar artery; 9, arcuate artery; 10, interlobular artery; 11, capillary plexus.
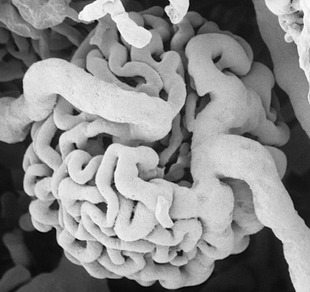
The remaining part of the nephron forms a long tubule differentiated into several successive segments. The first, the proximal convoluted tubule, is very tortuous and is located close to the corpuscle from which it arises (Figure 5–27/2). This part gradually straightens and enters one of the narrow rays that penetrate the cortex from the medulla. The tubule then forms a long hairpin loop (formerly known as the loop of Henle) within the medulla. The first part of the loop, the descending limb, is relatively narrow and runs through the medulla to approach the papilla before turning back. The ascending limb is generally thicker, although the change in caliber need not coincide with the change in direction, and runs back to regain the medullary ray. On leaving this, the tubule forms a second or distal convoluted part that is also placed close to the corpuscle of origin (Figure 5–27/4). A short junctional section then runs to join a collecting tubule within the medullary ray. Each collecting tubule (Figure 5–27/5), which serves many nephrons, runs through the medulla before opening into a larger vessel, a papillary duct, close to the apex (Figure 5–27/6). Several score of papillary ducts drain into the renal pelvis. The papillary ducts can be clearly demonstrated in resin-injection specimens (see Figure 5–24). The perforated (cribriform) areas where they discharge are confined to the apices of independent papillae or to specific regions of a common crest.
Variations in the location of the corpuscles and in the overall length and proportions of the tubules have functional importance that cannot be discussed here.
Each kidney is supplied by a renal artery, a branch of the abdominal aorta, which may carry more than a tenth of the total output of the left ventricle! The renal artery divides into several interlobar arteries (Figure 5–27/8) that follow the divisions, former or extant, between the renal pyramids at the corticomedullary junction. These vessels are prominent in gross sections of the kidney. They give rise to branches known as arcuate arteries that curve over the bases of the pyramids (Figure 5–27/9). These in turn give origin to numerous interlobular arteries that supply the units or lobules into which the cortex is divided by the medullary rays (Figure 5–27/10). Each interlobular artery gives rise to many branches that supply individual glomeruli. The glomerular capillaries rejoin in one emissary vessel at the distal pole of the glomerulus, and this then supplies a further capillary plexus around the tubules (Figure 5–27/11). The flow of blood through this second capillary bed is countercurrent to the direction of the urine flow. The vessels that issue from the juxtomedullary corpuscles (those in the innermost layer of the cortex) have a particular importance in the supply of the medulla. The renal circulation is actually more complicated than is described here and provides opportunities for collateral circulation. However, the interlobular arteries are certainly functional end-arteries, and the interlobar arteries are possibly functional end-arteries.
The veins, which lead ultimately to the caudal vena cava, are broadly satellite. Lymphatic vessels drain to nodes of the lumbar series that accompanies the aorta. The sympathetic nerves to the kidneys are routed through the celiacomesenteric plexus and thence along the renal arteries. The synapses may be located within the major ganglia or within smaller (aorticorenal) ganglia within peripheral parts of the plexus. The vagus contributes the parasympathetic supply.
THE RENAL PELVIS AND URETER
In cattle the ureter is formed by the coming together of the short passages that lead from the calices that enclose individual renal papillae (Figure 5–24, B and Figure 28–27). In most domestic species the ureter begins in a common expansion, the renal pelvis, into which all the papillary ducts open—although in different ways in different species (see Figures 5–24 and 21–23). Few differences in pelvic anatomy are of practical significance. However, in the dog and cat the form of the renal pelvis obtains an importance lacking in the other species from its ready depiction in radiographs. The renal pelvis of these animals is molded on the renal crest and extends flanges dorsal and ventral to this. Each flange shows a number of local expansions or recesses that are divided from each other by projections of renal tissue (Figure 5–29). Neighboring recesses are also separated by the interlobar vessels.
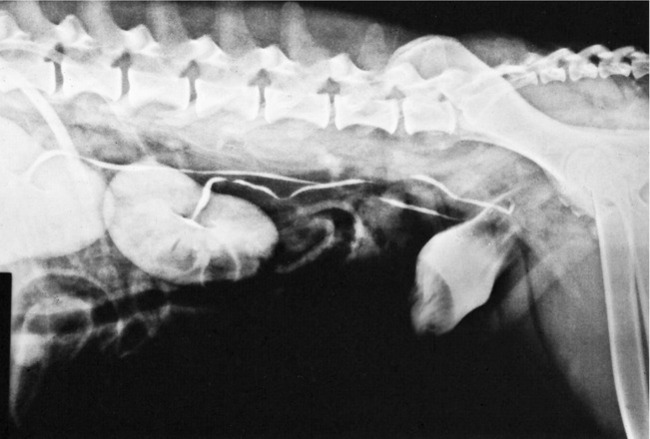
The remaining tubular part of each ureter has a fairly even caliber. It follows a broadly sagittal course against the abdominal roof, although it may exhibit occasional sharp changes in direction. On reaching the pelvic cavity the ureter bends medially to enter the genital fold in the male or the broad ligament in the female; this carries the ureter over the dorsal surface of the bladder, into which it opens near the neck (Figure 5–30). In the male the ureter passes dorsal to the corresponding deferent duct.
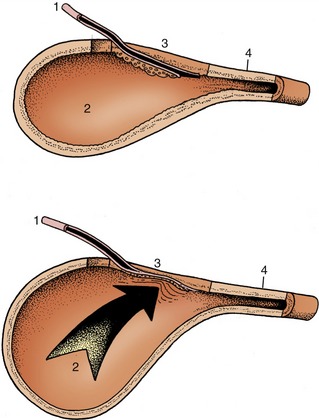
The ureter penetrates the bladder wall very obliquely. The length of the intramural course guards against reflux of urine into the ureter when the pressure is raised within the bladder (Figure 5–31). It does not prevent further filling of the bladder because the resistance is overcome by peristaltic contractions of the ureteric wall. The wall of the renal pelvis and ureter possesses an external adventitia, a middle muscularis, and an internal mucosa. The muscle coat is well developed, and although its peristalsis helps move urine to the bladder, it can enter spasm when provoked by local irritation such as is provided by a urinary calculus.
THE URINARY BLADDER
The bladder is a distensible storage organ and thus can have no constant size, position, or relationships. It is small and globular when fully contracted and is then remarkable for the great thickness of its walls and the negligible extent of its lumen. The contracted bladder rests on the pubic bones; it is confined to the pelvic cavity in the larger species but extends into the abdomen in carnivores. When the bladder enlarges it becomes pear-shaped, presenting a cranial vertex (apex), an intermediate body, and a caudal neck that narrows to the internal urethral orifice at the junction with the urethra. Although continuing distention carries an ever-increasing portion of the bladder into the abdomen, the neck remains fixed within the pelvis through its continuity with the urethra (Figure 5–32/11).
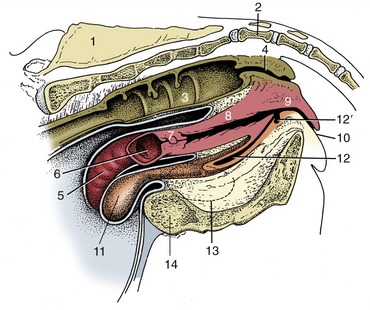
Figure 5–32 Median section of the bovine pelvis. 1, Sacrum; 2, first caudal vertebra; 3, interior of rectum; 4, anal canal; 5, exterior of right uterine horn; 6, interior of stump of left uterine horn; 7, cervix; 8, vagina; 9, vestibule; 10, vulva; 11, exterior of bladder; 12, urethra; 12′, suburethral diverticulum; 13, obturator foramen; 14, pelvis symphysis.
No immediate increase in internal pressure occurs when the bladder begins to fill. However, once a certain, quite considerable volume has been attained, the pressure rises sharply; this creates the urge to void urine, an urge that is obeyed without hesitation in many species. In house-trained animals the urge may temporarily disappear if resisted, although discomfort and, later, pain may be experienced if the bladder becomes overfull. In the well-trained dog the distention may be very great, carrying the apex cranial to the umbilicus and stretching the walls to paper thinness with risk of rupture. Though the outline of the grossly distended bladder is smooth, that of the more modestly distended organ is irregular as the low internal pressure allows it to be indented by its firmer neighbors (see Figure 5–30).
In the larger species the contracted bladder is largely retroperitoneal, but most of the surface becomes intraperitoneal when the organ is even moderately expanded. Three folds continue this serosal covering onto the abdominal and pelvic walls (Figure 5–33). Paired lateral vesical folds convey the round ligaments of the bladder; these vestiges of the umbilical arteries retain narrow lumina through which some blood reaches the cranial part of the bladder. The third, median vesical fold, is empty in the adult, but in the fetus it supports the urachus, the constricted cranial continuation of the bladder that passes forward to leave the abdomen through the umbilical foramen before expanding externally into the allantoic sac. Urachus and umbilical arteries rupture at birth; the urachus survives as a scar on the bladder vertex, while the umbilical arteries are transformed into the round ligaments. The folds in the adult bound the ventral pair of the several excavations into which the pelvic peritoneal cavity is divided (see Figures 22–6 and 22–7).
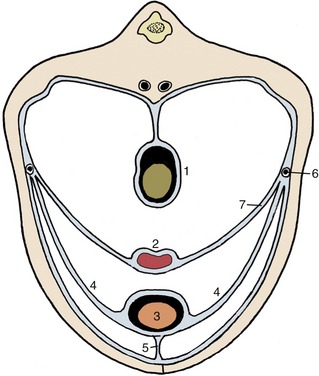
Figure 5–33 Peritoneal disposition in the caudal part of the abdomen. 1, Colon; 2, uterus; 3, bladder; 4, lateral vesical ligaments; 5, median vesical ligament; 6, ureter; 7, broad ligament of uterus (mesometrium).
The constant dorsal relations of the bladder are to the reproductive organs and their supporting folds: the uterus and vagina within the broad ligament in the female and the deferent duct (and perhaps the vesicular glands) within the genital fold in the male. The bladder may also make indirect contact with the rectum through these folds. The ventral surface touches the pelvic and abdominal floor. Other relations of the intraabdominal part of the bladder are less predictable and may be numerous when the bladder is greatly enlarged.
The loose attachment of the bladder mucosa and its ability to stretch allow marked change in the appearance of the interior with altered physiological status. The surface, much folded when the lumen is small, becomes generally smooth when the bladder fills. However, two particular folds resist effacement. These run from the slitlike orifices of the ureters, converge at the exit from the bladder, and fuse to form a median urethral crest that continues into the pelvic urethra (Figure 5–34/5). The triangle bounded by the ureteric and urethral openings is termed the trigone; it appears to have a different origin from the remainder of the bladder wall (p. 169) and is believed to have an enhanced sensitivity (Figure 5–34/4). The bladder epithelium is of the transitional kind.
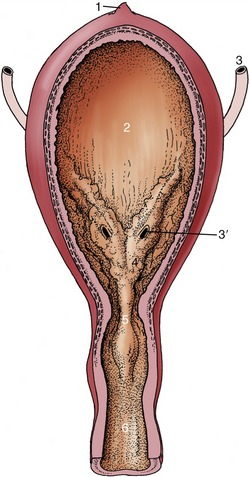
Figure 5–34 The interior of the urinary bladder. 1, Scar of urachus; 2, bladder; 3, ureter; 3′, ureteric orifice; 4, trigone of bladder; 5, urethral crest; 6, urethra.
The bladder muscle is arranged in three sheets that exchange fascicles. The muscle is probably entirely detrusor—available to squeeze and empty the bladder—and fails to form an internal sphincter, although one is often described. Many now believe that, in place of this, some muscle bundles form a series of arcades whose summits are directed toward the orifice; they therefore dilate rather than occlude the exit when they contract. If this is so, continence depends on the tension passively exerted by the elastic elements within the mucosa and on the action of the external sphincter, the striated urethralis. This interpretation is consistent with the demonstration that in certain species (dog, goat) the proximal part of the urethra forms part of the urine reservoir, expanding as the bladder fills. The functional boundary between bladder and urethra would thus appear to be represented by the cranial limit of the urethralis in these species.
Autonomic fibers reach the bladder through the sympathetic hypogastric and parasympathetic pelvic nerves; the latter innervate the detrusor muscle. Sensory fibers are routed through the pudendal nerve. The main blood supply is from the vaginal (or prostatic) artery, but, as has been mentioned, it is supplemented by the reduced umbilical arteries.
THE FEMALE URETHRA
The female urethra runs caudally on the pelvic floor below the reproductive tract. It passes obliquely through the vaginal wall to open ventrally at the junction of vagina and vestibule (Figure 5–35). Its length and breadth vary considerably among species; it is conspicuously short and wide in mares. In some animals, such as the cow and sow, it opens together with a suburethral diverticulum (Figure 5–32/12′) and in others, such as the bitch, on a hummock. Both arrangements create difficulties when catheterization of the bladder is attempted.
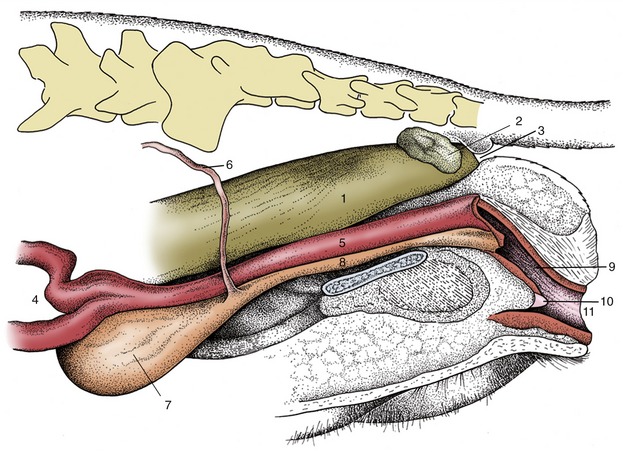
Figure 5–35 Pelvic organs of the bitch. The lateral pelvic wall and the lateral wall of the vestibule have been removed. 1, Rectum; 2, anal sac; 3, anus; 4, uterus; 5, vagina; 6, ureter; 7, bladder; 8, urethra; 9, vestibule; 10, clitoris; 11, vulva.
When a diverticulum is present, it is enclosed within the urethralis, which surrounds the urethra along most of its length. The cranial fascicles of this muscle encircle the urethra, while the caudal ones support it within U-shaped loops that arise and end on the vaginal wall. Contraction of this part of the muscle closes the urethra by pressing the two organs together; it also narrows the vagina. The urethralis obtains a somatic innervation through the pudendal nerve, but sympathetic and parasympathetic involvement is also described.
The urethral submucosa contains many veins that constitute a form of erectile tissue that may contribute to continence by assisting mucosal apposition. These features apart, the structure of the urethra continues that of the bladder.
THE MALE REPRODUCTIVE ORGANS
The male reproductive organs include paired gonads, the testes, which produce both male gametes (sperm) and hormones; paired gonadal duct systems, each consisting of an epididymis and deferent duct (ductus deferens), which convey the exocrine products of the testes to the urethra; a suite of accessory glands, which contributes the bulk of the semen; the male urethra, which extends from the bladder to the free extremity of the penis and serves for the passage of both urine and semen; the penis, the male copulatory organ, which deposits the semen within the reproductive tract of the female; and skin adaptations, the scrotum and the prepuce, developed in relation to the testes and the penis.
THE TESTES AND THEIR ADNEXA
The Testis
The testis combines endocrine and exocrine components within a common capsule. The endocrine component functions normally at the core temperature of the body, but in most mammals the successful production of the male gametes requires a temperature a few degrees lower than that within the abdomen. Hence, although the testes develop within the abdomen, they later migrate, descending through the inguinal canals to come to lie within the scrotum (see p. 189), a pouch of skin and underlying fasciae variously placed between the groin and perineum. That plausible though rather facile explanation of the descent fails to account for the ability of spermatogenesis to occur normally at the core temperature in a few mammals (described as testicond, e.g., elephants, hyraxes) in which the testes remain within the abdomen throughout life. It is consistent with the periodic changes exhibited in many small mammals (chiefly found among rodents, insectivores, and bats) in which the testes descend into the scrotum for the breeding season, after which they return to the abdomen. This is brought about by contraction of the cremaster muscle sac found in these species.
The testes are solid ellipsoidal organs whose bulk bears no fixed proportion to the body size. Among domestic species they are conspicuously small in cats and impressively large in sheep and goats. Their orientation also varies. They are carried with their long axes vertical in ruminants (necessitating a deep and pendulous scrotum), horizontal in horses and dogs, and tilted toward the anus in pigs and cats. These differences are broadly correlated with the position of the scrotum, which is below the caudal part of the abdomen in ruminants, perineal in pigs and cats, and intermediate in position in horses and dogs (Figure 5–36). Each testis is separately suspended within the scrotum by a spermatic cord, a bundle of structures that includes the deferent duct and the supplying vessels and nerves enclosed within a double covering of peritoneum.
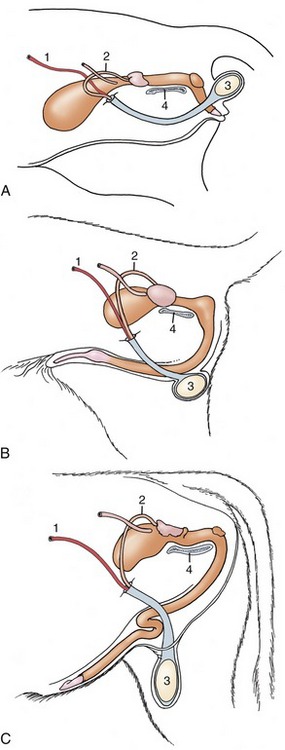
Figure 5–36 The perineal, intermediate, and inguinal positions of the scrotum exhibited by the tomcat (A), dog (B), and bull (C). 1, Testicular artery; 2, deferent duct; 3, testis; 4, pelvic symphysis.
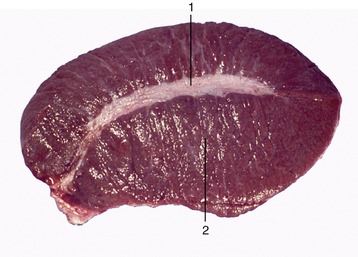
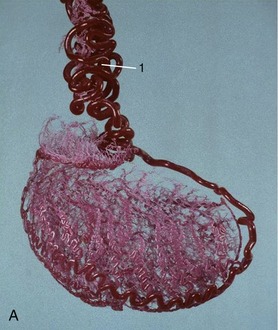
The outer surface of the testis is made smooth by the direct peritoneal investment, except at the poles and along one margin where the testis is attached to the epididymis, a structure formed by the coiled initial portion of the external duct system. The peritoneum covers a thickish capsule (tunica albuginea) mainly composed of dense connective tissue but sometimes including smooth muscle. The larger branches of the testicular artery and vein run within the capsule, where they are visible in a pattern that is species characteristic. The parenchyma is contained under moderate pressure, which accounts for its pouting through any incision of the capsule. It is probable that slight swelling of the parenchyma can be accommodated by the testis assuming a more globular form, but any significant expansion raises the intratesticular pressure and produces pain, which may be severe when the testis is inflamed (orchitis).* The capsule detaches septa and trabeculae that divide the parenchyma into lobules. The septa are not always conspicuous, but in those species in which they are well developed, they may be seen to converge on a substantial thickening (mediastinum testis); this may be axial or displaced toward the side bordering the epididymis.
The soft, yellowish or brownish parenchyma consists of intermingled seminiferous tubules and interstitial tissue (Figure 5–38). The latter consists of massed interstitial (Leydig) cells supported by a delicate connective tissue framework in which run small blood and lymphatic vessels (Figure 5–39). The interstitial cells are the principal producers of the steroid androgenic hormones. The greater part (60% in boars and stallions, 90% in rams and bulls) of the parenchyma is formed by the tubules in which the process of spermatogenesis is conducted.
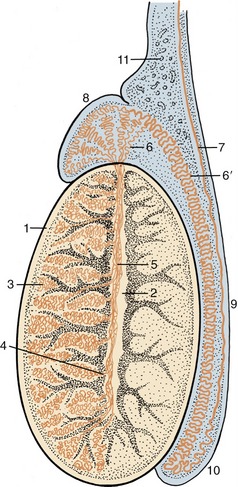
Figure 5–38 Longitudinal section of a testis and epididymis, schematic. 1, Tunica albuginea; 2, mediastinum; 3, seminiferous tubules; 4, straight tubules; 5, rete testis; 6, efferent ductules; 6′, epididymal duct; 7, deferent duct; 8, head of epididymis; 9, body of epididymis; 10, tail of epididymis; 11, pampiniform plexus.
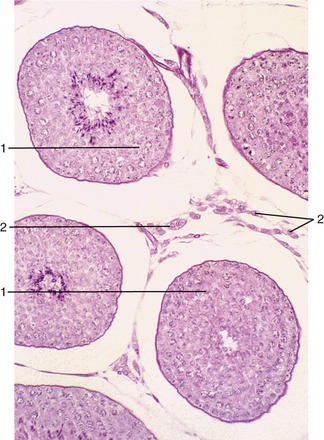
Figure 5–39 Testis (dog) (140×). 1, seminiferous tubules (showing spermatogenesis); 2, interstitial tissue with androgen-producing (Leydig) cells.
Each seminiferous tubule (Figure 5–38) is much contorted and also looped so that both ends open into the rete testis (Figure 5–38/5), a plexus of spaces within the mediastinum. Within the seminiferous tubules two cell types can be discerned: the Sertoli cells, which support and nourish the germ cells by the production of hormones and growth factors, and the seminiferous epithelium (Figure 5–39). The rete drains by a dozen or so efferent ductules (Figure 5–38/6) that pierce the capsule to join the head of the epididymis.
The endocrine functions of the testis are performed by the interstitial (Leydig) cells, responsible for androgen production, and the sustentacular (Sertoli) cells, responsible for inhibin production. Both types are normally under the pulsatile but more or less tonic control of gonadotropins (luteinizing hormone [LH] and follicle-stimulating hormone [FSH], respectively) produced in the pituitary (p. 217). Among other functions, the sustentacular cells produce activin and inhibin, whose names clearly indicate their effects upon the synthesis and release of FSH through mechanisms that may be direct or mediated via the hypothalamus. Androgens clearly have distinct local function but are also responsible for secondary sex characteristics such as the maturation of the accessory sex glands, male skeletomuscular development, skin characteristics, and even the prenatal differentiation of certain brain and spinal cord nuclei. They are also partly responsible for the behavior typical of the male. They also exert a negative feedback on pituitary gonadotropin secretion; part of this feedback is effected at the level of the hypothalamus. In the fetal period, active production of androgens may take place without pituitary control. The interstitial cells in this period are also responsible for the production of the insulin-like factor 3 that is associated with gubernacular outgrowth and thus with testicular descent. In the fetal period the sustentacular cells produce the AMH that exerts an inhibitory effect on the paramesonephric ducts (p. 171), causing the disappearance of most of the female duct system.
THE EPIDIDYMIS
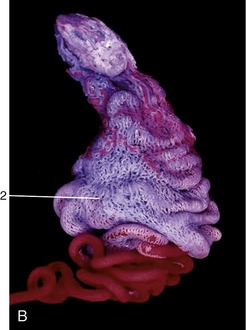
The epididymis is a firm organ that is largely formed by the numerous convolutions of the single epididymal duct within a connective tissue matrix. It is attached along one of the longer borders—dorsal in the dog, caudomedial in the bull—of the testis and usually spreads some distance over both poles (Figure 5–40). It is conventionally divided into three parts—head, body, and tail—but these rather arbitrary divisions do not always correspond to functional distinctions.
The head (Figure 5–38/8) is firmly attached to the testicular capsule. It receives the efferent ductules, which immediately or after some coiling join to form the wider epididymal duct (Figure 5–38/6′). The body may be less completely attached to the surface of the testis, and in that case an intervening space (testicular bursa, homologous with the ovarian bursa) is created (see Figure 5–41/3). The tail is firmly attached to the testis by a ligament (proper ligament of the testis) and also to the parietal layer of the enveloping peritoneal sac by the ligament of the tail of the epididymis (Figure 5–41/7,8). The tail finally tapers, and the duct emerges to continue as the deferent duct (see Figure 5–41/4). The epididymis appears spongy in section because the coiled duct is inevitably cut across many times.
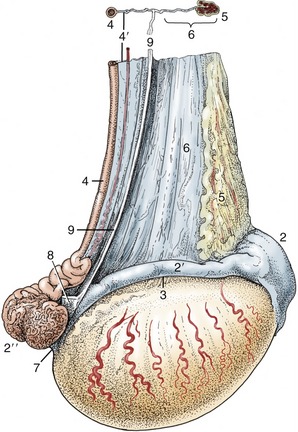
Figure 5–41 Lateral view of the right testis of a stallion. 1, Testis; 2, head of epididymis; 2′, body of epididymis; 2″, tail of epididymis; 3, testicular bursa; 4, deferent duct; 4′, mesoductus deferens; 5, pampiniform plexus; 6, mesorchium; 7, proper ligament of testis; 8, ligament of tail of epididymis; 9, cut edge of fold connecting visceral and parietal layers of the vaginal tunic.
THE DEFERENT DUCT
The deferent duct is undulating where it emerges but gradually straightens when followed toward the abdomen (Figure 5–42). It first runs medial to the epididymis as it heads toward the testicular vessels that form the bulkier components of the spermatic cord. The constituents of the cord remain together as they pass through the inguinal canal but disperse at the vaginal ring (see Figure 5–36 and Figure 22–19). The duct here turns caudomedially to pass under the ureter before gaining the dorsal surface of the bladder (see Figure 5–36); it penetrates the prostate before finally entering the urethra a little way beyond the urethra’s origin from the bladder. The abdominal part continues to be supported by a peritoneal fold (mesoductus), which joins its contralateral partner to produce a horizontal genital fold above the bladder. The greater part of the duct is of uniform appearance and structure; its lumen is rather narrow in relation to the thick muscular wall. In most species the subterminal stretch lying on the bladder exhibits a fusiform enlargement, the ampulla of the deferent duct or ampullary gland (see Figure 5–51/4). Although the term suggests a widening of the lumen, the thickening is mainly due to glandular proliferation in the wall of the duct, largely in the locally folded mucosa.
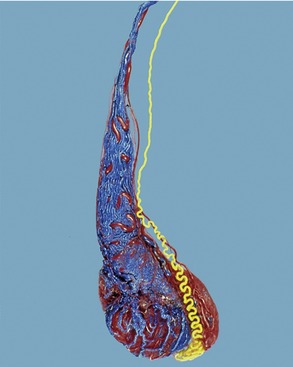
Figure 5–42 Corrosion cast (dog) of testicular artery (red), pampiniform plexus (blue), and deferent duct (yellow).
In most domestic mammals a second accessory gland grows from the duct close to its termination. This, the vesicular gland, is described in a later section, but it may be noted in the meantime that the short, shared passage is known as the ejaculatory duct.
The Vaginal Tunic and Spermatic Cord
The peritoneal process (vaginal tunic) that encloses the testis is an evagination of the lining of the abdomen through the inguinal canal. The narrow proximal part that surrounds the spermatic cord widens distally to form a flasklike expansion within the scrotum that encloses the testis and epididymis. The parietal and visceral layers of the tunic are connected by a fold that extends from the vaginal ring to the tail of the epididymis (see Figure 5–41).* The cavity between the parietal and visceral layers (Figure 5–43/9) normally contains only a minute amount of serous fluid. It communicates with the peritoneal cavity of the abdomen through the vaginal ring, a narrow slitlike opening placed within the internal opening of the inguinal canal. Sometimes a loop of small intestine or another abdominal organ herniates into the peritoneal process through the vaginal ring; this complication is often encountered at castration. It is worth mentioning that in human infants the neck of the peritoneal process usually becomes obliterated shortly after birth, which isolates the cavity about the testis.
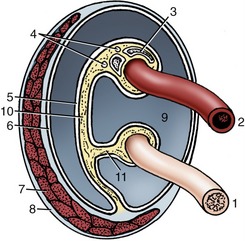
Figure 5–43 Transverse section of the spermatic cord and its immediate investments, schematic. 1, Deferent duct; 2, testicular artery (coiled); 3, pampiniform plexus; 4, testicular nerves and lymph vessels; 5, visceral layer of vaginal tunic; 6, parietal layer of vaginal tunic; 7, cremaster muscle; 8, external spermatic fascia; 9, vaginal cavity; 10, mesorchium; 11, mesoductus.
The spermatic cord varies in length and shape according to the position and orientation of the testis. It is shortest and most compact in those species in which the testis hangs vertically in the inguinal region. The bulk of the cord is provided by the testicular artery and veins, both remarkably modified. The artery branches from the abdominal aorta and first pursues a fairly direct course toward the vaginal ring, where the constituents of the spermatic cord are assembled. The more distal part is extraordinarily convoluted—one account describes no less than 7 m of artery packed within a 10-cm stretch of cord (Figures 5–44 and 5–45, A-B). These particular figures perhaps exaggerate the usual arrangement but serve to emphasize its extravagance. The testicular veins constitute a very elaborate close-meshed pampiniform plexus in which the contortions of the artery are embedded (Figure 5–45, B); the plexus ultimately reduces to a single vein that runs to the caudal vena cava. Arteriovenous anastomoses are present between the coiled testicular artery and its epididymal branches and the veins of the pampiniform plexus (Figure 5–46). A generous lymphatic drainage passes to lymph nodes placed about the bifurcation of the aorta. In some species a small lymph node is present near the inguinal canal. The lymph conveys a substantial fraction of the hormone production of the testis. The inconspicuous testicular nerves are of sympathetic origin.
The Scrotum
Variations in the location and form of the scrotum have been noted (see Figure 5–36). Externally, a median groove marks the division into right and left compartments; it often betrays a striking asymmetry of the testes. The lower part of the scrotum is molded on the testes and adjusts as their position varies with the ambient temperature (Figure 5–47).
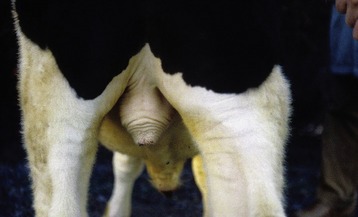
Figure 5–47 Scrotum of a bull. The musculature in tunica dartos is contracted causing elevation of the scrotum.
The relatively thin scrotal skin is well provided with both sweat and sebaceous glands. It is sometimes rather bare, but this is not a constant feature; indeed, the scrotum is hidden by hair in the cat and densely covered by fleece in sheep of certain breeds. When bare, it is often pigmented. The scrotal skin adheres to a tough fibromuscular layer (tunica dartos), which also extends as a septum between the compartments that separately lodge the testes. Internal to the dartos, a (spermatic) fascia is present that may be resolved into several layers, which are believed to correspond to the layers of the abdominal wall. The predominant layer is the external spermatic fascia, which can be clearly separated from the dartos (Figure 5–48). The loose intermediate stratum allows the vaginal tunic independent movement within the scrotal sac; in addition to its functional significance (see further on), this facilitates castration by the closed method (in which the testis is brought to the exterior within the vaginal tunic before the cord is severed proximally). The dense external spermatic fascia that supports the vaginal tunic also invests the cremaster, a slip of muscle that passes onto the cord on detachment from the caudal margin of the internal oblique muscle of the abdomen.
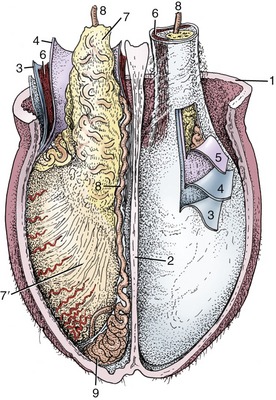
Figure 5–48 Cranial view of the opened scrotum of a bull; the investments of the testis have been partly dissected. 1, Scrotal skin and dartos; 2, scrotal septum; 3, external spermatic fascia; 4, parietal layer of vaginal tunic; 5, visceral layer (dissected from surface of testis); 6, cremaster muscle; 7, visceral layer of vaginal tunic covering structures in spermatic cord; 7′, visceral layer on testis; 8, deferent duct; 9, tail of epididymis.
Testicular Function
In most wild mammals the breeding period is seasonal, and this is reflected by changes in the morphology and activity of the reproductive organs of both sexes. Little of this seasonality remains among male domestic animals, in which the seminiferous epithelium is active throughout the year with at most only slight variation in sperm output. Although the process of spermatogenesis is not described, the reader is reminded that the serial cell divisions and maturation processes that constitute the cycle are not synchronous in every part of the seminiferous epithelium. Instead, adjacent segments show successive stages so that a “lucky” longitudinal section of a tubule displays the different stages of the process occurring as a wave spreading along its length (see Figure 5–39).
The process of spermatogenesis is influenced by temperature, and as already stated, it cannot proceed normally at the core temperature of the body. The seminiferous epithelium is damaged in testes that fail to descend into the scrotum (the “cryptorchid condition”), and these do not produce sperm. Similar changes are evident in testes that, having descended successfully, are later returned to the abdomen and, indeed, in scrotal testes that are overheated by an unusually thick covering of hair or fleece. Because the interstitial tissue is less susceptible to temperature, it follows that libido and potency may be normal in cryptorchid animals that are infertile.
Many factors help maintain the appropriate endotesticular temperature. The exposed position of the scrotum, the absence of fat within the scrotal fascia, and the intracapsular situation of large testicular vessels all favor heat loss by radiation (Figure 5–49); the generous supply of sweat glands allows additional loss through evaporation from the skin surface. Perhaps more importantly, the extensive contact between the vessels within the cord precools the blood within the artery as this follows its winding course in relation to the venous plexus (see Figure 5–45). The opportunities for heat loss are such that the testicular temperature could be lowered excessively in colder climates. Countermeasures are available. Contraction of the tunica dartos, directly sensitive to temperature change, tightens and bunches the scrotum, thereby reducing the exposed surface and also drawing the testes toward the warmer trunk (see Figure 5–47). The testes may also be separately raised within the scrotum by contraction of the cremaster muscles, which pull on the vaginal tunics; being striated, these muscles react briskly to pull the testes away from potentially harmful stimuli.
Castration of surplus male animals has long been practiced to make them more manageable or to promote particular carcass qualities. Modern husbandry, the effects of selective breeding, and changes in consumer requirements now make it possible to bring food animals to slaughter at earlier ages than before, and the necessity for and economic advantage of routine castration are beginning to be questioned. The direct influence of castration on the reproductive organs is considered in some detail for cattle, the species about which most is known, on page 719.
THE PELVIC REPRODUCTIVE ORGANS
The Male Urethra
The male urethra extends from an internal orifice at the bladder neck to an external orifice at the free extremity of the penis. It is thus divisible into an internal or pelvic part and an external or spongy part; here, spongy refers to the very vascular tissue that surrounds the urethra on its leaving the pelvic cavity. The spongy part is largely incorporated within the penis and is appropriately considered as a component of that organ. The pelvic part is joined by the deferent and vesicular (or combined ejaculatory) duct(s) a short distance from its origin from the bladder; by far the greater part of the urethra thus serves to discharge both urine and semen.
Although the pelvic urethra shows regional and specific variations, it consists essentially of a mucosal tube successively invested by a vascular submucosa and a muscular tunic. The mucous membrane is thrown into longitudinal folds in the inactive state. The initial part also carries a dorsal crest that continues from the urethral orifice to end in a thickening (colliculus seminalis). The colliculus displays on its sides the slitlike orifices of the deferent ducts and the much smaller openings through which the many prostatic ducts discharge (Figure 5–50/7). Similar but more distal openings mark the entry of the ducts of other accessory glands (Figure 5–50/8). The submucosa contains a rather inconspicuous system of connecting blood spaces that is continuous with the vastly more generous spongy investment of the second part of the urethra. The major component of the muscle coat is the striated urethralis that encircles the tube.
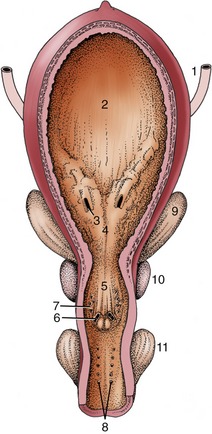
Figure 5–50 Ventral view of the opened bladder and urethra of a stallion. 1, Ureter; 2, bladder; 3, ureteric orifice; 4, trigone of bladder; 5, urethral crest and seminal colliculus; 6, opening of ejaculatory duct; 7, multiple openings of prostatic ducts; 8, multiple openings of bulbourethral ducts; 9, vesicular gland; 10, prostate; 11, bulbourethral gland.
The urethra is embedded in fat and other connective tissues where it lies on the pelvic floor. The dorsal surface is related to the rectum and, with species differences, to various accessory reproductive glands; usually only a narrow median strip that faces directly into the rectogenital pouch is covered by peritoneum. The urethra is easily palpated per rectum, a procedure that may stimulate rhythmic activity of its muscle.
The Accessory Reproductive Glands
The full set comprises ampullary, vesicular, prostate, and bulbourethral glands, although not all of these are present in every species (Figure 5–51). The ampullary glands have been sufficiently described.
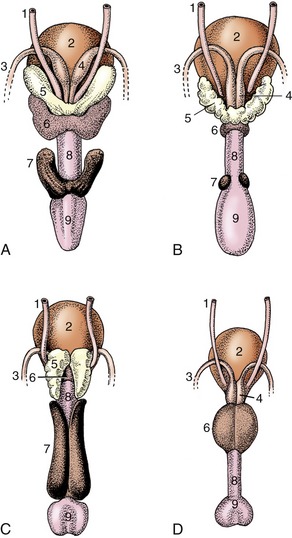
Figure 5–51 Accessory reproductive glands of the stallion (A), bull (B), boar (C), and dog (D); dorsal view. 1, Ureter; 2, bladder; 3, deferent duct; 4, ampullary gland; 5, vesicular gland; 6, body of prostate; 7, bulbourethral gland; 8, urethra, 9, bulb of penis.
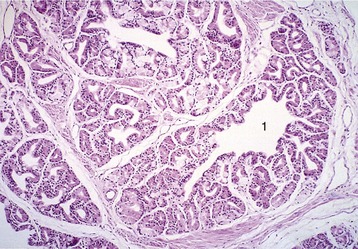
Figure 5–52 Bulbourethral gland (goat) (HE; 70×), a compound tubular gland lined with a columnar secretory epithelium. 1, Collecting duct.
Paired vesicular glands (Figure 5–51/5) are present in all domestic species except the dog and cat. Each buds from the distal part of the deferent duct in the embryo, and this relationship commonly persists; in the pig the later absorption of the ejaculatory duct into the urethra causes the vesicular gland to open separately. These glands vary greatly in appearance; in the horse they are large, externally smooth, and bladder-like, resembling the human organs that were formerly known as seminal vesicles. This term is inappropriate because in most species the glands are knobby and thick-walled with rather narrow, branched lumina. The vesicular glands lie wholly or partly within the genital fold, each lateral to the corresponding deferent duct.
A prostate (Figure 5–51/6) is present in all domestic species. In some it consists of two parts: one is diffusely spread within the wall of the pelvic urethra, and the other is a compact body placed external to the urethralis. Both parts drain by many small ducts. The small ruminants have only the diffuse or disseminate part, and the horse only the compact part. The disseminate part is vestigial in the dog and cat, but the compact part is very large and globular and so well developed that it surrounds the urethra entirely (dog) or almost so (cat).
Paired bulbourethral glands (Figures 5–51/7 and 5–52), compound tubular glands with a secretory epithelium, lie on the dorsal aspect of the urethra close to the pelvic exit. They are found in all species other than the dog (although they are vestigial in the cat). They are of moderate size in horses and ruminants but are very substantial in the pig, in which they appear as rather irregular elongated cylinders placed to each side of the urethra. They may drain by one or by several ducts.
All the larger glands possess well-developed capsules and internal septa in which much smooth muscle is present that expels the secretion at the appropriate time.
THE PENIS AND PREPUCE
The penis is suspended below the trunk and is partly contained between the thighs, where it is anchored to the floor of the pelvis by a suspensory ligament in the large species. In the quiescent state, the free extremity is concealed within an invagination of the abdominal skin, the prepuce, which opens at a variable site behind the umbilicus. The organ is mainly constructed of three columns of erectile tissue (Figure 5–53). These are independent caudally where they constitute the root of the penis, but their major parts are combined in the body of the penis.
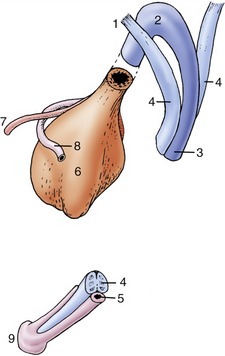
Figure 5–53 Schematic drawing of the components that constitute the equine penis at its root and at its apex. 1, Crus penis; 2, bulb; 3, corpus spongiosum; 4, corpus cavernosum; 5, urethra; 6, bladder; 7, ureter; 8, deferent duct; 9, glans.
The paired dorsal columns are known as the crura of the penis (Figure 5–53/1) at their widely separated origins from the ischial arch. They converge, bend forward, and run below the pelvic floor before joining. Each consists of a core of cavernous tissue enclosed within a thick connective tissue casing (tunica albuginea), and the complex is known as a corpus cavernosum (Figure 5–53/4). A septum exists between the two corpora cavernosa in the proximal part of the body, but in most species this will be found to weaken and ultimately disappear when traced distally toward the apex of the penis. In carnivores the septum is complete. The combined structure is grooved ventrally to accommodate the third component, the urethra within its enveloping vascular sleeve, the corpus spongiosum (Figure 5–53/3). The blood spaces within the crura and corpus cavernosum communicate freely.
The corpus cavernosum does not extend to the apex of the penis, which is formed by an expansion of the corpus spongiosum. The corpus spongiosum commences at the pelvic outlet with the sudden enlargement of the meager spongy tissue of the pelvic urethra. The expansion constitutes the bulb of the penis (Figure 5–53/2), a bilobed structure that tapers to continue as a more uniform sleeve. The corpus spongiosum is more delicate than the corpus cavernosum, having larger blood spaces separated by thinner septa. Its cranial expansion over the distal end of the corpus cavernosum, usually known as the glans (Figure 5–53/9), forms the apex of the whole organ. Since the corpus spongiosum surrounds the urethra, the urethral orifice is brought to the very extremity of the penis; indeed, in small ruminants a free urethral process prolongs the urethra well beyond this.
Other pronounced species differences in penis structure exist. In the dog and cat the distal part of the corpus cavernosum is transformed into bone, the os penis. The glans has very different forms. It is minimally developed in the pig, insubstantial in the ruminants, but large and mushroom-shaped in the horse. It is most specialized in the dog, in which it presents bulbar proximal and long cylindrical distal parts. The penis of the cat is unique (among domestic species) in pointing caudoventrally from the ischial arch; this retention of the embryonic posture affects the manner of copulation.
The construction of the corpus cavernosum also exhibits major differences. In some species it contains small blood spaces enclosed within and divided by substantial amounts of tough fibroelastic tissue. Relatively little additional blood need be retained to make this fibroelastic type of penis become erect (Figure 5–54, A); this construction is found in the penis of the boar and ruminant species in which the quiescent organ exhibits a sigmoid flexure of that part of its body carried between the thighs. In the other type, the blood spaces are relatively larger, and the enclosure and intervening septa more delicate and more muscular (Figure 5–54, B); a relatively much greater quantity of blood is required to achieve erection, which involves significant increases in both length and girth. This musculocavernous type of penis is found in the stallion and, in atypical form, in the dog.
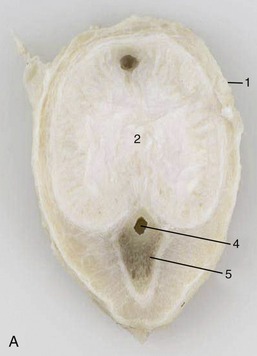
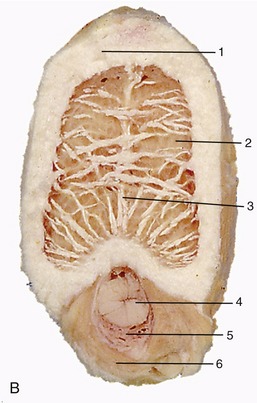
Figure 5–54 Transverse sections of the fibroelastic penis of a bull (A) and the musculocavernous penis of a stallion (B). 1, Tunica albuginea; 2, corpus cavernosum; 3, septum; 4, urethra; 5, corpus spongiosum; 6, bulbospongiosus.
The prepuce or sheath is a tubular fold consisting of an external layer (lamina externa), continuous with the general integument, and an internal layer (lamina interna) that faces the free end of the penis; the internal layer continues as the covering of the free part of the penis after reflection in the depth of the preputial cavity. Both the internal layer and the penile covering are hairless but often well provided with smegma-secreting glands and lymphoid tissue. In the newborn male the penis and sheath are fused, and separation is gradually achieved during the period before puberty (p. 719). The attachments of the adult prepuce are sufficiently loose to allow the internal lamina to be reflected onto the erect penis when this is protruded through the preputial orifice.
Certain muscles are associated with the penis. The bulbospongiosus is the thick extrapelvic continuation of the urethralis. It begins abruptly and extends distally to end on the surface of the corpus spongiosum at a variable distance beyond the point at which this is incorporated within the penis.
The powerful paired ischiocavernosi arise from the ischial arch, almost enclose the crura, and follow them to their fusion.
The retractor penis is also paired. It arises from the caudal vertebrae and descends through the perineum, bending laterally to pass around the anal canal, to reach the penis. Unlike the other muscles associated with the penis, the retractor is mainly composed of smooth muscle fibers.
Narrow slips of striated muscle (cranial and caudal preputial) may pass onto the prepuce and attach near its opening. The caudal muscles are less frequently encountered and retract the prepuce, thus uncovering the extremity of the penis. The cranial muscles protract the prepuce. Both caudal and cranial muscles must be regarded as detachments of the cutaneous trunci; they are best developed in the bull but lacking in the stallion.
The penis obtains its exclusive (in the horse, principal) blood supply from the artery of the penis, a terminal branch of the internal pudendal. The artery of the penis has a very short course, and at the ischial arch it quickly divides to form an artery of the bulb, which enters the bulb of the penis and supplies the corpus spongiosum; a deep artery, which pierces the tunica albuginea to supply the corpus cavernosum; and the dorsal artery, which passes apically on the dorsal border of the organ to supply the free end. The dorsal artery may be reinforced by anastomosis with the obturator artery (horse) and generally by anastomosis with the external pudendal artery for the supply of the prepuce. The veins are broadly satellite. Interspecific details are considered in the later chapters when they are significant.
The nerves to the penis accompany the vessels. The motor fibers are predominantly parasympathetic and from the pelvic nerves.
SPERM TRANSPORT IN THE MALE TRACT; ERECTION OF THE PENIS
The sperm are immotile when released into the lumen of the seminiferous tubules, where they float in fluid secreted by the sustentacular (Sertoli) cells of the epithelial lining. Their passage through the rete testis into the head of the epididymis is effected by the current generated by the combination of the testicular secretory pressure and the resorption of fluid by the lining of the efferent ductules. Onward progress through the epididymis appears to depend on several factors, among which spontaneous peristalsis of the muscular epididymal duct is probably most important. Hydrostatic pressure may continue to play a part, and in many species the sperm have themselves acquired the capacity for coordinated movement by the time they reach the tail of the epididymis. Many aspects of the process remain obscure, and it is not clear whether the physiological maturation of the sperm—which take some days to complete their passage through the epididymis—is merely the result of aging or whether it is due to specific features of the milieu. Fertilization with epididymal sperm has been achieved under experimental conditions, most readily when utilizing sperm removed from the tail. Secretory activity of the lining of the epididymal duct is maintained by androgens, and it is possible that these also have a direct influence on sperm. The deferent duct also exhibits peristalsis, which gradually moves the sperm toward the ampullary region. In sexually inactive animals, sperm are lost from here by seepage into the urethra whence they are flushed away by urine. A few may be resorbed by the lining of the duct system.
This regular but slow emission of sperm contrasts with the vigorous ejaculation that occurs during coitus. Erection of the penis is a necessary preliminary to this and is brought about by the engorgement of the cavernous and spongy spaces. This engorgement both stiffens and enlarges the penis, causing its free extremity to protrude from the prepuce, which makes possible intromission, the introduction of the penis into the vagina. The details of the process, which differs significantly among species, are largely dependent on the structure of the penis. In species in which the penis is fibroelastic, little additional blood need be retained to distend the cavernous spaces fully; the penis therefore does not increase greatly in size, and its protrusion is largely due to effacement of the preexisting sigmoid flexure. Moreover, because relatively little additional blood is required, full erection may be achieved rapidly. The cavernous spaces are much larger and more dilatable in the musculocavernous penis possessed by horses and dogs. In these species a much greater increase in both length and girth occurs. The process requires more time for its completion.
Two distinct phases of erection are recognized. In the first stages of sexual excitement, blood flow into the penis increases as the walls of the supplying arteries relax; at the same time the venous outflow is obstructed. The pressure within the cavernous spaces rises rapidly and soon equals that within the arteries that deliver blood to the corpus cavernosum via the crura and to the corpus spongiosum via the bulb.
The venous outflow is restricted at the proximal extremity of the organ, where the veins are compressed against the ischial arch; this has more effect on the drainage of the crura and corpus cavernosum than on that of the corpus spongiosum, whose more distal outlet is as yet unaffected (see Figure 15–20).
The process continues and intensifies after intromission. Rhythmic contractions of the ischiocavernosus and bulbospongiosus muscles now begin, impelling blood forward through the corpus cavernosum and corpus spongiosum. The internal pressures fluctuate in time with this activity. The additional blood pumped distally within the corpus cavernosum cannot escape because the emissary veins are compressed; the pressure therefore rises further. In contrast, the contractions of the bulbospongiosus produce only intermittent rises in pressure because some blood continues to escape at the free extremity of the penis; the effect of this flow is to massage the urethra, which supplies a further impulse to the forward movement of semen when ejaculation takes place.
In most species the pressures drop rapidly after ejaculation, first reaching that within the arteries and then dropping to the resting pressure (a mere 15 to 20 mm Hg). As the blood escapes, the penis shrinks, becomes more flaccid, and is returned to the prepuce. The return is brought about by the active involvement of the retractor penis muscles (Figure 29–34).
The volume and composition of the ejaculate vary with the species and also with recent sexual activity. Only a small part of the semen is provided by the sperm-rich fraction emanating from the testes and epididymides; most comes from the accessory reproductive glands. Because semen volume is dependent on the bulk of these glands, it could be anticipated that the ejaculate would be greatest in the boar. The various contributions to the semen are very imperfectly mixed when expelled into the urethra, but information on the sequence of discharge and on the specific proportions and function of the different glandular secretions must be sought elsewhere. The semen is moved through the urethra by the activity of striated muscles (urethralis, bulbospongiosus), and its ejaculation into the vagina or cervix (according to the species) is therefore forceful.
THE FEMALE REPRODUCTIVE ORGANS
The female reproductive organs include paired female gonads, or ovaries, which produce both female gametes (ova) and hormones; paired uterine tubes, which capture the ova on their release from the ovaries and convey them to the uterus; the uterus, in which the fertilized ova are retained and nourished until prenatal development is complete; the vagina, which serves both as copulatory organ and as birth canal; and the vestibule, which continues the vagina to open externally at the vulva but which also doubles as a urinary passage (see Figure 5–2).
Age and functional changes are particularly obtrusive where these organs are concerned. Age changes include the rapid growth and maturation associated with puberty and also the regression that occurs as the capacity for reproduction wanes with increasing age. Functional changes include those that are relatively transient and recur with each reproductive cycle as well as other, more lasting ones that are associated with pregnancy and giving birth. Unnecessary complications will be avoided if this initial account concentrates on the description of the organs of the mature nonpregnant animal; growth and functional changes are left for later comment. Even so, a few general terms are introduced at this point to help the reader.
Female mammals generally accept the male only close to the time of ovulation, a period characterized by various structural changes and by general excitability as well as specific behavioral features; the period is known as “heat” or “season” in lay language and as “estrus” more technically. Estrus recurs with varying frequency according to a program that is characteristic for each species although subject to environmental modification. In certain wild mammals the breeding season is confined to a certain part of the year, and sexual receptivity, with the concomitant structural and behavioral changes, occurs only once (monestrous species) or perhaps several times (seasonally polyestrous species) within this period. In other (truly polyestrous) species the cycle is repeated throughout the year; the adoption of the polyestrous mode often distinguishes domestic and laboratory species from their wild progenitors. The condition in which female receptivity is continuous and not linked to ovulation occurs only in women and some higher primate species (e.g., bonobo); in most of the latter it appears to be more common among, if not confined to, menagerie specimens.
The estrous cycle is divided into several phases. Estrus, the climax, is prefaced by proestrus, a period of follicular development; it is followed by a period of luteal activity divided between metestrus and diestrus. In monestrous species a lengthy period of sexual inactivity (anestrus) occurs before the cycle is renewed with a preparatory period of proestrus. In polyestrous species, proestrus follows directly after diestrus.
Proestrus and estrus together represent the follicular phase, when the reproductive condition is predominantly determined by the rising levels of estrogen produced in the batch of ovarian follicles then rapidly developing to maturity and rupture. Metestrus and diestrus represent the luteal phase, when the dominant hormonal influence is exerted by progesterone, the hormone produced by the corpora lutea, the transient endocrine glands that replace the ruptured follicles.
Other helpful terms tell whether a female animal has or has not borne young. Animals that have borne young are said to be parous, those that have not are nulliparous, and uniparous and multiparous extend this terminology in obvious fashion. Other terms refer to the number of young habitually carried by the gravid female. A mare with its (generally) single foal is monotocous; a sow with its litter of piglets is polytocous.*
THE OVARIES
The ovaries possess both gametogenic and endocrine functions. Each ovary is a solid, basically ellipsoidal body, although commonly made irregular by the projection from the surface of large follicles and corpora lutea (Figure 5–55, A-F). The irregularity is naturally greatest in polytocous species, in which follicles ripen in batches. The ovaries are much smaller than the testes of conspecific males but, like these, bear no constant proportion to body size. Those of the mare are relatively large and also peculiar in being kidney-shaped. Ovaries are usually found in the dorsal part of the abdomen, close to the tips of the horns of the uterus, as they do not shift far from their place of development. This migration, generally modest, occurs in the absence of any apparent endocrine influence: it is most considerable in ruminants in which the ovaries come to lie close by the pelvic inlet. Each ovary is suspended within the cranial part (mesovarium) of the broad ligament, the common suspension of the female reproductive tract.
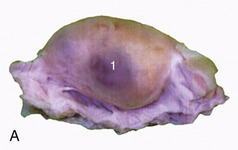
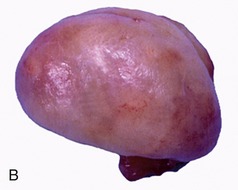
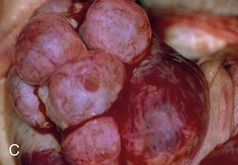
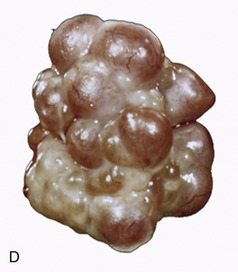
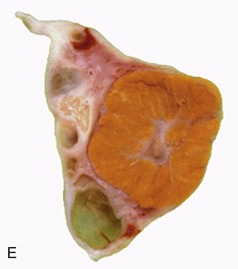
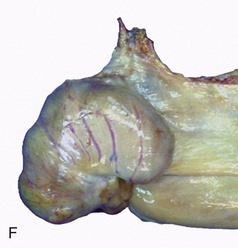
Figure 5–55 Specific and functional variations in ovarian morphology. A, Ovary of a cow (monotocous). B, Ovary of a bitch in a quiet stage. C, Ovary of a bitch exhibiting several mature follicles. D, Ovary of sow (polytocous) exhibiting mature follicles. E, Sectioned ovary of a cow containing a large corpus luteum. F, Ovary of a mare, with ovulation fossa. 1, Mature follicle.
A section through the ovary of a mature animal shows it to consist of a central looser and more vascular part contained within a denser shell. The parenchymatous zone (cortex) is bounded by a tunica albuginea directly below the peritoneum and is strewn with follicles in various stages of development and regression. Each follicle contains a single ovum; the stages through which it passes are shown in schematic fashion in Figure 5–56. The rapid enlargement undergone by those follicles selected to come to maturity in the current cycle is mainly due to the accumulation of the fluid by which the ova are swept out on ovulation. The cavity within the ruptured follicle, though it may initially fill with blood, is soon occupied by hypertrophy of the granulosa and theca cells that originally lined the space. This produces a solid body, known as the corpus luteum (yellow body) on account of its color (Figure 5–55, E). Corpora lutea are transient structures that wax and wane between one estrous period and the next (assuming pregnancy does not ensue) (Figure 5–57, A-C). Degeneration of the corpora lutea is characterized by vacuolization of the cytoplasm of the luteal cells due to lipid accumulation and nuclear shrinkage. Although transient, they are important as the source of progesterone, just as the ripening follicles are the source of estrogen. Corpora lutea finally regress and are replaced by connective tissue scars, corpora albicantes (white bodies). The alternation in the levels of estrogen and progesterone determines the changes in the behavior pattern and in the morphology and activity of the reproductive tract.
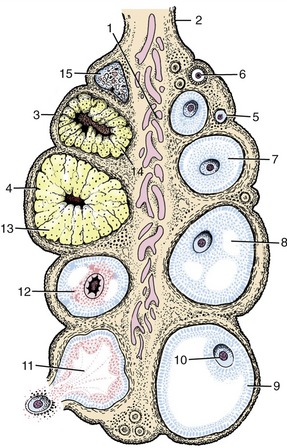
Figure 5–56 Schematic representation of the different functional stages in ovarian activity. 1, Medulla; 2, mesovarium; 3, surface epithelium; 4, tunica albuginea (poorly developed); 5, primordial follicle; 6, primary follicle; 7, secondary follicle; 8, early tertiary follicle; 9, mature follicle; 10, oocyte; 11, ruptured follicle; 12, atretic follicle; 13, corpus luteum; 14, atretic corpus luteum; 15, corpus albicans.
THE UTERINE TUBES
The uterine tubes* are narrow and generally very flexuous. They capture the ova released from the ovaries and convey them toward the uterus; because they also convey the sperm in their ascent, fertilization normally occurs within the tubes.
The free cranial extremity takes the form of a thin-walled funnel (infundibulum; Figure 5–58/2) placed close to the cranial pole of the ovary. The free edge of the funnel is ragged, and the tags (fimbriae) come into contact with and sometimes adhere to the surface of the ovary. A small (abdominal) orifice in the depth of the funnel leads to the longer tubular part that is divided into two more or less equal segments. The proximal one, known as the ampulla, is followed by the more convoluted and narrower isthmus, but the distinction between these segments is not equally obvious in all species or at all phases of the cycle (Figure 5–58/3,4). The isthmus joins the apex of the horn of the uterus at the uterotubal (salpingouterine) junction, a region of very variable appearance. The junction is gradual in ruminants and pigs and abrupt in horses and carnivores; indeed, in the mare and to a lesser extent also in the bitch and cat, the terminal part of the tube is thrust into the apex of the horn to raise a small papilla perforated by the (uterine) orifice of the tube. Too much should not be made of these differences as, regardless of its appearance, the junction always represents a real barrier, impeding both the ascent of sperm and the descent of ova. The tube wall consists of external serosal, middle muscular, and internal mucosal tunics. The mucosa is folded longitudinally along its whole length from infundibulum to isthmus; secondary and even tertiary folds reduce the lumen of the ampulla to a series of narrow branching clefts. The tube is carried in a side-fold (mesosalpinx) of the part of the broad ligament that supports the ovary.
THE UTERUS
The uterus,* the womb in popular speech, is the enlarged part of the tract in which embryos come to rest, where they establish a means of physiological exchange with the mother’s bloodstream, and where they are protected and nourished until ready to be delivered to the outside world. It is the part of the tract that displays the most striking specific differences, although the most extreme forms do not occur among domestic species. These differences find a ready explanation in the manner of formation of the reproductive tract (p. 172) from two paramesonephric ducts that grow caudally to meet and fuse with each other and with the median urogenital sinus, the ventral division of the cloaca (see Figures 5–15 and 5–16). In some species, including many rodents, fusion of the ducts is limited to the most caudal portions, which contribute to the vagina; the more cranial parts remain distinct, and the uterus thus consists of paired tubes that open separately into the vagina (double uterus—uterus duplex). In contrast, in women and most other primates, fusion is much more extensive and only the uterine tubes remain paired; a median uterus with a simple undivided lumen is present. In the intermediate variety (bicornuate uterus) found in all major domestic species, the uterus comprises a caudal median part from which paired horns diverge cranially to continue as the uterine tubes.
In all domestic mammals the median part of the uterus has two segments. The caudal, very thick-walled segment, the cervix (Figure 5–59/8), provides a sphincter controlling access to and from the vagina. A part of the cervix (Figure 5–59/9) (portio vaginalis) usually projects into the vaginal lumen with which it communicates at the external ostium. The lumen of the cervix (cervical canal) is constricted and often almost occluded by mucosal folds; it opens into the body of the uterus (Figure 5–59/6) at the internal ostium. The body is generally a rather small segment in domestic species, although the proportions vary (see Figure 5–16); it is largest in the mare. The division of the interior is not always obvious externally because an internal septum may partially divide an apparently single space. Although visual inspection generally fails to reveal the extent of the cervix, this is easily discovered on rectal palpation as it is much firmer than the adjacent parts.

Figure 5–59 The reproductive tract of a cow, opened dorsally. 1, Ovary; 2, infundibulum; 3, uterine tube; 4, horn of uterus; 5, intercornual ligaments; 6, body of uterus; 7, caruncles; 8, cervix; 9, vaginal part of cervix; 10, vagina; 10′, fornix; 11, vestibule; 12, external urethral opening; 13, opening of major vestibular gland; 14, clitoris; 15, vulva.
The horns (cornua) vary greatly in length, and it is hardly surprising that they are longest in polytocous species. Their disposition also varies; they are characteristically wound in ruminants, straight and divergent in mares and bitches, and cast into intestine-like loops in sows. The cervix generally lies within the pelvic cavity, interposed between the rectum and the bladder (Figure 5–32/7), but the body and horns of the uterus typically lie within the abdomen above the mass of intestines.
The uterus possesses serosal, muscular, and mucosal coats that are known as the perimetrium, myometrium, and endometrium, respectively. The serosal covering reaches the uterus by extension from the supporting broad ligament (mesometrium; Figure 5–33/7). The muscle is arranged as weak external longitudinal and thicker internal circular layers that are separated by a very vascular stratum of connective tissue. The tissues, especially the external muscle layer, extend (as parametrium) into the supporting broad ligaments. Dense connective tissue intermingles with the muscle of the cervix and makes this a very indistensible part of the tract at most times (Figure 5–60).
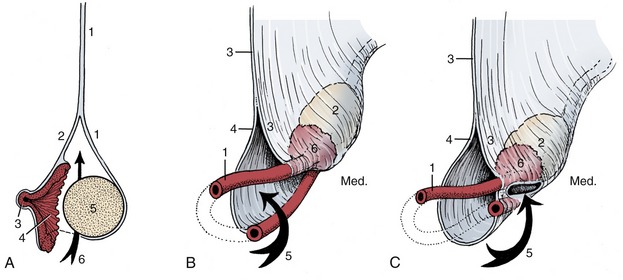
Figure 5–60 A, Schematic representations of the ovary and the suspensory system of the ovary and uterine tube and of the varying form of the ovarian bursa. 1, Mesovarium; 2, mesosalpinx; 3, abdominal opening of uterine tube; 4, infundibulum; 5, ovary; 6, the arrow is in the ovarian bursa. Schematic representation of the suspensory system of the ovary and uterine tube and of the varying form of the ovarian bursa. B, Spacious bursa with large entrance (cow, mare). C, Bursa with constricted entrance and entrapped ovary (bitch). 1, Uterine tube; 2, ovary; 3, mesovarium; 4, mesosalpinx; 5, arrow entering the ovarian bursa; 6, infundibulum.
The endometrium is thick. Its surface relief varies among species and is most remarkable in ruminants, in which numerous permanent elevations (caruncles) mark the sites where the embryonic membranes firmly attach during pregnancy (Figure 5–59/7). Numerous tubular glands open on the surface, which is generally lined by a simple columnar epithelium. The mucosa within the cervix is prominently modeled by both longitudinal and circular folds whose interdigitation helps close the passage (Figure 5–59/8). Mucus secreted by cervical glands plugs the canal at most times and so helps seal the uterus from the vagina. The passage is open only at estrus and immediately before, during, and, for a short time, after parturition.
THE VAGINA
The remainder of the female tract, although sometimes loosely termed the vagina, consists of two parts. The cranial part, the vagina in the strict sense (Figure 5–59/10), is a purely reproductive passage that runs from the cervix to the entrance of the urethra. The caudal part, the vestibule, extends from the urethral orifice to the external vulva and combines reproductive and urinary functions. The two parts together constitute the female copulatory organ and birth canal.
The vagina is a relatively long, thin-walled tube that is distensible in length and width. It occupies a median position within the pelvic cavity, related to the rectum dorsally and the bladder and urethra ventrally (Figure 5–32/8). It is mostly retroperitoneal, although peritoneum does cover the cranial parts of both the dorsal and the ventral surfaces to a variable extent. Incision of this part of the dorsal wall, a relatively easy procedure to perform from within the vagina in larger species, provides a convenient access to the peritoneal cavity (see Figure 22–6/2,8). The corresponding ventral approach is prohibited by the presence of a plexus of veins draining the uterus and vagina.
The vaginal muscle, although weaker, has a similar disposition to that of the uterus. The mucosa is lined by a stratified squamous epithelium that reacts, more emphatically in some species than in others, to changes in hormone levels throughout the estrous cycle. Glands are confined to the cranial part of the vagina, although the moisture may diffuse more widely. The surface is smooth but circular, and longitudinal folds may form when the walls of the inactive organ collapse inward. The intrusion of the cervix into the cranial part of the vagina reduces the lumen of this part to a (generally) ringlike space known as the fornix (Figure 5–59/10′).
The junction of vagina and vestibule is supposedly marked in virgin animals by a transverse mucosal fold (hymen). This is best developed in the filly and the gilt, but even in these it is rarely very prominent. It does not survive coitus. The junctional region is less distensible than the parts of the tract cranial and caudal to it.
THE VESTIBULE AND VULVA
The vestibule, much shorter than the vagina, lies mainly if not entirely caudal to the ischial arch, which is a circumstance that permits it to slope ventrally to its opening at the vulva. The amount of “drop” is variable, both among species and individuals (Figure 5–61). The resulting inflection of the axis of the genital passage must be borne in mind when introducing a vaginal speculum or other instrument.
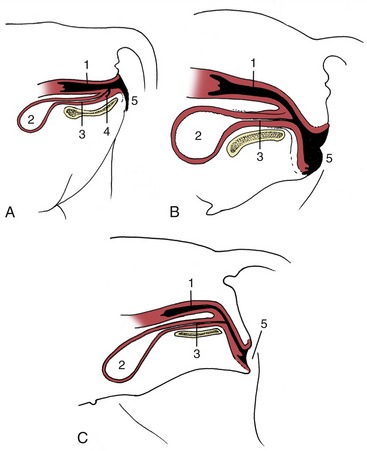
Figure 5–61 Variation in the position of the vestibule in relation to the ischial arch (A, cow; B, mare; C, bitch). 1, Vagina; 2, bladder; 3, urethra; 4, suburethral diverticulum; 5, vulva.
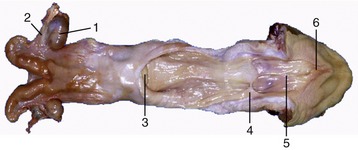
Figure 5–62 Uterus and opened vagina of the cow. 1, Ovary; 2, uterine tube; 3, cervix; 4, hymen; 5, vestibule; 6, glans of clitoris.
The walls of the vestibule are less elastic than those of the vagina and come together at rest, reducing the lumen to a vertical cleft. The urethra opens on the floor, directly caudal to whatever indication of a hymen (Figure 5–62/4) may exist. In some animals, for example, the bitch, the urethral opening is raised above the general level of the vestibular floor (Figure 5–35); in others, such as the cow, it is associated with a suburethral diverticulum (Figure 5–32/12′). More caudally, the vestibular walls are marked by the entrances of the ducts of vestibular glands. In certain species (e.g., bitch) the glands are small but numerous and the duct orifices form linear series; in others (e.g., cow) a large glandular mass to each side drains by a single duct (Figure 5–59/13). In a few species (e.g., ewe) both minor and major vestibular glands are present. These glands produce a mucous secretion that lubricates the passage at coitus and at parturition. At estrus the odor of the secretion has a sexually stimulating effect on the male. The vestibular wall is exceptionally well vascularized with a concentration of veins forming a lateral patch of erectile tissue known as the vestibular bulb and regarded as the homologue of the bulb of the penis.
The vestibule opens to the exterior at the vulva. The vertical vulvar opening is bounded by labia that meet at dorsal and ventral commissures. Except in the mare, the dorsal commissure is rounded, the ventral one pointed and raised above the level of the surrounding skin. The labia correspond to the (inner) labia minora of human anatomy; the (outer) labia majora are suppressed in domestic species.
The clitoris, the female homologue of the penis, lies just within the ventral commissure (Figure 5–59/14). It is formed of two crura, a body and a glans, in the same fashion as its much larger male homologue. Without dissection, only the glans is visible where it projects within a fossa on the vestibular floor, partly enveloped by a mucosal fold constituting a prepuce.
THE ADNEXA
The broad ligaments, the principal attachments of the female reproductive tract, are bilateral sheets that take extended origin from the abdominal roof and pelvic walls. The cranial part of each hangs vertically and suspends the ovary, uterine tube, and horn of the uterus. The caudal part passes more horizontally to attach to the side of the body of the uterus, cervix, and cranial part of the vagina; the right and left caudal parts with their visceral inclusion thus divide the pelvic cavity into dorsal and ventral spaces (Figures 5–33/7, 22–6, and 29–7). Different parts of the broad ligaments obtain the specific designations already mentioned (e.g., mesovarium). These ligaments are unlike most peritoneal folds because the serosal membranes are held apart by considerable amounts of tissue, mainly smooth muscle; this sometimes makes it difficult to point to the exact boundary between the uterus and its adnexa. The muscle enables the ligaments to take an active part in the support and disposition of the reproductive organs in addition to conveying vessels and nerves.
When followed distally from its attachment to the abdominal roof, the mesovarium, which supports the ovary, releases a lateral fold (mesosalpinx) that passes onto the uterine tube (Figures 5–58/7 and 5–60, A). Mesosalpinx and mesovarium enclose a pouch, the ovarian bursa, into which the ovary projects. The bursa may be shallow and unable to hold the ovary (mare; Figure 5–58/9) or deep and so enclosed by the fusion of apposed serosal surfaces that the ovary is permanently trapped (bitch; Figure 5–60, C). In certain nondomestic species (e.g., mouse) fusion is so complete that the space within the bursa no longer communicates with the peritoneal cavity. The walls of the bursa may contain so much fat that the ovary is quite hidden. The mesovarium also supports a fibromuscular band, the proper ligament of the ovary, which extends from the caudal pole of the ovary to the adjacent tip of the horn of the uterus.
The large part of the broad ligament that passes onto the horn and body of the uterus helps to give the organ the shape characteristic of the species. The two serosal membranes are very widely separated by fat where they attach to the cervix and especially to the vagina; the lateral part of the vagina is therefore retroperitoneal (see Figure 29–8). A cord of fibrous tissue and smooth muscle, the round ligament of the uterus, passes from the tip of the horn of the uterus toward (and in the bitch, through) the inguinal canal, supported by a special fold of peritoneum detached from the lateral surface of the broad ligament.
The muscles and fasciae associated with the female reproductive organs are best considered in topographical contexts for those animals in which they have special importance (p. 705). It will be recalled that the pelvic outlet is closed by a musculofascial partition of complicated form and structure. The dorsal part, the pelvic diaphragm, closes the outlet about the anus. The ventral part, the urogenital diaphragm (membrana perinei), closes the outlet about the vestibule. Muscle forms the principal component of the pelvic diaphragm, while the fasciae predominate in the urogenital diaphragm.
The blood supply to the female reproductive organs is obtained from several sources. The ovarian artery, a direct branch of the aorta, supplies the ovary and branches to the uterine tube and cranial part of the horn of the uterus; the pattern of branching varies in detail. The ovarian artery assumes an extraordinarily convoluted course and, depending on species, is more or less closely related to the ovarian vein. The uterine branch anastomoses with the uterine artery within the broad ligament (Figure 5–63/1′,2).
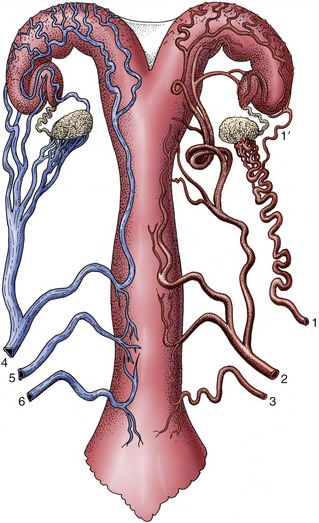
Figure 5–63 Semischematic ventral view of the blood supply to the reproductive tract of the cow. The arteries are depicted on the right side, the veins on the left. 1, Ovarian artery; 1′, uterine branch; 2, uterine artery; 3, vaginal artery; 4, ovarian vein; 5, accessory vaginal vein; 6, vaginal vein.
The uterine artery arises as an indirect branch of the internal iliac artery (except in the mare) and runs forward within the broad ligament. It detaches a series of anastomosing branches to the body and horn of the uterus; the most cranial anastomoses with the ovarian artery, the most caudal with the vaginal artery. Thus, an arterial arcade is established, running the length of the uterus and supplied from both ends (Figure 5–64). Much rather inconclusive discussion has occurred on the significance of this arrangement in determining the generosity of the blood supply to different parts of the uterus—and therefore to particular implantation sites in the pregnant animal. Some believe that differences in arterial pressure disfavor certain sites and that this explains the location of the runts that are so common in the pig.
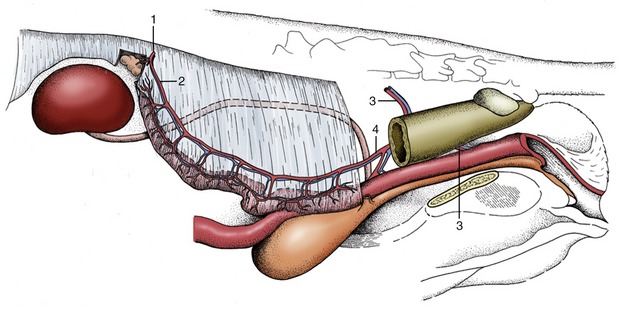
Figure 5–64 Semischematic drawing of the blood supply of the female reproductive tract (bitch). 1, Ovarian artery; 2, uterine branch of the ovarian artery; 3, vaginal artery; 4, uterine artery.
The more caudal parts of the tract are variously supplied by branches of the internal pudendal and vaginal arteries; some more important species differences are mentioned elsewhere.
The veins, broadly satellite to the arteries, do not correspond to their companions in relative importance. The plexiform ovarian vein is relatively much larger, the uterine vein relatively much smaller, than the accompanying artery (see Figure 5–63). A prominent and elaborate venous plexus present on the ventral aspect of the uterus and vagina drains both organs; it allows blood to escape by any of the paired ovarian, uterine, and vaginal veins. The close relation between the artery and ovarian vein, best seen in ruminants and sows, provides a means for the countercurrent transfer of the luteolytic hormone (prostaglandin) from venous to arterial blood (p. 211).
The lymphatics from the ovaries and more cranial parts of the tract pass to the aortic and medial iliac nodes; those from more caudal parts pass to the medial iliac and other nodes within the pelvis.
Innervation of the female reproductive organs is provided by both sympathetic and parasympathetic fibers, by routes that have yet to be fully clarified. Sympathetic fibers run to the ovary together with the ovarian artery, but although they reach ripening follicles, their significance is unclear as denervation hardly disturbs ovarian function. The fibers to the uterine tube, uterus, and vagina mainly follow the other arteries to form plexuses within the broad ligaments and the genital organs themselves. In the caudal part of the broad ligaments these fibers are augmented by other sympathetic fibers that travel by way of the plexus located in the retroperitoneal pelvic tissue.
The parasympathetic fibers branch from the pelvic nerves and reach the genital organs via the pelvic plexus. A large proportion goes to erectile tissue.
Both sympathetic and parasympathetic fibers seem to be concerned with uterine activity, although their precise roles in stimulation and inhibition are still controversial. The uterus is able to coordinate contractions and accomplish a normal birth even after denervation.
AGE AND FUNCTIONAL CHANGES IN THE FEMALE TRACT
Only a general account of the important age and functional changes is presented in this chapter, which glosses over the many species differences that affect all aspects but particularly the timing and duration of events.
Age and Cyclic Changes
The juvenile reproductive organs are disproportionately small. At birth the ovaries provide no evidence of their future endocrine role, which is not established until shortly before puberty when ripening follicles, and the corpora lutea that replace them, produce the hormones that stimulate the growth, tissue differentiation, and activity of the reproductive tract, and the manifestation of female behavior. In contrast, the gametogenic or exocrine function was established in the young fetus with the migration of primordial germ cells into the ovary. These immigrant cells proliferate rapidly to produce a population of perhaps 3 million at its maximum, but this number soon begins to be progressively reduced in a process that continues to puberty and beyond. Only a few hundred thousand generally survive at birth and, because no later accession to their number is possible, this determines the later, much more niggardly release of female gametes compared with male gametes. Each surviving oocyte is initially surrounded by a single layer of flattened epithelial (granulosa) cells to form the structure known as the primordial follicle. Most primordial follicles remain in arrested development, or undergo atresia, but some transform into primary follicles that are distinguished by the enlargement of the oocyte and its enclosure within a covering of granulosa cells that have assumed a cuboidal conformation.
Growth of reproductive organs is initially isometric, keeping pace with general somatic growth. After puberty the actions of ovarian hormones, cumulative over the first few cycles, bring about a rapid enlargement and a better differentiation of the component tissues. Follicles in all stages of development may now be found within the ovaries together with corpora lutea and replacement scars (see Figure 5–56).
There is a continuous slow growth of many follicles within the adult ovaries. In the ovary of anestrous animals the follicles grow to the early antral stage (see Figure 5–57, A) but then degenerate. The onset of the breeding season is heralded by a more rapid development of a few, which are chosen from this larger population according to obscure criteria. These favored follicles enlarge at an exponential rate under the influence of FSH of the pituitary. Their growth is explained by the proliferation of granulosa and theca cells and the accumulation of follicular fluid. This fluid increasingly distends a central vesicle (antrum) into which the ovum projects, raised on a mound of cells (cumulus oophorus) and enclosed within a cellular covering (corona radiata). The follicle is bounded by a two-layered capsule (theca interna and externa) differentiated from the surrounding stroma (see Figure 5–56). As each follicle grows it shifts toward the surface of the ovary, where it forms an increasingly salient projection. The granulosa cells of the ripening follicle produce estrogen, and it is the peak level of production of this hormone that induces both the behavioral pattern and the structural changes that characterize the animal in heat.
Estrogen has an epitheliotropic effect most evident in promoting proliferation of the vaginal epithelium and simple lengthening of the uterine glands. It also produces edema and hyperemia of the tissues of the reproductive tract; edema may produce a visible swelling of the vulva, while congestion of the endometrium may lead in some species (notably the bitch) to the appearance of blood in the external discharge. It also enhances the irritability of the myometrium that is detectable through the uterus, including the cervix, which becomes more responsive to manipulation.
Ovulation occurs late in estrus or shortly after its termination and is stimulated by LH, also of pituitary origin. Ovulation is spontaneous in most species, but in some, including the cat, the mechanical stimulus of coitus is necessary to set in train the events that culminate in follicular rupture (Table 5–1). Once shed into the peritoneal cavity, an ovum is soon gathered into the expanded end of the uterine tube. How this is effected is uncertain, although it is clear that the nonmotile ovum can play no active part. The most likely mechanisms are the production of a current in the suspending fluid by the ciliary beat of the tubal epithelium and grasping movements of the muscular fimbriae, which are closely applied to the surface of the ovary at this time. Both mechanisms would be assisted by the surface irregularity provided by adherent corona cells.
The space within the vacated follicle fills with blood when rupture has been attended by considerable hemorrhage, but any clot is soon replaced by proliferation of the surviving granulosa and internal theca cells to form a solid body, the corpus luteum (see Figure 5–57, B). This structure grows rapidly and may soon equal the follicle that it replaces. It produces progesterone, the hormone that continues the preparation of the uterus for the reception of the embryo and for the maintenance of pregnancy. In animals that become pregnant it survives well into or throughout pregnancy (according to species), but it regresses quite rapidly in cycles that are infertile (see Figure 5–57, C). Responsibility for its regression rests with a luteolytic hormone (prostaglandin) produced by the “empty” uterus. The effects of progesterone reinforce those produced by previous exposure to estrogen and stimulate further growth of the uterine glands, which now become branched, tortuous, and more active, secreting the so-called uterine milk that nourishes the embryo before implantation. Progesterone also dampens the activity of the myometrium.
The transport of ova within the tube is achieved by the combination of ciliary and muscular activity. If mating has occurred the ova rendezvous with the sperm within the ampulla. Although sperm may reach this site within a few minutes of coitus, a longer sojourn within the female tract is required before they become capable of fertilization. According to species, semen is initially deposited within the vagina or the cervix, where it forms a coagulum from which some sperm soon emerge. Even when the semen is deposited in the vagina, churning movements soon bring some sperm into contact with the cervical mucus, which provides a more hospitable environment than the acid secretion of the vagina. The physicochemical properties of the cervical mucus at this time help align the sperm, directing them on their upward path. Even so, the movement of sperm would be slow if they depended on their own puny efforts; transport is mainly effected by muscular contractions, evoked by prostaglandin within the semen, and by oxytocin reflexly released into the bloodstream at coitus. Though sperm are produced with great prodigality, only a small proportion, 1% or 2% of the many millions within an ejaculate deposited within the vagina, succeed in passing the cervical barrier. The uterotubal junction, the next major impediment, is successively negotiated by even fewer sperm (and these necessarily of normal motility). In species in which intrauterine deposition of sperm takes place, the uterotubal junction is the first barrier. Movement within the tube is more erratic because the muscular contractions on which it depends are ill-coordinated. In most species sperm remain fertile for a day or two after coitus, and many apparently find temporary refuge in cervical glands and other niches.
Fertilization activates the ovum, and cleavage begins within a short time. Its later fate is considered in the following section.
The Course of Pregnancy
The evolution of the gravid uterus affects its size, position, form, and relations; these changes naturally become increasingly evident as pregnancy advances. Even so, it will be convenient to take brief note of certain of them now, before resuming the history of the fertilized ovum. The principles effecting change in size are more or less the same in all animals, but the other aspects vary among species and are best considered separately for each (see the appropriate later chapters). The increase in size may ultimately be as much as 100-fold (as in the cow), but the greater part of this is represented by the contents of the uterus, which comprise the fetal membranes and fluids in addition to the conceptus(es). The more modest growth of the organ involves all its components. The endometrium remains hyperemic and edematous, and the myometrium enlarges owing to a vast increase in the size of individual muscle cells. Despite this hypertrophy, the uterine wall is unable to keep pace with the growth of the contents and it stretches markedly—so much so that in rats and other species of similar size it becomes transparent. The broad ligaments share in the increase and come to contain large amounts of muscle. The arteries enlarge greatly as it becomes necessary to satisfy an ever-increasing demand for blood. Activity of the cervical glands continually renews the mucous plug that seals the cervical canal.
Implantation involves reaction from the apposed epithelial layers of the blastocyst and endometrium, and in some species considerable erosion of maternal tissue takes place as the attachment develops (see later). This erosion occurs mainly in species in which the blastocyst remains small before implantation and either seeks out a nidus (nest) in a cleft of the endometrium or burrows into its substance. The blastocysts of domestic species grow considerably before implantation and remain centrally within the lumen and thus related to the whole circumference of the endometrium. In some species it is not always easy to decide when the closeness of association amounts to implantation, and in domestic ungulates the event is probably significantly longer delayed than the 2 weeks after coitus suggested for many other mammals.
Implantation and the initial development of the fetal membranes may be regarded as concluding the preembryonic period, the first of the three periods into which development is conventionally divided. Its principal features may be summarized as follows: the intrauterine migration and eventual settlement of the blastocyst, and its rapid transformation from a spherical to a threadlike form in many species (which include the ruminants and pig but not the horse).
The second or embryonic period is occupied by the establishment of a fully functional placenta, the differentiation of the various tissues and organ systems (for which the reader should see the relevant systematic chapters), and the initiation of various functions, most notably an embryonic circulation. Overall growth is still relatively modest, but by the end of this period the external conformation is sufficiently developed to identify the major taxon—order and, perhaps, family—to which the embryo belongs, though not yet the particular species.
The remaining part of the intrauterine development is assigned to a third or fetal period, although the determination of the boundary is necessarily somewhat arbitrary and imprecise.* Organogenesis continues throughout the fetal period and, for many organs, well into postnatal life, but the changes that now bring the different systems into the degree of structural and functional competence necessary for survival after birth are less dramatic than those that took place earlier. The rapid growth, which is the foremost characteristic of the fetal period, continues into postnatal life without significant interruption around the time of birth (Figure 5–65).
Figure 5–65 The growth of lambs. A, B, and C record the growth in weight of lambs during fetal and early postnatal life. D, Schematic summary of metrical and other features of the development of the fetal lamb and its adnexa.
The early transformations and the complexities of organogenesis provide ample opportunity for development to go awry, and death or malformation is common in the first two periods. This is probably true for all mammals, although data are most reliably available for the human and the pig. Some losses and abnormalities are due to intrinsic defects of the conceptus, some to an unreceptive state of the uterus, and some to exposure of the mother to any of a variety of environmental insults. It is known, for example, that chromosomal abnormality of structure or number is demonstrable in about 10% of clinically detectable human pregnancies, including spontaneous early abortions, and it is believed to be even more common in conceptuses lost at earlier stages before there was awareness of pregnancy. In contrast, chromosomal abnormality is identified in a much smaller proportion, perhaps 0.5%, of human infants delivered at term. Although the fertilization rate in pigs is high, possibly exceeding 95% in some herds, it has been estimated that only 60% or so of conceptuses come to term. Most deaths occur within the first 40 days (of a gestation period of 114 days), but because conceptuses lost at an early stage are generally resorbed and leave no trace, the figure must be interpreted with caution. The rates for fertilization and delivery at term for other species vary too much from herd to herd and stud to stud to be susceptible to convenient or safe summary.
The environmental insults that may affect development adversely cannot be comprehensively listed, much less adequately considered here. They include ionizing radiation, viral infections, inorganic and organic chemicals, including some that are constituents of plants (e.g., clover, soya and certain other legumes, Veratrum californicum) potentially present in pasture or other feedstuffs. Many of these agents are better known from their effects in the laboratory than in the field, and while some are lethal, others are more likely to produce abnormalities that are survivable, if only for a time. Such agents (teratogens) are most likely to produce abnormality when exposure occurs during the embryonic period, when so many complicated and critically timed procedures are under way; earlier exposure is more likely to result in death.
Familiar examples of indictable infective agents are provided by bovine viral diarrhea (BVD), hog cholera (swine fever) (HCV), border disease virus (BDV), and human rubella and cytomegalovirus. These viruses are notorious for causing fetal death resulting in abortion or stillbirth and for producing defects of brain and eye especially or growth retardation in young born to mothers infected in early pregnancy. Fetuses infected at a later stage with BVD or HCV become immunotolerant to these viruses and may be born apparently healthy. Because they are persistently infected, they represent a real danger to other livestock on the farm.
Fetal Membranes and Placentation
We have insufficient space to describe the formation of the embryonic or fetal membranes but include diagrams (Figure 5–66) as reminders of the principal points. The definitive gross arrangement is shown for the dog (Figure 5–67, A), horse (Figure 5–67, B), and ruminant (Figure 5–67, D-E) conceptuses. These membranes concur with the endometrium in the formation of the placenta, an organ that may be defined as an apposition or fusion of fetal and maternal tissue for the purposes of physiological exchange and hormone production. A provisional placenta, furnished by a vascularized yolk sac, may provide a useful organ of exchange in early pregnancy. This omphaloplacenta is important in the first third or so of equine pregnancy (Figure 5–68), but in most species the chorioallantoic placenta, the definitive placenta of eutherian mammals, becomes competent at a relatively earlier stage. In the definitive arrangement, the chorion, intimately associated with the endometrium, is vascularized by vessels that reach it by following the allantoic outgrowth from the hindgut. The stalk of the allantois (urachus), the accompanying vessels that become the umbilical arteries and veins, and the ensheathing connective tissue (the fetal variety known as Wharton’s jelly) constitute the umbilical cord, which persists as the communication between fetus and placenta until ruptured in the course of birth or shortly thereafter.
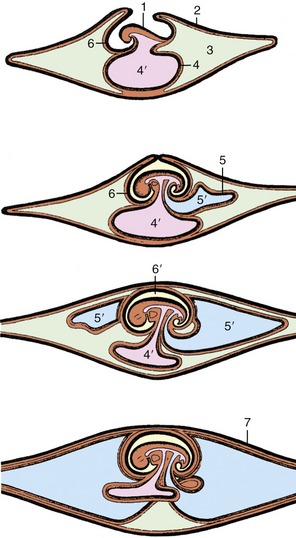
Figure 5–66 Schematic representation of the formation of extraembryonic membranes. 1, Embryo; 2, chorion; 3, extraembryonic celom; 4, yolk sac; 4′, yolk sac cavity; 5, allantois; 5′, allantoic cavity; 6, amnion; 6′, amniotic cavity; 7, chorioallantois.
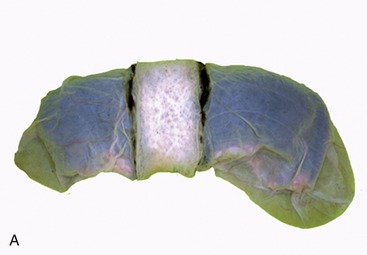
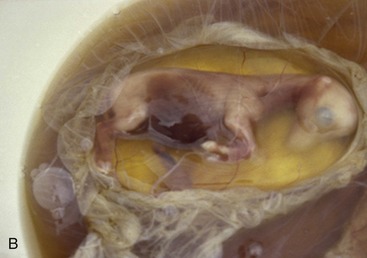
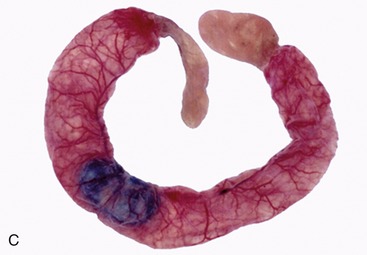

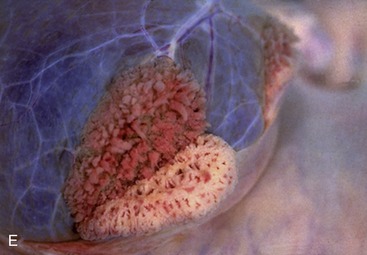
Figure 5–67 Placentation in a number of species. The most intense interaction between the fetal membranes and the endometrium in domesticated species is found in carnivores with the zonary placenta (A). The placenta of the horse fetus is not very complex. The villi do not penetrate deep into the endometrium (B). A similar situation exists in the pig (C). The placenta of ruminants is a cotyledonary placenta (D) with many placentomes (cow). The partial separation of the maternal and fetal part of the placentome is demonstrated (E).
The chorioallantoic placenta takes many forms that may be classified in several complementary ways. The first system refers to the gross distribution of the chorionic villi, minute outgrowths of the chorionic surface that engage with depressions of the endometrial surface to provide the areas of exchange. In the horse and the pig these villi are spread in small clumps (microcotyledons) over virtually the entire surface of the chorion (see Figure 5–67, B-C); such placentas are diffuse. In ruminants the villi develop in scattered patches or cotyledons opposite the endometrial caruncles; each cotyledon and associated caruncle forms a separate unit or placentome, and these collectively constitute a cotyledonary placenta (see Figure 5–67, D-E). In the dog and the cat the villi develop in a band of chorion that encircles the trunk of the embryo, forming a zonary placenta* (see Figure 5–67, A). The fourth and last type does not occur in domestic species; in this, the common pattern in primates and rodents, the villi are concentrated in one large patch, forming a discoidal placenta (Figure 5–69).
The second system refers to the tissue layers that separate the fetal and maternal bloodstreams. Initially, six layers are present: chorionic capillary endothelium, connective tissue; epithelium; endometrial epithelium, connective tissue, and capillary endothelium. The tissue barrier at the areas of exchange is always later reduced, sometimes only by the closer approach of the two sets of capillaries but often by tissue loss. In theory, the six layers persist in the epitheliochorial placenta seen in the mare and sow. They are reduced to four, by the loss of the endometrial epithelium and connective tissue, in the endotheliochorial placenta seen in dogs and cats (Figure 5–70), and they suffer the ultimate reduction to one layer, embryonic endothelium, in the hemoendothelial placenta of bats. Ruminants were long described as having a syndesmochorial placenta, in which only the uterine epithelium had been lost; modern studies discount this loss, and it is now believed that these animals also have epitheliochorial placentas.
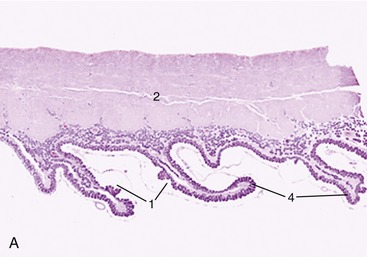
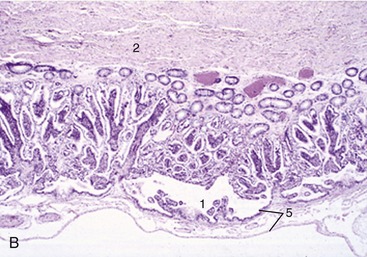
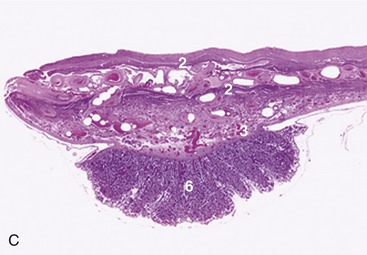
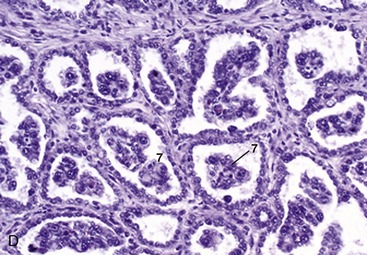
Figure 5–70 A to H, Placental histology (see H for label descriptions). A, Diffuse folded villous placenta (pig) (4×). B, Diffuse villous placenta (horse) (28×). C and D, Cotyledonary villous placenta (cow) (4×; 140×). E and F, Zonary labyrinthine placenta (cat) (4×; 279×). G and H, Zonary labyrinthine placenta (dog) (4×; 279×). 1, areola; 2, myometrium; 3, endometrial glands; 4, primary fold; 5, allantochorion; 6, placentome; 7, trophoblastic giant cells; 8, uterine septum; 9, chorionic villi; 10, placental labyrinth; 11, decidual cell; 12, maternal capillaries; 13, fetal capillaries; 14, marginal hematomas; 15, trophoblast cells.
The third system refers to the loss of maternal tissue that occurs at birth. In some species the fetal and maternal layers part cleanly, no maternal tissue is shed, and the description nondeciduate is appropriate. When implantation is interstitial, considerable maternal loss may be expected; the human placenta is of this deciduate type. Minor loss of uterine tissue occurs in an intermediate semideciduate type found in ruminants (Table 5–2).
The histological system appears to define different degrees of placental permeability. While this is broadly true, histological differences provide an incomplete explanation of variations in permeability. It must be borne in mind that the barrier may not be exactly as implied by the description; moreover, the placenta evolves and changes in structure during pregnancy, and significant regional differences may exist side by side. Freely diffusible molecules cross from one circulation to the other according to their relative concentrations, and in this respect the human hemochorial placenta certainly allows more rapid passage than the “thicker” epitheliochorial placenta of the larger domestic species. The transport of larger molecules depends on other factors, including specialized unidirectional mechanisms.
Differences in the barrier to the passage of immunoglobulin G (IgG) are of particular veterinary significance. In some species a mechanism exists for the transfer to the fetus of maternal antibodies produced in response to infection; these may confer some immediate protection on the newborn, possibly delivered into an environment contaminated by the same infective agent. This prenatally acquired immunity is denied to offspring of species (including horses and farm animals) with epitheliochorial placentas; their neonates rely on colostrum, the milk first produced as the source of antibodies that may provide temporary protection.
Fetal antigens, present in plasma or borne by blood cells, may leak into the maternal bloodstream with potentially damaging consequences. The classic illustration is furnished by the hemolytic disease (erythroblastosis fetalis) of human infants; this condition develops in a second or later child confronted by antibody produced by a Rhesus-negative mother in reaction to the incompatibility of her Rhesus factor with that of a previous Rhesus-positive child. Antibody production by the mother develops so slowly that the child (usually the first) provoking the response generally escapes serious harm. Similar conditions occur in other species, including horses and pigs, but damage to their offspring can be prevented by denying them access to colostrum, which contains the relevant antibody.
The endocrine functions of the placenta are both complex and lacking in uniformity among species, even between those that are closely related. The horse is of unusual interest in this context since equine chorionic gonadotropin is produced from structures unique to Equidae: the endometrial cups (Figure 5–71 and p. 576).
Figure 5–71 Endometrial cups (mare) during early pregnancy. These cups are responsible for the production of pregnant mare’s serum gonadotropin (PMSG).
Elevated levels of steroid hormones in blood and urine provide the basis for diagnostic tests for pregnancy in women; even the do-it-yourself tests now widely marketed are generally reliable, if not infallible. Tests of comparable reliability, simplicity, and economy are not yet available for domestic species for which reliance is still largely placed on clinical procedures, including ultrasonography.
The placenta is one (though not the only) source of other hormones relevant to pregnancy. Lactogen, a hormone related to growth hormone, acts with other hormones to develop the mammary glands for the approaching lactation, while relaxin, which is also secreted by the corpus luteum, helps prepare the reproductive tract and pelvic parietes for parturition (p. 214); later, acting in synergy with oxytocin, it stimulates the expulsive activity of the myometrium.
Although prostaglandin is not a product of the placenta, it may be convenient to take notice of it here. This hormone is manufactured by the endometrium of the “empty” uterus; production is delayed for 2 weeks (or so) after the corpus luteum first forms. It leaves the uterus in the uterine vein, and in some species, including ruminants, it reaches the ipsilateral ovary after countercurrent transfer to the ovarian artery; in others, for example, the horse, in which contact between artery and vein is less close, it reaches the ovaries only after diffusion through the general circulation. Within the ovary, prostaglandin promotes luteolysis (regression of the corpus luteum) with consequent decline and eventual cessation of progesterone secretion and release. The production of prostaglandin is stimulated by oxytocin, but in pregnancy the conceptus produces a factor that blocks endometrial receptivity to oxytocin, thus indirectly protecting the corpus luteum, whose integrity is now required.
Before concluding the account of the fetal membranes and placenta, brief attention may be given to the fluids contained within the amniotic and allantoic cavities. These fluids, whose main claim to notice sometimes appears to be their rather dramatic release at the time of birth—the “breaking of the waters”—actually have rather important functions to perform throughout gestation, and at certain stages they account for a very considerable fraction of the total content of the gravid uterus. The fluid within the amniotic cavity immediately surrounds and supports the embryo or fetus, cushioning it against compression and protecting it against chance blows to the mother’s abdomen. This protection is most required by the young embryo whose skeleton is still largely unformed and whose external covering—hardly yet to be called skin—is delicate and vulnerable to trauma. Later, when these structures are better developed, the amniotic fluid tends to be reduced in amount (see Figure 5–65), relative to the size of the fetus in large species and in absolute terms in small mammals, although the precise amount is always rather variable. At its maximum, it measures about 3 to 5 L in cattle and perhaps a little more in horses; in pigs it varies around 100 mL, and it is about 10 to 30 mL in dogs and cats. It is often assumed that amniotic fluid is more or less stagnant, but there is a brisk turnover: production and resorption are roughly matched in the short term. In early stages, the fluid is a dialysate from vessels of the embryonic skin and amnion; later, once rupture of the urogenital membrane has opened a passage from the bladder, it consists largely of urine and, as more is added, the fluid already present is reduced by being swallowed. Deficient and excessive amounts of this fluid (oligohydramnios and polyhydramnios, respectively) are possible complications of pregnancy, the former often indicating anomalous development of the kidneys, the latter potentially open to correction by the addition of a “sweetener” to encourage deglutition. This fluid is not normally a significant contributor to that present in the respiratory passages of the fetus and newborn, although this is sometimes suggested. Being slightly mucoid, amniotic fluid has additional value as a lubricant of the birth canal at parturition.
The allantoic cavity is large in all domestic species, but the human allantois fails to expand and is soon reduced to a negligible vestige. It is possibly the consequent lack of medical interest that explains the relative paucity of information concerning the formation, turnover, and role of allantoic fluid. The allantoic cavity does, of course, receive urine through the urachus before the urethral route is established, and this helps maintain the osmotic pressure of the fetal plasma at a level that prevents fluid loss to the maternal bloodstream. A second function may be to maintain sufficient radial pressure to hold the chorion tight against the endometrium in those species in which the placental attachment is less firm. There is rather more allantoic than amniotic fluid in the large species and about the same amount in dogs and cats. Although there is about 100 mL in the pig at midterm, the quantity is reduced to very little at full term. However, the quantities are rather variable in all species.
PARTURITION AND THE PUERPERAL PERIOD: THE NEONATE
Parturition is mainly initiated by the fetus, although the mother is not without all influence; mares, for example, tend to give birth when conditions in the stable are quiet and settled. The endocrine control of birth is complicated and beyond the scope of this book, but certain preparatory changes in the tissues may be mentioned. These take some time to develop, affect many structures, and largely comprise an increase in their water content and loosening of the larger collagen accumulations. The most familiar effect is the insinking of the tailhead of cows to the side as parturition impends. Similar but concealed changes soften the caudal reproductive tract, including, most significantly, the cervix. In some species there is considerable weakening of the pelvic symphysis, but articular changes in domestic animals are limited to some loosening of the sacroiliac joints. After parturition the reproductive organs tend to return toward their former condition, although the restoration after the first pregnancy is never complete. The uterine muscle contracts directly after delivery, and this organ loses much of the weight it gained during pregnancy within a few days.
Before this chapter is concluded, a few sentences may be devoted to the status of the newborn, which exhibits interspecific differences that are both striking and important. Neonates of so-called precocial species possess a remarkable ability to fend for themselves more or less at once (Figure 5–72), while those of altricial species are initially much more reliant on maternal care and the warmth and protection of a nest (Figure 5–73). The young of the ungulate orders, both perissodactyls and artiodactyls, are generally precocial; those of carnivores and primates, including human infants, are predominately less developed. Young rodents are divided between the two categories; those like rats (myomorphs) are born naked, unable to maintain body temperature independently, barely capable of struggling to reach the dam’s teats, and have their eyelids joined and external ear canals closed by epithelial fusion; in contrast, guinea pigs and their close relatives (caviomorphs) are born fully haired, mobile, equipped with vision and hearing, and have the ability to seek and ingest solid food within hours of being born (although they may take milk during the first 2 or 3 weeks). The differences among domestic species are significant if less extreme. Foals, like most newborn ungulates, are able to stand and attend their mothers almost at once; their skeletons are well developed, and most secondary ossification centers are not only present but also well advanced in modeling toward their adult form. Relatively efficient locomotor coordination allows them to follow the herd or flock within a short time. Kittens and puppies, on the other hand, have skeletons that are less mature, and many ossification centers have yet to make their appearance (Figure 5–74); the forelimb musculature is sufficiently developed and controlled to enable them to scramble toward the teats, but that of the hindlimbs is less competent and contributes little to this progress. The development of the sense organs is somewhat retarded, and the eyelids do not part until the tenth day or shortly thereafter. These differences in neonatal status are gradually “ironed out” and most mammals—ourselves excluded—show comparable maturity by the end of the usual lactation period.


Figure 5–72 Developmental status shortly after birth. A, Neonatal foal with mother (the mare has yet to discharge the fetal membranes [after birth]). B, Newborn guinea pigs, which are born in a more developed state.