35 The Pelvis and the Reproductive Organs of the Pig
The thick layer of subcutaneous fat almost completely hides the junction between abdomen and pelvis, which is indicated only by a slight indentation above the fold of the flank. The landmarks of the pelvic skeleton are not immediately visible, but the positions of the coxal and ischial tubers are readily discoverable on palpation, which reveals the small size of the girdle in relation to the overall dimensions of the hindquarters. The body and tuber of the ischium unite in very few pigs, and while the tuber remains unfused, there is a risk of its becoming detached by the pull of the powerful hamstring muscles that arise from it. Young sows are most commonly affected and are unable to rise when this happens; the condition is very painful, and there is no cure but slaughter.
Interest in the bony pelvis is inevitably concentrated on aspects relevant to parturition. From a lateral view, the pelvic floor and the iliac shaft meet at an angle that approaches 180° (Figure 35–1). This brings the pelvic inlet, which is large and oval, into a plane that faces almost directly ventrally into the abdomen; it also carries the “vertical diameter” caudally, to intersect the part of the sacrum composed of bones not yet fused and therefore allowed some mobility. The pelvic floor slopes caudoventrally. The pelvic canal is a little higher than it is wide (Figure 35–1); the spines of the ischia are bent slightly inward to narrow the passage. If the birth canal is actually somewhat less than the skeleton suggests on account of soft tissue structures also occupying space, some compensation is to be found in the slackening of the sacrosciatic ligament, which completes the lateral wall of the pelvic cavity, and in the slackening of the joints of the girdle about the time of farrowing.

Figure 35–1 A, Median section of the sow’s pelvis. B, Transverse section of the pelvis near the level of the vertical diameter. 1, Coxal tuber; 2, ischial spine; 3, ischial tuber; 4, obturator foramen; 5, pelvic symphysis; 6, S4; 7, promontory; 8, acetabulum; 9, sacrosciatic ligament; 10, angle between pelvic floor and conjugata; 11, plane of pelvic floor; 12, conjugata; 13, vertical diameter; 14, transverse diameter; 15, pelvic axis.
THE RECTUM AND ANUS
The shortness of its mesentery is the only additional point that need be made concerning the rectum. Congenital absence of the anus (atresia ani) once was of frequent occurrence; perhaps surprisingly, it may allow afflicted piglets to survive for 3 or 4 weeks without treatment. If the rectum ends blindly at no great distance from the skin, a passage may be created by simple surgery.
Prolapse of the rectum, encountered in somewhat older pigs, requires more sophisticated surgery, especially if the everted bowel has been mutilated by pen mates, as so often happens. Effective surgery requires some knowledge of the muscles associated with the anus. These are more or less as in other species (see Figure 3–47): bundling together of the longitudinal muscle of the rectum creates the rectococcygeus, and thickening of the circular muscle creates the internal anal sphincter. The striated external sphincter presents no features of note; the levator ani runs between the sacrosciatic ligament and the lateral aspect of the anal canal; and the retractor penis (or clitoridis) passes lateral to the rectum and with its fellow forms a sling below the rectum before continuing to the penis (or clitoris).
THE BLADDER AND FEMALE URETHRA
The empty bladder is a small, firm ovoid placed over the pubic pecten (Figure 35–4/5,5′). As it fills, it extends over the abdominal floor, perhaps reaching as far forward as the umbilicus. It assumes a spherical form when grossly distended. The bladder is wholly covered in peritoneum, which continues into paired recesses below the urethra. A small suburethral diverticulum (Figure 35–4/6), associated with the opening of the urethra into the vestibule, may interfere with catheterization of the bladder.
THE FEMALE REPRODUCTIVE ORGANS
THE OVARY AND UTERINE TUBE
The ovaries, about 5 cm long, are distinguished by the many follicles and corpora lutea that project from the entire surface (Figure 35–2). They are usually found hidden among the intestines, slightly ventrolateral to the pelvic inlet. The relatively long mesovaria commonly allow both ovaries to lie against the one flank, and consequently, both may be removed through a single incision.
The uterine tube (Figure 35–3/4) is about 20 cm long and is carried in the wall of the cone-shaped ovarian bursa; it meets the horn of the uterus at a tapering junction. Obstruction of the tube (the origin of hydrosalpinx) can cause infertility in sows.
THE UTERUS
The sow’s uterus is distinguished by its short body and long, intestiniform horns (Figure 35–3/5,8). The body, about 5 cm long, is shorter than first appears because the immediately adjacent parts of the horns lie side by side within common investments (as in ruminants). In the nongravid state each horn measures about 1 m, and as it is suspended by a fairly generous broad ligament (Figure 35–3/6), it enjoys considerable freedom of position, relations, and arrangement, although it fails to reach the abdominal floor. Some parts become mingled with coils of small intestine and can be confused with these. The cervix, which lies half within the abdomen and half within the pelvis, is peculiar for its length (ca. 25 cm) and for the rows of mucosal prominences (Figure 35–3/11) that project into the lumen, interdigitate, and thus close the canal, except at estrus and parturition. Its junctions with the uterine body and the vagina taper and are ill defined.
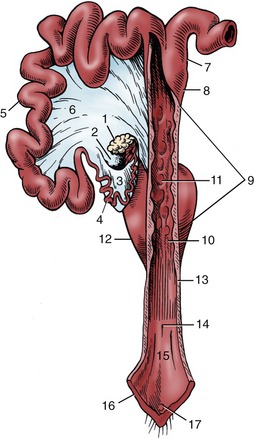
Figure 35–3 The reproductive tract of the sow opened dorsally in part; the right uterine horn and ovary are not shown. 1, Left ovary; 2, ovarian bursa; 3, mesosalpinx; 4, uterine tube; 5, uterine horn; 6, broad ligament; 7, parallel segments of uterine horns; 8, body of uterus; 9, cervix; 10, external uterine orifice; 11, mucosal prominences; 12, bladder; 13, vagina; 14, external urethral orifice; 15, vestibule; 16, vulva; 17, glans of clitoris.
THE VAGINA, VESTIBULE, AND VULVA
The vagina is unremarkable, and the vestibule is relatively long. The conical vulva slopes so that it faces rather obliquely upward (Figure 35–4, A/7); it is sometimes so upturned that the cleft is inaccessible to the boar. Gilts with an infantile vulva are common and undesirable as breeding stock because the defect hints at poor development of the reproductive organs with a consequently greater risk of infertility. The clitoris is normally barely visible (Figure 35–3/17). Clitoral enlargement is common and is associated with intersexuality (female pseudohermaphroditism).
The uterine artery, the principal supply to the uterus, is supplemented by branches of the ovarian and vaginal arteries (Figure 35–5/2,7). The ovarian vein, which drains most of the uterus in addition to the ovary, forms a plexus around the uterine and ovarian arteries that facilitates the transfer of luteolytic prostaglandins.
FUNCTIONAL ASPECTS
Gilts attain puberty around 6 months. The species is polyestrus: the cycle repeats at intervals of about 21 days. Fertilization takes place in the ampullae, where the conceptuses are detained for a few days before being admitted to the uterus. Cleavage continues there, creating blastocysts that are initially spherical and randomly placed. By the end of 2 weeks they have become filamentous and greatly lengthened—up to 60 cm—and have adopted permanent, regularly spaced stations that make full use of both horns, which is an arrangement that may have required some conceptuses to migrate from one horn to the other. The conception rate is high, but so also is prenatal mortality—40% or more. The placenta is of the diffuse epitheliochorial type. Antibody transfer does not occur in utero, and the newborn is dependent on the ingestion of colostrum for its initial immunological protection.
During pregnancy, the horns increase greatly in diameter, and their length may double. Growth of the tissues within the broad ligaments allows the horns to sink into the ventral half of the abdomen, where they push the intestines craniodorsally and make contact with the stomach and liver; they carry the ovaries with them, taking them out of reach of a hand within the rectum. Confirmation of pregnancy at this stage is provided by the firmness of the cervix and, more reliably, by the characteristic fremitus of the enlarged uterine artery. An alternative, less troublesome method of pregnancy diagnosis is available in ultrasonography, with the use of either a transabdominal or transrectal approach (Figure 35–4, B-C).
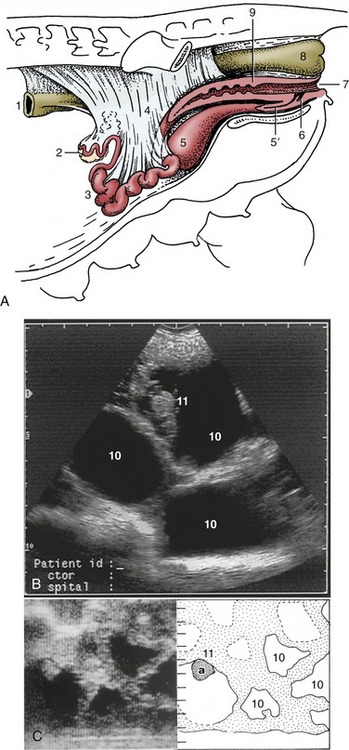
Figure 35–4 A, The reproductive organs of the sow in situ. (The presence of the intestines in the intact animal causes the ovaries and uterine horns to lie more dorsally than shown here.) Transrectal (B) and transabdominal (C) ultrasonographic images of 30-day gravid porcine uteruses. (Scales in centimeters.) 1, Descending colon; 2, ovary; 3, uterine horns; 4, broad ligament; 5, bladder; 5′, urethra; 6, suburethral diverticulum; 7, vulva; 8, rectum; 9, cervix; 10, allantoic fluid-filled spaces; 11, (a), embryo.

Figure 35–5 The principal arteries supplying the left side of the female reproductive tract, schematic. 1, Aorta; 2, ovarian a. with cranial uterine branch; 3, internal iliac a.; 4, external iliac a. continued by femoral into left thigh; 5, umbilical a.; 6, left uterine a. crossing medial surface of external iliac; 7, vaginal a. with caudal uterine branch; 8, left uterine horn; 9, bladder; 10, urethra; 11, vagina; 12, rectum.
Gestation lasts 114 days (on average), and farrowing is preceded by the usual relaxation of the joints and tissues of the pelvic region, although this may not be apparent to an observer. The considerable number in most litters, distributed between the two horns, suggests that should fetuses from both sides arrive together at the entrance to the body they might have to jostle for priority of passage. The risk of collision is prevented by the arrangement of the circular muscle of the uterus, which is able to close the exit from one horn while simultaneously securing maximal enlargement of the exit from the other. The mechanism is so effective that a hand exploring the interior of the uterus in these circumstances is unable to locate the entry to the horn that is temporarily shut off. The arrangement does not operate at all times; both horns open freely into the body of the atonic uterus, which allows fetuses to be transferred from one horn to the other at cesarean section.
After the first piglets have been expelled, those remaining may travel more freely possibly because they are now able to move through the lubricated tube provided by a succession of already-vacated embryonic membrane sacs and because the umbilical cords provide rather loose tethers. They may shift quite far and even slip past their neighbors while still attached to their placentas.
Some criteria that may be used for estimating the age of pig fetuses are provided in Table 35–1.
Table 35–1 Guide to the Aging of Pig Fetuses
| Weeks | Crown-Rump Length (cm) | External Features |
|---|---|---|
| 2.5 | ≈1 | Limb buds forming |
| 4 | ≈2 | Tactile hair follicles appear; mammary primordial present |
| 5 | ≈3.5 | Palate fused; facial clefts closed |
| 6 | ≈6.5 | Prepuce and scrotum, or labia and clitoris present |
| 7 | ≈9 | Eyelids fused; intestines returned to abdomen |
| 13 | ≈24 | Eyelids separated |
| Full term | On average 114 days |
From Evans HE, Sack WO: Prenatal development of domestic and laboratory animals. Growth curves, external features, and selected references. Anat Histol Embryol 2:11–45, 1973.
THE MALE REPRODUCTIVE ORGANS
THE SCROTUM AND TESTES
The scrotum is perineal in position. The tail of the epididymis and the less salient associated pole of the testis point dorsocaudally by the anus and are readily palpable. The free border of the testis faces caudoventrally, and the attached border is closely applied to the surface of the thigh (Figure 35–6).

Figure 35–6 Schema of the boar’s reproductive organs. 1, Scrotum; 2, left testis; 3, tail of epididymis; 4, deferent duct; 5, bladder; 6, rudimentary teat; 7, vesicular gland covering the small body of the prostate; 8, bulbourethral gland; 9, prepuce; 10, penis; 11, preputial diverticulum; 12, right hip bone.
It has been the established custom to castrate male pigs when they are 2 to 4 days old in the belief that this prevents the development of the taint that characterizes the flesh of boars. It is now increasingly appreciated, in some countries at least, that the taint does not appear until after the usual age at slaughter and that castration is therefore pointless. Both the open and closed methods of castration are used with young pigs. In the former, the tunica vaginalis is incised, the ligament joining it to the epididymis divided, and the cord severed. This is the method employed with old boars. In the closed method (Figure 35–7, B), the scrotum is opened, the tunica vaginalis is left intact but freed from attachments, and the cord is transected close to the external inguinal opening. The situation of the scrotum explains the unusual length of the cord.
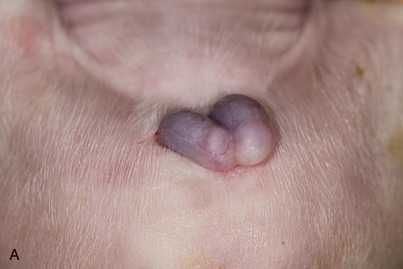

Figure 35–7 A, Open castration method of a newborn piglet. (Note: The parietal layer of vaginal tunic is still intact.) B, The closed castration method in a 5-week-old piglet (also performed in case of an inguinal hernia).
In pigs, descent of the testis commences about the 60th day of gestation, and regression of the extraabdominal gubernaculum creates the conditions in which the testis is able to leave the inguinal canal by approximately the 90th day. After a period of uncertainty, when the testis may move back and forth between the canal and the groin, a permanent position in the scrotum is adopted by full term. Abnormalities of gubernacular development and regression are common. Both excessive swelling and delayed regression may widen the canal abnormally, allowing a loop of intestine to slip into the vaginal cavity and thus creating an indirect inguinal or, should it reach so far, scrotal hernia. Surgical correction of this defect is generally combined with castration by the closed method. (The inguinal hernias occasionally seen in young gilts are associated with abnormal genital tracts that resemble those of bovine freemartins.)
THE PELVIC REPRODUCTIVE ORGANS
The deferent ducts take their usual courses to penetrate the body of the prostate before opening into the urethra on the summit of a low papilla (Figure 35–8/5). They do not expand to form ampullae and in the last part of their courses are covered by the very large vesicular glands that open beside them (Figure 35–8/7). Only small parts of these glands are contained within the pelvic cavity; the bulk protrudes into the abdomen, beyond the neck of the bladder (Figure 35–6/7), and is enclosed within the genital folds. In addition to a modest irregular body, the prostate (Figure 35–8/8) possesses a large disseminate part spread within the wall of the pelvic urethra.
The bulbourethral glands are remarkable for their shape and size. They lie dorsolateral to the pelvic urethra and are sufficiently long to touch the vesicular glands (Figure 35–8, A/11 and Figure 35–6/8). Each drains through a dilated sometimes duplicated duct that opens onto the thickening that separates a dorsal diverticulum from the lumen of the urethra where this bends around the ischial arch. The glands are covered by the bulboglandularis muscles, whose contraction secures their evacuation (Figure 35–8, A/12). The caudal ends of the glands may be palpated per rectum. The ability to touch the urethra between them is diagnostic of the castrate (Figure 35–8, B); inability to do this in the absence of palpable testes suggests cryptorchidism.
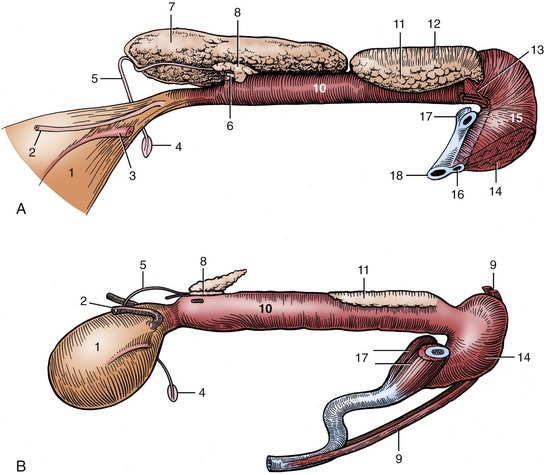
Figure 35–8 Pelvic urethra and associated organs of an 8-month-old boar (A) and a 6-month-old castrate (B), left lateral views. The left vesicular gland has been removed to expose the prostate. 1, Bladder; 2, left ureter; 3, left umbilical artery; 4, right vaginal ring; 5, right deferent duct; 6, left deferent duct, cut at prostate; 7, right vesicular gland; 8, body of prostate; 9, retractor penis; 10, pelvic urethra, surrounded by urethralis; 11, left bulbourethral gland; 12, bulboglandularis covering dorsal half of bulbourethral gland; 13, excretory duct of left bulbourethral gland; 14, bulbospongiosus; 15, bulb of penis; 16, urethra and corpus spongiosum; 17, right and left crura, cut; 18, corpus cavernosum.
THE PENIS AND PREPUCE
The penis, broadly similar to that of the bull, is relatively thin, exhibits a prescrotal sigmoid flexure, and is about 60 cm long (when flaccid) (Figure 35–6/10). A thick tunica albuginea encloses the corpus cavernosum (Figure 35–9/1). The corpus spongiosum lies first on the ventral surface of the corpus cavernosum, but more distally it is recessed in a deep groove that brings it to a central position (Figure 35–9, B/6). Apart from the sigmoid flexure, the shaft is twisted on its longitudinal axis a full turn counterclockwise (when viewed from behind). The direction of the twist is the same as that of the spiral of the apex (Figure 35–9, C).
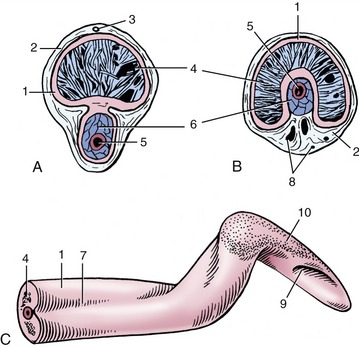
Figure 35–9 Transverse sections of the penis. A, Proximal to the sigmoid flexure. B, Distal to the sigmoid flexure. C, Free end of penis. 1, Tunica albuginea; 2, connective tissue surrounding penis; 3, dorsal artery of penis; 4, corpus cavernosum; 5, urethra; 6, corpus spongiosum; 7, urethral groove; 8, blood vessels; 9, external urethral orifice; 10, thin glans penis.
The relatively long prepuce houses the free part of the penis in its narrow caudal half. The wider cranial half communicates with a dorsal diverticulum, a pouch containing an evil-smelling fluid consisting of cell debris soaked in urine (Figure 35–6/9,11). The diverticulum is covered by the cranial preputial muscle, which empties it before copulation (Figure 35–10, A/1). The fluid contains a pheromone that encourages the sow to assume the immobile mating stance. If the contents of the diverticulum collect excessively, the appearance may mimic umbilical hernia. An infected diverticulum may be opened and drained through a dorsolateral incision that inevitably includes the muscle. The diverticulum is sometimes removed in boars used for artificial insemination so that contamination of the semen is reduced. Although the tip of the penis occasionally becomes entrapped in the diverticulum, it is readily freed.
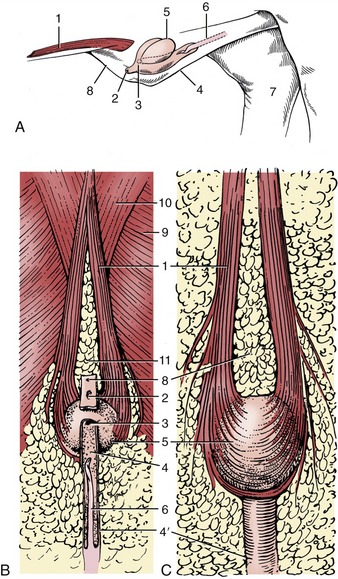
Figure 35–10 Prepuce and preputial diverticulum. A, In situ, schematic, craniolateral view. B, Ventral view. C, Dorsal view. 1, Cranial preputial muscle, in A cut at both ends; 2, preputial orifice; 3, orifice between prepuce and diverticulum; 4, 4′, wide cranial and narrow caudal parts of preputial cavity; 5, preputial diverticulum; 6, penis; 7, medial surface of right hock; 8, umbilicus; 9, cutaneous trunci; 10, pectoralis profundus; 11, preputial fat.
FUNCTIONAL ASPECTS
The size of the accessory glands is related to the large volume of the ejaculate, at least 200 mL. Despite their great size, the vesicular and bulbourethral glands together contribute rather less than half the seminal fluid; the bulk is provided by the prostate and urethral glands.
During erection the blood pressure in the cavernous spaces rises sharply, straightening the sigmoid flexure and increasing the length of the penis by about a quarter. The single longitudinal twist of the shaft increases to six turns, while the corkscrew spiral of the free part becomes much more pronounced. During coitus, a slow process that may last for as long as 30 minutes, the boar is said to “soak” because of the absence of obvious activity on his part. However, forward and backward twisting movements of the penis do occur under the influence of the retractor muscle. There is no substance to the persistent belief that the prominences of the cervical mucosa form a canal with a left-hand thread matching that of the spiraled end of the penis. The end of the penis is considered to almost enter the uterus.
THE LYMPHATIC STRUCTURES OF THE PELVIS
The medial iliac lymph nodes grouped about the terminal branches of the aorta have been described on page 770 and in Figure 34–12. They are continued into the pelvic cavity by sacral nodes below the sacrum and anorectal nodes below the tailhead. The latter nodes drain the rectum, anus, and tail; their efferents pass to the medial iliac nodes. Ischial nodes receiving lymph from the perineum, caudal thigh, and popliteal nodes and gluteal nodes draining the gluteal region lie lateral to the sacrosciatic ligament. Both sets also drain to the medial iliac nodes.
THE ANATOMY OF RECTAL EXPLORATION
Rectal palpation is possible in sows weighing 150 kg or more without great difficulty or ill effects on the animal. It is generally found that the small diameter and short suspension of the descending colon are greater impediments to these examinations than constriction of the pelvic canal. With ample lubrication and sufficient cooperation, the arm can be introduced almost to the elbow; however, because the forearm is solidly wedged in the pelvic canal, the scope for exploration depends entirely on the length of and the mobility that may be exercised by the hand. The procedure allows examination of the pelvic inlet and bladder and, more importantly, the ovaries, cervix, and uterine artery for pregnancy diagnosis. The right kidney and the spiral colon, recognized through its coarse, granular content, may also be identified; the colon prevents access to the left kidney. Examination of the more confined pelvic cavity of boars is not feasible; the intrusion causes obvious pain.
