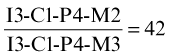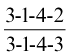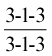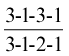3 The Digestive Apparatus
The digestive apparatus* comprises the organs concerned with the reception, mechanical reduction, chemical digestion, and absorption of food and drink and with the elimination of unabsorbed residues. It consists of the alimentary tract, extending from the mouth to the anus, and certain glands—the salivary glands, pancreas, and liver—that drain by ducts that open into the tract. The parts of the alimentary tract in proper sequence are the mouth, pharynx, esophagus, stomach, small intestine, and large intestine (Figure 3–1). Some of the digestive organs have other sometimes just as vital functions that are quite distinct from the processing of food intake.
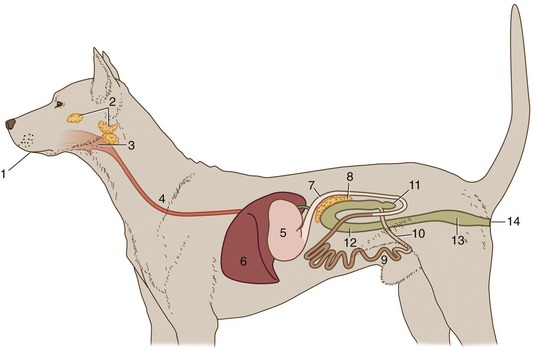
Figure 3–1 Schematic representation of the digestive apparatus in the dog. 1, Mouth; 2, salivary glands; 3, pharynx; 4, esophagus; 5, stomach; 6, liver; 7, duodenum; 8, pancreas; 9, jejunum; 10, ileum; 11, cecum; 12, colon; 13, rectum; 14, anus.
These organs are primarily formed of endoderm, the germ layer that lines the yolk sac, although the muscle and connective tissues that support the epithelium are of mesodermal origin, as elsewhere.
The separation of the digestive tube from the yolk sac is achieved in the folding process that converts the flat embryonic disk into a more or less cylindrical body. The folding is the result of the disk growing more rapidly than the extraembryonic tissue with which it is continuous; as a consequence of the constraint exerted at the periphery, the disk buckles upward while its edges are folded or rolled under. Because growth is most rapid along the longitudinal axis, the folding is more pronounced at the head and tail extremities than along the lateral margins. This ensures that the part of the yolk sac taken into the body presents two horns extending cranially and caudally from a middle region that retains free communication with the larger part of the yolk sac remaining outside the embryo. The included part of the yolk sac is known as the gut, and its three regions are the foregut, midgut, and hindgut. The midgut joins the other regions through tapering parts known as the cranial and caudal intestinal portals (Figure 3–2).
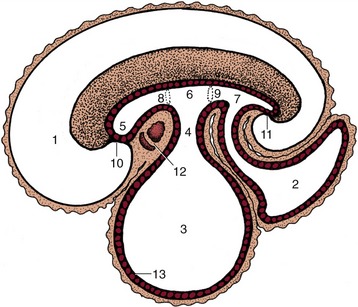
Figure 3–2 Sagittal section of an early embryo. Part of the yolk sac is taken into the body in the folding process. 1, Amniotic cavity; 2, allantoic cavity; 3, yolk sac; 4, stalk of yolk sac; 5, foregut; 6, midgut; 7, hindgut; 8, cranial intestinal portal; 9, caudal intestinal portal; 10, oral plate; 11, cloacal plate; 12, heart and pericardial cavity; 13, endoderm.
THE MOUTH
The term mouth (os, gen. oris) designates not only the cavity and its walls but also the accessory structures that project (teeth, tongue) and drain (salivary glands) into it. The mouth has as its main functions the prehension, mastication, and insalivation of food. It may also play a role in aggression and defense, while in ourselves it is important in the formulation of the sounds of speech. In most species it functions as an airway when flow through the nose is impaired.
The mouth (oral) cavity is entered between the lips and continues into the pharynx (Figure 3–3) through a caudal narrowing at the level of the palatoglossal arches (see further on). It is divided by the teeth and margins of the jaws into an outer vestibule, bounded by the lips and cheeks externally, and the central mouth cavity proper. When the mouth is closed, these divisions communicate through gaps behind and between the teeth. The vestibule extends caudally toward the ramus of the mandible and the masseter muscle. The proportion of its walls formed by the lips varies with feeding habits; a wide gape is necessary in species that feed greedily or use their teeth to seize prey or in fight, whereas a smaller opening suffices in most herbivores and rodents.
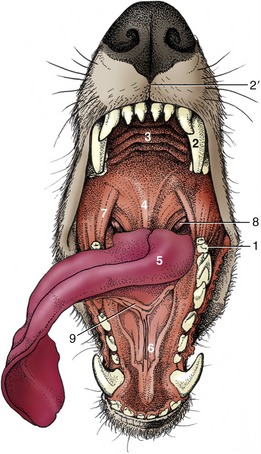
Figure 3–3 General view of the oral cavity of the dog. 1, Vestibule; 2, canine tooth; 2′, philtrum; 3, hard palate; 4, soft palate; 5, tongue; 6, sublingual caruncle; 7, palatoglossal arch; 8, palatine tonsil; 9, frenulum.
Diet and feeding habits also determine the form of the lips (labia oris). In some species, such as the horse, the lips are employed in collecting food and introducing it to the mouth; for this purpose they must be both sensitive and mobile. When other parts are more important in prehension the lips can be less mobile and reduced in size (e.g., cat) or thickened and insensitive (e.g., ox). The lips of the dog are extensive but thin, and although they can be drawn back from the teeth, they are not capable of other purposeful movements. Lip posture is an important factor in communication in this species and can signal aggressive intent or submission. In newborn animals the lips form the seal about the teat that is necessary for successful sucking.
The lips are composed of skin, an intermediate layer of muscle, tendon, and glands, and the oral mucosa. The skin and mucosa usually meet along the margin of the lips, though the boundary can be displaced in either direction. The muscles that make up the greater part of the lips belong to the mimetic musculature, which is the field of the facial nerve. They include an orbicular muscle encircling the opening and, with some species variation, others that raise, depress, and retract the lips. Small salivary glands are scattered among the muscle bundles below the mucosa, especially toward the angles (commissures) where the two lips meet.
There is rarely anything remarkable in the arrangement of the lower lip. In the dog it is rather loose but fastened to the lower jaw at the level of the canine tooth and has a thin, serrated margin. Modifications of the upper lip are more frequent. Sometimes a median naked area is present continuous with the modified skin around the nostrils. The extensive moist and glandular nasolabial plate of the ox and the rostral disk of the pig are good examples of this. The area of modified skin is often much narrower and may be divided by a median groove (philtrum) as in the dog. Dog breeders refer to this modified region as the “nose leather” (see Figure 3–3). In man and in the horse a hairy integument extends across the entire upper lip.
The cheeks (buccae), which tend to be most capacious in herbivores, have a similar structure. The principal support is the buccinator muscle, which has the important function of returning to the central cavity any food that has escaped into the vestibule. There are additional salivary glands, sometimes aggregated in quite large masses: the zygomatic gland of the dog (see Figure 3–12/8), concealed below the zygomatic arch, has its origin in this way. The buccal mucosa must be sufficiently loose to allow the occasional maximal opening of the mouth while avoiding large folds that would at other times invite injury from the teeth (Figure 3–4); it tends, therefore, to be tightly anchored in some places. In ruminants, whose food may be dry and rough, additional protection is required; because a very thick and much cornified epithelium would limit flexibility, protection is provided by large, closely spaced, pointed papillae (see Figure 3–7). A small papilla (in ourselves easily found with the tongue tip) carries the opening of the duct of the parotid gland.
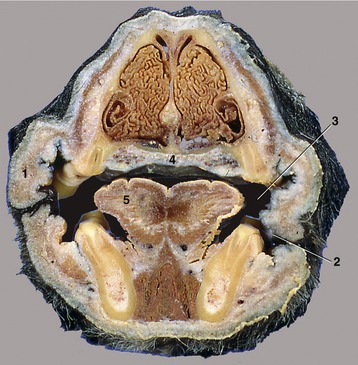
Figure 3–4 Transverse section of the head of the dog at the level of P2. 1, Cheek (with buccal folds); 2, vestibule; 3, oral cavity proper; 4, hard palate (with venous plexus); 5, tongue.
Diverticula of the oral vestibule (cheek pouches) occur in certain rodents and monkeys. These pouches have a storage function and enable the animal to harvest its food rapidly, stowing it away for later mastication. They attain a considerable size in hamsters, reaching well onto the thorax; when developed to this degree, the pouches have their own supporting musculature.
The cavity within the dental arcades—the mouth cavity proper—is roofed by the palate; bounded laterally by the teeth, gums, and margins of the jaws; and floored by the tongue and the small area of mucosa left uncovered by the tongue. Most of the walls are rigid, and when the mouth is closed the size of the cavity can be altered only by raising or lowering the tongue and floor.
The larger, rostral part of the roof is based on a bony shelf formed of the palatine processes of the incisive, maxillary, and palatine bones and is known as the hard palate (palatum durum). This is continued caudally, without external demarcation, by the soft palate, in which a connective tissue aponeurosis replaces the bone.
The hard palate is usually flat (though vaulted in ourselves) and is covered by a thick mucosa fashioned into a series of more or less transverse ridges (rugae), which may guide the food backward (Figure 3–5). In general, they are most prominent and their covering epithelium most heavily keratinized in herbivores. A small median swelling, the incisive papilla, is commonly found behind the incisor teeth, flanked by the orifices of small (incisive) ducts that perforate the palate. These ducts branch and lead to the nasal cavity and to the vomeronasal organ (Figure 3–6). They convey small amounts of the fluid from the mouth for appraisal by the olfactory mucosa of the vomeronasal organ (p. 352).
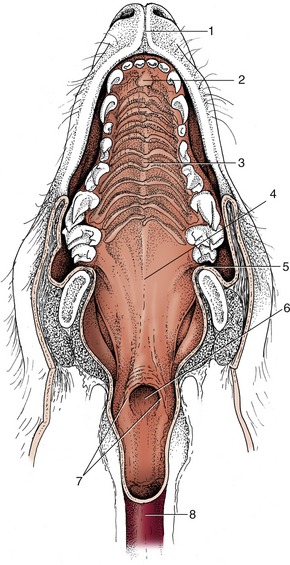
Figure 3–5 The hard and soft palate of the dog. 1, Philtrum; 2, incisive papilla; 3, hard palate with rugae; 4, soft palate; 5, palatoglossal arch; 6, intrapharyngeal ostium; 7, palatopharyngeal arches; 8, esophagus.
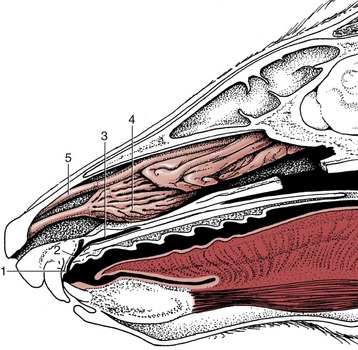
Figure 3–6 Paramedian section of the rostral part of the head of the dog. The plane of section fails to demonstrate the opening of the incisive duct into the nasal cavity. 1, Incisive papilla; 2, incisive duct; 3, vomeronasal organ; 4, ventral nasal concha; 5, dorsal nasal concha.
A striking peculiarity in ruminants is the dental pad, a tough but yielding cushion in the position generally occupied by upper incisor teeth (lacking in these animals); the pad acts as a counterpart to the lower incisors in grazing (Figure 3–7). A dense, richly vascularized tissue beneath the palatine epithelium functions both as the lamina propria of the mucosa and as the periosteum of the bone, attaching so tightly that not even the most vigorous mastication shifts it. Peripherally, the hard palate blends with the gums, the rather insensitive mucosa along the alveolar margins of the jaws.
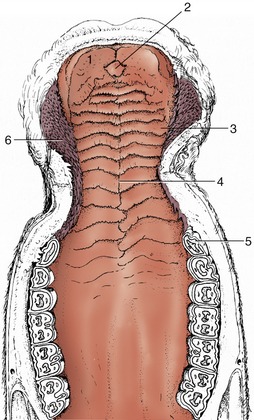
Figure 3–7 The hard palate of a cow. 1, Dental pad; 2, incisive papilla; 3, rugae of hard palate; 4, palatine raphe; 5, P2; 6, buccal papillae.
The soft palate is described with the pharynx (p. 119).
THE TONGUE
The tongue (lingua) occupies the greater part of the oral cavity but also extends into the oropharynx (Figure 3–8). It has an attached root and body and a free apex and is a highly muscular organ capable of both vigorous and precise movements, as in prehension, lapping, grooming, and manipulating the food within the mouth on the one hand and speech articulation on the other. The mobility is achieved by restricting the attachments to the more caudal part, which leaves the apex free to roam both within and beyond the mouth. The attachment of the root is to the hyoid bone, and that of the body is to the symphysial region of the mandible. The tongue is also supported by paired mylohyoideus muscles that sling it between the lower jaws. In the dog especially, the tongue is used to procure heat loss by panting, which is a process facilitated by the very generous supply of blood and the numerous arteriovenous anastomoses (p. 240).
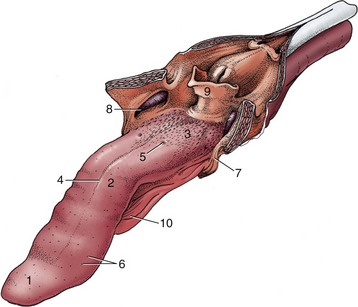
Figure 3–8 The tongue of the dog. The soft palate and the esophagus are sectioned in the median plane. 1, Apex; 2, body; 3, root, forming floor of oropharynx; 4, median groove; 5, vallate papilla; 6, fungiform papillae; 7, palatoglossal arch; 8, palatine tonsil in tonsillar fossa; 9, epiglottis; 10, frenulum.
In general shape, the tongue corresponds to the oral cavity. The apex is dorsoventrally compressed, the succeeding middle portion is somewhat triangular in section (being joined to the oral floor by a mucosal fold or frenulum), and the root is uniformly wide to allow entry to the muscles passing forward from the hyoid bone. Mucosal reflections (palatoglossal arches; Figure 3–8/7) also pass from each side of the root to join the soft palate; they demarcate the exit from the mouth.
The mucosa is tough and tightly adherent where repeated contact with abrasive food occurs but looser and less heavily keratinized where a softer diet or a more protected position allows. Much of the surface is covered by a variety of papillae. Some, like the soft threadlike (filiform) papillae that are scattered widely over the human tongue, provide additional protection; the harsh conical papillae that make the cat’s tongue so efficient a rasp are a larger version of these. Other papillae carry taste buds and have a more restricted distribution, characteristic for each species (Figure 3–9): their names—fungiform, foliate, and vallate papillae—give good indications of their shapes. A few small salivary glands lie below the epithelium.
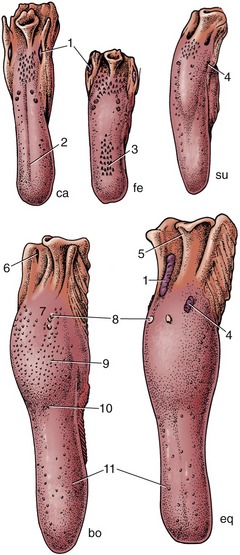
Figure 3–9 Dorsal view of the tongue and epiglottis of the dog (ca), cat (fe), pig (su), cattle (bo), and horse (eq). 1, Palatine tonsil; 2, median groove; 3, filiform papillae; 4, foliate papillae; 5, epiglottis; 6, tonsillar sinus; 7, root of tongue; 8, vallate papillae; 9, torus linguae; 10, fossa linguae; 11, fungiform papillae.
The bulk of the tongue consists of muscle, usually divided into intrinsic and extrinsic groups. Four pairs of extrinsic muscles exist (Figure 3–10). One, the geniohyoideus, lies somewhat apart and passes from the incisive part of the mandible to the body of the hyoid bone; it therefore lies below the tongue rather than within it. It is able to draw the hyoid and thus the tongue forward. The genioglossus arises more dorsally than the geniohyoideus and first runs back below the floor of the mouth before dividing into bundles that fan upward in the sagittal plane. Those bundles that turn forward to the apex of the tongue retract this part; those that pass toward the root draw the whole tongue forward. The middle group passes toward the upper surface (dorsum), which it may depress. The other two muscles arise from the hyoid apparatus. The hyoglossus takes origin from the basihyoid and runs forward, lateral to the genioglossus; the styloglossus takes origin from the stylohyoid but farther to the side. Both draw the tongue back but in rather different fashions; the styloglossus also tends to elevate it. The intrinsic muscle is disposed in bundles that run longitudinally, transversely, and vertically (see Figure 4–2). Simultaneous contraction of the transverse and vertical bundles stiffens the tongue.
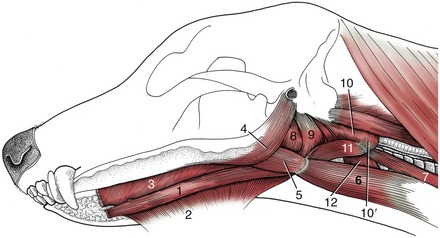
Figure 3–10 Muscles of the tongue and pharynx of the dog. 1, Geniohyoideus; 2, mylohyoideus; 3, genioglossus; 4, styloglossus; 5, hyoglossus; 6, sternohyoideus; 7, sternothyroideus; 8,9, hyopharyngeus (two parts); 10, thyropharyngeus; 10′, cricopharyngeus; 11, thyrohyoideus; 12, cricothyroideus.
The muscle bundles are interspersed with considerable amounts of fat, which is an arrangement that imparts a unique consistency and flavor to the cooked tongue. This fat is very resistant to mobilization in starvation.
In the dog, alone among the domestic species, the ventral part of the tongue contains a prominent fibrous condensation, the lyssa, easily recognized on palpation. A fibrous septum that extends from this is responsible for the conspicuous median groove on the upper surface.
The innervation accurately reflects the origin of the tongue as an unpaired swelling of the pharyngeal floor (see Figure 3–58, C) that is later extended by contributions from the ventral parts of the adjacent pharyngeal (branchial) arches. The mucosa retains a sensory innervation from the corresponding arch nerves. The lingual branch of the mandibular nerve is responsible for general sensation over the rostral two thirds of the tongue; the chorda tympani, a branch of the facial nerve, is responsible for the special sensation of taste in the same area. Both general and special sensation of the root region are the responsibility of the glossopharyngeal and, to a small extent, the vagus nerves. The extrinsic and intrinsic muscles are all supplied by the hypoglossal nerve, although it is probable that the sensory fibers emanating from spindles and other receptors in these muscles travel mainly in the lingual nerve.
Relatively little of the floor of the mouth is left accessible rostral and lateral to the attachments of the tongue. The largest free area lies ventral to the apex, behind the incisor teeth. The mucosa here covers the incisive part of the mandible directly, but elsewhere it lies on muscle and the floor is yielding. The most prominent features are fleshy protuberances or caruncles behind the central incisors; these carry the common openings of the mandibular and major sublingual salivary ducts (see Figure 3–3). In some species, much smaller serial elevations to each side of the frenulum mark the openings of the lesser ducts of the sublingual gland. The mylohyoideus muscle passes below the mucosa and tongue from a linear attachment on the medial aspect of the mandible to meet its fellow of the other side in a median raphe; the two together suspend the tongue in a muscular hammock (see Figure 3–21/4). This muscle is supplied by the mandibular nerve and plays an important part in initiating swallowing (p. 121).
THE SALIVARY GLANDS
Numerous salivary glands drain into the oral cavity. Their secretion, the saliva, keeps the interior of the mouth moist, and when mixed with food, saliva facilitates mastication. When the food is eventually formed into a bolus for swallowing, the saliva lubricates its passage.
Small salivary glands have been mentioned as features of the lips, cheeks, and tongue; others are present in the soft palate, pharynx, and esophagus. Although individually unimportant, their collective contribution must be considerable. However, most saliva comes from certain larger glands situated at a greater distance from the mouth cavity into which they drain through longer ducts (Figure 3–11). Unlike the minor glands, which mostly produce a mucous secretion, some of these major glands produce a more watery (serous) fluid containing the enzyme ptyalin, which plays a minor role in carbohydrate digestion.
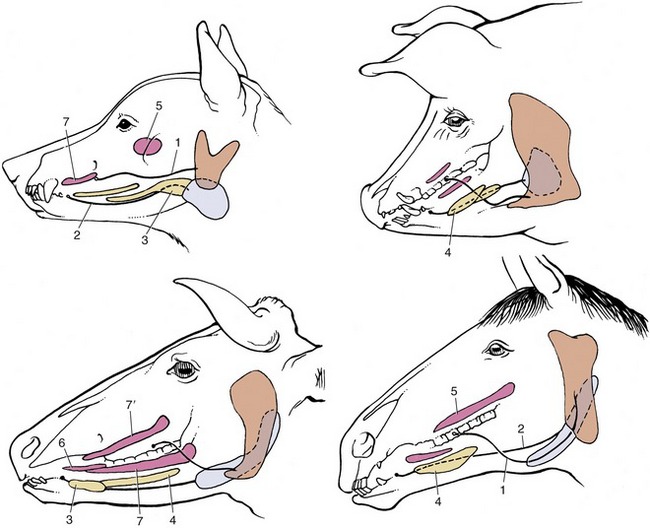
Figure 3–11 The major salivary glands of the dog, pig, cattle, and horse. Orange: parotid gland; white: mandibular gland; yellow: sublingual glands; red: buccal glands. 1, Parotid duct; 2, mandibular duct; 3, compact (monostomatic) part of sublingual gland; 4, diffuse (polystomatic) part of sublingual gland; 5, dorsal buccal glands (zygomatic gland in the dog); 6, middle buccal glands; 7, ventral buccal glands; 7′, middle buccal gland.
The parotid gland, which is purely serous in most species (though not in the dog), obtains its name from its relationship to the ear, being molded around the ventral part of the auricular cartilage (Figure 3–12). In the dog it is small and confined to the vicinity of the cartilage. Because the serous parotid secretion is important in moistening and softening food, the gland is larger and the flow more copious in herbivores. In these species the parotid gland extends rostrally onto the masseter muscle, ventrally toward the angle of the jaw, and caudally toward the atlantal fossa. In all species it is enclosed within a fascial covering that sends trabeculae inward to divide the gland into obvious lobules.
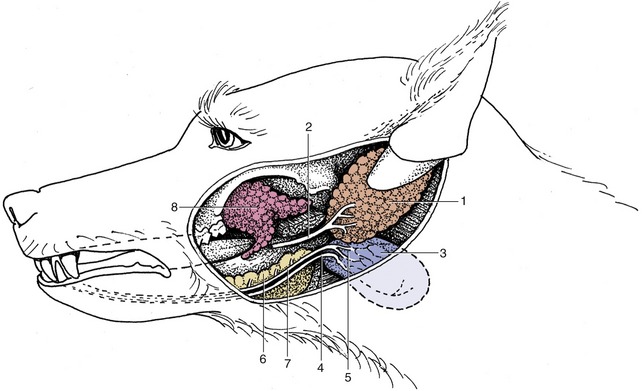
Figure 3–12 The salivary glands of the dog. 1, Parotid gland; 2, parotid duct; 3, mandibular gland; 4, mandibular duct; 5, caudal part of compact sublingual gland; 6, rostral part of compact sublingual gland; 7, major sublingual duct; 8, zygomatic gland.
The major collecting ducts run within these trabeculae and eventually join to form a single duct that leaves the cranial aspect. In the dog this duct takes the shortcut across the lateral surface of the masseter to open into the vestibule of the mouth opposite the fourth upper premolar tooth. In the large domestic animals the duct takes the longer but more protected route medial to the angle of the jaw and winds below the mandible to enter the face along the rostral margin of the masseter.
The mandibular gland produces a mixed mucous and serous secretion. Generally smaller than the parotid, it is more compact and is placed close to the angle of the jaw. It is a moderately large, very regular ovoid structure in the dog. It too is much larger in herbivores, in which it has a deeper position. This gland also drains by a single large duct that runs ventral to the mucous membrane of the floor of the mouth, close to the frenulum of the tongue, to open on the sublingual caruncle.
The sublingual gland is also commonly mixed and sometimes consists of parts: one is compact (monostomatic) and drains by a single duct, and the other is diffuse (polystomatic) and opens by several small ducts. In the dog the compact part fits over the rostral extremity of the mandibular gland, which it appears to continue. The duct that leaves this part runs close to the mandibular duct and discharges alongside this or through a common opening. The diffuse part, the only part present in the horse, is a thin strip lying below the mucosa of the oral floor; its many ducts open beside the frenulum.
The flow of saliva is normally continuous, although the rate is influenced by many factors. It is depressed by anxiety or fear and may be wholly suspended when the body is dehydrated: the resulting dryness of the mouth contributes to the sensation of thirst. It is increased when substances—even inedible ones—are introduced into the mouth, although food is most effective, as was demonstrated by the classic experiments of Pavlov. Events indicating that feeding is imminent are also effective. The rate of secretion is controlled by the innervation. The salivary glands receive both sympathetic and parasympathetic supplies, the latter being vastly more important. The parasympathetic fibers come from the two salivatory nuclei of the brainstem and first travel in the facial and glossopharyngeal nerves; later the fibers pass into various branches of the trigeminal nerve that convey them to their destinations. The preganglionic fibers synapse close to the gland, and the postganglionic fibers terminate in direct contact with the secretory cells. Stimulation is followed by copious flow accompanied by vasodilation. Sympathetic stimulation produces vasoconstriction, which slows the rate of production and alters the composition of the saliva.
In addition to its cleansing, lubricant, and digestive functions, saliva serves as a route for the excretion of certain substances, some of which may accumulate as a deposit (tartar) on the teeth.
THE MASTICATORY APPARATUS
The masticatory apparatus comprises the teeth and gums, the temporomandibular and symphysial joints of the jaws, and the masticatory muscles.
DENTITION
The mammalian dentition* possesses certain characters that in combination, if not individually, are diagnostic of the class. The complement of teeth is limited to a fairly small number, rarely exceeding 44 in the permanent dentition, which is determined for each species—although minor variations may occur. Unlike those of most other vertebrates, the teeth are very differently developed in different regions of the mouth for better performance of special tasks; this character, known as heterodonty, allows the recognition of incisor, canine, premolar, and molar groups. A single replacement of the teeth first erupted is provided by a second, stronger set that is better adapted to the larger jaws and to the more vigorous mastication of the adult. The sequence is known as diphyodonty in contrast to the polyphyodonty (multiple succession) of most other vertebrates. Finally, the teeth are implanted in sockets set along the margins of the jaws, which is an arrangement described as thecodont.
The number and classification of the teeth in a particular species are conveniently represented by a formula. For the dog, the formula of the permanent dentition may be written
or, more succinctly and no less clearly,
The temporary (milk or deciduous) dentition of the same animal may be represented
without risk of confusion, as molar teeth are always lacking in the milk set. There are various notations for the identification of individual teeth. According to the most convenient, P1 may stand for the first permanent upper premolar, i2 for the secondary temporary lower incisor, and so forth, precision being achieved by the use of upper and lower case letters and superscript and subscript numerals.
The term diastema is used for a considerable gap between teeth in the one jaw, most usually for that between the incisors and premolars.
The description of a simple tooth may be considered before returning to the features of the different types of teeth. A tooth (dens) consists of crown and root, and each is easily distinguished. The crown is encased in enamel, a very resistant, calcified, slightly opalescent, white material, while the root is encased in cement, a softer, less shiny, yellowish tissue. The part of the tooth between root and crown is termed the neck (Figure 3–13). Certain variations in structure may occur at the neck: the cement and enamel commonly abut, but the cement may overlie the enamel or sometimes the two tissues fail to meet, exposing a narrow strip of dentine, the third calcified tissue of the tooth. The dentine, which is also known as ivory, provides the greater part of the substance of the tooth and contains a small central cavity that houses the connective tissue pulp. The pulp continues through a canal in the root of the tooth to merge with the connective tissue in the depth of the tooth socket (alveolus).
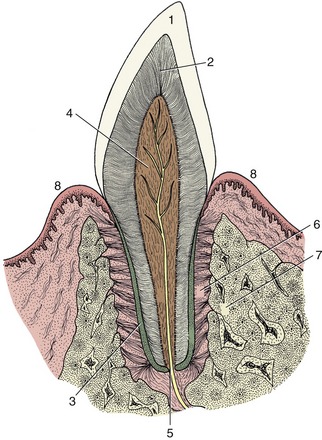
Figure 3–13 Schematic longitudinal section of a simple tooth. 1, Enamel; 2, dentine; 3, cement; 4, pulp; 5, apical foramen; 6, periodontal ligament; 7, socket (alveolus); 8, gum.
Figure 3–13 depicts the idealized condition in which both the gum (gingiva) embraces the neck and the crown corresponds to the exposed part of the tooth. The gums may recede with advancing age, exposing the cervical part of the root, which is a condition familiar in many older people who are said, on this account, to be “long in the tooth.” The opposite condition, in which part of the enamel-covered crown is concealed below the gum line, occurs in many mammals; in some a large portion of the crown is initially held in reserve to be extruded gradually in compensation for the attrition at the masticatory surface. Such high-crowned teeth are said to be hypsodont (or hypseledont) and are characteristic of herbivores, which feed on abrasive food. Even in species such as primates or dogs with low-crowned (brachydont) teeth suited to a softer diet that produces less wear, it is common for part of the enamel-covered region to lie below the gum when the tooth first comes into use. For these reasons it is useful to distinguish the “clinical crown” from the anatomical crown: the first term specifies the exposed part of the tooth regardless of its structure, and the second specifies the enamel-covered part regardless of its location (Figure 3–14).
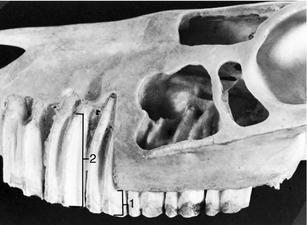
Figure 3–14 Premolar teeth exposed in the upper jaw of a horse. The part protruding above the gum is the clinical crown (1); the whole enamel-covered part is the anatomical crown or body (2) of the tooth.
The detailed description of the crown requires some system for indicating its various surfaces. The usual terms of relative position are inadequate for this purpose because the curved line followed by the tooth row (arcade) alters the orientation of equivalent surfaces of successive teeth in the series. Less ambiguous terms are vestibular (labial, buccal) and lingual, and mesial and distal; their usage is indicated in Figure 3–18. Where adjacent teeth touch, the appropriate mesial and distal surfaces may both be termed contact surfaces. The working area, if extensive and not a mere cutting edge, is known as the occlusal or masticatory surface.
Enamel is a densely calcified tissue of ectodermal origin. It is acellular and therefore unable to react to injury: it cannot regenerate to patch a hole or repair a fracture. Because it is exposed to rough treatment, it is necessarily very hard, indeed uniquely so for a biological material. Despite this, the enamel casing may eventually be breached, and the softer dentine that wears away more rapidly would then be exposed. The thickness and the resistance of the enamel therefore largely determine the working life of the brachydont tooth. In species in which the tooth crown is high and only gradually passed above the gum line, the enamel may be folded in a very complicated fashion; this increases the efficiency of the masticatory surface, as the unequal resistance of the tissues exposed on opening the enamel casing results in an irregular ridged arrangement (see Figures 3–19 and 18–20).
Cement is the least hard of the calcified tissues of the tooth and resembles bone in structure, although it lacks so regular an organization. The initial deposit over the root is thin, but as deposition continues throughout life it may eventually form quite a thick crust. Collagen fibers extend from the cement into the periodontal ligament or membrane (periodontium), the specialized connective tissue that fastens the tooth in its socket. Although broadly comparable to bone in structure and development, cement differs in one important respect: it is relatively immune to pressure erosion. Orthodontists make use of this characteristic when they adjust the position of a tooth in the jaw by fitting an appliance that presses the tooth against the alveolar wall. If the adjustment is performed correctly, the pressure produces an erosion of the bone but leaves the tooth unaffected and free to shift into the space created. This lack of response to pressure is relative, not absolute, and excessive pressure causes resorption; indeed, the roots of the temporary teeth are resorbed under pressure from their permanent replacements thrusting against them.
Dentine is also similar to bone in having a calcified, collagen-rich matrix. In bone the osteoblasts become imprisoned in the matrix, but the dentine-producing cells (odontoblasts) recede from the newly formed dentine and remain as a continuous layer on the surface lining the dental (pulp) cavity. The odontoblasts retain their productive capacity throughout life, and a slow but continuous production of secondary dentine, with corresponding reduction of the dental cavity, continues into old age. This process may be accelerated when local damage or abrasion of the crown threatens to expose the pulp. Secondary dentine is easily recognized by its darker color. Although once disputed, it is generally believed that fine nerve processes enter a short distance into the dentine from the pulp.
The dental cavity reflects the external form of the tooth, sending a branch into each major elevation of the crown and through a narrow passage in the root where it opens at the apical foramen; when more than one root is present, each contains a channel that joins the central cavity.
The pulp that fills this space is a very delicate connective tissue margined by the odontoblast layer and richly vascularized. A lymphatic plexus also exists, although this is difficult to demonstrate. Numerous nerves run within the pulp; some are vasomotor, although most are sensory and possess endings that can be stimulated in various ways. Whatever the stimulus, thermal, mechanical, or chemical, the sensation perceived is pain; because the pulp is contained within unyielding walls, even a slight inflammatory swelling is quickly appreciated.
Each tooth is implanted in a separate socket in the margin of a jaw. The form of the socket corresponds to that of the root and is therefore often branched and irregular. Where the teeth lie close together the septa between adjacent sockets may be very delicate or even defective. Typically, the socket is lined by a thin lamina of compact bone perforated for the passage of the vessels and nerves that supply both the socket and the tooth. The outer surface of the lamina may be braced by trabeculae of spongy bone extending toward the surface of the jaw or radiating into surrounding parts; where the alveolar margin is narrow, however, the lamina merges with the external compacta of the jaw. The tooth is attached to the socket by means of the tough fibrous periodontal ligament. This is particularly rich in collagen fibers that attach to both the cement and the alveolar bone and are so oriented that the tooth is suspended in a sling; masticatory forces that tend to drive the tooth deeper into the socket are thus transformed into tension on the socket wall. The arrangement allows the tooth a certain (though usually very limited) mobility, and slight rotation and tilting are normal during mastication.
The vessels and nerves that supply the teeth are derived from the major trunks (superior and inferior alveolar arteries, veins, and nerves) that course through canals in the jaws.
Tooth eruption is a complicated and controversial process involving a number of factors: root growth, bone growth, pulpal proliferation, tissue pressure, and periodontal traction. Their relative importance is disputed, but the last factor is probably the most significant. The temporary teeth rise in the jaws after the crown is completed but before the root is formed; this process carries the tooth closer to the surface and provides the space necessary for the formation of the root. The movement of the crown is facilitated by a loosening of the connective tissue of the dental follicle (p. 142) and gum and by the presence of remnants of the epithelium of the dental lamina, which define the line of passage. However, if these remnants are large and cystic, as sometimes happens, they may obstruct rather than facilitate the movement of the tooth, divert it from its true path, and give rise to troublesome anomalies of site and spacing. The retention of an epithelial covering over the unerupted crown ensures that no breach of continuity occurs when the tooth breaks through to the surface, as this remnant of the enamel organ fuses with the epithelium of the gums embracing the tooth (Figure 3–15).
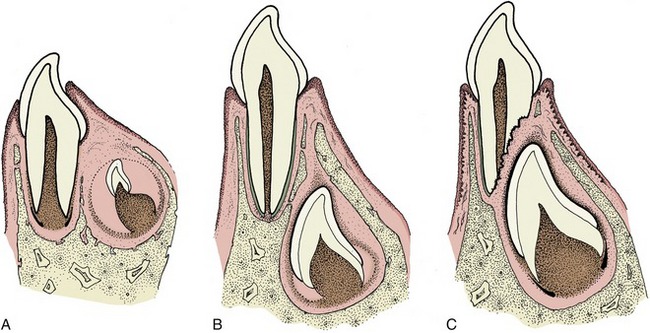
Figure 3–15 Schematic drawings representing tooth eruption and replacement. A, Eruption of a deciduous tooth. The primordium of the permanent tooth is located on the lingual side of the deciduous tooth. B, The fully developed deciduous tooth within a bony alveolus. The crown of the permanent tooth has already formed. C, The permanent tooth is ready to break through. The root of the deciduous tooth has been resorbed; formation of the root of the permanent tooth is in progress.
The eruption of the permanent teeth is more complicated. These develop in bony crypts deep to the roots of the equivalent teeth of the temporary set. To erupt they must escape from this confinement and displace their predecessors. The erosion of the roof and the continuous adjustment of the walls of the embedded alveolus involve the usual processes of bone remodeling, and it is hardly too fanciful to say that the permanent tooth and its alveolus migrate as a unit through the jaw to enter the alveolus of the temporary tooth. The replacement tooth then presses on the root of the temporary tooth, causing its resorption. The attachment of the temporary tooth is loosened, which allows it to shift and become increasingly mobile during mastication; it is soon shed, and the permanent tooth then rises in its place. Proper eruption of the permanent tooth depends on the temporary teeth holding places ready for them; if the latter are prematurely lost, the filling of the alveoli by bone may make it difficult for the permanent teeth to establish their proper occlusal relationships.
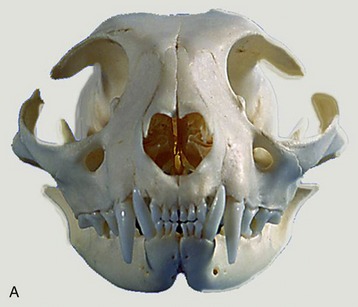
The dentition of the dog, although relatively simple, is well adapted to the feeding habits of the animal (Figure 3–16). The incisor teeth are small and peglike and are crowded together in the rostral part of each jaw. On eruption, each upper incisor presents a trilobed crown with a labial cutting edge. The lower incisors are bilobed. These features are lost as wear reduces the tooth to a simple prismatic peg. The name incisor suggests that these teeth are used for dividing food before it is taken into the mouth, but in this species a second and more efficient shear is provided by teeth farther back in the mouth. The incisors in the dog are employed mainly in nibbling and grooming.
The canine teeth are particularly well developed, so much so that the generic name (Canis) for doglike animals provides the term by which these teeth are known in all mammals. Canines are large, curved, and laterally compressed teeth of simple form and are capable of inflicting a deep wound; they are used for aggressive and holding purposes. A large part of each canine tooth is implanted in the jaw; the extent and position of the embedded part of the upper canine are revealed by a bony ridge over the alveolus.
The premolar and molar teeth together constitute the cheek teeth, a term more common and more useful in descriptions of the dentition of herbivorous species, in which the two groups have become assimilated to each other in form and function. In all mammals the first few (maximally four) cheek teeth are represented in both dentitions and are assigned to the premolar group; the remainder (maximally three) are represented only in the permanent dentition and are known as molar teeth. The premolars of the dog form an irregular but fairly closely spaced series of increasing size and complexity. The cusps or projections of the individual crowns are aligned one behind the other to form a discontinuous serrated cutting edge rather resembling that of the pinking shears of a dressmaker and effective for the same reason: the elongation of the blade makes possible a more rapid and cleaner division while the notches help hold the food in place. The more caudal molars also possess a cutting potential but are principally developed for crushing and are distinguished by their broader and more extensive masticatory surfaces. The cusps or elevations that they carry are arranged in a pattern that is faithfully reproduced on the teeth of all members of the species; their homologues can be recognized, although sometimes only with great difficulty, in the teeth of other mammals.
Most of the cheek teeth, unlike the incisors and canines, have more than one root. Multiple roots, especially if divergent, provide firmer anchorage but make extraction difficult, if not impossible, without previous division of the crown into portions corresponding to the individual roots.
The dentition of the cat is reduced to
in the permanent set (Figure 3–17). It is even more closely adapted to a fleshy diet, as the reduction of the molar series has largely eliminated the crushing potential presented by the dog’s dentition. The cutting action of the cat’s cheek teeth earns them the description secodont; the dual-purpose structure of the dog’s molars is better described as tuberculosectorial. The incisors of cats are remarkably small and the canine teeth relatively large.
In other domestic species, the diet is much more abrasive and requires considerably more crushing and grinding. The dentition is modified accordingly. The details are presented in the later chapters; here it is sufficient to note only the most conspicuous features.
In the dentition of the pig the broad crowns of the cheek teeth carry an elaborate formation of blunt cusps that make them very effective crushing instruments; teeth of this sort are said to be bunodont (Figure 3–18). The canine teeth of this species remain open at the embedded end (root) so that accretion of dental tissues continues throughout the animal’s life. This persistent growth, coupled with their curved form, allows them to assume very striking forms in older individuals, particularly in boars.
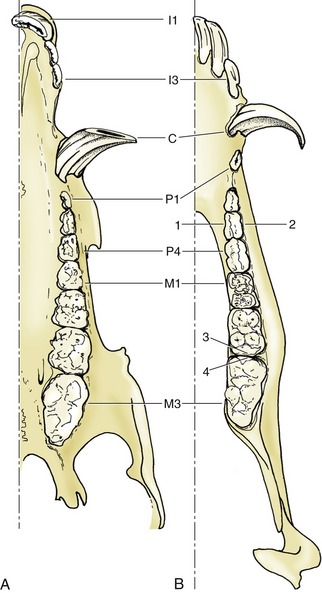
Figure 3–18 Permanent dentition of the pig, upper (A) and lower (B) jaws. 1, Lingual surface; 2, vestibular surface; 3, distal surface; 4, mesial surface.
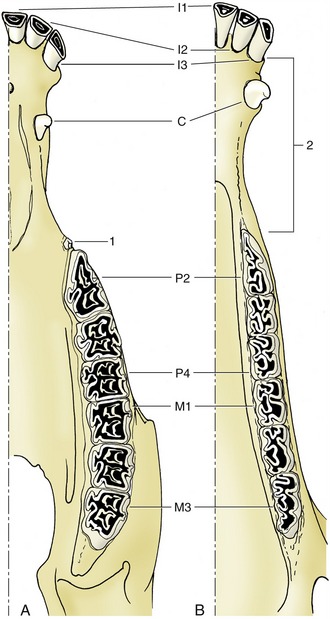
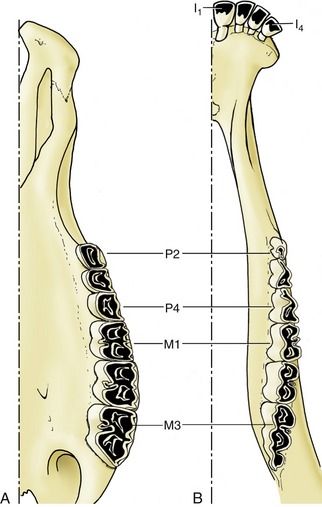
The other species are more restricted to a herbivorous diet than the omnivorous pig, and the dentition of horses and ruminants must allow for continuous and considerable wear at the masticatory surfaces. This requirement is met by the enlargement of these surfaces, by the increase in height of the crowns, which are only gradually extruded (the delayed development of the roots allows growth to continue for some years after the teeth have come into wear), and, above all, by complicated folding of the enamel. This folding has two important consequences. It increases the amount of the hardest and most durable component of the tooth that is exposed and so reduces the rate of attrition. It provides an alternation of harder and softer materials, which, wearing at different rates, produces an unevenness of the masticatory surface that gives it a rasplike quality (Figures 3–19 and 3–20).
THE ARTICULATIONS OF THE JAWS
Although it is customary to describe two temporomandibular joints, these may be regarded as the widely separated halves of a single condylar joint (p. 21). Clearly, movement at one side must be accompanied by a movement, not necessarily identical, at the other side.
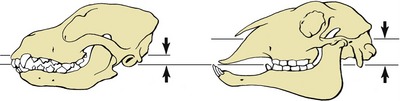
The articular surfaces are provided by the head, carried on a dorsal process of the ramus of the mandible, and the mandibular fossa of the skull, a facet mainly formed by the squamous temporal bone, although sometimes extending beyond it. The forms of the two surfaces reflect the feeding habits, and in species such as the dog, in which hingelike movements of the lower jaw predominate, the head takes the form of a transverse condyle to which the fossa provides a corresponding gutter. Backward dislocation of the jaw is opposed by the prominent retroarticular process placed directly behind the mandibular fossa. A peculiarity of the joint is the presence of a fibrous or fibrocartilaginous articular disk that divides the cavity into upper and lower compartments. Although the phylogenetic origin of this structure is disputed, its functional significance may lie in its resolving the complex movements of the joint into simpler components; a hinge movement occurs between the mandible and the disk, while gross sliding movements (translations) of the mandible relative to the skull occur at the upper level. It is perhaps because the movements of the dog’s jaw are so simple that the disk is rather thin and poorly developed in this species. In species in which lateral grinding movements predominate, the mandibular head is larger, the surface more plateau-like, and the disk thicker, although the details differ considerably.
In most species the halves of the mandible are firmly fused together, but in the dog (and in ruminants) they articulate by means of a symphysis, providing a third joint. This much neglected joint allows small movements that may be important in securing more precise adjustment of the upper and lower tooth rows and therefore a more effective cutting or crushing mechanism. Two types of movement appear to be possible: a spreading movement, altering the angle between the halves of the mandible, and one in which each half rotates about its own long axis so that the tooth cusps alter their inclination to the vertical. The dog appears to make use of these possibilities when adjusting the position of a bone between the teeth before attempting to crack it.
THE MUSCLES OF MASTICATION
The muscles that provide the masticatory forces are derived from the first pharyngeal arch, and in keeping with this, they are supplied by the mandibular nerve. They comprise the temporalis, masseter, pterygoideus medialis, and pterygoideus lateralis (Figure 3–21). Other muscles that play some part in jaw movements, particularly in opening the mouth, are not normally included under the term muscles of mastication.
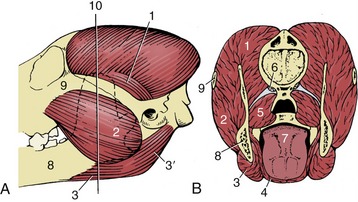
Figure 3–21 The muscles of mastication of the dog, left lateral aspect (A), in section (B). 1, Temporalis; 2, masseter; 3, 3′, rostral and caudal bellies of digastricus; 4, mylohyoideus; 5, medial pterygoid; 6, origin of lateral pterygoid; 7, tongue; 8, mandible; 9, zygomatic arch; 10, level of transection (B).
The temporalis arises from an extensive area on the lateral surface of the cranium and converges to an insertion on the coronoid process of the mandible. On contraction the resultant force pulls the mandible upward; the muscle is especially large in those species, such as the dog and cat, in which the chief jaw movement is scissorlike. A measure of its development is provided by the salience of the zygomatic arch: a well-sprung arch provides more room for this muscle. Although the main action is to raise the mandible, some fibers tend to draw it forward, while others tend to pull the condyle against the retroarticular process.
The masseter lies lateral to the mandible. It takes its origin from the maxillary region of the skull and the zygomatic arch and has a wide insertion on the more caudal part of the mandible. It is frequently a multipennate muscle intersected by strong tendon plates. The fibers in the different strata do not all run parallel; different parts may have contrasting functions. Some may protrude the mandible, and others may retract it; however, the general effect is to raise the mandible and draw it toward the active side, for mastication is restricted to one side at a time in domestic species. The masseter muscle is therefore rather small in the dog; it is proportionately better developed in herbivorous species that make lateral and rotational movements when chewing.
The pterygoid mass of muscle lies medial to the mandible and passes to this bone from the pterygopalatine region of the skull. Generally the mass is clearly divided into a small lateral and a larger medial muscle. Some fibers of the lateral pterygoid muscle attach to the articular disk and help to control its movements, but the principal function of the mass is to raise the mandible and draw it inward with some simultaneous protrusion. In species in which transverse movements are important the masseter and contralateral pterygoid muscles may form a functional pair.
Opening the mouth is assisted by gravity, but certain muscles are also available for the performance of this movement. The digastricus passes from the skull, caudal to the temporomandibular joint, to the ventral margin of the mandible and opens the mouth. The muscle consists of two parts arranged in tandem. The rostral portion is supplied by the mandibular nerve, the caudal portion by the facial, which is an indication that the muscle has a composite origin in the mesoderm of the first two pharyngeal arches. In species in which the sternocephalicus has a mandibular attachment, it may open the mouth.
In most mammals the mouth is held closed at rest; the mandible is supported by the tonic activity of the masticatory muscles and possibly assisted by the hermetic seal created by the application of the dorsum of the tongue to the palate. The jaws are symmetrically placed in relation to the median plane, and the upper and lower tooth rows are slightly separated or in gentle, interrupted contact. The arcade formed by the upper teeth is generally wider than its counterpart, and the tooth rows are superimposed for only part of their widths. In some species, such as the rat, simultaneous occlusion is impossible in both incisor and molar regions; in them, the lower jaw must be advanced and dropped to bring the incisor tips together and withdrawn and raised for molar contact. Such animals generally favor an intermediate position of the lower jaw at rest.
A slight increase in muscular activity brings the teeth into more extensive contact, which is known as centric occlusion. The relationships between the teeth in this position are variable, even in the same individual at different ages since the teeth come together in altered fashion as wear reduces the more salient projections (and in some species also by migration of teeth within the jaws). It is usual to find that each cheek tooth engages with two teeth of the opposite series, and the lower teeth are generally a little mesial to their upper counterparts. In the dog, the largest teeth, the last upper premolar and the first lower molar, bite together and constitute the sectorial (or carnassial) teeth, the principal shear (see Figure 3–16). The teeth in front of the sectorials do not meet but leave open a carrying space, while the last cheek teeth make extensive contact. The lower canine engages in front of the upper canine, filling the space between this and the third incisor.
The relationship between the teeth is a dynamic one, as is readily seen from the so frequently defective human dentition. A tooth deprived of normal support may drift under the influence of the masticatory forces; the pressures exerted by the lips, cheek, and tongue are also important in maintaining normal contact and alignment. It is evident from developmental studies that these associations are established before eruption and that common factors control the growth of the two jaws and the development of the teeth so that a harmonious relationship normally exists at all stages of development. However, anomalies are not uncommon, and the “undershot” and “overshot” jaw are well illustrated by Bulldogs and by many Afghan Hounds.
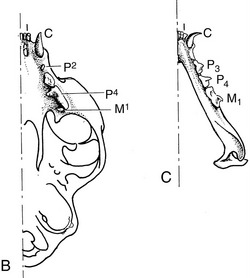
The simplest activity that is common to all species, regardless of their masticatory habits, is the gaping that occurs on depression of the lower jaw. Gaping is achieved by slackening or cessation of activity in the masticatory muscles, by contraction of their antagonists, and by gravity. As the jaw is lowered the mandibular head rolls on the articular disk while the disk itself slides forward in the mandibular fossa, probably assisted by those lateral pterygoid fibers that attach to it. Closure of the mouth requires the reversal of these processes and must at times be vigorous enough to detach a morsel. Sometimes, the detachment is achieved by the incisors, and in certain species the hinge movement is complicated by a preliminary protrusion of the lower jaw to bring the incisor edges into alignment. When the cheek teeth are employed in biting, the action is unilateral. Herbivores employ the cheek teeth for grinding food already taken into the mouth, and the active (closing) movement is preceded by lateral displacement. The temporomandibular joint of these animals is situated high above the occlusal plane, and the lower teeth are drawn forward over their upper fellows as they approach. This contributes a grinding component that is absent when the joint and occlusal surfaces are more nearly level. The sheep and dog, typical examples of herbivore and carnivore, illustrate these differences in the position of the joint in relation to the teeth (Figure 3–22).
THE PHARYNX AND SOFT PALATE
The pharynx lies behind the mouth and continues into the esophagus. It is a funnel-shaped chamber contained between the base of the skull and the first couple of cervical vertebrae dorsally, the larynx ventrally, and the pterygoid muscles, the mandible, and the dorsal part of the hyoid apparatus laterally. Because it communicates freely with other cavities in the head, it is rather difficult to form a clear conception of its boundaries and extent; a first impression may be obtained from Figures 3–23 and 4–2. Figure 3–27 illustrates the crossing of the air and food pathways and is a reminder that the pharynx possesses a respiratory function as well as an alimentary function.
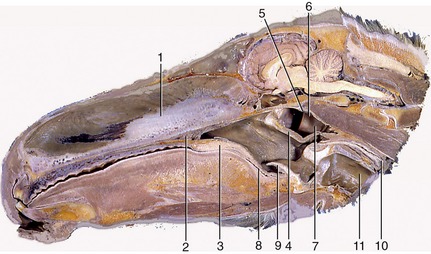
Figure 3–23 Paramedian section through the equine head. 1, Nasal septum; 2, hard palate; 3, soft palate; 4, palatopharyngeal arch; 5, roof of nasopharynx; 6, nasopharynx; 7, entrance to auditory tube; 8, oropharynx; 9, epiglottis; 10, esophagus; 11, trachea.
The key to understanding the pharynx is provided by the soft palate, already encountered as the continuation of the hard palate beyond the choanal margin. In repose the soft palate lies on the tongue, but when the animal swallows, the soft palate is raised into a more horizontal position and then more obviously divides the pharynx into dorsal and ventral parts. Two pairs of arches connect the soft palate to adjacent structures. The palatopharyngeal arches pass onto the lateral wall of the pharynx and may be long enough to meet above the entrance to the esophagus (see Figure 3–23). Together with the free margin of the palate they circumscribe the constriction of the lumen—the intrapharyngeal ostium—that marks the separation of the pharynx into dorsal and ventral compartments. The dorsal compartment is known as the nasopharynx. The more rostral palatoglossal arches pass onto the sides of the tongue at its root; they demarcate the passage from the mouth to the oropharynx (see Figure 3–3). The oropharynx is somewhat arbitrarily divided from the third subdivision, the laryngopharynx, at the level of the epiglottis. The laryngopharynx lies above the larynx and corresponds with this in extent.
Functional considerations suggest that the nasopharynx could well be regarded as a part of the nasal cavity. Food does not enter it, it takes no part in the swallowing process, and it serves passively to convey air. The topography of the connection with the nasal cavity varies much among species; a single ductlike communication is present in the dog. In addition to the major connections, the nasopharynx communicates with the cavities of the middle ears through the auditory (Eustachian) tubes. The paired tubal openings are placed on the summits of small pimple-like elevations in the dog. Small muscle bundles radiate over the pharyngeal wall from the opening and provide a mechanism for dilating the orifice, thus allowing air to pass to or from the middle ear so that the pressure on the two sides of the eardrum may be equalized (Figure 3–24). Much of the wall of the nasopharynx is reduced to a thin mucosa that finds support by attaching to neighboring structures, mainly the base of the skull and the ventral straight muscles of the head. The mucosa possesses a typical respiratory epithelium and contains numerous mucous glands and much lymphoid tissue, of which some is scattered and some is massed. The lymphoid masses that form elevations visible to the naked eye are known as the pharyngeal tonsils (adenoids in ourselves) and form part of the ring of lymphoid tissue that guards the passage from the nose and mouth to the pharynx and beyond (Figure 3–25); like other lymphoid developments they are larger in infancy than later. Excessively enlarged tonsils impair the airflow.
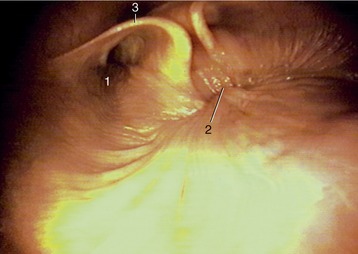
Figure 3–24 Caudal part of nasopharynx (horse). 1, Entrance to auditory tube; 2, closure between the rostral and caudal parts of the nasopharynx (during swallowing); 3, cartilage flange supporting the auditory tube.
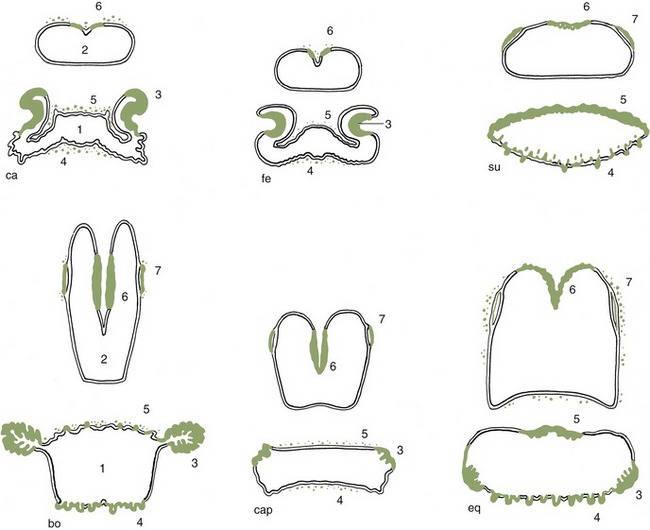
Figure 3–25 Tonsils in the wall of oropharynx and nasopharynx; ca, dog; fe, cat; su, pig; bo, cattle; cap, goat; eq, horse. 1, Oropharynx; 2, nasopharynx; 3, palatine tonsil; 4, lingual tonsil; 5, tonsil of the soft palate; 6, pharyngeal tonsil; 7, tubal tonsil.
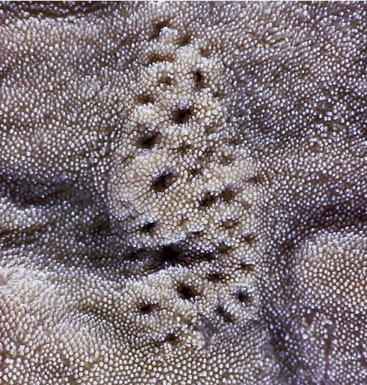
The narrowness of the oropharynx limits the size of the morsels that can be swallowed. Its lateral walls are supported by a fascia and are the site of the palatine tonsils. These are very differently arranged in different species; in some (e.g., the horse) they are diffuse (though raised slightly), whereas in others they constitute a compact mass that may project away from or toward the lumen, as in the ox and dog, respectively (see Figure 3–25). Tonsils that project into the lumen are overlain by flaps of mucosa that partly hide them from inspection through the open mouth (Figure 3–8/8 and Figure 3–26).
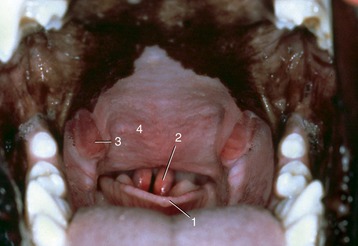
Figure 3–26 View into the oropharynx of a dog. 1, epiglottis; 2, cuneiform process of arytenoid cartilages; 3, palatine tonsils; 4, soft palate.
The laryngopharynx is the largest part of the pharynx. It is wide in front but narrows before joining the esophagus at a boundary that is well defined by a mucosal fold in the dog but more difficult to recognize in most other species. At rest, the lumen of the caudal part of the laryngopharynx is closed by the apposition of the lateral walls and roof to the floor. The floor is largely occupied by the entrance to the larynx, which presents the epiglottis, the arytenoid cartilages, and the aryepiglottic folds. The epiglottis serves as a breakwater to deflect fluids to the side, into gutters (piriform recesses) that run beside the projection of the larynx (Figure 3–27).
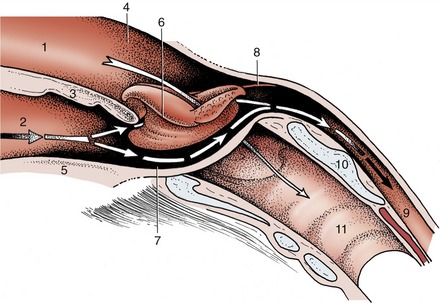
Figure 3–27 Schematic drawing of the pharynx showing its rostral connection with the nasal and oral cavities and caudal connection with the esophagus and larynx. 1, Nasal cavity; 2, oral cavity; 3, soft palate; 4, nasopharynx; 5, root of tongue; 6, larynx (protruding through pharyngeal floor); 7, laryngopharynx (piriform recess); 8, caudal end of palatopharyngeal arch; 9, esophagus; 10, lamina of cricoid cartilage; 11, trachea.
Below an external fascia, the greater part of the pharyngeal wall is covered by a set of striated muscles. These fall into three groups—constrictor, dilator, and shortener—although no individual muscle has an action quite so simple as these terms suggest (Figure 3–28). The constrictor muscles arise from certain fixed points conveniently placed to each side and run onto the roof of the pharynx; with their fellows they form a series of arches that enclose the lumen on its lateral and dorsal aspects. For most purposes it is sufficient to recognize rostral, middle, and caudal constrictor muscles, although each may be divided into lesser units. The rostral constrictor arises from the pterygoid region of the skull (pterygopharyngeus) and the aponeurosis of the soft palate (palatopharyngeus) and embraces the pharynx at the level of the palatopharyngeal arch; many fibers take an almost longitudinal course and thus also assist in shortening the pharynx, drawing it onto and over a bolus received from the mouth. The middle constrictor (hyopharyngeus) arises from neighboring parts of the hyoid bone. The caudal constrictor arises in two parts, from the thyroid (thyropharyngeus) and cricoid (cricopharyngeus) cartilages. When the three constrictors contract in succession, they hurry the bolus distally into the esophagus. The dilator muscle (stylopharyngeus caudalis) also arises from the hyoid apparatus but runs more transversely to fan out in the pharyngeal wall; when active it widens the rostral part of the pharynx, enabling it to accept the bolus more easily.
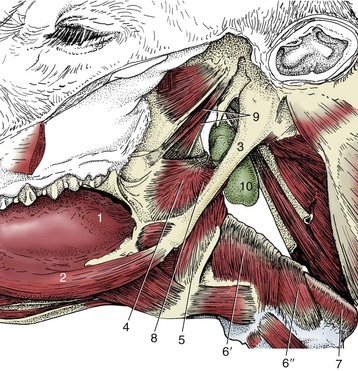
Figure 3–28 Lateral view of the connection of the pharynx with the base of the bovine skull. 1, Root of tongue; 2, styloglossus; 3, stylohyoid; 4, rostral pharyngeal constrictor; 5, middle pharyngeal constrictor; 6, caudal pharyngeal constrictor (6′, thyropharyngeus, 6″, cricopharyngeus); 7, esophagus; 8, pharyngeal dilator (stylopharyngeus caudalis); 9, tensor and levator veli palatini; 10, medial retropharyngeal lymph node.
A fibroelastic aponeurosis internal to the muscles supports the mucosa. It also provides a median raphe to which many fibers of the paired muscles insert and which, continuing to the skull, serves to fix the whole organ in position. The mucous membrane of the oral and laryngeal parts of the pharynx is covered by a stratified squamous epithelium and possesses many small salivary glands that provide additional lubrication to the passage of food.
The soft palate (velum palatinum) is bounded by a respiratory mucosa on its dorsal surface and an oral mucosa ventrally. It is braced by a stout aponeurosis below the dorsal mucosa; the part ventral to the aponeurosis mainly consists of close-packed salivary glands, interrupted toward the midline by the longitudinally disposed palatinus muscle, which shortens the palate. Two small muscles that arise from the muscular process of the temporal bone insert into the lateral part of the aponeurosis after following slightly different courses. As their names indicate, the muscles, the tensor veli palatini and the levator veli palatini, tense the soft palate by exerting lateral traction and raise the soft palate, respectively. The mucous membrane of the pharynx and soft palate and the muscles, except the tensor, which is supplied by the mandibular nerve, obtain their innervation from a plexus to which the vagus nerve makes the chief contribution and the glossopharyngeal nerve a minor contribution.
THE ESOPHAGUS
The esophagus (or gullet) conveys food from the pharynx to the stomach. This relatively narrow tube begins dorsal to the cricoid cartilage of the larynx and follows the trachea down the neck, at first inclining to the left but regaining a median position above the trachea before or shortly after entering the thorax (Figure 3–29). Within the thorax it runs in the mediastinum (p. 158), and, continuing beyond the tracheal bifurcation, it passes over the heart before penetrating the esophageal hiatus of the diaphragm. It then makes its way over the dorsal border of the liver to join the stomach at the cardia. It thus consists of cervical, thoracic, and abdominal portions, although the last is very short.
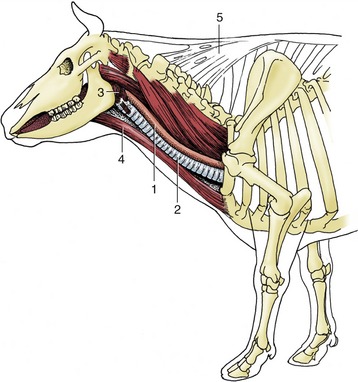
Figure 3–29 Lateral view of the bovine neck. In midneck the esophagus lies on the left dorsolateral aspect of the trachea. 1, Esophagus; 2, trachea; 3, pharyngeal musculature; 4, sternocephalicus muscle; 5, nuchal ligament.
Only a few of the more important features of its topography are mentioned here. The cervical part runs within the visceral space of the neck, related to the subvertebral muscles dorsally and the left side of the trachea medioventrally (see Figure 3–29). For much of its length it is accompanied by the left common carotid artery and vagosympathetic and recurrent laryngeal nerves.
The thoracic part crosses to the right of the aortic arch, which may deflect it from its sagittal course; more caudally its dorsal and ventral borders are followed by the trunks into which the fibers of the right and left vagus nerves are regrouped.
The structure of the esophagus conforms to a pattern that is common to the remainder of the alimentary canal. The outer coat is a loose connective tissue (adventitia) in the neck, but this is largely replaced by serosa* in the thorax and abdomen. The muscle is striated at the origin of the esophagus, but in some species (e.g., cat, pig, and horse) the striated muscle is replaced by smooth muscle at some point within the thorax. It is usual to describe two muscle strata. Both are spiral, and they wind in opposite directions in the first part of the esophagus; closer to the stomach the outer coat becomes more longitudinal and the inner one more circular (Figure 3–30). The arrangement is quite complicated in detail and reveals considerable interlacing of muscle bundles that exchange between the two layers. Although morphological evidence for their existence is unconvincing, a number of sphincters are suggested by functional studies. They include a cranial sphincter, probably provided by fibers of the cricopharyngeus muscle, and possibly others within the thorax, where the passage of food tends to be delayed. A thickening suggestive of a sphincter occurs at the junction of the esophagus with the stomach, although the flow of food is more obviously impeded at a slightly more cranial level, immediately in front of the diaphragm. However, no anatomical evidence exists for a prediaphragmatic sphincter.
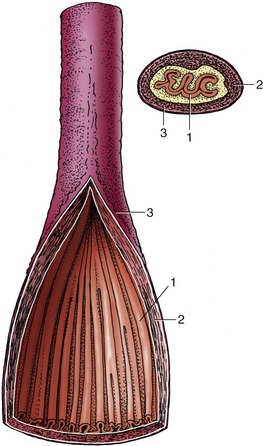
Figure 3–30 Semischematic drawing of the structure of the esophagus, sectioned longitudinally and transversely. 1, Mucosa; 2, muscular layer (longitudinal and circular); 3, adventitia.
The inner part of the wall is divided between submucosa and mucosa by a fenestrated muscularis mucosae, usually more prominent in the thoracic esophagus (Figure 3–31, B); it helps throw the lining of the empty organ into longitudinal folds. The surface epithelium is generally stratified squamous, and the degree of keratinization reflects the relative harshness of a species’ habitual diet. This is nicely illustrated when the esophageal epithelium of the dog (Figure 3–31, A) is compared with the thicker epithelium of the goat, which has a much rougher diet (Figure 3–31, B). Another striking difference between these species is provided by the many mucus-secreting tubuloacinar glands present in the submucosa of the canine esophagus. The boundary between esophageal and gastric epithelia is sharp and may be displaced to either side of the cardia. In humans, prolonged or repeated exposure to gastric juice (e.g., heartburn) may provoke transformation of the stratified epithelium of the lower esophagus into the columnar gastric variety.
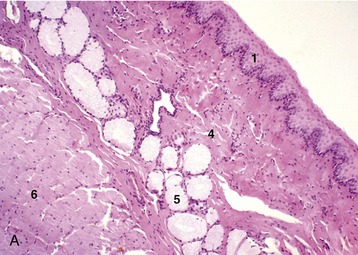
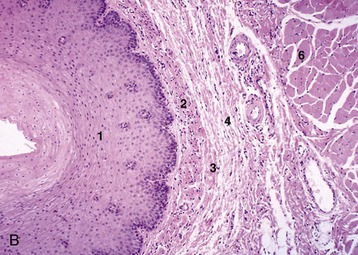
Figure 3–31 Esophagus, in the dog (A) and goat (B) (70×). 1, stratif. squam. epithelium; 2, lam. propria; 3, lam. muscularis mucosae; 4, submucosa; 5, mucus-secreting tubuloacinar glands; 6, muscularis interna.
The esophagus receives its innervation from the sympathetic and vagus nerves, including the recurrent laryngeal branches. The vagal supply is the more important. The striated muscle arises from the mesoderm of the pharyngeal arches and is under control of the general visceral motor neurons of the vagus, whereas the smooth muscle portions are under direct control of the intrinsic nervous system and indirect control of the autonomic nervous system. A myenteric plexus extends the length of the esophagus.
The blood supply from various local arteries presents no features of special interest.
DEGLUTITION
The first stage of deglutition is a voluntary act, but once the food has left the mouth its progress is not under control of the will.
Food that has been sufficiently prepared by mastication and insalivation is collected in a recess formed when the dorsal surface of the tongue is cupped; it is then isolated when the apex of the tongue is pressed against the palate. The jaws are closed, and brisk contraction of the mylohyoid, hyoglossal, and styloglossal muscles raises the tongue and impels the bolus into the oropharynx. Inevitably the food touches the pharyngeal mucosa, and this contact initiates the reflex that completes the act. The afferent nerves include branches of the mandibular, glossopharyngeal, and vagal trunks. As the food passes caudally, the soft palate is raised, and its free margin is drawn toward the dorsocaudal pharyngeal wall. Closure of the intrapharyngeal ostium prevents dissipation of the pressure generated in the mouth and ensures that the food is carried toward the esophagus by denying escape into the nasopharynx. This stage is accompanied by brief inhibition of breathing, with the glottis closed. The hyoid apparatus and the larynx are simultaneously drawn forward, and the epiglottis, meeting the tongue, is tilted back to provide some cover to the laryngeal entrance; however, no question of it fitting into the opening (as is often assumed) exists, and it is known that surgical resection of most of the human epiglottis does not seriously impair swallowing efficiency. The food passes over the epiglottis, or to the side of this, with the impetus maintained by the coordinated successive and rapid contraction of the constrictor muscles. The pharynx, which was dilated for reception of the bolus by the caudal stylopharyngeus muscle, is then shortened and in effect drawn onto and over the bolus by the longitudinal fibers of the constrictor muscles. The caudal end of the pharynx relaxes to receive the food, which is then hastened through the esophagus by a wave of peristalsis that commences just beyond the cricopharyngeal fibers. This last movement is probably coordinated by a local reflex, unlike the preceding events, which are controlled by a deglutition center in the brainstem.
Fluid is swallowed in essentially the same way. It passes mainly through the piriform recesses, and the initial impetus may be sufficient to project it well into the esophagus.
THE ABDOMINAL CAVITY
Some general observations concerning the abdominal cavity are necessary before continuing the description of the digestive system.
The abdomen is the portion of the trunk that lies caudal to the diaphragm (p. 32). It contains the largest of the body cavities, which is continuous at a plane passing through the sacral promontory and the pubic brim with the more caudal and very much smaller pelvic cavity (see Figure 2–2). The more cranial (intrathoracic) part of the abdominal cavity is protected by the hindmost ribs and costal cartilages and is rather restricted in the variations in size that it may experience; the more caudal part is supported by the skeleton only on its dorsal aspect and is therefore more variable. The pelvic cavity has the most extensive bony support and the most constant size, although even here a certain latitude is allowed by changes in the soft tissue components of its walls (see Figure 29–25, A-B).
The structure of the abdominal and pelvic walls has been described with the locomotor apparatus. Comparative features, including conformation and the factors that influence this in different species, are considered in later chapters. The abdominal and pelvic cavities contain the peritoneal sac; the stomach, small and large intestines, and associated liver and pancreas; the spleen; the kidneys, ureters, bladder, and urethra (in part); the ovaries and most of the reproductive system in the female and a smaller part of the reproductive tract in the male; the adrenal glands; and many nerves, blood vessels, and lymph nodes and vessels.
PERITONEAL STRUCTURES
An incision through the whole thickness of the abdominal wall enters the peritoneal cavity, which is a division of the celom that is bounded by a delicate serous membrane, the peritoneum. The peritoneal cavity is completely enclosed in the male, but in the female a potential communication with the exterior exists at the abdominal opening of each uterine tube. The peritoneal cavity contains only a small amount of serous fluid because the abdominal organs are excluded from the space by their peritoneal covering. Nonetheless, it is common to designate as intraperitoneal those organs that are suspended from the abdominal roof within the peritoneal reflections. Although misleading, the term is useful in emphasizing the difference between this and the alternative retroperitoneal arrangement of other organs that are directly joined to the abdominal wall. A diagram (Figure 3–32) may make the distinction plain. The same diagram illustrates the division of the peritoneum into a parietal part lining the walls (parietes), a visceral part directly enshrouding the organs (viscera), and a series of double folds connecting the parietal to the visceral parts. These folds are often collectively known as mesenteries, but properly this term is restricted to the fold suspending the small intestine (and more specifically only the jejunum and ileum); certain similar folds are conveniently named mesocolon, mesovarium, and so on, according to the organ that they support. Others, for example, the greater omentum, have names less immediately revealing.
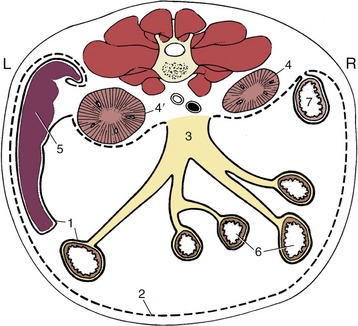
Figure 3–32 Schematic transverse section through the abdomen of the dog. 1, Visceral peritoneum (continuous line); 2, parietal peritoneum (broken line); 3, root of mesentery; 4, 4′, right and left kidneys (retroperitoneal); 5, spleen; 6, jejunum; 7, descending duodenum.
A small outpouching (infracardiac bursa) of the parietal peritoneum extends a little way into the mediastinum within the thorax along the right face of the esophagus where this penetrates the diaphragm.
The peritoneum consists of a single layer of flattened mesothelial cells supported by a fibroelastic tissue that attaches, more or less firmly according to position, to the underlying structures. A considerable amount of fat is often stored below the peritoneum, and some locations are especially favored. In the healthy animal the peritoneal cavity is reduced to a series of clefts between the closely packed abdominal organs. Most clefts are of capillary dimensions, and the total volume of the peritoneal fluid is therefore small—a few milliliters in the dog. The fluid is nonetheless of vital importance, for it lubricates the viscera, allowing them to slip freely over each other or against the abdominal wall in the performance of their own functions or when displaced by other activities. The fluid is constantly turned over, although the mechanism of resorption is disputed. Whatever its nature, the large surface area (2 m2 in humans) of the peritoneum aids rapid removal, and drugs are sometimes administered by intraperitoneal injection. Toxins are also readily absorbed, and because the warm and moist peritoneal cavity affords ideal conditions for bacterial growth, inflammation of the peritoneum is never regarded lightly.
Inflamed serous sheets have a tendency to stick together, and in the course of time these adhesions may become organized and permanent. For this reason the surgeon often turns in the edges of the wound, bringing serosal surfaces together, when closing an incision. Adhesion between organs that are normally free to move over each other is a possible and undesirable sequel to infection or trauma of the peritoneum. Clearly, any attachment that limits mobility may interfere with normal function. However, it must also be noted that adhesion of apposed serosal surfaces (with the obliteration of the intervening space) is commonplace in development and explains the definitive position and arrangement of many organs and mesenteries.
In early development the gastrointestinal tract pursues a sagittal course through the body cavity. It is attached along its whole length to the roof of the embryonic trunk by a primitive dorsal “mesentery,” but only a portion of the foregut (that which becomes the stomach and first part of the duodenum) and a short caudal portion of the hindgut have similar ventral attachments. The parts of the dorsal mesentery associated with the differentiating organs are assigned appropriate names and may be listed in succession: (dorsal) mesogastrium, mesoduodenum, mesojejunum, mesoileum, mesocolon, and mesorectum. The ventral connection to the stomach is known as the ventral mesogastrium. The mesojejunum and mesoileum together constitute the (great) mesentery of adult anatomy. Most portions of the dorsal mesentery persist in more or less unmodified form (at least in the dog), but the mesogastria have a more complicated fate dictated by the later development of the stomach.
The dorsal mesogastrium becomes drawn out and folded on itself during development and is then known as the greater omentum. The folding creates a pouch, the omental bursa, enclosing a portion of the peritoneal cavity. However, the pouch is flattened and its walls brought into close contact so that the cavity is potential, not actual. The greater omentum of the dog is turned caudally between the viscera and the abdominal floor, and its walls are described as parietal (ventral) and visceral (dorsal) because of their relationship to the abdominal wall and viscera. It is the first structure to appear when the abdominal floor is opened. The later growth of the liver reduces access to the interior of the bursa to a narrow opening known as the epiploic (omental) foramen, through which the cavity of the omental bursa remains in open, if restricted, communication with the major part of the peritoneal cavity. The main features of the arrangement are shown in Figures 3–33 and 3–61. The differential growth and the secondary attachments that determine the adult arrangement vary considerably between species, and those details that possess a practical importance are mentioned in context. In most species the greater omentum is lacelike, which is an effect produced by the deposition of fat in strands along the course of the blood vessels; in ruminants so much fat may be present that the omentum appears to consist entirely of this tissue. The omentum has no intrinsic capacity for movement but is liable to be shifted about the abdomen by the movements of other structures. Because it possesses the common tendency of serous membranes to adhere when inflamed, it is often found attached in regions of infection and helps to wall these off. The surgeon may stitch the greater omentum over a closed incision of a viscus as extra insurance against leakage.
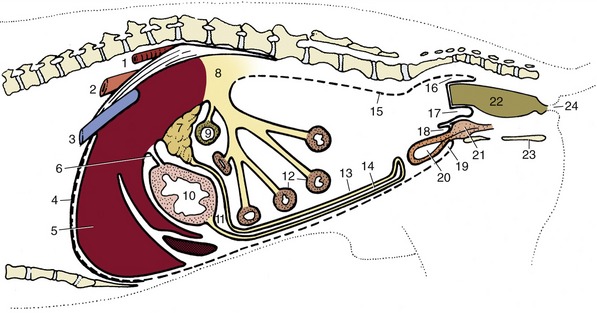
Figure 3–33 Paramedian section of the abdominal cavity of a dog to show the disposition of the peritoneum (schematic). 1, Aorta; 2, esophagus; 3, caudal vena cava; 4, diaphragm; 5, liver; 6, lesser omentum; 7, pancreas; 8, root of mesentery; 9, transverse colon; 10, stomach; 11, omental bursa; 12, small intestine; 13, deep wall of greater omentum; 14, superficial wall of greater omentum; 15, parietal peritoneum; 16, pararectal fossa; 17, rectogenital pouch; 18, vesicogenital pouch; 19, pubovesical pouch; 20, bladder; 21, prostate; 22, rectum; 23, ischium; 24, anus.
The no less complicated arrangement of peritoneal folds that develops, mainly in the pelvic cavity, in association with the urogenital organs is best described with these organs (p. 184).
Visceral Topography
The general disposition of the viscera is determined by the form of the cavity in which they are retained; their detailed arrangement is influenced by individual features of attachment, motility, and distention. Because the peritoneal cavity is hermetically sealed and most abdominal contents are incompressible, it follows that any change in the position or contours of one organ must be followed by adjustment of the abdominal wall or by a reciprocal change in a neighboring organ. In this way a quite trivial change in one organ may set in motion a chain reaction extending into all parts of the abdomen. The weight of the abdominal contents is considerable, especially in the larger herbivores. They “float” within the serous fluid, and the gravitational forces are opposed by the tension actively and passively developed by the structures of the abdominal wall, by the cranial pull on the diaphragm exerted by the negative pressure within the thorax, and, to a lesser and uncertain extent, by the mesenteries and vessels that support particular organs.
The essence of the situation can be conveyed schematically (Figure 3–34). It is seen that the internal pressure varies at different heights within the abdomen; it is less than the ambient pressure in the most dorsal part, equal to this at one particular level, and increasingly greater than this toward the abdominal floor. This explains the concavity of the upper part of the flank very evident in cattle and also the tendency for air to rush into the rectum when exploration of this part is clumsily performed. Clearly, the local internal pressures also vary with respiratory changes in intrathoracic pressure and with posture.
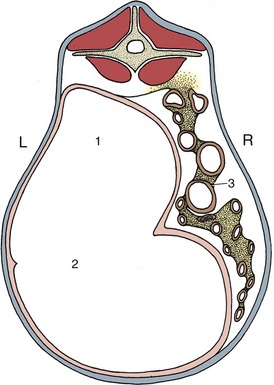
Figure 3–34 Section through the abdomen of a goat. The greater pressure in the lower part of the abdomen causes the convex form of the lower part of the abdominal wall. The pressure within the upper part of the abdomen is below that of the atmosphere, and the flank is sunken. 1, Gas in upper part of rumen; 2, ingesta in the lower part of rumen; 3, intestines.
The significance of the mesenteries and other attachments in determining visceral topography is disputed. Some of the more robust attachments, for example, those between the liver and the diaphragm, anchor organs quite firmly; others are too frail to play a significant role, and the organs to which they attach must be held in place by mutual contact and by the “lift” of the diaphragm. Certainly, they drop as soon as air is introduced into the peritoneal cavity. The potbellied appearance familiar in many older people is alleged to be in part a consequence of the loss of elasticity in the lungs with resulting reduction of the diaphragmatic “pull.” Some of the arteries that branch from the aorta to supply abdominal organs possess an unusually thick adventitia, and this may allow them to bear some weight when the enclosing mesenteries are fully stretched.
In the dead animal the viscera commonly conform to a fixed pattern. If allowance is made for such obvious factors as the recent consumption of a meal, a tolerably accurate forecast of their disposition can be made before the abdomen is opened, although this introduces air and hence some sagging is inevitable. Therefore, good reason once existed for believing that each of the hollow organs possessed a fairly constant “normal” form. The introduction of radiography destroyed this comfortable illusion, although not before many patients had their organs “tailored” to fit the preconceptions of surgeons reared on traditional anatomy. It can hardly be stressed too strongly that detailed assertions of normal form and position have no place in the description of the hollow organs.
When the positions of the abdominal organs need to be described, it is generally sufficient to relate them to the abdominal wall by means of everyday expressions.
THE STOMACH
The stomach, interposed between the esophagus and small intestine, is the dilated part of the digestive tract in which the processes of digestion are initiated. It is succeeded by the intestine, which consists of a proximal small intestine (the principal organ of digestion and absorption in most species) and a distal large intestine (generally much shorter and especially concerned with the dehydration of the food residue).
However, among mammals there exists considerable diversity in the form and structure of these two parts of the digestive system, which are closely associated in function and which are collectively known as the gastrointestinal tract. Much of this diversity is clearly adaptive and reflects the habitual diet of the various groups. The concentrated diet of carnivores is most easily digested, and these animals have a small and simple stomach (Figure 3–35, A) and a relatively short and uncomplicated intestine. The fodder of herbivores is less easily managed; it has a lower nutritive value and must be consumed in large amounts. Moreover, a major part consists of celluloses and other complex carbohydrates that are not susceptible to the action of mammalian digestive enzymes. These substances can be utilized only if they are first broken down by symbiotic microorganisms; this is a relatively slow process that requires the provision of a large fermentation chamber where food may be held in an environment favorable to the multiplication and activity of the microorganisms. In some herbivorous species, such a chamber is supplied by a greatly enlarged and subdivided stomach, in others by a voluminous and complicated large intestine. Ruminants illustrate the first alternative, the horse the second. Some indication of the range of variation of gastrointestinal anatomy among domestic species is provided by Figure 3–36. Detailed accounts are found in the chapters concerned with individual species; the description that follows is largely confined to the simple organs of the dog and cat.
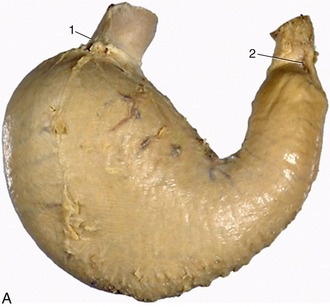
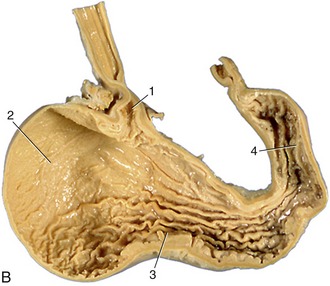
Figure 3–35 A, Visceral surface of stomach (dog). 1, cardia; 2, pylorus. B, Interior of stomach (dog). 1, cardiac opening; 2, fundus; 3, body; 4, pyloric antrum.
Figure 3–36 Gastrointestinal tracts of the dog (A), of the horse (B), and of cattle (C) laid out in one plane. 1, Stomach; 2, small intestine; 3, cecum; 4, ascending colon; 5, descending colon.
The stomach (ventriculus)* receives food from the esophagus and retains it for a time before discharging it into the duodenum, the first part of the small intestine. The stomach of the dog has a relatively modest capacity, ranging from 0.5 to 6.0 L according to breed, and conforms to a pattern that is common to most carnivores and indeed to many other mammals, including ourselves. It consists of two distinct parts that converge and join at a ventral angle (Figure 3–37). The larger part, into which the esophagus opens at the cardia, lies mainly to the left of the median plane, well forward under cover of the ribs and in direct contact with the liver and the diaphragm; it is relatively distensible and rapidly expands to accommodate a meal. The second part is narrower, has thicker walls, and is more constant in appearance since it is less affected by the presence of a meal; it passes to the right to continue into the duodenum at the pylorus (Figure 3–35, B). The cranial (parietal) aspect of both parts is mainly in contact with the liver, while the more numerous relations of the caudal (visceral) surface include the intestinal mass, left kidney, pancreas, and greater omentum. The left part of the margin is applied to the hilar region of the spleen.
Figure 3–37 The tunica muscularis of the canine stomach. A, Parietal surface after removal of the serosa. B, Stomach turned inside out with the mucosa removed. The tunica muscularis comprises outer longitudinal, middle circular, and inner oblique layers. The longitudinal layer clothes the curvatures (1) and the pyloric part (1′) but is thin over the body. The circular layer surrounds the body (2) and is especially prominent on the pyloric part (2′), where it furnishes the pyloric sphincters (2″). The oblique layer (3) is thickest along the lesser curvature, where it forms two lips that fuse over the cardia (cardiac loop); it is thin where it lines the fundus and body (3′).
Other terms are available when it is necessary to refer to particular regions of the stomach more precisely. The large left sac is divided between a blind dome (fundus) rising above the cardia and a body (corpus) extending from the cardia to the ventral angle. The more tubular right or pyloric part is divided between a more proximal pyloric antrum and a more distal pyloric canal; the distinction is based on the terminal muscular thickening (see Figure 3–35, B). The margin separating the two surfaces is divided between greater and lesser curvatures, each of which runs between the cardiac and pyloric openings. The convex greater curvature gives attachment to the greater omentum, of which a part (gastrosplenic ligament) connects the spleen with the stomach. The shorter, concave lesser curvature is connected with the liver by the lesser omentum. This curvature is marked by a sharp change in direction known as the angular notch (incisura).
The stomach wall is composed of layers corresponding to those of the esophagus and intestine. The external peritoneum or serosa covers the entire organ, adhering to the underlying muscle, except along the curvatures, where it is reflected to continue into the omenta; its absence from the curvatures makes them the parts most likely to burst when the organ is excessively distended.
The next coat is of smooth muscle and is arranged in three layers, each of which is incomplete but with its deficiencies compensated by the others. The external layer is more or less longitudinal and continues the outer muscle of the esophagus; it is concentrated along the curvatures, although it spreads more widely over the pyloric part. The middle layer is disposed in hoops, and those most proximal form a weak sphincter around the cardia; beyond this the pattern is interrupted by the projection of the fundus, but it is resumed at a lower level. It then continues to the pyloric canal, where the hoops are bunched together on the lesser curvature, forming a muscular knot (that in some species produces an obvious projection into the lumen) and fanning out on the greater curvature; the edges of this “fan” are sometimes held to constitute proximal and distal pyloric sphincters. The innermost layer is very incomplete but compensates for the deficiencies in the circular muscle; particularly stout fascicles arch above the cardia before continuing distally to each side of the lesser curvature, extending toward, but not beyond, the angular notch (see Figure 3–37).
The thin submucosa internal to the muscle is separated from the mucosa proper by a plexiform muscularis mucosae. It contains major arterial and venous plexuses and also a wealth of elastic fibers that help the muscularis mucosae throw the mucosa of the empty organ into the folds (rugae) that provide the characteristic surface relief (Figure 3–37 and Figure 3–38, A). These folds are predominantly longitudinal in orientation, although individually tortuous; they are completely effaced only when the stomach is grossly distended.
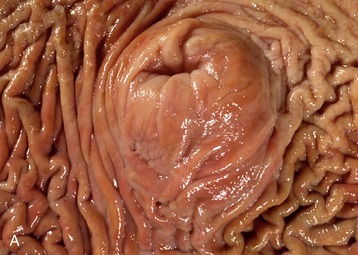
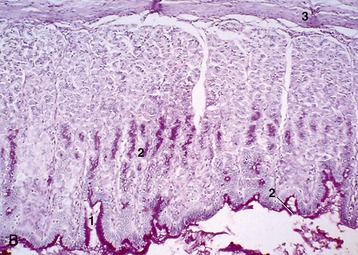
Figure 3–38 A, Protruding cardia surrounded by longitudinal folds. B, Mucosa of stomach (PAS-H; 70×) (dog). 1, gastric pit; 2, mucopolysaccharide-secreting cells; 3, lam. muscularis mucosae.
The entire gastric mucosa is densely pockmarked by innumerable tiny depressions. These so-called gastric pits (many would be better described as crevices) are invisible to the naked eye but account for the surface folding seen in histological sections (Figure 3–38, B). The surface epithelium of columnar, mucus-secreting cells continues into the pits and even extends into the uppermost parts of the gastric glands that deliver their products into the depth of the pits. This epithelium is largely responsible for the protective coat that makes gastric mucosa slimy to the touch. The gastric glands are of three varieties, termed cardiac, proper gastric (fundic), and pyloric, although it must be stressed that in many species, including the dog, their distribution does not exactly coincide with the gross regions that bear the same names. The cardiac and pyloric glands produce additional mucus, whereas the proper gastric glands are alone responsible for the gastric juice active in digestion by virtue of its pepsin and hydrochloric acid content. The enzyme is the product of its most numerous (chief) cell type, the acid of the fewer parietal cells; there is also a further contingent of mucus-secreting cells. It is claimed that the proper gastric glandular region has a somewhat darker hue than the remainder of the mucosa.
The blood supply to the stomach comes from all three chief branches of the celiac artery and is particularly generous along the two curvatures (Figure 3–39). The arteries anastomose quite freely externally and also within the stomach wall. For the most part, the arteries that penetrate the wall pass to the submucosa before branching to form an elaborate plexus from which both the muscular and the mucosal coats are fed. The mucosal branches supply unusually wide-bored capillaries below the epithelium and about the glands.
Figure 3–39 Distribution of the celiac artery of the dog (ventral view). 1, Aorta; 2, celiac artery; 3, hepatic artery; 4, splenic artery; 5, left gastric artery; 6, left gastroepiploic artery; 7, gastroduodenal artery; 8, right gastric artery; 9, cranial mesenteric artery; 10, pancreas; 11, spleen; 12, stomach; 13, liver.
The veins are similarly arranged and ultimately combine to form trunks that join the portal vein. Numerous arteriovenous anastomoses provide a means of regulating mucosal blood supply, and much blood is diverted from the capillary bed of the fasting organ.
Lymph vessels are present in profusion, particularly in the submucosa. They lead to several gastric nodes, each charged with the drainage of a particular territory.
The stomach is innervated by parasympathetic fibers within the two vagal trunks and by sympathetic fibers that reach the organ with the arteries. The efferent fibers of both sets are accompanied by more numerous afferent fibers. Parasympathetic fibers of the vagus synapse on ganglion cells in intramural plexuses within the submucosa and between the muscle coats and exert a high measure of control over gastric motility. The effects of vagal stimulation on the proximal and distal regions of the stomach are dissimilar: in the proximal stomach, vagal activity suppresses muscular contraction and leads to adaptive relaxation, whereas in the distal stomach, vagal stimulation causes intense peristaltic activity. Vagal stimulation of distal antral motility is mediated by acetylcholine, but the identity of the inhibitory mediator is not well established; it may be vasoactive intestinal peptide. The intramural plexuses are involved in the local reflexes in which the stomach wall reacts to direct stimulation. Sympathetic and parasympathetic fibers also innervate the surface epithelium and glands, but only parasympathetic fibers end on the intragastric endocrine cells.* Division of the vagal nerves, either the main trunks or selected branches, reduces gastric activity and secretion.
The topography and the form of the living stomach of the dog are much influenced by functional changes. The empty stomach is small and contracted toward the fixed point of the esophageal entrance. It lies entirely within the rib cage and fails to reach the abdominal floor. The wall is generally inert except for occasional weak peristaltic contractions, and little secretion from the glands occurs. Any residual peristaltic activity ceases as soon as food is offered (or anticipated). Secretion increases as a reflex response to the taste of food or the effort of mastication; it appears to be independent of food actually reaching the stomach. When food does arrive, it first collects in layers (because as yet no mixing movements are present) and largely occupies the body, which expands in all directions but principally ventrally and caudally. A motor response is delayed, and when it begins, it is relatively slow in building to a peak. Peristaltic contractions commence near the cardia and course distally, accelerating and becoming more vigorous when they reach the muscular pyloric antrum. The terminal segment contracts en masse, and the injection of ingesta into the duodenum therefore occurs when the wave is still some distance from the pylorus. Radiographic studies suggest that the pylorus is open for about one third of the time; it is probable that emptying is more dependent on intermittent increase of the intragastric pressure than on the regular peristaltic activity.
The effects of feeding on topography and relations are considerable, especially in animals kept under regimens that allow them to feed seldom but to repletion. The fully distended stomach may extend almost to the umbilicus—or even beyond this in the puppy—pushing the intestinal mass dorsally and caudally. The liver is pushed to the right while the spleen, tethered to the left part of the greater curvature, follows the expansion of that side of the stomach.
THE INTESTINE
The intestine* commences at the pylorus and continues to the anus. It is divided between the proximal small intestine (intestinum tenue) and the distal large intestine (intestinum crassum), which are parts that do not always differ as much in caliber as their names suggest. However, the boundary is made obvious by the outgrowth of a blind diverticulum, the cecum, at the origin of the large intestine (Figure 3–40). The small intestine consists of three parts: an initial duodenum, which is short and rather closely fixed in position, and the jejunum and ileum, which are carried by the great mesentery. The large intestine also comprises three parts; recognition of the blind-ending cecum presents no problem, but the separation of colon from rectum is arbitrarily put at the pelvic inlet. The rectum joins the short anal canal that leads to the exterior, but this canal is not part of the intestine in the strict sense.
Figure 3–40 Intestinal tract of the dog (schematic). 1, Stomach; 2, descending duodenum; 3, caudal flexure; 4, ascending duodenum; 5, jejunum; 6, ileum; 7, cecum; 8, ascending colon; 9, transverse colon; 10, descending colon; 11, rectal ampulla; 12, jejunal lymph nodes.
The length of the intestine may be given in absolute terms or, more usefully, in measures of body length. Unfortunately, the figures commonly quoted cannot be taken too seriously, as formidable difficulties in measurement are present in life and uncertainty is introduced by relaxation of the gut after death. The dog, in keeping with its diet, has a relatively short gut; it is perhaps some three or four times its body length in life. Intestinal length in herbivores varies with the nature of the gastrointestinal adaptation but may be as much as 25 times the body length in sheep.
THE SMALL INTESTINE
The duodenum is short and closely attached to the abdominal roof by a short mesoduodenum. The initial portion continues from the pyloric part of the stomach and passes toward the right body wall before being deflected caudally to descend to a point between the right kidney and the pelvic inlet. It then passes medially, behind the root of the mesentery, before ascending a short distance; it ends by bending ventrally to enter the mesentery, where it is continued as the jejunum. The more constant relations of the dog’s duodenum are to the liver at its origin, thereafter to the right body wall laterally, to the pancreas and later the right kidney medially, and, overall, to other parts of the intestinal mass. Although the first part of the duodenum is not expanded to form a distinct “duodenal bulb” or “cap” (so commonly the site of ulcers in people), its functional independence is retained.
The jejunum and ileum are less closely fixed in position, but, although the arrangement of individual coils continually adjusts, this gut as a whole occupies a more or less constant position in the ventral part of the abdominal cavity (Figure 3–41). The coils are carried by the mesentery, which conveys the vessels and nerves; the mesentery is bunched at its root around the origin of the cranial mesenteric artery from the aorta and widens to the length of the gut at its other margin. The initial and final portions of the mesentery are shortest and ease the transitions with the relatively fixed duodenum at one end and with the ascending colon at the other (see Figure 3–40). The distinction between jejunum and ileum is arbitrary and perhaps unnecessary, for although certain progressive structural changes occur, these do not allow recognition of a sharp boundary. The convention that we follow limits the ileum to a short, relatively more muscular (and hence firmer) final portion with a direct peritoneal connection with the cecum. Many anatomists from English-speaking countries assume a more or less equal division between the two parts.
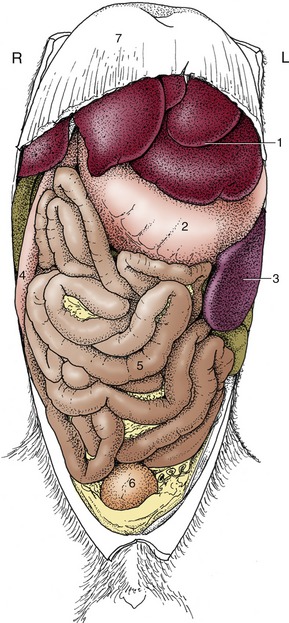
Figure 3–41 Ventral view of the abdominal organs of the dog after removal of the greater omentum. 1, Liver; 2, stomach; 3, spleen; 4, descending duodenum; 5, jejunum; 6, bladder; 7, diaphragm.
The jejunum fills those parts of the abdomen that are not preempted by other viscera. In the dog, in which the large intestine is relatively small, it lies more or less symmetrically about the midline, between the liver and stomach cranially and the urinary bladder caudally. It lies on the abdominal floor, though separated from the parietal peritoneum by the intervention of the greater omentum. The coils are quite mobile, and at first sight their disposition appears to be haphazard; closer inspection shows that there is some pattern to the arrangement. The mainly sagittal coils of the proximal part lie largely cranial to the more transverse coils of the distal part (see Figure 3–41). The ileum pursues a rather direct cranial, dorsal, and dextral course toward its junction with the large intestine. In life the intestine is not uniformly full, and at any moment most parts are flattened and molded by the pressures of adjacent viscera. The lumen may be locally obliterated, and when a passage is retained, it is more often than not reduced to a narrow channel along one margin: a “keyhole” form is seen when viewed in section. This explains the narrow streaks that are the common representation of the small intestine in radiographs obtained after the administration of a barium suspension. Segmental and peristaltic movements continually alter the configuration in life.
The intestine is composed of the usual four tunics (Figure 3–42). The luminal surface has a velvety appearance because of the innumerable tiny but densely packed projections known as the intestinal villi. These are fingerlike in the dog and horse but broader and leaflike in many species (Figure 3–43). In addition to the interspecific differences, variations in form and dimension may be present at different locations along the length of the small intestine. The appearance and the detailed morphology may be profoundly influenced by changes in diet (early weaning) or disease (microbial infections). The villi greatly increase the area of epithelium available for absorption; the efficiency of the process is enhanced by very generous subepithelial capillary plexuses (Figure 3–43, B). Microscopic intestinal glands (crypts) open to the surface between the bases of the villi. The crypts produce a mucous secretion, which coats the surface of the bowel, and various enzymes that contribute to the further digestion of carbohydrate and protein breakdown products.
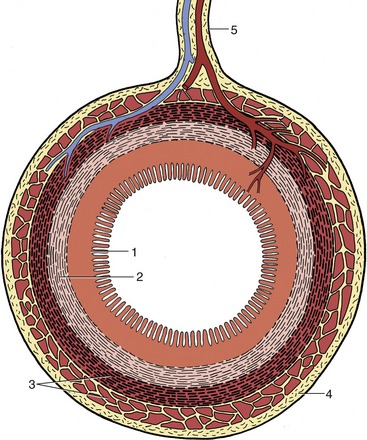
Figure 3–42 Transverse section through the gut. The artery and vein reach the gut via the mesentery; the larger branches fail to reach the antimesenteric border. 1, Mucosa; 2, submucosa; 3, muscle layer; 4, serosa; 5, mesentery.
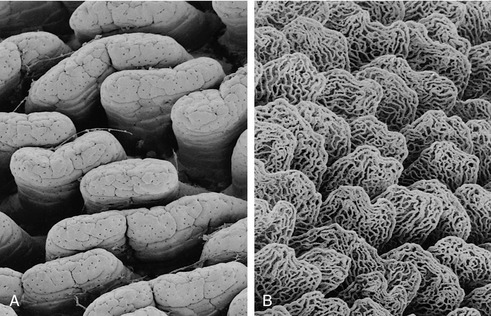
Figure 3–43 Scanning electron micrographs of rat duodenal villi (A) and of a vascular cast of the same tissue demonstrating subepithelial capillary plexuses (B).
Larger (Brunner’s) glands confined to the submucosa of the duodenum, especially its initial part, also secrete a protective mucus. A proportion of the cells lining the crypts, perhaps 1% of the total population, belong to the enteroendocrine (enterochromaffin) system (p. 222). Of several varieties, these cells form a series, commencing with the gastrin-producing cells of the stomach and extending through the small into the large intestine, that produces a number of hormones that influence various aspects of gastrointestinal activity. The intestinal components of the series, unlike that of the stomach, are under regulation by intrinsic nerves of the organ wall and largely outweigh the influence of the extrinsic nerve supply to the gut. Cholecystokinin, which provokes contraction of the gallbladder, is an important member of the set.
The great length and the villous surface of the small intestine combine to increase the absorptive area. In some species the absorptive area is also increased by permanent longitudinal and spiral folds; these are not pronounced in the dog, and the mucosal relief sometimes visible in radiographs is produced by temporary ridges.
The mucosa is rich in nodules of lymphoid tissue, both solitary and clumped; the larger aggregations (Peyer’s patches*; [Figure 3–44]) cause visible depressions and elevations of the mucosa that may become more obvious by the absence of a covering pile of villi. These aggregations tend to be more numerous and individually larger toward the junction with the large intestine.
Attention must be directed, however briefly, to the remarkable cycle of epithelial renewal exhibited by the lining of the small intestine throughout life. The epithelium is renewed by the mitotic division of cells in the depths of the crypts. The cells lining the crypts, continuously recruited in this way, gradually ascend to the surface, spread to embrace the bases of the villi, and continue up these to the summits where they are finally shed into the gut lumen. The passage from the bottom of a crypt to the summit of a villus takes about 3 days and involves a prodigious wastage—one calculation suggests a loss of about 1 g of epithelial cells for every centimeter stretch of the human small intestine every day. The process has the fortunate consequence of permitting rapid renewal of the integrity of the gut lining after extensive damage, such as the necrosis and loss by sloughing of the surface layer that occurs in certain infections in various domestic species. While repair is in train, the villi are reduced in size; they are not fully restored until a sufficiency of epithelial cells has again become available to clothe villi of normal height and proportions.
Both the liver and the pancreas discharge into the duodenum. The arrangement in the dog is for the bile duct and one pancreatic duct to discharge by separate openings on a (major duodenal) papilla a few centimeters beyond the pylorus, while the second larger pancreatic duct discharges on a smaller papilla a little farther on. Neither papilla is conspicuous.
THE LARGE INTESTINE
In its most elementary form the mammalian large intestine is a short tube, little wider than the small intestine from which it arises to pursue a direct course to the anus. The canine large intestine is somewhat more complicated, though still simple if compared with that of herbivores (Figure 3–45). As in most species, it is clearly divided into cecum, colon, and rectum, while the colon is itself differentiated into ascending, transverse, and descending parts (Figure 3–45/3,4,5). The cecum is a blind-ending piece of gut that arises at the junction of the ileum and colon. The division of the colon follows from the rotation of the embryonic gut imposing a conformation on the adult organ that resembles a question mark (when viewed from below; Figure 14–15).
Figure 3–45 Schematic drawing of the large intestine of the domestic mammals: carnivores (Car), the pig (su), ruminants (Ru), and the horse (eq). Cranial is to the upper right. 1, Ileum; 2, cecum; 3, ascending colon; 4, transverse colon; 5, descending colon; 6, rectum and anus; 7, aorta; 8, celiac artery; 9, 9′, cranial and caudal mesenteric arteries; 10, 10′, dorsal diaphragmatic and pelvic flexures of ascending colon; 11, 11′, proximal and distal loops of ascending colon.
The canine cecum is unusual in having no direct connection with the ileum; however, because it is conventional to regard the cecum as the first part of the large intestine, the description commences with it. The cecum of the dog is short and at first sight appears even shorter because it is drawn into a spiral and held against the ileum by folds of peritoneum. It is only slightly wider than the small intestine and tapers slightly toward its rounded blind extremity. The lumen communicates with the interior of the colon, immediately beyond the ileocolic junction, through an opening that is guarded by an inner, circular, muscular ring (the cecocolic sphincter) (Figure 3–46).
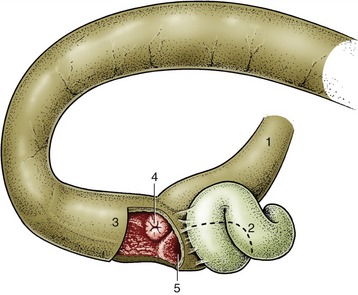
Figure 3–46 The ileocolic junction and its relation to the cecum in the dog. 1, Ileum; 2, cecum; 3, ascending colon; 4, ileal orifice surrounded by annular fold; 5, cecocolic orifice.
The smooth, externally featureless colon has a caliber that is uniformly and significantly, though not remarkably, greater than that of the small bowel. It is suspended throughout its length by a moderately long mesocolon, which allows it some mobility, and its position and relations vary within certain limits; the flexures that divide it into ascending, transverse, and descending parts are not precisely fixed. The short ascending part continues the axis of the ileum from a junction defined internally by an ileocolic opening of similar appearance and construction to that at the origin of the cecum. The transverse part runs across the abdomen from right to left, between the stomach cranially and the mass of small intestine and cranial mesenteric artery caudally. The descending part is the longest; it follows the left flank before edging medially to enter the pelvic cavity, where it is continued as the rectum without other visible demarcation than the passage across the abdominopelvic boundary. The term rectum implies a straight course, but often this part of the bowel is deflected to one side by pressure from other viscera, most usually a distended bladder. The rectum is the most dorsal of the pelvic viscera and lies above the reproductive organs, bladder, and urethra. Its cranial part has the same relationship to the peritoneum as the colon, but this changes as the mesorectum shortens and the serosal covering is reflected laterally to continue into the parietal peritoneum of the pelvic cavity and ventrally to continue over the urogenital organs. The terminal part is wholly retroperitoneal and is directly attached to the vagina in the female, to the urethra in the male, and to the pelvic diaphragm in both sexes.
The mucosa of the large intestine is generally smooth because villi are lacking. No permanent mucosal folds are present, but there are numerous scattered lymph nodules, especially in the rectum, where they tend to be conspicuous; this is because the summits of the swellings are here depressed, leading to tiny pits. In many species, including the horse and pig among domestic animals, the outer muscle coat of the large intestine is mainly concentrated in a number of bands (teniae), which, on shortening, pucker the gut so that a linear series of sacculations (haustra) is produced (see Figure 21–11). Such bands are not present on the intestine of the dog and cat.
The anal canal joins the bowel to the exterior. It is a short passage that is derived from the proctodeum, the invagination of the surface ectoderm. The lumen is constricted at the rectoanal junction where the mucosa is thrown into longitudinal folds, normally pressed together to occlude the orifice (Figure 3–47). Anal continence, however, depends primarily on the presence of two sphincters; the internal anal sphincter is merely a thickening of the circular smooth muscle of the gut, but the external sphincter is striated, of somatic origin, and under voluntary control (Figure 3–48).
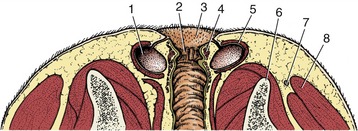
Figure 3–47 Dorsal (horizontal) section through the canine anal canal. 1, Anal sac; 2, columnar zone of the anal canal; 3, cutaneous zone; 4, internal anal sphincter; 5, external anal sphincter; 6, ischium; 7, sacrotuberous ligament; 8, gluteus superficialis.
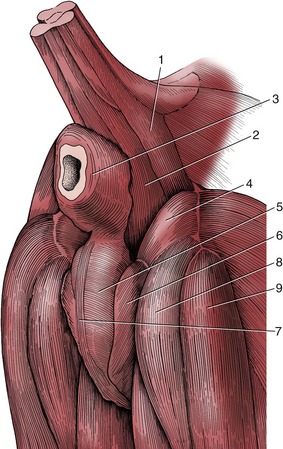
Figure 3–48 The muscles of the perineal region of the male dog. 1, Coccygeus; 2, levator ani; 3, external anal sphincter; 4, internal obturator; 5, bulbospongiosus; 6, ischiocavernosus; 7, retractor penis; 8, semimembranosus; 9, semitendinosus.
Many glands are always present in the anal region, both in the mucosa and in the surrounding skin. Most are small, but the dog and cat also possess two so-called anal sacs (sinus paranales). Each is roughly the size of a hazelnut (in the dog) and is located ventrolateral to the anus between the internal and external sphincters (see Figures 3–47 and 15–4). The fundus of the sac secretes an evil-smelling fluid that drains through a single duct to an opening near the anocutaneous junction. The sac is compressed at defecation, expelling the secretion, which probably serves as a territorial marker. Such sacs are found in most carnivores and are most notorious in the skunk.
The blood supply to the intestinal tract is mainly provided by the cranial and caudal mesenteric arteries; however, the initial part of the duodenum is supplied through the hepatic branch of the celiac artery and the caudal part of the rectum by rectal branches of the internal pudendal artery. The cranial mesenteric artery supplies the bulk of the small intestine, the ileocecocolic junctional region, and the midpart of the colon through its three primary divisions; the details of branching vary among species and also, though to a lesser extent, among individuals. The smaller caudal mesenteric artery has a distribution restricted to the descending colon and cranial part of the rectum. The arrangement in the dog is illustrated (Figures 3–42 and 3–49); although its relevance in surgery suggests that the pattern of arterial branching should be known, the richness of the anastomoses is of even greater importance. These ensure that the intestine can normally survive the complete obstruction of a major supplying vessel. The chain of anastomoses continues beyond the territories of the mesenteric arteries to connect with those of the celiac and internal pudendal arteries.
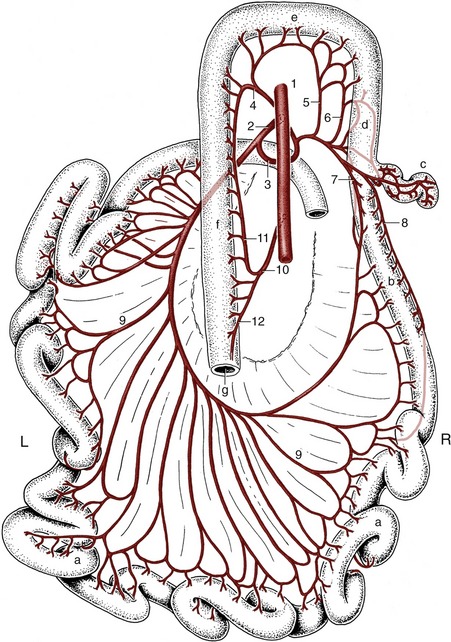
Figure 3–49 Distribution of the cranial and caudal mesenteric arteries to the intestines of the dog (dorsal view). a, Jejunum; b, ileum; c, cecum; d, ascending colon; e, transverse colon; f, descending colon; g, rectum. 1, Aorta; 2, cranial mesenteric artery; 3, ileocolic artery; 4, middle colic artery; 5, right colic artery; 6, colic branch of ileocolic artery; 7, mesenteric ileal branch; 8, antimesenteric ileal branch; 9, jejunal arteries; 10, caudal mesenteric artery; 11, left colic artery; 12, cranial rectal artery.
The veins are broadly comparable and join to form the cranial and caudal mesenteric veins, two of the main radicles (the splenic vein is the third) of the portal vein (Figure 3–50). Certain tributary veins connect with systemic veins at the extremities of their territories, which are the thoracic esophagus and anal canal, parts that normally drain by systemic routes. Congestion within the portal circulation (p. 137) may lead to enlargement of submucosal veins in both these (and other) parts but is much more important in human than in veterinary medicine. The gut wall contains a considerable proportion of the lymphocyte population and represents an important component of the body’s defense mechanism, one capable of barring entry to a variety of antigens.
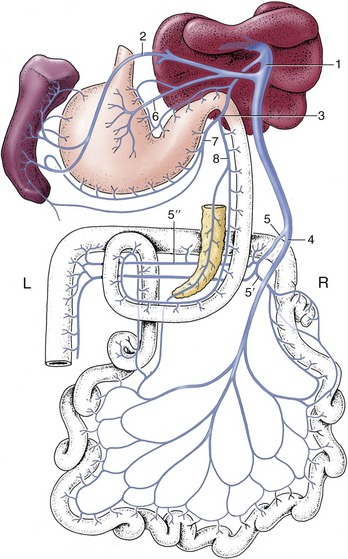
Figure 3–50 Semischematic dorsal view of the formation of the portal vein (dog). 1, Portal vein; 2, splenic vein; 3, gastroduodenal vein; 4, cranial mesenteric vein; 5, caudal mesenteric vein; 5′, ileocolic vein; 5″, middle colic vein; 6, left gastric vein; 7, right gastroepiploic vein; 8, cranial pancreaticoduodenal vein.
The lymphatic drainage of the small intestine, in particular, is copious because some of the products of digestion are absorbed by this route. When these products include fat, the lymph is milky and the intestinal lymphatic vessels (“lacteals”) are unusually conspicuous. The flow is directed toward certain nodes through which the lymph percolates before joining the cisterna chyli, the dilated origin of the thoracic duct, the most important lymphatic vessel (p. 260). In the dog these nodes are large but few and are centralized toward the root of the mesentery (see Figure 3–40); in other species they may be more numerous and more widely scattered and may include many that are peripheral, close to the gut itself.
The intestine receives both sympathetic and parasympathetic nerves. The sympathetic pathways lead through the celiac, cranial mesenteric, and caudal mesenteric ganglia, and the postganglionic fibers enmesh the relevant arteries (see Figure 8–76). The parasympathetic pathways involve both vagal and pelvic nerves. The former supply the intestine to the junction of the transverse and descending parts of the colon; the latter supply the descending colon and rectum. The parasympathetic nerves augment peristalsis, but the effects of intestinal denervation are far less striking than those of gastric denervation.
Under stress, vasoconstriction may close the capillary bed of the intestinal wall, leading to abnormal permeability that allows large molecules to overcome the gut barrier; septic shock is then an eventual possibility.
THE LIVER
The liver (hepar) is located in the most cranial part of the abdomen, immediately behind the diaphragm. It is by far the largest gland in the body and performs many functions essential for life. The most obvious is the production of bile, but the parts it plays in protein, carbohydrate, and fat metabolism are even more important and depend on the liver’s situation astride the bloodstream draining the gastrointestinal tract. This ensures that the products of digestion, which are conveyed in the bloodstream after absorption, are presented to the hepatic cells before entering the general circulation.
The metabolic functions of the liver explain the wide interspecific variation in size: average values are about 3% to 5% of body weight in carnivores, 2% to 3% in omnivores, and as little as 1% to 1.5% in herbivores. The liver is substantially heavier in the young animal than in the adult; it often shows considerable atrophy in old age. Usually brownish-red, the fresh liver is soft and has a characteristic friable consistency.
The adult liver intervenes between the diaphragm cranially and the stomach and intestinal mass caudally. Although extended across the median plane, the bulk lies to the right in all species (Figure 3–51). It is not so very asymmetrical in the dog: the proportions to the right and left of the median plane are about 3:2. In most species, including the dog, the liver is grossly divided into lobes by a series of fissures that extend inward from the ventral margin (Figure 3–52). The lobation pattern shows many features of resemblance among different mammals, and considerable effort has been given to determining the homologies of individual lobes and fissures. The theoretical pattern, which accords the dog’s liver left lateral, left medial, right lateral, right medial, quadrate, and caudate lobes, of which the last is enlarged by papillary and caudate processes, is illustrated (Figure 3–53). It should not be regarded as more than a convenient fiction that facilitates description. Modern studies minimize the significance of the external fissuration and rely more on the internal ramifications of the vessels to establish homologies. Such studies have had the useful by-product of providing the surgeon with the detailed knowledge of the vascular architecture necessary for the safe removal of diseased parts of the human liver.
Figure 3–51 Caudal surface of the liver of the dog (A), pig (B), horse (C), and cattle (D). The median planes are indicated. The liver is asymmetrical, less so in the dog, more so in the pig and horse, and most in cattle, in which the bulk of the organ is displaced to the right. Note the absence of a gallbladder from the horse liver.
Figure 3–52 A, Visceral surface of liver (dog). B, Visceral surface of liver (pig). 1, Gallbladder; 2, hepatic ducts.
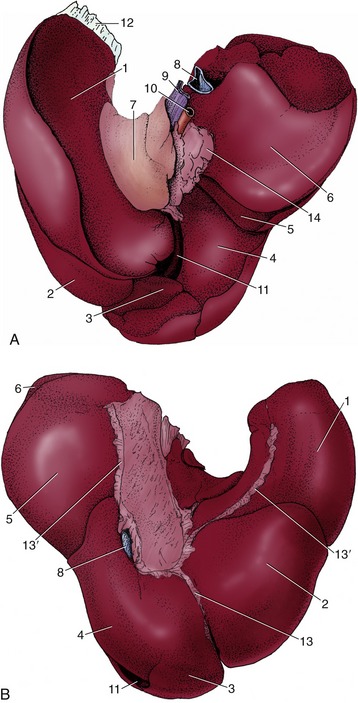
Figure 3–53 Visceral (A) and diaphragmatic (B) surfaces of the canine liver. 1, Left lateral lobe; 2, left medial lobe; 3, quadrate lobe; 4, right medial lobe; 5, right lateral lobe; 6, caudate process (of caudate lobe); 7, papillary process (of caudate lobe); 8, caudal vena cava; 9, portal vein; 10, hepatic artery; 11, gallbladder; 12, left triangular ligament; 13, falciform ligament; 13′, coronary ligaments; 14, lesser omentum.
In life the liver adapts to the form of neighboring organs, and when fixed in situ, it retains the conformation and impressions these impose. The rather large liver of the dog is therefore bluntly conical, and its cranial surface matches the curvature of the diaphragm against which it is pressed. The caudal surface is concave; to the left it exhibits a large excavation for the stomach, which is then extended over the median plane into a narrow duodenal groove. The dorsal border extends more caudally and reaches farther dorsally on the right side, where it is further extended by the caudate process, which carries a deep impression for the cranial pole of the right kidney. Toward the median plane, this border carries a groove for the passage of the caudal vena cava and, to the left of this, a notch for the esophagus. The gallbladder lies between the quadrate and right medial lobes; it is partly attached, partly free, and in some dogs so deeply embedded that it reaches the parietal surface, thus making contact with the diaphragm (see Figure 3–53).
The liver is clothed in peritoneum except for relatively small areas at the porta (hilus), in the fossa for the gallbladder, and at the origin of certain peritoneal reflections. The right and left triangular, the coronary, and the falciform ligaments that pass to the diaphragm from the parietal surface have fibrous cores and attach the liver firmly; the lesser omentum, which passes from the visceral surface to the stomach and duodenum, is more fragile. A tunica fibrosa encloses the parenchyma beneath the serosa; it enters the substance at the porta and detaches extensions that convey the blood vessels inward, dividing where the vessels divide and thinning at each division. The finer trabeculae pervade the entire organ and divide the liver into innumerable small units, the hepatic lobules of the classic description. Although particularly marked in the pig’s liver (Figure 3–54), the lobular pattern is also quite obtrusive in that of the dog, in which the lobules appear as hexagonal areas (about 1 mm across) on the intact surface and in gross and histological sections.
Figure 3–54 A, Surface of liver (enlarged) with clearly defined hepatic lobules (pig). B, Liver (pig) (28×). 1, central v.; 2, interlobular a.; 3, hepatic lobule; 4, interlobular connective tissue; 5, centrolobular venule.
C, Scanning electron microscopy of corrosion cast of hepatic vessels (rat); note valve within central v.
The liver receives a very generous blood supply through the hepatic artery, a branch of the celiac artery, and the portal vein. The relative importance of these two supplies varies among species. The proportions are not known with certainty for the dog; the artery supplies the human liver with only one fifth of the blood but about three fifths of the oxygen. The branches of the hepatic artery that actually enter the liver are effectively end-arteries. However, provision exists for a collateral circulation outside the liver, between the hepatic artery and the other branches of the celiac artery that supply the stomach and duodenum (see Figure 3–39). The intrahepatic arteries divide in company with branches of the portal vein and tributaries of the hepatic duct. They supply the connective tissue structures en route to the hepatic sinusoids into which both they and the branches of the portal vein eventually discharge.
The portal vein is formed by the union of tributaries draining the digestive tract, pancreas, and spleen (see Figure 3–50). It is connected to systemic veins in the cardioesophageal and rectoanal regions at the extremities of its territory. These connections provide alternative outlets for portal blood when the flow through the liver is obstructed or impaired. The effects of obstruction vary between species and reflect the varying effectiveness of the hepatic artery in supplying oxygen. In the dog complete obstruction is rapidly fatal.
All blood delivered to the liver is collected by a single set of veins of which the central veins of the hepatic lobules are the smallest radicles. These eventually form the few large hepatic veins that open into the caudal vena cava as this tunnels through the liver substance. The circulation through the liver possesses numerous anastomoses—interarterial, intervenous, and arteriovenous; it is also controlled by various sphincter mechanisms, and together these features make it capable of very subtle regulation. A relatively rare congenital defect allows portal blood to pass directly to the caudal caval vein.
The liver receives sympathetic and parasympathetic nerves by way of periarterial plexuses and the vagal trunks, respectively.
The hepatic duct system begins with microscopic canaliculi within the lobules. These open into larger ductules that ultimately form a few large hepatic ducts by successive unions within the connective tissue between the lobules. Before or shortly after leaving the liver at the porta these combine in a single trunk that runs to the duodenum (Figure 3–55). A tortuous side branch (cystic duct) that arises from the common trunk leads to the pear-shaped gallbladder. The part of the common trunk that is distal to the origin of the cystic duct is known as the bile duct (ductus choledochus). Variation in the duct system is frequent; some hepatic ducts may enter the gallbladder directly, while others may join the main outlet distal to the cystic duct. The gallbladder not only stores the bile but also concentrates it by absorption through the folded mucosa. As is well known, a gallbladder is not essential; it is lacking in the horse, the rat, and certain other species, which compensate by enlargement of the duct system (see Figure 3–51).

Figure 3–55 The bile drainage system of the dog. 1, Gallbladder; 2, bile duct; 3, cystic duct; 4, hepatic ducts.
The muscle of the bladder wall and duct, including the sphincter at the entrance to the duodenum, is supplied by parasympathetic nerves. Pain arising from the duct system, common in human patients, is abolished by section of the (sympathetic) splanchnic nerves.
THE PANCREAS
The pancreas is a much smaller gland closely related to the duodenum in the dorsal part of the abdominal cavity. It is yellowish and bears some resemblance to a salivary gland, although it is softer and more loosely knit than most of these. It combines exocrine and endocrine functions.
The exocrine component is by far the larger; it produces a digestive juice that is discharged into the proximal part of the duodenum through one or two ducts. The juice contains enzymes that break down protein, carbohydrates, and fats. The endocrine component comprises the pancreatic islets, which are cell clumps that are scattered between the exocrine acini and are the source of insulin, glucagon, and gastrin; the islets are therefore of prime importance in carbohydrate metabolism (p. 222).
The pancreas is conventionally regarded as consisting of a body and two lobes, which is a description that suits the canine pancreas but is less apt for those of some other species (Figure 3–56). When hardened in situ, the canine pancreas is acutely flexed: the apex of the V nestles close to the cranial flexure of the duodenum. The slender right lobe runs within the mesoduodenum; the thicker but shorter left lobe extends over the caudal surface of the stomach toward the spleen, within the greater omentum (see Figure 3–33/7).
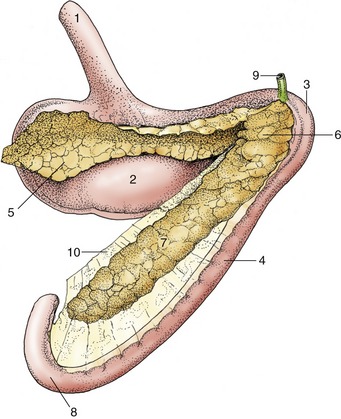
Figure 3–56 The pancreas of the dog (caudal view). 1, Esophagus; 2, stomach; 3, cranial flexure of duodenum; 4, descending duodenum; 5, left lobe of pancreas; 6, body; 7, right lobe; 8, caudal flexure of duodenum; 9, bile duct; 10, mesoduodenum.
The pancreas arises from two primordia that bud from the proximal part of the duodenum. The buds later merge, but in many species evidence of the dual origin of the pancreas is provided by its duct system. A greater pancreatic duct commonly drains the part of the pancreas that arises from the ventral primordium and opens into the duodenum together with, or just beside, the bile duct. A lesser (accessory) duct emerges from the part of the pancreas formed by the dorsal primordium and opens on the opposite aspect of the gut. This is the arrangement usually found in the dog, although the terminal part of one duct sometimes regresses; because the duct systems of the two lobes communicate within the gland, the absence of one or the other outlet is of no significance. In some species only one duct commonly survives.
The generous blood supply is from the cranial and caudal pancreaticoduodenal arteries, of which the former branches from the celiac and the latter from the cranial mesenteric artery. The veins drain to the portal vein. The gland is supplied by both sympathetic and parasympathetic nerves.
THE DEVELOPMENT OF THE DIGESTIVE APPARATUS
The foregut and hindgut end blindly at the oral and cloacal membranes, circumscribed median areas where the endoderm and ectoderm are in direct contact, with no intervening mesoderm (see Figure 3–2). These membranes form the floors of surface depressions known as the stomodeum and proctodeum. The depressions are deepened by the relatively rapid growth of the surrounding tissue; when the membranes break down the depressions become confluent with the gut, extending it at each end by a short passage lined with ectoderm. The cranial extension forms the larger part of the mouth, the caudal one the anal canal.
The foregut differentiates to form the pharynx, esophagus, stomach, and first part of the duodenum together with the structures formed by outgrowth from these parts. The midgut forms the remainder of the small intestine, the cecum, and the larger part of the colon. The hindgut forms the distal part of the colon, the rectum, and, after partitioning, part of the urogenital tract.
THE MOUTH
The stomodeum, carried ventrally in the folding process, comes to lie between the swelling of the forebrain dorsally and that over the developing heart ventrally. The oral membrane soon breaks down; with its disappearance it is no longer possible to recognize the extent of the ectodermal contribution to the lining of the mouth.
The mouth is built up by the forward growth of certain processes that appear around the margins of the oral plate. Dorsally, a frontal process appears as the result of a spurt in growth of the paraxial mesoderm around the forebrain. Laterally and ventrally, the margin is formed by the mandibular arch, the first of the thickenings (see further on) that develop in the mesoderm lateral to the presumptive pharynx.
The frontal process is initially a simple prominence. Soon bilateral thickenings, olfactory placodes, appear in the covering ectoderm immediately bounding the oral depression. These placodes sink below the surface when growth of the surrounding mesoderm throws up a rim around each. The rim has the form of a horseshoe with a ventral interruption leading to a groove extending to the mouth. The interruption divides the lateral and medial parts of the rim, which are known hereafter as the lateral and medial nasal processes. The mandibular arches also expand and grow toward each other at this time; they soon fuse ventral to the oral depression, forming the continuous shelf of the lower jaw and mouth floor. In addition, the upper end of each mandibular arch detaches a maxillary process that extends forward between the frontal and mandibular processes to enclose the mouth laterally. The various swellings gradually merge.
The depressions in which the olfactory placodes are contained originally communicate with the oral cavity, but these connections are lost when the placodes sink more deeply within blind pits, the nasal fossae, that now excavate the upper jaw. The tissue that remains between these pits and the mouth constitutes the primary palate. Communication between nose and mouth is regained when the pits eventually break through into the mouth cavity at two openings known as the primitive choanae (Figure 3–57). The disruption is considerable, and only the most rostral part of the primary palate survives.

Figure 3–57 Sagittal section through the nasal and oral cavity of a young embryo. 1, Lower lip; 2, tongue; 3, nasal cavity; 4, primitive choana (future incisive duct); 5, position of future secondary palate; 6, primary palate.
The definitive nasal cavities arise from a fresh subdivision of the temporarily combined nasal and oral spaces. The inner aspect of each maxillary process sends out a flange, the palatine process, which first hangs ventrally to the side of the developing tongue. At a certain stage it undergoes a very rapid reorientation in which it is swung inward and upward to meet its fellow of the other side (Figure 3–58, A-B). It fuses with this, with the residue of the primary palate, and with the lower edge of the septum between the nasal fossae; a horizontal shelf is thus formed between the nasal fossae and the mouth. Fusion of the residual primary palate (the region of the incisive papilla) with the palatine processes is almost complete but leaves open the small passages that become the incisive ducts. The shelf that now divides the nasal and oral cavities constitutes the secondary (definitive) palate, which later differentiates into rostral hard and caudal soft parts. The mechanism of its formation is not wholly understood; the timing is critical because the stage at which the secondary palate forms is normally soon followed by a marked widening of the head. If reorientation of the palatine processes is delayed, they are too short to bridge the gap and fail to fuse with each other and with the ventral edge of the nasal septum, which leaves the secondary palate divided by a median fissure through which the nasal and oral cavities communicate. The consequences of this anomaly (cleft palate) can be severe, not least because of resulting difficulties in feeding from the teat.
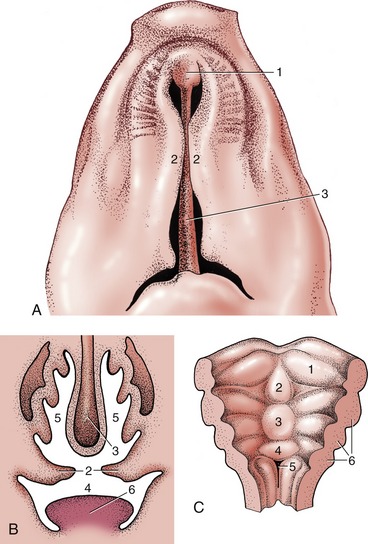
Figure 3–58 A, Ventral view of the development of the palate (pig). B, Transverse section through oral and nasal cavity before closure of the secondary palate. 1, primary palate; 2, palatine processes (secondary palate); 3, nasal septum; 4, oral cavity; 5, nasal cavity; 6, tongue.
C, Development of the tongue in the floor of the oral cavity. 1, Distal (lateral) tongue swelling; 2, median tongue swelling; 3, proximal tongue swelling; 4, primordium of epiglottis; 5, laryngeal entrance; 6, pharyngeal arches.
The division of the mouth cavity into its vestibular and central parts is foreshadowed by the appearance of ectodermal thickenings that run parallel to the margins of both the maxillary and the mandibular processes. These thickenings are soon transformed into grooves, known as labiogingival grooves, as they mark the division of the lips from the outer aspect of the gums; deepening of the grooves creates and then enlarges the vestibular space. A second, similar formation internal to the labiogingival groove of the mandibular process separates the gum from the tongue now developing in the floor of the mouth.
The salivary glands, both major and minor, are formed from solid outgrowths of epithelium that push into the underlying mesenchyme. These branch repeatedly and become canalized to form both gland acini and ducts. It is tempting to suppose that their sites of origin correspond with the points of entry of the adult ducts; however, some evidence suggests that the openings may be relocated when grooves in the oral epithelium are bridged over, extending the ducts.
The tongue develops in the floor of the mouth. It has a complicated origin, being formed by the mergence of several swellings (see Figure 3–58, C). One, a median (distal) tongue swelling, appears on the pharyngeal floor between the lower ends of the mandibular arches and later fuses with more lateral swellings that appear over the adjacent parts of these arches. A more caudal (proximal) swelling extends from the floor onto the ventral parts of the second, third, and, possibly, fourth pharyngeal arches. The caudal swelling divides as follows: the caudal part becomes the epiglottis and the rostral part blends with the other contributions to the tongue. The thyroid gland develops from the pharyngeal floor between the median and proximal swellings. The substance of the tongue is supposed to derive mainly from myotomes of occipital somites. It is alleged that material from these myotomes migrates forward under the floor of the mouth, and although the evidence is not wholly convincing, the theory satisfactorily accounts for the innervation of the lingual muscles by the hypoglossal nerve, which is the nerve specific to the occipital somites. The sensory supply to the lingual epithelium involves the mandibular, fascial, glossopharyngeal, and vagus nerves, which are the nerves associated with the first, second, third, and fourth arches.
The separation of the tongue from the floor is gradual; it is more complete for the part that forms the body than for that that forms the root.
The first indications of the teeth are ribbonlike thickenings of epithelium internal to the labiogingival thickenings. The thickenings extend as plates, dental laminae, into the subjacent mesenchyme (Figure 3–59); quite soon a linear series of knoblike swellings buds from the deep margin of each. The swellings represent the enamel organs of the temporary teeth, and their number corresponds to the dental formula of the species. Occasionally it is greater; the disparity occurs when primordia appear (and possibly develop quite far) for teeth that later regress without erupting. The upper incisors of ruminants are examples of teeth whose development is aborted in this way.
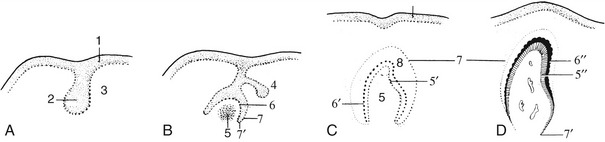
Figure 3–59 A, Development of dental plate. B, Development of an enamel organ. C, Enamel organ. D, Deciduous tooth before eruption. 1, Epithelium of oral cavity; 2, dental plate; 3, mesenchyme; 4, bud of a permanent tooth; 5, dental papilla; 5′, odontoblasts (differentiated from the outer cell layer of the papilla); 5″, dentine; 6, inner dental epithelium (future ameloblasts); 6′, ameloblasts; 6″, enamel; 7, outer dental epithelium; 7′, transition of inner and outer dental epithelia (where root formation occurs); 8, enamel reticulum.
The mesenchyme condenses against the free surfaces of each bud; when the bud shortly invaginates, the mesenchyme now known as the dental papilla fills the resulting cup. The whole tooth germ, the enamel organ together with the dental papilla, is enclosed by a mesenchymal thickening that merges with the papilla at its base, forming the dental sac or follicle.
The enamel organ consists of an inner epithelium (over the concave surface applied to the dental papilla), an outer epithelium (over the convex surface facing the dental follicle), and an intervening sparsely cellular tissue (enamel reticulum) (see Figure 3–59). The cells of the inner dental epithelium are known as ameloblasts because they produce enamel. Enamel formation begins over the center of the crown but soon spreads outward from this focus. As the layer thickens, the ameloblasts retreat in a centrifugal direction until finally they meet and fuse with the outer dental epithelium to form an epithelial cuticle over the crown.
Meanwhile, certain cells of the mesodermal papilla have become arranged in a sheet facing the ameloblasts. Because they produce dentine, they are known as odontoblasts. The first dentine also appears toward the center of the crown, a little later than the first deposition of enamel. Thereafter dentine deposition also spreads out in all directions. As the layer thickens the odontoblasts withdraw in a centripetal direction, and when dentine production has ceased, they remain as a covering to the pulp, which is the surviving less differentiated portion of the original papilla.
The root of the tooth is initially ensheathed by a prolongation of the enamel organ not producing enamel. The sheath later breaks down when the follicular tissue produces cement to encase the dentine of the root.
After the enamel organs of the temporary teeth have appeared the dental lamina undergoes extensive destruction. However, its free edge remains to produce a second crop of buds, the enamel organs of the replacement teeth; these remain dormant until activated to replicate the sequence that created the temporary teeth.
THE PHARYNX
Many details of the development of the pharyngeal region are more appropriately considered in Chapters 2 and 6. The pharynx is initially dorsoventrally flattened and widest immediately behind the oral plate, but the initial form is altered by the unequal growth of the mesoderm flanking the endodermal tube (Figure 3–60). This mesoderm forms serial thickenings, the pharyngeal (branchial) arches, which protrude into the pharyngeal lumen and bulge on the surface of the neck. The internal modeling of the lumen defines a series of pouches with which corresponding grooves coincide externally (see Figure 3–60). The number of arches (and therefore of pouches) is disputed. It is most commonly assumed that five arches exist, representing the first four and the sixth of the somewhat longer series found in other vertebrates. Each arch develops an internal skeleton and musculature with which a particular cranial nerve is associated; the fates of these are tabulated elsewhere (p. 57). Each pouch has a specific fate (see Figure 6–5). The features of immediate interest include the contributions of the first and, possibly, the second pouches to the cavity of the middle ear, which is a fate revealed in the adult by the site of entry of the auditory tube into the nasopharynx. The ventral part of the second pouch forms the tonsillar sinus, a landmark providing some clue to the former position of the oral plate.
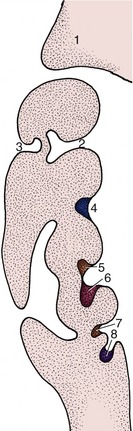
Figure 3–60 Dorsal section of the left side of the pharynx showing the development of the pharyngeal arches and pouches. 1, Maxillary process; 2, pharyngotympanic tube (future auditory tube); 3, external auditory meatus; 4, palatine tonsil (in tonsillar sinus); 5, parathyroid gland III; 6, thymus; 7, parathyroid gland IV; 8, ultimobranchial body.
The outgrowth of the lower respiratory tract at the caudal limit of the pharynx is considered in the following chapter.
THE CAUDAL PART OF THE FOREGUT
A fusiform enlargement identifies the stomach at an early stage. The foregut between this and the pharynx becomes the esophagus, which is initially very short but elongates as the heart descends from the neck into the thorax. The esophagus is involved in the origin of the lower respiratory tract (p. 165) but, apart from this, presents little of interest. At one stage, the proliferation of the endodermal lining obstructs the lumen, but the passage is later restored.
The development of the stomach involves displacement, reorientation, and differential enlargement. The displacement carries it to a position ventral to the caudal thoracic segments. Reorientation appears to involve rotations about two axes. Rotation about the long axis of the stomach spindle carries the originally dorsal aspect to the left, where it is later distinguished as the greater curvature. The dorsal megogastrium, which becomes the greater omentum, shares in the process. Rotation about a vertical axis swings the cranial (cardiac) extremity to the left and the caudal (pyloric) one to the right (Figure 3–61). In most species the most conspicuous change in shape is an asymmetrical enlargement to the left of the cardia that produces the fundus; a much more radical reshaping is required in ruminants. In the human fetus the gastric glands are capable of secretion by midterm.
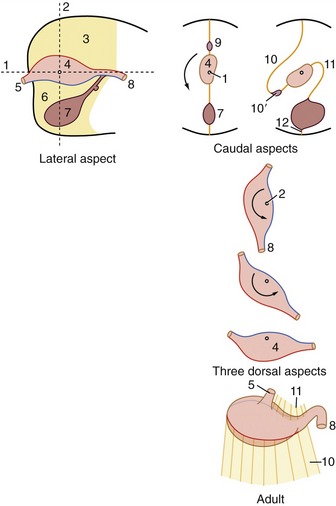
Figure 3–61 The reorientation of the developing simple stomach. It rotates counterclockwise (as seen from behind) around a longitudinal axis (caudal aspects [1]) and continues counterclockwise (as seen from above) around a dorsoventral axis (three dorsal aspects [2]). 1, Longitudinal axis; 2, dorsoventral (vertical) axis; 3, dorsal mesogastrium; 4, stomach primordium; 5, esophagus; 6, ventral mesogastrium; 7, developing liver; 8, duodenum; 9, developing spleen; 10, greater omentum; 10′, omental bursa; 11, lesser omentum; 12, developing ligaments of the liver.
The short portion of foregut between the gastric spindle and the midgut forms the initial part of the duodenum that terminates at the entrance of the bile and pancreatic ducts.
The Liver and Pancreas
The liver appears as an endodermal diverticulum at the junction of the foregut and midgut. It quickly divides into a cranial branch, which forms the gland tissue and hepatic ducts, and a caudal branch, which forms the gallbladder and cystic duct (Figure 3–62).
Figure 3–62 Development of the liver. A, Early development: a cranial branch (1) of the endodermal diverticulum invades the septum transversum; a caudal branch (1′) forms the gallbladder and cystic duct. B, A later stage, in which the developing liver expands caudally into the abdominal cavity. 1, liver; 1′, gallbladder; 2, pericardium and heart; 3, dorsal primordium of pancreas; 4, tongue; 5, tracheobronchial diverticulum; 6, stomach; 7, loop of midgut; 8, vitelline duct; 9, hindgut; 10, cloacal membrane; 11, allantoic stalk.
The cranial branch extends fingerlike processes into the splanchnic mesoderm of the adjacent septum transversum, carried here with the formation of the head fold. As the processes penetrate the mesoderm, they engage with the vitelloumbilical system of veins, which arrive here from the extraembryonic membranes. Very soon a three-dimensional spongework of hepatic cell-cords and plates is formed, surrounded on all sides by thin-walled blood vessels, which is a precocious realization of the adult arrangement. Attenuation of the connection between the liver and the gut forms the lesser omentum.
The growth of the liver, extremely rapid in younger embryos, is a major factor in the temporary herniation of the midgut (see further on). Although its growth slows later, the liver remains disproportionately large (by comparison with that of the adult) until well after birth. One relevant factor is the exercise of an erythropoietic activity before birth that is later relinquished. The secretory and metabolic functions are established by midterm in the human fetus.
The pancreas arises from the same portion of the foregut as the liver. There are initially two primordia: one is dorsal and the second is ventral and associated with the hepatic outgrowth (Figure 3–63). These later fuse, allowing combination of the two duct systems, following which one or the other may lose its connection with the gut. The islet tissue develops by budding from the ducts. Both endocrine and exocrine components are competent well before birth.
Figure 3–63 Development of the pancreas. A, Early stage. B, A later stage showing separate duct systems in the two primordia. C, The two primordia have fused after the migration of the ventral pancreas. The dorsal pancreas now drains mainly via the ventral duct system. 1, Liver primordium; 1′, hepatic ducts; 1″, bile duct; 2, gallbladder; 3, ventral primordium of pancreas; 4, dorsal primordium of pancreas; 5, stomach; 6, duodenum.
The celiac artery is associated with the postpharyngeal part of the foregut.
THE MIDGUT
The midgut forms the intestine, from the entry of the bile duct to the junction of the transverse and descending parts of the colon. Its initial wide connection with the yolk sac is quickly lost.
The early growth of the midgut is very rapid, causing it to hang in a loop from an elongated mesentery in which the midgut (cranial mesenteric) artery runs. The expanding liver claims so large a part of the abdominal cavity that insufficient room remains for the intestine. The long mesentery then permits the midgut to slip out of the abdominal cavity into the umbilical cord, which is a process known as physiological herniation, where growth continues. The cranial limb of the herniated loop becomes the small intestine; the appearance of a diverticulum, the future cecum, indicates the division of the caudal limb into the terminal part of the small intestine and the initial part of the colon. The cranial limb grows more rapidly and soon becomes much coiled. The key event is the rotation of the loop about the arterial axis (Figure 3–64), which is a rotation that carries the originally caudal limb forward on the left, then across the abdomen before it passes caudally on the right side, completing a rotation through approximately 270°. This rotation, clockwise when viewed from above, brings the intestines more or less into their adult disposition when they are returned to the abdomen (Figure 3–65). The return is possible because the rate of liver increase slows and falls behind the general growth of the embryo. The final arrangement may depend on local shortenings of the mesentery and fusions of apposed peritoneum-clad surfaces.
Figure 3–64 Three stages in the growth and rotation of the canine midgut, in left lateral views. 1, Cranial mesenteric artery; 2, caudal mesenteric artery; 3, dorsal mesogastrium; 3′, greater omentum, fenestrated in C to expose stomach; 4, ventral mesogastrium with developing liver; 5, vitelline duct; 6, cecal primordium; 7, ileocecal fold.
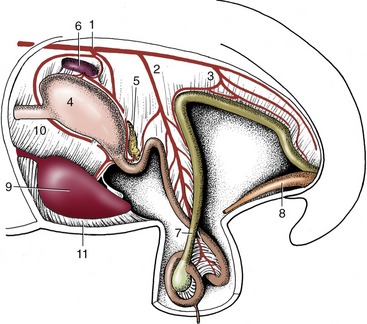
Figure 3–65 Development of the intestinal tract during the rotation process. The midgut loop is herniated into the extraembryonic celom. 1, Celiac artery; 2, cranial mesenteric artery; 3, caudal mesenteric artery; 4, stomach; 5, pancreas; 6, spleen; 7, loop of midgut; 8, bladder expansion of the urogenital sinus; 9, liver; 10, lesser omentum; 11, falciform ligament.
THE HINDGUT
The hindgut develops into the descending colon and the rectum, parts supplied by the caudal mesenteric artery in the adult. Initially the gut ends blindly against the cloacal plate. Except in the horse and ruminants, in which the descending colon shows a secondary increase in length, significant changes affect only the terminal part of the hindgut. A bud, the allantois, grows from its ventral aspect toward and through the umbilical opening in the abdominal wall; once outside the embryo it enlarges to form the capacious allantoic sac (Figure 5–66). A wedge of tissue (urorectal septum) enlarging in the angle between the gut and this diverticulum thrusts toward the cloacal membrane (Figure 3–66). When it meets this, it divides the gut into two separate tubes: the dorsal one is continuous with the descending colon, and the ventral one is continuous with the allantois and destined to form the lower urogenital tract. Meanwhile, proliferation of mesoderm beneath the ectoderm around the proctodeum has deepened the pit; when the dorsal part (anal membrane) of the cloacal membrane breaks down, this deepening is added to the gut, which provides it with the anal canal that leads to the exterior.
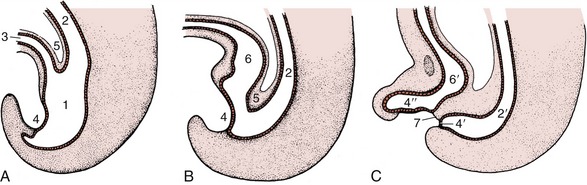
Figure 3–66 Division of the distal part of the hindgut into rectum and urogenital sinus. A, Formation of the allantois and beginning of the caudal extension of the urorectal septum (5). B, The urorectal septum now approaches the cloacal membrane. C, Complete division of urogenital sinus and anorectal canal. 1, Cloaca; 2, hindgut; 2′, anorectal canal; 3, allantois; 4, cloacal membrane; 4′, anal membrane; 4″, urogenital membrane; 5, urorectal septum; 6, primitive urogenital sinus; 6′, urogenital sinus; 7, tissue bridge ventral to future anus.