CHAPTER 10 Heartworm Disease
GENERAL CONSIDERATIONS
HEARTWORM LIFE CYCLE
The heartworm (Dirofilaria immitis) is transmitted by various species of mosquitoes, which act as its obligate intermediate host. A mosquito initially ingests the microfilariae, or first-stage larvae (L1), which circulate in the blood of an infected host animal. The L1 develops into an L2 and then enters the infective L3 stage within the mosquito over a period of approximately 2 to 2.5 weeks. Infective larvae enter the new host when the mosquito takes another blood meal. L3 larvae migrate subcutaneously within the new host, molting into an L4 stage within 9 to 12 days, and then entering the L5 stage. The juvenile L5 worms enter the vasculature about 100 days after infection, where they migrate preferentially to the peripheral pulmonary arteries of the caudal lung lobes. It takes at least 5 and usually more than 6 months before these worms develop into adults, at which time gravid females release microfilariae and the infection becomes patent.
Microfilariae passed to another animal by blood transfusion or across the placenta do not develop into adult worms because the mosquito host is required to complete the parasite’s life cycle. Therefore puppies younger than 6 months of age that have circulating microfilariae most likely received them transplacentally and do not have patent heartworm disease (HWD).
HWD is widespread throughout the United States, especially along the eastern and gulf coasts and in the Mississippi River valley. Sporadic cases occur in other areas of the country and Canada; the disease is prevalent in other regions of the world as well. Heartworm transmission is limited by climate. An average daily temperature of >64° F for about a month is needed for the L1 larvae to mature within a mosquito to the infective stage. Heartworm transmission peaks during July and August in temperate regions of the Northern Hemisphere.
Dogs and other canids are the preferred host species. Although cats are also affected by HWD, they are more resistant to infection than dogs. The overall prevalence of heartworm disease in cats is thought to be 5% to 20% of that in dogs in the same geographic area. Reported prevalences range from 0% to > 16%. In the United States, cases have been identified in most of the midwest and eastern states and in California.
TESTS FOR HEARTWORM DISEASE
Serologic Tests
Antigen tests.
Adult heartworm antigen (Ag) tests are recommended as the main screening test for HWD in dogs. Currently available Ag test kits are highly accurate. Because monthly heartworm preventive drugs promote occult infections by virtually eliminating circulating microfilariae, Ag testing provides higher overall sensitivity for diagnosing HWD. Circulating Ag is usually detectable by about 6.5 to 7 months after infection but not sooner than 5 months. There is no reason to test puppies younger than 7 months. Testing of adults is recommended at about 7 months after the preceding transmission season. Depending on the climate, monthly heartworm prophylaxis may have been started (or continued) before that time.
Commercially available test kits are immunoassays that detect circulating heartworm Ag from the adult female reproductive tract. Most are enzyme-linked immunosorbent assays (ELISAs), although hemagglutination and immunochromatographic test methods also are available. These tests are generally very specific and have a good sensitivity. Positive results are consistently obtained when at least three female worms 7 to 8 months or older are present. Most kits do not detect infections less than 5 months old, and male worms are not detected. Most serum/plasma kits often can identify infections with one live female worm. Microwell-format ELISA tests in general are slightly more sensitive than the rapid assay, membrane-format tests. Of the latter the SNAP test (Idexx Laboratories, Westbrook, Maine) reportedly is more sensitive for detecting infections with 1 or 2 female worms. A weak positive or ambiguous test result may be rechecked using a different test kit or repeated after a short time with the same type of kit; microfilaria testing and chest radiographs can also help determine whether infection is present. A false-positive Ag test result can usually be traced to a technical error. False-negative results may occur with a low worm burden, immature female worms only, male unisex infection, or a cold test kit. Because the adult worm burden is low in cats and there is greater probability of male unisex infections, false-negative test results are more likely in this species.
Antibody tests.
Heartworm antibody tests are marketed for cats. The ELISA antibody (Ab) tests use either recombinant Ag or heartworm Ag extracted and purified from male and female worms. These tests are used to screen for feline heartworm disease. The Ab tests have minimal to no cross-reactivity with gastrointestinal (GI) parasitic infections. Ab tests provide greater sensitivity than Ag tests because larvae of either sex can provoke a host immune response. The specificity of the Ab tests for HWD is of some concern, however. Serum Ab to both immature and adult worms is detected as early as 60 days after infection, and some immature heartworm larvae never develop into adults. Therefore a positive Ab test indicates exposure to migrating larvae as well as adults, not the presence of adult heartworms specifically. When the Ab test is positive, other evidence also should be sought to support a diagnosis of HWD. This can include a positive heartworm Ag test or findings consistent with HWD on thoracic radiography and echocardiography. The concentration of Ab does not appear to correlate well with an individual cat’s worm burden, nor with the severity of clinical disease or radiographic signs. High Ab titers are associated with heartworm death as well as heavy infection. It is unclear how long circulating Ab remain after elimination of heartworm infection.
False negative Ab tests also occur fairly frequently (in up to approximately 14% of cases). These are usually related to infection with a single worm and are a matter of concern because the feline worm burden is often low. Therefore a negative heartworm Ab test suggests one of the following: (1) the cat does not have heartworm infection, (2) the cat has an infection less than 60 days old, or (3) the cat produced a concentration of IgG Ab against the Ag used in making the test that is too low to be detected. When clinical findings suggest HWD but the Ab test is negative, serological testing should be repeated using a different Ab test and a heartworm Ag test. Chest radiographs and an echocardiogram are also recommended. The Ab test may also be repeated in a few months.
Microfilaria Identification
Tests for circulating microfilariae are no longer recommended for routine heartworm screening. They are useful in identifying patients that are reservoirs of infection and to assess whether high numbers of microfilariae are present before a monthly preventive drug is administered. Microfilaria testing is mandatory if diethylcarbamazine (DEC) is to be used as a heartworm preventive. The macrocyclic lactone preventive drugs, administered monthly, reduce and eliminate microfilaremia by impairing the reproductive function of female and possibly also male worms. Most dogs become amicrofilaremic by the sixth monthly dose with these drugs. However, up to 90% of heartworm-positive dogs that are not treated monthly with a macrolide have circulating microfilariae. The remaining so-called occult infections, in which there are no circulating microfilariae, can result from an immune response that destroys the microfilariae within the lung (true occult infection), unisex infection, sterile adult heartworms, or the presence of only immature worms (prepatent infection). Occult infections are frequently associated with severe signs of disease. Low numbers of microfilariae and diurnal variations in the number of circulating microfilariae in peripheral blood can also cause false-negative microfilaria test results. Circulating microfilariae are rarely found in cats with HWD.
Microfilaria concentration tests that use at least 1 ml of blood are recommended for detecting circulating microfilariae. The nonconcentration tests are more likely to miss low numbers of microfilariae, although they do allow observation of mirofilarial motility. Dirofilaria have a stationary rather than a migratory movement pattern. Nonconcentration tests include examination of a fresh wet blood smear or the buffy coat of a spun hematocrit tube.
Concentration tests are done using either a millipore filter or the modified Knott’s centrifugation technique. Both techniques lyse the red blood cells and fix any existing microfilariae. The modified Knott test is preferred for measuring larval body size and differentiating D. immitis from non pathogenic filarial larvae, such as Acanthocheilonema (formerly Dipetalonema) reconditum (Table 10-1). An occasional false-positive microfilaria test result occurs in animals with microfilariae but no live adult heartworms.
 TABLE 10-1 Morphologic Differentiation of Microfilaria
TABLE 10-1 Morphologic Differentiation of Microfilaria
| SMEAR | DIROFILARIA IMMITIS | DIPETALONEMA RECONDITUM |
|---|---|---|
| Fresh smear | ||
| Stained smear* |
(modified Knott’s test); microfilariae tend to be smaller with lysate of filter tests. Width and morphology are the best discriminating factors.
HEARTWORM DISEASE IN DOGS
Pathophysiology
Heartworm disease is an important cause of pulmonary hypertension (cor pulmonale) in regions where the disease is endemic. Increased pulmonary vascular resistance raises pulmonary arterial pressure according to the relationship: cardiac output = Δ pressure/resistance. The presence of adult worms in the pulmonary arteries provokes reactive vascular lesions sthat reduce vascular compliance and lumen size. Within days after young heartworms enter the pulmonary arteries, pathologic changes begin in these vessels. The host-parasite interaction is thought to be more important than the worm number alone in the development of clinical signs, although a large worm burden may be associated with severe disease. The pathogenesis of HWD may be modulated by obligate intracellular bacteria (genus Wolbachia) that are harbored by the worms. This may involve bacterial endotoxins as well as the host immune response to a major Wolbachia surface protein, which is thought to contribute to pulmonary and renal inflammation. Little correlation has been found between pulmonary vascular resistance and the number of worms present. A low worm burden can produce serious lung injury and a greater rise in pulmonary vascular resistance if the cardiac output is high. The increase in pulmonary blood flow associated with exercise exacerbates the pulmonary vascular pathology.
Villous myointimal proliferation of the pulmonary arteries containing heartworms is the characteristic lesion. The heartworm-induced changes begin with endothelial cell swelling, widening of intercellular junctions, increased endothelial permeability, and periarterial edema. Endothelial sloughing leads to the adhesion of activated white blood cells and platelets. Various trophic factors stimulate smooth muscle cell migration and proliferation within the media and into the intima. Villous proliferations consist of smooth muscle and collagen with an endothelium-like covering. These proliferative changes of the intima occur 3 to 4 weeks after adult worms arrive. They cause luminal narrowing of the smaller pulmonary arteries and also induce further endothelial damage and more proliferative lesions. Hypersensitivity pneumonitis may contribute to parenchymal lung lesions. Endothelial damage promotes thrombosis as well as a perivascular tissue reaction and periarterial edema. Interstitial and alveolar infiltrates may become radiographically apparent; partial lung consolidation develops in some animals. Hypoxic vasoconstriction can also play a role in the vascular changes that increase pulmonary vascular resistance and consequently cause pulmonary hypertension. Dead worms stimulate greater host response and worsen the pulmonary disease. Worm fragments and thrombi cause embolization and a more intense reaction, which eventually leads to fibrosis.
The worm distribution, and accompanying villous proliferation, is most severe in the caudal and accessory lobar arteries. Affected pulmonary arteries lose their normal tapered peripheral branching appearance and appear blunted or pruned. Aneurysmal dilation and peripheral occlusion may occur. The vessels become tortuous and proximally dilated as the increased pulmonary vascular resistance demands higher perfusion pressures.
Right ventricular dilation and concentric hypertrophy are the responses to a chronic requirement for increased systolic pressure. Chronic pulmonary hypertension can lead to right ventricular (RV) myocardial failure, increased RV diastolic pressure, and signs of right-sided congestive heart failure (CHF), especially in conjunction with secondary tricuspid insufficiency. Cardiac output progressively declines as the RV fails. When cardiac output becomes inadequate during exercise, exertional dyspnea, fatigue, and syncope may occur.
Chronic hepatic congestion secondary to HWD may lead to permanent liver damage and cirrhosis. Circulating immune complexes or possibly microfilarial antigens provoke glomerulonephritis. Renal amyloidosis has also been associated with HWD in dogs in rare cases. Occasionally, aberrant worms can cause embolization of the brain, eye, or other systemic arteries.
Although the caudal pulmonary arteries are the preferred site, worm migration to the caudal vena cava is associated with heavy worm burdens. A massive number of worms can cause mechanical occlusion of the RV outflow tract, pulmonary arteries, tricuspid valve region, or venae cavae. This is known as the caval syndrome. Cases of systemic arterial migration causing hindlimb lameness, paresthesia, and ischemic necrosis are sporadically described.
PULMONARY HYPERTENSION WITHOUT HEARTWORM DISEASE
A number of diseases besides HWD are associated with pulmonary hypertension in dogs, including hypoxic pulmonary disease and vascular obstructive disease (e.g., pulmonary thromboembolism). Vascular obstruction reduces total cross-sectional pulmonary vascular area by mechanically obstructing vessels and provoking local hypoxic pulmonary vasoconstriction as well as other reactive changes. Associated pulmonary parenchymal disease can contribute to reduced vascular area.
Chronic elevations in pulmonary venous pressure (as from mitral regurgitation) may increase pulmonary artery pressure but usually only mildly to moderately. Pulmonary edema or congestion associated with high venous pressure can contribute to increased pulmonary vascular resistance by reducing lung compliance and increasing resistance to air flow. Pulmonary overcirculation caused by a congenital cardiac shunt can cause vascular injury and pulmonary arterial remodeling leading to high vascular resistance, pulmonary hypertension and shunt reversal (Eisenmenger’s physiology; see p. 109).
Clinical Features
There is no specific age or breed predilection for HWD in dogs. Although most affected dogs are between 4 and 8 years old, HWD is also diagnosed in dogs <1 year (but >6 months) of age as well as in geriatric animals. Males are affected two to four times as often as females. Large-breed dogs and those living mainly outdoors are at much greater risk of infection than small-breed and indoor dogs. The length of the haircoat does not appear to affect infection risk.
Dogs diagnosed by a positive routine screening test are often asymptomatic. Dogs with occult disease and those not routinely tested are more likely to have advanced pulmonary arterial disease and clinical signs. Dogs with clinical disease often have a history of fatigue, shortness of breath or exertional dyspnea, syncope, cough, hemoptysis, weight loss, or signs of right-sided CHF. A change in or loss of the dog’s bark has sometimes been reported.
Physical examination findings may be normal in patients with early or mild disease. Severe disease is frequently associated with poor body condition, tachypnea or dyspnea, jugular vein distention or pulsations, ascites, or other evidence of right-sided CHF. Increased or abnormal lung sounds (wheezes and crackles), a loud and often split second heart sound (S2), an ejection click or murmur at the left heartbase, a murmur of tricuspid insufficiency, or cardiac arrhythmias are variably heard on auscultation. Severe pulmonary arterial disease and thromboembolism can lead to disseminated intravascular coagulation (DIC), thrombocytopenia, epistaxis, and possibly hemoglobinuria. Hemoglobinuria is also associated with caval syndrome. Aberrant worm migration to the central nervous system, eye, femoral arteries, subcutis, peritoneal cavity, and other sites occurs occasionally and causes related signs.
RADIOGRAPHY
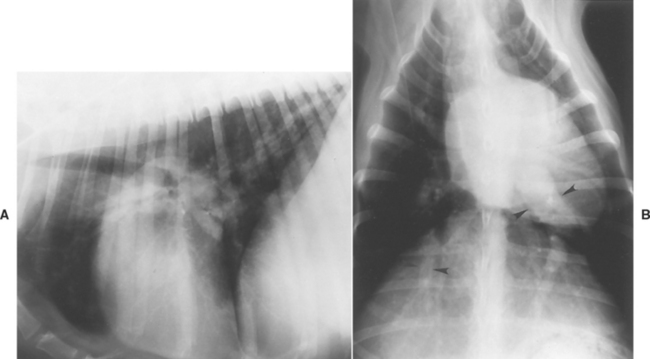
Radiographic findings are often normal early in the disease, although marked changes can develop rapidly in dogs with heavy worm burdens. Characteristic findings include RV enlargement, a pulmonary trunk bulge, and centrally enlarged and tortuous lobar pulmonary arteries with peripheral blunting (Fig. 10-1 and p. 15). The caudal lobar arteries, which are usually the most severely affected, are best evaluated on a dorsoventral (DV) view; the width of these vessels is normally no larger than the ninth rib (at its intersection with the vessels). Enlargement of lobar pulmonary arteries (without concurrent venous distention) is strongly suggestive of HWD or other cause of pulmonary hypertension. An enlarged caudal vena cava also may be seen (see p. 16). Patchy pulmonary interstitial or alveolar infiltrates suggestive of infarction, edema, pneumonia, or fibrosis also are common. These pulmonary opacities may be mainly perivascular. Right-sided CHF caused by HWD is associated with radiographic evidence of severe pulmonary arterial disease and right heart enlargement.
ELECTROCARDIOGRAPHY
Electrocardiographic (ECG) findings are usually normal, although advanced disease can cause a right axis deviation or an arrhythmia. Dogs with heartworm-induced CHF almost always have ECG criteria for RV enlargement. Tall P waves, suggesting right atrial (RA) enlargement, are sometimes found.
ECHOCARDIOGRAPHY
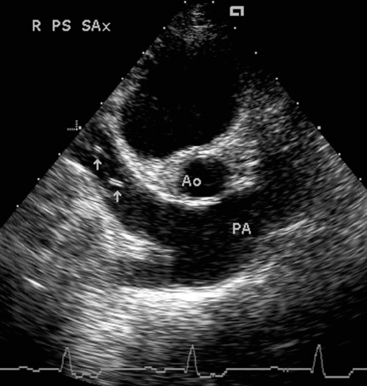
Echocardiographic findings in dogs with advanced HWD include RV and RA dilation, RV hypertrophy, paradoxical septal motion, a small left heart, and pulmonary artery dilation. Heartworms located in peripheral pulmonary arteries cannot be seen on echocardiogram. Heartworms within the heart, the main pulmonary artery and its bifurcation, and venae cavae appear as small, bright parallel echos (Fig. 10-2). Suspected caval syndrome can be quickly confirmed by echocardiography. Secondary right-sided CHF may be demonstrated by pleural or pericardial effusion or ascites. Color-flow Doppler can be used in the identification of tricuspid regurgitation even when an audible murmur is absent. Spectral Doppler measurement of maximum tricuspid or pulmonary regurgitant jet velocity allows estimation of pulmonary hypertension severity (see p. 45).
CLINICOPATHIC FINDINGS
Eosinophilia, basophilia, and monocytosis are inconsistent hematologic findings. However, fewer than half of dogs with HWD have eosinophilia. Mild regenerative anemia, thought to result from hemolysis, occurs in less than a third of affected dogs. Thrombocytopenia may result from platelet consumption in the pulmonary arterial system, especially after adulticide treatment. DIC also develops in some in dogs with advanced disease. The immune response to heartworms produces a polyclonal gammopathy. Mild to moderate elevations in liver enzyme activity and azotemia may occur. Proteinuria is found in 20% to 30% of affected dogs and is more likely with advanced disease. Hypoalbuminemia may develop in severely affected animals.
PRETREATMENT EVALUATION
As a general rule, adulticide treatment is recommended for dogs infected with heartworms. The withholding of adulticide treatment in some asymptomatic cases remains controversial. Although continuous monthly treatment with prophylactic ivermectin does eventually kill late precardial larvae and young adult worms, this effect occurs over a prolonged time (over 1 to 2 years). Older worms are more resistant to ivermectin and can still cause clinical disease. Furthermore, progression of pulmonary arterial changes, pulmonary disease, and other heartworm-induced effects (e.g., glomerulonephritis) may increase the risk of adulticide treatment should this be undertaken in the future. If adulticide therapy is not given, the dog should at least be treated continuously with ivermectin or possibly with selamectin or moxidectin, which also have some adulticidal effects. Use of heartworm prophylaxis is also important to prevent disease transmission to other animals (by reducing the microfilaremia).
Heartworm-infected dogs should have a thorough his-tory and physical examination. Pretreatment thoracic radiographs provide the best overall assessment of pulmonary arterial and parenchymal disease status. The risk of postadulticide pulmonary thromboembolism is increased in dogs with preexisting clinical and radiographic signs of severe pulmonary vascular disease, especially in those with right-sided CHF or a high worm burden. Other pretreatment tests should include a complete blood count (CBC), serum biochemical profile, and urinalysis. A platelet count is important in animals with severe pulmonary arterial disease. If hypoalbuminemia or proteinuria is detected, a urine protein-creatinine ratio or urine protein loss quantification is advised. Mildly to moderately increased liver enzyme activity may be associated with hepatic congestion, but it does not preclude therapy with melarsomine. Liver enzyme activities usually normalize within 1 to 2 months of heartworm treatment. Some dogs with HWD develop azotemia and/or severe proteinuria. Prerenal azotemia is treated with fluid therapy before adulticide is given. Severe glomerular disease, with loss of antithrombin as well as other proteins, may increase the risk for thromboembolism. Aspirin is not recommended as a routine preadulticide treatment in most dogs because convincing evidence of a beneficial antithrombotic effect is lacking.
The use of prophylactic monthly doses of ivermectin for up to 6 months before the administration of an adulticide in dogs that are clinically stable may be useful. This strategy can reduce heartworm Ag mass by decreasing or eliminating circulating microfilariae and tissue-migrating larvae, stunting immature worm growth, and damaging the adult female reproductive system. Delaying melarsomine for several months also allows any late-stage larvae to mature further, which should increase susceptibility to the adulticidal effect. Microfilaria-positive dogs should be observed in the hospital after the first ivermectin dose in case of adverse reaction. Specific microfilaricide treatment is not necessary before using adulticide.
ADULTICIDE THERAPY IN DOGS
Melarsomine dihydrochloride (Immiticide, Merial) is the adulticide of choice. It is effective against both immature and mature heartworms; male worms are more susceptible than females. The worm kill can be controlled by adjusting the dose. An alternative dosing protocol is advised for dogs with more severe disease to promote a more gradual worm kill.
Melarsomine is rapidly absorbed from the intramuscular (IM) injection site. Unchanged drug and a major metabolite are rapidly eliminated in the feces; a minor metabolite is excreted in urine. The drug should be given by deep IM injection into the epaxial lumbar muscles (L3 to L5 region), exactly as recommended by the manufacturer. The lumbar muscle site provides good vascularity and lymphatic drainage with minimal fascial planes. Furthermore, gravity may help prevent the drug from leaking into subcutaneous tissues, where it can cause more irritation. The drug does cause a local reaction at the injection site; this is clinically noticeable in about a third of treated dogs. Melarsomine is available as a sterile lyophilized powder in 50-mg vials. The rehydrated product is fully stable for 24 hours if kept refrigerated in the dark.
Coughing or gagging and (less often) dyspnea after treatment may be related to the HWD itself, although pulmonary congestion is reported as a toxic effect of overdosing. Most clinical signs noted in dogs treated with melarsomine have been behavioral (e.g., tremors, lethargy, unsteadiness and ataxia, restlessness), respiratory (e.g., panting, shallow breathing, labored respirations, crackles), or injection-site related (e.g., edema, redness, tenderness, vocalization, increased aspartate aminotransferase and creatine kinase activities). Injection site reactions are generally mild to moderate and resolve within 4 (to 12) weeks. Occasionally these reactions are severe. The manufacturer reports that firm nodules may persist indefinitely at the sites. General signs of lethargy, depression, and anorexia occur in about 15% or fewer dogs; other adverse effects, including fever, vomiting, and diarrhea, occur occasionally. Adverse effects are generally mild at recommended doses. Hepatic and renal changes have not proved clinically relevant in animals receiving recommended doses of melarsomine. Overall melarsomine causes less systemic toxicity than its predecessor, thiacetarsamide. Nevertheless, melarsomine has a low margin of safety. Overdose may cause collapse, severe salivation, vomiting, respiratory distress resulting from pulmonary inflammation and edema, stupor, and death. Some clinical reversal of melarsomine toxicity may be achieved with BAL (British Anti-Lewisite or dimercaprol) at a dose of 3 mg/kg, administered intramuscularly. This also decreases adulticide activity.
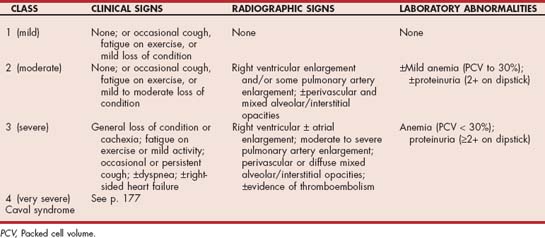
The HWD severity is used to guide melarsomine therapy (Table 10-2). Standard therapy is used for dogs with mild (class 1) to moderate (class 2) disease. Standard therapy (Box 10-1) involves two doses of 2.5 mg/kg given intramuscularly, 24 hours apart. The manufacturer’s administration instructions should be followed carefully. Dogs with severe disease (class 3) or those in class 2 in which a more conservative approach is desired are treated with the alternative dosing regimen. This is designed to partially reduce the worm burden with an initial injection, followed by the standard adulticide regimen 1 month later. The risk of massive pulmonary thromboembolism and death resulting from an initially heavy worm kill is reduced with this protocol. Dogs with caval syndrome (class 4) should not be given adulticide treatment until worms are surgically removed (see p. 177).
 BOX 10-1 Checklist for Melarsomine (Immiticide) Adulticide Therapy in Dogs
BOX 10-1 Checklist for Melarsomine (Immiticide) Adulticide Therapy in Dogs
Standard treatment protocol (for class 1 and many class 2 dogs)
Alternate treatment protocol (for class 3 and some class 2 dogs)
Strict rest should be enforced for 4 to 6 weeks after adulticide therapy to reduce the effects of adult worm death and pulmonary thromboembolism (see p. 176). The rest period for working dogs should probably be longer because increased pulmonary blood flow in response to exercise exacerbates pulmonary capillary bed damage and subsequent fibrosis.
Heartworm Ag testing is recommended 6 months after adulticide treatment; results should be negative with successful treatment. Many dogs are heartworm Ag–negative by 4 months after adulticide therapy. Incomplete worm kill is associated with persistent antigenemia. The decision to repeat adulticide therapy is guided by the patient’s overall health, performance expectations, and age. Complete worm kill is probably not necessary; even if some adult heartworms survive, pulmonary arterial disease improves considerably after adulticide therapy.
Thiacetarsamide is an older adulticide agent that may still be available. It has no advantages and several disadvantages compared with melarsomine. Likewise, the use of other drugs, such as levamisole or stibophen, as adulticides is not recommended. Levamisole does not consistently kill adult heartworms, although it is somewhat effective against male worms and may sterilize adult female worms.
Postadulticide Pulmonary Thromboembolic Complications
Pulmonary arterial disease worsens from 5 to 30 days after adulticide therapy and is especially severe in previously symptomatic dogs. It occurs because dead and dying worms lead to thrombosis and pulmonary artery obstruction, with exacerbation of platelet adhesion, myointimal proliferation, villous hypertrophy, granulomatous arteritis, perivascular edema, and hemorrhage. Pulmonary blood flow obstruction and increased vascular resistance further strain the right ventricle and increase oxygen demand. Poor cardiac output, hypotension, and myocardial ischemia may result. Severe ventilation-perfusion mismatch may result from pulmonary hypoperfusion, hypoxic vasoconstriction and bronchoconstriction, pulmonary inflammation, and fluid accumulation. Pulmonary thromboembolization is most likely to occur 7 to 17 days after adulticide therapy. As expected, the caudal and accessory lung lobes are most commonly and severely affected.
Depression, fever, tachycardia, tachypnea or dyspnea, and cough are common clinical signs. Hemoptysis, right-sided CHF, collapse, or death may also occur. Interstitial and alveolar pulmonary inflammation and fluid accumulation cause pulmonary crackles on auscultation. Focal lung consolidation may cause areas of muffled lung sounds. Thoracic radiographs show patchy alveolar infiltrates with air bronchograms, especially near the caudal lobar arteries. Thrombocytopenia or neutrophilia with a left shift may be seen on CBC.
Treatment of pulmonary thromboembolism includes strict rest (i.e., cage confinement) and glucocorticoid therapy to reduce pulmonary inflammation (prednisone, 1 to 2 mg/kg/day by mouth initially, then tapering). Supplemental oxygen therapy is recommended to reduce hypoxiamediated pulmonary vasoconstriction. A bronchodilator (e.g., oral aminophylline, 10 mg/kg IM or IV q8h; or oral theophylline, 9 mg/kg q6-8h), judicious fluid therapy (if there is evidence of cardiovascular shock), and cough suppressants may be useful. Antibiotics have been given empirically, but they are of questionable benefit unless there is evidence of concurrent bacterial infection. Hydralazine has reduced pulmonary vascular resistance experimentally, and some dogs seem to respond clinically to diltiazem. Systemic hypotension and tachycardia must be avoided when using a vasodilator. Aspirin is not recommended because there is no convincing evidence that it prevents thrombosis or reduces pulmonary arteritis. Heparin (200 to 400 U/kg sodium heparin administered subcutaneously q8h, or 50 to 100 U/kg calcium heparin administered subcutaneously q8-12h) may be considered for severe cases of thromboembolism. However, excessive bleeding is a possible serious adverse effect. Low-molecular-weight heparin might provide a safer alternative to unfractionated heparin, but definitive recommendations are not yet available.
Endothelial changes in survivors regress within 4 to 6 weeks. Pulmonary hypertension and arterial disease, along with radiographic changes, diminish over the next several months. Eventually, pulmonary arterial pressure and the contour of the proximal pulmonary arteries normalize, although some fibrosis may remain.
PULMONARY COMPLICATIONS
Immune-mediated pneumonitis occurs in some dogs. Allergic or eosinophilic pneumonitis develops in a minority of dogs with occult HWD. Clinical manifestations of heartworm pneumonitis include a progressively worsening cough, crackles heard on auscultation, tachypnea or dyspnea, and sometimes cyanosis, weight loss, and anorexia. Eosinophilia, basophilia, and hyperglobulinemia are inconsistent findings. Heartworm Ag tests are usually positive. Diffuse interstitial and alveolar infiltrates, especially in the caudal lobes, are common on radiographs; these can be similar to those in dogs with pulmonary edema or blastomycosis. There is often no clinically relevant cardiomegaly or pulmonary lobar artery enlargement. Tracheal wash cytology usually reveals a sterile eosinophilic exudate with variable numbers of well-preserved neutrophils and macrophages. Therapy with a glucocorticoid (prednisone, 1-2 mg/kg/day by mouth initially) usually results in rapid and marked improvement. Prednisone may be continued as needed, in gradually tapered doses (to 0.5 mg/kg every other day) and does not appear to adversely affect the adulticide efficacy of melarsomine.
Pulmonary eosinophilic granulomatosis is an uncommon syndrome that has been associated with HWD, although some affected dogs have negative heartworm tests. Its pathogenesis is thought to involve a hypersensitivity reaction to heartworm Ag or immune complexes, or both. Pulmonary granulomas comprise a mixed mononuclear and neutrophilic cell population, with many eosinophils and macrophages. A proliferation of bronchial smooth muscle within granulomas and an abundance of alveolar cells in the surrounding area are common findings. Lymphocytic and eosinophilic perivascular infiltrates may also occur. Eosinophilic granulomas involving the lymph nodes, trachea, tonsils, spleen, GI tract, and the liver or kidneys may occur concurrently. The clinical signs of pulmonary eosinophilic granulomatosis are similar to those of eosinophilic pneumonitis. Clinicopathologic findings variably include leukocytosis, neutrophilia, eosinophilia, basophilia, monocytosis, and hyperglobulinemia. In some cases an exudative, primarily eosinophilic pleural effusion develops. Radiographic findings include multiple pulmonary nodules of varying size and location with mixed alveolar and interstitial pulmonary infiltrates; hilar and mediastinal lymphadenopathy may also be present. Eosinophilic granulomatosis is treated initially with prednisone (1 to 2 mg/kg q12h); however, additional cytotoxic therapy may be needed as well. Not all dogs respond completely, and relapses are common, especially when therapy is reduced or discontinued. The response to immunosuppressive drugs after relapse may be poor. Therapy for adult heartworms is given when pulmonary disease improves.
Severe pulmonary arterial disease is more common in dogs with long-standing heartworm infection, in those with many adult worms, and in active dogs. Severe cough, exercise intolerance, tachypnea or dyspnea, episodic weakness, syncope, weight loss, and ascites are common clinical signs; death sometimes occurs. Typical radiographic findings include markedly enlarged, tortuous, and blunted pulmonary arteries. Pulmonary parenchymal infiltrates leading to hypoxemia are seen in some cases; these are treated with prednisone as described in the preceding paragraph. Throm bocytopenia and hemolysis may occur in dogs with severe pulmonary arterial disease and thromboembolism. Monitoring of platelet count and packed cell volume is recommended. DIC develops in some dogs. Conservative therapy with oxygen, prednisone, and a bronchodilator (e.g., theophylline), as for postadulticide pulmonary thromboembolism, should help improve oxygenation and reduce pulmonary artery pressures. Alternate-day, low-dose prednisone (e.g., 0.5 mg/kg orally) is thought to have beneficial antiinflammatory effects, although long-term use of high corticosteroid doses may reduce pulmonary blood flow, increase risk of thromboembolism, and inhibit vascular disease resolution.
After the animal’s condition is stabilized, the alternative melarsomine protocol may be used. Use of aspirin is discouraged, especially with hemoptysis. Prophylactic antibiotics are sometimes recommended because of the potential for secondary bacterial infections in devitalized pulmonary tissue.
RIGHT-SIDED CONGESTIVE HEART FAILURE
Severe pulmonary arterial disease and pulmonary hypertension can cause CHF. Jugular venous distention or pulsation, ascites, syncope, exercise intolerance, and arrhythmias are typical signs. Pleural or pericardial effusion as well as other signs secondary to pulmonary arterial and parenchymal disease may also occur. Treatment is the same as for dogs with severe pulmonary arterial disease, with the addition of furosemide (e.g., 1-2 mg/kg/day), an angiotensin-converting enzyme inhibitor (ACEI; e.g., enalapril 0.5 mg/kg q12-24 h by mouth), and a sodium-restricted diet. Use of digoxin in these cases is controversial; pimobendan has not been evaluated in this setting but could be useful.
CAVAL SYNDROME
The (vena) caval syndrome occurs in heavily infected animals when venous inflow to the heart is obstructed by a mass of worms, leading to low-output cardiovascular shock. Other terms for this condition include postcaval syndrome, acute hepatic syndrome, liver failure syndrome, dirofilarial hemoglobinuria, and vena cava embolism. As the heartworm burden increases, adult worms migrate to the right atrium and caudal vena cava from their preferred locations in the pulmonary artery and right ventricle. Factors other than worm burden alone are probably also involved in the development of the caval syndrome, including degree of pulmonary hypertension. Caval syndrome occurs more often in geographic areas where HWD is enzootic; up to 20% of dogs with HWD are estimated to be affected in some areas.
Most dogs that develop caval syndrome have no history of heartworm-related signs. Acute collapse is common, often accompanied by anorexia, weakness, tachypnea or dyspnea, pallor, hemoglobinuria, and bilirubinuria. A tricuspid insufficiency murmur, jugular distention and pulsations, weak pulses, a loud and possibly split S2, and a cardiac gallop rhythm are often found. Sometimes coughing or hemoptysis and ascites occur. Tricuspid insufficiency and partial occlusion of RV inflow caused by a mass of worms, in conjunction with pulmonary hypertension, lead to the development of right-sided congestive signs and poor cardiac output.
Clinicopathologic findings may include microfilaremia, Coombs-negative fragmentation hemolytic anemia (from red blood cell trauma), azotemia, abnormal liver function, and increased liver enzyme activities; DIC is common. Intravascular hemolysis results in hemoglobinemia and hemoglobinuria. Thoracic radiographs indicate right heart and pulmonary artery enlargement. The ECG usually suggests RV enlargement. Ventricular or supraventricular premature complexes are common. Echocardiography reveals a mass of worms entangled at the tricuspid valve and in the right atrium and venae cavae (Fig. 10-3). RV dilation and hypertrophy, paradoxical septal motion, and a small left ventricle are also typical.
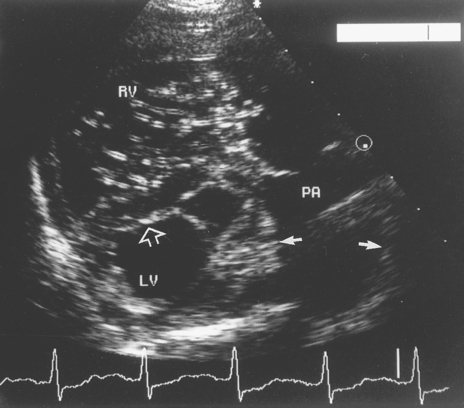
FIG 10-3 Echocardiogram from a 9-year-old male mixed-breed dog with caval syndrome. The transducer is in the right parasternal short-axis position at a level just below the aorta. The image shows the enlarged and hypertrophied right ventricle and its outflow tract. Many small, bright parallel echoes are apparent in the body of the right ventricle (RV) in this diastolic frame and are caused by a clump of heartworms entangled in the tricuspid valve apparatus. Note also the widened main pulmonary artery segment typical of pulmonary hypertension (small arrows). The interventricular septum is flattened and pushed toward the left ventricle (LV) by high right ventricular pressure (open arrow). The LV itself is small because the heartworms obstruct blood flow through the right heart. PA, Main pulmonary artery.
Most dogs die within 24 and 72 hours as a result of cardiogenic shock complicated by metabolic acidosis, DIC, and anemia unless they are aggressively treated. Worms must be surgically removed from the vena cava and right atrium as soon as possible. The dog is lightly sedated, if necessary, and local anesthesia is used. A right jugular venotomy with the dog restrained in left lateral recumbency is the usual approach. Long alligator forceps, an endoscopic basket retrieval instrument, or horsehair brush device are used to grasp and withdraw the heartworms through the jugular vein incision. The instrument is gently passed down the vein into the right atrium; repositioning of the animal’s head and neck may be necessary to pass the instrument beyond the thoracic inlet. The goal is to retrieve as many worms as possible; generally, five to six unsuccessful attempts in sequence is the end point. Resistance to instrument withdrawal from the vein may occur if too many worms are grasped at once or a cardiovascular structure is grabbed by forceps. Survival rates of 50% to 80% have been reported for dogs undergoing this procedure. Another technique that has been used in very small dogs is right auricular cannulation performed via a thoracotomy to remove worms. (See Suggested Readings for more information on this technique.)
Cautious intravenous (IV) fluid administration with other supportive care is provided during and after surgical worm removal. Central venous pressure monitoring helps the clinician assess the effectiveness of worm removal and fluid therapy. Treatment with a positive inotrope or sodium bicarbonate is usually not necessary, but a broad spectrum antibiotic is recommended. Monitoring for anemia, thrombocytopenia, DIC, and organ dysfunction is important; treatment is given as indicated. Severe pulmonary thromboembolism and renal or hepatic failure are associated with poor outcome. Dogs that survive acute caval syndrome can be treated with adulticide within a few weeks after stabilization to eliminate remaining worms. The use of a flexible alligator forceps with fluoroscopic or transesophageal echo guidance has been advocated as a way to reduce the worm burden in the main pulmonary artery and lobar branches before adulticide therapy. This can reduce the risk for postadulticide thromboembolism in heavily infected dogs, although technical issues and the need for heavy sedation or anesthesia may be limitations.
MICROFILARICIDE THERAPY
Specific microfilaricidal therapy for dogs with circulating microfilariae may be given 3 to 4 weeks after adulticide therapy, but the gradual microfilaricidal effect of monthly preventive drugs has largely replaced the need for this treatment. Oral ivermectin (at 50 μg/kg) and milbemycin oxime (at standard preventive dose) can rapidly reduce microfilariae. Ivermectin at this dose is safe for Collies. The rapid death of many microfilariae can cause systemic effects within 3 to 8 (and occasionally 12) hours of the first dose; these include lethargy, inappetence, excessive salivation, retching, defecation, pallor, and tachycardia. Such adverse effects are usually mild, but dogs with a high number of circulating microfilariae may experience circulatory collapse. This condition generally responds to glucocorticoid therapy (e.g., prednisolone sodium succinate, 10 mg/kg, or dexamethasone, 1 mg/kg, administered intravenously) and IV fluid administration (e.g., 80 ml/kg over 2 hours) if these are instituted promptly. All cases should be closely observed for 8 to 12 hours after initial microfilaria treatment with either macrolide. An additional benefit is protection against new infection. Moxidectin and selamectin are also known to be microfilaricidal, but clinical experience for this purpose is lacking. Other drugs used as microfilaricides in the past (e.g., levamisole and fenthion) are not recommended because of lower efficacy and frequent adverse effects.
HEARTWORM PREVENTION
Heartworm prophylaxis is indicated for all dogs living in endemic areas. The time of year that infection can occur is limited in many geographic areas, because sustained warm, moist conditions are needed for transmission of the disease. Transmission can occur only during a few months in the most northern parts of the United States and Canada; year-round transmission is likely only in the far south of the continental United States. Although monthly preventive therapy may be necessary only during June through November in most of the United States, continuous chemoprophylaxis throughout the year may be more practical in locations where transmission is likely during more than half the year.
Several drugs are currently available for preventing heartworm disease: the avermectins (ivermectin, selamectin) and the milbemycins (milbemycin oxime, moxidectin). Diethycarbamazine (DEC) is another choice, but it must be given daily. The avermectins and milbemycins induce neuromuscular paralysis and death in nematode (and arthropod) parasites by interacting with membrane chloride channels. They are effective against third- and fourth-stage larvae and sometimes young adult worms as well as microfilariae; however, milbemycin is least effective against adult D. immitis. Retroactive efficacy (reachback) with these agents lasts at least 1 and possible more than 2 months after a single dose. These agents are quite safe in mammals when used as directed, even in sensitive Collies. Cases of clinical toxicity have usually been related to dosage miscalculation using a concentrated livestock preparation.
The avermectins and milbemycins are packaged in monthly dose units according to body weight ranges. Dosing should begin within 1 month of the start of the heartworm transmission season and continue to within 1 month after the transmission season ends. Year-round administration may be preferable depending on location. Drugs available for monthly oral administration include ivermectin (6-12 μg/kg; Heartgard, Merial), milbemycin oxime (0.5-1.0 mg/kg; Interceptor, Novartis Animal Health), and moxidectin (3 μg/kg; ProHeart, Fort Dodge Animal Health). Selamectin (Revolution, Pfizer Animal Health) is applied to the skin between the shoulder blades at a monthly dose range of 6-12 mg/kg; efficacy is not affected if bathing or swimming is delayed at least 2 hours after application. Some of these agents are effective against other parasites at the doses used for heartworm prevention (e.g., hookworms with milbemycin; fleas, earmites, and ticks with selamectin). These drugs are also sometimes marketed in combination with other antiparasitic agents for broader protection against endoparasites and ectoparastites.
DEC (at 3 mg/kg, or 6.6 mg/kg of the 50% citrate, by mouth once daily) has been used for decades to prevent HWD. The drug is thought to affect the heartworm’s L3 to L4 molting stage at 9 to 12 days after infection. The drug may be discontinued 2 months after a killing frost in regions with cold winters and reinstituted 1 month before mosquito season in the spring. Before beginning (or restarting) DEC treatment, dogs must be negative for microfilariae (see p. 170). Puppies 6 months of age and older also should be tested for microfilariae. Annual microfilaria tests are strongly recommended, even in areas where the drug is given year-round. To be effective, DEC must be given daily. If a lapse in DEC administration of <6 weeks has occurred, one dose of a monthly preventive drug should restore protection. For longer lapses, monthly chemoprophylaxis should be extended for a year. Microfilaria-positive dogs should not be given DEC. Adverse reactions of variable severity may occur, especially in dogs with higher numbers of microfilariae. These may include lethargy progressing to vomiting, diarrhea, and bradycardia; some patients develop hypovolemic shock, with tachypnea, tachycardia, recumbency, hypersalivation, and eventually death. IV dexamethasone (at least 2 mg/kg), fluids, and other supportive measures have been used to treat the hypovolemia and shock; atropine is used for severe bradycardia. Dogs with this microfilaria-induced reaction that do not show clinical improvement within 3 to 5 hours are likely to die. Dogs without circulating microfilariae may be given DEC. Those on DEC prophylaxis that are subsequently discovered to have circulating microfilariae may be continued on the drug without interruption during adulticide and microfilaricide therapy to prevent reinfection.
Preventive therapy can begin at 6 to 8 weeks of age. Dogs old enough to have been previously infected should be tested for circulating Ag and (if DEC is to be used) microfilariae before chemoprophylaxis is initially begun. Retesting for heartworm Ag every 2 to 3 years is probably adequate when monthly preventive agents are used. When DEC is chosen as a preventive, yearly microfilaria testing is important before DEC is reinstituted.
HEARTWORM DISEASE IN CATS
Pathophysiology
In cats the pathophysiologic changes associated with HWD occur in two stages. Approximately 3 to 6 months after infection, immature worms arrive, and may die, in the pulmonary arteries. This stimulates pulmonary intravascular macrophage activation. These specialized phagocytic cells are located in the pulmonary capillary beds of cats but not dogs. Activation of these macrophages leads to acute inflammation in the pulmonary arteries and lung tissue. Adventitial and perivascular inflammatory cell infiltrates of eosinophils and neutrophils are seen as well. Cats also have more extensive alveolar type 2 (surfactant-producing) cell hyperplasia than dogs, which can interfere with alveolar O2 exchange. The parenchymal lesions are thought to play an important role in the development of acute respiratory distress in cats 3 to 9 months after infection. The acronym HARD (heartworm-associated respiratory disease) has been proposed for the lesions and subsequent clinical signs that may result from the death of L5 larvae in the lungs of these cats. Although some cats recover, this phase is fatal in others. Sudden death can occur.
In cats that survive, the acute inflammation subsides. Vascular injury leads to myointimal proliferations and muscular hypertrophy in affected pulmonary arteries. These lesions tend to be focal. This may be why clinically relevant pulmonary hypertension, secondary RV hypertrophy, and right-sided CHF are uncommon in cats. Dead and degenerating worms cause recrudescence of pulmonary inflammation and thromboembolism. Disease is most severe in the caudal lung lobes. Caudal lobar arterial obstruction can be caused by villous proliferation, thrombi, or dead heartworms. Adult worms are more likely to obstruct the pulmonary arteries of cats (compared with dogs) by virtue of their relative size. The bronchopulmonary circulation in cats is thought to prevent pulmonary infarction.
Vomiting is common in cats with HWD. The mechanism for this may involve central stimulation (of the chemoreceptor trigger zone) by inflammatory mediators. Antiinflammatory doses of a glucocorticoid often control this sign.
Infected cats generally have fewer adult worms than do infected dogs. Heartworms mature more slowly, fewer numbers of infective larvae mature to adults, and the adult life span is shorter in cats. However, live worms can persist for 2 to 3 years. Heartworm-infected cats generally have fewer than eight adult worms in the RV and pulmonary arteries, and most cats have only one or two worms. Nevertheless, even one adult worm can cause death. Unisex infection is common. Most cats have no or only a brief period of microfilaremia. Aberrant worm migration is also more common in cats than dogs and complicates necropsy confirmation of infection. Aberrant sites have included the brain, subcutaneous nodules, body cavities, and occasionally a systemic artery.
Clinical Features
Most reported cases have occurred in cats 3 to 6 years of age, although cats of any age are susceptible. Domestic Shorthair cats seem to be overrepresented. Male cats are overrepresented in some but not all studies. Cats living strictly indoors are not protected from infection. Infection is self-limiting in some cats. Some researchers have noted an increase in HWD diagnosis during fall and winter, presumably after infection in the spring, but others have found fewer cases in the latter part of the year.
Clinical signs are variable and may be transient or nonspecific. Respiratory signs occur in more than half of symptomatic cats, especially dyspnea and/or paroxysmal cough, which can mimic feline asthma. Other client complaints include lethargy, anorexia, vomiting, syncope, other neurological signs, and sudden death. Vomiting, usually unrelated to eating, is common and may be the only sign in some infected cats. Severe clinical signs are usually associated with the arrival of L5 worms in the pulmonary arteries (and HARD surrounding the death of some L5) and also with thromboembolism after the death of one or more adult worms. The sudden onset of neurologic signs, with or without anorexia and lethargy, is common during aberrant worm migration. Such signs include seizures, dementia, apparent blindness, ataxia, circling, mydriasis, and hypersalivation. Only rarely do cardiopulmonary and neurologic signs co-exist. Although heartworms can cause significant pulmonary disease, some cats have no clinical signs.
Auscultation may reveal pulmonary crackles, muffled lung sounds (either from pulmonary consolidation or pleural effusion), tachycardia, and sometimes a cardiac gallop sound or murmur. Pleural effusion caused by right-sided CHF, as well as syncope, is less common in cats than in dogs with HWD. However, chylothorax and ascites are occasionally associated with HWD in cats, and pneumothorax occurs rarely. There are sporadic reports of caval syndrome in cats.
Peracute respiratory distress, ataxia, collapse, seizures, hemoptysis, or sudden death may occur.
Diagnosis
Definitive diagnosis is more difficult in cats than dogs. A combination of serologic testing (see p. 170), thoracic radiographs, and echocardiography is used. Microfilaria testing is only occasionally helpful.
TESTS FOR HEATWORM DISEASE IN CATS
Serologic Tests
Feline heartworm Ab tests are often used for screening; however, although they are fairly sensitive, they are not specific for adult heartworms. The ELISA-based Ag tests are highly specific in detecting adult heartworm infection, but their sensitivity depends on the gender, age, and number of worms. Serologic test results may be negative early in the infection, although the cat may have clinical signs. Ag test results are negative during the first 5 months after infection and may be variably positive at 6 to 7 months; infections with mature female worms should be detected after 7 months. False-negative heartworm Ag test results are more likely in cats because worm burden is typically low; also, a longer time is required for cats to become Ag positive. Acute death and severe clinical signs may occur in Ag-negative cats. Furthermore, postmortem diagnosis may be difficult if the worms are located in distal pulmonary arteries or aberrant sites. Occasionally, a positive Ag test result occurs but no worms are found on postmortem examination. Spontaneous worm death, worms overlooked during pulmonary evaluation, and ectopic infection are likely reasons for this finding.
RADIOGRAPHY
Radiographic findings that suggest HWD include pulmonary artery enlargement with or without visible tortuosity and pruning, RV or generalized cardiac enlargement, and diffuse or focal pulmonary bronchointerstitial infiltrates (Fig. 10-4). Pulmonary hyperinflation is sometimes evident. The pulmonary artery and right heart changes are typically more subtle in cats than dogs. Radiographic findings may not correlate with clinical signs or results of serologic tests. Pulmonary artery distention may be greatest within the first 7 months of infection; some regression may occur subsequently, especially in cranial arteries. The DV view is best for evaluating caudal lobar arteries; these are more frequently abnormal on radiographs. The right caudal lobar artery may be more prominent; however, a left caudal pulmonary artery ≥1.6 multiplied by the width of the ninth rib at the ninth intercostal space was reported as the most discriminating radiographic finding for separating heartworm-infected from non-infected cats (Schafer et al., 1995). The main pulmonary artery segment is not usually visible on DV or ventrodorsal views in cats because its location is more medial than it is in dogs. Marked right heart enlargement is more likely when signs of right-sided CHF (e.g., pleural effusion) exist. Thoracocentesis may be necessary to evaluate the heart, pulmonary vasculature, and lung parenchyma when there is pleural effusion. Ascites occurs in some cats with HWD, but it is rare in cats with heart failure resulting from cardiomyopathy.
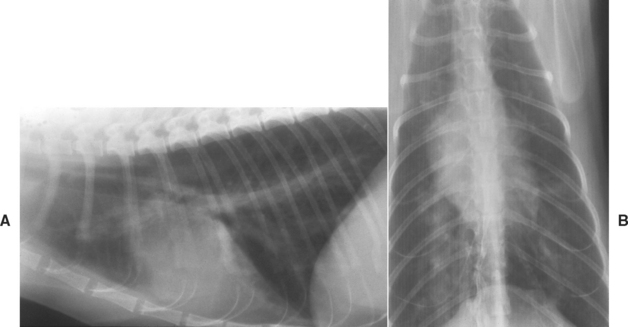
FIG 10-4 Lateral (A) and dorsoventral (B) radiographs from a cat with heartworm disease. There are interstitial infiltrates throughout the lung fields and enlarged pulmonary arteries seen on both views.
Both heartworm-associated pneumonitis as well as pulmonary thromboembolism produce pulmonary infiltrates; focal perivascular and interstitial opacities are more common than diffuse infiltrates. Radiographs are normal in a small minority of heartworm-infected cats.
Pulmonary arteriography may confirm a suspected diagnosis of HWD in a cat with a false-negative Ag test result and normal echocardiogram. The study may be performed using a large-bore jugular catheter. Morphologic changes in the pulmonary arteries are outlined, and worms appear as linear filling defects.
ECHOCARDIOGRAPHY
Echocardiographic findings may be normal unless worms are located in the heart, main pulmonary artery segment, or proximal left and right pulmonary arteries. However, heartworms may be visualized in about one half to three fourths of infected cats. Higher numbers of worms increase the likelihood of identification with echocardiography. Because worms are seen more often in the pulmonary arteries than in right heart chambers, an index of suspicion and careful interrogation of these structures are important.
ELECTROCARDIOGRAPHY
ECG findings are often normal, but most cats with heartworm-induced CHF have changes suggesting RV enlargement. Arrhythmias appear to be uncommon. Advanced pulmonary arterial disease and CHF are more likely to cause ventricular tachyarrhythmias.
OTHER TESTS
Between one and two thirds of infected cats have peripheral eosinophilia, usually from 4 to 7 months after infection. Many times the eosinophil count is normal; basophilia is uncommon. About one third of the cases have mild nonregenerative anemia. Advanced pulmonary arterial disease and thromboembolism may be accompanied by neutrophilia (sometimes with a left-shift), monocytosis, thrombocytopenia, and DIC. Hyperglobulinemia, the most common biochemical abnormality, occurs inconsistently. The prevalence of glomerulopathies in cats with HWD is unknown, but it does not appear to be high.
Tracheal wash or bronchoalveolar lavage specimens may show an eosinophilic exudate that suggests allergic or parasitic disease, similar to that found with feline asthma or pulmonary parasites. This finding usually occurs between 4 and 8 months after infection. Later in the disease, tracheal wash findings may be unremarkable or indicate nonspecific chronic inflammation. Pleural effusion resulting from heartworm-induced CHF is generally a modified transudate, although chylothorax occasionally develops.
At around 6.5 to 7 months after infection, a transient (1 to 2 months in duration), low-grade microfilaremia occurs in about half of infected cats. Therefore microfilaria concentration tests are usually negative. Nevertheless, a concentration test may still prove valuable in some individual cats. Between 3 and 5 ml, rather than 1 ml, of blood should be used to increase the probability of detecting microfilariae.
MEDICAL THERAPY AND COMPLICATIONS
Adulticide therapy is not recommended in most cases because the likelihood of severe complications in this species is high. Also, spontaneous cure is possible in cats because of the shorter heartworm life span, and cats are not significant reservoirs for HWD transmission to other animals. On the basis of a retrospective study (Atkins et al., 2000), cats treated with thiacetarsamide had no survival advantage over those that were not treated with adulticide.
The recommended, and more conservative, approach for infected cats is to use prednisone as needed for respiratory signs and radiographically evident pulmonary interstitial infiltrates. A monthly heartworm preventive drug is also advised but not a heartworm adulticide. Serologic tests (for heartworm Ab and Ag) are obtained every 6 to 12 months to monitor infection status. Ag-positive cats usually become negative within 4 to 5 months of worm death. It is unclear how long Ab tests remain positive. Serial thoracic radiographs and echocardiograms also can be useful for monitoring cats that have had abnormal findings. Interstitial pulmonary infiltrates usually respond to prednisone (e.g., 2 mg/kg/day by mouth, reduced gradually over 2 weeks to 0.5 mg/kg qod, then discontinued after 2 more weeks). Prednisone therapy may be repeated periodically if respiratory signs recur.
The possibility of severe respiratory distress and death is always present, especially after spontaneous or adulticide-induced worm death. Pulmonary thromboembolism is more likely to produce a fatal outcome in cats than dogs. Clinical findings with pulmonary thromboembolism include fever, cough, dyspnea, hemoptysis, pallor, pulmonary crackles, tachycardia, and hypotension. Radiographic signs include poorly defined, rounded or wedge-shaped interstitial opacities that obscure associated pulmonary vessels. Alveolar infiltrates are seen in some cases. Cats with acute disease are given supportive care, which may include an IV glucocorticoid (e.g., 100 to 250 mg prednisone sodium succinate), fluid therapy, a bronchodilator, and supplemen-tal oxygen. Diuretics are not indicated. Aspirin is currently not recommended for cats with HWD. Aspirin and other nonsteroidal antiinflammatory drugs have not been shown to produce benefit and may exacerbate pulmonary disease.
Right-sided CHF develops in some cats with severe pulmonary arterial disease. Cough and other signs of pulmonary interstitial disease or a thromboembolic event occur inconsistently. Dyspnea (caused by pleural effusion) and jugular venous distention or pulsation are common. Radiographic and ECG findings usually suggest RV enlargement. Therapy is directed at controlling the signs of heart failure. This includes thoracocentesis as needed, cage rest, and cautious furosemide therapy (e.g., 1 mg/kg q12-24h). An ACEI may be helpful. Digoxin is not usually recommended. Pimobendan might be considered, but clinical experience is lacking. The cat’s clinical progress and clinicopathologic abnormalities are used to guide supportive therapy.
Caval syndrome occurs rarely in cats. Successful removal of adult worms through a jugular venotomy is possible.
Adulticide therapy may be considered for cats that continue to manifest clinical signs despite prednisone treatment. Potentially fatal thromboembolism can occur, even with only one worm present. About a third of adulticide-treated cats are expected to have thromboembolic complications. The risk is expected to be higher for heavily infected cats. An adulticide should never be given only on the basis of a positive Ag, Ab, or microfilaria test result. There is little clinical experience with melarsomine (Immiticide) in cats. Doses of >3.5 mg/kg appear to be toxic in this species. IV thiacetarsamide (Caparsolate) has been used successfully at the same doses used in dogs (2.2 mg/kg q12h for 2 days) in combination with prednisone and extremely close monitoring for 2 weeks. Acute respiratory failure and death may occur as a result of dying worms or toxic effects of the arsenical drug. Profound depression and GI side effects also are common after each dose. Pretreatment with an antihistamine and soluble glucocorticoid before thiacetarsamide administration is of unknown efficacy. The effectiveness of chronic ivermectin at the recommended prophylactic dose against juvenile worms in cats is not known. Results of adult worm Ag tests should be negative within 3 to 4 months of successful adulticide therapy; the time required for Ab titers to become negative is likely much longer.
SURGICAL THERAPY
Several approaches are described for removing adult heartworms from cats, although they are technically challenging. A right jugular venotomy may be used to reach worms in the right atrium and vena cava with small alligator forceps, endoscopic grasping or basket retrieval forceps, or another device. Worm removal via thoracotomy and right atriotomy has also been done successfully. A left thoracotomy and pulmonary arteriotomy may permit worm extraction from within the pulmonary artery. A potentially fatal anaphylactic reaction associated with worm breakage could occur during such procedures. Presurgical treatment with a glucocorticoid and antihistamine has been suggested. It is not known whether pretreatment with heparin for several days can reduce thromboembolism associated with surgical worm removal.
MICROFILARICIDE THERAPY
Microfilaricide therapy is rarely necessary because microfilaremia is brief. However, ivermectin and milbemycin should be effective in this setting.
Heartworm Prevention in Cats
Heartworm prophylaxis is recommended for cats in endemic areas. Selamectin (Revolution), ivermectin (Heartgard for cats), and milbemycin oxime (Interceptor Flavor Tabs for Cats) are effective preventive drugs in cats. Selamectin is used at the same dose as for dogs (6-12 mg/kg, topically). Selamectin also is useful for controlling fleas and earmites as well as hookworm and roundworm infections in cats. Ivermectin is administered orally at 24 μg/kg monthly (four times the dose used in dogs). The minimum recommended dose for milbemycin is 2 mg/kg (about twice the dose used in dogs). All these agents are safe in kittens 6 weeks or older. A heartworm Ag test is recommended before beginning prophylaxis if infection could have occurred 8 months or more in the past. These agents may be used in seropositive cats. The efficacy of moxidectin or DEC for heartworm prevention in cats is not known.
Bazzocchi C, et al. Immunological role of the endosymbionts of Dirofilaria immitis: the Wolbachia surface protein activates canine neutrophils with production of IL-8. Vet Parasitol. 2003;117:73.
Datz C. Update on canine and feline heartworm tests. Compend Cont Educ Pract Vet. 2003;25:30.
Kellum HB, Stepien RL. Sildenafil citrate therapy in 22 dogs with pulmonary hypertension. J Vet Intern Med. 2007;21:1258-1264.
Litster A, et al. Radiographic cardiac size in cats and dogs with heartworm disease compared with reference values using the vertebral heart scale method: 53 cases. J Vet Cardiol. 2005;7:33.
McCall JW. The safety-net story about macrocyclic lactone heartworm preventives: a review, an update, and recommendations. Vet Parasitol. 2005;133:197.
American Heartworm Society: 2005 Guidelines for the diagnosis, prevention, and management of heartworm (Dirofilaria immitis) infection in dogs, retrieved on 1/27/08, American Heartworm Society; www.heartwormsociety.org. Accessed 1/27/2008.
Atkins CE, Miller MW. Is there a better way to administer heartworm adulticidal therapy? Vet Med. 2003;98:310.
Frank J, et al. Systemic arterial dirofilariasis in five dogs. J Vet Intern Med. 1997;11:189.
Hettlich BF, et al. Neurologic complications after melarsomine dihydrochloride treatment for Dirofilaria immitis in three dogs. J Am Vet Med Assoc. 2003;223:1456.
Hopper K, Aldrich J, Haskins SC. Ivermectin toxicity in 17 collies. J Vet Intern Med. 2002;16:89.
Kitagawa H, et al. Comparison of laboratory test results before and after surgical removal of heartworms in dogs with vena caval syndrome. J Am Vet Med Assoc. 1998;213:1134.
Kitoh K, et al. Role of histamine in heartworm extract-induced shock in dogs. Am J Vet Res. 2001;62:770.
Kuntz CA, et al. Use of a modified surgical approach to the right atrium for retrieval of heartworms in a dog. J Am Vet Med Assoc. 1996;208:692.
Lok JB, et al. Activity of an injectable, sustained-release formulation of moxidectin administered prophylactically to mixed breed dogs to prevent infection with Dirofilaria immitis. Am J Vet Res. 2001;62:1721.
Rawlings CA, et al. Surgical removal of heartworms. Semin Vet Med Surg. 1994;9:200.
American Heartworm Society: 2007 Guidelines for the diagnosis, prevention, and management of heartworm (Dirofilaria immitis) infection in cats, retrieved on 1/27/08, American Heartworm Society; www.heartwormsociety.org. Accessed 1/27/2008.
Atkins C, et al. Prevalence of heartworm infection in cats with signs of cardiorespiratory abnormalities. J Am Vet Med Assoc. 1998;212:517.
Atkins C, et al. Heartworm infection in cats: 50 cases (1985–1997). J Am Vet Med Assoc. 2000;217:355.
Borgarelli M, et al. Surgical removal of heartworms from the right atrium of a cat. J Am Vet Med Assoc. 1997;211(1):68.
Browne LE, et al. Pulmonary arterial disease in cats seropositive for Dirofilaria immitis but lacking adult heartworms in the heart and lungs. Am J Vet Res. 2005;66:1544.
DeFrancesco TC, et al. Use of echocardiography for the diagnosis of heartworm disease in cats: 43 cases (1985–1997). J Am Vet Med Assoc. 2001;218:66.
Dillon AR, et al. Feline heartworm disease: correlations of clinical signs, serology, and other diagnostics—results of a multi-center study. Vet Ther. 2000;1:176.
Morchon R, et al. Specific IgG antibody response against antigens of Dirofilaria immitis and its Wolbachia endosymbiont bacterium in cats with natural and experimental infections. Vet Parasitol. 2004;125:313.
Snyder PS, et al. Performance of serologic tests used to detect heartworm infection in cats. J Am Vet Med Assoc. 2000;216:693.