CHAPTER 5 Congenital Cardiac Disease
GENERAL CONSIDERATIONS
Common congenital cardiac malformations, as well as some that occur more sporadically, are described in this chapter. Most congenital heart defects produce an audible murmur (Fig. 5-1), although some serious malformations do not. Murmurs caused by congenital disease range in intensity from very loud to very soft depending on the type and severity of the defect and on hemodynamic factors. In addition to murmurs of congenital disease, clinically insignificant “innocent” murmurs are relatively common in puppies and kittens. Innocent murmurs are usually soft systolic ejection–type murmurs heard best at the left heart base; their intensity may vary with heart rate or body position. Innocent murmurs tend to get softer and usually disappear by about 4 months of age. Murmurs caused by congenital disease usually persist and may get louder with time, although this is not always the case. Careful examination and auscultation are important, not only in animals intended for breeding but also in working dogs and pets. Puppies and kittens with a soft murmur and no other clinical or radiographic signs can be ausculted repeatedly as they grow to determine if the murmur disappears. Further diagnostic tests are indicated in animals with a persistent or loud murmur, those that manifest other signs, and those for which economic or breeding-potential decisions are pending. Adult dogs and cats with a previously undiagnosed congenital defect may or may not manifest clinical signs of disease at presentation.
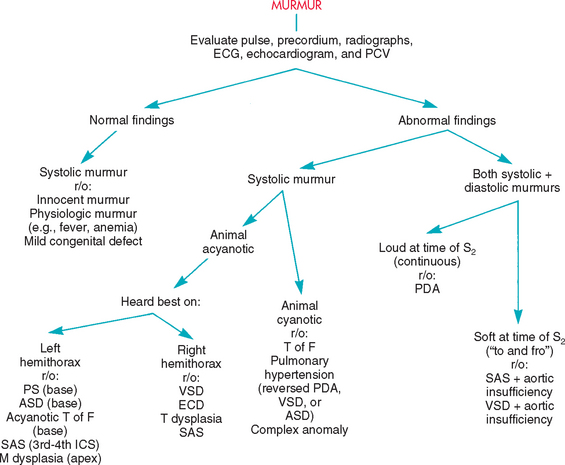
FIG 5-1 Flow chart for differentiating murmurs in puppies and kittens. ASD, Atrial septal defect; ECD, endocardial cushion defect; ECG, electrocardiogram; ICS, intercostal space; M, mitral valve; PCV, packed cell volume; PDA, patent ductus arteriosus; r/o, rule out; SAS, subaortic stenosis; T, tricuspid valve; T of F, tetralogy of Fallot; VSD, ventricular septal defect.
Congenital heart defects most often involve either a valve (or valve region) or an abnormal communication between the systemic and pulmonary circulations. Abnormally formed valves can be insufficient, stenotic, or both. Other malformations can exist, and multiple anomalies occur in some patients. Congenital malformations vary widely in type and severity. The patient’s prognosis and options for therapy depend on the definitive diagnosis as well as severity. Initial noninvasive testing usually includes thoracic radiographs, an electrocardiogram (ECG), and echocardiographic studies (M-mode, 2-dimensional [2-D], and Doppler). A packed cell volume (PCV) documents erythrocytosis in some cases with right-to-left shunting. Cardiac catheterization with selective angiocardiography can be useful to define some structural abnormalities or severity and is needed during transvascular interventional procedures. Surgical repair or palliation, balloon valvuloplasty, transcatheter shunt occlusion, or other interventional techniques may be helpful for some cases.
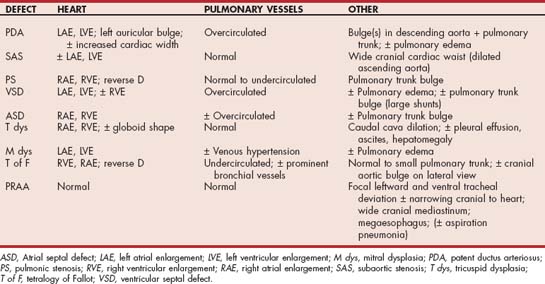
Patent ductus arteriosus (PDA) and subaortic stenosis (SAS) have been identified in different surveys as the most common congenital cardiovascular anomaly in the dog; pulmonic stenosis (PS) is also quite common. Persistent right aortic arch (a vascular ring anomaly), ventricular septal defect (VSD), malformations (dysplasia) of the atrioventricular (AV) valves, atrial septal defect (ASD), and tetralogy of Fallot (T of F) occur less frequently but are not rare. An AV septal (endocardial cushion) defect consists of all or some of the following: a high VSD, a low ASD, and malformations of one or both AV valves. The most common malformations in cats are AV valve dysplasias and atrial or ventricular septal defects; other lesions include SAS, PDA, T of F, and PS. Endocardial fibroelastosis, mainly in Burmese and Siamese cats, also has been reported. Congenital malformations are more prevalent in male than female cats. Congenital malformations in both species can occur as isolated defects, which is most often the case, or in various combinations.
The prevalence of congenital defects is higher in purebred animals than in mixed-breed animals. In some studies a polygenic inheritance pattern has been suggested, although there is more recent focus on a single major gene effect influenced by other modifying genes. Recognized breed predispositions are listed in Table 5-1; animals of other breeds can also be affected with any of these defects as well.
 TABLE 5-1 Breed Predispositions for Congenital Heart Disease
TABLE 5-1 Breed Predispositions for Congenital Heart Disease
| DISEASE | BREED |
|---|---|
| Patent ductus arteriosus | Maltese, Pomeranian, Shetland Sheepdog, English Springer Spaniel, Keeshond, Bichon Frise, Toy and Miniature Poodles, Yorkshire Terrier, Collie, Cocker Spaniel, German Shepherd Dog; Chihuahua, Kerry Blue Terrier, Labrador Retriever, Newfoundland; female > male |
| Subaortic stenosis | Newfoundland, Golden Retriever, Rottweiler, Boxer, German Shepherd Dog, English Bulldog, Great Dane, German Short-Haired Pointer, Bouvier des Flandres, Samoyed |
| Pulmonic stenosis | Bulldog (male > female), Mastiff, Samoyed, Miniature Schnauzer, West Highland White Terrier, Cocker Spaniel, Beagle, Airedale Terrier, Boykin Spaniel, Chihuahua, Scottish Terrier, Boxer, Fox Terrier(?) |
| Ventricular septal defect | English Bulldog, English Springer Spaniel, Keeshond; cats |
| Atrial septal defect | Samoyed, Doberman Pinscher, Boxer |
| Tricuspid dysplasia | Labrador Retriever, German Shepherd Dog, Boxer, Weimaraner, Great Dane, Old English Sheepdog, Golden Retriever; other large breeds; (male > female?) |
| Mitral dysplasia | Bull Terrier, German Shepherd Dog, Great Dane, Golden Retriever, Newfoundland, Mastiff, Rottweiler(?); cats; (male > female) |
| Tetralogy of Fallot | Keeshond, English Bulldog |
| Persistent right aortic arch | German Shepherd Dog, Great Dane, Irish Setter |
EXTRACARDIAC ARTERIOVENOUS SHUNT
The most common congenital arteriovenous shunt is PDA. Rarely, similar hemodynamic and clinical abnormalities are caused by an aorticopulmonary window (a communication between the ascending aorta and pulmonary artery) or some other functionally similar communication in the hilar region.
PATENT DUCTUS ARTERIOSUS
Etiology and Pathophysiology
Functional closure of the ductus arteriosus normally occurs within hours after birth and is followed by structural changes that occur over several months, which cause permanent closure. The ductal wall in animals with an inherited PDA is histologically abnormal and unable to constrict. When the ductus fails to close, blood shunts through it from the descending aorta into the pulmonary artery. Shunting occurs during both systole and diastole because aortic pressure normally is higher than pulmonic pressure throughout the cardiac cycle. This left-to-right shunt causes a volume overload of the pulmonary circulation, left atrium (LA), and left ventricle (LV). The shunt volume is directly related to the pressure difference (gradient) between the two circulations and the diameter of the ductus.
Hyperkinetic arterial pulses are characteristic of PDA. Blood runoff from the aorta into the pulmonary system allows diastolic aortic pressure to decrease below normal. The widened pulse pressure (systolic minus diastolic pressure) causes palpably stronger arterial pulses (Fig. 5-2).
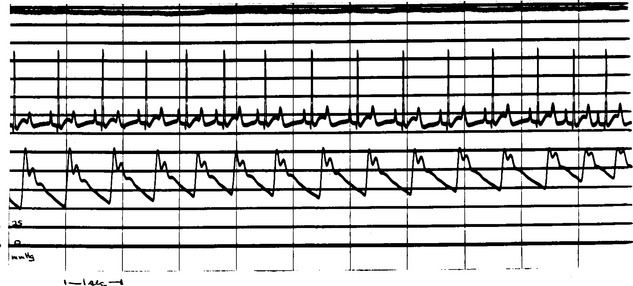
FIG 5-2 Continuous femoral artery pressure recording during surgical ligation of a patent ductus arteriosus in a Poodle. The wide pulse pressure (left side of trace) narrows as the ductus is closed (right side of trace). Diastolic arterial pressure rises because blood runoff into the pulmonary artery is curtailed.
(Courtesy Dr. Dean Riedesel.)
Compensatory mechanisms (e.g., increased heart rate, volume retention) maintain adequate systemic blood flow. However, the LV is subjected to a great hemodynamic burden, especially when the ductus is large, because the increased stoke volume is pumped into the relatively high pressure aorta. LV and mitral annulus dilation in turn cause mitral regurgitation and further volume overload. Excess fluid retention, declining myocardial contractility stemming from the chronic volume overload, and arrhythmias contribute to the development of congestive heart failure (CHF).
In some cases, excessive pulmonary blood flow leads to pulmonary vascular changes, increased resistance, and pulmonary hypertension (see p. 109). If pulmonary artery pressure rises to equal aortic pressure, very little blood shunting occurs. However, if pulmonary artery pressure exceeds aortic pressure, shunt reversal (right-to-left flow) occurs. Approximately 15% of dogs with inherited PDA develop a reversed shunt; female Cocker Spaniels may be at increased risk for reversed PDA.
Clinical Features
A left-to-right shunting PDA (discussed here) is by far the most common form; clinical features of reversed PDA are described on p. 110. The prevalence of PDA is higher in certain breeds of dogs, and a polygenic inheritance pattern is thought to be responsible. The prevalence is approximately three times greater in female than male dogs. Reduced exercise ability, tachypnea, or cough is present in some cases, but many animals are asymptomatic when first diagnosed. Typical findings include a continuous murmur heard best high at the left base (see p. 9), often with a precordial thrill, hyperkinetic (bounding, “waterhammer”) arterial pulses, and pink mucous membranes.
Diagnosis
Radiographs usually show cardiac elongation (left heart dilation), left atrial and auricular enlargement, and pulmonary overcirculation (Table 5-2). A bulge often is evident in the descending aorta (“ductus bump”) or main pulmonary trunk, or both (Fig. 5-3). The triad of all three bulges (i.e., pulmonary trunk, aorta, and left auricle), located in that order from the 1 to 3 o’clock position on a dorsoventral (DV) radiograph, is a classic finding but not always seen. There is also evidence of pulmonary edema in animals with left-sided heart failure. Characteristic ECG findings include wide P waves, tall R waves, and often deep Q waves in leads II, aVF, and CV6LL. Changes in the ST-T segment secondary to LV enlargement may occur. However, the ECG is normal in some animals with PDA.
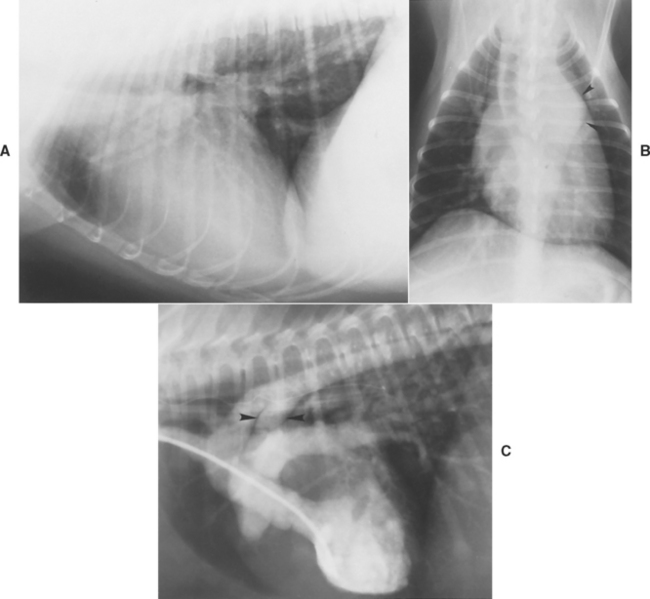
FIG 5-3 Lateral (A) and dorsoventral (DV) (B) radiographs from a dog with a patent ductus arteriosus. Note the large and elongated heart and prominent pulmonary vasculature. A large bulge is seen in the descending aorta on the DV view (arrowheads in B). C, Angiocardiogram obtained using a left ventricular injection outlines the left ventricle, aorta, patent ductus (arrowheads), and pulmonary artery.
Echocardiography also shows left heart enlargement and pulmonary trunk dilation. LV fractional shortening can be normal or decreased, and the E point–septal separation is often increased. The ductus itself may be difficult to visualize because of its location between the descending aorta and pulmonary artery. Views from the cranial left parasternal position are useful. Doppler interrogation documents continuous, turbulent flow into the pulmonary artery (Fig. 5-4). The maximum aortic-to-pulmonary artery pressure gradient should be estimated. Cardiac catheterization is generally unnecessary for diagnosis, although it is important during interventional procedures. Catheterization findings include higher oxygen content in the pulmonary artery compared with the right ventricle (oxygen “step-up”) and a wide aortic pressure pulse. Angiocardiography shows left-to-right shunting through the ductus (see Fig. 5-3, C).
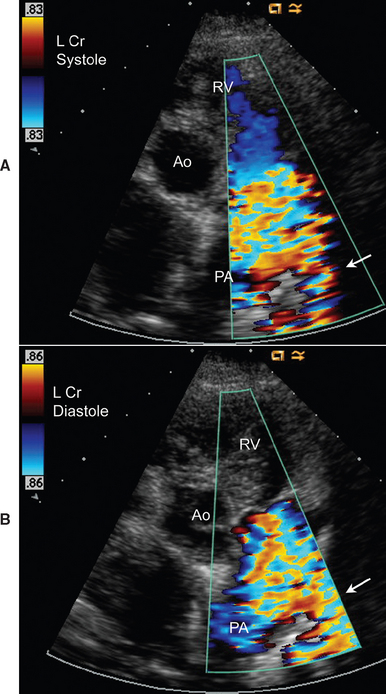
FIG 5-4 Continuous turbulent flow into the pulmonary artery from the area of the patent ductus (arrow) is illustrated by systolic (A) and diastolic (B) color flow Doppler frames from the left cranial parasternal position, in an adult female Springer Spaniel. Ao, Ascending aorta; PA, main pulmonary artery; RV, right ventricle.
Treatment and Prognosis
Closure of the left-to-right ductus, usually performed as soon as is feasible, is recommended by either a transcatheter or surgical occlusion method. Surgical ligation is successful in most cases, although a perioperative mortality of about 10% has been reported. Patient age or weight does not appear to affect the outcome of surgery. Several methods of transcatheter PDA occlusion are available. These involve placing a vascular occluding device (e.g., the Amplatz canine ductal occluder) or wire coils with attached thrombogenic tufts within the ductus. Vascular access is usually via the femoral artery, although some have used a venous approach to the ductus. Where available, transcatheter PDA occlusion offers a much less invasive alternative to surgical ligation. Complications can occur (including aberrant coil embolization and residual ductal flow, among others), and not all cases are suitable for transcatheter occlusion. A normal life span can be expected after uncomplicated ductal closure. The concurrent mitral regurgitation usually resolves after ductus ligation or occlusion if the valve is structurally normal.
Animals with CHF are treated with furosemide, an angiotensin-converting enzyme inhibitor (ACEI), rest, and dietary sodium restriction (see Chapter 3). Because contractility tends to decline over time, pimobendan or digoxin may be indicated as well. Arrhythmias are treated as necessary.
If the ductus is not closed, prognosis depends on its size and the level of pulmonary vascular resistance. CHF is the eventual outcome for most patients that do not undergo ductal closure. More than 50% of affected dogs die within the first year. In animals with pulmonary hypertension and shunt reversal, ductal closure is contraindicated because the ductus acts as a “pop-off” valve for the high right-sided pressures. Ductal ligation in animals with reversed PDA produces no improvement and can lead to right ventricular (RV) failure.
VENTRICULAR OUTFLOW OBSTRUCTION
Ventricular outflow obstruction can occur at the semilunar valve, just below the valve (subvalvular), or above the valve in the proximal great vessel (supravalvular). SAS and PS are most common in dogs and cats. Stenotic lesions impose a pressure overload on the affected ventricle, requiring higher systolic pressure as well as a slightly longer time to eject blood across the narrowed outlet. This generates a systolic pressure gradient across the stenosis because pressure downstream of the stenosis is normal. The magnitude of this gradient is related to the severity of the obstruction.
Concentric myocardial hypertrophy typically develops in response to a systolic pressure overload; some dilation of the affected ventricle can also occur. Ventricular hypertrophy can impede diastolic filling (by increasing ventricular stiffness) or lead to secondary AV valve regurgitation. Heart failure results when ventricular diastolic and atrial pressures are elevated. Cardiac arrhythmias can contribute to the onset of CHF. Furthermore, the combination of outflow obstruction, paroxysmal arrhythmias, and/or inappropriate bradycardia reflexly triggered by ventricular baroreceptor stimulation can result in signs of low cardiac output. These are often associated with severe outflow tract obstruction and include exercise intolerance, syncope, and sudden death.
SUBAORTIC STENOSIS
Etiology and Pathophysiology
Subvalvular narrowing caused by a fibrous or fibromuscular ring is the most common type of aortic stenosis in dogs. Certain larger breeds of dog are predisposed to this defect. SAS is thought to be inherited as an autosomal dominant trait with modifying genes that influence its phenotypic expression. SAS also occurs in cats; supravalvular lesions have been reported in this species as well.
The spectrum of SAS severity varies widely; three grades of SAS have been described in Newfoundland dogs. The mildest (grade I) is associated with no clinical signs or murmur and only subtle subaortic fibrous tissue ridging seen on postmortem examination. Moderate (grade II) SAS consists of mild clinical and hemodynamic evidence of the disease, with an incomplete fibrous ring below the aortic valve found at postmortem. Dogs with grade III SAS have severe disease and a complete fibrous ring around the outflow tract. Some cases have an elongated, tunnel-like obstruction. There may also be malformations of the mitral valve apparatus. Outflow tract narrowing and dynamic obstruction with or without a discrete subvalvular ridge have also been noted in Golden Retrievers. A component of dynamic LV outflow tract obstruction may be important in other dogs as well.
The obstructive lesion of SAS develops during the first several months of life, and there may be no audible murmur at an early age. In some dogs no murmur is detected until 1 to 2 years of age, and the obstruction may continue to worsen beyond that. Murmur intensity usually increases with exercise or excitement. Because of such factors, as well as the presence of physiologic murmurs in some animals, definitive diagnosis and genetic counseling to breeders can be difficult.
The severity of the stenosis determines the degree of LV pressure overload and resulting concentric hypertrophy. Coronary perfusion is easily compromised in animals with severe SAS and left ventricular hypertrophy. Capillary density may become inadequate as hypertrophy progresses, and high systolic wall tension with coronary narrowing can cause systolic flow to be reversed in small coronary arteries. These factors contribute to the development of myocardial ischemia and fibrosis. Clinical sequelae include arrhythmias, syncope, and sudden death. Many animals with SAS also have aortic or mitral valve regurgitation because of related malformations or secondary changes; this imposes a volume overload on the LV. Left-sided CHF develops in some cases. Animals with SAS are thought to be at higher risk for devel oping aortic valve endocarditis because of jet lesion injury to the underside of the valve (see p. 121 and Figure 6-4).
Clinical Features
Historical signs of fatigue, exercise intolerance or exertional weakness, syncope, or sudden death occur in about a third of dogs with SAS. Low-output signs can result from severe outflow obstruction, tachyarrhythmias or sudden reflex bradycardia, and hypotension resulting from the activation of ventricular mechanoreceptors. Left-sided CHF can develop, usually in conjunction with concurrent mitral or aortic regurgitation, other cardiac malformations, or acquired endocarditis. Dyspnea is the most commonly reported sign in cats with SAS.
Characteristic physical examination findings in dogs with moderate-to-severe stenosis include weak and late-rising femoral pulses (pulsus parvus et tardus) and a precordial thrill low at the left heartbase. A harsh systolic ejection murmur is heard at or below the aortic valve area on the left hemithorax. This murmur often radiates equally or more loudly to the right heartbase because of the orientation of the aortic arch. The murmur frequently is heard over the carotid arteries, and it may even radiate to the calvarium. In mild cases a soft, poorly radiating ejection murmur at the left and sometimes right heartbase may be the only abnormality found on physical examination. Functional aortic stenosis murmurs that are not associated with SAS are common in Greyhounds and other sight hounds. Aortic regurgitation can produce a diastolic murmur at the left base or may be inaudible. Severe aortic regurgitation can increase the arterial pulse strength. There may be evidence of pulmonary edema or arrhythmias.
Diagnosis
Radiographic abnormalities (see Table 5-2) can be subtle, especially in dogs and cats with mild SAS. The LV can appear normal or enlarged. Poststenotic dilation in the ascending aorta can cause a prominent cranial waist in the cardiac silhouette (especially on a lateral view) and cranial mediastinal widening. The ECG is often normal, although evidence of LV hypertrophy (left axis deviation) or enlargement (tall complexes) can be present. Depression of the ST segment in leads II and aVF can occur from secondary myocardial ischemia or to hypertrophy; exercise induces further ischemic ST-segment changes in some animals. Ventricular tachyarrhythmias are common.
Echocardiography reveals the extent of LV hypertrophy and subaortic narrowing. A discrete tissue ridge below the aortic valve is evident in many animals with moderate-to-severe disease (Fig. 5-5). Premature closure of the aortic valve, systolic anterior motion of the anterior mitral leaflet, and increased LV subendocardial echogenicity (probably from fibrosis) are common in animals with severe obstruction. Ascending aorta dilation, aortic valve thickening, and LA enlargement with hypertrophy may also be seen. In mildly affected animals 2-D and M-mode findings may be unremarkable. Doppler echocardiography reveals systolic turbulence originating below the aortic valve and extending into the aorta, as well as high peak systolic outflow velocity (Fig. 5-6). Some degree of aortic or mitral regurgitation is common. Spectral Doppler studies are used to estimate the stenosis severity. Doppler-estimated systolic pressure gradients in unanesthetized animals are usually 40% to 50% higher than those recorded during cardiac catheterization under anesthesia. Severe SAS is associated with peak estimated gradients >100 to 125 mm Hg. The LV outflow tract should be interrogated from more than one position to achieve the best possible alignment with blood flow. The subcostal (subxiphoid) position usually yields the highest-velocity signals, although the left apical position is optimal in some animals. The Doppler-estimated aortic outflow velocity may be only equivocally high in animals with mild SAS, especially with suboptimal Doppler beam alignment. With optimal alignment, aortic root velocities of <1.7 m/sec are typical in normal unsedated dogs; velocities over ∼2.25 m/sec are generally considered abnormal. Peak velocities in the equivocal range between these values may indicate the presence of mild SAS, especially if there is other evidence of disease, such as disturbed flow in the outflow tract or ascending aorta and aortic regurgitation. This is mainly of concern when selecting animals for breeding. In some breeds (e.g., Greyhound, Boxer, Golden Retriever), outflow velocities in this equivocal range (1.8-2.25 m/sec) are common. This may reflect breed-specific variation in LV outflow tract anatomy or response to sympathetic stimulation, rather than SAS. A limitation of using the estimated pressure gradient to assess outflow obstruction severity is that this gradient depends on blood flow. Factors causing sympathetic stimulation and increased cardiac output (e.g., excitement, exercise, fever) will increase outflow velocities, whereas myocardial failure, cardiodepressant drugs, and other causes of reduced stroke volume will decrease recorded velocities. Cardiac catheterization and angiocardiography are rarely used now to diagnose or quantify SAS, except in conjunction with balloon dilation of the stenotic area.
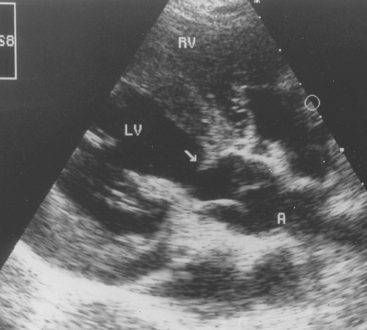
FIG 5-5 Echocardiogram from a 6-month-old German Shepherd Dog with severe subaortic stenosis. Note the discrete ridge of tissue (arrow) below the aortic valve, creating a fixed outflow tract obstruction. A, Aorta; LV, left ventricle; RV, right ventricle.
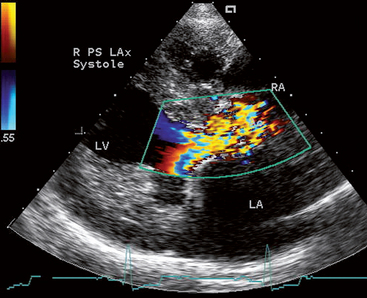
FIG 5-6 Color flow Doppler frame of the left ventricular outflow region in systole from a 2-year-old female Rottweiler with severe subaortic stenosis. Note the turbulent flow pattern originating below the aortic valve, as well as the thickened septum, papillary muscle, and left ventricular free wall. Right parasternal long axis view; Ao, aorta; LA, left atrium; LV, left ventricle; RA, right atrium.
Treatment and Prognosis
Several palliative surgical techniques have been used in dogs with severe SAS, with limited success. Cardiopulmonary bypass and open-heart surgery are necessary to reach the lesion directly. Although resection of the stenotic area can significantly reduce the LV systolic pressure gradient and possibly improve exercise ability, a long-term survival advantage appears lacking. Transvascular balloon dilation of the stenotic area reduces the measured gradient in some dogs, although narrowing may partially recur. Likewise, no survival benefit has been documented with this procedure.
Medical therapy with a β-blocker is advocated in patients with moderate to severe SAS to reduce myocardial oxygen demand and minimize the frequency and severity of arrhythmias. Animals with a high pressure gradient, marked ST-segment depression, frequent ventricular premature beats, or a history of syncope may be more likely to benefit from this therapy. Whether β-blockers prolong survival is unclear. Exercise restriction is advised for animals with moderate-to-severe SAS. Prophylactic antibiotic therapy is recommended for animals with SAS before the performance of any procedures with the potential to cause bacteremia (e.g., dentistry).
The prognosis in dogs and cats with severe stenosis (catheterization pressure gradient >80 mm Hg or Doppler gradient >100-125 mm Hg) is guarded. More than half of dogs with severe SAS die suddenly within their first 3 years. The overall prevalence of sudden death in dogs with SAS appears to be just over 20%. Infective endocarditis and CHF may be more likely to develop after 3 years of age. Atrial and ventricular arrhythmias and worsened mitral regurgitation are complicating factors. Dogs with mild stenosis (e.g., catheterization gradient <35 mm Hg or Doppler gradient <60-70 mm Hg) are more likely to survive longer and without clinical signs.
PULMONIC STENOSIS
Etiology and Pathophysiology
PS is more common in small breeds of dogs. Some cases of valvular PS result from simple fusion of the valve cusps, but valve dysplasia is more common. Dysplastic valve leaflets are variably thickened, asymmetric, and partially fused, with a hypoplastic valve annulus. Right ventricular pressure overload produces right ventricle (RV) hypertrophy as well as secondary dilation. Severe ventricular hypertrophy promotes myocardial ischemia and its sequelae. Excessive muscular hypertrophy below the valve (infundibular area) can create a dynamic subvalvular component to the stenosis. Other variants of PS, including supravalvular stenosis and RV muscular partition (double chamber RV) occur rarely.
High-velocity blood flow across the stenotic orifice creates turbulence leading to poststenotic dilation in the main pulmonary trunk. Right atrial dilation from secondary tricuspid insufficiency and high RV filling pressure predisposes to atrial tachyarrhythmias and CHF. The combination of PS and a patent foramen ovale or ASD can allow right-to-left shunting at the atrial level, but this is rare in dogs and cats.
A single anomalous coronary artery has been described in some Bulldogs and Boxers with PS and is thought to contribute to the outflow obstruction. In such cases, palliative surgical procedures and balloon valvuloplasty may cause death secondary to transection or avulsion of the major left coronary branch.
Clinical Features
Many dogs with PS are asymptomatic when diagnosed, although right-sided CHF or a history of exercise intolerance or syncope may exist. Clinical signs may not develop until the animal is several years old, even in those with severe stenosis. Physical examination findings characteristic of moderate-to-severe stenosis include a prominent right precordial impulse; a thrill high at the left base; normal to slightly diminished femoral pulses; pink mucous membranes; and, in some cases, jugular pulses. A systolic ejection murmur is heard best high at the left base on auscultation. The murmur can radiate cranioventrally and to the right in some cases but usually is not heard over the carotid arteries. An early systolic click is sometimes identified; this probably is caused by abrupt checking of a fused valve at the onset of ejection. A murmur of tricuspid insufficiency or arrhythmias can be heard in some cases.
Diagnosis
Radiographic findings typically seen in animals with PS are outlined in Table 5-2. Marked RV hypertrophy shifts the cardiac apex dorsally and to the left. The heart may appear as a “reverse D” shape on a DV or ventrodorsal (VD) view. A variably sized pulmonary trunk bulge (poststenotic dilation) is best seen at the 1 o’clock position on a DV or VD view (Fig. 5-7). The size of the poststenotic dilation does not correlate with the severity of the pressure gradient. A dilated caudal vena cava is also seen in some animals.
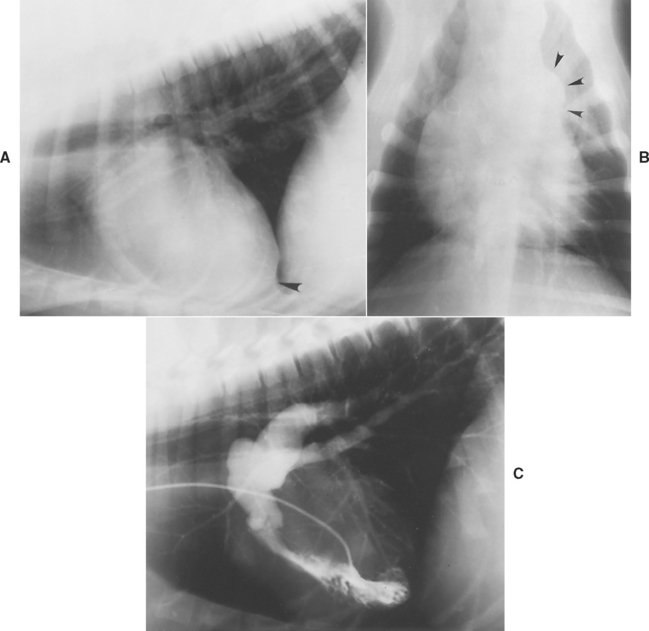
FIG 5-7 Lateral (A) and dorsoventral (DV) (B) radiographs from a dog with pulmonic stenosis, showing right ventricular enlargement (apex elevation on lateral view [arrowhead in A] and reverse D configuration on DV view) along with a pulmonary trunk bulge (arrowheads in B) seen on a DV view. C, Angiocardiogram using a selective right ventricular injection demonstrates poststenotic dilation of the main pulmonary trunk and pulmonary arteries. The thickened pulmonic valve is closed in this diastolic frame.
ECG changes are more common in patients with moderate to severe stenosis. These include an RV hypertrophy pattern, right axis deviation, and sometimes an RA enlargement pattern (P pulmonale) or tachyarrhythmias. Echocardiographic findings characteristic of moderate-to-severe stenosis include RV hypertrophy and enlargement. The interventricular septum often appears flattened as high RV pressure pushes it toward the left (Figure 5-8, A). RA enlargement is often seen as well. A thickened, asymmetrical, or otherwise malformed pulmonic valve usually can be identified (Fig. 5-8, B), although the outflow area may be narrow and difficult to visualize clearly. Poststenotic dilation of the main pulmonary trunk is expected. Pleural effusion and marked right heart dilation often accompany secondary CHF. Paradoxical septal motion is likely in such cases as well. Doppler evaluation along with anatomic findings provides an estimate of PS severity. Cardiac catheterization and angiocardiography also can be used to assess the pressure gradient across the stenotic valve, the right heart filling pressure, and other anatomic features. Doppler-estimated systolic pressure gradients in unanesthetized animals are usually 40% to 50% higher than those recorded during cardiac catheterization. PS is generally considered mild if the Doppler-derived gradient is <50 mm Hg and severe if it is >80 to 100 mm Hg.
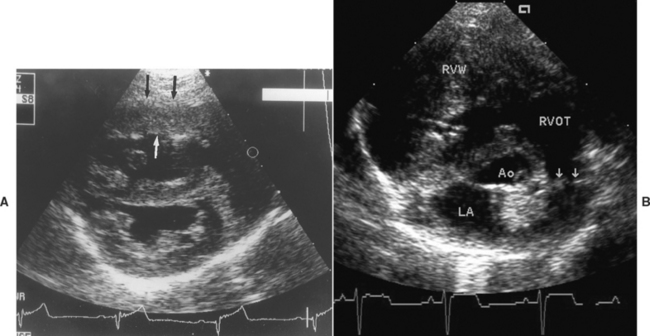
FIG 5-8 Echocardiograms from two dogs with severe pulmonic stenosis. (A) Right parasternal short-axis view at the ventricular level in a 4-month-old male Samoyed shows right ventricular hypertrophy (arrows) and enlargement; high right ventricular pressure flattens the septum toward the left in this diastolic frame. (B) Thickened, partially fused leaflets of the malformed pulmonary valve (arrows) are seen in a 5-month-old male Pomeranian. Ao, Aortic root; LA, left atrium; RVOT, right ventricular outflow tract; RVW, right ventricular wall.
Treatment and Prognosis
Balloon valvuloplasty is recommended for palliation of severe (and sometimes moderate) stenosis, especially if infundibular hypertrophy is not excessive. This procedure reduces or eliminates clinical signs and appears to improve long-term survival in severely affected animals. Balloon valvuloplasty, done in conjunction with cardiac catheterization, involves passing a specially designed balloon catheter across the valve and inflating the balloon to enlarge the stenotic orifice. The procedure is most successful in dogs with simple fusion of the pulmonic valve cusps. Dysplastic valves are more difficult to dilate effectively, but good results are possible in some cases. Various surgical procedures also have been used to palliate moderate-to-severe PS in dogs. Balloon valvuloplasty generally is attempted before a surgical procedure because it is less risky. Animals with a single anomalous coronary artery should not undergo balloon or surgical dilation procedures. Coronary anatomy can be verified using echocardiography or angiography.
Exercise restriction is generally advised for animals with moderate-to-severe stenosis. A β-blocker may be helpful, especially in those with prominent RV infundibular hypertrophy. Signs of CHF are managed medically (see Chapter 3). The prognosis in patients with PS is variable and depends on the severity of the lesion. Life span can be normal in those with mild PS, whereas animals with severe PS often die within 3 years of diagnosis. Sudden death or the onset of CHF is common. The prognosis is considerably worse in animals with tricuspid regurgitation, atrial fibrillation or other tachyarrhythmias, or CHF.
INTRACARDIAC SHUNT
Blood flow volume across an intracardiac shunt depends on the size of the defect and the pressure gradient across it. In most cases, flow direction is from left to right, causing pulmonary overcirculation. Compensatory increases in blood volume and cardiac output occur in response to the partial diversion of blood away from the systemic circulation. A volume overload is imposed on the side of the heart doing the most work. If right heart pressures increase as a result of pulmonary hypertension or a concurrent PS, shunt flow may equilibrate or reverse (i.e., become right-to-left).
VENTRICULAR SEPTAL DEFECT
Etiology and Pathophysiology
Most VSDs are located in the membranous part of the septum, just below the aortic valve and beneath the septal tricuspid leaflet. VSDs sporadically occur in other septal locations also. A VSD may be accompanied by other AV septal (endocardial cushion) malformations, especially in cats. Usually, VSDs produce a volume overload on the lungs, LA, LV, and RV outflow tract. Small defects may be clinically unimportant. Moderate-to-large defects tend to cause left heart dilation and can lead to left-sided CHF. A very large VSD causes both ventricles to function as a common chamber and induces RV dilation and hypertrophy. Pulmonary hypertension secondary to overcirculation is more likely to develop in animals with a large shunt. Some animals with VSD also have aortic regurgitation, with diastolic prolapse of a valve leaflet. Presumably this occurs because the deformed septum provides inadequate anatomic support for the aortic root. Aortic regurgitation places an additional volume load on the LV.
Clinical Features
The most common clinical manifestations of VSD are exercise intolerance and signs of left-sided CHF. Many animals are asymptomatic at the time of diagnosis. The characteristic auscultatory finding is a holosystolic murmur, heard loudest at the cranial right sternal border (which corresponds to the direction of shunt flow). A large shunt volume can produce a murmur of relative or functional PS (systolic ejection murmur at the left base). With concurrent aortic regurgitation, the corresponding diastolic decrescendo murmur is heard at the left base.
Diagnosis
Radiographic findings associated with VSD vary with thesize of the defect and the shunt volume (see Table 5-2). Large shunts cause left heart enlargement and pulmonary overcirculation. However, large shunts that increase pulmonary vascular resistance and pressure lead to RV enlargement. A large shunt volume (with or without pulmonary hypertension) also can increase main pulmonary trunk size.
The ECG may be normal or suggest LA or LV enlargement. In some cases, disturbed intraventricular conduction is suggested by “fractionated” or splintered QRS complexes. An RV enlargement pattern usually indicates a very large defect, pulmonary hypertension, or a concurrent RV outflow tract obstruction, although sometimes a right bundle-branch block causes this pattern.
Echocardiography reveals left heart dilation (with or without RV dilation) when the shunt is large. The defect often can be visualized just below the aortic valve in the right parasternal long-axis LV outflow view. The septal tricuspid leaflet is located to the right of the defect. Because echo “dropout” at the thin membranous septum can mimic a VSD, the area of a suspected defect should be visualized in more than one plane. Supporting clinical evidence and a murmur typical of a VSD also should be present before the diagnosis is made. Doppler (or echo-contrast) studies usually demonstrate the shunt flow (Fig. 5-9).
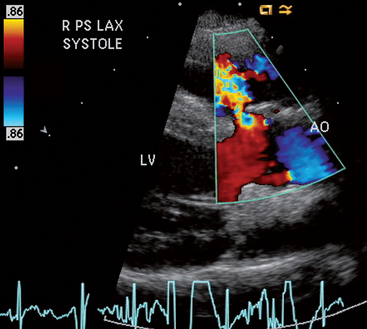
FIG 5-9 Color flow Doppler frame in systole showing turbulent flow (from left to right) through a small membranous ventricular septal defect just below the aortic root in a 1-year-old male terrier. Right parasternal long axis view; AO, aortic root; LV, left ventricle.
Cardiac catheterization, oximetry, and angiocardiography allow measurement of intracardiac pressures, indicate the presence of an oxygen step-up at the level of the RV outflow tract, and show the pathway of abnormal blood flow.
Treatment and Prognosis
A small-to-moderate defect usually allows a relatively normal life span. In some cases, the defect closes spontaneously within the first 2 years of life. Closure can result from myocardial hypertrophy around the VSD or a seal formed by the septal tricuspid leaflet or a prolapsed aortic leaflet. Left-sided CHF is more likely in animals with a large septal defect, although pulmonary hypertension with shunt reversal develops in some instead, usually at an early age.
Definitive therapy for VSD usually requires cardiopulmonary bypass or hypothermia and intracardiac surgery, although transcatheter delivery of an occlusion device may be possible in some cases. Large left-to-right shunts are sometimes palliated by surgically placing a constrictive band around the pulmonary trunk to create a mild supravalvular PS. This raises RV systolic pressure in response to the increased outflow resistance. Consequently, less blood shunts from LV to RV. An excessively tight band can cause right-to-left shunting (functionally analogous to a T of F), however. Left-sided CHF is managed medically. Palliative surgery should not be attempted in the presence of pulmonary hypertension and shunt reversal.
ATRIAL SEPTAL DEFECT
Etiology and Pathophysiology
Several types of ASD exist. Those located in the region of the fossa ovalis (ostium secundum defects) are more common in dogs. An ASD in the lower interatrial septum (ostium primum defect) is likely to be part of the AV septal (endocardial cushion or common AV canal) defect complex, especially in cats. Sinus venosus–type defects are rare; these are located high in the atrial septum near the entry of the cranial vena cava. Animals with ASD commonly have other cardiac malformations as well. In most cases of ASD, blood shunts from LA to RA and results in a volume overload to the right heart. However, if PS or pulmonary hypertension is present, right-to-left shunting and cyanosis may occur.
Clinical Features
The clinical history in animals with an ASD is usually rather nonspecific. Physical examination findings associated with an isolated ASD are often unremarkable, although large left-to-right shunts can cause a murmur of relative PS. Fixed splitting (i.e., with no respiratory variation) of the second heart sound (S2) is the classic auscultatory finding. Rarely, a soft diastolic murmur of relative tricuspid stenosis might be audible.
Diagnosis
Right heart enlargement, with or without pulmonary trunk dilation, is found radiographically in patients with severe shunts (see Table 5-2). The pulmonary circulation may appear to be increased unless pulmonary hypertension has developed. Left heart enlargement is not seen unless another defect, such as mitral insufficiency, is present. The ECG may be normal or show evidence of RV and RA enlargement. Cats with an AV septal defect may have RV enlargement and a left axis deviation.
Echocardiography is likely to show RA and RV dilation, with or without paradoxical interventricular septal motion. Large ASDs can be visualized. Care must be taken not to confuse the thinner fossa ovalis region of the interatrial septum with an ASD because echo dropout also occurs here. Doppler echocardiography allows identification of smaller shunts that cannot be clearly visualized on 2-D exam, but venous inflow streams may complicate this. Cardiac catheterization shows an oxygen step-up at the level of the RA. Abnormal flow through the shunt may be evident after the injection of contrast material into the pulmonary artery.
ATRIOVENTRICULAR VALVE MALFORMATION
MITRAL DYSPLASIA
Congenital malformations of the mitral valve apparatus include shortened or overly elongated chordae tendineae, direct attachment of the valve cusp to a papillary muscle, thickened or cleft or shortened valve cusps, prolapse of valve leaflets, upwardly displaced or malformed papillary muscles, and excessive dilation of the valve annulus. Mitral valve dysplasia (MD) is most common in large-breed dogs and also occurs in cats. Valvular regurgitation is the predominant functional abnormality, and it may be severe; the pathophysiology and sequelae resemble those of acquired mitral regurgitation (see p. 121). Mitral valve stenosis is uncommon. Obstruction to ventricular filling increases LA pressure and can precipitate the development of pulmonary edema. Mitral regurgitation usually accompanies stenosis.
The clinical signs seen in patients with MD are similar to those in dogs with degenerative mitral valve disease, except for the younger patient age. Reduced exercise tolerance, respiratory signs of left-sided CHF, inappetence, and atrial arrhythmias (especially atrial fibrillation) are common in affected animals. Mitral regurgitation typically causes a systolic murmur heard best at the left apex.
The radiographic, ECG, echocardiographic, and catheterization findings are similar to those found in patients with acquired mitral insufficiency. Echocardiography can depict the specific mitral apparatus malformations as well as the degree of chamber enlargement and functional changes.
Therapy consists of medical management for CHF. Animals with mild to moderate mitral valve dysfunction may do well clinically for years. However, for those with severe mitral regurgitation or stenosis, the prognosis is poor. Surgical valve reconstruction or replacement may be possible in some cases.
TRICUSPID DYSPLASIA
Animals with tricuspid dysplasia (TD) have malformations of the tricuspid valve and related structures that are similar to those of MD. The tricuspid valve can be displaced ventrally into the ventricle (an Ebsteinlike anomaly) in some cases; ventricular preexcitation may be more likely in these animals. Tricuspid dysplasia is identified most frequently in large-breed dogs, particularly in Labrador Retrievers, and in males.
The pathophysiologic features of TD are the same as those of acquired tricuspid regurgitation. Severe cases result in marked enlargement of the right heart chambers. Progressive increase in RA and RV end-diastolic pressures eventually result in right-sided CHF. Tricuspid stenosis is rare.
The historical signs and clinical findings likewise are similar to those of degenerative tricuspid disease. Initially, the animal may be asymptomatic or mildly exercise intolerant. However, exercise intolerance, abdominal distention resulting from ascites, dyspnea resulting from pleural effusion, anorexia, and cardiac cachexia often develop. The murmur of tricuspid regurgitation is characteristic but not always clearly audible. Jugular pulsations are common. Additional signs that accompany CHF include jugular vein distention, muffled heart and lung sounds, and ballotable abdominal fluid.
Radiographs demonstrate RA and RV enlargement. The round appearance of the heart shadow in some cases is similar to that seen in patients with pericardial effusion or dilated cardiomyopathy. A distended caudal vena cava, pleural or peritoneal effusion, and hepatomegaly are common.
RV and occasionally RA enlargement patterns are seen on ECG. A splintered QRS complex configuration may be seen. Atrial fibrillation or another atrial tachyarrhythmias occur commonly. Some patients exhibit signs of ventricular preexcitation.
Echocardiography reveals right heart dilation, which can be massive. Valve apparatus malformations also may be clear in several views (Fig. 5-10), although the left apical four-chamber view is especially useful. Intracardiac electrocardiography is necessary to confirm an Ebstein’s anomaly, which is suggested by ventral displacement of the tricuspid valve annulus. A ventricular electrogram recorded on the RA side of the valve is diagnostic, although this technique is rarely done in the clinic.
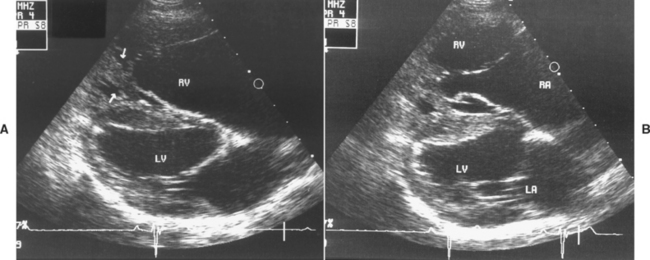
FIG 5-10 Right parasternal long-axis echo images from a 1-year-old male Labrador Retriever with tricuspid valve dysplasia in diastole (A) and systole (B). The valve annulus appears to be ventrally displaced; the leaflet tips are tethered to a malformed, wide papillary muscle (arrows in A). Wide leaflet tip separation in systole (B) caused severe tricuspid regurgitation and clinical congestive heart failure. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
CHF and arrhythmias are managed medically. Periodic thoracocentesis may be needed in animals with pleural effusion that cannot be controlled with medication and diet. The prognosis is guarded to poor, especially when cardiomegaly is marked. Nevertheless, some dogs survive for several years. Balloon dilation has been used successfully to treat tricuspid stenosis.
CARDIAC ANOMALIES CAUSING CYANOSIS
Malformations that allow deoxygenated blood to reach the systemic circulation result in hypoxemia. Visible cyanosis occurs when the desaturated hemoglobin concentration is >5 g/dl. Arterial hypoxemia stimulates increased red blood cell production, which increases oxygen carrying capacity. However, blood viscosity and resistance to flow also rise with the increase in PCV. Severe erythrocytosis (PCV ≥65%) can lead to microvascular sludging, poor tissue oxygenation, intravascular thrombosis, hemorrhage, stroke, and cardiac arrhythmias. Erythrocytosis can become extreme, with a PCV >80% in some animals. The possibility of a venous embolus crossing the shunt to the systemic circulation poses another danger in these cases.
Anomalies that most often cause cyanosis in dogs and cats are T of F and pulmonary arterial hypertension secondary to a large PDA, VSD, or ASD. Other complex but uncommon anomalies, such as transposition of the great vessels or truncus arteriosus, also send deoxygenated blood to the systemic circulation. Some collateral blood flow to the lungs develops from the bronchial arteries of the systemic circulation. These small tortuous vessels may increase the overall radiographic opacity of the central pulmonary fields.
Physical exertion tends to exacerbate right-to-left shunting and cyanosis because greater blood flow to skeletal muscle reduces total peripheral vascular resistance. Despite the pressure overload on the right heart, CHF is rare. The shunt provides an alternate pathway for high pressure flow.
TETRALOGY OF FALLOT
Etiology and Pathophysiology
The four components of the T of F are a VSD, PS, a dextropositioned aorta, and RV hypertrophy. The VSD can be quite large. The PS can involve the valve or infundibular area; in some cases, the pulmonary artery is hypoplastic or not open at all (atretic). The large aortic root extends over the right side of the interventricular septum and facilitates RV-to-aortic shunting. Aortic anomalies exist in some animals as well. RV hypertrophy occurs in response to the pressure overload imposed by the PS and systemic arterial circulation. The volume of blood shunted from the RV into the aorta depends on the balance of outflow resistance caused by the fixed PS compared with systemic arterial resistance, which can vary. Exercise and other causes of decreased arterial resistance increase right-to-left shunt volume. Pulmonary vascular resistance is generally normal in animals with T of F. A polygenic inheritance pattern for T of F has been identified in the Keeshond. The defect also occurs in other dog breeds and in cats.
Clinical Features
Exertional weakness, dyspnea, syncope, cyanosis, and stunted growth are common in the history. Physical examination findings are variable, depending on the relative severity of the malformations. Cyanosis is seen at rest in some animals. Others have pink mucous membranes, although cyanosis usually becomes evident with exercise in these cases. The precordial impulse is usually of equal intensity or stronger on the right chest wall than on the left. Inconsistently, a precordial thrill is palpable at the right sternal border or left basilar area. Jugular pulsation may be noted. A holosystolic murmur at the right sternal border consistent with a VSD, or a systolic ejection murmur at the left base compatible with PS, or both may be heard on auscultation. However, some animals have no audible murmur because hyperviscosity associated with polycythemia diminishes blood turbulence and therefore murmur intensity.
Diagnosis
Thoracic radiographs depict variable cardiomegaly, usually of the right heart (see Table 5-2). The main pulmonary artery may appear small, although a bulge is evident in some cases. Reduced pulmonary vascular markings are common, although a compensatory increase in bronchial circulation can increase the overall pulmonary opacity. The malpositioned aorta creates a cranial bulge in the heart shadow on lateral view. RV hypertrophy displaces the left heart dorsally and can simulate left heart enlargement. The ECG typically suggests RV enlargement, although a left axis deviation is seen in some affected cats.
Echocardiography depicts the VSD, a large aortic root shifted rightward and overriding the ventricular septum, some degree of PS, and RV hypertrophy. Doppler studies reveal the right-to-left shunt and high-velocity stenotic pulmonary outflow jet. An echo-contrast study also can document the right-to-left shunt. Typical clinicopathologic abnormalities include increased PCV and arterial hypoxemia.
Treatment and Prognosis
Definitive repair of T of F requires open-heart surgery. Palliative surgical procedures can increase pulmonary blood flow by creating a left-to-right shunt. Anastomosis of a subclavian artery to the pulmonary artery and the creation of a window between the ascending aorta and pulmonary artery are two techniques that have been used successfully.
Severe erythrocytosis and clinical signs associated with hyperviscosity (e.g., weakness, shortness of breath, seizures) can be treated with periodic phlebotomy (see p. 111) or alternatively, hydroxyurea (see p. 111). The goal is to maintain PCV at a level where clinical signs are minimal; further reduction of PCV (into the normal range) can exacerbate signs of hypoxia. A β-blocker may help reduce clinical signs in some dogs with T of F. Decreased sympathetic tone, RV contractility, RV (muscular) outflow obstruction, and myocardial oxygen consumption, along with increased peripheral vascular resistance, are potential benefits, although the exact mechanism is not clear. Exercise restriction is also advised. Drugs with systemic vasodilator effects should not be given.
The prognosis for animals with T of F depends on the severity of PS and erythrocytosis. Mildly affected animals and those that have had a successful palliative surgical shunting procedure may survive well into middle age. Nevertheless, progressive hypoxemia, erythrocytosis, and sudden death at an earlier age are common.
PULMONARY HYPERTENSION WITH SHUNT REVERSAL
Etiology and Pathophysiology
Pulmonary hypertension develops in a relatively small percentage of dogs and cats with shunts. The defects usually associated with development of pulmonary hypertension are PDA, VSD, AV septal defect or common AV canal, ASD, and aorticopulmonary window. The low-resistance pulmonary vascular system normally can accept a large increase in blood flow without marked increase in pulmonary arterial pressure. It is not clear why pulmonary hypertension develops in some animals, although the defect size in those animals is usually quite large. It is possible that the high fetal pulmonary resistance may not regress normally in these animals or their pulmonary vasculature may react abnormally to an initially large left-to-right shunt flow. In any case, irreversible histologic changes occur in the pulmonary arteries that increase vascular resistance. These include intimal thickening, medial hypertrophy, and characteristic plexiform lesions.
As pulmonary vascular resistance rises, pulmonary artery pressure increases and the volume of blood shunted from left-to-right diminishes. If the right heart and pulmonary pressures exceed those of the systemic circulation, the shunt reverses direction and deoxygenated blood flows into the aorta. These changes appear to develop at an early age (e.g., by 6 months), although exceptions are possible. The term Eisenmenger’s physiology refers to the severe pulmonary hypertension and shunt reversal that develop.
Right-to-left shunts that result from pulmonary hypertension cause pathophysiologic and clinical sequelae similar to those resulting from T of F. The major difference is that the impediment to pulmonary flow occurs at the level of the pulmonary arterioles rather than at the pulmonic valve. Hypoxemia, RV hypertrophy and enlargement, erythrocytosis and its consequences, increased shunting with exercise, and cyanosis can occur. Likewise, right-sided CHF is uncommon but can develop in response to secondary myocardial failure or tricuspid insufficiency. The right-to-left shunt permits venous emboli to cross into the arterial system, potentially resulting in stroke or other arterial embolization.
Clinical Features
The history and clinical presentation of animals with pulmonary hypertension and shunt reversal are similar to those associated with T of F. Exercise intolerance, shortness of breath, syncope (especially in association with exercise or excitement), seizures, and sudden death are common. Cough and hemoptysis may also occur. Cyanosis may be evident only during exercise or excitement. Intracardiac shunts cause equally intense cyanosis throughout the body. Cyanosis of the caudal mucous membranes alone (differential cyanosis) is typically caused by a reversed PDA. Here, normally oxygenated blood flows to the cranial body by way of the brachycephalic trunk and left subclavian artery (from the aortic arch), and the rest of the body receives desaturated blood through the ductus, located in the descending aorta (Fig. 5-11). Rear limb weakness is common in animals with reversed PDA.
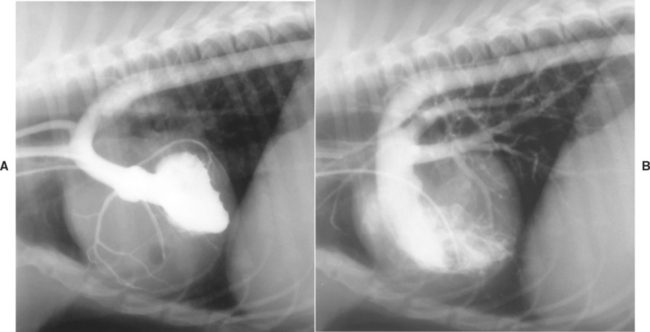
FIG 5-11 Angiocardiograms from an 8-month-old female Cocker Spaniel with patent ductus arteriosus, pulmonary hypertension, and shunt reversal. Left ventricular injection (A) shows dorsal displacement of the left ventricle by the enlarged right ventricle. Note the dilution of radiographic contrast solution in the descending aorta (from mixing with nonopacified blood from the ductus) and the prominent right coronary artery. Right ventricular injection (B) illustrates right ventricular hypertrophy and pulmonary trunk dilation secondary to severe pulmonary hypertension. Opacified blood courses through the large ductus into the descending aorta.
A murmur typical of the underlying defect(s) may be heard, but often no murmur or only a very soft systolic murmur is audible because blood viscosity is increased by polycythemia. There is no continuous murmur in patients with reversed PDA. Pulmonary hypertension often causes the S2 sound to be loud and “snapping” or split. A gallop sound is occasionally heard. Other subtle physical examination findings include a pronounced right precordial impulse and jugular pulsations.
Diagnosis
Thoracic radiographs typically reveal right heart enlargement; a prominent pulmonary trunk; and tortuous, proximally widened pulmonary arteries. A bulge in the descending aorta may be seen in dogs with reversed PDA. The left heart in animals with a reversed PDA or VSD may be enlarged as well. The ECG usually suggests RV and sometimes RA enlargement, with a right axis deviation.
Echocardiography reveals the RV hypertrophy and intracardiac anatomic defects (and sometimes a large ductus), as well as pulmonary trunk dilation. Doppler or echo-contrast study can confirm an intracardiac right-to-left shunt. Reversed PDA flow can be shown by imaging the abdominal aorta during venous echocontrast injection. Peak RV (and in the absence of PS, pulmonary artery) pressure can be estimated by measuring the peak velocity of a tricuspid regurgitation jet. Pulmonary insufficiency flow can be used to estimate diastolic pulmonary artery pressure. Cardiac catheterization also can confirm the diagnosis and quantify the pulmonary hypertension and systemic hypoxemia.
Treatment and Prognosis
Therapy is aimed at managing secondary erythrocytosis to minimize signs of hyperviscosity and attempting to reduce pulmonary arterial pressure, if possible. Exercise restriction is also advised. Erythrocytosis can be managed by periodic phlebotomy or use of oral hydroxyurea. It is unclear whether PCV alone should be used to guide treatment, although maintaining PCV at about 62% has previously been recommended. The ideal PCV for a patient would seem to be that associated with minimal physical manifestations of hyperviscosity (e.g., rear limb weakness, shortness of breath, lethargy). Surgical closure of the shunt is contraindicated. The prognosis is generally poor in animals with pulmonary hypertension and shunt reversal, but some patients have done well for years with medical management.
Phlebotomy can be done when necessary. One method is to remove 5 to 10 ml blood/kg body weight and administer an equal volume of isotonic fluid. Another technique (described by Cote and Ettinger, 2001) involves removing 10% of the patient’s circulating blood volume initially without giving replacement fluid. This volume (ml) is calculated as 8.5% × body weight (kg) × 1000 g/kg × 1 ml/g. After 3 to 6 hours of cage rest, an additional volume of blood is removed if the patient’s initial PCV was >60%. This additional volume would be 5% to 10% of the circulating blood volume if initial PCV was 60% to 70%, or an additional 10% to 18% if initial PCV was >70%.
Hydroxyurea therapy (40 to 50 mg/kg by mouth q48h or 3×/week) can be a useful alternative to periodic phlebotomy in some patients with secondary erythrocytosis. A CBC and platelet count should be monitored weekly or biweekly to start. A PCV between approximately 55% to 60% is the suggested target. Possible adverse effects of hydroxyurea include anorexia, vomiting, bone marrow hypoplasia, alopecia, and pruritus. The dose can be divided q12h on treatment days, or administered twice weekly, or at <40 mg/kg depending on the patient’s response.
Sildenafil citrate is a selective phosphodiesterase-5 inhibitor that may reduce pulmonary resistance via nitric oxide–dependent pulmonary vasodilation. It appears to improve clinical signs and exercise tolerance in some dogs with pulmonary hypertension, although experience in animals is limited. Doses of 0.5 to 2(or 3) mg/kg q12h or q8h seem to be well-tolerated and produce some reduction in Doppler-estimated pulmonary artery pressure. Lower initial doses are suggested, with gradual up-titration. Adverse effects of sildenafil can include possible nasal congestion, hypotension, or sexual adverse effects, especially in intact animals. Other vasodilator drugs tend to produce systemic effects that are similar to or greater than those on the pulmonary vasculature; therefore they are of little benefit and possibly detrimental. Low-dose aspirin (e.g., 5 mg/kg) therapy may also be useful in animals with pulmonary hypertension and reversed shunt, but this is not well-studied.
OTHER CARDIOVASCULAR ANOMALIES
VASCULAR RING ANOMALIES
Various vascular malformations originating from the embryonic aortic arch system can occur. These can entrap the esophagus and sometimes the trachea within a vascular ring at the dorsal heart base. Persistent right aortic arch is the most common vascular ring anomaly in the dog. This developmental malformation surrounds the esophagus: dorsally and to the right with the aortic arch, to the left with the ligamentum arteriosum, and ventrally with the base of the heart. Different vascular ring anomalies can occur as well. In addition, other vascular malformations, such as a left cranial vena cava or PDA, may accompany a vascular ring anomaly. Vascular ring anomalies are rare in cats.
The vascular ring prevents solid food from passing normally through the esophagus. Clinical signs of regurgitation and stunted growth commonly develop within 6 months of weaning. Esophageal dilation occurs cranial to the ring; food may be retained in this area. Sometimes the esophagus dilates caudal to the stricture as well, indicating that altered esophageal motility coexists. Respiratory signs including coughing, wheezing, and cyanosis usually signal secondary aspiration pneumonia. However, in some cases a double aortic arch can cause stridor and other respiratory signs secondary to tracheal stenosis.
The animal’s body condition score may be normal initially, but progressive debilitation ensues. A palpably dilated cervical esophagus (containing food or gas) is evident at the thoracic inlet in some cases. Fever and respiratory signs suggest aspiration pneumonia. Vascular ring anomalies by themselves do not result in abnormal cardiac sounds.
Thoracic radiographs show a leftward tracheal deviation near the cranial heart border on DV view. Other common signs include a widened cranial mediastinum, focal narrowing and/or ventral displacement of the trachea, air or food in the cranial thoracic esophagus, and sometimes evidence of aspiration pneumonia. A barium swallow allows visualization of the esophageal stricture over the heartbase and cranial esophageal dilation (with or without caudal esophageal dilation).
Surgical division of the ligamentum arteriosum, or other vessel if the anomaly is not a persistent right aortic arch, is the recommended therapy. In some cases a retroesophageal left subclavian artery or left aortic arch is also present and must be divided to free the esophagus. Medical management consists of frequent small, semisolid, or liquid meals eaten in an upright position. This feeding method may be necessary indefinitely. Persistent regurgitation occurs in some dogs despite successful surgery, suggesting a permanent esophageal motility disorder.
COR TRIATRIATUM
Cor triatriatum is an uncommon malformation caused by an abnormal membrane that divides either the right (dexter) or the left (sinister) atrium into two chambers. Cor triatriatum dexter occurs sporadically in dogs; cor triatriatum sinister has been described only rarely. Cor triatriatum dexter results from failure of the embryonic right sinus venosus valve to regress. The caudal vena cava and coronary sinus empty into the RA caudal to the intra-atrial membrane; the tricuspid orifice is within the cranial RA “chamber.” Obstruction to venous flow through the opening in the abnormal membrane elevates vascular pressure in the caudal vena cava and the structures that drain into it.
Large- to medium-size breeds of dog are most often affected. Persistent ascites that develops at an early age is the most prominent clinical sign. Exercise intolerance, lethargy, distended cutaneous abdominal veins, and sometimes diarrhea are reported also. Neither a cardiac murmur nor jugular venous distention are features of this anomaly.
Thoracic radiographs indicate caudal vena caval distention without generalized cardiomegaly. The diaphragm may be displaced cranially by massive ascites. The ECG is usually normal. Echocardiography reveals the abnormal membrane and prominence of the caudal RA chamber and vena cava. Doppler studies show the flow disturbance within the RA and allow the intra-RA pressure gradient to be estimated.
Successful therapy requires enlarging the membrane orifice or excising the abnormal membrane to remove flow obstruction. A surgical approach using inflow occlusion, with or without hypothermia, can be used to excise the membrane or break it down using a valve dilator. A much less invasive option is percutaneous balloon dilation of the membrane orifice. This works well as long as a sufficiently large balloon is used. Several balloon dilation catheters placed simultaneously may be needed for effective dilation in larger dogs.
ENDOCARDIAL FIBROELASTOSIS
Diffuse fibrosis and elastic thickening of the endocardium characterize the congenital abnormality endocardial fibroelastosis. It is reported more commonly in cats, especially Burmese and Siamese, but has been observed rarely in dogs. Left-sided or biventricular heart failure commonly develops early in life. A mitral regurgitation murmur may be present. Criteria for LV and LA enlargement are seen on radiographs, ECG, and echocardiogram. Evidence for reduced LV myocardial systolic and diastolic function may be present. Definitive antemortem diagnosis is difficult.
OTHER VASCULAR ANOMALIES
A number of venous anomalies have been described. Many are not clinically important. The persistent left cranial vena cava is a fetal venous remnant that courses lateral to the left AV groove and empties into the coronary sinus of the caudal RA. Although it causes no clinical signs, its presence may complicate surgical exposure of other structures at the left heartbase. Portosystemic venous shunts are common and can lead to hepatic encephalopathy as well as other signs. These malformations are thought to be more prevalent in the Yorkshire Terrier, Pug, Miniature and Standard Schnauzers, Maltese, Pekingese, Shih Tzu, and Lhasa Apso breeds.
Bonagura JD, Lehmkuhl LB. Congenital heart disease. In: Fox PR, Sisson D, Moise NS, editors. Textbook of canine and feline cardiology. ed 2. Philadelphia: WB Saunders; 1999:471-535.
Buchanan JW: Prevalence of cardiovascular disorders. In Fox PR, Sisson D, Moise NS, editors: Textbook of canine and feline cardiology, ed 2, Philadelphia, 1999, pp. 457-470.
Tidholm A. Retrospective study of congenital heart defects in 151 dogs. J Small Anim Pract. 1997;38:94.
Ventricular Outflow Obstruction
Belanger MC, et al. Usefulness of the indexed effective orifice area in the assessment of subaortic stenosis in the dog. J Vet Intern Med. 2001;15:430.
Buchanan JW. Pulmonic stenosis caused by single coronary artery in dogs: four cases (1965–1984). J Am Vet Med Assoc. 1990;196:115.
Buchanan JW. Pathogenesis of single right coronary artery and pulmonic stenosis in English bulldogs. J Vet Intern Med. 2001;15:101.
Bussadori C, et al. Balloon valvuloplasty in 30 dogs with pulmonic stenosis: effect of valve morphology and annular size on initial and 1-year outcome. J Vet Intern Med. 2001;15:553.
Estrada A, et al. Prospective evaluation of the balloon-to-annulus ratio for valvuloplasty in the treatment of pulmonic stenosis in the dog. J Vet Intern Med. 2006;20:862.
Falk T, Jonsson L, Pedersen HD. Intramyocardial arterial narrowing in dogs with subaortic stenosis. J Small Anim Pract. 2004;45:448.
Fingland RB, Bonagura JD, Myer CW. Pulmonic stenosis in the dog: 29 cases. J Am Vet Med Assoc. 1986;189:218.
Kienle RD, Thomas WP, Pion PD. The natural history of canine congenital subaortic stenosis. J Vet Intern Med. 1994;8:423.
Koplitz SL, et al. Aortic ejection velocity in healthy Boxers with soft cardiac murmurs and Boxers without cardiac murmurs: 201 cases (1997-2001). J Am Vet Med Assoc. 2003;222:770.
Meurs KM, Lehmkuhl LB, Bonagura JD. Survival times in dogs with severe subvalvular aortic stenosis treated with balloon valvuloplasty or atenolol. J Am Vet Med Assoc. 2005;227:420.
Orton EC, et al. Influence of open surgical correction on intermediate-term outcome in dogs with subvalvular aortic stenosis: 44 cases (1991–1998). J Am Vet Med Assoc. 2000;216:364.
Pyle RL. Interpreting low-intensity cardiac murmurs in dogs predisposed to subaortic stenosis (editorial). J Am Anim Assoc. 2000;36:379.
Stafford Johnson M, et al. Pulmonic stenosis in dogs: balloon dilation improves clinical outcome. J Vet Intern Med. 2004;18:656.
Birchard SJ, Bonagura JD, Fingland RB. Results of ligation of patent ductus arteriosus in dogs: 201 cases (1969–1988). J Am Vet Med Assoc. 1990;196:2011.
Buchanan JW, Patterson DF. Etiology of patent dutus arteriosus in dogs. J Vet Intern Med. 2003;17:167.
Bureau S, Monnet E, Orton EC. Evaluation of survival rate and prognostic indicators for surgical treatment of left-to-right patent ductus arteriosus in dogs: 52 cases (1995-2003). J Am Vet Med Assoc. 2005;227:1794.
Campbell FE, et al. Immediate and late outcomes of transarterial coil occlusion of patent ductus arteriosus in dogs. J Vet Intern Med. 2006;20:83.
Cote E, Ettinger SJ. Long-term clinical management of right-to-left (“reversed”) patent ductus arteriosus in 3 dogs. J Vet Intern Med. 2001;15:39.
Fox PR, Bond BR, Sommer RJ. Nonsurgical transcatheter coil occlusion of patent ductus arteriosus in two dogs using a preformed Nitinol snare delivery technique. J Vet Intern Med. 1998;12:182.
Fujii Y, et al. Transcatheter closure of congenital ventricular septal defects in 3 dogs with a detachable coil. J Vet Intern Med. 2004;18:911.
Guglielmini C, et al. Atrial septal defect in five dogs. J Small Anim Pract. 2002;43:317.
Hogan DF, et al. Transarterial coil embolization of patent ductus arteriosus in small dogs with 0.025 inch vascular occlusion coils: 10 cases. J Vet Intern Med. 2004;18:325.
Moore KW, Stepien RL. Hydroxyurea for treatment of polycythemia secondary to right-to-left shunting patent ductus arteriosus in 4 dogs. J Vet Intern Med. 2001;15:418.
Orton EC, et al. Open surgical repair of tetralogy of Fallot in dogs. J Am Vet Med Assoc. 2001;219:1089.
Saunders AB, et al. Pulmonary embolization of vascular occlusion coils in dogs with patent ductus arteriosus. J Vet Intern Med. 2004;18:663.
Saunders AB, et al. Echocardiographic and angiocardiographic comparison of ductal dimensions in dogs with patent ductus arteriosus. J Vet Intern Med. 2007;21:68.
Schneider M, et al. Transvenous embolization of small patent ductus arteriosus with single detachable coils in dogs. J Vet Intern Med. 2001;15:222.
Schneider M, et al. Transthoracic echocardiographic measurement of patent ductus arteriosus in dogs. J Vet Intern Med. 2007;21:251.
Stafford Johnson M, et al. Management of cor triatriatum dexter by balloon dilatation in three dogs. J Small Anim Pract. 2004;45:16.
Stokhof AA, Sreeram N, Wolvekamp WTC. Transcatheter closure of patent ductus arteriosus using occluding spring coils. J Vet Intern Med. 2000;14:452.
Van Israel N, et al. Review of left-to-right shunting patent ductus arteriosus and short term outcome in 98 dogs. J Small Anim Pract. 2002;43:395.
Adin DB, Thomas WP. Balloon dilation of cor triatriatum dexter in a dog. J Vet Intern Med. 1999;13:617.
Buchanan JW. Tracheal signs and associated vascular anomalies in dogs with persistent right aortic arch. J Vet Intern Med. 2004;18:510.
Famula TR, et al. Evaluation of the genetic basis of tricuspid valve dysplasia in Labrador Retrievers. Am J Vet Res. 2002;63:816.
Fossum TW, Miller MW. Cor triatriatum and caval anomalies. Semin Vet Med Surg. 1994;9:177.
Isakow K, Fowler D, Walsh P. Video-assisted thoracoscopic division of the ligamentum arteriosum in two dogs with persistent right aortic arch. J Am Vet Med Assoc. 2000;217:1333.
Kornreich BG, Moise NS. Right atrioventricular valve malformation in dogs and cats: an electrocardiographic survey with emphasis on splintered QRS complexes. J Vet Intern Med. 1997;11:226.
Lehmkuhl LB, Ware WA, Bonagura JD. Mitral stenosis in 15 dogs. J Vet Intern Med. 1994;8:2.
Muldoon MM, Birchard SJ, Ellison GW. Long-term results of surgical correction of persistent right aortic arch in dogs: 25 cases (1980–1995). J Am Vet Med Assoc. 1997;210:1761.