CHAPTER 27 Ancillary Therapy: Oxygen Supplementation and Ventilation
OXYGEN SUPPLEMENTATION
Oxygen supplementation is generally indicated to maintain arterial blood oxygen pressures (Pao2) at more than 60 mm Hg. Oxygen supplementation is indicated in every dog or cat with signs of respiratory distress or labored breathing. Cyanosis is another clear indication. Whenever possible, the cause of hypoxemia should be identified and specific treatment initiated as well. Assisted ventilation is indicated for animals with an inadequate arterial oxygen concentration despite supplementation and for animals with arterial carbon dioxide pressures exceeding 60 mm Hg (see Chapter 20).
The inhaled concentration of oxygen can be supplemented by the administration of 100% oxygen by mask, hood, nasal catheter, transtracheal catheter, endotracheal tube, tracheal tube, or oxygen cage. Administration of oxygen by nasal catheter is very well suited to most practices.
When administering 100% oxygen to an animal, the clinician must consider the anhydrous nature of pure oxygen and the toxic effects of oxygen in a high concentration. Because oxygen from tanks contains no water, drying of the airways can occur quickly, particularly if the nasal cavity has been completely bypassed by catheters or tubes. All animals with respiratory tract diseases should be systemically hydrated. Moisture must be added to the airways of animals receiving oxygen by catheter or tube for longer than a few hours. Ventilators designed for long-term use have a heated humidifier incorporated into their design. Humidity exchange filters, which can also be attached to tracheal and endotracheal tubes, function by retaining moisture from exhaled air and adding it to inhaled air. These filters can support bacterial growth and must be replaced daily. Nebulization can also be used to add moisture to the airways. Less effective methods of hydration can be used if other options are not available, such as instillation of sterile 0.9% sodium chloride solution directly into tubes or catheters. Some water vapor can also be added to the oxygen by incorporating pass-over or bubble humidifiers in the system.
The inhalation of air with greater than 50% oxygen is toxic to the pulmonary epithelium. Pulmonary function deteriorates, and death can result. Air with greater than 50% oxygen is therefore not provided for longer than 12 hours. If higher concentrations are necessary to maintain adequate arterial oxygen concentrations, ventilatory support is initiated.
OXYGEN MASKS
Oxygen masks are useful for short-term supplementation. The animal experiences minimal stress, and manipulations such as venous catheter placement and thoracocentesis can be performed. A snug fit is desirable to decrease the volume of dead space, and a relatively high flow rate is necessary (Table 27-1). Sterile eye ointment is applied to prevent desiccation of the corneas.
 TABLE 27-1 Maximum Achievable Oxygen Concentrations and Associated Flow Rates for Various Methods of Supplementation
TABLE 27-1 Maximum Achievable Oxygen Concentrations and Associated Flow Rates for Various Methods of Supplementation
| METHOD OF ADMINISTRATION | MAXIMUM OXYGEN CONCENTRATION (%) | FLOW RATE |
|---|---|---|
| Mask | 50-60 | 8-12 L/min |
| Nasal catheter | 50 | 6-8 L/min or 50-150 mL/kg/min |
| Transtracheal catheter | 30-40 | 1-2 L/min |
| Endotracheal tube | 100 | 0.2 L/kg/min |
| Tracheal tube | 100 | 0.2 L/kg/min |
| Oxygen cage | 60 | 2-3* |
* After cage is filled, flow is adjusted based on oxygen concentration as measured by oxygen sensor.
From Court MH et al: Inhalation therapy: oxygen administration, humidification, and aerosol therapy, Vet Clin North Am Small Anim Pract 15:1041, 1985.
OXYGEN HOODS
Oxygen hoods that can be placed over the animal’s head are available. With some, the animals must be laterally recumbent and still, limiting the use of hoods to animals recovering from anesthesia, those that are severely depressed, and those that are heavily sedated (Fig. 27-1). Others are designed to completely surround the animal’s head and are attached around the neck. One design is an adaptation of an Elizabethan collar (OxyHood, Jorgensen Laboratories, Inc.). In some situations oxygen hoods may be better tolerated than oxygen masks, and it may take less manpower to care for an animal for which one is being used than an animal with an oxygen mask. A means for escape of exhaled air must always be provided to prevent the buildup of CO2 within the hood.
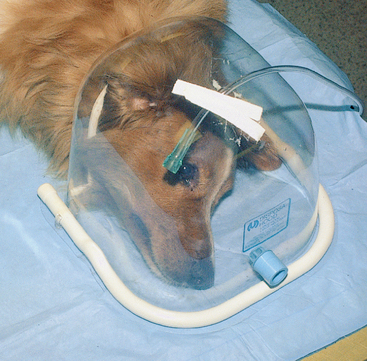
FIG 27-1 An oxygen hood can be used for recumbent animals as a substitute for an oxygen mask. In this patient oxygen is being delivered through an opening in the top of the hood, and the light blue opening that will accommodate standard anesthesia tubing is left open for circulation of air. Regardless of the method used to increase the oxygen in inspired air, a means for escape of expired CO2 is essential. (Disposa-Hood, Utah Medical Products, Inc., Midvale, Utah.)
NASAL CATHETERS
Nasal catheters can be used for long-term oxygen supplementation (Fig. 27-2). The animal is relatively free to move and is accessible for evaluation and treatment. Most animals tolerate the catheter well. Catheters can become obstructed with nasal secretions, however. Soft red rubber or infant feeding tubes or polyurethane catheters can be used. Tube size is based on patient size. In general, a 3.5 to 5 French tube is used for cats, and a 5 to 8 French tube is used for dogs.

FIG 27-2 Dog with intranasal catheter in place for delivery of oxygen. The catheter is sutured to the muzzle less than 1 cm from its exit from the naris and is further anchored with sutures to the face so that it exits behind the animal’s head. An Elizabethan collar is routinely used to prevent the animal from removing the catheter.
The method of placement has been described by Fitzpatrick et al. (1986). First, the length of tubing to be inserted into the nasal cavity is measured against the head of the animal. The tubing should reach the level of the carnassial tooth. Sedation is rarely necessary. A water-soluble lubricant or 0.2% lidocaine jelly is applied to the length of the catheter that will be within the nasal cavity. Next, 0.2% lidocaine is dripped gently into the nasal cavity through the naris with the animal’s nose pointed upward. The catheter is then passed through the naris, initially aimed dorsomedially through the naris, then immediately ventromedially. Once the correct length of catheter has been inserted, it is gently bent beneath the lateral cartilage and sutured to the muzzle no farther than 1 cm caudal to the exit from the naris. The catheter can be further anchored to the face with sutures, traveling between the eyes to behind the animal’s head. An Elizabethan collar is placed on the patient to prevent the animal from removing the catheter.
A sterile intravenous set can be connected to the catheter. The intravenous line can be attached to a half-filled bottle of sterile saline solution and positioned above the fluid level. Oxygen is then delivered through the bottle, below the fluid level, providing some moisture as the oxygen bubbles through the saline.
TRANSTRACHEAL CATHETERS
Oxygen can be administered through a jugular catheter placed with a sterile technique through the trachea. This approach is particularly useful for the emergency stabilization of animals with an upper airway obstruction. Branditz et al. (1989) have described a method for cardiopulmonary resuscitation that can be performed by one person by administering oxygen at a high flow rate of 15 L/ min through a tracheal catheter. In this method a large jugular catheter is placed as described for transtracheal washing (see Chapter 20).
ENDOTRACHEAL TUBES
Endotracheal tubes are used to administer oxygen during surgical procedures and cardiopulmonary resuscitation. They can be used to bypass most upper airway obstructions for emergency stabilization. Pure oxygen can be administered for short periods. Longer supplementation requires the mixing of 100% oxygen with room air. Ventilation can be provided with a cuffed endotracheal tube. Trauma to the trachea is decreased through the use of high-volume, low-pressure cuffs and by inflating the cuff with the least amount of pressure necessary to create a seal. If positive-pressure ventilation is not being used, the cuff can remain deflated.
Because endotracheal tubes are not tolerated by alert animals, tracheal tubes are preferred for long-term management. Conscious animals in which endotracheal tubes are used must be given sedatives, analgesics, paralyzing agents, or a combination of these drugs. The combination of hydromorphone and diazepam is adequate in some animals. Pentobarbital, administered intravenously to effect, can be added if necessary. The combination of ketamine and valium may be safer for the initial intubation of patients that are hypoxemic. Following intubation and improvement in hypoxemia, morphine and pancuronium can be given.
The cuff should be deflated when possible to minimize tracheal damage. The tube must be cleaned periodically to remove secretions (see the recommendations for tracheal tube cleaning), and frequent flushing of the oral cavity is performed. Moisture must be added to the inspired gases, as previously discussed.
TRACHEAL TUBES
Tracheal tubes are placed through the tracheal rings and are readily tolerated by conscious animals. It is rare that an animal requires an emergency tracheostomy. Nearly all such animals can be stabilized using other techniques. Thus tracheal tubes can be placed using a careful, sterile surgical technique. Tracheal tubes are generally used for the management of animals with an upper airway obstruction. Room air often contains adequate oxygen for use in animals with an upper airway obstruction once the obstruction has been bypassed.
The tube itself should have a diameter nearly as wide as the tracheal lumen and a length of 5 to 10 rings. It is necessary to use high-volume, low-pressure cuffs to prevent tracheal damage and subsequent stricture. Double-lumen tubes are ideal for this method. The inner tube can be removed for cleaning and replaced easily. Single-lumen tubes also work and may be necessary in small animals.
Tracheal tubes are usually placed with the animal anesthetized with a short-acting agent. The trachea is exposed through a ventral midline incision made just beneath the larynx. The trachea is entered through an incision made a few rings below the cricoid cartilage, parallel to the trachea and perpendicular to the rings, and through just enough rings to allow passage of the tube. Either end of the incision can be widened with a small transverse incision. Stay sutures are placed on each side of the incision to facilitate initial placement of the tube as well as later replacement if the tube is accidentally or intentionally removed. The tube is then inserted into the opening. With minimal pressure on the airway, it is tied with gauze around the neck of the animal. Few or no sutures are used to close the incision to prevent the collection of air subcutaneously. A gauze sponge with a slit cut in it and coated with antiseptic ointment can be placed over the incision and around the tube.
The tube must be monitored for obstruction and cleaned. The inner tube of double-lumen tubes can be easily removed for this purpose. The tube is cleaned every 30 to 60 minutes initially, with the interval increased as less secretions accumulate. Sterile technique is used when handling the tubes, and they must be replaced if they become contaminated.
Single-lumen tubes are difficult to remove and replace safely for the first few days unless stay sutures are left in place. Periodic cleaning can be performed with the tube in place. Sterile saline solution is instilled into the tube for this purpose. To perform suctioning, a sterile urinary catheter with several openings at the end is attached to a suction unit and passed through the tube. The trachea and tracheal tube are then suctioned to remove secretions. Suctioning is performed for short intervals to allow the lungs to reinflate. Cleaning is performed every few hours initially, then less frequently if secretions are not accumulating.
A smaller tube can be used once the animal is able to oxygenate adequately with room air. The tube can be removed when the animal can oxygenate by breathing around a small tube with the lumen obstructed. The incision is allowed to heal without suturing. The tip of the tube is cultured for bacteria.
Antibiotics are not administered prophylactically. Any existing infection or infections that occur during therapy are treated on the basis of culture and sensitivity information.
OXYGEN CAGES
Oxygen cages provide an oxygen-enriched environment with minimal stress to animals. However, the animal is isolated from direct contact, which can be a disadvantage. Other environmental factors, such as humidity, temperature, and carbon dioxide concentration, must be monitored and controlled or extreme stress and even death can occur. The animal is totally dependent on proper cage function. The ability of the cage to maintain the correct environment varies with the specific cage as well as with each animal. Commercial cages are available for veterinary use. Incubators from human hospitals can be modified for small animals.
VENTILATORY SUPPORT
The purposes of ventilatory support are to decrease the retention of carbon dioxide and to improve oxygenation. Ventilatory support is labor intensive and associated with complications, however. It is used when other means of respiratory support are not adequate.
The retention of carbon dioxide, or hypercapnia, occurs in animals that are unable to ventilate adequately. Spontaneous ventilation can be impaired by neurologic dysfunction, such as that which occurs with severe head trauma, polyneuropathies, and some toxicities. Ventilatory support is recommended in such animals if the PaCO2 level increases to more than 60 mm Hg. Hypoventilation caused by a pleural effusion or pneumothorax is treated by removing the fluid or air, not by positive-pressure ventilation. Hypoventilation caused by an upper airway obstruction is treated by establishing a patent airway.
Animals with cerebral edema, usually caused by trauma, may benefit from ventilatory support to maintain the PaCO2 within 20 to 30 mm Hg. The resultant decrease in blood flow to the brain may decrease the total intracranial volume, thereby decreasing pressure on the brain.
Animals with severe lung disease may be unable to maintain adequate oxygenation without ventilatory support. Positive-pressure ventilation is routinely necessary for the management of patients with acute respiratory distress syndrome (ARDS; see Chapter 22, p. 319). As previously noted, the long-term administration of air with an oxygen concentration greater than 50% results in serious lung damage. If the Pao2 cannot be maintained at greater than 60 mm Hg without excessive oxygen supplementation, ventilatory support is indicated.
The delivery of air by positive pressure is different from the normal inhalation of air by negative pressure. With positive pressure, the distribution of ventilation within the lungs is altered. The intrathoracic pressure increases each time the lungs are filled with air, which results in decreased venous return to the heart. Along with other effects, systemic hypotension results and can be severe enough to cause acute renal failure. Compliance of the lungs also decreases over time in animals receiving positive-pressure ventilation. As the lungs become stiffer, greater pressures are necessary to expand them. Careful monitoring of animals is essential during ventilation. Important variables to monitor include blood gas values, compliance, mucous membrane color, capillary refill time, pulse quality, arterial blood pressure, central venous pressure, lung sounds, and urine output. The extensive nursing care and monitoring required for these patients usually limit the use of long-term ventilatory support to large referral hospitals.
Branditz FK, et al. Continuous transtracheal oxygen delivery during cardiopulmonary resuscitation: an alternative method of ventilation in a canine model. Chest. 1989;95:441.
Court MH, et al. Inhalation therapy: oxygen administration, humidification, and aerosol therapy. Vet Clin North Am Small Anim Pract. 1985;15:1041.
Fitzpatrick RK, et al. Nasal oxygen administration in dogs and cats: experimental and clinical investigations. J Am Anim Hosp Assoc. 1986;22:293.
McKiernan BC. Principles of respiratory therapy. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders; 1983:216.
Moon PF, et al. Mechanical ventilation. In: Kirk RW, et al, editors. Current veterinary therapy XI. Philadelphia: WB Saunders; 1992:98.
 Drugs Used in Respiratory Disorders
Drugs Used in Respiratory Disorders