CHAPTER 22 Disorders of the Pulmonary Parenchyma and Vasculature
VIRAL PNEUMONIAS
CANINE INFLUENZA
Etiology
The canine influenza virus appears to be a recent adaptation from an equine influenza virus (Crawford et al., 2005). Serologic evidence has been found to support its existence among racing greyhounds since 1999 (Anderson et al., 2007). Therefore most dogs are susceptible to infection regardless of age, and spread among dogs in contact with one another, especially those housed together, is rapid. The virus is transmitted through respiratory secretions that are aerosolized or contaminate objects, including hands, clothing, bowls, and kennels. Dogs are thought to shed the virus for up to 10 days after the first appearance of clinical signs, and shedding can also occur from the nearly 20% of infected dogs that never develop clinical signs (Crawford, 2005).
Clinical Features
The disease is most frequently identified during outbreaks among dogs in group housing, such as race tracks and animal shelters. Individual pets often have a recent history (usually in the previous week) of exposure to other dogs. Clinical signs of canine influenza in most dogs are similar to those of infectious tracheobronchitis (see p. 285). This mild form of the disease causes a cough that can be harsh and loud, as typically heard with infectious tracheobronchitis, but that is more often soft and moist. Some dogs may have concurrent mucopurulent nasal discharge, a less common finding in infectious tracheobronchitis.
Dogs with the severe form of disease develop overt pneumonia, peracutely or after having acough for up to 10 days (Crawford, 2005). Secondary bacteria infection is common. Presenting signs can include fever, increased respiratory rate progressing to respiratory distress, and auscultable crackles.
Diagnosis
A diagnosis of canine influenza should be considered in all dogs with acute cough until proven otherwise because it is highly transmissible to susceptible dogs. The diagnosis of pneumonia is made by the radiographic detection of a bronchointerstitial or bronchoalveolar pattern or both in dogs showing appropriate clinical signs. A tracheal wash is recommended to determine the types of bacteria involved and their antibiotic sensitivity.
Confirmation of the diagnosis of influenza is possible through several methods: serology, ELISA for antigen detection, virus isolation, and polymerase chain reaction (PCR) for viral RNA. Serology has several advantages compared with the other methods because blood is simple to collect, the resultant serum is stable, and infection can be detected even after viral shedding has ceased. However, rapid confirmation of the diagnosis is not possible through serology because rising antibody titers are required to confirm the diagnosis. More timely results are possible with antigen detection (Directigen Flu A, Becton, Dickinson and Company) and PCR. Preliminary data by Spindel et al. (2007) using nasal swabs for specimens indicate that PCR is much more sensitive in detecting virus than antigen detection by ELISA or virus isolation. Other specimens that can be submitted for virus isolation or PCR are pharyngeal swabs, tracheal wash fluid, or lung tissue. Results from any test for viral detection can be falsely negative because of the relatively short period of shedding after the development of signs in many patients. For best results, samples are collected from febrile dogs very early in the course of disease.
Treatment
In dogs with the mild form of disease, cough will generally persist for several weeks even when treated with antibiotics and cough suppressants. Mucopurulent nasal discharge can be a result of secondary bacterial infection and may respond to antibiotics.
Dogs with pneumonia require aggressive supportive care, including intravenous fluid therapy if needed to maintain systemic (and therefore airway) hydration. A variety of bacteria have been isolated from infected dogs, including Streptococcus equi subsp. zooepidemicus and gram-negative organisms that are resistant to commonly prescribed antibiotics. Broad spectrum antibiotics should be prescribed initially and can be modified later on the basis of culture and sensitivity results and response to therapy. Initial choices include the combination of ampicillin with sulbactam and either a fluoroquinolone or an aminoglycoside or meropenem. (For additional information on treating bacterial pneumonia, see p. 304)
Prognosis
Most dogs that are exposed to the influenza virus will become infected. Dogs with the mild form of the disease fully recover, although cough may persist for as long as a month. The prognosis is more guarded for dogs that develop the severe form of the disease. Overall mortality has been reported to be <5% (Yoon et al., 2005).
Prevention
Vaccination is the most promising approach for prevention, but no vaccines are currently available. In veterinary hospitals, animal shelters, and other kenneling facilities, immediate isolation of dogs with signs of influenza is indicated and strict isolation protocols must be followed. The virus is readily killed by routine disinfectants. Successful prevention of spread of organisms depends on careful cleaning and disinfection of tables, cages, bowls, and any other objects in contact with infected dogs. In addition, strict attention to detail is necessary regarding hand cleaning after contact with any animal and using disposable barrier protection (e.g., gloves, booties, outerwear) when working with infected dogs or contaminated areas. Recommendations for managers and workers of kennel facilities are provided by the American Veterinary Medical Association (www.avma.org/public_health/influenza/canine_guidelines.asp).
OTHER VIRAL PNEUMONIAS
Several other viruses can infect the lower respiratory tract, but rarely do signs of viral pneumonia predominate. The role of canine adenovirus 1 and parainfluenza virus in canine infectious tracheobronchitis has already been discussed (see Chapter 21). In dogs canine distemper virus can also infect the respiratory epithelium. Clinical signs of pneumonia usually result from a secondary bacterial pneumonia. Infection of the gastrointestinal tract or central nervous system can also occur in dogs with distemper (see Chapter 97). In cats, calicivirus can cause pneumonia, but this manifestation of infection is rare. The dry form of feline infectious peritonitis can affect the lungs, but cats are generally seen because of signs of involvement of other organs. Feline infectious peritonitis is discussed in Chapter 97.
BACTERIAL PNEUMONIA
Etiology
A wide variety of bacteria can infect the lungs. Common bacterial isolates from dogs and cats with pulmonary infections include Bordetella bronchiseptica, Streptococcus spp., Staphylococcus spp., Escherichia coli, Pasteurella spp., Klebsiella spp., Proteus spp., and Pseudomonas spp. Anaerobic organisms can be part of mixed infections, particularly in animals with aspiration pneumonia or with lung lobe consolidation. Mycoplasma organisms have been isolated from dogs and cats with pneumonia, but their exact role is not known.
Bacteria can colonize the airways, alveoli, or interstitium. The term pneumonia means inflammation of the lung, but the term is not specific for bacterial disease. Infection that clinically appears to be limited to the airways and peribronchial tissues is called bacterial bronchitis. If all three regions are involved, the disease is called either bacterial bronchopneumonia or bacterial pneumonia. Most cases of bacterial pneumonia result from bacteria of the oral cavity and pharynx entering the lungs via the airways, which causes a bronchopneumonia involving primarily the gravitydependent cranial and ventral lung lobes (see Fig. 20-5). Bacteria that enter the lung through the hematogenous route usually cause pneumonia that assumes a caudal or diffuse pattern and marked interstitial involvement.
Bacterial pneumonia is a common lung disease, particularly in dogs. Community-acquired infectious pneumonia has been described in puppies (Radhakrishnan et al., 2007), most often caused by Bordetella bronchiseptica (49% of cases). However, consideration should also be given for predisposing abnormalities. In adult dogs, a predisposing abnormality usually exists. Abnormalities to consider in all patients include the aspiration of ingested material or gastric contents because of cleft palate, megaesophagus, or other causes of aspiration pneumonia (p. 309); decreased clearance from the lungs of normally inhaled debris, particularly in animals with chronic bronchitis, ciliary dyskinesia, or bronchiectasis; immunosuppression resulting from drugs, malnutrition, stress, or endocrinopathies; other infections, including canine influenza, canine distemper, feline leukemia virus infection, or feline immunodeficiency virus infection; the inhalation or migration of foreign bodies; and, rarely, neoplasia or fungal or parasitic infections.
Clinical Features
Dogs and cats with bacterial pneumonia are evaluated because of respiratory signs, systemic signs, or both. Respiratory signs can include cough (which is usually productive and soft), a bilateral mucopurulent nasal discharge, exercise intolerance, and respiratory distress. Cough is less common in cats with pneumonia. Systemic signs include lethargy, anorexia, fever, and weight loss. The animal may have a history of chronic airway disease or regurgitation. Cats, particularly kittens, from stressful housing situations (e.g., overcrowding) appear predisposed to develop pneumonia as a result of Bordetella infections. Dogs with complicated infectious tracheobronchitis may have a recent history of harsh cough and a history consistent with exposure, as described in Chapter 21. Other potential predisposing factors, as listed in the preceding paragraph, are pursued through careful history taking.
Fever may be present on physical examination but is identified in only about half of patients. Crackles and occasionally expiratory wheezes may be auscultated, with the abnormal lung sounds often prominent over the cranioventral lung fields.
Diagnosis
Bacterial pneumonia is diagnosed on the basis of the complete blood count (CBC), thoracic radiograph findings, and the results from tracheal wash fluid cytologic analysis and bacterial culture. A CBC showing neutrophilic leukocytosis with a left shift, neutropenia with a degenerative left shift, or moderate-to-marked neutrophil toxicity is supportive of bacterial pneumonia. A normal or stress leukogram is as likely to be found.
Abnormal patterns on thoracic radiographs vary with the underlying disease. The typical abnormality is an alveolar pattern, possibly with consolidation, that is most severe in the dependent lung lobes (see Fig. 20-5). Increased bronchial and interstitial markings are also often present. Infections secondary to foreign bodies can be localized to any region of the lung. An interstitial pattern alone may be present in animals with early or mild disease or in those with infections of hematogenous origin. A bronchial pattern alone may be present in animals with a primarily bronchial infection. Radiographs are also evaluated for the presence of megaesophagus and other extrapulmonary disease.
Pulmonary specimens are evaluated cytologically and microbiologically (bacterial and ideally mycoplasmal cultures) to establish a definitive diagnosis and provide guidance in antibiotic selection. To maximize the diagnostic yield, specimens should be collected before antibiotic therapy is initiated. A tracheal wash specimen is generally sufficient. Septic neutrophilic inflammation is typically found in animals with bacterial pneumonia, and growth of organisms on bacterial culture is expected. Examination of a gram-stained preparation will provide early guidance in antibiotic selection pending results of culture and will also assist in the identification of anaerobes or unusual organisms (e.g., Mycobacteria and filamentous organisms).
A conscientious effort is also made to identify any underlying problems. In some animals, such as those with megaesophagus, the initiating cause is obvious. Further diagnostic tests are indicated in other animals, depending on the results of the clinicopathologic evaluation. These may include bronchoscopy to search for airway abnormalities or foreign bodies, conjunctival scrapings to look for distemper virus, serologic tests to determine whether the animal has a fungal infection, tests for influenza virus, and hormonal assays to determine whether the animal has hyperadrenocorticism. Ciliary dyskinesia is discussed briefly in Chapter 21. The diagnostic evaluation for aspiration pneumonia is discussed on p. 309.
Antibiotics
The treatment of bacterial pneumonia consists of antibiotics and supportive care, with follow-up evaluation (Box 22-1). The antibiotic sensitivity of the involved organisms is diffi cult to predict. Gram-negative infections and infections with multiple organisms are common. Antibiotics are initially selected on the basis of severity of clinical signs and the cytologic characteristics (i.e., morphology and gram-staining) of organisms found in pulmonary specimens. Antibiotic selection is subsequently modified, as needed, according to clinical response and sensitivity data from bacterial cultures of pulmonary specimens.
 BOX 22-1 Therapeutic Considerations for Bacterial Pneumonia
BOX 22-1 Therapeutic Considerations for Bacterial Pneumonia
Antibiotics
Selected on basis of results from gram staining and culture and sensitivity testing of pulmonary specimens
The extent to which an antibiotic can penetrate into the airway secretions does not need to be a major consideration in patients with bacterial pneumonia. Antibiotics generally achieve concentrations within the pulmonary parenchyma equal to those in plasma. Nebulization of antibiotics is rarely indicated.
For animals with mild or moderate clinical signs, antibiotics that can be initiated before sensitivity results are available include amoxicillin-clavulanate (20 to 25 mg/kg q8h), cephalexin (20 to 40 mg/kg q8h), or chloramphenicol (dogs, 50 mg/kg q8h; cats, 10 to 15 mg/kg q12h). Fluoroquinolones are reserved for animals with resistant gram-negative infections. Kittens from stressful environments suspected of having Bordetella-induced pneumonia should be treated with amoxicillin-clavulanate, doxycycline (5 to 10 mg/kg q12h; followed by a bolus of water), or fluoroquinolones while awaiting results of cultures. Doxycycline or a fluoroquinolone is more likely to be effective but has a greater potential for side effects in young kittens.
Animals with severe clinical signs or possible sepsis should be treated initially with intravenous antibiotics. Broad-spectrum coverage in animals with life-threatening infections can be achieved with meropenem (8 mg/kg q8h) or the combination of either ampicillin with sulbactam (22 mg/kg of ampicillin q8h) and a fluoroquinolone or ampicillin with sulbactam and an aminoglycoside (e.g., amikacin, 5 to 10 mg/kg q8h). Sulbactam is a beta-lactamase inhibitor, as is clavulanate, and the combination of ampicillin with sulbactam provides a drug with similar activity as amoxicillin-clavulanate in an intravenous formulation. If Toxoplasma infection is among the differential diagnoses, the combination of a fluoroquinolone and clindamycin or a fluoroquinolone and azithromycin can be used (see Chapter 99).
Antibiotic treatment should be continued for at least 1 week after the clinical signs resolve. Guidelines for patient monitoring are provided on p. 306.
Airway Hydration
The drying of secretions results in increased viscosity and decreased ciliary function, which interfere with the normal clearance mechanisms of the lung. Thus the water content of airway secretions must be maintained and airways must be hydrated in animals with pneumonia. Animals with any evidence of dehydration should receive fluid therapy. Diuretics can cause dehydration, and their use is contraindicated in such animals.
Additional moisture for the airways can be provided through humidification or nebulization. Such therapy is particularly recommended for animals with areas of consolidation or with suspected decreased airway clearance, such as those with bronchiectasis. Humidification refers to the saturation of air with water vapor. Depending on the temperature, the volume of water that remains as vapor is limited. The moisture reaches only the nasal cavity and the proximal trachea. Vaporization is not effective in hydrating deeper regions of the lungs. However, the more proximal effect can still provide some relief, particularly in animals with nasal discharge. Humidification is convenient and can be achieved simply by placing the animal in a steamy bathroom or in a small room with an inexpensive vaporizer, which is readily available at pharmacies.
Nebulization is necessary to provide moisture deeper into the airways. Nebulizers generate small, variably sized droplets, with a diameter ranging from 0.5 to 5 μm required to reach the deeper airways. Several types of nebulizers are available. Disposable jet nebulizers are readily available and inexpensive, and they can be attached to bottled oxygen or an air compressor (Fig. 22-1). Effective, inexpensive portable compressors are commercially available if needed for home use. The nebulized oxygen is delivered to the animal through a face mask. The particles can be seen as a mist.
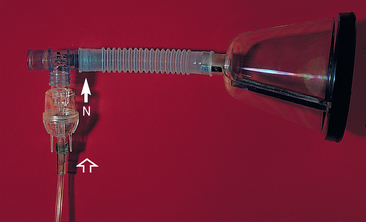
FIG 22-1 Disposable jet nebulizers are readily available and inexpensive. Sterile saline solution is placed in the nebulizer (N). Oxygen enters the bottom of the nebulizer (open arrow), and nebulized air exits the top (closed arrow). Nebulized air is delivered to the animal with a face mask, as shown here, or it can be delivered into an enclosed cage.
Sterile saline solution is used as a nebulizing solution because it has mucolytic properties and is relatively nonirritating. Premedication with bronchodilators has been suggested as a way to reduce the bronchospasms, although use of saline alone in dogs does not usually cause problems. It is recommended that nebulization be performed two to six times daily for 10 to 30 minutes each time. Nebulization should be followed immediately by physiotherapy to promote the expectoration of exudate that may have increased in volume with rehydration. Nebulizers and tubing should be replaced after no more than 24 hours of use in actively infected patients, and face masks should be cleaned and disinfected.
Physiotherapy
Lying in one position impairs airway clearance, and lung consolidation can occur if one side remains dependent for prolonged periods. Therefore animals that are recumbent must be turned at least every 2 hours. Because activity causes animals to take deeper breaths and to cough, which promotes airway clearance, animals that are in a sufficiently stable condition and can tolerate the oxygen demands should be mildly exercised.
Physiotherapy is indicated after nebulization to promote coughing and facilitate the clearance of exudate from the lungs. Mild exercise is used when possible. Otherwise, coupage is performed. To perform coupage, the clinician strikes the animal’s chest over the lung fields with cupped hands. The action should be forceful but not painful and should be continued for 5 to 10 minutes if tolerated by the patient. Coupage may also be beneficial for animals with lung consolidation that are not receiving nebulization.
Bronchodilators
Bronchospasm can occur secondary to inflammation, particularly in cats. Bronchodilators are used in animals showing increased respiratory efforts, particularly if expiratory wheezes are auscultated. Patient status should be monitored closely because bronchodilators may worsen ventilation:perfusion (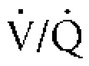 ) mismatching, exacerbating hypoxemia. They are discontinued if clinical signs worsen or do not improve. Bronchodilators are discussed in Chapter 21 (cats, p. 290; dogs, p. 296).
) mismatching, exacerbating hypoxemia. They are discontinued if clinical signs worsen or do not improve. Bronchodilators are discussed in Chapter 21 (cats, p. 290; dogs, p. 296).
Other Treatment
Expectorants are of questionable value in dogs and cats. Glucocorticoids are relatively contraindicated in animals with bacterial pneumonia. Oxygen therapy (see Chapter 27) is provided if the clinical signs, arterial blood gas measurements, or pulse oximetry measurements indicate a need for it.
Monitoring
Dogs and cats with bacterial pneumonia should be closely monitored for signs of deteriorating pulmonary function. Respiratory rate and effort and mucous membrane color are monitored at least twice daily. Thoracic radiographs and the CBC are evaluated every 24 to 72 hours. If the animal’s condition does not improve within 72 hours, it may be necessary to alter treatment or perform additional tests. Animals showing improvement are sent home and reevaluated every 10 to 14 days. Once clinical and radiographic signs have resolved, antibiotic treatment is continued for an additional week.
The evidence of infection on initial radiographs can obscure that of focal disease processes such as neoplasia or foreign bodies, and focal opacities may not be apparent while an animal is receiving antibiotics. Therefore radiographs should be reevaluated approximately 1 week after antibiotic therapy has been discontinued in animals with recurrent infection or suspected localized disease. Persistence of localized disease after long-term antibiotic therapy is an indication for bronchoscopy, thoracoscopy, or thoracotomy.
Prognosis
Bacterial pneumonia responds readily to appropriate therapy. The prognosis is more guarded in animals with underlying problems that predispose them to infection, and the likelihood of eliminating these problems must be taken into consideration.
Pulmonary abscess formation is an uncommon complication of bacterial pneumonia. Abscesses are seen as focal lesions on radiographs, and entire lobes may be involved. Horizontal-beam radiographs can be useful in determining whether the lesions are filled with fluid. Ultrasonography can also be helpful in characterizing areas of consolidation. Abscesses resolve in response to prolonged medical therapy in some animals, but if improvement is not observed or radiographic evidence of disease reappears after the discontinuation of therapy, surgical excision (i.e., lobectomy) is indicated.
TOXOPLASMOSIS
The lungs are a common site of involvement in cats with toxoplasmosis. Thoracic radiographs typically show fluffy alveolar and interstitial opacities throughout the lungs in such animals. Less often, a nodular interstitial, diffuse interstitial or bronchial pattern, lung lobe consolidation, or pleural effusion is seen. Organisms are rarely recovered from the lungs by tracheal wash. Bronchoalveolar lavage is more likely to retrieve organisms (see Fig. 20-17). Toxoplasmosis is a multisystemic disease and is discussed in detail in Chapter 99.
FUNGAL PNEUMONIA
The common mycotic diseases that can involve the lungs are blastomycosis, histoplasmosis, and coccidioidomycosis. In most cases, the organisms enter the body through the respiratory tract. The infection may be successfully eliminated without the animal showing clinical signs, or the animal may show only transient respiratory signs. The infection may also progress to cause disease involving the lungs alone or spread systemically to various target organs, or both processes may occur. Cryptococcal organisms also enter the body through the respiratory tract and can infect the lungs, particularly in cats. However, the presenting signs in cats are generally those of nasal infection. Pulmonary signs are most often the primary presenting complaint in dogs with blastomycosis and cats with histoplasmosis.
Pulmonary mycoses are considered in the differential diagnoses of dogs or cats with progressive signs of lower respiratory tract disease, especially if they occur in conjunction with weight loss, fever, lymphadenopathy, chorioretinitis, or other evidence of multisystemic involvement. Thoracic radiographs typically show a diffuse, nodular, interstitial pattern of the lungs (see Fig. 20-6). The nodules are often miliary. The presence of this pattern in dogs with suspicious clinical signs supports a diagnosis of mycotic infection, but other diseases, including neoplasia, parasitic, or atypical bacterial (e.g., mycobacterial) infections and eosinophilic lung disease, can also produce similar patterns, so these must be borne in mind as well. Other potential radiographic abnormalities include alveolar and bronchointerstitial patterns and consolidated regions of lung. Hilar lymphadenopathy can occur, most commonly in animals with histoplasmosis. The lesions caused by histoplasmosis can also be calcified.
Organisms can occasionally be retrieved by tracheal wash. However, because of the interstitial nature of these diseases, bronchoalveolar lavage and lung aspiration are more likely to be successful (see Figs. 20-15 and 20-16). Fungal culture is probably more sensitive than cytologic analysis alone. An inability to find organisms in pulmonary specimens does not rule out the diagnosis of mycotic disease, however. A complete discussion of systemic mycoses is provided in Chapter 98.
PULMONARY PARASITES
Several parasites can cause lung disease. Certain intestinal parasites, especially Toxocara canis, can cause transient pneumonia in young animals, usually those younger than a few months of age, as the larvae migrate through the lungs. Infection with Dirofilaria immitis can result in severe pulmonary disease through inflammation and thrombosis (see Chapter 10). Oslerus osleri resides at the carina and mainstem bronchi of dogs and is discussed in Chapter 21. The other primary lung parasites that are most commonly diagnosed are Capillaria (Eucoleus) aerophila and Paragonimus kellicotti in dogs and cats, Aelurostrongylus abstrusus in cats, and Crenosoma vulpis in dogs.
Infection occurs as a result of the ingestion of infective forms, often within intermediate or paratenic hosts, that subsequently migrate to the lungs. An eosinophilic inflammatory response often occurs within the lungs, causing clinical signs in some, but not all, infected animals. The definitive diagnosis is made by the identification of the characteristic eggs or larvae in respiratory or fecal specimens (see Chapter 20).
CAPILLARIA (EUCOLEUS) AEROPHILA
Capillaria aerophila, also known as Eucoleus aerophila, is a small nematode. Adult worms are located primarily beneath the epithelial surfaces of the large airways. Clinical signs develop in very few animals with Capillaria infections, and the disease is most often identified through the fortuitous identification of characteristic eggs during routine fecal examinations.
The rare animal that displays signs has signs of allergic bronchitis. Thoracic radiograph findings are generally normal, although a bronchial or bronchointerstitial pattern may be seen. Tracheal wash fluid can show eosinophilic inflammation. Capillaria is diagnosed by the finding of characteristic eggs in tracheal wash fluid or fecal flotation material (see Fig. 20-12, C).
The treatment of choice for dogs and cats is fenbendazole (50 mg/kg orally q24h for 14 days). Levamisole (8 mg/kg orally for 10 to 20 days) has also been used successfully in dogs. Ivermectin has been suggested for treatment, but a consistently effective dosage has not been established. The prognosis in animals with the disease is excellent.
PARAGONIMUS KELLICOTTI
Paragonimus kellicotti is a small fluke. Snails and crayfish are both necessary intermediate hosts, thus limiting the disease to animals that have been in the region of the Great Lakes, in the Midwest, or in the southern United States. Pairs of adults are walled off by fibrous tissue, usually in the caudal lung lobes, with a connection to an airway to allow for the passage of eggs. A local granulomatous reaction may occur around the adults, or a generalized inflammatory response to the eggs may occur.
Infection is more common in cats than in dogs. Some dogs and cats have no clinical signs. When clinical signs are present, they may be the same as those seen in animals with allergic bronchitis. Alternatively, signs of spontaneous pneumothorax can result from the rupture of cysts.
The classic radiographic abnormality is single or multiple solid or cavitary mass lesions, most commonly present in the right caudal lobe (see Fig. 20-10). Other abnormal patterns seen on thoracic radiographs can be bronchial, interstitial (reticular or nodular), or alveolar in nature, depending on the severity of the inflammatory response (see Fig. 20-11).
Infection is diagnosed definitively through the identification of the ova in fecal specimens (using the sedimentation technique described in Chapter 20), tracheal wash fluid, or bronchoalveolar lavage fluid (see Fig. 20-12, D). Multiple fecal specimens should be examined in suspected cases because the eggs are not always present. A presumptive diagnosis is necessary in some cases. Note that ova from the tapeworm Spirometra spp. can be mistakenly identified as ova from Paragonimus (Fig. 22-2).
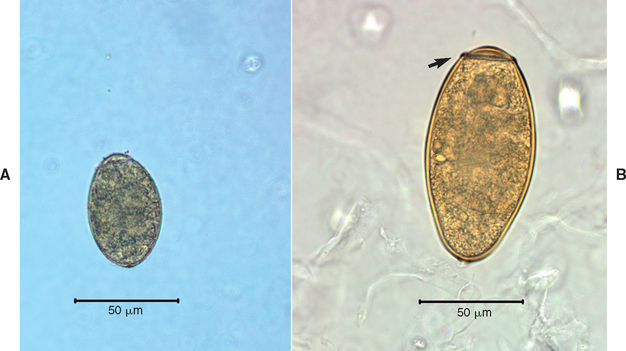
FIG 22-2 The operculated ova from Spirometra tapeworms (A) can be misdiagnosed as Paragonimus ova (B). The Spirometra ova are smaller and more pale than the yellow-brown Paragonimus ova. Most notably, Paragonimus ova have a distinctly visible shoulder (arrow) at the operculated end.
(Courtesy James R. Flowers.)
Fenbendazole is used to treat paragonimiasis at the same dosage as that recommended for the treatment of capillariasis. Alternatively, praziquantel can be used at a dosage of 23 mg/kg orally every 8 hours for 3 days.
Thoracocentesis should be used to stabilize the condition of animals with pneumothorax. If air continues to accumulate within the pleural space, however, it may be necessary to place a chest tube and perform suction until the leak has been sealed (see Chapter 24). Surgical intervention is rarely required.
The response to treatment is monitored by thoracic radiographs and periodic fecal examinations. Treatment may have to be repeated in some cases. The prognosis is excellent.
AELUROSTRONGYLUS ABSTRUSUS
Aelurostrongylus abstrusus is a small worm that infects the small airways and pulmonary parenchyma of cats. Snails or slugs serve as intermediate hosts. Most cats with infection have no clinical signs. Those cats that do are usually young. The clinical signs are those of bronchitis. The abnormalities seen on radiographs may also reflect bronchitis, although a diffuse miliary or nodular interstitial pattern is present in some cats. Eosinophilic inflammation may be apparent in peripheral blood and airway specimens.
A definitive diagnosis is made through the identification of larvae, which may be present in fecal specimens prepared using the Baermann technique (see Fig. 20-12, A) or in airway specimens obtained by tracheal washing or bronchoalveolar lavage. Multiple fecal specimens should be examined in suspected cases because the larvae are not always present.
Cats should be treated with fenbendazole at the same dosage as that used for the treatment of capillariasis. In one study, the dosage of 50 mg/kg orally q24h for 15 days was effective in eliminating infection in all four cats treated (Grandi et al., 2005). In contrast with a previous report, ivermectin (0.4 mg/kg, administered subcutaneously) was not effective in one cat treated. The response to treatment is monitored by thoracic radiographs and periodic fecal examinations. Treatment may have to be repeated in some cases.
Antiinflammatory therapy with glucocorticoids alone often causes the clinical signs to resolve. However, eliminating the underlying parasitic disease is a preferable treatment goal, and glucocorticoid therapy may interfere with the effectiveness of the antiparasitic drugs. Bronchodilators may provide symptomatic relief and presumably do so without interference with antiparasitic drug action. The prognosis in animals with the infection is excellent.
CRENOSOMA VULPIS
Crenosoma vulpis is a lungworm of foxes that can also infect dogs. Dogs living in Atlantic Canada and parts of Europe are most commonly diagnosed with this disease, while the diagnosis remains rare in the United States. However, it is possible that with increased residential development into fox habitats, the frequency of cases in this country will increase. The worm resides in the airways (i.e., trachea, bronchi, bronchioles). Snails or slugs serve as intermediate hosts. The clinical signs are those of allergic or chronic bronchitis. Thoracic radiographs may have a bronchointerstitial or patchy alveolar pattern or occasionally a nodular pattern. Infection is diagnosed definitively through the identification of the larvae in fecal specimens using the Baermann technique described in Box 20-8, tracheal wash fluid, or bronchoalveolar lavage fluid (see Fig. 20-12, B). Multiple fecal specimens should be examined in suspected cases because the larvae are not always present. A single oral dose of milbemycin oxime (0.5 mg/kg) was effective in resolving clinical signs and elimination of larvae from feces collected 4 to 6 weeks after treatment in 32 dogs (Conboy, 2004). This treatment may not be effective against immature larvae. As with other pulmonary parasites, the response to treatment is monitored with thoracic radiographs and periodic fecal examinations.
ASPIRATION PNEUMONIA
Etiology
A small amount of fluid and bacteria is aspirated from the oropharynx into the airways of healthy animals, but normal airway clearance mechanisms prevent infection. Organisms from the oropharynx are thought to be the source of bacteria in many animals with bacterial pneumonia, specifically bacterial bronchopneumonia (see p. 303). In people such infection is termed aspiration pneumonia. In veterinary medicine the term aspiration pneumonia is generally used to refer to the inflammatory lung disease that occurs as a result of the inhalation of overt amounts of solid or liquid material into the lungs. The materials that are usually aspirated are stomach contents or food. Normal laryngeal and pharyngeal function prevents aspiration in healthy animals, although occasionally an excited puppy or a dog running through tall grass aspirates a foreign body. Otherwise, the presence of aspiration pneumonia in an animal of any age indicates an underlying predisposing abnormality (Box 22-2).
 BOX 22-2 Underlying Causes of Aspiration Pneumonia in Dogs and Cats*
BOX 22-2 Underlying Causes of Aspiration Pneumonia in Dogs and Cats*
Esophageal Disorders
Megaesophagus, Chapter 31
Reflux esophagitis, Chapter 31
Esophageal obstruction, Chapter 31
Myasthenia gravis (localized), Chapter 71
Localized Oropharyngeal Abnormalities
Cricopharyngeal motor dysfunction, Chapter 31
Laryngoplasty, Chapter 17
Brachycephalic airway syndrome, Chapter 17
Systemic Neuromuscular Disorders
Myasthenia gravis, Chapter 71
Polyneuropathy, Chapter 71
Polymyopathy, Chapter 72
Aspiration pneumonia is a common complication of animals with regurgitation. Megaesophagus is the most common cause of regurgitation (see Chapter 31). Other causes of regurgitation (e.g., reflux esophagitis, esophageal obstruction) are less common. Another cause of aspiration pneumonia is localized or systemic neurologic or muscular disease affecting the normal swallowing reflexes of the larynx or pharynx. These reflexes can also be depressed in dogs or cats with abnormal levels of consciousness or in those that are anesthetized. Laryngeal paralysis does not always lead to the development of aspiration pneumonia, but aspiration is a potential complication of therapeutic laryngoplasty. It can also occur in animals with abnormal pharyngeal anatomy resulting from mass lesions, brachycephalic airway syndrome, or cleft palate. Bronchoesophageal fistulae are a rare cause of aspiration pneumonia.
Aggressive force-feeding, especially in mentally depressed animals, and improper placement of stomach tubes into the trachea are iatrogenic causes of aspiration pneumonia. Mineral oil administered to prevent hairballs can be a cause of aspiration pneumonia in cats because the tasteless and odorless oil is poorly handled by the pharynx.
The damage to the lung resulting from aspiration may stem from chemical damage, obstruction of the airways, infection, and the resulting inflammatory response to each of these factors. Gastric acid causes severe chemical injury to the lower airways. Tissue necrosis, hemorrhage, edema, and bronchoconstriction ensue, and a marked acute inflammatory response is initiated. Hypoxemia resulting from decreased alveolar ventilation and compliance can be fatal.
Severe respiratory distress can occur from physical obstruction of the airways by the aspirated material. In most cases only small airways are obstructed, but rarely a large piece of food will obstruct a major airway. Obstruction is subsequently exacerbated by reflex bronchoconstriction and inflammation. Inhaled solid material initiates an inflammatory reaction that includes an abundance of macrophages. This response can become organized, resulting in the formation of granulomas.
Bacterial infection may result from the aspiration of contaminated material, such as ingesta that remained in the esophagus. Acidic gastric contents are probably sterile, although in people the contents are considered contaminated if antacids have been taken, if an intestinal obstruction is present, or with periodontal disease. Note that many veterinary patients have periodontal disease. Regardless of the sterility of the aspirated material, the resultant damage to the lungs by gastric acid greatly predisposes the animal to the development of a secondary infection.
The inhalation of mineral oil elicits a chronic inflammatory response. The clinical signs in this setting are often mild, but in rare instances they may be severe. Radiographic abnormalities persist and can be erroneously interpreted as representing neoplastic lesions.
Clinical Features
Dogs and cats with aspiration pneumonia are frequently presented for acute, severe respiratory signs. Systemic signs such as anorexia and depression are common, and these patients may even present in shock. Vomiting, regurgitation, or eating may have preceded the onset of distress. Other patients are seen because of chronic intermittent or progressive signs of coughing or increased respiratory efforts. Occasionally, patients show only signs of depression or the predisposing disease. A thorough history is obtained, with all organ systems carefully reviewed. The owners are specifically questioned about force-feeding and medication administration.
Fever may be present, but it is an inconsistent finding. Crackles are often auscultated, particularly over the dependent lung lobes. Wheezes are heard in some cases. Once a patient is in stable condition, a thorough neuromuscular examination is performed. The ability of the patient to prehend and swallow food and water should also be observed.
Diagnosis
Aspiration pneumonia is usually diagnosed on the basis of the suggestive radiographic findings in conjunction with evidence of a predisposing condition. Thoracic radiographs typically show diffuse, increased interstitial opacities with alveolar flooding (air bronchograms) and consolidation of the dependent lung lobes (see Fig. 20-5). Radiographic abnormalities may not be apparent until 12 to 24 hours after aspiration, however. Occasionally, nodular interstitial patterns are seen in chronic cases. Large nodules can form around solids; miliary nodules often form in animals that have aspirated mineral oil. Large airway obstruction is suspected if radiographs show a soft-tissue mass within a large airway, but this is an unusual finding. A marked, diffuse alveolar pattern can be seen in dogs that have severe secondary edema (see the section on pulmonary edema, p. 319).
The peripheral blood count can reflect the pulmonary inflammatory process, but it is often normal. Neutrophils are examined for the presence of toxic changes suggestive of sepsis.
Tracheal wash is indicated for all animals that can tolerate the procedure to identify complicating bacterial infection and obtain antibiotic sensitivity data. A marked inflammatory response characterized by a predominance of neutrophils is seen in cytologic specimens. Blood resulting from hemorrhage may be seen in specimens from animals in the acute period after aspiration. Bacteria may also be seen. Bacterial cultures should always be performed.
Bronchoscopy can be used to grossly examine the airways and detect and remove large solids. However, the likelihood of a large airway obstruction is very small, so bronchoscopy is performed only if there are clear signs of large airway obstruction (see Chapter 26) or if the animal is not conscious and therefore does not require general anesthesia for the procedure.
Blood gas analysis can be helpful in differentiating hypoventilation from ventilation-perfusion abnormalities (see Chapter 20), although a combination of abnormalities exists in most animals with aspiration pneumonia. Animals with evidence of profound hypoventilation may have either a large airway obstruction or muscle weakness secondary to an underlying neuromuscular disorder such as myasthenia gravis. Blood gas analysis also assists in the therapeutic management of these animals and can be used effectively to monitor the response to therapy.
Diagnostic evaluation is indicated to identify potential underlying diseases (see Box 22-2). This may include a thorough oral and pharyngeal examination, contrast-enhanced radiographic studies to evaluate the esophagus, or specific neuromuscular tests.
Treatment
Suctioning of the airways is helpful only for animals that aspirate in the hospital while already anesthetized or unconscious, when it can be performed immediately after aspiration. If a bronchoscope is immediately available, suctioning can be performed through the biopsy channel, which affords visualized guidance. Alternatively, a sterile soft rubber tube attached to a suction pump can be passed blindly into the airways through an endotracheal tube. Excessive suction may result in lung lobe collapse. Therefore low-pressure, intermittent suction is used, followed by expansion of the lungs with several positive-pressure ventilations using an anesthetic or Ambu bag. Airway lavage is contraindicated.
Animals in severe respiratory distress should be treated with fluid therapy, oxygen supplementation, bronchodilators, and glucocorticoids. Fluids are administered intravenously at high rates to treat shock (see Chapter 30) and should be continued after initial stabilization of the animal’s condition to maintain systemic hydration, which is necessary to maximize the effectiveness of airway clearance mechanisms. However, overhydration must be avoided because of a tendency for pulmonary edema.
Oxygen supplementation (see Chapter 27) is initiated immediately in compromised animals. Positive-pressure ventilation is required for animals in severe respiratory distress that is unresponsive to oxygen therapy.
Bronchodilators can be administered to decrease bronchospasms and ventilatory muscle fatigue. They are most likely to be effective in cats. Bronchodilators can worsen ventilation:perfusion (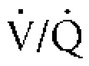 ) mismatching, exacerbating hypoxemia. They are discontinued if no improvement is seen or clinical signs appear to worsen after their administration.
) mismatching, exacerbating hypoxemia. They are discontinued if no improvement is seen or clinical signs appear to worsen after their administration.
Rapid-acting glucocorticoids are administered for the treatment of shock. Their use in the absence of shock is controversial. The antiinflammatory effects of glucocorticoids can be beneficial, but glucocorticoids can interfere with normal host defense mechanisms in tissues that have already been severely compromised. This author reserves the use of glucocorticoids for patients that have severe respiratory compromise and a deteriorating clinical picture despite appropriate antibiotic therapy and supportive care. Low (antiinflammatory) doses of short-acting preparations are administered for up to 48 hours.
Animals with a large airway obstruction can benefit from bronchoscopy and foreign body removal. However, routine bronchoscopy is not indicated because of the risk of the general anesthesia needed during the procedure and the infrequency of large airway obstructions.
Antibiotics are administered immediately in animals that are presented in severe distress or with overt systemic signs of sepsis. Selected antibiotics should have a broad spectrum of activity and be administered intravenously. Such drugs include meropenem or combinations of either ampicillin with sulbactam and a fluoroquinolone or ampicillin with sulbactam and an aminoglycoside (see the section on bacterial pneumonia, p. 303).
A tracheal wash is performed in stable patients before initiation of antibiotics to document the presence of infection and obtain antibiotic sensitivity data. This information is particularly valuable because prolonged treatment is often needed and also because research in human medicine has amply demonstrated that resistant secondary infections can develop after aspiration in patients given antibiotics initially or on an empirical basis. As discussed for bacterial pneumonia, the high incidence of gram-negative and mixed infections make assumptions regarding antibiotic sensitivity prone to error. Pending results of culture, it is reasonable to initiate treatment with a penicillin with a beta-lactamase inhibitor (e.g., amoxicillin-clavulanate or ampicillin with sulbactam). Because infection can occur as a later complication in these patients, frequent monitoring with physical examination, CBC, and thoracic radiographs is necessary to detect any deterioration consistent with secondary infection. Tracheal wash is repeated if infection is suspected.
Further therapeutic and monitoring considerations are discussed in the section on bacterial pneumonia (p. 303). Underlying diseases are treated to prevent recurrence.
EOSINOPHILIC LUNG DISEASE (PULMONARY INFILTRATES WITH EOSINOPHILS AND EOSINOPHILIC PULMONARY GRANULOMATOSIS)
Eosinophilic lung disease is a broad term describing inflammatory lung disease in which the predominant infiltrating cell is the eosinophil. Eosinophilic inflammation can involve primarily the airways or the interstitium. Allergic bronchitis and idiopathic bronchitis are by far the most common eosinophilic lung diseases seen in cats and are discussed in Chapter 21. Interstitial infiltration, with or without concurrent bronchitis, is sometimes referred to as pulmonary infiltrates with eosinophils (PIE) and is typically seen in dogs. Eosinophilic pulmonary granulomatosis is a severe type of PIE seen in dogs and is characterized by the development of nodules and often hilar lymphadenopathy. It must be differentiated from a mycotic infection and neoplasia. The term eosinophilic bronchopneumopathy is also used to describe eosinophilic lung disease. These names are descriptive only and likely encompass a variety of hypersensitivity disorders of the lung.
Because eosinophilic inflammation is a hypersensitivity response, an underlying antigen source is actively pursued in affected animals. Considerations include heartworms, pulmonary parasites, drugs, and inhaled allergens. Food allergy could play a role in these disorders, but this association has not been explored. Potential allergens are discussed further in the section on allergic bronchitis, Chapter 21. Bacteria, fungi, and neoplasia can also induce a hypersensitivity response, but this response often is not the predominant finding. In many cases no underlying disease can be found. Eosinophilic pulmonary granulomatosis is strongly associated with heartworm disease.
Clinical Features
Eosinophilic lung diseases are seen in young and older dogs. Affected dogs are evaluated because of progressive respiratory signs, such as cough, increased respiratory efforts, and exercise intolerance. Systemic signs such as anorexia and weight loss are usually mild. Lung sounds are often normal, although crackles or expiratory wheezes are possible.
Diagnosis
The finding of peripheral eosinophilia is included in some definitions of PIE, but it is not present in all animals with the disease, nor is it a specific finding. A diffuse interstitial pattern is seen on thoracic radiographs. Eosinophilic pulmonary granulomatosis results in the formation of nodules, usually with indistinct borders. These nodules can be quite large, and hilar lymphadenopathy may also be present. A patchy alveolar opacity and consolidation of the lung lobes can occur as well.
Pulmonary specimens must be examined to establish a diagnosis of PIE. In some cases of PIE, evidence of eosinophilic inflammation may be found in tracheal wash fluid. More aggressive techniques for collecting pulmonary specimens, such as bronchoalveolar lavage, lung aspiration, or lung biopsy, are required to identify the eosinophilic response in other cases. Other inflammatory cell populations are frequently present in lesser numbers in such specimens.
Potential antigen sources should be considered, and pulmonary specimens should be carefully examined for the presence of infectious agents and features of malignancy. Heartworm tests and fecal examinations for pulmonary parasites are indicated in all cases.
Treatment
Any primary disease identified during the diagnostic evaluation of these animals is treated directly. Eliminating the source of the antigen that may be triggering the excessive immune response may result in a cure.
Antiinflammatory therapy with glucocorticoids is indicated for dogs in which an antigen source cannot be identified and for dogs with heartworm disease if the eosinophilic inflammation is causing respiratory compromise (see Chapter 10). Dogs with eosinophilic granulomatosis often require more aggressive immunosuppressive therapy.
Dogs are typically treated with glucocorticoids, such as prednisone, at an initial dosage of 1 to 2 mg/kg orally every 12 hours. Clinical signs and thoracic radiographs are used to monitor the animal’s response to therapy, and initially these should be assessed every week. Once the clinical signs have resolved, the dosage of glucocorticoids is decreased to the lowest effective one. If signs have remained in remission for 3 months, discontinuation of therapy can be attempted. If signs are exacerbated by glucocorticoid therapy, immediate reevaluation to search for underlying infectious agents is indicated.
Dogs with large nodular lesions (eosinophilic granulomatosis) should be treated with a combination of glucocorticoids and a cytotoxic agent. Prednisone is administered to these animals at a dosage of 1 mg/kg orally every 12 hours, in combination with cyclophosphamide at a dosage of 50 mg/m2 orally every 48 hours. Clinical signs and thoracic radiographs are evaluated every 1 to 2 weeks until remission is achieved. CBCs are also done every 1 to 2 weeks to detect excessive bone marrow suppression resulting from the cyclophosphamide. Attempts to discontinue therapy can be made after several months of remission. It may be necessary to discontinue the cyclophosphamide earlier than this because long-term treatment is associated with sterile hemorrhagic cystitis. (See Chapter 78 for further discussion of the adverse effects of cyclophosphamide therapy.) The effectiveness of other immunosuppressive drugs, such as cyclosporine, have not been reported.
IDIOPATHIC INTERSTITIAL PNEUMONIAS
The term idiopathic interstitial pneumonia generally denotes inflammatory and/or fibrotic infiltration of the lungs involving primarily the alveolar septa. Small airways, alveoli, and the pulmonary vasculature may also be affected. The alveolar septa include alveolar epithelium, epithelial basal lamina, capillary endothelial basal lamina, and capillary endothelium. Other cells include fibroblasts and alveolar macrophages. To make a diagnosis of idiopathic disease, the known etiologies of interstitial lung disease must be ruled out as completely as possible. Etiologies of interstitial lung disease are numerous and include many infectious agents and some toxins and neoplasia.
Idiopathic pulmonary fibrosis is the most well-described idiopathic interstitial pneumonia in dogs and cats. Some of the eosinophilic lung diseases (not including allergic or idiopathic feline bronchitis) may also be part of this group of diseases (see p. 311). Other inflammatory lung diseases of the interstitium in which a cause cannot be identified are occasionally seen in dogs and cats. The lesions may represent a form of vasculitis, a component of systemic lupus erythematosus, immune complex disease, or some other hypersensitivity response. These diseases are rare, however, and not well documented. A lung biopsy must be performed for a definitive diagnosis to be made. A clinical diagnosis is made only after extensive testing has been done to rule out more common causes of lung disease, particularly infectious agents and neoplasia, and after a prolonged positive response to immunosuppressive therapy. Lymphomatoid granulomatosis is a nodular interstitial disease that exhibits clinical signs similar to those seen in animals with eosinophilic pulmonary granulomatosis. It was initially considered to be an inflammatory lung disease but is currently considered to be lymphoproliferative neoplasia of the lung (see p. 314).
IDIOPATHIC PULMONARY FIBROSIS
In people idiopathic pulmonary fibrosis is the clinical diagnosis that is defined by the histopathologic diagnosis of usual interstitial pneumonia. However, the histopathologic pattern of usual interstitial pneumonia can be seen as a result of other diseases, and according to the American Thoracic Society/European Respiratory Society consensus statement (2002), the diagnosis of idiopathic pulmonary fibrosis also requires (1) the exclusion of other known causes of interstitial lung diseases including drug toxicities, environmental exposures, and collagen vascular diseases; (2) characteristic radiographic or computed tomographic abnormalities; and (3) characteristic pulmonary function abnormalities. In veterinary medicine the latter criterion may be difficult to apply, but attention should be paid to the other criteria.
The characteristic lesions that result in the histopathologic pattern of usual interstitial pneumonia are as follows: fibrosis, areas of fibroblast proliferation, metaplasia of the alveolar epithelium, and mild to moderate inflammation. Honeycomb change may occur as a result of enlarged airspaces lined by abnormal alveolar epithelium. The lungs are heterogeneously affected, with areas of normal lung intermixed with abnormal regions. The abnormal regions are often subpleural. A defect in wound healing has been hypothesized as the cause.
Idiopathic pulmonary fibrosis has been recently described in cats on the basis of histologic lesions which are quite similar to those in people (Cohn et al., 2004; Williams et al., 2006; Fig. 22-3). Unlike the disease that affects people and cats, the disease in dogs has been associated with the primary lesion of collagen deposition in the alveolar septa with no fibroblastic foci (Norris et al., 2005).
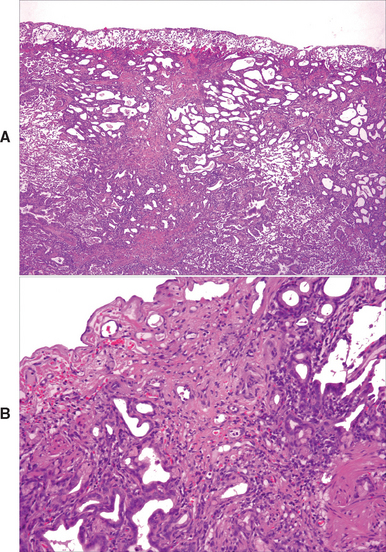
FIG 22-3 Photomicrographs of a lung biopsy from a cat with idiopathic pulmonary fibrosis. At lower power (A) there is distortion and obliteration of the normal pulmonary architecture because of replacement of the parenchyma with disorganized bands of fibrous tissue and scattered mononuclear inflammatory cells. There are few recognizable alveoli in this section. The alveolar septae are thickened, and metaplasia of the alveolar epithelium is present. At higher power (B) subpleural alveoli show marked distortion with marked septal fibrosis and type 2 epithelial hyperplasia. Although normal areas of the lung are not shown, the disease is characterized by heterogeneity of lesions within the lung.
(Photomicrographs courtesy Stuart Hunter.)
Neoplasia can occur concurrently with idiopathic pulmonary fibrosis in people and was reported in 6 of 23 cats (Cohn et al., 2004). The lesions of pulmonary fibrosis can also be misinterpreted as carcinoma, and 4 of 23 cats considered to have pulmonary fibrosis were initially given a pathologic diagnosis of carcinoma.
Clinical Features
A breed predisposition is seen in dogs with pulmonary fibrosis. West Highland White Terriers are most frequently reported, with fewer cases documented among Staffordshire Bull Terriers, Jack Russell Terriers, Cairn Terriers, and Schipperkes. Both dogs and cats tend to be middle-aged or older at the time of presentation, although characteristic signs have been found in patients as young as 2 years of age.
Signs are most often slowly progressive over months. In cats the duration of signs may be shorter, with 6 of 23 cats having shown signs for only 2 days to 2 weeks (Cohn et al., 2004). Respiratory compromise is the most prominent clinical sign of pulmonary fibrosis, manifested as exercise intolerance and/or rapid, labored breathing. Cough often occurs, but if it is the predominant sign, higher consideration for a diagnosis of bronchitis should be given. Syncope may occur in dogs.
Crackles are the hallmark auscultatory finding in dogs and are noted in some cats. Wheezes are heard in approximately half of dogs and some cats. The abnormal breathing pattern is typically tachypnea with relatively effortless expiration.
Diagnosis
Thoracic radiographs of dogs with pulmonary fibrosis typically show a diffuse interstitial pattern. The abnormal densities generally must be moderate to severe to be distinguished from age-related change. A bronchial pattern is often noted concurrently, contributing to the overlap in signs between pulmonary fibrosis and chronic bronchitis. Evidence of pulmonary hypertension may be seen (see p. 316). Radiographs of cats with this disease may show diffuse or patchy infiltrate (Fig. 22-4). Patterns may be interstitial, bronchial, alveolar, or mixed but are often quite severe. Bronchiectasis, caused by traction on the airways, may be noted in either species with advanced disease.
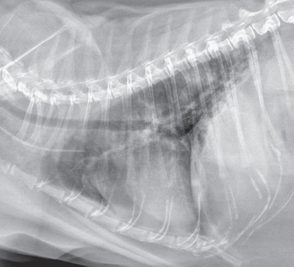
FIG 22-4 Lateral thoracic radiograph from a cat with idiopathic pulmonary fibrosis showing a diffuse interstitial pattern with patchy areas of alveolar disease in the caudal lung lobes. Pericardial and mediastinal fat are also seen. Radiographic abnormalities in cats with fibrosis are quite variable, including the range of interstitial, bronchial, alveolar, or mixed patterns.
Results of the CBC, serum biochemistry panel, and urinalysis are generally unremarkable. Polycythemia may be present secondary to chronic hypoxemia. Screening tests to identify other etiologies of interstitial lung disease include fecal examinations for parasites, heartworm tests, and appropriate infectious disease serology.
Airway specimens should be collected in sufficiently stable patients, primarily to assist in the identification of other causes of lung disease. Mild to moderate inflammation may be seen in patients with pulmonary fibrosis, but this is a nonspecific finding. Bronchoscopy may also be useful in some patients for identifying other causes of lung disease, such as chronic bronchitis.
Typical lesions identified by computed tomography are often used in making a presumptive diagnosis of idiopathic pulmonary fibrosis in people. Similar lesions may be seen in some dogs with the disease (Johnson et al., 2005). Results of computed tomography in cats have not been reported.
A definitive diagnosis of pulmonary fibrosis requires a lung biopsy obtained by thoracotomy or thoracoscopy. The expense and invasiveness of biopsy preclude its use in some patients. Furthermore, the lack of specific treatment recommendations for pulmonary fibrosis is a deterrent. However, biopsy should be considered in patients that are stable and whose owners have sufficient resources. The less invasive tests cannot completely rule out the existence of a different, directly treatable disease (e.g., atypical bacterial infection, fungal disease, parasitism), and more aggressive treatment for pulmonary fibrosis could be recommended with histologic confirmation of the diagnosis.
Treatment
Even in people, large, well-controlled studies have not been performed to determine the ideal treatment strategy for idiopathic pulmonary fibrosis (Hoyles et al., 2006). Most individuals are treated with prednisone at low dosages and azathioprine. Cyclophosphamide is used routinely by some physicians and only during exacerbations by others. Corticosteroids alone are not considered to be effective. Many other drugs, including colchicine and penicillamine, have been tried or investigated, but thus far none have been proved convincingly effective. Survival rates 5 years after the diagnosis remain only 20% to 30% with treatment.
Most dogs and cats have been treated with corticosteroids and bronchodilators. Theophylline derivatives have the theoretical potential to provide some benefit through potentiation of steroid activity. On the basis of clinical experience with people, the addition of azathioprine or cyclophosphamide may be of benefit. Animals with severe pulmonary hypertension may benefit from treatment of this complication (p. 316).
Prognosis
The prognosis for idiopathic pulmonary fibrosis in dogs and cats is poor, with relentless progression of disease expected. Nevertheless, individual patients, particularly dogs, can survive for longer than a year. The mean survival time in dogs in one study was 18 months from the onset of signs, with survival up to 3 years (Corcoran et al., 1999). The prognosis in cats is poorer. Of 23 cats, 14 died or were euthanized within weeks of the onset of signs and only 7 of 23 survived longer than 1 year (Cohn et al., 2004).
PULMONARY NEOPLASIA
Primary pulmonary tumors, metastatic neoplasia, and multicentric neoplasia can all involve the lungs. Most primary pulmonary tumors are malignant. Carcinomas predominate and include adenocarcinoma, bronchoalveolar carcinoma, and squamous cell carcinoma. Sarcomas and benign tumors are much less common. Small cell carcinoma, or oat cell tumor, which occurs frequently in people, is rare in dogs and cats.
The lungs are a common site for the metastasis of malignant neoplasia from other sites in the body and even from primary pulmonary tumors. Neoplastic cells can be carried in the bloodstream and trapped in the lungs, where there is low blood flow and an extensive capillary network. Lymphatic spread or local invasion can also occur.
Multicentric tumors can involve the lungs. Such tumors include lymphoma, malignant histiocytosis, and mastocytoma. An unusual lymphoproliferative tumor limited to involvement of the lung is lymphomatoid granulomatosis. This neoplasm is characterized by the infiltration of pleomorphic lymphoreticular and plasmacytoid cells around and into blood vessels, with accompanying eosinophils, neutrophils, lymphocytes, and plasma cells.
Multiple tumors of different origins can occur in the same animal. In other words, the presence of a neoplasm in one site of the body does not necessarily imply that the same tumor is also present in the lungs.
Clinical Features
Neoplasms are most common in older animals but also occur in young adult animals. Tumors involving the lungs can produce a wide spectrum of clinical signs. These signs are usually chronic and slowly progressive, but peracute manifestations such as pneumothorax or hemorrhage can also occur.
Most signs reflect respiratory tract involvement. Infiltration of the lung by the tumor can cause interference with oxygenation, leading to increased respiratory efforts and exercise intolerance. Mass lesions can compress airways, provoking cough and obstructing ventilation. Erosion through vessels can result in pulmonary hemorrhage. The blood loss can be sudden, resulting in acute hypovolemia and anemia in addition to respiratory compromise. Edema, nonseptic inflammation, or bacterial infection can occur secondary to the tumor. Erosion through the airways can result in pneumothorax. Pleural effusion of nearly any character can form. In rare cases, the caudal or cranial venae cavae are obstructed, resulting in the development of ascites or head and neck edema, respectively.
Nonspecific signs in dogs and cats with pulmonary neoplasms include weight loss, anorexia, depression, and fever. Gastrointestinal signs may be the primary complaint. Vomiting and regurgitation may be the presenting signs in cats in particular. Lameness may be the presenting sign in patients with hypertrophic osteopathy secondary to thoracic mass lesions and in cats with metastasis of carcinoma to their digits.
Some animals with lung neoplasia have no clinical signs at all, and the tumor is discovered as an incidental finding on thoracic radiographs or at postmortem examination. Animals with metastatic or multicentric lung neoplasia can be seen because of signs of tumor involvement in another organ.
Lung sounds may be normal, decreased, or increased. They are decreased over all lung fields in animals with pneumothorax or pleural effusion. Localized decreased or increased lung sounds can be heard over regions that are consolidated. In a few patients, crackles and wheezes can be auscultated. There may be evidence of other organ involvement or hypertrophic osteopathy.
Diagnosis
Neoplasia is definitively diagnosed through the histologic or cytologic identification of criteria of malignancy in populations of cells in pulmonary specimens (Fig. 22-5). Thoracic radiographs are commonly evaluated initially, and findings can support a tentative diagnosis of neoplasia. Radiographs can also be used to identify the location of disease, and this information helps the clinician select the most appropriate technique for specimen collection.
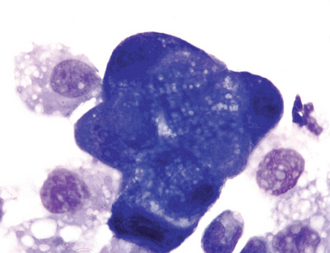
FIG 22-5 Bronchoalveolar lavage fluid from the dog whose lateral thoracic radiograph showing a severe, unstructured interstitial pattern is depicted in Fig. 20-8. Many clumps of deeply staining epithelial cells showing marked criteria of malignancy were seen. One such clump is shown here. A diagnosis of carcinoma was made. Note that a cytologic diagnosis of carcinoma should not be made if there is concurrent inflammation. The surrounding lighter-staining cells are alveolar macrophages, the normal predominant cell type in bronchoalveolar lavage fluid.
Good-quality radiographs, including both left and right lateral projections, should be evaluated. Primary pulmonary tumors can cause localized mass lesions (see Figs. 20-7 and 20-10) or the consolidation of an entire lobe (see Fig. 20-9, A). Tumor margins are often distinct but can be ill-defined as a result of associated inflammation and edema. Cavitation may be evident. Metastatic or multicentric disease results in a diffuse reticular, nodular, or reticulonodular interstitial pattern (see Fig. 20-8). In cats primary lung tumors often have a diffuse distribution by the time of presentation, and the radiographic pattern may be suggestive of bronchitis, edema, or pneumonia.
Pulmonary neoplasia is occasionally associated with hemorrhage, edema, inflammation, infection, or airway occlusion that can contribute to the formation of alveolar patterns and consolidation. Lymphadenopathy, pleural effusion, or pneumothorax can also be identified by radiography in some patients with neoplasia.
Nonneoplastic disease, including fungal infection, lung parasites, the aspiration of mineral oil, eosinophilic granulomatosis, atypical bacterial infections, and inactive lesions from previous disease, can produce similar radiographic abnormalities. Pulmonary specimens must be evaluated to establish a diagnosis. Tracheal wash fluid cytology rarely results in a definitive diagnosis. It is generally necessary to evaluate lung aspirates, bronchoalveolar lavage fluid, or lung biopsy specimens.
It may be appropriate to delay the collection of pulmonary specimens in asymptomatic animals with multifocal disease or animals with significant unrelated problems. Rather, radiographs are obtained again in 4 to 6 weeks to document the progression of lesions. Such delay is never recommended in dogs or cats with potentially resectable disease.
The confirmation of malignant neoplasia in other organs in conjunction with typical thoracic radiographic abnormalities is often adequate for making a presumptive diagnosis of pulmonary metastases. Overinterpretation of subtle radiographic lesions should be avoided. Conversely, the absence of radiographic changes does not eliminate the possibility of metastatic disease.
Evaluation of the thorax by computed tomography should be considered in patients with known or suspected neoplasia. Computed tomography is much more sensitive than thoracic radiography in the detection of metastatic disease (see Chapter 20). In patients with localized disease for whom surgical excision is being planned, computed tomography provides more detailed anatomic information regarding the involvement of adjacent structures and is also more accurate in identifying involvement of tracheobronchial lymph nodes, compared with radiography (Paoloni et al., 2006).
Treatment
Solitary pulmonary tumors are treated by surgical resection. To obtain clear margins, usually the entire lung lobe that is involved must be excised. Lymph node biopsy specimens, as well as biopsy specimens from any grossly abnormal lung, are obtained for histologic analysis.
In animals with a large mass lesion, respiratory signs may abate after excision, even if metastatic lesions are present throughout the lungs. If the lesions cannot be removed surgically, chemotherapy can be attempted (see Chapter 77). No protocol is uniformly effective for the treatment of primary lung tumors.
Metastatic neoplasms of the lungs are treated with chemotherapy. In most animals the initial protocol is determined by the expected sensitivity of the primary tumor. Unfortunately, metastatic neoplasms do not always have the same response to specific agents as the primary tumor.
Multicentric tumors are treated with standard chemotherapeutic protocols, regardless of whether the lungs are involved. Multicentric tumors are discussed in Chapter 79. Lymphomatoid granulomatosis is treated with chemotherapy designed to treat lymphoma (see Chapter 80).
Prognosis
The prognosis for animals with benign neoplasms is excellent, but these tumors are uncommon. The prognosis for animals with malignant neoplasia is potentially related to several variables, which include tumor histology, presence of regional lymph node involvement, and presence of clinical signs. Survival times of several years are possible after surgical excision. Ogilvie et al. (1989) reported that of 76 dogs with primary pulmonary adenocarcinoma, surgical excision resulted in remission (i.e., elimination of all macroscopic evidence of tumor) in 55 dogs. The median survival time of dogs that went into remission was 330 days, whereas the survival time in dogs that did not achieve remission was 28 days. At the completion of the study, 10 dogs remained alive. McNiel et al. (1997) found that the histologic score of the tumor, presence of clinical signs, and regional lymph node metastases were significantly associated with the prognosis in 67 dogs with primary lung tumors. Median survival times for dogs with and without clinical signs were 240 and 545 days, respectively. Median survival times for dogs with and without lymph node involvement were 26 and 452 days, respectively. Median survival times for dogs with papillary carcinoma were 495 days, compared with 44 days for dogs with other histologic tumor types. Survival times ranged from 0 to 1437 days. A report of 21 cats with primary lung tumors found a median survival time of 115 days after surgery (Hahn et al., 1998). Cats with moderately differentiated tumors had a median survival time of 698 days (range of 13 to 1526 days), whereas cats with poorly differentiated tumors had a median survival time of 75 days (range of 13 to 634 days). The prognosis for animals with multicentric neoplasms is not known to depend on the presence or absence of pulmonary involvement.
PULMONARY HYPERTENSION
Etiology
Increased pulmonary arterial pressure (i.e., pulmonary systolic pressure >30 mmHg) is called pulmonary hypertension. The diagnosis is most accurately made by direct pressure measurements via cardiac catheterization, a procedure rarely performed in dogs or cats. An estimation of pulmonary artery pressure can be made by Doppler echocardiography in patients with pulmonary or tricuspid valvular insufficiency (see Chapter 6). The increasing availability of this technology has increased awareness of the existence of pulmonary hypertension in veterinary medicine. Causes of pulmonary hypertension include obstruction to venous drainage as can occur with heart disease (see Chapter 6), increased pulmonary blood flow caused by congenital heart lesions (see Chapter 5), and increased pulmonary vascular resistance. Genetic factors may influence the occurrence of pulmonary hypertension in some individuals but not in others with the same disease. When no underlying disease can be identified to explain the hypertension, a clinical diagnosis of primary (idiopathic) pulmonary hypertension is made.
Pulmonary vascular resistance can be increased as a result of pulmonary thromboembolism (see p. 317) or heartworm disease (see Chapter 10). Vascular resistance can also be increased as a complication of chronic pulmonary parenchymal disease, such as canine chronic bronchitis (see p. 287) and idiopathic pulmonary fibrosis (see p. 312). A simplistic explanation for increased vascular resistance as a complication of pulmonary disease is the adaptive response of the lung to improve the matching of ventilation and perfusion (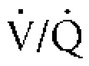 ) through hypoxic vasoconstriction. However, in people other factors are thought to contribute significantly to the development of hypertension associated with pulmonary disease, including endothelial dysfunction, vascular remodeling, and possibly thrombosis in situ.
) through hypoxic vasoconstriction. However, in people other factors are thought to contribute significantly to the development of hypertension associated with pulmonary disease, including endothelial dysfunction, vascular remodeling, and possibly thrombosis in situ.
Clinical Features and Diagnosis
Pulmonary hypertension is diagnosed more commonly in dogs than cats. Clinical signs include those of progressive hypoxemia and can be difficult to distinguish from any underlying cardiac or pulmonary disease. Signs of pulmonary hypertension include exercise intolerance, weakness, syncope, and respiratory distress. Physical examination may reveal a loud split S2 heart sound (see Chapter 6). Radiographic evidence of pulmonary hypertension may be present in severely affected patients and includes pulmonary artery enlargement and right-sided cardiomegaly. Radiographs are evaluated closely for underlying cardiopulmonary disease. The diagnosis of pulmonary hypertension is most often made through Doppler echocardiography. Use of this modality to estimate pulmonary artery pressure requires the presence of pulmonary or tricuspid regurgitation and a highly skilled echocardiographer.
Treatment
Pulmonary hypertension is best treated by identifying and aggressively managing the underlying disease process. In people pulmonary hypertension associated with chronic bronchitis is usually mild and not directly treated. Long-term oxygen therapy is often provided, but this treatment is rarely practical for veterinary patients. Direct treatment can be attempted in patients showing clinical signs of pulmonary hypertension if no underlying disease is identified or management fails to improve pulmonary arterial pressures. Unfortunately, little is known about the treatment of pulmonary hypertension in animals, and adverse consequences can occur through worsening of 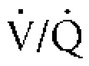 matching or other drug-related side effects. Therefore careful monitoring of clinical signs and pulmonary artery pressures is indicated. The drug most commonly used to treat pulmonary hypertension in dogs is sildenafil citrate (Viagra, Pfizer), a phosphodiesterase V inhibitor that causes vasodilation through a nitric oxide pathway. Dosage and toxicity studies have not been published, but a dosage range between 0.5 and 2.7 mg/kg (median 1.9 mg/kg) orally every 8 to 24 hours has been reported (Bach et al., 2006). A dosage of 0.5 mg/kg orally every 12 hours can be used initially and increased to effect. Long-term anticoagulation with warfarin or heparin is often prescribed for people with primary pulmonary hypertension to prevent small thrombi formation. Its potential benefits for veterinary patients are not known (see the next section, on the treatment of pulmonary thromboembolism).
matching or other drug-related side effects. Therefore careful monitoring of clinical signs and pulmonary artery pressures is indicated. The drug most commonly used to treat pulmonary hypertension in dogs is sildenafil citrate (Viagra, Pfizer), a phosphodiesterase V inhibitor that causes vasodilation through a nitric oxide pathway. Dosage and toxicity studies have not been published, but a dosage range between 0.5 and 2.7 mg/kg (median 1.9 mg/kg) orally every 8 to 24 hours has been reported (Bach et al., 2006). A dosage of 0.5 mg/kg orally every 12 hours can be used initially and increased to effect. Long-term anticoagulation with warfarin or heparin is often prescribed for people with primary pulmonary hypertension to prevent small thrombi formation. Its potential benefits for veterinary patients are not known (see the next section, on the treatment of pulmonary thromboembolism).
PULMONARY THROMBOEMBOLISM
The extensive low-pressure vascular system of the lungs is a common site for emboli to lodge. It is the first vascular bed through which thrombi from the systemic venous network or right ventricle pass. The respiratory signs can be profound and even fatal in dogs and cats. Hemorrhage, edema, and bronchoconstriction, in addition to the decreased blood flow, can contribute to the respiratory compromise. The attendant increased vascular resistance secondary to the physical obstruction by emboli and vasoconstriction results in pulmonary hypertension, which can ultimately lead to the development of right-sided heart failure.
Thromboemboli generally form as a result of disease in organs other than the lungs, and a search for the underlying cause of clot formation is therefore essential. Abnormalities predisposing to clot formation include venous stasis, turbulent blood flow, endothelial damage, and hypercoagulation. In addition to emboli originating from thrombi, emboli can consist of bacteria, parasites, neoplasia, or fat. Conditions that have been associated with the development of pulmonary emboli, and the pages where they are discussed, are listed in Box 22-3. The remainder of this discussion is limited to pulmonary thromboembolism (PTE).
 BOX 22-3 Abnormalities Potentially Associated with Pulmonary Thromboembolism*
BOX 22-3 Abnormalities Potentially Associated with Pulmonary Thromboembolism*
Hyperadrenocorticism, Chapter 53
Immune-mediated hemolytic anemia
Hyperlipidemia, Chapter 54
Glomerulopathies, Chapter 43
Dirofilariasis and adulticide therapy, Chapter 10
Cardiomyopathy, Chapters 7 and 8
Endocarditis, Chapter 6
Pancreatitis, Chapter 40
Disseminated intravascular coagulation, Chapter 87
* Discussions of these abnormalities can be found in the given chapters.
Clinical Features
In many instances, the predominant presenting sign of animals with PTE is peracute respiratory distress. Cardiovascular shock and sudden death can occur. As awareness of PTE has increased, the diagnosis is being made with greater frequency in patients with milder and more chronic signs of tachypnea or increased respiratory efforts. Historic or physical examination findings related to a potential underlying disease increase the index of suspicion for a diagnosis of PTE. A loud or split-second heart sound (see Chapter 1) may be heard on auscultation and is indicative of pulmonary hypertension. Crackles or wheezes are heard in occasional cases.
Diagnosis
Routine diagnostic methods do not provide information that can be used to make a definitive diagnosis of PTE. A high index of suspicion must be maintained because this disease is frequently overlooked. The diagnosis is suspected on the basis of clinical signs, thoracic radiography, arterial blood gas analysis, echocardiography, and clinicopathologic data. A definitive diagnosis requires spiral (helical) computed tomography, angiography, or nuclear perfusion scanning, but spiral (helical) computed tomography is becoming the routine modality for diagnosis in people.
PTE is suspected in dogs and cats with severe dyspnea of acute onset, particularly if there are minimal or no radiographic signs of respiratory disease. In many cases of PTE the lungs appear normal on thoracic radiographs in spite of the severe lower respiratory tract signs. When radiographic lesions occur, the caudal lobes are most often involved. Blunted pulmonary arteries, in some cases ending with focal or wedge-shaped areas of interstitial or alveolar opacities resulting from the extravasation of blood or edema, may be present. Areas of lung without a blood supply can appear hyperlucent. Diffuse interstitial and alveolar opacities and right-sided heart enlargement can occur. Pleural effusion is present in some cases and is usually mild. Echocardiography may show secondary changes (e.g., right ventricular enlargement, increased pulmonary artery pressures), underlying disease (e.g., heartworm disease, primary cardiac disease), or residual thrombi.
Arterial blood gas analysis can show hypoxemia to be mild or profound. Tachypnea leads to hypocapnia, except in severe cases, and the abnormal alveolar-arterial oxygen gradient (A-a gradient) supports the presence of a ventilation-perfusion disorder (see Chapter 20). A poor response to oxygen supplementation is supportive of a diagnosis of PTE.
Clinicopathologic evidence of a disease known to predispose animals to thromboemboli further heightens suspicion for this disorder. Unfortunately, measurements of clotting parameters are not helpful in making the diagnosis. In people measurement of circulating d-dimers (a degradation product of cross-linked fibrin) is used as an indicator of the likelihood of PTE. It is not considered a specific test, so its primary value has been in the elimination of PTE from the differential diagnoses. However, even a negative result can be misleading in certain disease states and in the presence of small subsegmental emboli.
Measurement of d-dimer concentrations is available for dogs through commercial laboratories. A study of 30 healthy dogs, 67 clinically ill dogs without evidence of thromboembolic disease, and 20 with thromboembolic disease provides some guidance for interpretation of results (Nelson et al., 2003). A d-dimer concentration of >500 ng/ml was able to predict the diagnosis of thromboembolic disease with 100% sensitivity but with a specificity of only 70% (i.e., having 30% false-positive results). A d-dimer concentration of >1000 ng/ml decreased the sensitivity of the result to 94% but increased the specificity of the result to 80%. A d-dimer concentration >2000 ng/ml decreased the sensitivity of the result to 36% but increased the specificity to 98.5%. Thus the degree of elevation in d-dimer concentration must be considered in conjunction with other clinical information.
Spiral (helical) computed tomography is commonly used in people to confirm a diagnosis of PTE and is being used increasingly to confirm the diagnosis in veterinary medicine. One limitation of thoracic computed tomography in dogs and especially cats is patient size. In addition, veterinary patients will not hold their breath. Patients must be anesthetized and positive pressure ventilation applied during scanning for maximal resolution. A high quality computed tomography scanner and an experienced radiologist are required for accurate interpretation.
Angiography can provide a definitive diagnosis of PTE. Sudden pruning of pulmonary arteries or intravascular filling defects and extravasation of dye are characteristic findings. However, these changes may be apparent for only a few days after the event, so this test must be done early in the disease. Nuclear scans can provide evidence of PTE with minimal risk to the animal. Unfortunately, this technology is for the most part available only at academic institutions.
Pulmonary specimens for histopathologic evaluation are rarely collected, except at necropsy. However, evidence of embolism is not always found at necropsy because clots may dissolve rapidly after death. Therefore such tissue should be collected and preserved immediately after death. The extensive vascular network makes it impossible to evaluate all possible sites of embolism, and the characteristic lesions may also be missed.
Treatment
Shock therapy may be needed for patients in severe distress, including high doses of rapid-acting glucocorticoids (e.g., prednisolone sodium succinate, up to 10 mg/kg intravenously). Animals should also receive immediate oxygen therapy (see Chapter 27).
Animals with suspected hypercoagulability are likely to benefit from anticoagulant therapy. Large-scale clinical studies of the response of dogs or cats with PTE to anticoagulant therapy have not been published. Anticoagulant therapy is administered only to animals in which the diagnosis is highly probable. Dogs with heartworm disease suffering from postadulticide therapy reactions usually are not treated with anticoagulants (see Chapter 10). Potential surgical candidates should be treated with great caution. Clotting times must be monitored frequently to minimize the risk of severe hemorrhage. General guidelines for anticoagulant therapy are provided here. However, more complete descriptions of anticoagulant therapy are available in the literature, and a current pharmacology text should be consulted before anticoagulants are used.
Initially, heparin (200 to 300 U/kg subcutaneously q8h) is administered for anticoagulant therapy. The goal of heparin therapy is to maintain the partial thromboplastin time (PTT) at 1.5 to 2.5 times normal, which corresponds to approximately a 1.2 to 1.4 times increase above the normal activated clotting time (ACT). Clotting times are evaluated before and 2 hours after the administration of heparin, and the dosage is adjusted on the basis of the results.
Hemorrhage is a potential complication of heparin therapy. Protamine sulfate is a heparin antagonist that can be administered if bleeding is not adequately controlled after heparin treatment is discontinued. Some clinicians advocate gradually tapering the dosage of heparin over several days when discontinuing treatment to avoid rebound hypercoagulation.
Heparin can be administered by the owner at home, but long-term anticoagulation is usually maintained with oral warfarin. Animals receiving warfarin therapy require frequent monitoring, and dosage adjustments are common. The potential for drug interactions with all concurrent medications being administered must be considered. An initial dosage of 0.1 to 0.2 mg/kg by mouth every 24 hours is pre scribed for dogs, and a total of 0.5 mg every 24 hours is prescribed for most cats. The goal of therapy is to maintain a prothrombin time (PT) of 1.5 to 2 times normal or an international normalization ratio (INR) of 2.0 to 3.0. It appears that it is safer to use the INR than the PT for monitoring anticoagulation. The INR is calculated from the measured PT and corrects for the variable strength of the thromboplastin reagent used in the assay. The INR or the formula to calculate it can be obtained from the commercial laboratory or the supplier of in-office test kits. Heparin therapy can be discontinued once the desired prolongation has been reached. It may be possible to decrease the frequency of administration of oral warfarin to every 48 hours after several days of treatment.
Until the PT has stabilized, which takes a minimum of 5 days, clotting times are assessed daily. Subsequent examination of the animal and evaluation of clotting times are performed at least every 5 days, with the interval gradually increasing to every 4 to 6 weeks if consistent and favorable results are obtained.
Excessive hemorrhage is the primary complication of warfarin therapy. Plasma or vitamin K1 (2 to 5 mg/kg q24h) can be used to treat uncontrollable hemorrhage. However, if vitamin K is used, further attempts at anticoagulation using warfarin cannot be made for several weeks.
The use of fibrinolytic agents for the treatment of PTE in animals has not been well established. Recombinant tissue plasminogen activator has shown promise because it acts locally at sites of fibrin deposition.
Because of the serious problems and limitations associated with anticoagulant therapy, eliminating the predisposing problem must be a major priority.
Prevention
No methods of preventing PTE in at-risk patients have been objectively studied in veterinary medicine. Treatments that have potential benefit include the long-term administration of low molecular weight heparin, aspirin, or clopidagrel. Aspirin for the prevention of PTE remains controversial because aspirin-induced alterations in local prostaglandin and leukotriene metabolism may be detrimental.
PULMONARY EDEMA
Etiology
The same general mechanisms that cause edema elsewhere in the body cause edema in the pulmonary parenchyma. Major mechanisms are decreased plasma oncotic pressure, vascular overload, lymphatic obstruction, and increased vascular permeability. The disorders that can produce these problems are listed in Box 22-4.
 BOX 22-4 Possible Causes of Pulmonary Edema
BOX 22-4 Possible Causes of Pulmonary Edema
Increased Vascular Permeability
Disseminated intravascular coagulation
Inflammation (infectious or noninfectious)*
* Inflammation is usually the prominent clinical abnormality, not edema.
The fluid initially accumulates in the interstitium. However, because the interstitium is a small compartment, the alveoli are soon involved. When profound fluid accumulation occurs, even the airways become filled. Respiratory function is further affected as a result of the atelectasis and decreased compliance caused by compression of the alveoli and decreased concentrations of surfactant. Airway resistance increases as a result of the luminal narrowing of small bronchioles. Hypoxemia results from ventilation-perfusion abnormalities.
Clinical Features
Animals with pulmonary edema are seen because of cough, tachypnea, respiratory distress, or signs of the inciting disease. Crackles are heard on auscultation, except in animals with mild or early disease. Blood-tinged froth may appear in the trachea, pharynx, or nares immediately preceding death from pulmonary edema. Respiratory signs can be peracute, as in acute respiratory distress syndrome (ARDS), or subacute, as in hypoalbuminemia. However, a prolonged history of respiratory signs (e.g., months) is not consistent with a diagnosis of edema. The list of differential diagnoses in Box 22-4 can often be greatly narrowed by obtaining a thorough history and performing a thorough physical examination.
Diagnosis
Pulmonary edema in most dogs and cats is based on typical radiographic changes in the lungs in conjunction with clinical evidence (from the history, physical examination, radiography, echocardiography, and serum biochemical analysis [particularly albumin concentration]) of a disease associated with pulmonary edema.
Early pulmonary edema assumes an interstitial pattern on radiographs, which progresses to become an alveolar pattern. In dogs edema caused by heart failure is generally more severe in the hilar region. In cats the increased opaci-ties are more often patchy and unpredictable in their distribution. Edema resulting from increased vascular permeability tends to be most severe in the dorsocaudal lung regions.
Radiographs should be carefully examined for signs of heart disease, venous congestion, PTE, pleural effusion, and mass lesions. Echocardiography is helpful in identifying primary cardiac disease if the clinical signs and radiographic findings are ambiguous.
Decreased oncotic pressure can be identified by the serum albumin concentration. Concentrations less than 1 g/dl are usually required before decreased oncotic pressure is considered to be the sole cause of the pulmonary edema. Pulmonary edema resulting purely from hypoalbuminemia is probably rare. In many animals volume overload or vasculitis is a contributing factor. Plasma protein quantitation using a refractometer can indirectly assess albumin concentration in emergency situations.
Vascular permeability edema, or noncardiogenic pulmonary edema, can result in the full range of compromise, from minimal clinical signs that spontaneously resolve to the frequently fatal, fulminant process of ARDS. ARDS, or “shock lung,” describes a syndrome of acute, rapidly progressive pulmonary edema. In a review of 19 dogs with ARDS by Parent et al. (1996), the time of onset of dyspnea ranged from 0.5 to 48 hours (mean 4.5 hours) before admission, and the duration of dyspnea before death in dogs not mechanically ventilated ranged from 8 to 76 hours (mean 16 hours).
Pulmonary specimens from patients with vascular permeability edema are not cytologically unique, showing a predominantly neutrophilic response.
Arterial blood gas analysis and pulse oximetry in dogs and cats with pulmonary edema are useful in selecting and monitoring therapy. Hypoxemia is present, usually in conjunction with hypocapnia and a widened A-a gradient.
Treatment
It is easier for the body to prevent edema fluid from forming than it is to mobilize existing fluid. The initial management of pulmonary edema should be aggressive. Once the edema has resolved, the body’s own compensatory mechanisms become more effective and the intensity of therapeutic interventions can often be decreased.
All animals with pulmonary edema are treated with cage rest and minimal stress. Dogs and cats with significant hypoxemia should receive oxygen therapy (see Chapter 27). Positive-pressure ventilation is required in severe cases. Methylxanthine bronchodilators (see pp. 290 and 296) may also be beneficial in some patients. They are mild diuretics and also decrease bronchospasms and possibly respiratory muscle fatigue. However, in some patients bronchodilators exacerbate ventilation: perfusion (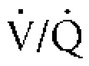 ) mismatching. The patient’s response to bronchodilators should be carefully observed.
) mismatching. The patient’s response to bronchodilators should be carefully observed.
Furosemide is indicated for the treatment of most forms of edema but is not used in hypovolemic animals. Animals with hypovolemia actually require conservative fluid supplementation. If this is necessary to maintain the vascular volume in animals with cardiac impairment or decreased oncotic pressure, then positive inotropic agents or plasma infusions, respectively, are necessary.
Edema caused by hypoalbuminemia is treated with plasma or colloid infusions. However, it is not necessary for the plasma protein concentrations to reach normal levels for edema to decrease. Furosemide can be administered to more quickly mobilize the fluid from the lungs, but clinical dehydration and hypovolemia must be prevented. Diagnostic and therapeutic efforts are directed at the underlying disease.
The treatment of cardiogenic edema is discussed in Chapter 3.
Overhydration is treated by the discontinuation of fluid therapy. Furosemide is administered if respiratory compromise is present. If excessive volumes of fluid were not administered inadvertently, causes of fluid intolerance, such as oliguric renal failure, heart failure, and increased vascular permeability, must be sought.
Edema caused by increased vascular permeability is difficult to treat. In some cases, pulmonary compromise is mild and the edema transient. Routine supportive care with oxygen supplementation may be sufficient, but mechanical ventilation is often required. Any active underlying problem should be identified and corrected.
ARDS responds poorly to management. Ventilator therapy with positive end-expiratory pressure is indicated, and even with such aggressive support the mortality rate is high. Furosemide is generally ineffective in treating edema caused by increased vascular permeability, but because of limitations in our diagnostic capabilities, it is reasonable to include this drug in the initial management of these patients. Glucocorticoids are of no clear benefit in these patients, but they are frequently given to animals with moderate to severe signs. Many novel therapies for ARDS have been studied in people, although to date none has been shown to be consistently effective in improving outcome. Studies are ongoing. Examples of such therapies include endotoxin blockers, inhibitors of specific inflammatory mediators, inhaled nitrous oxide, antioxidant drugs, and surfactant replacement.
Prognosis
The prognosis for an animal with pulmonary edema depends on the severity of the edema and the ability to eliminate or control the underlying problem. Aggressive management early in the course of edema formation improves the prognosis for an animal with any given disease. Animals with ARDS have a guarded to grave prognosis.
American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002;165:277.
American Veterinary Medical Association. Control of canine influenza in dogs-questions, answers, and interim guidelines. www.avma.org/public_health/influenza/canine_guidelines.asp. Retrieved on Feb. 12, 2008.
Anderson TC, et al. Serological evidence for canine influenza virus circulation in racing greyhounds from 1999 to 2003. Abstr. J Vet Intern Med. 2007;21:576.
Bach JF, et al. Retrospective evaluation of sildenafil citrate as a therapy for pulmonary hypertension in dogs. J Vet Intern Med. 2006;20:1132.
Barsanti JA, et al. Parasitic diseases of the respiratory tract. In: Kirk RW, editor. Current veterinary therapy VIII. Philadelphia: WB Saunders, 1983.
Berry CR, et al. Pulmonary lymphomatoid granulomatosis in seven dogs (1976–1987). J Vet Intern Med. 1990;4:15.
Bidgood T, et al. Comparison of plasma and interstitial fluid concentrations of doxycycline and meropenem following constant rate intravenous infusion in dogs. Am J Vet Res. 2003;64:1040.
Bowman DD, et al. Evaluation of praziquantel for treatment of experimentally induced paragonimiasis in dogs and cats. Am J Vet Res. 1991;52:68.
Bowman DD, et al. Georgis’ parasitology for veterinarians, ed 6. Philadelphia: WB Saunders, 1995.
Calvert CA, et al. Pulmonary and disseminated eosinophilic granulomatosis in dogs. J Am Anim Hosp Assoc. 1988;24:311.
Clercx C, et al. Eosinophilic bronchopneumopathy in dogs. J Vet Intern Med. 2000;14:282.
Cohn LA, et al. Identification and characterization of an idiopathic pulmonary fibrosis-like condition in cats. J Vet Intern Med. 2004;18:632.
Conboy G. Natural infections of Crenosoma vulpis and Angiostronylus vasorum in dogs in Atlantic Canada and their treatment with milbemycin oxime. Vet Record. 2004;155:16.
Corcoran BM, et al. Chronic pulmonary disease in West Highland white terriers. Vet Record. 1999;144:611.
Crawford PC, et al. Transmission of equine influenza virus to dogs. Science. 2005;310:482.
Crawford C. Canine influenza virus (canine flu), University of Florida College of Veterinary Medicine Veterinary Advisory. www.vetmed.ufl.edu/pr/nw_story/CANINEFLUFACTSHEET.htm. Retrieved on Feb. 12, 2008.
DeMonye W, et al. Embolus location affects the sensitivity of a rapid quantitative d-dimer assay in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med. 2002;165:345.
Drobatz KJ, et al. Noncardiogenic pulmonary edema. Compend Contin Educ Pract Vet. 1994;16:333.
Drobatz KJ, et al. Noncardiogenic pulmonary edema in dogs and cats: 26 cases (1989–1993). J Am Vet Med Assoc. 1995;206:1732.
Grandi G, et al. Aelurostrongylus abstrusus (cat lungworm) infection in five cats from Italy. Vet Parasitol. 2005;25:177.
Hahn KA, et al. Primary lung tumors in cats: 86 cases (1979–1994). J Am Vet Med Assoc. 1997;211:1257.
Hahn KA, et al. Prognosis factors for survival in cats after removal of a primary lung tumor: 21 cases (1979–1994). Vet Surg. 1998;27:307.
Hoyles RK, et al. Treatment of idiopathic pulmonary fibrosis. Clin Pulm Med. 2006;13:17.
Johnson LR, et al. Pulmonary thromboembolism in 29 dogs: 1985-1995. J Vet Intern Med. 1999;13:338.
Johnson LR, et al. Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992-1996. J Vet Intern Med. 1999;13:440.
Johnson VS, et al. Thoracic high-resolution computed tomographic findings in dogs with canine idiopathic pulmonary fibrosis. J Small Anim Pract. 2005;46:381.
Keyes ML, et al. Pulmonary thromboembolism in dogs. Vet Emerg Crit Care. 1993;3:23.
LaRue MJ, et al. Pulmonary thromboembolism in dogs: 47 cases (1986–1987). J Am Vet Med Assoc. 1990;197:1368.
McNiel EA, et al. Evaluation of prognostic factors for dogs with primary lung tumors: 67 cases (1985–1992). J Am Vet Med Assoc. 1997;211:1422.
Nelson OL, et al. The utility of plasma d-dimer to identify thromboembolic disease in dogs. J Vet Intern Med. 2003;17:830.
Norris AJ, et al. Interstitial lung disease in West Highland white terriers. Vet Pathol. 2005;42:35.
Norris CR, et al. Pulmonary thromboembolism in cats: 29 cases (1987–1997). J Am Vet Med Assoc. 1999;215:1650.
Norris AJ, et al. Interstitial lung disease in West Highland white terriers. Vet Pathol. 2005;42:35.
Ogilvie GK, et al. Prognostic factors for tumor remission and survival in dogs after surgery for primary lung tumor: 76 cases (1975–1985). J Am Vet Med Assoc. 1989;195:109.
Paoloni MC, et al. Comparison of results of computed tomography and radiography with histopathologic findings in tracheobronchial lymph nodes in dogs with primary lung tumors: 14 cases (1999-2002). J Am Vet Med Assoc. 2006;228:1718.
Parent C, et al. Clinical and clinicopathologic findings in dogs with acute respiratory distress syndrome: 19 cases (1985–1993). J Am Vet Med Assoc. 1996;208:1419.
Quinn DA, et al. D-dimers in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med. 1999;159:1445.
Radhakrishnan A, et al. Community-acquired infectious pneu-monia in puppies: 65 cases (1993-2002). J Am Vet Med Assoc. 2007;230:1493.
Reinemeyer CR. Parasites of the respiratory system. In: Bonagura JD, et al, editors. Current veterinary therapy XII. Philadelphia: WB Saunders, 1995.
Roudebush P. Bacterial infections of the respiratory system. In: Greene CE, editor. Infectious diseases of the dog and cat. Philadelphia: WB Saunders, 1990.
Schermerhorn T, et al. Pulmonary thromboembolism in cats. J Vet Intern Med. 2004;18:533.
Speakman AJ, et al. Antimicrobial susceptibility of Bordetella bronchiseptica isolates from cats and a comparison of the agar dilution and E-test methods. Vet Microbiol. 1997;54:63.
Spindel ME, et al. Detection and quantification of canine influenza virus by one-step real-time reverse transcription PCR. Abstr. J Vet Intern Med. 2007;21:576.
Urquhart GM, et al. Veterinary parasitology, ed 2. Oxford: Blackwell Science, 1996.
Williams K, et al. Identification of spontaneous feline idiopathic pulmonary fibrosis. Chest. 2006;125:2278.
Yoon K-J, et al. Influenza virus in racing greyhounds. Emerg Infect Dis. 2005;11:1974.