CHAPTER 20 Diagnostic Tests for the Lower Respiratory Tract
THORACIC RADIOGRAPHY
GENERAL PRINCIPLES
Thoracic radiographs play an integral role in the diagnostic evaluation of dogs and cats with clinical signs related to the lower respiratory tract. They are also indicated for the evaluation of animals with vague, nonspecific signs of disease to detect occult pulmonary disease. Thoracic radiographs can be helpful in localizing disease processes, narrowing and prioritizing the differential diagnoses, determining the extent of disease involvement, and monitoring the progression of disease and response to treatment.
A minimum of two views of the thorax should be taken in all dogs and cats. Right lateral and ventrodorsal (VD) views usually are preferred. The sensitivity of radiographs in the detection of lesions is improved if both right and left lateral views are obtained. These are indicated if disease of the right middle lung lobe, metastatic disease, or other subtle changes are suspected. The side of the lung away from the table is more aerated, thereby providing more contrast for soft-tissue opacities, and is slightly magnified compared with the side against the table. Dorsoventral (DV) views are taken to evaluate the dorsal pulmonary arteries in animals with suspected heartworm disease, pulmonary thromboembolism, or pulmonary hypertension. The combination of DV and VD views has the same advantages as the combination of right and left lateral views in detecting subtle changes in the dorsally oriented vessels. DV, rather than VD, views are taken to minimize stress in animals in respiratory distress. Horizontal-beam lateral radiographs with the animal standing can be used to evaluate animals with suspected cavitary lesions or pleural effusion.
Careful technique is essential to ensure that thoracic radiographs are obtained that yield useful information. Poor technique can lead to either underinterpretation or overinterpretation of abnormalities. Appropriate film, settings, and development procedures should be used, and the films should be interpreted using proper lighting. The settings used are recorded so that the same technique can be used when obtaining future films, which allows for more critical comparison of the progression of disease. The dog or cat should be restrained adequately to prevent movement, and a short exposure time is used.
Radiographs should be taken during maximum inspiration. Fully expanded lungs provide the most air contrast for soft-tissue opacities, and motion is also minimized during this phase of the respiratory cycle. Radiographic indications of maximum inspiration include widening of the angle between the diaphragm and vertebral column (representing maximal expansion of caudal lung lobes); a lucent region in front of the heart shadow (representing maximal expansion of the cranial lung lobes); flattening of the diaphragm; minimal contact between the heart and the diaphragm; and a well-delineated, nearly horizontal vena cava. Radiographs of the lungs obtained during phases of respiration other than peak inspiration are difficult to interpret. For example, incomplete expansion of the lungs can cause increased pulmonary opacities to be seen that appear pathologic, resulting in misdiagnosis.
Animals that are panting should be allowed to calm down before thoracic radiographs are obtained. A paper bag can be placed over the animal’s muzzle to increase the concentration of carbon dioxide in the inspired air, causing the animal to take deeper breaths. It may be necessary to sedate some animals.
All structures of the thorax should be evaluated systematically in every animal to enhance diagnostic accuracy. Extrapulmonary abnormalities may develop secondary to pulmonary disease and may be the only radiographic finding (e.g., subcutaneous emphysema after tracheal laceration). Conversely, pulmonary disease may occur secondary to other evident thoracic diseases, such as mitral valve insufficiency, megaesophagus, and neoplasia of the body wall.
TRACHEA
The trachea and, in young animals, the thymus are recognizable in the cranial mediastinum. Radiographs of the cervical trachea must also be taken in dogs and cats with suspected upper airway obstruction or primary tracheal disease, most notably tracheal collapse. During evaluation of the trachea, it is important to obtain radiographs of the cervical portion during inspiration and of the thorax during both inspiration and expiration.
Only the inner wall of the trachea should be visible. If the outer wall of the trachea is identified, this is suggestive of pneumomediastinum. The trachea normally has a uniform diameter and is straight, deviating ventrally from the vertebral bodies on lateral views as it progresses toward the carina. It may appear elevated near the carina if the heart is enlarged or pleural effusion is present. Flexion or extension of the neck may cause bowing of the trachea. On VD views the trachea may deviate to the right of midline in some dogs. The tracheal cartilage becomes calcified in some older dogs and chondrodystrophic breeds.
The overall size and continuity of the tracheal lumen should also be evaluated. The normal tracheal lumen is nearly as wide as the laryngeal lumen. Hypoplastic tracheas have a lumen less than half the normal size (Fig. 20-1). Strictures and fractured cartilage rings can cause an abrupt, localized narrowing of the air stripe. Mass lesions in the tissues adjacent to the trachea can compress the trachea, causing a more gradual, localized narrowing of the air stripe. In animals with extrathoracic tracheal collapse, the tracheal air stripe is narrowed in the cervical region during inspiration. In animals with intrathoracic tracheal collapse, the air stripe is narrowed on thoracic films during expiration. Fluoroscopy, available primarily through referral centers, provides a more sensitive assessment of tracheal collapse. Finally, the air contrast of the trachea sometimes allows foreign bodies or masses to be visualized within the trachea. Most foreign bodies lodge at the level of the carina or within the bronchi. The inability to radiographically identify a foreign body does not rule out the diagnosis, however.
LUNGS
The clinician must be careful not to overinterpret lung abnormalities on thoracic radiographs. A definitive diagnosis is not possible in most animals, and microscopic examination of pulmonary specimens, further evaluation of the heart, or testing for specific diseases is necessary. The lungs are examined for the possible presence of four major abnormal patterns: vascular, bronchial, alveolar, and interstitial. Mass lesions are considered with the interstitial patterns. Lung lobe consolidation, atelectasis, pulmonary cysts, and lung lobe torsions are other potential abnormalities. Animals with severe respiratory distress but normal thoracic radiograph findings usually have thromboembolic disease or have suffered a very recent insult to the lungs, such as trauma or aspiration (Box 20-1).
 BOX 20-1 Common Lower Respiratory Tract Differential Diagnoses for Dogs and Cats with Respiratory Signs and Normal Thoracic Radiographs
BOX 20-1 Common Lower Respiratory Tract Differential Diagnoses for Dogs and Cats with Respiratory Signs and Normal Thoracic Radiographs
* Gastroesophageal reflux is a common cause of cough in people. Documentation in dogs and cats is limited, but the possibility should also be considered.
Vascular Pattern
The pulmonary vasculature is assessed by evaluating the vessels to the cranial lung lobes on the lateral view and the vessels to the caudal lung lobes on the VD or DV view. Normally, the blood vessels should taper gradually from the left atrium (pulmonary vein) or right ventricle (pulmonary arteries) toward the periphery of the lungs. Companion arteries and veins should be similar in size. Arteries and veins have a consistent relationship with each other and the associated bronchus. On lateral radiographs the pulmonary artery is dorsal and the pulmonary vein is ventral to the bronchus. On VD or DV radiographs the pulmonary artery is lateral and the pulmonary vein is medial to the bronchus. Vessels that are pointed directly toward or away from the X-ray beam are “end-on” and appear as circular nodules. They are distinguished from lesions by their association with a linear vessel and adjacent bronchus.
Abnormal vascular patterns generally involve an increase or decrease in the size of arteries or veins (Box 20-2). The finding of arteries larger than their companion veins indicates the presence of pulmonary hypertension or thromboembolism, most commonly caused by heartworm disease, a finding seen in both dogs and cats (Fig. 20-2). The pulmonary arteries often appear tortuous and truncated in such animals. Concurrent enlargement of the main pulmonary artery and right side of the heart may be seen in affected dogs. There may also be interstitial, bronchial, or alveolar infiltrates in cats and dogs with heartworm disease as a result of concurrent inflammation, edema, or hemorrhage.
 BOX 20-2 Differential Diagnoses for Dogs and Cats with Abnormal Pulmonary Vascular Patterns on Thoracic Radiographs
BOX 20-2 Differential Diagnoses for Dogs and Cats with Abnormal Pulmonary Vascular Patterns on Thoracic Radiographs
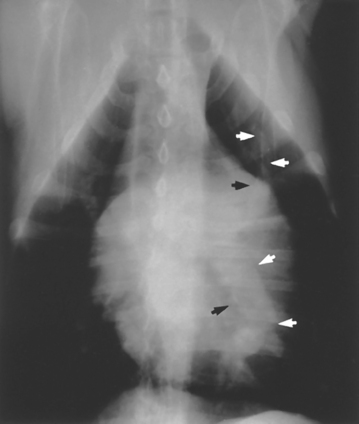
FIG 20-2 Dilation of pulmonary arteries is apparent on this ventrodorsal view of the thorax in a dog with heartworm disease. The artery to the left caudal lung lobe is extremely enlarged. Arrowheads delineate the borders of the arteries to the left cranial and caudal lobes.
Veins larger than their companion arteries indicate the presence of congestion resulting from left-sided heart failure. Pulmonary edema may also be present.
Dilation of both arteries and veins is an unusual finding, except in young animals. The finding of pulmonary overcirculation is suggestive of left-to-right cardiac or vascular shunts, such as patent ductus arteriosus and ventricular septal defects.
The finding of smaller-than-normal arteries and veins may indicate the presence of pulmonary undercirculation or hyperinflation. Undercirculation most often occurs in combination with microcardia resulting from hypoadrenocorticism or other causes of severe hypovolemia. Pulmonic stenosis may also cause radiographically visible undercirculation in some dogs. Hyperinflation is associated with obstructive airway disease, such as allergic or idiopathic feline bronchitis.
Bronchial Pattern
Bronchial walls are normally most easily discernible radiographically at the hilus. They should taper and grow thinner as they extend toward the periphery of each lung lobe. Bronchial structures are not normally visible radiographically in the peripheral regions of the lungs. The cartilage may be calcified in older dogs and chondrodystrophic breeds, making the walls more prominent but still sharply defined.
A bronchial pattern is caused by thickening of the bronchial walls or bronchial dilation. Thickened bronchial walls are visible as “tram lines” and “doughnuts” in the peripheral regions of the lung (Fig. 20-3). Tram lines are produced by airways that run transverse to the X-ray beam, causing the appearance of parallel thick lines with an air stripe in between. Doughnuts are produced by airways that are pointing directly toward or away from the beam, causing a thick circle to be seen radiographically, with the airway lumen creating the “hole.” The walls of the bronchi tend to be indistinct. The finding of thickened walls indicates the presence of bronchitis and results from an accumulation of mucus or exudate along the walls within the lumens, an infiltration of inflammatory cells within the walls, muscular hypertrophy, epithelial hyperplasia, or a combination of these changes. Potential causes of bronchial disease are listed in Box 20-3.
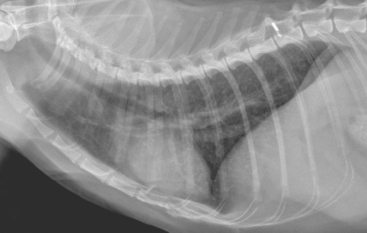
FIG 20-3 A bronchointerstitial pattern is present in this lateral radiograph from a cat with idiopathic bronchitis. The bronchial component results from thickening of the bronchial walls and is characterized by “doughnuts” and “tram lines.” In this radiograph the bronchial changes are most apparent in the caudal lung lobes.
 BOX 20-3 Differential Diagnoses for Dogs and Cats with Bronchial Patterns on Thoracic Radiographs*
BOX 20-3 Differential Diagnoses for Dogs and Cats with Bronchial Patterns on Thoracic Radiographs*
Chronic bronchial disease can result in irreversible dilation of the airways, which is termed bronchiectasis. It is identified radiographically by the presence of widened, nontapering airways (Fig. 20-4). Bronchiectasis can be cylindrical (tubular) or saccular (cystic). Cylindrical bronchiectasis is characterized by fairly uniform dilation of the airway. Saccular bronchiectasis additionally has localized dilations peripherally that can lead to a honeycomb appearance. All major bronchi are usually affected.
Alveolar Pattern
Alveoli are not normally visible radiographically. Alveolar patterns occur when the alveoli are filled with fluid-dense material (Box 20-4). The fluid opacity may be caused by edema, inflammation, hemorrhage, or neoplastic infiltrates, which generally originate from the interstitial tissues. The fluid-filled alveoli are silhouetted against the walls of the airways they surround. The result is a visible stripe of air from the airway lumen in the absence of definable airway walls. This stripe is an air bronchogram (Fig. 20-5). If the fluid continues to accumulate, the airway lumen will eventually also become filled with fluid, resulting in the formation of solid areas of fluid opacity, or consolidation.
 BOX 20-4 Differential Diagnoses for Dogs and Cats with Alveolar Patterns on Thoracic Radiographs*
BOX 20-4 Differential Diagnoses for Dogs and Cats with Alveolar Patterns on Thoracic Radiographs*
* Any of the differential diagnoses for interstitial patterns (Boxes 20-5 and 20-6) can cause an alveolar pattern if associated with severe inflammation, edema, or hemorrhage.
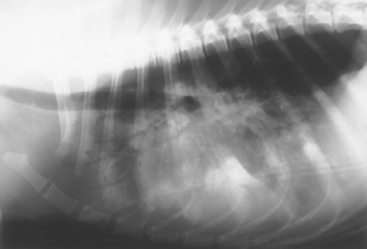
FIG 20-5 Lateral view of the thorax of a dog with aspiration pneumonia. An alveolar pattern is evident by the increased soft-tissue opacity with air bronchograms. Air bronchograms are bronchial air stripes without visible bronchial walls. In this radiograph the pattern is most severe in the ventral (dependent) regions of the lung, consistent with bacterial or aspiration pneumonia.
Edema most often results from left-sided heart failure (see Chapter 22). In dogs the fluid initially accumulates in the perihilar region, and eventually the entire lung is affected. In cats patchy areas of edema can be present initially throughout the lung fields. The finding of enlarged pulmonary veins supports the cardiac origin of the infiltrates. Noncardiogenic edema is typically most severe in the caudal lung lobes.
Inflammatory infiltrates can be caused by infectious agents, noninfectious inflammatory disease, or neoplasia. The location of the infiltrative process can often help establish a tentative diagnosis. For example, diseases of airway origin, such as most bacterial and aspiration pneumonias, primarily affect the dependent lung lobes (i.e., the right middle and cranial lobes and the left cranial lobe). In con trast, diseases of vascular origin, such as dirofilariasis and thromboemboli, primarily affect the caudal lung lobes. Localized processes involving only one lung lobe suggest the presence of a foreign body, neoplasia, abscess, granuloma, or lung lobe torsion.
Hemorrhage usually results from trauma. Thromboembolism, neoplasia, coagulopathies, and fungal infections can also cause hemorrhage into the alveoli.
Interstitial Pattern
The pulmonary interstitial tissues confer a fine, lacy pattern to the pulmonary parenchyma of many dogs and cats as they age, in the absence of clinically apparent respiratory disease. They are not normally visible on inspiratory radiographs in young adult animals.
Abnormal interstitial patterns are reticular (unstructured), nodular, or reticulonodular in appearance. A nodular interstitial pattern is characterized by the finding of roughly circular, fluid-dense lesions in one or more lung lobes. However, the nodules must be nearly 1 cm in diameter to be routinely detected. Interstitial nodules may represent active or inactive inflammatory lesions or neoplasia (Box 20-5).
Active inflammatory nodules often have poorly defined borders. Mycotic infections typically result in the formation of multiple, diffuse nodules. The nodules may be small (miliary; Fig. 20-6) or large and coalescing. Parasitic granulomas are often multiple, although paragonimiasis can result in the formation of a single pulmonary nodule. Abscesses can form as a result of foreign bodies or as a sequela to bacterial pneumonia. Nodular patterns may also be seen on the radiographs obtained in animals with some eosinophilic lung diseases and idiopathic interstitial pneumonias.
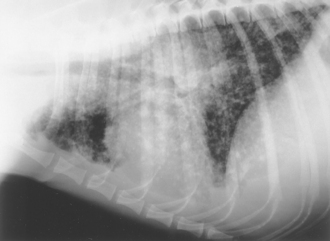
FIG 20-6 Lateral view of the thorax in a dog with blastomycosis. A miliary, nodular interstitial pattern is present. Increased soft-tissue opacity above the base of heart may be the result of hilar lymphadenopathy.
Inflammatory nodules can persist as inactive lesions after the disease resolves. In contrast to active inflammatory nodules, however, the borders of inactive nodules are often well demarcated. Nodules may also become mineralized in some conditions, such as histoplasmosis. Well-defined, small, inactive nodules are sometimes seen in healthy older dogs without a history of disease. Radiographs taken several months later in these animals typically show no change in the size of these inactive lesions.
Neoplastic nodules may be singular or multiple (Fig. 20-7). They are often well defined, although secondary inflammation, edema, or hemorrhage can obscure the margins. There is no radiographic pattern that is diagnostic for neoplasia. Lesions caused by parasites, fungal infections, and some eosinophilic lung diseases or idiopathic interstitial pneumonias may be indistinguishable from neoplastic lesions. In the absence of strong clinical evidence, malignant neoplasia must be confirmed cytologically or histologically. If this is not possible, radiographs can be obtained again 4 weeks later to evaluate for progression of disease.
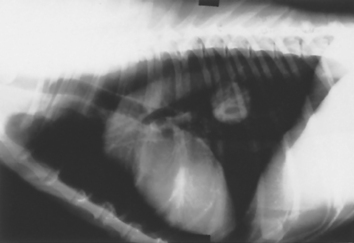
FIG 20-7 Lateral view of the thorax of a dog with malignant neoplasia. A well-circumscribed, solid, circular mass is present in the caudal lung field. Papillary adenocarcinoma was diagnosed after surgical excision.
Neoplastic involvement of the pulmonary parenchyma cannot be totally excluded on the basis of thoracic radiograph findings because malignant cells are present for a while before lesions reach a radiographically detectable size. The sensitivity of radiography in identifying neoplastic nodules can be improved by obtaining left and right lateral views of the thorax.
The reticular interstitial pattern is characterized by a diffuse, unstructured, lacy increase in the opacity of the pulmonary interstitium, which partially obscures normal vascular and airway markings. Reticular interstitial patterns frequently occur in conjunction with nodular interstitial patterns (also called reticulonodular patterns) and alveolar and bronchial patterns (Fig. 20-8).
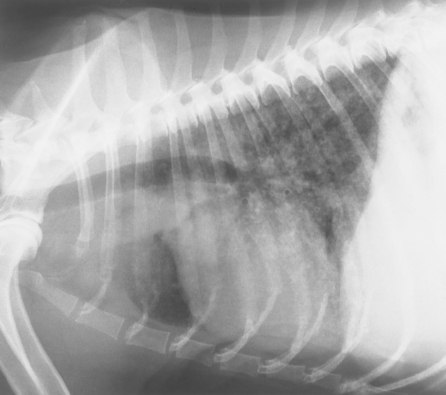
FIG 20-8 Lateral radiograph of a dog with pulmonary carcinoma. An unstructured pattern is present as well as an increased bronchial pattern.
The increased reticular interstitial opacity can result from edema, hemorrhage, inflammatory cells, neoplastic cells, or fibrosis within the interstitium (Box 20-6). The interstitial space surrounds the airways and vessels and is normally extremely small in dogs and cats. With the continued accumulation of fluid or cells, however, the alveoli can be-come flooded, which produces an alveolar pattern. Visible focal interstitial accumulations of cells, or nodules, can also develop with time. Any of the diseases associated with alveolar and interstitial nodular patterns can cause a reticular interstitial pattern early in the course of disease (see Boxes 20-4 and 20-5). This pattern is also often seen in older dogs with no clinically apparent disease, presumably as a result of pulmonary fibrosis; this further decreases the specificity of the finding.
Lung Lobe Consolidation
Lung lobe consolidation is characterized by a lung lobe that is entirely soft-tissue opacity (Fig. 20-9, A). Consolidation occurs when an alveolar or interstitial disease process progresses to the point at which the entire lobe is filled with fluid or cells. Common differential diagnoses for lung lobe consolidation are severe bacterial or aspiration pneumonia (essentially resulting in an abscess of the entire lobe), neoplasia, lung lobe torsion, and hemorrhage. The inhalation of plant material can also result in consolidation of the involved lung lobe as a result of the inflammatory reaction to the foreign material and secondary infection. This differential diagnosis should be considered especially in regions of the country where foxtails are prevalent.
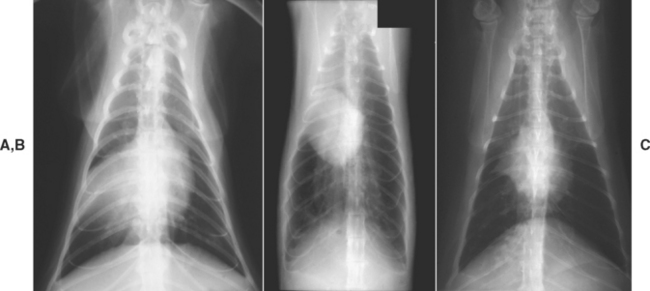
FIG 20-9 Thoracic radiographs from three different patients, ventrodorsal projections. Radiograph A shows consolidation of the right middle lung lobe caused by neoplasia. Note that the soft tissue density of the lung silhouettes with the shadow of the heart. Radiograph B shows atelectasis of the middle region of the right lung and marked hyperinflation of the remaining lungs in a cat with idiopathic bronchitis. Note the shift of the heart shadow toward the collapsed region. Radiograph C shows atelectasis of the right middle lung lobe in another cat with idiopathic bronchitis. In this patient the adjacent lung lobes have expanded into the area previously occupied by the right middle lobe, preventing displacement of the heart.
Atelectasis
Atelectasis is also characterized by a lobe that is entirely soft-tissue opacity. In this instance the lobe is collapsed as a result of airway obstruction. All the air within the lobe has been absorbed and not replaced. It is distinguished from consolidation by the small size of the lobe (Fig. 20-9, B). Often the heart is displaced toward the atelectatic lobe. Atelectasis is most commonly seen involving the right middle lobe of cats with bronchitis (Fig. 20-9, C). Displacement of the heart may not occur in these cats.
Cavitary Lesions
Cavity lesions describe any abnormal air accumulation in the lung. They can be congenital, acquired, or idiopathic. Specific types of cavitary lesions include bullae, which result from ruptured alveoli due to congenital weakness of tissues and/or small airway obstruction, such as in some cats with idiopathic bronchitis; blebs, which are bullae located within the pleura; and cysts, which are cavitary lesions lined by airway epithelium. Parasitic “cysts” (not lined by epithelium) can form around Paragonimus worms. Thoracic trauma is a common cause of cavitary lesions. Other differential diagnoses include neoplasia, lung infarction (from thromboembolism), abscess, and granuloma. Cavitary lesions may be apparent as localized accumulations of air or fluid, often with a partially visible wall (Fig. 20-10). An air-fluid interface may be visible using standing horizontal-beam projections. Bullae and blebs are rarely apparent radiographically.
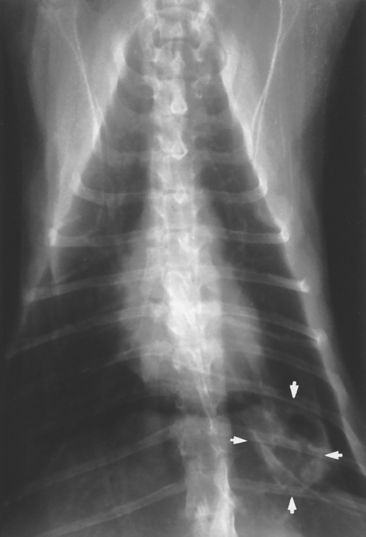
FIG 20-10 Ventrodorsal view of the thorax in a cat showing a cystic lesion (arrowheads) in the left caudal lung lobe. Differential diagnoses included neoplasia and Paragonimus infection.
Cavitary lesions may be discovered incidentally or on thoracic radiographs of dogs and cats with spontaneous pneumothorax. If pneumothorax is present, surgical excision of the lesion is usually indicated (see Chapter 25). If inflammatory or neoplastic disease is suspected, further diagnostic testing is indicated. If the lesion is found incidentally, animals can be periodically reevaluated radiographically to determine whether the lesion is progressing or resolving. If the lesion does not resolve during the course of 1 to 3 months, surgical removal is considered for diagnostic purposes and to prevent potentially life-threatening spontaneous pneumothorax.
Lung Lobe Torsion
Lung lobe torsion can develop spontaneously in deep-chested dogs or as a complication of pleural effusion or pneumonectomy in dogs and cats. The right middle and left cranial lobes are most commonly involved. The lobe usually twists at the hilus, obstructing the flow of blood into and out of the lung lobe. Venous drainage is obstructed before arterial flow, causing the lung lobe to become congested with blood. Over time, air is absorbed from the alveoli and atelectasis can occur.
Lung lobe torsion is difficult to identify radiographically. Severe bacterial or aspiration pneumonia resulting in consolidation of these same lobes is far more common and produces similar radiographic changes. The finding of pulmonary vessels or bronchi traveling in an abnormal direction is strongly suggestive of torsion. Unfortunately, pleural fluid, if not present initially, often develops and obscures the radiographic image of the affected lobe. Ultrasonography is often useful in detecting a torsed lung lobe. Bronchoscopy, bronchography, computed tomography, or thoracotomy is necessary to confirm the diagnosis in some animals.
ANGIOGRAPHY
Angiography can be used to confirm a diagnosis of pulmonary thromboembolism. Obstructed arteries are blunted or do not show the normal gentle taper and arborization. Arteries may appear dilated and tortuous. There also may be localized areas of extravasated contrast agent. If several days have elapsed since the embolization occurred, however, lesions may no longer be identifiable; therefore angiography should be performed as soon as the disorder is suspected and the animal’s condition is stabilized. Angiography may also be used as a confirmatory test in cats with presumptive dirofilariasis but negative adult anti-gen blood test results and echocardiographic findings (see Chapter 10).
ULTRASONOGRAPHY
Ultrasonography is used to evaluate pulmonary mass lesions adjacent to the body wall, diaphragm, or heart and also consolidated lung lobes (Fig. 20-11). Because air interferes with the sound waves, aerated lungs and structures surrounded by aerated lungs cannot be examined. However, some patients with a reticular interstitial pattern on thoracic radiographs have sufficient infiltrates to be visualized where they abut the body wall. The consistency of lesions often can be determined to be solid, cystic, or fluid filled. Some solid masses are hypolucent and appear to be cystic on ultrasonograms. Vascular structures may be visible, particularly with Doppler ultrasound, and this can be helpful in identifying lung lobe torsion. Ultrasonography can also be used to guide needles or biopsy instruments into solid masses for specimen collection. It is used for evaluating the heart in animals with clinical signs that cannot be readily localized to either the cardiac or the respiratory systems. Ultrasonographic evaluation of patients with pleural disorders is discussed in Chapter 24.
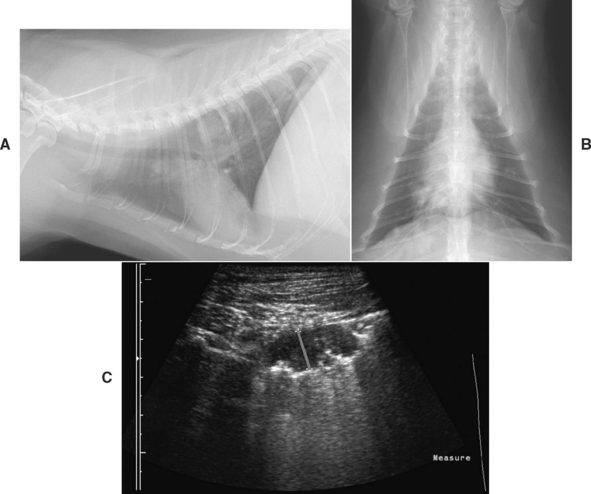
FIG 20-11 Multiple pulmonary nodules are easily visible on the lateral radiograph (A) from a cat with a one year history of cough and recent episodes of respiratory distress with wheezing. Nodules do not obviously extend to the chest wall based on the ventrodorsal radiograph (B). However, a 1-cm mass was found on ultrasonagraphic examination of the right thorax (C; a red line has been positioned between the ultrasound markers to indicate site of measurement). An ultrasound-guided aspirate was performed. The presence of eosinophils in the aspirate prompted the performance of fecal examinations for pulmonary parasites, and a diagnosis of paragonimiasis was made through the identification of characteristic ova.
COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING
Computed tomography (CT) and magnetic resonance imaging (MRI) are used routinely in human medicine for the diagnostic evaluation of lung disease. The accessibility of CT in particular has led to its increased use in dogs and cats. The resultant three-dimensional images are more sensitive and specific for the identification of certain airway, vascular, and parenchymal diseases as compared with thoracic radiography. In a study of dogs with metastatic neoplasia, only 9% of nodules detected by CT were identified by thoracic radiography (Nemanic et al., 2006). Examples of cases that may benefit from CT include those with possible metastatic disease; possible pulmonary thromboembolism; idiopathic interstitial pneumonias, including idiopathic pulmonary fibrosis; or potentially excisable disease (to determine the extent and location of disease and the potential involvement of other structures, such as the major vessels). The application of CT and MRI to the diagnosis of specific canine and feline pulmonary diseases requires further investigation.
NUCLEAR IMAGING
Mucociliary clearance can be measured by placing a drop of technetium-labeled albumin at the carina and observing its movement with a gamma camera to assist in the diagnosis of ciliary dyskinesia. Nuclear imaging can be used for the relatively noninvasive measurement of pulmonary perfusion and ventilation, valuable for the diagnosis of pulmonary thromboembolism. Restrictions for handling radioisotopes and the need for specialized recording equipment limit the availability of these tools to specialty centers.
PARASITOLOGY
Parasites involving the lower respiratory tract are identified by direct observation, blood tests, cytologic analysis of respiratory tract specimens, or fecal examinations. Oslerus osleri reside in nodules near the carina, which can be identified bronchoscopically. Rarely, other parasites may be seen. Blood tests are often used to diagnose heartworm disease (see Chapter 10).
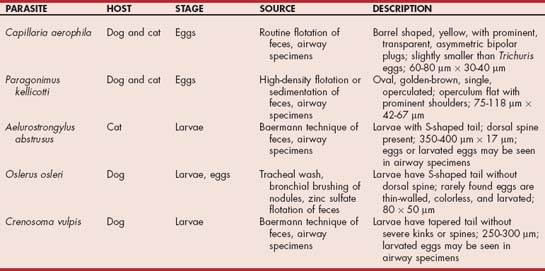
Larvae that may be present in fluid from tracheal or bronchial washings include O. osleri, Aelurostrongylus abstrusus (Fig. 20-12, A), and Crenosoma vulpis (Fig. 20-12, B). Eggs that may be present include those of Capillaria (Eucoleus) aerophila and Paragonimus kellicotti (Fig. 20-12, C and D). Larvated eggs or larvae from Filaroides hirthi or Aelurostrongylus milksi can be present but are rarely associated with clinical signs. The more common organisms are described in Table 20-1.
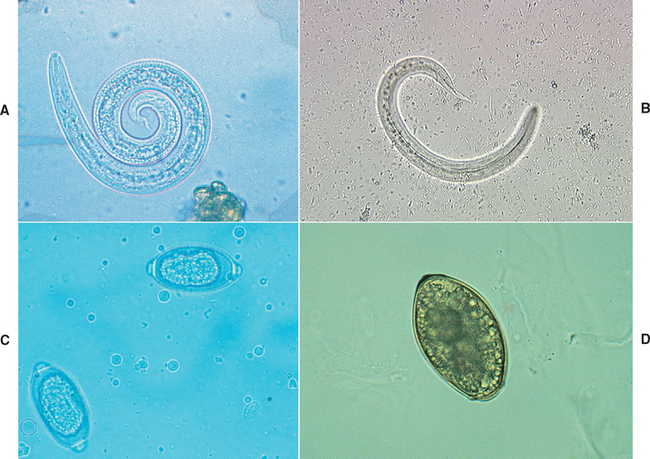
FIG 20-12 A, Larva of Aelurostrongylus abstrusus. B, Larva of Crenosoma vulpis. C, Double operculated ova of Capillaria sp. D, Single operculated ova of Paragonimus kellicotti.
The hosts of lung parasites generally cough up and swallow the eggs or larvae, which are then passed in the feces to infect the next host or an intermediate host. Fecal exami nation for eggs or larvae is a simple, noninvasive tool for the diagnosis of such infestations. However, because shedding is intermittent, parasitic disease cannot be included solely on the basis of negative fecal examination findings. Multiple (at least three) examinations should be performed in animals that are highly suspected of having parasitic disease. If possible, several days should be allowed to elapse between each collection of feces.
Routine fecal flotation can be used to concentrate eggs from C. aerophila. High-density fecal flotation (specific gravity [s.g.], 1.30 to 1.35) can be used to concentrate P. kellicotti eggs. Sedimentation techniques are preferred for concentrating and identifying P. kellicotti eggs, particularly if few eggs are present. Larvae are identified through the use of the Baermann technique. However, O. osleri larvae are insufficiently motile for reliable identification with this technique, and zinc sulfate (s.g., 1.18) flotation is recommended. Even so, false-negative results are common in cases with O. osleri. All of these techniques can be readily performed at minimal expense. Methods for sedimentation and the Baermann technique are described in Boxes 20-7 and 20-8.
 BOX 20-7 Sedimentation of Feces for Concentration of Eggs
BOX 20-7 Sedimentation of Feces for Concentration of Eggs
Data from Urquhart GM et al: Veterinary parasitology, ed 2, Oxford, 1996, Blackwell Science.
 BOX 20-8 Baermann Technique for Concentration of Larvae
BOX 20-8 Baermann Technique for Concentration of Larvae
Data from Urquhart GM et al: Veterinary parasitology, ed 2, Oxford, 1996, Blackwell Science.
Toxoplasma gondii occasionally causes pneumonia in dogs and cats. Dogs do not shed Toxoplasma organisms in the feces, but cats may. However, the shedding of eggs is part of the direct life cycle of the organisms and does not correlate with the presence of systemic disease resulting from the indirect cycle. Infection is therefore diagnosed by finding tachyzoites in pulmonary specimens or indirectly on the basis of serologic findings.
Migrating intestinal parasites can cause transient pulmonary signs in young animals. Migration most often occurs before the mature adults develop in the intestine, and thus eggs may not be found in feces.
SEROLOGY
Serologic tests can detect a variety of pulmonary pathogens. Antibody tests provide only indirect evidence of infection, however. In general, they should be used only to confirm a suspected diagnosis, not to screen for disease. Whenever possible, identification of the infectious organisms is the preferred method of diagnosis. Tests available for common pulmonary pathogens include those for Histoplasma, Blastomyces, Coccidiodomyces, Toxoplasma, and feline coronavirus. These tests are discussed fully in Chapter 92. Antibody tests for canine influenza are discussed further in Chapter 22. Serum antigen tests for Cryptococcus (see Chapter 98) and adult heartworms are also available (see Chapter 10). Antibody tests for dirofilariasis are available and used primarily to support the diagnosis of feline heartworm disease (see Chapter 10).
TRACHEAL WASH
Indications and Complications
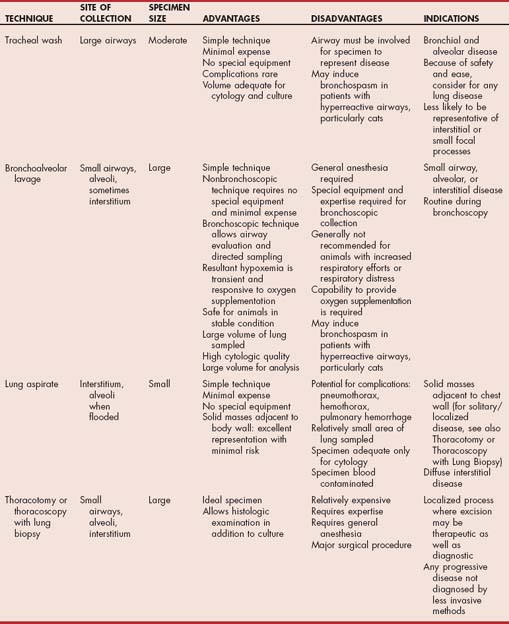
Tracheal wash can yield valuable diagnostic information in animals with cough or respiratory distress resulting from disease of the airways or pulmonary parenchyma and in animals with vague presenting signs and pulmonary abnormalities detected on thoracic radiographs (i.e., most animals with lower respiratory tract disease). Tracheal wash is generally performed after results of the history, physical examination, thoracic radiography, and other routine components of the database are known.
Tracheal wash provides fluid and cells that can be used to identify diseases involving the major airways while bypassing the normal flora and debris of the oral cavity and pharynx. The fluid obtained is evaluated cytologically and microbiologically and therefore should be collected before the initiation of antibiotic treatment whenever possible. Tracheal wash is likely to provide a representative specimen in patients with bronchial or alveolar disease (Table 20-2). It is less likely to identify interstitial and small focal disease processes. However, the procedure is inexpensive and minimally invasive, and this makes it reasonable to perform in most animals with lower respiratory tract disease if the risks of other methods of specimen collection are deemed too great. Potential complications are rare, and they include tracheal laceration, subcutaneous emphysema, and pneumomediastinum. Bronchospasm may be induced by the procedure in patients with hyperreactive airways, particularly cats with bronchitis.
TECHNIQUES
Tracheal wash is performed using transtracheal or endotracheal techniques. Transtracheal wash is performed by passing a catheter into the trachea to the level of the carina through the cricothyroid ligament or between the tracheal rings in an awake or sedated animal. Endotracheal wash is performed by passing a catheter through an endotracheal tube in an anesthetized animal. The endotracheal technique is preferred in cats and very small dogs, although either technique can be used in any animal. Patients with airways that may be hyperreactive, particularly cats, are treated with bronchodilators (see the section on endotracheal technique).
Transtracheal Technique
Transtracheal wash fluid is collected using an 18- to 22-gauge through-the-needle intravenous catheter (e.g., Intracath; Becton, Dickinson and Company). The catheter should be long enough to reach the carina, which is located at approximately the level of the fourth intercostal space. The longest intravenous catheter available may be 12 inches (30 cm), which is long enough to reach from the cricothyroid ligament to the carina in most dogs. However, it may be necessary to insert the catheter between tracheal rings in giant-breed dogs to ensure that it reaches the carina. Alternatively, a 14-gauge, short, over-the-needle catheter is used to enter the trachea at the cricothyroid ligament and a 3.5F polypropylene male dog urinary catheter is passed through the catheter into the airways. The ability of the urinary catheter to pass through the 14-gauge catheter should be tested each time before the procedure is performed.
The dog can sit or lie down, depending on what position is more comfortable for the animal and clinician. The dog is restrained with its nose pointing toward the ceiling at about 45 degrees from horizontal (Fig. 20-13, A). Overextension of the neck causes the animal to be more resistant. Dogs that cannot be restrained should be tranquilized. If tranquilization is needed, premedication with atropine or glycopyrrolate is recommended to minimize contamination of the trachea with oral secretions. Narcotics are avoided to preserve the cough reflex, which can facilitate the retrieval of fluid.
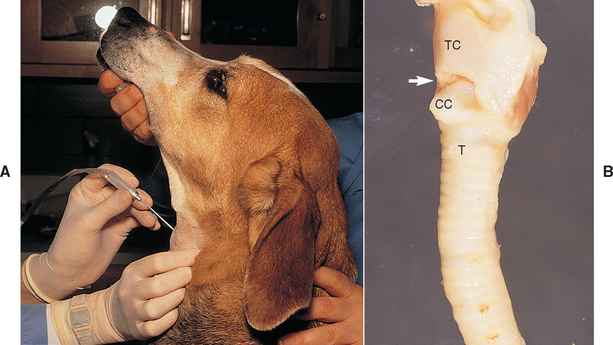
FIG 20-13 A, To perform a transtracheal wash, the animal is restrained in a comfortable position with the nose pointed toward the ceiling. The ventral neck is clipped and scrubbed, and the clinician wears sterile gloves. The cricothyroid ligament is identified as described in B. After an injection of lidocaine, the needle of the catheter is placed through the skin. The larynx is grasped firmly with the fingers and thumb at least 180 degrees around the airway. The needle can then be inserted through the cricothyroid ligament into the airway lumen. B, The lateral view of this anatomic specimen demonstrates the trachea and larynx in a position similar to that of the dog in A. The cricothyroid ligament (arrow) is identified by palpating the trachea (T) from ventral to dorsal until the raised cricoid cartilage (CC) is palpated. The cricothyroid ligament is the first depression above the cricoid cartilage. The cricothyroid ligament attaches cranially to the thyroid cartilage (TC). The palpable depression above the thyroid cartilage (not shown) should not be entered.
The cricothyroid ligament is identified by palpating the trachea in the ventral cervical region and following it dorsally toward the larynx to the raised, smooth, narrow band of the cricoid cartilage. Immediately above the cricoid cartilage is a depression, where the cricothyroid ligament is located (Fig. 20-13, B). If the trachea is entered above the cricothyroid ligament, the catheter is passed dorsally into the pharynx and a nondiagnostic specimen is obtained. Such dorsal passage of the catheter often results in excessive gagging and retching.
Lidocaine is always injected subcutaneously at the site of entry. The skin over the cricothyroid ligament is prepared surgically, and sterile gloves are worn to pass the catheter. The needle of the catheter is held with the bevel facing ventrally. The skin over the ligament is then tented, and the needle is passed through the skin. The larynx is stabilized with the nondominant hand. To properly stabilize it, the clinician should grasp at least 180 degrees of the circumference of the airway between the fingers and thumb. Failure to hold the airway firmly is the most common technical mistake made. Next, the tip of the needle is rested against the cricothyroid ligament and inserted through the ligament with a quick, short motion.
The hand stabilizing the trachea is then used to pinch the needle at the skin, with the hand kept firmly in contact with the neck, while the catheter is threaded into the trachea with the other hand. By keeping the hand holding the needle against the neck of the animal so that the hand, needle, and neck can move as one, the clinician prevents laceration of the larynx or trachea and inadvertent removal of the needle from the trachea. Threading the catheter provokes coughing. There should be little or no resistance to the passage of the catheter. Elevating the hub of the needle slightly so that the tip points more ventrally or retracting the needle a few millimeters facilitates passage of the catheter if it is lodged against the opposite tracheal wall. The catheter itself should not be pulled back through the needle because the tip can be sheared off within the airway by the cutting edge of the needle.
Once the catheter is completely threaded into the airway, the needle is withdrawn and the catheter guard is attached to prevent shearing of the catheter. The person restraining the animal now holds the catheter guard against the neck of the animal so that movement of the neck will not dis-lodge the catheter. The head can be restrained in a natural position.
It is convenient to have four to six 12-ml syringes ready, each filled with 3 to 5 ml of 0.9% sterile preservative-free sodium chloride solution. The entire bolus of saline in one syringe is injected into the catheter. Immediately after this, many aspiration attempts are made. After each aspiration, the syringe must be disconnected from the catheter and the air evacuated without losing any of the retrieved fluid. Aspirations should be forceful and repeated at least five or six times so that small volumes of airway secretions that have been aspirated into the catheter are pulled the entire length of the catheter into the syringe.
The procedure is repeated using additional boluses of saline until a sufficient amount of fluid is retrieved for analysis. A total of 1.5 to 3 ml of turbid fluid is adequate in most instances. The clinician does not need to be concerned about “drowning” the animal with the infusion of the modest volumes of fluid described because the fluid is rapidly absorbed into the circulation. Failure to retrieve adequate volumes of visibly turbid fluid can be the result of several technical difficulties, as outlined in Figure 20-14.
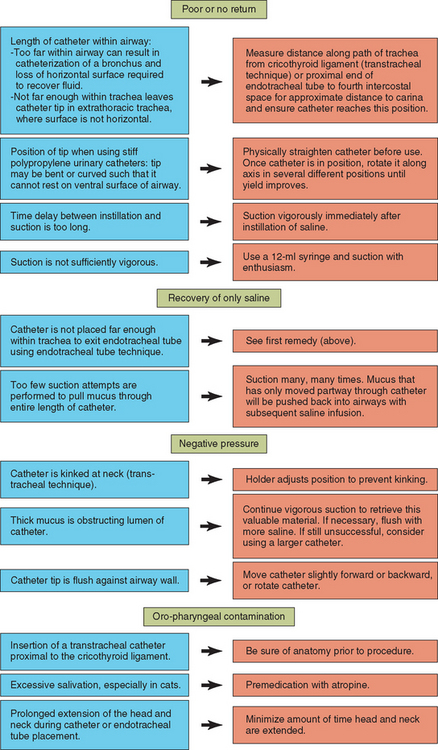
FIG 20-14 Overcoming problems with tracheal wash fluid collection. Green boxes indicate problems, blue boxes indicate possible causes, and orange boxes indicate remedies.
The catheter is removed after sufficient fluid is collected. A sterile gauze sponge with antiseptic ointment is then immediately placed over the catheter site, and a light bandage is wrapped around the neck. This bandage is left in place for several hours while the animal rests quietly in a cage. These precautions minimize the likelihood that subcutaneous emphysema or pneumomediastinum will develop.
Endotracheal Technique
The endotracheal technique is performed by passing a 3.5-5F male dog urinary catheter through a sterilized endotracheal tube. The animal is anesthetized with a short-acting intravenous agent to a sufficient depth to allow intubation. A short-acting barbiturate, propofol, or, in cats, a combination of ketamine and acepromazine or diazepam is effective. Premedication with atropine, particularly in cats, is recommended to minimize contamination of the trachea with saliva. Cats with lower respiratory tract disease may have airway hyperreactivity and generally should be administered a bronchodilator before the tracheal wash. Terbutaline (0.01 mg/kg) can be given subcutaneously to cats not already receiving oral bronchodilators. It is also prudent to keep a metered dose inhaler of albuterol at hand to administer through the endotracheal tube or by mask if breathing becomes labored.
A sterilized endotracheal tube should be passed without dragging the tip through the oral cavity. The animal’s mouth is opened wide with the tongue pulled out, a laryngoscope is used, and, in cats, sterile topical lidocaine is applied to the laryngeal cartilages to ease passage of the tube with minimal contamination.
The urinary catheter is passed through the endotracheal tube to the level of the carina (approximately the fourth intercostal space), maintaining sterile technique. The wash procedure is performed as described for the transtracheal technique. Slightly larger boluses of saline may be required, however, because of the larger volume of the catheter. Use of a catheter larger than 5F seems to reduce the yield of the wash except when secretions are extremely viscous.
SPECIMEN HANDLING
The cells collected in the wash fluid are fragile. The fluid is ideally processed within 30 minutes of collection, with minimal manipulation. Bacterial culture is performed on at least 0.5 to 1 ml of fluid. Fungal cultures are performed if mycotic disease is a differential diagnosis, and Mycoplasma cultures are considered for cats and dogs with signs of bronchitis. Cytologic preparations are made both from the fluid and from any mucus within the fluid. Both fluid and mucus are examined because infectious agents and inflammatory cells can be concentrated in the mucus, but the proteinaceous material causes cells to clump and interferes with evaluation of the cell morphology. Mucus is retrieved with a needle, and squash preparations are made. Direct smears of the fluid itself can be made, but such specimens are often hypocellular. Sediment or cytocentrifuge preparations are generally necessary to make adequate interpretation possible. Straining the fluid through gauze to remove the mucus is discouraged because infectious agents may be lost in the process. Routine cytologic stains are used.
Microscopic examination of slides includes the identification of cell types, qualitative evaluation of the cells, and an examination for infectious agents. Cells are evaluated qualitatively for evidence of macrophage activation, neutrophil degeneration, lymphocyte reactivity, and characteristics of malignancy. Epithelial hyperplasia secondary to inflammation should not be overinterpreted as neoplasia, however. Infectious agents such as bacteria, protozoa (Toxoplasma gondii), fungi (Histoplasma, Blastomyces, and Cryptococcus organisms), and parasitic larvae or eggs may be present (see Fig. 20-12, and Figs. 20-15 through 20-17). Because only one or two organisms may be present on an entire slide, a thorough evaluation is indicated.
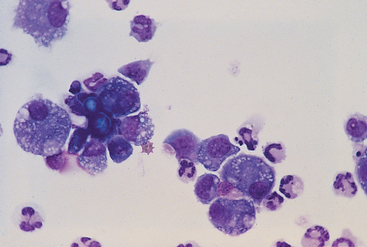
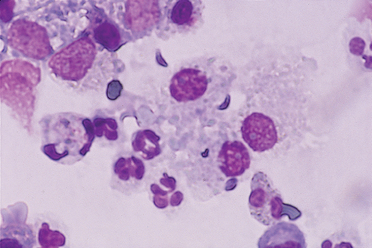
FIG 20-15 Photomicrograph of a Blastomyces organism from the lungs of a dog with blastomycosis. The organisms stain deeply basophilic, are 5 to 15 μm in diameter, and have a thick refractile cell wall. Often, as in this figure, broad-based budding forms are seen. The cells present are alveolar macrophages and neutrophils. (Bronchoalveolar lavage fluid, Wright stain.)
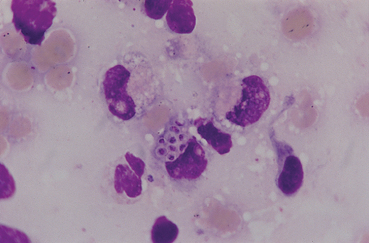
FIG 20-16 Photomicrograph of Histoplasma organisms from the lungs of a dog with histoplasmosis. The organisms are small (2 to 4 μm) and round, with a deeply staining center and a lighter-staining halo. They are often found within phagocytic cells: in this figure, an alveolar macrophage. (Bronchoalveolar lavage fluid, Wright stain.)
INTERPRETATION OF RESULTS
Normal tracheal wash fluid contains primarily respiratory epithelial cells. Few other inflammatory cells are present (Fig. 20-18). Occasionally, macrophages are retrieved from the small airways and alveoli because the catheter was extended into the lungs beyond the carina or because relatively large volumes of saline were used. Most macrophages are not activated. In these instances the presence of macrophages does not indicate disease but rather reflects the acquisition of material from the deep lung (see the section on nonbronchoscopic bronchoalveolar lavage).
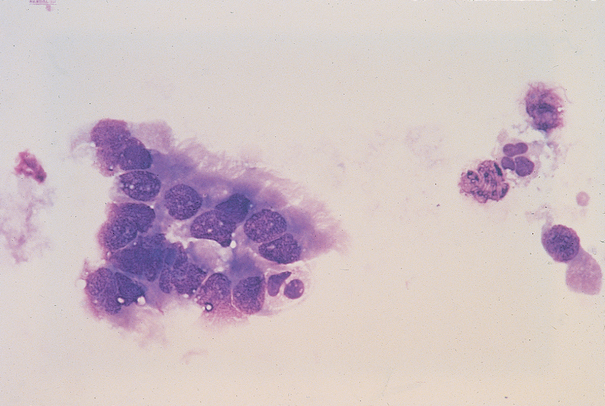
FIG 20-18 Tracheal wash fluid from a healthy dog showing ciliated epithelium and few inflammatory cells.
Slides are examined for evidence of overt oral contamination, which can occur during transtracheal washing if the catheter needle was inadvertently inserted proximal to the cricothyroid ligament. Rarely, dogs can cough the catheter up into the oropharynx. Oral contamination can also result from drainage of saliva into the trachea, which usually occurs in cats that hypersalivate or dogs that are heavily sedated, particularly if the head and neck are extended more than briefly for the passage of the endotracheal tube or transtracheal catheter. Oral contamination is indicated by the finding of numerous squamous epithelial cells, often coated with bacteria, and Simonsiella organisms (Fig. 20-19). Simonsiella organisms are large basophilic rods that are frequently found stacked uniformly on one another along their broad side. Specimens with overt oral contamination generally do not provide accurate information about the airways, particularly with regard to bacterial infection.
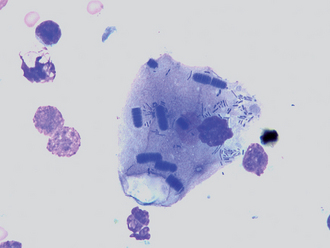
FIG 20-19 Tracheal wash fluid showing evidence of oropharyngeal contamination. The numerous, uniformly stacked basophilic rods are Simonsiella organisms, normal inhabitants of the oral cavity. These organisms, as well as many other bacteria, are adhering to a squamous epithelial cell. Squamous epithelium is another indication of contamination from the oral cavity.
Cytologic results of tracheal wash fluid are most useful when pathogenic organisms or malignant cells are identified. The presence of pathogens such as Toxoplasma gondii, systemic fungal organisms, and parasites provide a definitive diagnosis. The finding of bacterial organisms in cytologic preparations without evidence of oral contamination indicates the presence of infection. The growth of any of the systemic mycotic agents in culture is also clinically significant, whereas the growth of bacteria in culture may or may not be significant because low numbers of bacteria can be present in the large airways of healthy animals. In general, the cytologic identification of bacteria and their growth in culture without multiplication in enrichment broth are significant findings. Bacteria that are not seen cytologically and that grow only after incubation in enrichment media can result from several situations. For example, the bacteria may be causing infection without being present in high numbers because of the prior administration of antibiotics or because of the collection of a nonrepresentative specimen. The bacteria may also be clinically insignificant and represent normal tracheal inhabitants or result from contamination during collection. Other clinical data must therefore be considered when interpreting such findings. The role of Mycoplasma sp. in respiratory disease of the dog and cat is not well understood. These organisms cannot be seen on cytologic preparations and are difficult to grow in culture. Specific transport media is necessary. Growth of Mycoplasma organisms from tracheal wash fluid may indicate primary or secondary infection or be an insignificant finding. Treatment is generally recommended.
Criteria of malignancy for making a diagnosis of neoplasia must be interpreted with extreme caution. Overt characteristics of malignancy must be present in many cells in the absence of concurrent inflammation for a definitive diagnosis to be made.
The type of inflammatory cells present in tracheal wash fluid can assist in narrowing the differential diagnoses, although a mixed inflammatory response is common. Neutrophilic (suppurative) inflammation is common in bacterial infections. Before antibiotic therapy is initiated, the neutrophils may be (but are not always) degenerative, and organisms can often be seen. Neutrophilic inflammation may be a response to a variety of other diseases. For instance, it can be caused by other infectious agents or seen in patients with canine chronic bronchitis, idiopathic pulmonary fibrosis or other idiopathic interstitial pneumonias, or even neoplasia. Some cats with idiopathic bronchitis have neutrophilic inflammation rather than the expected eosinophilic response (see Chapter 21). The neutrophils in these instances are generally nondegenerative.
Eosinophilic inflammation reflects a hypersensitivity response, and common diseases resulting in eosinophilic inflammation include allergic bronchitis, parasitic disease, and eosinophilic lung disease. Parasites that affect the lung include primary lungworms or flukes, migrating intestinal parasites, and heartworms. Over time, mixed inflammation can occur in patients with hypersensitivity. It is occasionally possible for nonparasitic infections or neoplasia to cause eosinophilia, usually as part of a mixed inflammatory response.
Macrophagic (granulomatous) inflammation is characterized by the finding of increased numbers of activated macrophages, generally present as a component of mixed inflammation along with increased numbers of other inflammatory cells. Activated macrophages are vacuolated and have increased amounts of cytoplasm. This response is nonspecific unless an etiologic agent can be identified.
Lymphocytic inflammation alone is uncommon. Viral or rickettsial infection, idiopathic interstitial pneumonias, and lymphoma are considerations.
True hemorrhage can be differentiated from a traumatic specimen collection by the presence of erythrophagocytosis and hemosiderin-laden macrophages. An inflammatory response is also usually present. Hemorrhage can be caused by neoplasia, mycotic infection, heartworm disease, thromboembolism, foreign body, lung lobe torsion, or coagulopathies. Evidence of hemorrhage is seen occasionally in animals with congestive heart failure or severe bacterial pneumonia.
NONBRONCHOSCOPIC BRONCHOALVEOLAR LAVAGE
Indications and Complications
Bronchoalvelolar lavage (BAL) is considered for the diagnostic evaluation of patients with lung disease involving the small airways, alveoli, or interstitium that are not in respiratory distress (see Table 20-2). A large volume of lung is sampled by BAL (Figs. 20-20 and 20-21). The collected specimens are of large volume, providing more than adequate material for routine cytology, cytology involving special stains (e.g., Gram stains, acid-fast stains), multiple types of cultures (e.g., bacterial, fungal, mycoplasmal), or other specific tests that might be helpful in particular patients (e.g., flow cytometry, polymerase chain reaction [PCR]). Cytologic preparations from BAL fluid are of excellent quality and consistently provide large numbers of well-stained cells for examination.
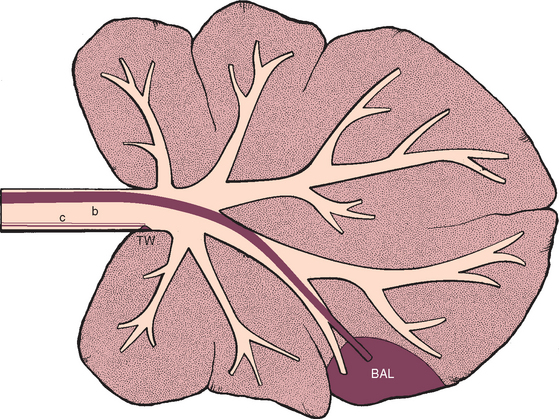
FIG 20-20 The region of the lower respiratory tract that is sampled by bronchoalveolar lavage (BAL) in comparison with the region sampled by tracheal wash (TW). The solid red line (b) within the airways represents a bronchoscope or modified feeding tube. The open lines (c) represent the tracheal wash catheter. Bronchoalveolar lavage yields fluid representative of the deep lung, whereas tracheal wash yields fluid representative of processes involving major airways.
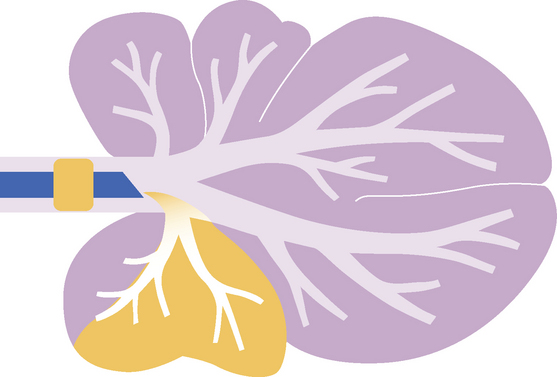
FIG 20-21 The region of the lower respiratory tract presumed to be sampled by nonbronchoscopic bronchoalveolar lavage in cats using an endotracheal tube.
Although general anesthesia is required, the procedure is associated with few complications and can be performed repeatedly in the same animal to follow the progression of disease or observe the response to therapy. The primary complication of BAL is transient hypoxemia. The hypoxemia generally can be corrected with oxygen supplementation, but animals exhibiting increased respiratory efforts or respiratory distress in room air are not good candidates for this procedure. Patients with hyperreactive airways, particularly cats, are treated with bronchodilators, as described previously, for endotracheal washing. For patients with bacterial or aspiration pneumonia, tracheal washing routinely results in an adequate specimen for cytologic and microbiologic analysis and avoids the need for general anesthesia in these patients.
BAL is a routine part of diagnostic bronchoscopy, during which visually guided BAL specimens can be collected from specific diseased lung lobes. However, nonbronchoscopic techniques (NB-BAL) have been developed that allow BAL to be performed with minimal expense in routine practice settings. Because visual guidance is not possible using these methods, they are used primarily for patients with diffuse disease. However, the technique described for cats probably samples the cranial and middle regions of the lung on the side of the cat placed against the table, whereas the technique described for dogs consistently samples one of the caudal lung lobes.
TECHNIQUE FOR NB-BAL IN CATS
A sterile endotracheal tube and syringe adapter are used in cats to collect lavage fluid (Fig. 20-22; see also Fig. 20-21). Cats, particularly those with signs of bronchitis, should be treated with bronchodilators before the procedure, as described previously for tracheal wash (endotracheal technique), to decrease the risk of bronchospasm. The cat is premedicated with atropine (0.05 mg/kg subcutaneously) and anesthetized with ketamine and acepromazine or diazepam, given intravenously. The endotracheal tube is passed as cleanly as possible through the larynx to minimize oral contamination. To achieve sufficient cleanliness, the tip of the tongue is pulled out, a laryngoscope is used, and sterile lidocaine is applied topically to the laryngeal mucosa. The cuff is then inflated sufficiently to create a seal, but overinflation is avoided to prevent tracheal rupture (i.e., use a 3-ml syringe and inflate cuff in 0.5-ml increments only until no leak is audible when gentle pressure is placed on the oxygen reservoir bag).
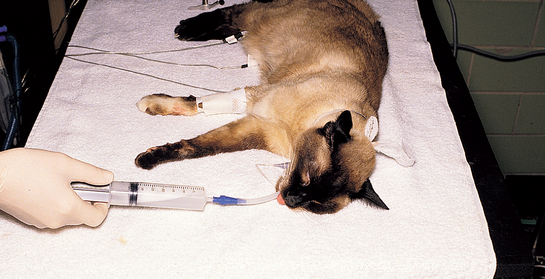
FIG 20-22 Bronchoalveolar lavage using an endotracheal tube in a cat. The fluid retrieved is grossly foamy because of the surfactant present. The procedure is performed quickly because the airway is completely occluded during the infusion and aspiration of fluid.
The cat is placed in lateral recumbency with the most diseased side, as determined by physical and radiographic findings, against the table. Oxygen (100%) is administered for several minutes through the endotracheal tube. The anesthetic adapter is then removed from the endotracheal tube and replaced with a sterile syringe adapter, using caution to avoid contamination of the end of the tube or adapter. Immediately, a bolus of warmed, sterile 0.9% saline solution (5 ml/kg body weight) is infused through the tube over approximately 3 seconds. Immediately after infusion, suction is applied by syringe. Air is eliminated from the syringe, and several aspiration attempts are made until fluid is no longer recovered. The procedure is repeated using a total of two or three boluses of saline solution. The cat is allowed to expand its lungs between the infusions of saline solution. After the last infusion, the syringe adapter is removed (because it greatly interferes with ventilation) and excess fluid is drained from the large airways and endotracheal tube by elevating the caudal half of the cat a few inches off of the table. At this point, the cat is cared for as described in the section on recovery of patients after BAL.
TECHNIQUE FOR NB-BAL IN DOGS
An inexpensive 122-cm 16F Levin-type polyvinyl chloride stomach tube (Argyle stomach tube, Tyco Healthcare Group LP) can be used in dogs to collect lavage fluid. The tube must be modified for successful NB-BAL. Sterile technique is maintained throughout. The distal end of the tube is cut off to remove the side openings. The proximal end is cut off to remove the flange and shorten the tube to a length slightly greater than the distance from the open end of the dog’s endotracheal tube to the last rib. A syringe adapter is placed within the proximal end of the tube (Fig. 20-23).
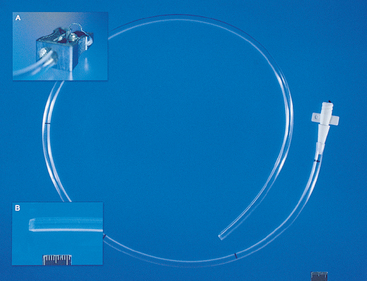
FIG 20-23 The catheter used for nonbronchoscopic bronchoalveolar lavage in dogs is a modified 16F Levin-type stomach tube. The tube is shortened by cutting off both ends. A simple pencil sharpener (inset A) is used to taper the distal end of the tube (inset B). A syringe adapter is added to the proximal end. Sterility is maintained throughout.
Recovery of BAL fluid can be improved by tapering the distal end of the tube. Tapering is readily achieved using a metal, single-blade, handheld pencil sharpener that has been autoclaved and is used only for this purpose (see Fig. 20-23, A and B).
The dog is premedicated with atropine (0.05 mg/kg subcutaneously) or glycopyrrolate (0.005 mg/kg subcutaneously) and anesthetized using a short-acting protocol that will allow intubation, such as with propofol, a short-acting barbiturate, or the combination of medetomidine and butorphanol. If the dog is of sufficient size to accept a size 6 or larger endotracheal tube, the dog is intubated with a sterile endotracheal tube placed as cleanly as possible to minimize oral contamination of the specimen. The modified stomach tube will not fit through a smaller endotracheal tube, so the technique must be performed without an endotracheal tube or a smaller stomach tube must be used. If no endotracheal tube is used, extreme care must be taken to minimize oral contamination in passing the modified stomach tube, and an appropriate-sized endotracheal tube should be available to gain control of the airway in case of complications and for recovery.
Oxygen (100%) is provided through the endotracheal tube or by face mask for several minutes. The modified stomach tube is passed through the endotracheal tube using sterile technique until resistance is felt. The goal is to wedge the tube snugly into an airway rather than have it abut an airway division. Therefore the tube is withdrawn slightly, then passed again, until resistance is consistently felt at the same depth. Rotating the tube slightly during passage may help achieve a snug fit. Remember that if the endotracheal tube is not much larger than the stomach tube, ventilation is restricted at this point and the procedure should be completed expediently.
For medium-size dogs and larger, two 35-ml syringes are prepared in advance, each with 25 ml of saline and 5 ml of air. While the modified stomach tube is held in place, a 25-ml bolus of saline is infused through the tube, followed by the 5 ml of air, by holding the syringe upright during infusion (Fig. 20-24). Gentle suction is applied immediately after infusion, using the same syringe. It may be necessary to withdraw the tube slightly if negative pressure is felt. The tube should not be withdrawn more than a few millimeters. If it is withdrawn too far, air will be recovered instead of fluid. The second bolus of saline is infused and recovered in the same manner, with the tube in the same position. The dog is cared for as described in the next section.
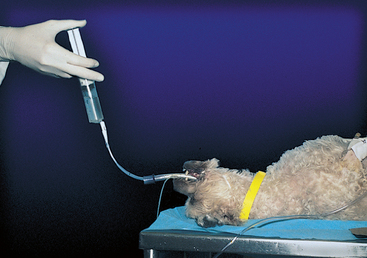
FIG 20-24 Bronchoalveolar lavage using a modified stomach tube in a dog. The tube is passed through a sterile endotracheal tube and lodged in a bronchus. A syringe preloaded with saline and air is held upright during infusion so that the saline is infused first, followed by the air.
In very small dogs, it is prudent to reduce the volume of saline used in each bolus, particularly if a smaller diameter stomach tube is used. Overinflation of the lungs with excessive fluid volumes should be avoided.
RECOVERY OF PATIENTS FOLLOWING BAL
Regardless of the method used, BAL causes a transient decrease in the arterial oxygen concentration, but this hypoxemia responds readily to oxygen supplementation. Where possible, patients are monitored with pulse oximetry (p. 283) before and throughout the procedure and during recovery. After the procedure, 100% oxygen is provided through an endotracheal tube for as long as the dog or cat will allow intubation. Several gentle “sighs” are performed with the anesthesia bag to help expand any collapsed portions of lung. Bronchospasms are a reported complication of BAL in people, and increased airway resistance has been documented in cats after bronchoscopy and BAL (Kirschvink et al., 2005). Albuterol in a metered dose inhaler should be on hand to administer through the endotracheal tube or by spacer and mask if needed.
After extubation the mucous membrane color, pulses, and character of respirations are monitored closely. Crackles can be heard for several hours after BAL and are not cause for concern. Treatment with oxygen supplementation is continued by mask, oxygen cage, or nasal catheter if there are any indications of hypoxemia. Oxygen supplementation is rarely necessary for more than 10 to 15 minutes after BAL, even in animals with diseased lungs; however, the ability to provide supplementation for longer periods is a prerequisite for the performance of this procedure.
SPECIMEN HANDLING
Successful BAL yields fluid that is grossly foamy, a result of the surfactant from the alveoli. Approximately 50% to 80% of the total volume of saline instilled is expected to be recovered. Less will be obtained from dogs with tracheobronchomalacia (collapsing airways). The fluid is placed on ice immediately after collection and is processed as soon as possible, with minimum manipulation to decrease cell lysis. For convenience, retrieved boluses can be combined for analysis; however, fluid from the first bolus usually contains more cells from the larger airways, and fluid from later boluses is more representative of the alveoli and interstitium.
The BAL fluid is analyzed cytologically and microbiologically. Nucleated cell counts are performed on undiluted fluid using a hemocytometer. Cells are concentrated onto slides for differential cell counts and qualitative analysis using cytocentrifugation or sedimentation techniques. Excellent-quality slides result that are stained using routine cytologic procedures. Differential cell counts are performed by counting at least 200 nucleated cells. Slides are scrutinized for evidence of macrophage activation, lymphocyte reactivity, neutrophil degeneration, and criteria of malignancy. All slides are examined thoroughly for possible etiologic agents, such as fungi, protozoa, parasites, and bacteria (see 17-Figs. 20). As described for tracheal wash, visible strands of mucus can be examined for etiologic agents by squash preparation.
Approximately 5 ml of fluid is used for bacterial culture. Additional fluid is submitted for fungal culture if mycotic disease is among the differential diagnoses. Mycoplasma cultures are considered in cats and dogs with signs of bronchitis.
INTERPRETATION OF RESULTS
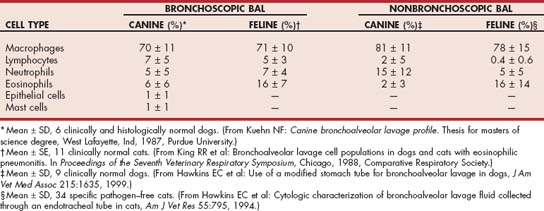
Normal cytologic values for BAL fluid are inexact because of inconsistency in the techniques used and variability among individual animals of the same species. In general, total nucleated cell counts in normal animals are less than 400 to 500/μl. Differential cell counts from healthy dogs and cats are listed in Table 20-3.
Interpretation of BAL fluid cytology and cultures is essentially the same as that described for tracheal wash fluid, although the specimens are more representative of the deep lung than the airways. In addition, the normal cell population of macrophages must not be misinterpreted as being indicative of macrophagic or chronic inflammation (Fig. 20-25). As for all cytologic specimens, definitive diagnoses are made through the identification of organisms or abnormal cell populations. Fungal, protozoal, or parasitic organisms may be present in extremely low numbers in BAL specimens; therefore the entire concentrated slide preparation must be carefully scanned. Profound epithelial hyperplasia can occur in the presence of an inflammatory response and should not be confused with neoplasia.
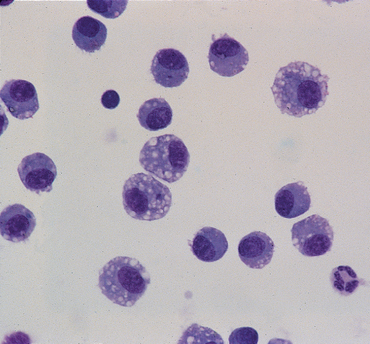
FIG 20-25 Bronchoalveolar lavage fluid from a normal dog. Note that alveolar macrophages predominate.
If quantitative bacterial culture is available, growth of organisms at greater than 1.7 × 103 colony-forming units (CFU)/ml has been reported to indicate infection (Peeters et al., 2000). In the absence of quantitative numbers, growth of organisms on a plate directly inoculated with BAL fluid is considered significant, whereas growth from fluid that occurs only after multiplication in enrichment broth may also be a result of normal inhabitants or contamination. Patients that are already receiving antibiotics at the time of specimen collection may have significant infection with few or no bacteria by culture.
DIAGNOSTIC YIELD
A retrospective study of BAL fluid cytologic analysis in dogs at referral institutions showed that BAL findings provided the basis for a definitive diagnosis in 25% of cases and were supportive of the diagnosis in an additional 50%. Only dogs in which a definitive diagnosis was obtained by any means were included. Definitive diagnoses were possible on the basis of BAL only in those animals in which infectious organisms were identified or in those cases in which overtly malignant cells were present in specimens in the absence of marked inflammation. BAL has been shown to be more sensitive than radiographs in identifying pulmonary involvement with lymphosarcoma. Carcinoma was definitively identified in 57% of cases, and other sarcomas were not found in BAL fluid. Fungal pneumonia was confirmed in only 25% of cases, although organisms were found in 67% of cases in a previous study of dogs with overt fungal pneumonia.
TRANSTHORACIC LUNG ASPIRATION AND BIOPSY
Indications and Complications
Pulmonary parenchymal specimens can be obtained by transthoracic needle aspiration or biopsy. Although only a small region of lung is sampled by these methods, collection can be guided by radiographic findings or ultrasonography to improve the likelihood of obtaining representative specimens. As with tracheal wash and BAL, a definitive diagnosis will be possible in patients with infectious or neoplastic disease. Patients with non-infectious inflammatory diseases require thoracoscopy or thoracotomy with lung biopsy for a definitive diagnosis.
Potential complications of transthoracic needle aspiration or biopsy include pneumothorax, hemothorax, and pulmonary hemorrhage. The procedures are not recommended in animals with suspected cysts, abscesses, pulmonary hypertension, or coagulopathies. Severe complications are uncommon, but these procedures should not be performed unless the clinician is prepared to place a chest tube and otherwise support the animal if necessary.
Lung aspirates and biopsy specimens are indicated for the nonsurgical diagnosis of intrathoracic mass lesions that are in contact with the thoracic wall. The risk of complications in these animals is relatively low because the specimens can be collected without disrupting aerated lung. Obtaining aspirates or biopsy specimens from masses that are far from the body wall and near the mediastinum carries the additional risk of lacerating important mediastinal organs, vessels, or nerves. If a solitary localized mass lesion is present, thoracotomy and biopsy should be considered rather than transthoracic sampling because this permits both the diagnosis of the problem and the potentially therapeutic benefits of complete excision.
Transthoracic lung aspirates can be obtained in animals with a diffuse interstitial radiographic pattern. In some of these patients, solid areas of infiltrate in lung tissue immediately adjacent to the body wall can be identified ultrasonographically even though they are not apparent on thoracic radiographs (see Fig. 20-11). Ultrasound guidance of the aspiration needle into the areas of infiltrate should improve diagnostic yield and safety. If areas of infiltrate cannot be identified ultrasonographically, BAL should be considered before lung aspiration in animals that can tolerate the procedure because it yields a larger specimen for analysis and, in this author’s opinion, carries less risk than transthoracic aspiration in patients that are not experiencing increased respiratory efforts or distress. Tracheal wash (if BAL is not possible) and appropriate ancillary tests are also generally indicated before lung aspiration in these patients because they carry little risk.
TECHNIQUES
The site of collection in animals with localized disease adjacent to the body wall is best identified with ultrasonography. If ultrasonography is not available or the lesion is surrounded by aerated lung, the site is determined on the basis of two radiographic views. The location of the lesion during inspiration in all three dimensions is identified by its relationship to external landmarks: the nearest intercostal space or rib, the distance from the costochondral junctions, and the depth into the lungs from the body wall. If available, fluoroscopy or CT also can be used to guide the needle or biopsy instrument.
The site of collection in animals with diffuse disease is a caudal lung lobe. The needle is inserted between the seventh to ninth intercostal spaces, approximately two thirds of the distance from the costochondral junctions to the spine.
The animal must be restrained for the procedure, and sedation or anesthesia is necessary in some. Anesthesia is avoided, if possible, because the hemorrhage created by the procedure is not cleared as readily from the lungs in an anesthetized dog or cat. The skin at the site of collection is shaved and surgically prepared. Lidocaine is injected into the subcutaneous tissues and intercostal muscles to provide local anesthesia.
Lung aspiration can be performed with an injection needle, spinal needle, or a variety of thin-walled needles designed specifically for lung aspiration in people. Spinal needles are readily available in most practices, are sufficiently long to penetrate through the thoracic wall, and have a stylet. A 22-gauge, 1.5- to 3.5-inch (3.75- to 8.75-cm) spinal needle is usually adequate.
The clinician wears sterile gloves. The needle with stylet is advanced through the skin several rib spaces from the desired biopsy site. The needle and skin are then moved to the biopsy site. This is done because air is less likely to enter the thorax through the needle tract following the procedure if the openings in the skin and chest wall are not aligned. The needle is then advanced through the body wall to the pleura. The stylet is removed, and the needle hub is immediately covered by a finger to prevent pneumothorax until a 12-ml syringe can be placed on the hub. During inspiration the needle is thrust into the chest to a depth predetermined from the radiographs, usually about 1 inch (2.5 cm), while suction is applied to the syringe (Fig. 20-26). To keep from inserting the needle too deeply, the clinician may pinch the needle shaft with the thumb and forefinger of the nondominant hand at the desired maximum depth of insertion. During insertion the needle can be twisted along its long axis in an attempt to obtain a core of tissue. The needle is then immediately withdrawn to the level of the pleura. Several quick stabs into the lung can be made along different lines to increase the yield.
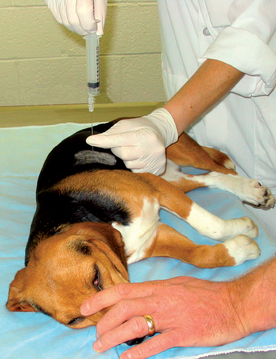
FIG 20-26 Transthoracic lung aspiration performed with a spinal needle. Note that sterile technique is used. The needle shaft can be pinched with a finger and thumb at the maximum depth to which the needle should be passed. The finger and thumb thus act as a guard to prevent overinsertion of the needle. Although this patient is under general anesthesia, this is not usually indicated.
Each stab should take only a second. Prolonging the time that the needle is within the lung tissue increases the likelihood of complications. The lung tissue will be moving with respirations, resulting in laceration of tissue, even if the needle is held steady.
The needle is withdrawn from the body wall with a minimal amount of negative pressure maintained by the syringe. It is unusual for the specimen to be large enough to have entered the syringe. The needle is removed from the syringe, the syringe is filled with air and reattached to the needle, and the contents of the needle are then forced onto one or more slides. Grossly, the material is bloody in most cases. Squash preparations are made. Slides are stained using routine procedures and then evaluated cytologically. Increased numbers of inflammatory cells, infectious agents, or neoplastic cell populations are potential abnormalities. Alveolar macrophages are normal findings in parenchymal specimens and should not be interpreted as representing chronic inflammation. They should be carefully examined for evidence of phagocytosis of bacteria, fungi, or red blood cells and for signs of activation. Epithelial hyperplasia can occur in the presence of inflammation and should not be confused with neoplasia. Sometimes the liver is aspirated inadvertently, particularly in deep-chested dogs, yielding a population of cells that may resemble those from adenocarcinoma. However, hepatocytes typically contain bile pigment. Bacterial culture is indicated in some animals, although the volume of material obtained is quite small.
Transthoracic lung core biopsies can be performed in animals with mass lesions. They are collected after an aspirate has proved to be nondiagnostic. Needle biopsy instruments can be used to biopsy lesions adjacent to the chest wall (e.g., EZ Core biopsy needles, Products Group International). Smaller-bore, thin-walled lung biopsy instruments can be obtained from medical suppliers for human patients. These instruments collect smaller pieces of tissue but are less disruptive to normal lung. Ideally, sufficient material is collected for histologic evaluation. If not, squash preparations are made for cytologic studies.
BRONCHOSCOPY
Indications
Bronchoscopy is indicated for the evaluation of the major airways in animals with suspected structural abnormalities, for visual assessment of airway inflammation or pulmonary hemorrhage, and as a means of collecting specimens in animals with undiagnosed lower respiratory tract disease. Bronchoscopy can be used to identify structural abnormalities of the major airways, such as tracheal collapse, mass lesions, tears, strictures, lung lobe torsions, bronchiectasis, bronchial collapse, and external airway compression. Foreign bodies or parasites may be identified. Hemorrhage or inflammation involving or extending to the large airways may also be seen and localized.
Specimen collection techniques performed in conjunction with bronchoscopy are valuable diagnostic tools because they can be used to obtain specimens from deeper regions of the lung than is possible with the tracheal wash technique, and visually directed sampling of specific lesions or lung lobes is also possible. Animals undergoing bronchoscopy must receive general anesthesia, and the presence of the scope within the airways compromises ventilation. Therefore bronchoscopy is contraindicated in animals with severe respiratory tract compromise unless the procedure is likely to be therapeutic (e.g., foreign body removal).
Technique
Bronchoscopy is technically more demanding than most other endoscopic techniques. The patient is often experiencing some degree of respiratory compromise, which poses increased anesthetic and procedural risk. Airway hyperreactivity may be exacerbated by the procedure, particularly in cats.(Kirschvink et al., 2005) A small-diameter, flexible endoscope is needed and should be sterilized before use. The bronchoscopist should be thoroughly familiar with normal airway anatomy to ensure that every lobe is examined. BAL is routinely performed as part of diagnostic bronchoscopy after thorough visual examination of the airways. The reader is referred to chapters in other textbooks for details about performing bronchoscopy and bronchoscopic BAL (Kuehn, 2004; McKiernan, 2005; Hawkins, 2004). Bronchoscopic images of normal airways are shown in Fig. 20-27. Reported cell counts from bronchoscopically collected BAL fluid are provided in Table 20-3.
FIG 20-27 Bronchoscopic images of normal airways. The labels for the lobar bronchi are from a useful nomenclature system for the major airways and their branches by Amis et al. (1986). A, Carina, the division between the right (R) and left (L) mainstem bronchi. B, Right mainstem bronchus. The carina is off the right side of the image. The openings to the right cranial (RB1), right middle (RB2), accessory (RB3), and right caudal (RB4) bronchi are visible. C, Left mainstem bronchus. The carina is off the left side of the image. The openings to the left cranial (LB1) and left caudal (LB2) bronchi are visible. The left cranial lobe (LB1) divides immediately into cranial (narrow arrow) and caudal (broad arrow) branches.
(Amis TC et al: Systematic identification of endobronchial anatomy during bronchoscopy in the dog, Am J Vet Res 47:2649, 1986.)
Abnormalities that may be observed during bronchoscopy and their common clinical correlations are listed in Table 20-4. A definitive diagnosis may not be possible on the basis of the findings yielded by gross examination alone. Specimens are collected through the biopsy channel for cytologic, histopathologic, and microbiologic analysis. Bronchial specimens are obtained by bronchial washing, bronchial brushing, or pinch biopsy. Material for bacterial culture can be collected with guarded culture swabs. The deeper lung is sampled by BAL or transbronchial biopsy. Foreign bodies are removed with retrieval forceps.
 TABLE 20-4 Bronchoscopic Abnormalities and Their Clinical Correlations
TABLE 20-4 Bronchoscopic Abnormalities and Their Clinical Correlations
| ABNORMALITY | CLINICAL CORRELATION |
|---|---|
| Trachea | |
| Hyperemia, loss of normal vascular pattern, excess mucus, exudate | Inflammation |
| Redundant tracheal membrane | Tracheal collapse |
| Flattened cartilage rings | Tracheal collapse |
| Uniform narrowing | Hypoplastic trachea |
| Strictures | Prior trauma |
| Mass lesions | Fractured rings, foreign body granuloma, neoplasia |
| Tears | Usually caused by excessive endotracheal tube cuff pressure |
| Carina | |
| Widened | Hilar lymphadenopathy, extraluminal mass |
| Multiple raised nodules | Oslerus osleri |
| Foreign body | Foreign body |
| Bronchi | |
| Hyperemia, excess mucus, exudate | Inflammation |
| Collapse of airway during expiration | Chronic inflammation, bronchomalacia |
| Collapse of airway, inspiration and expiration, ability to pass scope through narrowed airway | Chronic inflammation, bronchomalacia |
| Collapse of airway, inspiration and expiration, inability to pass scope through narrowed airway | Extraluminal mass lesions (neoplasia, granuloma, abscess) |
| Collapse of airway with “puckering” of mucosa | Lung lobe torsion |
| Hemorrhage | Neoplasia, fungal infection, heartworm, thromboembolic disease, coagulopathy, trauma (including foreign body related) |
| Single mass lesion | Neoplasia |
| Multiple polypoid masses | Usually chronic bronchitis; at carina, Oslerus |
| Foreign body | Foreign body |
THORACOTOMY OR THORACOSCOPY WITH LUNG BIOPSY
Thoracotomy and surgical biopsy are performed in animals with progressive clinical signs of lower respiratory tract disease that has not been diagnosed using less invasive means. Although thoracotomy carries a greater risk than the previously mentioned diagnostic techniques, the modern anesthetic agents, surgical techniques, and monitoring capabilities now available have made this procedure routine in many veterinary practices. Analgesic drugs are used to manage the postoperative pain, and complication-free animals are discharged as soon as 2 to 3 days after surgery. Surgical biopsy provides excellent-quality specimens for histopathologic analysis and culture. Abnormal lung tissue and accessible lymph nodes are biopsied.
Excisional biopsy of abnormal tissue can be therapeutic in animals with localized disease. Removal of localized neoplasms, abscesses, cysts, and foreign bodies can be curative. The removal of large localized lesions can improve the matching of ventilation and perfusion, even in animals with evidence of diffuse lung involvement, thereby improving the oxygenation of blood and reducing clinical signs.
In practices where thoracoscopy is available, this less invasive technique can be used for initial assessment of intrathoracic disease. Similarly, a “mini” thoracotomy through a relatively small incision can be performed. If disease is obviously disseminated throughout the lungs such that surgical intervention will not be therapeutic, biopsies of abnormal tissue can be obtained with these methods via small incisions. For patients with questionable findings or apparently localized disease, thoracoscopy or “mini” thoracotomy can be transitioned to a full thoracotomy during the same anesthesia.
BLOOD GAS ANALYSIS
Indications
The measurement of partial pressures of oxygen (Pao2) and carbon dioxide (Paco2) in arterial blood specimens provides information about pulmonary function. Venous blood analysis is less useful because venous blood oxygen pressures are greatly affected by cardiac function and peripheral circulation. Arterial blood gas measurements are indicated to document pulmonary failure, to differentiate hypoventilation from other causes of hypoxemia, to help determine the need for supportive therapy, and to monitor the response to therapy. Respiratory compromise must be severe for abnormalities to be measurable because the body has tremendous compensatory mechanisms.
TECHNIQUES
Arterial blood is collected in a heparinized syringe. Dilution of specimens with liquid heparin can alter blood gas results. Therefore commercially available syringes preloaded with lyophilized heparin are recommended (e.g., Micro ABGTM, 1-ml luer slip syringe with 25-g needle and 50 U heparin, Vital Signs, Inc). Alternatively, the procedure for heparinizing syringes as described by Hopper et al. (2005) should be followed: 0.5 ml of liquid sodium heparin is drawn into a 3-ml syringe with a 25 g needle. The plunger is drawn back to the 3 ml mark. All air is then expelled from the syringe. This procedure for expelling air and excess heparin is repeated three times.
The femoral artery is commonly used (Fig. 20-28). The animal is placed in lateral recumbency. The upper rear limb is abducted, and the rear limb resting on the table is restrained in a partially extended position. The femoral artery is palpated in the inguinal region, close to the abdominal wall, using two fingers. The needle is advanced into the artery between these fingers. The artery is thick walled and loosely attached to adjacent tissues; thus the needle must be sharp and positioned exactly on top of the artery. A short, jabbing motion facilitates entry.
FIG 20-28 Position for obtaining an arterial blood specimen from the femoral artery. The dog is in left lateral recumbency. The right rear limb is being held perpendicular to the table to expose the left inguinal area. The pulse is palpated in the femoral triangle between two fingers to accurately locate the artery. The needle is laid directly on top of the artery, then stabbed into it with a short, jabbing motion.
The dorsal pedal artery is useful for arterial collection in medium-sized and large dogs. The position of the artery is illustrated in Fig. 20-29.
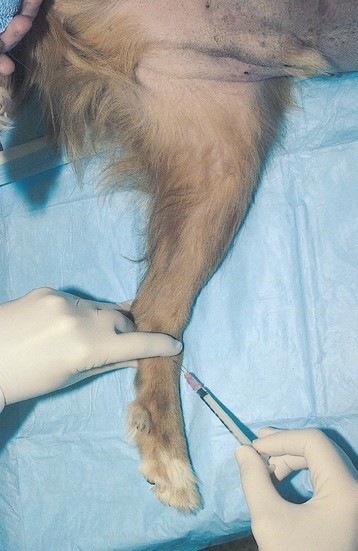
FIG 20-29 Position for obtaining an arterial blood specimen from the dorsal pedal artery. The dog is in left lateral recumbency, with the medial surface of the left leg exposed. A pulse is palpated just below the tarsus on the dorsal surface of the metatarsus between the midline and the medial aspect of the distal limb.
Once the needle has penetrated the skin, suction is applied. On entry of the needle into the artery, blood should enter the syringe quickly, sometimes in pulses. Unless the animal is severely compromised, the blood will be bright red compared with the dark red of venous blood. Dark red blood or blood that is difficult to draw into the syringe may be from a vein. Mixed samples from both the artery and vein can also be collected accidentally, particularly from the femoral site.
After removal of the needle, pressure is applied to the puncture site for 5 minutes to prevent hematoma formation. Pressure is applied even after unsuccessful attempts if there is any possibility that the artery was entered.
All air bubbles are eliminated from the syringe. The needle is covered by a cork or rubber stopper, and the entire syringe is placed in crushed ice unless the blood specimen is to be analyzed immediately. Specimens should be analyzed as soon as possible after collection. Minimal alterations occur in specimens stored on ice during the few hours required to transport the specimen to a human hospital if a blood gas analyzer is not available on site. Because of the availability of reasonably priced blood gas analyzers, point-of-care testing is now possible.
INTERPRETATION OF RESULTS
Approximate arterial blood gas values for normal dogs and cats are provided in Table 20-5. More exact values should be obtained for normal dogs and cats using the actual analyzer.
 TABLE 20-5 Approximate Ranges of Arterial Blood Gas Values for Normal Dogs and Cats Breathing Room Air
TABLE 20-5 Approximate Ranges of Arterial Blood Gas Values for Normal Dogs and Cats Breathing Room Air
| MEASUREMENT | ARTERIAL BLOOD |
|---|---|
| Pao2 (mm Hg) | 85-100 |
| Paco2 (mm Hg) | 35-45 |
| Hco3 (mmol/L) | 21-27 |
| pH | 7.35-7.45 |
Pao2 and Paco2
Abnormal Pao2 and Paco2 values can result from technical error. The animal’s condition and the collection technique are considered in the interpretation of blood gas values. For example, an animal in stable condition with normal mucous membrane characteristics being evaluated for exercise intolerance is unlikely to have a resting Pao2 of 45 mm Hg. The collection of venous blood is a more likely explanation for this abnormal value.
Hypoxemia is present if the Pao2 is below the normal range. The oxyhemoglobin dissociation curve describing the relationship between the saturated hemoglobin level and Pao2 is sigmoid in shape, with a plateau at higher Pao2 values (Fig. 20-30). Normal hemoglobin is almost totally saturated with oxygen when the Pao2 is greater than 80 to 90 mm Hg, and clinical signs are unlikely in animals with such values. The curve begins to decrease more quickly at lower Pao2 values. A value of less than 60 mm Hg corresponds to a hemoglobin saturation that is considered dangerous, and treatment for hypoxemia is indicated. (See the section on oxygen content, delivery, and utilization [p. 282] for further discussion.)
In general, animals become cyanotic when the Pao2 reaches 50 mm Hg or less, which results in a concentration of nonoxygenated (unsaturated) hemoglobin of 5 g/dl or more. Cyanosis occurs as a result of the increased concentration of nonoxygenated hemoglobin in the blood and is not a direct reflection of the Pao2. The development of cyanosis depends on the total concentration of hemoglobin, as well as the oxygen pressure; cyanosis develops more quickly in animals with polycythemia than in animals with anemia. Acute hypoxemia resulting from lung disease more often produces pallor in an animal than cyanosis. Treatment for hypoxemia is indicated for all animals with cyanosis.
Determining the mechanism of hypoxemia is useful in selecting appropriate supportive therapy. These mechanisms include hypoventilation, inequality of ventilation and perfusion within the lung, and diffusion abnormality. Hypoventilation is the inadequate exchange of gases between the outside of the body and the alveoli. The Pao2 and Paco2 are both affected by a lack of gas exchange, and hypercapnia occurs in conjunction with hypoxemia. Causes of hypoventilation are listed in Box 20-9.
The ventilation and perfusion of different regions of the lung must be matched for the blood leaving the lung to be fully oxygenated. The relationship between ventilation ( ) and perfusion (
) and perfusion ( ) can be described as a ratio (
) can be described as a ratio ( /
/ ). Hypoxemia can develop if there are regions of lung with either a low or a high
). Hypoxemia can develop if there are regions of lung with either a low or a high  /
/ .
.
Poorly ventilated portions of lung with normal blood flow have a low  /
/ . Regionally decreased ventilation occurs in most pulmonary diseases for reasons such as alveolar flooding, alveolar collapse, or small airway obstruction. The flow of blood past totally nonaerated tissue is known as a venous admixture or shunt (
. Regionally decreased ventilation occurs in most pulmonary diseases for reasons such as alveolar flooding, alveolar collapse, or small airway obstruction. The flow of blood past totally nonaerated tissue is known as a venous admixture or shunt ( /
/ of zero). The alveoli may be unventilated as a result of complete filling or collapse, resulting in physiologic shunts, or the alveoli may be bypassed by true anatomic shunts. Unoxygenated blood from these regions then mixes with oxygenated blood from ventilated portions of the lung. The immediate result is a decreased Pao2 and an increased Paco2. The body responds to the hypercapnia by increasing ventilation, effectively returning the Paco2 to normal or even lower than normal. However, the increased ventilation cannot correct the hypoxemia because blood flowing by ventilated alveoli is already maximally saturated.
of zero). The alveoli may be unventilated as a result of complete filling or collapse, resulting in physiologic shunts, or the alveoli may be bypassed by true anatomic shunts. Unoxygenated blood from these regions then mixes with oxygenated blood from ventilated portions of the lung. The immediate result is a decreased Pao2 and an increased Paco2. The body responds to the hypercapnia by increasing ventilation, effectively returning the Paco2 to normal or even lower than normal. However, the increased ventilation cannot correct the hypoxemia because blood flowing by ventilated alveoli is already maximally saturated.
Except where shunts are present, the Pao2 can be improved in dogs and cats with lung regions with low  /
/ by providing supplemental oxygen therapy administered by face mask, oxygen cage, or nasal catheter. Positive-pressure ventilation may be necessary to combat atelectasis (see Chapter 27).
by providing supplemental oxygen therapy administered by face mask, oxygen cage, or nasal catheter. Positive-pressure ventilation may be necessary to combat atelectasis (see Chapter 27).
The ventilation of areas of lung with decreased circulation (a high  /
/ ) occurs in dogs and cats with thromboembolism. Initially there may be little effect on arterial blood gas values because blood flow is shifted to unaffected regions of the lung. However, blood flow in the normal regions of the lungs increases with increasing severity of disease, and
) occurs in dogs and cats with thromboembolism. Initially there may be little effect on arterial blood gas values because blood flow is shifted to unaffected regions of the lung. However, blood flow in the normal regions of the lungs increases with increasing severity of disease, and  /
/ is decreased enough in those regions that a decreased Pao2 and a normal or decreased Paco2 occur, as described previously. Both hypoxemia and hypercapnia are seen in the setting of extremely severe embolization.
is decreased enough in those regions that a decreased Pao2 and a normal or decreased Paco2 occur, as described previously. Both hypoxemia and hypercapnia are seen in the setting of extremely severe embolization.
Diffusion abnormalities alone do not result in clinically significant hypoxemia but can occur in conjunction with  /
/ mismatching in diseases such as idiopathic pulmonary fibrosis and noncardiogenic pulmonary edema. Gas is normally exchanged between the alveoli and the blood by diffusing across the respiratory membrane. This membrane consists of the fluid lining the alveolus, alveolar epithelium, alveolar basement membrane, interstitium, capillary basement membrane, and capillary endothelium. Gases must also diffuse through plasma and red blood cell membranes. Functional and structural adaptations that facilitate diffusion between the alveoli and red blood cells provide an efficient system for this process, which is rarely affected significantly by disease.
mismatching in diseases such as idiopathic pulmonary fibrosis and noncardiogenic pulmonary edema. Gas is normally exchanged between the alveoli and the blood by diffusing across the respiratory membrane. This membrane consists of the fluid lining the alveolus, alveolar epithelium, alveolar basement membrane, interstitium, capillary basement membrane, and capillary endothelium. Gases must also diffuse through plasma and red blood cell membranes. Functional and structural adaptations that facilitate diffusion between the alveoli and red blood cells provide an efficient system for this process, which is rarely affected significantly by disease.
A-a Gradient
Hypoventilation is differentiated from  /
/ abnormalities by evaluating the Paco2 in conjunction with the Pao2. Qualitative differences are described in the preceding paragraphs: hypoventilation is associated with hypoxemia and hypercap nia, and
abnormalities by evaluating the Paco2 in conjunction with the Pao2. Qualitative differences are described in the preceding paragraphs: hypoventilation is associated with hypoxemia and hypercap nia, and  /
/ abnormalities are generally associated with hypoxemia and normocapnia or hypocapnia. It is possible to quantify this relationship by calculating the alveolar-arterial oxygen gradient (A-a gradient), which factors out the effects of ventilation and the inspired oxygen concentration on Pao2 (Table 20-6).
abnormalities are generally associated with hypoxemia and normocapnia or hypocapnia. It is possible to quantify this relationship by calculating the alveolar-arterial oxygen gradient (A-a gradient), which factors out the effects of ventilation and the inspired oxygen concentration on Pao2 (Table 20-6).
 TABLE 20-6 Relationships of Arterial Blood Gas Measurements
TABLE 20-6 Relationships of Arterial Blood Gas Measurements
| FORMULA | DISCUSSION |
|---|---|
| Pao2 ∝ Sao2 | Relationship is defined by sigmoid oxygen-hemoglobin dissociation curve. Curve plateaus at greater than 90% Sao2 with Pao2 values greater than 80 mm Hg. Curve is steep at Pao2 values of between 20 and 60 mm Hg. (Assuming normal hemoglobin, pH, temperature, and 2,3-diphosphoglycerate concentrations.) |
| Cao2 = (Sao2 × Hgb × 1.34) + (0.003 × Pao2) | Total oxygen content of blood is greatly influenced by Sao2 and hemoglobin concentration. In health, more than 60 times more oxygen is delivered by hemoglobin than is dissolved in plasma (Pao2). |
| Paco2 = PAco2 | These values are increased with hypoventilation at alveolar level and decreased with hypoventilation. |
| PAo2 = FIo2 (PB - PH2O) - Paco2/R on room air at sea level: PAo2 = 150 mm Hg - Paco2/0.8 | Partial pressure of oxygen in alveolar air available for exchange with blood changes directly with inspired oxygen concentration and inversely with Paco2. R is assumed to be 0.8 for fasting animals. With normally functioning lungs. (minimal  / / mismatch), alveolar hyperventilation results in increased PAo2 and subsequently increased Pao2, whereas hypoventilation results in decreased PAo2 and decreased Pao2. mismatch), alveolar hyperventilation results in increased PAo2 and subsequently increased Pao2, whereas hypoventilation results in decreased PAo2 and decreased Pao2. |
| A-a = PAo2 - Pao2 | A-a gradient quantitatively assesses  / / mismatch by eliminating contribution of alveolar ventilation and inspired oxygen concentration to measured Pao2. Low Pao2, with a normal A-a gradient (10 mm Hg in room air) indicates hypoventilation alone. Low Pao2 with a wide A-a gradient (>15 mm Hg in room air) indicates a component of mismatch by eliminating contribution of alveolar ventilation and inspired oxygen concentration to measured Pao2. Low Pao2, with a normal A-a gradient (10 mm Hg in room air) indicates hypoventilation alone. Low Pao2 with a wide A-a gradient (>15 mm Hg in room air) indicates a component of  / / mismatch. mismatch. |
| Paco2 ∝ 1/pH | Increased Paco2 causes respiratory acidosis; decreased Paco2 causes respiratory alkalosis. Actual pH depends on metabolic (HCO3) status as well. |
A-a, Alveolar-arterial oxygen gradient (mm Hg); Cao2, oxygen content of arterial blood (ml of O2/dl); Flo2, fraction of oxygen in inspired air (%); Hgb, hemoglobin concentration (g/dl); Paco2, partial pressure of CO2 in arterial blood (mm Hg); pa co2, partial pressure of O2 in alveolar air (mm Hg); Pa o2, partial pressure of O2 in arterial blood (mm Hg); pao2, partial pressure of O2 in alveolar air (mm Hg); Pb, barometric (atmospheric) pressure (mm Hg); pH2O, partial pressure of water in alveolar air (100% humidified) (mm Hg); pH, negative logarithm of H+ concentration (decreases with increased H+); r,respiratory exchange quotient (ratio of O2 uptake per CO2 produced); Sao2, amount of hemoglobin saturated with oxygen (%);  /
/ , ratio of ventilation to perfusion of alveoli.
, ratio of ventilation to perfusion of alveoli.
The premise of the A-a gradient is that Pao2 (a) is nearly equal (within 10 mm Hg in room air) to the partial pressure of oxygen in the alveoli, PAo2 (A), in the absence of a diffusion abnormality or  /
/ mismatch. In the presence of a diffusion abnormality or
mismatch. In the presence of a diffusion abnormality or  /
/ mismatch, the difference widens (greater than 15 mm Hg in room air). Examination of the equation reveals that hyperventilation, resulting in a lower Paco2, results in a higher PAo2. Conversely, hypoventilation, resulting in a higher Paco2, results in a lower PAo2. Physiologically the Pao2 can never exceed the PAo2, however, and the finding of a negative value indicates an error. The error may be in one of the measured values or in the assumed R value (see Table 20-6).
mismatch, the difference widens (greater than 15 mm Hg in room air). Examination of the equation reveals that hyperventilation, resulting in a lower Paco2, results in a higher PAo2. Conversely, hypoventilation, resulting in a higher Paco2, results in a lower PAo2. Physiologically the Pao2 can never exceed the PAo2, however, and the finding of a negative value indicates an error. The error may be in one of the measured values or in the assumed R value (see Table 20-6).
Clinical examples of the calculation and interpretation of the A-a gradient are provided in Box 20-10.
 BOX 20-10 Calculation and Interpretation of A-a Gradient: Clinical Examples
BOX 20-10 Calculation and Interpretation of A-a Gradient: Clinical Examples
Example 1: A healthy dog breathing room air has a PaO2 of 95 mm Hg and a Paco2 of 40 mm Hg. His calculated PAo2 is 100 mm Hg. (PAo2 = FIO2 [PB - PH2O] - Paco2/R = 0.21 [765 mm Hg - 50 mm Hg] - [40 mm Hg/0.8].) The A-a gradient is 100 mm Hg - 95 mm Hg = 5 mm Hg. This value is normal.
Example 2: A dog with respiratory depression due to an anesthetic overdose has a PaO2 of 72 mm Hg and a Paco2 of 56 mm Hg in room air. His calculated PAo2 is 80 mm Hg. The A-a gradient is 8 mm Hg. His hypoxemia can be explained by hypoventilation.
Later the same day, the dog develops crackles bilaterally. Repeat blood gas analysis shows a PaO2 of 60 mm Hg and a Paco2 of 48 mm Hg. His calculated PAo2 is 90 mm Hg. The A-a gradient is 30 mm Hg. Hypoventilation continues to contribute to the hypoxemia, but hypoventilation has improved. The widened A-a gradient indicates  /
/ mismatch. This dog has aspirated gastric contents into his lungs.
mismatch. This dog has aspirated gastric contents into his lungs.
Oxygen Content, Delivery, and Utilization
The commonly reported blood gas value Pao2 reflects the pressure of oxygen dissolved in arterial blood. This value is critical for assessing lung function. However, the clinician must remember that other variables are involved in oxygen delivery to the tissues besides Pao2 and that tissue hypoxia can occur in spite of a normal Pao2. The formula for calculating the total oxygen content of arterial blood (Cao2) is provided in Table 20-6. The greatest contribution to Cao2 in health is oxygenated hemoglobin. In a normal dog (Pao2, 100 mm Hg; hemoglobin, 15 g/dl), oxygenated hemoglobin accounts for 20 ml of O2/dl, whereas dissolved oxygen accounts for only about 0.3 ml of O2/dl.
The quantity of hemoglobin is routinely appraised by the complete blood count. It can also be estimated on the basis of the packed cell volume (by dividing the packed cell volume by 3). The oxygen saturation of hemoglobin (SaO2) is dependent on the Pao2, as depicted by the sigmoid shape of the oxygen-hemoglobin dissociation curve (see Fig. 20-30). However, the SaO2 is also influenced by other variables that can shift the oxygen-hemoglobin dissociation curve to the left or right (e.g., pH, temperature, or 2,3-diphosphoglycerate concentrations) or interfere with oxygen binding with hemoglobin (e.g., carbon monoxide toxicity or methemoglobinemia). Some laboratories measure SaO2.
Oxygen must also be successfully delivered to the tissues, and this depends on cardiac output and local circulation. Ultimately, the tissues must be able to effectively utilize the oxygen—a process interfered with in the presence of toxicities such as carbon monoxide or cyanide poisoning. Each of these processes must be considered when interpreting the blood gas values in an individual animal.
Acid-Base Status
The acid-base status of an animal can also be assessed using the same blood sample as that used to measure blood gases. Acid-base status is influenced by the respiratory system (see Table 20-6). Respiratory acidosis results if carbon dioxide is retained as a result of hypoventilation. If the problem persists for several days, compensatory retention of bicarbonate by the kidneys occurs. Excess removal of carbon dioxide by the lungs caused by hyperventilation results in respiratory alkalosis. Hyperventilation is usually an acute phenomenon, potentially caused by shock, sepsis, severe anemia, anxiety, or pain; therefore compensatory changes in the bicarbonate concentration are rarely seen.
The respiratory system partially compensates for primary metabolic acid-base disorders, and this can occur quickly. Hyperventilation and a decreased Paco2 occur in response to metabolic acidosis. Hypoventilation and an increased Paco2 occur in response to metabolic alkalosis.
In most cases, acid-base disturbances can be identified as primarily respiratory or primarily metabolic in nature on the basis of the pH. The compensatory response will never be excessive and alter the pH beyond normal limits. An animal with acidosis (pH of less than 7.35) has a primary respiratory acidosis if the Paco2 is increased and a compensatory respiratory response if the Paco2 is decreased. An animal with alkalosis (pH of greater than 7.45) has a primary respiratory alkalosis if the Paco2 is decreased and a compensatory respiratory response if the Paco2 is increased.
If both the Paco2 and the bicarbonate concentration are abnormal, such that both contribute to the same alteration in pH, a mixed disturbance is present. For instance, an animal with acidosis, an increased Paco2, and a decreased HCO3 has a mixed metabolic and respiratory acidosis.
PULSE OXIMETRY
Indications
Pulse oximetry is a method of monitoring the oxygen saturation of blood. The saturation of hemoglobin with oxygen is related to the Pao2 by the sigmoid oxygen-hemoglobin dissociation curve (see Fig. 20-30). Pulse oximetry is noninvasive, can be used to continuously monitor a dog or cat, provides immediate results, and is affordable for most practices. It is a particularly useful device for monitoring animals with respiratory disease that must undergo procedures requiring anesthesia. It can also be used in some cases to monitor the progression of disease or the response to therapy. More and more clinicians are using these devices for the routine monitoring of animals under general anesthesia, particularly if the number of personnel is limited, because alarms can be set to warn of marked changes in values.
METHODOLOGY
Most pulse oximeters have a probe that is attached to a fold of tissue, such as the tongue, lip, ear flap, inguinal skin fold, toe, or tail (Fig. 20-31). This probe measures light absorption through the tissues. Other models measure reflected light and can be placed on mucous membranes or within the esophagus or rectum. Artifacts resulting from external light sources are minimized in the latter sites. Arterial blood is identified by the oximeter as that component which changes in pulses. Nonpulsatile absorption is considered background.
INTERPRETATION
Values provided by the pulse oximeter must be interpreted with care. The instrument must record a pulse that matches the palpable pulse of the animal. Any discrepancy between the actual pulse and the pulse received by the oximeter indicates an inaccurate reading. Common problems that can interfere with the accurate detection of pulses include the position of the probe, animal motion (e.g., respirations, shivering), and weak or irregular pulse pressures (e.g., tachycardia, hypovolemia, hypothermia, arrhythmias).
The value measured indicates the saturation of hemoglobin in the local circulation. However, this value can be affected by factors other than pulmonary function, such as vasoconstriction, low cardiac output, and the local stasis of blood. Other intrinsic factors that can affect oximetry readings include anemia, hyperbilirubinemia, carboxyhemoglobinemia, and methemoglobinemia. External lights and the location of the probe can also influence results. Pulse oximetry readings of oxygen saturation are less accurate below values of 80%.
These sources for error should not discourage the clinician from using this technology, however, because changes in saturation in an individual animal provide valuable information. Rather, results must be interpreted critically.
The examination of the oxygen-hemoglobin dissociation curve (see Fig. 20-30) in normal dogs and cats shows that animals with Pao2 values exceeding 85 mm Hg will have a hemoglobin saturation greater than 95%. If Pao2 values decrease to 60 mm Hg, the hemoglobin saturation will be approximately 90%. Any further decrease in Pao2 results in a precipitous decrease in hemoglobin saturation, illustrated by the steep portion of the oxygen-hemoglobin dissociation curve. Ideally, then, hemoglobin saturation should be maintained at more than 90% by means of oxygen supplementation or ventilatory support (see Chapter 27) or the specific treatment of the underlying disease. However, because of the many variables associated with pulse oximetry, such strict guidelines are not always valid. In practice, a baseline hemoglobin saturation value is measured and subsequent changes in that value are then used to assess improvement or deterioration in oxygenation. Ideally, the baseline value is compared with the Pao2 obtained from an arterial blood sample collected concurrently to ensure the accuracy of the readings.
Bauer TG. Lung biopsy. Vet Clin North Am Small Anim Pract. 2000;30:1207.
Bowman DD, et al. Georgis’ parasitology for veterinarians, ed 7. Philadelphia: WB Saunders, 1999.
Faunt KK, et al. Evaluation of biopsy specimens obtained during thoracoscopy from lungs of clinically normal dogs. Am J Vet Res. 1998;59:1499.
Hardie EM, et al. Tracheal rupture in cats: 16 cases (1983–1998). J Am Vet Med Assoc. 1999;214:508.
Hawkins EC, et al. Bronchoalveolar lavage in the evaluation of pulmonary disease in the dog and cat. J Vet Intern Med. 1990;4:267.
Hawkins EC, et al. Cytologic characterization of bronchoalveolar lavage fluid collected through an endotracheal tube in cats. Am J Vet Res. 1994;55:795.
Hawkins EC, et al. Cytological analysis of bronchoalveolar lavage fluid in the diagnosis of respiratory tract disease in dogs: a retrospective study. J Vet Intern Med. 1995;9:386.
Hawkins EC, et al. Use of a modified stomach tube for bronchoalveolar lavage in dogs. J Am Vet Med Assoc. 1999;215:1635.
Hawkins EC. Bronchoalveolar lavage. In: King LG, editor. Textbook of respiratory disease in dogs and cats. St Louis: Elsevier, 2004.
Hendricks JC, et al. Practicality, usefulness, and limits of pulse oximetry in critical small animal patients. Vet Emerg Crit Care. 1993;3:5.
Hopper K, et al. Assessment of the effect of dilution of blood samples with sodium heparin on blood gas, electrolyte, and lactate measurements in dogs. Am J Vet Res. 2005;66:656.
Kirschvink N, et al. Bronchodilators in bronchoscopy-induced airflow limitation in allergen-sensitized cats. J Vet Intern Med. 2005;19:161.
Kneller SK. Thoracic radiography. In: Kirk RW, editor. Current veterinary therapy IX. Philadelphia: WB Saunders, 1986.
Kuehn NF, et al. Bronchoscopy. In: King LG, editor. Textbook of respiratory disease in dogs and cats. St Louis: Elsevier, 2004.
McKiernan BC. Bronchoscopy. In: McCarthy TC, et al, editors. Veterinary endoscopy for the small animal practitioner. St Louis: Elsevier, 2005.
Neath PJ, et al. Lung lobe torsion in dogs: 22 cases (1981–1999). J Am Vet Med Assoc. 2000;217:1041.
Nemanic S, et al. Comparison of thoracic radiographs and single breath-hold helical CT for detection of pulmonary nodules in dogs with metastatic neoplasia. J Vet Intern Med. 2006;20:508.
Norris CR, et al. Use of keyhole lung biopsy for diagnosis of interstitial lung diseases in dogs and cats: 13 cases (1998-2001). J Am Vet Med Assoc. 2002;221:1453.
Peeters DE, et al. Quantitative bacterial cultures and cytological examination of bronchoalveolar lavage specimens from dogs. J Vet Intern Med. 2000;14:534.
Reinemeyer CR. Parasites of the respiratory tract. In: Bonagura JD, et al, editors. Current veterinary therapy XII. Philadelphia: WB Saunders, 1983.
Shaw DH, et al. Eosinophilic bronchitis caused by Crenosoma vulpis infection in dogs. Can Vet J. 1996;37:361.
Suter PF. Thoracic radiography. Wettswil, Switzerland: Peter F Suter, 1984.
Teske E, et al. Transthoracic needle aspiration biopsy of the lung in dogs with pulmonic disease. J Am Anim Hosp Assoc. 1991;27:289.
Urquhart GM, et al. Veterinary parasitology, ed 2. Oxford: Blackwell Science, 1996.
West JB. Respiratory physiology: the essentials, ed 7. Baltimore: Lippincott, Williams & Wilkins, 2004.
West JB. Pulmonary pathophysiology: the essentials, ed 6. Baltimore: Lippincott, Williams & Wilkins, 2003.