CHAPTER 46 Canine Urolithiasis
GENERAL CONSIDERATIONS
Canine urine is a complex solution in which salts (e.g., calcium oxalate, magnesium ammonium phosphate) can remain in solution under conditions of supersaturation. However, supersaturated urine has a potential energy of precipitation, or the tendency to form solids from the dissolved salts. Crystalluria is a consequence of urine supersaturation, and uroliths may form if crystals aggregate and are not excreted. Uroliths may damage the uroepithelium and result in urinary tract inflammation (hematuria, pollakiuria, dysuria-stranguria). They may also predispose the animal to the development of a bacterial urinary tract infection (UTI). If uroliths lodge in the ureters or urethra, urine flow may be obstructed.
Most uroliths in dogs are found in the bladder or urethra; only about 5% are located in the kidneys or ureters. Uroliths are usually named according to their mineral content. Recent data collected at the College of Veterinary Medicine of the University of Minnesota have shown that approximately 38% of canine uroliths are struvite (magnesium ammonium phosphate), 42% are calcium oxalate, 5% are urate, 1% are silicate, 1% are cystine, and 14% are mixed or compound uroliths (i.e., the urolith contains less than 70% of any one mineral type). Crystalline aggregates constitute approximately 95% of the urolith weight, and an organic matrix composed of protein and mucoprotein complexes may constitute as much as 5%. Factors associated with particular types of uroliths are summarized in Table 46-1.
Etiology and Pathogenesis
Conditions that contribute to the crystallization of salts and the formation of uroliths include a sufficiently high concentration of salts in the urine, adequate time in the urinary tract (urinary retention of salts and crystals), a urine pH favorable for salts to crystallize, a nucleation center or nidus on which crystallization can occur, and decreased concentrations of crystallization inhibitors in the urine. The combination of a high dietary intake of minerals and protein and the ability of dogs to produce relatively highly concentrated urine contributes to the supersaturation of urine with salts. In some cases decreased tubular resorption (e.g., calcium, cystine, uric acid) or an increased production secondary to bacterial infection (e.g., ammonium and phosphate ions) also contributes to this supersaturation.
Several theories exist concerning the pathogenesis of uroliths. In the precipitation-crystallization theory, the supersaturation of urine with salts is thought to be the primary factor responsible for initiating nidus formation and sustaining the growth of the urolith. Normal canine urine is supersaturated with several salts. However, the greater the concentration of salts in urine and the less often voiding occurs (e.g., decreased water intake), the greater the chance of urolith formation. Supersaturated urine has a potential energy of precipitation, or a driving force that favors crystal formation. The greater the magnitude of the supersaturation, the greater the potential for crystallization to occur. Conversely, undersaturated solutions have a potential energy of dissolution, such that previously formed crystals dissolve at a rate proportional to the degree of undersaturation.
In other theories of urolith formation, it is thought that substances in urine may promote or inhibit crystal formation. For example, in the matrix nucleation theory an organic matrix substance in urine is thought to promote initial nidus formation. This matrix substance may be albumin, globulin, Tamm-Horsfall mucoprotein, or an immunologically unique hydroxyproline-deficient protein called matrix substance A. The proteinaceous matrix substance may promote crystallization by providing a surface where crystallization can occur and by binding crystals together, which may increase their urinary retention. According to another theory, the crystallization inhibitor theory, the absence of a critical inhibitor of crystal formation is considered to be the primary factor that allows initial nidus formation. Examples of crystallization inhibitors are citrates, glycosaminoglycans, and pyrophosphates. Decreased concentrations of these substances in urine may facilitate spontaneous crystallization and urolith growth. The extent to which promoters and inhibitors of crystallization are involved in urolith formation in dogs is unknown. In all cases, however, supersaturation of the urine with urolith constituents is essential for uroliths to form.
Struvite uroliths.
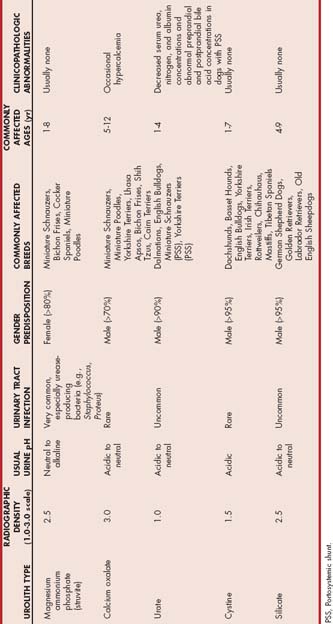
Struvite or magnesium ammonium phosphate uroliths are common uroliths in dogs (Fig. 46-1). Uroliths that predominantly consist of struvite may also contain a small amount of calcium phosphate (hydroxyapatite) or calcium carbonate. Because most canine diets are rich in minerals and protein, canine urine frequently becomes supersaturated with magnesium, ammonium, and phosphate; however, a UTI is an important factor predisposing to the formation of struvite uroliths in dogs and Staphylococcus and Proteus are commonly associated pathogens. These bacteria contain urease and are capable of splitting urea into ammonia and carbon dioxide. Hydroxyl and ammonium ions are formed by the hydrolysis of ammonia, which decreases hydrogen ion concentrations in urine, resulting in an alkaline urine and decreased struvite solubility. The hydrolysis of urea increases the urine concentrations of ammonium and phosphate (a result of the increased dissociation of phosphorus) ions, which augments urine supersaturation. High urine ammonia concentrations may also damage glycosaminoglycans that prevent bacteria from adhering to the urinary mucosa. Bacterial cystitis also increases the amount of organic debris available as a crystallization surface. Because of their high association with UTIs, struvite uroliths are more common in female dogs (80% to 97% of uroliths in female dogs are struvite). Uroliths in dogs younger than 1 year of age are usually struvite and are also frequently associated with a UTI.

FIG 46-1 A, Typical appearance of struvite stones, although struvite stones may also be jack shaped (B).
(B courtesy Dr. Howard Seim, Colorado State University.)
The factors involved in the pathogenesis of struvite uroliths in sterile urine are not known; however, the struvite uroliths that form in cats usually do so in the absence of a UTI. A greater urine-concentrating ability, and therefore a greater degree of urine supersaturation, may be partially responsible for causing uroliths to form in cats and in those dogs without UTIs. In addition, a consistently high urine pH in the absence of a UTI (potentially caused by drugs, diet, or renal tubular disorders) may facilitate struvite urolith formation.
Although struvite uroliths may occur in any breed, those most commonly affected include Miniature Schnauzers, Miniature Poodles, Bichon Frises, and Cocker Spaniels. The high prevalence of struvite uroliths in Cocker Spaniels has led to the suggestion that there is a familial predisposition in this breed (see Table 46-1). Uroliths larger than 1 cm in any dimension are likely to be struvite. In addition, struvite uroliths found in the urinary bladder are most likely to be smooth, blunt-edged or faceted, or pyramidal.
Calcium oxalate uroliths.
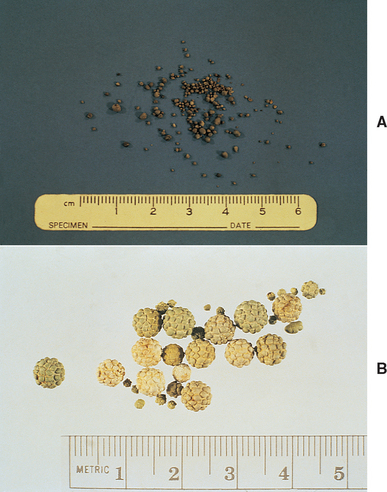
Calcium oxalate uroliths in dogs are often the monohydrate (whewellite) form (Fig. 46-2, A; see also Fig. 41-3) rather than the dihydrate (weddellite) form (see Figs. 41-4 and 46-2, B). The factors involved in the pathogenesis of calcium oxalate urolithiasis in dogs are not completely understood but frequently involve increased concentrations of calcium in the urine. Hypercalciuria probably occurs most commonly in dogs postprandially and is associated with increased absorption of calcium from the gut. Another potential cause of hypercalciuria is the defective tubular resorption of calcium. Hypercalciuria may also occur secondary to overt hypercalcemia (e.g., that resulting from primary hyperparathyroidism, neoplasia, or vitamin D intoxication); however, this is thought to be an infrequent cause of calcium oxalate uroliths. Treatment with certain drugs (e.g., glucocorticoids, furosemide) as well as dietary supplementation with calcium or sodium chloride may also result in hypercalciuria. An association between hyperadrenocorticism and the development of calcium-containing uroliths has also been identified in dogs. Finally, decreased urine concentrations of glycosaminoglycans, Tamm-Horsfall protein, osteopontine, and/or citrate, which are calcium oxalate crystallization inhibitors, or defective urinary nephrocalcin or increased dietary intake of oxalate (e.g., vegetables, grass, vitamin C) may play a role in the pathogenesis of calcium oxalate urolithiasis in some dogs. The overall prevalence of calcium oxalate uroliths in dogs has increased significantly over the past 10 years and may be related to the increased use of urine-acidifying diets or other unidentified environmental factors.
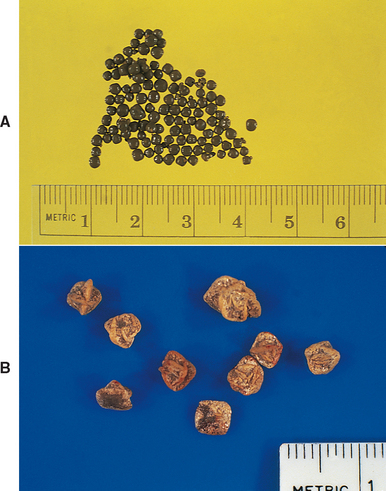
FIG 46-2 Typical appearance of monohydrate calcium oxalate stones (A) and dihydrate calcium oxalate stones (B).
Approximately 70% of calcium oxalate uroliths are found in male dogs, and Miniature and Standard Schnauzers, Miniature Poodles, Yorkshire Terriers, Lhasa Apsos, Bichon Frises, and Shih Tzus are the breeds commonly affected. Obesity also appears to increase the risk of calcium oxalate urolithiasis. The increased prevalence in male dogs may be related to an increase in the hepatic production of oxalate mediated by testosterone. Conversely, estrogens in female dogs may increase the urinary excretion of citrate. Calcium oxalate uroliths frequently occur in older dogs (mean age: 8 to 12 years), and a concurrent UTI appears to be rare. Calcium oxalate solubility is increased in urine with a pH above 6.5, whereas a urine pH of less than 6.5 favors calcium oxalate crystal formation.
Urate uroliths.
Most urate uroliths are composed of ammonium acid urate; 100% uric acid and sodium urate uroliths are relatively rare (Fig. 46-3). Uric acid is derived from the metabolic degradation of endogenous purine ribonucleotides and dietary nucleic acids. It is hypothesized that the hepatic transport of uric acid is defective in Dalmatians and some English Bulldogs because uric acid conversion to allantoin has been found to be decreased in them, even though hepatocyte uricase activities are often adequate. The decreased production of allantoin seen in these breeds results in the increased urinary excretion of uric acid. Normally, allantoin, which is produced through the oxidation of uric acid by uricase, is the major metabolite generated during purine metabolism. In comparison with uric acid, allantoin is quite soluble in urine.
In addition to a decreased hepatic metabolism of uric acid, the proximal tubular resorption of uric acid appears to be decreased in Dalmatians. This increases the uric acid and sodium urate (the salt of uric acid) concentrations in urine. Although urinary uric acid excretion in Dalmatians is approximately 10 times that of other dogs, urate stones form in only a small percentage. For unknown reasons, male Dalmatians are at greater risk of having urate stones than are female Dalmatians. In one published study the male : female ratio for urate stone–forming Dalmatians was reported to be 16.4 : 1. Approximately 60% of urate uroliths occur in Dalmatians, and, conversely, approximately 75% of the uroliths in Dalmatians are urate uroliths. In addition to Dalmatians, English Bulldogs have an increased incidence of urate uroliths.
Another possible cause of urate stone formation is a decreased glycosaminoglycan concentration in the urine. Glycosaminoglycans in urine may combine with urate salts, resulting in an overall negative charge and reduced crystallization. High dietary protein is usually associated with an increase in the urinary excretion of both uric acid and ammonium ions. Ammonia, which is produced by renal tubular cells from glutamine, diffuses into the tubular lumen and serves as a buffer for secreted hydrogen ions, thereby forming ammonium ions. Ammonium ions are relatively lipid insoluble and therefore become trapped within the tubular fluid. Uric acid crystallization is facilitated in acidic urine, whereas an alkaline urine appears to favor ammonium urate crystallization. Ammonium acid urate stones may also form in any dog with hepatic insufficiency (e.g., hepatic cirrhosis, microvascular dysplasia, or portosystemic shunt [PSS]) as a result of increased renal excretion of ammonium urates. PSSs are common in Miniature Schnauzers, Yorkshire Terriers, and Pekingese dogs; therefore ammonium acid urate uroliths are more common in these breeds. UTIs, especially those with urease-producing bacteria, may facilitate ammonium acid urate crystallization by increasing urine ammonia concentrations. A UTI may also occur secondary to urolith-induced mucosal irritation.
Silicate uroliths.
Silicate uroliths were first reported in the United States in 1976 in association with crystallographic analysis of uroliths. Silicate uroliths frequently, but not always, have a jack shape (Fig. 46-4), although not all jackstones are silicates (ammonium urate and struvite uroliths may also be jack shaped; see Fig. 46-1, B). The factors responsible for the pathogenesis of silicate uroliths are unknown, but their formation is probably related to the dietary intake of silicates, silicic acid, or magnesium silicate. There appears to be a link between the formation of silicate uroliths and the consumption of large amounts of corn gluten or soybean hulls, which can be high in silicates. Many of the reported silicate uroliths in the United States have occurred in male German Shepherd Dogs, Old English Sheepdogs, and Golden and Labrador Retrievers. Most silicate uroliths are diagnosed in dogs 6 to 8 years of age. Alkaline urine appears to increase silicate solubility, and secondary UTIs may occur as a result of mucosal irritation caused by these jack-shaped uroliths.
Cystine uroliths.
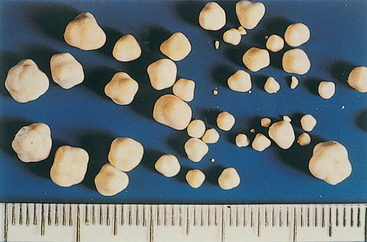
Cystinuria, an inherited disorder of renal tubular transport, is thought to be the primary cause of cystine uroliths. The tubular resorptive defect involves cystine and, in some cases, other amino acids (tubular resorption of cysteine, the immediate precursor of cystine, glycine, ornithine, carnitine, arginine, and lysine, may also be decreased). Although the plasma cystine concentrations are normal in these dogs, the concentration of plasma methionine, a precursor of cystine, may be increased. Plasma cystine is freely filtered through the glomeruli and is actively resorbed by proximal tubular epithelial cells in normal dogs. Were it not for the relative insolubility of cystine in urine and the potential for uroliths to form, cystinuria would be of little consequence. Cystine is most soluble in alkaline solutions; therefore cystine stones usually form in acidic urine. Interestingly, cystine uroliths do not form in all dogs with cystinuria; therefore cystinuria is a predisposing, rather than a primary, causative factor. Cystine uroliths (Fig. 46-5) are most frequently observed in male dogs, and Dachshunds are the breed principally affected, but Basset Hounds, Tibetan Spaniels, English Bulldogs, Yorkshire Terriers, Irish Terriers, Chihuahuas, Mastiffs, and Rottweilers also appear to be at increased risk for cystine urolithiasis.
For unknown reasons, cystine uroliths usually do not form in young dogs; the average age at detection is 3 to 6 years. The prevalence of cystine urolithiasis in dogs in the United Kingdom has been reported to be much higher than that seen in dogs in the United States, probably reflecting the increased popularity of affected breeds in the United Kingdom. UTIs may occur secondarily; however, infection is not thought to play a primary role in the pathogenesis of cystine uroliths.
Clinical Features and Diagnosis
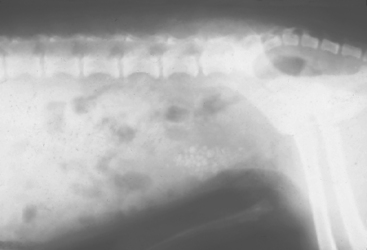
The clinical features of urolithiasis depend on the number, type, and location of the stones in the urinary tract. Because most uroliths are located in the urinary bladder, clinical signs of cystitis (hematuria, pollakiuria, dysuria-stranguria) are frequently observed. Mucosal irritation is relatively severe in dogs with jack-shaped uroliths, as opposed to that seen in dogs with solitary, smooth stones. Incomplete voiding (i.e., urine retention), mucosal hyperplasia leading to polyp formation, and sequestration of bacteria within the stone are additional complications associated with urolithiasis. In male dogs smaller uroliths may pass into the urethra, causing partial or complete obstruction with signs of bladder distention, dysuria-stranguria, and postrenal azotemia (depression, anorexia, vomiting). Uroliths frequently lodge in the male urethra at the caudal aspect of the os penis (Fig. 46-6). Occasionally, the urinary bladder or urethra may rupture and result in an abdominal effusion or subcutaneous perineal fluid accumulation and postrenal azotemia. Animals with unilateral renal uroliths may be asymptomatic, or they may have hematuria and chronic pyelonephritis. Frequently, chronic kidney disease develops in animals with bilateral renal uroliths, especially if pyelonephritis is also present. Dogs with ureteral uroliths may also be asymptomatic, or they may have hematuria and abdominal pain. Unilateral obstruction of a ureter often results in unilateral hydronephrosis without evidence of decreased renal function.
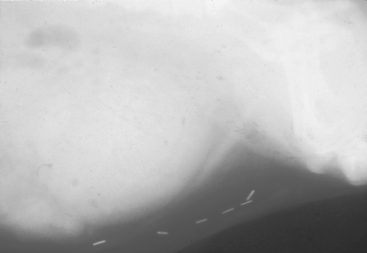
FIG 46-6 Radiograph of a male dog with an opaque urethral calculus at the caudal aspect of the os penis. Note the distended bladder associated with the obstructive uropathy and the staples from a previous cystotomy for urolith removal.
Canine urolithiasis is usually diagnosed on the basis of a combination of historical, physical examination, and radiographic or ultrasonographic findings (Fig. 46-7). In male dogs with dysuria and stranguria caused by urethral stones, attempted passage of a urinary catheter will often be met with a “gritty feeling” of resistance. Regardless of the ease of catheter passage, the diagnosis can usually be confirmed with retrograde positive contrast–enhanced urethrography. In some cases cystouroliths can be detected during abdominal palpation in dogs with signs of cystitis. Plain film radiographs will usually confirm the presence of cystouroliths unless the stones are radiolucent or very small. Doublecontrast–enhanced cystography is a more sensitive diag-nostic tool for detecting radiolucent cystouroliths. Finally, ultrasonography can be used to visualize radioopaque or radiolucent uroliths and is the imaging method of choice for diagnosing renoliths and hydronephrosis-hydroureter that can be associated with renoliths.
Treatment
General principles for the treatment of urolithiasis include the relief of any urethral obstruction and decompression of the bladder, if necessary. This can usually be accomplished by the passage of a small-bore catheter, cystocentesis, or dislodgment of the urethral calculi by retrograde hydropulsion. Only rarely will an emergency urethrotomy be necessary. Fluid therapy should be initiated to restore water and electrolyte balance if postrenal azotemia exists. Hyperkalemia is a potentially life-threatening electrolyte disturbance that may occur in association with postrenal azotemia caused by urethral obstruction or rupture of the urinary bladder or urethra. The serum potassium concentration as well as the blood urea nitrogen and creatinine concentrations should be measured in patients with a suspected obstruction. Alternatively, bradycardia and electrocardiographic findings of flattened P waves, a prolonged PR interval, widened QRS complexes, and tall or spiked T waves are suggestive of hyperkalemia and indicate the need for aggressive treatment to lower the serum potassium concentration. Hyperkalemia should be promptly treated according to the regimen outlined in Box 46-1.
 BOX 46-1 Electrocardiographic Findings and Treatment Recommendations for Dogs and Cats with Hyperkalemia
BOX 46-1 Electrocardiographic Findings and Treatment Recommendations for Dogs and Cats with Hyperkalemia
ECG, Electrocardiogram; IV, intravenous.
The medical dissolution of struvite, urate, and cystine uroliths has been shown to be effective (Table 46-2); however, the choice between the surgical removal of uroliths and medical dissolution is not always clear. Disadvantages of surgery include the need for anesthesia, the invasiveness of the procedure (potential surgical complications), the possibility of incomplete removal of uroliths, and the persistence of underlying causes. Inasmuch as the underlying cause is usually not eliminated, surgery typically does not lead to a decrease in the rate of urolith recurrence. Advantages of surgery include the fact that the urolith type can be definitively diagnosed, any concurrent or predisposing anatomic abnormalities (e.g., urachal remnants, urinary bladder polyps) can be corrected, and urinary bladder mucosal samples can be obtained for bacterial culture if the urine yields no growth on culture.
 TABLE 46-2 Treatment and Prevention of Urolithiasis in Dogs
TABLE 46-2 Treatment and Prevention of Urolithiasis in Dogs
| UROLITH TYPE | TREATMENT OPTIONS | PREVENTION |
|---|---|---|
| Struvite | ||
| Calcium oxalate | Surgical removal | Hill’s u/d diet |
| Urate | ||
| Silicate | Surgical removal | |
| Cystine |
BUN, Blood urea nitrogen.
Medical treatment decreases the concentration of calculogenic salts in the urine, increases salt solubility in urine, and increases urine volume, which produces urine with a lower concentration of calculogenic salts. The major disadvantage of the medical treatment of urolithiasis is that considerable owner compliance is required for several weeks to months. The cost of medical dissolution is comparable to the cost of surgery because multiple urinalyses, bacterial cultures, and frequent radiographs are required for follow-up. Animals with urolith-induced obstructive uropathy cannot be treated medically, and some uroliths (calcium oxalate, calcium phosphate, silicate, and mixed-composition uroliths) do not respond to medical dissolution. In addition to the medical dissolution of uroliths, voiding urohydropropulsion or catheter urolith retrieval can be used to remove cystouroliths nonsurgically in some animals (Box 46-2; see also Lulich et al., 1992, 1993, for detailed instructions). Lithotripsy, available at some referral centers, has also been used successfully to treat nephroliths and, less commonly, ureteroliths in dogs.
 BOX 46-2 Guidelines for Urohydropropulsion
BOX 46-2 Guidelines for Urohydropropulsion
Uroliths must be smaller than the smallest urethral diameter.
Smooth uroliths will pass more readily than those with irregular surfaces.
General preventive measures to be taken in addition to the surgical or medical management of uroliths include the induction of diuresis and the eradication of UTIs. Diuresis is important because it lowers the urine specific gravity and the urinary concentration of calculogenic salts. Feeding canned food will help increase water intake. In general, the maintenance of a urine specific gravity of less than 1.020 is ideal, and dogs should be allowed frequent opportunities to void. The urine sediment and pH should be monitored routinely, and UTIs should be treated pro-mptly on the basis of bacterial culture and sensitivity results (see specific instructions in discussion of each type of urolith).
Struvite uroliths.
Struvite uroliths can usually be dissolved by feeding the animal a struvite dissolution diet (e.g., Hill’s Canine Prescription Diet s/d and Royal Canin canine URINARY SO). It takes an average of 8 to 10 weeks (range: 2 weeks to 7 months) for struvite uroliths to be dissolved in this way. The rate at which uroliths dissolve is proportional to the surface area of the urolith exposed to the undersaturated urine and the presence or absence of a UTI (sterile struvite uroliths will dissolve more rapidly than those associated with a UTI). These diets should not be fed routinely as a maintenance diet and should not be used in pregnant, lactating, or growing animals or after surgery because wound healing may be compromised as a result of the restricted protein in the diet. In addition, because of its high salt content, struvite dissolution diets should not be fed to dogs with congestive heart failure, hypertension, or nephrotic syndrome. In Miniature Schnauzers, the high fat content of the s/d diet may exacerbate any lipid abnormalities and increase the risk of pancreatitis; in this case Hill’s Prescription Diet w/d may be used. The dissolution diet should be fed for a minimum of 30 days after the calculi are no longer visible radiographically. It should be noted that these diets will not dissolve nonstruvite uroliths and will not be effective if a UTI persists or if the animal is fed anything in addition to the dissolution diet. Lack of owner compliance with the dietary recommendations (i.e., instructions to feed the dissolution diet only) is indicated if the serum urea nitrogen concentrations remain greater than 10 mg/dl after the diet has been initiated.
In addition to decreasing the concentration of crystalloids in the urine, the elimination of any bacterial UTI is an essential part of the medical treatment of struvite urolithiasis. If infection is present at the start of treatment, antibiotics should be continued throughout the course of the medical dissolution treatment to destroy viable bacteria that may be liberated from the urolith as it dissolves. Antibiotics should be selected on the basis of urine culture and sensitivity findings; in cases of severe or persistent UTIs caused by urease-producing bacteria, the urease inhibitor acetohydroxamic acid (Lithostat; Mission Pharmacal, San Antonio, Texas) may be added to the treatment, but it is rarely needed. At a dose of 12.5 mg/kg, administered orally q12h, it may help dissolve struvite uroliths that are resistant to antibiotic and dietary treatment. Adjunctive treatment with urinary acidifiers in conjunction with the struvite dissolution diets is usually not recommended. The most common causes of alkaline urine during diet treatment are a persistent bacterial infection and lack of dietary compliance. The medical treatment of sterile struvite uroliths is the same as that described in previous paragraphs, except that antibiotics are not necessary.
Measures to prevent the recurrence of struvite uroliths include preventing and controlling UTIs, maintaining an acidic urine, and decreasing the dietary intake of calculogenic salts. Hill’s Canine Prescription Diet c/d is a good maintenance diet to prevent sterile struvite urolith recurrence because the protein, magnesium, calcium, and phosphorous content is only moderately restricted and it produces an acidic urine. In dogs with recurrent UTIs, predisposing abnormalities (e.g., urachal remnant, urinary bladder polyp) should be identified or ruled out with double-contrast–enhanced cystography or ultrasonography. Otherwise, silent hyperadrenocorticism may also result in recurrent UTI (see Chapter 45). Occasionally, long-term, lower-dose prophylactic antibiotic treatment may be necessary to prevent recurrent UTIs. Routine urinalyses should be performed every 2 to 4 months in asymptomatic animals, and follow-up urine cultures performed in animals with clinical signs of lower urinary tract inflammation.
Calcium oxalate uroliths.
A medical treatment for the dissolution of oxalate urolithiasis has not yet been developed. A moderate restriction of protein, calcium, oxalate, and sodium intake, with a normal intake of phosphorus, magnesium, and vitamins C and D, is recommended to prevent recurrence of calcium oxalate uroliths after surgical removal (e.g., Hill’s Canine Prescription Diet u/d is recommended for this). Increased dietary sodium intake may result in an increase in the urinary excretion of calcium and therefore should be avoided. Potassium citrate, given orally, may help prevent recurrence of calcium oxalate uroliths because citrate complexes with calcium, thereby forming a relatively soluble calcium citrate. In addition, it results in mild urine alkalinization, which increases the solubility of calcium oxalate. However, because overzealous urine alkalinization may result in the formation of calcium phosphate uroliths, this should be avoided. The recommended dose of potassium citrate is 40 to 75 mg/kg, administered orally q12h. Thiazide diuretics have also been recommended to decrease the urinary excretion of calcium; hydrochlorothiazide (2 mg/kg, administered orally q12h) has been shown to reduce urine calcium excretion in dogs. This effect was enhanced by combining the treatment with the u/d diet.
Urate uroliths.
The medical dissolution of urate uroliths that are not associated with hepatic insufficiency (e.g., PSSs) should include a diet low in protein and nucleic acids, alkalinization of the urine, xanthine oxidase inhibition, and the elimination of UTIs. Hill’s Canine Prescription Diet u/d has a reduced protein and purine content and produces alkaline urine; therefore it is recommended for the dissolution and prevention of urate uroliths. The u/d diet decreases the hepatic formation of urea and hence renal medullary hypertonicity and urine-concentrating ability. In addition, allopurinol, a competitive inhibitor of the enzyme xanthine oxidase, which converts hypoxanthine to xanthine and xanthine to uric acid (Fig. 46-8), should be administered orally at a dose of 10 to 15 mg/kg q12h or once daily, and, if necessary, sodium bicarbonate or potassium citrate should be administered orally to maintain a urine pH of 7.0. The dose of the urine alkalinizer has to be individualized for each animal. Potassium citrate is available in a wax matrix tablet (Urocit-K; Mission Pharmacal, San Antonio, Texas). Treatment can be started with a one-quarter tablet q8h and the dosage adjusted up or down based on the urine pH. Higher doses of allopurinol especially if combined with higher protein diets, increase the risk of xanthine urolith formation. It is unknown if the long-term use of allopurinol to prevent the recurrence of urate uroliths increases the risk of xanthine uroliths. The benefits of allopurinol may, however, outweigh the risks in animals that have had multiple episodes of urate urolithiasis. Just as in the management of struvite uroliths, any UTI should be appropriately treated because urease-producing organisms will increase the urine ammonium ion concentration and potentiate ammonium urate crystal production.
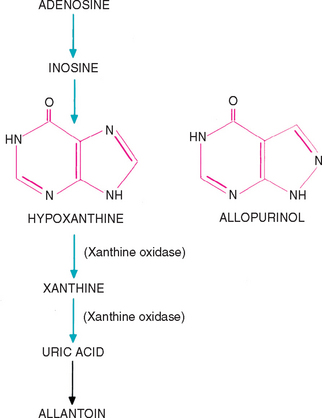
FIG 46-8 Metabolism of purine adenosine and a comparison of the structures of hypoxanthine and allopurinol.
In dogs with urate urolithiasis secondary to severe hepatic insufficiency, the underlying disorder should be corrected if possible. If hepatic function can be improved (e.g., surgical correction of a PSS) and the urine becomes undersaturated with ammonium and urate ions, uroliths may dissolve spontaneously. Even though spontaneous dissolution after surgical correction of a PSS is possible, it is usually recommended that a cystotomy be performed to remove uroliths at the time of PSS correction. In dogs with inoperable PSS or microvascular dysplasia, the k/d or l/d diet may be used to help decrease urine saturation with ammonium urate and reduce signs of hepatoencephalopathy.
Silicate uroliths.
Although the medical dissolution of silicate uroliths is not yet feasible, recommended ways to decrease recurrence after surgical removal include a dietary change, increasing the urine volume, and urine alkalinization. Hill’s Canine Prescription Diet u/d may be beneficial because it contains low amounts of silicates and produces alkaline urine. In addition, in certain regions soil may contain high concentrations of silicate; therefore the consumption of dirt and grass should be discouraged.
Cystine uroliths.
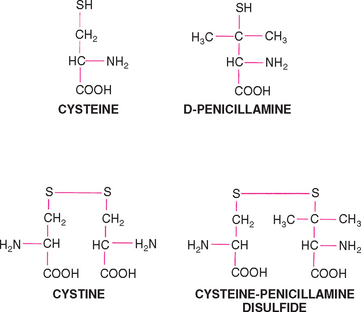
Recommendations for the medical dissolution and prevention of cystine uroliths include a reduction in the dietary intake of protein and methionine, alkalinization of the urine, and the administration of thiol-containing drugs. Hill’s Canine Prescription Diet u/d is appropriate because it has a very low protein content, produces alkaline urine, and decreases the urine-concentrating ability. Urine pH should be maintained at approximately 7.5, with potassium citrate given orally if necessary. Treatment can be started with a one-quarter tablet q8h and the dosage adjusted up or down depending on the urine pH (see urate section above). Sodium bicarbonate or sodium chloride supplementation should be avoided because the resulting natriuresis may enhance cystinuria. d-Penicillamine forms a disulfide compound with cysteine and therefore decreases the cystine content of the urine (Fig. 46-9). This disulfide compound is approximately 50 times more soluble than cystine in urine. d-Penicillamine may interfere with surgical wound healing, and treatment should not be initiated earlier than 2 weeks after surgery. Other possible infrequent or rare adverse effects of d-penicillamine include immune complex glomerulonephritis, fever, and skin hypersensitivity. Another thiol-containing drug, N-(2-mercaptopropionyl)-glycine (MPG), increases the solubility of cystine in urine by means of a disulfide exchange reaction similar to that produced by d-penicillamine and may have fewer adverse effects. The dose of MPG recommended for dogs for urate urolith dissolution is 15 to 20 mg/kg, administered orally q12h. Thiol-containing drugs should be used along with Hill’s Canine Prescription Diet u/d if necessary to prevent cystine urolith formation.
MONITORING THE PATIENT WITH UROLITHIASIS
Whenever medical dissolution of uroliths is being attempted, the patient should be reexamined at least monthly. A complete urinalysis should be performed, and abdominal radiographs or ultrasonography should be done to assess urolith size. If urinalysis findings are suggestive of a UTI, bacterial culture and sensitivity testing should be performed and antibiotic treatment initiated or adjusted accordingly. If the urolith has not decreased in size after 2 months of dissolution treatment, the clinician should reassess owner compliance, the control of infection, and urolith type and consider removing the urolith surgically.
Uroliths recur in up to 25% of dogs, and it is not uncommon for individual dogs to have three or more episodes of urolithiasis in their lifetimes. The likelihood of recurrence appears to be greatest in dogs with metabolic uroliths (calcium oxalate, urate, and cystine uroliths) or a familial predisposition (e.g., Miniature Schnauzers with struvite uroliths). Therefore appropriate preventive measures and frequent reevaluations are important in such dogs.
Adams LG, Syme HM. Canine lower urinary tract diseases. In Ettinger SJ, Feldman EC, editors: Textbook of veterinary internal medicine, ed 6, St Louis: Elsevier/Saunders, 2005.
Aldrich J, et al. Silica-containing urinary calculi in dogs (1981–1993). J Vet Intern Med. 1997;11:288.
Bartges JW, et al. Prevalence of cystine and urate uroliths in Bulldogs and urate uroliths in Dalmatians. J Am Vet Med Assoc. 1994;204:1914.
Bartges JW, et al. Influence of four diets on uric acid metabolism and endogenous acid production in healthy Beagles. Am J Vet Res. 1996;57:324.
Bartges JW, et al. Bioavailability and pharmacokinetics of intravenously and orally administered allopurinol in healthy Beagles. Am J Vet Res. 1997;58:504.
Bartges JW, et al. Influence of two diets on pharmacokinetic parameters of allopurinol and oxypurinol in healthy Beagles. Am J Vet Res. 1997;58:511.
Bartges JW, et al. Ammonium urate uroliths in dogs with portosystemic shunts. In: Bonagura JD, editor. Current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
Hess RS, et al. Association between hyperadrenocorticism and development of calcium-containing uroliths in dogs with urolithiasis. J Am Vet Med Assoc. 1998;212:1889.
Hoppe A, et al. Cystinuria in the dog: clinical studies during 14 years of medical treatment. J Vet Intern Med. 2001;15:361.
Lulich JP, et al. Evaluation of urine and serum metabolites in Miniature Schnauzers with calcium oxalate urolithiasis. Am J Vet Res. 1991;52:1583.
Lulich JP, et al. Prevalence of calcium oxalate uroliths in Miniature Schnauzers. Am J Vet Res. 1991;52:1579.
Lulich JP, et al. Catheter-assisted retrieval of urocystoliths from dogs and cats. Am J Vet Med Assoc. 1992;201:111.
Lulich JP, et al. Nonsurgical removal of urocystoliths by voiding urohydropropulsion. Am J Vet Med Assoc. 1993;203:660.
Lulich JP, et al. Effects of hydrochlorothiazide and diet in dogs with calcium oxalate urolithiasis. J Am Vet Med Assoc. 2001;218:1583.
Lulich JP, Osborne CA. Management of urolithiasis. In Elliott JA, Grauer GF, editors: BSAVA manual of canine and feline nephrology and urology, ed 2, Gloucester, England: British Small Animal Veterinary Association, 2007.
Sanderson SL, et al. Evaluation of urinary carnitine and taurine excretion in 5 cystinuric dogs with carnitine and taurine deficiency. J Vet Intern Med. 2001;15:94.
Seaman R, et al. Canine struvite urolithiasis. Compend Contin Educ Pract Vet. 2001;23:407.
Stevenson AR, et al. Effects of dietary potassium citrate supplementation on urine pH and urinary relative supersaturation of calcium oxalate and struvite in healthy dogs. Am J Vet Res. 2000;61:430.
Weichselbaum RC, et al. Evaluation of the morphologic characteristics and prevalence of canine urocystoliths from a regional urolith center. Am J Vet Res. 1998;59:379.
 TABLE 46-1
TABLE 46-1