CHAPTER 48 Disorders of Micturition
Micturition is the normal process of the passive storage and active voiding of urine. Disorders of micturition encompass problems with urine storage (incontinence) and bladder emptying (urine retention). Urinary incontinence is the inappropriate passage of urine during the storage phase of micturition. The most common forms of urinary incontinence occur secondary to either increased detrusor contractility or decreased urethral outflow resistance. Conversely, decreased detrusor contractility or increased urethral outflow resistance can result in urine retention. Armed with an understanding of bladder and urethral neuroanatomy, as well as the mechanism of action of currently available drugs, clinicians are able to effectively control many disorders of micturition.
PHYSIOLOGY OF MICTURITION
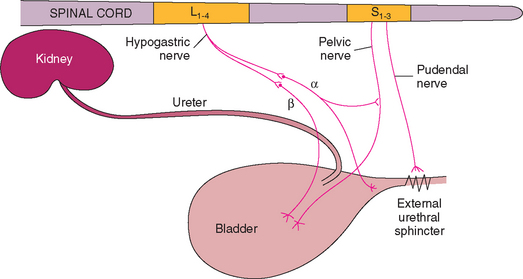
Micturition is controlled by a combination of autonomic and somatic innervation (Fig. 48-1). Parasympathetic innervation to the bladder is provided by the sensory and motor portions of the pelvic nerve that arises from sacral spinal cord segments S1 to S3 (vertebral body L5). The sensory portion relays the sensation of bladder fullness as the stretch receptors associated with detrusor muscle fibers are activated. The motor portion of this parasympathetic innervation predominates during the voiding phase of micturition, with stimulation of the pelvic nerve resulting in the depolarization of pacemaker fibers throughout the detrusor muscle. The subsequent spread of excitation to adjoining muscle fibers through tight junctions of smooth muscle cells leads to contraction of the detrusor muscle.
The S1 to S3 spinal cord segments are also the source of the somatic innervation to the external urethral sphincter via the pudendal nerve. The motor portion of the pudendal nerve causes contraction of the skeletal muscle of the external urethral sphincter under voluntary control. The external urethral sphincter is located predominantly in the midportion of the female urethra and in the membranous portion of the male urethra. The pudendal nerve also has sensory and motor function to the perineal region, including the anal sphincter, vulva, and prepuce.
Sympathetic innervation to the bladder is provided by the hypogastric nerve and is composed of preganglionic fibers exiting spinal cord segments L1 to L4 in the dog (vertebral bodies L1 to L3) and L2 to L5 in the cat (vertebral bodies L2 to L4) and synapsing in the caudal mesenteric ganglion. β-Adrenergic fibers terminate in the detrusor muscle; stimulation of these fibers results in detrusor muscle relaxation, which facilitates urine storage. α-Adrenergic fibers innervate the smooth muscle fibers in the trigone and urethra; stimulation of these fibers causes contraction and formation of the functional internal urethral sphincter. α-Adrenergic receptors also have a modulating effect on the external urethral sphincter.
The normal storage phase of micturition is governed by sympathetic autonomic domination, which causes the detrusor muscle to relax as a result of β-adrenergic stimulation and the internal urethral sphincter to contract as a result of α-adrenergic stimulation. Voiding is also consciously inhibited by the contraction of striated urethral muscles distal to the bladder and involuntarily inhibited by a spinal reflex that tightens the external urethral sphincter when there is a sharp increase in intraabdominal pressure (e.g., during abdominal palpation or bladder expression, barking, coughing, sneezing, retching). Urinary incontinence occurs if the intravesi-cal pressure exceeds the pressure exerted by the urethral sphincters.
Stretch receptors in the bladder send impulses through the pelvic nerve and spinal cord pathways to the thalamus and cerebral cortex when the urinary bladder fills and intramural tension exceeds the threshold. Voluntary control of voiding is mediated by the cerebral cortex through the pons (main micturition center), the cerebellum, and the reticulospinal tracts to the sacral nuclei. The voiding phase of micturition is characterized by parasympathetic activity. In this phase the detrusor muscle contracts secondary to cholinergic stimulation of the motor portion of the pelvic nerve. It is important to note that during this cholinergic-mediated detrusor contraction, the α and β-adrenergic input to the internal and external urethral sphincters is reflexly inhibited at the level of the pons. When the bladder is empty, the normal sympathetic domination resumes and the detrusor muscle relaxes to allow filling to occur. The normal residual volume of urine after complete voiding is approximately 0.2 to 0.4 ml/kg (with a maximum of 10 ml) in both dogs and cats.
Etiology and Clinical Features of Disorders of Micturition
Disorders of micturition can be divided into two major categories: those associated with a large or distended bladder and those associated with a small or normal-sized bladder (Table 48-1). Urine retention disorders associated with distended bladders include neurogenic disorders (upper [UMN] and lower [LMN] motor neuron disease, functional urethral obstruction, reflex dyssynergia) and anatomic obstructive disorders. Neurologic disorders may be caused by any condition that produces compression, damage, or degeneration of the spinal cord or pelvic nerve. Overdistention of the bladder for a prolonged time may also cause a neurogenic incontinence by decreasing bladder detrusor muscle tone (a type of LMN disorder). Dysautonomia in dogs and cats, an autonomic polyganglionopathy, also produces an LMN incontinence that is associated with weak and ineffective detrusor activity. On the other hand, urine leakage or incontinence disorders are usually associated with a small or normal-size bladder caused by increased detrusor contractility or decreased urethral outflow resistance. Congenital abnormalities of the urinary system (e.g., ectopic ureters, vaginal strictures) can also result in urinary incontinence associated with a small or normal-sized urinary bladder. It should be noted that urine leakage can occur with urine retention disorders when intravesical pressure exceeds outflow resistance. This type of urine leakage is referred to as paradoxic or overflow incontinence (discussed in greater detail later).
 TABLE 48-1 Disorders of Micturition
TABLE 48-1 Disorders of Micturition
| DISORDER | CAUSES |
|---|---|
| Distended Bladder | |
| Neurogenic | |
| Lower motor neuron disease | |
| Upper motor neuron disease | |
| Reflex dyssynergia (detrusor-urethral dyssynergia) | Unknown |
| Functional urethral obstruction | Urethral muscular spasm, often associated with urethral inflammation or trauma |
| Anatomic outflow tract obstruction | Urethral stricture, neoplasia, cystic or urethral calculi, granulomatous urethritis, prostatic disease |
| Small or Normal-Sized Bladder | |
| Urethral sphincter mechanism incompetence | Deficient bladder/urethral support, hormone-responsive |
| Detrusor hyperreflexia or instability | Bladder irritation, urethral irritation |
| Congenital incontinence | Ectopic ureters, patent urachus, urethral fistula (rectal or vaginal), pseudohermaphroditism, vaginal strictures |
DISTENDED BLADDER
Big, distended urinary bladders are usually easily palpated on physical examination, and the ease of bladder expression is an important part of patient assessment. If the distended bladder is easy to express, the underlying problem is usually decreased detrusor contractility. Conversely, if the bladder is difficult to express, increased outflow resistance should be suspected. Both functional (e.g., increased urethral tone caused by increased sympathetic tone or urethral spasm) and anatomic (e.g., urethral uroliths or trigonal masses) problems can cause increased outflow resistance. Urethral catheterization and/or positive contrast urethrography can be used to differentiate functional and anatomic causes of increased outflow resistance.
If neurologic lesions or deficits are detected during neurologic examination, the status of the bladder helps localize the lesion and classify the injury as either a UMN lesion (above the fifth lumbar vertebral body) or an LMN lesion (at or below the fifth lumbar vertebral body). The most characteristic sign of an LMN lesion affecting the bladder is a distended bladder that is easily expressed. An LMN injury affecting the bladder causes both sphincter and detrusor hyporeflexia; if the lesion involves spinal cord segments S1 to S3, both perineal and bulbospongiosus reflexes of the pudendal nerve are usually absent.
UMN lesions affecting the bladder result in a large, distended bladder that is difficult to express but easy to catheterize. Thoracolumbar spinal cord lesions causing paresis or paralysis are frequent causes of UMN bladder disorders. An animal with a UMN lesion has no voluntary control of micturition, and the urethral sphincter shows reflex hyperexcitability because the somatic efferents in the pudendal nerve are not inhibited, making expression difficult.
Reflex dyssynergia, or detrusor-urethral dyssynergia, is seen primarily in large-breed male dogs. The cause is usually difficult to determine but may include any of several neurologic lesions of the spinal cord or autonomic ganglia. Pathophysiologically, reflex dyssynergia results from the active contraction of the detrusor without relaxation of the internal or external urethral sphincters. Characteristic signs of reflex dyssynergia include normal or near-normal initiation of voiding, followed by a narrowed urine stream. Urine may be delivered in spurts, or flow may be completely disrupted and the dog will often strain to produce urine. After a while the dog will lower its leg and then often begins dribbling urine while walking away. It is difficult to express urine from the bladder of a dog with reflex dyssynergia, but urethral catheterization is usually easily accomplished. With reflex dyssynergia, increased outflow resistance occurs when the dog tries to initiate voiding. A similar type of functional urethral obstruction has been described in three male dogs in which resting outflow resistance was increased (Lane, 2000). Prostatitis and a history of urethral calculi were associated with the functional urethral obstruction in two cases, respectively; the third case was diagnosed as idiopathic.
Anatomic outflow obstruction results in a big, distended bladder that is usually both difficult to express and catheterize. In some cases a catheter may be passed around an anatomic urethral lesion relatively easily, and a positive contrast retrograde urethrogram may be necessary to confirm the presence of a lesion.
Incontinence in an animal with a primary urine retention problem is called paradoxic or overflow incontinence. Urine leakage occurs in this case when intravesical pressure exceeds outflow resistance. Clinical signs associated with a functional or anatomic urethral obstruction include dribbling of urine, straining to urinate without producing urine, restlessness, and abdominal pain. The most common causes of anatomic urethral obstruction are calculi and neoplasia in dogs, and struvite/mucous plugs in cats; however, trigonal masses, urethral strictures, and granulomatous urethritis can also create obstructions to urine flow. Any type of prostatic disease in dogs may produce an outflow tract obstruction. Older male dogs with benign prostatic hyperplasia may be evaluated because of stranguria and tenesmus; however, bacterial prostatitis, prostatic neoplasia, and prostatic abscesses are more likely causes of a urinary outflow tract obstruction. In patients with decreased detrusor contractility, paradoxic incontinence occurs earlier and at lower intravesicular pressures compared with patients that have either functional or anatomic outflow resistance problems.
SMALL OR NORMAL-SIZE BLADDER
Causes of urinary incontinence associated with a small or normal-size bladder include increased detrusor contractility and decreased outflow resistance. Increased detrusor contractility is generally associated with bladder and or urethral irritation/inflammation that creates an urge to void that overcomes normal house-trained behavior. These patients often exhibit pollakiuria, dysuria, and stranguria and have inflammatory or hemorrhagic urine sediment findings. Conversely, in patients with decreased urethral outflow resistance, urine leakage is often most pronounced when the animal is asleep or relaxed. The voiding phase of micturition is usually normal in these patients, as is the urinalysis (unless complicated by an ascending urinary tract infection).
Detrusor muscle hypercontractility (also referred to as detrusor instability or urge incontinence) is the inability to control voiding owing to a strong urge to urinate. Inflammation of the bladder or urethra may trigger the voiding reflex by creating a sensation of bladder fullness. Clinical signs of this type of incontinence include pollakiuria, dysuria-stranguria, and frequently hematuria. A bacterial urinary tract infection is the most common cause in the dog, and sterile inflammation of the lower urinary tract is the most common cause in cats. Evidence of a urinary tract infection or inflammation revealed by urinalysis (e.g., bacteriuria, pyuria, or hematuria) initially supports the tentative diagnosis of urge or inflammatory incontinence. If clinical signs persist after appropriate treatment for the urinary tract inflammation has been initiated, further diagnostic studies, including ultrasonography, contrast-enhanced radiography, and cystoscopy, are indicated because infiltrative disease of the bladder (e.g., neoplasia, chronic cystitis), polyps, uroliths, or urachal remnants can result in pollakiuria and stranguria. It should also be noted that detrusor hyperreflexia/instability may be a primary or idiopathic disorder that is not associated with bladder or urethral inflammation.
The preferred terminology for decreased urethral outflow resistance is urethral sphincter mechanism incompetence (USMI). This urethral sphincter dysfunction is most often observed in spayed, medium- to large-breed female dogs. Decreased tone in collagenous supporting structures of the urogenital tract caused by aging and/or decreased estrogen concentrations is thought to be the primary cause of USMI. Additional causes/complications may include abnormal bladder/urethral position (e.g., pelvic bladder), decreased responsiveness of α-adrenergic urethral receptors, and obesity. Recently, abnormal caudad bladder movement with the dog under anesthesia has been identified in bitches with USMI. This is thought to be due to deficient bladder and urethral support mechanism in these dogs. Estrogen and testosterone are believed to contribute to the integrity of urethral muscle tone by augmenting its responsiveness to α-adrenergic innervation. Thus middle-age to older, spayed female dogs are prone to incontinence because of decreased estrogen concentrations. This incontinence is most pronounced when the animal is asleep or relaxed and often responds to estrogen replacement or α-adrenergic therapy. Less frequently, incontinence develops in male dogs after castration; the condition seems to occur most commonly in dogs castrated at an older age and often responds to α-adrenergic treatment or hormone replacement. Both processes are diagnosed on the basis of history, physical examination findings, urinalysis (lack of evidence of lower urinary tract inflammation), and the animal’s response to therapy. Frequently, α-adrenergic treatment (e.g., phenylpropanolamine) may be combined with hormone replacement treatment in severe cases of USMI.
Urinary incontinence in a young animal with a small or normal-size bladder may be associated with a variety of congenital defects of the urinary or genital systems. The most common defects are ectopic ureters and vaginal strictures, but patent urachus, urethrorectal and urethrovaginal fistulae, and female pseudohermaphroditism have also been associated with urinary incontinence. Ectopic ureters are most commonly observed in female dogs. Breeds in which the prevalence of ectopic ureters is high include Siberian Huskies, Miniature and Toy Poodles, Labrador Retrievers, Fox Terriers, West Highland White Terriers, Collies, and Cardigan and Pembroke Welsh Corgis. Ectopic ureters are rarely seen in cats, but the gender predisposition is reversed (i.e., the prevalence is higher in male than in female cats). USMI is a frequent concurrent problem in dogs with ectopic ureters or vaginal strictures.
The most common clinical sign associated with ectopic ureters is constant dribbling of urine, although dogs and cats with a unilateral ectopic ureter also may void normally. Because 70% of ectopic ureters in dogs terminate in the vagina, vaginoscopy may allow visualization of the opening of the ureter; however, the orifice may be difficult to see even if the vagina is fully distended with air. Intravenous urography and retrograde vaginourethrography are excellent diagnostic tests for characterizing the defect, although a recent study suggested that contrast computed tomography (CT) is the test of choice for the diagnosis of ectopic ureters. In contrast to the incontinence seen in animals with ectopic ureters, incontinence associated with a vaginal stricture is often intermittent, occurring with changes in body position. Vaginal strictures can be diagnosed by digital vaginal examination, vaginoscopy, or contrast-enhanced vaginography.
Incontinence may also be caused by cognitive disorders (CDs), decreased bladder capacity, or decreased mobility in senior animals. Polyuric-polydipsic disorders, such as chronic kidney disease (CKD) in senior animals, also often exacer bate incontinence. Likewise, use of diuretic and corticosteroid medications should be avoided, if possible, in incontinent animals because of their negative effects on urineconcentrating ability.
Diagnosis
Clinical features of disorders of micturition often help the clinician discern the underlying problem. For example, if continuous urinary incontinence has been present from birth, the likely underlying problem is a congenital anomaly. Incontinence associated with hematuria, pollakiuria, and dysuria-stranguria usually indicates the presence of inflammation of the bladder, urethra, or both. Inappropriate dribbling of urine during sleep or relaxation indicates USMI, and leakage of urine in female dogs associated with postural changes may point to the pooling of urine behind a vaginal stricture. Dogs with pelvic bladders, which is a more caudal abdominal location in which the bladder neck is caudal to the pecten of the pubic bone (Fig. 48-2), can also have urethral sphincter incompetence that results in urinary incontinence. All these forms of incontinence are usually associated with a small or normal-size bladder.
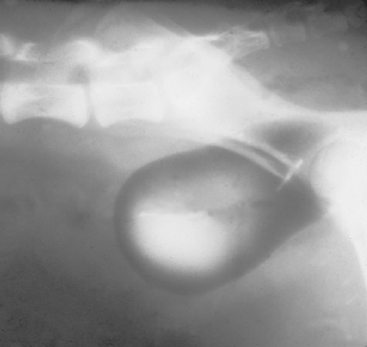
FIG 48-2 Double-contrast–enhanced cystogram showing a pelvic bladder in a 2-year-old spayed female Doberman Pinscher with urethral sphincter mechanism incompetence.
Dysuria and stranguria that occur in association with an abnormal or absent urine stream are typical of an obstructive uropathy. Urethral obstructions may be caused by anatomic (e.g., uroliths, tumors) or functional (e.g., reflex dyssynergia) problems. Urinary incontinence that occurs in association with trauma or pelvic surgery is usually neurogenic in origin (LMN disease); if paresis or paralysis is present, the lesion is usually above the fifth lumbar vertebral body and is a UMN lesion. Obstructive uropathies and UMN and LMN disorders result in large, distended bladders.
As noted earlier, incontinence in senior animals may be caused by CDs, a decreased bladder capacity, or decreased physical control. Physical problems in such animals, especially polyuric disorders and disabilities that impair mobility, should be identified and treated. Polyuria and polydipsia can trigger urge incontinence by placing continual stress on the bladder wall and urethral sphincter; however, in these cases the urine volume is large. A normally completely housebroken animal with polyuria and polydipsia may start urinating in the house if it does not have frequent access to the outdoors. If increased thirst and large urine volume are described by the owner, appropriate diagnostic tests should be performed to identify conditions that cause polydipsia and polyuria (e.g., diabetes mellitus, pyometra, CKD, hyperadrenocorticism, hypercalcemia).
Owners frequently mistake submissive urination, which may be a normal behavioral pattern of young dogs, with urinary incontinence. Other voiding patterns that are construed by some owners as incontinence are the urine marking used by male and occasionally female animals and inappropriate elimination behavior problems. The owner’s description of the animal’s voiding pattern may reveal a behavioral basis for the abnormal micturition, although a complete physical examination and a urinalysis should always be performed to identify or rule out a urinary tract disorder.
INITIAL EVALUATION
The age of onset, reproductive status of the animal, age at neutering, current medications, and history of trauma or previous urinary tract disorders are important anamnestic points to cover during the history-taking in an animal with any disorder of micturition. The physical examination should include evaluation of the perineum for evidence of urine scalding or staining. A thorough palpation of the bladder to assess its size and wall thickness and a rectal examination to assess anal tone, the prostate gland, the pelvic urethra, and the trigone region of the bladder should be performed in all cases. A digital vaginal examination is also indicated, and vaginoscopy may be used to help identify congenital defects (e.g., vaginal strictures, ectopic ureters) in larger female dogs.
A neurologic examination should include evaluation of the perineal and bulbospongiosus reflexes. The perineal reflex causes the anal sphincter to contract and the tail to ventroflex in response to pinching the perineal skin. The bulbospongiosus reflex causes the anal sphincter to contract in response to gentle compression of the bulb of the penis or the vulva. Both these reflexes depend on an intact pudendal nerve (sensory and motor) and spinal cord segments S1 to S3. If both reflexes are normal, the pudendal reflex arc is intact. Because of their common origin, injury to the pudendal nerve may also affect the pelvic nerve.
Dogs should be walked outside so that the voiding posture and urine stream size and character can be observed. Imme diately after the animal has attempted to void, the bladder should be palpated to determine the residual volume (normal residual volume is approximately 0.2 to 0.4 ml/kg). Catheterization is indicated to quantify the residual volume if a large bladder is palpable after voiding (in male dogs, however, behavioral urine marking can make assessment of residual urine volume difficult).
Urinalysis should be performed in all animals with urinary incontinence. If a urine culture is indicated, cystocentesis is the preferred method of collection; however, animals with a distended bladder should be catheterized instead to empty the bladder and prevent the problem of urine leaking from the cystocentesis site. Additional diagnostic testing that can be accomplished at many referral centers includes cystoscopy and urethral pressure profilometry (UPP). Cystoscopy allows direct visualization of the urethral and bladder mucosa and the ability to obtain mucosal specimens for culture and histology. The functional length of the urethral sphincter and the urethral closure pressure can be determined via UPP, which is usually performed in conscious patients. A flexible catheter with a side port is passed through the urethra, and after the bladder has been emptied, the catheter is connected to a pressure transducer and a withdrawal arm (that pulls catheter back through the urethra at a constant rate). Saline is then infused through the catheter as it is withdrawn, and the resistance to flow (pressure) is recorded versus distance traveled. (See additional descriptions of bladder and urethral function testing in Chapter 42.)
PHARMACOLOGIC TESTING
Frequently, the diagnosis of disorders of micturition is based to some degree on the animal’s response to pharmacologic testing or therapy. For example, detrusor hypocontractility should improve in response to a parasympathomimetic drug (e.g., bethanechol), and decreased urethral tone should respond to α-adrenergic agents (e.g., phenylpropanolamine) or hormone replacement therapy. Increased urethral tone is treated with α-sympatholytics (e.g., phenoxybenzamine) and striated muscle relaxants (e.g., diazepam). Detrusor hypercontractility often responds to treatment of the underlying inflammatory process, such as bacterial cystitis or urolithiasis; however, smooth muscle antispasmodics (e.g., oxybutynin) and parasympatholytics (e.g., propantheline) may be useful in cases of severe inflammation.
LOWER MOTOR NEURON DISORDERS
Animals with LMN diseases resulting from sacral spinal cord lesions or dysautonomia require expression or strict aseptic catheterization of their bladder at least three times per day. Urinalysis or examination of the urine sediment should be performed weekly, and a urine bacterial culture should be performed if there is any evidence of a urinary tract infection. Care should be taken to prevent urine scalding by applying petroleum jelly to the perivulvar or peripreputial and abdominal skin. Bethanechol may be administered to increase detrusor contractility if the urethra is confirmed to be patent by bladder expression (5–15 mg/dog PO q8h; 1.25–5 mg/cat PO q8h). Adverse effects of bethanechol include salivation, vomiting, diarrhea, or coliclike signs that indicate intestinal cramping. These signs usually appear within 1 hour of drug administration; if they are observed, the dose of bethanechol should be decreased.
To manage detrusor atony, the bladder must be expressed or urinary catheterization done intermittently to keep the bladder empty for a period of days to weeks. A closed urine-collection system should always be used with indwelling catheters. Urinalysis should be performed every 3 or 4 days and a urine bacterial culture and antibiotic sensitivity testing done if there is any evidence of urinary tract inflammation. Bethanechol may be administered to increase detrusor contractility but only after increased outflow resistance has been ruled out.
UPPER MOTOR NEURON DISORDERS
The nature of the management of animals with a UMN lesion affecting the bladder depends on whether the animal has an autonomic bladder. A reflex, or autonomic, bladder often develops 5 to 10 days after a spinal cord injury, and it occurs because stretching of the bladder wall stimulates a local reflex arc that results in detrusor contraction. There is no cortical perception or voluntary control, and initially voiding is usually incomplete, resulting in a large urine residual volume. Treatment in an animal before an autonomic bladder develops should include aseptic catheterization three times per day. The use of corticosteroids for the treatment of neurologic disease may cause polyuria, necessitating more frequent catheterization to prevent overdistention of the bladder. Corticosteroids also predispose animals to urinary tract infections. During the initial stages of treatment, urinalysis or urine sediment examination should be performed every 3 or 4 days, and urine bacterial culture and antibiotic sensitivity testing should be performed if there is evidence of urinary tract inflammation (corticosteroids frequently mask signs of inflammation). Because these animals are usually in pain and reluctant to move, it is important to prevent urine scalding. The use of elevated racks or absorbent bedding is indicated, and petroleum jelly applied around the perineum or prepuce may minimize urine scalding.
After an autonomic bladder develops, the bladder should be palpated after urination to determine the residual urine volume. It may still be necessary to catheterize the bladder two or three times per day to minimize urine stasis. Urinalyses should continue to be done on a monthly schedule (weekly if the animal is receiving corticosteroids), and owners should be instructed to bring in a urine sample if a change in urine color or odor is noted. Nursing care to prevent urine scalding should be continued.
REFLEX DYSSYNERGIA
Reflex dyssynergia often responds to pharmacologic management; however, a therapeutic response may not be seen for several days. Drugs commonly used include an α-blocker (e.g., prazosin or phenoxybenzamine), a somatic muscle relaxant (e.g., diazepam), and occasionally bethanechol. Intermittent urinary catheterization should be performed as necessary to keep the bladder small and combat detrusor atony that may be caused by overdistention of the bladder.
Phenoxybenzamine has a slow onset of action, and the dose should be increased only at 3- to 4-day intervals. The urine stream should be evaluated to gauge drug effectiveness. If the stream is weak but continuous and of normal diameter, bethanechol may be used to increase detrusor contractility; however, it must not be used until the functional urethral obstruction has been relieved. If the urine stream is intermittent or narrowed, increased doses of diazepam or phenoxybenzamine or both may be required. Because diazepam has a very short duration of action (approximately 1 to 2 hours when administered orally), administering it 30 minutes before walking the animal sometimes aids in the management of reflex dyssynergia. It may be several weeks before a correct combination of drugs is determined, however, and drug dosages may have to be modified over time. Periodic urinalyses are indicated to detect urinary tract inflammation or infection at an early stage.
Hypotension is the major adverse effect of phenoxybenzamine, and the dose should be decreased immediately if the animal shows any indication of lethargy, weakness, or disorientation. In most cases the dosage of phenoxybenzamine should be increased only if a favorable response is not observed after 3 or 4 days; rapid dose changes should be avoided. Nausea is an adverse effect that can be minimized by administering the medication with a small meal. Glaucoma is a rare complication of phenoxybenzamine treatment in people; it is unknown if this occurs in dogs.
FUNCTIONAL URETHRAL OBSTRUCTION
Nonneurogenic functional urethral obstruction, in which resting as well as voiding urethral pressures are abnormally high, has been associated with prostatic disease; urinary tract infection; urethral muscular spasm; and urethral inflammation, hemorrhage, or edema in dogs and cats. Affected animals have clinical signs and histories similar to those in dogs with reflex dyssynergia. Resting urethral pressure profilometry is usually necessary to differentiate these two syndromes. When treatment of the underlying disorder fails to decrease the increased outflow resistance, α-blockers (e.g., prazosin or phenoxybenzamine) and skeletal muscle relaxants (e.g., diazepam) can be used.
URETHRAL SPHINCTER MECHANISM INCOMPETENCE
The treatment of urinary incontinence associated with decreased sphincter tone includes hormone replacement or α-adrenergic drugs (or both). The usual induction therapy for estrogen-responsive incontinence consists of diethylstilbestrol (DES; 0.1 to 1.0 mg total administered orally q24h for 3 to 5 days). The frequency of administration is then decreased to the lowest possible dose that will maintain continence. Some dogs can be successfully tapered to a very low maintenance schedule (e.g., 0.1 to 1.0 mg per dog every 7 to 10 days). Phenylpropanolamine (1.5 to 2.0 mg/kg administered orally q8h) may be used as an alternative drug or in addition to DES. Owners of dogs receiving phenylpropanolamine should be cautioned to observe their dog for hyperexcitability, panting, or anorexia and to decrease the dose if these signs develop. Although initially administered three times per day, in some animals the dosing frequency of timed-release or precision-release phenylpropanolamine can be decreased to a once- or twice-daily schedule. Careful observation by the owner for recurrence of signs usually reveals when the dose needs to be increased. Dogs with increasing resistance to DES pose the greatest worry because the development of estruslike signs and bone marrow toxicity are possible adverse effects of higher-dose DES therapy. Endocrine alopecia is another possible adverse effect. If DES-resistant dogs are not concurrently receiving phenylpropanolamine, a trial of it should be instituted before the DES dose exceeds recommended levels. α-Adrenergic drugs are contraindicated in patients with systemic hypertension, mitral regurgitation, and anxiety disorders.
Urethral sphincter incompetence in neutered male dogs is best treated with α-adrenergic drugs. If testosterone is to be used, it should be parenterally administered because most testosterone administered orally undergoes rapid hepatic degradation. Depository forms injected intramuscularly may be effective for 4 to 6 weeks. Male dogs receiving testosterone should have regular rectal examinations to evaluate prostate size. Testosterone should not be used in dogs that were previously neutered because of a testosterone-responsive disease (e.g., benign prostatic hypertrophy, perianal adenomas) or behavioral disorders (e.g., aggression).
In those patients with USMI refractory to hormone replacement and/or α-adrenergic therapy, alternative treatments include gonadotropin-releasing hormone (GnRH) analogues and urethral bulking and surgical procedures. Increased concentrations of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) have been documented in spayed dogs, and GnRH analogues will downregulate production/secretion of LH and FSH. Submucosal collagen injections at the level of the internal urethral sphincter via urethroscopy can also be used as an adjunct treatment to increase urethral sphincter tone. Finally, surgical procedures such as colposuspension, cystourethropexy, and formation of seromuscular urethral slings may benefit patients with USMI that is nonresponive to medical management.
DETRUSOR HYPERCONTRACTILITY
Smooth muscle relaxants and anticholinergics (e.g., dicyclomine, oxybutynin, propantheline bromide, imipramine, flavoxate) have been used to decrease inappropriate, involuntary detrusor contractions associated with lower urinary tract inflammation, but their use should be reserved for those animals that do not respond to treatment of the primary disorder (e.g., antibiotics for bacterial urinary tract infections). Animals with chronic or recurrent cystitis require a thorough evaluation of the cause of the urinary tract infection (see Chapter 45). Antispasmodics may provide a small degree of relief; however, the identification and elimination of the underlying inflammatory disorder should be the priority. When the detrusor hypercontractility is primary or idiopathic, anticholingeric agents may be beneficial.
CONGENITAL DISORDERS
The correction of congenital defects depends on the nature and extent of the defect. For example, a patent urachus or urachal diverticulum is surgically correctable, as are many forms of ectopic ureters. However, because USMI may occur in conjunction with an ectopic ureter, surgical reimplantation of the ureter does not guarantee continence. The use of α-adrenergic drugs after surgery increases the likelihood of success. Urethral pressure profilometry can be used to detect USMI and measure the response to α-adrenergic drugs before surgery.
ANATOMIC URETHRAL OBSTRUCTION
In animals with an anatomic urethral obstruction, the size and nature of the lesion can usually be determined by retrograde positive-contrast urethrography. The prevention of renal damage secondary to urinary obstruction and the relief of urinary obstruction to prevent detrusor atony resulting from overdistention are the main priorities in dogs and cats with urine outflow tract obstructions. If the obstruction is created by a urethral urolith, retropulsion of the urolith into the bladder may be successful. If the urolith cannot be moved by retropulsion, a temporary or permanent perineal urethrostomy may be necessary.
In dogs with benign prostatic hyperplasia resulting in urethral obstruction, castration usually leads to a rapid decrease in the size of the prostate. The use of estrogens to decrease prostatic size is not recommended because of the potential for systemic adverse effects and the development of squamous metaplasia of the prostate. Surgical drainage and marsupialization may be necessary to manage prostatic abscesses or prostatic cysts. In some cases of prostatic neoplasia, partial or complete prostatectomy or radiotherapy may be beneficial; however, prostatectomy is difficult and frequently results in neurologic damage and USMI.
Prognosis
In general, the prognosis for animals with neurogenic forms of urinary incontinence is poor. The long-term prognosis for animals with most types of spinal cord lesions is unfavorable, unless an intervertebral disk protrusion can be successfully decompressed or an extradural mass successfully removed or treated with chemotherapy or radiotherapy. Even if the spinal cord is decompressed, normal micturition may not completely return because the central nervous system has a minimal capacity for regeneration. Damage to the pudendal nerve, pelvic nerve, or sacral nerve roots is associated with a more favorable prognosis because peripheral nerves have a greater capacity to regenerate.
Most of the time, reflex dyssynergia responds to pharmacologic management, but occasionally the underlying disease worsens, making pharmacologic management ineffective. Drug doses should be reevaluated and increased if this happens, but this is not always successful. Diagnostic procedures such as myelography, an epidurography, CT, or magnetic resonance imaging (MRI) may be indicated in these refractory cases. Catheterization using aseptic techniques may be necessary for the long-term management of these animals.
Periodic urinalyses to identify or rule out urinary tract infections constitute an important aspect of follow-up care in an animal with any disorder of micturition. The frequency of the urinalyses depends on the nature of the disorder. Owners can be instructed to evaluate the color and odor of the urine and to bring in a urine sample immediately if they suspect an infection; however, routine monitoring is the cornerstone of the prevention of severe urinary tract infections. The prognosis for animals with USMI is usually good, although some dogs require multiple drugs for management.
Dogs treated for urge or inflammatory incontinence secondary to a urinary tract infection should undergo follow-up urinalysis or urine bacterial culture studies to confirm that the urinary tract infection has been eliminated. Long-term dietary management may help prevent recurrences in animals with urolithiasis.
The prognosis for dogs and cats with trigonal or urethral neoplasia is usually poor. In most cases, urethral neoplasia is inoperable because the clinical signs (dysuria, stranguria, hematuria, urethral obstruction) are usually not observed until the tumor is invasive. In contrast, most female dogs with granulomatous (chronic active) urethritis respond well to a combination of prednisolone, cyclophosphamide, and antibiotics.
Adams LG, Syme HM. Canine lower urinary tract diseases. In Ettinger SJ, Feldman EC, editors: Textbook of veterinary internal medicine, ed 6, St Louis: Elsevier/Saunders, 2005.
Arnold S, et al. Urethral sphincter mechanism incompetence in male dogs. In: Bonagura JD, editor. Current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
Atalan G, et al. Ultrasonographic assessment of bladder neck mobility in continent bitches and bitches with urinary incontinence attributable to urethral sphincter mechanism incompetence. Am J Vet Res. 1998;53:673.
Barch A, et al. Evaluation of long-term effects of endoscopic injection of collagen into the urethral submucosa for treatment of urethral sphincter incompetence in female dogs: 40 cases (1993-2000). J Am Vet Med Assoc. 2005;226:73.
Byron JK, et al. Comparison of the effect of propofol and sevoflurane on the urethral pressure profile in healthy female dogs. Am J Vet Res. 2003;64:1288.
Carofiglio F, et al. Evaluation of the urodynamic and hemodynamic effects of orally administered phenlypropanolamine and ephedrine in female dogs. Am J Vet Res. 2006;67:723.
Fischer JR, et al. Urethral pressure profile and hemodynamic effects of phenoxybenzamine and prazosin in non-sedated male beagle dogs. Can J Vet Res. 2003;67:30.
Hamaide AJ, et al. Urodynamic and morphologic changes in the lower portion of the urogenital tract after administration of estriol alone and in combination with phenylpropanolamine in sexually intact and spayed female dogs. Am J Vet Res. 2006;67:901.
Lane IF, et al. Functional urethral obstruction in 3 dogs: clinical and urethral pressure profile findings. J Vet Intern Med. 2000;14:43.
Lane IF. Urinary obstruction and functional urine retention. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 5, Philadelphia: WB Saunders, 2000.
Lane IF. Use of anticholinergic agents in lower urinary tract disease. In: Bonagura JD, editor. Current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
Fischer JR, Lane IF. Incontinence and urine retention. In Elliott JA, Grauer GF, editors: BSAVA manual of canine and feline nephrology and urology, ed 2, Gloucester, England: British Small Animal Veterinary Association, 2007.
Reichler IM, et al. The effect of GnRH analogs on urinary incontinence after ablation of the ovaries in dogs. Theriogenology. 2003;60:1207.
Samii VF, et al. Digital fluoroscopic excretory urography, digital fluoroscopic urethrography, helical computed tomography, and cystoscopy in 24 dogs with suspected ureteral ectopia. J Vet Intern Med. 2004;18:271.
Wood JD, et al. Use of particulate extracellular matrix bioscaffold for treatment of acquired urinary incontinence in dogs. J Am Vet Med Assoc. 2005;226:1095.
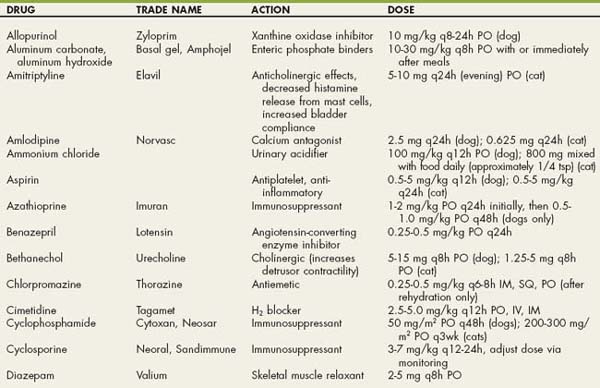
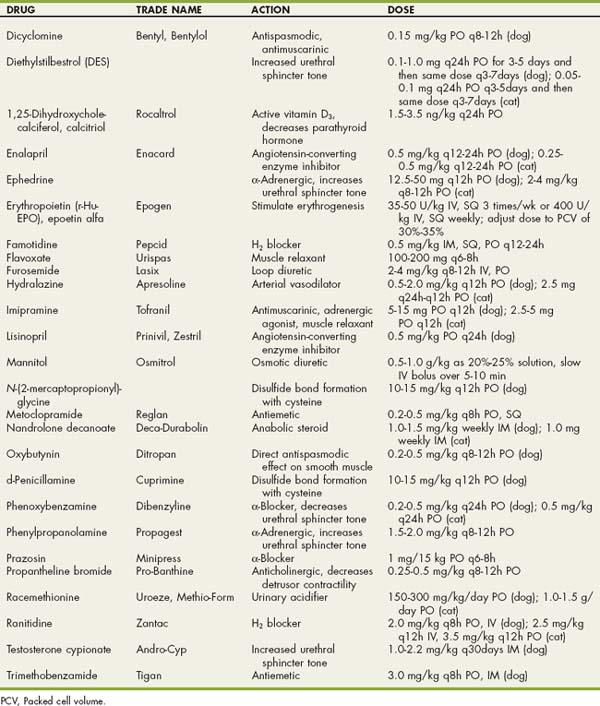
 Drugs Used in Dogs and Cats with Urinary Tract Disorders
Drugs Used in Dogs and Cats with Urinary Tract Disorders