CHAPTER 60 Disorders of Male Fertility
NORMAL SEXUAL DEVELOPMENT AND BEHAVIOR
DEVELOPMENT
Sexual differentiation into a male or female has three components: chromosomal, gonadal, and phenotypic sex. The major genetic components that direct the development of a male are located on the short arm of the Y chromosome in the sex determining region, or Sry gene. Together with certain factors elsewhere on the sex chromosomes and on some autosomes, the Sry gene directs the development of the sexually indifferent fetal gonads into testes. Testicular differentiation is observed in the canine fetus at day 36 of gestation. The many hormones produced by the fetal testis cause continued sexual differentiation into the male phenotype. In the absence of these influences, the phenotype will become female. Fetal Sertoli cells produce Müllerian inhibiting substance, which causes regression of the Müllerian ducts that would otherwise have developed into the oviduct, uterus, and cranial vagina. Müllerian duct regression is complete by day 46 of gestation in dogs. Fetal Leydig cells produce testosterone, which causes the Wolffian ducts to develop into the ductuli deferentes and epididymes. Dihydrotestosterone, a metabolite of testosterone, causes the urogenital sinus, the genital tubercle, and the genital swelling to differentiate into the urethra and prostate, penis, and scrotum, respectively. The male external genitalia can be recognized by ultrasound between gestation days 38 to 43 in the feline fetus (Zambelli et al., 2006a). Factors produced by the fetal testis also cause the testis to descend from its fetal position near the caudal pole of the kidney, through the inguinal canal, into the scrotum. Normal testicular descent is a prenatal event in cats and is normally complete by 10 to 42 days of age in dogs. Although later descent is possible, a diagnosis of cryptorchidism should be considered if the testes are not palpable within the scrotum by 8 to 10 weeks of age.
The Leydig (interstitial) cells are stimulated by luteinizing hormone (LH) to produce testosterone and, in much lesser amounts, estradiol. In addition to the development of the ductus deferens and epididymis, testosterone initiates and maintains all aspects of spermatogenesis; supports libido; and, via negative feedback, regulates hypothalamic secretion of gonadotropin releasing hormone (GnRH) and pituitary secretion of LH and follicle-stimulating hormone (FSH). Testosterone is also the prohormone for dihydrotestosterone (DHT) and estradiol, which are formed in the testis and also in peripheral tissues, by the action of the enzymes 5α-reductase and aromatase, respectively. DHT promotes the maturation of the prostate and the external genitalia and the development of the secondary sexual characteristics at puberty (see Fig 56-4). Only a small amount of estradiol is secreted by the testes. Most of the circulating estradiol in adult males is formed in the extragonadal tissues by the aromatization of circulating testosterone.
The Sertoli cells and spermatogonia (germ cells) are located along the basement membrane of the seminiferous tubules. Cytoplasmic processes from Sertoli cells extend from the lamina propria of the seminiferous tubules to the tubular lumen and surround the developing germ cells. Sertoli cells are regulated by FSH and testosterone to produce several substances that are necessary for spermatogenesis and normal spermatid maturation. In fact, spermatogenesis is regulated via the effects of FSH on Sertoli cells, not by direct effects of FSH on germ cells. Specific functions of Sertoli cells vary according to the developmental stage of the germ cells they surround. These may also vary depending on the species of animal.
Sertoli cells convert testosterone, produced by the Leydig cells, to estradiol. This is especially apparent in prepubertal animals. Most of the testicular estradiol in adults appears to originate from Leydig cells. The role of estradiol in male reproduction is unclear. Along with testosterone, it is involved in the regulation of gonadotropin secretion. In some instances, estrogens augment the effects of androgens, such as in the canine prostate, where estradiol regulates the number of DHT receptors. In the mammary gland estrogens seem to have antiandrogenic effects. Sertoli cells also produce a substance known as androgen-binding protein, which is thought to moderate the effects of testosterone. It may also be involved in the transport of testosterone within the testis and epididymis. Sertoli cells also produce the hormones inhibin and activin. Inhibin causes a decrease in FSH secretion by the pituitary. Activin has the opposite effect on FSH secretion.
Spermatogenesis refers to the maintenance of spermatogonia and the differentiation of spermatogonia into spermatozoa within the seminiferous tubules. Both proliferating and noncommitted spermatogonia are located nearest the tubular basement membrane. As the germ cells mature into spermatocytes and eventually spermatids, they move toward the tubular lumen, such that the most differentiated cells are nearest the lumen. Eight to 10 stages of sperm development, or cellular associations, have been identified in dogs. They represent maturation of spermatogonia, then several stages of maturing spermatocytes, and finally several stages of maturing spermatids. A cross-section of any given normal seminiferous tubule contains all stages of the developing spermatozoa.
In addition to the proliferating spermatogonia, there is a population of noncommitted spermatogonia that are called A0-spermatogonia. These spermatogonia remain in reserve and, unlike proliferating spermatogonia, are quite resistant to damage by toxins and radiation. The recovery of spermatogenesis that occurs after testicular injury results from the repopulation of the germinal epithelium by the progeny of the A0-spermatogonia. In dogs approximately 62 days elapse from the time A0-spermatogonia begin to differentiate into mature spermatogonia until the time mature spermatozoa are released into the tubular lumen. It then takes approximately 14 days for spermatozoa to become fully mature and motile in the epididymis. Frequent ejaculation does not influence daily sperm production in the testes, but it does diminish the number of sperm ejaculated by depleting the extragonadal reserves from the epididymis and ductus deferens.
Sexual maturity and physical maturity are closely related. Puberty occurs around 9 to 10 months of age in tomcats. The same is generally true for dogs, but the large and giant breeds mature more slowly. The onset of puberty is signaled by the development of masculine physical characteristics, such as heavier muscling and thicker skin on the jowls, and sexual behavior, such as territorial urine marking and mounting of other colony members. Semen quality and serum concentrations of testosterone gradually approach those of mature males, although prepubescent males may be fertile. Semen quality and libido tend to decline with advancing age, especially in geriatric males. Although males are fertile long past 6 years of age, they often are less active in breeding programs. This is primarily because they are replaced by younger males with similar or preferred phenotypic and genotypic qualities. Because other health problems often develop in older animals, their physical ability to mate may decline. Undesirable behavior is cited as a common reason for retiring older tomcats from breeding colonies.
BREEDING BEHAVIOR
Much of normal breeding behavior is learned by dogs and cats. Consequently, early breeding experiences help determine a male’s future success as a stud. There is usually one dominant male in any specific territory. Even if all males are given equal opportunity, the dominant male will do most of the breedings. Establishing dominance and territory is usually a prerequisite to mating; therefore the standard practice is to bring the female to the male. Because successful studs must be physically, sexually, and socially mature, it is usually recommended that males not enter a breeding program until they are at least 12 months old, even though puberty may have occurred months earlier. Dogs that are used in an artificial insemination program may become so accustomed to semen collection that they are no longer interested in natural service.
Ideally, a sexually inexperienced male should first be exposed to a docile, experienced female in his own territory. Virgin males usually make many unsuccessful attempts to mount before achieving intromission. This first encounter should be short and well supervised so that the male does not become frustrated or exhausted by his unsuccessful attempts to copulate or, worse, become intimidated or actually injured by an aggressive female. The tomcat typically grasps the queen’s neck near her shoulders and straddles her with his front feet. The neck grasp is thought to be necessary to restrain the female and to properly position both animals’ rear quarters to allow intromission to occur. The rear quarters are then straddled, and intromission occurs (Fig. 60-1). To insure adequate copulatory stimulation to induce ovulation in the queen, three breedings per day for the first 3 days of estrus are recommended (see Chapter 56). When semen is collected from cats three times per week, the volume and number of sperm are fairly constant from collection to collection. When semen is collected daily, by the fourth day the volume and number of sperm drop to less than half of that on the first day and then remain fairly constant at 14 to 45 million sperm per ejaculate. Libido and sperm motility and morphology did not change with frequent collection, other than an increase in the number of immature spermatozoa (Zambelli, 2006b).
Because the canine os penis maintains rigidity, intromission can and does occur before the penis is actually erect. During erection the bulbus glandis of the canine penis swells twofold to threefold, filling the vestibule and preventing separation of the breeding pair. Ejaculation of the first two seminal fractions begins shortly after intromission, during the rapid pelvic thrusting. Soon after its pelvic thrusting subsides, the dog dismounts and faces away from the bitch, but the erect penis, having turned 180 degrees in a horizontal plane, remains in the bitch. This is known as the postcoital lock or tie (see Fig. 56-2). Ejaculation, primarily of the third fraction, which consists of prostatic fluid, continues during the tie. The tie persists until the dog’s erection subsides 15 to 30 minutes or more later. If the dog’s penis is erect before intromission, the size of the bulbus glandis prevents complete intromission and a tie will not occur when the dog dismounts. Some dog owners refer to this as an outside tie. In this situation the entire ejaculate may not have been deposited in the bitch.
Two breedings during the fertile period are recommended for dogs because doing so has been shown to increase both whelping rates and litter size. When breeding is performed at the most optimal time during the fertile period, whelping rates are not significantly higher with two breedings than with one, although litter size is larger. Three breedings during the fertile period do not increase whelping rates above two breedings, but there may be a slight increase in litter size. Daily ejaculation diminishes sperm numbers dramatically by the second day, whereas semen collection every other day does not have such a profound effect on sperm numbers. On average 4 to 10 days of ejaculation deplete the extragonadal sperm reserves in the epididymis and ductus deferens. Thereafter the number of sperm per ejaculate is substantially less than half the number collected on the first day. This could be an important consideration for a very popular stud and when semen is being collected for freezing. Results from breeding during the fertile period with semen of good quality are optimized when bitches are 6 years of age or younger and dogs are 8 years of age or younger.
Artificial Insemination
Artificial insemination (AI) is used in dogs primarily when natural breeding cannot be accomplished. Transporting semen, rather than live animals, to distant geographic locations is a great advantage of AI over natural service. AI is also used when behavioral problems, such as partner preference, or physical problems, such as vaginal prolapse, prevent copulation of the desired pair. Some dog breeders prefer AI because they believe that the risk of breeding trauma is minimized and that the stud is less likely to be exposed to infectious diseases carried by the bitch. In addition, a single ejaculate with sufficient numbers of spermatozoa can be divided and used to inseminate several bitches. The success of AI is determined by several factors, including the reproductive health of the animals, the quality of the semen, the timing and the number of inseminations, intravaginal versus intrauterine insemination, and the technical skills of the person performing the insemination.
It is important to document semen quality before insemination because the success of the insemination will be no better than the quality of the semen. Knowing that semen quality is poor, the owner may wish to use a different stud. Although pregnancies are occasionally achieved using fresh semen of inferior quality, the litter size is usually smaller. Usually, no litters result if frozen-thawed semen of poor quality is used. In addition, once inseminated, the bitch should not be bred to a different male during that cycle because paternity is uncertain unless DNA testing is done. Two inseminations should be performed at the optimal time during the fertile period, as discussed in Chapter 56. It has been shown that pregnancy rates from AI are improved when the semen is deposited directly into the uterus rather than into the cranial vagina. This is especially important when frozen-thawed semen is used because, relative to fresh or chilled semen, the frozen-thawed spermatozoa have a very short life span and poor ability to transverse the cervix. It has been estimated that to achieve similar pregnancy rates with frozen-thawed semen, at least 10 times the number of viable sperm are needed for vaginal AI than for intrauterine AI. Intrauterine insemination can be accomplished transcervically using specially developed catheters (Norwegian catheter; Norske Pelsdyrforlag A/L) or endoscopically. These transcervical insemination techniques are commonly referred to as TCI. Intrauterine insemination can also be accomplished via laparoscopy or through a mini laparotomy.
While the semen is being handled, it must be protected from sudden changes in temperature. Freshly ejaculated canine semen is most effectively protected against temperature shock by working at room temperature and inseminating promptly after collection. Pregnancy rates of 84% have been achieved with vaginal AI of fresh semen. Chilled and frozen semen are protected against damage during processing by the addition of a protective “semen extender” to the sample and by careful attention to the cooling, freezing, and thawing rates. Semen extenders have been formulated to provide nutritional support for the sperm cells, buffer pH changes that occur because of continued metabolic activity, maintain physiologic osmotic pressure, prevent bacterial growth, protect cells from cold shock during chilling, and limit cell damage during freezing and thawing.
The advantage of chilled extended semen over natural breeding or AI with fresh semen is that the semen, rather than the animals, can be transported. The advantage of chilled semen over frozen semen is that the pregnancy rates are better. Pregnancy rates and the longevity of chilled semen vary tremendously according to the type of extender and processing methods used. Properly extended and cooled semen of good quality can be stored at 5°C for 12 to 24 hours and usually longer. Recently, new extenders have been developed that maintain sperm viability for 1 to 2 weeks at 5°C (www.canirep.com). It is important to follow the instructions for warming chilled semen before insemination because the methods will vary according to the extender used. Pregnancy rates of 50% to 70% are reported for the use of various extenders with chilled semen and vaginal insemination. Whelping rates can be improved from about 45% with vaginal AI to about 65% with intrauterine AI using chilled semen. Extender, equipment, and instructions for their use and for collecting, chilling, and inseminating semen are available from several commercial sources (Fresh Express ICG, Synbiotics; Cryogenetics Laboratory of New England; International Canine Semen Bank; Canine Cryobank; www.canirep.com).
The greatest advantage of frozen semen is that cryopreservation is the only way in which the genetic potential of valuable male animals can be saved indefinitely. Using frozen semen, litters can be sired by a dog long after his death. Another advantage is that semen can be collected and stored whenever it is convenient to do so, in contrast to fresh or chilled semen, in which the timing is determined by the availability of cycling females. Conception rates achieved using frozen semen vary according to the extender and the sperm-processing techniques used (pellets versus straws, freezing rates, thawing rates). Pregnancy rates achieved with vaginal insemination using frozen-thawed semen of good quality have been about 30%, whereas pregnancy rates of 50% to 80% have been achieved when intrauterine insemination has been performed. The frozen semen should be accompanied by information from the collection and storage facility regarding the number of sperm in each straw or vial and the recommendations for thawing the semen. The latter is important because the ingredients in the extender influence the ideal thawing rates and temperatures, which have a significant effect on the postthaw motility. For additional information on AI and regulations for international shipment of chilled and frozen canine semen, the reader should consult the International Veterinary Information Service (www.IVIS.org).
DIAGNOSTIC TECHNIQUES TO ASSESS REPRODUCTIVE FUNCTION
SEMEN COLLECTION AND EVALUATION
Indications
Semen is collected and evaluated as a routine part of a breeding soundness examination, for evaluation of male infertility, when artificial insemination is to be performed, and when semen is to be preserved by chilling or freezing. Cytologic evaluation of the ejaculate is also used to evaluate diseases of the prostate, testes, and epididymides.
Technique
Many factors affect semen quality, including the animal’s age, testicular size, frequency of ejaculation, degree of sexual arousal, collection technique, and the amount of seminal fluid collected. Semen is easily collected from dogs, especially those with previous breeding experience. The collection area should be quiet and free from distractions, with secure footing for the animals. The dog is encouraged to ejaculate by rapid massage of the bulbus glandis through the prepuce. The presence of an estrous bitch will improve the quality of the ejaculate and the ease with which semen is collected (libido). It has been shown that prostaglandin F2α (Lutalyse®, Pfizer) 0.1 mg/kg, administered subcutaneously 15 minutes before collection, increases the number of sperm ejaculated, similar to the presence of an estrous bitch. It may also have a positive effect on libido. When prostaglandin F2α and an estrous teaser bitch are both used, the effects are additive and the number of sperm ejaculated may be increased by nearly 300% (Root Kustritz et al., 2007). Side effects of prostaglandin F2α are transient and mild but also common. They include salivation, defecation, and vomiting. Semen can also be collected by electroejaculation, but this is rarely necessary in domestic dogs. Semen is usually collected from cats by electroejaculation under general anesthesia, although some cats can be trained to accept an artificial vagina. Feline semen collection and evaluation are not usually performed in clinical practice, but assisted reproduction techniques are used extensively in the study of endangered feline species.
Canine semen is ejaculated in three fractions. The first fraction, or presperm fraction, is composed of a few drops of clear fluid that originates from the prostate. Although this is uncommon, certain dogs may ejaculate several ml of the presperm fraction. The second fraction is the sperm-rich fraction. The volume of the sperm-rich fraction varies from 0.5 to 5 ml, depending on testicular size and individual variation. The sperm-rich fraction appears cloudy and opalescent. Usually, no attempt is made to separate the first two fractions. The third and largest fraction is prostatic fluid, of which there may be as much as 30 ml. Normal prostatic fluid is clear and easily distinguished from the milky, sperm-rich fraction. For routine semen evaluation and artificial insemination, it is sufficient to collect only enough prostatic fluid to ensure that the entire sperm-rich fraction has been obtained.
There are a variety of sophisticated methods that can be used to assess the structure and function of spermatozoa. These include various staining techniques to evaluate viability, the integrity of the plasma membrane, the capacitation status, and the acrosome reaction. There are in vitro assays to assess sperm functions, such as the ability to stimulate the acrosome reaction, measuring the ability of sperm to bind to the zona pellucida, or determining the ability to penetrate the oocyte. Computer-assisted sperm analysis programs have been adapted for use with canine semen. These programs can assess sperm motility and morphology. At this time uniform laboratory standards for adapting these procedures to canine semen have not been established, and the correlation of specific findings with in vivo fertility has not been determined. Nevertheless, these methods have important potential application, especially for semen freezing centers. Scanning and transmission electronmicroscopy continue to be useful for evaluation of sperm morphology.
In clinical practice semen evaluation focuses primarily on sperm numbers, morphology, and motility. These parameters are often referred to as the spermiogram. A complete cytologic evaluation of other cells (e.g., white blood cells, red blood cells, epithelial cells) in the seminal fluid is also important. In cases of azoospermia determination of seminal fluid alkaline phosphatase is indicated. Because feline semen is rarely evaluated in clinical practice, the interested reader is referred to Zambelli (2006b) and Johnston (2001) for additional information (see Suggested Readings). Semen must be handled carefully. All equipment should be clean and free of contaminants, including water and excessive lubricant. The sample must be protected against sudden changes in temperature. Normal dog semen can usually be handled at room temperature for 10 to 15 minutes without adverse effects. Nevertheless, the sample should be processed promptly. Slides and coverslips should be maintained at 37°C.
Motility
Assessment of motility is usually the first step in semen evaluation because it must be assessed promptly after collection so that changing temperature does not slow motility. Although not a precise measure, assessment of motility is considered a critical part of semen evaluation because it gauges spermatozoal function and viability. Very poor samples can be distinguished from very good ones. A decrease in the percentage of motile sperm is one of the first detectable changes after testicular injury. Asthenozoospermia is the term used to denote low motility. The percentage of motile sperm and the vigor of the movement can be spuriously diminished if the sample is exposed to excessive heat or cold, contaminated equipment, inflammatory cells, or bacteria. Poor motility may also be found in the setting of incomplete ejaculation. The motility of sperm ejaculated after a long sexual rest may also be poor because of aging of the cells. In addition, sperm in semen that has been chilled or frozen usually does not regain its original motility when warmed. In the case of chilled semen, the percentage of motile sperm may be similar to that seen in the fresh semen, but the individual sperm usually move with less vigor. Both the percentage and the speed of motility are usually diminished in frozen-thawed semen. Side-to-side oscillation may be a reflection of cooling, or it may be an artifact, representing the jostling of nonmotile sperm by motile ones.
To assess spermatozoal motility, a drop of undiluted semen is placed on a warm slide, covered with a warm coverslip, and examined by phase-contrast or light microscopy using the 40× and 100× objectives. Very concentrated samples should be diluted with warm 2.9% sodium citrate or phosphate-buffered saline solution to permit careful evaluation of individual spermatozoa. The percentage of motile sperm and the vigor of their movement are then estimated. In the normal dog more than 70% of the sperm should show rapid, steady, progressively forward motility. Although normal parameters for the feline spermiogram have not yet been adopted, reports of semen collected by electroejaculation and artificial vagina describe 60% to 85% progressively motile sperm. Spermatozoa that move in circles or in another nonlinear manner usually do so because of morphologic defects in the tail or midpiece.
Morphology
The sperm head is composed of the nucleus, which is covered proximally by the acrosome. The equatorial segment of the head represents thinning of the acrosome. The postacrosomal sheath and cell membrane cover the sperm head distally. The sperm tail is composed of the neck, midpiece, principal piece, and end piece. Often, the principal piece and end piece are collectively referred to as the tail. The neck is composed of laminated fibers and implantation plates that connect the midpiece to the head at the implantation fossa. A mitochondrial helix surrounds the axoneme of the midpiece. The axoneme is composed of nine microtubule doublets surrounding a central pair of singlet microtubules. A fibrous sheath of nine outer dense fibers surrounds the axoneme of the principal piece. The outer dense fibers gradually dissipate, and the end piece begins where the fibrous sheath ends. During spermatogenesis residual cytoplasm is extruded at the level of the midpiece.
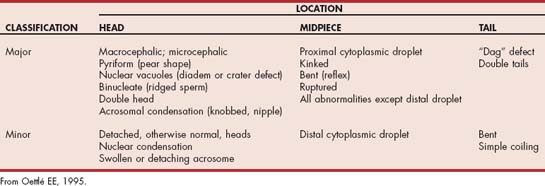
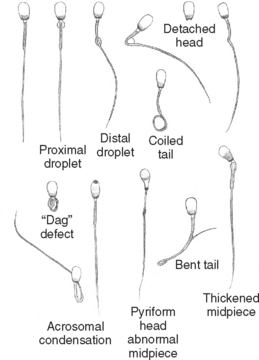
Teratozoospermia refers to abnormal spermatozoal morphology. Morphologic abnormalities are sometimes classified as being primary or secondary or as being major or minor. Primary abnormalities are usually attributed to abnormal spermatogenesis in the testicle, whereas secondary abnormalities are attributed to errors in epididymal maturation or improper sample handling. Because the morphologic abnormality does not always reflect the site of the lesion, the abnormality should be described specifically (e.g., bent tail). Abnormalities are also classified according to their likely effect on fertility as being of either major or minor importance (Table 60-1). Abnormalities in the size and shape of the head, acrosome, midpiece, or proximal tail, and proximal droplets are usually considered the most severe (Fig. 60-2). Loose or detached heads, or acrosomes that are otherwise normal, distal droplets, and bent tails are considered less severe, although they may be the first abnormalities noted after a testicular insult. These classifications were developed for evaluation of fresh semen. The relative significance of some abnormalities, such as the acrosomal reaction, may differ in frozen-thawed semen because they may then reflect irreparable damage during processing.
Morphology can be assessed in unstained samples using phase-contrast microscopy. Many abnormalities can also be seen in unstained samples using routine light microscopy with properly adjusted light intensity and low condenser position, but more information can be gained when the samples are stained. Although many stains can be used to evaluate sperm morphology, in veterinary medicine the most common are eosin-nigrosin stains (e.g., Society for Theriogenology morphology stain). In dogs and cats modified Wright’s stain (Diff-Quik®, VWR Scientific Products) is a better choice because there is less chance of stain-induced artifact in canine semen, and the staining characteristics using Diff-Quik correlate well with biomarkers of feline spermatozoal health (Mota et al., 2006). Another advantage over eosin-nigrosin stains is that other cells, such as red blood cells and white blood cells, are also stained and easily recognized. Stained slides are examined microscopically under oil immersion. A minimum of 100, but preferably 200, spermatozoa is classified as being normal or abnormal (Figs. 60-2 to 60-4). Fewer than 20% of the spermatozoa in semen samples from normal dogs have morphologic abnormalities. Electron microscopy may provide additional useful information in selected cases.
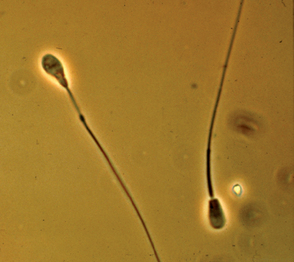
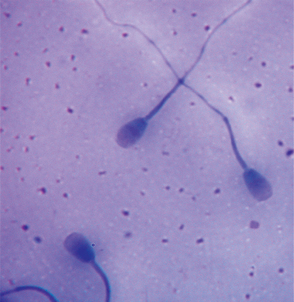
FIG 60-4 Canine spermatozoa with pyriform head and abnormal midpiece; phase-contrast microscopy.
(Courtesy Dr. Patricia Olson, Morris Animal Foundation, Englewood, Colo.)
Bent tails deserve special mention because they may be artifactually induced by cold shock and by stain or diluent of improper pH or osmolality. Finding excessive numbers of bent tails in a sample previously assessed as having had normal motility strongly suggests the possibility that the bent tails were caused by the stain because sperm with bent tails usually cannot move with a straight, forward progression. Stain-induced abnormalities and those resulting from improper sample handling should not be found in subsequent, properly handled aliquots of the sample. Persistence of morphologic abnormalities is an indication for further diagnostic evaluation, including semen culture.
Spermatozoa Concentration
The number of normal motile sperm has a direct effect on fertility. Although pregnancies can be achieved with fewer sperm, a minimum of 200 million motile sperm are usually recommended for intravaginal artificial insemination in dogs. Doing so is expected to yield normal pregnancy rates and litter size. Fewer numbers of sperm or samples with high numbers of abnormal sperm yield lower pregnancy rates and/or smaller litter size. The negative effects of some sperm defects can be overcome (compensated for) by increasing the number of sperm inseminated, whereas others cannot. In dogs the volume of the ejaculate and thus the concentration of sperm are influenced most by the volume of sperm-free prostatic fluid. For this reason the number of sperm in the total ejaculate, rather than the number of sperm per ml of semen, is assessed. The number of sperm is determined using a hemocytometer. The sample to be counted is usually diluted 1 : 100 using white blood cell/platelet dilution pipettes (Unopette; Becton-Dickinson and Co.). It may be unnecessary to dilute oligozoospermic samples before counting. To calculate the number of sperm per ejaculate, the number of sperm per ml as determined by the hemocytometer is multiplied by the total volume (ml) of the sample collected. Spectrophotometric methods may also be used to determine the sperm count. The number of sperm per ejaculate in normal dogs is 300 × 106 to 2000 × 106. Average counts of 30 × 106 to 300 × 106 sperm per ejaculate are reported for cats.
There is great interdog and intradog variation in the number of sperm in any given ejaculate from normal fertile dogs. The breed of dog, size of the testes, degree of arousal, and frequency of ejaculation affect the number of sperm ejaculated. Because the spermatogenic potential is directly related to testicular size, smaller breeds of dogs are expected to have fewer sperm per ejaculate than large breeds of dogs. Although it has been stated that sperm production is also directly related to body weight, this holds only for animals in normal body condition. The relationship between sperm production and body weight is lost in obese dogs. Daily ejaculation depletes extragonadal reserves from the epididymis and ductus deferens, causing an immediate and dramatic reduction in the number of sperm ejaculated, but in normal males the numbers are often still within the normal range. To minimize this effect, at least 1 day, and preferably 3 to 4 days, should elapse between collections. This is especially important for semen being collected for freezing and for subfertile males in any setting. As a general rule, a total number of less than 200 × 106 sperm in any sample from a mature dog should be considered abnormally low (oligozoospermic), regardless of the breed of dog.
Volume
The volume of seminal fluid, and therefore the concentration of the sperm, varies according to collection technique. Typically, electroejaculation yields higher volume and lower concentration than does the use of an artificial vagina. The seminal volume is determined directly from the calibrated tube into which the sample is collected. Volumes tend to be smaller in young dogs than in mature dogs. Volume does not usually correlate with fertility unless the animal fails to ejaculate an adequate amount of the sperm-rich fraction. For intrauterine AI in bitches, a volume of 4 ml or less is used. This is accomplished by centrifugation of the sample to concentrate the number of sperm/unit volume.
Color
The color is assessed by direct visualization. Dog semen is normally white to opalescent and opaque. Inflammatory cells and squamous epithelial cells may also cause the seminal fluid to be opalescent. Inflammatory cells can originate from anywhere in the urinary or genital tracts, including smegma from the preputial cavity. Yellow seminal fluid usually indicates the presence of urine. To avoid urine contamination, dogs should not be allowed to micturate immediately before semen collection. Some males urinate during ejaculation, which is not normal. They are usually subfertile. Red or red-brown semen usually contains blood. Blood in a canine ejaculate usually originates from the prostate, or it results from damage to the surface of the penis during collection. The latter source of hemorrhage can easily be excluded by prompt inspection of the penile surface. Complete cytologic evaluation of the semen is important, especially in cases of infertility and when the sample has an abnormal color.
Cytology
In addition to the spermatozoa, all cells in the semen sample should be evaluated. Cytologic examination of the third fraction of the ejaculate is very helpful in the evaluation of canine prostatic disorders. A finding of red blood cells indicates hemorrhage, whereas a finding of white blood cells and macrophages indicates inflammation somewhere in the urogenital tract. Fewer than 2000 white blood cells per ml, or up to 7 white blood cells per high power field, is considered normal in canine semen. Dogs with leukospermia should be tested for Brucella canis (see Chapter 58), and the semen should be cultured for aerobes, anaerobes, and Mycoplasma. Some epithelial cells are normally present in dog semen, and their numbers increase with sexual rest. They are also present in the prepuce and on skin. Crystals may be found in samples contaminated with urine or with talc from the collection equipment. When excessive numbers of cells other than spermatozoa are found, further diagnostic assessment of the urogenital tract may be warranted.
Seminal Alkaline Phosphatase
Alkaline phosphatase is produced in the canine epididymis and the feline testis and/or epididymis. Therefore the enzyme can be used as an indicator that epididymal fluid, which should contain high numbers of motile sperm, is present in the sample. The seminal alkaline phosphatase activity in whole semen from normal dogs may be as high as 4000 to 5000 IU/L or greater and greater than 100,000 IU/L in cat seminal plasma. Azoospermia with low seminal alkaline phosphatase activity may indicate bilateral obstruction distal to the epididymes, or ejaculation may have been incomplete. Azoospermia with high alkaline phosphatase indicates failure of sperm production or bilateral obstruction at the rete testis. Alkaline phosphatase activity in seminal fluid is determined by the same methods used to determine alkaline phosphatase activity in serum.
Seminal pH
In some species changes in the seminal pH have diagnostic significance. The pH of canine seminal fluid and of prostatic fluid normally ranges from 6.3 to 7.0 and from 6.0 to 7.4, respectively, even in the presence of genital tract disease. Therefore determination of the seminal or prostatic fluid pH is rarely of diagnostic importance in dogs. Feline seminal fluid has a pH of about 6.6.
Interpretation of Semen Evaluation
The seminal characteristics thought to correlate best with fertility are the total number of sperm per ejaculate and the motility and morphology of spermatozoa. The quality of a canine semen sample is a reflection of (1) spermatozoal production during the past 62 days; (2) epididymal maturation during the past 14 days; (3) the extragonadal sperm reserves, which may take up to 7 days to be replenished in normal dogs; and (4) the spermatozoal output of that particular ejaculation. The finding of normal semen is not proof of normal fertility, however, because the male must also have normal libido and normal mating ability. Nevertheless, a dog with normal semen is expected to successfully impregnate a bitch if other factors are favorable. Likewise, the finding of abnormal semen does not necessarily indicate sterility unless there is persistent azoospermia or complete, true necrozoospermia. Even males with normal fertility, as demonstrated by breeding trials, may on occasion have a sample that is not within the expected normal ranges, particularly with regard to total sperm count. However, evidence shows that when fewer than 60% to 80% of the sperm are morphologically normal or when progressive motility is less than 50%, canine pregnancy rates are poor. Although it has been stated that prolonged abstinence contributes to poor semen quality because of spermatozoa senescence, it is unlikely that abstinence alone causes the quality of previously normal semen to diminish to the point of oligozoospermia and less than 60% normal morphology and motility. To help establish a prognosis or to resolve doubt about the cause of an unsatisfactory sample, the dog should be reevaluated several times over a period of at least 2 months. Recovery from a testicular insult may not be reflected by improved seminal quality for more than 3 to 5 months.
BACTERIAL CULTURE OF SEMEN
Quantitative and qualitative culture of the semen is indicated (1) as a routine part of the diagnostic evaluation of infertility in the male; (2) when excessive numbers of inflammatory cells are identified in the semen; and (3) in dogs with suspected bacterial prostatitis, epididymitis, or orchitis. Clinically significant growth of >105 colony-forming units (CFUs) of aerobic and anaerobic bacteria and Mycoplasma are recovered from more than half of normal fertile dogs that have no cytologic evidence of inflammation in the seminal fluid. Conversely, 30% of aerobic cultures from dogs with large numbers of white blood cells in the seminal fluid yielded no growth. For these reasons it has been recommended that semen culture be included as a routine part of the evaluation of canine infertility, irrespective of cytologic findings suggestive of infection. For culture results to be meaningful, an aseptic technique and sterile collection devices should be used. To limit contamination from the preputial cavity, the preputial orifice should be cleansed and smegma flushed off the surface of the penis before semen is collected. The sperm-rich fraction and the prostatic fraction of the canine ejaculate should yield fewer than 100 CFUs of bacteria per milliliter, whereas normal feline seminal plasma may contain more than 10,000 CFUs of bacteria. Results of semen cultures must be interpreted in conjunction with the clinical signs, the results of the cytologic evaluation of the ejaculate, and the variety of species of bacteria grown.
The normal bacterial florae of the prepuce and distal urethra are the same organisms most frequently isolated from normal canine and feline semen and from dogs with bacterial prostatitis, orchitis, or epididymitis. The normal florae of the distal urethra and prepuce consist predominantly of aerobic organisms, but anaerobic organisms are also found. Pasteurella multocida, β-hemolytic Streptococci, and Escherichia coli are the organisms most commonly isolated from dogs, whereas β-hemolytic E. coli, Pseudomonas aeruginosa, and Proteus mirabilis are the most common in cats (Boxes 60-1 and 60-2). The number of CFUs per milliliter of semen attributable to urethral contamination reportedly varies from 100 to 10,000. A separate culture of the material from a urethral swab, obtained before ejaculation, can be used to identify urethral organisms. Alternatively, the first fraction and the initial portion of the second fraction of the canine ejaculate can be discarded (i.e., not submitted for culture). The number of urethral organisms contained in the later seminal fractions then tends to be reduced. Separately culturing each fraction of the canine ejaculate may help show whether the infection is in the testes and epididymis (second fraction) or in the prostate gland (third fraction). In most dogs the first fraction consists of only a few drops of fluid and is difficult to separate from the second fraction.
 BOX 60-1 Bacterial Isolates from the Prepuce and Semen of Stud Dogs
BOX 60-1 Bacterial Isolates from the Prepuce and Semen of Stud Dogs
| Prepuce | Semen | Semen |
|---|---|---|
| Mycoplasma present in 11% of samples and 80% of dogs | Mycoplasma present in 3% of samples and 27% of dogs | Mycoplasma present in 58% of samples |
| No bacterial growth in 14% of samples | No bacterial growth in 70% of samples | No bacterial growth in 18% of samples |
 BOX 60-2 Bacterial Isolates from the Prepuce and Semen of Tomcats with Normal Semen
BOX 60-2 Bacterial Isolates from the Prepuce and Semen of Tomcats with Normal Semen
From Johnston SD et al: Ovarian and testicular function in the domestic cat: clinical management of spontaneous reproductive disease, Anim Reprod Sci 42:261, 1996.
| Prepuce | Semen |
|---|---|
| No aerobic bacterial growth in 10% of samples | No aerobic bacterial growth in 3% of samples |
In dogs with epididymitis or orchitis, a culture specifically for Brucella canis should be requested (see Chapter 58). Specimens for anaerobic, Mycoplasma, and B. canis culture must be handled promptly and carefully because these organisms are sometimes more difficult to isolate than aerobes. The veterinarian should contact the microbiology laboratory to obtain specific recommendations concerning the submission of these samples. Special media, such as Anaerobic Culturette (Becton-Dickinson), is often recommended. Despite widespread concern among dog breeders, the role of Mycoplasma as a spontaneous cause of canine infertility remains to be fully clarified (see Chapter 58).
DIAGNOSTIC IMAGING
In the evaluation of male reproductive disorders, radiography is used primarily to assess the size of the prostate gland and identify metastatic lesions in dogs with suspected prostatic adenocarcinoma. Ultrasonography is very useful to identify and characterize lesions within the prostate, testis, and epididymis; to help determine the cause of testicular or scrotal swelling; to assess the character of the spermatic cord in suspected cases of torsion; and to help establish the location of undescended testes. Ultrasonography is routinely used to guide biopsy needles for obtaining specimens from the prostate gland and focal lesions within the testis or epididymis.
HORMONAL EVALUATION
Testosterone
Testosterone is produced by the interstitial (Leydig) cells of the testes, under the control of LH and GnRH. It is secreted in a pulsatile manner that occurs about every 80 minutes in male dogs. In normal male cats the nadir concentrations may be below the levels of detection of some assay systems. There is also a diurnal rhythm, with the lowest serum concentrations in the morning and the highest at night. The serum testosterone concentration is most frequently measured to determine the presence and functional status of the testes. It is useful to differentiate previously castrated males from those with bilateral cryptorchidism or those in which an intraabdominal testis was left after castration of a scrotal testis. It may also be of interest in evaluation of intersex animals. A single random determination is often not helpful because the nadir concentrations can be very low and values from intact animals may overlap with those for castrated males. Provocative testing is necessary to adequately assess testosterone production. This is done by measuring the serum testosterone concentration before and after the administration of human chorionic gonadotropin (hCG) or gonadotropin releasing hormone (GnRH).
Because the preferred protocols and the reference ranges vary greatly among laboratories, consultation with the laboratory is important. For example, in hCG stimulation protocols the serum testosterone concentration is typically measured before and 2 to 4 hours after the administration of hCG (40 to 50 IU/kg, administered intramuscularly in dogs and cats; or 250 IU per cat, administered intramuscularly). In GnRH stimulation protocols samples are typically obtained before and 1 hour after administration of GnRH (0.22 μg/kg, administered intravenously in dogs; 1.0 to 2.2 μg/kg, administered intramuscularly in dogs; 25 μg/cat, administered intramuscularly). Reference ranges for intact male dogs from a large veterinary diagnostic laboratory are 2.6 to 13.9 nmol/L and 13 to 17.3 nmol/L, before and after GnRH, respectively. Castrated male dogs and cats have serum testosterone concentrations of <0.5 nmol/L. In contrast, another veterinary diagnostic laboratory’s ranges for intact male dogs are 0.19 to 26.3 ng/ml and 0.46 to 22.1 ng/ml and for castrated dogs 0.01 to 0.24 ng/ml and 0.02 to 0.42 ng/ml, before and after hCG, respectively; and the reference range for serum testosterone concentrations in castrated cats overlaps that of intact male cats (0.10 to 2.3 ng/ml versus <0.5 ng/ml, respectively).
Finding high serum concentrations of testosterone, with or without the administration of hCG or GnRH, indicates the presence of at least one testicle. Animals with only one testis and intersex animals may have testosterone values between those typically found in castrated and intact males after hCG or GnRH administration. Testosterone is sometimes measured in the evaluation of infertile males, but testosterone deficiency is rarely documented as a cause, or effect, of acquired infertility in dogs or cats. Congenital hypogonadism is characterized by abnormally small testes, which lack normal spermatogenesis and testosterone production.
Finding low serum concentrations of testosterone after hCG or GnRH administration in an otherwise healthy male almost always indicates previous castration because Leydig cells are quite resistant to thermal injury, toxic injury such as that caused by chemotherapeutic agents, and infectious agents, unless the entire testicle is destroyed by the process. Low testosterone concentrations could also indicate exposure to drugs such as ketoconazole that interfere with steroid hormone production or drugs that suppress GnRH or LH, such as gonadal steroids or GnRH antagonists (See the section on contraception in Chapter 56). Severe malnutrition can damage Leydig cells.
Because the development and maturation of the penile spines in cats and the prostate gland in dogs are androgen dependent, they serve as bioassays for the presence of DHT. Penile spines begin to appear in intact male cats at about 12 weeks of age; they regress by 6 weeks after castration. The finding of penile spines indicates the presence of testicular tissue and justifies a presumptive diagnosis of cryptorchidism in tomcats that do not have palpable testes in the scrotum. Finding a prostate gland in a supposedly castrated dog that is of normal size for a sexually intact dog indicates either prostatic neoplasia or the presence of a retained testicle. Administration of exogenous androgens would be another possible explanation in both species.
Gonadotropins: Follicle-Stimulating Hormone and Luteinizing Hormone
The gonadotropins FSH and LH are produced by the pituitary under the control of hypothalamic GnRH (see Chapter 56; Fig. 56-4). They are secreted in a pulsatile manner. LH pulses occur about every 100 minutes during daylight hours and approximately every 80 minutes during darkness. FSH supports Sertoli cell function and spermatogenesis. LH stimulates testosterone secretion by the Leydig cells of the testis. The gonadal hormones, in turn, feed back to the hypothalamus and pituitary. After gonadectomy this negative feedback control of the gonadotropins is lost, and serum concentrations of LH and FSH are persistently elevated. This could also occur with the rare condition of gonadal dysgenesis. Serum LH concentration in normal male dogs reportedly ranges from 0.2 ng/ml to less than 20 ng/ml. Normal FSH concentrations in healthy dogs reportedly range from 20 to 293 ng/ml.
To avoid unnecessary laparotomy, serum concentrations of LH can be measured to determine the presence or absence of gonads in animals with unknown reproductive status, such as those newly acquired by shelters or private owners. Males with very high LH have been castrated. Males with low LH have one or both testicles. If the testes are not in the scrotum, the male is cryptorchid. Much less likely causes of low LH would be exposure to exogenous sex hormones or a hypothalamic-pituitary lesion causing hypogonadotropic hypogonadism. Males with hypogonadism have abnormally small testes with diminished (or absent) spermatogenesis and testosterone production. The secretory capacity of the pituitary gonadotropins can be assessed by determining LH and/or FSH before and after administration of GnRH. A point-of-care, semiquantitative immunochromogenic assay for LH (ICG Status-LH®, Synbiotics) has been intermittently available. Few commercial laboratories offer validated quantitative assays for LH or FSH for veterinary species at this time.
TESTICULAR ASPIRATION AND BIOPSY
Testicular biopsy or aspiration and epididymal aspiration are usually reserved for animals that have been thoroughly investigated by other noninvasive means but in which no cause of infertility has been identified. Aspiration, biopsy, or both are indicated early in the evaluation of animals with discrete, focal lesions or in those with marked changes in the consistency of the testis or epididymis. The cytologic evaluation of testicular aspirates can identify inflammatory cells, sperm, neoplastic cells, and infectious agents. Testicular aspiration is usually reserved for the evaluation of discrete lesions rather than the assessment of spermatogenesis because tissue architecture is not preserved and the progression of spermatogenesis cannot be assessed. Fine-needle (i.e., 25 gauge) aspiration of the testes is performed in a manner similar to the aspiration of other masses. Sedation may be required in some dogs and is usually recommended for animals undergoing epididymal aspiration. In the absence of equipment to collect semen from cats, cytologic evaluation of a testicular aspirate could be used to confirm the presence of sperm.
Seminiferous tubule architecture, the progression of spermatogenesis, and interstitial and Sertoli cell numbers can be evaluated in specimens obtained by biopsy. Histopathology can also be used to determine whether there is inflammation or neoplasia within the testicular parenchyma. It has been shown that biopsy of a normal testis has no deleterious effect on semen quality in normal dogs. General anesthesia is required for animals undergoing testicular biopsy. The initial surgical approach is similar to that used for open castration except that the testis is not lifted out through the skin incision. When the proper vaginal tunic and the adherent tunica albuginea are incised, normal testicular tissue promptly bulges through the incision site. This bulging testicular tissue is excised for histopathologic and microbiologic evaluation. The proper vaginal tunic/tunica albuginea is closed. Then the common vaginal tunic is closed, the testis is replaced in the scrotum, and the closure is as in a routine castration. Alternatively, the skin is incised with a scalpel, the testis is immobilized, and a biopsy needle is pushed through the tunic into testicular tissue. Incisional biopsy provides larger tissue specimens than does needle biopsy, but this method also causes greater damage to testicular parenchyma. Testicular tissue for histopathologic evaluation must not be fixed in formalin because artifacts are produced. Zenker’s, Bouin’s, glutaraldehyde, and Karnovsky’s fixative are recommended, depending on whether tissues are to be embedded in paraffin or plastic. Some clinicians prefer glutaraldehyde over Bouin’s for the epididymis. It is recommended that the pathologist be consulted regarding the preferred fixative before obtaining the specimen.
Complications from testicular aspiration or biopsy are not common if a careful, gentle, and aseptic technique is used. However, some of the potential complications could adversely affect the future fertility of the dog. These include swelling, local hyperthermia, infection, hemorrhage, and the formation of sperm granulomas. We routinely rinse away residual scrub solutions and apply an ice pack to the biopsy site while the dog is recovering from anesthesia to minimize swelling and local skin irritation. All pertinent noninvasive tests should be performed before biopsy is considered.
Noninflammatory, degenerative conditions of the testes vary in severity from diminished spermatogenesis to a complete absence of germ cells and collapse of the seminiferous tubules. Sometimes, only Sertoli cells remain. The less severe lesions are potentially reversible if the underlying cause can be eliminated. Unfortunately, the histologic appearance of testicular specimens obtained from animals with degenerative conditions of the testes rarely indicates the initiating cause. Chemical toxins and thermal and radiation injury can all cause testicular degeneration that may progress to testicular atrophy. The Leydig and Sertoli cells may be spared. Libido is maintained if the Leydig cells are not affected. Chronic testicular infection can also result in testicular degeneration. In this event evidence of the etiologic agents and inflammation may no longer be present.
Suppurative inflammation of the testes is characterized by infiltration of neutrophils. Macrophages and giant cells may also be found. Bacterial or mycotic infections are the usual cause. Viral orchitis, which occurs in some species, has not been reported in dogs. Immune-mediated reactions to sperm, mycotic infection, and Brucella canis infection are the most common causes of granulomas in the canine testicle.
If lymphocytes and plasma cells are found, the orchitis is usually thought to be immune mediated, but this does not exclude the possibility that infection was the initiating cause. For example, chronic B. canis infection is characterized by lympho-plasmacytic inflammation that is thought to be caused by antisperm antibodies produced as a result of the infection. Because the antigens that are unique to spermatozoa are usually not accessible to immune surveillance, any thing that disrupts the integrity of the seminiferous tubules or the blood-testis barrier has the potential to expose sperm antigens and incite an immune response. By this mechanism, testicular trauma, infection, or neoplasia may cause lymphocytic orchitis. Often, the cause of canine lymphocytic orchitis is not found, and sterility ultimately occurs. Foci of lymphocytes can be found in testicular biopsy specimens from apparently normal cats of all ages. The significance of these is unknown, but they are most prevalent in cats older than 8 to 9 years of age.
DIAGNOSTIC APPROACH TO INFERTILITY
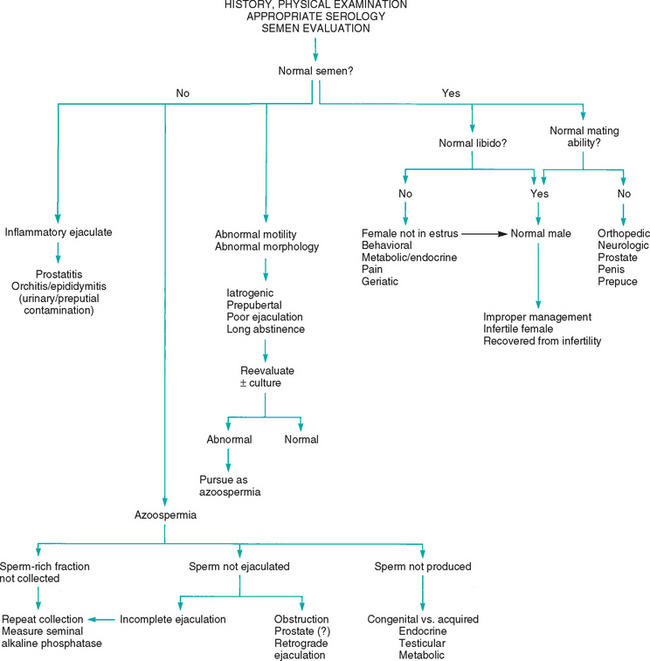
Normal seminal quality, normal desire to breed (libido), and normal ability to mate are all necessary for normal fertility in males. Therefore the diagnostic approach to infertility must investigate all three of these factors (see Fig. 60-5). Dogs achieving pregnancy rates of less than 75% when bred to apparently normal females using proper breeding management should probably be evaluated for subfertility because pregnancy rates of 85.4% ± 12.4% have been reported for privately owned, fertile stud dogs in which two matings per estrus were done. Pregnancy rates of greater than 90% are expected in well-managed commercial breeding colonies because individual animals with poor fertility are likely to be promptly culled.
The diagnostic approach begins with a complete history and physical examination. The history should assess the male’s past breeding performance, breeding management, fertility of the females to which he has been bred, and current or previous health problems (Box 60-3). Some common drugs and metabolic disorders that are known to affect male fertility are listed in Box 60-4.
Assessment of the male’s libido and mating ability can help narrow the differential diagnoses. A normal male may appear to lack libido if it is not in its established territory, if it is less dominant than the female or another male in the immediate vicinity, if it is inexperienced or frightened, or if it prefers a different partner. Some normal males show no interest until the female is actually in estrus, as opposed to proestrus. Dogs that are accustomed to semen collection may no longer be interested in natural service despite normal arousal and a willingness to ejaculate. Daily ejaculation, especially over a week or two, and ejaculation more often than once a day are other factors that can diminish the libido of normal male dogs. Frequent ejaculation does not diminish libido in tomcats. Excessive endogenous or exogenous glucocorticoids, stress, and pain also cause decreased libido in dogs. Libido also appears to decrease with advancing age.
Generally, mating ability is determined by physical, mechanical, and neurologic factors governing mounting, erection, intromission, and ejaculation. Orthopedic disorders of the rear legs; spine; and, less commonly, the front legs may prevent mounting or intromission but do not usually affect libido and ejaculatory ability unless they are painful. Semen collection and artificial insemination could be used in such animals. Some animals may exhibit normal arousal and mount only to dismount before attempting intromission. It is often difficult to determine whether this behavior is caused by inadequate libido or by inadequate mating ability. This behavior is also often exhibited when a vaginal abnormality is encountered and also in some males accustomed to semen collection. Painful conditions often diminish libido as well as interfere with mating ability.
A complete physical examination should be performed to assess the animal’s overall health and identify congenital or heritable abnormalities that should be grounds for excluding the male from the breeding program. Many metabolic and physical abnormalities can adversely affect spermatogenesis, libido, and mating ability. The testes and epididymes are palpated to determine their size, shape, consistency, and location. In situations of unilateral disease, there is urgency to establish and correct the cause before the condition affects the contralateral testis. This can occur by direct extension of the disease process itself or as a result of local swelling, pressure, and hyperthermia, all of which are deleterious. Finding abnormally small testes in an infertile male justifies a guarded prognosis for recovery of fertility, irrespective of the underlying cause. Testicular atrophy is common in dogs older than 10 years of age. The American Kennel Club will not register puppies sired by dogs 12 years of age or older without documentation of semen quality. The canine prostate is palpated per rectum and transabdominally. The penis and prepuce are palpated and inspected. Because the penis must be extruded from the prepuce for a thorough examination to be performed, as well as for semen to be collected in dogs, the two are often performed together. This is contraindicated if the history indicates the animal may have a penile lesion that could be aggravated by sexual arousal.
Anatomic abnormalities reported to cause difficulty in mating include phimosis, a persistent penile frenulum, an abnormally short os penis in dogs, and entanglement of the penis in preputial hair in cats. Tomcats that fail to grasp the queen’s neck in the proper location may not be in the correct position for intromission. This is seen in some inexperienced males and in mating pairs with disparate body lengths. Male dogs are often reluctant to breed bitches with anatomic abnormalities of the vulva or vagina. Usually, neither shows outward signs of discomfort other than failure to mate; thus it may be difficult to discern whether intromission does not occur because of a female or a male problem.
A thorough neurologic and orthopedic examination should be performed. Neurologic disorders can interfere with mounting, erection, intromission, and ejaculation. For example, motor nerve dysfunction can cause difficulty with mounting and intromission, but semen collection for AI may be possible in such animals. Sensory or autonomic disturbances can cause difficulty with erection (and therefore with intromission in cats) and ejaculation. Semen collection by electroejaculation may or may not be possible in such animals, depending on the location of the lesion.
Semen evaluation is a crucial part of evaluating male infertility (Fig. 60-5). In addition, the semen of infertile males should be submitted for aerobic, anaerobic, and Mycoplasma culture, and B. canis testing should be performed in dogs. Males with a history of infertility but that currently have normal semen, normal libido, and normal mating ability are now normal. Such males may have recovered from their previous infertility, the breeding management (e.g., timing of insemination) could have been inappropriate, or the female may have been infertile. Normal males should be bred again to fertile females using optimal breeding management. If the semen is abnormal, further evaluation of the reproductive tract is indicated. Semen is judged to be abnormal if inadequate numbers of sperm are found, if the motility of sperm is inadequate, if sperm morphology is abnormal, or if the semen contains excessive numbers of other cells (white blood cells, macrophages, red blood cells).
Abnormal motility (asthenozoospermia) and morphology (teratozoospermia) are often the first indicators of gonadal damage, irrespective of the cause. Morphologically abnormal sperm often do not have normal motility. Causes include primary testicular disease, metabolic and endocrine disorders, transient insults (e.g., fever), incomplete ejaculation, and iatrogenic causes. Sperm in semen from young dogs and dogs that have not mated for a long time may show poor motility, and the semen may contain more than the usual number of morphologically abnormal sperm. Iatrogenic causes include temperature shock, exposure of the semen sample to a stain of improper pH and osmolality, and exposure of the sample to latex rubber, plastics, and other spermicidal agents.
Infertile animals with abnormal semen should be reevaluated in 4 to 7 days or sooner if an iatrogenic cause is suspected. Care should be taken at that time to ensure that the entire sperm-rich fraction is collected and that improper handling does not damage the sample. If abnormalities persist, semen culture and ultrasound of the reproductive tract are indicated. A metabolic evaluation (e.g., complete blood count, serum biochemistry panel, urinalysis) is also appropriate. If no other abnormalities are identified, semen should be reevaluated in 2 to 3 months before additional testing is done. If the problem persists, additional testing is indicated, as discussed in the section on acquired infertility.
OLIGOZOOSPERMIA AND AZOOSPERMIA
A decrease in the total number of sperm per ejaculate may occur with or without abnormalities in sperm morphology or motility. Sperm numbers may be less than normal (oligozoospermia), or sperm may be completely absent (azoospermia). The concentration of sperm per ejaculate may decline because of abnormalities in spermatogenesis or ejaculation. The clinician must always exclude the possibility that the entire sperm-rich fraction was not collected before proceeding further. This is ensured by repeat semen collection. In dogs an estrous bitch and the administration of prostaglan din F2α should be used to maximize the number of sperm ejaculated (see p. 953). In cats another series of electrical stimulation should be given because the occasional azoospermic sample happens in normal males.
Spermatogenesis is a complex process that can be affected by environmental factors such as scrotal temperature; metabolic disorders, especially endocrinopathies; toxins and drugs; and infection. A thorough history taking and physical examination, ultrasound examination of the reproductive tract, semen culture, and standard laboratory tests help identify these possibilities. In addition, oligozoospermia and azoospermia may result from primary testicular failure or bilateral obstruction of the ductus deferens or epididymes. Because bilateral obstruction could occur at the level of the prostate gland, the prostate should be carefully evaluated. Measuring the seminal alkaline phosphatase activity (see p. 957) should help determine whether epididymal fluid was collected. High seminal alkaline phosphatase activity indicates that epididymal fluid, which should contain high numbers of motile sperm, was collected and that obstruction to flow from the epididymes is apparently not the cause of the low sperm count.
Retrograde ejaculation of semen into the urinary bladder rather than out the urethra is another cause of oligozoospermia. This condition is thought to be neurogenic in origin, perhaps resulting from inadequate pressure in the proximal urethra or neck of the bladder. Some spermatozoa normally pass retrograde during ejaculation but substantially more do so during electroejaculation than during natural copulation. In the event of pathologic retrograde ejaculation, the volume of semen or the number of spermatozoa discharged is lower than normal. Retrograde ejaculation is diagnosed on the basis of finding excessive numbers of sperm in urine after ejaculation. Urine may be obtained by catheterization or cystocentesis. Some sperm are normally found in urine, but large numbers, especially approaching those in discharged semen, are considered abnormal. Treatment with α-adrenergic drugs (e.g., pseudoephedrine, 4 to 5 mg/kg, administered orally q8h or twice, 3 hours and 1 hour, before breeding) to increase urethral tone in dogs with retrograde ejaculation has been recommended, but experience with this treatment is limited.
The treatment of oligozoospermia and azoospermia depends on finding and eliminating the cause. Unfortunately, this is not always possible. Oligozoospermic males may be subfertile rather than infertile. It is assumed that sperm reserves and spermatogenesis are poor in oligozoospermic males; therefore they should be bred judiciously. This means allowing for adequate time between breedings so that sperm reserves can be replenished, performing insemination at the optimal time determined by ovulation timing, and breeding only to healthy, fertile females. Dogs with as few as 20 × 106 to 100 × 106 sperm per ejaculate have been reported to successfully impregnate normal, fertile bitches when ejaculation has been limited to twice, done at a 2-day interval. Intrauterine, rather than intravaginal, insemination may also be considered.
Oligozoospermia may or may not progress to azoospermia, depending on the cause. As a general rule, azoospermic males tend to remain azoospermic, especially if testicular size is abnormally small. The finding of small testes in an infertile male suggests the presence of congenital hypoplasia or acquired testicular atrophy or fibrosis, none of which is likely to be reversible. Because recovery from a testicular insult is slow and because canine spermatogenesis takes 62 days, the animal could reasonably be evaluated every 2 months for 6 to 12 months.
CONGENITAL INFERTILITY
Congenital causes of infertility should be considered in azoospermic animals that have no history of siring a litter or other reproductive activity. Abnormalities of the hypothalamic-pituitary-gonadal axis, such as hypogonadotropic hypogonadism; anatomic abnormalities of the Wolffian duct system, such as atresia; and disorders of sexual differentiation, such as intersex, are possible causes. Successfully correcting the congenital causes of infertility is unlikely. The diagnostic evaluation necessary to confirm a specific cause of congenital infertility can be quite extensive unless the external genitalia are abnormal or an infertile tomcat happens to have a calico or tortoiseshell coat color. The black and orange coat color each require an X chromosome to be expressed. Because male cats normally have only one X, they cannot express both coat colors. Therefore a male cat with calico or tortoiseshell color has either XXY or one of several reported chimeric states. Some of these cats are phenotypically normal males with normal spermatogenesis, presumably because of chimerism with the normal feline 38 XY karyotype in the gonadal tissue.
The phenotypic, gonadal, and chromosomal sex of the animal can be determined. The chromosomal sex can be determined by karyotyping. The phenotypic sex is determined by physical examination of the external genitalia. The internal genitalia (Müllerian and Wolffian duct derivatives) can be examined by ultrasonography, laparoscopy, or laparotomy. The gonadal sex can be assessed by endocrinologic evaluation (serum testosterone and LH concentrations) and gonadal biopsy. Otherwise, the diagnostic plan is the same as that for males with acquired infertility.
ACQUIRED INFERTILITY
Animals with acquired infertility are known, or at least thought, to previously have had functionally and anatomically normal reproductive tracts. In some instances the onset of infertility or subfertility may be identified by a review of the breeding record, looking for a diminution in litter sizes and pregnancy/whelping rates. In other cases the time of onset of infertility is never determined. A thorough history should pay special attention to the possibility of toxin- or drug-induced infertility, excessive stress, or excessive frequency of ejaculation. A complete physical examination, semen evaluation and culture, ultrasonographic evaluation of the reproductive tract, and B. canis serology in dogs should be done. If these fail to establish the diagnosis, a thorough metabolic and endocrine evaluation should be done before more invasive procedures, such as testicular biopsy, are performed.
Bacterial infection of the testes, epididymides, or scrotum can cause alterations in spermatogenesis as a result of the destructive properties of the organisms themselves and as a result of local swelling and hyperthermia. B. canis, Mycoplasma, and herpes virus infections are discussed in Chapter 58. The role of bacterial prostatitis in canine infertility is unclear, but most theriogenologists consider bacterial prostatitis to be a common, potentially reversible cause of infertility. Appropriate antibiotic therapy should be initiated if the semen culture is positive for pathologic numbers of bacteria. Appropriate antimicrobial therapy should continue for a minimum of 2 to 4 weeks, or longer in the case of chronic bacterial prostatitis.
A variety of pharmaceutical agents have been empirically used to enhance semen quality in many species. The effect of drugs and nutriceuticals on the spermiogram depends on the cause of the problem. As discussed earlier, prostaglandin F2α has been shown to increase the number of sperm ejaculated and the ease with which semen is collected from normal dogs. In oligozoospermic and azoospermic dogs with pathologically high serum concentrations of estradiol and low serum concentrations of testosterone, treatment with an aromatase inhibitor corrected the hormonal concentrations and improved semen quality, although not to expected normal values. Treatment with vitamin E improved the spermiogram in dogs being given dexamethasone, presumably by protecting against oxidative stress. Whether vitamin E would improve semen quality in otherwise normal animals is not known because studies in rabbit, ram, and boar have yielded conflicting results.
There are anecdotal reports that treatment with GnRH or prolactin improved semen quality in dogs with abnormal spermiograms, but these reports lack evidence of the underlying pathophysiology, such as GnRH or gonadotropin deficiency or hyperprolactinemia. Glycosaminoglycans, omega-3 fatty acids, and vitamin C have also been tried. A dietary supplement containing docosahexaenoic acid (DHA), vitamin E, and selenium (PROSPERM®, Minitube America) has been used to improve semen quality in stallions and boars. Another antioxidant supplement (CellAdvance®, Vetri-Science Products) and a carnitine supplement (Motility Plus®, Platinum Performance) are also available. With few exceptions, there is not yet evidence of efficacy of these products in the treatment of canine infertility. Were these to be used, the duration of treatment would probably be at least 4 to 8 weeks because the canine spermatogenic cycle is about 60 days.
Testicular aspiration or biopsy should be considered when the abnormalities in the spermiogram have not improved after several months, especially in animals with normal testicular size. Testicular biopsy may be unwarranted in animals with testes that are already substantially smaller than normal because the testicular degeneration, atrophy, or fibrosis that cause small testicles are usually considered irreversible. Testicular biopsy specimens from cats older than 7 years show diminished spermatogenesis and degeneration of seminiferous tubules compared with the findings in younger cats. These are considered typical age-related changes. Testicular atrophy is common in dogs older than 10 years. A portion of the biopsy specimen should also be submitted for bacterial culture. The following histologic lesions have been identified in dog testes: neoplasia, suppurative and nonsuppurative inflammation, mycotic orchitis, lymphocytic orchitis, granulomatous orchitis, spermatogenic arrest, and testicular degeneration. There is limited information available on cats other than information on the testicular changes associated with aging.
Bjurström L, et al. Long-term study of aerobic bacteria in the genital tract in stud dogs. Am J Vet Res. 1992;53:670.
Davidson AP. Clinical theriogenology. Vet Clin North Am. 2001;31:2.
Greene CE, editor. Infectious diseases of the dog and cat, ed 3, Philadelphia: WB Saunders, 2006.
Hatamoto L, et al. Effects of dexamethasone treatment (to mimic stress) and vitamin E oral supplementation on the spermiogram and on seminal plasma spontaneous lipid peroxidation and antioxidant enzyme activities in dogs. Theriogenology. 2006;66:1610.
Hess M. Documented and anecdotal effects of certain pharmaceutical agents used to enhance semen quality in dogs. Theriogenology. 2006;66:613.
International Veterinary Information Service (www.IVIS.org): Recent advances in small animal reproduction, 2001.
Johnston SD, et al. Canine and feline theriogenology. Philadelphia: WB Saunders, 2001.
Johnston SD, et al. Ovarian and testicular function in the domestic cat: clinical management of spontaneous reproductive disease. Anim Reprod Sci. 1996;42:261.
Kawakami E, et al. Improvement in spermatogenic function after subcutaneous implantation of a capsule containing an aromatase inhibitor in four oligozoospermic dogs and one azoospermic dog with high plasma estradiol-17 β concentrations. Theriogenology. 2004;62:165.
Linde-Forsberg C: Hints on semen freezing, cryoextenders and frozen semen artificial insemination, Proceedings of the Annual Conference of the Society of Theriogenology and the American College of Theriogenology, Colorado Springs, Colo, 2002.
Mota P, et al. Comparison between different markers of sperm quality in the cat: Diff-Quik as a simple optical technique to assess changes in the DNA of feline epididymal sperm. Theriogenology. 2006;65:1360.
Oettlé E. Sperm abnormalities and fertility in the dog. In: Bonagura JD, editor. Kirk’s current veterinary therapy XII. Philadelphia: WB Saunders, 1995.
Peters MAJ, et al. Aging, testicular tumours and the pituitary-testis axis in dogs. J Endocrinol. 2000;166:153.
Rijsselaere T, et al. New techniques for the assessment of canine semen quality: a review. Theriogenology. 2005;64:706.
Root Kustritz, et al. Relationship between inflammatory cytology of canine seminal fluid and significant aerobic bacterial, anaerobic bacterial or Mycoplasma cultures of canine seminal fluid: 95 cases (1987-2000). Theriogenology. 2005;64:1333.
Root Kustritz, et al. Effect of administration of prostaglandin F2alpha or presence of an estrous teaser bitch on characteristics of the canine ejaculate. Theriogenology. 2007;67:255.
Thomassen R, et al. Artificial insemination with frozen semen in dogs: a retrospective study of 10 years using a non-surgical approach. Theriogenology. 2006;66:1645.
Wilson MS. Transcervical insemination techniques in the bitch. Vet Clin North Am. 2001;31:291.
Zambelli D, et al. Ultrasonography for pregnancy diagnosis and evaluation in queens. Theriogenology. 2006;66:135.
Zambelli D, et al. Semen collection in cats: techniques and analysis. Theriogenology. 2006;66:159.