CHAPTER 56 Disorders of the Estrous Cycle
NORMAL ESTROUS CYCLE
THE BITCH
The average age at the time of puberty in bitches is 9 to 10 months, and the range is 6 to 24 months of age. The interval from the beginning of one cycle to the beginning of the next, or the interestrous interval, varies from 4 to 12 months and averages 7 months. Therefore bitches have only one or two cycles per year. The interestrous interval is extremely variable within individual bitches, more so than it is among bitches. Because of this variability, the past interestrous interval does not accurately predict the next cycle in an individual bitch. Although a few bitches are very consistent, in most there is more than a month’s variation from cycle to cycle. The interestrous interval is not influenced by pregnancy or the photoperiod, although breeds such as the Basenji cycle only once each year, indicating a possible effect of the photoperiod in some individuals.
The estrous cycle in the bitch is divided into four components: proestrus, estrus, diestrus, and anestrus. Proestrus and estrus together are often referred to as heat or season. Together they constitute the follicular phase of the reproductive cycle. The luteal phase of the cycle is referred to as diestrus. The canine estrous cycle is distinctly different from that of other domestic species in several regards. These include the very long anestrus (months as opposed to days or weeks), the long proestrus and estrus (days to weeks as opposed to hours or days), the fact that the corpora luteal lifespan is independent of the presence (or absence) of pregnancy, ovulation of an immature oocyte, and long viability (days as opposed to hours) of oocyte and sperm within the female tract.
Proestrus
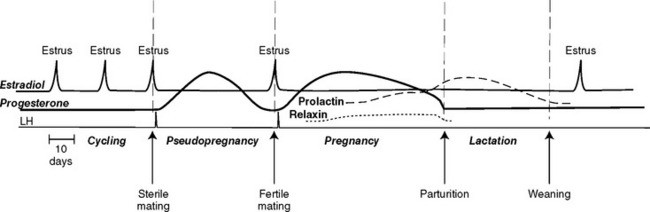
Proestrus is considered to begin when vulvar swelling and a sanguineous discharge are first observed. It ends when the bitch allows copulation. The average duration of proestrus is 9 days, and the range is 3 to 17 days. Attractiveness and receptivity to male dogs gradually increase throughout proestrus. The factors that end anestrus and initiate a new follicular phase in the bitch are poorly understood. Throughout anestrus follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are secreted concomitantly, in a pulsatile pattern. The FSH pulses are of lesser magnitude but longer duration than are the LH pulses. Basal concentrations of FSH increase as anestrus progresses, whereas basal LH concentrations are unchanged. The increase in FSH concentrations is considered crucial to initiate ovarian follicular development and the onset of proestrus. The developing ovarian follicles are 1.5 to 5 mm in diameter. They produce estrogens, the most important of which is estradiol 17-β. Estradiol causes the vulvar swelling, vaginal edema and cornification, and uterine bleeding that is recognized by a serosanguineous vulvar discharge. Estradiol serum concentrations gradually increase during early proestrus. They increase sharply just before the preovulatory LH surge and rapidly decline to basal levels thereafter (Fig. 56-1).
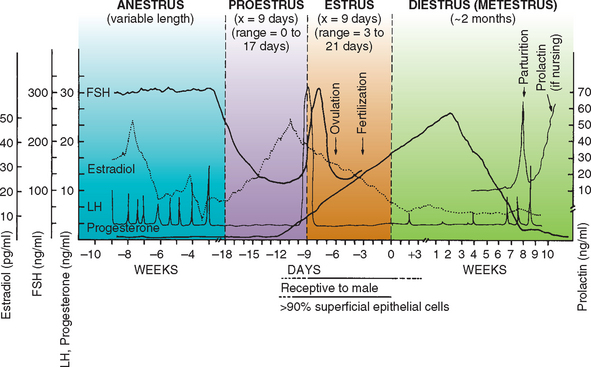
FIG 56-1 The canine estrous cycle.
(From Morrow DA, editor: Current therapy in theriogenology, ed 2, Philadelphia, 1986, WB Saunders.)
Under the influence of estrogen the vaginal epithelial cells proliferate and mature (cornification). The stratified squamous epithelium increases in thickness from a few cell layers in anestrus to 20 to 30 cell layers in late proestrus. The degree of estrogenic influence and therefore the stage of the estrous cycle with respect to the serum estrogen concentration can be monitored by vaginal cytology (see p. 891).
Estrus
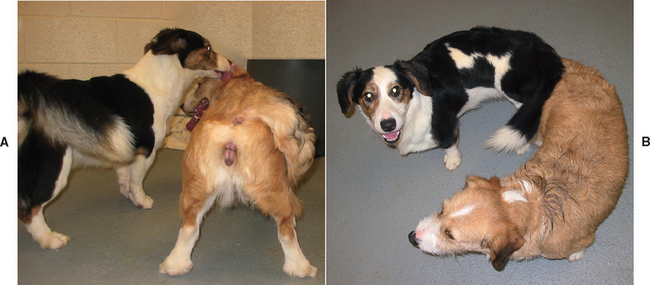
Behavioral estrus is characterized by acceptance of mating. The bitch’s feet are firmly planted to allow the male to mount—hence the term standing heat (Fig. 56-2). The tail is deviated to the side to allow intromission; this behavior has been referred to as flagging. Stroking the perineum may occasionally elicit flagging, which would be an indication that the bitch is in estrus. The average duration of estrus is 9 days, and the range is 3 to 21 days. The swollen vulva is less turgid than during proestrus. The vulvar discharge of estrus is usually less bloody than that of proestrus, but normal bitches often have a sanguineous discharge throughout both. Therefore changes in the gross appearance of the discharge are not necessarily indicators of the transition from proestrus to estrus.
The preovulatory follicles have reached 3 to 8 mm in diameter. The increase in estradiol concentrations during proestrus, via positive feedback to the hypothalamus, initiates the LH surge, which in turn causes ovulation and the subsequent formation of corpora lutea (CL) and progesterone secretion by the ovary (Fig. 56-1). In the bitch the initial increase in progesterone secretion coincides with the LH surge. Although the onset of behavioral estrus usually occurs within a day or two of the LH surge, behavioral estrus may occur as early as 4 days before or as late as 6 days after the LH surge. Therefore the day on which a bitch allows copulation is not closely associated with the LH surge or ovulation.
In most bitches ovulation occurs within 48 hours of the LH surge (the range is 0 to 96 hours). Ovulation from both ovaries is apparently completed within 24 hours. Primary oocytes (prophase I) are ovulated and resume meiosis during tubal transport. By 2 to 3 days after ovulation, oocytes have matured (metaphase II) and fertilization can occur. Mature oocytes have a fertile life of 2 to 4 days, perhaps longer. The time during which mature oocytes are available for fertilization has been referred to as the fertile period. Although semen is initially deposited in the cranial vagina during copulation, the large volume of prostatic fluid and the postcoital tie force semen through the cervix (see Fig. 56-2). For that reason dogs are considered among the species having intrauterine semen deposition during natural mating. Fertilization occurs in the uterine tubes. Sperm transport is enhanced by vaginal and uterine contractions that spontaneously occur with natural mating but not during artificial insemination. Freshly ejaculated canine sperm bind to the uterine crypts and glands and to the distal uterine tube, which serves as the sperm reservoir. The sperm reservoir maintains sperm viability between insemination and ovulation, regulates sperm capacitation to synchronize sperm function with ovulation, and controls sperm transport in the uterine tube. Canine sperm remain capable of fertilization in the female tract for 3 to 4 days and occasionally for as long as 6 days. Some sperm can be found in the female tract up to 11 days. The end of the fertile period is thought to be due to oocyte aging, but changes in the cervix and uterine tube environment also play a role.
Breeding Management
Because of the importance of territorial and social dominance to canine reproduction, the usual practice is to take the bitch to the stud for breeding. To optimize conception rates and litter size, viable sperm that are capable of fertilization and mature oocytes that are capable of being fertilized must be present simultaneously. This can be accomplished by a number of different strategies. A common practice is to begin breeding on a predetermined day of the cycle and to breed every other day for as long as behavioral estrus lasts or for at least two breedings. Often, day 10 to day 12 after the onset of proestrus is chosen. Because the average length of proestrus is 9 days, bitches experiencing an “average” cycle would be in estrus at that time. Because the LH surge usually occurs close to the onset of behavioral estrus, because ovulation usually occurs 2 days after the LH surge, because ova would be fertilizable 2 days later, and because freshly ejaculated semen is capable of fertilization for 4 days, this method of breeding management is usually successful. On the basis of data from artificial insemination programs, two breedings during the fertile period increase conception rates and litter size. Breeding every other day is certainly acceptable but probably unnecessary for animals with normal fertility, provided that at least two breedings are done during the fertile period.
The management scheme of breeding on a predetermined day of the cycle is often modified according to the behavior of the bitch and occasionally according to the behavior of the stud. Bitches not in estrus will not allow copulation. Putting the breeding pair together for supervised periods (15 to 60 minutes) and observing their behavior, a practice called teasing, will enable the manager to identify the first day of behavioral estrus; breeding can be done every 2 to 3 days thereafter throughout estrus. Certain males will occasionally show distinctly greater interest in breeding on a particular day during estrus than on other days of that cycle. Some kennel managers believe that such behavior in a male signals the optimal time for insemination, citing excellent conception rates and large litters from these males as validation.
Vaginal cytology is a very useful adjunct to these management schemes, especially in instances in which the female does not exhibit strong behavioral estrus or in which the breeding pair is separated geographically, necessitating transportation of the animals or the semen. The changes in the exfoliated cells reflect the effects of estrogen on the vaginal epithelium. Under the influence of estrogen, the vaginal epithelium changes in thickness from a thin layer (2 or 3 cells) of stratified squamous cells without cornification to many cell layers in depth with prominent cornification and rete pegging. The epithelial cells exfoliate easily. Vaginal cytology is an excellent bioassay for estrogen that can be used to monitor the follicular phase of the ovarian cycle. As the cytologic changes in proestrus approach those characteristic of estrus, the animal or the semen should be shipped to ensure safe arrival for insemination during the fertile period. Females that do not show normal behavioral estrus during the time that the findings of exfoliative vaginal cytology are consistent with estrus (i.e., greater than 90% superficial cells) could be bred using artificial insemination.
The success of these management methods is predicated on the assumptions that ovulation will occur sometime during behavioral and cytologic estrus and that multiple inseminations will ensure that viable sperm, capable of fertilization, are present whenever ovulation and oocyte maturation actually do occur. When the LH surge is identified and used in conjunction with the other management tools, the certainty that insemination is performed during the optimal fertile period is enhanced. The practice of using the LH surge to determine when to breed has been referred to as ovulation timing. Ovulation timing is especially helpful in situations in which gamete viability is less than optimal, such as with aged bitches or when frozen-thawed semen is used. The LH surge can be identified by measuring serum LH concentrations daily or by identifying the preovulatory increase in the serum concentrations of progesterone that coincides with the LH surge in bitches. Inseminations should be done 4 to 6 days after the LH surge. Interpretation of LH and progesterone results is discussed in greater detail in the section on the assessment of reproductive hormones.
Unlike the situation with queens, breeding a bitch several times during the same day appears to offer no advantage over breeding a single time on a given day. The day of insemination with respect to the occurrence of ovulation is more important than the number of inseminations per day. As the time between insemination and the fertile period lengthens, both conception rates and pups per litter decrease. Conception rates and litter size are also affected by maternal age. Conception rates, litter size, and neonatal survival are greatest for Beagle bitches between 2 and 3.5 years of age. After 5 years of age the conception rate and litter size decline, and neonatal mortality begins to increase. Similarly, in Labrador Retriever, Golden Retriever, and German Shepherd Dog bitches studied from 1 to 10 years of age, it was found that the number of pups born declines when bitches are 7 years of age or older. Litter size differs among breeds, with the bitches of smaller breeds tending to have fewer pups per litter because they produce fewer ova.
Diestrus
There are no external signs to mark the onset of diestrus other than the cessation of the signs of estrus. The beginning of diestrus is marked by an abrupt change in vaginal cytology. It is characterized by a sudden reduction in the number of superficial cells and the reappearance of intermediate cells, neutrophils, and background debris. Diestrus represents the luteal phase of the cycle. The serum progesterone concentration increases rapidly during the first 2 weeks after ovulation (see Fig. 56-1). It peaks at 15 to 80 ng/ml (approximately 47 to 250 nmol/L) by 15 to 30 days after ovulation. The luteal secretion of progesterone depends on pituitary LH and prolactin. The plasma progesterone concentration remains elevated but gradually declines during the next 2 months regardless of whether pregnancy occurs. In pregnant bitches there is a rapid prepartum drop in the progesterone concentration to less than 2 ng/ml (approximately 6.4 nmol/L). This occurs approximately 64 days after the LH surge and approximately 24 hours before the onset of parturition. The decline in the progesterone concentration may be more gradual in nonpregnant bitches and may not reach basal levels of 0.2 to 0.5 ng/ml (approximately 0.6 to 1.6 nmol/L) for 75 to 90 days. Specific luteotropic or luteolytic factors produced by the canine uterus or placenta that regulate ovarian CL function have yet to be identified. For example, the canine endometrium produces prostaglandin during pregnant and nonpregnant states, but this does not cause earlier CL regression in nonpregnant bitches. Although LH and prolactin are luteotropic in bitches, luteal regression appears to occur after a predetermined life span irrespective of the continuing availability of LH. Parturition and signs of false pregnancy (see Chapter 58) are the only clinical evidence of the end of diestrus. Endocrinologically, diestrus ends when the serum progesterone concentrations decline to less than 1 ng/ml (approximately 3 nmol/L).
Anestrus
Anestrus follows diestrus and ends with the onset of proestrus of the next cycle. The interval from the end of diestrus, as defined by basal serum progesterone concentrations, to the onset of proestrus is quite variable but averages 4.5 months. Because there are no external signs associated with anestrus, this phase of the cycle has been described erroneously as a period of sexual quiescence. In fact, the pituitary-ovarian axis and the uterus are active during anestrus. Pulsatile secretion of the pituitary hormones LH and FSH continue throughout. During anestrus the endometrium sloughs. The size and activity of the endometrial glands and the thickness of the myometrium and endometrium all decrease, although not to the parameters seen in prepubertal bitches. Endometrial repair continues for about 120 days after nonpregnant cycles and for slightly longer (150 days) after a pregnant cycle. The duration of anestrus per se is rarely determined in clinical practice because anestrus has no external indicators. Rather, the interestrous interval, the onset of proestrus of one cycle to the onset of proestrus of the next cycle, is usually described. The interestrous interval is not lengthened by pregnancy or lactation.
THE QUEEN
Female cats are seasonally polyestrous. Cyclicity is controlled by the photoperiod, which must be approximately 12 to 14 hours of light with an intensity of 50 foot-candles. Melatonin appears to be the signal of photoperiod in domestic cats. Cats exposed to natural light usually cease cycling during short days of winter, whereas cats in equatorial photoperiods or maintained under artificial light often cycle throughout the year. It has been shown that maintaining 14 : 10 to 16 : 8 hour light : dark schedules maximizes the number of cycling queens in the colony. In the presence of adequate light, sexual maturity and the first estrous cycle normally occur at 6 to 9 months of age, with a range of 5 to 12 months. Unlike bitches, which ovulate spontaneously, queens are induced to ovulate by coital stimulation of the vagina. In addition to coitus-induced ovulation, many domestic cats also have cycles in which spontaneous ovulation occurs.
The follicular phase of the cycle is characterized by increasing serum concentrations of estradiol 17-β associated with the onset of proestrus and estrus. Because there is negligible vulvar swelling or discharge in queens compared to bitches, proestrus and estrus are usually recognized by behavioral changes. When it is observed, the vulvar discharge is a clear fluid. Proestrus is characterized by rubbing, treading with the rear feet, vocalization, and decreasing hostility toward the male, although queens will continue to strike at the tom. Proestrus may be so short as to be unrecognized, but more typically it lasts 1 to 2 days.
Estrus is characterized by increased vocalization, rolling, lordosis, holding the tail to one side, and allowing copulation. The characteristic estrual posture can sometimes be elicited by stroking the perineum (Fig. 56-3). Tremors of the body or tail may also be seen. The cytologic appearance of exfoliated vaginal epithelial cells during the estrous cycle is similar to that of bitches, except that red blood cells are much less common. The duration of estrus among queens is quite variable but averages 5 to 8 days. Its duration is not influenced by copulation. Anovulatory cycles occur every 2 to 3 weeks (average 18 days with 12 hours of light) as long as light is adequate. There may or may not be a short interestrous period of a few days.
Ovulation occurs as a result of a neuroendocrine reflex that is initiated by the mechanical stimulation of sensory receptors in the vagina and cervix. This sensory input causes a surge of LH to be released from the pituitary gland (Fig. 56-4), which in turn causes ovulation. A high level of estradiol is also required for ovulation. The precise intensity of the copulatory stimulation necessary to induce the LH surge is unknown but varies among queens. The frequency of coital stimulation is apparently the single most important determinant of ovulation in cats. A single copulation induces the LH surge necessary for ovulation in approximately 50% of cats, whereas more than 90% of normal domestic shorthair cats ovulate if bred 3 times daily for the first 3 days of estrus. The day of estrus on which mating occurs and the duration of estrus have no apparent effect on ovulation, except insofar as the concentration of estradiol varies. Once the LH surge occurs, hormonal responses to additional copulatory stimuli are diminished. Ovulation occurs approximately 48 hours after the LH surge. Although cats continue to be referred to as induced ovulators, it is also clear that many cats (35% to 60%) also ovulate spontaneously, in the absence of coital stimulation or direct physical contact with other cats.
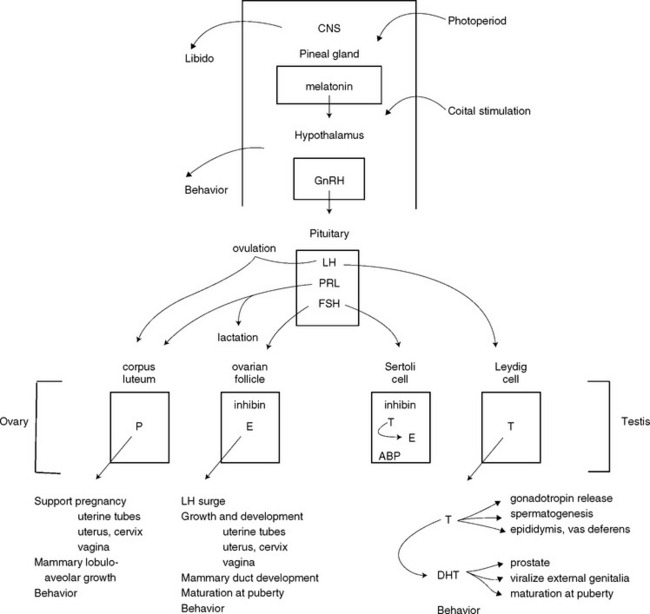
FIG 56-4 Hypothalamic-pituitary-gonadal axis. ABP, Androgen-binding protein; DHT, dihydrotestosterone; E, estrogen; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; P, progesterone; PRL, prolactin; T, testosterone.
After intromission and ejaculation, the queen emits a characteristic scream that signals to the male to dismount. Despite willing acceptance of copulation moments before, queens will attack the tom at this time. Because cats often prefer seclusion, breeding may not be witnessed by the owner. The queen’s scream may be the only evidence that mating has occurred. The queen then begins frenzied rolling and grooms her perineum for several minutes and aggressively rebuffs the male. When this “after-reaction” subsides, the queen allows another mating, by either the same tom or another one. Mating frequency is greatest during the first 2 hours (average of five copulations per hour), after which the frequency decreases to about one copulation per hour for the next 3 days. To ensure adequate copulatory stimulation to induce ovulation, three breedings per day for the first 3 days of estrus are recommended. Semen is deposited in the vagina during copulation. The cervix and uterotubal junction are barriers to sperm transport in the cat. The cervix is open on the first day of estrus in both ovulatory and nonovulatory cycles. It is closed when estradiol concentrations fall and when progesterone concentrations rise. As with the bitch, the queen’s uterine contractions during mating promote sperm transport. The uterotubal junction and uterine crypts are sperm reservoirs before ovulation, and the tubal isthmus is the reservoir near ovulation.
Because of the territorial nature of cats, especially males, the queen should be brought to the stud. The two should be placed together for short periods so that their behavior can be observed. In this way the manager can be confident that matings have occurred; conversely, the cats can be separated if fighting occurs. This supervised mating scheme may be the best way to optimize conception, but it is labor intensive. Some managers prefer to house the queen and tom together and allow mating to occur ad libitum, without direct observation. In some large breeding colonies, harem, rather than individual, mating schemes are used. In the harem scheme, one or two toms are housed with several queens. Even though both toms have equal access to the queens, the dominant male usually does most of the breeding. This method is the least labor intensive but has the disadvantages of unknown breeding dates, unknown paternity if more than one tom is involved, and delayed recognition of subfertility in individual animals.
After ovulation the follicles luteinize and produce progesterone. This is the luteal phase of the cycle. Serum concentrations of progesterone rise 24 to 48 hours after ovulation and peak 25 to 30 days later. As with bitches, luteal progesterone is necessary for the maintenance of pregnancy. The corpora lutea continue to produce progesterone throughout the approximately 65-day gestation (Fig. 56-5), with the serum concentrations gradually declining during the second half of pregnancy. Contrary to what was previously thought, the feline placenta either does not secrete progesterone or does so in amounts insufficient to maintain pregnancy. Serum concentrations of estradiol increase in late pregnancy in cats. Although estrous behavior has been observed in pregnant queens, true superfetation has not been proved.
There apparently are pregnancy-specific luteotropic hormones from the feline placenta or pituitary that control the life span of the corpus luteum. After the nonfertile induction of ovulation (i.e., when the animal is not pregnant), the corpora lutea persist for about 30 to 40 days. The next cycle may begin any time thereafter, usually within 10 days. In one colony the average interestrous interval was 61 days in queens that were bred but did not conceive, whereas the average interestrous interval for nonbred, anovulatory queens was 22 days. Serum concentrations of progesterone were not determined in those cats.
Litters typically consist of two to five kittens. Queens usually do not resume cycling while they are nursing a litter. Estrous behavior is usually evident 2 to 3 weeks after weaning, although this is quite variable. The postpartum estrus is shorter in duration and less fertile than others. Litter size and neonatal survival are best for queens age 1 to 5 years, provided that first parity occurs before 3 years of age. Litter size and neonatal survival usually improve after the first parity. However, if the first parity occurs after 3 years of age, litter size and neonatal survival usually remain poor. Reproductive performance declines after 6 years of age. Because of decreased fertility, decreased litter size, increased neonatal losses, and the increased prevalence of other illnesses in older queens, most should be retired from breeding after 8 years of age.
DIAGNOSTIC TESTS FOR THE REPRODUCTIVE TRACT
VAGINAL CYTOLOGY
The importance of exfoliative vaginal cytology in breeding management and in the evaluation of females with reproductive disorders cannot be overemphasized. Vaginal cytology is used to determine the stage of the estrous cycle, determine breeding and whelping (see Chapter 58) dates, and identify the nature of certain abnormal processes within the reproductive tract (see Chapter 57). Specimens may be obtained with a moistened, cotton-tipped swab or by flushing and aspirating a small volume of saline solution from the vagina. Specimens can be stained with any number of commercially available stains, including Wright’s, Wright-Giemsa, modified Wright-Giemsa (Diff-Quik; Baxter Scientific), trichrome, or new methylene blue. The number and morphologic characteristics of vaginal epithelial cells are evaluated. The preparations are also examined for the presence of other material, such as bacteria, white blood cells, red blood cells, mucus, cellular debris, endometrial cells, or neoplastic cells.
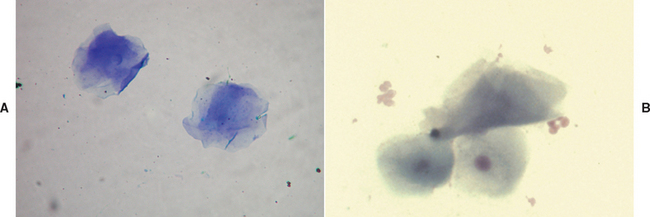
The vaginal epithelium changes dramatically under the influence of estrogen, a process known as cornification. During early proestrus the noncornified parabasal and intermediate vaginal epithelial cells are the predominant cells (more than 80%). As proestrus progresses, the population of exfoliated cells gradually matures; parabasal and intermediate cells disappear as superficial (cornified) cells increase in number. At the end of proestrus superficial and anuclear squamous cells account for 70% to 80% of the epithelial cells. White blood cells decrease in number. Extracellular bacteria may be present throughout proestrus and estrus (Fig. 56-6).
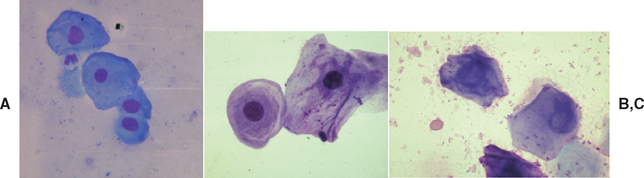
FIG. 56-6 Vaginal cytology of estrus. A, parabasal and intermediate cells. B, Intermediate and superficial cells. C, Anuclear squamous cells.
A predominance of superficial cells, an absence of neutrophils, and a clear background characterize vaginal cytologic specimens obtained during estrus. During estrus 90% or more of the epithelial cells are superficial and anuclear squamous cells. White blood cells are normally absent during estrus. Red blood cells and extracellular bacteria are often present (see Fig. 56-6). The beginning of diestrus is marked by an abrupt change in vaginal cytology. Diestrus is characterized by a sudden reduction in the number of superficial cells and the reappearance of intermediate cells, neutrophils, and background debris. On the first day of cytologic diestrus, parabasal and intermediate cells outnumber the superficial and anuclear squamous cells. Sheets of intermediate cells are also often observed. White blood cells return in high numbers during the first day or two of diestrus. Red blood cells and bacteria disappear. The initial dramatic change in cytologic appearance is followed by a gradual change to the anestrual cytologic appearance. On the basis of the examination of only a single cytologic specimen, proestrus cannot necessarily be distinguished from diestrus. The vaginal cytology of anestrus is quite acellular; it contains primarily parabasal cells and a few small intermediate epithelial cells. The transition from proestrus, through estrus, and into diestrus is usually adequately monitored by cytologic studies done every 2 or 3 days.
VAGINOSCOPY
Vaginoscopy is useful for evaluating animals with lower urinary tract signs or urinary incontinence, vulvar discharge, infertility, and anatomic abnormalities; for determining the nature and extent of lesions within the vestibule and vagina; and for identifying the stage of the estrous cycle. Samples for cytologic, microbiologic, and histopathologic studies can easily be obtained through the endoscope. Assuming that the clinician has access to the proper equipment, laser surgery can also be performed. Complications resulting from vaginoscopy, which include hemorrhage, laceration, and introduction of infection, are uncommon with proper technique. Endoscopic findings are assessed by comparing them to the normal anatomic features of the vagina, often in conjunction with vaginal cytology. The endoscopic appearance varies tremendously with the stage of the estrous cycle.
The canine vagina is quite long. In Beagles, for example, it measures 10 to 14 cm in length and 1.5 cm in diameter, whereas in Newfoundlands the length may be up to 29 cm. The endoscopic equipment must be of the appropriate size for the particular female. Proctoscopes and cystoscopes designed for human pediatric or adult patients or flexible fiberoptic endoscopic equipment of appropriate diameter can be used. Pediatric anoscopes or veterinary otoscopes may be narrow enough for use in queens and small bitches but are too short for examination of the cranial vagina and cervix.
In bitches that are in heat, vaginoscopy is usually performed with the animal awake and standing and without sedation or anesthesia unless a biopsy is planned. Anesthesia is necessary for vaginoscopy in queens, small bitches, and puppies and when cystoscopes with saline infusion to distend the vagina will be used. The perineum is inspected and cleansed. The endoscope is then lubricated with warm saline solution or with sterile, water-soluble lubricant. The clitoris and clitoral fossa must be avoided. Therefore the endoscope is passed in a dorsal direction through the dorsal commissure of the vulva. There will be increased resistance at the narrow vestibulovaginal junction (Fig. 56-7) in all but estrual bitches. It is especially narrow in prepubertal and neutered animals. The angle of the speculum is adjusted to be more parallel with the spine after it passes through the vestibulovaginal junction.
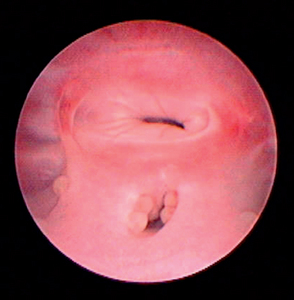
FIG 56-7 Vaginoscopy demonstrating the vestibulovaginal junction in a 1-year-old, spayed female retriever with lympho-nodular urethritis causing persistent pollakiuria.
During proestrus the longitudinal folds of the vagina are edematous, round, and smooth. As new folds develop, the vaginal lumen becomes filled with folds. A clear, bright-red fluid is seen in the vaginal lumen, sometimes in large amounts. As estrus approaches, the vaginal folds become lower and wrinkled. During estrus the folds appear sharp, angular, and crinkled. The mucosa is pale, and the vaginal lumen is wide. There is less luminal fluid than there is during proestrus. This fluid is clear and usually straw colored; however, it may continue to be bright red throughout estrus.
During diestrus (the luteal phase) the vaginal folds are low, round, and soft. The folds in the cranial vagina have a characteristic rosette appearance and may be mistaken for the cervix. Clear or opalescent mucus is present in the vaginal lumen during diestrus. The vaginal mucosa has streaks of hyperemia. During anestrus and in neutered bitches, the vaginal folds are low and round and do not fill the lumen. There is a thin mucous coating that gives the mucosa a translucent, pink-red appearance. In these animals the mucous membranes are thin and easily traumatized. Pinpoint submucosal hemorrhages may develop in response to seemingly gentle contact with the endoscope. During anestrus and in neutered animals there is usually some resistance to the passage of the endoscope unless the instrument is very well lubricated.
In bitches one of the vaginal folds, known as the dorsal median postcervical fold, is often mistaken for the cervix. This fold extends from the caudal-dorsal edge of the vaginal portion of the cervix along the dorsal midline and eventually blends into lesser folds of the vagina. It is composed of longitudinal and oblique smooth muscle bundles and irregularly arranged collagen. Unlike other folds of the vagina, the dorsal median fold has no elastic fibers. In Beagle-size bitches, this fold is 15 to 42 mm long and 2 to 10 mm wide, compared with the average vaginal length in the same bitches of 158 ± 30 mm. The lumen of the cranial vagina in this area is quite narrow. Because of its length, location, and inelastic nature, the dorsal median postcervical fold often prevents visualization and catheterization of the canine cervix.
The vaginal portion of the cervix is tubular, with small furrows radiating from the os, which give it the appearance of a star or rosette. The cervical os is not obviously “open,” even if fluid is seen flowing through it, except during the puerperium. The vaginal lumen around the cervix and the cranial aspect of the dorsal median postcervical fold is quite narrow, and except during estrus the use of small-diameter (0.5 cm) instruments is usually necessary to visualize the cervix. The narrow pericervical vaginal lumen with the dorsal median postcervical fold and the rosette appearance of the cranial vagina can be confused with the cervix.
VAGINAL BACTERIAL CULTURES
Bacterial infections of the reproductive tract are relatively common. Bacterial culture is indicated for the evaluation of many reproductive disorders, including infertility, vulvar discharge, pyometra, metritis, abortion, and stillbirth. Because the uterus is usually sterile, except in some bitches during proestrus and estrus, the interpretation of uterine culture results is relatively straightforward. Unfortunately, because of the difficulty in catheterizing the cervix in the bitch or queen, uterine samples are usually obtained only during laparotomy. Vaginal cultures are usually performed in lieu of uterine cultures. To minimize contamination from the vestibule and caudal vagina, samples for bacterial culture should be obtained from the cranial vagina using a guarded culture swab (e.g., those manufactured by Kalayjian Industries and Nasco) or through a sterile speculum.
The canine vagina has normal bacterial florae, which are listed in Box 56-1. Only 2% of 826 specimens obtained from intact bitches were negative for bacterial growth (Bjurström et al., 1992), whereas 23% of 66 specimens from queens were negative (Ström-Holst et al., 2003). In order of reported frequency, the most commonly isolated organisms from bitches are Pasteurella, Streptococci, and Escherichia coli. With the exception of Mycoplasma, anaerobic organisms are much less commonly isolated than aerobic. Cultures from bitches usually yield mixed populations of bacteria; however, in Bjurström’s study 18% were a growth of only one organism. The florae vary within and among individuals and throughout the cycle. The normal florae of the feline vagina are similarly diverse. E. coli, Staphylococcus, and Streptococus canis are the most common organisms recovered from queens. Unlike the situation in bitches, a single organism (most often E. coli) is isolated from 41% of cats. Anaerobic organisms are uncommon in queens. Even in normal bitches and queens, organisms may be recovered in large numbers.
Most of the organisms that make up the normal vaginal florae are also potential pathogens. Several studies have shown that there are no differences among the bacterial isolates from normal fertile bitches, infertile bitches, and bitches with evidence of genital disease. Isolation of opportunistic pathogens from the vagina is therefore not proof of infection. Thus the results of vaginal cultures and the potential role of the isolated organisms in the pathogenesis of the clinical signs must be interpreted cautiously. Brucella canis (see Chapter 58) is always considered a pathogen, even in the absence of clinical signs. The role of Mycoplasma spp. and Ureaplasma spp. in reproductive disorders in cats and dogs remains unclear.
VIROLOGY
Viral diseases may cause reproductive problems by directly affecting reproductive organs or because of the systemic illness they cause in the pregnant female. Respiratory disease and neonatal death are the most common manifestations of canine and feline herpes infection. Canine herpes virus also causes apparent failure to conceive; abortion; and, less commonly, genital lesions. Rarely, vesicular lesions may be found on the mucosa of the vestibule or prepuce of infected dogs. The most important route of transmission is oronasal contact with infected secretions. Transplacental and venereal transmission are much less important. The virus may be isolated from nasal, conjunctival, tracheal, vaginal, or preputial scrapings for 2 to 3 weeks after acute infection. Thereafter virus isolation and polymerase chain reaction (PCR) are usually negative. Because canine herpesvirus is poorly immunogenic, virus-neutralizing antibodies are present in small amounts for short periods. The finding of any detectable titer in the presence of compatible clinical signs is therefore considered significant. Fetal and neonatal necropsy findings are generalized, multifocal hemorrhages in kidney, lung, and liver and necrotic foci with intranuclear inclusion bodies. In queens panleukopenia, calici virus, feline infectious peritonitis, and feline leukemia virus infection are reported to be potential causes of infertility, abortion, and neonatal death.
ASSESSMENT OF REPRODUCTIVE HORMONES
Measurement of serum concentrations of reproductive hormones can be useful in evaluating animals with suspected or known reproductive disorders. The reproductive hormones are released in cyclic, episodic, or pulsatile manners; therefore the results of a single determination often are not diagnostic because the phase of the cyclic release at the time of sample collection is unknown. For that reason, repetitive determinations performed over the course of hours, days, or weeks, or provocative testing, may be necessary. Most hormone assays, such as radioimmunoassays (RIA), chemiluminescent, and enzyme-linked immunosorbent assays (ELISA), depend on immunologic reactions. Errors can result if antibodies or antigens in homologous assay systems are not species specific and if species-specific interference with antibody binding occurs in heterologous systems. For these reasons, it is critical that each laboratory validate its procedures and determine reference ranges for each species and each hormone to be tested.
Progesterone
As the time of ovulation approaches during estrus, ovarian follicular cells transform from estrogen-producing to progesterone-producing cells. LH causes ovulation and thus is responsible for this transformation. After ovulation, the follicles become CLs and produce progesterone. The stage of the ovarian cycle during which progesterone concentrations are high is called diestrus. If conception occurred, the length of diestrus will be the length of gestation. Gestation averages 65 days after breeding in the queen and 63 days after breeding in the bitch. If conception did not occur in a queen that did ovulate, the CLs will regress in 30 to 40 days. The bitch, on the other hand, is unique among common domestic animals in that the CLs persist and produce progesterone for 60 or more days, irrespective of pregnancy status. The CLs are the only significant source of progesterone in the pregnant bitch and queen and are required to maintain pregnancy throughout. Progesterone concentration must drop to basal levels for parturition to occur. It remains at basal levels through anestrus, until ovulation during the next estrous cycle (see Fig. 56-1).
In the bitch progesterone concentrations begin to increase above basal levels as a preovulatory event. This initial rise occurs simultaneously with the LH surge. Therefore progesterone can be used to approximate the LH surge and predict impending ovulation in the bitch. In the queen the initial rise above basal progesterone concentration occurs after the LH surge. In both the bitch and queen, high progesterone concentrations are indicative that ovulation did occur. The next cycle will not begin until sometime after progesterone has returned to basal levels.
There are a wide variety of laboratory methods used to detect progesterone. These include RIA, which is considered to be the gold standard, and chemiluminescent immunoassay (CLIA). These are available from several commercial laboratories with “same-day” results. Lower values are obtained using CLIA than RIA. Results may be reported in ng/ml or nmol/L. The conversion from one unit to the other is (ng/ml)(3.18) = (nmol/L) of progesterone. It is essential to use the reference ranges established by the laboratory for its methodology and validated for use in the particular species. The advantage of RIA and CLIA is quantitative results. There are point-of-care tests based on ELISA and rapid immunomigration (RIM) method (Ovucheck® Premate, Synbiotics Corp.). These provide semiquantitative results in three ranges. The low range is usually less than 3 ng/ml (less than approximately 9.5 nmol/L), the midrange is from approximately 3 ng/ml to 10 ng/ml, and the high range is greater than approximately 10 ng/ml (approximately 31.8 nmol/L), depending on the kit manufacturer. The midrange of the kit is designed to correlate with the LH surge in the bitch and is used to predict that ovulation will occur in 3 to 6 days. Compared with RIA or CLIA, the semiquantitative, in-house kits have been found to be 80% to 90% accurate in determining progesterone concentrations in dogs and cats. Nevertheless, some practitioners find them useful. Storage time, temperature, contact with red blood cells, contact with serum separator gel, and anticoagulants affect the results. Therefore the laboratory or kit manufacturer’s recommendations for sample handling must be followed. Samples for progesterone determination must never be drawn into serum separator tubes because the results will be spuriously decreased.
One of the most common reasons to measure progesterone in bitches is to determine the optimal time to breed. It is used in two ways. One is to approximate the time of the LH surge, a practice known as ovulation timing. It is based on the fact that in bitches the serum progesterone concentration increases to more than 1 to 2 ng/ml (approximately 3 to 6 nmol/L) at or shortly before the preovulatory LH surge. Therefore serial determinations (every 2 to 3 days) of the serum progesterone concentration during proestrus, to identify the initial increase above 2 ng/ml, can be used to estimate the time of ovulation, which follows the LH surge by about 2 days. As discussed earlier, fertilization could occur about 2 days after ovulation. Therefore the recommendation is to breed 3 to 6 days after the initial rise in progesterone (i.e., the LH surge) is detected. The other way progesterone concentrations are used to determine breeding day is based on the knowledge that fertilization could occur about 2 days after ovulation, during which time progesterone concentration has been rapidly increasing. Serum concentrations of progesterone greater than 8 ng/ml (25.4 nmol/L) are interpreted to indicate that ovulation has occurred. Analysis of several independent breeding trials in which serum concentrations of progesterone were determined on the days of insemination revealed that pregnancy rates were best when insemination was performed on days that serum progesterone concentrations were greater than 8 ng/ml (greater than 25.4 nmol/l) and up to 19 to 26 ng/ml (approximately 60 to 80 nmol/L). Two inseminations, 48 hours apart, are recommended, unless the initial progesterone is already near 19 ng/ml (60 nmol/L), in which case the second insemination is done the next day.
Finding the increased serum concentrations of progesterone indicative of ovulation would be of interest in females suspected of having ovulation failure. In the case of queens this may be due to inadequate copulatory stimulation to induce the LH surge. Finding high progesterone would also confirm ovulation in an animal suspected of having had a “silent” or unobserved heat, or it could confirm the presence of an ovary in an animal suspected of having an ovarian remnant after being spayed. The adequacy of luteal function during pregnancy can be monitored by determining serum progesterone concentrations once weekly for about 9 weeks after breeding or until parturition. This would be of interest in females in which inadequate luteal function (premature luteolysis; hypoluteoidism) was the suspected cause of unexplained abortion. It would also be useful in monitoring the effectiveness of certain abortifacient drugs. In pregnant bitches (but not necessarily in pregnant queens), parturition normally occurs within 24 hours after serum progesterone concentration decreases below 1 to 2 ng/ml (approximately 3 to 6 nmol/L). Therefore impending parturition can be predicted by monitoring the serum progesterone concentration. This information would be of use in the management of dystocia and in the planning of cesarean sections (Box 56-2).
Estradiol
Estradiol-17β is the main estrogen in circulation. The primary source of estradiol in sexually intact females is the ovarian follicle. In both males and females estradiol is also derived in peripheral tissue by the aromatization of testosterone and androstenedione. In sexually intact males the testis produces small amounts of estradiol, but this accounts for only about 20% of estradiol production in dogs. The majority is derived from aromatization of circulating androgens, testosterone and androstenedione. Androstenedione is of adrenal origin. Typical mean serum estradiol concentrations in the bitch are 5 to 10 pg/ml during anestrus, 10 to 20 pg/ml during early proestrus, and 50 to 100 pg/ml during late proestrus. Estradiol concentrations decline through estrus (see Fig. 56-1). During estrus in queens, estradiol is also typically >25 pg/ml to above 50 pg/ml. It returns to basal levels of <15 pg/ml in between cycles and during the seasonal anestrus.
Unfortunately, estradiol concentrations are often at or below the limits of detection of the assays used by many commercial endocrine laboratories. Estradiol concentrations also fluctuate widely and rapidly, and the high concentrations that occur during proestrus may be detectable for only a day or two. Deficiencies in circulating concentrations of estradiol are rarely documented in dogs and cats. Pathologic increases in estradiol production, such as those that occur in animals with ovarian follicular cysts or Sertoli cell tumors, may still be less than the detectable limits of many assays. For these reasons the measurement of estradiol concentrations often does not yield diagnostic results. A simple, accurate means of gauging estrogenic activity in the female is to evaluate vaginal epithelial cells for signs of cornification (see Fig. 56-6). All things considered, vaginal cytology is often preferable to determination of serum concentrations of estradiol in females. The preputial epithelium is also responsive to estrogen, exhibiting changes similar to those of the vaginal epithelium (Fig. 56-8). The paraneoplastic syndromes associated with excessive estrogen in dogs include alopecia, gynecomastia, pendulous prepuce, and bone marrow suppression. In bitches and queens cystic follicles in intact or remnant ovaries may continuously produce estradiol and cause persistent signs of heat and, much less commonly, alopecia. Assessing estradiol or its influence on vaginal epithelium is indicated for determining the stage of the estrus cycle for breeding management and for evaluating females suspected of having an ovarian remnant after being spayed. Finding cornification of vaginal epithelium or very high estradiol concentrations in a supposedly spayed queen or bitch that is displaying characteristic physical or behavioral signs of heat would be consistent with a diagnosis of ovarian remnant. Finding very high estradiol concentrations or the influence of estradiol on vaginal or preputial epithelium in an animal displaying estrogen-induced paraneoplastic syndromes justifies a search for a gonadal source (estrogen-producing testicular tumor, cystic ovarian follicles) or an exogenous source of estrogen. These would be far more likely than an adrenal source of estrogen in species other than the ferret.
Gonadotropins: Follicle-Stimulating Hormone and Luteinizing Hormone
The gonadotropins, FSH and LH, are produced by the pituitary, under the control of hypothalamic gonadotropin-releasing hormone (GnRH; see Fig. 56-4). As discussed earlier in the chapter, they are secreted in a pulsatile manner, in ever-increasing magnitude until a so-called surge occurs. The increasing concentrations of FSH at the end of anestrus initiate ovarian follicular development and the onset of the next estrus cycle. The surge of LH causes maturation and ovulation of ovarian follicles, which luteinize and produce progesterone. The duration of the LH surge is relatively short, usually occurring within a 24-hour window, although it may remain elevated for somewhat longer. Additionally, in queens a neuroendocrine reflex initiated by coital stimulation of the vagina also causes the LH surge. In males FSH supports Sertoli cell function and spermatogenesis. LH stimulates testosterone secretion by the Leydig cells of the testis. The gonadal hormones, in turn, feed back to the hypothalamus and pituitary. Following gonadectomy this negative feedback control of LH is lost, and serum concentrations of LH and FSH are persistently elevated. This could also occur with the rare condition of gonadal dysgenesis. The secretory capacity of the pituitary gonadotropins can be assessed by determining LH and/or FSH before and after administration of GnRH. A point-of-care, semiquantitative immunochromogenic assay for LH has been intermittently available (ICG Status-LH®, Synbiotics). Few commercial laboratories offer quantitative assays for LH or FSH for veterinary patients at this time.
As discussed earlier, identification of the preovulatory LH surge is a useful tool in canine breeding management; however, the LH surge lasts only 24 to 72 hours. Therefore frequent sampling (i.e., at least once q24h) is essential to ensure that it is not missed. Because pulses of LH other than the surge may be of sufficient magnitude to be detected by the assay, some clinicians recommend measuring serum concentrations of progesterone several days after the surge. Progesterone concentrations above 2 ng/ml (6 nmol/L) differentiate the actual pre-ovulatory LH surge from the normal proestrus pulses of LH. Trying to determine optimal breed ing time with such precision is most applicable when frozen semen is to be used because the life span of thawed spermatozoa is short, perhaps only 24 hours. Because the frequent blood sampling necessary to identify the LH surge is inconvenient and expensive, progesterone concentrations are often assessed in lieu of LH itself to estimate the surge.
To avoid unnecessary laparotomy, serum concentrations of LH can be measured to determine the presence or absence of gonads in animals with unknown reproductive status, such as those newly acquired by shelters or private owners. High concentrations of LH are consistently found from 5 days to as long as 5 years after ovariectomy in bitches. This is because negative feedback from the gonadal hormones to the pituitary is lost. Conversely, LH is also helpful for evaluating females suspected of having ovarian remnants after being spayed. In this situation feedback loops are still intact and LH concentrations will be low except during heat. Finding high serum LH concentrations is sensitive for detecting animals that have been spayed (sensitivity: 100% in 50 queens; 98% in 300 bitches). However, it is not as specific, especially in bitches, because high LH is also normally found in cycling females (specificity: 92% in queens; 78% in bitches). The proportion of animals with high LH that are spayed—in other words, the probability that high LH correctly predicts a spayed animal—is fairly high (positive predictive value: 92% in queens; 90% in bitches) but not perfect, again because intact females also have high LH at some times during the estrus cycle. Therefore females with high LH are either spayed or in heat, which can easily be differentiated by physical examination, vaginal cytology, or measurement of serum progesterone. Males with high LH have been castrated. The proportion of animals with low LH that are actually spayed is very low (negative predictive value: 100% in queens; 96% in bitches). In other words, the probability that finding low LH will correctly predict an intact animal is very high. Females with low LH have not been spayed or have ovarian remnants and are not presently in heat. Males with low LH have one or both testicles. If they are not in the scrotum, the male is cryptorchid. A much less likely cause of low LH in males and females would be exposure to exogenous sex hormones.
FSH is rarely measured in small animal practice, primarily because appropriate assays are usually not commercially available. However, it has been shown that FSH is a more specific indicator of neuter status than is LH in bitches because FSH concentrations are consistently higher in spayed bitches than intact bitches, even during heat.
Gonadotropin-Releasing Hormone
GnRH, which is secreted by the hypothalamus, controls pituitary secretion of FSH and LH in both male and female animals. GnRH assays are not readily available, and GnRH is rarely measured in the small animal practice. However, exogenous GnRH administration can be used to evaluate the pituitary-gonadal axis. After the administration of GnRH to normal dogs and cats, there is a prompt (within 30 minutes) increase in the serum concentrations of LH. The magnitude of the response is influenced by the stage of the reproductive cycle and the dose of the drug. After the serum concentration of gonadotropins increases in response to GnRH, serum concentrations of gonadal hormones also increase. The degree of gonadal responsiveness understandably varies with the stage of the reproductive cycle in females and whether the male has one, two, or no testes. Failure of serum LH concentrations to increase after GnRH administration points to the possibility of a pituitary problem. Failure of gonadal sex hormones to increase appropriately after GnRH administration indicates either pituitary dysfunction (no increase in LH), gonadal dysfunction, or prior gonadectomy. Administration of GnRH can also be used to induce estrus in the bitch and queen.
Relaxin
Relaxin is produced primarily by the placenta; therefore it is pregnancy specific in bitches and queens. In pregnant bitches and queens, relaxin reaches detectable levels in serum or plasma as early as 20 days after the LH surge and peaks 30 to 35 days after the LH surge. It remains high throughout pregnancy, until parturition or abortion, when it declines precipitously. Low levels may be detectable for 4 days postpartum in bitches. Although the manufacturer suggests that the test can be useful 21 days after breeding, it is a more sensitive indicator of pregnancy when performed 30 or more days after breeding. There may be an influence of litter size on relaxin concentrations. Finding high concentrations of relaxin in serum or plasma confirms pregnancy. Declining or undetectable concentrations are found in cases of spontaneous or induced abortion and after parturition. Relaxin is undetectable in pseudopregnant and nonpregnant bitches and queens. There are two commercially available point-of-care assays for relaxin. Witness Relaxin®, a rapid immunomigration assay, can be used for dogs and cats. ReproCHEK® is an ELISA system for use in dogs. (Both assays are from Synbiotics Corp.)
DIAGNOSTIC IMAGING
Radiology and ultrasonography are useful for evaluating the ovaries, uterine wall, and intrauterine contents; confirming pregnancy; and assessing fetal viability. The normal uterus and ovaries in a nonpregnant animal are not detected by routine abdominal radiography (see Fig. 56-9). During normal anestrus they may be difficult to identify by ultrasonography. Increased size and density and an abnormal shape of the uterus may be detected by either technique. Ultrasonography can be used to evaluate the uterine wall and the intrauterine contents. Ultrasonography may also help identify ovarian remnants, ovarian cysts in animals with persistent estrus and hyperestrogenism (follicular cysts), or persistent anestrus (nonfunctional or luteal cysts). It may be able to identify ovarian neoplasia as well. In males diagnostic imaging is very helpful in evaluating the prostate and testes (see Chapters 61 and 62). Negative findings with diagnostic imaging do not necessarily exclude disease in the reproductive tract, especially in females.
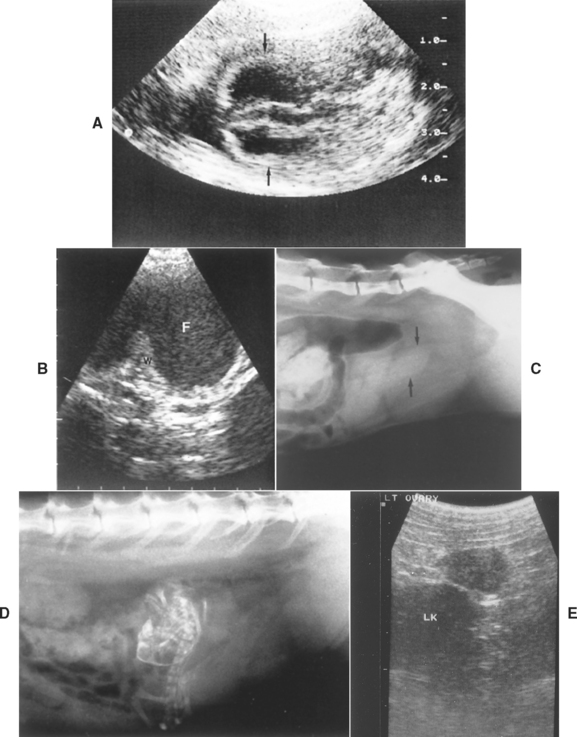
FIG 56-9 A, Sonogram of canine gestational sac (arrows) at 29 days. Scale is in centimeters. B, Sonogram of canine pyometra showing thickened uterine wall (W) and lumen distended with fluid (F). C, Radiograph of feline pyometra showing fluid-filled uterus (arrows). D, Radiograph of mummified fetus. E, Sonogram of 1.8 × 1.2-cm ovary with corpora lutea in a normal 3-year-old Weimaraner 30 days after estrus. The serum progesterone concentration was 64 nmol/L. LK, Left kidney.
(A Courtesy Dr. Tom Bell, East Lansing, Mich.)
Because of the difficulty involved in catheterizing the cervix, contrast studies of the uterus and uterine tubes (i.e., hysterosalpingography) are rarely done in bitches and queens. During estrus contrast material deposited in the cranial vagina may enter the uterus and provide a hysterogram, but at other stages of the cycle the cervix is normally closed. Positive-contrast vaginography, using a Foley catheter and a water-soluble contrast agent (e.g., diatrizoates such as Renografin®), is easily performed, but general anesthesia is necessary. Vaginography can be considered if vaginoscopy fails to clearly identify strictures, anatomic defects, masses, or foreign material in the vagina.
KARYOTYPING
Some intersex conditions and developmental abnormalities of the reproductive tract may be associated with chromo somal anomalies (e.g., XXX, XO). These animals are usually seen because of abnormal external genitalia, infertility, or persistent anestrus. Karyotype analysis can be performed if a congenital rather than an acquired cause is suspected and if routine diagnostic tests have failed to identify the cause of the reproductive dysfunction. Cells from any tissue can theoretically be used for chromosomal analysis, but lymphocytes from heparinized blood samples are the usual specimen. (e.g., University of Minnesota Veterinary Cytogenetics Laboratory, Department of Veterinary Pathobiology).
LAPAROSCOPY AND CELIOTOMY
Exploratory celiotomy is often the most cost-effective way to diagnose and treat intersex animals. In all other circumstances, however, diagnostic laparoscopy or exploratory celiotomy should not be done until a noninvasive diagnostic evaluation of the bitch or queen with a reproductive disorder has been completed. Laparoscopy and celiotomy allow gross visualization of the reproductive tract, bacterial culture of the uterine lumen, and full-thickness biopsy of the uterus. The patency of the uterine horn and uterine tubes might be determined by infusion of sterile saline solution, using the techniques developed for in vitro fertilization and embryo transfer. Laparoscopy and celiotomy are best performed during anestrus to fully appreciate persistent pathologic changes in the uterus.
FEMALE INFERTILITY
An accurate history is critical to the evaluation of a female animal suspected to be infertile. When taking the history, the clinician should investigate the details of previous cycles, including the dates of onset of each cycle, the female’s behavior during estrus, the dates and methods of previous inseminations, the fertility of the studs used, and the events following breeding (Box 56-3). A complete physical examination should be performed to identify (1) potential causes of infertility outside the reproductive tract, (2) other abnormalities that might adversely affect the health of the female or the pregnancy itself should conception occur, and (3) congenital and heritable defects that should exclude this female from a breeding program.
 BOX 56-3 Historical Information for Female Infertility
BOX 56-3 Historical Information for Female Infertility
The reproductive tract is then examined. Mammary glands are carefully palpated to assess their size and consistency and the character of any secretions. The vulva is inspected to determine if there are structural abnormalities or any discharge. The labia are separated so that the vestibular mucosa and clitoris (in bitches) can be visualized. The uterus is palpated transabdominally. A vulvar discharge may be more apparent after abdominal palpation. The vestibule and posterior vagina should be palpated with a gloved finger in bitches of adequate size. Rectal palpation may help determine the extent of abnormal structures within the vestibule and caudal vagina.
The history and physical examination findings determine the nature of any additional diagnostic tests to be performed. Historic or physical abnormalities outside the reproductive tract should be investigated. All dogs should be tested for Brucella canis (see Chapter 58) before breeding and before infertility is evaluated further. A complete blood count (CBC), serum biochemistry panel, and urinalysis provide excellent information regarding the overall metabolic health of the animal and could reasonably be included as a routine part of the evaluation of infertility. Only normal, healthy animals in excellent body condition should be bred.
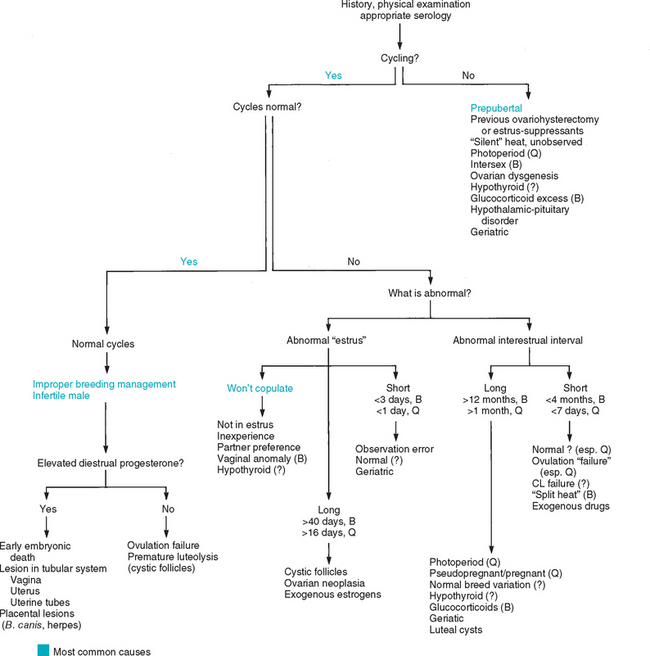
The reproductive history often dictates the nature of the diagnostic approach. Perhaps most important are characterizing proestrus-estrus and the interestrous interval of the female, identifying the criteria used to determine when the female is bred, and determining the female’s behavior during mating (see Fig. 56-10). Typically, one of the following four descriptions applies: failure to cycle, abnormal interestrous interval, abnormal proestrus-estrus, or normal cycles.
FAILURE TO CYCLE
There are two subcategories of animals with persistent anestrus. Primary anestrus refers to females 24 months of age or older that have never cycled. Secondary anestrus applies to females that have previously cycled but are no longer doing so. An animal that has never cycled may be a normal prepubertal animal younger than 24 months of age, may be experiencing “silent” heats, may have a congenital gonadal or chromosomal anomaly, or may have a concurrent disorder that is preventing estrous cycles. Exposure to light may be inadequate to initiate and maintain cyclicity in queens with persistent anestrus. Gonadal dysfunction, concurrent metabolic disorders or medications, and advancing age should be considered in females with secondary anestrus.
Diagnostic tests for persistent anestrus are usually delayed until a female is 2 years of age because of the probability that she is a normal prepubertal animal. Some veterinarians believe that an initial undetected or “silent” first heat cycle is common in bitches. If so, this could explain why some young bitches appear to have persistent anestrus. Unobserved or silent heats may be detected retrospectively by measuring the serum progesterone concentration. If the concentration is greater than basal anestrus levels (>2 ng/ml, or >6.4 nmol/L) in a bitch, a cycle has occurred within the previous 60 to 90 days. The finding of high serum concentrations of progesterone in a supposedly anestrous queen indicates that unobserved estrus has occurred and also that either unobserved mating or spontaneous ovulation occurred within the past 30 to 40 days. Clinical signs of false pregnancy (see Chapter 58) would also indicate that an undetected cycle occurred approximately 60 days earlier. Silent cycles could be detected prospectively by examining vaginal cytology every 1 to 2 weeks. Noncycling females should be housed with cycling females whenever possible because the pheromones from cycling females may induce noncycling females to cycle. Queens should be exposed to at least 12 hours of light for at least 2 months before further testing is done.
Persistent anestrus may result from suppression of function of the hypothalamic-pituitary-ovarian axis. Hypothyroidism, exogenous glucocorticoid therapy, and concurrent metabolic disease are commonly reported but rarely confirmed causes in bitches. Thyroid function is assessed by measuring serum concentrations of the thyroid hormones and canine thyroid-stimulating hormone (cTSH; see Chapter 51). The role of hypothyroidism in infertility in the bitch has not yet been thoroughly evaluated. Exogenous glucocorticoids are commonly administered to animals and cause many alterations in reproductive function, including prolonged anestrus and abortion. The history should be reviewed to determine if the animal could have received glucocorticoid treatment. In mature bitches increased serum alkaline phosphatase activity in conjunction with relatively normal alanine aminotransferase activity is suggestive of supraphysiologic amounts of glucocorticoids. If there is still doubt about excess endogenous or exogenous glucocorticoids, adrenocortical function can be assessed with an adrenocorticotropic hormone (ACTH) stimulation test. The presence of other concurrent metabolic disease is determined with a CBC, serum biochemistry panel, and urinalysis.
Persistent anestrus may also result from a primary abnormality anywhere within the hypothalamic-pituitary-gonadal axis, including intersex conditions, ovarian dysgenesis, progesterone-secreting luteal cysts, or ovarian tumor. It may also result from previous ovariohysterectomy. Females with ovarian dysgenesis or that have undergone oophorectomy are expected to have chronically increased serum concentrations of LH, which can be measured. Serum progesterone concentrations can be determined to assess functional luteal cysts. The functional status of the hypothalamic-pituitary-ovarian axis can be evaluated by measuring serum LH and progesterone concentrations before and after GnRH administration. Ultrasonographic evaluation of the ovaries may identify ovarian abnormalities such as cysts or neoplasia. On close inspection, many apparently female intersex animals have detectable anatomic abnormalities of the clitoris, vestibule, and/or vagina that result from exposure to androgens. A GnRH stimulation or human chorionic gonadotropin (hCG) test, done to assess the serum concentrations of testosterone, could be used to demonstrate the presence of testicular tissue. Because protocols vary among laboratories, the laboratory should be consulted for dosages and sampling times. Karyotyping can also be performed, although intersex animals may have normal karyotypes. Abnormal karyotypes have been found in bitches and queens with ovarian dysgenesis.
Induction of estrus may be tried in otherwise normal, healthy females if other diagnostic tests have failed to identify the cause of persistent anestrus. Exploratory celiotomy or laparoscopy, done to assess the gross appearance of the reproductive tract and to obtain biopsy specimens of the internal genitalia, should be considered only after all noninvasive diagnostic methods have been tried.
PROLONGED INTERESTROUS INTERVAL
Interestrous intervals of greater than 12 months in bitches and greater than 1 month in cycling queens are usually considered abnormal, although long interestrous intervals may also be a normal breed variation, as seen in the Basenji, Tibetan Mastiff, and Dingo dogs, which often cycle only once a year. Many of the causes of persistent anestrus, such as glucocorticoid administration in bitches and inadequate photoperiods in queens, may also cause a prolonged interestrous interval. Pregnancy, pseudopregnancy, and early embryonic death are causes of prolonged interestrous intervals in queens but not in bitches. This difference is because the CL life span in bitches is 60 to 70 days, irrespective of pregnancy status. Lack of hiding places and irregular feeding times have been shown to disrupt normal cycles in cats (Pelican, 2006). Prolonged interestrous intervals may also occur with increasing age or may signify an underlying disorder. Silent or unobserved heats should also be considered. The diagnostic workup in animals with prolonged interestrous intervals should include a thorough review of the estrus identification techniques used by the owner, identification of medications being administered to the animal, assessment of the overall metabolic health of the animal (i.e., CBC, biochemistry panel, urinalysis), and an evaluation of thyroid gland and adrenocortical function in bitches.
SHORT INTERESTROUS INTERVAL
Abnormally short interestrous intervals of less than 4 months are occasionally seen in bitches and are usually associated with infertility. Infertility in these animals presumably results from implantation failure because the endometrium has not recovered from the previous cycle, a process that takes 120 to 150 days, although ovulation failure may be involved. In some breeds, most notably the German Shepherd Dog, and in some individual animals, an interestrous interval of 4 to 4.5 months may be normal and may not interfere with fertility. Cystic ovarian follicles might cause frequent cycling (i.e., short interestrous interval) but most commonly are associated with persistent estrus. The administration of gonadotropins, prostaglandin F2α, prolactin antagonists, or estrogen can artificially shorten the interestrous interval. In most bitches, however, the cause of short interestrous intervals is not discovered.
A short interestrous interval must be differentiated from a split heat cycle in bitches. Split heats are characterized by normal proestrus that stops abruptly before progressing to estrus. Two to 4 weeks later, proestrus begins again and progresses through normal, fertile estrus. Split heats are a normal phenomenon that can occur in any bitch during any estrus. Split heats are seen most often in pubertal bitches that have normal proestrus and estrus during subsequent cycles. Rarely do split heats occur repeatedly in an individual bitch. Split heats do not cause infertility, except in the sense that the initial proestrus frustrates breeding management.
Additional diagnostic tests are often not performed in bitches with confirmed short interestrous intervals, although ovarian ultrasound would be reasonable. Another diagnostic consideration would be to monitor the changes in serum progesterone concentrations during estrus and diestrus to assess whether the short cycles may be related to ovulation failure. Administration of an androgen such as mibolerone or methyltestosterone to prevent estrus for at least 6 months has been considered, but there is little published evidence of efficacy. Even though estrus can easily be delayed with androgen treatment, affected bitches usually remain subfertile. Interrupting the short cycle by administering a progestin during proestrus has enabled 10 previously infertile bitches to conceive on the next cycle (Wanke, 2006). Previous interestrous intervals for the bitches were 2 to 4 months (mean 3.2 months). Treatment with megestrol acetate (2 mg/kg, orally) or clormadinone acetate (0.5 mg/kg, orally) for 8 days, beginning within the first 3 days of proestrus, stopped the cycle before ovulation. Progesterone concentrations remained at basal levels. The next cycles occurred 1.5 to 3.5 months (mean 2.7 months) after treatment and were fertile. Although subsequent cycles were not discussed in this report, breeding on the first estrus occurring after the discontinuation of therapy has previously been recommended because short interestrous intervals frequently resume. The role of genetics in this problem is not known.
ABNORMAL PROESTRUS AND ESTRUS
The most common abnormalities of proestrus and estrus are refusal to allow mating, prolonged estrus, and abnormally short estrus. Females that are not in estrus refuse mating. An occasional bitch or queen exhibits partner preference by refusing to mate with one male but readily mating with another. Inexperienced and timid females may also be reluctant to breed. In bitches physical abnormalities of the vulva or vagina are common causes of refusal to mate (see Chapter 57). Physical abnormalities include vaginal strictures; congenital defects in the vulva and vagina; vaginal hyperplasia/prolapse; and, rarely, vaginal neoplasia.
Vaginal cytologic studies should be performed to identify the present stage of the cycle (see Fig. 56-6). As just mentioned, females that are not in estrus will not accept mating. Digital palpation of the vulva, vestibule, and vagina in animals of adequate size can identify vaginal prolapse and most vaginal strictures and congenital defects. Vaginoscopy should be performed if digital palpation fails to identify a cause for the refusal to allow mating.
Vaginal strictures that are identified during anestrus should always be palpated again during estrus to determine their actual significance. Annular vaginal strictures are usually located immediately cranial to the external urethral orifice, at the anatomic junction between the vestibule and the vagina. The vestibulovaginal junction is normally the narrowest part of the posterior tract (see Fig. 56-7). During anestrus this normal narrowing may be mistaken for an annular stricture. The diameter of the vestibulovaginal junction normally increases significantly during proestrus and estrus, making differentiation from a true stricture easy at this stage of the cycle. Strictures of the vulva or vestibule usually do not change as dramatically during estrus. Similarly, normal vaginal examination findings during anestrus do not exclude the possibility of vaginal hyperplasia/prolapse, which occurs only at times of estrogenic stimulation, as a potential cause for reluctance to mate. Artificial insemination can be used to breed otherwise normal estrual females that refuse to mate as well as those with vaginal hyperplasia/prolapse. With the exception of vaginal hyperplasia/prolapse, physical abnormalities should be surgically corrected if the female is to remain in the breeding program. Surgery is best performed during anestrus. The heritability of congenital vaginal and vulvar anomalies is unknown.
Prolonged or Persistent Estrus
Although proestrus and estrus each last an average of 9 days, proestrus lasting as long as 17 days and estrus lasting 21 days have been observed in normal, fertile bitches. Understandably, many owners become concerned if a season (proestrus plus estrus) lasts longer than 3 weeks. Nevertheless, a season is not considered abnormally long in bitches until it reaches 35 to 40 days. In queens estrus lasting longer than 16 days is considered abnormal. This must not be confused with the normal, multiple cycles that occur in queens.
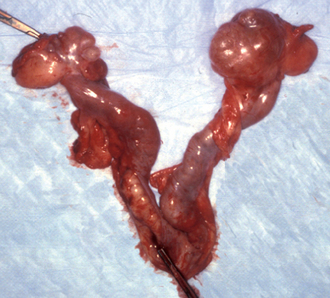
Prolonged proestrus/estrus is usually caused by functional follicular cysts (Fig. 56-11), which occur in intact ovaries and also in ovarian remnants in spayed bitches and queens. Ovarian neoplasia and exogenous estrogen administration may also cause persistent signs of estrus. Vaginal cytology should be performed to confirm that estrogenic stimulation is present and thus could reasonably be considered the cause of the behavioral and physical signs. Usually, the diagnosis of ovarian follicular cysts is based on the historic, physical, vaginal cytologic, and ultrasound findings. Serum concentrations of estrogen could also be determined. Because spontaneous regression of follicular cysts may occur, watchful waiting for 2 to 4 weeks is often the initial therapeutic approach. If clinical signs do not promptly resolve, treatment is indicated. Induction of ovulation can be attempted using GnRH (Cystorelin®; 2.2 μg/kg, adminstered intramuscularly q24h for 3 days); however, the results have been variable. If mature follicles are present and induced to ovulate, signs of estrus should resolve in 5 to 7 days. The cysts can be manually ruptured via laparoscopy or celiotomy. In cases of unilateral ovarian cysts, unilateral oophorectomy can be performed. Ovariohysterectomy should be considered for those females that fail to respond promptly to medical management for cystic ovaries because the prognosis for fertility is guarded and continued estrogenic stimulation may be harmful to the uterus and bone marrow. Ovarian neoplasia is uncommon in bitches and queens. Surgical excision is the treatment of choice. If exposure to exogenous estrogenic drugs is the cause of persistent signs of estrus, it should be discontinued.
Short Estrus
Abnormally short estrus of less than 3 days in bitches or less than 1 day in queens is most often the result of an error in observation or recognition of estrus. Females older than 6 to 8 years of age may experience erratic cycles, including short estrus. A split heat cycle should also be considered in bitches with an apparently short estrus. Short estrus may be normal in some animals. Methods of proestrus and estrus detection should be changed in females with a truly short estrus so that they can be bred at the appropriate time. This usually entails beginning vaginal cytologic studies or teasing with a stud well before the expected transition from proestrus to estrus and continuing this until the first day of estrus is identified. Combining this with ovulation timing, as determined by serum progesterone or LH concentrations, may be helpful in identifying the optimal time for insemination.
NORMAL CYCLES
Infertility in a female otherwise normal in all aspects of the reproductive cycle may result from improper breeding management; infertility in the male; abnormalities in the ovary, uterine tubes, uterus, or vagina; early embryonic death; or advancing age. A history of false pregnancy occurring after previous cycles strongly suggests that the hypothalamic-pituitary-gonadal axis was intact during those cycles. Therefore the investigation should initially focus elsewhere. Conception rates and litter size are greatest and neonatal mortality is lowest in bitches (Beagles) between 2 and 3.5 years of age. Reproductive performance in queens is best between 1 and 6 years of age. After 5 years of age in Beagles and 6 years of age in queens, conception rates and litter size decline and neonatal mortality begins to increase. Because of this age-related decrease in fertility, an extensive diagnostic evaluation of older females may not be warranted.
The most common causes of infertility in females with normal estrous cycles are improper timing of insemination and poor semen quality. Because male fertility can be so easily evaluated (see Chapter 60), the male should be evaluated before an extensive diagnostic evaluation of the female is undertaken. A solid history that the male sired litters that were born shortly before and shortly after mating with the bitch in question would provide good circumstantial evidence against male infertility. Semen evaluation would provide information about the male’s current status. The process of freezing and thawing canine semen substantially decreases its quality. Because the post-thaw life span may be only 24 hours and because its ability to transverse cervical mucus is so diminished, pregnancy rates using frozen semen are very poor unless ovulation timing and intrauterine, not intravaginal, insemination are used. Although the effects of chilling semen are far less deleterious, freshly ejaculated semen retains the best quality.
In well-managed colonies of normal dogs, conception rates of better than 90% are expected. A thorough history concerning breeding management, particularly how the owner determines when to breed, is imperative. Canine breeding management is discussed on p. 887. Feline breeding management is discussed on p. 889. A common practice for dog breeders is to simply begin breeding on a predetermined day after the onset of proestrus (commonly day 10) and continue breeding every other day for as long as the bitch is receptive. This method works well for normal bitches that have a very typical cycle. However, if a particular bitch has a short proestrus, for example, day 10 may actually be at the end of estrus rather than at the beginning. In queens the frequency of mating during estrus is a more important determinant of ovulation, and thus conception, than is the specific day of the cycle on which mating occurs.
Vaginal cytology can be used to identify estrus. Obtaining the specimen for cytologic evaluation may induce ovulation in some estrous queens, but this is apparently not a common occurrence. Monitoring serum concentrations of LH or progesterone can be used to determine ovulation and the fertile period in bitches. It has been shown in bitches in artificial insemination (AI) programs that two inseminations, done 48 hours apart during the fertile period, improve pregnancy rates and increases the number of pups per litter compared with only one insemination. If the first insemination happens to occur late in the fertile period, the second is done 24 hours later. Unlike the situation in queens, breeding a bitch several times during the same day appears to offer no advantage over breeding a single time on a given day. Intrauterine insemination with fresh, chilled, or frozen-thawed semen increases pregnancy rates over vaginal insemination. Also, the mean litter size was larger with intrauterine AI than with vaginal AI.
After the female has been bred using optimal protocols and semen of excellent quality, it should be examined 20 to 30 days later to determine whether pregnancy has occurred. Pregnancy can be diagnosed on the basis of abdominal palpation, ultrasonographic findings, or by finding high serum concentrations of relaxin. Ultrasonography should be performed in animals found not pregnant because the ovaries and uterus can be evaluated for a potential cause. If the female is not pregnant, the serum progesterone concentration should be measured to determine if ovulation occurred. Low serum progesterone concentrations (less than 2 to 5 ng/ml or approximately 6.4 to 16 nmol/L) suggest ovulation failure or premature luteolysis. The cause of ovulation failure may be an ovarian abnormality or, in the case of queens, inadequate coital stimulation. Premature luteolysis, or failure of the corpora lutea to maintain progesterone production, results in fetal resorption with no outward clinical signs when it occurs before day 35 of gestation. Premature luteolysis is rarely documented in bitches or queens; however, to differentiate ovulation failure from premature luteolysis, serum progesterone concentrations are serially determined using quantitative methods (e.g., RIA) from the time of proestrus through diestrus. Progesterone concentrations that never exceed 8 ng/ml (approximately 25 nmol/L) suggest ovulation failure, whereas premature luteolysis is reflected by a more rapid or earlier decline than normal from high postovulatory concentrations of progesterone. Hypothalamic or pituitary dysfunction would be unlikely causes of ovulation failure in the female that is otherwise cycling normally. More likely, hypothalamic or pituitary malfunction would be manifest as abnormal cycles.
When a female with normal cycles is known to have been bred appropriately during estrus to a male that is known to be fertile and when ovulation has been confirmed by the finding of elevated serum progesterone concentrations during diestrus, the hypothalamic-pituitary-gonadal axis is considered intact (see Fig. 56-4). Abnormalities in the vagina, uterus, uterine tubes, placenta, or the conceptus itself are then likely to be the source of the infertility. The diagnostic approach should begin with a review of the history to identify potential causes of early embryonic death. Special attention should be paid to infectious disease and medications administered to the female. Early embryonic death is difficult to confirm in bitches and queens, but in queens a prolonged interestrous interval provides a clue. Attempts to confirm pregnancy by ultrasound or measuring serum concentrations of relaxin can be done as early as day 14 to 20, although false-negative results are understandably common at that stage.
Infectious agents are an important cause of early embryonic death. Although many agents are capable of causing placentitis or fetal death, B. canis in bitches and calici and herpes viruses in queens are the foremost such agents. In lieu of cultures of specimens from the uterine lumen, cultures of the cranial vagina should be performed. Some clinicians recommend that cultures be obtained during estrus because the cervix is open at that time and fluid in the cranial vagina may have originated from the uterus. Bacterial infections should be treated appropriately before breeding. The uterus may be incapable of supporting implantation or pregnancy because of disorders such as bacterial endometritis or cystic endometrial hyperplasia. Ultrasound of the reproductive tract is indicated. Vaginal lesions can easily be excluded as the cause of infertility by vaginoscopy and vaginal cytology. The potential teratogenic or abortifacient effects of medications should always be considered. Many commonly used medications, such as glucocorticoids and certain antibiotics, also cause embryonic death. In addition, some congenital fetal anomalies cause early embryonic death because they are incompatible with continued survival.
The presence of antisperm antibodies in the female has not yet been documented as a cause of infertility in dogs or cats. However, were they to occur, breeding with a different male could circumvent the problem. Finally, exploratory celiotomy can be performed during anestrus to visualize the reproductive tract, assess the patency of the uterus and uterine tubes, obtain uterine specimens for culture, and procure full-thickness uterine biopsy specimens for histologic assessment.
ESTRUS SUPPRESSION, CONTRACEPTION, AND POPULATION CONTROL
SURGICAL METHODS
In the United States and Canada ovariohysterectomy and castration are the most common methods of population control for dogs and cats. They are permanent and relatively expensive, invasive procedures. Ovariohysterectomy can be accomplished by midline or flank approaches or via laparoscopy. Laparoscopy has also been used for castration of cryptorchid testicles. Some clinicians have recommended ovariectomy over ovariohysterectomy, although this has not been widely accepted among veterinarians in the United States. Ovariohysterectomy has traditionally been recommended at 5 to 8 months of age, just before the animal reaches puberty. Doing so dramatically reduces the risk that the animal will develop mammary cancer in the future, in addition to preventing estrus and unwanted pregnancy. To reduce the number of unwanted (i.e., relinquished by their owners) and stray animals euthanized at animal shelters, many shelters mandate surgical sterilization as part of the adoption agreement. Because postadoption compliance with sterilization agreements has universally been poor, preadoption “early spay-neuter” policies at 6 to 8 weeks of age have been advocated. Safe and effective anesthetic and surgical techniques have been developed. Several studies have shown that the physical and behavioral traits of animals neutered at 7 weeks of age are the same as those in animals neutered at the more conventional age of 7 months. However, there is an increased rate of urinary incontinence in female dogs spayed at 6 to 8 weeks of age compared with those spayed at 6 to 8 months. Whereas the risks associated with early spay-neuter of male dogs and of both male and female cats are minimal, it may be prudent to delay spaying female dogs until they are older. Gonadectomy at any age results in decreased metabolic rate and decreased caloric requirements, irrespective of any change in physical activity. It has also been reported that food intake actually increases after neutering in male and female cats fed ad libitum. Neutering has been shown to cause hyperleptinemia in male cats, but not female cats, after neutering. Unless caloric intake is diminished to match the changed metabolic rate, animals will gain weight after gonadectomy. Gonadectomized animals have delayed physeal closure, and less developed genitalia and secondary sex characteristics than do age-matched, sexually intact controls. Tubal ligation and vasectomy can be used to prevent pregnancy in dogs and cats without circumventing the physical and behavioral changes associated with sexual maturation. Owners may or may not consider this desirable.
NON-SURGICAL METHODS FOR CONTRACEPTION OR STERILIZATION
Less expensive, permanent, nonsurgical methods of sterilization would be ideal for preadoption programs at humane societies and animal shelters and for large-scale application in trap-neuter-release programs to control feral populations. The injection of sclerosing agents into the testis, epididymis, or ductus deferens has been investigated in dogs and cats. These agents have included various concentrations of zinc, formalin, chlorhexidine in DMSO, ethanol, silver nitrate, potassium permagnate, Freund’s Complete Adjuvant, BCG, methallibure, dexamethasone, metopiron, niridazol, αchlorohydrine, and danazol. Treated animals may remain fertile for 6 to 8 weeks after treatment. Some animals never become sterile, whereas in others the effects are transient. There appears to be some species variation as well because intratesticular 70% glycerol was ineffective in dogs, whereas it has consistently resulted in azoospermia in monkeys, rabbits, rats, and hamsters. Some studies have reported minimal signs of discomfort. Others have reported swelling, scrotal ulceration and mutilation, local granulomatous reactions, vomiting, diarrhea, leukocytosis, and lethargy. A zinc gluconate solution (Neutersol®; Addison Biological Laboratory) for intratesticular injection is marketed for chemical castration of puppies between 3 and 10 months of age. At this time there is no published evidence that the use of sclerosing agents has resulted in lifelong sterility in pet dogs or cats or that there has been an impact on control of feral populations.
The concept of immunizing animals against GnRH, LH, LH receptors, sperm antigens, and the zona pellucida of oocytes has appeal for both permanent sterilization and temporary contraception. Because GnRH and LH control gonadal function, blocking their effects would theoretically suppress estrus cycles and ovulation in females, spermatogenesis in males, and sexual behavior in males and females. These effects might be maintained permanently or temporarily, depending on the duration of adequate antibody titers. GnRH is highly conserved across species and therefore is poorly immunogenic unless conjugated with other molecules. A GnRH vaccine (Improvac®; CSL Animal Health) is available in Australia for use in mares. An antigonadotropin releasing factor (GnRF) product (Canine Gonadotropin Releasing Factor Immunotherapeutic®; Pfizer) is marketed in the United States for treatment of benign prostatic hyperplasia in dogs. Although not marketed for these effects, it does cause the testes to shrink and serum testosterone concentrations to decline, both of which would be deleterious to spermatogenesis. GnRH has also been conjugated with cytotoxic agents designed to destroy the GnRH receptors. Although this markedly suppressed reproductive activity in peripubertal male dogs, the response was variable. Induction of antibodies against LH and LH receptors results in impaired reproduction; however, the responses have been so variable that a consistent vaccination protocol has not been established for dogs or cats. Bitches and queens have resumed normal cycling after antibody titers declined. Attempts to immunize animals against sperm antigen have not had satisfactory results for sterilization or contraception. The zona pellucida (ZP) is the acellular coating around ova, and vaccination with porcine ZP has been found to consistently produce high antiZP titers and prevent conception in bitches. Significant ovarian abnormalities also develop, including ovarian cysts of various types and prolonged proestrus/estrus. Vaccination of queens against ZP has not resulted in contraception. An antiporcine ZP vaccine (SpayVac®; ImmunoVaccine Technologies, Nova Scotia) is commercially available for immunocontraception in some captive wild species.
Intravaginal spermacides have not yet been developed for use in bitches or queens. An intravaginal mechanical barrier was marketed for use in bitches, but the failure rate was high. An intrauterine contraceptive device is available for bitches (Biotumer, Argentina). Although it was quite effective in a small clinical trial (Volpe, 2001), it is somewhat impractical because of the difficulty in transcervical placement. High intensity ultrasound suppresses spermatogenesis when applied to the testes and causes luminal occlusion when applied to the epididymides and ductus deferentia in dogs and cats. However, skin burns occur in approximately 20% of treated animals. Bisdiamines are amebicidal drugs that have been found to cause spermatogenic arrest in all species studied thus far, including dogs and cats. They have generated great interest for contraception in zoo animals because, when administered daily in food, they cause spermatogenic arrest before the spermatid stage but spermatogonia are preserved. Therefore the effects are reversible. Side effects in dogs and men have been only slight weight loss and slight decrease in red blood cell count. In cats there was no change in red blood cell counts, but testosterone concentrations declined during treatment. The drug must be administered daily to be effective. Females must not be exposed to bisdiamines because they are highly teratogenic.
CONTRACEPTION
Contraception may be defined as a reversible method of blocking fertility. Contraception is particularly desirable for animals that eventually will be bred but for which estrus will interfere with their work (e.g., racing, hunting) or show career. Progestins and androgens can inhibit the release or synthesis of gonadotropins in dogs and cats and thereby prevent estrus. GnRH analogues reversibly inhibit reproductive function in males and females by downregulating LH and FSH receptors, thereby suppressing the pituitary-gonadal axis.
Progestins
Megestrol acetate (Ovaban®; Pfizer) is the only progestin approved for estrus control in bitches in the United States. It is not labeled for use in cats. It is intended for short-term (2 years), temporary use. In some European countries oral and injectable progestins such as medroxyprogesterone acetate (MPA; 3 mg/kg, administered intramuscularly every 5 to 6 months) and proligestone are commonly used to prevent estrus. Progestins are most reliable in preventing estrus when treatment is initiated during anestrus and are less reliable if initiated during early proestrus. The synthetic progestin levonorgestrel (Norplant®; Wyeth-Ayerst) is a slow-release, subdermal implant. It is effective in suppressing estrus for 12 months in cats, with no adverse effects except the development of cystic endometrial hyperplasia. The return to normal cycling after discontinuing progestin therapy is variable but typically is within 2 and 9 months. Progestins have many dose-dependent undesirable effects that commonly occur at therapeutic doses. Progestins routinely cause cystic endometrial hyperplasia, which may predispose the animal to the development of pyometra. Twelve weeks after initiating MPA treatment at 10 mg/kg subcutaneously, every 3 weeks, there was atrophy of the endometrium and dramatic reduction in estrogen and progesterone receptors. However, by 24 weeks, most uterine cell types had escaped progestin downregulation, estrogen and progesterone receptors had returned to normal, and cysts of endometrial glands had developed (De Bosschere, 2002). Progestins are also associated with mammary hyperplasia and an increased incidence of mammary tumors. When progestin therapy is discontinued, signs of false pregnancy may develop (see Chapter 58). Other adverse effects in bitches and queens include diabetes mellitus, acromegaly, and adrenocortical suppression. Conversely, some progestins (MPA and proligestone) act as glucocorticoid agonists in the bitch, and long-term treatment with high doses may result in iatrogenic hyperadrenocorticism, including steroid hepatopathy. Alopecia, thinning of the skin, and discoloration of the hair at the site of progestin injection have also been observed. For these reasons, the benefit of progestins for estrus suppression should be carefully weighed against the risks. Medroxyprogesterone is used for the treatment of benign prostatic hyperplasia in dogs (see Chapter 62) with no apparent adverse effects on semen quality. At higher doses (20 mg/kg), MPA decreases sperm output and motility and increases morphologic abnormalities.
Androgens
Mibolerone (Cheque Drops®; Pfizer) was the only androgen approved for estrus suppression in bitches in the United States. The dose varied according to body weight and breed. Daily doses of 50 μg were required to suppress estrus in queens, but doses of 60 μg caused heptotoxicity. Although not labeled for this use, various forms of testosterone (testosterone propionate, 110 mg, administered intramuscularly or subcutaneously, once weekly; methyltestosterone, 25 to 50 mg, administered orally, twice weekly) are routinely administered to Greyhound bitches during training and racing. Prolonged anestrus occurs in some of these bitches after androgen therapy is discontinued. Adverse effects of androgens are clitoral hypertrophy (sometimes with permanent ossification), mucopurulent vulvar discharge, and vaginitis. Liver enzyme activity may also be increased. These effects are usually reversible after androgens are discontinued, but they may persist for months to years. Irreversible masculinization of the female fetuses occurs when androgens are administered to pregnant females. Additional androgenic effects include thickening of the skin on the neck of queens and an apparent increase in muscle mass and aggressiveness in bitches. Subcutaneous administration of 0.6 mg/kg testosterone propionate to dogs caused marked decline in sperm motility that persisted for 3 months. Oral administration of 50 mg of methyltestosterone for 1 month decreased sperm output. Chronic administration of the synthetic testosterone danazol causes azoospermia in dogs.
GnRH Agonists
The GnRH agonist deslorelin has been shown to safely and effectively suppress reproductive function for up to 2 years in adult male and female dogs and cats when administered as a slow-release subcutaneous implant. (Ovuplant®, Fort Dodge; Suprelorin®; Peptech Animal Health). Depending on the age of the bitch and the stage of the cycle at which a GnRH agonist is implanted, it can initially induce an estrus cycle. This unwanted effect can be overcome by the simultaneous short-term administration of a progestin such as megestrol acetate. After an initial stimulatory effect on the pituitary gonadotropins, GnRH agonists suppress further LH and FSH release by downregulation of receptors. Other GnRH agonists, nafarelin, leuprolide, and buserelin, are also effective contraceptives in bitches and dogs. A formulation of nafarelin safely and effectively delayed puberty when administered to prepubertal bitches (mean age 5 months) as a slow-release subcutaneous implant for the duration of the 1-year study. Spontaneous cycling occurred 1 to 13 months after the implants were removed. The implants were difficult to find and remove from two obese bitches.
OVARIAN REMNANT SYNDROME
Occasionally, queens and bitches resume cycling or continue to exhibit behavioral and physical signs of estrus after oophorectomy. This may happen weeks or as long as 5 years after oophorectomy. The signs may be cyclic in nature, or there may be persistent signs of hyperestrogenism, including alopecia, hyperpigmentation, and lichenification. The cause is remnant ovarian tissue that has regained folliculogenesis and estrogen production. The presence of high concentrations of estrogen can be detected using vaginal cytology to identify cornification of epithelial cells (see Fig. 56-6). The history should be reviewed to ensure that the patient is not being exposed to exogenous estrogens (e.g., agents prescribed to treat urinary incontinence in the patient) or estrogen-containing creams, contraceptives, or hormone replacement therapy used by owner. In the absence of exogenous estrogens, the findings of clinical signs typical of estrus, along with vaginal cytology consistent with estrus, confirm the presence of ovarian remnants and justify a recommendation for exploratory celiotomy to find and remove the ovarian remnants. If additional confirmation of the ovarian remnant syndrome is desired before exploratory surgery, the remnant ovary’s ability to ovulate and produce progesterone can be evaluated. Measuring serum concentrations of progesterone 5 to 7 days after expected ovulation achieves this goal. In bitches progesterone increases during heat, coincident with the LH surge, and remains elevated for about 60 days thereafter. Therefore progesterone could be measured while the bitch is showing the clinical signs of heat or for several weeks thereafter. Queens generally are considered to be induced to ovulate by coital stimulation. However, many also ovulate spontaneously. Progesterone remains elevated for only 30 to 40 days after ovulation in nonpregnant queens. Therefore progesterone is measured a week or two after the signs of heat in queens. Progesterone concentrations greater than 2 ng/ml (6.4 nmol/L) are indicative of spontaneous ovulation and the presence of CL in the ovarian remnants. The clinician should keep in mind that ovarian remnants may not be “cycling” in a normal manner and ovulation may not occur. Some ovarian remnants develop cystic follicles that produce estrogen but do not ovulate. The finding of high progesterone confirms the presence of ovarian remnant, but low progesterone does not exclude it. Another approach is to attempt to induce ovulation while the female is in heat by administering hCG (10 IU/kg, intramuscularly, in bitches; 250 IU/queen, intramuscularly) or GnRH (0.5 μg/kg, intramuscularly, in bitches; 25 μg/cat, intramuscularly). Approximately 5 to 7 days later the serum progesterone concentration should exceed 2 ng/ml if responsive ovarian tissue is present. The finding of low serum concentrations of LH indicates the presence of ovarian tissue. However, it is possible that exogenous estrogens could also suppress LH.
Ovarian remnants are often bilateral. They are typically found in the usual anatomic location for ovaries, not in aberrant or ectopic places. The cause of ovarian remnant syndrome is evidently the surgical technique. Treatment consists in the surgical removal of the ovarian remnants. Ovarian remnants are often small, despite the magnitude of the clinical signs they cause. They may be obscured by periovarian fat, especially in bitches. Some veterinarians have suggested that ovarian remnants may be easier to identify if follicles (i.e., when the female is in heat) or CL (i.e., shortly after ovulation) are present rather than during the interestrous period (i.e., anestrus). However, in our experience, the most important determinant of success is adequate surgical exposure and dissection.
OVARIAN NEOPLASIA
Ovarian neoplasia is rare in bitches and queens. Granulosa cell tumors are reported to occur in ovarian remnants as well as in intact females (Fig. 56-12). Bitches with estrogenproducing ovarian tumors may have clinical signs of estrus, bone marrow toxicity, dermatologic changes, or a combination of these. Estrogen-producing ovarian tumors would not be expected to respond to exogenous hCG or GnRH administration. Bitches with progesterone-producing granulosa cell tumors may show mammary gland development and may have cystic endometrial hyperplasia.
INDUCTION OF ESTRUS AND OVULATION
Estrus induction has been attempted in bitches in clinical settings to shorten the normal interestrous interval, to treat primary and secondary anestrus, and to time pregnancy and parturition for the owners’ convenience. In research settings estrus induction has been used for in vitro fertilization (IVF) and to synchronize estrus for embryo transfer (ET). Although some estrus-induction protocols are associated with superovulation, as revealed by examination of ovarian follicles, the superovulation is apparently not reflected in increased litter size. Induction of ovulation may be indicated for females with ovulation failure as shown by low diestrous serum concentrations of progesterone or for those with persistent estrus caused by ovarian follicular cysts.
THE QUEEN
The photoperiod can be manipulated to induce estrus in queens. Continuous exposure to 12 to 14 hours of light of at least 50 foot-candles and exposure to 10 to 12 hours of dark per day causes normal, mature queens to begin cycling within 4 to 8 weeks. Housing anestrous queens with cycling queens also helps to induce estrus. If the hormonal induction of estrus is to be attempted, the queen should first be exposed for several months to a minimum of 12 hours of light per 24 hours. Induction of ovulation may be indicated for cycling queens in AI or IVF/ET programs or to induce pseudopregnancy, which would temporarily prolong the interestrous interval when frequent cycles are troubling the owners. The intramuscular administration of 250 IU of hCG or 25 μg of GnRH (Cystorelin; Ceva) on the first 2 days of estrus is recommended, although a single 25-μg dose of GnRH is known to cause an LH surge in normal cats. Probing the vagina with a smooth rod such as a thermometer or with a cotton swab four to eight times, at 5- to 20-minute intervals, may also stimulate an LH surge in normal cycling queens. The probing need last only 2 to 5 seconds.
Estrus induction has primarily involved using domestic cats as models for AI and IVF/ET in endangered wild felids. The most common hormones used to induce estrus (i.e., folliculogenesis) in cats are porcine FSH and equine chorionic gonadotropin (eCG). A disadvantage of pFSH is the need for daily administration until the onset of estrus. Because of its longer half-life, a single injection of eCG (100 IU, intramuscularly) is often sufficient. Ovulation is then induced with LH (hCG; 75, 100, or 250 IU, adminis-tered intramuscularly or intravenously, depending on the formulation), or mating with a tomcat. A commonly used protocol for artificial insemination regimens is eCG followed by hCG 80 to 84 hours later. Protocols using GnRH for estrus induction have also been successful, but to date, pregnancy rates have been lower. Although there is much variation in response to estrus induction protocols among feline species, a common problem is hyperstimulation of the ovaries. This has resulted in excessive or prolonged estrogen production, premature or prolonged progesterone production, or premature luteolysis, all of which disrupt rather than enhance reproduction. It has been shown in cattle that follicular cysts develop following luteal phases with inadequate progesterone levels. Therefore the use of progestins before gonadotropins to help prevent ovarian hyperstimulation is being investigated (Pelican, 2006).
THE BITCH
Many drugs have been used to induce estrus—in other words, to shorten the interestrual interval—in bitches. Prostaglandin F2α, which is used to treat pyometra, causes luteolysis and decreased progesterone production by the CL. This shortens the normally long diestrous period and thereby shortens the interestrual interval by 1 to 2 months.
The mechanisms by which the dopamine agonists cabergoline and bromocriptine induce estrus are multifactorial. One mechanism is by suppression of prolactin, which is luteotropic. Suppressing luteal function decreases progesterone, shortens diestrus, and increases LH pulsatility in bitches. However, this is not the sole explanation because metergoline, a seratonin antagonist that also decreases prolactin, does not induce estrus in bitches. Furthermore, even at low doses that do not suppress prolactin nor shorten diestrus, bromocriptine significantly shortens the interestrous interval. Bromocriptine (Lactafal®, Eurovet), given orally q12h at doses of 5, 20 and 50 μg, beginning in mid-diestrus until the onset of the next cycle, resulted in mean interestrual intervals of 136 ± 16 days, 96 ± 6 days, and 92 ± 11 days, respectively, compared with untreated controls’ mean interval of 216 ± 9 days (Beijerink et al., 2003). The higher doses suppressed prolactin and progesterone, whereas the low dose did not. Other researchers have reported that some bromocriptine-induced cycles are not fertile, despite hormonal variations similar to those in normal cycles. Cabergoline (Galastop®, Ceva Vetem; Dostinex®, Pfizer) at a dosage of 5 μg/kg orally, q24h, through the first 2 days of proestrus has induced fertile estrus in bitches with primary and secondary anestrus. The duration of treatment ranged from 4 to 34 days, with a mean of 16 days. Other researchers have suggested that continuing treatment through the first 4 to 5 days of proestrus is important for success.
GnRH agonists, administered as slow-release implants, induce estrus in bitches. The effectiveness of estrus induction and the fertility of the induced cycle are dose dependent and age dependent and vary according to the stage of the cycle during which the drug is administered and perhaps the anatomic location of the implant. These variations are apparently the result of competing upregulation and downregulation of LH release. The GnRH agonist deslorelin has been studied for both estrus induction and estrus suppression in bitches. High doses induced fertile estrus, but abortions occurred during the ensuing diestrus. Lower doses of 1.05 mg or 2.1 mg deslorelin (Ovuplant®, Fort Dodge) implanted in Beagle bitches beneath the vestibular submucosa in the ventral commissure of the vulva reliably induced proestrus within 3 to 5 days of administration, and the LH surge occurred 9 to 17 days later (Volkmann et al., 2006). All the bitches implanted during anestrus ovulated, and 69% became pregnant when bred to the same dogs as were the untreated controls. All the untreated controls also ovulated, and 67% became pregnant. However, although bitches implanted during diestrus also came into estrus and experienced an LH surge, only 69% ovulated and the pregnancy rate was only 15%. Furthermore, 15% of those treated in diestrus developed pyometra during the diestrus after the induced estrus.
Induction of ovulation might be indicated for cycling bitches in which ovulation is failing to occur or bitches with follicular ovarian cysts. Potential agents include hCG (22 IU/kg, intramuscularly) and GnRH (50 to 100 μg, intramuscularly; or 2.2 μg/kg, intramuscularly GnRH; Cystorelin®, Ceva). It has been recommended that hCG and GnRH be given on the first day of estrus, as determined by the behavior of the bitch and vaginal cytology. Success is confirmed by the finding of a serum progesterone concentration of greater than 8 ng/ml in early diestrus. For the treatment of follicular cystic ovarian disease in the bitch, 2.2 μg/kg of GnRH is administered intramuscularly for 3 days. However, the results of this treatment have been disappointing.
Ball B, et al. Effects of a GnRH cytotoxin on reproductive function in peripubertal male dogs. Theriogenology. 2006;66:766.
Beijerink N, et al. Low doses of bromocriptine shorten the interestrous interval in the bitch without lowering plasma prolactin concentration. Theriogenology. 2003;60:1379.
Bjurström L, et al. Long-term study of aerobic bacteria of the genital tract in breeding bitches. Am J Vet Res. 1992;53:665.
Chatdarong K, et al. Distribution of spermatozoa in the female reproductive tract of the domestic cat in relation to ovulation induced by natural mating. Theriogenology. 2004;62:1027.
Chatdarong K, et al. Cervical patency during non-ovulatory and ovulatory estrus cycles in domestic cats. Theriogenology. 2006;66:804.
Corrada Y, et al. Short-term progestin treatments prevent estrous induction by a GnRH agonist implant in anestrous bitches. Theriogenology. 2006;65:366.
Davidson AP, editor. Clinical theriogenology. Vet Clin North Am Small Anim Pract. 2001;31:2.
De Bosschere H, et al. Changes in sex hormone receptors during administration of progesterone to prevent estrus in the bitch. Theriogenology. 2002;58:1209.
England G, et al. Relationship between the fertile period and sperm transport in the bitch. Theriogenology. 2006;66:1410.
Gobello C. Dopamine agonists, anti-progestins, anti-androgens, long-term-release GnRH agonists and anti-estrogens in canine reproduction: a review. Theriogenology. 2006;66:1569.
Gobello C, et al. Use of cabergoline to treat primary and secondary anestrus in dogs. J Am Vet Med Assoc. 2002;220:1653.
Greene C, editor. Infectious diseases of the dog and cat, ed 3, Philadelphia: WB Saunders, 2006.
Handelman C et al: Evaluation of a test for plasma relaxin in pregnant and nonpregnant bitches, Proceedings of the 18th ACVIM Forum, Seattle, 2000, p. 722.
Harper E, et al. Effects of feeding regimens on bodyweight, composition and condition score in cats following ovariohysterectomy. J Small Anim Pract. 2001;42:433.
Hoenig M, et al. Effects of neutering on hormonal concentrations and energy requirements in male and female cats. Am J Vet Res. 2002;63:634.
Howe L. Surgical methods of contraception and sterilization. Theriogenology. 2006;66:500.
Immegart H, et al. Evaluation of intratesticular injection of glycerol for nonsurgical sterilization of dogs. Am J Vet Res. 2000;61:544.
Johnston S, et al, editors. Canine and feline theriogenology. Philadelphia: WB Saunders, 2001.
Kutzler M, et al. Non-surgical methods of contraception and sterilization. Theriogenology. 2006;66:514.
Levy J, et al. Evaluation of the effect of a long-term trap-neuter-return and adoption program on a free-roaming cat population. J Am Vet Med Assoc. 2003;222:42.
Löfstedt R, et al. Evaluation of a commercially available luteinizing hormone test for its ability to distinguish between ovariectomized and sexually intact bitches. J Am Vet Med Assoc. 2002;220:1331.
Morresey P. Reproductive effects of canine herpesvirus. Compendium. 2004;4:804.
Munson L, et al. Efficacy, safety and reversibility of bisdiamine as a male contraceptive in cats. Theriogenology. 2004;62:81.
Pelican M. Ovarian control for assisted reproduction in the domestic cat and wild felids. Theriogenology. 2006;66:37.
Phillips T, et al. Selective control of the estrous cycle of the dog through suppression of estrus and reduction of the length of anestrus. Theriogenology. 2003;59:1441.
Reynaud K, et al. In vivo canine oocyte maturation, fertilization and early embryogenesis: a review. Theriogenology. 2006;66:1685.
Rubion S, et al. Treatment with a subcutaneous GnRH agonist containing controlled release device reversibly prevents puberty in bitches. Theriogenology. 2006;66:1651.
Scott K, et al. Characteristics of free-roaming cats evaluated in a trap-neuter-return program. J Amer Vet Med Assoc. 2002;221:1136.
Silva T, et al. Sexual characteristics of domestic queens kept in a natural equatorial photoperiod. Theriogenology. 2006;66:1476.
Spain C, et al. Long-term risks and benefits of early-age gonadectomy in cats. J Am Vet Med Assoc. 2004;224:372.
Spain C, et al. Long-term risks and benefits of early-age gonadectomy in dogs. J Am Vet Med Assoc. 2004;224:380.
Ström-Holst B, et al. Characterization of the bacterial population of the genital tract of adult cats. Am J Vet Res. 2003;64:963.
Volkmann D, et al. The use of deslorelin implants for the synchronization of estrous in diestrous bitches. Theriogenology. 2006;66:1497.
Volpe P, et al. Intrauterine device for contraception in dogs. Vet Rec. 2001;149:77.
Wanke M. Progestin treatment for infertility in bitches with short interestrous interval. Theriogenology. 2006;66:1579.