CHAPTER 58 False Pregnancy, Disorders of Pregnancy and Parturition, and Mismating
FALSE PREGNANCY
Etiology
False pregnancy is a clinical phenomenon in which a female that was not pregnant displays maternal behavior such as nesting, the adoption of inanimate objects or other animals, mammary gland development, and lactation. False pregnancy occurs commonly in intact, cycling bitches and is considered to be normal. It occurs after diestrus (i.e., luteal phase), when serum concentrations of progesterone decline. The terms false pregnancy, pseudopregnancy, and psuedocyesis are often used interchangeably, but none accurately reflects the situation in bitches in which the clinical signs occur during what would have been the postpartum period, not during what would have been the pregnant period (i.e., luteal phase) of the cycle. Progesterone causes mammary gland development and weight gain, irrespective of pregnancy status. The drop in serum concentrations of progesterone at the end of diestrus causes an abrupt increase in prolactin secretion, which causes lactation and the behavioral changes of false pregnancy. Because the bitch ovulates spontaneously and always enters a long luteal phase, false pregnancy is a common phenomenon in cycling bitches. It is uncommon in queens. In bitches false pregnancy also occurs after the withdrawal of exogenous progestins and after oophorectomy performed during diestrus.
False pregnancy is considered a normal phenomenon in bitches. It is not associated with any reproductive abnormalities, including cycle irregularities, pyometra, or infertility. To the contrary, the occurrence of false pregnancy provides evidence that ovulation took place during the preceding cycle and that the hypothalamic-pituitary-gonadal axis is intact. Why some bitches are prone to developing clinical signs and why the severity of the clinical signs vary from cycle to cycle are not known. Although serum concentrations of prolactin do increase when progesterone is withdrawn, they are not always elevated to the same degree, nor are they always found to remain elevated by the time bitches are evaluated for false pregnancy. This may be due in part to the 6-hour pulsatile secretion pattern of prolactin, which makes interpretation of the results of a single blood sample less reliable. Nevertheless, at similar prolactin concentrations some bitches show clinical signs of false pregnancy and others do not. Some individual predisposition toward the development of false pregnancy evidently exists. In addition, factors relating to nutrition influence the occurrence of false pregnancy. Thin bitches are less likely to experience false pregnancy than bitches of the same breed in ideal body condition.
Clinical Features
The most common clinical signs of false pregnancy are mammary gland enlargement and lactation. The mammary secretion varies from a small amount of clear or brownish fluid to large amounts of milk that may drip spontaneously from the glands. Nesting behavior is the next most common clinical sign of false pregnancy. Many bitches will “adopt” things. Some animals also experience restlessness, irritability, abdominal enlargement, anorexia, and vomiting. The diagnosis is based on the historical and physical findings in a nonpregnant bitch or, less commonly in a queen, at the end of diestrus. It may also occur after oophorectomy during diestrus or when exogenous progestins are discontinued. Before treatment of false pregnancy is undertaken, it is essential that the evaluation, such as diagnostic imaging, be sufficient to rule out pregnancy because all treatments for false pregnancy will be deleterious to pregnancy, should it exist.
Treatment
False pregnancy is a normal, self-limiting phenomenon in bitches that usually does not require treatment. The clinical signs usually resolve after 2 or 3 weeks. Stimulation to the mammary glands, such as licking, can promote lactation. Withholding food for 24 hours, followed by a gradual (i.e., 3 to 5 days) increase back to usual quantities, helps to reduce lactation. When treatment is needed, drugs that inhibit prolactin release, such as dopamine agonists and serotonin antagonists, are effective in ameliorating the behavioral and physical signs of false pregnancy in bitches. These drugs are not labeled for veterinary use in the United States at this time. The dopamine antagonist cabergoline (Galastop®; Ceva Vetem; Dostinex®, Pfizer), 5 μg/kg orally, once daily, causes improvement in 3 to 4 days, with the signs resolving by 7 days. Dostinex® can be compounded to the appropriate concentration. Cabergoline may cause vomiting and, rarely, increased aggression. The serotonin antagonist metergoline (Contralac®; Virbac Laboratories) also inhibits prolactin secretion. The suggested dose is 0.1 to 0.2 mg/kg twice daily for 8 days. It does not cause vomiting but can cause hyperexcitability, aggression, and whining. Mild tranquilization can be considered for bitches showing aggressive behavior, keeping in mind that phenothiazines can increase prolactin secretion.
Progestins, such as megestrol acetate (Ovaban®, Shering-Plough), and androgens also suppress prolactin secretion and can diminish the clinical manifestations of false pregnancy. As would be expected, however, clinical signs often recur after progestins are withdrawn. Therefore although labeled for this use, Ovaban® is not recommended. Ovariohysterectomy should not be performed during mid- to late diestrus because false pregnancy can occur as a result of removing the ovarian source of progesterone, particularly in those animals with a prior history. When false pregnancy does occur after ovariohysterectomy, it may be more persistent than in intact bitches. Furthermore, in bitches spayed during an episode of false pregnancy, the condition may be greatly prolonged. Spaying during false pregnancy is therefore not recommended. Cabergoline treatment has been beneficial in the majority of these cases of prolonged false pregnancy.
If any signs of false pregnancy become recurrent in a spayed animal, the likely possibility of an ovarian remnant should be considered. If signs of false pregnancy persist for longer than the expected 2 to 3 weeks, bitches should be evaluated for hypothyroidism (see Chapter 51). Primary hypothyroidism is associated with increased hypothalamic thyrotopin-releasing hormone (TRH), which can stimulate prolactin release. In some hypothyroid bitches an increased secretion of prolactin, presumably in response to increased TRH secretion, may result in excessive lactation if false pregnancy occurs. Thyroid hormone replacement therapy causes the lactation to resolve in these hypothyroid bitches.
NORMAL EVENTS IN PREGNANCY AND PARTURITION
In the bitch and queen fertilization occurs in the uterine tubes (oviduct), where the fertilized ova then develop into morulae before entering the uterus. Early canine blastocysts enter the uterus about 8 to 10 days after ovulation. From 12 to 17 days after ovulation, embryos migrate within the uterus, ultimately becoming equally spaced within both uterine horns. Implantation is completed within 18 to 21 days after ovulation. In the queen morulae enter the uterine horns 5 or 6 days after ovulation and migrate within the uterus from days 6 to 8. Implantation is complete 12 to 14 days after ovulation.
Functional corpora lutea (CLs) are essential throughout pregnancy in the bitch and queen. The serum progesterone concentration can be used to assess corpora luteal function. After ovulation it should be greater than 5 to 8 ng/ml (approximately 16 to 25 nmol/L) and should continue to increase for the next 15 to 25 days (see Fig. 56-1). The serum progesterone concentration remains at peak levels for 7 to 14 days and then gradually declines throughout the remainder of pregnancy. In pregnant bitches a rapid, prepartum drop in the concentration to less than 2 ng/ml (approximately 6.4 nmol/L) is consistently found within 48 hours of whelping. This abrupt decline in progesterone is the result of an acute rise in prostaglandin F2α concentrations, which does not occur during the nonpregnant cycle. The luteal secretion of progesterone depends on both pituitary luteinizing hormone (LH) and prolactin. During the second half of the canine pregnancy, prolactin is the main luteotropic factor. A similar trend in the corpora luteal secretion of progesterone is observed in queens. As in the bitch, prolactin is luteotropic. Serum concentrations of prolactin and relaxin increase during the second half of pregnancy in bitches and queens.
Body weight and caloric needs steadily increase throughout pregnancy, especially during the last trimester, in both bitches and queens. Body weight steadily increases through weeks 4 to 7, with as much as a 40% increase in caloric intake. Appetite often declines during the last 2 weeks of pregnancy, but body weight continues to increase because of fetal and mammary growth. Weight loss does not occur during normal pregnancy. Animals that are underweight may have difficulty maintaining body condition and milk production after parturition. Conversely, obesity is known to contribute to the development of dystocia and increased neonatal mortality. In bitches the packed cell volume (PCV) declines to 40% by day 35 and to less than 35% at term. Mild, mature neutrophilia is common in pregnant bitches. Red blood cell (RBC) numbers, the hemoglobin concentration, and PCV decline throughout pregnancy in queens as well, but the absolute numbers are often still within the normal range.
FECUNDITY
Overall health, body condition, nutrition, and age greatly influence fecundity. Conception rates and litter size are greatest and neonatal mortality is lowest in Beagles between 2 and 3.5 years of age. After 5 years of age, conception rate and litter size decline and neonatal mortality begins to increase. Litter size also varies with parity, with the largest litters at third and fourth parity. In the bitch litter size varies according to breed, with smaller breeds tending to have smaller litters than larger breeds. Analysis of litters registered by the American Kennel Club showed that litter size for Labrador Retrievers and Golden Retrievers ranged from five to ten pups, with 70% of the litters containing seven or more pups. Conversely, litter size for Chihuahuas and Yorkshire terriers ranged from two to five pups, with 80% of the litters having four pups or less (Kelley, 2002). In the queen litters typically consist of two to five kittens, with an average of four, irrespective of breed. Litter size and neonatal survival are best in queens 1 to 5 years of age, provided that first parity occurs before 3 years of age. Litter size and neonatal survival usually improve after first parity. If first parity occurs after 3 years of age, however, litter size and neonatal survival usually remain poor. Reproductive performance of queens declines after 6 years of age. Superfecundation, in which litter mates have different sires, commonly occurs in queens and bitches. When it does, DNA tests for paternity can be performed by various laboratories (examples: VetGen.com; VGL.ucdavis.edu).
PREGNANCY DIAGNOSIS
Pregnancy can be confirmed by palpating the abdomen, performing diagnostic imaging, and detecting the hormone relaxin in blood. Abdominal palpation is easily and quickly performed, especially in cats. Although this is the most subjective method of pregnancy diagnosis, it is a reliable method for those skilled in palpation. Palpably distinct uterine swellings that represent uterine edema, embryonic membranes, and early placental development are about 1 cm in diameter at 20 days after breeding and about 2.5 cm by day 25. By 30 to 35 days the gestational sacs are becoming elongated and the uterus is more diffusely enlarged, making it more difficult to detect pregnancy by palpation at that time. Uterine enlargement caused by pregnancy cannot be accurately differentiated from uterine enlargement caused by some other process, such as pyometra, on the basis of abdominal palpation findings alone.
Ultrasonography is an excellent method of pregnancy detection in bitches and queens. It has the advantage of also assessing fetal viability because cardiac activity and fetal movements are evident. Pregnancy can be diagnosed when the gestational sac or fetal structures are identified (see Fig. 56-9, A; Fig. 58-1). The gestational sac appears as a spherical, anechoic structure surrounded by a hyperechoic wall composed of the uterine wall and placenta. Hyperechoic fetal structures are seen within the gestational sac. Although it is possible to identify the gestational sac as early as 10 days after breeding in the bitch and queen, pregnancy is more reliably detected 24 to 28 days after breeding in bitches and 20 to 24 days after breeding in queens. At that time fetal structures and cardiac activity are detected within the gestational sacs. Fetal heart rates range from about 200 to 250 beats per minute. Fetal movement characterized by dorsiflexion of the head and extension of the limbs is common in both species after day 33 to 39. By days 40 to 50 fetal anatomy is obvious (Fig. 58-2). Nonviable fetuses show no motion and lose identifiable morphology within 1 day of death. After death the fetal size decreases, and the fetus assumes the appearance of an ovoid mass of heterogeneous echogenicity (Fig. 58-3).
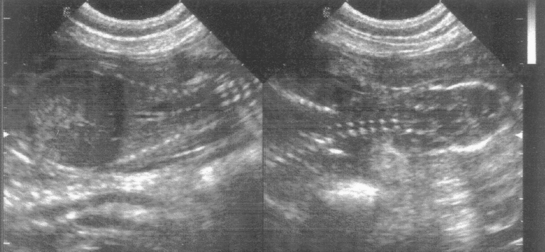
FIG 58-1 Sonograms of canine pregnancy, 40 days after first breeding (dorsal view). Fetal spine and ribs appear on left image. On right image cervical spine and outline of fetal skull are shown.
Because the hormone relaxin is produced primarily by the placenta, it is pregnancy specific in bitches and queens. In pregnant females, relaxin reaches detectable levels in serum or plasma as early as 20 days after the LH surge and peaks 30 to 35 days after the LH surge. It remains high throughout pregnancy, until parturition or abortion, when it declines precipitously. Although relaxin can be detected 21 days after breeding, it is a more sensitive indicator of pregnancy when performed 30 or more days after breeding. Finding high concentrations of relaxin in serum or plasma confirms pregnancy. Declining or undetectable concentrations are found in cases of spontaneous or induced abortion and after parturition. Relaxin is undetectable in pseudopregnant and nonpregnant bitches and queens.
Abdominal radiography can be used to confirm pregnancy after the fetal skeleton has calcified sufficiently to be detected on radiographs. This usually happens approximately 40 to 45 days after breeding in the bitch and 35 to 40 days after breeding in the queen. Before that time the enlarging uterus appears as a tubular fluid density. Because abdominal radiographs are taken later, they are usually not used for pregnancy diagnosis per se. They are used to estimate fetal numbers, identify problems that might lead to dystocia, and confirm the remaining presence of fetuses in the bitch or queen examined because of dystocia.
GESTATION LENGTH
Gestation length, defined as the interval from a fertile mating to parturition, averages 66 days (range 64 to 71 days) in queens. Because the bitch ovulates spontaneously at any time during estrus, determining gestation length on the basis of breeding date is more variable (see Chapter 56). The average gestation length is 63 ± 7 days if calculated from the date of first breeding to parturition. It is 65 ± 1 days if calculated from the LH peak and 57 ± 3 days if calculated from the first day of cytologically confirmed diestrus. Gestation length appears to vary somewhat according to breed of dog and the size of the litter as well. In a group of 308 large dogs (Hounds, Retrievers, German Shepard Dogs), litters of four or fewer pups averaged 1 day longer gestation than litters with five or more pups (Eilts et al., 2005). Conversely, in 36 Beagles litter size, which ranged from two to eleven pups, had no significant effect on gestation length (Tsutsui et al., 2006b).
PARTURITION
Physiologically, parturition may be thought of as a release from inhibitory effects on the uterus and the recruitment of factors promoting uterine activity. Factors that maintain uterine quiescence during pregnancy include progesterone and relaxin. Factors that stimulate uterine activity include prostaglandin and oxytocin. In the bitch maternal cortisol (and probably also fetal cortisol) concentration and maternal prostaglandin PGF2α concentration increase before parturition. PGF2α causes luteolysis and a subsequent decrease in the serum progesterone concentration to less than 1 ng/ml (approximately 3 nmol/L) 24 hours before parturition. Although a similar prepartum decline in the serum progesterone concentration is seen in queens, basal concentrations are apparently not necessary for parturition to be initiated. Prostaglandin also stimulates uterine contractions, as does oxytocin. Among other mechanisms, oxytocin is released in response to pressure against the cervix. The decrease in progesterone and increase in prostaglandin cause the placenta to separate. Relaxin, which is produced by the placenta, abruptly declines at parturition. In both bitches and queens a prepartum increase in the prolactin concentration is seen, which is probably also a result of the decreased serum progesterone concentration. Postpartum, prolactin secretion is stimulated by suckling. In queens the serum estradiol concentration increases before parturition, but in the bitch PGF2α increases without an increase in estradiol.
PREDICTING LABOR
Accurate prediction of the due date is helpful in planning for normal deliveries, scheduling cesarean sections, and evaluating females with suspected prolonged gestation. Because queens are induced to ovulate by coitus, breeding date can be used to predict parturition within ±1 day of the average 66-day gestation. Using breeding dates alone, the clinician can predict parturition within ± 7 days of the average 63-day gestation in bitches. A range of 14 days is too imprecise to be helpful in managing problem pregnancies. In bitches identifying the first day of diestrus on the basis of vaginal cytologic findings (see Chapter 56) can be used to predict when labor should occur because most bitches whelp 57 ± 3 days after day 1 of diestrus. Parturition occurs 65 ± 1 days after the LH surge in the bitch. The LH surge can be measured directly, or estimated by the concomitant initial rise above basal serum progesterone concentrations that occurs during estrus (Chapter 56). Because the serum concentrations of progesterone decrease from more than 3 ng/ml (approximately 9 nmol/L) to less than 1 ng/ml (approximately 3 nmol/L) during the 24 hours before labor in bitches, determining the prepartum progesterone concentration is very useful to determine that a bitch has reached full term.
Alternatively, because the decrease in the serum progesterone concentration just before whelping causes a transient drop in the rectal temperature in most bitches, measuring the rectal temperature is a useful way to predict impending labor. The usual recommendation is for owners to monitor rectal temperature two to three times daily during the last 2 weeks of gestation to establish a baseline. Temperature decreases below baseline by 2° to 3° F (1.1° to 1.7° C) 6 to 18 hours before parturition. In small breeds it may drop as low as 95° F (35° C), in medium-size breeds as low as 96.8° F (36° C), and in large breeds to 98.6° F (37° C). When the drop in temperature is identified, it is usually a reliable indication that parturition will soon occur. In some bitches the temperature fluctuates. In a study of 100 canine pregnancies in which rectal temperature was taken approximately every 12 hours, the prepartum drop in rectal temperature was not detected in 19 animals before the delivery of the first pup. A prepartum drop in the rectal temperature of queens is an inconsistent finding. Many, but not all, queens refuse to eat during the last 24 to 48 hours of gestation. Loss of appetite usually is a good indicator of impending parturition.
If obvious signs of labor are not present within 24 hours of the rectal temperature drop in near-term bitches or of the loss of appetite in near-term queens, the gravid female should be examined. Unfortunately, diagnostic imaging does not add precision when estimating impending parturition. However, it is very useful for assessing fetal development and viability. Using the extent of fetal skeletal mineralization on radiographs, including the recognition of teeth and phalanges, to predict parturition was only accurate to within 3 days in 75% of cats. Using the diameter of the inner chorionic cavity and the biparietal (skull) diameter on ultrasound to predict parturition was only accurate to within 2 days in 86% of bitches.
STAGES OF LABOR
Three stages of labor exist in bitches and queens. Stage I is characterized by nesting behavior, restlessness, shivering, and anorexia. Bitches usually pant. The cervix dilates during stage I. No external signs of uterine or abdominal contractions exist. However, uterine contractions can be documented using ultrasound or external pressure transducers (tocodynamometers) that are strapped around the belly. During pregnancy, uterine contractions are slow and tonic in nature. During stage I of parturition, uterine contractions increase in frequency, duration, and strength. These changes are coincident with the decline in progesterone concentrations, the decline in rectal temperature, and the change in behavior of the bitch. As determined by changes in rectal temperature and change in the dam’s behavior, stage I normally lasts for 6 to 12 hours. As determined by the change in uterine contractions until the delivery of the first pup, the duration of stage I was reported to be 13 to 24 hours in one study (n = 5 bitches) and to average 12 hours in another (n = 100 bitches) (Copley, 2002).
Stage II is characterized by obvious abdominal contractions, passage of amnionic fluid, and delivery of the puppy or kitten. Rectal temperature is normal or slightly above normal. Stage II is usually accomplished in 3 to 6 hours. It may last as long as 12 hours in some normal bitches. In some normal queens it may rarely last 24 hours. There may be intermittent, active abdominal straining for several hours before the birth of the first neonate. Constant, unrelenting straining is not normal. Usually less than 1 hour passes between the delivery of subsequent puppies or kittens. The dam may rest for as long as 1 hour or so between births, with no active straining during that time. Occasionally, 12 to 24 hours pass between the births of apparently healthy kittens, but this is not normal for puppies and may be associated with neonatal mortality in both species.
The placenta is normally passed within 5 to 15 minutes of the birth of each neonate. This is stage III. The dam removes the amniotic membranes and cleans the neonate, severing the umbilical cord and eating the placenta. If the dam fails to remove the fetal membranes from the neonate’s face, the owner should do so. Cleaning the neonate is important maternal behavior necessary for bonding between the dam and her offspring; thus the dam should be encouraged to do it. All placentas should be passed within 4 to 6 hours. If the owner is attending, the umbilical cord should be clamped and cut about 1 cm from the body wall. If bleeding occurs, the cord can be ligated.
DYSTOCIA
Dystocia, or difficult birth, has an estimated overall prevalence of approximately 5% to 6% of pregnancies in bitches and queens. In certain breeds, however, the prevalence is much higher, approaching 18% in Devon Rex cats in the United Kingdom and 100% in English Bulldogs in the United States. With the exception of those breeds at high risk, dystocia might be considered a relatively uncommon cause of morbidity or mortality in bitches and queens, accounting for fewer than 1% of emergency admissions. However, it is the most common periparturient problem requiring emergency care and a major cause of neonatal mortality in puppies and kittens. Overall mortality rates from birth to weaning average 12% (range 10% to 30%) in puppies and 13 % in kittens, but 65% of those losses occur at parturition and during the first week of life as a result of stillbirth, fetal stress, and hypoxia related to parturition.
There appears to be an increased risk of dystocia in aged bitches, but no relationship between age and dystocia has been found in queens. In both dogs and cats purebred animals are more likely to have dystocia than are mixed breeds. Dolicocephalic (e.g., Siamese type) and brachycephalic (e.g., Persian type) are at greater risk for dystocia than mesocephalic (e.g., domestic shorthair type) cats. In dogs chondrodysplastic breeds and those selected for large heads are at greater risk. When normal parturition is used as a criterion in selection of breeding bitches or queens, the occurrence of dystocia within the colony can be decreased, demonstrating that breed alone is not the determinant. The majority (71%) of privately owned queens presented for dystocia have experienced dystocia during more than one pregnancy, whereas in a large commercial colony of domestic shorthair cats, the incidence of dystocia was only 0.4%. This could reflect different husbandry practices or genetics of brood stock selected on the basis of reproductive performance.
The two most common causes of dystocia in small animals are (1) uterine inertia and (2) fetal malpresentation. Of these, uterine inertia is by far the most common, accounting for about 60% of all cases. Uterine inertia is the failure to develop and maintain uterine contractions sufficient for normal progression of labor. Uterine inertia has a variety of potential causes (e.g., genetic factors, age, nutrition, metabolic factors), but the specific cause for a particular case usually is not identified. The exception is mechanical obstruction that results in myometrial exhaustion and secondary uterine inertia. Fetal malpresentation accounts for approximately 15% of dystocia cases in bitches and queens.
Maternal causes of obstructive dystocia relate primarily to abnormalities in size or shape of the pelvic canal. These abnormalities may be congenital or acquired, involving the bony or soft tissue structures. Within breeds, certain individuals are at greater risk than others. For example, in both the Boston Terrier and Scottish Terrier, breeds with distinctly different head conformation, bitches with a dorsoventral flattening (i.e., vertical diameter ≤ horizontal diameter) of the pelvic canal are more likely to have obstructive dystocia than bitches with normal pelvic conformation (i.e., vertical diameter > horizontal diameter). Cephalopelvic disproportion, in which the fetal head is too large for the small maternal pelvic canal, also can occur. Uterine torsion is also a cause of obstruction (Fig. 57-9). Malpresentation is the most common fetal cause of obstruction. Fetal oversize or congenital deformities causing large abnormal shape (Fig. 58-4) may also cause obstruction.
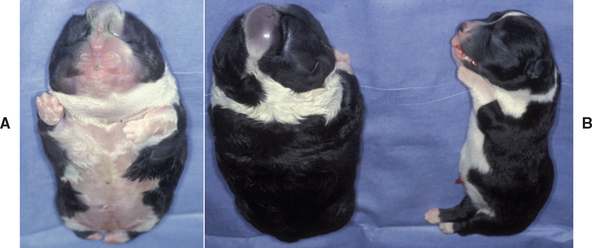
FIG 58-4 A, Cause of dystocia: newborn Cardigan Welsh Corgi puppy with anasarca (ventral view). B, Dorsal view of same puppy with normal litter mate.
Small litter size predisposes to dystocia in bitches for a variety of reasons. The fetal signals that initiate parturition may be insufficient in very small litters, which may lead to prolonged gestation. A negative correlation between litter size and puppy size exists: the smaller the litter, the larger the individual pup. This may increase the likelihood of obstruction. Conversely, a very large litter may overstretch the uterus and lead to inertia. Litter size has no apparent bearing on the occurrence of dystocia in queens. Fetal death accounts for 1% to 4.5% of dystocia in bitches and queens, respectively. Extreme anxiety reportedly inhibits normal progression of labor. How often this contributes to dystocia in dogs and cats is not known.
History
Early recognition and correction of dystocia is crucial to the successful management and optimal neonatal health. The first things that should be determined are the presence of placental membranes or fetal parts at the vulva, and the presence and character of any vulvar discharge. A partially delivered puppy or kitten needs immediate attention. The history should continue with an investigation of the length of gestation, known predisposition to or previous occurrence of dystocia, the progression through the stages of labor, and any indication of illness in the dam. This would include information on rectal temperature monitoring, behavior of the dam, presence and characterization of contractions, number of puppies or kittens already born, and the duration of each of these events. Breeders should be asked if they have already administered any drugs or performed any obstetric procedures. Any sign of illness in the pregnant female is reason to recommend that she be examined. On the other hand, a common error made by owners and veterinarians is to delay intervention because the dam does not appear to be in trouble. The decision to delay is usually made without regard to the well-being of the fetuses, which are often severely stressed long before the dam shows clinical signs relating to the demise of her fetuses. The dam should be examined if the expected due date has arrived and no signs of labor exist, irrespective of a lack of maternal discomfort or illness. This is to ensure that all is well with the fetuses and to determine if continued watchful waiting is a reasonable approach.
If stage I has not progressed to stage II within 12 hours, the dam should be examined, even if other signs of labor or maternal illness are lacking. Exercise often stimulates abdominal contractions. For that reason, some veterinarians have recommended that the owners walk the bitch up and down the stairs or around the house before loading her in the car for the drive to the veterinary hospital. The onset of stage II of labor is recognized by the return of rectal temperature to normal, the presence of strong abdominal contractions, and the passage of amnionic fluid. The passage of amnionic fluid is an indication of stage II labor, irrespective of contractions. The first pup should be born within 2 to 3 hours of amnionic fluid. Other findings of concern are the presence of a vulvar discharge, fetal membranes, or a partially delivered fetus (Box 58-1). Partially delivered puppies or kittens need prompt attention if they are to survive. A dark-green discharge in bitches or red-brown discharge in queens originates from the placenta. Its presence indicates that at least one placenta has begun to separate. If a pup or kitten has not been delivered within 2 to 4 hours, the dam should be examined. A bright yellow vulvar discharge is meconium. Passage of meconium is indicative of severe fetal stress. It is often associated with fetal aspiration of amnionic fluid and a grave prognosis for neonatal survival. A purulent discharge may be found if uterine infection or fetal maceration exists. Viable fetuses may also still be present.
It has been shown in dogs that neonatal mortality is directly correlated to duration of labor (Linde-Forsberg, 2005). For example, one study found that if delivery was complete within 1 to 4.5 hours of the onset of stage II labor, puppy mortality was 5.8%, whereas neonatal mortality was 13.7% after 5 to 24 hours of stage II labor. The outcome for the bitch and the puppies is favorable when the dam is healthy, the fetal heart rates are normal (>200 bpm), stage I is less than 6 hours in duration, and the duration of stage II is less than 12 hours. When stage II lasts longer than 12 hours but less than 24 hours, the prognosis for puppy survival is poor, although the prognosis for the bitch is still fine. If stage II lasts longer than 24 hours, the puppies are likely to die and morbidity for the bitch is increased. Fetal heart rates less than 150 to 160 bpm or illness in the bitch is also associated with worsening prognosis. In a different study puppy mortality from birth to 7 days of age decreased from 33% to 6% as a result of fetal monitoring and early intervention during parturition (Davidson, 2001). Among the multiparous bitches in that study, neonatal mortality decreased from 42% to 12%.
Weak, intermittent straining lasting more than 2 to 4 hours before the first puppy or kitten is born or lasting longer than 1 hour between births is cause for concern. Strong, persistent straining lasting longer than 20 to 30 minutes without delivery of a pup or kitten is not normal. If more than 12 hours of stage II have elapsed or, conversely, if labor appears to have stopped before the entire litter is delivered, the dam should be examined. Cats have been observed to deliver live kittens over 24 to 40 hours, with no obvious straining or discomfort between kitten births. Even though live kittens are often born, such prolonged delivery is associated with increased neonatal morbidity and mortality and therefore should probably not be considered normal. The average duration of labor was reported to be 16 hours in one colony, but kitten mortality was 29%.
Diagnosis
The historical and physical findings are diagnostic of dystocia. The first step is to examine the perineum for evidence of a partially delivered fetus, which requires immediate attention. There may be a bulge in the perineum dorsal to the vulva, or there may be fetal limbs or tail protruding from the vulva. When it is determined that no partially delivered fetus is present, the complete physical examination of the dam proceeds as usual. Systemic illness in the dam should be pursued as usual for any ill animal. For example, hyperthermia may be caused by the exertion of labor, but infection, especially of the mammae or uterus, should be considered. A complete blood count (CBC) and biochemical panel would be reasonable. Regardless of cause, dehydration must be corrected.
The abdomen is palpated to evaluate uterine size, tone, and the presence of fetuses. Fetal movement and uterine contractions may be felt, but their adequacy cannot be assessed by palpation alone. The inability to detect movement or contractions via abdominal palpation is not necessarily cause for concern. The perineum is examined for the presence and character of any discharge. In bitches of adequate size a digital vaginal exam should be performed to assess for the presence of a fetus in the birth canal. If one is found, it should be delivered immediately. If none is found, the dorsal wall of the vagina should be stroked because doing so often stimulates abdominal contractions. This procedure has been referred to as feathering. The cervix is not palpable per vaginum. Puppies or kittens stuck in the vagina may be delivered by obstetric manipulation or with the aid of episiotomy. The mammary glands are palpated to assess the presence and character of secretions. Some primiparous bitches may not have obvious milk. Lactation begins within 24 hours of parturition. Multiparous bitches and queens may lactate during the last week of gestation.
After assessing maternal health by physical examination, the clinician assesses the fetuses using radiology and ultrasonography. The number, size, shape, location, posture, and presentation of any remaining fetuses are often best determined by radiographs. A cause for obstruction, such as large fetus, an abnormal pelvic canal, or fetal malposition may be identified. Fetal viability is difficult to assess on radiographs because postmortem changes are not detectable for hours or days after death (Box 58-2). Intrafetal gas may be detectable as early as 6 hours after death. The bones of the fetal skeleton and head may collapse as early as 48 hours after death. However, the absence of those radiographic signs is not diagnostic of life or death. The number of fetuses remaining cannot be accurately determined with ultrasonography; however, ultrasonography is ideal for assessment of fetal viability on the basis of heart rate and fetal movement.
As determined by ultrasound, normal canine fetal heart rates during labor are 170 to 230 bpm. Fetal kittens’ heart rates are 190 to 250 bpm. Fetal movement is observed from about day 40 of gestation onward. Normal fetuses are quite active near term. Subjectively, this activity seems to increase during ultrasonographic examination. Fetal movement and heart rates are decreased as a result of stress and hypoxemia. In fetal pups heart rates below normal are associated with poor neonatal survival unless pups are delivered promptly. It has been shown that heart rates < 150 to 160 bpm indicate fetal stress. When heart rates are less than 130 bpm, there is poor survival unless pups are delivered within 1 to 2 hours. There is high neonatal mortality among pups with fetal heart rates less than 100 bpm unless they are immediately delivered. We have also observed that lack of fetal movement, irrespective of heart rate, is also a poor prognostic indicator. Presumably, the situation is similar in cats, taking the normally faster feline heart rate into account. The precise gestational age cannot be determined on the basis of ultrasonographic findings, but fetal maturity and impending fetal death can be assessed by the development, or lack thereof, of fetal organs (see Fig. 58-2). Previously recognizable fetal anatomy begins to be lost within 24 hours of fetal death. The overall size of the fetal mass decreases and condenses into a heterogeneous echotexture (see Fig. 58-3).
Treatment
A partially delivered fetus should be delivered within 10 minutes. Care must be taken to avoid disarticulating the extremities. Liberal amounts of lubrication should be used. Rotating the fetus 45 degrees to take advantage of the widest diagonal part of the pelvic canal may be helpful. Gently alternating traction from left to right (i.e., rocking) may help relieve shoulder or hip lock. Traction should be applied in a ventral direction that follows the natural conformation of the vestibule. It may be helpful to lift the vulvar lips upward while pressing the pup downward. A vaginal exam should be performed in all dams of adequate size to determine whether a fetus is lodged in the vagina and to stimulate the vagina (i.e., feathering) in hopes of initiating abdominal contractions. If the dam is extremely nervous, mild sedation should be considered.
When the clinician has determined that an “overdue” bitch is healthy and the fetuses are healthy (as determined by the presence of fetal movement and normal heart rates), serum concentrations of progesterone may be determined. This would be especially helpful when information by which the actual length of gestation might be calculated is lacking. The finding of progesterone that is greater than 3 ng/ml (9 nmol/L) in a bitch would indicate that the pregnancy has not yet reached full term. Intervention should be delayed, and watchful waiting should continue for several hours. If 24 hours pass with no progression of labor, all parameters should be reassessed. Aglepristone, 15 mg/kg, given subcutaneously twice on 1 day, safely and effectively induced parturition in Beagle bitches (Baan et al., 2005). Progesterone concentrations were still elevated when treatment began. Parturition occurred 32 to 56 hours (mean 41 hours) after the first injection. Puppy survival rates were no different from those of control bitches that whelped naturally. The only side effect was irritation at the injection site.
Animals in stage I of labor are expected to progress to stage II in less than 12 to 24 hours. When that does not happen, watchful waiting no longer applies, nor does it apply to dams already in stage II of labor. Sometimes, all other parameters are found to be normal except one of the fetuses is not moving or has a heart rate of 150 to 160 bpm or less. The dam and the other fetuses are healthy. In that situation the benefits of immediate intervention in an attempt to save all the fetuses should be weighed against the cost and risks. For example, the decisions made in a situation in which all but one of 10 puppies are apparently normal might be different from the decisions made under identical circumstances but a litter size of only two. The owner’s attitude about the relative value of each puppy or kitten in the litter and about stillbirth or neonatal death must be considered. It is common for bitches and queens to carry healthy fetuses to term despite the death of some litter mates.
The type of treatment is dictated by the presence or absence of obstruction and by the health of the dam and fetuses. If obstruction or serious fetal compromise exists, cesarean section is indicated without delay. If no obstruction exists, medical management may be attempted in healthy dams with no signs of fetal stress. Several studies have found that 65% to 80% of bitches and queens presented for dystocia were eventually treated with cesarean section. Medical management was successful in resolving the dystocia in only 20% to 30% of canine and feline cases. The maternal mortality rate is reported to be about 1% among bitches undergoing cesarean section. In addition to maternal survival, the goal of managing dystocia is to achieve puppy and kitten survival beyond the most critical first week of life.
When the dam and the fetuses are healthy and no obstruction exists, medical management of dystocia can be considered. The goal of medical management is to reestablish a normal labor pattern of uterine contractions. This is done with oxytocin and calcium. Typically, oxytocin increases the frequency of uterine contractions and calcium increases the strength. High doses and/or frequent administration of oxytocin are contraindicated because they cause sustained uterine contractions that delay the expulsion of fetuses and compromise placental blood flow. This causes placental separation, fetal hypoxia, and fetal acidosis. These actions contribute to fetal and neonatal mortality. The goal of oxytocin therapy is to increase the frequency of uterine contractions to a normal labor pattern. This is best accomplished while the uterine contractions are being monitored. Unfortunately, this is often not done in veterinary medicine. Studies in which uterine monitoring was done have demonstrated that the large doses of oxytocin that have traditionally been recommended are not necessary. Current recommendations are to administer small doses, 0.25 to 4.0 U per dog, intramuscularly. In our colony of mixed-breed dogs weighing 35 to 45 lb, we administer 0.25 U. We do not monitor uterine pressure. Labor should progress (i.e., straining begins) within 30 minutes, and a pup should soon be delivered. If so, the clinician may repeat administration of oxytocin as needed to perpetuate normal parturition. Repeated doses should not be administered if a normal labor pattern is not established. In studies that monitored uterine contractions of whelping bitches, the mean total cumulative doses of oxytocin needed were 4 to 7.7 U per bitch. When the animal does not respond to oxytocin administration within 30 to 45 minutes, it is unlikely that further treatment with single agent oxytocin will be beneficial.
Myometrial contraction depends on the influx of calcium ions. Generally speaking, calcium administration increases the strength of uterine contractions even in the absence of documented hypocalcemia. For this reason some clinicians have recommended the routine administration of calcium gluconate in the management of nonobstructive dystocia. It has been recommended by some that 10% calcium gluconate be administered before the administration of oxytocin. If normal labor does not resume, oxytocin is added. Calcium gluconate 10%, 0.2 ml/kg or less, or 1 to 5 ml/dog, is administered subcutaneously or intravenously depending on the preparation and the label directions. Some preparations are too irritating to be administered by routes other than intravenous (IV). If the IV route is chosen, calcium is administered slowly (1 ml/min) while monitoring the heart. Administration should be immediately discontinued if bradycardia or dysrhythmia occurs. If labor progresses (i.e., straining begins), calcium may be repeated as needed or continued with oxytocin. In a study using uterine monitoring as a guide, the mean total cumulative dose of 10% Ca gluconate administered to bitches was 3 ml. Conversely, before uterine monitoring was available, doses of 1.5 to 20 ml were reported. Higher doses or bolus IV administration of Ca gluconate should be reserved for animals with documented clinical signs or laboratory evidence of hypocalcemia. When medical management fails to initiate a normal labor pattern, cesarean section should be performed without delay.
Cesarean section is indicated, without delay, in the following circumstances: obstruction, such as fetal oversize, fetal malposition, or uterine torsion; existence of fetal compromise; failure of medical management with calcium and oxytocin administration; the possibility that continued pregnancy or labor might be harmful to the bitch or queen; or preexisting maternal illness. At the time of this writing, at least one company provides fetal and uterine monitoring services for veterinarians: Veterinary Perinatal Specialties (www.whelpwise.com).
PREGNANCY LOSS
Etiology
Infectious disease is an important cause of pregnancy loss in dogs and cats. Infectious diseases can cause early embryonic death, resorption, or abortion through their effects on the dam, the fetus, or the placenta. Other than interrupting pregnancy, many of these pathogens cause minimal clinical signs of maternal illness. Bacteria reported to cause fetal death and abortion in bitches include Brucella canis, Escherichia coli, β-hemolytic Streptococcus, Leptospira, Campylobacter, Salmonella, Mycoplasma spp., and Brucella abortus. Bacterial causes of pregnancy loss are uncommonly reported in cats. Experimental infection with Toxoplasma gondii has also been found to cause abortion in bitches and queens.
Clinical Features
Embryonic and fetal death can result from maternal disorders, fetal disorders, or placental disorders. Queens and bitches often lose one or more fetuses and yet carry the rest of the litter to term and deliver normal healthy puppies or kittens. Anything that adversely affects the health of the dam and medications used for treatment have the potential to adversely affect the pregnancy. Other than a disorder that causes overt clinical illness in the dam, the signs associated with fetal death depend primarily on the stage of gestation at which the loss occurs.
When early embryonic death occurs, there are no clinical signs of the bitch having been pregnant. Therefore the bitch is likely to be presented for (apparent) failure to conceive rather than for pregnancy loss. In pregnant queens, early embryonic death will be reflected by a prolonged interestrual interval of 30 to 50 days rather than the usual nonovulatory cycles every 14 to 21 days. Pregnancy loss has no effect on the canine interestrual interval because the canine CLs persist for more than 60 days regardless of whether the bitch is pregnant. Progesterone, produced by the CLs, causes mammary development and weight gain regardless of whether pregnancy exists. Therefore bitches may continue to appear pregnant for 60 or more days. If early pregnancy is lost in queens, the CLs regress in 30 to 50 days; thus any appearance of pregnancy diminishes after that time. Other than the loss of mammary development in queens, usually there are no physical signs, such as vulvar discharge, when embryonic death occurs during the first 30 days of gestation in bitches and queens. Resorption occurs. When fetal death occurs after about day 30 of pregnancy, uterine contents are passed (abortion). The first clinical sign of abortion is usually a blood-tinged vulvar discharge. The character of the discharge is variable, according to the underlying cause of the abortion. The quantity is variable from scant to substantial. The later in gestation fetal death occurs, the more obvious it becomes that fetal parts are being expelled.
MYCOPLASMA
Mycoplasma and Ureaplasma are members of the normal florae in the canine vagina, prepuce, and distal urethra. Mycoplasma has been isolated from 59% of vaginal cultures, 80% of preputial samples, and 27% of semen samples from normal dogs in kennels with excellent pregnancy rates of 88% to 90%. Mycoplasma infection has been reported to cause conjunctivitis, polyarthritis, abscesses, and urinary tract infection in cats. In dogs pneumonia, urinary tract infection, colitis, and reproductive disorders have been associated with Mycoplasma and Ureaplasma infection. Although experimental inoculation of the reproductive tract with Mycoplasma canis causes endometritis in bitches and orchitis and epididymitis in dogs, the significance of Mycoplasma in spontaneously occurring canine reproductive disease is unclear because there is no difference between the prevalence of Mycoplasma isolated from normal animals and the prevalence of Mycoplasma isolated from animals with reproductive disorders.
Because Mycoplasma and Ureaplasma are members of the normal canine genital florae and because they are isolated with equal frequency from normal dogs and dogs with reproductive disorders, Mycoplasma or Ureaplasma infection should not be diagnosed on the basis of culture results alone. The clinical signs and cytologic findings should also be consistent with an infectious process. Mycoplasma and Ureaplasma are fragile organisms. A special medium such as Amies should be used for culture studies, and samples should be placed on ice and arrive at the laboratory within 24 hours. Susceptibility testing is rarely available. Usually, the organisms are susceptible to tetracycline, chloramphenicol, and fluoroquinolones. Unfortunately, many of these antibiotics are contraindicated during pregnancy and lactation. Isolation and even culling of infected animals have been recommended for the control of Mycoplasma infection in a kennel, but such extreme measures are not usually necessary.
BRUCELLA CANIS
Brucella canis is a small, gram-negative coccobacillus. Dogs are the definitive host for B. canis infection. They are much less susceptible to Brucella abortus and Brucella suis. Cats are resistant to B. canis but can be infected under experimental conditions. Compared to B. abortus and Brucella mellitensus infection, people are relatively resistant to B. canis. The source of infection is usually the person’s own pet. Laboratory personnel have also acquired the disease from infected specimens. Biohazard precautions should be taken when handling specimens from suspect animals. The prevalence of human B. canis infection in the United States is not known because although human brucellosis is a notifiable disease, the Centers for Disease Control (CDC) does not require speciation. In one study B. canis infection accounted for 4 of the 331 people with brucellosis in a 10-year period.
B. canis readily crosses all mucous membranes. Although venereal transmission occurs, the most common routes of infection are oronasal and conjunctival. Neutered and “virgin” animals can become infected as well as sexually intact animals. The greatest numbers of organisms are shed in aborted material and postabortion vaginal discharge, which readily contaminate the environment. Large numbers are shed in semen, particularly during the first 6 to 8 weeks of infection, but shedding persists for 60 weeks to 2 years. Organisms are also shed in urine, especially from males. Urinary shedding persists for at least 3 months. Urine is especially important in transmission when animals are housed in groups. B. canis is shed in milk, and transplacental transmission occurs. It can also be transmitted on contaminated fomites.
Tissue macrophages and other phagocytic cells carry the organism to lymphoid tissue, bone marrow, and the reproductive tract, where they multiply. Organisms persist in mononuclear phagocytes, bone marrow, lymph node, spleen, and prostate. Persistence of the organism in the prostate is thought to explain the greater number of organisms recovered from the urine of infected males than from females. Bacteremia is present 1 to 4 weeks after infection and persists for 6 months to 5.5 years. Nonprotective antibodies develop within weeks of infection but may not be detectable until 8 to 12 weeks after inoculation. Titers persist for as long as the bacteremia is present. Titers decline after the bacteremia subsides, even though the organism is still present in tissues.
Clinical Features
B. canis infection primarily affects reproduction. Transient lymphadnopathy may be observed. Animals are afebrile. Placentitis caused by B. canis results in fetal death. Abortion after about day 45 is the most commonly reported clinical sign of B. canis infection in females. However, fetal death may occur at any time during gestation, and early embryonic death would go unnoticed or could be misinterpreted as conception failure. Occasionally, a litter is carried to term, but the pups usually die within a few days of birth.
The most common clinical sign of B. canis infection in males is infertility. Scrotal and epididymal enlargement are usually transient early in infection. Testicular enlargement is uncommon. Abnormalities in seminal quality occur within 5 weeks of infection and become pronounced by 8 weeks. White blood cells, macrophages, sperm agglutination, and abnormal sperm morphology are found. By 20 weeks of infection, more than 90% of the sperm may be abnormal. Eventually, testicular atrophy and azoospermia develop, and inflammatory cells are no longer found in semen. Other than reproductive signs, dogs are healthy. B. canis may infect nonreproductive organs, most notably the eye and intervertebral disk. In such cases there are clinical signs associated with uveitis and discospondylitis. Osteomyelitis, dermatitis, meningoencephalitis, and glomerulonephropathy are less common.
Diagnosis
The diagnosis of B. canis infection is suggested by the history of abortion in females, infertility and seminal abnormalities in the male, and the relative absence of physical abnormalities. The diagnosis of B. canis is confirmed by identification of the organism by culture or polymerase chain reaction (PCR). Positive serologic results must be confirmed by these methods. Blood, postabortion vaginal discharge, and semen are the best specimens for culture. Blood culture or PCR is the best method for identifying early (2 to 8 weeks) infection. The number of bacteria in blood usually remains very high for at least 6 months after infection. Bacteremia subsides as the infection becomes chronic; thus blood cultures are not always positive. Semen cultures are most helpful during the first 3 months of infection, when the number of organisms in semen is high. Urine cultures may be positive, especially in males. The organism can also be recovered from lymph nodes, spleen, liver, bone marrow, prostate, epididymis, placenta, and the lumen of the gravid or postabortion uterus. B. canis is rarely recovered from the nonpregnant uterus or the vagina except after abortion.
Although isolation of the organism is the definitive diagnosis, it is impractical for the routine screening of asymptomatic animals. For this reason serologic testing is the most frequently used screening diagnostic procedure for B. canis infection. Antibodies to cell wall (somatic) lipopolysaccharide (LPS) antigens of B. canis cross-react with many other organisms including Pseudomonas aeruginosa, Staphylococcus, Actinobacillus equuli, and Brucella ovis. Therefore any of the serologic tests using cell wall LPS antigens have high false-positive rates, some as high as 60%. The addition of 2-mercaptoethanol (2-ME) eliminates the less specific reactions of IgM antibodies, but false-positive results are still common. Internal cytoplasmic protein antigens (CPAg), on the other hand, are highly specific for Brucella infection.
Serologic tests using cell wall antigens include the following: 2-ME rapid slide agglutination test (RSAT), 2-ME tube agglutination test (TAT), indirect fluorescent antibody (IFA), agar gel immunodiffusion (AGID), and enzyme-linked immunosorbent assay (ELISA). The serologic tests that include the more specific cytoplasmic protein antigen are the AGID (CPAg) at NYS Diagnostic Laboratory, Cornell University, Ithaca, New York, and an ELISA (CPAg) that has limited availability. Unfortunately, laboratory reagents and/or methods have not been standardized for any of these tests except 2-ME RSAT and 2-ME TAT. Availability of the standardized reagents for 2-ME TAT is sporadic. Therefore the reliability of test results and the accuracy of interpretation are extremely variable among laboratories.
Despite its lack of specificity the RSAT (D-Tec CB®; Synbiotics) has the tremendous advantage of being easy, quick to perform, and highly sensitive. Negative RSAT results are rare (1%) in animals that have been infected long enough to develop detectable antibodies (8 to 12 weeks). Treatment with antibiotics causes negative culture and serology results, despite persistence of the organism in tissues. Titers decline in chronic infection, but they may persist for months after the bacteremia has ceased.
Treatment
Antibiotic therapy rarely, if ever, results in a cure for B. canis infection. The results of cultures and serologic testing become negative in animals with chronic infection and also in those receiving antibiotic therapy, despite the persistence of B. canis in tissues; thus it is difficult to ascribe declining titers or negative culture findings to treatment rather than to the natural progression of the disease. Bacteremia and positive serologic results often recur days to months after treatment. Minocycline, tetracycline, dihydrostreptomycin, trimethoprim sulfadiazine, gentamicin, doxycycline, enrofloxacin, and various combinations thereof have been used to treat B. canis. The vast majority of treated dogs remained infected. Evidence shows that, despite therapy, the organism is not cleared from the prostate. Testicular damage is usually irreversible. Treated dogs are readily susceptible to reinfection. Because the chance of successful treatment is so unlikely and because infected animals remain a source of infection for other dogs and people, treatment is ill advised. If treatment is attempted, infected animals should be neutered to minimize the shed of organisms. No vaccine exists.
Prevention and Control
B. canis is insidious. No readily recognizable signs appear until animals have been infected for weeks or months, during which time they have exposed other members of the colony to the infection. Eventually, B. canis infection will devastate the reproductive performance of the individual animal and the kennel. In kennels with infected animals, conception rates can decline to as low as 30%; the proportion of pregnancies ending in abortion can reach 80%; litter size (Beagles) can decline from a previous average of six pups to one pup per litter; and the number of pups surviving to weaning age can reach zero. Obviously, the risk of inadvertent exposure to asymptomatic, infected animals that are brought into the colony, even briefly, is too great to leave to chance. All animals should be tested before breeding. New members to be added to the colony should be quarantined for 8 to 12 weeks until the results of at least two tests performed at 4-week intervals are negative. Animals with any of the symptoms of B. canis infection should never be admitted to the colony for any reason until B. canis infection is positively excluded as the cause. As with asymptomatic animals, it may take as long as 3 months to ensure that the animal is not infected.
The RSAT is recommended for the routine screening of asymptomatic animals because it is so sensitive. If the animal has been infected for 8 to 12 weeks so that antibodies have reached detectable levels, if the animal is not so chronically infected that the titers have declined, and if no antibiotics have been administered, animals that do not have the infection should be correctly identified by a negative test result. Positive test results must be confirmed with other methods because the RSAT lacks specificity and false-positive results are common.
When an animal is found to be positive on the basis of the RSAT or other screening test, especially if clinical signs compatible with B. canis infection are seen, the animal should be isolated from the rest of the colony and the entire kennel should be quarantined until the results can be verified. The definitive diagnosis can be made only on the basis of the isolation and identification of the organism from culture or PCR of appropriate specimens. An AGID test that uses CPAg, but not those using LPS antigen, may also be helpful to confirm the diagnosis.
When the infection is confirmed, the positive animal should be eliminated from the colony and all other colony members tested monthly. All positive animals are eliminated. Monthly colony-wide testing of all remaining animals, including those with negative results to the previous month’s test, continues until all results are negative in all the remaining animals for 3 consecutive months. Because of the biologic behavior of the infection, it is expected that additional positive animals will be found for several months. Therefore the prevalence of infected animals in the colony is usually not significantly lowered until testing and culling have continued for 4 to 5 months.
Testing and culling are time-consuming and expensive, even in small colonies. Many are tempted to try treating the disease rather than to accept the immediate losses incurred by culling. Treatment is made all the more attractive by reports of apparent success. Bacteremia and serologic titers diminish in response to antibiotic therapy, and many treated bitches successfully conceive and carry a healthy litter to term during that time. However, evidence from studies in which animals were evaluated by culturing internal organs or blood 6 or more months after treatment shows that many still harbor the organism despite negative serologic test results. Thus far, the evidence of all the studies of spontaneously occurring infection have shown that B. canis is not eliminated from the colony, even when infected animals are strictly isolated and regardless of treatment, until infected animals are actually culled.
A different approach might be considered for a household pet than for a breeding animal. Antibiotic therapy plus neutering should essentially eliminate genital secretions and the shedding of organisms by this route, but not necessarily others. Treatment and neutering would not absolutely exclude the possibility that the animal might remain a source of infection for other dogs or human members of the household. Owners of pets or kennels should be informed of the zoonotic potential. All people exposed to infected or suspect animals should practice good hygiene.
HERPES VIRUS
Herpes virus has been implicated as a cause of abortion, stillbirths, and infertility in dogs and cats. Canine herpes virus (CHV) has been suggested as the causative organism of vesicular lesions of the vagina and prepuce, but isolation of the virus from spontaneously occurring genital cases is rarely reported. Mild respiratory tract disease is by far the most common clinical sign of herpes virus infection in dogs and cats older than 12 weeks of age. The lesions are usually limited to the mucosal surfaces of the oropharynx. Occasionally, the manifestations of feline herpes virus (FHV) type I (i.e., rhinotracheitis) may be severe and include conjunctivitis, corneal ulceration, and fatal pneumonia. In neonates herpes virus infection causes fulminant multiple-organ failure and death. Neonates become infected in utero, through exposure to infected secretions of the dam, or through postnatal exposure to infected older members of the colony. Neonatal herpes virus infection is one of the most common manifestations of CHV infection in a breeding colony. Neonates nursing from seropositive bitches are resistant to infection.
Because herpes viruses are spread primarily by aerosolization and direct contact with oronasal secretions, the population density, segregation of life stages, and sanitation of the facility influence the severity of disease within the colony. The prevalence of CHV is estimated to be 10% to 15% in single-pet households and as high as 85% in kennels. Once infected, animals are considered infected for life. The infection may remain latent or be expressed at any time. Nasal secretions, even from asymptomatic carriers, are considered epizootiologically the most important routes of transmission. Venereal transmission of CHVs and FHVs is rare.
Diagnosis
The most common clinical signs of herpes virus infection in dogs and cats are respiratory. From the standpoint of reproductive disease, herpes virus infection should be considered in cases of acute neonatal death, as a potential cause of abortion in dogs and cats, as a potential cause of infertility in cats, and as a potential cause of vesicular lesions of the mucosal surfaces of the genitalia in adult dogs. The diagnosis can be confirmed by the finding of the characteristic intranuclear inclusion bodies in tissue sections, by serologic studies, and by virus isolation and PCR.
Swabs from the affected area (genital lesion, conjunctiva, nasal) should be submitted on ice for virus isolation. Some laboratories have found that herpes viruses are more easily recovered from rayon-Dacron swabs (Dacron-tipped applicators; Baxter) than from wooden cotton-tipped swabs. This is especially important if the virus concentration is low. Herpes virus has usually not been isolated beyond 2 to 3 weeks after the primary infection. Therefore virus isolation is not a very useful diagnostic test for chronic infection, unless viral recrudescence has occurred. Herpes viruses induce a weak systemic humoral response in the host, with antibody titers rising and falling quickly (4 to 8 weeks) after infection. If seropositive animals also show typical clinical signs, this is considered diagnostic for herpes virus infection. Suspected herpes-induced genital lesions can be biopsied. Histopathologic findings typical of herpes virus infection include the vesicles, degeneration of epithelial cells, and marked acantholysis. Intranuclear inclusions may be found but are less common in the material from genital lesions than in nasal epithelium or kidney tissue
The diagnosis of CHV infection is most easily established in cases of neonatal death because the clinical signs and postmortem lesions are very characteristic. Grossly, the lesions consist of multifocal, diffuse hemorrhages and gray discoloration of parenchymal organs, especially the kidney, liver, and lungs. Microscopically, multifocal, necrotizing lesions are found. The virus can be isolated from many organs, especially the adrenals, lung, liver, kidneys, and spleen. In cases of neonatal death, chilled (not frozen) samples from the liver, kidney, and spleen should be submitted for virus isolation and formalin fixed for histopathologic examination. The whole abortus or placenta can be submitted chilled for virus isolation. Although FHV infection causes abortion in pregnant cats, the virus is usually not recoverable from aborted material. Intranuclear inclusions are found in histologic specimens from the uterus, placenta, and aborted fetus of infected queens.
Herpes virus infection is prevented and controlled by changing management practices. Crowded conditions should be eliminated. Herpes viruses are very labile, and commonly available disinfectants are effective in destroying them. Sanitation and hygiene should be improved. Animals should be segregated according to life stages. Pregnant females and neonates should be isolated from all other colony members to prevent exposure to asymptomatic carriers. Although a bitch infected late in pregnancy is likely to suffer neonatal losses, she is also likely to acquire some immunity, which will protect her subsequent litters. For that reason, neonatal CHV usually is not a recurrent problem in an individual bitch. Neonatal CHV may remain a colony problem, however, unless management practices are changed. Vaccines are available.
OTHER CAUSES OF PREGNANCY LOSS
Viral agents are the most commonly reported infectious cause of abortion in queens. Calici virus is one of the most important. In addition to calici and herpes viruses, parvo virus (panleukopenia), feline leukemia virus, feline immunodeficiency virus, and feline infectious peritonitis have been implicated as causes of abortion in cats. Canine distemper is reported to cause bitches to abort.
Apparent luteal insufficiency is discussed as a cause of resorption and abortion, but it is rarely documented in bitches or queens. Determination of serial serum progesterone concentrations would be the first step in documenting this problem. Certain drugs that may be used to treat or prevent maternal illness are also known to be toxic to pregnant females, to be teratogenic, to cause fetal death, or to cause abortion (Box 58-3). Nutritional imbalances can cause pregnancy loss. This can be prevented by feeding high-quality commercial pet foods that are labeled for reproduction and lactation or labeled for use in all life stages.
 BOX 58-3 Examples of Drugs with Probable or Known Risk to Pregnancy in Dogs and Cats
BOX 58-3 Examples of Drugs with Probable or Known Risk to Pregnancy in Dogs and Cats
Fetal anomalies and chromosomal aberrations are reported to be a major cause of spontaneous abortion in women. Anatomic abnormalities are found in 20% of kittens that are stillborn or that die during the first 3 days of life. Most congenital fetal anomalies have no identifiable cause. Some are known to be heritable. Some are caused by environmental factors, such as exposure to teratogens. Chromosomal anomalies have been poorly investigated as a cause of spontaneous abortion in domestic animals, but they have been identified in some stillborn kittens and puppies. When normal-appearing, full-term puppies or kittens are stillborn, the most likely cause is fetal distress during parturition. Subsequent pregnancies and labor should be monitored more closely for signs of fetal stress.
Diagnosis of Resorption and Abortion
The diagnostic efforts are directed toward finding the cause of resorption and abortion so that (1) the dam and any remaining viable fetuses can be treated properly, (2) the problem can be avoided during the subsequent pregnancies of this particular female, and (3) the rest of the colony can be protected from similar occurrences. The diagnostic approach should begin with a thorough history taking that includes such factors as changes in the bitch’s or queen’s environment, the recent addition of new animals to the house or kennel, the vaccination status of the animal, current drug therapy being given, and dietary supplements being administered. This should provide clues to possible exposure to infectious agents and teratogens. Many of the potential causes of fetal resorption-abortion can be excluded or identified during a careful history taking.
The dam should be thoroughly examined for signs of illness and the presence of remaining fetuses. Bitches and queens may abort part of a litter and carry the rest to term. Diagnostic imaging should be performed to determine the status of the uterine contents. Radiographs are most useful for identifying and counting fetal skeletons. Ultrasound is most helpful in assessing the viability of any remaining fetuses and assessing the character of other uterine contents, such as fluid or retained placentas. The metabolic status of the dam or queen should be determined with appropriate laboratory tests, such as a CBC, a serum biochemistry panel, and urinalysis. A sample of the uterine discharge obtained from the anterior vagina should be submitted for bacterial culture and antibiotic sensitivity testing. Appropriate serologic tests (e.g., Brucella titer, feline calicivirus) should also be performed on the dam. The abortus and placenta should be submitted for gross, microscopic, and microbiologic examinations. This complete postmortem examination of the abortus is the single most helpful procedure when attempting to identify the causes of abortion.
Hereditary causes of fetal anomalies may be difficult to prove. Knowledge of the hereditary defects common to the breed is an important aspect of such investigations. The breeding records of related animals should be scrutinized to determine whether there have been similar occurrences. If any are found, hereditary causes become more likely. If birth defects occur in subsequent litters from the same dam and sire, both should be eliminated from the breeding program. If hereditary causes and environmental causes (i.e., exposure to teratogens) can be ruled out, the dam and sire can reasonably be bred again because most birth defects have no identifiable cause, occur sporadically as isolated events, and do not recur in subsequent pregnancies.
Treatment
Therapy for the aborting female is supportive and symptomatic unless a cause can be found. If viable fetuses remain, the pregnancy can be allowed to continue. If not, any remaining contents of the uterus should be removed by ovariohysterectomy or through the administration of ecbolic agents as described for the treatment of pyometra in Chapter 57. Antibiotics should be administered as soon as appropriate specimens for microbiologic and serologic studies have been obtained. In many bitches and queens fetal resorptionabortion is an isolated event with no identifiable cause or treatment. Subsequent breedings are often uneventful.
The next pregnancy should be monitored closely with ultrasonography, beginning about day 10 for queens and about day 15 for bitches, to differentiate failure to conceive from early embryonic death and to recognize impending resorption by the delay in development of specific structures or a slow fetal growth rate. Fetal death will be recognized by lack of cardiac activity and fetal movement. The status of the CL (possible luteal insufficiency) and the placenta can be monitored with serial serum concentrations of progesterone and relaxin, respectively. To evaluate the possibility of premature labor, uterine activity can also be monitored (WhelpWise.com).
OTHER PREGNANCY DISORDERS
With the availability of uterine monitoring, premature labor has now been identified in bitches. Its prevalence and causes are unknown. At this time, treatment recommendations follow those for women but experience is limited so far. Uterine rupture is uncommon in the dog and cat. It occurs during or after labor. Typically, the animal presents with an acutely painful abdomen. Other causes of acute abdominal pain are excluded by diagnostic imaging and biochemical evaluation. The diagnosis is confirmed by exploratory surgery. Treatment is ovariohysterectomy. Ectopic pregnancy rarely occurs in bitches and queens. The clinical signs are usually nonspecific abdominal discomfort or the finding of an abdominal mass. Diagnostic imaging usually reveals a mummified fetus (Fig. 56-9). Treatment is surgical excision. The gravid uterus occasionally is incarcerated in an abdominal wall hernia. Presumably, this is the result of blunt abdominal trauma. Severe electrolyte and glucose abnormalities have been reported in the occasional pregnant bitch and queen and in association with retained fetuses. Treatment is aggressive fluid therapy appropriate to the specific metabolic derangement. Some pregnant animals responded well enough to carry their litters to term. Others were spayed as a part of the treatment plan.
MISMATING (ABORTIFACIENTS)
Queens and bitches may occasionally mate at an undesirable time or with an undesirable male. The dilemma is then whether and how to prevent the birth of unwanted puppies or kittens without offending the moral sensibilities of the owner and veterinarian or threatening the health of the dam and her future reproductive capabilities. If continued reproductive function is not important, ovariohysterectomy can be performed when the female goes out of heat. Ovariohysterectomy should be performed during the first 3 to 4 weeks of diestrus because doing so is less likely to cause galactorrhea than when performed after 30 days in diestrus.
If continued reproduction is important, a question is whether to intervene immediately or wait until pregnancy is confirmed, at about 25 days. A single mating does not always result in pregnancy. As many as 26% to 62% of bitches examined 25 to 40 days after a misalliance are found to be not pregnant. Therefore an option is to do nothing until pregnancy has been confirmed. The risk that this misalliance might result in conception could be assessed by vaginal cytology and serum progesterone concentration, although this is not commonly done. Spermatozoa may sometimes be found on vaginal cytology during the first 24 hours after breeding. Their absence, however, does not preclude the possibility that insemination has occurred. Finding basal serum concentrations of progesterone (<1 ng/ml) indicates that ovulation has not yet occurred, whereas finding progesterone concentrations around 10 ng/ml to 20 ng/ml indicates optimal fertility. Some owners may elect to intervene early, especially if the risk of conception seems high, rather than to wait until pregnancy is confirmed. At this time, our recommendation is to wait until pregnancy is confirmed rather than to treat unnecessarily because the abortifacients currently available in the United States have side effects.
As with the treatment of pyometra, a variety of luteolytic and uterotonic drugs can be used to induce abortion (Box 58-4). Luteolysis is important to stop continued progesterone production, which is necessary to maintain the endometrium for implantation, maintain the health of the placenta, and suppress myometrial activity. Myometrial contractions are necessary to expel the uterine contents. Dopamine agonists such as bromocriptine and cabergoline suppress luteal activity by suppressing prolactin, which is luteotropic in bitches. Prostaglandins, such as prostaglandin F2α and cloprostenol, cause luteolysis via apoptosis, and they also cause myometrial contractions. Competitive antagonists of the progesterone receptor, such as aglepristone, block the effects of progesterone on the uterus and cervix. All these drugs will cause the next interestrous interval to be shortened by 1 to 3 months. None of these drugs is labeled for use in dogs and cats in the United States, although veterinary preparations are available in many other countries. Women who might be pregnant should handle all these drugs with great care.
 BOX 58-4 Therapeutic Options for Canine Misalliance
BOX 58-4 Therapeutic Options for Canine Misalliance
SC, Subcutaneous; PO, oral; LH, luteinizing hormone.
ESTROGENS
Estrogens cause delayed transport of the embryo through the uterine tube, which results in degeneration of the embryo. Although some estrogens are effective in preventing pregnancy, their use has been discouraged for the following reasons. Pyometra develops in some bitches given estrogen during diestrus. Estrogens can prolong the duration of behavioral estrus, predispose to cystic ovarian follicle formation, and cause aplastic anemia. These effects are dose dependent, but aplastic anemia develops in some bitches given the recommended dose. If estrogens are used, they should not be administered during diestrus, as determined by vaginal cytologic findings. Estradiol cypionate (ECP) is no longer approved for use in dogs in the United States. Estradiol benzoate, 0.2 mg/kg, given intramuscularly 2 days after mating, prevented pregnancy in all 10 bitches in which it was administered. Animals were monitored for 75 days posttreatment. No uterine abnormalities were detected on ultrasound examination performed every 5 days, and no changes were found on CBCs performed every 12 days.
PROSTAGLANDINS
After pregnancy has been confirmed, prostaglandins are administered until abortion is complete. This is determined by abdominal ultrasound. If treatment is stopped before the entire litter is aborted, the remaining fetuses may be carried to term. Prostaglandin F2α (0.1 to 0.25 mg/kg; Lutalyse, Pfizer) is administered to bitches subcutaneously, q12h to q8h daily, beginning 30 to 35 days after breeding and continuing until abortion is complete, which is accomplished in 3 to 9 days. In queens 0.2 mg/kg of natural PGF2α administed subcutaneously, q12h for 5 days, beginning about day 45 of gestation, was effective in three of four queens. However, treatment was discontinued after 5 days because of side effects. Side effects include panting, salivation, emesis, defecation, urination, mydriasis, and nesting behavior. Intensive grooming behavior and vocalization may also be seen in the queen. Adverse reactions usually develop within 5 minutes of PGF2α administration and last for 30 to 60 minutes. The severity of reactions is directly related to the dose administered and inversely related to the number of days of therapy. Adverse reactions tend to become milder with subsequent injections.
Fewer side effects are reported for cloprostenol (Estrumate®, Schering-Plough); however, gastrointestinal signs still occur in 30% to 54% of bitches given the drug. In bitches cloprostenol, 1.0 microgram/kg, is administered subcutaneously, q48h, beginning 25 to 42 days after breeding and continuing until abortion is complete. This occurs in 1.5 to 10 days, with a mean of 4 days. In those treated early, pregnancy ends by resorption. In those treated later, abortion occurs. A mucoid sanguineous discharge is present for 3 to 10 days. Given the fewer side effects and fewer injections, cloprostenol may be a better choice than prostaglandin F2α.
The dopamine antagonist cabergoline may be used with cloprostenol at a dose of 5 μg/kg orally, q24h. Gastrointestinal signs are the most common side effect of cabergoline (Galastop®, Ceva Vetem; Dostinex®, Pfizer).
Another protocol involves administration of one of the prostaglandins to bitches early in diestrus, before it is known if pregnancy exists. The advantage of early treatment is that fetuses are resorbed rather than expelled. Postabortion sequelae such as vulvar discharge are also minimal compared with those in bitches treated after day 30 to 35 of gestation. The disadvantage is that some animals will be subjected needlessly to treatment side effects. The protocol is begun no sooner than day 5 of cytologic diestrus, up to day 15 of diestrus. PGF2α is administered q12h for 4 days, or cloprostenol is administered q12h for 5 days using the dosages previously discussed. The serum progesterone concentrations are determined at the end of treatment. Fetal death is likely if the serum progesterone concentration declines and remains below 2 ng/ml (approximately 6.4 nmol/L) for 48 hours. If the progesterone concentration is greater than 2 ng/ml after treatment, luteolysis is not complete, in which case the pregnancy status should be assessed with ultrasonography or a second course of treatment should be administered. In either case reevaluation by ultrasound and/or progesterone determination at 20 days is recommended. When treatment is stopped without confirming the success of pregnancy termination, about 15% of treated bitches will carry their litters to term.
ALTERNATIVE TREATMENTS
Cabergoline can be used as a single agent. When given after 7 weeks of gestation in bitches, cabergoline (5 μg/kg, administered orally q24h for 5 days) causes abortion in 3 to 5 days with few side effects. Unfortunately, at this late stage of gestation recognizable fetal parts or live fetuses that die shortly thereafter may be passed. Parturition (i.e., full-term pregnancy) was prevented in 12 of 14 feral cats given cabergoline in their feed at a dose of either 25 μg/day for 5 days or 50 μg/day for 3 days.
A single course of aglepristone (Alizine®, Virbac, Carros, France) has an efficacy of 97% in bitches and 87 % in queens, when administered at any time from the day of copulation to day 45. The dose for bitches is 10 mg/kg, given subcutaneously twice, 24 hours apart. The dose for cats is 10 or 15 mg/kg, given subcutaneously twice, 24 hours apart. The only side effect reported for aglepristone is transient pain or swelling at the injection site. Massaging the injection site to help disperse the drug can minimize this reaction. Resorption or abortion occurs 1 to 7 days after treatment. On the day of fetal expulsion animals may be somewhat lethargic. The postabortion discharge typically lasts for 1 to 5 days. The animals in which pregnancy is not terminated may deliver a live litter at term, give birth to dead fetuses, or retain them. For this reason the effect of treatment should be determined by posttreatment ultrasound. If fetuses remain, another dose of aglepristone may be tried or prostaglandins can be administered. Given its efficacy and safety, aglepristone is a good choice for early and midgestation pregnancy termination in dogs and cats.
Baan M, et al. Induction of parturition in the bitch with the progesterone-receptor blocker aglépristone. Theriogenology. 2005;63:1958.
Beccaglia M, et al. Comparison of the accuracy of two ultrasonographic measurements in predicting the parturition date in the bitch. J Small Anim Pract. 2006;47:670.
Copley K: Comparison of traditional methods for evaluating parturition in the bitch versus using external uterine and fetal monitors, Proceedings of the Annual Conference of the Society for Theriogenology and American College of Theriogenology, Nashville, Tenn, 2002, p 375.
Cortese L, et al. Hyperprolactinemia and galactorrhea associated with primary hypothyroidism in a bitch. J Small Anim Pract. 1997;38:572.
Davidson AP, editor. Clinical theriogenology. Vet Clin North Am. 2001;31:209.
Eilts B, et al. Factors affecting gestation duration in the bitch. Theriogenology. 2005;64:242.
England G, et al. Ultrasonographic characteristics of early pregnancy failure in bitches. Theriogenology. 2006;66:1694.
Fieni F, et al. Clinical, biological and hormonal study of midpregnancy termination in cats with aglepristone. Theriogenology. 2006;66:1721.
Georgiev P, et al. Mid-gestation pregnancy termination by the progesterone antagonist aglepristone in queens. Theriogenology. 2006;65:1401.
Gobello C, et al. Dioestrous ovariectomy: a model to study the role of progesterone in the onset of canine pseudopregnancy. J Reprod Fertil Suppl. 2001;57:55.
Gobello C, et al. Use of prostaglandins and bromocriptine mesylate for pregnancy termination in bitches. J Am Vet Med Assoc. 2002;220:1017.
Haney D, et al. Use of fetal skeletal mineralization for prediction of parturition date in cats. J Am Vet Med Assoc. 2003;223:1614.
Johnston SD, et al, editors. Canine and feline theriogenology. Philadelphia: WB Saunders, 2001.
Kelley R: Canine reproductive management: factors affecting litter size, Proceedings of the Annual Conference of the Society for Theriogenology and American College of Theriogenology, Nashville, Tenn, 2002, p 291.
Lee W, et al. Ovariectomy during the luteal phase influences secretion of prolactin, growth hormone, and insulin-like growth factor-I in the bitch. Theriogenology. 2006;66:484.
Linde-Forsberg C, et al. Abnormalities in pregnancy, parturition and the periparturient period. In: Ettinger SJ, et al, editors. Textbook of veterinary internal medicine. ed 6. Philadelphia: WB Saunders; 2005:1655.
Luz M, et al. Plasma concentrations of 13, 14-dihydro-15-keto prostaglandin F2-alpha (PGFM), progesterone and estradiol in pregnant and nonpregnant diestrus cross-bred bitches. Theriogenology. 2006;66:1436.
Norwitz E, et al. The control of labor. N Engl J Med. 1999;341:660.
Peña F, et al. Mismating and abortion in bitches: the preattachment period. Compendium. 2002;24:400.
Reynaud K, et al. In vivo canine oocyte maturation, fertilization and early embryogenesis: A review. Theriogenology. 2006;66:1685.
Root Kustritz M. Clinical management of pregnancy in cats. Theriogenology. 2006;66:145.
Tsutsui T, et al. Plasma progesterone and prolactin concentrations in overtly pseudopregnant bitches: a clinical study. Theriogenology. 2007;67:1032.
Tsutsui T, et al. Estradio benzoate for preventing pregnancy in mismated dogs. Theriogenology. 2006;66:1568.
Tsutsui T, et al. Relation between mating or ovulation and the duration of gestation in dogs. Theriogenology. 2006;66:1706.
Wanke M, et al. Use of enrofloxacin in the treatment of canine brucellosis in a dog kennel (clinical trial). Theriogenology. 2006;66:1573.
Zambelli D, et al. Ultrasonography for pregnancy diagnosis and evaluation in queens. Theriogenology. 2006;66:135.
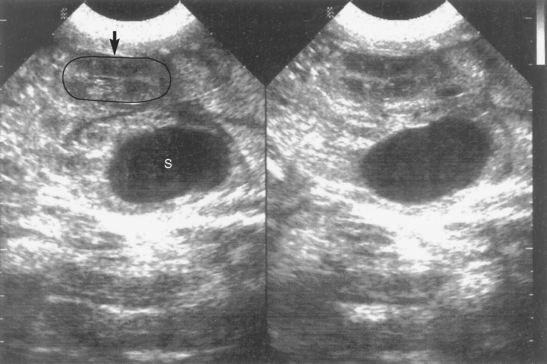
 BOX 58-1
BOX 58-1