CHAPTER 66 Loss of Vision and Pupillary Abnormalities
GENERAL CONSIDERATIONS
Loss of vision or pupillary abnormalities may be detected during the physical examination of an animal examined because of neurologic dysfunction or may be the primary reason for presentation. Owners rarely recognize a visual deficit until it is bilateral and complete, at which time the animal is brought in because of an apparently sudden onset of blindness. When an animal is evaluated because of loss of vision, it is important first to determine whether or not the animal is actually blind and to perform a complete ocular and neuroophthalmological examination.
NEUROOPHTHALMOLOGICAL EVALUATION
VISION
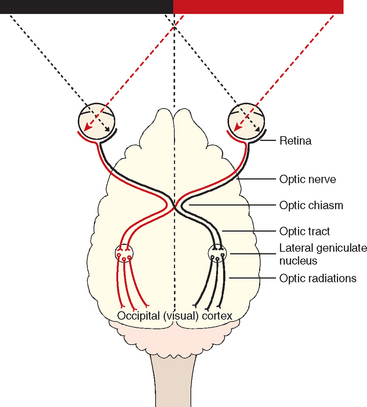
Vision should initially be assessed by observing the animal’s response to the environment, including its ability to negotiate doorways and stairs and the attention it pays to rolling or falling silent objects such as cotton balls. If unilateral vision loss is suspected, the normal eye should be covered during testing. For vision to be present the entire visual pathway must be intact. This includes the retina; the optic nerve, which passes through the optic chiasm to the optic tract to synapse in the lateral geniculate nucleus (LGN) in the diencephalon; and axons projecting to the visual cortex in a band of fibers called the optic radiation. Most of the optic nerve axons cross in the optic chiasm (particularly those carrying information from the lateral visual field) and are continued in the contralateral optic tract, LGN, and optic radiations to the visual cortex (Fig. 66-1). The visual cortex must be functional for the animal to process and respond appropriately to visual cues.
MENACE RESPONSE
The menace response is a cortically mediated blink produced by a threatening gesture (Fig. 66-2). The sensory part of this response involves each of the components of the visual pathway (see Fig. 66-3). Normally, the visual stimulus is directed at the nasal retina (i.e., the menacing gesture is in the lateral visual field coming from the side), and because almost all of the optic nerve axons that originate in the nasal retina cross in the optic chiasm, primarily the contralateral visual cortex is assessed. The information interpreted in the visual cortex is forwarded to the motor cortex to initiate a blink response, requiring a functional facial nerve (CN7). The menace response is also coordinated in the cerebellum, with unilateral cerebellar lesions causing ipsilateral loss of the menace response but no loss of vision. The absence of a menace response could therefore be a result of ocular, retinal, or optic nerve disease; damage to the contralateral forebrain; an altered mental state; cerebellar disease; or an inability to blink (CN7 deficit; Box 66-1). This learned response may not be present in puppies and kittens younger than 12 weeks of age.

FIG 66-2 The menace response is performed by making a threatening movement toward each eye in turn. The expected response is a blink. The stimulus is primarily directed toward the nasal retina, assessing the contralateral visual cortex.
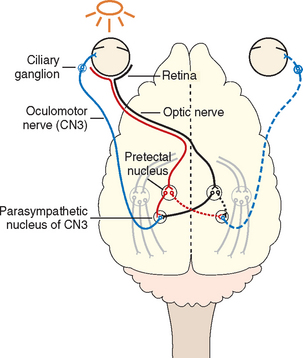
PUPILLARY LIGHT REFLEX
The pupillary light reflex (PLR) should always be assessed, whether or not an animal is able to see. A bright light is directed into the pupil, and the pupil is assessed for constriction (direct reflex). The opposite pupil should simultaneously constrict (consensual response). The sensory visual pathway is the same as that described for the menace response except that some optic tract axons synapse before the LGN in the pretectal nucleus located at the junction between the midbrain and the thalamus. Most of the axons arising from this nucleus cross midline again and synapse in the parasympathetic component of the oculomotor nucleus ipsilateral to the eye being stimulated. Stimulation of the parasympathetic axons of the oculomotor nerve (CN3) results in pupil constriction. Because some of the axons leaving the pretectal nucleus do not cross, there is also stimulation of the contralateral oculomotor nucleus, resulting in a somewhat weaker consensual pupillary response. The pupillary response to light can be minimal if the light used is not bright enough, if the animal is nervous and has high resting sympathetic tone, or if there is ocular disease (iris atrophy or greatly increased intraocular pressure) preventing pupillary constriction. The pupillary light response requires fewer functional photoreceptors and optic nerve axons than vision, so partial lesions of the proximal visual pathways (retina, optic nerve, optic chiasm, optic tract) can sometimes cause loss of vision with normal PLRs, similar to lesions of the forebrain (Table 66-1).
DAZZLE REFLEX
The dazzle response is the generation of a rapid blink when a very bright light is directed into the eye. The sensory visual pathway is as described for the PLR in that this is a subcortical ipsilateral reflex that does not require the visual cortex, but the motor pathway is mediated by the facial nerve (CN7) rather than the oculomotor nerve. A negative dazzle response in a blind eye suggests retinal or optic nerve disease. A positive dazzle response in a blind eye supports central (brain) disease.
PUPIL SIZE AND SYMMETRY
Pupil size and symmetry should be assessed in room light as well as in darkness to evaluate the ability of the pupils to constrict (parasympathetic function) and to dilate (sympathetic function). Pupil abnormalities causing dilation (mydriasis) or constriction (miosis) of only one pupil will result in anisocoria. If the abnormal pupil is unable to constrict, the anisocoria caused by mydriasis in the affected eye will be most apparent in bright light. Anisocoria caused by a single miotic pupil, such as is seen in animals with Horner’s syndrome, will be most apparent in a darkened room as the normal pupil dilates. A complete ophthalmic examination should be performed to ascertain whether pupillary abnormalities can be explained by nonneurologic abnormalities of the eye. Iris atrophy, iris hypoplasia, and glaucoma will cause mydriasis, whereas uveitis and painful conditions of the cornea commonly cause miosis. Hippus, a condition in which there are exaggerated oscillations of pupillary size in response to light, can be an indication of central nervous system disease.
DISORDERS OF EYEBALL POSITION AND MOVEMENT
During the neurologic examination it is important to evaluate eye position and movement. The extraocular muscles are innervated by the oculomotor nerve (CN3), the trochlear nerve (CN4), and the abducent nerve (CN6), with lesions resulting in an abnormal eye position (strabismus) or failure of the eye to move appropriately when the head is moved during evaluation of the vestibulo-ocular reflex (see Chapter 63). Strabismus can occur with lesions of individual nerves, but most often paralysis of all of the extraocular muscles (external ophthalmoplegia) occurs with a mass in the region of the paired cavernous sinuses on the floor of the calvarium adjacent to the pituitary gland (cavernous sinus syndrome). Mass lesions in this area typically also damage the parasympathetic pupillary fibers in the oculomotor nerve (CN3), causing a fixed midrange or mydriatic pupil with normal vision (internal ophthalmoplegia). Ipsilateral damage to the ophthalmic and maxillary branches of the trigeminal nerve result in diminished corneal and medial palpebral sensation and occasionally atrophy of the ipsilateral masticatory muscles.
LACRIMAL GLAND FUNCTION
The lacrimal gland and the lateral nasal gland are innervated by the parasympathetic portion of the facial nerve. Normal function is assessed by performing a Schirmer tear test and examining the ipsilateral nostril for dryness. Facial nerve lesions result in a loss of the palpebral reflex because of an inability to blink, decreased basal tear production, and a dry nose. Sensory innervation of the cornea is provided by the trigeminal nerve (CN5), and corneal stimulation by touch, cold, wind, or other irritants normally results in a blink response and increased reflex tear production. Lesions of the ophthalmic branch of the trigeminal nerve (CN5) result in decreased reflex tear production and decreased blink frequency, which may lead to keratitis and corneal ulceration.
LOSS OF VISION
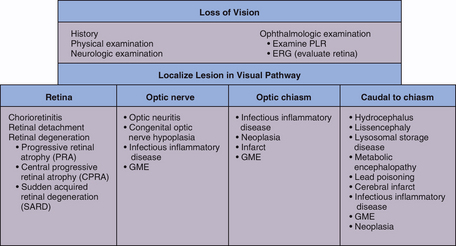
LESIONS OF THE RETINA, OPTIC DISK, AND OPTIC NERVE
Concurrent loss of vision and diminished or absent PLR indicate the presence of a lesion affecting both the visual and PLR pathways. Unilateral severe lesions of the retina, optic disk, or optic nerve before the optic chiasm result in impaired vision and loss of the direct PLR in the affected eye as well as a loss of the PLR in the opposite eye (the consensual response) when light is directed in the affected eye (see Table 66-1). The direct and consensual response to light directed in the unaffected eye should be normal. Ocular or optic nerve disease must be very severe to cause complete loss of PLRs. Whenever an animal is evaluated for blindness, the retina should be carefully examined to rule out disorders such as progressive retinal atrophy, retinal dysplasia, retinal detachment, retinal hemorrhage, and chorioretinitis. Optic nerve atrophy secondary to glaucoma or trauma must also be eliminated as a cause of blindness and PLR loss.
Sudden Acquired Retinal Degeneration
Sudden acquired retinal degeneration syndrome (SARDS) is an idiopathic syndrome causing sudden bilateral degeneration of retinal photoreceptors in dogs. Middle-aged and old dogs of any breed can be affected, with females and obese individuals predisposed. The primary presenting complaint is loss of vision, with complete blindness occurring over a period of hours to weeks and often overnight. Pupils are dilated and PLRs are sluggish in dogs examined shortly after vision loss and absent in dogs with advanced disease. Many affected dogs have concurrent polyuria, polydipsia, panting, weight gain, and lethargy. Clinical, serum biochemical, and urinalysis findings may be typical of hyperadrenocorticism, but endocrine tests and advanced imaging of the pituitary and adrenal glands rarely confirm that disorder. In the early stages of SARDS both fundi appear normal, but with time the retinal changes become indistinguishable from chronic retinal degeneration caused by other conditions. SARDS is differentiated from retrobulbar optic neuritis by its extinguished (flat-line) electroretinogram (ERG). The pathogenesis of the disorder appears to be localized production of antibodies directed against retinal neurons. No consistent response to treatment has been reported, but the administration of intravenous immunoglobulin infusions may be of some benefit early in the course of SARDS. Systemic signs are usually transient and resolve without treatment, but the blindness is permanent.
Optic Neuritis
Inflammation of the optic nerves causes blindness and loss of PLRs (Fig. 66-4). Fundoscopic evaluation may reveal optic disk swelling and discoloration (red) with or without associated retinal detachment and hemorrhage. When optic neuritis occurs posterior to the globes (i.e., retrobulbar), the visible portion of the optic nerves will be normal. In dogs with blindness and loss of PLRs with a normal fundus, ERG is required to differentiate bilateral retrobulbar optic neuritis (normal ERG) from SARDS (flat-line ERG).
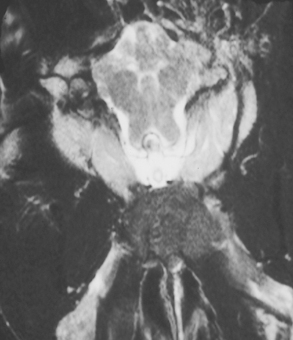
Optic neuritis is most commonly seen as an isolated idiopathic immune-mediated disorder affecting one or both optic nerves, but it may also be a manifestation of systemic disease (Box 66-2), especially canine distemper, ehrlichiosis, mycotic disease, and granulomatous meningoencephalitis (GME). Diagnosis of idiopathic (immune-mediated) optic neuritis is made only after infectious and neoplastic disorders are ruled out during a thorough workup for systemic and intracranial disease, including a complete blood count (CBC), serum chemistry profile, urinalysis, heartworm antigen test, serologic screening for infectious diseases, thoracic radiography, and cerebrospinal fluid (CSF) collection and analysis. Magnetic resonance imaging (MRI) can be used to eliminate mass lesions of the optic chiasm. If all test results are normal, primary immune-mediated optic neuritis is tentatively diagnosed.
Treatment of idiopathic optic neuritis should be initiated with orally administered corticosteroids (prednisone 1 to 2 mg/kg/day). If a favorable response is seen (i.e., improved vision and PLRs), then the dose of steroids should be gradually decreased over 2 to 3 weeks until alternate-day therapy is achieved. If there is no initial response to steroid therapy, then the prognosis for return of vision is poor. Untreated optic neuritis leads to irreversible optic nerve atrophy and permanent blindness. Even with appropriate therapy, many cases will progress or relapse.
Papilledema
Edema of the optic disk usually indicates that there is increased intracranial pressure caused by a cerebral tumor or inflammatory mass lesion. This is seen as an enlarged optic disk with indistinct or fluffy margins and kinking of blood vessels as they pass over the disk. Papilledema may be difficult to distinguish on fundoscopic evaluation from optic neuritis, although patients with a significant forebrain lesion causing papilledema should have clinical evidence of forebrain disease, including abnormal mentation, behavior change, and seizures. Despite reports that papilledema does not affect vision, most patients with papilledema caused by increased intracranial pressure are cortically blind.
LESIONS OF THE OPTIC CHIASM
Lesions of the optic chiasm result in a failure of transmission of the visual image and the light stimulus, causing blindness, normal fundic examination, normal ERG, bilateral mydriasis, and loss of the direct and consensual PLRs in both eyes. Neoplasia and other space-occupying masses can occur at this location, especially lymphoma (cats), pituitary macroadenomas, meningiomas, and primary nasal tumors extending into the brain (Fig. 66-5; see also Fig. 66-4). Vascular lesions such as hemorrhage and infarction, infectious inflammatory granulomas, and granulomatous meningoencephalitis can also affect the optic chiasm. Evaluation should include a search for evidence of extraneural infectious or neoplastic disease followed by MRI, CSF collection and analysis, and endocrinologic testing as warranted.
LESIONS CAUDAL TO THE OPTIC CHIASM
Lesions in the lateral geniculate nucleus, optic radiations, or visual cortex prevent interpretation of the image, resulting in a normal fundic examination, normal ERG, normal PLRs (direct and consensual), and blindness in the eye opposite the side of the lesion. With unilateral lesions of the optic tracts or optic radiations the visual deficits are most com plete in the lateral visual field of the contralateral eye and the medial visual field of the ipsilateral eye. Other clinical signs of forebrain disease, such as seizures, circling, and decreased consciousness, are expected with forebrain lesions severe enough to cause visual deficits. Causes of intracranial blindness (i.e., central or cortical blindness) include trauma-induced hemorrhage and edema, vascular infarcts, GME, infectious encephalitis, central nervous system neoplasia, congenital disorders (e.g., hydrocephalus, lissencephaly), and degenerative disorders (lysosomal storage diseases). Animals with functional disturbances of the forebrain caused by metabolic encephalopathies, lead intoxication, hypoxia, or postictal depression may also present with cortical blindness. Diagnostic evaluation for intracranial blindness should follow guidelines outlined in Chapter 65 and should include thorough physical, ophthalmologic, and neurologic examinations; a laboratory database; screening thoracic and abdominal radiographs; CSF analysis; and CT or MRI evaluation.
HORNER’S SYNDROME
Lesions affecting the sympathetic innervation to the eye result in Horner’s syndrome. This condition causes miosis (constriction of the affected pupil), drooping of the upper eyelid (ptosis), and an inward sinking of the eyeball (enophthalmos). The third eyelid (nictitating membrane) is often partially protruded (Box 66-3; Fig. 66-6).
Horner’s syndrome can result from injury to the sympathetic innervation to the eye anywhere along its pathway (Box 66-4; Fig. 66-7). Lesions are classified as first order (central), second order (preganglionic), or third order (postganglionic) according to the level of the lesion along the sympathetic pathway.
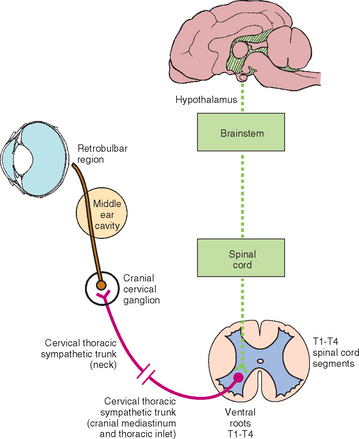
FIG 66-7 Sympathetic innervation to the eye. An injury anywhere along this pathway will result in Horner’s syndrome.
First order neurons originate in the hypothalamus and rostral midbrain and travel down the tectotegmental spinal tract, coursing through the brainstem and cervical spinal cord to terminate at the preganglionic cell bodies in the thoracic spinal cord. Upper motor neuron lesions in the brainstem or cervical spinal cord are a relatively rare cause of Horner’s syndrome but may occur secondary to trauma, infarction, neoplasia, or inflammatory disease. Ipsilateral hemiplegia and other concurrent neurologic abnormalities are expected in these animals (see Box 66-4).
The preganglionic cell bodies of the second order neurons are located in the lateral horn of the spinal cord gray matter at the level of the first three thoracic spinal cord segments (T1-T3). The second order axons leave the spinal cord with the T1 to T3 ventral nerve roots. The sympathetic axons then leave the spinal nerves to form the thoracic sympathetic trunk, which courses cranially within the thorax. The axons continue to course cranially within the vagosympathetic trunk in the cervical region and synapse in the cranial cervical ganglion, ventral and medial to the tympanic bulla at the base of the skull. Injury to second order neurons can occur when there is damage to the spinal cord at the cervical intumescence (C6-T2) caused by trauma, infarcts, neoplasia, or inflammatory disease. Affected animals will exhibit lower motor neuron signs in the affected forelimb, upper motor neuron signs in the ipsilateral rear limb, and Horner’s syndrome. In animals with brachial plexus avulsion there will be complete lower motor neuron paralysis of the affected limb and an ipsilateral Horner’s syndrome that may be partial (miosis only) because of sparing of the T3 (and sometimes T2) nerve roots (Fig. 66-8). Horner’s syndrome can also occur when the second order neurons are damaged by thoracic surgery, mediastinal masses (lymphoma or thymoma), bite wounds to the neck, strangulation injuries, invasive thyroid carcinoma, or errors made during thyroidectomy or surgery for cervical intervertebral disk disease. Physical and neurologic findings are often useful in localizing preganglionic Horner’s syndrome.
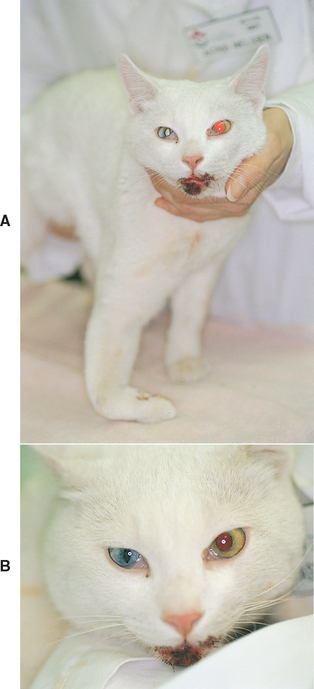
FIG 66-8 Horner’s syndrome (A) in a domestic short-haired cat with traumatic right brachial plexus avulsion (B).
Most dogs and cats with Horner’s syndrome have postganglionic lesions. The postganglionic (third order) axons for ocular sympathetic innervation course rostrally through the tympanooccipital fissure into the middle ear and enter the cranial cavity with the glossopharyngeal nerve (CN9), leaving the cranial cavity via the orbital fissure for distribution to the smooth muscle of the orbit, the upper and lower eyelids, the third eyelid, and the iris muscles. Third order Horner’s syndrome is common in patients with otitis media or neoplasia within the middle ear, often accompanied by evidence of peripheral vestibular (CN8) disturbance and sometimes facial nerve (CN7) paralysis. Rarely, retrobulbar injury, neoplasia, or abscessation will result in a third order Horner’s syndrome.
Pharmacologic testing has been recommended to help localize the cause of Horner’s syndrome in dogs and cats (Table 66-2). This testing can be used to help determine the most likely site of the lesion. When the Horner’s syndrome has been present for at least 2 weeks, denervation hypersensitivity will occur secondary to the loss of sympathetic innervation. A single drop of a very dilute concentration of a direct-acting sympathomimetic (0.1% phenylephrine: stock 10% solution diluted 1:100 with saline solution) is applied to both eyes, using the unaffected eye as a control. Because this dilute solution does not normally induce pupillary dilation, the pupil in the normal eye should not dilate. Dilation of the affected pupil will occur within 20 minutes in an animal with a postganglionic (third order Horner’s syndrome) lesion. Although, theoretically, pharmacologic testing should be helpful in localizing the site of neuron injury in animals with Horner’s syndrome, results of pharmacologic testing can be equivocal and may not always contribute practical information regarding the cause or the prognosis.
The diagnostic approach in an animal with Horner’s syndrome should include a complete physical examination and ophthalmologic, neurologic, and otoscopic examinations. Further tests should be recommended after lesion localization depending on neurologic examination findings and pharmacologic testing. Thoracic, spinal, and cervical radiography should be performed and advanced diagnostic imaging (e.g., myelography, CT, MRI) should be considered if a first or second order lesion is suspected. When a postganglionic lesion is suspected, skull radiographs, CT, or MRI should be performed to evaluate the middle ear for signs of otitis media, neoplasia, or trauma. In dogs and cats with Horner’s syndrome, at least 50% have no other neurologic abnormalities and a cause is not identified; these animals are classified as having idiopathic disease. Idiopathic Horner’s syndrome resolves spontaneously within 6 months in most dogs. Idiopathic second order Horner’s syndrome is especially common in Golden Retrievers.
PROTRUSION OF THE THIRD EYELID
In dogs and cats the third eyelid may protrude over the corneal surface in the presence of corneal or conjunctival irritation or space-occupying retroorbital disease. This may also occur if the animal experiences a decrease in periorbital mass as a result of dehydration, a loss of retrobulbar fat or muscle (Fig. 66-9), or a loss of volume within the eye (i.e., microphthalmos, phthisis bulbi).

FIG 66-9 Dramatic muscle atrophy in a dog with masticatory muscle myositis has resulted in retraction of the globes into the orbits and protrusion of the third eyelid over most of the corneal surface.
Protrusion of the third eyelid is a conspicuous feature of Horner’s syndrome (with miosis) and also of dysautonomia (with mydriasis). Systemic illness or tranquilization can also result in third eyelid protrusion in some dogs and cats. A peculiar syndrome (e.g., Haw’s syndrome) has been observed in cats, and occasionally in dogs, in which a dramatic bilateral third eyelid protrusion of no obvious cause is observed. Affected cats are usually younger than 2 years of age and in good health otherwise, although digestive disturbances or heavy intestinal parasite loads have occasionally been documented. The instillation of sympathomimetic drops (phenylephrine 10%) causes the membrane to rapidly retract. The condition resolves spontaneously within several weeks or months.
Boydell P. Idiopathic horner syndrome in the golden retriever. J Neuroophthalmol. 2000;20:288.
Cottrill NB. Differential diagnosis of anisocoria. In: Bonagura JD, editor. Current veterinary therapy XIII small animal practice. Philadelphia: WB Saunders, 2000.
Cullen CL, Grahn BH. Diagnostic ophthalmology. Acute prechiasmal blindness due to sudden acquired retinal degeneration syndrome. Can Vet J. 2002;43:729.
Grahn BH, Cullen CC, Peiffer RL. Neuro-Ophthalmology. In: Grahn BH, Cullen CL, Peiffer RL, editors. Veterinary ophthalmology essentials. Philadelphia: Elsevier, 2004.
Hamilton HL, et al. Diagnosis of blindness. Current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
Mattson A, et al. Clinical features suggesting hyperadrenocorticism associated with sudden acquired retinal degeneration syndrome in a dog. J Am Anim Hosp Assoc. 1992;28:199.
Morgan RV, et al. Horner’s syndrome in dogs and cats: 49 cases (1980–1986). J Am Vet Med Assoc. 1989;194:1096.
Penderis J. Disorders of eyes and vision. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004.
 BOX 66-1
BOX 66-1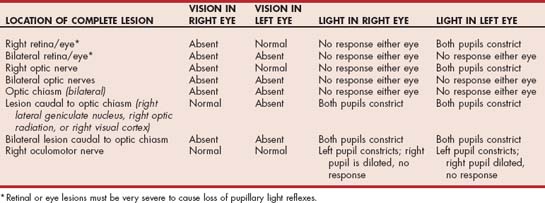

 BOX 66-4
BOX 66-4 TABLE 66-2
TABLE 66-2