CHAPTER 63 Lesion Localization and the Neurologic Examination
FUNCTIONAL ANATOMY OF THE NERVOUS SYSTEM AND LESION LOCALIZATION
The most important step in the diagnostic evaluation of dogs or cats with neurologic signs is establishing an accurate anatomic diagnosis (Box 63-1). A basic understanding of nervous system structure and function is essential to correctly interpret neurologic examination findings and localize lesions to clinically significant regions.
BRAIN
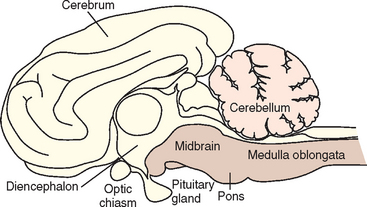
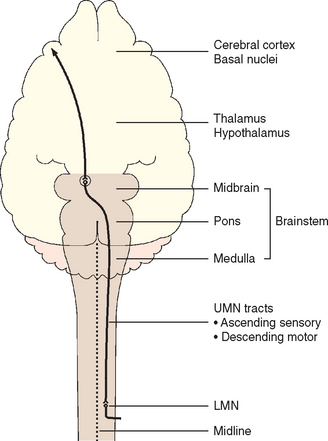
The brain consists of the cerebrum, the brainstem, and the cerebellum. The brainstem is further subdivided from rostral to caudal into the diencephalon (thalamus and hypothalamus), midbrain, pons, and medulla oblongata (Fig. 63-1). Neurologic abnormalities within the brain can usually be localized on the basis of clinical findings to one of three clinically important regions. These include (1) the forebrain (the cerebrum and diencephalon), (2) the pons and medulla, and (3) the cerebellum (Box 63-2).
Forebrain
The forebrain includes the cerebral cortex, cerebral white matter, basal nuclei, and the diencephalon. The cerebral cortex is important for behavior; vision; hearing; fine motor activity; and conscious perception of touch, pain, temperature, and body position (proprioception). The cerebral white matter transmits ascending sensory information and descending motor signals, and the basal nuclei are involved in maintaining muscle tone and the initiation and control of voluntary motor activity. Unilateral forebrain lesions result in a relatively normal gait but postural reaction deficits and increased muscle tone in limbs on the contralateral (opposite) side of the body. The diencephalon is important in the integration of sensory input, maintenance of consciousness and attention, and control of autonomic and endocrine functions such as appetite, thirst, temperature, electrolyte, and water balance. The olfactory nerve, cranial nerve 1 (CN1), projects onto the hypothalamus, and the optic nerve (CN2) and optic chiasm are on the ventral surface of the hypothalamus; therefore lesions in this region can result in loss of the sense of smell or contralateral visual deficits with normal pupillary light reflexes. Neurologic examination findings associated with forebrain lesions are listed in Box 63-3.
Pons and Medulla
The pons and medulla compose the portion of the brainstem that contains the regulatory centers for consciousness (ascending reticular activating system) and normal respiration. This area provides a link between the spinal cord and the cerebral cortex through ascending sensory and descending motor tracts. These tracts cross in the rostral midbrain, such that while unilateral forebrain lesions result in contralateral limb deficits, unilateral lesions of the pons, medulla, or spinal cord cause ipsilateral (same side) deficits. Ten pairs of cranial nerves (3 to 12) originate in this region, with lesions reflecting motor or sensory dysfunction of individual nerves. Because vestibular nuclei are located in the medulla oblongata and the flocculonodular lobe of the cerebellum, lesions at this site commonly result in head tilt, disequilibrium, and nystagmus (see Chapter 68). Box 63-3 lists common neurologic examination abnormalities in patients with lesions of the pons and medulla.
Cerebellum
The cerebellum controls the rate, range, and force of movements. It serves to coordinate muscular activity, regulate fine movement, and modulate muscle tone. Lesions of the cerebellum result in a wide-based stance, ataxia (incoordination) with normal strength, and increased muscle tone (spasticity). The gait is hypermetric or exaggerated, with each limb being raised excessively during protraction and then returned more forcefully than normal to weight bearing. Cerebellar lesions may also result in a fine tremor of the head that becomes more pronounced during voluntary movement such as reaching for food (intention tremor). Severe lesions of the rostral cerebellum result in opisthotonus with rigid extension of the forelimbs (decerebellate posture) (see the discussion of posture, p. 989). Box 63-3 lists the clinical signs caused by lesions of the cerebellum. Cerebellar disorders are discussed in Chapter 65.
SPINAL CORD
The spinal cord resides entirely within the bony vertebral column. It is composed of a central H-shaped core of gray matter surrounded by white matter. Spinal cord gray matter contains the cell bodies of interneurons and lower motor neurons (LMNs). White matter is composed of nerve fibers organized into columns of ascending and descending tracts. These long tracts transmit ascending sensory information (proprioception, touch, temperature, pressure, and pain) and descending motor signals between higher centers in the brain and spinal cord neurons.
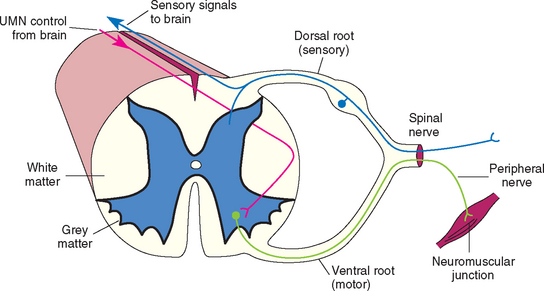
The spinal cord can be functionally divided into segments, with each spinal cord segment giving rise to one pair of spinal nerves (left and right), each of which has a dorsal (sensory) and ventral (motor) root (Fig. 63-2). The cell bodies for the LMNs supplying the thoracic limbs are in the ventral gray matter within a thickened region of the cord called the cervical intumescence (segments C6-T2), whereas the LMNs supplying the pelvic limbs originate in the lumbar intumescence (segments L4-S3; Fig. 63-3). After a neurologic examination, each limb should be characterized as normal or as having upper motor neuron (UMN) or LMN signs. This will allow localization of spinal cord lesions to one of four functional anatomical regions: spinal cord segments C1-C5, C6-T2, T3-L3, or L4-S3 (Box 63-4). Because the ascending and descending tracts to the rear limbs are located peripherally in the cord, it is common for dogs and cats with compressive lesions of the cervical (C1-C5) cord to have more pronounced UMN deficits in the rear limbs than in the forelimbs. Also, lesions that affect only the center of the cord (central cord syndrome) in the cranial cervical (C1-C5) or caudal cervical (C6-T2) region will often produce profound UMN (C1-C5) or LMN (C6-T2) deficits in the forelimbs with minimal UMN deficits in the rear limbs.
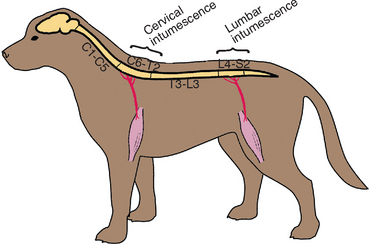
FIG 63-3 Spinal cord segments at the cervical intumescence (C6-T2) and the lumbar intumescence (L4-S3) give rise to the important peripheral nerves of the limbs.
 BOX 63-4 Localization of Spinal Cord Disease
BOX 63-4 Localization of Spinal Cord Disease
UMN, Upper motor neuron; LMN, lower motor neuron.
Lower Motor Neuron signs
The LMN is the efferent neuron that directly connects the central nervous system (CNS) to a muscle or gland (Fig. 63-4). Components of spinal LMNs include the nerve cell bodies within the ventral gray matter, the axons leaving the spinal canal as ventral nerve roots and spinal nerves, and the peripheral nerves formed by the spinal nerves that terminate at the neuromuscular junction in the muscle to produce contraction (see Fig 63-2). Damage to any component of the LMN will result in the appearance of LMN signs in the muscles normally innervated by that particular LMN. LMN signs include flaccid paresis (weakness) or paralysis (loss of motor function), decreased or absent muscle tone, rapid muscle atrophy, and decreased or absent spinal reflexes (Table 63-1). When there is damage to the sensory component of the LMN (the peripheral nerve, spinal nerve, or dorsal nerve root), there may also be a loss of sensation in the skin and limb directly supplied by that LMN. Spinal cord lesions causing focal LMN signs are discussed in Chapter 70. Disorders affecting peripheral nerves and disorders causing diffuse LMN paralysis are discussed in Chapter 71.
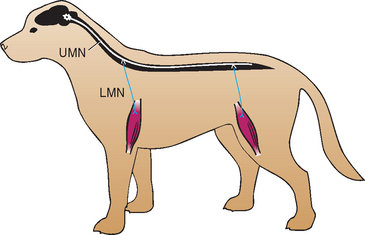
FIG 63-4 The upper motor neuron (UMN) and lower motor neuron (LMN) systems are responsible for mediating normal motor function.
 TABLE 63-1 Summary of Upper Motor Neuron and Lower Motor Neuron Signs
TABLE 63-1 Summary of Upper Motor Neuron and Lower Motor Neuron Signs
| CHARACTERISTIC | UPPER MOTOR NEURON | LOWER MOTOR NEURON |
|---|---|---|
| Motor function | Spastic paresis to paralysis in all limbs caudal to lesion | Flaccid paresis or paralysis at site of lesion |
| Postural reactions (knuckling) | Often delayed | Normal unless severe lesion |
| Gait | Wide-based stance, ataxic, long strides, delayed limb protraction | Short strides, limbs maintained under center of gravity |
| Muscle tone | Normal or increased | Decreased |
| Muscle atrophy | Late and mild—disuse | Rapid and severe—neurogenic |
| Spinal reflexes | Normal or increased | Decreased or absent |
Upper Motor Neuron Signs
Those motor systems originating in the brain to control the LMN are UMNs (see Fig. 63-4). UMNs are responsible for initiating and maintaining normal movement, regulating the muscle tone used to support the body against gravity, and inhibiting myotactic reflexes. Components of the UMN include nerve cell bodies in the cerebral cortex, basal nuclei, and brainstem as well as the motor tracts in the brainstem and spinal cord white matter, which relay information from the higher centers to the LMN. These pathways cross the midline in the rostral brainstem so that forebrain lesions result in contralateral deficits in the limbs, whereas UMN lesions of the spinal cord, pons, or medulla oblongata result in ipsilateral deficits in the limbs (Fig. 63-5). Damage to the UMN nuclei or tracts will cause loss of voluntary motor function and a release of the inhibitory effect of UMNs on all LMNs caudal to the level of injury. The resultant UMN signs in all muscles caudal to the site of the lesion include spastic paresis or paralysis, increased extensor muscle tone, and normal to increased spinal reflexes (see Table 63-1). Associated sensory signs such as ataxia and decreased sensation in the skin and limbs caudal to the lesion reflect inter ruption of the UMN sensory tracts responsible for mediating proprioception (position sense) and pain perception.
Spinal Cord Sensory Pathways
Sensory nerves that detect touch, temperature, and pain are distributed to the surface of the body and limbs. There are also sensory nerves responsible for proprioception that originate in the skin, muscles, tendons, and joints. The nerve cell bodies of most of these sensory nerves are located in the ganglia of dorsal nerve roots entering the spinal cord (see Fig. 63-2). Sensory tracts responsible for mediating sensation and conscious and unconscious proprioception ascend the spinal cord and brainstem to the brain. Most of these tracts ascend the ipsilateral spinal cord and cross over in the rostral brainstem to reach the contralateral cerebrum (see Fig 63-5). Patients with a unilateral forebrain lesion will typically experience hypalgesia (decreased sensation) in the limbs, trunk, and face on the opposite side. Damage to the sensory tracts in the spinal cord will disrupt the transmission of sensory and proprioceptive information to the brain (UMN), resulting in signs of ataxia, or incoordination, and the loss of conscious proprioception in all limbs caudal to the site of the lesion. With unilateral spinal cord lesions the deficits will be ipsilateral. If UMN spinal cord lesions are severe, there may also be some loss of superficial skin sensation caudal to the lesion. In addition to the sensory tracts responsible for relaying information to UMN centers regarding superficial sensation and proprioception, there are multisynaptic, small-diameter, bilateral crossing tracts deep in the white matter of the spinal cord that project to the cerebral cortex and are involved in the conscious perception of noxious stimuli (nociception, deep pain). The small diameter and deep location of these tracts make them very resistant to compressive injury, so loss of the ability to perceive a noxious stimulus (loss of deep pain perception) in the rear limbs of an animal with a T3-L3 lesion usually indicates a very severe transverse spinal cord injury.
Loss of sensation caused by damage to spinal cord dorsal gray matter, dorsal nerve roots, or the sensory portion of a peripheral nerve allows the LMN lesion to be precisely localized on the basis of skin sensation mapping. When there is a compressive or irritative lesion of the nerve root or peripheral nerve, there will sometimes be hyperesthesia (pain) at the site.
NEUROMUSCULAR SYSTEM
Peripheral Nerves
The peripheral nervous system consists of 12 pairs of cranial nerves originating in the brainstem and 36 pairs of spinal nerves originating in the spinal cord. Nerve fibers from the spinal nerves in the cervical and lumbar intumescences join together to form the peripheral nerves that innervate the muscles of the limbs. Spinal nerve or peripheral nerve lesions result in LMN motor signs in affected muscles and limbs and sometimes decreased, absent, or altered sensation. Box 63-5 lists the clinical signs caused by peripheral nerve lesions. Peripheral nerve disorders are discussed in Chapter 71.
Neuromuscular Junction
At the neuromuscular junction (NMJ) electrical activity is transmitted from nerve axons to muscle fibers, resulting in muscular contraction. This process is mediated through the calcium-dependent release of the neurotransmitter acetylcholine (ACh) from the nerve terminal into the synaptic cleft. ACh diffuses across the synaptic cleft and binds to ACh receptors on the postsynaptic (muscle) membrane, inducing a conformational change and ion flux that result in muscular contraction. ACh is then rapidly removed from the synapse by acetylcholinesterase, readying the synapse for the next nerve impulse. Disorders that interfere with ACh release or inactivation and disorders that alter postsynaptic cholinergic receptor function will adversely affect neuromuscular transmission. Presynaptic neuromuscular junction disorders causing decreased release of ACh result in flaccid tetraparesis and decreased spinal reflexes (see Box 63-5) similar to diffuse peripheral nerve disorders. Myasthenia gravis is a postsynaptic disorder with reduction in the number of functional ACh receptors. The result is partial failure of NMJ transmission and exercise-induced weakness that may improve with rest but normal muscle tone and spinal reflexes. Disorders that interfere with acetylcholinesterase, the enzyme that normally inactivates ACh in the synapse, cause autonomic nervous system overstimulation and neuromuscular weakness. Myasthenia gravis and other disorders of neuromuscular transmission are discussed in Chapter 71.
Muscle
Skeletal muscle functions to maintain body posture and produce movement. Generalized weakness (tetraparesis), a stiff and stilted gait, and exercise intolerance are common clinical features in patients with muscle disease (see Box 63-5). Postural reactions and reflexes are normal. Some disorders cause muscle pain and muscle swelling, whereas others cause muscle atrophy and/or fibrosis. Muscle disorders are discussed in Chapter 72.
NEUROLOGIC CONTROL OF MICTURITION
The physiologic control of micturition is complex and integrated centrally. The pelvic nerves originate in sacral segments S1-S3 and supply parasympathetic innervation to the bladder. Stimulation causes detrussor muscle contraction, bladder contraction, and bladder emptying. The striated skeletal muscle of the external urethral sphincter is under conscious and reflex control and is innervated by the pudendal nerve arising from sacral segments S1-S3. Sympathetic innervation to the bladder is supplied through the hypogastric nerves arising in the lumbar segments (L1-L4 in dogs, L2-L5 in cats). Sympathetic stimulation causes detrusor muscle relaxation (β-adrenergic) and contraction of the internal urethral sphincter (α-adrenergic). Sympathetic tone dominates during urine storage, allowing the bladder to distend with urine. As the bladder enlarges, sensory information from bladder wall stretch receptors is transmitted via the sensory portion of the pelvic nerve through ascending spinal cord pathways to the thalamus and cerebral cortex. When it is appropriate to void, impulses are sent from the cerebral cortex to the pons and then down the reticulospinal tract to the sacral spinal cord segments. Parasympathetic stimulation results in detrusor muscle contraction. There is normally simultaneous inhibition of sympathetic tone in the internal urethral sphincter and somatic (pudendal) input to the external urethral sphincter, allowing urine to flow. Damage to any component of this complex system or the connection with UMN centers will result in disorders of micturition.
Sacral spinal cord, nerve root, or pudendal nerve lesions typically result in urinary incontinence and a large bladder that is easily expressed and leaks continuously (LMN bladder). Perineal and bulbocavernosus reflexes are decreased or absent. Mild or moderate UMN lesions (spinal cord above the sacral segments) cause increased urethral tone, making it difficult for patients to void completely. With relatively mild lesions a syndrome of detrussor-urethral dyssynergia may result, wherein involuntary contraction of the urethral sphincter occurs during detrussor contraction, halting urine flow during voiding. Severe UMN spinal cord lesions causing severe paresis or paralysis typically result in a bladder that is enlarged and very difficult or impossible to express manually (UMN bladder). Occasionally, a reflex or automatic bladder will develop 5 to 10 days after acute UMN spinal cord injury, resulting in reflex detrussor contraction and spontaneous partial emptying of the bladder without cortical perception or voluntary control.
SCREENING NEUROLOGIC EXAMINATION
A screening neurologic examination takes only a few minutes (Box 63-6). Abnormalities of mentation, posture, and gait are initially evaluated. Postural reactions are then evaluated. If abnormalities are detected, evaluation of muscle tone, spinal reflexes, urinary tract function, and sensory perception aids in lesion localization. Finally, cranial nerves are evaluated, and if necessary, localization of a lesion within the brain is attempted.
MENTAL STATE
Owners should always be asked if they have noticed any changes in their pet’s behavior because subtle changes are often not apparent to the examiner. A decreased level of consciousness, such as depression, stupor, or coma (Table 63-2), may occur with a metabolic disturbance or damage or disease affecting the cerebrum or brainstem. Delirium, confusion, or agitation suggests either cerebral cortical disease or a metabolic encephalopathy. Seizures occur with forebrain lesions or functional disturbances secondary to metabolic encephalopathies or intoxications. Aggression, compulsive pacing, loss of housebreaking, vocalizing, and head pressing can all be seen with a forebrain lesion. A behavioral syndrome in which animals with a structural unilateral forebrain lesion ignore all sensory input from the contralateral half of their environment has been called hemi-inattention syndrome.
 TABLE 63-2 Disorders of Consciousness
TABLE 63-2 Disorders of Consciousness
| STATE | CHARACTERISTIC |
|---|---|
| Normal | Alert; responds appropriately to environmental stimuli |
| Depressed | Quiet or drowsy, responds to environmental stimuli; obtunded |
| Delirious | Alert; responds inappropriately to stimuli; agitated or confused |
| Stuporous | Unconscious, except when aroused by strong (often painful) stimuli |
| Comatose | A state of deep unconsciousness from which the animal cannot be aroused, even with noxious stimuli |
POSTURE
A normal upright posture is maintained through the integration of multiple CNS pathways and spinal reflexes. Abnormal postures reflect a disruption of this normal integration. A wide-based stance is common in ataxic animals, particularly those with cerebellar or vestibular disease (Fig. 63-6). A continuous head tilt with resistance to straightening is usually associated with an abnormality of the vestibular system (Fig. 63-7). In recumbent animals posture and other neurologic findings aid in lesion localization.
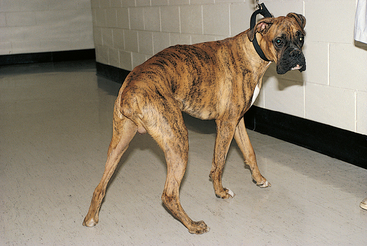
FIG 63-6 Wide-based stance and excessive limb abduction indicative of ataxia in a 2-year-old Boxer with Neospora caninum meningoencephalomyelitis affecting the cervical spinal cord and cerebellum.

FIG 63-7 Right-sided head tilt in an adult cat with right-sided peripheral vestibular disease caused by otitis media/interna.
Schiff-Sherrington Posture
The Schiff-Sherrington posture is observed in dogs when an acute, severe thoracic or cranial lumbar spinal cord lesion (usually a fracture/luxation, infarction, or hemorrhage) interferes with the normal ascending inhibition of thoracic limb extensor motor neurons by border cells in the spinal cord from L1 to L7 (most from L2 to L4). Forelimbs exhibit increased extensor tone with normal voluntary motion, strength, and conscious proprioception (Fig. 63-8). The rear limbs are paralyzed with normal to increased reflexes (UMN). This posture suggests severe spinal cord damage but does not have prognostic significance.

FIG 63-8 Schiff-Sherrington posture in a 9-year-old Lhaso Apso caused by traumatic fracture and luxation of the spine at T11-T12, with damage to the spinal cord at that site. There was a loss of proprioception, loss of voluntary motion, and loss of deep pain in the rear limbs, with increased reflexes. The forelimbs were neurologically normal except for increased extensor tone.
Decerebrate Rigidity
This posture is most commonly observed when there is a rostral brainstem (midbrain) lesion. Affected animals are stuporous or comatose, all limbs are rigidly extended, and there is dorsal extension of the head and neck (opisthotonus; Fig. 63-9A).
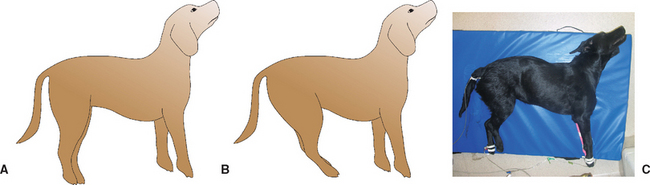
Decerebellate Rigidity
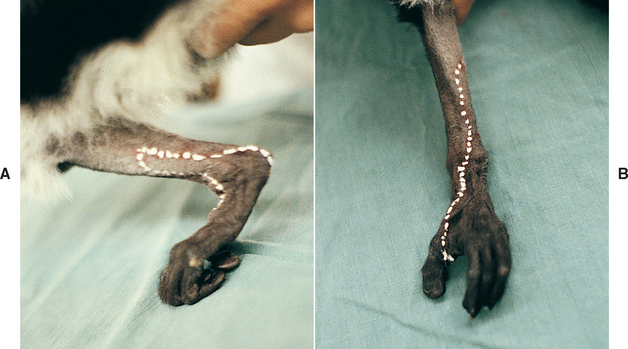
The rostral portion of the cerebellum is responsible for inhibition of excessive extensor muscle tone. A lesion in this region will result in increased thoracic limb extensor muscle tone, opisthotonus, and normal mentation. Rear limbs typically have the hips flexed forward as a result of increased iliopsoas muscle tone. This posturing can be episodic (see Fig. 63-9B and C).
GAIT
Clinical evaluation of gait involves observation of the animal’s movements during walking on a flat, nonslippery surface, with frequent turns and circling. If the animal is unable to walk unassisted, it should be supported with a harness or sling so that voluntary movement and gait can be better assessed. Each patient must be evaluated for paresis (weakness), ataxia, lameness, and circling.
Paresis
Paresis is defined as weakness or inability to support weight or generate a normal gait. Paralysis is the term used to describe paresis so severe that all voluntary movement is lost (Table 63-3). Paresis occurs with LMN or UMN lesions, but the gait that results is markedly different between the two. Animals with LMN disease are usually profoundly weak, and they take small steps, always maintaining their feet under their center of gravity. Their short-strided gait is commonly mistaken for an orthopedic lameness, and they may tremble or collapse with minor exertion. Attempts to move quickly may result in a bunny-hopping gait. In contrast, animals with UMN lesions have a delay in the onset of protraction (the swing phase) and a longer than normal stride with a variable degree of spasticity or stiffness of the limbs. Animals with UMN lesions are ataxic as a result of disruption of the general proprioceptive (sensory) tracts that accompany the UMN tracts.
 TABLE 63-3 Localizing Lesions Causing Paresis and Paralysis
TABLE 63-3 Localizing Lesions Causing Paresis and Paralysis
| Tetraparesis/Tetraplegia: Paresis or Paralysis of All Four Limbs | |
| Normal conscious proprioception and spinal reflexes | |
| LMN fore and rear | Generalized disorders of spinal cord ventral gray matter, ventral nerve roots, peripheral nerves or neuromuscular junction |
| LMN forelimbs, UMN rear limbs | C6-T2 spinal cord |
| UMN forelimbs, UMN rear limbs | C1-C5 or brainstem |
| Paraparesis/Paraplegia: Paresis or Paralysis of Rear Limbs | |
| Normal forelimbs, LMN rear limbs | L4-S3 spinal cord |
| Normal forelimbs, UMN rear limbs | T3-L3 spinal cord |
| Monoparesis/Monoplegia: Paresis or Paralysis of One Limb | |
| LMN | Lesion of the LMN directly innervating the affected limb (motor neuron cell body in ventral spinal cord gray matter, ventral nerve roots, spinal nerves, peripheral nerves) |
| Rear limb UMN | Ipsilateral T3-L3 spinal cord |
| Hemiparesis/Hemiplegia: Paresis or Paralysis of Both Limbs on One Side | |
| LMN fore, UMN rear | C6-T2 ipsilateral spinal cord |
| UMN fore, UMN rear | C1-C5 ipsilateral spinal cord; ipsilateral brainstem; contralateral forebrain lesion |
LMN, Lower motor neuron; UMN, upper motor neuron.
Ataxia
Ataxia, or incoordination, is caused by lesions of the cerebellum, vestibular system, or the general proprioceptive (GP) sensory tracts in the spinal cord and caudal brainstem (Box 63-7). Animals with GP ataxia lose awareness of where their limbs are in space. They have a wide-based stance, long strides, excessive abduction of limbs during turning, exaggerated limb movements, and a tendency to scuff or knuckle affected limbs while walking. When affected animals are walking, their limbs may cross and the weight-bearing phase may be prolonged because of delayed protraction of affected limbs. Vestibular ataxia is manifested primarily as a loss of balance, reflected in a head tilt and a wide-based, crouched stance with a tendency to lean, drift, fall, or roll to the side. Vestibular ataxia is often accompanied by an abnormal nystagmus (see Chapter 68). Cerebellar ataxia reflects an inability to control the rate, range, and force of movement. Affected animals will have a wide-based stance, swaying of the body from side to side (truncal ataxia), and exaggerated (hypermetric) limb movements with normal strength and increased muscle tone (Fig. 63-10). A fine head tremor may be present (see Chapter 65).
Lameness
Animals are lame when normal movement causes discomfort. If all limbs are equally painful, they may develop a stiff, short-strided gait, as seen in animals with polyarthritis. Animals with lameness affecting one limb have a short weight-bearing phase in the affected limb and a longer than normal weight-bearing phase in the contralateral limb. In some cases the painful limb will be elevated or carried. Lameness affecting one limb is common in animals with orthopedic disease but can also be a prominent feature in animals with entrapment (pinching) of a spinal nerve or nerve root by a lateralized disk extrusion or nerve root tumor.
Circling
Circling can be caused by lesions of the forebrain or the vestibular system. Dogs with unilateral forebrain lesions will usually walk or pace in wide circles toward the side of the lesion. Tight circling toward the side of the lesion is more often associated with vestibular disorders (Fig. 63-11). Most animals with vestibular disease also exhibit head tilt and nystagmus.
POSTURAL REACTIONS
The complex series of responses that maintain an animal in an upright position are called postural reactions. Postural reaction testing is used to determine whether animals can recognize the position of their limbs in space (conscious proprioception). Sensory receptors for proprioception originate in the muscles, tendons, and joints, and spinal cord proprioceptive tracts relay this sensory information to the cerebral cortex. Most proprioceptive tracts ascend the ipsilateral spinal cord and cross midline in the rostral brainstem (see Fig. 63-5). Abnormalities detected during the manipulations performed to test postural reactions do not provide precise localizing information but are sensitive indicators that suggest the presence of neurologic dysfunction somewhere along the neurologic pathway. A careful and systematic evaluation of postural reactions may permit the examiner to detect subtle deficits not observed during routine gait examination and to determine whether each limb is neurologically normal or abnormal. Postural reaction testing should include knuckling, hopping, wheelbarrowing, and hemiwalking (Fig. 63-12). When performed by an experienced clinician comparing the right and left limbs in an animal that has voluntary movement, hopping is the most sensitive and reliable postural reaction test. In animals with significant weakness it is important to support most of the body weight during postural reaction testing. Animals with neuromuscular disorders that still have normal sensation and the ability to voluntarily move their limbs will hop quickly (normal) as long as their weight is supported because their proprioception is normal. For the purpose of lesion localization, abnormalities of postural reaction testing are usually interpreted as UMN signs, which must then be confirmed with testing of muscle tone and spinal reflexes (see Box 63-4 and Table 63-1).
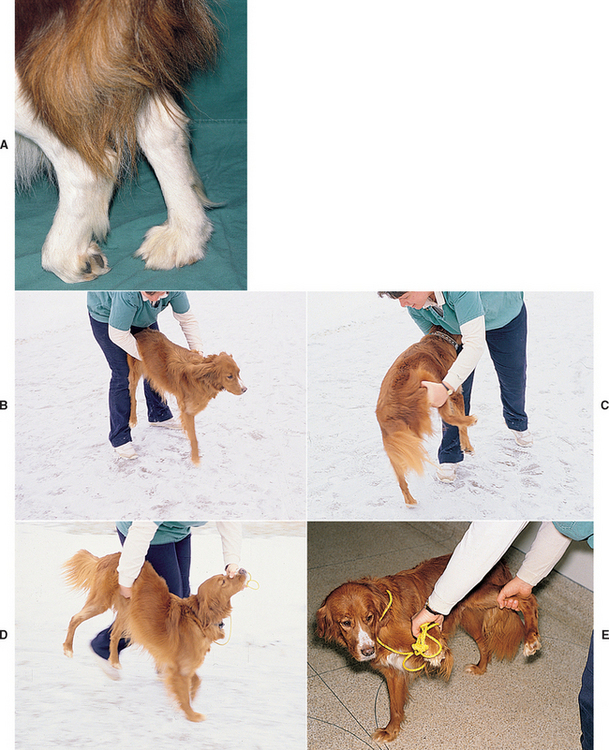
FIG 63-12 Postural reaction testing. A, Conscious proprioception (knuckling) is evaluated by placing the dorsal surface of the animal’s paw on the floor while the animal’s weight is supported. The normal response is an immediate return to a normal position. B, Forelimb hopping. The animal is supported under the abdomen, and one thoracic limb is lifted from the ground. The animal is leaned and moved laterally toward the limb being evaluated. The normal animal responds by quickly lifting and replacing the limb under its body as it moves laterally. C, Pelvic limb hopping. The animal is supported under the chest, and one pelvic limb is lifted. The animal is leaned and moved laterally toward the limb being evaluated. The normal animal responds by quickly lifting and replacing its limb under the body as it moves laterally. D, Wheelbarrowing. The animal is supported under the abdomen and moved forward. The head may be elevated to remove visual input and accentuate proprioceptive abnormalities, as shown here. E, Hemiwalking. The front and rear limbs on one side are lifted, and forward and lateral walking movements are evaluated.
MUSCLE SIZE/TONE
Muscle atrophy and muscle tone should be assessed by careful palpation and movement of each limb through a range of motion. Muscle atrophy can occur slowly as a result of disuse or rapidly as a result of a lesion of the LMN supplying a muscle (neurogenic atrophy). If focal muscle atrophy is detected in a limb, this can be used to precisely localize lesions of the peripheral nerve, nerve roots, or spinal cord gray matter because the spinal cord segments and peripheral nerves responsible for innervating each of the individual limb muscles are well known. Muscle swelling or enlargement is a feature of some myopathies. Muscle tone is generally decreased in animals with significant lesions of the LMN, whereas extensor muscle tone is usually increased with UMN lesions (see Table 63-1). Extreme alterations in muscle tone can be seen in animals with Schiff Sherrington syndrome and with decerebrate and decerebellar rigidity (see Figs. 63-8 and 63-9).
SPINAL REFLEXES
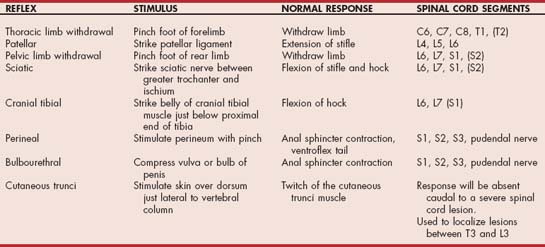
Spinal reflex evaluation is the most reliable way to classify a neurological disorder as being UMN or LMN. Spinal reflexes and muscle tone will be diminished to absent in LMN disorders and normal to increased in UMN disease. Spinal limb reflexes are best assessed in a relaxed animal restrained in lateral recumbency. Each reflex is judged to be absent (0), decreased (+1), normal (+2), or increased (+3 or +4). Significant LMN lesions will reliably cause an absent or decreased reflex. UMN lesions cause an increased reflex that will not always be distinguishable from normal. In the absence of other neurologic deficits an exaggerated reflex means little and can be observed in an excited or nervous animal. The limb reflexes that are most useful in dogs and cats include the patellar reflex, the sciatic reflex, the pelvic limb withdrawal (flexor) reflex, and the thoracic limb withdrawal (flexor) reflex. Because other reflexes are found inconsistently in normal animals, they are not routinely evaluated. The spinal reflexes and the spinal cord segments responsible for mediating each reflex are listed in Table 63-4.
Patellar Reflex
With the animal restrained in lateral recumbency, the examiner evaluates the upper (nonrecumbent) limb by holding the stifle in partial flexion and striking the patellar ligament with the flat surface of the reflex hammer (pleximeter), stretching the fibers of the quadriceps muscle (Fig. 63-13). The normal response is a reflex contraction of the quadriceps muscle. This is a monosynaptic myotactic (stretch) reflex, with both sensory and motor components contained in the femoral nerve and the L4, L5, and L6 spinal nerves; nerve roots; and spinal cord segments. A weak or absent patellar reflex indicates a lesion of the femoral nerve or the L4-6 spinal cord segments or nerve roots. A lesion cranial to the L4 spinal cord segment will typically cause an exaggerated reflex. Although this is the most reliable tendon reflex for evaluation, it is sometimes difficult to interpret the response. Occasionally, a lesion of the sciatic nerve or the L6-S2 spinal cord segments will cause the patellar reflex to appear increased by decreasing tone in the muscles opposing stifle extension (pseudohyperreflexia). The patellar reflex can be difficult to elicit in animals with significant orthopedic disease of the stifle, and rarely it is decreased or absent in normal dogs (especially large-breed puppies). In tense patients the reflex may be decreased or absent in the upper limb but normal in the relaxed recumbent limb.
Pelvic Limb Withdrawal (Flexor) Reflex
The examiner squeezes a digit with enough pressure to elicit flexion of the hip, stifle, hock, and digits (see Fig. 63-14A and B). If manual pressure is not adequate, the examiner squeezes the base of a toenail with a pair of forceps. The pelvic limb withdrawal reflex is complex. Sensory input is through the peroneal (dorsal, lateral) and tibial (ventral) branches of the sciatic nerve and the saphenous branch of the femoral nerve (medial). Motor output is through the sciatic nerve and its branches, the tibial nerve (digital flexion), and the peroneal nerve (tarsal flexion). Because hip flexion is mediated by the femoral nerve and the lumbar spinal nerves, this reflex can occur when the medial toe is stimulated even if the sciatic nerve and its branches have been destroyed. A decreased pelvic limb withdrawal response indicates an LMN lesion affecting the sciatic nerve (or branches) or the L6-S2 spinal cord segments or nerve roots. A lesion cranial to L6 results in a normal to increased reflex response. The withdrawal response is a segmental reflex that is not dependent on the animal’s conscious perception of the noxious stimulus; functional transection of the spinal cord cranial to L6 will result in an increased reflex (UMN) but no ability to feel the stimulus.
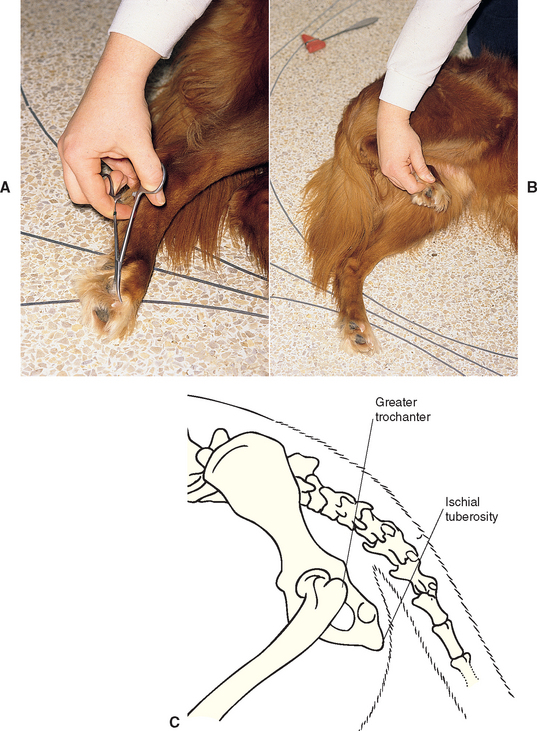
FIG 63-14 Assessing the sciatic nerve and spinal cord segments L6-S2. Pelvic limb withdrawal reflex: Pinch the toe (A), resulting in limb flexion (B). Assess flexion in all of the joints of the limb. It may be necessary to apply a forceps to the nail base to provide adequate stimulation. Sciatic reflex: Strike the sciatic nerve in the notch between the greater trochanter of the femur and the ischial tuberosity, resulting in limb flexion (C).
Sciatic Reflex
With the animal in lateral recumbency, the examiner palpates the notch formed by the greater trochanter of the femur and the ischial tuberosity. Using the tapered end of the pleximeter to tap in this notch, the examiner elicits a brief flexion of the hock (Fig. 63-14C). The sciatic reflex requires that the sciatic nerve, spinal cord segments L6-S2, and the peroneal nerve (branch of the sciatic nerve) be intact. The reflex will be decreased with lesions of those components and normal to increased with UMN lesions cranial to L6.
Thoracic Limb Withdrawal (Flexor) Reflex
The only reliable forelimb reflex is the withdrawal reflex. Because multiple nerves are involved, this reflex is used as a crude test of the entire brachial plexus (nerve roots and peripheral nerves) and cervical intumescence (C6-T2). The examiner squeezes a digit to elicit flexion of the shoulder, elbow, carpus, and digits (Fig. 63-15). Lesions involving the peripheral nerves, nerve roots, or spinal cord segments at that site will result in a decreased or absent reflex. Lesions above C6 in the spinal cord will cause a normal to increased (UMN) reflex response.
Crossed Extensor Reflex
When the withdrawal (flexor) reflexes are elicited in an animal in lateral recumbency, a reflex extension of the limb opposite the one being stimulated is termed a crossed extensor reflex. The presence of this reflex in an animal that is not trying to rise or get away indicates that there is a UMN lesion to the limb being evaluated.
Perineal Reflex and Bulbourethral Reflex
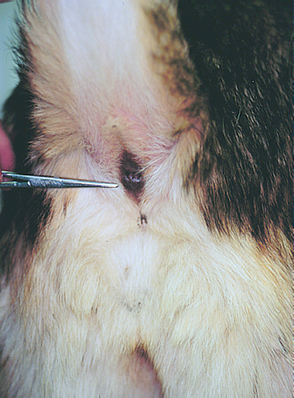
The perineal and bulbocavernosus reflexes are used to assess the pudendal nerve (sensory and motor) and sacral spinal cord segments S1-S3. In the perineal reflex the perineal skin is pinched with a hemostat, causing the anal sphincter to contract and the tail to ventroflex (Fig. 63-16). The same response should occur during digital rectal examination. The bulbourethral reflex causes anal sphincter contraction in response to gently squeezing the bulb of the penis or the vulva. LMN damage to the pudendal nerve or the S1-S3 spinal cord segments will cause a loss of both of these reflexes, urinary incontinence (LMN bladder), loss of tone in the internal and external anal sphincters, and resultant anal dilation and fecal incontinence.
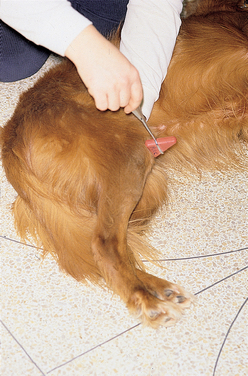
Cutaneous Trunci Reflex (Panniculus)
Pinching the skin of the dorsum causes a reflex contraction of the cutaneous trunci muscles bilaterally, producing a twitch of the overlying skin. This reflex can be very useful in the evaluation of patients with a severe spinal cord lesion localized to the T3-L3 region. Affected patients will have UMN signs in the rear limbs and normal forelimbs, but unless they have a painful site, it can be difficult to localize the lesion more precisely. When skin along the dorsum is pinched, the stimulated sensory nerve from that site enters the spinal cord and afferent sensory information ascends the spinal cord in sensory tracts. If the spinal cord is intact between the site of stimulation and the C8-T1 segments, a synapse occurs bilaterally at the C8-T1 spinal cord segments, stimulating motor neurons of the lateral thoracic nerve, which causes the cutaneous trunci muscle to contract. In spinal cord lesions causing paralysis the ascending pathway will be disrupted such that no panniculus reflex is elicited when the skin is pinched caudal to the level of the lesion, but stimulation of the skin cranial to the lesion elicits a response (Fig. 63-17). Testing is started at the level of the ilial wings, although in many normal animals the reflex cannot be elicited until stimulation is applied at the midlumbar region. If a twitch occurs at the most caudal aspect, then the entire pathway is intact. If there is no response, then systematic stimulation of the skin just lateral to each vertebral body should be performed, progressing anteriorly until a twitch is observed. Because the sensory nerves that supply the skin enter the spinal cord one or two vertebrae cranial to the dermatome stimulated, the cord lesion is predictably slightly cranial to the site where the panniculus reflex is lost. The cutaneous trunci reflex can be lost unilaterally when there is a lesion of the ipsilateral brachial plexus or C8-T1 spinal cord segments, ventral nerve roots, or spinal nerves. In rare cases this reflex cannot be elicited in a normal dog.
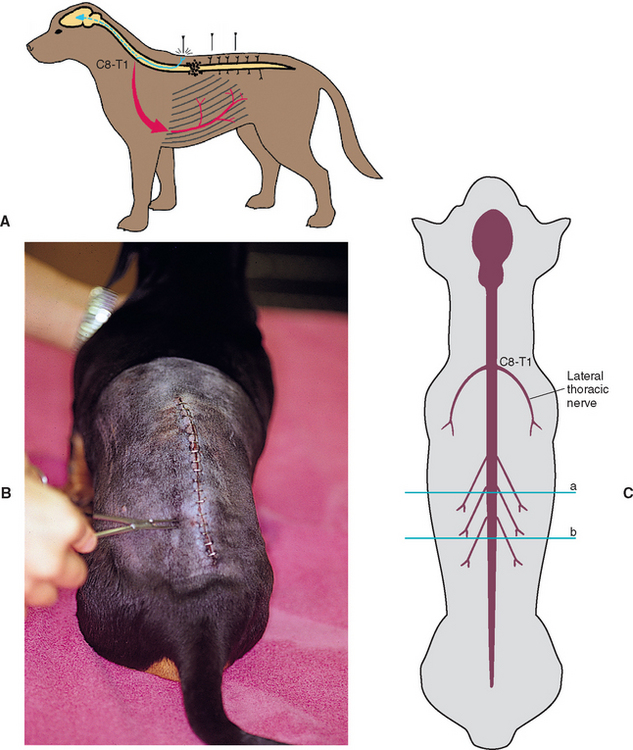
FIG 63-17 Cutaneous trunci reflex. A and B, Pinch the dorsal skin with a hemostat just lateral to the spine. If the spinal cord is not injured between the site of stimulation and the C8-T1 spinal cord segments, this will lead to a bilateral twitch of the cutaneous trunci muscle. The reflex may be absent caudal to a severe spinal cord lesion. C, The spinal sensory nerves course caudally so that the dermatomes for skin sensation lateral to the vertebral column are caudal to their own vertebral bodies. A spinal cord lesion at site a will therefore result in loss of the panniculus response caudal to site b.
SENSORY EVALUATION
Evaluation of an animal’s ability to feel a noxious stimulus such as a pinch (nociception) can be helpful in the localization of UMN and LMN lesions. When there is a transverse UMN spinal cord lesion, the animal’s ability to feel a painful stimulus (skin or pinch with fingers or hemostat) may be decreased in the skin of the trunk and in the limbs caudal to the lesion because the ascending sensory tracts are disrupted in the damaged spinal cord. If minor stimulation (superficial pain assessment) in a paralyzed animal does not elicit a behavioral response such as turning the head, vocalizing, or trying to bite, then the animal’s ability to perceive a more severe noxious stimulus such as a hemostat applied to the nail base (deep pain) should be tested. The spinal tracts that carry deep pain sensation are small, bilateral, and multisynaptic and located deep in the spinal cord white matter. Only a very severe bilateral spinal cord lesion will completely disrupt these tracts, making the ability to perceive deep pain an important prognostic indicator in animals with severe spinal cord injury (Fig. 63-18). It is important to remember that withdrawal of the limb indicates only an intact reflex arc (peripheral nerve and spinal cord segments), whereas a behavioral response requires that the sensory spinal cord tracts ascending to the brain also be intact.
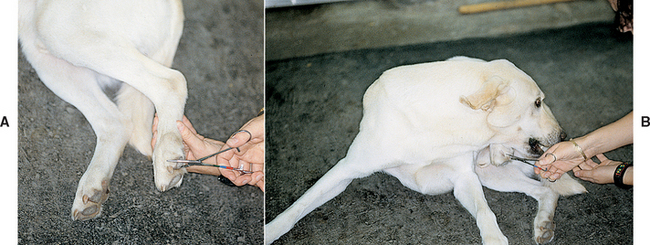
FIG 63-18 Evaluation of deep pain. Pinch the toe (A) to assess whether this elicits a behavioral response (B). The absence of deep pain sensation indicates the presence of severe spinal cord damage.
When LMN paralysis of a limb is evident, mapping the boundaries of normal and diminished sensation can aid in lesion localization to specific peripheral nerves, dorsal nerve roots, or spinal cord segments. The skin should be pinched with a hemostat and regions of local anesthesia or decreased sensation identified (Fig. 63-19). These results can be compared with established maps of cutaneous regions deriving sensory innervation from individual nerves (dermatomes), allowing the LMN neurologic defect to be precisely localized (see Chapter 73).
PAIN/HYPERPATHIA
The neck, spine, limbs, muscles, bones, and joints should be palpated and manipulated to detect painful areas or restricted mobility. Pain is usually most intense directly over a lesion, making this part of the neurologic examination important in lesion localization. Traumatic and inflammatory conditions are most likely to be painful, whereas degenerative and congenital conditions are rarely painful. Neoplastic condi tions causing distortion of tissues (meninges, nerve roots, or bone) will also be painful.
The animal’s posture and gait should be observed. Animals with neck pain maintain a low head carriage, with their head and neck extended, and are unwilling to turn their neck to look to the side; they will instead pivot their entire body. Animals with pain of the thoracic or lumbar spine stand with an arched back (Fig. 63-20). Animals with painful bones, joints, or muscles typically have a short-strided, stiff gait and are reluctant to exercise.
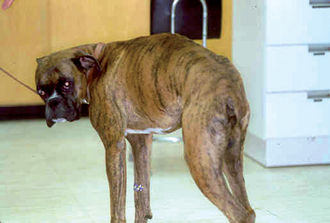
FIG 63-20 This 1-year-old Boxer stands with an arched back because of pain associated with diskospondylitis.
Neck pain is a sign commonly associated with compressive or inflammatory diseases of the cervical spinal cord, cervical spinal roots, or meninges. The neck should be gently manipulated in dorsal, lateral, and ventral flexion and resistance to movement or pain assessed. Deep palpation of the vertebrae and cervical spinal epaxial muscles may also be performed (Fig. 63-21). Anatomic structures that can cause neck pain include the meninges, nerve roots, intervertebral disks, facetal joints, bones, and muscles (Box 63-8). Neck pain has also been recognized as a clinical symptom of intracranial disease, particularly of forebrain mass lesions.
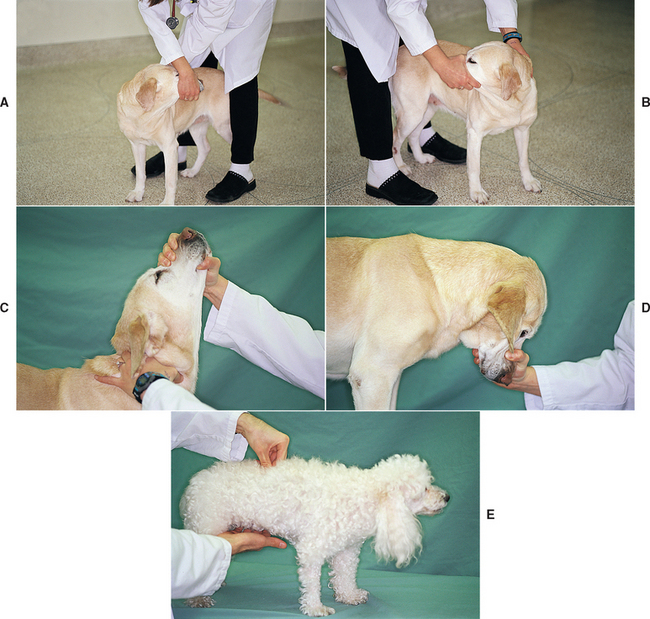
FIG 63-21 Testing for cervical and thoracolumbar spinal pain by (A to D) manipulating the neck through a full range of motion and (E) applying pressure through deep palpation of the vertebral bodies and spinal epaxial muscles.
Pain in other regions of the vertebral column may help localize lesions caused by intervertebral disk disease, diskospondylitis, or neoplasia. Dogs and cats with disease of the thoracolumbar spine may experience pain when pressure is applied over the affected vertebrae. Because these animals may also resist abdominal palpation, vertebral or spinal hyperpathia may be misinterpreted as abdominal pain. Cauda equina compression that is caused by a tumor, disk, or ligamentous proliferation typically causes pain in the lumbosacral region (see Chapter 70). This can be demonstrated in affected dogs by applying direct pressure over the lumbosa cral junction or applying dorsal traction to the tail (see Fig. 70-20).
Muscular pain should be assessed by manipulating the limbs and palpating individual muscle groups. During palpation it is important to attempt to differentiate pain that originates within the muscle from that caused by bone or joint abnormalities. Muscle disorders that are associated with pain are primarily the inflammatory diseases, such as immune-mediated polymyositis, masticatory myositis, and infectious myositis caused by the protozoal organisms Toxoplasma and Neospora. Ischemic myopathy, as occurs in animals with thrombosis affecting the arterial blood supply to a muscle group, can also result in severe muscular cramping and pain on palpation.
URINARY TRACT FUNCTION
Severe lesions of the spinal cord are often associated with urinary tract dysfunction. Bladder function should be assessed on the basis of the owner’s or the clinician’s observations of micturition, palpation of the bladder, and attempts to express urine. A flaccid, easily expressed bladder with absent or diminished perineal and bulbocavernosus reflexes and reduced anal tone is expected with lesions of the LMN (S1-S3 spinal cord segments, pudendal nerve, pelvic nerve). UMN lesions cranial to the sacral segments cause diminished voluntary control of urination and reflex hyperexcitability of the urethral sphincter. There can be incomplete voiding or detrussor-urethral dyssynergia. Severe UMN lesions will result in a tense distended bladder that is difficult to express.
CRANIAL NERVES
Cranial nerve dysfunction may result from a disorder affecting a single nerve; a diffuse polyneuropathy affecting multiple nerves; or a cluster of abnormalities, as is commonly seen in animals with a disease affecting the middle and inner ear or the brainstem. Animals with brainstem disease causing cranial nerve dysfunction usually have additional signs such as postural reaction deficits, hemiparesis, quadriparesis or altered mentation.
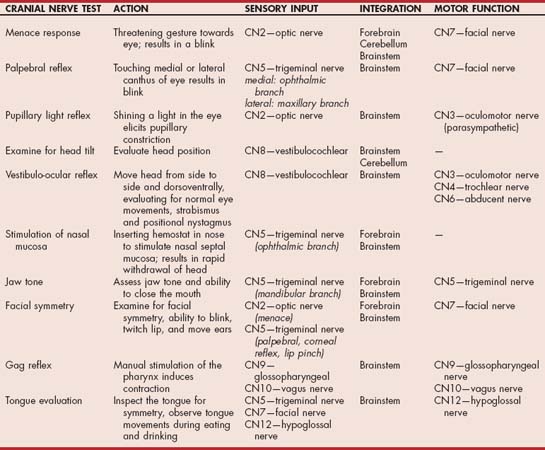
Cranial nerve examination is not difficult. The cranial nerves that are most often affected can be evaluated quickly with a rapid regional neurologic examination (Table 63-5). If findings yielded by the preliminary examination indicate the presence of an abnormality, a more thorough examination of each individual cranial nerve can be undertaken (Table 63-6; see also Suggested Readings).
 TABLE 63-6 Cranial Nerve Function
TABLE 63-6 Cranial Nerve Function
| CRANIAL NERVE | SIGNS OF LOSS OF FUNCTION |
|---|---|
| I (olfactory) | Loss of ability to smell |
| II (optic) | Loss of vision, dilated pupil, loss of pupillary light reflex (direct and consensual when light shone in affected eye) |
| III (oculomotor) | Loss of pupillary light reflex on affected side (even if light shone in opposite eye), dilated pupil, ventrolateral strabismus |
| IV (trochlear) | Slight dorsomedial eye rotation |
| V (trigeminal) | Atrophy of temporalis and masseter muscles, loss of jaw tone and strength, dropped jaw (if bilateral), analgesia of innervated areas (face, eyelids, cornea, nasal mucosa) |
| VI (abducent) | Medial strabismus, impaired lateral gaze, poor retraction of globe |
| VII (facial) | Lip, eyelid, and ear droop; loss of ability to blink; loss of ability to retract lip; possibly decreased tear production |
| VIII (vestibulocochlear) | Ataxia, head tilt, nystagmus, deafness |
| IX (glossopharyngeal) | Loss of gag reflex, dysphagia |
| X (vagus) | Loss of gag reflex, laryngeal paralysis, dysphagia |
| XI (accessory) | Atrophy of trapezius, sternocephalicus, and brachiocephalicus muscles |
| XII (hypoglossal) | Loss of tongue strength |
Evaluation of Menace Response, Vision, and Pupils
The optic nerve (CN1) is an important component of the afferent pathways for the menace response, vision, and the pupillary light reflex. The examiner covers one eye and assesses the menace response in the opposite eye. Next, the examiner advances a hand or finger toward the eye being evaluated, taking care to avoid touching the eyelid or whiskers or generating an air current that will stimulate the cornea, which is innervated by the sensory portion of the trigeminal nerve (CN5). The menace response is a learned response and will not be present until 10 to 12 weeks of age in puppies and kittens. In addition to the menace response, the examiner should observe the animal’s response to its environment by making sudden movements and dropping cotton balls to see if the animal follows the movement. It may be necessary to set up a maze of objects to assess vision in each eye. Pupil size should be examined at rest in a well-lighted room and then in a dimly lit room and the two eyes compared. The examiner evaluates the ability of each pupil to constrict (parasympathetic function) and to dilate (sympathetic function) by shining a bright light in one eye, then swinging the light into the other eye to observe the response, and then swinging it back again. The parasympathetic axons of the oculomotor nerve (CN3) are responsible for pupil constriction. Loss of vision and pupillary abnormalities are discussed in Chapter 66.
Examine for Strabismus, Nystagmus, and Head tilt
To check for strabismus, nystagmus, and head tilt, the examiner must determine whether the eyes are normally positioned in the orbits and whether there is any abnormal resting (spontaneous) nystagmus. Spontaneous nystagmus indicates either a central vestibular (medullary) lesion, a lesion of the vestibular portion of CN8, or a lesion of the cerebellum. A head tilt is common with a lesion in any of these locations. Abnormal eye position (strabismus) may indicate a vestibular disorder or damage to the innervation of the extraocular muscles (CN3, 4, 6) (Fig. 63-22). Oculomotor nerve (CN3) dysfunction can result in a ventrolateral strabismus and an inability to rotate the eye dorsally, ventrally, or medially. Lesions of the abducent (CN6) nerve cause a medial strabismus and an inability to look laterally, and lesions of the trochlear nerve (CN4) cause a dorsolateral rotation of the eye. Lesions of these nerves (CN3, 4, 6) usually occur together, producing complete external ophthalmoplegia. Vestibular disorders commonly cause a ventral strabismus (eye drop) on the side of the lesion that may be evident only during head and neck extension. A quick assessment of the function of all these nerves can be accomplished by moving the head from side to side and eliciting the vestibulo-ocular reflex. As the head is turned slowly to the right, the gaze of both eyes should slowly drift left before jerking to the right to resume a central position. The examiner assesses these normal vestibular eye movements (physiologic nystagmus) while moving the head in each direction.
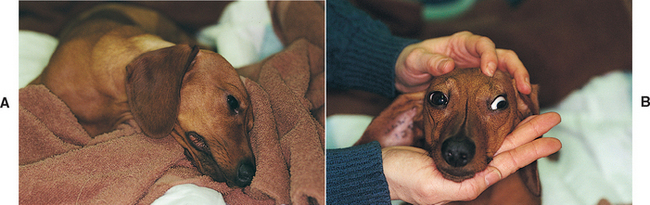
FIG 63-22 Head tilt (A) and ventrolateral strabismus (B) in a 2-year-old Dachshund after needle trauma to the brainstem during cervical myelography.
In addition to moving the head from side to side to determine whether the eye movements are normal, the examiner should hold the animal’s head still in each lateral position to determine whether an abnormal (positional) nystagmus develops. The head and neck should then be extended and held in that position while the eyes are evaluated for a ventral strabismus and the development of nystagmus. When the head of a normal animal is held still, there will be no nystagmus. In most animals with severe or acute central or peripheral vestibular lesions, there will be a resting (spontaneous) nystagmus regardless of the position of the head. In less severe or compensated vestibular disorders the examiner will be able to elicit only a few beats of abnormal nystagmus when the animal’s head is held in a certain position; this is called positional nystagmus, and it is abnormal. Positional nystagmus will sometimes be evident only when the animal is placed in dorsal recumbency with the head and neck extended (Fig. 63-23). The direction of nystagmus is defined as the direction of the fast phase of eye movements.
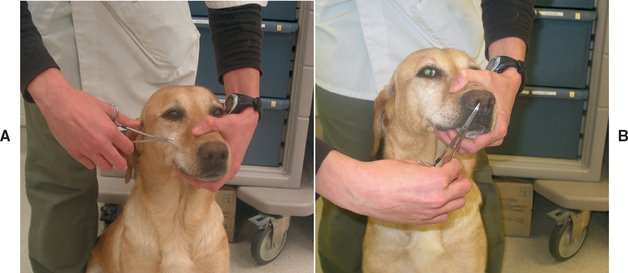
Evaluation of Trigeminal (CN5) Nerves
The trigeminal nerve (ophthalmic and maxillary branches) supplies the sensory innervation to the skin of the face, the cornea, the mucosa of the nasal septum, the nasopharyngeal mucous membranes, and the teeth and gingiva of the upper jaw. The mandibular branch supplies sensory innervation to the mandibular portion of the face and oral cavity as well as motor function to the muscles of mastication. Sensory function is tested by assessing the corneal and palpebral reflexes, assessing the response to stimulation of the nasal septal mucosa, and pinching the skin of the face with a hemostat (Fig. 63-24). Motor function is assessed by evaluating the size and symmetry of the masticatory muscles and testing the resistance of the jaw when opening the mouth. Bilateral trigeminal motor paralysis results in a dropped jaw and inability to close the mouth (Fig. 63-25). Loss of corneal sensation will decrease reflex release of tears and trophic factors, leading to keratitis (neurotrophic keratitis) and corneal ulceration in some dogs.
Evaluation of the Facial Nerves (CN7)
The facial nerve provides motor innervation to the muscles of the face and sensory innervation to the rostral two thirds of the tongue (for taste) and palate. Parasympathetic fibers innervate the lacrimal glands and the mandibular and sublingual salivary glands and can be assessed with a Shirmer tear test. Motor function is assessed by examining the face for symmetry and observing spontaneous blinking and ear movements as well as by eliciting the corneal and palpebral reflexes, the menace response, and the ability to twitch the face in response to a pinch (sensory CN5). Because the facial nerve courses through the middle ear before distribution to the muscles of the face, middle ear lesions can cause dysfunction.
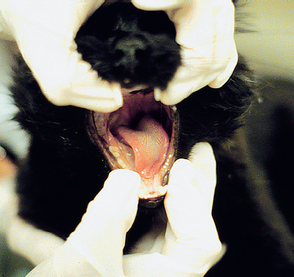
Evaluation of the Glossopharyngeal (CN9), Vagus (CN10), and Hypoglossal (CN12) Nerves
The glossopharyngeal, vagus, and hypoglossal nerves are usually evaluated together as components of the gag reflex and normal eating and drinking. The glossopharyngeal nerve (CN9) provides motor innervation to the pharynx and palate and sensory innervation to the caudal third of the tongue and pharynx. It also provides parasympathetic stimulation to the parotid and zygomatic salivary glands. The vagus nerve (CN10) provides motor and sensory innervation to the larynx, pharynx, and esophagus and sensory innervation to the thoracic and abdominal viscera. The parasympathetic portion of the vagus provides motor innervation to most thoracic and abdominal viscera. The hypoglossal nerve (CN12) provides motor innervation to the tongue.
The swallowing or gag reflex (CN9 and CN10) can be evaluated by applying external pressure in the hyoid region to induce swallowing or by stimulating the pharynx with a finger to induce the gag reflex. This can also be evaluated by watching the animal eat and drink. The parasympathetic portion of CN10 can be tested by measuring the reflex bradycardia that normally occurs when applying digital pressure to both eyeballs (oculocardiac reflex). The hypoglossal nerve (CN12) can be evaluated by inspecting the tongue for atrophy or asymmetry (Fig. 63-26) and observing tongue movement during eating and drinking or when licking food paste placed on the nose.
LESION LOCALIZATION
After the neurologic examination is completed, an animal’s mentation, cranial nerves, posture, gait, forelimbs, rear limbs, perineum, anus, and bladder can be characterized as normal or abnormal. If disease above the foramen magnum is present, clinical findings should allow a lesion to be localized to a specific region of the brain. In patients with spinal cord disease determining whether the neurologic abnormality in each limb is UMN or LMN in origin allows localization to a region of the spinal cord or specific spinal cord segments (see Box 63-4). When LMN signs are present in a single limb, the lesion can often be even more precisely localized by determining the muscles affected and, if sensory nerves are also affected, by testing sensation in dermatomes. Focal hyperpathia may also help to precisely localize a lesion. Whenever possible, the clinician should be able to explain all detected neurologic abnormalities on the basis of a single lesion. Occasionally, however, this will be impossible because the animal has multiple foci of disease or a diffuse disorder.
DIAGNOSTIC APPROACH
Once a neurologic lesion has been localized, it is necessary to generate a list of likely differential diagnoses. This list should take into account the signalment, historical data, the neuroanatomic location of the lesion, and the nature of the onset and progression of neurologic signs. It is important to consider all possible mechanisms or causes of disease that can affect the nervous system (Box 63-9). Once a list of likely differential diagnoses has been developed, diagnostic tests can be performed to confirm or exclude each.
 BOX 63-9 DAMNIT-VP Scheme: Mechanisms of Disease
BOX 63-9 DAMNIT-VP Scheme: Mechanisms of Disease
| D | Degenerative |
| A | Anomalous |
| M | Metabolic, malformation |
| N | Neoplastic, nutritional |
| I | Infectious, inflammatory, immune, iatrogenic, idiopathic |
| T | Traumatic, toxic |
| V | Vascular |
| P | Parasitic |
ANIMAL HISTORY
Patient age, gender, breed, and lifestyle may provide clues regarding the underlying disease. Young animals are most likely to be seen because of congenital or hereditary disorders; they are also at highest risk for intoxications and infectious diseases. Older animals are more susceptible to neoplastic diseases and many of the known degenerative disorders. Certain breeds are predisposed to particular disorders, and there are many congenital and inherited disorders that have been seen in only one or a few breeds. Dogs engaging in particular competitive or working activities (e.g., hunting, herding, racing, jumping) may be at increased risk for specific activity-related injuries. Potential exposure to trauma, toxins, and infectious disorders should be ascertained through careful history taking.
DISEASE ONSET AND PROGRESSION
Evaluation of the onset and progression of neurologic signs is of primary importance in prioritizing the list of differential diagnoses (Box 63-10). The signs may be peracute and nonprogressive, or they may become progressively more severe with time. In peracute disorders the time of onset of the neurologic signs can be pinpointed exactly, with the animal going from being normal to abnormal within minutes or hours. Signs reach maximal intensity very rapidly and then remain static or improve over time. Examples include external trauma, internal trauma from intervertebral disk extrusion, vascular disorders such as infarcts or hemorrhage, and some rapid-acting intoxications such as strychnine. Rarely, animals with a typically slowly progressive disorder such as a tumor present with a peracute exacerbation of their signs as a result of hemorrhage or fracture at the site of the tumor. A thorough history will often reveal that these animals were not entirely normal before the acute deterioration.
 BOX 63-10 Characterization of Disease Processes Based on Onset and Progression
BOX 63-10 Characterization of Disease Processes Based on Onset and Progression
Neurologic disorders with fairly rapid deterioration over days to weeks are classified as subacute and progressive. Infectious and noninfectious inflammatory diseases and some of the more rapidly progressive neoplasms (e.g., lymphomas, metastatic malignancies) usually fall into this category. Metabolic and nutritional disorders and some intoxications can also cause subacute progressive signs. Animals with chronic progressive signs that develop very slowly over many weeks or months are most likely to have neoplastic or degenerative disease.
SYSTEMIC SIGNS
Identification of concurrent systemic abnormalities may aid in the diagnosis of neoplastic, metabolic, or inflammatory nervous system disorders. A complete physical examination and ophthalmologic evaluation, including a funduscopic examination, should be performed in every animal with suspected neurologic disease. Laboratory tests and imaging modalities useful for specific evaluation of nervous system disorders are limited, so identification and characterization of associated abnormalities in other tissues can facilitate diagnosis. Ancillary diagnostic tests can then be performed to further evaluate animals with neurologic disease and thereby arrive at a specific diagnosis.
Chrisman CL, et al. Neurology for the small animal practitioner. Jackson: Teton NewMedia, 2003.
DeLahunta A. Neurologic examination. Braund KG, editor. Clinical neurology in small animals: localization, diagnosis and treatment. Ithaca, N.Y.: IVIS. 2001. www.ivis.org.
DeLahunta A. Veterinary anatomy and clinical neurology, ed 2. Philadelphia: WB Saunders, 1983.
Garosi L. The neurological examination. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004.
Garosi L. Lesion localization and differential diagnosis. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004.
Lane IF. Diagnosis and management of urinary retention. Vet Clin North Am Small Anim Pract. 2000;30:24.
Sharp NJH, Wheeler SJ. Small animal spinal disorders. Philadephia: Elsevier, 2005.



 BOX 63-8
BOX 63-8