Patient Monitoring
After completion of this chapter, the reader should be able to:
• Perform complete monitoring of the patient under anesthesia, assessing cardiovascular, respiratory, and neurologic parameters.
• Discuss the special anesthetic considerations regarding monitoring of various surgical procedures and patient positions.
• List examples of anesthesia monitoring devices.
• Describe the functions of anesthesia monitoring devices.
• Discuss cause and effect of various physiologic changes that may occur during anesthesia.
Role of the Veterinary Technician Anesthetist
For surgical procedures it is desirable to have a veterinary technician anesthetist, whose sole responsibility is to monitor the patient under anesthesia, as well as a circulating nurse to help transport the patient from the surgery prep area to the surgical suite. This team approach allows the surgeon and surgical assistant to be scrubbing while the anesthetist and circulating nurse arrange the patient, anesthesia machine, and monitoring devices in the surgery room. When the circulating nurse takes responsibility for positioning the patient on the surgery table, attaching the monitoring devices to the patient, and performing the final skin prep and paint in the surgery room, the anesthetist is able to focus on the most important task, monitoring the patient. Without the help of a circulating nurse, the veterinary technician would need to perform all these tasks alone in rapid succession. If the technician is working alone, the primary task is careful and constant monitoring of the surgical patient. This may mean delaying the final surgical scrub (or other, less critical tasks) until the veterinary technician is confident that the patient is stable.
Monitoring
Constant monitoring of the animal under general anesthesia or sedation is an extremely important responsibility. The patient that is monitored closely is the patient that is safely anesthetized. Close and constant monitoring allows the anesthetist to detect dangerous trends and to make appropriate adjustments. If the patient is showing signs of waking up, the anesthetist can increase the concentration of anesthetic. If the patient is showing signs of becoming too deeply anesthetized, the anesthetist can decrease the concentration of gas delivered. Table 5-1 summarizes the stages of anesthesia.
TABLE 5-1
CRT, capillary refill time; min, minute.
From McKelvey D, Hollingshead KW: Veterinary anesthesia and analgesia, ed 3, St Louis, 2003, Mosby.
Constant monitoring by a technician responsible solely for that patient is the preferred situation. Recommendations include checking the patient every 5 minutes and recording the findings on an anesthesia form. When gas anesthetic agents that provide quick induction and recovery are used, such as sevoflurane, more frequent monitoring may be needed. The observant surgeon and surgical assistant can supplement intermittent and frequent monitoring by nonsterile personnel.
Several patient parameters should be monitored every 5 minutes, including heart rate and rhythm, respiratory rate and rhythm, capillary refill time, color of mucous membranes, eye position, muscle tone, and reflexes. The patient’s temperature should be monitored at least every 15 minutes if possible. Several anesthetic and treatment parameters should be tabulated every 5 to 15 minutes, including volume of fluid administered, oxygen flow rate, oxygen tank pressure, and anesthetic gas concentration.
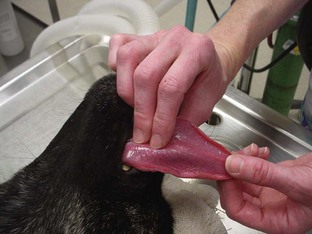
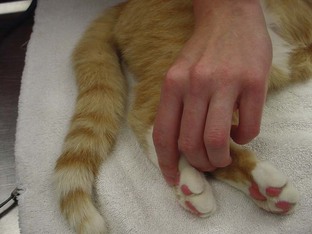
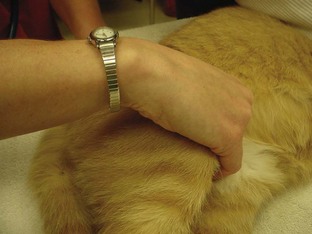
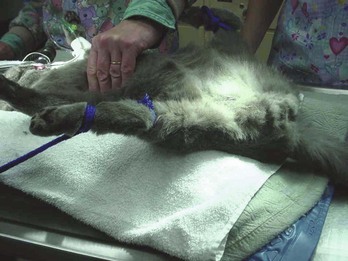
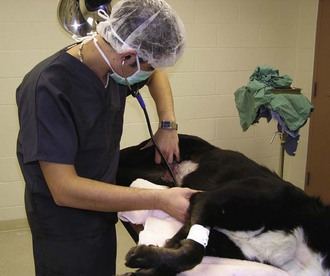
The most reliable way to monitor the patient is through personal, direct contact. The technician can listen for the heart rate and respiratory rate through the stethoscope. The pulse can be palpated to obtain a general impression of pulse strength and therefore of blood pressure (Figures 5-1 to 5-5). The reservoir bag can be observed for frequency and extent of movement. The color of the mucous membranes can be visually assessed, and the capillary refill time is directly measured. The technician’s sense of smell can detect possible anesthetic leakage problems.
Heart Rate and Rhythm
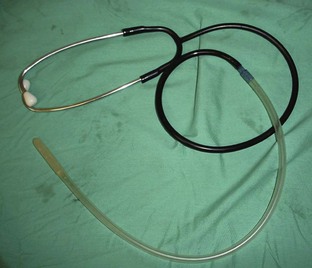
The heart rate can be monitored through direct palpation of the thoracic wall or by listening through a stethoscope. The stethoscope can be the conventional type that is placed on the thoracic wall. However, the location of the surgical site may prevent the use of a conventional stethoscope. An esophageal stethoscope is a more convenient and less intrusive means of monitoring heart rate intraoperatively.
In positioning the esophageal stethoscope, special sensory plastic tubing is placed in the esophagus next to the heart. The tubing is passed through the mouth into the esophagus. The endotracheal (ET) tube prevents the esophageal stethoscope tubing from entering the trachea. The heart sounds can be heard through ear pieces, or an amplifier can be attached to broadcast the sound (Figures 5-6 and 5-7). If the end of the tubing is adjacent to the lung fields, breath sounds will be heard.
The heart rate of an anesthetized dog or cat is expected to be slower than the animal’s rate when awake and excited about being examined by a stranger. Most anesthetic drugs have cardiac depressant effects. Hypothermia can also cause a decreased heart rate. When the heart rate falls below an acceptable limit, the surgeon should be notified. Suggested guidelines for acceptable lower limits are 50 beats per minute (beats/min) for a large dog, 70 beats/min for a small dog, and 100 beats/min for a cat. Rates lower than these warrant notifying the surgeon immediately.
Anticholinergic drugs such as atropine sulfate and glycopyrrolate are administered to help maintain the heart rate above the acceptable lower limits (see Chapter 2). Dissociative anesthetics such as ketamine and tiletamine can also elevate the heart rate. When an animal is in shock or pain, the heart rate is usually rapid. Regardless of the reason, once the heart rate exceeds the acceptable limits, the surgeon should be notified. Suggested guidelines for acceptable upper limits are 180 beats/min for a large dog, 200 beats/min for a small dog, and 220 beats/min for a cat.
An irregular heart rhythm may be detected through auscultation of the thorax or on an electrocardiogram (ECG). Some anesthetic agents sensitize the heart muscle to arrhythmias. When the heart beats arrhythmically, the heart is inefficient at pumping the blood through the body. This inefficiency leads to poor tissue perfusion, acidosis, poor oxygen delivery to the tissues, and buildup of waste products in the body tissues. These irregular beats may spontaneously disappear. If the arrhythmic beats are frequent or continuous, the veterinarian should be alerted.
Detection of a pulse deficit is cause for concern. A pulse deficit occurs when the heart contracts (beats) but does not generate enough push to produce a palpable peripheral pulse. Pulse deficits are detected when the heartbeat is arrhythmic; tissue perfusion is poor, and the patient may quickly go into shock. The veterinarian should be alerted immediately. “Skipped” beats are detected when one is listening to the heartbeat with a stethoscope but is unable to palpate that individual beat simultaneously as a peripheral pulse. In this case the single skipped beat is a result of a single premature depolarization of the heart muscle.
Tissue Perfusion
To maintain healthy and functioning tissues and organs, adequate tissue perfusion must be maintained. If not, adverse long-term effects may be sustained. The kidneys are particularly sensitive to states of low perfusion. During inadequate perfusion states, waste products from cellular metabolism are not removed, and nutrients such as oxygen and glucose are not delivered to the cells.
Mucous Membranes
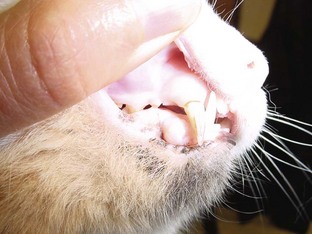
The color of the mucous membranes is a general indicator of tissue perfusion. The pink color of the mucous membranes results from the color of the blood in the capillaries seen through the nonpigmented areas. Mucous membrane color may be evaluated by looking at the gums, conjunctiva, and mucosal surface of the vulva or prepuce.
Pale mucous membranes may indicate a state of poor tissue perfusion or anemia (Figure 5-8). Preanesthetic diagnostic blood tests, such as complete blood count (CBC) and measurements of hematocrit and hemoglobin, will identify the anemic patient. Preferably, the severely anemic patient will not go to surgery before receiving appropriate blood component therapy. Pale mucous membrane color can also be an indicator of shock. Efforts must be made before the anesthetic episode to identify and begin correction of the poor color.
Dark-red mucous membranes may be seen if an animal is septic, meaning that the patient has a severe blood infection. Bacteria may be cultured from the blood. The immune system is reacting to the infection by releasing vasoactive chemicals that cause postcapillary venous dilation and increased permeability in the vessel walls. This can lead to congestion in the capillary beds and thus the brick-red color of the mucous membranes.
A bluish color to the mucous membranes (cyanosis) suggests that the red blood cells are not carrying adequate oxygen. The red cells may not have exchanged oxygen in the lungs, suggesting a respiratory problem such as obstruction of oxygen flow to the lungs or lack of breathing. The ET tube should be evaluated immediately for a kink or obstruction. A bluish color in the buccal mucosa may also result if a tie on the ET tube is too tight around the cheeks, causing stagnant blood flow. The buccal mucosa should be compared with the conjunctival mucosa to determine whether a tight tie could be the cause of the bluish color and needs to be loosened.
Capillary Refill Time
The capillary refill time (CRT) normally should be less than 2 seconds. The same areas used to assess the color of mucous membranes may be used to assess CRT. When CRT is longer than 2 seconds, perfusion in these tissues is poor. This condition may be caused by low arterial blood pressure or peripheral vasoconstriction. Release of epinephrine can cause peripheral vasoconstriction, and slow CRT is often seen in nervous and frightened animals. α2-adrenergic agonist drugs such as medetomidine cause significant peripheral vasoconstriction, thus slowing the CRT. A normal CRT can be detected shortly after some animals are deceased, reinforcing the importance of constant monitoring of several vital signs, not just one.
Pulse and Blood Pressure
Pulse strength can be defined as the ease or difficulty of palpating the blood flowing through the artery. Pulse strength is a general indicator of the blood pressure. If the patient’s pulse becomes more difficult to palpate, the blood pressure has fallen. If the patient’s pulse becomes more easily palpable or bounding, the blood pressure has increased. Pulses can be monitored at the following arteries: lingual, femoral, carotid, dorsal metatarsal, and digital (see Figures 5-1 to 5-3). Additionally the heart may be palpated directly through the chest wall. In cats the examiner’s thumb and fingers are placed on either side of the chest, and light pressure is applied to feel the beating heart (see Figure 5-4). Obese patients and larger patients, such as medium-sized to large dogs, are more difficult to palpate.
An ultrasonic Doppler or oscillometric device is needed to assess arterial blood pressure accurately (see Chapter 1). Normal mean arterial pressure (MAP) ranges in awake animals are from 85 to 120 mm Hg. MAP should be maintained above 70 mm Hg while the patient is under anesthesia and never allowed to drop below 60 mm Hg.
Hypotension
Hypotension (low blood pressure) in an anesthetized patient may be caused by the following factors:
Hypovolemia: Excessive blood loss or dehydration reduces the volume of the vascular space. With little blood to pump through the vessels, the blood pressure will be low.
Cardiac insufficiency: Patients with preexisting heart disease may have a weak myocardium or leaking heart valves, which can lead to inefficient pumping action.
Excessive vasodilation: Many preanesthetic and anesthetic drugs cause vasodilation to varying degrees. The effects can be additive and significant. When the blood vessels dilate, the blood pools, venous return to the heart is decreased, and blood pressure drops.
Anesthetic depth: In general, as the patient goes deeper under anesthesia, the blood pressure drops. The correlation between blood pressure and anesthetic depth is quite accurate.
Respiratory Rate and Rhythm
One inhalation and one exhalation are considered one breath. Counting only the inhalations or only the exhalations is necessary to determine the correct respiratory rate (RR). The number of breaths counted in 15 seconds is multiplied by 4 to calculate the breaths per minute, or the respiratory rate. A dog or cat in the surgical plane of anesthesia has a rate of 8 to 20 breaths/min. A respiratory rate of less than 8 breaths/min is cause for concern and most likely indicates an excessive anesthetic depth. As anesthetic depth becomes dangerously deep, respiration ceases before the heart stops beating.
The respiratory rate can be determined by watching the patient’s chest movement. The reservoir bag on the anesthesia machine can also be observed. The reservoir bag collapses and fills synchronously with the patient’s breathing. The anesthetist can listen for breath sounds through the stethoscope and should note their character and intensity. An increase in intensity, crackles, or wheezing indicates pulmonary airway narrowing through constriction or the presence of fluid. Devices such as the capnograph display the respiratory rate on a digital display.
The depth and rhythm of the respirations should also be assessed. The inspiratory phase is about half as long as the expiratory phase. A short pause occurs before the next inspiration. If the animal feels pain during the surgery, the respiratory rate will increase. Dissociative anesthetic agents will cause the animal to inhale, hold its breath, then exhale. This irregular breathing pattern is characteristic of dissociative anesthetics. As anesthetic depth increases, respiratory rate usually decreases. Gasping or labored breathing is of particular concern. An obstruction of the trachea or ET tube or a lack of fresh gas flow can cause exaggerated respiratory effort. Check the patency of the ET tube, check for the appropriate oxygen flow rate on the anesthesia machine, and confirm that the pressure in the reservoir bag is not too high. If the ET tube is not patent, it may need to be suctioned, or the patient may need to be re-intubated. If the desired setting on the oxygen flowmeter is not being maintained, the oxygen tank supplying the anesthesia machine may need to be changed. If the reservoir bag is overinflated and has a high pressure, assuming the pop-off valve is open, the anesthetist can try decreasing the oxygen flow rate on the anesthesia machine.
The animal that spasmodically contracts its diaphragm may be exhibiting agonal (“death agony”) breathing. Agonal breathing, or agonal gasping, is not “real” breathing; that is, no physiologic exchange of gases is occurring in the lungs. Agonal gasps can be mistaken for deep breaths if the respiratory rate has not been sufficiently monitored. Agonal gasps typically occur after a long period of apnea and are often detected as rapid movement in the patient’s diaphragm, jaw, or larynx. Agonal gasps occur after cardiac arrest, and the veterinarian must be alerted immediately of their presence.
The practice of manually ventilating the patient every 5 to 10 minutes during the anesthetic period is recommended. Assisted ventilation (“bagging”) opens up collapsed alveoli, preventing atelectasis. Anesthetized patients tend to breathe more shallowly; combined with the weight of other internal organs on the lungs, the shallow breathing contributes to atelectasis (Box 5-1).
While administering a ventilating breath, the anesthetist should listen at the animal’s mouth for any sound of air rushing past the ET tube. Some anesthetic agents, such as isoflurane, have a pungent odor, which indicates leakage if detected. If a sound is heard or the odor of gas detected, the cuff seal is incomplete. The cuff on the ET tube should be further inflated or the patient re-intubated with a larger ET tube.
Body Temperature
Body temperature is monitored before and during the anesthetic period. Monitoring every 15 minutes is common practice, although unless an esophageal thermometer probe or a rectal probe is used, this schedule may prove challenging.
In rare instances, hyperthermia may develop when inhalation anesthetics are used. Dangerously high body temperatures spontaneously manifest in patients that develop malignant hyperthermia. If the high temperature goes unnoticed, life-threatening cerebral edema can develop. Genetic predisposition may play a role in the phenomenon of malignant hyperthermia, which has been documented in humans, pigs, and dogs. If an abnormally high body temperature is detected, or if a temperature significantly higher than the preanesthetic body temperature is recorded, the anesthetic procedure should be terminated as soon as possible.
The development of hypothermia is common and a constant concern in patients undergoing general anesthesia. The following factors contribute to the decrease in body temperature:
• Anesthetic drugs relax muscles, significantly diminish the shivering response, and decrease the overall metabolic rate. Production of body heat decreases.
• The surgical site is shaved, washed with soap and water, then rinsed with alcohol. Evaporation from the skin surface has a cooling effect on the body.
• Room-temperature fluids are administered.
• A body cavity may be opened, and internal organs are exposed to ambient temperatures.
Measures to prevent body heat loss should be taken, as discussed earlier.
Neurologic Parameters
As anesthetic depth increases, reflex activity diminishes and eventually disappears. A reflex is an automatic reaction to a stimulus made by the animal without conscious awareness of the reaction. These reflex actions protect the animal from harm. For example, the swallowing reflex is triggered by the presence of saliva or other matter in the pharynx. The animal swallows the material into the gastrointestinal system rather than aspirating it into the respiratory system. Most reflexes monitored during anesthesia are located around the face, eyes, and throat. The return of the reflex or increasing strength of the reflex response indicates that the level of anesthesia is becoming lighter. The technician anesthetist may monitor the following reflexes:
• Auricular (ear flick) reflex: The fully conscious animal flicks its ear when the pinna is lightly touched or tickled. The intensity of the reaction varies among individuals. If the reflex is tested in rapid succession, the reaction to the stimulus may diminish quickly. Although this reflex can persist well into a moderate level of anesthesia, its variable nature renders it somewhat unreliable. It is best to monitor all available reflexes.
• Pedal (withdrawal) reflex: When pressure is applied to a toe, the limb is withdrawn. The pressure applied should be significant. The withdrawal reflex is useful when the animal is being masked. The mask interferes with the ability to monitor other reflexes around the face. Once the pedal reflex disappears, the animal may be sufficiently anesthetized to attempt intubation.
• Palpebral (blink) reflex: The blink reflex protects the globe from injury by the eyelids quickly closing. The reflex can be elicited by lightly touching the medial canthus of the eye. The reflex disappears or is significantly diminished as the animal enters the surgical plane of anesthesia. The palpebral reflex usually returns just before the laryngeal reflex as the animal is recovering.
• Corneal reflex: The animal blinks or retracts the globe when the cornea is touched. This reflex persists well into the deep levels of anesthesia. The corneal reflex is seldom used in monitoring anesthesia; it is used more often to help assess the point of death during euthanasia.
• Laryngeal (swallow) reflex: When saliva, food, or water is in the pharynx, the epiglottis and arytenoid cartilage close the entrance to the trachea, and the animal swallows. The laryngeal reflex can also be initiated by rubbing the ventral throat. The swallow reflex must be lost in order for intubation to proceed easily.
Cats tend to retain the laryngeal reflex longer than dogs, so they need to be more deeply under anesthesia before intubation can be accomplished. If intubation is attempted too soon, the larynx may go into a spasm and remain closed. Cats also are more likely to develop laryngeal spasm than dogs during the intubation process. See Chapter 2 for a list of methods to avoid or reduce the likelihood of these spasms.
Muscle Tone
Muscles usually relax as the animal goes deeper under anesthesia. Dissociative anesthetics cause muscle rigidity; however, some muscle relaxants, such as benzodiazepines (e.g., diazepam, zolazepam), are usually added when these anesthetics are used. The most important muscles to relax are those that control the opening of the mouth. Once the mouth can be easily opened, intubation can be performed more easily.
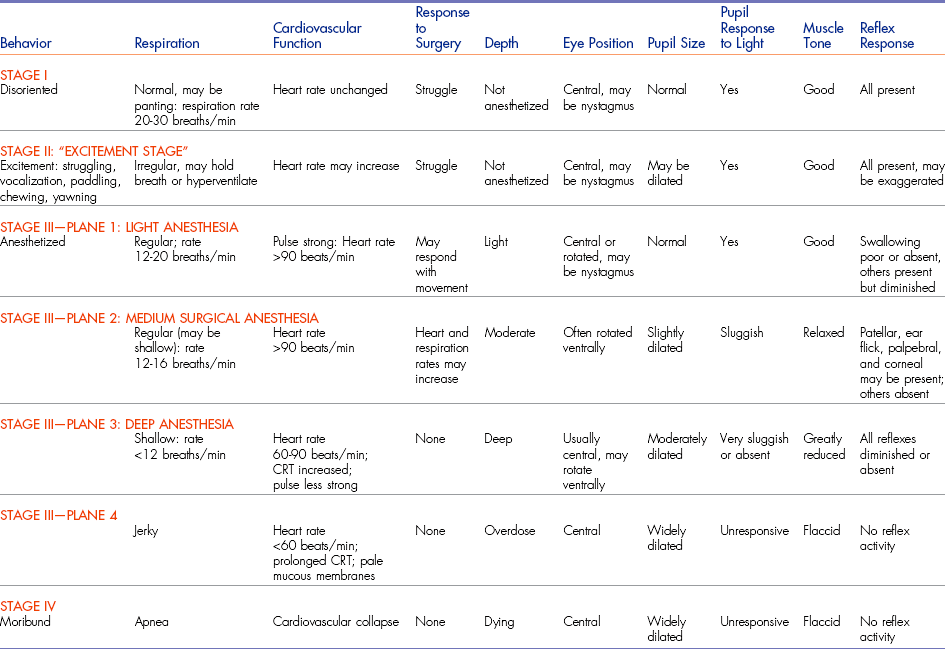
It is important to monitor the intensity of the jaw tone during anesthesia. When jaw tone increases, the animal’s depth of anesthesia is becoming lighter. The anesthetist can test the resistance to opening the mouth by carefully prying apart the upper and lower teeth with just two fingers (Figure 5-9).
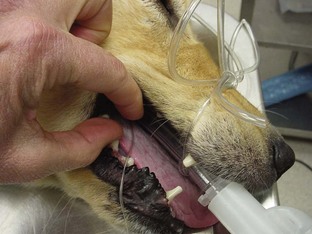
FIGURE 5-9 Method for testing jaw tone involves prying the mouth open with just two of the examiner’s fingers barely inserted into the mouth.
Depending on the procedure being performed, jaw tone may not be available for monitoring by the anesthetist. If the animal is undergoing a dental procedure or oral surgery, a mouth speculum may be placed in the mouth to keep it open. In such cases the anal opening may be inspected because it can indicate the degree of muscle relaxation the patient is experiencing. In the conscious animal, the anal opening is closed. In general, as the animal goes deeper under anesthesia, the anal tone becomes more relaxed and the anus opens more.
Eyes
The position of the eye in the socket, the pupil size, and the eye’s responsiveness to light can be used to assess anesthesia depth. Although the responses may vary with the individual animal and the drugs given, the following general observations can be useful in assessing anesthetic depth:
• The eye rotates ventrally when the animal is in a moderate or surgical level of anesthesia (Figure 5-10).
• At light and deep levels of anesthesia, the eye is in a central position.
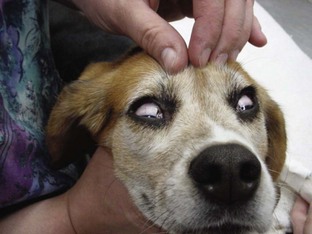
• When dissociative anesthetics are given, the eye position may never change (Figure 5-11).

FIGURE 5-11 Glycopyrrolate and Terazol (tiletamine/zolazepam) were administered intramuscularly to this cat 15 minutes ago. Eye ointment should be applied as soon as possible to protect the eyes from drying out. Tear production and the blink reflex are diminished. Note the widely dilated pupils.
• As the anesthetic depth increases, the pupil constricts more slowly in response to a light shined at it (pupillary light response [PLR]).
• Deeper stages of anesthesia and anticholinergic drugs cause pupillary dilation (mydriasis). The use of these drugs in the preanesthetic protocol interferes with the reliability of this reflex.
It should be noted here as well that when a patient undergoes cardiac and respiratory arrest and expires, the pupils are fully dilated.
Special Surgical Case Considerations
Although parameters to monitor for surgical patients are fairly similar for all cases, certain anesthetic complications, reactions, and considerations must be given in special situations. Young, healthy animals that are undergoing an elective procedure are still at risk for anesthetic complications, although the risk is significantly lower. Patients experiencing a trauma or a disease process pose more of a challenge. An animal that is experiencing dystocia and now requires a cesarean section is compromised differently from a patient that has been hit by a car and now requires surgery to repair a fractured tibia.
Cranial Procedures
In most surgical procedures, the eyes are available for palpebral reflex evaluation, the jaw is available for muscle tone assessment, and the eye position is evaluated throughout the procedure. Mucous membrane color and capillary refill time are monitored by means of the gingiva for appropriate monitoring. Procedures that require the surgeon to be working on or near the head present unique monitoring challenges. When surgical procedures necessitate the removal of these options, technicians must redefine the options for monitoring. Ophthalmic, aural, or mouth procedures require the anesthetist to “rethink” the approach to anesthesia monitoring—using the vulva or prepuce to monitor mucous membrane color, for example. Attaching a reflectance probe to the underside of the base of the tail instead of on the tongue is another option. Using the femoral artery for pulse assessment instead of the lingual artery is also another method to continue to effectively monitor the patient, despite obstacles to the traditional methods used.
Respiratory Compromise
Patients that are experiencing dyspnea, for whatever reason, create a challenge for the technician anesthetist. For the patient in which thoracic trauma is the source of dyspnea, the use of a ventilator extremely may be beneficial. Once the chest cavity is opened, ventilatory support needs to be provided anyway, so employing it earlier will do no harm. Patients in which abdominal issues are the cause of the respiratory compromise also benefit from ventilatory support. Pressure on the aorta, for whatever reason (gastric dilatation, gravid uterus, hemoabdomen, etc.), compromises return of venous supply as well as respiratory issues.
Patient Positioning Factors
Depending on the surgery being performed, certain necessary patient positions can create challenges for the anesthetist. Neurologic procedures that require the patient to be in ventral recumbency risk the development of respiratory problems. Animals lying on their abdomen and chest are not able to breathe as easily and effectively as if they were positioned in a different way. A patient in dorsal recumbency, with the neck in full extension or hyperextension (e.g., for thyroidectomy, ventral slot), will have respiratory issues due to the decreased ability to expand their chest. Animals undergoing perineal surgery (perineal urethrostomy, anal sac ectomy, etc.) also present a respiratory challenge. With their hind legs draped over the end of the surgery table, much pressure is on their abdominal organs and diaphragm, inhibiting normal respiratory function.
Anesthesia Monitoring Devices
The monitoring of a patient that is under the effects of anesthetic drugs is a highly involved and critically important responsibility of the veterinary technician. The veterinary technician must be willing and prepared to anticipate and troubleshoot potential problems to provide the safest anesthetic episode for the patient. Although there is no ideal monitoring device, multiple devices used together can provide useful information on the patient’s status.
A well-trained, educated, sensitive, alert technician is probably the best “device.” The senses of the technician are constantly relied on to monitor a patient. The technician’s sense of sight can watch for changes in mucous membrane color or patient movement or changes in respirations; the sense of sound is applied to listen to heart rate and rhythm; the sense of smell can detect the presence of anesthetic gas; and the sense of touch can feel for changes in peripheral pulses indicating changes in blood pressure.
Even the best technician has limitations, however, so the use of auxiliary monitoring devices is extremely helpful. Many types of monitors are available with varying capabilities and limitations. Some monitoring devices combine multiple capabilities into one unit for convenience and optimal anesthetic evaluation.
Table 5-2 lists normal values for anesthetic monitoring of dogs and cats.
TABLE 5-2
Anesthetic Monitoring: Normal Parameters for Cats and Dogs
| Sao2 (arterial oxygen saturation) | 95%-99% |
| ETco2 (end-tidal carbon dioxide level) | 35-45 mm Hg |
| Blood pressure: | |
| Systolic | 90-160 mm Hg |
| Diastolic | 50-90 mm Hg |
| MAP (mean arterial pressure): | |
| Awake | 85-120 mm Hg |
| Under anesthesia | 70-99 mm Hg |
Pulse Oximeter
Pulse oximetry is based on the ability to evaluate the level of oxygen saturation in the blood to help assess tissue perfusion. Pulse oximeters also monitor pulse rates. Oxygen in the blood is carried by the hemoglobin in the red blood cells. The pulse oximeter measures how much (or the percentage) of the available hemoglobin is saturated with oxygen. This measurement is achieved using two different wavelengths of light transmitted through tissue with a pulsatile blood flow. The sensors (or probes) emit wavelengths of both red light and infrared light. The infrared light determines oxygen saturation, and the red light determines the pulse rate. The light is transmitted through the tissue, and the photodetector (located opposite the transmitter) senses the light. The software within the unit compares the absorption ratio of the two different wavelengths. Oxygen-rich blood (arterial) absorbs less light, so more of the light wavelength is sensed by the detector; therefore a higher arterial oxygen saturation (Sao2) reading is displayed.
Models
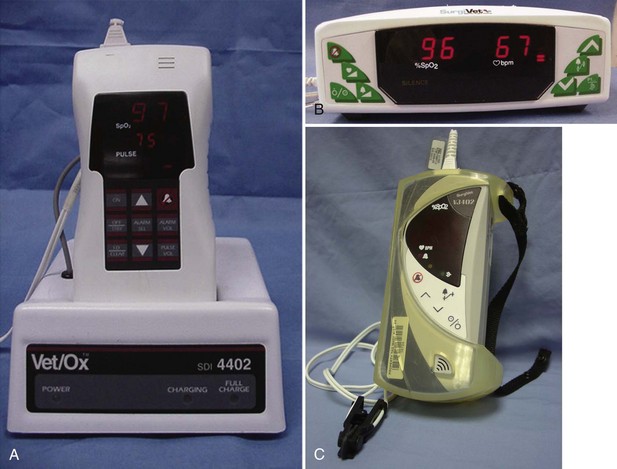
Some pulse oximeters are single-function units that measure only Sao2 and pulse. Units can be small handheld versions, whereas other versions are larger and more cumbersome (Figure 5-12). Most models allow the user to set alarms for high and low limits of acceptable readings. These alarms emit an audio tone when the high or low limits of Sao2 or pulse are reached, and they can alert the person monitoring the patient to potential problems. Other models are capable of monitoring multiple parameters, including Sao2, heart rate, respiratory rate, and end-tidal CO2 (ETco2).
Sensors
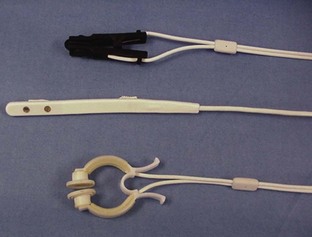
The choice of sensor depends on the placement site and species of animal being monitored (Figure 5-13). One of the most common sensors is the lingual sensor, which resembles a clothespin in design and is available in large and small versions. Other common sensors are the reflectance probe and universal C clamp. The lingual sensor, as the name implies, is most frequently used on the tongue but can be used on the ear pinna, toe webbing, external genitalia (vulva or prepuce), or any body region with no hair. Using this probe on sites other than the tongue does cause increased pressure on the probe spring and can result in premature degradation of the probe. The reflectance probe can be used with a protective sleeve and placed in the rectum or used alone on the underside of the tail, at the base of the tail. The universal C clamp sensor can be used on feet, hocks, and other areas. Regardless of what sensor is used, certain factors can affect the efficacy of the unit and the reliability of the information. Some factors are related to patient wellness, some to patient anatomy, and others to machine function. If at any time the information being displayed by the unit appears unreasonable, the factors listed here should be evaluated to ascertain the true status of the patient.
Unit Options
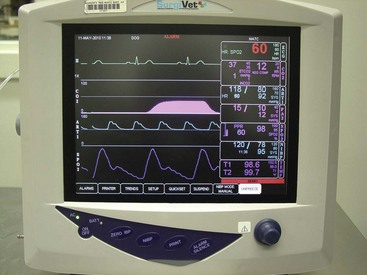
Some Sao2 units provide only basic information, that is, a pulse rate and an Sao2 readout. Even these basic units, however, also have a pulse bar graph to indicate the strength of the peripheral pulse being evaluated. Other models include additional information, such as noninvasive blood pressure (NIBP) monitoring, ETco2 readings (capnography), and electrocardiograph (ECG) readouts (Figure 5-14). The more parameters measured by the device, the more information acquired by the anesthetist, but there is also greater potential for machine malfunction.
Troubleshooting
Maintaining an appropriate level of oxygen in the patient’s blood is paramount. With a patient attached to the anesthesia machine with 100% O2 running, the Sao2 reading should be 95% or greater. If the reported data fall below this level, the veterinary technician needs to evaluate the situation to determine whether the machine is malfunctioning or whether in fact the patient’s Sao2 levels are dropping. Assessing the patient and the equipment quickly is important to correct the problem appropriately. Sometimes the sensor has slipped from its original position, and simply repositioning it may correct the problem. Sometimes with the lingual probe, wetting the tongue with tap water can aid in obtaining a more accurate reading. Also, it has been found that when the lingual probe is used with felines, folding the tongue can be beneficial. Folding the tongue lengthwise provides a thicker tissue mass and generally a more accurate reading. If the animal is not exchanging gases well even though a normal respiration rate is present, ventilation of the animal may need to be provided manually. Artificial ventilation helps expand the lungs more completely, thereby allowing better gas exchange.
Capnometer or Capnograph
Capnometry is the measurement and evaluation of the level of CO2 in the patient’s exhaled breath, or the ETco2. This component of anesthesia is important because it can help the anesthetist evaluate the patient’s respiratory rate and the quality of the respirations, therefore allowing better interpretation of the patient’s depth of anesthesia. An arterial blood gas sample evaluation is the best method to determine blood CO2 levels, but this is not usually performed in most practices. The alternative, ETco2 monitoring, is an option that accurately reflects the arterial CO2 levels.
Measurement
The capnometer can measure ETco2 by one of two methods (Figure 5-15). The sensors used are either a mainstream or a sidestream device. A mainstream device evaluates the patient’s CO2 levels as the breath passes through the airway. This sensor requires the patient to be intubated and correlates to the patient’s breathing pattern. A sidestream device uses a vacuum to draw a portion of the exhaled breath down a tiny tube to the main unit for evaluation. The delay in the collection of the sample translates into a displayed pattern that is “out of sync” with the patient’s breathing pattern.
Display Wave
The display on the capnometer is a digital readout of the ETco2. The display of a capnograph is in the form of a wave. The incline of the wave indicates the exhaled portion of a breath. The plateau at the peak height of the wave indicates the ETco2 value. As the patient begins to inhale, the wave begins the decline because inspired CO2 levels should be close to zero (Figure 5-16). Some monitoring devices are capnometers, whereas others are capnographs. Either can combine the measurement of ETco2 with other monitoring parameters to provide the anesthetist with the most complete picture of the patient and the anesthetic episode.
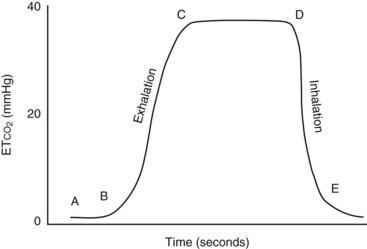
FIGURE 5-16 Capnograph tracing. (A) start of expiration; (B) dead space gas replaced by alveolar gas; (C-D) pure alveolar gas; (E) Dilution of alveolar gas by inspired fresh gas. (From Wright B, Hellyer P: Respiratory monitoring during anesthesia: pulse oximetry and capnography. Compendium 18:1083, 1996.)
Normal Values
Normal levels of expired CO2 are 35 to 45 mm Hg for both dogs and cats. Levels that depart from the accepted range require quick, efficient evaluation by the anesthetist to determine the appropriate course of action. Values below 35 mm Hg, or hypocarbia, can be attributed to overzealous artificial ventilation, increased respiratory rate, too light a plane of anesthesia, pain, or hypoxia. Administering analgesics, easing up on ventilation, increasing anesthesia depth, or treating an underlying hypoxia may help resolve the hypocarbia. Values above 45 mm Hg are caused by hypoventilation, which leads to higher levels of CO2. If more CO2 is being produced than removed, a dangerous situation can quickly develop. Hypercarbia can be the result of a decreased respiratory rate, decreased respiratory minute volume, exhausted soda lime, malfunction of one of the unidirectional valves on the anesthesia machine, or a kinked endotracheal tube. Elevated CO2 levels generally imply that some or all of the gas that was just exhaled has been rebreathed. Corrections to lower the CO2 include checking the machine and increasing ventilation to remove the excess CO2.
Blood Pressure Monitor
Blood pressure monitors are used to assess the blood pressure of a patient that is either awake or under anesthesia. It is important to monitor all aspects of physiology while an animal is anesthetized. Using only one monitoring device may provide information on only one parameter. Blood pressure monitors should be used in conjunction with other devices to enable the anesthetist to provide the best anesthetic care possible.
Indirect Monitoring
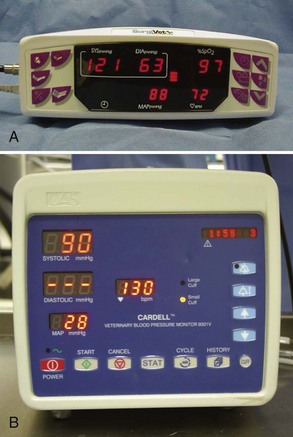
In private small animal hospitals, blood pressure is most often monitored by indirect methods (Figure 5-17). Doppler crystals are placed on the skin over arteries to produce an auditory assessment of pulse quality and blood pressure trends (Figure 5-18). Common arteries that are used with the Doppler crystal include the coccygeal, dorsal pedal, and digital. Theoretically any artery can be used, but these are usually the most accessible. A sphygmomanometer and a cuff can be added to the ensemble to allow estimated numeric assessment of diastolic and systolic pressures (Figure 5-19).
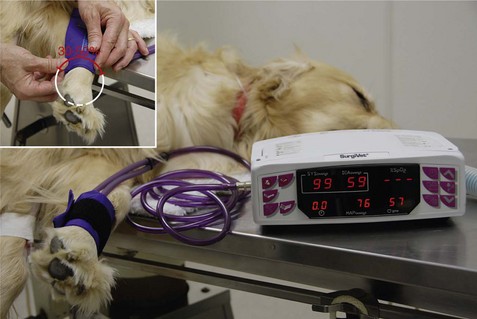
FIGURE 5-19 Oscillometric blood pressure monitor with a cuff placed on the metacarpus. The following measurements are indicated: systolic BP, 99 mm Hg; diastolic BP, 59 mm Hg; mean arterial blood pressure (MAP), 76 mm Hg; pulse, 57 bpm. Inset, Selecting an appropriately sized blood pressure cuff, the width of which should be 30% to 50% of the circumference of the extremity.
Noninvasive blood pressure machines, (Figure 5-20), which eliminate the need for a manual sphygmomanometer, are available. The units have cuffs that are placed on the patient, and the cuff inflation line is attached to the machine. The machine inflates the cuff at either preset intervals or manually determined intervals, the cuff is deflated, and a blood pressure measurement is acquired. The reliability of the numeric value displayed depends on the use of an appropriately sized cuff. Cuffs that are either too large or too small give inaccurate results. To ensure that the proper-sized cuff is being used, the following guidelines should be used: (1) the cuff width should be 40% of the circumference of the limb on which it is to be used and (2) the length should be appropriate so as to fall into the securing range indicated on the cuff.
Direct Monitoring
Direct blood pressure monitoring is performed by placing a catheter in an artery and then attaching a transducer to the catheter. The transducer is connected to an oscilloscope that can display the information being received through the transducer. Usually, numeric values for diastolic pressure, systolic pressure, and mean arterial pressure (MAP) and a waveform for the pulses are displayed. This procedure is more technically challenging than indirect monitoring and requires more expensive equipment. The advantage of direct monitoring is that a “true” evaluation of the pressure can be made. With indirect monitoring, only trends can be observed because accurate numbers are not available.
Electrocardiograph
Continuous ECG monitoring of anesthetized patients provides valuable information. In addition to providing a heart rate and wave formation tracing, many ECG machines are capable of monitoring other parameters, including pulse oximetry readings, temperature, and respiratory rate. “High-end” electrocardiographs can also provide direct blood pressure monitoring options and tracing printouts. Regardless of the additional functions provided with an ECG unit, the basic ECG tracing (electrocardiogram) is a valuable source of information for the anesthetist. Continuous, or even intermittent, monitoring of the heart rate and rhythm with an ECG can alert the anesthetist to current or impending problems that may be avoided. For example, the appearance of a premature ventricular contraction (PVC) alone may be an isolated incident, but repeated multiple PVCs can be a sign of impending, severe cardiac distress. Other situations, such as atrial dysfunction (evident as changes in the P wave), myocardial hypoxia, and electrolyte disturbances (evident as changes in the T wave), can be observed with continuous ECG monitoring.
Key Points
1. “Light and lively” is better than “deep and dead.” It is safer to keep the patient in a light to moderate plane of anesthesia.
2. Hands-on monitoring can be more reliable than mechanical device monitoring. Mechanical devices can register incorrect readings. Do not rely exclusively on mechanical monitoring devices.
3. For a full evaluation of the patient’s condition and stage of anesthesia, the anesthetist should assess multiple parameters, including reflexes.
4. There is a direct correlation between progression of hypotension and progression of anesthetic depth.
5. To determine a decreasing or increasing trend in body temperature, pulse, and respiratory rate (TPR), the technician must be sure to measure these preanesthetic values of the patient. Preanesthetic TPR is invaluable information.
6. Patients undergoing long surgical procedures may need intraoperative doses of analgesia. “Turning up the gas” is often not sufficient.
7. Pulse oximeters measure the level of oxygen saturation of the blood.
8. Capnometry is the measurement of the level of exhaled CO2; a normal reading is 35 to 45 mm Hg.
9. Blood pressure can be monitored by direct or indirect methods.
1. How frequently should physiologic parameters be recorded in the anesthesia log during a surgery?
2. Which of the following contributes to hypothermia in the anesthetized patient?
a. Anesthetic-induced muscle relaxation.
b. The surgical prep using water and alcohol.
3. Hypotension can be caused by:
4. Which reflex significantly diminishes or is absent when the patient enters the surgical plane of anesthesia?
5. Which class of drugs causes muscle rigidity?
6. Which of the following surgical procedures may create challenges for monitoring the patient owing to patient positioning?
7. Which monitoring device measures exhaled CO2 levels?
8. A change in which wave of an electrocardiogram is likely when myocardial hypoxia is occurring?
9. To ensure the most accurate blood pressure reading, the cuff should be what percentage of the width of the limb?
Edwards, NJ. ECG manual for veterinary technicians. Philadelphia: Saunders; 1993.
Muir, WW, III., Hubbell, JAE, Bednarski, RM. Handbook of veterinary anesthesia, ed 4. St Louis: Mosby; 2007.
Simpson, K. Let’s talk about capnography. Surgivet, August 2003. Available at http://www.surgivet.com.
Thomas, JA, Lerche, P. Anesthesia and analgesia for veterinary technicians, ed 4. St Louis: Mosby; 2011.