Chapter 33 Colouring and flavouring agents
In addition to those materials essential to the pharmacological action of medicaments there is a range of others that are present in formulations for either ethical or technical reasons. Included here are colouring matters, flavourings, stabilizers, emulsifiers, thickeners, preservatives, antioxidants and tablet disintegrants and coatings. In the food industry these are classed as additives and for the EU there is a list of permitted substances that may be used in some of the above categories; each substance is given a number prefaced by the letter E. Under EU rules, for appropriate foods, such additives must be included in the labelling. In the UK consumers can obtain further information from the Food Standards Agency and from commercially produced booklets.
For medicinal purposes these additives, which are often identical to those used in foods, are controlled by the Medicines Act and not all manufacturers’ data sheets provide information on the nature of the additives present. Thus, if a patient requires a medicament free of gluten or tartrazine it may be necessary for the pharmacist to make enquiries of the manufacturer. In recent years there has been an increasing demand for materials of natural origin and, particularly regarding colouring agents, the toxic nature of many of the synthetic dyes is becoming widely recognized. A considerable number of the additives used in standard medical practice are covered by the monographs of national pharmacopoeias which give standards for purity etc. Others, not so covered, and used in herbal preparations, may be included in the EU list. In some instances e.g. Raspberry Syrup BP 1988 and Cherry Syrup the preparation may have the dual role of colourant and flavouring. Also, as in the case of some flavouring and emulsifying agents, there may be an overlap with medicinal action. Thus oils of clove, and peppermint are used as flavours but the former has antibacterial, and the latter, carminative properties. Similarly, natural gums which are widely used as thickening, emulsifying and suspending agents have, in larger doses, a therapeutic action.
COLOURING AGENTS
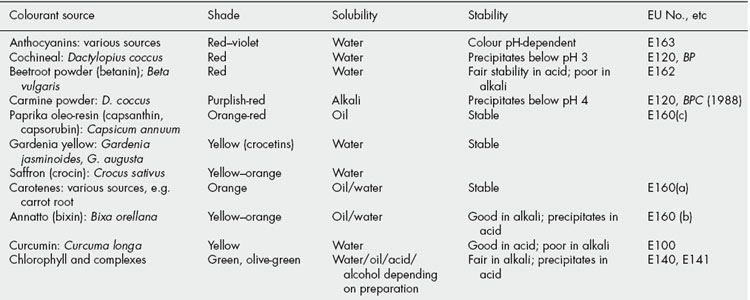
The essential subsidiary requirements of a medicinal colourant are nontoxicity and stability. Specific factors to be considered are the effect of pH on colour (many natural pigments are pH indicators), solubility in water and oils, and stability to light, heat and sugars. Table 33.1 lists a range of some of the more important natural colourants used in food and medicinals and Fig. 33.1 shows the chemical structures.
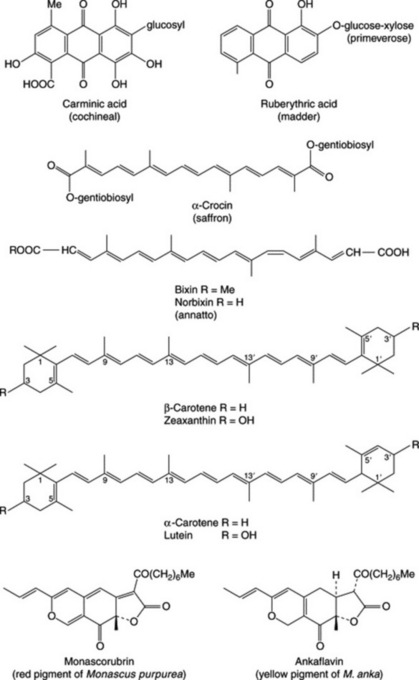
Fig. 33.1 Chemical structures of some natural pigments of pharmaceutical significance (for anthocyanidins see Table 21.6).
For a report covering the legal aspects appropriate to Europe and Japan, see ‘Further reading’ (Henry 2000).
RED POPPY PETALS
Red poppy petals of the BP/EP consist of the dried whole or fragmented petals of Papaver rhoeas L. (field poppy, corn poppy) family Papaveraceae. The annual plant is found throughout Europe apart from the far north, N. Africa, temperate Asia and by introduction in N. America, Australia and New Zealand. Once a colourful sight as a weed in cornfields but now, due to the use of selective weed-killers, largely confined in its habit to waste areas and disturbed ground.
When harvested, the petals are a bright scarlet in colour with a dark violet claw and a smooth and shiny upper surface. The dried commercial product is dingy violet, crumpled or broken and often in clumps. Each petal is broadly ovate, about 6 cm long with an entire margin and veins arising from the base and anastomozing just below the margin.
Microscopy of the powder shows sinuously walled epidermal cells, small anomocytic stomata, vascular vibrous tissue, the remains of anthers and pollen grains about 30 μm in diameter with three pores.
The taste is mucilaginous and slightly bitter.
The colour of red poppy petals is due to anthocyanidins, including the gentiobioside of cyanidin (mecocyanin; see Table 21.6). On treatment with acid the drug becomes scarlet, whereas alkalis turn it a greenish-blue. The colour and blotching of the petals is variable and the BP/EP specifies a colouring capacity of not less than 0.6 when determined by absorbance measurements on an acid ethanolic extract at 525 nm.
Alkaloids with little toxicity (e.g. rhoeadine) and mucilage are also present. For a report on the isolation of two new depsides, (esters composed of two phenolic acids) and other known compounds see M. Hillenbrand et al., Planta Med., 2004, 70, 380.
Red poppy petals were traditionally employed as an anodyne and expectorant but are now used principally as a colouring for infusions and syrups.
COCHINEAL
Cochineal is the dried female insect Dactylopius coccus Costa (Coccus cacti Linné) (order Hemiptera), containing eggs and larvae. Cochineal insects are indigenous to Central America. Commercial supplies are derived principally from Peru (85%) amounting in 1998 to 6.99 × 105 kg; other producers are the Canary Islands, Chile, Bolivia and Mexico.
History
Cochineal was used by the Greeks and Romans and was an important dye in 15th century England. It was derived from the swollen females of two scale insects Kermes vermilio and K. ilicis close relatives of D. coccus. These species use as host the kermes oak, Quercus coccifera, a native of the Mediterranean coast (for further details see J. Compton, The Garden, 1990, 115, 385).
Culture and life history
Each year eggs from the previous crop, which are protected during the rainy season by shelters placed over the plants, are ‘sown’ on the cacti (usually species of Opuntia) on which it is intended to breed. Both male and female insects emerge. The males are about 1 mm long and possess wings, while the females are about 2 mm long and without wings. After fertilization the females attach themselves to the cacti by means of their probosces, which become embedded in the tissues of the plant; the males then die. The females swell to about twice their former size, owing to the presence of developing larvae, and develop red colouring matter. The larvae mature in about 14 days and escape from the now dead body of the parent. Only a small proportion of the larvae develop into males. For the next fortnight the young females crawl about the plant and the males fly. The sequence of events described above is then repeated. The life cycle thus takes about 6 weeks and three to five generations of the insects may be produced in a season.
Collection and preparation
The insects are brushed from the plants with small brooms and killed, a certain number being left to provide for subsequent crops. The first crop of the season usually contains the most colouring matter. The insects are killed by plunging in boiling water, by stove heat or by exposure to the fumes of burning sulphur or charcoal. If heat is used, the insects change to a purplish-black colour and are known as ‘black grain’, while the fume-killed purplish-grey ones are known as ‘silver grain’. Small immature insects and larvae which can be separated by sieves are sold as ‘granilla’ or siftings.
Characters
Cochineal insects are 3.5–5.5 mm long and somewhat oval in outline. The convex dorsal surface shows from nine to 11 segments, but there are no constrictions between head, thorax and abdomen. The insect has a pair of seven-jointed antennae and three pairs of very inconspicuous legs. The surface bears tubular glands which secrete wax, the melting of which by heat accounts for the difference in colour between the silver grain and black grain varieties.
Cochineal should be examined microscopically after removing the colouring matter by means of solution of ammonia. Within each insect will be found from 60 to 450 eggs and larvae. For illustrations, see previous editions of this book.
Constituents
Cochineal contains about 10% of carminic acid, (Fig. 33.1), a brilliant purple, water-soluble colouring matter; it is a C-glycoside, anthraquinone derivative. The insects also contain about 10% of fat and 2% of wax. Recent research has shown that irradiation, even at the lowest level tested (1 KGy), is effective in eliminating the microbial count and has no significant effect on the stability of the pigment. The BP describes a test of absence of salmonellae andEscherichia coli and a colour value test in which the extinction of a diluted extract of pH 8.0 is measured at 530 nm.
Carmine, an aluminium lake, is prepared by precipitation by adding aluminium and calcium ions to an extract of cochineal; it contains about 50% of carminic acid. ‘Carmines’ are produced which vary enormously in shades and tinting strengths.
Adulteration
The weight of cochineal may be increased by ‘dressing’ it with inorganic matter, the colour of which is chosen so as to blend with the variety of insect being adulterated. If genuine, no insoluble matter should separate when the insects are placed in water and the ash should not exceed 7%.
Saffron
Saffron consists of the dried stigmas and tops of the styles of Crocus sativus (Iridaceae). The drug is prepared in Spain (70% of world supply); other producers are China, Iran and Kashmir. It is included in the EP. Saffron was prized by the ancients and was cultivated in Greece, Asia Minor and Persia. Cultivation of the plant in Spain appears to date from the tenth century and in England from the fourteenth century. In 1728 quite large quantities of English saffron were being grown, particularly in the area between Saffron Walden and Cambridge.
The corms are planted in July or August in soil carefully prepared during the previous autumn. The first flowering takes place in September or October of the following year, after which each corm replaces itself by one or more daughter corms. After three harvests of flowers, the corms, which have at least doubled in number, are dug up in May or June. The best of these are reserved for planting in fresh ground in July or August. Saffron culture is labour-intensive. Collection is very much family-orientated. The flowers are gathered in the early morning, placed in baskets or hampers and conveyed to the picking house. The picker takes each flower in turn in the left hand and breaks the style just below the stigmas with the nail of the right thumb. The detached stigmas are dried by artificial heat, usually charcoal stoves, over which they are placed in hair sieves. After about 30–45 min the drug is cooled and stored in a dry place. About 90 000–100 000 flowers give 5000 g of fresh stigmas or about 1000 g of the dried drug.
Saffron or hay-saffron, as it is often called, occurs in loose masses consisting of reddish-brown stigmas among which yellowish pieces, the tops of the styles, can usually be seen. It has a sweetish aromatic odour and a bitter taste. When chewed the saliva is coloured orange–yellow. If the soaked drug is examined under a lens or microscope, the stigmas will be found either separate or united in threes to the apex of the yellowish styles. Each stigma is about 25 mm long and has the shape of a slender funnel, the rim of which is dentate or fimbricate.
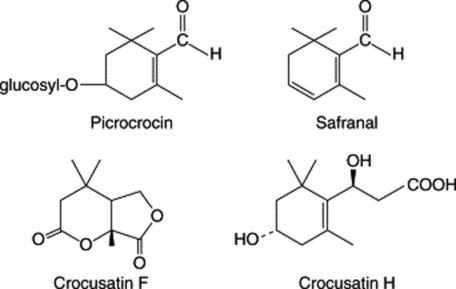
Saffron contains a number of carotenoid pigments. A hypothetical protocrocin of the fresh plant is decomposed on drying into one molecule of crocin (a coloured glycoside) (Fig. 33.1) and two molecules of picrocrocin (a colourless bitter glycoside). Crocin on hydrolysis yields gentiobiose and crocetin, while picrocrocin yields glucose and safranal. The latter substance is largely responsible for the characteristic odour and together with picrocrocin the taste of saffron. Other related crocins (crocin-2, -3 and -4) have been described. Five new monoterpenoids, crocusatins A–E, have been obtained from the pollen and a further five, crocusatins F–I, reported from the stigmas (C.-Y. Li and T.-S. Wu, Chem. Pharm. Bull., 2002, 50, 1305; J. Nat. Prod., 2002, 65, 1452). Crocusatin H and crocins 1 and 3 were shown to have significant tyrinase inhibitory activity. The same authors (J. Nat. Prod., 2004, 67, 437) have subsequently described further monoterpenoids, crocusatins J–L, a new naturally occurring acid and 31 known compounds from a methanolic extract of the petals of C. sativa. The essential oil from the stigmas and petals contains 34 or more components, mainly terpenes, terpene alcohols and esters.
By the culture of C. sativus stigmas on suitable media, stigma-like and style-like tissues which contain crocin, picrocrocin and other pigments have been obtained. Callus cultures at pH 7.0–7.6 with added uridine-diphospho-glucose are able to transform all-trans-crocetin into its related glycosides (D. Dufresne et al., Planta Medica, 1997, 63, 150). An antioxidant, 3,8-dihydroxy-1-methylanthraquinone-2-carboxylic, claimed to be superior to Vitamin E in its inhibition of oxidation of linoleic acid, has been isolated from callus stem tissue of saffron.
Although orthodox medicine has generally considered saffron to exert no appreciable therapeutic effects, recent work has demonstrated anticancer, antiarthritic, antihypertensive and other activities which probably arise from the powerful antioxidant properties of the constituents.
Saffron is used in Chinese medicine and in the West it is employed to a limited extent as a colouring and flavouring agent. In Cornwall, UK, it is used for making saffron cakes.
Annatto
Annatto seeds are those of Bixa orellana (Bixaceae) and are characterized by having on their surface an edible carotenoid pigment.
The plant is a shrub or small tree, native to northern South America and widely cultivated for the seeds or as an ornamental in the West Indies, tropical Asia and Africa; there are white- and pink-flowered varieties which can be propagated by seeds or cuttings. The estimated world annual production of seeds is 4000 tonnes; Ecuador, India, Kenya and Peru are the principal producers. Annatto usage as a colourant dye is lost in antiquity but with the advent of synthetic dyes at the beginning of the twentieth century its use declined dramatically. However, from the late 1950s, corresponding with the quest for safer food additives, its importance in the food industry has again steadily increased. The solubility of the dye in fixed oil, e.g. castor oil, makes it ideally suitable for use in the dairy industry.
Bixin, a C24-apocarotenoid (Fig. 33.1), is the principal component of the dye and it normally constitutes about 2.5% (dry wt) of the seeds although varieties containing higher proportions are being developed in Ecuador. Isolated for the first time in 1875, it was not until 1961 that its structure was fully established; it belongs to a small group of compounds which also includes crocetin (see Saffron) and abscisic acid (q.v.). Removal of the methyl ester group of bixin yields the dicarboxylic acid norbixin (Fig. 33.1) which forms the basis of the water-soluble annatto dyes. Various semi-synthetic derivatives of bixin also find use as food colourants.
Due largely to the work of A. Z. Mercadante and colleagues (Phytochemistry, 1999, 52, 135 and references cited therein) a considerable number of minor pigments of the seeds have now been characterized. These include C30 and C32 apocarotenoids; C19, C22, C24 and C25 diapocarotenoids, three of these being the first examples of geranylgeraniol serving as the esterifying alcohol with a carotenoid carboxylic acid; also isolated were a number of known C40 carotenes.
The castor-oil extract of the seeds contains, in addition to the pigments, a small amount of essential oil, the principal component of which is the sesquiterpene hydrocarbon ishwarane.
Chromatographic and spectrophotometric methods are available for the quality control of bixin.
Although used chiefly in the food industry, annatto and bixin have been employed in the production of coloured coating materials for tablets, pills, granules and herbal medicine preparations.
Marigold flowers
Tagetes erecta (Compositae), known commonly as the African marigold, is grown commercially in Mexico, Peru and Ecuador for extraction of xanthophyll pigments from the florets. There is an estimated world area of 7600 ha given over to cultivation; each plant produces on average about 330 mg of xanthophylls. With the exception of flavoxanthin [E161(a)] the xanthophylls have the same carbon skeletons as the carotenes, thus lutein (formerly known as xanthophyll) is 3, 3′-dihydroxy-α-carotene (Fig. 33.1). Lutein finds commercial use as an additive of chicken feed to give colour to egg yolks. The role of lutein in dietary supplements and its pharmacological properties have already been mentioned (Chapter 32).
Note: the common English garden marigold, not to be confused with the above, is Calendula officinalis, a well-established herbal remedy.
Red beetroot
Powdered red beetroot, Beta vulgaris (Chenopodiaceae), and the isolated red pigment betanin are widely used non-toxic food and pharmaceutical colourants. Betanin is a nitrogen-containing glycoside (Table 33.1) which on hydrolysis gives the aglycone betanidin and glucose. Hairy root cultures of the plant (Agrobacterium or Rhizobium spp. transformed) release red pigment to the culture medium, which is substantially the same as that contained within the hairy roots and in the original plant cells (M. Taya et al., J. Ferment Bioeng., 1992, 73, 31).
Additional to its value as a colourant and food, various medicinal activities have been ascribed to red beetroot, including that of a free-radical scavenger; for a report on its potential hepatoprotective value, see M. Agarwal et al., Fitoterapia, 2006, 77, 91.
Monascus
Monascus purpureus is a mould which, when rown on cooked or autoclaved rice and then the whole dried and pulverized, gives a food colourant that has long been used in Chinese cooking. There is now interest in widening the use of this pigment. Strains of mould have been selected to give various shades and that producing the dark red monascorubrin (Fig. 33.1) is particularly important. Considerable work has been carried out on the production of various pigments from chemical and u.v.-mutant strains of the mould using continuous production methods rather than batch processing. With continuous fermentation it is important that the desired product is released to the medium. New chemically defined media have been described which give an increase in the OD500 measurements and a reversal of pigment location from predominantly cell-bound to extracellular. It is envisaged that the red pigment will serve as an edible, non-toxic substitute for expensive cochineal.
For recent work on the isolation of alanine or aspartate derivatives of monascorubrin and rubropunctatin see K. Sato et al., Chem. Pharm. Bull., 1997, 45, 227.
Other species of Monascus also produce prigments and Korean workers have described strains of M. anka, developed by u.v.-mutation and natural selection, which produce enhanced levels of the pigment ankaflavin (Fig. 33.1). From the same species, a new series of pigments (monankarins A-F) having a conjugated pyrano-coumarin skeleton and exhibiting monoamine oxidate inhibitory activity has been isolated (C. F. Hossain et al., Chem. Pharm. Bull., 1996, 44, 1535.
Red rose petals
The unexpanded petals of the Provence rose, Rosa gallica, were used for preparing the acid infusions of rose of the BPC 1949. The drug is mildly astringent and for this reason, and also for the colouring principles, the infusions served as a convenient vehicle for gargles containing alum or tannin. The anthocyanine constituents made the petal extracts unsuitable for prescribing with alkaline salts.
DYESTUFFS
Natural products were at one time of prime importance to the dyeing industry and remain so today in some native societies. Three well-known examples with pharmaceutical links are alkanna, henna and madder. Alkanna and henna contain naphthoquinone derivatives and are described in more detail in Chapter 21. Madder, the root of Rubia tinctorum (Rubiaceae) formerly grown in large quantity in the area of Avignon contains anthraquinone derivatives including ruberythric acid (Fig. 33.1). On hydrolysis the latter yields primeverose and alizarin, the pigment responsible for Turkey Red colour. Towards the end of the nineteenth century the use of the natural product was superseded by synthetic material.
For an interesting article on the production of woad, one of the most ancient of dyes known to man, see P. John, ‘Further reading,’ below.
Evans WC. Annatto: a natural choice. Biologist. 2000;47(4):181-184.
John P. Indigo reduction in the woad vat: a medieval biotechnology revealed. Biologist. 2006;53(1):31-35.
Lauro GL, Francis FJ, editors. Natural food colorants. Vol 14 of a Basic Symposium Series of the Institute of Food Technologists, Chicago. Marcel Dekker, New York, 2000.
This volume includes articles on the following: Carmine, pp 1–9 (J Schul), The betalains, pp 11–30 (JH von Elbe, IL Goldman), Monascus pp 31–85 (RE Mudgett), Paprika pp 97–113 (CL Locey, JA Guzinski), Annatto pp 115–152 (LW Levy, DM Rivadeneira), Lycopene pp 153–192 (ML Nguyen, SJ Schwartz), Turmeric pp 205–226 (R Buescher, L Yang), Chlorophylls pp 227–236 (GAF Hendry), Anthocyanins pp 237–252 (RE Wrolstad), Color measurement pp 273–287 (K Loughrey), Health aspects pp 288–314 (G Mazza), Regulations in Europe and Japan pp 314–327 (BS Henry)
Negbi M, Hardman R, editors. Medicinal and aromatic plants—industrial profiles, Vol. 8. Amsterdam: L. Harwood Academic, 2000. Saffron: Corcus sativus
FLAVOURING AGENTS
Natural flavours are often complex mixtures of compounds such as are found in essential oils and may contain over 100 components, all blending to give a characteristic flavour. Alternatively, a flavouring agent may contain a single compound only, such as vanillin. The design of regulations for flavours for the food industry is obviously a complex task and the EU is currently considering this. Flavours used in medicaments are at present covered by the Medicines Act.
Although food and medicinal flavourings have aspects in common their role in a medicine is different to that in a food. In the former case they are used to disguise an unpleasant taste resulting from the active constituents of the medicine rather than, as in the latter case, to make more attractive an already palatable material. It is questionable whether medicines should be formulated to make them so pleasant to the taste that they are no longer distinguishable as such. Helliwell and Jones discussed this aspect (Pharm. J., 1994, 253, 181) in an article ‘How good should a medicine taste?’ mentioning conceptual pharmaceuticals in which a postprandial OTC medicine could be formulated as a liqueur so that the particular brand would become associated with a pleasant after-meal experience.
Certain natural flavours traditionally have been formulated as syrups for addition to the active medicament, a practice now somewhat discouraged on dental health grounds. A number have, in addition to flavour, some medicinal properties. The following, for example, are also, together with their oils, carminatives: citrus peels, ginger, peppermint leaf, fennel fruits, dill fruits, coriander fruits, caraway fruits, cardamom, nutmeg and cinnamon. Liquorice extract is used to disguise the taste of nauseous medicines and Wild Cherry Syrup BPC 1988, although employed in cough preparations, is primarily used for flavouring. Raspberry and blackcurrant syrups have no therapeutic value although the juice of the latter is used for its vitamin C content. Similarly saffron (q.v. above) and oil of rose (used to flavour lozenges) have no medicinal effect.
Sweetening agents
There is a need for alternatives to sucrose as a sweetening agent for medical purposes (e.g. for diabetics) and for diet improvement. Although saccharin is the most widely used substitute two natural products are also noteworthy.
Sorbitol
Sorbitol (D-glucitol), USP/NF 1995, is a polyhedric alcohol which was first isolated from mountain ash berries (Sorbus aucuparia, Rosaceae) and is now known to occur in other members of the family and widely throughout the plant kingdom. It is prepared synthetically by the catalytic hydrogenation of glucose. Sorbitol solution (Sorbitol Liquid) BPC 1988 contains 70% of mainly sorbitol and is used as a sweetening agent and vehicle in elixirs, linctuses and mixtures; it has about half the sweetening power of syrup.
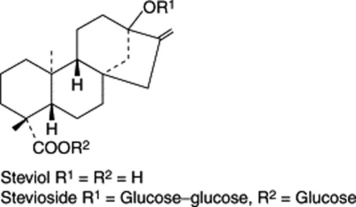
Stevioside
A group of ent-kaurane glycosides, derivatives of steviol, have sweetening properties some three hundred times that of sucrose. Stevioside, the most important, is obtained from Stevia rebaudiana (Compositae) a plant native to N.E. Paraguay. Although first isolated in 1931 its structure was not elucidated until 1963 and then some 10 years later it was produced commercially in Japan. New non-glycosidic labdane diterpenoids (sterebins) continued to be isolated (B. D. McGarvey et al., J. Nat. Prod., 2003, 66, 1395). Now, some 700–1000 tons of plant material are processed annually by Japan, Brazil and other countries. The product is used in the soft drinks and food industries. For a report on the pharmacological and physiological effects of S. rebaudiana on animals and humans see M. S. Melis, J. Ethnopharm., 1999, 67, 157.