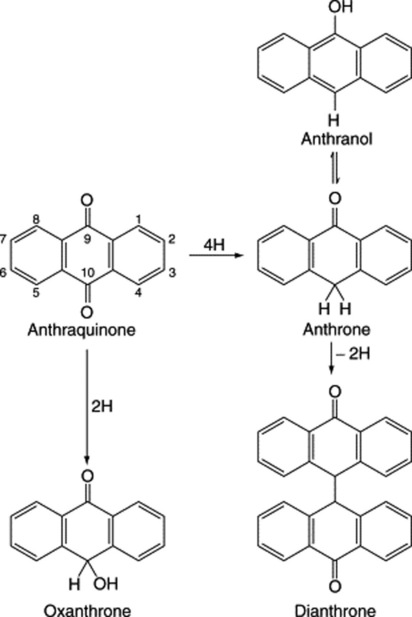
Chapter 21 Phenols and phenolic glycosides
Phenols probably constitute the largest group of plant secondary metabolites. Widespread in Nature, and to be found in most classes of natural compounds having aromatic moieties, they range from simple structures with one aromatic ring to highly complex polymeric substances such as tannins and lignins. Phenols are important constituents of some medicinal plants and in the food industry they are utilized as colouring agents, flavourings, aromatizers and antioxidants. This chapter mainly deals with those phenolic classes of pharmaceutical interest, namely: (1) simple phenolic compounds, (2) tannins, (3) coumarins and their glycosides, (4) anthraquinones and their glycosides, (5) naphthoquinones, (6) flavone and related flavonoid glycosides, (7) anthocyanidins and anthocyanins, (8) lignans and lignin. The biosynthetic origin of some of these compounds involving the shikimic acid pathway is shown in Fig. 21.2. Phenols may also have aromatic rings derived by acetate condensation (Fig. 18.9.).
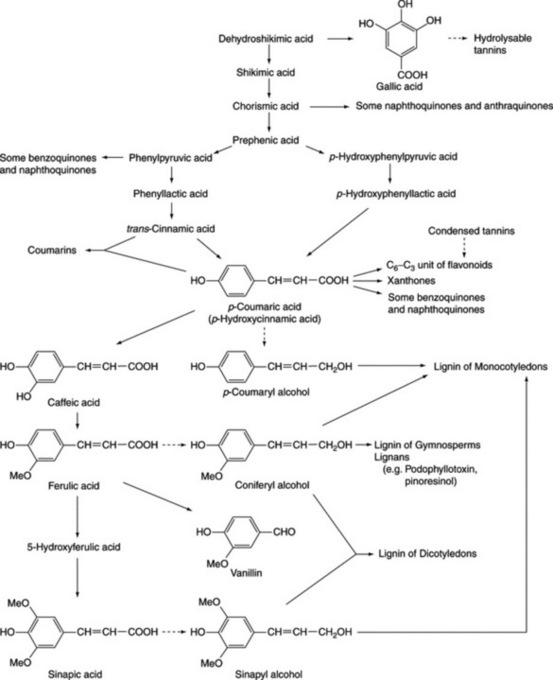
Fig. 21.2 Phenolic compounds originating from shikimic acid (see Fig. 18.8 for details of shikimic acid pathway).
SIMPLE PHENOLIC COMPOUNDS
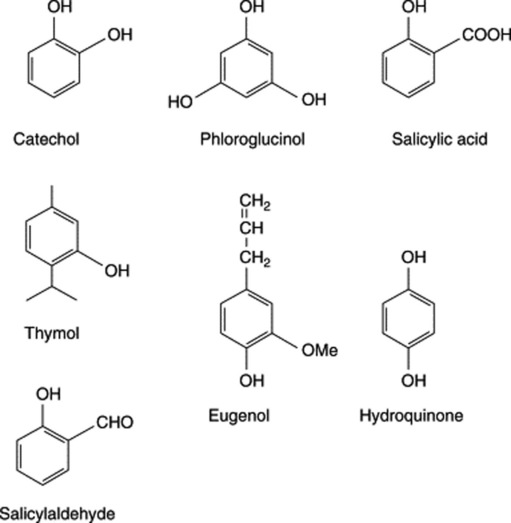
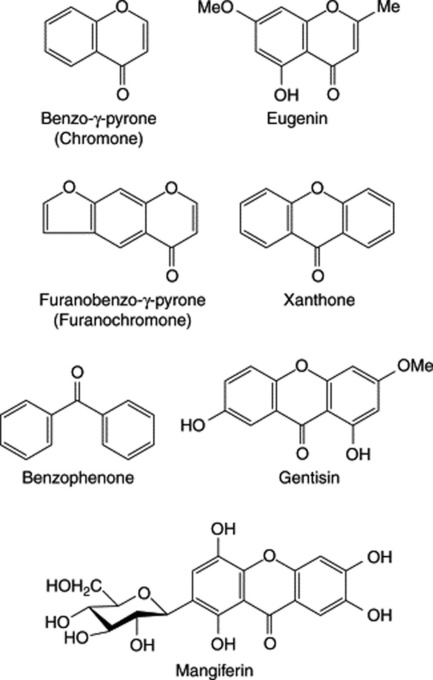
Catechol (o-dihydroxybenzene) occurs free in kola seeds and in the leaves of Gaultheria spp. and its derivatives are the urushiol phenols of the poison oak and poison ivy (q.v.). Derivatives of resorcinol (m-dihydroxybenzene) constitute the narcotic principles of cannabis and the glucoside arbutin involves quinol (hydroquinone, p-dihydroxybenzene). The taenicidal constituents of male fern, the bitter principles of hops and the lipophilic components of hypericum (q.v.) are phloroglucinol derivatives.
The phenolic compounds in this group often also possess alcoholic, aldehydic and carboxylic acid groups; they include eugenol (a phenolic phenylpropane), vanillin (a phenolic aldehyde) and various phenolic acids, such as salicylic, ferulic and caffeic acids. Glycoside formation is common, and the widely distributed glycoside coniferin and other derivatives of phenolic cinnamic alcohols are precursors of lignin. Some of the best-known simple phenolic glycosides are listed in Table 21.1.
Table 21.1 Examples of phenolic glycosides.
| Name | Examples of sources | Products of hydrolysis |
|---|---|---|
| Salicin | Salix and Populus spp. Viburnum prunifolium | Salicyl alcohol, glucose |
| Populin (benzoyl-salicin) | Populus tremula | Salicyl alcohol, benzoic acid, glucose |
| Arbutin | Ericaceae and Rosaceae | Hydroquinone, glucose |
| Phloridzin | Rosaceae, including spp. of Malus | Phloretin, glucose |
| Trilobatin | Malus, Spiraea | Phloretin, glucose |
| Coniferin | Coniferae | Coniferyl alcohol, glucose |
| Gaultherin | Gaultheria, Betula and Monotropa | Methyl salicylate, primeverose |
| Syringin | Particularly in Oleaceae | Methoxyconiferyl alcohol, glucose |
| Glucovanillin | Vanilla spp. and some Gramineae | Vanillin, glucose |
| Gein | Geum spp. | Eugenol, vicianose (glucose + arabinose) |
| Glucogallin | Rheum spp. | Gallic acid, glucose |
| Hamamelitannin | Hamamelis virginiana | Gallic acid (2 mols), hamamelose |
MEADOWSWEET
Meadowsweet BP/EP, Filipendula BHP 1983 consists of the dried flowering tops of Filipendula ulmaria (L.) Maxim. [Spirea ulmaria L.], family Rosaceae.
This well-known perennial plant is found in wet meadows, marshes, by rivers, etc. throughout most of Europe, temperate Asia and as an escape in the eastern US and Canada. It is up to 120 cm in height with numerous radical longish petioled leaves. Each leaf is composed of up to five pairs of ovate serrated leaflets. Numerous aromatic cream-coloured flowers form irregular cymose panicles, which are particularly dense on the terminal branches of the leafy stems.
The commercial chopped drug occurs as clumps of broken leaflets dark green on the upper surface, paler and tormentose on the lower. Also brown fragmented flowers, unopened flower buds and small, more or less spirally twisted fruits containing brown seeds. Angular,greenish-brown longitudinally ridged hollow stems up to 5 mm in diameter constitute a considerable portion of the drug.
Among the complex mixture of structures in the powder the following can be noted: leaves and sepals having lower epidermis with slightly sinuous anticlinal walls, anomocytic stomata and cluster crystals of calcium oxalate up to 40 μm diameter in the mesophyll; papilose epidermis of petals; pollen grains with three pores and a smooth to slightly pitted exine; numerous trichomes, occasionally glandular with a one- to three-celled stalk and multicellular head with brown contents but principally clothing trichomes of various size, often twisted together; vascular tissue of the stem and veins.
Constituents
The BP/EP requires a minimum concentration of 0.1% for the steam volatile fraction of Meadowsweet; the flowers have recorded higher values. The major component of the oil (up to ca 70%) is salicylaldehyde (Fig. 21.1) together with methyl salicylate, benzaldehyde, benzyl alcohol, and smaller amounts of other components such as vanillin. In 1839, Löwingand and Weidmann, working on meadowsweet, were the first to report salicylic acid as a natural product. Other constituents of the drug are the phenolic glycosides gaultherin (Table 21.1) and spiraein (salicyl alcohol + primerose), various flavonoids, e.g. hyperoside (Fig. 21.18), tannins and mucilage.
The pharmacopoeial TLC test for identity indicates the required presence of methyl salicylate and salicylaldehyde in the test sample. The permitted maximum for stems with a diameter greater than 5 mm is 5% and for foreign matter, 3%.
Oil of wintergreen
Natural oil of wintergreen was formerly obtained from the leaves of Gaultheria procumbens (Ericaceae), but is now distilled from the bark of Betula lenta (Betulaceae). Gaultheria oil of the Indian Pharmacopoeia is obtained from the fresh plant of Gaultheria fragrantissima and contains not less than 98% of esters calculated as methyl salicylate.
WILLOW BARK
Various species of Salix which include S. purpurea L., (purple willow) S. daphnoides Vill. and S. fragilis L. (crack willow) are sources of the official drug (BP/EP, BHP, ESCOP, Complete German Commission E). There are about 300 species of Salix showing much hybridization and unusual forms. They are distributed in all parts of the North Temperate Zone, the Arctic Zone and the South Temperate Zone. Identification can present difficulties. Species range from tall trees to tiny shrubs. The commercial drug is obtained principally from S.E. Europe but also from Britain and other European countries.
The commercial drug occurs as thin, channelled pieces of varying length, about 1.5 cm wide and 1.5 mm thick. It easily fractures longitudinally and, transversely, shows an inner inconspicuous fibrous fracture. The outer surface is brown, grey or greenish, glossy and smooth or dull and rugged; the inner surface is lightish brown and finely longitudinally striated. The powder is characterized by cork cells, parenchymatous cells containing cluster crystals of calcium oxalate and lignified fibre groups with crystal sheaths of calcium oxalate.
Willow bark is a source of salicin (Table 21.1), a phenolic glycoside now seldom used but generally regarded as the natural forerunner of aspirin. The composition of the glycoside mixture is variable in the bark depending on species, age of bark and time of collection. The latter is usually made in spring when the bark is easily removed from the branches. Other phenolic glycosides are salicortin (an ester of salicin), acetylated salicin (fragilin) and salicortin. Salicin is easy to prepare (see 15th edition of this book) and is a suitable compound with which to introduce students to this class of glycoside.
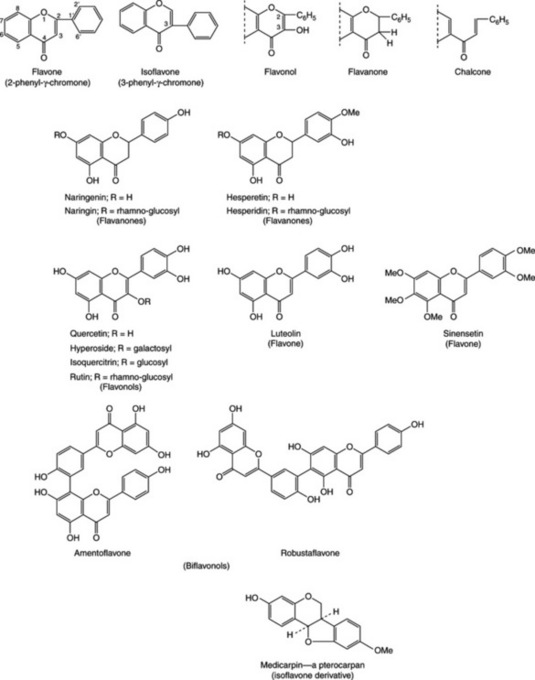
Flavonoids of the bark (to over 4%) include the 5- and 7-glucosides of naringenin, isoquercitrin and chalcone (see Fig. 21.18). Tannins are of the condensed types (q.v.).
The BP requires the dried drug to contain a minimum of 1.5% total salicylic acid derivatives, calculated as salicin. Liquid chromatography with spectrophotometric determination at 270 nm is used for the assay.
Willow is employed as an anti-inflammatory in the treatment of rheumatism, arthritis and muscular pains.
Black haw bark
The root bark of Viburnum prunifolium (Caprifoliaceae) was formerly official in most pharmacopoeias, but its use for dysmenorrhoea, threatened abortion and asthma has gradually decreased. It contains about 0.2% of salicin, volatile oil and isovaleric acid, tannin and resin.
HOPS
Hops are the dried strobiles of Humulus lupulus L. (Cannabinaceae). Only the pistillate plants are cultivated, large quantities being produced in England (particularly Kent), Germany, Belgium, France, Russia and California. The strobiles are collected, dried in kilns and pressed into bales known as ‘pockets’. They are sometimes exposed to the fumes of burning sulphur, which modifies the sulphur components already in the hops but which is said to stabilize the aroma and colour.
Hops are included in the EP, BP, BHP and in monographs of the British Herbal Compendium, ESCOP and German Commission E.
The hop strobiole consists of external and internal sessile bracts which overlap one another and enclose the ovary. Together they form a petiolate greenish-yellow inflorescence 2–5 cm in length. The odour is characteristically aromatic.
On the fruits and bases of the bracts are numerous shining glands. These, when separated, constitute the drug lupulin. The commercial product is generally very impure, owing to the fact that it is obtained by sieving the sweepings of the hop room floors. It occurs as a granular, reddish-brown powder with a characteristic odour and bitter aromatic taste.
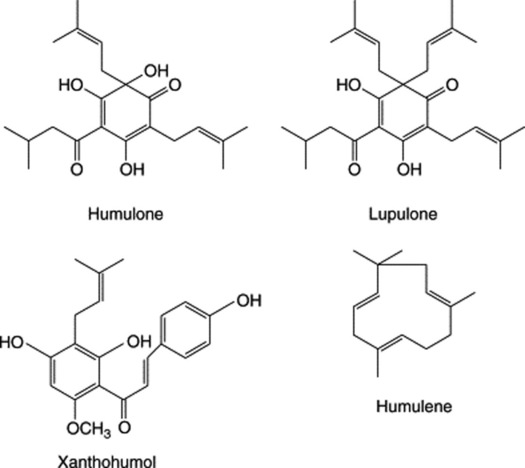
The bracts and stipules of the hop contain tannin but the odour and taste of the drug are mainly due to the very complex secretion contained in the lupulin glands. On distillation the fruits yield 0.30–1.0% of an oil composed of well over 100 components and containing terpenes, sesquiterpenes including humulene (Fig. 21.3) and compounds such as 2-methyl-but-3-ene-2-ol and 3-methylbutanoic acid. The two latter, and related substances, increase significantly during processing of the fresh hops. The bitterness is due to crystalline phloroglucinol derivatives known as α-acids (e.g. humulone), β-acids (e.g. lupulone) and also about 10% of resins. 2,3,4-Trithiapentane, S-methylthio-2-methylbutanoate, S-methylthio-4-methyl-pentanoate and 4,5-epithiocaryophyllene have been isolated from the volatile oil of unsulphurated hops.
There has been considerable recent interest in the wide-ranging biological activities of the constituents of hops. Thus prenylated compounds such as xanthohumol and the recently isolated acylphloroglucinol-glucopyranosides have been variously reported to have cytotoxic effects on human cancer cell lines together with antiproliferative, antioxidant and oestrogenic properties. For details, see L. R. Chadwick et al., J. Nat. Prod., 2004, 67, 2024; G. Bohr et al., J. Nat. Prod. 2005, 68,1545. The mildly sedative properties of hops are ascribed, in part, to 2-methyl-3-buten-2-ol; their principal use is as an aromatic bitter in the preparation of beer.
Male fern.
Male fern (Filix Mas) consists of the rhizome, frond bases and apical bud of Dryopteris filix-mas agg. (Polypodiaceae). The taxonomy of the genus is complicated and the aggregate is composed of a complex of three related species—D. filix-mas (L.) Schott. s. str., D. borrei Newm. and D. abbreviata (Lam and D.-C.) Newm. Other ferns may also be involved in extracts produced globally.
Male fern samples that have not deteriorated in activity due to long storage, etc. should have an internal green colour. The active constituents are an interesting range of phloroglucinol derivatives, which have been thoroughly investigated.
Extracts of male fern were traditionally employed as taenicides, particularly for tape worms, but safer drugs are now available and used in preference.
A full account involving history, characters, constituents and allied drugs is given in the previous edition of this book pp. 214–217.
Kamala
Kamala consists of the trichomes and glands separated from the fruits of Mallotus philippinensis (Euphorbiaceae), a tree found in India, Pakistan and the East Indies. It occurs as a dull reddish-brown powder without odour or taste. Under the microscope it is seen to consist of very characteristic globular glands containing red resin, and radiating groups of unicellular curved trichomes. It contains the anthelminthic phloroglucinol derivatives rottlerin and isorottlerin, resins and wax. It is used in India for the treatment of tapeworm infestation; also for treating poultry.
Tar (Pix Liquida)
Wood tar is known in commerce as Stockholm tar. It is prepared by the destructive distillation of various trees of the family Pinaceae. In addition to the tar, an aqueous distillate is obtained from which acetic acid, methyl alcohol and acetone are prepared. A residue of wood charcoal remains in the retorts. Wood tar is a blackish semiliquid with a characteristic odour and taste.
The constituents include the following phenols and phenolic ethers: phenol, C6H5OH; cresols, C6H4(CH3)OH; methyl cresols; catechol or pyrocatechin, C6H4(OH)2; guaiacol (methyl catechol) and its homologues. Also the hydrocarbons benzene, toluene (methylbenzene), xylenes (dimethylbenzenes), mesitylene and pseudocumene (trimethylbenzenes), styrene (phenylethylene), naphthalene (C10H8), retene (m-methylisopropylphenanthrene), chrysene (C18H12) and paraffins.
Pine tar is characterized by the large amount of guaiacol and its homologues which are present. Other tars, such as those of the birch and beech, show considerable differences in composition. Wood tar is acid in reaction, whereas coal tar, which is also official, is alkaline and in light petroleum gives a blue fluorescence. Creosote is obtained from wood tar by distillation. Tar is mainly used externally, in the form of ointment or tar parogen, as a stimulating antiseptic in certain skin diseases.
Wood tar, when shaken with water, gives an aqueous layer that is acid to litmus (cf. coal tar below) (BP test for identity).
COAL TAR
Coal tar is prepared by the destructive distillation of bituminous coal; it is a nearly black viscous liquid and when shaken with water gives an aqueous alkaline solution. A petroleum spirit extract has a blue fluorescence enhanced by UV light. The upper ash limit for the BP product is 2.0%.
Both coal tar and wood tar are used in the treatment of psoriasis.
VANILLA AND VANILLIN
Vanilla (Vanilla Pods) consists of the carefully cured fully grown but unripe fruits of Vanilla fragrans (Salis.) Ames (syn. V. planifolia Andrews) (Orchidaceae) (Mexican or Bourbon vanilla) and of V. tahitensis (Tahiti vanilla). The fruits of other species, such as V. pompona (West Indian vanilla), are also used but to a much more limited extent.
Vanilla fragrans is grown, in a semi-wild state, in the woods of eastern Mexico, its natural home. Vanilla is cultivated in Réunion (or Bourbon), Mauritius, Seychelles, Madagascar, Java, Ceylon, Tahiti, Guadeloupe, Martinique and Indonesia. China and India are now major producers and due to oversupply prices have fallen dramatically over the past few years.
History
Vanilla was found in Mexico by the Spaniards, where it was used for flavouring chocolate, a use to which it is still put. It found a place in the London Pharmacopoeia of 1721.
Cultivation
Vanilla requires a warm and fairly moist climate. Propagation is simple: cuttings 1–3 m long are attached to trees (e.g. Casuarina equisetifolia), where they soon strike roots on the bark. The plant is an epiphyte. It flowers at the end of 2 or 3 years and continues to produce fruit for 30–40 years. The flowers are usually pollinated by women and children, a pointed stick being introduced into one flower after another. Clonal propagation of the vanilla plant has been described together with in vitro multiplication using axillary bud explants (P. S. George and G. A. Ravishankar, Plant Cell Rep., 1997, 16, 490).
Collection and curing
The fruits are collected when the upper part of the pod changes in colour from green to yellow. The characteristic colour and odour of the commercial drug are only developed as a result of enzyme action during the curing. The details of the latter process vary somewhat in different countries, but frequently it consists of slow drying in sheds which are kept at carefully regulated temperatures.
Packing and grading
Before grading, any pods showing a tendency to mould are picked out. The remainder are sorted to size and packed in bundles of 50 pods. Traditionally, these were packed in tin cases or boxes holding about 10–12 kg, soldered up and packed in wooden cases. On arrival in London the tins were opened and the pods were examined. UK supplies now arrive via France or Germany, with some from Madagascar. During storage crystals frequently develop on the surface of the pods.
Characters
Vanilla pods are 15–25 cm long, 8–10 mm diameter and somewhat flattened. The surface is longitudinally wrinkled, dark brown to violet-black in colour, and frequently covered with needle-like crystals of vanillin (‘frosted’). The fruits are very pliable and have a very characteristic odour and taste.
Constituents
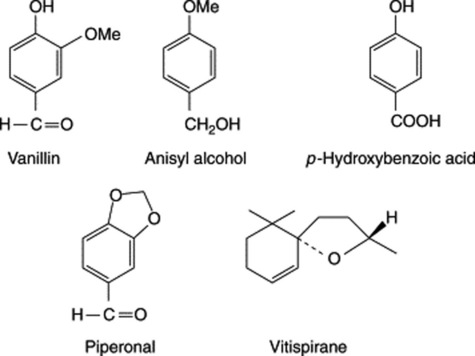
Green vanilla contains glycosides, namely glucovanillin (vanilloside) and glucovanillic alcohol. During the curing these are acted upon by an oxidizing and a hydrolysing enzyme which occur in all parts of the plant. Glucovanillic alcohol yields on hydrolysis glucose and vanillic alcohol; the latter compound is then by oxidation converted into vanillic aldehyde (vanillin). Glucovanillin, as its name implies, yields on hydrolysis glucose and vanillin (Fig. 21.4).
The three species given above differ in their relative contents of anisyl alcohol, anisaldehyde (also anisyl ethers and anisic acid esters), piperonal and p-hydroxybenzoic acid. These minor components, together with the two diastereoisomeric vitispiranes, add to the flavour of the pods.
Vanillin BP/EP
Vanillin BP is the aldehyde corresponding to methyl-protocatechuic acid and has been synthesized in a number of ways. Large quantities of it are prepared from eugenol isolated from oil of cloves (q.v.) or from guaiacol (methyl catechol). It can also be produced by microbial oxidation of eugenol. In the plant glucovanillin is biosynthesized via ferulic acid (see Fig. 21.2). Synthesis begins when elongation of the fruit ceases, which is about 8 months after pollination; before this, other phenolic glycosides predominate.
Adulteration
Extracts of Mexican origin may be adulterated by coumarin, probably arising from the use of tonka beans (q.v.). A capillary GC assay has been described for such products (see R. J. Marles et al., Economic Bot., 1987, 41, 41).
Uses
Vanilla pods are widely used in confectionery and in perfumery. They have been replaced to some extent, but by no means completely, by synthetic vanillin. About 0.07 parts of vanillin are approximately equivalent to 1 part of the bean, but an essence so prepared fails to represent the odour and flavour of the whole pods.
For a review of natural vanillin, covering biosynthesis, biotechnological production, cell and organ culture and metabolic engineering, see N. J. Walton et al., Phytochemistry, 2003, 63, 505–515.
BEARBERRY LEAVES
Bearberry leaf EP/BP/BHP consists of the dried leaves of Arctostaphylos uva-ursi, Ericaceae; ESCOP and German Commission E monographs on the drug are also available.
A. uva-ursi is a small evergreen shrub found in central and northern Europe and in North America. The leaves are dark green to brownish-green, 2–3 cm long, obovate or spathulate, gradually narrowing to a very short petiole, apex obtuse or retuse. They are coriaceous in texture and almost glabrous. The upper surface is shiny and marked with sunken veinlets; the lower surface is lighter and marked with a network of dark veinlets. The drug is odourless but has an astringent and somewhat bitter taste.
Microscopical features include: an upper epidermis of polygonal cells with a thick cuticle; lower epidermis with anomocytic stomata and surrounded by 5–11 subsidiary cells; scars of trichome bases, occasional conical trichomes, crystal fibres.
Bearberry contains the glycosides arbutin (Table 21.1) and methylarbutin, about 6–7% of tannin, (+)-catechol, ursone and the flavone derivative quercetin. Some 14 phenolic acid constituents, including gallic and ellagic acids, have been recorded.
The pharmacopoeial drug is required to contain at least 7.0% of hydroquinone derivatives calculated as arbutin. These are assayed by liquid chromatography of an aqueous extract of the leaves with arbutin as a reference and absorbance measurement at 280 nm. The official TLC chromatographic test for identity distinguishes arbutin, gallic acid and hydroquinone. Bearberry is diuretic and astringent and during excretion it exerts an antiseptic action on the urinary tract.
Propolis or bee glue
This is the material with which the honey bee seals cracks and crevices, and varnishes surfaces within the hive. Its composition varies according to geographical source. It is collected by worker bees from the leaf buds and is enriched by wounded plant exudates such as mucilages, gums and resins; bee secretions and enzymes are then mixed in. Like honey, the composition varies according to geographical source.
Propolis has a long history, it being used by the Egyptians in the embalming process (antiputrefactive), by the Greeks and Romans in wound treatment (antiseptic), by the Incas (antipyretic) and by inclusion in the London pharmacopoeias of the 17th century. Today it is used by medical herbalists and has become a popular medicament (S. Castaldo and F. Capasso, Fitoterapia, 2002, 73, S1). It also features in apitherapy—an old tradition that has experienced a recent revival.
Over 160 compounds have been shown to be involved and one analysis gave phenolics (58%), beeswax (24%), flavonoids (6%), terpenes (0.5%), lipids and wax (8%) and bioelements, e.g. Mn, Cu, Zn (0.5%). In temperate regions of Europe the resinous coating of poplar buds (Populus nigra, P. italica, P. tremula) forms a major collection source for the bees and the natural phenolic content of the resin, e.g. esters of caffeic and ferulic acids, vanillin, eugenol, flavonoids, etc., can be used to identify the natural source.
Latterly there have been numerous reports concerning the analysis and biological activity of propolis originating from various regions and especially from Latin American countries. In these areas species of Araucaria (Araucariaceae), Baccharis (Compositae) and Clusia (Guttiferae) have been established as biological sources. In addition to the constituents listed previously, prenylated cinnamic acid and chromane derivatives, diterpenoid acids, lignans and components of the volatile oil have been identified.
Notwithstanding the differences in chemical composition of propolis depending on geographical source, a pronounced antibacterial property is common to all. In temperate regions flavonoid and phenolic esters have been shown to exert bacterial activity. New polyisoprenylated benzophenones have recently been reported as antibacterial agents in propolis of Venezuelan origin (B. Trusheva et al., Fitoterapia, 2004, 75, 683), and similar compounds (propolones) have been found in that of Cuban origin together with garcinelliptone and hyperibone (I. M. Hernández et al., J. Nat. Prod., 2005, 68, 931). Neoflavonoids with anti-nitric oxide production activity occur in propolis from Nepal (S. Awale et al., J. Nat. Prod., 2005, 68, 858).
Readers requiring further information on this interesting substance can refer to the references on p. 219 in the 15th edition of this book, and to Fitoterapia, Supplement 1, 2002, 73, S1–S64, devoted entirely to propolis; V. Bankova, J. Ethnopharmacol., 2005, 100, 114; Y. Lu et al., Fitoterapia, 2004, 75, 267.
CAPSICUM
The BP/EP drug (Chillies; Red Peppers) consists of the dried, ripe fruits of Capsicum annuum var. minimum (Miller) Heiser, and small-fruited varieties of C. frutescens L. (Solanaceae). In commerce the description given applies to various African commercial varieties (principally from Zimbabwe and Malawi) and these are sold in England as chillies, while the larger but less pungent Bombay and Natal fruits are known as capsicums. Very large Capsicum fruits, resembling tomatoes in texture and practically non-pungent, are widely grown in southern Europe as vegetables.
History
Capsicums appear to be of American origin and were referred to in 1494 by Chanca, a physician who accompanied Columbus on his second voyage to the West Indies. The plants were introduced into India at a very early date, possibly by the Portuguese. ‘Ginnie Pepper’ was well known in England in 1597 and was grown by Gerarde.
Macroscopical characters
African Chillies are oblong-conical in shape, 12–25 mm long and up to 7 mm wide. The five-toothed calyx and straight pedicel are together about 20–30 mm long. The pericarp is glabrous, shrivelled and orange-red; the Sierra Leone and Zambian chillies usually have a better colour than those from Zanzibar.
Internally the fruits are divided into two cells by a membranous dissepiment to which the seeds were originally attached. The latter, usually about 10–20 in each fruit, are of a flattened reniform shape and are about 3–4 mm long. Like other solanaceous seeds, they have a coiled embryo and oily endosperm. African chillies are very sternutatory and have an intensely pungent taste.
Constituents
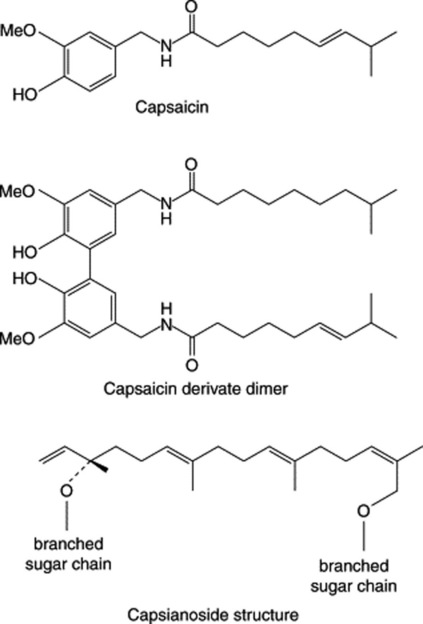
In 1876, Thresh extracted the drug with petroleum, treated the extract with aqueous alkali, and by passing carbon dioxide through the alkaline liquid precipitated crystals of an intensely pungent compound, capsaicin. As may be inferred from the method of preparation, capsaicin is of phenolic nature.
The pungent phenolic fraction of capsicum also contains a proportion of 6,7-dihydrocapsaicin. The capsaicin content of fruits varies appreciably in a range up to 1.5% and is much influenced by environmental conditions and age of the fruit. It occurs principally in the dissepiments of the fruits—for example, entire fruit 0.49, pericarp 0.10, dissepiment 1.79, seed 0.07. The pungency of capsicum is not destroyed by treatment with alkalis (distinction from gingerol, which also contains the vanillyl group) but is destroyed by oxidation with potassium dichromate or permanganate. Chillies also contain ascorbic acid (0.1–0.5%), thiamine, red carotenoids such as capsanthin and capsorubin (see ‘Carotenoids’) and fixed oil (about 4–16%). They yield about 20–25% of alcoholic extract (capsicin) and about 5% (official limit 10.0%) of ash. Hungarian capsicums or ‘Paprika’ are derived from a mild race of C. annuum and are a convenient source of ascorbic acid. According to Bennett and Kirby, the pungent principle of C. annuum is composed of capsaicin 69%, dihydrocapsaicin 22%, nordihydrocapsaicin 7%, homocapsaicin (C11 acid) 1% and homodihydrocapsaicin 1%. A number of minor components of this class have been recorded.
In a study of the water-soluble constituents of the fruits of three varieties of C. annuum, Izumitani et al. (Chem. Pharm. Bull., 1990, 38, 1299) isolated twelve novel acyclic glycosides (geranyllinalool derivatives) named capsianosides A–F (dimeric esters of acyclic diterpene glycosides) and capsianosides I–V (monomeric compounds of acyclic diterpene glycosides). Further capsianosides have now been reported by J.-H. Lee et al., (Chem. Pharm. Bull., 2006, 54, 1365). T. Ochi et al., (J. Nat. Prod., 2003, 66, 1094) record a dimeric capsaicin having almost the same antioxidant activity as capsaicin but with no pungent taste (Fig. 21.5).
Biogenesis of capsaicin
Work by Leete and Louden on C. frutescens and by Bennett and Kirby on C. annuum demonstrated that phenylalanine is incorporated into the C6–C1 vanillyl unit of capsaicin, the C-3 of phenylalanine giving the methylene group of the vanillylamine residues; the incorporation probably proceeds via cinnamic, p-coumaric, caffeic and protocatechuic acids. Tyrosine did not appear to be a probable precursor. Leete’s feeding experiments with [U-14C]-valine gave incorporations consistent with the hypothesis that the C10 isodecanoic acid is formed from isobutyryl coenzyme A and three acetate units. More recent work showed that the homo derivatives (C11 acid) are formed from leucine and isoleucine.
The ontogenetic formation of capsaicinoids in the fruits of C. frutescens involves a prior active accumulation of p-coumaroyl, caffeoyl and 3,4-dimethoxycinnamoyl glycosides, 3-O-rhamnosylquercetin and 7-O-glucosylluteolin. When the fruit ceases to increase in length the amount of these compounds falls and capsaicinoid synthesis commences together with that of the glycosides of vanillic acid and p-hydroxybenzaldehyde (see N. Sukrasno and M. M. Yeoman, Phytochemistry, 1993, 32, 839).
Cell cultures
The biogenetic potential for capsaicin production is reported (1991) as 10 times greater in immobilized cell cultures (alginate entrapment) than in control suspension cultures.
Tests and standards
The official TLC test for identity establishes the presence of capsaicin and dihydrocapsaicin in the sample. The synthetic equivalent of capsaicin, nonivamide (pelargonyl vanillylamide), a commercial product used as a flavour in the food industry and in medicine as a topical rubifacient, is limited by a liquid chromatographic assay to a maximum of 5% of the total capsaicinoid content. Liquid chromatography is also used to determine the total capsaicinoid content (minimum 0.4%). A number of colorimetric assays can be used for the quantitative determination of capsaicin (see Table 16.5); the BPC 1973 utilized ultraviolet absorption at 248 and 296 nm for the ointment and oleoresin. Foreign matter should not exceed a maximum of 2%; fruits of C. annuum L. var. longum (Sendtn.) (see ‘Bombay capsicums’ below) should be absent.
Allied drugs
Japanese Chillies are probably derived from C. frutescens and are about 3–4 cm long. They possess about one-quarter of the pungency of the African Chillies, but are now no longer commercially relevant.
Bombay Capsicums are ascribed to C. annuum L. The pericarp is thicker and tougher than in the chillies, and the pedicel is frequently bent. They are much less pungent than African chillies.
Natal Capsicums are larger than the Bombay variety, being up to 8 cm long. They have a very bright red, transparent pericarp. They are much less pungent than chillies.
Uses
Capsicums are used as a condiment under the name of Cayenne pepper. The drug is given internally in atonic dyspepsia and flatulence. It is used externally as a counter-irritant, in the form of ointment, plaster, medicated wool, etc., for the relief of rheumatism, lumbago, etc. Capsaicin creams are available for the relief of pain in osteoarthritis, post-herpetic neuralgia and painful diabetic neuropathy (Pharm. J., 1998, 260, 692).
TANNINS
The term ‘tannin’ was first applied by Seguin in 1796 to denote substances present in plant extracts which were able to combine with protein of animal hides, prevent their putrefaction and convert them into leather. On this basis a tannin is a substance which is detected qualitatively by a tanning test (the goldbeater’s skin test) and is determined quantitatively by its adsorption on standard hide powder. This definition excludes simpler phenolic substances, often present with tannins, such as gallic acid, catechins and chlorogenic acid, although they may under certain conditions give precipitates with gelatin and be partly retained by hide powder. Such substances of relatively low molecular weight are called ‘pseudo-tannins’. Most true tannins have molecular weights of from about 1000 to 5000. To be effective for tannage the polyphenol molecule most be neither so large as to be unable to enter the interstices between the collagen fibrils of the animal skin nor so small that it is unable to cross-link between the protein molecules of adjacent fibrils at several points. Many tannins are glycosides. The definition of a tannin as given above is an old, essentially practical one which may be purely fortuitous and, in the light of further research, could prove misleading from the point of view of plant metabolism and plant biochemistry. Indeed, modern authors often treat tannins not as a specific phytochemical group but as examples of polyphenols illustrating particular aspects of gallic acid and flavan-3-ol phytochemistry. The characteristic properties of tannins derive from the accumulation within a moderately sized molecule of a substantial number (1–2 per 100 mol. wt.) of phenolic groups many of which are associated with o-dihydroxy and o-trihydroxy orientation within a phenyl ring.
The above tannin-protein co-precipitation is important not only in the leather industry but also in relation to the physiological activity of herbal medicines, taste of foodstuffs and beverages, and in the nutritional value of feeds for herbivores. Environmental factors affecting this process have been studied by H. Kawamoto and F. Nakatsubo (Phytochemistry, 1997, 46, 479).
Two main groups of tannins are usually recognized; these are the hydrolysable tannins and the condensed tannins (proanthocyanidins).
Hydrolysable tannins
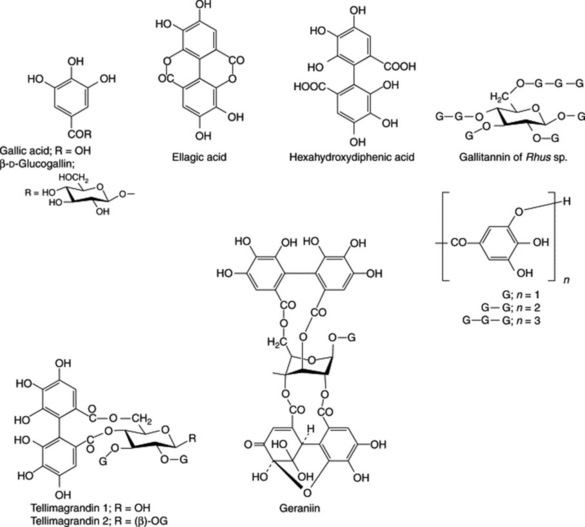
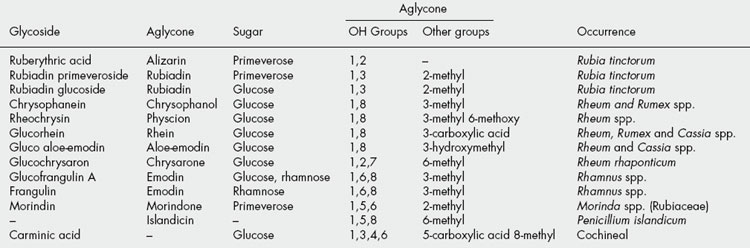
These may be hydrolysed by acids or enzymes such as tannase. They are formed from several molecules of phenolic acids such as gallic and hexahydroxydiphenic acids which are united by ester linkages to a central glucose molecule. A simple tannin illustrating this point is one derived from a species of sumac (Rhus), with a possible structure as shown in Fig. 21.6. Like gallic acid their solutions turn blue with iron salts. They were formerly known as pyrogallol tannins, because on dry distillation gallic acid and similar components are converted into pyrogallol. Two principal types of hydrolysable tannins are gallitannins and ellagitannins which are, respectively, composed of gallic acid and hexahydroxy-diphenic acid units. Ellagic acid (the depside of gallic acid) can arise by lactonization of hexahydroxydiphenic acid during chemical hydrolysis of the tannin; thus, the term ellagitannin is a misnomer.
Ellagitannins found in plants of medicinal interest, and for which structures have been elucidated include geraniin (Herb Robert and American cranesbill) and tellimagrandins 1 and 2 (Oak bark, Pomegranate and Meadowsweet); Fig. 21.6.
Modern methods of analysis have made considerable advances in the study of tannin chemistry of medicinal plants as evidenced by the work of Okuda on oriental drugs. In 1982 agromoniin, the first of a new class of oligomeric hydrolysable tannins was isolated from Agromonia. These tannins are composed of two, three or four monomeric units. Something less than 20 of these units including geraniin and tellimagrandins 1 and 2 are known to be involved in the production of over 150 compounds.
As an example, many plants of the Onagraceae e.g. Oenothera spp. contain in addition to tellimagrandin, the dimer oenothein B and trimer oenothein A; these macrocyclic ellagitannins are also produced in callus cultures of O. lacinata and are of interest for their anticancer and polygalacturonase-inhibiting properties (S. Taniguchi et al., Phytochemistry, 1998, 48, 981).
C-glucosidic ellagitannins are common in a number of families including the Myrtaceae, Hamamelidaceae, Punicaceae and Rosaceae and several have also been recorded as moieties of more than 10 oligomeric ellagitannins.
For an article on the classification of oligomeric hydrolysable tannins and the specificity of their occurrence in plants see Okuda et al., Phytochemistry, 1993, 32, 507.
In a series of enzymatic studies Gross and colleagues indicated the central position of β-D-glucogallin in the early stages of tannin synthesis in Quercus robur leaves. This compound appears to act as both donor and acceptor of the galloyl group in the enzymatic formation of 1,6-digalloyl-D-glucose; the responsible enzyme is β-glucogallin: β-glucogallin 6-O-galloyl-transferase.
The presumed immediate precursor of the two subclasses of hydrolysable tannins (gallotannins and ellagitannins) is 1,2,3,4,6-pentagalloylglucose, and in a continuation of their enzyme studies the above group have purified (×500) the enzyme responsible for the conversion of the precursor to the gallotannin 3-O-digalloyl-1,2,4,6-tetra-O-galloyl-β-D-glucose. The source of the enzyme was Rhus typhina (staghorn sumac) and its designation is β-glucogallin: 1,2,4,6-pentagalloyl-β-D-glucose galloyl-transferase (R. Niemetz and G. G. Gross, Phytochemistry, 1998, 49, 327).
Examples of drugs containing hydrolysable tannins are:
Gallitannins: rhubarb, cloves, red rose petals, bearberry leaves, Chinese galls, Turkish galls, hamamelis, chestnut and maple.
Ellagitannins: pomegranate rind, pomegranate bark, myrobalans, eucalyptus leaves, kousso, some Australian kinos, chestnut (Castanea spp.) and oak bark.
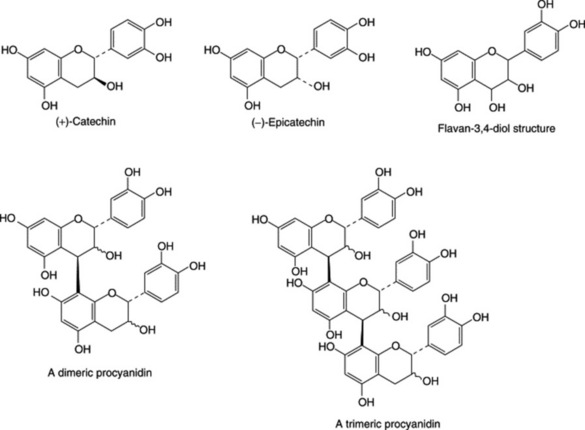
Condensed tannins (proanthocyanidins)
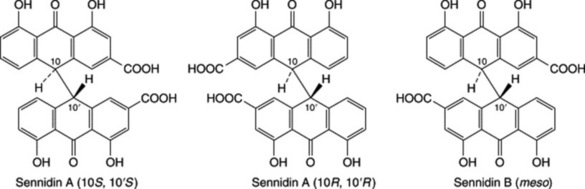
Unlike hydrolysable tannins, these are not readily hydrolysed to simpler molecules and they do not contain a sugar moiety. They are related to the flavonoid pigments and have polymeric flavan-3-ol structures. Catechins, which also occur with the tannins and flavan-3,4-diols (leucoanthocyanidins) are intermediates in the biosynthesis of the polymeric molecules. Stereochemical variations add to the variety of possible structures. Monomeric, dimeric and trimeric forms are illustrated in Fig. 21.7. Work by Japanese phytochemists has exploited modern techniques for separating and determining the structures of these oligomers and polymers including those of cassia bark, Cassia fistula, cinchona, Quercus and rhubarb.
On treatment with acids or enzymes condensed tannis are converted into red insoluble compounds known as phlobaphenes. Phlobaphenes give the characteristic red colour to many drugs such as red cinchona bark, which contain these phlobatannins and their decomposition products. On dry distillation they yield catechol and these tannins are therefore sometimes called catechol tannins. Like catechol itself, their solutions turn green with ferric chloride.
Some drugs (e.g. tea, hamamelis leaves and hamamelis bark) contain both hydrolysable and condensed tannins. The following are rich in condensed tannins:
‘Complex tannins’
This term has been applied by Okuda to a newly-discovered group of tannins which are biosynthesized from both a hydrolysable tannin (mostly a C-glucoside ellagitannin) and a condensed tannin. The union occurs through a C–C bond between the C-1 of the glucose unit of the ellagitannin and the C-8 or C-6 of the flavan-3-ol derivative. The monomers are also involved in oligomer formation.
To date, complex tannins have not great relevance to mainstream pharmacognosy; monomers have been isolated from the Combretaceae, Fagaceae (Quercus, Castanea), Myrtaceae, Polygonaceae (Rheum) and Theaceae (Camellia). It is anticipated that many more compounds of this group will be discovered.
Pseudotannins
As already mentioned, pseudotannins are compounds of lower molecular weight than true tannins and they do not respond to the goldbeater’s skin test. Examples:
Occurrence of tannins
Tannins are of wide occurrence in plants and are usually found in greatest quantity in dead or dying cells. They exert an inhibitory effect on many enzymes due to protein precipitation and, hence, they may contribute a protective function in barks and heartwoods. Commercial tannins, as used in the leather industry, are obtained from quebracho, wattle, chestnut and myrobalans trees. Pharmaceutical tannin is prepared from oak galls (q.v.) and yields glucose and gallic acid on hydrolysis; many commercial samples contain some free gallic acid.
Some plants (clove, cinnamon, etc.) contain tannin in addition to the principal therapeutic constituents. This may complicate extraction or produce incompatibilities with other drugs (many alkaloids, for example, are precipitated by tannins).
Properties and tests
Tannins are soluble in water, dilute alkalis, alcohol, glycerol and acetone, but generally only sparingly soluble in other organic solvents. Solutions precipitate heavy metals, alkaloids, glycosides and gelatin. With ferric salts, gallitannins and ellagitannins give blue-black precipitates and condensed tannins brownish-green ones. If a very dilute ferric chloride solution is gradually added to an aqueous extract of hamamelis leaves (which contains both types of tannin), a blue colour is produced which changes to olive-green as more ferric chloride is added. Other tests are the following.
In practice, these tests have to some extent been superseded by the use of TLC, particularly for the identification of crude drugs.
Medicinal and biological properties
Tannin-containing drugs will precipitate protein and have been used traditionally as stypics and internally for the protection of inflamed surfaces of mouth and throat. They act as antidiarrhoeals and have been employed as antidotes in poisoning by heavy metals, alkaloids and glycosides. In Western medicine their use declined after World War II when it was found that absorbed tannic acid can cause severe central necrosis of the liver. Recent studies have concentrated on the antitumour activity of tannins (M. Ken-ichi et al., Biol. Pharm. Bull., 1993, 16, 379) and it has been shown that, to exhibit a strong activity, ellagitannin monomer units having galloyl groups at the O-2 and O-3 positions on the glucose core(s), as in the tellimagrandins (Fig. 21.6) are required. Anti-HIV activity has also been demonstrated.
Proanthocyanidins (condensed tannins) are associated with the beneficial effects of various herbs and infusions produced from them. The antitumour activity of green and black tea has been extensively researched in recent years with positive findings. Of the components of tea, epigallocatechin-3-gallate, specifically, has been shown to prevent angiogenesis in mice. Cranberry juice has long been used for reducing bacterial infections of the bladder and these claims have now been supported by a randomized, double-blind, placebo-controlled trial carried out on 153 elderly women (J. Avorn et al., J. Amer. Med. Assoc., 1994, 271, 751). Fructose has been implicated in this activity but recently, proanthocyanidins prepared from cranberries by reverse-phase and adsorption chromatography were shown to inhibit the adherence of P-fimbriated E. coli to uroepithelial-cell surfaces; other Vaccinium spp., including blueberries had similar bioactivity, suggesting their contribution to the salutary effects in urinary tract infections (A. Howell et al., New Engl. J. Med., 1998, 339, 1085).
OAK BARK
Oak bark is the cut and dried bark from the fresh young branches of Quercus robur L. (English oak, Common oak), Q. petraea Liebl. (Sessile or Durmast oak) and Q. pubescens Willd. (Downy oak), family Fagaceae. The three species are recognized by the BP/EP and the first two by the BHP. The distribution of the species is widespread in Europe and W. Asia. Q. alba L. (White oak) is used in the USA.
The commercial bark, obtained principally from E. and S.E. Europe, occurs as channelled pieces, 3–4 mm thick and of various lengths. Younger, thinner pieces have a smooth, greyish-green cork with lenticels, older pieces have a greyish-brown rhytidome and show a fracture, granular in the outer part and fibrous and splintery in the inner part. Conspicuous features of the reddish-brown powder include cork cells, lignified fibres with crystal sheaths of calcium oxalate, pitted sclereids and cluster crystals of calcium oxalate in parenchymatous cells.
Principal constituents are phlobatannins, ellagitannins and gallic acid, a minimum of 3.0% calculated as pyrogallol [C6H3(OH)3 (1:2:3)] being specified by the BP/EP.
Oak bark is used medicinally for its astringent properties and industrially for tanning and dyeing.
GALLS AND TANNIC ACID
Turkish galls (Turkey Galls; Galla) are vegetable growths formed on the young twigs of the dyer’s oak, Quercus infectoria (Fagaceae), as a result of the deposition of the eggs of the gall-wasp Adleria gallaetinctoriae.
The dyer’s oak is a small tree or shrub about 2 m high which is found in Turkey, Syria, Persia, Cyprus and Greece. Abnormal development of vegetable tissue round the larva is due to an enzyme-containing secretion, produced by the young insect after it has emerged from the egg, which by the rapid conversion of starch into sugar stimulates cell division. As starch disappears from the neighbourhood of the insect, shrinkage occurs and a central cavity is formed in which the insect passes through the larval and pupal stages. Finally, if the galls are not previously collected and dried, the mature insect or imago bores its way out of the gall and escapes. During these changes the colour of the gall passes from a bluish-grey through olive-green to almost white.
Galls are collected by the peasants of Turkey and Syria. After drying they are graded according to colour into three grades, blue, green and white, which are found on the London market.
History
Galls were well known to the ancient writers and Pliny records the use of their infusion as a test for sulphate of iron in verdigris, possibly the earliest mention of an attempt to detect adulteration by chemical means.
Characters
Aleppo galls are globular in shape and from 10 to 25 mm in diameter. They have a short, basal stalk and numerous rounded projections on the surface. Galls are hard and heavy, usually sinking in water. The so-called ‘blue’ variety are actually of a grey or brownish-grey colour. These, and to a lesser extent the olive-green ‘green’ galls, are preferred to the ‘white’ variety, in which the tannin is said to have been partly decomposed. White galls also differ from the other grades in having a circular tunnel through which the insect has emerged. Galls without the opening have insect remains in the small central cavity. Galls have a very astringent taste.
Sections through a gall show a very large outer zone of thin-walled parenchyma, a ring of sclerenchymatous cells, and a small, inner zone of rather thick-walled parenchyma surrounding the central cavity. The parenchymatous tissues contain abundant starch, masses of tannin, rosettes and prisms of calcium oxalate, and the rounded so-called ‘lignin bodies’, which give a red colour with phloroglucinol and hydrochloric acid.
Constituents
Galls contain 50–70% of the tannin known as gallotannic acid (Tannic Acid BP/EP); this is a complex mixture of phenolic acid glycosides varying greatly in composition. It is prepared by fermenting the galls and extracting with water-saturated ether. Galls also contain gallic acid (about 2–4%), ellagic acid, sitosterol, methyl betulate, methyl oleanolate, starch and calcium oxalate. Two new compounds, derivatives of ellagic acid and pentahydroxynaphthalene, isolated from the alcoholic extract of galls have been shown to have nitricoxide- and superoxide-inhibiting activity (H. Hamid et al., Pharm. Biol., 2005, 43, 317). Nyctanthic, roburic and syringic acids have more recently been identified and syringic acid has been identified as the CNS-active component of the methanolic extract of galls. (For the isolation of flavonoids of oak galls see M. Ahmed et al., Fitoterapia, 1991, 62, 283.)
Tannic acid is a hydrolysable tannin (see above) yielding gallic acid and glucose and having the minimum complexity of pentadigalloyl glucose. Solutions of tannic acid tend to decompose on keeping with formation of gallic acid, a substance which is also found in many commercial samples of tannic acid. It may be detected by the pink colour produced on the addition of a 5% solution of potassium cyanide.
Allied drugs
Many different kinds of galls are known. They are generally produced on plants, but sometimes on animals. In addition to the large number produced by insects, particularly of the genera Cynips and Aphis, some are produced by fungi.
Chinese and Japanese galls are of considerable commercial importance. They are produced by an aphis, Schlectendalia chinensis, on the petioles of the leaves of Rhus chinensis (Anacardiaceae). These galls, which the Chinese call ‘wu-pei-tzu’, meaning ‘five knots’, are irregular in shape and partly covered with a grey, velvety down, the removal of which discloses a reddish-brown surface. They break easily and show a large, irregular cavity containing insect remains. They contain 57–77% of tannin and have been valued in China as astringents and styptics for at least 1250 years.
Crowned Aleppo galls are sometimes found in samples of ordinary Aleppo galls. They are about the size of a pea, are stalked, and bear a crown of projections near the apex. The insect producing them is Cynips polycera.
Hungarian galls are produced by Cynips lignicola on Quercus robur growing in former Yugoslavia. They are used in tanning. English oak galls, formed by Adleria kollari on Quercus robur, contain about 15–20% of tannin.
HAMAMELIS LEAF
Hamamelis leaf (witch hazel leaves) consists of the dried leaves of Hamamelis virginiana L. (Hamamelidaceae), a shrub or small tree 2–5 m high, which is widely distributed in Canada and the USA. It is official in the BP/EP and is the subject of an ESCOP monograph.
Macroscopical characters
The leaves are shortly petiolate, 7–15 cm long, and broadly oval to ovate in shape; base asymmetrically cordate, apex acute. The lamina is dark brownish-green to green in colour and very papery in texture. The venation is pinnate and the margin crenate or sinuate-dentate. The veins are very conspicuous on the lower surface; they leave the mid-rib at an acute angle and run straight to the margin, where they terminate in a marginal crenation. Odour, slight; taste, astringent and bitter.
The BP/EP drug is required to contain not more than 7% of stems and not more than 2% of other foreign matter.
Microscopical characters
The drug has very distinctive microscopical characters. These include characteristic stomata present on the lower surface only; very large lignified idioblasts, crystal cells accompanying the pericyclic fibres, tannin-containing cells and, especially in young leaves, stellate hairs. The calcium oxalate is in monoclinic prisms 10–35 μm long. The stellate hairs (Fig. 42.1H) consist of 4–12 cells united at the base. Each cell is thick-walled and up to 500 μm long.
Constituents
Hamamelis contains gallitannins, ellagitannins, free gallic acid, proanthocyanidins, bitter principles and traces of volatile oil. With ferric chloride solution the gallitannins and the free gallic acid give a blue colour and the ellagitannins, green.
The pharmacopoeia requires the leaves to contain not less than 3.0% tannins; these tannins represent the difference between the total poly-phenol content of the leaf and the polyphenol content not absorbed by hide powder. Reagents employed in the assay are Phosphomolybdotungstic reagent and sodium carbonate solution with absorbance measurements made at 760 nm; pyrogallol is used as a test solution. The leaves appear to contain no hamamelitannin (see ‘Hamamelis Bark’, below).
Volatile compounds, although present in small amounts only, have been studied by GC-MS analysis. Some 175 compounds have been distinguished and classified as homologous series of alkanes, alkenes, aliphatic alcohols, related aldehydes, ketones and fatty acid esters; distinctive monoterpenoids were evident (R. Engel et al., Planta Medica, 1998, 64, 251).
A procedure for the identification and assay involving TLC, HPLC, plate densitometry and spectrophotometry for the proanthocyanidins, phenolic acids and flavonoids in leaf extracts has been described (B. Vennat et al., Pharm. Acta Helv., 1992, 67, 11).
Allied drugs
Hamamelis bark occurs in curved or channelled pieces which seldom exceed 10 cm long or 2 cm wide. The bark is silvery grey and smooth, or dark grey and scaly. The inner surface is pinkish and often bears fragments of whitish wood. Sections show a cortex containing prismatic crystals of calcium oxalate, a complete ring of sclerenchymatous cells, and groups of phloem fibres. The bark contains a mixture of hamamelitannin and condensed tannin; the former has recently been demonstrated to be a potent oxygen scavenger (H. Masaki et al., Phytochemistry, 1994, 37, 337). Three separate hamamelitannins, α-, β- and γ-, are now known. The most important, β-hamamelitannin, is formed from two gallic acid molecules and one molecule of the sugar hamamelose. Newer galloylhamameloses and proanthocyanidins have now been identified (C. Haberland and H. Kolodziej, Planta Medica, 1994, 60, 464; C. Hartisch and J. Kolodziej, Phytochemistry, 1996, 42, 191). For the fractionation of those polymeric proanthocyanidins having similar structures but different molecular weights, see A. Dauer et al., Planta Med., 2003, 69, 89.
Uses
Hamamelis owes its astringent and haemostatic properties to the tannins. Hamamelitannin and the galloylated proanthocyanidins isolated from H. virginiana are reported to be potent inhibitors of 5-lipo-oxygenase, supporting the anti-inflammatory action of the drug (C. Hartisch et al., Planta Medica, 1997, 63, 106). The above compounds are presumably not present in Hamamelis Water or Distilled Witch Hazel, which is, however, widely used as an application to sprains, bruises and superficial wounds and as an ingredient of eye lotions. It contains safrole and other volatile components.
TORMENTIL
There are over 300 spp. of Potentilla family Rosaceae of which several, including P. anserina, (silverweed), P. reptans (creeping cinquefoil) and P. erecta (common tormentil), find medicinal use. Tormentil BP/EP consists of the whole or cut dried rhizome, freed from roots of P. erecta (L.) Raeusch. (P. tormentilla Stokes). This perennial plant is widely spread throughout central and northern Europe, favouring the acidic soils of marshes, meadows, open woods and hills. Commercial supplies come from East European countries.
Plants are up to 30 cm tall with several loosely pilosed stems bearing leaves consisting of three- to five-toothed finely haired leaflets. Yellow flowers in loose terminal cymes have long pedicels and, unusually for the genus, four petals.
The rhizomes are dark brown on the outer corky layer and white on the inside when freshly broken, but turning red on exposure to the air. The chopped dried drug consists of hard pieces of rhizome with the remains of roots attached. Depressed pale scars from the stems are visible and some remains of stems in the form of fine, branching strands, less than 1 mm in diameter, may also be attached to the rhizome. The fracture is granular, odour faint but not unpleasant and the taste strongly astringent.
Characteristic features of the powder include brown cork cells, parenchymatous tissue containing tannin, sclerenchymatous tissues, vascular elements, starch in conglomerates or as single grains up to about 20 μm in length, and abundant cluster crystals of calcium oxalate up to about 50 μm in diameter.
Constituents
The rhizome contains a mixture of both hydrolysable and condensed tannins (proanthocyanidins). Among the former is agrimoniin, a dimeric ellagitannin found also in Agrimonia and Alchemilla, and belonging to the same biosynthetic group, ellagic acid and catechol gallates. Other components are flavan-3-ols, the pseudosaponin tormentoside, quinovic acid and various phenylpropanes together with a trace of volatile oil.
HAWTHORN
The leaves, flowers and false fruits are all medicinally useful, the leaves and flowers being used principally for the preparation of infusions, etc. with the fruits employed in the manufacture of prepared medicaments. The dried false fruits of Crataegus monogyna and C. laevigata, family Rosaceae, together with their hybrids are official in the EP, BP and BHP; similarly the leaf and flower, for which there is also an ESCOP monograph.
The thorny, deciduous trees are native to Europe and have a long medical and ethnobotanical history. Commercial supplies of the dried fruits, required to contain not less than 1.0% procyanidins, originate from Eastern Europe.
Characters
Characteristic of a number of genera of the family Rosaceae, so-called hawthorn berries are false fruits (pomes, and not in the strict botanical sense berries) in which the carpels become adherent to the hollow, fleshy receptacle and the sepals, petals and stamens become situated at the upper end of the fruit. The carpels become stony so that the pome comes rather to resemble a drupe (Ch. 41). The false fruits of C. monogyna with one carpel contain a single stony true fruit whereas those of C. laevigata with two or three carpels contain two or three fruits.
The dried reddish-brown to dark red fruits have a slight odour and mucilaginous, slightly acid taste; with C. monogyna they are up to 10 mm in length and slightly larger for C. laevigata. At the upper end of the false fruit are the remains of the five reflexed sepals which surround a shallow depression from the base of which arise stiff lignified tufts of trichomes and the remains of the style (two styles with C. laevigata). The base of the fruit may be either attached to a pedicel or show the scar of attachment of the latter.
In addition to the long, lignified, tapering clothing trichomes of the inner surface of the receptacle other microscopical features include: cells of the outer receptacle with red pigmentation; sclereids; calcium oxalate as clusters and in files of cells as prisms; seed fragments showing a mucilaginous testa and embryo cells containing aleurone grains and fixed oil. A more detailed description will be found in the pharmacopoeias.
Constituents
The fruits contain 1–3% oligomeric procyanidins, the structures of which appear to be only partially ascertained together with flavonoids, principally hyperoside about 1%. The leaves in contrast contain less hyperoside and more vitexin rhamnoside.
Thin layer chromatography of a methanolic extract of the drug and fluorescence visualization at 365 nm is used as a test for identity. Procyanidins are evaluated by acid hydrolysis of an alcoholic extract followed by absorbance measurements at 545 nm of the butanol-soluble procyanidins produced.
The leaves and flowers, in contrast to the fruits, contain less hyperoside and more vitexin rhamnoside. In a study of important factors for the use of monitored commercial material, W. Peschel et al. (Fitoterapia, 2008, 79, 1) have examined the variability of total flavonoid content of the drug in relation to wild trees, age of cultivation site, sun exposure and harvest time.
AGRIMONY
Agrimony BP/EP, BHP family Rosaceae consists of the dried flowering tops of Agrimonia eupatoria L.
This erect, chalk-loving perennial herb is common throughout southern Europe and is indigenous to the British Isles, except for northern Scotland. Related species are found across North America. Hungary and Bulgaria are commercial suppliers of the drug.
The leaves are compound imparipinnate, with four to six opposite pairs of leaflets and a terminal leaflet. Larger leaflets are up to 6 cm in length with coarsely serrate or serrate–dentate margins, usually densely villous and often greyish on the lower surface. The golden flowers, 5–8 mm in diameter, are arranged spirally as terminal spikes. The pendulous fruits, 4–6 mm long, are deeply grooved with small projecting hooked bristles.
Characteristic microscopical features include stiff, thick-walled trichomes (500 μm) often with spiral thickenings and abundant clusters and prisms of calcium oxalate in the leaf mesophyll. Stomata are mainly of the anomocytic, occasionally anisocytic type. Pollen grains are ovoid to subspherical (up to 60 μm × 35 μm) with three pores and a smooth, thin exine.
The BP drug is required to contain a minimum of 2.0% tannins, expressed as pyrogallol when assayed by the official ‘determination of tannins in herbal drugs’. The TLC test of identification exploits the flavonoid content (rutin and isoquercitroside as test substances). Vitamins, triterpenes, volatile oil have also been reported as components of the drug.
Among other herbal uses, agrimony is employed as a mild astringent, internally and externally, against inflammation of the throat and for gastroenteritis.
ALCHEMILLA
The flowering and aerial parts of the lady’s mantle, Alchemilla xanthochlora (A. vulgaris sensu latiore), family Rosaceae, are described in the BP/EP and BHP 1996. The plant is widespread in Europe, North America and Asia; commercial supplies are obtained principally from Eastern Europe. In addition to the identification by macroscopic and microscopic characters the pharmacopoeias include thin-layer chromatographic tests providing characteristic fluorescent zones.
The BP/EP drug is required to contain not less than 6.0% of tannins expressed as pyrogallol when determined by the official method (cf. Hamamelis and Rhatany). The characterized ellagitannins are pedunculagin and the dimeric alchemillin. Other constituents are flavonoids, quercetin 3-O-β-D-glucoside having been isolated as the major flavonoid in leaves of French origin.
Alchemilla acts as an astringent against bleeding and diarrhoea and has a long tradition of use for gynaecological conditions such as menorrhagia.
RHATANY
Rhatany of the BP and EP (Krameria) is the dried root of Krameria triandra (Krameriaceae, a small family related to the Leguminosae), a small shrub with decumbent branches about 1 m long. The drug is collected in Bolivia and Peru and is known in commerce as Peruvian rhatany.
The root has a knotty crown several centimetres in diameter and gives off numerous branch roots some of which attain a length of 60 cm. The roots are nearly cylindrical and are covered with a reddish-brown cork, which is scaly except in very young roots. A transverse section shows a reddish-brown bark which occupies about one-third of the radius and encloses a yellowish, finely radiate wood. A small, deeply coloured heartwood is sometimes present in the larger species. The bark readily separates from the wood. The former is astringent but the latter almost tasteless.
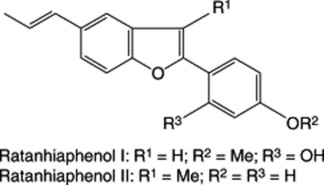
The tannins of krameria root (krameria-tannic acid) are entirely of the condensed (proanthocyanidin) type having a ‘polymeric’ flavin-3-ol structure. In this instance there is a procyanidin:propelargonidin ratio of 35:65 as determined by acid hydrolysis. Astringency of the root is due to compounds with a degree of polymerization of more than five. (For further details see E. Scholz and H. Rimpler, Planta Med., 1989, 55, 379). A phlobaphene (krameria-red), starch and calcium oxalate are also present. Stahl and Ittel (1981) reported the isolation of two benzofuran derivatives, ratanhiaphenols I and II, from the root. Both compounds are effective u.v. light filters and could be useful in sun-protection preparations. The BP and EP include an assay for tannins (polyphenols) of not less than 5.0% based on the colour reaction involving alkaline sodium phosphomolybdotungstate (absorbance measured at 760 nm). Polyphenols not adsorbed by hide powder, also determined with the same reagent, are excluded from the calculation.
The drug is used as an astringent and the significant antimicrobial activity of the extract gives rational support for its use in mouth and throat infections.
Allied species
The roots of several other species are occasionally encountered in commerce, but the Peruvian drug is the only one generally available. Krameria cystisoides of Mexican origin has indigenous medicinal uses. It contains over 20 compounds of the lignan, neolignan and norneolignan type. Similar constituents are reported for K. lanceolata; see H. Achenbach et al., Phytochemistry, 1987, 26, 1159; 1989, 28, 1959.
Pomegranate rind
The pomegranate fruit is one of the oldest known to man and has featured in mythology, and as a food and medicine from ancient civilizations of the Middle East to its present wide cultivation in India and surrounding countries, Turkey, southern Europe and California.
Pomegranate rind consists of the dried pericarp of the fruit of Punica granatum (Punicaceae). It occurs in thin, curved pieces about 1.5 mm thick, some of which bear the remains of the woody calyx or a scar left by the stalk. The outer surface is brownish-yellow or reddish. The inner surface bears impressions left by the seeds. Pomegranate rind, used in India as a herbal remedy for non-specific diarrhoea, is very astringent and contains about 28% of tannin (ellagitannins) and colouring matters. It should be distinguished from the root bark, which contains alkaloids.
For a discussion of the biochemistry, health effects, commercialization, plant growth and improvement of the pomegranate fruit, see N. P. Seeram et al. (eds), R. Hardman (series ed.) 2006 Medicinal and aromatic plants – industrial profiles, Vol 43. Pomegranate. CRC Press, Taylor and Francis Group. Boca Raton, FL., 244 pp.
Aspidosperma barks
The bark of Aspidosperma quebracho-blanco (Apocynaceae), which is used as a tanning material, was formerly official in several pharmacopoeias.
Myrobalans
Myrobalans are the dried fruits of Terminalia chebula (Combretaceae), a tree common in India. The immature fruits are black, ovoid and about 1–3 cm long. They contain about 20–40% of tannin, β-sitosterol, anthraquinones and a fixed oil containing principally esters of palmitic, oleic and linoleic acids. The tannin and anthraquinone constituents make the drug both astringent and cathartic in action. Microbiological tests support the Indian use of an aqueous extract of the fruit as an anticaries agent (A. G. Jagtap and S. G. Karkera, J. Ethnopharmacology, 1999, 68, 299).
CATECHU
Gambir or pale catechu of the BP 1989; BP (Veterinary), 2007 is a dried, aqueous extract prepared from the leaves and young twigs of a climbing shrub, Uncaria gambir (Rubiaceae). It must be carefully distinguished from black catechu or cutch. The plant is a native of Malaya and it is largely cultivated for the production of the drug in Indonesia and Malaya for marketing through Singapore.
History
The catechu described by Barbosa (1514) was black catechu or cutch, and the first account of gambir appears to be that of a Dutch trader in 1780. In addition to the cube gambir used in pharmacy, large blocks of the extract are imported for use in dyeing and tanning. Other forms are used in the East for chewing with betel leaf.
Preparation
The preparation of catechu in Johore differs only slightly from the procedure adopted in Indonesia. It consists of extracting the leaves and young twigs with boiling water, evaporating the extract to a pasty consistency and dividing it into cubes, which are then sun-dried. Fuller details of the preparation are given in the 10th edition.
Many different forms of catechu are used in the East, and the drug for the Eastern market frequently has 20–50% of fine rice husks added as the liquid coagulates in the tubs. Such catechu is, of course, unofficial, and contains starch.
Macroscopical characters
Catechu occurs in cubes, which are very friable and may be broken up in transit or, if incompletely dried, may be more or less agglutinated. Of the samples available, those from Indonesia measure 17–22 mm and have a reddish-brown surface, often stamped with a maker’s mark, while those from Johore measure 24–29 mm and have a blackish exterior and the faces of the cube are depressed. Internally, both varieties are cinnamon-brown and porous. Odourless; taste, very astringent and at first somewhat bitter, afterwards sweetish.
Microscopical characters
When mounted in water, catechu shows minute, acicular crystals of catechin, many of which are branched and interlacing. They dissolve on warming and a considerable amount of vegetable debris is left. The leaves, particularly the stipules, bear simple, unicellular hairs up to about 350 mu;m long, with smooth, moderately thick, lignified walls. The twigs have lignified pericyclic fibres, wood fibres, and spiral, annular and pitted vessels. Minute starch grains are commonly present, particularly in the Indonesian drug, but the amount should be strictly limited. Rice husks have been observed in some samples.
Chemical tests
Constituents
Gambir contains about 7.33% of catechins, 22–50% of catechutannic acid, catechu red, quercitin and gambir-fluorescin.
BP (Vet.) 2007 standards include a loss on drying of not more than 15.0% and a water-insoluble matter of not more than 33.0% with reference to the dried material.
Catechin forms white, acicular crystals which are soluble in hot water and alcohol and give a green colour with ferric salts. Catechutannic acid is an amorphous phlobatannin which appears to be formed from catechin by loss of the elements of water. It readily yields the phlobaphene catechu-red. If the drug is carefully prepared, it will contain a high proportion of catechin and correspondingly smaller amounts of catechutannic acid and catechu-red.
Allied drug
Cutch or black catechu is an extract prepared from the heartwood of Acacia catechu (Leguminosae). Cutch occurs in black, somewhat porous masses. The taste resembles that of gambir. Microscopical examination of the water-soluble residue shows wood fibres and large vessels and sometimes fragments derived from the leaves on which the drug is spread.
Cutch contains 2–12% of catechins, 25–33% of phlobatannin, 20–30% of gummy matter, quercitrin, quercitin, moisture, etc. It yields 2–3% of ash. The catechin (acacatechin) is not identical with that in gambir. The drug may be distinguished from gambir as it gives no reaction for gambir-fluorescin.
Kinos
The name ‘kino’ has been applied to a number of dried juices, rich in phlobatannins and formerly used for their astringent properties. They include Malabar kino from Pterocarpus marsupium (Leguminosae), butea gum or Bengal kino from Butea frondosa (B. monosperma) (Leguminosae) and eucalyptus kino or red gum from Eucalyptus rostrata (Myrtaceae).
Croton lechleri
The bark of this and related euphorbiaceous trees yield, when slashed, a blood-red sap commonly known in South American folk medicine as Sangre de Grado, Sangre de Drace, or dragon’s blood (not to be confused with the dragon’s blood obtained from species of Daemonorops palms, q.v.). It is used locally for its anti-infective, antitumour and wound- healing properties. Cai et al. (Phytochemistry, 1991, 30, 2033; 1993, 32, 755) have shown proanthocyanidins to be the principal constituents (c. 90%) which vary from monomers to heptamers. These polyphenols possess oxygen free-radical scavenging activity and may assist in wound healing (C. Desmarchelier et al., J. Ethnopharmacology, 1997, 58, 103). Minor components isolated are phenols, alcohols, sterols and four diterpenoids, two of the latter being of the clerodane type. An alkaloid, tapsine, has been ascribed as the wound healing constituent; it could also account for the antitumour activity claimed for the latex (Z. Chen et al., Planta Medica, 1994, 60, 541).
COUMARINS AND GLYCOSIDES
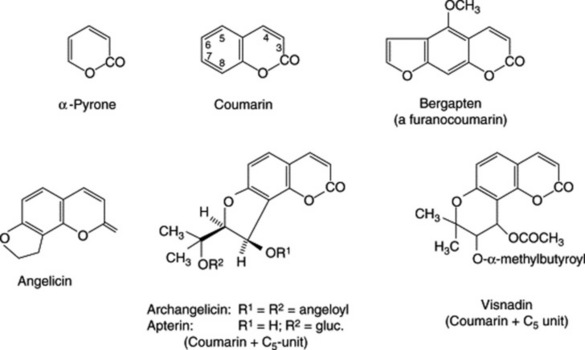
Derivatives of benzo-α-pyrone such as coumarin (the lactone of O-hydroxycinnamic acid), aesculetin, umbelliferone and scopoletin are common in plants both in the free state and as glycosides. Not all are phenolic but they are included here with the phenolic derivatives for convenience. Some 1000 natural coumarins have been isolated. Coumarin itself has been found in about 150 species belonging to over 30 different families, although it is probably present in the undamaged plant as trans-O-glucosyloxycinnamic acid. Enzyme activity in the damaged tissue leads to a loss of glucose and a trans → cis isomerization followed by ring closure. Coumarin gives a characteristic odour of new-mown hay and occurs in many Leguminosae such as sweet clover, melitot and tonco beans; the latter contain about 1–3% of coumarin. It is also recorded in woodruff, Asperula odorata (Rubiaceae) and cassia oil.
In ammoniacal solution these compounds have a blue, blue–green or violet fluorescence, which has long been used as a qualitative test for certain umbelliferous resins such as asafoetida and galbanum. The fluorescence is, of course, more marked if examined in filtered ultra-violet light and is used for the chromatographic visualization of the compounds.
The substitution patterns of some common hydroxy and methoxy coumarins are given in Table 21.2. Structurally more complex coumarins such as the calanolides and inophyllums have received recent attention as potent HIV-1-RT inhibitors (see Chapter 32).
Table 21.2 Hydroxy and methoxy coumarins.
| Compound | Additional groupings | Occurrence |
|---|---|---|
| Umbelliferone | HO at 7 (above) | Belladonna and stramonium (Solanaceae): Daphne mezereum (Thymeliaceae); Ferula species yielding asafoetida and galbanum, and many other Umbelliferae, chicory leaves (Compositae) |
| Herniarin | CH3O at 7 | Lavandula spica (Labiatae), Ruta graveolens (Umbelliferae) and certain Compositae |
| Aesculetin | HO at 6, HO at 7 | Horse-chestnut (Hippocastanaceae), certain Rosaceae and Fraxinus (Oleaceae) |
| Scopoletin | CH3O at 6, HO at 7 | Roots of gelsemium, oat, jalap, scammony, scopolia and belladonna; leaves of tobacco, stramonium, chicory and many others |
| Fraxin | CH3O at 6, HO at 7, O-glucose at 8 | Fraxinus spp. (Oleaceae) |
| Chicoriin | CH3O at 6, O-glucose at 7 | Cichorium intybus herb |
The furanocoumarins are closely related to the above and occur particularly in the Rutaceae and Umbellifeare. For example, celery fruits contain rutaretin and its dehydrated derivative apiumetin. Bergapten occurs in bergamot oil and in the Chinese root-drug derived from Peucedanum decursivum (Umbelliferae) which also contains the less-common pyranocoumarins. Marmesin derivatives (Fig. 13.2) and archangelicin have a reduced furanocoumarin structure consisting of coumarin and a C5 sub-unit. Other prenylated compounds are the 3-iso-prenylcoumarins, as illustrated by rutamarin of the genus Ruta; for recent research on R. graveolens see S. D. Srivastava et al., Fitoterapia, 1998, 69, 80. A wide range of biological activities has been demonstrated for these metabolites (see R. H. Galán et al., Phytochemistry, 1990, 29, 2053).
Furanocoumarins are responsible at least in part for the unpredictable and variable effects on drug availability resulting from the consumption of grapefruit juice. Two components of the juice (6′, 7′-dihydroxybergamottin and FC26) inactivate cytochrome P450 enzymes (specifically CYP3A4 and CYP3A5) resulting in an increased oral bioavailability of various drugs used to treat cancer, hypertension, heart disease and allergies. However, unnamed constituents of the juice have recently been shown to activate the efflux pump controlling P-glycoprotein-mediated drug transport which secretes absorbed drugs back into the gut. In vitro studies have demonstrated reduced absorption of vinblastine, cyclosporin, losartan, digoxin and fexofenadine. The two effects are therefore antagonistic and explain the unpredictable action of grapefruit juice on drug bioavailability. For reports on this research see The Lancet, 1999, 353, 1335; Pharm. J., 1999, 262, 573; HerbalGram, 1998, No. 43, 22.
Ammi species contain furanomethoxycoumarins but are more important for their content of furanobenzo-γ-pyrones (q.v. under ‘Flavones’).
Bicoumarins are formed from two coumarin moieties and the linkage may occur in a number of ways. Dicoumarol is formed at C3–C3′ through a methylene group and was, in 1941, the first of the series to be isolated. It is a constituent of fermenting hay and is formed by microbial action of coumarin. It is a powerful anticoagulant and haemorrhagic and can cause the death of animals consuming the spoiled fodder.
ANGELICA ROOT
The root of the official drug (BP, EP, BHP) consists of the rhizome and root of Angelica archangelica L. (A. officinalis Haffm.) (Umbelliferae), whole or cut and carefully dried. It is required to contain not less than 0.2% of volatile oil. The North American root is derived from A. atropurpurea and the Chinese from a number of species under the name man-mu or tangkuei.
The rhizomes are vertical and up to 5 cm in diameter, greyish-brown or reddish-brown in colour, bearing leaf and stem scars at the apex. Entwined, longitudinally furrowed, roots occur on the lower surface. The fracture is uneven and the transverse surface shows brown spots, indicating secretory cells, situated in the spongy, radiate, off-white bark. Microscopy of the powder shows, among other features, numerous simple starch grains 2–4 μm, yellowish-brown secretory canals, cork cells and lignified reticulately thickened vessels.
Considerable recent work on the genus has resulted in the isolation of a number of furanocoumarins and their glycosides; the formulae of bergapten, angelicin, archangelicin (a diester) and apterin are given in Fig. 21.8, and those of marmesin and psoralen in Fig. 13.2. These compounds are reported to have potent coronary vasodilator effects and are calcium antagonists. Monoterpenes constitute the major components (80–90%) of the volatile oil.
There are official limits for foreign matter, loss on drying ( 10%), total ash (
10%), total ash ( 10%) and acid-insoluble ash (
10%) and acid-insoluble ash ( 2.0%).
2.0%).
In herbal medicine the root is indicated in the treatment of bronchitis associated with vascular deficiency, and dyspeptic conditions.
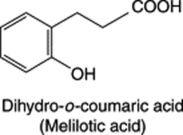
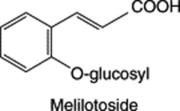
MELILOT
Melilot BP/EP, BHP 1996 consists of the dried flowering tops of Melilotus officinalis (L.) Lam, (common melilot, ribbed melilot, king’s clover, yellow sweet clover), family Leguminosae/Papilionaceae. It is found throughout Europe and eastwards to western China, N. America, except the far north, and elsewhere often as a weed of cultivation, probably introduced into Britain, together with other melilots (of which there are three common species), in the 16th century. Habitats include fields, hedgerows and waste places.
Melilot is an erect or decumbent branched biennial up to 100 cm tall. The finely ridged glabrous stems bear alternate stalked trifoliate leaves with two stipules joined to the base of the petiole. The leaflets of the upper leaves are oblong–elliptic, each with acute apex and base, margin entire. The yellow papillionaceous flowers occur in racemes up to 5 cm in length and give rise to almost straight glabrous pods, brown when ripe and transversely rugose. Seeds are wrinkled giving the ‘ribbed’ of the common name.
Features of the powdered drug include numerous anisocytic stomata with between three and six subsiduary cells on both epidermi; uniseriate covering trichomes composed of two small basal cells and a longer, bent, somewhat warty terminal cell; a few glandular trichomes with a two- to three-celled stalk and biseriate head of four cells; prismatic crystals of calcium oxalate associated with the vascular tissue; papillose epidermal cells of the petals; lignified fibrous anther fragments; spherical–ovoid pollen grains about 25 μm across with three pores and a smooth exine.
Constituents
Coumarin derivatives occur in melilot although coumarin itself is not present to any extent in the living plant. It arises when the plant is crushed, or the dried material treated with water, by the action of a β-gluconidase enzyme specific to cis-o-hydroxycinnamic acid glucoside giving first the unstable hydrolytic product coumarinic acid, which then cyclizes to coumarin producing the well-known ‘new-mown hay’ odour. The trans-isomer remains unchanged and is isolated as melilotoside (see F. Bourgoud et al., Phytochem. Anal., 1994, 5, 127; P. Bradley British Herbal Compendium,
Vol. 2, 2006, p. 270). Other acids isolated from melilot include dihydro-o-coumaric acid (melilotic acid), caffeic acid and other minor acids. Various oleanene saponins, volatile compounds and flavonoids have also been reported.
The pharmacopoeial TLC identification test for melilot indicates the presence of coumarin and possibly o-coumaric acid in the genuine drug. Assay of the coumarin content, minimum 0.3%, involves absorbance measurements at 275 nm on a boiled methanolic extract of the powder.
The various traditional medical uses of the drug have yet to be firmly established by clinical trials.
Tonco seed
Tonco seed or Tonquin beans are the dried seeds of Dipteryx odorata Willd. (Coumarouna odorata Aubl.) and Dipteryx oppositifolia (Leguminosae). The former plant is a native of Guiana and Brazil and is extensively cultivated in Venezuela, while the latter is found in Guiana and northern Brazil. Both are large trees bearing single-seeded fruits about 3–5 cm long.
The fruits are collected when ripe (May and June), they are opened and the seeds are dried in the sun. If sold without further treatment, they are known as ‘black’ beans. The seeds produced in Venezuela and near its border are larger than those produced in northern Brazil and parts of Guiana. The former, which are more highly valued, are known as ‘Angostura’ and the latter as ‘Para’ beans. Large quantities of both Angostura and Para beans are sent to Trinidad, where they are macerated for 24 h in rum and dried in the open air. This treatment causes a crystalline deposit of coumarin to be formed on the testa and the seeds are said to be ‘frosted’. Angostura and Para beans thus occur in commerce both black and frosted.
Tonco beans are up to 40 mm long, 10 mm wide and 5 mm thick. They are rounded at one end and bluntly pointed at the other. The surface is black and deeply wrinkled longitudinally, a crystalline encrustation being present in the frosted variety. A transverse section shows a very thin, black testa and two yellowish-brown, planoconvex, oily cotyledons. Odour, very fragrant; taste, aromatic and bitter.
Tonco beans contain 1–3% of coumarin, 25% of fat (containing unsaponifiable sitosterin and stigmasterin) and a larger amount of starch. Ash about 3.5%. Tonco beans are used in tobacco manufacture and in perfumery. Synthetic coumarin has, to some extent, replaced the natural product.
Celery fruit. Apium; Apii Fructus
The drug consists of the dried ripe fruits of Apium graveolens (Umbelliferae).
The cremocarp is brown, subspherical and about 1–1.5 mm long. The mericarps are mostly separate in the drug and each shows five straight primary ridges. A transverse section is almost pentagonal and shows 6–9 vittae, two on the commissural surface and four to seven in the grooves of the dorsal surface. Odour and taste, aromatic. Celery fruits contain 2–3% of oil consisting of terpenes with smaller quantities of the anhydride of sedanonic acid, the lactone of sedanolic acid and phenols. The fruits also contain a number of coumarins, furanocoumarins and coumarin glycosides.
Celery fruits are official in the BHP and have a long-standing use in the treatment of rheumatic diseases; the therapeutic action appears to be potentiated by Taraxacum (q.v.).
ANTHRAQUINONES AND GLYCOSIDES
Long before anything was known of their chemistry, rhubarb, aloes, senna and cascara were recognized as forming a natural group of purgative drugs. Also certain vegetable and animal dyestuffs such as madder and cochineal were of great economic importance before the introduction of synthetic dyestuffs. Later the chemical similarity of these purgative drugs and dyestuffs became apparent, as illustrated by the formulae for emodin (the aglycone of a number of purgative glycosides of Rhamnus spp.) and alizarin (the aglycone of a dyestuff of the madder plant).
Substances of the anthraquinone type were the first to be recognized, both in the free state and as glycosides. Further work showed that natural products also contained reduced derivatives of the anthraquinones (oxanthrones, anthranols and anthrones) and compounds formed by the union of two anthrone molecules (i.e. the dianthrones).
Because glycosides are often easily hydrolysed, the earlier workers tended to isolate products of hydrolysis rather than the primary glycosides. The following aglycones have long been established: chrysophanol or chrysophanic acid from rhubarb and cascara; aloe-emodin from rhubarb and senna; rhein from rhubarb and senna; emodin or frangula-emodin from rhubarb and cascara. Improved extraction methods, developed by Stoll and his colleagues, led to the isolation of the main senna glycosides, sennosides A and B, in 1941. Since this date many new glycosides including C-glycosides and various stereoisomers have been isolated and their structures determined.
In monocotyledons, anthraquinone derivatives are found only in the Liliaceae, in the form of the unusual C-glycoside barbaloin. Among dicotyledons they occur in the Rubiaceae, Leguminosae, Polygonaceae, Rhamnaceae, Ericaceae, Euphorbiaceae, Lythraceae, Saxifragaceae, Scrophulariaceae and Verbenaceae. They appear to be absent from the Bryophyta, Pteridophyta and Gymnosperms but occur in certain fungi and lichens. The fungal anthraquinone pigments are nearly all chrysophanol or emodin derivatives.
As indicated in Chapter 18, natural anthraquinones are synthesized either via the acetate–malonate pathway or they are derived from shikimate and mevalonate. The medicinally important purgative anthraquinones are formed by the former route and all have a 1,8-dihydroxy substitution. Conversely, compounds such as alizarin which have one of the rings unsubstituted arise by the second pathway. The relationships between the oxidized and reduced forms of the anthraquinone nucleus are shown in Fig. 21.9.
Modern research indicates that the 1,8-dihydroxyanthraquinone derivatives frequently occur with 1,8-dihydroxynaphthalene glycosides.
Anthraquinones
The derivatives of anthraquinone present in purgative drugs may be dihydroxy phenols such as chrysophanol, trihydroxy phenols such as emodin or tetrahydroxy phenols such as carminic acid. Other groups are often present, for example, methyl in chrysophanol, hydroxymethyl in aloe-emodin and carboxyl in rhein and carminic acid. When such substances occur as glycosides, the sugar may be attached in various positions. See formulae for carminic acid and glucofrangulin. Some examples of anthraquinone derivatives are given in Table 21.3.
Anthraquinone derivatives are often orange-red compounds, which may sometimes be observed in situ (e.g. in the medullary rays of rhubarb and cascara). They are usually soluble in hot water or dilute alcohol. Bornträger’s test is often used for their detection. The powdered drug is macerated with an immiscible organic solvent, ether is recommended, and after filtration aqueous ammonia or caustic soda is added, when a pink, red or violet colour in the aqueous layer after shaking indicates the presence of free anthraquinone derivatives. If glycosides only are present, the test should be modified by first hydrolysing with alcoholic potassium hydroxide solution or 2 M acid. When alkali is added to powdered drugs or to sections, the red colour produced serves to locate the anthraquinone derivatives in the tissues (e.g. in the medullary rays of cascara bark). If the drug being tested contains either very stable anthraquinone glycosides or reduced derivatives of the anthranol type, Bornträger’s test will be negative.
Anthraquinones containing a free carboxylic acid group (e.g. rhein) can be separated from other anthraquinones by extraction from an organic solution with sodium bicarbonate solution.
Anthranols and anthrones
These reduced anthraquinone derivatives occur either free or combined as glycosides. They are isomeric and one may be partially converted to the other in solution. The parent substance, anthrone, is a pale yellow, non-fluorescent substance which is insoluble in alkali; its isomer, anthranol, is brownish-yellow and forms a strongly fluorescent solution in alkali. Anthranol derivatives, such as are found in aloes, have similar properties, and the strong green fluorescence which aloes give in borax or other alkaline solution has long been used as a test for its identification. Anthranols and anthrones are the main constituents of chrysarobin, a mixture of substances prepared by benzene extraction from the material (araroba) found in the trunk cavities of the tree Andira araroba. If a little chrysarobin is treated on a white tile with a drop of fuming nitric acid, the anthranols are converted into anthraquinones. A drop of ammonia allowed to mix gradually with the acid liquid produces a violet colour. This modification of Bornträger’s test had been used as a test for identity before the underlying chemistry was known.
Oxanthrones
The formula given shows that these are intermediate products between anthraquinones and anthranols. They give anthraquinones on oxidation and Fairbairn’s modification of the Bornträger test accomplishes this by means of hydrogen peroxide. An oxanthrone has been reported as a constituent of cascara bark.
Dianthrones
These are compounds derived from two anthrone molecules, which may be identical or different; they readily form as a result of mild oxidation of the anthrone or mixed anthrones (e.g. a solution in acetone and presence of atmospheric oxygen). They are important aglycones in species of Cassia, Rheum and Rhamnus; in this group the sennidins, aglycones of the sennosides (see formula), are among the best-known examples. Reidin A, B and C which occur in senna and rhubarb are heterodianthrones, i.e. composed of unlike anthrones, and involve aloe-emodin, rhein, chryophanol or physcion.
It will be noted that two chiral centres (at C-10 and C-10′) are present in the dianthrones, and for a compound having two identical anthrone moieties, e.g. sennidin A, two forms (the 10S, 10′S and 10R, 10′R) are possible together with the meso form (sennidin B). These compounds also occur in the plant as their 1,1′-diglucosides.
Aloin-type or C-glycosides
The aloin obtained from species of Aloe, although one of the first glycosides to be isolated, was a problem for investigators for a long time. It is strongly resistant to normal acid hydrolysis but may be oxidized with ferric chloride. A study of its degradation products and infrared spectrum indicated a sugar-like chain and the structure shown, in which the sugar is joined to the aglycone with a direct C–C linkage (a C-glycoside). Two aloins (A and B) are known and arise from the chiral centre at C-10; their separation by high-speed countercurrent chromatography (see Chapter 17) has been recently described (C. XueLi et al., J. Chrom. and Rel. Technol., 2007, 30, 12).
Pharmacological action
The action of the anthraquinone laxatives is restricted to the large bowel; hence their effect is delayed for up to 6 h or longer. The nature of the peristaltic initiation is not known for certain but it has been suggested that the common anthraquinone and anthranol derivatives influence the ion transport across colon cells by inhibition of Cl− channels (J. Hönig et al., Planta Med., 1992, 58 (Suppl. 1), A586).
SENNA LEAF
Senna (Sennae Folium) consists of the dried leaflets of Cassia senna L. (C. acutifolia Delile), which are known in commerce as Alexandrian or Khartoum senna, and of Cassia angustifolia Vahl, which are known in commerce as Tinnevelly senna. The senna plants are small shrubs of the family Leguminosae, about 1 m high, with paripinnate compound leaves. C. senna is indigenous to tropical Africa and is cultivated in the Sudan (Kordofan, Sennar). C. angustifolia is indigenous to Somaliland, Arabia, Sind and the Punjab, and is cultivated in South India (Tinnevelly). The botanical validity for distinguishing between the above two plants has been called in question (Brenan, Kew Bull., 1958, 231), but Fairbairn and Shrestha (Lloydia, 1967, 30, 67) reinvestigated the well-established character differences between the two commercial types (see below) and concluded that the distinction remains valid; any further investigation on the two varieties grown under identical conditions does not appear to have been reported.
History
Senna appears to have been used since the ninth or tenth century, its introduction into medicine being due to the Arabian physicians, who used both the leaves and the pods. It was formerly exported through Alexandria, from where the name of the Sudanese drug is derived.
Collection and preparation
Alexandrian senna is collected mainly in September, from both wild and cultivated plants. The branches bearing leaves and pods are dried in the sun and conveyed to Omdurman. Here the pods and large stalks are first separated by means of sieves (see ‘Senna Fruit’). That which has passed through the sieves is then ‘tossed’ in shallow trays, the leaves working to the surface and heavier stalk fragments and sand to the bottom. The leaves are then graded, partly by means of sieves and partly by hand-picking into (1) whole leaves, (2) whole leaves and half-leaves mixed, and (3) siftings. The whole leaves are those usually sold to the public, while the other grades are used for making galenicals. The drug is packed, somewhat loosely, in bales and sent by rail to Port Sudan, from where it is exported.
Tinnevelly senna is obtained from cultivated plants of Cassia angustifolia grown in South India, N.W. Pakistan and Jammu, where the plants are more luxuriant than those found wild in Arabia. It may be grown either on dry land or in wetter conditions as a successor to rice. Being a legume, it usefully adds nitrogen to the soil. Owing to the careful way in which the drug is collected and compressed into bales, few leaflets are usually broken.
Macroscopical characters
Senna leaflets bear stout petiolules. The lamina has an entire margin, an acute apex, and a more or less asymmetric base. The surfaces are pubescent. Odour, slight but characteristic; taste, mucilaginous, bitterish and unpleasant.
Typical senna leaflets are shown in Fig. 21.10. The main differences between the two varieties are given in Table 21.4.
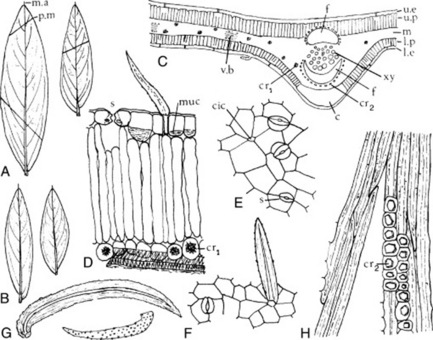
Fig. 21.10 Senna leaflets. A, Indian senna; B, Alexandrian senna (both ×1); C, transverse section of leaflet (×80); D–H, elements of the powder (all ×200); D, leaflet fragment in transverse section; E, F, epidermal fragments in surface view; G, isolated trichomes; H, portion of fibre group with crystal sheath, c, collenchyma; cic, cicatrix; cr1, cr2, calcium oxalate crystals of the cluster and prismatic type respectively; f, fibre groups; l.e, lower epidermis; l.p, lower palisade layer; m, mesophyll; muc, mucilage; m.a, mucronate apex; p.m, press mark; s, stoma (paracytic type); u.e, upper epidermis; u.p, upper palisade layer; xy, xylem.
Table 21.4 Comparison of Alexandrian and Indian senna leaves.
| Alexandrian senna | Tinnevelly senna |
|---|---|
| Macroscopical characters | |
| Seldom exceed 40 mm in length | Seldom exceed 50 mm in length |
| Greyish-green | Yellowish-green |
| More asymmetric at base | Less asymmetric at base |
| Rather more broken and curled at the edges | Seldom broken and usually flat owing to compression |
| Few press markings | Often shows impressions due to the midvein of other leaflets |
| Microscopical characters | |
| Hairs more numerous, the average distance between each being about three epidermal cells | Hairs less numerous, the average distance between each being about six epidermal cells |
| Most of the stomata have two subsidiary cells only | The stomata having two or three subsidiary cells respectively are in the ratio of about 7:3 |
| Vein-islet number 25–29.5 | Vein-islet number 19.5–22.5 |
| Stomatal index 10.0–15.0, usually 12.5 | Stomatal index 14.0–20.0, usually 17.5 |
| Chemical tests* | |
| Ether extract of hydrolysed acid solution of drug gives with methanolic magnesium acetate solution: | The same test: |
| a pink colour in daylight, | an orange colour in daylight, |
| a pale greenish-orange in filtered ultraviolet light | a yellowish-green in filtered ultraviolet light |
| TLC test for distinctive naphthalene derivatives† | |
| 6-Hydroxymusizin glycoside present | Tinnevellin glycoside present |
* For full details, see Nandy and Santra, J. Ind. Pharm. Manuf., 1968, 6, 235.
Microscopical characters
Senna leaflets have an isobilateral structure (see Fig. 41.4). The epidermal cells have straight walls, and many contain mucilage. Both surfaces bear scattered, unicellular, non-lignified warty hairs up to 260 mu;m long (Fig. 21.10D, G). The stomata have two cells with their long axes parallel to the pore and sometimes a third or fourth subsidiary cell (Fig. 21.10E, F). The mesophyll, consisting of upper and lower palisade layers and median spongy mesophyll, contains cluster crystals about 15–20 μm in diameter. The midrib is biconvex. Below the midrib bundle is a zone of collenchyma. The midrib bundle and larger veins are almost surrounded by a zone of lignified pericyclic fibres and a sheath of parenchymatous cells containing prisms of calcium oxalate 10–20 μm long (Fig. 21.10 C).
Vein-islet numbers and stomatal indices can be used to distinguish the two species (see Table 21.4) and the BP/EP utilizes stomatal index.
Constituents
Since Tutin first isolated aloe-emodin and rhein in 1913, many other compounds based on these two have been obtained. Stoll et al. (1941) isolated two active crystalline glycosides, sennoside A and sennoside B. They both hydrolyse to give two molecules of glucose and the aglycones sennidin A and B. Sennidin A is dextrorotatory and B is its mesoform formed by intramolecular compensation (Fig. 21.12).
The activity of senna was still not fully explained by the isolation of these constituents, and later work, notably by Fairbairn, Friedrich, Friedmann, Lemli and their associates demonstrated the presence of many other (some pharmacologically active) components. These include: sennosides C and D, which are the glycosides of heterodianthrones involving rhein and aloe-emodin; palmidin A (see ‘Rhubarb’); aloe-emodin dianthrone-diglycoside, rhein-anthrone- 8-glycoside, rhein-8-diglucoside, aloe-emodin-8-glucoside, aloe- emodin-anthrone-diglucoside, possibly rhein-1-glucose, and a primary glycoside having greater potency than sennosides A and B and distinguished from them by the addition of two glucose molecules. A new anthraquinone glycoside, emodin-8-O-sophoroside (a diglucoside), has been isolated in 0.0027% yield from dried Indian senna leaves (J. Kinjo et al., Phytochemistry, 1994, 37, 1685).
Two naphthalene glycosides isolated from senna leaves and pods (Lemli et al., Planta Med., 1981, 43, 11) are 6-hydroxymusizin glucoside and tinnevellin glucoside. The former is found in Alexandrian senna and the latter in Indian senna; this difference has been used as a
distinguishing feature of the commercial varieties, see Table 21.4. Senna also contains the yellow flavonol colouring matters kaempferol (3,4′,5,7-tetrahydroxyflavone), its glucoside (kaempferin) and isorhamnetin; also a sterol and its glucoside, mucilage, calcium oxalate and resin. The structures of water-soluble polysaccharides and a lignan have been reported.
Although senna is not noted for its volatile components, Tutin in his 1913 publication had observed the ‘strongly aromatic dark-coloured essential oil’. Over 80 years later W. Schulz et al. (Planta Medica, 1996, 62, 540) have again examined the volatiles of senna leaf and recorded (GC-MS) more than 200 components afforded by aqueous distillation. 122 constituents were identified including monoterpenes, phenylpropanes, fatty acids and esters, etc. Hexadecanoic acid was a significant component in addition to many of the more common constituents of volatile oils.
Formation and distribution of anthraquinone derivatives. In young senna seedlings chrysophanol is the first anthraquinone formed, then aloe-emodin appears and finally rhein; this ontogenetic sequence is in keeping with the expected biogenetic order, which involves the successive oxidation of the 3-methyl group of chrysophanol (Table 21.3). In the presence of light glycosylation follows and later the glycosides are translocated to the leaves and flowers. During fruit development the amounts of aloe-emodin glycoside and rhein glycoside fall markedly, and sennosides accumulate in the pericarp.
Lemli and Cuveele (Planta Med., 1978, 34, 311) considered that fresh leaves of Cassia senna contain anthrone glycosides only. By drying between 20 and 50°C these are enzymatically converted to dianthrone forms (sennosides). However, Zenk and coworkers (Planta Med., 1981, 41, 1) maintained that sennoside formation is not entirely an artefact arising through drying but that these compounds together with the monoanthrones, and their oxidized forms (anthraquinones), are part of a redox system of possible significance to the living cell.
The distribution of sennoside B (determined by Zenk and coworkers by immunoassay) was for a C. angustifolia plant (sample dried at 60°C): flowers 4.3%, leaves 2.8%, pericarp 2.4%, stems 0.2%, roots 0.05%. Within the flowers the anthers and filaments contained 7.2%, carpels and ovaries 5.8%, petals 5.2%, sepals 4.7% and flower stalks 3.2%.
Evaluation
It is difficult to remove all fragments of rachis, petiole and stalk from the drug, but the amount of these structures is limited by the BP to 3%. In the whole drug the percentage of these is determined by hand-picking and weighing, but with the powdered drug recourse has to be made to quantitative microscopy.
C. senna is cultivated in Russia and the leaves are harvested mechanically; this leads to unavoidable mixture with petioles and stems but, because the active constituents are similar in all parts of the plant, this does not affect the quality of the glycosidal extracts.
Lack of knowledge of the precise active principles of senna coupled with the synergistic action of various compounds hampered the development of a satisfactory chemical assay for the drug. The BP/EP determines the total senna leaf glycosides in terms of sennoside B (not less than 2.5%). This involves extraction of the glycosides and free anthraquinones from the leaves, removal of the free aglycones and hydrolysis and oxidation of the remaining sennosides and other glycosides to give rhein and some aloe-emodin, which are then determined spectrophotometrically. Chromatographic tests for the leaf are given in the BP and EP.
The leaves are officially required to give an acid-insoluble ash of not more than 2.5%.
Allied drugs
Bombay, Mecca and Arabian Sennas are obtained from wild plants of C. angustifolia grown in Arabia. Some of the leaflets are shipped to Port Sudan and are graded like the Alexandrian drug, while some are sent to Bombay and frequently arrive in England with shipments of the Tinnevelly.
The leaflets resemble those of Tinnevelly senna but are somewhat more elongated and narrower, and of a brownish or brownish-green colour. Levin (1929) states that they may be distinguished microscopically from other sennas by their vein islet number.
Dog senna, a variety formerly much esteemed and still used in France, is derived from Cassia obovata. The plant is indigenous to Upper Egypt, but was cultivated in Italy in the sixteenth century. The leaves are obovate and quite different in appearance from the official leaflets. When in powder they may be distinguished by the papillose cells of the lower epidermis. Maurin found them to contain 1.0–1.15% of anthraquinone derivatives.
Palthé senna, derived from Cassia auriculata, has been found in Indian senna. It may be distinguished by the long hairs, the crimson colour given when boiled with chloral hydrate solution or treated with 80% sulphuric acid and the absence of anthraquinone derivatives. The leaves of other parts of the plant are widely used in Ayurvedic medicine for rheumatism and diabetes. The antioxidant activity of the flowers has been recently demonstrated (L. Pari and M. Latha, Pharm. Biol., 2002, 40, 512; A. Kumaran and R. J. Karunakaran, Fitoterapia, 2007, 78, 46).
The leaflets of other species of Cassia have also been imported, but may be distinguished from the genuine drug by the characters given above.
For Nigeria, the leaves of the local Cassia podocarpa have been suggested as a substitute for the official senna; bioassays have given an equivalent activity (A. A. Elujoba and G. O. Iweibo, Planta Med., 1988, 54, 372).
C. alata produces anthraquinone derivatives and has been used traditionally in Thailand as a laxative. Root cultures have been studied for their anthraquinone-producing properties (N. Chatsiriwej et al., Pharm. Biol., 2006, 44, 416).
Substitute
Argel leaves, which are derived from Solenostemma arghel (Asclepiadaceae), were at one time regularly mixed in a definite proportion with Alexandrian senna. The plant occurs in the Sudan, but the leaves are now seldom seen in commerce. If used to adulterate senna powder, it may be distinguished by the two- or three-celled hairs, each of which is surrounded by about five subsidiary cells.
SENNA FRUIT
Senna pods (Sennae Fructus) are the dried, ripe fruits of C. senna and C. angustifolia (Leguminosae), which are known as Alexandrian and Tinnevelly senna pods, respectively. Both have separate monographs in the BP/EP.
Collection
The pods are collected with the leaves and dried as described above. After separation from the leaves they are hand-picked into various qualities, the finer being sold in cartons and the inferior ones used for making galenicals.
Characters
The characteristic sizes and shapes of the two varieties are shown in Fig. 21.11. The Tinnevelly pods are longer and narrower than the Alexandrian and the brown area of pericarp surrounding the seeds is greater. The remains of the style are distinct in the Tinnevelly but not in the Alexandrian.
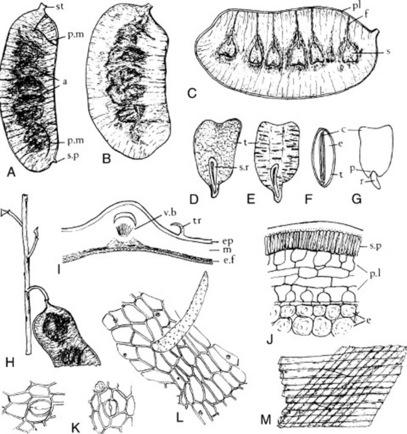
Fig. 21.11 Senna fruits. A, Tinnevelly fruit; B, Alexandrian fruit; C, Alexandrian pod opened to show seeds (all ×1); D, seed of Alexandrian fruit; E, seed of Tinnevelly fruit; F, transverse section of seed; G, isolated embryo with one cotyledon removed (all ×4); H, stem with Tinnevelly fruit attached (×1); I, transverse section of pericarp (×90;) J, transverse section of seed coat; K, fragments of epidermis with stomata; L, fragment of epidermis with trichome; M, fibrous layers from endocarp in surface view (all ×200); a, brown areas of pericarp covering seeds; c, cotyledons; e, endosperm; e.f, fibrous endocarp; ep, epicarp; f, funiculus; m, mesocarp; p, plumule; pl, placenta; p.l, parenchymatous layers of testa; p.m, press marks from other pods; r, radicle; s, seed; st, stalk; s.p, (J) subepidermal palisade; s.p, (A), stylar point; s.r, spathate ridge; tr, trichome; v.b, vascular bundle partially enclosed by fibres.
After soaking in water the pods are readily opened and about six wedge-shaped seeds are disclosed, each attached to the dorsal surface of the pod by a thin funicle (Fig. 21.11C). Under a lens the testas ofthe Tinnevelly show a general reticulation and wavy, transverse ridges, while the Alexandrian show a general reticulation only (Fig. 21.11D, E). The pericarp of the pod bears unicellular hairs and stomata of a type similar to those found on senna leaves; portions of the fibrous layer of the endocarp are very evident in the powder (Fig. 21.11K, L, M).
Constituents
The active constituents of the pods are located in the pericarp; they are similar to those of the leaves, together with sennoside A, which constitutes about 15% of the sennoside mixture. The seeds contain very little sennoside but Zenk’s group reported the cotyledons of 3-day-old seedlings to contain amounts equivalent to those in the leaves. Sennoside content varies from about 1.2 to 2.5% in the Tinnevelly (BP/EP 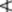 2.2%) and from about 2.5 to 4.5% in the Alexandrian (BP/EP
2.2%) and from about 2.5 to 4.5% in the Alexandrian (BP/EP  3.4%). C. Terreaux et al. (Planta Medica, 2002, 68, 349) have reported the isolation of kaempferol and tinnevellin 8-glucoside from an extract of the Tinnevelly pods together with two new carboxylated benzophenone glucosides. Preparations of the powdered pericarp, e.g. Senna Tablets BP, standardized in terms of sennoside B, are now commonly prescribed.
3.4%). C. Terreaux et al. (Planta Medica, 2002, 68, 349) have reported the isolation of kaempferol and tinnevellin 8-glucoside from an extract of the Tinnevelly pods together with two new carboxylated benzophenone glucosides. Preparations of the powdered pericarp, e.g. Senna Tablets BP, standardized in terms of sennoside B, are now commonly prescribed.
Uses
The use of laxatives is increasing and senna constitutes a useful purgative for either habitual constipation or occasional use. It lacks the astringent after-effect of rhubarb. Despite the availability of a number of synthetics, sennoside preparations remain among the most important pharmaceutical laxatives.
Cassia pods
Cassia pods are the dried ripe fruits of Cassia fistula (Leguminosae), a large tree thought to be indigenous to India but now widely cultivated in the tropics. The drug is chiefly obtained from the West Indies (Dominica and Martinique) and Indonesia.
The fruit is a cylindrical indehiscent pod about 25–30 cm long and 20–25 mm diameter. It is dark chocolate brown to black in colour and contains from 25 to 100 oval, reddish-brown seeds separated by membranous dissepiments. In the fresh pods the seeds are completely embedded in black pulp, which, however, gradually dries on the septa. For this reason pods which do not rattle when shaken are usually preferred. The pulp has a prune-like odour and a sweetish taste.
The pulp is dissolved from the crushed fruit by percolation with water. The percolate is strained and evaporated to a soft extract. The most important anthraquinone derivatives of the pulp appear to be rhein and combined sennidin-like compounds. Anthraquinones have been detected in non-differentiating callus cultures. Cassia pulp also contains about 50% of sugars, colouring matter and a trace of volatile oil. The leaves of C. fistula contain free and combined rhein, sennidins and sennosides A and B; these compounds exhibit a marked seasonable fluctuation. The heartwood is reported to contain barbaloin and rhein together with a leucoanthocyanidin.
Cassia pulp was formerly used in the form of Confection of Senna. In Ayurvedic medicine the plant is used to treat a variety of ailments. Its antifungal, antibacterial and laxative properties have been established and more recently (T. Bhakta et al., Pharm. Biol., 1998, 36, 140) its antitussive activity has been demonstrated.
CASCARA BARK
Official cascara sagrada (Sacred Bark, Chittern Bark) is the dried bark of Rhamnus purshianus DC (Frangula purshiana (DC) A. Gray ex J. C. Cooper) (Rhamnaceae). The bark is collected from wild trees, which are 6–18 m high, growing on the Pacific coast of North America (British Columbia, Washington and Oregon). Depleted wild US sources encouraged cultivation of the tree in western Canada, the USA and Kenya but these efforts do not appear to be completely successful.
History
Cascara is a drug of comparatively recent introduction into modern medicine. According to tradition, a cascara, probably R. californica, was known to early Mexican and Spanish priests of California; Rhamnus purshianus, however, was not described until 1805 and its bark was not introduced into medicine until 1877.
The common, European buckthorn was well known to the Anglo- Saxons; its berries were official in the London Pharmacopoeia of 1650.
Collection and storage
The bark is collected from mid-April to the end of August, when it separates readily from the wood. Longitudinal incisions about 5–10 cm apart are first made in the trunk and the bark removed. The tree is then usually felled and the branch bark separated. The pieces are dried in the shade with the cork uppermost. Such material is referred to as ‘natural’ cascara but commercial supplies are now comminuted to give small even fragments known as ‘evenized’, ‘processed’ or ‘compact’ cascara. During preparation and storage the bark must be protected from rain and damp, or partial extraction of the constituents may occur or the bark may become mouldy. That the bark must be kept for at least 1 year before use is no longer a BP requirement but the bark appears to increase in medicinal value and price until it is about 4 years old. Many companies prefer to use bark which has been stored for considerably more than 1 year. To reduce freight and handling charges on the bark, large quantities of the extract are now imported directly.
Macroscopical characters
The bark occurs in quills, or channelled or nearly flat pieces. All of these forms may attain 20 cm in length and are 1–4 mm thick, the thinner bark being most esteemed. The flat strips from the trunk are usually much wider (up to 10 cm) than the quills or channelled pieces (about 5–20 mm) obtained from the branches.
Cascara (Fig. 21.13A) bears a somewhat patchy, silvery-grey coat of lichens. Pieces bearing moss are also quite common. Between the patches of lichen may be seen a smooth, dark purplish-brown cork marked with lighter-coloured, transversely elongated lenticels. On scraping the cork, no bright purple inner cork is disclosed (distinction from R. alnus). The inner surface is dull purplish-brown to black, striated longitudinally and somewhat corrugated transversely. The fracture is short and granular in the outer part, but somewhat fibrous in the phloem. In the yellowish-brown cortex and phloem lighter groups of sclerenchymatous cells and phloem fibres may be seen with a lens. They may be made more distinct by staining with phloroglucinol and hydrochloric acid. The medullary rays, which tend to curve together in groups, are well seen in sections mounted in potash. Odour, slight but characteristic; taste bitter.
Fig. 21.13 Cascara bark. A, single quill (×0.66); B, general diagram of transverse section of bark (×20); C, transverse section of outer tissues; D, ditto of phloem (both × 200); E, cork cells in surface view; F, fragment of phloem from powder (both × 250); c, collenchyma; ck, cork; c.p, cortical parenchyma; I, lenticel; li, lichen patch; I.f, longitudinal furrows; m, moss; m.r, medullary ray; m.s, mussel scale; ox1, ox2, prismatic and cluster crystals of calcium oxalate respectively; p.f, pericyclic fibres; ph.f, phloem fibres; ph.p, phloem parenchyma; s, sclereids; s.p, sieve plate; s.t, sieve tube; t, scar of twig.
Microscopical characters
A transverse section of cascara bark (Fig. 21.13B–D) shows a partial coat of whitish lichen, some 10–15 layers of flattened cork cells with reddish-brown contents and a cortex composed of collenchyma, parenchyma and groups of sclereids. The collenchymatous cells show thickened pitted walls and contain chloroplasts filled with starch. Some of the parenchymatous cells also contain chloroplasts and starch; many of them contain a yellow substance coloured violet by alkalis and rosette crystals of calcium oxalate usually 6–10 μm diameter, but occasionally up to 45 μm diameter. The parenchymatous cells abutting on the groups of sclereids contain prisms of calcium oxalate. The sclereids are irregular or ovoid in shape, are variable in size, and have thick lignified walls sometimes showing stratification and traversed by funnel-shaped pits. A pericycle is not clearly delimited, but the zone immediately outside the phloem in which sclereids and occasional fibres occur is regarded as representing this region. The phloem is composed of zones of tangentially elongated groups of phloem fibres, enclosed in a sheath of parenchymatous cells containing prisms of calcium oxalate which alternate with sieve tubes and phloem parenchyma (Fig. 21.13F). The individual fibres are yellow in colour, are 8–15 μm in diameter, and have thick lignified walls showing stratification and pit canals. The sieve tubes show sieve plates, each with several sieve fields, on the radial walls. The sieve plates are usually covered with a deposit of callus and can be identified after staining with alkaline solution of corallin. The phloem parenchyma resembles that of the cortex, containing plastids, starch, material coloured violet by alkali, and rosettes of calcium oxalate. The medullary rays are 1–5 cells wide and 15–25 cells deep. The medullary ray cells are parenchymatous, somewhat radially elongated and with similar contents to those of the parenchyma; their content of material stained violet by alkali is often high. Fragments of moss leaves and liverworts are usually found in the powder.
Constituents
It has long been recognized that cascara bark stored for at least 1 year gave galenicals which were better tolerated but as effective as those prepared from more recently collected bark. This is presumably due to hydrolysis or other changes during storage. It was also found at an early date that the very bitter taste of cascara is reduced by treating extracts with alkalis, alkaline earths or magnesium oxide. Proprietary extracts of this type became very popular and pharmacopoeias followed the same idea to produce such preparations.
Cascara contains about 6–9% anthracene derivatives which are present both as normal O-glycosides and as C-glycosides. The following groups of constituents are now manifest.
The primary glycosides are more active than the aloins whereas the free anthraquinones and dimers have little purgative activity. The cascarosides have a sweet and more pleasant taste than the aloins. The BP/EP requires the bark to contain not less than 8.0% of hydroxyanthracene glycosides of which not less than 60% consists of cascarosides, calculated as cascaroside A. A two-point spectro-photometric assay is employed with absorbance measurements at 515 nm and 440 nm.
Experiments by Betts and Fairbairn in 1964, although based on a single fresh plant, suggested that free anthraquinones are formed by the leaves and that they are stored in the bark mainly as C-glycosides, the older bark containing the most C-glycosides. Rhamnus purshianus cell suspension cultures will produce anthracene derivatives in which the accumulation of these compounds, particularly emodin, is significantly raised by a 12 h light/dark cycle; continuous illumination of the cultures suppresses anthraquinone formation.
Substitutes
Several species of Rhamnus have a similar geographical distribution to that of R. purshianus. These include R. alnifolia, which is too rare to be a likely substitute; R. crocea, whose bark bears little resemblance to the official drug, and R. californica Esch. The latter is so closely related to R. purshianus that some botanists do not divide them into separate species. The plant appears to have a much more southerly distribution than the typical R. purshianus and is therefore unlikely to occur in bark of Canadian origin. It has a more uniform coat of lichens and wider medullary rays than the official species, but resembles the latter in having sclerenchymatous cells. The bark of R. fallax has been recorded as a cascara substitute. European frangula bark, distinguished by the BP/EP TLC test, is described below.
FRANGULA BARK
Frangula bark, alder buckthorn, is obtained from Rhamnus frangula L. (Frangula alnus Mill) (Rhamnaceae), a shrub 3–5 m high and found in Britain and Europe. Commerical supplies are available from Balkan countries and a little from Russia. The plant differs from the common buckthorn, R. cathartica, in that it does not possess thorns; it bears dark-purple berries whose medicinal properties have long been accepted. Although much used in England, the demand decreased with the increased popularity of cascara; on the Continent, particularly in France, cascara has not replaced it to the same extent.
The bark, included in the BP/EP, is required to contain not less than 7.0% glucofrangulins calculated as glucofrangulin A.
The bark occurs in single or double quills which are usually of smaller size than those of cascara and about 0.5–2 mm thick. It has a purplish cork and transversely elongated, whitish lenticels. On removing the outer cork cells by scraping, a dark crimson inner cork is exposed. The transverse section closely resembles that of cascara but groups of sclerenchymatous cells are absent.
Frangula contains anthraquinone derivatives present mainly in the form of glycosides. The rhamnoside franguloside, or frangulin, was isolated in 1857. This is now known to consist of two isomers, frangulosides A and B, formed by partical hydrolysis of the corresponding rhamnoglucosides, glucofrangulins A and B (Table 21.3). The fresh bark also contains anthranols and anthrones, which are unstable and readily oxidize to the corresponding anthraquinones; Lemli (1965, 1966) detected emodin-dianthrone, palmidin C (see ‘Rhubarb’), palmidin C monorhamnoside and emodin-dianthrone monorhamnoside. Wagner et al. characterized frangulin B as 6-O-(D-apiofuranosyl)-1,6,8-trihydroxy-3-methylanthraquinone and more recently reported the new glycoside emodin-8-O-β-gentiobioside.
Allied drugs
The common buckthorn, Rhamnus cathartica, has a glossy reddish-or greenish-brown cork and does not possess sclereids. It contains frangula-emodin and a glycoside, rhamnicoside, which yields on hydrolysis rhamnicogenol (an anthraquinone derivative), glucose and xylose. Rauwald and Just (Planta Med., 1981, 42, 244) reported the isolation of the anthraquinone glycoside alaternin; 1,2,6,8- tetrahydroxy-3-methyl-anthraquinone; physcion; chrysophanol; and frangula-emodin. The bark also contains a number of blue-fluorescent substances which in the chromatograms produced by the BP/EP tests of identity for Cascara and Frangula serve to distinguish this adulterant. The fluorescent substances have recently been identified as naphtholide glycosides of the sorigenin type, as below.
The bark of R. carniolica has a dull reddish cork and differs from frangula bark in that it possesses sclerenchymatous cells and has wider medullary rays. In recent years the barks of a number of Turkish species of Rhamnus have been systematically examined for their anthraquinone and flavonoid contents (see M. Koskun, Int. J. Pharmacognosy, 1992, 30, 151 and references cited therein).
RHUBARB
Rhubarb (Chinese Rhubarb) consists of the dried underground parts of Rheum palmatum L. (Polygonaceae) or R. officinale Baillon or hybrids of these two species, or mixtures of these. The drug appears still to be obtained from both wild and cultivated plants grown on the high plateaux of Asia from Tibet to south-east China. The BP/EP drug is required to contain not less than 2.2% of hydroxyanthraquinone derivatives calculated as rhein.
Species and commercial grades
The genus Rheum comprises about 50 species, which may be classified into two sections, the first including R. palmatum and R. officinale, and the second R. rhaponticum, R. undulatum and R. emodi. A systematic study is made unusually difficult by geography and by the tendency of cultivated plants to form hybrids such as R. palmatum × R. undulatum and R. palmatum × R. emodi. The exact morphological and chemical characters of such hybrid rhizomes appear not to have been described. Formerly most of the drug was derived from R. palmatum L. var. tanguticum Maximowicz and R. officinale H. Br. and was traditionally known in commerce as Shensi, Canton and high-dried rhubarb. R. palmatum and R. palmatum var. tanguticum now appear to be the chief sources. The best grade of the present-day drug corresponds to that formerly known as Shensi rhubarb. Another present-day grade is similar to the old Canton. In addition, some inferior drug is exported, much of which fails to give the pink fracture characteristic of good-quality rhubarb.
In practice the grading system is more complex than the above might imply, and currently about a dozen grades of rhubarb are recognized by merchants. The grades commonly listed are: ‘flat’, ‘common round’, ‘small round’, ‘extra small round’, ‘sticks’, ‘third grade’ and lower qualities. The flat and round are further categorized on a percentage basis (e.g. ‘flat 90%’ or ‘common round 80%’), depending on the pinkness and quality of the fracture. However, not all of these grades are necessarily available at any one time. Currently (2000) it is almost impossible to obtain rhubarb from China which meets BP/EP requirements for hydroxyanthraquinone derivatives. Demand for the better grades (CR & Flat 80 & 90%) is now almost exclusively for the beverage industry.
History
Chinese rhubarb has a long history. It is mentioned in a herbal of about 2700 BC and subsequently formed an important article of commerce on the Chinese trade routes to Europe. Today it still holds a place in medicine. The first international symposium on the drug was held in Chengde, China in 1990 under the title ‘Rhubarb 90’.
Collection and preparation
Provided that the older accounts are still substantially correct, the rhizomes are grown at a high altitude (over 3000 m) dug up in autumn or spring when about 6–10 years old, decorticated and dried. The decorticated rhizomes are when whole roughly cylindrical (‘rounds’) or if cut longitudinally are in planoconvex pieces (‘flats’). Pieces used often to show a hole indicating that they had been threaded on cords for drying.
The drug is exported from Shanghai to Tientsin, often via Hong Kong. The better qualities are packed in tin-lined wooden cases containing either 280 lb or 50 kg, and inferior quality in hessian bags.
Botanical characters of rhizome
The rhizomes of R. palmatum and R. officinale are similar in structure except for the size and distribution of the abnormal vascular bundles, ‘star spots’, of the pith. Transverse sections of both, after peeling, show phloem on the outside, cambium, radiate wood and a pith with ‘star sports’ (Fig. 21.14A, B). In R. palmatum the latter are relatively small (about 2.5 mm) and most of them are arranged in a continuous ring; in R. officinale the ‘star spots’ are larger (about 4 mm) and are irregularly scattered.
Fig. 21.14 Chinese rhubarb. A, common round (×0.5); B, star spot in transverse section (×20); C–E, fragments of the powder (×200); C, portions of reticulate vessels; D, calcium oxalate crystals; E, starch; c, cambium; cr, crystals; f, facets produced by peeling; m.r, medullary ray; ph, phloem; st, star spot; v, vessel; x.p, xylem parenchyma.
Macroscopical characters
Despite the large number of commercial grades, it is convenient to describe the various rhubarb types under three headings.
The drug has a firm texture, non-shrunken appearance and a bright yellow surface showing whitish reticulations. These reticulations are due to the fusiform or lozenge-shaped cut ends of the closely arranged medullary rays (which are reddish-brown) seen against the white background of the phloem parenchyma. In the palmatum type the medullary rays are only about 6 cells deep, but in the officinale type they may be as much as 200 cells deep. This difference accounts for the fact that the surface of the officinale type gives the apperance of parallel red and white lines rather than a reticulation. In both species the appearance of the transverse surface varies according to the depth of peeling, which may extend into the radiate wood or even into the pith.
The best rhubarb breaks with a marbled or ‘nutmeg’ fracture, the freshly broken surface showing a bright pink colour—this is one character used in grading—see above. Such drug gives the bright yellow powder favoured by buyers. Particular attention is paid by the buyer not only to the colour of the fracture, but also to absence of signs of decay or insect attack. Odour, aromatic; taste, bitter and slightly astringent.
Microscopy of powder
Powdered rhubarb (Fig. 21.14) is easily identified. It shows abundant calcium oxalate rosettes up to 200 μm in diameter; simple two to five compound starch grains; reticulate vessels and other wood elements which give no reaction for lignin. The yellow contents of the medullary ray cells (anthraquinone derivatives) become reddish-pink with ammonia solution and deep red with caustic alkalis.
Constituents
As with other anthraquinone-containing drugs, the chemical complexity of rhubarb was not fully appreciated by the earlier research workers. Free anthraquinones were the first substances to be isolated: chrysophanol, aloe-emodin, rhein, emodin and emodin monomethylether or physcion (1844–1905). Glycosides of some of the above were also separated. These substances did not account satisfactorily for the action of the drug, and modern methods of investigation have established the presence of the following types of anthraquinones in rhubarb.
In addition to the above purgative compounds, rhubarb contains astringent compounds such as glucogallin, free gallic acid, (–)-epicatechin gallate and catechin. Other derivatives of gallic acid include glycerol gallate, gallic acid glucoside gallates and isolindleyin (a methyl p-hydroxyphenylpropionate derivative of a glycogallate). A new class of gallototannins has a sucrose core and chromone glucosides have also been identified (Y. Kashiwada et al., Phytochemistry, 1988, 27, 1469; 1990, 29, 1007).
Rhubarb also contains starch and calcium oxalate. The total ash is very variable, as the amount of calcium oxalate varies from about 5 to 40%. The acid-insoluble ash should not exceed 1%. The BP assay for anthraquinone derivatives is a spectrophotometric method and replaces the former standard for alcohol-soluble extractive.
Ontogenetic production of anthraquinone derivatives
As the drug is collected in autumn, variations in constituents arising from seasonable changes should present no problem. Nevertheless, considerable research has been devoted to this aspect over many years. Work by Lemli and colleagues (1982) indicated that oxidized compounds, the anthraquinones, are the major components of the anthracene mixture in the summer months and the reduced forms, the anthrones, in winter. The conversions occur within a time lapse of about 3 weeks, and just before each, the anthrone diglycoside content increases markedly. Experiments showed that the anthraquinone → anthrone conversion could be artificially induced by decreasing the ambient temperature. Earlier reports by Schmid (1951) suggested that the age of the rhizome also affected the ratio of reduced:oxidized glycosides. Chinese workers have also addressed the problem (Chem. Abs., 1992, 117, 66652; 66653).
Constituents of rhapontic rhubarb
Rhapontic rhubarb contains a glycoside, rhaponticin, which is a stilbene (diphenylethylene) derivative of the formula
This substance and desoxyrhaponticin (glycoside of 3,5-dihydroxy-4′-methoxystilbene) account for the difference in fluorescence between official and rhapontic rhubarbs. Rhapontic rhubarb does contain anthraquinone derivatives, although these differ from those in the official drug. One is the glucoside glucochrysaron (see Table 21.3).
Chemical tests
Other rhubarbs
A considerable number of the anthraquinone derivatives present in R. palmatum have also been reported in Indian rhubarb. L. Krenn et al. (J. Nat. Prod., 2003, 66, 1107; Chem. Pharm. Bull., 2004, 52, 391) have identified a new sulphated anthraquinone glycoside (sulfemodin 8-O-β-D-glucoside) together with new 10-hydroxycascarosides C and D and, 10R-chrysaloin 1-O-β-D-glucopyranoside; some phenolic compounds have antioxidant properties.
ALOES
Aloes is the solid residue obtained by evaporating the liquid which drains from the transversely cut leaves of various species of Aloe (Liliaceae). The juice is usually concentrated by boiling and solidifies on cooling.
The official (BP, EP, USP) varieties of aloes are the Cape from South Africa and Kenya, and the Barbados (Curaçao) from the West Indian Islands of Curaçao, Aruba and Bonaire. There are separate pharmacopoeial monographs for each type. Socotrine and Zanzibar varieties are no longer official.
Plants
Of about 180 known species of Aloe, the drug is mainly obtained from the following: Cape variety from Aloe ferox and its hybrids; Curaçao variety from Aloe barbadensis; Socotrine and Zanzibar varieties from Aloe perryi. The genus Aloe includes herbs, shrubs and trees, bearing spikes of white, yellow or red flowers. Aloe ferox is an example of the arborescent type and A. barbadensis of the herbaceous type. Aloe leaves are fleshy, are strongly cuticularized and are usually prickly at the margins.
It has been suggested that if natural stocks of A. ferox became exhausted then A. classenii and A. turkanensis would be preferable for cultivation because chemical races would not be a problem and their production of sideshoots would make vegetative propagation easier. However, some problems have arisen concerning the commerce in the African aloes because the Washington Conference of the Convention on International Trade in Endangered Species (CITES) placed all species of Aloe, with the exception of A. vera (a cultivar of A. barbadensis), on the protected list.
Leaf structure
Transverse sections of an Aloe leaf usually show the following zones: (1) a strongly cuticularized epidermis with numerous stomata on both surfaces; (2) a region of parenchyma containing chlorophyll, starch and occasional bundles of needles of calcium oxalate; (3) a central region which frequently occupies about three-fifths of the diameter of the leaf, consisting of large, mucilage-containing parenchymatous cells; (4) a double row of vascular bundles which lie at the junction of the two previous zones and have a well-marked pericycle and endodermis. The aloetic juice from which the drug is prepared is contained in the large, pericyclic cells and sometimes in the adjacent parenchyma. When the leaves are cut, the aloetic juice flows out. No pressure should be applied or the aloes will be contaminated with mucilage. The mucilage, contained in zone 3 as above is used in the cosmetic and herbal industries in ‘aloe vera’ preparations (see Chapter 19).
History
According to legend, Socotrine aloes was known to the Greeks as early as the fourth century BC; the Greek colonists were sent to the island by Alexander the Great solely to preserve and cultivate the aloe plant. The drug was apparently known in England in the tenth century, and from the seventeenth century records of the East India Company it would appear that they frequently purchased the whole stock of aloes of the ‘King of Socotra’. Socotrine and Zanzibar aloe were for many years the only official aloes, but they have now been replaced by the Cape and Curaçao varieties. Cape aloes was first exported about 1780 and became official in Britain in 1932. Barbados aloes was produced from about 1650 and lapsed about the beginning of the present century. The production of Curaçao (also called Barbados) aloes was started by the Dutch in the Islands of Curaçao, Aruba and Bonaire about 1817; recently aloes of similar type has been exported from nearby Venezuela.
Preparation and characters of Cape aloes
Cape aloes is prepared from wild plants of A. ferox and its hybrids. The leaves are cut transversely near the base and about 200 of them are arranged round a shallow hole in the ground, which is lined with plastic sheeting or more traditionally a piece of canvas or a goatskin. The leaves are arranged so that the cut ends overlap and drain freely into the canvas. After about 6 h all the juice has been collected and it is transferred to a drum or paraffin tin in which it is boiled for about 4 h on an open fire. The product is poured while hot into tins, each holding 25 kg, where it solidifies. For export the tins are placed in cases holding either two, four or eight tins.
The drug occurs in dark-brown or greenish-brown, glassy masses. Thin fragments have a deep olive colour and are semitransparent. The powder is greenish-yellow, and when pieces of the drug have rubbed against one another, patches of powder are found on the surface. The drug has a very characteristic, sour odour (the so-called rhubarb or apple-tart odour), which is particularly noticeable if one breathes on the drug before smelling. Taste, nauseous and bitter. The powder when examined under the microscope in lactophenol is usually amorphous.
Preparation and characters of Barbados (Curaçao) aloes
Curaçao aloes is produced from cultivated plants of A. barbadensis. The cut leaves are stacked in V-shaped troughs arranged on a slope so that the juice flows from a hole at one end of the trough into a collecting vessel. When sufficient juice has been collected, it is evaporated in a copper vessel. The temperature used is generally lower than in the case of Cape aloes and the product is, therefore, usually opaque, although some which is semi-transparent may be produced and is known in commerce as ‘Capey Barbados’. Originally Barbados and Curaçao aloes were packed in gourds, now seen only in museums. The present-day drug is exported in cases each holding about 58.5 kg.
Typical Barbados aloes varies in colour from yellowish-brown to chocolate-brown, but poorer qualities that have been overheated may be almost black. The drug is opaque and breaks with a waxy fracture. The semi-transparent ‘Capey Barbados’ becomes more opaque on keeping. Curaçao has a nauseous and bitter taste and a characteristic odour recalling iodoform. Mounted in lactophenol, it shows small acicular crystals.
Substitutes and adulterants
Socotrine and Zanzibar aloes are now rare in the British market, and Natal aloes from A. candelabrum is no longer imported. Socotrine is yellowish-brown to blackish-brown, opaque and breaks with a porous fracture. Zanzibar is similar but has a waxy fracture and may be packed with leaves or skins (so-called ‘monkey-skin aloes’). All may be distinguished from official aloes by chemical tests.
Chemical tests
A chromatographic test is included in the BP/EP together with assays for hydroxyanthracene derivatives. Of the latter Barbados aloes should contain not less than 28% and Cape aloes not less than 18% calculated as barbaloin.
Constituents
Aloes contain C-glycosides and resins. The crystalline glycosides known as ‘aloin’ were first prepared by T. and H. Smith of Edinburgh, UK, from Barbados aloes in 1851; Aloin (BP, 1988) contains not less than 70% anhydrous barbaloin. The main crystalline glycoside, barbaloin, is found in all the commercial varieties (Leger, 1907). Leger showed that on heating to about 160°C barbaloin is partly converted into amorphous beta;-barbaloin. This substance is said to be absent from the Barbados variety, but present to the extent of about 8% in the Cape.
Barbaloin is a C-glycoside—a 10-glucopyranosyl derivative of aloe-emodin-anthrone. Unlike O-glycosides, it is not hydrolysed by heating with dilute acids or alkalis. It can, however, be decomposed by oxidative hydrolysis, with reagents such as ferric chloride, when it yields glucose, aloe-emodin anthrone and a little aloe-emodin. It will be seen from the formula of barbaloin that stereoisomerism is possible at C-10; in 1979 both isomers were obtained by HPLC of a methanolic extract of Aloe ferox and in 1980 Auterhoff et al. separated commercial aloin into its stereoisomers. The absolute configuration of the two aloins was independently elucidated by Rauwald et al. (Angew. Chem., Int. Ed. Engl., 1989, 28, 1528) and Manitto et al. (J. Chem. Soc., Perk. Trans. I, 1990, 1297); aloin A is (10S)-barbaloin and aloin B the (10R)-epimer (Fig. 21.15). The two are interconvertible via the corresponding anthranol form. All varieties of aloes give a strong greenish fluorescence with borax, a characteristic of anthranols, which are readily formed from anthrones by isomeric change. This has long been used as a general test for aloes.
Small quantities of aloe-emodin are sometimes present in aloes, and Cape aloes also contains aloinosides A and B, which are O-glycosides of barbaloin; aloinoside B has rhamnose attached via an oxymethyl group at C-3. In A. barbadensis free and esterified 7-hydroxyaloins A and B are characteristic 10-C-glucosyl-anthrones. These compounds are responsible for the violet-purple colours given in various specific tests for Barbados aloes (see H. W. Rauwald et al., Planta Med., 1991, 57, Suppl. 2, A129).
The resin of aloes, reputed to have a purgative action, has been periodically investigated from the end of the nineteenth century onwards. In South African spp. (e.g. A. ferox) aloesin (now often referred to asaloeresin B) was identified in 1970 by Haynes et al., and was the first C-glucosyl-chromone to be described. Other 5-methylchromones isolated from Cape aloes include aloeresin A and C which are p-coumaroyl derivatives linked via a hydroxyl of the glucose. Two non-glucosylated 5-methylchromones present in smaller amounts than the aloesins were reported in 1997. A glycosidic 6-phenylpyran-2-one derivative (aloenin A) was isolated and characterized from A. arborescens leaves in 1974 by Japanese workers. Aloenin B has now been obtained from Kenya aloes (see formulae). (For research on these and related constituents see G. Speranza et al., Phytochemistry, 1993, 33, 175; J. Nat. Prod., 1992, 55, 723; 1993, 56, 1089; 1997, 60, 692. Two aloesol derivatives (Fig. 21.14) have been isolated: 8-C-β-D-glucopyranosyl-7-O-methyl-(R)-aloesol (L. Duri et al., Fitoterapia, 2004, 75, 520) from a commercial sample (Kenya) and the 10S diastereoisomer from A. vera (N. Okamura et al., Phytochemistry, 1996, 43, 495).
Three new naphtho[2,3-c]furan derivatives have recently been isolated from a commercial sample of Cape aloes (J. Koyama et al., Phytochemistry, 1994, 37, 1147).
As with other anthraquinone-producing plants, in Aloe species the content of anthraquinones is subject to seasonal variation, and these compounds are implicated in the active metabolism of the plant. McCarthy and coworkers in South Africa have shown that the anthraquinone derivatives are confined to the leaf juices and that aloin reaches a maximum concentration in the dried leaf juices of A. ferox and A. marlothi in the summer (24.1% in November) and is lowest in winter (14.8% in July).
Uses
Aloes is employed as purgative. It is seldom prescribed alone, and its activity is increased when it is administered with small quantities of soap or alkaline salts, while carminatives moderate its tendency to cause griping. It is an ingredient of Compound Benzoin Tincture (Friars’ Balsam).
There appears to be little variation of the major constituents of the leaf exudate of A. ferox depending on geographical location of the plant but selection of high-yielding strains giving a high production of aloin (25%) is recommended for commercial cultivation (B.-E. van Wyk et al., Planta Medica, 1995, 61, 250).
‘Aloe vera’ products See Chapter 20.
Chrysarobin
Chrysarobin is a mixture of substances obtained from araroba or Goa powder by extraction with hot benzene. Araroba is extracted from cavities in the trunk of Andira araroba (Leguminosae). Chrysarobin contains chrysophanol anthranol, the corresponding anthrone and other similar constituents; it gives a strong green fluorescence in alkaline solution. Chrysarobin was formerly much used for skin diseases and is still occasionally prescribed.
Madder
The root of Rubia tinctorum (Rubiaceae) was formerly grown in large quantities as a dyestuff, but has been almost completely replaced by synthetic dyes. It contains several anthraquinone glycosides, the chief of which, ruberythric acid (Table 21.3) yields on hydrolysis alizarin and primeverose. Twenty compounds have been isolated from the roots and their mutagenicity studied (Y. Kawasaki et al., Chem. Pharm. Bull., 1992, 40, 1504), and three hydroxymethylanthraquinone glycosides have been described (N. A. El-Emary and E. Y. Backheet, Phytochemistry, 1998, 49, 277).
HYPERICUM—ST JOHN’S WORT
Hypericum consists of the dried aerial parts of Hypericum perforatum, family Hypericaceae (Clusiaceae) gathered usually at the time of flowering or shortly before. Commercial extracts are standardized on their naphthodianthrone content, expressed as hypericin.
The plant is abundant throughout Europe in grassland, woodlands and hedges, extending to the Himalayas and Central and Russian Asia, except in Arctic regions. It was introduced into N.E. America and Australia at an early stage of colonization where it has since become a noxious weed. It is a herbaceous perennial, usually forming a colony with a spreading root system. The bright yellow flowers are in handsome terminal corymbs.
History
The plant was known in ancient Greece for its medicinal attributes and since the Middle Ages has been used for its anti- inflammatory and healing properties. It also became highly regarded for the treatment of mental illness. The generic name derives from the Greek hyper—above, and icon (eikon)—picture, referring to the ancient practice of hanging the plant above religious pictures to ward off evil spirits. The common name St John’s wort is attributed to the fact, among others, that it comes into flower around St John’s Day (June 24th).
The drug is now included in the BP/EP, a number of European pharmacopoeias, the British Herbal Pharmacopoeia, the American Herbal Pharmacopoeia, and as monographs for the German Commission E and ESCOP.
Collection
Collection is from wild and cultivated plants and increased demand has meant that farmers in the US and Australia who battled to eradicate it as a weed now harvest it as a viable crop. Care should be taken during collecting as contact photosensitivity has been reported. Drying at 70° for 10 hours is recommended.
Macroscopy
The drug consists of green leaf fragments and stems, unopened buds and yellow flowers. Oil glands are visible in the leaves as transparent areas, hence the specific name perforatum, and as small black dots on the lower surface. The opposite, sessile leaves are 1.5–4.0 cm in length, elliptical to ovate in outline, glabrous with an entire margin. Pieces of hollow stem are cylindrical with two faint ribs on either side.
The odour is distinct and the taste slightly sweet and astringent.
Microscopy
The upper epidermal cells of the leaf are sinuous in outline with beaded anticlinal walls; the lower epidermis possesses anomocytic and paracytic stomata. The mesophyll has large hypericin-containing oil glands, some with red contents, and these are also found in the petals and sepals. Pollen grains are ellipsoidal, 20–25 μm in diameter, with three pores and a smooth exine. Trichomes and calcium oxalate are absent.
In an innovative study, Rapisarda et al. (Pharm. Biol., 2003, 41, 1) have used scanning electron microscopy and image analysis involving size and shape parameters of leaf epidermal cells to provide a quantitative morphological analysis of the three Italian Hypericum spp.—H. perforatum L., H. hircinum L. and H. perfoliatum. The markers obtained provided key factors for the identification and selection of these species and their hybrids.
Constituents
Hypericum contains a variety of constituents with biological activity.
Anthraquinones. Principally hypericin and pseudohypericin; also iso-hypericin and emodin-anthrone. The BP/EP requires not less than 0.08% of total hypericins expressed as hypericin calculated with reference to the dried drug. The extracted hypericins are assayed by absorption measurement at 590 nm.
Prenylated phloroglucinol derivatives. Hyperforin (2.0–4.5%), adhyperforin and furohyperforin (L. Verotta et al., J. Nat. Prod., 1999, 62, 770), the latter at concentrations of about five per cent of the hyperforin content. These phloroglucinols constitute the principal components of the lipophilic extract of the plant and are considered to be the most important active constituents regarding antibiotic and antidepressant properties. Unfortunately, they are very prone to oxidative transformations and a number of such degradation products have been identified, see L. Verotta et al., J. Nat. Prod., 2000, 63, 412; V. Vajs et al., Fitoterapia, 2003, 74, 439. For an article, with many references, on the wide-ranging aspects of hyperforin, see L. Beerhues, Phytochemistry, 2006, 67, 2201.
The involvement of branched-chain amino acids in the biosynthesis of hyperforin and adhyperforin has been demonstrated with shoot cultures of H. perforatum: L-[U-13C5] valine and L-[U-13C6] isoleucine, when fed to the shoots, were incorporated respectively into the side-chains of hyperforin and adhyperforin. Production of the former was not increased by the administration of unlabelled L-valine, whereas the latter was enhanced by the feeding of the unlabelled L-isoleucine (K. Karppinen et al., Phytochemistry, 2007, 68, 1038). Two phloroglucinols, hyperfirin and adhyperfirin, previously reported to be precursors of hyperforin and adhyperforin, respectively, have now been detected in the plant (E. C. Tatsis et al., Phytochemistry, 2007, 68, 383).
Flavonoids. These include flavonols such as kaempferol, luteolin and quercetin, the flavanol glycosides quercitrin, isoquercitrin and hyperoside. The biflavonoid amentoflavone (Fig. 21.18) is confined principally to the flowers (A. Umek et al., Planta Medica, 1999, 65, 388).
Selected formulae for the above are shown in Figs 21.16 and 21.18.
Volatile oil. Up to 0.35% consisting principally of saturated hydrocarbons including alkanes and alkanols in the range C16–C29.
Other constituents. Many other components of hypericum have been reported including various plant acids (caffeic, chlorogenic, etc.), amino acids, vitamin C, tannins and carotenoids.
Many reports have appeared concerning the distribution of the above constituents in different organs of the plant and generally on a weight for weight basis it is the flowers, particularly the petals, that possess the highest concentrations. According to an Australian report (I. A. Southwell et al., Phytochemistry, 1991, 30, 475) it is the narrow-leaved varieties, both in Europe and Australia, that possess a relatively high proportion of oil glands and give a higher yield of hypericin compared with the broad-leaved forms. Hypericin concentrations determined on twelve samples of herb collected throughout Oregon varied widely (0.01–0.38%) (G. H. Constantine and J. Karchesy, Pharm. Biol., 1998, 36, 365). B. Buter et al. (Planta Medica, 1998, 64, 431) have suggested that a key factor for successful future field production will rest in the selection of genetically superior strains giving increased secondary metabolite production, together with improvements in agrotechnological methods. In this connection R. J. Percifield et al. (Planta Medica, 2007, 73, 1525), studying 50 Hypericum accessions, have demonstrated the value of amplified fragment-length polymorphism analysis for the characterization of closely related samples.
Cell culture of Hypericum spp. and their chemotypes has proved extremely variable in naphthadianthrone yields with pseudohypericin production exceeding that of hypericin (T. Kartnig et al., Planta Medica, 1996, 62, 51). Flavonoids, completely different to those of the intact plant and including the new compound 6-C-prenylluteolin, have been identified in callus cultures of H. perforatum (A. P. C. Dias et al., Phytochemistry, 1998, 48, 1165).
Other Hypericum spp
The genus contains some 400 spp.; most of the more common ones have lower or nil hypericin contents and are often distinguishable in the dried condition by the nature of the ridges on the stem. H. maculatum (Imperate St John’s wort) is similar in constituents to H. perforatum but contains less; it may be distinguished by the slightly quadrangular stem and larger leaves. H. hirsutum, H. tetrapterum and H. montana are other common European species.
The essential oils of two Turkish species (H. hyssopifolium and H. lysimachioides) are rich in sesquiterpene hydrocarbons, but unlike some other species investigated, poor in monoterpene hydrocarbons. Caryophyllene oxide is a major component. Both oils possess antimicrobial activities (Z. Toker et al., Fitoterapia, 2006, 77, 57). Eight species from S. Brazil showed no detectable amounts of hypericin or pseudohypericin (A. Ferraz et al., Pharm. Biol., 2002, 40, 294). H. chinense finds use in Japanese folk medicine for the treatment of female disorders. It contains acyl phloroglucinols and spirolactones; six new xanthones have been reported (N. Tanaka and Y. Takaishi, Chem. Pharm. Bull., 2007, 55, 19).
Action and uses
An explosion in the popularity of St John’s wort related to its unregulated availability for the treatment of mild to moderate depression. In the USA, for the first eight months of 1999, it ranked second to ginkgo as the best-selling product of the herbal mainstream market, with retail sales valued at over 78 million (M. Blumenthal, HerbalGram, 1999, 47, 64). In Germany, it represented 25% of all antidepressant prescriptions. It was described as ‘nature’s Prozac’, without the disadvantageous side-effects of the latter. However, a cautionary warning was struck by two reports (S. Piscitelli et al., Lancet, 2000, 355, 547; F. Ruschitzka et al., Lancet, 548). In the first, St John’s wort was observed to lower plasma concentrations of the protease inhibitor indinavir. In the second report, heart transplant rejection, as a result of the lowering of ciclosporin plasma concentrations below therapeutic levels, followed St John’s wort therapy. It has subsequently transpired that St John’s wort will adversely affect the performance of a number of common drugs by causing their rapid elimination from the body, either by enhanced metabolism or as a result of increased action of the drug transporter P-glycoprotein. Among common drugs so affected are anticoagulants such as warfarin, digoxin, tricyclic antidepressant agents, simvastatin and others.
In the UK, there are currently (2007) no specific restrictions on the sale of St John’s wort as a herbal preparation but it is recommended that professional advice be sought if it is to be taken in conjunction with other medicines.
COCHINEAL
Cochineal is an important colourant and indicator and consists of the dried female insects, Dactylopius coccus, containing eggs and larvae. It contains about 10% of carminic acid which is a C-glycoside anthraquinone derivative (Table 21.3). The insects are described in detail in Chapter 33.
NAPHTHOQUINONES AND GLYCOSIDES
The nature of these compounds was indicated earlier; they are produced by higher plants, fungi and actinomycetes and exhibit a broad range of biological actions including fungicidal, antibacterial, insecticidal, phytotoxic, cytostatic and anticarcinogenic. In plants they commonly occur in the reduced and glycosidic forms as illustrated by the 4β-D-glucoside of α-hydrojuglone, a constituent of walnut tree leaves (Juglans regia, Juglandaceae).
On extraction and work-up, or in the soil, the compounds are oxidatively converted to the coloured naphthoquinone. In some heart-woods, e.g. Diospyros spp. (Ebenaceae) napthoquinones occur as monomers, complex dimers and trimers. In addition to timber usage (ebony) many species of Diospyros are used world-wide in the traditional medicine of countries where they grow. S. Ganapaty et al. (Phytochemistry, 2006, 67, 1950) have reported on the antiprotozoal properties of various naphthoquinones, viz two naphthaldehydes, diospyrin, 8′-hydroxydiospyrin and plumbagin isolated from the roots of D. assimilis. Plumbago zeylanica (Plumbaginaceae) grows throughout tropical Africa and Asia and the root is used in Indian medicine; it contains juglone in addition to pentacyclic triterpenes.
Naphthoquinones have been shown to be biosynthesized via a variety of pathways including acetate and malonate (plumbagin of Plumbago spp.), shikimate/succinyl CoA combined pathway (lawsone) and shikimate/mevalonate combined pathway (alkannin).
Henna
Henna consists of the dried leaves of Lawsonia inermis (Lythraceae), a shrub cultivated in north Africa including Egypt, India and Ceylon. The leaves are greenish-brown to brown and about 2.5–5 cm long. The apex is mucronate, the margin entire and revolute, and venation pinnate. Henna contains a colouring matter, lawsone (a hydroxynaphthoquinone), various phenolic glycosides, coumarins, xanthones, quinoids, β-sitosterol glucoside, flavonoids including luteolin and its 7-O-glucoside, fats, resin and henna-tannin. (For a report on new glucosides see Y. Takeda and M. O. Fatope, J. Nat. Prod., 1988, 51, 725.) Henna is commonly used as a dye for the hair, and wool washed in a dilute solution of ammonia and boiled in a decoction of the drug should be dyed Titian red.
The astringent stem-bark of L. inermis is traditionally used in India for the treatment of jaundice, enlargement of the liver and spleen, and for various skin diseases. Isoplumbagin, exhibiting significant anti-inflammatory activity, has been isolated from the bark in 0.05% yield (M. Ali and M. R. Grever, Fitoterapia, 1998, 69, 181). For a recent report on the hepatoprotective activity of the bark see S. Ahmed et al., J. Ethnopharmacology, 2000, 69, 157.
Lithospermums
The genus Lithospermum (60 spp.) (Boraginaceae) contains plants with hormonal activity. The seeds of the European L. officinale (gromwell) were formerly official in several pharmacopoeias.
The reported constituents of the herb are shikonin, a naphthoquinone derivative; scyllitol, a cyclitol; a cyanoglucoside-lithospermocide; caffeic, chlorogenic and ellagic acids; and catechin-type tannins. Shikonin, the enantiomer of alkannin (found in Anchusa Root, see below) is also a constituent of L. erythrorhizon root and is produced for the cosmetic and pharmaceutical industries in Japan by cell culture of the plant. Among the many publications on this subject, Tani et al. (Phytochemistry, 1992, 31, 690) reported on the structure of an endogenously produced oligogalacturonide necessary for the induction of shikonin biosynthesis in the culture. For investigations relating to the biosynthesis of shikonin from p-hydroxybenzoic acid and geranyl pyrophosphate in L. erythrorhizon see T. Okamoto et al., Phytochemistry, 1995, 38, 83.
In the Far East, preparations of the purple roots have long been used for the treatment of burns, inflammations, wounds and ulcers. In Europe, alkanna root has been similarly employed and it has now been shown in laboratory tests that shikonin and alkannin have no significant difference in anti-inflammatory activity. The occurrence of these naphthoquinones is of interest, since similar compounds occur in the related families Rubiaceae (Galium, Rubia), Verbenaceae and Bignoniaceae. Lithospermum arvense is used as an oral contraceptive in Central Europe, as it suppresses the oestrus cycle. The North American Lithospermum ruderale has similar hormonal activity.
Alkanna root
Alkanet or Anchusae Radix is the dried root of Alkanna tinctoria (Boraginaceae), a herb found in Hungary, southern Europe and Turkey. It consists of reddish-purple roots about 10–15 cm long and 1–2 cm diameter near the crown. The surface is deeply fissured and readily exfoliates. Attached to the crown are the remains of leaves having whitish, bristly hairs. Alkanna is used for colouring oils and tars and in the form of a tincture for the microscopical detection of oils and fats. The pigments are naphthoquinone derivatives of the formulae below.
Alkannin itself may be an artefact arising from various esters. Most of the pigment compounds produced in cell culture appear to give alkannin on KOH hydrolysis (TLC, Rf values) and root cultures give pigments identical to those extracted from normal roots (G. Mita et al., Plant Cell Rep., 1994, 13, 406).
Other members of the Boraginaceae—for example, Macrotomia cephalotes (Syrian Alkanet)—produce similar red naphthoquinones.
CHROMONES AND XANTHONES
These compounds are structural derivatives of benzo-γ-pyrone and although not of great pharmaceutical importance a few compounds are worthy of mention.
Chromones are isomeric with the coumarins. A simple derivative is eugenin (Fig. 21.17) found in the clove plant, Syzygium aromaticum. More complex are the furanochromones, the active constituents of the fruits of Ammi visnaga (q.v.).
Xanthones are found mainly in the Gentianaceae and Guttiferae, otherwise scattered sporadically throughout the plant kingdom as in the Moraceae and Polygalaceae. The characteristic oxygenation pattern of these compounds derived from higher plants indicated that they were of mixed shikimate–acetate origin whereas xanthones derived from fungi show a characteristic acetate derivation. An important step in their biosynthesis appears to be the oxidative coupling of hydroxylated benzophenones. Simple oxygenated derivatives, such as gentisin which contributes to the yellow colour of fermented Gentian Root (q.v.), are found in both the Gentianaceae and Guttiferae. More highly oxygenated compounds and O-glycosylxanthones are found in the former family whereas prenylated xanthones, several of which have antimicrobial properties, are widely distributed in the latter. For studies on the antifungal xanthones from the roots of Marila laxiflora, Guttiferae, see J.-R. Ioset, Pharm. Biol., 1998, 36, 103. The C-glycosyl xanthone mangiferin (Fig. 21.17) is found in several species of Hypericum and in Cratoxylem pruniflorum and Chiretta (Swertia chirata). Mangiferin has anti-inflammatory, antihepatotoxic and antiviral properties. In contrast to its CNS-stimulant properties other xanthones exhibit CNS depressive properties in rats and mice.
The mycotoxin pigments of Claviceps purpurea (ergot) are complex xanthones called secalonic acids. They contribute, with the ergot alkaloids, to the toxic properties of the whole drug.
FLAVONE AND RELATED FLAVONOID GLYCOSIDES
The flavonoids which occur both in the free state and as glycosides are the largest group of naturally occurring phenols. More than 2000 of these compounds are now known, with nearly 500 occurring in the free state. They are formed from three acetate units and a phenylpropane unit as has already been outlined (Fig. 18.11) and are typed according to the state of oxygenation of the C3 unit, i.e. C-2,3,4 (see Fig. 21.18 and Table 21.5). Examples given in this section all have a γ-pyrone moiety with the exception of the chalcones, which although not strictly flavonoids are biosynthetically related. The anthocyanins are described later.
The flavones and their close relations are often yellow (Latin flavus, yellow). They are widely distributed in nature but are more common in the higher plants and in young tissues, where they occur in the cell sap. They have been used extensively as chemotaxonomic markers and are abundant in the Polygonaceae, Rutaceae, Leguminosae, Umbelliferae and Compositae (see Table 21.5).
They occur both in the free state and as glycosides; most are O-glycosides but a considerable number of flavonoid C-glycosides are known. Dimeric compounds with, for example, a 5′-8-carbon–carbon linkage are also known (biflavonoids). The glycosides are generally soluble in water and alcohol, but insoluble in organic solvents; the genins are only sparingly soluble in water but are soluble in ether. Flavonoids dissolve in alkalis, giving yellow solutions which on the addition of acid become colourless.
Although the original high hopes for the therapeutic usefulness of flavonoids were not immediately realized, recent researches have demonstrated their involvement in the medicinal action of drugs such as liquorice root, Roman chamomile and gingko. It is very probable that a number of herbal remedies, whose constituents are as yet unknown, will be shown to contain active flavonoids. Of the 84 drugs described in the BHP, Vol 1, 1991, some 36 contain flavonoids but not necessarily as the active constituents. A number of flavonoid-containing herbs have now been included in the BP/EP, examples are Birch Leaf, Calendula Flower, Elder Flower, Horsetail, Lime Flower, Motherwort and Passiflora. The group is known for its anti-inflammatory and antiallergic effects, for antithrombitic and vasoprotective properties, for inhibition of tumour promotion and as a protective for the gastric mucosa. Some of these pharmacological properties can be explained on the basis of antioxidant activity as has recently been shown for tiliroside (see Lime Flower) and the related gnaphaliine isolated from the aerial parts of Helichrysum italicum (G. R. Schinella et al., Fitoterapia, 2007, 78, 1). Many flavonoid-containing plants are diuretic (e.g. buchu and broom) or antispasmodic (e.g. liquorice and parsley). Some flavonoids have antitumour, antibacterial or antifungal properties. E.-A. Bae et al. (Planta Medica, 1999, 65, 442) have recently investigated the in vitro anti-Helicobacter pylori activity of a number of flavonoids (hesperidin, hesperetin, naringin, naringenin, diosmin, diosmetin) and suggest that even if not potent inhibitors of, they may contribute to the prevention of gastritis. Others, e.g. fustic (from the wood of Morus tinctoria) and sumac (leaves of Rhus spp.) are colouring and tannin materials.
Pure flavone, which is colourless, occurs on the surface of some species of Primula. As shown in Table 21.5, many flavones are phenolic or methoxyl derivatives and form sap-soluble glycosides. The intensity of their yellow colour increases with the number of hydroxyl groups and with increase of pH.
Isoflavonoids
Isoflavones are found in the heartwood of species of Prunus and in species of Iris, and are particularly abundant in the Leguminosae (e.g. in dyer’s broom, Genista tinctoria). The latter contains genistin (not to be confused with the gentisin of gentian), the 7-glucoside of genistein. Rotenone contained in the roots of Derris and Lonchocarpus species (see Chapter 40) is an isoflavonoid in which the 2,3 double bond of an isoflavone is reduced.
Phyto-oestrogens
Isoflavones, along with coumestans (also flavonoids) and lignans (q.v.), belong to a class of substances known as non-steroidal phyto-oestrogens. Both structurally and functionally they are similar to oestradiol (see Fig. 23.4) and related sex hormones and exert weak oestrogenic effects. They are present in certain foods and herbal remedies and are well-documented as producing infertility in animals as, for example, clover disease in sheep grazing on clovers containing a high phyto-oestrogen content.
Studies provoking much medical and general press attention have centred on the role of phyto-oestrogens as dietary constituents having positive effects in the prevention of cancer, heart disease and postmenopausal symptoms.
Foods containing appreciable quantities of isoflavones are soya beans, soy products and other legume crops; they are also present in the herbs Red Clover Flower BHP (Trifolium pratense) and broomtops (Cytisus scoparius). In the plant they occur free or in the glycosidic form, in the latter case being hydrolysed by colonic bacteria to give the active aglycone; genistein and daidzein are the principal examples, the latter being formed from formononetin (Table 21.5). As plant nutraceuticals, these compounds are more fully discussed in Chapter 32.
A new class of non-steroidal phyto-oestrogens are the prenylated flavonoids. Many of these compounds are known and this activity has been described for 8-isopentenylnaringenin.
Biflavonoids
The first biflavonoid to be isolated was gingetin, in 1929. Now more than 100 are known with a variety of biological activities being reported. Amentoflavone is of wide distribution, e.g. species of Ginkgo, Hypericum, Rhus and together with robustaflavone has been shown to have activity against influenza A virus, HSV-1 and HSV-2 viruses (Y.-M. Lin et al., Planta Medica, 1999, 65, 120).
Andersen M, Markham KR. Flavonoids, chemistry biochemistry and application. CRC Press/Taylor and Francis. Boca Raton, FL, 2006;xiv:1237
Cos P, et al. Phytoestrogens and recent developments. Planta Medica. 2003;69(7):589-599.
Gross M. Flavonoids and cardiovascular disease. Pharmaceutical Biology. 2004;42(Supplement):121-135.
Neuhouser ML. Flavonoids and cancer prevention: what is the evidence in humans? Pharmaceutical Biology. 2004;42(Supplement):36-45.
Pietta P-G. Flavonoids as antioxidants. Journal of Natural Products. 2000;63(7):1035-1042.
AGNUS CASTUS FRUIT
The BP/EP, BHP drug consists of the whole, ripe, dried fruit of Vitex agnus castus L., family Verbenaceae. Synonyms include Chaste tree, Chaste berry and Monk’s pepper, alluding to its association with chastity. The plant is a shrub or small tree found in the Mediterranean regions of southern Europe; Morocco and Albania are important commercial suppliers.
The small dark berries are collected from the wild in autumn and dried. As such, they are blackish-brown in colour with a diameter of up to 5 mm, quadrilocular and four-seeded. A persistent toothed calyx covers up to three-quarters of the fruit. Features of the powder include covering and glandular trichomes from the calyx and numerous diverse fragments from the pericarp and seeds—all detailed in the official monographs.
Extensive studies on the phytochemistry have involved flavonoids (of which the BP specifies a minimum content of 0.08% calculated as casticin), also vitexin, penduletin and kaempferol; diterpenes including rotundifuran (Fig. 21.19) and vitexilactone; and various iridoids including aucubin (Fig. 24.1). The latter is used as a reference in the TLC test of identity and casticin is assayed by liquid chromatography. Volatile oil (ca 0.5%) consists mainly of mono- and sesquiterpenes.
The drug has a long history of use in various menstrual problems and in deficient lactation. In 2001 it was recommended as a therapeutic option for premenstrual syndrome where no cause could be identified (R. Schellenberg et al., Br. Med. J., 2001, 322, 134) and further supported in 2006 by research demonstrating its activation of the μ-opiate receptor (D. E. Webster et al., J. Ethnopharmacol, 2006, 106, 216).
BIRCH LEAF
Birch leaf has been included in several European pharmacopoeias for many years. The BP/EP drug consists of the dried whole or broken leaves of Betula pendula Roth. and/or B. pubescens Ehrh. and hybrids, family Betulaceae, containing not less than 1.5% of flavonoids calculated as hyperoside. B. pendula (silver birch) is common throughout Europe and B. pubescens (downy or white birch) is Eurasian in origin. Commercial supplies derive largely from Eastern Europe, China and the former USSR.
Leaves from B. pendula are 2.5–6.0 cm in length and 2–5 cm wide, glabrous, green on the upper surface, lighter green on the lower; in shape rhomboidal, triangular or ovoid in outline with a broadly tapering or cuneate base attached to a long petiole. The margin is biserrate, the apex long and acuminate. Larger veins are pinnate with an overall reticulation.
B. pubescens has similar, somewhat smaller, more rounded and often slightly pubescent leaves. The apex is neither long nor acuminate and the marginal teeth are smaller and less obviously apically directed than with B. pendula.
The microscopical characteristics of both species are similar, the lower epidermi having, in surface view, straight-walled cells and numerous anomocytic stomata with four to eight subsidiary cells. Calcium oxalate crystals occur as clusters in the mesophyll cells and as crystal fibres near the larger veins. Peltate sessile glands situated in shallow depressions are numerous on both surfaces. B. pubescens possesses unlignified unicellular thick-walled covering trichomes 80–100–200–600 μm in length.
The flavonoid compositions of both B. pendula and B. pubescens are similar with total flavonoids (about 3%) including hyperoside (up to 0.8%), quercitrin (up to 0.14%), myricetin galactoside (up to 0.37%) and other glycosides of quercetin (e.g. the arabinoside avicularin), kaempferol and myricetin. For a detailed analysis of 14 batches of B. pendula and three batches of B. pubescens see A. Carnat et al., Ann. Pharm. Franc., 1996, 54, 231. This group also found the flavonoid level of older leaves of B. pendula to be lower than that of young leaves. The small proportion (0.1%) of essential oil contains sesquiterpene oxides. Other constituents of the leaves are (+)-catechin, monoterpene glycosides, triterpene alcohols and esters of the dammarene type. The mineral content (c. 4%) is particularly rich in potassium.
The official assay involves an acid hydrolysis of the powdered sample followed by extraction of the flavonoids, and their determination by absorption measurements at 425 nm.
Preparations of the leaf are used as an irrigant of the urinary tract, especially in cases of inflammation and renal gravel. Birch leaf is also an antirheumatic and has been employed to treat gout; as an astringent it is used as a mouthwash.
Birch bark has found traditional use in medicine for the topical treatment of skin diseases; it contains proanthocyanidins and considerable amounts of triterpenoid derivatives of the lupane (betulin, betulinic acid, lupeol) and oleane (oleanolic acid) types. Recently, M. Laszczyk et al. (Planta Medica, 2006, 72 1353) have described the isolation and the physical, chemical and pharmacological properties of a gel extract from the outer bark containing triterpenes which can be applied topically to the skin; its cytotoxic properties were demonstrated experimentally. Betulinic acid is a potential agent for the treatment of human melanoma.
CALENDULA FLOWER
Source
Calendula flower derives from the marigold Calendula officinalis L., family Compositae, and is not to be confused with ‘marigold’ referring to Tagetes spp. The EP and BP specify the whole or cut, dried, and fully opened flowers detached from the receptacle and obtained from cultivated varieties. The BHP allows also the whole composite flowers which includes the involucre of bracts.
C. officinalis is a native of Central, Eastern and Southern Europe and commercial supplies are obtained largely from Eastern Europe and Egypt.
Characters
Detailed descriptions for the ligulate florets and the composite flower heads will be found in the BP and BHP respectively. Diagnostic features are the morphology of the ligulate and tubular florets, various biseriate clothing and glandular trichomes (see e.g. Fig. 42.4), pollen grains with a spiny exine, and corolla fragments with yellow oil-droplets, calcium oxalate and fairly large anomocytic stomata.
Constituents
Flavonoids, triterpenoids, essential oil and polysaccharides are the principal constituents of calendula flowers. All groups have been shown to exhibit pharmacological activity and serve to illustrate the difficulty of devising an assay which represents the true therapeutic activity of the drug. The EP and BP determine the flavonoid content, expressed as hyperoside (not less than 0.4%), utilizing the same method as for Birch Leaf. Other assays based on triterpenoid assessment have been described.
The flavonoid mixture involves quercetin and isorhamnetin derivatives. Triterpenoid saponins (calendulosides A–F, see Table 23.5) are glycosides based on oleanolic acid-3-O-β-D-glucuronide and are present in variable proportions (2–10%) depending on time of harvesting and chemotype. The roots are a richer source than the flowers. These saponins have haemolytic and anti-inflammatory activity. Polysaccharides include a rhamnoarabinogalactan (Mr 15 000; rhamnose 24.8%, arabinose 34.2%, galactose 41.0%) and two arabinogalactans with Mr’s of 25 000 and 35 000 (J. Varljen et al., Phytochemistry, 1989, 28, 2379). Antitumour and phagocytosis stimulation properties have been reported for the polysaccharide fraction. At least 15 compounds have been identified in the essential oil.
Other constituents of the flowers are triterpene alcohols (e.g. α- and β-amyrin, calenduladiol, etc.), sesquiterpenes and carotenoids.
ELDER FLOWER
Elder flower consists of the dried flowers of Sambucus nigra L., family Caprifoliaceae. This shrub or small tree is native throughout Europe and Western and Central Asia; commercial supplies of the flowers come principally from Eastern Europe, small quantities are collected in the UK.
Characters
The elder inflorescence consists of small regular flowers arranged in compound umbel-like cymes; calyx superior, 5-toothed; corolla flat, rotate, deeply 5-lobed, creamy white with 5 stamens inserted in the tube; anthers yellow. The flowers have a slightly bitter taste and a sweet, not altogether agreeable odour.
The microscopical characters of the corolla include numerous small oil globules and an upper epidermis with cells having slightly thickened, beaded walls and a striated cuticle. Epidermal cells of the calyx also have striated walls and those at the basal end exhibit unicellular marginal teeth. Calcium oxalate is seen as idioblasts of sandy crystals. There are numerous pollen grains about 30 μm in diameter (as measured in a chloral hydrate mountant) with a faintly pitted exine and three germinal pores and furrows.
Constituents
The drug contains a small proportion (up to c. 0.2%) of a semi-solid volatile oil consisting of free acids, principally palmitic acid, and C14–C31 n-alkanes. By 1985 over 80 components had been identified in the oil.
Flavonoids (up to 3.0%) are predominantly flavonols and their glycosides: rutin predominates with smaller quantities of isoquercetrin, astragalin and hyperoside together with the aglycones quercetin and kaempferol.
Other constituents are triterpenes (α- and β-amyrin principally as esters of fatty acids), triterpene acids (ursolic, oleanolic and 20β-hydroxyursolic acids), various other plant acids (chlorogenic, p-coumaric, caffeic and ferulic acids, (Fig. 19.5), and their beta;- glucosides), sterols, mucilage, tannin and traces of sambunigrin (Table 25.1).
Standards
There are BP/EP limits for discoloured, brown flowers (15%) and for fragments of coarse pedicels and other foreign matter (8%). Thin-layer chromatography is employed as a test for identity with further modification to detect adulteration with Sambucus ebulus. Flavonoids, calculated as isoquercetrin are determined by absorbance measurements at 425 nm.
Allied species
Sambucus ebulus (danewort) is a perennial, foetid glabrous herb with a creeping rhizome and upright little-branched stems. It occurs throughout Europe and apart from habit, is distinguished from S. nigra by obvious ovate stipules. S. canadensis, American elder, is a somewhat smaller tree than S. nigra and is widely spread throughout North America; it is used similarly to S. nigra.
Uses
Elder flowers are administered principally as an infusion or herbal tea for the treatment of feverish conditions and the common cold; it acts as a diaphoretic but the mechanism and constituents involved are unclear. The flowers also have diuretic properties.
It may be noted that the sialic acid-binding lectin present in elder stem-bark extracts finds considerable current use in certain biochemical procedures.
HORSETAIL
Equisetum BHP 1996, Horsetail BP/EP consists of the dried sterile aerial parts of Equisetum arvense L. (common horsetail). The plant is found throughout Europe, common in Britain, central China and parts of N. America, preferring the moist sandy or loamy soil of hedgebanks, fields or waste places. Classed within the Pteridophyta (p. 21) it is a flowerless perennial, 20–80 cm in height producing in the spring chlorophyll-free jointed stems each terminating in a sporangia-producing cone. Apparent in the summer are jointed green stems with grooved, toothed sheaths at the nodes together with whorls of many-jointed, spreading branches. It is these sterile structures that constitute the medicinal drug.
Macroscopically, the drug consists of broken green stems and branches, the larger pieces being up to 80 cm in length and 5 cm in diameter. The surface is rough to the touch and the fracture short, exposing a large central cavity. The internodes of the main stem have up to 15 vertical grooves and at the nodes a sheath with as many triangular lanceolate teeth as grooves on the internodes. The branches are solid, again with internodes, the lowest of which on each branch is longer than the sheath with which it is associated.
Diagnostic features of the powder include: characteristic epidermis with paracytic stomata overlapped by the two adjacent subsidiary cells; two-celled non-lignified protuberances on the ridged areas; large-celled parenchyma with many lacunae; non-lignified fibres up to 1 mm long with narrow lumens; small spirally or annularly thickened lignified vessels.
Constituents
Various flavonoids occur in horsetail to the extent of 1.0%, the BP/EP requiring a content of at least 0.3% total flavonoids expressed as isoquercitroside. A major component is quercetin 3-glucoside, also luteolin glycosides in some samples (Fig. 21.18). Chemical races of horsetail involve flavonoids, see M. Veit et al., Planta Medica, 1989, 55, 214. Horsetail also contains a naturally high mineral content, with silicic acid and silicates comprising about 8% of the drug; also present are potassium and aluminium chlorides, all contributing to a high ash value for the drug for which, unusually for crude drugs, the pharmacopoeia sets a minimum, as well as higher limit, viz: total ash within 12–27%, acid-insoluble ash within 3–15%. Alkaloids are usually absent or present in small amounts, but see below for poisonous adulterants. Phenolic acids, saponins and phytosterols are among other reported constituents.
Uses
At one time the high siliceous mineral content of horsetail rendered it useful for the abrasive cleaning of copper and bare wooden objects. Traditionally, it has been used medicinally for its diuretic, haemostatic and astringent properties in particular for genitourinary complaints and externally to assist wound healing.
Allied species
A number of Equisetum spp. grow in similar damp localities to that of E. arvense, including E. sylvaticum the wood horsetail, E. palustre the marsh horsetail and E. fluviatile the water horsetail and might be mistaken or substituted for the genuine drug. As these are known to cause animal poisoning due to the alkaloids, e.g. palustrine and saponins, which have been reported, correct identification of the drug is essential. This is addressed by the pharmacopoeial macroscopic and microscopic descriptions of the drug, briefly covered above, and the TLC test for ‘Other Equisetum species and hybrids’.
JAVA TEA
Java tea BP/EP, BHP consists of the dried leaves and tops of stems of Orthosiphon stamineus Benth. (O. aristatus Miq., O. spicatus Bak.), family Labiatae/Laminiaceae.
The plant is a perennial shrub up to 1 m in height with a four-angled stem bearing pointed leaves and lilac-coloured flowers arranged in whorls with four very long blue–violet stamens. It is native to S.E. Asia and Australia. Commercial supplies come from plants cultivated in Indonesia; the leaves and tips are collected shortly before flowering.
Characters
The shortly petiolate leaves are 2–7 cm long with pointed apex, cuneate base, coarsely serrate margin, deep green to yellowish-green upper surface, greenish-grey lower surfaces and, often pigmented pinnate venation.
Microscopical characteristics include wavy-walled, slightly beaded epidermal cells, diacytic stomata more numerous on the lower surface, laminaceous glandular trichomes having in contrast to many other species only four secretory cells, multicellular uniseriate conical clothing trichomes often with reddish contents. The stems afford considerable non-specific vascular tissue.
Constituents
Flavonoids as represented by sinensetin (Fig. 21.18) and various derivatives; diterpenes as illustrated by orthosiphols A–J and other highly oxygenated diterpenes (see S. Awale et al., Chem. Pharm. Bull., 2003, 51, 268); various benzochromenes, including methylripariochromene A in variable amounts and others; volatile oil up to 0.7% and containing β-caryophyllene and its oxide, α-humulene, β-elemene, etc.; caffeic acid and its derivatives, particularly rosmarinic acid; phytosterols such as β-sitosterol; and inorganic salts, particularly potassium at around 3.0%.
The BP/EP requires a minimum content of 0.05% sinensetin for the drug determined by liquid chromatography using sinensetin as the reference compound.
LIME FLOWER
Lime Flower BP/EP consists of the dried inflorescences of Tilia cordata Miller (small-leaved lime), T. platyphyllos Scop. (broad-leaved lime), T. × vulgaris Heyne or a mixture of these, family Tiliaceae. The trees are native throughout Europe and extensively planted. Commercial supplies of the flowers come from China, the Balkans, Turkey and Hungary, the latter exporting (1997) over 100 tonnes.
The inflorescences consist of pendulous long-peduncled cymes consisting of yellowish-green flowers, the peduncles being adnated to almost glabrous, strap-shaped bracts for about half their lengths. Each flower has five petals, five sepals, numerous stamens forming five groups, and a five-lobed stigma. The odour is faintly aromatic and the taste sweet and mucilaginous.
Features of the microscopy are the mucilaginous cells of the sepals and petals; small clusters of calcium oxalate crystals throughout the parenchymatous tissues; oval to slightly triangular pollen grains, 30–40 μm in diameter and having three germinal pores and a finely granulated exine; other general features of the sepals and petals.
The flavonoid constituents comprise quercetin glycosides (rutin, hyperoside, quercitrin, etc.) and kaempferol glycosides (tiliroside, astragalin). Mucilage, present chiefly in the bracts consists largely of galactomannans, and a small proportion of volatile oil (c. 0.02–0.1%) containing farnesol, farnesyl acetate, geraniol and eugenol gives the drug its characteristic faint odour, more pronounced with the fresh flowers. Phenolic acids and proanthocyanidins are also present.
The official upper limit for foreign matter in Lime Flower is 2.0% with observations for the absence of T. americana (basswood) and T. tomentosa based on flower structure. No assay is given but methods have been published in the German literature.
As with most herbal remedies lime flowers have a multiplicity of applications. In action they are diaphoretic, antispasmodic and expectorant and as such are used, often in conjunction with other herbs, as a nerve tonic, for the treatment of catarrh and indigestion, and for the alleviation of headaches.
MOTHERWORT
The dried aerial parts of Leonurus cardiaca L. family Labiatae/Lamiaceae constitute the drug Motherwort BP/EP, Leonurus BHP 1983 (common name lion’s tail). The herb is collected during the flowering period.
L. cardiaca is native to Siberia and found generally throughout Europe from Scandinavia to the N. Mediterranean countries; it is rare in Britain, occurring on waste land, in hedges, ditches etc., and has become naturalized in N. America. A perennial herb 60–150 cm in height, it has square stems and alternate leaves. The lower and middle leaves are distinctly divided into five to seven pointed, dentate lobes, whereas the upper leaves are three-lobed, dark green on the upper surface with few trichomes, paler green and felted on the lower surface. Labiate flowers occur in well-separated whorls in the axils of the upper leaves, the general form giving rise to the generic and one of the common names of the plant (see above). The pubescent flower is white or pink, often spotted on the lower lip of the corolla; the angled calyx has five teeth the two lower ones being sharply recurved.
The microscopic features of the green powder are characteristic of the family and include: straight-walled cells of the upper epidermis with striated cuticles, the lower epidermis with anisocytic stomata, glandular trichomes with short unicellular stalks and multicellular heads, numerous covering trichomes occasionally up to 1500 μm in length, vascular tissue of stems and veins, small clusters and single crystals of calcium oxalate.
For the dried drug the BP/EP limits brown or yellow leaves to 2% and other foreign matter to 2%.
Constituents
Apart from the earlier isolation of stachydrine (formula, see Fig. 26.2), it was in the period 1970–1985 that the major constituents of motherwort were elucidated; these include flavonoids, iridoids, terpenoids and tannins.
Flavonoids include hyperoside, kaemferol-3-D-glucoside, quercitrin and rutin (for formulae of these see Fig. 21.18 and Table 21.5). Iridoids include leonuride (see Fig. 24.1), ajugol and others. Minor alkaloids in addition to the major alkaloid stachydrine include the stereoisomers of its 4-hydroxy derivative (turicin) and betonicine (see formula under ‘Yarrow’). Diterpenes of the labdane type include leocardin and a diterpene similar to marrubiin (q.v.). For the structural determination of three new labdane diterpenes see K. Vijai et al., Planta Medica, 2008, 74, 1288.
The BP/EP requires a minimum flavonoid content for the drug of 0.2% expressed as hyperoside and assayed by absorbance measurements at 425 nm on a hydrolysed extract.
OLIVE LEAVES
The olive (Olea europaea L., family Oleaceae), best known medicinally for its expressed oil (q.v.), is also used in continental Europe for
the antiseptic, astringent and sedative properties of the leaves. It can be employed both internally and externally.
The dried, leathery leaves, 30–50 mm long and 10–15 mm wide are elliptic, oblong or lanceolate in shape. The apex is mucronate, base shortly petiolate and tapering; margin entire and somewhat recurved. The upper surface is dark green, the lower paler due to a covering of silvery trichomes.
Microscopy of the powder shows thick walled polygonal epidermal cells with small anomocytic stomata on the lower surface together with large peltate trichomes, often broken. Sclereids are apparent.
Flavonols, including rutin (Fig. 21.18) and oleuropein are the principal components. Both compounds are used in the BP/EP TLC test for identity and there is a minimum requirement of 5.0% for oleuropein determined by liquid chromatography using a standard solution of it as reference. The pentacyclic triterpenoid maslinic acid occurs in the petioles and has various biological activities; it may offer advantages in the resistance to oxidative stress in animals (Montilla M. P. et al., Planta Med., 2003, 69, 472).
Triterpene acids have been isolated from plant-cell suspension cultures; they include six ursane-type acids and two oleane-type acids (oleanolic and maslinic) with the ursane-type predominant (H. Saimaru et al., Chem. Pharm. Bull., 2007, 55, 784).
Olive leaves are used as an infusion for their tranquillizing effect in nervous tension and for their antiseptic, astringent and febrifuge properties.
PASSIFLORA
Passiflora (Passion Flower) consists of the dried aerial parts of Passiflora incarnata L. collected during the flowering and fruiting period. The drug is described in the BP, EP, BHP, the British Herbal Compendium, Vol. 1 and in an ESCOP monograph; it is also official in the French, German and Swiss pharmacopoeias. The genus is native to South America and species are widely cultivated as ornamentals. P. incarnata is imported from the USA, India and to some extent from the West Indies.
By the nature of its definition, the macroscopical and microscopical characteristics of the drug are numerous and for these the reader should consult one of the sources mentioned above.
Constituents of the genus include flavonoids, mainly C-glycosides of apigenin and luteolin such as vitexin, isovitexin, orientin, iso-orientin and their 2′′-β-D-glucosides. The BP drug is required to contain not less than 1.5% total flavonoids calculated as vitexin (measurement at 401 nm) after treatment of a dry extract with methanol in glacial acetic acid followed by boric acid and oxalic acid in anhydrous formic acid. Other constituents include traces of volatile oil, cyanogenetic glycosides and possibly traces of alkaloids of the harman type. Species other than D. incarnata (P. coerulea, P. edulis) are eliminated by thin-layer chromatography.
Passiflora has sedative actions; it is a popular ingredient of herbal preparations designed to counteract sleeplessness, restlessness and irritability.
For a review update covering all aspects of the drug (over 200 refs). see K. Dhawan et al., J. Ethnopharmacol., 2004, 94, 1–23.
SPINY RESTHARROW ROOT
Spiny restharrow, Ononis spinosa L., family Leguminosae/Papilionaceae (syn. Restharrow, Cammock, Stayplough) is widely distributed throughout most of Europe except for the mountainous regions and the extreme north, also western Asia and North Africa; in England it is less common than the related species O. repens (Common restharrow). The plant is cultivated in Europe for medicinal purposes and harvested in the autumn. It is principally the roots that are used for medicinal purposes.
The dried drug consists of brown, longitudinally grooved roots, somewhat flattened and twisted and showing in transverse section a distinct radial arrangement of the xylem vascular tissue. The fracture is short and fibrous. Microscopical features include rounded starch grains and vascular tissue having vessels with small bordered pits.
Constituents
Isoflavones include the pterocarpan medicarpin (Fig. 21.18) (also a constituent of lucerne), homopterocarpin-7-O-glucoside and trifolirhizin (maackianin-7-O-glucoside); possibly the isoflavone formonoetin and its 7-O-glucoside. Tannins, lectins and triterpenoids, including α-onocerin (α-onoceradiendiol) have also been recorded. Volatile oil (0.02–0.29%), occurring in the entire plant, gives the drug a somewhat unpleasant odour and contains principally anethole, carvone and menthol; among other constituents are methone, camphor and estragole.
Hesperidin and rutin
Although flavonoid preparations such as hesperidin and rutin are used in medicine, they do not appear to have justified the high hopes which followed the work of Szent–Györgyi in 1935 on the ‘citrin’ (sometimes known as vitamin P) of paprika and lemon peel. Citrus and other fruits have long been included in the human diet and, in addition to ascorbic acid and other compounds, provide flavonoids which decrease capillary fragility and are therefore employed in cases of hypertension and radiation injuries. The substance formerly known as ‘citrin’ is now known to be a mixture of the rhamnoglucosides of eriodictyol (a tetrahydroxyflavone) and methyl eriodictyol (hesperetin). Among the commercially available products of this type are some produced by the citrus industry containing hesperidin. A similar glycoside, rutin, (Fig. 21.18) the rhamnoglucoside of quercetin, is found in many plants, and commercial supplies are made from tobacco residues, Sophora and Eucalyptus spp. or buckwheat (Fagopyrum esculentum), which yields about 3–4%. Hairy root cultures of F. esculentum are reported to give a higher flavonol production than normal root cultures (F. Trotin et al., Phytochemistry, 1993, 32, 929). The flowers of Sambucus nigra (elder) have long been used in domestic and veterinary medicine, particularly in the form of ointment. They contain p-coumaric acid, rutin and kaempferol.
Rutin occurs as a yellow crystalline powder, soluble in alkali but only slightly soluble in water. Rutin on hydrolysis yields quercetin, rhamnose and glucose, while hesperidin yields hesperetin (or methyl eriodictyol), rhamnose and glucose.
BUCKWHEAT HERB
The drug consists of the dried aerial parts of Fagopyrum esculentum Moench, family Polygonaceae, collected when the plant is flowering and prior to fruiting.
Fagopyrum esculentum originated in Central Asia and by the Middle Ages was being cultivated in Europe as a source of grain and green fodder. It has been cultivated in the UK and is now found wild on wasteground as an escape. The fresh plant contains a photosensitizing agent, which, if consumed by animals exposed to sunlight, can cause them damage.
The plant is a little-branched, glabrous herb often with reddish stems and producing a cymose paniculate inflorescence with pink or white flowers. Dark green leaves, paler on the lower surface, are broadly triangular in outline, cordate–saggitate and acuminate. Features of the powder include anomocytic stomata, epidermal papilla-like projections over the veins, numerous calcium oxalate cluster crystals up to 100 μm and small prismatic crystals, vascular tissue of leaf and stem, spherical pollen grains and corolla fragments.
Rutin (see above) is the most important therapeutic constituent of the herb and the BP/EP requires a minmum content of 4.0% determined by liquid chromatography with absorbance measurements at 350 nm.
Buckwheat is used in the treatment of various circulatory disorders, including varicose veins, chilblains and retinal bleeding.
Silybin and silymarin
A number of flavonolignans—for example, silybin and silymarin (a 1,4-dioxan produced by the oxidative combination of taxifolin and coniferyl alcohol)—have antihepatotoxic properties, and extracts of plants containing them—for example, Silybum marianum (Carduus marianus), Compositae—are widely used in Germany for the treatment of liver ailments. The fruits of S. marianum contain silybin, silydianin and silychristin. For further details and structure of silybin see Chapter 29.
Visnaga
The drug consists of the dried ripe fruits of Ammi visnaga (Umbelliferae), an annual plant about 1–1.5 m high. It grows in the Middle East and is collected, particularly in Egypt. The greyish-brown mericarps are usually separate but are sometimes attached to the carpophore. Each mericarp is broadly ovoid and about 0.5 mm long. It has five prominent primary ridges and six vittae. Odour, slightly aromatic; taste, very bitter.
Khellin, the most important active constituent, is crystalline and has been synthesized. It is 2-methyl-5,8-dimethoxyfuranochrome. It occurs to the extent of about 1%, the highest concentration being reported in the immature fruits, and is accompanied by two other crystalline compounds, visnagin (about 0.1%) and khellol glucoside (about 0.3%). The fruits contain a minute amount (less than 0.03%) of volatile oil. Contrary to previous ideas that khellin and visnagin are located in the vittae, Franchi et al. (Int. J. Crude Drugs. Res., 1987, 25, 137) have shown that, for fruits collected in Southern Tuscany, the furanochromones are present in the large secretory canals of the primary ribs and in the endosperm.
The drug has long been used in Egypt. Khellin, which is now commercially available in tablets and injection, is a potent coronary vasodilator. It has been employed in the treatment of angina pectoris and bronchial asthma, but its use appears to be limited by undesirable side-reactions.
ANTHOCYANIDINS AND GLYCOSIDES
Anthocyanidins are flavonoids structurally related to the flavones. Their glycosides are known as anthocyanins. These names are derived from the Greek antho-, flower, and kyanos, blue. They are sap pigments and the actual colour of the plant organ is determined by the pH of the sap. For example, the blue colour of the cornflower and the red of roses is due to the same glycosides and both of these plants on hydrolysis with hydrochloric acid yield cyanidin hydrochloride.
Table 21.6 gives a few examples of these numerous and very widely distributed compounds. The most common anthocyanidin, cyanidin, occurs in about 80% of permanently pigmented leaves, 69% of fruits and 50% of flowers. Cyanidin is followed in order of frequency by delphinidin and pelargonidin.
Anthocyanidins are precipitated from aqueous solutions as lead salts or as picrates. After hydrolysis with 20% hydrochloric acid, anthocyanidin hydrochlorides, being only slightly soluble, often crystallize out. Chromatographic methods are widely used for the separation and identification of both the aglycones and sugars.
The sugar components are usually attached in the 3- or (more rarely) 5-position. It may be noted that in flavone glycosides the attachment is usually in the 7-position. They may be monosaccharides (glucose, galactose, rhamnose or arabinose); disaccharides (e.g. the rhamnoglucoside of Antirrhinum spp.); or trisaccharides (e.g. the 5-glucoside-3-rutinoside of certain Solanaceae such as Atropa and Solanum). Diglucosides, in which separate glucose molecules are attached in both the 3- and 5-positions, are common (e.g. Campanula and Dahlia spp.).
Despite their considerable biological importance the anthocyanidins are of little pharmaceutical significance as such, but as previously considered they constitute the monomers of the polymeric condensed tannins (q.v.).
For a review covering the analysis and biological activities of anthocyanins, see J.-M. Kong et al., Phytochemistry, 2003, 64, 923–933.
BILBERRY FRUIT
Bilberry (Vaccinium myrtillus L., family Vacciniaceae/Ericaceae) also known as blaeberry, whortleberry and huckleberry, is distributed throughout Europe, N. Asia and N. America including Canada. It grows on the acid soils of mountainous regions, heaths and moorlands and is found in most, particularly northern, regions of the British Isles. The plant is a glabrous, deciduous shrub up to 60 cm tall with creeping rhizomes and numerous erect stems and branches. It bears ovate, bright green leaves, 1–3 cm in length, pitcher-shaped greenish-pink flowers and globose berries about 8 mm in diameter, blue–black when ripe with a glaucous bloom. The quadri- or quinque-locular mesocarp of the fruit contains many ovoid, small, brown seeds. The edible sweet-tasting berries are collected from July to September. Both leaves and fruits were recognized in the Middle Ages for their medicinal value and separate monographs for fresh and dried fruits are now included in the BP/EP.
Constituents of the fruits
Anthocyanins, particularly glucosides and galactosides of cyanidin, peonidin, delphinidin, petunidin and malvidin (Table 21.6) are responsible for the final colour of the berries. These pigments increase in quantity during ripening whereas that of the polyphenols (−)-epicatechin, (+)-catechin and dimeric proanthocyanidins (Fig. 21.7) decrease. Other constituents include a number of common phenolic acids, vitamin C and volatile compounds. Over 100 volatiles have been identified, the principal ones that afford the characteristic odour of the berries being trans-2-hexenal, ethyl 3-methylbutyrate and ethyl 2-methylbutyrate.
For the dried fruits, the BP/EP specifies a minimum tannin content of 1.0% expressed as pyrogallol and for the fresh fruits a minimum of 0.30% anthocyanins expressed as cyanidin-3-glucoside chloride. The latter is determined by absorption measurements at 528 nm on an acidified aqueous extract of the dried fresh berries. The loss on drying of the fresh berries is 80–90%.
Frozen fresh berries should be stored at or below −18°C. It is important to inspect the dried drug for insect and mouldiness.
Uses and actions
Bilberry has many traditional medicinal uses, a number of which have been supported by fairly extensive pharmacological research. For detailed references, the reader should consult J. Barnes et al., Herbal Medicines, 2nd edn. 2002, p. 73, The Pharmaceutical Press, London. See also P. Morazzoni et al., Fitoterapia, 1996, 66, 3 for an overall review of bilberry.
STILBENES
Two stilbenes of pharmacognostical interest are rhaponticin and resveratrol. The former is a glycosidal constituent of rhapontic rhubarb (q.v.) and due to its fluorescence in u.v. light has long been used to detect adulteration of the official rhubarb. Resveratrol is a constituent of species of Arachis, Cassia, Eucalyptus, Polygonum and Veratrum. Recent interest has centred on its occurrence in grape preparations including red wine, and its therapeutic properties as an antioxidant, anti-inflammatory, anti-PAF and anticancer agent. It may also reduce the risk of coronary heart disease (J. S. Soleas et al., Clinical Biochemistry, 1997, 30, 91). As a constituent of ‘darakchasava’, it has long featured in Indian medicine (B. Paul et al., J. Ethnopharmacology, 1999, 68, 71).
Pinosylvin, a natural stilbene of the heartwood of Pinus spp. is related to, and has similar antibacterial properties to, resveratrol. S. K. Lee et al., Fitoterapia, 2005, 76, 258.
Stilbenes are biosynthesized from hydroxycinnamic acids and acetate.
For further discussion, see Chapter 32: The plant nutraceuticals.