Chapter 34 Miscellaneous products
There are a few miscellaneous pharmaceutical materials of natural origin which are not included in the preceding chapters; these are considered below.
KIESELGUHR OR DIATOMITE
Large deposits of diatomite are found in Aberdeenshire in the UK, Virginia and California in the USA, Germany and North Africa. The crude product contains about 65–87% of SiO2, together with organic matter, clay, iron oxide and about 5–15% of water. The silica is mainly amorphous, being present in the siliceous walls of minute, unicellular plants belonging to a number of families of the Bacillariophyceae. A much smaller percentage of silica occurs in the walls of spicules of siliceous sponges and, in a crystalline form, as sand. Depending on the geographical origin of the diatomite, the diatoms may be either freshwater or marine forms.
The material is dried and crushed, ignited to remove organic matter, boiled with dilute hydrochloric acid to remove impurities such as iron, washed with water and dried. It is then sifted or ‘air-blown’, the finest grades used in face powders being obtained by the latter method.
Characters
Purified kieselguhr is a fine, white or pale-buff odourless powder. For microscopical examination it may be mounted in cresol or olive oil. In the latter medium the amorphous silica of the diatoms becomes almost invisible, while the crystalline particles of sand remain clear. Only small amounts of sand (Fig. 34.1H) should be present.
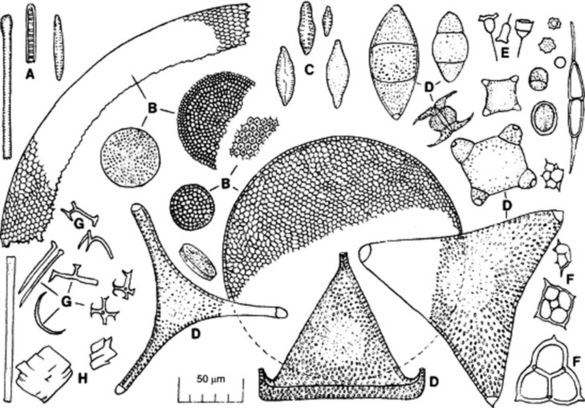
Fig. 34.1 Diatomite (various sources) showing the shells of diatoms and other constituents. A, Diatom skeletons of the Fragilariaceae (e.g. Synedra, Fragilaria); B, entire or broken portions of Coscinodiscus spp.; C, shells of the Naviculaceae (e.g. Navicula); D, various forms belonging to the Biddulphioideae (Biddulphia, Trinacria, etc.); E, fragments of Asterionella spp.?; F, silicoflagellates; G, sponge spicules; H, sand particles.
The diatoms (Fig. 34.1) consist of two halves or valves which fit together like a pill-box. The two positions from which they may be studied are known as the valve-view and the girdle-view. The valves show considerable variation in shape, some samples of kieselguhr showing numerous discoid types resembling that of the Arachnoidiscus found in agar, while other samples consist largely of pennate forms. A mixture of both types is usually most suitable for filtration. In many diatoms a median cleft is found in the valves, known as the raphe. The valves also show dots and lines, which vary in the different species and are due to minute cavities in the wall.
Kieselguhr is insoluble in all acids except hydrofluoric, but is soluble after fusion with alkalis. It is used for the filtration of oils, fats, syrups, etc., and in the form of the Berkefeld filter for sterilization. Highly purified material is used as an inactive support in column, gas and thin-layer chromatography; the powder will hold up to its own weight of water and still retain its powdery consistency. Diatomite is also employed in face powders, pills, polishing powders and soaps, and to absorb nitroglycerin in the manufacture of dynamite; it is a component of the BP (Vet) pyrethrum dusting powder.
Extant species of diatoms form an important component of plankton and are involved in the food chains of seas and rivers. (For a wide-ranging illustrated introduction to these single-celled plants see The Diatoms; Biology and Morphology of the Genera by F. E. Round et al. (1990), Cambridge University Press.)
PREPARED CHALK
Chalk is a whitish or greyish rock which is widely distributed in north-western Europe. It consists mainly of the shells of unicellular animals known as the Foraminifera. Chalk as quarried often contains about 97 or 98% of calcium carbonate, the remainder being largely siliceous and therefore insoluble in acids. The impure chalk is finely ground with water and freed from most of the heavier siliceous impurities by elutriation. The coarser product is sold as ‘whiting’ and the finer elutriated product is allowed to settle and while still pasty is poured into a funnel-shaped trochiscator. The latter is tapped on a porous chalk slab and ejects the chalk to form ‘cones’, which are allowed to dry giving Prepared Chalk BP. These cones (‘crab’s eyes’) may be powdered.
Characters
For examination chalk should be mounted in cresol, warmed and examined microscopically (Fig. 34.2). Most of the foraminiferous shells have been broken but a number of whole ones usually remain. The whole shells may be concentrated in a small bulk by removing the broken ones by elutriation and examining the residue. Note the following:
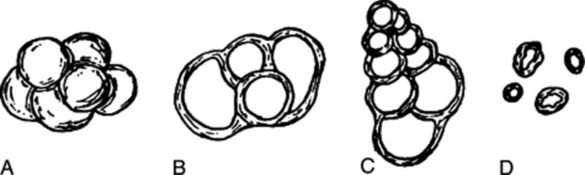
Fig. 34.2 Shells from prepared chalk. Globigerina in (A) water, (B) cresol (×200). C, Textularia in cresol (×200). D, coccoliths (×400).
Globigerina. In these the shell is of calcite and is perforated by large canals. Each consists of a few lobular chambers arranged in a plane or helicoid spiral. The size varies from about 35 to 80 μm.
Textularia. In these the shell is composed of grains of sand cemented together by calcareous matter. They are usually conical or cuneiform in shape and are composed of numerous chambers in two alternating parallel series. The size varies from about 50 to 180 μm.
Remains of fossil algae. Small rings or discs about 4–9 μm in diameter, termed coccoliths or morpholites.
Prepared chalk is assayed by acid–alkali back-titration; there are pharmacopoeial limits for heavy metals and arsenic; limits for aluminium, iron, phosphate and matter insoluble in hydrochloride acid are determined gravimetrically.
Precipitated chalk
Precipitated chalk (calcium carbonate BP/EP is made by the interaction of a soluble calcium salt and a solublecarbonate. The precipitate varies considerably with the method of preparation. When precipitated at about 0 °C, the product is very light and almost entirely amorphous; at about 30 °C a denser precipitate of minute rhombohedra is formed, and if boiling solutions are used, the precipitate consists of prismatic rhombohedra with a higher specific gravity than either of the previous forms. The BP assay involves a complexometric titration of calcium; there are limits for various metals, etc.
GELATIN
Gelatin is a mixture of reversible gel-forming proteins derived from certain animal tissues, particularly skin and bones, with hot water. The process converts insoluble collagens into soluble gelatin, the solution of which is then purified and concentrated to a solid form.
The initial stages of the preparation vary with the starting material, bones, for example, being defatted with an organic solvent and sometimes decalcified by treatment with acid. Two types of gelatin are characterized in the BP/EP—type A is obtained by partial acid hydrolysis of animal collagen and type B by partial alkaline hydrolysis; mixtures of both types are also permitted.
Characters
Sheet gelatin prepared as above may be cut into strips or made into a granular powder. Gelatin is colourless or pale yellow, istranslucent and has little odour or taste. It is insoluble in cold water but absorbs a considerable volume of liquid; it dissolves on heating and a 2% solution forms a jelly on cooling. The gelatinizing power of gelatin is reduced by long boiling. The quality of gelatin is largely judged by its ‘jelly strength’ or ‘Bloom strength’ which is determined by a Bloom gelometer. The two types of gelatin (A and B) have isoelectric points in the ranges pH 6.0–9.5 (A) and pH 4.7–5.6 (B). Type B is compatible with anionic substances (e.g. the natural gums), whereas type A is not; for some specific purposes, narrower tolerance limits than above may be required. Note the BP/EP limit tests and standards.
Constituents
Gelatin consists mainly of the protein glutin and therefore gives the usual tests for proteins. Thus, it evolves ammonia when heated with soda lime (distinction from agar); with mercuric nitrate solution gives a white precipitate that turns brick-red on warming; it gives a precipitate with a solution of trinitrophenol.
Uses
Gelatin is used in the preparation of pastilles, pastes, suppositories, pessaries, capsules, pill-coatings and gelatin sponge. Specially purified and pyrogen-free gelatins are available for intravenous injection, and a grade with high ‘Bloom strength’ is used for making gelatin capsules and for bacteriological culture media.
Gelatin sponge
Gelatin sponge can be conveniently mentioned here as an absorbable, water-insoluble haemostatic material. It may be prepared by whisking a warm solution of gelatin to a foam of uniform porosity and drying. After cutting into pieces it is sterilized by dry heat. Note the BP standards for this material. It is used in a similar manner to oxidized cellulose.
FISH BODY OILS
The oils expressed from the bodies of a number of ‘oily’ fish of the families cited on p. 43 contain esters of omega-3 fatty acids. As such, they have become important dietary supplements and two such oils are included in the BP/EP. For an explanation of the structural representation of the various acids, as cited below, see Chapter 19, ‘Fatty Acids’.
Fish oil, rich in omega-3-acids
The expressed oil is processed in much the same way as for cod-liver oil (q.v.) and involves winterization and deodorization. The esterifying ω-3 acids, as exemplified in the pharmacopoeia, are: α-linolenic acid (C18:3 n-3), moroctic acid (C18:4 n-3), eicosatetraenoic acid (C20:4 n-3), timnodonic (eicosapentaenoic) acid (C20:5 n-3; EPA), heneicosapentaenoic acid (C21:5 n-3), clupanodonic acid (C22:5 n-3) and cervonic (docosahexaenoic) acid (C22:6 n-3; DHA).
It will be noted that up to six double bonds may be involved in these acids; all are ω-3-acids and the positions of the remaining double bonds occur in sequence, separated by one methylene group (see α-linolenic acid, Table 19.3).
The total omega-3-acids, expressed as triglycerides, should be 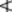 28.0%; that of EPA
28.0%; that of EPA 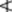 13.0% and DHA
13.0% and DHA 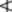 9.0%. Oligomers, determined by size exclusion chromatography (p. 143), should not exceed 1.5%. The maximum permitted anisidine value (p. 180) is 30.0. An antioxidant may be added to the oil.
9.0%. Oligomers, determined by size exclusion chromatography (p. 143), should not exceed 1.5%. The maximum permitted anisidine value (p. 180) is 30.0. An antioxidant may be added to the oil.
Farmed Salmon Oil
BP/EP is obtained from salmon, Salmo salar, family Salmonidae, which have been fed in accordance with EU or other applicable regulations. The oil is expressed mechanically at below 100 °C either from whole fish or fish from which the fillets have been removed; it is centrifuged and winterized. The pale pink oil contains the important polyunsaturated acids DHA, EPA and moroctic acid (see above); the two former constitute 10.0–28.0% of the oil, expressed as triglycerides. Chromatography is used to identify the acid components and 13C-NMR for their further evaluation. The anisidine value is maximized at 10.0, considerably less than for the Fish Oil described above; similarly with the peroxide value. These figures indicate the extent of secondary oxidation of the oils.
Preparations derived from fish body oils
The following modifications of fish body oils are included in the pharmacopoeia.
Omega-3-marine triglyceride contains a mixture of the glyceryl esters prepared from the purified concentrated acids or from the omega-3-acid ethyl esters. It contains a minimum 60.0% of total omega-3 acids expressed as triglycerides and a minimum 45% of EPA and DHA, also expressed as triglycerides. The maximum permitted peroxide value is 10.0 and that for the anisidine value 30.0.
Omega-3-acid ethyl esters 60 and omega-3-acid ethyl esters 90 contain higher minimum concentrations of EPA and DHA than the above, as indicated in their names. They are prepared by transesterification of the body oil of ‘oily’ fishes with subsequent purification, fractionation and molecular distillation.
These preparations are used to treat such conditions as hypertriglyceridaemia, to reduce the risk of CHD, thrombosis and for other disorders, still under evaluation.
SILK
Silk is the prepared fibre from the cocoons of Bombyx mori, the mulberry silkworm, and other species of Bombyx and of Antheraea (order Lepidoptera). It is produced in China, Japan, India, Asia Minor, Italy, France and many other countries. While the silk of B. mori forms the greater part of that used, considerable quantities of the so-called wild silks are produced by Antheraea mylitta (India), A. assama (India), A. pernyi (China) and A. yama-mai (Japan).
Before the silkworm passes from the caterpillar to the chrysalis or pupal stage, it secretes around itself an oval cocoon about 2–5 cm long, consisting of a continuous thread up to 1200 m long. This thread consists of two silk or fibroin fibres cemented together by a layer of silk glue or sericin. Strands of semiliquid fibroin, produced by two glands in the insect, flow into a common exit-tube in the head, where they meet the secretion of silk glue produced by another pair of glands. The double fibre with its coating of sericin emerges from a spinneret in the head of the worm, coagulates and hardens on contact with the air and is spun into the cocoon by figure-of-eight movements of the head. If the chrysalis were allowed to mature, the silk would be damaged by the escaping insect. It is therefore killed by heating at 60–80 °C for a few hours or by a short exposure to steam. The cocoons are then graded, placed in hot water and beaten to facilitate removal of the outer layer of fibre, which is only of secondary value, and to soften the silk glue.
The double fibre in the cocoon is known as a bave and its constituent fibres are known as brins. The reeler takes the loose ends of the fibres of 2–15 cocoons and twists and reels them into a single thread. Most raw silk is reeled from about five cocoons and therefore has 10 brins, fibres containing less than six brins being too fine for commercial purposes. Silk is then usually scoured by treatment with hot soap solution to remove the sericin.
Microscopy
Examine some fibres of raw silk mounted in water. The diameter of these is several times that of a single brin; the individual brins may be seen although difficult to count; and flakes of silk glue may be seen on the surface. If a little of this raw silk is now boiled with soap solution or dilute sodium carbonate solution, the sericin completely dissolves and the constituent brins may be mounted and examined.
The lack of cellular structure and the breadth of the brins are distinguishing characters of mulberry silk. Brins of mulberry silk measure 10–21 μm (mostly about 16 μm), whereas those of wild silks are 30–60 μm. The latter often show well-marked longitudinal striations.
Silk gives the general tests for animal fibres, and the following:
Chemical nature
Natural silk is composed of the protein fibroin. Fibroin on hydrolysis gives mainly glycine (44%) and alanine (27%) together with smaller amounts of serine (11%), tyrosine (5%) and other amino acids. The molecule is a chain-like structure, with a repeating unit 0.7 nm long. This repeating unit, as revealed by radiograph analysis, corresponds in length to that of two fully extended amino acid residues.
Surgically, silk is used as a non-absorbable suture and as such must comply with the BP requirements for such materials. (For a general article on silk see M. L. Ryder, Biologist, 1995, 42, 52.)
WOOL, ANIMAL WOOL, SHEEP’S WOOL
Wool is prepared from the fleece of the sheep, Ovis aries (order Ungulata), by cleansing and washing. The length and quality of the hair varies not only from animal to animal, but also in different parts of the same fleece. In order to get more or less uniform grades, the wool-sorter spreads each fleece on a frame covered with wire-netting and separates it into wool of different qualities. At the same time he beats much dust and dirt through the netting and picks out burrs, etc. The wool is washed in tanks of warm, soft, soapy water, being squeezed between rollers as it passes from tank to tank.
The approximate composition of raw wool is as follows: wool fibre, 31%; ‘wool sweat’ or ‘suint’, consisting mainly of the potassium salts of fatty acids, 32%; earthy matter removable by washing, 26%; and ‘wool grease’.
From the washings of the scouring process ‘wool grease’ may be separated by mechanical means or by the use of organic solvents. When purified it is known as wool fat or anhydrous lanolin (q.v.). Potassium salts may also be recovered. After washing, the wool is dried, and the fibres are mechanically loosened, carded, and spun into yarn.
Microscopical
The hairs originate in relatively deep pits or hair follicles in the skin and the ‘wool grease’ is secreted by neighbouring sebaceous glands. If fibres of raw wool are examined under the microscope, they are seen to be covered with irregular masses of grease, the structure of the hair itself being indistinct. If raw wool is to be mounted for microscopical examination, it should be defatted by ether or chloroform, as it will not otherwise wet with water; even with scoured wool, it is advisable to moisten the threads with alcohol before mounting in water, dilute glycerin or solution of picric acid.
Wool hairs are 2–50 cm long and 5–100 μm, usually 13–40 μm, diameter. As the fleeces are removed by shearing, the bases of the hairs are lacking, and tapering ends, known as ‘lamb ends’ are only found in wool from the first shearing. Three regions of the hair, known as the cuticle, cortex and medulla, are distinguishable.
Cuticle
This consists of imbricated, flattened, more or less translucent epithelial scales. The shape and arrangement of the scales varies in different breeds of sheep, edges being smooth and straight in some and serrated and wavy in others. The number of scales in a 100 μm length is fairly constant, averaging about 9.7–12.1, in different wools. Such counts may be used to distinguish sheep’s wool from angora wool, etc.
Tests
Chemical nature of wool
Wool fibres are composed of the protein keratin. They show elasticity, in contrast to the cellulose and silk fibres. X-ray examination of stretched and unstretched fibre shows that the elasticity arises from a reversible intramolecular transformation of the fibre substance. The radiograph of the stretched fibre closely resembles that given by fibres, such as silk, with fully extended polypeptide chains. In this condition each amino acid residue is 0.34 nm long. This unstable form of keratin is known as β-keratin. The stable form, α-keratin is contracted and the structural unit, corresponding to three amino acid residues, is 0.51 nm long. The chemical relationship between these two forms and its importance in conferring elasticity properties on wool fibres is illustrated in former editions of this work.
Leech
The medicinal leech, Hirudo medicinalis, is about 6–10 cm long. The sucker at the anterior end has three radiating jaws provided with ‘teeth’. Placed in contact with the skin, the animal produces a triradiate cut and can draw about 4–8 ml of blood. The salivary glands secrete hirudin, an acidic polypeptide of molecular weight around 7000; it retards coagulation of blood and allows bleeding to continue after the leech has been removed. Preparations containing hirudin for the treatment of bruises are manufactured commercially. Other enzymes isolated from the leech include hementin, an antithrombin agent, and orgelase, which degrades hyaluronic acid.
Some 12 000 kg of leeches are used annually in Europe and are exported from France, Italy, Portugal and Central Europe. The animal, classed as a threatened species, is officially protected in some countries including Britain. Future supplies of leech products may need to be met by commercial farms (one currently operates in S. Wales) and by genetically engineered organisms. The cloning and expression of a recombinant gene for hirudin in yeast and bacteria has been reported (1988).
Although used less than formerly, leeches are often the least painful way to reduce inflammation. They have also staged a medical revival by their use in skin grafting for the removal of coagulated blood from beneath the new skin. Unfortunately, the leech is host to Aeromanas hydrophila, an organism on which it depends to digest the blood consumed. This is a potential source of infection of wounds and, according to a report in the British Medical Journal (11 April 1987), three types of infection, including diarrhoea, have been reported in patients receiving leech treatment. However, the problem can be eliminated by the use of suitable antibiotics.
(For a general review article (20 refs) on the medicinal leech see J. M. Elliot and P. A. Tullett, Biologist, 1992, 39, 153.)
SHELLAC (LAC)
Shellac (lac) is a resinous substance prepared from a secretion that encrusts the bodies of a scale insect Karria lacca (Lucifer lacca), order Hemiptera. Lac is produced in India, Thailand and to a lesser extent in China (5% of world production). In India the chief plants are members of the Leguminosae (Acacia spp., Butea frondosa), Euphorbiaceae (Aleurites laccifera), Moraceae (Ficus spp.), Dipterocarpaceae (Cajanus indivus, Shorea talura), Rhamnaceae (Ziziphus jujuba) and Sapindaceae (Schleichera trijuga). In China the host trees are mainly species of Ficus and Dalbergia (Leguminosae) (C. Saint-Pierre and O. Binrong, Econ. Bot., 1994, 48, 21). The insects resemble cochineal insects in structure and life history.
Lac is found most abundantly on the smaller branches and twigs. These are broken off and constitute stick lac. Usually, however, the lac is not exported in this form but is scraped from the twigs by means of curved knives. The lac is usually ground in India and the colouring matter extracted with water or dilute soda solution. The solution evaporated to dryness constitutes lac dye, and the exhausted lac when dried seed lac. From the latter the four types of shellac recognized in the EP/BP are prepared (Table 34.1). Other commercial grades are also utilized. Button lac is the molten lac poured into circular moulds and stamped with the maker’s name. Flake shellac having a brownish-yellow colour is known in commerce as orange shellac and the darker, reddish-brown varieties are known as ruby or garnet shellac. A number of varieties, required to conform to a table for acid value, loss on drying and wax content, are including in the USP/NF 1995. Lac contains about 6% of wax, 6.5% of red water-soluble colouring matter, laccaic acid, 70–85% of resin and a few insect remains, vegetable debris, etc. The resin, composed of two parts, a hard and a soft fraction, is formed from hydroxy fatty acids and sesquiterpenes. An example of the former is aleuritic acid (9,10,16-trihydroxypalmitic acid) and of the latter, a cedrene-type sesquiterpene acid; a water-insoluble yellow pigment is erythrolaccin, a tetrahydroxy-4-methylanthraquinone. The BP/EP includes tests for colophony (TLC), arsenic, heavy metals, etc. Shellac is classified as a pharmaceutical aid and is also used in varnishes, polishes, sealing wax, etc.
Table 34.1 Pharmacopoeial types of shellac.
| Type | Preparation | Characters |
|---|---|---|
| Wax-containing shellac | From molten seedlac by filtration through bags or by hot solvent extraction. When sufficiently cool the product is stretched into a large sheet and then broken into flakes | Flakes, brownish-orange or yellow. Almost insoluble in water and partly soluble in ether. With alcohol it gives an opalescent solution |
| Bleached shellac | Seedlac is dissolved in hot soda solution, bleached with hypochlorite or chlorine and precipitated by acid. It is ‘pulled’ under water into sticks and dried | A cream to brownish-yellow powder. An opalescent solution is given with alcohol |
| Dewaxed shellac | From seedlac or wax-containing shellac by treatment with a suitable solvent and removal of the wax by filtration | Flakes as wax-containing shellac. With alcohol it gives a clear solution |
| Bleached dewaxed shellac | Seedlac or wax-containing shellac is treated with hot soda solution and bleached with hypochlorite; the insoluble wax is removed by filtration, the product precipitated from solution with dilute acid, and dried | Appearance as bleached shellac. With alcohol it gives a clear solution |
Isinglass
Russian isinglass or ichthyocolla consists of the dried prepared swimming bladder of the sturgeon, Acipenser huso. The fish are caught in South Russian rivers and in the Black and Caspian seas. Isinglass consists chiefly of collagen and resembles gelatin in its properties. Brazilian isinglass is a similar product but derived from fish of different genera.
Ambergris
This very expensive substance used in perfumery is a pathological product found in the intestines of sperm whales or cast by them into the sea. It occurs in streaky grey or brown waxy masses which, exceptionally, may weigh up to 45 kg. It is associated with the beaks of squids on which the whales feed. Ambergris contains about 25% of ambrein. It has a fragrant musk-like odour but its main value lies in the fact that it has a subtle effect on fine perfumes and gives them great tenacity or persistence of odour.
Musk
Musk is the dried secretion from the preputial follicles of the musk deer, Moschus moschiferus. This small deer is found in China andthe Himalayas. The musk-containing sacs are known as ‘pods’. They are about 5–7 cm diameter, weigh up to 30 g and contain about half their weight of musk. When distilled, musk yields about 1.4% of dark brown volatile oil, the chief odorous constituent of which is muskone. This is a cyclic ketone having a closed chain of 15 carbon atoms. Other constituents of musk are steroidal hormones, muscopyridine and other alkaloids and peptides. A synthetic compound, which differs from muskone only in the absence of a methyl group, is cyclopentadecanone. Most other synthetic musk substitutes have little chemical similarity to the natural product. Musk acts as a fixative and is an important ingredient of many high-class perfumes.
Civet
This product, which resembles musk, is obtained from the perineal follicles of African or Indian civet cats, Viverra spp. It contains civetone, a cyclic ketone closely related to muskone but having a closed chain of 17 carbon atoms.
Royal jelly/Queen bee jelly
This hive product of Chinese origin consists of the milky fluid produced by the salivary glands of worker bees and used as essential nourishment for the development of the queen bee larvae. It contains a mixture of amino acids, vitamins (including most of the vitamin B complex and vitamin C), lipids, fatty acids, carbohydrates and minerals. The fresh material is unstable and requires refrigeration. It may also be freeze-dried but more satisfactory preparations are stated to be capsules containing royal jelly stabilized by the addition of honey. Royal jelly is an expensive dietary supplement recommended in health magazines for counteracting the effects of ageing and for the treatment of myalgic encephalomyelitis, depression, dermatitis and other conditions. Its value, which has yet to be clinically proved, may arise from the biologically favourable relative proportions of the many constituents rather than from their quantity.